Abstract
Abeliophyllum distichum (Oleaceae), which is the only species in the monotypic genus and is grown only on the Korean peninsula, has a high scarcity value. Its five variants (white, pink, round, blue, and ivory) have different morphological characteristics in terms of the color of petals and sepals or shape of the fruits. Despite its high value, there has been no study on variant classification except in terms of their morphological characteristics. Thus, we performed a volatile component analysis of A. distichum flowers and multivariate data analyses to reveal the relationship between fragments emitted from five variants of A. distichum flowers with their morphological characteristics. As a result, 66 volatile components of this plant were identified by headspace solid-phase microextraction gas chromatography–mass spectrometry (HS-SPME-GC-MS), showing unique patterns for each set of morphological characteristics, especially the color of the petals. These results suggest that morphological characteristics of each variant are related to the volatile composition.
Keywords: Abeliophyllum distichum, HS-SPME-GC-MS, morphological characteristics, scents, volatile components analysis
1. Introduction
Flower fragrances and pigments are characteristics of various insect-pollinated flowers; they serve as a major signal to lure pollinating insects to the reproductive organs. [1,2]. The fragrances of various flowers (e.g., lilac, rose, jasmine) have been used in perfumery due to their pleasant effects on the human sensory system, and some of them have been synthesized for production of artificial perfumes [3]. Thus, much research has focused on identifying and characterizing their odors and flavors.
After approval of the Nagoya Protocol, research using domestic native species became a key issue [4]. Among the various Korean plants, Abeliophyllum distichum (Oleaceae), which is the only species in a monotypic genus (Abeliophyllum) and is grown only in the Korean peninsula, has high scarcity value [5]. For this reason, the Korean government designated A. distichum as an endangered species until recently, and have even designated some of its habitats as natural monuments to preserve them [6]. Due to its high scarcity and ecological and geographic value, A. distichum has rarely been studied compared with other plants. However, in 2017, A. distichum was removed from the list of endangered species by the Ministry of the Environment [6] due to the development of mass breeding techniques [7,8]. Previous phytochemical studies of these plants have focused on just the leaves, and they reported that four phenylethanoid glycosides and two flavonoids are components of these plants [9,10,11]. These compounds have been reported to have anti-oxidation, anti-inflammation, anti-hypertension, anti-diabetes, and neuroprotection effects [10,11,12,13].
Moreover, this plant is known to have attractive and strong fragrances [14,15]. Even though A. distichum has a good fragrance, few studies have reported analyses of the associated volatiles. If such data were available, these volatiles could be manufactured as fragrance oil for various applications such as in aromatherapy, perfumes, and cosmetics. Thus, the aim of our study was to reveal the biological effects of A. distichum volatiles and standardize its content for use by the cosmeceutical industry. Accordingly, volatile analytes of A. distichum have high value from both the research and industrial perspectives.
Various quantitative and qualitative protocols for determining the volatile compositions in the flowers have been developed using their essential oils (such as Soxhlet extraction) [16,17]. However, these protocols have several disadvantages, such as loss of fragrance, production of artificial volatiles during solvent extraction, and consumption of a large number of organic solvents and time [16,17].
To compensate for these shortcomings, headspace analysis is used as a preferred analytical protocol for the volatile composition of natural materials [18]. However, there are some problems associated with headspace methods such as memory effects on traps and dynamic purging. For this reason, solid-phase micro extraction (SPME), which is a simple and rapid sample preparation technique, was developed by Arthur in 1990 [19]. This method has the powerful advantages of solvent-free extraction as well as concentration in a single step method [20]. Thus, it has been widely used for fragrance analysis [21]. Therefore, we performed a volatile analysis of A. distichum flowers using HS-SPME coupled with gas chromatography–mass spectrometry (GC-MS). To identify volatile components, we measured mass spectra for each peak and compared them with a mass spectral database (NIST library) and LRI (Linear retention indices) values [22,23].
Moreover, there have been some reports showing that flower volatiles are related to environmental factors, biological recognition, and morphological characteristics [24,25]. At present, five variants of A. distichum have been reported: white miseon (A. distichum Nakai), pink miseon (A. distichum for. lilacinum Nakai), ivory miseon (A. distichum for. eburneum T. B. Lee), blue miseon (A. distichum for. viridicalycinum T. B. Lee), and round miseon (A. distichum var. rotundicarpum T. B. Lee) [26,27,28]. These variants have different morphological characteristics in terms of the color of petals and sepals or fruit shapes, respectively.
In this study, HS-SPME-GC-MS analysis was performed to estimate the differences in fragrance composition of volatiles such as monoterpenes, alcohols, and aromatics on the basis of the morphological characteristics of the five variants of A. distichum flowers (white, pink, ivory, blue, and round miseon). In addition, data of volatiles emitted from five variants were further interpreted on the basis of multivariate data analyses to visually characterize the dissimilarities associated with morphological characteristics (especially the color of the petals). These analyses included principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA).
2. Results and Discussion
2.1. Profiling of Volatile Components from Five Variants of the Flowers in Five Variants of A. distichum Using HS-SPME-GC-MS Analysis
Identification of volatiles was conducted on the basis of the total ion chromatogram (TIC) from the measurement of five variants of A. distichum flowers using HS-SPME (Thermo Scientific Trace™ 1300, Thermo Fisher Scientific Inc., Sunnyvale, CA, USA) coupled with triple quadrupole mass spectrometer (QqQ-MS, TSQ 8000, Thermo Fisher Scientific Inc., USA) (Figure S1). The intensities of peaks, especially those eluted from 30–35 and 40–45 min, were significantly different depending on the sample. As shown in Figure S1 and Table 1, each variant had different fragrance compositions as well as different morphological characteristics (such as color of petals and sepals and fruit shape). The TIC spectra for each variant were deconvoluted using Xcalibur 3.1 software from the Thermo Finnigan Corporation, San Jose, CA, USA. Each peak was identified by matching its spectrum with the NIST library (>85% similarity) and by analyzing the retention indices calculated against n-alkanes (C7–C30) (i.e., retention time and relative retention time). The resulting identifications of peaks were confirmed through an analysis of fragmentation patterns in the mass spectra. A total of 66 volatiles were identified and quantified in the headspace of 5 variants of A. distichum flowers, including 16 oxygenated monoterpenes, 1 oxygenated sesquiterpene, 5 monoterpene hydrocarbons, 12 aromatic alcohols, 5 aromatics, 13 alcohols, 10 aldehydes and carboxyls, and 4 miscellaneous components grouped as “others” (Table 1 and Table S1). The structure types and the odor notes of each scent are illustrated in Table S2.
