Abstract
Significance: The kidney plays an important role in the long-term control of blood pressure. Oxidative stress is one of the fundamental mechanisms responsible for the development of hypertension. Dopamine, via five subtypes of receptors, plays an important role in the control of blood pressure by various mechanisms, including the inhibition of oxidative stress.
Recent Advances: Dopamine receptors exert their regulatory function to decrease the oxidative stress in the kidney and ultimately maintain normal sodium balance and blood pressure homeostasis. An aberration of this regulation may be involved in the pathogenesis of hypertension.
Critical Issues: Our present article reviews the important role of oxidative stress and intrarenal dopaminergic system in the regulation of blood pressure, summarizes the current knowledge on renal dopamine receptor-mediated antioxidation, including decreasing reactive oxygen species production, inhibiting pro-oxidant enzyme nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase, and stimulating antioxidative enzymes, and also discusses its underlying mechanisms, including the increased activity of G protein-coupled receptor kinase 4 (GRK4) and abnormal trafficking of renal dopamine receptors in hypertensive status.
Future Directions: Identifying the mechanisms of renal dopamine receptors in the regulation of oxidative stress and their contribution to the pathogenesis of hypertension remains an important research focus. Increased understanding of the role of reciprocal regulation between renal dopamine receptors and oxidative stress in the regulation of blood pressure may give us novel insights into the pathogenesis of hypertension and provide a new treatment strategy for hypertension.
Keywords: dopamine receptors, hypertension, kidney, oxidative stress
Introduction
It is well accepted that hypertension is a complex trait determined by genetic, epigenetic, behavioral, and environmental factors and their intricate interactions. The kidney plays an important role in the long-term control of blood pressure and is the major organ involved in the regulation of sodium homeostasis (60). An inappropriate sodium retention in hypertension results from enhanced renal sodium transport per se or a failure to respond appropriately to signals that decrease renal sodium transport in the face of increased sodium intake. Sodium excretion is regulated by numerous humoral or hormone factors. Among these, dopamine has been shown to be an important regulator of sodium balance and blood pressure through an independent renal dopaminergic system (187a, 195). Oxidative stress has gained attention as one of the fundamental mechanisms involved in the development of hypertension (42, 101). Our studies and those of others have shown that the function of renal dopamine receptors is impaired in hypertension, in part, because of oxidative stress (96, 116, 165). In this article, we review the role of oxidative stress and intrarenal dopaminergic system in the regulation of blood pressure, the abnormality of this interaction in hypertension, and the current knowledge of the mechanisms involved in this interaction. These may advance our understanding of the role of reciprocal regulation between renal dopamine receptors and oxidative stress in the control of blood pressure and highlight potential and novel strategies for the prevention and treatment of hypertension.
Reactive Oxygen Species and Hypertension
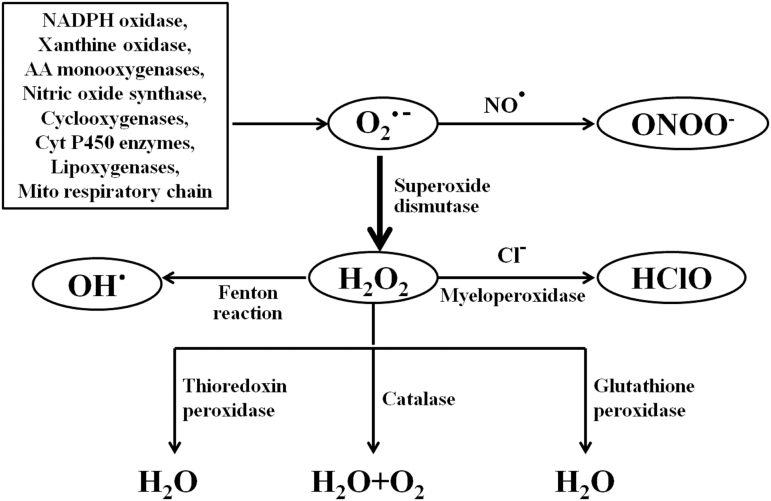
The term oxidative stress can be defined as a serious disturbance of the balance between the production of reactive oxygen species (ROS) and the antioxidant defense systems, and has been shown in a wide range of studies to contribute to the pathogenesis of many diseases, such as hypertension (34). ROS are a class of oxygen atoms that contain unpaired electrons, including superoxide anion (O2−), hydrogen peroxide (H2O2), hydroxyl anion (−OH) formed from H2O2, and singlet oxygen (1O2), hypochlorous acid (HClO), among others, together with secondary products of their interaction with nitric oxide (NO) and lipids. ROS are naturally generated by specific oxidases, such as the reduced form of nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase, xanthine/xanthine oxidase, various arachidonic acid monooxygenases, and enzymes of the mitochondrial respiratory chain (50, 85) (Fig. 1). Apart from the above, numerous other enzymes such as NO synthase, cyclooxygenases, cytochrome P450 enzymes, and lipoxygenases, as well as some cell organelles such as the peroxisome and endoplasmic reticulum, also contribute to intracellular ROS production (37, 66). Moreover, there is cross talk among these pro-oxidant systems, which leads to increased levels of ROS (38). Mitochondrial respiration is a major site of ROS generation, whereas membrane-associated NADPH oxidase is the most important source of O2− outside the mitochondria in nonphagocytic cells, including vascular and renal mesangial and tubular cells (39, 127). However, the levels of ROS depend not only on their production but also on their degradation by antioxidant enzymes. While superoxide dismutase (SOD) is one of the major defense systems to remove ROS, catalase (CAT), peroxiredoxins (Prxs), glutathione peroxidase (GSH-Px), glutathione transferase (GST), glutathione reductase (GR), and thioredoxin (Trx) reductase are all important in the metabolism of H2O2 (72, 112). In addition, some nonenzymatic compounds such as ascorbate, β-carotene, glutathione (GSH), nicotinamide, and tocopherol/vitamin E also counteract ROS (129). Once oxidation exceeds antioxidation, the balance between the oxidation system and antioxidant system is broken, oxidative stress occurs, causing deleterious effects, including tissue damage.
FIG. 1.
Schematic diagram of ROS production and metabolism. Multiple sources can produce superoxide (O2•−), which can rapidly react with NO to form peroxynitrite (ONOO•) or be converted to H2O2 via SOD. H2O2 can be converted to water (H2O) and O2 via catalase, glutathione peroxidase, or thioredoxin peroxidase. H2O2 can also produce hydroxyl radical (OH•) through the Fenton reaction, or generate hypochlorous acid (HClO) via the enzyme myeloperoxidase. AA, arachidonic acid; Cyt, cytochrome; H2O2, hydrogen peroxide; Mito, mitochondrial; NADPH, nicotinamide-adenine dinucleotide phosphate; NO, nitric oxide; ROS, reactive oxygen species; SOD, superoxide dismutase.
Role of oxidative stress in hypertension
In 1990, Schneider et al. first reported that hypertensive patients had lower glutathione peroxide activity and higher SOD and serum GR activity compared with normotensive healthy subjects (126). In 1991, oxidative stress was implicated to play an important role in the pathogenesis of hypertension. Nakazono et al. reported that the intravenous injection of a fusion protein consisting of human Cu/Zn-type SOD and a C-terminal basic peptide, targeted to endothelial cells, decreased the blood pressure of spontaneously hypertensive rats (SHRs), but not normotensive rats (104). Subsequently, a number of studies have shown the vital role of ROS in the development of hypertension (1, 148). Excessive ROS generation and/or impaired antioxidant mechanisms are commonly observed in various animal models of genetic and experimental hypertension (26, 49, 163). These were confirmed in specific ROS generating gene-knockout mice, or animal models of ROS-mediated hypertension, in which ROS production is suppressed. Mice models with genetic deficiency of superoxide-generating enzymes, such as NADPH oxidase NOX1 or NOX2, have decreased blood pressure (51, 128). Inhibition of ROS generation with apocynin, a NADPH oxidase inhibitor, or allopurinol, a xanthine oxidase inhibitor, and radical scavenging with antioxidants such as resveratrol or SOD mimetics decrease blood pressure and prevent the development of hypertension in rodent models of hypertension (31, 57, 118). Moreover, the association between ROS and increased blood pressure is also demonstrated in hypertensive human subjects. Clinical studies have shown increased ROS production in patients with various types of hypertension, including essential hypertension, renovascular hypertension, malignant hypertension, and preeclampsia (64, 142, 144). All of these reports contribute to the accumulating evidence that increased oxidative stress is involved in the pathogenesis of hypertension.
Studies have shown that oxidative stress induces hypertension through numerous mechanisms in some systems associated with the regulation of blood pressure. In the cardiovascular system, oxidative stress, induced by some factors such as angiotensin II (Ang II), endothelin-1, and urotensin I, promotes vascular contraction, endothelial dysfunction, and vascular remodeling, leading to vascular damage and subsequent hypertension via different mechanisms, including altering NO bioavailability or signaling, increasing intracellular calcium concentration (5, 119). In the kidney, oxidative stress leads to abnormal renal physiological functions associated with increased blood pressure via some mechanisms, including increasing renal vasoconstriction, causing dysfunction of glomerular cells and proteinuria, disrupting water and sodium homeostasis (6, 56). In the central nervous system (CNS), oxidative stress causes hypertension via some neural mechanisms, including activating the sympathetic nervous system, underpinning the processing of the cardiovascular reflexes in the medulla oblongata (65, 80). In addition, oxidative stress also induces hypertension via other mechanisms such as increasing inflammation and activating the renin/angiotensin/aldosterone system (6, 59).
The kidney is essential for the long-term regulation of arterial blood pressure. NADPH oxidase is the predominant source of ROS outside the mictochondria in the kidney (127). It is an enzyme complex comprising five subunits: membrane component protein cytochrome b588 (22phox and gp91phox); cytosolic components p40phox, p47phox, and p67phox; and a low-molecular-weight G protein, Rac1 or Rac2. The NADPH oxidase family has seven members, Nox1, Nox2 (aka gp91phox), Nox3, Nox4, Nox5, Duox1, and Duox. The NADPH oxidase subunits are expressed in almost all of the nephron segments, including the macula densa, mesangial cells, podocytes, and renal arterioles, including endothelial cells (92). Antioxidant enzymes, for example, SOD, the major scavenging system for removing ROS, are also well expressed in the kidney where they restrict the levels of ROS and limit hypertensive tissue injury (120, 164). Several studies have shown that excess renal production of ROS from the imbalance of pro-oxidant and antioxidant systems can initiate the development of hypertension. Renal ROS are increased in various hypertensive animal models (71, 92c, 99). Studies using several methods, including germ line gene deletion, specific RNA silencing, and pharmacological inhibitors, support the role of increased renal ROS production in the development of hypertension. For example, extracellular SOD (eSOD) gene knockout mice have high blood pressure, as well as increased renal cortical p22phox expression, and NADPH oxidase activity (164). Specific small interfering RNA (siRNA) silencing of p22phox in the kidney cortex is associated with reduced systemic oxidative stress and attenuation in Ang II-induced high blood pressure (103). Treatment with the NADPH oxidase inhibitor apocynin or the SOD mimetic tempol decreases systolic blood pressure, accompanied with the inhibition of renal NADPH oxidase activity in SHRs (27). Increased renal superoxide anion production is associated with the inactivation of NO, which increases afferent arteriolar tone and sodium reabsorption, and impairs tubuloglomerular feedback response, all of which are involved in the regulation of blood pressure (166). However, it should be noted that the overall effect of ROS on overall renal sodium transport is still difficult to assess because ROS can decrease or increase sodium transport, which may due to the nephron-specific effects of ROS on tubular Na+ transport and fluid reabsorption (111, 132, 139).
Intrarenal Dopaminergic System
Dopamine, an endogenous catecholamine, is well known as a neurotransmitter in the CNS (102). There is increasing evidence that dopamine is also an important regulator of renal function and ultimately blood pressure (14, 55). Dopamine is synthesized not only in noradrenergic and dopaminergic nerves, but also in non-neural tissues such as the kidney (141, 194, 195). Both the renal synthesis and metabolism of dopamine differ from that in neurons. Renal proximal tubules (RPTs) synthesize dopamine from the circulating or filtered dopamine precursor l-dihydroxy-phenylalanine (l-DOPA), which is then converted to dopamine by aromatic amino acid decarboxylase (AADC) (75, 141, 194, 195). Unlike neurons, RPT cells do not express dopamine β-hydroxylase, and therefore, synthesized dopamine is not metabolized to norepinephrine and epinephrine (194). Moreover, dopamine produced in the RPT is not stored, but transported across the basolateral and apical membranes and into the peritubular space and tubular lumen, respectively, where it acts on its receptors locally and in more distal nephron segments.
Dopamine, via its receptors, plays an important role in the regulation of sodium balance and blood pressure. It should be noted that the affinity of dopamine to its receptors is in the nanomolar range; higher concentrations occupy other G protein-coupled receptors (GPCRs; e.g., α-adrenergic, β-adrenergic, and serotonin receptors). Although normal circulating concentrations of dopamine (in the picomolar range) are not sufficiently high to activate dopamine receptors, high nanomolar concentrations can be attained in dopamine-producing tissues (e.g., the RPT and jejunum) (63, 194). There is evidence that intrarenal dopamine synthesis/release is modulated by alterations in dietary salt intake and intracellular sodium. Conversely, intrarenal dopamine production regulates sodium excretion. Under conditions of moderate sodium excess, locally generated dopamine acts on renal tubular and jejunal cells to decrease sodium transport. When sodium intake is enhanced, renal dopamine, acting in an autocrine/paracrine manner, is responsible for over 50% of the increase in sodium excretion (63, 187a, 194, 195).
Dysfunction of Renal Dopamine Receptors and Hypertension
The dopaminergic system exerts an autocrine/paracrine regulatory role on renal sodium transport in the kidney via its five receptor subtypes. Dopamine receptors, belonging to the α-group of the rhodopsin family of seven transmembrane GPCRs, are classified into two families: D1-like receptors (dopamine D1 receptor [D1R] and dopamine D5 receptor [D5R]) couple to stimulatory G protein GαS and stimulate adenylyl cyclase (AC) activity, whereas D2-like receptors (dopamine D2 receptor [D2R], dopamine D3 receptor [D3R], and dopamine D4 receptor [D4R]) couple to inhibitory G protein Gαi/Gαo and inhibit AC activity (63, 187a, 194, 195).
