Abstract
Malignant mesotheliomas (MMs) are aggressive therapy-resistant tumors that generally have a poor prognosis. We previously reported the establishment of four new monoclonal antibodies (mAbs) for the diagnosis and treatment of MM. In this report, we characterized one of these antibodies, JMAM-1. The molecules whose antibodies were calibrated were picked up, transfected assuming CD10, and elucidated by fluorescence activated cell sorter. Survival experiments were performed using tumor-bearing mice model. JMAM-1 mAb was found to bind with CD10 antigen. The Kaplan–Meier survival curve showed a small but prolonged survival effect. JMAM-1 mAb-treated MSTO-211H cells showed increased cell cycle arrest involved by cyclin-dependent-kinase. JMAM-1 antibody has cytostatic effect and may be a candidate for the treatment of MM. Among mesothelioma, CD10-positive cases have been reported to have a poorer prognosis than negative cases, which can be used as a tool for diagnosis.
Keywords: monoclonal antibody, malignant mesothelioma, CD10, JMAM-1
Introduction
Malignant mesothelioma (MM) is an uncommon but aggressive tumor with a very poor prognosis. Despite improvements in surgical management, chemotherapy, and radiotherapy, its prognosis remains poor, with a median survival of <2 years.(1–7)For clinical diagnosis, patients with the sarcomatoid subtype have the poorest prognosis with a remarkably short survival.(8) Even among patients with epithelioid mesothelioma, survival outcomes are variable. Therefore, further prognostic factors are necessary to optimize treatment options and to better stratify patients in clinical trials.(9,10)
CD10 (neutral endopeptidase), a zinc-dependent metalloproteinase, is expressed in various normal tissues and is capable of efficiently degrading various peptides and cytokines.(11–14) CD10 is also expressed in malignant tumors and has been identified as a predictor of tumor biological aggressiveness through extracellular enzymatic degradation and intracellular signaling crosstalk.(15–25) CD10 is expressed in MM,(26) and patients present with a poorer prognosis than negative cases.
Recently, CD10 has been demonstrated to be a novel marker of cisplatin resistance and cancer stem cells using cell lines from other solid malignancies.(27) In addition, CD10 has been reported to cleave and activate a peptidic prodrug of doxorubicin,(28,29) and recent clinical trials suggest that chemotherapy with doxorubicin improves quality of life with an acceptable level of toxicity.(30,31) Therefore, CD10 is a potential marker for investigating chemotherapy sensitivity or resistance in patients with MM.(26) These results indicate that CD10 is closely related with tumorigenicity and self-renewal ability. Furthermore, tumoral CD10 expression correlates with aggressive histological types and higher mitotic activity, and it is an independent prognostic factor for patients with MM.
In the first report, we determined the establishment of four antibodies against MM. However, at that time, the antigen molecules of each antibody had not been identified.(1) Herein we report the identification of the antigen molecule and other studies on the JMAM-1 antibody, which had the highest cell growth inhibitory effect, among the four antibodies.
Materials and Methods
Ethics approval and consent to participate
Animal experiments were conducted following protocols approved by the Animal Care Committee of the Juntendo University of Medicine. The Ethics Review Committee for Animal Experimentation at the Juntendo University Faculty of Medicine approved all animal experiments (Project Number 260105).
Animals
Female BALB/c nu/nu mice of 4 weeks of age were obtained from SLC (Hamamatsu, Japan) and housed in a specific pathogen-free facility in microisolator cages. The Animal Care and Use Committee of Juntendo University approved all animal experiments.
Cell lines
NCI-H226 and MSTO-211H mesothelioma cell lines and Huh-7 hepatoma cell lines were purchased from American Type Culture Collection. Cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS; Thermo Fisher) in standard conditions (5% CO2 at 37°C). Cells undergoing exponential proliferation were used for all experiments.
Reagents and antibodies
Mouse anti-human leukocyte antigen (HLA) class I (HLA-A, -B, and -C) monoclonal antibody (mAb; clone: W6/32) was purchased from BioLegend (San Diego, CA). Alexa Fluor 488-conjugated goat anti-mouse IgG was purchased from Invitrogen (CA). Mouse IgG was purchased from Abcam (Cambridge, United Kingdom). Anti-CD26 mAb (clone 1F7) and ERC-mesothelin were established in our laboratory.(32,33) Anti-CD10 mAb (clone 56C6) was purchased from LSI Medience. EnVision™+DualLink (DAKO) and 3,3′-diamin-obenzidine (Dojindo Laboratories) were used as the chromogens. Alexa 488 conjugate was purchased from Thermo Fisher.
