Abstract
The LuxS quorum sensing system is considered as the main system that most of the oral bacteria use to communicate in order to create biofilms. Here we identified 11 of the most important biofilm formers that utilize the LuxS system and presented current and recent information regarding this system. Though different bacterial species are able to communicate thorough the LuxS system, it was also found that cross kingdom communication can occur between bacteria and fungi and bacteria and epithelial cells. Immune response also plays and important role in mitigating the effects of biofilms. Here we identified 6 of the most important molecules that are involved in the immune response to biofilms. These immune molecules maintain the stability in the oral cavity by preventing bacteria from overwhelming the space and simultaneously minimizing the immune response in order not to cause tissue damage. Here we also discuss current research being done in order to maintain the balance in the oral cavity via inhibiting biofilm formation without eradicating oral bacteria in order to prevent the overgrowth of other organisms such as Candida albicans. One approach being used is inhibiting AI-2 intermediates which leads to lack of quorum sensing communication between bacteria through the use of intermediate analogues. Another approach that found success is the utilization of D forms of sugars where D-ribose and D-galactose have been proven to inhibit the LuxS system and subsequently preventing the process of quorum sensing leading to the reduction in biofilm formation.
Keywords: LuxS, Quorum sensing, Biofilm, Oral microflora, Oral immune response
1. Introduction
Oral microbiology, though harmless in most cases, tend to form biofilms in the oral cavity. Studies conducted on oral cavity mucosa have shown that the body responds to the presence of bacteria by triggering an immune response in addition to various pathways contributing to survivability of cells and apoptotic behavior (Ebersole et al., 2019). One of the major tools that bacteria utilize to communicate with each other is Quorum sensing. It is essentially the language that bacteria communicate with (Shao and Demuth, 2010). The language is communicated through releasing of proteins known as autoinducers. It was found that when growing bacteria in liquid broth, removing bacteria will leave behind a trail of proteins that could send messages to other bacteria in order to communicate (Nealson and Hastings, 1979). This communication is believed to be cross species (Lewenza et al., 2002, Riedel et al., 2001). While Pseudomonas and Burkholderia bacterial species are known to be able to cross communicate utilizing N-acylhomoserine lactone dependent quorum sensing, encoded by LuxI and LuxR, such product have not been identified in oral bacterial biofilms. Even though the research field on oral biofilm has been studied extensively, the detection of such compounds has been overlooked in addition to the fact that genetic material studies did not indicate the presence of N-acylhomoserine, however, saliva studies did show that it contained some N-acylhomoserine indicating the presence of the protein (Kumari et al., 2006, Kumari et al., 2008). The main challenge is that only half of the bacteria in the oral cavity are cultivable, while the other half can be detected only through the presence of their DNA which greatly hinders the ability to study the whole microbiota of the oral cavity (Chen et al., 2010, Dewhirst et al., 2010).
Later it was found that there were other communication proteins designated as autoinducer-2 (AI-2), encoded by LuxS (Surette et al., 1999). It was also found that the AI-2 system contributes to antibiotic resistance in bacteria giving it even further importance medically due to the dire situation with wide spread antibiotic resistance (Ju et al., 2018). Even with the current knowledge, there is still a lot that is unknown about quorum sensing in biofilms.
It was found that the communication between bacteria in order to form and co-exist in biofilms is extremely important for the survival of the bacteria, but also a major source for oral diseases. The fight to control oral bacterial biofilm has long existed even before the current knowledge in biofilms. Biofilms are communities of bacteria tightly growing together on surfaces and providing shelter and nutrient to each other by secreting extracellular sugars and other materials. As a result, bacteria become a very tight knit community that is hard to get rid of. Many studies have been done to break-down bacterial biofilms yet nothing has reached satisfactory results and physical removing of bacteria via tooth brushing remains as the hallmark for oral hygiene (Darby, 2009, Hellström et al., 1996). But the problem remains with deep pocket infections within the oral cavity that physical removal of bacteria is not possible. With periodontal diseases being classified as a serious health problem, 5 to 20 of adults suffer from such diseases (Petersen et al., 2005). With biofilms remaining as the predominant problem with oral cavity infections, little is known about chemical and genetic interaction of bacteria to coexist with each other in biofilm communities where they remain protected and continue to cause harm when present in unwanted areas throughout the oral cavity. Though short term presence of bacterial biofilms in the oral cavity may not be harmful, long term presence will lead to sever illnesses.
In this article, we will summarize the current knowledge about oral bacteria quorum sensing and its relationship to biofilm formation while emphasizing the major known genes responsible for these actions and the immune response genes that are activated as a result of being exposed to bacterial biofilms.
