Abstract
Background
Dengue is one of the most common vector-borne diseases globally, however, its burden is poorly quantified. Hence, we aimed to report the dengue burden in 195 countries and territories between 1990 and 2017, using data from the Global Burden of Diseases, Injuries, and Risk Factors Study 2017.
Methods
Following the methodology framework and analytical strategies used in the Global Burden of Disease Study 2017, we analysed the incidence, mortality, and disability-adjusted life years (DALYs) of dengue in geographically defined populations worldwide between 1990 and 2017. We also determined the association between development levels and dengue burden. All estimates were reported as numbers and rates per 100 000 population, with 95% uncertainty intervals.
Findings
Globally, the total number of dengue cases increased from 23 283 274 (95% UI 453 180.7–51 840 670) in 1990 to 104 771 911 (95% UI 63 759 019–158 870 031) in 2017. The age-standardised incidence rate increased from 431.6 (8.4–961.0) per 100 000 population in 1990 to 1371.3 (834.5–2079.3) per 100 000 population in 2017. In addition, the number of deaths due to dengue increased from approximately16 957 (7 613–30 091) in 1990 to 40 467 (17 620–49 778) in 2017. Meanwhile, the global age-standardised death rate increased from 0.31 (0.14–0•56) per 100 000 population in 1990 to 0.53 (0.23–0•65) per 100 000 population in 2017. Overall, there were 2 922 630 DALYs (1 629 424–3 967 492) attributed to dengue in 2017 globally, an increase of 107.6% since 1990 (1 407 571 DALYs [624 016.4–2 510 025]), and the age-standardised DALY rate increased from 26.10 (11.57–46.53) per 100 000 population to 38.25 (21.33–51.93) per 100 000 population between 1990 and 2017. The association between socio-demographic index (SDI) and dengue-related DALYs suggested that the lowest age-standardised DALY rates were found in countries in the low and high-SDI quintile in 2017, and from 1990 to 2017, the age-standardized DALY rate tended to increase in regions with the lowest SDI but declined in regions with the highest SDI. There was a nonlinear association between the socio-demographic index and the healthcare access and quality index and age-standardised DALY rates.
Interpretation
Dengue is a major public health challenge worldwide. While there is remarkable international variation in its incidence, the dengue burden is increasing globally. The results of this study could be useful for policy makers to implement cost-effective interventions and reduce the dengue burden, particularly in countries with high incidence or increasing burden.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (NSFC) (grant numbers 81,800,041 and 82,000,078).
Keywords: Dengue, GBD 2017, Burden, Incidence
Research in context.
Evidence before this study
Dengue is the most rapidly spreading mosquito-borne viral disease worldwide. The burden of dengue has been investigated in previous research using data from GBD 2013, however, this dataset had several limitations. Importantly, the GBD 2017 incorporated additional data sources and applied new methodologies compared with GBD 2013. To our knowledge, no study has provided detailed estimates of dengue-related incidence, mortality, DALYs in 195 countries and territories around the world for several years.
Added value of this study
We used data from the GBD 2017 to provide the most up-to-date estimates on a wide range of health measures related to dengue at the global, regional, and country-specific levels for 21 regions and, 195 countries and territories as well as, by sex, age group, and socio-demographic index (SDI). We report the burden of dengue, including incidence, mortality and DALYs, by age, sex, and SDI from 1990 to 2017, using all available data based on standardised GBD methods at the global, regional, and national levels. We believe this analysis presents the most comprehensive picture of dengue burden to date.
Implications of all the available evidence
Dengue remains a substantial public health challenge worldwide. Our results suggest that there were about 100 million dengue infections resulting in about 40 000 deaths in 2017. Between 1990 and 2017, age-standardised incidence, mortality and DALYs rates increased in most countries. The highest burden of dengue is in South Asia, Southeast Asia and the Caribbean. The information provided in this study could be crucial for researchers, public health officials and health policy makers to implement cost-effective interventions to be able to confront the increasing dengue burden.
Alt-text: Unlabelled box
1. Introduction
Dengue is the most rapidly spreading mosquito-borne viral disease and is caused by any one of four single-stranded, positive-sense RNA viruses (DENV-1 to DENV-4) [1]. In recent decades, the incidence of dengue has grown dramatically worldwide; it has been estimated to cause 390 million infections yearly, and approximately 20 000 deaths [2]. Moreover, the number of dengue cases reported to the World Health Organization has increased over eight fold in the last two decades from 505,430 cases in 2000, to over 2.4 million in 2010 and 4.2 million in 2019. Meanwhile, the reported deaths between 2000 and 2015 increased from 960 to 4032 [3]. These reports and disease modeling estimates suggest that dengue is greatly underreported.
