Abstract
Significance: Perivascular adipose tissue (PVAT), which is present surrounding most blood vessels, from the aorta to the microvasculature of the dermis, is mainly composed of fat cells, fibroblasts, stem cells, mast cells, and nerve cells. Although the PVAT is objectively present, its physiological and pathological significance has long been ignored.
Recent Advances: PVAT was considered as a supporting component of blood vessels and a protective cushion to the vessel wall from the neighboring tissues during relaxation and contraction. Nonetheless, further extensive research found that PVAT actively regulates blood vessel tone through PVAT-derived vasoactive factors, including both relaxing and contracting factors. In addition, PVAT contributes to atherosclerosis through paracrine secretion of a large number of bioactive factors such as adipokines and cytokines. Thereby, PVAT regulates the functions of blood vessels through various mechanisms operating directly on PVAT or on the underlying vessel layers, including vascular smooth muscle cells (VSMCs) and endothelial cells (ECs).
Critical Issues: PVAT is a unique adipose tissue that plays an essential role in maintaining the vascular structure and regulating vascular function and homeostasis. This review focuses on recent updates on the various PVAT roles in hypertension and atherosclerosis.
Future Directions: Future studies should further investigate the actual contribution of alterations in PVAT metabolism to the overall systemic outcomes of cardiovascular disease, which remains largely unknown. In addition, the messengers and underlying mechanisms responsible for the crosstalk between PVAT and ECs and VSMCs in the vascular wall should be systematically addressed, as well as the contributions of PVAT aging to vascular dysfunction.
Keywords: perivascular adipose tissue, hypertension, atherosclerosis
Introduction
There are two main types of adipose tissues in the body: white adipose tissue (WAT) and brown adipose tissue (BAT). WAT is widely distributed in the subcutaneous and the visceral cavity of the body, and is the main form of fat storage in the body. Its main function is to store excess energy in the form of fatty acids for the body to use when needed. However, visceral WAT contributes to atherosclerosis development by secreting proinflammatory adipokines, such as resistin, which lead to insulin resistance, dysfunction of vascular endothelial cells (ECs), and proliferation of vascular smooth muscle cells (VSMCs) (12, 58). Conversely, BAT promotes heat generation in the body by decomposing the lipids and burning them through uncoupling oxidation by the mitochondrial electron transport chain (77).
Currently, obesity is highly prevalent worldwide due to an imbalance between energy intake and energy expenditure. Due to the energy expenditure characteristics of BAT, activation of BAT has been explored as one of the strategies to treat obesity and associated diseases (125). However, the BAT amount is very limited in adult humans, and activation of BAT upon cold stimulation could only be found in 3% of males and 7.5% of females, respectively, by positron emission tomography-computed tomography (24, 71). Interestingly, cold stimulation can convert WAT into beige adipose tissue (BeAT) through a browning process (16). Similar to adipocytes in classic BAT, some clusters of adipocytes in BeAT express uncoupling protein 1 (UCP-1), which promotes energy expenditure (111). Thus, inducing BeAT instead of BAT is emerging as a strategy to treat obesity and associated diseases (23).
Of significance, and relevant to cardiovascular diseases (CVDs), the blood vessels such as the aorta, coronary, and carotid arteries are surrounded by adipose tissue, referred to as perivascular adipose tissue (PVAT). The fat attenuation index (FAI), which is the average reduction in the signal of adipose tissue within a volume of interest as measured from reconstructed computerized tomography scanning, in PVAT was inversely associated with the size of adipocytes and positively correlated with atherosclerotic plaque burden in the coronary artery (7). A recent large cohort study involving 3912 clinical patients further documented that high values of perivascular FAI were associated with a significantly higher adjusted risk of all-cause cardiac mortality (78).
However, PVAT displays heterogeneity according to its location. The PVAT from the thoracic aortic area is BAT-like, and the PVAT from the abdominal aortic area is BeAT-like in morphology and gene expression profile (103). Compared with visceral WAT, PVAT is irregular in shape and scattered (19). The expression levels of FABP4, FAS, GPDH, and LPSR are significantly lower in PVAT than in visceral WAT (19). Functionally, PVAT secretes a large number of angiogenic factors such as TSP-1, serpinE1, and IGFBP, which regulate vascular angiogenesis (87).
PVAT also secretes other vasoactive substances described in the sections below that contribute to vascular tone regulation and atherosclerosis development. In addition, adipocyte precursors and stromal cells in PVAT contribute to vascular remodeling (43, 81). Therefore, in addition to visceral WAT, PVAT should be included in the study of the roles of adipose tissues in the cardiovascular system and their contribution to cardiovascular health and disease.
PVAT and Hypertension
Hypertension is a major risk factor for stroke, aortic aneurysm, and coronary heart disease. Hypertension is a progressive chronic clinical syndrome characterized by elevated systemic arterial blood pressure. Apart from contributions to hypertension from the neurohemal fluid system, including the heart, kidney, and nervous system, this disease is prevalent in obese subjects, suggesting that changes in adipose tissue also contribute to hypertension development and progression.
PVAT produces many biologically active molecules including adipokines (e.g., leptin, adiponectin, and omentin), cytokines/chemokines (e.g., interleukin [IL]-6, tumor necrosis factor-α [TNF-α], monocyte chemoattractant protein-1 [MCP-1]), gaseous molecules (e.g., nitric oxide [NO] and hydrogen sulfide [H2S]), prostacyclin, angiotensin-1 to 7 (Ang 1–7), angiotensin II (Ang II), methyl palmitate, and reactive oxygen species (ROS) (16, 105). All these molecules contribute to vascular homeostasis. Adiponectin, NO, H2S, prostacyclin, Ang 1–7, and methyl palmitate induce vasodilation by targeting the underlying ECs and VSMCs in the blood vessel wall. Ang II, ROS, and other yet undetermined factors cause vasoconstriction. Thus, PVAT-derived factors synergistically and concurrently regulate vascular tone (105, 115).
PVAT mass is markedly increased in obesity. Compared with lean PVAT, obese PVAT secretes more inflammatory adipokines/cytokines, including TNF-α, leptin, IL-6, plasminogen activator, and resistin, which may further alter PVAT characteristics and secretomes, eventually affecting vascular homeostasis. Thus, the contribution of PVAT to hypertension has received increased attention in the last decade. Here, we provide an update on recent research progress regarding the roles of PVAT in blood pressure regulation.
Renin–angiotensin–aldosterone system in PVAT and hypertension
The mechanisms underlying hypertension development and progression are complex. Abnormal activation of the renin–angiotensin–aldosterone system (RAAS) is critical to the process of hypertension from the initial to the end stage. Interestingly, angiotensinogen (Agt) levels were found to be significantly elevated in adipose tissue of rats with essential hypertension and obesity. Also, the concentrations and activities of Agt, plasma rennin, and angiotensin-converting enzyme in adipose tissue were positively associated with the degree of obesity (29, 75, 120).
The adipose tissue might be the main source of RAAS in hypertensive patients with obesity (22). Locally, Ang II is derived from Agt, which is also found in PVAT. Angiotensin receptors including angiotensin type 1 receptor (AT1R), angiotensin type 2 receptor (AT2R), angiotensin type 3 receptor (AT3R), and angiotensin type 4 receptor (AT4R) are expressed throughout the vascular wall. It is known that AT1R activation can cause vasoconstriction, aldosterone synthesis, and secretion, increase vasopressin secretion and cardiac hypertrophy, and it regulates sympathetic activity.
Activation of AT1R in PVAT promotes vascular inflammation and endothelial dysfunction (48, 91). Aldosterone may directly act on PVAT to promote proinflammatory phenotypes. Aldosterone receptor antagonists have been shown to have beneficial effects on oxidative stress, endothelial function, blood pressure regulation, weight loss, and systemic insulin sensitivity (29, 50, 96). Angiotensin receptor blockers (ARBs) can reduce release of Ang II and aldosterone from PVAT, and prompt the release of perivascular-derived relaxing factors, which induce vasodilation by opening voltage-dependent K+ channels on VSMCs (11, 48, 60).
We recently reported that pretreatment of blood vessel rings with ARBs markedly blocked their contraction in response to PVAT extracts (24). Knockout of Agt in PVAT significantly reduced local Ang II production in PVAT from mice. The Agt-deficient mice showed hypotension during the circadian resting phase. In fact, Agt expression in PVAT showed a circadian pattern, suggesting that Ang II production in PVAT was controlled by the circadian clock. Indeed, knockout of Bmal1 in PVAT significantly blunted the circadian expression pattern of Agt and recapitulated the blood pressure phenotype of PVAT-Agt-deficient mice, showing hypotension during the resting phase. Circadian control of Agt expression in PVAT appears to involve direct transcriptional control of Agt by Bmal1 (Fig. 1) (18).
FIG. 1.
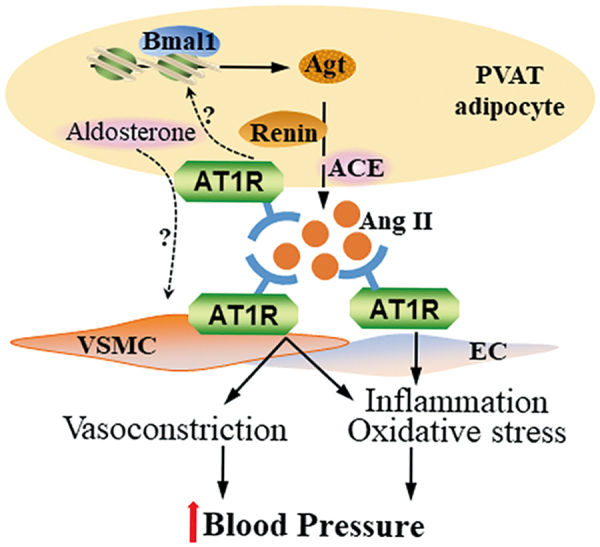
RAAS in PVAT and blood pressure regulation. Local Agt in PVAT is transcriptionally regulated by Bmal1 resulting in local production of Ang II in a circadian pattern, and increasing blood pressure by inducing contraction of VSMC and promoting inflammation and oxidative stress in EC. It is yet unknown whether Ang II binds the AT1 receptors on PVAT adipocytes to negatively feedback on Bmal1 and Agt expression. In addition, PVAT adipocytes produce aldosterone. Currently, there is no direct evidence demonstrating that PVAT-derived aldosterone regulates VSMC function. Agt, angiotensinogen; Ang II, angiotensin II; AT1, angiotensin type 1; EC, endothelial cell; PVAT, perivascular adipose tissue; RAAT, renin–angiotensin–aldosterone system; VSMC, vascular smooth muscle cell. Color images are available online.