The expression of the dopamine receptor subtypes in specific nephron segments is species-dependent. For example, in humans, D1R is expressed in all segments of the proximal tubule, thick ascending limb, distal convoluted tubule, and all segments of the collecting duct but not in the mesangial cell, podocyte, juxtaglomerular cell, or macula densa. By contrast, rats and mice express the D1R in juxtaglomerular cell and macula densa; rats express D1R in mesangial cell while mice express D1R in the podocyte (63, 187a, 194, 195). No dopamine receptor is expressed in thin descending and thin ascending limb, regardless of species. Activation of renal dopamine receptors decreases renal electrolyte and water transport and is affected by many conditions, including oxidative stress (63, 187a, 194, 195). Importantly, disruption of any of the dopamine receptor genes in mice results in hypertension, the pathogenesis of which is specific for each receptor subtype, indicating the contribution of dopamine receptor on the regulation of blood pressure.
An increase in endogenous renal dopamine or the intrarenal infusion of specific dopamine receptor agonists increases sodium and water excretion; however, these effects are impaired in hypertensive states (167b, 187). Although all of the mechanisms causing the impairment are not known, impaired dopamine production or dopamine receptor dysfunction may be involved (28, 33, 48, 58, 123, 134, 136). First, an abnormal renal synthesis of dopamine may be involved in the development of hypertension (28, 33, 123, 134). Black salt-sensitive hypertensive subjects have decreased renal dopamine production (134). Caucasians with mild, untreated essential hypertension have decreased renal dopamine production, as related to the amount of sodium ingested (33). However, there are reports of increased urinary dopamine excretion in young hypertensive patients and patients with borderline hypertension, due to the elevated conversion of DOPA to dopamine in the kidney (28, 123). The reasons leading to the different results in various reports are not known. These studies indicate that age, race, and duration of hypertension affect renal dopamine production. It should be noted that the exact causal association between the hypertensive patients and abnormal dopamine metabolism is still unclear because all the above reports only observed the simple phenomenon that hypertensive patients have abnormal dopamine metabolism. Renal dopamine production is also species-dependent. For example, daily dopamine excretions are similar among Dahl salt-sensitive rats, Dahl salt-resistant rats, and Sprague-Dawley (SD) rats, but salt loading increases urinary dopamine to a greater extent in Dahl salt-sensitive and salt-resistant rats than SD rats (58). The decreased urinary dopamine in SD rats is related to a decreased conversion of DOPA to dopamine; however, urinary dopamine is similar in Dahl salt-sensitive and salt-resistant rats (58). Urinary dopamine excretion is also not different between Wistar Kyoto (WKY) rats and SHR in the basal state, and is greater in SHR than WKY rats during high-salt intake (136). Nevertheless, the natriuretic effect of D1-like receptor agonist and the antinatriuretic effect of D1-like receptor antagonist are impaired in the SHR (48, 68). Therefore, the renal dopamine dysfunction in hypertension starts at the receptor level. Oxidative stress may participate in the impairment of dopamine receptors (154, 175, 177). This review describes the association between dopamine receptors and oxidative stress in hypertension.
Renal D1 receptor and hypertension
Renal-selective activation of D1-like receptors increases sodium excretion (52). Renal-selective silencing of D1R reduced urinary sodium excretion and urine flow in SD rats on low- or high-sodium diet, but blood pressure was not affected (162). However, the renal-selective impairment of D1R localization in the plasma membrane with β-methyl cyclodextrin impaired the ability of the renal interstitial infusion of D1-like receptor agonist, fenoldopam, and increased blood pressure in SD rats fed a high-sodium diet (52). Moreover, the germ line deletion of the Drd1 gene causes hypertension (4). The natriuretic effect of D1R may be due to its ability to inhibit directly the sodium hydrogen exchanger type 3 (NHE3), sodium phosphate cotransporter type 2 (NaPi2), chloride bicarbonate (Cl−/HCO3−) exchanger, and the epithelial sodium channel (ENaC) at the luminal membrane, as well as Na+-K+-ATPase (NKA) and the sodium bicarbonate cotransporter at the basolateral membrane (15, 23, 187a, 195). The D1R can also indirectly exert its natriuretic effect by interacting with other hormonal factors or receptors, including Ang II, angiotensin 1–7, gastrin, Ang II types 1 and 2 receptor, and cholecystokinin B receptor (CCKBR), and other dopamine receptor subtypes (14, 30, 44, 53, 106, 110).
The D1R function, as with other receptors, is affected by its expression in the cell membranes and compartments. For example, the D1R is mainly located at the microvillous brush border and apical membranes in WKY rats, whereas the D1R is mainly in the cytosol with minimal expression at the brush border and apical membrane in the SHRs. Indeed, more D1Rs are located in endocytic vesicles in the SHR than WKY rat (183). The internalization of D1R in renal tubules of SHR and Dahl salt-sensitive rats, may explain, in part, the diminished ability of D1-like receptors to inhibit renal sodium transporter/channel activity in hypertension (77, 107). Impaired D1R-mediated inhibition on sodium transports is also observed in humans with essential hypertension (108). In addition to the altered renal cellular localization of D1R in hypertension, its expression is also decreased in obese Zucker rats, which are hypertensive (156).
Renal D5 receptor and hypertension
The D5R, by itself, or together with D1R, also mediates the diuretic and natriuretic effects of D1-like receptor; D1R and D5R interact in the inhibition of NHE3 and NKA activity, the D1R primarily by cAMP, whereas the D1R/D5R heteromer modulates the D1R effect through a phospholipase C pathway in RPT cells (53, 187a). The importance of the renal D5R in the regulation of blood pressure has been proven in Drd5 knockout mice and cross-transplantation studies (13, 87). Disruption of the Drd5 gene in mice results in hypertension that is aggravated by a high-salt diet (13, 87). Blood pressure in Drd5−/− mice transplanted with kidneys from wild-type (Drd5+/+) mice is lower than in Drd5−/− mice transplanted with Drd5−/− kidneys, while blood pressure is higher in Drd5+/+ mice transplanted with Drd5−/− kidneys than Drd5+/+ mice transplanted with Drd5+/+ kidneys (13). The natriuresis in Drd5−/− mice is decreased, which is due to the upregulation of renal sodium transporters (158). These may be partly related to the D5R regulation of the AC isoform in lipid rafts and nonlipid rafts (186). The D5R also interacts with Gα12 and Gα13, which is related to the inhibition of NHE3 activity (199). Similar to the D1R, the natriuretic effect of D5R may also be caused via interactions with other factors or receptors, including D1R, angiotensin II type 1 receptor (AT1R), and renalase (53, 54, 153).
Basal D5R expression is decreased in RPT cells and renal brush border membranes of SHR (193). Renal D5R expression is also decreased in obese Zucker rats (156). Moreover, the dysregulation of D5R internalization and trafficking is involved in the higher blood pressure and blunted natriuretic response to agonist in salt-loaded mice (151). In the hypertensive state, the interactions between D5R and other factors or receptors are disturbed, for example, activation of D5R increased renalase protein expression and function in WKY rat RPT cells, but decreased them in SHR cells (69, 153). Such aberrant D5R mediated-regulation may be involved in the pathogenesis of hypertension.
Renal D2 receptor and hypertension
Activation of renal D2R has many effects, including the inhibition of noradrenaline release from the kidney, protection against ischemia/reperfusion injury to RPTs, and maintenance of normal blood pressure (105, 117, 122). The D2R modulates renal dopamine synthesis and protects the kidney by limiting the inflammatory reaction (84, 109). Disruption of the Drd2 gene increases blood pressure in mice that is associated with salt sensitivity, depending on the genetic background (91, 145). It should be noted that D2R can stimulate or inhibit NKA (25, 167). The stimulatory effect of D2-like receptors on NKA activity changes to inhibition in the presence of D1-like receptors (79). Indeed, the natriuretic effect of dopamine depends on the activation of both D1-like and D2-like dopamine receptors (43, 76).
There is no difference in the renal D2R expression and RPT cellular distribution between WKY rats and SHR. However, the D2R is also expressed in the glomeruli in WKY rats but not SHR (131). In obese Zucker rats, renal D2R protein expression is decreased, which induces salt sensitivity and elevates blood pressure, and can be normalized by candesartan, an AT1R blocker (156).
Renal D3 receptor and hypertension
Disruption of the Drd3 gene decreases urine flow rate and sodium excretion, and causes hypertension (12). However, a disruption of the Drd3 gene does not increase blood pressure regardless of salt intake in another strain of mice (135). This differential effect on blood pressure occurs despite the decreased ability of these two strains of Drd3−/− mice to increase sodium excretion after an acute or a chronic sodium chloride load. Whether the difference in blood pressure phenotype is related to the difference in the genetic background remains to be determined. Dahl salt-resistant rats on a high-sodium diet and chronically treated with a highly selective D3R antagonist BSF-135170 have increased blood pressure (95). Stimulation of the D3R induces natriuresis and diuresis in Wistar rats (172). This may be partly due to the D3R-mediated inhibition of renal NHE and NKA activity (7, 113, 114, 172). D3R activation also modulates renal hemodynamics and tubular function, and increases amino acid-induced glomerular hyperfiltration (93, 94). In addition, stimulation of D3R attenuates renal ischemia/reperfusion injury via increased linkage with Gα12 (161).
D3R expression is decreased in the renal cortex and RPT cell of the SHR, relative to the WKY rat (131, 188). This is reflected in in vivo studies, in which selective renal D3R activation produces diuresis and natriuresis in the WKY rats, but not in SHRs (82, 187).
Renal D4 receptor and hypertension
The D4R inhibits vasopressin-dependent transepithelial sodium transport in the rat cortical collecting duct. This is secondary to the ability of D4R to inhibit vasopressin-mediated stimulation of cAMP production (89, 138). This is exerted mainly at the basolateral membrane despite a greater expression of D4R at the luminal membrane (124). The D4R agonist PD168077 inhibits NKA activity in RPT cells from WKY rats but not from SHRs (140). D4R agonist stimulation with PD168077 also decreases the stimulatory effect of insulin on NKA activity in RPT cells from WKY rats but not SHRs (198). However, renal cortical and medullary NKA activities are not altered in Drd4 knockout mice (24). The renal cortical expression of the D4R is increased in SHRs, relative to WKY rats, although renal medullary D4R expression is not different between these two rat strains (131). We also found that the D4R decreases AT1R expression in RPT cells from WKY rats but not SHRs (29). Disruption of the Drd4 gene in mice produces hypertension, which is associated with increased renal AT1R expression (24). Thus, the D4R, as with the other dopamine receptor subtypes, participates in the regulation of blood pressure, due, in part, to negative interaction with the AT1R.
Disruption of the Drd4 gene does not affect body weight in mice (24). However, renal D4R is decreased in obese Zucker rats (156). The D4R decreases insulin receptor expression in RPT cells from WKY rats but not SHRs (198). Furthermore, the inhibition of the D4R on AT1R and insulin receptor is also aberrant in SHRs, which might be also involved in the pathogenesis of essential hypertension (29, 198).
Dysregulation of Dopamine Receptors in Oxidative Stress in Hypertension
D1-like receptors
As aforementioned, the D1-like receptor family includes at least two subtypes: D1R and D5R, also called D1A receptor and D1B receptor in rodents, which stimulate AC activity (63, 187a, 194, 195). D1-like, not D2-like, receptors are the major determinants of dopaminergic-mediated regulation of salt and water reabsorption induced by dopamine. Indeed, during conditions of moderate sodium balance, more than 50% of renal sodium excretion is regulated by D1-like receptors. It should be noted that there are no agonists that can distinguish D1R from D5R. LE-PM436 is a selective D5R antagonist but there is no specific D1R agonist (53). Therefore, in our present review, the term “D1-like receptor” is used when the study did not determine if the activation of D1-like receptors is specifically attributable to the D1R or D5R.
Renal D1R and oxidative stress
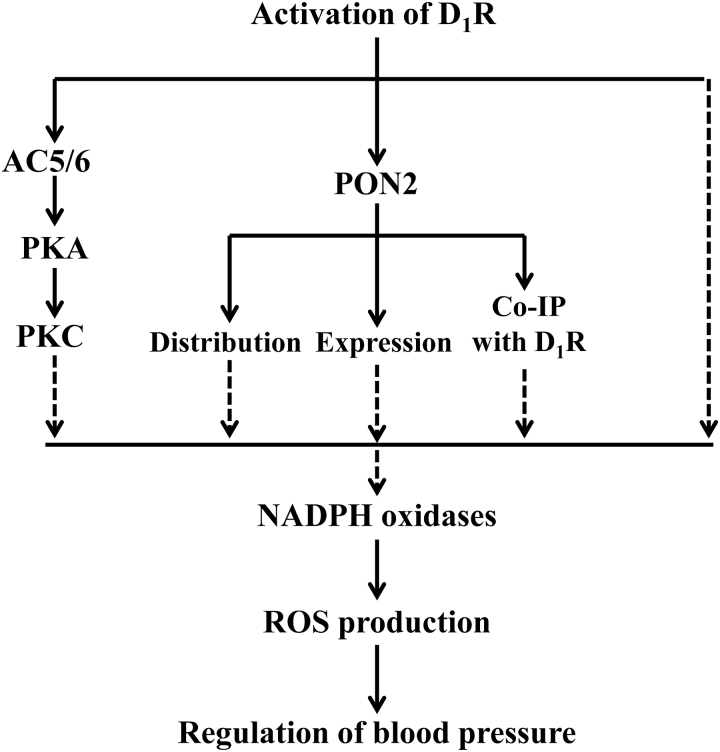
Most of studies reported that stimulation with D1-like receptor in low concentrations reduces oxidative stress in RPT cells, not only by decreasing the generation of ROS but also by increasing their degradation (62, 88, 185). The antioxidant effect of D1-like receptor is mainly exerted by inhibiting the pro-oxidant enzyme, NADPH oxidase, and stimulating some antioxidant enzymes such as paraoxonase 2 (PON2). Fenoldopam, a D1-like receptor agonist, disperses Nox subunits within lipid rafts and nonlipid rafts, and decreases the expression of p22phox and Rac1 in lipid rafts and Nox2 and Nox4 in nonlipid rafts. These actions reduce NADPH oxidase activity in human RPT cells (62). The basal NADPH oxidase activity in RPT cells is much higher in hypertensive than normotensive humans and rats but is inhibited by fenoldopam to a greater extent in the former than in the latter (88, 185). In HEK-293 cells heterologously expressing human D1R (HEK-hD1R), the D1R-mediated inhibition of NADPH oxidase activity is via a protein kinase A (PKA) and protein kinase C (PKC) cross talk (184). This cross talk is due, in part, to the regulation of AC isoforms in lipid and nonlipid rafts by D1R. D1R, but not D5R, increases AC activity in lipid rafts but decreases it in nonlipid rafts. Fenoldopam increases AC 5/6 isoform expression in lipid rafts but has little effect in nonlipid rafts (186).
