Abstract
Almost 50% of infertility cases are due to male factors, and spermatogenesis failure is one of the most severe forms of male infertility. Sertoli cell-only syndrome (SCOS) also known as germ cell aplasia is characterized by azoospermia in which the seminiferous tubules of testicular biopsy are lined only with Sertoli cells. The definitive diagnosis of SCOS is by diagnostic testicular biopsy. Although SCOS may be a result of Klinefelter syndrome, most of the SCOS men have a normal karyotype. Along with genetic aberrations, signaling pathways and endocrine processes might be major factors in the development of SCOS. Sperm retrieval and intracytoplasmic sperm injection (ICSI) are available treatments for SCOS. However, some SCOS patients do not have therapeutic options to help them having a biological child. This review aims to summarize our present knowledge about SCOS and to highlight the importance of future researches in the diagnosis and treatment of this disorder.
Keywords: Sertoli cell only syndrome, SCOS, Germ cell aplasia, Azoospermia, Male infertility
Introduction
Infertility is considered a major public health issue of recent decades and affects about 15% of couples trying to conceive [1]. Approximately 50% of infertility cases are caused by a male factor [2], that often caused by spermatogenesis failures, problems related to sperm quality (e.g., morphology and/ or motility), and a reduced number of spermatozoa in the semen. Azoospermia, the complete absence of sperm in the ejaculate, is a more severe form of male infertility occurring in 10–15% of males seeking medical care for infertility [3]. Azoospermia can be classified as obstructive azoospermia (OA) and non-obstructive azoospermia (NOA). OA results from any obstruction along the male reproductive tract that prevents spermatozoa to reach the ejaculate, while NOA is characterized by severely impaired spermatogenesis due to endogenous or exogenous abnormalities. Based on histological evaluation of the testicular biopsy, NOA is classified into hypospermatogenesis (HS), maturation arrest (MA), and SCOS [4]. The prevalence of SCOS in azoospermic patients has been reported about 26.3–57.8% [5, 6]. There are various etiologies of human SCOS, which include Y chromosome microdeletions, chromosome disorders, undescended testis, radiation, cytotoxic drugs, and virus infection [7]. However, the causes of it have not been fully understood. SCOS is also known as germ cell aplasia and Del Castillo syndrome, which is characterized by azoospermia in which the seminiferous tubules of testicular biopsy samples are lined only with Sertoli cells. According to the initial description by Del Castillo in 1947, SCOS was characterized by the total absence of germ cells, Sertoli cells with a normal cytoplasm within seminiferous tubules, lack of histological degeneration in the testes, and reduced testicular volume with normal secondary sexual characteristics. The focal and complete SCOS is the two types of this disorder. The focal SCOS is characterized by residual areas of normal spermatogenesis within the testis. On the other hand, complete SCOS is identified with gonocytes that lose their way during migration to the embryonic gonads and non-formation of their germinal epithelium [8]. In the focal SCOS, spermatozoa could be retrieved from the testis by surgical procedures. By harvesting a single, viable sperm from the testis, in combination with ICSI, men with SCOS had this opportunity to father their genetically own offspring [9]. In this review, we present the research findings, including histopathology, genetics, epigenetics, signaling pathways, and endocrinology of SCOS, and focus on clinical management. The study of the molecular causes of SCOS has increased greatly in the past decade, enabling understanding of the pathophysiological mechanisms underlying the features of the disorder. This review aimed to provide an overview of the current knowledge regarding SCOS etiology and emphasize the need for future investigation for the management of this disorder.
Methods
PubMed and Google Scholar databases were systematically searched for peer-reviewed original articles identified by relevant keywords, such as “Sertoli cell only syndrome,” “SCOS,” “SCO,” “germ cell aplasia,” and “Del Castillo syndrome.” Keywords were used in multiple combinations to identify those papers relevant to the Sertoli cell-only syndrome. Further articles were identified by the exact analysis of reference lists from relevant publications. The non-English language articles were excluded, and only original articles published in English that were available online until the end of April 2020 were included in the search. The most relevant publications, for example, those concerning the molecular genetic basis of SCOS were assessed and discussed critically to offer a conclusion for the etiology of the disease. In total, 824 studies were identified through database searching following the search for the keywords. Titles and abstracts from the electronic databases were evaluated, and after full-text papers screening, 155 articles were included in the review. Concerning animal studies, priority was given to publications relevant to the human.
Histopathology
Histological diagnosis of SCOS is confirmed when the examined testicular biopsy sample reveals that all seminiferous tubules are lined by only Sertoli cells, without any germ cells. Because of the testicular tissue heterogeneity, observation of SCO histological pattern in one or more biopsies does not disprove the fact that focal spermatogenesis may exist in a different location within the testis [10]. Two distinct histological patterns of SCO were introduced: primary and secondary SCO [11]. The primary, congenital, or pure SCO is caused by a prenatal defect in the migration of gonocytes into the male gonads, resulting in sterility. On the other hand, secondary or mixed SCO occurs as a result of postnatal damage to the healthy testes that may result in a focal SCO pattern in testicular tissue. The difference between primary and secondary SCO based on the histological evaluation, include histology of seminiferous tubules walls, morphology and function of Sertoli cells, and the interstitial tissue appearance [10, 12]. In primary SCO, the diameter of seminiferous tubules was narrower than normal, with no thickening of the tubular wall, and no thickening of inner collagen layers was observed. Sertoli cells had ovoid or round nuclei with a regular outline and columnar cytoplasm that vimentin distributes in subnuclear basal areas similar to fetal Sertoli cells. On the contrary, in the secondary SCO, seminiferous tubules were small in diameter, tubular wall hyalinization and thickening with peritubular fibrosis, and irregular thickening of the inner collagen layer was observed. Normal Sertoli cells had cytological features similar to post-pubertal testis with irregularly shaped nuclei and diffuse cytoplasmic distribution of vimentin. In some tubules, germ cells were observed [11]. The thickened tubular wall of the seminiferous tubules may impair the relationship between the Sertoli cells population and the interstitial tissue and consequently, affect hormone permeability. This phenomenon may affect inhibin B and follicle-stimulating factor (FSH) levels in the peripheral blood [13]. The lipid content of Sertoli cells is different in two types of SCOS. There are many lipid granules in Sertoli cells cytoplasm of mixed SCOS form due to reabsorption of degenerated germ cells. However, pure SCOS contains a very small amount of lipid and glycogen [10].
It has been reported that four types of Sertoli cells exist in testicular biopsies of SCO tubules that can be identified with light and electron microscopy: (i) normal mature cells with an intended nucleus, triangular shape and with a prominent tripartite nucleolus, (ii) immature cells showing round regularly outlined nuclei and immature cytoplasm, which formed a pseudostratified epithelium similar to those of prepubertal testes (iii) dysgenetic cells with immature nuclei and almost mature cytoplasm with less-developed cytoplasmic organelles and (iv) involuting cells with very irregular outlined nuclei and a mature cytoplasm showing characteristic organelles of the normal mature Sertoli cells and atypical inter-Sertoli junctional specializations [12]. The state of differentiation of Sertoli cells can be determined by the presence or absence of Sertoli cell-specific maturation markers [14, 15].
Ooba et al. (2008) reported that the basement membrane thickness in patients with SCO was significantly greater and testicular tubule diameter was smaller than in those with OA. Besides, in patients with SCO, expression of an extracellular matrix component, laminin, was increased and an overabundance of laminin-specific chains in seminiferous tubules may result from inhibition of spermatogenesis. They also showed a significant decrease of seminal laminin in the SCO patients, so assessing seminal laminin levels could lead to the prediction of the SCO pathology in the infertile men [16].
Endocrinology
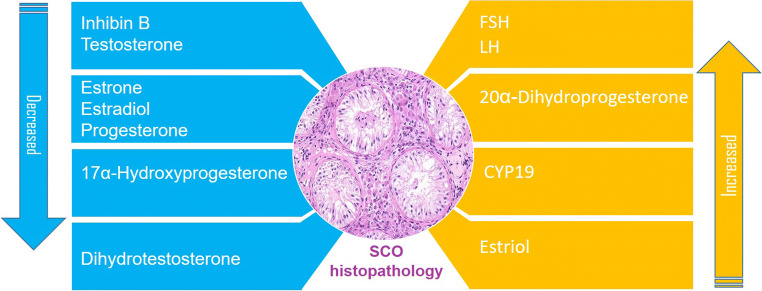
Abnormalities in the paracrine regulation of spermatogenesis may contribute to the SCOS pathophysiology. However, it has been reported a unique case of SCOS with normal gonadotropins [17]. Also, it has been reported a significant decrease in serum estrone, estradiol, progesterone, 17a-hydroxyprogesterone, and dihydrotestosterone, and a significant increase in serum estriol and testosterone (T) in men with SCOS [18]. A selective increase in FSH levels has been reported in SCOS [19]. FSH stimulates inhibin B secretion by Sertoli cells. This process also is modulated by interactions between Sertoli and germ cells [20]. It has been reported that in SCOS patients basal FSH serum levels were higher, and basal luteinizing hormone (LH) concentration was similar to normal adult males [21]. Endocrinological abnormalities in patients with SCOS were mild compared to those in patients with Klinefelter syndrome. Elevated levels of serum LH, as well as FSH and lowered levels of serum testosterone, suggested Leydig and germ cell failure [22]. In another study, the serum FSH and LH levels in patients with SCOS were significantly higher than in normal males. The elevated basal serum LH levels and normal serum testosterone levels indicated that there was a dysfunction in the Leydig cells in SCOS [23]. It has been reported that all SCOS patients presented with elevated serum FSH levels suggesting Sertoli dysfunction or reduced production of inhibin due to the absence of germ cells [24]. The hormonal profile of SCOS patients has been investigated. Elevated FSH level and decreased Inhibin B levels were observed in SCOS patients. The decrease in the number of functioning Sertoli cells and a large percentage of ghost tubules in SCO can explain the low level of Inhibin B. On the other hand, a thickened tubular wall of the seminiferous tubules probably impairs hormone permeability and levels in the peripheral blood [13].
It has been demonstrated that spermatogenic failure observed in SCOS patients cannot be explained by a change in FSH bioactivity or FSHR mutations [25]. Testicular FSH and hCG receptors were measured in SCOS patients. FSH receptors were found in only 4 of 10 SCOS patients, and human chorionic gonadotrophin (hCG) receptors were detected in all patients with SCOS. The serum levels of LH and FSH in the SCOS group were higher than those in the control group, the level of testosterone in the SCOS group was lower than the control group [26]. Androgen receptor (AR) immunoexpression in Sertoli and Leydig cells of SCOS was higher than spermatogenesis arrest and normal testis samples. This result was associated with the lack of germ cells in the testis and suggested that germ cells had a regulatory effect on AR expression [27]. It has been concluded that the progesterone receptor (PR) and estrogen receptor α (ERα) may play a role in the pathogenesis of the MA and SCO phenotype in infertile men. The expression of PR was reduced in all cell types, and only truncated isoform of PR (52 KDa) was expressed in SCO as compared with that in the OA patients. The expression of ERα was enhanced in the Leydig cells of the SCO testes [28].
