Abstract
Drought stress, being the inevitable factor that exists in various environments without recognizing borders and no clear warning thereby hampering plant biomass production, quality, and energy. It is the key important environmental stress that occurs due to temperature dynamics, light intensity, and low rainfall. Despite this, its cumulative, not obvious impact and multidimensional nature severely affects the plant morphological, physiological, biochemical and molecular attributes with adverse impact on photosynthetic capacity. Coping with water scarcity, plants evolve various complex resistance and adaptation mechanisms including physiological and biochemical responses, which differ with species level. The sophisticated adaptation mechanisms and regularity network that improves the water stress tolerance and adaptation in plants are briefly discussed. Growth pattern and structural dynamics, reduction in transpiration loss through altering stomatal conductance and distribution, leaf rolling, root to shoot ratio dynamics, root length increment, accumulation of compatible solutes, enhancement in transpiration efficiency, osmotic and hormonal regulation, and delayed senescence are the strategies that are adopted by plants under water deficit. Approaches for drought stress alleviations are breeding strategies, molecular and genomics perspectives with special emphasis on the omics technology alteration i.e., metabolomics, proteomics, genomics, transcriptomics, glyomics and phenomics that improve the stress tolerance in plants. For drought stress induction, seed priming, growth hormones, osmoprotectants, silicon (Si), selenium (Se) and potassium application are worth using under drought stress conditions in plants. In addition, drought adaptation through microbes, hydrogel, nanoparticles applications and metabolic engineering techniques that regulate the antioxidant enzymes activity for adaptation to drought stress in plants, enhancing plant tolerance through maintenance in cell homeostasis and ameliorates the adverse effects of water stress are of great potential in agriculture.
Keywords: drought stress, plants, mitigation, abiotic stress
1. Introduction
Plants are exposed to various environmental stresses during growth and development under natural and agricultural conditions. Among these, drought is one the most severe environmental stresses affecting plant productivity. About 80–95% of the fresh biomass of the plant body is comprised of water, which plays a vital role in various physiological processes including many aspects of plant growth, development, and metabolism [1,2]. As a result, some consider drought as the main environmental stress for different plants, particularly in drought prone areas [3,4], the single most critical threat to world food security in the future and the catalyst of important famines in the past [5]. The effects of drought in agriculture are aggravated due to the depletion of water resources and the increased food demand from an alarming world population growth [6]. The unpredictable nature of the drought is dependent upon various factors such as uneven and undependable distribution of rainfall, evapotranspiration, and water holding capacity around the rhizosphere [7,8]. Moreover, in some cases plants are unable to uptake water from the soil, even though enough moisture is present in the root zone [9], a phenomenon known as physiological drought or pseudo-drought [10].
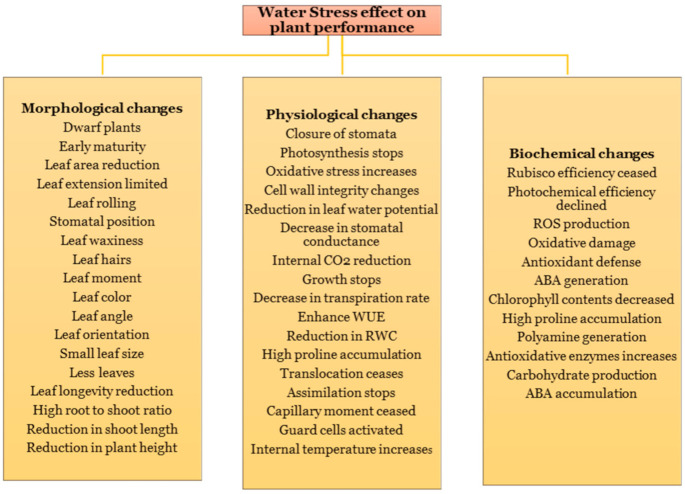
Different molecular, biochemical, physiological, morphological and ecological traits and processes (Figure 1) of the plants are impaired under drought stress conditions [11]. Plant yield and quality are adversely affected in water deficit environments [12]. Growth stages, age, plant species and drought severity and duration are the key factors that influence the plant responses to drought [13]. The resistance mechanism to drought, in turn, varies among plant species. Plants, therefore, have the ability to reduce their resource utilization and adjust their growth to cope against adverse environmental conditions like drought [14,15]. Various networks at the molecular level, such as those involved in signal transduction, are responsible for enhancing these responses against drought stress [16,17]. The stomatal regulation of plants through enhanced ion transport, transcription factor activities and abscisic acid (ABA) signaling are also involved in the molecular mechanisms of plant response to drought stress [18,19].
Figure 1.
Morphological, physiological and biochemical dynamics of plants affected by water stress.
Under certain changing circumstances, there is a need to improve the drought tolerance of the plant. For enhancement of water-use efficiency, when physical adaptation of roots and leaves are not enough to cope with certain drought molecular signals including the gene coding regularity protein that expresses many other genes and signals through crosstalk according to different regulatory mechanisms [20,21]. To meet future food demand, fostering more work on drought-tolerant plants and the use of economical and beneficial agriculture practices will be of paramount importance [22,23].
2. Causes of Drought Stress in Plants
Global climate change is expected to accelerate in the future because of the continuous rising of air temperature and atmospheric CO2 levels that ultimately alters the rainfall patterns and its distribution [24,25]. Although deficient water input from rainfall is usually the main driver for drought stress, the loss of water from soils through evaporation, which is driven by high temperature events, high light intensity and dry wind, can further aggravate an existing drought stress event [26]. Global climate change typically results in prevalent drought stress conditions over vast areas at a global scale. Alongside drought, salinity stress is also considered a primary cause of water deficit in plants [27,28,29]. Certain factors responsible for drought stress are briefly highlighted.
2.1. Global Warming
Some of the consequences derived from climate change could be beneficial for agricultural productivity. For example, higher rates of photosynthesis have been reported under elevated CO2, hence its presence in the atmosphere in elevated concentrations could enhance grain yields in the future [30]. However, in most cases, climate change has detrimental consequences both in natural and agricultural ecosystems. Increases in air temperatures can result in the melting of glaciers and potential flooding of agricultural lands with low or null slope [31]. Additionally, the loss of glaciers is causing the shrinkage of water reservoirs which limits the water availability to crops, a trend that is increasing with time. In fact, in various rain-fed agricultural areas around the world, the annual accumulated precipitation has decreased because of global warming [32]. Loss of water due to global warming is not only occurring in the soil, but also at the plant level. Internal water in plants up to great extent are lost to the atmosphere driven by the increased temperatures resulting from global warming, a phenomenon that further exacerbates the already existing water deficit problems in various agricultural systems around the world [33]. If expected increases in air temperature around 2 °C greater than present levels occur by the end of this century, approximately one fifth of the world population will be affected by severe water deficit [34].
2.2. Rainfall Anomalies
More stress is expected in areas where crop production is solely dependent on rainfall compared to areas that are being irrigated through canals, rivers and the water channel [35]. Thus, in rain-fed areas drought episodes are strongly correlated with the rainfall distribution across the year and high chances of water stress are observed in some years over a certain period of time [35]. Industrialization, deforestation and urbanization are the prominent anthropogenic activities that affect rainfall patterns, and thus water availability to plants, through its influence in climate change [36]. In Pakistan, erratic and more frequent rainfall occurs in early spring and winter, while more frequently drier and hotter seasons take place due to less and/or no rainfall in early fall and summer seasons. In summer in particular, the combination of greater atmospheric water demand for the plants, higher evaporation and transpiration rates, and less rainfall availability associated with this season amplifies the detrimental effects of drought stress in plant growth and development. However, rainfall distribution and intensity within and across the years play a prominent role in both the management of the water resources for plants and the occurrence of drought stresses in most cases [37,38].
2.3. Shifts in Monsoon Patterns
During the summer season, the monsoon system is considered as a source of rainfall in various areas of the world. Its occurrence is interlinked with temperature being the driving force [39]. It is expected that in rain-fed areas the amount of summer precipitations will decrease by 70% by the beginning of the XXII century if the prevailing situations continue [40]. According to estimations, high rainfall is expected due to linear increment in CO2 concentration in atmosphere that will affect crop production adversely and will lead to massive floods and massive economic losses in the agriculture sector of densely populated countries [41,42]. Under such circumstances, monsoon rainfall variability is and will continue to affect the moisture level of the rhizosphere, thereby affecting plant productivity in particular areas of the world through dynamics in rainfall intensity, occurrence and duration. Remarkably, two thirds of the world population are currently facing food insecurity due to extreme variation between dry and wet seasonal rainfalls as a result of changes in the monsoon shifts [43]. Added to the intrinsically random and unpredicted nature of the rainfall patterns, and due to recent climate changes, shortening or extendibility of the rainy season may exacerbate present and future scenarios with both water deficit and/or water excess problems in some climatic zones [37]. Being agricultural, crop production practices need to be adopted accordingly during the monsoon behavior and shifted to sustainable crop production. Proper management and crop planning are two strategies to cope with quantitative shifts going from deficient to excessive and, vice versa, to monsoon patterns.
3. Effect of Drought Stress on Plants
Depending on the dynamics in the environmental conditions, plants could face various stresses that may severely affect their growth and development [44,45]. Certain metabolic changes and gene expressions occur to enable the plants to survive under these circumstances [27,46]. Grain quality and yield could be greatly affected by drought stress, known as the most limiting stress in agriculture. Thus, investigating the plants’ ability to cope with water limitation is of great value and should continue to receive attention in the near future, especially in arid and semi-arid environments [47]. Currently, major staple crops are being intensively studied to identify the drought-responsive mechanisms to harvest maximum grain yields and quality, but future work should focus on the combined effect of both heat and drought stress impacts at the reproductive stages of main grain crops [48].
The optimal level of water availability is necessary for plants growth and development, fluctuation in soil moisture beyond optimal can affect grain yield and quality. On the other hand, less than optimal water availability in the rhizosphere hampers the plant growth, thereby inhibiting the plant nutrient uptake [49]. The latter has recently been responsible of huge reductions in the production of grain crops, and is only expected to become more severe due to global warming and variability in climate [50,51].
Water scarcity outbreaks are due to the occurrence of less or the absence of rainfall resulting in low soil moisture content and low water potential in aerial parts of the plant such as leaves and stems [52]. When this occurs, the rate of loss of water through transpiration from leaves surpasses the water uptake rate through roots in dry environments [53]. The roots strive to uptake more water through their expansion and this ultimately adapts plants to minimize stomatal loss of water when there is a water deficit [54]. Typical drought stress symptoms in plants include leaf rolling, stunning plants, yellowing leaves, leaf scorching, permanent wilting [55]. Moreover, plant response to a given water deficit is strongly dependent on the previous occurrence and intensity of other drought stress events [28,56,57] and the presence of other stresses [58].
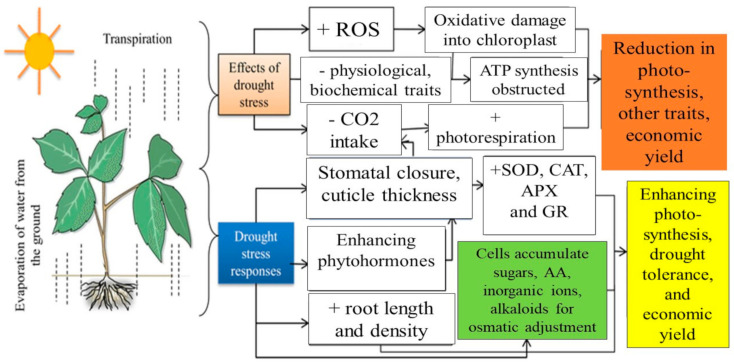
Despite the adverse effects that water deficit has on plant performance, plants have the ability to respond to varying degrees of water deficit (Figure 2). There is a strong correlation between plant growth and water availability as cell enlargement is more affected by water deficits than cell division [59]. Under these conditions, the growth of the plants is inhibited as a result in the reduction of the cell wall extensibility and turgor [60]. When drought conditions are severe, respiration can also decrease, although increments in respiration were observed under mild stress [61]. To cope against the water deficit, the osmotic adjustment of stressed plants is maintained through an increase in sugar content of roots and leaves, and relatively greater growth in roots compared to shoots has been observed in plants subjected to drought stress in the past [62].
Figure 2.
Adverse effects and adaptations of plants to drought stress, modified from Ullah et al. [63]—means reduce; + means increase.
Environmental factors including drought duration, intensity and frequency, soil characteristics, growth conditions and stages, and plant species strongly influence the extent and duration of drought-related symptoms in plants [64]. Increases in the rate of leaves senescence and drooping, scorching and limp leaves, leaf rolling and brittleness, closed flowers and flower sagging, etiolation, wilting, turgidity, premature fall, senescence and yellowing of leaves are among the most ubiquitous symptoms of drought stress in plants [65,66]. Although less usual, twig cracks, branch dieback, necrosis, stunted growth, bark crack, shrub canopy and tree thinning represent other symptoms displayed by plants under drought conditions [67]. In some cases, plants may die under extreme drought stress. Whereas water deficiency typically has a profound impact on plant growth and development, water excess also affects plant performance and hampers growth and final yield [68]. When this occurs, excess water stress symptoms are soft fleshy leaves, leaves with rotten patches, fungus affected and moldy plant parts.
4. Plant Responses to Drought Stress
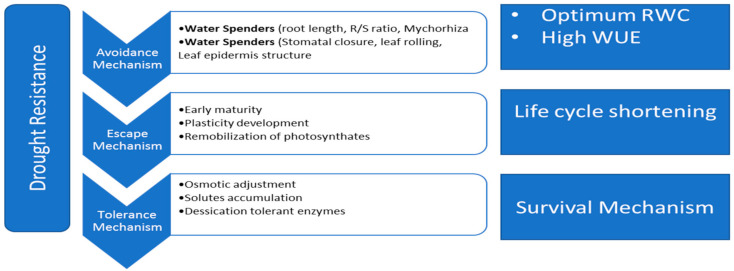
Different adaptive mechanisms that make plants more tolerant to the adverse effects of drought stress have been developed through evolution [69]. Stress avoidance, escape and tolerance are the three main survival strategies that plants utilize when exposed to drought stress. Thus, plants responses to drought stress vary from the molecular up to plant level [70]. The mechanisms of plant escape, avoidance and tolerance (Figure 3) against drought stress are discussed in the following sections.
Figure 3.
Schematic diagram of drought resistance mechanism in plants. RWC = relative water contents; WUE = water-use efficiency.
4.1. Escape Mechanism
To escape the detrimental effects of drought stress on plant productivity, some plants utilize mechanisms involving rapid plant development and shortening of the life cycle, self-reproduction, and seasonal growth before the beginning of the driest part of the year [71]. Among these mechanisms, early flowering is perhaps the best possible escape adaptive mechanism in plants [72], although this mechanism can imply a considerable reduction in the length of the plant growing period and the final plant productivity in some cases [73].