2. Biofilms and the LuxS system of oral bacteria:
Oral bacteria form biofilms in the oral cavity in order to protect from harmful agents in addition to forming communities to feed and nourish themselves (Hall-Stoodley et al., 2004). The bacteria involved in most known pathogenic oral infections are listed in Table 1 and biofilm capabilities discussed in this segment. Oral bacteria communicate with a quorum sensing systems to send protein signals between each other. These signals affect multiple aspects of the oral biofilm including carbohydrate metabolism, biofilm structure and biofilm composition (Brambilla et al., 2016, Tong et al., 2011). One of the most well studied bacteria in terms of cariogenic activities is Streptococcus mutans (Fumes et al., 2018, Salli and Ouwehand, 2015). Recent studies have shown that the bacterium could communicate with other streptococci utilizing the LuxS system. Recently, it was found by Wang et al that S. mutans and S. gordonii quorum sensing mechanism affected multiple aspects of the dual bacteria biofilm and also increased susceptibility to chlorohexidine (Wang et al., 2017). Gene expression analysis of genes involved in S. mutans biofilm formation was also assayed in this study. It was found that spaP, fruA, gtfB, gtfC and gtfD were all upregulated when 0.1uM of AI-2 was added to the culture. spaP is onvolved in adhesion of bacteria while fruA is involved in biofilm formation and gtfB, gtfC and gtfD are involved in both adhesion and biofilm formation and are all considered to be cariogenic genes. The result in biofilm formation complemented the results of the gene expression study. However, when adding 10uM of AI-2, it was found that there was no significant increase of gene expression when compared to the negative control indicating the importance of AI-2 in the formation of biofilms for S. mutans. This is a significant finding in understanding the role of quorum sensing in biofilm formation. It was also found the AI-2 affected biofilm formation between S. gordonii and P. gingivalis (Mcnab et al., 2003). The authors identified the presence of the LuxS gene in S. gordonii and proceeded to create a knock out of the LuxS gene; the resulting bacteria were unable to produce AI-2 which resulted in almost no reduction in the S. gordonii biofilm formation capabilities. However, when analyzing biofilm capabilities for P. ginigivalis, it was found that when co-culturing with S. gordonii, only the strain that had the LuxS was able to lead to a co-culture biofilm. This indicated that LuxS and AI-2 from S. gordonii are essential for the ability of P. ginigivalis to produce biofilms. The authors were able to also show their results via confocal laser microscope. Visualizing the bacteria in the co-cultured biofilm had shown that P. gingivalis only stayed on the surface of the S. gordonii biofilm layer. It was also reported that AI-2 involvement in the co-culture biofilm affected carbohydrate metabolism as well. Four proteins involved in carbohydrates metabolism were overproduced as a result of AI-2 stimulation including GTF, YlbN-like protein, fructanase and tagatose 1,6-diphosphate aldolase (Mcnab et al., 2003). The breakdown of carbohydrates will ultimately lead to the contribution of biofilm formation and eventually dental caries (Pitts et al., 2017, Yu et al., 2017).
Table 1.
Overview of the most important oral biofilm bacteria that have the Lux quorum sensing system.
| No. | Bacterium | Quorom Lux system | Protein | Important notes |
|---|---|---|---|---|
| 1 | Burkholderia Spp. (Riedel et al., 2001) | LuxI/LuxR | N-acyl homoserine (AHL) | Transient oral bacteria that uses the AHL system found in gram negative bacteria and found in saliva |
| 2 | Pseudomonas aeruginosa (Riedel et al., 2001) | LuxI/LuxR | (AHL) | |
| 3 | Vibrio harveyi (Bassler, 1999, Nealson et al., 1970) | LuxI/LuxR/LuxS | AHL and AI-2 | Not an oral bacterium, but able to use both quorum sensing systems and main model used to understand the Lux system |
| 4 | Porphyromonas gingivalis (Frias et al., 2001) | LuxS | Autoinducer-2 (AI-2) | Three important oral bacteria that belong to the same genera |
| 5 | Fusobacterium nucleatum (Frias et al., 2001) | LuxS | AI-2 | |
| 6 | Prevotella intermedia (Frias et al., 2001) | LuxS | AI-2 | |
| 7 | Streptococus mutans (Sztajer et al., 2008) | LuxS | AI-2 | Oral gram positive and gram negative bacteria notorious for forming oral biofilms |
| 8 | Streptococcus gordonii (Cuadra-Saenz et al., 2012) | LuxS | AI-2 | |
| Streptococcus oralis (Cuadra-Saenz et al., 2012) | LuxS | AI-2 | ||
| 9 | Agregatibacter actinomycetemcomitans (Fong et al., 2001) | LuxS | AI-2 | |
| 10 | Eikenella corodens (Azakami et al., 2006) | LuxS | AI-2 | |
| 11 | Enterococcus faecalis (Laganenka and Sourjik, 2018) | LuxS | AI-2 | |
| 12 | Escherichia coli (Li et al., 2007) | LuxS | AI-2 | Transient oral bacteria |