Table 1.
Relative contents of the detected volatile components of fresh petal tissue in five variants of Abeliophyllum distichum flowers using headspace solid-phase microextraction gas chromatography–mass spectrometry (HS-SPME-GC-MS) analysis.
| No. | Compound | RT a | LRI b | Relative Content c (%) ± SD d | ||||
|---|---|---|---|---|---|---|---|---|
| White e | Pink f | Ivory g | Blue h | Round i | ||||
| 1 | isovaleraldehyde | 11.50 | 885 | 1.763 ± 0.073 | 3.472 ± 0.240 | 3.641 ± 0.116 | 3.418 ± 0.024 | 2.973 ± 0.244 |
| 2 | ethanol | 11.91 | 891 | 6.688 ± 0.231 | 1.016 ± 0.039 | 1.133 ± 0.027 | 0.905 ± 0.055 | 2.305 ± 0.103 |
| 3 | decane | 15.26 | 1000 | 1.475 ± 0.103 | 2.023 ± 0.128 | 3.730 ± 0.299 | 1.282 ± 0.052 | 2.364 ± 0.187 |
| 4 | 5-ethyl-2,2,3-trimethylheptane | 16.82 | 1040 | 0.716 ± 0.031 | 1.022 ± 0.129 | 1.453 ± 0.094 | 2.452 ± 0.054 | 1.191 ± 0.186 |
| 5 | n-hexanal | 17.67 | 1061 | 0.694 ± 0.022 | 0.735 ± 0.100 | 1.536 ± 0.011 | 1.104 ± 0.040 | 0.852 ± 0.057 |
| 6 | 2-butyl-3-octanol | 19.22 | 1101 | 0.148 ± 0.046 | 0.279 ± 0.018 | 0.418 ± 0.054 | 0.218 ± 0.051 | 0.227 ± 0.129 |
| 7 | β-myrcene | 20.94 | 1146 | 4.232 ± 0.132 | 0.572 ± 0.046 | 0.448 ± 0.008 | 0.326 ± 0.005 | 0.610 ± 0.011 |
| 8 | isoamylalcohol | 21.98 | 1174 | 0.854 ± 0.013 | 0.951 ± 0.028 | 1.301 ± 0.053 | 0.687 ± 0.025 | 1.461 ± 0.139 |
| 9 | limonene | 22.62 | 1191 | 0.793 ± 0.031 | 0.176 ± 0.015 | 0.180 ± 0.006 | 0.243 ± 0.004 | 0.283 ± 0.007 |
| 10 | 2-hexenal | 22.91 | 1198 | 4.831 ± 0.109 | 4.124 ± 0.424 | 8.376 ± 0.140 | 3.335 ± 0.098 | 3.407 ± 0.232 |
| 11 | ocimene-1 | 23.54 | 1215 | 1.013 ± 0.045 | 0.162 ± 0.010 | 0.579 ± 0.007 | 0.072 ± 0.002 | 0.164 ± 0.004 |
| 12 | ocimene-2 | 24.21 | 1234 | 3.020 ± 0.101 | 0.744 ± 0.058 | 4.792 ± 0.136 | 0.314 ± 0.006 | 0.636 ± 0.014 |
| 13 | n-hexyl acetate | 24.85 | 1251 | 1.027 ± 0.061 | 0.196 ± 0.019 | 3.825 ± 0.096 | 0.267 ± 0.032 | 0.695 ± 0.048 |
| 14 | isohexanol | 25.95 | 1281 | 0.210 ± 0.007 | 0.429 ± 0.014 | 0.760 ± 0.057 | 0.330 ± 0.007 | 0.403 ± 0.001 |
| 15 | 3-methyl-1-pentanol | 26.43 | 1294 | 0.083 ± 0.005 | 0.101 ± 0.009 | 0.115 ± 0.023 | 0.078 ± 0.013 | 0.089 ± 0.013 |
| 16 | 2-hexenyl acetate | 26.92 | 1307 | 0.133 ± 0.002 | 0.033 ± 0.002 | 0.611 ± 0.023 | 0.027 ± 0.001 | 0.108 ± 0.008 |
| 17 | 6-methyl-5-hepten-2-one | 27.19 | 1315 | 0.102 ± 0.001 | 0.239 ± 0.018 | 0.266 ± 0.016 | 0.246 ± 0.002 | 0.287 ± 0.002 |
| 18 | 1-hexanol | 27.32 | 1319 | 3.809 ± 0.006 | 2.367 ± 0.097 | 6.383 ± 0.250 | 1.794 ± 0.067 | 3.570 ± 0.180 |
| 19 | E-3-eicosene | 27.49 | 1324 | 0.110 ± 0.005 | 0.132 ± 0.011 | 0.159 ± 0.029 | 0.105 ± 0.018 | 0.118 ± 0.019 |
| 20 | 2-methyl-1-decanol | 27.80 | 1333 | 0.329 ± 0.009 | 0.436 ± 0.019 | 1.341 ± 0.068 | 0.475 ± 0.012 | 0.513 ± 0.001 |
| 21 | Z-3-hexenol | 28.47 | 1352 | 1.488 ± 0.050 | 1.493 ± 0.057 | 3.262 ± 0.189 | 0.724 ± 0.006 | 1.150 ± 0.012 |
| 22 | allo-ocimene | 28.60 | 1355 | 0.555 ± 0.029 | 0.089 ± 0.007 | 0.334 ± 0.006 | 0.039 ± 0.001 | 0.088 ± 0.003 |
| 23 | p-cymene | 28.65 | 1357 | 0.089 ± 0.000 | 0.110 ± 0.010 | 0.198 ± 0.006 | 0.114 ± 0.007 | 0.157 ± 0.013 |
| 24 | 2-hexenol | 29.14 | 1371 | 2.015 ± 0.019 | 1.551 ± 0.254 | 5.163 ± 0.262 | 0.733 ± 0.030 | 2.553 ± 0.275 |
