Abstract
Aims
One of the comorbidities associated with severe outcome and mortality of COVID-19 is dyslipidemia. Statin is one of the drugs which is most commonly used for the treatment of dyslipidemic patients. This study aims to analyze the association between statin use and composite poor outcomes of COVID-19.
Data synthesis
We systematically searched the PubMed and Europe PMC database using specific keywords related to our aims until November 25th, 2020. All articles published on COVID-19 and statin were retrieved. Statistical analysis was done using Review Manager 5.4 and Comprehensive Meta-Analysis 3 software.
Results
A total of 35 studies with a total of 11, 930, 583 patients were included in our analysis. Our meta-analysis showed that statin use did not improve the composite poor outcomes of COVID-19 [OR 1.08 (95% CI 0.86–1.35), p = 0.50, I2 = 98%, random-effect modelling]. Meta-regression showed that the association with composite poor outcomes of COVID-19 was influenced by age (p = 0.010), gender (p = 0.045), and cardiovascular disease (p = 0.012). Subgroup analysis showed that the association was weaker in studies with median age ≥60 years-old (OR 0.94) compared to <60 years-old (OR 1.43), and in the prevalence of cardiovascular disease ≥25% (RR 0.94) compared to <25% (RR 1.24).
Conclusion
Statin use did not improve the composite poor outcomes of COVID-19. Patients with dyslipidemia should continue taking statin drugs despite COVID-19 infection status, given its beneficial effects on cardiovascular outcomes.
Keywords: Coronavirus disease 2019, COVID-19, Statin, Dyslipidemia, Treatment
Introduction
In March 2020, the World Health Organization (WHO) has declared the coronavirus disease 2019 (COVID-19) as global pandemic disease, and now nine months after that, the number of positive and death cases from COVID-19 is still increasing. The reported manifestation of COVID-19 can range from mild respiratory symptoms such as fever and cough to severe and potentially lethal symptoms such as sepsis, arrhythmia, heart failure, and loss of consciousness [1]. Several meta-analysis have demonstrated that patients' comorbid conditions such as dyslipidemia was associated with the development of severe outcomes and mortality from COVID-19 [[2], [3], [4], [5], [6], [7], [8]]. Most of the patients with dyslipidemia will take statin as their daily medications. Statin belong to a HMG-CoA reductase inhibitor drugs that has been long known as an effective cholesterol-lowering agent. Previous studies have proposed that statin may be beneficial in improving the outcome of COVID-19 [9,10]. The beneficial effects of statin may be related to its pleiotropic properties. This pleiotropic property of statin is believed to reduce the burden of obesity, cardiovascular disease, and dyslipidemia which are associated with poor outcomes of COVID-19. Statin can impair the virus's ability to infect cells and reducing its infectivity through down-regulation of CD147 in human cells, including pulmonary cells [9]. Statin can also prevent or reverse host cell lipid raft alterations induced by COVID-19 infection, which can reduce both cell infection and viral replication. Moreover, pleiotropic properties of statin can exert anti-inflammatory effects by inhibiting NLRP3 inflammasome through the TLR4/MyD88/NF-κB pathway, therefore restraining the uncontrolled inflammation which can be fatal in COVID-19 patients [9]. In silico study by Reiner et al. [10] showed that statin may be an effective SARS-CoV-2 Mpro inhibitors based on its binding energy which is higher than protease or polymerase inhibitors. However, all of these arguments are not yet supported by sufficient in human studies. This study aims to analyze the association between statin use and outcomes from COVID-19.
Methods
Eligibility criteria
Studies were included in this review if met the following inclusion criteria: representation for clinical questions (P: positive/confirmed cases of COVID-19; I: a group of patients who take statin as their medications; C: a group of patients who did not use statin; O: composite poor outcomes which comprise of risk COVID-19, severe COVID-19, and mortality), type of study was a randomized control trial, cohort, clinical trial, case-cohort, and cross-over design, and if the full-text article was available. The following types of articles were excluded: articles other than original research (e.g., review articles, letters, or commentaries); case reports; articles not in the English language; articles on research in pediatric populations (17 years of age or younger); and articles on research in pregnant women.
Search strategy and study selection
A systematic search of the literature was conducted on PubMed and Europe PMC using the keywords “statin” OR “lipid-lowering drugs” OR “lipid-lowering agents” AND “coronavirus disease 2019″ OR “COVID-19″, between 2019 and present time (October 25th, 2020) with language restricted to English only. The title, abstract, and full text of all articles identified that matched the search criteria were assessed, and those reporting the rate of statin use in COVID-19 patients with a clinically validated definition of each component of the outcomes of interest were included in this meta-analysis. The references of all identified studies were also analyzed (forward and backward citation tracking) to identify other potentially eligible articles. The study was carried out per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11].
Data extraction and quality assessment
Data extraction was performed independently by two authors, we used standardized forms that include author, year, study design, number of participants, age, gender, hypertension, diabetes, cardiovascular disease, statin use, and proportion of patients with each outcome of COVID-19.
The outcome of interest was the composite poor outcomes that comprised of risk of COVID-19 infection, severe COVID-19, and mortality. The risk of COVID-19 infection was defined as the likelihood of someone getting contracted by COVID-19. Severe COVID-19 was defined as patients who had any of the following features at the time of, or after, admission: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤93%; (3) ratio of the partial pressure of arterial oxygen (PaO2) to a fractional concentration of oxygen inspired air (fiO2) ≤300 mmHg; or (4) critical complication (respiratory failure, septic shock, and or multiple organ dysfunction/failure) or admission into ICU. Mortality outcome from COVID-19 was defined as the number of patients who were dead because of COVID-19 infection.
Two investigators independently evaluated the quality of the included cohort and case–control studies using the Newcastle–Ottawa Scale (NOS) [12]. The selection, comparability, and exposure of each study were broadly assessed and studies were assigned a score from zero to nine. Studies with scores ≥7 were considered of good quality.
