Abstract
Simple Summary
The diamondback moth, Plutella xylostella, is the most destructive pest for Brassica vegetable crops worldwide. The management of this pest was estimated to cost about United States dollars (USD) 4–5 billion annually. Prolonged and unsupervised insecticide exposures have led to not only the emergence of insecticide resistance in P. xylostella, but also negative impacts on human health, environmental pollution, and nontargeted organisms. Therefore, the development of new safer, environmentally friendly, and target-specific insecticides is vital in order to combat this pest. In this study, we evaluated the potential of selected farnesyl derivative compounds that could act as biorational insecticides targeting the juvenile hormone biosynthesis of P. xylostella. Out of five farnesyl derivatives tested, farnesyl acetate showed the highest mortality percentage of P. xylostella. Then, the sublethal effects of farnesyl acetate on biological characteristics of P. xylostella were determined. The results demonstrated that farnesyl acetate had negative effects on development, pupal weight, pupation, adult emergence, female ratio, fecundity, egg hatching rate, and oviposition period of P. xylostella. Farnesyl acetate also induced abnormalities in pupal and adults of P. xylostella. These findings indicate that farnesyl acetate can reduce the population number and reproductive success of P. xylostella, possibly leading to the effective management of this pest.
Abstract
The diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae), is the most important pest of cruciferous vegetables worldwide. In this study, we evaluated the properties of selected farnesyl derivative compounds against P. xylostella. The toxicity and sublethal concentration (LC50) of farnesyl acetate, farnesyl acetone, farnesyl bromide, farnesyl chloride, and hexahydrofarnesyl acetone were investigated for 96 h. The leaf-dip bioassays showed that farnesyl acetate had a high level of toxicity against P. xylostella compared to other tested farnesyl derivatives. The LC50 value was 56.41 mg/L on the second-instar larvae of P. xylostella. Then, the sublethal effects of farnesyl acetate on biological parameters of P. xylostella were assessed. Compared to the control group, the sublethal concentration of farnesyl acetate decreased pupation and emergence rates, pupal weight, fecundity, egg hatching rate, female ratio, and oviposition period. Furthermore, the developmental time of P. xylostella was extended after being exposed to farnesyl acetate. Moreover, the application of farnesyl acetate on P. xylostella induced morphogenetic abnormalities in larval–pupal intermediates, adults that emerged with twisted wings, or complete adults that could not emerge from the cocoon. These results suggested that farnesyl acetate was highly effective against P. xylostella. The sublethal concentration of farnesyl acetate could reduce the population of P. xylostella by increasing abnormal pupal and adults, and by delaying its development period.
Keywords: Plutella xylostella, farnesyl derivatives, sublethal concentration, biological parameters, pest management, morphological abnormalities
1. Introduction
The diamondback moth, Plutella xylostella, is a damaging pest for Brassica vegetable crops all over the world [1,2]. The management of this pest was estimated to cost about United States dollars (USD) 4–5 billion annually [3,4]. Relying on temperature, humidity, and food availability, the life cycle of P. xylostella takes between 18 and 51 days [5]. Due to the voracious appetite of the larvae, P. xylostella infestation can eradicate the entire cropping systems and render regions inadequate for production [6]. Rapid insect life cycle, high fecundity, high reproduction rate, and high selection pressure with insecticides are the major factors that contribute to the resistance of this pest to various insecticides [6,7]. Furthermore, its mobility and rapid resistance evolution against many insecticides become the ultimate drawbacks in managing this pest [8]. To boost crop production, farmers usually conduct more frequent sprays of insecticide, increase the doses, or apply mixtures of several insecticides. Prolonged and unsupervised insecticide exposures have led to not only the emergence of insecticide resistance in P. xylostella, but also negative impacts on human health, environmental pollution, and nontargeted organisms [9].
Juvenile hormones (JHs) are critical insect hormones that regulate a large diversity of physiological processes and adult reproduction in insects. Due to these roles in insects, JHs have gained attention as safe and specific targets for environmentally friendly and biorational insecticide discovery [10]. The juvenile hormone analogues (JHA), which are insect growth regulators (IGRs) that affect insect development and reproduction, have been developed and used in integrated pest management (IPM). JHAs are potent inhibitors of embryogenesis, metamorphosis, and adult formation of most insects. JHAs insecticides such as methoprene, fenoxycarb, and pyriproxyfen are commonly found in flea treatments for dogs and cats, mosquito control products, and insecticides against Lepidopteran pests [11,12,13] The application of JHAs such as fenoxycarb and pyriproxyfen have shown effectiveness on P. xylostella by suppressing adult emergence, fecundity, egg hatching, and abnormality in the pupal and adult insects [14,15,16]. However, previous studies reported that these insecticides also affected the development, behavior, and reproduction in beneficial insect and nontarget organisms [17,18,19,20]. Thus, a different strategy has to be envisaged for the development of new, safer, and efficient JH-based control products targeting P. xylostella. For this reason, interfering with JH biosynthesis has been considered as a promising strategy for the insecticide discovery [21]. The early steps in the biosynthetic pathway of juvenile hormone III (JHIII) follow the mevalonate pathway to form farnesyl pyrophosphate from acetyl-CoA [22]. The later steps involve the conversion of farnesyl pyrophosphate to farnesol through hydrolysis. Subsequently, farnesol is converted to farnesal and farnesoic acid via oxidation reactions. Farnesoic acid then undergoes an epoxidation and methyl transfer to form a JHIII [22]. Recent studies reported the characterization and inhibition of several enzymes from the JH biosynthesis pathway in insects, such as Aedes aegypti (L.), Diploptera punctata (Eschscoltz), Helicoverpa armigera (Hubner), and Manduca sexta (L.). Characterization of recombinant juvenile hormone acid methyl transferase (JHAMT), which is an enzyme that converts juvenile hormone acid into JHIII, has shown that knockdown of JHAMT in D. punctata results in decreased oocyte and vitellin content as a consequence of reduction in JH biosynthesis [23]. In H. armigera, the knockdown of farnesyl diphosphate synthase caused the reduction of JH titer and, thus, disrupted the molting process in larvae [24]. Meanwhile, the characterization of enzymatic activity of JHAMT in A. aegypti revealed that several juvenile hormone acid analogues were potent inhibitors of JHAMT [25]. An enzyme-inhibitory activity of farnesol dehydrogenase in M. sexta was also observed, and geranylgeraniol caused a reduction in JH biosynthesis in vitro and mortality of larvae in a feeding toxicity test [26]. Previously, we characterized the farnesol dehydrogenase from P. xylostella and several farnesyl derivatives shown as potent analogue inhibitors for P. xylostella farnesol dehydrogenase when tested in vitro [27]. Farnesol dehydrogenase, an intermediate enzyme in the JHIII biosynthesis pathway, catalyzes the oxidation of farnesol to farnesal [28]. Farnesol dehydrogenase activity was observed in several insects [27,29] and plants [28,30,31]. A few studies reported that geranyl and farnesyl derivatives showed inhibitory effects on several insect pests [32,33,34,35]. Therefore, the objective of this study was to evaluate the toxicity of selected farnesyl derivatives against P. xylostella and the effects of sublethal concentration on developmental time, oviposition period, pupal weight, pupation, adult emergence, female ratio, fecundity, and egg hatchability. The findings from this study are expected to help develop new effective and safe insecticides to manage P. xylostella populations in particular and Lepidopteran pests in general.