D1R also decreases ROS production by increasing the expression/activity of antioxidant enzymes. Short- but not long-term stimulation with fenoldopam increases the expression of PON2 in both lipid rafts and nonlipid rafts and its physical interaction with D1R in HEK-hD1R (173). The ability of D1-like receptors to stimulate antioxidant enzymes, such as GSH-Px, SOD-1, and glutamylcysteine transferase, involves Nfr-2, a transcription factor, involved in antioxidant homeostasis (20). Figure 2 illustrates the effects of renal D1R on the negative regulation of oxidative stress.
FIG. 2.
Schematic summary of the regulation of renal D1R on oxidative stress. The broken lines indicate inhibitory effects, whereas the solid lines indicate stimulatory effects. AC, adenylyl cyclase; Co-IP, coimmunoprecipitation; D1R, dopamine D1 receptor; PKA, protein kinase A; PKC, protein kinase C; PON2, paraoxonase 2.
It should be noted that, although renal D1R inhibits oxidative stress, the function of D1R is, in turn, directly or indirectly impaired by oxidative stress. Treatment with H2O2 stimulates PKC leading to the activation of G protein-coupled receptor kinase 2 (GRK2), which causes serine phopshorylation of D1R and receptor G-protein uncoupling in RPT cells, resulting in impairment in D1-like receptor function (9). During oxidative stress, D1R function is defective, and there is mild hypertension. However, in the presence of oxidative stress, high salt intake causes marked elevation in blood pressure in SD rats, which results from a defective renal D1R function leading to the failure of dopamine to inhibit NKA and promote sodium excretion, indicating that oxidative stress is involved in renal D1R dysfunction and salt-sensitive hypertension (18). Other conditions such as inflammation and hyperglycemia can also impair the function of D1R via decreasing antioxidant enzyme and increasing oxidative stress (10, 100). However, treatment with the antioxidant tempol decreases renal D1R hyperphosphorylation and restores D1R-G-protein coupling and function by reducing oxidative stress in hyperglycemic rats and obese Zucker rats (10, 22). Another antioxidant sulforaphane mitigates oxidative stress, preserves D1R function, and preventes hypertension via activation of the NRF2-phase II antioxidant enzyme pathway (20). Exercise also increases antioxidant defense and D1R function via increasing the nuclear levels of NRF2 and decreasing oxidative stress in old rats (11).
Other mechanisms are involved in the regulation of oxidative stress on D1R. For example, oxidative stress causes nuclear translocation of nuclear factor-κB (NF-κB) in the RPT, which contributes to defective D1R-G-protein coupling and function via mechanisms involving PKC, membranous translocation of GRK2, and subsequent D1R phosphorylation (16, 19). Oxidative stress also directly suppresses the transcription of D1R gene and subsequent D1R signaling via activation of transcription factors AP1 and SP3 (21). Both of these effects can be reversed by treatment with tempol (16, 19, 21).
Renal D5R and oxidative stress
Compared with other dopamine receptors, the D5R has generated significant interest for its specific characteristics. The D5R has a higher affinity for dopamine than the D1R and exhibits constitutive activity (139a). The D5R also has a trafficking profile distinct from that of other dopamine receptors, which is related to the third intracellular loop of the D5R that is necessary for PKC-mediated D5R endocytosis. The endocytosis of D5R, in the basal state, is dependent on clathrin and dynamin, while β-arrestin 2 is needed in the case of agonist-induced endocytosis (115, 143).
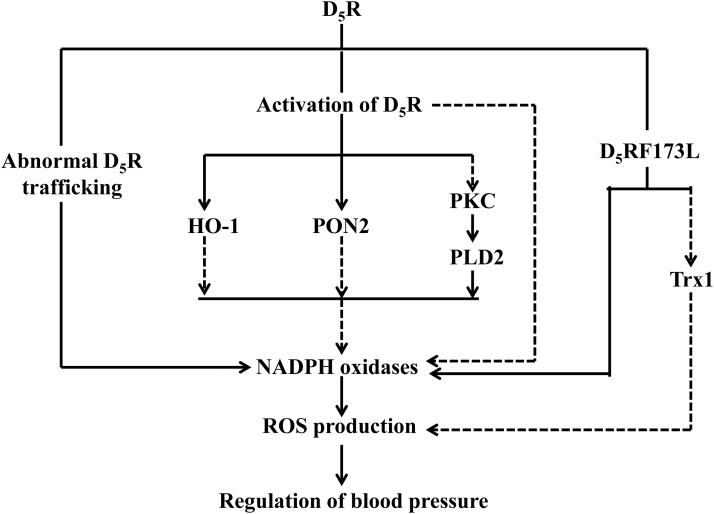
Stimulation of the D5R results in an antioxidant response that is mediated by the inhibition of NADPH oxidase activity in the short term, and inhibition of NADPH oxidase expression, in the long term. D5R-deficient mice are hypertensive, salt-sensitive, and in a state of systemic oxidative stress, which is related to increased renal NADPH oxidase expression and activity (177). Cross-transplantation studies showed that transplantation of a wild-type D5R kidney into a nephrectomized Drd5−/− mouse decreases renal Nox-2 expression. Conversely, transplantation of a Drd5−/− kidney into a nephrectomized D5R wild-type mouse increases renal Nox-2 expression (13). The increased blood pressure in D5R-deficient mice may be mediated by increased ROS production because apocynin normalizes increased blood pressure. Thus, the ability of D5R stimulation to decrease ROS production may explain, in part, the antihypertensive effect of D5R activation (177).
The inhibition of D5R on the NADPH oxidase activity may be partly due to D5R-mediated inhibition of phospholipase D (PLD). PLD is an ubiquitous enzyme stimulated by many cell surface receptors that hydrolyze phospholipids to form phosphatidic acid and the free polar head group of the phospholipid substrate. PLD is involved in the pathophysiology of hypertension by increasing the formation of ROS (147, 176). Total renal PLD activity is increased in Drd5−/− mice. PLD2, not PLD1, expression is greater in Drd5−/− mice. Moreover, activation of the D5R by fenoldopam in HEK-293 cells heterologously expressing human D5R (HEK-hD5R) cells decreases PLD2 expression and activity, indicating that the antioxidative effects of D5R are, in part, caused by the inhibition of PLD2 (176). Dysregulation of D5R trafficking is also associated with its impaired antioxidant function. Our studies demonstrated that the silencing of sorting nexin 1 (SNX1), involved in trafficking of internalized GPCRs, expression in RPT cells results in the failure of D5R to internalize and bind GTP, blunting of the agonist-induced increase in cAMP production and decrease in sodium transport (151). In vivo studies showed that depletion of renal SNX1 in C57BL/6J or BALB/cJ mice increases blood pressure. The natriuretic effect of the D1-like receptor agonist, fenoldopam, is also blunted in acutely saline-loaded BALB/cJ mice (151). Basal ROS, NOX activity, and blood pressure are higher in SNX1-deficient mice, which are normalized by apocynin, a drug that prevents NOX assembly (168).
Some protective factors against oxidative stress, such as PON2 and heme oxygenase-1 (HO-1), are also altered in the Drd5−/− mice. Short-term stimulation with fenoldopam increases PON2 protein in nonlipid rafts, but not lipid rafts, in HEK-hD5R; long-term fenoldopam stimulation increases PON2 protein in HEK-hD5R, not in HEK-hD1R cells. Moreover, renal PON2 protein is decreased in Drd5−/− mice. In human RPT cells, silencing the Drd5 decreases PON2 expression and increases ROS production. These indicate that D5R inhibits ROS production by regulating PON2 distribution in membrane microdomains and its renal expression (173). We also found that the activity of HO, an antioxidant enzyme, and the renal HO-1 protein expression are decreased in Drd5−/− mice, which can be restored by the administration of hemin, an HO-1 inducer (92b). Furthermore, cellular NADPH oxidase activity is decreased by 35% in HEK-hD5R that is abrogated with silencing of the heme oxygenase 1 (HMOX1) gene. HMOX1 siRNA also impairs the ability of fenoldopam to decrease NADPH oxidase activity in HEK-hD5R cells (92b). These indicate that the high blood pressure and increased ROS production in Drd5−/− mice may be caused by decreased HO-1 expression and activity.
D5R single-nucleotide polymorphisms (SNPs) are reported to be involved in oxidative stress in hypertension. Humans carry SNPs in the DRD5 gene, some of which confer diminished D5R function and abnormal coupling with AC (67). The human D5R173F>L (hD5R173F>L) mutation markedly impairs stimulation of cAMP production (92a, 154). On normal salt diet, the blood pressure, renal NADPH oxidase activity, and AT1R expression are higher in hD5R173F>L than hD5RWT transgenic mice. However, after 2 weeks on high-salt diet, the blood pressure and renal NADPH oxidase activity, but not AT1R expression, are increased in hD5R173F>L but not in hD5RWT transgenic mice. These can be reversed by the AT1R antagonist candesartan, indicating that the ability of the hD5R to negatively regulate the renal NADPH oxidase activity and AT1R function may have important implications in the pathogenesis of salt-sensitive blood pressure (92a). Our other studies also demonstrated the role of thioredoxin 1 (Trx1), an antioxidant enzyme, in the impaired sodium excretion of hD5R173F>L transgenic mice (154). hD5R173F>L transgenic mice have higher blood pressure, lower basal urine flow and sodium excretion, and impaired agonist (fenoldopam)-mediated natriuresis and diuresis, which are due, in part, to decreased renal Trx1 expression and increased ROS production. Hyperphosphorylation of hD5R173F>L, with its dissociation from Gαs and Gαq, is the key factor in the impaired renal D5R function and increased blood pressure of hD5R173F>L mice (154). Figure 3 illustrates the effects of renal D5R on the regulation of oxidative stress.
FIG. 3.
Schematic view of effects of renal D5R on the regulation of oxidative stress. The broken lines indicate inhibitory effects, whereas the solid lines indicate stimulatory effects. D5R, dopamine D5 receptor; HO-1, heme oxygenase-1; PLD2, phospholipase D2; Trx1, thioredoxin 1.
D2-like receptors
Renal D2R and oxidative stress
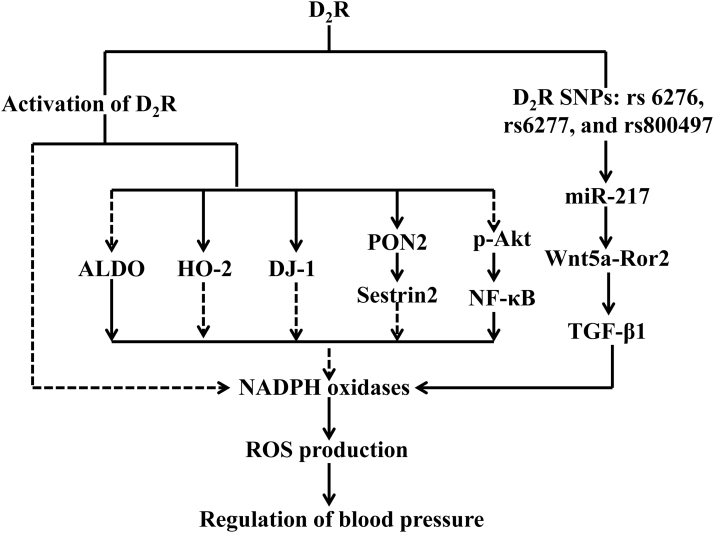
Decreasing oxidative stress is one of the mechanisms by which D2R regulates blood pressure. Disruption of Drd2, the D2R gene, increases ROS production and causes ROS-dependent hypertension (8). Drd2−/− mice have increased urinary excretion of 8-isoprostane, a product of lipid peroxidation, NADPH activity, and expression of Nox1, Nox2, and Nox4, but decreased expression of the antioxidant enzyme heme oxygenase-2 (HO-2) in the kidney (8, 36). Apocynin, an inhibitor of NADPH oxidase, normalizes the blood pressure in Drd2−/− mice (8). Thus, the D2R negatively regulates ROS production by inhibiting pro-oxidant but enabling antioxidant systems (8, 36). The D2R in the adrenal gland is also implicated in aldosterone regulation because urinary aldosterone is increased in Drd2−/− mice. However, spirolactone, a competitive antagonist of the mineralocorticoid receptor, only normalized the renal expression of Nox1 and Nox4, but had no effects on the excretion of 8-isoprostane and the expression of Nox2 or HO-2, indicating that the increased ROS production in Drd2−/− mice is only partially mediated by impaired aldosterone regulation (8). Similar to the results in Drd2 knockout mice, silencing the D2R in mouse RPT cells increases ROS production (36). The results from the germ line deletion of Drd2 or silencing of Drd2 in mouse RPT cells are supported by studies stimulating the D2R. Dopamine and D2R agonists have free radical scavenging and antioxidant activities. Stimulation of D2R in mouse or human RPT cells decreases hyperoxidized peroxiredoxins and reduces ROS production, accompanied by a decrease in Nox-4 expression and NADPH oxidase activity (36, 175).
The antioxidant effect of D2R involves its interaction with other proteins, including PON2, DJ-1 (also known as Park 7), and sestrin2 (36, 174, 175). PON2, which belongs to the paraoxonase gene family, is expressed in the kidney, acting to protect against cellular oxidative stress. Renal PON2 is involved in the inhibition of renal NADPH oxidase activity and ROS production and contributes to the maintenance of normal blood pressure. Our studies found that D2R colocalizes with PON2 in the brush border of mouse RPTs; both D2R and PON2 are found in nonlipid rafts and lipid rafts and physically interacts with each other (175). Renal PON2 protein is decreased in Drd2−/− mice. Treatment of human RPT cells with the D2R agonist quinpirole decreases ROS production that is related to D2R agonist stimulation of PON2 (175). These indicate that PON2 is positively regulated by D2R and may, in part, mediate the inhibitory effect of renal D2R on NADPH oxidase activity and ROS production.
The D2R also interacts with another antioxidant protein DJ-1. DJ-1 is a multifunctional oxidative stress response protein that defends cells against ROS and mitochondrial damage (36, 40). D2R and DJ-1 are expressed in the mouse kidney and colocalize and coimmunoprecipitate in mouse RPT cells. Mice with germ line deletion of Drd2 or with renal-selective Drd2 silencing are hypertensive and have increased ROS production and renal cortical expression of NOX4, but decreased expression of DJ-1. Conversely, treatment of mouse RPT cells with a D2R agonist increases DJ-1 expression and decreases NOX4 expression and NADPH oxidase activity. Renal-selective silencing of DJ-1 in mice increases blood pressure, renal Nox4 expression, and NADPH oxidase activity (36). Silencing DJ-1 or Drd2 in mouse RPT cells and kidneys also decreases Nrf2 expression and activity and increases ROS production (35). These studies suggest that the inhibitory effect of D2R on renal ROS production is at least, in part, mediated by the positive regulation of DJ-1.
