Abstract
Chronic exposure to manganese (Mn) may lead to a movement disorder due to preferential Mn accumulation in the globus pallidus and other basal ganglia nuclei. Iron (Fe) deficiency also results in increased brain Mn levels, as well as dysregulation of other trace metals. The relationship between Mn and Fe transport has been attributed to the fact that both metals can be transported via the same molecular mechanisms. It is not known, however, whether brain Mn distribution patterns due to increased Mn exposure vs. Fe deficiency are the same, or whether Fe supplementation would reverse or inhibit Mn deposition. To address these questions, we utilized four distinct experimental populations. Three separate groups of male Sprague–Dawley rats on different diets (control diet [MnT], Fe deficient [FeD], or Fe supplemented [FeS]) were given weekly intravenous Mn injections (3 mg Mn/kg body mass) for 14 weeks, whereas control (CN) rats were fed the control diet and received sterile saline injections. At the conclusion of the study, both blood and brain Mn and Fe levels were determined by atomic absorption spectroscopy and magnetic resonance imaging. The data indicate that changes in dietary Fe levels (either increased or decreased) result in regionally specific increases in brain Mn levels compared with CN or MnT animals. Furthermore, there was no difference in either Fe or Mn accumulation between FeS or FeD animals. These data suggest that dietary Fe manipulation, whether increased or decreased, may contribute to brain Mn deposition in populations vulnerable to increased Mn exposure.
Keywords: Iron deficiency (ID), iron supplementation, brain Mn accumulation, MMT, MRI
Manganese (Mn) intoxication is known to cause a movement disorder, manganism, which may phenotypically resemble Parkinson’s disease, but is distinct in both pathology and disease progression (Pal et al., 1999). Manganism has most often been associated with occupations involving exposures to high concentrations of Mn dust (> 5 mg Mn/m3), such as Mn mining (Myers et al., 2003; Rodier, 1955) and steel production (Yu et al., 2003). More recent, but still controversial, data also suggest a possible link between Parkinson’s disease and Mn exposures in welders (Racette et al., 2001, 2005). Increased brain Mn accumulation in the absence of dystonia, however, has been also documented in numerous patient populations, including those suffering from iron (Fe) deficiency (ID), on parenteral nutrition and welders (reviewed by Fitsanakis et al., 2006b). Several animal studies have further confirmed that ID alone (Erikson et al., 2002, 2004; Kim et al., 2005) or ID coupled with high levels of Mn (IDMn) (Garcia et al., 2007) also lead to increased brain Mn accumulation. Few studies, though, have examined whether increased dietary Fe might provide protective measures for vulnerable populations.
It is important to examine possible prophylactic measures in light of the fact that an antiknocking agent containing Mn, methylcyclopentadienyl Mn tricarbonyl (MMT), may soon be added to gasoline in this and other countries. Indeed, in Canada, where MMT is already available, the amount of Mn in the air near heavily trafficked areas has increased drastically over the past several years (Boudia et al., 2006; Zayed et al., 2003). This suggests that populations without previous exposure to Mn may now breathe Mn-laden fumes throughout the course of a life-time. This is particularly disturbing considering that up to 60% of the world’s population, including people in developed countries, are mildly to severely Fedeticiant (Umbreit, 2005; WHO, 2003). Many of these are women, infants, young children, and the elderly, who are historically recognized as particularly vulnerable populations to toxic insult. Although Fe supplementation is a valid treatment for ID, it is unknown as to whether this would also reverse or prevent brain Mn accumulation.
Although brain Mn accumulation and ID may seem unrelated, in fact they are not (Erikson and Aschner, 2006; Malecki et al., 1999). It is known that in numerous tissues (Garrick et al., 2003; Picard et al., 2000) Mn and Fe share the same transporters, namely the divalent metal transporter-1 (DMT-1) and the transferrin (Tf)/transferrin receptor (TfR) system (Aschner and Gannon, 1994; Crossgrove et al., 2003; Deane et al., 2004). It has been hypothesized, therefore, that one of the reasons brain Mn deposition increases during periods of Fe depletion relates to the fact that less Fe is available for transport by these proteins, which are then upregulated during ID (Connor et al., 2001). This transport imbalance results in regionally specific Mn accumulation in the brain. It would seem to follow, then, that increased dietary Fe should limit or prevent brain Mn deposition during periods of increased Mn exposure. If this were the case, it would suggest a relatively simple treatment for, or prevention of, the onset of manganism.
In order to test the hypothesis that Fe supplementation in the presence of increased Mn exposure may lead to decreased brain Mn accumulation, we examined one control (CN) and three treated cohorts of adult male rats: CN and Mn-treated (MnT) animals were given a diet with normal Fe levels (30 mg Fe/kg food), but received weekly intravenous saline or Mn injections, respectively. Two other groups also received weekly Mn injections, but were either given an Fe-deficient (FeD; 3 mg Fe/kg chow) or Fe-supplemented (FeS; 300 mg Fe/kg chow) diet. Brain Mn and Fe levels were determined at the conclusion of the study with both atomic absorption spectroscopy (AAS) and magnetic resonance imaging (MRI).
MATERIALS AND METHODS
Animals.
All animal protocols were approved by the Vanderbilt University Medical Center Institutional Animal Care and Use Committee. Male Sprague–Dawley rats (240–250 g) were ordered from Harlan (Indianapolis, IN). Rats had free access to food and water during the 14-week experimental protocol. CN (n = 5) and MnT (n = 6) rats received normal rat chow with 30 mg Fe/kg chow. Two additional groups received diets with modified Fe levels: the FeD (n = 6) group was fed a specially formulated diet with reduced levels of Fe (3 mg Fe/kg chow); the FeS (n = 4) group received a diet with 300 mg Fe/kg chow. Both Fe-modified diets were formulated and assayed for Fe content by BioServe (Frenchtown, NJ), and were designed to provide 10× less (FeD) or 10× more (FeS) Fe than the normal daily allowances for rodents. In addition to dietary modifications, animals from the three treatment groups (MnT, FeD, and FeS) received weekly intravenous tail vessel injections of a sterile, isotonic Mn solution (3 mg Mn/kg body mass) of MnCl2 for a total of 14 weeks. This dose roughly corresponds to a body burden of 20 ppb (assuming 10 ml of blood from a rat with a body mass of 250–300 g), which is well within the limits observed in humans exposed to environmental Mn and exhibiting symptoms of Mn intoxication (Mergler et al., 1999), and those observed in animal models (Park et al., 2003). Control rats received injections of similar volumes of sterile, isotonic saline.
Rats were weighed weekly as a gross measure of general health. Additionally, blood was collected from tail vessels at the conclusion of the study to verify red blood cell (RBC) Mn and Fe levels.
