Abstract
Background
The safety and effectiveness of intramuscular olanzapine or haloperidol compared to midazolam as the initial pharmacological treatment for acute agitation in emergency departments (EDs) has not been evaluated.
Methods
A pragmatic, randomised, double-blind, active-controlled trial was conducted from December 2014 to September 2019, in six Hong Kong EDs. Patients (aged 18–75 years) with undifferentiated acute agitation requiring parenteral sedation were randomised to 5 mg intramuscular midazolam (n = 56), olanzapine (n = 54), or haloperidol (n = 57). Primary outcomes were time to adequate sedation and proportion of patients who achieved adequate sedation at each follow-up interval. Sedation levels were measured on a 6-level validated scale (ClinicalTrials.gov Identifier: NCT02380118).
Findings
Of 206 patients randomised, 167 (mean age, 42 years; 98 [58·7%] male) were analysed. Median time to sedation for IM midazolam, olanzapine, and haloperidol was 8·5 (IQR 8·0), 11·5 (IQR 30·0), and 23·0 (IQR 21·0) min, respectively. At 60 min, similar proportions of patients were adequately sedated (98%, 87%, and 97%). There were statistically significant differences for time to sedation with midazolam compared to olanzapine (p = 0·03) and haloperidol (p = 0·002). Adverse event rates were similar across the three arms. Dystonia (n = 1) and cardiac arrest (n = 1) were reported in the haloperidol group.
Interpretation
Midazolam resulted in faster sedation in patients with undifferentiated agitation in the emergency setting compared to olanzapine and haloperidol. Midazolam and olanzapine are preferred over haloperidol's slower time to sedation and potential for cardiovascular and extrapyramidal side effects.
Funding
Research Grants Council, Hong Kong.
Research in context.
Evidence before this study
Rapid sedation via the intramuscular route is often preferred in patients with acute agitation in emergency settings. Whether intramuscular olanzapine is effective and safe compared to conventional treatment options (including benzodiazepines and first-generation antipsychotics) in emergency settings, where the aetiology of acute agitation is usually undifferentiated and clinical endpoints are urgent, is uncertain.
We searched PubMed from inception to July 22, 2020, without language restrictions, for randomised trials, systematic reviews, meta-analyses, and observational studies, using the terms “agitation or acute agitation or violence” and “parenteral or intramuscular” and “emergency”. During the study period, we identified four randomised trials (published in 2007, 2011, 2013, and 2016) conducted in psychiatry settings that evaluated the effectiveness and safety of intramuscular olanzapine. We did not find any randomised trials comparing intramuscular olanzapine with other sedatives for the treatment of acute agitation in emergency departments.
Added value of this study
We conducted a randomised, active-controlled trial in six Hong Kong emergency departments comparing time to sedation in patients receiving 5 mg of intramuscular midazolam, olanzapine or haloperidol. All three treatments provided adequate sedation at 60 min and midazolam resulted in faster sedation compared to the others. The proportion of patients observed to be asleep after treatment with midazolam was higher compared with olanzapine and haloperidol. Overall adverse event rates were similar across the three arms while extrapyramidal syndrome (n = 1) and fatal cardiac arrest (n = 1) episodes were reported in the haloperidol arm.
Implications of all the available evidence
In patients with undifferentiated agitation admitted to emergency departments, intramuscular midazolam and olanzapine are preferred over haloperidol in view of haloperidol's slower time to sedation and the potential for cardiovascular and extrapyramidal side effects.
Alt-text: Unlabelled box
1. Introduction
Undifferentiated acute agitation presenting at the emergency department (ED) often stems from various aetiologies, including underlying mental illness, drug or alcohol intoxication, or a combination of diagnoses [1], [2], [3], [4]. Failed de-escalation strategies or oral medication necessitates parenteral sedation to prevent subsequent harm [5], with intramuscular sedation generally considered prior to intravenous cannulation [2].
A range of medications are currently used for rapid intramuscular sedation to manage acute agitation in the emergency setting, including benzodiazepines (e.g. midazolam [6], [7], [8], [9], [10], [11], [12], [13], lorazepam) [11,14,15], first-generation antipsychotics (e.g. haloperidol [[6], [7], [8],[11], [12], [13],[15], [16], [17], [18], [19], [20]], droperidol) [9,10,16,20], and second-generation antipsychotics (e.g. olanzapine [7,8,18], risperidone [17], ziprasidone) [7,8,10]. Recently, interest in second-generation antipsychotics to manage acute agitation has grown due to the lower incidence of extrapyramidal side effects (EPS) [7,8], over-sedation [8], and need for further rescue medications [10].
Intramuscular olanzapine and other sedatives have not been directly compared using randomised clinical trials in the general ED setting. Studies to date have used observational [21], [22], [23] or open-label study designs [18]. Previous randomised controlled trials have focused on intravenous olanzapine [4,24] or were conducted in psychiatric emergency settings [7,8,18]. The intravenous route provides rapid onset of action but may be inappropriate in some contexts as adequate staff and resources are required to manage potential emergencies. Our survey of emergency physicians in Hong Kong EDs reported a preference for intramuscular sedation over intravenous sedation, whereas the intravenous route was generally preferred in Australasia [25], suggesting regional differences in prescribing culture, and differences in the perceived need for aggressive management. Other studies investigated intramuscular olanzapine in psychiatric settings where patients with agitation were predominantly homogeneous with a history of mental illness [7,8,18] rather than “undifferentiated”, with an undiagnosed cause of agitation. Study results from psychiatric settings are not directly generalisable to inform acute ED practice due to differences in measured outcomes and perceived accept time to adequate sedation in different clinical contexts. To date, evidence of intramuscular olanzapine use in the general ED is scant as all have been observational studies [[21], [22], [23],26]. Therefore, comparing intramuscular olanzapine with haloperidol or midazolam, the two most commonly selected sedating agents in local setting [25], is important in determining a safe and effective choice of an initial agent for acute agitation in the ED setting. This study compares a second-generation antipsychotic (olanzapine) with a conventional antipsychotic (haloperidol) and a benzodiazepine (midazolam) in acute agitation management in the ED.