Statistical analysis
A meta-analysis was performed using Review Manager 5.4 (Cochrane Collaboration) and Comprehensive Meta-Analysis version 3 software. We used the Generic Inverse Variance formula with random-effects models to calculate each outcome's risk. The heterogeneity was assessed by using the I2 statistic with a value of <25%, 26–50%, and >50% were considered as low, moderate, and high degrees of heterogeneity, respectively. The effect estimate was reported as odds ratio (OR) along with its 95% confidence intervals (CIs). P-value was two-tailed, and the statistical significance was set at ≤0.05. Random effects meta-regression was performed using a restricted-maximum likelihood for pre-specified variables including age, gender, hypertension, diabetes, and cardiovascular disease. Subgroup analysis was performed for each component of composite poor outcomes. We performed Begg's funnel-plot analysis to qualitatively assess the risk of publication bias.
Results
Study selection and characteristics
A total of 1528 records were obtained through systematic electronic searches. After the removal of duplicates, 1510 records remained. A total of 1455 records were excluded after screening the titles/abstracts because they did not match our inclusion and exclusion criteria. After evaluating 55 full-texts for eligibility, 10 full-text articles were excluded because they do not have the outcome of interest (risk of COVID-19, severe COVID-19, and mortality), 7 full-text articles were excluded because they do not have the control/comparison group, 3 full-text articles were excluded because the articles were not in English, and finally, 35 studies [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]] with a total of 11, 930, 583 sample sizes were included in the meta-analysis (Fig. 1 ). Of a total of 35 included studies, 31 were retrospective cohort, 2 studies were prospective cohort, while the remaining 2 study were case–control study. The essential characteristics of the included studies are summarized in Table 1 .
Figure 1.
PRISMA flowchart.
Table 1.
Characteristics of included studies.
| Study and country | Sample size | Design | Overall age mean ± SD | Male n (%) | Hypertension n (%) | Diabetes n (%) | Cardiovascular disease n (%) | Statin use n (%) |
|---|---|---|---|---|---|---|---|---|
| Alamdari NM et al. [12] 2020 (Iran) | 459 | Retrospective cohort | 61.7 ± 11.8 | 320 (69.7%) | 214 (46.6%) | 119 (25.1%) | 185 (40.3%) | 117 (25.5%) |
| An C et al. [13] 2020 (Korea) | 10,237 | Retrospective cohort | 44.9 ± 19.7 | 4088 (39.9%) | 7090 (69.3%) | 1021 (10%) | 511 (5%) | 1074 (10.5%) |
| Argenziano MG et al. [14] 2020 (USA) | 1000 | Retrospective cohort | 62.6 ± 18.5 | 596 (59.6%) | 601 (60.1%) | 372 (37.2%) | 233 (23.3%) | 361 (36.1%) |
| Ayed M et al. [15] 2020 (Kuwait) | 103 | Retrospective cohort | 53.3 ± 14 | 88 (85.5%) | 36 (35%) | 40 (39.2%) | 12 (11.8%) | 10 (9.8%) |
| Bifulco M et al. [16] 2020 (Italy) | 541 | Retrospective cohort | 65 ± 11.3 | 341 (63%) | 273 (50.4%) | 130 (24%) | 143 (26.4%) | 117 (21.6%) |
| Cariou B et al. [17] 2020 (France) | 1317 | Retrospective cohort | 69.8 ± 13 | 855 (64.9%) | 1003 (77.2%) | 1317 (100%) | 140 (11.6%) | 627 (47.6%) |
| Daniels LB et al. [18] 2020 (USA) | 170 | Retrospective cohort | 59 ± 19 | 98 (57.6%) | 75 (44.1%) | 34 (20%) | 56 (32.9%) | 46 (27%) |
| De Spiegeleer AD et al. [19] 2020 (Belgium) | 154 | Retrospective cohort | 85.9 ± 7.2 | 51 (33.1%) | 39 (25.3%) | 28 (18.2%) | N/A | 31 (20.1%) |
| Dreher M et al. [20] 2020 (Germany) | 50 | Retrospective cohort | 66.3 ± 13.3 | 33 (66%) | 35 (70%) | 29 (58%) | 7 (14%) | 18 (36%) |
| Gupta A et al. [21] 2020 (USA) | 2626 | Retrospective cohort | 62.3 ± 20 | 1497 (57%) | 1430 (54.4%) | 968 (36.8%) | 1052 (40%) | 876 (33.3%) |
| Higuchi T et al. [22] 2020 (Japan) | 57 | Retrospective cohort | 52.1 ± 25.5 | 32 (56.1%) | 16 (28.1%) | 13 (22.8%) | 5 (8.8%) | 12 (21.1%) |