Recent studies have showed that cross communication between different species of microorganisms in the oral cavity may occur. The communication between cross species of S. mutans and C. albicans has been established (Koo et al., 2018). Bachtiar et al have shown that sending messages between Agregatibacter actinomycetemcomitans bacterium and Candida albicans, which is an opportunistic fungal species found in the oral cavity, was possible through activation of the LuxS gene (Bachtiar and Bachtiar, 2020). The releasing of the AI-2 from A. actinomycetemcomitans lead to a decrease in biofilm formation by the C. albicans and an increase in the biofilm formation by S. mutans. This indicated that AI-2 from A actinomycetemcomitans is required to push S. mutans to produce more biofilm while causing C. albicans to reduce biofilm production. This is another example of the cross species talk that could occur as a result of the quorum sensing activity of oral bacteria. Another role for the AI-2 has been shown in A actinomycetemcomitans and P. gingivalis where it was found that AI-2 also regulates iron chelation and acquisition (Fong et al., 2003, James et al., 2006). Iron deficient media have shown to reduce the growth of bacteria indicating the importance of iron for cell to cell signaling and the survivability of oral bacteria (James et al., 2016). This supports the idea that bacteria survive in the oral cavity by providing support to each other utilizing cross species quorum sensing communication. In most cases the LuxS system is released in connection to environmental stress as a signal for survivability measures including DNA repair and nutritional acquisition (Yuan et al., 2005). Laganenka et al were also able to show that AI-2 from E. faecalis was able to significantly increase biofilm formation activity in the bacterium E. coli (Laganenka and Sourjik, 2018). Through measuring of cell viability in a static fashion, it was shown that cell numbers were higher when E. coli and E. faecalis were co-cultured together. Utilizing fluorescence microscopy, the authors were able to show that E. coli aggregates were 3 times larger after just 2 h of co-culturing. It was also shown that the increase in biofilm activity was specifically due to the presence of AI-2. Interestingly, the increase in biofilm capabilities of E. coli was irrelevant of bacterial contact indicating that the message was sent via AI-2. When measuring the response of co-cultured E. coli and E. faecalis to oxidative stress, it was reported that co-cultured biofilms were 17% more resistant than single bacteria biofilm indicating stress response is an inducer to AI-2 release (Laganenka and Sourjik, 2018). Although E. coli is not known as the other oral bacteria, it is present in the oral cavity in a transient fashion, other Gram positive bacteria my increase their virulence by quorum sensing communication through the LuxS system. With the presence of other Gram negative bacteria that are considered enterobactereacae, such as Klebsiella, such co-culture mechanism may be of significant importance in oral diseases, especially in Gram negative organism.
3. Immune response
The immune response of the oral mucosa to the presence of oral biofilms is important to keeping bacterial activity in check in the oral cavity. Many genes have been identified as being essential for a normal immune response (Mancl et al., 2013). Little information is known about the extensiveness of the immune response for the constant presence of the naturally occurring oral bacteria. Though many genes have been identified, the constitutive expression nature of these genes has not been studied extensively. Here we will summarize some of the most important immune response molecules that are responsible for controling oral microbiom. Most of proteins encoded by these genes have been identified either in the oral mucosa or saliva. Table 2 demonstrates the most important molecules that will be discussed in this section.
Table 2.
Most important biofilm immune response molecules.
| No. | Immune molecule | Role |
|---|---|---|
| 1 | slgA | Dimeric IgA produced in large amounts in saliva |
| 2 | Hsp70 | Heath shock chaperone protein |
| 3 | oPMN | Circulating neutrophils capable of rapid mobility |
| 4 | MMp | Enzyme involved in destruction of extracellular matrix |
| 5 | TNF | Cytokine involved in cell destruction and bone remolding |
| 6 | IL-1 | Interleukin involved in tissue and bone destruction |
The slgA immunoglobulin is one of the most important antibodies produced in the mucosal membranes to protect against bacteria in general. It is composed of two IgA type immunoglobulins, 1 and 2, found in dimeric form (Macpherson and Slack, 2007). This immunoglobulin is not only found in the mucosa, but it is also secreted in saliva and found to be stimulated by the important cariogenic bacterium S. mutans (Van Nieuw Amerongen et al., 2004). The important function of immunoglobulins is to prevent massive overgrowth of biofilms and not the eradication of the bacterium indicating the importance of growth control rather than the elimination of bacteria (Van Nieuw Amerongen et al., 2004). There is no work to be found that has connected bacterial AI-2 to the production of slgA. It is expected there will be a correlation between the slgA protein and AI-2 if such study is conducted.
Hsp70 is a stress response protein known in cellular heat shock response. It is a chaperone protein and is believed to be constitutively expressed and involved in the immune-response of the oral cavity (Fábián et al., 2007). It has extracellular cytoprotective properties and stimulates the production of natural killer cells leading to increased cell defense (Fabian et al., 2004). Although there is very little research done to connect oral biofilm and AI-2 to the production of Hsp70, Tsakmakidis et al have shown that common oral bacteria such as Agregatibacter actinomycetemcomitans and S. oralis enhance the production of Hsp70 peri-implant mucosal cells defense mechanism against these bacteria (Ingendoh-Tsakmakidis et al., 2019).
