Abstract
Background
Therapeutic hypothermia may need prolonged duration for the patients with severe traumatic brain injury (sTBI).
Methods
The Long-Term Hypothermia trial was a prospective, multicenter, randomized, controlled clinical trial to examine the safety and efficacy in adults with sTBI. Eligible patients were 18–65, Glasgow Coma Scale score at 4 to 8, and initial intracranial pressure (ICP) ≥ 25 mm Hg, randomly assigned to the long-term mild hypothermia group (34–35 °C for 5 days) or normothermia group at 37 °C. The primary outcome was the Glasgow outcome scale (GOS) at 6 months. Secondary outcomes included ICP control, complications and laboratory findings, the length of ICU and hospital stay, and GOS at 6 months in patients with initial ICP ≥ 30 mm Hg. This trial is registered with ClinicalTrials.gov, NCT01886222.
Findings
302 patients were enrolled from June 25, 2013, to December 31, 2018, with 6 months follow-up in 14 hospitals, 156 in hypothermia group and 146 in normothermia group. There was no difference in favorable outcome (OR 1·55, 95%CI 0·91–2·64; P = 0·105) and in mortality (P = 0·111) between groups. In patients with an initial ICP ≥ 30 mm Hg, hypothermic treatment significantly increased favorable outcome over normothermia group (60·82%, 42·71%, respectively; OR 1·861, 95%CI 1·031–3·361; P = 0·039). Long-term mild hypothermia did not increase the incidences of complications.
Interpretation
Long-term mild hypothermia did not improve the neurological outcomes. However, it may be a potential option in sTBI patients with initial ICP ≥ 30 mm Hg.
Funding
: Shanghai municipal government and Shanghai Jiao Tong University/School of Medicine.
Keywords: Traumatic brain injury, Randomized controlled trial, Prolonged hypothermia, Efficacy, Safety
Research in context.
Evidence before this study
We performed a systematic review and searched Cochrane, clinical trial register, MEDLINE, Embase, PubMed, and other biomedical databases for randomized controlled trials that recruited patients with brain injury and randomly allocated them to the hypothermia group and the normothermia group. A recent Cochrane review of hypothermia for treatment of severe brain injury, identified 37 randomized clinical trials involving 3110 patients. We further identified one trial that compared the efficacy of hypothermia treatment with control in 387 patients. The results of these clinical trials have not demonstrated the effectiveness of short term (<72 h) hypothermia in the treatment of patients with severe traumatic brain injury (sTBI). The evidence suggests that short term hypothermia treatment does not reduce the incidence of death or severe disability or increase the incidences of complications in patients with TBI.
Added value of this study
To our knowledge, this is the first multicenter randomized controlled trial to evaluate the safety and efficacy of prolonged (5 days) mild hypothermia for sTBI with severe intracranial hypertension. In this trial we found that long-term mild hypothermia did not improve neurological outcomes at 6 months in patients with sTBI. In addition, long-term mild hypothermia did not increase the incidences of complications. Notably, hypothermic treatment significantly increased the favorable outcomes of patients with an initial ICP ≥ 30 mm Hg compared to normothermia group, which indicates that prolonged mild hypothermia may be an option with potential for the sTBI patients with initial ICP ≥ 30 mm Hg in the future.
Implications of all the available evidence
Based on the evidence, prolonged mild hypothermia failed to improve functional recovery as compared with the normothermia in the overall population of sTBI patients. When taken in the context of previous clinical trials, the results of this study do not support the application of mild hypothermia therapy for the management of sTBI patients. However, there was a notable finding of an improved outcome in sTBI patients with initial ICP ≥ 30 mm Hg, which deserves further study.
Alt-text: Unlabelled box
Introduction
Millions of people have a traumatic brain injury (TBI) annually worldwide. About one million new cases with TBI occur each year in China [1]. Severe TBI (sTBI) leads to a huge economic burden to society and families throughout the world [2]. Mild hypothermia is commonly used in patients with sTBI in many areas including China, but the evidence underpinning its use is weak, and uncertainty exists on the required duration of treatment [3]. The use of therapeutic hypothermia for TBI was first reported in 1897 [4]. Randomized controlled trials (RCTs), of short duration (48–72 h) mild hypothermia treatment have failed to show benefit: NABIS: H I [5] and NABIS: H II [6] did not show improvement in outcomes and reported major complications of low blood pressure or rebound intracranial pressure (ICP) increase during early rewarming [7,8]. The Eurotherm3235 trial in Europe more recently showed that hypothermia sustained for 48 h successfully reduced ICP but led to a higher mortality rate and worse functional outcome [9]. Two other randomized trials of mild hypothermia, the POLAR trial [10] and B-HYPO study, [11] in which hypothermia was sustained for 72 h, indicated that hypothermia did not improve neurologic outcomes at 6 months compared with normothermia. Almost all reported clinical trials of hypothermia therapy have failed to show efficacy in patients with sTBI [12].
Despite the failure of trials on short duration hypothermia, prolonged mild hypothermia (>72 h), which targets the period of greatest risk for raised ICP following TBI, may be effective in treating sTBI patients with severe intracranial hypertension. Hypothermic treatment for sTBI was proved by laboratory evidence in protecting the blood-brain barrier function and cerebral functions as well due to the reduction of ICP, but failed to be translated into clinical effecacy [13]. Numerous studies [14], [15], [16], [17] have confirmed that cerebral edema begins from minutes to hours after injury, peaks at 3–5 days and lasts for more days [18,19]. Reported evidence indicates that 5 days long-term mild hypothermia significantly decreases ICP and may improve outcome of patients with sTBI [20]. However, the therapeutic efficacy of long-term (5 days) mild hypothermia has not been examined yet in large-scale multicenter RCTs. In addition, the possible increase in complications and alteration in laboratory findings following hypothermic intervention, [21], [22], [23] also should be considered when long-term mild hypothermia is applied. In this multicenter randomized controlled trial, we thus aimed to explore the safety and efficacy of 5 days mild hypothermia in sTBI with severe intracranial hypertension.
