Abstract
Relapse to addictive drug use remains a major medical problem worldwide. In rodents, glutamate release in the nucleus accumbens core triggers reinstated drug seeking in response to stress, and drug-associated cues and contexts. Glutamatergic dysregulation in addiction results in part from long-lasting adaptations in accumbens astroglia, including downregulation of the glutamate transporter GLT-1 and retraction from synapses after withdrawal from psychostimulants and opioids. While their capacity to clear glutamate is disrupted by drug use and withdrawal, accumbens astrocytes undergo rapid, transient plasticity in response to drug-associated cues that reinstate seeking. Cued reinstatement of heroin seeking, for example, restores synaptic proximity of astrocyte processes through ezrin phosphorylation, and enhances GLT-1 surface expression. These adaptations limit drug seeking behavior and largely occur on non-overlapping populations of astroglia. Here we review the growing literature supporting a critical role for accumbens astrocytes in modulating glutamate transmission during drug seeking in rodent models of relapse.
Keywords: addiction, relapse, self-administration, reinstatement, astroglia, glutamate, synapse, GLT-1
Introduction
Glutamate spillover in the nucleus accumbens core (NAcore) is a characteristic feature of reinstatement induced by drug-associated cues and contexts in rodent models of relapse [1]. Many years of work have demonstrated adaptations in the NAcore induced by multiple classes of addictive drugs, but not by natural rewards such as sucrose, that increase the likelihood of glutamate spillover in response to reward-associated cues that stimulate seeking [2]. While much of the work in the field has focused on neural signaling and its contribution to reinstated seeking, NAcore astroglia contribute to aberrant glutamate homeostasis after chronic drug intake, and astrocytes in the NAcore undergo constitutive adaptations that facilitate glutamate spillover after self-administration and withdrawal from various classes of addictive drugs. Recent studies also demonstrate that NAcore astroglia are dynamic during drug cue exposure and undergo plasticity that serves to attenuate reinstated drug seeking. Thus, accumbens astrocytes support cue reactivity, an important indication of relapse vulnerability in humans [3], through long-term drug-induced changes and also retain mechanisms for suppressing drug-seeking behavior, making this cell type a compelling target for interventions aimed at preventing relapse in human patients. Here we integrate the recent literature supporting a role for nucleus accumbens astrocytes in glutamate regulation and dysregulation in rodent models of drug addiction and relapse.
Glutamatergic dysregulation and drug seeking
While drugs of abuse derive their rewarding properties through increasing dopamine transmission in the striatum [4], long-lasting reactivity to drug-associated cues and contexts characteristic of drug addiction relies upon glutamatergic signaling within the NAcore [1]. Relapse vulnerability is modeled in rodents through operant training with addictive substances that are delivered coincident with environmental cues (i.e. lights, tones). After a withdrawal period, re-introduction of these cues stimulates robust drug seeking behavior [5]. Previous studies using such models point to glutamate release from prefrontal cortical terminals in the NAcore as a causative trigger of cue-reinstated drug seeking behavior [6]. Furthermore, the inability to update behaviors based on new contingencies, such as apparent lack of drug availability or emergence of negative consequences, is a behavioral feature of addiction, and follows from repeated intake of addictive drugs, but not natural rewards. For example, rats that have undergone a similar training schedule described above using palatable reward delivery in place of intravenous drug delivery are able to more rapidly update their behavior when no reward is present [7]. Likewise many aspects of glutamatergic dysregulation noted in rodents after self-administration and withdrawal from addictive drugs are not observed after similar training using sucrose. The cellular machinery that controls glutamate transmission is altered by drugs of abuse (but not sucrose) such that drug-paired cues and contexts that trigger synaptic glutamate release lead to glutamate spillover at NAcore synapses and robust and perseverative behavioral responding [2]. Thus, efforts to understand adaptations underlying glutamate spillover have been an important goal of drug addiction research.