The relative contribution of other potential underlying mechanisms and the extent and role of the PVAT-dependent regulation of circadian rhythmicity of blood pressure and hypertension are still unknown, including their potential association with adverse outcomes in superdipper and nondipper phenotypes in humans, as observed in the clinic (69). Nevertheless, inhibition of RAAS by ARB and aldosterone inhibitors may reduce blood pressure and provide other cardiovascular benefits through direct effects on PVAT, beyond those on their well-established targets, such as kidney and heart (68).
Sympathetic nervous system in PVAT and hypertension
Electrophysiological studies confirmed the contribution of the sympathetic nervous system (SNS) in adipose tissues under conditions of glucose deficiency, cold stimulation, and free fatty acids (FFAs) release (95). SNS activation promotes lipolysis in adipose tissues. Local denervation of SNS resulted in increased adipose tissue expansion and inhibition of lipolysis (10), underscoring the contribution of adipose tissue SNS to hypertension. In the clinic, α/β-adrenergic receptor blockers, which reduce SNS activity, are used for the treatment of obesity-associated hypertension to reduce blood pressure (49).
It is unclear whether the activation of SNS in PVAT is associated with hypertension. Recent studies have shown that SNS activation is an important factor in PVAT-dependent vasoconstriction in vitro (9). Activation of the sympathetic nerves improves the contractility of the small mesenteric resistance arteries with PVAT, which can be eliminated by removing the sympathetic nerves in the adipose tissue. Stimulation of the sympathetic nerves has a diastolic effect on the superior mesenteric artery. However, in the aorta, there is a significant contraction-promoting effect by stimulation of the SNS (4). PVAT contains 5-hydroxytryptamine (5-HT). Fenfluramine, a 5-HT releaser, induced a greater contraction of aortic rings with PVAT than rings without PVAT. The α-1 adrenoreceptor antagonist prazosin and the norepinephrine transporter inhibitor nisoxetine significantly blocked fenfluramine-induced aortic ring contraction, indicating that PVAT-derived 5-HT or norepinephrine resulted in vessel ring contraction (56).
In addition, electrophysiological studies demonstrated that perivascular nerve activation by electrical field stimulation elicited a frequency-dependent contractile response in arterial rings, in which the amplitude of contraction was higher in arterial rings with PVAT than in those after PVAT removal. Electrical field stimulation of nerves in PVAT increased the release of O2•−, Ang II, and chemerin, which induced vessel contraction (25, 38, 65, 108). Also, electrical field stimulation induced PVAT release of adiponectin, calcitonin gene-related peptide and methyl palmitate, which elicited endothelium-independent anticontractile effects on arterial rings (15, 94). These results suggest that PVAT releases both contractile and anticontractile factors in response to perivascular sympathetic nerve stimulation to maintain the physiological tone (Fig. 2).
FIG. 2.
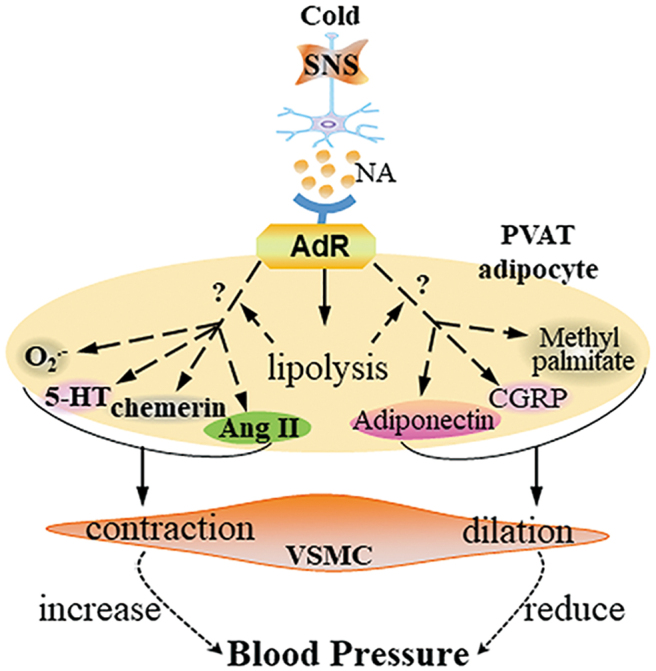
SNS in PVAT and blood pressure regulation. Cold or other stimuli activate the SNS in PVAT and release NA. Through binding to its receptors on PVAT adipocytes, NA induces lipolysis, which may lead to the release of PVAT-derived contracting factors such as Ang II, chemerin, serotonin (5-HT), and O2•− or PVAT-derived relaxing factors such as adiponectin, CGRP, and methyl palmitate. Those factors coordinately regulate VSMC contraction or dilation. 5-HT, 5-hydroxytryptamine; CGRP, calcitonin gene-related peptide; NA, noradrenalin; SNS, sympathetic nervous system. Color images are available online.
Furthermore, renal sympathetic activity affects blood pressure in part by increasing renovascular resistance via release of norepinephrine from sympathetic nerves onto the renal arteries. PVAT surrounding the renal blood vessels contains a pool of norepinephrine (86). Sympathomimetic tyramine-induced vessel contraction was greater in the renal artery with PVAT versus that without PVAT, an effect that was reduced by nisoxetine. Renal denervation significantly reduced norepinephrine contents in renal PVAT but did not modify tyramine-induced contraction of renal arteries with PVAT (86).
Thus, these data support the hypothesis that activation of SNS in PVAT might coordinately regulate blood pressure through PVAT-derived factors. More studies are required to determine whether the activity of the SNS in PVAT of hypertensive subjects is altered, the roles in blood pressure regulation of the substances released specifically from PVAT upon SNS activation, and the underlying mechanisms.
Adipokines and immune cells in PVAT and hypertension
Hypertension is more prevalent in obese subjects than in lean subjects. The progression of hypertension is related to immune responses in adipose tissues (26). Under the obese condition, hypertrophy of adipose tissues causes alterations of the normal profiles of adipokines (36). Adiponectin is one of the most abundant adipokines that dilate the vascular wall. The plasma adiponectin level is reduced in hypertensive patients with obesity, and shows a significant upward trend after treatment and control of hypertension, indicating protective effects of adiponectin in hypertension (8, 121). The presence of PVAT reduced the contractile response to norepinephrine in blood vessel rings; however, PVATs anticontractile effect was significantly reduced in obese subjects and obese mice (44).
Multiple signaling pathways contribute to the anticontractile effects of PVAT-derived adiponectin. PVAT from knockout mice with loss of either adiponectin or mitochondrial Ca2+-activated large-conductance K+ (BKCa) channel did not show an anticontractile response in blood vessel rings, suggesting that BKCa channels are involved in adiponectin-induced vasorelaxation in arteries (66). cGMP-dependent protein kinase (PKG) was necessary for the anticontractile effects of PVAT on ECs and VSMCs, evidenced by the absence of anticontractile effects from PVAT of PKG−/− mice. The absence of PKG in PVAT was associated with reduced adipocyte adiponectin expression, suggesting that vasorelaxation induced by PVAT-derived adiponectin was associated with PKG (66).
AMP-activated protein kinase (AMPK) is a key mediator of cellular energy balance and may mediate the vascular effects of adiponectin (Fig. 3). High-fat-diet feeding reduced the anticontractile effect of PVAT as well as AMPK phosphorylation (6). PVAT from AMPKα1 knockout mice secreted significantly less adiponectin. PVAT from wild-type mice augmented relaxation of vessel rings without PVAT in response to cromakalim, a potassium channel-opening vasodilator, but not PVAT from AMPKα1 knockout mice. Addition of adiponectin to AMPKα1-deficient vessel rings without PVAT augmented relaxation in response to cromakalim (5).
FIG. 3.
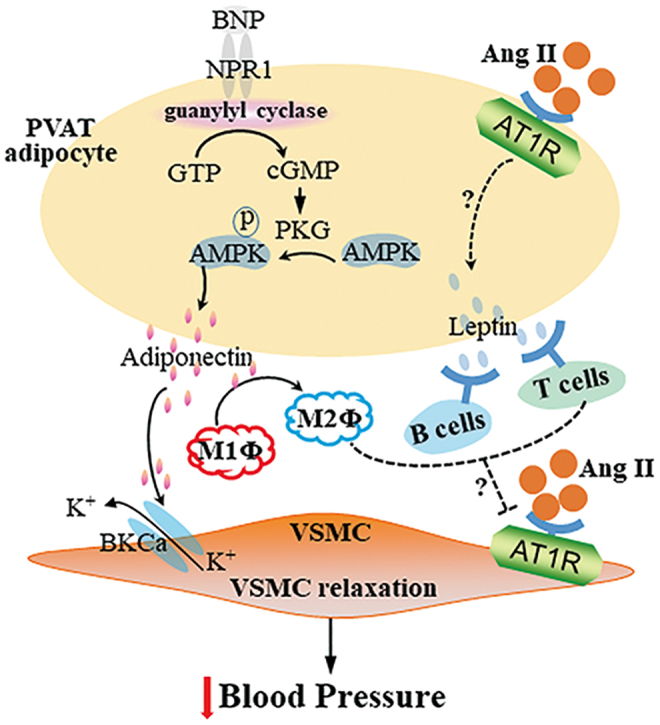
Adipokines and immune cells in PVAT and blood pressure regulation. Vasodilators, such as BNP, bind to receptors on PVAT adipocytes and activate the guanylyl cyclase cascade signaling via GTP-cGMP-PKG, which induces secretion of adiponectin through phosphorylation of AMPK. Adiponectin from PVAT adipocytes dilates VSMC by opening Ca2+-activated K+ channels (BKCa) on VSMC. Also, adiponectin promotes M1 macrophage (M1Φ) to M2Φ polarization, which, together with activation of B cells and T cells by PVAT-derived leptin, blocks Ang II-induced VSMC contraction. The mechanisms underlying the regulation of the Ang II effects by those immune cells remain unclear. AMPK, AMP-activated protein kinase; BNP, brain natriuretic peptide; PKG, cGMP-dependent protein kinase. Color images are available online.