4.2. Avoidance and Tolerance Mechanisms
Under the avoidance strategy, plant water potential is maintained high through a reduction in the stomatal transpiration losses and the increase of water uptake from well-established root systems [74]. In other cases, xeromorphic characteristics such as the presence of hairy leaves and cuticles may help to maintain high water potentials in plant tissues [75]. However, overdevelopment of these structures has a value for the plant in terms of reductions in plant productivity and reduced average size of vegetative and reproductive parts of the plant [76].
On the other hand, an adaptive tolerance mechanism at the photosynthetic machinery level includes reductions in the plant leaf area and limitations in the expansion of new leaves. Similarly, trichomes production on either side of the leaves are exomorphic attributes that allow the plant to tolerate water deficits in dry environments [77]. These structures reduce the leaf temperature by increasing the rate of light reflection in the leaf and also by adding another extra layer of resistance to the water loss. Hence the rate of water loss through leaf transpiration is reduced [78]. However, it is broadly accepted that changes in the root system, including root size, density, length, proliferation, expansion and growth rate, represent the main strategy for drought-tolerant plants to cope against water deficits [79]. Other mechanisms like osmotic adjustment, antioxidant defense mechanism, solute accumulation, metabolic and biochemical dynamics of stomatal closure and increment in root shoot ratio are other common strategies that allow plants to tolerate the adverse effect of drought stress [80].
5. Approaches to Alleviate the Adverse Effects of Drought Stress
Use of best management practices related to sowing time, plant population, plant genotype, and soil and nutrient management can help to reduce grain yield losses in field crops subjected to drought stress [81,82]. However, use of transgenic plants with drought-tolerant events is perhaps the drought stress mitigation approach most heavily publicized and the one receiving more attention at present. Several efforts like breeding, molecular and genomic approaches are being undertaken to develop drought-tolerant plants through usual conventional breeding methods [83], with the focus to improve water extraction efficiency, water use efficiency, stomatal conductance, and osmotic adjustments, among others [84]. Other strategies include use of modern and more effective methods of irrigation, good planting practices, mulching, contouring, osmoprotectants and plants inoculations with certain microorganisms that enhance drought tolerance [85].
5.1. Selection and Breeding Strategies
Conventional and traditional breeding methods used up to the present were based on the empirical selection of yield [86]. The low heritability, on the one hand, and high genotype and environment interaction on the other, are the main factors defining the quantitative yield trait in major staple crops [87]. Thus, conventional breeding is in practice for yield improvement [88]. Knowledge of plant physiological processes is the prerequisite for selecting quantitative trait loci, locating gene sequences and quantitative trait loci introgression [89]. Due to irregular, undependable and unpredictable response of the drought, screening resistant cultivars is not possible in open conditions, however, it is manageable in sheltered and/or controlled conditions [90]. Conversely, the expression of randomly selected progenies for improved drought stress tolerance in diverse environments is an effective approach known as classical breeding [91]. The cultivars with low transpiration rates and unchanged WUE under non-stress conditions have no effect on final harvest [92]. Scientists are working on the genetic analysis of the root architecture, relative water contents, and osmotic potentials [93]. Focus need to be given to the yield contributing traits which are highly heritable that affects the grain yield under drought conditions but not under optimal conditions based on their feasibility to measure [94]. Nevertheless, they exhibit broad sense heritability for yield in water-limited agriculture systems and have often no interaction with grain yield [95]. When plants are subjected to drought stress, the most important factor that appears first under such circumstances is hampering of WUE which differs for varieties and cultivars [96]. Under these circumstances, plants decrease the stomatal density and leaf size thereby minimizing water loss and maintains the internal water balance [97]. Hence certain genotypes and cultivars, which are drought susceptible and unable to adjust to environmental conditions, resulted in low WUE [98]. Therefore, through a breeding approach, WUE could be enhanced for sustainable crop product in biomass per unit of water utilized [99].
Drought resistance is induced directly or indirectly in the crop species through traits’ genetic variability and thus has the improvement capability through selection in breeding. Marker assisted selection (MAS) and genomic selection (GS) are the two main approaches of genomic assisted breeding. For the prior approach, an initial step is to identify the molecular markers associated with the trait of interest, the prerequisite for selection in breeding programs. However, GS depends on progress of selection models based on genetic markers present on the whole genome and selection of genome estimated breeding values (GEBVs) in breeding populations through phenotyping training population. The MAS is a key part for many crops breeding programs over a few decades, GS being relatively new because it has only recently been applied to crops.
Molecular markers are involved in MAS that map close to quantitative trait loci (QTL) or specific genes that are linked with the particular target trait and could be used identify the individual with desirable alleles [100]. The QTL mapping or genome-wide association approaches are used to select marker trait association through accurate, reliable trait evaluation and dense molecular markers. Through these methods, QTLs for the traits linked with drought resistance are identified in various crops i.e., wheat [101], maize [102], sorghum [103], rice [104], soybean [105], pearl millet [106] and many other crops.
The genomic selection uses all the markers available for a population of GEBVs and GS models are used for selection of elite lines without phenotyping [100]. Contrary to MAS, the knowledge of QTLs is not the prerequisite for GS [107]. However, GS needs higher density marker data than MAS. This is possible through availability of low cost and genome wide marker coverage genotyping approaches [108]. GS is being applied for drought resistance induction breeding in maize by the international maize and wheat improvement center (CIMMYT) [109]. Research efforts through this approach are on course in other crops i.e., sugarcane, legumes and wheat [110,111,112].
5.2. Molecular and Genomic Perspective
Biochemical and molecular factors involved in the induction of processes to ameliorate the negative impacts of water stress include transcription, stress responsive genes (Table 1) and abscisic acid [113]. Concurrently to the increased tolerance to drought deficits, breeding programs are also interested to kept other stresses under control through transgenic expression of different stress responsive genes [114,115]. However, the increased expression of these genes is frequently associated with a deceleration in the plant growth rate, this could narrow down its practical use. Thus, the molecular and genetic bases for drought resistance still needs attention to successfully contend with these circumstances [116]. In this sense, genomic and related technological tools could highlight the genes that mitigate the stress effect so that efforts are conducive to maintain those genes in successive breeding programs [117]. The molecular level of stress-tolerant genes is in cross talk quantitative loci traits showing their interaction and cloning of the genes that are related to stress [118]. In general, it is accepted that a combination of selection through marker assessment, molecular and traditional breeding as an integrated approach is the best alternative for the improvement of the abiotic stress tolerance in plants genetic engineering [119,120].
Table 1.
Genes responsible for drought tolerance in plants.
| Host Plant | Gene Responsible | Function | Reference |
|---|---|---|---|
| Wheat | TaNAC69 | Increased tolerance to drought | [121] |
| Maize | NF-YB2 | Under drought it enhances yield and photosynthetic rate | [122] |
| Rice | AP37, OSNAC10 | Drought tolerance and grain yield increased | [123] |
| Soybean | P5C5 | Improvement in drought tolerance | [124] |
| Sugarcane | SodEFF3 | Drought tolerance increased | [125] |
| Tobacco | HSP70-1 | Drought stress tolerance mechanisms enhanced | [120] |
6. Drought-Resistance Induction
Plants adopt various approaches and strategies to alleviate the adverse effects of drought stress. Agriculturists are also using various strategies for drought stress tolerance, among which the application of exogenous regulators, chemicals, synthetic hormones and compounds are of great value to increase drought resistance at different plant growth stages.
6.1. Seed Priming
Seed priming has been referred to as the most important short-term approach to alleviate the adverse effect of drought on plants [126] (Table 2). The objective of this pre-sowing technique is to initiate the germination process in the metabolic machinery of the seed and prepare the seed for radicle protrusion without radicle emergence taking place during the process [127]. The germination process of prime seeds is more efficient, which results in higher germination rates and uniformity compared to non-primed seeds [128]. In crops like wheat, maize and chickpea seed priming is used to alleviate the adverse effect of drought stress [126,127,128]. Recently, the directly seeded rice (DSR) method used in rice grown in aerobic conditions resulted in an increment in the drought severity and frequency [129]. Under water scarce conditions, different osmotica were used for DSR with the result that CaHPO4 and KCL osmopriming enhanced crop yield and productivity. Better germination and stands were observed in primed seeds in water scant areas [128,130]. Optimal stand, better yield, ability to withstand drought, early and synchronized germination followed by emergence are linked with seed priming. It is reported that primed seed enhanced WUE by 44% in wheat crop than non-primed seeds under water stress conditions. High grain yield with early emergence and flowering resulted in primed seeds in water limited environments. Similarly, osmopriming with KNO3 and hydropriming enhanced yield of certain crops in water scarcity [131].
Table 2.
Tolerance mechanisms in different field crops through seed-priming treatments.
| Priming Method | Crop | Protective Effects | References |
|---|---|---|---|
| Cacl2 Hydro- and Osmopriming | Rice | Phenols, falovnoida accumulation, antioxidant system and enhances stand establishment | [132] |
| SNP Osmopriming | Compatible solutes accumulation enhances RWC, photosynthetic capacity, membrane stability and antioxidant enzymes | [133] | |
| On-farm priming | Maize | Sustains optimal temperature for germination and less emergence time | [134] |
| Hydropriming | Canola | Growth of seedling, root shoot ratio and germination enhanced | [135] |
| Molecular priming | Plants | Induce tolerance against oxidative stress | [136] |
| KNO3 Priming | Soybean | Number of grains and pods per plant increased | [137] |
| Hydropriming | Cotton | Seed vigor and germination enhancement, thermal time reduction for emergence of radical | [138] |
| Bio- and Osmopriming | Increment in LA, phenols, clorophyll contents, grain yield and quality | [139] | |
| Osmopriming | Sunflower | Catalase synthesis and immunocytolocalization increased | [138] |
| Osmopriming | Wheat | Maintains RWC, enhances proline accumulation, chlorophyll contents and emergence of leaf | [140] |
| CaCl2 Osmopriming | LPO reduction, osmolyte accumulation, increment in LA, RWC and grain yield | [141] | |
RWC: Relative water contents, LPO: Lipid peroxidation, LA: Leaf area, SNP: sodium nitroprusside.
6.2. Plant Growth Regulators
Application of natural and synthetic plant growth regulators (Table 3) can improve drought tolerance in plants [142]. The reduction in the length and weight of the hypocotyl in seedlings due to water stress can be mitigated with the application of gibberellic acid (GA), which helps to maintain the internal water balance and the protein synthesis in drought stressed plants [143]. The stomatal conductance, as well as the photosynthesis and the respiration rates in wheat and cotton and maize were increased in water-scant areas following application of GA, and this resulted in higher grain yields compared to treatments where GA was not applied [143,144]. Exogenous application of abscisic acid, uniconazole, brassinolide and jasmonic acid can also improve crop productivity under drought [145,146]. Another active cytokinin, benzyladenine, is a hormone that regulates the drought resistance mechanism in various plants, including maize, wheat, cotton, chickpea barley and rice soluble sugar, soluble protein content, and the activities of superoxide dismutase, peroxidase, and catalase in the leaves were increased by uniconazole and brassinoloide in drought stress conditions [147].
Table 3.
Tolerance mechanisms to drought enhancement through phytohormones in different field crops.
| Host Plant | Phytohormone | Mechanism | Yield Dynamics | References |
|---|---|---|---|---|
| Potato | Auxin | ROS and water loss reduction | Increased 10% | [152] |
| Jasmonic Acid (JA) | Root and shoot length increased, water loss decreased, plant defense and oxidative stress changed | [153] | ||
| Soybean | Abscisic Acid (ABA) | Stress genes regulated, proline and antioxidative enzyme activity increased and reduction in stomatal size | 21% increment | [154] |
| Rice | Gibberellic Acid (GA) | Maximum WUE, photosynthesis, APX, CAT, proline contents, expanded roots, and dwarf plants | 10–30% increased | [155] |
| Cotton | ABA | SOD, CAT, chlorophyll and proline increases | 46% increase | [156] |
| Rice | ABA | Longer roots, reduced stomatal density, size and leaf area, while ABA, proline, soluble sugar and SOD increased | 16% increased | [157] |
| Maize | ABA | Increased ABA accumulation and drought tolerance | Increased | [158] |
| Barley | Cytokinins | Transgenic barley plants showed better drought tolerance via better dehydration avoidance | Increased | [159] |
| Tomato | GA | Reduced whole-plant transpiration, smaller and reduced stomatalpores | Increased | [160] |
Abbreviations: WUE: Water use efficiency, APX: Ascorbate peroxidase, CAT: Catalase, SOD; Superoxidase dismutase, ROS: Reactive oxygen species.
Salicylic acid, an exogenously applied substance also improves drought tolerance and enhances growth and final harvest of the plants under water scarcity [148]. An enhancement in the catalase activity of wheat was observed through salicylic acid application under water-scarce conditions [149]. Use of salicylic acid and its derivatives in foliar and seed treatment applications increased the drought tolerance mechanism in wheat crop subjected to drought stress. Research shows that application of salicylic acid in wheat indirectly increased the accumulation of proline through an increment in the abscisic acid content [148,149]. In maize (Zea mays L.), polyamines contents are increased under drought stress conditions. Phytohormones such as ethylene and brassinolide (BR) are also of great importance to cope with various environmental stresses, especially drought stress. It enhances plant tolerance to biotic and abiotic stresses, through a complex pathway to regulate the plant defense system, by activating BZR1/BES1 transcription factors. It also regulates reactive oxygen species (ROS) production in plants under stress, and unbalancing of ROS scavenging leads to oxidative bursts, which have adverse effects on plants [150,151].
6.3. Osmoprotectants
The multiple range of plant stresses that reduce plant growth and productivity are regulated (Table 4) by osmoprotectants signaling. These substances accumulate during the time when growing conditions are not suitable for plant growth and development, and are responsible for maintaining the internal physiological processes that ensure plant survival under optimal conditions such as water scarcity [161,162]. Among others, important osmoprotectants in plants subjected to water stress include proline, trehalose, mannitol, fruton, and glycinebetaine [163]. These compounds, typically used for seed treatment or exogenously applied at different growth stages of established crops, protect the subcellular structure, increase the activity of antioxidant enzymes and mediate the osmotic adjustment in water-stressed plants [164,165]. Foliar application of proline also enhances the internal free proline in plants thereby increasing their drought tolerance [166]. Finally, use of polyamines like spermidine have also demonstrated to be efficient to increase plant tolerance to water stress in crops like barley (Hordeum vulgare L.) and wheat [167].
Table 4.