2. Methods
2.1. Study design
This was a multi-centre, double-blind, randomised, active-controlled pragmatic trial at EDs in six public hospitals under the Hong Kong Hospital Authority (Table S1 and S7). Between April 2014 to March 2019, patient attendances at these 24-h EDs comprised 39% of the Hospital Authority's overall ED visits. The Institutional Review Board or Clinical Research Ethics Committee approval was given at all study sites (Supplementary Table S1). An independent Data Safety and Monitoring Board for the study comprised a Biostatistician (BJC), Clinical Pharmacologist (BMYC), Toxicologist (MLT), Emergency Physician (HFH), Psychiatrist (CWL). This study was registered in ClinicalTrials.gov (NCT02380118).
2.2. Patients
Patients were enrolled from December 24, 2014 to September 6, 2019. Inclusion criteria were: patients aged 18–75 years; and requiring parenteral drug sedation for acute agitation at the treating physician's discretion. Patients who had received oral or parenteral sedative drug(s) within 12 h, either as usual medications or pre-hospital acute agitation management, were eligible. Exclusion criteria were known hypersensitivity or contraindication to any of the study drugs, immediately reversible aetiology for agitation (e.g. hypotension, hypoxia, hypoglycaemia), known pregnancy, or acute alcohol withdrawal (as it is amendable to benzodiazepines alone) [27]. All eligible patients were initially treated according to the study protocol (Appendix 1).
Written informed consent was secured from either the patient following recovery, if they had capacity to understand the study and give informed consent, or the patient's authorised representative. Inherent challenges in obtaining informed consent from highly agitated patients attending emergency settings have been acknowledged [3,10,11,22]. Application for patient consent waiver, as used in previous clinical trials internationally [1,3,4,10,11,19], was submitted but not approved. As requested by local ethics committees, patient consent was obtained after intervention or from an authorised representative.
2.3. Randomisation and masking
Eligible patients were assigned to the next sequential study pack pre-assembled by independent pharmacists (not involved in the patient's care during acute agitation episode) from the participating hospital's pharmacy department according to a computer-generated randomisation list. Study packs contained the assigned study drug, data collection tools, an unblinding envelope, and other necessary documents. All study packs and unblinding envelopes were opaque, sealed and tamper-proof. At each site, patients were randomised to “permuted blocks of six”, each containing two packs of haloperidol, olanzapine, and midazolam to ensure each arm had similar allocated numbers. Six randomisation lists were generated independently for each study site.
As the appearance of each study drug (midazolam/haloperidol: clear liquid ampule; olanzapine: yellow powder vial) could compromise double-blinding, an independent nurse (not involved in the participant's care) prepared and administered all study drugs. Blinding was maintained with all staff involved in patient care, monitoring, data collection, and statistical analysis.
2.4. Procedures
Patients were randomised to receive an initial 5 mg intramuscular injection of olanzapine, haloperidol, or midazolam with a ratio of 1:1:1, with an optional additional 5 mg dose of the same medication (maximum total dose=10 mg). Study drug selection and doses were based on local Hong Kong clinical practice and survey results [25] on prescribing preferences for acute agitation management, which reported haloperidol and midazolam monotherapy as the sedating agents most frequently selected for undifferentiated agitation in ED. The intramuscular route was the most common choice (63·9%) regardless of drug chosen; median initial and cumulative doses were 5 mg and 10 mg respectively. Although the product monograph recommends a starting dose of 10 mg intramuscular olanzapine, this is seldom prescribed in the local ED setting. Investigators concurred that both the initial (5 mg) and one additional dose (5 mg) would be included in each study pack to allow for flexibility in dosing escalation. To achieve initial or maintain adequate sedation, patients were eligible for alternative sedative drug(s) in addition to the assigned study drug according to clinical response and at the treating physician's discretion.
Agitation/sedation level was measured on a 6-point validated sedation scale: (5=highly aroused, violent; 4=highly aroused, possibly distressed, or fearful; 3=moderately aroused, unreasonable, or hostile; 2=mildly aroused, willing to talk reasonably; 1=minimal agitation; and 0=asleep) [28]. Adequate sedation was defined as a score ≤2. This scale was applied in previous clinical trials that studied sedation in ED [1,3,4,29]. Sedation scores and actual time of measurement were recorded at baseline (immediately before initial dose, t = 0), at first observed adequate sedation, and at 10, 20, 30, 45, and 60 min after the first dose regardless of observed time to sedation. Measurements were recorded by the treating physician, nurses (other than the independent nurse), or research staff. All study participants were given standard sedation care including 1:1 nursing and regular monitoring of sedation level, vital signs, cardiac rhythm, protocol-specified common adverse events, and any other untoward medical occurrence whether or not the occurrence is related to or considered to have a causal relationship with the study drug. Common adverse events related to study drugs were listed in the data collection tool, including airway management (jaw thrust, oral, nasal airway), need for assisted ventilation (bag & mask, intubation), oxygen desaturation <90%, systolic BP<90 mmHg, dystonic reactions, seizures, vomiting or aspiration. If possible, a 12-lead electrocardiogram (ECG) was obtained within 30 min of adequate sedation. However, ECGs are challenging to perform in highly agitated patients and therefore were not always obtained.
Other participant data, including sex, age, and medication history were collected on a standardised Case Report Form. ED presentation details were also collected at triage including perceived possible causes at current presentation such as drug/substance abuse, alcohol intoxication, underlying mental illness, non-compliance to usual medication, and suicidal ideation. Concurrent prescriptions of any antipsychotics, antidepressants, or hypnotics/anxiolytics were also retrieved from the Hospital Authority's computerised medical records system wherever possible.
