Abstract
Background
Serious pain commonly occurs after posterior spinal surgery. This study aims to evaluate the effect of preemptive and multimodal analgesia using celebrex, pregabalin and ropivacaine on pain control after this surgery.
Methods
Ninety-three patients undergoing posterior spinal surgery were enrolled in this prospective, randomized, double-blind, placebo-controlled clinical trial. All patients were treated with patient- controlled analgesia (PCA, intravenous tramadol hydrochloride and flurbiprofen) as required. They were randomized to combination analgesia intervention (oral celebrex, pregabalin and subcutaneous infiltration of ropivacaine), ropivacaine intervention (only subcutaneous infiltration of ropivacaine), and control intervention (placebo). We compared postoperative visual analog scale (VAS) scores and PCA dose among the three groups.
Results
The VAS scores were significantly lower in the combination analgesia group than in the control group at 0 h, 2 h, 12 h, 24 h, 3 d, 5 d, 7 d and 14 d after posterior spinal surgery, while combination analgesia was also superior to ropivacaine in terms of VAS scores at 24 h and 14 d postoperatively. The combination analgesia group was also associated with significantly reduced PCA consumption compared with the control group, but there was no statistical difference in PCA consumption between the ropivacaine group and control group.
Conclusion
Combination analgesia using celebrex, pregabalin and ropivacaine is effective and safe to alleviate pain after posterior spinal surgery.
Clinical Trial Registration
Chinese Clinical Trial Registry No. ChiCTR2000031236.
Keywords: preemptive analgesia, multimodal analgesia, pain control, posterior spinal surgery, randomized trial
Introduction
With the aggravation of aging and an increase in accidents, the number of patients with degenerative lumbar diseases (e.g. lumbar disc herniation and spondylolisthesis) and spinal fracture has significantly increased, and posterior spinal surgery has been widely performed to treat these diseases.1,2 However, severe postoperative pain commonly occurs in these patients. The pain negatively affects postoperative satisfaction, hinders postoperative recovery and prolongs the hospitalization time.3,4 Various approaches have been developed to relieve postoperative pain, improve rapid recovery and the quality of life. Perioperative analgesia mainly includes local infiltration, nerve block, intravenous and oral analgesics.5,6 In accordance with the pathological mechanisms of acute pain, preemptive and multimodal analgesia has shown important potential in alleviating postoperative pain.7
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used for pain control through inhibiting cyclooxygenase and subsequent synthesis of prostaglandin E2.8 NSAIDs are documented to effectively inhibit the production of prostaglandins in the spinal cord and surrounding tissues, and thus relieve postoperative pain.9 Celebrex, a NSAID, serves as an important analgesic in osteoarthritis, hand surgery, hip and knee replacement, etc.10 As an antiepileptic drug, pregabalin can reduce central sensitivity by acting on the α-2-δ subunit of voltage-gated calcium channels, thereby decreasing neurotransmitters associated with spinal pain. Pregabalin was reported to reduce the pain and opioid consumption after spinal surgery.11,12 NSAIDs and pregabalin can achieve a synergistic effect on analgesia.13,14 In addition, the local subcutaneous infiltration of ropivacaine has shown some ability of postoperative pain control.15,16
In this study, based on the hypothesis that preemptive and multimodal analgesia using celebrex, pregabalin and ropivacaine can reduce postoperative pain of posterior spinal surgery, we examined the impact of preemptive and multimodal analgesia (combination analgesia: oral celebrex and pregabalin in combination with subcutaneous infiltration of ropivacaine) on pain intensity and PCA dose after posterior spinal surgery. The aim of this double-blind, randomized, placebo-controlled study was to establish a therapeutic approach in reducing the pain intensity following posterior spinal surgery.
Methods
Study Design and Population
Ninety-three patients undergoing posterior spinal surgery were enrolled in this prospective, double-blind, randomized placebo-controlled clinical trial. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (IRB #20190701). Written informed consent was obtained from all subjects participating in the trial. The trial was registered prior to patient enrollment at the Chinese Clinical Trial Registry (ChiCTR2000031236, Principal investigator: Bin He, Date of registration: March 25, 2020).