Sestrin2, another antioxidant protein, is involved in the effect of renal D2R on blood pressure. We found that the antioxidant action of D2R is mediated, in part, by a positive regulation of sestrin2 expression. Drd2 germ line deletion in mice or Drd2 silencing in human RPT cells decreases, whereas D2R stimulation increases sestrin2 expression (174). Sestrin2 decreases ROS production and prevents cellular damage from oxidative stress by catalyzing the reduction of hyperoxidized peroxiredoxins. D2R-induced sestrin2 activation is dependent on PON2, and depletion of PON2 increases peroxiredoxin hyperoxidation and sestrin2 degradation (174). In vivo renal selective silencing of sestrin2 in mice increases renal oxidative stress and blood pressure (174). These results suggest that the D2R, via PON2/DJ-1/sestrin2 pathways, keeps a normal renal redox balance, which contributes to the maintenance of normal blood pressure.
D2R-mediated inhibition on oxidative stress may also be associated with decreasing inflammation. Oxidative stress is positively linked to inflammation and vice versa. D2R protects the kidney by limiting the inflammatory reaction; impaired D2R function results in renal inflammation and damage (196). The decrease or lack of D2R function enhances Akt phosphorylation by decreasing the expression of the protein phosphatase 2A (PP2A) regulatory subunit PPP2R2C. This activates NF-κB, a proinflammatory transcription factor that can be repressed or activated by oxidative stress and vice versa (167a, 197). However, renal-selective D2R rescue by the retrograde renal infusion of adeno-associated virus (AAV) vector carrying D2R reduces the expression of proinflammatory factors and kidney injury, preserves renal function, and normalizes the blood pressure (81). Moreover, RPT cells of subjects bearing D2R SNPs (rs6276, rs6277, and rs1800497), via the miR-217/Wnt5a/Ror2 pathway, decrease D2R expression and express a proinflammatory phenotype with increased TNF-α, cytokines, and chemokines and a profibrotic phenotype with increased transforming growth factor-beta 1 (TGF-β1) (61, 74), which are associated with increased ROS production. However, the mechanisms involved in the renal D2R-mediated inhibition of inflammation and oxidative stress need to be determined. Figure 4 illustrates the effects of renal D2R on the regulation of oxidative stress.
FIG. 4.
Schematic presentation of the role of renal D2R on the regulation of oxidative stress. The broken lines indicate inhibitory effects, whereas the solid lines indicate stimulatory effects. ALDO, aldosterone; D2R, dopamine D2 receptor; HO-2, heme oxygenase-2; NF-κB, nuclear factor-κB; TGF-β1, transforming growth factor-beta 1.
Renal D3R/D4R and oxidative stress
Everett and Senogles reported that the D3R, via a complex with RhoA, stimulates PLD activity in human HEK293 cells overexpressing the rat D3R (45). However, in rat nerve cells (oligodendrocytes), the D3R has antioxidant effects. D3R activation protects oligodendrocyte injury caused by glutamate oxidative stress (121); pramipexole, a D2R/D3R agonist but with a much higher affinity to the D3R (pKi = 8.4–8.7) than D2R (pKi = 5.1–7.4), protects against free radical-mediated lipid peroxidation in dopaminergic neurons (200). The hypertension in Drd3−/− mice is mild and not associated with increased ROS production, but may be due to the compensatory mechanism that occurs with deletion of the Drd3 gene. For example, disruption of the Drd3 gene in mice leads to the increased expression of D5R (155), which has antioxidant activity (13, 177). In addition, activation of D3R increases D1R, D5R, and endothelin B receptor expression and their mediated inhibitory effect on NKA activity in RPT cells; all of these receptors have antioxidant activity (69, 182, 192). It is not known if endothelin B receptor expression is increased in the kidney of Drd3−/− mice, but D1R expression is not. Whether or not D3R has a direct antioxidant activity remains to be determined.
Similar to the renal D3R, whether the D4R has an antioxidative effect in the kidney or not is still unknown. However, D4R exerts an antioxidant effect in the nervous system (70, 130). We can speculate that renal D4R may have an indirect antioxidative activity in the kidney. The D4R can negatively regulate the renal and vascular expressions of AT1R and insulin receptor (29, 198), which are pro-oxidants (83, 180). Activation of AT1R increases D4R expression in rat RPT cells and renal AT1R expression is increased in Drd4−/− mice (24, 140). As with the D3R, a direct antioxidant effect of D4R remains to be determined.
Aberrant Regulation of Renal Dopamine Receptors on Oxidative Stress in Hypertensive Status
Oxidative stress, whether systemic or renal, is present in animal models of hypertension and human essential hypertension (3, 6). The inhibitory effect of dopamine receptors on oxidative stress is impaired in hypertensive animal models. The phosphorylation of renal dopamine receptors is increased and persistent in various hypertensive animal models. This may be the main reason for the impaired renal dopamine receptor-mediated inhibition on oxidative stress in hypertensive status (2, 32, 92c, 146, 157, 181). For example, the long-term exposure of pregnant SD rats to fine particulate matter induces hypertension accompanied by persistent phosphorylation of the renal D1R. The D1R antioxidant effect is decreased, resulting in an increased ROS production (92c). In the hypertensive offspring of these rats, the inhibition of ROS production by tempol, an oxygen free radical scavenger, reduces blood pressure and increases sodium excretion, accompanied by a normalization of D1R function that is related to phosphorylation (181). Indeed, the hyperphosphorylation and dysfunction of renal D1R and increased ROS, found in hypertensive offspring, induced by adverse maternal exposures, such as maternal diabetes mellitus, fine particulate matter, and lipopolysaccharide, are normalized by tempol (96, 157, 181).
In obese Zucker rats, which manifest with moderate degree of hypertension, both hyperglycemia and hyperinsulinism, via the upregulation of mitogen-activated protein kinase (MAPK), reduce renal D1R affinity and G protein coupling, leading to increased oxidative stress, which can be ameliorated by antioxidants (17). In the other hypertensive animal models such as old age-associated hypertension or hyperinsulinemia-induced hypertension, increased oxidative stress has also been shown to contribute to the hyperphosphorylation and defective function of renal D1R, which can be restored by antioxidants (2, 32, 146). The age-associated hypertension may be due to the different mechanisms that affect the renal D1R response to high-salt diet. In FBN rats, high-salt diet increases renal D1R expression in adult rats, but decreases D1R expression and increases renal gp91phox and NHE3 expression in aged rats (116).
Abnormal intracellular localization of the dopamine receptor may be also involved in the impaired renal dopamine receptor-mediated inhibition of oxidative stress in hypertensive states. For example, D1R expression is decreased at the RPT cell surface membrane, accompanied by increased expression in early or sorting endosome, in SHRs, relative to WKY rats. These findings indicate that in the SHR, the majority of D1Rs in RPTs are internalized and fail to be recycled to the cell surface membrane, resulting in defective D1R function (183). Additional factors, including GPCRs, may be also involved in the impaired renal dopamine receptor-mediated inhibition of oxidative stress in hypertension. For example, stimulation of CCKBR increases the cell membrane expression of the D1R in WKY rat RPT cells, but not in SHRs (30). Short-term Ang II treatment decreases cell surface membrane D1R protein in WKY rats but not in SHR RPT cells. Moreover, D1R and AT1R colocalization and coimmunoprecipitation are greater in WKY rats than in SHRs (191). The dopamine receptor exerts its physiological functions, including antioxidant effects, via its proper internalization and reinsertion to the plasma membrane after activation with its agonist. Thus, the abnormal intracellular localization of dopamine receptor causes inactivation, impaired reactivation, and function, and results in increased ROS production in hypertension.
Mechanisms involved in the abnormal regulation of renal dopamine receptors on oxidative stress
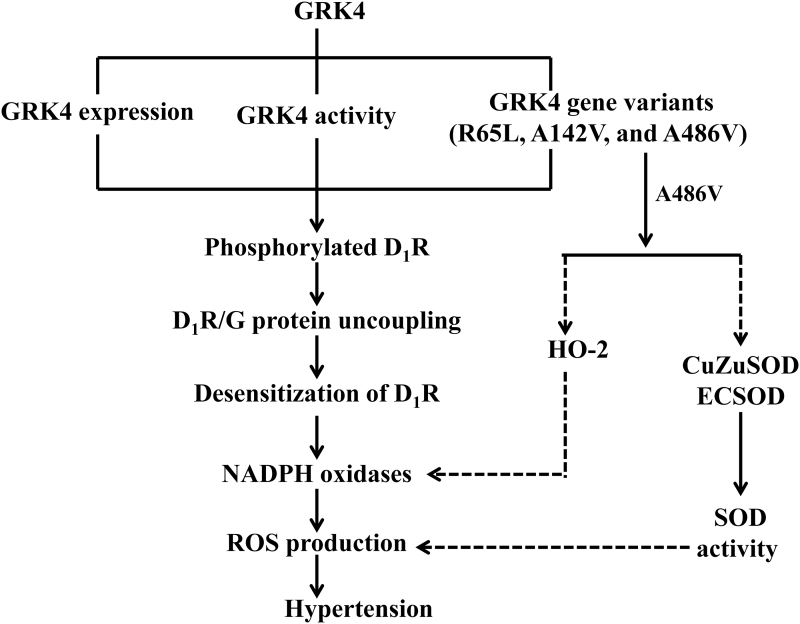
There are several mechanisms involved in the abnormal regulation of renal dopamine receptors, resulting in oxidative stress. G protein-coupled receptor kinase 4 (GRK4), a subtype in a family of seven serine/threonine protein kinases characterized by their ability to specifically recognize and phosphorylate agonist-activated GPCRs, plays a crucial role in the regulation of renal D1R phosphorylation and cellular trafficking (169, 170). Increased GRK4 activity due to increased expression or GRK4 gene variants impairs renal D1R function in hypertension. Basal renal GRK4 and serine-phosphorylated D1R levels are higher in SHRs than WKY rats. The renal-selective silencing of GRK4 decreases the quantity of serine-phosphorylated D1R and increases urine flow and sodium excretion to a greater extent in SHRs than WKY rats (125).
The impaired renal dopamine receptor-mediated inhibition on oxidative stress in hypertension may be due to the phosphorylation of D1R because the D1R expression is not changed, but its phosphorylation is increased in hypertension, which is associated with the increased activity of GRK2 and GRK4 (47, 78, 157). Studies of others and ours have also shown that the increased expression and activity of GRK4 enhance D1R phosphorylation, decrease D1R function, leading to the increased ROS production. These effects can be reversed by antioxidants in some hypertensive animal models, including age-related hypertension, and hypertensive offspring induced by adverse maternal exposures such as fine particulate matter and lipopolysaccharide (2, 32, 92c, 157, 181).
GRK4 gene variants, including GRK4 486V, 142V, and 65L, play important roles in the pathogenesis of hypertension and may provide novel therapeutic antihypertensive strategies (149, 170, 190). Decreasing renal GRK4 expression attenuates the increase in blood pressure with age and decreases the protein excretion in SHRs (125). Indeed, expressing human (h) GRK4γ142A>V but not the human GRK4γ (hGRK4γ) gene in transgenic mice produces hypertension and impairs the diuretic and natriuretic effects of D1-like agonist stimulation (47). Our meta-analysis revealed a significant association of GRK4 486V with hypertension, with an odds ratio of 1.5 (95% confidence interval: 1.2–1.9) (190). Depending on the genetic background of the mouse, hGRK4γ wild type prevents salt-sensitive hypertension, whereas hGRK4γ 486V converts a salt-resistant phenotype to a salt-sensitive phenotype (160). The elevated blood pressure of hGRK4γ142A>V transgenic mice is not associated with increased ROS production (159). By contrast, salt-sensitive hGRK4γ486A>V transgenic mice have increased renal oxidative stress (41). The hypertension of salt-sensitive hGRK4γ486V transgenic mice can be partially prevented by chronic tempol treatment (41). Figure 5 illustrates the regulation of D1R by GRK4 in the kidney. In addition, we have also reported that GRK4 regulates the phosphorylation and function of the D3R in human proximal tubule cells (152). However, the role of this regulation in the modulation of renal oxidative stress needs further study. Moreover, the role of GRK4 on the other renal dopamine receptors still remains unclear.
FIG. 5.
Regulation of D1R by GRK4 in the kidney. The increased expression and activity of GRK4 and its GRK4 gene variants (R65L, A142V, and A486V) cause D1R phosphorylation and D1R/G protein uncoupling, followed with inactivity of D1R and increased the activity of NADPH oxidases, leading to the increased ROS production and blood pressure. The broken lines indicate inhibitory effects, whereas the solid lines indicate stimulatory effects. D1R, dopamine receptor type 1; GRK4, G protein-coupled receptor kinase 4.
GRK2, another subtype of G protein-coupled receptor kinase, also phosphorylates D1R and uncouples D1R from signaling proteins. Increased expression of GRK2 also impairs D1R function that can result in increased oxidative stress and hypertension, including age-associated hypertension, hyperinsulinemia-mediated hypertension, and hypertensive offspring induced by adverse maternal exposures such as lipopolysaccharide and maternal diabetes mellitus (15, 16, 46, 96, 157). As with GRK4-related hypertension, GRK2-related hypertension can be normalized by the antioxidants (46).
The abnormal cellular trafficking of dopamine receptors may be another important mechanism involved in the abnormal regulation of renal dopamine receptors that can result in oxidative stress and hypertension. The SNX family consists of a diverse group of cytoplasmic and membrane-associated proteins orchestrating the process of cargo sorting, through the endosomal network (171). We reported that sorting nexin 5 (SNX5), a component of the mammalian retromer, is critical to D1R internalization and trafficking to the plasma membrane (150). However, renal SNX5 expression is decreased in SHRs and RPT cells from hypertensive humans, which causes aberrant D1R trafficking and impaired D1R function, including D1R-mediated antioxidative effects and sodium excretion (86, 150). Depletion of renal SNX5 expression in SHRs causes a further increase in systolic blood pressure and a decrease in sodium excretion (150). Moreover, renal-selective silencing of Snx5 in C57Bl/6J mice increases blood insulin and glucose levels, decreases urinary insulin excretion, and causes insulin resistance, which are associated with impaired D1R function (86, 150). Insulin can increase the production of ROS.