Magnetic resonance imaging
Upon arrival at Vanderbilt, rats were allowed to adjust to their new environment for 4–6 days prior to any handling or testing. Following this, all animals were imaged prior to the beginning of their respective treatment and again at week 14, the last week of the study. In order to maintain consistency during the study, animals were always imaged 24 h after that week’s injection.
For the scans, animals were initially anesthetized with 2% isoflurane and placed in a stereotaxic support cradle with their head secured with tape. The cradle was put in the volume coil to ensure the rat’s head was located in the coil’s center. Isoflurane was then lowered to 1.5–1.75% and maintained for the duration of the scanning protocol, usually 1.5 h. During the scan, body temperature was maintained at 37°C with warm air controlled by a rectal temperature probe (SA Instruments, Stony Brook, NY). Respiration was monitored throughout, and maintained at 50–70 breaths per minute.
All experiments were acquired using a 4.7 T, 31-cm-bore Varian INOVA magnet with actively shielded gradients (40 G/cm, rise time full amplitude of 130 μs) and 63 mm transmit/receive quadrature imaging volume coil. Rat brains were scanned from both axial (field of view [FOV] = 40 × 40 mm, 30 slices) and coronal directions (FOV = 40 × 50 mm, 20 slices) with 0.75 mm slice thickness. T1 was measured using two-dimensional fast low angle shot sequence with parameters as following: time repetition/time echo = 489/6.59 ms; flip angles = 10°, 30°, 55°, or 70°; two acquisitions; image matrix = 256 × 256.
Blood collection and brain dissection.
At the conclusion of the study (week 14), rats were anesthetized with ketamine (80 mg/kg body mass) and xylazine (12 mg/kg body mass). When animals no longer responded to deep toe pinch, they were rapidly decapitated. Trunk blood was collected in heparanized tubes, centrifuged (1000 × g) at 4°C for 30 min to separate RBCs from plasma and stored at −80°C until metal analysis. Brains were removed and dissected into the following regions: cerebellum, brain stem (medulla and pons), midbrain, hippocampus, striatum, and cortex. Regions were quick-frozen in dry ice, and stored at −80°C until analysis by AAS.
Brain metal analysis by AAS.
Tissue Mn and Fe concentrations were, measured with graphite furnace AAS (Varian AA240, Varian, Inc., Palo Alto, CA). Brain regions were digested in ultrapure nitric acid (1:10 wt/vol dilution) for 48–72 h in a sand bath (60°C). One hundred microliters of digested tissue was brought to 1 ml of total volume with 2% nitric acid and analyzed for Mn and Fe. For RBCs, a 400-μl aliquot was vortexed with 100 μl of 0.5% Triton-X for 30 s. This was brought up to 1 ml of total volume with 2% nitric acid for analysis. The mixture was then centrifuged and the clear supernatant was used for analysis (100-μl aliquot brought up to a 1-ml volume with 2% nitric acid). Bovine liver (10 μg Mn/l) was digested in ultrapure nitric acid and used as an internal standard for analysis.
Image analysis.
T1 maps were calculated by fitting the series of T1-dependent images to the appropriate theoretical expressions using two-parameter least squares fits. The parametric maps were then coregistered to a high-resolution rat template and resliced using statistical parametric mapping (http://www.fil.ion.ucl.ac.uk/spm). Statistical comparisons were made between the coregistered parametric maps using two-tailed Student t-test (Matlab, MathWorks, Inc., Natick, MA). The statistical maps (p < 0.05, corrected by false discovery rate) were generated, and were later overlaid on a standard rat brain template (http://www.fil.ion.ucl.ac.uk/spm).
Statistical analyses.
One-way analysis of variance (ANOVA), with Bonferroni’s post-test, was used to compare brain and blood metal data from all four groups. Statistically significant differences in body mass over the course of the studies were determined using linear regression analysis, followed by one-way ANOVA comparison of the regression lines. Both one-way ANOVAs and linear regression analysis were done with GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego, CA, www.graph-pad.com). Data were accepted as statistically significantly different when p ≤ 0.05. Two-tailed Student’s t-test (p < 0.05, corrected for false discovery rate) was used to compare T1 values between all four groups and to further generate statistical maps.
RESULTS
Body Mass
The general health of each animal was grossly monitored by weekly measurement of body mass throughout the course of the study. In addition, animals were visually inspected several times per week to determine whether any overt behavioral abnormalities (unsteadiness, tremor or gait disturbances) were present. Consistent with the low dose of Mn administered weekly (3 mg Mn/kg body mass), no behavioral changes were noted. Control animals (CN) generally doubled their body mass compared over the 14-week experiment (Fig. 1). This was not the case for animals on a control diet, but receiving Mn injections (MnT), or animals fed FeS or FeD chow that were also injected weekly with Mn. MnT and FeD rats did not gain as much body mass compared with CN (p < 0.001 for both). Interestingly, both FeS and FeD animals gained statistically significantly more body mass than the MnT rats (p < 0.001). All treated animals, however, were smaller than their CN counterparts. Food intake was monitored, and changes in body mass could not be attributed to differences in food consumption among the groups.
FIG. 1.