Patients were enrolled between March 2020 and July 2020. The inclusion criteria of this study were as follows: (1) ages 18–90, (2) single segment and double segments of posterior spinal fusion surgery because of lumbar disc herniation, spinal stenosis or spondylolisthesis, and (3) posterior reduction and fixation of thoracolumbar fracture. The exclusion criteria included allergy to amide anesthetics or sulfonamide, severe hepatic and renal dysfunction, bronchospasm, asthma, active gastrointestinal ulcer or bleeding, severe heart failure or creatinine clearance rate < 60 mL/min.
After submission of written informed consent, patients were randomized (1:1:1 ratio) to either combination analgesia intervention (oral celebrex [G.D. Searle LLC, USA], pregabalin [Qilu Pharmaceutical (Hainan) Co., Ltd, China] and subcutaneous infiltration of ropivacaine [Aspen Pharmacare Australia Pty Ltd, Australia]), ropivacaine intervention (only subcutaneous infiltration of ropivacaine), or control intervention (placebo) using a web-based computer-generated block randomization procedure (block size of six). Randomization was stratified by the kind and number of operation segments.
Allocation concealment was achieved by enclosing the assigned protocols in sealed, opaque, sequentially numbered envelopes, which were opened only after the arrival of the patient in the operating theatre. Blinding of research personnel and patients was maintained throughout the entire observation period including all postoperative follow-ups. Ye Zhang and Jinqiu Zhao generated the random allocation sequence. Qinsong Ren and Zhengxue Quan enrolled participants. Wei Zhang and Shuai Xu assigned participants to interventions. Bin He and Muzi Zhang uniformly prepared the study drugs. Ye Zhang, Jinqiu Zhao and Yunsheng Ou collected the data. Anesthesiologists’ team was random for the surgeries.
Study Intervention
The typical analgesia methods for all patients mainly included 1.5–2 mg/kg of propofol followed by 4–12 mg/kg/h, 1 mg/kg of rocuronium and remifentanil 0.1–0.2 μg/kg/min. Patients in the combination analgesia group obtained oral 400 mg celebrex and 150 mg pregabalin 2 h before surgery, then celebrex 200 mg and 75 mg pregabalin twice daily after surgery for 7 days plus subcutaneous infiltration of 0.75% ropivacaine (total 150 mg, 20 mL) at the end of surgery. Oral placebo capsule and subcutaneous infiltration of 0.75% ropivacaine at the end of surgery were administered in the ropivacaine group. Patients in the control group received oral placebo capsule and subcutaneous infiltration of 0.9% normal saline solution using the identical application scheme. 0.75% ropivacaine was prepared for the subcutaneous infiltration.
Outcome Measures and Safety
We recorded the baseline characteristics of each patient, including age, sex, height, weight, intraoperative bleeding volume, operative time and preoperative pain.
Primary outcome was visual analog scale (VAS) scores at 12 h after surgery. Secondary outcomes included: (i) VAS scores at 0 h, 2 h, 24 h, 3 d, 5 d, 7 d and 14 d after surgery; (ii) the required dose of patient-controlled analgesia (PCA, continuous infusion of tramadol hydrochloride 800 mg [Grunenthal GmbH, Germany] and flurbiprofen 100 mg [TIDE Pharmaceutical Co., China] in 84 mL saline solution); (iii) walking time, indicating the day on which patients could get up and walk; (iv) hospital stay after surgery; (v) adverse events such as nausea, vomiting, fever and infection. All patients were closely monitored for the occurrence of adverse events. Pain intensity levels were evaluated by VAS scores with a range of 0–10, and 10 indicated the most serious pain.
Statistical Analysis
Sample Size Calculation
The sample size estimation was based on data from our clinical routine. In 30 arbitrarily chosen patients undergoing posterior spinal surgery before the start of the study, VAS scores at 12 h postoperatively were documented. The pilot data (VAS score at 12 h postoperatively) from 30 patients were as follows: combination analgesia group, 2.7 (standard deviation [SD], 0.65); ropivacaine group, 3.3 (SD, 0.65); and control group, 4.2 (SD, 1.03).