| 25 | nonaldehyde | 29.39 | 1378 | 1.091 ± 0.031 | 1.498 ± 0.041 | 1.598 ± 0.037 | 1.975 ± 0.079 | 1.844 ± 0.036 |
| 26 | acetic acid | 30.42 | 1408 | 0.196 ± 0.034 | 0.173 ± 0.017 | 0.8233 ± 0.076 | 0.268 ± 0.019 | 0.214 ± 0.012 |
| 27 | 1-octen-3-ol | 30.61 | 1413 | 0.495 ± 0.024 | 0.614 ± 0.009 | 0.959 ± 0.051 | 0.650 ± 0.003 | 0.782 ± 0.013 |
| 28 | 1,3-ditertarybutylbenzene | 30.75 | 1418 | 3.921 ± 0.288 | 4.228 ± 0.323 | 5.504 ± 0.873 | 3.374 ± 0.595 | 5.148 ± 0.624 |
| 29 | linalool oxide | 31.04 | 1426 | 12.646 ± 0.180 | 12.625 ± 0.381 | 3.441 ± 0.073 | 2.136 ± 0.037 | 2.094 ± 0.060 |
| 30 | benzaldehyde | 33.71 | 1507 | 1.397 ± 0.043 | 2.843 ± 0.086 | 2.826 ± 0.166 | 2.946 ± 0.156 | 1.929 ± 0.099 |
| 31 | l-linalool | 33.88 | 1512 | 15.727 ± 0.187 | 3.754 ± 0.081 | 1.948 ± 0.079 | 2.758 ± 0.035 | 4.788 ± 0.068 |
| 32 | 1-octanol | 34.21 | 1522 | 0.451 ± 0.012 | 0.495 ± 0.026 | 0.626 ± 0.033 | 0.604 ± 0.019 | 0.683 ± 0.034 |
| 33 | lilac aldehyde A | 34.32 | 1526 | 1.002 ± 0.010 | 1.119 ± 0.003 | 0.321 ± 0.005 | 1.588 ± 0.024 | 2.436 ± 0.024 |
| 34 | lilac aldehyde B | 34.84 | 1542 | 0.956 ± 0.007 | 1.097 ± 0.020 | 0.310 ± 0.005 | 1.561 ± 0.031 | 2.310 ± 0.041 |
| 35 | lilac aldehyde C | 35.06 | 1549 | 0.779 ± 0.016 | 0.886 ± 0.001 | 0.256 ± 0.007 | 1.289 ± 0.026 | 1.919 ± 0.010 |
| 36 | hotrienol | 35.80 | 1573 | 0.163 ± 0.018 | 4.198 ± 0.400 | 0.036 ± 0.021 | 2.832 ± 0.149 | 0.144 ± 0.013 |
| 37 | lilac aldehyde D | 35.88 | 1575 | 0.430 ± 0.005 | 0.583 ± 0.010 | 0.138 ± 0.002 | 0.761 ± 0.016 | 1.030 ± 0.008 |
| 38 | β-cyclocitral | 37.20 | 1618 | 0.032 ± 0.001 | 0.054 ± 0.001 | 0.109 ± 0.007 | 0.074 ± 0.002 | 0.070 ± 0.001 |
| 39 | phenylacetaldehyde | 37.36 | 1623 | 1.099 ± 0.072 | 2.441 ± 0.057 | 4.018 ± 0.043 | 2.208 ± 0.048 | 1.966 ± 0.067 |
| 40 | 2-methylbutrate | 37.48 | 1627 | 0.023 ± 0.002 | 0.112 ± 0.010 | 0.294 ± 0.020 | 0.043 ± 0.002 | 0.064 ± 0.004 |
| 41 | p-methylbenzaldehyde | 37.76 | 1636 | 0.264 ± 0.038 | 0.598 ± 0.101 | 0.784 ± 0.135 | 0.572 ± 0.114 | 0.509 ± 0.153 |
| 42 | Z-3-hexenyl angelate | 38.13 | 1649 | 0.192 ± 0.004 | 0.043 ± 0.003 | 0.103 ± 0.004 | 0.048 ± 0.001 | 0.068 ± 0.005 |
| 43 | salicylic aldehyde | 38.58 | 1664 | 0.072 ± 0.004 | 0.195 ± 0.002 | 0.054 ± 0.001 | 0.461 ± 0.008 | 0.116 ± 0.004 |
| 44 | 4-oxoisophorone | 39.05 | 1679 | 0.739 ± 0.022 | 0.414 ± 0.022 | 0.534 ± 0.018 | 1.048 ± 0.018 | 0.992 ± 0.053 |
| 45 | lilac alcohol A | 39.87 | 1707 | 0.623 ± 0.035 | 0.590 ± 0.050 | 0.189 ± 0.004 | 0.342 ± 0.006 | 0.930 ± 0.076 |
| 46 | epoxylinalool | 39.98 | 1710 | 1.346 ± 0.039 | 1.284 ± 0.083 | 0.329 ± 0.009 | 0.210 ± 0.003 | 0.203 ± 0.012 |
| 47 | lilac alcohol B | 40.51 | 1729 | 0.964 ± 0.040 | 1.299 ± 0.100 | 0.388 ± 0.008 | 0.525 ± 0.016 | 1.555 ± 0.114 |
| 48 | lilac alcohol C | 41.49 | 1763 | 0.852 ± 0.035 | 0.535 ± 0.030 | 0.217 ± 0.002 | 0.770 ± 0.019 | 1.376 ± 0.105 |
| 49 | methyl salicylate | 41.56 | 1766 | 2.125 ± 0.131 | 3.034 ± 0.058 | 2.555 ± 0.017 | 12.811 ± 0.298 | 4.683 ± 0.115 |
| 50 | β-phenethyl acetate | 42.37 | 1794 | 0.132 ± 0.001 | 0.281 ± 0.012 | 0.278 ± 0.009 | 0.908 ± 0.026 | 0.349 ± 0.006 |
| 51 | lilac alcohol D | 42.55 | 1800 | 1.797 ± 0.104 | 2.280 ± 0.205 | 1.044 ± 0.020 | 0.891 ± 0.033 | 3.379 ± 0.147 |
