Abstract
Objective
To estimate the SARS-CoV-2 antibody seroprevalence in healthcare workers (HCWs) at a university hospital in Mallorca, Spain.
Methods
All HCWs received an e-mail inviting them to take part in the study. Participants had a nasopharyngeal swab test performed for reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) and serological tests to detect SARS-CoV-2 antibodies (primary study). Additionally, they were invited to complete a questionnaire on their exposure to COVID-19 individuals and their COVID-19-related symptoms (secondary study). Prevalence of antibodies (IgG, IgM, or both) and 95% confidence intervals (CIs) were calculated.
Results
Seventy-nine percent of the hospitalʼs HCWs (N = 2210) took part in the primary study. Antibodies were detected in 61 participants, a prevalence of 2.8% (95% CI: 2.5–3.1). The prevalence was slightly higher in nurses (3.4%), registrars (3.9%), and wardens (3.4%). Thirty-nine percent of the primary study participants completed the secondary study questionnaire. Those with positive antibody test results had closer contact with COVID-19 individuals (60% vs. 92%; p < 0.001).
Conclusion
After the first wave of the COVID-19 pandemic in Spain, the seroprevalence of SARS-CoV-2 antibodies in our university hospital HCWs was around 2.8%, which is slightly higher than the seroprevalence in the general population in our region. We believe it would be advisable to perform additional seroprevalence studies during the second wave of the epidemic.
Keywords: Healthcare workers, COVID-19, Seroprevalence, Hospital infection
Introduction
COVID-19 (coronavirus disease, 2019) is a novel viral disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first identified in December 2019 in Wuhan, China (Zhu et al., 2020). Due to its alarming spread, disease severity, number of affected countries, and number of deaths, the World Health Organization (WHO) declared COVID-19 a pandemic on 11th March 2020 (World Health Organization, 2020). To date (15th February 2021), there have been more than 106 million confirmed cases and more than two million deaths worldwide (European Centre for Disease Prevention and control, 2020). Spain has been one of the most affected countries (Instituto de Salud Carlos III, 2020).
Most of the available data on SARS-CoV-2 infection is from symptomatic patients, with symptoms that vary from mild, such as fever, cough, diarrhea, or anosmia, to severe, pneumonia, respiratory distress, and death (Martin-Sanchez et al., 2020, Aguila et al., 2020, Vargas-Gandica et al., 2020, Guan et al., 2020). However, it has been demonstrated that some patients with a SARS-CoV-2 infection remain asymptomatic. In these cases, seroprevalence studies can be helpful to determine the proportion of individuals who have antibodies against SARS-CoV-2 and can be used to estimate the actual number of individuals who have been infected. In this line of research, several population-based studies estimating the burden of SARS-CoV-2 infection in France, Switzerland, and Spain have been published (Salje et al., 2020, Stringhini et al., 2020, Pollan et al., 2020). In the latter, in a sample of more than 61 000 participants across Spain, the estimated seroprevalence determined by immunoassay was 4.6% (95% CI: 4.3–5.0) (Pollan et al., 2020). In the Balearic Islands, one of the regions in Spain with fewer cases during the first wave of the epidemic, probably due to an islands-effect, the mean seroprevalence was 1.8% (Pollan et al., 2020). This study was performed at the end of April and the beginning of May, which is considered the end of the first wave of the pandemic in Spain (Pollan et al., 2020).
Hospital healthcare workers (HCWs) are a population particularly at risk of infection since they have been on the frontline of COVID-19 management from the beginning of the epidemic. Studies have been carried out on this specific population to estimate whether the risk of infection is higher in this group than in the general population. In a sample of 316 HCWs in direct contact with COVID-19 patients in a German hospital, the proportion of participants with antibodies against SARS-CoV-2 was 1.6% (Korth et al., 2020). However, in a similar study in a Belgian hospital, the proportion was 12.6% (Martin et al., 2020). Other prevalence studies have been performed in China and the Netherlands, but they were based on symptomatic HCWs (Lai et al., 2020, Kluytmans-van den Bergh et al., 2020). In our country, a seroprevalence study was performed on a random sample of 578 participants from a tertiary hospital in Barcelona (Garcia-Basteiro et al., 2020). The prevalence in HCWs was 9.3%, higher than the seroprevalence in Barcelona's general population (6.8%) (Pollan et al., 2020).
