Abstract
Purpose
To evaluate the relationship of retinal layer thickness with age and age-related macular degeneration (AMD) in the Carotenoids in Age-Related Eye Disease Study 2.
Methods
Total retinal thickness within the macular area, and individual layer thickness was determined for CAREDS2 participants (n = 906 eyes, 473 women) from the Women's Health Initiative using Heidelberg optical coherence tomography (OCT). Mean measurements within the OCT grid were compared across age tertiles (69–78, 78–83, and 83–101 years) and AMD outcomes.
Results
Mean retinal thickness in the central circle, inner ring, and outer ring were 277 ± 34 µm, 326 ± 20 µm, and 282 ± 15 µm, respectively. Thickness did not vary by age in the central circle, but decreased with age in the inner and outer circles (P ≤ 0.004). Specifically, ganglion cell (GCL), inner plexiform, and outer nuclear (ONL) layer thickness decreased with age (P ≤ 0.003). Age-adjusted retinal thickness in all three circles did not vary by AMD outcomes (486 without AMD and 413 with AMD). However, individual layers showed changes with GCL and photoreceptor thinning and retinal pigment epithelial thicknening in eyes with late AMD. After controlling for age and AMD, higher ONL thickness was associated with better visual acuity.
Conclusions
In this cohort of older women, a decrease in perifoveal thickness was associated with increasing age, particularly in the inner retinal layers. Variabilty in thickness in AMD eyes was primarily due to outer retinal layers. Among all retinal layers, the ONL plays an important role in preserving visual acuity.
Translational Relevance
The study provides a deeper understanding of age related changes to the retinal layers and their effect on visual loss.
Keywords: age-related macular degeneration; carotenoids in age-related eye disease study 2; macular thickness; optical coherence tomography, women's health initiative, postmenopausal, women
Introduction
Optical coherence tomography (OCT) provides noninvasive in vivo cross-sectional imaging of the retina. Spectral domain OCT (SD-OCT) devices permit the demarcation of individual retinal layers at higher acquisition speeds and image resolution from previous devices.1 In addition, SD-OCT enables automated segmentation and quantification of individual retinal layers through the application of various software and segmentation algorithms. Previous studies have demonstrated that SD-OCT segmentation software produces reproducible thickness measurements.2
SD-OCT is used extensively in clinical practice and clinical trials to measure the thickness of the retina and identify anatomic changes that occur with diseases, including those related to aging. For example, in age-related macular degeneration (AMD), SD-OCT segmentation has been used to monitor drusen, choroidal neovascularization, and geographic atrophy.3 SD-OCT segmentation is also used to identify thinning of the nerve fiber layer in neurodegenerative diseases, such as Alzheimer's disease.4 The description of SD-OCT changes in diseases of aging have been constrained by small sample sizes of populations younger than 75 years and an overrepresentation of severe disease. In addition, normative thickness data from proprietary SD-OCT manufacturers are not publicly available.
Previous studies that have used SD-OCT segmentation to evaluate the distribution of retinal layer thickness measurements across population subgroups were vital to understanding the epidemiological associations and pathophysiological mechanisms of disease, as well as to define normal ranges.5–12 However, these studies have been limited to population studies without retinal diseases. We used data from the Carotenoids in Age-Related Eye Disease Study 2 (CAREDS2), a unique, and well-characterized cohort of women aged 69 to 101 years at the time of their participation in 2016 to 2019, to provide the macular thickness measurements and to study the relationship with age and AMD. We also explored relationships between the thickness of individual retinal layers and best-corrected visual acuity. CAREDS2 is an ancillary study of the Women's Health Initiative Observation Study (WHI-OS).
Methods
Participants
The details of the CAREDS study population has been previously published.13 In summary, the WHI-OS, a prospective cohort study at 40 sites throughout the United States investigating the most common causes of morbidity and mortality among postmenopausal women aged 50 to 79 years (n = 3143).14 Eligibility criteria included having high or low dietary lutein plus zeaxanthin intake levels (>78th or <28th percentiles) at WHI enrollment (1994–1998). Women from three WHI-OS study sites were invited to participate in CAREDS: the University of Wisconsin (Madison, WI), the University of Iowa (Iowa City, IA), and the Kaiser Center for Health Research (Portland, OR), were recruited for CAREDS baseline (2001–2004) (n = 2005). Of the CAREDS cohort, 685 participated in the CAREDS2 follow-up (2016–2019), of which 487 participated in study visits in-person. The analysis dataset for this project includes 473 women who completed SD-OCT scans. The age of the participants at the time of OCT assessment in CAREDS2 ranged from 69 to 101 years. The study was approved by the institutional review boards associated with each center. All participants provided written informed consent, and the study adhered to the tenets of the Declaration of Helsinki.
SD-OCT Imaging
SD-OCT scans were acquired by certified photographers with a Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany) OCT machine following the CAREDS2 reading center (Fundus Photograph Reading Center, University of Wisconsin–Madison) approved protocol. Volume scans centered on the macula were obtained from both eyes for all study participants. The macular scan protocol includes a 20° × 20° volume scan at high speed with 97 B scans, interscan distance of 60 µm and an image averaging of five frames.
Retinal Layer Segmentation
Retinal layers were automatically segmented using Heidelberg Spectralis software (version 1.9.13.0). Layers were defined using the boundaries generated by the software. The layers, including internal limiting membrane (ILM), retinal nerve fiber layer, ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer, outer plexiform layer, outer nuclear layer (ONL), photoreceptor layer (PR), and retinal pigment epithelium (RPE), are shown in Figure 1. The boundaries of the total retina were defined as ILM to BM. The total retina was divided into the inner and outer retinal layers by the software. The inner retinal layers were defined as ILM to external limiting membrane and the outer retinal layers as external limiting membrane to BM, respectively.
Figure 1.