Methods
Study design and participants
This study was a prospective multicenter randomized controlled trial to investigate the safety and efficacy of long-term (5 days) mild hypothermia versus normothermia on the outcome of patients following sTBI. The trial was conducted in 14 hospitals affiliated to universities in China: Renji hospital, Shanghai Jiao Tong University/School of Medicine; The First Affiliated Hospital, College of Medicine, Zhejiang University; West China Hospital of Sichuan University; The Second School of Medicine, Wenzhou Medical University; Taihu Hospital, Wuxi; South Taihu Hospital, Huzhou; Shenzhen Second People's Hospital; Nanfang Hospital, Southern Medical University; Tianjin Wujing Brain Hospital; Xiangya Hospital, Central South University; Taizhou First People's Hospital; Tangdu Hospital, Xi'an; Qilu Hospital of Shandong University; and The Affiliated Hospital of Southwest Medical University.
Eligible patients were adults (aged 18–65 years) with closed head injury, presenting within 24 h post injury with a Glasgow Coma Scale (GCS) score [24] of 4 to 8 after resuscitation. Additional inclusion criteria were an initial intracranial pressure (ICP) ≥ 25 mm Hg [25], and serious traumatic abnormalities on the initial head computed tomographic (CT) scan, including contusion, hematoma, midline shift, compressed ambient cistern or brain swelling. Patients were excluded if they exhibited any of the following issues: life-threatening or severe extracranial injury; serious pre-existing medical conditions; systolic blood pressure less than 90 mm Hg or oxygen saturation less than 95% after resuscitation; women who were pregnant or breast-feeding; inability to obtain informed consent; or unable to initiate hypothermia therapy within 24 h post-injury.
The study was approved by the Ethics Committee of Renji Hospital (no: Renji Lunshen [2013] 101 K, and amended version V1.1 in 2014, final version V1.2 in 2017) and local institutional review boards of each participating site. The trial is registered on ClinicalTrial.gov with the ID number NCT01886222. The trial was performed in accordance with the Declaration of Helsinki and the guidelines of Good Clinical Practice. The trial protocol [26] was published before recruitment stopped, without awareness of any unblinded data. Written witnessed informed consent was sought from a legally acceptable representative of each patient prior to randomization.
Randomization and masking
Participants in each site were randomly assigned in a 1:1 ratio, stratified by center, to the long-term hypothermia or normothermia group using a block-randomization scheme, 4 patients in a block. The randomization sequence was generated by an independent statistician with SAS 9·2 who was not involved in the determination of eligibility. The secretary at the trial coordinating center (Renji Hospital, Shanghai Jiao Tong University/School of medicine) placed the randomization sequence and treatment assignment together into opaque envelops, sealed and mailed these envelops to each participating center. Randomization was done at each trial center by study physicians after opening sequentially numbered envelopes. Participant recruitment was done under the supervision of study investigators at each site.
Investigators who assessed the outcome measures were excluded from the randomization and treatment process, i.e., masked to treatment allocation. Emergency service personnel, study nurses involved in randomization, and personnel who managed the patients were by necessity unmasked to treatment allocation.
Procedures
After completion of the initial assessment in the Emergency Department, patients firstly underwent implantation of a ventricular or intraparenchymal ICP monitor in the operation room before any further surgical intervention if necessary, e.g., hematoma removal, decompressive craniectomy. Those with an initial ICP, defined as the first measured value once the ICP monitor in place, less than 25 mm Hg were excluded from the trial. All patients with an initial ICP ≥ 25 mm Hg were considered eligible for the trial. Following surgery, patient was transferred to the neurosurgical intensive care unit (ICU) and treated with the ICP continuously monitored. Patients were then randomly assigned. Those who were assigned to the mild hypothermia group received cooling blankets (MTRE Advanced Technologies Ltd, Israel) to achieve the target temperature of 34–35 °C immediately after randomization. Thereafter hypothermia was maintained for 5 days from the start of hypothermia and during this period other ICP-lowering therapies were also used as needed to reduce and maintain ICP < 25 mm Hg. The patients were then re-warmed slowly at a rate of no greater than 0·5 °C every 4 h. The rewarming rate was also individualized by the patient's ICP level to prevent rebound intracranial hypertension. Patients randomized to normothermia were maintained at 37 °C during the entire study period.
During the ICU period, patients with increased ICP (≥ 25 mm Hg) were treated progressively using a standard, stepwise strategy including head elevation, ventilation control, sedation and analgesia, and hyperosmolar therapy. Barbiturates were not used in either group. Rectal temperature was used to guide temperature management [27]. To prevent shivering during cooling and rewarming phases, the patients were sedated with continuous infusions of chlorpromazine and promethazine, and paralyzed with tracrium or vecuronium; the infusing rate of these drugs was adjusted according to the vital signs and other physiologic measurements. Patients in the normothermia group with temperature above 37 °C were treated with ice packs around the neck and limbs, sponging the body with alcohol, and/or use of paracetamol. Corticosteroids were not used. In addition, imbalance of serum electrolytes were also corrected intravenously or orally. The main goal of sTBI management was to avoid hypotension, hypoxia, disturbed internal environment and intracranial hypertension by maintaining systolic blood pressure above 90 mm Hg, peripheral oxygen saturation greater than 95%, ICP less than 25 mm Hg and cerebral perfusion pressure at a level of 50–70 mm Hg. All treatment procedures and specific operating protocols for the management of sTBI in the trial were standardized, based on guidelines and consensus at the time [28].
The entry form was used to collect baseline information including the following variables: demographics, time from injury to admission, cause of injury, vital signs, body temperature, GCS score, results of neurological tests, the initial head CT scan, and information on the implantation of ICP monitor and the need of surgery. Once a patient had been randomized, relevant data were collected daily over the first 7 days of admission to hospital by the responsible study physician. Specifically, physiological parameters including blood pressure, heart rate, rectal temperature, and ICP were collected each hour in the first 6 h after induction of hypothermia and then every 2 h. GCS score, results of laboratory data including routine blood tests, the function of the liver and kidney, level of serum electrolytes and blood glucose, and arterial blood gas analysis were evaluated once a day. Urine output, types and volumes of intravenous fluid administered, and use and dosages of co-interventions were recorded each day for the first 7 days after admission. Control CT scans during ICU care were performed as clinically indicated and routine follow-up scans scheduled for all surviving patients at 1 and 6 months after injury. Information on complication was observed and recorded till 6 months post-injury. Outcome assessment at 6 months was performed by a blinded investigator of the local study center who was unaware of the patient's allocation using a structured interview with the Glasgow Outcome Scale [29,24]. The investigator might visit the patients in the ward who were still in the hospital to go through the evaluation. The patients, who had been discharged, were told to come back in due time for assessment when leaving the hospital. For patients who did not return for follow-up, the investigator contacted the patients or their family members by telephone or other possible methods to explain the situation and complete the outcome assessment. The patient was recorded as loss to follow-up, if all attempts for follow-up failed or no further contact was made. Any death occurring either in or out of the hospital was recorded and explored for possible causes.