This study’s objectives were to determine the seroprevalence of SARS-CoV-2 antibodies in HCWs at a university hospital in Mallorca, Spain, and to examine the potential relationship with onset symptoms and exposure to COVID-19 subjects.
Methods and materials
Study design, participants, and settings
This cross-sectional seroprevalence study was performed between 28th April 2020 and 11th June 2020. The target population was all HCWs registered in the Human Resources database of the University Hospital Son Llatzer, including Hospital Joan March, on the island of Mallorca, Spain. The HCWs were classified as: physicians, registrars, nurses, nursing assistants, wardens, and other healthcare and non-healthcare workers, including psychologists, physiotherapists, optometrists, nutritionists, social workers, administrative staff, and kitchen workers. For the primary analysis, the inclusion criteria required being registered in the human resources database as a hospital worker and giving consent for a blood draw and a nasopharyngeal swab. All external workers who were not in this database and all participants with no serological results were excluded. The primary study participants who had also completed a questionnaire collecting information on onset symptoms and exposure to COVID-19 subjects (hospital patients, co-workers, or family and friends) were included in the secondary analysis.
The University Hospital Son Llatzer, which includes Joan March Hospital, is the second biggest hospital in Mallorca, with more than 400 beds, and provides health services to a population of around 250 000 (Ministerio de Sanidad). All the participants provided their consent for a blood draw and a nasopharyngeal swab. We obtained approval from the local ethics committee to use the data generated in this study for research purposes (Protocol IB4299/20PI).
Procedures
An e-mail was sent on 20th April 2020 to all HCWs, inviting them to participate in this study. After consenting to participate, information on age, sex, and professional category was recorded. A blood draw for immunoglobulin detection, and a nasopharyngeal swab for reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) to detect SARS-CoV-2 RNA were obtained from all participants and stored at −80 °C until their analysis. Both procedures were performed by trained nurses using appropriate personal protective equipment. When a positive RT-qPCR result was obtained, the patient was immediately contacted and isolated until obtaining a negative result.
Participants who took both tests were also invited to complete the above-mentioned questionnaire online.
Laboratory assessment
RNA was extracted from the nasopharyngeal swab samples using the QIAamp Viral RNA Mini Kit (Qiagen). RT-qPCR was performed for SARS-CoV-2 RdRp and N genes detection (Argene, SARS-CoV-2 GENE). The kit also contains an internal control for validation; a positive and negative control are also included in each analysis.
Serum IgG and IgM antibodies against SARS CoV-2 were performed on the Abbott Architect platform using the Abbott SARS-CoV-2 IgG and IgM assay following the manufacturer's instructions. The assay is a chemiluminescent microparticle immunoassay for the qualitative detection of IgG against the SARS-CoV-2 Nucleocapside protein and IgM against the SARS-CoV-2 Spike protein (San Tang et al., 2020).
More than 60,000 samples have been evaluated with Abbott's SARS-CoV-2 IgG assays in more than 20 publications/evaluations (San Tang et al., 2020, Nicol et al., 2020, Paiva et al., 2021). The Sensitivity reported was between 80% and 100% more than 14 days post symptom onset and specificity between 95% and 100%.
The manufacturer reported a sensitivity for IgG of 86.4% after seven days from symptom onset and 100% after 14 days, and a specificity of 99.6%, using RT-PCR as the gold standard. They reported a sensitivity for IgM of 85.7% after seven days from symptom onset and 96.7% after 14 days, and a specificity of 99.6%, using RT-PCR as the gold standard.
