Abstract
This cross-sectional study investigated the Staphylococcus aureus (S. aureus) and methicillin-resistant S. aureus (MRSA) nasopharyngeal carriage epidemiology in Accra approximately five years post-pneumococcal conjugate vaccines introduction in the country. Archived nasopharyngeal swabs collected from 410 children aged under five years old were bacteriologically cultured. The resultant S. aureus isolates were subjected to antimicrobial susceptibility testing and screening for carriage of the mecA and LukF-PV (pvl) genes, following standard procedures. The data obtained were analyzed with Statistical Products and Services Solutions (SPSS) using descriptive statistics and Chi square tests of associations. The isolated bacteria decreased across coagulase-negative Staphylococci (47.3%, n = 194), S. aureus (23.2%, n = 95), Diphtheroids (5.4%, n = 22), Micrococcus species (3.7%, n = 15), Klebsiella pneumoniae (3.2%, n = 13), Moraxella species and Citrobacter species (1.5% each, n = 6), Escherichia coli, Enterobacter species, and Pseudomonas species (0.9% each, n = 2). The MRSA carriage prevalence was 0.49% (n = 2). Individuals aged 37–48 months recorded the highest proportion of S. aureus carriage (32.6%, 31/95). Resistance of S. aureus to the antibiotics tested were penicillin G (97.9%, n = 93), amoxiclav (20%, n = 19), tetracycline (18.9%, n = 18), erythromycin (5.3%, n = 5), ciprofloxacin (2.1%, n = 2), gentamicin (1.1%, n = 1), cotrimoxazole, clindamycin, linezolid, and teicoplanin (0% each). No inducible clindamycin resistance was observed for the erythromycin-resistant isolates. Three (3.2%) of the isolates were multidrug resistant, of which 66.7% (2/3) were MRSA. The pvl gene was associated with 59.14% (55/93) of the methicillin-sensitive S. aureus (MSSA) isolates, but was not detected among any of the MRSA isolates.
Keywords: nasopharyngeal carriage, Staphylococcus aureus, MRSA, children
1. Introduction
The human nasopharynx and oropharynx are anatomical sites colonized by a wide array of microorganisms—from commensals to potential pathogens—and a higher risk of transmission of these organisms occurs among children [1]. Staphylococcus aureus, Moraxella catarrhalis, Haemophilus influenzae, and Streptococcus pneumoniae are examples of these organisms and have been reported to occasionally cause local and disseminated infections—such as pneumonia, bacteraemia, endocarditis, osteomyelitis, meningitis, and skin and soft tissue infections—as a sequel to colonization of these and other anatomical sites [2,3]. Of these commensals, S. aureus and S. pneumoniae (also called pneumococcus) are the most clinically significant, given their high capacity to cause invasive diseases [4,5,6,7] concurrent with their predisposition to developing multidrug resistance [8,9,10,11,12,13,14]. However, of the two, S. pneumoniae is the predominant nasopharyngeal colonizer, and it frequently colonizes children below the age of five; this probably accounts for the high rates of its invasive infections in this population relative to adults [15,16]. As pneumococcal infections are preceded by their carriage, it was deemed necessary to develop and administer vaccines aimed at protecting against pneumococcal carriage [17,18,19]. One of these vaccines, which is currently widely used, is the Pneumococcal Conjugate Vaccine-13 (PCV-13) [17], and its introduction led to a decline in pneumococcal carriage [20].
The introduction of pneumococcal conjugate vaccines, notwithstanding its antecedent positive results recorded in relation to vaccine-captured S. pneumoniae serotypes, has been derailed by an upsurge in colonization with non-vaccine S. pneumoniae serotypes [21], as well as a perturbation of the nasopharyngeal microbial homeostasis of vaccinated persons and their associates [22,23,24]. A prominently feared perturbation is the insurgence of S. aureus in the nasopharynx based on its antagonistic relationship with S. pneumoniae [25,26,27,28,29,30], and as a consequence, an escalation of S. aureus respiratory tract infections (RTIs) and other diseases [31]. These fears are not unfounded, as S. aureus-related acute otitis media has been more frequently reported among PCV-vaccinated individuals [21,25,30]. Moreover, globally, RTIs are recognized key causes of death among adults and children [32]. In addition, S. aureus is a significant source of RTIs, and its carriage in the nasopharynx is noted as an imperative precursor of its invasive infections [33]. Of principal concern is the substantial proportion of S. aureus infections accounted for by the multidrug-resistant methicillin-resistant S. aureus (MRSA)—projected at 30.1% of community-acquired and 74.1% of nosocomial infections [34]. Infections with MRSA often occur in tandem with extended hospital stays, increased healthcare costs, and high mortality rates [35,36,37]. One report estimated that MRSA caused 80,000 invasive infections and more than 11,000 deaths in 2011 in the United States of America (USA) [38]. Consequently, PCV-13 introduction could potentially increase the population of reservoirs of S. aureus, MRSA, and other multidrug-resistant pathogens, who could serve as a conduit for transmission of these pathogens.
In May 2012, Ghana introduced PCV-13 in the Expanded Immunization Programme as part of efforts to lessen the menace of pneumococcal infections among children aged less than five years [39]; the epidemiology of pneumococcal nasopharyngeal carriage in relation to PCV-13 introduction in Ghana is widely documented [11,15,20,40,41,42]. These reports, however, failed to capture how these PCV-induced dynamics of pneumococcal epidemiology have influenced the nasopharyngeal epidemiology of other colonizers, particularly the S. pneumoniae-antagonistic S. aureus. It is noteworthy that an in-depth understanding of the PCV-induced evolution of nasopharyngeal S. aureus epidemiology is an important step in mounting a robust public health strategy to combat both S. aureus and S. pneumoniae infections, the brunt of which is borne by children with young immune systems [16,43,44,45]. In Ghana, this is very significant given the several MRSA outbreaks in the country since 2012 [46], the same year of inception of PCV-13 vaccinations in the country. Hence, the aim of this study was to investigate the nasopharyngeal carriage of S. aureus and MRSA among children below five years of age in Accra in the conjugate vaccine era, focusing on the prevalence, antibiogram, and Panton-Valentine leukocidin (pvl) gene carriage of S. aureus and MRSA.
2. Methods
2.1. Study Site, Design, and Sample Processing
This study was carried out in the Accra metropolis of the Greater Accra Region of Ghana. The metropolis falls within the coastal belt, which has humid and warm climatic conditions. Accra has the highest population density when compared to other districts in the country. According to the 2010 population and housing census, the inhabitants of Accra numbered 1,665,086, representing 42% of the region’s total inhabitants (http://www.statsghana.gov.gh/docfiles). In this study, archived nasopharyngeal swab (NPS) specimens were bacteriologically cultured; these specimens were from a previous cross-sectional survey conducted about five years into the inception of PCV-13 vaccination in the country (from September to December 2016) among 410 children aged under five years [47]. The participants of that study were enrolled from seven randomly selected schools in the metropolis, spanning across the suburbs Kaneshie, Mamprobi, Korle-Gonno, and Palladium (Ashiedu Keteke).
