Abstract
The amygdala in mammals plays a key role in emotional processing and learning, being subdivided in pallial and subpallial derivatives. Recently, the cortical ring model and the pallial amygdalar radial model (Puelles et al. 2019; Garcia-Calero et al. 2020) described the pallial amygdala as an histogenetic field external to the allocortical ring, and subdivided it in five major radial domains called lateral, basal, anterior, posterior and retroendopiriform units. The anterior radial unit, whose cells typically express the Lhx9 gene (see molecular profile in Garcia-Calero et al. 2020), is located next to the pallial/subpallial boundary. This radial domain shows massive radial translocation and accumulation of its derivatives into its intermediate and superficial strata, with only a glial palisade representing its final periventricular domain. To better understand the development of this singular radial domain, not described previously, we followed the expression of Lhx9 during mouse amygdalar development in the context of the postulated radial subdivisions of the pallial amygdala and other telencephalic developmental features.
Keywords: Radial amygdalar model, Pallium, Ventral pallium, Pallio-subpallial boundary, Pallial amygdala, Medial amygdala
Introduction
The telencephalic amygdala of mammals is a mixed pallial/subpallial nuclear complex located at the tip of the temporal lobe (Burdach 1819–1822; Johnston 1923; Loo 1930, 1931; De Olmos et al. 1985, 2004; Alheid et al. 1995; Swanson and Petrovich 1998; Martínez-García et al. 2012; Olucha-Bordonau et al. 2015, Medina et al. 2017). It is implicated in evaluation of combined external stimuli, processing of fear and other emotions, and consequent learning (Weiskranft 1956; Amaral et al. 2003; Phelps and Ledoux 2005; Ledoux, 2007; Rolls 2014, 2015). It contains a variety of nuclei and superficial corticoid structures with different pallial or subpallial embryonic origins, which are strongly interconnected (Johnston 1923; Krettek and Price 1978; Pitkänen et al. 1997; Smith-Fernandez et al. 1998; Puelles et al. 2000, 2016a, 2019; Medina et al. 2004; Tole et al. 2005; García-López et al. 2008; Hirata et al. 2009; Waclaw et al. 2010; Olucha-Bordonau et al. 2015; Medina et al. 2017; Desfilis et al. 2018). The subpallial amygdala includes the anterior, central and medial amygdalar nuclei, characterized by a high number of GABA-ergic cells (Swanson and Petrovich 1998). The pallial amygdala encompasses various amygdalar superficial corticoid masses (CxA, ACo, PLCo, PMCo) together with the classical basolateral/basomedial complex (lateral, basolateral and basomedial nuclei) and the amygdalo-hippocampal area (AHi) (Swanson and Petrovich 1998; Puelles et al. 2000, 2016a; Medina et al. 2004, 2017; Hirata et al. 2009; Waclaw et al. 2010). Corticoid and nuclear pallial amygdalar areas are often studied as separate entities.
A number of modern developmental studies which attended to the amygdalar molecular profile contributed significantly to our present partial understanding of pallial amygdalar patterning and progenitor sources (e.g., Smith-Fernandez et al. 1998; Puelles et al. 2000, 2016a; Medina et al. 2004, 2017; Remedios et al. 2004, 2007; Tole et al. 2005; Bielle et al. 2005; García-López et al. 2008; Hirata et al. 2009; Waclaw et al. 2010; Ruiz-Reig et al. 2018; Garcia-Calero et al. 2020). For instance, expression of the Emx1 transcription factor and other regulatory genes suggested a subdivision of the pallial amygdala into molecularly distinct ventral pallial and lateral pallial territories (Smith-Fernández et al. 1998; Puelles et al. 2000; Gorski et al. 2002; Medina et al. 2004; Cocas et al. 2011; Martínez-García et al. 2008, 2012; Olucha-Bordonau et al. 2015). In addition, Remedios et al. (2007) conjectured that the ectopically migrated amygdalar nucleus of the lateral olfactory tract (NLOT) originates from a caudal part of the dorsal pallium. Other studies proposed a common embryonic source for basolateral and corticoid amygdalar structures in the ventral pallium (Stenman et al. 2003; Hirata et al. 2009; Waclaw et al. 2010). Dbx1 was considered a selective marker of ventropallial progenitors in the telencephalic pallium (Yun et al. 2001; Medina et al, 2004; Bielle et al. 2005; Puelles et al. 2016a). Study of Dbx1-derived lineage suggested that the ventral pallium was only a partial source of excitatory neurons populating the basolateral amygdalar nuclear complex and the cortical amygdala (Hirata et al. 2009; Waclaw et al. 2010). Indeed, Puelles et al. (2016a) mapped Dbx1-LacZ-labelled neuronal derivatives, and identified the Dbx1-positive ventral pallium as the origin of anterior parts of the basolateral amygdalar complex. The observation that some caudal parts of the same complex were apparently largely devoid of Dbx1-derived cell populations was not easy to explain. It was suggested as one possibility that a dorsal part of the ventral pallium progenitors might not express this marker (thus leading to lack of LacZ signal in the corresponding neuronal derivatives; this notion was proposed by LP in Puelles et al. 2016a). Another possible explanation adduced was that there might exist an extra amygdalar pallial subdivision, i.e., not ventropallial in nature, which was devoid of Dbx1-positive progenitors. Such hypothetic non-ventropallial domain, which would occupy the caudal amygdala, proposed originally by L.Medina, was tentatively identified as a ventrolateral caudal pallium (or ventrocaudal pallium) (Puelles et al. 2016a; see also Abellán et al. 2014; Medina et al. 2017; Desfilis et al. 2018). Ruiz-Reig et al. (2018) defined an apparently different ‘caudoventral’ amygdalar sector, which reportedly contributes cells to the subpallial medial amygdala.
On the other hand, Puelles (2014, 2017) and Puelles et al. (2016b) analysed critically the classic notion of a claustroamygdalar complex (Kuhlenbeck 1924, 1927; Holmgren 1925), which had been assumed to exist by Puelles et al. (2000) and Medina et al. (2004). The selective claustral marker Nr4a2 was examined throughout development in the mouse, concluding that the resulting updated lateral pallium sector (represented by a claustro-insular radial histogenetic complex; see also Puelles et al. 2019) does not reach the amygdalar domain. According to these new data, the pallial amygdala has no part of lateral pallium, contrarily to what was concluded by Medina et al. (2004).
Finally, Abellán et al. (2014) ascribed the periventricular AHi area and the posteromedial corticoid nucleus (PMCo) to the medial pallium on the basis of a number of shared molecular markers. This brief abstract of relevant literature shows that distinct ventral, ventrolateral caudal, caudoventral, lateral, dorsal and medial pallial portions have been ascribed to the pallial amygdala, though some of the early proposals have been subsequently negated (case of lateral pallium), qualified (case of ventral pallium), or doubted (case of dorsal pallium; see below). The new ventrolateral caudal and caudoventral subdivisions are still being tested.
Recently, other approaches threw new light on this difficult topic. First, Puelles et al. (2019) and Garcia-Calero et al. (2020) deduced from the concentric ring model of the telencephalic cortical pallium and correlative molecular mappings that the pallial amygdala is wholly external in nature to the whole cortex-like pallium and thus should be considered histogenetically independent as a separate amygdalar pallium field (a point that was unclear before; most authors tended to assume that amygdalar populations were produced within given cortical pallial sectors and thereafter migrated tangentially to their final amygdalar sites, though such migrations were not demonstrated; see Deussing and Wurst 2007). Puelles et al. (2019) and Garcia-Calero et al. (2021) expressed doubts about a dorsopallial origin of the amygdalar NLOT nucleus (postulated by Remedios et al. 2007) due to inconsistency of this notion within the concentric ring model (i.e., there is no dorsal pallium next to the separate amygdalar pallial neighbourhood). Secondly, we recently examined the radial (glial) dimension of the mouse pallial amygdala, aiming to identify its intrinsic radial histogenetic subdivisions, and clarify the ascription of individual amygdalar nuclei relative to periventricular, intermediate or superficial strata within these units (Garcia-Calero et al. 2020). Our results revealed five major radial units and some radial subdivisions side by side, totalling nine radial modules (see summary of radial amygdalar units, subunits and derived nuclei in Table1; a 3D schema representing these amygdalar units is found in Garcia-Calero et al. 2020 and Garcia-Calero and Puelles 2020).
Table 1.
Amygdalar pallial nuclei distributed in periventricular, mantle and superficial strata in the amygdalar radial units (lateral, basal, anterior, posterior and retroendopiriform) or subunits (for basal and posterior units). For abbreviations, see list
| Units | Lateral | Basal | Anterior | Posterior | Retroendopiriform | ||||
|---|---|---|---|---|---|---|---|---|---|
| Subunits | Baso-lateral | Basomedio-lateral | Basomedio-medial | Rostro-lateral | Rostro-medial | Caudo-lateral | |||
| Periventricular stratum | L | BLP | BMPL | BMPM | Glial palisade | AHiRL | AHiRM | AHiCL | REP |
| Intermediate stratum | LI | BLA/BLI | BMIL | BMIM | BMA | PMCoRLi | PMCoRMi | PMCoCLi | REPI |
| Superficial stratum | CxAR | CxAC | PLCoC | PLCoR | ACo | PMCoRLs | PMCoRMs | PMCoCLs | REPCo |
The new status of the entire pallial amygdala field as topologically external to the telencephalic cortex (i.e., producing all its nuclei, rather than receiving them via migrations, irrespective of its contact with some cortical areas and the existence of shared gene markers) draws new attention to amygdalar borders (Puelles et al. 2019). It was proposed that the pallial amygdala lies intercalated between the alar hypothalamus, ventrally, and caudo-ventral parts of the outer allocortical ring, dorsally. These include the continuum formed by the caudal periamygdalar piriform cortex, with its amygdalo-piriform specialized area, the entorhinal schizocortex and the hippocampal complex. The amygdalar pallial field in addition contacts the subpallium (its striatal, pallidal and diagonal domains, where central, medial and anterior amygdala subpallial domains arise; Puelles et al. 2013, 2016b; Garcia-Calero et al. 2020). The pallial amygdala also contacts caudally the prethalamic eminence (rostrodorsal diencephalon; Puelles et al. 2019; Alonso et al. 2020). These multiple planar relationships still need to be examined in more detail to assess their relevance for causal developmental explanation of amygdalar pattern.
The present report is centred on the anterior radial unit, which was first defined in our radial model of the pallial amygdala. It lies next to the pallio-subpallial border and thus might relate to cortical ventral pallium represented in the neighbouring olfactory cortex (i.e., as suggested by Dbx1-LacZ-labelled progeny; Puelles et al. 2016a). Garcia-Calero et al. (2020) already concluded from selected developmental data and other results from the literature that this pallial amygdalar subdivision uniquely displays accumulation of its neuronal derivatives at its intermediate and superficial strata, developing thus a depopulated periventricular zone. This does not occur in the other eight pallial amygdalar radial modules, which retain periventricular derivatives. The intermediate and superficial components of this anterior radial domain (formed by the conventional anterior basomedial nucleus, BMA, and the anterior corticoid nucleus, ACo, respectively) separate the subpallium from the neighbouring lateral and basal radial units. Radial glia DiI-labelling experiments indicated that the exclusively glial periventricular zone of the anterior radial unit is next to the lateral (L) and the posterior basolateral (BLP) nuclei, laterally, and the pallio-subpallial boundary, medially, thus maintaining an equivalent border-related topologic position (Garcia-Calero et al. 2020). Analysis of some 80 amygdalar gene expression patterns mined from the Allen Developing Mouse Brain Atlas database (http://www.developingmouse.brain-map.org) demonstrated that 14 among the 20 genes found to be expressed in this unit (70%) distinguish the anterior radial domain from the other pallial amygdalar units (Garcia-Calero et al. 2020). The transcription factor gene Lhx9 employed in the present work emerged as one of the selective markers for the whole anterior radial unit.
Lhx9 is a Lim-homeodomain gene which is expressed in the telencephalon of vertebrates during development (Retaux et al. 1999; Bachy et al. 2001; Moreno et al. 2004; Tole et al. 2005; García-López et al. 2008; Abellán et al. 2009, 2013; Medina et al. 2017; Desfilis et al. 2018). Several analyses of the Lhx9 expression pattern were published in recent years in mouse and chicken telencephalon, reportedly showing some overlap with the expression of its paralog Lhx2 in the BMA and ACo nuclei, plus some other more caudal nuclei (Remedios et al. 2004; Tole et al. 2005; García-López et al. 2008; Abellán et al. 2009, 2010, 2013; Medina et al. 2017; Garcia-Calero et al. 2020). Lhx9 expression was generally described by these authors as marking in general the ventral pallium; interestingly, its early radially distributed signal in the amygdala coincides topographically with a Tbr1-poor area found next to the subpallium (Tole et al. 2005). However, its distribution does not seem to coincide with that of Dbx1-LacZ-labelled progeny, supposed to represent the ventral pallium mantle (Puelles et al. 2016a). Lhx9 gene transcripts were also observed in subpallial amygdalar regions such as the anterior amygdala (AA), parts of the medial amygdala, and the bed nucleus of the accessory olfactory bulb (BAOT) (García-López et al. 2008; Abellán et al. 2009, 2013; Medina et al. 2017; Garcia-Calero et al. 2020).
In the present work, we studied in more detail the developmental expression of Lhx9 as a marker of the anterior amygdalar radial unit, and eventually of other amygdalar nuclei. We compared the expression of this gene with other significant cortical and amygdalar gene markers such as the Tbr1 protein (a general pallial marker; Puelles et al. 2000; Hevner et al. 2001; Medina et al. 2004), the subpallial marker Dlx5 (Puelles et al. 2000; Medina et al. 2004; Cobos et al. 2006), the Lim-homeodomain gene Lhx2 (Bulchand et al. 2003; Tole et al. 2005), and the kelch family gene Enc1 (Garcia-Calero and Puelles 2005; Garcia-Calero 2005).
Our early results at E12.5 corroborate the previously described complete ventriculo-pial radial distribution of the Lhx9-labelled domain, identified by us as the primordium of the anterior amygdalar radial unit, whereas no such signal was found at the neighbouring lateral and basal radial units, which contrasted by expressing instead selectively the Enc1 gene marker. This already raised questions about the apparent heterogeneity of amygdalar components thought to be ventropallial (Puelles et al. 2016a). Subsequently, at mid- and late-embryonic, or perinatal, stages, there is a progressive decrease in the number of Lhx9 positive cells found in periventricular and deep intermediate strata of the anterior radial amygdalar unit, accompanied by clear-cut accumulation of Lhx9 transcripts at correlative superficial intermediate and cortical anterior strata (BMA and ACo nuclei). Moreover, other Lhx9-positive amygdalar formations gradually emerge at the caudomedial amygdalar pole. The posterior radial unit (AHi/PMCo), held to be a derivative of the medial pallium (see above) becomes strongly labelled, as well as the likewise caudal (and novel) retroendopiriform radial unit (Garcia-Calero et al. 2020; Table 1). Both are spatially separated from the anterior radial unit.