The clinical profile and presentation of patients with dengue infection may differ from asymptomatic infection to dreadful complications,such as dengue shock syndrome [4,5]. Since the clinical presentation of dengue is similar to that of other febrile illnesses caused by more than ten pathogens, misdiagnosis is common even among experienced physicians [1]. In addition, due to economic and technological limitations, some potential dengue cases remain undetected. Moreover, official passive surveillance systems are not designed to capture subclinical infections. Hence, a large disparity exists between the number of reported cases and the estimates of actual cases.
Currently, treatment remains supportive and no effective antiviral agents exist [4]. Although Dengvaxia, the only FDA-approved dengue vaccine, is licenced in 20 countries, the WHO did not recommend its use in seronegatives [6]. Thus, current dengue control relies mainly on combinations of chemical and biological targeting against larval or adult mosquitoes [7]. Mosquito reduction campaigns have been very successful but they are difficult to sustain, mainly because they are labor intensive, require discipline and diligence, and are plagued by diminishing returns [8]. Nevertheless, the dengue incidence is high in resource-constrained countries where restricted health budgets are divided between control and treatment [9]. Therefore, it is essential for public health policymakers, vaccine developers, vector control specialists, and physicians to accurately provide robust estimates of the current and future burden of dengue.
Recently, Stanaway and colleagues reported the global burden of dengue by utilizing the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2013 [10], and found a trend of increasing global incidence and dengue deaths in the past two decades. However, they did not examine the association between the burden of dengue and the sociodemographic status of countries. Moreover, aside from this study, there are no updated estimates on the burden of dengue. Hence, we examined data from the GBD 2017 to determine the global, regional, and national incidence of dengue as well as deaths and disability adjusted life-years (DALYs) in terms of counts and age-standardised rates from 1990 to 2017 by age, sex, and socio-demographic index (SDI) to provide a comprehensive and comparable analysis of dengue burden.
2. Methods
2.1. Data sources
Data on dengue burden in 195 countries and territories from 1990 to 2017 were obtained from the Global Health Data Exchange GBD Results Tool (http://ghdx.healthdata.org/gbd-results-tool) (date of data extraction, 15 July 2020). The GBD 2017 uses all the available up-to-date sources of epidemiological data and improved standardised methods to provide a comprehensive assessment of health loss across 359 diseases and injuries, 282 causes of death and 84 risk factors [11]. The general methodology of the GBD 2017, including its main changes compared with previous years, has been described in previous publications [12], [13], [14]. Briefly, the mortality-to-incidence ratio (MIR) estimation was updated from GBD 2016, with the use of the Healthcare Access and Quality Index (HAQ Index) [15] rather than the SDI in the data cleaning and modeling process, and the spatiotemporal Gaussian process regression approach was also updated. Covariate inputs for the Cause of Death Ensemble model (CODEm) were updated and changed on the basis of recommendations from GBD collaborators. The rates were standardised according to the GBD world population and were reported per 100 000 person-years. The GBD 2017 uses various interrelated metrics to measure population health loss, including number of deaths and mortality, number of cases and prevalence, years of life lost (YLL) due to premature death, years lived with disability (YLD), and DALYs. For this report, we used the GBD Results Tool to extract estimates and their 95% uncertainty intervals (UIs) for deaths, prevalence of cases, and DALYs as measures of dengue burden from 1990 to 2017 by region and country.
The SDI is a composite indicator of a geographical location's development status. In GBD 2017, the SDI was calculated based on the total fertility rate among females younger than 25 years, educational attainment for those aged 15 years or older, and lag distributed income per capita [16]. The SDI ranged from 0 to 1, where 0 represents the fewest years of schooling, lowest income per capita, and highest fertility, and 1 represents most years of schooling, highest income per capita, and lowest fertility. The geometric mean of these values for each location-year was recorded [17], and the 195 countries and territories were classified into 5 groups according to the SDI quintile (low-SDI, low-middle-SDI, middle-SDI, high-middle-SDI, and high-SDI quintile).
The Healthcare Access and Quality (HAQ) Index is an indicator of health system performance for 195 countries and territories and was calculated on the basis of amenable mortality [15]. The HAQ Index ranges from 0 (worst) to 100 (best).
2.2. Data analysis
To characterize the dengue burden by age, sex, year, and location, a descriptive analysis was conducted. The number of cases and deaths as well as age-standardised incidence, age-adjusted mortality, and age-standardised DALYs per 100 000 population for both sexes combined were calculated and compared at the global, regional, and country levels. UIs were calculated from 1000 draw-level estimates for each parameter, and 95% UIs were defined by the 25th and 975th values of the ordered 1000 estimates; a 95% UI excluding 0 was considered to be statistically significant. Finally, we examined the shape of the association of dengue in terms of age-standardised prevalence and DALYs-with the SDI using the fit spline models [18]. The maps were made using ECharts software. All statistical analyses were performed using GraphPad Prism 8 software.