Osmoprotectants significance in drought tolerance mechanisms different plants species.
| Osmolytes | Plants | Plants Mechanism | References |
|---|---|---|---|
| Glycine betaine | Maize, Rice, Barley | Photosynthetic efficiency maintenance, thalakoid membrane protection and osmotic adjustment | [168] |
| GA& ABA | Tobacco | Improves stress tolerance, scavenging of ROS and carbon nitrogen balance | [169] |
| Fructan | Sugar Beet | ROS scavenger, protein and membrane stabilization and osmotic adjustment | [170] |
| Mannitol | Maize | Scavenge the stress induce oxygen radicals and osmotic adjustment | [171] |
| D-Ononitol | Arabidopsis | Prevent water loss in plants | [172] |
ROS: Reactive oxygen species.
6.4. Silicon, An Abundant Element on Earth
The most abundant element on the earth surface, silicon, could be used as a mineral nutrient to increase plant resistance (Table 5) to various levels and degrees of stresses [173] and the overall mechanical strength of both stressed and non-stressed plants [174]. Moreover, exogenous application of silicon has demonstrated the capacity to increase the relative water contents in sorghum and sunflower [173,174]. Additionally, and compared to the unfertilized control, wheat plants applied with silicon not only maintained higher relative water contents but also increased the shoot dry matter when exposed to water stress conditions. Thus, silicon application decreased the shoot to root ratio through root growth facilitation. Furthermore, silicon application to wheat increased the photosynthesis rate, stomatal conductance and the antioxidant defense compared to plants with no silicon application [175]. Thus, silicon application in crops exposed to drought conditions can play an important role in maintaining the growth of roots and transport of water under drought stress [173,176].
Table 5.
Silicon activates the antioxidants activity and improves drought tolerance mechanisms plants.
| Crop Plant | Activity | Reference |
|---|---|---|
| Tomato | CAT, SOD and GR activity increased | [177] |
| Tomato | Increment in CAT and SOD activity while reduction in POD activity | [178] |
| Wheat | CAT, SOD and GR activity increased | [179] |
| Sunflower | APX and MDA activity reduction | [180] |
| Wheat | Increment in ascorbate contents | [181] |
Abbreviations: CAT: Catalase, SOD; Superoxidase dismutase, GR: Glutathione reductase, POD: Peroxidase, APX: Ascorbate peroxidase, MDA: Malondialdehyde.
6.5. Selenium As An Antioxidative Protectant
The plants exposed to water stress deficit produce ROS that can cause and oxidative damage to the biomolecules such as carbohydrates, proteins, lipids and nucleic acids; and therefore, reducing the photosynthesis, respiration and growth of plants [182]. Selenium (Se) application can result in compatible solutes in the plants grown under water deficit; and thereby reducing the oxidative stress in plants. The cellular dehydration of the plants is reduced through the accumulation of these osmolytes [183]. Senescence is stimulated in the plants as a result of an oxidative stress protection that produces the ROS enzymes under Se application to the plants [184,185]. Protective enzymatic activities are also activated through Se application in plants [186]. The application of Se enhances the production and synthesis of proline and peroxidase through antioxidant effect. Its application in plants can decrease the membrane degradability and enhance ROS enzymes activity [184,185,187]. Moreover, Se application can enhance plant growth, reduce oxidative stress damage, increases oxidative stress under light stress, antioxidants production due to senescence and regulating water balance of the plants to tolerate drought stress [188].
6.6. Potassium: A Vital Regulator
Potassium (K) application under drought stress condition ameliorates the adverse effect (Table 6) of the water deficit and maintains the plant productivity. Under drought stress condition, the plants uptake more potassium for their internal regulatory mechanism [189]. The increase of K by plants cause an oxidative damage, and therefore can form ROS during the photosynthesis process [190]. Thus, the reason of the high K demand by plants grown under stress is to maintain the CO2 fixation during photosynthesis process. Under plant stress, the increment of ROS in plants can be due to CO2 reduction [191]. The photosynthesis process was impaired, and carbohydrate metabolism was also affected through ROS production when plants were grown under water deficit conditions [192]. The low photosynthesis rate was observed in plants grown under drought stress with the lower dose of K application than higher dose of K [193]. Therefore, adequate K is needed for plants to maintain their physiological processes. It is also observed that the low grain yield of crops grown under water deficit condition could be enhanced through K application. The application of K as soil amendment or as foliar application is beneficial for the optimal physiological processes of plants [194,195]. Consequently, K application is of great importance for getting optimal yield production of crops grown under rained and/or water deficit environments [196].
Table 6.
Potassium application mitigates the adverse effects in plants subjected to water deficit stress.
| Plant Species | Water Stress Level and Time | Potassium Rate | Advantages | References |
|---|---|---|---|---|
| Wheat | PEG @ 15% | 10 mM K2O | Proline contents, chlorophyll a, b and carotenoids increased | [197] |
| Sunflower | Withholding irrigation at grain filling | 100 kg ha−1 | shoot dry matter and biomass increased | [198] |
| Rice | 30 DAP for 10 days | 120 kg ha−1 | shoot dry matter increased and osmolyte synthesis enhanced | [199] |
| Maize | 65% of FC water holding | 0.42 g kg−1 of soil | K+, glycine betaine and osmotic nitrides accumulation increased | [200] |
| Barley | 50% of soil moisture | 10 mM K2CO3 | Reduction I soluble carbohydrate and enhanced K in plants | [201] |
6.7. Plant Microbes Crosstalk
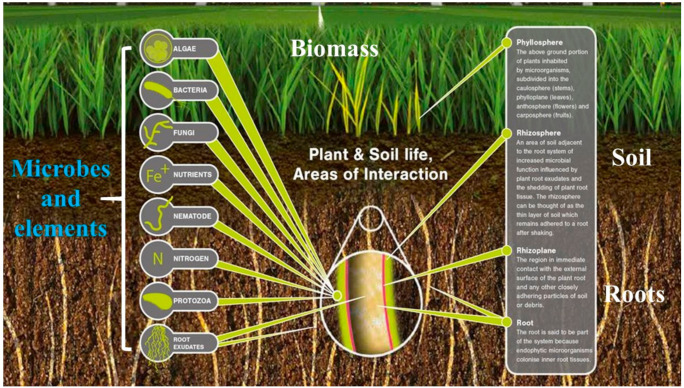
The microorganisms also play a vital role in reducing the adverse effects of drought stress (Table 7) and thereby improving plant productivity [202]. The oxidative damage in the plants grown under different environmental stresses can be reduced through the microorganisms (Figure 4) and enabling the cereals to cope with drought conditions. Among them, plant growth-promoting rhizobacteria (PGPR) is responsible for drought stress effect mitigation in dry environments [203]. The PGPR inoculation into the plants can increase the drought tolerance of those crops [204], because these PGPR make colonies in the root-zones and enhance the plant growth under different circumstances [205]. They also can solubilize various micronutrients to make them available for the plant uptake [202,206]. PGPR also enhances the plant resistance to different abiotic stresses [207]. The Bacillus species assembles solutes that enable maize plants to cope with drought and prevent degeneration [208]. In rice plants, the biotic and abiotic stresses were mitigated through phyllosphere bacteria inoculation [209]. The inoculation of Bacillus amyloliquefaciens, Azospirillum brasilense, Rhizobium leguminosarum, Mesorhizobium cicero bacterials strains improved homeostasis in plants and increased growth, biomass and drought tolerance index [210]. Similarly, Trichoderma sp. was reported to be a beneficial for drought stress [211], particularly Trichoderma harizianum was noted to be a beneficial application for rice drought tolerance [212].
Table 7.
Effect of microbes on plant adaptive mechanism for mitigation of drought stress.
| Specie/Plant Name | Microbes | Activity | Ref |
|---|---|---|---|
| Maize | Azospirillum lipoferum | Increase accumulation of soluble sugar, free amino acids and proline. Affect the growth of root length, shoot fresh weight, shoot dry weight, root fresh weight and root dry | [214] |
| Bacillus spp. | Increased accumulation of proline, sugars, free amino acids and decrease electrolyte leakage. It also reduce the activity of antioxidants enzyme (catalase, glutathione peroxidase) | [215] | |
| Helianthus annuus | Pseudomonas putida strain GAP-P45 | Epoxy polysaccharide production | [216] |
| Capsicum annum | Bacillus licheformis strain K11 | Stress related genes and proteins | [217] |
| Rice | Trichoderma harzianum | promote root growth independent of water status and delay drought response | [218] |
| Phaseolus vulgaris |
Rhizobium tropici and Paenibacillus polymyxa |
Upregulation of genes involved in stress tolerance | [219] |
| Medicago truncatula | Sinorhizobium medicae | Root nodulation and nutrient acquisition of nutrient during drought stress | [220] |
| Wheat | Bacillus amyloliquefaciens 5113 | Bacterial mediated plant attenuated transcript level and improves homeostasis | [221] |
| Azospirillumbrasilense NO40 | |||
| Brassica oxyrrhina |
Pseudomonas libanensis TR1 and Pseudomonas reactans Ph3R3 |
Increased plant growth, leaf relative water and pigment content and decreased concentrations of proline and malondialdehyde in leaves | [222] |
| Cicer arietinum L. | Pseudomonas putida MTCC5279 (RA) | Osmolyte accumulation, ROS scavenging ability and stress-responsive gene expressions | [223] |
| Lettuce | Azospirillum sp. | Promoted aerial biomass, chlorophyll and ascorbic acid content, as well as enhanced overall visual quality, hue, chroma and antioxidant capacity, and reduced the browning intensity | [224] |
| Arabidopsis | Piriformospora indica | Drought tolerance | [225] |
| Soybean | Pseudomonas putida H-2–3 | Reduce the level of abscisic acid and salicylic acid and increase level of jasmonic acid content. Modulated antioxidants by declining superoxide dismutase, flavonoids and radical scavenging activity | [226] |
| Wheat | Azospirillum brasilense NO40 | Catalase, exopolysaccharides and IAA produced by the Rhizobia improved the growth, biomass and drought tolerance index | [227] |
| Mesorhizobium ciceri (CR-30 and CR39), and Rhizobium phaseoli (MR-2) |
Figure 4.
Schematic representation of the interaction among microbes, soil and plant, modified from Andreote et al. [213].
6.8. Hydrogel: A Water Absorbing Polymer
Hydrogel is a polymer, and its application to the soil in agriculture systems can reduce the need for frequent irrigation [228]. Plants can survive and sustain their life cycle through hydrogel conditioning in arid and semi-arid environments, where the shortage of water is a serious issue [229]. The water limitation is not covered with the rainfall occurrence, and hence there is a demand to protect the available soil moisture from damage and loss to overcome soil degradation [230]. Due to hydrogel soil amendment, soil physical, chemical and biological traits are enhanced with positive effects on the plant growth and development [231]. Through its application to soil, it increases the plant survival time under drought stress, which was decreased due to the loss of the water and the hydraulic conductance in soil [232]. The survival time of the plants was increased with the hydrogel application since it resulted in sufficient soil moisture. Therefore, its application into the soil, particularly in arid and semi-arid environments and drought-affected areas, is beneficial for water saving in rhizosphere [233]. Apart from this, the hydraulic conductivity of the polymer amended soil is less than the plain soil. Similarly, water loss through evaporation in polymer-amended soil was lower than the soil with no hydrogel amendment [234].
6.9. Nanoparticles; Coping Drought Stress
Nanoparticles (NPs) are characterized by its particle shape, tunable pore size, potential reactivity and high surface area [235]. In plants, the cellular organelles are targeted, and certain contents are released through the nanoparticle target [235,236]. The activity of antioxidants enzymes i.e., SOD, CAT and POD were regulated and enhanced (Table 8) by the application of nanoparticles [237]. For example, the activity of SOD in plants was increased by the application of TIO2 NPs [238]. In agriculture, different trace elements and their oxides of NPs were used for enhancing drought stress resistance in different plants (Table 8). The negative effects of abiotic stress such as drought, chilling stress, salinity and heavy metal toxicity were mitigated through silicon nanoparticles (Si-NPs) application [235,239]. Growth and physio- and biochemical traits such as proline, chlorophyll, carbohydrates, carotenoids and relative water contents were significantly improved in different plant species when NPs were applied such as silica and ZnO nanoparticles [235]. Si-NPs also enhanced the drought resistance in wheat plants [240,241]. Similarly, the salinity and drought stress in plants were also mitigated by ZnO nanoparticles application (235). During the early stage of growth, the application of ZnO NPs stimulated the seed reservoirs for sapling and enhanced the drought resistance in plants [242]. Ferrous in combination with Zn were also reported to have a beneficial effect on plant resistance to drought stress. Plants grown under drought stress were mitigated through TIO2 nanoparticles, consequently activated different compounds and ameliorated the adverse effects of water deficit [243,244]. To improve drought stress in plants, other NPs such as silver (Ag) and copper (Cu) were used in lentil for mitigated drought stress negative effects. Nano-silica could also enhance the drought tolerance in different plants [235]. The increase of SOD and POD activity in wheat crop as drought resistance mechanism was observed through ZnO NPs. The drought resistance in wheat was also enhanced under Zn and Cu NPs [241,245].
Table 8.
Drought stress tolerance enhancement in plants through Nanoparticles application.
| Nanoparticles | Mechanism | References |
|---|---|---|
| Iron | Drought stress impacts on safflower yield components and oil percentage were mitigated through foliar spray of iron nanoparticles (Fe-NPs) | [246] |
| Silica | Si-NPs enhanced drought tolerance in plants | [247] |
| Titanium | Seed gluten and starch contents of wheat were improved through foliar application of titanium | [248] |
| Thiol-gated mesoporous silica | The encapsulated ABA release was controlled that enhances AtGALK2 gene thereby improved drought resistance in Arabidopsis seedlings | [249] |
| Zinc oxide | Germination rate and percentage of soybean were improved by the application of ZnO NPs | [250] |
| Zinc and copper | MDA accumulation was decreased with the increment in antioxidative enzymes and RWC under water deficit in the presence of Zn and Cu NPs applications | [251] |
6.10. Metabolic Engineering and Stress Tolerance Strategy
One of the most optimal solutions for coping drought stress is the drought tolerant crops development [252]. Thus, a great challenge is to enhance the drought tolerance without a significant effect on grain yield. The drought tolerance induction in plants through metabolic engineering, thereby enhancing stress related metabolites, is considered as an optimal strategy [253]. In arid and semi-arid regions, the successful breeding for drought tolerance through raffinose biosynthesis engineering pathway is one of the classic strategies. The accumulation of raffinose and galactinol in plants grown under water deficit is stimulated through galactinol synthase (AtGolS) gene with specific gene AtGolS2 that is stimulated under drought stress in particular [254]. The expression of this gene in plants enhances the raffinose and galactinol level, thereby enhancing drought tolerance in plants as well as protecting them from an oxidative stress. Both the raffinose and galactinol exhibits the potential to protect cell under environmental stresses through ROS scavenger and compatible solutes [254]. In this respect, the increment in raffinose and galactinol levels under metabolome analysis of rice and soybean indicated their response to drought stress. Crop plant transformation through AtGolS2 application activates the plants’ resistance to stress under dry environments. Different studies suggest that the application of AtGolS2 in transgenic plants not only increase drought tolerance but improves also grain yield [255]. Thus, AtGolS2 metabolic engineering is considered a useful approach and a significant tool to increase grain yield under water deficit conditions [256].