The present results, together with correlative Enc1 and Tbr1 data, plus a re-evaluation of the Dbx1-derived progeny data of Puelles et al. (2016a), corroborate the molecular singularity of the mode of development of each of the diverse amygdalar radial units (Garcia-Calero et al. 2020), all of which seem in retrospect to derive from Dbx1-positive neuroepithelium. This leads us to discuss the issue whether it is helpful to extrapolate cortical pallial sectors into the separate pallial amygdalar field, concluding that it may be advantageous not to do so. We also discuss minimally the apparent functional role of the anterior amygdalar unit within the amygdalar system.
Results
Lhx9 expression in the amygdalar domain at early developmental stages
We examined the changes in Lhx9 expression during amygdalar development (Figs. 1, 2) within the conceptual context of our recently proposed radial model of the pallial amygdala, wherein 5 amygdalar radial units were defined (Table 1; Garcia-Calero et al. 2020). Summary reference to late-embryonic Lhx9 expression was already made in that report; present results explore this aspect in more depth. Apart standard section planes, we also used the amygdalar radial plane (loc.cit.), which fits optimally the radial disposition of amygdalar glial bundles (the section planes used were indicated in the figures). Briefly, the amygdalar radial section plane intersects jointly the ventricle and the pial surface of the pallial amygdala with an obliquity of 30–45 degrees relative to conventional coronal sections; this angle was calculated while embedding the brain, by orienting the block’s basis and cutting surface relative to a reference plane tangential to the relatively flat entorhinal cortex; see Fig. 1 in Garcia-Calero et al. 2020). Comparison of Lhx9 signal with Enc1 transcripts helped us understand the disposition of the Lhx9-positive domain within the amygdala (Fig. 2) (Garcia-Calero and Puelles 2005; García-Calero 2005).
Fig. 1.
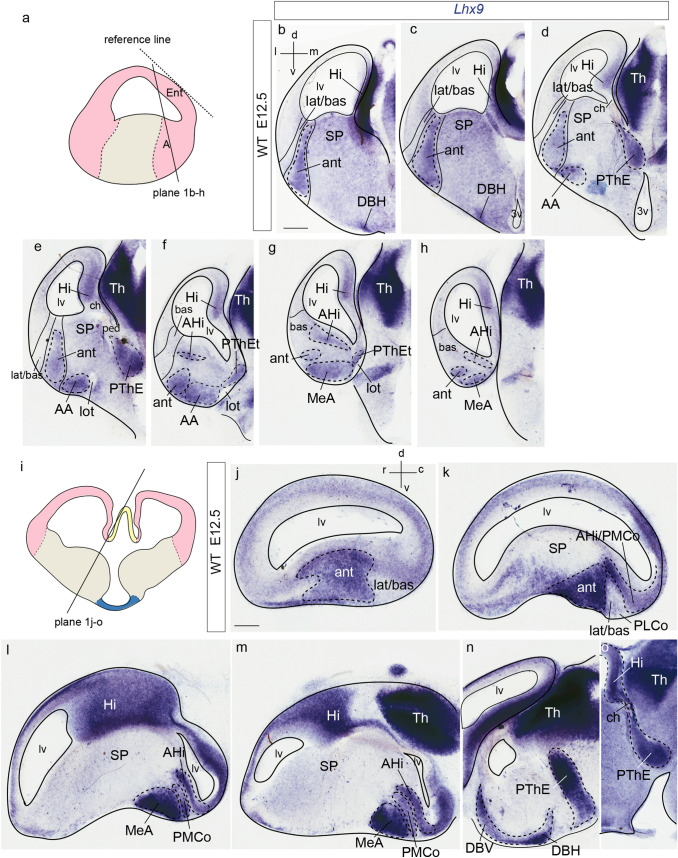
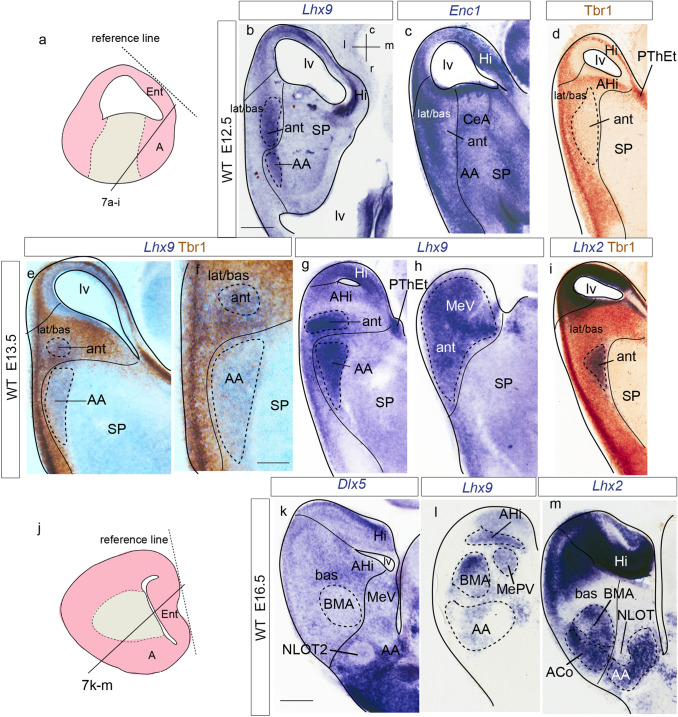
Amygdalar Lhx9 expression in stage E12.5 mouse embryos. a, i Schematic representation of section planes: a amygdalar radial plane for figures (b–h); i oblique sagittal plane for figures (j–o). b–h Lhx9 expression images ordered from rostral to caudal levels; b orientation in upper left-hand corner. The limits between the lateral/basal complex and the olfactory cortex, as well as between the lateral/basal complex and the anterior radial unit, or the latter and subpallium are indicated with thin black lines. j–o Sagittal Lhx9 expression images ordered from lateral to medial levels; j orientation in upper right-hand corner. For abbreviations, see list. Scale bars in b–o represent 300 µm
Fig. 2.
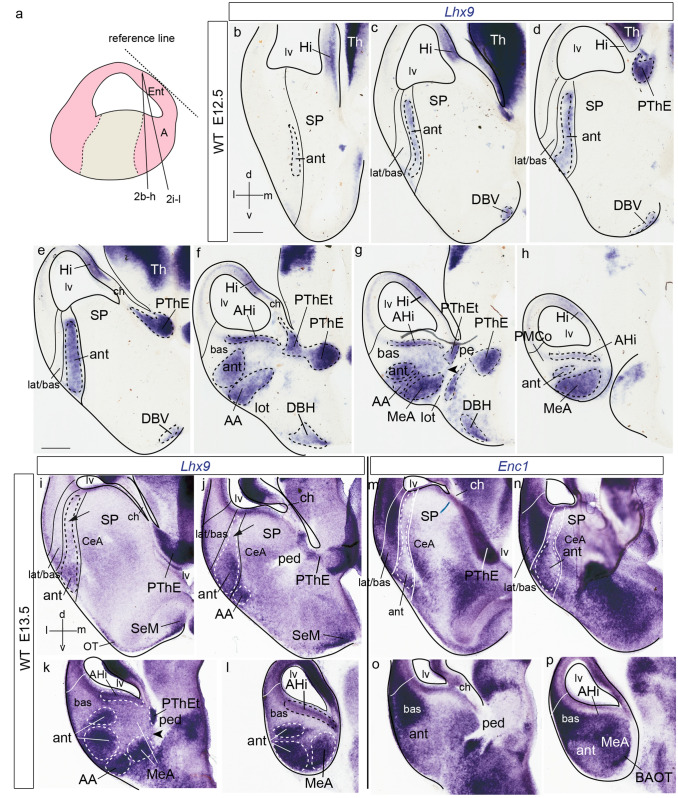
Lhx9 and Enc1 expression in mouse amygdalar region at embryonic stages E12.5 and E13.5. a Schematic representation of section plane. b–h Lhx9 expression at stage E12.5 ordered from rostral to caudal levels; b orientations indicated at the bottom left-hand corner. i–l Lhx9 expression at stage E13.5 in a slightly different amygdalar radial plane (see a), ordered from rostral to caudal levels. m–p Enc1 expression at stage E13.5, ordered from rostral to caudal levels, in alternate sections from the same embryo as in (i–l); i, j black arrow indicates a Lhx9-negative gap in the deep strata of the anterior amygdalar radial unit; orientation indicated in the bottom left-hand corner of (i). g, k black arrowhead point to positive Lhx9 cells in the medial amygdalar surface with possible source in PThEt. The limits between the lateral/basal complex and olfactory cortex, between the lateral/basal complex and the anterior radial unit, or the latter and subpallium are indicated with black or white lines. For abbreviations, see list. Scale bars represent 300 µm (b–h) and 350 µm (i–p)
At E12.5, Lhx9 expression adopts a full radial distribution in the amygdalar pallial region, with transcripts extending uniformly from periventricular levels to the brain surface, always next to the pallio-subpallial boundary (ant; Figs. 1b–h, j, k; 2b–h). The lateral and basal radial units are respectively disposed lateral and latero-caudal to the anterior unit, and do not show Lhx9 expression (lat/bas in the same images). The subpallium is largely Lhx9-negative, with exception of some Lhx9-positive cells which spread subpially out of the anterior unit into the anterior amygdala, and positive cells observed at the vertical and horizontal nuclei of the diagonal band (lat/bas; bas; ant; SP; AA; DBV and DBH; Figs.1b–f, j-l, n; 2c–g; note PLCo is the superficial structure of the basal amygdalar unit; see also Fig. 2i–p). At caudal superficial levels, there is also an expansion of Lhx9-positive cells that invades parts of the prospective medial amygdala (probably by tangential migration; Bupesh et al. 2011; Garcia-Calero et al. 2020). There is separate labelling of the posterior radial unit, mainly at its periventricular zone, the amygdalo-hippocampal area (AHi; MeA; Figs.1f–h, k-m; 2f–h). On the whole, there is abundant expression of Lhx9 at the amygdalar caudal pole, presided by the superficial ACo structure of the anterior radial unit. It is note-worthy that the Lhx9-positive BMA lacks contact with the periventricular or ventricular zones and displays a rounded form (Figs. 1f–h; 2f–h). The caudal Lhx9 expression separately limits medially with the caudal end of the lateral olfactory tract, an area where the Lhx9-positive BAOT nucleus will later become distinct (lot; Figs. 1e-g; 2f, g; Garcia-Calero et al. 2020; their Figs. 8d, e).
Fig. 8.
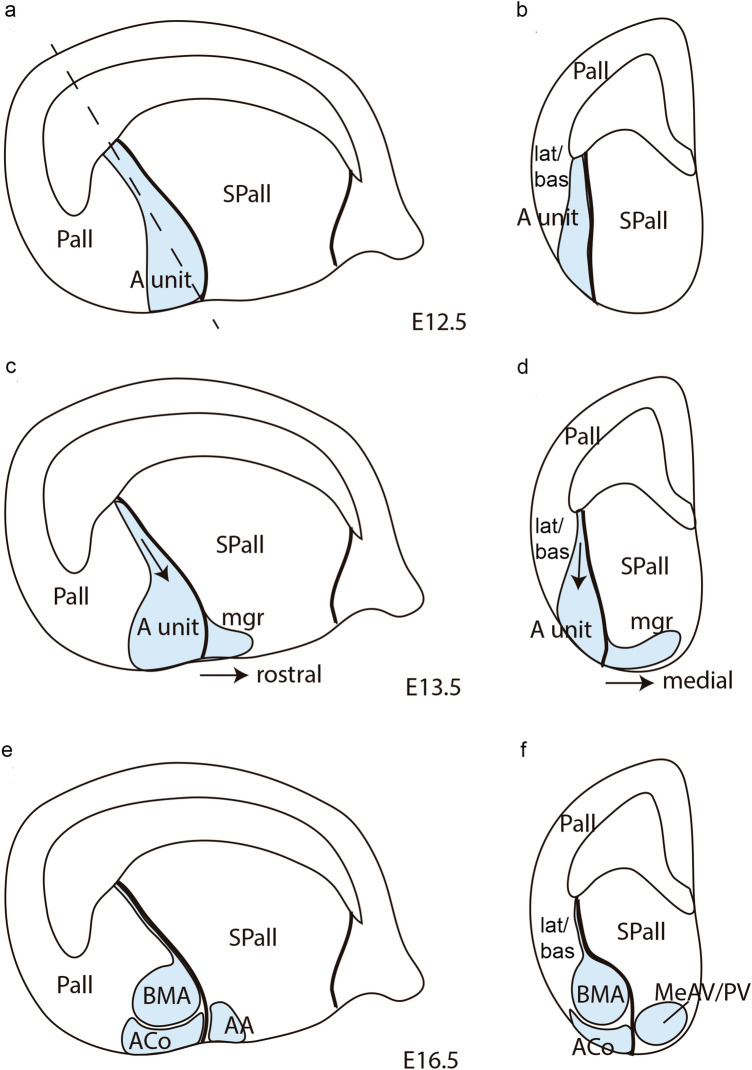
Schema illustrating in sagittal and radial section views the main developmental shape changes shown by the mouse anterior amygdalar radial unit (A. unit, light blue), highlighting the latter’s relationship with the telencephalic subpallium and other radial amygdalar domains, such as the lateral and basal units. a, b Anterior radial unit initial appearance in sagittal and radial amygdalar section planes in mouse embryos at stage E12.5. The anterior radial unit appears as a compact structure extending radially from the ventricle to the pial surface, close to the pallial/subpallial boundary. The lateral and basal radial units are located lateral to the anterior radial unit in (b). The dash line in a shows the radial amygdalar section plane used in b, d, f. c, d Anterior radial unit shape and postulated intrinsic radial cell migration movements in mouse embryo sagittal and radial amygdalar sections at stage E13.5. The anterior radial unit shape shows a narrowing in the periventricular stratum, presumably due to radial cell migration from this region to the intermediate and superficial strata (black arrow). There is also an apparent tangential cell migration of identically labeled cells spreading from the unit’s superficial stratum into rostrally and medially adjacent amygdalar subpallium (mgr). e, f Definitive shape of the anterior radial unit in sagittal and radial amygdalar sections of mouse embryos at stage E16.5, after full depopulation of its periventricular stratum (where only a thin radial glial palisade remains) and definition of its derivatives, the intermediate basomedial nucleus (BMA) and the superficial anterior cortical nucleus (ACo). The derivatives of the previous tangential migrations into the anterior and medial amygdalar subpallium are also represented (anterior amygdalar nucleus, or AA, and anteroventral and posteroventral medial amygdalar nuclei, or MeAV/MePV)
A separate forebrain region in this area which also expresses Lhx9 is the prethalamic eminence (PThE). This shows at E12.5 intense Lhx9 signal (PThE; or its telencephalic subarea PThEt; Figs. 1d–g, n, o; 2d–g). The Lhx9-positive PThE has its topologically dorsal end at the fissural chorioidal tela (a derivative of the roof plate), which attaches on its other side to the hippocampal fimbrial hem (also a Lhx9 positive area) (PThE; PThEt; ch; Hi; Figs. 1c–e, m, o; 2d–f). No contact was observed between the chorioidal tela and the amygdala proper.
