Abstract
Background:
(Macro)autophagy is an important process of self-degradation of macromolecules and organelles that ensures cellular homeostasis and energy preservation during stressful conditions. Dysregulated placental autophagy has been implicated in a wide range of pregnancy complications. Recent studies identified hypoxia as a key regulator of trophoblast autophagy in vitro; however, its effects on placental autophagy in vivo remain incompletely understood. In this study, we evaluated the monochorionic twin anemia-polycythemia sequence (TAPS) placenta as model of discordant placental oxygenation to determine the effects of hypoxia on placental autophagy in utero.
Methods:
We performed a retrospective comparative analysis of tissue oxygenation and autophagy in anemic and polycythemic territories of TAPS placentas (N = 12). Archival tissues were subjected to immunohistochemical, immunofluorescence and Western blot analyses of carbonic anhydrase (CA) IX (hypoxia marker) and key autophagy/lysosomal markers.
Results:
CAIX protein levels were significantly higher in anemic twin territories than in corresponding polycythemic territories, consistent with relative tissue hypoxia. Anemic placental shares further displayed significantly higher levels of LC3I/II (autophagosome markers) and LAMP1/2 (lysosome markers), associated with upregulated expression of lysosome/autophagosome activity-associated markers, transcription factor EB and cathepsin D. The accumulation of autophagosomes and lysosomes in anemic shares was accompanied by elevated p62 protein expression, suggestive of inhibition of the downstream autophagy pathway.
Conclusions:
TAPS placentas display striking intertwin discordance in tissue oxygenation and autophagic activity and may provide a suitable model for study of the interrelationship between hypoxia, autophagy, and pregnancy outcome in a monochorionic twin setting.
Keywords: Hypoxia, Monochorionic, p62, Growth restriction, Preeclampsia
1. Introduction
Autophagy (also known as ‘macroautophagy’) is a catalytic process by which cells self-degrade long-lived or damaged macromolecules and organelles during stressful conditions in order to maintain cellular homeostasis [1,2]. During autophagy, cellular components, such as proteins, lipids, and organelles, are sequestered in double-membrane vesicles, termed autophagosomes, and transferred to lysosomes or endosomes [1]. Following fusion with lysosomes and creation of autolysosomes, the autophagic cargos are digested to their basic components (i.e. amino acids and fatty acids) by lysosomal hydrolases, providing new building blocks for cellular recycling. Degradation of dysfunctional organelles such as ribosomes, peroxisomes, and mitochondria, ensures critical quality control of cellular organelles [3]. Autophagy thus serves to maintain cellular homeostasis and to provide an alternative energy source to cells under stress [2,4].
The role of autophagy is well recognized in development and aging, and autophagy has been implicated in an ever-expanding array of diseases, including cancer, infection/inflammation, metabolic diseases, and neurodegenerative disorders, [5–8]. More recently, clinical and research interest in the field of autophagy has been directed towards the placenta [9–16]. Several lines of evidence suggest placental or trophoblast autophagy is dysregulated in a wide range of pregnancy complications, including spontaneous abortion [17], inflammation-induced preterm labor [18], idiopathic fetal growth restriction [19–21], preeclampsia [10,11,14] and placental malaria [22].
Most studies have emphasized the role of hypoxia as key regulator of placental and trophoblast autophagy in pregnancy complications [summarized in Ref. [9]]. In vitro studies have shown that hypoxia modulates autophagic activity in primary trophoblastic cells, trophoblast cell lines, and chorionic plate-derived mesenchymal stromal cells [10,14,19,20,23,24]. However, the exact effects of hypoxia on placental autophagy in utero and the clinical implications of altered placental autophagy remain to be elucidated [reviewed in Ref. [9]].
Studies focused on placental autophagy in singletons are inherently at risk of being confounded by pregnancy-specific variables known to affect placental or trophoblast autophagy, including: gestational age [25], maternal prepregnancy body mass index [26], parturition type [11,26–29], and pregnancy complications such as preeclampsia and fetal growth restriction [4,9,19,30,31].
