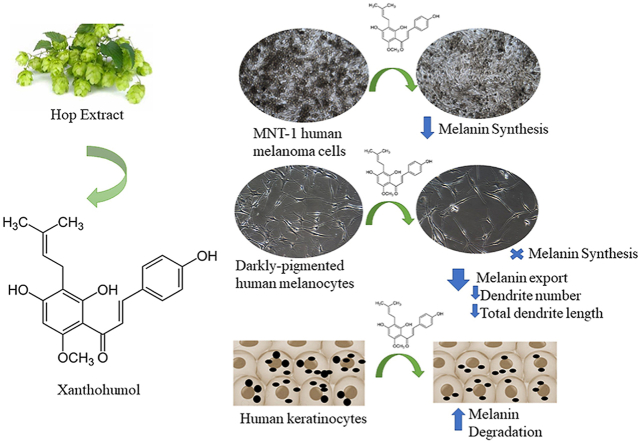
Abstract
Xanthohumol (XH) is the most abundant prenylated flavonoid found in the hop plant (Humulus lupulus L.) and has previously been shown to have depigmenting effects in B16F10 mouse melanoma cells; however, studies of its depigmenting efficacy in human melanocytes are still lacking. In this work, we explored the effects of XH on melanogenesis in MNT-1 human melanoma cells and normal human melanocytes from darkly-pigmented skin (HEM-DP). XH was screened for cytotoxicity over 48 h, and subsequently tested on melanogenesis in MNT-1 cells. XH was further tested in HEM-DP cells for melanin synthesis and melanosome export; dendricity was quantitated to assess effects on melanosome export. Melanosome degradation was studied in human keratinocytes (HaCaT). Our results showed that XH inhibited melanin synthesis in MNT-1 cells at 30 μM but increased intracellular tyrosinase activity without affecting ROS levels. In HEM-DP cells, XH robustly suppressed cellular tyrosinase activity at nontoxic concentrations (2.5–5 μM) without any effect on melanin synthesis. However, XH inhibited melanosome export by reducing dendrite number and total dendrite length. Further testing in HaCaT cells demonstrated that XH induced melanosome degradation at low micromolar concentrations without any cytotoxicity. In summary, our results demonstrate that XH at low micromolar concentrations might hold promise as a potent inhibitor of human pigmentation by primarily targeting melanin export and melanin degradation. Further studies to elucidate the signaling mechanisms of action of melanosome export inhibition by XH and in vivo efficacy are warranted.
Keywords: Xanthohumol, Human melanocytes, Anti-melanogenic, Dendricity, Melanosome degradation
Graphical abstract
Highlights
-
•
Xanthohumol (XH) inhibited melanin synthesis in MNT-1 human melanoma cells.
-
•
XH did not inhibit melanin synthesis in primary human melanocytes but significantly suppressed both dendrite number and total dendrite length at low micromolar concentrations.
-
•
Reduction of melanosome export by reduction in dendricity was correlated with the inhibition of intracellular tyrosinase activity.
-
•
XH induced melanosome degradation in human keratinocytes.
-
•
XH is a candidate for skin-lightning which inhibits human melanogenesis by targeting later steps in melanogenesis.
List of abbreviations
- XH
Xanthohumol
- DMSO
Dimethyl Sulfoxide;
- l-DOPA
3,4-Dihydroxy-l-phenylalanine;
- DMEM
Dulbecco's Modified Eagle's Medium
- PBS
Phosphate Buffered Saline;
- MEM
Minimum Essential Medium
- MTS
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
- BCA
Bicinchoninic Acid
- HI-FBS
Heat-Inactivated Fetal Bovine Serum
- HMGS
Human Melanocyte Growth Supplement
- DP
Darkly-Pigmented
- TDL
Total Dendrite Length
- PNG
P-Nitrophenyl-α-d-glucopyranoside;
- ROS
Reactive Oxygen Species
- HBSS
Hank's Buffered Salt Saline
- DCF
Dichlorofluorescein
1. Introduction
Ultraviolet radiation (UVR) activates the central neuroendocrine system to maintain body homeostasis and also induces upregulation of synthesis of melanin pigment [1]. The pigment melanin in the skin provides UV photoprotection, quenches free radicals, and chelates toxic ions, however an aberrant overproduction of melanin causes disorders of hyperpigmentation such as age spots, freckles, melasma, drug-induced pigmentation and post-inflammatory hyperpigmentation (PIH) [2,3]. These affect social life and pose cosmetic concerns, especially for female populations who desire a flawless complexion. Melanin is synthesized by neural-crest derived cells -melanocytes located at the basement membrane of the skin; inside melanocytes the pigment is synthesized in specialized organelles called melanosomes. Post-synthesis, melanin is exported from the melanocyte dendrite tips into keratinocytes; these cells then migrate towards the upper layers of skin laden with melanosomes, causing the visual appearance of skin pigmentation [4]. Tyrosinase, a glycoprotein, is the key rate-limiting enzyme which catalyzes two early steps in the synthesis of melanin; it catalyzes the conversion of l-Tyrosine to l-DOPA, which is oxidized to Dopaquinone and polymerized to melanin [5]. In melanocytes, l-Tyrosine is transported into melanosomes from the extracellular space and initiates the melanogenic pathway in vivo [6]. Apart from regulating melanogenic pathway, l-Tyrosine and l-DOPA are also known to regulate cellular functions in a direct or indirect manner via non-receptor mediated processes [7].