At E13.5, amygdalar Lhx9 expression again labels the anterior radial domain next to the pallio-subpallial boundary, as in the previous stage (ant; Fig. 2i–l). However, there is hardly any Lhx9 signal at the anterior periventricular stratum, thus creating a clear-cut separation between the still weakly Lhx9-positive anterior ventricular stratum and the corresponding, more strongly positive intermediate and superficial strata of the anterior mantle (arrow; Fig. 2i, j). We compared this pattern with amygdalar Enc1 expression in adjacent sections, which identifies the lateral/basal complex (Garcia-Calero et al. 2020). Strong Enc1 signal at this complex extends fully radially to its superficial component, the rostral amygdalo-cortical transition area (lat/bas; Fig. 2m, n). The lateral/basal complex appears clearly delimited medially by an Enc1-negative gap which separates that complex from the central amygdalar nucleus, a part of the striatal subpallium shows weak Enc1 expression (CeA; Fig. 2m, n). This unlabelled gap is the site of the increasingly depopulated deep intermediate and periventricular anterior unit, already much reduced in width at this stage (ant; lat/bas; bas; CeA; Figs. 2m–p). The Lhx9-positive intermediate and superficial anterior strata are instead wider than at E12.5, and show superficial contiguity with a larger mass of positive elements apparently invading the anterior amygdala, and maybe even the olfactory tuberculum (OT; AA; Fig. 2i, j). At more caudal levels, the Enc1 signal at the lateral/basal complex starts to expand medialwards, but is still separated at this locus from the posterior unit (AHi) by a deep Lhx9-positive portion of the anterior unit mantle; the latter seems here less separated from the corresponding ventricular zone than more rostrally (bas; ant; AHi; Figs. 2o, p). A definite separation is nevertheless observed slightly more caudally between the Enc1-positive basal complex, which has finally become continuous medially with the posterior unit (AHi), and the caudal end of the anterior unit (lat/bas; ant; AHi; Fig. 2l, p). The medial amygdala and AHi also show Lhx9 signal, consistent with that observed already at E12.5 (MeA; AHi; Fig. 2k, l). The stretched telencephalic endpart of the Lhx9 positive PThE domain, now seen separated from the main non-telencephalic PThE by the incipient cerebral peduncle, can be distinguished at this stage as a small Lhx9-positive patch at the medial telencephalic surface, next to the chorioidal fissure. The PThEt seems tenuously in contact with Lhx9-positive cells in the MeA possibly including cells spread over the lot (PThEt; MeA; Fig. 2k, l; arrowhead; Figs. 1g, 2k).
Lhx9 transcripts at intermediate and perinatal stages
We next examined amygdalar Lhx9 expression at intermediate and perinatal stages (E14.5, E16.5 and E18.5) in different section planes (horizontal in Figs. 3 and 6, amygdalar radial in Fig. 4, sagittal in Fig. 5). For comparative purposes, we included Enc1 expression studied in the amygdalar radial plane at E17.5 (Fig. 4i–l). The text below refers to these three stages analysed together, adding specific stage details when required. The results at perinatal stages were partially advanced in Garcia-Calero et al. (2020).
Fig. 3.
Lhx9 expression in mouse amygdalar region at embryonic stage E14.5, in nearly horizontal sections ordered from dorsal to ventral levels. Note lack of continuity of anterior unit signal with the ventricle. a Schematic representation of section plane for Figs.3 and 4; b orientation indicated in the upper right-hand corner. The limits between the posterior radial unit and the hippocampus, the lateral/basal complex and the cortex, as well as between the lateral/basal complex and the anterior radial unit, or the latter versus the subpallium are indicated with black lines. For abbreviations, see list. Scale bar represent 250 µm (b–g)
Fig. 6.
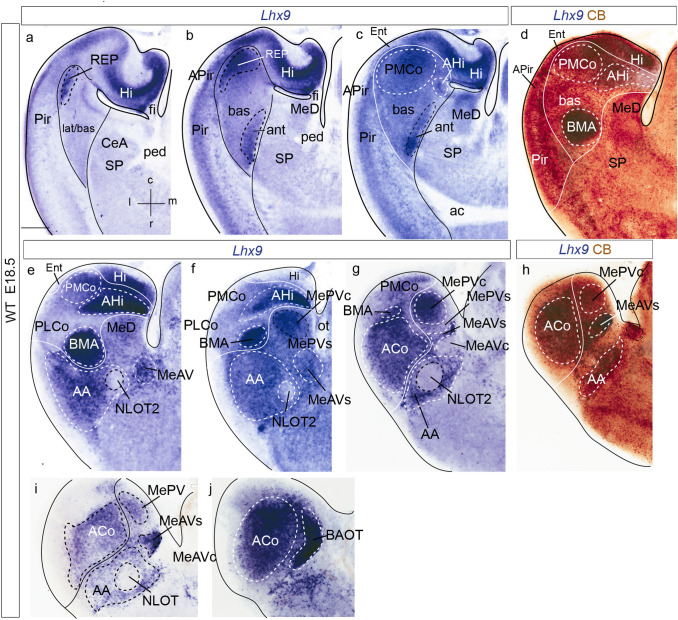
Lhx9 expression in mouse amygdalar region at embryonic stage E18.5, in horizontal section planes a–h left side details ordered from dorsal to ventral levels; i, j mirror-inverted right side details ordered from dorsal to ventral levels, a orientation indicated in the bottom right-hand corner; d, h Lhx9 expression counterstained with CB. The limits between the posterior radial unit and the hippocampus, the lateral/basal complex and the cortex, as well as between the lateral/basal complex and the anterior radial unit, or the latter versus the subpallium are indicated with black lines. For abbreviations, see list. Scale bars represent 300 µm (a–j)
Fig. 4.
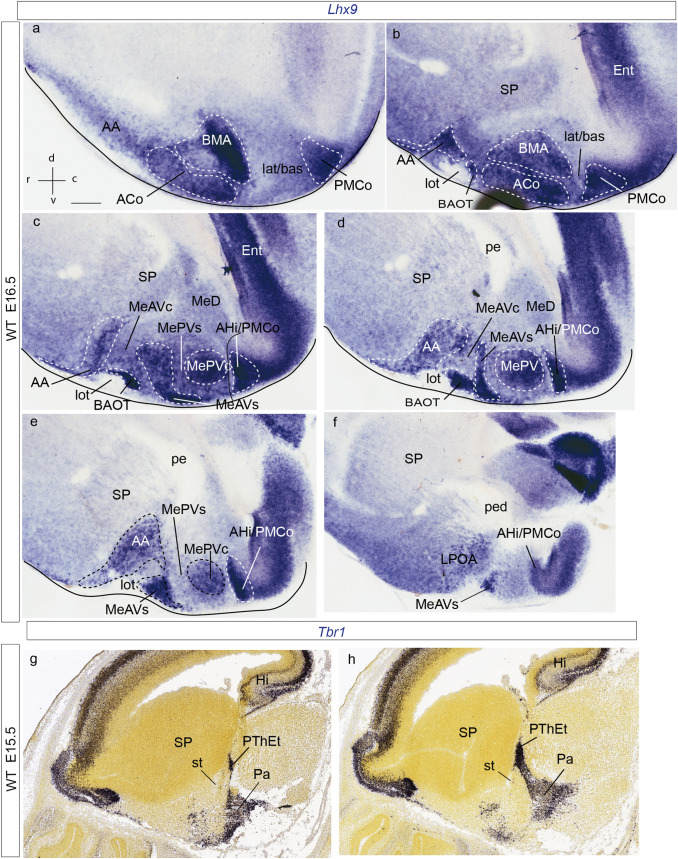
Lhx9 and Enc1 expressions in amygdalar radial plane sections in mouse at embryonic stage E16.5, ordered from rostral to caudal; a orientation indicated in the bottom left-hand corner. a–h Expression of Lhx9 in the amygdalar region. Note in c the indentation of Lhx9-positive population in AA due to the arrival of the NLOT Lhx9-negative migrating stream. i–l Enc1 expression counterstained with RC2 (showing radial glia) in mouse amygdalar region. The limits between the lateral/basal complex and the cortex, as well as between the lateral/basal complex and the anterior radial unit, or the latter versus the subpallium are indicated with black or white lines. For abbreviations, see list. Scale bars represent 350 µm (a–l)
Fig. 5.
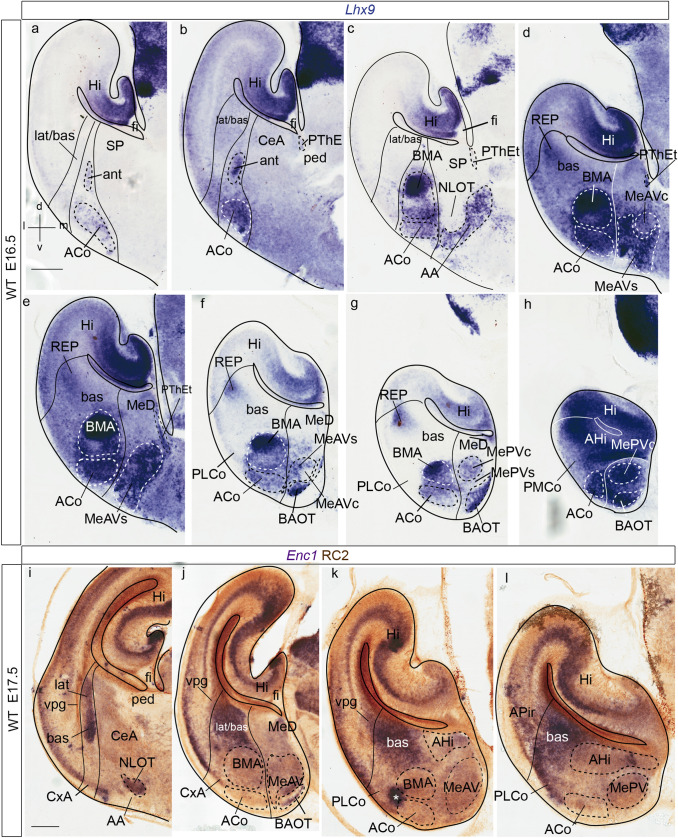
Lhx9 and Tbr1 expression in mouse amygdalar region at embryonic stage E16.5 and E15.5 respectively, in sagittal section planes ordered from lateral to medial level; a orientation indicated in the down left-hand corner. a–f Lhx9 expression at stage E16.5. g, h Illustration of evaginated (telencephalic) part of prethalamic eminence (PThEt) defined by Tbr1 expression at stage E15.5 from Website: ©2013 Allen Institute for Brain Science. Allen Developing Mouse Brain Atlas. http://developingmouse.brain-map.org). For abbreviations, see list. Scale bars represent 300 µm (a–h)
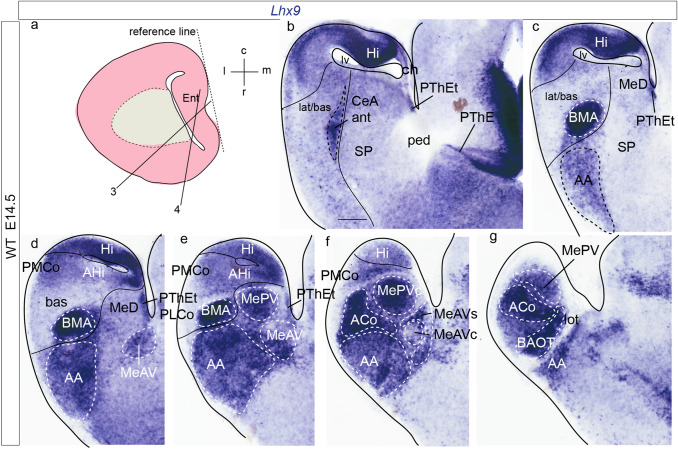
At intermediate and perinatal stages, the periventricular stratum of the anterior radial unit, presumed to be located next to the pallio-subpallial boundary, is Lhx9-negative. Lhx9 expression within the anterior unit starts at the deep part of the intermediate stratum with a few scattered Lhx9 positive cells, and expands importantly at the outer part of the intermediate stratum (site of BMA) and the superficial stratum (ACo) (ant; BMA; ACo; Figs. 3b–g; 4a–g; 5a, b; 6b–j). Coinciding with the reduction of the periventricular and deep intermediate anterior radial domain there is a corresponding increase of the periventricular volume of the neighbouring Enc1-labelled lateral and basal radial units (ant; lat/bas; bas; PLCo; Figs. 3b–e; 4a–g, i–l; 5a, b; 6a–f). In addition, numerous Lhx9-positive cells spread rostrally from the anterior radial unit into the anterior amygdala, more markedly than observed at earlier stages (ant; BMA; AA; Figs. 3c–g; 4c, d; 5a–e; 6e–i).
At the caudal telencephalic pole, the posterior radial unit appears also Lhx9-labelled, mainly at its periventricular zone, AHi; however, at perinatal stages Lhx9 transcripts also emerge at the posterior intermediate and cortical strata, which form the PMCo primordium; in contrast, the PLCo, the superficial component of the basomedial lateral and medial subunits, lacks any Lhx9 signal (AHi; PMCo; PLCo; Figs. 3d–f; 4f–h; 5a–f; 6c–g). At stage E16.5 additional Lhx9 signal emerges selectively at the periventricular stratum of the retroendopiriform radial unit, placed lateral to the negative BLP periventricular nucleus of the basal unit (REP; Figs.4d–g; 6a, b). The ventral part of the medial amygdala also shows many positive Lhx9-positive cells, in contrast to its negative dorsal part (MeD; MeAV; MePV; Figs. 3c–g; 4d–h; 5c–f; 6b–i). Lhx9 expression in MeV delineates from E16.5 onwards core and shell regions within the MeAV and MePV nuclei, with a negative core versus positive shell subdivision in MeAV, in contrast to MePV, which displays a Lhx9-positive core nucleus and a negative shell (MeAVs; MeAVc; MePVs; MePVc; Figs. 4d-h; 5c–f; 6f–i). There is also a Lhx9-positive cell population at the MeD pial surface which we think may be related to the earlier Lhx9 positive cells dispersing from the PThEt (Figs. 3c-e; 4b–e). This PThEt-related Lhx9-positive band extends superficially over superficial MeAV (MeAVs) and may reach the incipient BAOT nucleus (PThEt; MeAVs; BAOT; Figs. 3b–g; 4b–h); this relationship is also visible in sagittal sections (Figs. 5b–f). Tbr1 expression characterizes the complete PThE domain in the dorsalmost prethalamic area, continuously with apparent tangential migration into superficial MeV and, eventually, BAOT (Figs. 5g, h; Huilgol et al. 2013; Ruiz-Reig et al. 2017; Ruiz-Reig and Studer 2017). At E18.5 the Lhx9/Tbr1-positive PThEt patch was not clearly observed. At E16.5, the NLOT migration stream is detectable as a wide Lhx9-negative corridor, which starts to indent the caudal limit of the Lhx9-positive AA (NLOT; AA; Fig. 4c). At E18.5 the definitive postmigratory Lhx9 negative NLOT nucleus appears surrounded all around by Lhx9 positive cells of the AA subpallial region (Fig. 6e–g, i), which also express Lhx2 (Fig. 7m).
