Abstract
An enigma in studies of neuropsychiatric disorders is how to translate polygenic risk into disease biology. For schizophrenia, where > 145 significant GWAS loci have been identified and only a few genes directly implicated, addressing this issue is a particular challenge. We used a combined cellomics and proteomics approach to show that polygenic risk can be disentangled by searching for shared neuronal morphology and cellular pathway phenotypes of candidate schizophrenia risk genes. We first performed an automated high-content cellular screen to characterize neuronal morphology phenotypes of 41 candidate schizophrenia risk genes. The transcription factors Tcf4 and Tbr1 and the RNA topoisomerase Top3b shared a neuronal phenotype marked by an early and progressive reduction in synapse numbers upon knockdown in mouse primary neuronal cultures. Proteomics analysis subsequently showed that these three genes converge onto the syntaxin-mediated neurotransmitter release pathway, which was previously implicated in schizophrenia, but for which genetic evidence was weak. We show that dysregulation of multiple proteins in this pathway may be due to the combined effects of schizophrenia risk genes Tcf4, Tbr1, and Top3b. Together, our data provide new biological functions for schizophrenia risk genes and support the idea that polygenic risk is the result of multiple small impacts on common neuronal signaling pathways.
Subject terms: Neuroscience, Cell biology
Introduction
Schizophrenia (SCZ) is a severe neuropsychiatric disorder characterized by persistent delusions and hallucination and abnormal social behavior. SCZ has a high heritability and genetic risk factors have an important role in disease pathogenesis [1, 2]. Despite many years of research, few genes have been identified that may be directly involved. Whole-exome sequencing led to the identification of SETD1A [3] and RBM12 [4] and three rare copy number variants (CNVs) have been identified that impact single genes (i.e., NRXN1 [5], TOP3B [6], and VIPR2 [7]). Other CNVs have been identified [6, 8–11], but these are difficult to interpret functionally because they change the dosages of many genes and often present with complex, multi-system clinical phenotypes.
In contrast, common variation in the genome seems to have a large impact on the development of SCZ [2]. More than 145 independent genomic risk loci have been associated with SCZ or in genome-wide association studies (GWAS) [12, 13]. Some of these loci include associated gene variants that can modify the expression of large numbers of other genes or proteins. Examples include miR137 [14] genes whose transcripts are bound by the fragile X mental retardation protein (FMRP) [15], and several transcription factors (e.g., TCF4 [16]), providing an extra layer of genetic complexity to the disease. Together, all evidence suggests that SCZ is a highly polygenic disorder with many genes contributing to its development and symptomatology.
The high number of candidate risk loci poses an unprecedented biological challenge. It is currently not understood how this polygenic risk translates into common patterns of brain alterations underlying the disease. Are there many different cellular and molecular pathways that lead to disease, or do risk genes converge onto a small number of shared pathways underlying SCZ? We addressed this issue by performing a combination of cellular phenotyping (cellomics) and proteomics analysis. We used RNA interference in combination with automated high-content screening to identify neuronal phenotypes associated with reduced expression of 41 SCZ risk genes, and subsequent proteomics analysis to identify cellular pathways for genes sharing similar phenotypes. Our data show that inhibition of Tcf4, Tbr1, and Top3b each cause a similar synaptic morphological phenotype. The three genes converge onto a neurotransmitter release pathway that was previously associated with SCZ, and molecular changes induced by each gene are needed to significantly impact this pathway. Our data suggest that polygenic risk in SCZ may at least in part converge onto common cellular disease pathways that cannot be detected by computational pathway enrichment analyses alone, and that the high polygenic risk challenge in SCZ may be solved at the level of experimentally defined pathway sharing.
Materials and methods
Primary neuron culture
Hippocampal primary neuron cultures from E18 wildtype C57Bl/6 mouse embryos were prepared as previously described [17]. Hippocampal tissue was incubated 25 min at 37 °C in a Hanks balanced salt solution (Sigma) containing 1% HEPES buffer solution (1 m; Gibco) and 10% trypsin (Gibco). After three washes, the tissue was placed in Neurobasal medium (Gibco) completed with 2% B27 (Gibco), 2% HEPES solution, 0.25% glutamine (200 mm; Gibco) and 0.1% Pen/Strep (Gibco) and triturated with a fire-polished Pasteur pipette. Cells were counted in a Fuchs-Rosenthal chamber and plated in multi-well plates (Greiner Bio-one) that were previously coated with poly-d-lysine (Sigma). Cells were plated at 12.5K/well in 96-well glass bottom plates for morphological analyses, at 125K/well in 24-well plates for RNA extraction, or at 300K/well in 12-well plates for protein extraction.
Lentivirus production
Bacterial glycerol stock (MISSION library, Sigma; Supplementary Table S1) were grown in agar plates with LB medium and 1% ampicillin. Single colonies were picked and expanded for DNA extraction (QIAprep spin mini prep kit; Qiagen). HEK 293T cells were transfected with the small hairpin RNA (shRNA) plasmid DNA together with envelope and packaging plasmids. One day after transfection medium was replaced with Optimem medium (Gibco) completed with 1% Pen/Strep and 1% glutamine. On the third day, the medium was collected and centrifuged at 1000 ×g for 5 min; the supernatant containing the viral particles was filtered (0.45 μm pore size) and aliquoted.
Virus infection efficiency test
Primary hippocampal neurons were plated and infected at DIV1 with 1, 3, or 6 μl virus in 200 μl culture medium per well. At DIV2, puromycin (0.2 mg/ml; Gibco) was added. Cells were fixed at DIV7 and stained with Hoechst (1:10,000; Invitrogen) and anti-MAP2 (1:5000; Bio-connect). Infection efficiency was determined as the percentage of living cells compared with untreated non-infected control wells.
Immunocytochemistry
Hippocampal neurons were infected with shRNAs at DIV1 and cultures were fixed and stained at DIV7, DIV14, or DIV21. Cells were fixed with 4% paraformaldehyde and 4% sucrose in phosphate-buffered salie (PBS; pH 7.4) followed by permeabilization with PBS containing 0.5% Triton X-100. Cells were incubated at room temperature (RT) with PBS containing 0.1% Triton X-100 and 1% BSA and then for two nights at 4 °C in PBS containing 0.1% Triton X-100, 1% BSA, anti-synapsin 1 (1:1000; Chemicon/Millipore: #AB1543P), anti-PSD-95 (1:250; Thermo Scientific; #MA1046) and anti-MAP2 (1:5000; Chemicon/Millipore, #AB5543). After washing twice with PBS, neurons were incubated for 90 min at RT in Alexa-488-conjugated goat anti-mouse (1:400; Abcam, #A11001), Alexa-568-conjugated goat anti-rabbit (1:400; Abcam, #A11011) and Alexa-647-conjugated goat anti-chicken (1:400; Abcam, #A21449). After washing twice with PBS and once with distilled H2O, the neurons were incubated for 10 min at RT with Hoechst (1:10000; Invitrogen).
Imaging and image analysis
Images were acquired on an Opera™ LX (PerkinElmer) automated confocal microscopy system at × 10 and at × 40 magnification and were analyzed using Columbus image data storage and analysis software (v2.5.2.124862; PerkinElmer). Exposure and image analysis settings were kept constant throughout the entire screen. At × 10 magnification the entire well was imaged and quantitative parameters related to cell numbers and the length and complexity of dendritic trees were extracted based on Hoechst and anti-MAP2 staining. Glial nuclei were excluded based on their larger size and dimmer Hoechst staining compared with neurons and average MAP2 intensity per soma (i.e., in a circle around each nucleus) was used to select neurons. Dendrites were traced based on MAP2 staining. Dendrite selection parameters were set such that background MAP2 staining (often glial cells or debris) did not contribute to dendrite length measurements. Dendrite roots (primary dendrite origins) and nodes (branch points) were collected as measures of dendrite complexity. At × 40 magnification quantitative parameters related to synaptic development were extracted based on anti-MAP2, anti-synapsin and anti-PSD-95 staining. Synapsin-positive presynaptic puncta and PSD-95-positive postsynaptic puncta were traced specifically on MAP2-positive dendrites. Ten relevant measures of neuronal survival and network development were calculated: total number of nuclei per well, total number of neurons per well, number of primary neurites per neuron, number of dendritic branch points per neuron, total dendrite length per neuron, number of presynaptic puncta per dendrite length, presynaptic puncta average intensity, number of postsynaptic puncta per dendrite length, postsynaptic puncta average intensity, and number of colocalized pre- and postsynaptic puncta per dendrite length.