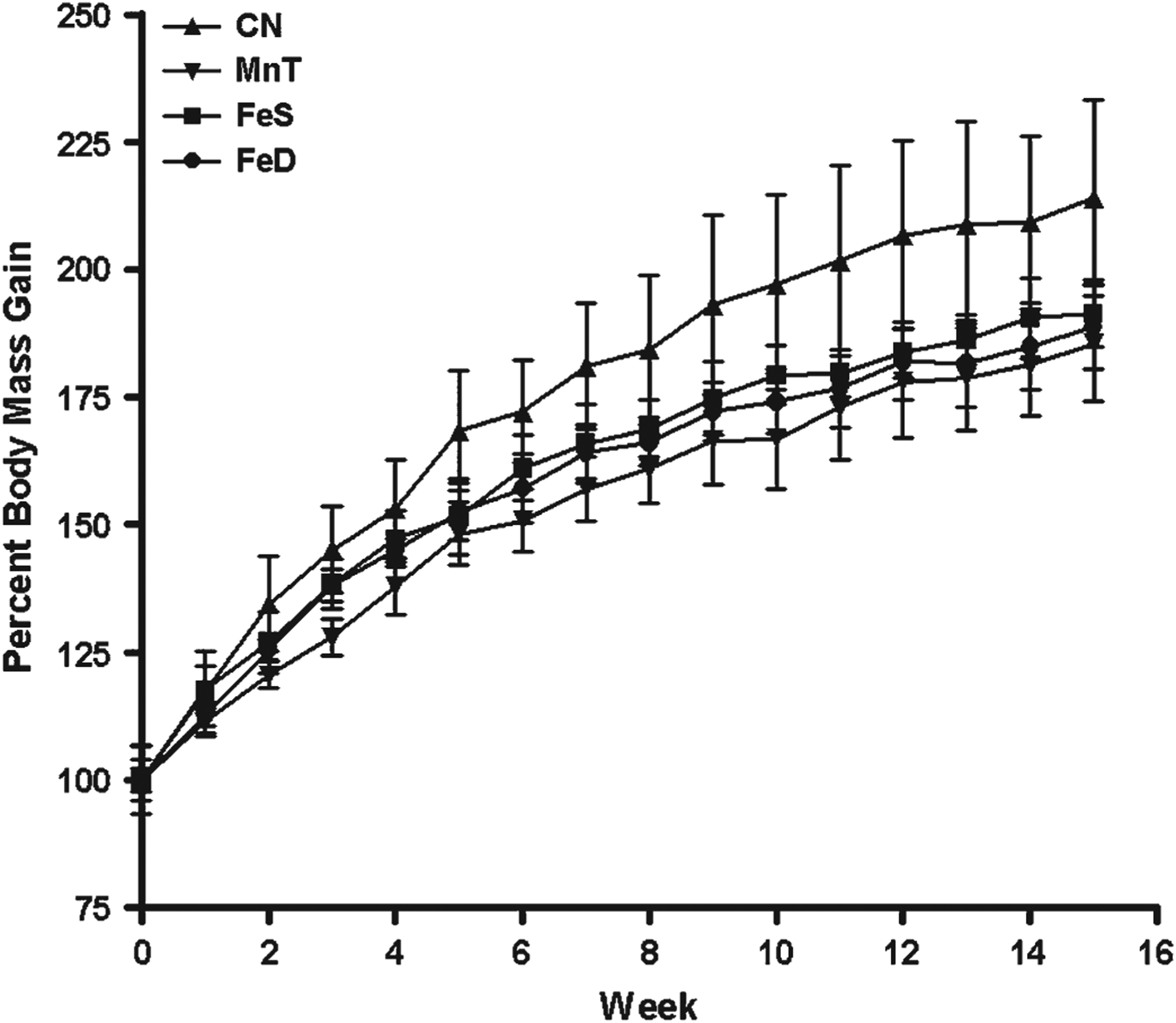
Weekly body mass gain—Rats in each group were weighed weekly as a general assessment of overall health. All treatment groups gained statistically significantly less mass than the CN animals (p < 0.001). Although there was no difference between MnT and FeS or FeS and FeD, FeD animals were significantly lighter than MnT animals (p < 0.001). Abbreviations for treatment groups appears in the text.
Blood Mn and Fe Levels (AAS)
Although Mn is transported throughout the body by various plasma proteins, it is also highly concentrated in RBCs. Additionally, although approximately 98% of the plasma Mn can be cleared by the liver, the metal is thought to remain bound to hemoglobin for up to 20 days (Mahoney and Small, 1968). Consistent with the low Mn concentrations that the animals received, there was no statistically significant change in the amount of Mn in RBC of treated groups compared with CN (Fig. 2).
FIG. 2.
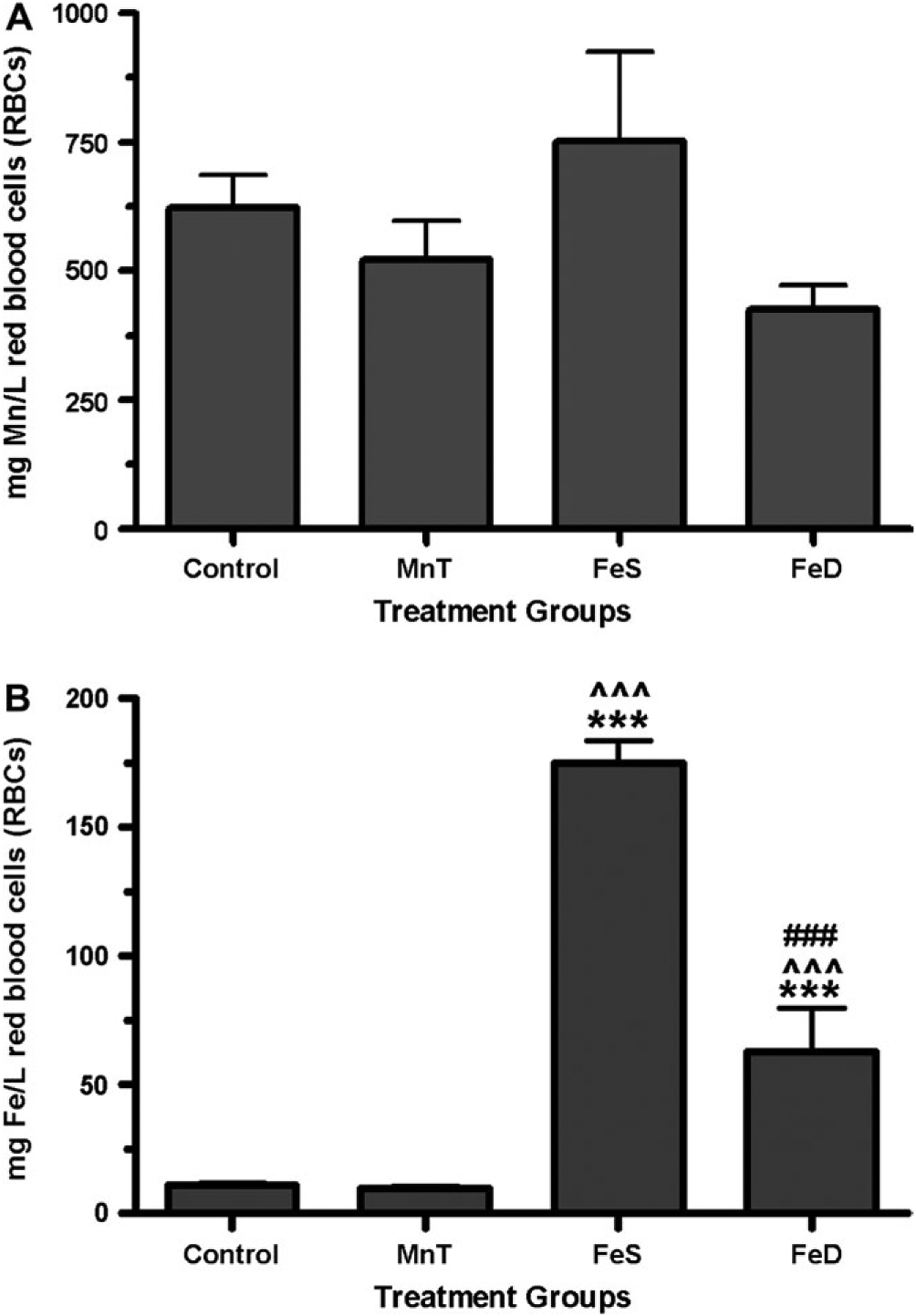
RBC metal content—AAS was used to determine the amount of (a) Mn or (b) Fe in RBCs isolated from rats at week 14, the last week of the study. There was no statistically significant difference in RBC Mn content among any of the groups. RBC Fe content was statistically significantly increased in FeS and FeD animals compared with CN (***p < 0.001) and MnT (p < 0.001). Additionally, the FeS group had more RBC-associated Fe compared with the FeD animals (###p < 0.001).