| 52 | geraniol | 42.74 | 1807 | 0.024 ± 0.000 | 0.009 ± 0.000 | 0.008 ± 0.001 | 0.009 ± 0.000 | 0.009 ± 0.001 |
| 53 | benzenemethanol | 43.71 | 1843 | 1.905 ± 0.037 | 1.978 ± 0.122 | 3.094 ± 0.052 | 2.124 ± 0.065 | 3.362 ± 0.092 |
| 54 | benzeneethanol | 44.79 | 1882 | 10.502 ± 0.364 | 25.032 ± 0.669 | 14.782 ± 0.212 | 31.453 ± 0.480 | 25.039 ± 0.972 |
| 55 | benzyl nitrile | 45.41 | 1905 | 0.008 ± 0.000 | 1.239 ± 0.022 | 0.027 ± 0.001 | 0.020 ± 0.001 | 0.013 ± 0.001 |
| 56 | 2-phenylbut-2-enal | 45.62 | 1913 | 0.107 ± 0.003 | 0.265 ± 0.008 | 0.120 ± 0.001 | 0.704 ± 0.020 | 0.384 ± 0.008 |
| 57 | β-lonone | 46.14 | 1933 | 0.070 ± 0.004 | 0.109 ± 0.004 | 0.214 ± 0.006 | 0.130 ± 0.003 | 0.140 ± 0.005 |
| 58 | 2-methylphenol | 46.75 | 1956 | 0.068 ± 0.003 | 0.083 ± 0.002 | 0.107 ± 0.002 | 0.090 ± 0.001 | 0.068 ± 0.002 |
| 59 | E-nerolidol | 47.92 | 2001 | 0.017 ± 0.001 | 0.030 ± 0.002 | 0.004 ± 0.000 | 0.016 ± 0.000 | 0.118 ± 0.003 |
| 60 | dimethyl salicylate | 48.83 | 2037 | 0.044 ± 0.004 | 0.031 ± 0.001 | 0.491 ± 0.031 | 0.212 ± 0.003 | 0.218 ± 0.008 |
| 61 | ethyl linalool | 50.58 | 2112 | 0.432 ± 0.007 | 0.461 ± 0.009 | 0.158 ± 0.004 | 0.595 ± 0.004 | 1.017 ± 0.016 |
| 62 | eugenol | 51.23 | 2154 | 0.155 ± 0.010 | 0.156 ± 0.020 | 0.623 ± 0.013 | 0.078 ± 0.001 | 0.126 ± 0.006 |
| 63 | 8-hydroxy-6,7-dihydrolinalool | 51.62 | 2180 | 0.355 ± 0.036 | 0.246 ± 0.016 | 0.046 ± 0.007 | 0.893 ± 0.066 | 0.442 ± 0.044 |
| 64 | methyl palmitate | 52.69 | 2267 | 0.094 ± 0.022 | 0.148 ± 0.027 | 0.288 ± 0.032 | 0.192 ± 0.015 | 0.318 ± 0.019 |
| 65 | 2-hydroxylinalool | 53.14 | 2307 | 0.408 ± 0.044 | 0.274 ± 0.023 | 0.160 ± 0.026 | 0.431 ± 0.029 | 0.744 ± 0.107 |
| 66 | 1,3-diacetylbenzene | 55.68 | 2496 | 0.115 ± 0.026 | 0.214 ± 0.050 | 0.191 ± 0.045 | 0.112 ± 0.025 | 0.213 ± 0.043 |
a Retention time (min); b linear retention indices calculated against n-alkanes (C7–C30); c relative contents (%); d standard error; e white miseon (A. distichum Nakai); f pink miseon (A. distichum for. lilacinum Nakai); g ivory miseon (A. distichum for. eburneum T.B. Lee); h blue miseon (A. distichum for. viridicalycinum T.B. Lee); i round miseon (A. distichum var. rotundicarpum T.B. Lee).
2.2. Multivariate Data Analyses of Volatile Components from Five Variants of A. distichum Flowers
To understand how the classification of volatile components emitted from five variants of A. distichum flowers could be expressed and correlated to morphological characteristics, we performed multivariate data analyses (PCA and PLS-DA) for the scents identified in Section 2.1. Volatile component fingerprinting of five variants of A. distichum flowers (white miseon, pink miseon, ivory miseon, blue miseon, and round miseon) (Figure S1) was carried out with PCA, a method of unsupervised multivariate projection, to effectively describe the dissimilarities on the basis of the volatile data patterns [29]. Mathematical methods of par-scaling and mean-centering the datasets resulting from the above samples to unit variance were performed using the SIMCA-P 14.1 software. Before multivariate data analyses, each peak with an identified volatile (Table S1) was normalized to the internal standard (1,2-dichlorobenzene-d4).
