Abstract
Simple Summary
Trissolcus japonicus, an important natural enemy of brown marmorated stink bug in Asia, was first detected in the USA in 2014. To investigate when and where T. japonicus is found in the field, yellow sticky traps were deployed in the canopy of tree of heaven growing at the edge of small isolated patches, windbreaks, and woodlots in 2018 and 2019. In both years, captures occurred from May to September, with peaks in July and August. Captures of T. japonicus were recorded from all three habitats but were not consistently associated with a particular habit. In 2017 and 2018, T. japonicus captures were compared between tree of heaven paired with several other H. halys host trees growing at the woods edge, and in 2019, captures in tree of heaven, black walnut, and black locust growing in the same windbreaks were compared. Trissolcus japonicus and several native H. halys parasitoids were captured in all hosts, but there was not a consistent effect of host tree species on T. japonicus captures. These results can be used to inform and optimize future surveillance efforts for detecting T. japonicus as it continues to expand its range in the USA.
Abstract
Trissolcus japonicus, an important egg parasitoid of Halyomorpha halys in Asia, was first detected in the USA in 2014. To evaluate the effect of habitat and the seasonality of T. japonicus detections in the USA, yellow sticky traps were placed in the canopy of Ailanthus altissima growing at the edge of isolated patches of trees, windbreaks, and woodlots in northern Virginia in 2018 and 2019. In both years, captures occurred from May to September, and peaked in July and August. While T. japonicus was detected in all habitats, there was not a consistent effect of habitat type on capture frequency. To evaluate tree species effects on T. japonicus captures, in 2017 and 2018, yellow sticky traps deployed in the canopy of A. altissima bordering apple orchards were paired with a nearby trap in one of several wild tree species along a common woods edge. In 2019, these traps were deployed in A. altissima, black walnut, and black locust growing in the same windbreaks. No consistent association between captures of T. japonicus or native parasitoids of H. halys and the tree species sampled was observed among years. Results are discussed in relation to the ecology and sampling optimization of T. japonicus.
Keywords: biological control, parasitoid, Halyomorpha halys, Ailanthus altissima
1. Introduction
Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), is a polyphagous invasive stink bug from Asia that has been a severe agricultural and nuisance pest in many parts of the USA since the late 2000s [1]. A widespread outbreak in 2010 resulted in major losses to the apple and peach crops in the Mid-Atlantic USA [2]. To manage H. halys, many American tree fruit producers increased their use of broad-spectrum insecticides [3], but this is certainly not considered a sustainable, long-term solution. Ultimately, biological control may play an important role in sustainable H. halys management [1]. Surveys of native H. halys parasitoids in North America [4] revealed that the species detected most commonly from sentinel egg masses included members of the genera Anastatus Motschulsky (Hymenoptera: Eupelmidae), Telenomus Haliday (Hymenoptera: Scelionidae), and Trissolcus Ashmead (Hymenoptera: Scelionidae). However, field studies have suggested that endemic parasitoids and predators in the USA are not yet regulating H. halys populations adequately [4,5].
Detections of North American H. halys parasitoids have been somewhat habitat dependent [4]. Trissolcus species tended to be more prevalent in ornamental, semi-natural/urban, and forest habitats than in other systems [4], and Herlihy et al. [6] found that this genus predominated in wooded habitats. Parasitization of sentinel H. halys eggs did not differ between exotic or native host plants but was greatest at the edge of unmanaged woods [7]. Anastatus species were among the most common parasitoids in ornamental systems [8].
Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae) is one of the most important natural enemies of H. halys in Asia [9,10]. The discovery of an adventive population of T. japonicus in Maryland in 2014 [11] prompted more intensive and extensive surveillance for H. halys parasitoids in North America and Europe, resulting in T. japonicus detections in 13 states, Washington, DC [6,12,13,14], Canada, Italy, and Switzerland [15,16,17,18]. Although Abram et al. [19] noted that the effects of egg parasitoids, including T. japonicus, on H. halys populations have not been quantified, intensified sampling for T. japonicus in the USA to track changes in its spread and abundance is warranted and would benefit from a better understanding of its temporal and spatial distributions.
Zhang et al. [10] documented the seasonal parasitism of H. halys eggs by T. japonicus in Beijing, China, although its seasonal phenology elsewhere has not been reported. In the USA, it has been detected in woodland [6,20,21] and lightly wooded residential habitats [13], and Herlihy et al. [6] reported that it parasitized significantly more H. halys sentinel eggs in wooded habitats than in soybean or apple plantings. In both the USA [20] and China [10], T. japonicus has also been detected in peach orchards. However, there have not been systematic comparisons of T. japonicus detection frequency and relative abundance among the wooded habitats in which it has been found, particularly those adjacent to crops at risk from H. halys attack. Moreover, although T. japonicus detections in the USA have been most common in arboreal habitats [6,11,21,22,23], its presence and abundance among different H. halys tree hosts has not been examined.
Optimizing the efficiency of sampling to track changes in the range and abundance of T. japonicus in the USA can be achieved and informed by a greater understanding of potential habitat and plant species effects on its detection frequency, and by documenting its seasonal phenology. Here, studies conducted in northern Virginia, USA, where T. japonicus has been present since at least 2015 [21], address adventive T. japonicus captures in relation to habitat type, plant species, and seasonality.
2. Materials and Methods
2.1. Seasonal Phenology and Habitat Type
Study sites were unmanaged, wooded habitats adjacent to commercial and experimental orchards in the counties of Frederick (14 sites) and Shenandoah (1 site), Virginia, USA, where T. japonicus has been detected consistently since 2015. All sites were within 10 km of Virginia Tech’s Alson H. Smith Jr. Agricultural Research and Extension Center (AHSAREC) (39.112867, −78.284029) in Frederick county, and the same sites were used for trapping in 2018 and 2019.
Tree of heaven, Ailanthus altissima (Mill.) Swingle, is an invasive Asian species [24] that often grows prolifically in disturbed or semi-disturbed locations in Virginia and elsewhere in the USA [25]. In this area, it was the most abundant deciduous tree species at the edge of woodlands bordering tree fruit orchards [26] and supports all H. halys life stages [27]. In addition, inspection of the foliage of felled tree of heaven yielded H. halys egg masses parasitized by T. japonicus [20]. The prevalence of this tree and the occurrence of both H. halys and T. japonicus on it led to the selection of female tree of heaven for standardized sampling.
