Key Points
Question
What clinical outcomes are associated with preoperative and postoperative exercise training for hip joint replacement?
Findings
In this systematic review and meta-analysis including 32 studies, there was very low– to moderate-quality evidence relevant to preoperative and postoperative supervised exercise interventions. Compared with usual care or no or minimal intervention, supervised exercise interventions were not associated with improved self-reported physical function.
Meaning
These findings suggest that supervised preoperative and postoperative exercise interventions was probably not necessary for patients with total hip replacement.
This systematic review and meta-analysis explores clinical outcomes associated with exercise training before and after hip arthroplasty.
Abstract
Importance
Preoperative and postoperative exercise interventions are commonly used in patients with total hip arthroplasty despite a lack of established efficacy.
Objective
To explore clinical outcomes associated with exercise training before and after hip arthroplasty.
Data Sources
PubMed, Cochrane Central Register of Controlled Trials, Cumulative Index to Nursing and Allied Health Literature, EMBASE, and Google Scholar were searched from their inception to March 2020. Reference lists of included trials and related reviews were also searched.
Study Selection
Randomized clinical trials of land-based exercise interventions before or after total hip arthroplasty were included.
Data Extraction and Synthesis
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. Data extraction was independently performed in duplicate. Random-effects meta-analyses with restricted maximum likelihood were performed for pooling the data.
Main Outcomes and Measures
The primary prespecified outcome was self-reported physical function. Secondary prespecified outcomes were self-reported pain intensity, quality of life, gait speed, lower body muscle strength, lower body flexibility, anxiety, hospital length of stay, and adverse events.
Results
A total of 32 randomized clinical trials with 1753 patients were included in the qualitative synthesis, and 26 studies with 1004 patients were included in the meta-analysis. Compared with usual care or no or minimal intervention, postoperative exercise training was not associated with improved self-reported physical function, with a moderate level of certainty, at 4 weeks (standardized mean difference [SMD], 0.01; 95% CI, −0.18 to 0.20), 12 weeks (SMD, −0.08; 95% CI, −0.23 to 0.07) and 26 weeks (SMD, −0.04; 95% CI, −0.31 to 0.24) postoperatively, and low level of certainty at 1 year after surgical treatment (SMD, 0.01; 95% CI, −0.09 to 0.12). For preoperative exercise interventions, there was no association of exercised training with self-reported physical function compared with the control at the 12-week (SMD, −0.14; 95% CI, −0.61 to 0.32) or 1-year follow-ups (SMD, 0.01; 95% CI, −0.37 to 0.40) with very low certainty, and no association with length of stay (mean difference, −0.21; 95% CI, −0.74 to 0.31) at moderate certainty. Results for postoperative hip muscle strength were rated at very low certainty, with no statistical significance. Meta-analysis could not be performed for other outcomes.
Conclusions and Relevance
This systematic review and meta-analysis found low- to moderate-quality evidence that postoperative exercise interventions were not associated with improved self-reported physical function compared with usual care or no or minimal intervention. Furthermore, there was very low–quality evidence that preoperative exercise programs were not associated with higher self-reported physical function and hospital length of stay compared with usual care or no or minimal intervention.
Introduction
Osteoarthritis is one of the leading causes of pain, disability, and health care resource use worldwide.1 While the disease can affect any joint, the hip is among the most common sites.2,3 There is a growing concern that the incidence, in part owing to the aging population, and subsequent financial burden at both the individual and societal level will continue to increase.1,3
Joint replacement surgery of the hip is cost-effective and clinically relevant in appropriately selected patients.4 The use of primary total hip joint replacement is expected to increase by 71% from 2018 to 2030 (ie, approximately 635 000 total procedures).5 The typical hip joint replacement surgery within the United States costs a patient between $17 763 and $23 9696 and cumulatively costs the US health care system $15 billion per year.7 Notably, this expenditure only represents a proportion of costs associated with this procedure, as subsequent rehabilitation was estimated to cost in excess of $180.4 million per year.8
Preoperative health status (eg, greater muscle strength and capacity to complete activities of daily living) is a factor associated with favorable perioperative outcomes after total joint replacements.9,10 Health-related quality of life has been reported to decrease during the preoperative period,11 which may be further complicated when patient expectations do not align with physical function and quality of life immediately after arthroplasty.12 Furthermore, it is known that patients may still have functional deficits (eg, compromised muscle strength, postural stability, gait speed) up to 2 years after total hip arthroplasty.13,14,15
Preoperative and postoperative care for patients with total hip arthroplasty is generally considered effective for reducing pain intensity and disability; however, robust evidence is lacking.16,17,18 A systematic review by Wang et al,19 including studies up to November 2015, reported minimal improvements associated with preoperative rehabilitation and low levels of certainty of the evidence, although Wang et al analyzed data for rehabilitation of hip and knee joint arthroplasty together. Wang et al19 stated that this result could be explained by the heterogeneity of the included studies owing to types of prehabilitation programs, control group intervention adherence, and fidelity within the programs, which could have impacted the ability to detect any existing differences. This highlights the lack of consensus that preoperative and postoperative exercise interventions are beneficial for patients with total hip arthroplasty. Therefore, we aimed to conduct a systematic review and meta-analysis to determine which clinical outcomes were associated with preoperative and postoperative exercise training after hip arthroplasty when compared with active control and usual care or minimal intervention. We specifically assessed land-based therapy and not hydrotherapy because it is the form of therapy that is mostly applied in physiotherapy clinics owing to lack of access to pools.16 Specifically, we examined the following patient-reported outcomes: disability, pain intensity, gait speed, lower body muscle strength, range of motion of the hip joint, hospital length of stay (LOS), and adverse events. Our primary hypothesis was to determine if there was an association of preoperative or postoperative exercise training after hip arthroplasty with patient-reported outcomes compared with active control and usual care or no or minimal intervention.
Methods
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.17 This systematic review and meta-analysis was prospectively registered with the PROSPERO database.
Search Strategy
Studies were identified by searching multiple databases, including PubMed, Cochrane Central Register of Controlled Trials, Cumulative Index to Nursing and Allied Health Literature, EMBASE, and Google Scholar from their inception to March 2020. The search terms were identified after preliminary searches of the literature and by comparing them against previous systematic reviews.18,20 The search strategy with search terms is presented in eAppendix 1 in the Supplement. This search strategy was modified and applied to the other searched databases. Additional studies were searched through manual research of reference lists of relevant literature reviews16,18,19,20 and via citation tracking of the included studies. Two of us (T.S. and J.Z.) searched the different databases, considering the prespecified inclusion and exclusion criteria, to select potentially relevant trials.
Trials were initially evaluated based on title and abstract, and full-text versions of the relevant studies were obtained and independently evaluated by 2 of us (T.S. and M.H.). Disagreements were settled through discussion among reviewers (T.S. and M.H.). A third reviewer (J.Z.) adjudicated any disagreement.
Inclusion and Exclusion Criteria
Inclusion criteria were patients waiting for total hip arthroplasty or who had already received a total hip arthroplasty, underwent postoperative therapy started after leaving the hospital, performed only a land-based exercise intervention, had preintervention and postintervention measures for all study groups, and had at least 1 measured outcome (ie, self-reported physical function, pain intensity, quality of life, gait speed, muscle strength or range of motion, adverse events, LOS, anxiety). Studies that performed partial hip arthroplasties, revision surgery, or hip resurfacing operations were excluded. As the focus of the review was on exercise training, nonexercise modalities (eg, manual therapy, osteopathy, electrical stimulation, water-based therapy) were excluded. The intervention of interest was defined as land-based exercise training, defined as any program of exercises (eg, aerobic, range-of-motion, resistance, or activity requiring physical effort) prescribed using sets and repetitions.21 Comparators included no intervention, usual or standard care, placebo, and other forms of physiotherapeutic interventions (eg, neuromuscular stimulation, water-based interventions). Studies that analyzed only muscle morphology or architecture as outcomes were not included. Eligible follow-up time points were closest to after the intervention, closest to 4 weeks, closest to 12 weeks, closest to 26 weeks, and closest to 1 year. We included randomized clinical trials (RCTs) in German or English, as prior work has shown adding non-English studies does not significantly impact the effect size estimates.22 Quasi-RCTs and nonrandomized clinical trials were excluded, given that they do not offer an unbiased estimate of the effect size.23
Data Extraction
Study information was extracted by 2 of us (T.S. and J.Z.), with disagreement settled via discussion. Reviewers were not blinded to information regarding the authors, journal, or outcomes for each article reviewed. Reviewers extracted author, sample size, age and sex of patients, type of intervention, setting, frequency, exercise prescription details (ie, volume, duration, effort or exertion, load, progression), start of the intervention (ie, time after operation when the intervention began), follow-up time points, and outcome measures. If a study did not report relevant data for extraction, the corresponding author was contacted.
Risk of Bias Assessment and GRADE
Risk of bias was assessed via the Cochrane Risk of Bias Tool version 2.024 for all 5 domains: randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of the reported result. An overall risk of bias judgement was made for each outcome and each time point. Two independent assessors (T.S. and M.H.) performed the assessment. Disagreements were resolved through discussion or by a third reviewer (J.Z.) if necessary.
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method to appraise the certainty of evidence.25,26 All ratings started at a high level of certainty given guidelines for meta-analyses including RCTs only. Two of us (T.S. and J.Z.) downgraded evidence based on risk of bias, inconsistency, indirectness, imprecision, and publication bias. A GRADE assessment was completed for each individual meta-analysis.
Statistical Analysis
For data analysis, we created 2 categories of comparators: usual care or no or minimal intervention and active control (eg, combined different types of interventions such as conventional rehabilitation, pool-based exercises, stretching and mobility exercises, neuromuscular stimulation, and isometric exercises with a progressive and supervised character). Our primary outcome measure was self-reported physical function, such as the Harris Hip Score, Hip disability and Osteoarthritis Outcome Score, Western Ontario and McMaster Universities Osteoarthritis Index, and Oxford Hip and Knee Scores. Secondary outcomes were self-reported pain intensity (eg, Visual Analog Scale, Numeric Rating Scale for pain), quality of life (eg, 36-item Short-Form Health Survey, 12-item Short-Form Health Survey, European Quality of Life–5 Dimensions), gait speed, lower body muscle strength (eg, stair climbing in seconds, sit to stand in seconds), lower body flexibility (degrees of joint range of motion), anxiety, hospital LOS, and adverse events. If more than 1 outcome measure was reported for each type of outcome in the same study, only 1 was considered for further analysis.