2.5. Outcomes
The primary outcome measure was time to achieve adequate sedation. Three study arms were compared according to: 1) time required to achieve adequate sedation following drug administration; and 2) proportion of patients adequately sedated at 10, 20, 30, 45, and 60 min. Secondary outcome measures included: 1) proportion of patients requiring a second dose of study drug and/or alternative drug(s) to achieve initial adequate sedation; 2) proportion of patients with corrected QT interval (QTc) prolongation on the ECG (defined as QTc interval over 450 ms and 470 ms, respectively, for males and females) [30]; 3) adverse events reported after study drug administration; 4) the proportion of patients with a sedation score of 0 (observed asleep) after study drug administration [28]; and 5) ED length of stay (LOS).
2.6. Statistical analysis
Sample size calculation was based on Isbister et al., where intramuscular droperidol and midazolam were compared head-to-head in an Australian ED [9]. Assuming sedation times were similar to the Australian study and to demonstrate a difference in the mean time to sedation of 20 versus 25 min (standard deviation=12) between the two arms (haloperidol vs. midazolam), 91 patients were required in each arm (2-sided, statistical power 0·8). Thus, we aimed to enrol 282 patients (rounded up from 273 to account for patient loss).
The modified intention-to-treat principle was applied in the primary analysis. Thirty-six patients received treatment but were excluded due to lack of informed consent. As required by the IRB and stated in the protocol, a primary analysis of patients with informed consent was undertaken according to the allocated study arm, regardless of any unblinding or protocol deviations. The proportion of patients adequately sedated over time were plotted with Kaplan-Meier curves. Time from drug administration to adequate sedation was analysed based on the Kaplan-Meier estimate allowing for interval-censored data. Statistical tests for comparison of every two Kaplan-Meier curves were conducted with asymptotic two-sample log-rank tests. Median time to sedation was calculated for each arm with 95% confidence intervals (CI). Further, the relationship between drug exposure and time to sedation was investigated by fitting Weibull accelerated failure time models adjusted for sex, age, concurrent psychotropic medications, and perceived possible cause of agitation.
Differences in the median ED LOS from study drug administration to discharge/transfer were tested with the log-rank test. Differences in descriptive data of secondary outcomes were tested using Fisher's exact test/Chi-squared test (categorical variables) and log-rank test (continuous variables). A two-sided p-value <0·05 was considered statistically significant. Interim analysis was conducted at recruitment of 130 patients as detailed in Supplementary-Interim Analysis. Data was analysed using R version 3·4·3 (R Foundation for Statistical Computing, Vienna, Austria) by independent Data Safety and Monitoring Board member BJC and statistician VJF.
2.7. Role of the funding source
This study was funded by Research Grants Council, Hong Kong (789813). The study's funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author has full access to all data in the study and has final responsibility for the decision to submit for publication.
3. Results
3.1. Participants
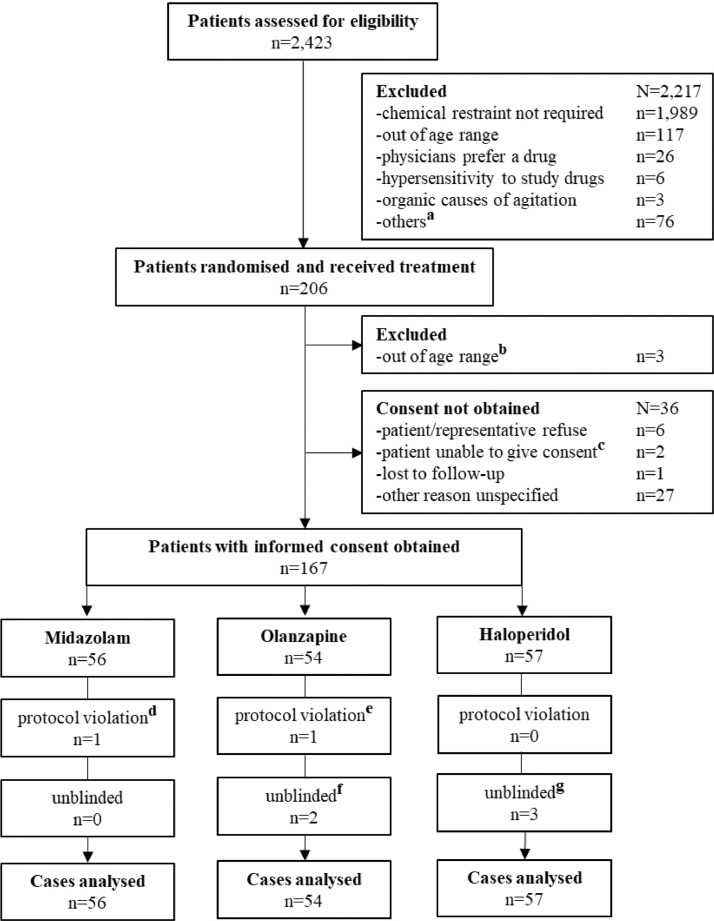
During the study period 2423 patients were screened, of which 206 received study drugs and 167 provided informed consent (Fig. 1), provided by patients and representatives in 25·1% and 74·9% of cases, respectively. After receiving study drugs, 39 patients were excluded due to failure to consent (n = 36) or found out of age range (n = 3) (Fig. 1). Fifty-six patients were allocated to midazolam arm, 54 to olanzapine, and 57 to haloperidol. The actual sample size was less than planned as the study concluded prematurely due to factors detailed in Supplementary-Early Termination. Patient baseline characteristics were generally balanced, although the haloperidol arm (42%) had a lower proportion of males compared with the midazolam (61%) and olanzapine (70%) arms (Table 1). Use of any antidepressants, hypnotics, and anxiolytics were similar across all arms. Results of interim analysis are listed in Supplementary Table S2–5 and Figure S1.
Fig. 1.