Leydig cell dysfunction was found in patients with SCOS that may be due to a disturbing influence from the damaged tubules. 17α-hydroxylation was significantly lower, and the production of 20α-dihydroprogesterone was significantly higher compared to the control group [29]. Altered intra-testicular estradiol/testosterone ratio in some patients with complete SCOS suggests that P450-aromatase encoded by CYP19 is increased and might contribute to Leydig cell impairment [30]. Increased expression of aromatase in Leydig cells leads to higher E2 production and may account for the Leydig cell dysfunction in patients with SCOS [31].
An unusual case of insulinoma was reported that his testicular biopsy showed SCOS. This report emphasized clinicians’ attention to the possibility of an association between insulinoma and primary testicular failure [32]. Schematic representation of the hormone levels alternation in SCOS is presented in Fig. 1.
Fig. 1.
Schematic representation of the hormones level alternation in SCOS, based on available studies. Abnormalities in the hormonal regulation of spermatogenesis may contribute to the SCOS pathophysiology
Etiology
Even though SCOS was first described in 1947, its exact etiology remains enigmatic in some cases. SCOS may be a result of Klinefelter syndrome but most of the SCOS men have a normal karyotype, and some have smaller testis and higher levels of FSH [33, 34]. Along with genetic aberrations, signaling pathways and endocrine processes might be major factors in the development of SCOS. In this review, we present the research findings related to SCOS etiology, including genetics, epigenetics, signaling pathways, and endocrinology of this disorder.
Genetic causes of SCOS
Genetic disorders associated with male infertility include chromosomal abnormality, Y chromosome microdeletions, mutations, polymorphisms, and epigenetic disorders. Known genetic abnormalities contribute to 15–20% of the most severe forms of male infertility [35]. Stouffs et al. (2016) have investigated possible causes of SCOS with a specific focus on genetic alternations. Their results showed in a large part of the patients (>23%), abnormality in the sex chromosomes is involved. Klinefelter syndrome was present in the majority of patients, followed by patients with Yq microdeletions [33]. In addition to these genetic disorders, other genetic modifications have been identified in association with SCOS as follows below (Table 1).
Table 1.
Genetic abnormalities observed in human Sertoli cell-only syndrome
| Genetic abnormality | References |
|---|---|
| Chromosome abnormalities | |
| Klinefelter syndrome (47, XXY) | [34] |
| 45, X/46, XY mosaicism | [36] |
| 46, XX male | [37] |
|
Chromosome rearrangements Isochromosome Yp |
[38] |
| Paracentric inversion of chromosome 7 (q22-31) and 12 (p12q12) | [39, 40] |
| Duplication in chromosome 19p13.3 | [41] |
| Y chromosome microdeletions | |
| AZFa | [42] |
| AZFb | [43] |
| AZFc | [44] |
| Gene mutations | |
| USP9Y | [45] |
| USP26 | [46] |
| DBY | [45] |
| HOXD9 | [7] |
| SYCE1 | [7] |
| COL1A1 | [7] |
| H19 | [7] |
| KCNQ1 | [7] |
| PLK-4 | [47] |
| CX43 | [48] |
| FANCM | [49] |
| ETV5 | [50] |
| H-MSI | [51] |
| MMR | [51] |
| CAG repeat in AR gene | [52] |
| SNPs | |
| ETV5 (+48845 G>T) | [53] |
| FGF9 (c.-712 C/T) | [54] |
| LRWD1 (3 coding SNPs) | [55] |
| SEPTIN 12 (8 coding SNPs) | [56] |
| RAD21L (coding SNP) | [57] |
Chromosomal abnormality
Chromosomal alterations are one of the common genetic causes of male infertility. The Klinefelter syndrome (47, XXY) is the most frequent abnormality, that is related to sex chromosomes aberration. The prevalence of Klinefelter syndrome is about 2/1000 male births [58] and usually is associated with SCO histopathology. However, autosomal anomalies like Robertsonian and reciprocal translocations and inversions are also involved in male infertility [59]. 45, X/46, XY mosaicism is one of the rare chromosomal alterations with a wide phenotypic spectrum. There is a subgroup of this mosaicism that has a normal male phenotype and is usually diagnosed during infertility investigation [60]. It has been reported that testicular biopsy of males with this mosaicism showed SCO histopathology [61]. Another chromosomal disorder that is associated with SCOS is 46, XX male, in which an unequal crossing over occurs between the short arms of the X and Y sex chromosomes during the gametogenesis, results in an abnormal X chromosome containing the SRY gene [36]. The SRY positive XX male syndrome is usually diagnosed in adult infertile men during infertility investigations. A rare case of 46, XX male with ambiguous genitalia and SCOS was reported [62]. Chromosomal rearrangement appears to impact spermatogenesis. Very rarely structural rearrangements of the Y-chromosome, the isochromosome of Yp observed in a man with SCOS [37]. It has been reported that duplications in chromosome 19p13.3 had the highest frequency in patients with SCOS [38]. Paracentric inversion of chromosome 7 (q22–31) [41] and chromosome 12 (p12q12) [39] were reported in association with SCO histopathology.
Mutations
It has been demonstrated that a large percentage of SCOS may be related to Yq11 (AZFa sub-region) microdeletions [40, 42, 61, 63–66]. The massive deletions in the AZFb or AZFb+c regions are one of the important genetic causes of SCO [67]. It has been demonstrated that a very high incidence of AZFc deletions exists in incomplete SCOS [43]. The absence of ubiquitin-specific protease 9Y (USP9Y) gene that is located in the AZFa region of the Y chromosome is often associated with blockage of spermatogonial cell proliferation causing SCOS in adults. However, the ubiquitin-specific protease 26 (USP26) gene is an X-linked gene specifically expressed in testis and its alternation has been reported in SCOS [44]. Patients with deletion of both USP9Y and DBY (DEAD/H box polypeptide, Y chromosome) had azoospermia with testicular histology of SCO [63].
Investigation of autosomal genes defects in SCOS revealed amplifications and deletions in several genes including HOXD9, SYCE1, COL1A1, H19, and KCNQ1 are associated with SCOS and might be implicated in this disorder [7]. A heterozygous 13-bp deletion in the Ser/Thr kinase domain of Polo-like kinase 4 (PLK-4) has been reported in a man with SCOS [46]. This kinase is one of the key regulators of centriole duplication. Connexin 43 (CX43) is a gap junction protein expressed in the blood-testis barrier that affects the maturation of Sertoli cells and the process of spermatogenesis [45, 68]. The absence of CX43 expression in human Sertoli cells is associated with SCOS and impaired spermatogenesis [47], but the responsible mechanism is unclear. Using in vitro hiSC model, Liang et al. revealed that the absence of CX43 disrupts multiple molecular pathways, including lipid metabolism and nucleobase catabolism in Sertoli cells [69].
It has been reported that the human male Bi-allelic Recessive Loss-of-Function Variants in FANCM (Fanconi anemia complementation group M) is possibly linked to SCOS [70]. ETS (E twenty-six) variant gene 5 (ETV5; alias ETS-related molecule) belongs to a family of transcription factors that regulate promoter activities of genes involved in many cellular functions [71]. It has been demonstrated that ETV5 gene mutations are associated with SCOS [48]. Maduro et al. (2003) reported for the first time significance of high microsatellite instability (H-MSI) and DNA mismatch repair (MMR) protein defects are present in azoospermia, predominantly in SCOS [49].
Mutations in the androgen receptor (AR) gene cause a variety of defects related to male infertility. The investigation of androgen receptor CAG and GGN repeat lengths in men with spermatogenic impairment showed that the CAG 21 allele seems to increase the risk of idiopathic SCOS [72]. Copy number variants (CNV) were evaluated by array-CGH in patients with SCOS which was the first CNV study in male infertility. They found sex-chromosomal, mostly private CNVs were significantly overrepresented in SCOS and 4 additional candidate genes, and two regions without known genes were related to SCOS [50]. On the other hand, no known infertility-related copy number variations were found by array comparative genomic hybridization technique in a selected group of idiopathic SCOS [33].
It has been found that loss of the Nupr1 protein leads to testicular development of a SCOS-like phenotype in the mouse model [51]. The transcription factor FOXJ3 (Forkhead box J3) is highly expressed in spermatogonia and meiotic spermatocytes in the mice testes. Foxj3 knockout mice that Foxj3 was deleted from spermatogonia exhibited complete sterility and SCOS in males [52]. CUL4B belongs to the CRL4 subfamily, and the CRL complex plays a critical role in the survival of germ cells [73]. The CUL4B-null mice had SCOS phenotype, but Miyamoto et al. reported no significant mutations in CUL4B in these patients with SCOS [74]. So mutation in some genes in animal models could not be applied to humans.
Epigenetic alternations
Misregulation of epigenetic modification has been detected in association with SCOS. It has been reported that the presence of highly acetylated histone H4 in testicular sections with SCO pathologic pattern [75]. In an epigenetic disorder, Prader-Willi syndrome, testicular histology in childhood sometimes showed SCOS [76, 77].
Single nucleotide polymorphisms
Several single nucleotide polymorphisms (SNP) have been identified in association with the SCOS. The homozygous TT allele of the +48845 G>T variant in the ETV5 gene confers a higher risk of male infertility associated with SCO and NOA in Australian men [78]. Studies from fibroblast growth factor 9 (Fgf9) knockout mice confirmed the crucial roles of FGF9 in male sex determination and gonadal development [79]. An association between aberrant expression of Fgf9 gene with SCOS was reported. Fgf9 expression was significantly decreased in patients with SCOS as a result of a promoter polymorphism (c.-712C/T) of the FGF9 gene, which could be one of the causes of its low expression [80]. The human LRWD1 (leucine-rich repeats and WD repeat domain containing 1) gene is critical for the initiation of pre-replication complex assembly. The association of defects in this gene with SCOS was investigated. No mutations were detected in LRWD1; however, three coding SNPs were found in the SCOS patients [53].
Septin12 (SEPTIN12) is a member of the septin family of cytoskeletal GTPases that form filamentous structures in interphase cells and express specifically in the testis. The association of SEPTIN12 gene defects with SCOS was investigated. They found no mutations; however, 8 coding SNPs were detected in the patients with SCOS [81]. RAD21L gene is suggested to be a canonical cohesion subunit that is transcribed in the testis. It has been reported that coding SNPs in this gene was notably higher in the patient with SCOS in comparison to the control group [54]. However, testis-specific cytoplasmic poly(A) polymerase beta (PAPOLB) is known as a critical factor for spermatogenesis. A study suggested a lack of association of PAPOLB polymorphisms with SCOS in humans [55].