Commercially used skin-whitening agents, such as hydroquinone, arbutin, and kojic acid have exhibited serious side-effects [8,9] and are no longer deemed safe. Additionally, rhododendrol, a natural skin-whitening cosmetic was recalled after it caused adverse reactions of leukoderma and vitiligo in the Japanese population [10]. Recently, natural bioactives have emerged which do not exhibit these serious side-effects; there have been reports of melanogenesis inhibition by many natural product extracts or purified bioactives. Moreover, since the deadly skin cancer, melanoma, is characterized by a high melanin content [11,12], the identification of a natural compound that may inhibit melanogenesis is also significant as such compounds could be used as adjuvants in the therapy of melanotic melanomas. Melanogenesis inhibitors are not only limited to compounds which reduce melanin synthesis in cells, but also compounds which can suppress melanosome export, as after being synthesized in the cells, melanin is secreted from melanocytes and phagocytosed by keratinocytes via various pathways [13]. The traditional tyrosinase-based inhibitors of melanogenesis which function by reducing melanin accumulation in the cells are not very appealing, partly due to a rise in the search of novel compounds which can target different steps in melanogenesis pathway, in particular, melanosome export. Furthermore, it has been documented that post-transfer, melanosomes are degraded in keratinocytes; melanosomes in keratinocytes from lightly-pigmented skin are degraded faster as compared to those from darkly-pigmented skin [14]. This has also spurred research into cosmetic agents which can target melanosome degradation in keratinocytes.
Xanthohumol (XH; chemical structure shown in Fig. 1A) is a bioactive prenylated flavonoid derived from the female inflorescence of the hop plant (Humulus lupulus L.) and constitutes up to 0.1–1% of dry weight of hops [15]. XH has been shown to exhibit a broad spectrum of biological activities such as anticancer activity [16], antibacterial activity [17], and anti-inflammatory activity; it inhibits both elastase and matrix metalloproteinase (MMP) activity [18]. The biological benefits of XH on skin health have been reviewed previously [19]. XH showed neuroprotective effects in ischemia-reperfusion injury model [20] and has recently demonstrated anti-Alzheimer's activity by inhibiting aggregation of amyloid-β [21] and tau proteins [22]. Furthermore, XH-enriched functional foods and extracts can reach pharmacologically relevant concentrations [23] and XH has been demonstrated to have a well-tolerated safety profile for in vivo consumption as reported in a previous study [24].
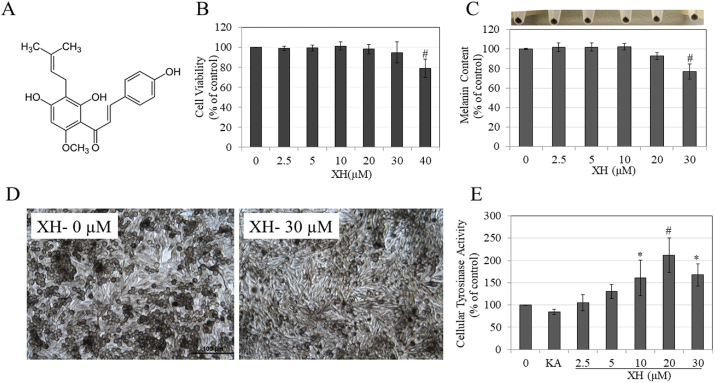
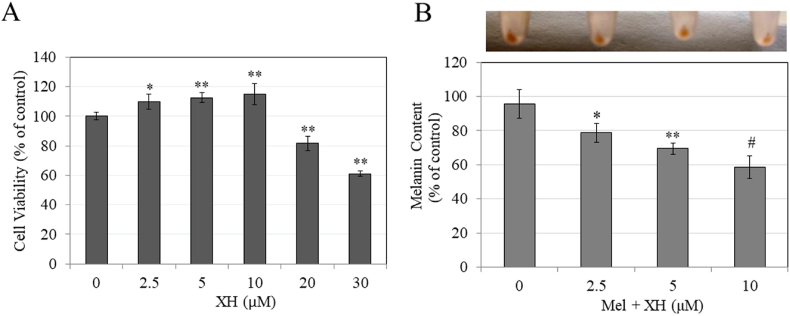
Fig. 1.
A) Chemical structure of XH; B) Viability of MNT-1 human melanoma cells treated with XH at different concentrations for 48 h; #p < 0.01 vs. control; One-Way ANOVA with Dunnett's test; C) Melanin assay in MNT-1 human cells treated with XH for 48 h with corresponding panel showing photos of cell pellets; #p < 0.01 vs. control; One-Way ANOVA with Dunnett's test; D) Representative bright-field micrographs showing control and XH (30 μM) group. Scale bar corresponds to 100 μm; E) Cellular tyrosinase activity in lysates of MNT-1 cells treated with XH (0–30 μM) and Kojic acid (KA) at 1 mM as a positive control; *p < 0.05; #p < 0.0001 vs. control; One-Way ANOVA with Tukey's test; Data is Mean ± SD of at least three independent experiments for all except E) which is Mean ± SD of four independent experiments.
Previous studies have reported that XH inhibited mushroom tyrosinase activity in an acellular system [25] and inhibited melanin synthesis in B16F10 mouse melanoma cells. The authors concluded that XH is a skin-whitening agent [26]. However, in the latter study, the effect of XH on melanosome export was never reported. Moreover, at this time there are no reports if XH can inhibit melanogenesis in human melanocytes. Hence, in this work, we have studied the effects of XH on melanogenesis in MNT-1 human melanoma cells and primary human melanocytes and delineated a novel mechanism of anti-melanogenic activity of this compound at low micromolar concentrations. Our findings support the potential of XH as a skin-lightning agent for diminution of human melanogenesis.
2. Materials and methods
2.1. Materials
XH (>98% purity) were purchased from Enzo Life Sciences (NY, USA). Tyrosinase from mushroom (Cat#: T3824-25KU), l-DOPA (Cat#: D9628) and synthetic melanin (Cat#: M8631) were purchased from Sigma-Aldrich (St. Louis, MO). 2′, 7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) reagent was purchased from Molecular Probes.
2.2. Cell culture
MNT-1 human melanoma cells were obtained as a kind gift from Dr. Michael Marks, University of Pennsylvania, and were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 18% heat-inactivated fetal bovine serum (HI-FBS), 1% minimum essential medium (MEM), 1% antibiotics (penicillin and streptomycin), and 10% AIM-V media (Invitrogen). Human primary epidermal melanocytes from darkly-pigmented (HEM-DP) neonatal donor was obtained from Cascade Biologics (Portland, OR) and were cultured in Medium 254 (Cascade Biologics) supplemented with 1% human melanocyte growth supplement (HMGS, Cascade Biologics) and 1% antibiotics. Human keratinocytes (HaCaT) were purchased from AddexBio (San Diego, CA) and cultured in DMEM medium supplemented with 1% antibiotics and 10% HI-FBS. All cells were cultured at 37 °C in a humidified atmosphere with 95% air and 5% CO2.