Flow Diagram of Patient Inclusion (Modified CONSORT Diagram)
a, including other exclusion criteria, patients’ preference, profound risk of adverse event, and multiple exclusion reasons; b, the age of these 3 patients was unknown at recruitment, two patients were found to be over 75 years old, one below 18 years old after treatment; c. two patients were unconscious during the length of stay at Emergency Department and not accompanied by any representative; d, one dose of study drug was discarded due to contamination; e, one dose of study drug was given intravenously; f, allocation of two patients was unblinded due to protocol violation (intravenous route; n = 1) and for informing of the procedural sedation for endoscopy after transfer (5 mg given in Emergency Department; n = 1); g, allocation of three patients was unblinded due to adverse event (n = 2) and for informing of further sedation (10 mg given in Emergency Department; n = 1).
Table 1.
Baseline characteristics of patients.
| Midazolam (N = 56) |
Olanzapine (N = 54) |
Haloperidol (N = 57) |
||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Age (median, IQR) | 44 | (34, 54) | 40 | (30,54) | 42 | (33, 55) |
| Male | 34 | (61) | 38 | (70) | 24 | (42) |
| Perceived possible cause | ||||||
| Drug/substance abuse | 16 | (31) | 14 | (27) | 19 | (37) |
| Alcohol intoxication | 15 | (28) | 12 | (23) | 13 | (25) |
| Underlying mental illnesses | 47 | (87) | 45 | (83) | 46 | (84) |
| Non-compliance to usual medication | 24 | (47) | 22 | (43) | 18 | (35) |
| Suicidal ideation/attempt | 18 | (34) | 17 | (33) | 18 | (35) |
| Prior sedative drug | 1a | (2) | 1b | (2) | 1c | (2) |
| Concurrent psychotropic medications (any antipsychoticsd, antidepressantse, or hypnotics and anxiolyticsf) | 19 | (34) | 17 | (31) | 13 | (23) |
| Baseline sedation score | ||||||
| 3 | 13 | (23) | 16 | (30) | 14 | (25) |
| 4 | 17 | (30) | 21 | (39) | 17 | (30) |
| 5 | 26 | (46) | 16 | (30) | 25 | (44) |
IQR, interquartile range; atramadol 50 mg; bhaloperidol 5 mg; chaloperidol 10 mg dantipsychotics were medications under the British National Formulary (BNF) category 4·2·1 and 4·2·2; eantidepressants were defined as medications under the BNF category 4·3; fhypnotics and anxiolytics were defined as medications under the BNF category 4·1.
3.2. Primary outcomes
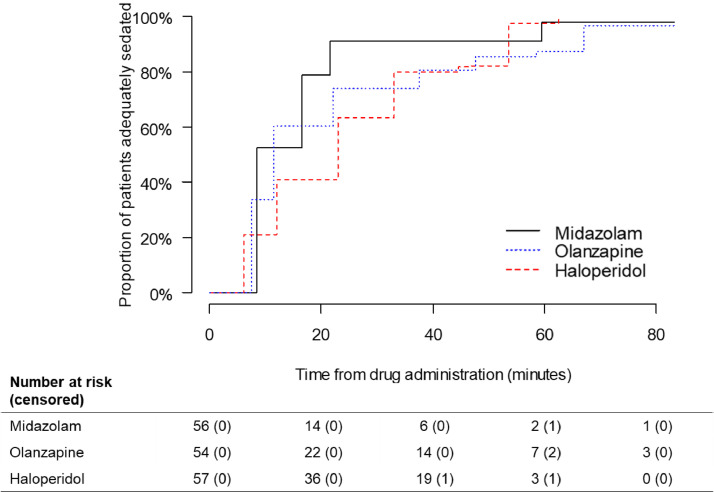
The median time to sedation estimated by the Kaplan-Meier function was 8·5 (95% CI 8·5–59·5, IQR 8·0), 11·5 (95% CI 7·5–67·0, IQR 30·0), and 23·0 min (95% CI 6·0–53·5, IQR 21·0) for midazolam, olanzapine, and haloperidol, respectively. At 10 min after the initial dose, 52%, 34%, and 21% of patients were adequately sedated in the midazolam, olanzapine, and haloperidol arms, respectively. At 60 min, the proportion of patients sedated increased to 98%, 87%, and 97%, respectively (Table 2). The proportion of patients sedated by time was plotted on Kaplan-Meier curves (Fig. 2). Significant differences were detected in the Kaplan-Meier curves for midazolam compared with olanzapine (p = 0·03) and haloperidol (p = 0·002); however, this was not observed for haloperidol compared with olanzapine (p = 0·78).
Table 2.
Proportion of patients adequately sedated at each time point.
| Study group |
||||||
|---|---|---|---|---|---|---|
| Midazolam (N = 56) |
Olanzapine (N = 54) |
Haloperidol (N = 57) |
||||
| n | (%) | n | (%) | n | (%) | |
| Proportion sedated, min | ||||||
| At 10 | 29·3 | (52) | 18·2 | (34) | 12·0 | (21) |
| At 20 | 44·0 | (79) | 32·7 | (60) | 23·3 | (41) |
| At 30 | 51·0 | (91) | 40·0 | (74) | 36·2 | (63) |
| At 45 | 51·0 | (91) | 43·6 | (81) | 46·5 | (82) |
| At 60 | 54·8 | (98) | 47·2 | (87) | 55·5 | (97) |
Interval-censored data was applied in this analysis.
Fig. 2.
Proportion of Patients Adequately Sedated by Time in Kaplan-Meier Curve
No included patient was censored during observation. p-values derived by using asymptotic log-rank two-sample test for comparison of midazolam vs olanzapine, midazolam vs haloperidol, and haloperidol vs olanzapine were 0.03, 0.002 and 0.78, respectively.