2.3. Role of funding source
This work was supported by a grant from the National Natural Science Foundation of China (NSFC) (grant numbers 81,800,041 and 82,000,078).
3. Results
3.1. Incidence
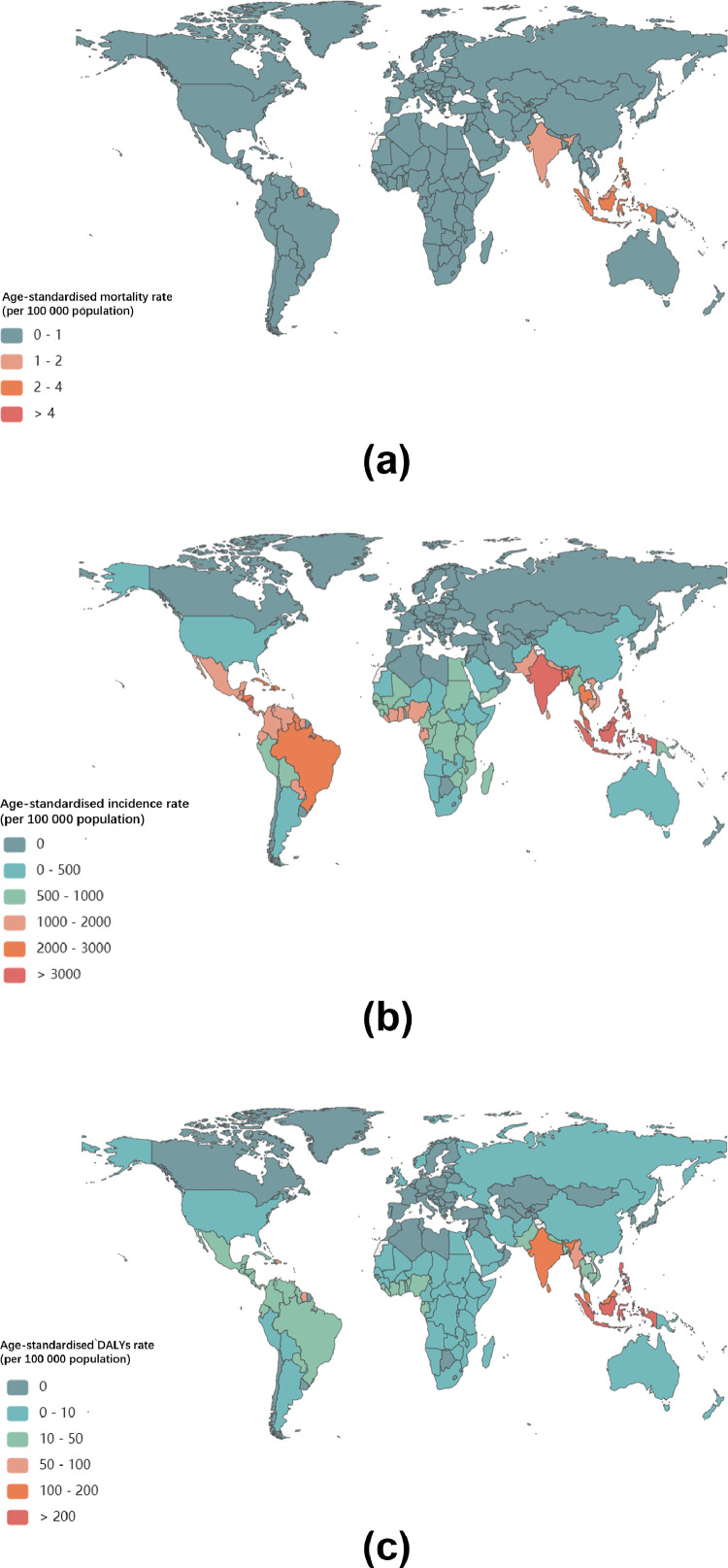
In 2017 an estimated 104 771 911 (95% UI; 63 759 019–158 870 031) individuals had dengue worldwide with 52 357 259 (31 887 948–79 333 365) occurring among females and 52 414 653 (31 871 071–79 536 666) among males, compared with 23 283 274 (453 180.7–51 840 670) in 1990 (Supplementary Table 1). The age-standardised incidence rate per 100 000 population-at the global level increased from 431.6 (8.4–961.0) in 1990 to 1371.3 (834.5–2079.3) in 2017 (Supplementary Table 1). At the country level, the age-standardised incidence rate per 100 000 population for dengue was highest in Barbados (4179.9 [2619.9–6185.4]), followed by Dominica (4152.1 [2680.9–6012.3]), Indonesia (4117.1 [2427.4–6378.5] per 100 000 population) and India (4072.9 [2409.4–6295.3]). By contrast, 81 countries and territories had no dengue data in 2017 (Fig. 1A and Supplementary Table 2). Moreover, the GBD 2017 reported the first dengue cases in South Africa and Namibia, with estimated incidence rates per 100 000 population of 121.2 (45.2–263.8) and 9856.9 (6209.5–14,576.6), respectively (Supplementary Table 3).
