Abstract
BNN27 is a novel 17‐spiroepoxy derivative of the neurosteroid Dehydroepiandrosterone with neuroprotective properties. The purpose of this study was the detection and quantification of BNN27 after single intraperitoneal administration, in the serum and retina of normal rodents. Forty‐two C57BL/6 mice and 48 Sprague–Dawley rats were used for the quantification of BNN27 in the blood serum and retina, respectively. BNN27 was injected intraperitoneally (i.p.) at concentrations of 100 and 30 mg/kg of body weight (b.w.), respectively. The blood was collected with retro‐orbital bleeding and the retina was isolated after enucleation at various time points. The molecule concentrations were measured with Liquid chromatography‐mass spectrometry (LC‐MS). Non‐compartmental analysis was used to determine pharmacokinetic parameters. BNN27 was found to have an elimination constant k el = 0.465 h−1 and mean residence time (MRT) 2.154 h in the mouse serum. The maximum concentration (C max) in the retina was detected at 2 h () after intraperitoneal administration and was equal to 1100 ng/g. BNN27 is rapidly eliminated from both blood and retina. In the retina specifically, it is undetectable 6 h after injection. BNN27 shows a rapid systemic elimination as anticipated by its small size and lipophilicity. It is measurable in small peripheral tissues such as the rat retina, after one single i.p. injection, using a simple method such as LC‐MS. Its detection in the retina corroborates the existing biological data that the molecule crosses the blood–retinal barrier, highlighting it as a potential neuroprotective agent for retinal disease.
Keywords: blood–retinal barrier, BNN27, LC‐MS, neuroprotection, pharmacokinetics, steroids
The pharmacokinetics of the microneurotrophin BNN27, a synthetic DHEA exhibiting neuroprotective actions but lacking endocrinal activity, have been calculated in this study. BNN27 shows a rapid absorption and elimination from the blood of the C57BL/6 mouse after ip administration as expected by its small size and lipophilic nature. Bioavailability in the retina of albino rats was also documented, demonstrating the ability of the molecule to cross blood‐retina barriers (retina data provided in the article)..

Abbreviations
- AUC
area under the time curve
- AUMC
area under the first moment curve
- b.w.
body weight
- BBB
blood–brain barrier
- BRB
blood–retinal barrier
- Cltot
total body clearance
- Cmax
maximum measured concentration
- CNS
central nervous system
- Cp
measured concentration
- DHEA
dehydroepiandrosterone
- Do
administered dose
- IS
internal standard
- Kel'
apparent elimination rate constant
- LC‐MS
liquid chromatography‐mass spectrometry
- LOD
limit of determination
- LOQ
limit of quantification
- MNT
microneurotrophin
- MRT
mean residence time
- NGF
nerve growth factor
- p75NTR
p75 neurotrophin receptor
- R‐L
Ringer's‐Lactate
- S‐D
Sprague–Dawley
- SEM
standard error of the mean
time after administration when C max is measured
- Trk
tropomyosin‐related kinase
- Vss
apparent volume of distribution at steady state
- WFI
water for injection
1. INTRODUCTION
A new family of small synthetic molecules, C17‐spiro derivatives of dehydroepiandrosterone (DHEA) has been synthesized recently, 1 named microneurotrophins (MNTs) due to their ability to mimic some of the endogenous neurotrophins effects through the activation of specific neurotrophin receptors. Two of them, BNN27 [(R)‐3β,21‐ dihydroxy‐17R,20‐epoxy‐5‐pregnene] and BNN20 (17β‐spiro [5‐androstene‐17 2'oxiran]3β‐ol) (Figure 1), have exhibited anti‐apoptotic properties, in vitro 1 , 2 and in vivo. 2 , 3 , 4 , 5 BNN27 is the molecule that has demonstrated specific properties regarding its binding to neurotrophin receptor and the subsequent biological actions, making it the lead molecule for the future development for potential therapeutic use.
FIGURE 1.
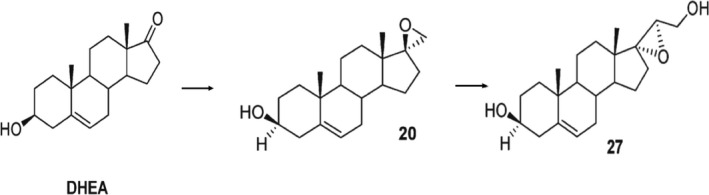
Chemical structure of DHEA and its derivates BNN20 and BNN27
The molecule seems to selectively bind to both NGF (Nerve Growth Factor) receptors, namely TrkA and p75 NTR neurotrophin receptors, 6 , 7 and thus demonstrate anti‐inflammatory, neurotrophic, and anti‐apoptotic activities in cell cultures 8 , 9 and animal models 8 , 9 , 10 , 11 , 12 , 13 for CNS (central nervous system) and retinal disease. 5 , 14 , 15 , 16 Additionally, it is highly lipophilic with proven facility to cross artificial membranes that mimic the blood–brain barrier (BBB). 2
Its main advantage compared to its predecessor molecule, the DHEA, is the lack of the classic hormone receptor activity 1 exhibited by DHEA itself. DHEA's metabolism to estrogens and androgens results in various important side effects, such as increased risk of hormone‐dependent carcinomas (breast, prostate), neuropsychiatric disorders, heart disease, and impaired insulin sensitivity 17 , 18 , 19 , 20 , 21 , 22 , 23 that limit its use as long‐term therapeutic agent despite its proven neuroprotective action. BNN27 seems to lack this hormone‐receptor activation, while preserving its anti‐apoptotic, neurotrophic properties. This highly interesting profile for potential pharmacological use in the CNS as well as the retina has led to the initiation of this study, which targets on the detection and quantification of the molecule after systemic administration.