MicroRNAs in SCOS
MicroRNAs (miRNA), a new class of endogenous small RNA molecules, are important posttranscriptional regulators of gene expression. A little is known about their involvement in human spermatogenesis. In the animal model, it has been demonstrated that miRNAs play critical roles in male germ cell development [56]. It has been reported that patients with SCOS showed a large number of deregulated miRNAs that might be due to the absence of germ cells in these patients. Members of the miR-517a/b/c family which is known to be a regulator of genes that control cell proliferation and survival [57] were deregulated in SCOS patients [82]. It has been reported that 174 miRNAs were distinctly expressed in Sertoli cells of SCOS compared with OA. They suggested that these miRNAs may be novel insights associated with the pathogenesis of SCOS [83]. In another study, miR-202-3p was upregulated in Sertoli cells of SCOS patients compared with OA patients. This microRNA directly targets the Wnt/b-catenin signaling pathway, induced Sertoli cell apoptosis, and inhibited cell proliferation [84].
Signaling pathways
Signaling pathways, a series of chemical reactions that control cell functions, and their disorders might be involved in SCOS pathophysiology. Summary of signaling pathways involved in SCOS pathophysiology is presented in Table 2. Caspases are a family of proteins involved in the apoptotic pathway. When Caspase-3 is activated, there is no return in the apoptotic process and will be disassembled [99]. Increased active caspase-3 was found in SCOS and might be involved in its etiology [100]. It has been demonstrated that the expression of FasL is upregulated in the testis samples of patients with SCOS compared with normal spermatogenesis. Expression of Fas, FasL, and active caspase-3 was detected in Sertoli cells and hyperplastic interstitial cells of SCOS patients. This led to trigger apoptotic depletion of Fas-expressing germ cells in SCOS [101]. Phosphoribosylpyrophosphate synthetases 2 (PRPS2) expression in patients with SCOS was significantly greater than normal spermatogenesis. PRPS2 overexpression significantly inhibited cell apoptosis via p53/Bcl-2/caspases signaling pathway and promoted cell cycle transition. So, PRPS2 might be one of the potential novel proteins associated with SCOS [102]. Expression of SAM68 (Src-associated substrate in mitosis of 68 kD, also known as KH-DRBS1) at mRNA and protein levels was absent or barely detectable in testicular tissues of all patients with SCOS. This protein regulates a range of processes and decreased expression of Sam68 may suppress germ cell proliferation and induced apoptosis [85]. The expression of galectin-l and -3 (vertebrate lectins interacting with B-galactosides) and galectin-specific binding sites were found to be increased in Sertoli cells in SCOS compare to normal human testis might have a connection to the control of apoptosis [86].
Table 2.
List of signaling pathways involved in SCOS pathophysiology
| Proteins | Expression status | Signaling pathway/function | Reference(s) |
|---|---|---|---|
| Caspase 3 | ↑ | Apoptosis | [85] |
| FasL | ↑ | Apoptosis | [86] |
| PRPS2 | ↑ | Inhibition of apoptosis in Sertoli cells | [87] |
| SAM68 | ↑ | Apoptosis | [88] |
| Galectin 1, 3 | ↑ | Apoptosis | [89] |
| FGF5 | ↓ | Cell proliferation | [90] |
| TGF-β | TGF-β/EGFR signaling | [91] | |
| GDNF, FGF8, BMP4 | ↓ | Proliferation and differentiation of SSCs | [92] |
| RAB20, RAB3D, RAB40B,RRAS2, HRAS, and catalytic subunit of PI3CA and PI3K | ↓ | Vesicular traffickingR | [92] |
| RohB | ↓ | Cell signaling | [93] |
| APC/C | ↓ | Cell cycle | [94] |
| IGF1/PI3K/AKT | ↑ | Cell cycle | [94] |
| S-COMT | ↑ | Metabolizing of estrogen | [95] |
| SOX9, AMH | ↑ | Androgen/AR | [96] |
| AR | ↓ | Androgen/AR | [96] |
| CYP19A1 | ↑ | Androgen/estrogen level regulation | [97] |
| CYP17A1 | ↓ | Androgen/estrogen level regulation | [31] |
| SOAT, OATP6A1, OSCP1 | ↓ | Androgen/estrogen level regulation | [98] |
Fibroblast growth factor-5 (FGF5) is a growth factor that could promote spermatogonial stem cell (SSC) proliferation via ERK and AKT activation. FGF5 was downregulated in SCOS compared to OA patients [87]. The TGF-β pathway and the interaction between TGF-β and EGFR signaling may play a role in the dysfunction of Sertoli cells in SCOS and contribute in the pathogenesis of this disorder [88]. It has been suggested that overexpression of BMP4 might be related to the SCOS. They identified BMP4 as the first autocrine factor controlling the development and function of adult human Sertoli cells [89]. Paduch et al. (2019) provided the first detailed comparison of the transcriptomes of human testes with normal spermatogenesis to the testes with SCOS and identified deficits in gene expression by Sertoli cells. They focused on 244 transcripts as human Sertoli cell signature transcripts and found that 31% were expressed at significantly lower levels. They concluded that Sertoli cells of SCO testes express abnormally low levels of GDNF, FGF8, and BMP4 that regulate replication and differentiation of SSCs. Reduced expression of genes that act in vesicular trafficking regulation pathways and proteins that polarize and organize the Sertoli cell plasma membrane. This data indicated that SCOS is associated with multiple deficits in Sertoli cell gene expression [90].
Ras homologous B protein (RhoB) belongs to the Ras homologous subfamily are regulatory molecules involved in various cell signaling pathways. Decreased expression of RhoB protein was observed in the Sertoli and Leydig cells in the testis of SCOS patients and suggests the involvement of RhoB in the human spermatogenesis [91]. SCOS pathogenesis could be due to defects in the cell cycle or hormone-mediated pathways in Sertoli and Leydig cells. Recently, it has been suggested that SCOS may be caused by disordered APC/C-mediated cell cycle progression and PI3K/AKT signaling. It has been reported that downregulation of APC/C-mediated cell cycle progression in the spermatogonia and upregulation of IGF1/PI3K/AKT signaling in Sertoli cells is a driver of SCOS development [103].
It has been reported that testicular expression of S-COMT, the estrogen-metabolizing enzyme, was elevated in seminiferous tubules of SCOS patients. Increased expression of this enzyme may be related to high intratesticular 2OHE2 and 2ME2 concentrations, which could negatively affect the function of Sertoli cells and spermatogenesis [92]. Upregulation of SOX9 and AMH proteins but downregulation of AR proteins in Sertoli cells due to defect of androgen/AR signaling is detected in patients with SCOS. This finding explains the immaturity of Sertoli cells in SCOS patients with low serum testosterone levels and demonstrates a role for SOX9 and the regulation of SOX9/AMH by AR signaling in the pathogenesis of SCOS [93]. It has been shown that SCOS patients with low T/LH ratios and Leydig cell hyperplasia have Leydig cells that overexpress CYP19A1, leading to increased intratesticular E2 levels and E2/T ratios [94]. So impaired androgenic production in SCOS is associated with depressed CYP17A1 expression, possibly regulated by E2. It has been demonstrated that Leydig cells of men with SCOS and testicular steroidogenic dysfunction show discordance between transcriptional and protein expression of CYP17A1, associated with impaired T production and elevated intratesticular E2 levels. Thus, Leydig cell dysfunction is associated with post-transcriptional deregulation of CYP17A1 in patients with SCOS [95]. It has been reported mRNA expression of steroid sulfate carriers to include the Sodium-dependent Organic Anion Transporter (SOAT), the Organic Anion Transporting Polypeptide 6A1 (OATP6A1), and the Organic Solute Carrier Partner 1 (OSCP1) in human testis with SCOS were significantly lower or even absent. Significantly lower expression of SOAT may cause a disturbed transport of sulfated steroids and consequently, disrupt the local supply of androgens and estrogens [96].
Other etiologies
Environmental agents such as temperature during testicular development, as in cryptorchidism situation, could cause the disappearance of the germ cell and create SCOS [97]. Some of the other involved factors are the destruction of germ cells by the action of X-ray irradiation [98] or chemical agents such as hormonal treatment [104] and chemotherapy in cancer treatment. Estrogen therapy reduces serum concentrations of testosterone and gonadotropins [105] and causes the transformation of mature Sertoli cells to undifferentiated Sertoli cells. It has been suggested neural elements (neuronlike cells) may be involved in SCOS pathogenesis; however, the underlying mechanisms were unknown [106]. SCO may be the final stage of persistent, longstanding testicular parenchymal hypoxia, which deteriorates sperm production over time [107]. So impairment in venous drainage of the male reproductive system can cause SCOS. Alcohol induces disorders in the human testis, and it has been suggested some cases of heavy alcohol drinkers had SCOS [108].
It has been reported that varicocele in rats can cause SCOS. Laboratory varicocele caused progressive impairment of the testes, and SCOS could be induced when the damage was severe [109]. Hypothermic testicular ischemia also could produce SCOS in animal models [110].
Diagnosis and clinical management
Azoospermia occurs in 10–15% of males seeking medical care for infertility [3]. After confirmation of azoospermia based on the examination of multiple semen specimens, clinically distinguishing NOA from OA can be done by the analysis of diagnostic parameters, including history, physical examination, and hormonal analysis. These parameters provide a >90% prediction of whether azoospermia is obstructive or non-obstructive [111]. The gold-standard diagnostic test for distinguishing between NOA subtypes (HS, MA, SCOS, tubular sclerosis, and mixed patterns) is a testicular biopsy and subsequent histopathologic examination of biopsy specimens. Each of these subtypes has a different prognosis for infertility treatment. The diagnosis and management of SCOS, which is the subject of this review, are described in more detail below.
Clinical parameters and genetic testing
The clinical characteristics of SCOS patients vary widely regarding history, physical examination, and hormonal profile. On physical examination, except for Klinefelter syndrome, other patients have normal secondary male sexual characteristics, and no gynecomastia is seen. For all patients with azoospermia, serum level of FSH, LH, and total T is obtained. The testes volume can be normal or smaller in size, and some patients have higher levels of FSH than normal [33]. In a study, it has been shown that when testicular biopsies showed bilateral SCO, the patient had azoospermia (86%) or oligozoospermia (14%). The testicular size was normal in 36%, and the FSH level was normal (43%), raised (21%), or grossly elevated (more than twice normal, 36%) [112]. So the clinical features associated with the SCO histology are extremely variable. On the other hand, when one biopsy shows SCO, the opposite testis appearance could vary from impaired spermatogenesis to almost normal spermatogenesis [112].