2. Materials and Methods
2.1. Insect Rearing
The larvae of P. xylostella were collected from vegetable farms in Cameron Highlands, Pahang, Malaysia (4°34′55″ N, 101°24′41″ E) and brought to the laboratory of Institute of Systems Biology, Universiti Kebangsaan Malaysia for rearing. Larvae were reared on mustard plant, Brassica rapa, as food in a screen cage (35 cm × 35 cm × 47 cm) at 25 ± 1 °C with a photoperiod of 12 h/12 h (light (L)/darkness (D)). The newly emerged adults were transferred to new mustard plants for oviposition, and cotton wool soaked in 10% honey water placed inside the cage was added as a dietary supplement [36]. The experiments were started after the third generations of the moth.
2.2. Bioassay and Determination of Sublethal Concentration (LC50) of Farnesyl Derivatives
The leaf-dip method was used for bioassay experiments [14] using five farnesyl derivatives: farnesyl acetate, farnesyl acetone, farnesyl bromide, farnesyl chloride, and hexahydrofarnesyl acetone. These farnesyl derivatives were selected because they bear a similar structure to farnesol and acted as potent analogue inhibitors for P. xylostella farnesol dehydrogenase when tested in vitro [27]. Four concentrations (12.5, 25, 50, and 100 mg/L) of each derivative were prepared by mixing with 0.02% Tween-20 and distilled water. Mustard leaf discs (4.5 cm diameter) were dipped in each concentration of farnesyl derivative solutions for 10 s. For the control treatment, the leaf discs were dipped in distilled water mixed with 0.02% Tween-20. The treated leaf discs were air-dried at room temperature for 2 h and then placed in a petri dish. A total of 10 second-instar larvae were released on the leaf discs inside the dish. The treatments (farnesyl derivatives and control) were replicated three times and arranged following a complete randomized design (CRD). The mortality of the larvae was observed and recorded after 96 h exposure to the treatments. The insects were considered dead when they were unable to move after being prodded with a soft paint brush [37].
2.3. Sublethal Treatment and Effects on P. xylostella
A farnesyl acetate solution at an LC50 concentration of 96 h (56.41 mg/L) (determined as above) was prepared and sprayed onto fresh mustard plants. After being air-dried for about 2 h, 100 second-instar larvae of P. xylostella, which were starved for about 2 h earlier, were released on farnesyl acetate-treated plants as mentioned above. For the control treatment, the mustard plants were sprayed with distilled water. Each treatment (LC50 of farnesyl acetate and control) was replicated three times, and the experiment was arranged as a CRD. The surviving larvae were transferred to new untreated mustard plants after 96 h exposure to the treated plants and allowed to continue their development until the pupal stage. The number of pupae was recorded daily until all larvae pupated. Pupae were placed individually in Petri dishes, and the 2 day old age pupae were weighed individually. The number of emerged pupae were recorded daily until all pupae emerged as adults. After adult emergence, 10 pairs of adults of each treatment were selected, and each pair (male and female emerged on the same day) was put in a new cage. For oviposition, adults were allowed to mate and lay eggs on mustard leaves placed in each cage. The number of eggs laid by each female adult was recorded daily, and the leaves were replaced every day until the death of the adults. Egg hatching was also recorded daily. The pre-oviposition period (time from adult emergence and first egg laying), oviposition period (time from first egg laying to last egg laying), and post-oviposition period (time from last egg laying to death) were also recorded. The pupation, emergence, female ratio, fecundity (total number of eggs laid per female), and egg hatching rate were calculated as described in Table 1.
Table 1.
Calculation of sublethal effects properties of Plutella xylostella.
2.4. Data Analysis
Percentage larval mortality was corrected following Abbott’s formula [42]. Data were transformed using arcsin to get normality before being analyzed with two-way analysis of variance (ANOVA). Probit analysis was performed using EPA Probit Analysis Program (version 1.5) to estimate the sublethal concentration (LC50) of farnesyl derivatives at 96 h. A t-test was carried out to analyze the effect of farnesyl acetate on the development, pupation, emergence, female ratio, oviposition period, fecundity, and hatching rate of P. xylostella. Significance was accepted at p < 0.05.