Plasmid
RG223013 (Qiagen, Stockholm, Sweden) and FuGENE® 6 reagent (Promega, Japan) were used.
Transfection of chimeric construct and establishment of stable transfected cell lines
Twenty-four hours before transfection, 2 × 105/mL Huh-7 cells were seeded in a 60-mm plate. The RG223013 construct was prepared using Plasmid Maxiprep (Qiagen), and then, the Huh-7 cells were transfected with FuGENE 6 reagent (Promega) according to the manufacturer's instructions. At 8 hours, the culture medium was replaced with RPMI-1640 medium containing 10% FBS. After 48 hours, the supernatant of the transfected cells were confirmed by flow cytometry analysis using a BD LSRFortessa cell analyzer (BD Biosciences). The cells were collected and analyzed for transient expression of CD10 mAb through western blot analysis.
Western blot analysis
MSTO-211H, Huh-7 cells, and transfected Huh-7 cells (3 × 106 cells/well) incubated, with or without rfhSP-D (20 μg/mL), in a serum-free Roswell Park Memorial Institute (RPMI) medium for 12 and 24 hours. The cells were lysed in a lysis buffer (50 mM Tris-HCL pH 7.5, 150 mM NaCl, 1% Triton X, 1 mM sodium orthovanadate, 10 mM β-glycerophosphate, 2 mM ethylenediaminetetraacetic acid (EDTA), and 10 mM sodium pyrophosphate) and analyzed by western blotting. Lysate proteins (30 μg) were separated by 15% sodium dodecyl sulfate - polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred onto poly vinylidene di fluoride (PVDF) membranes (Pall Corporation, NY, USA). Protein samples were separated by SDS-PAGE and transferred to a PVDF membrane. After treatment with Pierce Fast Blocking Buffer (Pierce Biotechnology, Inc., Tokyo, Japan), the membranes were incubated with a buffer containing JMAM-1 mAb (1 μg/mL), followed by a horseradish peroxidase-conjugated secondary antibody. The membrane was treated with an enhanced chemiluminescent reagent (Amersham, Tokyo, Japan), and the reactive protein bands were visualized using a Fujifilm image analyzer.
Cell viability assay
MSTO-211H cells were seeded in triplicate in 6-well plates at a density of 3 × 105 cells/well. After 24 hours, JMAM-1 mAb was added to each well at a concentration of 5 μg/mL. The percentage of viable cells after treatment was calculated assuming 100% as the number of untreated cells. We counted the number of viable cells 24, 48, and 72 hours after antibody administration. Cell viability was then determined by a dose–response curve using GraphPad Prism 7 Software.
Statistical analysis
All experiments were conducted in triplicates, and the data are expressed as mean ± standard deviation (SD). The resulting mean values were <10% SD. Statistical analyses were performed using the SPSS 14.0 software (SPSS, Inc., NY). The data sets were compared by Student's t-tests, and p < 0.05 was considered statistically significant.