3.3. Secondary outcomes
The proportion of patients given the second dose of study drug or alternative sedative(s) was similar across all arms (Table 3). Fully-adjusted accelerated factors for olanzapine and haloperidol were compared with midazolam at 1·72 (95% CI 1·16–2·55) and 1·89 (95% CI 1·28–2·80), respectively (Supplementary Table S6), indicating significantly faster sedation for midazolam. The Weibull accelerated failure time model found a minimal effect of sex on time to adequate sedation with an accelerated factor of 0·96 (95% CI 0·49–1·88) for male (compared with female).
Table 3.
Patients given second dose of study drug or alternative sedatives, with adverse event report, and observed asleep.
| Study group |
P value | ||||||
|---|---|---|---|---|---|---|---|
| Midazolam (N = 56) |
Olanzapine (N = 54) |
Haloperidol (N = 57) |
|||||
| n | (%) | n | (%) | n | (%) | ||
| Administered second dose of study drug or alternative sedatives | 18 | (32) | 16 | (30) | 23 | (40) | 0·46 |
| Administered second dose | 13 | (23) | 15 | (28) | 18 | (32) | 0·61 |
| Administered alternative sedatives | 9a | (16) | 6 | (11) | 7b | (12) | 0·72 |
| Midazolam | 6 | (67) | 2 | (33) | 3 | (43) | |
| Haloperidol | 2 | (22) | 4 | (67) | 2 | (29) | |
| Diazepam | 3 | (33) | 0 | – | 2 | (29) | |
| Lorazepam | 1 | (11) | 0 | – | 1 | (14) | |
| With adverse event | 2 | (4) | 3 | (6) | 3 | (5) | 0·91 |
| Oxygen desaturation (<90%) | 2 | (4) | 1 | (2) | 1 | (2) | |
| Dry month | 0 | – | 2 | (4) | 0 | – | |
| Dystonia | 0 | – | 0 | – | 1 | (2) | |
| Cardiac arrest | 0 | – | 0 | – | 1 | (2) | |
| ECG obtained | (N = 52) | (N = 52) | (N = 56) | ||||
| QTc prolongation | 12 | (23) | 9 | (17) | 13 | (23) | 0·59 |
| Fell asleep after treatment | 28 | (50) | 10 | (19) | 17 | (30) | <0·01 |
*ECG, 12-lead electrocardiogram; QTc, corrected QT interval.
An adverse event is any untoward medical occurrence in a patient after administration of a medicinal product, which does not necessarily have a causal relationship with this treatment. An adverse event can therefore be any unfavourable and unintended sign (for example, an abnormal laboratory finding), symptom, or disease temporally associated with the use of study drug, whether or not considered related to study drug.
Three patients were given two alternative sedative drugs.
One patient was given two alternative sedative drugs.
Overall, the adverse event rate was similar for midazolam, olanzapine, and haloperidol at 4%, 6%, and 5%, respectively (Table 3). The most common adverse event was oxygen desaturation (midazolam, n = 2; olanzapine, n = 1; haloperidol, n = 1). Two patients in the olanzapine arm reported dry mouth. One patient reported dystonia after one dose of haloperidol (5 mg), which resolved fully with 2 mg intramuscular benztropine. In the single severe adverse event, the patient had a cardiac arrest three hours after two doses of intramuscular haloperidol (10 mg in total; second dose given 33 min after initial dose) and died 8 days later (see Supplementary-Severe Adverse Event).
Similar proportions of ECG completion and QTc prolongation were observed across the three arms (Table 3). The proportion of patients observed to be asleep after treatment with midazolam was higher compared with olanzapine and haloperidol (p<0·01) (Table 3). LOS data was estimated as 4·52 (95% CI 2·63–8·69), 4·01 (95% CI 2·31–6·42), and 4·02 (95% CI 2·32–6·87) hours for midazolam, olanzapine and haloperidol, respectively. No significant difference in LOS was detected (p = 0·73).
4. Discussion
Intramuscular midazolam was more effective compared with antipsychotic drugs (olanzapine and haloperidol), as shown by the shorter time to adequate sedation and the higher proportion of patients sedated at any time point. No differences in outcomes were observed between olanzapine and haloperidol. All study drug dosages were effective in managing acute agitation in ED settings within 60 min.
No significant differences in adverse event rates were found across the three arms. One episode of extrapyramidal syndrome (dystonia) and one severe adverse event (fatal cardiac arrest) were reported in the haloperidol arm. Due to the low number of adverse events, the probability of type II error cannot be ruled out as the study was insufficiently powered to identify such differences. The high risk of extrapyramidal syndrome associated with haloperidol is well recognised and the event was considered to be causally associated with haloperidol [31]. The Data Safety Monitoring Board assessment concluded that the cause of death was associated with potential psychostimulants and unlikely to be solely associated with the study drug.
Additionally, significant differences were found in the proportion of patients asleep after study drug administration, with the highest percentage in the midazolam arm. Previous studies reported that midazolam posed a higher risk of over-sedation [9], considered as an undesirable clinical outcome [32]. Although no extended ED LOS was detected, patient assessment and referrals to other medical specialties may be delayed, requiring logistical considerations. Although no incident of respiratory depression was observed, midazolam is reported to cause respiratory depression [10] and careful monitoring of vital signs remains important, especially with high or rapidly escalating doses. Although differences in adverse event rate was not observed, intramuscular olanzapine was reported to be associated with a preferred safety profile compared to haloperidol [33]. Our times to sedation by midazolam and haloperidol were consistent with previously reported results. Intramuscular midazolam 2·5/5 mg was reported to achieve sedation within 5·1–18·3 min [6,10,11,13]. The mean time to sedation by intramuscular haloperidol 5 mg was reported to vary from 5 to 3 min [6,11,13]. Data exists concerning time to sedation in EDs by intramuscular olanzapine in randomised clinical trials and the product information suggests that the peak plasma concentration of 5 mg is expected within 15–45 min after administration. Raveendran et al. reported that 87% of violent patients with mental illnesses were sedated within 15 min with 10 mg intramuscular olanzapine in the psychiatry emergency setting [18].