One NPS specimen was collected from each of the study participants by means of FlOQSwabs (Copan Flock Technologies, Italy) swab sticks. Each of these was directly inoculated into its corresponding uniquely labeled vial containing skim milk tryptone glucose glycerol (STGG) transport medium (Oxoid, Basingstoke, UK) and transported to the Department of Medical Microbiology’s research laboratory for storage at −80 °C. The samples were analyzed for the presence of S. pneumoniae and archived at −80 °C for use in the current study.
In the current study, which focused on isolating S. aureus and bacteria other than S. pneumoniae, these archived samples were retrieved from the −80 °C freezer, thawed, brought to room temperature, and vortexed. For each specimen, a loopful was inoculated into sterile tryptic soy broth (Oxoid, Ltd. Basingstoke, UK) and incubated for 48 h at 37 °C. After 48 h of incubation, a loopful of the suspension was plated on sterile blood agar supplemented with 5% sheep blood, MacConkey agar, and mannitol salt agar (Oxoid, Ltd. Basingstoke, UK) and incubated aerobically for 18–24 h.
2.2. Isolation and Phenotypic Identification of Bacteria
Identification of the bacteria isolated was done using standard methods, including colony morphology, Gram staining, catalase testing, tube coagulase testing in rabbit-citrate-plasma (Becton and Dickinson ®; Heidelberg, Germany), and growth on mannitol salt agar. Gram-positive small to large yellow colonies from mannitol salt agar (Oxoid, Ltd. Basingstoke, UK) were sub-cultured onto blood agar (Oxoid, Hampshire, UK) plates, followed by incubation under aerobic conditions at 37 °C for 18 to 24 h. Catalase testing was performed on large, round, golden-yellow colonies, most of which displayed β-hemolysis on the blood agar plates, to identify staphylococcal isolates. Differentiation of the catalase-positive isolates into S. aureus and coagulase-negative Staphylococci was done via coagulase testing. Catalase- and coagulase-positive mannitol-fermenting Gram-positive cocci were identified as S. aureus and confirmed as such via spa gene screening.
2.3. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility tests were performed on the S. aureus isolates using Kirby Bauer’s disc diffusion method [28]. A 0.5% McFarland equivalent suspension of each isolate was inoculated on a Muller-Hinton agar (MHA) (Oxoid, Hampshire, UK) plate, followed by incubation at 37 °C for 18–24 h. For each S. aureus isolate, within 15 min following the adjustment of the turbidity of the inoculum to 0.5 McFarland suspension using a nephelometer (BD Phoenix Spec TM, Becton, Dickinson and Company, Franklin Lakes, NJ, USA), a sterile cotton swab was dipped into the adjusted suspension and evenly streaked across the entire surface of a sterile and dry Muller-Hinton agar plate with the purpose of obtaining a semi-confluent growth post-incubation.
The antimicrobials used for susceptibility testing included erythromycin (15 μg), clindamycin (2 μg), penicillin G (10 Units), tetracycline (30 μg), cefoxitin (30 μg), cotrimoxazole (1.25/23.75 μg), ciprofloxacin (5 μg), amoxiclav (20/10 μg), teicoplanin (30 μg), linezolid (30 μg), and gentamicin (10 μg), all of BD BBLTM Sensi-Disc Antimicrobial Susceptibility Test Disc. MRSA were screened using cefoxitin (30 μg) discs by the disc diffusion technique [28]. Cefoxitin zones of inhibition greater than or equal to 22 mm and less than 22 mm were considered phenotypically to indicate methicillin-sensitive S. aureus (MSSA) and MRSA (confirmation of which was made via screening for mecA gene carriage), respectively. The results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) [28] guidelines. S. aureus ATCC 25923 was taken as the positive control strain. Multidrug resistance (MDR) attribute determination was based on the resistance of an organism to three or more different classes of antimicrobials [13]. Isolates that were erythromycin-resistant and clindamycin-sensitive were confirmed for inducible resistance by use of the double disc-zone test (D-zone test). Clindamycin and erythromycin discs were placed at proximities of 15–26 mm from one another on the MHA plates, and these were inspected after 18 h at 37 °C. Flattening of the inhibition zone (D shape) around clindamycin was considered to indicate inducible clindamycin resistance. This test permits the identification of three different phenotypes:
(a). Inducible MLSB (macrolide-lincosamide-streptogramin B) phenotype: iMLSB S. aureus isolates show resistance to erythromycin (zone size of ≤13 mm) and sensitivity to clindamycin (zone size of ≥21 mm), giving a D-shaped zone of inhibition around clindamycin with flattening near the erythromycin disc (D test positive).
(b). Constitutive MLSB phenotype: cMLSB S. aureus isolates show resistance to both erythromycin (zone size of ≤13 mm) and clindamycin (zone size of ≤14 mm).
(c). Methicillin-sensitive (MS (macrolide–streptogramin)) phenotype: These S. aureus isolates show resistance to erythromycin (zone size of ≤13 mm) and sensitivity to clindamycin (zone size of ≥21 mm), but without the D-shaped zone indicative of iMLSB (D test negative).
Additional information on the phenotypic groupings of the D test is presented in Table 1.
Table 1.
Phenotypic groupings and their features in the D test.
| Phenotype | Resistance Phenotype | CLI Result | ERY Result | Double Disc Diffusion Test Description |
|---|---|---|---|---|
| D+ | Inducible MLSB | S | R | Flattened, D-shaped clear zone around the CLI disc close to resistant ERY disc |
| D− | MS | S | R | Susceptible zone around the CLI disc and resistant zone around ERY disc |
| R | Constitutive MLSB | R | R | Resistance zones around the CLI and ERY discs |
| S | No Resistance | S | S | Susceptible clear zones around both discs |
S, sensitive; R, resistant; D+, D test positive; D−, D test negative; CLI, clindamycin; ERY, erythromycin; MLSB = macrolide-lincosamide-streptogramin B; MS, macrolide-streptogramin.
2.4. Molecular Analysis
Pure S. aureus colonies were put in 50 µL of phosphate-buffered saline (PBS) in Eppendorf tubes. The isolates in the Eppendorf tubes were stored at −80 °C until DNA was extracted. DNA was extracted from 95 S. aureus isolates using commercial kits from Zymo Research Quick-DNATM Fungal/Bacterial Mini-prep (Zymo Research Corp., Irvine, CA, USA), following the manufacturer’s instructions.
Multiplex PCR was performed to detect mecA, spa, and pvl genes. This was done following the method described by Larsen et al. [48]. Each PCR reaction contained 10 mM mecA primers, 10 mM spa primers, and 10 mM pvl primers, multiplex PCR Master Mix (New England BioLabs One Tag Quick-Load 2 × Master Mix with Standard Buffer, Beverly, MA, USA), and 1 lL of DNA template preparation. The reaction was performed in an MJ Research PTC-200 Peltier Thermal Cycler. The PCR amplicons were visualized using 2% Agarose, and band size was compared to a 100 bp DNA marker (New England BioLabs, Beverly, MA, USA). The positive controls used were MRSA ATCC 33591, which is positive for mecA and spa genes, and MSSA ATCC 25923, which is also positive for spa and pvl genes; nuclease-free water was used as a negative control. Table 2 provides details of the primers used in the multiplex PCR.
Table 2.
