Abstract
Introduction
To assess the frequency of allergic reactions to asparaginase (ASP) and possible risk factors for reactions in a cohort of pediatric patients.
Method
The study was performed based on retrospective data from patients under acute lymphoid leukemia treatment in a general university hospital located in southern Brazil. Information on patients who used ASP from 2010 to 2017 was collected. Allergic reactions were identified in electronic medical records.
Results
Among the 98 patients included in the study, 16 (16.3 %) experienced an allergic reaction to native l-asparaginase (L-ASP). Of the 22 patients (22.4 %) that received only intravenous (IV) administration of l-ASP, 10 (62.5 %) had allergic reactions, while 48 patients (49 %) received intramuscular (IM) administration and 28 (28.6 %) received IV and IM administrations. The occurrence of allergic reactions differed between the groups (p < 0.001), and IV administration was associated with allergic reactions. Association was also observed between the severity of the reaction and the route of administration, with the IM route associated with grade 2 and IV route associated with grade 3. Occurrence of allergic reactions was higher when the commercial formulation of l-ASP, Leuginase®, was used (p = 0.0009 in the analysis per patient and p = 0.0003 in the analysis per administration).
Conclusions
The IV administration and commercial Leuginase® presentation were associated with more allergic reactions in the study population, which corroborates the findings in the literature. The IV route was also associated with higher severity of reactions in the present study.
Keywords: Acute lymphoblastic leukemia, Asparaginase, Hypersensitivity, Children
Introduction
Acute lymphoid leukemia (ALL) is the most common type of cancer in children, accounting for approximately 25 % of diagnoses in patients under 15 years of age.1 Combined with other medications, asparaginase (ASP) is critical in the treatment of ALL. Its action occurs by the depletion of asparagine, an essential amino acid for neoplastic cells. As serum asparagine levels are depleted, RNA, DNA and protein levels decrease, resulting in the death of neoplastic cells.2, 3, 4
Three types of ASP are available: Native l-asparaginase (L-ASP), derived from Escherichia coli (Elspar®, Aginasa® and Leuginase®), pegylated asparaginase (PEG-ASP), derived from Escherichia coli conjugated with polyethylene glycol (Oncaspar)® and Erwinia-ASP (Erwinase®), derived from bacterial strains of Erwinia chrysanthemi.5 The main limitation for continued use of ASP is hypersensitivity reactions with consequent development of anti-asparaginase antibodies and reduced ASP enzyme activity.5, 6, 7 Immune reactions caused by ASP may be divided into clinical hypersensitivity, with allergic reactions ranging from localized erythema to anaphylaxis and subclinical hypersensitivity, with the occurrence of neutralizing antibodies and reduced ASP activity, in the absence of clinical signs.8
The l-ASP, a formulation currently used in Brazil, can have intravenous (IV) or intramuscular (IM) administration. The IV administration is preferred in some cases because drug infusion may be interrupted in the event of an anaphylactic reaction, which is not possible with the IM administration. The IM administration is painful and requires multiple high-dose administrations.9, 10 The development of hypersensitivity reactions may be influenced by factors such as the type of ASP used, the concomitant use of corticosteroids and the route of administration. In addition, the risk of reaction may increase with new exposure to ASP after a period without using it, and reactions occur most commonly in the post-induction phases.9, 11
Pre-medication with corticosteroids and antihistamines is not recommended, as it may mask the presence of an allergic reaction and the formation of anti-asparaginase antibodies.12
The present study aimed to evaluate the frequency of allergic reactions to ASP in a cohort of pediatric patients from a Brazilian health service, as well as to characterize risk factors for their development.
Method
This is a retrospective study conducted by the analysis of medical records of patients undergoing ALL treatment at a general university hospital located in southern Brazil. The study was approved by the institution's Research Ethics Committee (CAAE 83670018.8.0000.5327).