Fig. 7.
Lhx9, Enc1, Lhx2, Tbr1, Dlx5 expressions variously compared in mouse telencephalon during development (from E12-5 to E16.5 embryonic stages). a, j Schematic representation of section planes; b orientation indicated in the upper right-hand corner. b Lhx9 expression at anterior radial unit (ant) at stage E12.5. c Enc1 expression restricted to lat/bas complex at stage E12.5, laterally to ant. d Low Tbr1 protein expression at the ant at stage E12.5. e, f Lhx9 expression counterstained with Tbr1 immunoreaction at stage E13.5; note lack of Tbr1 coinciding with the ant radial derivatives (including migrated cells in AA); f is a higher magnification detail of the anterior radial domain in (e); g, h Lhx9 expression at E13.5 ordered from dorsal to ventral. i Lhx2 expression restricted to ant radial unit at stage E13.5; section counterstained with Tbr1. k Dlx5, l Lhx9, m Lhx2 expressions at stage E16.5. Note in k and m the advance of the negative NLOT migration stream into the mass of Dlx5-positive (subpallial) and Lhx2-positive (pallial) AA cells; this section also shows Lhx2 expression at the BMA and ACo. For abbreviations, see list. Scale bars represent 250 µm (b–e; g–i), 150 µm (f) and 300 µm (k–m)
Lhx9 amygdalar expression in the molecular context of the developing mouse telencephalon
We compared amygdalar expression of Lhx9 with pallial mantle Tbr1 protein and subpallial Dlx5 gene expression in horizontal sections at early and intermediate developmental stages (E12.5, E13.5, E16.5), in order to test its postulated restriction to the pallium (e.g., Tole et al. 2005). Comparison with the presence of Enc1 and Lhx2 transcripts at embryonic and perinatal stages was also useful to complete this analysis (Fig. 7).
At stages E12.5/E13.5, the Lhx9-positive mantle of the anterior radial unit lies within the pallium, even if strictly adjacent to the pallio-subpallial boundary; remarkably, though, this unit displays weaker Tbr1-positive reaction in its mantle layer than other pallial amygdalar regions such as the lateral, basal and posterior radial units; the latter are selectively identified using the Enc1 marker, but this does not label the anterior radial unit (ant; lat/bas;bas; Fig.7b–g). Similar results were reported previously as regards Lhx9 and Tbr1 (Tole et al. 2005; García-López et al. 2008). There is accordingly a qualitative molecular difference between the singular anterior radial unit and the other pallial amygdalar units, which remarkably affects among other molecular differences the supposedly fundamental Tbr1 signal (see Tables 1–5 and Suppl. Tables 1 of Garcia-Calero et al. 2020).
Whereas the anterior radial unit starts at the ventricle at E12.5, at E13.5 the periventricular and deep intermediate strata of this domain are devoid of positive cells. The latter first appear within the outer part of the intermediate stratum as a small tail-like deep zone medially adjacent to the BLA cell mass, which then expands superficially into the prospective BMA nucleus (ant, BMA; Figs. 7g, h). Lhx2 expression studied in combination with Tbr1 signal shows an expression pattern similar to that of Lhx9 at early and late stages, labelling the anterior radial unit in overlap with the Tbr1-poor pallial mantle area (Figs. 7i, m), as well as cells in AA which appear to have migrated tangentially from the anterior superficial stratum. No signal is found at the NLOT nucleus (Figs. 7m).
At E12.5/E13.5 all the pallial amygdalar radial units are uniformly Dlx5-negative areas (data not shown), whereas the AA expresses abundantly Dlx5 at least up to E16.5 (Fig. 7k). On the other hand, the whole MeA was moderately Dlx5-positive up to E14.5 (less than neighboring central amygdala and other striato-pallidal areas; not shown), but appeared to lose this expression at E16.5, possibly as a result of increased numbers of migrated pallial neurons (MeA; Fig. 7k).
Another amygdalar relationship of interest is that with the diencephalic Lhx9-positive PThE domain, which shares some pallial markers (e.g., ventricular Pax6, and mantle Tbr1 and Lhx9). The latter is a partly evaginated but is essentially an extratelencephalic and hyperdorsal diencephalic area (see Puelles et al. 2020) which contacts the caudal telencephalic pole next to the caudal end of the hippocampus; its dorsal evaginated telencephalic portion (PThEt) finishes attached to the tela at the end of the telencephalic chorioidal fissure (PThE; PThEt; Figs. 7d, g). Some of our data suggest that Lhx9-positive eminential cells may invade the superficial stratum of the MeA, possibly reaching the BAOT.
At stage E16.5 we detected some Dlx5 transcripts in the mantle of the lateral, basal and posterior pallial radial units, apparently corresponding to migrated subpallial cells (Marín and Rubenstein, 2001; AHi; Fig. 7k; results not shown for lat). At the anterior radial unit, represented by BMA/ACo, there are less Dlx5 transcripts than at other amygdalar pallial units (ant; Figs. 7k, l).
Discussion
Our main goal in this work was a detailed description of Lhx9 expression in the amygdalar area as a means to explore its molecular partitions, and in particular the derivatives of the anterior radial unit. We consider this gene a partially selective marker of the anterior radial unit (since it labels also other amygdalar structures) on the basis of our recent genoarchitectural analysis of the pallial amygdala (Garcia-Calero et al. 2020; summary of radial units in Table 1). The anterior radial unit is singular in that it lacks a populated periventricular stratum due to superficial translocation of all its derivatives. We followed the progress of this phenomenon during telencephalic development, and noted changing relationships with surrounding pallial structures, while retaining the primary contact with the subpallium (Fig. 8). We also illustrated the development of other amygdalar regions found labelled by Lhx9 signal outside the anterior radial unit, such as the posterior radial unit (AHi/PMCo), the periventricular retroendopiriform nucleus (REP) lying lateral to the BLP nucleus, the bed nucleus of the accessory olfactory tract (BAOT), and parts of the anterior and medial subpallial amygdala. We will discuss below the relationships of Lhx9 transcripts with other molecular markers potentially distinguishing a ventral pallium-like sector in the pallial amygdala and also consider in the context of our recent amygdalar radial model the conventional notion of a migratory cortical origin of diverse amygdalar pallial sectors (Medina et al. 2004; Deussing and Wurst 2007). Our conclusion is that it is not meaningful to extrapolate subdivisions of the cortical pallium by merely assumed migrations to the topologically separate amygdalar pallium, irrespective of shared gene markers. Both the cortex and the amygdala have distinct patterns of intrinsic subdivision with differential molecular profiles.
Lhx9 labels the anterior radial domain in the mouse pallial amygdala
Lhx9 gene belongs to the LIM-homeodomain gene family, a group of transcription factors which play important roles during embryonic development in vertebrates, including CNS formation (Tuschida et al. 1994; Retoux et al. 1999; Bertuzzi et al. 1999; Nakagawa and O’Leary 2001; Bachy et al. 2001; Shirasaki and Pfaff 2002; Bulchand et al. 2003; Remedios et al. 2004; Tole et al. 2005; García-López et al. 2008; Abellán et al. 2009, 2010, 2013, 2014; Desfilis et al. 2018). Combinatorial expression of some LIM genes defines cell, nuclear and regional identities during development of spinal cord, thalamus and telencephalon (Nakawaga and O´Leary 2001; Shirakassi and Pfaff 2002; Remedios et al. 2004; Tole et al. 2005; García-López et al. 2008; Abellán et al. 2009, 2010, 2013, 2014).
A role has been proposed for LIM-genes also in mouse amygdalar parcellation, mainly as regards its pallio-subpallial subdivision: Lhx9 and Lhx2 transcripts are described as characteristic of pallial amygdala, whereas Lhx6 is expressed in the subpallial amygdala at embryonic stages (Remedios et al. 2004; Tole et al. 2005; Choi et al. 2005; García-López et al. 2008; Abellán et al. 2009; 2013; Medina et al. 2017). Moreover, the whole cortical ventral pallium (i.e., the olfactory pallial sector; Puelles, 2014, 2017; Puelles et al. 2019) and a hypothetic ventropallial amygdalar subregion have been co-defined by selective Lhx9 expression (Tole et al. 2005; García-López et al. 2008; Abellán et al. 2009; Medina et al. 2017). Our present results lead us to discrepate with these two conclusions. First, we find both Lhx9 and Lhx2 signals not only in the pallial amygdala, but also, independently, in parts of the subpallial amygdala (AA, MeA). Secondly, our data suggest that Lhx9 does not label initially the entire cortical ventral pallium, nor all possible ‘ventropallial’ parts of the amygdala according to the Puelles et al. (2016a) analysis. Indeed, its amygdalar signal is always restricted over time, first to the anterior unit primordium at early stages, and then to the mature derivatives of the anterior radial amygdalar unit, some of which apparently invade tangentially the AA and MeA subpallial regions.
Lhx9 labels several nuclei in the amygdalar domain, namely BMA/ACo in the anterior radial unit, AHi/PMCo in the posterior radial unit; the periventricular REP part of the retroendopiriform radial unit, and various possibly tangentially migrated cell groups in the subpallial AA and MeA (Remedios et al. 2004; García-López et al. 2008; Abellán et al. 2009, 2013; Garcia-Calero et al. 2020). Our developmental study corroborates previous reports on the existence of an early domain with full radial Lhx9 expression, found next to the pallio-subpallial boundary (e.g., Tole et al. 2005; García-López et al. 2008; Fig. 8). However, we think that this labelling represents only the anterior radial unit of Garcia-Calero et al. (2020), together with some tangentially migrated cells, rather than the cortical ventral pallium in general. That is, the Lhx9 signal shown by these authors is always restricted to a particular radial unit of the amygdalar pallium, and is not significantly present in cortical ventral pallium.
Interestingly, Tole et al. (2005) reported that the Lhx9-positive radial cell stream they identified adjacent to the pallio-subpallial boundary overlaps with a mantle domain showing low Tbr1 expression at early mouse embryonic stages; sagittal sections of the Tbr1 E13.5 specimen illustrated at the Allen Developing Mouse Brain Atlas clearly show that this low Tbr1-signal area is restricted to amygdalar pallium. Guided by its position bordering the subpallium, and possibly following notions developed by Medina et al. (2004) in search of amygdalar correspondences, these authors interpreted that this peculiar Lhx9-positive and low Tbr1 band represented the ventropallial migratory stream, that is, the postulated active movement of ventropallial cells born at the cortical ventral pallium sector of the hemisphere (VPall as defined by Puelles et al. 2000 and Medina et al. 2004) into the amygdala. However, the cortical VPall shows at all early embryonic stages strong Tbr1 expression. For similar reasons, the Lhx9-negative mantle region found lateral to the amygdalar Lhx9 domain, which shows discrete Emx1 and Cdh8 expression, was wrongly ascribed to the lateral pallium (LPall), given that recent additional research with the claustro-insular markers Nr4a2 and Cyp26b indicated that the updated claustro-insular LPall does not extend into the amygdalar field (Puelles 2014, 2017). This implies that this lateral amygdalar pallial locus must be a separate and molecularly distinct portion of the pallial amygdala, presently estimated to include the lateral and basal radial units.
Following similar early and imperfect criteria, other authors proposed that early ventropallial expression of Lhx9 extended to caudal ventral pallium levels encompassing the whole, or a large part, of the pallial amygdala domain (García-López et al. 2008; Abellán et al. 2009; Medina et al. 2017). Since it was becoming evident by then that cortical sectors of VPall did not express Lhx9 at middle and late embryonic stages, it was implicitly assumed by those interested in this topic that Lhx9 possibly was downregulated during development in the whole rostral VPall, as well as in the basolateral amygdalar nuclear complexes (also thought to belong to VPall at least in part; Puelles et al. 2016a), while only some nuclear entities such as BMA and ACo retained Lhx9 expression (García-López et al. 2008; Abellán et al. 2009; Medina et al. 2017).
We also observed that full radial Lhx9 transcripts typically appear next to the pallio-subpallial boundary at E12.5, in a region which is poor in Tbr1 protein (as first shown by Tole et al. 2005; Fig.8a, b). However, our morphologic interpretation differs, because we consider the data under the novel light of the radial model of the pallial amygdala (Garcia-Calero et al. 2020; Table 1). This postulates a distinct anterior radial unit which is primarily independent from other amygdalar pallial partitions, both in terms of glial architecture and molecular profile, and lies intercalated between the lateral/basal amygdalar radial units and the local subpallium. As shown by present results, this radial region accumulates its cells via radial migration restricted to the pallial amygdala in its outer-intermediate and superficial layers as of E13.5 (Figs. 8c, d), producing the BMA and ACo, respectively (Figs. 8e, f). The corresponding periventricular domain remains represented only by a glial palisade, an aspect hardly visualized in Nissl-stained material or in ISH genoarchitectural material (see corresponding experimental DiI labeling of radial glia in Garcia-Calero et al. 2020). We compared from E12.5 onwards the amygdalar Lhx9-positive anterior radial unit with selective Enc1 labelling of the adjacent lateral/basal radial unit complex, discovering that the lateral and basal amygdalar radial unit primordia already exist next to the amygdalar Lhx9-positive domain, which coincides with low Tbr1 signal at E12.5, and can be recognized steadily at the same site subsequently. Eventually the lateral and basal radial units develop massive periventricular nuclei (L, BLP and BMPM/BMPL), jointly with intermediate populations (LI, BLA, BLI, BMIM, BMIL) and superficial aggregates (CxAR, CxAC, PLCo) in contrast to the superficialized anterior unit (BMA, ACo; data from Garcia-Calero et al. 2020). Such relationships of Lhx9+/low Tbr1 and Enc1+ labelled fields only obtain at amygdalar levels, in contrast with the cortical pallium. These results imply that the full radial early Lhx9-positive domain observed at E12.5 represents only the prospective anterior radial unit, and not all potentially ventropallial analogs of the pallial amygdala, which include at least part of the lateral/basal complex according to Dbx1-related findings (Medina et al. 2004, 2017; Puelles et al. 2016a). Moreover, we conclude that probably no part of (cortical) ventral pallium ever expresses Lhx9, given that this domain strongly co-expresses Tbr1 throughout development (Puelles et al. 2000).
