Abstract
Background
Tuberous Sclerosis Complex (TSC) is a genetic disorder characterised by the development of benign tumours secondary to loss of inhibitory regulation of the mTOR (mechanistic Target of Rapamycin) intracellular growth pathway. Metformin inhibits the mTOR pathway. We investigated whether metformin would reduce growth of hamartomas associated with tuberous sclerosis complex.
Methods
In this multicentre randomized, double-blind, placebo-controlled trial, patients with a clinical diagnosis of tuberous sclerosis, aged over 10 years and with at least one renal angiomyolipoma of greater than 1 cm in diameter were enrolled. Participants were randomly allocated (1:1) by a secure website to receive metformin or placebo for 12 months. The primary outcome was percentage volume change of renal angiomyolipomas (AML) at 12 months compared to baseline. Secondary outcomes were percentage change at 12 months from baseline in volume of cerebral Subependymal Giant Cell Astrocytomas (SEGA); appearance of facial and ungual hamartomas; frequency of epileptic seizures; and adaptive behaviour. The trial is registered with The International Standard Randomised Controlled Trial Number (ISRCTN), number 92545532, and the European Union Drug Regulating Authorities Clinical Trials (EUDRACT), number 2011-001319-30.
Findings
Between 1 November 2012 and 30 September 2015 72 patients were screened and 55 were randomly assigned to metformin (28) or placebo (27). Four participants withdrew between randomisation and starting treatment. All 51 patients who started therapy completed the trial and were assessed for outcome at 12 months. The median percentage change in angiomyolipoma (AML) volume was +7.6% (IQR -1.8% to +42.6%) for the placebo group and +8.9% (IQR 1.3% to 19.5%) for the metformin group (p = 0.28). Twenty-seven patients had SEGAs: 13 received placebo and 14 metformin. The median percentage change in SEGA volume was +3.0% (IQR -22.8% to +27.7%) for the placebo group and – 20.8% (IQR – 47.1% to - 5.0%) for the metformin group (p = 0.03). Twenty-one patients were assessed for seizure frequency: 9 received placebo and 12 received metformin. In the metformin group, a mean reduction of 43.7% from baseline in seizures was observed and in the placebo group a 3.1% mean reduction was observed, with a difference in response of 40.6% (95% CI -3.1% to +84.2%, p = 0.03). There were no significant differences between metformin and placebo groups for the other secondary outcomes. There were no deaths. Three serious adverse events (SAEs) occurred during the trial (all patients on metformin).
Interpretation
Metformin did not reduce AML volume. Metformin did reduce SEGA volume and seizure frequency compared with placebo. There may be a role for metformin in slowing or reversing growth of some life-threatening hamartomas in TSC and for reducing seizure frequency. Further study is justified.
Funding
This study was funded by the National Institute for Health and Research (NIHR) through the The Research for Patient Benefit Programme (RfPB).
Research in Context.
Evidence before this study
We performed a literature search on the treatment of Tuberous Sclerosis Complex and have continued to update this review until 1st May 2020. In identifying research in the area, we searched the HDAS (Healthcare Database Advanced Search) including EMBASE, AMED, BNI, CINAHL, EMCARE, HMIC, Medline, PsycINFO and PubMed (up to May 1, 2020), and the reference lists of all relevant retrieved articles. We searched with the terms “Tuberous Sclerosis Complex”, “Tuberous Sclerosis”, “TSC”, “TS”, “mTOR”, “mTOR inhibit*”, “metformin”, “randomized controlled trial”, “controlled clinical trial”, and “clinical trial” with no language restrictions.
We found no randomised-controlled trial investigating metformin in TSC. We identified studies, which have shown that mTOR inhibitors, specifically rapamycin and everolimus, can reduce TSC-related lesions such as renal angiomyolipomas (AMLs), Subependymal Giant Cell Astrocytomas (SEGAs), facial angiofibromas and improve epilepsy. We identified that metformin inhibits the mTOR pathway and has a safer side effect profile compared with other mTOR inhibitors. We hypothesized that metformin may reduce the volume of TSC related lesions.
Added value of this study
Our study is the only treatment trial of metformin in TSC until 1st May 2020. It is the first trial to assess the safety and efficacy of metformin in individuals with TSC. Our study showed that metformin did not reduce AML volume but did reduce SEGA volume and seizure frequency compared with placebo. There may be a role for metformin in slowing or reversing the growth of some life-threatening hamartomas in TSC and for reducing seizure frequency.
Implications of all the available evidence
This study suggests treatment with metformin may be effective in reducing SEGA volume and frequency of epileptic seizures in children and adults with TSC. Metformin appears to be a less potent inhibitor of mTOR compared to everolimus and rapamycin, but it has a more benign side-effect profile and is less expensive. Further research into the use of metformin in TSC is indicated.
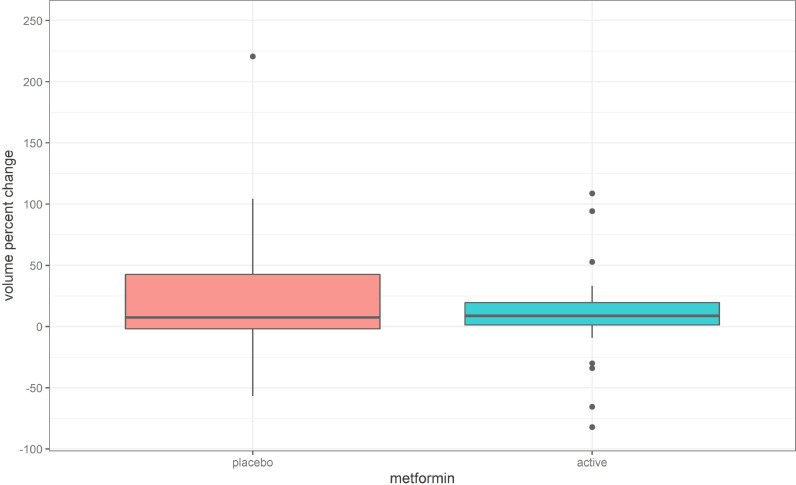
Alt-text: Unlabelled box
1. Introduction
Tuberous Sclerosis Complex (TSC) is a relatively common genetic disorder with a prevalence between 4 and 9 per 100,000. [1]. Based on the revised diagnostic criteria the estimated incidence rate of definite or possible TSC is between 1 in 6760 and 1 in 13,520 live births [2]. It is characterized by the development of tumours (hamartomas) throughout the body. Tumours affecting the heart (cardiac rhabdomyomas), kidneys (renal angiomyolipomas) and brain (subependymal giant cell astrocytomas) can cause life threatening complications [1]. Tumours on the skin and nails can be significantly disfiguring. TSC is associated with epilepsy in approximately 75% of patients and with learning difficulties in approximately 50%. Individuals with TSC may also have a range of psychological and behavioural problems including autism [3]. Many adults with TSC are unable to live independently and require state or family care [4].