Astrocyte regulation of synaptic glutamate transmission
Presynaptic glutamate release has the capacity to increase glutamate concentration to >1 mM in the synaptic space [8], and since glutamate cannot be degraded extracellularly, its uptake through transporters terminates glutamatergic neurotransmission. The bulk of glutamate uptake in the adult rat brain occurs through GLT-1 (EAAT-2 in humans), which is expressed largely by astrocytes [9] that shape glutamate transmission through both expression and perisynaptic proximity of GLT-1 [10]. While high affinity GLT-1 captures glutamate in ~1 ms [11, 12], the time course of glutamate uptake through a single transporter is relatively slow (~70 ms [13]), requiring a high density and rapid surface diffusion of GLT-1 near excitatory synapses [10, 14]. Quantum dot tracking of GLT-1 in mixed hippocampal cultures shows that clusters of GLT-1 are remodeled within milliseconds in response to neural activity and that surface diffusion of GLT-1 toward and away from synapses impacts the time course of excitatory transmission [10].
Astrocytes in the striatum also modulate synaptic transmission through glutamate release. Perhaps the most well-studied and undisputed mechanism of glutamatergic transmission by astrocytes is through the cystine-glutamate antiporter, system xc- [15]. System xc- releases glutamate in a 1:1 stoichiometric exchange for cystine, which is used to maintain intracellular levels of the antioxidant glutathione and preserve redox homeostasis [16, 17]. In the NAcore, roughly 60% of basal extracellular glutamate derives from cystine-glutamate exchange [18]. System xc- is expressed most highly by astrocytes [19, 20] and exerts a measure of control over presynaptic glutamate release by providing glutamatergic tone on presynaptic mGluR2/3, which induces autoinhibition of transmitter release during excitatory transmission [21, 22].
A critical factor in the efficacy of astroglial glutamate transporters and exchangers is the perisynaptic localization of astroglial peripheral processes. While synaptic proximity of GLT-1 and system xc- would be expected to facilitate control of glutamatergic transmission, studies indicate that synaptic insulation by astrocyte processes is highly variable in the striatum [23, 24]. Technical advances allowing high throughput assessment of synaptic proximity of astrocyte processes on a nm scale demonstrate that approximately half of synapses in the dorsolateral striatum receive astrocyte insulation at <10nm. The nearest astroglial contact for the remaining synapses is anywhere between 10 and 400 nm [24], raising important questions regarding causes and consequences of high vs. low synaptic insulation by astrocytes. Perisynaptic astrocyte processes are morphologically plastic and exhibit rapid actin-dependent remodeling in response to neural activity through Gq-coupled [25], and perhaps other astroglial receptors [26]. Importantly, theoretical models estimate that astrocyte insulation along ~50% of the synaptic perimeter effectively doubles glutamate concentration within the glial sheath through spatial buffering [27]. Additionally, the glial sheath is biased towards the postsynapse in brain regions like the hippocampus and cerebellum, facilitating spillover towards the presynapse where inhibitory autoreceptors are located and negatively regulate presynaptic release probability [27]. Thus, through their synaptic proximity and expression of glutamate transporters and exchangers, astrocytes play an unmistakable role in maintaining synaptic glutamate homeostasis.
Astrocyte adaptations that facilitate the spillover of synaptic glutamate after drug use
Glutamatergic dysregulation in the NAcore after chronic self-administration of a number of drugs of abuse, including pscyhostimulants and opioids, have been tied directly to disrupted astroglial mechanisms of glutamate regulation in the NAcore. The most well-characterized mechanism whereby drugs of abuse promote cue reactivity in animal models is through downregulation of GLT-1 on NAcore astrocytes [28] leading to reduced glutamate uptake capacity. In general, it appears that GLT-1 downregulation in the NAcore is more often a consequence of self-administered, rather than experimenter-administered drug paradigms (as in [29]), and reductions in GLT-1 expression appear to be strongly influenced by drug intake and duration of withdrawal, with both increased drug intake and extended withdrawal durations exacerbating GLT-1 downregulation [30]. Importantly, pharmacological treatments that upregulate expression of GLT-1 and system xc- serve to attenuate cue-reinstated drug seeking in animal models [31, 32] and measures of craving in human patients [33]. It is possible that GLT-1 downregulation follows from reduced extracellular glutamate tone, perhaps through reduced expression, function, or synaptic proximity of system xc-. Cocaine [18] or nicotine [29], but not heroin-induced [34] downregulation of system xc- expression or function contribute to drug-induced reductions in basal extracellular glutamate, which is largely regulated through cystine-glutamate exchange [18]. The downregulation of system xc- would also be expected to enhance synaptic glutamate release through reduced tone on presynaptic mGluR2/3 [35].