Outcomes
The primary outcome was the Glasgow Outcome Scale (GOS) score 6 months after injury [29,24]. The GOS scores were dichotomized into favorable outcomes for good recovery and moderate disability or unfavorable outcomes for who die or survive with severe disability or in a vegetative state. Secondary outcomes included ICP control, length of ICU and hospital stay, frequency of complications in the first 6 days after randomization, and GOS at 6 months in patients with initial ICP ≥ 30 mm Hg. Safety was assessed by the frequency of complications recorded during hospital stay after injury. Similar to the complications observed in NABIS:H II trial, [6] we specifically summarized the complications of pneumonia, hypokalemia, gastrointestinal bleeding, coagulation disorder and intracranial infection in the first 6 days.
Statistical analysis
We estimated the required sample size based on results of our previous two trials of exploring the use of long-term hypothermia in 2000 and 2006 [14,20]. In the former one, the proportions of patients with favorable outcomes in normothermia and long-term hypothermia were found to be 27·27% and 46·51%, respectively; while in the latter one the proportions of patients with favorable outcomes in short-term hypothermia and long-term hypothermia were 29·0% and 43·5%, respectively. Thus, to detect a difference of not less than 16·23% (from 43·5% to 27·27%) with an α value of 0·05 and a power of 0·8 between long-term hypothermia group and normothermia group, 272 patients would be needed. To allow for some loss to follow-up, a sample size of 300 in total (150 for each group) was determined therefore, which could give an 84% power to detect the same difference at the same significance level.
Categorical variables were described as numbers and percentage, normally distributed continuous variables were presented as mean and standard deviation (SD), non-normally distributed variables were expressed as median and interquartile ranges. A two-sided significance level of 0·05 was used. A p value of less than 0·05 was deemed significant. Statistical analyses were conducted with R Statistical Software (version 3·6·1).
Analysis was performed in accordance with the intention-to-treat principle. All randomized patients were included in their originally assigned group regardless of the actual treatment approach they received. The primary outcome was measured by Wilcoxon test and logistic regression, with admission age, sex, baseline Glasgow coma scale score and pupillary reactivity as covariates.
For secondary outcomes, the frequencies of categorical outcome events were compared using chi-squared test or Fisher's exact test; for continuous outcomes, the median values were compared with Wilcoxon test and Hodges–Lehmann Estimation; for GOS in patients with ICP ≥30 mm Hg, Logistic regression was performed to adjust for unbalanced covariates between groups.
Role of the funding source
The sponsor of the study had no role in the data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
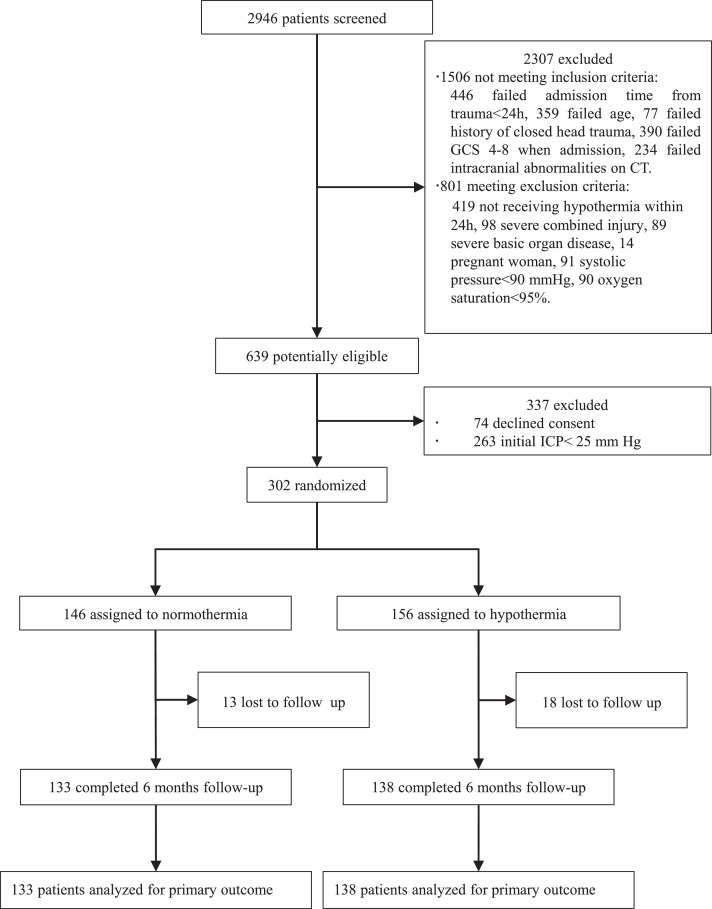
A total of 2946 patients at 14 centers were assessed for trial eligibility. Enrolment occurred from June 25, 2013, to December 31, 2018, with 6-months follow-up after recruitment. 2307 patients were excluded by the first set of inclusion criteria. Specifically, 446 patients due to the admission time from trauma beyond 24 h, 359 patients due to age criteria, 77 patients due to open head injury, 390 due to GCS score out the range of 4 to 8, 234 due to injury category on computed tomographic scan, 419 due to hypothermia initiated later than 24 h, 98 due to severe combined injury, 89 due to severe basic organ disease, 14 female patients due to pregnancy, 91 due to very low systolic blood pressure (SBP), and 90 due to very low oxygen saturation, were excluded. 639 patients were then potentially eligible, among whom 74 patients declined consent. Before randomization, 263 patients were lastly excluded because of the initial ICP < 25 mm Hg. Thus, 302 patients were finally randomly assigned to normothermia group (n = 146) or hypothermia group (n = 156) and subsequently analyzed. 13 and 18 patients, respectively in two groups, were lost to follow-up (Fig. 1).
