Abstract
Background
Heart failure (HF) together with type 2 diabetes (T2D) and chronic kidney disease (CKD) are major pandemics of the twenty first century. It is not known in people with new onset HF, what the distinct and combined associations are between T2D and CKD comorbidities and cause-specific hospital admissions and death, over the past 20 years.
Methods
An observational study using the UK Clinical Practice Research Datalink linked to the Hospital Episode Statistics in England (1998–2017). Participants were people aged ≥30 years with new onset HF. Exposure groups were HF with: (i) no T2D and no CKD (reference group); (ii) CKD-only (estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2); (iii) T2D-only; (iv) T2D and CKD. CKD severity groups were: CKD-3a (eGFR 45–59); CKD-3b (30–44); CKD-4 (15–29); CKD-5 (<15). Outcomes were cardiovascular and non-cardiovascular hospitalisations and all-cause death.
Findings
In 87,709 HF patients (mean age, 78 years; 49% female), 40% had CKD-only, 12% T2D-only, and 16% both. Age-standardised first-year CVD hospitalisation rates were significantly higher in HF patients with CKD-only (46.4; 95% CI 44.9,47.9 per 100 person years) and T2D-only (49.2; 46.7,58.8) than in the reference group (35.1; 34.0,36.1); the highest rate was in patients with T2D-CKD-5: 89.1 (65.8,112.4). Similar patterns were observed for non-CVD hospitalisations and deaths. Group differences remained significant after adjustment for potential confounders. Median survival was highest in the reference (4.4 years) and HF-T2D-only (4.1 years) groups, compared to HF-CKD-only (2.2 years). HF-T2D-CKD group survival ranged from 2.8 (CKD-3a) to 0.7 years (CKD-5). Over time, CVD hospitalisation rates significantly increased for HF-CKD-only (+26%) and reduced (-24%) for HF-T2D-only groups; no reductions were observed in any of the HF-T2D-CKD groups. Trends were similar for non-CVD hospitalisations and death: whilst death rates significantly reduced for HF-T2D-only (-37%), improvement was not observed in any of the T2D-CKD groups.
Interpretation
In a cohort of people with new onset HF, hospitalisations and deaths are high in patients with T2D or CKD, and worst in those with both comorbidities. Whilst outcomes have improved over time for patients with HF and comorbid T2D, similar trends were not seen in those with comorbid CKD. Strategies to prevent and manage CKD in people with HF are urgently needed.
Funding
NIHR fellowship [reference: NIHR 30011]
Keywords: Heart failure, Chronic kidney disease, Type 2 diabetes, Hospitalisation, Mortality, Cardiovascular
Research in context.
Evidence before this study
In heart failure (HF) populations, type 2 diabetes (T2D) and chronic kidney disease (CKD) are common and are associated with increased risk of hospitalisations and death. Prior evidence has reported individual associations between these diseases and all-cause outcomes, but evidence on trends and the distinct and combined associations between T2D and CKD comorbidities and cause-specific hospital admissions and death in HF is scarce. We searched PubMed for papers using the keywords; heart failure, trend*, hospital’, death and mortality, up to December 14th, 2020.
Added value of this study
In people with new HF, hospitalisation and death rates were higher in patients with T2D or CKD, and worst in those with both comorbidities. Whilst outcomes have improved over time for patients with HF and comorbid T2D, this improvement was attenuated in the presence of CKD at all stages of renal dysfunction.
Implications of all the available evidence
People with HF and CKD are a high risk group. Strategies to prevent and manage CKD in people with HF are urgently required to improve outcomes.
Alt-text: Unlabelled box
1. Introduction
Globally, heart failure (HF) affects at least 26 million people and is increasing in prevalence [1]. HF is diagnosed in 1–2% of the general population, increasing to 5–10% in people over 65 years [2]. Even with improved therapies for HF, the mortality rate remains high, with approximately 50% mortality within 5 years of diagnosis [2]. HF together with type 2 diabetes (T2D) and diabetic chronic kidney disease (CKD) are major pandemics of the twenty first century [3]. These chronic diseases often occur together, with complex inter-relations, which calls for more clarity on the relative associations between each of the three conditions and adverse outcomes.
Due to the system based specialisation in the medical model of care, current strategies for managing most chronic diseases such as HF, CKD and T2D, encourage clinicians to adopt evidence-based guidelines for these conditions separately [4]. In recent years however, due to improvements in diagnosis and treatments resulting in more people living longer with these chronic long-term conditions, strategies that are more complex are often needed [5]. The emergence of therapeutic agents that provide cardio-reno-protective benefits [6,7]. are shifting the treatment paradigm to a broader goal of reducing morbidity, mortality and end-organ complications in these chronic conditions. Despite this knowledge, little contemporary information is available on mortality or cardiovascular and non-cardiovascular hospitalisation trends in people with HF in relation to the presence of T2D and CKD.
In a recent large multi-national population-based study, it was shown that HF and renal disease manifestation were the first reasons for admission in people with prior diabetes and no cardio-renal disease [8]. In another study using registry data of people with T2D, CKD was associated with higher risk of cardiovascular events and all-cause mortality [9].
The aim of the current analysis was to build on the findings of these previous studies, to investigate the distinct and combined associations between T2D and CKD comorbidities and cause-specific hospital admissions and death in people with HF in England between 1998 and 2017.
2. Methods
2.1. Study population
All patients with a new onset of HF recorded in the Clinical Practice Research Datalink (CPRD) or the Hospital Episode Statistics (HES) (Supplementary Figure 1) between 1st January 1998 and 31st July 2017. The CPRD, as the world's largest database of routinely recorded clinical data from primary care, has been validated for epidemiological research, [10] and includes an age and sex representative sample of the UK general population (approximately 7%). The HES database includes all admissions to NHS hospitals in England.
Patients from CPRD or HES were aged ≥ 30 years with a first code for HF (Supplementary Table 1), recorded in their clinical record during the study time window and were eligible for data linkage. Patients in HES were included if they had a first HF ICD-10 diagnostic code (Supplementary Table 2) in the primary discharge position and had linked CPRD data. Where patients appeared in both datasets, the first code was used as the HF index date. All patients required a minimum of 12-months of ‘up to standard’ CPRD data, prior to study entry. ‘Up-to-Standard’ is a quality marker indicating that patient data is continuous and complete. Follow-up was until the first of death or 31st July 2017.
2.2. Exposures
T2D was identified before or on the index date using an algorithm based on a detailed set of clinical codes, medications, age of diabetes onset and BMI, [11] to differentiate between type 1 and type 2 diabetes mellitus (Supplementary Figure 2). Patients with type 1 diabetes were excluded.
CKD was defined as estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2, calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [12] including the most recent eGFR measure before or on the index date (median time between the most recent eGFR measure and the index date was 91 days [IQR 23 to 286]). CKD was further stratified by four severity groups, based on the Kidney Disease: Improving Global Outcomes guidelines [13] as follows: CKD-3a (eGFR 45–59, ‘mild to moderate’ kidney disease); CKD-3b (eGFR 30–44, ‘moderate to severe’); CKD-4 (eGFR 15–29, ‘severe’) and CKD-5 <15 (‘kidney failure’ or dialysis).
HF patients were categorised by the presence of T2D and CKD, as follows: (i) T2D 0 CKD 0 (reference group), (ii) T2D 0 CKD 1 (HF-CKD-only), (iii) T2D 1 CKD 0; (HF-T2D-only) and (iv) T2D 1 CKD 1 (HF-T2D-CKD). To assess the influence of CKD severity, we further stratified the CKD group by the four CKD severity categories (CKD-3a to CKD-5).