In this area, three common habitat types in which tree of heaven grows are: (1) spatially isolated, often roughly circular patches typically associated with rock breaks in otherwise cultivated or fallow fields; (2) windbreaks or hedgerows; (3) the edge of woodlands (Figure 1). All of these habitat types are commonly associated with commercial tree fruit orchards and thus were selected for sampling (n = 5 sites per type). The mean (± SE) distance (km) between isolated patch, windbreak, and woodlot sites was, 3.8 ± 0.5, 6.5 ± 0.7, and 4.2 ± 0.5, respectively, and the mean (± SE) distance (km) between a given site and the site nearest to it was 0.84 ± 0.24. The mean (± SE) area (m2) comprised of trees at these sites was; (1) isolated patches 278.6 ± 88.1 (17.3 ± 3.6 m at widest point), (2) windbreaks 2629.2 ± 827.5 (10.7 ± 1.7 m at widest point), and (3) woods 649,866.4 ± 254,141.6 (977.8 ± 130.0 m at widest point).
Figure 1.
Representative habitat types in which Trissolcus japonicus was sampled using yellow sticky traps in the mid-canopy of mature female tree of heaven near orchards in Frederick and Shenandoah counties, VA in 2018 and 2019: (A) Isolated patches of predominantly tree of heaven, (B) Long, narrow windbreak of mixed wild trees and shrubs, (C) Contiguous, unmanaged woodland with wild trees and shrubs. Marker indicates trap location in trees of at the edge of each habitat.
As described in Quinn et al. [22], backfolding yellow sticky traps (23 × 28 cm, Alpha Scents, West Linn, OR, USA) deployed atop 4.8 m bamboo poles were used for sampling. Holes were punched through both halves of folded traps, at about 2.54 cm on either side of the center point and about 1.9 cm from the edge. A 45.7 cm length of twist tie was inserted through the holes, the top of the pole was inserted between the trap sides, and the twist tie was used to secure the trap by wrapping and twisting it around the pole and finally to the shank of a wire hook affixed to the pole below the trap. Interlocking tabs on the trap corners ensured that both sides were closely appressed to the pole when deployed. One trap was deployed in the mid-canopy of a mature female tree of heaven growing at the habitat edge at each site by suspending the pole from a lateral branch via the wire hook. Traps were deployed on 3 May and 20 April in 2018 and 2019, respectively, and replaced at 7 ± 2-day intervals through 21 or 30 September in the respective years.
2.2. Host Plant Comparisons
Mature trees at the edge of woodlands (2017 and 2018) and windbreaks (2019) adjacent to tree fruit orchards within 10 km of the AHSAREC were used for trapping. The height and diameter at breast height (DBH) of each sample tree was recorded using a Nikon Forestry Pro Hypsometer (Nikon Corporation, Tokyo, Japan) and measuring tape, respectively. As in the previous study, female tree of heaven (11.2 ± 1.3 m tall, 0.2 ± 0.02 m DBH) was the standard species used in all paired host comparisons. The endemic species used were black walnut, Juglans nigra, L. (Fagales: Juglandaceae) (12.4 ± 1.5 m tall, 1.3 ± 0.8 m DBH), black locust, Robinia pseudoacacia L. (Fabales: Fabaceae) (8.5 ± 0.9 m tall, 0.13 ± 0.03 m DBH), hackberry, Celtis occidentalis L. (Rosales: Cannabaceae) (16.1 ± 5.5 m tall, 0.3 ± 0.06 m DBH), and black cherry Prunus serotina Ehrh. (Rosales: Rosaceae) (8.2 m ± 3.7 tall, 0.2 m ± 0.01 DBH), all of which were also among the most common wild trees recorded in this region [26] and are known hosts of H. halys [27]. In addition to representing a diversity of plant families, these trees differ in leaf structure (i.e., simple versus complex). The same tree of heaven, black walnut, black locust, and hackberry trees were used in 2017 and 2018, and black cherry was added in 2018.
Backfolding yellow sticky traps were deployed as described previously. At each site, a trap in female tree of heaven was paired with a trap in one of the aforementioned species (n = 5 per species pairing). Trees within pairs were 10 to 25 m apart and the mean distance between pairs was 3.4 ± 0.2 km. Traps were replaced at 7 ± 2-day intervals from 31 July until 29 August 2017 and 13 June until 20 September 2018.
In 2019, sampling was conducted at five windbreaks, separated by 5.6 ± 0.7 km. At each site, a single yellow sticky trap was deployed as described previously in one tree of heaven (8.5 ± 1.0 m tall, 0.1 ± 0.01 m DBH), one black walnut (10.1 ± 1.02 m tall, 0.4 ± 0.01 m DBH), and one black locust (7.1 ± 1.6 m tall, 0.1 ± 0.0.2 m DBH). Adjacent sampling trees were 23.7 ± 8.6 m apart and the distance between trees at the ends of the sampling area was 47.4 ± 15.9 m. Traps were replaced at 7 ± 1-day intervals from 17 June until 11 August.
2.3. Parasitoid Identification
For all studies, all parasitoids of interest captured (i.e., those considered to be potential H. halys parasitoids) were tentatively identified in situ in the laboratory, following Talamas et al. [28]. With the exception of Anastatus spp., all specimens were sent in situ on a small piece of the trap to E.J. Talamas for species confirmation. Male and female T. japonicus captured in the 2018 and 2019 habitat type study were differentiated based on antennal morphology [29].
2.4. Statistical Analysis
For each year, seasonal detections of T. japonicus are presented as total male and female T. japonicus from pooled captures across all habitat types by week. To compare T. japonicus captures among habitat types, data from each year were pooled across sample dates and analyzed using the Kruskal-Wallis test followed by the Bonferroni corrected Dunn’s test (SAS Institute, Cary, NC, USA; SAS Institute Inc. 2018). For the 2017 and 2018 paired host study, captures of T. japonicus were compared by tree species pair using the Wilcoxon signed-rank test. In 2019, captures were compared among the three host species using the Kruskal–Wallis test followed by the Bonferroni corrected Dunn’s test. All statistical comparisons used SAS 9.4 [30] and were considered significant at p < 0.05.