Herein, we investigate the quantitative/pharmacokinetic characteristics of BNN27 after systemic administration (single intraperitoneal [i.p.] injection) in normal rodents. Initially, the plasma pharmacokinetics of BNN27 were followed after single i.p. injection in normal mice, in order to verify that it is systemically available after intraperitoneal administration, as expected due to its highly lipophilic nature and its small size. After verifying the previous point, the retinal bioavailability of BNN27 was investigated in rats, because the small size of the mouse retina was inadequate for the selected methodology. For the detection of BNN27 in retinal tissues a LC‐MS methodology was setup. The retinal detection of BNN27 after i.p. injection would also verify that the molecule crosses the blood–retinal barrier (BRB) as suggested by its biological actions investigated so far.
2. MATERIALS AND METHODS
2.1. Animals and housing
Animal experimentation was conducted in accordance with the ARRIVE guidelines 24 and was carried out in compliance with the EU Directive 2010/63/EU, the Greek Government guidelines and the guidelines of our ethics committee.
All animals were housed one to two animals per cage in animal room maintained at 22°C, with an alternating 12‐h light–dark cycle. Food and water were available ad libitum. Euthanasia was performed with ether inhalation.
2.2. Experimental/study design
Prior to initiation of each experiment, all animals were weighed and randomized by b.w. and gender.
The study was divided in two parts: Initially, in order to understand if i.p. administration provides rapid absorption and systemic availability of BNN27, as anticipated by its high lipophilicity and small size, the quantification of BNN27 was carried out in the serum of C57BL/6 mice (RRID:MGI:5656552). The measurements were made at various time‐points, after single i.p. dose administration in adult male and female C57BL/6 mice weighting between 23 and 26 g.
After verifying rapid systemic availability of BNN27 following i.p. administration in mice, in the second part of the study we investigated the retinal bioavailability of BNN27 after single i.p. administration in Sprague–Dawley (S‐D) rats (RRID:MGI:5651135). The switch of the experimental rodent was necessary in order to increase the sample tissue available for quantification in each time point. In addition, the retina of both eyes of each animal was extracted and used as one sample. Adult male and female S‐D rats, weighting between 250 and 350 g, were included in this second part of the study.
2.3. Systemic availability study‐blood sampling
Forty‐two C57BL/6 mice were randomized in seven (7) time points. In each group, five animals (n = 5) received i.p. 100 mg/kg of b.w. of the BNN27 solution and one animal served as blind control and received i.p. the respective volume of the solvent only (water for injection [WFI], see “preparation of the BNN27 solution”). The blood collection was performed at the time points selected which were: 0, 30 min, 1 h, 2, 4, 6, and 24 h after injection. The blood was collected under light anesthesia in heparinized tubes from the orbital sinus with a sterile Pasteur pipette (retro‐orbital bleeding) and the animals were killed. The samples were centrifuged at 6810 g for 20 min at 4°C (Centrifuge 5804 R, Eppendorf AG) and the supernatant was collected and stored at 4–8°C until analysis.
2.4. Retina availability study‐retinal tissue sampling
Forty‐eight S‐D rats were randomized in eight (8) time points. In each group, five animals received i.p. 30 mg/kg of b.w. of the BNN27 solution and one animal served as blind control and received i.p. the respective volume of the solvent only (WFI). A lower dosage was selected, to be close to the common therapeutic dosages used in the in vivo experiments so far. The retinas were collected at the selected time points: 0, 15, 30 min, 1 h, 2, 4, 6, and 24 h after administration.
The retina isolation was performed with the following procedure. The eyes were enucleated after euthanasia. After enucleation, the eyeball was placed in a sterile glass Petri dish filled with chilled PBS after two washings with the same solution. The procedure was performed under the microscope. The eyeball was cut with sterile micro‐scissors and the anterior segment (together with the crystal lens) was removed. Next, the sclera was fixed on a solid surface with micro‐forceps and with the tip of a sterile surgical micro‐sponge spear, the retina was carefully detached from the RPE and the rest of the eyecup with one maneuver. This assumes a total detachment with minimal tissue loss. The retina was then immediately washed off the micro‐sponge with sterile PBS back into the dish. The retinas of both eyes of each animal were elaborated together, as one sample (finally, n = 5 per time point).
At the time point of the maximum retinal concentration (C max) after injection (), six more animals were added in the study and underwent a blood perfusion procedure. This time point was selected after the collection of the retinal concentration data (over time) and the determination of the C max. At the given time point, five animals (n = 5) were injected i.p. with the BNN27 solution (30 mg/kg of b.w.) and one was injected i.p. with the respective volume of WFI. All six animals underwent transcardial perfusion with heparinized Ringer's‐Lactate (R‐L) solution for 10 min (or until liver is cleared of blood) under deep anesthesia, followed by euthanasia and retina collection as described earlier. A single time point was selected, because of the low concentrations expected after perfusion. The inversion of the concentration gradient between the intracellular and the intravascular space during the blood elimination would induce a “wash out” phenomenon for the BNN27 as it passively diffuses towards the decreasing intravascular concentration during the procedure. Therefore, the detection of any concentration of BNN27 after perfusion would be a positive confirmation to the results of the main method (as opposed to the blind samples that were expected with zero concentration). Choosing the ensured the initial maximal intracellular concentration.