In this study, we evaluated the monochorionic twin anemia-polycythemia sequence (TAPS) placenta as model of discordant placental oxygenation to determine the effects of hypoxia on placental autophagy in utero. Selection of the monochorionic twin placenta as model system for the current study allowed elimination of most maternal, pregnancy-related, and genetic confounding factors, as twins are exposed to the same maternal and uterine milieu and, in addition, monochorionic (monozygotic) twins (and their placentas) have a near-identical genetic makeup. Autophagy was compared between the anemic and polycythemic placental territories from pregnancies complicated by TAPS, a recently described form of chronic and slow intertwin blood transfusion that results in large intertwin differences in hemoglobin and reticulocyte levels without associated (severe) twin oligohydramnios-polyhydramnios sequence [32,33]. The aim of this study was to test the hypothesis that placentas of TAPS pregnancies, characterized by striking intertwin hemoglobin discordance, show corresponding intertwin differences in placental oxygenation and autophagy.
2. Materials and methods
2.1. Patient population
We performed a retrospective analysis of a consecutive series of diamniotic-monochorionic twin placentas from pregnancies complicated by TAPS, examined at the Department of Pathology at Women and Infants Hospital of Rhode Island between 2010 and 2018. The diagnosis of twin anemia-polycythemia sequence was based on the following proposed criteria for postnatal diagnosis of TAPS: intertwin Hb difference >8 g/dL and very small intertwin anastomoses [34,35]. Placentas with fetal demise and placentas from pregnancies complicated by twin-to-twin transfusion syndrome (TTTS, twin oligohydramnios-polyhydramnios sequence) were excluded from this study. The accompanying charts were reviewed for relevant maternal and fetal/neonatal information. Relevant neonatal information included birth weight, Apgar scores, gender, and first hematologic blood count values (hemoglobin and hematocrit, reticulocyte count, obtained immediately after delivery). Findings in the anemic twin territory were compared with those of the adjacent polycythemic twin territory.
2.2. Processing of the placenta
Immediately upon receipt in the Department of Pathology, placental parenchyma from four randomly selected quadrants of each twin territory was sampled for subsequent molecular (Western blot) analyses. To avoid inclusion of shared cotyledons, these samples were obtained from villous parenchyma lateral to the respective cord insertions, at a distance from the vascular equator. Areas of placental parenchyma with calcification, infarction, fibrin deposition or hemorrhage were avoided. Placental samples were placed in RNAlater (Amnion Inc., Austin, TX) and stored at −20 °C. Further examination of the placenta, including injection and categorization of the chorionic vasculature, was performed as previously described in detail [36–38]. For histological, immunohistochemical and immunofluorescence studies, tissues were obtained from at least 5 randomly selected areas per twin territory. These tissues were formalin-fixed and paraffin-embedded according to standard methods.
2.3. Immunohistochemical and immunofluorescence analysis
Immunohistochemical staining was performed using a Dako Autostainer (Dako, Carpenteria, CA), as previously described in detail [39]. For this peroxidase-based staining we used rabbit polyclonal anti-carbonic anhydrase IX (CAIX) antibody (Abcam, Cambridge, MA) as marker of tissue hypoxia. The following antibodies were used as markers of tissue autophagy: anti-LC3 (which recognizes both LC3-I and LC3-II) and anti-p62/SQSTM1 (both from MBL International, Woburn, MA), anti-LAMP1 (D2D11) and anti-LAMP2 (D5C2P) (both from Cell Signaling Technology, Danvers, MA). Placental sections from corresponding twin territories were prepared and immunostained in one single session. Controls for specificity consisted of incubation with isotype IgG instead of the respective primary antibodies, which abolished all immunoreactivity.