List of primers used for the multiplex PCR detection of spa, pvl, and mecA genes.
| Gene | Primer | Sequence (5′–3′) | Size (bp) | Reference |
|---|---|---|---|---|
| mecA |
mecA P4 mecA P7 |
5′-TCCAGATTACAACTTCACCAGG-3′ 5′-CCACTTCATATCTTGTAACG-3′ |
162 | Oliveira and de Lencastre [49] |
| spa * |
spa 113F spa 1514R |
5′-TAAAGACGATCCTTCGGTGAGC-3′ 5′-CAGCAGTAGTGCCGTTTGCTT-3′ |
180–600 | Harmsen et al. [50] |
| pvl |
pvlF pvlR |
5′-GCTGGACAAAACTTCTTGGAATAT-3′ 5′-GATAGGACACCAATAAATTCTGGATTG-3′ |
83 | Deurenberg et al. [51] |
* Its amplicons are of variable sizes (180–600 bp) [52].
2.5. Data Analysis
Data were entered into a Microsoft Excel spreadsheet, then imported into Statistical Products and Services Solutions (SPSS) software version 22 and analyzed. Descriptive statistics were used in computing the S. aureus carriage prevalence per age group and gender, as well as antibiotic resistance rates. Tests of associations between S. aureus carriage and factors such as age and gender were performed using independent-samples Chi-square tests.
2.6. Ethical Approval
Ethical approval for this study was obtained from the Ethical and Protocol Review Committee of the College of Health Sciences, University of Ghana (Protocol Identification Number: CHS-Et/M.9-P4.3/2015-2016).
3. Results
3.1. Demographics of the Study Population
A total of 410 specimens collected from children under five years of age, who were recruited in a previous study focused on the epidemiology of S. pneumoniae nasopharyngeal colonization among the study participants [47], were cultured for the isolation of S. aureus. As presented in Table 3, the study participants comprised 51.2% (210/410) males and 48.8% (200/410) females. The mean age of the children sampled was 38.8 months. All 410 participants had been vaccinated with the PCV-13 at ages 6, 10, and 14 weeks from birth, through the Ministry of Health/Ghana Health Service Expanded Programme on Immunization. In the previous study [47], their claims of vaccination were verified by inspection of their vaccination cards. In total, only 61 of the total 410 children were aged ≤2 years.
Table 3.
Carriage prevalence of Staphylococcus aureus by age group in children aged ≤5 years attending nursery and kindergarten facilities in Accra.
| Age Group (Months) | Age Group (Years) | Number of Children | Carriage Prevalence of S. aureus | ||
|---|---|---|---|---|---|
| Males | Females | Total (%) | |||
| 0–12 | 0–1 | 2 | 3 | 5 (1.2) | 3 (3.2%) |
| 13–24 | 1.1–2 | 31 | 25 | 56 (13.7) | 14 (14.7%) |
| 25–36 | 2.1–3 | 86 | 86 | 172 (42.0) | 28 (29.5%) |
| 37–48 | 3.1–4 | 70 | 59 | 129 (31.5) | 31 (32.9%) |
| 49–60 | 4.1–5 | 21 | 27 | 48 (11.7) | 19 (20%) |
| Total | 210 | 200 | 410 | 95 | |
3.2. Nasopharyngeal Staphylococcus aureus Carriage
The S. aureus and MRSA nasopharyngeal carriage prevalence rates among the study participants were 23.2% (95/410) and 0.49% (2/410), respectively. Females recorded a higher S. aureus carriage prevalence of 24.5% (49/200) than did males, with 21.90% (46/210), but this difference was not statistically significant (χ2[1, N = 410] = 0.388, p = 0.534). The youngest S. aureus carrier was 6 months old, while the oldest was 60 months old. When S. aureus carriage was stratified by age group, differences in carriage were statistically significant (χ2[4, N = 410] = 15.82, p = 0.003), and the age group of 37–48 months (3.1–4.0 years) recorded the highest carriage prevalence of 32.6% (n = 31). The two MRSA isolates were found in females aged 36 months (3 years) and 52 months (4 years). The isolation, identification, and characterization of Streptococcus pneumoniae present in these samples were reported previously [47]. Table 3 above describes the carriage prevalence and distribution of S. aureus per age group and gender, and Table 4 describes the prevalence of pathogens (other than S. pneumoniae) isolated from the nasopharyngeal swab samples.
Table 4.
Bacterial pathogens isolated from the nasopharyngeal swab samples.
| Bacterial Pathogen | Number | Prevalence (%) |
|---|---|---|
| Coagulase negative Staphylococci | 194 | 47.3 |
| Staphylococcus aureus | 95 | 23.2 |
| Diphtheroids | 22 | 5.4 |
| Micrococcus species | 15 | 3.7 |
| Moraxella species | 6 | 1.5 |
| Klebsiella pneumoniae | 13 | 3.2 |
| Citrobacter species | 6 | 1.5 |
| Escherichia coli | 2 | 0.9 |
| Enterobacter species | 2 | 0.9 |
| Pseudomonas species | 2 | 0.9 |
3.3. Antimicrobial Susceptibility Profile of the S. aureus Isolates
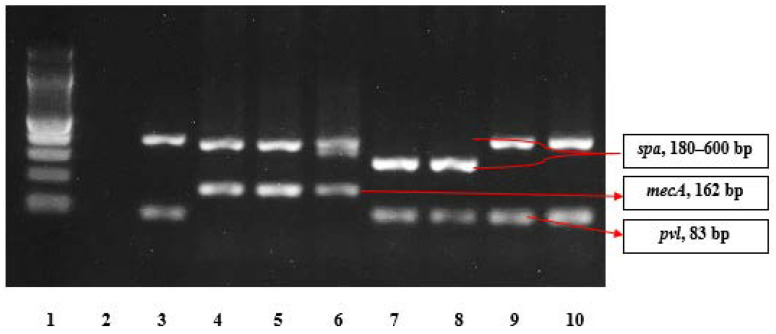
The resistance of S. aureus to the antimicrobials tested decreased across penicillin G (97.9%, n = 93), amoxiclav (20%, n = 19), tetracycline (18.9%, n = 18), erythromycin (5.3%, n = 5), cefoxitin and ciprofloxacin (2.1% each, n = 2), and gentamycin (1.1%, n = 1). All the isolates were susceptible to cotrimoxazole, clindamycin, linezolid, and teicoplanin. The two MRSA isolates were resistant to penicillin G, amoxiclav, tetracycline, and cefoxitin. With the MSSA isolates, resistance was observed against penicillin G (97.8%, n = 91), amoxiclav (18.3%, n = 17), tetracycline (17.2%, n = 16), erythromycin (5.4%, n = 5), ciprofloxacin (2.2%, n =2), and gentamicin (1.1%, n = 1). Multidrug resistance (MDR) was detected in 3.2% (n = 3) of the isolates; these exhibited resistance to tetracycline, penicillin, and amoxiclav. Of the three MDR isolates, 66.7% (n = 2) were MRSA, and 33.3% (n = 1) were MSSA. All the erythromycin-resistant isolates lacked inducible clindamycin resistance and showed the phenotypic D test phenomenon of the macrolide–streptogramin phenotype. The two MRSA isolates were mecA gene-positive, but pvl gene-negative; 59.14% (n = 55) of the MSSA isolates were pvl gene-positive; and all 95 S. aureus isolates were spa gene-positive. The results of the molecular analysis are presented in Figure 1.