3. Results
3.1. Sublethal Concentration of Selected Farnesyl Derivatives on P. xylostella
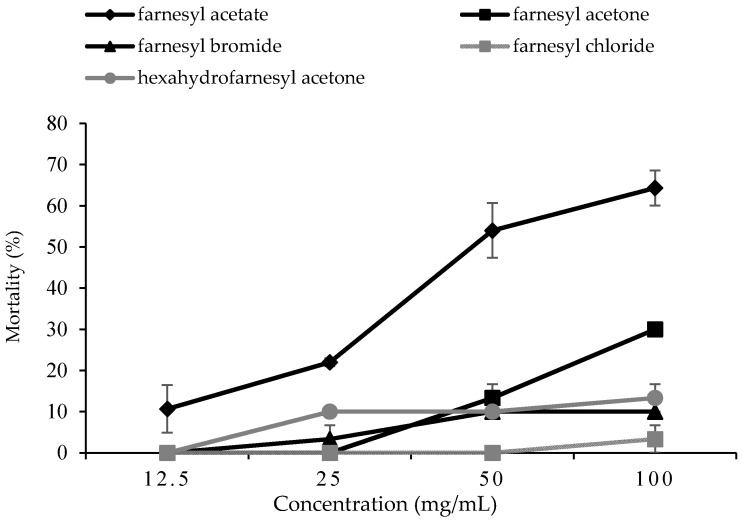
The results of the bioassay showed that treatment of farnesyl derivatives significantly caused various levels of larval mortality (F = 118.309, degrees of freedom (df) = 4, p < 0.0001) (Figure 1). The larval mortality of each treatment was corrected to control mortality (Supplementary Data S1). The highest larval mortality (64%) was observed when P. xylostella were treated with 100 mg/L of farnesyl acetate, followed by farnesyl acetone (30%), hexahydrofarnesyl acetone (13.3%), farnesyl bromide (10%), and farnesyl chloride (3.3%). The larval mortality also significantly increased with increasing concentrations of farnesyl derivatives (F = 70.202, df = 3, p < 0.0001). A significant interaction (F = 14.73, df = 12, p < 0.0001) occurred between farnesyl derivatives and concentration in influencing the larval mortality. The estimated LC50 and LC90 at 96 h of farnesyl acetate were 56.41 and 272.56 mg/L, respectively (Table 2). Meanwhile, the LC50 and LC90 of farnesyl acetone were 142.87 and 407.67 mg/L, respectively. However, other farnesyl derivatives (farnesyl bromide, farnesyl chloride and hexahydrofarnesyl acetone) showed no toxicity effects on P. xylostella larvae.
Figure 1.
Percentage mortality of P. xylostella treated with farnesyl derivatives at various concentrations at 96 h. Data are shown as the mean ± standard error (SE).
Table 2.
Toxicity of farnesyl derivatives on the second instar larvae of P. xylostella. LC50, sublethal concentration.
| Farnesyl Derivatives | N 1 | LC50 | LC90 | Slope ± SE | χ2 | p-Value |
|---|---|---|---|---|---|---|
| Farnesyl acetate | 150 | 56.41 (36.963–92.523) |
272.562 (141.988–1696.881) |
1.87 ± 0.487 | 0.943 | >0.05 |
| Farnesyl acetone | 150 | 142.87 (94.116–849.911) |
407.67 (186.36–23,669.26) |
2.814 ± 0.991 | 1.019 | >0.05 |
| Farnesyl bromide | 150 | - | - | - | ||
| Farnesyl chloride | 150 | - | - | - | ||
| Hexahydrofarnesyl acetone | 150 | - | - | - |
1 Number of larvae tested.
3.2. Sublethal Effects of Farnesyl Acetate
3.2.1. Developmental Time and Pupal Weight
Larval developmental time was significantly extended from 8.4 days (control) to 11.85 days in farnesyl acetate treatment (Table 3). Meanwhile, the development time of pupae was also significantly extended by the sublethal dose of farnesyl acetate compared to the control (p < 0.0001, t = −4.43, df = 58). However, the adulthood period was slightly shorter than that in the control treatment (p = 0.001, t = 3.55, df = 58). Treatment of farnesyl acetate caused a decrease in pupal weight of P. xylostella (Table 4). The pupal weight was significantly reduced by 1.41-fold compared to the control group.
Table 3.
Effects of sublethal concentration of farnesyl acetate on the developmental period of P. xylostella. df, degrees of freedom.
| Developmental Time (Mean ± SE) (Days) | df | t | p | ||
|---|---|---|---|---|---|
| Control | Treatment | ||||
| Second instar larvae | 1.6 ± 0.08 | 2.6 ± 0.09 | 58 | −8.06 | <0.0001 |
| Third instar larvae | 1.6 ± 0.09 | 2.4 ± 0.09 | 58 | −6.22 | <0.0001 |
| Fourth instar larvae | 3.2 ± 0.07 | 4.85 ± 0.14 | 58 | −10.65 | <0.0001 |
| All larvae | 8.4 ± 0.17 | 11.85 ± 0.21 | 58 | −12.00 | <0.0001 |
| Pupa | 5.17 ± 0.17 | 6.17 ± 0.18 | 58 | −4.43 | <0.0001 |
| Adult | 21.4 ± 0.55 | 20.8 ± 0.45 | 58 | 3.55 | 0.001 |
Table 4.
Effects of sublethal concentration of farnesyl acetate on pupal weight, pupation rate, adult emergence rate, female ratio, fecundity, and egg hatching rate (mean ± SE).
| Pupal Weight (mg) | Pupation (%) | Emergence (%) | Female Ratio (%) | Fecundity (Egg/Female) | Egg Hatching (%) | |
|---|---|---|---|---|---|---|
| Control | 7.4 ± 0.07 | 90 ± 1.15 | 92 ± 2.11 | 45.3 ± 1.17 | 104.78 ± 4.4 | 84.17 ± 1.12 |
| Treated | 5.23 ± 0.21 | 66.67 ± 7.69 | 58.7 ± 9.96 | 27.47 ± 3.3 | 25.11 ± 0.98 | 76.64 ± 1.62 |
3.2.2. Pupation and Adult Emergence
The percentage pupation in P. xylostella treated with farnesyl acetate was significantly lower than that in the control group. The pupation rate was only 66.67%, 1.35-fold lower than the control group (Table 4). The adult emergence rate also reduced with farnesyl acetate treatment, which was 1.57-fold lower than the control (p = 0.03, t = 3.27, df = 4) (Table 4).
3.2.3. Female Ratio, Fecundity, and Hatchability
A sublethal concentration of farnesyl acetate significantly reduced the P. xylostella female ratio (p = 0.007, t = 5.11, df = 4), fecundity (p < 0.0001, t = 17.68, df = 16), and egg hatching rate (p = 0.002, t = 3.82, df = 16) (Table 4). The female ratio decreased from 45.3% in the control to 27.47% in the treated group. Meanwhile, the fecundity and egg hatching rate reduced by 4.17- and 1.10-fold, respectively.
3.2.4. Ovipositional Period
Farnesyl acetate at LC50 significantly delayed the pre-oviposition period (p = 0.018, t = −2.64, df = 16), which was 0.45 days longer than the control group (Table 5). Meanwhile, both the oviposition and the post-oviposition periods were significantly shorter compared with those of the control group. The oviposition and post oviposition durations were 3.11 days and 0.75 days shorter than the control group.
Table 5.