Data reported as nonparametric variables (eg, median and interquartile range), or measure of spread reported as 95% CI or SE of the mean, were converted to mean and SD using established formulae.25,27,28,29 Transformations of the median were calculated via a web-based calculator.30 Data that were not reported numerically were extracted via Graph Digitizer software version 2.26 (GetData)30 from published figures. Missing SDs were imputed using established methods.25,31 Change from baseline data were transformed as recommended by the Cochrane Collaboration25 and Morris and DeShon.31 When only change from baseline data were reported we assumed a conservative correlation coefficient of r = 0.9, between the SD of the change scores and the preintervention and postintervention SDs (which we assumed were equal) to calculate a standardized mean difference (SMD) as SDchange = SDpreintervention/postintervention × √(2 × [1 − r]).31 Meta-analysis was conducted if at least 3 studies were available for an outcome. A random effects meta-analysis was used for all continuous outcomes with a restricted maximum likelihood estimator for the between study variance T2. We used the Hartung-Knapp-Sidik-Jonkman method for estimating the variance of the pooled effect. This method substantially outperforms the DerSimonian-Laird method,32 especially if the number of studies is small or there is substantial heterogeneity.33,34,35 Measures of heterogeneity used were Cochrane Q and the resulting χ2 statistic and I2. We used 95% prediction intervals (PIs) to assess the amount of heterogeneity if there were at least 10 studies in the meta-analysis.36 Publication bias was assessed via funnel plots, Egger test, trim and fill methods, and P curve analysis if at least 10 studies were included in the meta-analysis.37 We performed sensitivity analysis via outlier identification and influence analysis38,39 and also performed a sensitivity analysis40 by conducting all meta-analytic summaries with the standard approach of calculating the 95% CI for the pooled effect. We incorporated all findings of the sensitivity analyses in GRADE (ie, imprecision). If meta-analysis was not possible, we used the structured reporting of effects and calculated effect sizes with a 95% CI and rated the evidence according to their risk of bias.25
Effect size measures for continuous outcomes were either the SMD25 or mean difference (MD) with 95% CIs. We used SMDs because they allow comparison of different outcome measurement scales adopted by each study. SMD effect size was interpreted as small, 0.20; medium, 0.50; or large, 0.80.41 Effect sizes were calculated from final means with SDs and sample sizes for the intervention and control groups. A negative value signified an advantage for the intervention group. Cluster RCTs were handled according to the Cochrane handbook by calculating a design effect.25 Selected studies for which these or other crucial data were not directly reported or obtainable by contacting authors were not included in the review. All calculations and graphics were performed with the statistical and computing environment R version 4.0.2 (R Project for Statistical Computing)42 and the extension packages Meta,43 Metafor44 and dmetar.45 Two-sided 95% CIs were used. Statistical significance was set at α = .05 for all analyses. Data were analyzed in August 2020.
Results
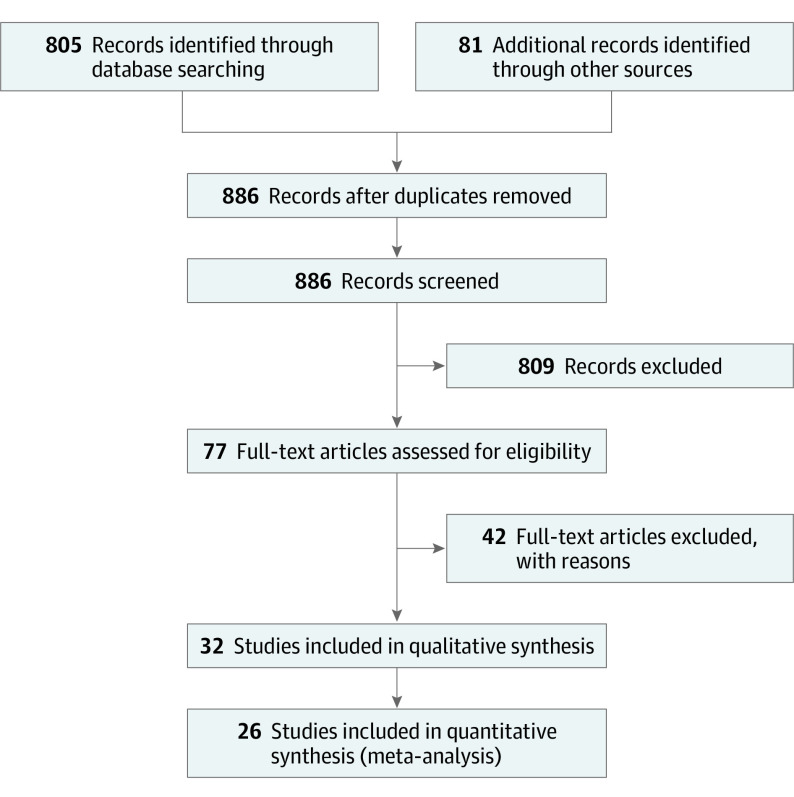
We identified 886 study records through database searches and manual research of reference lists of relevant literature reviews. After removing duplicates and screening titles and abstracts of all remaining unique articles, 77 full text articles were assessed for eligibility. We included 35 records, with 9 records for preoperative interventions46,47,48,49,50,51,52,53,54 and 26 records for postoperative interventions,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80 and we excluded 42 records (Figure 1). Literature sources and reasons for exclusion of ineligible studies are reported in eAppendix 2 in the Supplement. Two records for Husby et al61,62 and 3 records for Maire et al65,66,67 were considered the same study for all analyses. Subsequently, 32 studies (9 preoperative studies and 23 postoperative studies) were included in the qualitative synthesis, and 26 studies were included in the quantitative synthesis (meta-analysis).
Figure 1. Study Inclusion Flowchart.
eAppendix 2 in the Supplement provides reasons for the exclusion of 42 full-text articles.
Study Characteristics
The characteristics of included articles are shown in Table 1. Sample sizes for preoperative studies ranged from 6 to 43 patients, with a mean (SD) sample size of 26 (14) patients and total sample across all studies of 501 patients. For postoperative studies, the sample size ranged from 7 to 80 patients, with a mean (SD) sample size of 28 (19) patients and total sample across all studies of 1252 patients. Preoperative programs had a typical duration of 4 to 12 weeks (range, 1-12 weeks) and were performed for 2 to 7 days per week (mean [SD], 6.6 [1.7] days per week). Postoperative training interventions had a typical duration of 6 to 12 weeks (range, 3-12 weeks) and were performed for 2 to 7 days per week (mean [SD], 3.9 [1.3] days per week).
Table 1. Characteristics of the Included Studies.
| Source | Intervention group/control group | Intervention group | Control group(s) | Startc | Follow-upd | Outcome measure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size, No. | Age, mean (SD), y | Women, % | Type of intervention | Settinga | Frequency | Volume and durationb | Type of intervention | Settinga | Frequency | Volume and durationb | ||||
| Preoperative studies | ||||||||||||||
| Bitterli et al,54 2011 | 41/39 | 65.4 (10.8)/68.4 (9.7) | 46/31 | Sensorimotor training | HB | 2×/d for 2-6 wk | 6 Exercises for 10 reps each/NR | None | NR | NR | NA | 2-6 wks BO | 4 mo and 1 y | SF-36, WOMAC, BBS |
| Doiron-Cadrin et al,46 2019 | 6/5/6 | 69.9 (9.1)/61.3 (8.1)/66.7 (9.2) for hip and knee combined | 64/83/73 | Telerehabilitation (resistance, mobility, and proprioceptive exercise) | HB | 2×/wk for 12 wk | 6 Exercise for 2 sets of 10 reps/NR | C1: rehabilitation C2: usual care | C1: S; C2: S | C1: exercise 2×/wk for 12 wk ; C2: 1 education session | C1: 6 exercise for 2 sets of 10 reps; C2: NA | NR | ≥12 wk | LEFS, WOMAC, SF-36, SPW, TST, TUG |
| Ferrara et al,47 2008 | 11/12 | 63.8 (9.0)/63.1 (6.9) | 64/58 | Group and individual exercise (resistance, flexibility, and cardiovascular exercise) | S | 5 d/wk for 4 wk | 3-4 Sets of 8-12 reps, 10-15 min cardiovascular exercise/40 min in groups, 20 min individual therapy | None | S | NR | NR | 1 mo BO | 3 mo | BMRC, WOMAC, SF-36, BI, VAS, hip-ROM |
| Gill et al,48 2009 | 40/42 | 71.6 (8.9)/69.2 (10.5) | 57/67 | Land-based group exercise + HB exercise (cardiovascular or other exercise) | S | 2 d/wk + 3 d HB exercise for 6 wk | 4 Exercises for 2 sets of 10 reps/1 h + 30 min HB exercise | Pool-based group exercise + HB exercise (cardiovascular or other exercise) | S and HB | S: 2 d/week + 3 d HB: 6 wk |
1.5 h HB exercise, 4 exercise for 2 sets of 10 reps | NR | 7 wk and 5 wk | WOMAC, 50FTW, 30CST, SF36 MCS |
| Gocen et al,49 2004 | 30/30 | 46.9 (11.5)/55.5 (14.4) | 43/27 | Lower body stretching + upper body strengthening | PS | 3×/d, duration NR | 1 Set of 10 reps/NR | None | NA | NA | NA | 8 wk BO | Discharge, 3 mo, and 2 y | HHS, VAS, hip-ROM |
| Holsgaard-Larsen et al,50 2020 | 40/40 | 70.0 (7.7)/70.8 (7.5) | 68/63 | Explosive resistance training for lower body | S | 2×/wk for 10 wk | 10-min warm-up; 4 exercise for 3 sets of 8-12 reps/1 h | Usual care | NA | NA | NA | NR | Postintervention (BO), 3 mo, 6 mo, 9 mo, and 12 mo | HOOS, gait speed, chair raise test, muscle strength, body composition |
| Oosting et al,51 2012 | 15/15 | 76.9 (6.3)/75.0 (6.3) | 93/67 | Functional exercise + walking capacity + encouragement for HB exercise | S | 2×/wk for 3-6 wk | NR/encouraged to walk ≤30 min/d | Usual care | NA | NA | NA | 3-6 wk BO | 6 wk | Feasibility, TUG, CRT, 6MWT, VAS, HOOS, LAPAQ |
| Vukomanović et al,53 2008 | 23/22 | 60.1 (11.0)/56.2 (18.5) | 70/80 | Functional exercise | S | 2 classes | NR/NR | None | NA | NA | NA | NR | Discharge and 15 mo | VAS, HHS, JOA |
| Villadsen et al,52 2014 | 43/41 | 68.7 (8.4)/68.6 (7.1) | 51/51 | Neuromuscular exercise program | S | 2×/wk for 8 wk | 2-3 Sets of 10-15 reps/1 h | Preoperative education | NA | NA | NA | 8 wk BO | 4 wk and 12 wk | HOOS, EQ5D |