Patients up to 18 years old who started using ASP from January 2010 to December 2017 were included. Patients who started or completed treatment at other institutions and those whose information was not available, making data collection impossible, were excluded from the study.
The variables age at diagnosis, gender, ALL type (B x T) and treatment protocols were obtained from the institution's electronic medical records. According to the National Cancer Institute (NCI) classification, patients were classified according to their risk of leukemia.13 For all ASP administrations, the following information was obtained: protocol and phase of treatment, route of administration, asparaginase formulation and trade name, use of corticosteroids or antihistamines prior to administration, concomitant use of corticosteroids and total number of doses received per patient.
Allergic reactions were identified in the electronic medical records. In this case, the number of doses received prior to the reaction was verified and the reaction was classified according to its severity, using common adverse event terminology criteria (CTCAE v5) for allergic reactions.14
Statistical analysis
Continuous variables were assessed for their distribution with the Shapiro-Wilk normality test. Continuous variables with asymmetric distribution were expressed as medians and 25th and 75th percentiles. Qualitative variables were summarized as absolute and relative frequencies. The ages of patients with and without reactions were compared by the Mann-Whitney test. Categorical variables, on the other hand, were compared by the text Yates χ2 or Fisher's exact test. The significance level adopted was 0.05. The data were compiled in a Microsoft Excel® spreadsheet and later submitted and analyzed in the SPSS® version 18.0 and Winpepi version 11.65.
Results
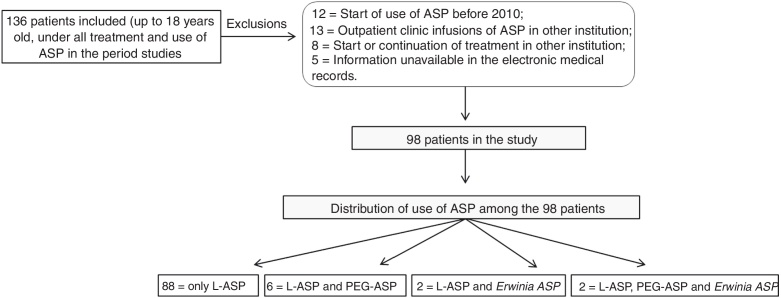
During the study period, 136 patients with ASP administration were identified, of which 38 met the exclusion criteria, resulting in 98 patients included in the study (Fig. 1). The clinical and demographic data of the patients are described in Table 1.
Fig. 1.
Patients selected for the study and distribution of use of ASP among patients.
Table 1.
Patient clinical and demographic characteristics.
| Characteristics | All patients | Without allergic reaction | Allergic reaction | p-value | |
|---|---|---|---|---|---|
| Continuous variable: median (interquartile interval) | |||||
| Age at diagnose (years) | 4.5 [3.0; 8.25] | 4.0 [2.75; 8.25] | 5.0 [4.0; 8.5] | 0.406 | |
| Categorical variables: n (%) | |||||
| Sex | |||||
| Male | 60 (61.2) | 45 (54.9) | 15 (93.8) | 0.008 | |
| Female | 38 (38.7) | 37 (45.1) | 1 (6.3) | ||
| Diagnose | |||||
| B- ALL | 86 (88.7) | 72 (87.1) | 14 (87.5) | >0.999 | |
| T-ALL | 11 (11.3) | 9 (11) | 2 (12.5) | ||
| NCI risk classification of leukemia | |||||
| Low risk | 61 (62.9) | 51 (62.2) | 10 (62.5) | >0.999 | |
| High risk | 36 (37.1) | 30 (36.6) | 6 (37.5) | ||
| Concomitant use of corticosteroids – total administrations of ASP: n (%) | |||||
| Yes | 1331 (87.45) | 1319 (87.64) | 12 (70.59) | 0.052 | |
| No | 191 (12.55) | 186 (12.36) | 5 (29.41) | ||
| Use of pre-medication - total administrations of ASP: n (%) | |||||
| Yes | 1192 (78.89) | 1176 (78.71) | 16 (94.12) | 0.145 | |
| No | 319 (21.11) | 318 (21.29) | 1 (5.88) | ||
*p-value was calculated using Mann-Whitney test (age); Yates χ² test (sex, NCI risk classification of leukemia) and Fisher’s exact test (type of leukemia, concomitant use of corticosteroids and use of pre-medication).