Results
JMAM-1 mAb binds with CD10 antigen
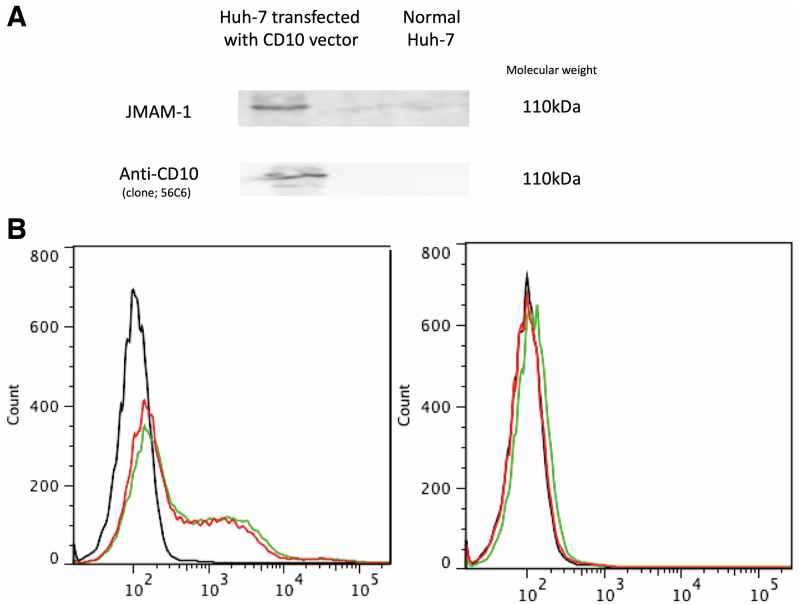
When a comprehensive analysis of the antigen molecule was requested for JMAM-1 mAb, the possibility of CD10 was suggested, and it was necessary to prove it by transfecting nonexpressing cells. When examined by fluorescence activated cell sorter (FACS), Huh-7 cells usually do not bind to the JMAM-1 antibody. When FACS was subsequently performed by introducing the CD10 plasmid into the cells, binding to the JMAM-1 antibody was observed. Therefore, it was suggested that the JMAM-1 mAb was anti-CD10 mAb (Fig. 1). Western blot analysis indicated same molecular weight.
FIG. 1.
JMAM-1 mAb recognized CD10 transfectant. Right side indicates normal Huh-7 cells, and left side indicates CD10 vector-transfected Huh-7 cells (A, B). (A) Western blotting indicated anti-CD10 expression by transfected Huh-7 cells. (B) FACS proved that JMAM-1 mAb recognized anti-CD10. Black line, control mouse IgG. Red line, anti-CD10 mAb. Green line, JMAM-1 mAb. FACS, fluorescence activated cell sorter; mAb, monoclonal antibody.
JMAM-1 mAb-treated MSTO-211H cells showed increased cell cycle arrest
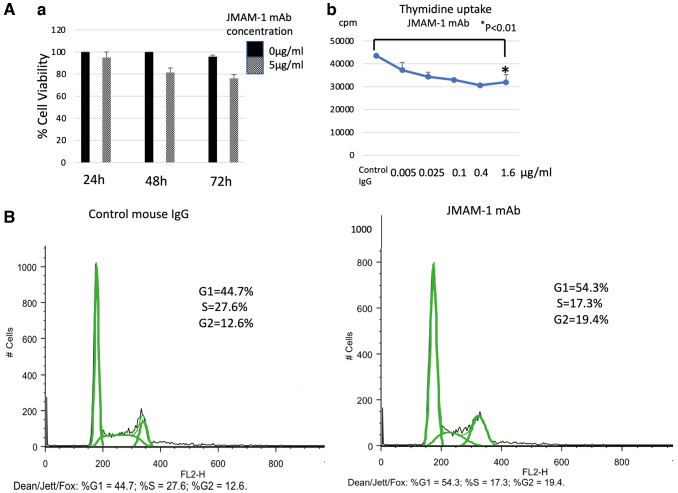
MSTO-211H cells (2 × 105/well in tissue culture plate) starved for 18 hours in RPMI medium, and then treated with JMAM-1 mAb (5 μg/mL) for 48 hours. MSTO-211H cells were incubated in the presence of JMAM-1 (10 μg/mL) or in a culture medium alone (control) for 24 hours and were subjected to FACS cell cycle analysis. The total cell population was divided into G1, S, and G2 phases and subsequently analyzed (Fig. 2).
FIG. 2.
JMAM-1 mAb-treated MSTO-211H cells showed increased cell cycle arrest. (A) (a) Effect of JMAM-1 mAb on cell viability. MSTO-211H cell viability was analyzed by JMAM-1 mAb treatment (24–72 hours). (b) Effect of JMAM-1 mAb on thymidine uptake assay. MSTO-211H cells were incubated with different doses of JMAM-1 mAb (0–1.6 μg/mL) for 72 hours in a CO2 incubator. (B) JMAM-1 mAb-treated MSTO-211H cells showed increased cell cycle arrest. MSTO-211H cell was incubated in the presence of JMAM1 (5 μg/mL) or in culture medium alone (control) for 48 hours and was subjected to FACS cell cycle analysis. The total population of cells was divided into the G1, S, and G2 phases and analyzed. Control mouse IgG (%G1 = 44.7, %S = 27.6, %G2 = 12.6). JMAM-1 mAb (%G1 = 54.3, %S = 17.3, %G2 = 19.4). Analyzed by Dean-Jett-Fox.