Anatomic colocalization of autophagosomes and lysosomes, a characteristic of autophagy, was assessed by combining anti-LC3 (as marker of autophagosomes) with anti-LAMP1 (as marker of lysosomes) immunofluorescence staining. For these double immunofluorescence studies, tissue sections were incubated sequentially with polyclonal rabbit anti-LC3, Alexa Fluor 594-conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), monoclonal mouse anti-LAMP1 (Santa Cruz Biotechnology, Dallas, TX), and Alexa Fluor 488-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.). Sections were covered with aqueous mounting medium containing 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI, Vector Laboratories, Inc., Burlingame, CA). Controls consisted of omission of one or both primary antibodies, which abolished the respective immunoreactivities.
Confocal images were acquired with a Nikon C1si confocal microscope (Nikon Inc., Melville, NY) using diode lasers 402, 488 and 561. Serial optical sections were obtained sequentially with the lambda setting of EZ-C1 computer software (Nikon). Z series sections were collected at 0.2 μm with a 60× Plan Apo, 1.4 numerical aperture lens and a scan zoom of 3. Colocalization analysis was performed on deconvolved, 3D acquisitions with Elements software (Elements version 3.2, Nikon). In each Z stack, regions of interest were outlined and analyzed with a colocalization macro (Nikon). The presence of colocalization of the red and green fluorophores was verified by Pearson’s correlation coefficient in manually selected regions of interest [40]. A minimum threshold of Pearson’s correlation coefficient of r2 > 0.5 was used to indicate positive colocalization.
2.4. Western blot analysis
Hypoxia- and autophagy-related protein expression was assessed by Western blot analysis of placental lysates, according to methods described in detail elsewhere [41], using the antibodies described above, and, in addition: rabbit monoclonal anti-cathepsin D (Abcam, Cambridge, MA), and rabbit polyclonal anti-TFEB antibodies (Cell Signaling Technology, Danvers, MA). The housekeeping gene GAPDH (Santa Cruz Biotechnology) served as internal loading control. Band intensity was expressed as the integrated optical density (IOD) normalized to the IOD of GAPDH.
2.5. Data analysis
Values are expressed as mean ± standard deviation (SD) or standard error of mean (SEM). The significance of differences between groups was determined by Student t-test, Mann-Whitney U test, ANOVA with post-hoc Scheffe test, Fisher’s exact test, or Wilcoxon matched-pairs signed rank test, where applicable. Data were analyzed and graphically represented using GraphPad Prism 5 software (GraphPad Prism; GraphPad Software, Inc., San Diego, CA). Data depicted in modified (Tukey) box plots reflect group median, upper and lower quartiles (box), maximum and minimum values excluding outliers (whiskers), and outliers (more than 3/2 times upper quartile). The significance level was set at P < 0.05. The study was approved by the Institutional Review Board.
3. Results
3.1. General clinical and placental data
Autophagy was studied in a consecutive cohort of 12 TAPS placentas. TAPS was spontaneous in all cases (i.e. not iatrogenic following laser treatment for TTTS). Relevant clinical and placental data are summarized in Table 1. The gestational age ranged between 26 and 37 weeks’ gestation (median age: 33 weeks). The mean birth weight of polycythemic twins was significantly larger than that of anemic twins (P < 0.05); in 11/12 TAPS twin sets, the polycythemic twin was the heavier of the two. Delivery was by cesarean section in 9/12 cases. Twins were of the male gender in 7/12 pregnancies. Apgar scores were equivalent for polycythemic and anemic twins. The intertwin Hb difference was >8 g/dL in all cases, fulfilling the proposed hematologic criteria for TAPS, and ranged from 8.9 g/dL to 19.0 g/dL (median: 12.8 g/dL). Reticulocyte counts obtained soon after birth (prior to transfusion) were only available in one case (reticulocyte count ratio 4.0 (12.5%/3.1%)).
Table 1.