Figure 1.
The agarose gel electrophoresis pattern for amplification products of spa, mecA, and pvl genes using multiplex PCR (Lane 1, 100 bp ladder; Lane 2, negative control; Lane 3, positive control for methicillin-sensitive S. aureus (MSSA) (ATCC 25923); Lane 4, positive control for methicillin-resistant S. aureus (MRSA) (ATCC 33591); Lanes 5–10, Staphylococcus aureus isolates).
4. Discussion
The human nasopharynx is colonized by a wide array of microorganisms, including Staphylococcus aureus, Moraxella catarrhalis, Haemophilus influenzae, and Streptococcus pneumoniae, of which S. aureus and S. pneumoniae seem the most clinically significant [2,3,4,5,6,7,12,13,14]. Attempts to control infections of the predominant nasopharyngeal colonizer, S. pneumoniae, via vaccination with PCVs have been met with concerns regarding potential disruption of the nasopharyngeal microbial homeostasis to favor S. aureus colonization [22,23,24,25,26,27,29,53]. To contribute data to guide deliberations on the matter, our aims were to investigate the nasopharyngeal carriage of S. aureus and MRSA (in the context of other nasopharyngeal colonizers) among children below five years of age in Accra approximately five years post-PCV introduction in the country and to report on the prevalence, antibiogram, and pvl gene carriage of S. aureus and MRSA.
The overall S. aureus nasopharyngeal carriage prevalence of 23.2% is comparable to what has been reported previously in Ghana in the nasopharynx of HIV-infected children below five years of age (22.03%) [12] and in the anterior nares of the general population (21%) [54] and children with sickle cell disease (33.3%) [14], but it is heterogeneously lower (44.9%) [13] and higher (8%) [55] than the prevalence among HIV-infected patients post-PCV introduction. It is also comparable to what has been reported for nasopharyngeal carriage in other countries: in The Gambia (20–25.9%) [56,57], Portugal (21.6%) [58], and the Netherlands (23.2%) [25]. However, the post-vaccination carriage prevalence recorded among apparently healthy children in the current study is slightly lower than what was reported in Iran (29.6%) [59], Belgium (34%) [60], Vietnam (30.6%) [61], and The Gambia (30.6%) [62], but higher than that reported in Ethiopia (10.3%) [3]. In the Ethiopian study, Assefa et al. [3] used a relatively smaller sample size of 234, and that might account for the difference in the carriage prevalence reported. As the S. aureus nasopharyngeal carriage prevalence recorded in the current study is comparable to the nasal carriage prevalence of the organism recorded in a number of studies conducted in the country post-PCV introduction, it could be discerned that the proportion of S. aureus in the nasopharynx seems to be increasing, and this is suggestive that PCV-13 introduction may have played a role, even if infinitesimal. This is because it is the anterior nares that is the ecological niche for S. aureus, and not the nasopharynx; hence, the nasopharyngeal carriage prevalence of the organism is expected to be strikingly lower than its carriage in the anterior nares. In fact, PCV-13 introduction seems a reasonable hypothesis to explain the 21% nasopharyngeal carriage prevalence reported in a study by Sampane-Donkor et al. [12] among HIV-infected children post-PCV introduction, despite the fact that HIV infection is a risk factor for S. aureus carriage [13]. Even though additional studies would be needed to exhaustively ascertain whether S. aureus has become established in the nasopharynx, the extrapolation made is further guided by the report of another study conducted by Adiku et al. [63] prior to PCV-13 introduction in Ghana. The researchers reported a 14.8% nasopharyngeal carriage rate of S. aureus among children under five years of age. However, five years after PCV-13 was introduced in Ghana, this study observed a marked increase in S. aureus prevalence in the nasopharynx (23.2%). This finding is consistent with what has been reported in areas where PCV-13 has been introduced [29,64]. The phenomenon could be attributable to the removal of the vaccine serotypes of S. pneumoniae which regulate the normal flora in the nasopharynx [25,64]. This regulation is carried out through the production of H2O2, which is bactericidal to S. aureus [29,65,66]. There are 94 serotypes of S. pneumoniae, but only 13 serotypes are present in the current vaccine. Past studies have shown that vaccine-type strains are those found to correlate with S. aureus carriage, legitimizing the elevated concern that the introduction of the vaccine would indirectly lead to a rise in S. aureus carriage and infections [4,5,29,67]. These concerns notwithstanding, as PCV-13 has been instrumental in controlling pneumococcal carriage and fatal infections in this immunologically vulnerable population, it would be premature to call for its withdrawal. Rather, there is a need to brainstorm on how best to fuse its use with curbing S. aureus establishment in the nasopharynx.
In this study, high resistance rates to penicillin (98%), amoxiclav (20%), and tetracycline (18.9%) were observed. The high penicillin non-susceptibility observed in this study is consistent with what has been reported previously in Ghana (96–100%) [12,13,14,54,55]. This high prevalence might be due to the fact that HIV-infected individuals and sickle cell disease patients are more exposed to antimicrobials, as well as resistant microbes, owing to their health status [32]. Ampicillin, like its contemporary beta-lactam antibiotic, penicillin, had a high resistance prevalence (100%), which is consistent with what has been reported elsewhere [3]. This observation may be due to the fact that penicillin and ampicillin have been on the market for a long time, are inexpensive, and could easily be obtained over the counter (although being prescription drugs); hence, they may have been misused greatly. In fact, MSSA isolated from urban areas in Africa are known to be highly penicillin-resistant (73.7–100%) [68,69]. The rate of resistance to amoxiclav observed in the current study exceeded what had been reported previously by Risk et al. [70] in The Gambia. The difference could be due to the use of amoxiclav as a first-line presumptively prescribed antibiotic in Ghana [71]. It is noteworthy that there was a marked reduction in tetracycline resistance (18.9%) in this study as compared to a higher resistance rate of 82% reported previously by Donkor et al. [72]. In addition, the 100% susceptibility of S. aureus to cotrimoxazole, clindamycin, linezolid, and teicoplanin observed agrees with findings from preceding studies carried out in Ghana [54,73]. This could be due to low or no exposure of the study participants to cotrimoxazole and clindamycin, and also because the drugs linezolid and teicoplanin are not easily accessible and affordable. Sedighi et al. [59] recommended that clindamycin or cotrimoxazole (trimethoprim–sulfamethoxazole) could be used in mild to moderately severe diseases caused by community-acquired MRSA. The double disc diffusion test (D test) was negative for erythromycin-resistant strains in this study, which is in contrast with the report by Sedighi et al. [59]. It is recommended that with erythromycin-resistant strains of S. aureus, D testing should always be carried out for the detection of inducible clindamycin resistance.