This was not the case, however, when RBC Fe content was determined. Compared with CN animals, there was no statistically significant difference in RBC Fe concentrations in rats in the MnT group. On the other hand, rats from both FeD and FeS groups had blood Fe levels that were statistically significantly higher than that of both CN and MnT (p < 0.001). Additionally, FeD rats have significantly less Fe associated with their RBCs than FeS rats (p < 0.001). These data are consistent with other reports demonstrating that FeD rats have increased [Fe]blood compared with CN animals, likely due to an upregulation of Fe-transport proteins (Crowe and Morgan, 1992; Taylor et al., 1991).
Regional Brain Metal Accumulation (AAS)
At the conclusion of the study, brains from each animal were removed and dissected into the following regions: cerebellum, brain stem (pons and medulla), midbrain, hippocampus, striatum and cortex. Brain Mn content was determined using AAS. As depicted in Figure 3a, brain Mn levels were statistically significantly higher in the cerebellum and brain stem for all treated animals compared with CN (p < 0.05). Other brain regions showed differential metal dysregulation compared with CN animals. For example, Mn content was increased in the hippocampus of MnT animals (p < 0.01), but decreased in animals receiving FeD chow (p < 0.05). Additionally, striatal and cortical Mn concentrations were greater in FeD and FeS (p < 0.05) compared with control animals, whereas MnT animals had similar cortical Mn concentrations relative to CN (Fig. 3a).
FIG. 3.
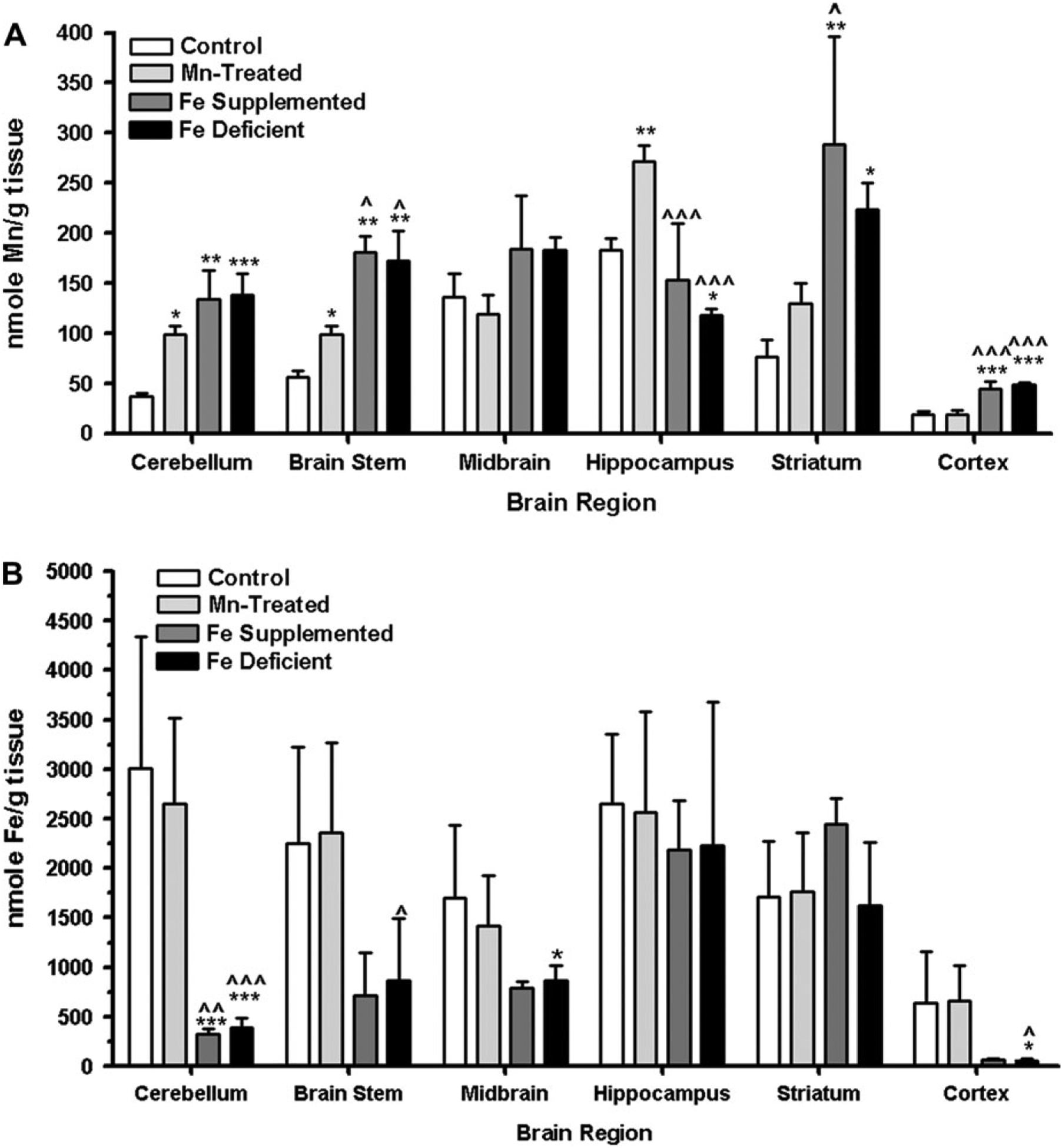
Regional brain metal content—AAS was used to determine the amount of (a) Mn or (b) Fe in discrete brain regions. Both Mn and Fe levels were differentially altered in each treatment group and for each discrete brain region. For both graphs in the figure, the following symbols are used: *p < 0.05, **p < 0.01, and ***p < 0.001 compared with CN; p < 0.05, p < 0.01, and p < 0.001 compared with MnT.