Tumor xenograft experiments
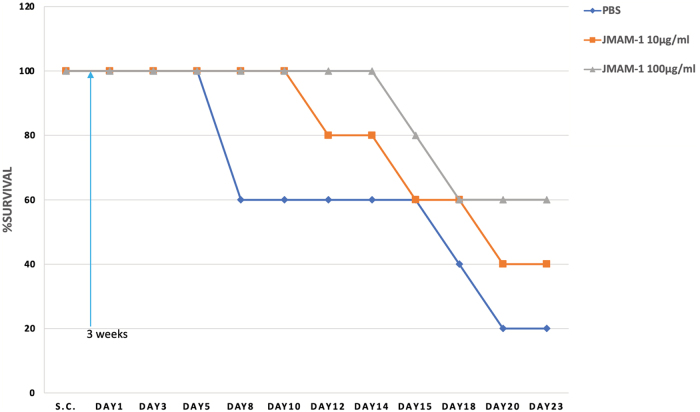
Female BALB/c nu/nu mouse of 4 weeks of age were obtained from SLC. Thirty BALB/c mouse were injected intraperitoneally with NCI-H226 cells (1 × 106/head) suspended in PBS. Three weeks later, the mice were injected intraperitoneally with JMAM-1 mAb (10 or 100 μg/mL/head) or with control PBS 11 times between days 1 and 23. A slight but prolonged survival effect was observed in the group administered 100 μg/head compared with the group administered 10 μg/head and the PBS group (Fig. 3).
FIG. 3.
In vivo efficacy of JMAM-1 mAb in the NCI-H226 CDX model BALB/c nude mice were injected S.C. with NCI-H226 cells. After 3 weeks, 10 and 100 μg/mL JMAM-1 mAb and control PBS, respectively, were administered intraperitoneally from days 1 to 23, every 2 or 3 days for 11 times. PBS, phosphate-buffered saline.
Comparison between JMAM-1 and other mAbs by XTT assay
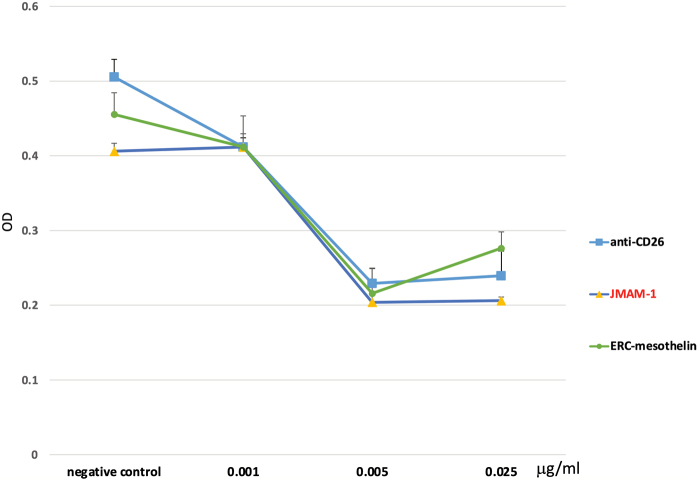
NCI-H226 cells (3 × 103 cells/well) were seeded in a 96-well plate and XTT assay was performed. JMAM-1 concentrations used for dose–response analyses were 0.001, 0.005, 0.025, 0.1, 0.4, and 2 μM. After 48 hours, ERC-mesothelin and anti-CD26 were prepared using the same concentrations and absorbance was read at 450 nm using an ELISA plate reader (BioTek Instruments, Northern Vermont). Data were analyzed using Prism 5 statistical software (n = 3; p < 0.05) through one-way analysis of variance, followed by Tukey test. JMAM-1 mAb showed almost the same cell growth inhibitory effects as anti-CD26 and mesothelin (Fig. 4).
FIG. 4.