The 3.2% multidrug resistance (MDR) recorded in this study was below the previously reported 6.0% in Ghana by Egyir et al. [54] and 16.7% by Sampane-Donkor et al. [12] among HIV-infected children (which could be due to frequent exposure to antimicrobial agents as prophylaxes). The low prevalence of MRSA carriage (0.49%) and low proportion of MRSA among S. aureus isolates (2.1%, n = 2) are consistent with the low carriage rates reported in the country (0–3.4%) [12,13,14,54,55]. This could be ascribed to the low intake of antimicrobial agents like fluoroquinolones (such as levofloxacin or ciprofloxacin, exposure to which enhances MRSA isolation) [26,74] and third-generation cephalosporins (ceftazidime) in the community setting in Ghana, owing to their relatively high costs and characteristic prescription as therapeutic agents for acute infections [54,72]. Cumulative occurrence of MRSA with increasing use of ceftazidime, fluoroquinolones, and co-amoxiclav has also been reported [75].
Sub-Saharan Africa is a known hotspot for pvl-carrying MSSA at rates ranging from 17% to 74% [76], thus predisposing populations in these regions to S. aureus that could cause polymorphonuclear leucocyte lysis and tissue necrosis. In the present study, 59.14% of the MSSA isolates harbored the pvl gene, and this is consistent with the 58% prevalence reported previously in Kumasi, Ghana [73]. Nonetheless, the finding in this study is in sharp contrast to what have been reported in Europe (0.9–1.4%) [60,77]. The two MRSA isolates recovered in this study did not harbor the pvl gene, and this mirrors what was reported previously by Egyir et al. [54] in Ghana.
One possible limitation of the current study is that as its parent study primarily focused on S. pneumoniae carriage, sampling was done from the nasopharynx, the ecological niche of S. pneumoniae, without sampling from the anterior nares (the ecological niche of S. aureus), consequently narrowing the robustness with which inferences could be drawn from the data on both nasal and nasopharyngeal S. aureus carriage for each study participant. Moreover, a control group was not recruited. However, the extent of these limitations is minimal, as somewhat contemporary data on nasal carriage were available from other studies in the region, which allowed for a measure of extrapolation with the results of the study.
5. Conclusions
It is concluded that the nasopharyngeal carriage prevalence of S. aureus was high, with a small proportion of these colonizers being MRSA, indicating a possible establishment of S. aureus in the nasopharynx of individuals vaccinated with PCV-13. Also, antimicrobial resistance was generally low among the isolates, and inducible clindamycin resistance was absent. Furthermore, a high proportion of the isolates harbored the Panton-Valentine leukocidin gene. The findings of this study highlight the need to study the possible effects of PCV-13 introduction on nasopharyngeal carriage of S. aureus among other at-risk populations, such as HIV-infected and sickle cell disease patients.
Acknowledgments
The authors acknowledge the contributions of the study participants, as well as the technical staff of the Department of Medical Microbiology and the assistance received from Elizabeth Y. Tettey, Georgina Tetteh-Ocloo, Prince J. Pappoe-Ashong, Marjorie Quarchie, and Derrick Opoku.
Author Contributions
Conceptualization, N.T.K.D.D.; methodology, N.T.K.D.D., J.A.O., M.-M.O., P.B.T.-Q., F.C.N.K., B.E. and E.S.D., software, N.T.K.D.D., J.A.O., M.-M.O., P.B.T.-Q., F.C.N.K., B.E. and E.S.D.; validation and formal analysis, N.T.K.D.D., M.-M.O., J.A.O., F.C.N.K., P.B.T.-Q., J.A., K.K.A.-O., B.E. and E.S.D.; investigation, M.-M.O., N.T.K.D.D. and J.A.O.; resources, N.T.K.D.D., M.-M.O., J.A.O., P.B.T.-Q., J.A., K.K.A.-O., E.S.D. and B.E.; data curation, N.T.K.D.D., J.A.O., P.B.T.-Q., M.-M.O., F.C.N.K. and E.S.D.; writing—original draft preparation, N.T.K.D.D., M.-M.O., J.A.O., F.C.N.K., E.S.D., P.B.T.-Q., J.A., K.K.A.-O. and B.E.; writing—review and editing, N.T.K.D.D., M.-M.O., J.A.O.; F.C.N.K., E.S.D.; P.B.T.-Q., J.A., K.K.A.-O. and B.E.; visualization, N.T.K.D.D., J.A.O., P.B.T.-Q., F.C.N.K., E.S.D. and B.E.; supervision, N.T.K.D.D. and J.A.O.; project administration, N.T.K.D.D. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this work was obtained from ORID (University of Ghana, Legon) and the Hospital Acquired Infection (HAI) Ghana Project.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethical and Protocol Review Committee of the College of Health Sciences, University of Ghana (Protocol Identification Number: CHS-Et/M.9-P4.3/2015-2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author via ntkddayie@ug.edu.gh.
Conflicts of Interest
The authors have no conflicting interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thapa S., Gokhale S., Sharma A.L., Sapkota L.B., Ansari S., Gautam R., Shrestha S., Neopane P. Burden of bacterial upper respiratory tract pathogens in school children of Nepal. BMJ Open Respir. Res. 2017;4:e000203. doi: 10.1136/bmjresp-2017-000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leiberman A., Dagan R., Leibovitz E., Yagupsky P., Fliss D.M. The bacteriology of the nasopharynx in childhood. Int. J. Pediatr. Otorhinolaryngol. 1999;49:S151–S153. doi: 10.1016/S0165-5876(99)00151-2. [DOI] [PubMed] [Google Scholar]
- 3.Assefa A., Gelaw B., Shiferaw Y., Tigabu Z. Nasopharyngeal carriage rate and antimicrobial susceptibility pattern of potential pathogenic bacteria among pediatric outpatients at Gondar University teaching hospital, Ethiopia. J. Infect. Dis. Ther. 2013;21:109. doi: 10.4172/jidt.1000109. [DOI] [PubMed] [Google Scholar]
- 4.Brogden K.A., Guthmiller J.M., E Taylor C. Human polymicrobial infections. Lancet. 2005;365:253–255. doi: 10.1016/S0140-6736(05)70155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pettigrew M.M., Gent J.F., Revai K., Patel J.A., Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg. Infect. Dis. 2008;14:1584–1591. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Q., Almudervar A., Casey J.R., Pichichero M.E. Nasopharyngeal bacterial interactions in children. Emerg. Infect. Dis. 2012;18:1738–1745. doi: 10.3201/eid1811.111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thevaranjan N., Whelan F.J., Puchta A., Ashu E., Rossi L., Surette M.G., Bowdish D.M.E. Streptococcus pneumoniae colonization disrupts the microbial community within the upper respiratory tract of aging mice. Infect. Immun. 2016;84:906–916. doi: 10.1128/IAI.01275-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han L.L., McDougal L.K., Gorwitz R.J., Mayer K.H., Patel J.B., Sennott J.M., Fontana J.L. High frequencies of clindamycin and tetracycline resistance in methicillin-resistant Staphylococcus aureus Pulsed-Field Type USA300 isolates collected at a Boston ambulatory health center. J. Clin. Microbiol. 2007;45:1350–1352. doi: 10.1128/JCM.02274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Bambeke F., Reinert R.R., Appelbaum P.C., Tulkens P.M., E Peetermans W. Multidrug-resistant Streptococcus pneumoniae infections. Drugs. 2007;67:2355–2382. doi: 10.2165/00003495-200767160-00005. [DOI] [PubMed] [Google Scholar]