2.3. Other characteristics
Based on the most prevalent ethnic groups in the 2011 census in England and Wales, ethnicity was categorised into 3 distinct groups: White, South Asian or Black. People coded as ‘mixed’ ‘other’ or ‘unknown’ were categorised as ‘other’. The 2010 patient level Index of multiple Deprivation (IMD) score [14] was used to define socio-economic status which was ranked into quintiles (the most affluent group; quintile 1, to the most deprived group; quintile 5). Cardiovascular medications were identified by at least one prescription in a 4-month time-window prior to HF diagnosis. For patients with T2D, glucose-lowering medications at baseline included metformin, sulphonylureas, thiazolidinediones, incretins, ‘other’ oral medications, and insulin. Cardiovascular and non-cardiovascular comorbidities were identified using Read and ICD-10 codes in CPRD and HES, respectively, recorded up to and including the index date. We also collected information on other risk factors using the most recent measure prior to study entry, including smoking and alcohol status, body mass index (BMI), systolic blood pressure, total cholesterol and haemoglobin concentrations.
2.4. Outcomes
All unplanned hospitalisations with at least one overnight stay that occurred after the index date were included. Admissions during follow-up were counted for each patient and further stratified, according to the primary discharge code, into cardiovascular (ICD-10 chapter 9 codes) and non-cardiovascular admissions (other ICD-10 chapters). In a sensitivity analysis, we further stratified CVD admissions by HF and other-CVD causes. The Office of National Statistics database was used to ascertain date of death from any cause.
2.5. Statistical analysis
Baseline characteristics were first presented by exposure group as number (%) or mean with 95% confidence intervals, estimated using 1000 bootstrap samples. Outcome rates during the follow-up were calculated for each calendar year of HF diagnosis and for two 4-year summary time periods at the start (1998–2002) and towards the end (2012–2015) of the study time-window. As data was over dispersed, negative binomial models were used. An interaction term between calendar year as a categorical variable and comorbidity severity group was entered into the model, which also included age. Outcome rates in HF are not constant over time and are significantly higher during the first year after diagnosis; therefore, to improve accuracy, rates were predicted separately for the first year of follow-up and for all subsequent years, in those surviving their first year. For the mortality outcome only, given the high number of in-hospital and immediate post discharge deaths, we also reported first month deaths separately. Rates were predicted for each comorbidity group and for each calendar year, at the mean population age (78 years). We investigated the difference in trends among comorbidity groups using the P value of an interaction term between calendar year as a continuous variable and comorbidity groups entered into the model adjusted for age. Two sensitivity analyses were conducted. First, given the potential higher outcome rates in people with HF diagnosed in the hospital setting compared to the community, we stratified our results by place of diagnosis. Second, we used Joinpoint Regression to identify any significant change in trend lines over time.
Next, overall differences in hospitalisation rates among comorbidity groups were investigated using negative binomial models to estimate incidence rate ratios (IRR) with 95% confidence intervals adjusting for age, gender, ethnicity, socioeconomic status, calendar year of diagnosis, place of diagnosis (primary care or hospital), cardiovascular medications, smoking, alcohol, comorbidities, BMI, blood pressure and cholesterol and haemoglobin levels. Overall difference in time to death among comorbidity groups were investigated using Royston-Parmar-Lambert flexible parametric survival models to estimate hazard ratios with 95% confidence intervals adjusted by the same variables. The reference group for both outcomes was the HF group without T2D and CKD. Age-standardised survival at 1, 3 and 5-years, stratified by comorbidity status and adjusted for calendar year, were also estimated. Survival curves were calculated using the Stata command stpm2_standsurv [15].
All analyses were performed in Stata-MP 16.0. To account for missing data in the multivariable models, multiple imputations using chained equations were performed using MI Impute in Stata (Supplementary Table 3): results were obtained using Rubin's rules combining 10 imputed datasets [16]. Three sensitivity analyses were performed: CVD admissions were stratified by cause (HF or other CVD) and by gender and we conducted a complete case analysis.
2.6 Patient involvement
This study used available data on CPRD and HES and no direct patient involvement or engagement took place as part of the study. However, in our recent review on priorities of patients with cardiometaboilic multimorbidities prior to the study, we found that patients’ priorities are mainly driven by their illness experiences in preserving functional ability. Both cardiovascular and non-cardiovascular hospitalisations can adversely affect functional ability. The findings in this study will be disseminated to diabetes UK and the British Heart Foundations.
3. Results
3.1. Study population
There were 87,709 patients with new-onset HF, median follow-up 2.36 [IQR 0.46,5.67] years, mean age 78 years (SD 11.3), 49% female and 41% diagnosed with HF in the hospital setting (Table 1). 67,227 (77%) of the patients had eGFR data at baseline. Of these patients, 21,815 (32%) had no T2D and CKD (reference group), 26,881 (40%) had CKD only, 7893 (12%) had T2D only and 10,638 (16%) had both CKD and T2D. Compared to the reference group (mean age 74 years), the HF-CKD-only group were 9 years older, the HF-T2D-only were 1 year younger and the HF-T2D-CKD group were 6 years older. At baseline, All T2D and CKD comorbidity groups were more likely to have cardiovascular comorbidities than the reference group. All HF patients with T2D (with or without CKD) were more likely to be prescribed cardiovascular medications and have a lower cholesterol and systolic blood pressure, but higher BMI than the HF patients with CKD-only and these patterns were consistent across CKD severity groups (Supplementary Table 4). Prescription of angiotensin-converting enzyme (ACE) inhibitor reduced with increasing CKD severity (Supplementary Table 5). Between 1998 and 2017, prescribing of ACE or angiotensin receptor blocker (ARB) increased for patients diagnosed in the community, but reduced after 2011 for patients diagnosed first in the hospital (Supplementary Table 6). These patterns were consistent across all CKD severity groups. In those with T2D, prescription of insulin increased and Metformin decreased, with increasing CKD severity (Supplementary Table 5).
Table 1.
Patients’ characteristics by comorbidity status.