Genetic tests include cytogenetic karyotyping and molecular diagnosis and subtyping of Y chromosome microdeletions are usually recommended to all men with NOA. The karyotype of most SCOS men is normal, while SCOS may be a result of Klinefelter syndrome, microdeletions in the Y chromosome, and other genetic causes. Deletions that remove the entire AZFa are associated with the pure SCO with no residual areas of complete spermatogenesis. Although partial AZFa deletions may be associated with residual spermatogenesis in the testis [113]. However, for many of these patients, the possible genetic cause is unknown. Some genetic causes reported in associations with SCOS are described in the etiology section of this review but are not yet applicable in clinical practice.
Testicular biopsy
The exact diagnosis of SCOS is based on diagnostic testicular biopsy and histopathological examination. Histologic examination is performed on paraffin-embedded and hematoxylin and eosin-stained samples by examining at least 100 different sections of seminiferous tubules. When no germ cells are present in the seminiferous tubules except Sertoli cells, but the tubular architecture is not affected by fibrosis; SCOS is diagnosed [114]. Two distinct histologic patterns of SCO, namely primary and secondary, also can be identified by histologic examination. These two patterns of SCO have a different prognosis for sperm retrieval, and their differences are described in more detail in the histopathology section.
Clinical management
SCOS patients do not have therapy options to cure their disorder and help them to have a biological child. However, Paulis et al. (2017) reported an infertile couple case in whom the male has SCOS that with FSH hormone treatment, several sperms were observed in testicular biopsy achieved by testicular sperm extraction (TESE). After ICSI and subsequent embryo transfer, the live birth of a girl resulted [114]. Nonetheless, based on current knowledge, there is not enough evidence and this does not apply to all SCOS cases. Another intervention before sperm retrieval in NOA patients is varicocele repair. In NOA men with varicocele, varicocelectomy may offer an opportunity to have motile sperm via ejaculate. Significant improvement in testicular histology has been reported after varicocele repair in NOA patients [115]. There have been some studies regarding motile sperm obtained in SCOSpatients after varicocele repair [116, 117]. On the other hand, some studies showed no improvement in SCOS after varicocelectomy [118–120]. Therefore, varicocele repair in patients with SCOS, unlike other NOA subtypes such as HS and MA, has been very little if any effect on sperm recovery.
SCOS affects about 26.3–57.8% of azoospermic patients [5, 6], and sperm could be found in the seminiferous tubules of 20% of these men using testicular sperm extraction procedure [121]. Many SCOS patients have rare foci of complete spermatogenesis in the testes so, surgically testicular sperm retrieval combined with ICSI is an option for infertility treatment in these patients. Two different histological patterns of SCOS are the primary and the secondary forms with different prognoses to testicular sperm extraction. The definitive diagnosis of these patterns is by diagnostic testicular biopsy. Successful sperm retrieval rate in incomplete and complete germ cell aplasia has been reported 86.0% and 19.3%, respectively [122], which can be successfully used for ICSI. So, secondary forms of SCOS have a better prognosis in testicular sperm retrieval.
In addition to fertility issues, it has been reported that the prevalence of testicular nodules and cancer in men with complete SCOS is very high [123]. So, because of the increased risk of cancer in the testes with complete SCOS, a biopsy for the histological examination should always be performed. The future preconception genetic testing of parents, as mutation carriers, or preimplantation genetic testing of embryos may be needed for identifying possible carriers. This provides more information for couples about whether or not the syndrome could be passed on to their children. Also, there is not enough data on the health of SCOS patients’ offspring, and long-term follow-up studies are needed in this regard.
Sperm retrieval and prognostic factors
Patients with SCOS are infertile due to non-obstructive azoospermia. Before describing the microdissection testicular sperm extraction (micro-TESE) technique by Schlegel in 1999 [124], the preferred sperm retrieval method in NOA patients was conventional TESE. The removal of large fragments of testicular tissue in TESE may compromise androgen production [125] and had tough laboratory processing [126]. In the micro-TESE procedure, the surgeon is looking for a dilated and opaque seminiferous tubule that might be containing sperm. This method has fewer complications than TESE and more sperm retrieval rate, especially in SCOS patients. It has been demonstrated that the sperm retrieval rate in SCOS by TESE is lower than in patients with NOA in general, and micro-TESE increases the sperm retrieval rates. In a systematic review, it has been shown more favorable sperm retrieval in SCOS for micro-TESE when compared with conventional TESE [127]. Retrieved sperm in SCOS patients can be immediately used for ICSI or cryopreserved for future ICSI attempts. ICSI achieved similar live birth rates in patients with SCOS, like other patients with NOA [128].
After testicular sperm extraction, for most SCOS patients with failed sperm recovery, assisted reproductive techniques such as ICSI will be impossible. For men with complete AZFa, AZFb, or AZFbc, the chance of sperm retrieval success is virtually nonexistent, and usually proceeding with sperm retrieval is not recommended. Hormonal concentration, testicular volume, and histologic diagnosis are not informative enough to predict the presence of testicular sperm. Serum FSH level is not also a predictor of a successful sperm retrieval, but it can affect the outcome of sperm recovery. It was shown that those men with higher FSH had a more successful sperm retrieval by micro-TESE [129]. Telomerase enzyme activity has been detected in male germ cells. In testicular samples with haploid cells, higher telomerase activity was detected [130] while patients with SCOS showed no telomerase activity. In a retrospective cohort study that included 640 patients with pure SCOS who underwent micro-TESE, 44.5% of patients had successful sperm retrieval. No difference was observed in sperm retrieval rates based on testis volume, but patients with ≥ 15cc testicular volume and FSH 10-15 mU/mL had the worst prognosis, with a sperm retrieval rate of 6.7% [131]. Intraoperative measuring the diameter of seminiferous tubules during micro-TESE is a useful predictor for sperm retrieval in NOA patients. However, in SCOS patients, it has been reported that the sperm retrieval rate in men with a tubule diameter ≥100 μm was lower than that in those with <100 μm. The sperm retrieval rate from the contralateral testis in men with a tubule diameter ≥100 μm, also was lower than that in those with <100 μm [132]. This finding appears to conflict with previous reports that showed sperm recovery is higher in dilated tubules.
It still is a challenge to identify patients who have a better chance of successful testicular sperm extraction. Parameters include age, infertility time, serum FSH, LH, testosterone levels, and testicular volume were not useful to predict sperm retrieval. Therefore, it is necessary to use a suitable biomarker for predicting testicular sperm presence in testes and identifies those patients with real chances of a positive sperm recovery result on the future biopsy. This prevents unnecessary testis operation and its side effects. Specific markers in seminal plasma may reflect the cumulative yield of spermatogenesis in testes and thus be useful to detect focal spermatogenesis. Many studies have been done and are being done and gain insight into the origin of SCOS may be helping in this regard. In cases with no mature sperm retrieved, in vitro culture of preexisting immature germ cells has been proposed. On the other hand, some of the pure types of SCOS patients have no germ cells, the only possible way to have biological offspring would be the use of their somatic cells via induced pluripotency to generate germline and in vitro spermatogenesis [133]. Further studies are needed to develop this method, and there is a long way to go before it can be used clinically.
Future perspectives
Further studies are needed to understand the molecular pathways of genes involved in the SCOS. Recently, the iPSC cell line HUSTi002-A generated from fibroblasts of a SCOS patient who carries a homozygous PIWIL2 mutation offers an opportunity to study the roles of the PIWIL2 gene in the pathogenesis of SCOS [134]. In a functional proteomics study, it has been identified 13 differential proteins in testis samples of SCOS compared to normal spermatogenesis. Among these differential proteins, Heterogeneous nuclear ribonucleoprotein L (HnRNPL) was suggested as a key regulator of apoptosis, death, and growth of spermatogenic cells, by String and Pubgene bioinformatic programs. Further in vitro and in vivo studies revealed that knockdown of HnRNPL led to inhibited proliferation, increased apoptosis of spermatogenic cells but decreased apoptosis of Sertoli cells. So HnRNPL was suggested as a key regulator of spermatogenesis in SCOS [135]. Other proteomics studies revealed that cell cycle and proteolysis, and RNA splicing were the most significant biological processes impaired by suppression of related proteins in SCOS. This study provides novel candidate proteins associated with SCOS, including 298 and 76 down- and upregulated protein changes, respectively. From these altered proteins, 57 were highly downregulated and 3 were highly upregulated. It has been demonstrated that spliceosome, cell cycle, and proteasome proteins, as well as energy and metabolic involved proteins, are highly suppressed in SCOS patients [136].
Cell-free seminal mRNA (cfs-mRNA) contains testis-specific transcripts from bilateral testes and is a novel non-invasive approach for the classification of azoospermia. It has been reported the presence of DEAD-box polypeptide 4 (DDX4) in cfs-mRNA to identify and characterize the incidence of SCOS is more accurate than testicular histopathology [137].
To be the father of a biological child, in vitro maturation of SSCs isolated from SCOS without sperm in their testicular biopsies is a new approach for possible future infertility treatment. In vitro maturation of SSCs extracted from the testis of SCOS patients is one of the new suggested approaches to treat their infertility problem. For the first time, Abofoul-Azab et al. (2019) showed the presence of meiotic and/or postmeiotic cells in biopsies without the sperm of SCOS patients and examined the possibility of inducing spermatogenesis from isolated spermatogonial cells of these biopsies in vitro using 3D MCS. They indicated that isolated cells from some of these biopsies could be induced to meiotic and/or postmeiotic stages under in vitro culture conditions [138]. New researches with the aid of novel technologies will help to more accurately identify the etiology of SCOS and its clinical management in the future.
Conclusion
The available data are insufficient to conclude an accurate and non-invasive way for diagnosis and a definite method for the treatment of all SCOS cases. The clinical features of SCOS are extremely variable. Most of the SCOS patients have a normal karyotype, and some have smaller testes and normal or higher levels of FSH. So it is difficult and sometimes impossible to diagnose SCOS based on clinical characteristics. The exact diagnosis of this disorder is still based on a diagnostic bilateral testicular biopsy. This is an invasive method with complications such as pain and fibrosis. On the other hand, patients with SCOS are infertile due to non-obstructive azoospermia. For the treatment of these patients, testicular sperm extraction is the only option for these men to have their biological child. Frequent failure of sperm retrieval is not acceptable for the infertile couple. It still is a challenge to identify patients who have a better chance of successful testicular sperm extraction. So, there is a need to find a suitable biomarker to diagnose the SCO histopathology without using biopsy and predict testicular sperm presence in the testes. This prevents unnecessary testis operation and its side effects and identifies those patients with real chances of a positive sperm recovery result on the future biopsy. As a non-invasive marker, for example, molecular markers in seminal plasma may reflect the spermatogenesis status in the testes and can be useful to detect rare foci of spermatogenesis. This method does not need a testicular biopsy and will be patients friendly.