In addition, the complement 3 (C3) activation in macrophages in PVAT was negatively associated with adiponectin expression in PVAT of mice with hypertension induced by DOCA salt. PVAT macrophage-derived C3 resulted in C5a generation, which promoted macrophage polarization from M2 to M1. C5a further induced TNF-α secretion and inhibited adiponectin expression in PVAT adipocytes of hypertensive mice. Consistently, macrophage infiltration and C5a expression were increased, which was associated with decreased adiponectin expression in adipose tissue from patients with aldosterone-producing adenoma (89, 90).
In the clinical setting, it was observed that the anticontractile activity of PVAT, lost in obese patients before bariatric surgery when compared with healthy volunteers, was restored 6 months after surgery. The improvement in anticontractile function after surgery was accompanied by improvements in inflammatory cytokines, adipokine profiles, and systolic blood pressure together with increased PVAT adiponectin and reduced macrophage infiltration and inflammation (1).
In mice, high-fat-diet feeding caused an increased infiltration of macrophages in PVAT with increased expression of the M1 macrophage markers Nos2 and IL-1β and the M2 marker Chil3 (6). Adiponectin secreted by adipose tissues stimulates M2-type polarization in macrophages, exerting anti-inflammatory effects (124). Sustained weight loss in rats led to an improvement in PVAT function associated with reduced inflammation and normalization of plasma adipokine levels, including leptin (13).
Leptin is another abundant adipokine released by adipose tissues, including PVAT. Leptin mRNA and protein expression were significantly lower in PVAT from spontaneously hypertensive rats (SHRs) (37). Apart from targeting the central nervous system, leptin receptors are expressed on CD4+ and CD8+ T cells, B cells, and monocytes/macrophages. In the absence of leptin, CD4+ T cells showed decreased proliferation and immune responses (82). While Ang II administration increased leukocyte and T cell content in PVAT (74), T regulatory cells (Tregs) adoptive transfer prevented Ang II-induced hypertension and microvascular injury (73). In T and B cell-deficient mice with knockout of recombination-activating gene 1 (Rag1), Ang II-induced increases in systolic blood pressure (but not in diastolic blood pressure) could be rescued by infusion of Tregs.
Ang II-induced endothelial dysfunction and oxidative stress in PVAT were exacerbated in the Rag1 knockout mice, while T cell transplantation from wild-type mice rescued the phenotype. Ang II increased monocyte/macrophage infiltration and proinflammatory polarization in PVAT in the Treg-deficient mice (73). Administration to mice of 1,2,3,4,6-penta-O-kgalloyl-β-d-glucose (PGG), an anti-inflammatory compound, significantly decreased total leukocyte and all T cell subset infiltration in PVAT. PGG also decreased the content of T cells bearing CD25, CCR5, and CD44 receptors and the expression of MCP-1 and RANTES in PVAT. PGG administration also decreased the content of TNF+ and IFN-γ+ CD8 T cells and IL-17A+ CD4+ and CD3+ CD4− CD8− cells (74). These studies suggest an active role of the local immune responses in PVAT contributing to hypertension.
Apelin is widely found in the body, and is expressed in human and mouse adipocytes. Apelin levels are inversely associated with inflammation. Apelin-13 downregulates the expression of TNF-α and MCPs in macrophages (119, 123). Apelin-13 increased NO bioavailability, and reduced blood pressure in mice and rats (20, 63). Ang II also increased vasoconstriction in apelin-deficient mice (93). Acetylcholine-induced relaxation of arteries without PVAT was increased in the presence of exogenous apelin. Apelin mRNA level in PVAT was higher in obese SHRs than in control Wistar-Kyoto rats, perhaps reflecting a compensatory mechanism (47). Collectively, these findings suggest that apelin in PVAT might modulate blood pressure to promote vascular tone.
In addition, PVAT might mediate sex-dependent vascular function in hypertension. In the presence of PVAT, contraction in response to endothelin-1 and the thromboxane mimetic U46619 was significantly reduced in porcine coronary arteries from females, but not males. The adiponectin receptor agonist (adipoRon) produced greater relaxation in porcine coronary arteries from females compared with males (3). Vasorelaxation induced by cromakalim in rings with PVAT was significantly impaired in vessels from stroke-prone SHR males relative to females. A crossover study assessing the function of male PVAT on female vessels confirmed the reduced vasorelaxation response to cromakalim associated with male PVAT (99).
Further studies indicated that resistin expression was increased approximately twofold in PVAT from male stroke-prone SHR vessels. Preincubation with resistin significantly impaired the ability of female vessels with PVAT to relax in response to cromakalim (99). These findings suggest roles of PVAT-derived adipokines in mediating sex-dependent vascular function in hypertension and may underscore intrinsic differences in PVAT between the sexes, a possibility not yet addressed (110).
Overall, the findings summarized in this section highlight considerable progress in our understanding of the complexity behind the multifaceted roles of PVAT in the regulation of blood pressure and its contribution to hypertension, as well as define PVAT as a necessary target to be considered for clinical management of blood pressure.
PVAT and Atherosclerosis
Atherosclerosis is a chronic metabolic syndrome characterized by endothelial dysfunction, lipid deposition, and inflammatory infiltration (97). Endothelial dysfunction/injury caused by high shear stress promotes initiation of atherosclerosis, and subsequent adhesion of circulating inflammatory cells to the dysfunctional endothelium triggers accumulation of cholesterol in the arterial wall to further aggravate atherosclerosis (104). Recently, the roles of PVAT in atherosclerosis development have been extensively studied. Here, we review recent updates regarding the relationship between PVAT and atherosclerosis.
PVAT thermogenesis and atherosclerosis
The PVATs in different locations resemble different types of adipose tissues. The thoracic aortic PVAT is BAT-like, while abdominal aortic PVAT is BeAT in rodents (79). The human aortic and coronary artery PVAT is BeAT as well (19, 31). As described above, both BAT and BeAT have thermogenic capability due to high UCP-1 protein levels in mitochondria. Unlike WAT, BAT and BeAT have anti-inflammatory characteristics.
We previously reported that thermogenic aortic PVAT was able to improve endothelial function, which was impaired in aging mice. Activation of PVAT in ApoE−/− mice with intact PVAT by mild cold exposure improved endothelial function and prevented atherosclerosis development. ApoE−/− mice lacking PVAT showed severe atherosclerotic lesions that could not be reduced by mild cold exposure (17). We further demonstrated that lack of PVAT augmented macrophage infiltration in the perivascular area of the aorta and increased production of inflammatory cytokines, which resulted in vascular inflammation and increased atherosclerotic lesions in the aortic wall (117).
Consistently, deletion of insulin receptors in brown adipocytes resulted in lipoatrophy in BAT, including PVAT, and induced visceral adiposity in the gonadal depot. Insulin receptor-deficient ApoE−/− mice showed severe atherosclerosis and increased expression of TNF-α and leptin, while there was decreased adiponectin in adipose tissues, including thoracic PVAT (42). These results suggest that promoting thermogenesis in thermogenic PVAT may inhibit atherosclerosis development. Even though cold exposure significantly induced thermogenesis in PVAT (17), cold exposure significantly increased blood pressure and heart rate (44), which could increase the incidence rates of stroke and heart attack (35), thus making it a nonfeasible treatment in humans.
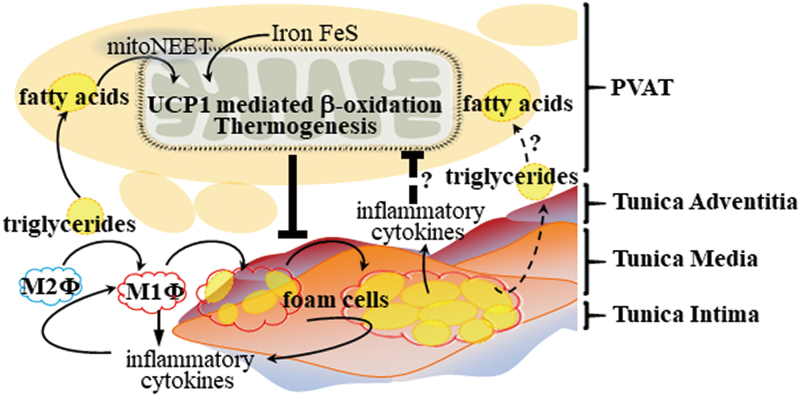
We found that CDGSH iron-sulfur domain 1 protein (referred to as mitoNEET), a mitochondrial outer membrane protein that promotes adipose tissue browning and thermogenesis, was highly expressed and significantly increased in PVAT upon cold stimulation. ApoE−/− mice with mitoNEET overexpression in PVAT were cold resistant and showed increased expression of thermogenic genes. Furthermore, they showed significant downregulation of inflammatory genes and reduced atherosclerosis development upon high-fat-diet feeding (116). Therefore, targeted pharmacological approaches to induce thermogenesis in PVAT could inhibit PVAT inflammation and atherosclerosis development, and represent a more feasible treatment compared with cold exposure (Fig. 4).
FIG. 4.