| All HF patients N = 87,709 | HF with no T2D and CKD (reference group) N = 21,815 | HF with CKD-only N = 26,881 | HF with T2D-only N = 7893 | HF with T2D and CKD N = 10,638 | CKD missing N = 20,482 | |
|---|---|---|---|---|---|---|
| Age in years | 77.8 (77.8,77.9) | 73.9 (73.8,74.1) | 82.6 (82.5,82.7) | 72.5 (72.3,72.7) | 79.7 (79.5,79.8) | 76.9 (76.8,77.1) |
| Female | 43,173 (49.2) | 9263 (42.5) | 15,158 (56.4) | 2983 (37.8) | 5297 (49.8) | 10,472 (51.1) |
| Most affluent group | 16,357 (18.7) | 4501 (20.7) | 5509 (20.5) | 1241 (15.7) | 1793 (16.9) | 3313 (16.2) |
| Most deprived group | 14,745 (16.8) | 3301 (15.1) | 3868 (14.4) | 1634 (20.7) | 1904 (17.9) | 4038 (19.8) |
| Diagnosis in hospital | 36,094 (41.2) | 8215 (37.7) | 11,811 (43.9) | 3776 (47.8) | 5847 (55.0) | 6445 (31.5) |
| Beta blocker | 26,421 (30.1) | 7076 (32.4) | 9125 (33.9) | 2853 (36.1) | 3981 (37.4) | 3386 (16.5) |
| ACE inhibitor | 32,732 (37.3) | 8175 (37.5) | 10,451 (38.9) | 3622 (45.9) | 4778 (44.9) | 5706 (27.9) |
| ARB | 9069 (10.3) | 2068 (9.5) | 3313 (12.3) | 1125 (14.3) | 1881 (17.7) | 682 (3.3) |
| ACE or ARB | 40,559 (46.2) | 9960 (45.7) | 13,388 (49.8) | 4585 (58.1) | 6374 (59.9) | 6252 (30.5) |
| AA | 5718 (6.5) | 1354 (6.2) | 2188 (8.1) | 600 (7.6) | 988 (9.3) | 588 (2.9) |
| Diuretic (loop) | 42,932 (48.9) | 8915 (40.9) | 15,027 (55.9) | 3390 (42.9) | 6211 (58.4) | 9389 (45.8) |
| Aspirin | 33,554 (38.3) | 7481 (34.3) | 11,012 (41.0) | 3395 (43.0) | 4877 (45.8) | 6789 (33.1) |
| Comorbidities Number; mean (SD) | 4.1 (4.1,4.1) | 3.4 (3.4,3.4) | 4.7 (4.6,4.7) | 4.9 (4.8,4.9) | 6.1 (6.1,6.2) | 2.7 (2.6,2.7) |
| IHD | 43,537 (49.6) | 9795 (44.9) | 13,846 (51.5) | 4479 (56.7) | 6568 (61.7) | 8849 (43.2) |
| MI | 23,197 (26.4) | 5290 (24.2) | 7288 (27.1) | 2534 (32.1) | 3663 (34.4) | 4422 (21.6) |
| AF | 34,730 (39.6) | 9011 (41.3) | 12,470 (46.4) | 3081 (39.0) | 4652 (43.7) | 5516 (26.9) |
| Hypertension | 56,403 (64.3) | 13,255 (60.8) | 18,983 (70.6) | 6217 (78.8) | 9186 (86.4) | 8762 (42.8) |
| Stroke | 10,585 (12.1) | 2152 (9.9) | 3555 (13.2) | 1027 (13.0) | 1799 (16.9) | 2052 (10.0) |
| Anaemia | 11,089 (12.6) | 2418 (11.1) | 4051 (15.1) | 1154 (14.6) | 2224 (20.9) | 1242 (6.1) |
| Obesity | 21,996 (25.1) | 5583 (25.6) | 5201 (19.3) | 3645 (46.2) | 4320 (40.6) | 3247 (15.9) |
| COPD | 16,357 (18.6) | 4350 (19.9) | 4707 (17.5) | 1754 (22.2) | 2145 (20.2) | 3401 (16.6) |
| Asthma | 16,396 (18.7) | 4551 (20.9) | 4600 (17.1) | 1882 (23.8) | 2187 (20.6) | 3176 (15.5) |
| Depression | 19,873 (22.7) | 5503 (25.2) | 5852 (21.8) | 2205 (27.9) | 2614 (24.6) | 3699 (18.1) |
| Osteoarthritis | 32,430 (37.0) | 8042 (36.9) | 11,362 (42.3) | 2979 (37.7) | 4522 (42.5) | 5525 (27.0) |
| Cancer | 20,399 (23.3) | 5040 (23.1) | 7524 (28.0) | 1663 (21.1) | 2736 (25.7) | 3436 (16.8) |
| Dementia | 3969 (4.5) | 829 (3.8) | 1574 (5.9) | 301 (3.8) | 537 (5.0) | 728 (3.6) |
| Smoking | 17,204 (19.6) | 4555 (20.9) | 4090 (15.2) | 1663 (21.1) | 1564 (14.7) | 5332 (26.0) |
| Alcohol | 53,401 (60.9) | 14,965 (68.6) | 16,651 (61.9) | 4951 (62.7) | 6131 (57.6) | 10,703 (52.3) |
| BMI (kg/m2) | 27.7 (27.7,27.8) | 27.5 (27.4,27.6) | 26.6 (26.6,26.7) | 30.7 (30.5,30.9) | 29.6 (29.5,29.8) | 26.9 (26.8,26.9) |
| Systolic BP (mm/Hg) | 137.9 (137.7,138.0) | 135.1 (134.9,135.4) | 136.2 (136.0,136.5) | 135.2 (134.8,135.7) | 136.5 (136.1,136.9) | 145.3 (145.0,145.6) |
| Cholesterol (mmol/L) | 4.7 (4.7,4.7) | 4.8 (4.8,4.8) | 4.8 (4.7,4.8) | 4.3 (4.3,4.3) | 4.3 (4.3,4.3) | 5.4 (5.4,5.5) |
Data are reported as number (%) for categorical variables and as means (standard deviation) for continuous data. T2D, type II diabetes mellitus; CKD, chronic kidney disease; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; AA, aldosterone antagonist (spironolactone or eplerenone). IHD, ischaemic heart disease; MI, myocardial infarction; AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; BMI, body mass index; BP, blood pressure.
3.2. Trends in hospitalisation rates
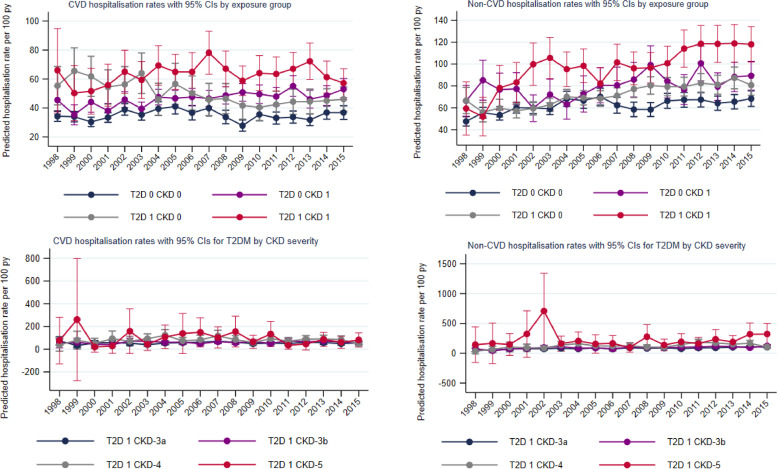
First-year rates: Compared to the reference group without T2D or CKD, age-adjusted CVD hospitalisation rates were significantly higher for HF patients with CKD-only and T2D-only (35.1; 95% CI 34.0, 36.1 per 100 person-years vs. 46.4; 44.9, 47.9 vs. 49.2; 46.7, 58.8 respectively). First-year rates increased with increasing CKD severity reaching 89.1 (65.8, 112.4) in the HF-T2D-CKD-5 group. Between 1998–2001 and 2012–2015, CVD hospitalisation rates increased significantly by 26% for the HF-CKD-only group but significantly reduced by 24% for the HF-T2D-only group (Table 2). When CVD admissions were stratified by specific type (HF admission and ‘other CVD’ admissions), patterns were similar, but the divergence between the HF-CKD-only and HF-T2D-only groups was greater for HF admissions (Supplementary Table 7). The reduction in CVD admissions in the HF-T2D-only group was lost for all HF-T2D-CKD severity groups (Table 2, Fig. 1) and was similar for both HF and other-CVD admissions (Supplementary Table 7). When stratified by place of diagnosis, rates of CVD admissions were between 50 and 100% higher for the HF groups diagnosed in the hospital, compared to the group diagnosed in the community. However, both settings experienced similar trends over time, with a significant reduction in rates for the HF-T2D-only group, which was not present for the groups with CKD (Supplementary Table 8). Men had slightly higher overall rates than women across all comorbidity groups (Supplementary Table 9). Whilst CVD admission rates remained stable or decreased over time for men, women experienced a significant increase in the reference group (interaction-P <0.001) and HF-CKD-only group (interaction-P 0.009).
Table 2.