3. Results
3.1. Seasonal Captures of Trissolcus japonicus
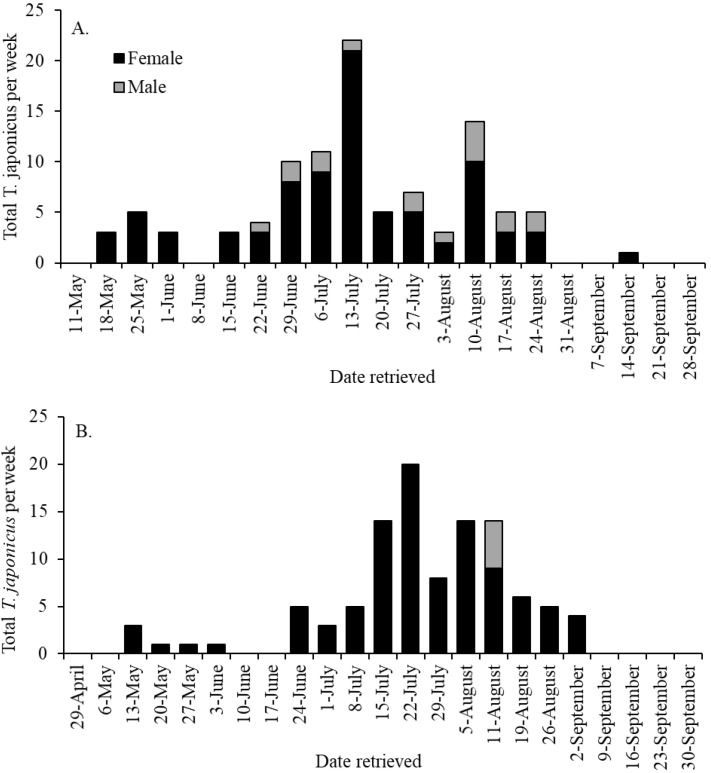
In 2018 and 2019, respectively, 101 (83.2% female) and 104 (95.2% female) T. japonicus were captured across all habitats sampled (Table 1 and Table 2). In 2018, the first capture was recorded on 18 May (Figure 2A) and captures occurred on most weeks through 14 September, with peak captures on 13 July and 10 August. The last detection of T. japonicus was recorded on 14 September. Interestingly, males were captured only between mid-June and late August. In 2019, the date of first T. japonicus capture, 13 May (Figure 2B), was similar to that in 2018. Again, T. japonicus was captured on most weeks between mid-May and early September, but males were recorded only on 11 August. Additionally, similar to 2018, peak captures in 2019 occurred on 22 July and between 5 and 11 August, and the last capture was recorded on 2 September.
Table 1.
Adventive and native parasitoids of Halyomorpha halys captured in yellow sticky traps in the mid-canopy of mature female tree of heaven in Frederick and Shenandoah counties, Virginia, USA 2018.
| Habitat | Trissolcus | Telenomus | Anastatus | Encyrtidae | |||||
|---|---|---|---|---|---|---|---|---|---|
| japonicus | brochymenae | thyantae | euschisti | edessae | podisi | spp. | spp. | ||
| Patch | 40 | 2 | 0 | 4 | 0 | 2 | 2 | 2 | 0 |
| Windbreak | 50 | 6 | 0 | 14 | 1 | 3 | 2 | 1 | 0 |
| Woods edge | 11 | 2 | 1 | 11 | 0 | 4 | 5 | 4 | 2 |
| Total | 101 | 10 | 1 | 29 | 1 | 9 | 9 | 7 | 2 |
Table 2.
Adventive and native parasitoids of Halyomorpha halys captured in yellow sticky traps in the mid-canopy of mature female tree of heaven in Frederick and Shenandoah counties, Virginia, USA 2019.
| Habitat | Trissolcus | Telenomus | Gryon | Anastatus | Encyrtidae | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| japonicus | brochymenae | thyantae | euschisti | podisi | persimilis | spp. | spp. | spp. | ||
| Patch | 43 | 2 | 5 | 18 | 20 | 0 | 9 | 1 | 5 | 2 |
| Windbreak | 35 | 4 | 7 | 25 | 17 | 1 | 2 | 1 | 5 | 0 |
| Woods edge | 26 | 4 | 7 | 15 | 6 | 2 | 10 | 0 | 4 | 1 |
| Total | 104 | 10 | 19 | 58 | 43 | 3 | 21 | 2 | 14 | 3 |
Figure 2.
Weekly captures of Trissolcus japonicus in yellow sticky traps in the mid-canopy of mature female tree of heaven in Frederick and Shenandoah counties, Virginia, USA in (A) 2018 and (B) 2019. Captures in isolated patches, windbreaks, and woodlands were pooled by week.
3.2. Effect of Habitat Type on T. japonicus Captures
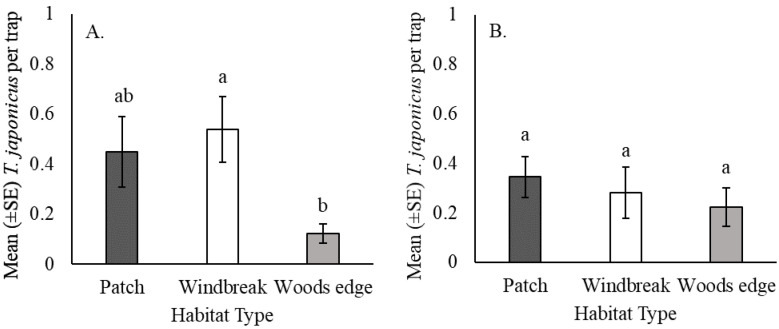
In 2018, there was a significant effect of habitat type on T. japonicus captures (χ2 = 8.31, df = 2, p < 0.05); significantly more were captured in windbreaks than at the woods edge (Zwindbreak = 7.9, Zwoods edge = 6.9, p < 0.05), with no difference between the woods edge and isolated patches (Zwoods edge = 6.9, Zpatch= 7.4, p > 0.05) or between windbreaks and isolated patches (Zwindbreak = 7.9, Zpatch = 7.4, p > 0.05) (Figure 3A). In 2019, however, habitat type did not significantly affect captures (χ2 = 0.25, df = 2, p > 0.05) (Figure 3B).
Figure 3.
Trissolcus japonicus captures in yellow sticky traps in the mid-canopy of mature female tree of heaven in Frederick and Shenandoah counties, Virginia, USA by habitat type in (A) 2018 and (B) 2019. Bars with the same letter are not significantly different (Kruskal–Wallis test and Bonferroni corrected Dunn’s test, p < 0.05).