Effects of sublethal concentration of farnesyl acetate on pre-oviposition, oviposition, and post-oviposition periods (day ± SE) of P. xylostella.
| Mean ± SE (Days) | df | t | p | ||
|---|---|---|---|---|---|
| Control | Treatment | ||||
| Pre-oviposition | 0.9 ± 0.05 | 1.35 ± 0.16 | 16 | −2.64 | 0.018 |
| Oviposition | 16.33 ± 0.44 | 13.22 ± 0.4 | 16 | 5.22 | <0.0001 |
| Post-oviposition | 3.05 ± 0.19 | 2.3 ± 0.17 | 16 | 2.8 | 0.0123 |
3.2.5. Abnormalities Caused by Farnesyl Acetate Treatment
Farnesyl acetate also induced malformation and abnormalities of P. xylostella morphology, including the formation of larval–pupal intermediates, complete adults that could not emerge from the cocoon, and adults with twisted wings (Figure 2).
Figure 2.
Morphological abnormalities in treated P. xylostella with sublethal concentration of farnesyl acetate at adult and pupal stages: (a) normal adult; (b,c) adult with twisted wings; (d) adult trapped in the cocoon; (e) larvae–pupae intermediates; scale bar: 1 mm.
4. Discussion
This study evaluated the sublethal concentration of selected farnesyl derivatives against P. xylostella. The leaf-dip bioassay revealed that farnesyl acetate showed the highest toxicity and lowest LC50 against P. xylostella compared to other tested farnesyl derivatives. The results also indicated that the LC50 of farnesyl acetate was 4 to 100-fold higher than that of several broad-spectrum insecticides and IGRs such as spinosad, fenvalerate, fipronil, methomyl, teflubenzuron and hexaflumuron [40,43,44,45,46,47] (Table 6). Unfortunately, P. xylostella has developed resistance to most of those insecticides. On the other hand, the LC50 of farnesyl acetate was 2 to 700-fold lower than methamidophos, diflubenzuron and dichlorodiphenyltrichloroethane (DDT) [46,48]. Similarly, the LC50 of farnesyl acetate was 1.66- and 35.63-fold lower than commercially available JHAs, fenoxycarb and pyriproxyfen, respectively [15,16]. The use of a lower concentration of insecticides with high sublethal effects constitutes an environmentally friendly alternative for improving IPM strategies [49]. Furthermore, a recent finding reported that farnesyl acetate exhibited larvicidal activity and induced inhibition of ovary growth in mosquito, Aedes albopictus (Skuse), by modulating the formation and expression of the JH receptor complex [35]. The LC50 concentration of farnesyl acetate was then selected for the study of the sublethal effects on P. xylostella.
Table 6.
Sublethal concentration (LC50) of insecticides on P. xylostella. JHA, juvenile hormone analogue; IGR, insect growth regulator.
| Class | Insecticides | LC50 (mg/L) |
References |
|---|---|---|---|
| Farnesyl acetate | 56.41 | In this study | |
| Farnesyl acetone | 142.87 | In this study | |
| JHA | Pyriproxyfen | 2010 | [15] |
| Fenoxycarb | 93.92 | [16] | |
| IGR | Teflubenzuron | 6.73–1440 | [46] |
| Diflubenzuron | 1907–2171 | [46] | |
| Hexaflumuron | 1.48 | [47] | |
| Broad spectrum | Spinosad | 0.28 | [40] |
| Fenvalerate | 13-2700 | [43] | |
| Fipronil | 7.57 | [44] | |
| Methomyl | 0.4-7.3 | [45] | |
| Methamidophos | 116.35-6755 | [46] | |
| DDT | 170-44200 | [48] |
The treatment with the LC50 of farnesyl acetate on P. xylostella affected the developmental period and process of P. xylostella. The larval and pupal periods of P. xylostella were extended after the application of farnesyl acetate. Previous studies reported that the treatment of JHAs, pyriproxyfen, and fenoxycarb extended the developmental period of larval and pupal stages of the P. xylostella [14,15,16]. On the other hand, the application of a sublethal concentration of hexaflumuron, an IGR, on P. xylostella larvae also prolonged larval and pupal developmental time [49]. Other studies of JHA effects on insects such as beet armyworm (Spodoptera exigua (Hubner)), obliquebanded leafroller (Choristoneura rosaceana (Harris)), Indian meal moth (Plodia Interpunctella (Hubner)), Asian citrus psyllid (Diaphorina citri (Kuwayama)), and lesser grain borer (Rhyzopertha dominica (Fabricius)) also revealed that JHAs extended the development time of the insects [50,51,52,53,54]. The extension of the larval or pupal period might be because of the prolonged presence of JH in the hemolymph [55]. Therefore, ecdysone may not be triggered, interrupting the formation of the next stage. The application of pyriproxyfen on Tenebrio molitor (L.) significantly inhibited the production of ecdysone and, therefore, interfered with the normal development of this insect [56]. Despite the extension of larval duration, the pupal weight decreased following the treatment of farnesyl acetate. The treatment of pyriproxyfen on P. xylostella also reduced pupal weight due to impaired amino-acid uptake, lipid synthesis, and catabolism [15]. Similarly, fenoxycarb severely interrupted lipid synthesis and catabolism, and it decreased food consumption and development rate in silkworms, Bombyx mori (L.) [57]. Hence, the reduction in pupal weight of P. xylostella in this study can perhaps be attributed to disturbed nutrition intake, lipid synthesis and catabolism.
A sublethal concentration of farnesyl acetate had a negative effect on pupation and adult emergence rate of P. xylostella. Treatment of JHA also showed a decreasing adult emergence rate of P. xylostella. The emergence rate in P. xylostella was reduced from 96% in the control group to 76% as a consequence of pyriproxyfen treatment [15]. In Mediterranean flour moth (Ephistea kuehniella (Zeller)), the pupation and emergence rates were very low when fenoxycarb was applied to the last instar of the insect larvae [58]. Treatment of cotton whitefly, Bemisia tabaci (Gennadius) with pyriproxyfen demonstrated a similar pupation rate to the control, but adult emergence was reported to be less than 10% [59]. Meanwhile, only 0–36% of early-instar and 25–74% of late-instar Asian citrus psyllid, D. citri, survived to adults after exposure to pyriproxyfen [53]. Adult emergence of stored grain pests, Oryzaephilus surinamensis (L.), Tribolium castaneum (Herbst), and Trogoderma granarium (Everts), was also reduced when exposed to pyriproxyfen [60].