| Hippisley-Cox J et al. [23] 2020 (England) | 8,275,949 | Prospective cohort | 48.4 ± 18.4 | 4,115,973 (49.7%) | 1,414,021 (17.1%) | 575,610 (6.9%) | 433,631 (5.24%) | 1,073,039 (12.9%) |
| Ho F et al. [24] 2020 (England) | 285,817 | Prospective cohort | 57.6 ± 8.4 | 131,589 (46%) | N/A | 13,658 (4.7%) | 13,819 (4.8%) | 44,250 (15.4%) |
| Holman N et al. [25] 2020 (England) | 3,138,410 | Retrospective cohort | 65.8 ± 14.3 | 1,756,110 (55.9%) | 734,965 (23.4%) | 3,138,410 (100%) | 196,305 (6.2%) | 2,218,500 (70.6%) |
| Huh K et al. [26] 2020 (Korea) | 65,149 | Case-control | 48.3 ± 15.3 | 32,183 (49.4%) | 21,368 (32.8%) | 17,981 (27.6%) | 13,533 (20.7%) | 11,103 (17%) |
| Inciardi RM et al. [27] 2020 (Italy) | 99 | Retrospective cohort | 67 ± 12 | 80 (81%) | 63 (64%) | 30 (31%) | 53 (54%) | 25 (26%) |
| Israel A et al. [28] 2020 (Israel) | 20,757 | Retrospective cohort | 59 ± 19.1 | 10,473 (50.4%) | 8511 (41%) | 11,076 (53.3%) | 6873 (33.1%) | 937 (4.5%) |
| Izzi-Engbeaya C et al. [29] 2020 | 889 | Retrospective cohort | 65.8 ± 17.5 | 534 (60%) | 418 (47%) | 337 (38%) | 373 (42%) | 180.9 ± 99.8 |
| Kibler M et al. [30] 2020 (France) | 702 | Retrospective cohort | 82 ± 6.9 | 313 (44%) | 587 (83.6%) | 213 (30.3%) | 318 (45.3%) | 344 (50.2%) |
| Lala A et al. [31] 2020 (USA) | 2736 | Retrospective cohort | 66.4 ± 15.8 | 1630 (59.6%) | 1065 (38.9%) | 719 (26.3%) | 935 (34.1%) | 984 (36%) |
| Luo P et al. [32] 2020 (China) | 283 | Retrospective cohort | 64.5 ± 10 | 156 (55.1%) | 164 (57.9%) | 283 (100%) | 43 (15.1%) | 55 (19.4%) |
| Maddaloni E et al. [33] 2020 (Italy) | 237 | Case-control | 75 ± 12.5 | 151 (63.7%) | N/A | 237 (100%) | 31 (13%) | 150 (63.2%) |
| Masana L et al. [34] 2020 (Spain) | 2157 | Retrospective cohort | 66.3 ± 17.7 | 1234 (57.3%) | 1081 (50.1%) | 501 (23.2%) | 620 (28.7%) | 581 (26.9%) |
| McCarthy CP et al. [35] 2020 (USA) | 247 | Retrospective cohort | 62.3 ± 19.2 | 143 (57.9%) | 128 (51.8%) | 68 (27.5%) | 114 (46.1%) | 107 (43.3%) |
| Ramachandran P et al. [36] 2020 (USA) | 295 | Retrospective cohort | 64.3 ± 14.8 | 162 (54.9%) | 209 (70.8%) | 132 (44.7%) | 45 (15.2%) | 114 (38.6%) |
| Rodriguez-Nava G et al. [37] 2020 (USA) | 87 | Retrospective cohort | 67 ± 12.5 | 56 (64.4%) | N/A | N/A | N/A | 47 (54%) |
| Saeed O et al. [38] 2020 (USA) | 4252 | Retrospective cohort | 65 ± 16 | 2255 (53%) | 3060 (72%) | 2266 (53%) | 1111 (26%) | 1355 (31.8%) |
| Song SL et al. [39] 2020 (USA) | 249 | Retrospective cohort | 62.6 ± 17.7 | 142 (57%) | 122 (49%) | 83 (33.3%) | 70 (28.1%) | 123 (49.3%) |
| Tan WYT et al. [40] 2020 (Singapore) | 717 | Retrospective cohort | 40.6 ± 28.1 | 410 (57.2%) | 139 (19.3%) | 76 (10.6%) | 50 (6.9%) | 151 (21%) |
| Ullah AZMD et al. [41] 2020 (England) | 15,586 | Retrospective cohort | 57.1 ± 18.2 | 6840 (43.9%) | 10,167 (65.2%) | 6047 (38.8%) | 4421 (28.4%) | 5221 (33.5%) |
| Vila-Corcoles A et al. [42] 2020 (Spain) | 34,936 | Retrospective cohort | 70.9 ± 11.3 | 16,805 (48.1%) | 34,936 (100%) | 9829 (28.1%) | 10,097 (28.9%) | 11,328 (32.4%) |
| Wang B et al. [43] 2020 (USA) | 58 | Retrospective cohort | 67 ± 12.5 | 30 (52%) | 37 (64%) | 16 (28%) | 20 (34%) | 27 (47%) |
| Yan H et al. [44] 2020 (China) | 49,245 | Retrospective cohort | 49.9 ± 16.6 | 23,799 (48.3%) | 9985 (20.2%) | 2977 (6%) | 637 (2.1%) | 458 (1.5%) |
| Zeng H et al. [45] 2020 (China) | 1031 | Retrospective cohort | 60.3 ± 14.3 | 538 (52.2%) | 384 (37.2%) | 189 (18.3%) | 84 (8.1%) | 38 (3.6%) |
| Zhang XJ et al. [46] 2020 (China) | 13,981 | Retrospective cohort | 56.3 ± 16.2 | 6830 (48.8%) | 4860 (34.7%) | 2282 (16.3%) | 1171 (8.3%) | 1219 (8.7%) |
Quality of study assessment
Studies with various study designs including cohort and case–control were included in this review and assessed accordingly with the appropriate scale or tool. Newcastle Ottawa Scales (NOS) were used to assess the cohort and case–control studies (Table 2 ). All included studies were rated ‘good’. In conclusion, all studies were seemed fit to be included in the meta-analysis.
Table 2.