Assuming this difference and aiming for a power of 75% and a risk of 0.05 for a type-1 error, 25 patients were required for each group. We selected 30 patients per group to allow for a 20% dropout rate.
Data Analysis
The statistical analysis was primarily conducted on an intention-to-treat basis using IBM SPSS Statistics v.25 (IBM Corp., USA). Variables were tested for normal distribution by the Kolmogorov–Smirnov test. Data were presented as mean (SD), medians (lower and upper quartiles) or frequencies, as appropriate. Data that followed a normal distribution (height) were compared using one-way analysis of variance (ANOVA). Kruskal–Wallis test was used for data that were not normally distributed (age, weight, bleeding volume, operative time, preoperative pain, VAS scores at 0 h, 2 h, 12 h, 24 h, 3 d, 5 d, 7 d and 14 d after the surgery, PCA dose, walking time, hospital stay after surgery). Categorical data were analyzed using the χ2 test or Fisher exact test if any cells expected counts less than 5. P-value of <0.05 was considered to indicate statistical significance.
Results
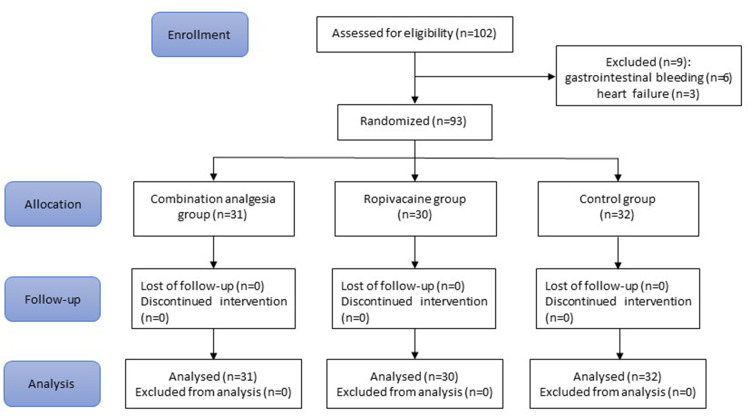
The study flow chart is shown in Figure 1. A total of 102 patients planned for elective posterior spinal surgery were screened; 93 patients were randomized to combination analgesia group (n = 31), ropivacaine group (n = 30) or control group (n = 32). All patients received their allocated treatment and were included in the intention-to-treat population.
Figure 1.
CONSORT flow chart.
Preoperative Assessment and Intraoperative Data
Patients in all groups did not differ significantly with respect to patient characteristics including age, sex, height, weight, intraoperative bleeding volume, operative time and preoperative pain (P>0.05, Table 1).
Table 1.
Demographic Data and Clinical Characteristics of the Patients
| Combination Analgesia Group | Ropivacaine Group | Control Group | Combination Analgesia vs Ropivacaine Group | Combination Analgesia vs Control Group | Ropivacaine Group vs Control Group | |
|---|---|---|---|---|---|---|
| Number | 31 | 30 | 32 | P value | ||
| Age (year) | 59 (50,68), median (lower, upper quartiles) | 59.5 (51,66.5) | 55.5 (48.25,66.5) | 0.793 | ||
| Sex (male/female) | 15/16 | 15/15 | 16/16 | 0.989 | ||
| Height (cm) | 164.55 (7.58) mean (SD) | 165.17 (7.32) | 164.72 (7.86) | 0.751 | 0.929 | 0.817 |
| Weight (kg) | 56 (48,70) | 58 (49,71) | 60 (52,70.25) | 0.708 | ||
| Bleeding volume (mL) | 100 (80,150) | 100 (80,150) | 100 (80,150) | 0.918 | ||
| Operative time (min) | 160 (120,180) | 162.5 (120,180) | 150 (120,180) | 0.717 | ||
| Preoperative pain (VAS) | 5 (4,5) | 5 (4,5) | 5 (4,5) | 0.621 | ||
Note: Data are expressed as mean (standard deviation, SD), median (lower and upper quartiles) or frequency (n).