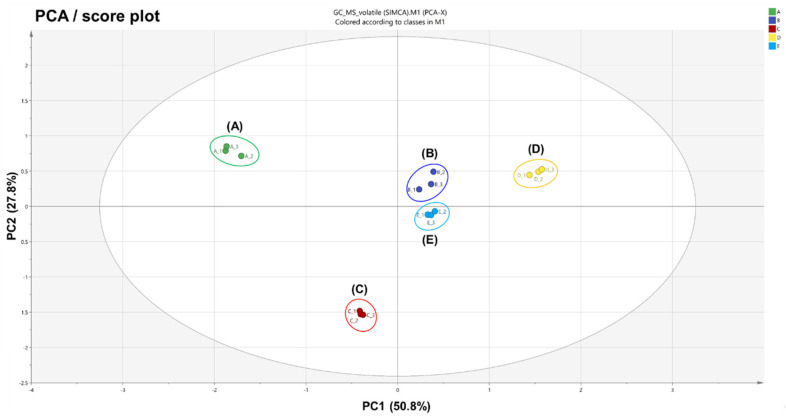
The PCA and PLS-DA results showed distinct separations between all five variants of A. distichum flowers, indicating that volatile components are related to the phenotype of each variant. The PCA result derived from the volatiles of each variant showed a score plot (Figure 1) together with a loading plot (Figure S1) consisting of PC1 (50.8%) and PC2 (27.8%). These described 78.6% of the total variance in the optimal separation of data. White miseon and ivory miseon and others were clearly separated by the principal PC1 and by the PC2, while white, pink, and blue miseon were clearly separated from the others. The loading plots (Figure S2) of PCA results were consistent with their respective score plots. The described dissimilarities of aroma characteristics relative to their correlative clusters highlighted variations in their fingerprints. On the basis of PC1 in the loading plot, white and ivory miseon showed higher contents of three oxygenated monoterpenes (l-linalool, linalool oxide, and epoxylinalool), two monoterpene hydrocarbons (β-myrcene and ocimene), and six alcohol derivatives (ethanol, 1-hexanol, 2-hexenal, 2-hexenol, (Z)-3-hexenol, and n-hexyl acetate) than others. On the other hand, one oxygenated monoterpene (hotrienol), three aromatic alcohols (benzeneethanol, methyl salicylate, and benzaldehyde), and one aldehyde (isovaleraldehyde) were higher in pink, blue, and round miseon compared with others. On the basis of PC2 in the loading plot, 10 oxygenated monoterpenes (epoxylinalool, hotrienol, lilac alcohol B–D, lilac aldehyde A–C, linalool oxide, and l-linalool), one monoterpene hydrocarbon (β-myrcene), two aromatic alcohols (benzeneethanol and methyl salicylate), and two alcohol derivatives (ethanol and nonaldehyde) were higher in white, pink, and blue miseon compared with others. In addition, two alcohol derivatives (2-hexenol and n-hexyl acetate) were highly concentrated in ivory miseon, round miseon, and blue miseon.
Figure 1.
Principal component analysis (PCA) score plot obtained from HS-SPME-GC-MS results on five variants of Abeliophyllum distichum flowers: (A) white miseon, (B) pink miseon, (C) ivory miseon, (D) blue miseon, (E) round miseon.
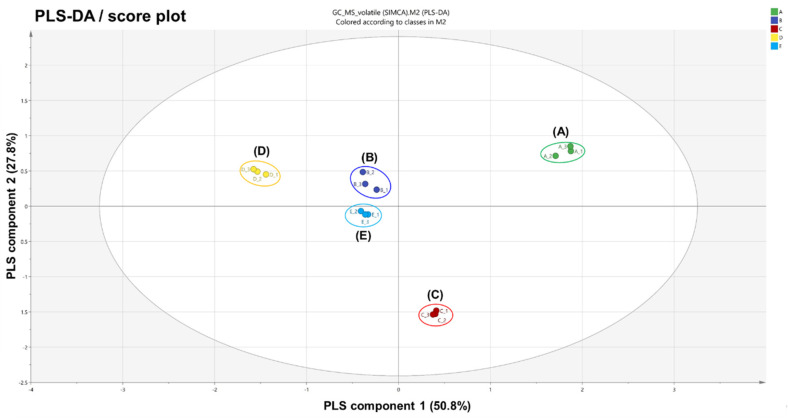
PLS-DA analysis was performed to further confirm the segregation of volatiles emitted from each variant [30]. Analogously, PLS-DA results (Figure 2 and Figure S3), in which the total variance was 78.6%, showed the same behavior as the PCA results. This alignment indicates that volatile fingerprints are deeply related to the morphological characteristics of A. distichum flowers.
Figure 2.
Partial least squares discriminant analysis (PLS-DA) score plot obtained from HS-SPME-GC-MS results on five variants of Abeliophyllum distichum flowers: (A) white miseon, (B) pink miseon, (C) ivory miseon, (D) blue miseon, (E) round miseon.
On the basis of the variable influence on projection (VIP; Table 2), we found that 30 volatiles from A. distichum flowers were higher than 0.7, and 18 scents showed VIPs larger than 1.0, which is the most influential value for this model [31]. Table 2 indicates that the scents of plants, especially oxygenated monoterpenes, were related to separation in each variant. These results suggest that morphological characteristics of each variant were related to the content of volatiles.
Table 2.
The variable influence on projection (VIP) values (>0.7) of the volatile components for the separation between the flowers in five variants of Abeliophyllum distichum in the PLS-DA-derived score plots.