2.5. Chemicals and solutions/ preparation of the BNN27 solution
The BNN compounds are proprietary and patented by the Bionature E.A. Ltd (http://www.bionature.net) (Patent Number: WO2008/155534 A2). They come in the form of lyophilized powders, and are manufactured in large‐scale under GMP conditions. The MNTs used in the study, BNN27 and BNN20, were kindly provided by Dr A. Gravanis, co‐founder of Bionature E.A. Ltd. Chemically these MNTs are spiro‐epoxy steroid derivatives of DHEA and are fully characterized for their in vitro activity. 1 , 6 , 7 In this study, BNN27 was used as study molecule and was injected in the animals, whereas BNN20 was used as an internal standard (IS) for quantification purposes.
The BNN27 solution for injection was prepared in two steps. The powder was first diluted in absolute ethanol at 60°C, and kept in sterile closed microtubes to avoid evaporation of the highly volatile solvent. Second, immediately before injection, the alcoholic solution was added to sterile WFI (ethanol 6% v/v), resulting in the formation of a fine dispersion, which was then injected directly to the animal. This methodology minimized the agglomeration of the hydrophobic molecules in the syringe, which could hinder the continuous administration of the drug in the intra‐peritoneal space of the animals.
Methanol (LC‐MS grade) was purchased by Sigma‐Aldrich (3050). Formic acid (98%–100%) was from Riedel‐de Haen (Sigma Aldrich, Laborchemikallen, GmbH, D‐30926); ultrapure water was produced by a water purification system (Direct‐Q 3UV, Merck). Bond Elut SPE columns (C18, 1 ml/100 mg) for HPLC, were obtained from Agilent Technologies.
2.6. Chromatographic analysis
A LC‐MS system was used for the analytical determination of BNN27. The system consisted of a binary LC pump (Shimadzu Prominence LC), a vacuum degasser, an auto‐sampler and a column oven. A solvent mixture of water methanol‐formic acid (89.7–10–0.3 v/v) (A) and methanol‐formic acid (99.7%–0.3%) (B), was selected as the mobile phase with a flow rate of 0.6 ml/min. Separation of the analyte was achieved on a Discovery C18 HPLC column (250 × 4.6 mm, 5 μm) at 30οC. Under the described conditions, BNN27 eluted at 8.93 min. A 30 μl volume of each sample was injected in the mobile phase flow (Figure 2).
FIGURE 2.
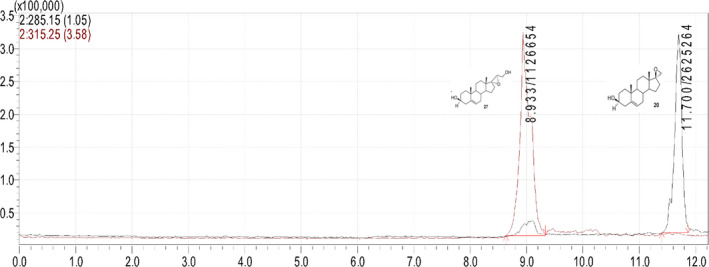
Typical chromatogram of BNN27 (8.93 min) and BNN20 (11.7 min)
A mass spectrometer (LCMS‐2010 EV Shimadzu), coupled with an atmospheric pressure chemical ionization interface and a single quadrupole mass filter, was used to detect and quantify BNN27 in the selected ion monitoring (SIM) mode, in positive mode, with ions m/z 315.15, 297.20, and 255.15 for BNN27 and 285.15, 267.15, and 255.15 for BNN20 (in bold the ions used for quantification) (Figure 3a,b).
FIGURE 3.
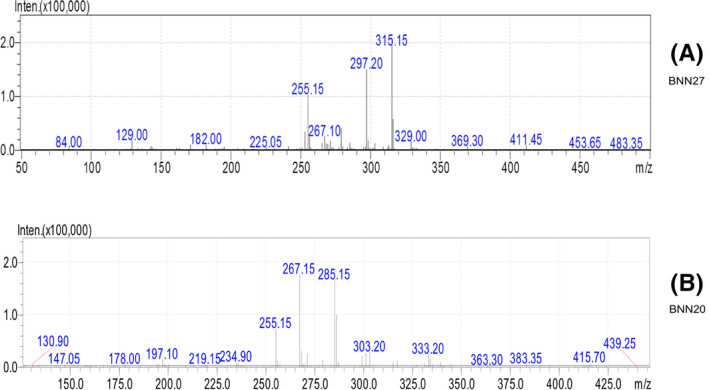
Full scan Spectra for BNN27 (A) and BNN20 (B)
The interface, CDL, and heat block temperatures were 400°C, 200°C, and 200°C, respectively. The detector voltage was 1.5 kV, the nebulizing gas flow was 2.5 L/min and the drying gas was set at 0.02 MPa.
2.7. BNN27 determination in serum
Blank serum samples were used for the preparation of the spiked samples at concentration levels 0, 25, 50, 100, 250, and 500 ng/ml. The extraction of BNN27 from serum was performed by SPE extraction as described in a previous published paper 25 with minor modifications. Briefly, spiked and serum samples (0.1–0.2 ml) diluted with 0.5 ml distilled water and 100 ng of BBN20 were added as IS. The diluted sample placed in an ultrasonic water bath for 20 min and then was loaded to a Bond Elut SPE column (C18, 1 ml/100 mg). SPE cartridges were activated with the addition of 1 ml of methanol and 1 ml of water. Analytes were eluted with 1 ml of diethylether. The final eluate was evaporated to dryness under a gentle nitrogen stream at 35°C, reconstituted in 100 μl methanol and 30 μl was injected in the LC‐MS system.
2.8. BNN27 determination in retina tissue
For the analysis of the retinal samples, an amount of 2 ml of methanol was added to (2x) 19 ± 0.5 mg of tissue, the tissues were mechanically homogenized and were sonicated for 60 s in an ultrasonic bath (30 kHz, UP100H, Hielscher US Tech). Then, the samples were centrifuged at 20 817 g (4°C) for 60 min. The extract was removed and evaporated to dryness under a stream of nitrogen. Hundred microliter of methanol was added to the residue and 30 μl of the final solution was injected to the LC‐MS system. Blank retinal samples were used for the preparation of spiked samples at concentration levels between 0 and 5260 ng/g.