Fig. 1.
Global age-standardised mortality, incidence, and DALY rate of Dengue Fever in 2017.
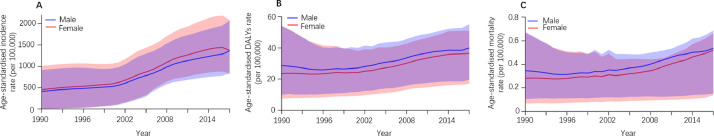
By sex, the pattern of age-standardised incidence rates per 100 000 population between 1990 and 2017 were similar among males and females, although the rates were consistently higher among females (Fig. 2A). Among all regions, dengue was most prevalent in South Asia (3546.9 [2128.5–5429.5]), Southeast Asia (2940.6 [1787.3–4457.0]) and the Caribbean (2510.4 [1656.1–3578.7]) in 2017 (Fig. 1A and Supplementary Table 4).
Fig. 2.
Age-standardised DALY rate, age-standardised mortality rate and age-standardised incidence rate per 100 000 people with dengue fever in males (men and boys), females (women and girls), and both sexes from 1990 to 2017.
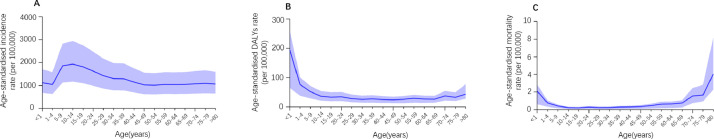
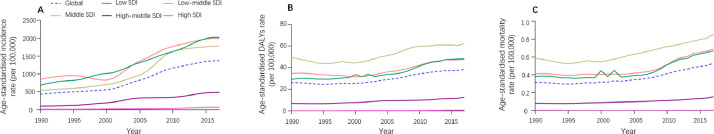
By age, dengue incidence peaked at 10–14 years (Fig. 3A and supplementary Table 5). Between 1990 and 2017, the age-standardised incidence rate due to dengue was highest in the low and low-middle SDI quintiles (Fig. 4A), and showed an upward trend for all five SDI regions (Fig. 4A and Supplementary Table 6).
Fig. 3.
Age-standardised DALYs rate, age-standardised mortality rate and age-standardised incidence rate per 100 000 people with dengue Fever in 2017.
Fig. 4.
Age-standardised DALY rate, age-standardised mortality rate and age-standardised incidence rate per 100 000 people grouped by SDI quintiles for dengue fever from 1990 to 2017.
3.2. DALYs
In 2017, there were 2 922 630 DALYs (95% UI 1 629 424–3 967 492) due to dengue globally, showing an increase of 107.6% since 1990 (1 407 571 DALYs [624 016.4–2 510 025]). On the one hand, the age-standardised DALY rate per 100 000 population increased from 26.10 [11.57–46.53] in 1990 to 38.25 [21.33–51.93] in 2017 (Supplementary Table 1). Indonesia (258.24 [117.91–318.35]), the Philippines (219.53 [108.83–307.08]), and Tonga (181.10 [107.07–282.15]) had the highest age-standardised DALY rates in 2017 (Fig. 1B and Supplementary Table 2). At the regional level, the age-standardised DALY rate of dengue per 100 000 population was found to be highest in Southeast Asia (154.24 [85.27–195.07]), South Asia (89.93 [48.07–127.03]) and the Caribbean (41.02 [20.70–62.52]) in 2017 (Fig. 1B and Supplementary Table 4). Between 1990 and 2017, the worldwide age-standardised DALYs rate was higher among males than females (Fig. 2B) and highest in the middle SDI quintile than in the other quintiles (Fig. 4B and supplementary Table 6); in 2017, it peaked at age 0–1 for both males and females (Fig. 3B and Supplementary Table 5).
3.3. Mortality
Compared to 1990, the number of deaths due to dengue increased from approximately 16 957 (7 613–30 091) to 40 467 (17 620–49 778) in 2017, an increase of around 24 000 deaths. Meanwhile, the global age-standardised death rate per 100 000 population increased from 0.31 (0.14–0•56) in 1990 to 0.53 (0.23–0•65) in 2017. The pattern of the age-standardised death rate by sex across age groups was relatively similar to the age-standardised DALY rate (Fig. 2C and Supplementary Table 1). Geographically, deaths attributable to dengue per 100 000 people were most frequent in the Southeast Asia superregion (1.97 deaths [0.97–2.35]) and South Asia superregion (1.46 deaths [0.61–1.90]) in 2017 (Fig. 1C and Supplementary Table 4). Moreover, the highest age-standardised death rate in 2017 was found among individuals aged 0–1 years and >80 years (Fig. 3C and supplementary Table 5) as well as in the middle SDI quintile; it was lower in the high and high-middle SDI quintiles than in other quintiles in 2017 (Fig. 4C and Supplementary Table 6).