Cellomics data analysis
Cellomics data (n = 3 cultures per shRNA) was normalized per plate against the scrambled control and log2 transformed. Log-normalized data were checked for normality of distribution and rank correlation was used to check for parameter correlation. Multi-level modeling was applied to the data to estimate the contribution of technical and experimental variation to the overall variance of each parameter. The R package lme4 [18] was used to fit a mixed-effect model for every parameter. Batch, plate, edge, DIV, and shRNA treatment were taken as potential sources of variation. A Mann–Whitney U test was performed per gene/parameter combination under the null hypothesis that the distribution of control wells and experimental wells behave the same. A null distribution of 10,000 p values was generated for each gene/parameter by a random shuffle of control and experimental well data within each plate. The Mann–Whitney U test was performed on this permutated parameter set per gene. The null-p value was then used to obtain an empirical p value by comparing the distribution of observed and null-p values. To correct for multiple testing, a second permutation analysis was performed and the minimum p value for all gene tests per permutated sample was acquired. Again, an empirical p value was obtained by comparing the observed and minimum p value distributions. This final empirical p value can be interpreted as the corrected p value and accounts for the number of tests as well as the correlation between test statistics. Cluster analysis of shRNA- or gene-level phenotypes was performed using a Pearson correlation distance matrix. Significance of shRNAs co-clustering was determined using a χ2-test.
Real-time qPCR
RNA was extracted from hippocampal neurons at DIV7 (n = 4 cultures per shRNA) using the RNAeasy mini kit (Qiagen). RNA concentration was determined using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). For cDNA synthesis, 200 ng RNA was mixed with hexanucleotide primers (25 pmol/μl), heated to 37 °C for 1 min, and then snap-cooled on ice. A mix with reverse transcriptase (200 units/μl; Promega) and dNTPs (10 mm) was added and samples were incubated for 45 min at 37 °C. Real-time qPCR was performed using SYBR green (GC Biotech) as the reporter dye. The following primers were used: Tcf4 (fwd: TGAACCCGGCAAACCCTGAA, rev: TCCCTAAGGCAGCCATTCGC), Top3b (fwd: GAGCCGCGTTTAGTGGGCA, rev: GCCAATGCTGACTCCTCGGG), Tbr1 (fwd: GGCGGATCCCAATCACTGGA, rev: AGACCCGGTTTCCTTGCACA). Hprt (fwd: ATGGGAGGCCATCACATTGT, rev: ATGTAATCCAGCAGGTCAGCAA) and Actb (fwd: GCTCCTCCTGAGCGCAAG, rev: CATCTGCTGGAAGGTGGACA) were used for normalization. Data were analyzed using the 2−ΔΔCp method [19].
Proteomics
Proteins were extracted from neuronal cultures at DIV7 (n = 3 cultures per shRNA). After washing twice with PBS at 4 °C, 500 μl of PBS with protease inhibitor (Roche) was added to each well. Cells were scraped and recovered in Eppendorf tubes, centrifuged at 3000 × g for 5 min at 4 °C, supernatant discarded, and cells resuspended in 15 μl of loading buffer. Samples were processed for mass spectrometry as described previously [20, 21]. Protein samples were run on an sodium dodecyl sulfate polyacrylamide gel electrophoresis gel and the gel was fixed and stained with Coomassie blue. Gel lanes were cut into small pieces, de-stained with two incubations with 50 nm ammonium bicarbonate (Fluka)/50% acetonitrile (JT Baker) and dried with 100% acetonitrile. Proteins were in-gel digested overnight at 37 °C with trypsin (Promega) and the digested peptides were extracted with two incubations with 0.1% trifluoroacetic acid (Applied Biosystems)/50% acetonitrile and one incubation with 0.1% trifluoroacetic acid/80% acetonitrile. The eluted peptide solution was dried in a speedvac and dissolved in 0.1% acetic acid solution before being loading into an Ultimate 3000 liquid chromatography system (Dionex, Thermo Scientific). Peptides were electro-sprayed into the TripleTOF 5600 mass spectrometer (Sciex), with a micro-spray needle voltage of 5500 V and analyzed by data independent acquisition. Each SWATH cycle consisted of a parent ion scan of 150 msec and 8 Da SWATH windows, with scan time of 80 msec, through 450–770 m/z mass range. The collision energy for each window was calculated for a 2+ ion centered upon the window (spread of 15 eV).
Proteomics data analysis
MS spectra were analyzed with Spectronaut software (Biognosys) and searched against a spectral library of cultured mouse primary hippocampal neurons for peptide identification. The optimal quality (q) value threshold for each proteomics data set was set on the average median q value of all samples. For protein identification, peptides were allowed to fail detection in only one replicate sample within groups and all but one samples between groups. Outlier analysis was performed when the sample coefficient of variance was higher than 0.12. In that case, replicates with median q values deviating > 10 times compared with other replicates were considered outliers and were removed from the data set. After removal of contaminant proteins (i.e., immunoglobulins, keratins, and trypsin), regulated proteins were selected based on two criteria: proteins had to show (i) a significant log2-fold change compared with scrambled control in at least one shRNA sample per gene (Student’s t test, p < 0.05), and (ii) a concordant log2-fold change (in the same direction, but not necessarily significant) in all other shRNA samples per gene. Protein–protein interaction analysis was performed using STRING [22]. “Interaction sources” was set to “experiments” only and “interaction score” was set to “high confidence” (0.7 or higher). Addition of a maximum of 10 1st shell interactors was allowed. Functional enrichment was tested for GO terms using gProfiler [23]. Enrichment was tested for “molecular function” GO terms only.
Results
We selected 41 neuronally expressed genes for which an association with SCZ has been demonstrated or suggested (Table 1). Most genes (25) are genetically associated with SCZ, others are associated with autism spectrum disorder (ASD; 19 genes) or bipolar disorder (BPD; 10 genes) and have been implicated in SCZ otherwise. For instance, many predicted miR137 and FMRP targets are included as these represent dysregulated pathways in SCZ [14, 15]. Full gene names, gene function summaries, and additional evidence for SCZ involvement for all genes are listed in Supplementary Table S2. For all genes, shRNA constructs were obtained for RNA interference. Given the variation in efficacy and the possibility of off-target effects, multiple shRNA constructs per gene were used (Supplementary Table S1). shRNA-containing lentiviral (LVV) particles were produced and tested for infection efficiency on mouse primary hippocampal neurons (Supplementary Fig. S1a). For each LVV production batch, a random selection of shRNAs was tested including at least one shRNA per target gene. At optimal virus concentrations, an average infection efficiency was observed of 80% ± 28% (Supplementary Fig. S1b). Real-time quantitative PCR demonstrated a knockdown efficiency of 64% ± 15% at 14 days post infection (n = 5; data not shown).
Table 1.