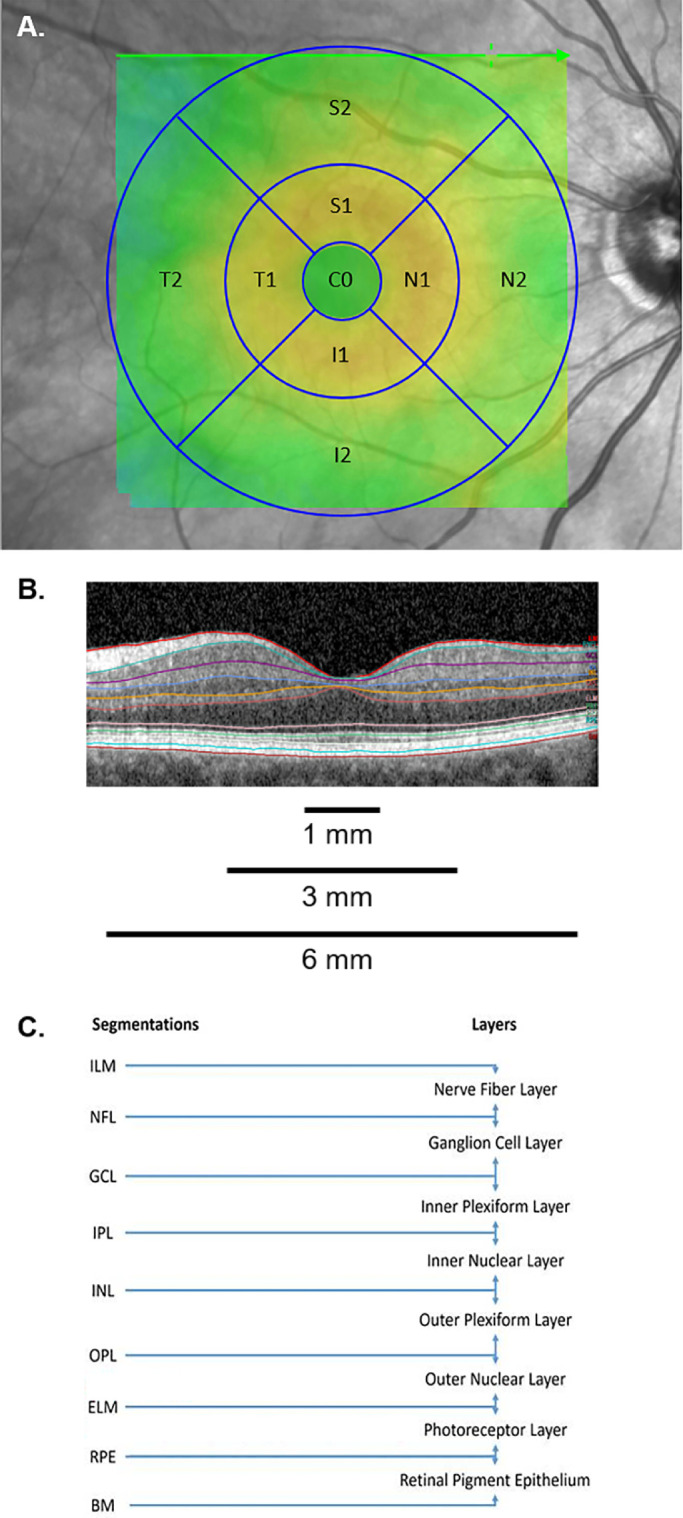
Representative SD-OCT scan and the nine sectors of the ETDRS grid with segmentation performed and retinal layers evaluated. (A) The nine sectors of the ETDRS grid, including the central circle cell (C0), inner nasal cell (N1), outer nasal cell (N2), inner superior cell (S1), outer superior cell (S2), inner temporal cell (T1), outer temporal cell (T2), inner inferior cell (I1), and outer inferior cell (I2). The nine sectors of the ETDRS grid were combined to form the central circle (1 mm diameter), inner ring (3 mm diameter), and outer ring (6 mm diameter). (B) Representative SD-OCT B-scan with default segmentation through the fovea, including the ILM (red), RNFL (teal), GCL (purple), IPL (blue), INL (orange), OPL (maroon), ONL (yellow), ELM (pink), PR (green), RPE (light blue), and BM (red). (C) Depiction of segmentation performed, and retinal layers evaluated.
The segmentation was reviewed throughout the entire Early Treatment in Diabetic Retinopathy Study (ETDRS) grid and segmentation errors were manually edited within the Heidelberg Spectralis software in all scans as needed. Segmentation review and editing was performed by masked, study certified graders at the CAREDS2 reading center. A total of 946 SD-OCT images from 473 CAREDS2 study participants were available for segmentation. ETDRS grid centration was assessed and scans with additional artifacts, such as z-offset (n = 3) and B-scan inversion (n = 2) were excluded. Additional SD-OCT scans were excluded because of grossly inaccurate segmentation throughout all retinal layers beyond that correctable by manual editing (n = 2). The remainder of excluded SD-OCT scans were due to confounding pathology, including macular hole (n = 8), significant intraretinal or subretinal fluid (n = 4), and severe macular degeneration (n = 21) prohibiting the identification of individual retinal layers.
Thickness maps were generated within Heidelberg Spectralis software, providing mean macular thickness (µm) and volume (mm3) measurements for the nine sectors of the ETDRS grid, which were combined to form three concentric circles, including the central circle (1 mm diameter), inner ring (3 mm diameter), and outer ring (6 mm diameter) as displayed in Figure 1.
AMD Assessment
AMD presence and severity at CAREDS baseline (2001–2004) was evaluated using 30° stereoscopic fundus photographs and at CAREDS2 follow-up (2016-2019) using 30° stereoscopic digital images. Of the 487 CAREDS2 participants, AMD status was determined from fundus photographs in 470 (96.5%) participants. AMD status of an additional five participants was determined from provider reports or medical records. Three field stereoscopic color photographs were evaluated for presence and severity of AMD using the Age-related Eye Disease Study (AREDS) severity scale.15 Based on CAREDS2 protocol defined outcomes, we grouped the original AREDS 12-step AMD scale into early AMD (AREDS levels 1–5), intermediate AMD (AREDS levels 6–8) and late AMD (AREDS levels 9–11b). Late AMD was further classified as geographic atrophy and neovascular AMD.