2.3. Cytotoxicity assay in MNT-1 human melanoma cells
In order to test the effects of XH on melanogenesis, we first screened nontoxic concentrations of the compounds using MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) cytotoxicity assay. Briefly, MNT-1 cells (3 × 104 cells/well) were cultured in a 96-well plate for 24 h at 37 °C in 5% CO2 incubator. After 24 h, the medium was replaced by fresh medium containing XH at various concentrations, and further maintained for 48 h. At the end of 48 h, the culture medium was aspirated and replaced by 100 μl of fresh medium with 20 μl of MTS reagent and incubated for 1 h. After that, the absorbance was read at 490 nm using a Versamax® microplate reader and cell viability was calculated from the absorbance values relative to control groups and expressed in %.
2.4. Melanin assay in MNT-1 cells
MNT-1 cells (2.3 × 105 cells/well) were seeded in 12-well plates and cultured for 48 h after which XH was added and cultures maintained for another 48 h. At the end of the treatments, cells were harvested, and cell pellets were washed in PBS. After aspiration, 250 μL of 1 N NaOH was added and heated to 70 °C for 45 min to solubilize melanin. Next, 200 μl aliquots of lysate were transferred to a 96-well plate and the absorbance was read at 475 nm using a microplate reader. A portion of the lysate was used to evaluate total protein content using BCA assay (Pierce BCA kit, Thermo Scientific). The absorbance was normalized to total protein contents and reported as relative melanin levels as % of control.
2.5. Tyrosinase assay in MNT-1 cells
We quantitated the cellular tyrosinase activity as l-DOPA oxidase activity which involves the conversion of DOPA to Dopachrome based on established method [2]. Briefly, MNT-1 cells were seeded in 12-well plates at a density of 2.3 × 105 cells/well. After 48 h, the medium was changed and XH was added, and further incubated for 48 h. At the end of treatments, cells were trypsinized and cell pellets were washed in PBS and lysed. 50 μl of lysates were then aliquoted in a 96-well microplate and 100 μl of 3 mM l-DOPA solution (freshly prepared in phosphate buffer, pH 6.5) was added. The absorbance was measured at 475 nm in the kinetic mode every 30 s for 40 min at 30 °C using a microplate reader. The % tyrosinase activity was calculated from the slope of the linear range of the velocities of inhibition and was normalized by the total protein contents assayed by BCA kit.
2.6. Intracellular α-glucosidase activity in MNT-1 cells
Previous reports have established that the activity of α-glucosidase enzyme regulates the early stages of tyrosinase maturation [[27], [28], [29]]. In order to test if XH's anti-melanogenic activity might be explained, at least in part, by the inhibition of α-glucosidase activity, MNT-1 cellular lysates were tested with p-nitrophenyl-α-d-glucopyranoside (PNG) substrate. This substrate reacts with α-glucosidase enzyme to produce p-nitrophenol which can be measured spectrophotometrically. MNT-1 cells (4.5 × 105 cells/well) were grown in 6-well plates for 48 h followed by addition of XH at various concentrations and incubated for a further 48 h. Cells were harvested, lysed and 50 μl of lysates were aliquoted in a 96-well plate and 100 μl of 2 mM PNG substrate was added. The rate of the formation of the reaction product was monitored at 405 nm in the kinetic mode for 45 min at 37 °C in a microplate reader. The intracellular α-glucosidase activity (%) was calculated as: (rate of sample reaction/rate of control reaction) × 100%.
2.7. Cellular ROS generation in MNT-1 cells
We next evaluated ROS production in MNT-1 cells treated with XH to test if reduced melanin production might be explained by a reduction in ROS generation since melanogenesis is accompanied by increased ROS generation and oxidative stress [30]. For this, we used the nonfluorescent H2DCFDA probe which is converted to highly fluorescent 2′,7′-dichlorofluorescein (DCF) upon oxidation by ROS. Briefly, MNT-1 cells (1 × 105 cells/well) were cultured in a 24-well plate for 48 h followed by replacement of medium containing XH and incubated for another 48 h. At the end of treatment, the medium was aspirated, cells were washed with HBSS buffer and then incubated in 50 μM DCFH-DA dye solution (prepared in serum-free, pyruvate-free, phenol-red free DMEM medium) for 45 min at 37 °C. Subsequently, the medium was aspirated, cells washed in buffer and the plate was read in a fluorescence microplate reader at excitation/emission of 485/535 nm using the well-scan mode. Results are reported as % relative fluorescence intensity.
2.8. Cytotoxicity in HEM-DP cells
MTS assay was used to screen XH for cytotoxicity in HEM-DP cells. Briefly, 3.2 × 104 cells/well were inoculated in a 48-well plate for 24 h and then XH was added and cultures were maintained for 48 h. MTS assay was conducted similar to method reported earlier, and cell viability reported as % normalized to negative control after blank subtractions.
2.9. Melanin and tyrosinase activity assay in HEM-DP cells
We next assayed the effects of XH on melanin synthesis levels and intracellular tyrosinase activity in HEM-DP cells similar to the method outlined before. For melanin assay, HEM-DP cells (1.9 × 105 cells/well) were seeded in a 12-well plate and cultured for 48 h followed by addition of XH and cultured maintained for 48 h. Cells were harvested, washed, and solubilized in 200 μl of NaOH at 70 °C and then 150 μl were aliquoted in a 96-well plate and absorbance was read at 475 nm; the absorbance was normalized to the total protein content and reported as % of control.
For assaying cellular tyrosinase activity, HEM-DP cells were cultured in 12-well plates (1.3 × 105 cells/well) and after 48 h, the medium was replaced with fresh medium containing various concentrations of XH and cultured for another 48 h. After the treatment, the cells were harvested, lysed, and 25 μl of lysates were incubated with 75 μl of 3 mM l-DOPA and the absorbance was measured at 475 nm for 20 min (with reading every 30 s at 30 °C) using a microplate reader. The % tyrosinase activity was calculated from the slope of the linear range of the velocities of inhibition and was normalized to the total protein contents assayed by BCA kit.