Statistical analysis
All participants with a positive result for IgM, IgG, or IgM and IgG antibodies were considered positive for statistical purposes. Seroprevalence of SARS-CoV-2 antibodies was reported as a percentage, and the 95% confidence interval (CI) was estimated using a finite population correction. Results were reported for the total study population and for each of the seven groups studied: physicians, registrars, nurses, nursing assistants, wardens, and other healthcare and non-healthcare workers. Other healthcare workers included psychologists, physiotherapists, optometrists, nutritionists, and social workers; other non-healthcare workers included administrative and management staff and kitchen workers. Categorical variables were expressed as counts and percentages. Continuous variables such as age were expressed as mean and standard deviation (SD).
Comparisons between groups (positive vs. negative serological results) were performed using Fisher’s exact test (categorical variables) or the independent Student t-test (continuous variables) after assessing for normality using histograms and the Shapiro–Wilk test.
Results
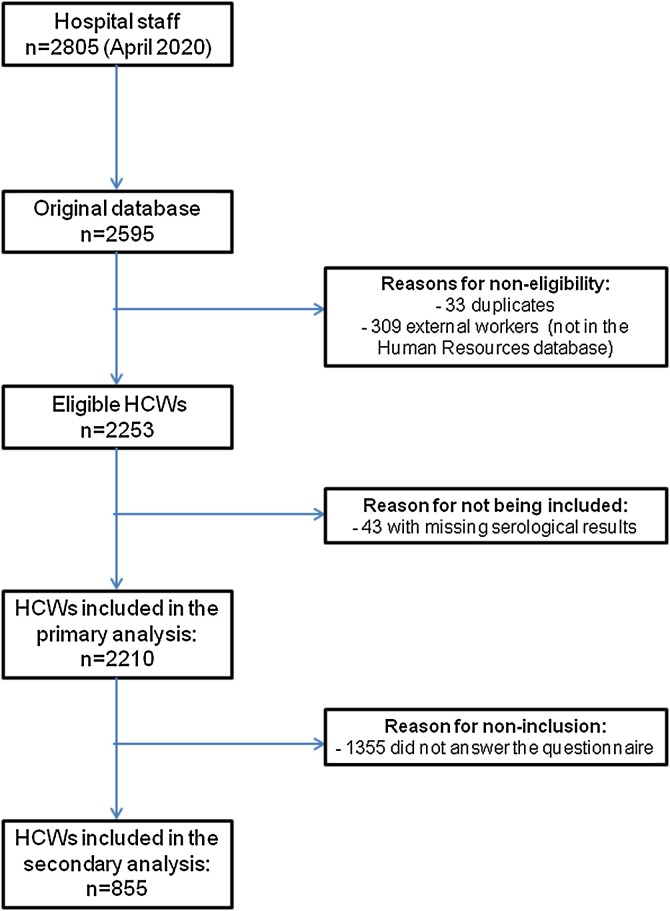
In April 2020, there were 2805 HCWs in the Human Resources database. Our original database was composed of 2595 participants. After an eligibility assessment, a total of 2210 participants were included in the primary analysis (Figure 1 ), with a total response rate of 79%. Mean (SD) age was 44 (10) years, and 74% (1637 participants) were women.
Figure 1.
Study participation flowchart.
Sixty-one participants tested positive for SARS-CoV-2 antibodies, a prevalence of 2.8% (95% CI: 2.5–3.1). Two were positive for IgM, three for IgM and IgG, and the remaining participants (56) were positive for IgG. According to the professional category (Table 1 ), the seroprevalence results show that registrars, nurses, and wardens were the HCWs with the highest seroprevalence. The five non-healthcare workers who tested positive were all from the administrative staff.
Table 1.