| Postoperative studies | ||||||||||||||
| Austin et al,55 2017 | 60/60 | 61.2 (8.4)/62.3 (12.7) | 38/52 | Physical therapy + HB exercise | S | 2× to 3×/wk for 10 wk | NR/NR | HB exercise with manual | HB | 10 wk | NR/NR | AO | 1 mo, and 6-12 mo | HHS, WOMAC, SF-36 |
| Beck et al,56 2019 | 80/80 | NR/NR | 53/64 | Sports therapy (resistance exercise, cardiovascular exercise, flexibility exercise) | S | 50 units | NR/45 min | None | NA | NA | NA | 6 wk AO | 6 (±1 mo), 12 (±3 mo) | Isokinetic strength of hip, postural stability, lactate threshold, WOMAC, HHS, EQ-5D |
| Bodén and Adolphson,57 2004 | 10/10 | 54 (NR)/55 (NR) | NR/NR | Full weight bearing (pressure sensor) and HB exercise | HB | NR | NA | Late weight-bearing | HB | NA | NA | AO | 2 y | HHS, bone density, bone remodeling, prostheses fixation |
| Coulter et al,58 2017 | 56/42 | 66 (NR)/63 (NR) | 64/50 | Physiotherapeutic exercise (resistance exercise + functional exercise) | S | 1×/wk for 4 wk + HB exercise | NR/NR | Manual of resistance exercise | HB | NR | NR/NR | AO | 5 wk, 12 wk, and 26 wk | WOMAC, SF-36, TUG, PSC, UCLA activity index |
| Galea et al,59 2008 | 11/12 | 68.6 (9.7)/66.6 (7.9) | 73/67 | Functional exercise + resistance exercise | S | 2×/wk for 8 wk | 7 Exercises/45 min | Functional exercise + resistance exercise | HB | 8 wk | NR | 8 wk AO | Postintervention | TUG, SCT, 6MWT, WOMAC, AQoL, gait parameters |
| Heiberg et al,60 2012 | 35/33 | 70.2 (66.5)/70.6 (68.4) | 35/33 | Functional exercise + resistance exercise | S | 2×/wk for 6 wk | NR/70 min | None | NA | NA | NA | 3 mo AO | 5 mo and 12 mo | SCT, 6MWT, hip-ROM, HOOS, HHS, self-efficacy, IMF |
| Husby et al, 200961 and 201062 | 12/12 | 58 (5)/56 (8) | 58/67 | Conventional rehabilitation + resistance exercise | S | 5 d/wk for 4 wk | Sling exercise + leg press and hip ABD/1 h and 10 min warmup and 4 sets of 5 reps | Conventional rehabilitation | S | 5 d/wk for 4 wk | Sling exercise /1 h/NR | 1 wk AO | 1 wk, 5 wk, 6 mo, and 12 mo | Strength, gait pattern, cardiovascular parameters, SF-36 |
| Jan et al,63 2004 | 13/13/27 | 58.8 (12.9)/59.3 (10.3)/57.0 (12.8) | 30/38/63 | Flexibility, resistance exercise, walking | HB | 7 d/wk for 12 wk | 6 Exercises for 2 sets of 10 reps/30 min walk | C1: same as I (low adherence); C2: No intervention | C1: S; C2: NA | C1: 7 d/wk for 12 wk; C2: NA | C1: 6 exercise for 2 sets of 10 reps/30 min walk; C2: NA | 1.5 y AO | 1 wk | Hip strength, gait speed, HHS |
| Johnsson et al,64 1988 | 14/16 | 70 (58-76)/66 (50-74) | NR | Resistance exercise, walking | NR | NR | NR/NR | None | NA | NA | 2 mo AO | 6 mo | Hip strength, hip ROM | |
| Maire et al, 2003,65 2004,66 and 200667 | 7/7 | 77 (NR)/77 (NR) | 86/86 | Traditional rehabilitation + arm interval ergometer | S | 3×/wk for 6 wk | Intervals 6 × 5 min (4 min base work 1 min peak work)/30 min | Traditional rehabilitation | S | NR | NR | 1 wk AO | 2 mo and 1 y | 6MWT, WOMAC, walking test distance |
| Mikkelsen et al,69 2012 | 25/21 | 67.7 (7)/66.8 (8) | 48/48 | Resistance + stretching exercise + mobility exercise | HB | 2×/d for 7 d/wk | 10 Reps per exercise/NR | Stretching + mobility exercise | H | 2×/d for 7×/wk | 10 Reps per exercise/NR | 1 d AO | 4 wk an 12 wk | Adherence, PAS, gait speed, hip strength, balance, WOMAC, EQ-5D |
| Mikkelsen et al,68 2014 | 32/30 | 64.8 (8)/65.1 (10) | 44/40 | Resistance exercise + HB exercise | S and HB | S:2×/wk; HB:5×/wk | 3 Sets with 10-12 reps (wk 1), 10 reps (wk 2-5), and 8 reps (wk 6-10)/35-50 min | Stretching + mobility exercise | H/ | 2×/d, 7 d/wk | 2 Sets of 10 reps/NR | 1 wk AO | 2 wk, 4 wk, 6 wk, 10 wk, 6 mo, and 12 mo | Leg extension power, 20MWT, 30CST, HOOS |
| Mitrovic et al,70 2016 | 35/35 | 69.2 (6.3)/68.1 (6.4) | 63/77 | Standard rehabilitation program (lower body exercise) + additional upper body exercise | PS and HB | PS: 2×/d for 5 d/wk for 6 wk; HB: 6 wk | NR/45 min | Standard rehabilitation program (lower body exercise) | PS and HB | 2×/d for 5 d/wk for 6 wk; HB: 6 wk exercise | NR/30 min | 1 d AO | 2 wk and 12 wk | HHS, Hand grip strength, SF-36, Program tolerance |
| Monhagan et al,71 2016 | 32/31 | 68 (8)/69 (9) | 37/26 | Functional exercise + usual care | S | 2×/wk for 6 wk | 12 Exercises for 15 reps/35 min | Usual care | S | 2×/wk for 6 wk | 15 Reps/exercise for 7 isometric exercise | 12 wk AO | 18 wk | WOMAC, VAS, 6MWT, SF-12 |
| Monticone et al,72 2014 | 50/50 | 69.5 (7.5)/68.8 (8.1) | 64/56 | Task oriented exercise + full weight bearing + ergometer cycling | S | 5×/wk for 3 wk | NR/90 min | Open chain exercise + partial weight bearing | S | 5×/wk for 3 wk | NR/90 min | 4-7 d AO | Posttreatment and 1 y | WOMAC, NRS, FIM, SF-36 |
| Morishima et al,73 2014 | 14/14 | 60.3 (7.4)/59.9 (5.4) | NR | Interval walking | HB | 60 min/wk of fast walking for 12 wk | ≥5 Sets of 2-3 min low intensity V̇o2 peak followed by 3 min high intensity | None | NA | NA | NA | 2 mo AO | 1 wk | Thigh muscle strength, V̇o2 peak, energy expenditure |
| Nanakaku et al,74 2016 | 14/14 | 60.5 (6.4)/60.8 (7.5) | 86/86 | Resistance exercise + hip external rotator strengthening | S | 5×/wk for 4 wk | 3 Sets of 8-12 reps/NR | Resistance exercise | S | 5×/wk for 4 wk | 3 Sets of 8-12 reps/NR | 3 d AO | Posttreatment | Hip pain, Hip muscle strength, Hip-ROM, TUG |
| Nelson et al,75 2019 | 35/35 | 62 (9)/67 (11) | 66/60 | Telerehabilitation (resistance exercise) | HB | 1× to 2×/wk for 30 min | NR/NR | In-person resistance exercise | S and HB | 3× for 30 min; HB: 6 wk exercise | NR/NR | 2 wk after discharge | 6 wk and 6 mo | HOOS, SF-12, EQ-5D |
| Okoro et al,76 2016 | 25/24 | 65.2 (9.1)/66.3 (11.0) | 60/42 | Resistance exercise | HB | 5×/wk for 6 wk | 3-10 Reps/NR | Standard rehabilitation | S | NR | NR/NR | 4-7 d AO | 9-12 mo | Strength, CRT, TUG, TST, 6MWT, lean mass |
| Suetta et al,77 2004 | 13/12/11 | 69 (NR)/68 (NR)/69 (NR) | 46/58/55 | Resistance exercise | S | 3×/wk for 12 wk | 3-5 Sets with 8-20 reps | C1: NMES; C2: HB exercise | C1: HB; C2: HB | C1: 1 h/d; C2: NR | C1: 1 h; C2: NR | 7 d AO | 5 wk and 12 wk | Gait speed, CRT in 5 s, TST, muscle strength |
| Trudelle-Jackson et al,78 2004 | 18/16 | 59.4 (10.8)/59.6 (12.1) | 43/64 | Weight-bearing exercise | HB | 3× to 4×/wk for 8 wk | 1-2 Sets with 15-20 reps/NR | Isometric exercise, flexibility exercise | HB | 3× to 4×/wk for 8 wk | 1-2 Sets with 15-20 reps/NR | 4-12 mo AO | Posttreatment | Hip muscle strength, postural stability, fear of falling, HQ-12 |
| Unlu et al,79 2007 | 9/8/9 | 45.4 (8.7)/57.8 (7.5)/52.6 (10.3) | 78/75/56 | Exercise program (stretching + resistance exercise) | HB | 2×/d for 6 wk | NR/NR | C1: Inpatient therapy; C2: no intervention | C1: S; C2: NA | C1: NR; C2: NA | NR/NR | 12-24 mo AO | Posttreatment | Hip muscle strength, gait parameters |
| Winther et al,80 2018 | 31/29 | 61 (NR)/66 (NR) | 45/52 | Resistance exercise | S | 3×/wk for 3 mo | 2 Exercises with 4 sets with 5 reps/NR | Conventional rehabilitation | S | NR | NR/NR | After discharge | 3 mo, 6 mo, and 12 mo | 6MWT, hip strength, NRS, HHS, HOOS-PS |
Abbreviations: ABD, abduction; AO, after operation; AQoL, assessment of quality of life; BBS, Biodex Balance System; BI, Barthel Index; BMRC, British Medical Research Council; BO, before operation; C1, control group 1; C2, control group 2; CRT, chair rise time; 30CST, 30-second chair stand test; FIM, Functional Independence Measure; 50FTW, 50-foot timed walk; HB, home-based; HHS, Harris Hip Score; HOOS, Hip disability and Osteoarthritis Outcome Score; HOOS-PS, HOOS Physical Function Short Form; HQ-12, 12-Item Hip Questionnaire; LAPAQ, Longitudinal Aging Study Amsterdam Physical Activity Questionnaire; IMF, Index of Muscle Function; JOA, Japanese Orthopaedic Association; LEFS, Lower Extremity Functional Scale; MCS, Mental Component Score; 6MWT, 6 minute walk test; NA, not applicable; NMES, neuromuscular electrical stimulation; NR, not reported; NRS, Numerical Rating Scale; PAS, Physical Activity Scale; PS, partially supervised; PSC, patient-specific concerns; rep, repetition; ROM, range of motion; S, supervised; SCT, stair climb test; SF-12, Short Form 12 item; SF-36, 36-Item Short-Form Health Survey; SPW, self-paced walk; TST, timed stair tests; TUG, timed up-and-go; VAS, visual analog scale; V̇o2, oxygen consumption; WOMAC, Western Ontario McMaster Osteoarthritis Index.