3.3. Trissolcus japonicus Captures in Paired Host Trees at the Woods Edge
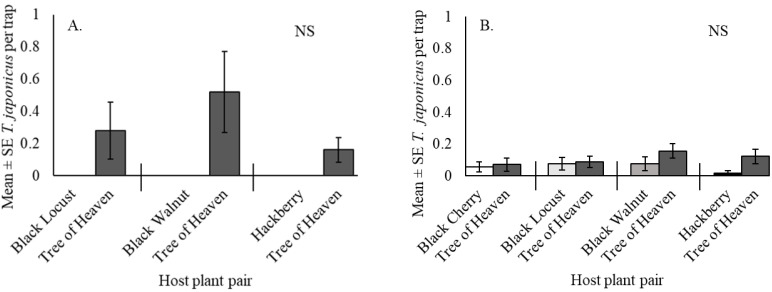
In 2017, 24 T. japonicus were captured during the four weeks of sampling in July and August (Table 3). All captures of T. japonicus were from tree of heaven, although captures did not differ significantly among the native tree species; tree of heaven vs black locust, S = −3, p > 0.05, tree of heaven vs black walnut, S = −5, p > 0.5, tree of heaven vs hackberry, S = −3, p > 0.05 (Figure 4A).
Table 3.
Adventive and native parasitoids of Halyomorpha halys captured in yellow sticky traps in the mid-canopy of paired H. halys host trees (n = 5 per host pairing) at the forest edge in Frederick Co., VA, from 31 July until 29 August 2017.
| Host Plant | Trissolcus | Telenomus | Gryon | ||
|---|---|---|---|---|---|
| japonicus | brochymenae | euschisti | spp. | spp. | |
| Black Locust | 0 | 1 | 1 | 3 | 1 |
| Tree of Heaven | 7 | 1 | 1 | 0 | 0 |
| Black Walnut | 0 | 0 | 0 | 0 | 0 |
| Tree of Heaven | 13 | 1 | 1 | 0 | 0 |
| Hackberry | 0 | 0 | 0 | 0 | 0 |
| Tree of Heaven | 4 | 0 | 2 | 0 | 0 |
| Total | 24 | 3 | 5 | 3 | 1 |
Figure 4.
Trissolcus japonicus captures in yellow sticky traps in the mid-canopy of paired tree hosts of Halyomorpha halys (n = 5 per host pairing) at the forest edge in Frederick Co., VA, from (A) 31 July until 29 August 2017 and (B) 13 June until 20 September 2018. NS signifies no significant difference within host plant pairs at p < 0.05.
In 2018, 42 T. japonicus were captured on traps during 12 weeks of sampling between June and September (Table 4). Of all T. japonicus captures, 66.7% were from tree of heaven, and captures among the native tree species did not differ significantly; tree of heaven vs black locust, S = −1.5, p > 0.05, tree of heaven vs black walnut, S = −10, p > 0.05, tree of heaven vs hackberry S = −11, p > 0.05, tree of heaven vs black cherry, S = −1, p > 0.05 (Figure 4B).
Table 4.
Adventive and native parasitoids of Halyomorpha halys captured in yellow sticky traps in the mid-canopy of paired H. halys host trees (n = 5 per host pairing) at the forest edge in Frederick Co., VA, from 13 June until 20 September 2018.
| Host Plant Pair | Trissolcus | Telenomus | Gryon | |||
|---|---|---|---|---|---|---|
| japonicus | thyantae | euschisti | podisi | spp. | spp. | |
| Black Cherry | 3 | 1 | 6 | 1 | 1 | 0 |
| Tree of Heaven | 4 | 0 | 1 | 0 | 0 | 0 |
| Black Locust | 5 | 0 | 4 | 1 | 1 | 0 |
| Tree of Heaven | 6 | 0 | 3 | 6 | 0 | 0 |
| Black Walnut | 5 | 0 | 3 | 0 | 0 | 0 |
| Tree of Heaven | 10 | 0 | 5 | 0 | 0 | 0 |
| Hackberry | 1 | 1 | 5 | 2 | 0 | 1 |
| Tree of Heaven | 8 | 0 | 8 | 1 | 1 | 0 |
| Total | 42 | 2 | 35 | 11 | 3 | 1 |
3.4. Trissolcus japonicus Captures Among Three Tree Species in Windbreaks
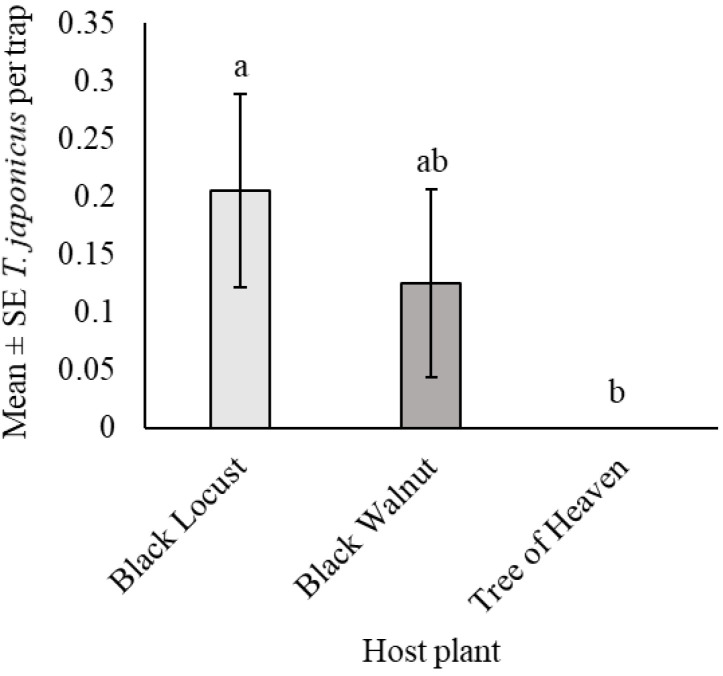
During the 8-week sampling period between June and August 2019, only 13 T. japonicus were captured (Table 5), 61.5, 38.5, and 0.0% of which were from black locust, black walnut, and tree of heaven, respectively. Captures of T. japonicus differed significantly among the tree species in which traps were deployed (χ2 = 6.32, df = 2, p < 0.05); significantly more were captured in black locust than in tree of heaven (z black locust = 8.4, z tree of heaven = 7.3, p < 0.05) (Figure 5), while captures in black walnut were not significantly different from those in black locust (z black locust = 8.4, z black walnut = 7.8, p > 0.05) or tree of heaven (z black walnut = 7.8, z tree of heaven = 7.3, p > 0.05).
Table 5.
