Abstract
Background
Isolated abdominal dissection (IAD) is an uncommon clinical problem that is less well understood than thoracic aortic dissection. We performed a population based assessment of the incidence, natural history and treatment outcomes of IAD to better characterize this disease.
Methods
We utilized the Rochester Epidemiology Project to identify all Olmsted County, MN residents with a diagnosis of aortic dissection, intramural hematoma or penetrating ulcer (1995–2015). Diagnostic imaging of all patients was reviewed to confirm the diagnosis of IAD for inclusion. Presentation, treatment, and outcomes were reviewed. Survival of IAD patients was compared to age- and sex-matched population controls 3:1.
Results
Of 133 residents with aortic syndrome (aortic dissection, intramural hematoma, or penetrating ulcer), 23 were initially diagnosed with IAD. Nine were reclassified as PAU and excluded, leaving 14 patients for review (10 male (71%), mean age 71 years). Three patients (21%) were symptomatic (abdominal/back pain/ hypertension) and none had malperfusion/rupture. Prior aortic dilatation was present in 8 (57%) and Marfan syndrome in 1 (7%). Two (14%) patients had iatrogenic IAD. Initial management was medical in 13 and EVAR in one (symptomatic subacute, infrarenal dissection with small aneurysm). The median clinical and imaging follow-up was 6.7 (range 0–17 years). An abdominal aortic aneurysm occurred in 8 (6 at the time of IAD diagnosis, one at 2.9 years, and one at 5.2 years after diagnosis). The average growth in the entire cohort was 0.9±0.4 cm, which translated to an average growth rate of 0.09 cm/year. Subsequent intervention was performed in 2; for severe aortic stenosis with claudication in one (infrarenal aortic stenting) and increasing aortic size in one (open repair). One patient required re-intervention (thrombolysis and stenting for EVAR limb thrombosis). Survival for IAD at 1, 3, and 5 years was 93%, 85% and 76% compared with population controls at 98%, 85% and 71% respectively (long rank p= 0.38). Mortality was due to cardiovascular causes in 3 (21%) and no deaths were aortic related. Major adverse cardiac events occurred in 5 (36%) due to heart failure.
Conclusions
Isolated abdominal dissection is rare. Initial management for asymptomatic patients is medical. The aortic growth rate is slow, with no aortic related mortality and a low rate of aortic intervention. Overall mortality is similar to population controls. Heart failure and cardiac related death are prevalent, suggesting close cardiovascular care is needed in this patient population.
Keywords: Aortic dissection, isolated abdominal aortic dissection, aneurysm, mortality, natural history
Table of Contents Summary
In this retrospective population-based study 14 patients had Isolated abdominal dissection (IAD) only three (21%) were symptomatic and none had malperfusion or rupture. Aortic growth rate was slow, averaged f 0.09 cm/year, 2 patients required intervention. There was no late aortic related mortality and overall mortality was similar to population controls.
Introduction
Isolated abdominal dissection (IAD) is a rare diagnosis. Among patients within the International Registry of Acute Aortic Dissection (IRAD), IAD was diagnosed in only 1.3% of patients (18/1417) (1). In comparison, the overall incidence of thoracic aortic dissection is 4.4 per 100 000 person-years. (2, 3) Recent systematic reviews by Wu and Liu et al identified less than 600 cases of IAD worldwide that have been presented as case series and reports (4–10). None of these prior studies are population-based or community based epidemiological surveys investigating the incidence and natural history of IAD. These reports are likely to represent reporting or referral bias by larger centers in the cases published. Thus, a comprehensive picture of all IAD cases is lacking. Rare diseases (typically affecting populations smaller than 200,000 individuals in the United States) can be a major public health issue(11, 12). Paucity of population-based data on rare diseases limits an assessment of the true health burden of the disease (13). This problem with studying management and outcomes of uncommon conditions can be tackled by multiple approaches. Firstly, epidemiological population based studies can generate estimates of prevalence in a geographic area using life-time population data. This is important information about their natural history that cannot be obtained from other sources. As small numbers are an obvious limitation, this information can then be utilized along with other databases/ registries like the vascular low frequency disease consortium to generate larger sample sizes to further improve the quality of evidence(14). The aim of this study was to analyze a population-based incident cohort of IAD and to assess the basic profile, risk factors, and imaging characteristics, natural history and treatment outcomes of the patients.