Previous studies on P. xylostella and S. exigua demonstrated that there were no differences in female ratio after the treatment of a JHA, pyriproxyfen [15,51]. However, the application of JHAs has been known to influence the reproductive ability of insects. The fecundity and egg hatchability of P. xylostella decreased after being treated with pyriproxyfen and fenoxycarb [14,15,16]. Similar effects were observed in C. rosaceana, S. exigua, D. citri and Platynota idaeusalis (Walker) [50,51,53,61] as a result of treatment with JHAs. The egg hatching suppression was also observed in T. castaneum and T. granarium due to pyriproxyfen treatment [62]. In this study, P. xylostella reproduction may have been negatively affected by smaller (underweight) pupae that failed to consume enough nutrients and underwent abnormal physiological processes for growth. The nutritional condition of the female can influence the development of ovaries and egg production [63]. The reduction in macronutrient compounds such as lipids, proteins, and carbohydrates consumed by the insects may result in abnormal oogenesis [64]. The incorporation of pyriproxyfen was reported to affect the ovary’s development and oogenesis, reducing the fecundity and egg hatching rate of P. interpunctella [52]. Another JHA, methoprene, also affected the ovarian growth of Blatta germanica L., causing a drastic decrease in oocytes in newly emerged adults [65].
The ovipositional period plays an important role in the reproductive success of insects. A decreased oviposition time may result in a decrease in fecundity and vice versa. In the present study, the pre-oviposition period of P. xylostella was extended but the oviposition period was shortened compared to the control. The results are similar to the previous study of P. xylostella, indicating that a JHA, pyriproxyfen, delayed the pre-oviposition period of P. xylostella but shortened the oviposition period [14]. The treatment of pyriproxyfen slowed the maturation of the ovaries, thereby postponing adult mating [14]. In contrast, another study reported that pyriproxyfen prolonged the oviposition period of P. xylostella compared to the control [15]. The treatment of P. xylostella with other insecticides such as hexaflumuron (IGR), lufenuron (IGR), and cantharidin (natural toxin from blister beetle) also reduced the oviposition period [46,66,67].
The abnormalities of P. xylostella morphology observed in this study are in accordance with the previous study on a JHA, pyriproxyfen. The treatment of P. xylostella larvae with a sublethal dose of pyriproxyfen triggered abnormalities in P. xylostella, such as untanned pupae, larvae–pupae intermediates, imperfectly sclerotized pupae, adults that were unable to emerge, and adults with twisted winged [15]. Similar morphogenetic abnormalities were reported in S. exigua and D. citri as a consequence of pyriproxyfen treatment [51,53]. At the pupal stage, only ecdysone is released by the insects and JH is absent to allow metamorphosis to occur [68]. Hence, the insect is unable to excrete the excess JH through the introduction of external JHA during the larval period. Consequently, the presence of JHA may induce inhibition of ecdysone production, thereby disturbing the normal larval–pupal molt commitment, and leading to larval–pupal or larval–adult intermediates and other morphological abnormalities [50,56].
5. Conclusions
Our results suggest that farnesyl acetate is highly effective against P. xylostella. A sublethal concentration of farnesyl acetate had negative effects on P. xylostella, such as development, pupal weight, pupation, emergence, fecundity, egg hatchability, and oviposition period. Farnesyl acetate also caused abnormalities in pupae and adults of P. xylostella. This led to low reproductive success and reduced the population number and possibly the infestation level, resulting in good IPM of P. xylostella. However, further works are required to assess the sublethal effects of farnesyl acetate on several generations, and intensive studies are needed to determine the sublethal effect on field populations of insects.
6. Patent
Farnesyl acetate has been filed for a patent (Malaysia Patent Office (MyIPO), Application No. PI2018002697 entitled “An Insect Composition”.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/12/2/109/s1, Data S1: Larval mortality.
Author Contributions
Conceptualization and hypothesis, M.H., I.A.G., and N.W.O.; methodology design and data collection, N.Y.; formal data analysis, N.Y.; first draft, N.Y.; review and editing, I.A.G., N.W.O., W.M.A., and M.H.; supervision, I.A.G., N.W.O., W.M.A., and M.H.; project administration and funding acquisition, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministry of Higher Education (MOHE) of Malaysia Government (Grant number: FRGS/2/2014/ST04/UKM/02/2).
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the study design, data collection, analysis, interpretation, writing of the manuscript, or decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Imran M. Brassica Germplasm-Characterization, Breeding and Utilization. Intechopen; London, UK: 2018. Economic insect pests of Brassica. [Google Scholar]
- 2.Furlong M.J., Wright D.J., Dosdall L.M. Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 2013;58:517–541. doi: 10.1146/annurev-ento-120811-153605. [DOI] [PubMed] [Google Scholar]
- 3.Zalucki M.P., Shabbir A., Silva R., Adamson D., Shu-Sheng L., Furlong M.J. Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): Just how long is a piece of string? J. Econ. Entomol. 2012;105:1115–1129. doi: 10.1603/EC12107. [DOI] [PubMed] [Google Scholar]
