Abstract
The demand for fresh, safe, and healthy fruits by consumers has increased, which concurrently occurs with an increase in initiatives on reducing food wastage. Starfruit consists of good nutrition and valuable sensory attributes, but its shelf life is short and can only be preseved for a few days at ambient storage. This research was conducted to determine the effectiveness of synergistic edible coatings (pectin [Pe] and maltodextrin [M] and 100, 200, and 300 ppm of sodium chloride [SC]) on the quality and safety criterion of starfruits throughout a shelf life analysis of 14 days at ambient temperature. Consumer acceptability of the edible-coated starfruit was also evaluated. The coating process was performed using a dipping method. The uncoated (control) and coated samples were evaluated for the characteristics of weight, pH, total soluble solids (TSS), water activity, color, texture, microbial growth, FTIR, and sensory evaluation. From the results, the starfruit coated with Pe + M + 100 ppm SC had a significantly lower weight-loss trend compared to the other samples. On day 14, pH of the coated starfruits were 3.02, 3.14, and 3.31 for 100, 200, and 300 ppm of SC, respectively, were found to be significantly different (p < 0.05) from the control (pH 3.49). The control had a significantly higher value of total soluble solids (6.00 ⁰Brix) compared to the coated starfruits (6.00, 5.47, and 5.33 ⁰Brix, respectively). The coated samples have significantly higher values of firmness than control especially in initial days of storage. It was observed that Pe + M + 100 ppm SC could minimize the spoilage of fruits by reducing the growth of yeast and mold, as well as bacteria, up to 0.86 and 2.02 log CFU/ml, respectively. FTIR results confirmed the presence of the coating on the starfruit. In the sensory evaluation, no significance different (p > 0.05) were obtained for all the sensory attributes and overall acceptability for day 0 and 3. In conclusion, starfruit coated with synergistic Pe + M + 100 ppm SC appeared to be the best sample in extending its shelf life and maintaining the physicochemical characteristics of starfruits up to more than 14 days.
Keywords: Synergistic, Edible coating, Maltodextrin, Pectin, Sodium chloride, Starfruit, Ambient
Synergistic; Edible coating; Maltodextrin; Pectin; Sodium chloride; Starfruit, Ambient.
1. Introduction
Consumption of food such as fruits could help to prevent undernutrition cases (Ghee et al., 2019) and noncommunicable diseases (NCDs) such as cancer, diabetes, chronic kidney disease and heart disease (Frank et al., 2019). Consequently, food safety and quality criterion are crucial in food industries. However, the deterioration of the fruit criterion may cause detrimental effects to the whole food supply chain. It is estimated that almost 33% of a total food supply lost due to food spoilage (Salunkhe et al., 1974). The worst part is it leads to food wastage that brings harm to the environment as it can become a platform for the growth of pathogenic microorganisms. The phenomenon of fruit deterioration is influenced by major factors such as surrounding temperature, atmospheric composition and relative humidity. These factors act as catalysts for the pathogenic and microbial growth, which subsequently become the source of unpleasant smell for the surrounding environment (Salunkhe et al., 1974).
The fruit criterion could be classified as a Safety Criterion Analysis and Quality Criterion Analysis. The analysis includes safety parameters such as pH, water activity, and microbial growth, while quality aspects consist of fruit's weight loss, color, texture, total soluble solid, FTIR and sensory evaluation (Kyriacou and Rouphael, 2018; Barrett et al., 2010). The pH and water activity of fruits are significant as they affect the probable conditions for the growth of pathogenic bacteria, yeast and mould (Sandulachi, 2016). Hot and humid-weather countries provide an environment that encourages microbial growth, which would then contribute to the deterioration of fruit (Sandulachi, 2016; Zagory and Kader, 1989).
The spoilage of fruits and vegetables occurs due to microbiological, chemical or physical changes. Microorganisms can cause softening of the fruit texture that could then lead to spoilage. Acinetobacter sp., Pseudomonas sp., Clostridium sp. and lactic acid bacteria are examples of microbes responsible for the spoilage of fruits (Batt, 2016). The presence of microorganisms that produces enzymes, which lead to undesirable changes in fruits and vegetables, is known as microbiological food spoilage. Chemical spoilage may occur due to sanitation chemicals as fruits usually undergo washing with a sanitizing chemical. Chlorine, sodium hypochlorite, chlorine gas and calcium hypochlorite are commonly used in the food industry, although when their amount exceeds 200 ppm, the chemicals are capable of causing damage to the product (Barth et al., 2019). This reaction will change the sensory characteristics of the food. Meanwhile, physical spoilage occurs when moist foods are dehydrated, or dried food absorbs excessive amount of moisture (Petruzzi et al., 2016).
Starfruit is preferable by consumers due to the sweet and sour varieties of its taste. It is also rich in natural antioxidants such as carotenoids, vitamin C, and some phenolic compounds (Gol et al., 2013). However, a starfruit is easily spoilt as it has a high level of moisture content, which could cause higher postharvest losses (Gol et al., 2013). Starfruit can only last within a few weeks before it is engulfed by mold and become completely spoiled. These spoilages cause the starfruit to have a shorter shelf life, as well as an undesirable color, texture, odor, and flavor compare to an uncontaminated starfruit.
Food preservation is important for nutrition supply to human. Prevention techniques, such as edible coating, could protect the fruits from deterioration. Edible coating is environmentally friendly and can provide additional protective layers to fruits. Several studies have been conducted to develop a method to prolong the shelf life of starfruits, including edible coating (Baraiya et al., 2013; Gol et al., 2013; Mohamad Zaki et al., 2012). In general, coating can minimize moisture loss, respiration, and transpiration rate, as well as maintaining a fruit's firmness (Mannozzi et al., 2017). According to Mohamad Zaki et al. (2012), an edible coating may consist of proteins, lipids, and polysaccharides. Carbohydrate-based coating such as pectin was able to extend the shelf life of the fruit up to 11 days by delaying the changes in weight loss, total soluble solids, pH, total acidity, firmness, and color (Menezes and Athmaselvi, 2016). Hence, the preservation method through edible coating is considered as necessary (Clayton, 2012). Meanwhile, maltodextrin could be used as texturizing agents and fat replacers, thus enhancing the processing and storage stability of solids, subsequently reducing caking, stickiness and increase flow ability (Nurhadi et al., 2015).