Patient and placental data of TAPS pregnancies.
| Polycythemic twin/placental side (12) | Anemic twin/placental side (12) | P | |
|---|---|---|---|
| Gestational age (wks) | 33 (26–37) | ||
| Birth weight (g) | 1726 ± 668 | 1433 ± 649 | <0.05 |
| Median Apgar 1 min | 7 (3–8) | 7 (1–9) | NS |
| Apgar <7 at 1 min | 4/12 | 6/12 | NS |
| Median Apgar 5 min | 8.5 (6–9) | 8 (6–9) | NS |
| Hgb (g/dL) | 22.2 ± 1.7 | 9.0 ± 2.5 | <0.01 |
| Hct (%) | 67.3 ± 4.0 | 28.6 ± 6.1 | <0.01 |
| Placenta weight (g) | 683 ± 129 | ||
| Placental share (%) | 48 (39–61) | 52 (45–61) | NS |
| Peripheral cord (M/V) | 5/12 | 10/12 | NS |
All placental weights were appropriate for gestational age. There was no obvious correlation between placental sharing and TAPS status: the polycythemic twin had the larger placental share in 5/12 cases. Anemic twins tended to have peripheral (marginal or velamentous) cord insertion more frequently than polycythemic twins (9/12 versus 5/12). Consistent with the proposed placental criteria for TAPS diagnosis, all TAPS placentas had few, relatively small intertwin choriovascular anastomoses (Fig. 1). To determine the putative direction of flow in the artery-to-vein (AV) anastomoses, the number of AV anastomoses in one direction was subtracted from the number of AV anastomoses in the opposite direction. In 9/12 cases, the putative direction of flow, derived from the net number of AV anastomoses visible on the chorionic surface, was directed from anemic twin share to polycythemic twin share, concordant with the TAPS status (Fig. 1). As expected, histologic examination of the TAPS placentas revealed variable degrees of congestion of the villous capillaries in the polycythemic twin share, and small, collapsed capillaries with increased numbers of nucleated erythroid precursors (erythroblastosis) in the anemic twin share (Fig. 1).
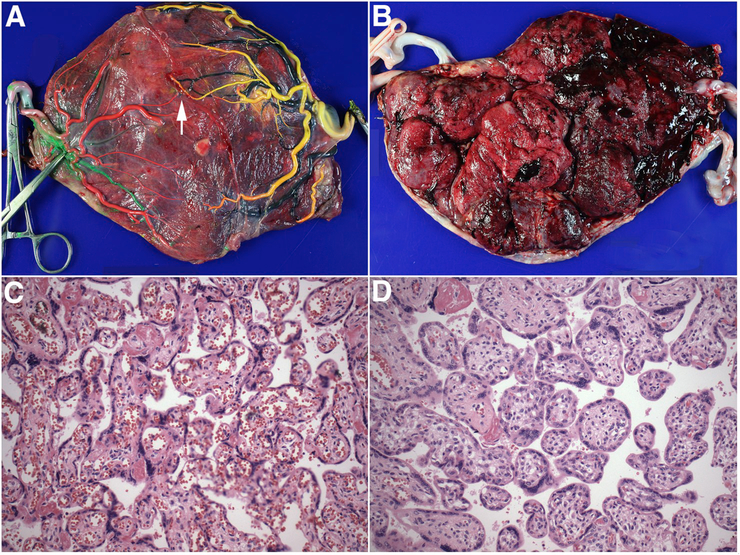
Fig. 1. Representative gross and microscopic appearance of TAPS placenta.
A. Fetal surface following removal of the intertwin membrane and injection of the chorionic vessels. Vascular injection highlights the near-complete separation of the choriovascular beds. A single artery-to-vein anastomosis from anemic (left) to polycythemic (right) territory is shown by arrow. Artery-to-artery and vein-to-vein anastomoses are absent. Color code: Left twin: artery: red, vein: green; right twin: artery: yellow, vein: black. B. Maternal surface demonstrating marked color (redness) discordance with sharp demarcation of anemic (left) and polycythemic (right) territories. C-D. Representative micrographs of plethoric and pale placental parenchyma, respectively (hematoxylin-eosin staining, original magnification ×200).