Fig. 1.
Trial profile. GCS=Glasgow coma scale. CT=computerized tomography. ICP=intracranial pressure. TBI=traumatic brain injury. Initial ICP refers to the ICP value firstly measured pre-operation.
The demographics and baseline characteristics of the 302 patients are shown in Table 1. The patients were predominantly male (76·71% and 81·41% in the normothermia and hypothermia group, respectively), in middle age (49·12 years old and 44·72, respectively, P = 0.003), and suffered from traffic incidents (62·33% and 59·61%, respectively). The initial ICP was high in both groups (36·75 and 36·15 mm Hg), and surgery of decompressive craniectomy (71·23% and 67·95%) and surgical hematoma removal (91·78% and 92·31%) were common in both groups. The initial ICP measured during operation showed no significant difference between the normothermia and hypothermia groups (P = 0·61). No statistical differences were found in GCS (P = 0·254), temperature (P = 0·604) at randomization, SBP (P = 0·347), HR (P = 0·706), pupillary response (P = 0·509), and laboratory findings: alanine transaminase (P = 0·410), aspartate aminotransferase (P = 0·591), creatinine (P = 0·617), blood urea nitrogen (P = 0·419), Ca2+ (P = 0·993), Cl− (P = 0·179), Na+ (P = 0·122), K+ (P = 0·854), buffer excess (P = 0·501), pH (P = 0·721), PaCO2 (P = 0·598), PaO2 (P = 0·928), blood platelet (P = 0·542), white blood cells (P = 0·656), hemoglobin (P = 0·845), glucose (P = 0·924).
Table 1.
Demographics and baseline characteristics of the intention-to-treat patients.
| Normothermia (n = 146) | Hypothermia (n = 156) | |
|---|---|---|
| Sex | ||
| Male | 112 (76·71%) | 127 (81·41%) |
| Female | 34 (23·28%) | 29 (18·59%) |
| Age (years) | 49·12 (12·82) | 44·72 (13·05) |
| Time from injury (hours) | 2·50 (1·27, 4·00) | 2 00 (1·15, 3·12) |
| Causes of TBI | ||
| Traffic incidents | 91 (62·33%) | 93 (59·61%) |
| Falls | 36 (24·66%) | 38 (24·36%) |
| Violence | 2 (1·37%) | 5 (3·21%) |
| Other causes | 17 (11·64%) | 20 (12·82%) |
| GCS | 6 (5, 7) | 6 (4, 7) |
| Temperature at randomization (°C) | 36·82 (0·60) | 36·79 (0·66) |
| SBP (mm Hg) | 139·10 (24·02) | 136·54 (23·01) |
| HR (Beats per minute) | 86·93 (21·28) | 86·03 (20·40) |
| Initial ICP (mm Hg) | 36·75 (10·40) | 36·15 (9·62) |
| Pupillary response | ||
| Both reacting | 77 (53·11%) | 86 (55·13%) |
| One reacting | 19 (13·10%) | 26 (16·67%) |
| Neither reacting | 49 (33·79%) | 44 (28·20%) |
| CT feature | ||
| Intracranial hematoma | 143 (97·95%) | 152 (97·44%) |
| Midline shift>5mm | 55 (37·67%) | 55 (35·26%) |
| Diffuse brain swelling | 35 (23·97%) | 43 (27·56%) |
| Surgery | ||
| Hematoma removal | 134 (91·78%) | 144 (92·31%) |
| Decompressive craniectomy | 104 (71·23%) | 106 (67·95%) |
| Laboratory findings | ||
| Alanine transaminase (U/L) | 27·50 (17·00, 43·75) | 28·00 (19·00, 38·00) |
| Aspartate aminotransferase (U/L) | 38·50 (26·25, 50·68) | 38·50 (29·00, 55·25) |
| Creatinine (μmol/L) | 65·00 (54·00, 76·00) | 64·00 (52·08, 80·00) |
| Blood urea nitrogen (mmol/L) | 5·10 (4·17, 6·70) | 5·30 (4·30, 6·55) |
| Ca2+ (mmol/L) | 1·85 (1·05, 2·08) | 1·89 (1·04, 2·08) |
| Cl− (mmol/L) | 105·23 (5·38) | 106·21 (6·87) |
| Na+ (mmol/L) | 140·25 (138·00, 143·00) | 140·30 (138·00, 143·10) |
| K+ (mmol/L) | 3·67 (0·60) | 3·68 (0·58) |
| pH | 7·39 (0·07) | 7·39 (0·07) |
| PaO2 (mm Hg) | 159·44 (89·35) | 160·35 (77·54) |
| PaCO2 (mm Hg) | 36·49 (8·07) | 36·99 (7·81) |
| Buffer excess (mmol/L) | −2·25 (3·91) | −2·55 (3·54) |
| Glucose (mmol/L) | 9·31 (7·78, 10·70) | 9·00 (7·70, 11·29) |
| White blood cells (10^9/L) | 13·90 (10·83, 19·03) | 14·50 (10·23, 19·34) |
| Hemoglobin (g/L) | 119·00 (97·25, 139·75) | 122·00 (97·00, 142·00) |
| Blood platelet (10^9/L) | 162·58 (70·56) | 168·03 (81·21) |
Data were represented as mean (SD), number (%) or median (IQR). TBI=traumatic brain injury. GCS=Glasgow coma scale. SBP=systolic blood pressure. HR=heart rate. ICP=intracranial pressure. CT=computerized tomography. Initial ICP refers to the ICP value firstly measured pre-operation.