2.9. Pharmacokinetic and statistical analysis
Non‐compartmental analysis can be used to determine pharmacokinetic parameters of actives without deciding on a particular compartmental model. Herein, pharmacokinetic parameters were determined by non‐compartmental analysis. Basic calculations are based on the area under the curve concentrations versus times curve (zero moment) and the first moment curve (AUMC). All concentrations that were less than the limit of quantification (LOQ) were treated as zero. The area under the time curve (AUC) and the area under the first moment curve (AUMC) were calculated by the trapezoidal rule, in all cases. For the serum data in mice, the final segment of the AUC curve is calculated as Cp(last)/k', where k' is the last exponential term calculated from the Cp versus time graph. However, due to the limited number of time points in the final exponential drug decay phase, the accuracy of the later estimation is questionable, and for this reason the pharmacokinetic parameters for serum were calculated both by AUC24 h and also AUC∞. For the retina data (in rats) this was not the case, since the concentrations measured at the last time points were already below detection limit, and therefore set as zero.
Mean residence time values were calculated as the ratio AUMC/AUC, and the apparent elimination rate constant K el' as 1/MRT, in both cases (data from serum in mice and retinal concentrations in rats). From the serum concentration data in mice, total body clearance (Cltot) was calculated as Do/AUC (where Do is the administered dose) by making the assumption that the bioavailability of BNN27 is 100% (F = 1) following i.p. injection.
Curve fitting and pharmacokinetic parameters and were calculated with GraphpadPrism 6.0 (GraphPad Software, Inc.) and PKfs of Microsoft Excel 2013 (Microsoft).
2.10. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 26 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. 27
3. RESULTS
3.1. Method validation
The recovery of the method was calculated at five spiked levels (10, 25, 50, 100, and 500 ng/ml) for blood analysis and calculated to be 87.3 ± 12.6% and for the retina at 0, 530, 1320, 2630, and 5260 ng/g and calculated to be 76.7 ± 11.1%.
The linearity of the method in standard solutions (r 2 = 0.999) and spiked samples (r 2 = 0.9975 for blood and r 2 = 0.9930 for retina) was found acceptable (r 2 > 0.99). The % accuracy and inter day precision (expressed as %RSD) were determined by analyzing spiked samples at different concentrations of 10, 25, 50, 100, and 500 ng/ml for blood, and 0, 530, 1320, 2630, and 5260 ng/g for retina, respectively. The mean % accuracy of the method was 101 ± 17.7 for blood analysis and 100.6 ± 14.6 for retina, whereas the inter day precision was 13.1 ± 3.2 for blood and 10.6 ± 2.1 for retina.
The low limits of determination (LOD) and LOQ were achieved that allowed us the determination of the BNN levels in the retinal tissues and blood samples. More specific, the LOD and LOQ values for the detection of BNN27 in retina samples were 17 and 55 ng/g, respectively. And the corresponding values for the determination in blood samples were 2.9 and 9.7 ng/ml, respectively.
3.2. Blood serum
The mean blood volume collected per animal was 0.5 ± 0.15 ml. The mean serum volume collected was 0.15 ± 0.05 ml.
Mean concentrations of BNN27 (and standard error of the mean, SEM) measured in the serum of C57BL/6 mice are presented in Table 1 and depicted in Figure 4. All blind samples were measured at zero BNN27 concentration.
TABLE 1.
BNN27 concentrations over time
| Time (h) | CSerum (ng/ml) in mice (dose = 100 mg/kg) |
AUC (ng×h/ml) |
AUMC (ng×h2/ml) |
CRetina (ng/g) in rats (dose = 30 mg/kg) |
AUC (ng×h/g) |
AUMC (ng×h2/g) |
|---|---|---|---|---|---|---|
| 0 | 9026.7 (744.08) | 0 | 0 | <LOD | ||
| 0.25 | – | – | – | <LOD | 0 | 0 |
| 0.5 | 7838.2 (312.87) | 4216.22 | 979.78 | 230 (100) | 28.75 | 14.38 |
| 1 | 6064.4 (393.4) | 7691.86 | 3475.64 | 730 (180) | 268.75 | 225.63 |
| 2 | 907.1 (83.31) | 11 177.6 | 7414.95 | 1.100 (130) a | 1183.75 | 1690.63 |
| 4 | 542.9 (180.48) | 12 627.64 | 11 400.81 | 170 (160) | 2453.75 | 4570.63 |
| 6 | 152.9 (16.88) | 13 323.44 | 14 489.8 | <LOD | 2623.75 | 5250.63 |
| 24 | 26.9 (0.44) | 14 941.64 | 28 556.8 | <LOD | 2623.75 | 5250.63 |
| ∞ | 15 107.97 | 32 548.76 | 2623.75 | 5250.63 |
Note: BNN27 concentration in C57BL/6 mice serum (Cserum, ng/ml) and in S‐D rat retina (Cretina, ng/g), following i.p. injection of 100 and 30 mg/kg doses, respectively, over time (h). Concentration values represent the mean (SEM). AUC and AUMC values are included for each set of values.
Abbreviations: AUC, area under the curve; AUMC, area under the first moment curve.
The corresponding value measured at this time point, after transcardial perfusion of 5 animals with Ringer's‐Lactate solution is 320 (70) ng/g.
FIGURE 4.