Adventive and native parasitoids of Halyomorpha halys captured in yellow sticky traps in the mid-canopy of H. halys host trees in windbreaks (n = 5 sites) in Frederick Co., VA, from 17 June until 11 August 2019.
| Host Plant | Trissolcus | Telenomus | |||||
|---|---|---|---|---|---|---|---|
| japonicus | brochymenae | thyantae | euschisti | edessae | podisi | spp. | |
| Tree of Heaven | 0 | 1 | 2 | 6 | 1 | 9 | 2 |
| Black Locust | 8 | 1 | 0 | 9 | 0 | 2 | 0 |
| Black Walnut | 5 | 1 | 0 | 6 | 0 | 6 | 2 |
| Total | 13 | 3 | 2 | 21 | 1 | 17 | 4 |
Figure 5.
Trissolcus japonicus captures in yellow sticky traps in the mid-canopy of three tree hosts of Halyomorpha halys trees in the same windbreaks (n = 5 sites) in Frederick Co., VA, from 17 June until 11 August 2019. Bars with the same letter are not significantly different (Kruskal–Wallis test and Bonferroni corrected Dunn’s test, p > 0.05).
3.5. Captures of Native Parasitoids of Halyomorpha halys
In the habitat type study, 61 and 159 specimens of native parasitoids of H. halys were captured across the habitats sampled in 2018 and 2019, respectively (Table 1 and Table 2). All of the native species captured and listed in Table 1 and Table 2 are known to develop successfully, to varying degrees, in H. halys sentinel eggs [4]. In 2018, these included Trissolcus brochymenae (Ashmead), Trissolcus euschisti (Ashmead), Trissolcus thyantae Ashmead, Trissolcus edessae Fouts, Telenomus podisi Ashmead, Telenomus spp., Anastatus spp., and Encyrtidae (Table 1). Captures in 2019 included Trissolcus brochymenae, Trissolcus thyantae, Trissolcus euschisti, Telenomus podisi, Telenomus persimilis Ashmead, Telenomus spp., Gryon spp. (Scelionidae), Anastatus spp., and Encyrtidae (Table 2).
In the paired host study, 12 and 52 specimens of native Scelionidae were captured in 2017 and 2018, respectively (Table 3 and Table 4). In 2017, these included Trissolcus brochymenae, Trissolcus euschisti, Telenomus spp., and Gryon spp. (Table 3). Captures in 2018 included Trissolcus thyantae, Trissolcus euschisti, Telenomus podisi Ashmead, Telenomus spp., and Gryon spp. (Table 4). In 2019, 48 specimens of native scelionids were captured on traps in the three tree species in windbreaks, including Trissolcus brochymenae, Trissolcus thyantae, Trissolcus euschisti, Trissolcus edessae, Telenonus podisi, and Telenomus spp. (Table 5).
4. Discussion
Phenological synchrony of parasitoids with their hosts influences parasitoid population size and rate of colonization, with phenological mismatch potentially reducing observed parasitism of H. halys eggs by T. japonicus in Beijing, China [31]. Sampling in two consecutive years revealed first detections of T. japonicus in mid-May, peak captures in mid-July and early August, a marked decline in late August, and last detections in early to mid-September. The onset of captures in May coincided with the period of peak H. halys emergence from overwintering sites in the eastern USA [32] and peak captures followed predicted periods of peak H. halys oviposition [33]. As it does for other species [34], this synchrony should increase the likelihood of the persistence of adventive T. japonicus, supported by consistent indications of its range expansion in the USA in recent years [1]. Importantly, the seasonality of T. japonicus captures aligned well with the seasonal parasitism of H. halys eggs by T. japonicus in China [10], based on sentinel egg mass deployments at regular intervals from May or June through August or September. Moreover, declining detections in late August aligned with the cessation of H. halys oviposition by approximately mid-August [35], despite highest annual H. halys populations from late August through much of September [36,37]. While the overwintering biology of T. japonicus remains poorly understood, declining captures starting in late August may indicate that T. japonicus enters overwintering sites during this period. Similar total captures in 2018 and 2019 were notable given that 2019 was much drier than 2018 [38], suggesting that the abundance of T. japonicus remained stable despite annual climate variation, also boding well for its persistence. Our documentation of seasonal changes in captures of adventive T. japonicus in the Mid-Atlantic USA can inform the timing and efficiency of T. japonicus surveillance in H. halys host trees, particularly given the concurrence with results from China [10].
It is generally believed that decreased habitat size and increased fragmentation reduce parasitoid abundance [39] and parasitism [40]. Thus, highest captures of T. japonicus might have been expected in our largest and most contiguous habitat type, woods edge. However, in 2018, the fewest T. japonicus were captured at the edge of woodlots and most were captured in windbreaks, with no significant differences in captures among all habitats in 2019. These findings suggest site-specific variability in T. japonicus abundance. Positive density-dependent responses of parasitoids to hosts have been documented for other Trissolcus species [41,42], and T. japonicus abundance was likely influenced by H. halys density. While we did not monitor H. halys populations at the study sites, simultaneous sampling of H. halys and T. japonicus in future research may prove instructive.
Given the broad host range of H. halys [27] and the diversity of feeding and reproductive hosts available in this region [26], in theory, T. japonicus foraging for egg masses should not be limited by tree species. Rather, T. japonicus foraging may be most strongly associated with the presence or density of H. halys and its egg masses. Counts of H. halys egg masses on ornamental trees in an urban landscape by Formella et al. [43] revealed no significant differences among hosts in egg mass numbers, confirming H. halys oviposition on many plant species. However, their ground-based counts via visual observations may have underestimated egg mass density, based on data [20] showing greater numbers of H. halys egg masses producing T. japonicus from those collected at mid-canopy compared with other tree strata. In ornamental tree nurseries, more H. halys egg masses were found on angiosperms than gymnosperms, spanning numerous plant species and families [44]. Boyle et al. [45] suggested that T. japonicus may respond primarily to kairomones left by gravid H. halys walking on plant surfaces. If the distribution of H. halys populations are stochastic and show spatial and temporal changes in relative density, the distribution of T. japonicus might be expected to show the same trend, thereby resulting in changing parasitoid densities in a given area over time [46]. The curious differences in T. japonicus captures between native hosts and tree of heaven in 2017–2018 and 2019 may reflect this stochastic process.