Best Corrected Visual Acuity
Best corrected visual acuity (BCVA) was measured using the standardized ETDRS protocol modified for the AREDS trials.16
Statistical Analysis
Macular thickness and volume measurements of the central circle (1 mm diameter), inner ring (3 mm diameter), and outer ring (6 mm diameter) were expressed as means ± standard deviation (SD) or least squares mean ± standard error (SE). Age was categorized into tertiles, and a P value for continuous trend over years was computed. AMD outcomes were expressed as a categorical variable, including no AMD, early AMD, intermediate AMD, and late AMD. We investigated the association between mean macular thickness and age tertile, as well as mean macular thickness and AMD outcomes, adjusted for age. Generalized estimating equations were used, which enabled the use of scans from both study eyes of each participant. Empirical standard error estimates were used for generalized estimating equations parameter estimation considering within person correlation. A two-tailed P value < 0.05 was considered significant. We also examined the age-adjusted associations between BCVA and individual layer thickness in the central circle, including sensitivity analyses, excluding participants with cataract and late AMD. Statistical analysis was performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Macular Thickness by Age
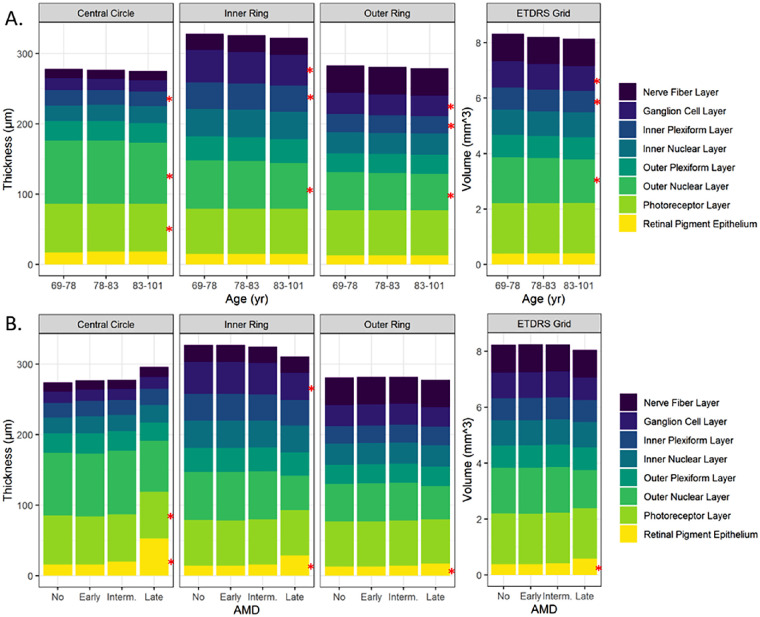
Overall, 946 SD-OCT scans of both eyes of 473 CAREDS2 participants were available for the current analysis. After excluding 40 scans, 906 scans were evaluated as presented in Table 1. Mean (SD) macular thickness overall, and in individual retinal layers are described by age tertile, within each of the nine ETDRS subfields (Fig. 2A and Table 2). In the central circle, we did not observe significant variations in the total retinal thickness by age. However, in the analysis of separate layers, the IPL, ONL, and PR layers were significantly thinner with age. In the more peripheral inner and outer rings, the overall thickness of the retina and some specific layers (GCL, IPL, and ONL) also significantly decreased with age.
Table 1.
Mean Macular Thickness and Volume by Retinal Layer
| Retinal Layers | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inner | Outer | Retinal | |||||||||
| Inner | Outer | Nerve | Ganglion | Plexi- | Inner | Plexi- | Outer | Photo- | Pigment | ||
| Total | Retinal | Retinal | Fiber | Cell | form | Nuclear | form | Nuclear | receptor | Epithe- | |
| Retina | Layers | Layers | Layer | Layer | Layer | Layer | Layer | Layer | Layer | lium | |
| Macular thickness (µm) | |||||||||||
| Central Circle | 277 (34) | 188 (26) | 86 (14) | 13 (3) | 16 (8) | 21 (4) | 23 (7) | 28 (6) | 89 (13) | 68 (5) | 18 (14) |
| Inner Ring | |||||||||||
| Nasal | 333 (23) | 252 (20) | 80 (5) | 23 (4) | 46 (7) | 39 (4) | 40 (4) | 35 (7) | 70 (12) | 65 (3) | 15 (4) |
| Temporal | 318 (21) | 237 (19) | 80 (8) | 19 (2) | 42 (8) | 38 (4) | 37 (4) | 31 (4) | 72 (9) | 65 (3) | 15 (7) |
| Superior | 329 (22) | 248 (19) | 79 (4) | 27 (5) | 47 (6) | 37 (4) | 39 (4) | 35 (8) | 65 (12) | 64 (2) | 15 (4) |
| Inferior | 325 (21) | 246 (20) | 79 (7) | 26 (4) | 46 (8) | 37 (4) | 39 (4) | 35 (8) | 65 (12) | 64 (2) | 15 (6) |
| Average | 326 (20) | 246 (19) | 80 (5) | 24 (3) | 45 (7) | 38 (4) | 39 (3) | 34 (5) | 68 (9) | 64 (2) | 15 (5) |
| Outer Ring | |||||||||||
| Nasal | 299 (20) | 221 (19) | 77 (3) | 53 (10) | 32 (4) | 25 (3) | 31 (3) | 28 (3) | 53 (9) | 64 (2) | 13 (2) |
| Temporal | 270 (15) | 192 (15) | 77 (3) | 22 (3) | 30 (8) | 28 (3) | 30 (3) | 27 (2) | 55 (7) | 64 (2) | 13 (2) |
| Superior | 283 (16) | 204 (15) | 78 (3) | 41 (8) | 29 (4) | 24 (3) | 30 (4) | 26 (3) | 56 (7) | 65 (2) | 14 (3) |
| Inferior | 274 (17) | 197 (16) | 76 (3) | 41 (9) | 28 (4) | 24 (3) | 30 (3) | 26 (3) | 49 (6) | 63 (2) | 13 (1) |
| Average | 282 (15) | 204 (15) | 77 (3) | 39 (6) | 30 (4) | 25 (3) | 30 (2) | 27 (2) | 53 (6) | 64 (2) | 13 (2) |
| Macular volume (mm3) | |||||||||||
| ETDRS grid | 8.2 (0.5) | 6.0 (0.4) | 2.2 (0.1) | 1.0 (0.2) | 0.9 (0.1) | 0.8 (0.1) | 0.9 (0.1) | 0.8 (0.1) | 1.6 (0.2) | 1.8 (0.1) | 0.4 (0.1) |
Numbers are presented as mean (SD).
Figure 2.
Mean macular thickness and volume by retinal layer. (A) Thickness and volume by age tertile (years). (B) Thickness and volume by AMD outcome, age adjusted. * indicates P < 0.05.
Table 2.