2.10. Dendricity analysis in HEM-DP cells
Melanocyte cytosolic projections known as dendrites are critical conduits for pigment export to keratinocytes and quantification of dendricity parameters were based on the parameters reported previously [31]. HEM-DP cells were cultured in 12-well plates at 5 × 104 cells/well for 24 h and then XH were added for 48 h. At the end of experiments, 5–6 random microscopic fields were imaged at 20× objective magnification and analyzed using a Nikon Labphot microscope with digital camera and computer-interfaced NIS Elements 5.0 imaging software. Dendrite lengths were measured from the center of each cell to the tip and these parameters were computed: i) Total dendrite length; ii) Number of dendrites; and iii) % of cells >3 dendrites.
2.11. Cytotoxicity assay in HaCaT cells and melanosome degradation assay in HaCaT
We first tested XH in HaCaT cells over a duration of 48 h for cytotoxicity before conducting the melanosome degradation assay. Briefly, 2 × 104 HaCaT cells were seeded in a 96-well plate for 24 h followed by replacement of medium with XH at various doses and incubation for another 48 h. At the end of treatments, MTS assay was conducted with similar protocol as outlined earlier with an incubation of 90 min.
2.12. Melanosome degradation assay in HaCaT
The degradation of melanosomes in keratinocytes was studied using a previously reported method with some modifications [32]. Briefly, HaCaT cells were seeded in 6-well plates and grown for 48 h followed by the addition of synthetic melanin and incubation was continued for another 48 h. Post incubations, the wells were extensively washed in HBSS to remove unbound melanin and XH was added at various doses and the cultures were maintained for 48 h. At the end of treatment, melanin content was analyzed using method outlined earlier and the results were normalized to total protein content and were reported as % of control after subtraction of background absorbance of negative control which consisted of cells without melanin.
2.13. Statistical analysis
One-way analysis of variance (ANOVA) with Dunnett's or Tukey's post-hoc test was run using GraphPad Prism software (version 8.0; La Jolla, California). Differences were considered statistically significant at p < 0.05. All data are reported as Mean ± SD.
3. Results
3.1. Effects of XH on MNT-1 cell viability
The results of viability of MNT-1 cells upon treatment with XH are summarized in Fig. 1B. XH was nontoxic in the concentration range 2.5–30 μM, but induced significant toxicity at 40 μM (p < 0.01). Hence, in subsequent experiments, we selected a concentration range of XH over 2.5–30 μM to test for effects on melanogenesis.
3.2. Effects of XH on melanin synthesis in MNT-1 cells
After screening nontoxic concentrations of XH, we next tested its effects on melanogenesis in MNT-1 cells over a 48 h duration; our results are summarized in Fig. 1C. Visual examination of cell pellets showed that the pellet of group treated with XH at 30 μM showed a slightly less intense coloration compared to untreated control group. Quantitation of melanin showed that XH significantly inhibited melanin synthesis by 23% (p < 0.01) at 30 μM. The images of cells showed no change in morphology after treatment with XH at 30 μM, except a visible reduction in intracellular melanin pigment was noted (Fig. 1D).
3.3. Effects of XH on intracellular tyrosinase activity in MNT-1 cells
Tyrosinase activity was assessed in cellular lysates of MNT-1 cells to study if reduction in melanogenesis could be explained, at least in part, by a reduction in the activity of tyrosinase enzyme. Our results showed that XH unexpectedly stimulated intracellular tyrosinase activity over a concentration range of 5–30 μM, with a peak at 20 μM (Fig. 1E). Tyrosinase activity was upregulated by 30.34%, 60.81%, 112% and 67.85%, by XH at concentrations of 5, 10, 20 and 30 μM, respectively; significance was achieved for concentrations 10, 20 and 30 μM. These results indicate that alterations in tyrosinase activity are not related to the anti-melanogenic action of XH. The auto-oxidation of DOPA was not corrected in control groups since that found to be negligible (Fig. S1).
3.4. Effects of XH on intracellular α-glucosidase activity
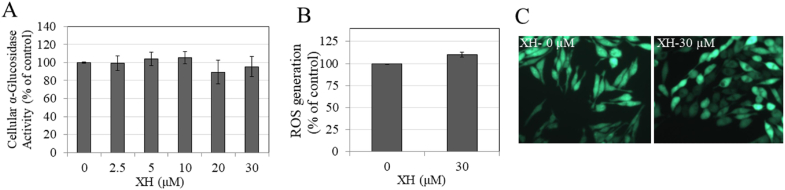
Since tyrosinase maturation is regulated by the activity of α-glucosidase enzyme, we next tested if the inhibition of melanin synthesis by XH could be explained, in part, by inhibition of intracellular glucosidase activity. The results showed that XH did not affect intracellular α-glucosidase activity at any concentration (Fig. 2A).
Fig. 2.
A) Intracellular α-glucosidase activity in MNT-1 cells treated with XH at various doses for 48 h; B) Intracellular ROS generation levels in MNT-1 cells and; C) Representative fluorescent images showing cells from control and XH (30 μM) group; All Data is Mean ± SD of at least two independent experiments.
3.5. Effects of XH on intracellular ROS generation in MNT-1 cells
Next, we estimated ROS generation in MNT-1 cells to evaluate if the anti-melanogenic activity of XH (30 μM) could be related to a reduction of intracellular ROS generation; however our results showed that XH had no effect on ROS levels as compared to control (Fig. 2B); fluorescent images also showed no changes in signal intensity (Fig. 2C).
3.6. Effects of XH on viability, melanin synthesis and cellular tyrosinase activity in HEM-DP cells
In order to confirm if XH can display anti-melanogenic activity in normal melanocytes, our next step consisted of testing XH for cytotoxicity in HEM-DP cells. As shown in Fig. 3A, XH induced significant cytotoxicity at 10 μM, with a 65.97% reduction of viability, while concentrations <10 μM were nontoxic and were thus selected for further study.
Fig. 3.