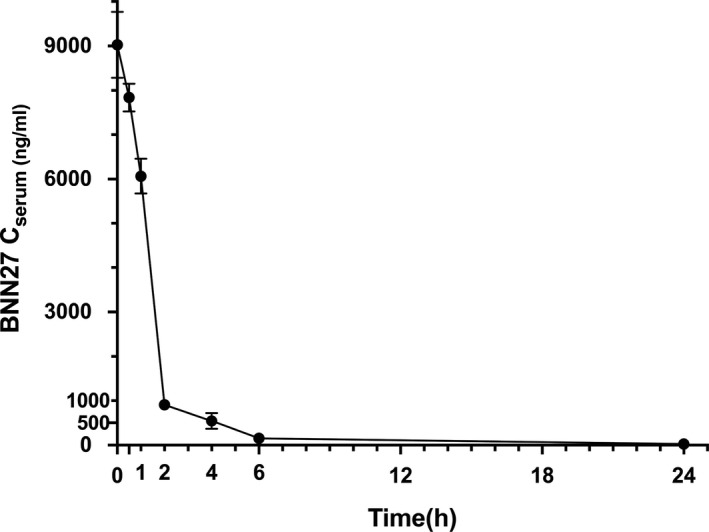
Mean concentration of BNN27 (ng/ml), with SEM, in mice serum, after single i.p dose administration of 100 mg/kg of body weight, over time (h). N = 5 at each time point. Error bars are not depicted when they are shorter than the height of the symbol
The Elimination Constant Rate was calculated as K el(h−1)= 0.465 h−1 and the MRT equal to 2.154 h, when AUC∞ was used (for calculations). Corresponding values of 0.523 h−1 (for K el) and 1.911 h (for MRT) were calculated when AUC24 was considered, which were similar with the previous ones. The other pharmacokinetic parameters are summarized in Table 2. The Do for the Clearance calculation was 2.45 mg of BNN27 (100 mg/kg for average weighting 24.5 g animals).
TABLE 2.
Pharmacokinetic parameters
| Parameters |
Serum (mice) [for AUC∞] |
Serum (mice) [for AUC24 h] |
Retina (rats) |
|---|---|---|---|
| C max | 9026.68 ng/ml | 9026.68 ng/ml | 1100 ng/g |
| AUC | 15 107.97 ng×h/ml | 14 941.63 ng×h/ml | 2623.75 ng×h/g |
| AUMC | 32 548.76 ng×h2/ml | 28 556.80 ng×h/ml | 5250.63 ng×h2/g |
| MRT (h) | 2.154 | 1.911 | 2.001 |
| K el (h−1) | 0.465 | 0.523 | 0.499 |
| Cl (L×h−1) [assuming F = 1] | 0.162 | 0.164 | – |
| V ss (L) | 0.394 | 0.313 | – |
Note: Pharmacokinetic parameters calculated from the serum BNN27 concentrations in mice and from the retinal BNN27 concentrations in rats, following i.p. injection of 100 and 30 mg/kg doses, respectively. Nonparametric pharmacokinetic analysis method was used. For parameters calculated from serum values, both, the parameters calculated from AUC24 h (up to 24 h) as well as those calculated from AUC∞, are reported (for comparison).
3.3. Retina
The mean weight of the rat retina was 19 ± 0.5 mg. The mean BNN27 concentration (and SEM) measured at each time point, as well as for the perfused animals, are presented in Table 1 and depicted in Figure 5. All blind samples were measured at zero BNN27 concentration. The maximum concentration as measured (C max) was 1100 ng/g, at = 2 h after i.p. administration. At the same time point (2 h post i.p. injection), the mean retinal BNN27 concentration in the perfused animals was 320 (70) ng/g. The rest of the parameters are presented in Table 2.
FIGURE 5.
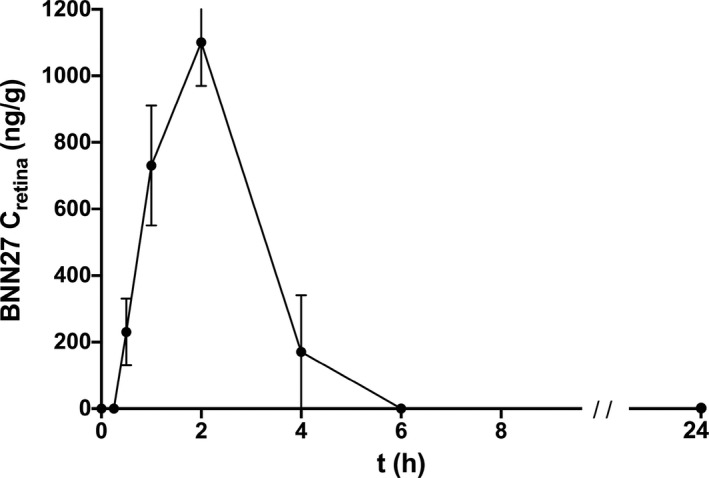
Mean concentration of BNN27 (ng/g), with SEM, in rat retinal tissues, after single i.p. dose administration of 30 mg/kg of body weight, over time (h). N = 5 at each time point
4. DISCUSSION
This study provides some quantitative/pharmacokinetic parameters of a novel small‐sized synthetic agonist of neurotrophin receptors, the MNT BNN27, after systemic (i.p.) administration in rodents. The pharmacokinetic properties in the mice blood are described, as well as the quantification of the MNT in the retina of S‐D rats, using LC‐MS.
Mass spectrometry has been frequently used for the quantification of various steroids in the blood and brain of experimental animals 2 , 28 , 29 and humans. 25 , 30 , 31 , 32 , 33 The method that is presented herein proved to be accurate and efficient in measuring the DHEA analog. It is characterized of good linearity (r 2 > 0.99), accuracy and precision, acceptable recoveries and LOQ values comparable to the literature 25 , 28 , 29 , 34 , 35 indicating it is as a satisfactory assay, effective for measuring the new molecule not only in serum, but also in small peripheral tissues such as the rat retina.