Mean Macular Thickness and Volume by Age Group
| Age Groups, Tertile (Years) | ||||
|---|---|---|---|---|
| Tertile 1 69–78 | Tertile 2 78–83 | Tertile 3 83–101 | P Value | |
| Total retina, number of eyes | 330 | 321 | 255 | |
| Macular thickness (µm) | ||||
| Central circle | 278 (33) | 277 (28) | 277 (42) | 0.89 |
| Inner ring | 330 (20) | 326 (18) | 323 (23) | 0.001 |
| Outer ring | 284 (16) | 280 (15) | 280 (15) | 0.004 |
| Macular volume (mm3) | ||||
| ETDRS grid | 8.3 (0.5) | 8.2 (0.4) | 8.2 (0.5) | 0.002 |
| Inner retinal layers, number of eyes | 312 | 307 | 236 | |
| Macular thickness (µm) | ||||
| Central circle | 190 (28) | 188 (23) | 187 (26) | 0.25 |
| Inner ring | 249 (19) | 245 (17) | 241 (19) | <0.0001 |
| Outer ring | 206 (15) | 203 (14) | 201 (14) | 0.001 |
| Macular volume (mm3) | ||||
| ETDRS grid | 6.1 (0.4) | 6.0 (0.4) | 5.9 (0.4) | <0.0001 |
| Outer Retinal Layers, number of eyes | 330 | 321 | 255 | |
| Macular thickness (µm) | ||||
| Central circle | 86 (11) | 86 (12) | 86 (20) | 0.40 |
| Inner ring | 80 (5) | 79 (3) | 80 (7) | 0.98 |
| Outer ring | 77 (3) | 77 (2) | 78 (3) | 0.10 |
| Macular volume (mm3) | ||||
| ETDRS grid | 2.2 (0.1) | 2.2 (0.1) | 2.2 (0.1) | 0.57 |
| Nerve fiber layer, number of eyes | 319 | 309 | 241 | |
| Macular thickness (µm) | ||||
| Central circle | 13 (3) | 13 (3) | 13 (2) | 0.32 |
| Inner ring | 23 (3) | 24 (3) | 24 (3) | 0.21 |
| Outer ring | 39 (6) | 39 (6) | 39 (7) | 0.83 |
| Macular volume (mm3) | ||||
| ETDRS grid | 1.0 (0.1) | 1.0 (0.2) | 1.0 (0.2) | 0.93 |
| Ganglion cell layer, number of eyes | 317 | 309 | 240 | |
| Macular thickness (µm) | ||||
| Central circle | 17 (13) | 16 (4) | 16 (4) | 0.08 |
| Inner ring | 46 (7) | 45 (6) | 44 (7) | <0.0001 |
| Outer ring | 30 (4) | 30 (4) | 29 (4) | <0.0001 |
| Macular volume (mm3) | ||||
| ETDRS grid | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | <0.0001 |
| Inner plexiform layer, number of eyes | 307 | 308 | 230 | |
| Macular thickness (µm) | ||||
| Central circle | 22 (4) | 21 (4) | 21 (4) | 0.04 |
| Inner ring | 38 (4) | 37 (4) | 37 (4) | <0.0001 |
| Outer ring | 26 (2) | 25 (3) | 25 (2) | 0.006 |
| Macular volume (mm3) | ||||
| ETDRS grid | 0.80 (0.07) | 0.78 (0.07) | 0.78 (0.07) | 0.0004 |
| Inner nuclear layer, number of eyes | 307 | 308 | 230 | |
| Macular thickness (µm) | ||||
| Central circle | 22 (7) | 23 (6) | 24 (7) | 0.10 |
| Inner ring | 39 (4) | 39 (3) | 39 (3) | 0.03 |
| Outer ring | 30 (3) | 30 (2) | 30 (2) | 0.13 |
| Macular volume (mm3) | ||||
| ETDRS grid | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 0.08 |
| Outer plexiform layer, number of eyes | 311 | 311 | 234 | |
| Macular thickness (µm) | ||||
| Central circle | 28 (6) | 28 (7) | 28 (6) | 0.61 |
| Inner ring | 34 (4) | 34 (5) | 34 (4) | 0.89 |
| Outer ring | 27 (2) | 27 (2) | 27 (2) | 0.16 |
| Macular volume (mm3) | ||||
| ETDRS grid | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.1) | 0.27 |
| Outer nuclear layer, number of eyes | 311 | 311 | 234 | |
| Macular thickness (µm) | ||||
| Central circle | 90 (12) | 90 (12) | 87 (15) | 0.01 |
| Inner ring | 69 (8) | 68 (9) | 65 (10) | 0.0001 |
| Outer ring | 54 (6) | 53 (7) | 52 (6) | 0.003 |
| Macular volume (mm3) | ||||
| ETDRS grid | 2 (0.2) | 2 (0.2) | 2 (0.2) | 0.001 |
| Photoreceptor layer, number of eyes | 330 | 321 | 255 | |
| Macular thickness (µm) | ||||
| Central circle | 69 (5) | 68 (5) | 68 (4) | 0.004 |
| Inner ring | 64 (2) | 64 (2) | 64 (2) | 0.65 |
| Outer ring | 64 (2) | 64 (2) | 64 (2) | 0.34 |
| Macular volume (mm3) | ||||
| ETDRS grid | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 0.83 |
| Retinal pigment epithelium, number of eyes | 330 | 321 | 255 | |
| Macular thickness (µm) | ||||
| Central circle | 17 (10) | 18 (11) | 18 (20) | 0.59 |
| Inner ring | 15 (5) | 15 (3) | 15 (6) | 0.77 |
| Outer ring | 13 (2) | 13 (1) | 13 (2) | 0.08 |
| Macular volume (mm3) | ||||
| ETDRS grid | 0.4 (0.1) | 0.4 (0.04) | 0.4 (0.1) | 0.25 |
Numbers are presented as mean (SD).
Macular Thickness by AMD Presence and Severity
The thickness of the total retina was similar in eyes with and without AMD in the central circle, as well as inner and outer rings (Fig. 2B and Table 3). Eyes with AMD were further classified into early AMD (n = 205), intermediate AMD (n = 185), and late AMD (n = 23). Of the 23 eyes with late AMD, five (22%) had geographic atrophy (both central and noncentral) and 18 (78%) had neovascular AMD. The thickness of the total retina did not vary by AMD severity in the central circle, as well as inner and outer rings (P = 0.35, P = 0.26, and P = 0.51). The thickness of the inner retinal layers was also similar across AMD outcomes in the central circle and outer ring (P = 0.11 and P = 0.25) but decreased significantly with increasing AMD severity in the inner ring (P = 0.003). The thickness of the outer retinal layers decreased significantly with increasing AMD severity in the central circle, as well as inner and outer ring (P = 0.009, P = 0.002, and P = 0.009). In the analysis of the individual retinal layers, the thickness of the RPE increased with AMD severity within the central circle, as well as inner and outer rings (P = 0.001, P = 0.0005, and P = 0.002). These results were likely due to 78% of late AMD eyes being neovascular compared to 22% having geographic atrophy. The PR layer in the central circle, but not the inner and outer rings, was thinner in eyes with AMD compared to those without AMD (P = 0.0008). The thickness of the GCL decreased by AMD severity in the inner ring (P = 0.05).
Table 3.