| No. | Compound | RT | VIP Value | No. | Compound | RT | VIP Value |
|---|---|---|---|---|---|---|---|
| 1 | linalool oxide | 31.04 | 2.89 | 16 | benzaldehyde | 33.71 | 1.01 |
| 2 | benzeneethanol | 44.79 | 2.59 | 17 | benzenemethanol | 43.71 | 1.01 |
| 3 | methyl salicylate | 41.56 | 2.56 | 18 | n-hexyl acetate | 24.85 | 1.00 |
| 4 | l-linalool | 33.88 | 2.48 | 19 | lilac aldehyde C | 35.06 | 0.99 |
| 5 | hotrienol | 35.80 | 1.80 | 20 | epoxylinalool | 39.98 | 0.92 |
| 6 | ethanol | 11.91 | 1.65 | 21 | lilac alcohol C | 41.49 | 0.89 |
| 7 | lilac alcohol D | 42.55 | 1.60 | 22 | 1-hexanol | 27.32 | 0.88 |
| 8 | β-myrcene | 20.94 | 1.34 | 23 | 2-hexenol | 29.14 | 0.87 |
| 9 | 2-hexenal | 22.91 | 1.26 | 24 | isovaleraldehyde | 11.50 | 0.87 |
| 10 | ocimene-2 | 24.21 | 1.21 | 25 | 1,3-ditertarybutylbenzene | 30.75 | 0.86 |
| 11 | benzyl nitrile | 45.41 | 1.18 | 26 | isoamylalcohol | 21.98 | 0.76 |
| 12 | lilac aldehyde A | 34.32 | 1.13 | 27 | 4-oxoisophorone | 39.05 | 0.76 |
| 13 | lilac aldehyde B | 34.84 | 1.08 | 28 | lilac alcohol A | 39.87 | 0.75 |
| 14 | lilac alcohol B | 40.51 | 1.07 | 29 | ethyl linalool | 50.58 | 0.75 |
| 15 | 5-ethyl-2,2,3-trimethylheptane | 16.82 | 1.02 | 30 | Z-3-hexenol | 28.47 | 0.75 |
3. Materials and Methods
3.1. Plant Materials
The flowers of A. distichum were provided by the Miseonnamu-maeul Agricultural Association Corporation (CEO: Jongtae Woo), Goesan-gun, Chungcheongbuk-do, Republic of Korea, in March 2017. Five variants, white miseon (A. distichum Nakai, KHU-NPCL-201704-01), pink miseon (A. distichum for. lilacinum Nakai, KHU-NPCL-201704-02), ivory miseon (A. distichum for. eburneum T. B. Lee, KHU-NPCL-201704-03), blue miseon (A. distichum for. viridicalycinum T. B. Lee, KHU-NPCL-201704-04), and round miseon (A. distichum var. rotundicarpum T. B. Lee, KHU-NPCL-201704-05), were identified by Prof. Dae-Keun Kim, College of Pharmacy, Woosuk University, Jeonju, South Korea. Voucher specimens (KHU-NPCL-201704-01-05) have been deposited at the Natural Products Chemistry Laboratory, Kyung Hee University.
3.2. Volatile Component Analysis from Five Variants of A. distichum Flowers
Approximately 30 g of raw floral tissue of 5 variants of A. distichum were collected between 10:00 a.m. to 10:30 a.m. on 25 March 2017 (12 ± 1 °C; 46 ± 2% relative humidity). The moistened samples were immediately transported to the laboratory. Fresh tissue of 5 variants of A. distichum flowers (1.0 g) were transferred into 20 mL glass vials sealed by metal screw-caps with pre-notched Teflon silicone septa. Approximately 10 flowers were used for each sample. Then, 5 μL of deuterated 1,2-dichlorobenzene-d4 (1000 ppm in pyridine) was added in each sample as an internal standard. Volatile components were extracted by HS-SPME using a 2 cm 50/30 μm DVB/CAR/PDMS StableFlex fiber (Supelco, Bellefonte, PA, USA). The samples were incubated and shaken for 20 min (using a 10 s on/off cycle) at 50 °C, with the fiber introduced halfway through this period (TriPlus RSH with SPME module; Thermo Scientific, USA). Other SPME parameters were set as follows: extraction time: 40 min, desorption time: 2 min, pre-conditioning time: 5 min, post-conditioning time: 20 min. Subsequently, the fiber was inserted into the injector port of the GC (Thermo Scientific Trace 1300, Thermo Fisher Scientific Inc., USA) for 1 min at 250 °C. A 1 μL of sample was injected in split mode (20:1, v/v). The volatile compounds were separated using a DB-WAX column (60 m × 0.25 mm internal diameter × 0.5 μm film thickness). Helium was used as the carrier gas at a constant flow rate of 3.0 mL/min. The oven temperature profile was started at 40 °C for 3 min, and then was programmed to reach 250 °C at a rate of 4 °C/min, staying at 250 °C for 5.5 min. The GC-MS transfer line temperature was set to 270 °C. A triple quadrupole mass spectrometer (TSQ 8000, Thermo Fisher Scientific Inc., USA) operated in scan mode at 70 eV with the electron ionization (EI) source kept at 250 °C. Five scans per second were recorded over the mass range m/z 35–550. The identification of volatile compounds was confirmed by comparing their spectra and retention times with standards from the library NIST 2.0. Quantitative analysis by the peak area normalization method was conducted to determine the relative amounts using internal standard. Analysis of each sample was performed three times. Linear retention indices (LRI) were calculated for each respective mass spectrum using the following equation: LRI = 100 × n + [100 × (tx − tn)]/(tn+1 − tn), where x is the targeted compound x, n is the number of carbon atoms of the n-alkane eluted before x, n + 1 is the number of carbon atoms of the n-alkane eluted after x, tx is the retention time of x, tn is the retention time of n, and tn+1 is the retention time of n+1 [32].
3.3. Multivariate and Statistical Analysis
Raw chromatographic data acquired from triple quadrupole GC-MS analyses were processed by Xcalibur 3.1 software (Thermo Finnigan Corporation, San Jose, CA, USA), in which automatic peak detection and mass spectrum deconvolution (compound identification) were performed with references to library NIST 2.0. PCA and PLS-DA were then chosen to create a prediction model. SIMCA version 14.1 (Umetrics, Umeå, Sweden) and MetaboAnalyst 4.0 (http://www.metaboanalyst.ca) were initially employed to comprehend the relationship in terms of similarity or dissimilarity among groups of multivariate data. Statistical analysis was performed using a GraphPad Prism software version 7.00 (GraphPad Software, Inc., San Diego, CA, USA). Significance was estimated using repeated one-way analysis of variance (ANOVA) followed by Tukey’s test. Data were presented as mean ± standard error.