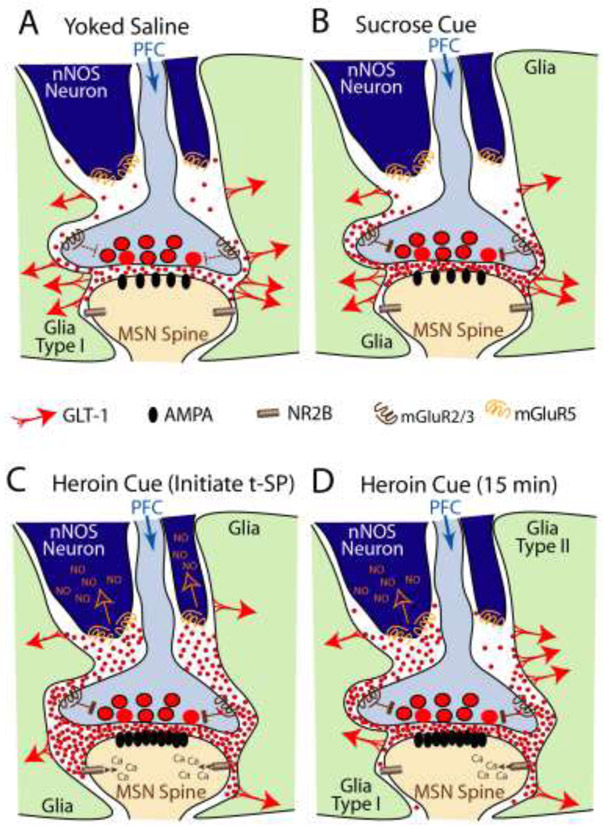
Finally, withdrawal from self-administration of psychostimulants and opioids produces a robust retraction of astroglial processes from NAcore synapses [36-38], exacerbating the downregulation of GLT-1 by delaying glutamate uptake and permitting glutamate escape from the synapse and perisynaptic annulus. Thus, the downregulation of GLT-1 and synaptic retraction by astroglial processes sets the stage for drug cue-induced synaptic glutamate release to stimulate perisynaptic and extrasynaptic glutamate receptors. As mentioned above, synaptic coverage by astroglia is biased towards the postsynapse, making drug-induced astroglial retraction especially impactful at the postsynaptic annulus where heroin use and withdrawal elevates the density of NMDA receptors containing GluN2B subunits (NR2B) [39]. Stimulating these receptors promotes synaptic potentiation and is required for cue-induced heroin and nicotine seeking [40]. Retraction of astrocyte processes may also lead to stimulation of glutamate receptors on neighboring synapses within the neuropil [41]. Indeed this has been observed in a number of studies using microdialysis, where cued and drug-induced reinstatement of seeking in animal models stimulates elevations in glutamate above baseline in drug-addicted animals relative to controls [6], and at least for cued heroin and cocaine seeking, activates mGluR5 on nearby nNOS-expressing interneurons. Recruitment of nNOS interneurons increases nitric oxide synthesis and promote transient post-synaptic potentiation at accumbens medium spiny neurons (MSNs), which is required for cue-induced reinstatement [42, 43]. Figure 1 illustrates the actions of synaptic glutamate spillover that contribute to cue-induced reinstatement of drug seeking and result from astroglial synaptic retraction and downregulation of GLT-1. As illustrated in Figure 1, the astroglial changes that permit glutamate spillover, including synaptic retraction of astrocyte processes and downregulation of GLT-1, have not been found to occur after operant training with sucrose [38, 44].
Figure 1. Hypothesized model of how astroglial adaptations impact reinstated heroin seeking.