Comparison between JMAM-1 and other mAbs by XTT assay. NCI-H226 cells (3 × 103 cells/well) were seeded in a 96-well plate and XTT assay was performed. JMAM-1 concentrations used for dose–response analyses were 0.001, 0.005, 0.025, 0.1, 0.4, and 2 μM. After 48 hours, ERC-mesothelin and anti-CD26 were prepared using the same concentrations and absorbance was read at 450 nm using an ELISA plate reader (Bio Tek Instruments, Northern Vermont, USA). Data were analyzed using Prism 5 statistical software (n = 3; p < 0.05) through one-way analysis of variance, followed by Tukey test. JMAM-1 mAb showed almost the same cell growth inhibitory effects as anti-CD26 and mesothelin.
Discussion
In our first report, we produced four antibodies (JMAM1, 2, 3, and 4)(1); when one of these antibodies was analyzed, it was possible to identify the molecule was CD10 that recognized. Kadota et al. reported that CD10 expression is positively cases in mesothelioma correlated with higher-grade histological types: CD10 expression was identified in 42% of epithelioid nonpleomorphic tumors, 57% of epithelioid pleomorphic tumors, 79% of biphasic tumors in the sarcomatoid area, and 93% of sarcomatoid tumors. Furthermore, mitotic counts were significantly higher in CD10-positive tumors than in CD10-negative tumors. Moreover, there is an association between tumoral CD10 expression and overall survival in all patients with epithelioid and nonepithelioid mesothelioma.(6,7)
For diagnosis of mesothelioma, it is necessary to use the new antibody together with the currently used antibody. However, because it stained both epithelial and sarcomatoid type MM, it seems to be useful for diagnosis. It must be noted that renal cancer was also stained. Thus, for the diagnosis of carcinoma, CD10 mAb is used to establish the origin. Moreover, for MM, it seems that the CD10-positive histological type has a poor prognosis. Thus, CD10 mAb is expected to be used not only for the diagnosis of mesothelioma but also as a prognostic marker for cancer cases.34
Currently, we do not know how the epitope of other companies' CD10 antibodies differ. Moreover, identification of epitopes is difficult but will be undertaken if required.
JMAM-1 mAb has an inhibitory effect on cell proliferation, and even Kaplan–Meier estimates showed a life-prolonging effect of JMAM-1 mAb compared with that of control PBS.
However, after repeated therapy, not all patients respond to therapeutic mAbs(35) because clones appear during the course of treatment that do not express the target. Therefore, additional therapeutic options are required to treat such patients. Common therapeutic mAbs against cell surface molecules exert their effects largely through immunological mechanisms, including complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC). When we examined whether there are steps that suppress cell growth involving factors other than ADCC and CDC, the cell cycle system was considered.
A cell cycle assay was performed to elucidate cell proliferation inhibition by JMAM-1 mAb, and it was found that fewer cells reached S phase compared with those in the control PBS group, demonstrating inhibition of proliferation. Through G1 to S phase, some cyclin-dependent kinase inhibitory proteins may act in the suppression of cell growth.(36) YAP protein is involved in the proliferation of MPM cells, whereas suppressing CDK7 reduces YAP protein as well as suppresses the infiltration and metastasis of MM.(37) Jinbai reported that CDK7 might be a therapeutic target for MM. CDK7 is the catalytic subunit of the CDK-activating kinase (CAK) complex that phosphorylates SPT5/SUPT5H, SF1/NR5A1, POLR2A, p53/TP53, CDK1, CDK2, CDK4, CDK6, and CDK11B/CDK11. CAK activates the cyclin-associated kinases CDK1, CDK2, CDK4, and CDK6 by threonine phosphorylation, thus regulating cell cycle progression.(38)
It may be necessary to examine the relationship between CDK and CD10 in the future.
Conclusions
JMAM-1 mAb was found to be a CD10 protein. The diagnosis of MM, expression of CD10 correlates with aggressive histological types and is an independent predictor of patient survival. JMAM-1 mAb may also predict the prognosis of MM. Our data show that JMAM-1 mAb inhibits the proliferation of MM cells. This is thus an effective treatment for MM.