Brain Fe content was also determined at the conclusion of the study. Brain Fe levels were similar in animals receiving Fe-modified diets regardless of whether animals were fed FeD or FeS chow. Additionally, cerebellar Fe was statistically significantly lower in FeS and FeD animals compared with either CN or MnT (p < 0.001 for both) (Fig. 3b). No differences in Fe concentration was observed in the hippocampus or striatum among the four groups. Finally, the FeD group demonstrated a statistically significant decrease in Fe deposition in the brain stem relative to MnT (p < 0.05), the midbrain compared with CN (p < 0.05) and the cortex compared with both the MnT and CN (p < 0.05 for both groups).
Brain Mn Accumulation (MRI)
Although AAS provides a good indicator for gross metal accumulation, the dissections and subsequent metal determination do not allow for detection of subtle changes in specific brain subregions. To supplement the AAS data, animals were also imaged using MRI at the beginning and end of the study. Figure 4 demonstrates that Mn accumulation also takes place to a statistically significant degree in the olfactory bulb of all animals relative to CN (Fig. 4a). Additionally, the pattern of Mn accumulation for both FeD and FeS animals is statistically significantly different from that of MnT animals. This is particularly evident in Figure 4b, panels labeled FeD versus MnT and FeS versus MnT. Here, brain Mn accumulation appears to involve more of the cortex and lateral nuclei of the brain than in the MnT animals. The images in Figures 4a, 4b, and 4c that compare the FeS versus FeD animals also strikingly reiterate the AAS data suggesting that brain Mn accumulation is not generally ameliorated by changes in dietary Fe levels. Indeed, Mn deposition is further exacerbated in most brain regions following modification Fe in the rodent chow.
FIG. 4.
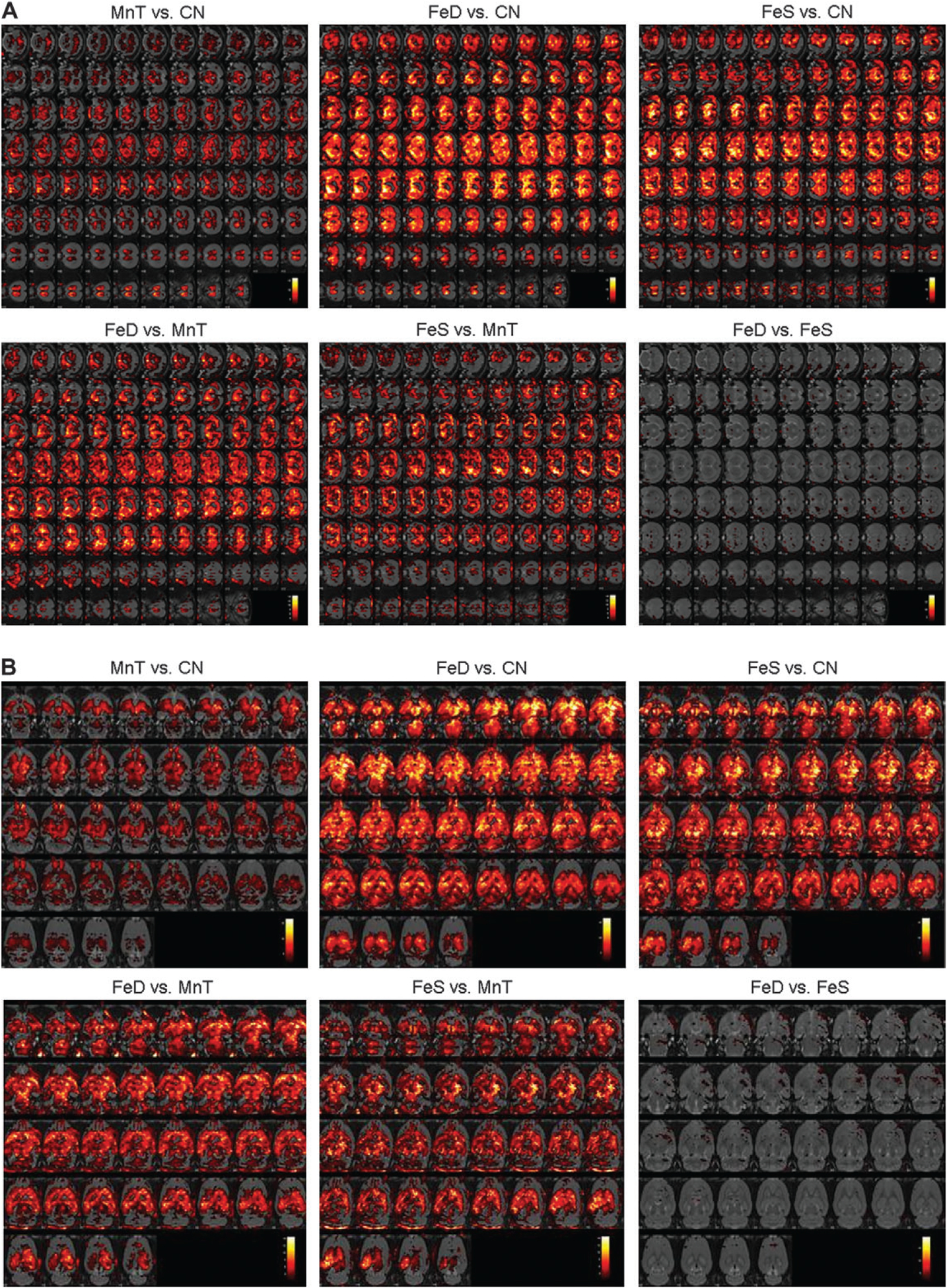
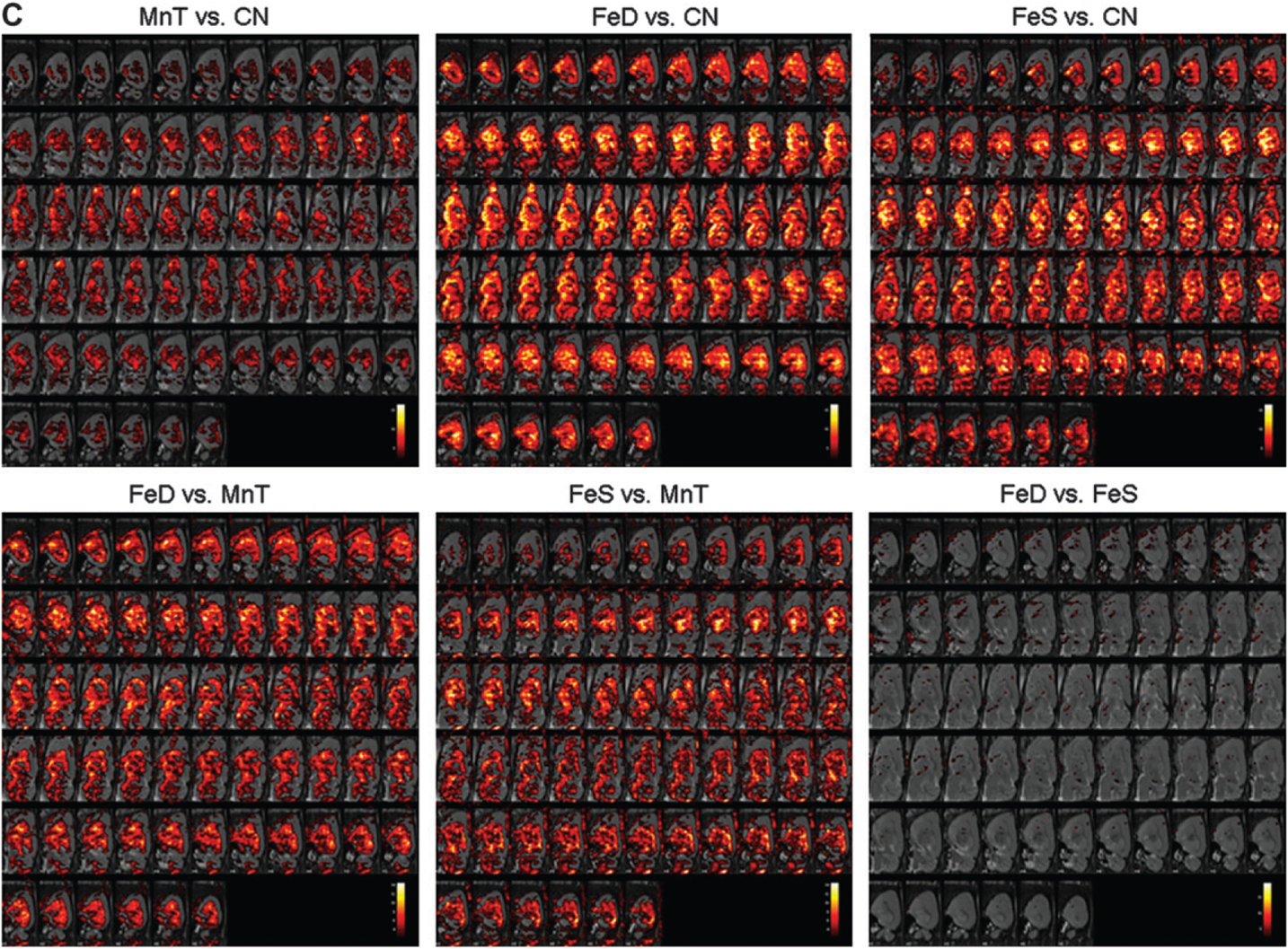
MR images depicting brain Mn accumulation—Animals were imaged at week 14 to determine regional changes in brain Mn deposition. T1 maps were compared across groups and the corresponding statistical maps were overlaid on high-resolution rat brain templates. Images are presented in the (a) frontal, (b) horizontal, and (c) sagittal planes. White indicates greater statistically significant differences in regional Mn amounts, whereas red faux coloring indicates the minimal threshold of p<0.05.
DISCUSSION
Although high brain Mn levels have more often been associated with occupational exposure (Levy and Nassetta, 2003), the proposed inclusion of methylcyclopentadienyl Mn tricarbonyl (MMT) in gasoline may lead to an increased risk of brain Mn accumulation in populations that are more prone to ID, that is, women and children (Kim et al., 2005). In order to reduce the brain Mn burden, occupational patients are often removed from the metal’s source. New treatments, such as chelation therapy, have more recently been tried with varying degrees of success (Discalzi et al., 2000). In addition to Mn exposure, it has been reported that some ID patients demonstrate both increased blood and brain Mn levels (Kim et al., 2005). These patients are often provided short-term Fe supplements, which results in a decreased pallidal index (an indication of brain Mn accumulation) following diagnostic MR imaging (Hernandez et al., 2002; Kim et al., 2005). As this Fe repletion model appeared to provide decrease brain Mn accumulation, we tested the hypothesis that animals receiving an Fe-fortified diet in the presence of weekly Mn injections should have lower brain Mn concentrations when compared with CN, Mn-treatment alone or Mn-injected animals on an FeD diet.