3.4. Burden of dengue by SDI and the HAQ index
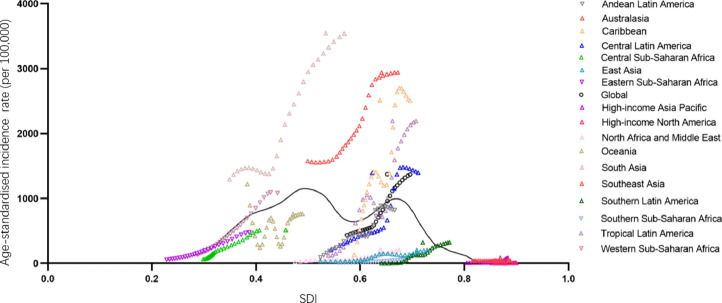
Fig. 5 presents the global and regional-level observed age-standardised DALY rates from 1990 to 2017 and their association with the SDI. The expected pattern was nonlinear in nature, peaking at SDI values of approximately 0.5 and 0.65 before decreasing with increasing SDI values. Southeast Asia and South Asia had a much higher age-standardised incidence rate than expected based on the SDI between 1990 and 2017. The lowest age-standardised incidence rate was seen at SDIs of approximately 0.2 and 0.8.
Fig. 5.
Age-standardised incidence rate of dengue globally and for 17 GBD regions by SDI, 1990–2017.
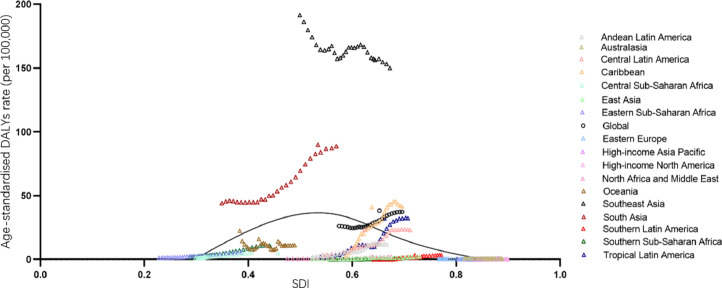
The patterns of the age-standardised DALY rate versus the SDI were similar to those of age-standardised prevalence rates. The exceptions were Southeast Asia and South Asia where observed levels were higher than expected during every year of the study period (Fig. 6).
Fig. 6.
Age-standardised DALY rate of dengue globally and for 17 GBD regions by SDI, 1990–2017.
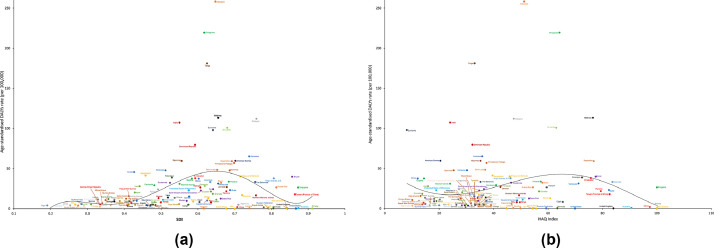
Fig. 7 shows the national-level observed age-standardised DALY rates and their association with the SDI and HAQ index. The expected patterns were nonlinear in nature, peaking at an SDI value of approximately 0•65 and an HAQ index value of approximately 67, before decreasing with increasing SDI and HAQ Index values. The age-standardised DALY rate was higher than the expected level based only on the SDI for a number of countries/territories, such as Indonesia, the Philippines, and Tonga. This pattern was also observed based on the HAQ Index.
Fig. 7.
Age-standardised DALY rates of dengue for 195 countries and territories by SDI and HAQ Index, 2017.
4. Discussion
In this paper, we presented the most up-to-date estimates of the incidence, mortality, and DALYs for dengue in 195 countries and territories from 1990 to 2017. An estimated 100 million dengue infections occured across more than 110 countries and territories in 2017, increasing from 23 million dengue infections in 1990, with potential for further spread. Our results show increasing trends in dengue incidence, mortality and DALYs in the past two decades globally. This is important for public health officials and policy makers worldwide to implement interventions to slow down the rising global burden of dengue.