Sodium chloride is considered due to its strong antimicrobial that could prevent the growth of microorganisms on fruits and vegetables (Guan and Fan, 2010). Sodium chloride is a good sanitizing agent for food and fresh-cut produce and has a stronger oxidizing agent (Mola et al., 2016). Besides, it can be used to sanitize tools and food preparation surfaces, bleach paper, and textiles (Xiao et al., 2011). Mola et al. (2016) found that the microbial activity and browning effect were reduced when sodium chloride mixed with calcium chloride and calcium ascorbate were used as a coating on fresh-cut rose apple. Sodium chloride-gum Arabic coating was applied to tomato fruits and able to improve their shelf life up to 14 days (Paladugu and Gunasekaran, 2017). Meanwhile, the development of pectin-sodium chloride coating provides beneficial impacts on banana such as maintaining quality attributes and prolonging the shelf life of the fruit (Sarduni et al., 2020).
However, the effects of sodium chloride concentration in edible coating have not been completely studied. Moreover, few studies have been recorded on the impact of maltodextrin as an edible coating on post-harvest fruit. Subsequently, the study on synergistic impacts of pectin-maltodextrin coating medium incorporated with sodium chloride on fruit safety and quality criterion at ambient shelf life remains to be scarce. In this research, pectin, maltodextrin, and sodium chloride were synergized as an edible coating matrix on starfruits. This research aimed to determine the effectiveness of synergistic edible coating of pectin-maltodextrin with the presence of 100, 200, and 300 ppm sodium chloride on the safety and quality criterion of starfruits at ambient temperature for a determined shelf life duration. The analysis performed in this study covered microbial enumeration, pH, water activity, texture, color, total soluble solids, and functional group analysis during a 14-days storage duration. Lastly, evaluation of consumer acceptability of the edible coated starfruit was evaluated.
2. Material and methods
2.1. Materials
Starfruits were obtained from Mydin Mall Pulau Sebang, Alor Gajah, Melaka, Malaysia. Starfruits at maturity index of 2 with approximately similar size and no external defects were selected (Hanani et al., 2012). The samples were washed and rinsed before coating process. Plate count agar, potato dextrose agar and peptone water (Oxoid) and sodium chloride, maltodextrin and pectin (R&M Chemicals) were used in this study.
2.2. Preparation of coating solutions
Three different coating solutions were prepared which consisted of 6% (w/v) pectin (Pe), 4% (w/v) maltodextrin (M) and 100, 200, and 300 ppm sodium chloride (SC). Then, each solution was stirred homogeneously until all coating materials were completely dissolved.
2.3. Coating process of starfruit
Starfruits were dipped in 100 ppm sodium chloride solution for 30 s and dried at ambient temperature for 30 min. Then, the starfruit was dipped in the mixture of pectin and maltodextrin solution for 30 s and dried in the air for 60 min. The same procedure was repeated using 200 and 300 ppm of sodium chloride. The samples were labelled as Pe + M + 100 ppm, SC Pe + M + 200 ppm SC and Pe + M + 300 ppm SC for samples using 100, 200, and 300 ppm, respectively. Uncoated samples were defined as control. Next, the samples were placed on trays and stored for 14 days at 28 ±°C. The samples were analysed for safety and quality criterion at day 0, 3, 5, 7, 10, and 14 of storage except for color, FTIR spectra and sensory evacuations were performed on day 0 only. All analysis were evaluated in triplicate and results were recorded in form of mean ± standard deviation.
2.4. Safety Criterion Analysis
2.4.1. pH analysis
The starfruit was converted into liquid form by using mortar and pestle. The pH was determined using pH meter (Mettler Toledo, Model: Delta 320). The pH meter was calibrated with buffers at pH 4 and pH 7 before use.
2.4.2. Water activity (aw) analysis
The samples were measured using water activity meter (Meter, Model: Aqualab4TE) at 25 °C. The analyser was calibrated before performing the analysis.
2.4.3. Microbial growth analysis
Plate count agar (PCA) and potato dextrose agar (PDA) were used to enumerate total plate count and yeast and mold, respectively. About 25 g of sample was placed in 225 ml of 1% sterile peptone water and homogenized for 1 min using stomacher. Then, ten-fold serial dilution was performed using sterile peptone water before plating technique was conducted. The PCA and PDA plates were incubated at 37 °C for 2 days and 30 °C for 5 days, respectively. The colony forming unit per ml of the sample was reported as log10 CFU/ml.
2.5. Quality Criterion Analysis
2.5.1. Determination of weight loss
Weight loss of the starfruits was determined by measuring using an analytical balance (B204S Mettler Toledo). The weight of samples on every sampling day were recorded (Mohamad Zaki et al., 2012).
2.5.2. Determination of colour
The colour of fruits was determined using chromameter (CR-400 Chroma Meter Konica Minolta). All data was taken as L∗, a∗, b∗ and h⁰ colour space values.
2.5.3. Determination of texture
TA.XT plus Texture Analyzer (Stable Micro System) was used to determine the texture of starfruits. In the analysis, force in compression mode was measured by using a cylinder stainless probe with diameter of 2 mm. The weight of the load used for this analysis is 5 kg. The control and coated starfruit were placed under the probe at the centre of the platform.
2.5.4. Measurement of total soluble solids
The total soluble solids (TSS) were measured using the refractometer (Master 50H Atago). About 1–2 drops of the starfruit were placed on the prism of the refractometer and the reading was recorded.
2.5.5. Fourier transform infrared spectroscopy (FTIR)
The starfruit was cut into smaller pieces before undergo FTIR analysis (Nicolet iS10 Thermo Scientific). On top of the crystal, the starfruits were placed and the clamp was tightened down. The sample was evaluated using a spectral range of 600–4000 cm־1 and the resolution used was 1.0 cm־1.
2.5.6. Sensory evaluation
Each sample was evaluated by 20 untrained panellists through sensory evaluation and their consent were obtained. A 5-point hedonic test was used in the sensory evaluation. Four different samples were placed into colourless plate and arranged according to the master sheet. Distilled water was prepared for each panellist to rinse their mouth before tasting each sample. The results of color, aroma, sweetness, texture, and overall acceptability were obtained and recorded.