Our studies using tree of heaven for standardized sampling have shown it to be a productive species for detecting T. japonicus. Across several studies in 2020, > 500 T. japonicus were captured in yellow sticky traps in female tree of heaven (Dyer, unpublished data). However, these data indicate that surveillance for T. japonicus need not be limited to specific H. halys host trees, thus enabling greater sampling flexibility. In other parts of the USA, sentinel H. halys eggs yielded T. japonicus detections from vine maple (Acer circinatum), Catalpa sp. [23], and English holly (Ilex aquifolium) [12].
Native parasitoids that attack H. halys eggs were captured, and in most studies, captures of T. japonicus were much higher than those of any other species. The community of native parasitoids observed was similar in composition to that reported by Tillman [47] in GA, USA, where T. japonicus has not yet been detected. Additionally, T. japonicus attacked more H. halys eggs than native stink bug eggs in field choice trials [23], suggesting that, as Konopka et al. [48] concluded, T. japonicus may be able to successfully coexist with native species in the biological control of H. halys. However, long-term effects of the addition of T. japonicus to the community of pentatomid parasitoids remain to be determined and warrant continued monitoring. Longitudinal studies using sticky traps alone or in concert with or other sampling methods, such as sentinel egg masses, may provide an indication of the impact of T. japonicus on the relative abundance of native H. halys parasitoids.
5. Conclusions
These studies further validate previous results [21] by showing that yellow sticky traps were effective for T. japonicus monitoring and surveillance. Detections of T. japonicus in several different habitats and BMSB host tree species indicated flexibility in the spatial component of T. japonicus sampling. Temporally, the consistently greatest captures from mid-June through early August can inform the timing of sampling and increase the likelihood of its detection. As discussed previously [21], yellow sticky traps are effective for addressing questions about the presence of T. japonicus, communities of native H. halys parasitoids, and the spread of T. japonicus in the invaded range of H. halys, but do not replace the use of sentinel or wild egg masses to assess H. halys egg parasitism or the impacts of parasitism on its populations.
Acknowledgments
We thank J. Engelman, W.T. Hadden, N. Brandt, B. Ruether, H. Aycock, J. Hardesty, C. Walls, T. Garrett, E. Craig, and A. Hagen for their assistance and the growers who allowed us to work on their property.
Author Contributions
Conceptualization, N.F.Q., T.C.L., and J.C.B.; methodology, N.F Q., T.C.L., and J.C.B.; software, N.F.Q., validation, N.F.Q., T.C.L., and J.C.B.; formal analysis, N.F.Q.; investigation, N.F.Q. and E.J.T.; resources, T.C.L. and J.C.B.; data curation, N.F.Q.; writing—original draft preparation, N.F.Q.; writing—review and editing, N.F.Q., E.J.T., T.C.L., and J.C.B.; visualization, N.F.Q., T.C.L., and J.C.B.; supervision, T.C.L. and J.C.B.; project administration, N.F.Q., T.C.L., and J.C.B.; funding acquisition, N.F.Q., T.C.L., and J.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by USDA Specialty Crop Block Grant 301-17-036 via the Virginia Department of Agriculture and Consumer Services, USDA NIFA SCRI 2016-51181-25409, and Southern Region SARE RD309-137/ S001521. Elijah Talamas was supported in part by the Florida Department of Agriculture and Consumer Services-Division of Plant Industry and USDA Farm Bill: Identification, monitoring, and redistribution of Trissolcus japonicus– Biological Control of Brown Marmorated Stink Bug 262 (BMSB).
Institutional Review Board Statement
Not applicable. Studies did not involve humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leskey T.C., Nielsen A.L. Impact of the invasive brown marmorated stink bug in North America and Europe: History, biology, ecology, and management. Annu. Rev. Entomol. 2018;63:599–618. doi: 10.1146/annurev-ento-020117-043226. [DOI] [PubMed] [Google Scholar]
- 2.Herrick C. Brown Marmorated Stink Bug Causes $37 Million in Losses to Mid-Atlantic Apple Growers. Growing Produce. [(accessed on 15 May 2018)];2011 Available online: https://www.growingproduce.com/fruits/apples-pears/brown-marmorated-stink-bug-causes-37-million-in-losses-to-mid-atlantic-apple-growers/
- 3.Leskey T.C., Lee D.-H., Short B.D., Wright S.E. Impact of insecticides on the invasive Halyomorpha halys (Hemiptera: Pentatomidae): Analysis of insecticide lethality. J. Econ. Entomol. 2012;105:1726–1735. doi: 10.1603/EC12096. [DOI] [PubMed] [Google Scholar]
- 4.Abram P.K., Hoelmer K.A., Acebes-Doria A., Andrews H., Beers E.H., Bergh J.C., Bessin R., Biddinger D., Botch P., Buffington M.L., et al. Indigenous arthropod natural enemies of the invasive brown marmorated stink bug in North America and Europe. J. Pest Sci. 2017;90:1009–1020. doi: 10.1007/s10340-017-0891-7. [DOI] [Google Scholar]
- 5.Dieckhoff C., Tatman K.M., Hoelmer K.A. Natural biological control of Halyomorpha halys by native egg parasitoids: A multi-year survey in northern Delaware. J. Pest Sci. 2017;90:1–16. doi: 10.1007/s10340-017-0868-6. [DOI] [Google Scholar]
- 6.Herlihy M.V., Talamas E.J., Weber D.C. Attack and success of native and exotic parasitoids on eggs of Halyomorpha halys in three Maryland habitats. PLoS ONE. 2016;11:e0150275. doi: 10.1371/journal.pone.0150275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelius M.L., Dieckhoff C., Hoelmer K.A., Olsen R.T., Weber D.C., Herlihy M.V., Talamas E.J., Vinyard B.T., Greenstone M.H. Biological control of sentinel egg masses of the exotic invasive stink bug Halyomorpha halys (Stål) in Mid-Atlantic USA ornamental landscapes. Biol. Control. 2016;103:11–20. doi: 10.1016/j.biocontrol.2016.07.011. [DOI] [Google Scholar]