We have originally measured the BNN27 levels in the serum of C57BL/6 mice after single intra‐peritoneal administration of 100 mg/kg of b.w. of the solution. A rapid elimination of the molecule was observed, the MRT was 2.154 h, whereas after 6 h only traces of the substance were detected. This suggests that the molecule is rapidly absorbed after i.p. administration as anticipated by its small size and lipophilic properties. The same rapid elimination characterizes its predecessor molecule, DHEA, when administered systematically as well. 36 , 37 , 38
Furthermore, we have detected and quantified BNN27 in the rat retina, after one single i.p. injection of 30 mg/kg of b.w. The retina is one of the target peripheral tissues of BNN27 in animal models, hence the concentration was adapted to be closer to the concentrations that have been used in the studies so far. The LC‐MS method that was used for the serum kinetics was modified and adjusted for the size and nature of the retinal tissue during the sample preparation. BNN27 was detected in low concentration levels with this method (minimum 170 ng/g), ascertaining our assay effective for small peripheral tissues and detection of minute quantities. A similar assay has been used by Ibán‐Arias et al 15 for a smaller series of animals (rats) after consecutive i.p. injections of a lower concentration of BNN27 with affirmative results as well.
According to our results, the MNT moves fast into and away from the retinal tissue. BNN27 is present in the retina of S‐D rats 30 min after a single i.p. administration of 30 mg/kg of b.w. It reaches the peak concentration in 2 h and disappears 4 h after that. This rapid distribution and elimination of the molecule enhances the theoretical assumption that a small and lipophilic molecule, like the BNN27, crosses biological membranes such as the BBB and the BRB with passive diffusion.
The presence of BNN27 in the rat retina is corroborated by its detection in the tissue after transcardial perfusion with a physiological solution. The blood extraction achieved by this technique asserts any detection of the compound to correspond to tissue accumulation only. The detection of the molecule in the tissue after perfusion proves that the molecule can cross the blood–retina barrier after parenteral administration, as suggested by the in vivo results in several animal‐model studies 5 , 14 , 15 , 16 as well. Consequently, considering the similarities between the BRB and the BBB, one could imply the penetration of the BBB by the molecule. The results of numerous in vivo experiments 2 , 8 , 9 , 10 , 11 , 12 , 13 as well as in vitro assays 1 , 2 , 8 , 9 can support this allegation.
In addition, the early detection of BNN27 in the retina is in line with data from the study of Bennett et al, for the rat brain, where the researchers report to have detected BNN27 in the rat brain 30 min after i.p. administration. 2 Considering that the retina is part of the CNS, this comes as no surprise and the numerous studies proving anti‐apoptotic and neuroprotective effect of BNN27 in CNS and retinal disease animal models enhance even further our hypothesis that this MNT crosses not only the BRB, but also the BBB.
Finally, the i.p. administration of both concentrations of the BNN27 did not show any macroscopic systemic or ocular toxicity. During this study (24 h after i.p. administration, the maximal time point), no acute steroidogenic or other side effect was described for both rodents, neither systemic nor topical at the sight of the injection or the ocular and periocular region. This is in accordance with all the animal studies where BNN27 was used so far. Unlike DHEA, that evokes significant steroid‐related side effects, BNN27 seems devoid of any similar activity. However, long‐term toxicity studies should be subjoined to corroborate the safety data.
A significant limitation of the study was the use of two different experimental animals for the two procedures. Once the rapid systemic absorption and elimination of BNN27 in mice was validated, we wanted to test the same simple method to a peripheral tissue, other than the brain (already tested by several researchers) that expresses NGF receptor activity. The retina was our candidate of choice, because of our special interest in retinal disease and the already existing in vivo results. However, the size of the mouse retina was insufficient for the application of the quantification method used in this study. Hence, we have chosen a bigger rodent, the Sprague–Dawley rat, in order to obtain more substantial sample tissue. Since the rat's BRB does not differ from the mouse's BRB, the use of both rodents was found acceptable for this study. We have extracted the retina of both eyes of each animal and used as one sample. This maximized the available tissue and optimized any distribution difference between the two eyes. Thus, it improved the performance of the LC‐MS and solidified our data.
Another remark for the study design is the different dose that was used for the two parts of the experiment. In the first part, a concentration of 100 mg/kg of b.w. was used, which is the maximum dilution of the BNN27 achieved at the ethanol solution used in our laboratory. In the second part, a concentration of 30 mg/kg of b.w. was chosen that was closer to the usual therapeutic dosage of BNN27 in animal models. The detection of the lower dosage would prove the presence of the molecule in peripheral tissue in “real life” therapeutic conditions, but also the safe use of the method in lower concentration for small tissues. With the reduction in the dose toward an already used therapeutic one, we have managed to give a direct reference to proof of the presence of the molecule in the rat retina, one of the target tissues of BNN27 in animal models.
Conclusively, the aim of the study was to investigate the presence of a novel synthetic DHEA analog in the serum and retina of rodents, after single dose i.p. administration. This was successfully achieved by a LC‐MS‐based method that proved to be sensitive and efficient for this purpose, even for a low concentration and a small biological sample such as the rat retina. The quantitative determination of BNN27 in the retina after perfusion enhances the data supporting that the molecule crosses the blood–retina barrier. The quantitative data provided with this study for serum and peripheral/retinal tissues for this novel synthetic MNT can contribute to the understanding of the molecule's in vivo properties and the adaptation of dose regimens in experimental use.