TSC is caused by mutations in the tumour suppressor genes TSC1 on chromosome 9 or TSC2 on chromosome 16 that encode for the proteins hamartin and tuberin respectively [5, 6]. Hamartin and tuberin function together within the cell as a complex, and have an inhibitory effect on the mechanistic Target Of Rapamycin (mTOR), a protein kinase that effects cell growth and division through the regulation of protein synthesis [7]. Mutations in either the TSC1 or TSC2 genes allows over-activation of the mTOR pathway, which leads to relatively uncontrolled cell growth and, in turn, the formation of hamartomas in multiple organs.
Recent studies have shown that drugs that inhibit mTOR, specifically rapamycin and everolimus, can reduce TSC-related lesions such as renal angiomyolipomas (AMLs), Subependymal Giant Cell Astrocytomas (SEGAs) and facial angiofibromas in humans [8]. Spontaneous regression of these hamartomas is not commonly seen [9]. Rapamycin has been shown to reverse learning deficits and improve epilepsy in mouse models of TSC [10]. The EXIST-3 trial has shown that everolimus therapy reduces seizure frequency in patients with TSC and treatment-resistant focal seizures and that it has a tolerable safety profile [11]. Rapamycin and everolimus have a relatively high incidence of adverse events such as mucositis, diarrhoea, and respiratory infections severe enough to require hospitalization in a third of patients [12]. Everolimus treatment in TSC patients has also rarely been associated with life-threatening outcomes, including sepsis and death [13]. Patients sometimes require periods off therapy and this interruption has been linked to the re-growth of tumours [8]. Little is known about the long-term (> 5 years) side effects of these drugs.
Metformin inhibits the mTOR pathway via activation of adenosine monophosphate-activated protein kinase (AMPK) and p53 [14, 15]. It has been used for over 50 years to aid glycaemic control in patients with type 2 diabetes, and has a benign side effect profile [16]. It does not lower blood sugar in non-diabetic people unless given in overdose [16]. We hypothesised that metformin will reduce TSC-related tumour size via the inhibition of mTOR. We also hypothesized that metformin may improve epilepsy and cognitive ability in patients with TSC. Therefore, we conducted a clinical experiment using metformin in the form of a randomised double-blinded placebo-controlled trial in patients with TSC.
2. Methods
2.1. Study design
The Metformin in Tuberous Sclerosis (MiTS) study was a randomised double-blind placebo-controlled trial. Three hospitals with specialist TSC services enrolled patients (Royal United Hospital [41 patients], Great Ormond Street Hospital [3 patients], and Bristol Royal Hospital for Sick Children [11 patients]). These are three TS clinics run by the study group. These clinics follow up the largest number of TSC patients in the UK. Prior to their standard clinic appointment, all clinic patients (or their parents/carers) were sent a letter introducing the study (including participant information sheets and study team contact details). They were offered the opportunity to discuss the study at an appointment directly after their next clinic visit.
Investigators (SA, and FO'C) who worked at all three sites, enrolled and managed patients in the trial. Treatment allocation was undertaken online through the Bristol Randomised Trials Collaboration Unit, School of Social and Community Medicine, University of Bristol. Our research protocol was approved by the UK Yorkshire and Humber Multicentre Research Ethics Committee (11/YH/0295) and all relevant local research ethics committees. The full protocol is available at https://bristolcns.org/research/
2.2. Participants
Inclusion criteria were a clinical diagnosis of tuberous sclerosis complex as defined by the International Tuberous Sclerosis Complex Consensus Group [17, 18], age 10 to 65 years, and the presence of at least one renal angiomyolipoma of ≥ one centimetre in diameter. Exclusion criteria were: a serious inter-current illness or an uncontrolled disease that could compromise participation in the study, pre-existing impairment of renal function, the use of x-ray contrast medium containing iodine within the last 30 days, multiple AMLs that cannot be distinguished separately on magnetic resonance imaging (MRI), renal haemorrhage within the preceding 12 months, presence of renal aneurysms > 10 mm in diameter, impaired liver function, acute or chronic disease which may cause tissue hypoxia and increase the risk of lactic acidosis (e.g. cardiac/respiratory failure, recent myocardial infarction), pre-existing diabetes, current treatment with injected or oral hypoglycaemic drugs, pregnancy, planning to become pregnant, or breastfeeding.
2.3. Randomisation and masking
Patients were randomly allocated (1:1) to placebo or metformin for 12 months. The randomisation was stratified by centre and minimised by age-group (10 to <20; 20 to <30; 30 to <40; and 40 to <65) and by the presence or absence of learning disabilities. The randomisation was concealed from investigators. The investigators randomised patients online and received a randomisation number and treatment pack number that they then wrote on the study prescription form. The prescriptions were sent to the main trial pharmacy at the sponsor's site. On receipt of the prescription forms, the Trial pharmacists identified the treatment pack number on their (provided) spreadsheet that listed the drug to dispense for each treatment pack number. The online randomisation system sent an automatic email to all pharmacists confirming the recruited patient's ID, centre and drug allocation. The pharmacists made up the treatment pack and dispensed the trial treatment. The online randomisation was performed by the (BRTC) Bristol Randomised Trials Collaboration Unit, School of Social and Community Medicine, University of Bristol.
2.4. Procedures
The study treatments were metformin (standard 500 mg tablets, manufactured by Relonchem Ltd, Widnes, Cheshire) and placebo (500 mg tablets, manufactured by Essential Nutrition Ltd, Brough, UK). Metformin and placebo tablets were matched for shape, size and colour. Both were labelled, and final quantitative pharmacology release was certified by the University Hospitals Bristol Pharmaceuticals.
For adult patients (> 16 years), the starting dose was 500 mg twice a day orally. At 6 months, the dose was escalated to 500 mg three times if the patient was tolerating the treatment. For children aged 10–16 years, the drug dosing started at 500 mg once a day. After two weeks the dose was escalated to 500 mg twice a day. At 6 months the dose was escalated to 500 mg three times a day if the patient was tolerating treatment.