PVAT thermogenesis and atherosclerosis. PVAT enhances lipid droplet depletion in adipocytes through thermogenesis in the mitochondria via UCP-1-mediated β-oxidation. Sustained thermogenesis in PVAT reduces plasma lipid levels and prevents macrophage (Φ) polarization and foam cell formation in the blood vessel walls, resulting in decreased release of inflammatory cytokines. Enhanced mitoNEET activity in the mitochondrial membrane induces transport of Fe-S into the mitochondria, which helps utilization of FA through UCP-1-mediated β-oxidation. It is unknown whether thermogenesis in PVAT adipocytes can reduce the lipid contents in foam cells, or whether inflammatory cytokines released from foam cells can inhibit thermogenesis in PVAT adipocytes. FA, fatty acids; UCP-1, uncoupling protein 1. Color images are available online.
PVAT and endothelial dysfunction in atherosclerosis development
Inflammatory response and oxidative stress in PVAT in obesity ultimately lead to endothelial dysfunction (51). Endothelial dysfunction can also reduce endothelial nitric oxide synthase (eNOS) expression, reduce NO production and bioavailability, with ensuing adverse consequences on vasodilation, and significantly increase peripheral resistance of blood vessels. Recent studies have shown that PVAT expresses eNOS (114), and removal of PVAT reduces basal NO production in small arteries from healthy individuals, suggesting that PVAT contributes to vascular NO production (107).
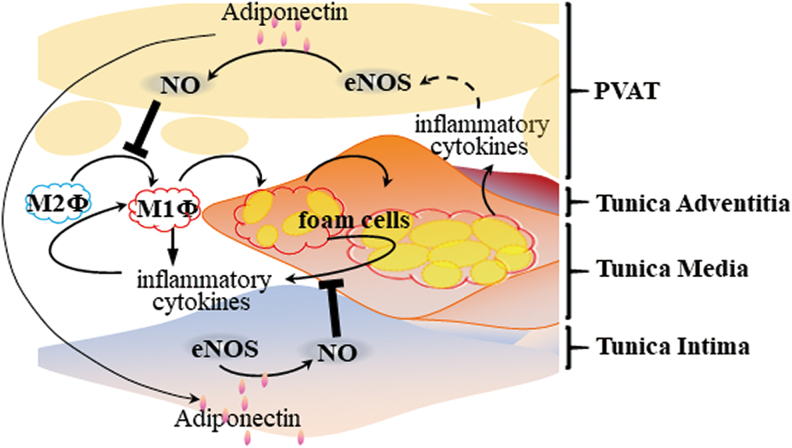
It is known that adiponectin normalizes endothelial function through a mechanism involving increased eNOS phosphorylation and controls blood pressure through an endothelial-dependent mechanism (52). Furthermore, PVAT-derived adiponectin inhibits plaque formation via a reduced vascular inflammatory response, as indicated above (Fig. 5). PVAT secretes biologically active H2S, which improves endothelial function (32). However, the relationship between PVAT-derived H2S and atherosclerosis development is unknown. Further investigation is required regarding the antiatherosclerotic effects of PVAT-derived factors such as adiponectin, NO, and H2S operating through improved endothelial function.
FIG. 5.
PVAT, endothelial dysfunction and atherosclerosis. PVAT-derived adiponectin promotes NO production by PVAT adipocytes and EC, which, in turn, inhibits macrophage polarization and subsequent foam cell formation in the vascular wall. Inflammatory cytokines released from foam cells may promote eNOS activity in PVAT adipocytes. eNOS, endothelial nitric oxide synthase; NO, nitric oxide. Color images are available online.
PVAT immune cells and atherosclerosis development
PVAT-derived inflammatory mediators may have adverse effects on atherosclerotic plaque formation and stability. Obesity increases the number and activation levels of the multiple resident immune cells in PVAT, including macrophages, dendritic cells (DCs), T cells, and B cells, which locally regulate the inflammatory status of the vascular wall.
Macrophages can be divided into classically activated M1 macrophages and selectively activated M2 macrophages depending on their activation state, function, and secreted factors. IL-4 and IL-13 secreted by eosinophils stimulate M2-type polarization in macrophages, exerting anti-inflammatory effects (21, 62). In adipose tissue of obese animals, the polarization state of macrophage cells is transformed from M2 to M1. Metabolically activated macrophages express classic M1-type polarizing markers (such as CD38, CD319, and CD274) and secrete large amounts of inflammatory mediators, such as IL-6, IFN-γ, and TNF-α (55). M2 macrophages in PVAT increased the expression of brown adipocyte-specific markers, which resulted in reduced inflammation and lower oxidative stress (64, 76).
Mas receptors are expressed in PVAT. AVE0991, a nonpeptide Ang-(1–7) receptor Mas agonist, has significant antiatherosclerotic properties in ApoE−/− mice. AVE0991 treatment reduced expression and production of the proinflammatory cytokines IL-1β, TNF-α, CCL2, and CXCL10, and differentiation to the M1 macrophage phenotype in PVAT (98). In addition, dysfunction of the low-density lipoprotein receptor-related protein-1 (LRP1) in liver and macrophages increased atherosclerotic risk (28, 72). Transplantation of adipose tissue from Lrp1−/− mice to the carotid artery regions of Ldlr−/− mice markedly increased atherosclerotic plaque in the carotid arteries, which was associated with increased expression of inflammatory genes such as IL6, TNF-α, and MCP-1 and infiltration of CD68+ macrophages in Lrp1−/− PVAT (53).
Macrophages also play an important role in regulating T cell activation through antigen presentation and costimulatory ligand expression, which lead to aggregation of other proinflammatory cells including CD4 T cells, CD8 T cells, B cells, and DCs (59). There are three types of T cells in PVAT; that is, CD4+ helper T cells (Th), CD8+ cytotoxic T cells (Tc), Treg (76). Th1, Tc1, and Th17 cells secrete proinflammatory cytokines such as IFN-γ, TNF-α, IL-17, and IL-6, which are related to atherosclerosis development (33, 112). In contrast, Treg have the ability to alter adipose tissue remodeling, improve insulin resistance, and reduce atherosclerosis and hypertension. They produce immunosuppressive factors, such as TGF-β and IL-10, regulating the immune response, reducing oxidative stress in blood vessels, endothelial dysfunction, infiltration of macrophages and T cells in the aorta, and proinflammatory cytokines in plasma (2, 41).
DCs are the most effective antigen-presenting cells found in PVAT (109). During PVAT inflammation, inflammatory mediators released from DCs stimulate T cells to produce proinflammatory cytokines by blocking the CD28/CD80/CD86 costimulatory axis between DCs and T cells (106). However, the contributions of DCs and T cells in PVAT to atherosclerotic lesion formation are still largely unclear.
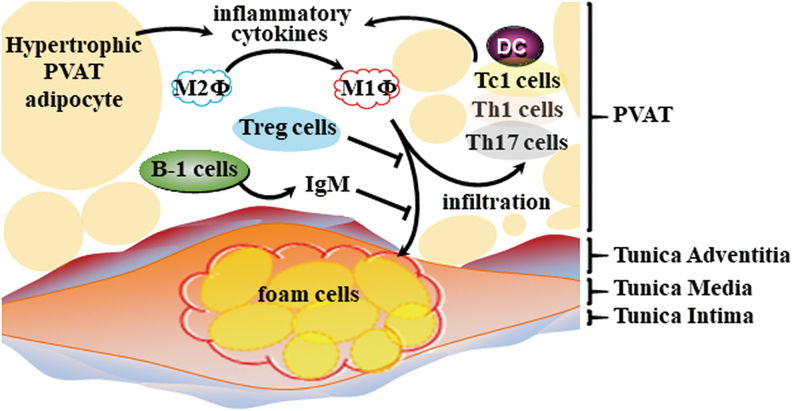
B cells have emerged as important immune cells in the regulation of visceral adipose tissue inflammation and atherosclerosis. B cell-mediated effects on atherosclerosis are subset dependent, with B-1 cells attenuating and B-2 cells aggravating atherosclerosis (14, 30, 57, 70, 100, 101). PVAT from human coronary arteries near the diseased region harbors B cells, including B-1 cells. Moreover, mouse PVAT harbors high numbers of atheroprotective IgM-secreting B-1 cells. IgM from B-1 cells blocks oxidized LDL-induced foam cell formation and inflammatory cytokine production. ApoE−/− mice with B cell-specific knockout of the helix-loop-helix factor Id3 had increased numbers of IgM-secreting B-1b cells in PVAT (101), further suggesting that inhibition of local inflammation in PVAT may serve to provide atheroprotection (Fig. 6).
FIG. 6.
PVAT immune cells and atherosclerosis. There are various PVAT resident immune cells, including macrophages, DCs, T cells (such as Tc1, Th1, and Th17), and B cells. Inflammatory cytokines released from hypertrophic PVAT adipocytes promote macrophage (Φ) polarization to the M1 type, which further induces T cells and DCs to produce inflammatory cytokines, thereby causing a positive feedback loop for foam cell formation in the vascular wall. However, Treg can prevent macrophage infiltration and foam cell formation. In addition, resident IgM-producing B-1 cells prevent atherosclerosis development. Thus, the overall imbalance of immune cells in PVAT can drive atherosclerosis development. DC, dendritic cell; Treg, regulatory T cell. Color images are available online.
Anti-inflammatory response in PVAT prevents atherosclerosis development
Teneligliptin is a DPP-4 inhibitor, which is used for the treatment of type 2 diabetes mellitus. Oral administration of teneligliptin to ApoE−/− mice significantly inhibited atherosclerotic lesions in the aortic arch, which was associated with reduced expression of macrophage marker Mac3 and Nox-4 in the PVAT surrounding the aortic arch (92). Treatment of ApoE−/− mice with another antidiabetic drug, pioglitazone, also significantly downregulated oxidative stress and proinflammatory markers in PVAT (84).