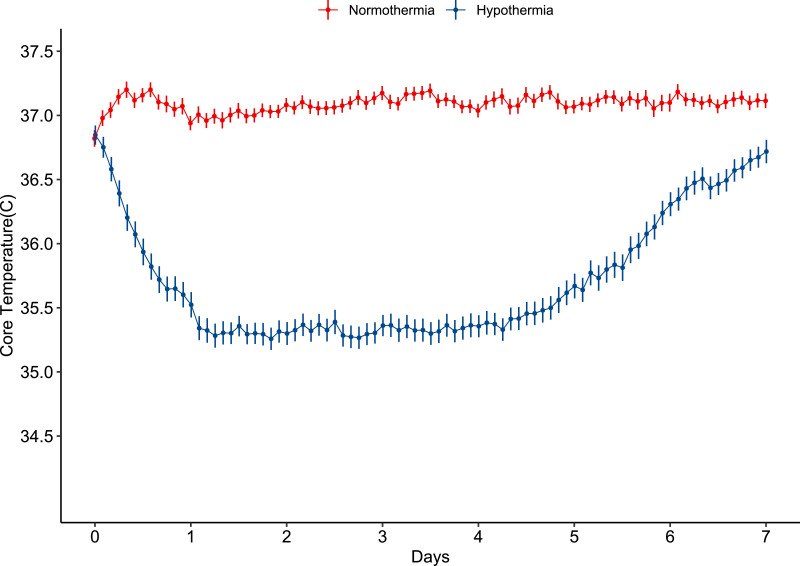
Analyzed in the hypothermia group, the median time between start of hypothermia and time of injury was 9·49 h (IQR 6·94–12·76), the temperature cooled towards the target (34–35 °C) within 24 h, 122 patients in hypothermia group reached target temperature. The mean temperature was 35·36 °C (1·10) during the 5 days of hypothermia treatment (Fig. 2). In the normothermia group, core temperature was 37·08 °C (0·65) during the 5 days of temperature intervention.
Fig. 2.
Core temperature for the first 7 days after randomization. The core temperature of the patients randomized to normothermia was maintained at 37 °C during the entire study period. While the patients assigned to hypothermia group were cooled to the target temperature of 34–35 °C after randomization for 5 days. Data were represented with mean and SE.
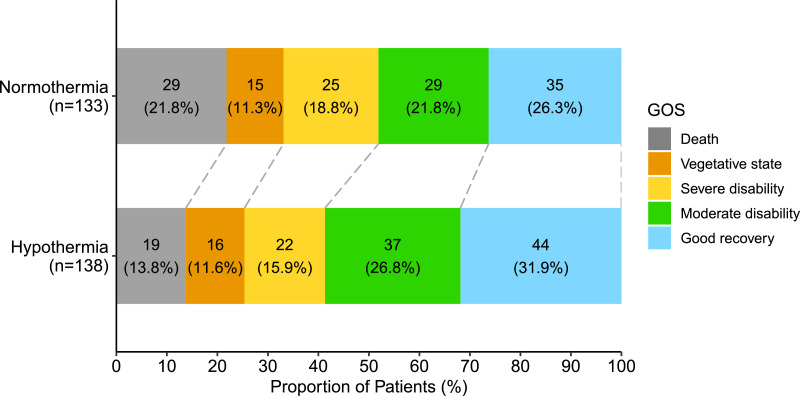
The primary outcome, the 6-month Glasgow outcome scale (GOS) score, was analyzed in 271 patients (Fig. 3). There was no significant difference between groups when analyzed without dichotomization (P = 0·081). The dichotomized GOS, classified into favorable and unfavorable outcomes, adjusted for admission age, sex, baseline Glasgow coma scale score and pupillary reactivity, was not statistically significant between the hypothermia group and the normothermia group (OR 1·55, 95%CI 0·91–2·64). However, the proportions of favorable outcomes were relatively higher in the hypothermia group compared to the normothermia group. In the hypothermia group, 58·7% had favorable outcome compared to 48·1% in the normothermia group (P = 0·105). The mortality was 13·77% in hypothermia and 21·80% in normothermia group (P = 0·111). The length of ICU stay in the hypothermia group (median 17·00 days, IQR 12·00–22·00) was significantly longer compared to the normothermia group (median 12·00 days, IQR 1·00–19·00) (P<0·001), whereas there was no statistical difference in length of hospital stay between the hypothermia and the normothermia groups (P = 0·201) (Table S1).
Fig. 3.
Distribution of GOS at 6 months after randomization. Each cell corresponded to a GOS score, and the width of the cell represented the proportion of patients. GOS=Glasgow outcome scale.
Complications reported for the first 6 days after randomization are shown in Table 2. There were no significant differences of the two groups in the percentage of patients with reported complications: pneumonia (P = 0·620), hypokalemia (P = 0·172), and gastrointestinal bleeding (P = 0·162). In the hypothermia group, there were 44 cases of pneumonia, 53 cases of hypokalemia, and two cases of gastrointestinal bleeding. In the normothermia group, there were 45 cases of pneumonia, 39 cases of hypokalemia, six cases of gastrointestinal bleeding, and four cases of coagulation disorder. No intracranial infection was reported in both groups.
Table 2.
Complications.
| Normothermia | Hypothermia | P value | |
|---|---|---|---|
| (n = 146) | (n = 156) | ||
| Pneumonia | 45 (30·82%) | 44 (28·21%) | 0·620 |
| Hypokalemia | 39 (26·71%) | 53 (33·97%) | 0·172 |
| Gastrointestinal bleeding | 6 (4·11%) | 2 (1·28%) | 0·162 |
| Coagulation disorder | 4 (2·74%) | 0 (0·00%) | .. |
| Intracranial infection | 0 (0·00%) | 0 (0·00%) | .. |
Data were presented as number (%). Complications were reported for the first 6 days after randomization. Hypokalemia referred to serum potassium levels ≤ 3·0 mmol/L.
Laboratory findings in the first 6 days after randomization are reported in Table 3. There was no difference between groups in liver and kidney function: alanine transaminase (P = 0·766), aspartate aminotransferase (P = 0·977), creatinine (P = 0·055), blood urea nitrogen (P = 0·248), electrolytes: Ca2+ (P = 0·271), Cl− (P = 0·919), Na+ (P = 0·840), K+ (P = 0·959), arterial blood gas: buffer excess (P = 0·912), pH (P = 0·361), PaCO2 (P = 0·173), PaO2 (P = 0·419), blood sugar (P = 0·917), white blood cells (P = 0·602), hemoglobin (P = 0·144) and platelets (P = 0·449).