Newcastle–Ottawa quality assessment of observational studies.
| First author, year | Study design | Selection | Comparability | Outcome | Total score | Result |
|---|---|---|---|---|---|---|
| Alamdari NM et al. [12] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| An C et al. [13] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Argenziano MG et al. [14] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Ayed M et al. [15] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Bifulco M et al. [16] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗ | 7 | Good |
| Cariou B et al. [17] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗∗ | 9 | Good |
| Daniels LB et al. [18] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| De Spiegeleer AD et al. [19] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Dreher et al. [20] 2020 | Cohort | ∗∗ | ∗∗ | ∗∗∗ | 7 | Good |
| Gupta A et al. [21] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗∗ | 9 | Good |
| Higuchi T et al. [22] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Hippisley-Cox J et al. [23] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Ho F et al. [24] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Holman N et al. [25] 2020 | Cohort | ∗∗∗∗ | ∗∗ | ∗∗∗ | 9 | Good |
| Huh K et al. [26] 2020 | Case-control | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Inciardi RM et al. [27] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Israel A et al. [28] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Izzi-Engbeaya C et al. [29] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Kibler M et al. [30] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Lala A et al. [31] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗∗ | 9 | Good |
| Luo P et al. [32] 2020 | Cohort | ∗∗ | ∗∗ | ∗∗∗ | 7 | Good |
| Maddaloni E et al. [33] 2020 | Case-control | ∗∗∗ | ∗∗ | ∗∗ | 7 | Good |
| Masana L et al. [34] 2020 | Cohort | ∗∗∗∗ | ∗∗ | ∗∗∗ | 9 | Good |
| McCarthy CP et al. [35] 2020 | Cohort | ∗∗∗∗ | ∗∗ | ∗∗∗ | 9 | Good |
| Ramachandran P et al. [36] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Rodriguez-Nava G et al. [37] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗ | 7 | Good |
| Saeed O et al. [38] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗∗ | 9 | Good |
| Song SL et al. [39] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Tan WYT et al. [40] 2020 | Cohort | ∗∗ | ∗∗ | ∗∗∗ | 7 | Good |
| Ullah AZMD et al. [41] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Vila-Corcoles A et al. [42] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗ | 7 | Good |
| Wang B et al. [43] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Yan H et al. [44] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Zeng H et al. [45] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗ | 8 | Good |
| Zhang XJ et al. [46] 2020 | Cohort | ∗∗∗ | ∗∗ | ∗∗∗∗ | 9 | Good |
Statin and outcomes
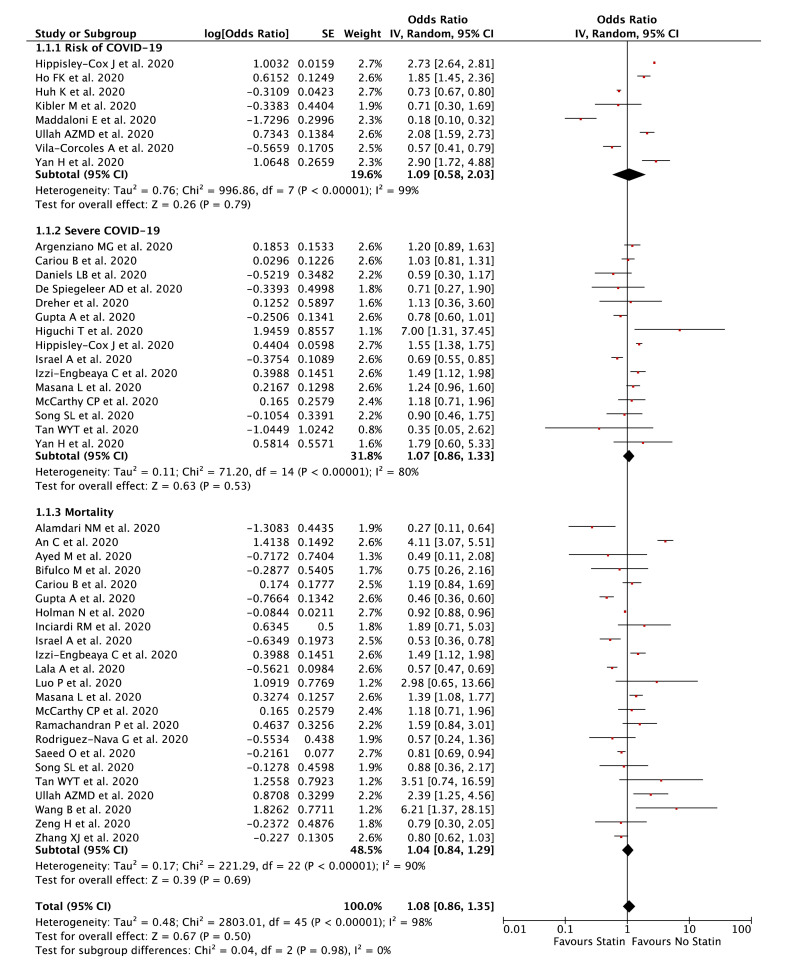
Our pooled analysis showed that statin was not associated with composite poor outcome [OR 1.08 (95% CI 0.86–1.35), p = 0.50, I 2 = 98%, random-effect modelling] (Fig. 2 ). Subgroup analysis showed that statin was not associated with risk of COVID-19 [OR 1.09 (95% CI 0.58–2.03), p = 0.79, I 2 = 99%, random-effect modelling], severe COVID-19 [OR 1.07 (95% CI 0.86–1.33), p = 0.53, I 2 = 80%, random-effect modelling], and mortality from COVID-19 [OR 1.04 (95% CI 0.84–1.29), p = 0.69, I 2 = 90%, random-effect modelling].
Figure 2.
Forest plot that demonstrates the association of statin with composite poor outcome and its subgroup which comprises of risk of COVID-19, severe COVID-19, and mortality.