Acknowledgments
The authors thank the staff of the Animal Care Committee for animal care, Juntendo University School of Medicine. The authors also thank the Laboratory of Molecular and Biomedical Research, the Laboratory of Cell Biology, Research Support Center Juntendo University Graduate School of Medicine.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported, in part, by Kakenhi. Shuji Matsuoka received a grant from the Japan Society for the Promotion of Science (web: https://kaken.nii.ac.jp/d/p/15K06880.en.html, grant no. 15K06880). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
References
- 1. Mizutani N, Abe M, Matsuoka S, Kajino K, Wakiya M, Ohtsuji N, Hatano R, Morimoto C, and Hino O: Establishment of anti-mesothelioma monoclonal antibodies. BMC Res Notes 2016;9:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oury TD, Roggli VL and Sporn TA: Pathology of Asbestos-Associated Diseases. Springer, Verlag Berlin Heidelberg, 2014 [Google Scholar]
- 3. Flores RM, Zakowski M, Venkatraman E, Krug L, Rosenzweig K, Dycoco J, LeeC, Yeoh C, Bains M, and Rush V: Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007;2:957–965 [DOI] [PubMed] [Google Scholar]
- 4. Rusch VW, Giroux D, Kennedy C, Ruffini E, Cangir AK, Rice D, Pass H, Asmura H, Waller D, Edwards J, Weller W, and Hoffnann H: Initial analysis of the International Association for the Study of Lung Cancer mesothelioma database. J Thorac Oncol 2012;7:1631–1639 [DOI] [PubMed] [Google Scholar]
- 5. Sugarbaker DJ, Flores RM, Jaklitsch MT, Richards WG, Strauss GM, Corson JM, Decamp MM Jr, Swanson SJ, Bueno R, Lukanich JM, Baldini EH, and Mentzer SJ: Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: Results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54–63; discussion 63–55. [DOI] [PubMed] [Google Scholar]
- 6. Kadota K, Suzuki K, Colovos C, Sima CS, Rusch VW, Travis WD, and Adusumilli PS: A nuclear grading system is a strong predictor of survival in epithelioid diffuse malignant pleural mesothelioma. Mod Pathol 2012;25:260–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kadota K, Suzuki K, Sima CS, Rusch VW, Adusumilli PS, and Travis WD: Pleomorphic epithelioid diffuse malignant pleural mesothelioma: A clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J Thorac Oncol 2011;6:896–904 [DOI] [PubMed] [Google Scholar]
- 8. Burt BM, Bader A, Winter D, Rodig SJ, Bueno R, and Sugarbaker DJ: Expression of interleukin-4 receptor alpha in human pleural mesothelioma is associated with poor survival and promotion of tumor inflammation. Clin Cancer Res 2012;18:1568–1577 [DOI] [PubMed] [Google Scholar]
- 9. Suzuki K, Kadota K, Sima CS, Sadelain M, Rusch VW, Travis WD, and Adusumill PS: Chronic inflammation in tumor stroma is an independent predictor of prolonged survival in epithelioid malignant pleural mesothelioma patients. Cancer Immunol Immunother 2011;60:1721–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kadota K, Kachala SS, Nitadori J, Suzuki K, Dunphy MP, Sima CS, Travis WD, Rush VW, and Adusumill PS: High SUVmax on FDG-PET indicates pleomorphic subtype in epithelioid malignant pleural mesothelioma: Supportive evidence to reclassify pleomorphic as nonepithelioid histology. J Thorac Oncol 2012;7:1192–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rusch VW, Giroux D, Kennedy C, Ruffini E, Cangir AK, Rice D, Pass H, Asamura H, Waller D, Edwards J, Weder W, Hoffmann H, and van Meerbeeck JP; Staging Committee IASLC: Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol 2012;7:1631–1639 [DOI] [PubMed] [Google Scholar]