2.6. Statistical analysis
The data were statistically analysed using IBM SPSS Statistics (64-bit edition). The statistical analysis was done by using a descriptive one-way analysis of variance (ANOVA). Mean separation was performed with the Duncan Multiple Range Test at (p ≤ 0.05). The data were presented as mean value and standard deviation among the samples.
3. Results and discussion
3.1. Safety Criterion Analysis
3.1.1. pH analysis
The fruits underwent the process of senescence when they were stored for longer periods. This would eventually lead to an increase in pH. As illustrated in Table 1, the value of pH for control increased from 3.02 (day 0) to 3.49 (day 14). A similar trend was observed for the Pe + M + 200 ppm SC sample where the value increased from 3.11 (day 0) to 3.14 (day 14). Meanwhile, the pH readings of Pe + M + 100 ppm SC sample and Pe + M + 300 ppm SC sample decreased from 3.15 to 3.02 and 3.33 to 3.31, respectively. In general, lower pH values were observed in the coated starfruits compared to control. This was due to the fact that the coatings were able to reduce the respiration and metabolic rates, hence decreasing the uptake of organic acids in the respiration process (Baraiya et al., 2013). Thus, the application of coating slowed down the pH changes of the fruit. According to a study conducted by Moalemiyan et al. (2011), pH of the control mango was higher than coated mangoes. In this study, the mango was coated with pectin and sorbitol before it was stored at room temperature. Furthermore, a similar result was also obtained by Mannozzi et al. (2017), where blueberries coated with pectin and alginate recorded lower pH readings compared to the control. Overall, the Pe + M + 100 ppm SC sample showed a lower value of pH than the other coated samples. It was also deduced that the coated starfruits with 100 ppm SC was able to maintain more acidity than the control fruit due to slow metabolic activities.
Table 1.
pH of control and pectin (Pe) and maltodextrin (M) and 100 ppm, 200 ppm and 300 ppm sodium chloride (SC) coated starfruit samples during 14 days of storage.
| pH | ||||||
|---|---|---|---|---|---|---|
| Day | D0 | D3 | D5 | D7 | D10 | D14 |
| Control | 3.02 ± 0.01a | 3.12 ± 0.01a | 3.12 ± 0.01a | 3.44 ± 0.02d | 3.42 ± 0.03c | 3.49 ± 0.02d |
| Pe + M + 100 ppm SC | 3.15 ± 0.01c | 3.24 ± 0.01c | 3.45 ± 0.00b | 2.76 ± 0.03b | 3.63 ± 0.04d | 3.02 ± 0.03a |
| Pe + M + 200 ppm SC | 3.11 ± 0.01b | 3.17 ± 0.02b | 3.53 ± 0.02d | 3.14 ± 0.02c | 2.79 ± 0.05a | 3.14 ± 0.01b |
| Pe + M + 300 ppm SC | 3.33 ± 0.01d | 3.33 ± 0.01d | 3.49 ± 0.00c | 2.72 ± 0.01a | 3.06 ± 0.05b | 3.31 ± 0.03c |
abcd Values with different letter within the column are significantly different (P < 0.05).
3.1.2. Water activity (aw) analysis
Water activity is the ratio of the water vapor pressure of the product to the vapor pressure of pure water at the same temperature. The water activity for most fruits and vegetables is in the range of 0.95 and above (Sandulachi, 2016). As microorganisms can grow on food products with a minimum level of water activity, the reduction of water activity can control the growth of microbes (Berk, 2018). As tabulated in Table 2, the water activity of all samples increased from day 0 to day 14. The control showed an increase from 0.994 to 1.008, while Pe + M + 100 ppm SC, Pe + M + 200 ppm SC and Pe + M + 300 ppm SC increased from 0.996 to 1.008, 0.992 to 1.008 and 0.989 to 1.009, respectively. However, the coated samples showed a slightly lower water activity than the control on day 5. The results of water activity on day 14 for coated starfruits were not significantly different (p > 0.05) from the control. The higher value of water activity maintained in all samples may support the growth of microbes.
Table 2.
Water activity (aw) of control and pectin (Pe) and maltodextrin (M) and 100 ppm, 200 ppm and 300 ppm sodium chloride (SC) coated starfruit samples during 14 days of storage.
| Aw | ||||||
|---|---|---|---|---|---|---|
| Day | D0 | D3 | D5 | D7 | D10 | D14 |
| Control | 0.994 ± 0.001bc | 0.993 ± 0.001b | 0.991 ± 0.002a | 0.989 ± 0.003a | 1.007 ± 0.001b | 1.008 ± 0.004a |
| Pe + M + 100 ppm SC | 0.996 ± 0.002c | 0.993 ± 0.001b | 0.987 ± 0.001a | 0.987 ± 0.002a | 1.001 ± 0.003a | 1.008 ± 0.001a |
| Pe + M + 200 ppm SC | 0.992 ± 0.002ab | 0.989 ± 0.002a | 0.987 ± 0.006a | 0.986 ± 0.001a | 1.004 ± 0.003ab | 1.008 ± 0.002a |
| Pe + M + 300 ppm SC | 0.989 ± 0.002a | 0.989 ± 0.002a | 0.987 ± 0.005a | 0.987 ± 0.003a | 1.004 ± 0.002ab | 1.009 ± 0.003a |
abcValues with different letter within the column are significantly different (P < 0.05).