Another SNX subtype, SNX1, the first mammalian SNX, is essential for the trafficking and function of D5R. Our studies showed that renal-selective silencing of SNX1 in C57Bl/6 and BALB/c mice impairs the agonist-activated D5R internalization, prevents GTP binding, blunts cAMP response, impairs natriuresis, and increases blood pressure (151). Moreover, germ line deletion of the Snx1 gene in mice increases blood pressure and enhances ROS production that may be related to an increase in the expression of NADPH oxidase components in the kidney. The oxidative stress associated with germ line deletion of the Snx1 gene in mice is due to the impaired D5R antioxidative function and normalized by the antioxidant apocynin (168). These studies indicate that abnormal regulation of the trafficking of dopamine receptors caused by decreased abundance of SNXs leads to impaired receptor function, increased oxidative stress, and hypertension. The targeting SNXs may represent a novel mechanism for the treatment of essential hypertension.
Conclusions
In summary, increasing evidence shows that renal dopamine receptors exert their antioxidative activity in the kidney and play an important role in the regulation of sodium excretion and blood pressure (Table 1). The aberration of such regulation may be involved in the pathogenesis of hypertension. Increased understanding of the role of reciprocal regulations between renal dopamine receptors and oxidative stress in the regulation of renal function and blood pressure may give us a novel insight into the pathogenesis of hypertension.
Table 1.
Summary of Renal Dopamine Receptors, Oxidative Stress, and Hypertension
| Receptor subtype | Effects of renal dopamine receptors on the physiological status | Functional deficits in hypertension | Manifestation in gene knockout/knockdown mice or gene mutant | References |
|---|---|---|---|---|
| D1R | Inhibits renal sodium transport; induces natriuresis and diuresis; disperses NOX subunits, decreases NADPH oxidase expression and activity; interacts with antioxidant enzymes such as PON2. | Decreased D1R-mediated natriuresis and diuresis in SHR and Dahl salt-sensitive rats; impaired D1R-mediated inhibition of sodium transport in hypertensive subjects; reduced D1R expression in obese Zucker rats; increased renal NADPH oxidase expression and activity in hypertensive rats and subjects. | Increased blood pressure after selective inhibition of the renal dopamine subtype D1R with AS-ODN in conscious SD rats; reduced urinary sodium excretion and urine output and elevated blood pressure in Drd1−/− mice. | (4, 15, 23, 63, 77, 88, 107, 108, 133, 156, 162, 173, 184) |
| D5R | Inhibits renal sodium transport; induces natriuresis and diuresis; inhibits NADPH oxidase expression and activity; increases PON2 expression. | Decreased D5R expression in RPT cells and renal brush border membranes of SHR; impaired D5R internalization and trafficking in salt-induced hypertension. | Elevated blood pressure, and increased expression of renal sodium transporters in Drd5−/− mice; enhanced systemic oxidative stress in Drd5−/− mice; increased renal PLD activity, NADPH oxidase expression and activity, decreased renal expression of PON2 and HO-1 protein, and impaired HO activity in Drd5−/− mice; increased blood pressure and renal NADPH oxidase activity, and decreased urine sodium excretion in hD5R173F>L transgenic mice. | (13, 73, 87, 92b, 151, 154, 158, 173, 176, 177, 193) |
| D2R | Inhibits noradrenaline release from the kidney; modulates renal local dopamine synthesis; limits renal inflammation; inhibits renal NKA activity in the presence of D1-like receptors; decreases hyperoxidized peroxiredoxins and reduces ROS production, accompanied with decreased NOX4 expression and NADPH oxidase activity; interacts with antioxidant enzymes, including PON2, DJ-1, and sestrin2. | Decreased D2R expression in obese Zucker rats. | Hypertension associated with salt sensitivity, and sodium retention in Drd2−/− mice; increased renal ROS production, NADPH oxidase expression and activity, and renal inflammation in Drd2−/− mice; renal inflammation and fibrosis induced by D2R SNPs (rs6276, rs6277, and rs1800497). | (8, 36, 61, 74, 79, 81, 84, 109, 122, 145, 156, 174, 175, 196) |
| D3R | Induces natriuresis and diuresis; inhibits renal NHE and NKA activity; modulates renal hemodynamics and tubular function, and increases amino acid-induced glomerular hyperfiltration. | Decreased renal D3R expression in the cortex and RPT cells of SHR; impaired D3R-imediated diuresis and natriuresis in SHR; impaired inhibition of sodium transport in SHRs and in hypertensive Dahl salt-sensitive rats. | Hypertension and decreased urine flow rate and sodium excretion in Drd3−/− mice. | (7, 12, 93–95, 113, 114, 131, 155, 172, 187, 188) |
| D4R | Inhibits vasopressin-dependent transepithelial Na+ transport and osmotic water permeability; inhibits NKA activity. | Impaired D4R-imediated NKA inhibiton in RPT cells of SHR; decreased D4R expression in obese Zucker rats. | Hypertension associated with increased renal AT1R expression in Drd4−/− mice. | (24, 138, 140, 156) |
AS-ODN, antisense oligodeoxynucleotide; AT1R, angiotensin II type 1 receptor; D1R, dopamine D1 receptor; D2R, dopamine D2 receptor; D3R, dopamine D3 receptor; D4R, dopamine D4 receptor; D5R, dopamine D5 receptor; HO-1, heme oxygenase-1; NADPH, nicotinamide-adenine dinucleotide phosphate; NHE, sodium hydrogen exchanger; NKA, Na+-K+-ATPase; PLD, phospholipase D; PON2, paraoxonase 2; ROS, reactive oxygen species; RPT, renal proximal tubule; SD, Sprague-Dawley; SHR, spontaneously hypertensive rat; SNP, single-nucleotide polymorphism.
However, there are still some questions that need to be studied in the future, for example, the exact causal relationship between increased oxidative stress and the impaired renal dopamine receptors is still unclear; the direct antioxidant activity of renal D3R and D4R still needs to be proved; more details of mechanisms involved in the abnormal regulation of renal dopamine receptors on oxidative stress are needed to be determined. Dopamine receptor-mediated inhibition of oxidative stress may be found to play a potential role in the antihypertensive treatment, which may represent a valuable pharmaceutical target for potential therapeutic approaches in the treatment of hypertension.
Abbreviations Used
- AC
adenylyl cyclase
- Ang II
angiotensin II
- AT1R
angiotensin II type 1 receptor
- CCKBR
cholecystokinin B receptor
- CNS
central nervous system
- D1R
dopamine D1 receptor
- D2R
dopamine D2 receptor
- D3R
dopamine D3 receptor
- D4R
dopamine D4 receptor
- D5R
dopamine D5 receptor
- GPCRs
G protein-coupled receptors
- GR
glutathione reductase
- GRK2
G protein-coupled receptor kinase 2
- GRK4
G protein-coupled receptor kinase 4
- GSH-Px
glutathione peroxidase
- GST
glutathione transferase
- H2O2
hydrogen peroxide
- HEK-hD1R
HEK-293 cells heterologously expressing human D1R
- HEK-hD5R
HEK-293 cells heterologously expressing human D5R
- hGRK4γ
human GRK4γ
- HMOX1
heme oxygenase gene
- HO-1
heme oxygenase-1
- HO-2
heme oxygenase-2
- l-DOPA
l-dihydroxy-phenylalanine
- NADPH
nicotinamide-adenine dinucleotide phosphate
- NF-κB
nuclear factor-κB
- NHE3
sodium hydrogen exchanger type 3
- NKA
Na+-K+-ATPase
- NO
nitric oxide
- PKC
protein kinase C
- PLD
phospholipase D
- PON2
paraoxonase 2
- ROS
reactive oxygen species
- RPTs
renal proximal tubules
- SD
Sprague-Dawley
- SHR
spontaneously hypertensive rat
- siRNA
small interfering RNA
- SNP
single-nucleotide polymorphism
- SNX1
sorting nexin 1
- SNX5
sorting nexin 5
- SOD
superoxide dismutase
- Trx1
thioredoxin 1
- WKY
Wistar Kyoto
Funding Information
These studies were supported, in part, by grants from the National Key R&D Program of China (2018YFC1312700), the National Natural Science Foundation of China (31730043, 81770425), Program of Innovative Research Team by National Natural Science Foundation (81721001), Key Program of The Third Affiliated Hospital of Chongqing Medical University (KY19024), the National Institutes of Health (R01-DK039308, P01-HL074940), and the Clinical Medical Research Talent Training Program of The Third Military Medical University (2018XLC10I2).
References
- 1. Abais-Battad JM, Lund H, Dasinger JH, Fehrenbach DJ, Cowley AW Jr., and Mattson DL. NOX2-derived reactive oxygen species in immune cells exacerbates salt-sensitive hypertension. Free Radic Biol Med 146: 333–339, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad Banday A and Lokhandwala MF.. Defective renal dopamine D1 receptor function contributes to hyperinsulinemia-mediated hypertension. Clin Exp Hypertens 28: 695–705, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Ahmad KA, Yuan Yuan D, Nawaz W, Ze H, Zhuo CX, Talal B, Taleb A, Mais E, and Qilong D. Antioxidant therapy for management of oxidative stress induced hypertension. Free Radic Res 51: 428–438, 2017 [DOI] [PubMed] [Google Scholar]
- 4. Albrecht FE, Drago J, Felder RA, Printz MP, Eisner GM, Robillard JE, Sibley DR, Westphal HJ, and Jose PA. Role of the D1A dopamine receptor in the pathogenesis of genetic hypertension. J Clin Invest 97: 2283–2288, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alves-Lopes R, Neves KB, Anagnostopoulou A, Rios FJ, Lacchini S, Montezano AC, and Touyz RM. Crosstalk between vascular redox and calcium signaling in hypertension involves TRPM2 (transient receptor potential melastatin 2) cation channel. Hypertension 75: 139–149, 2020 [DOI] [PubMed] [Google Scholar]
- 6. Araujo M and Wilcox CS. Oxidative stress in hypertension: role of the kidney. Antioxid Redox Signal 20: 74–101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armando I, Villar VA, Jones JE, Lee H, Wang X, Asico LD, Yu P, Yang J, Escano CS Jr., Pascua-Crusan AM, Felder RA, and Jose PA.. Dopamine D3 receptor inhibits the ubiquitin-specific peptidase 48 to promote NHE3 degradation. FASEB J 28: 1422–1434, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Armando I, Wang X, Villar VA, Jones JE, Asico LD, Escano C, and Jose PA. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension 49: 672–678, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Asghar M, Banday AA, Fardoun RZ, and Lokhandwala MF. Hydrogen peroxide causes uncoupling of dopamine D1-like receptors from G proteins via a mechanism involving protein kinase C and G-protein-coupled receptor kinase 2. Free Radic Biol Med 40: 13–20, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Asghar M, Chugh G, and Lokhandwala MF. Inflammation compromises renal dopamine D1 receptor function in rats. Am J Physiol Renal Physiol 297: F1543–F1549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asghar M, George L, and Lokhandwala MF. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am J Physiol Renal Physiol 293: F914–F919, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Asico LD, Ladines C, Fuchs S, Accili D, Carey RM, Semeraro C, Pocchiari F, Felder RA, Eisner GM, and Jose PA. Disruption of the dopamine D3 receptor gene produces renin-dependent hypertension. J Clin Invest 102: 493–498, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asico LD, Zhang X, Jiang J, Cabrera D, Escano CS, Sibley DR, Wang X, Yang Y, Mannon R, Jones JE, Armando I, and Jose PA. Lack of renal dopamine D5 receptors promotes hypertension. J Am Soc Nephrol 22: 82–89, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Banday AA, Diaz AD, and Lokhandwala M. Kidney dopamine D1-like receptors and angiotensin 1–7 interaction inhibits renal Na+ transporters. Am J Physiol Renal Physiol 317: F949–F956, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banday AA, Fazili FR, and Lokhandwala MF. Insulin causes renal dopamine D1 receptor desensitization via GRK2-mediated receptor phosphorylation involving phosphatidylinositol 3-kinase and protein kinase C. Am J Physiol Renal Physiol 293: F877–F884, 2007a [DOI] [PubMed] [Google Scholar]
- 16. Banday AA, Fazili FR, and Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and hypertension via mechanisms that involve nuclear factor-kappaB and protein kinase C. J Am Soc Nephrol 18: 1446–1457, 2007b [DOI] [PubMed] [Google Scholar]
- 17. Banday AA, Fazili FR, Marwaha A, and Lokhandwala MF. Mitogen-activated protein kinase upregulation reduces renal D1 receptor affinity and G-protein coupling in obese rats. Kidney Int 71: 397–406, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Banday AA, Lau YS, and Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and salt-sensitive hypertension in Sprague-Dawley rats. Hypertension 51: 367–375, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Banday AA and Lokhandwala MF. Oxidative stress reduces renal dopamine D1 receptor-Gq/11alpha G protein-phospholipase C signaling involving G protein-coupled receptor kinase 2. Am J Physiol Renal Physiol 293: F306–F315, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Banday AA and Lokhandwala MF. Transcription factor Nrf2 protects renal dopamine D1 receptor function during oxidative stress. Hypertension 62: 512–517, 2013 [DOI] [PubMed] [Google Scholar]