Meta-regression
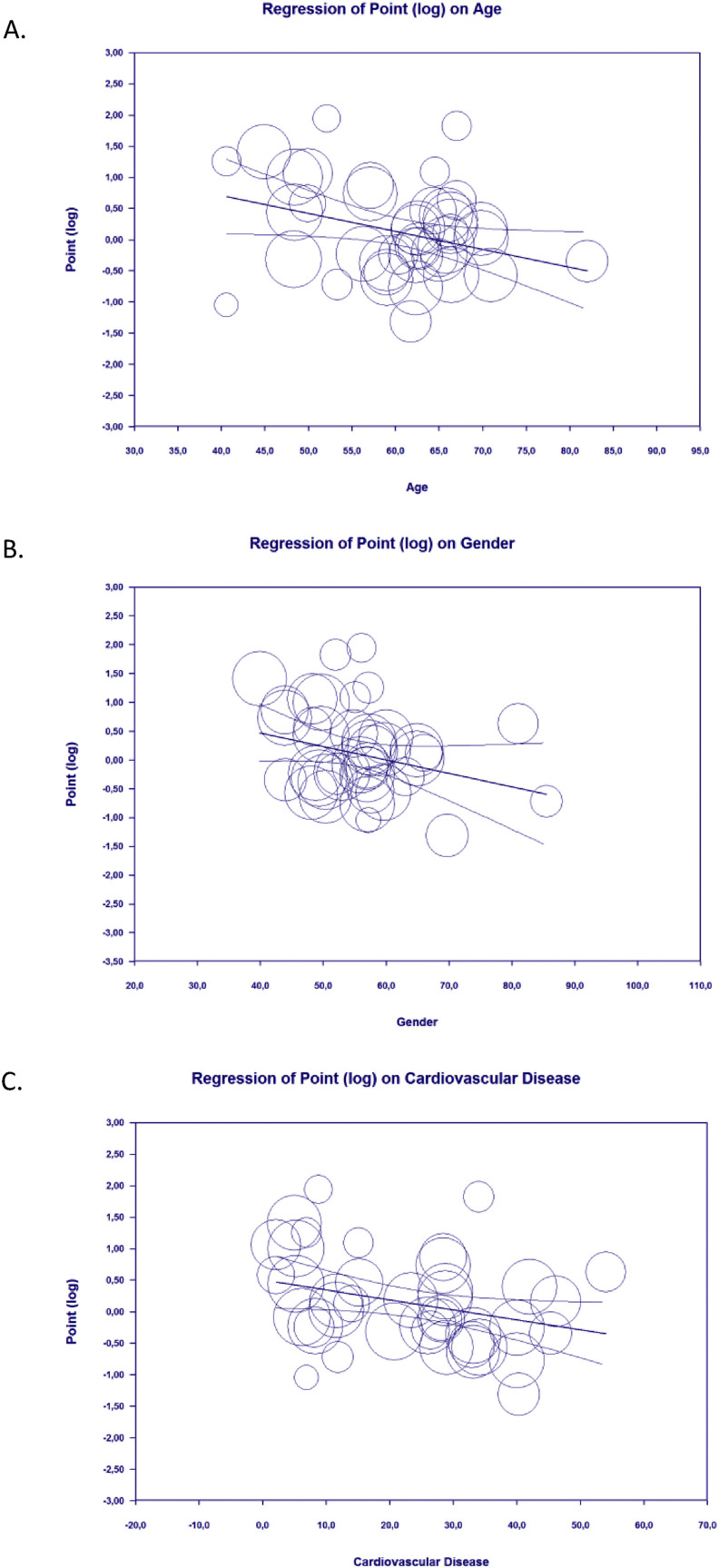
Meta-regression showed that the association between statin and composite poor outcome was affected by age (p = 0.010) (Fig. 3 A), gender (p = 0.045) (Fig. 3B), and cardiovascular disease (p = 0.012) (Fig. 3C), but not affected by hypertension (p = 0.610) and diabetes mellitus (p = 0.246).
Figure 3.
Bubble-plot for Meta-regression. Meta-regression analysis showed that the association between statin and composite poor outcome was affected by age [A], gender [B], and cardiovascular disease [C].
Subgroup analysis
Subgroup analysis for studies with median age ≥60 years [OR 0.94 (95% CI 0.80–1.09), p = 0.39, I 2 = 88%, random-effect modelling] showed a lower OR for composite poor outcome compared to <60 years [OR 1.43 (95% CI 0.91–2.24), p = 0.12, I 2 = 99%, random-effect modelling].
Subgroup analysis for studies with prevalence of male gender ≥50% [OR 0.91 (95% CI 0.79–1.04), p = 0.17, I 2 = 82%, random-effect modelling] showed a lower OR for composite poor outcome compared to <50% [OR 1.49 (95% CI 0.97–2.27), p = 0.07, I 2 = 99%, random-effect modelling].
Subgroup analysis for studies with prevalence of cardiovascular disease ≥25% [OR 0.94 (95% CI 0.76–1.16), p = 0.58, I 2 = 88%, random-effect modelling] showed a lower OR for composite poor outcome compared to <25% [OR 1.24 (95% CI 0.89–1.72), p = 0.20, I 2 = 99%, random-effect modelling].
Subgroup analysis for studies with USA population [OR 0.85 (95% CI 0.68–1.06), p = 0.15, I 2 = 77%, random-effect modelling] showed a lower OR for composite poor outcome compared to Asia population [OR 1.12 (95% CI 0.75–1.69), p = 0.57, I 2 = 93%, random-effect modelling] and Europe population [OR 1.16 (95% CI 0.81–1.68), p = 0.42, I 2 = 99%, random-effect modelling].
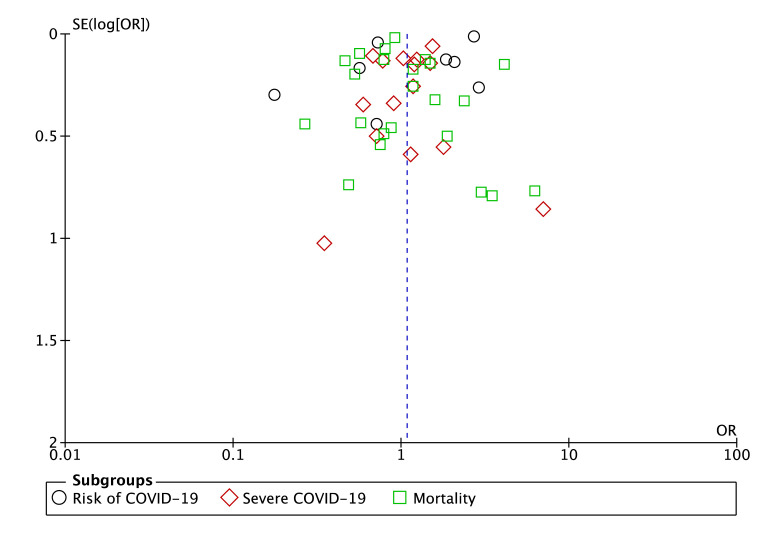
Publication bias
The funnel-plot analysis showed a qualitatively symmetrical inverted funnel-plot for the association between statin and composite poor outcome (Fig. 4 ), showing no indication of publication bias.