CONFLICT OF INTEREST
Tsika C, Tzatzarakis MN, Tsoka PA, Efstathopoulos P, Charalampopoulos I, and Tsilimbaris MK have no financial interest in this study. Gravanis A is co‐founder of Bionature E.A. Ltd. The BNN compounds are proprietary and patented by the Bionature E.A. Ltd (http://www.bionature.net) (Patent Number: WO2008/155534 A2).
AUTHOR CONTRIBUTION
CT, MKT, and MNT designed the experiments. CT, PT, and PE performed the experiments under supervision of TMN, MKT, and IC. CT and MNT collected the data. CT, MNT, and SGA analyzed the data and wrote the manuscript. IC, AG, MNT, SGA, and MKT revised the manuscript which was approved by all authors.
ACKNOWLEDGMENTS
The MNTs used in the study, BNN27 and BNN20, were kindly provided by Dr A. Gravanis, co‐founder of Bionature E.A. Ltd.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. All authors confirm adherence to this data policy.
REFERENCES
- 1. Calogeropoulou T, Avlonitis N, Minas V, et al. Novel dehydroepiandrosterone derivatives with antiapoptotic, neuroprotective activity. J Med Chem. 2009;52(21):6569‐6587. [DOI] [PubMed] [Google Scholar]
- 2. Bennett JP, Brien LCO, Brohawn DG, O’Brien LC, Brohawn DG. Pharmacological properties of microneurotrophin drugs developed for treatment of amyotrophic lateral sclerosis. Biochem Pharmacol. 2016;117:68‐77. [DOI] [PubMed] [Google Scholar]
- 3. Botsakis K, Mourtzi T, Panagiotakopoulou V, et al. BNN‐20, a synthetic microneurotrophin, strongly protects dopaminergic neurons in the “weaver” mouse, a genetic model of dopamine‐denervation, acting through the TrkB neurotrophin receptor. Neuropharmacology. 2017;121:140‐157. [DOI] [PubMed] [Google Scholar]
- 4. Panagiotakopoulou V, Botsakis K, Delis F, et al. Anti‐neuroinflammatory, protective effects of the synthetic microneurotrophin BNN‐20 in the advanced dopaminergic neurodegeneration of “weaver” mice. Neuropharmacology. 2020;165:107919. [DOI] [PubMed] [Google Scholar]
- 5. Lisa S, Iban‐Arias R, Kokona D, Charalampopoulos I, Gravanis A, Thermos K. Effects of novel synthetic microneurotrophins in diabetic retinopathy. Springerplus. 2015;4(sup1):L25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pediaditakis I, Kourgiantaki A, Prousis KC, et al. BNN27, a 17‐spiroepoxy steroid derivative, interacts with and activates p75 neurotrophin receptor, rescuing cerebellar granule neurons from apoptosis. Front Pharmacol. 2016;7:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pediaditakis I, Efstathopoulos P, Prousis KC, et al. Selective and differential interactions of BNN27, a novel C17‐spiroepoxy steroid derivative, with TrkA receptors, regulating neuronal survival and differentiation. Neuropharmacology. 2016;111:266‐282. [DOI] [PubMed] [Google Scholar]
- 8. Glajch KE, Ferraiuolo L, Mueller KA, et al. MicroNeurotrophins improve survival in motor neuron‐astrocyte co‐cultures but do not improve disease phenotypes in a mutant SOD1 mouse model of amyotrophic lateral sclerosis. PLoS One. 2016;11(10):e0164103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonetto G, Charalampopoulos I, Gravanis A, Karagogeos D. The novel synthetic microneurotrophin BNN27 protects mature oligodendrocytes against cuprizone‐induced death, through the NGF receptor TrkA. Glia. 2017;65(8):1376‐1394. [DOI] [PubMed] [Google Scholar]
- 10. Pitsikas N, Zoupa E, Gravanis A. The novel dehydroepiandrosterone (DHEA) derivative BNN27 counteracts cognitive deficits induced by the D1/D2 dopaminergic receptor agonist apomorphine in rats. Psychopharmacology. 2021;238:227‐237. [DOI] [PubMed] [Google Scholar]
- 11. Pitsikas N, Gravanis A. The novel dehydroepiandrosterone (DHEA) derivative BNN27 counteracts delay‐dependent and scopolamine‐induced recognition memory deficitsm in rats. Neurobiol Learn Mem. 2017;140:145‐153. [DOI] [PubMed] [Google Scholar]
- 12. Kokras N, Dioli C, Paravatou R, et al. Psychoactive properties of BNN27, a novel neurosteroid derivate, in male and female rats. Psychopharmacology. 2020;237(8):2435‐2449. [DOI] [PubMed] [Google Scholar]
- 13. Zoupa E, Gravanis A, Pitsikas N. The novel dehydroepiandrosterone (DHEA) derivative BNN27 counteracts behavioural deficits induced by the NMDA receptor antagonist ketamine in rats. Neuropharmacology. 2019;151:74‐83. [DOI] [PubMed] [Google Scholar]
- 14. Tsoka P, Matsumoto H, Maidana DE, et al. Effects of BNN27, a novel C17‐spiroepoxy steroid derivative, on experimental retinal detachment‐induced photoreceptor cell death. Sci Rep. 2018;8(1):10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ibán‐Arias R, Lisa S, Mastrodimou N, et al. The synthetic microneurotrophin BNN27 affects retinal function in rats with streptozotocin‐induced diabetes. Diabetes. 2018;67(2):321‐333. [DOI] [PubMed] [Google Scholar]
- 16. Ibán‐Arias R, Lisa S, Poulaki S, et al. Effect of topical administration of the microneurotrophin BNN27 in the diabetic rat retina. Graefes Arch Clin Exp Ophthalmol. 2019;257(11):2429‐2436. [DOI] [PubMed] [Google Scholar]