CD40/CD40L signaling exerts a critical role in the development of atherosclerosis. Treatment of ApoE−/− mice with siRNA against CD40 significantly reduced the extent and severity of the atherosclerotic lesions, as well as the number of F4/80+, galectin3+, macrophages, and NF-κB+ cells in the intima, which was associated with downregulation of transcription factor Taf3 and macrophage differentiation regulator Xpr1 in PVAT (46).
PVAT-derived adiponectin might be one of the anti-inflammatory adipokines able to inhibit the development of atherosclerosis. Indeed, ApoE−/− mice that received PVAT transplantation from adiponectin-deficient mice exhibited advanced atherosclerotic lesions in the carotid artery, and this was associated with attenuated autophagy in macrophages (61). Transplantation of thoracic PVAT from wild-type mice to the carotid artery of ApoE−/− mice nearly abrogated plaque macrophage content without affecting plaque size, while transplantation of thoracic PVAT from ApoE−/− mice showed higher mRNA levels of inflammatory cytokines compared with wild-type PVAT (85).
However, transplantation itself may cause PVAT inflammation and underlying vascular remodeling. In fact, transplantation of thoracic aortic PVAT to carotid arteries of ApoE−/− mice markedly increased the intraplaque macrophage infiltration, lipid core, intimal and vasa vasorum neovascularization, and expression of matrix metalloproteinase-2/9 in plaques while decreased smooth muscle cells and collagen in the atherosclerotic plaques (122).
These data were obtained at the endpoint of the animal study. The roles of PVAT in the plaque progression during development of the atherosclerotic lesion remain still unaddressed. Recently, a novel imaging technique using microcomputed tomography (microCT) to characterize the temporal and spatial development of aortic PVAT and luminal plaque soft tissue has been developed (94). Using microCT, the vascular wall volume was found to be larger, while the aortic PVAT volume was reduced in ApoE−/− mice compared with those in the C57BL/6J mice. Plaque development was colocalized with luminal ostia and origins of branching arteries, which traveled through areas of greatest PVAT volume in ApoE−/− mice. This technique provides a new strategy to assess the relationship between PVAT and pathology in vascular walls in the same animal during lesion development (34).
Mechanisms underlying PVAT roles in atherosclerosis development
Obesity promotes expansion of adipose tissues, including PVAT, and increases the rate of basal fat breakdown, which in turn increase release of FFA and secretion of proinflammatory factors into circulation. Hypertrophic PVAT may release abundant FFA into the perivascular area and other layers of the blood vessel wall.
FFA activate nuclear factor-κB (NF-κB), protein kinase C (PKC) and Toll-like receptors (TLRs) which promote phosphorylation of insulin receptor substrate 1 (IRS-1) in PVAT and other vascular cells (64, 88). Phosphorylation of IRS-1 reduces its ability to activate downstream PI3K/Akt signaling, resulting in inhibition of eNOS synthesis and NO production, accompanied by a decrease in NO bioavailability, triggering changes in vascular function and accumulation of ROS, resulting in endothelial dysfunction and, ultimately, the inevitable development of atherosclerosis. In addition, FFA activate TLR2 and TLR4, which regulate the NF-κB signaling pathway in macrophages (88).
TLR4-deficient Ldlr−/− mice showed more mitochondria and capillaries in PVAT and were protected against atherosclerosis development associated with reduced TNF-α expression in PVAT (64). Intracellular oxidation of stored FFA in PVAT adipocytes may produce ROS, leading to local vascular inflammation and PKC activation (83). Vascular local ROS can activate PKC and NF-κB-mediated inflammatory responses, as well as induce mitochondrial dysfunction leading to more ROS production (27). PKC also triggers eNOS coupling, which increases the synthesis of endothelin-1, leukocyte adhesion, and cytokine activation, and leads to vasoconstriction, platelet aggregation, and endothelial dysfunction (40).
In addition, in rats with high-fat-diet-induced obesity, PVAT dysfunction can promote endothelial dysfunction by modulating the AMPK/mammalian target of rapamycin kinase pathway (67). The AMPK signaling pathway is involved in the regulation of PVAT inflammation and oxidative stress (118). AMPK is an anti-inflammatory and antioxidative-stress regulator that exerts anti-inflammatory effects by upregulating IL-10 and downregulating TNF-α and IL-6 (39, 67). AMPK agonists AICAR, metformin, and resveratrol inhibited expression of the inflammatory cytokines (TNF-α, IL-6, MCP-1) and promoted expression of the anti-inflammatory factors, such as adiponectin and peroxisome proliferator-activated receptor-γ, which are associated with decrease of endoplasmic reticulum (ER) stress in PVAT (102, 113).
The NACHT, LRR, and PYD domains-containing protein 3 (NLRP3), also known as cryopyrin, is predominantly expressed in macrophages and is a component of the inflammasome. NLRP3 activation occurs in response to ER stress-related inflammation (45). NLRP3-dependent inflammasome activation decreased adiponectin while increased IL-6 secretion in PVAT, suggesting that inflammation induces PVAT dysfunction associated with ER stress (126).
The findings discussed in this section underscore a causative role of PVAT dysfunction in atherosclerosis and point to potential targets for intervention aimed at restoring PVAT homeostasis in the treatment of atherosclerosis.
Conclusion and Perspective
PVAT is a unique adipose tissue that plays an essential role in maintaining vascular structure and regulating vascular functions. Under physiological conditions, PVAT functions as an anti-inflammatory tissue, depicts heat production, improved FFA metabolism, and can regulate vasodilation. However, under pathophysiological conditions, such as obesity, PVAT becomes dysfunctional and promotes infiltration of inflammatory immune cells and local oxidative stress, triggering an “outside-to-inside” pathological signaling within the vessel wall, which leads to dysfunction of the underlying VSMCs and ECs. Eventually, dysfunctional PVAT promotes hypertension and atherosclerosis progression, thus suggesting an “outside-to-inside” paradigm for vascular disease development. Whether and how these PVAT effects act concurrently and synergistically with “inside-to-outside” signaling from VSMC and EC to PVAT remain to be addressed.
Future studies should further investigate the messengers responsible for the crosstalk between PVAT and underlying VSMCs and ECs in the vascular wall. We also need to better understand the balance of anticontractile and contractile systems in PVAT in homeostasis and in pathology as it pertains to blood pressure regulation. CVDs display sex differences. It is yet unclear whether and which intrinsic features of PVAT exhibit sex differences and what are their consequences on the development of CVDs. In addition, the actual contribution of alterations in PVAT metabolism to the overall systemic outcomes of CVDs remains largely unknown. Finally, aging contributes to vascular dysfunction. Single-cell RNA sequencing and bioinformatics analyses revealed that the brown adipogenic differentiation capacity of the stromal cells in PVAT was decreased during aging, which contributed to vascular remodeling (81).
In addition, old mice were resistant to cold-induced browning of perivascular adipocytes (80), and the browning levels of thoracic PVAT of aging SHRs were reduced significantly more than those in age-matched Wistar-Kyoto control rats (54). Therefore, aging might result in PVAT dysfunction and contribute to hypertension development. It is unclear whether dysfunctional PVAT also contributes to atherosclerosis development during aging. The new imaging techniques may provide a unique advantage for research investigating the dynamic alterations in PVAT during aging as well as the progression of vascular diseases such as hypertension and atherosclerosis.
Funding Information
This work was partially supported by National Institutes of Health grant HL122664 (Lin Chang) and HL068878 and HL137214 (Yuqing Eugene Chen).