- 4.Kermani N., Abu-Hassan Z.A., Dieng H., Ismail N.F., Attia M., Abd Ghani I. Pathogenicity of Nosema sp.(Microsporidia) in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) PLoS ONE. 2013;8:e62884. doi: 10.1371/journal.pone.0062884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bortoli S.A., Vacari A.M., Goulart R.M., Ferraudo A.S., Volpe H.X. Classification of crucifer cultivars based on the life-history of diamondback moth (Plutella xylostella) Int. J. Pest Manag. 2013;59:73–78. doi: 10.1080/09670874.2013.765057. [DOI] [Google Scholar]
- 6.Talekar N., Shelton A. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 1993;38:275–301. doi: 10.1146/annurev.en.38.010193.001423. [DOI] [Google Scholar]
- 7.Li Z., Feng X., Liu S.S., You M., Furlong M.J. Biology, ecology, and management of the diamondback moth in China. Annu. Rev. Entomol. 2016;61:277–296. doi: 10.1146/annurev-ento-010715-023622. [DOI] [PubMed] [Google Scholar]
- 8.Wee S.L. Effects of conspecific herbivory and mating status on host searching and oviposition behavior of Plutella xylostella (Lepidoptera: Plutellidae) in relation to its host, Brassica oleracea (Brassicales: Brassicaceae) Fla. Entomol. 2016;99:159–165. doi: 10.1653/024.099.sp119. [DOI] [Google Scholar]
- 9.Kumar V., Yadav C.S., Singh S., Goel S., Ahmed R.S., Gupta S., Grover R.K., Banarjee B.D. CYP 1A1 polymorphism and organochlorine pesticides levels in the etiology of prostate cancer. Chemosphere. 2010;81:464–468. doi: 10.1016/j.chemosphere.2010.07.067. [DOI] [PubMed] [Google Scholar]
- 10.López Ó., Fernández-Bolaños J.G., Gil M.V. New trends in pest control: The search for greener insecticides. Green Chem. 2005;7:431–442. doi: 10.1039/b500733j. [DOI] [Google Scholar]
- 11.Rust M.K., Lance W., Hemsarth H. Synergism of the IGRs methoprene and pyriproxyfen against larval cat fleas (Siphonaptera: Pulicidae) J. Med. Entomol. 2016;53:629–633. doi: 10.1093/jme/tjw010. [DOI] [PubMed] [Google Scholar]
- 12.Lawler S.P. Environmental safety review of methoprene and bacterially-derived pesticides commonly used for sustained mosquito control. Ecotoxicol. Environ. Saf. 2017;139:335–343. doi: 10.1016/j.ecoenv.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 13.Tunaz H., Uygun N. Insect growth regulators for insect pest control. Turk. J. Agric. For. 2004;28:377–387. [Google Scholar]
- 14.Mahmoudvand M., Moharramipour S., Iranshahi M. Effects of pyriproxyfen on life table indices of Plutella xylostella in multigenerations. Psyche. 2015;2015:453701. [Google Scholar]
- 15.Alizadeh M., Karimzadeh J., Rassoulian G.R., Farazmand H., Hosseini-Naveh V., Pourian H.R. Sublethal effects of pyriproxyfen, a juvenile hormone analogue, on Plutella xylostella (Lepidoptera:Plutellidae): Life table study. Arch. Phytopathol. Pflanzenschutz. 2012;45:1741–1763. doi: 10.1080/03235408.2012.706426. [DOI] [Google Scholar]
- 16.Mahmoudvand M., Moharramipour S. Sublethal effects of fenoxycarb on the Plutella xylostella (Lepidoptera: Plutellidae) J. Insect Sci. 2015;15:82. doi: 10.1093/jisesa/iev064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang E.C., Wu P.S., Chang H.C., Chen Y.W. Effect of sub-lethal dosages of insecticides on honeybee behavior and physiology; Proceedings of the International Seminar on Enhancement of Functional Biodiversity Relevant to Sustainable Food Production in ASPAC; Tsukuba, Japan. 8–12 November 2010; pp. 9–11. [Google Scholar]
- 18.Fourrier J., Deschamps M., Droin L., Alaux C., Fortini D., Beslay D., Decourtye A. Larval exposure to the juvenile hormone analog pyriproxyfen disrupts acceptance of and social behavior performance in adult honeybees. PLoS ONE. 2015;10:e0132985. doi: 10.1371/journal.pone.0132985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y.W., Wu P.S., Yang E.C., Nai Y.S., Huang Z.Y. The impact of pyriproxyfen on the development of honey bee (Apis mellifera L.) colony in field. J. Asia-Pac. Entomol. 2016;19:589–594. doi: 10.1016/j.aspen.2016.06.005. [DOI] [Google Scholar]
- 20.Arambourou H., Fuertes I., Vulliet E., Daniele G., Noury P., Delorme N., Barata C. Fenoxycarb exposure disrupted the reproductive success of the amphipod Gammarus fossarum with limited effects on the lipid profile. PLoS ONE. 2018;13:e0196461. doi: 10.1371/journal.pone.0196461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cusson M., Sen S.E., Shinoda T. Juvenile hormone biosynthetic enzymes as targets for insecticide discovery. In: Ishaaya I., Palli S.R., Horowitz A.R., editors. Advanced Technologies for Managing Insect Pests. Springer; Dordrecht, The Netherlands: 2013. pp. 31–55. [Google Scholar]
- 22.Noriega F.G. Juvenile hormone biosynthesis in insects: What is new, what do we know, and what questions remain? Int. Sch. Res. Not. 2014;2014:967361. doi: 10.1155/2014/967361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J., Marchal E., Hult E.F., Tobe S.S. Characterization of the juvenile hormone pathway in the viviparous cockroach, Diploptera punctata. PLoS ONE. 2015;10:e0117291. doi: 10.1371/journal.pone.0117291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W., Ma L., Xiao H., Liu C., Chen L., Wu S., Liang G. Identification and characterization of genes involving the early step of juvenile hormone pathway in Helicoverpa armigera. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-16319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Ekert E., Heylen K., Rougé P., Powell C.A., Shatters R.G., Jr., Smagghe G., Borovsky D. Aedes aegypti juvenile hormone acid methyl transferase, the ultimate enzyme in the biosynthetic pathway of juvenile hormone III, exhibits substrate control. J. Insect Physiol. 2014;64:62–73. doi: 10.1016/j.jinsphys.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Yin Y., Qiu Y.W., Huang J., Tobe S.S., Chen S.S., Kai Z.P. Enzymes in the juvenile hormone biosynthetic pathway can be potential targets for pest control. Pest Manag. Sci. 2020;76:1071–1077. doi: 10.1002/ps.5617. [DOI] [PubMed] [Google Scholar]