A) Following yoked saline delivery, synaptic glutamate release is easily accommodated by the presence of GLT-1 on astroglial processes near the synapse. Perisynaptic astroglial insulation favors the postsynapse, biasing glutamate spillover towards presynaptic mGluR2/3 autoreceptors that regulate release. B) Although operant training with sucrose reduces surface expression of GLT-1, synaptic proximity by astrocyte processes is maintained, leading to sufficient glutamate uptake and mGluR2/3 autoreceptor stimulation to prevent synaptic spillover from activating mGluR5 on nNOS interneurons or extrasynaptic NR2B on the postsynapse in response to sucrose-associated cues. C) Following heroin withdrawal there is both reduced GLT-1 and reduced astroglial proximity to the synapse. This allows for maximal spillover and glutamatergic stimulation of mGluR5 on nNOS interneurons and extrasynaptic NR2B, initiating postsynaptic potentiation through AMPA receptor insertion, and amplifying the behavioral motivation to reinstate heroin seeking. D) By 15 min of cued heroin reinstatement, astroglia have undergone two distinct adaptations that each partly dampen reinstated heroin seeking via distinct mechanisms. In type I astroglia, synaptic proximity returns, presumably to the postsynapse that biases access of synaptic glutamate spillover towards mGluR2/3 autoreceptors and away from postsynaptic NR2B. However, we hypothesize that more distant diffusion of glutamate to mGluR5 on nNOS interneurons to amplify reinstated heroin seeking is not prevented. Conversely, in type II astroglia GLT-1 on the surface is increased extrasynaptically, perhaps impairing diffusion of glutamate spillover to extrasynaptic mGluR5 on nNOS interneurons, limiting postsynaptic potentiation.
Astroglial plasticity in response to drug cues attenuates seeking
While astrocytes were long thought to be inert homeostatic support cells, recent evidence points to their dynamic role in shaping synaptic physiology and behavioral outputs [38, 45, 46]. For instance, extracellular glutamate dynamics are impacted by GLT-1 surface diffusion, which is uniquely high compared with other neurotransmitter transporters [10]. While GLT-1 is relatively immobile in cultures lacking neurons, it exhibits surface diffusion in the presence of glutamate and is stabilized near glutamatergic synapses [10]. Interestingly, while GLT-1 appears to target glutamate release sites, glutamate release events increase surface diffusion of GLT-1 away from the synaptic compartment [10]. This effect is thought to increase the efficiency of glutamate removal given the relatively slow kinetics of glutamate uptake through GLT-1 [13] by allowing synaptically released glutamate access to unbound transporter molecules. Perhaps related to the glutamate release-induced lateral diffusion of GLT-1 away from the synapse, presenting heroin-associated cues to reinstate heroin seeking transiently upregulates surface expression of GLT-1 despite its overall downregulation after withdrawal [34]. However, it appears that surface GLT-1 may not target synaptic sites during 15-min of cued reinstatement, since increased synaptic co-registration of GLT-1 is not observed at this time point in reinstated animals [44].
In addition to GLT-1 trafficking, astrocyte cytoarchitecture is highly plastic in response to drug-associated cues that reinstate seeking behavior [38]. In rats extinguished from heroin self-administration, 15-min exposure to cues that reinstate seeking produces a transient increase in synaptic proximity by astroglial processes in the NAcore. The cue-induced morphological plasticity requires phosphorylation of ezrin [38], an actin binding protein selective for astrocyte peripheral processes [47]. The enhanced synaptic insulation by astroglial processes serves to suppress seeking behavior, since its inhibition enhances the duration of cued reinstatement [38]. Research is ongoing to uncover the signaling cascade linking extracellular glutamate to ezrin phosphorylation and astrocyte process elongation [48]. Interestingly, while both forms of transient astroglial plasticity, enhanced morphological plasticity and increased surface expression of GLT-1, are expected to limit cued drug seeking [38, 44], both occur on largely non-overlapping subpopulations of NAcore astroglia during cued reinstatement [44], suggesting discrete mechanisms that trigger their initiation. Figure 1 illustrates these two types of astroglial plasticity and our hypotheses on how this drug cue-induced plasticity can dampen cued drug seeking. On one hand, type I plasticity, where cues produce morphological plasticity to temporarily re-associate astroglial processes with the synapse, may inhibit postsynaptic diffusion to NR2B. However, because there is no restoration of GLT-1, this plasticity may be less effective in preventing diffusion to extrasynaptic glutamate receptors, including mGluR5 on nNOS interneurons, a known target mediating cue-induced drug seeking [43]. A complementary strategy for blunting cued drug seeking is seen in type II plasticity where GLT-1 surface expression is elevated by cues outside of the synapse. In this situation, diffusion of synaptic glutamate would continue to have access to glutamate receptors on the postsynaptic annulus, but diffusion to more distant receptors would be blunted by the increase in GLT-1. Combined, these effects would synergize to dampen, but not prevent cue-induced drug seeking.