Sixteen patients (16.3 %) had an allergic reaction attributed to l-ASP and one patient had a reaction in two consecutive infusions. Two patients had reactions with the IM and the other 14 with the IV. Three patients had reactions in the induction phase and the others, in the post-induction phase, and in eight patients the reactions occurred in the re-induction phase. The median number of doses received before the reaction was 9 [9.0; 11.0] and the median time in months, from the first administration of l-ASP to the reaction, was 5 [3.0; 5.0].
The frequency of reactions was higher in male patients, compared to those in females (p = 0.008), and there was only one female patient who had a reaction to l-ASP. There was no statistically significant difference regarding age (p = 0.406), type of leukemia (p > 0.999) and risk classification (p > 0.999).
A high number of asparaginase administrations with concomitant corticosteroid use have been observed, and there is a tendency for a lower incidence of allergic reactions in patients using corticosteroids (p = 0.052) (Table 1).
Twenty-two patients (22.4 %) received l-ASP IV administration alone and 48 (49 %) received IM administration. Administration of l-ASP occurred by both IV and IM administrations in 28 (28.6 %) patients. The occurrence of allergic reactions differed between groups (p < 0.001), and IV administration was associated with the occurrence of an allergic reaction (Table 2).
Table 2.
Frequency of allergic reaction in patients with l-ASP administration by IV, IM routes or IV and IM routes (n = 98).
| Administration route | n (%) | Allergic reactions (%) | p-value* |
|---|---|---|---|
| IV | 22 (22.4) | 10 (62.5) | < 0.001 |
| IM | 48 (49) | 0 | |
| IV and IM | 28 (28.6) | 6 (37.5) |
p-value was calculated using Fisher’s exact test.
All allergic reactions were classified as grade 2 or 3, according to the CTCAE. Three patients had a grade 2 allergic reaction (18.6 %) and 12 patients had a grade 3 allergic reaction (75 %). In all patients with a grade 3 reaction (n = 12, 100 %), l-ASP was administered intravenously. For two (66.7 %) patients with a grade 2 reaction, l-ASP administration was via IM administration. The other patient (n = 1, 33.3 %) with a grade 2 allergic reaction had undergone intravenous administration of l-ASP. A statistically significant difference (p = 0.029) was observed between the severity of allergic reactions and the route of administration. The reaction degree and the administration route were associated, with the IM route associated with grade 2 and the IV route, with grade 3. For one patient, it was not possible to classify the reaction due to the lack of information.
In both per patient and total number of administrations, there was a statistically significant difference between the different commercial presentations of l-ASP used at the institution during the study period (Elspar®, Aginasa®, and Leuginase®) (Table 3).
Table 3.
Frequency of allergic reaction to the different commercial presentations of l-ASP per patient and per number of administrations.
| Type of l-asparaginase | TOTAL | Allergic reactions | p-value* | |
|---|---|---|---|---|
| n | n (%) | |||
| Analysis per patient (n = 98) | ||||
| Elspar | 29 | 4 (13.8) | a | 0.0009 |
| Aginasa | 44 | 4 (9.1) | a | |
| Leuginase | 9 | 6 (66.7 %) | b | |
| Elspar and Aginasa | 10 | 0 | a | |
| Leuginase and Aginasa | 6 | 2 (33.3 %) | ab | |
| Analysis per administration (n = 1457) | ||||
| Elspar | 457 | 4 (0.87) | a | 0.0003 |
| Aginasa | 853 | 5 (0.59) | a | |
| Leuginase | 157 | 8 (5.10) | b |
Different letters represent percentages statistically different, using an adjusted standardized residual analysis.
p-value was calculated using χ² test.