Methods
Data sources
This study was performed utilizing the Rochester Epidemiology Project (REP), a medical record linkage system that includes all residents and local health care providers in Olmsted County, Minnesota. As previously described in more detail, the database has been utilized to analyze the incidence of aortic syndromes (AS) in Olmsted County, Minnesota from 1995–2015 (3). All adult (age ≥ 18 years) residents with an incident diagnosis of aortic dissection (AD), intramural hematoma (IMH), and penetrating aortic ulcer (PAU) were identified from the REP using the International Classification of Disease (ICD), 9th and 10th revision, codes and Hospital Adaptation of the ICD, 2nd edition, codes. CT imaging was reviewed to confirm the diagnosis of IAD. IAD was defined as an isolated intimal tear within the abdominal aorta, not associated with thoracic pathology (such as dissection or IMH). Cases with unclear imaging diagnosis were reviewed with a vascular radiologist for final determination. IAD was defined as acute if symptomatic and diagnosed and/or treated within 14 days of the onset of symptoms. It was defined as sub-acute between 2 weeks upto 3 months and chronic after 3 months. Maximum aortic size was calculated based on CT imaging and the final aortic diameter change was calculated as the difference in aortic size between the index scan and last available scan. Clinical course and outcomes were reviewed. Mortality was assessed by review of death certificates for decedents and categorized as aortic related (rupture, ischemic complications, surgical complications), cardiovascular related (myocardial infarction [MI], heart failure [HF] or stroke) or from other causes. Charts of all IAD cases and population controls were reviewed. Imaging, procedures, diagnoses, major adverse cardiac cardiovascular events (new onset MI, HF or stroke) and hospitalizations were recorded for analysis.
Aortic aneurysm (abdominal) was defined as a largest transverse aortic diameter ≥ 3 cm, ≥ 4.5 cm for the ascending aorta (aortic valve to innominate artery), ≥ 4 cm for the aortic arch (innominate artery to left subclavian artery) and ≥ 3.7 cm for the descending aorta (left subclavian artery to diaphragm. Abnormal aortic diameter < 3 cm were recorded as aortic dilatation. The institutional review boards of Mayo Clinic and the Olmsted Medical Center, the two major health care providers within the REP, approved the present study as a minimal risk study that waived the requirement for a study specific consent. All individuals included in the present study had previously provided a general written informed consent for the use of their medical records in research according to Minnesota statutes. No patients with IAD were excluded because of lack of research authorization.
Statistical analysis
Summary statistics, including median (range), mean (standard deviation) and frequencies (percentages) were used to describe the baseline characteristics and descriptive outcomes. Patients were matched 3:1 to random age/sex population controls to detect a minimal HR for death of 1.95 with an alpha or 0.05 and power of 0.8. Survival was evaluated as time to event using life tables and Kaplan-Meier plots with log rank test to assess differences. Statistical analyses were performed using STATA (StataCorp, College Station, Tex) and SAS software (SAS Institute Inc., Cary, NC).