Matrix metalloproteinases (MMp), Tumor Necrosis factor (TNF) and interleukin 1 (IL1) are a part of one immune response system that is activated when lipopolysaccharides (LPS) is released from Gram negative oral bacteria (Page et al., 1997). MMp and TNF mediate epithelial cell destruction and extracellular matrix breakdown during inflammation allowing for new tissue growth and allowing for infiltration of leukocytes into the area of infection (Darby, 2009, Ohlrich et al., 2009). The role of Gram negative bacteria in oral biofilms may significantly increased as a result of activating this inflammatory pathway. On the other hand, IL1 together with TNF are involved in some minor aspects of mediating bone loss (Page et al., 1997). The increase in inflammation is believed to help with increase in biofilm formation by means of cellular secretions providing some nutrients to the bacteria and allowing for extensive growth (Darby, 2009)
It has been recently shown that the epithelial cells are capable of producing a mimic of AI-2 in order to communicate with the bacteria (Ismail et al., 2016). However, only AI-2 like activity was detected. Though the cell lines tested were not oral epithelia cell lines, it is feasible that oral epithelial cell lines will react in a similar manner. For example the bacterial genes that were found to be associated with stimulating such communication in Vibrio harveyi were VIBHAR_RS11610, VIBHAR_RS11600, VIBHAR_RS16700 and VIBHAR_RS04115. It was found that VIBHAR_RS11600 had a DNA binding site indicating potential role in regulation of AI-2 mimic gene expression. Running VIBHAR_RS11600 through Phyre 2 software for protein prediction demonstrated that the DNA binding domain (Fig. 1) is identical to many transcriptional regulatory protein in other bacterial species such as E. faecalis, S. epidermidis, S. aureus and K. pneumoniae. This indicates a potential role for these bacteria and the ability to induce host-bacteria communication and stimulating AI-2 mimic from various epithelial cells in the human body. However DNA sequence comparison did not show any homology with any other bacteria except for various species of Vibrio.
Fig. 1.
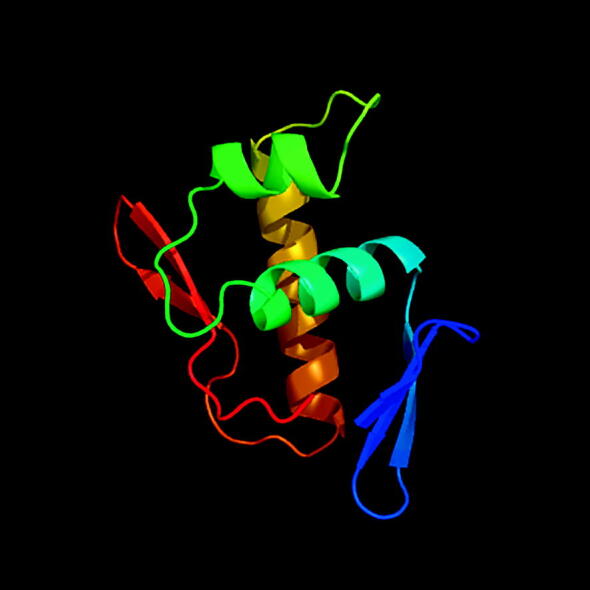
3D structure of the VIBHAR_RS11600 DNA binding protein as it was predicted by the Phyr2 protein prediction software.
Oral Polymorphonuclear Neutrophils (oPMNs) are activated by plaque biofilm as a defense mechanism (Rijkschroeff et al., 2018). Its main function is to balance the presence of oral bacteria and the health of the oral cavity. When oPMNs are over produced it leads to oral tissue damage and eventually increase inflammatory response. oPMN circulate in the blood system and is able to be rapidly mobilized to the oral cavity in response to inflammation inducers (Borregaard, 2010, Furze and Rankin, 2008). It was shown that oPMNs can be found in saliva and oral rinses of dental patients, and even a much larger increase in patients with periodontitis indicating its importance in the control of oral microbiology (Loos, 2016). Despite that, similar to previous immune-response proteins, there is little to no work done to connect the release of oPMNs to the production of the important quorum sensing molecule AI-2.
4. Inhibition of biofilms through discovery of AI-2 inhibitors
Due to the well-established role of AI-2 in biofilm formation and quorum sensing, the idea of inhibiting AI-2 as a preventive measure for reducing oral biofilms has been studied with various compounds. Weiland- Bräuer et al have done some metagenomics analysis in order to search for potential proteins that would inhibit both the AHL and AI-2 systems (Weiland-Bräuer et al., 2016). They were able to identify 142 for AHL and 13 for AI-2 as Quorum Quenching proteins (QQ). The QQ proteins were able to inhibit biofilms from E. coli, B. subtilis and S. aureus but not P. aeruginosa which uses the AHL system instead of the AI-2 system (Sauer et al., 2002). Although all four organisms are mostly transient residents of the oral cavity, the concept of AI-2 inhibition could be applied on the more permanent oral organisms as well. Muras et al tested extract of the marine bacterium Tenacibaculum sp. and found that the bacterial extract lead to reduction in AI-2 activity and biofilm formation in three important oral bacteria, S. mutans, S. oralis and S. dentisani by a significant margin (Muras et al., 2018). With this bacterium known to produce toxins and being pathogenic to marine life, the likelihood of utilizing the extract as treatment is not possible; however, identifying the particular compound causing inhibition of quorum sensing may be of significance for future studies (Jal and Khora, 2015). Another technique that has been used to inhibit AI-2 is the usage of analogues. Schramm et al have used an analogue for methylthioadenosine nucleosidase, MTAN, which acts by converting S-adenosylhomocysteine (SAH) to ribosylhomocysteine, which is an intermediate in AI-2 biochemical synthesis (Schramm, 2007). This finding indicates that such compounds are capable of interrupting the AI-2 quorum sensing system through provision of intermediate analogues that interfere with biochemical synthesis of AI-2 proteins and therefore reduce or even prevent the synthesis of biofilm. Interestingly, it has been reported that D-galactose inhibited biofilm formation in the notorious cariogenic bacterium S. mutans. Ryu et al showed that D-galactose reduced biofilm formation significantly at various concentrations with 20 mM being the maximum effective concentration in this particular study by reducing biofilm capabilities by more than 50%. At 200 mM D-galactose, the effect was nearly identical to that of 20 mM (Ryu et al., 2020). This was consistent with what the same group have reported earlier about D-galactose inhibiting AI-2 leading to the inhibition of biofilm formation of A actinomycetemcomitans and P. gingivalis. (Ryu et al., 2016). Choi et al even applied for a US patent for technology to use D-galactose as a method for inhibiting AI-2 and therefore inhibit biofilm formation in patients who need it (Choi et al., 2019). These finding show that D-galactose is a strong candidate for further studying and analysis for wide spread anti-biofilm use with relatively no toxicity.