A) Viability of HEM-DP cells in the presence of different concentrations of XH for 48 h measured by MTS assay; Data is Mean ± SD of at least two independent experiments; B) Cellular tyrosinase activity in HEM-DP treated with XH for 48 h; *p < 0.05; **p < 0.01 vs. control; One-way ANOVA with Dunnett's test; Data is Mean ± SD of at least two independent experiments; C) Melanin content assay in cultures of HEM-DP cells treated for 48 h with different concentrations of XH with corresponding panel showing photos of cell pellets (n = 4 per group).
XH inhibited tyrosinase activity in a dose-dependent manner by 36.57% and 47.61% at 2.5 and 5 μM, respectively (p < 0.01, Fig. 3B). However, XH did not affect melanin synthesis in cells at any concentration as detected visually or after quantitation (Fig. 3C).
3.7. Effects of XH on dendricity in HEM-DP cells
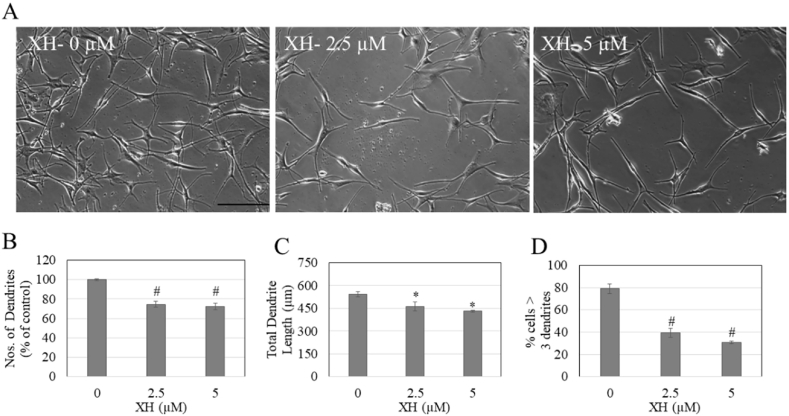
Since XH did not alter melanin biosynthesis in HEM-DP cells, we next evaluated if XH may inhibit melanosome export, which was evaluated by quantitation of dendricity. Cells treated with XH at concentrations of 2.5 and 5 μM showed an altered cellular morphology with less branched dendrites as compared to control cells which were multi-dendritic (Fig. 4A). For cells treated with XH at concentration of 2.5 and 5 μM, the dendrites appeared elongated, however the number of dendrites was visibly diminished. We next quantified the dendricity parameters to elucidate if XH had any inhibitory effect on melanosome export by inhibiting any or all of the three parameters. Our results showed that XH significantly reduced the dendrite number by 25.22% (p < 0.01) and 27.78% (p < 0.01) at 2.5 and 5 μM as compared to control, respectively (Fig. 4B). Similarly, both concentrations of XH showed a significant reduction in total dendrite length (TDL), where 2.5 μM reduced the TDL by 15.09% (p < 0.05) and 5 μM reduced the TDL by 20.5% (p < 0.05) (Fig. 4C). In the control group, 78.95% of cells were multi-dendritic (>3 dendrites); this percentage was significantly reduced by 39% and 48% by XH at concentrations of 2.5 μM and 5 μM, respectively (p < 0.01, Fig. 4D).
Fig. 4.
HEM-DP cell morphology and dendricity measurement. A) Representative phase-contrast micrographs of HEM-DP cells treated with XH at concentrations 0–5 μM; Scale bar corresponds to 100 μm; Quantification of dendricity by B) Number of dendrites; C) Total dendrite length; D) % of cells >3 dendrites; A total of ~60 cells were counted from duplicate wells for each treatment group; *p < 0.05; #p < 0.01; One-way ANOVA with Tukey's post-hoc test.
Taken together, the results demonstrate that XH effectively inhibited melanosome export by inhibiting dendricity parameters, without affecting melanin synthesis; this points to a novel mechanism of action of XH in human melanocytes.
3.8. Effects of XH on cell viability and melanosome degradation in HaCaT cells
Upon secretion of melansomes from the melanocytes, they are phagocytosed by keratinocytes to form a perinuclear cap around the nucleus of keratinocytes and eventually undergo degradation based on skin type [14]. An increase in melanosome degradation is a novel mode of action of melanogenesis inhibition. We first screened XH for cytotoxicity over a duration of 48 h in human keratinocytes (HaCaT) using MTS assay in order to exclude any contribution of cytotoxicity on melanosome degradation assay. Our results showed that XH significantly stimulated keratinocyte proliferation at 2.5, 5 and 10 μM by 9.75%, 12.66% and 15.11%, respectively. However, XH induced significant toxicity at higher concentrations of 20 μM and 30 μM (p < 0.01; Fig. 5A), hence concentrations <20 μM were selected for further testing.
Fig. 5.
A) Viability of HaCaT cells after treatment with XH for a duration of 48 h; B) Melanosome degradation assay in HaCaT cells treated with synthetic melanin (for 48 h) followed by treatment with XH at various nontoxic concentrations for another 48 h with corresponding panel showing photos of cell pellets; *p < 0.05; **p < 0.01 and #p < 0.001 compared to control; One-way ANOVA followed by Dunnett's test; All data is representative of Mean ± SD of one out of two independent experiments with n = 3 per group.
XH robustly suppressed phagocytosed melanin levels in keratinocytes in a dose-dependent manner with visibly lighter color of pellets (image panel, Fig. 5B). Quantitation of melanin levels showed reductions of 21.36% (p < 0.05), 30.42% (p < 0.01) and 41.44% (p < 0.001) at concentrations of 2.5, 5 and 10 μM, respectively (Fig. 5B). These results highlight that XH might induce degradation of phagocytosed melanosomes, which in turn, could contribute to the reduced melanin content in keratinocytes.