- 21. Banday AA and Lokhandwala MF. Transcriptional regulation of renal dopamine D1 receptor function during oxidative stress. Hypertension 65: 1064–1072, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Banday AA, Marwaha A, Tallam LS, and Lokhandwala MF. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes 54: 2219–2226, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Beheray S, Kansra V, Hussain T, and Lokhandwala MF. Diminished natriuretic response to dopamine in old rats is due to an impaired D1-like receptor-signaling pathway. Kidney Int 58: 712–720, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Bek MJ, Wang X, Asico LD, Jones JE, Zheng S, Li X, Eisner GM, Grandy DK, Carey RM, Soares-da-Silva P, and Jose PA. Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension 47: 288–295, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Bertorello A and Aperia A. Inhibition of proximal tubule Na+-K+-ATPase activity requires simultaneous activation of DA1 and DA2 receptors. Am J Physiol 259: F924–F928, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Camargo LL, Harvey AP, Rios FJ, Tsiropoulou S, Da Silva RNO, Cao Z, Graham D, McMaster C, Burchmore RJ, Hartley RC, Bulleid N, Montezano AC, and Touyz RM. Vascular nox (NADPH oxidase) compartmentalization, protein hyperoxidation, and endoplasmic reticulum stress response in hypertension. Hypertension 72: 235–246, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cao P, Ito O, Guo Q, Ito D, Muroya Y, Rong R, Mori T, Ito S, and Kohzuki M. Endogenous hydrogen peroxide up-regulates the expression of nitric oxide synthase in the kidney of SHR. J Hypertens 29: 1167–1174, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Castellano M, Beschi M, Agabiti-Rossi E, Muiesan ML, Romanelli G, Falo F, Malerba M, and Muiesan G. Renal noradrenergic and dopaminergic activity in patients with borderline essential hypertension. J Cardiovasc Pharmacol 8(Suppl 5): S116–S118, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Chen K, Deng K, Wang X, Wang Z, Zheng S, Ren H, He D, Han Y, Asico LD, Jose PA, and Zeng C. Activation of D4 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Hypertension 65: 153–160, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Y, Asico LD, Zheng S, Villar VA, He D, Zhou L, Zeng C, and Jose PA. Gastrin and D1 dopamine receptor interact to induce natriuresis and diuresis. Hypertension 62: 927–933, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW, Kim KC, Lee J, Lee MY, Lee SM, Kang DH, and Lee BH. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J 28: 3197–3204, 2014 [DOI] [PubMed] [Google Scholar]
- 32. Chugh G, Lokhandwala MF, and Asghar M. Altered functioning of both renal dopamine D1 and angiotensin II type 1 receptors causes hypertension in old rats. Hypertension 59: 1029–1036, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clark BA, Rosa RM, Epstein FH, Young JB, and Landsberg L. Altered dopaminergic responses in hypertension. Hypertension 19: 589–594, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Costantini D. Understanding diversity in oxidative status and oxidative stress: the opportunities and challenges ahead. J Exp Biol 222: jeb194688, 2019 [DOI] [PubMed] [Google Scholar]
- 35. Cuevas S, Yang Y, Konkalmatt P, Asico LD, Feranil J, Jones J, Villar VA, Armando I, and Jose PA. Role of nuclear factor erythroid 2-related factor 2 in the oxidative stress-dependent hypertension associated with the depletion of DJ-1. Hypertension 65: 1251–1257, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cuevas S, Zhang Y, Yang Y, Escano C, Asico L, Jones JE, Armando I, and Jose PA. Role of renal DJ-1 in the pathogenesis of hypertension associated with increased reactive oxygen species production. Hypertension 59: 446–452, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cyr A, Chambers L, Waltz PK, Whelan SP, Kohut L, Carchman E, Dyer M, Luciano J, Kautza B, Gomez HD, Otterbein LE, Rosengart MR, Shiva S, and Zuckerbraun BS. Endotoxin engages mitochondrial quality control via an iNOS-reactive oxygen species signaling pathway in hepatocytes. Oxid Med Cell Longev 2019: 4745067, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Daiber A, Di Lisa F, Oelze M, Kröller-Schön S, Steven S, Schulz E, and Münzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharmacol 174: 1670–1689, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dan Dunn J, Alvarez LA, Zhang X, and Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol 6: 472–485, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Miguel C, Hamrick WC, Sedaka R, Jagarlamudi S, Asico LD, Jose PA, and Cuevas S. Uncoupling protein 2 increases blood pressure in DJ -1 knockout mice. J Am Heart Assoc 8: e011856, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Diao Z, Asico LD, Villar VAM, Zheng X, Cuevas S, Armando I, Jose PA, and Wang X. Increased renal oxidative stress in salt-sensitive human GRK4γ486V transgenic mice. Free Radic Biol Med 106: 80–90, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dikalov SI and Dikalova AE. Crosstalk between mitochondrial hyperacetylation and oxidative stress in vascular dysfunction and hypertension. Antioxid Redox Signal 31: 710–721, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eklöf AC. The natriuretic response to a dopamine DA1 agonist requires endogenous activation of dopamine DA2 receptors. Acta Physiol Scand 160: 311–314, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Ennis RC, Asico LD, Armando I, Yang J, Feranil JB, Jurgens JA, Escano CS Jr., Yu P, Wang X, Sibley DR, Jose PA, and Villar VA.. Dopamine D1-like receptors regulate the α1A-adrenergic receptor in human renal proximal tubule cells and D1-like dopamine receptor knockout mice. Am J Physiol Renal Physiol 307: F1238–F1248, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Everett PB and Senogles SE. D3 dopamine receptor signals to activation of phospholipase D through a complex with Rho. J Neurochem 112: 963–971, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Fardoun RZ, Asghar M, and Lokhandwala M. Role of oxidative stress in defective renal dopamine D1 receptor-G protein coupling and function in old Fischer 344 rats. Am J Physiol Renal Physiol 291: F945–F951, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, Asico LD, Wang W, Zheng S, Yamaguchi I, Williams SM, Gainer J, Brown NJ, Hazen-Martin D, Wong LJ, Robillard JE, Carey RM, Eisner GM, and Jose PA. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci U S A 99: 3872–3877, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Felder RA, Seikaly MG, Cody P, Eisner GM, and Jose PA. Attenuated renal response to dopaminergic drugs in spontaneously hypertensive rats. Hypertension 15: 560–569, 1990 [DOI] [PubMed] [Google Scholar]
- 49. Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O'Connor PM, and Cowley AW Jr. Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab 15: 201–208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Förstermann U, Xia N, and Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res 120: 713–735, 2017 [DOI] [PubMed] [Google Scholar]
- 51. Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, and Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett 580: 497–504, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Gildea JJ, Kemp BA, Howell NL, Van Sciver RE, Carey RM, and Felder RA. Inhibition of renal caveolin-1 reduces natriuresis and produces hypertension in sodium-loaded rats. Am J Physiol Renal Physiol 300: F914–F920, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gildea JJ, Shah IT, Van Sciver RE, Israel JA, Enzensperger C, McGrath HE, Jose PA, and Felder RA. The cooperative roles of the dopamine receptors, D1R and D5R, on the regulation of renal sodium transport. Kidney Int 86: 118–126, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gildea JJ, Wang X, Jose PA, and Felder RA. Differential D1 and D5 receptor regulation and degradation of the angiotensin type 1 receptor. Hypertension 51: 360–366, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Gildea JJ, Xu P, Kemp BA, Carey RM, Jose PA, and Felder RA. The dopamine D1 receptor and angiotensin II type-2 receptor are required for inhibition of sodium transport through a protein phosphatase 2A pathway. Hypertension 73: 1258–1265, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gonzalez-Vicente A, and Garvin JL. Effects of reactive oxygen species on tubular transport along the nephron. Antioxidants (Basel) 6: 23, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Graton ME, Potje SR, Troiano JA, Vale GT, Perassa LA, Nakamune ACMS, Tirapelli CR, Bendhack LM, and Antoniali C. Apocynin alters redox signaling in conductance and resistance vessels of spontaneously hypertensive rats. Free Radic Biol Med 134: 53–63, 2019 [DOI] [PubMed] [Google Scholar]
- 58. Grossman E, Hoffman A, Tamrat M, Armando I, Keiser HR, and Goldstein DS. Endogenous dopa and dopamine responses to dietary salt loading in salt-sensitive rats. J Hypertens 9: 259–263, 1991 [DOI] [PubMed] [Google Scholar]
- 59. Hall JE, do Carmo JM, da Silva AA, Wang Z, and Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol 15: 367–385, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, and Hall ME. Hypertension: physiology and pathophysiology. Compr Physiol 2: 2393–2442, 2012 [DOI] [PubMed] [Google Scholar]
- 61. Han F, Konkalmatt P, Chen J, Gildea J, Felder RA, Jose PA, and Armando I. MiR-217 mediates the protective effects of the dopamine D2 receptor on fibrosis in human renal proximal tubule cells. Hypertension 65: 1118–1125, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Han W, Li H, Villar VA, Pascua AM, Dajani MI, Wang X, Natarajan A, Quinn MT, Felder RA, Jose PA, and Yu P. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension 51: 481–487, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Harris RC. Abnormalities in renal dopamine signaling and hypertension: the role of GRK4. Curr Opin Nephrol Hypertens 21: 61–65, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Higashi Y, Maruhashi T, Noma K, and Kihara Y. Oxidative stress and endothelial dysfunction: clinical evidence and therapeutic implications. Trends Cardiovasc Med 24: 165–169, 2014 [DOI] [PubMed] [Google Scholar]
- 65. Hirooka Y, Sagara Y, Kishi T, and Sunagawa K. Oxidative stress and central cardiovascular regulation. Pathogenesis of hypertension and therapeutic aspects. Circ J 74: 827–835, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Holmström KM, and Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15: 411–421, 2014 [DOI] [PubMed] [Google Scholar]
- 67. Housley DJ, Nikolas M, Venta PJ, Jernigan KA, Waldman ID, Nigg JT, and Friderici KH. SNP discovery and haplotype analysis in the segmentally duplicated DRD5 coding region. Ann Hum Genet 73: 274–282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huang H, Li X, Zheng S, Chen Y, Chen C, Wang J, Tong H, Zhou L, Yang J, and Zeng C. Downregulation of renal G protein-coupled receptor kinase type 4 expression via ultrasound-targeted microbubble destruction lowers blood pressure in spontaneously hypertensive rats. J Am Heart Assoc 5: e004028, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huang H, Ren H, Chen C, Wang X, Yang J, Han Y, He D, Zhou L, Asico LD, Jose PA, and Zeng C. D3 dopamine receptor regulation of D5 receptor expression and function in renal proximal tubule cells. Hypertens Res 35: 639–647, 2012 [DOI] [PubMed] [Google Scholar]
- 70. Ishige K, Chen Q, Sagara Y, and Schubert D. The activation of dopamine D4 receptors inhibits oxidative stress-induced nerve cell death. J Neurosci 21: 6069–6076, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jennings BL, Montanez DE, May ME Jr., Estes AM, Fang XR, Yaghini FA, Kanu A, and Malik KU.. Cytochrome P450 1B1 contributes to increased blood pressure and cardiovascular and renal dysfunction in spontaneously hypertensive rats. Cardiovasc Drugs Ther 28: 145–161, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jiang H, Wang H, and De Ridder M. Targeting antioxidant enzymes as a radiosensitizing strategy. Cancer Lett 438: 154–164, 2018 [DOI] [PubMed] [Google Scholar]
- 73. Jiang X, Chen W, Liu X, Wang Z, Liu Y, Felder RA, Gildea JJ, Jose PA, Qin C, and Yang Z. The synergistic roles of cholecystokinin B and dopamine D5 receptors on the regulation of renal sodium excretion. PLoS One 11: e0146641, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jiang X, Konkalmatt P, Yang Y, Gildea J, Jones JE, Cuevas S, Felder RA, Jose PA, and Armando I. Single-nucleotide polymorphisms of the dopamine D2 receptor increase inflammation and fibrosis in human renal proximal tubule cells. Hypertension 63: e74–e80, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jiang X, Zhang Y, Yang Y, Yang J, Asico LD, Chen W, Felder RA, Armando I, Jose PA, and Yang Z. Gastrin stimulates renal dopamine production by increasing the renal tubular uptake of l-DOPA. Am J Physiol Endocrinol Metab 312: E1–E10, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jose PA, Asico LD, Eisner GM, Pocchiari F, Semeraro C, and Felder RA. Effects of costimulation of dopamine D1- and D2-like receptors on renal function. Am J Physiol 275: R986–R994, 1998 [DOI] [PubMed] [Google Scholar]
- 77. Jose PA, Eisner GM, Drago J, Carey RM, and Felder RA. Dopamine receptor signaling defects in spontaneous hypertension. Am J Hypertens 9: 400–405, 1996 [DOI] [PubMed] [Google Scholar]
- 78. Jose PA, Soares-da-Silva P, Eisner GM, and Felder RA. Dopamine and G protein-coupled receptor kinase 4 in the kidney: role in blood pressure regulation. Biochim Biophys Acta 1802: 1259–1267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kirchheimer C, Mendez CF, Acquier A, and Nowicki S. Role of 20-HETE in D1/D2 dopamine receptor synergism resulting in the inhibition of Na+-K+-ATPase activity in the proximal tubule. Am J Physiol Renal Physiol 292: F1435–F1442, 2007 [DOI] [PubMed] [Google Scholar]
- 80. Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, and Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation 109: 2357–2362, 2004 [DOI] [PubMed] [Google Scholar]
- 81. Konkalmatt PR, Asico LD, Zhang Y, Yang Y, Drachenberg C, Zheng X, Han F, Jose PA, and Armando I. Renal rescue of dopamine D2 receptor function reverses renal injury and high blood pressure. JCI Insight 1: e85888, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ladines CA, Zeng C, Asico LD, Sun X, Pocchiari F, Semeraro C, Pisegna J, Wank S, Yamaguchi I, Eisner GM, and Jose PA. Impaired renal D1-like and D2-like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol 28: R1071–R1078, 2001 [DOI] [PubMed] [Google Scholar]
- 83. Lara LS, McCormack M, Semprum-Prieto LC, Shenouda S, Majid DS, Kobori H, Navar LG, and Prieto MC. AT1 receptor-mediated augmentation of angiotensinogen, oxidative stress, and inflammation in ANG II-salt hypertension. Am J Physiol Renal Physiol 302: F85–F94, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, and Liu F. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J 26: 2127–2136, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lejri I, Agapouda A, Grimm A, and Eckert A. Mitochondria- and oxidative stress-targeting substances in cognitive decline-related disorders: from molecular mechanisms to clinical evidence. Oxid Med Cell Longev 2019: 9695412, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li F, Yang J, Villar VAM, Asico LD, Ma X, Armando I, Sanada H, Yoneda M, Felder RA, Jose PA, and Wang X. Loss of renal SNX5 results in impaired IDE activity and insulin resistance in mice. Diabetologia 61: 727–737, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, Jones JE, Wang Z, Periasamy A, Lau YS, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Gildea JJ, Felder RA, and Jose PA. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest 118: 2180–2189, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li H, Han W, Villar VA, Keever LB, Lu Q, Hopfer U, Quinn MT, Felder RA, Jose PA, and Yu P. D1-like receptors regulate NADPH oxidase activity and subunit expression in lipid raft microdomains of renal proximal tubule cells. Hypertension 53: 1054–1061, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li L and Schafer JA. Dopamine inhibits vasopressin-dependent cAMP production in the rat cortical collecting duct. Am J Physiol 275: F62–F67, 1998 [DOI] [PubMed] [Google Scholar]