All patients had a renal MRI at baseline prior to initiation of treatment and 12 months after starting the treatment. Patients with learning disabilities, and some of the children, required general anaesthetic for the MRI. The same MRI protocol was used in the three centres. MRI (1.5 tesla) was used with gadolinium intravenous contrast agent. Fat saturated spoiled gradient echo sequences were performed in axial and coronal planes. Contrast bolus was adjusted by body weight according to manufacturer dose specifications. The scans were analysed by a radiologist (MLa) who was blind to treatment allocation. Images were reviewed on a work-station (Fuji Synapse PACS, Fujifilm, Japan). Lesions were measured in three dimensions with baseline AMLs required to measure at least ≥1 cm in diameter. The volume measurements were performed as an approximated ellipsoid using the formula, width × depth × length x 0.523. The volume of up to the five largest AMLs per patient were identified from the baseline and 12-month scans. The five largest lesions were chosen in those patients who had more than five lesions measuring ≥ 1 cm. The mean volume of all the measured lesions was calculated for each patient at baseline and 12 months.
Each patient in the trial also had a baseline cranial MRI and a follow-up cranial MRI 12 months after the initiation of treatment. Up to 2 SEGAs were identified per patient. The two largest SEGA lesions were chosen in patients who had more than two SEGA lesions. SEGA was defined as a lesion at the Foramen of Monro which enhances with contrast, and measures ≥ 0.5 cm in diameter. The volume of SEGAs was calculated at the baseline and 12-month scan. The scans were analysed by a neuroradiologist (MLi) who was blind to treatment allocation. Identical volumetric measurement methodology was used as for the AML lesions.
The facial angiofibromas and ungual fibromas were assessed at baseline and at 12 months after initiation of treatment using digital photography. These lesions were assessed objectively by a dermatologist blinded to treatment allocation using physician's global assessment (PGA) [19] of digital photographs and subjectively by patient/parent/carer report after 12 months of treatment. The PGA has a seven-point scale ranging from a score of 0 (no evidence of disease / 100% improvement) to 6 (Disease is worse than at baseline > 25% or more). Consequently, a lower score on the PGA indicates greater improvement. The same 10.2 mega pixels digital camera was used for all the patients, in all the three centres, by the same researcher (SA). Six photographs were taken from each patient at baseline and at the 12-month visit. Three photographs were taken with the camera flash on and the other three are taken with the flash in automatic mode. One photograph was taken in full-face view directly facing the camera and the other two were side profiles. Patients were advised not to wear makeup before having their photograph taken.
Patients or their caregivers recorded seizure events in a seizure diary for one month prior to initiating treatment and for one month prior to the 12-month assessment. Adaptive behaviour outcome was measured using the Vineland Adaptive Behaviour Scales (VABS) [20] Composite Score. The Vineland is a validated measure of adaptive behaviour that yields an age standardised score with a population mean of 100 and a standard deviation of 15. The Vineland was administered at baseline and 12 months in face-to-face interviews by the researcher (SA) who was blinded to treatment allocation. Health related Quality of Life (QOL) was assessed at baseline and at 12 months using the paediatric Quality of Life Inventory (PedsQL) for children (ages 10 to18 years), and the Short Form 36 Health Survey (SF-36) for adults without learning difficulty (i.e. > 18 years).
Consent was obtained from the parents or legal guardians of children. Assent from the children was also sought were possible. Consent was obtained from adults with normal intellect. Parents cannot give consent on behalf of their children once they are over 18, even if they have learning disabilities. For this group of patients, consent was obtained from all the parties who were involved in the patients’ care such as parents, guardians, carers, legal representatives, general practitioners, case workers, support workers and hospital specialists, via best interest decision. The investigators communicated with these parties by email, phone, or face to face meetings, to explain the study and obtain written consent from them. This was in line with ethical approval.
2.5. Pharmacovigilance
Toxicity was graded using the National Cancer Institute's Common Terminology Criteria for Adverse Events [21]. Participants recorded a daily diary for the first 14 days, then a monthly diary thereafter. Following any dose escalation there was a further 14 days of daily record keeping. Clinical assessment including measurement of renal and liver function and blood glucose levels occurred at baseline, 6 weeks after starting treatment, and then 6 weeks after any dose change at 6 months.
2.6. Outcomes
The primary outcome was percentage change from baseline in renal angiomyolipoma (AML) volume measured via magnetic resonance imaging (MRI). Secondary outcomes were percentage change from baseline in SEGA volume measured by MRI; change in appearance of facial angiofibromatosis and ungual fibromas as measured by Physician Global Assessment score and by patient report; change from baseline in the frequency of epileptic seizures as measured in the one month prior to the 12 month assessment (i.e. in the 12th month of treatment); change from baseline in Vineland Adaptive Behaviour Scales (VABS) composite score; and change from baseline in Peds QL and SF-36 quality of life scores.
2.7. Statistical analysis
Sample size calculations for this study were difficult as there is no previous evidence, either in the experimental or clinical sphere, of metformin use in tuberous sclerosis complex that could inform the investigators of likely effect size. However, at the beginning of the study it was calculated that 36 patients would be needed in each arm to achieve a statistical power of 90% to detect a mean reduction in renal tumour volume of 20% at a significance level of 5%.
All the outcome variables were continuous. Initial comparisons between the active treatment group and placebo for primary and secondary outcomes were made with using either t-tests or Wilcoxon-Mann-Whitney (WMW) tests depending upon whether the data was normally or non-normally distributed. One-sided alternatives were used as the study hypothesis was that metformin based upon its documented action on the mTOR pathway would result in reduction of hamartoma volume compared to placebo. We tested assumptions of normality using the Shapiro-Wilk's test and homogeneity of variances with Bartlett's test using the statistical programme R, version 3.4.2. All other statistical analyses were done with Stata IC, version 11.2.
The trial is registered with The International Standard Randomised Controlled Trial Number (ISRCTN), number 92545532, and the European Union Drug Regulating Authorities Clinical Trials (EUDRACT) number 2011-001319-30
2.8. Role of funding source
The sponsor and funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The senior authors (FJKO'C, SA, MCB) had full access to all the anonymised data in the study and had final responsibility for the decision to submit for publication.
3. Results
Between 1st November 2012 and 30th September 2015 72 patients were assessed for eligibility, of whom 55 met the inclusion criteria and were randomly assigned (see Fig. 1). 28 were allocated to metformin therapy and 27 to placebo. Three patients allocated to placebo and one allocated to metformin did not start therapy for social reasons, leaving 27 patients who started metformin therapy and 24 who started on placebo. All the patients who began treatment completed the 12-month treatment period and therefore 51 patients were analysed for primary and secondary outcomes.
Fig. 1.
Trial profile.
The demographic details of the participating cohort are shown in Table 1. There were no clinically important differences observed between treatment groups with regard to baseline characteristics.
Table 1.