The concept of inhibiting biofilm formation in the oral cavity has mostly been done in vitro, providing us with a proof of concept for such approaches. In order to examine the capability of reducing AI-2 in an in vivo model, Cho et al have tested known anti AI-2 molecules Furanone and D-ribose on periodontal mouse model (Cho et al., 2016). They were able to show that the addition of D-ribose reduced P. gingivalis numbers by more than 31% suggesting that adding D-ribose to drinking water would help with reduction of biofilms in the oral cavity. Suzuki et al have hypothesized that Fructanase binds to AI-2 and inhibits quorum sensing in S. mutans which in turn reduced biofilm formation significantly (Suzuki et al., 2017). They were able to show that inhibition occurred not only to the biofilm mass, but also reduced the production of other important biofilm ingredients such as eDNA and even extracellular insoluble carbohydrates such as glucans which are important in oral biofilm formation. They were able to show that the inhibition of biofilms occurred at many levels and not just AI-2 system. This indicates the strength of Fructanse as anti-biofilm agent as it was thoroughly studied by Suzuki et al.
5. Conclusion
The importance of AI-2 in oral bacterial biofilm is well established. With quorum sensing being a major contributor to biofilm formation, inhibition of quorum sensing would seemingly be an effective method of reducing oral diseases as most of them are caused by oral bacteria forming biofilms. Combating biofilms while making sure to not kill all the bacteria is a challenge, specifically when we know that fungal microorganisms, such as C. albicans, will replace bacteria and start growing in the oral cavity causing a lot of problems. Having a normal microflora with little disturbance is ideal in order to maintain the balance. With a lot of work being done on preventing the formation of oral bacterial biofilms, inhibition of the LuxS system through prevention of the AI-2 protein from sending messages would be ideal. With the available molecules, such as D-galactose, that have little toxicity on both the oral epithelium and bacteria but yet still have the capabilities to prevent biofilms, it would be ideal to have such compound investigated and presented to the public as an oral health preventive measure. Since most of the work being done on AI-2 being recent, a lot of work is still to be done to asses such AI-2 inhibitors. One area of research that was not covered well is the immune response towards the presence of AI-2. Though AI-2′s role in oral biofilms has been established, its importance to the immune response has not been demonstrated. It is expected to have a role in the immune response since it is found to be an abundantly used protein. Though this article demonstrated the most important immune response molecules to biofilms in the oral cavity, no article was able to show the direct response of the immune system towards the protein. This will be an interesting field to observe in the future. One aspect of utilizing anti-biofilm treatment is that it maintains the presence of oral bacteria which is needed for a healthy oral cavity, but prevents the harmful aspects of these bacteria. Two major strategies that have been implemented in order to counter act biofilm formation through inhibit of AI-2 mostly involve the use of analogues or metagenomics analysis to find natural proteins that could bind to the AI-2 protein and inhibit it. Though compounds like D-ribose and D-galactose have been shown to inhibit AI-2 communication, the exact method of the inhibition has not been fully understood. One important oral bacterium that was not talked about here is Treponema denticola. Though it is an important organism in pathogenic oral biofilms, no report of it having AI-2 has been presented indicating that it is a receiver of the AI-2 message, but unable to produce it. It would be interesting to see in the future a breakdown of bacteria that are capable of producing AI-2 and ones that are only capable of receiving it to get a better understanding on how oral bacteria biofilm communities work. Most of the work on AI-2 inhibitors focused on just the biofilm formation aspect, but Suzuki et al have toughly investigated the response and showed that anti-biofilm activity affect bacteria at many levels and not just its biofilm capabilities. Even with all the compounds and molecules that have been identified to interfere with biofilm formation, it expected that not only biofilm in these organisms would be affected, but rather a compounded effect is expected to be seen upon deeper investigations. With the development of current computational technology, many scientists have taken the rout to use in silico approaches to identify molecules that inhibit biofilm formation (Byeon et al., 2017). Such techniques are inexpensive and provide a lot of information that will allow scientist to work more comfortably before laboratory experimentation as the potential for these molecules have already been explored beforehand.
Ethical statement
Ethical statement is not needed due the fact this review article did not involve any subjects or experimentation.
Author statement
The author of the paper did the research, information/reference collection, writing and revision of the manuscript.
Declaration of Competing Interest
The author would like to declare that there are no conflict of interest to be disclosed.