Mean Macular Thickness and Volume by AMD Outcome, Age Adjusted
| AMD | |||||
|---|---|---|---|---|---|
| No AMD | With AMD | P Value | |||
| Total retina, number of eyes | 486 | 413 | |||
| Macular thickness (µm) | |||||
| Central circle | 277 (2) | 278 (2) | 0.60 | ||
| Inner ring | 327 (1) | 326 (1) | 0.34 | ||
| Outer ring | 282 (1) | 281 (1) | 0.35 | ||
| Macular volume (mm3) | 0.27 | ||||
| ETDRS grid | 8.2 (0.02) | 8.2 (0.02) | |||
| AMD | |||||
| No AMD | Early AMD | Intermediate AMD | Late AMD | P Value | |
| Total retina, number of eyes | 486 | 205 | 185 | 23 | |
| Macular thickness (µm) | |||||
| Central circle | 277 (2) | 276 (2) | 279 (3) | 285 (17) | 0.35 |
| Inner ring | 327 (1) | 326 (1) | 327 (1) | 317 (6) | 0.26 |
| Outer ring | 282 (1) | 281 (1) | 282 (1) | 278 (2) | 0.51 |
| Macular volume (mm3) | |||||
| ETDRS grid | 8.2 (0.02) | 8.2 (0.03) | 8.3 (0.03) | 8.1 (0.08) | 0.41 |
| Inner retinal layers, number of eyes | 457 | 194 | 177 | 21 | |
| Macular thickness (µm) | |||||
| Central circle | 189.1 (1.3) | 189.1 (1.6) | 189.0 (1.8) | 165.0 (8.1) | 0.11 |
| Inner ring | 246.5 (0.9) | 245.8 (1.1) | 245.5 (1.1) | 223.4 (4.6) | 0.003 |
| Outer ring | 203.7 (0.7) | 203.3 (0.9) | 203.8 (0.8) | 197.1 (2.3) | 0.25 |
| Macular volume (mm3) | |||||
| ETDRS grid | 6.02 (0.02) | 5.99 (0.03) | 6.01 (0.02) | 5.71 (0.08) | 0.05 |
| Outer retinal layers, number of eyes | 486 | 205 | 185 | 23 | |
| Macular thickness (µm) | |||||
| Central circle | 84.9 (0.4) | 84.1 (0.4) | 87.2 (0.9) | 119.7 (16.0) | 0.009 |
| Inner ring | 79.0 (0.2) | 78.7 (0.2) | 80.2 (0.3) | 93.1 (5.7) | 0.002 |
| Outer ring | 77.2 (0.1) | 77.1 (0.1) | 77.6 (0.2) | 79.6 (1.2) | 0.009 |
| Macular volume (mm3) | |||||
| ETDRS grid | 2.20 (0.004) | 2.19 (0.005) | 2.22 (0.006) | 2.37 (0.071) | 0.002 |
| Nerve fiber layer, number of eyes | 467 | 198 | 175 | 23 | |
| Macular thickness (µm) | |||||
| Central circle | 13.2 (0.2) | 13.2 (0.2) | 13.1 (0.2) | 13.8 (0.7) | 0.81 |
| Inner ring | 23.8 (0.2) | 23.7 (0.2) | 23.2 (0.2) | 23.7 (0.6) | 0.07 |
| Outer ring | 39.2 (0.4) | 39.0 (0.4) | 38.6 (0.4) | 40.7 (1.3) | 0.64 |
| Macular volume (mm3) | |||||
| ETDRS grid | 0.99 (0.01) | 0.98 (0.01) | 0.97 (0.01) | 1.02 (0.03) | 0.42 |
| Ganglion cell layer, number of eyes | 466 | 197 | 175 | 22 | |
| Macular thickness (µm) | |||||
| Central circle | 16.5 (0.5) | 16.4 (0.4) | 16.0 (0.3) | 16.7 (1.2) | 0.53 |
| Inner ring | 45.4 (0.3) | 45.1 (0.4) | 45.0 (0.4) | 41.8 (1.4) | 0.05 |
| Outer ring | 29.6 (0.2) | 29.7 (0.4) | 29.9 (0.2) | 28.4 (0.7) | 0.88 |
| Macular volume (mm3) | |||||
| ETDRS grid | 0.92 (0.01) | 0.92 (0.01) | 0.93 (0.01) | 0.85 (0.03) | 0.33 |
| Inner plexiform layer, number of eyes | 461 | 193 | 175 | 12 | |
| Macular thickness (µm) | |||||
| Central circle | 21.3 (0.2) | 21.4 (0.3) | 21.5 (0.3) | 21.6 (1.4) | 0.39 |
| Inner ring | 37.6 (0.2) | 37.5 (0.2) | 37.5 (0.2) | 36.4 (0.9) | 0.39 |
| Outer ring | 25.3 (0.1) | 25.2 (0.1) | 25.3 (0.1) | 25.2 (0.4) | 0.63 |
| Macular volume (mm3) | |||||
| ETDRS grid | 0.79 (0.004) | 0.79 (0.004) | 0.79 (0.004) | 0.78 (0.013) | 0.58 |
| Inner nuclear layer, number of eyes | 461 | 193 | 175 | 12 | |
| Macular thickness (µm) | |||||
| Central circle | 22.5 (0.3) | 23.5 (0.5) | 23.4 (0.6) | 23.2 (2.3) | 0.09 |
| Inner ring | 38.7 (0.2) | 38.7 (0.2) | 38.8 (0.3) | 37.4 (0.9) | 0.91 |
| Outer ring | 30.1 (0.1) | 30.2 (0.1) | 30.2 (0.2) | 30.4 (0.5) | 0.45 |
| Macular volume (mm3) | |||||
| ETDRS grid | 0.9 (0.004) | 0.9 (0.004) | 0.9 (0.005) | 0.9 (0.02) | 0.54 |
| Outer plexiform layer, number of eyes | 466 | 194 | 178 | 14 | |
| Macular thickness (µm) | |||||
| Central circle | 27.9 (0.3) | 28.7 (0.5) | 28.3 (0.5) | 26.3 (1.9) | 0.53 |
| Inner ring | 33.7 (0.2) | 33.8 (0.3) | 34.1 (0.3) | 33.7 (1.0) | 0.27 |
| Outer ring | 26.8 (0.1) | 26.8 (0.1) | 26.9 (0.1) | 27.1 (0.8) | 0.52 |
| Macular volume (mm3) | |||||
| ETDRS grid | 0.802 (0.003) | 0.801 (0.004) | 0.807 (0.005) | 0.804 (0.020) | 0.46 |
| Outer nuclear layer, number of eyes | 466 | 194 | 178 | 14 | |
| Macular thickness (µm) | |||||
| Central circle | 89.5 (0.7) | 87.8 (1.0) | 90.1 (0.9) | 73.7 (5.6) | 0.17 |
| Inner ring | 67.9 (0.5) | 67.8 (0.6) | 68.2 (0.6) | 53.3 (3.6) | 0.08 |
| Outer ring | 53.1 (0.3) | 53.5 (0.4) | 53.8 (0.4) | 49.7 (1.2) | 0.45 |
| Macular volume (mm3) | |||||
| ETDRS grid | 1.6 (0.01) | 1.6 (0.01) | 1.6 (0.01) | 1.5 (0.05) | 0.65 |
| Photoreceptor layer, number of eyes | 486 | 205 | 185 | 23 | |
| Macular thickness (µm) | |||||
| Central circle | 68.6 (0.2) | 67.8 (0.3) | 67.5 (0.3) | 66.8 (1.6) | 0.0008 |
| Inner ring | 64.4 (0.1) | 64.1 (0.1) | 64.2 (0.1) | 65.0 (1.0) | 0.52 |
| Outer ring | 64.0 (0.01) | 63.9 (0.1) | 64.1 (0.1) | 63.8 (0.4) | 0.75 |
| Macular volume (mm3) | |||||
| ETDRS grid | 1.82 (0.003) | 1.81 (0.004) | 1.81 (0.004) | 1.82 (0.014) | 0.47 |
| Retinal pigment epithelium, number of eyes | 486 | 205 | 185 | 23 | |
| Macular thickness (µm) | |||||
| Central circle | 16.1 (0.2) | 16.3 (0.2) | 19.9 (0.9) | 53.1 (16.0) | 0.001 |
| Inner ring | 14.5 (0.1) | 14.7 (0.1) | 16.0 (0.3) | 28.5 (5.6) | 0.0005 |
| Outer ring | 13.2 (0.1) | 13.2 (0.1) | 13.5 (0.1) | 16.0 (1.3) | 0.002 |
| Macular volume (mm3) | |||||
| ETDRS grid | 0.38 (0.002) | 0.385 (0.002) | 0.403 (0.004) | 0.564 (0.070) | 0.0003 |
Numbers are presented as least squared mean (SE).