4. Discussion
XH has previously been reported to possess inhibitory effects on melanogenesis in B16F10 mouse melanoma cells [26] while it inhibits activity of mushroom tyrosinase in a cell-free system [25]. However, to date, there are no reports on the effects of XH on melanogenesis in human melanocytes; this is a critical issue since most depigmenting candidates in the flavonoid group have shown opposite effects on melanogenesis in mouse and human cells in earlier studies [33,34]. Herein, we demonstrated that XH exhibits anti-melanogenic activity in human MNT-1 cells and primary melanocytes (HEM-DP) and that XH specifically; i) inhibited melanogenesis in MNT-1 human melanoma cells but unexpectedly stimulated cellular tyrosinase activity; ii) inhibited melanocyte dendricity (inhibited both the total dendrite length and dendrite number in HEM-DP cells, while it inhibited cellular tyrosinase activity without affecting melanin synthesis and; iii) induced melanosome degradation in keratinocytes, which is indicative of its capacity to target later steps in the melanogenesis cascade. To the best of our knowledge, the inhibition of melanosome export and degradation has not been reported yet for XH as a mechanism of anti-melanogenic activity.
Unexpectedly, our results of the effects of XH on cellular tyrosinase activity in MNT-1 cells and HEM-DP were contradictory; XH increased tyrosinase activity in MNT-1 cells but decreased tyrosinase activity in HEM-DP cells. This result may be attributable to the intrinsic differences in both cells; MNT-1 are immortalized human melanoma cells while HEM-DP are normal melanocytes. It can be speculated that this may also be a compound-specific effect which may differ in normal and malignant cells; however, that hypothesis warrants further testing. We also evaluated if XH might downregulate expression of tyrosinase protein, however our results (Fig. S2) showed that XH did not affect tyrosinase protein expression up to concentrations of 20 μM, but significantly stimulated protein levels at 30 μM (where it augmented cellular tyrosinase activity but inhibited melanin synthesis). Overall, it appears that in MNT-1 cells, the effects of XH on cellular tyrosinase activity and protein levels cannot explain the reduction in melanin synthesis at 30 μM. Previous reports have described that flavonoids can interfere in dopa-oxidase assay due to their chemical structure and redox activity [35]. A typical flavonoid, quercetin, has been shown to partially form adducts with dopaquinone via a Michael-type addition, which was identified by HPLC [36]. We speculate that XH might similarly form intermediate adducts since it also shares a Michael-type group in its structure; such an intermediate might be formed only in MNT-1 cells (in contrast to HEM-DP cells) due to presence of some endogenous activators. Identification of this apparent discrepancy was not further pursued as it was not the focus of this work, however, further investigation of mechanisms and study of intermediates formed can be achieved by employing HPLC method.
Since XH displayed anti-melanogenic activity in both types of cells, it can be concluded that the inhibitory effects of XH on melanin synthesis in MNT-1 cells are unrelated to tyrosinase activity and involve other pathways. XH has been shown to be an effective inhibitor of both monophenolase and diphenolase activity of mushroom tyrosinase enzyme in a cell-free system: the authors reported the IC50 values for anti-monophenolase and anti-diphenolase activity inhibition to be 15.4 μM and 37.1 μM, respectively [25]. In the present study, we did not test XH for any effects on mushroom tyrosinase activity as mushroom tyrosinase differs from human tyrosinase in several aspects [37] and thus is not a true indicator of response from cellular melanin levels; this was also corroborated in another study where plasma-activated compounds inhibited mushroom tyrosinase activity but stimulated melanogenesis [38]. XH is a potent inhibitor of direct α-glucosidase activity in vitro [39], hence the inhibition of melanogenesis may be mediated, at least partially, by the direct inhibition of activity of α-glucosidase, which is involved in glycosylation and maturation of tyrosinase [29,40]. Interestingly, the structure of XH contains α-β unsaturated keto group which is a Michael acceptor group reminiscent of the structure of curcumin which inhibits melanogenesis as demonstrated previously [41]. The mechanism of melanin inhibition by XH in MNT-1 human melanoma cells does not involve inhibition of intracellular tyrosinase activity since that was significantly higher at concentrations <30 μM where no change in melanin levels were noted. In our earlier report, we noted a similar finding with the compound casuarictin which inhibited cellular tyrosinase activity without having any effect on melanin production levels [42]. Even though tyrosinase is the rate-limiting step in melanogenesis, there are other pathways distal to tyrosinase which may account for the mechanisms of melanogenesis inhibition.
Melanocyte dendrites transfer melanin granules into keratinocytes by mechanisms which are hitherto unclarified. XH did not inhibit melanin synthesis in HEM-DP cells at the tested concentrations (2.5–5 μM); however, it significantly attenuated dendricity parameters, which suggests that the depigmenting action of XH is primarily attributable to steps in the melanogenesis pathway distal from melanin synthesis. In a previous study conducted in B16F10 cells [26], XH downregulated protein and mRNA expression of Microphthalmia Transcription Factor (MITF) which has a critical role in melanosome export and dendricity [43]. We thus speculate that a possible mechanism of reduced dendricity might be attributable to a reduction in MITF expression in HEM-DP cells; however, that putative mode of action needs further exploration. Our results on reduction of dendricity in the absence of any change in melanin synthesis in HEM-DP cells are somewhat reminiscent of the actions of piperine derivatives which were shown in a study on pro-melanogenic compounds to target a similar step in melanogenesis; in that study, the authors found that these derivatives stimulated dendricity but did not affect melanin content in the cell [44]. Several recent studies have started to exploit melanocyte dendricity as a novel target for skin-lightning agents which has appeal for the cosmetics market in addition to clinical usage. We did not test for the inhibition of melanosome export by XH in MNT-1 cells since they are not amenable to dendrite analysis due to their morphology: they do not possess the physiological dendrite network characteristic of primary human melanocytes from dark skin. The number of dendrites in darkly-pigmented (DP) melanocytes usually ranges from 3 to 10 per cell, which can extend hundreds of microns. In contrast, MNT-1 cells are bipolar (2 dendrites), and the dendrite length is similar to length of the cell body which is few microns. To the best of our knowledge, this is the first study to report inhibition of melanosome export by XH due to effects on dendricity.