Footnotes
Peer review under responsibility of King Saud University.
References
- Azakami H., Teramura I., Matsunaga T., Akimichi H., Noiri Y., Ebisu S., Kato A. Characterization of autoinducer 2 signal in Eikenella corrodens and its role in biofilm formation. J. Biosci. Bioeng. 2006;102(2):110–117. doi: 10.1263/jbb.102.110. [DOI] [PubMed] [Google Scholar]
- Bachtiar E.W., Bachtiar B.M. Effect of cell-free spent media prepared from Aggregatibacter actinomycetemcomitans on the growth of Candida albicans and Streptococcus mutans in co-species biofilms. Eur. J. Oral Sci. 2020 doi: 10.1111/eos.12725. [DOI] [PubMed] [Google Scholar]
- Bassler B.L. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 1999;2(6):582–587. doi: 10.1016/s1369-5274(99)00025-9. [DOI] [PubMed] [Google Scholar]
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Brambilla E., Ionescu A.C., Cazzaniga G., Ottobelli M., Samaranayake L.P. Levorotatory carbohydrates and xylitol subdue Streptococcus mutans and Candida albicans adhesion and biofilm formation. J. Basic Microbiol. 2016;56(5):480–492. doi: 10.1002/jobm.201500329. [DOI] [PubMed] [Google Scholar]
- Byeon J.Y., Sim J., Ryu E.J., Sim J., Lee H., Cho K.H., Choi B.K., Lee J. In Silico Development of Quorum-Sensing Inhibitors. Bull. Korean Chem. Soc. 2017;38(7):728–734. [Google Scholar]
- Chen T., Yu W.H., Izard J., Baranova O.V., Lakshmanan A., Dewhirst F.E. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database. 2010 doi: 10.1093/database/baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.J., Song H.Y., Ben Amara H., Choi B.K., Eunju R., Cho Y.A., Seol Y., Lee Y., Ku Y., Rhyu I.C., Koo K.T. In vivo inhibition of Porphyromonas gingivalis growth and prevention of periodontitis with quorum-sensing inhibitors. J. Periodontol. 2016;87(9):1075–1082. doi: 10.1902/jop.2016.160070. [DOI] [PubMed] [Google Scholar]
- Choi, B.K., Ryu, E.J., Sim, J.H., Kim, B.M., Lee, J., Sim, J., inventors, 2019. Seoul National University R&DB Foundation, Soongsil University. Foundation of University-Industry Cooperation, assignee, 2019. Method of inhibiting quorum sensing using d-galactose. United States patent application US 16/364,422.
- Cuadra-Saenz G., Rao D.L., Underwood A.J., Belapure S.A., Campagna S.R., Sun Z., Tammariello S., Rickard A.H. Autoinducer-2 influences interactions amongst pioneer colonizing streptococci in oral biofilms. Microbiology. 2012;158(Pt 7):1783. doi: 10.1099/mic.0.057182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby I. Non-surgical management of periodontal disease. Aust. Dent. J. 2009;54:S86–S95. doi: 10.1111/j.1834-7819.2009.01146.x. [DOI] [PubMed] [Google Scholar]
- Dewhirst F.E., Chen T., Izard J., Paster B.J., Tanner A.C., Yu W.H., Lakshmanan A., Wade W.G. The Human Oral Microbiome. J. Bacteriol. 2010;192(19):5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole J.L., Peyyala R., Gonzalez O.A. Biofilm-Induced Profiles of Immune Response Gene Expression by Oral Epithelial Cells. Mol. Oral Microbiol. 2019;34(1) doi: 10.1111/omi.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fábián T.K., Fejérdy P., Nguyen M.T., Sőti C., Csermely P. Potential immunological functions of salivary Hsp70 in mucosal and periodontal defense mechanisms. Archivum immunologiae et therapiae experimentalis. 2007;55(2):91. doi: 10.1007/s00005-007-0012-z. [DOI] [PubMed] [Google Scholar]
- Fabian T.K., Toth Z., Fejerdy L., Kaan B., Csermely P., Fejérdy P. Photo-acoustic stimulation increases the amount of 70 kDa heat shock protein (Hsp70) in human whole saliva. A pilot study. Int. J. Psychophysiol. 2004;52(2):211–216. doi: 10.1016/j.ijpsycho.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Fong K.P., Chung W.O., Lamont R.J., Demuth D.R. Intra-and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitansLuxS. Infect. Immun. 2001;69(12):7625–7634. doi: 10.1128/IAI.69.12.7625-7634.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong K.P., Gao L., Demuth D.R. luxS and arcB control aerobic growth of Actinobacillus actinomycetemcomitans under iron limitation. Infect. Immun. 2003;71(1):298–308. doi: 10.1128/IAI.71.1.298-308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias, J., Olle, E., Alsina, M., 2001. Periodontal pathogens produce quorum sensing signal molecules. Infect. Immunity.69(5):3431-4. [DOI] [PMC free article] [PubMed]
- Fume, A.C., da Silva Telles, P.D., Corona, S.A., Borsatto, M.C., 2018. Effect of aPDT on Streptococcus mutans and Candida albicans present in the dental biofilm: Systematic review. Photodiag. Photodynam. Therapy, vol. 21, pp. 363-366. [DOI] [PubMed]