This review aimed to provide an overview of the current knowledge regarding SCOS etiology and emphasize the need for future investigation for the management of this disorder. At present, there is no treatment for SCOS patients. SCOS patients with failed surgically sperm retrieval result does not have therapy options to help them having a biological child. In vitro maturation of SSCs isolated from SCOS men without sperm in their testicular biopsies and the use of somatic cells via induced pluripotency to generate germline, are new approaches for possible future infertility treatment in these patients. The reasons for many cases of SCOS are unclear. Recent studies have expanded our understanding of the etiology of the SCOS. There is a need for more research to identify the causes of idiopathic SCOS cases. Finally, more understanding of the etiology of SCOS can help to develop a suitable therapeutic method for the management of this disorder.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eisenberg ML, Lathi RB, Baker VL, Westphal LM, Milki AA, Nangia AK. Frequency of the male infertility evaluation: data from the national survey of family growth. J Urol [Internet]. Elsevier Inc. 2013;189:1030–1034. doi: 10.1016/j.juro.2012.08.239. [DOI] [PubMed] [Google Scholar]
- 2.Pan MM, Hockenberry MS, Kirby EW, Lipshultz LI. Male infertility diagnosis and treatment in the era of in vitro fertilization and intracytoplasmic sperm injection. Med Clin NA [Internet] 2017;10:1–7. doi: 10.1016/j.mcna.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Tournaye H, Krausz C, Oates RD. Male reproductive impairment 2 Concepts in diagnosis and therapy for male reproductive impairment. LANCET Diabetes Endocrinol [Internet] 2016;8587:1–11. doi: 10.1016/S2213-8587(16)30043-2. [DOI] [PubMed] [Google Scholar]
- 4.McLachlan RI, Rajpert-De Meyts E, Hoei-Hansen CE, de Kretser DM, Skakkebaek NE. Histological evaluation of the human testis - approaches to optimizing the clinical value of the assessment: Mini Review. Hum Reprod. 2007;22:2–16. doi: 10.1093/humrep/del279. [DOI] [PubMed] [Google Scholar]
- 5.Ammal PL, Das SK. Testicular biopsy in male infertility. J Med Sci Clin Res. 2017;05:20616–20619. [Google Scholar]
- 6.Franco G, Scarselli F, Casciani V, De Nunzio C, Dente D, Leonardo C, et al. A novel stepwise micro-TESE approach in non obstructive azoospermia. BMC Urol. 2016;16:20. doi: 10.1186/s12894-016-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koc G, Ozdemir AA, Girgin G, Akbal C, Kirac D, Avcilar T, Guney AI. Male infertility in Sertoli cell-only syndrome: An investigation of autosomal gene defects. Int J Urol. 2019;26:292–298. doi: 10.1111/iju.13863. [DOI] [PubMed] [Google Scholar]
- 8.Del Castillo EB, Trabucco A, DE la Balze FA. Syndrome produced by absence of the germinal epithelium without impairment of the Sertoli or Leydig cells. J Clin Endocrinol Metab. 1947;7:493–502. doi: 10.1210/jcem-7-7-493. [DOI] [PubMed] [Google Scholar]
- 9.Silber SJ. Microsurgical TESE and the distribution of spermatogenesis in non-obstructive azoospermia. Hum Reprod. 2000;15:2278–2284. doi: 10.1093/humrep/15.11.2278. [DOI] [PubMed] [Google Scholar]
- 10.Anniballo R, Ubaldi F, Cobellis L, Sorrentino M, Rienzi L, Greco E, et al. Criteria predicting the absence of spermatozoa in the Sertoli cell-only syndrome can be used to improve success rates of sperm retrieval. 2000;15:2269–77. [DOI] [PubMed]
- 11.Terada T, Hatakeyama S. Morphological evidence for two types of idiopathic ‘ Sertoli-cell-only ’ syndrome. Int J Androl. 1991;14:117–126. doi: 10.1111/j.1365-2605.1991.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 12.Nistal M, Jimenez F, Paniagua R. Sertoli cell types in the Sertoli-cell-only syndrome: relationships between Sertoli cell morphology and aetiology. Histopathology. 1990;16:173–180. doi: 10.1111/j.1365-2559.1990.tb01086.x. [DOI] [PubMed] [Google Scholar]
- 13.Weller O, Yogev L, Yavetz H, Paz G, Kleiman S, Hauser R. Differentiating between primary and secondary Sertoli-cell-only syndrome by histologic and hormonal parameters. Fertil Steril. 2005;83:1856–1858. doi: 10.1016/j.fertnstert.2004.11.074. [DOI] [PubMed] [Google Scholar]
- 14.Franke FE, Pauls K, Rey R, Marks A, Bergmann M, Steger K. Differentiation markers of Sertoli cells and germ cells in fetal and early postnatal human testis. Anat Embryol (Berl). 2004;209:169–177. doi: 10.1007/s00429-004-0434-x. [DOI] [PubMed] [Google Scholar]
- 15.Brehm R, Steger K. Regulation of Sertoli cell and germ cell differentation. Adv Anat Embryol Cell Biol [Internet] 2005;181:1–93. [PubMed] [Google Scholar]
- 16.Ooba T, Ishikawa T, Yamaguchi K, Kondo Y. Expression and distribution of laminin chains in the testis for patients with azoospermia. J Androl. 2008;29:147–152. doi: 10.2164/jandrol.107.003210. [DOI] [PubMed] [Google Scholar]
- 17.Bibro MC, Fourcroy JL, Charles EM. A unique case of Sertoli cell only syndrome with normal gonadotropins * t. Fertil Steril [Internet]. Elsevier Masson SAS. 1984;42:655–658. doi: 10.1016/S0015-0282(16)48156-2. [DOI] [PubMed] [Google Scholar]
- 18.Abdalla MI, Ibrahim II, Rizk AM, Agouz WTEL, Girgis SM. Endocrine studies of azoospermia. 1. Serum steroid levels in Sertoli cell only syndrome. Arch Androl. 1979;256:253–256. doi: 10.3109/01485017908987321. [DOI] [PubMed] [Google Scholar]
- 19.Heath H. Plasma gonadotropins in germinal cell aplasia ( Sertoli cell only syndrome) J Urol [Internet]. The American Urological Association Education and Research, Inc. 1973;109:847–849. doi: 10.1016/S0022-5347(17)60560-3. [DOI] [PubMed] [Google Scholar]
- 20.Pineau C, Sharpe RM, Saunders PT, Gérard N, Jégou B. Regulation of Sertoli cell inhibin production and of inhibin alpha-subunit mRNA levels by specific germ cell types. Mol Cell Endocrinol. Ireland. 1990;72:13–22. doi: 10.1016/0303-7207(90)90235-z. [DOI] [PubMed] [Google Scholar]
- 21.Zarate A, Garrido J, Canales ES, Soria J, Schally AV. Disparity in the negative gonadal feedback control for LH and FSH secretion in cases of germinal aplasia or Sertoli-cell-only syndrome. JCE M. 1974:1973–5. [DOI] [PubMed]
- 22.Ishida H, Isurugi K, Aso Y, Takayasu H, Tamaoki B-I. Endocrine studies in Sertoli-cell-only syndrome. J Urol [Internet]. The American Urological Association Education and Research, Inc.; 1976;116:56–58. Available from: 10.1016/S0022-5347(17)58673-5 [DOI] [PubMed]
- 23.Micic S, Ilic V, Micic M, Cenbacev O, Dotlic R. Endocrine Profile of 45 Patients with Sertoli cell only syndrome. Andrologia. 1983;15:228–232. doi: 10.1111/j.1439-0272.1983.tb00365.x. [DOI] [PubMed] [Google Scholar]
- 24.Kostakopoulos A, Protoyerou V, Tekerlekis P, Georgoulakis J, Louras G, Goulandris N. DNA flow-cytometric, histological and hormonal analysis of sertoli cell only syndrome (SECOS) Int Urol Nephrol. 2002;33:77–79. doi: 10.1023/a:1014417322540. [DOI] [PubMed] [Google Scholar]
- 25.Leifke E, Simoni M, Kamischke A, Gromoll J, Bergmann M, Nieschlag E. Does the gonadotrophic axis play a role in the pathogenesis of Sertoli-cell-only syndrome ? Int J Androl. 1997;36:29–36. doi: 10.1046/j.1365-2605.1997.00102.x. [DOI] [PubMed] [Google Scholar]
- 26.Okuyama A, Nonomura N, Koh E, Kondoh N, Nakamura M, Namiki M, et al. Testicular FSH and hCG receptors in sertoli cell only syndrome. Arch Androl. 1989;124:119–124. doi: 10.3109/01485018908986833. [DOI] [PubMed] [Google Scholar]
- 27.Loukil LH, Boudawara TS, Ayadi I, Bahloul A, Jlidi R, And HA, et al. High androgen receptor immunoexpression in Human " Sertoli cell only " testis. Arcbs Inst Pasteur Tunis. 2005;82:1–4. [PubMed] [Google Scholar]
- 28.Han Y, Feng HL, Ssandlow JI, Haines CJ. Comparing expression of progesterone and estrogen receptors in testicular tissue from men with obstructive and nonobstructive azoospermia. J Androl. 2009;30:127–133. doi: 10.2164/jandrol.108.005157. [DOI] [PubMed] [Google Scholar]
- 29.Hammar M, Berg AA. Impaired leydig cell function in vitro in testicular tissue from human males with “Sertoli Cell Only” syndrome. Andrologia. 1985;17:37–41. doi: 10.1111/j.1439-0272.1985.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 30.Lardone MC, Castillo P, Valdevenito R, Ebensperger M, Ronco AM, Pommer R. P450-aromatase activity and expression in human testicular tissues with severe spermatogenic failure. Int J Androl. 2009;33:650–660. doi: 10.1111/j.1365-2605.2009.01002.x. [DOI] [PubMed] [Google Scholar]
- 31.Lardone MC, Argando F, Florez M, Parada-Bustamante A, Ebensperger M, Palma C, et al. Overexpression of CYP19A1 aromatase in Leydig cells is associated with steroidogenic dysfunction in subjects with Sertoli cell-only syndrome. Andrology. 2016:1–8. [DOI] [PubMed]
- 32.Malabu UH, Gowda D, Tan YM. Case report insulinoma presenting with long-standing depression , primary hypogonadism , and Sertoli cell only syndrome. Case Rep Endocrinol. 2013;2013:12–14. doi: 10.1155/2013/926385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stouffs K, Gheldof A, Tournaye H, Vandermaelen D, Bonduelle M, Lissens W, et al. Sertoli Cell-only syndrome : behind the genetic scenes. Biomed Res Int. 2016;2016:6191307. doi: 10.1155/2016/6191307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paulis G. Chromosomic causes of infertility. Clin Manag Male Infertil. 8th ed. 2014:63–77.