Baseline patient demographic and disease characteristics.
| Metformin (n = 27) | Placebo (n = 24) | |
|---|---|---|
| Median age | 30 years | 26 years |
| Age | Number of patients (%) | |
| 10–20 years | 9 (33%) | 8 (33%) |
| >20–30 years | 6 (22%) | 6 (25%) |
| >30–40 years | 7 (25.9%) | 7 (29%) |
| >40–65 years | 5 (18.5) | 3 (12.5%) |
| Sex | ||
| Men | 16 (59%) | 9 (37.5%) |
| Women | 11 (40.7%) | 15 (62.5%) |
| Presence of learning disabilities | 17 (63%) | 17 (70%) |
| Presence of SEGA | 14 (51%) | 13 (54%) |
| Mean diameter angiomyolipoma lesion | ||
| ≥8 cm | 4 (14.8%) | 4 (16.6%) |
| ≥4 cm and <8 cm | 12 (44.4%) | 7 (29%) |
| ≥3 cm and <4 cm | 4 (14.8%) | 1 (4%) |
| <3 cm | 7 (25.9%) | 12 (50%) |
| Unilateral angiomyolipoma | 5 (18.5%) | 5 (20.8%) |
| Number of angiomyolipoma lesions | 89 | 78 |
Adverse events in the Trial were rare and are shown in Table 2. There were 3 adverse events recorded on placebo and 11 on metformin. There were 3 serious adverse events on metformin therapy that necessitated hospitalisation: two cases of haemorrhage from angiomyolipomas and one case of worsening seizure control. None of the serious adverse events were judged to be serious adverse reactions to treatment.
Table 2.
Shows adverse events in both groups.
| Placebo | Metformin |
|---|---|
| Backache (n = 1) | Diarrhoea (n = 2) |
| Dental infection (n = 1) | Food poisoning (n = 1) |
| Headache (n = 1) | Fall (n = 1) |
| Worsening seizures (n = 2)a | |
| Gastric upset (n = 1) | |
| Depression (n = 1) | |
| Urinary Tract Infection (n = 1) | |
| Angiomyolipoma bleeding (n = 2)b |
Serious adverse events:.
1 admission to hospital.
2 patients admitted to hospital.
3.1. Renal angiomyolipomas
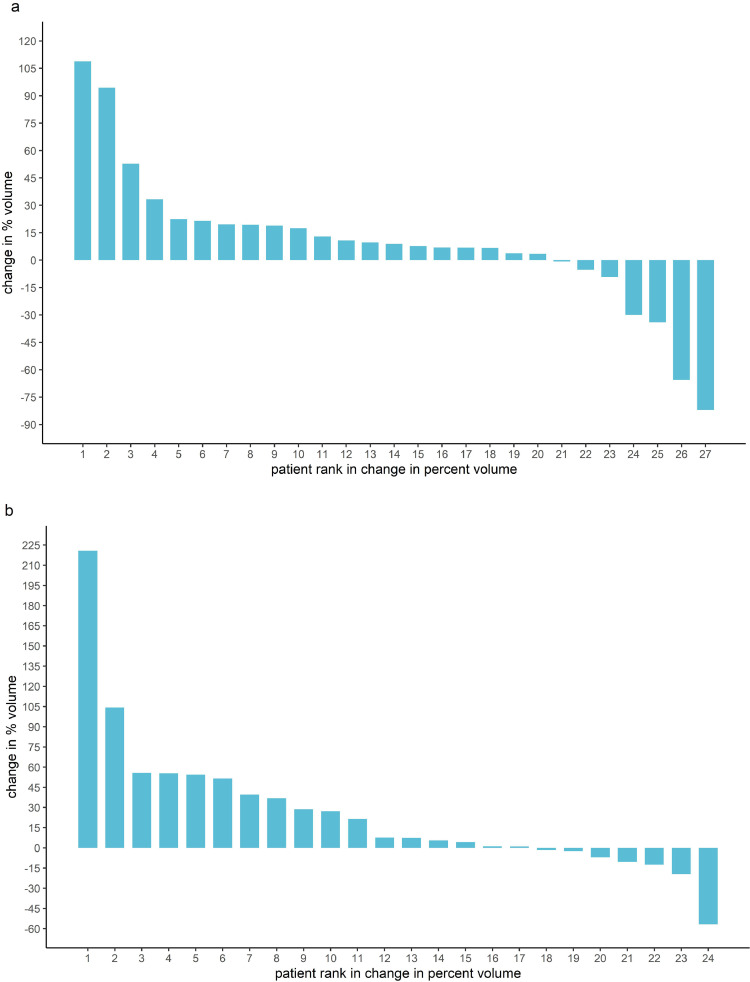
All the patients in the trial had at least one angiomyolipoma ≥ one centimetre in diameter. The distribution of the percentage volume changes in participants of the trial on both metformin and placebo is shown in Fig. 2 and the individual responses to treatment are depicted in the waterfall plots in Fig. 3. The median percentage change in AML volume was +7.6% (IQR −1.8% to +42.6%) for the placebo group and +8.9% (IQR 1.3% to 19.5%) for the metformin group (WMW test, z = 0.59, p = 0.28).
Fig. 2.
Distribution of percentage volume change in AMLs on placebo and metformin.
Fig. 3.
(a) Waterfall Plot: AML Volume Change on Metformin. (b) Waterfall Plot: AML Volume Change on Placebo.
3.2. Subependymal giant cell astrocytomas
Twenty-seven patients in the trial had at least one subependymal giant cell astrocytoma. 13 were randomised to placebo and 14 were randomised to metformin. The distribution of the percentage volume changes in SEGAs in participants of the trial on both metformin and placebo is shown in Fig. 4 and the individual responses to treatment are depicted in the waterfall plots in Fig. 5. The data was not normally distributed and therefore the difference in treatment effect was analysed using non-parametric statistics. The median percentage change in SEGA volume was +3.0% (IQR −22.8% to +27.7%) for the placebo group and −20.8% (IQR −47.1% to −5.0%) for the metformin group (WMW test, z = 1.89, p = 0.03). On a priori grounds we know that age is associated with risk of growth of SEGAs. SEGAs are known to grow in the first three decades of life and then become more quiescent such that current screening recommendations suggest screening only in the first three decades of life. In a retrospective analysis we sub-divided patients with SEGA into those who were either greater (n = 14) or less than (n = 13) 30 years of age in order to see whether metformin was more effective on SEGAs in younger patients, when lesions are more biologically and clinically active. In patients less than thirty years, the median percentage change in SEGA volume was + 11.4% (IQR −22.8% to +162.4%) for the placebo group and – 28.0% (IQR −61.1% to −11.4%) for the metformin group (WMW test, z = 1.86, p = 0.03). In those patients greater than thirty years, the median percentage change in SEGA volume was + 3.0% (IQR – 39.7% to + 14.4%) for the placebo group and – 10.3% (IQR – 49.9% to + 19.8%) for the metformin group (WMW test, z = 0.70, p = 0.24)
Fig. 4.