Results
Study cohort
Of 133 identified residents with AS (77 AD, 21 IMH, 35 PAU), fourteen patients (10%) were confirmed to have IAD [10 males (71%), median age 73 (range 44–90) years. Nine other patients initially diagnosed to have IAD were reclassified as PAU after imaging review and were excluded. Three patients (21%) were symptomatic. Two were acute with abdominal pain in one, back pain in one, and one was subacute with abdominal pain and hypertension. In those who were asymptomatic, imaging was performed for symptoms eventually diagnosed to be due to unrelated pathology. None had malperfusion or rupture on presentation. Risks factors for dissection included prior aortic dilatation in 8 (57%) and Marfan syndrome in 1 (7%). Those with aortic dilation had undergone prior imaging of the thoracic aorta for evaluation of unrelated conditions. None had family history of aortic dissection/ aneurysm. Two (14%) patients had an iatrogenic dissection, one from renal artery stenting (this patient was symptomatic) and one from transaortic bilateral renal artery endarterectomy (detected on routine follow-up imaging). Aneurysmal dilatation of the aortic root was present in 1 (7%, this had been diagnosed and repaired a year before the diagnosis of IAD in the patient with Marfan syndrome). None of the patients had a concomitant ascending/ arch/ descending thoracic aneurysm or dissection. The dissection flap was infrarenal in all patients. The average largest transverse abdominal aortic diameter at diagnosis was 2.6 ± 0.8 cm and dissection flap length was 6.4 ± 3.3 cm.
Early outcomes
The initial management was medical in 13 cases. At diagnosis, six patients (43%) presented with concomitant small infrarenal aortic aneurysms, with a size range of 3.1 −4.3 cm, four of these were managed conservatively. At presentation, endovascular aneurysm repair (EVAR, Medtronic AneuRx, 26×15×16.5 device) was performed in 1 symptomatic patient as primary treatment (subacute, infrarenal dissection with a small aneurysm, Fig 1). The early postoperative course was uncomplicated. One 56-year-old male presented with a rapid increase in size of his aortoiliac aneurysm (3.2cm to 4.2 cm in 6 months) and was then treated with open repair (Fig 2). One patient died one month after the diagnosis of an iatrogenic IAD due to pneumonia following prolonged hospitalization secondary to medical comorbidities after renal artery stenting). The overall 30-day and 90-day mortality rate was 0% and 7 % (Fig 1, supplemental).
Fig 1:
Subacute, infrarenal dissection with a small aneurysm treated by Endovascular aneurysm repair (EVAR, Medtronic AneuRx, 26×15×16.5 device)
Fig 2:
Open repair of an aortoiliac dissection with a metal probe is in the true channel with an aorto-right common-left external iliac artery bifurcated polyester Gelsoft (Vascutek graft), and follow up angiogram
Late outcomes
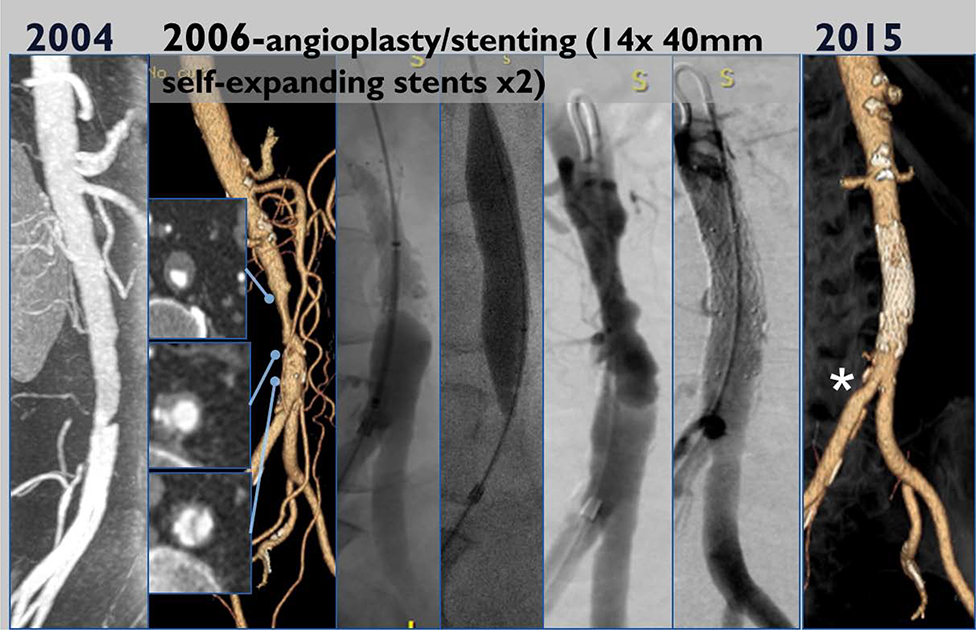