Our data show that the age-standardised incidence rate increased from 1990 to 2017. In addition to this long-term upward trend, previous studies conducted at numerous endemic sites worldwide have presented dynamic and intra-annual signature patterns of dengue incidence [19], [20], [21], [22], [23]. Dengue incidence is influenced by both climate and nonclimate drivers [19,24]. The local climate and the El Niño-Southern Oscillation (ENSO) are potentially important drivers of the interannual variability in dengue fever transmission [19]. The theoretical causal mechanisms linking ENSO to dengue are based on the connections between ENSO and local climate anomalies in certain regions of the world as well as the influence of climate on the dengue mosquito vector and virus [25,26]. Our results highlight non-climate factors (e.g. dengue virus serotype variation and strain-cross immunity) and other social-ecological drivers influencing vector populations and human exposure, such as vector control interventions; changes in land use and urban poverty and infrastructure; and human movement, which may be important determinants of dengue incidence variability.
Dengue primarily impacts tropical and subtropical countries. The GBD 2017 showed that dengue is most rampant in the regions of South Asia and Southeast Asia, similar to the GBD 2013 findings [10]. Our model shows that the age-standardised incidence rate is highest in South Asia, followed by the Southeast Asia, the Caribbean, tropical Latin America, and Central Latin America-a distributions, inconsistent with that the estimates of Stanaway and colleagues [10]. This difference may in part be explained by the additional data sources and new methodologies that were applied in the GBD 2017.
The age-standardised incidence rates of dengue are estimated to be highest in Barbados and, Dominica, followed by Indonesia and India. Dengue is reported to be hyperendemic in most English-speaking Caribbean countries, with an epidemic occurring every 4–5 years between 1997 and 2009 [27]. Among the ten countries with the highest age-standardised incidence of dengue in 2017, five were from the Caribbean (Barbados, Dominica, Trinidad and Tobago, Antigua and Barbuda and Saint Lucia). Most countries showed an increase in the age-standardised incidence rate of dengue during 1990–2017, while only Oceania experienced a dynamic change at the regional level. Differences in incidence in countries that are within the Oceania region should be noted. The age-standardised incidence rate of dengue increased steadily in Papua New Guinea and, declined in Fiji from 2008 to 2017.
Although no study has reported that the dengue virus is transmitted in a sex-dependent manner, the GBD 2017 showed that the age-standardised incidence rate was slightly higher in females than in males. This could be partly explained by sex-related physiology [28]. Conversely, the age-standardised mortality and DALY rates were found to be higher in males. Thus, more attention should be paid to the prevention and management of dengue in males. Moreover, our results suggest that the age-standardised incidence rate peaked around the ages of 10–14 among both sexes, which is consistent with the GBD 2013 estimates [10]. Moreover, the age-standardised mortality rate peaked in the oldest age group in GBD 2017, and in the post-neonatal age group in GBD 2013 [10]. The difference may in part be explained by the additional data sources and new methodologies applied in GBD 2017. Meanwhile, in both GBD 2013 and GBD 2017, the peak age-standardised DALY rate was in the youngest age group [10].
We also found that the increase in the age-standardised death and DALY rate paralleled that of the age-standardised incidence rate at the global and regional levels; however, the age-standardised death and DALY rate in the Southeast Asia region decreased from 1990 to 2017. Indonesia, the most populated country in Southeast Asia, witnessed a great increase in the age-standardised incidence rate over the study period, while in contrast, the age-standardised death and attributable DALY rate largely decreased. Indeed, data from the Indonesian National Disease Surveillance System have indicated an increasing trend of dengue incidence in Indonesia over the past 50 years [29]. In contrast, the case fatality rate has decreased by approximately half in each decade since 1980 [29,30]. This decline is mainly due to an improved management of dengue cases, leading to fewer deaths [31], from an increase in the milder cases captured by the surveillance system.
Our analysis of the association between the SDI and the burden of dengue has not been previously reported. As shown in the results, most countries with the greatest dengue burden were regions with a low to middle SDI. However, the dengue burden was relatively lower in the high-middle and high SDI regions. The differences in dengue burden among SDI levels could be expected due to urbanization. Urbanization resulted in rapid population growth and substantially increased the density, larval development rate, and adult survival time of Aedes mosquitoes [32,33]. Then, Aedes mosquitoes can bite several persons within one blood meal, thus amplifying dengue transmission dynamics [34]. We also found that the increase in age-standardised death rates paralleled that of the age-standardised DALY rate in different SDI regions. As DALYs are a composite index combining mortality and morbidity, the trends of DALYs related to dengue could be partly explained by dynamic changes in dengue mortality.
The main strength of the current study is that we used the data from the GBD 2017 estimates. Our study is the most comprehensive and up-to-date report of the estimates for dengue incidence, deaths and DALYs by age, sex, and location from 1990 to 2017. Inclusion of additional data in the GBD 2017 also allowed us to make national and regional-level estimates, and develop robust estimates for dengue.