We believe that the interpretive error incurred by the authors cited above in assuming that the early amygdalar Lhx9-positive anterior unit domain represented partly the preamygdalar (cortical) VPall sector was possibly due to the fact that at early developmental stages the amygdalar region occupies a larger proportional longitudinal extent of the hemisphere than at later stages, as is suggested by comparison of our early and late sagittal sections (see our Figs.1j, k and 5a–e); unfortunately, sagittal sections apparently were not examined by either Tole et al. (2005) or García-López et al. (2008). This morphogenetic change is probably due at least in part to differential growth of the ganglionic eminences and the cortex relative to the amygdalar complex.
It may be significant to note that the lateral/basal amygdalar complex does not contact the pallio-subpallial boundary, due to the physical intercalation of the anterior and posterior radial amygdalar units between the subpallium and the remaining amygdalar pallium (Garcia-Calero et al. 2020; remarkably, these two radial domains express separately Lhx9). A para-striatal topology is supposed to be a uniform characteristic of cortical VPall. The lateral/basal amygdalar complex (containing four full radial subunits; Table 1) would then represent topologically an unique Lhx9-negative and Enc1-positive region existing within amygdalar pallium, for which there is no apparent counterpart outside the amygdala (note a LPall component was excluded by Puelles, 2014, 2017).
Another Lim-homeodomain transcription factor, Lhx2, shows a quite similar expression pattern than its paralog Lhx9 at the anterior radial unit, though it was suggested to expand more laterally than Lhx9 (e.g., Fig. 1 in Tole et al. 2005). In our hands, strong Lhx2 signal observed next to the amygdalar subpallium was strictly restricted to the anterior radial unit nuclei or to anterior cells suspected to have migrated tangentially into amygdalar subpallium (Figs.7m, 8d–f; Garcia-Calero et al. 2020). This transcription factor may have redundant functions at this locus with respect to Lhx9 (Remedios et al. 2004). On the other hand, loss of function of Lhx2 reportedly produces lack of migration of the NLOT2 nucleus (Remedios et al. 2007; see also Garcia-Calero et al. 2021, where we deal specifically with this migration).
Lhx9 expression at the amygdalar caudal pole
Amygdalar Lhx9 transcripts also appear at caudal telencephalic levels, where they encompass a large part of the caudal telencephalic pole. Apart of standard intermediate/superficial elements derived from the anterior radial unit (ACo and BMA), the marker also appears at the posterior unit (particularly AHi of the AHi/PMCo complex), as well as in parts of the neighbouring hippocampal complex (medial pallium) and, separately, at the periventricular component of the retroendopiriform radial unit (Garcia-Calero et al. 2020) and the BAOT superficial nucleus. None of these extra expression domains seems close enough topographically to be considered an extension of the anterior radial unit.
On the other hand, there is visible labelling continuity between the ACo (superficial anterior pallial element) and the rostrally adjacent anterior amygdala (AA), which is widely held to be intrinsically subpallial in nature, as is corroborated by our Dlx5 mapping data. The Lhx9-positive AA cell population is accordingly presumably glutamatergic, secondarily mixed in with the local subpallial GABAergic neurons. The AA finally surrounds the separately migrated Lhx9/Lhx2-negative NLOT (Remedios et al. 2007). This apparent invasion of AA from the anterior pallial unit is already incipient at E12.5, and increases thereafter up to E16.5 (Figs. 8c, e).
Secondly, the Lhx9/Lhx2-positive ACo/AA cell population also shows marked continuity with similar cells found at the MeA area of the subpallial amygdala, incipient already at E12.5 and E13.5 (Figs. 1, 2; 8d, f). As development advances, the initially single positive MeA population separates into labelled cells at the MeAV and MePV nuclei (Fig. 3d–g; 4e–h; 5d, e; 6e–i; 7l). Whereas the MeAV shows cells with both Lhx9 and Lhx2 transcripts, the MePV does not contain Lhx2-positive cells (Fig. 7m). Once stabilized at the MeAV, Lhx9 cells predominantly surround its weakly labelled core subdivision, forming a positive shell around it. In contrast, the opposite pattern characterizes the MePV, where essentially a Lhx9-positive core and a weakly labelled shell are displayed.
We already conjectured before that this medial amygdala pattern probably needs to be interpreted as part of a tangential migration out of the anterior radial amygdalar unit into amygdalar subpallium (Garcia-Calero et al. 2020). Bupesh et al. (2011) previously postulated likewise a Lhx9-positive ventropallial migration into MeAV; however, we restrict its origin to the anterior amygdalar unit, without implicating an origin at the cortical VPall. The MePV nucleus must be distinctly heterogeneous in its cellular composition, since it reportedly is also tangentially invaded by Otp-positive cells from the paraventricular hypothalamic area (García-Moreno et al. 2010) and by Shh-positive cells from the septocommissural area (Hirata et al. 2009; Carney et al. 2010; Bupesh et al. 2011; Lischinsky et al. 2017).
A parallel observation was the development from intermediate embryonic stages onwards of the half-moon-shaped small superficial BAOT nucleus, which also expresses strongly the Lhx9 marker, and is found at the rostromedial border of the MeAV (Fig. 4f–h; 5c–e; 6j). However, lack of Lhx2 at the BAOT, and other differential molecular data (Table 4 and Suppl. Table 1 in Garcia-Calero et al. 2020) do not support an origin of this nucleus also at the amygdalar anterior radial unit. The nearest alternative Lhx9-positive forebrain regions are the posterior amygdalar radial unit and the evaginated or ‘telencephalic’ part of the prethalamic eminence (marked PThEt in our Figures). Resolving this issue will require a specific investigation.
Ruiz-Reig et al. (2018) observed that the caudal telencephalic pole was negative for subpallial markers such as Gsx2 and Ascl1, but displayed transcripts of the pallial genes Pax6 and Tbr2 protein at stage E12.5. This agrees with the conventional idea that the subpallial caudal ganglionic eminence (CGE) ends short of the caudal hemispheric pole proper, so that pallial tissue surrounds caudally the CGE (review in Puelles et al., 2013, 2016b). The pallial nature of the remnants of the basal, anterior and posterior radial units still present at the caudal telencephalic pole is well accepted in the literature (from Swanson and Petrovich 1998 to Garcia-Calero et al. 2020). In addition, Ruiz-Reig et al. (2018) presented evidence suggesting that an Ebf3+/Tbr1+ cell population migrates from an unidentified amygdalar pallial origin into to MePV (probably forming what we identified as MePVs). These authors also postulated an extra ‘caudo-ventral’ part within the amygdalar pallium which contributes cells to the MeAV (Ruiz-Reig et al. 2018); this should not be confused with the ‘ventro-caudal’ or ‘ventrolateral caudal’ amygdalar pallial part of Medina et al. (2017), nor with our anterior radial unit.
We now regard earlier tentative pallial partitions based on standard coronal sections (oblique to amygdalar radial glial structure) as insufficiently documented with regard to their molecular borders and derivatives, particularly as regards the amygdala. Significantly, the postulated migratory streams supposedly leading from various cortical pallial sectors into the amygdala (Medina et al. 2004; Tole et al. 2005; Remedios et al. 2004, 2007; Puelles et al. 2016a) have not received experimental corroboration. The amygdalar radial model (Garcia-Calero et al. 2020) offers a solid alternative schema of intrinsic amygdalar radial development for resolving this problem. In conclusion, most of the neural structures identified at the caudal telencephalic pole show a pallial molecular profile, eventually including Lhx9 expression (either via autochthonous differentiation within anterior, posterior or rep amygdalar pallium, or via tangential migration of pallium-originated cells into MeA).
Variety of molecular subdivisions in the amygdalar pallial field
Molecular pallial regionalization accompanied molecular distinction of pallium and subpallium domains in the telencephalon (Smith-Fernández et al. 1998; Puelles et al. 2000). Classic anatomic studies were based upon a tripartite pallium model (medial hippocampal pallium, dorsal neopallium, and lateral olfactory pallium; reviewed in Striedter 1997). This model clearly demanded correction, once two new molecular pallial sectors respectively positive and negative for Emx1 signal were discovered inside the old olfactory pallium (Smith-Fernández et al. 1998; Puelles et al. 2000); this led to the first tetrapartite pallium model (MPall, DPall, LPall, VPall, where the old ‘lateral’ pallium = was equated to the new LPall + Emx1-negative VPall; Puelles et al. 2000). It was assumed at the time that cortical and amygdalar pallium was unitary, implying that the new LPall and VPall sectors should extend into the amygdalar complex, forming a classic ‘claustroamygdalar’ continuum (e.g., Holmgren 1925; Kuhlenbeck 1973). Drawing on this assumption Medina et al. (2004) and Tole et al. (2005) explored in detail the amygdalar region, using a variety of subpallial and pallial markers (see also Martínez-García et al. 2008, 2012). Among a good number of solid findings, particularly on Dbx1, Ngn2, semaphorin5A, Emx2 expression at the cortical and amygdalar VPall, other results, notably on LPall, require in retrospect reinterpretation, given it was later discovered that neither the claustrum nor selective claustro-insular molecular markers extend into the amygdala (Puelles 2014, 2017; Puelles et al. 2019). Mapped markers whose amygdalar expression needs to be re-evaluated include Emx1, Lhx9, and Cdh8.
The Dbx1 gene was first reported as a ventropallial marker due to its labelling of a longitudinal ventricular microzone found next to the pallio-subpallial boundary throughout the hemisphere (Yun et al. 2001; Medina et al. 2004; Bielle et al. 2005). As determined by progeny analysis (see below) the mantle of this domain corresponds to the Emx1-poor, Sfrp2-positive, Ngn2-positive, semaphorin5A-positive and Emx2-positive VPall sector (Smith-Fernandez et al. 1998; Puelles et al. 2000; Kim et al. 2001; Gorski et al. 2002; Medina et al. 2004; Tole et al. 2005). Its cortical pallial derivatives were subsequently determined to include the olfactory cortex, the ventral endopiriform nucleus, the bed nucleus of the external capsule (Medina et al. 2004; Bielle et al. 2005; Hirata et al. 2009; Waclaw et al. 2010; Puelles et al. 2016a).
An analysis of Dbx1-related progeny studied by Puelles et al. (2016a) in Dbx1-LacZ transgenic mice gave the impression that amygdalar Dbx1-derivatives predominate in the BMA, CxA and ACo nuclei, as well as in anterior parts of the L and BLA nuclei. Such derivatives were relatively scarce in the corresponding posterior parts of the amygdala (i.e., caudal L and BLA, BLP and BMP, as well as PLCo, AHi and PMCo). In fact, what was shown at P60 was a caudally decreasing proportion of LacZ-positive neurons mixed with negative neurons at all section levels. In addition, the posterior amygdalar areas are rich in Emx1 transcripts and Emx1-cell linage derivatives, a gene marker poorly expressed in cortical VPall (Puelles et al. 2000; Gorski et al. 2002; Medina et al. 2004; Remedios et al. 2007; Cocas et al. 2011). Though distinct Emx1 signal was found at the BLA nucleus, leading to its initial ascription to lateral pallium (Medina et al. 2004), recently the lateral pallium was radically re-defined as the radial territory which encompasses selectively the molecularly distinct claustro-insular formation, which does not extend into the amygdalar area (Puelles 2014, 2017; Puelles et al. 2019). Taken together, these results reveal molecular similarity between parts of the pallial amygdala with three cortical pallial sectors. Namely, the rostral ant/lat/bas amygdalar subdivisions showing a higher proportion of Dbx1-LacZ-positive cells are comparable molecularly to the ventral pallium. In contrast, the Emx1-positive posterior (periventricular) and basolateral amygdalar parts showing a low proportion of Dbx1-LacZ-negative cells might be tentatively ascribed to a novel ventrolateral caudal or ventrocaudal amygdalar pallium sector (Puelles et al. 2016a). Finally, the posterior AHi/PMCo complex would be largely devoid of Dbx1 signal and might be compared on molecular grounds with the medial pallium (Abellán et al. 2014; Puelles et al. 2016a; Medina et al. 2017; Desfilis et al. 2018).
Ruiz-Reig et al. (2018) identified an exclusively amygdalar caudoventral subdivision characterized by Gdf10, Sfrp2 and Fgf15 expression, which they think is continuous but not identical with the amygdalar ventral pallium, and has no clear resemblance with any of the cortical pallial sectors. It was claimed by Ruiz-Reig et al. (2018) that their caudoventral amygdalar subdivision, whose ventricular zone does not express Dbx1, and contributes cells to a subregion of the MePV, does not correspond to the ventrocaudal pallium proposed in chicken, lizard, and mice by Abellán et al. (2014) and Medina et al. (2017).
Irrespective of the foregoing summary of available molecular data on pallial amygdala, any division system of the pallial amygdala which does not take into consideration its primary radial histogenetic organization, related to its intrinsic (rather than extrinsic cortical) ventricular progenitor partitions must be reexamined. Notably, all the notions of molecular amygdalar subdivision cited above were deduced from coronal sections oblique to radial amygdalar organization as demonstrated experimentally by Garcia-Calero et al. (2020). Indeed, previous anatomic work on amygdalar structure ranging back to Johnston (1923) was generally oblivious of where lies the amygdalar ventricular zone where distinct cell populations are produced over time (Garcia-Calero et al. 2020). This criticism applies also to work from our own laboratory (e.g., Puelles et al., 2000, 2016a; Medina et al. 2004; García-López et al. 2008). The so-called ‘posterior’ and ‘anterior’ amygdalar pallial parts discussed by Puelles et al. (2016a) consist of artefactually separated periventricular versus superficial parts of various radial amygdalar units (Garcia-Calero et al. 2020). This traditional but conceptually wrong approach introduces a gross error in the assumptions used regarding the position of the ventricular zone that generates the different amygdalar cell groups studied (distorting among other concepts the meaning of the descriptors ‘rostral/anterior’ and ‘caudal/posterior). Molecular profiles need to be examined in proper histogenetic context, which eventually implies stratified radial structure (Nieuwenhuys and Puelles 2016; Garcia-Calero and Puelles 2020). It does not make sense to continue using standard coronal sections to subdivide the pallial amygdala molecularly, and older work doing so needs to be reinterpreted.