The median clinical and imaging follow-up was 6.7 years (range 0–17 years). Imaging follow up ≥12 months after the index event (i.e. initial diagnosis or first intervention) of the abdominal aorta was available in all survivors (n=13). The total re-intervention rate was 33%. This was an iliac limb thrombosis at 1 year in the patient who underwent EVAR requiring thrombolysis and stenting, with no subsequent readmission/ reintervention after this at 17 years of follow up. The patient treated with open repair required no further re-intervention at 15 year follow up. Thrombosis of the false lumen occurred in 1 patient at 6 months after diagnosis of IAD. This 74-year-old female presented with lifestyle limiting claudication and was treated with infrarenal aortic stenting (two Protégé 14 × 40 mm self-expandable stents, Fig 3). She subsequently did well with no further re-intervention at last (8-year) follow up.
Fig 3:
Infrarenal aortic stenting for aortic stenosis (two Protégé 14 × 40 mm self-expandable stents) and follow up angiogram
Abdominal aortic diameter increased in 5 patients (36%) and remained stable in 5 (including 2 with a small aneurysm). The average growth in the entire cohort was 0.9±0.4 cm, which translated to an average growth rate of 0.09 cm/year. If the single patient whose aorta-iliac aneurysm increased in size by 1 cm. in 6 months were to be excluded, the growth rate of the abdominal aorta would be exceedingly small. Two patients without an aneurysm at presentation reached aneurysmal diameters (one at 2.9 years, and one at 5.2 years). Two of four small aortic aneurysms (including the patient with Marfan syndrome) demonstrated minimal growth, remained asymptomatic and did not require intervention. Imaging follow up for the thoracic aorta was available in 10 patients with a median follow up of 5 (1– 14) years. One patient with a descending thoracic diameter of 3.5 cm at the time of diagnosis of IAD progressed to become aneurysmal: 4.1 cm at 5-year follow-up. Another patient with a mild dilatation of the sinus of Valsalva of 4.2cm (initially considered normal when adjusted for body surface area, but kept on follow up due to family history of dissection), progressed to 4.4 cm in 12 years thereafter remained stable at 7 follow-up. Major adverse cardiac events occurred in 5 (36%) patients, at a median of 3 (1–7) years all with the development of heart failure. Freedom from major cardiovascular events after IAD compared to population controls at 5 years was 76% vs. 71% (log rank p=0.1). Mortality was due to cardiovascular causes in 3 patients (21%) and no deaths were aortic related. Survival after IAD at 1, 3 and 5 years was 93%, 85% and 76% compared with their population controls of 98%, 85% and 71% respectively (Fig 4, log rank p=0.38).
Fig 4:
Kaplan Meier analysis: Survival in patients with IAD compared with their population controls.
Discussion
Aortic dissection localized to the abdominal aorta is a rare diagnosis. Using a population based approach; we identified a cohort of 14 patients among 133 with newly diagnosed aortic syndromes (AD, IMH or PAU), representing only 10% of observed AS events over a 20 year period. This is the first population based epidemiological report of patients with IAD to date and our data highlight several insights into this uncommon patient population. Most patients remain asymptomatic, concomitant aortic aneurysms are prevalent, probably due to the underlying pathology that resulted in the dissection. However, the growth rate of these aneurysms is slow and late aortic related events are uncommon. In addition, cardiovascular mortality and heart failure are common, occurring in a third of patients. Despite these medical events, we observed a similar mortality in IAD patients compared to age and sex matched population controls.