Autophagy has been demonstrated to regulate skin pigmentation via degradation of melanosomes in keratinocytes [45,46]. Our results showed that XH also targets a further step in the melanogenesis pathway by inducing degradation of melanosomes in keratinocytes. Isoliquiritigenin, a dietary flavonoid which has a chalcone structure similar to XH has been shown to induce melanosome degradation in keratinocytes and the authors further assayed for activation of autophagy-related proteins [32]. We did not test if melanosome degradation may be attributable to autophagy but XH has shown to induce autophagy in cancer cells in earlier study [47]; hence it can be speculated that autophagy may be responsible for melanosome degradation, however the study of autophagy in detail and elucidation of proteins involved in autophagy are a subject of future research. We conducted our experiments on melanin degradation on HaCaT cells instead of primary keratinocytes, since the latter are known to exhibit limitations in terms of donor-dependent variability and difficulty to passage for longer period cultures. Although HaCaT cells are immortalized cells, they have been shown to mimic the functions of primary keratinocytes [48] and have been used as a convenient model of primary keratinocytes in several studies [49,50]. Nevertheless, future studies to evaluate XH's melanin degradation capacity in primary keratinocytes are warranted.
5. Conclusions
In summary, our results provide a proof-of-principle for the capacity of XH, a prenylated chalcone found in hop extract, to inhibit melanogenesis in human melanocytes at low micromolar concentrations. Our results further establish that XH inhibits melanogenesis by a dual-action mechanism of targeting melanin export in human melanocytes and melanin degradation in keratinocytes; these studies demonstrate that XH is an inhibitor of melanin export in the absence of effects on melanin synthesis. Additionally, our results indicate that XH may hold promise as a pigmentation inhibitor for treatment of hyperpigmentation disorders for cosmetic and clinical use that could be included in oral or topical formulations. Future studies to elucidate the molecular signaling mechanisms of melanogenesis inhibition and test the efficacy of XH in a human skin model are warranted.
Author contributions
SG conceptualized the idea, designed, and performed the experiments, analyzed the data, and wrote and edited the manuscript. SRS provided resources and both authors approved the final manuscript.
Funding
This research received no external funding.
Declaration of competing interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Acknowledgments
We would like to thank Dr. Michael Marks, University of Pennsylvania, for graciously providing MNT-1 cells.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.100955.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Slominski A.T. How UV light touches the brain and endocrine system through skin, and why. Endocrinology. 2018;159(5):1992–2007. doi: 10.1210/en.2017-03230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner M., Hearing V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008;84(3):539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costin G.E., Hearing V.J. Human skin pigmentation: melanocytes modulate skin color in response to stress. Faseb. J. 2007;21(4):976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 4.Byers H.R. Melanosome processing in keratinocytes. The Pigmentary System: Physiology and Pathophysiology. 2006:181–190. [Google Scholar]
- 5.Hearing V.J. Determination of melanin synthetic pathways. J. Invest. Dermatol. 2011;131(E1):E8–E11. doi: 10.1038/skinbio.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slominski A. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004;84(4):1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 7.Slominski A., Zmijewski M.A., Pawelek J. L‐tyrosine and L‐dihydroxyphenylalanine as hormone‐like regulators of melanocyte functions. Pigment cell & melanoma research. 2012;25(1):14–27. doi: 10.1111/j.1755-148X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGregor D. Hydroquinone: an evaluation of the human risks from its carcinogenic and mutagenic properties. Crit. Rev. Toxicol. 2007;37(10):887–914. doi: 10.1080/10408440701638970. [DOI] [PubMed] [Google Scholar]
- 9.Takizawa T. Hepatocellular tumor induction in heterozygous p53-deficient CBA mice by a 26-week dietary administration of kojic acid. Toxicol. Sci. 2003;73(2):287–293. doi: 10.1093/toxsci/kfg094. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa M. Clinical and epidemiological analysis in 149 cases of rhododendrol-induced leukoderma. J. Dermatol. 2017;44(5):582–587. doi: 10.1111/1346-8138.13694. [DOI] [PubMed] [Google Scholar]
- 11.Brożyna A.A. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget. 2016;7(14):17844. doi: 10.18632/oncotarget.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slominski R.M., Zmijewski M.A., Slominski A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015;24(4):258. doi: 10.1111/exd.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ando H. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Invest. Dermatol. 2012;132(4):1222–1229. doi: 10.1038/jid.2011.413. [DOI] [PubMed] [Google Scholar]
- 14.Ebanks J.P. Epidermal keratinocytes from light vs. dark skin exhibit differential degradation of melanosomes. J. Invest. Dermatol. 2011;131(6):1226–1233. doi: 10.1038/jid.2011.22. [DOI] [PubMed] [Google Scholar]
- 15.Nikolic D., van Breemen R.B. Analytical methods for quantitation of prenylated flavonoids from hops. Curr. Anal. Chem. 2013;9(1):71–85. [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens J.F. Xanthohumol and structurally related prenylflavonoids for cancer chemoprevention and control. Natural Products for Cancer Chemoprevention: Single Compounds and Combinations. 2020:319–350. [Google Scholar]
- 17.Bogdanova K. Antibiofilm activity of bioactive hop compounds humulone, lupulone and xanthohumol toward susceptible and resistant staphylococci. Res. Microbiol. 2018;169(3):127–134. doi: 10.1016/j.resmic.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Philips N. Direct inhibition of elastase and matrixmetalloproteinases and stimulation of biosynthesis of fibrillar collagens, elastin, and fibrillins by xanthohumol. J. Cosmet. Sci. 2010;61(2):125–132. [PubMed] [Google Scholar]