List of selected genes and evidence for their association with SCZ, ASD of BPD
| Gene | SCZ evidence | ASD evidence | BPD evidence |
|---|---|---|---|
| Adnp | Exome sequencing [44], FMRP target [45, 46] | De novo mutation [39] | |
| Ank2 | FMRP target [45, 46] | De novo mutation [39] | |
| Arid1b | FMRP target [45, 46] | SNP [47], de novo mutation [39] | SNP [48] |
| Atp2a2 | GWAS [12], FMRP target [45, 46] | ||
| Bcl11a | miR137 target [14] | ||
| C4a | GWAS [12] | ||
| Cacna1c | GWAS [12] | SNP [49] | SNP [50] |
| Cacna1d | miR137 target [14] | SNP [51] | |
| Cacna1i | GWAS [12], miR137 target [14], FMRP target [45, 46] | SNP [49] | |
| Cacna2d3 | Exome sequencing [52] | De novo mutation [39] | |
| Cacnb2 | GWAS [12], miR137 target [14] | SNP [53] | |
| Chd8 | De novo mutation [54], FMRP target [45, 46] | De novo mutation [39] | |
| Cntnap2 | SNP [55] | SNP [55] | |
| Csmd1 | GWAS [12], miR137 target [14] | SNP [56] | |
| Cttnbp2 | miR137 target [14] | ||
| Drd2 | GWAS [12] | SNP [50] | |
| Dyrk1a | De novo mutation [39] | ||
| Fmr1 | GWAS [15] | ||
| Gabrb3 | CNV [8] | SNP [57] | |
| Grm3 | GWAS [12] | SNP [58] | |
| Katnal2 | De novo mutation [39] | ||
| Kctd13 | GWAS [12] | ||
| Kmt2c | FMRP target [45, 46] | De novo mutation [59] | |
| Kmt5b | De novo mutation [39] | ||
| Kynu | Exome sequencing [15] | ||
| Mecp2 | De novo mutation [60], FMRP target [45, 46] | De novo mutation [60] | |
| Mib1 | miR137 target [14] | ||
| Nrxn1 | CNV [5], miR137 target [14], FMRP target [45, 46] | CNV [61] | |
| Pogz | CNV [62], de novo mutation [39] | ||
| Pten | miR137 target [14] | ||
| Reln | SNP [63], FMRP target [45, 46] | SNP [64] | |
| Scn2a | miR137 target [14], FMRP target [45, 46] | De novo mutation [39] | |
| Setd1a | Exome sequencing [3] | ||
| Shank3 | CNV [9], FMRP target [45, 46] | CNV [65] | |
| Stxbp1 | FMRP target [45, 46] | ||
| Stxbp5 | miR137 target [14] | ||
| Syngap1 | Exome sequencing [66], FMRP target [45, 46] | De novo mutation [39] | |
| Tbr1 | De novo mutation [39] | ||
| Tcf4 | GWAS [12], miR137 target [14], FMRP target [45, 46] | SNP [40] | |
| Top3b | CNV [6] | ||
| Vipr2 | CNV [7] |
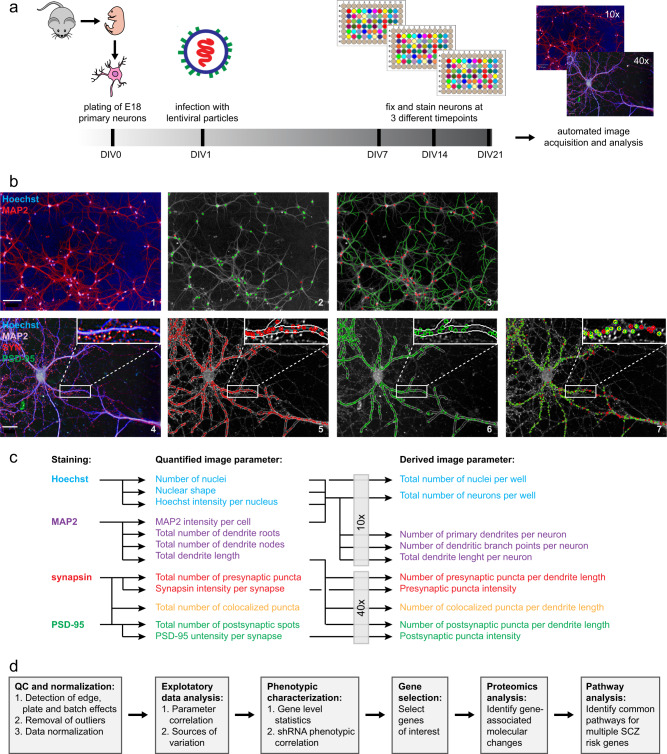
For RNA interference screening, neurons were transduced with shRNA-containing LVV particles at day 1 in vitro (DIV1) and plates were fixed and stained at three time points (DIV7, DIV14, DIV21) to collect morphological data at different stages of neuronal network development (Fig. 1a). Neurons were stained for nuclei (Hoechst), dendrites (anti-MAP2), presynaptic puncta (anti-synapsin), and postsynaptic puncta (anti-PSD-95). Images were acquired using automated confocal microscopy and analyzed using automated image analysis software (Fig. 1b; Supplementary Fig. S2). Ten relevant measures of neuronal survival and network development were calculated (Fig. 1c) and a combination of data analysis tools and proteomics discovery experiments was used to identify neuronal phenotypes and molecular pathways of interest (Fig. 1d).
Fig. 1.
High-content screening and data analysis workflow. a E18 embryonic mouse neurons were seeded in 96-well cell culture plates and infected with lentiviral particles containing shRNA constructs against the 41 genes of interest (4–5 shRNAs per gene). Neurons were only cultured in the inner 60 wells of a 96-well plate and infected on day 1 in vitro (DIV1). On each plate, at least three wells were infected with a scrambled control shRNA, three wells with a positive control shRNA (against NLGN1), and at least three wells were left uninfected. The remaining wells were infected with ~ 50 different experimental shRNAs. Controls and experimental shRNAs were always in a randomized order as to minimize plate position effects. Cultures were fixed at DIV7, DIV14, or DIV21. For each time point, shRNA replicates (n = 3) were divided over three different culture plates as to minimize plate effects. b Neurons were stained with a nuclear marker (Hoechst), a dendritic marker (anti-MAP2), a presynaptic marker (anti-synapsin) and a postsynaptic marker (anti-PSD-95) and imaged using automated confocal high-content microscopy. Neurons were first imaged at × 10 magnification (panel 1) to determine neuron numbers (panel 2; MAP2-positive neurons in green; MAP2-negative cells in red), and total dendrite length and numbers of primary dendrites and branch points (panel 3; selected neurons in red, traced dendrites in green). Neurons were subsequently imaged at × 40 magnification (panel 4) to quantify presynaptic puncta (panel 5; selected puncta in red), postsynaptic puncta (panel 6; selected puncta in green), and colocalized pre-and postsynaptic puncta (panel 7; colocalized puncta in yellow). Images are representative examples of DIV14 neurons. Examples of neurons at all DIV are included in Supplementary Fig. S2. Scale bars: 100 μm (panels 1–3), 20 μm (panels 4–7). c Based on these primary measurements, 10 core parameters were derived that measure relevant aspects of neuronal viability and survival, neuronal network formation, and synaptic connectivity. d Data were checked for batch, plate, and edge effects and outliers were removed. After normalization, multi-level exploratory data analysis was performed to determine the relative contribution of both experimental and technical sources of variation to overall variance in the data. Statistical analysis was then performed to detect significant gene-level effects in the data without prior removal of individual shRNAs. In parallel, robust biological effects were determined by filtering out individual shRNAs that produce inconsistent phenotypes that might represent off-target effects. Based on these two selection criteria candidate genes were selected for proteomics analysis and subsequent cellular pathway analysis
Inspection of plate heat maps revealed no major edge, plate, or batch effects on total cell numbers per well. When plotting all data per plate, one plate was a clear outlier with disproportionally high occurrence of low cell counts (Supplementary Fig. S3a), and these wells were removed. Data plots also revealed a consistent increase in total dendrite length per neuron and pre- and postsynaptic puncta densities with increasing DIV (Supplementary Fig. S3a), indicating that the image analysis algorithms reliably capture neuronal network maturation over time without saturation. To allow overall comparison, all data were normalized per plate against the median of the scrambled control and then log2 transformed. Untreated samples were found evenly distributed around zero, whereas shRNA-treated samples were skewed toward negative values, indicating that multiple shRNAs had negatively affected one or more neuronal network parameters (Supplementary Fig. S3b). Combining log-normalized data from all plates confirmed a normal distribution around zero for all 10 normalized and log2-transformed parameters with a slight skewedness toward negative values (Supplementary Fig. S4).
Correlation analysis showed that the 10 selected parameters cluster into nuclear, dendritic, and synaptic parameter sets (Fig. 2a). Within each set, most parameters show a relatively strong positive correlation. Interestingly, pre- and postsynaptic intensities are negatively correlated with the other synaptic parameters (i.e., the intensities of synapsin and PSD-95 staining decrease as synapse numbers increase). Multi-level modeling was used to estimate the relative contribution of technical and experimental variation to overall variance in the data set, and we found that the experimental manipulation of shRNA treatment accounted for most of the parameter variance. Technical sources of variation had little effect (i.e., batch number, plate number within batch, edge location, and DIV). For dendritic parameters, relatively high variation was observed owig to plate-within-batch effects (Fig. 2b; Supplementary Table S3).
Fig. 2.