- 12. McIntosh GG, Lodge AJ, Watson P, Hall AG, Wood K, Anderson JJ, Angus B, Home CH, and Milton ID: NCL-CD10-270: A new monoclonal antibody recognizing CD10 in paraffin-embedded tissue. Am J Pathol 1999;154:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shipp MA and Look AT: Hematopoietic differentiation antigens that are membrane-associated enzymes: Cutting is the key! Blood 1993;82:1052–1070 [PubMed] [Google Scholar]
- 14. Turner AJ and Tanzawa K: Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. FASEB J 1997;11:355–364 [DOI] [PubMed] [Google Scholar]
- 15. Iwaya K, Ogawa H, Izumi M, Kuroda M, and Mukai K: Stromal expression of CD10 in invasive breast carcinoma: A new predictor of clinical outcome. Virchows Arch 2002;440:589–593 [DOI] [PubMed] [Google Scholar]
- 16. Kim HS, Kim GY, Kim YW, Park YK, Song JY, and Lim SJ: Stromal CD10 expression and relationship to the E-cadherin/beta-catenin complex in breast carcinoma. Histopathology 2010;56:708–719 [DOI] [PubMed] [Google Scholar]
- 17. Desmedt C, Majjaj S, Kheddoumi N, Singhal SK, Haibe-Kains B, El Ouriaghli F, Chaboteaux C, Michiels S, Lallemand F, Jourme F, Duvillier H, Loi S, Quackenbush J, Dekoninck S, Blanpain C, Lagneaux L, Houhou N, Delorenzi M, Larsimont D, Piccart M, and Sotiriou C: Characterization and clinical evaluation of CD10 (+) stroma cells in the breast cancer microenvironment. Clin Cancer Res 2012;18:1004–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujimoto Y, Nakanishi Y, Sekine S, Yoshimura K, Akasu T, Moriya Y, and Shimoda T: CD10 expression in colorectal carcinoma correlates with liver metastasis. Dis Colon Rectum 2005;48:1883–1889 [DOI] [PubMed] [Google Scholar]
- 19. Kuniyasu H, Luo Y, Fujii K, Sasahira T, Moriwaka Y, Tatsumoto N, Sasaki T, Yamashita Y, and Ohmori H: CD10 enhances metastasis of colorectal cancer by abrogating the anti-tumoural effect of methionine-enkephalin in the liver. Gut 2010;59:348–356 [DOI] [PubMed] [Google Scholar]
- 20. Khanh do T, Mekata E, Mukaisho KI, Sugihara H, Shimizu T, Shiomi H, Murata S, Naka S, Yamamoto H, Endo Y, and Tani T: Prognostic role of CD10(+) myeloid cells in association with tumor budding at the invasion front of colorectal cancer. Cancer Sci 2011;102:1724–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dall'Era MA, True LD, Siegel AF, Porter MP, Sherertz TM, and Liu AY: Differential expression of CD10 in prostate cancer and its clinical implication. BMC Urol 2007;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fleischmann A, Rocha C, Saxer-Sekulic N, Zlobec I, Sauter G, and Thalmann GN: High CD10 expression in lymph node metastases from surgically treated prostate cancer independently predicts early death. Virchows Arch 2011;458:741–748 [DOI] [PubMed] [Google Scholar]
- 23. Ho ME, Quek SI, True LD, Morrissey C, Corey E, Vessella RL, Dulavimpit R, Nelson PS, Maresh EL, Mah V, Alavi M, Kim SR, Bagrvanova L, Horvath S, Chia D, Goodglick L, and Liu AY: Prostate cancer cell phenotypes based on AGR2 and CD10 expression. Mod Pathol 2013;26:849–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biasoli I, Morais JC, Scheliga A, Milito CB, Romano S, Land M, Pulcheri W, and Spector N: CD10 and Bcl-2 expression combined with the International Prognostic Index can identify subgroups of patients with diffuse large-cell lymphoma with very good or very poor prognoses. Histopathology 2005;46:328–333 [DOI] [PubMed] [Google Scholar]
- 25. Bilalovic N, Blystad AK, Golouh R, Nesland JM, Selak I, Trinh D, and Torlakovic E: Expression of bcl-6 and CD10 protein is associated with longer overall survival and time to treatment failure in follicular lymphoma. Am J Clin Pathol 2004;121:34–42 [DOI] [PubMed] [Google Scholar]
- 26. Butnor KJ, Nicholson AG, Allred DC, Zander DS, Henderson DW, Barrios R, Haque AK, Allen TC, Killen DE, and Cagle PT: Expression of renal cell carcinoma-associated markers erythropoietin, CD10, and renal cell carcinoma marker in diffuse malignant mesothelioma and metastatic renal cell carcinoma. Arch Pathol Lab Med 2006;130:823–827 [DOI] [PubMed] [Google Scholar]