We have previously published our results on the overall stable incidence of aortic dissection. (3, 15) The age- and sex-adjusted incidence of AS in this cohort was 7.7 per 100 000 person-years(3) Other authors also published similar stable rates based on autopsy studies (16) The 10% prevalence of IAD in our study is higher than the estimated 0.4% to 2% of all aortic dissections reported previously (7, 10, 17, 18). Two other reports, one from Greece (19) and another from South Korea(20), reported a greater than 10% incidence which is similar to our study but these were hospital based series, limiting direct comparisons to a population based report. It is possible that the true prevalence of IAD is underreported as asymptomatic patients are managed conservatively.
Overall, clinical and imaging characteristics among our cohort are similar to previous reports. Most clinical series have reported a male predominance of largely asymptomatic patients or with non-specific symptoms and no obvious clinical signs on examination (7, 21, 22). A recent systematic review that identified 491 patients (65 from North America) with IAD from hospital based series or registries worldwide also reported similar findings. Rare presentations like transient spinal ischemia have been reported but this is exceedingly uncommon (23, 24). Traditional atherosclerotic risk factors like hypertension are prevalent in those with IAD. Connective tissue disorders and a positive family history were rare, probably due to our defined population and geography.
Our data indicates that preexistent aortic dilatation is common but and subsequent aneurysmal degeneration is slow. There was limited need for late aortic related intervention. Faries et al. report that concomitant aortic aneurysms occur in 48.6% (14/37 patients) with an aneurysm growth rate of 1.2 mm/ year. (22) The growth rate in our study was lower (0.9mm/year). Trimarchi et al. advise aggressive surgical or endovascular management as half the long term deaths in their series were due to dissection related events.(1) We did not find a similar association in our study, probably as most of our patients did not have an acute presentation. Aortic dilatation was included as this is considered a risk factor for thoracic aortic dissection associated with connective tissue disorders. However, in our study, majority of those with aortic dilatation and IAD did not have connective tissue disorders. Thus, it is possible that aortic dilatation was a marker of the overall degenerative process and change in tissue characteristics affecting the aorta in this subgroup of patients. Our study would not be able to assess if aortic dilatation was a true risk factor for IAD.
We recommend that symptomatic patients may be treated early as they have good long-term outcomes. In our study, aortic size increased in 36%, this was higher than the Kang et al. who report an 8.7% rate of false lumen enlargement in 210 patients with IAD. They also advise intervention for ruptures, large concomitant aortic aneurysms, or underlying connective tissue disease with consideration of early repair in females, symptomatic patients or those with suprarenal IAD to reduce aorta related mortality (20). The one patient with Marfan syndrome in our study was managed conservatively. Although the overall rate of aortic growth was higher, we did not observe a corresponding increase in aortic related mortality or aortic rupture. This is probably reflective of the absence of the above poor prognostic factors in our patient cohort. The overall survival and freedom from major cardiovascular events were similar to population controls. This is significantly different from patients with aortic dissection, which is associated with a significantly increased risk of aortic and cardiovascular mortality and morbidity (15). Although our sample size was small, we feel that IAD, especially in asymptomatic patients with smaller aortic sizes has a better long term prognosis than thoracic dissection. These findings are consistent with a recent meta-analysis showing that appropriate initial treatment strategies (including conservative management, endovascular intervention or open repair) can all obtain acceptable clinical outcomes (7, 10). However, regular imaging surveillance and close cardiovascular care should be provided for these patients until more conclusive evidence on the benign course of this pathology is reached.
Our study has several limitations. Our overall number of patients with IAD is small, and the event rates are low. This limits our ability to draw robust clinical conclusions or analyze factors that would predict aneurysm growth or need for intervention. In addition, although our sample is a population based cohort, the demographics of Olmsted County, Minnesota may not be representative of the entire US. However, the data from Olmsted County have been shown to represent the demographics regionally in the Midwest. Imaging protocols and follow up frequency was not standardized, and imaging of the thoracic aorta was not available in all patients. Lastly, our review was retrospective and treatment determined by the individual providers over a 20 year period. However, our study is strengthened by its epidemiological foundation and is inclusive of all patients regardless of age or insurance and the clinical verification of all coding diagnoses.