Table 3.
Laboratory findings.
| Normothermia (n = 146) | Hypothermia (n = 156) | P value | |
|---|---|---|---|
| Alanine transaminase (U/L) | 30·35 (19·00, 44·25) | 28·25 (22·20, 45·63) | 0·766 |
| Aspartate aminotransferase (U/L) | 41·33 (27·96, 61·38) | 39·83 (29·87, 56·29) | 0·977 |
| Creatinine (μmol/L) | 63·00 (52·52, 76·75) | 59·61 (51·08, 71·29) | 0·055 |
| Blood urea nitrogen (mmol/L) | 6·81 (4·95, 4·58) | 6·16 (4·58, 8·97) | 0·248 |
| Ca2+ (mmol/L) | 1·78 (1·05, 2·06) | 1·90 (1·10, 2·09) | 0·271 |
| Cl− (mmol/L) | 109·65 (6·59) | 109·58 (6·28) | 0·919 |
| Na+ (mmol/L) | 143·82 (139·84, 147·77) | 143·64 (140·47, 147·50) | 0·840 |
| K+ (mmol/L) | 3·70 (0·31) | 3·71 (0·31) | 0·959 |
| pH | 7·41 (0·05) | 7·41 (0·04) | 0·361 |
| PaO2 (mm Hg) | 138·57 (40·91) | 142·24 (36·70) | 0·419 |
| PaCO2 (mm Hg) | 37·47 (5·25) | 38·31 (5·27) | 0·173 |
| Buffer excess (mmol/L) | −0·12 (2·91) | −0·15 (2·67) | 0·912 |
| Glucose (mmol/L) | 8·23 (7·26, 9·75) | 8·27 (7·33, 9·32) | 0·917 |
| White blood cells (10^9/L) | 11·72 (9·82, 14·54) | 11·62 (9·38, 14·69) | 0·602 |
| Hemoglobin (g/L) | 98·67 (89·92, 115·13) | 102·50 (92·00, 116·00) | 0·144 |
| Blood platelet (10^9/L) | 140·40 (54·28) | 135·44 (58·86) | 0·449 |
Data were presented as mean (SD) and median (IQR)· Data were calculated for the first 6 days after randomization.
There was an obvious interaction between therapeutic effects and initial ICP differentiated by initial ICP (Table S2). In the patients with an initial ICP ≥ 30 mm Hg, the favorable outcomes in the hypothermia group (59 of 97 patients, 60·82%) was significantly higher than that in the normothermia group (41 of 96 patients, 42·71%) (P = 0·018). There were also differences in age and SBP between the two groups (Table S2). Logistic regression was thus further performed to adjust for age and SBP, which still indicated the patients with initial ICP ≥ 30 mm Hg had significantly more favorable outcomes after hypothermia treatment (OR 1·861, 95%CI 1·031–3·361; P = 0·039) (Table S3).
Overall, there was no significant difference of ICP values between the normothermia and hypothermia groups (P = 0·45) (Figure S1), and no significant difference in dosage (P = 0·211) nor patient numbers (P = 0·312) of mannitol use (Figure S2). However, in patients not undergoing a decompressive craniectomy, ICP appeared better controlled in the hypothermia group (n = 49) compared to the normothermia group (n = 41) (Figure S1). Differences were, however, not significant (P = 0·32).
Discussion
In this multicenter randomized controlled trial, long-term mild hypothermia (targeted 34–35 °C for 5 days followed by slow rewarming) in sTBI patients with intracranial hypertension did not improve favorable neurological outcomes, evaluated by GOS at 6-month after injury compared with normothermia. The incidences of complications such as pneumonia, hypokalemia, gastrointestinal bleeding, coagulation disorder, and intracranial infection were not different between two groups. The length of ICU stay in the hypothermia group was significantly longer than that in the normothermia group, while the hospital stay showed no difference. On subgroup analysis, we found that long-term mild hypothermia significantly improved favorable outcome of sTBI patients with an initial ICP ≥ 30 mm Hg. This finding suggests that prolonged mild hypothermia might be an option with potential for the sTBI patients with initial ICP ≥ 30 mm Hg.
Five higher-quality multicenter randomized trials of hypothermia failed to provide any positive evidence for treatment efficacy [5,6,10,11,21]. One of the possible explanations was that the period of hypothermia might not be long enough to show any benefit in improving outcomes for sTBI patients, as studies have confirmed that cerebral edema begins from minutes to hours after injury, peaks at 3–5 days and may even last longer [18,19]. In addition, rebound ICP increase during rewarming after 48–72 h of hypothermia was found to have detrimental effects on outcomes [5]. Raised ICP after hypothermia treatment was found in NABIS: H II study, and the rewarming rate was 17 h in the study [6]. However, in our study, rebound increase of ICP was not observed in the hypothermia group. The 5-days prolonged hypothermia and slow re-warming rate at a steady pace of ICP control could contribute to prevent adverse effects of rebound ICP in the hypothermia group and may improve the benefit/risk ratio of hypothermia treatment.
In contrast to previous studies [21], [22], [23], long-term mild hypothermia did not increase the incidences of severe complications including pneumonia, hypokalemia, thrombocytopaenia, and arrhythmia in our study, compared with normothermic treatment. The incidence of pneumonia, hypokalemia, and other complications were shown to be similar between the two groups (Table 2), and laboratory examinations showed no differences in [K+], PaCO2, or blood platelet count between groups (Table 3). Post-hoc analyses of NABIS:H I and II trials suggested that in future trials the critical variable is to reach 35 °C or lower but not necessarily 33 °C [4,5]. The target temperature of 34–35 °C in our study, in contrast to previous trials (the final target temperature of 33 °C), may in combination with the slow and controlled rewarming have contributed to a relatively low incidence of the overall complications. We found that, although did not reach significance, prolonged mild hypothermia might reduce ICP in patients without decompressive craniectomy (DC) compared to those in the normothermia group in this study.