Figure 4.
Funnel plot analysis for the association of statin with composite poor outcome of COVID-19.
Discussion
Previous meta-analysis that showed the benefit of statin in reducing the disease severity and/or mortality from COVID-19 only involved 4 studies and did not elaborate the effects of the confounding factors which can affect the relationship between statin use and outcome of COVID-19, such as age, gender, and comorbid conditions, resulting in weak and preliminary conclusions [47]. Our systematic review and meta-analysis already involved 35 included studies and not only analyzes the association between statin use and composite poor outcomes of COVID-19, but also elaborate the effect of the confounding factors such as age, gender, and comorbid conditions.
This comprehensive meta-analysis of 35 studies showed that statin use was not associated with composite poor outcomes of COVID-19 which comprised of risk of COVID-19, severe COVID-19, and mortality from COVID-19. This association was influenced by age, gender, and cardiovascular disease. Further analysis based on meta-regression showed that magnitude of risk linked to statin use as a single factor was greater in studies with younger, non-male dominant, and low prevalence of cardiovascular disease patients, which is yet to be addressed by the existing literature.
Several reasons can be proposed to explain why statin use did not improve the outcomes from COVID-19. First, apart from its beneficial effects through pleiotropic properties, the action of statin on TLR and NF-κB signaling also carry the potential risk of exacerbating compensatory immune signals and poor disease outcome [48]. This theory is supported by a retrospective analysis of the findings from a multicenter clinical trial on the efficacy of rosuvastatin against infection-induced ARDS that showed higher IL-18 levels and mortality in statin-treated patients [49]. Second, based on the experimental studies in animal models, statin therapy may upregulate the expression of angiotensin-converting enzyme 2 (ACE2), which mediates the SARS-CoV-2 entry into host cells, thus can increase its viral load and infectivity, resulting in severe outcome of the disease [50,51]. Third, statin may cause hepatotoxicity and myotoxicity in some patients that can cause acute kidney injury. Moreover, this adverse effect of statin is higher in older patients and may be exacerbated when given concomitantly with antiviral agents for COVID-19 such as lopinavir and darunavir [52,53]. Statins are a substrate for cytochrome P450 (CYP) system, especially 3A isoenzymes and P-glycoproteins (P-gp), while protease inhibitors, such as lopinavir and darunavir are potent inhibitors of both CYP3A and P-gp, so their concomitant administration will result in markedly statin exposure and adverse effects [48,54]. Therefore, the beneficial effects of statin may be counter-balanced by its potentially harmful effects and causing a neutral effect toward COVID-19. Finally, the anti-inflammatory effects of statin are relatively lower than corticosteroids [55], making statin cannot make a significant alteration in the inflammation or cytokine storm that happened in COVID-19, thus the composite poor outcomes of COVID-19 was not altered by statin administration.
Patients with dyslipidemia and cardiovascular disease should hence be advised to still continue taking statin during the COVID-19 pandemic given its pleiotropic effects which offer benefit in reducing the cardiovascular adverse events and its neutral effects toward COVID-19 outcomes. Physicians should also still consider giving statin into the treatment regime of their patients who have dyslipidemia or cardiovascular disease if the patients have not been given it yet. However, in older patients, the benefit-risk balance of statin therapy should be carefully evaluated, either discontinuation of statin therapy and switching into other lipid-lowering therapies or continuation with caution and at lower doses is possible options.
This study has several limitations. First, data on the dosage and duration of statin therapy were lacking in the included studies, hence, cannot be analyzed. Second, we include some pre-print studies to minimize the risk of publication bias, however, the authors have made exhaustive efforts to ensure that only sound studies were included and we expect that most of those studies currently available in pre-print form will eventually be published and that we will identify them through ongoing electronic literature surveillances. We hope that this study can give further insight into statin therapy in COVID-19 patients.
Funding
None.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgment
None.