4. Conclusions
At present, five variants of this plant have been reported: white miseon (A. distichum Nakai), pink miseon (A. distichum for. lilacinum Nakai), ivory miseon (A. distichum for. eburneum T. B. Lee), blue miseon (A. distichum for. viridicalycinum T. B. Lee), and round miseon (A. distichum var. rotundicarpum T. B. Lee) [26,27,28]. The variants were classified only on the basis of morphological characteristics such as the color of the petals and sepals or the shapes of the fruit. There are many opinions on the taxonomic identities of variants of A. distichum, and some documents suggest that each variant was the same taxa [33]. Accordingly, a phytochemical investigation and chemical mapping of the variants is valuable.
In this study, we performed volatile component analysis of A. distichum flowers and multivariate data analyses to reveal the relation between fragments emitted from five variants of A. distichum flowers with their morphological characteristics. Sixty-six volatile components of this were plant identified and quantified by HS-SPME-GC-MS. Multivariate data analyses showed that almost all volatiles had unique patterns for each set of morphological characteristics, especially the color of the petals. These results suggest that the morphological characteristics of each variant were related to the content of the volatiles. Moreover, further studies are needed to understand the chemical origin of the morphological characteristics of each variant to reveal the dissimilarities of primary and secondary metabolites of these plants.
Acknowledgments
We thank the Miseonnamu-maeul agricultural association corporation (CEO: Jongtae Woo), Goesan-gun, Chungcheongbuk-do, Republic of Korea, for providing novel materials used for these experiments.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/10/2/224/s1: Table S1: The detected volatile components identified from the flowers in five variants of Abeliophyllum distichum using HS-SPME-GC-MS analysis. Table S2: Classification and odor notes of the detected volatile components identified from the flowers in five variants of Abeliophyllum distichum using HS-SPME-GC-MS analysis. Figure S1: Representative total ion chromatograms (TIC) for raw floral tissue of five variants of Abeliophyllum distichum: (A) white miseon, (B) pink miseon, (C) ivory miseon, (D) blue miseon, (E) round miseon. Figure S2: PCA score plot obtained from HS-SPME-GC-MS results on five variants of Abeliophyllum distichum flowers. Figure S3: PLS-DA score plot obtained from HS-SPME-GC-MS results on five variants of Abeliophyllum distichum flowers.
Author Contributions
Conceptualization: Y.-G.L. and N.-I.B.; methodology: Y.-G.L., W.-S.C., and S.-O.Y.; software: Y.-G.L., W.-S.C., and S.-O.Y.; validation: Y.-G.L.; formal analysis: Y.-G.L., W.-S.C., and S.-O.Y.; investigation: Y.-G.L., J.H.-B., and S.C.K.; resources: Y.-G.L., M.F., H.-G.K., J.H.-B., T.-H.Y., and Y.-H.L.; data curation: Y.-G.L. and S.C.K.; writing—original draft preparation: Y.-G.L.; writing—review and editing: N.-I.B.; visualization: Y.-G.L. and S.C.K.; supervision: N.-I.B.; project administration: Y.-G.L. and N.-I.B.; funding acquisition: N.-I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1A6A3A01100042).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Forbes A.M., Meier G.P., Haendiges S., Taylor L.P. Structure-Activity Relationship Studies of Flavonol Analogues on Pollen Germination. J. Agric. Food Chem. 2014;62:2175–2181. doi: 10.1021/jf405688d. [DOI] [PubMed] [Google Scholar]
- 2.Silva U.F.D., Borba E.L., Semir J., Marsaioli A.J. A simple solid injection device for the analyses of Bulbophyllum (Orchidaceae) volatiles. Phytochemistry. 1999;50:31–34. doi: 10.1016/S0031-9422(98)00459-2. [DOI] [Google Scholar]
- 3.Li Z.G., Lee M.R., Shen D.L. Analysis of volatile compounds emitted from fresh Syringa oblata flowers in different florescence by headspace solid-phase microextraction-gas chromatography-mass spectrometry. Anal. Chim. Acta. 2006;576:43–49. doi: 10.1016/j.aca.2006.01.074. [DOI] [PubMed] [Google Scholar]
- 4.Kamau E.C., Fedder B., Winter G. The Nagoya Protocol on Access to Genetic Resources and Benefit Sharing: What is new and what are the implications for provider and user countries and the scientific community. Law Env’t Dev. J. 2010;6:246–262. [Google Scholar]
- 5.Park J., Kim Y., Xi H., Jang T., Park J.H. The complete chloroplast genome of Abeliophyllum distichum Nakai (Oleaceae), cultivar Ok Hwang 1ho: Insights of cultivar specific variations of A. distichum. Mitochondrial DNA B. 2019;4:1640–1642. doi: 10.1080/23802359.2019.1605851. [DOI] [Google Scholar]
- 6.List of Endangered Wildlife, Ministry of Environment. [(accessed on 11 November 2019)]; Available online: https://species.nibr.go.kr/endangeredspecies.