Primary Outcome
Compared with the control group, there were significantly lower VAS scores at 12 h after posterior spinal surgery in the combination analgesia group (P<0.001) and ropivacaine group (P<0.001). The combination analgesia intervention showed a decrease in VAS scores compared with the ropivacaine group, but with no significant difference (P = 0.058, Table 2).
Table 2.
Postoperative VAS Scores Among the Three Groups
| Combination Analgesia Group | Ropivacaine Group | Control Group | Combination Analgesia vs Ropivacaine Group | Combination Analgesia vs Control Group | Ropivacaine Group vs Control Group | |
|---|---|---|---|---|---|---|
| Number | 31 | 30 | 32 | P value | ||
| Primary outcome | ||||||
| 12 h | 3 (2,3) | 3 (3,4) | 4 (4,5) | 0.058 | <0.001 | <0.001 |
| Secondary outcome | ||||||
| 0 h | 2 (2,2) | 2 (2,3) | 4 (3,4) | >0.9 | <0.001 | <0.001 |
| 2 h | 2 (2,3) | 2 (2,3) | 4 (4,4.75) | >0.9 | <0.001 | <0.001 |
| 24 h | 3 (2,3) | 4 (3,4) | 4 (3,5) | 0.005 | <0.001 | 0.148 |
| 3 d | 3 (2,3) | 3 (2,3.25) | 3.5 (3,4) | 0.117 | <0.001 | 0.025 |
| 5 d | 2 (2,3) | 3 (2,3) | 3 (3,3) | 0.271 | <0.001 | 0.107 |
| 7 d | 2 (1,2) | 2 (2,2) | 2 (2,3) | 0.193 | <0.001 | 0.091 |
| 14 d | 1 (1,1) | 2 (1,2) | 2 (1,2) | 0.005 | <0.001 | >0.9 |
| PCA dose | 40 (28,50) | 49 (42.25,55) | 55 (40,66) | 0.06 | 0.001 | 0.765 |
Note: Data are expressed as median (lower and upper quartiles).
Abbreviation: PCA, patient-controlled analgesia.
Secondary Outcomes
At 0 h, 2 h and 3 d after surgery, the VAS scores were significantly lower in the combination analgesia group and the ropivacaine group compared with the control group (P<0.001 for 0 h and 2 h, P = 0.025 for 3 d between ropivacaine group and control group), but there was no statistical difference in VAS scores between the combination analgesia group and the ropivacaine group (P > 0.05). At 24 h after surgery, the VAS scores were also significantly lower in the combination analgesia group than the ropivacaine group (P=0.005) and the control group (P<0.001), while no statistical difference was observed between the ropivacaine group and the control group (P = 0.148, Table 2).
At 5 d and 7 d after surgery, only patients in the combination analgesia group and ropivacaine group had markedly lower VAS scores than those in the control group (P<0.001). At 14 d postoperatively, the combination analgesia group had significantly lower VAS scores than the ropivacaine group and the control group (P≤0.005), while the ropivacaine group and the control group demonstrated similar VAS scores (P>0.9). Cumulative PCA consumption in the combination analgesia group was significantly lower than that in the control group (P = 0.001) (Table 2). There were similar walking times and hospital stays after surgery among the three groups (P>0.05, Table 3).
Table 3.
Postoperative Outcomes and Adverse Events
| Combination Analgesia Group | Ropivacaine Group | Control group | Combination Analgesia vs Ropivacaine Group | Combination Analgesia vs Control Group | Ropivacaine Group vs Control Group | |
|---|---|---|---|---|---|---|
| Number | 31 | 30 | 32 | P value | ||
| Walking time | 2 (2,2) | 2 (2,2) | 2 (2,3) | 0.207 | ||
| Hospital stay after surgery | 4 (4,5) | 5 (4,5) | 5 (4,5) | 0.388 | ||
| Adverse events | 1 | 2 | 3 | 0.610 | ||
| Nausea and vomiting | 1 | 0 | 2 | 0.771 | ||
| Fever | 0 | 1 | 0 | 0.323 | ||
| Infection | 0 | 1 | 1 | 0.768 | ||
| Secondary surgery | 0 | 0 | 1 | 1 | ||
| Urination disorders | 0 | 0 | 1 | 1 | ||
Note: Data are expressed as median (lower and upper quartiles) or frequency (n).