Seroprevalence of SARS-CoV-2 antibodies according to professional category.
| Category | HCWs in the HR database | Participants in the primary analysis | Positive cases | Prevalence (%) (95% CI) |
|---|---|---|---|---|
| Physicians | 410 | 380 | 10 | 2.6 (2.2–3.1) |
| Registrars | 110 | 77 | 3 | 3.9 (1.5–6.3) |
| Nurses | 894 | 684 | 23 | 3.4 (2.7–4.0) |
| Nursing assistant | 736 | 556 | 14 | 2.5 (1.9–3.2) |
| Wardens | 228 | 176 | 6 | 3.4 (2.1–4.7) |
| Other healthcare workers | 185 | 108 | 0 | 0 (0–2.2) |
| Other non-healthcare workers | 242 | 229 | 5 | 2.2 (1.7–2.6) |
| Total | 2805 | 2210 | 61 | 2.8 (2.5–3.1) |
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HCW, healthcare worker; HR, human resources; CI, confidence interval.
The mean (SD) age of the 61 participants who tested positive for SARS-CoV-2 antibodies was 42 (11) and 42 were women (69%). There were no statistical differences compared with the participants who had negative results: mean (SD) age was 44 (10), and 74% were women. Seven participants tested positive by RT-qPCR for the detection of SARS-CoV-2 RNA, all of whom also tested positive for IgG in the immunoglobulin assay.
Thirty-nine percent of the participants in the primary analysis (855) completed the questionnaire, 25 of whom had SARS-CoV-2 antibodies. Comorbidities for both groups are shown in Table 2 . No statistical comparisons have been performed between the two studied groups because of the low number of positive Ig group events. In general, the comorbidities rate was low in both groups, cardiovascular disease was the more prevalent one.
Table 2.
Participant comorbidities by immunoglobulin status.
| Comorbidity | Negative Ig (N = 830) | Positive Ig (N = 25) |
|---|---|---|
| Diabetes | 20 (2.4%) | 0 |
| Cardiovascular disease | 78 (9.4%) | 2 (8%) |
| Chronic hepatic disease | 6 (0.7%) | 1 (4%) |
| COPD | 31 (3.7%) | 1 (4%) |
| CKD | 4 (0.5%) | 0 |
| Immunodeficiency | 11 (1.3%) | 0 |
| Cancer | 2 (0.2%) | 0 |
| Pregnancy | 1 (0.1%) | 0 |
Abbreviations: Ig, immunoglobulin; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease.
Table 3 shows the percentage of participants who indicated they had been exposed to COVID-19 subjects. Participants with positive immunoglobulin results had also had more direct exposure to COVID-19 individuals, whether hospital patients, co-workers, or family members.
Table 3.
Relationship between SARS-CoV-2 antibody positivity and exposure to COVID-19 individuals.
| Exposure to COVID-19 individualsa | Negative Ig (N = 830) | Positive Ig (N = 25) | p-Value |
|---|---|---|---|
| Contact with infected people | 494 (59.5%) | 23 (92.0%) | <0.001 |
| Contact with patients | 351 (42.3%) | 15 (60.0%) | 0.10 |
| Contact with co-workers | 175 (21.1%) | 13 (52.0%) | <0.001 |
| Contact with family/friends | 30 (3.6%) | 5 (20.0%) | 0.003 |
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; Ig, immunoglobulin (either IgG, IgM, or both).
The same participant can have been included in more than one group.
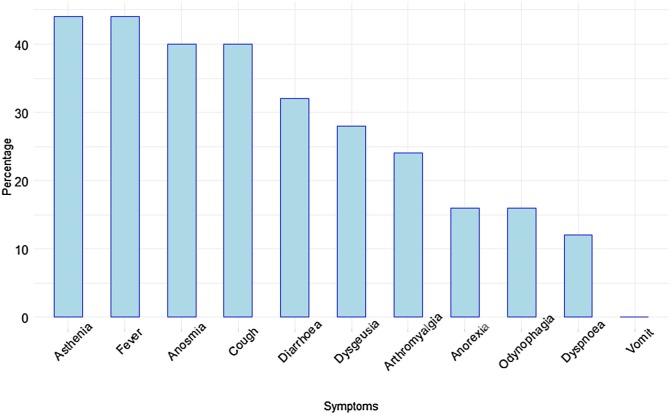
Finally, 15 of the 25 participants who tested positive for SARS-CoV-2 antibodies experienced COVID-19-compatible symptoms. Figure 2 summarizes the main symptoms experienced, with asthenia, fever, anosmia, and cough as the most common ones.