7. Conclusions
Under recent climatic changes, both the biotic and abiotic stresses are a serious threat for global food security and plant production sustainability. Among the abiotic stresses, drought stress is gaining attention due to its adverse effect on plant growth and development and significant reduction in plant yield and biomass causing global food insecurity. Drought stress affects plants through the life cycle i.e., from germination till maturity. Certain physiological, metabolic and biochemical processes are affected by drought stress that hampers plant productivity. To tackle the adverse effect of the drought stress on plants, certain mechanisms are adopted by the plants which enhance drought tolerance. Thus, there is need to explore the untapped adaptation characters in different plants and their incorporation to the genotypes that may tolerate the adverse effect of drought stress in order not to affect its productivity. Breeding technologies has greater potential for increasing plant performance and production under water deficit. Certain approaches are receiving greater attention for coping drought in arid and semi-arid environments.
Growth pattern and structural dynamics, reduction in transpiration loss through stomatal conductance altering and distribution, leaf rolling, root to shoot ratio dynamics, root length increment, accumulation of compatible solutes, enhancement in transpiration efficiency, osmotic and hormonal regulation and delayed senescence are the strategies that can be adopted by plants grown under water deficit.
To improve drought stress tolerance in plants, certain breeding strategies, molecular and genomics perspectives with special emphasis on the omics technology alteration i.e., metabolomics, proteomics, genomics, transcriptomics, glyomics and phenomics approaches are of great value. Other practices that include seed priming, growth hormones, osmoprotectants, silicon (Si), selenium (Se) and potassium application are worth using in scant water conditions in plants. Despites this, the beneficial effect of microbes, hydrogel, nanoparticles applications and metabolic engineering techniques also regulates the antioxidant enzymes activity for adaptation to drought stress in plants, enhancing plant tolerance through maintenance in cell homeostasis and ameliorates the adverse effects of water stress in plants. These innovative strategies provide better understanding of and potentially increase plant productivity in dry environments in order to reduce the threat to global food security.
Acknowledgments
The Deanship of Scientific Research at King Saud University through the research group number RG-1441-323 is acknowledged.
Author Contributions
Conceptualization: M.F.S. and M.A. (Mohammad Akmal) Data curation: M.F.S., N.A., M.A. (Mohammad Akmal), N.A-S., M.A. (Majed Alotaibi), Y.R., T.D. and M.L.B. Investigation: M.F.S., N.A., M.A. (Mohammad Akmal), N.A-S., M.A. (Majed Alotaibi), Y.R., T.D., H.H.A-W. and M.L.B. Resources: M.F.S., N.A., M.A. (Mohammad Akmal), N.A-S., M.A. (Majed Alotaibi), Y.R. and M.L.B. Software: M.F.S., N.A., M.A. (Majed Alotaibi). and M.L.B. Writing—original draft: M.F.S. and N.A. Writing—review and editing: M.F.S., N.A-S., N.A., M.A. (Mohammad Akmal), M.A. (Majed Alotaibi), Y.R., T.D. and M.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
The Deanship of Scientific Research at King Saud University through the research group number RG-1441-323 funded this work.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brodersen C.R., Roddy A.B., Wason J.W., McElrone A.J. Functional status of xylem through time. Annu. Rev. Plant Biol. 2019;70:407–433. doi: 10.1146/annurev-arplant-050718-100455. [DOI] [PubMed] [Google Scholar]
- 2.Abbasi T., Abbasi S.A. Biomass energy and the environmental impacts associated with its production and utilization. Renew. Sustain. Energy Rev. 2010;14:919–937. doi: 10.1016/j.rser.2009.11.006. [DOI] [Google Scholar]
- 3.Anjum S.A., Xie X.Y., Wang L.C., Saleem M.F., Man C., Lei W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011;6:2026–2032. [Google Scholar]
- 4.Diatta A.A., Fike J.H., Battaglia M.L., Galbraith J., Baig M.B. Effects of biochar on soil fertility and crop productivity in arid regions: A review. Arab. J. Geosci. 2020;13:595. doi: 10.1007/s12517-020-05586-2. [DOI] [Google Scholar]
- 5.Okorie V.O., Mphambukeli T.N., Amusan S.O. Exploring the political economy of water and food security nexus in BRICS. Afr. Insight. 2019;48:21–38. [Google Scholar]
- 6.O’Connell E. Towards adaptation of water resource Systems to climatic and socio-economic Chang. Water Resour. Manag. 2017;31:2965–2984. doi: 10.1007/s11269-017-1734-2. [DOI] [Google Scholar]
- 7.Passioura J.B., Angus J.F. Advances in Agronomy. Volume 106. Academic Press; Cambridge, MA, USA: 2010. Improving productivity of crops in water-limited environments; pp. 37–75. [Google Scholar]
- 8.Devincentis A.J. Ph.D. Thesis. University of California; Davis, CA, USA: 2020. Scales of Sustainable Agricultural Water Management. [Google Scholar]
- 9.Daryanto S., Wang L., Jacinthe P.A. Global synthesis of drought effects on cereal, legume, tuber and root crops production: A review. Agric. Water Manag. 2020;179:18–33. doi: 10.1016/j.agwat.2016.04.022. [DOI] [Google Scholar]
- 10.Salehi-Lisar S.Y., Bakhshayeshan-Agdam H. Agronomic Crops. Springer; Berlin/Heidelberg, Germany: 2020. Agronomic Crop Responses and Tolerance to Drought Stress; pp. 63–91. [Google Scholar]
- 11.Ortiz N., Armada E., Duque E., Roldán A., Azcón R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: Effectiveness of autochthonous or allochthonous strains. J. Plant Physiol. 2015;174:87–96. doi: 10.1016/j.jplph.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Battaglia M.L., Lee C., Thomason W. Corn yield components and yield responses to defoliation at different row widths. Agron. J. 2018;110:1–16. doi: 10.2134/agronj2017.06.0322. [DOI] [Google Scholar]
- 13.Gray S.B., Brady S.M. Plant developmental responses to climate Chang. Dev. Biol. 2016;419:64–77. doi: 10.1016/j.ydbio.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Osakabe Y., Osakabe K., Shinozaki K., Tran L.S.P. Response of plants to water stress. Front. Plant Sci. 2014;5:86. doi: 10.3389/fpls.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bielach A., Hrtyan M., Tognetti V.B. Plants under stress: Involvement of auxin and cytokinin. Int. J. Mol. Sci. 2017;18:1427. doi: 10.3390/ijms18071427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zandalinas S.I., Fritschi F.B., Mittler R. Signal transduction networks during stress combination. J. Exp. Bot. 2020;71:1734–1741. doi: 10.1093/jxb/erz486. [DOI] [PubMed] [Google Scholar]
- 17.Kaur G., Asthir B. Molecular responses to drought stress in plants. Biol. Plant. 2017;61:201–209. doi: 10.1007/s10535-016-0700-9. [DOI] [Google Scholar]
- 18.Prakash V., Singh V.P., Tripathi D.K., Sharma S., Corpas F.J. Crosstalk between nitric oxide (NO) and abscisic acid (ABA) signalling molecules in higher plants. Environ. Exp. Bot. 2019;161:41–49. doi: 10.1016/j.envexpbot.2018.10.033. [DOI] [Google Scholar]
- 19.Kumar M., Kesawat M.S., Ali A., Lee S.C., Gill S.S., Kim H.U. Integration of abscisic acid signaling with other signaling pathways in plant stress responses and development. Plants. 2019;8:592. doi: 10.3390/plants8120592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav S., Modi P., Dave A., Vijapura A., Patel D., Patel M. Effect of Abiotic Stress on Crops. In: Hasanuzzaman M., Filho M., Fujita M., Nogueira T., editors. Sustainable Crop Production. IntechOpen; Rijeka, Croatia: 2020. [DOI] [Google Scholar]
- 21.Shahid M.J., Ali S., Shabir G., Siddique M., Rizwan M., Seleiman M.F., Afzal M. Comparing the performance of four macrophytes in bacterial assisted floating treatment wetlands for the removal of trace metals (Fe, Mn, Ni, Pb, and Cr) from polluted river water. Chemosphere. 2020;243:125353. doi: 10.1016/j.chemosphere.2019.125353. [DOI] [PubMed] [Google Scholar]
- 22.Raza A., Razzaq A., Mehmood S.S., Zou X., Zhang X., Lv Y., Xu J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants. 2019;8:34. doi: 10.3390/plants8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diatta A.A., Thomason W.E., Abaye O., Thompson T.L., Battaglia M.L., Vaughan L.J., Lo M., Leme J.F.D.C. Assessment of nitrogen fixation by mungbean genotypes in different soil textures using 15N natural abundance method. J. Soil Sci. Plant Nutr. 2020;20:2230–2240. doi: 10.1007/s42729-020-00290-2. [DOI] [Google Scholar]
- 24.Yang H., Huntingford C., Wiltshire A., Sitch S., Mercado L. Compensatory climate effects link trends in global runoff to rising atmospheric CO2 concentration. Environ. Res. Lett. 2019;14:124075. doi: 10.1088/1748-9326/ab5c6f. [DOI] [Google Scholar]
- 25.Yin J., Gentine P., Zhou S., Sullivan S.C., Wang R., Zhang Y., Guo S. Large increase in global storm runoff extremes driven by climate and anthropogenic changes. Nat. Commun. 2018;9:1–10. doi: 10.1038/s41467-018-06765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen I., Zandalinas S.I., Huck C., Fritschi F.B., Mittler R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2021;171:66–76. doi: 10.1111/ppl.13203. [DOI] [PubMed] [Google Scholar]
- 27.Mostofa M.G., Ghosh A., Li Z.G., Siddiqui M.N., Fujita M., Tran L.S.P. Methylglyoxal–a signaling molecule in plant abiotic stress responses. Free Radic. Biol. Med. 2018;122:96–109. doi: 10.1016/j.freeradbiomed.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Adnan M., Fahad S., Zamin M., Shah S., Mian I.A., Danish S., Zafar-ul-Hye M., Battaglia M.L., Naz R.M.M., Saeed B., et al. Coupling Phosphate-Solubilizing Bacteria with Phosphorus Supplements Improve Maize Phosphorus Acquisition and Growth under Lime Induced Salinity Stress. Plants. 2020;9:900. doi: 10.3390/plants9070900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tariq M., Khan F., Shah A.H., Fahad S., Wahid F., Ali J., Adnan M., Ahmad M., Irfan M., Zafar-ul-Hye M., et al. Effect of micronutrients foliar supplementation on the production and eminence of plum (Prunusdomestica L.) Qual. Assur. Saf. Crop. 2020;12:32–40. [Google Scholar]
- 30.Brown S., Nicholls R.J., Lázár A.N., Hornby D.D., Hill C., Hazra S., Addo K.A., Haque A., Caesar J., Tompkins E.L. What are the implications of sea-level rise for a 1.5, 2 and 3 °C rise in global mean temperatures in the Ganges-Brahmaputra-Meghna and other vulnerable deltas? Regul. Environ. Chang. 2018;18:1829–1842. doi: 10.1007/s10113-018-1311-0. [DOI] [Google Scholar]
- 31.Cook B.I., Smerdon J.E., Seager R., Coats S. Global warming and 21st century drying. Clim. Dyn. 2014;43:2607–2627. doi: 10.1007/s00382-014-2075-y. [DOI] [Google Scholar]
- 32.Warner K., Afifi T. Where the rain falls: Evidence from 8 countries on how vulnerable households use migration to manage the risk of rainfall variability and food insecurity. Clim. Dev. 2014;6:1–17. doi: 10.1080/17565529.2013.835707. [DOI] [Google Scholar]
- 33.Sultan B., Defrance D., Iizumi T. Evidence of crop production losses in West Africa due to historical global warming in two crop models. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-49167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray D.K., West P.C., Clark M., Gerber J.S., Prishchepov A.V., Chatterjee S. Climate change has likely already affected global food production. PLoS ONE. 2019;14:217148. doi: 10.1371/journal.pone.0217148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konapala G., Mishra A.K., Wada Y., Mann M.E. Climate change will affect global water availability through compounding changes in seasonal precipitation and evaporation. Nat. Commun. 2020;11:1–10. doi: 10.1038/s41467-020-16757-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fatima A., Farid M., Safdar K., Fayyaz A., Ali S.M., Adnan S., Nawaz M., Munir H., Raza N., Zubair M. Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives. Springer; Berlin/Heidelberg, Germany: 2020. Loss of Agro-Biodiversity and Productivity Due to Climate Change in Continent Asia: A Review; pp. 51–71. [Google Scholar]
- 37.Leal W. Climate Change Adaptation in Africa and Asia: Challenges Ahead and Action Needed. United Nations, PIK Report 27.