Safety
Adverse events were found in six patients (one case in the combination analgesia group, two cases in the ropivacaine group, and three cases in the control group). Nausea and vomiting occurred in one patient in the combination analgesia group and two patients in the control group. One patient in the ropivacaine group had a fever. Superficial wound infection was observed in one patient in the ropivacaine group, and this patient obtained wound healing after debridement and suturing under local anesthesia. One case in the control group had a deep wound infection, and underwent secondary surgery for debridement under general anesthesia. Another case in the control group developed a urination disorder. There was no statistical difference in adverse events among the three groups and they were all well tolerable after immediate treatment (Table 3).
Discussion
The results of this study confirmed that postoperative VAS scores and PCA consumption after posterior spinal surgery were significantly reduced by preemptive and multimodal analgesia using celebrex, pregabalin and ropivacaine. However, this combination analgesia showed no significant impact on walking time or hospital stay after surgery. There was no increase in adverse events after combination analgesia intervention for posterior spinal surgery.
Preemptive and multimodal analgesia are two novel concepts to improve the efficacy of pain management.17,18 The pathophysiology of surgical pain includes peripheral sensitization initiated by inflammatory mediators and central sensitization resulting from hyperexcitability of the spinal neurons in the dorsal horn.18,19 Preemptive analgesia requires the administration of analgesics prior to surgery and aims to prevent this hyperexcitability of the central nervous system.20,21 Various analgesics are combined in multimodal analgesia and act through different mechanisms and at different sites in order to provide more effective analgesia.22,23
Celebrex, a cyclooxygenase (COX)-2 inhibitor, acts via suppressing COX-2-mediated production of prostaglandin E2 which is a product of arachidonic metabolism and promotes the pain and hyperalgesia associated with tissue trauma and inflammation.24 Celebrex is able to reduce the hyperalgesic state after surgical trauma through inhibiting the synthesis of prostaglandins in the spinal cord and the periphery.25,26 Pregabalin is developed as an anticonvulsant drug and inhibits central sensitization and the release of nociceptive neurotransmitters in the spinal cord through presynaptic and postsynaptic inhibition of calcium influx.27–29 Anticonvulsants have been documented to diminish postoperative pain and opioid requirements after spinal surgery.11
Celebrex and pregabalin act through different mechanisms and their combination shows a synergistic effect for postoperative pain control.23 In one RCT that enrolled patients undergoing total hip arthroplasty, celecoxib plus pregabalin was associated with remarkably improved pain relief and physical function.14 However, there have been limited studies exploring the combination of celecoxib and pregabalin for spinal surgery. Preoperative administration of pregabalin combined with celecoxib (preoperative pregabalin and celecoxib) resulted in reduced VAS scores and intravenous morphine consumption after lumbar surgery, but that study only reported the efficacy during 48 h.30 This study intended to investigate the effects of preemptive analgesia (preoperative oral administration of celebrex and pregabalin) and multimodal analgesia (celebrex and pregabalin combined with subcutaneous infiltration of ropivacaine) on postoperative pain up to 14 d after posterior spinal surgery.
Local wound infiltration of anesthetics has been developed into a useful and important component of the multimodality approach to postoperative pain control through reducing the sensitization of spinal dorsal horn neurons and inhibiting the transmission of noxious impulses from the incision.31 It can also suppress local inflammatory responses to incision injury.32 Local infiltration of anesthetics has shown some potential in pain relief after breast surgery and inguinal hernia repair.33,34 Ropivacaine, a pure levorotatory stereoisomer and long-acting amide local anesthetic agent, has been widely used for local anesthesia and postoperative analgesia. Ropivacaine has the same analgesic effects as bupivacaine and levobupivacaine, but shows lower incidence of motor block.35 There is decreased incidence of central nervous system toxicity and cardiotoxicity because of the reduced lipophilicity of ropivacaine.36
In this study, preemptive and multimodal analgesia using celebrex, pregabalin and ropivacaine significantly reduced VAS scores up to 14 d postoperatively and PCA consumption after posterior spinal surgery. This preemptive and multimodal analgesia is safe and well tolerable, showing no increase in adverse events. Our study is subject to a few limitations. Firstly, clinical pain in our patients is evaluated using the VAS score, instead of using quantitative sensory testing (QST) which is a well-validated experimental tool for evaluating and quantifying hyperalgesia with a stimulus-response gradient.37,38 Secondly, different procedures of operation are included in this study, and may have some influence on the analgesic evaluation. Thirdly, recent research has demonstrated sex differences with respect to pain perception,39 but we did not perform a subgroup analysis based on sex difference. Finally, PCA was discontinued if patients had severe nausea and vomiting, which may affect the comparison of PCA consumption among groups.