Figure 2.
Main symptoms experienced by participants with SARS-CoV-2 antibodies (N = 25). Results are expressed as percentages.
Discussion
The findings from this cross-sectional study suggest that the seroprevalence of SARS-CoV-2 antibodies in the HCWs of our university hospital in Mallorca, Spain, is around 2.8%. This seroprevalence appears to be slightly higher than in the general population of the Balearic Islands, which is estimated at 1.8% (Pollan et al., 2020).
There is some evidence that HCWs are at greater risk of SARS-CoV-2 infection. In a study carried out in China, 4% of all infected individuals were HCWs (Wu and McGoogan, 2020). This percentage was even higher in Los Angeles County in the United States, where positive HCWs represented 9% of all infections (Hartmann et al., 2020). Other countries have also found a higher seroprevalence of SARS-CoV-2 antibodies in HCWs than in the general population. For example, in a cross-sectional study in another region of Spain, seroprevalence in a tertiary Hospital in Barcelona was 9.3%, a higher percentage than in that region’s general population (6.8%) (Pollan et al., 2020). Furthermore, in London, where the general population seroprevalence was reported to be around 17% (Livemint, 2020), Portland Hospital estimated an HCW seroprevalence of 22% (Khalil et al., 2020). However, other studies have not found differences in the presence of antibodies against SARS-CoV-2 between HCWs and the general population. For instance, in New York, the seroprevalence was around 14%, a figure similar to the seroprevalence found in HCWs from the Northwell Health System (13.7%) (Rosenberg et al., 2020, Moscola et al., 2020).
In our hospital, nurses, wardens, and registrars were the HCWs with the highest seroprevalence. There is little evidence regarding seroprevalence in wardens and registrars in the literature. This may be because registrars are generally included in the same group as the physicians. However, the small sample of registrars in our study population makes an interpretation of this finding difficult. A higher seroprevalence among nurses compared with other HCWs has also been reported. A Chinese study found a higher seroprevalence among nurses than physicians (Lai et al., 2020), while in another study conducted in Los Angeles, infections in nurses accounted for almost half of all SARS-CoV-2 infections, although no prevalence was reported (Hartmann et al., 2020).
As expected, in our study, participants with a higher degree of exposure to COVID-19 individuals had higher levels of SARS-CoV-2 antibodies. Exposure to COVID-19 resulted from contact with co-workers, family, friends, and patients, although the relationship between the presence of anti-SARS-CoV-2 antibodies and exposure to patients was not statistically significant (Table 3; p = 0.10). However, despite not being statistically significant, a tendency towards a higher degree of exposure to COVID-19 patients was observed in HCWs who tested positive in the immunoglobulin assay (60%) compared with the group of HCWs who tested negative (42%).
Concerning self-reported COVID-19 symptoms in the 25 participants who tested positive for SARS-CoV-2 antibodies, asthenia, fever, cough, and anosmia were the symptoms that appeared in the majority of cases. In a study carried out in two Dutch Hospitals, the main symptoms were also asthenia (defined as general malaise), cough, and fever (Kluytmans-van den Bergh et al., 2020). In that study, anosmia was not one of the most common symptoms. However, in a brief report recently published from Emory University School of Medicine (Atlanta, United States), loss of taste and smell was found in 11% of participating HCWs, and fatigue, cough, and nasal congestion were the most common symptoms (Kempker et al., 2020). Thus, the symptoms described in our series are similar to those found in other published studies, except for anosmia, which was present in 40% of participants, a higher percentage than in the majority of studies.