- 38.Karandish F., Šimůnek J. Two-dimensional modeling of nitrogen and water dynamics for various N-managed water-saving irrigation strategies using HYDRUS. Agric. Water Manag. 2016;193:174–190. doi: 10.1016/j.agwat.2017.07.023. [DOI] [Google Scholar]
- 39.Ali N., Anjum M.M. Drought Stress: Major Cause of Low Yield and Productivity. Austin Environ. Sci. 2016;1:10–12. [Google Scholar]
- 40.Yu W., Yang Y.C., Savitsky A., Alford D., Brown C., Wescoat J., Debowicz D., Robinson S. The Indus Basin of Pakistan: The Impacts of Climate Risks on Water and Agriculture. The World Bank; Washington, DC, USA: 2013. [Google Scholar]
- 41.Reddy P.P. Climate Resilient Agriculture Ensuring Food Security. Springer; Berlin/Heidelberg, Germany: 2015. Impacts of climate change on agriculture; pp. 43–90. [Google Scholar]
- 42.Guo H.D., Zhang L., Zhu L.W. Earth observation big data for climate change research. Adv. Clim. Chang. Res. 2015;6:108–117. doi: 10.1016/j.accre.2015.09.007. [DOI] [Google Scholar]
- 43.Aryal J.P., Sapkota T.B., Khurana R., Khatri-Chhetri A., Jat M.L. Climate change and agriculture in South Asia: Adaptation options in smallholder production systems. Environ. Dev. Sustain. 2020;22:5045–5075. doi: 10.1007/s10668-019-00414-4. [DOI] [Google Scholar]
- 44.Bukhari S.A.H., Peerzada A.M., Javed M.H., Dawood M., Hussain N., Ahmad S. Agronomic Crops. Springer; Berlin/Heidelberg, Germany: 2019. Growth and Development Dynamics in Agronomic Crops Under Environmental Stress; pp. 83–114. [Google Scholar]
- 45.Battaglia M., Lee C., Thomason W., Fike J., Sadeghpour A. Hail damage impacts on corn productivity: A review. Crop Sci. 2019;59:1–14. doi: 10.2135/cropsci2018.04.0285. [DOI] [Google Scholar]
- 46.Ahanger M.A., Tomar N.S., Tittal M., Argal S., Agarwal R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plant. 2017;23:731–744. doi: 10.1007/s12298-017-0462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sobhanian H., Pahlavan S., Meyfour A. How does proteomics target plant environmental stresses in a semi-arid area? Mol. Biol. Rep. 2020;47:3181–3194. doi: 10.1007/s11033-020-05406-6. [DOI] [PubMed] [Google Scholar]
- 48.Seleiman M.F., Kheir A.M. Saline soil properties, quality and productivity of wheat grown with bagasse ash and thiourea in different climatic zones. Chemosphere. 2018;193:538–546. doi: 10.1016/j.chemosphere.2017.11.053. [DOI] [PubMed] [Google Scholar]
- 49.Elemike E.E., Uzoh I.M., Onwudiwe D.C., Babalola O.O. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019;9:499. doi: 10.3390/app9030499. [DOI] [Google Scholar]
- 50.Bal S.K., Minhas P.S. Abiotic Stress Management for Resilient Agriculture. Springer; Berlin/Heidelberg, Germany: 2017. Atmospheric stressors: Challenges and coping strategies; pp. 9–50. [Google Scholar]
- 51.Hafez E.H., Seleiman M.F. Response of barley quality traits, yield and antioxidant enzymes to water-stress and chemical inducers. Intern. J. Plant Prod. 2017;11:477–490. [Google Scholar]
- 52.Ristvey A.G., Belayneh B.E., Lea-Cox J.D. A Comparison of irrigation-water containment methods and management strategies between two ornamental production systems to minimize water security threats. Water. 2019;11:2558. doi: 10.3390/w11122558. [DOI] [Google Scholar]
- 53.Goche T., Shargie N.G., Cummins I., Brown A.P., Chivasa S., Ngara R. Comparative physiological and root proteome analyses of two sorghum varieties responding to water limitation. Sci. Rep. 2020;10:1–18. doi: 10.1038/s41598-020-68735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martínez-Vilalta J., Garcia-Forner N. Water potential regulation, stomatal behaviour and hydraulic transport under drought: Deconstructing the iso/anisohydric concept. Plant Cell Env. 2017;40:962–976. doi: 10.1111/pce.12846. [DOI] [PubMed] [Google Scholar]
- 55.Corso D., Delzon S., Lamarque L.J., Cochard H., Torres-Ruiz J.M., King A., Brodribb T. Neither xylem collapse, cavitation, or changing leaf conductance drive stomatal closure in wheat. Plant Cell Env. 2020;43:854–865. doi: 10.1111/pce.13722. [DOI] [PubMed] [Google Scholar]
- 56.Hafez E.H., Abou El Hassan W.H., Gaafar I.A., Seleiman M.F. Effect of gypsum application and irrigation intervals on clay saline-sodic soil characterization, rice water use efficiency, growth, and yield. J. Agric. Sci. 2015;7:208–219. doi: 10.5539/jas.v7n12p208. [DOI] [Google Scholar]
- 57.Battaglia M.L., Lee C., Thomason W., Van Mullekom J. Effects of corn row width and defoliation timing and intensity on canopy light interception. Crop Sci. 2020;59:1718–1731. doi: 10.2135/cropsci2018.05.0337. [DOI] [Google Scholar]
- 58.Thomason W.E., Battaglia M.L. Early defoliation effects on corn plant stands and grain yield. Agron. J. 2020;112:1–9. doi: 10.1002/agj2.20402. [DOI] [Google Scholar]
- 59.Humplík J.F., Bergougnoux V., Van Volkenburgh E. To stimulate or inhibit? That is the question for the function of abscisic acid. Trends Plant Sci. 2017;22:830–841. doi: 10.1016/j.tplants.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 60.Seleiman M.F., Refay Y., Al-Suhaibani N., Al-Ashkar I., El-Hendawy S., Hafez E.M. Integrative effects of rice-straw biochar and silicon on oil and seed quality, yield and physiological traits of Helianthus annuus L. grown under water deficit stress. Agronomy. 2019;9:637. doi: 10.3390/agronomy9100637. [DOI] [Google Scholar]
- 61.Birami B., Gattmann M., Heyer A.G., Grote R., Arneth A., Ruehr N.K. Heat waves alter carbon allocation and increase mortality of Aleppo pine under dry conditions. Front. For. Glob. Chang. Front. 2018;1:8. doi: 10.3389/ffgc.2018.00008. [DOI] [Google Scholar]
- 62.Miranda M.T., Da Silva S.F., Silveira N.M., Pereira L., Machado E.C., Ribeiro R.V. Root Osmotic Adjustment and Stomatal Control of Leaf Gas Exchange are Dependent on Citrus Rootstocks Under Water Deficit. J. Plant Growth Regul. 2020;285:1–9. doi: 10.1007/s00344-020-10069-5. [DOI] [Google Scholar]
- 63.Ullah A., Sun H., Yang X., Zhang X. Drought coping strategies in cotton: Increased crop per drop. Plant Biotechnol. J. 2017;15:271–284. doi: 10.1111/pbi.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zoghi Z., Hosseini S.M., Kouchaksaraei M.T., Kooch Y., Guidi L. The effect of biochar amendment on the growth, morphology and physiology of Quercuscastaneifolia seedlings under water-deficit stress. Eur. J. For. Res. 2019;138:967–979. doi: 10.1007/s10342-019-01217-y. [DOI] [Google Scholar]
- 65.Ruehr N.K., Grote R., Mayr S., Arneth A. Beyond the extreme: Recovery of carbon and water relations in woody plants following heat and drought stress. Tree Physiol. 2019;39:1285–1299. doi: 10.1093/treephys/tpz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan A., Pan X., Najeeb U., Tan D.K., Fahad S., Zahoor R., Luo H. Coping with drought: Stress and adaptive mechanisms, and management through cultural and molecular alternatives in cotton as vital constituents for plant stress resilience and fitness. Biol. Res. 2018;51:47. doi: 10.1186/s40659-018-0198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toscano S., Ferrante A., Romano D. Response of Mediterranean ornamental plants to drought stress. Horticulturae. 2019;5:6. doi: 10.3390/horticulturae5010006. [DOI] [Google Scholar]
- 68.Zargar S.M., Gupta N., Nazir M., Mahajan R., Malik F.A., Sofi N.R., Shikari A.B., Salgotra R.K. Impact of drought on photosynthesis: Molecular perspective. Plant Gene. 2017;11:154–159. doi: 10.1016/j.plgene.2017.04.003. [DOI] [Google Scholar]
- 69.Batool T., Ali S., Seleiman M.F., Naveed N.H., Ali A., Ahmend K., Abid M., Rizwan M., Shahid M.R., Alotaibi M., et al. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 2020;10:16975. doi: 10.1038/s41598-020-73489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galindo A., Collado-González J., Griñán I., Corell M., Centeno A., Martín-Palomo M.J., Girón I.F., Rodríguez P., Cruz Z.N., Memmi H., et al. Deficit irrigation and emerging fruit crops as a strategy to save water in Mediterranean semiarid agrosystems. Agric. Water Manag. 2020;202:311–324. doi: 10.1016/j.agwat.2017.08.015. [DOI] [Google Scholar]
- 71.Álvarez S., Rodríguez P., Broetto F., Sánchez-Blanco M.J. Long term responses and adaptive strategies of Pistacialentiscus under moderate and severe deficit irrigation and salinity: Osmotic and elastic adjustment, growth, ion uptake and photosynthetic activity. Agric. Water Manag. 2018;202:253–262. doi: 10.1016/j.agwat.2018.01.006. [DOI] [Google Scholar]
- 72.Tekle A.T., Alemu M.A. Drought tolerance mechanisms in field crops. World J. Biol. Med. Sci. 2016;3:15–39. [Google Scholar]
- 73.Blum A. Springer; Berlin/Heidelberg, Germany: 2011. Plant water relations, plant stress and plant production. In Plant Breeding for Water-Limited Environments; pp. 11–52. [Google Scholar]
- 74.Dobra J., Motyka V., Dobrev P., Malbeck J., Prasil I.T., Haisel D., Gaudinova A., Havlova M., Gubis J., Vankova R. Comparison of hormonal responses to heat, drought and combined stress in tobacco plants with elevated proline content. J. Plant Physiol. 2010;167:1360–1370. doi: 10.1016/j.jplph.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 75.Boulard T., Roy J.C., Pouillard J.B., Fatnassi H., Grisey A. Modelling of micrometeorology, canopy transpiration and photosynthesis in a closed greenhouse using computational fluid dynamics. Biosyst. Eng. 2017;158:110–133. doi: 10.1016/j.biosystemseng.2017.04.001. [DOI] [Google Scholar]
- 76.Wasaya A., Zhang X., Fang Q., Yan Z. Root phenotyping for drought tolerance: A review. Agronomy. 2018;8:241. doi: 10.3390/agronomy8110241. [DOI] [Google Scholar]
- 77.Zhang F., Wang P., Zou Y.N., Wu Q.S., Kuča K. Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress. Arch. Agron. Soil Sci. 2019;65:1316–1330. doi: 10.1080/03650340.2018.1563780. [DOI] [Google Scholar]
- 78.Tiwari P., Srivastava D., Chauhan A.S., Indoliya Y., Singh P.K., Tiwari S., Fatima T., Mishra S.K., Dwivedi S., Agarwal L., et al. Root system architecture, physiological analysis and dynamic transcriptomics unravel the drought-responsive traits in rice genotypes. Ecotoxicol. Environ. Safety. 2020;207:111252. doi: 10.1016/j.ecoenv.2020.111252. [DOI] [PubMed] [Google Scholar]
- 79.Tzortzakis N., Chrysargyris A., Aziz A. Adaptive response of a native mediterranean grapevine cultivar upon short-term exposure to drought and heat stress in the context of climate Chang. Agronomy. 2020;10:249. doi: 10.3390/agronomy10020249. [DOI] [Google Scholar]
- 80.López-Galiano M.J., García-Robles I., González-Hernández A.I., Camañes G., Vicedo B., Real M.D., Rausell C. Expression of miR159 is altered in tomato plants undergoing drought stress. Plants. 2019;8:201. doi: 10.3390/plants8070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parry M.A.J., Flexas J., Medrano H. Prospects for crop production under drought: Research priorities and future directions. Ann. Appl. Biol. 2005;147:211–226. doi: 10.1111/j.1744-7348.2005.00032.x. [DOI] [Google Scholar]
- 82.Adeyemi O., Keshavarz-Afshar R., Jahanzad E., Battaglia M.L., Luo Y., Sadeghpour A. Effect of wheat cover crop and split nitrogen application on corn yield and nitrogen use efficiency. Agronomy. 2020;10:1081. doi: 10.3390/agronomy10081081. [DOI] [Google Scholar]
- 83.Oliveira I.C.M., Guilhen J.H.S., de Oliveira Ribeiro P.C., Gezan S.A., Schaffert R.E., Simeone M.L.F., Pastina M.M. Genotype-by-environment interaction and yield stability analysis of biomass sorghum hybrids using factor analytic models and environmental covariates. Field Crop Res. 2020;257:107929. doi: 10.1016/j.fcr.2020.107929. [DOI] [Google Scholar]
- 84.Naeem M., Iqbal M., Shakeel A., Ul-Allah S., Hussain M., Rehman A., Zafar Z.U., Ashraf M. Genetic basis of ion exclusion in salinity stressed wheat: Implications in improving crop yield. Plant Growth Regul. 2020;92:479–496. doi: 10.1007/s10725-020-00659-4. [DOI] [Google Scholar]
- 85.Solis J., Gutierrez A., Mangu V., Sanchez E., Bedre R., Linscombe S., Baisakh N. Genetic mapping of quantitative trait loci for grain yield under drought in rice under controlled greenhouse conditions. Front. Chem. 2018;5:129. doi: 10.3389/fchem.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Galaitsi S.E., Russell R., Bishara A., Durant J.L., Bogle J., Huber-Lee A. Intermittent domestic water supply: A critical review and analysis of causal-consequential pathways. Water. 2016;8:274. doi: 10.3390/w8070274. [DOI] [Google Scholar]
- 87.Scopel E., Triomphe B., Affholder F., Da Silva F.A.M., Corbeels M., Xavier J.H.V., Lahmar R., Recous S., Bernoux M., Blanchart E., et al. Conservation agriculture cropping systems in temperate and tropical conditions, performances and impacts. A review. Agron. Sustain. Dev. 2013;33:113–130. doi: 10.1007/s13593-012-0106-9. [DOI] [Google Scholar]
- 88.Aslam M., Maqbool M.A., Cengiz R. Drought Stress in Maize (Zeamays L.) Effects, Resistance Mechanisms, Global Achievements and Biological Strategies for Improvement. Springer; Berlin/Heidelberg, Germany: 2015. [Google Scholar]
- 89.Medici L.O., Reinert F., Carvalho D.F., Kozak M., Azevedo R.A. What about keeping plants well watered? Environ. Exp. Bot. 2014;99:38–42. doi: 10.1016/j.envexpbot.2013.10.019. [DOI] [Google Scholar]
- 90.Ali F., Ahsan M., Ali Q., Kanwal N. Phenotypic stability of Zea mays grain yield and its attributing traits under drought stress. Front. Plant Sci. 2017;8:1397. doi: 10.3389/fpls.2017.01397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Araujo S.S., Beebe S., Crespi M., Delbreil B., Gonzalez E.M., Gruber V., Lejeune-Henaut I., Link W., Monteros M.J., Prats E., et al. Abiotic stress responses in legumes: Strategies used to cope with environmental challenges. Crit. Rev. Plant Sci. 2015;34:237–280. doi: 10.1080/07352689.2014.898450. [DOI] [Google Scholar]