- 17. Morris KT, Toth‐Fejel S, Schmidt J, Fletcher WS, Pommier RF. High dehydroepiandrosterone‐sulfate predicts breast cancer progression during new aromatase inhibitor therapy and stimulates breast cancer cell growth in tissue culture: a renewed role for adrenalectomy. Surgery. 2001;130(6):947‐953. [DOI] [PubMed] [Google Scholar]
- 18. Stoll BA. Dietary supplements of dehydroepiandrosterone in relation to breast cancer risk. Eur J Clin Nutr. 1999;53(10):771‐775. [DOI] [PubMed] [Google Scholar]
- 19. Arnold JT, Le H, McFann KK, Blackman MR. Comparative effects of DHEA vs. testosterone, dihydrotestosterone, and estradiol on proliferation and gene expression in human LNCaP prostate cancer cells. Am J Physiol Endocrinol Metab. 2005;288(3):E573‐E584. [DOI] [PubMed] [Google Scholar]
- 20. Karp G, Bentov Y, Masalha R, Ifergane G. Onset of late posttraumatic seizure after dehydroepiandrosterone treatment. Fertil Steril. 2009;91(3):931.e1‐2. [DOI] [PubMed] [Google Scholar]
- 21. Kline MD, Jaggers ED. Mania onset while using dehydroepiandrosterone [6]. Am J Psychiatry. 1999;156(6):971. [DOI] [PubMed] [Google Scholar]
- 22. Sahelian RBS. Dehydroepiandrosterone and cardiac arrhythmia. Ann Intern Med. 1998;129(7):588. [DOI] [PubMed] [Google Scholar]
- 23. Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SSC. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age‐advanced men and women. Clin Endocrinol (Oxf). 1998;49(4):421‐432. [DOI] [PubMed] [Google Scholar]
- 24. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Br J Pharmacol. 2020;177(16):3617‐3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magnisali P, Dracopoulou M, Mataragas M, Dacou‐Voutetakis A, Moutsatsou P. Routine method for the simultaneous quantification of 17alpha‐hydroxyprogesterone, testosterone, dehydroepiandrosterone, androstenedione, cortisol, and pregnenolone in human serum of neonates using gas chromatography‐mass spectrometry. J Chromatogr A. 2008;1206(2):166‐177. [DOI] [PubMed] [Google Scholar]
- 26. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46(D1):D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alexander SPH, Fabbro D, Kelly E, et al. The concise guide to pharmacology 2019/20: catalytic receptors. Br J Pharmacol. 2019;176(suppl 1):S247‐S296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakajima M, Yamato S, Shimada K. Determination of dehydroepiandrosterone sulphate in biological samples by liquid chromatography/atmospheric pressure chemical ionization‐mass spectrometry using [7,7,16,16–2H4]‐dehydroepiandrosterone as an internal standard. Biomed Chromatogr. 1998;12(4):211‐216. [DOI] [PubMed] [Google Scholar]
- 29. Li A, May MP, Bigelow JC. An LC/MS method for the quantitative determination of 7alpha‐OH DHEA and 7beta‐OH DHEA: an application for the study of the metabolism of DHEA in rat brain. Biomed Chromatogr. 2010;24(8):833‐837. [DOI] [PubMed] [Google Scholar]
- 30. Magnisali P, Chalioti M, Livadara T, Mataragas M, Paliatsiou S. 4‐androstenedione, cortisol and cortisone in newborn blood spots using liquid chromatography—tandem mass spectrometry. J Chromatogr B. 2011;879(19):1565‐1572. [DOI] [PubMed] [Google Scholar]
- 31. Cho S, Jung BH, Lee W, Chung BC. Rapid column‐switching liquid chromatography / mass spectrometric assay for DHEA‐sulfate in the plasma of patients with Alzheimer’ s disease. Biomed Chromatogr. 2006;1097:1093‐1097. [DOI] [PubMed] [Google Scholar]
- 32. Labrie F, Bélanger A, Labrie C, Candas B, Cusan L, Gomez JL. Bioavailability and metabolism of oral and percutaneous dehydroepiandrosterone in postmenopausal women. J Steroid Biochem Mol Biol. 2007;107(1–2):57‐69. [DOI] [PubMed] [Google Scholar]
- 33. Kushnir MM, Blamires T, Rockwood AL, et al. Liquid chromatography‐tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56(7):1138‐1147. [DOI] [PubMed] [Google Scholar]
- 34. Magnisali P, Chalioti MB, Livadara T, et al. Simultaneous quantification of 17α‐OH progesterone, 11‐deoxycortisol, Δ4‐androstenedione, cortisol and cortisone in newborn blood spots using liquid chromatography‐tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(19):1565‐1572. [DOI] [PubMed] [Google Scholar]
- 35. Liere P, Akwa Y, Pianos A, Robel P, Sjovall J. Validation of an analytical procedure to measure trace amounts of neurosteroids in brain tissue by gas chromatography—mass spectrometry. J Chromatogr B Biomed Sci Appl. 2000;739(2):301‐312. [DOI] [PubMed] [Google Scholar]
- 36. Longcope C. The metabolism of dehydroepiandrosterone sulfate and dehydroepiandrosterone. Aging Male. 1998;1(1):51‐55. [Google Scholar]
- 37. Longcope C. Dehydroepiandrosterone metabolism. J Endocrinol. 1996;150(suppl):S125‐S127. [PubMed] [Google Scholar]
- 38. Longcope C, Tast J. Dehydroepiandrosterone metabolism in the female rhesus monkey: oral versus intravenous administration. Steroids. 1996;61(1):7‐10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. All authors confirm adherence to this data policy.