- 8.Jones A.L., Jennings D.E., Hooks C.R.R., Shrewsbury P.M. Field surveys of egg mortality and indigenous egg parasitoids of the brown marmorated stink bug, Halyomorpha halys, in ornamental nurseries in the mid-Atlantic region of the USA. J. Pest Sci. 2017;90:1159–1168. doi: 10.1007/s10340-017-0890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z.Z.-Q., Yao Y.Y.-X., Qiu L.-F.L., Li Z.Z.-X. A new species of Trissolcus (Hymenoptera: Scelionidae) parasitizing eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with comments on its biology. Ann. Entomol. Soc. Am. 2009;102:39–47. [Google Scholar]
- 10.Zhang J., Zhang F., Gariepy T., Mason P., Gillespie D., Talamas E., Haye T. Seasonal parasitism and host specificity of Trissolcus japonicus in northern China. J. Pest Sci. 2017;90:1127–1141. doi: 10.1007/s10340-017-0863-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talamas E.J., Herlihy M.V., Dieckhoff C., Hoelmer K.A., Buffington M., Bon M.-C., Weber D.C. Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. J. Hymenopt. Res. 2015;43:119–128. doi: 10.3897/JHR.43.4661. [DOI] [Google Scholar]
- 12.Hedstrom C., Lowenstein D., Andrews H., Bai B., Wiman N. Pentatomid host suitability and the discovery of introduced populations of Trissolcus japonicus in Oregon. J. Pest Sci. 2017;90:1169–1179. doi: 10.1007/s10340-017-0892-6. [DOI] [Google Scholar]
- 13.Milnes J.M., Wiman N.G., Talamas E.J., Brunner J.F., Hoelmer K.A., Buffington M.L., Beers E.H. Discovery of an exotic egg parasitoid of the brown marmorated stink bug, Halyomorpha halys (Stål) in the Pacific Northwest. Proc. Entomol. Soc. Wash. 2016;118:466–470. doi: 10.4289/0013-8797.118.3.466. [DOI] [Google Scholar]
- 14.Morrison W.R., Blaauw B.R., Nielsen A.L., Talamas E., Leskey T.C. Predation and parasitism by native and exotic natural enemies of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) eggs augmented with semiochemicals and differing host stimuli. Biol. Control. 2018;121:140–150. doi: 10.1016/j.biocontrol.2018.02.016. [DOI] [Google Scholar]
- 15.Costi E., Haye T., Maistrello L. Surveying native egg parasitoids and predators of the invasive Halyomorpha halys in Northern Italy. J. Appl. Entomol. 2019;143:299–307. doi: 10.1111/jen.12590. [DOI] [Google Scholar]
- 16.Peverieri G.S., Talamas E., Bon M.C., Marianelli L., Bernardinelli I., Malossini G., Benvenuto L., Roversi P.F., Hoelmer K. Two Asian egg parasitoids of Halyomorpha halys (Stål) (Hemiptera, Pentatomidae) emerge in northern Italy: Trissolcus mitsukurii (Ashmead) and Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) J. Hymenopt. Res. 2018;67:37–53. doi: 10.3897/jhr.67.30883. [DOI] [Google Scholar]
- 17.Stahl J., Tortorici F., Pontini M., Bon M.C., Hoelmer K., Marazzi C., Tavella L., Haye T. First discovery of adventive populations of Trissolcus japonicus in Europe. J. Pest Sci. 2019;92:371–379. doi: 10.1007/s10340-018-1061-2. [DOI] [Google Scholar]
- 18.Abram P.K., Talamas E.J., Acheampong S., Mason P.G., Gariepy T.D. First detection of the samurai wasp, Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae), in Canada. J. Hymenopt. Res. 2019;68:29–36. doi: 10.3897/jhr.68.32203. [DOI] [Google Scholar]
- 19.Abram P.K., Mills N.J., Beers E.H. Review: Classical biological control of invasive stink bugs with egg parasitoids—what does success look like? Pest Manag. Sci. 2020;76:1980–1992. doi: 10.1002/ps.5813. [DOI] [PubMed] [Google Scholar]
- 20.Kaser J.M., Akotsen-Mensah C., Talamas E.J., Nielsen A.L. First report of Trissolcus japonicus parasitizing Halyomorpha halys in North American agriculture. Fla. Entomol. 2018;101:680–683. [Google Scholar]
- 21.Quinn N.F., Talamas E.J., Acebes-Doria A.L., Leskey T.C., Bergh J.C. Vertical sampling in tree canopies for Halyomorpha halys (Hemiptera: Pentatomidae) life stages and its egg parasitoid, Trissolcus japonicus (Hymenoptera: Scelionidae) Environ. Entomol. 2019;48:173–180. doi: 10.1093/ee/nvy180. [DOI] [PubMed] [Google Scholar]
- 22.Quinn N.F., Talamas E.J., Leskey T.C., Bergh J.C. Sampling methods for adventive Trissolcus japonicus (Hymenoptera: Scelionidae) in a wild tree host of Halyomorpha halys (Hemiptera: Pentatomidae) J. Econ. Entomol. 2019;112:1997–2000. doi: 10.1093/jee/toz107. [DOI] [PubMed] [Google Scholar]
- 23.Milnes J.M., Beers E.H. Trissolcus japonicus (Hymenoptera: Scelionidae) causes low levels of parasitism in three North American pentatomids under field conditions. J. Insect Sci. 2019;19:1–6. doi: 10.1093/jisesa/iez074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowarik I., Säumel I. Biological flora of central Europe: Ailanthus altissima (Mill.) Swingle. Perspectives in Plant Ecology. Perspect. Plant Ecol. Evol. Syst. 2007;8:207–237. doi: 10.1016/j.ppees.2007.03.002. [DOI] [Google Scholar]
- 25.Call L., Nilsen E. Analysis of spatial patterns and spatial association between the invasive tree-of heaven (Ailanthus altissima) and native black locust (Robinia pseudoacacia) Am. Midl. Nat. 2003;150:1–14. doi: 10.1674/0003-0031(2003)150[0001:AOSPAS]2.0.CO;2. [DOI] [Google Scholar]
- 26.Acebes-Doria A.L., Leskey T.C., Bergh J.C. Temporal and directional patterns of nymphal Halyomorpha halys (Hemiptera: Pentatomidae) movement on the trunk of selected wild and fruit tree hosts in the Mid-Atlantic region. Environ. Entomol. 2017;46:258–267. doi: 10.1093/ee/nvw164. [DOI] [PubMed] [Google Scholar]