Predicted outcome rates following HF diagnosis by population groups and calendar year.
| Predicted rate per 100 py (95% CI) | Relative diff. (%)a | P interactionb | |||||
|---|---|---|---|---|---|---|---|
| Overall | 1998–2001 | 2012–2015 | |||||
| Hospitalisation rates during the first year following heart failure diagnosis | |||||||
| CVD | |||||||
| T2D 0 CKD 0 | 35.1 (34.0–36.1) | 32.9 (31.2–34.6) | 34.6 (32.4–36.8) | 5.2 | ref | ||
| T2D 0 CKD 1 | 46.4 (44.9–47.9) | 40.2 (36.8–43.6) | 50.7 (47.2–54.1) | 26.1 | 0.001 | ||
| T2D 0 CKD-3a | 39.7 (38.0–41.5) | 33.2 (29.4–37.1) | 42.3 (38.3–46.3) | 27.4 | 0.011 | ||
| T2D 0 CKD-3b | 50.4 (47.7–53.1) | 43.6 (37.2–50.0) | 58.8 (52.0–65.6) | 34.9 | <0.001 | ||
| T2D 0 CKD-4 | 65.6 (59.8–71.4) | 65.3 (49.6–81.0) | 68.3 (55.4–81.3) | 4.6 | 0.9301 | ||
| T2D 0 CKD-5 | 77.8 (60.8–94.8) | 71.1 (35.8–106.4) | 64.2 (33.2–95.3) | −9.7 | 0.702 | ||
| T2D 1 CKD 0 | 49.2 (46.7–51.8) | 58.6 (51.9–65.3) | 44.8 (40.6–49.0) | −23.5 | <0.001 | ||
| T2D 1 CKD-3a | 56.5 (52.5–60.5) | 55.6 (43.7–67.5) | 57.6 (50.1–65.1) | 3.6 | 0.743 | ||
| T2D 1 CKD-3b | 63.2 (58.1–68.4) | 47.7 (33.5–61.8) | 65.3 (55.7–74.8) | 36.9 | 0.454 | ||
| T2D 1 CKD-4 | 83.7 (73.8–93.7) | 73.4 (36.3–110.5) | 78.9 (62.6–95.2) | 7.5 | 0.328 | ||
| T2D 1 CKD-5 | 89.1 (65.8–112.4) | 59.4 (−8.0–126.8) | 74.6 (40.6–108.6) | 25.6 | 0.388 | ||
| Non-CVD | |||||||
| T2D 0 CKD 0 | 62.2 (60.7–63.8) | 54.1 (51.8–56.5) | 66.1 (62.7–69.5) | 22.2 | ref | ||
| T2D 0 CKD 1 | 71.7 (69.8–73.6) | 59.4 (55.2–63.6) | 83.6 (78.9–88.3) | 40.7 | 0.001 | ||
| T2D 0 CKD-3a | 61.2 (59.0–63.4) | 51.6 (46.6–56.5) | 70.1 (64.7–75.6) | 35.9 | 0.017 | ||
| T2D 0 CKD-3b | 76.0 (72.6–79.4) | 62.0 (54.3–69.6) | 89.8 (81.3–98.4) | 44.8 | 0.032 | ||
| T2D 0 CKD-4 | 98.0 (90.7–105.2) | 83.2 (65.7–100.8) | 109.3 (92.2–126.4) | 31.4 | 0.378 | ||
| T2D 0 CKD-5 | 170.2 (140.8–199.6) | 117.7 (71.7–163.6) | 240.6 (152.0–329.2) | 104.4 | 0.008 | ||
| T2D 1 CKD 0 | 85.1 (81.3–88.9) | 75.6 (67.9–83.3) | 88.6 (81.7–95.4) | 17.2 | 0.765 | ||
| T2D 1 CKD-3a | 84.2 (79.1–89.2) | 66.9 (54.3–79.4) | 101.5 (90.6–112.4) | 51.7 | 0.054 | ||
| T2D 1 CKD-3b | 96.4 (89.9–103.0) | 71.1 (52.9–89.2) | 112.9 (99.3–126.5) | 58.8 | 0.227 | ||
| T2D 1 CKD-4 | 130.6 (117.6–143.5) | 85.6 (46.8–124.5) | 149.0 (124.6–174.1) | 74.1 | 0.437 | ||
| T2D 1 CKD-5 | 223.2 (177.0–269.3) | 215.7 (32.5–399.0) | 280.9 (184.8–377.1) | 30.2 | 0.876 | ||
| Deaths in first month N (%) | Mortality rates during the first year following heart failure diagnosis in those who survived the first month | ||||||
| 1998–2001 | 2012–2015 | Overall | 1998–2001 | 2012–2015 | |||
| T2D 0 CKD 0 | 1387 (13.0) | 567 (8.4) | 20.5 (19.8–21.2) | 22.3 (21.1–23.5) | 17.2 (15.9–18.5) | −22.9 | ref |
| T2D 0 CKD 1 | 155 (16.7) | 370 (12.7) | 24.9 (23.9–25.8) | 24.3 (22.3–26.3) | 25.4 (23.6–27.1) | 4.5 | <0.001 |
| T2D 0 CKD-3a | 321 (14.4) | 325 (11.6) | 19.8 (18.8–20.7) | 20.2 (17.9–22.5) | 19.4 (17.5–21.3) | −4.0 | 0.002 |
| T2D 0 CKD-3b | 176 (14.3) | 277 (15.1) | 26.1 (24.6–27.6) | 25.6 (22.1–29.1) | 29.2 (26.0–32.3) | 14.1 | <0.001 |
| T2D 0 CKD-4 | 99 (22.4) | 170 (23.3) | 40.4 (37.1–43.8) | 38.4 (30.2–46.6) | 36.8 (30.7–42.9) | −4.2 | 0.152 |
| T2D 0 CKD-5 | 31 (26.7) | 28 (25.2) | 74.1 (60.5–87.8) | 44.9 (26.0–63.8) | 63.8 (37.9 - 89.8) | 42.1 | 0.026 |
| T2D 1 CKD 0 | 219 (12.7) | 245 (9.3) | 25.1 (23.5–26.7) | 33.7 (29.5–37.9) | 21.2 (18.8–23.6) | −37.1 | 0.067 |
| T2D 1 CKD-3a | 68 (13.5) | 135 (10.6) | 25.8 (23.8–27.8) | 26.1 (20.3–31.9) | 25.9 (22.4–29.5) | −0.8 | 0.010 |
| T2D 1 CKD-3b | 53 (18.1) | 131 (13.0) | 30.4 (27.8–32.9) | 22.5 (15.4–29.6) | 29.2 (24.9–33.5) | 29.8 | 0.050 |
| T2D 1 CKD-4 | 26 (24.1) | 81 (16.0) | 46.6 (41.2–52.0) | 49.7 (27.4–71.9) | 42.3 (34.2–50.4) | −14.9 | 0.637 |
| T2D 1 CKD-5 | 8 (32.0) | 23 (19.7) | 78.6 (60.2–97.0) | 122.7 (22.4–222.9) | 68.5 (41.4–95.5) | −44.2 | 0.757 |
T2D, type II diabetes mellitus; CKD, chronic kidney disease (1: Estimated Glomerular Filtration Rate (eGFR) <60 ml/min/m2; 3a: Estimated Glomerular Filtration Rate (eGFR) 45–59 ml/min/m2; 3b: eGFR 30–44; 4: eGFR 15–29; 5 eGFR <15).
Crude death rates are reported for the first month following HF diagnosis as N (%); number and percentage. Age adjusted death rates are reported for the first year in people who survived the first month following diagnosis. All predictions are at the mean population age (78 years).