To the best of our knowledge, this is the first clinical report to explore the effect of preemptive analgesia (preoperative administration of celebrex and pregabalin) and multimodal analgesia (oral celebrex and pregabalin combined with subcutaneous infiltration of ropivacaine) on postoperative pain up to 14 d after posterior spinal surgery. Our results suggest that this preemptive and multimodal analgesia can effectively and safely alleviate postoperative pain for posterior spinal surgery.
Acknowledgments
This study was funded by Foundation of The First Affiliated Hospital of Chongqing Medical University (PYJJ2018-13) and Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0836).
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available in the ResMan Research Manager repository (http://www.medresman.org.cn/uc/sindex.aspx).
Ethics Statement
The study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University. The study has been registered in the Chinese Clinical Trial Registry (ChiCTR2000031236). Written informed consent was obtained from all patients.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Li D, Hai Y, Meng X, Yang J, Yin P. Topping-off surgery vs posterior lumbar interbody fusion for degenerative lumbar disease: a comparative study of clinical efficacy and adjacent segment degeneration. J Orthop Surg Res. 2019;14(1):197. doi: 10.1186/s13018-019-1245-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park MS, Ju YS, Moon SH, et al. Reoperation rates after posterior lumbar spinal fusion surgery according to preoperative diagnoses: a national population-based cohort study. Clin Neurol Neurosurg. 2019;184:105408. doi: 10.1016/j.clineuro.2019.105408 [DOI] [PubMed] [Google Scholar]
- 3.Hwang W, Lee J, Park J, Joo J. Dexmedetomidine versus remifentanil in postoperative pain control after spinal surgery: a randomized controlled study. BMC Anesthesiol. 2015;15(1):21. doi: 10.1186/s12871-015-0004-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HJ, Ahn HS, Nam Y, Chang BS, Lee CK, Yeom JS. Comparative study of the efficacy of transdermal buprenorphine patches and prolonged-release tramadol tablets for postoperative pain control after spinal fusion surgery: a prospective, randomized controlled non-inferiority trial. Eur Spine J. 2017;26(11):2961–2968. doi: 10.1007/s00586-017-5213-5 [DOI] [PubMed] [Google Scholar]
- 5.Panchamia JK, Amundson AW, Jacob AK, et al. A 3-arm randomized clinical trial comparing interscalene blockade techniques with local infiltration analgesia for total shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(10):e325–e338. doi: 10.1016/j.jse.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 6.Wilson MJA, MacArthur C, Hewitt CA, et al. Intravenous remifentanil patient-controlled analgesia versus intramuscular pethidine for pain relief in labour (RESPITE): an open-label, multicentre, randomised controlled trial. Lancet (London, England). 2018;392(10148):662–672. doi: 10.1016/S0140-6736(18)31613-1 [DOI] [PubMed] [Google Scholar]
- 7.Cooper HJ, Lakra A, Maniker RB, Hickernell TR, Shah RP, Geller JA. Preemptive analgesia with oxycodone is associated with more pain following total joint arthroplasty. J Arthroplasty. 2019;34(12):2878–2883. doi: 10.1016/j.arth.2019.07.021 [DOI] [PubMed] [Google Scholar]
- 8.Enthoven WTM, Roelofs PD, Koes BW. NSAIDs for chronic low back pain. JAMA. 2017;317(22):2327–2328. doi: 10.1001/jama.2017.4571 [DOI] [PubMed] [Google Scholar]
- 9.Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177–184. doi: 10.1016/j.annemergmed.2016.10.014 [DOI] [PubMed] [Google Scholar]