Setting can be HB, S, or PS.
Number of sets and reps, duration in minutes.
Start of the intervention.
Shown as time since total hip arthroplasty.
Data Synthesis
For the trial by Jan et al,63 we pooled the 2 intervention groups together, given that they were presented as low and high adherence groups for the same intervention. The trial by Mikkelsen et al69 was a cluster RCT. The sample size was not affected by the design of the trial, given that the design effect was 1 (intracluster correlation coefficient = 0).25 For 5 trials,50,52,55,78,80 we could not obtain all relevant trial data for our analyses, even after contacting the authors. Four trials56,65,69,78 reported the median values and either range or percentiles. Winther et al80 reported pain intensity outcomes only via a graph. Trudelle-Jackson et al78 reported muscle strength outcomes only via a graph. We imputed these values via Graph Digitizer. We imputed posttest SDs for 2 trials.52,55 In both trials, we used the pooled pretest SDs of the respective trial to calculate an SMD. The trial by Holsgaard-Larsen et al50 reported change from baseline data only, which we transformed assuming a conservative correlation coefficient between the preintervention and postintervention SD of r = 0.9. We also assumed that the preintervention and postintervention SDs of the change scores were equal.31 We performed a sensitivity analysis with a less conservative value of r = 0.5 to determine if the results of the meta-analysis would be markedly changed, and they were not (eAppendix 7 in the Supplement).
Preoperative studies were classified as usual care or no or minimal intervention46,47,49,50,51,52,53,54 vs active control.46,48 One study by Doiron-Cadrin et al46 contributed to both comparator categories. For postoperative studies we categorized the studies as active control57,59,65,66,67,70,72,75,76,78,79 and usual care or no or minimal intervention.55,56,58,59,60,61,62,63,64,68,69,71,73,74,77,79,80 Two studies77,79 contributed to 2 comparator categories. We did not calculate 95% PIs owing to the low number of studies.36 Assessment of publication bias was also not performed owing to the small number of studies.37
Risk of Bias and GRADE Assessment
We assessed the risk of bias of every outcome for every follow-up time point with the Cochrane Risk of Bias Tool 2.0 (eAppendix 3 in the Supplement). Summary risk of bias plots were created for meta-analytic outcomes only (eAppendix 4 in the Supplement). No study outcome was rated as low risk of bias. The study outcomes were rated overall with some concerns or with a high risk of bias. The certainty of the evidence was rated as very low for meta-analytic outcomes of self-reported physical function of preoperative studies, and the certainty of evidence for hospital LOS was rated as moderate. For the postoperative outcomes, the evidence was rated as very low to moderate for self-reported physical function and very low for hip muscle strength (eAppendix 5 in the Supplement). The main reasons for downgrading the evidence were risk of bias, inconsistency, and imprecision. Publication bias could not be assessed because the number of studies was fewer than 10. Indirectness was not a problem, as this review encompasses specific populations, types of interventions, and outcome measures.
Meta-analysis
A meta-analytic summary for the primary outcome (ie, self-reported physical function), hospital LOS, and hip muscle strength are shown in Table 2. The other secondary outcomes could not be pooled because of a lack of a sufficient number of studies for an outcome (ie, <3). This was owing to not reporting for a certain follow-up time point, belonging to a different comparator group, and not reporting the outcome for the analysis. These results were summarized with a risk of bias rating and calculated effect sizes for all studies not included in a meta-analytic summary (eAppendix 6 in the Supplement). The secondary outcome of anxiety could not be assessed, as this was not reported.
Table 2. Certainty of Evidence .
| Outcome | Studies included in meta-analysis | Standardized mean difference (95% CI) | I2 (95% CI), % | Studies, No. | Certainty rating | Reasons for downgrade |
|---|---|---|---|---|---|---|
| Preoperative exercise | ||||||
| Function, follow-up | ||||||
| Closest to 1-ya | Bitterli et al,54 2011; Gocen et al,49 2004; Holsgaard-Larsen et al,50 2020; Vukomanović et al,53 2008 | 0.01 to (−0.37 to 0.40) | 34 to (0 to 77) | 4 | Very low | Risk of bias, inconsistence, imprecision |
| Closest to 12-wka | Bitterli et al,54 2011; Doiron-Cadrin et al,46 2019; Ferrara et al,47 2008; Gocen et al,49 2004; Holsgaard-Larsen et al,50 2020; Villadsen et al,52 2014 | −0.14 to (−0.61 to 0.32) | 51 to (0 to 81) | 6 | Very low | Risk of bias, inconsistence, imprecision |
| Preoperative exercise | ||||||
| Length of staya | Bitterli et al,54 2011; Oosting et al,51 2012; Vukomanović et al,53 2008 | −0.21 (−0.74 to 0.31) | 0.0 (0.0 to 13.4) | 3 | Moderate | Risk of bias |
| Postoperative exercise | ||||||
| Function, follow-up | ||||||
| Closest to 1 ya | Austin et al,55 2017; Beck et al,56 2019; Heiberg et al,60 2012; Mikkelsen et al,68 2014; Winther et al,80 2018 | 0.01 to (−0.09 to 0.12) | 0 to (0 to 0) | 5 | Low | Risk of bias, imprecision |
| Closest to 26 weeksa | Beck et al,56 2019; Coulter et al,58 2017; Heiberg et al,60 2012; Mikkelsen et al,68 2014; Monhagan et al,71 2016 | −0.04 to (−0.31 to 0.24) | 0 to (0 to 79) | 5 | Moderate | Imprecision |
| Closest to 12 weeksa | Coulter et al,58 2017; Mikkelsen et al,69 2012; Mikkelsen et al,68 2014; Winther et al,80 2018 | −0.08 to (−0.23 to 0.07) | 0 to (0 to 0) | 4 | Moderate | Imprecision |
| Closest to 4 weeksa | Austin et al,55 2017; Coulter et al,58 2017; Mikkelsen et al,69 2012; Mikkelsen et al,68 2014 | 0.01 to (−0.18 to 0.20) | 0 to (0 to 37) | 4 | Moderate | Imprecision |
| Closest to 1 yb | Bodén and Adolphson,57 2004; Maire et al, 2003,65 2004,66 and 200667; Monticone et al,72 2014 | −0.68 to (−2.25 to 0.88) | 52 to (0 to 86) | 3 | Very low | Risk of bias, inconsistence, imprecision |
| Closest to after the interventionb | Mitrovic et al,70 2016; Monticone et al,72 2014; Trudelle-Jackson et al,78 2004 | −0.57 to (−1.44 to 0.30) | 38 to (0 to 81) | 3 | Very low | Inconsistency, imprecision |
| Hip abduction strength, follow-up | ||||||
| Closest to 1 ya | Beck et al,56 2019; Husby et al, 200961 and 201062; Winther et al,80 2018 | −0.19 to (−0.70 to 0.31) | 0 to (0 to 82) | 3 | Very low | Risk of bias, inconsistence, imprecision |
| Closest to 26 weeksa | Beck et al,56 2019; Husby et al, 200961 and 201062; Johnsson et al,64 1988; Mikkelsen et al,68 2014; Winther et al,80 2018 | −0.39 to (−0.91 to 0.13) | 55 to (0 to 83) | 5 | Very low | Risk of bias, inconsistence, imprecision |
| Hip flexion strength, follow-up closest to 26 weeksa | Beck et al,56 2019; Johnsson et al,64 1988; Mikkelsen et al,68 2014 | −0.20 to (−1.29 to 0.90) | 58 to (0 to 88) | 3 | Very low | Risk of bias, inconsistence, imprecision |
| Hip abduction strength, follow-up | ||||||
| Closest to 12 weeksa | Mikkelsen et al,69 2012; Mikkelsen et al,68 2014 Winther et al,80 2018 | −0.26 to (−1.28 to 0.76) | 54 to (0 to 87) | 3 | Very low | Inconsistence, imprecision |
| Closest to 4 weeksa | Husby et al, 200961 and 201062; Mikkelsen et al,69 2012; Mikkelsen et al,68 2014 | −0.49 to (−2.61 to 1.64) | 79 to (34 to 94) | 3 | Very low | Risk of bias, inconsistence, imprecision |
| Closest to after the interventiona | Husby et al, 200961 and 201062; Jan et al,63 2004; Nanakaku et al,74 2016; Unlu et al,79 2007 | −0.46 to (−1.57 to 0.65) | 65 to (0 to 88) | 4 | Very low | Risk of bias, inconsistence, imprecision |
Compared with usual care or no or minimal intervention.
Compared with an active control.