Handling Editor: J. Bella
References
- 1.Kwenandar F., Japar K.V., Damay V., Hariyanto T.I., Tanaka M., Lugito N.P.H., et al. Coronavirus disease 2019 and cardiovascular system: a narrative review. Int J Cardiol Heart Vasc. 2020;29:100557. doi: 10.1016/j.ijcha.2020.100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hariyanto T.I., Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(5):1463–1465. doi: 10.1016/j.dsx.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hariyanto T.I., Kurniawan A. Anemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Transfus Apher Sci. 2020 Aug;59(6):102926. doi: 10.1016/j.transci.2020.102926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hariyanto T.I., Kurniawan A. Thyroid disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(5):1429–1430. doi: 10.1016/j.dsx.2020.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hariyanto T.I., Putri C., Arisa J., Situmeang R.F.V., Kurniawan A. Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a Systematic Review and Meta-Analysis. Arch Gerontol Geriatr. 2021;93(March–April 2021) doi: 10.1016/j.archger.2020.104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hariyanto T.I., Kurniawan A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes Med. 2020 Sep;19:100290. doi: 10.1016/j.obmed.2020.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hariyanto T.I., Putri C., Situmeang R.F.V., Kurniawan A. Dementia is a predictor for mortality outcome from coronavirus disease 2019 (COVID-19) infection. Eur Arch Psychiatr Clin Neurosci. 2020 Oct 26:1–3. doi: 10.1007/s00406-020-01205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hariyanto T.I., Prasetya I.B., Kurniawan A. Proton pump inhibitor use is associated with increased risk of severity and mortality from coronavirus disease 2019 (COVID-19) infection. Dig Liver Dis. 2020 Oct 6;52(12):1410–1412. doi: 10.1016/j.dld.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues-Diez R.R., Tejera-Muñoz A., Marquez-Exposito L., Rayego-Mateos S., Santos Sanchez L., Marchant V., et al. Statins: could an old friend help in the fight against COVID-19? Br J Pharmacol. 2020;177(21):4873–4886. doi: 10.1111/bph.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiner Ž., Hatamipour M., Banach M., Pirro M., Al-Rasadi K., Jamialahmadi T., et al. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch Med Sci. 2020;16(3):490–496. doi: 10.5114/aoms.2020.94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D.G., Prisma Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alamdari N.M., Afaghi S., Rahimi F.S., Tarki F.E., Tavana S., Zali A., et al. Mortality risk factors among hospitalized COVID-19 patients in a major referral center in Iran. Tohoku J Exp Med. 2020 Sep;252(1):73–84. doi: 10.1620/tjem.252.73. [DOI] [PubMed] [Google Scholar]
- 13.An C., Lim H., Kim D.W., Chang J.H., Choi Y.J., Kim S.W. Machine learning prediction for mortality of patients diagnosed with COVID-19: a nationwide Korean cohort study. Sci Rep. 2020 Oct 30;10(1):18716. doi: 10.1038/s41598-020-75767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020 May 29;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayed M., Borahmah A.A., Yazdani A., Sultan A., Mossad A., Rawdhan H. Assessment of clinical characteristics and mortality-associated factors in COVID-19 Critical cases in Kuwait. Med Princ Pract. 2020 Nov 16 doi: 10.1159/000513047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bifulco M., Ciccarelli M., Bruzzese D., Dipasquale A., Lania A.G., Mazziotti G., et al. The benefit of statins in SARS-CoV-2 patients: further metabolic and prospective clinical studies are needed. Endocrine. 2021;71:270–272. doi: 10.1007/s12020-020-02550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020 Aug;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniels L.B., Sitapati A.M., Zhang J., Zou J., Bui Q.M., Ren J., et al. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am J Cardiol. 2020 Dec 1;136:149–155. doi: 10.1016/j.amjcard.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Spiegeleer A., Bronselaer A., Teo J.T., Byttebier G., De Tré G., Belmans L., et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID-19 infection among nursing home residents. J Am Med Dir Assoc. 2020 Jul;21(7):909–914. doi: 10.1016/j.jamda.2020.06.018. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreher M., Kersten A., Bickenbach J., Balfanz P., Hartmann B., Cornelissen C., et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int. 2020 Apr 17;117(16):271–278. doi: 10.3238/arztebl.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A., Madhavan M.V., Poterucha T.J., DeFilippis E.M., Hennessey J.A., Redfors B., et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Res Sq [Preprint] 2020 Aug 11 doi: 10.21203/rs.3.rs-56210/v1. rs.3.rs-56210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higuchi T., Nishida T., Iwahashi H., Morimura O., Otani Y., Okauchi Y., et al. Early clinical factors predicting the development of critical disease in Japanese patients with COVID-19: a single-center, retrospective, observational study. J Med Virol. 2020 Oct 14;93(4):2141–2148. doi: 10.1002/jmv.26599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hippisley-Cox J., Young D., Coupland C., Channon K.M., Tan P.S., Harrison D.A., et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020 Oct;106(19):1503–1511. doi: 10.1136/heartjnl-2020-317393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho F.K., Celis-Morales C.A., Gray S.R., Katikireddi V., Niedzwiedz C.L., Hastie C., et al. Modifiable and non-modifiable risk factors for COVID-19, and comparison to risk factors for influenza and pneumonia: results from a UK Biobank prospective cohort study. medRxiv. 2020;10(11) doi: 10.1101/2020.04.28.20083295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holman N., Knighton P., Kar P., O'Keefe J., Curley M., Weaver A., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020 Oct;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huh K., Ji W., Kang M., Hong J., Bae G.H., Lee R., et al. Association of previous medications with the risk of COVID-19: a nationwide claimsbased study from South Korea. medRxiv. 2020 May 18:2020. doi: 10.1101/2020.05.04.20089904. [DOI] [Google Scholar]
- 27.Inciardi R.M., Adamo M., Lupi L., Cani D.S., Di Pasquale M., Tomasoni D., et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020 May 14;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Israel A., Schaffer A., Cicurel A., Feldhamer I., Tal A., Cheng K., et al. Large population study identifies drugs associated with reduced COVID-19 severity. medRxiv [Preprint] 2020 Oct 18:2020. doi: 10.1101/2020.10.13.20211953. 10.13.20211953. [DOI] [Google Scholar]