Finally, as mentioned above, astrocytes also impact glutamate neurotransmission through their own mechanisms of glutamate release. Gq-coupled signaling in astroglia has been shown to induce astroglial glutamate release in the NAcore that suppresses cue-reinstated cocaine seeking through stimulation of presynaptic mGluR2/3 [45]. The same signaling in sucrose-trained rats does not impact cued seeking behavior [45]. Whether this or other mechanisms of gliotransmission are engaged in response to drug-associated cues is an open and fundamental question. Indeed there may be multiple mechanisms whereby a single neuron elicits a range of responses from a single astrocyte (as in [49]) and astrocyte responses may be highly variable within and between brain regions [23, 50]. For example, in a previous study, stimulation of astroglial mGluR5 in the nucleus accumbens was linked with NR2B receptor-dependent slow-inward currents in nearby medium spiny neurons (MSNs) through extrasynaptic receptors [51]. Although Gq-coupled GPCR stimulation of glutamate release from NAcore astroglia inhibits cued seeking [45, 52], it has not yet been linked to induction of morphological plasticity or changes in surface diffusion of GLT-1. Since signaling through group I mGluRs is required for astrocyte process extension toward hippocampal synapses [53], it seems a likely possibility that glutamate release by NAcore astrocytes may involve those astrocytes that exhibit type I morphological plasticity. Alternatively, astroglial glutamate release in response to drug cues may represent an independent mechanism whereby NAcore astrocytes modulate drug seeking behavior.
Conclusions and perspectives
Astroglia are important regulators of glutamatergic signaling, and drugs of abuse trigger glutamatergic dysregulation through astroglial mechanisms that promote reinstatement vulnerability. Despite constitutive changes that enhance cue reactivity in animal models, recent studies show that astroglia appear to be equipped with two or more discrete mechanisms enacted transiently by drug-associated cues to suppress reinstated seeking. Understanding the signaling cascades that lead to transient compensatory astroglial plasticity or restore the capacity of striatal astrocytes to regulate glutamate transmission after drug use remains a research priority. An important direction will be to understand how unique adaptations in subpopulations of astrocytes differentially signal within the NAcore. The NAcore is composed of mostly MSNs and is only ~5% interneurons. Despite their relatively low numbers, NAcore interneurons are capable of coordinating robust behavioral outputs, such as cued drug seeking [43]. However, astroglial signaling with striatal interneurons has not yet been demonstrated. On the other hand, astrocytes in the dorsal striatum signal uniquely with D1- or D2-type MSNs, but not both [54]. This poses the intriguing possibility that astroglial subpopulations engaging in distinct type I and type II astroglial plasticity in response to drug cues may segregate according to MSN subtype in the NAcore.
While it is clear that astrocytes are an important cell type to study and manipulate to restore glutamate homeostasis in the striatum, astroglial regulation of transmission has been less well characterized in the circuitry up and downstream from the NAcore that coordinate to produce drug seeking behaviors, though it is an important area of ongoing research [55]. Together, these studies will lead to an improved understanding of whether and how astroglia contribute to regulating other transmitter systems such as dopamine or GABA, if and how astroglial regulation of these transmitter systems is altered by use of addictive drugs, and whether there appear to be ways to therapeutically target these adaptations in animal models and in humans.
Highlights.
Astrocytes in the nucleus accumbens core undergo morphological remodeling after self-administration of addictive drugs, including cocaine, methamphetamine, and heroin, resulting in their reduced proximity at excitatory synapses.
Repeated administration of psychostimulants or opioids causes downregulation of the astroglial glutamate transporter GLT-1.
These long-lasting drug-induced astroglial adaptations potentiate glutamate spillover that drives drug seeking induced by drug-associated cues.
Cued reinstatement of drug seeking produces transient adaptations in nucleus accumbens astrocytes, including increased surface expression of GLT-1, phosphorylation of the actin binding protein ezrin, and increased synaptic proximity of astroglial processes. We predict that these adaptations dampen drug-seeking behavior by limiting synaptic glutamate spillover.
Acknowledgements
This work was supported by the National Institutes of Health (DA003906 and DA012513, PWK).