- 10.Stepan JG, London DA, Osei DA, Boyer MI, Dardas AZ, Calfee RP. Perioperative celecoxib and postoperative opioid use in hand surgery: a prospective cohort study. J Hand Surg. 2018;43(4):346–353. doi: 10.1016/j.jhsa.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L, Ran B, Li M, Shi Z. Gabapentin and pregabalin in the management of postoperative pain after lumbar spinal surgery: a systematic review and meta-analysis. Spine. 2013;38(22):1947–1952. doi: 10.1097/BRS.0b013e3182a69b90 [DOI] [PubMed] [Google Scholar]
- 12.Jiang HL, Huang S, Song J, Wang X, Cao ZS. Preoperative use of pregabalin for acute pain in spine surgery: a meta-analysis of randomized controlled trials. Medicine. 2017;96(11):e6129. doi: 10.1097/MD.0000000000006129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romano CL, Romano D, Bonora C, Mineo G. Pregabalin, celecoxib, and their combination for treatment of chronic low-back pain. J Orthop Traumatol. 2009;10(4):185–191. doi: 10.1007/s10195-009-0077-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmichael NM, Katz J, Clarke H, et al. An intensive perioperative regimen of pregabalin and celecoxib reduces pain and improves physical function scores six weeks after total hip arthroplasty: a prospective randomized controlled trial. Pain Res Manag. 2013;18(3):127–132. doi: 10.1155/2013/258714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kundra S, Singh RM, Singh G, Singh T, Jarewal V, Katyal S. Efficacy of magnesium sulphate as an adjunct to ropivacaine in local infiltration for postoperative pain following lower segment caesarean section. J Clin Diagn Res. 2016;10(4):Uc18–22. doi: 10.7860/JCDR/2016/17119.7683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen L, Husted H, Kristensen BB, Otte KS, Gaarn-Larsen L, Kehlet H. Analgesic efficacy of subcutaneous local anaesthetic wound infiltration in bilateral knee arthroplasty: a randomised, placebo-controlled, double-blind trial. Acta Anaesthesiol Scand. 2010;54(5):543–548. doi: 10.1111/j.1399-6576.2009.02196.x [DOI] [PubMed] [Google Scholar]
- 17.Kurd MF, Kreitz T, Schroeder G, Vaccaro AR. The role of multimodal analgesia in spine surgery. J Am Acad Orthop Surg. 2017;25(4):260–268. doi: 10.5435/JAAOS-D-16-00049 [DOI] [PubMed] [Google Scholar]
- 18.Kaka U, Rahman NA, Abubakar AA, et al. Pre-emptive multimodal analgesia with tramadol and ketamine-lidocaine infusion for suppression of central sensitization in a dog model of ovariohysterectomy. J Pain Res. 2018;11:743–752. doi: 10.2147/JPR.S152475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science (New York, NY). 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765 [DOI] [PubMed] [Google Scholar]
- 20.Steinberg AC, Schimpf MO, White AB, et al. Preemptive analgesia for postoperative hysterectomy pain control: systematic review and clinical practice guidelines. Am J Obstet Gynecol. 2017;217(3):303–313.e306. doi: 10.1016/j.ajog.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 21.Peng HM, Wang LC, Wang W, et al. Preemptive analgesia with parecoxib in total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Pain Physician. 2018;21(5):483–488. [PubMed] [Google Scholar]
- 22.Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329–334. doi: 10.1016/j.arth.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 23.Kim SI, Ha KY, Oh IS. Preemptive multimodal analgesia for postoperative pain management after lumbar fusion surgery: a randomized controlled trial. Eur Spine J. 2016;25(5):1614–1619. doi: 10.1007/s00586-015-4216-3 [DOI] [PubMed] [Google Scholar]
- 24.Angeli F, Trapasso M, Signorotti S, Verdecchia P, Reboldi G. Amlodipine and celecoxib for treatment of hypertension and osteoarthritis pain. Expert Rev Clin Pharmacol. 2018;11(11):1073–1084. doi: 10.1080/17512433.2018.1540299 [DOI] [PubMed] [Google Scholar]