Preoperative Exercise Interventions and the Primary Outcome of Self-Reported Function
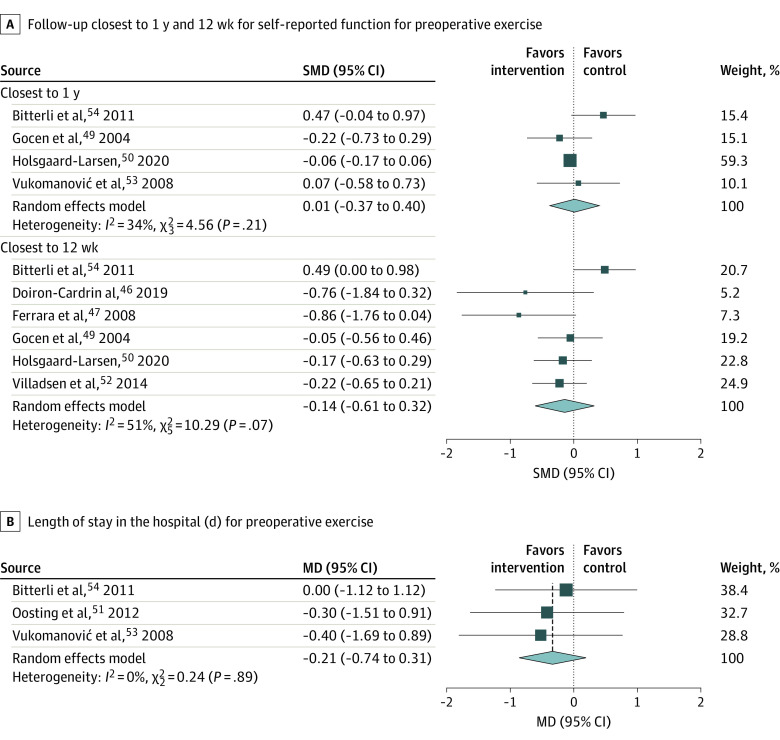
A total of 7 studies46,47,49,50,52,53,54 were included in the meta-analyses. Meta-analysis could be performed for self-reported physical function for exercise training compared with usual care or no or minimal intervention at the time points closest to 1 year49,50,53,54 and 12 weeks46,47,49,50,52,54 of follow-up (Figure 2A). There was no significant effect size in favor of the usual care or no or minimal control group (4 studies: SMD, 0.01 [95% CI, −0.37 to 0.40]; I2 = 34.2% [95% CI, 0% to 76.9%]) or in favor of the intervention group (6 studies: SMD, −0.14 [95% CI, −0.61 to 0.32]; I2 = 51.0% [95% CI, 0% to 80.6%]) at 1-year and 12-week follow-up. All estimates were rated at a very low level of certainty per GRADE (eAppendix 5 in the Supplement). A meta-analysis could not be performed for intervention vs active control owing to a paucity of studies.
Figure 2. Outcomes for Preoperative Exercise Intervention vs Usual Care or No or Minimal Intervention at Follow-ups .
Individual study outcomes are indicated with squares, and larger squares indicate more study weight. Diamonds indicate overall finding; MD, mean difference; and SMD, standardized mean difference.
Preoperative Exercise Interventions and the Secondary Outcome of Hospital LOS
Three51,53,54 studies reported on hospital LOS. Compared with usual care or no or minimal intervention, we found a no association of preoperative exercise with hospital LOS (3 studies: MD, −0.21 [95% CI, −0.74 to 0.31]; I2 = 0% [95% CI, 0% to 13.4%]) at a moderate rating of certainty (Figure 2B).
Postoperative Exercise Interventions and the Primary Outcome of Self-Reported Function
A total of 8 studies55,56,58,60,68,69,71,80 were included in the meta-analyses. We conducted 4 meta-analyses comparing exercise interventions with usual care or no or minimal intervention at the follow-up closest to 1 year,55,56,60,68,80 26 weeks,56,58,60,68,71 12 weeks,58,68,69,80 and 4 weeks55,58,68,69 (Figure 3). At the follow-up closest to 1 year, we found no statistically significant association of postoperative exercise with physical function (5 studies: SMD, 0.01 [95% CI, −0.09 to 0.12]; I2 = 0.0% [95% CI, 0% to 0%]) with a low level of certainty on GRADE (eAppendix 5 in the Supplement). At the follow-up closest to 26 weeks, we no statistically significant association of postintervention exercise with physical function for the intervention compared with the usual care or no or minimal intervention (5 studies: SMD, −0.04 [95% CI, −0.31 to 0.24]; I2 = 0% [95% CI, 0% to 79.1%]). There was no significant association for the intervention group at the follow-up closest to 12 weeks (4 studies: SMD, −0.08 [95% CI, −0.23 to 0.07]; I2 = 0% [95% CI, 0% to 0%]) or at the follow-up closest to 4 weeks (4 studies: SMD, 0.01 [95% CI, −0.18 to 0.20]; I2 = 0% [95% CI, 0% to 37.3%]). These results all had a moderate GRADE rating.
Figure 3. Outcomes for Postoperative Exercise Intervention vs Usual Care or No or Minimal Intervention at Follow-ups.
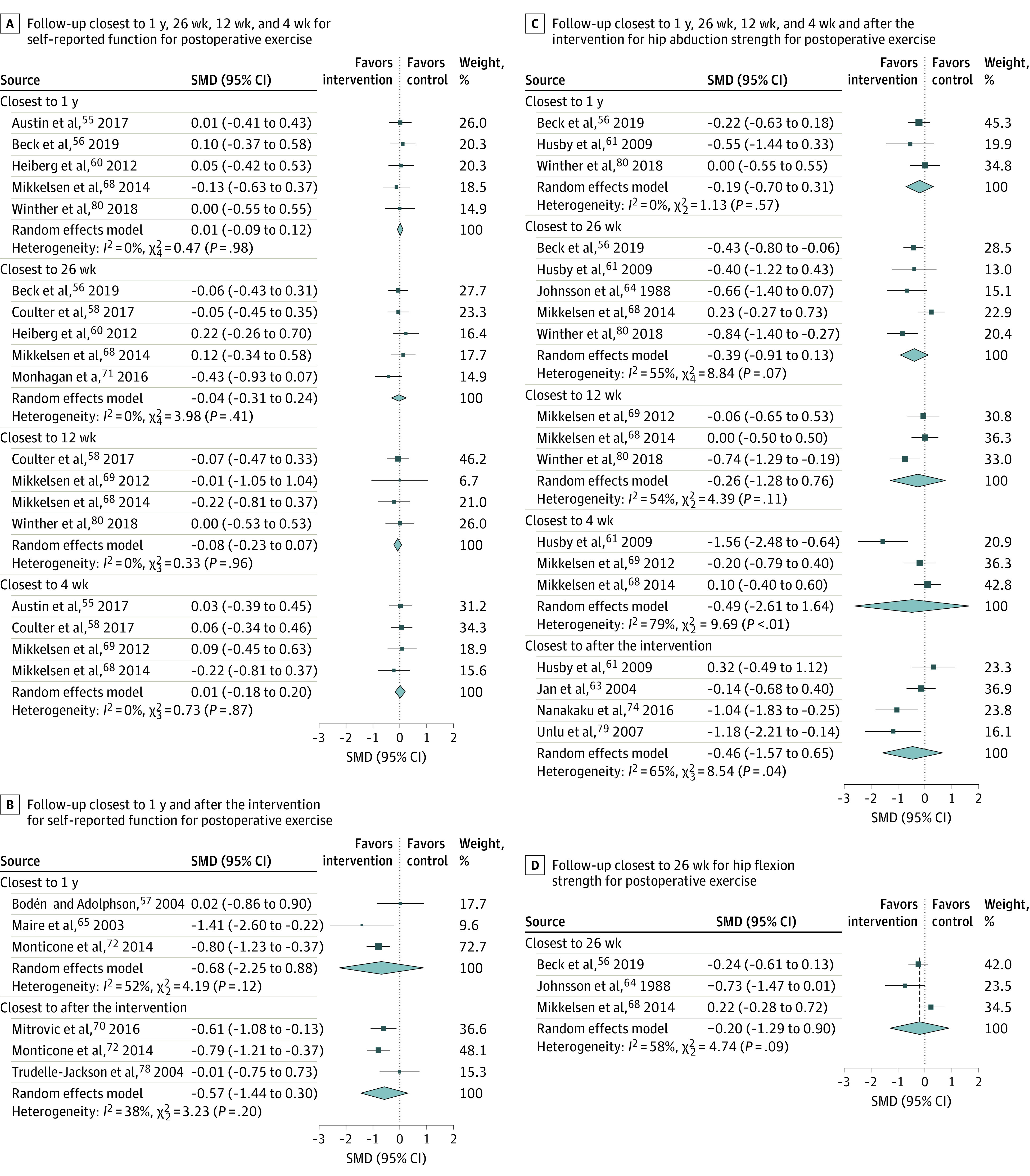
Individual study outcomes are indicated with squares, and larger squares indicate more study weight. Diamonds indicate overall finding; SMD, standardized mean difference.
A total of 5 studies57,65,66,67,70,72,78 reported on the outcome of self-reported physical function compared with an active comparator. We performed 2 meta-analyses comparing exercise interventions against active controls at the closest to 1-year follow-up57,65,66,67,72 and closest to after the intervention70,72,78 (Figure 3B). At the 1-year follow-up, we found no association of the intervention with self-reported physical function (3 studies: SMD, −0.68 [95% CI, −2.25 to 0.88]; I2 = 52.2% [95% CI, 0% to 86.3%]). At the follow-up closest to after the intervention, there was no association of the intervention with self-reported physical function (3 studies: SMD, −0.57 [95% CI, −1.44 to 0.30]; I2 = 38.2% [95% CI, 0% to 80.6%]). Both of these results had a rating of very low certainty (eAppendix 5 in the Supplement).