Footnotes
Conflicts of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* Denotes high relevance.
- 1.Kalivas PW, The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci, 2009. 10(8): p. 561–72. [DOI] [PubMed] [Google Scholar]
- 2.Bobadilla AC, et al. , Accumbens Mechanisms for Cued Sucrose Seeking. Neuropsychopharmacology, 2017. 42(12): p. 2377–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter BL and Tiffany ST, Meta-analysis of cue-reactivity in addiction research. Addiction, 1999. 94(3): p. 327–40. [PubMed] [Google Scholar]
- 4.Taber KH, et al. , Neuroanatomy of dopamine: reward and addiction. J Neuropsychiatry Clin Neurosci, 2012. 24(1): p. 1–4. [DOI] [PubMed] [Google Scholar]
- 5.Garcia Pardo MP, et al. , Animal models of drug addiction. Adicciones, 2017. 29(4): p. 278–292. [DOI] [PubMed] [Google Scholar]
- 6.LaLumiere RT and Kalivas PW, Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci, 2008. 28(12): p. 3170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Fardon R and Weiss F, Perseveration of craving: effects of stimuli conditioned to drugs of abuse versus conventional reinforcers differing in demand. Addict Biol, 2017. 22(4): p. 923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moussawi K, et al. , Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci, 2011. 5: p. 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danbolt NC, Glutamate uptake. Prog Neurobiol, 2001. 65(1): p. 1–105. [DOI] [PubMed] [Google Scholar]
- *10.Murphy-Royal C, et al. , Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat Neurosci, 2015. 18(2): p. 219–26.Here the authors use a combination of high-resolution imaging and electrophysiological approaches to assess the impact of GLT-1 surface dynamics on excitatory transmission. They first examine GLT-1 surface diffusion on rat hippocampal astrocytes in culture using quantum dot tracking and find that GLT-1 localizes near synaptic sites. The use of nanometer-sized particles to track GLT-1 diffusion in this study permits visualization over relatively short distances within and outside of the synaptic compartment, an advance over previous studies. Interestingly, glutamate uncaging increases GLT-1 diffusion away from glutamate release sites and impairing its displacement prolongs post-synaptic excitation. The authors conclude that surface diffusion of GLT-1 allows access of unbound transporters to synaptic glutamate and is an important factor in maintaining synaptic glutamate homeostasis.
- 11.Diamond JS and Jahr CE, Transporters buffer synaptically released glutamate on a submillisecond timescale. J Neurosci, 1997. 17(12): p. 4672–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clements JD, et al. , The time course of glutamate in the synaptic cleft. Science, 1992. 258(5087): p. 1498–501. [DOI] [PubMed] [Google Scholar]
- 13.Wadiche JI, et al. , Kinetics of a human glutamate transporter. Neuron, 1995. 14(5): p. 1019–27. [DOI] [PubMed] [Google Scholar]
- 14.Benediktsson AM, et al. , Neuronal activity regulates glutamate transporter dynamics in developing astrocytes. Glia, 2012. 60(2): p. 175–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewerenz J, et al. , The cystine/glutamate antiporter system x(c)(−) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal, 2013. 18(5): p. 522–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih AY, et al. , Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci, 2006. 26(41): p. 10514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bridges R, et al. , Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling. Pharmacol Rev, 2012. 64(3): p. 780–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker DA, Shen H, and Kalivas PW, Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids, 2002. 23(1-3): p. 161–2. [DOI] [PubMed] [Google Scholar]
- 19.Pow DV, Visualising the activity of the cystine-glutamate antiporter in glial cells using antibodies to aminoadipic acid, a selectively transported substrate. Glia, 2001. 34(1): p. 27–38. [DOI] [PubMed] [Google Scholar]
- 20.Sagara JI, Miura K, and Bannai S, Maintenance of neuronal glutathione by glial cells. J Neurochem, 1993. 61(5): p. 1672–6. [DOI] [PubMed] [Google Scholar]
- 21.Baker DA, et al. , The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci, 2002. 22(20): p. 9134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran MM, et al. , Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci, 2005. 25(27): p. 6389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Chai H, et al. , Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron, 2017. 95(3): p. 531–549 e9.In this study, the authors combine an impressive array of techniques, including RNA-seq, chemogenetics, electrophysiology, immunohistochemistry, Ca2+ and glutamate visualization, mass spectrometry, as well as 3D reconstruction of serial electron micrographs to compare characteristics of astrocytes in the hippocampus (CA1) and dorsolateral striatum of adult mice. Notable differences were observed in the morphology of astrocytes from the two brain regions, with striatal astrocytes being larger and thus covering a greater number of neuronal somata, but generally more distally apposed to synapses compared with astrocytes in the hippocampus. The authors demonstrate the high degree of variability in astrocyte proximity to striatal synapses. Interestingly, that data illustrate that striatal astrocytes are perhaps most proximal, but also most variable near mushroom-type spines.