Relative diff., relative difference; py, person-years; CI, confidence interval, Q; quintile.
a relative percentage difference in rates (per 100 person-years) between the first and second diagnosis calendar time periods, calculated by 100*([time-period 2 – time period 1] / time-period 1].
b P value for the difference in trend lines between groups. Estimated by fitting an interaction term between calendar year and exposure group in the Poisson models also containing age. As interaction tests have low power, p-values should be interpreted along with the graphical trends (Figs. 1 and 2).
Fig. 1.
Trends in estimated 1-year rates of cause-specific hospitalisations
Predicted admission rates at mean population age (78 years) per 100 person-years between 1998 and 2015. Follow up was until death or study end. Spikes indicate 95% CI.
Non-CVD hospitalisation rates were higher for the CKD-only group (71.7; 69.8, 73.6) and the T2D-only group (85.1; 81.3, 88.9) compared to the reference group (62.2; 60.7, 63.8) and were highest in the combined T2D-CKD group reaching 223 (177, 269) in T2D-CKD-s5. Over time, non-CVD admission rates significantly increased by 22% in the reference group and 41% in the CKD-only group (interaction-P<0.001) (Fig. 1). The T2D group had stable rates; however, such stability was lost in the T2D-CKD groups who experienced the highest increases, reaching 74% rise in the T2D-CKD-4 group. When stratified by place of diagnosis, trends over time were similar, with an increase in rates for the CKD-only group and stable rates for the T2D-only group. This stability was lost for the T2D-CKD group diagnosed in the hospital setting (Supplementary Table 8). Overall rates were similar between men and women across comorbidity groups (Supplementary Table 9). Over time rates remained stable in men but significantly increased for women across comorbidity groups. There was no significant deflection in the trend lines for first year hospitalisation rates, with the exception of non-CVD rates in the T2D-CKD group, which experienced the steepest increase prior to 2002.
Subsequent year rates: Hospitalisation rates after the first year of follow-up were lower than those observed during the first year rates but showed similar patterns by comorbidity groups (Supplementary Table 10). Over time, CVD admissions reduced for the reference group and T2D-only group but remained stable in all CKD and T2D-CKD groups whereas non-CVD admissions remained relatively stable.
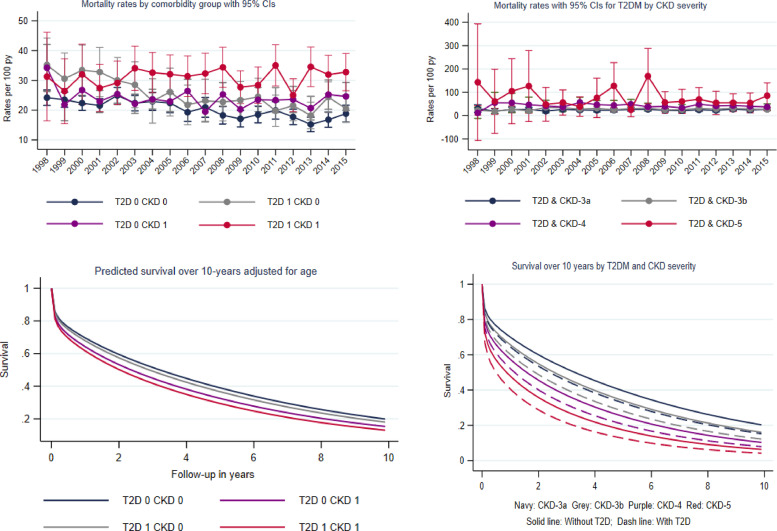
3.3. Trends in mortality rates
Compared to the rate in the reference group (20.5 per 100 person years; 19.8, 21.2), first year mortality rates were higher for the HF-CKD-only (24.9; 23.9, 25.8) and HF-T2D-only (25.1 (23.5, 26.7) groups (Table 2). Rates increased progressively with the severity of CKD and were highest in the HF-T2D-CKD-5 group (78.6; 60.2, 97.0). Over time mortality rates significantly decreased by 23% in the reference group whilst remaining stable in the HF-CKD-only group (interaction-P <0.001). The HF-T2D-only group experienced a 37% reduction in mortality rates over time, but for all HF-T2D-CKD severity groups, rates remained generally stable (Fig. 2). When stratified by place of diagnosis, mortality rates were twice as high in the group diagnosed in the hospital compared to the group diagnosed in the community (Supplementary Table 8). However, trends over time were similar between the groups with significant reductions in the reference and T2D-only groups, which were lost once CKD was present. Men had higher mortality rates than women across all comorbidity groups (Supplementary Table 9). Mortality rates in men reduced over time for most comorbidity groups but remained stable in the HF-T2D-CKD group. Rates in women were relatively stable overall, with the exception of the HF-T2D-only group where the rates reduced. There was no significant deflection in the trend lines for first year death rates, with the exception of the T2D-only group, which experienced the steepest reduction prior to 2006.
Fig. 2.
Trends in estimated mortality rates and survival by diabetes status
a) Estimated mortality rates at mean population age (78 years) per 100 person-years between 1998 and 2015. Spikes indicate 95% CI. Rates were calculated in survivors of the first month following HF diagnosis. b) 10-year age and calendar year standardised survival by comorbidity status.
Subsequent year rates after the first year of follow-up were lower but showed similar trends over time (Supplementary Table 10).
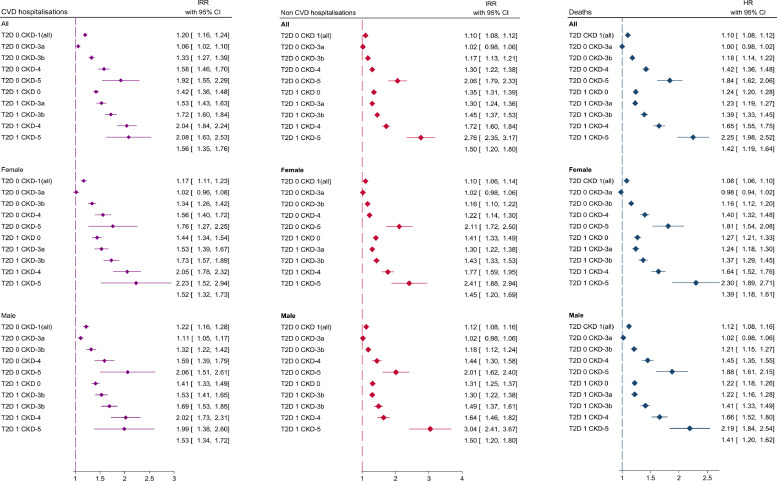
3.4. Overall differences in hospitalisation rates
In HF patients, compared to those without CKD and T2D, patients with HF-CKD-only (adjusted incident rate ratio (IRR): 1.20; 95% CI: 1.16, 1.24) and HF-T2D-only (1.42; 1.36, 1.48) showed higher CVD hospitalisation rates. The incidence rate ratio increased with increasing severity of CKD in both the HF-CKD-only and HF-T2D-CKD severity groups and was highest in the HF-T2D-CKD-5 group: IRR 2.08 (1.63, 2.53) (Supplementary Table 11; Fig. 3). These patterns were similar for non-CVD admissions: HF-CKD-only (adjusted IRR 1.34; 1.30, 1.39), HF-T2D-only (1.35; 1.31, 1.40), HF-T2D-CKD-5 (2.76; 2.37, 3.20). Incidence rate ratios stratified by gender were similar (Supplementary Table 12).
Fig. 3.
Adjusted associations between comorbidity groups and outcomes.