Postoperative Exercise Interventions for the Secondary Outcome of Hip Strength
A total of 9 studies56,61,62,63,64,68,69,74,79,80 were included in the meta-analyses of hip strength for intervention vs usual care or no or minimal intervention. We meta-analyzed 6 outcomes for hip muscle strength (Figure 3C and D). At the closest to 1-year follow-up,56,61,62,80 we found no association of the intervention with hip abduction muscle strength (3 studies: SMD, −0.19 [95% CI, −0.70 to 0.31]; I2 = 0% [95% CI, 0% to 81.6%]). At the follow-up closest to 26 weeks, there was no significant association of the intervention with hip abduction muscle strength56,61,62,64,68,80 (5 studies: SMD, −0.39 [95% CI, −0.91 to 0.13]; I2 = 54.7% [95% CI 0% to 83.3%]) or hip flexion muscle strength56,64,68 (3 studies: SMD, −0.20 [95% CI, −1.29 to 0.90]; I2 = 57.8% [95% CI, 0% to 88.0%]). There was also no significant effect size of the intervention with hip abduction muscle strength at the follow-up closest to 12 weeks68,69,80 (3 studies: SMD, −0.26 [95% CI, −1.28 to 0.76]; I2 = 54.0% [95% CI, 0% to 87.0%]). At the follow-up closest to 4 weeks,61,62,68,69 there was no significant effect size for hip abduction muscle strength (3 studies: SMD, −0.49 [95% CI, −2.61 to 1.64]; I2 = 79.0% [95% CI, 34.3% to 93.5%]), and at the follow-up closest to after the intervention,61,62,63,74,79 we found no significant effect size for hip abduction muscle strength (4 studies: SMD, −0.46 [95% CI, −1.57 to 0.65]; I2 = 57.8% [95% CI, 0% to 88.1%]). All estimates were rated at a very low level of certainty per GRADE. A meta-analysis for interventions compared with active controls could not be performed due to a shortage of studies.
Funding and Conflict of Interest
Among included studies, 13 were funded by private or professional organizations,48,50,51,54,56,57,59,61,64,68,69,75,78 7 were funded by government,46,60,63,67,71,73,76 and 1 study was funded by a combination of these entities.52 Additionally, 4 studies declared no funding source,55,58,70,72 and 7 studies did not report their funding source.47,49,53,74,77,79,80
The authors declared no conflict of interest in 22 studies,46,48,50,51,52,54,56,57,58,59,61,63,67,68,69,70,71,72,73,74,76,78 whereas the authors of 2 studies55,75 reported a conflict of interest, and 8 studies47,49,53,64,74,77,79,80 did not report on conflict of interest.
Adverse Events
In total, 11 studies48,50,51,52,57,60,68,69,70,72,73 (34%) reported on adverse events. Of 9 preoperative studies, 4 studies48,50,51,52 (44%) reported on adverse events. One study52 reported that 1 patient dropped out owing to increased pain. All other studies reported no serious adverse events. Of 23 postoperative studies, 7 studies (30%) reported on adverse events. The study by Mikkelsen et al68 reported 2 patients dropped out owing to adverse reactions to the exercises prescribed. All other studies reported no serious adverse events. We could not assess the treatment benefit-to-harm ratio given of limitations of reporting.
Sensitivity Analyses
We performed no subgroup analysis or meta-regression owing to the low number of studies (ie, <10). We performed sensitivity analysis for all meta-analytic outcomes via outlier identification. Studies were defined as outliers when their 95% CI was outside the 95% CI of the pooled effect.38 We did not identify any outliers. Influence analysis showed several influential studies for self-reported physical function at the follow-up closest to 1 year for preoperative exercise training (vs usual care or no or minimal intervention), self-reported physical function at the follow-up closest to 1 year and closest to after the intervention for postoperative exercise training (vs active control), and hip abduction muscle strength at the follow-ups closest to 26 weeks, 4 weeks, and closest to after the intervention (vs usual care or no or minimal intervention). We summarized the results of the influence analysis in eAppendix 7 in the Supplement. We also performed a sensitivity analysis by conducting all meta-analytic summaries with the standard DerSimonian and Laird approach for calculating the 95% CI for the pooled effect (eAppendix 7 in the Supplement). As expected, the Hartung-Knapp-Sidik-Jonkman correction gave wider 95% CIs with higher τ2 values (ie, more heterogeneity) and narrower 95% CIs if there was no heterogeneity. A real impact on the 95% CIs of the pooled effect size was only noted for the postoperative self-reported function outcome at the closest to 1 year follow-up and closest to 12 weeks follow-up.As there was no statistical heterogeneity (τ2 = 0; I2 = 0%) under this condition, the Hartung-Knapp-Sidik-Jonkman correction gave a smaller 95% CI for the pooled effect. We incorporated all results of the sensitivity analyses in the GRADE rating for imprecision. One trial50 reported change from baseline data only, which we transformed assuming a conservative correlation coefficient between the preintervention and postintervention standard deviation of r = 0.9. We also assumed that the preintervention and postintervention SDs of the change scores were equal.31 We performed a sensitivity analysis with a less conservative value of r = 0.5 to see if the results of the meta-analyses would be markedly changed, and they were not (eAppendix 7 in the Supplement).
Protocol Deviations Compared With PROSPERO Registration
We initially aimed to conduct a meta-analysis of protocol deviations compared with PROSPERO registration only if at least 5 studies could be included. However, owing to a low number of studies, we conducted a meta-analysis if only 3 studies were available. Through the application of the Hartung-Knapp-Sidik-Jonkman method for estimating the variance of the pooled effect, we hope to remedy the effects of a low number of studies on the 95% CI for these summary effects.25,36
Discussion
This meta-analysis and systematic review found that land-based preoperative exercise interventions vs usual care or no or minimal intervention was not associated with self-reported physical function, with very low certainty, or hospital LOS, with moderate certainty. For postoperative land-based exercise interventions compared with usual care or no or minimal intervention, there was no association of the intervention with self-reported physical function, with low (1 year after the operation) to moderate (4, 12, and 26 weeks after the operation) certainty. Moreover, there was no association of exercise intervention with hip abduction and flexion muscle strength, with very low certainty, compared with usual care or no or minimal intervention at the 4-week, 12-week, 26-week, or 1-year follow-ups. Our analysis comparing different active interventions of postoperative exercise with each other showed medium effect sizes for the intervention group with very low levels of certainty at the follow-ups closest to 1 year and closest to after the intervention.
Recent clinical practice guidelines81,82,83 for preoperative and postoperative rehabilitation after total hip arthroplasty give differing recommendations. The Royal Dutch Society for Physical Therapy (KNGF)81 and NICE83 guidelines do not universally recommend preoperative rehabilitation. While the NICE guidelines do not recommend preoperative rehabilitation, the KNGF guidelines recommend rehabilitation for patients who have an increased risk of delayed recovery after osteoarthritis-related hip joint replacement. The American Academy of Orthopaedic Surgeons (AAOS) guideline82 recommends preoperative rehabilitation, albeit with limited overall strength of evidence. Our results are in line with these recent guidelines and support the conclusion that preoperative exercise training may not be needed. The AAOS and KNGF guidelines recommend postoperative exercise therapy, with low to moderate certainty. NICE recommends supervised group or individual outpatient rehabilitation only for certain subgroups of patients who have difficulty managing their activities of daily living, have cognitive impairments, have specific rehabilitative needs, or find that self-directed rehabilitation does not meet their goals. Since there was no association with self-reported physical function or for hip muscle strength at any time point, our results support the recommendations made by NICE. We recommend integrating our results with guidelines of the AAOS and KNGF.
A key theme for further research should be the identification of potential subgroups who might gain an advantage by supervised group or individual outpatient rehabilitation.83 Of further interest is the assessment of digital or internet-based interventions (eg, smartphone apps) regarding cost-effectiveness compared with standard face-to-face interventions. Another area of interest is the comparison of different forms of exercise therapy with each other to determine a potentially superior mode of exercise training for rehabilitation. However, given the current paucity of literature, pairwise meta-analyses may be limited in drawing such conclusions. Network meta-analysis may be more suitable for determining potentially superior modes of exercise training and have recently gained traction in the field of sports medicine.84 Studies should focus on strong methodological rigor and larger sample sizes reduce the risk of bias and increase the certainty in observations. To ensure a low risk of bias, placebo- or sham-controlled trials should be considered.85,86,87 Furthermore, the studies should follow current guidelines for intervention description (eg, the template for intervention description and replication checklist86) to enable transparent evaluation and replication of intervention programs and should report factors potentially influencing the findings (eg, comorbidities and pain management). Moreover, reporting of adverse events needs to be strictly implemented, as this was lacking in approximately two-thirds of all included trials.
The strengths of our study include the overall assessment of preoperative and postoperative exercise intervention to give a concise overview of the whole rehabilitation process for total hip arthroplasty. Furthermore, we included a number of potential outcomes, as opposed to only pain or physical function. We also only combined studies that included hip arthroplasty, rather than those that included other joint replacements (eg, knee arthroplasty). Statistical sensitivity analyses were conducted to further check the robustness and validity of the results.
Limitations
This study has some limitations. There were no study outcomes with a low of risk of bias. We also could not assess the impact of publication bias owing to too few studies. Furthermore, we could not include all studies in the meta-analytic summaries owing to a lack of a sufficient number of studies for some outcomes (ie, <3). This may have impacted the conclusions of this review. We tried to remedy this by transparently reporting the study outcomes that could not be included in meta-analyses through structured reporting of effects, as recommended by the Cochrane Collaboration.25 We also could not assess the effect of physiotherapy on the time to return to work, because the included studies did not report on this important variable. A further limitation of our study was that we did not assess important covariates, such as the association of age with the outcomes, owing to the low number of studies to perform a robust meta-regression.25 It should also be noted that the results only apply to land-based interventions. We excluded water-based interventions, as access to appropriate pool-facilities is not readily available in most settings. Finally, although exercise training is considered relatively safe in general,88 adverse events could not be adequately assessed given insufficient reporting.