- 27.Zifruddin A.N., Mohamad-Khalid K.A., Suhaimi S.A., Mohamed-Hussein Z.A., Hassan M. Molecular Characterization and Enzyme Inhibition Studies of a Novel NADP+- Farnesol Dehydrogenase from Diamondback Moth, Plutella Xylostella (Lepidoptera:Plutellidae) Manuscript in preparation. [DOI] [PubMed] [Google Scholar]
- 28.Ahmad-Sohdi N.A.S., Seman-Kamarulzaman A.F., Mohamed-Hussein Z.A., Hassan M. Purification and characterization of a novel NADP+-farnesol dehydrogenase from Polygonum minus leaves. PLoS ONE. 2015;10:e0143310. doi: 10.1371/journal.pone.0143310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayoral J.G., Nouzova M., Navare A., Noriega F.G. NADP+-dependent farnesol dehydrogenase, a corpora allata enzyme involved in juvenile hormone synthesis. Proc. Natl. Acad. Sci. USA. 2009;106:21091–21096. doi: 10.1073/pnas.0909938106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue H., Tsuji H., Uritani I. Characterization and activity change of farnesol dehydrogenase in black rot fungus-infected sweet potato. Agric. Biol. Chem. 1984;48:733–738. doi: 10.1080/00021369.1984.10866210. [DOI] [Google Scholar]
- 31.Bhandari J., Fitzpatrick A.H., Crowell D.N. Identification of a novel abscisic acid-regulated farnesol dehydrogenase from Arabidopsis. Plant Physiol. 2010;154:1116–1127. doi: 10.1104/pp.110.157784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain A., Rizwan-Ul-Haq M., AlJabr A.M., Al-Ayedh H. Lethality of sesquiterpenes reprogramming red palm weevil detoxification mechanism for natural novel biopesticide development. Molecules. 2019;24:1648. doi: 10.3390/molecules24091648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Govindarajan M., Benelli G. Artemisia absinthium-borne compounds as novel larvicides: Effectiveness against six mosquito vectors and acute toxicity on non-target aquatic organisms. Parasitol. Res. 2016;115:4649–4661. doi: 10.1007/s00436-016-5257-1. [DOI] [PubMed] [Google Scholar]
- 34.Dancewicz K., Gliszczynska A., Halarewicz A., Wawrzenczyk C., Gabrys B. Effect of farnesol and its synthetic derivatives on the settling behaviour of the peach potato aphid Myzus persicae (Sulz.) Pestycydy. 2010;1–4:51–57. [Google Scholar]
- 35.Park D.H., Choi J.Y., Lee S.H., Kim J.H., Park M.G., Kim J.Y., Wang M., Kim H.J., Je Y.H. Mosquito larvicidal activities of farnesol and farnesyl acetate via regulation of juvenile hormone receptor complex formation in Aedes mosquito. J. Asia-Pac. Entomol. 2020;23:689–693. doi: 10.1016/j.aspen.2020.05.006. [DOI] [Google Scholar]
- 36.Kandil M.A., Abdel-kerim R.N., Moustafa M.A. Lethal and sub-lethal effects of bio-and chemical insecticides on the tomato leaf miner, Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae) Egypt. J. Biol. Pest Control. 2020;30:1–7. doi: 10.1186/s41938-020-00278-1. [DOI] [Google Scholar]
- 37.Hassan M., Yusoff N., Aizat W.M., Othman N.W., Abd Ghani I. Optimization method for proteomic analysis of the larva and adult tissues of Plutella xylostella (L.) (Lepidoptera: Plutellidae) Sains Malays. 2018;47:2975–2983. doi: 10.17576/jsm-2018-4712-06. [DOI] [Google Scholar]
- 38.Hui W., Juan W., Li H.S., Dai H.G., Gu X.J. Sub-lethal effects of fenvalerate on the development, fecundity, and juvenile hormone esterase activity of diamondback moth, Plutella xylostella (L.) Agric. Sci. China. 2010;9:1612–1622. [Google Scholar]
- 39.Amarasekare K.G., Shearer P.W. Laboratory bioassays to estimate the lethal and sublethal effects of various insecticides and fungicides on Deraeocoris brevis (Hemiptera: Miridae) J. Econ. Entomol. 2013;106:776–785. doi: 10.1603/EC12432. [DOI] [PubMed] [Google Scholar]
- 40.Yin X.H., Wu Q.J., Li X.F., Zhang Y.J., Xu B.Y. Sublethal effects of spinosad on Plutella xylostella (Lepidoptera: Yponomeutidae) Crop Prot. 2008;27:1385–1391. doi: 10.1016/j.cropro.2008.05.008. [DOI] [Google Scholar]
- 41.Xu C., Zhang Z., Cui K., Zhao Y., Han J., Liu F., Mu W. Effects of sublethal concentrations of cyantraniliprole on the development, fecundity and nutritional physiology of the black cutworm Agrotis ipsilon (Lepidoptera: Noctuidae) PLoS ONE. 2016;11:e0156555. doi: 10.1371/journal.pone.0156555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abbott W.S. A method of computing effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–270. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 43.Liu M.Y., Tzeng Y.J., Sun C.N. Diamondback moth resistance to several synthetic pyrethroids. J. Econ. Entomol. 1981;74:393–396. doi: 10.1093/jee/74.4.393. [DOI] [Google Scholar]
- 44.Sayyed A.H., Wright D.J. Fipronil resistance in the diamondback moth (Lepidoptera: Plutellidae): Inheritance and number of genes involved. J. Econ. Entomol. 2004;97:2043–2050. doi: 10.1093/jee/97.6.2043. [DOI] [PubMed] [Google Scholar]
- 45.Santos V.C., De Siqueira H.A.A., Da Silva J.E., De Farias M.J.D.C. Insecticide resistance in populations of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae), from the state of Pernambuco, Brazil. Neotrop. Entomol. 2011;40:264–270. doi: 10.1590/S1519-566X2011000200017. [DOI] [PubMed] [Google Scholar]
- 46.Syed A.R. Insecticide resistance in diamondback moth in Malaysia; Proceedings of the Second International Workshop on the Management of Diamondback Moth and Other Crucifer Pests; Tainan, Taiwan. 10–14 December 1990; Shanhua, Taiwan: Asian Vegetable Research and Development Center; 1992. pp. 437–442. [Google Scholar]