- 92.Tejero I.F.G., Moriana A., Pleguezuelo C.R.R., Zuazo V.H.D., Egea G. Water Scarcity and Sustainable Agriculture in Semiarid Environment. Academic Press; Cambridge, MA, USA: 2018. Sustainable Deficit-Irrigation Management in Almonds (Prunusdulcis L.): Different Strategies to Assess the Crop Water Status; pp. 271–298. [Google Scholar]
- 93.Bertolino L.T., Caine R.S., Gray J.E. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 2019;10:225. doi: 10.3389/fpls.2019.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shavrukov Y., Kurishbayev A., Jatayev S., Shvidchenko V., Zotova L., Koekemoer F., de Groot S., Soole K., Langridge P. Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017;8:1950. doi: 10.3389/fpls.2017.01950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Curin F., Severini A.D., González F.G., Otegui M.E. Water and radiation use efficiencies in maize: Breeding effects on single-cross Argentine hybrids released between 1980 and 2012. Field Crop Res. 2020;246:107683. doi: 10.1016/j.fcr.2019.107683. [DOI] [Google Scholar]
- 96.Vishwakarma K., Upadhyay N., Kumar N., Yadav G., Singh J., Mishra R.K., Kumar V., Verma R., Upadhyay R.G., Pandey M., et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017;8:161. doi: 10.3389/fpls.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ding Z., Ali E.F., Elmahdy A.M., Ragab K.E., Seleiman M.F., Kheir A.M.S. Modeling the combined impacts of deficit irrigation, rising temperature and compost application on wheat yield and water productivity. Agric. Water Manag. 2021;244:106626. doi: 10.1016/j.agwat.2020.106626. [DOI] [Google Scholar]
- 98.Tardieu F., Simonneau T., Muller B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Annu. Rev. Plant Biol. 2018;69:733–759. doi: 10.1146/annurev-arplant-042817-040218. [DOI] [PubMed] [Google Scholar]
- 99.Fang Y., Xiong L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015;72:673–689. doi: 10.1007/s00018-014-1767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Varshney R.K., Terauchi R., McCouch S.R. Harvesting the promising fruits of genomics: Applying genome sequencing technologies to crop breeding. PLoS Biol. 2014;10:6. doi: 10.1371/journal.pbio.1001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kollers S., Rodemann B., Ling J., Korzun V., Ebmeyer E., Argillier O., Hinze M., Plieske J., Kulosa D., Ganal M.W., et al. Whole genome association mapping of Fusarium head blight resistance in European winter wheat (Triticum aestivum L.) PLoS ONE. 2013;22:57500. doi: 10.1371/journal.pone.0057500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brown P.J., Upadyayula N., Mahone G.S., Tian F., Bradbury P.J., Myles S., Holland J.B., Flint-Garcia S., McMullen M.D., Buckler E.S., et al. Distinct Genetic Architectures for Male and Female Inflorescence Traits of Maize. PLoS Genet. 2011;7:e1002383. doi: 10.1371/journal.pgen.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang X., Wei X., Sang T., Zhao Q., Feng Q., Zhao Y., Li C., Zhu C., Lu T., Zhang Z., et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 2010;42:961–967. doi: 10.1038/ng.695. [DOI] [PubMed] [Google Scholar]
- 104.Morris G.P., Ramu P., Deshpande S.P., Hash C.T., Shah T., Upadhyaya H.D., Riera-Lizarazu O., Brown P.J., Acharya C.B., Mitchell S.E., et al. Population genomic and genome-wide association studies of agroclimatic traits in sorghum. Proc. Natl. Acad. Sci. USA. 2012;110:453–458. doi: 10.1073/pnas.1215985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hwang E.-Y., Song Q., Jia G., Specht J.E., Hyten D.L., Costa J., Cregan P.B. A genome-wide association study of seed protein and oil content in soybean. BMC Genom. 2014;15:1. doi: 10.1186/1471-2164-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bidinger F.R., Nepolean T., Hash C.T., Yadav R.S., Howarth C.J. Quantitative Trait Loci for Grain Yield in Pearl Millet under Variable Postflowering Moisture Conditions. Crop. Sci. 2007;47:969–980. doi: 10.2135/cropsci2006.07.0465. [DOI] [Google Scholar]
- 107.Nakaya A., Isobe S.N. Will genomic selection be a practical method for plant breeding? Ann. Bot. 2012;110:1303–1316. doi: 10.1093/aob/mcs109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hayes B.J., Goddard M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics. 2001;157:1819–1829. doi: 10.1093/genetics/157.4.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crossa J., Perez P.E.O., Hickey J., Burgueno J., Ornella L., Ceronrojas J.J., Zhang X., Dreisigacker S., Babu R., Li Y., et al. Genomic prediction in CIMMYT maize and wheat breeding programs. Heredity. 2014;112:48–60. doi: 10.1038/hdy.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gouy M., Rousselle Y., Bastianelli D., LeComte P., Bonnal L., Roques D., Efile J.-C., Rocher S., Daugrois J.-H., Toubi L., et al. Experimental assessment of the accuracy of genomic selection in sugarcane. Theor. Appl. Genet. 2013;126:2575–2586. doi: 10.1007/s00122-013-2156-z. [DOI] [PubMed] [Google Scholar]
- 111.Varshney R.K., Mohan S.M., Gaur P.M., GangaRao N., Pandey M.K., Bohra A., Sawargaonkar S.L., Chitikineni A., Kimurto P.K., Janila P., et al. Achievements and prospects of genomics-assisted breeding in three legume crops of the semi-arid tropics. Biotechnol. Adv. 2013;31:1120–1134. doi: 10.1016/j.biotechadv.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 112.Rutkoski J.E., Heffner E.L., Sorrells M.E. Genomic selection for durable stem rust resistance in wheat. Euphytica. 2010;179:161–173. doi: 10.1007/s10681-010-0301-1. [DOI] [Google Scholar]
- 113.Osakabe Y., Yamaguchi-Shinozaki K., Shinozaki K., Tran L.S.P. ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytologist. 2020;202:35–49. doi: 10.1111/nph.12613. [DOI] [PubMed] [Google Scholar]
- 114.Rai K.K., Rai A.C. Sustainable Agriculture in the Era of Climate Change. Springer; Berlin/Heidelberg, Germany: 2020. Recent Transgenic Approaches for Stress Tolerance in Crop Plants; pp. 533–556. [Google Scholar]
- 115.Liu Y., Liu X., Wang X., Gao K., Qi W., Ren H., Hu H., Sun D., Bai J., Zheng S. Heterologous expression of heat stress-responsive AtPLC9 confers heat tolerance in transgenic rice. BMC Plant Biol. 2020;20:1–11. doi: 10.1186/s12870-020-02709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hussain H.A., Hussain S., Khaliq A., Ashraf U., Anjum S.A., Men S., Wang L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018;9:393. doi: 10.3389/fpls.2018.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Medina S., Vicente R., Amador A., Araus J.L. Interactive effects of elevated [CO2] and water stress on physiological traits and gene expression during vegetative growth in four durum wheat genotypes. Front. Plant Sci. 2016;7:1738. doi: 10.3389/fpls.2016.01738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakashima K., Yamaguchi-Shinozaki K., Shinozaki K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014;5:170. doi: 10.3389/fpls.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bhatnagar-Mathur P., Vadez V., Sharma K.K. Transgenic approaches for abiotic stress tolerance in plants: Retrospect and prospects. Plant Cell Rep. 2008;27:411–424. doi: 10.1007/s00299-007-0474-9. [DOI] [PubMed] [Google Scholar]
- 120.Cho E.K., Hong C.B. Over-expression of tobacco NtHSP70-1 contributes to drought-stress tolerance in plants. Plant Cell Rep. 2006;25:349–358. doi: 10.1007/s00299-005-0093-2. [DOI] [PubMed] [Google Scholar]
- 121.Budak H., Kantar M., Yucebilgili Kurtoglu K. Drought tolerance in modern and wild wheat. Sci. World. J. 2013;4:66–75. doi: 10.1155/2013/548246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vandenbroucke K., Metzlaff M. Sustainable Food Production. Springer; Berlin/Heidelberg, Germany: 2013. Abiotic stress tolerant crops: Genes, pathways and bottlenecks; pp. 1–17. [Google Scholar]
- 123.Oh S.J., Kim Y.S., Kwon C.W., Park H.K., Jeong J.S., Kim J.K. Overexpression of the transcription factor AP37 in rice improves grain yield under drought conditions. Plant Physiol. 2013;150:1368–1379. doi: 10.1104/pp.109.137554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Valliyodan B., Nguyen H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol. 2006;9:189–195. doi: 10.1016/j.pbi.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 125.Trujillo L.E., Sotolongo M., Menendez C., Ochogavia M.E., Coll Y., Hernandez I., Hernandez L. SodERF3, a novel sugarcane ethylene responsive factor (ERF), enhances salt and drought tolerance when over expressed in tobacco plants. Plant Cell Physiol. 2008;49:512–525. doi: 10.1093/pcp/pcn025. [DOI] [PubMed] [Google Scholar]
- 126.Khan M.N., Zhang J., Luo T., Liu J., Rizwan M., Fahad S., Xu Z., Hu L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crop. Prod. 2019;140:111597. doi: 10.1016/j.indcrop.2019.111597. [DOI] [Google Scholar]
- 127.Nawaz J., Hussain M., Jabbar A., Nadeem G.A., Sajid M., Subtain M.U., Shabbir I. Seed priming a technique. Int. J. Agric. Crop Sci. 2013;6:1373. [Google Scholar]
- 128.Moosavi A., TavakkolAfshari R., Sharif-Zadeh F., Aynehband A. Effect of seed priming on germination characteristics, polyphenoloxidase, and peroxidase activities of four amaranth cultivars. J. Food Agric. Environ. 2009;7:353–358. [Google Scholar]
- 129.Farooq M., Siddique K.H., Rehman H., Aziz T., Lee D.J., Wahid A. Rice direct seeding: Experiences, challenges and opportunities. Soil Tillage Res. 2013;111:87–98. doi: 10.1016/j.still.2010.10.008. [DOI] [Google Scholar]
- 130.Nawaz A., Farooq M., Ahmad R., Basra S.M.A., Lal R. Seed priming improves stand establishment and productivity of no till wheat grown after direct seeded aerobic and transplanted flooded rice. Eur. J. Agron. 2016;76:130–137. doi: 10.1016/j.eja.2016.02.012. [DOI] [Google Scholar]
- 131.Delač D., Gršić K., Ninčević T., Carović-Stanko K., Varga F., Grdiša M. The influence of hydropriming and osmopriming with KNO3 on seed germination of dalmatian pyrethrum (Tanacetum cinerariifolium/Trevir./ Sch. Bip.) Agric. Conspec. Sci. 2018;83:205–211. [Google Scholar]
- 132.Hussain M., Farooq M., Lee D.J. Evaluating the role of seed priming in improving drought tolerance of pigmented and non-pigmented rice. J. Agron. Crop Sci. 2017;203:269–276. doi: 10.1111/jac.12195. [DOI] [Google Scholar]
- 133.Farooq M., Basra S.M.A., Wahid A., Rehman H. Exogenously applied nitric oxide enhances the drought tolerance in fine grain aromatic rice (Oryzasativa L.) J. Agron. Crop Sci. 2009;195:254–261. [Google Scholar]
- 134.Finch-Savage W.E., Dent K.C., Clark L.J. Soak conditions and temperature following sowing influence the response of maize (Zea mays L.) seeds to on-farm priming (pre-sowing seed soak) Field Crops Res. 2017;90:361–374. doi: 10.1016/j.fcr.2004.04.006. [DOI] [Google Scholar]
- 135.Omidi H., Khazaei F., Alvanaghand S.H., Heidari-Sharifabad H. Improvement of seed germination traits in canola (Brassica napus L.) as affected by saline and drought stresses. Plant Ecophysiol. 2009;3:151–158. [Google Scholar]
- 136.Kerchev P., van der Meer T., Sujeeth N., Verlee A., Stevens C.V., Van Breusegem F., Gechev T. Molecular priming as an approach to induce tolerance against abiotic and oxidative stresses in crop plants. Biotechnol. Adv. 2020;1:107503. doi: 10.1016/j.biotechadv.2019.107503. [DOI] [PubMed] [Google Scholar]
- 137.Ghassemi-Golezani K., Farshbaf-Jafari S., Shafagh-Kolvanagh J. Seed priming and field performance of Soybean (Glycine max L.) in response to water limitation. Not. Bot. Horti Agrobot. Cluj-Napoca. 2011;39:186–189. doi: 10.15835/nbha3926122. [DOI] [Google Scholar]
- 138.Casenave E.C., Toselli M.E. Hydropriming as a pre-treatment for cotton germination under thermal and water stress conditions. Seed Sci. Technol. 2007;35:88–98. doi: 10.15258/sst.2007.35.1.08. [DOI] [Google Scholar]
- 139.Tabassum T., Ahmad R., Farooq M., Basra S.M.A. Improving the drought tolerance in barley by osmopriming and biopriming. Int. J. Agric. Biol. 2018;20:1597–1606. [Google Scholar]
- 140.Farooq M., Irfan M., Aziz T., Ahmad I., Cheema S.A. Seed priming with scorbicacid improves drought resistance of wheat. J. Agron. Crop Sci. 2013;199:12–22. doi: 10.1111/j.1439-037X.2012.00521.x. [DOI] [Google Scholar]
- 141.Tabassum T., Farooq M., Ahmad R., Zohaib A., Wahid A., Shahid M. Terminal drought and seed priming improves drought tolerance in wheat. Physiol. Mol. Biol. Plants. 2018;24:845–856. doi: 10.1007/s12298-018-0547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ashraf M., Akram N.A., Al-Qurainy F., Foolad M.R. Advances in Agronomy. Volume 111. Academic Press; Cambridge, MA, USA: 2011. Drought tolerance: Roles of organic osmolytes, growth regulators, and mineral nutrients; pp. 249–296. [Google Scholar]
- 143.Javid M.G., Sorooshzadeh A., Moradi F., Modarres Sanavy S.A.M., Allahdadi I. The role of phytohormones in alleviating salt stress in crop plants. Aust. J. Crop Sci. 2011;5:726. [Google Scholar]
- 144.Nawaz F., Naeem M., Zulfiqar B., Akram A., Ashraf M.Y., Raheel M., Shabbir R.N., Hussain R.A., Anwar I., Aurangzaib M. Understanding brassinosteroid-regulated mechanisms to improve stress tolerance in plants: A critical review. Environ. Sci. Poll. Res. 2017;24:15959–15975. doi: 10.1007/s11356-017-9163-6. [DOI] [PubMed] [Google Scholar]
- 145.Ahmad I., Kamran M., Ali S., Cai T., Bilegjargal B., Liu T., Han Q. Seed filling in maize and hormones crosstalk regulated by exogenous application of uniconazole in semiarid regions. Environ. Sci. Pollut. Res. 2018;25:33225–33239. doi: 10.1007/s11356-018-3235-0. [DOI] [PubMed] [Google Scholar]
- 146.Fahad S., Nie L., Chen Y., Wu C., Xiong D., Saud S., Hongyan L., Cui K., Huang J. Sustainable Agriculture Reviews. Springer; Berlin/Heidelberg, Germany: 2015. Crop plant hormones and environmental stress; pp. 371–400. [Google Scholar]
- 147.Jan S., Abbas N., Ashraf M., Ahmad P. Roles of potential plant hormones and transcription factors in controlling leaf senescence and drought tolerance. Protoplasma. 2019;256:313–329. doi: 10.1007/s00709-018-1310-5. [DOI] [PubMed] [Google Scholar]