Conclusions
This systematic review and meta-analysis found that there was very low to moderate certainty evidence that supervised land-based postoperative exercise interventions were not associated with benefit compared with usual care or no or minimal intervention for self-reported function and hip strength after hip arthroplasty. There was also very low certainty that different forms of exercise training were associated with better outcomes compared with each other. Furthermore, there was very low quality evidence that preoperative exercise programs were not associated with better results than usual care or no or minimal intervention for self-reported physical function and moderate quality evidence for the lack of association with hospital LOS.
eAppendix 1. Search Strategy
eAppendix 2. Summary of Excluded Studies With Reason
eAppendix 3. Risk of Bias Assessment for All Outcomes
eAppendix 4. Summary Plots for Risk of Bias Assessment for Meta-analytic Results
eAppendix 5. GRADE Assessment of Meta-analytic Results
eAppendix 6. Structured Reporting of Effects
eAppendix 7. Sensitivity Analyses
References
- 1.Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2):160-167. doi: 10.1097/BOR.0000000000000479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105(1):185-199. doi: 10.1093/bmb/lds038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745-1759. doi: 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 4.Higashi H, Barendregt JJ. Cost-effectiveness of total hip and knee replacements for the Australian population with osteoarthritis: discrete-event simulation model. PLoS One. 2011;6(9):e25403. doi: 10.1371/journal.pone.0025403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100(17):1455-1460. doi: 10.2106/JBJS.17.01617 [DOI] [PubMed] [Google Scholar]
- 6.Miller LE, Martinson MS, Gondusky JS, Kamath AF, Boettner F, Bhattacharyya SK. Ninety-day postoperative cost in primary total hip arthroplasty: an economic model comparing surgical approaches. Clinicoecon Outcomes Res. 2019;11:145-149. doi: 10.2147/CEOR.S196545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Current Opinion in Orthopaedics. 2007;18(1):23-27. doi: 10.1097/BCO.0b013e328011a270 [DOI] [Google Scholar]
- 8.Ong KL, Lotke PA, Lau E, Manley MT, Kurtz SM. Prevalence and costs of rehabilitation and physical therapy after primary TJA. J Arthroplasty. 2015;30(7):1121-1126. doi: 10.1016/j.arth.2015.02.030 [DOI] [PubMed] [Google Scholar]
- 9.Fortin PR, Clarke AE, Joseph L, et al. Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999;42(8):1722-1728. doi: [DOI] [PubMed] [Google Scholar]
- 10.Mizner RL, Petterson SC, Stevens JE, Axe MJ, Snyder-Mackler L. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J Rheumatol. 2005;32(8):1533-1539. [PubMed] [Google Scholar]
- 11.Ackerman IN, Bennell KL, Osborne RH. Decline in health-related quality of life reported by more than half of those waiting for joint replacement surgery: a prospective cohort study. BMC Musculoskelet Disord. 2011;12(1):108. doi: 10.1186/1471-2474-12-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawker G, Bohm ER, Conner-Spady B, et al. Perspectives of Canadian stakeholders on criteria for appropriateness for total joint arthroplasty in patients with hip and knee osteoarthritis. Arthritis Rheumatol. 2015;67(7):1806-1815. doi: 10.1002/art.39124 [DOI] [PubMed] [Google Scholar]
- 13.Rasch A, Dalén N, Berg HE. Muscle strength, gait, and balance in 20 patients with hip osteoarthritis followed for 2 years after THA. Acta Orthop. 2010;81(2):183-188. doi: 10.3109/17453671003793204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sicard-Rosenbaum L, Light KE, Behrman AL. Gait, lower extremity strength, and self-assessed mobility after hip arthroplasty. J Gerontol A Biol Sci Med Sci. 2002;57(1):M47-M51. doi: 10.1093/gerona/57.1.M47 [DOI] [PubMed] [Google Scholar]
- 15.van Baar ME, Assendelft WJJ, Dekker J, Oostendorp RAB, Bijlsma JWJ. Effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a systematic review of randomized clinical trials. Arthritis Rheum. 1999;42(7):1361-1369. doi: [DOI] [PubMed] [Google Scholar]
- 16.Lowe CJ, Davies L, Sackley CM, Barker KL. Effectiveness of land-based physiotherapy exercise following hospital discharge following hip arthroplasty for osteoarthritis: an updated systematic review. Physiotherapy. 2015;101(3):252-265. doi: 10.1016/j.physio.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wijnen A, Bouma SE, Seeber GH, et al. The therapeutic validity and effectiveness of physiotherapeutic exercise following total hip arthroplasty for osteoarthritis: a systematic review. PLoS One. 2018;13(3):e0194517. doi: 10.1371/journal.pone.0194517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi: 10.1136/bmjopen-2015-009857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moyer R, Ikert K, Long K, Marsh J. The value of preoperative exercise and education for patients undergoing total hip and knee arthroplasty: a systematic review and meta-analysis. JBJS Rev. 2017;5(12):e2. doi: 10.2106/JBJS.RVW.17.00015 [DOI] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services . 2008 Physical activity guidelines for Americans. Accessed January 21, 2021. https://health.gov/our-work/physical-activity/previous-guidelines/2008-physical-activity-guidelines
- 22.Nussbaumer-Streit B, Klerings I, Dobrescu AI, et al. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol. 2020;118:42-54. doi: 10.1016/j.jclinepi.2019.10.011 [DOI] [PubMed] [Google Scholar]
- 23.Herbert R, Jamtvedt G, Hagen KB, Mead J, Chalmers I. Practical Evidence-Based Physiotherapy. Elsevier Health Sciences; 2011. [Google Scholar]
- 24.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. doi: 10.1002/9781119536604 [DOI] [Google Scholar]
- 26.Atkins D, Best D, Briss PA, et al. ; GRADE Working Group . Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785-1805. doi: 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 28.Shi J, Luo D, Weng H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11(5):641-654. doi: 10.1002/jrsm.1429 [DOI] [PubMed] [Google Scholar]
- 29.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GetData . Graph digitizer. Accessed July 14, 2020. http://getdata-graph-digitizer.com/
- 31.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7(1):105-125. doi: 10.1037/1082-989X.7.1.105 [DOI] [PubMed] [Google Scholar]
- 32.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14(1):25. doi: 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartung J. An alternative method for meta-analysis. Biomet J. 1999;41(8):901-916. doi: [DOI] [Google Scholar]
- 34.Hartung J, Knapp G. On tests of the overall treatment effect in meta-analysis with normally distributed responses. Stat Med. 2001;20(12):1771-1782. doi: 10.1002/sim.791 [DOI] [PubMed] [Google Scholar]
- 35.Makambi KH. The effect of the heterogeneity variance estimator on some tests of treatment efficacy. J Biopharm Stat. 2004;14(2):439-449. doi: 10.1081/BIP-120037191 [DOI] [PubMed] [Google Scholar]
- 36.Borenstein M. Common Mistakes in Meta-Analysis: And How to Avoid Them. Biostat; 2019. [Google Scholar]
- 37.Mavridis D, Salanti G. How to assess publication bias: funnel plot, trim-and-fill method and selection models. Evid Based Ment Health. 2014;17(1):30-30. doi: 10.1136/eb-2013-101699 [DOI] [PubMed] [Google Scholar]
- 38.Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing Meta-Analysis in R: A Hands-On Guide. PROTECT Lab; 2019. [Google Scholar]
- 39.Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112-125. doi: 10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 40.Jackson D, Law M, Rücker G, Schwarzer G. The Hartung-Knapp modification for random-effects meta-analysis: a useful refinement but are there any residual concerns? Stat Med. 2017;36(25):3923-3934. doi: 10.1002/sim.7411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Erbaum Press; 1988. [Google Scholar]
- 42.R Project for Statistical Computing . A language and environment for statistical computing. Accessed January 21, 2021. https://www.r-project.org/
- 43.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153-160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36(1):1-48. doi: 10.18637/jss.v036.i0320808728 [DOI] [Google Scholar]
- 45.Harrer M, Cuijpers P, Furukawa T, Ebert D. Dmetar: doing meta-analysis in R. 2019. Accessed January 21, 2021. https://dmetar.protectlab.org/
- 46.Doiron-Cadrin P, Kairy D, Vendittoli P-A, Lowry V, Poitras S, Desmeules F. Feasibility and preliminary effects of a tele-prehabilitation program and an in-person prehablitation program compared to usual care for total hip or knee arthroplasty candidates: a pilot randomized controlled trial. Disabil Rehabil. 2020;42(7):989-998. doi: 10.1080/09638288.2018.1515992 [DOI] [PubMed] [Google Scholar]
- 47.Ferrara PE, Rabini A, Maggi L, et al. Effect of pre-operative physiotherapy in patients with end-stage osteoarthritis undergoing hip arthroplasty. Clin Rehabil. 2008;22(10-11):977-986. doi: 10.1177/0269215508094714 [DOI] [PubMed] [Google Scholar]
- 48.Gill SD, McBurney H, Schulz DL. Land-based versus pool-based exercise for people awaiting joint replacement surgery of the hip or knee: results of a randomized controlled trial. Arch Phys Med Rehabil. 2009;90(3):388-394. doi: 10.1016/j.apmr.2008.09.561 [DOI] [PubMed] [Google Scholar]
- 49.Gocen Z, Sen A, Unver B, Karatosun V, Gunal I. The effect of preoperative physiotherapy and education on the outcome of total hip replacement: a prospective randomized controlled trial. Clin Rehabil. 2004;18(4):353-358. doi: 10.1191/0269215504cr758oa [DOI] [PubMed] [Google Scholar]
- 50.Holsgaard-Larsen A, Hermann A, Zerahn B, Mejdahl S, Overgaard S. Effects of progressive resistance training prior to total HIP arthroplasty—a secondary analysis of a randomized controlled trial. Osteoarthritis Cartilage. 2020;28(8):1038-1045. doi: 10.1016/j.joca.2020.04.010 [DOI] [PubMed] [Google Scholar]
- 51.Oosting E, Jans MP, Dronkers JJ, et al. Preoperative home-based physical therapy versus usual care to improve functional health of frail older adults scheduled for elective total hip arthroplasty: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2012;93(4):610-616. doi: 10.1016/j.apmr.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 52.Villadsen A, Overgaard S, Holsgaard-Larsen A, Christensen R, Roos EM. Postoperative effects of neuromuscular exercise prior to hip or knee arthroplasty: a randomised controlled trial. Ann Rheum Dis. 2014;73(6):1130-1137. doi: 10.1136/annrheumdis-2012-203135 [DOI] [PubMed] [Google Scholar]