Our hypothesis was based on both clinical data and molecular evidence suggesting that Mn and Fe use and compete for similar transporters, such as the divalent metal transporter-1 (DMT-1) and the transferrin (Tf)–transferrin receptor (TfR) system (Aschner and Aschner, 1990; Erikson and Aschner, 2006; Malecki et al., 1999). Indeed, the Belgrade rat, which lacks a functional DMT-1 transporter due to a mutation (Burdo et al., 1999), and the hypotransferrinemic mouse, which has reduced transferrin levels (Dickinson et al., 1996), have provided excellent opportunities to study the in vivo interdependency of these two metals. These animals demonstrate dysregulation of both Mn and Fe (Burdo et al., 1999; Chua and Morgan, 1997). Additionally, animals fed an FeD diet from birth show, at weaning, severe changes in numerous metal concentrations in the brain (Garcia et al., 2007). We were thus surprised to observe with independent analytical techniques (AAS and MRI) that animals fed Fe-modified diets (either increased or decreased Fe) while receiving Mn injection accumulated Mn in a regionally specific pattern that was indistinguishable from each other, but quite distinct from the brains of MnT rats (Figs. 3 and 4). This is not unprecedented in the literature, however. Some in vitro experiments involving cultured primary astrocytes have also demonstrated similarity in Mn transport regardless of whether culture media was FeS or FeD (Fitsanakis et al., 2006a). It has also been reported by Chua and Morgan (1996) that Fe overload and deficiency lead to increased brain Mn.
The experimental design for these studies was such that animals never became severely ID or pathologically Fe supplemented. Although both the MnT and FeD groups had decreased body mass compared with CN or FeS animals (Fig. 1), these animals did not have overt health problems. This is consistent with what occurs in human populations, i.e. most people are ID without significant anemia (Umbreit, 2005), and is thus a considerable strength of our current model. Additionally, by extending the study to 14 weeks, we were able to allow animals’ homeostatic mechanisms to readjust to our dietary alterations. This should have also been sufficient time for brain metal levels to equilibrate to the new concentration, allowing us to measure more static amounts of Mn or Fe rather than these metals during any early periods of compensation.
In general, treated animals in our study had regionally specific increased (Fig. 2) brain Mn levels (Figs. 3 and 4). These differences may be related to normal variations in the expression levels of metal-transporting proteins (Connor et al., 2001). Thus, in contrast to the cerebellum and brain stem, the striatum, hippocampus and midbrain generally have higher basal levels of Fe due the increased density of Fe-transporting proteins, which also transport Mn (Fig. 3a). The differences may also help explain the absence of Mn accumulation in areas that are normally metal-rich under basal conditions, such as the striatum and hippocampus. It is likely that the increased Mn levels in cerebellum and brain stem were more easily detectable due to the normally low levels of these metals in the respective regions. It is also known that changes in dietary metal levels can lead to alterations in DMT-1 and TfR under conditions of ID or Fe supplementation throughout an animal’s lifespan (Lin et al., 2006; Siddappa et al., 2003). Additionally, DMT-1 can be upregulated in response to increases in Mn concentrations (Wang et al., 2006).