Our recent study conducted in Australian EDs reported that after intravenous olanzapine 10 mg monotherapy (up to 20 mg in total if required), the median time to sedation and the proportion sedated at 60 min were 11 min and 90·8%, respectively [4]. In the current study, after intramuscular olanzapine 5 mg was given (up to 10 mg in total as required), the median time to sedation and the proportion sedated at 60 min were 11·5 min and 87%, respectively. In our previous randomised clinical trial of intravenous olanzapine, we reported that following intravenous midazolam 2·5–5 mg monotherapy, the median time to sedation was 10 min, and proportion of patients sedated by 60 min was 87·0% [1]. In the current study, after intramuscular midazolam 5 mg (up to 10 mg in total if required), the median time to sedation and the proportion sedated at 60 min were 8·5 min and 98%, respectively. These results suggest that the effectiveness of intramuscular olanzapine or midazolam (5 mg or 10 mg) in our predominantly Southern Chinese patient cohort might be comparable to intravenous administration of olanzapine or midazolam at similar doses. However, intramuscular sedation may provide more rapid initiation of emergency sedation, and potentially reduce the risk of injury to patients and staff, particularly during acute behavioural disturbance and in the context of mechanical restraint often being initially required. IV access in these circumstances may be fraught with difficulty and could be dangerous to both patients and staff members, so a less complex alternative route of administration (intramuscular) may provide many potential advantages if it is effective. The initial dose of intramuscular olanzapine (5 mg) may be considered relatively low compared to the manufacturer's recommendation (10 mg). In future studies, effectiveness and safety of higher doses of olanzapine in this clinical scenario could be further investigated.
The proportion of additional sedatives used in the haloperidol arm was numerically higher than the two other arms despite not reaching statistical significance. Consistently, higher proportions of additional sedative doses were required in patients treated with haloperidol vs. olanzapine as reported in observational studies by Klein et al. (18% vs. 11%)[26] and MacDonald et al. (43% vs. 29%), respectively [23].
Our study has several limitations. Firstly, the study concluded prematurely prior to attaining the full sample size mainly due to study fatigue and several episodes of social unrest in Hong Kong [34]. Early termination factors are detailed in Supplementary-Early Termination. The study period was extended from the initial planned 24 to 58 months. Despite the lower sample size, our study attained sufficient power to identify differences in the primary outcome measure among the three study drugs. Secondly, an imbalance in sex distribution was observed between the study arms, specifically, the haloperidol arm had more females than the other groups. However, the Weibull accelerated failure time model found a minimal effect of sex on time to adequate sedation given the accelerated factor by sex was close to one. Therefore, based on our results, the imbalance in sex distribution across the study arms is unlikely to affect the main results and conclusion. However, the possibility of imbalance in unmeasured baseline characteristics cannot be ruled out. Thirdly, while the sedation scale may introduce measurement bias due to subjectivity, it has been validated and applied in several studies conducted in similar settings [1,3,4]. Fourthly, patients were possibly excluded if physicians had a personal drug choice preference (e.g. antipsychotics preference for patients with known psychotic illness). However, exclusions of screened patients due to physician preference contributed only 1·2% of all excluded patients. Lastly, external validity may be impacted by the exclusion of some patients due to failure to obtain informed consent despite successful consent from 81·1% of those recruited. This discrepancy reflects immense challenges in undertaking pragmatic randomised clinical trials on acute agitation and behavioural emergencies in the ED setting and has been reported by researchers in ED settings elsewhere [22].
Our study indicates that intramuscular midazolam was more effective at 5–10 mg doses compared with olanzapine or haloperidol for acute agitation in ED settings, regardless of aetiology. Intramuscular midazolam's faster sedation time may deem it preferable as an initial sedation agent. Intramuscular midazolam, olanzapine, and haloperidol effectively sedated ED patients with acute agitation within 60 min. Although adverse event rates did not differ significantly, the potential risk of extrapyramidal side effects for haloperidol should be noted. Given the potential risk of adverse events, intramuscular midazolam or olanzapine should be considered over haloperidol for undifferentiated acute agitation in EDs. As this study includes only three of the most commonly prescribed sedatives in a local setting, further investigation to compare other sedatives is warranted.
Contributors
EWC, ICKW, and KSJL had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. EWC, KSJL, ICKW, CAG, JCK, DMT, DCMK, and LPL to developed the study design. EWC, KSJL, ICKW, BJC, JCK, DMT, DCMK, and SHT were responsible for the acquisition, analysis, and interpretation of data. EWC and KSJL drafted the manuscript. ICKW, CAG, JCK, DMT, and DCMK critically revised the manuscript. BJC and VJF contributed to statistical analysis. EWC, KSJL, LL, SHT, CTL, CPW, CAG, CHC, TSC, HFL, and SMT contributed in study site establishment and patient enrolment
Funding
This study was funded by Research Grants Council (Early Career Scheme), Hong Kong (789813).
Data sharing statement
Due to institutional review board restrictions associated with the trial, participant data is not available to external sources. Proposals to access the de-identified individual participant data (excluding any trial-specific participant opt-outs) that underlie the results reported in this article for secondary research purposes will be considered 12 months after publication. Proposal should be directed to the corresponding author, with approval by EWC and KSJL. Only proposals that are clearly in the public interest and compatible with the original purpose of the study will be considered. Qualified researchers will need to sign a data access agreement before data would be released.