Distribution of percentage volume changes in SEGAs on placebo and metformin.
Fig. 5.
(a) Waterfall Plot: SEGA Volume Change on Metformin. (b) Waterfall Plot: SEGA Volume Change on Placebo.
3.3. Epilepsy
Twenty-nine patients had active epilepsy, of whom 14 were on placebo and 15 on metformin. Eight patients (3 on metformin and 5 on placebo) failed to complete the seizure diary and therefore data on seizure frequency was available in 21 patients. A mean reduction of 43.7% from baseline in seizures was observed in the metformin group and 3.1% in the placebo group, with a difference in response of 40.6% (95% CI −3.1% to +84.2%, t = 1.95, p = 0.03). Nine out of twelve patients on metformin had a reduction in seizure frequency versus three out of nine patients reporting a reduction in seizure frequency on placebo. Three patients in the metformin group became seizure free at the 12-month assessments versus zero patients in the placebo group becoming seizure-free.
3.4. Facial angiofibromas
Nineteen patients had facial angiofibromas in the placebo group and 23 in the metformin group. The mean PGA (Physician Global Assessment) score for facial angiofibroma at 12 months for the placebo group was 3.4 (95% CI 3.0 to 3.9) and 3.1 (95% CI 2.5 to 3.6) for the metformin group. A lower score indicates greater improvement but there was no meaningful difference between the two treatment groups (t = 1.07, p = 0.3). Two out of 23 patients/carers/parents reported improvement on metformin. One out of 19 reported improvement on placebo. There were no reports of worsening rash in either group.
3.5. Ungual fibromas
Thirty-eight patients had ungual fibromas, 18 were on placebo and 20 on metformin. The mean PGA scores at 12 months were 4.4 (95% CI 4.1 to 4.8) for the placebo group and 4.2 (95% CI 3.7 to 4.6) for the metformin group indicating that there was no clinically meaningful difference between the two groups (t = 1.19, p = 0.24). None of the patients, carers or parents reported worsening or improving ungual fibromas in either group.
3.6. Vineland
There was no appreciable change in Vineland scores over the course of the trial in either treatment group. The total mean adaptive behaviour scores for the placebo group at baseline was 53.5 (95% CI 38.4 to 68.6), and 54.2 (95% CI 37.4 to 71.0) at 12 months. The total mean adaptive behaviour scores for the metformin group was 52.7 (95% CI 36.4 to 69.0) at baseline, and 49.5 (95% CI 34.5 to 64.4) at 12 months. The change in adaptive behaviour scores over the 12 months was + 0.7 (95% CI −3.7 to +5.1) for the placebo group and – 3.2 (95% CI −5.8 to −0.7) for the metformin group (t = 1.7, p = 0.1).
3.7. Quality of life
Health related quality of life was assessed in the trial at baseline and at 12 months using the paediatric Quality of Life Inventory (PedsQL) for children (ages 10 to 18 years). The PedsQL can be self-reported or completed by a proxy (i.e. parent). Self-reported PedsQL scores were obtained in 6 out of 12 children in the study aged 18 years or below. Proxy reported PedsQL scores were obtained in 9 out of 12 children. Three families failed to return PedsQL forms. The mean self-reported Peds QL scores for the placebo group at baseline was 79.9 (95% CI 18.2 to 141.5) and 71.7 (95% CI −10.9 to 154.3) at 12 months. The mean self-reported PedsQL score for the metformin group at baseline was 76.6 (95% CI 51.3 to 101.9) and 79.0 (95% CI 60.4 to 97.6) at 12 months. The change in self-reported PedsQL scores over the 12 months was −8.2 (95% CI −152.4 to +136.1) for those receiving placebo and + 2.4 (95% CI −10.0 to +14.9) for those on metformin (t = 1.2, p = 0.3). The mean proxy-reported Peds QL scores for the placebo group at baseline was 46.6 (95% CI 20.0 to 73.2) and 46.3 (95% CI 14.0 to 78.5) at 12 months. The mean proxy-reported PedsQL score for the metformin group at baseline was 66.0 (95% CI 39.2 to 92.8) and 72.2 (95% CI 55.9 to 88.6) at 12 months. The change in proxy-reported PedsQL scores over the 12 months was −0.3 (95% CI −29.7 to +28.9) for those receiving placebo and + 6.2 (95% CI −10.5 to +23.0) for those on metformin (t = 0.5, p = 0.6).
Health related quality of life in adults was measured using the Short Form Health Survey (SF-36). The SF-36 is a self-report form and it was therefore not possible to collect data on the 26 adults with learning difficulties in the study. SF-36 scores were obtained for 9 out of 13 adults in the study who did not have learning difficulties. Four of the adult patients did not return their SF-36 forms. The SF-36 generates a mental health and physical health summary score. The mean physical health score for the patients taking placebo at baseline was 39.8 (95% CI 18.3 to 61.3) and 44.8 (95% CI 19.9 to 65.8) at 12 months. The mean physical health score for the metformin group at baseline was 61.5 (95% CI 56.2 to 66.8) and 58.3 (95% CI 60.4 to 97.6) at 12 months. The change in physical health scores over the 12 months was +5 (95% CI −2.9 to +12.9) for those receiving placebo and −3.3 (95% CI −6.8 to +0.3) for those on metformin (t = 2.5, p = 0.04). The mean SF36 mental health summary score for the placebo group at baseline was 50.8 (95% CI 39.9 to 61.7) and 45.8 (95% CI 35.5 to 56.1) at 12 months. The mean mental health score for the metformin group at baseline was 43.3 (95% CI 23.1 to 63.4) and 47.8 (95% CI 40.2 to 55.3) at 12 months. The change in mental health scores over the 12 months was −5 (95% CI −15.0 to +5.0) for those receiving placebo and +4.5 (95% CI −20.0 to +29.0) for those on metformin (t = 1.2, p = 0.3).
4. Discussion
This randomised, double-blind, parallel group, placebo-controlled trial of metformin is the first to investigate the safety and efficacy of metformin in children and adults with TSC. Metformin was well tolerated and did not appear to cause any significant safety issues in patients during the course of the trial. Metformin did not reduce AML volume over the course of the trial. However, patients on metformin had a reduction in SEGA volume compared with placebo and also had a reduction in epileptic seizure frequency. There was no significant difference between the treatments with respect to the other secondary outcomes. The serious adverse events that occurred during the trial were apparently unrelated to the trial medication.