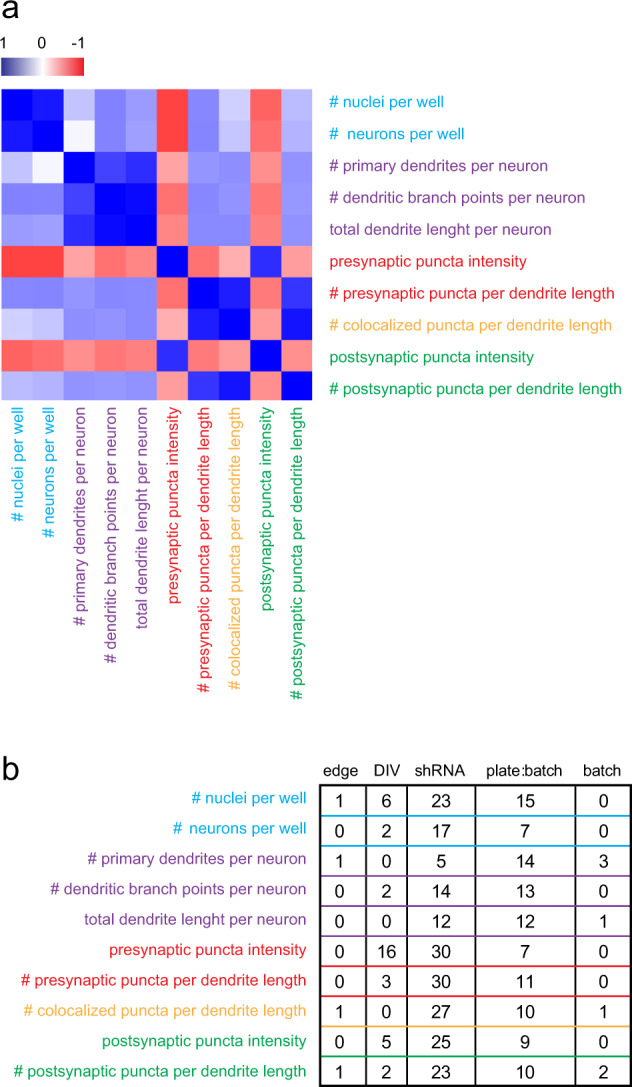
Exploratory analysis of normalized cellomics data. a Correlation analysis of 10 core parameters across all data reveals separation of cell number, dendritic, and synaptic parameter sets and strong correlations within each of these parameter sets. b Multi-level modeling was used to estimate variance in the data owing to technical variation (i.e., batch effects, plate-within-batch effects, and edge effects) or experimental variation (i.e., DIV and shRNA treatment). Normalized parameters are affected primarily by shRNA treatment, however, plate effects are observed for dendrite length, dendrite roots, and dendrite nodes. A complete overview of explained variances with confidence intervals is provided in Supplementary Table S3
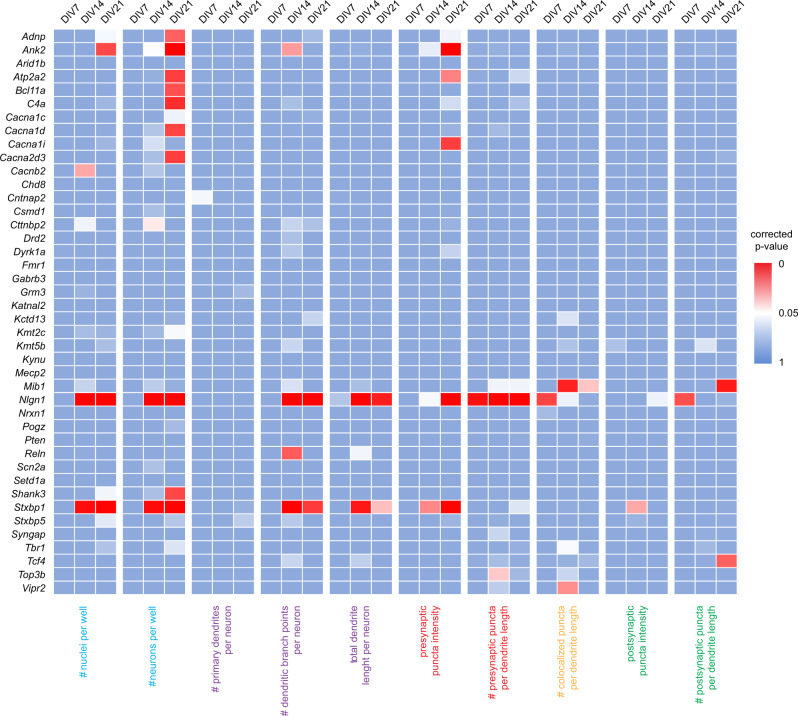
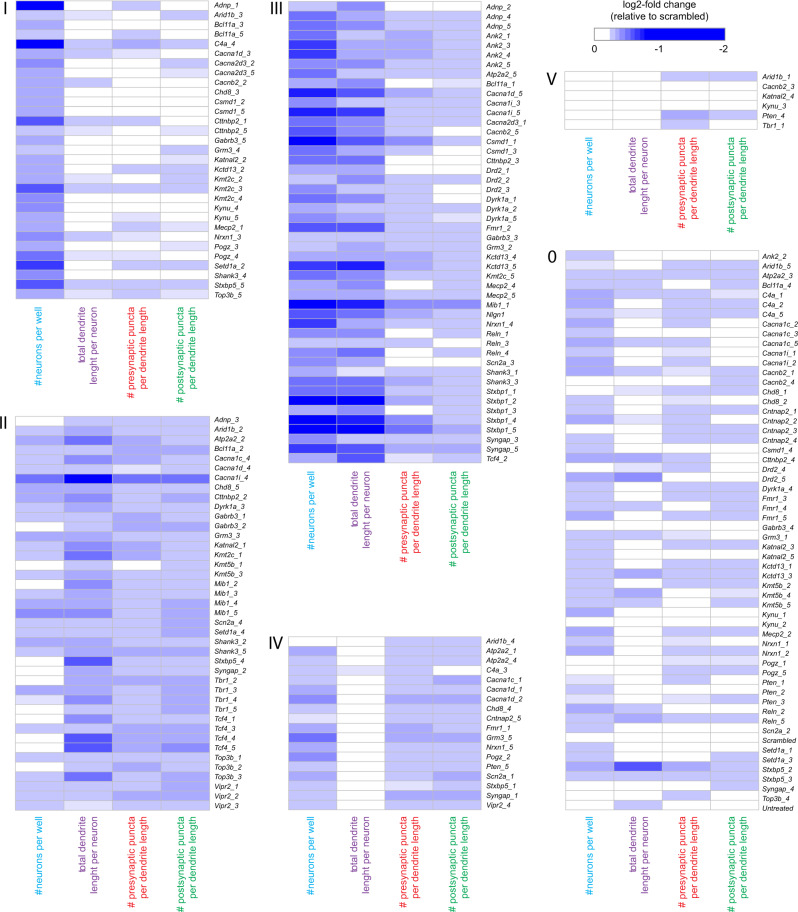
To determine gene-level effects in the data, we first performed an unbiased statistical analysis of the averaged normalized values of all shRNAs per gene across all 10 parameters. Gene-level averaged parameter values were tested against the average of the scrambled control values for each time point separately. Statistically significant changes in any parameter other than “number of nuclei” or “number of neurons” were detected for Ank2, Atp2a2, Cacna1i, Mib1, Nlgn1, Stxbp1, Tcf4, Top3b, and Vipr2 and (Mann–Whitney U, p < 0.05, corrected for multiple testing; Fig. 3). However, unbiased statistical analysis considers all shRNAs per gene as equally effective, which is unlikely and would result in underestimation of true biological effects. We therefore performed a cluster analysis to detect similarities in phenotypes across individual shRNAs and determined which shRNAs produce consistent, biologically relevant phenotypes. We selected four representative parameters: total number of neurons, total dendrite length per neuron, presynaptic puncta density, and postsynaptic puncta density. shRNAs with effect sizes less than twice the standard deviation of the scrambled controls for all four parameters at all three time points were excluded and were considered the no-effect group (Fig. 4; cluster “0”). Individual shRNAs were then clustered using a Pearson correlation distance matrix, resulting in five distinct clusters (Supplementary Fig. S5). These five clusters were characterized by: a reduction in neuron numbers (cluster “I”; 31 shRNAs); a reduction in dendrite length and synapse densities with little or no effect on neuron numbers (cluster “II”; 41 shRNAs); an overall reduction in all parameters (cluster “III”; 48 shRNAs); a reduction in synapse densities with little or no effect on other parameters (cluster “IV”; 18 shRNAs); and a small cluster with no effect across all four parameters (cluster “V”; 6 shRNAs) (Fig. 4). Twelve genes were identified with at least three shRNAs in the same phenotypic cluster: Adnp, Ank2, Drd2, Dyrk1A, Kmt2c, Mib1, Reln, Stxbp1, Tbr1, Tcf4, Top3b, and Vipr2. The co-clustering of three or more shRNAs for a single gene in the same phenotypic cluster was significantly different from chance (χ2 test, p = 1.6 × 10−5). When combining the effects of the co-clustered shRNAs per gene, the following phenotypes were identified: reduced neuron numbers (Kmt2c, Adnp, Dyrk1a), reduced dendrite length followed by a reduction in neuron numbers (Drd2, Ank2, Stxbp1, Mib1, Reln), and reduced dendrite length and synapse densities (Tcf4, Top3b, Tbr1, Vipr2) (Fig. 5a).
Fig. 3.
Statistical analysis of normalized data. A Mann–Whitney U test was performed per time point including all ten core parameters. Gene-level effects were calculated per parameter by averaging the normalized effects of all 4–5 gene-specific shRNAs. A permutation analysis was performed to correct for multiple testing. Heat maps show empirical p values for ten parameters across 41 genes at DIV7, DIV14, and DIV21
Fig. 4.