3.1.3. Microbial growth
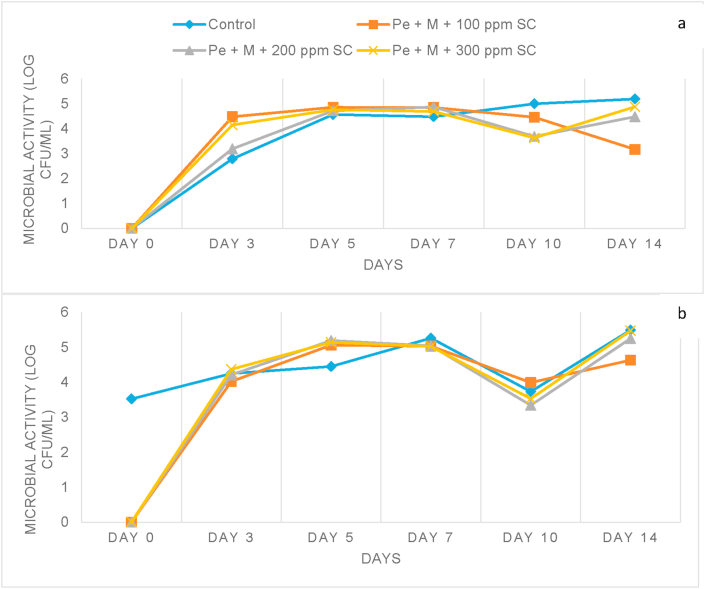
Figure 1 indicated that yeasts and molds were detected in the coated samples after 3 days of storage. Furthermore, the control showed the highest yeast and mold count, followed by Pe + M + 300 ppm SC and Pe + M + 200 ppm SC. The lowest yeast and mold count were observed in the starfruit coated with Pe + M + 100 ppm SC. The yeast and mold count for the control, Pe + M + 100 ppm SC, Pe + M + 200 ppm SC and Pe + M + 300 ppm SC were 5.49, 4.63, 5.25 and 5.47 log CFU/ml, respectively. For the detection of bacteria, the highest count was observed in the control, with a value of 5.19 log CFU/ml. Among the coated samples, Pe + M + 100 ppm SC showed the lowest value of bacteria, where the total bacteria count of Pe + M + 100 ppm SC, Pe + M + 200 ppm SC and Pe + M + 300 ppm SC were recorded to be 3.17, 4.48 and 4.88 log CFU/ml, respectively. The microbiological results indicated that coating of the starfruit, especially with Pe + M + 100 ppm SC, reduced the growth of yeast, mold and bacteria on the fruit, which may subsequently minimize its spoilage. It was noted that the lower concentration of SC (100 ppm) obtained better results compared to the other coated samples. The inclusion of sodium chloride may have caused changes in the morphology of the coating structure, especially when higher concentration of SC was applied. This may have resulted in disabling the function of the coating by disrupting the coating structure, thus allowing the penetration of air and moisture that subsequently supports the growth of microbes.
Figure 1.
Yeast and mold (a) and bacteria (b) count of control and pectin (Pe) and maltodextrin (M) and 100 ppm, 200 ppm and 300 ppm sodium chloride (SC) coated starfruit samples during 14 days of storage.
Overall, the high value of water activity in all the samples may have supported the growth of microorganisms on the fruits (Table 2). The coating process could minimize the growth of microbes as it can act as a gas barrier that causes the fruit to have a low oxygen permeability. This then causes the slowdown of respiration rate, making it difficult for microbes to grow (Sapper and Chiralt, 2018). Additionally, the presence of calcium chloride and higher acidic condition in the coated samples supported the inhibition of microbial growth. A similar result was reported by Mannozzi et al. (2017) and Mola et al. (2016) where yeast and bacteria count of their control sample was higher compared to the coated samples. Figure 2 showed the appearance of uncoated and coated starfruits during the 14 days of storage. These observations supported the microbial results observed in Figure 1 (a) and (b), which shown Pe + M + 100 ppm SC sample preserved the physical appearance at its best after 14 days of storage.
Figure 2.
The appearance of control and coated starfruits at different days of storage. Arrangement of starfruit from left to right represents sample of control and pectin (Pe) + maltodextrin (M) + 100 ppm, 200 ppm and 300 ppm sodium chloride (SC), respectively.
3.2. Quality Criterion Analysis
3.2.1. Determination of weight loss
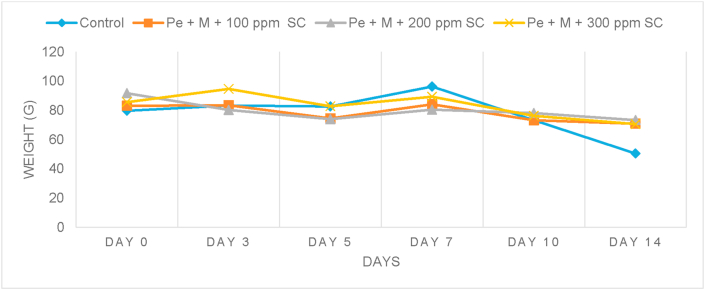
As shown in Figure 3, the weight of the starfruits decreased within 14 days of storage for all the samples. The control showed a significant reduction in weight, whereas the coated samples showed a slight decrease in weight. This indicated that the coating solution may have formed a compact network that caused the water permeability of the coating to decrease, thus reducing the weight loss of the starfruits (Mohamad Zaki et al., 2012). Among the coated samples, the starfruit coated with pectin-maltodextrin and 100 ppm sodium chloride (Pe + M + 100 ppm SC) showed a lower loss of weight compared to those coated with pectin-maltodextrin and 200 ppm sodium chloride (Pe + M + 200 ppm SC) and pectin-maltodextrin and 300 ppm sodium chloride (Pe + M + 300 ppm SC). This was probably due to the disruption in the coating integrity with the presence of a higher concentration of sodium chloride. The findings correlated with the microbial growth and textural results in Section 3.1.3 and 3.2.3, respectively.
Figure 3.
Weight of coated and uncoated starfruit stored at ambient temperature.
The loss of weight that occurs during fruit storage is due to the transfer of water from the fruit to its surroundings. The study demonstrated that edible coating could help in reducing the loss of weight in starfruits by acting as a moisture and gas barrier between a fruit and its surrounding (Rao et al., 2016). Studies conducted by Sarduni et al. (2020), Menezes and Athmaselvi (2016) and Gol et al. (2013) obtained similar result trends, where the loss of weight in the control was higher than the coated sample.