There is now an extensive literature describing the beneficial effects of metformin on a variety of different cancers. [22, 23] The beneficial effect is thought to be mainly due to metformin's inhibitory effect on the mechanistic target of rapamycin (mTOR) signalling pathway via activation of adenosine monophosphate-activated protein kinase (AMPK). [24] We formulated the hypothesis that treatment with metformin would result in reduction in size of renal angiomyolipomas in tuberous sclerosis patients. We chose to look at reduction in volume of angiomyolipomas as our primary outcome because they are one of the most prevalent hamartomas in TSC, they are associated with significant morbidity and mortality, [25] and previous trials of mTOR inhibitors such as rapamycin and everolimus have shown an effect on these lesions. [12] This trial did not show metformin to have a significant effect on AMLs in this patient population. One possible explanation for this negative result is that metformin simply does not have a clinically significant inhibitory effect on the mTOR pathway in TSC patients. Previous studies in a mouse model of TSC did not demonstrate any therapeutic effect of metformin in reducing the size of renal cystadenomas that form in this model after loss of expression of the TSC2 gene. The authors speculate that the complete absence of TSC2 in the renal cystadenoma lesions in this model leads to strong activation of mTORC1 due to high levels of RHEB-GTP, which makes the lesions resistant to the lesser inhibitor effects of metformin. [26] However, recently Fang et al. reported that metformin treatment effectively prevented aberrant kidney enlargement and cyst growth, inhibited inflammatory response, attenuated interstitial fibrosis, and protected renal function in a mouse model of renal proximal tubule-specific TSC1 gene-knockout. [27]
The effect of metformin on SEGAs seen in this study argues that metformin may indeed have a clinically significant effect on some of the hamartomas in TSC. It may be that the dose of metformin used in this study is not adequate to produce a significant inhibitory effect in the renal lesions. There is no published information to suggest an optimal dose of metformin when the objective is to reduce tumour or hamartoma size. The dose used in this study was chosen because it was the dose that is used, shown to be effective, and tolerated when treating type 2 diabetes patients which is the current main indication for metformin use. Further studies using different dosing regimens may well be justified.
The effect of metformin in reducing the volume of SEGAs in TSC patients in this study is striking. SEGAs are another important cause of morbidity, and occasional mortality, in TSC patients [25, 28]. They are hamartomas that grow at the foramen of Monro and can cause obstruction to flow of cerebrospinal fluid and consequent hydrocephalus. They occur in up to 30% of TSC patients [29]. Metformin has been shown to have a beneficial effect in more aggressive brain tumours such as high grade gliomas [30]. Metformin effectively crosses the blood-brain barrier and is distributed in multiple brain regions after oral dosing [31]. The apparent effect of metformin in reducing SEGA volume in the patients in this study is clinically meaningful and is potentially important for the future treatment of TSC patients. It is certainly a finding that needs replication and further research. SEGAs are known to grow in the first three decades of life and then become more quiescent such that current screening recommendations suggest screening only up to the age of 25 [32]. In a post-hoc analysis we subdivided the patients in this study into those less than 30 years and therefore at an age when SEGA growth would be most likely and those greater than 30 years who might be less likely to have active lesions. The effect of metformin on the SEGAs of those patients less than 30 years was more marked than in the older patients.
There is emerging evidence from animal work that metformin may have an anti-epileptic and anti-epileptogenic effect. Metformin may have an anti-epileptic effect via a number of different pathways: inhibition of MTOR, activation of AMPK and prevention of oxidative damage induced by seizure activity. Metformin has been shown to suppress seizures in some rodent models and Bruegeman et al. have recently demonstrated that metformin significantly suppressed seizure behaviour in a zebrafish PTZ-induced seizure model [33], [34], [35]. Metformin has not previously been studied in any clinical trial for human seizures or epilepsy. Consequently, the reduction of seizure activity seen in patients treated with metformin in this study is interesting. However, our study data is undermined by the failure to obtain seizure data in eight patients and we did not control for alterations in other anti-epileptic drugs. Nevertheless, the findings are sufficiently intriguing to justify further study of the possible anti-epileptic effects of metformin in tuberous sclerosis complex in an adequately powered clinical trial.
Everolimus and rapamycin are two mTOR inhibitors that have been shown to be effective at both reducing hamartoma size and improving refractory epilepsy in TSC [11, 36, 37]. However, these agents have significant side-effects. The most common side-effects of these agents are mouth ulceration and stomatitis but there is a risk of immunosuppression and severe infection. Two deaths in the recently reported EXIST-3 study were attributed to treatment [38]. Metformin inhibits mTOR via a different mechanism than everolimus and rapamycin and it has a significantly more benign side-effect profile. At the dose used in this study, it does not appear to be as potent an mTOR inhibitor as rapamycin or everolimus and does not have such a dramatic effect on the hamartomas associated with TSC. However, given its better side-effect profile it may prove to be a more attractive option for TSC patients who may benefit from long-term mTOR inhibition to prevent the development of symptomatic SEGAs and to improve their long-term epilepsy control. It is also possible that it could be given in conjunction with other mTOR inhibitors possibly having a synergistic effect and possibly allowing use of lower doses of more toxic mTOR inhibitors and thus reducing the incidence of severe side-effects. These questions need to be explored in future research.
Metformin has the advantage of not interacting with the cytochrome p450 system and therefore it is unlikely to interfere with the metabolism of other mTOR inhibitors. For the same reason, and in contrast to the other mTOR inhibitors everolimus and rapamycin, the metabolism of metformin will not be disturbed by antiepileptic drugs such as cannabidiol or carbamazepine that many TSC patients may be taking [39].
The obvious strengths of this trial are that treatment was randomised and that participants, families and carers, and investigators were blind to treatment allocation and therefore outcomes were assessed objectively and without bias. All the patients who started treatment in the trial were assessed for the primary outcome and all those who had SEGAs were assessed for SEGA growth at the end of the trial. There are, however, limitations with this study. The trial was small with just fifty-one participants. Although it was not possible to do a meaningful power calculation in this study because of a complete lack of data to support any assumptions regarding effect size, we had initially aimed to recruit 72 patients. Recruitment was difficult for several reasons. Firstly, the trial took place at the same time as industry-sponsored trials of the mTOR inhibitor everolimus that were looking at the same population of patients. Secondly, the trial required annual MRI scans of brain and kidney and these required general anaesthesia in learning disability patients and sometimes carers were reluctant to submit individuals for general anaesthesia for a research study. Thirdly, the limited finance for the study prevented extension of the study to multiple sites beyond where the investigators worked. It is possible that the small numbers in the study has precluded seeing a significant difference between metformin and placebo with respect to angiomyolipoma growth but there was certainly no evidence from this data to suggest metformin was causing angiomyolipomas to shrink. It is more likely that greater numbers in the study may have enabled us to see even more convincing effects of metformin on SEGA growth and on epilepsy control. Consequently, we think there is a strong case for doing a larger study looking at these two outcomes in particular.