Differences in hospitalisation rates among comorbidity groups were investigated using negative binomial models to estimate incidence rate ratios (IRR) with 95% confidence intervals. Overall difference in time to death among comorbidity groups were investigated using Royston-Parmar-Lambert flexible parametric survival models to estimate hazard ratios with 95% confidence intervals. All models were adjusted for adjusting for age, gender, ethnicity, socioeconomic status, place of diagnosis (primary care of hospital), cardiovascular medications, smoking, alcohol, comorbidities, BMI, blood pressure, cholesterol and haemoglobin concentrations.
3.5. Overall differences in mortality rates
The median survival was 50% lower in the HF-CKD-only group (2.2 years; 95% CI 2.2, 2.3) compared to the reference group without CKD and T2D (4.4; 4.3,4.5). The HF-T2D-only group had a median survival of 4.1 years (4.0, 4.3) years, which was significantly smaller with each increasing stage of CKD, reaching 0.7 years (0.5, 1.1) in the HF-T2D-CKD-5 group (Supplementary Table 13). Compared to the reference group, the HF-CKD-only group (HR 1.10; 95% CI 1.08, 1.12) and the HF-T2D-only group (1.24, 1.20, 1.28) had an increased risk of death. This risk increased for both comorbidity groups from CKD-3b to CKD-5 and was highest in the HF-T2D-CKD-5 group (2.25, 1.98, 2.52) (Fig. 3). The age standardised risk at 1, 3 and 5 years was 28%, 45% and 58% respectively in the reference group (Supplementary Table 14). The risk progressively increased with increasing CKD severity and was highest in the HF-T2D-CKD severity groups (Fig. 2). 1,3 and 5 year risk estimates in the HF-T2D-CKD-5 group were 56%, 76%, 86%.
3.6. Complete case analysis
Comorbidity associations with both outcomes were similar in the complete case analysis (Supplementary Tables 15 and 16)
4. Discussion
As people age, changes in their physiology and cardiovascular structure causes an increased susceptibility to CVD resulting in older people being more likely to be faced with a constellation of chronic conditions such as diabetes, HF and CKD [10]. In this cohort of older people with new-onset HF with a mean age of 78 years, high rates of mortality, CVD and non-CVD hospitalisations were observed at one year,. The presence of CKD was associated with worsening outcomes over the past 2 decades, whilst the presence of diabetes conferred an improvement in these outcomes over time. However, when CKD was present, these trends in improvement were lost and outcomes for people with HF and T2D worsened for each stage of increasing CKD severity, pointing to an urgent need for strategies to prevent and manage CKD in people with HF.
In people with T2D and new onset HF, the first-year rates of cardiovascular and non-cardiovascular morbidity and mortality increased with increasing comorbid CKD severity, peaking at CKD-stage 5. Between 1998–2001 and 2012–2015, whereas we noted a 26% increase in CVD hospitalisation rates for people with HF and CKD, there was a 24% reduction in those with HF and T2D. This reduction was attenuated in the presence of CKD at all stages. Over the same time-frame, non-CVD admission rates significantly increased in people with HF alone, and even more in people with HF and CKD. The presence of T2D seemed to stabilize the non-CVD admission rate until CKD was present, when rates then increased for all severity groups. Similarly, whilst reductions of 23% in mortality were seen in people with only HF over time, these reductions were even greater at 37% in people with HF who also had T2D. These reductions in mortality trends were not observed in people with CKD or with T2D and CKD. In general, despite the CVD and non-CVD hospitalisations and mortalities all improving over time in people with new onset HF and comorbid T2D, presence of comorbid CKD was associated with worse outcomes for each stage of increasing CKD severity.
T2D, CKD and HF are multifactorial diseases of considerable heterogeneity, but with many common risk factors and shared pathophysiological pathways [17]. Multiple target organ damage from physiological stresses in people with HF may synergistically increase their susceptibility for worsening diabetes control and CKD and increase their mortality [18,19]. It is known that the same age-associated pathological and biological changes involved in the development of CKD and insulin resistance in T2D, also predispose people to other chronic disease such as HF [20] and cardiorenal syndrome is the most frequent first comorbidity in people with T2D [21]. When these multiple diseases are present together, there is a bidirectional relationship with one condition accelerating the presentation and progression of the other [22]. Insulin resistance ultimately leads to hyperglycaemia which activates the renin-angiotensin-aldosterone system (RAAS) and this turn leads to efferent vaso-constriction through the neuro-hormonal effects on the efferent arterioles of the glomerulus leading to increase in hyper-filtration [23]. Hyper-filtration has been noted to be the main driver of the pathophysiological changes in diabetes nephropathy [24]. In the initial stages of diabetes kidney disease, the kidneys increase in size due to the hyper-filtration. This is followed by progressively increasing glomerular damage resulting in the ever-increasing excretion of urine albumin until there is overt proteinuric renal impairment and subsequent rise in blood pressure and anaemia. These place extra burdens on the heart, which by this stage is already weakened through the macrovascular complications of long-standing diabetes. The development of HF causes activation the RAAS the sympathetic nervous system, thereby completing the vicious cycle [25].
Furthermore, sometimes there can be therapeutic complexities such that a medication for one disease can inadvertently worsen the control of another; for example, the use of pioglitazone [26] or saxagliptin [27,28] in the management of T2D has been associated with an increased risk of hospitalisation for HF. Beta-blockers for HF with the exception of carvedilol can lead to slight hyperglycaemia [29], thus potentially worsening long-term glycaemic control. Conversely, angiotensin converting enzyme inhibitors used in HF and CKD can cause hypoglycaemia [30], which is associated with cardiac arrhythmias [31].
The decreasing trends in cardiovascular and non-cardiovascular hospitalisations and mortality in subjects with T2D are not dissimilar to previous findings [32,33]. Gregg et al. reported that between 1971 and 1986 and 1988 to 2000, the all-cause mortality rate reduced by 18.2 deaths per 1000 person-years in men with diabetes [32]. In a recent analysis, reducing rates were also observed in subjects with T2D for nonfatal myocardial infarction, total stroke and nonfatal stroke [26]. Several explanatory factors for these trends range from the improved control of cardiovascular risk factors in people with T2D to the use of improved lifesaving technology [34], [35], [36], [37]. There is now a focus on the multi-factorial control of people with T2D and CVD with emphasis on advice on smoking cessation, lipid levels, blood pressure and glycaemic control and appropriate use of aspirin and influenza vaccination [38], [39], [40], [41].
In general, our observation of worsening trends on adverse outcomes in people with HF and CKD is a consistent finding [42,43]. A possible explanation of this trend is that the declines in both the CVD and non-CVD adverse events in people with T2D could lead to a proportionately higher numbers of people living with CKD [44]. Despite the cardiovascular benefits of targeting the renin-angiotensin-aldosterone-system for over the past two decades [45,46], there is a still a residual risk of progression to end-stage kidney disease associated with increased morbidity, hospitalisation and death rates [47,48]. Conversely, progression of CKD in patients with HF limits the use of the same HF guideline-directed disease modifying therapies (inhibitors of the renin-angiotensin-aldosterone-system). In people with T2D, evidence points to a high proportion of people not being tested appropriately for CKD, resulting in significant under diagnosis [49]. This lack of awareness among patients and health care professionals could potentially lead to more severe CKD and act as a counterbalance to any benefits gained through earlier cardiovascular disease prevention in T2D. The high risk of poor outcomes associated with CKD in HF in our study, increased in the presence of T2D and highlights the need for multi-faceted interventions. Optimising cardiovascular drug therapies is important combined with risk factor management including lowering blood pressure and cholesterol and improving anaemia and exercise [50,51]. The introduction of sodium-glucose cotransporter-2 inhibitors with proven benefits in T2D, [52], [53], [54] HF, [8, 55] and CKD [7] in routine clinical practice will hopefully address the residual risk of worsening of these cardio-reno-metabolic conditions.