- 7.Lee N.N., Moon H.K., Choi Y.E. Effect of LEDs on shoot multiplication and rooting of rare plant Abeliophyllum distichum Nakai. J. Plant Biotechnol. 2014;41:94–99. doi: 10.5010/JPB.2014.41.2.94. [DOI] [Google Scholar]
- 8.Lee N.N., Kim J.A., Kim Y.W., Choi Y.E., Moon H.K. Effect of explant’s position and culture method on shoot proliferation and micro-cuttings for a rare and endangered species, Abeliophyllum distichum Nakai. J. Plant Biotechnol. 2015;42:228–234. doi: 10.5010/JPB.2015.42.3.228. [DOI] [Google Scholar]
- 9.Kuwajima H., Takahashi M., Ito M., Wu H.X., Takaishi K., Inoue K. A quinol glucoside from Abeliophyllum distichum. Phytochemistry. 1993;33:137–139. doi: 10.1016/0031-9422(93)85409-K. [DOI] [Google Scholar]
- 10.Oh H., Kang D.G., Kwon T.O., Jang K.K., Chai K.Y., Yun Y.G., Chung H.T., Lee H.S. Four glycosides from the leaves of Abeliophyllum distichum with inhibitory effects on angiotensin converting enzyme. Phytother. Res. 2003;17:811–813. doi: 10.1002/ptr.1199. [DOI] [PubMed] [Google Scholar]
- 11.Li H.M., Kim J.K., Jang J.M., Cui C.B., Lim S.S. Analysis of the inhibitory activity of Abeliophyllum distichum leaf constituents against aldose reductase by using high-speed counter current chromatography. Arch. Pharm. Res. 2013;36:1104–1112. doi: 10.1007/s12272-013-0127-1. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y.G., Lee H., Jung J.W., Seo K.H., Lee D.Y., Kim H.G., Ko J.H., Lee D.S., Baek N.I. Flavonoids from Chionanthus retusus (Oleaceae) flowers and their protective effects against glutamate-induced cell toxicity in HT22 cells. Int. J. Mol. Sci. 2019;20:3517. doi: 10.3390/ijms20143517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee Y.G., Seo K.H., Lee D.S., Gwag J.E., Kim H.G., Ko J.H., Park S.H., Lee D.Y., Baek N.I. Phenylethanoid glycoside from Forsythia koreana (Oleaceae) flowers shows a neuroprotective effect. Braz. J. Bot. 2018;41:523–528. doi: 10.1007/s40415-018-0468-6. [DOI] [Google Scholar]
- 14.Kim Y.S., Maunder M. Plants in Peril, 24: Abeliophyllum distichum. Curtis’s Bot. Mag. 1998;15:141–146. doi: 10.1111/1467-8748.00155. [DOI] [Google Scholar]
- 15.Hong S.P., Han M.J. The floral dimorphism in the rare endemic plant, Abeliophyllum distichum NAKAI (Oleaceae) Flora. 2002;197:317–325. doi: 10.1078/0367-2530-00047. [DOI] [Google Scholar]
- 16.de Castro M.L., Priego-Capote F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A. 2010;1217:2383–2389. doi: 10.1016/j.chroma.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Waheed A., Hamid F.S., Madiha B., Seemab A., Naveed A., Nadia K., Sohail A., Saqib M., Hina G. GC-MS analysis of chemical components seed oil of Raphanus sativus L. MOJ Toxicol. 2019;5:112–118. [Google Scholar]
- 18.Schlossman M.L. The Chemistry and Manufacture of Cosmetics. Allured Publishing Corporation; Westport, CT, USA: 2009. p. 851. [Google Scholar]
- 19.Arthur C.L., Pawliszyn J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990;62:2145–2148. doi: 10.1021/ac00218a019. [DOI] [Google Scholar]
- 20.Zhu F., Xu J.Q., Ke Y.Y., Huang S.M., Zeng F., Luan T.G., Ouyang G.F. Applications of in vivo and in vitro solid-phase microextraction techniques in plant analysis: A review. Anal. Chim. Acta. 2013;794:1–14. doi: 10.1016/j.aca.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Romeo V., Verzera A., Zino M., Condurso C., Tripodi G. Headspace Volatiles of Vicia sativa L. (Leguminoseae) by Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry. J. Essent. Oil Res. 2009;21:33–35. doi: 10.1080/10412905.2009.9700101. [DOI] [Google Scholar]
- 22.Flavornet and Human Odor Space. [(accessed on 12 November 2019)]; Available online: http://www.flavornet.org/flavornet.html.
- 23.The Good Scents Company. [(accessed on 12 November 2019)]; Available online: http://www.thegoodscentscompany.com.
- 24.Baldwin I.T. Plant volatiles. Curr. Biol. 2010;20:R392–R397. doi: 10.1016/j.cub.2010.02.052. [DOI] [PubMed] [Google Scholar]
- 25.Zini C.A., Augusto F., Christensen E., Smith B.P., Caramao E.B., Pawliszyn J. Monitoring biogenic volatile compounds emitted by Eucalyptus citriodora using SPME. Anal. Chem. 2001;73:4729–4735. doi: 10.1021/ac0103219. [DOI] [PubMed] [Google Scholar]
- 26.Nakai T. Genus novum Oleaseaerum in Corea media inventum. Bot. Mag. Tokyo. 1919;33:153–154. doi: 10.15281/jplantres1887.33.392_153. [DOI] [Google Scholar]
- 27.Nakai T. Notulae ad plantas Japoniae et Koreae. Bot. Mag. Tokyo. 1922;36:19–27. doi: 10.15281/jplantres1887.36.422_en19. [DOI] [Google Scholar]
- 28.Lee T.B. New forms of Abeliophyllum distichum. Korean J. Plant Taxon. 1976;7:21–22. doi: 10.11110/kjpt.1976.7.1.021. [DOI] [Google Scholar]
- 29.Jonsson J., Eriksson L., Hellberg S., Lindgren F., Sjostrom M., Wold S. A multivariate representation and analysis of DNA sequence data. Acta Chem. Scand. 1991;45:186–192. doi: 10.3891/acta.chem.scand.45-0186. [DOI] [PubMed] [Google Scholar]
- 30.Xia Q., Mei J., Yu W.J., Li Y.F. High hydrostatic pressure treatments enhance volatile components of pre-germinated brown rice revealed by aromatic fingerprinting based on HS-SPME/GC-MS and chemometric methods. Food Res. Int. 2017;91:103–114. doi: 10.1016/j.foodres.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Eriksson L., Johansson E., Kettaneh-Wold N., Trygg J., Wikström C., Wold S. Multi-and Megavariate Data Analysis. 3rd ed. Umetrics Academy; Umeå, Sweden: 2006. Process analytical technology (PAT) and quality by design (QBD) pp. 323–355. [Google Scholar]
- 32.Zellner B.D.A., Bicchi C., Dugo P., Rubiolo P., Dugo G., Mondello L. Linear retention indices in gas chromatographic analysis: A review. Flavour Fragr. J. 2008;23:297–314. doi: 10.1002/ffj.1887. [DOI] [Google Scholar]
- 33.Kim D.K., Park K.R., Kim J.H. A taxonomic study of Abeliophyllum Nakai (Oleaceae) based on RAPD analysis. Korean J. Plant Res. 2002;15:26–35. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.