Macular Thickness and Visual Acuity
Mean ± SD BCVA with each age tertile was 82.5 ± 7.9, 80.1 ± 11.9, and 76.5 ± 15.4; BCVA significantly declined with age (P < 0.0001), and the presence of late AMD (P < 0.0001) when adjusted for age. The association of RPE thickness to BCVA was no longer significant (P = 0.61), after excluding women with late AMD. In the age-adjusted model, increased thickness of the ONL was associated with better visual acuity whereas increased thickness of the RPE was associated with reduced BCVA (Table 4). The association of ONL thickness to BCVA weakened but remained statistically significant after excluding patients with cataract. Further excluding participants with late AMD attenuated the association further. The beta-coefficients (P values) for the association of ONL thickness to BCVA were 0.096 (0.001) with age-adjustment, 0.070 (0.01) after excluding women with cataract, and 0.047 (0.08) after further excluding women with late AMD.
Table 4.
Association Between Retinal Layer Thickness and Best Corrected Visual Acuity
| Eye Without | Eyes Without Cataract | |||||
|---|---|---|---|---|---|---|
| All Eyes | Cataract | and Late AMD | ||||
| Retinal Layers, (µm) | N | Beta Estimate | N | Beta Estimate | N | Beta Estimate |
| Nerve fiber layer | 865 | −0.128 | 730 | −0.061 | 710 | −0.053 |
| Ganglion cell layer | 863 | 0.008 | 730 | 0.010 | 710 | 0.004 |
| Inner plexiform layer | 818 | 0.108 | 691 | 0.125 | 680 | 0.112 |
| Inner nuclear layer | 818 | −0.035 | 691 | −0.027 | 680 | −0.030 |
| Outer plexiform layer | 824 | 0.040 | 697 | 0.041 | 684 | 0.034 |
| Outer nuclear layer | 824 | 0.096a | 697 | 0.079a | 684 | 0.047 |
| Photoreceptor layer | 865 | −0.023 | 730 | −0.067 | 710 | −0.040 |
| Retinal pigment epithelium | 865 | −0.062 | 730 | −0.061 | 710 | −0.024 |
P < 0.05.
Discussion
The histologic correlates of OCT images have been studied extensively,17–19 and results of histological20–22 and OCT studies23–25 indicate that many regions of the macula thin with age and neurodegenerative diseases. In the current analysis, we provide normative data on the macular thickness of women age 69 to 101 years and describe the variations in macular thickness across age and AMD outcomes. Results of this report corroborate other population-based studies demonstrating reduced retinal thickness with age, particularly outside the central circle.5–8,26
The difference in outer retinal thickness between AMD and non-AMD eyes was expected considering AMD is a disease primarily affecting the RPE. However, the difference in thickness between no AMD and early AMD was not discernible, which makes this measurement inadequate for identifying the transition. Outer retinal quantification in advanced AMD is an important biomarker for disease monitoring.27,28 Our data showed increasing outer retinal thickness in all three circles from early to intermediate AMD indicating the role of quantitative monitoring of disease severity.
The positive association between the thickness of the ONL and BCVA, adjusted for age, is noteworthy. Previously, individual retinal layers and impact on visual acuity has mostly been evaluation in relation to epiretinal membrane surgery or other retinal diseases such diabetes and glaucoma.29–31 Histopathologic studies have shown degeneration of ONL with loss of nuclei due to aging.32 The current study shows a thinning of ONL with age and that the age-adjusted association between ONL and BCVA persists after excluding participants with cataract, indicating that vision is preserved in those with delayed aging effects on retinal layers. This is explained, only in part, by the presence late AMD in some women.
Vital considerations when evaluating retinal thickness include the accuracy of the SD-OCT segmentation algorithms applied and the boundaries of the individual retinal layers used.33 Our study used the Heidelberg Spectralis SD-OCT device and its proprietary segmentation software. We noted errors in the automated segmentation that required manual correction throughout most scans (>80%). Errors in boundary lines, particularly ILM can occur because of commonly seen age-related pathologies, such as epiretinal membranes and thickened vitreous. The graders ensured that the line followed the ILM and made corrections as needed. The boundaries for Heidelberg's segmentation software differ from other devices and algorithms, such as those performed by Cirrus (Carl Zeiss Meditex, Dublin, CA, USA).34 These discrepancies likely contribute to the variation in measurements across population-based studies, even among similar population subgroups. For example, despite a similar cohort of elderly Caucasian individuals, our results differed slightly from those obtained by the Beaver Dam Eye Study, which used Topcon 3D-OCT software.7
Other factors that likely contribute to differences in retinal thickness measurements across population-based studies include the differences in thickness by gender5–7,35 and race.36,37 For example, the Beaver Dam Eye Study population (also predominantly Caucasian) was not gender specific,7 whereas the CAREDS2 population consisted of only women. We observed thinning of the total retina with increasing age, mainly because of reduced thickness of the inner retinal layers, particularly the GCL, IPL, and ONL. Thinning of these layers was only observed in the inner (3 mm diameter) and outer (6 mm diameter) rings and not in the central circle (1 mm diameter). Our results corroborate other population-based studies demonstrating reduced retinal thickness with age, particularly outside the central circle.5–7,35
Our study has some limitations. The CAREDS cohort includes only women, of primarily (97%) white race, and higher levels of socioeconomic status than similarly aged women in the American population. For this reason the results may lack generalizability to men, and to women of other races and socioeconomic status (Mares et al., unpublished data). Another limitation is the cross-sectional study design, which limits inferences about causal relationships between the observed associations. In addition, we did not control for axial length and refractive error, whereas previous studies reported that both axial length and refractive errors can influence the macular thickness measurements, particularly in eyes with high axial lengths.5–7,35,38,39 The mean axial length in the population was 23.6 (SD 1.1) and percentage of women with axial length >26 mm was small at 3% and not expected to affect study data. In addition, unlike other SD-OCT devices, the Heidelberg software applies model eye parameters to correct for ocular magnification.40 Last, we did not control for other diseases that may affect thickness measurements, such as neurodegenerative diseases.4
Despite these limitations, our study has several strengths. CAREDS2 involved a unique and well-characterized cohort of older women, for the study of age-related changes in the eye. An important strength is the age range of CAREDS2 study participants, with few studies containing participants with a mean age of 83 years and age range up to 101 years. We did manual editing of all segmented layers throughout the entire ETDRS grid, which provides the most accurate retinal thickness measurements. In addition, expert graders evaluated each individual retinal layer for confounding pathology that may alter the thickness measurements.