- 27. Fukusumi T, Ishii H, Konno M, Yasui T, Nakahara S, Takenaka Y, Yamamoto Y, Nishikawa S, Kano Y, Hasegawa S, Hamabe A, Haraguchi N, Doki Y, Mori M, and Inohara H: CD10 as a novel marker of therapeutic resistance and cancer stem cells in head and neck squamous cell carcinoma. Br J Cancer 2014;111:506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ravel D, Dubois V, Quinonero J, Meyer-Losic F, Delord J, Rochaix P, Nicolazzi C, Ribes F, Mazerolles C, Assouly E, Vialatte K, Hor I, Kearsey J, and Trouet A: Preclinical toxicity, toxicokinetics, and antitumoral efficacy studies of DTS-201, a tumor-selective peptidic prodrug of doxorubicin. Clin Cancer Res 2008;14:1258–1265 [DOI] [PubMed] [Google Scholar]
- 29. Pan C, Cardarelli PM, Nieder MH, Pickford LB, Gangwar S, King DJ, Yarranton GT, Buckman D, Roscoe W, Zhou F, Salles A, Chen TH, Horgan K, Wang YH, Nguyen T, and Bebbington CR: CD10 is a key enzyme involved in the activation of tumor-activated peptide prodrug CPI-0004Na and novel analogues: Implications for the design of novel peptide prodrugs for the therapy of CD10(+) tumors. Cancer Res 2003;63:5526–5531 [PubMed] [Google Scholar]
- 30. Scherpereel A, Berghmans T, Lafitte JJ, Colinet B, Richez M, Bonduelle Y, Meert AP, Dhalluin X, Leclercq N, Paesmans M, Willems L, and Sculier JP: Valproate-doxorubicin: Promising therapy for progressing mesothelioma: A phase II study. Eur Respir J 2011;37:129–135 [DOI] [PubMed] [Google Scholar]
- 31. Kadota K, Villena-Vargas J, Nitadori J, Sima CS, Jones DR, Travis WD, and Adusmilli PS: Tumoral CD10 expression correlates with aggressive histology and prognosis in patients with malignant pleural mesothelioma. Ann Surg Oncol 2015;22:3136–3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hatano R, Yamada T, Matsuoka S, Iwata S, Yamazaki H, Komiya E, Okamoto T, Dang NH, Ohnuma K, Morimoto C: Establishment of monoclonal anti-human CD26 antibodies suitable for immunostaining of formalin-fixed tissue. Diagn Pathol 2014;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hagiwara Y, Hamada Y, Kuwahara M, Maeda M, Segawa T, Ishikawa K, and Hino O: Establishment of a novel specific ELISA system for rat N- and C-ERC/mesothelin. Rat ERC/mesothelin in the body fluids of mice bearing mesothelioma. Cancer Sci 2008;99:666–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uehara K, Ikehara F, Tanabe Y, Nakazato I, Oshiro M, Inamine M, and Kinjo T: CD10 expression in the neuroendocrine carcinoma component of endometrial mixed carcinoma: Association with long survival. Diagn Pathol 2016;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Brien SM, Kantarjian H, Thomas DA, Giles FJ, Freireich EJ, Cortes J, Lerner S, and Keating MJ: Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol 2001;19:2165–2170 [DOI] [PubMed] [Google Scholar]
- 36. Lioyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, and Scheithauer BW: p27kip1: A multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol 1999;154:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miao J, Kyoyama H, Liu L, Chan G, Wang Y, Urisman A, Yang YL, Liu S, Xu Z, Bin H, Li H, Jablons DM, and You L: Inhibition of cyclin-dependent kinase 7 down-regulates yes-associated protein expression in mesothelioma cells. J Cell Mol Med 2020;24:1087–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schachter MM, Merrick KA, Larochelle S, Hirschi A, Zhang C, Shokat KM, Rubin SM, and Fisher RP: A Cdk7-Cdk4 T-loop phosphorylation cascade promotes G1 progression. Mol Cell 2013;50:250–260 [DOI] [PMC free article] [PubMed] [Google Scholar]