Our finding that favorable outcome was significantly improved in the patients with an initial ICP ≥ 30 mm Hg in prolonged mild hypothermic treatment compared with normothermia may be considered highly relevant. It is likely that patients with extremely high ICP are at high risk for brain swelling and may benefit most from hypothermia therapy, aiming to prevent and mitigate brain swelling. It may well be that the potential risks of hypothermia demand more deranged physiology before the risk: benefit ratio becomes favourable [3]. We suggest that prolonged mild hypothermia may be an option with potential for the sTBI patients with initial ICP ≥ 30 mm Hg.
Compared to previous mild hypothermia trials, the proportions of patients receiving DC (hypothermia group 67·95%, normothermia group 71·23%) and/or surgical hematoma removal (hypothermia group 92·31%, normothermia group 91·78%) were markedly high in this study (Table 1) [6]. We deliberately targeted this patient group with our inclusion criteria, as NABIS: H II has shown that in the patients who underwent surgical removal of intracranial hematomas, those with hypothermic treatment had significant fewer poor outcomes than those who had normothermia [5]. Our data confirm these findings for patients with a high initial ICP, indicating that hypothermia may be beneficial in high risk patients following surgical removal of intracranial lesions.
One limitation of this clinical trial is the target temperature control. Patients in the hypothermia group could not all be cooled to the target temperature of 34–35 °C, which was mainly due to the clinical practices, e.g., the priority of sustaining stable haemodynamics. The second limitation is that the primary outcome was evaluated with the score of GOS, but not the Extended Glasgow Outcome Scale (GOS-E) at 6-month after injury. GOS is a five-category scale used for assessing the neurologic outcome, while GOS-E is an eight-category one [24,30]. Thus GOS might lack the sensitivity to detect statistical changes in outcome and consequently, potential gains of the treatment could be lost. However, GOS was relatively simple and clear which contributed to a reliable clinical evaluation at 6-month follow up.
In conclusion, long-term (5 days) mild hypothermia therapy for severe TBI patients with severe intracranial hypertension did not improve the outcomes as compared with the normothermia treatment. However, prolonged mild hypothermia may be an option with potential for the sTBI patients with initial ICP ≥ 30 mmHg in the future, but the evidence is not conclusive yet and requires further study.
Author contributions
GG, JF, JL, and JJ participated in the concept and design of the study. All author participated in analysis, writing, or revision of the manuscript. All authors participated in the reported analyses and interpretation of results relevant to their domain of interest. GG, JH, YT, WZ, CZ, ML, JF, and JJ prepared the draft manuscript and coordinated its finalization. GG, JH, WZ, JF, and XW did the statistical analyses and drafting of tables and figures. ML, JF, and JJ revised the manuscript and gave support in statistical analyses and drafting of figures. All authors approved the final manuscript.
Declaration of Competing Interest
GG, JH, JF, RM, XW, JL, and JJ declare support from the Shanghai municipal government and Shanghai Jiao Tong University/School of Medicine. All other authors declare no competing interests.
Acknowledgments
Data sharing
Researchers who submit a methodologically sound study proposal that is approved by the author group can have access to the study protocol, individual participant data, data dictionary, analytic code, and analysis scripts. Proposals can be submitted online (https://clinicaldata.shsmu.edu.cn). A data access agreement is required, and all access must comply with regulatory restrictions imposed on the original study.
Acknowledgments
We are immensely grateful to our patients with TBI and their families for helping us in our efforts to improve care and outcomes for patients with TBI. The coordinating centre (Department of Neurosurgery, Renji Hospital, Shanghai Jiao Tong University/School of Medicine) received support from the Shanghai municipal government and Shanghai Jiao Tong University/School of Medicine.
Funding
This research was supported by grants Shanghai municipal government and Shanghai Jiao Tong University/School of Medicine. The sponsor of the study had no role in the data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100732.
Contributor Information
Guoyi Gao, Email: gao3@sina.com.
Jiyao Jiang, Email: jiyaojiang@126.com.
Appendix: List of Group Contributors
Qing Mao1, Sai Zhang2, Hairui Linghu2, Weiping Li2, Likun Yang3, Xialiang Li4, Zhonghua Wu4, Wei Wang4, Zhicheng Xin4, Chaochao Jiang4, Song Lan5, Xin Chen5, Zhongyi Sun5, Xiangying Luo5, Changlong Bi5, Jian Wu6, Zhichen Huang6, Danlong Chen6, Weili Hong6, Li Liu6, Wanwan Pan6, Xin Li6, Yong Ying6, Guoliang Shen6, Shaoyang Li6, Yun Bao7, Juan Mao7, Weiping Li8, Liang Wen9, Hao Wang9, Tianxiang Zhan9, Min Li10, Yanlong Yang10, Tao Chang10, Qingbao Guo10, Zhenghua Shi10, Minghao Man10
1. Head Trauma Center, Department of Neurosurgery, Renji Hospital, Shanghai Jiao Tong University/School of Medicine, Shanghai Institute of Head Trauma, Shanghai, China
2. Neurological Intensive Care Unit, Beijing Chaoyang Integrative Medicine Emergency Medical Center, Beijing, China
3. Department of Neurosurgery, Taihu Hospital, Wuxi, China
4. Department of Neurosurgery, South Taihu Hospital, Huzhou, China
5. Department of Neurosurgery, Xiangya Hospital, Central South University, Changsha, China
6. Department of Neurosurgery, Taizhou First People's Hospital, Zhejiang, China
7. Department of Neurosurgery, Nanfang Hospital, Southern Medical University, Guangzhou, China
8. Department of neurosurgery, Shenzhen Second People's Hospital, Shenzhen, China
9. Emergency and Trauma Center, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
10. Department of Neurosurgery, Tangdu Hospital, Xi'an, China
Appendix B. Supplementary materials
References
- 1.Jiang J.Y., Gao G.Y., Feng J.F. Traumatic brain injury in China. Lancet Neurol. 2019;18(3):286–295. doi: 10.1016/S1474-4422(18)30469-1. [DOI] [PubMed] [Google Scholar]