We accordingly have reinterpreted the Puelles et al. (2016a) data consistently with the new amygdalar radial model (Garcia-Calero et al. 2020). The first step would be to correlate early amygdalar expression of Dbx1 at E10.5-E11.5 with observations at later stages. Mantle derivatives of the Dbx1-labelled microzone can be studied in a second step. Work by Medina et al. (2004), Bielle et al. (2005), Teissier et al. 2010 and Puelles et al. (2016a) (e.g., see their Fig. 1a–c) showed that the early weakly Dbx1-positive cortical ventricular stripe found adjacent to the subpallial striatal territory (representing the cortical VPall) extends into amygdalar territory and finally bends medialwards around the end of the caudal ganglionic eminence (CGE), finally contacting the dorsal extension of the hypothalamic paraventricular area (Pa); the latter is represented early on by a longitudinal alar hypothalamic band of Dbx1 signal (see the Allen Developing Mouse Brain Atlas E11.5 data for Dbx1). The dorsal Pa displays at its caudal end, next to the bordering prethalamic eminence, a spike-like dorsal expansion which penetrates the hemispheric stalk through the floor of the interventricular foramen, then follows the floor of the terminal sulcus, until it meets the amygdalar Dbx1-labelled band at its caudal end (this hypothalamic spike reaching the evaginated amygdalar region was observed already at E9.5 by Fan et al. 1996, who used Sim1 mapping of the paraventricular area; it was illustrated schematically in Fig. 3 of Puelles and Rubenstein, 2003, and Fig. 10 of Puelles and Rubenstein 2015; recently we proposed to call it the hypothalamo-amygdalar corridor or HyA; Garcia-Calero et al. 2021). Note Dbx1-derived progeny clearly appears periventricularly at the floor of the terminal sulcus in E14.5 embryos, as shown by Puelles et al. (2016a) in their Figs. 2a–c. A similar para-subpallial distribution of an amygdalar stripe expressing Sfrp2 was previously found to contour caudally the CGE and reach the alar hypothalamus via the bottom of the terminal sulcus, that is, the HyA (Kim et al. 2001). These data jointly indicate that there exists an early molecularly distinct neuroepithelial band that accompanies the pallio-subpallial boundary along both the cortical and amygdalar parts of the pallium, and also connects finally with the dorsal part of the alar hypothalamus, which limits with the overlying telencephalic subpallium (first lateral to the LGE, and then around or under CGE and MGE). Given this band of tissue primarily positive for both Dbx1 and Sfrp2, there is no need for a cortical migration to produce corresponding amygdalar cell populations. One expects no change in this early topology, irrespective of morphogenetic deformations due to advancing telencephalic growth.
The next step is to approach the subsequent fate (derivatives/progeny) of the amygdalar part of the Dbx1-positive band. Given the existence of five pallial amygdala radial units (ant/lat/bas/post/rep; see Table 1; Garcia-Calero et al. 2020), it would be possible in principle that the amygdalar part of the Dbx1/Sfrp2-positive band corresponds solely to the anterior and posterior radial units, which are the ones contacting directly the subpallium; this would leave the lateral, basal and rep amygdalar units outside the topological para-subpallial position. Following this notion, one would expect accordingly Dbx1-progeny to be restricted to the BMA/ACo (ant derivatives) and AHi/PMCo (post derivatives). However, this possibility is apparently negated by the Dbx1-LacZ progeny data of both Waclaw et al. (2010) and Puelles et al. (2016a).
The latter authors indeed reported Dbx1-labelled anterior unit progeny (BMA/ACo), but also abundant labelled progeny at some parts of the lateral and basal radial units (L, CxA and the anterior part of BLA). Remarkably, they did not recognize Dbx1 progeny at nuclei derived from the posterior unit (AHi/PMCo). However, with more experience of amygdalar structure than we had then (due to our work in Garcia-Calero et al. 2020, 2021, Garcia-Calero and Puelles 2020, and present report), we would reinterpret the labelled cell mass which Puelles et al. (2016a) tagged as ‘MePV’ in their Fig. 2d (E14.5) as corresponding to the AHi/PMCo complex, found caudal to the MePV proper. Their Fig. 5b (E18.5), illustrating a section caudal to the MeA seen in Fig. 5a that shows ventricular labelling, may be reinterpreted similarly as corresponding to the AHi/PMCo posterior complex). At E18.5, Puelles et al (2016a) did not observe labelling of PLCo, the superficial subunit of the basomediolateral/basomediomedial radial bas subunits (see present Table 1), but the corresponding periventricular formation appears strongly labelled (tagged BM) in their Fig. 4d, e, and radial streams of labelled cells spread out from the periventricular stratum into a superficial locus tagged ‘CxA’ (same Figures). We think that this actually corresponds to the missing PLCo. The periventricular BLP nucleus of the basolateral radial bas subunit was described as unlabelled, but its periventricular mass at E18.5 was also labelled distinctly, misidentified as ‘L’ (Puelles et al. 2016a; their Fig. 4d, e). Interestingly, ventricular labelling at the caudal BLP locus is continuous with the extra-amygdalar ventricular zone of the cortical olfactory VPall (tagged in the cited Figs. as ‘VPne’), thus necessarily including also the intercalated rep domain (Garcia-Calero et al. 2020).
Such reappraisal of the Dbx1 progeny material (which is also roughly consistent with data from Waclaw et al. 2010), plus the observation that the postnatal pallial amygdala shows Dbx1-labelled cells in all its nuclear derivatives (mixed in a varying proportion with Dbx1-negative cells), raises doubts about the necessity of the extra Dbx1-negative ‘caudoventral’ or ‘ventrolateral caudal’ amygdalar pallial portion conceived theoretically to account for negative halves of nuclei (Puelles et al. 2016a), a notion still recently contemplated by Medina et al. (2017) and Desfilis et al. (2018). The mixture of Dbx1-LacZ positive and negative cells in all amygdalar pallial nuclei, including the ‘caudal’ (actually periventricular) ones, is consistent with the fact that the marker seems to be at least partly present at all the pallial amygdalar progenitor zones at E18.5, as it was at E10.5. This result is further consistent with the alternative interpretation offered in Puelles et al. (2016a) that the pallial ventricular sector producing the Dbx1-derived mantle both at cortical and amygdalar levels is apparently accompanied in parallel by a still undefined Dbx1-negative neuroepithelial domain that contributes its derivatives to the same amygdalar and cortical formations populated by Dbx1-derived progeny (note that also part of the olfactory cortex and the whole olfactory bulb are devoid of Dbx1-LacZ-positive cells; Puelles et al. 2016a). The Dbx1-derived component predominates at amygdalar levels (as suggested in wholemount preparations in Bielle et al. 2005) and diminishes significantly in a rostralward gradient along cortical VPall levels (a point already underlined by Puelles et al. 2016a). However, at amygdalar levels varying proportions of each derived cell population lack the Dbx1-LacZ marker. We postulate that this is due to the mixture of similar neurons born at the corresponding Dbx1-negative matrix component. The latter may include or correspond to the Dbx1-negative ‘ventrocaudal’ amygdalar ventricular portion contributing cells to the MeA of Ruiz-Reig et al. (2018), if it truly belongs to the pallium, as held by these authors (a doubt arises, though, because the MeA is generally assumed to be a part of the subpallium). However, relatively abundant Dbx1-positive cells were actually detected by Puelles et al. (2016a) also at the MeA. This perhaps suggests, jointly with the cited data of Ruiz-Reig et al. (2018), that some part of the MeA is perhaps pallial, and accordingly also shows a mixture of Dbx1-positive and negative cells.
We conclude from our reappraisal of the Dbx1 labelling evidence that the whole set of amygdalar pallial derivatives, similarly as the neighboring ventropallial olfactory cortex, are formed from a progenitor domain that combines Dbx1-positive and Dbx1-negative mother cells. This includes as well the posterior radial unit derivatives (AHi/PMCo), which were previously compared (or ascribed) to the MPall (Abellán et al., 2014; Medina et al. 2017).
The posterior radial unit /AHi/PMCo) would be the amygdalar portion which is in a position to contact behind the subpallial MeA (we do believe that at least some part of MeA is subpallial) the hypothalamo-amygdalar corridor that extends dorsalward (topologically) the alar hypothalamic paraventricular area, which was detected in Dbx1-LacZ material all along the bottom of the terminal sulcus.
Should the concept of cortical pallial sectors (VPall or others) be applied to amygdalar pallium?
The foregoing argument raises the point whether a typical cortical ventropallial subregion (VPall) can be said to extend within the pallial amygdala, just because some gene markers are shared between the structurally very distinct cortical and amygdalar parts of the pallium. We already argued against the idea that amygdalar populations originate from several cortical sectors and migrate separately into the amygdalar complex. This hypothesis was refuted by evidence mentioned above that ventral pallium markers such as Dbx1 and Sfrp2 are present independently at both the cortical and amygdalar pallial domains before neurogenesis takes place, and the same applies to markers of the medial pallium such as Emx2 and Lhx2. This makes local production of the respective cortical and amygdalar derivatives parsimonius, and we can forget the non-demonstrated cortico-amygdalar migration streams. Even accepting the available molecular evidence apparently supporting a ‘ventropallial’ similarity of given parts of the pallial amygdala (Medina et al. 2004; Bielle et al. 2005; Teissier et al. 2010; Puelles et al. 2016a), the presently corrected combined Dbx1/ Lhx9/ Lhx2/ Emx2/ Tbr1 data in the context of a variety of radial units indicate that, due to its molecular and structural heterogeneity, the hypothetic amygdalar VPall would be very different from the homogeneously ‘olfactory’ cortical VPall (Puelles 2014, 2017). Indeed, the Lhx9-negative lateral/basal units need to be distinguished from the Lhx9-positive anterior and posterior units, apart of between themselves (lateral versus basal; basolateral versus basomedial, basomediolateral versus basomediomedial; Table 1), on the basis of some 80 variously distributed gene patterns (Garcia-Calero et al. 2020). None of this amygdalar molecular complexity is found in the cortical VPall.
It should be noted that the primitive concept of ventral pallium stood originally on a particular apparently homogeneous molecular combination (Pax6/Dbx1-positive ventricular zone, plus a Tbr1-positive/Emx1-negative mantle zone; Smith-Fernández et al. 1998; Puelles et al. 2000, 2016a; Medina et al. 2004; Bielle et al. 2005; Remedios et al. 2004; Tole et al. 2005; García-López et al. 2008; Hirata et al. 2009; Waclaw et al. 2010). The recently discussed concentric ring model of the pallium (Puelles et al. 2019) suggests that this initial molecular definition of VPall applies primarily to the olfactory allocortex domain placed within the lateral part of the outer cortical ring (see also Puelles 2017). The pallial amygdala is instead a physically separate nuclear formation of substantial complexity, which lies topologically outside the outer cortical ring. As such it is probably exposed during development to a differential patterning atmosphere of organizing agents (positional signals), insuring intrinsic non-cortical differential pattern and histogenetic fates, which does not preclude the sharing of some developmental transcription factors.
In retrospect, we question the advantages of unifying the olfactory cortical VPall (or any other cortical sector) with the largely different overall structure and singularly varied molecular profile of the nuclearly-structured amygdala (Garcia-Calero et al. 2020). We recently pointed out that, as regards neurogenetic/histogenetic pattern, the cortical pallium is arranged in an inside-out pattern, whereas the amygdalar pallium adopts an outside-in pattern (Garcia-Calero and Puelles 2020). There is no obviously satisfying common causal explanation springing out of such traditional conceptual unification, either for the cortex or the amygdala. Modern causal models of cortical patterning normally leave aside the amygdala (review in Puelles et al. 2019). If we define an amygdalar (non-cortical) part of VPall (or other such sectors), which imply the existence of a particular molecular causal explanation of its adult structure), we immediately require ad hoc added causal explanations to account for the majoritary differential nuclear structure of the pallial amygdala (if the causes are the same, how is it that they develop differently?). The amygdala does receive olfactory input (in some of its parts, not all of them), but nevertheless displays important histogenetic variation with respect to the olfactory cortex (this is even more significative when a straightforward periamygdalar olfactory cortex is recognized just outside of the pallial amygdala, as shown in Garcia-Calero et al. 2020). We do not seem to have such ad hoc explanations at the moment. The same argument applies to DPall with respect to amygdalar NLOT (Remedios et al. 2007), and to hippocampal cortex structure (MPall) relative to the adjoining differently structured posterior amygdalar unit (AHi/PMCo; Abellán et al. 2014).
We conclude that it may be simpler to restrict the concepts of VPall, LPall, DPall and MPall to the cortical pallium, as conceived either in the updated tetrapartite pallial model (Puelles 2014, 2017), or in the more comprehensive and now preferred concentric ring cortical model (Puelles et al. 2019; note this model adds orbital, cingulate, postrhinal and entorhinal neighbourhoods not contemplated before, and maps as well known secondary organizers). The pallial amygdala obviously can be subdivided in its turn as seems most convenient (either according to present radial units proposed by Garcia-Calero et al. 2020, or by any other strictly amygdalar regionalization system attentive to radial topology, i.e., not based on conventional coronal sections). We do not need to achieve amygdalar consistency with cortical pallial sectors, since surely these adjacent but distinct pallial fields have significantly different patterning mechanisms, given the well-known structural differences. The different overall profile of the amygdalar pallial field compared to the cortical pallial field clearly does not impede that a number of genes cross the mutual boundary in various ways, perhaps also due to some shared patterning effects. These shared signals may even create some shared neuronal properties (e.g., the capacity to receive olfactory projections).
Is the conventional functional structure of the ‘BM’ amygdala illuminated by the new radial model?
A reviewer objected to our initially purely developmental morphological report, in which we mainly discussed predicted differential causal mechanisms in cortical versus amygdalar parts of the telencephalic pallium. This reviewer alluded to the higher interest of functional analysis for most readers (particularly in a journal whose title comprises ‘Structure and Function’). This assessment is no doubt true, but we think it is premature to construe at this stage from non-functional data a variant functional hypothesis of the amygdalar system. However, in brain science new structural concepts tend sooner or later to suggest novel possibilities of interpretation of available functional data, and we accordingly contribute our modest grain to this enterprise in this final section.
This report centers on the anterior amygdalar radial unit, whose novel definition in our model dissociates conceptually the BMA/ACo nuclei showing differential Lhx9 expression from the classic Lhx9-negative BMP nucleus. The latter is thought to derive from the separate basal amygdalar radial unit (Garcia-Calero et al. 2020). In contrast, much hodological and functional literature refers indistinctly to ‘the basomedial amygdala’ (‘BM’), a concept that lumps together ACo, BMA, and BMP. We will thus examine whether the correction we suggest illuminates in any way the current schema of functional amygdalar circuitry, as revealed by the connectivity of these three amygdalar centers. We will use as a data source the account of amygdalar connections given recently by Olucha-Bordonau et al. (2015).