Phenotypic clustering of individual shRNAs. Hierarchical clustering was performed based on time point (DIV7, DIV14, and DIV21)-averaged data for four representative core parameters, total neuron number per well, total dendrite length per neuron, presynaptic puncta density, and postsynaptic puncta density. Before clustering, shRNAs with log-normalized effect sizes of ≤ 2 × the SD of the scrambled controls were excluded from the data and designated as the no-effect cluster (cluster “0”). For the remaining shRNAs, five phenotypic clusters were identified that are characterized by primarily a reduction in neuron numbers (cluster “I”), primarily a reduction in dendrite length and synapse densities (cluster “II”), an overall reduction in all parameters (cluster “III”), a reduction in synapse densities with little or no effect on other parameters (cluster “IV”), or the absence of a marked phenotype (cluster “V”)
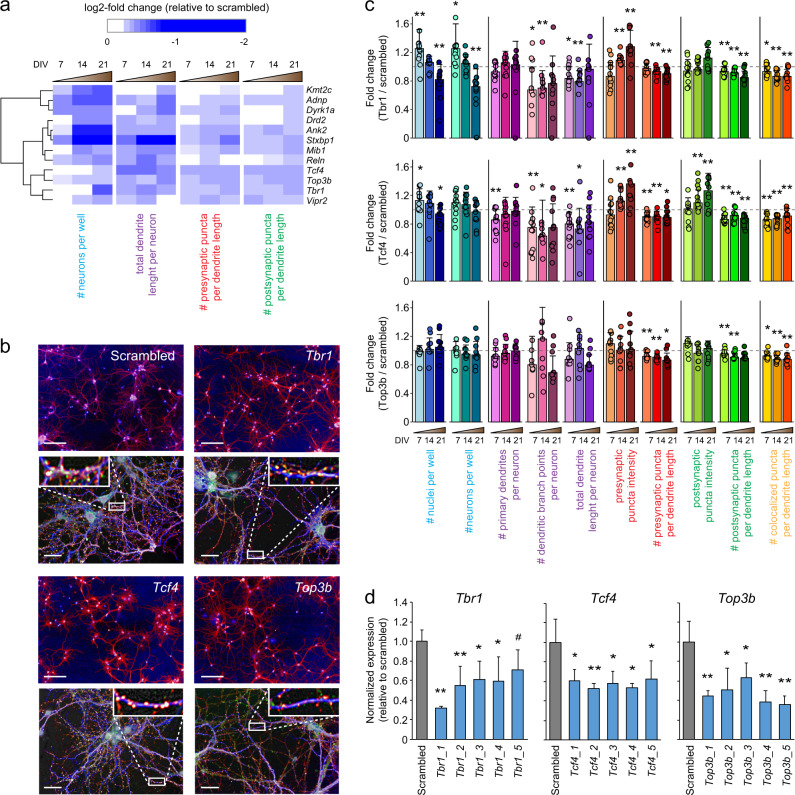
Fig. 5.
Tcf4−, Tbr1−, and Top3b-associated neuronal phenotypes. a Cluster analysis revealed 12 genes that show distinct and progressive neuronal phenotypes based on three or more co-clustering shRNAs per gene. Tcf4, Tbr1, and Top3b showed early or progressive synapse loss with a relatively small effect on neuron numbers. b Example images illustrate the neuronal morphology phenotypes of Tcf4, Tbr1, and Top3b knockdown cultures at DIV14 compared with a scrambled control culture. Scale bars: 100 μm (top panels), 20 μm (bottom panels). c Quantification of the Tcf4, Tbr1, and Top3b knockdown phenotypes across all 10 measured parameters demonstrates a significant reduction in pre-, post-, and/or colocalized synaptic puncta densities starting at DIV7, whereas neuronal cell numbers are not reduced until DIV21. Error bars represent SD; two-sided Student’s t test, n = 12 (Tbr1 and Tcf4) or 9 (Top3b), *p < 0.05, **p < 0.01. d Quantitative real-time PCR shows that shRNAs targeting Tcf4, Tbr1, and Top3b reduce the corresponding mRNA levels by 30–70% relative to scrambled controls. Expression levels are normalized to the averaged expression levels of Actb and Hprt. Error bars represent SD; two-sided Student’s t test, n = 4, * p < 0.05, **p < 0.01, #p < 0.01
Given the polygenic nature of SCZ and the assumption that combined risk of many genes with small effect size is needed to develop SCZ, it is possible that risk genes whose knockdown produce similar cellular phenotypes converge on similar molecular pathways underlying those phenotypes. To test this hypothesis, we selected Tcf4, Top3b, and Tbr1 as all showed a relatively strong and specific synaptic phenotype upon knockdown (Fig. 5a). Knockdown of all three genes resulted in a significant reduction of pre-, post-, and colocalized synaptic puncta densities starting at DIV7, whereas neuronal cell numbers were not reduced until DIV21 (Fig. 5b, c). Real-time quantitative PCR confirmed that all shRNAs producing the synaptic phenotype had knockdown efficiencies of 40–55% at DIV7, except for shRNA #5 targeting Tbr1, which was therefore excluded from further analysis (Fig. 5d). Noteworthy, the four shRNAs that did not cluster to the same phenotype (i.e., Tcf4 #2, Tbr1 #1, Top3b #4, and Top3b #5) did significantly reduce the corresponding mRNA levels. Phenotypically, Tcf4 #2 and Top3b #5 showed in addition to a synapse loss also reduced cell viability, indicating potential off-target effects; Tbr1 #1 and Top3b #4 showed a very mild synaptic phenotype but were classified as negatives, suggesting that they might actually be false negatives (Fig. 4).
To identify molecular pathways affected by Tcf4, Tbr1, or Top3b, we next performed a quantitative, label-free SWATH proteomics analysis of hippocampal cultures after knockdown of each gene with each shRNA separately. Proteomics analysis was performed at DIV7 when the synaptic phenotype was observed without significant changes in other cellular parameters. Peptide intensity distributions revealed high quality across all samples (Supplementary Fig. S6a, b) and, after setting optimal q value cutoffs for each experiment, we identified 2059 proteins in Tbr1 knockdown samples, 2570 in Tcf4 knockdown samples, and 2561 in Top3b knockdown samples (Supplementary Table S4). We next selected all proteins that showed a significant log2-fold change compared with scrambled shRNA-treated samples for at least one shRNA per gene (two-sided Student’s t test, p < 0.05) and a concordant change (i.e., in the same direction, but not necessarily statistically significant) for all other shRNAs targeting that gene. This analysis identified 94 consistently regulated proteins for Tcf4, 61 for Tbr1, and 67 for Top3b (Supplementary Fig. S6c; Supplementary Table S5). Only 2–5 proteins were regulated in common between any two genes, and only one protein between all three genes (Supplementary Fig. S6d).
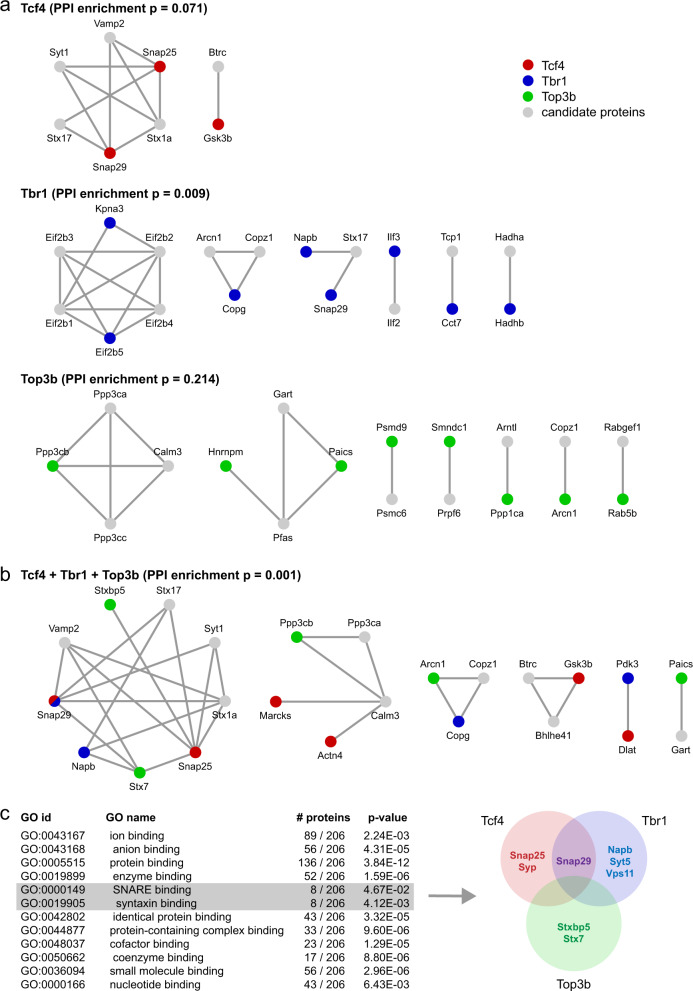
Protein–protein interaction (PPI) analysis was performed to predict cellular pathways that are dysregulated owing to either Tcf4, Tbr1, or Top3b knockdown. High-confidence PPI networks were extracted from STRING [22] based on experimentally validated interactions only using and allowing the addition of maximally 10 network candidate proteins. Significant PPI enrichment was detected in the TBR1-regulated protein set (p = 0.009), but not in the TCF4- or TOP3B-regulated protein sets (p = 0.071 and p = 0.214, respectively), and no detected network contained more than two regulated proteins (Fig. 6a). To identify PPI networks that may be dysregulated owing to the combined effect of Tcf4, Tbr1, and Top3b knockdown we also performed an analysis of all 210 regulated proteins together. PPI enrichment in this combined protein set was highly significant (p = 0.001), and the largest PPI network that was identified consisted of SNARE proteins involved in neurotransmitter release and contained five dysregulated proteins (SNAP25, SNAP29, NAPB, STX7, and STXBP5) from all three data sets (Fig. 6b). Smaller networks contained no more than three proteins from maximally two data sets.