- 10.Chambers H.F., DeLeo F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Genet. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dayie N.T.K.D., Arhin R.E., Newman M.J., Dalsgaard A., Bisgaard M., Frimodt-Møller N., Slotved H.-C. Penicillin resistance and serotype distribution of Streptococcus pneumoniae in Ghanaian children less than six years of age. BMC Infect. Dis. 2013;13:490. doi: 10.1186/1471-2334-13-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampane-Donkor E., Badoe E.V., Annan J.A., Nii-Trebi N.I. Colonisation of antibiotic-resistant bacteria in a cohort of HIV infected children in Ghana. Pan Afr. Med. J. 2017;26:1–7. doi: 10.11604/pamj.2017.26.60.10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donkor E.S., Kotey F.C.N., Dayie N.T.K.D., Duodu S., Tetteh-Quarcoo P.B., Osei M.-M., Tette E.M.A. Colonization of HIV-infected children with methicillin-resistant Staphylococcus aureus. Pathogens. 2019;8:35. doi: 10.3390/pathogens8010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appiah V.A., Pesewu G.A., Kotey F.C.N., Boakye A.N., Duodu S., A Tette E.M., Nyarko M.Y., Donkor E.S. Staphylococcus aureus Nasal colonization among children with sickle cell disease at the Children’s Hospital, Accra: Prevalence, risk factors, and antibiotic resistance. Pathogens. 2020;9:329. doi: 10.3390/pathogens9050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donkor E.S., Newman M.J., Oliver-Commey J., Bannerman E., Dayie N.T.K.D., Badoe E.V. Invasive disease and paediatric carriage of Streptococcus pneumoniae in Ghana. Scand. J. Infect. Dis. 2010;42:254–259. doi: 10.3109/00365540903490000. [DOI] [PubMed] [Google Scholar]
- 16.Donkor E.S., Kotey F.C.N. Studies on Components of Blood & Their Functions. Volume 1. Open Access eBooks; Las Vegas, NV, USA: 2020. Bacterial bloodstream infections; pp. 1–17. Chapter 5. [Google Scholar]
- 17.Sucher A.J., Chahine E.B., Nelson M., Sucher B.J. Prevnar 13, the new 13-valent pneumococcal conjugate vaccine. Ann. Pharmacother. 2011;45:1516–1524. doi: 10.1345/aph.1q347. [DOI] [PubMed] [Google Scholar]
- 18.Hampton L.M., Farley M.M., Schaffner W., Thomas A., Reingold A., Harrison L.H., Lynfield R., Bennett N.M., Petit S., Gershman K., et al. Prevention of antibiotic-nonsusceptible Streptococcus pneumoniae with conjugate vaccines. J. Infect. Dis. 2011;205:401–411. doi: 10.1093/infdis/jir755. [DOI] [PubMed] [Google Scholar]
- 19.Halasa N.B., Grijalva C.G., Arbogast P.G., Talbot T.R., Craig A.S., Griffin M.R., Schaffner W. Nearly complete elimination of the 7-valent pneumococcal conjugate vaccine serotypes in Tennessee. Pediatr. Infect. Dis. J. 2013;32:604–609. doi: 10.1097/INF.0b013e318287fe0d. [DOI] [PubMed] [Google Scholar]
- 20.Donkor E.S., Dayie N.T.K.D., Badoe E.V. Vaccination against pneumococcus in West Africa: Perspectives and prospects. Int. J. Gen. Med. 2013;6:757–764. doi: 10.2147/IJGM.S45842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinberger D.M., Malley R., Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–1973. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiertsema S.P., Kirkham L.-A.S., Corscadden K.J., Mowe E.N., Bowman J., Jacoby P., Francis R.W., Vijayasekaran S., Coates H., Riley T., et al. Predominance of nontypeable Haemophilus influenzae in children with otitis media following introduction of a 3+0 pneumococcal conjugate vaccine schedule. Vaccine. 2011;29:5163–5170. doi: 10.1016/j.vaccine.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 23.Davis S.M., Deloria-Knoll M., Kassa H.T., O’Brien K.L. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: Review of evidence on indirect effects. Vaccine. 2013;32:133–145. doi: 10.1016/j.vaccine.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Shak J.R., Vidal J.E., Klugman K.P. Influence of bacterial interactions on pneumococcal colonization of the nasopharynx. Trends Microbiol. 2013;21:129–135. doi: 10.1016/j.tim.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogaert D., Van Belkum A., Sluijter M., Luijendijk A., De Groot R., Rümke H.C., A Verbrugh H., Hermans P.W.M. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363:1871–1872. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 26.Regev-Yochay G., Dagan R., Raz M., Carmeli Y., Shainberg B., Derazne E., Rahav G., Rubinstein E. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in children. JAMA. 2004;292:716. doi: 10.1001/jama.292.6.716. [DOI] [PubMed] [Google Scholar]
- 27.Quintero B., Araque M., Jongh C.V.D.G.-D., Escalona F., Correa M., Morillo-Puente S., Vielma S., Hermans P.W.M. Epidemiology of Streptococcus pneumoniae and Staphylococcus aureus colonization in healthy Venezuelan children. Eur. J. Clin. Microbiol. Infect. Dis. 2010;30:7–19. doi: 10.1007/s10096-010-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphries R.M., Ambler J., Mitchell S.L., Castanheira M., Dingle T., Hindler J.A., Koeth L., Sei K., Hardy D., Zimmer B., et al. CLSI Methods Development and Standardization Working Group best practices for evaluation of antimicrobial susceptibility tests. J. Clin. Microbiol. 2018;56:e01934-17. doi: 10.1128/JCM.01934-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiss-Mandel A., Regev-Yochay G. Staphylococcus aureus and Streptococcus pneumoniae interaction and response to pneumococcal vaccination: Myth or reality? Hum. Vaccines Immunother. 2015;12:351–357. doi: 10.1080/21645515.2015.1081321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navne J.E., Koch A., Slotved H.-C., Andersson M., Melbye M., Ladefoged K., Børresen M. Effect of the 13-valent pneumococcal conjugate vaccine on nasopharyngeal carriage by respiratory pathogens among Greenlandic children. Int. J. Circumpolar Health. 2017;76:1309504. doi: 10.1080/22423982.2017.1309504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nzenze S.A. Effect of introduction of pneumococcal conjugate vaccine immunization on nasopharyngeal colonization of Streptococcus pneumoniae in South Africa (Doctoral dissertation) Wits Inst. Environ. D-Space. 2015;212:386. [Google Scholar]
- 32.World Health Organization Global antimicrobial resistance surveillance system (GLASS) report: Early implementation 2017–2018. [(accessed on 15 June 2018)]; Available online: https://www.who.int/docs/default-source/searo/amr/global-antimicrobial-resistance-surveillance-system---glass-report-early-implementation-2017-2018.pdf?sfvrsn=7e629fec_6.