- 90. This reference has been deleted
- 91. Li XX, Bek M, Asico LD, Yang Z, Grandy DK, Goldstein DS, Rubinstein M, Eisner GM, and Jose PA. Adrenergic and endothelin B receptor-dependent hypertension in dopamine receptor type-2 knockout mice. Hypertension 38: 303–308, 2001 [DOI] [PubMed] [Google Scholar]
- 92. Li Y and Pagano PJ. Microvascular NADPH oxidase in health and disease. Free Radic Biol Med 109: 33–47, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92a. Liu X, Wang W, Chen W, Jiang X, Zhang Y, Wang Z, Yang J, Jones JE, Jose PA, and Yang Z. Regulation of blood pressure, oxidative stress and AT1R by high salt diet in mutant human dopamine D5 receptor transgenic mice. Hypertens Res 38: 394–399, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92b. Lu Q, Yang Y, Villar VA, Asico L, Jones JE, Yu P, Li H, Weinman EJ, Eisner GM, and Jose PA. D5 dopamine receptor decreases NADPH oxidase, reactive oxygen species and blood pressure via heme oxygenase-1. Hypertens Res 36: 684–690, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92c. Lu X, Ye Z, Zheng S, Ren H, Zeng J, Wang X, Jose PA, Chen K, and Zeng C. Long-term exposure of fine particulate matter causes hypertension by impaired renal D1 receptor-mediated sodium excretion via upregulation of G-protein-coupled receptor kinase type 4 expression in Sprague-Dawley rats. J Am Heart Assoc 7: e007185, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Luippold G, Küster E, Joos TO, and Mühlbauer B. Dopamine D3 receptor activation modulates renal function in anesthetized rats. Naunyn Schmiedebergs Arch Pharmacol 358: 690–693, 1998 [DOI] [PubMed] [Google Scholar]
- 94. Luippold G, Pech B, Schneider S, Drescher K, Müller R, Gross G, and Mühlbauer B. Absence of amino acid-induced glomerular hyperfiltration in dopamine D3 receptor knockout mice. Naunyn Schmiedebergs Arch Pharmacol 372: 284–290, 2006 [DOI] [PubMed] [Google Scholar]
- 95. Luippold G, Zimmermann C, Mai M, Kloor D, Starck D, Gross G, and Mühlbauer B. Dopamine D3 receptors and salt-dependent hypertension. J Am Soc Nephrol 12: 2272–2279, 2001 [DOI] [PubMed] [Google Scholar]
- 96. Luo H, Chen C, Guo L, Xu Z, Peng X, Wang X, Wang J, Wang N, Li C, Luo X, Wang H, Jose PA, Fu C, Huang Y, Shi W, and Zeng C. Exposure to maternal diabetes mellitus causes renal dopamine D1 receptor dysfunction and hypertension in adult rat offspring. Hypertension 72: 962–970, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. This reference has been deleted
- 98. This reference has been deleted
- 99. Martins-Oliveira A, Guimaraes DA, Ceron CS, Rizzi E, Oliveira DMM, Tirapelli CR, Casarini DE, Fernandes FB, Pinheiro LC, and Tanus-Santos JE. Direct renin inhibition is not enough to prevent reactive oxygen species generation and vascular dysfunction in renovascular hypertension. Eur J Pharmacol 821: 97–104, 2018 [DOI] [PubMed] [Google Scholar]
- 100. Marwaha A and Lokhandwala MF. Tempol reduces oxidative stress and restores renal dopamine D1-like receptor-G protein coupling and function in hyperglycemic rats. Am J Physiol Renal Physiol 291: F58–F66, 2006 [DOI] [PubMed] [Google Scholar]
- 101. Massaro M, Scoditti E, Carluccio MA, and De Caterina R. Oxidative stress and vascular stiffness in hypertension: a renewed interest for antioxidant therapies? Vasc Pharmacol 116: 45–50, 2019 [DOI] [PubMed] [Google Scholar]
- 102. McCutcheon RA, Abi-Dargham A, and Howes OD. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci 42: 205–220, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, and Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension 47: 238–244, 2006 [DOI] [PubMed] [Google Scholar]
- 104. Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, and Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci U S A 88: 10045–10048, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Narkar V, Kunduzova O, Hussain T, Cambon C, Parini A, and Lokhandwala M. Dopamine D2-like receptor agonist bromocriptine protects against ischemia/reperfusion injury in rat kidney. Kidney Int 66: 633–640, 2004 [DOI] [PubMed] [Google Scholar]
- 106. Natarajan AR, Eisner GM, Armando I, Browning S, Pezzullo JC, Rhee L, Dajani M, Carey RM, and Jose PA. The renin-angiotensin and renal dopaminergic systems interact in normotensive humans. J Am Soc Nephrol 27: 265–279, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nishi A, Eklöf AC, Bertorello AM, and Aperia A. Dopamine regulation of renal Na+, K+-ATPase activity is lacking in Dahl salt-sensitive rats. Hypertension 21: 767–771, 1993 [DOI] [PubMed] [Google Scholar]
- 108. O'Connell DP, Ragsdale NV, Boyd DG, Felder RA, and Carey RM. Differential human renal tubular responses to dopamine type 1 receptor stimulation are determined by blood pressure status. Hypertension 29: 115–122, 1997 [DOI] [PubMed] [Google Scholar]
- 109. Ozono R, Ueda A, Oishi Y, Yano A, Kambe M, Katsuki M, and Oshima T. Dopamine D2 receptor modulates sodium handling via local production of dopamine in the kidney. J Cardiovasc Pharmacol 42(Suppl 1): S75–S79, 2003 [DOI] [PubMed] [Google Scholar]
- 110. Padia SH, Kemp BA, Howell NL, Keller SR, Gildea JJ, and Carey RM. Mechanisms of dopamine D1 and angiotensin type 2 receptor interaction in natriuresis. Hypertension 59: 437–445, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Panico C, Luo Z, Damiano S, Artigiano F, Gill P, and Welch WJ. Renal proximal tubular reabsorption is reduced in adult spontaneously hypertensive rats: roles of superoxide and Na+/H+ exchanger 3. Hypertension 54: 1291–1297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Parascandolo A and Laukkanen MO. Carcinogenesis and reactive oxygen species signaling: interaction of the NADPH oxidase NOX1–5 and superoxide dismutase 1–3 signal transduction pathways. Antioxid Redox Signal. 30: 443–486, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pedrosa R, Gomes P, Hopfer U, Jose PA, and Soares-da-Silva P. Gialpha3 protein-coupled dopamine D3 receptor-mediated inhibition of renal NHE3 activity in SHR proximal tubular cells is a PLC-PKC-mediated event. Am J Physiol Renal Physiol 287: F1059–F1066, 2004 [DOI] [PubMed] [Google Scholar]
- 114. Pedrosa R, Gomes P, Zeng C, Hopfer U, Jose PA, and Soares-da-Silva P. Dopamine D3 receptor-mediated inhibition of Na+/H+ exchanger activity in normotensive and spontaneously hypertensive rat proximal tubular epithelial cells. Br J Pharmacol 142: 1343–1353, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Plouffe B, Yang X, and Tiberi M. The third intracellular loop of D1 and D5 dopaminergic receptors dictates their subtype-specific PKC-induced sensitization and desensitization in a receptor conformation-dependent manner. Cell Signal 24: 106–118, 2012 [DOI] [PubMed] [Google Scholar]
- 116. Pokkunuri I, Chugh G, Rizvi I, and Asghar M. Age-related hypertension and salt sensitivity are associated with unique cortico-medullary distribution of D1R, AT1R, and NADPH-oxidase in FBN rats. Clin Exp Hypertens 37: 1–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Polakowski JS, Segreti JA, Cox BF, Hsieh GC, Kolasa T, Moreland RB, and Brioni JD. Effects of selective dopamine receptor subtype agonists on cardiac contractility and regional haemodynamics in rats. Clin Exp Pharmacol Physiol 31: 837–841, 2004 [DOI] [PubMed] [Google Scholar]
- 118. Prysyazhna O, Wolhuter K, Switzer C, Santos C, Yang X, Lynham S, Shah AM, Eaton P, and Burgoyne JR. Blood pressure-lowering by the antioxidant resveratrol is counterintuitively mediated by oxidation of cGMP-dependent protein kinase. Circulation 140: 126–137, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rodrigo R, González J, and Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res 34: 431–440, 2011 [DOI] [PubMed] [Google Scholar]
- 120. Romero M, Caniffi C, Bouchet G, Costa MA, Elesgaray R, Arranz C, and Tomat AL. Chronic treatment with atrial natriuretic peptide in spontaneously hypertensive rats: beneficial renal effects and sex differences. PLoS One 10: e0120362, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rosin C, Colombo S, Calver AA, Bates TE, and Skaper SD. Dopamine D2 and D3 receptor agonists limit oligodendrocyte injury caused by glutamate oxidative stress and oxygen/glucose deprivation. Glia 52: 336–343, 2005 [DOI] [PubMed] [Google Scholar]
- 122. Rump LC, Schwertfeger E, Schaible U, Schuster MJ, Frankenschmidt A, and Schollmeyer P. Dopamine receptor modulation of noradrenaline release by carmoxirole in human cortical kidney slices. Eur J Clin Pharmacol 44(Suppl 1): S47–S49, 1993 [DOI] [PubMed] [Google Scholar]
- 123. Saito I, Itsuji S, Takeshita E, Kawabe H, Nishino M, Wainai H, Hasegawa C, Saruta T, Nagano S, and Sekihara T. Increased urinary dopamine excretion in young patients with essential hypertension. Clin Exp Hypertens 16: 29–39, 1994 [DOI] [PubMed] [Google Scholar]
- 124. Saito O, Ando Y, Kusano E, and Asano Y. Functional characterization of basolateral and luminal dopamine receptors in rabbit CCD. Am J Physiol Renal Physiol 281: F114–F122, 2001 [DOI] [PubMed] [Google Scholar]
- 125. Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, Xu J, Luo Y, Wang X, Zeng C, Armando I, Felder RA, and Jose PA. Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension 47: 1131–1139, 2006 [DOI] [PubMed] [Google Scholar]
- 126. Schneider R, Iscovitz H, Ilan Z, Bernstein K, Gros M, and Iaina A. Oxygen free radical scavenger system intermediates in essential hypertensive patients before and immediately after sublingual captopril administration. Isr J Med Sci 26: 491–495, 1990 [PubMed] [Google Scholar]
- 127. Schreck C and O'Connor PM. NAD(P)H oxidase and renal epithelial ion transport. Am J Physiol Regul Integr Comp Physiol 300: R1023–R1029, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Schulz R, Murzabekova G, Egemnazarov B, Kraut S, Eisele HJ, Dumitrascu R, Heitmann J, Seimetz M, Witzenrath M, Ghofrani HA, Schermuly RT, Grimminger F, Seeger W, and Weissmann N. Arterial hypertension in a murine model of sleep apnea: role of NADPH oxidase 2. J Hypertens 32: 300–305, 2014 [DOI] [PubMed] [Google Scholar]
- 129. Senoner T and Dichtl W. Oxidative stress in cardiovascular diseases: still a therapeutic target? Nutrients 11: 2090, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shimada S, Hirabayashi M, Ishige K, Kosuge Y, Kihara T, and Ito Y. Activation of dopamine D4 receptors is protective against hypoxia/reoxygenation-induced cell death in HT22 cells. J Pharmacol Sci 114: 217–224, 2010 [DOI] [PubMed] [Google Scholar]
- 131. Shin Y, Kumar U, Patel Y, Patel SC, and Sidhu A. Differential expression of D2-like dopamine receptors in the kidney of the spontaneously hypertensive rat. J Hypertens 21: 199–207, 2003 [DOI] [PubMed] [Google Scholar]
- 132. Silva GB, Ortiz PA, Hong NJ, and Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension 48: 467–472, 2006 [DOI] [PubMed] [Google Scholar]
- 133. Siragy HM, Felder RA, Howell NL, Chevalier RL, Peach MJ, and Carey RM. Evidence that intrarenal dopamine acts as a paracrine substance at the renal tubule. Am J Physiol 257: F469–F477, 1989 [DOI] [PubMed] [Google Scholar]
- 134. Sowers JR, Zemel MB, Zemel P, Beck FW, Walsh MF, and Zawada ET. Salt sensitivity in blacks. Salt intake and natriuretic substances. Hypertension 12: 485–490, 1988 [DOI] [PubMed] [Google Scholar]
- 135. Staudacher T, Pech B, Tappe M, Gross G, Mühlbauer B, and Luippold G. Arterial blood pressure and renal sodium excretion in dopamine D3 receptor knockout mice. Hypertens Res 30: 93–101, 2007 [DOI] [PubMed] [Google Scholar]
- 136. Stier CT Jr., Itskovitz HD, and Chen YH.. Urinary dopamine and sodium excretion in spontaneously hypertensive rats. Clin Exp Hypertens 15: 105–123, 1993 [DOI] [PubMed] [Google Scholar]
- 137. This reference has been deleted
- 138. Sun D and Schafer JA. Dopamine inhibits AVP-dependent Na+ transport and water permeability in rat CCD via a D4-like receptor. Am J Physiol 271: F391–F400, 1996 [DOI] [PubMed] [Google Scholar]
- 139. Sun P, Yue P, and Wang WH. Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. Am J Physiol Renal Physiol 302: F679–F687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139a. Sunahara RK, Guan HC, O'Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HH, and Niznik HB. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature 350: 614–649, 1991 [DOI] [PubMed] [Google Scholar]
- 140. Tang L, Zheng S, Ren H, He D, Zeng C, and Wang WE. Activation of angiotensin II type 1 receptors increases D4 dopamine receptor expression in rat renal proximal tubule cells. Hypertens Res 40: 652–657, 2017 [DOI] [PubMed] [Google Scholar]
- 141. Taveira-da-Silva R, da Silva Sampaio L, Vieyra A, and Einicker-Lamas M. L-Tyr-induced phosphorylation of tyrosine hydroxylase at ser40: an alternative route for dopamine synthesis and modulation of Na+/K+-ATPase in kidney cells. Kidney Blood Press Res 44: 1–11, 2019 [DOI] [PubMed] [Google Scholar]
- 142. Taysi S, Tascan AS, Ugur MG, and Demir M. Radicals, oxidative/nitrosative stress and preeclampsia. Mini Rev Med Chem 19: 178–193, 2019 [DOI] [PubMed] [Google Scholar]
- 143. Thompson D and Whistler JL. Trafficking properties of the D5 dopamine receptor. Traffic 12: 644–656, 2011 [DOI] [PubMed] [Google Scholar]
- 144. Togliatto G, Lombardo G, and Brizzi MF. The future challenge of reactive oxygen species (ROS) in hypertension: from bench to bed side. Int J Mol Sci 18: 1988, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ueda A, Ozono R, Oshima T, Yano A, Kambe M, Teranishi Y, Katsuki M, and Chayama K. Disruption of the type 2 dopamine receptor gene causes a sodium-dependent increase in blood pressure in mice. Am J Hypertens 16: 853–858, 2003 [DOI] [PubMed] [Google Scholar]