- 35.De Kretser DM. Male infertility genetics - The future. J Androl. 2001;22:738–746. [PubMed] [Google Scholar]
- 36.Jain M, Chaudhary I, Halder A. The Sertoli cell only syndrome and glaucoma in a sex – determining region Y ( SRY ) positive XX infertile male. J Clin Diagnostic Res. 2013;7:1457–1459. doi: 10.7860/JCDR/2013/5186.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin YH, Chuang L, Lin YM, Lin YH, Teng YN, Kuo PL. Isochromosome of Yp in a man with Sertoli-cell-only syndrome. Fertil Steril. 2005;83:764–766. doi: 10.1016/j.fertnstert.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 38.Singh V, Bala R, Chakraborty A, Rajender S, Trivedi S, Singh K. Duplications in 19p13.3 are associated with male infertility. J Assist Reprod Genet. Journal of Assisted Reproduction and Genetics. 2019;36:2171–2179. doi: 10.1007/s10815-019-01547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghorbel M, Baklouti-gargouri S, Elghazel H, Zribi N, Ben F, Cherif M, et al. Biochemical and Biophysical Research Communications Pericentric inversion of chromosom 12 [ Inv ( 12 ) ( p12q12 )] associated with idiopathic azoospermia in one infertile Tunisian man. Biochem Biophys Res Commun [Internet] 2013;432:472–474. doi: 10.1016/j.bbrc.2013.01.110. [DOI] [PubMed] [Google Scholar]
- 40.De Rosa M, De Brasi D, Zarrilli S, Paesano L, Agostino RPAD, Longobardi S, et al. Short stature and azoospermia in a patient with Y chromosome long arm deletion. J Endocrinol Invest. 1997;20:623–628. doi: 10.1007/BF03346921. [DOI] [PubMed] [Google Scholar]
- 41.Ichioka K, Yoshimura K, Honda T, Takahashi A. Paracentric inversion of chromosome 7 ( q22 – 31 ) associated with nonobstructive azoospermia. Fertil Steril. 2005;83:2004–2005. doi: 10.1016/j.fertnstert.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 42.Kamp C, Huellen K, Fernandes S, Sousa M, Schlegel PN, Mielnik A, et al. High deletion frequency of the complete AZFa sequence in men with Sertoli-cell-only syndrome. Mol Hum Reprod. 2001;7:987–994. doi: 10.1093/molehr/7.10.987. [DOI] [PubMed] [Google Scholar]
- 43.Ferras C, Fernandes S, Marques CJ, Carvalho F, Alves C, Silva J, et al. AZF and DAZ gene copy-specific deletion analysis in maturation arrest and Sertoli cell-only syndrome. Mol Hum Reprod. 2004;10:755–761. doi: 10.1093/molehr/gah104. [DOI] [PubMed] [Google Scholar]
- 44.Steirteghem V, Stouffs K, Lissens W, Tournaye H, Liebaers I. Possible role of USP26 in patients with severely impaired spermatogenesis. Eur J Hum Genet. 2005;26:336–340. doi: 10.1038/sj.ejhg.5201335. [DOI] [PubMed] [Google Scholar]
- 45.Gerber J, Heinrich J, Brehm R. Blood-testis barrier and Sertoli cell function: Lessons from SCCx43KO mice. Reproduction. 2016;151:R15–R27. doi: 10.1530/REP-15-0366. [DOI] [PubMed] [Google Scholar]
- 46.Miyamoto T, Bando Y, Koh E, Tsujimura A, Miyagawa Y, Iijima M, Namiki M, Shiina M, Ogata K, Matsumoto N, Sengoku K. A PLK4 mutation causing azoospermia in a man with Sertoli cell-only syndrome. Andrology. 2016;4:75–81. doi: 10.1111/andr.12113. [DOI] [PubMed] [Google Scholar]
- 47.Defamie N, Berthaut I, Mograbi B, Chevallier D, Dadoune JP, Fénichel P, Segretain D, Pointis G. Impaired gap junction connexin43 in Sertoli cells of patients with secretory azoospermia: a marker of undifferentiated Sertoli cells. Lab Investig. 2003;83:449–456. doi: 10.1097/01.lab.0000059928.82702.6d. [DOI] [PubMed] [Google Scholar]
- 48.Yatsenko AN. ETV5 mutations: revisiting Sertoli cell only syndrome. Fertil Steril. 2012;98:5–6. doi: 10.1016/j.fertnstert.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maduro MR, Niederberger C, Lipshultz LI, Casella R, Kim E, Le N, et al. Microsatellite instability and defects in mismatch repair proteins : a new aetiology for Sertoli cell-only syndrome. Mol Hum Reprod. 2003;9:61–68. doi: 10.1093/molehr/gag013. [DOI] [PubMed] [Google Scholar]
- 50.Tuttelmann F, Simoni M, Kliesch S, Ledig S, Dworniczak B, Tu F. Copy number variants in patients with severe oligozoospermia and Sertoli-cell-only syndrome. PLoS One. 2011;6:e19426. doi: 10.1371/journal.pone.0019426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Passe CMM, White CR, King MW, Quirk PL, Iovanna JL, Quirk CC. Loss of the Protein NUPR1 ( p8 ) Leads to delayed LHB expression , delayed ovarian maturation , and testicular development of a Sertoli-Cell-only syndrome-like phenotype in mice 1. Biol Reprod. 2008;79:598–607. doi: 10.1095/biolreprod.108.068304. [DOI] [PubMed] [Google Scholar]
- 52.Ni L, Xie H, Tan L, Province H. Multiple roles of FOXJ3 in spermatogenesis : a lesson from Foxj3 conditional. Mol Reprod Dev. 2016;83:1–21. doi: 10.1002/mrd.22750. [DOI] [PubMed] [Google Scholar]
- 53.Miyamoto T, Koh E, Tsujimura A, Miyagawa Y, Saijo Y, Namiki M, et al. Single-nucleotide polymorphisms in the LRWD1 gene may be a genetic risk factor for Japanese patients with Sertoli cell-only syndrome. Andrologia. 2012;46:273–276. doi: 10.1111/and.12077. [DOI] [PubMed] [Google Scholar]
- 54.Minase G, Miyamoto T, Miyagawa Y, Iijima M, Ueda H, Saijo Y, et al. Single-nucleotide polymorphisms in the human RAD21L gene may be a genetic risk factor for Japanese patients with azoospermia caused by meiotic arrest and Sertoli cell-only syndrome. Hum Fertil [Internet]. Informa UK Limited, trading as Taylor 8 Francis Group. 2017;0:000. doi: 10.1080/14647273.2017.1292004. [DOI] [PubMed] [Google Scholar]
- 55.Miyamoto T, Shin T, Iijima M, Minase G, Okada H, Saijo Y, et al. The poly ( A ) polymerase beta gene may not be associated with azoospermia caused by Sertoli- cell-only syndrome in Japanese patients by comparing patients and normal controls. J Obstet Gynaecol (Lahore) [Internet]. Taylor & Francis. 2019;0:1–3. doi: 10.1080/01443615.2018.1504205. [DOI] [PubMed] [Google Scholar]
- 56.Hayashi K, Chuva de Sousa Lopes SM, Kaneda M, Tang F, Hajkova P, Lao K, et al. MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS One. 2008;3:1–9. doi: 10.1371/journal.pone.0001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W, Tan D, Zenali MJ, Brown RE. Constitutive activation of nuclear factor-kappa B (NF-κB) signaling pathway in fibrolamellar hepatocellular carcinoma. Int J Clin Exp Pathol. 2010;3:238–243. [PMC free article] [PubMed] [Google Scholar]
- 58.Morris JK, Alberman E, Scott C, Jacobs P. Is the prevalence of Klinefelter syndrome increasing? Eur J Hum Genet. 2008;16:163–170. doi: 10.1038/sj.ejhg.5201956. [DOI] [PubMed] [Google Scholar]
- 59.Griffin DK, Finch KA. The genetic and cytogenetic basis of male infertility. Hum Fertil. 2005;8:19–26. doi: 10.1080/14647270400016407. [DOI] [PubMed] [Google Scholar]
- 60.Layman LC, Tho SPT, Clark AD, Kulharya A, McDonough PG. Phenotypic spectrum of 45,X/46,XY males with a ring Y chromosome and bilaterally descended testes. Fertil Steril. 2009;91:791–797. doi: 10.1016/j.fertnstert.2007.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akinsal E, Baydilli N. Bayramov, Ruslan Ekmekcioglu O. A Rare Cause of Male Infertility : Urol Int. 2017;101:481–485. doi: 10.1159/000484615. [DOI] [PubMed] [Google Scholar]
- 62.Gurbuz F, Ceylaner S, Erdogan S, Topaloglu AK, Yuksel B. Sertoli cell only syndrome with ambiguous genitalia. J Pediatr Endocrinol Metab. 2016;29:3–6. doi: 10.1515/jpem-2015-0458. [DOI] [PubMed] [Google Scholar]
- 63.Foresta C, Moro E, Rossi A, Rossato M, Garolla A, Ferlin A. Role of the AZFa candidate genes in male infertility. J Endocrinol Invest. 2000;23:646–651. doi: 10.1007/BF03343788. [DOI] [PubMed] [Google Scholar]
- 64.Blagosklonova O, Fellmann F, Clavequin M, Roux C, Bresson J. AZFa deletions in Sertoli cell-only syndrome : a retrospective study. Mol Hum Reprod. 2000;6:795–799. doi: 10.1093/molehr/6.9.795. [DOI] [PubMed] [Google Scholar]
- 65.Fujisawa M, Shirakawa T, Kanzaki M. Y-chromosome microdeletion and phenotype in cytogenetically normal men with idiopathic azoospermia. Fertil Steril. 2001;76:491–495. doi: 10.1016/s0015-0282(01)01955-0. [DOI] [PubMed] [Google Scholar]
- 66.Hadjkacem-loukil L, Hadj-kacem H, Salem IH, Bahloul A, Fakhfakh F. Genotyping of Tunisian azoospermic men with Sertoli cell-only and maturation arrest. Andrologia. 2004:1–7. [DOI] [PubMed]
- 67.Yang Y, Ma MY, Xiao CY, Li L, Li SW, Zhang SZ. Massive deletion in AZFb / b + c and azoospermia with Sertoli cell only and / or maturation arrest. Int J Androl. 2007;6:573–578. doi: 10.1111/j.1365-2605.2007.00808.x. [DOI] [PubMed] [Google Scholar]
- 68.Weider K, Bergmann M, Giese S, Guillou F, Failing K, Brehm R. Altered differentiation and clustering of Sertoli cells in transgenic mice showing a Sertoli cell specific knockout of the connexin 43 gene. Differentiation [Internet]. Elsevier. 2011;82:38–49. doi: 10.1016/j.diff.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Liang J, Wang N, He J, Du J, Guo Y, Li L, et al. Induction of Sertoli-like cells from human fibroblasts by NR5A1 and GATA4. Elife. 2019;8:1–27. doi: 10.7554/eLife.48767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kasak L, Punab M, Nagirnaja L, Grigorova M, Minajeva A, Lopes AM, et al. Bi-allelic recessive loss-of-function variants in FANCM cause non-obstructive azoospermia. Am J Hum Genet [Internet]. ElsevierCompany. 2018;103:200–212. doi: 10.1016/j.ajhg.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- 72.Castro-nallar E, Bacallao K, Parada-bustamante A, Madariaga M, Piottante A, Ebensperger M, et al. Androgen Receptor Gene CAG and GGN Repeat Polymorphisms. J Androl. 2010;31:552–559. doi: 10.2164/jandrol.109.008821. [DOI] [PubMed] [Google Scholar]