3.2.2. Determination of colour
Table 3 reported the results of lightness (L∗), redness-greenness (a∗), yellowness-blueness (b∗) and hue color (h⁰) of the control and the coated starfruits. From the results, lightness (L∗) of the control, which was 54.53, showed a higher value than the coated samples. The lightness of Pe + M + 200 ppm SC sample (52.80) was slightly lower than the control, followed by Pe + M + 100 ppm SC (48.36) and Pe + M + 300 ppm SC (43.34). The results showed that the coating caused a lower lightness in the fruits. The results for coating with 100 ppm and 300 ppm were significantly different (p < 0.05) from the control. The a∗ values (redness-greenness) of the starfruits did not vary significantly (p < 0.05). According to the CIELAB graph, the coated sample had a higher value of greenness than the control (Appendix). Starfruit coated with Pe + M + 100 ppm SC was significantly different (p < 0.05) from the control, which had a higher value of greenness. The b∗ values (yellowness-blueness) were not significantly different (p < 0.05) between the control and coated samples. Pe + M + 100 ppm SC showed a higher value of yellowness compared to the control and the other coated solutions. In contrast, the hue color of the control was lower than the coated samples. Pe + M + 300 ppm SC recorded the higher value of hue color with a reading of 117.22, followed by Pe + M + 100 ppm SC, Pe + M + 200 ppm SC and the control with readings of 116.47, 115.85 and 114.89, respectively. In general, the coatings may have slowed the ripening process of the fruits, which promotes them to have a more intense green hue color than the control. The result showed that the ripening process of the starfruit was greatly slowed when coated with Pe + M + 100 ppm SC. The result was also in line with the physical appearance and total soluble solid (TSS) results (Figure 2 and Section 3.2.4, respectively) that demonstrated the increasing trend of TSS values due to ripening that occurred throughout the storage period. A similar result was observed by Mannozzi et al. (2017), where blueberry coated with pectin and alginate showed lower lightness compared to control, while the hue color value of the coated blueberry was higher than control.
Table 3.
Color characteristics of control and pectin (Pe) and maltodextrin (M) and 100 ppm, 200 ppm and 300 ppm sodium chloride (SC) coated starfruit samples.
| Color | ||||
|---|---|---|---|---|
| L∗ | a∗ | b∗ | h⁰ | |
| Control | 54.53 ± 1.13c | -10.94 ± 0.09b | 23.59 ± 0.78a | 114.89 ± 0.89a |
| Pe + M + 100 ppm SC | 48.36 ± 3.61ab | -12.94 ± 1.56a | 26.08 ± 4.12a | 116.47 ± 0.99bc |
| Pe + M + 200 ppm SC | 52.80 ± 2.29bc | -12.13 ± 0.63ab | 25.03 ± 1.44a | 115.85 ± 0.22ab |
| Pe + M + 300 ppm SC | 43.34 ± 3.87a | -11.79 ± 0.26ab | 22.92 ± 0.68a | 117.22 ± 0.18c |
abcValues with different letter within the column are significantly different (P < 0.05).
3.2.3. Determination of texture
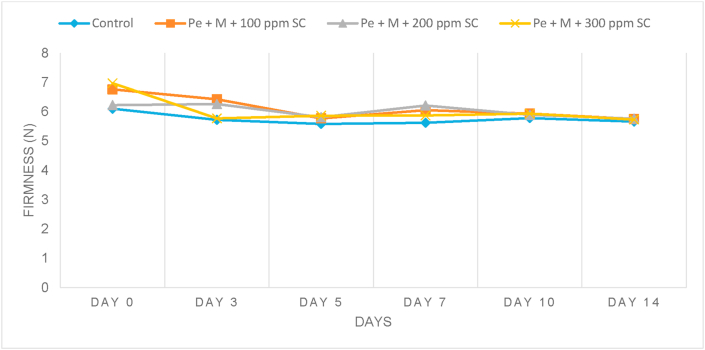
Texture is one of the important attributes that contributed to the acceptance of fruits by consumers. The texture of a fruit is dependent on the biochemical constituents, moisture content, cellular organelles, and composition of cell wall of the fruit. The change in texture during storage can deteriorate the appearance of a product and cause undesirable changes to its quality. The loss of firmness can cause a textural change in fruits (Nawab et al., 2017). Based on Figure 4, the values for firmness for all samples decreased from day 0 to day 14. However, the firmness for the coated samples was higher compared to the control in the early days of storage. The higher firmness of the coated samples was assumed to be due to the application of edible coating that may have increased the structural rigidity of the fruit's surface (Mannozzi et al., 2017). The results were in line with findings obtained by Menezes and Athmaselvi (2016) and Sarduni et al. (2020), where in one of the studies, a sapota fruit coated with pectin had higher firmness compared to control. The firmness for control and coated samples also showed a reduction throughout storage. On day 3, Pe + M + 100 ppm SC sample maintained the firmness of the fruit better compared to the other coated samples. This was supported by the weight loss results, where Pe + M + 100 ppm SC recorded a lower loss of weight than the other coated samples and maintaining the firmness of the sample.
Figure 4.
Firmness of control and pectin (Pe) and maltodextrin (M) and 100 ppm, 200 ppm and 300 ppm sodium chloride (SC) coated starfruit samples during 14 days of storage.
3.2.4. Measurement of total soluble solids (TSS)
The values of total soluble solids (TSS) for all the samples showed an increase (Table 4). The increment in TSS was due to metabolic processes that involve conversion of starch and acids into sugar (Mannozzi et al., 2017). The TSS value for control increased from 5.22 (day 0) to 6.00 ⁰Brix (day 14), while the Pe + M + 100 ppm SC sample increased from 5.20 (day 0) to 6.00 ⁰Brix (day 14). Similar results were observed for Pe + M + 200 ppm SC and Pe + M + 300 ppm SC samples, which increased from 5.07 to 5.47 ⁰Brix and 5.20 to 5.33 ⁰Brix, respectively. Among all the samples, control and Pe + M + 100 ppm SC samples recorded the highest value of TSS. The respiration rate and ethylene production were reduced with the application of edible coating as coating can modify the internal atmosphere by preventing the exchange of gases. The slow rate of respiration would then cause the TSS to have a lower value (Nawab et al., 2017). Similar results have been observed by Mannozzi et al. (2017) and Sarduni et al. (2020), where the values of TSS for control and coated samples increased during storage. The study involved the coating of blueberries with either pectin, sodium alginate or a sodium alginate/pectin combination. Menezes and Athmaselvi (2016) also found that TSS values for their control and coated sapota fruits increased throughout the storage. The sapota fruits coated with pectin had lower TSS compared to control.