- Furze R.C., Rankin S.M. Neutrophil mobilization and clearance in the bone marrow. Immunology. 2008;125(3):281–288. doi: 10.1111/j.1365-2567.2008.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Hellström M.K., Ramberg P., Krok L., Lindhe J. The effect of supragingival plaque control on the subgibgival microflora in human periodontitis. J. Clin. Periodontol. 1996;23(10):934–940. doi: 10.1111/j.1600-051x.1996.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Ingendoh-Tsakmakidis A., Mikolai C., Winkel A., Szafrański S.P., Falk C.S., Rossi A., Walles H., Stiesch M. Commensal and pathogenic biofilms differently modulate peri-implant oral mucosa in an organotypic model. Cell. Microbiol. 2019;21(10) doi: 10.1111/cmi.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail A.S., Valastyan J.S., Bassler B.L. A host-produced autoinducer-2 mimic activates bacterial quorum sensing. Cell Host Microbe. 2016;19(4):470–480. doi: 10.1016/j.chom.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jal S., Khora S.S. An overview on the origin and production of tetrodotoxin, a potent neurotoxin. J. Appl. Microbiol. 2015;119(4):907–916. doi: 10.1111/jam.12896. [DOI] [PubMed] [Google Scholar]
- James C.E., Hasegawa Y., Park Y., Yeung V., Tribble G.D., Kuboniwa M., Demuth D.R., Lamont R.J. LuxS involvement in the regulation of genes coding for hemin and iron acquisition systems in Porphyromonas gingivalis. Infect. Immun. 2006;74(7):3834–3844. doi: 10.1128/IAI.01768-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju X., Li J., Zhu M., Lu Z., Lv F., Zhu X., Bie X. Effect of the luxS gene on biofilm formation and antibiotic resistance by Salmonella serovar Dublin. Food Res. Int. 2018;107:385–393. doi: 10.1016/j.foodres.2018.02.039. [DOI] [PubMed] [Google Scholar]
- Koo H., Andes D.R., Krysan D.J. Candida–streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 2018;14(12) doi: 10.1371/journal.ppat.1007342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A., Pasini P., Deo S.K., Flomenhoft D., Shashidhar H., Daunert S. Biosensing systems for the detection of bacterial quorum signaling molecules. Anal. Chem. 2006;78(22):7603–7609. doi: 10.1021/ac061421n. [DOI] [PubMed] [Google Scholar]
- Kumari A., Pasini P., Daunert S. Detection of bacterial quorum sensing N-acyl homoserine lactones in clinical samples. Anal. Bioanal. Chem. 2008;391(5):1619–1627. doi: 10.1007/s00216-008-2002-3. [DOI] [PubMed] [Google Scholar]
- Laganenka L., Sourjik V. Autoinducer 2-dependent Escherichia coli biofilm formation is enhanced in a dual-species coculture. Appl. Environ. Microbiol. 2018;84(5) doi: 10.1128/AEM.02638-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewenza S., Visser M.B., Sokol P.A. Interspecies communication between Burkholderia cepacia and Pseudomonas aeruginosa. Can. J. Microbiol. 2002;48(8):707–716. doi: 10.1139/w02-068. [DOI] [PubMed] [Google Scholar]
- Li J., Attila C., Wang L., Wood T.K., Valdes J.J., Bentley W.E. Quorum sensing in Escherichia coli is signaled by AI-2/LsrR: effects on small RNA and biofilm architecture. J. Bacteriol. 2007;189(16):6011–6020. doi: 10.1128/JB.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos B.G. Severe periodontitis may result in higher numbers of oral polymorphonuclear (oPMN) leukocytes in oral rinse samples. J. Evid. Based Dental Pract. 2016;16(1):73–74. doi: 10.1016/j.jebdp.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Macpherson A.J., Slack E. The functional interactions of commensal bacteria with intestinal secretory IgA. Curr. Opin. Gastroenterol. 2007;23(6):673–678. doi: 10.1097/MOG.0b013e3282f0d012. [DOI] [PubMed] [Google Scholar]
- Mancl K.A., Kirsner R.S., Ajdic D. Wound biofilms: lessons learned from oral biofilms. Wound Repair Regene. 2013;21(3):352–362. doi: 10.1111/wrr.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab R., Ford S.K., El-Sabaeny A., Barbieri B., Cook G.S., Lamont R.J. LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J. Bacteriol. 2003;185(1):274–284. doi: 10.1128/JB.185.1.274-284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muras, A., Mayer, C., Romero, M., Camino, T., Ferrer, M.D., Mira, A., Otero, A., 2018. Inhibition of Steptococcus mutans biofilm formation by extracts of Tenacibaculum sp. 20J, a bacterium with wide-spectrum quorum quenching activity. J. Oral Microbiol., vol. 10, 1, pp. 1429788. [DOI] [PMC free article] [PubMed]
- Nealson K.H., Platt T., Hastings J.W. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 1970;104(1):313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K.H., Hastings J.W. Bacterial bioluminescence: its control and ecological significance. Microbiol. Rev. 1979;43(4):496. doi: 10.1128/mr.43.4.496-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrich E.J., Cullinan M.P., Seymour G.J. The immunopathogenesis of periodontal disease. Aust. Dent. J. 2009;54:S2–S10. doi: 10.1111/j.1834-7819.2009.01139.x. [DOI] [PubMed] [Google Scholar]