- 47.Mahmoudvand M., Abbasipour H., Garjan A.S., Bandani A.R. Sublethal effects of hexaflumuron on development and reproduction on the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae) Insect Sci. 2011;18:689–696. doi: 10.1111/j.1744-7917.2011.01411.x. [DOI] [Google Scholar]
- 48.Tabashnik B.E., Cushing N.L., Johnson M.W. Diamondback moth (Lepidoptera: Plutellidae) resistance to insecticides in Hawaii: Intra-island variation and cross-resistance. J. Econ. Entomol. 1987;80:1091–1099. doi: 10.1093/jee/80.6.1091. [DOI] [Google Scholar]
- 49.Mahmoudvand M., Abbasipour H., Garjan A.S., Bandani A.R. Decrease in pupation and adult emergence of Plutella xylostella (L.) treated with hexaflumuron. Chil. J. Agric. Res. 2012;72:206–211. doi: 10.4067/S0718-58392012000200007. [DOI] [Google Scholar]
- 50.Sial A.A., Brunner J.F. Lethal and sublethal effects of an insect growth regulator, pyriproxyfen, on obliquebanded leafroller (Lepidoptera: Tortricidae) J. Econ. Entomol. 2010;103:340–347. doi: 10.1603/EC09295. [DOI] [PubMed] [Google Scholar]
- 51.Moadeli T., Hejazi M.J., Golmohammadi G. Lethal effects of pyriproxyfen, spinosad, and indoxacarb and sublethal effects of pyriproxyfen on the 1st instars larvae of beet armyworm, Spodoptera exigua Hübner (Lepidoptera: Noctuidae) in the Laboratory. J. Agric. Sci. Technol. 2014;16:1217–1227. [Google Scholar]
- 52.Ghasemi A., Sendi J., Ghadamyari M. Physiological and biochemical effect of pyriproxyfen on Indian meal moth Plodia interpunctella (Hübner)(Lepidoptera: Pyralidae) J. Plant Pro. Res. 2010;50:416–422. doi: 10.2478/v10045-010-0070-9. [DOI] [Google Scholar]
- 53.Boina D.R., Rogers M.E., Wang N., Stelinskia L.L. Effect of pyriproxyfen, a juvenile hormone mimic, on egg hatch, nymph development, adult emergence and reproduction of the Asian citrus psyllid, Diaphorina citri Kuwayama. Pest Manag. Sci. 2010;66:349–357. doi: 10.1002/ps.1880. [DOI] [PubMed] [Google Scholar]
- 54.Jangir H., Kumawat K.C., Sharma P., Yadav D., Ranawat Y.S. Bioefficacy of insect growth regulators (IGRs) as seed protectant against lesser grain borer, Rhyzopertha dominica (Fab.) on wheat. J. Entomol. Zool. Stud. 2018;6:1641–1646. [Google Scholar]
- 55.Kuwano E., Fujita N., Furuta K., Yamada N. Synthesis and biological activity of novel anti-juvenile hormone agents. J. Pestic. Sci. 2008;33:14–16. doi: 10.1584/jpestics.R07-08. [DOI] [Google Scholar]
- 56.Aribi N., Smagghe G., Lakbar S., Soltani-Mazouni N., Soltani N. Effects of pyriperoxyfen, a juvenile hormone analog, on development of the mealworm, Tenebrio molitor. Pestic. Biochem. Physiol. 2006;84:55–62. doi: 10.1016/j.pestbp.2005.05.008. [DOI] [Google Scholar]
- 57.Leonardi M.G., Marciani P., Montorfono P.G., Cappellozza S., Giordana B., Monticalli G. Effects of fenoxycarb on leucine uptake and lipid composition of midgut brush border membrane in the silkworm, Bombyx mori (Lepidoptera: Bombycidae) Pestic. Biochem. Physiol. 2001;70:42–51. doi: 10.1006/pest.2001.2537. [DOI] [Google Scholar]
- 58.Moreno J., Hawlitzky N., Jimenez R. Effect of the juvenile hormone analog fenoxycarb on the last larval instar of Ephestia kuehniella Zell.(Lep., Pyralidae) J. Appl. Entomol. 1992;114:118–123. doi: 10.1111/j.1439-0418.1992.tb01105.x. [DOI] [Google Scholar]
- 59.Ishaaya I., Horowitz R. Pyriproxyfen, a novel insect growth regulator for controlling whiteflies. Mechanism and resistance management. Pestic. Sci. 1995;43:227–232. doi: 10.1002/ps.2780430308. [DOI] [Google Scholar]
- 60.Yasir M., Hasan M., Sagheer M., Fiaz M., Serrão J.E. Residual efficacy of pyriproxyfen on grain commodities against stored product insect pests. Gesunde Pflanz. 2020;72:265–272. doi: 10.1007/s10343-020-00509-3. [DOI] [Google Scholar]
- 61.Biddinger D.J., Hull L.A. Sublethal effects of selected insecticides on growth and reproduction of a laboratory susceptible strain of tufted apple bud moth (Lepidoptera: Tortricidae) J. Econ. Entomol. 1999;92:314–324. doi: 10.1093/jee/92.2.314. [DOI] [Google Scholar]
- 62.Ali Q., Ranjha M.H., Sahi G.M., Faisal M., Shakir H.U., Anjum N.A., Hanif M.S., Albaayit S.F.A. Ovicidal effect of insect growth regulators against eggs of Trogoderma granarium (Everts) and Tribolium castaneum (Herbst) Pak. J. Agric. Sci. 2020;57:73–79. [Google Scholar]
- 63.Harburguer L., Zerba E., Licastro S. Sublethal effect of pyriproxyfen released from a fumigant formulation on fecundity, fertility, and ovicidal action in Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 2014;51:436–443. doi: 10.1603/ME13137. [DOI] [PubMed] [Google Scholar]
- 64.Kanost M.R., Kawooya J.K., Law J.H., Ryan R.O., van Heusden M.C., Ziegler R. Insects haemolymph proteins. Adv. Insect Physiol. 1990;22:299–396. [Google Scholar]
- 65.Maiza A., Kilani S., Fraine J.P., Aribi N., Soltani N. Reproductive effects in German cockroach by ecdysteroid agonist RH- 0345, juvenile hormone analogue methoprene and carbamate benfuracarb. Commun. Agric. Appl. Biol. Sci. 2004;69:257–266. [PubMed] [Google Scholar]
- 66.Josan A., Singh G. Sublethal effects of lufenuron on the diamondback moth, Plutella xylostella (Linnaeus) Int. J. Trop. Insect Sci. 2004;20:303–308. doi: 10.1017/S1742758400015666. [DOI] [Google Scholar]
- 67.Huang Z., Wang Y., Zhang Y. Lethal and sublethal effects of cantharidin on development and reproduction of Plutella xylostella (Lepidoptera: Plutellidae) J. Econ. Entomol. 2015;108:1054–1064. doi: 10.1093/jee/tov057. [DOI] [PubMed] [Google Scholar]
- 68.Chapman R.F. The Insects Structure and Function. Cambridge University Press; New York, NY, USA: 1998. p. 750. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.