Our study has several strengths. First, to our knowledge, this is the largest sample-sized SARS-CoV-2 seroprevalence study conducted on HCWs in Spain (Garcia-Basteiro et al., 2020, Olalla et al., 2020, Valdivia et al., 2020). Other retrospective studies have reported the percentage of HCWs with symptomatic COVID-19 (Suarez Garcia et al., 2020), but their objective was not to estimate the seroprevalence in HCWs. Furthermore, in addition to the RT-qPCR and serological test results, we also provide information on the degree of exposure to COVID-19 individuals and onset symptoms. Another key aspect of this study is the high response rate achieved (almost 80%).
However, our study is not without limitations. First, we could not classify participants into groups according to their degree of exposure (high vs. low) to COVID-19 patients because information on the precise location of where some of them, such as wardens and assistant nurses, had been working was not available. Although the response rate in the primary study (seroprevalence and PCR) was high, fewer participants completed the questionnaire (39% of those included in the primary analysis). As a result, only 25 participants with positive serological results were included in the secondary analysis. Another key aspect is that this study’s results should be interpreted in the context of the first wave of the pandemic, given that a higher number of individuals are being infected in the Balearic Islands during the current second wave, and therefore seroprevalence may vary.
Furthermore, participants included at the beginning of the recruitment period may have contracted the infection lately during the study, in which case the seroprevalence may be underestimated. There is also a possibility of memory bias when answering the questionnaire. Lastly, there is a possibility that some participants may have been infected with SARS-CoV-2, but by the time this study was performed, their antibody concentrations may have become undetectable, thus testing negative in our study.
Conclusions
SARS-CoV-2 antibody seroprevalence in HCWs in University Hospital Son Llatzer in Mallorca, Spain, is around 2.8% and is slightly higher in nurses, wardens, and registrars. Study participants with antibodies against SARS-CoV-2 had a higher degree of exposure to COVID-19 subjects than those who tested negative. We believe it would be advisable to perform further seroprevalence studies during the second wave of the pandemic in our region.
Author contribution
AR: Study design, Data preparation, Data analysis, Manuscript draft
MA: Study design, Data preparation, Manuscript draft, Manuscript revision
VFB: Laboratory analysis, Manuscript revision
MPL: Sample handling, Data preparation, Manuscript revision
ZAN: Study design, Manuscript revision
JDG: Study design, Manuscript revision
AP: Study design, Manuscript draft, Manuscript revision
All authors have approved the final version of the article.
Funding sources
None.
Conflict of interest statement
None declared.
Acknowledgments
We thank all the personnel from our hospital who participated in the study and all the nurses, physicians, and other workers from the occupational health department.
References
- Aguila E.J.T., Cua I.H.Y., Fontanilla J.A.C., Yabut V.L.M., Causing M.F.P. Gastrointestinal manifestations of COVID-19: impact on nutrition practices. Nutr Clin Pract. 2020;35:800–805. doi: 10.1002/ncp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalogo nacional de hospitals. Ministerio de Sanidad. https://www.mscbs.gob.es/ciudadanos/prestaciones/centrosServiciosSNS/hospitales/home.htm.
- European Centre for Disease Prevention and Control . 2020. COVID-19 situation update worldwide, as of 29th September 2020.https://www.ecdc.europa.eu/en/covid-19-pandemic [Google Scholar]
- Garcia-Basteiro A.L., Moncunill G., Tortajada M., Vidal M., Guinovart C., Jimenez A. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann S., Rubin Z., Sato H., OYong K., Terashita D., Balter S. Coronavirus 2019 (COVID-19) infections among healthcare workers, Los Angeles County, February-May 2020. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto de salud Carlos III. Covid-19%20in%20Spain.%20https:/cnecovid.isciii.es/covid19/.