Conclusion
Isolated abdominal dissection is rare. Initial management for asymptomatic patients is medical. The aortic growth rate is slow, with no late aortic related mortality in this cohort and a low rate of aortic intervention. Overall mortality is similar to population controls. Heart failure and cardiac related death are prevalent, suggesting close cardiovascular care is needed in this patient population.
Supplementary Material
Article Highlights.
Type of research
Retrospective population-based cohort study
Key findings
Of 14 patients with isolated abdominal dissection (IAD), three (21%) were symptomatic and none had malperfusion or rupture. Aortic growth rate was slow, averaged f 0.09 cm/year, only 2 patients required intervention. There was no late aortic related mortality and overall mortality was similar to population controls, secondary to heart failure and cardiac causes.
Take home message
Isolated abdominal dissection is rare, in 14 patients the growth rate of the aorta was 0.09 cm/year. Long-term prognosis of patients with IAD is better than those with thoracic dissection.
Acknowledgments
Sources of funding: This study was supported by the American Heart Association (16SDG27250043). It was conducted using the resources of the Rochester Epidemiology Project, which is supported by the National Institutes of Health National Institute on Aging under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Data storage was performed with REDCap (UL1TR002377)
Conflict of Interest: Gustavo S. Oderich: Consulting fees, research grants from Cook Medical Inc., WL Gore, GE Healthcare (All paid to Mayo), no relation to this paper; Other co-authors: no conflicts
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trimarchi S, Tsai T, Eagle KA, Isselbacher EM, Froehlich J, Cooper JV, et al. Acute abdominal aortic dissection: insight from the International Registry of Acute Aortic Dissection (IRAD). Journal of vascular surgery. 2007;46(5):913–9. [DOI] [PubMed] [Google Scholar]
- 2.Clouse WD, Hallett JW Jr., Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clinic proceedings. 2004;79(2):176–80. [DOI] [PubMed] [Google Scholar]
- 3.DeMartino RR, Sen I, Huang Y, Bower TC, Oderich GS, Pochettino A, et al. Population-Based Assessment of the Incidence of Aortic Dissection, Intramural Hematoma, and Penetrating Ulcer, and Its Associated Mortality From 1995 to 2015. Circ Cardiovasc Qual Outcomes. 2018;11(8):e004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shu C, Liu Z, Li Q, Li X, Li M, Wang L. Endovascular treatment of isolated abdominal aortic dissection. J Cardiovasc Surg (Torino). 2018;59(3):490–2. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Ma T, Guo D, Xu X, Chen B, Jiang J, et al. Endovascular treatment of acute and chronic isolated abdominal aortic dissection. Vascular. 2018;26(4):418–24. [DOI] [PubMed] [Google Scholar]
- 6.Zhou M, Cai H, Li Z, Zhang Y, Liu Z, Tang H, et al. Contemporary Results of Endovascular Repair of Isolated Abdominal Aortic Dissection with Unibody Bifurcated Stent Grafts. Ann Vasc Surg. 2018;49:99–106. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Zafar M, Qiu J, Huang Y, Chen Y, Yu C, et al. A systematic review and meta-analysis of isolated abdominal aortic dissection. Journal of vascular surgery. 2019. [DOI] [PubMed] [Google Scholar]
- 8.Farber A, Lauterbach SR, Wagner WH, Cossman DV, Long B, Cohen JL, et al. Spontaneous Infrarenal Abdominal Aortic Dissection Presenting as Claudication: Case Report and Review of the Literature. Annals of Vascular Surgery. 2004;18(1):4–10. [DOI] [PubMed] [Google Scholar]
- 9.Jonker FH, Schlosser FJ, Moll FL, Muhs BE. Dissection of the abdominal aorta. Current evidence and implications for treatment strategies: a review and meta-analysis of 92 patients. J Endovasc Ther. 2009;16(1):71–80. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Han M, Zhao J, Kang L, Ma Y, Huang B, et al. Systematic Review and Meta-analysis of Current Literature on Isolated Abdominal Aortic Dissection. European Journal of Vascular and Endovascular Surgery. 2020;59(4):545–56. [DOI] [PubMed] [Google Scholar]
- 11.López-Bastida J, Oliva-Moreno J. Cost of illness and economic evaluation in rare diseases. Adv Exp Med Biol. 2010;686:273–82. [DOI] [PubMed] [Google Scholar]
- 12.https://www.govinfo.gov/content/pkg/PLAW-107publ280/html/PLAW-107publ280.htm. RARE DISEASES ACT OF 2002. 1988.