The median of ASP administrations per patient, including all formulations, l-ASP, PEG-ASP, and Erwinia ASP was 12, with a minimum of 4 and a maximum of 35 administrations. Patients using PEG-ASP and Erwinia ASP had no allergic reactions.
The dosage summary and administration schedule of l-asparaginase in the two most common treatment protocols among study patients, ALL-IC BFM 2009 and GBTLI LLA 2009, and a descriptive table containing the characteristics of each patient who had an allergic reaction are available in supplemental Table 1, Table 2.
Discussion
The present study evaluated the frequency of allergic reactions to ASP in pediatric patients treated for ALL, as well as the risk factors for the development of reactions. Possible risk factors were male gender, IV route of administration and the commercial formulation of l-ASP, Leuginase®.
The analyzed population seems representative because there was a predominance of male patients, diagnosed with type B ALL, of low risk and a median age of 4.5 years, characteristics also observed in previous studies with similar populations.15, 16
The frequency of allergic reactions to asparaginase is variable, with reported rates ranging from 3 to 45 % in ALL patients.10 Different formulations of asparaginase have distinct immunogenic profiles. With the native formulation of l-ASP, the frequency of allergic reactions is 13–30%. For PEG-ASP, the reaction rate is 10 % in patients who did not have previous hypersensitivity to l-ASP.17 The 16.3 % reaction rate found in this study is in line with the literature.
A study by Santos et al. in a pediatric hospital in Rio de Janeiro found that a high-risk classification and a younger age are risk factors for the occurrence of allergic reactions related to the IV administration of l-ASP. These results differ from those found in the present study, as there was no statistically significant difference regarding the frequency of reactions, age and risk classification of patients. The type of leukemia was not associated with the risk for allergic reaction, neither in the study by Santos et al. nor in the present study.18
Gender was associated with a significant difference in the frequency of l-ASP allergic reactions in this study. Male patients had a higher frequency of reactions, when compared to female patients. Although studies show that there was no difference in the risk for ASP allergic reaction between male and female patients,15, 16, 18 high levels of anti-asparaginase antibodies were identified in boys.19
Treatment-related factors may also be associated with the risk of developing an allergic reaction. In most ASP administrations in this study, the use of premedication was observed. The use of corticosteroids and antihistamines before ASP administration is associated with a reduction in the rate of reactions. Although the use of premedication may reduce clinical symptoms of hypersensitivity, it does not prevent antibody formation and ASP inactivation. In this sense, the monitoring of ASP activity is essential, as it leads to the identification of ASP inactivation, even when the patient has no clinical signs of hypersensitivity.8, 9, 11 Therefore, the use of premedication is not recommended in the absence of the verification of ASP activity levels8. There was no significant difference regarding the use of premedication between patients who had reactions to ASP and those who did not.