- Page, R.C., Offenbacher, S., Schroeder, H.E., Seymour, G.J., Kornman, K.S., 1997. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontology 2000. 14(1):216-48. [DOI] [PubMed]
- Petersen P.E., Bourgeois D., Ogawa H., Estupinan-Day S., Ndiaye C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005;83:661–669. [PMC free article] [PubMed] [Google Scholar]
- Pitts N.B., Zero D.T., Marsh P.D., Ekstrand K., Weintraub J.A., Ramos-Gomez F., Tagami J., Twetman S., Tsakos G., Ismail A. Dental caries. Nat. Rev. Disease Primers. 2017;3(1):1–6. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- Riedel K., Hentzer M., Geisenberger O., Huber B., Steidle A., Wu H., Høiby N., Givskov M., Molin S., Eberl L. N-acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology. 2001;147(12):3249–3262. doi: 10.1099/00221287-147-12-3249. [DOI] [PubMed] [Google Scholar]
- Rijkschroeff P., Loos B.G., Nicu E.A. Oral polymorphonuclear neutrophil contributes to oral health. Curr. Oral Health Rep. 2018;5(4):211–220. doi: 10.1007/s40496-018-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu E.J., An S.J., Sim J., Sim J., Lee J., Choi B.K. Use of d-galactose to regulate biofilm growth of oral streptococci. Arch. Oral Biol. 2020;111 doi: 10.1016/j.archoralbio.2020.104666. [DOI] [PubMed] [Google Scholar]
- Ryu E.J., Sim J., Sim J., Lee J., Choi B.K. D-Galactose as an autoinducer 2 inhibitor to control the biofilm formation of periodontopathogens. J. Microbiol. 2016;54(9):632–637. doi: 10.1007/s12275-016-6345-8. [DOI] [PubMed] [Google Scholar]
- Salli K.M., Ouwehand A.C. The use of in vitro model systems to study dental biofilms associated with caries: a short review. J. Oral Microbiol. 2015;7(1):26149. doi: 10.3402/jom.v7.26149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer K., Camper A.K., Ehrlich G.D., Costerton J.W., Davies D.G. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 2002;184(4):1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm V.L. Enzymatic transition state theory and transition state analogue design. J. Biol. Chem. 2007;282(39):28297–28300. doi: 10.1074/jbc.R700018200. [DOI] [PubMed] [Google Scholar]
- Shao H., Demuth D.R. Quorum sensing regulation of biofilm growth and gene expression by oral bacteria and periodontal pathogens. Periodontology. 2010;2000(52):53–67. doi: 10.1111/j.1600-0757.2009.00318.x. [DOI] [PubMed] [Google Scholar]
- Surette M.G., Miller M.B., Bassler B.L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. 1999;96(4):1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Nagasawa R., Senpuku H. Inhibiting effects of fructanase on competence-stimulating peptide-dependent quorum sensing system in Streptococcus mutans. J. Infect. Chemother. 2017;23(9):634–641. doi: 10.1016/j.jiac.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Sztajer H., Lemme A., Vilchez R., Schulz S., Geffers R., Yip C.Y., Levesque C.M., Cvitkovitch D.G., Wagner-Döbler I. Autoinducer-2-regulated genes in Streptococcus mutans UA159 and global metabolic effect of the luxS mutation. J. Bacteriol. 2008;190(1):401–415. doi: 10.1128/JB.01086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H., Zeng L., Burne R.A. The EIIABMan phosphotransferase system permease regulates carbohydrate catabolite repression in Streptococcus gordonii. Appl. Environ. Microbiol. 2011;77(6):1957–1965. doi: 10.1128/AEM.02385-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nieuw Amerongen A., Bolscher J.G., Veerman E.C. Salivary proteins: protective and diagnostic value in cariology? Caries Res. 2004;38(3):247–253. doi: 10.1159/000077762. [DOI] [PubMed] [Google Scholar]
- Wang X., Li X., Ling J. Streptococcus gordonii LuxS/autoinducer-2 quorum-sensing system modulates the dual-species biofilm formation with Streptococcus mutans. J. Basic Microbiol. 2017;57(7):605–616. doi: 10.1002/jobm.201700010. [DOI] [PubMed] [Google Scholar]
- Weiland-Bräuer N., Kisch M.J., Pinnow N., Liese A., Schmitz R.A. Highly effective inhibition of biofilm formation by the first metagenome-derived AI-2 quenching enzyme. Front. Microbiol. 2016;7:1098. doi: 10.3389/fmicb.2016.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu O.Y., Zhao I.S., Mei M.L., Lo E.C., Chu C.H. Dental biofilm and laboratory microbial culture models for cariology research. Dentistry J. 2017;5(2):21. doi: 10.3390/dj5020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Hillman J.D., Progulske-Fox A. Microarray analysis of quorum-sensing-regulated genes in Porphyromonas gingivalis. Infect. Immun. 2005;73(7):4146–4154. doi: 10.1128/IAI.73.7.4146-4154.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