- Kempker R.R., Kempker J.A., Peters M., Rebolledo P.A., Carroll K., Toomer L. Loss of smell and taste among healthcare personnel screened for coronavirus 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A., Hill R., Wright A., Ladhani S., O’Brien P. SARS-CoV-2-specific antibody detection in healthcare workers in a UK maternity hospital: correlation with SARS-CoV-2 RT-PCR results. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluytmans-van den Bergh M.F.Q., Buiting A.G.M., Pas S.D., Bentvelsen R.G., van den Bijllaardt W., van Oudheusden A.J.G. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai X., Wang M., Qin C., Tan L., Ran L., Chen D. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livemint: https://www.livemint.com/news/world/-17-londoners-5-of-uk-residents-have-covid-19-antibodies-11590148840735.html.
- Martin C., Montesinos I., Dauby N., Gilles C., Dahma H., Van Den Wijngaert S. Dynamic of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk health care workers and hospital staff. J Hosp Infect. 2020;106:102–106. doi: 10.1016/j.jhin.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Sanchez F.J., Del Toro E., Cardassay E., Valls A., Cuesta F., Vigara M. Clinical presentation and outcome across age categories among patients with COVID-19 admitted to a Spanish Emergency Department. Eur Geriatr Med. 2020;11:829–841. doi: 10.1007/s41999-020-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscola J., Sembajwe G., Jarrett M., Farber B., Chang T., McGinn T. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324:893–895. doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol T., Lefeuvre C., Serri O., Pivert A., Joubaud F., Dubée V. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech) J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva K.J., Grisson R.D., Chan P.A., Huard R.C., Caliendo A.M., Lonks J.R. Validation and performance comparison of three SARS-CoV-2 antibody assays. J Med Virol. 2021;93(2):916–923. doi: 10.1002/jmv.26341. [DOI] [PubMed] [Google Scholar]
- Pollan M., Perez-Gomez B., Pastor-Barriuso R., Oteo J., Hernan M.A., Perez-Olmeda M. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olalla J., Correa A.M., Martin-Escalante M.D., Hortas M.L., Martin-Sendarrubias M.J., Fuentes V. Search for asymptomatic carriers of SARS-CoV-2 in healthcare workers during the pandemic: a Spanish experience. MedRxiv preprint. 2020 doi: 10.1101/2020.05.18.20103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E.S., Tesoriero J.M., Rosenthal E.M. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. 2020;48:23–29. doi: 10.1016/j.annepidem.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salje H., Kiem C.T., Lefrancq N., Courtejoie N., Bosetti P., Paireau J. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369:208–211. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Tang M., Hoch K.G., Logsdon N.M., Hayes J.E., Gronowski A.N., Anderson N.W. Clinical performance of two SARS-CoV-2 serologic assays. Clin Chem. 2020;66(8):1055–1062. doi: 10.1093/clinchem/hvaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoC-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez Garcia I., Martinez de Aramayona M.J., Saez A., Lobo P. SARS- CoV-2 infection among healthcare workers in a hospital in Madrid, Spain. J Hosp Infect. 2020;106:357–363. doi: 10.1016/j.jhin.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia A., Torres I., Huntley D., Alcaraz M.J., Albert E., Solano de la Asuncion C. Caveats in interpreting SARS-CoV-2 IgM+/IgG- antibody profile in asymptomatic health care workers. J Med Virol. 2020;93:634–636. doi: 10.1002/jmv.26400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Gandica J., Winter D., Schnippe R., Rodriguez-Morales A.G., Mondragon J., Escalera-Antezana J.P. Ageusia and anosmia, a common sign of COVID-19? A case series from four countries. J Neurovirol. 2020;26:785–789. doi: 10.1007/s13365-020-00875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. WHO Director-general’s opening remark at the media briefing on COVID-19.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:724–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]