Our study is the first to our knowledge to assess not only the relative associations between HF, T2D and CKD severity and cardiovascular and non-cardiovascular outcomes, but also the trends over 2 decades. A notable strength of this study is the inclusion of only new onset HF, in an attempt to tease apart the ‘chicken or egg’ relationships among T2D, CKD and HF. The increased frequency of adverse outcomes in people with HF, T2D and CKD, highlight the need for evidence-based treatment strategies that addresses all three conditions.
Our study is limited first by lack of ejection fraction data, thus limiting the possibility to differentiate between phenotypes of HF i.e. with preserved or reduced ejection fraction which may have different rates of adverse events. Second, whilst our definitions were based on validated coding, there is also a possibility of misclassification of comorbidities with the use of routinely collected data and we did not account for new comorbidities developing after the onset of HF. That said, given the strong association between CKD, T2D and worse outcomes, any residual effect from new comorbidities developing during follow-up, would only serve to weaken and not strengthen the associations reported. Third, we did not have albuminuria data and eGFR was missing for 23% of the cohort, however we used a multiple imputation approach to account for missing data. Some of the covariates extracted from the databases for analysis such as smoking and alcohol are reliant on updated clinical recording of self-reported information. We used the most recent recording and imputation for missing data, but could not account for any inaccuracies of information. It is also possible that some of these covariates might be on the causal pathway between TD2 or CKD and HF.
Authors contribution
Claire A Lawson: study design, data collection, data analysis, data interpretation, figures, writing; Samuel Seidu: literature search, data collection, figures, study design, data interpretation, writing; Francesco Zaccardi: figures, data collection, study design, data interpretation, writing; Gerry Mccann: figures, study design, data interpretation, writing; Umesh T Kadam:data interpretation, writing; Melanie J Davies: figures, literature search, study design, data interpretation, writing; Carolyn SP Lam: figures, literature search, study design, data interpretation, writing; Hidde L. Heerspink: figures, literature search study design, data interpretation, writing; Kamlesh Khunti: study design, literature search, data collection, data interpretation, figures, writing.
The study protocol was approved by The Independent Scientific Advisory Committee (ISAC) for data access (Protocol 18_037R). Ethics approval for use of CPRD data following approval from ISAC is granted by a national research ethics committee (05/MRE04/87/AM06). Whilst individual patient consent is not required, all data is de-identified and patients can opt out of data contribution.
Declaration of Competing Interest
Dr. Khunti reports personal fees from Amgen, personal fees from Astrazeneca, personal fees from Bayer, personal fees from NAPP, personal fees from Lilly, personal fees from Merck Sharp & Dohme, personal fees from Novartis, personal fees from Novo Nordisk, personal fees from Roche, personal fees from Berlin-Chemie AG / Menarini Group, personal fees from Sanofi-Aventis, personal fees from Servier, personal fees from Boehringer Ingelheim, grants from Pfizer, grants from Boehringer Ingelheim, grants from AstraZeneca, grants from Novartis, grants from Novo Nordisk, grants from Sanofi-Aventis, grants from Lilly, grants from Merck Sharp & Dohme, grants from Servier, outside the submitted work; .
Dr. Seidu reports personal fees from Amgen, personal fees from Astrazeneca, personal fees from NAPP, personal fees from Lilly, personal fees from Merck Sharp & Dohme, personal fees from Novartis, personal fees from Novo Nordisk, personal fees from Roche, personal fees from Sanofi-Aventis, personal fees from Boehringer Ingelheim, grants from AstraZeneca, grants from Sanofi-Aventis, grants from Servier, grants from Janssen, outside the submitted work;
Dr. Heerspink reports other from Consultant to Abbvie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL-Pharma, Janssen, Gilead, MundiPharma, Mitsubishi Tanabe, Merck and Retrophin, grants from Research Grants from Abbvie, AstraZeneca, Boehringer Ingelheim and Janssen, outside the submitted work; .
Professor Davies reports personal fees from Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca and Janssen, personal fees from Servier and Gilead Sciences Ltd, personal fees from NAPP, Mitsubishi Tanabe Pharma Corporation and Takeda Pharmaceuticals International Inc., grants from Novo Nordisk, Sanofi-Aventis, Lilly, Boehringer Ingelheim, Astrazeneca and Janssen, outside the submitted work; .
Dr. Gerry McCann reports and Research grants from the BHF and NIHR related to T2D and Heart failure.
Carolyn SP Lam is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic and Vifor Pharma; has served as consultant or on the Advisory Board/ Steering Committee/ Executive Committee for Boston Scientific, Bayer, Roche, Diagnostics, AstraZeneca, Medtronic, Vifor Pharma, Novartis, Amgen, Merck, Janssen Research & Development LLC, Menarini, Boehringer Ingelheim, Novo Nordisk, Abbott Diagnostics, Corvia, Stealth BioTherapeutics, JanaCare, Biofourmis, Darma, Applied Therapeutics, MyoKardia, Cytokinetics, WebMD Global LLC, Radcliffe Group Lted and Corpis; and serves as co-founder & non-executive director of eKo.ai
All the other authors have nothing to disclose.
Acknowledgments
Role of the funding source
This publication presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. The study sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data sharing
The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The interpretation and conclusions contained in this study are those of the author/s alone. This study is based in part on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. Access to data is via application to the CPRD and can be shared with permission from CPRD. HES data, Copyright © (2017), was re-used with the permission of The Health & Social Care Information Centre. All rights reserved. The data that support the findings of this study are available from the corresponding author upon reasonable request
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100739.
Appendix. Supplementary materials
References
- 1.Savarese G., Lund L.H. Global public health burden of heart failure. Cardiac Fail Rev. 2017;3(1):7. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D. Executive summary: heart disease and stroke statistics—2015 update: a report from the American heart association. Circulation. 2015;131(4):434–441. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Deedwania P., Acharya T. Cardiovascular protection with anti-hyperglycemic agents. Am J Cardiovasc Drugs. 2019;19(3):249–257. doi: 10.1007/s40256-019-00325-9. [DOI] [PubMed] [Google Scholar]
- 4.Birkeland K.I. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes, Obes Metab. 2020 doi: 10.1111/dom.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowrick C. Sage Publications Sage UK; London, England: 2005. What is chronic illness? [Google Scholar]
- 6.Mahaffey K.W. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups: results from the randomized credence trial. Circulation. 2019;140(9):739–750. doi: 10.1161/CIRCULATIONAHA.119.042007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMurray J.J. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 8.Majid S. Adopting evidence-based practice in clinical decision making: nurses' perceptions, knowledge, and barriers. J Med Lib Assoc: JMLA. 2011;99(3):229. doi: 10.3163/1536-5050.99.3.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherney D.Z. Impact of cardio-renal-metabolic comorbidities on cardiovascular outcomes and mortality in type 2 diabetes mellitus. Am J Nephrol. 2020;51(1):74–82. doi: 10.1159/000504558. [DOI] [PubMed] [Google Scholar]
- 10.Herrett E., Gallagher A.M., Bhaskaran K., Forbes H., Mathur R., van Staa T. Data resource profile: clinical practice research datalink (CPRD) Int J Epidemiol. 2015;44(3):827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lusignan S., Khunti K., Belsey J. A method of identifying and correcting miscoding, misclassification and misdiagnosis in diabetes: a pilot and validation study of routinely collected data. Diabet Med. 2010;27(2):203–209. doi: 10.1111/j.1464-5491.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 12.Valente M., Hillege H., Navis G., Voors A., Dunselman P., van Veldhuisen D., Kevin Damman. The chronic kidney disease epidemiology collaboration equation outperforms the modification of diet in renal disease equation for estimating glomerular filtration rate in chronic systolic heart failure. Eur J Heart Fail. 2014;16(1):86–94. doi: 10.1093/eurjhf/hft128. [DOI] [PubMed] [Google Scholar]
- 13.Kidney disease improving global outcomes. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl (2011). 2013;3(1):1-150.hodology. 2005;5(1):1-6.