- 146. Umrani DN, Banday AA, Hussain T, and Lokhandwala MF. Rosiglitazone treatment restores renal dopamine receptor function in obese Zucker rats. Hypertension 40: 880–885, 2002 [DOI] [PubMed] [Google Scholar]
- 147. Usatyuk PV, Gorshkova IA, He D, Zhao Y, Kalari SK, Garcia JG, and Natarajan V. Phospholipase D-mediated activation of IQGAP1 through Rac1 regulates hyperoxia-induced p47phox translocation and reactive oxygen species generation in lung endothelial cells. J Biol Chem 284: 15339–15352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Vaka VR, McMaster KM, Cunningham MW Jr., Ibrahim T, Hazlewood R, Usry N, Cornelius DC, Amaral LM, and LaMarca B.. Role of mitochondrial dysfunction and reactive oxygen species in mediating hypertension in the reduced uterine perfusion pressure rat model of preeclampsia. Hypertension 72: 703–711, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Vandell AG, Lobmeyer MT, Gawronski BE, Langaee TY, Gong Y, Gums JG, Beitelshees AL, Turner ST, Chapman AB, Cooper-DeHoff RM, Bailey KR, Boerwinkle E, Pepine CJ, Liggett SB, and Johnson JA. G protein receptor kinase 4 polymorphisms: β-blocker pharmacogenetics and treatment-related outcomes in hypertension. Hypertension 60: 957–964, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Villar VA, Armando I, Sanada H, Frazer LC, Russo CM, Notario PM, Lee H, Comisky L, Russell HA, Yang Y, Jurgens JA, Jose PA, and Jones JE. Novel role of sorting nexin 5 in renal D1 dopamine receptor trafficking and function: implications for hypertension. FASEB J 27: 1808–1819, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Villar VA, Jones JE, Armando I, Asico LD, Escano CS Jr., Lee H, Wang X, Yang Y, Pascua-Crusan AM, Palmes-Saloma CP, Felder RA, and Jose PA.. Sorting nexin 1 loss results in D5 dopamine receptor dysfunction in human renal proximal tubule cells and hypertension in mice. J Biol Chem 288: 152–163, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Villar VA, Jones JE, Armando I, Palmes-Saloma C, Yu P, Pascua AM, Keever L, Arnaldo FB, Wang Z, Luo Y, Felder RA, and Jose PA. G protein-coupled receptor kinase 4 (GRK4) regulates the phosphorylation and function of the dopamine D3 receptor. J Biol Chem 284: 21425–21434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wang S, Lu X, Yang J, Wang H, Chen C, Han Y, Ren H, Zheng S, He D, Zhou L, Asico LD, Wang WE, Jose PA, and Zeng C. Regulation of renalase expression by D5 dopamine receptors in rat renal proximal tubule cells. Am J Physiol Renal Physiol 306: F588–F596, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wang S, Tan X, Chen P, Zheng S, Ren H, Cai J, Zhou L, Jose PA, Yang J, and Zeng C. Role of thioredoxin 1 in impaired renal sodium excretion of hD5RF173L transgenic mice. J Am Heart Assoc 8: e012192, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wang X, Escano CS, Asico L, Jones JE, Barte A, Lau YS, Jose PA, and Armando I. Upregulation of renal D5 dopamine receptor ameliorates the hypertension in D3 dopamine receptor-deficient mice. Hypertension 62: 295–301, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Wang X, Li F, Jose PA, and Ecelbarger CM. Reduction of renal dopamine receptor expression in obese Zucker rats: role of sex and angiotensin II. Am J Physiol Renal Physiol 299: F1164–F1170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wang X, Luo H, Chen C, Chen K, Wang J, Cai Y, Zheng S, Yang X, Zhou L, Jose PA, and Zeng C. Prenatal lipopolysaccharide exposure results in dysfunction of the renal dopamine D1 receptor in offspring. Free Radic Biol Med 76: 242–250, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wang X, Luo Y, Escano CS, Yang Z, Asico L, Li H, Jones JE, Armando I, Lu Q, Sibley DR, Eisner GM, and Jose PA. Upregulation of renal sodium transporters in D5 dopamine receptor-deficient mice. Hypertension 55: 1431–1437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wang Z, Armando I, Asico LD, Escano C, Wang X, Lu Q, Felder RA, Schnackenberg CG, Sibley DR, Eisner GM, and Jose PA. The elevated blood pressure of human GRK4gamma A142V transgenic mice is not associated with increased ROS production. Am J Physiol Heart Circ Physiol 292: H2083–H2092, 2007 [DOI] [PubMed] [Google Scholar]
- 160. Wang Z, Asico L, Wang X, Escano C, and Jose P. Human G protein-coupled receptor kinase type 4γ (GRK4γ) 486V-promoted salt sensitivity in transgenic mice is related with increased AT1 receptor (AT1R). J Am Soc Nephrol 18: 148A, 2007 [Google Scholar]
- 161. Wang Z, Guan W, Han Y, Ren H, Tang X, Zhang H, Liu Y, Fu J, He D, Asico LD, Jose PA, Zhou L, Chen L, and Zeng C. Stimulation of dopamine D3 receptor attenuates renal ischemia-reperfusion injury via increased linkage With Gα12. Transplantation 99: 2274–2284, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wang ZQ, Felder RA, and Carey RM. Selective inhibition of the renal dopamine subtype D1A receptor induces antinatriuresis in conscious rats. Hypertension 33: 504–510, 1999 [DOI] [PubMed] [Google Scholar]
- 163. Wei J, Xu L, Du YN, Tang XF, Ye MQ, Wu YJ, Han WQ, and Gao PJ. Membrane raft redox signalling contributes to endothelial dysfunction and vascular remodelling of thoracic aorta in angiotensin II-infused rats. Exp Physiol 104: 946–956, 2019 [DOI] [PubMed] [Google Scholar]
- 164. Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill PS, Aslam S, Wang X, Ji H, Sandberg K, Jose P, and Wilcox CS. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension 48: 934–941, 2006 [DOI] [PubMed] [Google Scholar]
- 165. White BH and Sidhu A. Increased oxidative stress in renal proximal tubules of the spontaneously hypertensive rat: a mechanism for defective dopamine D1A receptor/G-protein coupling. J Hypertens 16: 1659–1665, 1998 [DOI] [PubMed] [Google Scholar]
- 166. Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005 [DOI] [PubMed] [Google Scholar]
- 167. Yamaguchi I, Walk SF, Jose PA, and Felder RA. Dopamine D2L receptors stimulate Na+/K+-ATPase activity in murine LTK-cells. Mol Pharmacol 49: 373–378, 1996 [PubMed] [Google Scholar]
- 167a. Yan S, Wang P, Wang J, Yang J, Lu H, Jin C, Cheng M, and Xu D. Long non-coding RNA HIX003209 promotes inflammation by sponging miR-6089 via TLR4/NF-κB signaling pathway in rheumatoid arthritis. Front Immunol 10: 2218, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167b. Yao LP, Li XX, Yu PY, Xu J, Asico LD, and Jose PA. Dopamine D1 receptor and protein kinase C isoforms in spontaneously hypertensive rats. Hypertension 32: 1049–1053, 1998 [DOI] [PubMed] [Google Scholar]
- 168. Yang J, Asico LD, Feranil JB, Jones JE, Armando I, Weinman EJ, Jose PA, and Villar VAM. Uncovering the molecular mechanisms underlying the hypertension in snx1 knockout mice. Hypertension 64(Suppl 1): A573, 2014 [Google Scholar]
- 169. Yang J, Villar VA, Armando I, Jose PA, and Zeng C. G protein-coupled receptor kinases: crucial regulators of blood pressure. J Am Heart Assoc 5: e003519, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Yang J, Villar VA, Jones JE, Jose PA, and Zeng C. G protein-coupled receptor kinase 4: role in hypertension. Hypertension 65: 1148–1155, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Yang J, Villar VAM, Rozyyev S, Jose PA, and Zeng C. The emerging role of sorting nexins in cardiovascular diseases. Clin Sci (Lond) 133: 723–737, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Yang S, Han Y, Zheng S, Kou X, Asico LD, Huang H, Gao Z, Jose PA, and Zeng C. Enhanced natriuresis and diuresis in wistar rats caused by the costimulation of renal dopamine D3 and angiotensin II type 2 receptors. Am J Hypertens 28: 1267–1276, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Yang S, Yang Y, Yu P, Yang J, Jiang X, Villar VA, Sibley DR, Jose PA, and Zeng C. Dopamine D1 and D5 receptors differentially regulate oxidative stress through paraoxonase 2 in kidney cells. Free Radic Res 49: 397–410, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Yang Y, Cuevas S, Yang S, Villar VA, Escano C, Asico L, Yu P, Jiang X, Weinman EJ, Armando I, and Jose PA. Sestrin2 decreases renal oxidative stress, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of reactive oxygen species production. Hypertension 64: 825–832, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Yang Y, Zhang Y, Cuevas S, Villar VA, Escano C, D Asico L, Yu P, Grandy DK, Felder RA, Armando I, and Jose PA. Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Radic Biol Med 53: 437–446, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Bai RK, Sibley DR, Felder RA, and Jose PA. D5 dopamine receptor regulation of phospholipase D. Am J Physiol Heart Circ Physiol 288: H55–H61, 2005 [DOI] [PubMed] [Google Scholar]
- 177. Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, Wang X, Quinn MT, Sibley DR, Romero GG, Felder RA, and Jose PA. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol 290: R96–R104, 2006 [DOI] [PubMed] [Google Scholar]
- 178. This reference has been deleted
- 179. This reference has been deleted
- 180. Yarube I, Ayo J, Magaji R, and Umar I. Insulin treatment increases brain nitric oxide and oxidative stress, but does not affect memory function in mice. Physiol Behav 211: 112640, 2019 [DOI] [PubMed] [Google Scholar]
- 181. Ye Z, Lu X, Deng Y, Wang X, Zheng S, Ren H, Zhang M, Chen T, Jose PA, Yang J, and Zeng C. In utero exposure to fine particulate matter causes hypertension due to impaired renal dopamine D1 receptor in offspring. Cell Physiol Biochem 46: 148–159, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Yu C, Yang Z, Ren H, Zhang Y, Han Y, He D, Lu Q, Wang X, Wang X, Yang C, Asico LD, Hopfer U, Eisner GM, Jose PA, and Zeng C. D3 dopamine receptor regulation of ETB receptors in renal proximal tubule cells from WKY and SHRs. Am J Hypertens 22: 877–883, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Yu P, Asico LD, Luo Y, Andrews P, Eisner GM, Hopfer U, Felder RA, and Jose PA. D1 dopamine receptor hyperphosphorylation in renal proximal tubules in hypertension. Kidney Int 70: 1072107–1072109, 2006 [DOI] [PubMed] [Google Scholar]
- 184. Yu P, Han W, Villar VA, Li H, Arnaldo FB, Concepcion GP, Felder RA, Quinn MT, and Jose PA. Dopamine D1 receptor-mediated inhibition of NADPH oxidase activity in human kidney cells occurs via protein kinase A-protein kinase C cross talk. Free Radic Biol Med 50: 832–840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Yu P, Han W, Villar VA, Yang Y, Lu Q, Lee H, Li F, Quinn MT, Gildea JJ, Felder RA, and Jose PA. Unique role of NADPH oxidase 5 in oxidative stress in human renal proximal tubule cells. Redox Biol 2: 570–579, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Yu P, Sun M, Villar VA, Zhang Y, Weinman EJ, Felder RA, and Jose PA. Differential dopamine receptor subtype regulation of adenylyl cyclases in lipid rafts in human embryonic kidney and renal proximal tubule cells. Cell Signal 26: 2521–2529, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Zeng C, Asico LD, Yu C, Villar VA, Shi W, Luo Y, Wang Z, He D, Liu Y, Huang L, Yang C, Wang X, Hopfer U, Eisner GM, and Jose PA. Renal D3 dopamine receptor stimulation induces natriuresis by endothelin B receptor interactions. Kidney Int 74: 750–759, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187a. Zeng C and Jose PA.. Dopamine receptors: important antihypertensive counterbalance against hypertensive factors. Hypertension 57: 11–17, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, and Jose PA. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res 99: 494–500, 2006 [DOI] [PubMed] [Google Scholar]
- 189. This reference has been deleted
- 190. Zeng C, Villar VA, Eisner GM, Williams SM, Felder RA, and Jose PA. G protein-coupled receptor kinase 4: role in blood pressure regulation. Hypertension 51: 1449–1455, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Zeng C, Wang Z, Hopfer U, Asico LD, Eisner GM, Felder RA, and Jose PA. Rat strain effects of AT1 receptor activation on D1 dopamine receptors in immortalized renal proximal tubule cells. Hypertension 46: 799–805, 2005 [DOI] [PubMed] [Google Scholar]
- 192. Zeng C, Wang Z, Li H, Yu P, Zheng S, Wu L, Asico LD, Hopfer U, Eisner GM, Felder RA, and Jose PA. D3 dopamine receptor directly interacts with D1 dopamine receptor in immortalized renal proximal tubule cells. Hypertension 47: 573–579, 2006 [DOI] [PubMed] [Google Scholar]
- 193. Zeng C, Yang Z, Wang Z, Jones J, Wang X, Altea J, Mangrum AJ, Hopfer U, Sibley DR, Eisner GM, Felder RA, and Jose PA. Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension 45: 804–810, 2005 [DOI] [PubMed] [Google Scholar]
- 194. Zeng C, Zhang M, Asico LD, Eisner GM, and Jose PA. The dopaminergic system in hypertension. Clin Sci (Lond) 112: 583–597, 2007 [DOI] [PubMed] [Google Scholar]
- 195. Zhang MZ and Harris RC. Antihypertensive mechanisms of intra-renal dopamine. Curr Opin Nephrol Hypertens 24: 117–122, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Zhang Y, Cuevas S, Asico LD, Escano C, Yang Y, Pascua AM, Wang X, Jones JE, Grandy D, Eisner G, Jose PA, and Armando I. Deficient dopamine D2 receptor function causes renal inflammation independently of high blood pressure. PLoS One 7: e38745, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zhang Y, Jiang X, Qin C, Cuevas S, Jose PA, and Armando I. Dopamine D2 receptors' effects on renal inflammation are mediated by regulation of PP2A function. Am J Physiol Renal Physiol 310: F128–F134, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zhang Y, Ren H, Lu X, He D, Han Y, Wang H, Zeng C, and Shi W. Inhibition of D4 dopamine receptors on insulin receptor expression and wffect in renal proximal tubule cells. J Am Heart Assoc 5: e002448, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zheng S, Yu P, Zeng C, Wang Z, Yang Z, Andrews PM, Felder RA, and Jose PA. Galpha12- and Galpha13-protein subunit linkage of D5 dopamine receptors in the nephron. Hypertension 41: 604–610, 2003 [DOI] [PubMed] [Google Scholar]
- 200. Zou L, Xu J, Jankovic J, He Y, Appel SH, and Le W. Pramipexole inhibits lipid peroxidation and reduces injury in the substantia nigra induced by the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice. Neurosci Lett 281: 167–170, 2000 [DOI] [PubMed] [Google Scholar]