- 2.Injury GBDTB Spinal Cord Injury C. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stocchetti N., Carbonara M., Citerio G. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol. 2017;16(6):452–464. doi: 10.1016/S1474-4422(17)30118-7. [DOI] [PubMed] [Google Scholar]
- 4.C P. Traumatic injuries of the brain and its membranes. D. Appleton & Co; New York, NY: 1897. Principles of treatment. [Google Scholar]
- 5.Clifton G.L., Miller E.R., Choi S.C. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344(8):556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- 6.Clifton G.L., Valadka A., Zygun D. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: hypothermia II): a randomised trial. Lancet Neurol. 2011;10(2):131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clifton G.L. A review of clinical trials of hypothermia treatment for severe traumatic brain injury. Ther Hypothermia Temp Manag. 2011;1(3):143–149. doi: 10.1089/ther.2011.0009. [DOI] [PubMed] [Google Scholar]
- 8.Clifton M.G., Valadka P.A., Aisuku I.P., Okonkwo D.O. Future of rewarming in therapeutic hypothermia for traumatic brain injury: a personalized plan. Ther Hypothermia Temp Manag. 2011;1(1):3–7. doi: 10.1089/ther.2010.1500. [DOI] [PubMed] [Google Scholar]
- 9.Andrews P.J., Sinclair H.L., Rodriguez A. Therapeutic hypothermia to reduce intracranial pressure after traumatic brain injury: the Eurotherm3235 RCT. Health Technol Assess. 2018;22(45):1–134. doi: 10.3310/hta22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper D.J., Nichol A.D., Bailey M. Effect of early sustained prophylactic hypothermia on neurologic outcomes among patients with severe traumatic brain injury: the POLAR randomized clinical trial. JAMA. 2018;320(21):2211–2220. doi: 10.1001/jama.2018.17075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maekawa T., Yamashita S., Nagao S. Prolonged mild therapeutic hypothermia versus fever control with tight hemodynamic monitoring and slow rewarming in patients with severe traumatic brain injury: a randomized controlled trial. J Neurotrauma. 2015;32(7):422–429. doi: 10.1089/neu.2013.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis S.R., Evans D.J., Butler A.R., Schofield-Robinson O.J., Alderson P. Hypothermia for traumatic brain injury. Cochrane Database Syst Rev. 2017;9 doi: 10.1002/14651858.CD001048.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang J.Y., Lyeth B.G., Kapasi M.Z., Jenkins L.W., Povlishock J.T. Moderate hypothermia reduces blood-brain barrier disruption following traumatic brain injury in the rat. Acta Neuropathol. 1992;84(5):495–500. doi: 10.1007/BF00304468. [DOI] [PubMed] [Google Scholar]
- 14.Jiang J., Yu M., Zhu C. Effect of long-term mild hypothermia therapy in patients with severe traumatic brain injury: 1-year follow-up review of 87 cases. J Neurosurg. 2000;93(4):546–549. doi: 10.3171/jns.2000.93.4.0546. [DOI] [PubMed] [Google Scholar]
- 15.Qiu W., Shen H., Zhang Y. Noninvasive selective brain cooling by head and neck cooling is protective in severe traumatic brain injury. J Clin Neurosci. 2006;13(10):995–1000. doi: 10.1016/j.jocn.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Zhi D., Zhang S., Lin X. Study on therapeutic mechanism and clinical effect of mild hypothermia in patients with severe head injury. Surg Neurol. 2003;59(5):381–385. doi: 10.1016/s0090-3019(03)00148-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhi D.S., Zhang S., Zhou L.G. Continuous monitoring of brain tissue oxygen pressure in patients with severe head injury during moderate hypothermia. Surg Neurol. 1999;52(4):393–396. doi: 10.1016/s0090-3019(99)00101-9. [DOI] [PubMed] [Google Scholar]
- 18.Jiang J.Y. Mild-to-Moderate Hypothermia for Management of Severe Traumatic Brain Injury in China: history, Current Status, and Future. Ther Hypothermia Temp Manag. 2013;3(3):120–121. doi: 10.1089/ther.2013.0008. [DOI] [PubMed] [Google Scholar]
- 19.Stocchetti N., Colombo A., Ortolano F. Time course of intracranial hypertension after traumatic brain injury. J Neurotrauma. 2007;24(8):1339–1346. doi: 10.1089/neu.2007.0300. [DOI] [PubMed] [Google Scholar]
- 20.Jiang J.Y., Xu W., Li W.P. Effect of long-term mild hypothermia or short-term mild hypothermia on outcome of patients with severe traumatic brain injury. J Cereb Blood Flow Metab. 2006;26(6):771–776. doi: 10.1038/sj.jcbfm.9600253. [DOI] [PubMed] [Google Scholar]
- 21.Andrews P.J., Sinclair H.L., Rodriguez A. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med. 2015;373(25):2403–2412. doi: 10.1056/NEJMoa1507581. [DOI] [PubMed] [Google Scholar]
- 22.Geurts M., Macleod M.R., Kollmar R., Kremer P.H., van der Worp H.B. Therapeutic hypothermia and the risk of infection: a systematic review and meta-analysis. Crit Care Med. 2014;42(2):231–242. doi: 10.1097/CCM.0b013e3182a276e8. [DOI] [PubMed] [Google Scholar]
- 23.Polderman K.H. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 Suppl):S186–S202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 24.Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 25.CCoN Surgeons. Chinese surgical guidelines for management of traumatic brain injury. Chin J Neurosurg. 2009;25:100–101. [Google Scholar]
- 26.Lei J., Gao G., Mao Q. Rationale, methodology, and implementation of a nationwide multicenter randomized controlled trial of long-term mild hypothermia for severe traumatic brain injury (the LTH-1 trial) Contemp Clin Trials. 2015;40:9–14. doi: 10.1016/j.cct.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Marion D.W., Penrod L.E., Kelsey S.F. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336(8):540–546. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- 28.Brain Trauma F. American Association of Neurological S, Congress of Neurological S. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 29.Wilson J.T., Pettigrew L.E., Teasdale G.M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 30.Weir J., Steyerberg E.W., Butcher I. Does the extended Glasgow Outcome Scale add value to the conventional Glasgow Outcome Scale? J Neurotrauma. 2012;29(1):53–58. doi: 10.1089/neu.2011.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.