- 13.Nguengang Wakap S, Lambert DM, Olry A, Rodwell C, Gueydan C, Lanneau V, et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. European Journal of Human Genetics. 2020;28(2):165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence PF, Baril DT, Woo K. Investigating uncommon vascular diseases using the Vascular Low Frequency Disease Consortium. Journal of Vascular Surgery. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss S, Sen I, Huang Y, Killian JM, Harmsen WS, Mandrekar J, et al. Cardiovascular morbidity and mortality after aortic dissection, intramural hematoma, and penetrating aortic ulcer. Journal of vascular surgery. 2019;70(3):724–31.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huynh N, Thordsen S, Thomas T, Mackey-Bojack SM, Duncanson ER, Nwuado D, et al. Clinical and pathologic findings of aortic dissection at autopsy: Review of 336 cases over nearly 6 decades. Am Heart J. 2019;209:108–15. [DOI] [PubMed] [Google Scholar]
- 17.Beigi AA, Samani RE. Acute spontaneous isolated dissection of abdominal aorta. J Res Med Sci. 2009;14(5):323–5. [PMC free article] [PubMed] [Google Scholar]
- 18.Mozes G, Gloviczki P, Park WM, Schultz HL, Andrews JC. Spontaneous dissection of the infrarenal abdominal aorta. Semin Vasc Surg. 2002;15(2):128–36. [DOI] [PubMed] [Google Scholar]
- 19.Kouvelos GN, Vourliotakis G, Arnaoutoglou E, Papa N, Avgos S, Peroulis M, et al. Endovascular treatment for isolated acute abdominal aortic dissection. Journal of vascular surgery. 2013;58(6):1505–11. [DOI] [PubMed] [Google Scholar]
- 20.Kang JH, Kim YW, Heo SH, Woo SY, Park YJ, Kim DI, et al. Treatment strategy based on the natural course of the disease for patients with spontaneous isolated abdominal aortic dissection. Journal of vascular surgery. 2017;66(6):1668–78.e3. [DOI] [PubMed] [Google Scholar]
- 21.Becquemin JP, Deleuze P, Watelet J, Testard J, Melliere D. Acute and chronic dissections of the abdominal aorta: clinical features and treatment. Journal of vascular surgery. 1990;11(3):397–402. [PubMed] [Google Scholar]
- 22.Faries CM, Tadros RO, Lajos PS, Vouyouka AG, Faries PL, Marin ML. Contemporary management of isolated chronic infrarenal abdominal aortic dissections. Journal of vascular surgery. 2016;64(5):1246–50. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed M Acute infra-renal aortic dissection presenting as back pain and transient paralysis of the lower limbs. Int J Surg Case Rep. 2012;3(2):39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holper P, Hyhlik-Durr A, Kotelis D, von Tengg-Kobligk H, Bockler D. Paraplegia after spontaneous dissection of the abdominal aorta. Vasa. 2009;38(3):254–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.