Table 4.
Total soluble solids (TSS) of control and pectin (Pe) and maltodextrin (M) and 100 ppm, 200 ppm and 300 ppm sodium chloride (SC) coated starfruit samples during 14 days of storage.
| TSS (⁰Brix) | ||||||
|---|---|---|---|---|---|---|
| Day | D0 | D3 | D5 | D7 | D10 | D14 |
| Control | 5.22 ± 0.02b | 4.93 ± 0.12a | 5.00 ± 0.00a | 5.80 ± 0.00b | 6.00 ± 0.00a | 6.00 ± 0.00b |
| Pe + M + 100 ppm SC | 5.20 ± 0.00b | 4.83 ± 0.06a | 5.00 ± 0.00a | 6.00 ± 0.00c | 6.47 ± 0.12b | 6.00 ± 0.00b |
| Pe + M + 200 ppm SC | 5.07 ± 0.12a | 5.33 ± 0.12b | 5.00 ± 0.00a | 6.00 ± 0.00c | 6.00 ± 0.00a | 5.47 ± 0.12a |
| Pe + M + 300 ppm SC | 5.20 ± 0.00b | 6.00 ± 0.00c | 5.20 ± 0.00b | 5.20 ± 0.00a | 6.40 ± 0.00b | 5.33 ± 0.12a |
abcValues with different letter within the column are significantly different (P < 0.05).
3.2.5. Fourier transform infrared (FTIR)
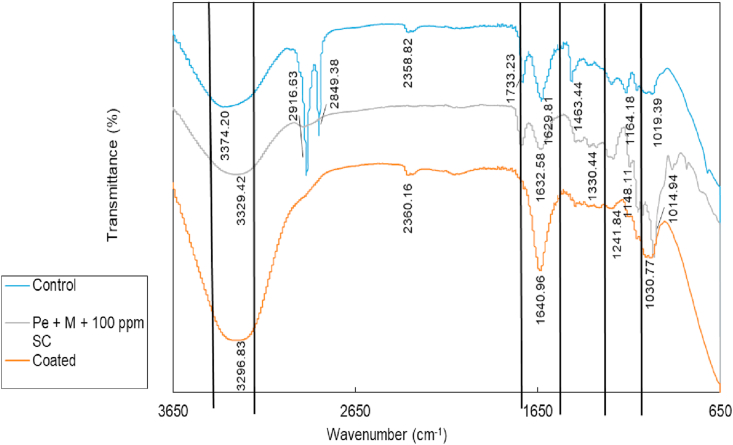
Fourier transform infrared (FTIR) was used to study the type of compound present in the coating solution and the starfruit. The FTIR spectra of pectin-maltodextrin and 100 ppm sodium chloride (Pe + M + 100 ppm SC), uncoated starfruit and coated starfruit were recorded in an IR spectrometer where the wave numbers ranged from 4000 to 400 cm−1. The FTIR spectra for Pe + M + 100 ppm SC, coated and uncoated starfruits are illustrated in Figure 5. There were three main peaks in the spectra that have the same group, which was in the range of 3400–3200 cm−1, 1650 cm−1 and 1300-1000 cm−1. The control that was in the range of 3400–3200 cm−1 had a reading of 3374.20 cm−1. The value indicated that it is in an aliphatic primary amine with medium intensity. Alternatively, the spectra for Pe + M + 100 ppm SC and coated starfruit were 3329.42 and 3296.83 cm−1, respectively. Both spectra belonged to a group of aliphatic primary amine, which was the same group as the control.
Figure 5.
FTIR spectra of pectin-maltodextrin and 100 ppm sodium chloride (Pe + M + 100 ppm SC), uncoated starfruit and coated starfruit.
However, the peaks in the range of 3000–2800 cm−1, with spectra of 2916.63 and 2849.38 cm−1, that were present in the control were absent in the coated sample. Both spectra belonged to a group of alkanes with medium intensity. The peak disappeared when the starfruit was coated with a coating solution. The absence of an alkane group on the coated starfruit was probably due to the interaction between the fruit and the coating solution. Additionally, spectra 2358.82 cm−1 that was present in the control was found to have shifted to 2360.16 cm−1 for the coated sample. Both spectra belonged to a group of carbon dioxide with strong intensity. The next peak value was in the range of 1650 cm−1. The spectra for control was 1629.81 cm−1. The spectra belonged to a group of alkenes with medium intensity. Both the Pe + M + 100 ppm SC with spectra 1632.58 cm−1 and the coated starfruit with spectra 1640.96 cm−1 were also from an alkene group with medium intensity. In contrast, spectra 1330.44 cm−1 that was present in the coating solution was absent in the coated starfruit. The spectra belonged to a group of aromatic amines with strong intensity. The application of coating caused the peak to disappear in the coated sample. The third peak value was in the range of 1300–1000 cm−1. The control had a medium intensity of amine group at spectra 1164.18 cm−1. Both the Pe + M + 100 ppm SC and coated starfruit also recorded a medium amine group with a spectra value of 1148.11 cm−1 and 1241.84 cm−1, respectively.
3.2.6. Sensory evaluation
Results for sensory analysis are reported in Table 5. Sensory analysis was conducted to determine whether the coating affected the overall acceptability of the fruits. The test was performed according to five attributes which were color, aroma, sweetness, texture, and overall acceptability based on a 5-point hedonic scale. The test was conducted using coated starfruits that were stored for 0 and 3 days. No significant difference (p > 0.05) in color was observed in all the samples for samples stored at day 0. The control ranked as the highest in all the attributes on day 0. The results for color showed that the panelists preferred the control, followed by Pe + M + 100 ppm SC and Pe + M + 200 ppm SC that scored the same values. The least preferable was the starfruits coated with Pe + M + 300 ppm SC. The results for color was not significantly different (p > 0.05) between the control and the coated samples. No significant difference (p > 0.05) was found in all the samples for aroma, texture, and overall acceptability, though the panelists preferred the control compared to coated starfruits. For sweetness, the control scored the highest value, followed by Pe + M + 300 ppm SC, Pe + M + 200 ppm SC and Pe + M + 100 ppm SC. The results for Pe + M + 100 ppm SC was significantly different (p < 0.05) from the control. This was probably due to the presence of sodium chloride. However, the panelists found all samples, whether coated or uncoated, acceptable.