Abbreviations Used
- 5-HT
5-hydroxytryptamine
- Agt
angiotensinogen
- AMPK
AMP-activated protein kinase
- Ang 1–7
angiotensin-1 to 7
- Ang II
angiotensin II
- ARBs
angiotensin receptor blockers
- AT1R
angiotensin type 1 receptor
- AT2R
angiotensin type 2 receptor
- AT3R
angiotensin type 3 receptor
- AT4R
angiotensin type 4 receptor
- BAT
brown adipose tissue
- BeAT
beige adipose tissue
- BKCa
Ca2+-activated large-conductance K+
- C3
complement 3
- CGRP
calcitonin gene-related peptide
- Chil3
chitinase-like protein 3
- CVDs
cardiovascular diseases
- DCs
dendritic cells
- DOCA
desoxycorticosterone acetate
- DPP-4
dipeptidyl peptidase-4
- ECs
endothelial cells
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- FABP4
fatty acid binding protein 4
- FAI
fat attenuation index
- FAS
fatty acid synthase
- FFA
free fatty acids
- GPDH
phosphoglucose dehydrogenase
- H2S
hydrogen sulfide
- IGFBP
insulin-like growth factor binding protein
- IL
interleukin
- IRS-1
insulin receptor substrate 1
- LPSR
lipopolysaccharide receptor
- LRP1
low-density lipoprotein receptor-related protein-1
- MCP-1
monocyte chemoattractant protein-1
- microCT
microcomputed tomography
- NF-κB
nuclear factor-κB
- NLRP3
NACHT, LRR and PYD domains-containing protein 3
- NO
nitric oxide
- Nos2
nitric oxide synthase 2
- PGG
1,2,3,4,6-penta-O-kgalloyl-β-d-glucose
- PKC
protein kinase C
- PKG
cGMP-dependent protein kinase
- PVAT
perivascular adipose tissue
- RAAS
renin–angiotensin–aldosterone system
- Rag1
recombination-activating gene 1
- ROS
reactive oxygen species
- serpinE1
serine protease inhibitor E1
- SHR
spontaneously hypertensive rats
- SNS
sympathetic nervous system
- TLRs
Toll-like receptors
- TNF-α
tumor necrosis factor-α
- Treg
T regulatory cells
- TSP-1
thrombospondin 1
- UCP-1
uncoupling protein 1
- VSMCs
vascular smooth muscle cells
- WAT
white adipose tissue
References
- 1. Aghamohammadzadeh R, Greenstein AS, Yadav R, Jeziorska M, Hama S, Soltani F, Pemberton PW, Ammori B, Malik RA, Soran H, and Heagerty AM. Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol 62: 128–135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agita A and Alsagaff MT. Inflammation, immunity, and hypertension. Acta Med Indones 49: 158–165, 2017 [PubMed] [Google Scholar]
- 3. Ahmad AA, Randall MD, and Roberts RE. Sex differences in the regulation of porcine coronary artery tone by perivascular adipose tissue: a role of adiponectin? Br J Pharmacol 174: 2773–2783, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmad MF, Ferland D, Ayala-Lopez N, Contreras GA, Darios E, Thompson J, Ismail A, Thelen K, Moeser AJ, Burnett R, Anantharam A, and Watts SW. Perivascular adipocytes store norepinephrine by vesicular transport. Arterioscler Thromb Vasc Biol 39: 188–199, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Almabrouk TAM, Ugusman AB, Katwan OJ, Salt IP, and Kennedy S. Deletion of AMPKalpha1 attenuates the anticontractile effect of perivascular adipose tissue (PVAT) and reduces adiponectin release. Br J Pharmacol 174: 3398–3410, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Almabrouk TAM, White AD, Ugusman AB, Skiba DS, Katwan OJ, Alganga H, Guzik TJ, Touyz RM, Salt IP, and Kennedy S. High fat diet attenuates the anticontractile activity of aortic PVAT via a mechanism involving AMPK and reduced adiponectin secretion. Front Physiol 9: 51, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, Margaritis M, Shirodaria C, Kampoli AM, Akoumianakis I, Petrou M, Sayeed R, Krasopoulos G, Psarros C, Ciccone P, Brophy CM, Digby J, Kelion A, Uberoi R, Anthony S, Alexopoulos N, Tousoulis D, Achenbach S, Neubauer S, Channon KM, and Antoniades C. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med 9: eaal2658, 2017 [DOI] [PubMed] [Google Scholar]
- 8. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, and Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79–83, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Ayala-Lopez N and Watts SW. New actions of an old friend: perivascular adipose tissue's adrenergic mechanisms. Br J Pharmacol 174: 3454–3465, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bartness TJ, Liu Y, Shrestha YB, and Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol 35: 473–493, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Correa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, and Touyz RM. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 59: 1069–1078, 2012 [DOI] [PubMed] [Google Scholar]
- 12. Briot A, Decaunes P, Volat F, Belles C, Coupaye M, Ledoux S, and Bouloumie A. Senescence Alters PPARgamma (peroxisome proliferator-activated receptor gamma)-dependent fatty acid handling in human adipose tissue microvascular endothelial cells and favors inflammation. Arterioscler Thromb Vasc Biol 38: 1134–1146, 2018 [DOI] [PubMed] [Google Scholar]
- 13. Bussey CE, Withers SB, Aldous RG, Edwards G, and Heagerty AM. Obesity-related perivascular adipose tissue damage is reversed by sustained weight loss in the rat. Arterioscler Thromb Vasc Biol 36: 1377–1385, 2016 [DOI] [PubMed] [Google Scholar]
- 14. Caligiuri G, Nicoletti A, Poirier B, and Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest 109: 745–753, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang HH, Shei-Dei Yang S, and Chang SJ. Perivascular adipose tissue modulation of neurogenic vasorelaxation of rat mesenteric arteries. J Cardiovasc Pharmacol 75: 21–30, 2019 [DOI] [PubMed] [Google Scholar]
- 16. Chang L, Garcia-Barrio MT, and Chen YE. Brown adipose tissue, not just a heater. Arterioscler Thromb Vasc Biol 37: 389–391, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, and Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 126: 1067–1078, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang L, Xiong W, Zhao X, Fan Y, Guo Y, Garcia-Barrio M, Zhang J, Jiang Z, Lin JD, and Chen YE. Bmal1 in perivascular adipose tissue regulates resting-phase blood pressure through transcriptional regulation of angiotensinogen. Circulation 138: 67–79, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, and Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res 104: 541–549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chun HJ, Ali ZA, Kojima Y, Kundu RK, Sheikh AY, Agrawal R, Zheng L, Leeper NJ, Pearl NE, Patterson AJ, Anderson JP, Tsao PS, Lenardo MJ, Ashley EA, and Quertermous T. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J Clin Invest 118: 3343–3354, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chung HS, Lee BS, and Ma JY. Ethanol extract of Mylabris phalerata inhibits M2 polarization induced by recombinant IL-4 and IL-13 in murine macrophages. Evid Based Complement Alternat Med 2017: 4218468, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cook S, Weitzman M, Auinger P, Nguyen M, and Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med 157: 821–827, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Cypess AM and Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes 17: 143–149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, and Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Darios ES, Winner BM, Charvat T, Krasinksi A, Punna S, and Watts SW. The adipokine chemerin amplifies electrical field-stimulated contraction in the isolated rat superior mesenteric artery. Am J Physiol Heart Circ Physiol 311: H498–H507, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeMarco VG, Aroor AR, and Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol 10: 364–376, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Di Meo S, Iossa S, and Venditti P. Improvement of obesity-linked skeletal muscle insulin resistance by strength and endurance training. J Endocrinol 234: R159–R181, 2017 [DOI] [PubMed] [Google Scholar]
- 28. Ding Y, Xian X, Holland WL, Tsai S, and Herz J. Low-density lipoprotein receptor-related protein-1 protects against hepatic insulin resistance and hepatic steatosis. EBioMedicine 7: 135–145, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dinh Cat AN, Friederich-Persson M, White A, and Touyz RM. Adipocytes, aldosterone and obesity-related hypertension. J Mol Endocrinol 57: F7–F21, 2016 [DOI] [PubMed] [Google Scholar]
- 30. Doran AC, Lipinski MJ, Oldham SN, Garmey JC, Campbell KA, Skaflen MD, Cutchins A, Lee DJ, Glover DK, Kelly KA, Galkina EV, Ley K, Witztum JL, Tsimikas S, Bender TP, and McNamara CA. B-cell aortic homing and atheroprotection depend on Id3. Circ Res 110: e1–e12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Efremova A, Senzacqua M, Venema W, Isakov E, Di Vincenzo A, Zingaretti MC, Protasoni M, Thomski M, Giordano A, and Cinti S.. A large proportion of mediastinal and perirenal visceral fat of Siberian adult people is formed by UCP1 immunoreactive multilocular and paucilocular adipocytes. J Physiol Biochem 2019. [Epub ahead of print]; DOI: 10.1007/s13105-019-00721-4 [DOI] [PubMed] [Google Scholar]
- 32. Emilova R, Dimitrova D, Mladenov M, Daneva T, Schubert R, and Gagov H. Cystathionine gamma-lyase of perivascular adipose tissue with reversed regulatory effect in diabetic rat artery. Biotechnol Biotechnol Equip 29: 147–151, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Engelbertsen D, Rattik S, Wigren M, Vallejo J, Marinkovic G, Schiopu A, Bjorkbacka H, Nilsson J, and Bengtsson E. IL-1R and MyD88 signalling in CD4+ T cells promote Th17 immunity and atherosclerosis. Cardiovasc Res 114: 180–187, 2018 [DOI] [PubMed] [Google Scholar]
- 34. Faight E, Verdelis K, Ahearn JM, and Shields KJ. 3D MicroCT spatial and temporal characterization of thoracic aorta perivascular adipose tissue and plaque volumes in the ApoE-/- mouse model. Adipocyte 7: 156–165, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fares A. Winter Hypertension: potential mechanisms. Int J Health Sci (Qassim) 7: 210–219, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fuster JJ, Ouchi N, Gokce N, and Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res 118: 1786–1807, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Galvez-Prieto B, Somoza B, Gil-Ortega M, Garcia-Prieto CF, de Las Heras AI, Gonzalez MC, Arribas S, Aranguez I, Bolbrinker J, Kreutz R, Ruiz-Gayo M, and Fernandez-Alfonso MS. Anticontractile effect of perivascular adipose tissue and leptin are reduced in hypertension. Front Pharmacol 3: 103, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, and Lee RM. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res 71: 363–373, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Gauthier MS, O'Brien EL, Bigornia S, Mott M, Cacicedo JM, Xu XJ, Gokce N, Apovian C, and Ruderman N. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem Biophys Res Commun 404: 382–387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Geraldes P and King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 106: 1319–1331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gilleron J, Bouget G, Ivanov S, Meziat C, Ceppo F, Vergoni B, Djedaini M, Soprani A, Dumas K, Jacquel A, Yvan-Charvet L, Venteclef N, Tanti JF, and Cormont M. Rab4b deficiency in T cells promotes adipose Treg/Th17 imbalance, adipose tissue dysfunction, and insulin resistance. Cell Rep 25: 3329–3341.e3325, 2018 [DOI] [PubMed] [Google Scholar]