- 14.Department for Communities and Local Government . 2020. English indices of deprivation 2010: guidance document.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/6871/1871208.pdf [online]. Updated 2011. Accessed June 29th. [Google Scholar]
- 15.Putter H., Fiocco M., Geskus R.B. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 16.Rubin D.B. Wiley; New York: 1987. Multiple imputation for nonresponse in surveys. New York: Wiley. [Google Scholar]
- 17.Zimmet P., Alberti K., Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 18.Barnett K. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 19.Fortin M. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10(2):142–151. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes P.J. Mechanisms of development of multimorbidity in the elderly. Eur Respir J. 2015;45(3):790–806. doi: 10.1183/09031936.00229714. [DOI] [PubMed] [Google Scholar]
- 21.Birkeland K., Bodegard J., Eriksson J. Cardiorenal disease is the most common first CV manifestation in type 2 diabetes and associated with increased mortality: a large multinational observational study. Presented at: European Association for the Study of Diabetes 55th Annual Meeting; September 16-20; Barcelona, Spain; 2019. Abstract 126. [Google Scholar]
- 22.Rangaswami J., Bhalla V., Blair J.E.A., Chang T.I., Costa S., Lentine K.L., Lerma E.V., Mezue K., Molitch M., Mullens W., Ronco C., Tang W.H.W., McCullough P.A. on behalf of the American heart association council on the kidney in cardiovascular disease and council on clinical cardiology. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement for healthcare professionals from the American heart association. Circulation. 2019;139:e840–e878. doi: 10.1161/CIR.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 23.Tang S.C., Chan G.C., Lai K.N. Recent advances in managing and understanding diabetic nephropathy. F1000 Res. 2016;5 doi: 10.12688/f1000research.7693.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magee G.M., Bilous R.W., Cardwell C.R. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52:691. doi: 10.1007/s00125-009-1268-0. [DOI] [PubMed] [Google Scholar]
- 25.Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Progr Cardiovasc Dis. 2019;62(Issue 4):298–302. doi: 10.1016/j.pcad.2019.07.003. July–August. [DOI] [PubMed] [Google Scholar]
- 26.Erdmann E. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive study (PROactive 08) Diabetes Care. 2007;30(11):2773–2778. doi: 10.2337/dc07-0717. [DOI] [PubMed] [Google Scholar]
- 27.Scirica B.M. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 28.Scirica B.M. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130(18):1579–1588. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 29.Bell D.S. Use of beta blockers in the patient with diabetes. Endocrinologist. 2003;13(2):116–123. [Google Scholar]
- 30.Herings R. Hypoglycaemia associated with use of inhibitors of angiotensin converting enzyme. Lancet. 1995;345(8959):1195–1198. doi: 10.1016/s0140-6736(95)91988-0. [DOI] [PubMed] [Google Scholar]
- 31.Fitzpatrick C. Association of hypoglycaemia and risk of cardiac arrhythmia in patients with diabetes mellitus: a systematic review and meta-analysis. Diabetes, Obes Metab. 2018;20(9):2169–2178. doi: 10.1111/dom.13348. [DOI] [PubMed] [Google Scholar]
- 32.Gregg E.W. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147(3):149–155. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 33.Vetrone L.M. Cardiovascular and mortality events in type 2 diabetes cardiovascular outcomes trials: a systematic review with trend analysis. Acta Diabetol. 2019;56(3):331–339. doi: 10.1007/s00592-018-1253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaede P., Lund-Andersen H., Parving H.-H., Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 35.Ergin A. Secular trends in cardiovascular disease mortality, incidence, and case fatality rates in adults in the United States. Am J Med. 2004;117(4):219–227. doi: 10.1016/j.amjmed.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Loria C.M., Sempos C.T., Vuong C. 1992. Plan and operation of the nhanes ii mortality study. 1999. [PubMed] [Google Scholar]
- 37.Statistics, N.C.f.H., Plan and operation of the second National Health and Nutrition Examination Survey, 1976-1980. 1981: US Department of Health and Human Services, Public Health Service, Office of ….
- 38.Imperatore G. Thirty-year trends in cardiovascular risk factor levels among US adults with diabetes: national Health and Nutrition Examination Surveys, 1971–2000. Am J Epidemiol. 2004;160(6):531–539. doi: 10.1093/aje/kwh232. [DOI] [PubMed] [Google Scholar]
- 39.Saaddine J.B. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med. 2006;144(7):465–474. doi: 10.7326/0003-4819-144-7-200604040-00005. [DOI] [PubMed] [Google Scholar]
- 40.Saydah S.H., Fradkin J., Cowie C.C. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291(3):335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 41.Khunti K., Kosiborod M., Ray K.K. Legacy benefits of blood glucose, blood pressure and lipid control in individuals with diabetes and cardiovascular disease: time to overcome multifactorial therapeutic inertia? Diabetes Obes Metab. 2018;20(6):1337–1341. doi: 10.1111/dom.13243. [DOI] [PubMed] [Google Scholar]
- 42.Roberts M.A. Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am J Kidney Dis. 2011;58(1):64–72. doi: 10.1053/j.ajkd.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 43.Herzog C.A. Impact of congestive heart failure, chronic kidney disease, and anemia on survival in the Medicare population. J Card Fail. 2004;10(6):467–472. doi: 10.1016/j.cardfail.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Harding J.L. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62(1):3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- 45.Brenner B.M. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 46.Lewis E.J. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 47.Liyanage T. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 48.Naylor K.L. Mortality in incident maintenance dialysis patients versus incident solid organ cancer patients: a population-based cohort. Am J Kidney Dis. 2019;73(6):765–776. doi: 10.1053/j.ajkd.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Szczech L.A., Stewart R.C., Su H.-.L., DeLoskey R.J., Astor B.C., Fox C.H. Primary care detection of chronic kidney disease in adults with type-2 diabetes: the ADD-CKD study (Awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease) PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.House A, Wanner C, Sarnak M, Piña I, McIntyre C, Komenda P, Bertram L, Kasiske P, Deswal A, deFilippi C, Cleland J, Anker S, Herzog C, Cheung M, Wheeler D, Winkelmayer W, McCullough P. Heart failure in chronic kidney disease: conclusions from a Kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int, Vol. 95, Issue 6, 1304–1317 [DOI] [PubMed]
- 51.Hama T., Oikawa K., Ushijima A. Effect of cardiac rehabilitation on the renal function in chronic kidney disease - Analysis using serum cystatin-C based glomerular filtration rate. Int J Cardiol Heart Vasc. 2018;19:27–33. doi: 10.1016/j.ijcha.2018.04.001. Published 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cefalu W.T. Dapagliflozin's effects on glycemia and cardiovascular risk factors in high-risk patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. Diabetes Care. 2015;38(7):1218–1227. doi: 10.2337/dc14-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cefalu W.T. Effects of canagliflozin on body weight and relationship to HbA 1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58(6):1183–1187. doi: 10.1007/s00125-015-3547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fioretto P., Giaccari A., Sesti G. Efficacy and safety of dapagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in diabetes mellitus. Cardiovasc Diabetol. 2015;14(1):142. doi: 10.1186/s12933-015-0297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zinman B. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.