- 27.Bakken A.J., Schoof S.C., Bickerton M., Kamminga K.L., Jenrette J.C., Malone S., Abney M.A., Herbert D.A., Reisig D., Kuhar T.P., et al. Occurrence of brown marmorated stink bug (Hemiptera: Pentatomidae) on wild hosts in nonmanaged woodlands and soybean fields in North Carolina and Virginia. Environ. Entomol. 2015;44:1011–1021. doi: 10.1093/ee/nvv092. [DOI] [PubMed] [Google Scholar]
- 28.Talamas E.J., Johnson N.F., Buffington M. Key to Nearctic species of Trissolcus Ashmead (Hymenoptera, Scelionidae), natural enemies of native and invasive stink bugs (Hemiptera, Pentatomidae) J. Hymenopt. Res. 2015;43:45–110. doi: 10.3897/JHR.43.8560. [DOI] [Google Scholar]
- 29.Yang S.Y., Zhong Y.Z., Zhang J.P., Wang X.P., Zhang F. A comparative scanning electron microscopy study on antennal sensilla of Trissolcus japonicus and Trissolcus plautiae, egg parasitoids of stink bugs (Pentatomidae) Ann. Entomol. Soc. Am. 2016;109:112–120. [Google Scholar]
- 30.SAS Institute Inc. SAS Software. The SAS Institute; Cary, NC, USA: 2018. version 9.4. [Google Scholar]
- 31.Van Nouhuys S., Lei G. Parasitoid-host metapopulation dynamics: The causes and consequences of phenological asynchrony. J. Anim. Ecol. 2004;73:526–535. doi: 10.1111/j.0021-8790.2004.00827.x. [DOI] [Google Scholar]
- 32.Bergh J.C., Morrison W.R., III, Joseph S.V., Leskey T.C. Characterizing spring emergence of adult Halyomorpha halys using experimental overwintering shelters and commercial pheromone traps. Entomol. Exp. Appl. 2017;162:336–345. doi: 10.1111/eea.12539. [DOI] [Google Scholar]
- 33.Nielsen A.L., Chen S., Fleischer S.J. Coupling developmental physiology, photoperiod, and temperature to model phenology and dynamics of an invasive heteropteran, Halyomorpha halys. Front. Physiol. 2016;7:165. doi: 10.3389/fphys.2016.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godfray H.C.J., Hassel M.P., Holt R.D. The population dynamic consequences of phenological asynchrony between parasitoids and their hosts. J. Anim. Ecol. 1994;63:1–10. doi: 10.2307/5577. [DOI] [Google Scholar]
- 35.Nielsen A.L., Hamilton G.C., Matadha D. Developmental rate estimation and life table analysis for Halyomorpha halys (Hemiptera: Pentatomidae) Environ. Entomol. 2008;37:348–355. doi: 10.1093/ee/37.2.348. [DOI] [PubMed] [Google Scholar]
- 36.Acebes-Doria A.L., Agnello A.M., Alston D.G., Andrews H., Beers E.H., Bergh J.C., Bessin R., Blaauw B.R., Buntin G.D., Burkness E.C., et al. Season-long monitoring of the brown marmorated stink bug (Hemiptera: Pentatomidae) throughout the United States using commercially available traps and lures. J. Econ. Entomol. 2020;113:159–171. doi: 10.1093/jee/toz240. [DOI] [PubMed] [Google Scholar]
- 37.Philips C.R., Kuhar T.P., Dively G.P., Hamilton G., Whalen J., Kamminga K. Seasonal abundance and phenology of Halyomorpha halys (Hemiptera: Pentatomidae) on different pepper cultivars in the Mid-Atlantic (United States) J. Econ. Entomol. 2017;110:192–200. doi: 10.1093/jee/tow256. [DOI] [PubMed] [Google Scholar]
- 38.NEWA. [(accessed on 1 November 2019)];2019 Available online: http://newa.cornell.edu/
- 39.Amir-Maafi M., Parker B.L. Density dependence of Trissolcus spp. (Hymenoptera: Scelionidae) on eggs of Eurygaster integriceps Puton (Hemiptera: Scutelleridae) Arab J. Plant Prot. 2002;20:62–64. [Google Scholar]
- 40.Elzinga J.A., van Nouhuys S., van Leeuwen D.J., Biere A. Distribution and colonisation ability of three parasitoids and their herbivorous host in a fragmented landscape. Basic Appl. Ecol. 2007;8:75–88. doi: 10.1016/j.baae.2006.04.003. [DOI] [Google Scholar]
- 41.Valladares G., Salvo A., Cagnolo L. Habitat fragmentation effects on trophic processes of insect-plant food webs. Conserv. Biol. 2006;20:212–217. doi: 10.1111/j.1523-1739.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 42.Meats A., Pando M.S.C. Ratio-dependent parasitism with Trissolcus basalis (Wollaston) (Hymenoptera: Scelionidae) on egg rafts of Nezara viridula (Linnaeus) (Hemiptera: Pentatomidae): Effect of experimental variables and compatibility of “ratio” and “Holling” models. Aust. J. Entomol. 2002;41:243–252. [Google Scholar]
- 43.Formella A., Dorman S.J., Taylor S.V., Kuhar T.P. Effects of aggregation lure and tree species on Halyomorpha halys (Hemiptera: Pentatomidae) seasonal oviposition. J. Econ. Entomol. 2019;113:203–210. doi: 10.1093/jee/toz281. [DOI] [PubMed] [Google Scholar]
- 44.Bergmann E.J., Venugopal P.D., Martinson H.M., Raupp M.J., Shrewsbury P.M. Host plant use by the invasive Halyomorpha halys (Stål) on woody ornamental trees and shrubs. PLoS ONE. 2016;11:e1049975. doi: 10.1371/journal.pone.0149975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyle S.M., Weber D.C., Hough-Goldstein J., Hoelmer K.A. Host kairomones influence searching behavior of Trissolcus japonicus (Hymenoptera: Scelionidae), a parasitoid of Halyomorpha halys (Heteroptera: Pentatomidae) Environ. Entomol. 2020;49:15–20. doi: 10.1093/ee/nvz155. [DOI] [PubMed] [Google Scholar]
- 46.Morrison G. Stochastic aggregative responses and spatial patterns of parasitism in patchy host-parasitoid interactions. Oecologia. 1986;70:402–410. doi: 10.1007/BF00379503. [DOI] [PubMed] [Google Scholar]
- 47.Tillman P.G. Diversity of stink bug (Hemiptera: Pentatomidae) egg parasitoids in woodland and crop habitats in southwest Georgia, USA. Fla. Entomol. 2016;99:286–291. doi: 10.1653/024.099.0220. [DOI] [Google Scholar]
- 48.Konopka J.K., Haye T., Gariepy T.D., McNeil J.N. Possible coexistence of native and exotic parasitoids and their impact on control of Halyomorpha halys. J. Pest Sci. 2017;90:1119–1125. doi: 10.1007/s10340-017-0851-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available upon request.