- 148.Miura K., Tada Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014;5:4. doi: 10.3389/fpls.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sedaghat M., Tahmasebi-Sarvestani Z., Emam Y., Mokhtassi-Bidgoli A. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant Physiol. Biochem. 2017;119:59–69. doi: 10.1016/j.plaphy.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 150.Sewelam N., Oshima Y., Mitsuda N., Ohme-Takagi M. A step towards understanding plant responses to multiple environmental stresses: A genome-wide study. Plant Cell Environ. 2014;37:2024–2035. doi: 10.1111/pce.12274. [DOI] [PubMed] [Google Scholar]
- 151.Tanveer M., Shahzad B., Sharma A., Khan E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019;135:295–303. doi: 10.1016/j.plaphy.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 152.Im-Kim J., Baek D., Park H.C. Overexpression of Arabidopsis YUCCA6inpotatoresultsinhigh-auxin developmental phenotypes and enhanced resistance to water deficit. Mol. Plant. 2013;6:337–349. doi: 10.1093/mp/sss100. [DOI] [PubMed] [Google Scholar]
- 153.Lo S., Ho T.D., Liu Y. Ectopic expression of specific GA2 oxidase mutants promotes yield and stress tolerance in rice. Plant Biotechnol. J. 2017;15:850–864. doi: 10.1111/pbi.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li Y., Zhang J., Zhang J., Hao L., Hua J., Duan L., Zhang M., Li Z. Expression of an Arabidopsis molybdenum cofactor sulphurase gene in soybean enhances drought tolerance and increases yield under field conditions. Plant Biotechnol. J. 2013;11:747–758. doi: 10.1111/pbi.12066. [DOI] [PubMed] [Google Scholar]
- 155.Wu J., Kim S.G., Kang K.Y., Kim J.G., Park S.R., Gupta R., Kim Y.H., Wang Y., Kim S.T. Overexpression of apathogenesis-related protein 10 enhances biotic and abiotic stress tolerance in rice. Plant Pathol. J. 2016;32:552–562. doi: 10.5423/PPJ.OA.06.2016.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liang C., Meng Z., Meng Z., Malik W., Yan R., Lwin K.M., Lin F., Wang Y., Sun G., Zhou T., et al. GhABF2, a bZIP transcription factor.; confers drought and salinity tolerance in cotton (Gossypium hirsutum L.) Sci. Rep. 2016;6:35040. doi: 10.1038/srep35040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu L., Chen X., Wang Z., Wang S., Wang Y., Zhu Q., Li S., Xiang C. Arabidopsis enhanced drought tolerance1/HOMEODOMAIN GLABROUS11 confers drought tolerance in transgenic rice without yield penalty. Plant Physiol. 2013;162:1378–1391. doi: 10.1104/pp.113.217596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lu Y., Li Y., Zhang J., Xiao Y., Yue Y., Duan L., Zhang M., Li Z. Overexpression of Arabidopsis molybdenum cofactor sulfurase gene confers drought tolerance in maize (Zea mays L.) PLoS ONE. 2013;10:e52126. doi: 10.1371/journal.pone.0052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pospíšilová H., Jiskrova E., Vojta P., Mrizova K., Kokáš F., Čudejková M.M., Bergougnoux V., Plíhal O., Klimešová J., Novák O., et al. Transgenic barley overexpressing a cytokinin dehydrogenase gene shows greater tolerance to drought stress. New Biotechnol. 2016;33:692–705. doi: 10.1016/j.nbt.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 160.Nir I.D., Moshelion M., Weiss D. The Arabidopsis GIBBERELLIN METHYL TRANSFERASE 1 suppresses gibberellin activity, reduces whole-plant transpiration and promotes drought tolerance in transgenic tomato. Plant Cell Environ. 2014;37:113–123. doi: 10.1111/pce.12135. [DOI] [PubMed] [Google Scholar]
- 161.Brito C., Dinis L.T., Moutinho-Pereira J., Correia C.M. Drought stress effects and olive tree acclimation under a changing climate. Plants. 2019;8:232. doi: 10.3390/plants8070232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Seleiman M. Use of plant nutrients in improving abiotic stress tolerance in wheat. In: Hasanuzzaman M., Nahar K., Hossain M.A., editors. Wheat Production in Changing Environments. Springer; Singapore: 2019. pp. 481–495. [Google Scholar]
- 163.Zouari M., Hassena A.B., Trabelsi L., Rouina B.B., Decou R., Labrousse P. Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants. Springer; Berlin/Heidelberg, Germany: 2019. Exogenous Proline-Mediated Abiotic Stress Tolerance in Plants: Possible Mechanisms; pp. 99–121. [Google Scholar]
- 164.Yang Y., Guo Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytologist. 2018;217:523–539. doi: 10.1111/nph.14920. [DOI] [PubMed] [Google Scholar]
- 165.Elkelish A., Qari S.H., Mazrou Y.S., Abdelaal K.A., Hafez Y.M., Abu-Elsaoud A.M., Batiha G.E.S., El-Esawi M.A., El Nahhas N. Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants Through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins. Plants. 2020;9:431. doi: 10.3390/plants9040431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Semida W.M., Abdelkhalik A., Rady M.O., Marey R.A., Abd El-Mageed T.A. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Scientiahorticulturae. 2020;272:109580. [Google Scholar]
- 167.Sallam A., Alqudah A.M., Dawood M.F., Baenziger P.S., Börner A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019;20:3137. doi: 10.3390/ijms20133137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ashraf M., Foolad M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007;59:206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- 169.Liu C., Zhao L., Yu G. The dominant glutamic acid metabolic flux to produce gamma-amino butyric acid over proline in Nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J. Integ. Plant Biol. 2011;53:608–618. doi: 10.1111/j.1744-7909.2011.01049.x. [DOI] [PubMed] [Google Scholar]
- 170.Pilon-Smits E.A.H., Terry N., Sears T., Van-Dun K. Enhanced drought resistance in fructan-producing sugar beet. Plant Physiol. Biochem. 1999;37:313–317. doi: 10.1016/S0981-9428(99)80030-8. [DOI] [Google Scholar]
- 171.Kaya C., Sonmez O., Aydemir S., Ashraf M., Dikilitas M. Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L.) J. Plant Interact. 2013;3:234–344. doi: 10.1080/17429145.2012.725480. [DOI] [Google Scholar]
- 172.Ahn C., Park U., Park P.B. Increased salt and drought tolerance by D-ononitol production in transgenic Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2011;415:669–674. doi: 10.1016/j.bbrc.2011.10.134. [DOI] [PubMed] [Google Scholar]
- 173.Yan G.C., Nikolic M., Ye M.J., Xiao Z.X., Liang Y.C. Silicon acquisition and accumulation in plant and its significance for agriculture. J. Integ. Agric. 2018;17:2138–2150. doi: 10.1016/S2095-3119(18)62037-4. [DOI] [Google Scholar]
- 174.Hurtado A.C., Chiconato D.A., de Mello Prado R., Junior G.D.S.S., Gratão P.L., Felisberto G., Viciedo D.O., Dos Santos D.M.M. Different methods of silicon application attenuate salt stress in sorghum and sunflower by modifying the antioxidative defense mechanism. Ecotox. Environ. Safety. 2020;203:110964. doi: 10.1016/j.ecoenv.2020.110964. [DOI] [PubMed] [Google Scholar]
- 175.Ali S., Rizwan M., Hussain A., urRehman M.Z., Ali B., Yousaf B., Wijaya L., Alyemeni M.N., Ahmad P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticumaestivum L.) Plant Physiol. Biochem. 2019;140:1–8. doi: 10.1016/j.plaphy.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 176.Chen D., Wang S., Yin L., Deng X. How does silicon mediate plant water uptake and loss under water deficiency? Front. Plant Sci. 2018;9:281. doi: 10.3389/fpls.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shi Y., Zhang Y., Han W., Feng R., Hu Y., Guo J. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 2016;7:196. doi: 10.3389/fpls.2016.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shi Y., Zhang Y., Yao H., Wu J., Sun H., Gong H. Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato under water deficit stress. Plant Physiol. Biochem. 2014;78:27–36. doi: 10.1016/j.plaphy.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 179.Gong H., Zhu X., Chen K., Wang S., Zhang C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005;169:313–321. doi: 10.1016/j.plantsci.2005.02.023. [DOI] [Google Scholar]
- 180.Gunes A., Pilbeam D.J., Inal A., Coban S. Influence of silicon on sunflower cultivars under drought stress, I: Growth, antioxidant mechanisms, and lipid peroxidation. Commun. Soil Sci. Plant Anal. 2008;39:1885–1903. doi: 10.1080/00103620802134651. [DOI] [Google Scholar]
- 181.Ma J.F., Tamai K., Yamaji N., Mitani N., Konishi S., Katsuhara M. A silicon transporter in rice. Nature. 2006;440:688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- 182.Dar M.I., Naikoo M.I., Khan F.A., Rehman F., Green I.D., Naushin F., Ansari A.A. Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress. Springer; Berlin/Heidelberg, Germany: 2017. An introduction to reactive oxygen species metabolism under changing climate in plants; pp. 25–52. [Google Scholar]
- 183.Ebeed H.T., Hassan N.M., Aljarani A.M. Exogenous applications of polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Biochem. 2017;118:438–448. doi: 10.1016/j.plaphy.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 184.Jung H.I., Kong M.S., Lee B.R., Kim T.H., Chae M.J., Sung J.K., Lee C.H., Lee E.J., Jung G.B., Kim Y.H. Exogenous glutathione increases arsenic translocation into shoots and alleviates arsenic-induced oxidative stress by sustaining ascorbate-glutathione homeostasis in rice seedlings. Front. Plant Sci. 2019;10:1089. doi: 10.3389/fpls.2019.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sharma A., Shahzad B., Kumar V., Kohli S.K., Sidhu G.P.S., Bali A.S., Handa N., Kapoor D., Bhardwaj R., Zheng B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9:285. doi: 10.3390/biom9070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Martinez V., Nieves-Cordones M., Lopez-Delacalle M., Rodenas R., Mestre T.C., Garcia-Sanchez F., Rubio F., Nortes P.A., Mittler R., Rivero R.M. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules. 2018;23:535. doi: 10.3390/molecules23030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mostofa M.G., Rahman M.M., Siddiqui M.N., Fujita M., Tran L.S.P. Salicylic acid antagonizes selenium phytotoxicity in rice: Selenium homeostasis, oxidative stress metabolism and methylglyoxal detoxification. J. Hazard. Mater. 2020;394:122572. doi: 10.1016/j.jhazmat.2020.122572. [DOI] [PubMed] [Google Scholar]
- 188.Kaya C., Okant M., Ugurlar F., Alyemeni M.N., Ashraf M., Ahmad P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere. 2019;225:627–638. doi: 10.1016/j.chemosphere.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 189.Hasanuzzaman M., Bhuyan M.H.M., Nahar K., Hossain M., Mahmud J.A., Hossen M., Masud A.A.C., Fujita M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy. 2018;8:31. doi: 10.3390/agronomy8030031. [DOI] [Google Scholar]
- 190.Foyer C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018;154:134–142. doi: 10.1016/j.envexpbot.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Seleiman M.F., Semida W.M., Rady M.M., Mohamed G.F., Hemida K.A., Alhammad B.A., Hassan M.M., Shami A. Sequential Application of Antioxidants Rectifies Ion Imbalance and Strengthens Antioxidant Systems in Salt-Stressed Cucumber. Plants. 2020;9:1783. doi: 10.3390/plants9121783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xin L., Zheng H., Yang Z., Guo J., Liu T., Sun L., Xiao Y., Yang J., Yang Q., Guo L. Physiological and proteomic analysis of maize seedling response to water deficiency stress. J. Plant Physiol. 2018;228:29–38. doi: 10.1016/j.jplph.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 193.Zahoor R., Zhao W., Dong H., Snider J.L., Abid M., Iqbal B., Zhou Z. Potassium improves photosynthetic tolerance to and recovery from episodic drought stress in functional leaves of cotton (Gossypiumhirsutum L.) Plant Physiol. Biochem. 2017;119:21–32. doi: 10.1016/j.plaphy.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 194.Hawrylak-Nowak B., Dresler S., Rubinowska K., Matraszek-Gawron R., Woch W., Hasanuzzaman M. Selenium biofortification enhances the growth and alters the physiological response of lamb’s lettuce grown under high temperature stress. Plant Physiol. Biochem. 2018;127:446–456. doi: 10.1016/j.plaphy.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 195.Brestic M., Zivcak M., Hauptvogel P., Misheva S., Kocheva K., Yang X., Li X., Allakhverdiev S.I. Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photosynth. Res. 2018;136:245–255. doi: 10.1007/s11120-018-0486-z. [DOI] [PubMed] [Google Scholar]
- 196.Kumar A., Nayak A.K., Das B.S., Panigrahi N., Dasgupta P., Mohanty S., Kumar U., Panneerselvam P., Pathak H. Effects of water deficit stress on agronomic and physiological responses of rice and greenhouse gas emission from rice soil under elevated atmospheric CO2. Sci. Total Environ. 2019;650:2032–2050. doi: 10.1016/j.scitotenv.2018.09.332. [DOI] [PubMed] [Google Scholar]
- 197.Jatav K.S., Agarwal R.M., Singh R.P., Shrivastava M. Growth and yield responses of wheat (Triticum aestivum L.) to suboptimal water supply and different potassium doses. J. Funct. Environ. Bot. 2021;2:39–51. doi: 10.5958/j.2231-1742.2.1.005. [DOI] [Google Scholar]
- 198.Soleimanzadeh H., Habibi D., Ardakani M.R., Paknejad F., Rejali F. Effect of potassium levels on antioxidant enzymes and malondialdehyde content under drought stress in sunflower (Helianthus annuus L.) Am. J. Agric. Biol. Sci. 2010;5:56–61. doi: 10.3844/ajabssp.2010.56.61. [DOI] [Google Scholar]
- 199.Zain N.A.M., Ismail M.R., Puteh A., Mahmood M., Islam M.R. Drought tolerance and ion accumulation of rice following application of additional potassium fertilizer. Commun. Soil Sci. Plant Anal. 2014;45:2502–2514. doi: 10.1080/00103624.2014.932374. [DOI] [Google Scholar]
- 200.Premachandra G.S., Saneoka H., Ogata S. Cell membrane stability and leaf water relationsas affected potassium nutrition of water-stressed maize. J. Exp. Bot. 1991;42:739–745. doi: 10.1093/jxb/42.6.739. [DOI] [Google Scholar]