- 53.Vukomanović A, Popović Z, Durović A, Krstić L. The effects of short-term preoperative physical therapy and education on early functional recovery of patients younger than 70 undergoing total hip arthroplasty. Vojnosanit Pregl. 2008;65(4):291-297. doi: 10.2298/VSP0804291V [DOI] [PubMed] [Google Scholar]
- 54.Bitterli R, Sieben JM, Hartmann M, de Bruin ED. Pre-surgical sensorimotor training for patients undergoing total hip replacement: a randomised controlled trial. Int J Sports Med. 2011;32(9):725-732. doi: 10.1055/s-0031-1271696 [DOI] [PubMed] [Google Scholar]
- 55.Austin MS, Urbani BT, Fleischman AN, et al. Formal physical therapy after total hip arthroplasty is not required: a randomized controlled trial. J Bone Joint Surg Am. 2017;99(8):648-655. doi: 10.2106/JBJS.16.00674 [DOI] [PubMed] [Google Scholar]
- 56.Beck H, Beyer F, Gering F, et al. Sports therapy interventions following total hip replacement. Dtsch Arztebl Int. 2019;116(1-2):1-8. doi: 10.3238/arztebl.2019.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bodén H, Adolphson P. No adverse effects of early weight bearing after uncemented total hip arthroplasty: a randomized study of 20 patients. Acta Orthop Scand. 2004;75(1):21-29. doi: 10.1080/00016470410001708040 [DOI] [PubMed] [Google Scholar]
- 58.Coulter C, Perriman DM, Neeman TM, Smith PN, Scarvell JM. Supervised or unsupervised rehabilitation after total hip replacement provides similar improvements for patients: a randomized controlled trial. Arch Phys Med Rehabil. 2017;98(11):2253-2264. doi: 10.1016/j.apmr.2017.03.032 [DOI] [PubMed] [Google Scholar]
- 59.Galea MP, Levinger P, Lythgo N, et al. A targeted home- and center-based exercise program for people after total hip replacement: a randomized clinical trial. Arch Phys Med Rehabil. 2008;89(8):1442-1447. doi: 10.1016/j.apmr.2007.11.058 [DOI] [PubMed] [Google Scholar]
- 60.Heiberg KE, Bruun-Olsen V, Ekeland A, Mengshoel AM. Effect of a walking skill training program in patients who have undergone total hip arthroplasty: followup one year after surgery. Arthritis Care Res (Hoboken). 2012;64(3):415-423. doi: 10.1002/acr.20681 [DOI] [PubMed] [Google Scholar]
- 61.Husby VS, Helgerud J, Bjørgen S, Husby OS, Benum P, Hoff J. Early maximal strength training is an efficient treatment for patients operated with total hip arthroplasty. Arch Phys Med Rehabil. 2009;90(10):1658-1667. doi: 10.1016/j.apmr.2009.04.018 [DOI] [PubMed] [Google Scholar]
- 62.Husby VS, Helgerud J, Bjørgen S, Husby OS, Benum P, Hoff J. Early postoperative maximal strength training improves work efficiency 6-12 months after osteoarthritis-induced total hip arthroplasty in patients younger than 60 years. Am J Phys Med Rehabil. 2010;89(4):304-314. doi: 10.1097/PHM.0b013e3181cf5623 [DOI] [PubMed] [Google Scholar]
- 63.Jan M-H, Hung J-Y, Lin JC-H, Wang S-F, Liu T-K, Tang P-F. Effects of a home program on strength, walking speed, and function after total hip replacement. Arch Phys Med Rehabil. 2004;85(12):1943-1951. doi: 10.1016/j.apmr.2004.02.011 [DOI] [PubMed] [Google Scholar]
- 64.Johnsson R, Melander A, Onnerfält R. Physiotherapy after total hip replacement for primary arthrosis. Scand J Rehabil Med. 1988;20(1):43-45. [PubMed] [Google Scholar]
- 65.Maire J, Dugué B, Faillenet-Maire A-F, et al. Recovery after total hip joint arthroplasty in elderly patients with osteoarthritis: positive effect of upper limb interval-training. J Rehabil Med. 2003;35(4):174-179. doi: 10.1080/16501970306127 [DOI] [PubMed] [Google Scholar]
- 66.Maire J, Faillenet-Maire A-F, Grange C, et al. A specific arm-interval exercise program could improve the health status and walking ability of elderly patients after total hip arthroplasty: a pilot study. J Rehabil Med. 2004;36(2):92-94. doi: 10.1080/16501970310021383 [DOI] [PubMed] [Google Scholar]
- 67.Maire J, Dugué B, Faillenet-Maire A-F, et al. Influence of a 6-week arm exercise program on walking ability and health status after hip arthroplasty: a 1-year follow-up pilot study. J Rehabil Res Dev. 2006;43(4):445-450. doi: 10.1682/JRRD.2005.03.0058 [DOI] [PubMed] [Google Scholar]
- 68.Mikkelsen LR, Mechlenburg I, Søballe K, et al. Effect of early supervised progressive resistance training compared to unsupervised home-based exercise after fast-track total hip replacement applied to patients with preoperative functional limitations: a single-blinded randomised controlled trial. Osteoarthritis Cartilage. 2014;22(12):2051-2058. doi: 10.1016/j.joca.2014.09.025 [DOI] [PubMed] [Google Scholar]
- 69.Mikkelsen LR, Mikkelsen SS, Christensen FB. Early, intensified home-based exercise after total hip replacement—a pilot study: intensified home-based exercise after THR. Physiother Res Int. 2012;17(4):214-226. doi: 10.1002/pri.1523 [DOI] [PubMed] [Google Scholar]
- 70.Mitrovic D, Davidovic M, Erceg P, Marinkovic J. The effectiveness of supplementary arm and upper body exercises following total hip arthroplasty for osteoarthritis in the elderly: a randomized controlled trial. Clin Rehabil. 2017;31(7):881-890. doi: 10.1177/0269215516655591 [DOI] [PubMed] [Google Scholar]
- 71.Monaghan B, Cunningham P, Harrington P, et al. Randomised controlled trial to evaluate a physiotherapy-led functional exercise programme after total hip replacement. Physiotherapy. 2017;103(3):283-288. doi: 10.1016/j.physio.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 72.Monticone M, Ambrosini E, Rocca B, Lorenzon C, Ferrante S, Zatti G. Task-oriented exercises and early full weight-bearing contribute to improving disability after total hip replacement: a randomized controlled trial. Clin Rehabil. 2014;28(7):658-668. doi: 10.1177/0269215513519342 [DOI] [PubMed] [Google Scholar]
- 73.Morishima Y, Mizushima T, Yamauchi K, Morikawa M, Masuki S, Nose H. Effects of home-based interval walking training on thigh muscle strength and aerobic capacity in female total hip arthroplasty patients: a randomized, controlled pilot study. PLoS One. 2014;9(9):e108690. doi: 10.1371/journal.pone.0108690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nankaku M, Ikeguchi R, Goto K, So K, Kuroda Y, Matsuda S. Hip external rotator exercise contributes to improving physical functions in the early stage after total hip arthroplasty using an anterolateral approach: a randomized controlled trial. Disabil Rehabil. 2016;38(22):2178-2183. doi: 10.3109/09638288.2015.1129453 [DOI] [PubMed] [Google Scholar]
- 75.Nelson M, Bourke M, Crossley K, Russell T. Telerehabilitation is non-inferior to usual care following total hip replacement—a randomized controlled non-inferiority trial. Physiotherapy. 2020;107:19-27. doi: 10.1016/j.physio.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 76.Okoro T, Whitaker R, Gardner A, Maddison P, Andrew JG, Lemmey A. Does an early home-based progressive resistance training program improve function following total hip replacement: results of a randomized controlled study. BMC Musculoskelet Disord. 2016;17(1):173. doi: 10.1186/s12891-016-1023-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suetta C, Magnusson SP, Rosted A, et al. Resistance training in the early postoperative phase reduces hospitalization and leads to muscle hypertrophy in elderly hip surgery patients—a controlled, randomized study. J Am Geriatr Soc. 2004;52(12):2016-2022. doi: 10.1111/j.1532-5415.2004.52557.x [DOI] [PubMed] [Google Scholar]
- 78.Trudelle-Jackson E, Smith SS. Effects of a late-phase exercise program after total hip arthroplasty: a randomized controlled trial. Arch Phys Med Rehabil. 2004;85(7):1056-1062. doi: 10.1016/j.apmr.2003.11.022 [DOI] [PubMed] [Google Scholar]
- 79.Unlu E, Eksioglu E, Aydog E, Aydog ST, Atay G. The effect of exercise on hip muscle strength, gait speed and cadence in patients with total hip arthroplasty: a randomized controlled study. Clin Rehabil. 2007;21(8):706-711. doi: 10.1177/0269215507077302 [DOI] [PubMed] [Google Scholar]
- 80.Winther SB, Foss OA, Husby OS, Wik TS, Klaksvik J, Husby VS. A randomized controlled trial on maximal strength training in 60 patients undergoing total hip arthroplasty. Acta Orthop. 2018;89(3):295-301. doi: 10.1080/17453674.2018.1441362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Doormaal MCM, Meerhoff GA, Vliet Vlieland TPM, Peter WF. A clinical practice guideline for physical therapy in patients with hip or knee osteoarthritis. Musculoskeletal Care. 2020;18(4):575-595. doi: 10.1002/msc.1492 [DOI] [PubMed] [Google Scholar]
- 82.American Academy of Orthopaedic Surgeons . Osteoarthritis of the hip: clinical practice guideline on the management of osteoarthritis of the hip. Accessed January 21, 2021. https://www.aaos.org/quality/quality-programs/lower-extremity-programs/osteoarthritis-of-the-hip/
- 83.National Institute for Health and Care Excellence . Joint replacement (primary): hip, knee and shoulder. Accessed August 27, 2020. https://www.nice.org.uk/guidance/ng157 [PubMed]
- 84.Owen PJ, Miller CT, Mundell NL, et al. Which specific modes of exercise training are most effective for treating low back pain: network meta-analysis. Br J Sports Med. 2020;54(21):1279-1287. doi: 10.1136/bjsports-2019-100886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bennell KL, Hinman RS, Metcalf BR, et al. Efficacy of physiotherapy management of knee joint osteoarthritis: a randomized, double blind, placebo controlled trial. Ann Rheum Dis. 2005;64(6):906-912. doi: 10.1136/ard.2004.026526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 87.Krauß I, Steinhilber B, Haupt G, Miller R, Martus P, Janßen P. Exercise therapy in hip osteoarthritis—a randomized controlled trial. Dtsch Arztebl Int. 2014;111(35-36):592-599. doi: 10.3238/arztebl.2014.0592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niemeijer A, Lund H, Stafne SN, et al. Adverse events of exercise therapy in randomised controlled trials: a systematic review and meta-analysis. Br J Sports Med. 2020;54(18):1073-1080. doi: 10.1136/bjsports-2018-100461 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search Strategy
eAppendix 2. Summary of Excluded Studies With Reason
eAppendix 3. Risk of Bias Assessment for All Outcomes
eAppendix 4. Summary Plots for Risk of Bias Assessment for Meta-analytic Results
eAppendix 5. GRADE Assessment of Meta-analytic Results
eAppendix 6. Structured Reporting of Effects
eAppendix 7. Sensitivity Analyses