Apart from GBD research, Bhatt et al. study presented the map of dengue risk and estimates of apparent and inapparent infections worldwide based on the global population in 2010 [2]. Bhatt et al. study and our study have different original data sources. GBD study input data were restricted to sources available at the time of analysis, either individual-level vital registration or hospitalization data with multiple ICD codes. Bhatt et al. study comprised of point or polygon locations of confirmed dengue infection presence derived from both peer-reviewed literature and HealthMap alerts. Bhatt et al. study is therefore more comprehensive than GBD study when estimating dengue incidence. However, Bhatt et al. study did not consist of data relative to dengue mortality and DALYs. Thus, we need to be cautious when interpreting the results of the two documents alone.
The limitations of the methods of the GBD impose biases on our estimates in the current study, as with all GBD research. First, the limited availability and quality of surveillance data from high-burden countries is an important limitation. Data about dengue were only available in limited regions and countries, and we have no data from much of Western Europe, Eastern Europe, Central Europe, and Central Asia. Existing surveillance systems are not sensitive, and mild febrile illnesses are less likely to be diagnosed and reported. Thus, there might be substantial underreporting of dengue cases even in many dengue-endemic countries [35]. Second, dengue symptoms are often confused or misdiagnosed with other febrile illnesses such as malaria, influenza, typhoid and Zika as their clinical manifestations are similar [1]. Additionally, due to the lack of DENV confirmatory testing capacity, the dengue misdiagnosis rate could be as high as 10% [36]. Finally, our modeling framework is restricted to predefined GBD age groups, which precludes estimation of burden for finer age categories that might be informative for vaccine policy.
In summary, the global burden of dengue is substantial and increasing despite the variation between countries in incidence, mortality and DALYs. The results of the GBD 2017 can be valuable for public health officials and policy makers implementing cost-effective interventions to address the future dengue burden.
Declaration of Competing Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
Funding
This work was supported by agrant from the National Natural Science Foundation of China (NSFC) (grant numbers 81800041 and 82000078).
Acknowledgements
We thank the institute for Health Metrics and Evaluation staff and its collaborators who prepared these publicly available data.
Author contributions
ZLZ and JZ contributed equally to this paper. JZ and HLC designed the study and accessed the database and that they confirm the accuracy of the database used. ZLZ and JZ contributed to data collection, data analysis, data interpretation and literature search. ZLZ and HLC drafted the manuscript. SC and LYC revised the final draft of the manuscript. All authors contributed to data acquisition, data analysis, or data interpretation, and all reviewed and approved the final version of the manuscript.
Data sharing
Any personally identifiable data cannot be made publicly available to protect participant privacy. All other relevant data are available upon request to the corresponding author.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100712.
Appendix. Supplementary materials
References
- 1.Simmons C.P., Farrar J.J., Nguyen v.V., Wills B. Dengue. N Engl J Med. 2012;366(15):1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt S., Gething P., Brady O. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Dengue and severe dengue. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. 2020.
- 4.World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. https://www.who.int/neglected_diseases/resources/9789241547871/en/. 2009. [PubMed]
- 5.Wilder-Smith A., Ooi E.E., Horstick O., Wills B. Dengue. Lancet. 2019;393(10169):350–363. doi: 10.1016/S0140-6736(18)32560-1. [DOI] [PubMed] [Google Scholar]
- 6.Idris F., Ting D., Alonso S. An update on dengue vaccine development, challenges, and future perspectives. Expert Opin Drug Discov. 2020:1–12. doi: 10.1080/17460441.2020.1811675. [DOI] [PubMed] [Google Scholar]
- 7.Hemingway J., Beaty B., Rowland M., Scott T., Sharp B. The Innovative Vector Control Consortium: improved control of mosquito-borne diseases. Trends Parasitol. 2006;22(7):308–312. doi: 10.1016/j.pt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Unlu I., Klingler K., Indelicato N., Faraji A., Strickman D. Suppression of Aedes albopictus, the Asian tiger mosquito, using a 'hot spot' approach. Pest Manag. Sci. 2016;72(7):1427–1432. doi: 10.1002/ps.4174. [DOI] [PubMed] [Google Scholar]