Health related quality of life was one of the secondary outcomes of this study. Unfortunately, the data for this outcome is incomplete mainly due to the impossibility of obtaining quality of life scores in learning disabled adult patients using the SF-36 but also due to the incomplete return of questionnaires to the study team. Quality of life scores were only obtained for 9 out of 12 (75%) children in the study and 9 out of 39 (23%) adults. The paucity of data for this outcome cautions against definitive conclusions in this area. The quality of life data that was collected did not suggest that there was a meaningful change during the course of the study in either group. The statistically significant difference in change of the SF-36 physical health scores between the metformin and placebo groups is curious and probably relates to the very low baseline score in the patients taking placebo compared to the metformin patients.
As with all clinical trials there is a possible issue with respect to external validity with this data. Sixty-eight per cent of the participants in this trial had learning disability compared with a rate of approximately 50% seen in epidemiological studies of tuberous sclerosis complex patients. However, the increased representation of learning disability patients in this trial may reflect the fact that more severe renal disease is more common in learning disabled TSC patients. [25] We should be aware, however, that the results from this trial may not map directly onto a general population of TSC patients.
One definite issue for the study is that seizure diary data was available for just 73% of the patients with epilepsy. The major reason for this was that some carers of individuals with learning disabilities in residential care homes were unable to complete the daily seizure diary due to short staffing issues. The results from this study suggest that metformin may have had a beneficial effect on seizure control, but the incompleteness of the dataset means that this result should be treated with caution. However, it strengthens the need to look at the effect of metformin on epilepsy in TSC in a larger trial in which epilepsy control is a primary outcome.
Metformin was well tolerated by children and adults in this study. Gastric upset is not uncommonly reported in diabetic patients. We encountered only one patient who complained of gastric upset and this settled quickly. This side effect was not commonly reported in our study probably because we did not use high doses and we gradually built the dose up over 6 months. The possible side effect of lactic acidosis with metformin use is still controversial. None of our patients developed lactic acidosis. In addition, none of the patients who were taking metformin developed hypoglycaemia. Metformin increases the sensitivity to insulin rather than insulin level, thus hypoglycaemia is not expected to occur due to metformin administration [16].
Metformin is apparently safe and well tolerated in children and adults with TSC when used in the doses used in this trial. Metformin did not reduce AML size. Patients on metformin had a reduction in SEGA volume compared with placebo, which was more marked in younger (< 30 years of age) patients. Patients on metformin also appeared to have fewer epileptic seizures, although seizure diary data was complete for just 73% of patients. There may be a role for metformin in slowing or reversing growth of life-threatening hamartomas in TSC and for helping to control the frequency of epileptic seizures. Further study is justified.
Author contributions
All authors made meaningful contributions to manuscript preparation, review, and editing. In addition, the authors made contributions as follows:
Finbar O'Callaghan conceived the study and devised the protocol. He recruited patients and analysed the study data. He co-wrote the manuscript. He maintains overall responsibility for the MiTS project.
Sam Amin led the execution of the trial. He analysed the data and co-wrote the manuscript.
Hannah Edwards co-wrote the protocol (with FO'C and AAM) and assisted with study management and setting up of randomisation
Andrew Mallick co-wrote the protocol (with FO'C and HE) and assisted with study management and patient recruitment.
Mario Cortina Borja provided statistical advice, analysed the study data and helped create study figures/illustrations.
Matthew Laugharne helped devise imaging protocols and analysed radiological images
Marcus Likeman helped devise imaging protocols and analysed radiological images
Declaration of Competing Interest
All other authors have no interests to declare.
Acknowledgments
Funding
This study was funded by the National Institute for Health and Research (NIHR) through the The Research for Patient Benefit Programme (RfPB), and supported by the NIHR Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. It was also supported by the Bath Unit for Research in Paediatrics and the Research and Development Departments at the Royal United Hospital Bath NHS Trust and University Hospital Bristol NHS Trust.
Data sharing statement
Anonymised patient data that underlie the results (tables, figures) reported in this study are available upon reasonable request from qualified researchers to the corresponding author.
References
- 1.O'Callaghan F.J., Shiell A.W., Osborne J.P., Martyn C.N. Prevalence of tuberous sclerosis estimated by capture-recapture analysis. Lancet. 1998;351(9114):1490. doi: 10.1016/S0140-6736(05)78872-3. [DOI] [PubMed] [Google Scholar]
- 2.Ebrahimi-Fakhari D., Mann L.L., Poryo M., Graf N., von Kries R., Heinrich B. Incidence of tuberous sclerosis and age at first diagnosis: new data and emerging trends from a national, prospective surveillance study. Orphanet J Rare Dis. 2018;13(1):117. doi: 10.1186/s13023-018-0870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulsifer M.B., Winterkorn E.B., Thiele E.A. Psychological profile of adults with tuberous sclerosis complex. Epilepsy Behav. 2007;10(3):402–406. doi: 10.1016/j.yebeh.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson A.P., McKinlay I.A., Hunt A. Care of adolescents with severe learning disability from tuberous sclerosis. Dev Med Child Neurol. 2002;44(4):256–262. doi: 10.1017/s0012162201002031. [DOI] [PubMed] [Google Scholar]
- 5.van Slegtenhorst M., de Hoogt R., Hermans C., Nellist M., Janssen B., Verhoef S. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277(5327):805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 6.Consortium E.C.T.S. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75(7):1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 7.Nellist M., van Slegtenhorst M.A., Goedbloed M., van den Ouweland A.M., Halley D.J., van der Sluijs P. Characterization of the cytosolic tuberin-hamartin complex. Tuberin is a cytosolic chaperone for hamartin. J Biol Chem. 1999;274(50):35647–35652. doi: 10.1074/jbc.274.50.35647. [DOI] [PubMed] [Google Scholar]
- 8.Franz D.N., Leonard J., Tudor C., Chuck G., Care M., Sethuraman G. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59(3):490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 9.Harabayashi T., Shinohara N., Katano H., Nonomura K., Shimizu T., Koyanagi T. Management of renal angiomyolipomas associated with tuberous sclerosis complex. J Urol. 2004;171(1):102–105. doi: 10.1097/01.ju.0000100100.36354.61. [DOI] [PubMed] [Google Scholar]
- 10.Ehninger D., Han S., Shilyansky C., Zhou Y., Li W., Kwiatkowski D.J. Reversal of learning deficits in a Tsc2+/- mouse model of tuberous sclerosis. Nat Med. 2008;14(8):843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French J.A., Lawson J.A., Yapici Z., Ikeda H., Polster T., Nabbout R. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388(10056):2153–2163. doi: 10.1016/S0140-6736(16)31419-2. [DOI] [PubMed] [Google Scholar]
- 12.Bissler J.J., McCormack F.X., Young L.R., Elwing J.M., Chuck G., Leonard J.M. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358(2):140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trelinska J., Dachowska I., Kotulska K., Fendler W., Jozwiak S., Mlynarski W. Complications of mammalian target of rapamycin inhibitor anticancer treatment among patients with tuberous sclerosis complex are common and occasionally life-threatening. Anticancer Drugs. 2015;26(4):437–442. doi: 10.1097/CAD.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 14.Dowling R.J., Zakikhani M., Fantus I.G., Pollak M., Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 15.Meric-Bernstam F., Gonzalez-Angulo A.M. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27(13):2278–2287. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krentz A.J., Bailey C.J. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65(3):385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- 17.Roach E.S., Gomez M.R., Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13(12):624–628. doi: 10.1177/088307389801301206. [DOI] [PubMed] [Google Scholar]
- 18.Northrup H., Krueger D.A., Group ITSCC. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr Neurol. 2013;49(4):243–254. doi: 10.1016/j.pediatrneurol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heald P., Mehlmauer M., Martin A.G., Crowley C.A., Yocum R.C., Reich S.D. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49(5):801–815. doi: 10.1016/s0190-9622(03)01475-0. [DOI] [PubMed] [Google Scholar]
- 20.Sparrow S.S., Balla D.A., Cicchetti D. American Guidance Service; Circle Pines: 1984. Vineland adaptive behavior scales. [Google Scholar]
- 21.https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. 2017.