ACo receives afferents from the main and accessory olfactory bulbs (Pro-Sistiaga et al. 2007; Gutierrez-Castellanos et al. 2010), the posterior intralaminar thalamic nuclei (non-chemosensory input), the posterior piriform cortex, and insular association areas (processed olfactory input), plus the medial prefrontal cortex (prelimbic and infralimbic areas apparently have roles in gating of fear responses and fear extinction, respectively). These connections are involved in the control of fear behavior, adjusting it to cognitive, contextual, mnemonic and internal state factors (Sotres-Bayon and Quirk 2010). Other connections with the orbitofrontal cortex relate to adjusting outcome expectancies, or emotional responses to unexpected outcomes. Relaxin afferents to ACo from the prepontine nucleus incertus (Olucha-Bordonau et al. 2003; Ryan et al. 2011, 2013) are held to relate this amygdalar centre to anxiety/depression. There are receptors for sexual steroids in the ACo, a feature held to allow integration of chemosensory and endocrine values in the control of socio-sexual behavior (Petrulis 2013). ACo projects contralaterally via the stria terminalis and the anterior commissure, and maintains reciprocal ipsilateral connections with other superficial amygdalar formations, such as the PMCo, PLCo, CxA, NLOT and BAOT (association of olfactory and vomeronasal inputs).
Interestingly, a number of intrinsic amygdalar connections interconnect these superficial centers with the BMA, BLA and La nuclei of the intermediate stratum, as well as with the BLP and BMP nuclei of the periventricular stratum. BMA (jointly with BLA/BLP and AHi) has massive output projections to the extended amygdala and other centers that control behavior, emotion and motivation (e.g., nucleus accumbens, medial amygdala and BST nuclei), and receives feedback from the extended amygdala. These interconnections allow functional interdependence between chemosensory (corticomedial) and non-chemosensory (basolateral) amygdalar divisions, underpinning integrated functional system properties of the amygdala. The amygdalar ‘BM’ outputs targetting the telencephalon (associative perirhinal and insular cortex; stratum lacunosum-moleculare of CA1, as opposed to preferent ‘BL’ outputs to prefrontal cortex and stratum oriens and radiatum of CA3/1). These ‘BM’ outputs are held to be involved in emotional memories and control of attention and motivation. The outputs reaching directly or indirectly the hypothalamus and brainstem control the expression of emotional behavior. The ‘BM’ amygdala participates jointly with the ‘BL’ counterpart in conditioned fear responses, receiving input from the La nucleus and projecting into the subpallial central nucleus (Ce), which spreads its projections to hypothalamus (autonomous response), periaqueductal grey (freezing response), brainstem (startle response) and VSt/DSt (escape response; instrumental learning). Canteras et al. (2001) put forward the hypothesis that ‘BM’ is involved in fear to live predators.
On the whole, we find in this brief course through amygdalar connections and functions related to the anterior radial unit studied by us in the present report, that the frequent reference in this literature to the ‘BM’ (sic), as if it were an integrated anatomo-functional complex, is not supported by clear evidence of preferential bidirectional connections between the Lhx9-positive BMA/ACo complex and the Lhx9-negative BMP nucleus (while BMA and ACo, members of the same radial unit, are indeed strongly interconnected). Recapitulative research is thus needed to discriminate which functions conventionally attributed to the lumped ‘BM’ on the basis of now obsolete anatomic assumptions, belong to each of the distinct ACo/BMA and BMP entities classified by us into different amygdalar radial units. A separate histogenetic origin does not preclude by itself that BMA/ACo collaborates functionally with BMP, but it can be recommended that researchers attend to each of these formations individually, and cease lumping them together automatically into the now anatomically imprecise ‘BM’ category. There is the possibility that such measures clarify the functional significance of these amygdalar components.
Experimental procedures
Animal preparation and tissue analysis
The day of the vaginal plug in female mice was counted as embryonic day (E) 0.5. The brains from mouse embryos (E12.5-E18.5) were dissected out and fixed overnight in 4% paraformaldehyde in pH 7.4 phosphate-buffered saline (PBS) at 4 °C. Next, these brains were embedded in 4% agarose in PBS and were sectioned with 100 µm thickness and cut in amygdalar radial plane (Garcia-Calero et al. 2020), horizontal, sagittal, and other obliquus planes with a Leica vibratome (VT1000 S) and processed for in situ hybridization and immunohistochemistry. The number of animals used in this work: 3–5 animals for every developmental stage analyzed.
In situ hybridization
We used restriction enzymes and polymerases in the presence of digoxigenin-11-UTP for riboprobe preparation. Mouse cDNA probes used for in situ hybridization analysis were Dlx5 (J.R. Rubenstein), Enc1 (M.C. Hernandez), Lhx2 and Lhx9 (our own lab). The hybridization protocol used in the present work was publish in Shimamura et al. (1994).
Immunohistochemistry
For immunohistochemistry experiments, we followed the protocol published in Garcia-Calero and Scharff (2013). The primary antibodies used in this study were: rabbit anti-calbindin (1:1000; CB-38, Swant, Bellinzona, Switzerland), rabbit anti-Tbr1 (1:200; sc-48816, Santa Cruz Biotechnology, Inc), mouse anti-RC2 (1:10; Developmental Studies Hybridoma Bank, Iowa City, IA).
Image capture, manipulation and figure assembly
Digital photo micrographs were acquired using Aperio CS2 and processed with Aperio ImageScope (Leica Microsystems GmbH, Mannheim, Germany), Adobe Photoshop and Adobe Illustrator softwares (Adobe Systems MountainView, CA, USA).
Acknowledgements
We thank the Allen Institute for Brain Science for public availability of the markers analysed (Website: ©2013 Allen Institute for Brain Science. Allen Developing Mouse Brain Atlas. http://developingmouse.brain-map.org).
Abbreviations
- 3v
Third ventricle
- AA
Anterior amygdala
- ACo
Anterior cortical nucleus
- AHi
Amygdalo-hippocampal area
- AHiCL
Amygdalo-hippocampal area, caudolateral part
- AHiRL
Amygdalo-hippocampal area, rostrolateral part
- AHiRM
Amygdalo-hippocampal area, rostromedial part
- ant
Anterior radial unit
- A. unit
Anterior radial unit
- APir
Amygdalo-piriform area
- BAOT
Bed nucleus of the accessory olfactory tract
- bas
Basal radial unit
- BLA
Anterior basolateral nucleus
- BLI
Intermediate basolateral nucleus
- BLP
Posterior basolateral nucleus
- BMA
Anterior basomedial nucleus
- BMP
Posterior basomedial nucleus
- BMIL
Lateral intermediate basomedial nucleus
- BMIM
Medial intermediate basomedial nucleus
- BMPL
Lateral posterior basomedial nucleus
- BMPM
Medial posterior basomedial nucleus
- c
Caudal
- CeA
Central amygdalar nucleus
- CxA
Cortex-amygdala transitional area
- CxAC
Cortex-amygdala transitional area, caudal part
- CxAR
Cortex-amygdala transitional area, rostral part
- ch
Chorioidal tela
- d
Dorsal
- DBV
Diagonal band, vertical limb
- DBH
Diagonal band, horizontal limb
- Ent
Entorhinal cortex
- fi
Fimbria of hippocampus
- Hi
Hippocampus
- l
Lateral
- L
Lateral nucleus
- lat
Lateral radial unit
- LI
Intermediate lateral nucleus
- lot
Lateral olfactory tract
- lv
Lateral ventricle
- m
Medial
- MeA
Medial amygdala
- MeD
Medial amygdala, dorsal part
- MeAV
Medial amygdala, anteroventral part
- MeAVc
Medial amygdala, anteroventral part, core
- MeAVs
Medial amygdala, anteroventral part, shell
- MePV
Medial amygdala, posteroventral part
- MePVc
Medial amygdala, posteroventral part, core
- MePVs
Medial amygdala, posteroventral part, shell
- NLOT
Nucleus of the lateral olfactory tract
- ot
Optic tract
- OT
Olfactory tuberculum
- Pal
Pallium
- ped
Peduncle
- Pir
Piriform cortex
- PLCo
Posterolateral cortical nucleus
- PLCoC
Posterolateral cortical nucleus, caudal part
- PLCoR
Posterolateral cortical nucleus, rostral part
- PMCo
Posteromedial cortical nucleus
- PMCoCLi
Posteromedial cortical nucleus, caudolateral part, intermediate layer
- PMCoRLi
Posteromedial cortical nucleus, rostrolateral part, intermediate layer
- PMCoRMi
Posteromedial cortical nucleus, rostromedial part, intermediate layer
- PMCoCLs
Posteromedial cortical nucleus, caudolateral part, superficial layer
- PMCoRLs
Posteromedial cortical nucleus, rostrolateral part, superficial layer
- PMCoRMs
Posteromedial cortical nucleus, rostromedial part, superficial layer
- PThE
Prethalamic eminence
- PThEt
Prethalamic eminence, telencephalic part
- post
Posterior radial unit
- r
Rostral
- REP
Retroendopiriform nucleus
- REPI
Retroendopiriform intermediate area
- REPCo
Retroendopiriform cortical area
- SP
Subpallium
- Spall
Subpallium
- Th
Thalamus
- v
Ventral
- vpg
Ventropallial glial packet
Funding
This work was supported by a Spanish Ministry of Economy and Competitiveness grant BFU2014-57516P (with European Community FEDER support), and a Seneca
Foundation (Autonomous Community of Murcia) Excellency Research contract, reference: 19904/GERM/15; project name: Genoarchitectonic Brain Development and Applications to Neurodegenerative Diseases and Cancer (to L.P.), by Seneca Foundation (5672 Fundación Séneca). University of Murcia, VAT: ESQ3018001B.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Human participants’ right statement
This article does not contain any studies involving human participants.
Research involving animals/ethical approval
All experimental protocols and handling, use, and care of laboratory animals were conducted in compliance with the current normative standards of the European Union (Directive 2010/63/EU), the Spanish Government (Royal Decree 1201/2005 and 53/2013; Law 32/107), and the approval of the University of Murcia committee for animal experimental ethics (No. A13170406).
Consent to participate and for publication
All authors consent to participate and publish the data included in this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abellán A, Legaz I, Vernier B, Rétaux S, Medina L. Olfactory and amygdalar structures of the chicken ventral pallium based on the combinatorial expression patterns of LIM and other developmental regulatory genes. J Comp Neurol. 2009;516:166–186. doi: 10.1002/cne.22102. [DOI] [PubMed] [Google Scholar]
- Abellán A, Vernier B, Rétaux S, Medina L. Similarities and differences in the forebrain expression of Lhx1 and Lhx5 between chicken and mouse: Insights for understanding telencephalic development and evolution. J Comp Neurol. 2010;518:3512–3528. doi: 10.1002/cne.22410. [DOI] [PubMed] [Google Scholar]
- Abellán A, Desfilis E, Medina L. The olfactory amygdala in amniotes: an evo-devo approach. Anat Rec (Hoboken) 2013;296:1317–1332. doi: 10.1002/ar.22744. [DOI] [PubMed] [Google Scholar]
- Abellán A, Desfilis E, Medina L. Combinatorial expression of Lef1, Lhx2, Lhx5, Lhx9, Lmo3, Lmo4, and Prox1 helps to identify comparable subdivisions in the developing hippocampal formation of mouse and chicken. Front Neuroanat. 2014;8:59. doi: 10.3389/fnana.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, de Olmos J, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system. San Diego: Academic Press; 1995. pp. 495–578. [Google Scholar]
- Alonso A, Trujillo CM, Puelles L. Longitudinal developmental analysis of prethalamic eminence derivatives in the chick by mapping of Tbr1 in situ expression. Brain Struct Funct. 2020;225:481–510. doi: 10.1007/s00429-019-02015-3. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: implications from nonhuman primate studies. Genes Brain Behav. 2003;2:295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Bachy I, Vernier P, Retaux S. The LIM-homeodomain gene family in the developing Xenopus brain: conservation and divergences with the mouse related to the evolution of the forebrain. J Neurosci. 2001;21:7620–7629. doi: 10.1523/JNEUROSCI.21-19-07620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuzzi S, Porter FD, Pitts A, Kumar M, Agulnick A, Wassif C, Westphal H. Characterization of Lhx9, a novel LIM/homeobox gene expressed by the pioneer neurons in the mouse cerebral cortex. Mech Dev. 1999;81:193–198. doi: 10.1016/s0925-4773(98)00233-0. [DOI] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Nême N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Bulchand S, Subramanian L, Tole S. Dynamic spatiotemporal expression of LIM genes and cofactors in the embryonic and postnatal cerebral cortex. Dev Dyn. 2003;226:460–469. doi: 10.1002/dvdy.10235. [DOI] [PubMed] [Google Scholar]
- Bupesh M, Abellán A, Medina L. Genetic and experimental evidence supports the continuum of the central extended amygdala and a mutiple embryonic origin of its principal neurons. J Comp Neurol. 2011;519:3507–3531. doi: 10.1002/cne.22719. [DOI] [PubMed] [Google Scholar]
- Burdach KF (1819–1822) Vom Baue und Leben des Gehirns, Leipzig
- Canteras NS, Ribeiro-Barbosa ER, Comoli E. Tracing from the dorsal premammillary nucleus prosencephalic systems involved in the organization of innate fear responses. Neurosci Biobehav Rev. 2001;25:661–668. doi: 10.1016/s0149-7634(01)00048-3. [DOI] [PubMed] [Google Scholar]
- Carney RS, Mangin JM, Hayes L, Mansfield K, Sousa VH, Fishell G, Machold RP, Ahn S, Gallo V, Corbin JG. Sonic hedgehog expressing and responding cells generate neuronal diversity in the medial amygdala. Neural Dev. 2010;5:14. doi: 10.1186/1749-8104-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Cobos I, Long JE, Thwin MT, Rubenstein JL. Cellular patterns of transcription factor expression in developing cortical interneurons. Cereb Cortex. 2006;1:i82–88. doi: 10.1093/cercor/bhk003. [DOI] [PubMed] [Google Scholar]
- Cocas LA, Georgala PA, Mangin JM, Clegg JM, Kessaris N, Haydar TF, Gallo V, Price DJ, Corbin JG. Pax6 is required at the telencephalic pallial-subpallial boundary for the generation of neuronal diversity in the postnatal limbic system. J Neurosci. 2011;31:5313–5324. doi: 10.1523/JNEUROSCI.3867-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Olmos J, Alheid GF, Beltramino C. Amygdala. In: Paxinos G, editor. The rat nervous system. Australia: Academic Press; 1985. pp. 223–334. [Google Scholar]