- *24.Octeau JC, et al. , An Optical Neuron-Astrocyte Proximity Assay at Synaptic Distance Scales. Neuron, 2018. 98(1): p. 49–66 e9.Here the authors introduce a neuron-astrocyte proximity assay (NAPA) that combines confocal co-registration and FRET detection of astrocyte and synaptic markers. Using this methodology, the authors are able to compare the proximity of astrocytes to different terminal types (excitatory vs. TH-positive) originating in different brain regions (cortex, thalamus, substantia nigra, and local collaterals) as well as distinct post-synaptic targets (D1- vs. D2-MSNs). The result is a veritable wealth of information on synaptic insulation by astrocytes crucial for subsequent analyses on normal or pathological conditions that may alter these measures. The authors are able to show that the nearest astroglial process is <10 nm from roughly half of striatal synapses, while the remaining half of striatal synapses are 10-400 nm from the most proximal astrocyte process, a distinction that would have been missed by both confocal microscopy alone as well by traditional 2D electron microscopy. Part of this variability may arise from their finding that astrocytes more closely associate with excitatory synapses compared with TH-positive terminals.
- 25.Bernardinelli Y, Muller D, and Nikonenko I, Astrocyte-synapse structural plasticity. Neural Plast, 2014. 2014: p. 232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavialle M, et al. , Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc Natl Acad Sci U S A, 2011. 108(31): p. 12915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehre KP and Rusakov DA, Asymmetry of glia near central synapses favors presynaptically directed glutamate escape. Biophys J, 2002. 83(1): p. 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts-Wolfe DJ and Kalivas PW, Glutamate Transporter GLT-1 as a Therapeutic Target for Substance Use Disorders. CNS Neurol Disord Drug Targets, 2015. 14(6): p. 745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knackstedt LA, et al. , The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry, 2009. 65(10): p. 841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim R, et al. , Regulation of glutamate transporter 1 (GLT-1) gene expression by cocaine self-administration and withdrawal. Neuropharmacology, 2018. 128: p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reissner KJ, et al. , Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol, 2015. 20(2): p. 316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaCrosse AL, et al. , Contrasting the Role of xCT and GLT-1 Upregulation in the Ability of Ceftriaxone to Attenuate the Cue-Induced Reinstatement of Cocaine Seeking and Normalize AMPA Receptor Subunit Expression. J Neurosci, 2017. 37(24): p. 5809–5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duailibi MS, et al. , N-acetylcysteine in the treatment of craving in substance use disorders: Systematic review and meta-analysis. Am J Addict, 2017. 26(7): p. 660–666. [DOI] [PubMed] [Google Scholar]
- 34.Shen HW, et al. , Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci, 2014. 34(16): p. 5649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moussawi K and Kalivas PW, Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol, 2010. 639(1–3): p. 115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scofield MD, et al. , Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biol Psychiatry, 2016. 80(3): p. 207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siemsen BM, et al. , Effects of Methamphetamine Self-Administration and Extinction on Astrocyte Structure and Function in the Nucleus Accumbens Core. Neuroscience, 2019. 406: p. 528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *38.Kruyer A, et al. , Heroin Cue-Evoked Astrocytic Structural Plasticity at Nucleus Accumbens Synapses Inhibits Heroin Seeking. Biol Psychiatry, 2019.The authors demonstrate that heroin self-administration and withdrawal reduces astrocyte proximity at NAcore synapses when compared with yoked saline or sucrose trained rats. Cues predictive of heroin delivery restore proximity of astrocytes to striatal synapses in heroin-trained animals and blocking this re-association enhances reinstatement of heroin seeking. The authors conclude that NAcore astrocytes are poised to respond to glutamate release triggered by drug cues with transient morphological plasticity that attenuates relapse.