- 29.Izzi-Engbeaya C., Distaso W., Amin A., Yang W., Idowu O., Kenkre J.S., et al. Severe COVID-19 and diabetes: a retrospective cohort study from three london teaching hospitals. BMJ Open Diabetes Res Care. 2021 Jan 6 doi: 10.1136/bmjdrc-2020-001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kibler M., Carmona A., Marchandot B., Matsushita K., Trimaille A., Kanso M., et al. Risk and severity of COVID-19 and ABO blood group in transcatheter aortic valve patients. medRxiv [Preprint] 2020 June 16:2020. doi: 10.1101/2020.06.13.20130211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020 Aug 4;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo P., Qiu L., Liu Y., Liu X.L., Zheng J.L., Xue H.Y., et al. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. 2020 Jul;103(1):69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maddaloni E., D'Onofrio L., Alessandri F., Mignogna C., Leto G., Coraggio L., et al. Clinical features of patients with type 2 diabetes with and without Covid-19: a case control study (CoViDiab I) Diabetes Res Clin Pract. 2020 Sep 21;169:108454. doi: 10.1016/j.diabres.2020.108454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masana L., Correig E., Rodríguez-Borjabad C., Anoro E., Arroyo J.A., Jericó C., et al. EFFECT oF STATIN THERAPY ON SARS-CoV-2 INFECTION-RELATED. Eur Heart J Cardiovasc Pharmacother. 2020 Nov 2 doi: 10.1093/ehjcvp/pvaa128. pvaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy C.P., Murphy S., Jones-O'Connor M., Olshan D.S., Khambhati J.R., Rehman S., et al. Early clinical and sociodemographic experience with patients hospitalized with COVID-19 at a large American healthcare system. EClinicalMedicine. 2020 Sep;26:100504. doi: 10.1016/j.eclinm.2020.100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramachandran P., Perisetti A., Gajendran M., Jean-Louis F., Bansal P., Dwivedi A.K., et al. Pre-hospitalization proton pump inhibitor use and clinical outcomes in COVID-19. Eur J Gastroenterol Hepatol. 2020 Nov 30 doi: 10.1097/MEG.0000000000002013. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Nava G., Trelles-Garcia D.P., Yanez-Bello M.A., Chung C.W., Trelles-Garcia V.P., Friedman H.J. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit Care. 2020 Jul 14;24(1):429. doi: 10.1186/s13054-020-03154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saeed O., Castagna F., Agalliu I., Xue X., Patel S.R., Rochlani Y., et al. Statin use and in-hospital mortality in diabetics with COVID-19. J Am Heart Assoc. 2020 Oct 23;9(24) doi: 10.1161/JAHA.120.018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song S.L., Hays S.B., Panton C.E., Mylona E.K., Kalligeros M., Shehadeh F., et al. Statin use is associated with decreased risk of invasive mechanical ventilation in COVID-19 patients: a preliminary study. Pathogens. 2020 Sep 17;9(9):759. doi: 10.3390/pathogens9090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan W.Y.T., Young B.E., Lye D.C., Chew D.E.K., Dalan R. Statin use is associated with lower disease severity in COVID-19 infection. Sci Rep. 2020 Oct 15;10(1):17458. doi: 10.1038/s41598-020-74492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ullah A.Z.M.D., Sivapalan L., Chelala C., Kocher H.M. COVID-19 in patients with hepatobiliary and pancreatic diseases in East London: a single-centre cohort study. medRxiv. 2020:2020. doi: 10.1101/2020.09.07.20189621. 09.07.20189621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vila-Corcoles A., Satue-Gracia E., Ochoa-Gondar O., Torrente-Fraga C., Gomez-Bertomeu F., Vila-Rovira A., et al. Use of distinct anti-hypertensive drugs and risk for COVID-19 among hypertensive people: a population-based cohort study in Southern Catalonia, Spain. J Clin Hypertens. 2020 Jul 25;22:1379–1388. doi: 10.1111/jch.13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang B., Van Oekelen O., Mouhieddine T.H., Valle D.M.D., Richter J., Cho H.J., et al. A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. J Hematol Oncol. 2020;13:94. doi: 10.1186/s13045-020-00934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan H., Valdes A.M., Vijay A., Wang S., Liang L., Yang S., et al. Role of drugs used for chronic disease management on susceptibility and severity of COVID-19: a large case-control study. Clin Pharmacol Ther. 2020 Dec;108(6):1185–1194. doi: 10.1002/cpt.2047. [DOI] [PubMed] [Google Scholar]
- 45.Zeng H., Zhang T., He X., Du Y., Tong Y., Wang X., et al. Impact of hypertension on progression and prognosis in patients with COVID-19: a retrospective cohort study in 1031 hospitalized cases in wuhan, China. medRxiv. 2020 July 24 doi: 10.1101/2020.06.14.20125997. 2020.06.14.20125997. [DOI] [Google Scholar]
- 46.Zhang X.J., Qin J.J., Cheng X., Shen L., Zhao Y.C., Yuan Y., et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metabol. 2020 Aug 4;32(2):176–187. doi: 10.1016/j.cmet.2020.06.015. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kow C.S., Hasan S.S. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020 Nov 1;134:153–155. doi: 10.1016/j.amjcard.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dashti-Khavidaki S., Khalili H. Considerations for statin therapy in patients with COVID-19. Pharmacotherapy. 2020;40(5):484–486. doi: 10.1002/phar.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers A.J., Guan J., Trtchounian A., Hunninghake G.M., Kaimal R., Desai M., et al. Association of elevated plasma interleukin-18 level with increased mortality in a clinical trial of statin treatment for acute respiratory distress syndrome. Crit Care Med. 2019;47(8):1089–1096. doi: 10.1097/CCM.0000000000003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tikoo K., Patel G., Kumar S., Karpe P.A., Sanghavi M., Malek V., et al. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem Pharmacol. 2015 Feb 1;93(3):343–351. doi: 10.1016/j.bcp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Lima Martínez M.M., Contreras M.A., Marín W., D'Marco L. Statins in COVID-19: is there any foundation? Clín Invest Arterioscler. 2020 Nov-Dec;32(6):278–281. doi: 10.1016/j.arteri.2020.06.003. English, Spanish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banach M., Penson P.E., Fras Z., Vrablik M., Pella D., Reiner Ž., et al. Brief recommendations on the management of adult patients with familial hypercholesterolemia during the COVID-19 pandemic. Pharmacol Res. 2020 Aug;158:104891. doi: 10.1016/j.phrs.2020.104891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cholesterol Treatment Trialists' Collaboration Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019 Feb 2;393(10170):407–415. doi: 10.1016/S0140-6736(18)31942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neuvonen P.J., Niemi M., Backman J.T. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006 Dec;80(6):565–581. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Vukovic P.M., Maravic-Stojkovic V.R., Peric M.S., Jovic MDj, Cirkovic M.V., Gradinac SDj, et al. Steroids and statins: an old and a new anti-inflammatory strategy compared. Perfusion. 2011;26(1):31–37. doi: 10.1177/0267659110385607. [DOI] [PubMed] [Google Scholar]