- De Olmos JS, Beltramino CA, Alheid G. Amygdala and extended amygdala of the rat: a cytoarchitectonical, fibroarchitectonical, and chemoarchitectonical survey. In: Paxinos G, editor. The rat nervous system. 3. Amsterdam: Elsevier-Academic Press; 2004. pp. 509–603. [Google Scholar]
- Desfilis E, Abellán A, Sentandreu V, Medina L. Expression of regulatory genes in the embryonic brain of a lizard and implications for understanding pallial organization and evolution. J Comp Neurol. 2018;526:166–202. doi: 10.1002/cne.24329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussing J, Wurst W. Amygdala and neocortex: common origins and shared mechanisms. Nat Neurosci. 2007;10:1081–1082. doi: 10.1038/nn0907-1081. [DOI] [PubMed] [Google Scholar]
- Fan CM, Kuwana E, Bulfone A, Fletcher CF, Copeland NG, Jenkins NA, Crews S, Martinez S, Puelles L, Rubenstein JL, Tessier-Lavigne M. Expression patterns of two murine homologs of Drosophila single-minded suggest possible roles in embryonic patterning and in the pathogenesis of Down syndrome. Mol Cell Neurosci. 1996;7:1–16. doi: 10.1006/mcne.1996.0001. [DOI] [PubMed] [Google Scholar]
- Garcia-Calero E (2005) Contribuciones a la regionalizacion telencefalica. Doctoral dissertation, University of Murcia, Spain
- García-Calero E, Puelles L (2005) Pallial expression of Enc1 RNA in postnatal mouse telencephalon. Brain Res Bull 66:445–448 [DOI] [PubMed]
- Garcia-Calero E, Puelles L (2020) Histogenetic Radial Models as Aids to Understanding Complex Brain Structures: The Amygdalar Radial Model as a Recent Example. Front Neuroanat 14:590011. 10.3389/fnana.2020.590011 [DOI] [PMC free article] [PubMed]
- Garcia-Calero E, Scharf C. Calbindin expression in developing striatum of zebra inches and its relation to the formation of area X. J Comp Neurol. 2013;521:326–341. doi: 10.1002/cne.23174. [DOI] [PubMed] [Google Scholar]
- Garcia-Calero E, Martínez-de-la-Torre M, Puelles L (2020) A radial histogenetic model of the mouse pallial amygdala. Brain Struct Funct. 10.1007/s00429-020-02097-4 [DOI] [PMC free article] [PubMed]
- Garcia-Calero E, López-González L, Martínez-de-la-Torre M, Fan CM, Puelles L (2021) Sim1-expressing cells illuminate the origin and course of migration of the nucleus of the lateral olfactory tract in the mouse amygdala. Brain Struct Funct. 10.1007/s00429-020-02197-1 [DOI] [PMC free article] [PubMed]
- García-López M, Abellán A, Legaz I, Rubenstein JL, Puelles L, Medina L. Histogenetic compartments of the mouse centromedial and extended amygdala based on gene expression patterns during development. J Comp Neurol. 2008;506:46–74. doi: 10.1002/cne.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Moreno F, Pedraza M, Di Giovannantonio LG, Di Salvio M, López-Mascaraque L, Simeone A, De Carlos JA (2010) A neuronal migratory pathway crossing from diencephalon to telencephalon populates amygdala nuclei. Nat Neurosci 13:680-689. 10.1038/nn.2556 [DOI] [PubMed]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Castellanos N, Martinez-Marcos A, Martínez-García F, Lanuza E. Chemosensory function of the amygdala. Vitam Horm. 2010;83:165–196. doi: 10.1016/S0083-6729(10)83007-9. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Hirata T, Li P, Lanuza GM, Cocas LA, Huntsman MM, Corbin JG. Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nat Neurosci. 2009;12:141–9. doi: 10.1038/nn.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren N. Points of view concerning forebrain morphology in higher vertebrates. Acta Zool. 1925;6:413–477. [Google Scholar]
- Huilgol D, Udin S, Shimogori T, Saha B, Roy A, Aizawa S, Hevner RF, Meyer G, Ohshima T, Pleasure SJ, Zhao Y, Tole S. Dual origins of the mammalian accessory olfactory bulb revealed by an evolutionarily conserved migratory stream. Nat Neurosci. 2013;16:157–165. doi: 10.1038/nn.3297. [DOI] [PubMed] [Google Scholar]
- Johnston JB. Further contributions to the study of the evolution of the forebrain. J Comp Neurol. 1923;35:337–481. [Google Scholar]
- Kim AS, Anderson SA, Rubenstein JRL, Lowenstein DH, Pleasure SJ. Pax-6 regulates expression of SFRP-2 and Wnt-7b in the developing CNS. J. Neurosci. 2001;21:RC132. doi: 10.1523/JNEUROSCI.21-05-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978;178:255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- Kuhlenbeck H (1924) Über die Homologien der Zellmassen im Hemi= sphärenhirn der Wirbeltiere. Folia Anatomica Japonica
- Kuhlenbeck H (1927) Vorlesungen über das Zentralnervensystem der Wirbeltiere. Jena: Fischer
- Kuhlenbeck H. The central nervous system of vertebrates. 3, Part II: overall morphological pattern. Basel: Karger; 1973. [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lischinsky JE, Sokolowski K, Li P, Esumi S, Kamal Y, Goodrich M, Oboti L, Hammond TR, Krishnamoorthy M, Feldman D, Huntsman M, Liu J, Corbin JG. Embryonic transcription factor expression in mice predicts medial amygdala neuronal identity and sex-specific responses to innate behavioral cues. Elife. 2017;6:e21012. doi: 10.7554/eLife.21012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo YT. The forebrain of the opossum, Didelphis virginiana: I. Gross morphology. J Comp Neurol. 1930;51:13–64. [Google Scholar]
- Loo YT. The forebrain of the opossum, Didelphis virginiana: II. Histology. J Comp Neurol. 1931;52:1–148. [Google Scholar]
- Marín O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- Martínez-García F, Novejarque A, Lanuza E. Two interconnected functional systems in the amygdala of amniote vertebrates. Brain Res Bull. 2008;75:206–213. doi: 10.1016/j.brainresbull.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Martínez-García F, Noverjarque A, Guitiérrez-Castellanos N, Lanuza E. Piriform cortex and amygdala. In: Watson C, Paxinos G, Puelles L, editors. The mouse nervous system. San Diego: Academic Press; 2012. pp. 140–172. [Google Scholar]
- Medina L, Legaz I, González G, de Castro F, Rubenstein JLR, Puelles L. Expression of Dbx1, neurogenin 2, semaphorin 5A, cadherin 8, and emx1 distinguish ventraland lateral pallial histogenetic divisions in the developing claustroamygdaloid complex. J Comp Neurol. 2004;474:504–523. doi: 10.1002/cne.20141. [DOI] [PubMed] [Google Scholar]
- Medina L, Abellán A, Vicario A, Castro-Robles B, Desfilis E. The Amygdala. In: Kaas J, editor. Evolution of nervous systems 2e. Oxford: Elsevier; 2017. pp. 427–478. [Google Scholar]
- Moreno N, Bachy I, Rétaux S, González A. LIM-homeodomain genes as developmental and adult genetic markers of Xenopus forebrain functional subdivisions. J Comp Neurol. 2004;472:52–72. doi: 10.1002/cne.20046. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, O'Leary DD. Combinatorial expression patterns of LIM-homeodomain and other regulatory genes parcellate developing thalamus. J Neurosci. 2001;21:2711–2725. doi: 10.1523/JNEUROSCI.21-08-02711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R, Puelles L. Towards a new neuromorphology. Switzerland: Springer International Publishing; 2016. [Google Scholar]
- Olucha-Bordonau FE, Teruel V, Barcia-Gonzalez J, Ruiz-Torner A, Valverde-Navarro AA, Martinez-Soriano F. Cytoarchitecture and efferent projections of the nucleus incertus of the rat. J Comp Neurol. 2003;464:62–97. doi: 10.1002/cne.10774. [DOI] [PubMed] [Google Scholar]
- Olucha-Bordonau FE, Fortes-Marco L, Otero-García M, Lanuza E, Martínez-García F. The rat nervous system. San Diego: Academic Press; 2015. Amygdala: structure and function; pp. 442–476. [Google Scholar]
- Petrulis A. Chemosignals, hormones and mammalian reproduction. Horm Behav. 2013;63:723–741. doi: 10.1016/j.yhbeh.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Del Mar A-J, Marcos P, Artacho-Perula E, Crespo C, Insausti R, Martinez-Marcos A. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol. 2007;504:346–362. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- Puelles L (2014) Development and evolution of the claustrum. In: The Claustrum. Elsevier
- Puelles L. Comments on the updated tetrapartite pallium model in the mouse and chick, featuring a homologous claustro-insular complex. Brain Behav Evol. 2017;90:171–189. doi: 10.1159/000479782. [DOI] [PubMed] [Google Scholar]
- Puelles L. Survey of midbrain, diencephalon, and hypothalamus neuroanatomic terms whose prosomeric definition conflicts with columnar tradition. Front Neuroanat. 2019;13:20. doi: 10.3389/fnana.2019.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JLR. A new scenario of hypothalamic organization: rationale of new hypotheses introduced in the updated prosomeric model. Front Neuroanat. 2015;9:27. doi: 10.3389/fnana.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JLR. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Puelles L, Harrison M, Paxinos G, Watson C. A developmental ontology for the mammalian brain based on the prosomeric model. Trends Neurosci. 2013;36:570–578. doi: 10.1016/j.tins.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Puelles L, Medina L, Borello U, Legaz I, Teissier A, Pierani A, Rubenstein JLR. Radial derivatives of the mouse ventral pallium traced with Dbx1-LacZ reporters. J Chem Neuroanat. 2016;75:2–19. doi: 10.1016/j.jchemneu.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Puelles L, Ayad A, Alonso A, Sandoval JE, MartÍnez-de-la-Torre M, Medina L, Ferran JL. Selective early expression of the orphan nuclear receptor Nr4a2 identifies the claustrum homolog in the avian mesopallium: impact on sauropsidian/mammalian pallium comparisons. J Comp Neurol. 2016;524:665–703. doi: 10.1002/cne.23902. [DOI] [PubMed] [Google Scholar]
- Puelles L, Alonso A, García-Calero E, Martínez-de-la-Torre M. Concentric ring topology of mammalian cortical sectors and relevance for patterning studies. J Comp Neurol. 2019;527:1731–1752. doi: 10.1002/cne.24650. [DOI] [PubMed] [Google Scholar]
- Puelles L, Diaz C, Stühmer T, Ferran JL, Martínez-de la Torre M, Rubenstein JLR (2020) LacZ-reporter mapping of Dlx5/6 expression and genoarchitectural analysis of the postnatal mouse prethalamus. J Comp Neurol. 10.1002/cne.24952 [DOI] [PMC free article] [PubMed]
- Remedios R, Subramanian L, Tole S. LIM genes parcellate the embryonic amygdala and regulate its development. J Neurosci. 2004;24:6986–6990. doi: 10.1523/JNEUROSCI.0001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedios R, Huilgol D, Saha B, Hari P, Bhatnagar L, Kowalczyk T, Hevner RF, Suda Y, Aizawa S, Ohshima T, Stoykova A, Tole S. A stream of cells migrating from the caudal telencephalon reveals a link between the amygdala and neocortex. Nature Neurosci. 2007;10:1141–1150. doi: 10.1038/nn1955. [DOI] [PubMed] [Google Scholar]
- Rétaux S, Rogard M, Bach I, Failli V, Besson MJ. Lhx9: a novel LIM-homeodomain gene expressed in the developing forebrain. J Neurosci. 1999;19:783–793. doi: 10.1523/JNEUROSCI.19-02-00783.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Emotion and decision-making explained. Oxford: Oxford University Press; 2014. [Google Scholar]
- Rolls ET. Limbic systems for emotion and for memory, but no single limbic system. Cortex. 2015;62:119–157. doi: 10.1016/j.cortex.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Ruiz-Reig N, Studer M. Rostro-Caudal and Caudo-Rostral Migrations in the Telencephalon: Going Forward or Backward? Front Neurosci. 2017;11:692. doi: 10.3389/fnins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Reig N, Andrés B, Huilgol D, Grove EA, Tissir F, Tole S, Theil T, Herrera E, Fairén A. Lateral thalamic eminence: a novel origin for mGluR1/Lot cells. Cereb Cortex. 2017;27:2841–2856. doi: 10.1093/cercor/bhw126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Reig N, Andres B, Lamonerie T, Theil T, Fairén A, Studer M. The caudo-ventral pallium is a novel pallial domain expressing Gdf10 and generating Ebf3-positive neurons of the medial amygdala. Brain Struct Funct. 2018;223:3279–3295. doi: 10.1007/s00429-018-1687-0. [DOI] [PubMed] [Google Scholar]
- Ryan PJ, Ma S, Olucha-Bordonau FE, Gundlach AL. Nucleus incertus—an emerging modulatory role in arousal, stress and memory. Neurosci Biobehav Rev. 2011;35:1326–1341. doi: 10.1016/j.neubiorev.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Ryan PJ, Buchler E, Shabanpoor F, Hossain MA, Wade JD, Lawrence AJ, Gundlach AL. Central relaxin-3 receptor (RXFP3) activation decreases anxiety- and depressive-like behaviours in the rat. Behav Brain Res. 2013;244:142–151. doi: 10.1016/j.bbr.2013.01.034. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Hirano S, McMahon AP, Takeichi M. Wnt-1-dependent regulation of local E-cadherin and alpha N-catenin expression in the embryonic mouse brain. Development. 1994;120:2225–2234. doi: 10.1242/dev.120.8.2225. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Smith-Fernandez A, Pieau C, Reperant J, Boncinelli E, Wassef M. Expression of the Emx-1 and Dlx-1 homeobox genes define three molecularly distinct domains in the telencephalon of mouse, chick, turtle and frog embryos: implications for the evolution of telencephalic subdivisions in amniotes. Development. 1998;125:2099–2111. doi: 10.1242/dev.125.11.2099. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Wang B, Campbell K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J Neurosci. 2003;23:10568–10576. doi: 10.1523/JNEUROSCI.23-33-10568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedter GF. The telencephalon of tetrapods in evolution. Brain Behav Evol. 1997;49:179–213. doi: 10.1159/000112991. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Teissier A, Griveau A, Vigier L, Piolot T, Borello U, Pierani A. A novel transient glutamatergic population migrating from the pallial-subpallial boundary contributes to neocortical development. J Neurosci. 2010;30(31):10563–10574. doi: 10.1523/JNEUROSCI.0776-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tole S, Remedios R, Saha B, Stoykova A. Selective requirement of Pax6, but not Emx2, in the specification and development of several nuclei of the amygdaloid complex. J Neurosci. 2005;25:2753–2760. doi: 10.1523/JNEUROSCI.3014-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Ehrman LA, Pierani A, Campbell K. Developmental origin of the neuronal subtypes that comprise the amygdalar fear circuit in the mouse. J Neurosci. 2010;30:6944–6953. doi: 10.1523/JNEUROSCI.5772-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]