- 19.Chen W. Beer and beer compounds: physiological effects on skin health. J. Eur. Acad. Dermatol. Venereol. 2014;28(2):142–150. doi: 10.1111/jdv.12204. [DOI] [PubMed] [Google Scholar]
- 20.Yen T.L. Neuroprotective effects of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus), in ischemic stroke of rats. J. Agric. Food Chem. 2012;60(8):1937–1944. doi: 10.1021/jf204909p. [DOI] [PubMed] [Google Scholar]
- 21.Wang X. Amyloid-beta aggregation inhibitory and neuroprotective effects of xanthohumol and its derivatives for alzheimer's diseases. Curr. Alzheimer Res. 2019;16(9):836–842. doi: 10.2174/1567205016666190827123222. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M. Xanthohumol inhibits tau protein aggregation and protects cells against tau aggregates. Food Funct. 2019;10(12):7865–7874. doi: 10.1039/c9fo02133g. [DOI] [PubMed] [Google Scholar]
- 23.Liu M. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus) Molecules. 2015;20(1):754–779. doi: 10.3390/molecules20010754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorn C. Xanthohumol feeding does not impair organ function and homoeostasis in mice. Food Chem. Toxicol. 2010;48(7):1890–1897. doi: 10.1016/j.fct.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 25.Kim D.W. Phenols displaying tyrosinase inhibition from Humulus lupulus. J. Enzym. Inhib. Med. Chem. 2016;31(5):742–747. doi: 10.3109/14756366.2015.1063621. [DOI] [PubMed] [Google Scholar]
- 26.Koo J.H. Effect of xanthohumol on melanogenesis in B16 melanoma cells. Exp. Mol. Med. 2008;40(3):313–319. doi: 10.3858/emm.2008.40.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi H. Influence of N-glycan processing disruption on tyrosinase and melanin synthesis in HM3KO melanoma cells. Exp. Dermatol. 2007;16(2):110–117. doi: 10.1111/j.1600-0625.2006.00515.x. [DOI] [PubMed] [Google Scholar]
- 28.Mikami M. Glycosylation of tyrosinase is a determinant of melanin production in cultured melanoma cells. Mol. Med. Rep. 2013;8(3):818–822. doi: 10.3892/mmr.2013.1602. [DOI] [PubMed] [Google Scholar]
- 29.Wang N., Hebert D.N. Tyrosinase maturation through the mammalian secretory pathway: bringing color to life. Pigm. Cell Res. 2006;19(1):3–18. doi: 10.1111/j.1600-0749.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 30.Urabe K. The inherent cytotoxicity of melanin precursors: a revision. Biochim. Biophys. Acta Mol. Cell Res. 1994;1221(3):272–278. doi: 10.1016/0167-4889(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 31.Jian Q. MicroRNA 340 is involved in UVB-induced dendrite formation through the regulation of RhoA expression in melanocytes. Mol. Cell Biol. 2014;34(18):3407–3420. doi: 10.1128/MCB.00106-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z. Autophagy participates in isoliquiritigenin–induced melanin degradation in human epidermal keratinocytes through PI3K/AKT/mTOR signaling. Biomed. Pharmacother. 2018;97:248–254. doi: 10.1016/j.biopha.2017.10.070. [DOI] [PubMed] [Google Scholar]
- 33.Liu-Smith F., Meyskens F.L. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. 2016;60(6):1264–1274. doi: 10.1002/mnfr.201500822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi M.-H., Shin H.-J. Anti-melanogenesis effect of quercetin. Cosmetics. 2016;3(2):18. [Google Scholar]
- 35.Gasowska-Bajger B., Wojtasek H. Reactions of flavonoids with o-quinones interfere with the spectrophotometric assay of tyrosinase activity. J. Agric. Food Chem. 2016;64(26):5417–5427. doi: 10.1021/acs.jafc.6b01896. [DOI] [PubMed] [Google Scholar]
- 36.Kubo I., Nitoda T., Nihei K.-i. Effects of quercetin on mushroom tyrosinase and B16-F10 melanoma cells. Molecules. 2007;12(5):1045–1056. doi: 10.3390/12051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mann T. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Invest. Dermatol. 2018;138(7):1601–1608. doi: 10.1016/j.jid.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Ali A. Influence of plasma-activated compounds on melanogenesis and tyrosinase activity. Sci. Rep. 2016;6:21779. doi: 10.1038/srep21779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu M. Xanthohumol, a prenylated chalcone from beer hops, acts as an α-glucosidase inhibitor in vitro. J. Agric. Food Chem. 2014;62(24):5548–5554. doi: 10.1021/jf500426z. [DOI] [PubMed] [Google Scholar]
- 40.Negroiu G. Protein specific N-glycosylation of tyrosinase and tyrosinase-related protein-1 in B16 mouse melanoma cells. Biochem. J. 1999;344(3):659–665. [PMC free article] [PubMed] [Google Scholar]
- 41.Tu C.X. Curcumin inhibits melanogenesis in human melanocytes. Phytother Res. 2012;26(2):174–179. doi: 10.1002/ptr.3517. [DOI] [PubMed] [Google Scholar]
- 42.Goenka S., Ceccoli J., Simon S.R. Anti-melanogenic activity of ellagitannin casuarictin in B16F10 mouse melanoma cells. Nat. Prod. Res. 2019:1–6. doi: 10.1080/14786419.2019.1636242. [DOI] [PubMed] [Google Scholar]
- 43.Chiaverini C. Microphthalmia-associated transcription factor regulates RAB27A gene expression and controls melanosome transport. J. Biol. Chem. 2008;283(18):12635–12642. doi: 10.1074/jbc.M800130200. [DOI] [PubMed] [Google Scholar]
- 44.Venkatasamy R. Effects of piperine analogues on stimulation of melanocyte proliferation and melanocyte differentiation. Bioorg. Med. Chem. 2004;12(8):1905–1920. doi: 10.1016/j.bmc.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 45.Murase D. Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J. Invest. Dermatol. 2013;133(10):2416–2424. doi: 10.1038/jid.2013.165. [DOI] [PubMed] [Google Scholar]
- 46.Ho H., Ganesan A.K. The pleiotropic roles of autophagy regulators in melanogenesis. Pigment Cell Melanoma Res. 2011;24(4):595–604. doi: 10.1111/j.1755-148X.2011.00889.x. [DOI] [PubMed] [Google Scholar]
- 47.Lu W.-J. Xanthohumol from Humulus lupulus L. induces glioma cell autophagy via inhibiting Akt/mTOR/S6K pathway. Journal of functional foods. 2015;18:538–549. [Google Scholar]
- 48.Boukamp P. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colombo I. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/7435621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mini C. Immortalized keratinocytes cells generates an effective model of Epidermal Human Equivalent for irritation and corrosion tests. Toxicol. Vitro. 2020:105069. doi: 10.1016/j.tiv.2020.105069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.