- 9.Rigau-Pérez J., Clark G., Gubler D., Reiter P., Sanders E., Vorndam A. Dengue and dengue haemorrhagic fever. Lancet. 1998;352(9132):971–977. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 10.Stanaway J., Shepard D., Undurraga E. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16(6):712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collaborators GDaIIaP Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collaborators GCC The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. The Lancet Gastroenterol Hepatol. 2019;4(12):913–933. doi: 10.1016/S2468-1253(19)30345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collaborators GI. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2019;7(1):69–89. doi: 10.1016/S2213-2600(18)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collaborators GRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collaborators GHAaQ Measuring performance on the Healthcare access and quality index for 195 countries and territories and selected subnational locations: a systematic analysis from the global burden of disease study 2016. Lancet. 2018;391(10136):2236–2271. doi: 10.1016/S0140-6736(18)30994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collaborators GPaF Population and fertility by age and sex for 195 countries and territories, 1950-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1995–2051. doi: 10.1016/S0140-6736(18)32278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collaborators GDaH Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collaborators GN. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. The Lancet Neurol. 2019;18(5):459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart-Ibarra A.M., Lowe R. Climate and non-climate drivers of dengue epidemics in southern coastal ecuador. Am. J. Trop. Med. Hyg. 2013;88(5):971–981. doi: 10.4269/ajtmh.12-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Méndez-Lázaro P., Muller-Karger F.E., Otis D., McCarthy M.J., Peña-Orellana M. Assessing climate variability effects on dengue incidence in San Juan, Puerto Rico. Int J Environ Res Public Health. 2014;11(9):9409–9428. doi: 10.3390/ijerph110909409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoddard S.T., Wearing H.J., Reiner R.C., Jr. Long-term and seasonal dynamics of dengue in Iquitos, Peru. PLoS Negl Trop Dis. 2014;8(7):e3003. doi: 10.1371/journal.pntd.0003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thai K.T., Cazelles B., Nguyen N.V. Dengue dynamics in Binh Thuan province, southern Vietnam: periodicity, synchronicity and climate variability. PLoS Negl Trop Dis. 2010;4(7):e747. doi: 10.1371/journal.pntd.0000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell K.M., Lin C.D., Iamsirithaworn S., Scott T.W. The complex relationship between weather and dengue virus transmission in Thailand. Am. J. Trop. Med. Hyg. 2013;89(6):1066–1080. doi: 10.4269/ajtmh.13-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilder-Smith A., Gubler D.J. Geographic expansion of dengue: the impact of international travel. Med Clin North Am. 2008;92(6):1377–1390. doi: 10.1016/j.mcna.2008.07.002. , x. [DOI] [PubMed] [Google Scholar]
- 25.Tun-Lin W., Burkot T.R., Kay B.H. Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in north Queensland, Australia. Med. Vet. Entomol. 2000;14(1):31–37. doi: 10.1046/j.1365-2915.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- 26.Rueda L.M., Patel K.J., Axtell R.C., Stinner R.E. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: culicidae) J Med Entomol. 1990;27(5):892–898. doi: 10.1093/jmedent/27.5.892. [DOI] [PubMed] [Google Scholar]
- 27.Kumar A., Gittens-St Hilaire M., Nielsen A. Long-term epidemiological dynamics of dengue in Barbados - one of the English-speaking Caribbean countries. Epidemiol Infect. 2018;146(8):1048–1055. doi: 10.1017/S0950268818000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Getahun A., Batikawai A., Nand D., Khan S., Sahukhan A., Faktaufon D. Dengue in Fiji: epidemiology of the 2014 DENV-3 outbreak. Western Pac Surveill Response J. 2019;10(2):31–38. doi: 10.5365/wpsar.2018.9.3.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harapan H., Michie A., Yohan B. Dengue viruses circulating in Indonesia: a systematic review and phylogenetic analysis of data from five decades. Rev Med Virol. 2019;29(4):e2037. doi: 10.1002/rmv.2037. [DOI] [PubMed] [Google Scholar]
- 30.Harapan H., Michie A., Mudatsir M., Sasmono R.T., Imrie A. Epidemiology of dengue hemorrhagic fever in Indonesia: analysis of five decades data from the national disease surveillance. BMC Res Notes. 2019;12(1):350. doi: 10.1186/s13104-019-4379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayurasakorn S., Suttipun N. The impact of a program for strengthening dengue hemorrhagic fever case management on the clinical outcome of dengue hemorrhagic fever patients. Southeast Asian J. Trop. Med. Public Health. 2010;41(4):858–863. [PubMed] [Google Scholar]
- 32.Struchiner C.J., Rocklöv J., Wilder-Smith A., Massad E. Increasing dengue incidence in singapore over the past 40 years: population growth, climate and mobility. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0136286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y., Kamara F., Zhou G. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl Trop Dis. 2014;8(11):e3301. doi: 10.1371/journal.pntd.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott T.W., Clark G.G., Lorenz L.H., Amerasinghe P.H., Reiter P., Edman J.D. Detection of multiple blood feeding in Aedes aegypti (Diptera: culicidae) during a single gonotrophic cycle using a histologic technique. J Med Entomol. 1993;30(1):94–99. doi: 10.1093/jmedent/30.1.94. [DOI] [PubMed] [Google Scholar]
- 35.Kakkar M. Dengue fever is massively under-reported in India, hampering our response. BMJ. 2012;345:e8574. doi: 10.1136/bmj.e8574. [DOI] [PubMed] [Google Scholar]
- 36.Utama I., Lukman N., Sukmawati D. Dengue viral infection in Indonesia: epidemiology, diagnostic challenges, and mutations from an observational cohort study. PLoS Negl Trop Dis. 2019;13(10) doi: 10.1371/journal.pntd.0007785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.