- 25.Shin JJ, McCrum CL, Mauro CS, Vyas D. Pain management after hip arthroscopy: systematic review of randomized controlled trials and cohort studies. Am J Sports Med. 2018;46(13):3288–3298. doi: 10.1177/0363546517734518 [DOI] [PubMed] [Google Scholar]
- 26.Puljak L, Marin A, Vrdoljak D, Markotic F, Utrobicic A, Tugwell P. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017;5(5):Cd009865. doi: 10.1002/14651858.CD009865.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derry S, Bell RF, Straube S, Wiffen PJ, Aldington D, Moore RA. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1(1):Cd007076. doi: 10.1002/14651858.CD007076.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rai AS, Khan JS, Dhaliwal J, et al. Preoperative pregabalin or gabapentin for acute and chronic postoperative pain among patients undergoing breast cancer surgery: a systematic review and meta-analysis of randomized controlled trials. J Plast Reconstr Aesthet Surg. 2017;70(10):1317–1328. doi: 10.1016/j.bjps.2017.05.054 [DOI] [PubMed] [Google Scholar]
- 29.Goodman CW, Brett AS. Gabapentin and pregabalin for pain - is increased prescribing a cause for concern? N Engl J Med. 2017;377(5):411–414. doi: 10.1056/NEJMp1704633 [DOI] [PubMed] [Google Scholar]
- 30.Kien NT, Geiger P, Van Chuong H, et al. Preemptive analgesia after lumbar spine surgery by pregabalin and celecoxib: a prospective study. Drug Des Devel Ther. 2019;13:2145–2152. doi: 10.2147/DDDT.S202410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brennan TJ, Zahn PK, Pogatzki-Zahn EM. Mechanisms of incisional pain. Anesthesiol Clin North Am. 2005;23(1):1–20. doi: 10.1016/j.atc.2004.11.009 [DOI] [PubMed] [Google Scholar]
- 32.Kawamata M, Takahashi T, Kozuka Y, et al. Experimental incision-induced pain in human skin: effects of systemic lidocaine on flare formation and hyperalgesia. Pain. 2002;100(1):77–89. doi: 10.1016/S0304-3959(02)00233-6 [DOI] [PubMed] [Google Scholar]
- 33.Scott NB. Wound infiltration for surgery. Anaesthesia. 2010;65(Suppl 1):67–75. doi: 10.1111/j.1365-2044.2010.06241.x [DOI] [PubMed] [Google Scholar]
- 34.Byager N, Hansen MS, Mathiesen O, Dahl JB. The analgesic effect of wound infiltration with local anaesthetics after breast surgery: a qualitative systematic review. Acta Anaesthesiol Scand. 2014;58(4):402–410. doi: 10.1111/aas.12287 [DOI] [PubMed] [Google Scholar]
- 35.Li M, Wan L, Mei W, Tian Y. Update on the clinical utility and practical use of ropivacaine in Chinese patients. Drug Des Devel Ther. 2014;8:1269–1276. doi: 10.2147/DDDT.S57258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuthiala G, Chaudhary G. Ropivacaine: a review of its pharmacology and clinical use. Indian J Anaesth. 2011;55(2):104–110. doi: 10.4103/0019-5049.79875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez V, Fletcher D, Bouhassira D, Sessler DI, Chauvin M. The evolution of primary hyperalgesia in orthopedic surgery: quantitative sensory testing and clinical evaluation before and after total knee arthroplasty. Anesth Analg. 2007;105(3):815–821. doi: 10.1213/01.ane.0000278091.29062.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilder-Smith Oliver HGPD, Tassonyi EPD, Crul Ben JPPD, Arendt-Nielsen LPD. Quantitative sensory testing and human surgery: effects of analgesic management on postoperative neuroplasticity. Anesthesiology. 2003;98(5):1214–1222. doi: 10.1097/00000542-200305000-00025 [DOI] [PubMed] [Google Scholar]
- 39.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–58. doi: 10.1093/bja/aet127 [DOI] [PMC free article] [PubMed] [Google Scholar]