In addition to being used as a premedication, corticosteroids may also be used concurrently with ASP administrations, which usually occur because of their inclusion in the ALL treatment protocols. In this study, a high percentage of corticosteroids were used concomitantly with ASP administrations. Although there was no statistically significant difference (p = 0.052) in the use of corticosteroids between the reaction group and the unreacted group, the percentage of corticosteroid use was higher in patients who did not have an allergic reaction (used in 87.64 % of the patients) than in those who had an allergic reaction (used in 70.59 % of administrations). The literature suggests reduced rates of hypersensitivity reaction when corticosteroids are used.10, 20 However, as with premedication, concomitant use of corticosteroids may mask the clinical signs of reactions. In addition, the development of anti-asparaginase antibodies may occur, which reinforces the importance of monitoring this drug activity.21
A higher frequency of allergic reactions in the post-induction phases was observed in this study. This corroborates with other studies indicating that reactions are more common in the post-induction phases after periods of time without using these drugs.9, 12
Regarding the different commercial presentations of l-ASP, Leuginase® was associated with a higher frequency of reactions, and there was a statistically significant difference between Leuginase® and the other presentations, both in the number of administrations and patient analysis. This result may suggest that this presentation possibly represents a risk factor for the development of allergic reactions. Zenatti et al., based on the lack of clinical studies on Leuginase®, evaluated its purity, immunogenicity and bioavailability, compared to Aginasa®. Mass spectrometric analysis showed the presence of different contaminating host cell proteins, which may be associated with increased immunogenicity. In vivo mouse analysis showed three-fold lower plasma bioavailability for Leuginase® and increased development of anti-asparaginase antibodies, compared to Aginasa®.22
Regarding the route of administration, a previous study showed results of an increased incidence of allergic reactions following the IV administration of the native formulation of l-ASP, compared to the IM administration.23, 24 Traditionally, l-ASP is administered via the IM route, due to the risk of allergic reactions,25 however in recent years, intravenous use has been used, as it is less painful and reduces patient anxiety.26 In this study, due to the treatment protocols used in the institution during the study period, administrations by IV and IM routes occurred. The intravenous route was associated with the occurrence of the reaction, which may suggest this route as a risk factor for the development of allergic reactions. Reactions whose route of administration was IV were more severe than the IM reactions, a statistically significant result. For PEG-ASP, a meta-analysis showed a reduction in hypersensitivity reactions when administration occurs by the IM route, in comparison to the IV route, in pediatric patients under treatment for ALL.27
In patients who have had an allergic reaction to l-ASP, treatment may be continued with Erwinia ASP. There is cross-reactivity between antibodies against l-ASP and its pegylated formulation, which does not apply to the Erwinia-derived formulation.28 The combination of Escherichia coli and polyethylene glycol (PEG-ASP) results in a less immunogenic formulation that can be administered with lower frequency due to its longer half-life. This fact, added to the discontinuation of l-ASP provision in some countries, such as the United States of America, results in the use of PEG-ASP as a first choice in many pediatric protocols for ALL.11, 21 In the institution of the present study, l-ASP has been replaced by PEG-ASP since 2018, signifying that the patients under treatment had l-ASP replaced by PEG-ASP. Some patients who experienced an allergic reaction to the native form also received PEG-ASP as an alternative, while the Erwinia-derived formulation was not available.
The present study has some limitations, such as the small sample size, when compared to other studies, and the high number of patients excluded from the study. As this is a retrospective study in which allergic reactions were identified through medical records, the number of reactions may have been underestimated, especially in the case of milder allergic reactions (grades 1 and 2). In addition, due to the retrospective design of the study, there is a limitation of the recording of information in the medical records, with possible variations and inaccuracies in the data documentation.
Conclusion
In this study, males were associated with a higher occurrence of reactions, compared to females. The IV route of administration was associated with a higher occurrence of allergic reactions and greater severity of reactions, suggesting caution in the use of this route, preferred for l-ASP administration. Higher occurrence of allergic reactions was also observed when the commercial presentation of l-ASP Leuginase® was used. This fact reinforces the importance of monitoring the quality of medicines. Due to the high use of pre-administration drugs, protocols for their use are suggested, as well as the monitoring of the activity of pre-medication ASP, in order to optimize the results obtained with the use of ASP, which plays a central role in ALL treatment.