Table 5.
Sensory evaluation of control and pectin (Pe) and maltodextrin (M) and 100 ppm, 200 ppm and 300 ppm sodium chloride (SC) coated starfruit sample at day 0 and day 3.
| Sensory evaluation (D0) | |||||
|---|---|---|---|---|---|
| Attribute | Color | Aroma | Sweetness | Texture | Overall acceptability |
| Control at day 0 | 3.40 ± 0.94a | 3.30 ± 1.08a | 3.50 ± 1.19b | 3.90 ± 1.02a | 3.75 ± 0.85a |
| Control at day 3 | 3.00 ± 0.92a | 2.70 ± 0.73a | 3.00 ± 1.07a | 3.30 ± 1.03a | 3.10 ± 0.72a |
| Pe + M + 100 ppm SC at day 0 | 3.15 ± 0.99a | 2.95 ± 0.94a | 2.55 ± 1.15a | 3.40 ± 1.09a | 3.15 ± 0.99a |
| Pe + M + 100 ppm SC at day 3 | 3.65 ± 1.23ab | 2.95 ± 0.83a | 2.95 ± 0.99a | 3.00 ± 0.97a | 3.25 ± 0.85a |
| Pe + M + 200 ppm SC at day 0 | 3.15 ± 1.09a | 2.70 ± 1.03a | 2.85 ± 1.23ab | 3.30 ± 0.92a | 3.15 ± 0.67a |
| Pe + M + 200 ppm SC at day 3 | 3.35 ± 0.75ab | 3.15 ± 0.75a | 2.75 ± 0.91a | 3.15 ± 1.09a | 3.10 ± 0.72a |
| Pe + M + 300 ppm SC at day 0 | 2.90 ± 0.85a | 3.00 ± 1.03a | 3.05 ± 1.09ab | 3.60 ± 1.14a | 3.40 ± 0.99a |
| Pe + M + 300 ppm SC at day 3 | 3.75 ± 1.16b | 3.15 ± 0.81a | 2.95 ± 1.05a | 3.19 ± 1.01a | 3.15 ± 0.88a |
abValues with different letter within the column are significantly different (P < 0.05).
For the sensory evaluation of starfruit after 3 days of storage, there was no significant difference (p > 0.05) in aroma between the coated samples and the control. The control was recorded as the least preferable among the panelists. The panelists preferred the coated starfruit than control in terms of color. The most preferable was the starfruit coated with Pe + M + 300 ppm SC. The value of color for Pe + M + 300 ppm SC was significantly different (p < 0.05) from the control. This was probably due to the ability of coating in preserving the color of the starfruits even after 3 days of storage (Sharma et al., 2019). Based on the data of sweetness and texture, the results were not significantly different (p > 0.05) between the control and the coated samples. Panelists preferred the control compared to the coated starfruits for both attributes. Overall, panelists preferred the starfruits coated with Pe + M + 100 ppm SC, followed by Pe + M + 300 ppm SC, while both the Pe + M + 200 ppm SC and the control were the least preferable. This finding was in line with the TSS, textural and physical appearance results, which demonstrated the coated samples obtained higher values compare to control especially for Pe + M + 100 ppm SC sample. Similar trend of results were obtained in a study where starfruits were coated with alginate, chitosan and gum Arabic. In the study, the panelists preferred the coated starfruits more than control (Gol et al., 2013).
4. Conclusion
Three samples of synergistic coating solution containing 1% pectin-maltodextrin at ratio 60:40 and sodium chloride (100, 200 and 300 ppm) were prepared as coatings for the starfruits. The effects of a combination coating with sodium chloride on fruits were evaluated. The results showed that all the coated starfruits have lower weight loss compared to control. The pH of a Pe + M + 100 ppm SC coated starfruit was the lowest compared to the other samples. The results of TSS for the control was slightly higher than the coated samples. The water activity for the control and coated samples also showed a slight increase throughout the storage. Based on the results of color, Pe + M + 100 ppm SC has lower lightness, higher values of greenness and yellowness, as well as a more intense green hue color from the control. As the firmness of the control was lower than the coated samples, it was deduced that the coating may have delayed the reduction of firmness in the starfruit. For the microbial growth analysis, the starfruit coated with Pe + M + 100 ppm SC had a lower count of yeast, mold and bacteria throughout the 14 days of storage. The FTIR spectra change occurred in the functional group obtained before and after the coating process. For the sensory evaluation, there were no significant differences (p > 0.05) between the uncoated and coated samples on day 0 and day 3. This newly developed synergistic coating solution provides an alternative approach in extending the shelf life and maintaining the safety and quality criterion of a starfruit up to 14 days at ambient storage.
Declarations
Author contribution statement
Nurul Izzati Mohd Suhaimi: Performed the experiments; Analyzed and interpreted the data.
Anis Alysha Mat Ropi: Analyzed and interpreted the data; Wrote the paper.
Shahrulzaman Shaharuddin: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This research was supported by Universiti Kuala Lumpur Branch Campus Malaysian Institute of Chemical and Bioengineering Technology (UniKL MICET), Malaysia especially Section of Food Engineering Technology.