It is quite surprising to note that FeS and FeD diets did not have opposite effects in brain Mn deposition. As mentioned above, Mn and Fe compete for the same transporter systems. It is, thus, reasonable that under conditions of low Fe, such as times of ID, more Mn could be transported to the brain due to a decrease in Fe availability and a subsequent increase in the number of metal transporters. Indeed, this correlation has been observed by others (Erikson et al., 2002; Garcia et al., 2006, 2007). Therefore, it seemed likely that the converse would hold true: under conditions of high Fe, brain Mn transport would decrease, resulting in a protective effect. This was not the case.
As depicted by both the AAS and MR data (Figs. 3 and 4), there was no statistically significant difference in the regional brain Mn accumulation in the FeD or FeS rats. There could be several reasons for this phenomenon: (1) the affinity of DMT-1 and other transporters, excluding Tf/TfR, is greater for that of Mn than Fe (Gunshin et al., 1997); (2) increased Fe in the FeS population likely lead to downregulation of Fe-transporting proteins, but did not affect other proteins or carriers more specific to Mn transport (Crossgrove et al., 2003); (3) it is known that increases in Fe availability lead to an increase in DMT expression in vitro (Erikson and Aschner, 2006; Roth et al., 2002), which could result in increased Mn transport and deposition. (4) Increased intracellular Fe countrations may reduce the extracellular transport of Ms. Regardless of the reasons, it is important to note that dietary Fe supplementation does not alleviate brain Mn accumulation in this experimental paradigm. In fact, under our experimental conditions, mild ID and Fe repletion both lead to increased brain Mn deposition. Although more work needs to be completed in order to more fully understand the molecular basis for these observations, our data suggest that it may be important to monitor patients or populations receiving high levels of dietary Fe supplementations.
With the potential necessity of following relevant biomarkers, we examined metal RBC levels by AAS, and brain metal deposition via MR technology. Indeed, one unique and timely aspect of incorporating MRI to map the areas of brain Mn accumulation is that it provided us with the opportunity to examine the metal deposition in multiple slices and orientations throughout the entire brain. The ability to generate statistically significant p-maps to compare the various groups presents a novel way to examine these brains (although other regions could have also been studies), and to make comparisons of specific regions in ways that were previously impossible. As the availability of MR increases and becomes more routine, particularly involving populations vulnerable to Mn exposure, it may be important to assess dietary metal consumption as well.
In conclusion, this work has demonstrated that changes in dietary Fe levels result in statistically significant increases in brain Mn accumulation compared with CN animals or those only exposed to Mn. Additionally, we found MRI to be a useful tool for determining the relative changes in the deposition of this paramagnetic metal throughout the entire brain. Further work is needed to examine the relationships between metal dysregulation or dietary Fe manipulation, and the transport of both Mn and Fe to the brain.
FUNDING
Department of Defense Manganese Health Research Project (04149002) to M.A.; and National Institute of Environmental Health and Safety (ES 007331 to MA; R15 ES013791-01) to K.E.
REFERENCES
- Aschner M, and Aschner J (1990). Manganese transport across the blood-brain barrier: Relationship to iron homeostasis. Brain Res. Bull 24, 857–860. [DOI] [PubMed] [Google Scholar]
- Aschner M, and Gannon M (1994). Manganese (Mn) transport across the rat blood-brain barrier: Saturable and transferrin-dependent transport mechanisms. Brain Res. Bull 33, 345–349. [DOI] [PubMed] [Google Scholar]
- Boudia N, Halley R, Kennedy G, Lambert J, Gareau L, and Zayed J (2006). Manganese concentrations in the air of the Montreal (Canada) subway in relation to surface automobile traffic density. Sci. Total Environ 366, 143–147. [DOI] [PubMed] [Google Scholar]
- Burdo J, Martin J, Menzies S, Dolan K, Romano M, Fletcher R, Garrick M, Garrick L, and Connor JR (1999). Cellular distribution of iron in the brain of the Belgrade rat. Neuroscience 93, 1189–1196. [DOI] [PubMed] [Google Scholar]
- Chua A, and Morgan E (1996). Effects of iron deficiency and iron overload on manganese uptake and deposition in the brain and other organs of the rat. Biol. Trace Elem. Res 55, 39–54. [DOI] [PubMed] [Google Scholar]
- Chua A, and Morgan E (1997). Manganese metabolism is impaired in the Belgrade laboratory rat. J. Comp. Physiol. B 167, 361–369. [DOI] [PubMed] [Google Scholar]
- Connor JR, Menzies SL, Burdo JR, and Boyer PJ (2001). Iron and iron management proteins in neurobiology. Pediatr. Neurol 25, 118–129. [DOI] [PubMed] [Google Scholar]
- Crossgrove J, Allen D, Bukaveckas B, Rhineheimer S, and Yokel R (2003). Manganese distribution across the blood-brain barrier. I. Evidence for carrier-mediated influx of manganese citrate as well as manganese and manganese transferrin. Neurotoxicology 24, 3–13. [DOI] [PubMed] [Google Scholar]
- Crowe A, and Morgan E (1992). Transferrin and iron uptake by the brain: Effects of altered iron status. J. Neurochem 57, 1584–1592. [DOI] [PubMed] [Google Scholar]
- Deane R, Zheng W, and Zlokovic B (2004). Brain capillary endothelium and choroid plexus epithelium regulate transport of transferrin-bound and free iron into the rat brain. J. Neurochem 88, 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson T, Devenyi A, and Connor J (1996). Distribution of injected iron 59 and manganese 54 in hypotransferrinemic mice. J. Lab. Clin. Med 128, 270–278. [DOI] [PubMed] [Google Scholar]
- Discalzi G, Pira E, Herrero Hernandez E, Valentini C, Turbiglio M, and Meliga F (2000). Occupational Mn parkinsonism: Magnetic resonance imaging and clinical patterns following CaNa2-EDTA chelation. Neurotoxicology 21, 863–866. [PubMed] [Google Scholar]