- 33.Pizzutto S.J., Hare K.M., Upham J.W. Bronchiectasis in children: Current concepts in immunology and microbiology. Front. Pediatr. 2017;5:123. doi: 10.3389/fped.2017.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Z., Gu F.-F., Guo X.-K., Ni Y.-X., Xe P., Han L.-Z. Antimicrobial resistance and molecular characterization of Staphylococcus aureus causing childhood pneumonia in Shanghai. Front. Microbiol. 2017;8:455. doi: 10.3389/fmicb.2017.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elixhauser A., Steiner C. Healthcare Cost and Utilization Project (HCUP) Statistical Brief #35. 2007 July. Agency for Healthcare Research and Quality (US); Rockville, MD, USA: 2007. [(accessed on 13 August 2020)]. Infections with methicillin-resistant Staphylococcus aureus (MRSA) in US Hospitals, 1993–2005. Available online: https://www.ncbi.nlm.nih.gov/books/NBK61977. [Google Scholar]
- 36.Klevens R.M., Edwards J.R., Richards C.L., Horan T.C., Gaynes R.P., Pollock D.A., Cardo D.M. Estimating health care-associated infections and deaths in U.S. Hospitals, 2002. Public Health Rep. 2007;122:160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Kraker M.E., Wolkewitz M., Davey P.G., Koller W., Berger J., Nagler J., Icket C., Kalenic S., Horvatic J., Seifert H., et al. Clinical impact of antimicrobial resistance in European hospitals: Excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections. [(accessed on 13 August 2020)];Antimicrob. Agents Chemother. 2011 55:1598–1605. doi: 10.1128/AAC.01157-10. Available online: https://www.um.edu.mt/library/oar/handle/123456789/45465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dantes R., Mu Y., Belflower R., Aragon D., Dumyati G., Harrison L.H., Lessa F.C., Lynfield R., Nadle J., Petit S., et al. Emerging infections program–active bacterial Core surveillance MRSA surveillance investigators. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Int. Med. 2013;173:1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Gargasson J.B., Nyonator F.K., Adibo M., Gessner B.D., Colombini A. Costs of routine immunization and the introduction of new and underutilized vaccines in Ghana. Vaccine. 2015;33:A40–A46. doi: 10.1016/j.vaccine.2014.12.081. [DOI] [PubMed] [Google Scholar]
- 40.Denno D.M., Frimpong E., Gregory M., Steel R.W. Nasopharyngeal carriage and susceptibility patterns of Streptococcus pneumoniae in Kumasi, Ghana. West Afr. J. Med. 2004;21:233–236. doi: 10.4314/wajm.v21i3.28038. [DOI] [PubMed] [Google Scholar]
- 41.Leimkugel J., Forgor A.A., Gagneux S., Pflüger V., Flierl C., Awine E., Naegeli M., Dangy J., Smith T., Hodgson A., et al. An Outbreak of Serotype 1 Streptococcus pneumoniae Meningitis in northern Ghana with features that are characteristic of Neisseria meningitides meningitis epidemics. J. Infect. Dis. 2005;192:192–199. doi: 10.1086/431151. [DOI] [PubMed] [Google Scholar]
- 42.Donkor E.S. Molecular typing of the pneumococcus and its application in epidemiology in sub-Saharan Africa. Front. Cell. Infect. Microbiol. 2013;3:12. doi: 10.3389/fcimb.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grundmann H., Aanensen D.M., Wijngaard v.d.C.C., Spratt B.G., Harmsen D., Friedrich A.W., the European Staphylococcal Reference Laboratory Working Group Geographic Distribution of Staphylococcus aureus causing invasive infections in Europe: A molecular-epidemiological analysis. PLoS Med. 2010;7:e1000215. doi: 10.1371/journal.pmed.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown A.F., Leech J.M., Rogers T.R., McLoughlin R.M. Staphylococcus aureus Colonization: Modulation of host immune response and impact on human vaccine design. Front. Immunol. 2014;4:507. doi: 10.3389/fimmu.2013.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddinger R.M., Luke-Marshall N.R., Sauberan S.L., Hakansson A.P., Campagnari A.A. Streptococcus pneumoniae modulates Staphylococcus aureus biofilm dispersion and the transition from colonization to invasive disease. mBio. 2018;9:e02089–17. doi: 10.1128/mbio.02089-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donkor E.S., Jamrozy D., Mills R.O., Dankwah T., Amoo P.K., Egyir B., Badoe E.V., Twasam J., Bentley S.D. A genomic infection control study for Staphylococcus aureus in two Ghanaian hospitals. Infect. Drug Resist. 2018;11:1757–1765. doi: 10.2147/IDR.S167639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dayie N.T.K.D., Tettey E.Y., Newman M.J., Bannerman E., Donkor E.S., Labi A.-K., Slotved H.-C. Pneumococcal carriage among children under five in Accra, Ghana, five years after the introduction of pneumococcal conjugate vaccine. BMC Pediatr. 2019;19:1–11. doi: 10.1186/s12887-019-1690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsen A.R., Stegger M., Sørum M. spa typing directly from a mecA, spa and pvl multiplex PCR assay—a cost-effective improvement for methicillin-resistant Staphylococcus aureus surveillance. Clin. Microbiol. Infect. 2008;14:611–614. doi: 10.1111/j.1469-0691.2008.01995.x. [DOI] [PubMed] [Google Scholar]
- 49.Oliveira D.C., De Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002;46:2155–2161. doi: 10.1128/AAC.46.7.2155-2161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harmsen D., Claus H., Witte W., Rothgänger J., Turnwald D., Vogel U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 2003;41:5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deurenberg R.H., Vink C., Driessen C., Bes M., London N., Etienne J., Stobberingh E.E., Bes M. Rapid detection of Panton-Valentine leukocidin from clinical isolates of Staphylococcus aureus strains by real-time PCR. FEMS Microbiol. Lett. 2004;240:225–228. doi: 10.1016/j.femsle.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 52.Stegger M., Andersen P.S., Kearns A., Pichon B., Holmes M.A., Edwards G., Laurent F., Teale C., Skov R., Larsen A.R. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecA (LGA251) Clin. Microbiol. Infect. 2012;18:395–400. doi: 10.1111/j.1469-0691.2011.03715.x. [DOI] [PubMed] [Google Scholar]
- 53.Huang Y.-C., Chen C.-J. Nasal carriage of methicillin-resistant Staphylococcus aureus during the first 2 years of life in children in northern Taiwan. Pediatr. Infect. Dis. J. 2015;34:131–135. doi: 10.1097/INF.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 54.Egyir B., Guardabassi L., Esson J., Nielsen S.S., Newman M.J., Addo K.K., Larsen A.R. Insights into nasal carriage of Staphylococcus aureus in an urban and a rural community in Ghana. PLoS ONE. 2014;9:e96119. doi: 10.1371/journal.pone.0096119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egyir B., Oteng A.A., Owusu E., Newman M.J., Addo K.K., Rhod-Larsen A. Characterization of Staphylococcus aureus from Human Immunodeficiency Virus (HIV) patients in Accra, Ghana. J. Infect. Dev. Ctries. 2016;10:453–456. doi: 10.3855/jidc.7428. [DOI] [PubMed] [Google Scholar]