This research did not receive any specific grants from public, commercial or nonprofit funding agencies.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.htct.2019.10.007.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Egler R.A., Ahuja S.P., Matloub Y. L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J Pharmacol Pharmacother. 2016;7(2):62–71. doi: 10.4103/0976-500X.184769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizzari C., Conter V., Stary J., Colombini A., Moericke A., Schrappe Optimizing asparaginase therapy for acute lymphoblastic leukemia. Curr Opin Oncol. 2013;25(1):S1–S9. doi: 10.1097/CCO.0b013e32835d7d85. [DOI] [PubMed] [Google Scholar]
- 3.Schrey D., Borghorst S., Lanvers-Kaminsky C., Hempel G., Gerß J., Moricke A. Therapeutic Drug Monitoring of Asparaginase in the ALL-BFM 2000 Protocol Between 2000 and 2007. Pediatr Blood & Cancer. 2010:952–958. doi: 10.1002/pbc.22417. [DOI] [PubMed] [Google Scholar]
- 4.Zheng W., Ren H., Ke X., Xue M., Zhang Y., Xie Y. PEG-asparaginase in BFM-90 regimen improves outcomes in adults with newly diagnosed lymphoblastic lymphoma. Chin J Cancer Res. 2017;29(1):66–74. doi: 10.21147/j.issn.1000-9604.2017.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller H.J., Boss J. Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998;28(2):97–113. doi: 10.1016/s1040-8428(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 6.Andrade A.F., Borges K.S., Silveira V.S. Update on the Use of L-Asparaginase in Infants and Adolescent Patients with Acute Lymphoblastic Leukemia. Clin Med Insights Oncol. 2014;8:95–100. doi: 10.4137/CMO.S10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avramis V.I., Avramis E.V., Hunter W., Melissa C.L. Immunogenicity of Native or Pegylated E. coli and Erwinia Asparaginases Assessed by ELISA and Surface Plasmon Resonance (SPR-Biacore) Assays of IgG Antibodies (Ab) in Sera from Patients with Acute Lymphoblastic Leukemia (ALL) Anticancer Res. 2009;29:299–302. [PubMed] [Google Scholar]
- 8.Van der Sluis I.M., Vrooman L.M., Pieters R., Baruchel A., Escherich G., Goulden N. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica. 2016;101(3):279–285. doi: 10.3324/haematol.2015.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asselin B., Rizzari C. Asparaginase pharmacokinetics and implications of therapeutic drug monitoring. Leuk Lymphoma. 2015;56(8):2273–2280. doi: 10.3109/10428194.2014.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke M.J. How to manage asparaginase hypersensitivity in acute lymphoblastic leukemia. Future Oncol. 2014;10(16):2615–2617. doi: 10.2217/fon.14.138. [DOI] [PubMed] [Google Scholar]
- 11.Burke M.J., Rheingold S.R. Differentiating hypersensitivity versus infusion-related reactions in pediatric patients receiving intravenous asparaginase therapy for acute lymphoblastic leukemia. Leuk Lymphoma. 2016;58(3):540–551. doi: 10.1080/10428194.2016.1213826. [DOI] [PubMed] [Google Scholar]
- 12.Raetz E.A., Salzer W.L. Tolerability and Efficacy of L-Asparaginase Therapy in Pediatric Patients With Acute Lymphoblastic Leukemia. J Pediatr Hematol Oncol. 2010;32(7):554–563. doi: 10.1097/MPH.0b013e3181e6f003. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute (NCI) Risk Groups for Childhood Acute Lymphoblastic Leukemia [Internet] 2018. https://www.cancer.gov/types/leukemia/patient/child-all-treatment-pdq#section/_26 [cited 2018 november] Available from:
- 14.National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events v5.0 (CTCAE) [Internet] 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf [cited 2018 november] Available from:
- 15.Petersen W.C., Clark D., Senn S.L., Cash T., Gillespie S., McCracken C.E. Comparison of Allergic Reactions to Intravenous and Intramuscular Pegaspargase in Children with Acute Lymphoblastic Leukemia. Pediatr Hematol Oncol. 2014;31(4):311–317. doi: 10.3109/08880018.2013.876134. [DOI] [PubMed] [Google Scholar]