Declaration of Competing Interest
All authors declare that no other support has been received from any organisation for the submitted work; no other financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted study. Outside the submitted work, EWC has received honorarium from the Hospital Authority, research grants from Narcotics Division of the Security Bureau of HKSAR, National Health and Medical Research Council (NHMRC, Australia), National Natural Science Foundation of China (NSFC), Research Fund Secretariat of the Food and Health Bureau (HMRF, HKSAR), Research Grants Council (RGC, HKSAR), Wellcome Trust; Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Janssen, Pfizer, RGA and Takeda outside the submitted work.. KSJL reports personal fees from MSD China, outside the submitted work. ICKW received grants from the Research Grants Council (RGC, Hong Kong), Innovative Medicines Initiative (IMI), Shire, Janssen-Cilag, Eli-Lily, Pfizer, Bayer, European Union FP7 program. ICKW is a member of the National Institute for Health and Clinical Excellence (NICE) ADHD Guideline Group, the British Association for Psychopharmacology ADHD guideline group, and advisor to Shire.
Acknowledgments
We thank all patients who participated in this trial. In addition to the authors, we express our appreciation to the following colleagues for their support and participation in this study: Queen Mary Hospital, Hong Kong, Tuen Mun Hospital, Hong Kong, Pamela Youde Nethersole Eastern Hospital, Hong Kong, Prince of Wales Hospital, Hong Kong, Ruttonjee Hospital, Hong Kong, United Christian Hospital, Hong Kong; all pharmacists, physicians and nursing staff in participating sites; research associates and students who participated in this study: X Li, TY Ko, KY Yip, YT Hui, CY Ching, SY Kwok, C Tang, HK Tse, YK Ho, CY Lim, TY Leung, WH Chui, SY Ng, HW Chan, Y Chen, and all summer and exchange students who joined the team to learn about clinical trials. We express our thanks to members of Data Safety and Monitoring Board: BJ Cowling, HF Ho, ML Tse, BMY Cheung, CW Law; Statistician: VJ Fang; Proofreading: L Lam, J Blais, V Geall and KC Yan. Lastly, we are grateful for the support from Ms McShirley Leung who would have been pleased to know about our study findings.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100751.
Contributor Information
Esther W. Chan, Email: ewchan@hku.hk.
Ian C.K. Wong, Email: wongick@hku.hk.
Appendix. Supplementary materials
References
- 1.Chan E.W., Taylor D.M., Knott J.C., Phillips G.A., Castle D.J., Kong D.C. Intravenous droperidol or olanzapine as an adjunct to midazolam for the acutely agitated patient: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Ann Emerg Med. 2013;61(1):72–81. doi: 10.1016/j.annemergmed.2012.07.118. [DOI] [PubMed] [Google Scholar]
- 2.Knott J.C., Isbister G.K. Sedation of agitated patients in the emergency department. Emerg Med Australas. 2008;20(2):97–100. doi: 10.1111/j.1742-6723.2008.01063.x. [DOI] [PubMed] [Google Scholar]
- 3.Knott J.C., Taylor D.M., Castle D.J. Randomized clinical trial comparing intravenous midazolam and droperidol for sedation of the acutely agitated patient in the emergency department. Ann Emerg Med. 2006;47(1):61–67. doi: 10.1016/j.annemergmed.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Taylor D.M., Yap C.Y.L., Knott J.C. Midazolam-droperidol, droperidol, or olanzapine for acute agitation: a randomized clinical trial. Ann Emerg Med. 2017;69(3):318–326. doi: 10.1016/j.annemergmed.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Downey L.V., Zun L.S., Gonzales S.J. Frequency of alternative to restraints and seclusion and uses of agitation reduction techniques in the emergency department. Gen Hosp Psychiatry. 2007;29(6):470–474. doi: 10.1016/j.genhosppsych.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Esmailian M., Ahmadi O., Taheri M., Zamani M. Comparison of haloperidol and midazolam in restless management of patients referred to the emergency department: a double-blinded, randomized clinical trial. J Res Med Sci. 2015;20(9):844–849. doi: 10.4103/1735-1995.170598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani C., Labate C.M., Sponholz A., Jr. Are low doses of antipsychotics effective in the management of psychomotor agitation? A randomized, rated-blind trial of 4 intramuscular interventions. J Clin Psychopharmacol. 2013;33(3):306–312. doi: 10.1097/JCP.0b013e3182900fd6. [DOI] [PubMed] [Google Scholar]
- 8.Baldacara L., Sanches M., Cordeiro D.C., Jackoswski A.P. Rapid tranquilization for agitated patients in emergency psychiatric rooms: a randomized trial of olanzapine, ziprasidone, haloperidol plus promethazine, haloperidol plus midazolam and haloperidol alone. Braz J Psychiatry. 2011;33(1):30–39. doi: 10.1590/s1516-44462011000100008. [DOI] [PubMed] [Google Scholar]
- 9.Isbister G.K., Calver L.A., Page C.B., Stokes B., Bryant J.L., Downes M.A. Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study. Ann Emerg Med. 2010;56(4):392–401. doi: 10.1016/j.annemergmed.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 10.Martel M., Sterzinger A., Miner J., Clinton J., Biros M. Management of acute undifferentiated agitation in the emergency department: a randomized double-blind trial of droperidol, ziprasidone, and midazolam. Acad Emerg Med. 2005;12(12):1167–1172. doi: 10.1197/j.aem.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Nobay F., Simon B.C., Levitt M.A., Dresden G.M. A prospective, double-blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med. 2004;11(7):744–749. doi: 10.1197/j.aem.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 12.TREC Collaborative Group Rapid tranquillisation for agitated patients in emergency psychiatric rooms: a randomised trial of midazolam versus haloperidol plus promethazine. BMJ. 2003;327(7417):708–713. doi: 10.1136/bmj.327.7417.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isenberg D.L., Jacobs D. Prehospital Agitation and Sedation Trial (PhAST): a randomized control trial of intramuscular haloperidol versus intramuscular midazolam for the sedation of the agitated or violent patient in the prehospital environment. Prehosp Disaster Med. 2015;30(5):491–495. doi: 10.1017/S1049023X15004999. [DOI] [PubMed] [Google Scholar]