- 39.Shen H, et al. , Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci U S A, 2011. 108(48): p. 19407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gipson CD, et al. , Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A, 2013. 110(22): p. 9124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henneberger C B. L; Panatier A; Reynolds JP; Medvedev NI; Minge D; Herde MK; Anders S; Kraev I; Zheng K; Jensen T; Sanchez-Romero I; Janovjak H; Ottersen OP; Nagelhus EA; Oliet SHR; Stewart MG; Nagerl UV; Rusakov DA, Astroglia withdraw from potentiated synapses boosting inter-synaptic cross-talk. bioRxiv, 2018. [Google Scholar]
- 42.Gipson CD, et al. , Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron, 2013. 77(5): p. 867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith ACW, et al. , Accumbens nNOS Interneurons Regulate Cocaine Relapse. J Neurosci, 2017. 37(4): p. 742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kruyer A K. PW, Heroin Cues Reveal Astroglial Heterogeneity in the Nucleus Accumbens Core. bioRxiv, 2020. [Google Scholar]
- *45.Scofield MD, et al. , Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol Psychiatry, 2015. 78(7): p. 441–51.Here the authors drive Gq-DREADD expression in astrocytes of the NAcore and find that its stimulation prior to reinstatement not only suppresses reinstated lever pressing for cocaine (but not sucrose), but also results in astroglial glutamate release. They ultimately show that astroglial glutamate release in this case stimulates autoinhibitory mGluR2/3 expressed on presynaptic terminals, suppressing further synaptic glutamate release.
- 46.Corkrum M, et al. , Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron, 2020. 105(6): p. 1036–1047 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Derouiche A and Frotscher M, Peripheral astrocyte processes: monitoring by selective immunostaining for the actin-binding ERM proteins. Glia, 2001. 36(3): p. 330–41. [DOI] [PubMed] [Google Scholar]
- 48.Derouiche A and Geiger KD, Perspectives for Ezrin and Radixin in Astrocytes: Kinases, Functions and Pathology. Int J Mol Sci, 2019. 20(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Covelo A and Araque A, Neuronal activity determines distinct gliotransmitter release from a single astrocyte. Elife, 2018. 7.Here the authors seek to understand whether astrocytes are tuned to respond to neural activity using a single gliotransmitter, or whether each astrocyte can instead signal through multiple gliotransmitters, depending on local neural activity. They focus their study on the CA3-CA1 hippocampal synapse and find that following either depolarization or high frequency stimulation, interneurons signal to astrocytes, which then release glutamate or ATP/adenosine to trigger synaptic potentiation or depression, respectively. Moreover, the authors show that a single neurotransmitter, GABA in this case, which is released at low levels from interneurons following low frequency or short duration stimulation, and at higher levels after high frequency or prolonged stimulation, can trigger a single astrocyte to releasing glutamate alone or glutamate and ATP/adenosine, respectively. The release of glutamate and ATP/adenosine serves to stimulate synaptic potentiation followed by synaptic depression. Altogether, the authors beautifully demonstrate that astroglia are quite flexible in their capacity to respond to local neurotransmission and that their flexibility allows them to contribute to a range of synaptic outcomes.
- 50.Durkee CA and Araque A, Diversity and Specificity of Astrocyte-neuron Communication. Neuroscience, 2019. 396: p. 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D'Ascenzo M, et al. , mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A, 2007. 104(6): p. 1995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bull C, et al. , Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology, 2014. 39(12): p. 2835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernardinelli Y, et al. , Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr Biol, 2014. 24(15): p. 1679–88. [DOI] [PubMed] [Google Scholar]
- 54.Martin R, et al. , Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science, 2015. 349(6249): p. 730–4. [DOI] [PubMed] [Google Scholar]
- 55.Testen A, et al. , Region-Specific Reductions in Morphometric Properties and Synaptic Colocalization of Astrocytes Following Cocaine Self-Administration and Extinction. Front Cell Neurosci, 2018. 12: p. 246. [DOI] [PMC free article] [PubMed] [Google Scholar]