- 73.Yu C, Zhang Y-L, Pan W-W, Li X-M, Wang Z-W, Ge Z-J, Zhou J-J, Cang Y, Tong C, Qing-Yuan Sun H-YF CRL4 complex regulates mammalian oocyte survival and reprogramming by activation of TET proteins (Science (2013) (1518)) Science (80- ) 2014;344:470. doi: 10.1126/science.1244587. [DOI] [PubMed] [Google Scholar]
- 74.Miyamoto T, Iijima M, Shin T, Minase G, Ueda H, Okada H, et al. CUL4B mutations are uncommon in Japanese patients with Sertoli-cell-only syndrome and azoospermia. J Obstet Gynaecol (Lahore) [Internet] Informa UK Limited, trading as Taylor & Francis Group. 2017;0:1–2. doi: 10.1080/01443615.2017.1336755. [DOI] [PubMed] [Google Scholar]
- 75.Faure AK, Kerjean A, Hazzouri M, Pelletier R, Pe M, Khochbin S, et al. Misregulation of histone acetylation in Sertoli cell-only syndrome and testicular cancer. Mol Hum Reprod. 2003;9:757–763. doi: 10.1093/molehr/gag101. [DOI] [PubMed] [Google Scholar]
- 76.Matsuyama S, Matsui F, Matsuoka K, Iijima M. Gonadal function and testicular histology in males with Prader - Willi syndrome. Endocrinol Diabetes Metab. 2018;2:1–8. doi: 10.1002/edm2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bakker NE, Wolffenbuttel KP, Looijenga LHJ. Testes in Infants with Prader-Willi Syndrome : Human Chorionic Gonadotropin Treatment , Surgery and Histology. J Urol [Internet]. Elsevier Ltd. 2015;193:291–298. doi: 10.1016/j.juro.2014.07.113. [DOI] [PubMed] [Google Scholar]
- 78.O’Bryan MK, Ph D, Grealy A, Sc B, Stahl PJ, Schlegel PN. Genetic variants in the ETV5 gene in fertile and infertile men with nonobstructive azoospermia associated with Sertoli cell – only syndrome. Fertil Steril [Internet]. Elsevier Inc. 2012;98:827-835.e3. doi: 10.1016/j.fertnstert.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 79.Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM. Male-to-Female Sex Reversal in Mice Lacking Fibroblast Growth Factor 9 the coelomic lining of the gonad (the coelomic epithe-lium) occurs between E11.3 and E12.1. This proliferation gives rise to Sertoli cells (a supporting cell lineage) early. Cell. 2001;104:875–889. doi: 10.1016/s0092-8674(01)00284-7. [DOI] [PubMed] [Google Scholar]
- 80.Chung C, Sc M, Lu C, Ph D, Cheng Y, Lin C, et al. Association of aberrant expression of sex-determining gene fi broblast growth factor 9 with Sertoli cell – only syndrome. Fertil Steril [Internet]. Elsevier Inc. 2013;100:1547-1554.e4. doi: 10.1016/j.fertnstert.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Miyakawa H, Miyamoto T, Koh E, Tsujimura A, Miyagava Y, Saijo Y, et al. Single-nucleotide polymorphisms in the SEPTIN12 gene may be. 2012;33:483–7. [DOI] [PubMed]
- 82.Noveski P, Popovska-Jankovic K, Kubelka-Sabit K, Filipovski V, Lazarevski S, PT and P-KD MicroRNA expression profiles in testicular biopsies of patients with impaired spermatogenesis. Andrology. 2016;4:1–8. doi: 10.1111/andr.12246. [DOI] [PubMed] [Google Scholar]
- 83.Yao C, Sun M, Yuan Q, Niu M, Chen Z, Wang H, et al. MiRNA-133b promotes the proliferation of human Sertoli cells through targeting GLI3. Oncotarget. 2016;7:2201–2219. doi: 10.18632/oncotarget.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang C, Yao C, Tian R, Zhu Z, Zhao L, Li P, et al. miR-202-3p regulates Sertoli cell proliferation , synthesis function , and apoptosis by targeting LRP6 and cyclin D1 of Wnt / b -Catenin Signaling. Mol Ther Nucleic Acid [Internet]. Elsevier Ltd. 2019;14:1–19. doi: 10.1016/j.omtn.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li L, Zhang F, Liu S, Tian Y, Le F. Decreased expression of SAM68 in human testes with spermatogenic defects. Fertil Steril [Internet]. Elsevier Inc. 2014;102:61-67.e3. doi: 10.1016/j.fertnstert.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 86.Wollinal U, Schreiberl G, Gornigl M, Feldrappel S, Gabius MBH. Sertoli cell expression of galectin-l and -3 and accessible binding sites in normal human testis and Sertoli cell only-syndrome. Histol Histopathol. 1999;14:779–784. doi: 10.14670/HH-14.779. [DOI] [PubMed] [Google Scholar]
- 87.Ruhui T, Chencheng Y, Chao Y, Zijue Z, Chong L, Erlei Z, et al. Fibroblast growth factor-5 promotes spermatogonial stem cell proliferation via ERK and AKT activation. Stem Cell Res Ther. 2019;10:1–14. doi: 10.1186/s13287-019-1139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun T, Xin ÆZ, Jin ÆZ. Effect of TGF- b / Smad signaling on sertoli cell and possible mechanism related to complete sertoli cell-only syndrome. Mol Cell Biochem. 2008;319:1–7. doi: 10.1007/s11010-008-9869-3. [DOI] [PubMed] [Google Scholar]
- 89.Hai Y, Sun M, Niu M, Yuan Q, Guo Y, Li Z, et al. BMP4 promotes human Sertoli cell proliferation via Smad1/5 and ID2/3 pathway and its abnormality is associated with azoospermia. Discov Med. United States. 2015;19:311–325. [PubMed] [Google Scholar]
- 90.Paduch DA, Hilz S, Grimson A, Schlegel PN, Jedlicka E, Wright WW. Aberrant gene expression by Sertoli cells in infertile men with Sertoli cell-only syndrome. PLoS One. 2019;14:1–27. doi: 10.1371/journal.pone.0216586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adly MA, Rezk M, Hussein A. Immunohistological profile of the ras homologous B Protein ( RhoB ) in human testes showing normal spermatogenesis , spermatogenic arrest and Sertoli cell only syndrome. Pathol Oncol Res. 2010;16:427–433. doi: 10.1007/s12253-009-9232-3. [DOI] [PubMed] [Google Scholar]
- 92.Parada-Bustamante A, Molina C, Valencia C, Florez M, Lardone MC, Argandona F, et al. Disturbed testicular expression of the estrogen-metabolizing enzymes CYP1A1 and COMT in infertile men with primary spermatogenic failure : possible negative implications on Sertoli cells. Andrology. 2017;5:486–494. doi: 10.1111/andr.12346. [DOI] [PubMed] [Google Scholar]
- 93.Lan K, Chen Y, Chang C, Chang Y, Lin H. Up-regulation of SOX9 in Sertoli cells from testiculopathic patients accounts for increasing anti- mullerian hormone expression via impaired androgen receptor signaling. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0076303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lardone MC, Argandoña F, Flórez M, Parada-Bustamante A, Ebensperger M, Palma C, Piottante A, Castro A. Overexpression of CYP19A1 aromatase in Leydig cells is associated with steroidogenic dysfunction in subjects with Sertoli cell-only syndrome. Andrology. 2017;5:41–48. doi: 10.1111/andr.12289. [DOI] [PubMed] [Google Scholar]
- 95.Lardone MC, Argandoña F, Lorca M, Piottante A, Flórez M, Palma C, Ebensperger M, Castro A. Leydig cell dysfunction is associated with post-transcriptional deregulation of CYP17A1 in men with Sertoli cell-only syndrome. Mol Hum Reprod. 2018;24:203–210. doi: 10.1093/molehr/gay006. [DOI] [PubMed] [Google Scholar]
- 96.Fietz D, Bakhaus K, Wapelhorst B, Grosser G, Gu S, Kliesch S, et al. Membrane transporters for sulfated steroids in the human testis - cellular localization , expression pattern and functional analysis. PLoS One. 2013;8:e62638. doi: 10.1371/journal.pone.0062638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rommerts FF, de Jong FH, Grootegoed JA, van der MHJ Metabolic changes in testicular cells from rats after long-tern exposure to 37 degrees C in vivo or in vitro. J Endocrinol. 1980;85:471–479. doi: 10.1677/joe.0.0850471. [DOI] [PubMed] [Google Scholar]
- 98.Hacker U, Schumann J, Göhde W, Müller K. Mammalian spermatogenesis as a biologic dosimeter for radiation. Acta Oncol (Madr). 1981;20:279–282. doi: 10.3109/02841868109130207. [DOI] [PubMed] [Google Scholar]
- 99.Chowdhury I, Tharakan B, Bhat GK. Caspases - an update. Comp Biochem Physiol - B Biochem Mol Biol. 2008;151:10–27. doi: 10.1016/j.cbpb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 100.Almeida C, Correia S, Rocha E. Angela Alves, Luís Ferraz, Joquina Silva, Mário Sousa AB. Caspase signalling pathways in human spermatogenesis. J Assist Reprod Genet. 2013;4:487–495. doi: 10.1007/s10815-013-9938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim S, Yoon Y, Park Y. Involvement of the Fas – Fas ligand system and active caspase-3 in abnormal apoptosis in human testes with maturation arrest and Sertoli cell – only syndrome. Fertil Steril. 2007;87:547–553. doi: 10.1016/j.fertnstert.2006.07.1524. [DOI] [PubMed] [Google Scholar]
- 102.Lei B, Wan B, Peng J, Yang Y, Lv D, Zhou X, et al. PRPS2 expression correlates with Sertoli cell-only syndrome and inhibits the apoptosis of TM4 Sertoli cells. J Urol [Internet]. Elsevier Ltd. 2015;15:04078-1. doi: 10.1016/j.juro.2015.04.116. [DOI] [PubMed] [Google Scholar]
- 103.Zhang H, Zhou D, Zhu F, Chen F, Zhu Y, Yu R, et al. Disordered APC / C - mediated cell cycle progression and IGF1 / PI3K / AKT signalling are the potential basis of Sertoli cell - only syndrome. Andrologia. 2019;51:1–8. doi: 10.1111/and.13288. [DOI] [PubMed] [Google Scholar]
- 104.Asbjørnsen G, Molne K, Klepp O, Aakvaag A. Testicular function after combination chemotherapy for Hodgkin’s disease. Scand J Haematol. 1976;16:66–69. doi: 10.1111/j.1600-0609.1976.tb01118.x. [DOI] [PubMed] [Google Scholar]