Fig. 6.
Tcf4−, Tbr1−, and Top3b-associated molecular pathways. a Proteomics analysis was used to identify dysregulated proteins owing to Tcf4, Tbr1, or Top3b knockdown. Dysregulated proteins were analyzed in STRING to identify PPI networks. Significant PPI enrichment was only detected in the Tbr1 knockdown protein set. Red, blue, and green circles are query proteins that are dysregulated upon Tcf4, Tbr1, or Top3b knockdown, respectively, gray circles are candidate proteins added by STRING. b The same analysis was performed for the combined dysregulated protein set, identifying the SNARE complex as a potential common target of Tcf4, Tbr1, and Top3b. c GO enrichment analysis of the combined dysregulated protein set (without addition of candidate interactors) confirms enrichment for proteins involved in “SNARE binding” and “syntaxin binding” contributed by TCF4−, TBR1−, and TOP3B-dysregulated proteins
To exclude the possibility that detection of the SNARE complex was owing to the addition of network candidates that are not part of the dysregulated protein set we also performed gene ontology (GO) enrichment analysis of dysregulated proteins only using gProfiler [23]. Significant enrichment was observed for “molecular function” GO classes “SNARE binding” (p = 0.047) and “syntaxin binding” (p = 0.004), and three proteins were identified (SYP, SYT5, and VPS11) in addition to the five found previously using PPI analysis (Fig. 6c). PPI and GO enrichment analysis together thus strongly suggest that reduced expression of SCZ risk genes Tcf4, Tbr1, and Top3b converges on a canonical neurotransmitter release pathway involving the SNARE protein complex.
Discussion
SCZ is a highly complex polygenic disorder for which > 145 genomic regions have been significantly associated in genetic studies [12, 13]. This poses the challenging question how this genetic variation converges onto common patterns of cellular and molecular alterations underlying the disorder. In-depth studies of individual risk genes, isolated from a spectrum of hundreds, have provided limited information on disease biology. In this study, we integrated cellomics and proteomics approaches to demonstrate that significant insight into disease biology can be obtained by combining neuronal cellular morphology and molecular pathway information for multiple risk genes and searching for shared characteristics.
RNA interference and subsequent high-content neuronal cellular phenotyping of 41 candidate SCZ risk genes resulted in the identification 12 genes showing robust phenotypic clustering and producing reliable phenotypes with multiple shRNAs targeting the same gene. Based on these findings, and assuming that polygenic risk is essential in SCZ, we hypothesized that SCZ risk genes that share a similar neuronal phenotype may each contribute to that phenotype in a synergistic manner. To test this, we selected Tbr1, Tcf4, and Top3b, which all showed a common knockdown phenotype characterized by a reduction in synapse densities, in line with the suggested developmental nature of SCZ [24]. Importantly, reduced synapse densities were observed early, before neuron numbers became affected, indicating that synaptic connectivity impairments were driving neuronal loss, and not the other way around. All three genes regulate gene expression, but none have been functionally linked yet. TBR1 is a T-box transcription factor involved in the development of cortical and amygdala neurons [25, 26] and impairments in hippocampal neurogenesis were shown to correlate with reduced TBR1 expression in an induced pluripotent stem cells (iPSC) model of SCZ [27]. TBR1 was shown to induce the expression of NMDA receptor subunit Grin2b in hippocampal and amygdala neurons in an activity-dependent manner [28]. TCF4 is a basic helix–loop–helix transcription factor previously associated with SCZ, ASD, and intellectual disability [29] and has been shown to regulate spine densities in the cortex and the hippocampus [30]. TOP3B is a topoisomerase that associates with FMRP in a direct, non-mRNA-mediated manner [6]. FMRP regulates the translation of synaptic mRNAs and the development and function of synapses [31], and genes encoding FMRP target mRNAs are significantly enriched for SCZ-associated rare mutations [15]. It is not clear whether TBR1, TCF4, and TOP3B regulate synaptic functions independently, or if they converge on shared synaptic pathways.
Our proteomics experiments and subsequent PPI and pathway analyses highlight syntaxin-mediated neurotransmitter release as a prominent pathway onto which TCF4-, TBR1-, and TOP3B-regulated protein expression converges. Central in this network is syntaxin 1A (STX1A), which is crucial for synaptic vesicle docking and neurotransmitter release [32]. A genetic association between STX1A and SCZ has been suggested [33] and STX1A mRNA and protein levels are decreased in post-mortem brain tissue from SCZ patients [34]. Importantly, STX1A itself was not dysregulated in our proteomics experiments. Instead, multiple nodes in the STX1A PPI network were affected by Tcf4, Tbr1, or Top3b knockdown. Other genes that were included in the screen may impact on the same pathway. Stxbp1 for instance is essential for syntaxin-mediated neurotransmitter release [35], and its knockdown resulted in a much stronger phenotype than Tcf4, Tbr1, or Top3b knockdown, including also reduced dendrite length and reduced neuronal viability. This suggests that neurotransmitter release in SCZ may be affected owing to subtle dysregulation via indirect upstream gene regulatory mechanisms rather than a dysregulation of proteins that are directly involved. In line with this, we could not find other SZC GWAS candidate genes that are part of the same neurotransmitter release pathway, nor did we observe significant enrichment for low p value GWAS hits among all proteins dysregulated by Tcf4, Tbr1, or Top3b knockdown (p = 0.44, 0.48, and 0.91, respectively; hypergeometric test). Together, these observations demonstrate that interpreting polygenic risk from a protein regulatory network perspective is able to uncover hidden aspects of disease biology.
Our study provides proof-of-principle that multi-level morphological and molecular phenotyping is able to extract disease-relevant pathway information about neuropsychiatric disorders, however, several limitations apply. For instance, stringent selection criteria (i.e., three or more shRNAs against the same gene producing a similar phenotype) led to the identification of only 12 candidate genes for follow up analysis. Although this prevented potential off-target effects from biasing the data towards false positive hits, it likely also increased false negative findings. Interestingly, another 10 genes (Atp2a, Cacna1d, Cacna1i, Csmd1, Gabrb3, Kctd13, Kmt5b, Mecp2, Shank3, and Syngap) were assigned with two shRNAs to one of the three phenotypic clusters marked by synaptic changes (i.e., clusters II, III, or IV in Fig. 4), including candidate synapse- or neurotransmission-modifying genes such as Cacna1d, Cacna1i, Gabrb3, Mecp2, Shank3, and Syngap. Likewise, additional pathways may be revealed when proteomics profiling is performed for more candidate genes in every phenotypic cluster. In particular, our screen included, in addition to Tcf4, Tbr1, or Top3b, nine more transcriptional regulators and chromatin modifiers that produced dendritic or synaptic changes (i.e., Adnp, Arid1b, Bcl11a, Chd8, Kmt2c, Kmt5b, Mecp2, Pogz, and Setd1a in clusters II, III of IV; Fig. 4). These are interesting candidate genes to discover additional converging pathways leading to neuronal connectivity changes underlying SCZ.
In this study, we used mouse primary neuronal cultures combined with RNA interference to study SCZ risk gene function. Given the fact that SCZ is a complex human disease and the underlying genetic variation cannot simply be modeled by reducing the expression of individual genes, this is a strongly reductionist approach. It is however unlikely that patient mutations that individually do not increase SCZ risk would produce detectable phenotypes in a cellular assay. RNA interference on the other hand allows functional characterization and clustering of candidate SCZ risk genes based on detectable phenotypes, and permits subsequent identification of shared molecular and cellular pathways. Our data show that using this approach, disease-relevant pathway information can be obtained and used to generate testable hypotheses with respect to multi-gene interactions that increase SCZ risk in humans. These hypotheses can then be tested in other models, for instance iPSC-derived human neurons, in which mutations that are predicted to converge onto common cellular pathways co-exist, either by nature or engineered.