- 22.Evans J.M., Donnelly L.A., Emslie-Smith A.M., Alessi D.R., Morris A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J., Li M., Song B., Jia C., Zhang L., Bai X. Metformin inhibits renal cell carcinoma in vitro and in vivo xenograft. Urol Oncol. 2013;31(2):264–270. doi: 10.1016/j.urolonc.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Zakikhani M., Dowling R., Fantus I.G., Sonenberg N., Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66(21):10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 25.Amin S., Lux A., Calder N., Laugharne M., Osborne J., O'callaghan F. Causes of mortality in individuals with tuberous sclerosis complex. Dev Med Child Neurol. 2017;59(6):612–617. doi: 10.1111/dmcn.13352. [DOI] [PubMed] [Google Scholar]
- 26.Auricchio N., Malinowska I., Shaw R., Manning B.D., Kwiatkowski D.J. Therapeutic trial of metformin and bortezomib in a mouse model of tuberous sclerosis complex (TSC) PLoS One. 2012;7(2):e31900. doi: 10.1371/journal.pone.0031900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang Y., Li F., Qi C., Mao X., Wang F., Zhao Z. Metformin effectively treats. Cell Death Discov. 2020;6:52. doi: 10.1038/s41420-020-0285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amin S., Carter M., Edwards R.J., Pople I., Aquilina K., Merrifield J. The outcome of surgical management of subependymal giant cell astrocytoma in tuberous sclerosis complex. Eur J Paediatr Neurol. 2013;17(1):36–44. doi: 10.1016/j.ejpn.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Jansen A.C., Belousova E., Benedik M.P., Carter T., Cottin V., Curatolo P. Newly diagnosed and growing subependymal giant cell astrocytoma in adults with tuberous sclerosis complex: results from the international TOSCA study. Front Neurol. 2019;10:821. doi: 10.3389/fneur.2019.00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seliger C., Genbrugge E., Gorlia T., Chinot O., Stupp R., Nabors B. Use of metformin and outcome of patients with newly diagnosed glioblastoma: pooled analysis. Int J Cancer. 2019 doi: 10.1002/ijc.32337. [DOI] [PubMed] [Google Scholar]
- 31.Łabuzek K., Suchy D., Gabryel B., Bielecka A., Liber S., Okopień B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep. 2010;62(5):956–965. doi: 10.1016/s1734-1140(10)70357-1. [DOI] [PubMed] [Google Scholar]
- 32.Amin S., Kingswood J.C., Bolton P.F., Elmslie F., Gale D.P., Harland C. The UK guidelines for management and surveillance of tuberous sclerosis complex. QJM. 2019;112(3):171–182. doi: 10.1093/qjmed/hcy215. [DOI] [PubMed] [Google Scholar]
- 33.Brueggeman L., Sturgeon M.L., Martin R.M., Grossbach A.J., Nagahama Y., Zhang A. Drug repositioning in epilepsy reveals novel antiseizure candidates. Ann Clin Transl Neurol. 2019;6(2):295–309. doi: 10.1002/acn3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubio Osornio M.D.C., Custodio Ramírez V., Calderón Gámez D., Paz Tres C., Carvajal Aguilera K.G., Phillips Farfán B.V. Metformin plus caloric restriction show anti-epileptic effects mediated by mTOR pathway inhibition. Cell Mol Neurobiol. 2018;38(7):1425–1438. doi: 10.1007/s10571-018-0611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y., Zhu B., Zheng F., Li Y., Zhang Y., Hu Y. Chronic metformin treatment facilitates seizure termination. Biochem Biophys Res Commun. 2017;484(2):450–455. doi: 10.1016/j.bbrc.2017.01.157. [DOI] [PubMed] [Google Scholar]
- 36.Bissler J.J., Franz D.N., Frost M.D., Belousova E., Bebin E.M., Sparagana S. The effect of everolimus on renal angiomyolipoma in pediatric patients with tuberous sclerosis being treated for subependymal giant cell astrocytoma. Pediatr Nephrol. 2018;33(1):101–109. doi: 10.1007/s00467-017-3806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franz D.N., Belousova E., Sparagana S., Bebin E.M., Frost M., Kuperman R. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381(9861):125–132. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 38.Franz D.N., Lawson J.A., Yapici Z., Ikeda H., Polster T., Nabbout R. Everolimus for treatment-refractory seizures in TSC: extension of a randomized controlled trial. Neurol Clin Pract. 2018;8(5):412–420. doi: 10.1212/CPJ.0000000000000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebrahimi-Fakhari D., Agricola K.D., Tudor C., Krueger D., Franz D.N. Cannabidiol elevates mechanistic target of rapamycin inhibitor levels in patients with tuberous sclerosis complex. Pediatr Neurol. 2020;105:59–61. doi: 10.1016/j.pediatrneurol.2019.11.017. [DOI] [PubMed] [Google Scholar]