- 42. Gomez-Hernandez A, Beneit N, Escribano O, Diaz-Castroverde S, Garcia-Gomez G, Fernandez S, and Benito M. Severe brown fat lipoatrophy aggravates atherosclerotic process in male mice. Endocrinology 157: 3517–3528, 2016 [DOI] [PubMed] [Google Scholar]
- 43. Gu W, Nowak WN, Xie Y, Le Bras A, Hu Y, Deng J, Issa Bhaloo S, Lu Y, Yuan H, Fidanis E, Saxena A, Kanno T, Mason AJ, Dulak J, Cai J, and Xu Q. Single-cell RNA-sequencing and metabolomics analyses reveal the contribution of perivascular adipose tissue stem cells to vascular remodeling. Arterioscler Thromb Vasc Biol 39: 2049–2066, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hong CH, Kuo TB, Huang BC, Lin YC, Kuo KL, Chern CM, and Yang CC. Cold exposure can induce an exaggerated early-morning blood pressure surge in young prehypertensives. PLoS One 11: e0150136, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoseini Z, Sepahvand F, Rashidi B, Sahebkar A, Masoudifar A, and Mirzaei H. NLRP3 inflammasome: its regulation and involvement in atherosclerosis. J Cell Physiol 233: 2116–2132, 2018 [DOI] [PubMed] [Google Scholar]
- 46. Hueso M, De Ramon L, Navarro E, Ripoll E, Cruzado JM, Grinyo JM, and Torras J. Silencing of CD40 in vivo reduces progression of experimental atherogenesis through an NF-kappaB/miR-125b axis and reveals new potential mediators in the pathogenesis of atherosclerosis. Atherosclerosis 255: 80–89, 2016 [DOI] [PubMed] [Google Scholar]
- 47. Kagota S, Maruyama-Fumoto K, Iwata S, Shimari M, Koyanagi S, Shiokawa Y, McGuire JJ, and Shinozuka K. Perivascular adipose tissue-enhanced vasodilation in metabolic syndrome rats by apelin and N-acetyl(-)l-cysteine-sensitive factor(s). Int J Mol Sci 20: 106, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kagota S, Maruyama-Fumoto K, Shimari M, McGuire JJ, and Shinozuka K. Angiotensin II type 1 receptor antagonist azilsartan restores vascular reactivity through a perivascular adipose tissue-independent mechanism in rats with metabolic syndrome. Cardiovasc Drugs Ther 33:501–509, 2019 [DOI] [PubMed] [Google Scholar]
- 49. Kalil GZ and Haynes WG. Sympathetic nervous system in obesity-related hypertension: mechanisms and clinical implications. Hypertens Res 35: 4–16, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kawarazaki W and Fujita T. The role of aldosterone in obesity-related hypertension. Am J Hypertens 29: 415–423, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ketonen J, Shi J, Martonen E, and Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J 74: 1479–1487, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Kim DH, Kim C, Ding EL, Townsend MK, and Lipsitz LA. Adiponectin levels and the risk of hypertension: a systematic review and meta-analysis. Hypertension 62: 27–32, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Konaniah ES, Kuhel DG, Basford JE, Weintraub NL, and Hui DY. Deficiency of LRP1 in mature adipocytes promotes diet-induced inflammation and atherosclerosis-brief report. Arterioscler Thromb Vasc Biol 37: 1046–1049, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kong LR, Zhou YP, Chen DR, Ruan CC, and Gao PJ. Decrease of perivascular adipose tissue browning is associated with vascular dysfunction in spontaneous hypertensive rats during aging. Front Physiol 9: 400, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, and Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab 20: 614–625, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kumar RK, Darios ES, Burnett R, Thompson JM, and Watts SW. Fenfluramine-induced PVAT-dependent contraction depends on norepinephrine and not serotonin. Pharmacol Res 140: 43–49, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kyaw T, Tay C, Hosseini H, Kanellakis P, Gadowski T, MacKay F, Tipping P, Bobik A, and Toh BH. Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PLoS One 7: e29371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lapid K, Lim A, Clegg DJ, Zeve D, and Graff JM. Oestrogen signalling in white adipose progenitor cells inhibits differentiation into brown adipose and smooth muscle cells. Nat Commun 5: 5196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee BC and Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta 1842: 446–462, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, Chen PY, Kuo JS, and Lee TJ. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation 124: 1160–1171, 2011 [DOI] [PubMed] [Google Scholar]
- 61. Li C, Wang Z, Wang C, Ma Q, and Zhao Y. Perivascular adipose tissue-derived adiponectin inhibits collar-induced carotid atherosclerosis by promoting macrophage autophagy. PLoS One 10: e0124031, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li Z, Xu F, Wang Z, Dai T, Ma C, Liu B, and Liu Y. Macrophages undergo M1-to-M2 transition in adipose tissue regeneration in a rat tissue engineering model. Artif Organs 40: E167–E178, 2016 [DOI] [PubMed] [Google Scholar]
- 63. Liakos CI, Sanidas EA, Perrea DN, Grassos CA, Chantziara V, Viniou NA, Barbetseas JD, and Papadopoulos DP. Apelin and visfatin plasma levels in healthy individuals with high normal blood pressure. Am J Hypertens 29: 549–552, 2016 [DOI] [PubMed] [Google Scholar]
- 64. Liu P, Huang G, Cao Z, Xie Q, Wei T, Huang C, Li Q, Sun M, Shen W, and Gao P. Haematopoietic TLR4 deletion attenuates perivascular brown adipose tissue inflammation in atherosclerotic mice. Biochim Biophys Acta Mol Cell Biol Lipids 1862: 946–957, 2017 [DOI] [PubMed] [Google Scholar]
- 65. Lu C, Su LY, Lee RM, and Gao YJ. Mechanisms for perivascular adipose tissue-mediated potentiation of vascular contraction to perivascular neuronal stimulation: the role of adipocyte-derived angiotensin II. Eur J Pharmacol 634: 107–112, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH, and Heagerty AM. Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol 304: H786–H795, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ma L, Ma S, He H, Yang D, Chen X, Luo Z, Liu D, and Zhu Z. Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens Res 33: 446–453, 2010 [DOI] [PubMed] [Google Scholar]
- 68. Ma TK, Kam KK, Yan BP, and Lam YY. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br J Pharmacol 160: 1273–1292, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mahabala C, Kamath P, Bhaskaran U, Pai ND, and Pai AU. Antihypertensive therapy: nocturnal dippers and nondippers. Do we treat them differently? Vasc Health Risk Manag 9: 125–133, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Major AS, Fazio S, and Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol 22: 1892–1898, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Marlatt KL and Ravussin E. Brown adipose tissue: an update on recent findings. Curr Obes Rep 6: 389–396, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. May P, Bock HH, and Nofer JR. Low density receptor-related protein 1 (LRP1) promotes anti-inflammatory phenotype in murine macrophages. Cell Tissue Res 354: 887–889, 2013 [DOI] [PubMed] [Google Scholar]
- 73. Mian MO, Barhoumi T, Briet M, Paradis P, and Schiffrin EL. Deficiency of T-regulatory cells exaggerates angiotensin II-induced microvascular injury by enhancing immune responses. J Hypertens 34: 97–108, 2016 [DOI] [PubMed] [Google Scholar]
- 74. Mikolajczyk TP, Nosalski R, Skiba DS, Koziol J, Mazur M, Justo-Junior AS, Kowalczyk P, Kusmierczyk Z, Schramm-Luc A, Luc K, Maffia P, Graham D, Kiss AK, Naruszewicz M, and Guzik TJ. 1,2,3,4,6-Penta-O-galloyl-beta-d-glucose modulates perivascular inflammation and prevents vascular dysfunction in angiotensin II-induced hypertension. Br J Pharmacol 176: 1951–1965, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Muller-Fielitz H, Lau M, Johren O, Stellmacher F, Schwaninger M, and Raasch W. Blood pressure response to angiotensin II is enhanced in obese Zucker rats and is attributed to an aldosterone-dependent mechanism. Br J Pharmacol 166: 2417–2429, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nosalski R and Guzik TJ. Perivascular adipose tissue inflammation in vascular disease. Br J Pharmacol 174: 3496–3513, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Oelkrug R, Polymeropoulos ET, and Jastroch M. Brown adipose tissue: physiological function and evolutionary significance. J Comp Physiol B 185: 587–606, 2015 [DOI] [PubMed] [Google Scholar]
- 78. Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, Thomas S, Herdman L, Kotanidis CP, Thomas KE, Griffin BP, Flamm SD, Antonopoulos AS, Shirodaria C, Sabharwal N, Deanfield J, Neubauer S, Hopewell JC, Channon KM, Achenbach S, and Antoniades C. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 392: 929–939, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Padilla J, Jenkins NT, Vieira-Potter VJ, and Laughlin MH. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol 304: R543–R552, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pan XX, Cao JM, Cai F, Ruan CC, Wu F, and Gao PJ. Loss of miR-146b-3p inhibits perivascular adipocyte browning with cold exposure during aging. Cardiovasc Drugs Ther 32: 511–518, 2018 [DOI] [PubMed] [Google Scholar]
- 81. Pan XX, Ruan CC, Liu XY, Kong LR, Ma Y, Wu QH, Li HQ, Sun YJ, Chen AQ, Zhao Q, Wu F, Wang XJ, Wang JG, Zhu DL, and Gao PJ. Perivascular adipose tissue-derived stromal cells contribute to vascular remodeling during aging. Aging Cell 18: e12969, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, and Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol 176: 7745–7752, 2006 [DOI] [PubMed] [Google Scholar]
- 83. Qi XY, Qu SL, Xiong WH, Rom O, Chang L, and Jiang ZS. Perivascular adipose tissue (PVAT) in atherosclerosis: a double-edged sword. Cardiovasc Diabetol 17: 134, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Quesada I, Cejas J, Garcia R, Cannizzo B, Redondo A, and Castro C. Vascular dysfunction elicited by a cross talk between periaortic adipose tissue and the vascular wall is reversed by pioglitazone. Cardiovasc Ther 36: e12322, 2018 [DOI] [PubMed] [Google Scholar]