It is still an open question whether genetic risk for SCZ converges onto one or more common cellular pathways. The fact that three non-synaptic genes show downstream convergence onto an important synaptic signaling pathway argues that the disease may be more homogeneous than suggested by its apparent genetic complexity, and is consistent with previous speculations based on genetic findings that synapses are an important substrate in SCZ pathology [36–38]. Another issue of debate is the genetic similarity between SCZ and other psychiatric disorders, in particular BPD and ASD. TBR1 has also been associated with ASD [39] and TCF4 with BPD [40], and TOP3B was shown to bind multiple mRNAs derived from ASD-linked genes [41], raising the possibility that STX1A-mediated neurotransmitter release is dysregulated in multiple psychiatric disorders. Indeed, genetic or functional associations exist between STX1A and BPD [42] or ASD [43]. Pathway analysis of additional risk genes may strengthen the hypothesis that neurotransmitter release pathways are common substrates for multiple psychiatric disorders.
Supplementary information
Acknowledgements
We thank M.A. Gonzalez-Lozano for technical assistance. M.R. was supported by the European grant EU-FP7 MC-ITN IN-SENS (#607616), and the Schizophrenia United Network (SUN) project. We gratefully acknowledge support from the Swedish Research Council (Vetenskapsrådet, award D0886501).
Author contributions
M.R., K.W.L., D.P., P.F.S., A.B.S. and R.E.v.K. designed the study, M.R., T.G. and I.P. performed all experiments, M.R. and R.E.v.K. performed data analysis, S.S. performed modeling and statistical analysis of cellomics data, M.R., P.F.S., A.B.S. and R.E.v.K. wrote the manuscript.
Compliance with ethical standards
Conflict of interest
P.F.S. reports the following potentially competing financial interests. Current: Lundbeck (advisory committee, grant recipient). Past 3 years: Pfizer (scientific advisory board), Element Genomics (consultation fee), and Roche (speaker reimbursement).
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Patrick F. Sullivan, August B. Smit, and Ronald E. van Kesteren
Contributor Information
Patrick F. Sullivan, Email: patrick_sullivan@med.unc.edu
Ronald E. van Kesteren, Email: ronald.van.kesteren@vu.nl
Supplementary information
The online version of this article (10.1038/s41380-019-0436-y) contains supplementary material, which is available to authorized users.
References
- 1.Gejman PV, Sanders AR, Duan J. The role of genetics in the etiology of schizophrenia. Psychiatr Clin North Am. 2010;33:35–66. doi: 10.1016/j.psc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giusti-Rodriguez P, Sullivan PF. The genomics of schizophrenia: update and implications. J Clin Invest. 2013;123:4557–63. doi: 10.1172/JCI66031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh T, Kurki MI, Curtis D, Purcell SM, Crooks L, McRae J, et al. Rare loss-of-function variants in SETD1A are associated with schizophrenia and developmental disorders. Nat Neurosci. 2016;19:571–7. doi: 10.1038/nn.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg S, Gudmundsdottir S, Sveinbjornsson G, Suvisaari J, Paunio T, Torniainen-Holm M, et al. Truncating mutations in RBM12 are associated with psychosis. Nat Genet. 2017;49:1251–4. doi: 10.1038/ng.3894. [DOI] [PubMed] [Google Scholar]
- 5.Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS, et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet. 2017;49:27–35. doi: 10.1038/ng.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoll G, Pietilainen OPH, Linder B, Suvisaari J, Brosi C, Hennah W, et al. Deletion of TOP3beta, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders. Nat Neurosci. 2013;16:1228–37. doi: 10.1038/nn.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin C, Zhou Z, Li W, Zhang F, Yuan J, Liu X, et al. Analysis of the association of VIPR2 polymorphisms with susceptibility to schizophrenia. Psychiatry Res. 2016;241:104–7. doi: 10.1016/j.psychres.2016.04.084. [DOI] [PubMed] [Google Scholar]
- 8.Huang CC, Cheng MC, Tsai HM, Lai CH, Chen CH. Genetic analysis of GABRB3 at 15q12 as a candidate gene of schizophrenia. Psychiatr Genet. 2014;24:151–7. doi: 10.1097/YPG.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 9.Failla P, Romano C, Alberti A, Vasta A, Buono S, Castiglia L, et al. Schizophrenia in a patient with subtelomeric duplication of chromosome 22q. Clin Genet. 2007;71:599–601. doi: 10.1111/j.1399-0004.2007.00819.x. [DOI] [PubMed] [Google Scholar]
- 10.Fryland T, Christensen JH, Pallesen J, Mattheisen M, Palmfeldt J, Bak M, et al. Identification of the BRD1 interaction network and its impact on mental disorder risk. Genome Med. 2016;8:53. doi: 10.1186/s13073-016-0308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, et al. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature. 2011;471:499–503. doi: 10.1038/nature09884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardinas AF, Holmans P, Pocklington AJ, Escott-Price V, Ripke S, Carrera N, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018;50:381–9. doi: 10.1038/s41588-018-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins AL, Kim Y, Bloom RJ, Kelada SN, Sethupathy P, Sullivan PF. Transcriptional targets of the schizophrenia risk gene MIR137. Transl Psychiatry. 2014;4:e404. doi: 10.1038/tp.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–90. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia H, Jahr FM, Kim NK, Xie L, Shabalin AA, Bryois J, et al. Building a schizophrenia genetic network: transcription factor 4 regulates genes involved in neuronal development and schizophrenia risk. Hum Mol Genet. 2018;27:3246–56. doi: 10.1093/hmg/ddy222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaudoin GM, 3rd, Lee SH, Singh D, Yuan Y, Ng YG, Reichardt LF, et al. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat Protoc. 2012;7:1741–54. doi: 10.1038/nprot.2012.099. [DOI] [PubMed] [Google Scholar]
- 18.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models Usinglme4. J Stat Softw. 2015;67:48830.
- 19.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 20.Koopmans F, Ho JTC, Smit AB, Li KW. Comparative analyses of data independent acquisition mass spectrometric approaches: DIA, WiSIM-DIA, and untargeted DIA. Proteomics. 2018;18. [DOI] [PMC free article] [PubMed]
- 21.Koopmans F, Pandya NJ, Franke SK, Phillippens I, Paliukhovich I, Li KW, et al. Comparative hippocampal synaptic proteomes of rodents and primates: differences in neuroplasticity-related proteinparative hippocampal synaptic proteomes of rodents and primates: differences in neuroplasticity-related proteins. Front Mol Neurosci. 2018;11:364. doi: 10.3389/fnmol.2018.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRINGv10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reimand J, Arak T, Adler P, Kolberg L, Reisberg S, Peterson H, et al. g:Profiler-a web server for functional interpretation of gene lists (2016 update) Nucleic Acids Res. 2016;44:W83–89. doi: 10.1093/nar/gkw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 2017;18:727–40. doi: 10.1038/nrn.2017.125. [DOI] [PubMed] [Google Scholar]
- 25.Bedogni F, Hodge RD, Elsen GE, Nelson BR, Daza RA, Beyer RP, et al. Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci USA. 2010;107:13129–34. doi: 10.1073/pnas.1002285107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang TN, Chuang HC, Chou WH, Chen CY, Wang HF, Chou SJ, et al. Tbr1 haploinsufficiency impairs amygdalar axonal projections and results in cognitive abnormality. Nat Neurosci. 2014;17:240–7. doi: 10.1038/nn.3626. [DOI] [PubMed] [Google Scholar]
- 27.Yu DX, Di Giorgio FP, Yao J, Marchetto MC, Brennand K, Wright R, et al. Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem Cell Rep. 2014;2:295–310. doi: 10.1016/j.stemcr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang HC, Huang TN, Hsueh YP. Neuronal excitation upregulates Tbr1, a high-confidence risk gene of autism, mediating Grin2b expression in the adult brain. Front Cell Neurosci. 2014;8:280. doi: 10.3389/fncel.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forrest MP, Hill MJ, Kavanagh DH, Tansey KE, Waite AJ, Blake DJ. The psychiatric risk gene transcription factor 4 (TCF4) regulates neurodevelopmental pathways associated with schizophrenia, autism, and intellectual disability. Schizophr Bull. 2017;44:1100–10. [DOI] [PMC free article] [PubMed]