- 201.Fayez K.A., Bazaid S.A. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J. Saudi Soc. Agric. Sci. 2014;3:45–55. doi: 10.1016/j.jssas.2013.01.001. [DOI] [Google Scholar]
- 202.Khan N., Bano A., Curá J.A. Role of Beneficial Microorganisms and Salicylic Acid in Improving Rainfed Agriculture and Future Food Safety. Microorganisms. 2020;8:1018. doi: 10.3390/microorganisms8071018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Niu X., Song L., Xiao Y., Ge W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018;8:2580. doi: 10.3389/fmicb.2017.02580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gontia-Mishra I., Sapre S., Sharma A., Tiwari S. Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol. 2016;18:992–1000. doi: 10.1111/plb.12505. [DOI] [PubMed] [Google Scholar]
- 205.Khan N., Bano A., Shahid M.A., Nasim W., Babar M.A. Interaction between PGPR and PGR for water conservation and plant growth attributes under drought condition. Biologia. 2018;73:1083–1098. doi: 10.2478/s11756-018-0127-1. [DOI] [Google Scholar]
- 206.Etesami H., Emami S., Alikhani H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects A review. J. Soil Sci. Plant Nutr. 2017;17:897–911. doi: 10.4067/S0718-95162017000400005. [DOI] [Google Scholar]
- 207.Khan N., Bano A., Ali S., Babar M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020;90:189–203. doi: 10.1007/s10725-020-00571-x. [DOI] [Google Scholar]
- 208.Alamgir A.N.M. Therapeutic Use of Medicinal Plants and Their Extracts. Springer; Berlin/Heidelberg, Germany: 2018. Phytoconstituents—Active and Inert Constituents, Metabolic Pathways, Chemistry and Application of Phytoconstituents, Primary Metabolic Products, and Bioactive Compounds of Primary Metabolic Origin; pp. 25–164. [Google Scholar]
- 209.Arun K.D., Sabarinathan K.G., Gomathy M., Kannan R., Balachandar D. Mitigation of drought stress in rice crop with plant growth-promoting abiotic stress-tolerant rice phyllosphere bacteria. J. Basic Microbiol. 2020;60:768–786. doi: 10.1002/jobm.202000011. [DOI] [PubMed] [Google Scholar]
- 210.Kumar A., Singh S., Gaurav A.K., Srivastava S., Verma J.P. Plant Growth-Promoting Bacteria: Biological Tools for the Mitigation of Salinity Stress in Plants. Front. Microbiol. 2020;11:1216. doi: 10.3389/fmicb.2020.01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hewedy O.A., Abdel Lateif K.S., Seleiman M.F., Shami A., Albarakaty F.M., El-Meihy R.M. Phylogenetic Diversity of Trichoderma Strains and Their Antagonistic Potential against Soil-Borne Pathogens under Stress Conditions. Biology. 2020;9:189. doi: 10.3390/biology9080189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Khadka R.B., Uphoff N. Effects of Trichoderma seedling treatment with System of Rice Intensification management and with conventional management of transplanted rice. PeerJ. 2019;7:5877. doi: 10.7717/peerj.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Andreote F.D., Gumiere T., Durrer A. Exploring interactions of plant microbiomes. Sci. Agric. 2014;71:528–539. doi: 10.1590/0103-9016-2014-0195. [DOI] [Google Scholar]
- 214.Hussain M.B., Zahir Z.A., Asghar H.N., Asgher M. Can catalase and exopolysaccharides producing rhizobia ameliorate drought stress in wheat? Int. J. Agric. Biol. 2014;16:1. [Google Scholar]
- 215.Vardharajula S., Zulfikar Ali S., Grover M., Reddy G., Bandi V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Int. 2011;6:1–14. [Google Scholar]
- 216.Sandhya V., Ali S.K.Z., Minakshi G., Reddy G., Venkateswarlu B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fertil. Soils. 2009;46:17–26. doi: 10.1007/s00374-009-0401-z. [DOI] [Google Scholar]
- 217.Sherameti I., Tripathi S., Varma A., Oelmuller R. The root-colonizing endophyte Pirifomospora indica confers drought tolerance in Arabidopsis by stimulating the expression of drought stress–related genes in leaves. Mol. Plant-Microbe Interact. 2008;21:799–807. doi: 10.1094/MPMI-21-6-0799. [DOI] [PubMed] [Google Scholar]
- 218.Kang S.M., Radhakrishnan R., Khan A.L., Kim M.J., Park J.M., Kim B.R., Shin D.H., Lee I.J. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014;84:115–124. doi: 10.1016/j.plaphy.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 219.Lim J.H., Kim S.D. Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in pepper. Plant Pathol. J. 2013;29:201–208. doi: 10.5423/PPJ.SI.02.2013.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shukla N., Awasthi R.P., Rawat L., Kumar J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 2012;54:78–88. doi: 10.1016/j.plaphy.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 221.Bano Q., Ilyas N., Bano A., Zafar N., Akram A., Hassan F. Effect of Azospirillum inoculation on maize (Zea mays L.) under drought stress. Pak J. Bot. 2013;45:13–20. [Google Scholar]
- 222.Staudinger C., Mehmeti-Tershani V., Gil-Quintana E., Gonzalez E.M., Hofhansl F., Bachmann G., Wienkoop S. Evidence for a rhizobia-induced drought stress response strategy in Medicagotruncatula. J. Proteom. 2016;136:202–213. doi: 10.1016/j.jprot.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 223.Ma Y., Rajkumar M., Zhang C., Freitas H. Inoculation of Brassica oxyrrhina with plant growth promoting bacteria for the improvement of heavy metal phytoremediation under drought conditions. J. Hazard. Mater. 2014;320:36–44. doi: 10.1016/j.jhazmat.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 224.Tiwari S., Lata C., Chauhan P.S., Nautiyal C.S. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol. Biochem. 2016;99:108–117. doi: 10.1016/j.plaphy.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 225.Fasciglione G., Casanovas E.M., Quillehauquy V., Yommi A.K., Goñi M.G., Roura S.I., Barassi C.A. Azospirillum inoculation effects on growth, product quality and storage life of lettuce plants grown under salt stress. Sci. Hortic. 2015;195:154–162. doi: 10.1016/j.scienta.2015.09.015. [DOI] [Google Scholar]
- 226.Figueiredo M.V., Burity H.A., Martínez C.R., Chanway C.P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008;40:182–188. doi: 10.1016/j.apsoil.2008.04.005. [DOI] [Google Scholar]
- 227.Kasim W.A., Osman M.E., Omar M.N., Abd El-Daim I.A., Bejai S., Meijer J. Control of drought stress in wheat using plant-growth-promoting bacteria. J. Plant Growth Regul. 2013;32:122–130. doi: 10.1007/s00344-012-9283-7. [DOI] [Google Scholar]
- 228.Al-Jabari M., Ghyadah R.A., Alokely R. Recovery of hydrogel from baby diaper wastes and its application for enhancing soil irrigation management. J. Environ. Manag. 2019;239:255–261. doi: 10.1016/j.jenvman.2019.03.087. [DOI] [PubMed] [Google Scholar]
- 229.Tu Y., Wang R., Zhang Y., Wang J. Progress and expectation of atmospheric water harvesting. Joule. 2018;2:1452–1475. doi: 10.1016/j.joule.2018.07.015. [DOI] [Google Scholar]
- 230.Yang M., Wang G., Lazin R., Shen X., Anagnostou E. Impact of planting time soil moisture on cereal crop yield in the Upper Blue Nile Basin: A novel insight towards. Agric. Water. Manag. 2020;243:106430. doi: 10.1016/j.agwat.2020.106430. [DOI] [Google Scholar]
- 231.Abobatta W. Impact of hydrogel polymer in agricultural sector. Adv. Agric. Environ. Sci. 2018;1:59–64. doi: 10.30881/aaeoa.00011. [DOI] [Google Scholar]
- 232.Jerszurki D., Couvreur V., Maxwell T., Silva L.D.C.R., Matsumoto N., Shackel K., de Souza J.L.M., Hopmans J. Impact of root growth and hydraulic conductance on canopy carbon-water relations of young walnut trees (Juglans regia L.) under drought. Sci. Hort. 2017;226:342–352. doi: 10.1016/j.scienta.2017.08.051. [DOI] [Google Scholar]
- 233.Ayangbenro A.S., Babalola O.O. Reclamation of arid and semi-arid soils: The role of plant growth-promoting archaea and bacteria. Curr. Plant Biol. 2020:100173. doi: 10.1016/j.cpb.2020.100173. in press. [DOI] [Google Scholar]
- 234.Saha A., Sekharan S., Manna U. Superabsorbent hydrogel (SAH) as a soil amendment for drought management: A review. Soil Tillage Res. 2020;204:104736. doi: 10.1016/j.still.2020.104736. [DOI] [Google Scholar]
- 235.Seleiman M.F., Almutairi K.F., Alotaibi M., Shami A., Alhammad B.A., Battaglia M.L. Nano fertilization as an emerging fertilization technique: Why modern agriculture can benefit from its use? Plants. 2020;10:2. doi: 10.3390/plants10010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cunningham F.J., Goh N.S., Demirer G.S., Matos J.L., Landry M.P. Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends Biotechnol. 2018;36:882–897. doi: 10.1016/j.tibtech.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Siddiqi K.S., Husen A. Engineered gold nanoparticles and plant adaptation potential. Nanoscale Res. Lett. 2016;11:400. doi: 10.1186/s11671-016-1607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ghasemlou F., Amiri H., Karamian R., Mirzaie-asl A. Alleviation of the effects of on drought stress Verbascum nudicuale by methyl jasmonate and titanium dioxide nanoparticles. Plant Physiol. 2019;9:2911–2920. [Google Scholar]
- 239.Siddiqui H., Ahmed K.B.M., Sami F., Hayat S. Sustainable Agriculture Reviews. Volume 41. Springer; Berlin/Heidelberg, Germany: 2020. Silicon nanoparticles and plants: Current knowledge and future perspectives; pp. 129–142. [Google Scholar]
- 240.Rizwan M., Ali S., Ali B., Adrees M., Arshad M., Hussain A., urRehman M.Z., Waris A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere. 2019;214:269–277. doi: 10.1016/j.chemosphere.2018.09.120. [DOI] [PubMed] [Google Scholar]
- 241.Khan Z.S., Rizwan M., Hafeez M., Ali S., Javed M.R., Adrees M. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. 2019;26:19859–19870. doi: 10.1007/s11356-019-05333-5. [DOI] [PubMed] [Google Scholar]
- 242.Seydmohammadi Z., Roein Z., Rezvanipour S. Accelerating the growth and flowering of Eustoma grandiflorum by foliar application of nano-ZnO and nano-CaCO3. Plant Physiol. Rep. 2019;25:140–148. doi: 10.1007/s40502-019-00473-9. [DOI] [Google Scholar]
- 243.Seleiman M.F., Alotaibi M., Alhammad B., Alharbi B., Refay Y., Badawy S. Effects of ZnO nanoparticles and biochar of rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agronomy. 2020;10:790. doi: 10.3390/agronomy10060790. [DOI] [Google Scholar]
- 244.Movafeghi A., Khataee A., Abedi M., Tarrahi R., Dadpour M., Vafaei F. Effects of TiO2 nanoparticles on the aquatic plant Spirodelapolyrrhiza: Evaluation of growth parameters, pigment contents and antioxidant enzyme activities. J. Environ. Sci. 2018;64:130–138. doi: 10.1016/j.jes.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 245.Maswada H.F., Mazrou Y.S., Elzaawely A.A., Alam-Eldein S.M. Nanomaterials. Effective tools for field and horticultural crops to cope with drought stress: A review. Span. J. Agric. Res. 2020;18:15. doi: 10.5424/sjar/2020182-16181. [DOI] [Google Scholar]
- 246.Davar F., Zareii A.R., Amir H. Evaluation the effect of water stress and foliar application of Fe nanoparticles on yield, yield components and oil percentage of safflower (Carthamus tinctorious L.) Int. J. Adv. Biol. Biomed. Res. 2014;2:1150–1159. [Google Scholar]
- 247.Ashkavand P., Tabari M., Zarafshar M., Tomaskova I., Struve D. Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. For. Res. 2015;76:350–359. doi: 10.1515/frp-2015-0034. [DOI] [Google Scholar]
- 248.Jaberzadeh A., Payam M., Hamid R., Tohidi M., Hossein Z. Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected towater deficit stress. Not. Bot. Horti Agrobot. Cluj-Napoca. 2013;41:201–207. doi: 10.15835/nbha4119093. [DOI] [Google Scholar]
- 249.Sun D., Hussain H.I., Yi Z., Rookes J.E., Kong L., Cahill D.M. Delivery of abscisic acid to plants using glutathione responsive mesoporous silica nanoparticles. J. Nanosci. Nanotechnol. 2018;18:1615–1625. doi: 10.1166/jnn.2018.14262. [DOI] [PubMed] [Google Scholar]
- 250.Sedghi M., Hadi M., Toluie S.G. Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. Ann. WUT-Ser. Biol. 2013;XVI:73–78. [Google Scholar]
- 251.Taran N., Storozhenko V., Svietlova N., Batsmanova L., Shvartau V., Kovalenko M. Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanoscale Res. Lett. 2017;12:60. doi: 10.1186/s11671-017-1839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ullah A., Manghwar H., Shaban M., Khan A.H., Akbar A., Ali U., Ali E., Fahad S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018;25:33103–33118. doi: 10.1007/s11356-018-3364-5. [DOI] [PubMed] [Google Scholar]
- 253.Naghshbandi M.P., Tabatabaei M., Aghbashlo M., Gupta V.K., Sulaiman A., Karimi K., Moghimi H., Maleki M. Progress toward improving ethanol production through decreased glycerol generation in Saccharomyces cerevisiae by metabolic and genetic engineering approaches. Renew. Sustain. Energy Rev. 2019;115:109353. doi: 10.1016/j.rser.2019.109353. [DOI] [Google Scholar]
- 254.Salvi P., Kamble N.U., Majee M. Ectopic over-expression of ABA-responsive Chickpea galactinol synthase (CaGolS) gene results in improved tolerance to dehydration stress by modulating ROS scavenging. Environ. Exp. Bot. 2020;171:103957. doi: 10.1016/j.envexpbot.2019.103957. [DOI] [Google Scholar]
- 255.Selvaraj M.G., Ishizaki T., Valencia M., Ogawa S., Dedicova B., Ogata T., Yoshiwara K., Maruyama K., Kusano M., Saito K., et al. Overexpression of an Arabidopsis thaliana galactinol synthase gene improves drought tolerance in transgenic rice and increased grain yield in the field. Plant Biotechnol. J. 2017;1511:1465–1477. doi: 10.1111/pbi.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Honna P.T., Fuganti-Pagliarini R., Ferreira L.C., Molinari M.D., Marin S.R., de Oliveira M.C., Farias J.R., Neumaier N., Mertz-Henning L.M., Kanamori N., et al. Molecular, physiological, and agronomical characterization, in greenhouse and in field conditions, of soybean plants genetically modified with AtGolS2 gene for drought tolerance. Mol. Breed. 2016;36:157. doi: 10.1007/s11032-016-0570-z. [DOI] [Google Scholar]