- 14.Alexander J., Tharyan P., Adams C., John T., Mol C., Philip J. Rapid tranquillisation of violent or agitated patients in a psychiatric emergency setting. Pragmatic randomised trial of intramuscular lorazepam v. haloperidol plus promethazine. Br J Psychiatry. 2004;185:63–69. doi: 10.1192/bjp.185.1.63. [DOI] [PubMed] [Google Scholar]
- 15.Battaglia J., Moss S., Rush J. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double-blind, emergency department study. Am J Emerg Med. 1997;15(4):335–340. doi: 10.1016/s0735-6757(97)90119-4. [DOI] [PubMed] [Google Scholar]
- 16.Calver L., Drinkwater V., Gupta R., Page C.B., Isbister G.K. Droperidol v. haloperidol for sedation of aggressive behaviour in acute mental health: randomised controlled trial. Br J Psychiatry. 2015;206(3):223–228. doi: 10.1192/bjp.bp.114.150227. [DOI] [PubMed] [Google Scholar]
- 17.Lim H.K., Kim J.J., Pae C.U., Lee C.U., Lee C., Paik I.H. Comparison of risperidone orodispersible tablet and intramuscular haloperidol in the treatment of acute psychotic agitation: a randomized open, prospective study. Neuropsychobiology. 2010;62(2):81–86. doi: 10.1159/000315437. [DOI] [PubMed] [Google Scholar]
- 18.Raveendran N.S., Tharyan P., Alexander J., Adams C.E., Group TR-IIC. Rapid tranquillisation in psychiatric emergency settings in India: pragmatic randomised controlled trial of intramuscular olanzapine versus intramuscular haloperidol plus promethazine. BMJ. 2007;335(7625):865. doi: 10.1136/bmj.39341.608519.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huf G., Coutinho E.S., Adams C.E., Group T.C. Rapid tranquillisation in psychiatric emergency settings in Brazil: pragmatic randomised controlled trial of intramuscular haloperidol versus intramuscular haloperidol plus promethazine. BMJ. 2007;335(7625):869. doi: 10.1136/bmj.39339.448819.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas H., Jr., Schwartz E., Petrilli R. Droperidol versus haloperidol for chemical restraint of agitated and combative patients. Ann Emerg Med. 1992;21(4):407–413. doi: 10.1016/s0196-0644(05)82660-5. [DOI] [PubMed] [Google Scholar]
- 21.Cole J.B., Moore J.C., Dolan B.J. A prospective observational study of patients receiving intravenous and intramuscular olanzapine in the emergency department. Ann Emerg Med. 2017;69(3):327–336. doi: 10.1016/j.annemergmed.2016.08.008. e2. [DOI] [PubMed] [Google Scholar]
- 22.Klein L.R., Driver B.E., Miner J.R. Intramuscular midazolam, olanzapine, ziprasidone, or haloperidol for treating acute agitation in the emergency department. Ann Emerg Med. 2018;72(4):374–385. doi: 10.1016/j.annemergmed.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald K., Wilson M., Minassian A. A naturalistic study of intramuscular haloperidol versus intramuscular olanzapine for the management of acute agitation. J Clin Psychopharmacol. 2012;32(3):317–322. doi: 10.1097/JCP.0b013e318253a2fe. [DOI] [PubMed] [Google Scholar]
- 24.Yap C.Y.L., Taylor D.M., Knott J.C. Intravenous midazolam-droperidol combination, droperidol or olanzapine monotherapy for methamphetamine-related acute agitation: subgroup analysis of a randomized controlled trial. Addiction. 2017;112(7):1262–1269. doi: 10.1111/add.13780. [DOI] [PubMed] [Google Scholar]
- 25.Chan E.W., Tang C., Lao K.S. Management of acute agitation in Hong Kong and comparisons with Australasia. Emerg Med Australas. 2015;27(6):542–548. doi: 10.1111/1742-6723.12499. [DOI] [PubMed] [Google Scholar]
- 26.Klein L.R., Driver B.E., Horton G., Scharber S., Martel M.L., Cole J.B. Rescue sedation when treating acute agitation in the emergency department with intramuscular antipsychotics. J Emerg Med. 2019;56(5):484–490. doi: 10.1016/j.jemermed.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 27.Perry E.C. Inpatient management of acute alcohol withdrawal syndrome. CNS Drugs. 2014;28(5):401–410. doi: 10.1007/s40263-014-0163-5. [DOI] [PubMed] [Google Scholar]
- 28.Alderton D., Bosanac P., Tran N., Castle D.J. Pharmacological and psychosocial treatments in schizophrenia. CRC Press; 2012. Management of acute behavioral disturbance in psychosis; pp. 134–151. Third Edition. [Google Scholar]
- 29.Riddell J., Tran A., Bengiamin R., Hendey G.W., Armenian P. Ketamine as a first-line treatment for severely agitated emergency department patients. Am J Emerg Med. 2017;35(7):1000–1004. doi: 10.1016/j.ajem.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 30.Surawicz B., Childers R., Deal B.J., Gettes L.S. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):976–981. doi: 10.1016/j.jacc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Geddes J., Freemantle N., Harrison P., Bebbington P. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ. 2000;321(7273):1371–1376. doi: 10.1136/bmj.321.7273.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garriga M., Pacchiarotti I., Kasper S. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatry. 2016;17(2):86–128. doi: 10.3109/15622975.2015.1132007. [DOI] [PubMed] [Google Scholar]
- 33.Kishi T., Matsunaga S., Iwata N. Intramuscular olanzapine for agitated patients: a systematic review and meta-analysis of randomized controlled trials. J Psychiatr Res. 2015;68:198–209. doi: 10.1016/j.jpsychires.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Ni M.Y., Yao X.I., Leung K.S.M. Depression and post-traumatic stress during major social unrest in Hong Kong: a 10-year prospective cohort study. Lancet. 2020;395(10220):273–284. doi: 10.1016/S0140-6736(19)33160-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.