References
- Barth M., Hankinson T.R., Zhuang H., Breidt F. Microbiological spoilage of fruits and vegetables. In: Sperber W.H., Doyle M.P., editors. Springer Science+Business Media; New York: 2019. (Compendium of the microbiological spoilage of foods and beverages, food microbiology and food safety). [Google Scholar]
- Baraiya N.S., Ramana Rao T.V., Thakkar V.R. Enhancement of storability and quality maintenance of carambola (Averrhoa carambola L.) fruit by using composite edible coating. J. Food Sci. Technol. 2013;69(3):195–205. [Google Scholar]
- Barrett D.M., Beaulieu J.C., Shewfelt R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010;50(5):369–389. doi: 10.1080/10408391003626322. [DOI] [PubMed] [Google Scholar]
- Batt C.A. Elsevier; 2016. Microbial Food Spoilage. Reference Module in Food Science. [Google Scholar]
- Berk Z. Food Process Engineering and Technology. third ed. 2018. Physical properties of food materials; pp. 1–29. [Google Scholar]
- Clayton K. 2012. Purdue Extension Food Entrepreneurship Series Department of Food Science the Role of Water Activity and Acidity in Preservation.www.foodsci.purdue.edu Retrieved from. [Google Scholar]
- Frank S.M., Webster J., McKenzie B., Geldsetzer P., Manne-Goehler J., Andall-Brereton G., Houehanou C., Houinato D., Gurung M.S., Bicaba B.W., McClure R.W., Supiyev A., Zhumadilov Z., Stokes A., Labadarios D., Sibai A.M., Norov B., Aryal K.K., Karki K.B., Jaacks L.M. Consumption of fruits and vegetables among individuals 15 years and older in 28 low- and middle-income countries. J. Nutr. 2019;149(7):1252–1259. doi: 10.1093/jn/nxz040. [DOI] [PubMed] [Google Scholar]
- Ghee Lim Kean. Med. J. Malaysia. 2019;74(Aug):701–719. 2121/01/2013 (031329) MITA(P) 124/1/91. [Google Scholar]
- Gol N.B., Chaudhari M.L., Rao T.V.R. Effect of edible coatings on quality and shelf life of carambola (Averrhoa carambola L.) fruit during storage. J. Food Sci. Technol. 2013;52:78–91. [Google Scholar]
- Guan W., Fan X. Combination of sodium chlorite and calcium propionate reduces enzymatic browning and microbial population of fresh-cut “Granny Smith” apples. J. Food Sci. 2010;75(2):M72–M77. doi: 10.1111/j.1750-3841.2009.01470.x. [DOI] [PubMed] [Google Scholar]
- Hanani M.Z.N., Zahrah M.S.H., Zaibunnisa A.H. Effect of chitosan-palm stearin edible coating on the post harvest life of star fruits (Averrhoa carambola L.) stored at room temperature. Int. Food Res. J. 2012;19(4):1433–1438. [Google Scholar]
- Kyriacou M.C., Rouphael Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018;234(September):463–469. [Google Scholar]
- Mannozzi C., Cecchini J.P., Tylewicz U., Siroli L., Patrignani F., Lanciotti R., Romani S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2017;85:440–444. [Google Scholar]
- Menezes J., Athmaselvi K.A. Study on effect of pectin based edible coating on the shelf life of Sapota fruits. Biosci. Biotechnol. Res. Asia. 2016;13(2):1195–1199. [Google Scholar]
- Moalemiyan M., Ramaswamy H.S., Maftoonazad N. Pectin-based edible coating for shelf-life extension of ataulfo mango. J. Food Process. Eng. 2011:1–29. [Google Scholar]
- Mohamad Zaki N.H., Mohamed Som H.Z., Abdul Haiyee Z. Application of palm stearin- chitosan edible coating on starfruits (averrhoa carambola L.) Malays. J. Anal. Sci. 2012;16(3):325–334. [Google Scholar]
- Mola S., Uthairatanakij A., Srilaong V., Aiamla-or S., Jitareerat P. Impacts of sodium chlorite combined with calcium chloride, and calcium ascorbate on microbial population, browning, and quality of fresh-cut rose apple. Agric. Nat. Res. 2016;50(5):331–337. [Google Scholar]
- Nawab A., Alam F., Hasnain A. Mango kernel starch as a novel edible coating for enhancing shelf- life of tomato ( Solanum lycopersicum ) fruit. Int. J. Biol. Macromol. 2017;103:581–586. doi: 10.1016/j.ijbiomac.2017.05.057. [DOI] [PubMed] [Google Scholar]
- Nurhadi B., Roos Y.H., Maidannyk V. Physical properties of maltodextrin DE 10: water sorption, water plasticization and enthalpy relaxation. J. Food Eng. 2015;19:1370–1380. [Google Scholar]
- Paladugu K., Gunasekaran K. Development of gum Arabic edible coating formulation through nanotechnological approaches and their effect on physico-chemical change in tomato (solanum lycopersicum l) fruit during storage. Int. J. Agric. Sci. 2017;9(8):3866–3870. [Google Scholar]
- Petruzzi L., Corbo M.R., Sinigaglia M., Bevilacqua A. The Microbiological Quality of Food: Foodborne Spoilers. Elsevier Ltd; 2016. Microbial spoilage of foods: fundamentals; pp. 212–215. [Google Scholar]
- Rao T.V.R., Baraiya N.S., Vyas P.B., Patel D.M. Composite coating of alginate-olive oil enriched with antioxidants enhances postharvest quality and shelf life of Ber fruit. Ziziphus mauritiana Lamk . Var . Gola. 2016;53(January):748–756. doi: 10.1007/s13197-015-2045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapper M., Chiralt A. Starch-based coatings for preservation of fruits and vegetables. Coatings. 2018;8(5):152. [Google Scholar]
- Sandulachi E. Water activity concept and its role in food preservation. 2016;15:44–47. [Google Scholar]
- Salunkhe D.K., Wu M.T., Rahman A.R. Developments in Technology of storage and handling of fresh fruits and vegetables. CRC Crit. Rev. Food Technol. 1974;5(1):15–54. [Google Scholar]
- Sarduni F.F.F., Hanafi S.N., Ibrahim S.F., Shaharuddin S. Effect of pectin production of healthy fried potato strips. Int. Food Res. J. 2020;22(4):1557–1563. [Google Scholar]
- Sharma P., Shehin V.P., Kaur N., Vyas P. Application of edible coatings on fresh and minimally processed vegetables: a review. Int. J. Veg. Sci. 2019;25(3):295–314. [Google Scholar]
- Xiao Z., Luo Y., Luo Y., Wang Q. Combined effects of sodium chlorite dip treatment and chitosan coatings on the quality of fresh-cut d’Anjou pears. Postharvest Biol. Technol. 2011;62(3):319–326. [Google Scholar]
- Zagory D., Kader A.A. 1989. Quality Maintenance in Fresh Fruits and Vegetables by Controlled Atmospheres; pp. 174–188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.