- 16.Abbott L.S., Zakova M., Shaikh F., Shewaramani N., Punnett A., Dupuis L.L. Allergic Reactions Associated with Intravenous Versus Intramuscular Pegaspargase: A Retrospective Chart Review. Paediatr Drugs. 2015;17(4):315–321. doi: 10.1007/s40272-015-0129-1. [DOI] [PubMed] [Google Scholar]
- 17.Narta U.K., Kanwar S.S., Azmi W. Pharmacological and clinical evaluation of l-asparaginase in the treatment of leukemia. Crit Rev Oncol Hematol. 2007;61(3):208–221. doi: 10.1016/j.critrevonc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Santos A.C., Land M.G., Silva N.P., Santos K.O., Lima-Dellamora E.C. Reactions related to asparaginase infusion in a 10-year retrospective cohort. Rev Bras Hematol Hemoter. 2017;39(4):337–342. doi: 10.1016/j.bjhh.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panosyan E.H., Seibel N.L., Martin-Aragon S., Gaynon P.S., Avramis I.A., Sather H. Asparaginase Antibody and Asparaginase Activity in Children With Higher-Risk Acute LymphoblasticLeukemia Children’s Cancer Group StudyCCG-1961. J Pediatr Hematol Oncol. 2004;26(4):217–226. doi: 10.1097/00043426-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Land V.J., Sutow W.W., Fernbach D.J., Lane D.M., Williams T.E. Toxicity of l-asparaginase in children with advanced leukemia. Cancer. 1972;30:339–347. doi: 10.1002/1097-0142(197208)30:2<339::aid-cncr2820300206>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Hijika N., Van der Sluis I.M. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2015;57(4):748–757. doi: 10.3109/10428194.2015.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zenatti P.P., Migita N.A., Cury N.M., Mendes-Silva R.A., Gozzo F.C., Campos-Lima P.O. Low Bioavailability and High Immunogenicity of a New Brand of E. coli l-Asparaginase with Active Host Contaminating Proteins. EBioMedicine. 2018;30:158–166. doi: 10.1016/j.ebiom.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nesbit M., Chard R., Evans A., Karon M., Hammond G.D. Evaluation of intramuscular versus intravenous administration of L-asparaginase in childhood leukemia. Am J Pediatr Hematol Oncol. 1979;1(1):9–13. [PubMed] [Google Scholar]
- 24.Evans W.E., Tsiatis A., Rivera G., Murphy S.B., Dahl G.V., Denison M. Anaphylactoid reactions to Escherichia coli and Erwinia asparaginase in children with leukemia and lymphoma. Cancer. 1982;49(7):1378–1382. doi: 10.1002/1097-0142(19820401)49:7<1378::aid-cncr2820490713>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 25.Place A.E., Stevenson K.E., Vroomann L.M., Harris M.H., Hunst S.K., O’Brien J.E. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): a randomised, open-label phase 3 trial. Lancet Oncol. 2015;16:1677–1690. doi: 10.1016/S1470-2045(15)00363-0. [DOI] [PubMed] [Google Scholar]
- 26.August K.J., Miller W.P., Dalton A., Schinnick S. Comparison of Hypersensitivity Reactions to PEG-Asparaginase in Children After Intravenous and Intramuscular Administration. J Pediatr Hematol Oncol. 2013;35(7):283–286. doi: 10.1097/MPH.0b013e31828e5471. [DOI] [PubMed] [Google Scholar]
- 27.Hasan H., Shaikh O.M., Rassekh S.R., Howard A.F., Goddard K. Comparison of hypersensitivity rates to intravenous and intramuscular PEG-asparaginase in children with acute lymphoblastic leukemia: A meta-analysis and systematic review. Pediatr Blood & Cancer. 2016;64(1):81–88. doi: 10.1002/pbc.26200. [DOI] [PubMed] [Google Scholar]
- 28.Zalewska-Szewczyk B., Gach A., Wyka K., Bodalski J., Młynarski W. The cross-reactivity of anti-asparaginase antibodies against different l-asparaginase preparations. Clin Exp Med. 2009;9(2):113–116. doi: 10.1007/s10238-008-0026-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.