Conclusions
We provide normative macular thickness data for a unique and well-characterized cohort of women, mostly white, aged 69 to 101 years in the CAREDS2 study, an ancillary study of the WHI-OS. The peripheral macula thinned with increasing age, particularly in the GCL, IPL, and ONL of the inner retina. Retinal thickness did not differ between eyes with AMD compared to without AMD in the neural retina (retina without the RPE layer). Greater thickness in the ONL may confer advantages for visual acuity, independent of age and AMD. Additional studies of retinal thickness in relation to vision function could enhance our understanding of the value in retinal thickness measurements in clinical practice.
With the population aging in the United States and a likely increase in the prevalence of age-related diseases, including AMD, our study will help to better understand AMD among older women. In addition, the results of the current analysis can be used in future epidemiological and clinical studies evaluating the associations between the macular thickness and age-related diseases.
Acknowledgments
The authors are grateful for the time and energy that the CAREDS2 participants devoted to collecting the data which informed this work. The authors also thank Elizabeth Showers, at the University of Wisconsin Photographic Reading Center, for her assistance with project management.
*The Second Carotenoids in Age-Related Eye Disease Study Research Group:
University of Wisconsin – Madison: Julie Mares, Barbara Blodi, Yao Liu, Amitha Domalpally, Corinne Engelman, Ronald Gangnon, Gloria Sarto; Oregon Health Sciences University: Steven Bailey, Erin LeBlanc (Kaiser-Permanente); University of Iowa: Karen Gehrs, Robert Wallace, Jennifer Robinson; Women's Health Initiative: Lesley Tinker; University of Texas: D. Max Snodderly; University of Georgia: Randy Hammond; University at Buffalo: Amy Millen; Tufts University: Elizabeth Johnson; CAREDS 2 Examiners and Clinical Coordinators: Portland, OR: Jennifer Maykoski, Ann Lundquist; Madison, WI: Chris Smith, Kim Wood, Jennie Perry-Raymond; Iowa City, IA: Heather Stockman, Jean Walshire, and Christine Sinkey.
CAREDS2 Coordinating Center Staff at the University of Wisconsin – Madison. Thomas Lawler, Courtney Blomme, Kim Wood, Kristen Hall, Diane Pauk, Esther Mezhibovsky; Scientists: Krista Christensen, and Marine Nalbandyan.
Short list of WHI investigators: Program Office: (National Heart, Lung, and Blood Institute, Bethesda, MD) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg.
Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Women's Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker. https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
Supported by National Eye Institute grants EY013018, EY016886 and EY025292, and a supplement to EY025292-01S1 from the Office of Dietary Supplements. This work was also supported in part by an unrestricted grant from Research to Prevent Blindness, Inc. to the UW Madison Department of Ophthalmology and Visual Sciences, and in part by a National Eye Institute Vision Research Core grant (P30 EY016665) to the UW Madison Department of Ophthalmology and Visual Sciences. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 2107-26, 42129-32, and 44221. The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Disclosure: T. Etheridge, None; Z. Liu, None; M. Nalbandyan, None; S. Cleland, None; B.A. Blodi, None; J.A. Mares, None; S. Bailey, None; R. Wallace, None; K. Gehrs, None; L.F. Tinker, None; R. Gangnon, None; A. Domalpally, None
References
- 1. Chen TC, Cense B, Pierce MC, et al.. Spectral domain optical coherence tomography: ultra-high speed, ultra-high resolution ophthalmic imaging. Arch Ophthalmol. 2005; 123: 1715–1720. [DOI] [PubMed] [Google Scholar]
- 2. Sohn EH, Chen JJ, Lee K, Niemeijer M, Sonka M, Abràmoff MD.. Reproducibility of diabetic macular edema estimates from SD-OCT is affected by the choice of image analysis algorithm. Invest Ophthalmol Vis Sci. 2013; 54: 4184–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keane PA, Patel PJ, Liakopoulos S, Heussen FM, Sadda SR, Tufail A.. Evaluation of age-related macular degeneration with optical coherence tomography. Surv Ophthalmol. 2012; 57: 389–414. [DOI] [PubMed] [Google Scholar]
- 4. Satue M, Obis J, Rodrigo MJ, et al.. Optical Coherence Tomography as a Biomarker for Diagnosis, Progression, and Prognosis of Neurodegenerative Diseases. J Ophthalmol. 2016; 2016: 8503859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duan XR, Liang YB, Friedman DS, et al.. Normal macular thickness measurements using optical coherence tomography in healthy eyes of adult Chinese persons: the Handan Eye Study. Ophthalmology. 2010; 117: 1585–1594. [DOI] [PubMed] [Google Scholar]
- 6. Gupta P, Sidhartha E, Tham YC, et al.. Determinants of macular thickness using spectral domain optical coherence tomography in healthy eyes: the Singapore Chinese Eye study. Invest Ophthalmol Vis Sci. 2013; 54: 7968–7976. [DOI] [PubMed] [Google Scholar]
- 7. Myers CE, Klein BE, Meuer SM, et al.. Retinal thickness measured by spectral-domain optical coherence tomography in eyes without retinal abnormalities: the Beaver Dam Eye Study. Am J Ophthalmol. 2015; 159: 445–456.e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. von Hanno T, Lade AC, Mathiesen EB, Peto T, Njølstad I, Bertelsen G.. Macular thickness in healthy eyes of adults (N = 4508) and relation to sex, age and refraction: the Tromsø Eye Study (2007-2008). Acta Ophthalmol. 2017; 95: 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Invernizzi A, Pellegrini M, Acquistapace A, et al.. Normative Data for Retinal-Layer Thickness Maps Generated by Spectral-Domain OCT in a White Population. Ophthalmol Retina. 2018; 2: 808–815.e801. [DOI] [PubMed] [Google Scholar]
- 10. Dai W, Tham YC, Chee ML, et al.. Normative pattern and determinants of outer retinal thickness in an Asian population: the Singapore Epidemiology of Eye Diseases Study. Br J Ophthalmol. 2019; 103: 1406–1412. [DOI] [PubMed] [Google Scholar]
- 11. Khawaja AP, Chua S, Hysi PG, et al.. Comparison of associations with different macular inner retinal thickness parameters in a large cohort: the UK Biobank. Ophthalmology. 2020; 127: 62–71. [DOI] [PubMed] [Google Scholar]
- 12. Wang Q, Wei WB, Wang YX, et al.. Thickness of individual layers at the macula and associated factors: the Beijing Eye Study 2011. BMC Ophthalmol. 2020; 20: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moeller SM, Parekh N, Tinker L, et al.. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): ancillary study of the Women's Health Initiative. Arch Ophthalmol. 2006; 124: 1151–1162. [DOI] [PubMed] [Google Scholar]
- 14. Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M.. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003; 13: S107–121. [DOI] [PubMed] [Google Scholar]
- 15. Davis MD, Gangnon RE, Lee LY, et al.. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005; 123: 1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Eye Institute (NEI) Age-Related Eye Disease Study (AREDS). Chapter 7: Examination Procedures, https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/document.cgi?study_id=phs000001.v3.p1&phd=7#Sec7.2.3.