- 56.Kwambana-Adams B.A., Barer M.R., Bottomley C., A Adegbola R., Antonio M. Early acquisition and high nasopharyngeal co-colonisation by Streptococcus pneumoniae and three respiratory pathogens amongst Gambian new-borns and infants. BMC Infect. Dis. 2011;11:175–178. doi: 10.1186/1471-2334-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bojang A., Kendall L., Usuf E., Egere U., Mulwa S., Antonio M., Greenwood B., Hill P.C., Roca A. Prevalence and risk factors for Staphylococcus aureus nasopharyngeal carriage during a PCV trial. BMC Infect. Dis. 2017;17:588. doi: 10.1186/s12879-017-2685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tavares D.A., Sá-Leão R., Miragaia M., De Lencastre H. Large screening of CA-MRSA among Staphylococcus aureus colonizing healthy young children living in two areas (urban and rural) of Portugal. BMC Infect. Dis. 2010;10:110–118. doi: 10.1186/1471-2334-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sedighi I., Moez H.J., Alikhani M.Y. Nasal carriage of methicillin resistant Staphylococcus aureus and their antibiotic susceptibility patterns in children attending day-care centers. Acta Microbiol. Et Immunol. Hung. 2011;58:227–234. doi: 10.1556/AMicr.58.2011.3.6. [DOI] [PubMed] [Google Scholar]
- 60.Blumental S., Deplano A., Jourdain S., De Mendonça R., Hallin M., Nonhoff C., Rottiers S., Vergison A., Denis O. Dynamic pattern and genotypic diversity of Staphylococcus aureus nasopharyngeal carriage in healthy pre-school children. J. Antimicrob. Chemother. 2013;68:1517–1523. doi: 10.1093/jac/dkt080. [DOI] [PubMed] [Google Scholar]
- 61.Van Nguyen K., Zhang T., Vu B.N.T., Dao T.T., Tran T.K., Nguyen D.N.T., Tran H.K.T., Nguyen C.K.T., Fox A., Horby P., et al. Staphylococcus aureus nasopharyngeal carriage in rural and urban northern Vietnam. Trans. R. Soc. Trop. Med. Hyg. 2014;108:783–790. doi: 10.1093/trstmh/tru132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bottomley C., Bojang A., Smith P.G., Darboe O., Antonio M., Foster-Nyarko E., Kampmann B., Greenwood B., D’Alessandro U., Roca A. The impact of childhood vaccines on bacterial carriage in the nasopharynx: A longitudinal study. Emerg. Themes Epidemiol. 2015;12:1. doi: 10.1186/s12982-014-0022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adiku T.K., Asmah R.H., Rodrigues O., Goka B.Q., Obodai E., A Adjei A., Donkor E.S., Armah G.E. Aetiology of acute lower respiratory infections among children under five years in Accra, Ghana. Pathogens. 2015;4:22–33. doi: 10.3390/pathogens4010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Devine V.T., Jefferies J.M., Clarke S.C., Faust S.N. Nasopharyngeal bacterial carriage in the conjugate vaccine era with a focus on pneumococci. J. Immunol. Res. 2015;2015:1–8. doi: 10.1155/2015/394368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLeod J.W., Gordon J. Production of hydrogen peroxide by bacteria. Biochem. J. 1922;16:499–506. doi: 10.1042/bj0160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Regev-Yochay G., Trzciński K., Thompson C.M., Malley R., Lipsitch M. Interference between Streptococcus pneumoniae and Staphylococcus aureus: In vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J. Bacteriol. 2006;188:4996–5001. doi: 10.1128/JB.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee G.M., Huang S.S., Rifas-Shiman S.L., Hinrichsen V.L., Pelton S.I., Kleinman K., Hanage W.P., Lipsitch M., McAdam A.J., Finkelstein J.A. Epidemiology and risk factors for Staphylococcus aureus colonization in children in the post-PCV7 era. BMC Infect. Dis. 2009;9:110. doi: 10.1186/1471-2334-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramdani-Bouguessa N., Bes M., Meugnier H., Forey F., Reverdy M.-E., Lina G., Vandenesch F., Tazir M., Etienne J. Detection of methicillin-resistant Staphylococcus aureus strains resistant to multiple antibiotics and carrying the Panton-Valentine Leukocidin genes in an Algiers Hospital. Antimicrob. Agents Chemother. 2006;50:1083–1085. doi: 10.1128/AAC.50.3.1083-1085.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kolawole D.O., Adeyanju A., Schaumburg F., Akinyoola A.L., Lawal O.O., Amusa Y.B., Köck R., Becker K. Characterization of colonizing Staphylococcus aureus isolated from surgical wards’ patients in a Nigerian university hospital. PLoS ONE. 2013;8:e68721. doi: 10.1371/journal.pone.0068721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Risk R., Naismith H., Burnett A., E Moore S., Cham M., Unger S. Rational prescribing in paediatrics in a resource-limited setting. Arch. Dis. Child. 2013;98:503–509. doi: 10.1136/archdischild-2012-302987. [DOI] [PubMed] [Google Scholar]
- 71.Asante K.P., Boamah E.A., Abdulai M.A., Buabeng K.O., Mahama E., Dzabeng F., Gavor E., Annan E.A., Owusu-Agyei S., Ghana Antimicrobial Resistance Working Group et al. Knowledge of antibiotic resistance and antibiotic prescription practices among prescribers in the Brong Ahafo Region of Ghana; a cross-sectional study. BMC Health Serv. Res. 2017;17:422. doi: 10.1186/s12913-017-2365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donkor E.S., Newman M.J., Frimpong E., A Opintan J., Asamoah-Adu A. Resistance to antimicrobial drugs in Ghana. Infect. Drug Resist. 2011;4:215–220. doi: 10.2147/IDR.S21769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eibach D., Nagel M., Hogan B., Azuure C., Krumkamp R., Dekker D., Gajdiss M., Brunke M., Sarpong N., Owusu-Dabo E., et al. Nasal carriage of Staphylococcus aureus among children in the Ashanti Region of Ghana. PLoS ONE. 2017;12:e0170320. doi: 10.1371/journal.pone.0170320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dziekan G., Hahn A., Thüne K., Schwarzer G., Schäfer K., Daschner F.D., Grundmann H. Methicillin-resistant Staphylococcus aureus in a teaching hospital: Investigation of nosocomial transmission using a matched case-control study. J. Hosp. Infect. 2000;46:263–270. doi: 10.1053/jhin.2000.0846. [DOI] [PubMed] [Google Scholar]
- 75.Crowcroft N.S., Ronveaux O., Monnet D.L., Mertens R. Methicillin-Resistant Staphylococcus aureus and antimicrobial use in Belgian hospitals. Infect. Control. Hosp. Epidemiol. 1999;20:31–36. doi: 10.1086/501555. [DOI] [PubMed] [Google Scholar]
- 76.Breurec S., Fall C., Pouillot R., Boisier P., Brisse S., Diene-Sarr F., Djibo S., Etienne J., Fonkoua M.C., Perrier-Gros-Claude J.D., et al. Epidemiology of methicillin-susceptible Staphylococcus aureus lineages in five major African towns: High prevalence of Panton-Valentine leukocidin genes. Clin. Microbiol. Infect. 2011;17:633–639. doi: 10.1111/j.1469-0691.2010.03320.x. [DOI] [PubMed] [Google Scholar]
- 77.Von Eiff C., Friedrich A.W., Peters G., Becker K. Prevalence of genes encoding for members of the staphylococcal leukotoxin family among clinical isolates of Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2004;49:157–162. doi: 10.1016/j.diagmicrobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author via ntkddayie@ug.edu.gh.