- 85. Ren L, Wang L, You T, Liu Y, Wu F, Zhu L, and Tang C. Perivascular adipose tissue modulates carotid plaque formation induced by disturbed flow in mice. J Vasc Surg 70: 927–936.e924, 2019 [DOI] [PubMed] [Google Scholar]
- 86. Restini CBA, Ismail A, Kumar RK, Burnett R, Garver H, Fink GD, and Watts SW. Renal perivascular adipose tissue: form and function. Vascul Pharmacol 106: 37–45, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rittig K, Dolderer JH, Balletshofer B, Machann J, Schick F, Meile T, Kuper M, Stock UA, Staiger H, Machicao F, Schaller HE, Konigsrainer A, Haring HU, and Siegel-Axel DI. The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia 55: 1514–1525, 2012 [DOI] [PubMed] [Google Scholar]
- 88. Rogero MM and Calder PC. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients 10: 432, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ruan CC, Ge Q, Li Y, Li XD, Chen DR, Ji KD, Wu YJ, Sheng LJ, Yan C, Zhu DL, and Gao PJ. Complement-mediated macrophage polarization in perivascular adipose tissue contributes to vascular injury in deoxycorticosterone acetate-salt mice. Arterioscler Thromb Vasc Biol 35: 598–606, 2015 [DOI] [PubMed] [Google Scholar]
- 90. Ruan CC, Ma Y, Ge Q, Li Y, Zhu LM, Zhang Y, Kong LR, Wu QH, Li F, Cheng L, Zhao AZ, Zhu DL, and Gao PJ. Complement-mediated inhibition of adiponectin regulates perivascular inflammation and vascular injury in hypertension. FASEB J 31: 1120–1129, 2017 [DOI] [PubMed] [Google Scholar]
- 91. Sakaue T, Suzuki J, Hamaguchi M, Suehiro C, Tanino A, Nagao T, Uetani T, Aono J, Nakaoka H, Kurata M, Sakaue T, Okura T, Yasugi T, Izutani H, Higaki J, and Ikeda S. Perivascular adipose tissue angiotensin II type 1 receptor promotes vascular inflammation and aneurysm formation. Hypertension 70: 780–789, 2017 [DOI] [PubMed] [Google Scholar]
- 92. Salim HM, Fukuda D, Higashikuni Y, Tanaka K, Hirata Y, Yagi S, Soeki T, Shimabukuro M, and Sata M. Teneligliptin, a dipeptidyl peptidase-4 inhibitor, attenuated pro-inflammatory phenotype of perivascular adipose tissue and inhibited atherogenesis in normoglycemic apolipoprotein-E-deficient mice. Vascul Pharmacol 96–98: 19–25, 2017 [DOI] [PubMed] [Google Scholar]
- 93. Sato T, Kadowaki A, Suzuki T, Ito H, Watanabe H, Imai Y, and Kuba K. Loss of Apelin augments angiotensin ii-induced cardiac dysfunction and pathological remodeling. Int J Mol Sci 20: 239, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Saxton SN, Ryding KE, Aldous RG, Withers SB, Ohanian J, and Heagerty AM. Role of sympathetic nerves and adipocyte catecholamine uptake in the vasorelaxant function of perivascular adipose tissue. Arterioscler Thromb Vasc Biol 38: 880–891, 2018 [DOI] [PubMed] [Google Scholar]
- 95. Saxton SN, Withers SB, and Heagerty AM. Emerging roles of sympathetic nerves and inflammation in perivascular adipose tissue. Cardiovasc Drugs Ther 33: 245–259, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schutten MT, Houben AJ, de Leeuw PW, and Stehouwer CD. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology (Bethesda) 32: 197–209, 2017 [DOI] [PubMed] [Google Scholar]
- 97. Singh RB, Mengi SA, Xu YJ, Arneja AS, and Dhalla NS. Pathogenesis of atherosclerosis: a multifactorial process. Exp Clin Cardiol 7: 40–53, 2002 [PMC free article] [PubMed] [Google Scholar]
- 98. Skiba DS, Nosalski R, Mikolajczyk TP, Siedlinski M, Rios FJ, Montezano AC, Jawien J, Olszanecki R, Korbut R, Czesnikiewicz-Guzik M, Touyz RM, and Guzik TJ. Anti-atherosclerotic effect of the angiotensin 1–7 mimetic AVE0991 is mediated by inhibition of perivascular and plaque inflammation in early atherosclerosis. Br J Pharmacol 174: 4055–4069, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Small HY, McNeilly S, Mary S, Sheikh AM, and Delles C. Resistin mediates sex-dependent effects of perivascular adipose tissue on vascular function in the Shrsp. Sci Rep 9: 6897, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Srikakulapu P and McNamara CA. B cells and atherosclerosis. Am J Physiol Heart Circ Physiol 312: H1060–H1067, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Srikakulapu P, Upadhye A, Rosenfeld SM, Marshall MA, McSkimming C, Hickman AW, Mauldin IS, Ailawadi G, Lopes MBS, Taylor AM, and McNamara CA. Perivascular adipose tissue harbors atheroprotective IgM-producing B cells. Front Physiol 8: 719, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sun Y, Li J, Xiao N, Wang M, Kou J, Qi L, Huang F, Liu B, and Liu K. Pharmacological activation of AMPK ameliorates perivascular adipose/endothelial dysfunction in a manner interdependent on AMPK and SIRT1. Pharmacol Res 89: 19–28, 2014 [DOI] [PubMed] [Google Scholar]
- 103. Tang Y, He Y, Li C, Mu W, Zou Y, Liu C, Qian S, Zhang F, Pan J, Wang Y, Huang H, Pan D, Yang P, Mei J, Zeng R, and Tang QQ. RPS3A positively regulates the mitochondrial function of human periaortic adipose tissue and is associated with coronary artery diseases. Cell Discov 4: 52, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tousoulis D, Charakida M, and Stefanadis C. Endothelial function and inflammation in coronary artery disease. Heart 92: 441–444, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Villacorta L and Chang L. The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Horm Mol Biol Clin Investig 21: 137–147, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, and Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation 122: 2529–2537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Virdis A, Duranti E, Rossi C, Dell'Agnello U, Santini E, Anselmino M, Chiarugi M, Taddei S, and Solini A. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue. Eur Heart J 36: 784–794, 2015 [DOI] [PubMed] [Google Scholar]
- 108. Watts SW, Dorrance AM, Penfold ME, Rourke JL, Sinal CJ, Seitz B, Sullivan TJ, Charvat TT, Thompson JM, Burnett R, and Fink GD. Chemerin connects fat to arterial contraction. Arterioscler Thromb Vasc Biol 33: 1320–1328, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wei Z, Spizzo I, Diep H, Drummond GR, Widdop RE, and Vinh A. Differential phenotypes of tissue-infiltrating T cells during angiotensin II-induced hypertension in mice. PLoS One 9: e114895, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. White UA and Tchoukalova YD. Sex dimorphism and depot differences in adipose tissue function. Biochim Biophys Acta 1842: 377–392, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, and Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wuttge DM, Zhou X, Sheikine Y, Wagsater D, Stemme V, Hedin U, Stemme S, Hansson GK, and Sirsjo A. CXCL16/SR-PSOX is an interferon-gamma-regulated chemokine and scavenger receptor expressed in atherosclerotic lesions. Arterioscler Thromb Vasc Biol 24: 750–755, 2004 [DOI] [PubMed] [Google Scholar]
- 113. Xia N, Forstermann U, and Li H. Effects of resveratrol on eNOS in the endothelium and the perivascular adipose tissue. Ann N Y Acad Sci 1403: 132–141, 2017 [DOI] [PubMed] [Google Scholar]
- 114. Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, Mikhed Y, Munzel T, Daiber A, Forstermann U, and Li H. Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet-induced obese mice. Arterioscler Thromb Vasc Biol 36: 78–85, 2016 [DOI] [PubMed] [Google Scholar]
- 115. Xia N and Li H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br J Pharmacol 174: 3425–3442, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xiong W, Zhao X, Garcia-Barrio MT, Zhang J, Lin J, Chen YE, Jiang Z, and Chang L. MitoNEET in perivascular adipose tissue blunts atherosclerosis under mild cold condition in mice. Front Physiol 8: 1032, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Xiong W, Zhao X, Villacorta L, Rom O, Garcia-Barrio MT, Guo Y, Fan Y, Zhu T, Zhang J, Zeng R, Chen YE, Jiang Z, and Chang L. Brown adipocyte-specific PPARgamma (peroxisome proliferator-activated receptor gamma) deletion impairs perivascular adipose tissue development and enhances atherosclerosis in mice. Arterioscler Thromb Vasc Biol 38: 1738–1747, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Xu X, Chen Y, Song J, Hou F, Ma X, Liu B, and Huang F. Mangiferin suppresses endoplasmic reticulum stress in perivascular adipose tissue and prevents insulin resistance in the endothelium. Eur J Nutr 57: 1563–1575, 2018 [DOI] [PubMed] [Google Scholar]
- 119. Yang F, Bai Y, and Jiang Y. Effects of Apelin on RAW264.7 cells under both normal and hypoxic conditions. Peptides 69: 133–143, 2015 [DOI] [PubMed] [Google Scholar]
- 120. Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, Daugherty A, and Cassis LA. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension 60: 1524–1530, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yilmaz MI, Sonmez A, Caglar K, Celik T, Yenicesu M, Eyileten T, Acikel C, Oguz Y, Yavuz I, and Vural A. Effect of antihypertensive agents on plasma adiponectin levels in hypertensive patients with metabolic syndrome. Nephrology (Carlton) 12: 147–153, 2007 [DOI] [PubMed] [Google Scholar]
- 122. Ying R, Li SW, Chen JY, Zhang HF, Yang Y, Gu ZJ, Chen YX, and Wang JF. Endoplasmic reticulum stress in perivascular adipose tissue promotes destabilization of atherosclerotic plaque by regulating GM-CSF paracrine. J Transl Med 16: 105, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yu S, Zhang Y, Li MZ, Xu H, Wang Q, Song J, Lin P, Zhang L, Liu Q, Huang QX, Wang K, and Hou WK. Chemerin and apelin are positively correlated with inflammation in obese type 2 diabetic patients. Chin Med J (Engl) 125: 3440–3444, 2012 [PubMed] [Google Scholar]
- 124. Yuan F, Ma J, Xiang X, Lan H, Xu Y, Zhao J, Li Y, and Zhang W. Improvement of adipose macrophage polarization in high fat diet-induced obese GHSR knockout mice. Biomed Res Int 2018: 4924325, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhang J, Chang L, Garcia-Barrio MT, and Chen YE. Revisiting vascular remodeling in the single-cell transcriptome era. Arterioscler Thromb Vasc Biol 39: 1896–1898, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zhu X, Zhang HW, Chen HN, Deng XJ, Tu YX, Jackson AO, Qing JN, Wang AP, Patel V, and Yin K. Perivascular adipose tissue dysfunction aggravates adventitial remodeling in obese mini pigs via NLRP3 inflammasome/IL-1 signaling pathway. Acta Pharmacol Sin 40: 46–54, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]