- Erikson KM, and Aschner M (2006). Increased manganese uptake by primary astrocyte cultures with altered iron status is mediated primarily by divalent metal transporter. Neurotoxicology 27, 125–130. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Shihabi ZK, Aschner JL, and Aschner M (2002). Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol. Trace Elem. Res 87, 143–156. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Syversen T, Steinnes E, and Aschner M (2004). Globus pallidus: A target brain region for divalent metal accumulation associated with dietary iron deficiency. J. Nutr. Biochem 15, 335–341. [DOI] [PubMed] [Google Scholar]
- Fitsanakis V, Piccola G, Aschner J, and Aschner M (2006a). Characteristics of manganese (Mn) transport in rat brain endothelial (RBE4) cells, an in vitro model of the blood-brain barrier. Neurotoxicology 27, 60–70. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Zhang N, Avison MJ, Gore JC, Aschner JL, and Aschner M (2006b). The use of magnetic resonance imaging (MRI) in the study of manganese neurotoxicity. Neurotoxicology 27, 798–806. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, and Aschner M (2006). A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicol. Sci 92, 516–525. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, and Aschner M (2007). Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol. Sci 95, 205–214. [DOI] [PubMed] [Google Scholar]
- Garrick M, Dolan K, Horbinski C, Ghio A, Higgins D, Porubcin M, Moore E, Hainsworth L, Umbreit J, Conrad M, et al. (2003). DMT1: A mammalian transporter for multiple metals. Biometals. 16, 41–54. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger U, Gunshin Y, Romero M, Boron W, Nussberger S, Gollan J, and Hediger M (1997). Cloning and characterization of a mammalian protoncoupled metal-ion transporter. Nature. 388, 482–488. [DOI] [PubMed] [Google Scholar]
- Hernandez EH, Valentini MC, and Discalzi G (2002). T1-weighted hyperintensity in basal ganglia at brain magnetic resonance imaging: Are different pathologies sharing a common mechanism? Neurotoxicology 23, 669–674. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park JK, Choi Y, Yoo CI, Lee CR, Lee H, Lee JH, Kim SR, Jeong TH, Yoon CS, et al. (2005). Blood manganese concentration is elevated in iron deficiency anemia patients, whereas globus pallidus signal intensity is minimally affected. Neurotoxicology 26, 107–111. [DOI] [PubMed] [Google Scholar]
- Levy BS, and Nassetta WJ (2003). Neurologic effects of manganese in humans: A review. Int. J. Occup. Environ. Health 9, 153–163. [DOI] [PubMed] [Google Scholar]
- Lin XM, Ji CY, Liu WJ, Long Z, and Shen XY (2006). Levels of serum transferrin receptor and its response to Fe-supplement in Fe-deficient children. Br. J. Nutr 96, 1134–1139. [DOI] [PubMed] [Google Scholar]
- Mahoney J, and Small W (1968). Studies on manganese III: The biological half0life of radiomanganese in man and factors which affect this half-life. J. Clin. Invest 47, 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecki E, Cook B, Devenyi A, Beard J, and Connor J (1999). Transferrin is required for normal distribution of 59Fe and 54Mn in mouse brain. J. Neurol. Sci 170, 112–118. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M, Belanger S, Larribe F, Beuter A, Bowler R, Panisset M, Edwards R, DeGeoffroy A, Sassine MP, et al. (1999). Manganese neurotoxicity, a continuum of dysfunction: Results from a community based study. Neurotoxicology 20, 322–327. [PubMed] [Google Scholar]
- Myers JE, teWaterNaude J, Fourie M, Zogoe HB, Naik I, Theodorou P, Tassel H, Daya A, and Thompson ML (2003). Nervous system effects of occupational manganese exposure on South African manganese mineworkers. Neurotoxicology 24, 649–656. [DOI] [PubMed] [Google Scholar]
- Pal P, Samii A, and Calne D (1999). Manganese neurotoxicity: A review of clinical features, imaging and pathology. Neurotoxicology 20, 227–238. [PubMed] [Google Scholar]
- Park NH, Park JK, Choi Y, Yoo CI, Lee CR, Lee H, Kim HK, Kim SR, Jeong TH, and Park J (2003). Whole blood manganese correlates with high signal intensities on T1-weighted MRI in patients with liver cirrhosis. Neurotoxicology 24, 909–915. [DOI] [PubMed] [Google Scholar]
- Picard V, Govoni G, Jabado N, and Gros P (2000). Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. J. Biol. Chem 275, 35738–35745. [DOI] [PubMed] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, and Perlmutter JS (2001). Welding-related parkinsonism: Clinical features, treatment, and pathophysiology. Neurology. 56, 8–13. [DOI] [PubMed] [Google Scholar]
- Racette BA, Tabbal SD, Jennings D, Good L, Perlmutter JS, and Evanoff B (2005). Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology 64, 230–235. [DOI] [PubMed] [Google Scholar]
- Rodier J (1955). Manganese poisoning in Moroccan miners. Br. J. Ind. Med 12, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J, Feng L, Dolan K, Lis A, and Garrick M (2002). Effect of the iron chelator desferrioxamine on manganese-induced toxicity of rat pheochromocytoma (PC12) cells. J. Neurosci. Res 68, 76–83. [DOI] [PubMed] [Google Scholar]
- Siddappa AJ, Rao RB, Wobken JD, Casperson K, Leibold EA, Connor JR, and Georgieff MK (2003). Iron deficiency alters iron regulatory protein and iron transport protein expression in the perinatal rat brain. Pediatr. Res 53, 800–807. [DOI] [PubMed] [Google Scholar]
- Taylor E, Crowe A, and Morgan E (1991). Transferrin and iron uptake by the brain: Effects of altered iron status. J. Neurochem 57, 1584–1592. [DOI] [PubMed] [Google Scholar]
- Umbreit J (2005). Iron deficiency: A concise review. Am. J. Hematol 78, 225–231. [DOI] [PubMed] [Google Scholar]
- Wang X, Li GJ, and Zheng W (2006). Upregulation of DMT1 expression in choroidal epithelia of the blood-CSF barrier following manganese exposure in vitro. Brain Res. 1097, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2003). Micronutrient Deficiencies: Battling Iron Deficiency Anaemia Vol. 2003. World Health Organization WHO/007. http://whglibdoc.who.int/hg/2000/who-nhd-00.7.pdf [Google Scholar]
- Yu IJ, Park JD, Park ES, Song KS, Han KT, Han JH, Chung YH, Choi BS, Chung KH, and Cho MH (2003). Manganese distribution in brains of Sprague-Dawley rats after 60 days of stainless steel welding-fume exposure. Neurotoxicology 24, 777–785. [DOI] [PubMed] [Google Scholar]
- Zayed J, Guessous A, Lambert J, Carrier G, and Philippe S (2003). Estimation of annual Mn emissions from MMT source in the Canadian environment and the Mn pollution index in each province. Sci. Total Environ 312, 147–154. [DOI] [PubMed] [Google Scholar]