- 30.Crux S, Herms J, Dorostkar MM. Tcf4 regulates dendritic spine density and morphology in the adult brain. PLoS ONE. 2018;13:e0199359. doi: 10.1371/journal.pone.0199359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–14. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vardar G, Chang S, Arancillo M, Wu YJ, Trimbuch T, Rosenmund C. Distinct functions of syntaxin-1 in neuronal maintenance, synaptic vesicle docking, and fusion in mouse neurons. J Neurosci. 2016;36:7911–24. doi: 10.1523/JNEUROSCI.1314-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong AH, Trakalo J, Likhodi O, Yusuf M, Macedo A, Azevedo MH, et al. Association between schizophrenia and the syntaxin 1A gene. Biol Psychiatry. 2004;56:24–29. doi: 10.1016/j.biopsych.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Sokolov BP, Tcherepanov AA, Haroutunian V, Davis KL. Levels of mRNAs encoding synaptic vesicle and synaptic plasma membrane proteins in the temporal cortex of elderly schizophrenic patients. Biol Psychiatry. 2000;48:184–96. doi: 10.1016/s0006-3223(00)00875-1. [DOI] [PubMed] [Google Scholar]
- 35.Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–9. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 36.Crabtree GW, Gogos JA. Synaptic plasticity, neural circuits, and the emerging role of altered short-term information processing in schizophrenia. Front Synaptic Neurosci. 2014;6:28. doi: 10.3389/fnsyn.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Focking M, Lopez LM, English JA, Dicker P, Wolff A, Brindley E, et al. Proteomic and genomic evidence implicates the postsynaptic density in schizophrenia. Mol Psychiatry. 2015;20:424–32. doi: 10.1038/mp.2014.63. [DOI] [PubMed] [Google Scholar]
- 38.Hall J, Trent S, Thomas KL, O'Donovan MC, Owen MJ. Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biol Psychiatry. 2015;77:52–58. doi: 10.1016/j.biopsych.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 39.De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–15. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter CJ. Multiple genes and factors associated with bipolar disorder converge on growth factor and stress activated kinase pathways controlling translation initiation: implications for oligodendrocyte viability. Neurochem Int. 2007;50:461–90. doi: 10.1016/j.neuint.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Xu D, Shen W, Guo R, Xue Y, Peng W, Sima J, et al. Top3beta is an RNA topoisomerase that works with fragile X syndrome protein to promote synapse formation. Nat Neurosci. 2013;16:1238–47. doi: 10.1038/nn.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunii Y, Hyde TM, Ye T, Li C, Kolachana B, Dickinson D, et al. Revisiting DARPP-32 in postmortem human brain: changes in schizophrenia and bipolar disorder and genetic associations with t-DARPP-32 expression. Mol Psychiatry. 2014;19:192–9. doi: 10.1038/mp.2012.174. [DOI] [PubMed] [Google Scholar]
- 43.Kofuji T, Hayashi Y, Fujiwara T, Sanada M, Tamaru M, Akagawa K. A part of patients with autism spectrum disorder has haploidy of HPC-1/syntaxin1A gene that possibly causes behavioral disturbance as in experimentally gene ablated mice. Neurosci Lett. 2017;644:5–9. doi: 10.1016/j.neulet.2017.02.052. [DOI] [PubMed] [Google Scholar]
- 44.Merenlender-Wagner A, Malishkevich A, Shemer Z, Udawela M, Gibbons A, Scarr E, et al. Autophagy has a key role in the pathophysiology of schizophrenia. Mol Psychiatry. 2015;20:126–32. doi: 10.1038/mp.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–61. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ascano M, Jr, Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature. 2012;492:382–6. doi: 10.1038/nature11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D'Gama AM, Pochareddy S, Li M, Jamuar SS, Reiff RE, Lam AN, et al. Targeted DNA sequencing from autism spectrum disorder brains implicates multiple genetic mechanisms. Neuron. 2015;88:910–7. doi: 10.1016/j.neuron.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ortega-Alonso A, Ekelund J, Sarin AP, Miettunen J, Veijola J, Jarvelin MR, et al. Genome-wide association study of psychosis proneness in the Finnish population. Schizophr Bull. 2017;43:1304–14. doi: 10.1093/schbul/sbx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu AT, Dai X, Martinez-Agosto JA, Cantor RM. Support for calcium channel gene defects in autism spectrum disorders. Mol Autism. 2012;3:18. doi: 10.1186/2040-2392-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y, Wang J, Rao S, Ritter M, Manor LC, Backer R, et al. An integrative computational approach to evaluate genetic markers for bipolar disorder. Sci Rep. 2017;7:6745. doi: 10.1038/s41598-017-05846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ross J, Gedvilaite E, Badner JA, Erdman C, Baird L, Matsunami N, et al. A rare variant in CACNA1D segregates with 7 bipolar i disorder cases in a large pedigree. Mol Neuropsychiatry. 2016;2:145–50. doi: 10.1159/000448041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Yoshikawa A, Brennan MD, Ramsey TL, Meltzer HY. Genetic predictors of antipsychotic response to lurasidone identified in a genome wide association study and by schizophrenia risk genes. Schizophr Res. 2018;192:194–204. doi: 10.1016/j.schres.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Lee MT, Chen CH, Lee CS, Chen CC, Chong MY, Ouyang WC, et al. Genome-wide association study of bipolar I disorder in the Han Chinese population. Mol Psychiatry. 2011;16:548–56. doi: 10.1038/mp.2010.43. [DOI] [PubMed] [Google Scholar]
- 54.Merner N, Forgeot d'Arc B, Bell SC, Maussion G, Peng H, Gauthier J, et al. A de novo frameshift mutation in chromodomain helicase DNA-binding domain 8 (CHD8): a case report and literature review. Am J Med Genet A. 2016;170A:1225–35. doi: 10.1002/ajmg.a.37566. [DOI] [PubMed] [Google Scholar]
- 55.Wang KS, Liu XF, Aragam N. A genome-wide meta-analysis identifies novel loci associated with schizophrenia and bipolar disorder. Schizophr Res. 2010;124:192–9. doi: 10.1016/j.schres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Xu W, Cohen-Woods S, Chen Q, Noor A, Knight J, Hosang G, et al. Genome-wide association study of bipolar disorder in Canadian and UK populations corroborates disease loci including SYNE1 and CSMD1. BMC Med Genet. 2014;15:2. doi: 10.1186/1471-2350-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, Li J, Shuang M, Lu T, Wang Z, Zhang T, et al. Association study and mutation sequencing of genes on chromosome 15q11-q13 identified GABRG3 as a susceptibility gene for autism in Chinese Han population. Transl Psychiatry. 2018;8:152. doi: 10.1038/s41398-018-0197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Brien NL, Way MJ, Kandaswamy R, Fiorentino A, Sharp SI, Quadri G, et al. The functional GRM3 Kozak sequence variant rs148754219 affects the risk of schizophrenia and alcohol dependence as well as bipolar disorder. Psychiatr Genet. 2014;24:277–8. doi: 10.1097/YPG.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koemans TS, Kleefstra T, Chubak MC, Stone MH, Reijnders MRF, de Munnik S, et al. Functional convergence of histone methyltransferases EHMT1 and KMT2C involved in intellectual disability and autism spectrum disorder. PLoS Genet. 2017;13:e1006864. doi: 10.1371/journal.pgen.1006864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piton A, Gauthier J, Hamdan FF, Lafreniere RG, Yang Y, Henrion E, et al. Systematic resequencing of X-chromosome synaptic genes in autism spectrum disorder and schizophrenia. Mol Psychiatry. 2011;16:867–80. doi: 10.1038/mp.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noor A, Lionel AC, Cohen-Woods S, Moghimi N, Rucker J, Fennell A, et al. Copy number variant study of bipolar disorder in Canadian and UK populations implicates synaptic genes. Am J Med Genet B Neuropsychiatr Genet. 2014;165B:303–13. doi: 10.1002/ajmg.b.32232. [DOI] [PubMed] [Google Scholar]
- 62.Stessman HAF, Willemsen MH, Fenckova M, Penn O, Hoischen A, Xiong B, et al. Disruption of POGZ is associated with intellectual disability and autism spectrum disorders. Am J Hum Genet. 2016;98:541–52. doi: 10.1016/j.ajhg.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Chen PL, McGrath J, Wolyniec P, Fallin D, Nestadt G, et al. Replication of an association of a common variant in the Reelin gene (RELN) with schizophrenia in Ashkenazi Jewish women. Psychiatr Genet. 2010;20:184–6. doi: 10.1097/YPG.0b013e32833a220b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen N, Bao Y, Xue Y, Sun Y, Hu D, Meng S, et al. Meta-analyses of RELN variants in neuropsychiatric disorders. Behav Brain Res. 2017;332:110–9. doi: 10.1016/j.bbr.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 65.Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xing J, Kimura H, Wang C, Ishizuka K, Kushima I, Arioka Y, et al. Resequencing and association analysis of six PSD-95-related genes as possible susceptibility genes for schizophrenia and autism spectrum disorders. Sci Rep. 2016;6:27491. doi: 10.1038/srep27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.