- 17. Spaide RF, Curcio CA.. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011; 31: 1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Staurenghi G, Sadda S, Chakravarthy U, Spaide RF, INfOCTIO Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN•OCT consensus. Ophthalmology. 2014; 121: 1572–1578. [DOI] [PubMed] [Google Scholar]
- 19. Cuenca N, Ortuño-Lizarán I, Pinilla I.. Cellular characterization of OCT and outer retinal bands using specific immunohistochemistry markers and clinical implications. Ophthalmology. 2018; 125: 407–422. [DOI] [PubMed] [Google Scholar]
- 20. Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976; 60: 324–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramrattan RS, Van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT.. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994; 35: 2857–2864. [PubMed] [Google Scholar]
- 22. Gao H, Hollyfield JG.. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992; 33: 1–17. [PubMed] [Google Scholar]
- 23. Sung KR, Wollstein G, Bilonick RA, et al.. Effects of age on optical coherence tomography measurements of healthy retinal nerve fiber layer, macula, and optic nerve head. Ophthalmology. 2009; 116: 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rao HL, Kumar AU, Babu JG, Kumar A, Senthil S, Garudadri CS.. Predictors of normal optic nerve head, retinal nerve fiber layer, and macular parameters measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 1103–1110. [DOI] [PubMed] [Google Scholar]
- 25. Demirkaya N, van Dijk HW, van Schuppen SM, et al.. Effect of age on individual retinal layer thickness in normal eyes as measured with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013; 54: 4934–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kelty PJ, Payne JF, Trivedi RH, Kelty J, Bowie EM, Burger BM.. Macular thickness assessment in healthy eyes based on ethnicity using Stratus OCT optical coherence tomography. Invest Ophthalmol Vis Sci. 2008; 49: 2668–2672. [DOI] [PubMed] [Google Scholar]
- 27. Schmidt-Erfurth U, Waldstein SM, Klimscha S, et al.. Prediction of Individual Disease Conversion in Early AMD Using Artificial Intelligence. Invest Ophthalmol Vis Sci. 2018; 59: 3199–3208. [DOI] [PubMed] [Google Scholar]
- 28. Abdelfattah NS, Zhang H, Boyer DS, et al.. Drusen volume as a predictor of disease progression in patients with late age-related macular degeneration in the fellow eye. Invest Ophthalmol Vis Sci. 2016; 57: 1839–1846. [DOI] [PubMed] [Google Scholar]
- 29. Pinilla I, Idoipe M, Perdices L, et al.. Changes in total and inner retinal thicknesses in type 1 diabetes with no retinopathy after 8 years of follow-up. Retina. 2020; 40: 1379–1386. [DOI] [PubMed] [Google Scholar]
- 30. Koo HC, Rhim WI, Lee EK.. Morphologic and functional association of retinal layers beneath the epiretinal membrane with spectral-domain optical coherence tomography in eyes without photoreceptor abnormality. Graefes Arch Clin Exp Ophthalmol. 2012; 250: 491–498. [DOI] [PubMed] [Google Scholar]
- 31. Kim JH, Lee HS, Kim NR, Seong GJ, Kim CY.. Relationship Between Visual Acuity and Retinal Structures Measured by Spectral Domain Optical Coherence Tomography in Patients With Open-Angle Glaucoma. Invest Ophthalmol Vis Sci. 2014; 55: 4801–4810. [DOI] [PubMed] [Google Scholar]
- 32. Gartner S, Henkind P.. Aging and degeneration of the human macula. 1. Outer nuclear layer and photoreceptors. Br J Ophthalmol. 1981; 65: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waldstein SM, Gerendas BS, Montuoro A, Simader C, Schmidt-Erfurth U.. Quantitative comparison of macular segmentation performance using identical retinal regions across multiple spectral-domain optical coherence tomography instruments. Br J Ophthalmol. 2015; 99: 794–800. [DOI] [PubMed] [Google Scholar]
- 34. Pierro L, Giatsidis SM, Mantovani E, Gagliardi M.. Macular thickness interoperator and intraoperator reproducibility in healthy eyes using 7 optical coherence tomography instruments. Am J Ophthalmol. 2010; 150: 199–204.e191. [DOI] [PubMed] [Google Scholar]
- 35. Song WK, Lee SC, Lee ES, Kim CY, Kim SS.. Macular thickness variations with sex, age, and axial length in healthy subjects: a spectral domain-optical coherence tomography study. Invest Ophthalmol Vis Sci. 2010; 51: 3913–3918. [DOI] [PubMed] [Google Scholar]
- 36. Girkin CA, McGwin G, Sinai MJ, et al.. Variation in optic nerve and macular structure with age and race with spectral-domain optical coherence tomography. Ophthalmology. 2011; 118: 2403–2408. [DOI] [PubMed] [Google Scholar]
- 37. Tariq YM, Li H, Burlutsky G, Mitchell P.. Ethnic differences in macular thickness. Clin Exp Ophthalmol. 2011; 39: 893–898. [DOI] [PubMed] [Google Scholar]
- 38. Lam DS, Leung KS, Mohamed S, et al.. Regional variations in the relationship between macular thickness measurements and myopia. Invest Ophthalmol Vis Sci. 2007; 48: 376–382. [DOI] [PubMed] [Google Scholar]
- 39. Röck T, Bartz-Schmidt KU, Bramkamp M, Röck D.. Influence of axial length on thickness measurements using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2014; 55: 7494–7498. [DOI] [PubMed] [Google Scholar]
- 40. Ctori I, Gruppetta S, Huntjens B.. The effects of ocular magnification on Spectralis spectral domain optical coherence tomography scan length. Graefes Arch Clin Exp Ophthalmol. 2015; 253: 733–738. [DOI] [PubMed] [Google Scholar]