- 105.Rodriguez-Rigau LJ, Tcholakian RK, Smith KD, Steinberger E. In vitro steroid metabolic studies in human testes I: Effects of estrogen on progesterone metabolism. Steroids. United States. 1977;29:771–786. doi: 10.1016/0039-128x(77)90121-0. [DOI] [PubMed] [Google Scholar]
- 106.Mayerhofer A, Frungieri MB, Fritz S, Bulling A, Jessberger BVHJ. Evidence for catecholaminergic, neuronlike cells in the adult human testis: changes associated with testicular pathologies. J Androl. 1999;20:341–347. [PubMed] [Google Scholar]
- 107.Gat Y, Gornish M, Perlow A, Chakraborty J, Levinger U, Pasqualotto F. Azoospermia and Sertoli-cell-only syndrome : hypoxia in the sperm production site due to impairment in venous drainage of male reproductive system. Andrologia. 2010;42:314–321. doi: 10.1111/j.1439-0272.2010.01047.x. [DOI] [PubMed] [Google Scholar]
- 108.Pajarinen JT, Karhunen PJ. Spermatogenic arrest and “Sertoli cell-only” syndrome- common alcohol-induced disorders of the human testis. Int J Androl. 1994;17:292–299. doi: 10.1111/j.1365-2605.1994.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 109.Liu J, Ding D, Liu J. Varicocele-caused progressive damage in bilateral testis and Sertoli Cell-only syndrome in homolateral testis in rats. Med Sci Monit. 2014;20:1931–1936. doi: 10.12659/MSM.891324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pei G, Youngf H, Goldstein M, Phillips DM, Sundaram K, Gunsalus GL, et al. Sertoli Cell-only syndrome produced by cold testicular. Endocrinology. 1988;122:3–4. doi: 10.1210/endo-122-3-1074. [DOI] [PubMed] [Google Scholar]
- 111.Schoor RA, Elhanbly S, Niederberger CS, Ross LS. The role of testicular biopsy in the modern management of male infertility. J Urol. 2002;167:197–200. [PubMed] [Google Scholar]
- 112.Bettocchp C, Parkinson MC, Ralph DJ, Pryor JP. Clinical aspects associated with Sertoli-cell-only histology. Br J Urol. 1998;82:534–537. doi: 10.1046/j.1464-410x.1998.00771.x. [DOI] [PubMed] [Google Scholar]
- 113.Tyler-Smith C, Krausz C. The will-o’-the-wisp of genetics—hunting for the azoospermia factor gene. N Engl J Med. Europe PMC Funders. 2009;360:925. doi: 10.1056/NEJMe0900301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paulis G, Paulis L, Concas C, Di Sarno M, Pagano R, Di Filippo A, et al. Pregnancy and live birth after follicle-stimulating hormone treatment for an infertile couple including a male affected by Sertoli cell-only syndrome. Res Reports Urol. 2017;9:203–208. doi: 10.2147/RRU.S148071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ustuner M, Yilmaz H, Yavuz U, Ciftci S, Saribacak A, Aynur BS, Yasar H, Culha MM. Varicocele repair improves testicular histology in men with nonobstructive azoospermia. Biomed Res Int. 2015;2015:1–6. doi: 10.1155/2015/709452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pasqualotto FF, Sobreiro BP, Hallak J, Pasqualotto EB, Lucon AM. Induction of spermatogenesis in azoospermic men after varicocelectomy repair: an update. Fertil Steril. Elsevier. 2006;85:635–639. doi: 10.1016/j.fertnstert.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 117.Lee JS, Park HJ, Seo JT. What is the indication of varicocelectomy in men with nonobstructive azoospermia? Urology. Elsevier. 2007;69:352–355. doi: 10.1016/j.urology.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 118.Kadioǧlu A, Tefekli̇ A, Cayan S, Kandirali E, Erdemi̇r F, Tellaloǧlu S. Microsurgical inguinal varicocele repair in azoospermic men. Urology. Elsevier; 2001;57:328–333. [DOI] [PubMed]
- 119.Cakan M, Altuğ U. Induction of spermatogenesis by inguinal varicocele repair in azoospermic men. Arch Androl. Taylor & Francis. 2004;50:145–150. doi: 10.1080/01485010490425250. [DOI] [PubMed] [Google Scholar]
- 120.Esteves SC, Glina S. Recovery of spermatogenesis after microsurgical subinguinal varicocele repair in azoospermic men based on testicular histology. Int braz j urol. SciELO Brasil. 2005;31:541–548. doi: 10.1590/s1677-55382005000600005. [DOI] [PubMed] [Google Scholar]
- 121.Esteves SC, Agarwal A. Re: Sperm retrieval rates and intracytoplasmic sperm injection outcomes for men with non-obstructive azoospermia and the health of resulting offspring. Asian J Androl [Internet]. 2014/04/22. Medknow Publications & Media Pvt Ltd; 2014;16:642. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4104100/
- 122.Tournaye H, Verheyen G, Nagy P, Ubaldi F, Goossens A, Silber S, et al. Are there any predictive factors for successful testicular sperm recovery in azoospermic patients? Hum Reprod. England. 1997;12:80–86. doi: 10.1093/humrep/12.1.80. [DOI] [PubMed] [Google Scholar]
- 123.Mancini M, Carmignani L, Gazzano G, Sagone P, Gadda F, Bosari S, Rocco F, Colpi GM. High prevalence of testicular cancer in azoospermic men without spermatogenesis. Hum Reprod. 2007;22:1042–1046. doi: 10.1093/humrep/del500. [DOI] [PubMed] [Google Scholar]
- 124.Schlegel PN. Testicular sperm extraction : microdissection improves sperm yield with minimal tissue excision. Hum Mol Genet. 1999;14:131–135. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 125.Eliveld J, van Wely M, Meißner A, Repping S, van der Veen F, van Pelt AMM. The risk of TESE-induced hypogonadism: a systematic review and meta-analysis. Hum Reprod Update. Oxford University Press. 2018;24:442–454. doi: 10.1093/humupd/dmy015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Esteves SC, Varghese AC. Laboratory handling of epididymal and testicular spermatozoa: what can be done to improve sperm injections outcome. J Hum Reprod Sci. Wolters Kluwer--Medknow Publications. 2012;5:233. doi: 10.4103/0974-1208.106333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deruyver Y, Vanderschueren D, Van der Aa F. Outcome of microdissection TESE compared with conventional TESE in non-obstructive azoospermia : a systematic review. Andrology. 2013;2:20–24. doi: 10.1111/j.2047-2927.2013.00148.x. [DOI] [PubMed] [Google Scholar]
- 128.Gul U, Turunc T, Haydardedeoglu B, Yaycioglu O, Kuzgunbay B. Sperm retrieval and live birth rates in presumed Sertoli-cell-only syndrome in testis biopsy : a single centre experience. Andrology. 2013;1:47–51. doi: 10.1111/j.2047-2927.2012.00003.x. [DOI] [PubMed] [Google Scholar]
- 129.Modarresi T, Sc M, Hosseinifar H, Sc M, Hampa AD, Chehrazi M, et al. Predictive factors of successful microdissection testicular sperm extraction in patients with presumed Sertoli cell-only syndrome. Int J Fertil Steril. 2013;9:107–112. doi: 10.22074/ijfs.2015.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yamamoto Y, Sofikitis N, Mio Y, Miyagawa I. Highly sensitive quantitative telomerase assay of diagnostic testicular biopsy material predicts the presence of haploid spermatogenic cells in therapeutic testicular biopsy in men with Sertoli cell-only syndrome. Hum Reprod. 1999;14:3041–3047. doi: 10.1093/humrep/14.12.3041. [DOI] [PubMed] [Google Scholar]
- 131.Berookhim BMGDP, Zaninovic N, Rosenwaks Z, Peter N, Schlegel M. Microdissection testicular sperm extraction in men with sertoli cell only testicular histology. Fertil Steril. 2014;102:1282–1286. doi: 10.1016/j.fertnstert.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu Y, Xi Q, Wang R, Zhang H. Intraoperative assessment of tubules in predicting microdissection testicular sperm extraction outcome in men with Sertoli cell-only syndrome. J Int Med Res. 2018;47:722–729. doi: 10.1177/0300060518809257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aponte PM, Schlatt IIS, Luiz III, De Franca R. Biotechnological approaches to the treatment of aspermatogenic men. Clinics. 2013;68(S1):157–167. doi: 10.6061/clinics/2013(Sup01)18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang X, Xie S, Li Z, Ye Z, Gu X, Zhou L, et al. Generation of an iPSC line ( HUSTi002-A ) from fi broblasts of a patient with Sertoli cell-only syndrome carrying c . 731 _ 732delAT in PIWIL2 gene. Stem Cell Res [Internet]. Elsevier. 2020;42:101703. doi: 10.1016/j.scr.2020.101703. [DOI] [PubMed] [Google Scholar]
- 135.Li J, Guo W, Li F, He J, Yu Q, Wu X, et al. HnRNPL as a key factor in spermatogenesis : Lesson from functional proteomic studies of azoospermia patients with sertoli cell only syndrome ☆. J Proteomics [Internet]. Elsevier B.V. 2012;75:2879–2891. doi: 10.1016/j.jprot.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 136.Alikhani M, Ali M, Gilani S, Zabet-moghaddam M, Parker L, Wo Y, et al. Quantitative proteomic analysis of human testis reveals system-wide molecular and cellular pathways associated with non-obstructive azoospermia. J Proteomics [Internet]. Failure:Problem in retrieving ISSN for S1874391917300568. 2017. 10.1016/j.jprot.2017.02.007. [DOI] [PubMed]
- 137.Yu Q, Gu X, Shang X, Li H, Xiong C. Discrimination and characterization of Sertoli cell-only syndrome in non-obstructive azoospermia using cell-free seminal DDX4. Reprod Biomed Online [Internet]. Elsevier Ltd. 2016. 10.1016/j.rbmo.2016.05.001. [DOI] [PubMed]
- 138.Abofoul-azab M, Lunenfeld E, Levitas E, Zeadna A. Identification of Premeiotic, Meiotic, and Postmeiotic Cells in Testicular Biopsies Without Sperm from Sertoli Cell-Only Syndrome Patients. Int J Mol Sci. 2019;20:470. doi: 10.3390/ijms20030470. [DOI] [PMC free article] [PubMed] [Google Scholar]