3.2. Analysis of tissue oxygenation in TAPS placentas
Oxygenation of the respective twin territories was estimated by studying the protein levels of the endogenous hypoxia marker, carbonic anhydrase IX (CAIX) [42], as described elsewhere [39]. The promoter region of the CA9 gene contains a hypoxia-response element that is a transcriptional target of the hypoxia inducible factor-1 (HIF-1) transcription factor. CAIX expression thus represents an indirect indicator of activation of the HIF-1 complex and can serve as cellular biomarker of chronic hypoxia in archival tissues [43,44]. Western blot analysis of CAIX protein expression in placental lysates demonstrated significantly higher CAIX protein levels in the anemic twin territories compared with the polycythemic twin territories (Fig. 2). Concordant with the Western blot data, protein levels of CAIX, immunolocalized to villous cytotrophoblastic cells, villous stromal cells, and extravillous trophoblast, were significantly higher in the anemic than in the corresponding polycythemic twin shares, consistent with relative tissue hypoxia (Fig. 2).
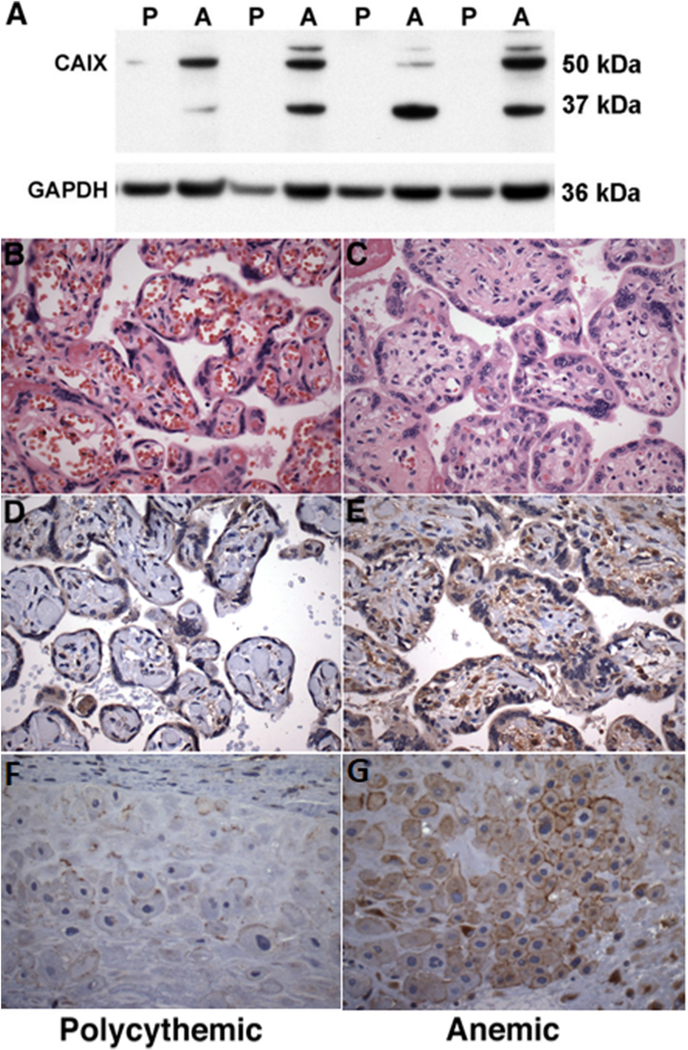
Fig. 2. Carbonic anhydrase IX expression in TAPS placentas.
A Western blot analysis of placental carbonic anhydrase (CA) IX expression in lysates of polycythemic (P) and anemic (A) territories of 4 representative TAPS placentas. GAPDH served as loading control. B-C. Representative micrographs of polycythemic and anemic placental parenchyma, respectively (hematoxylin-eosin staining, original magnification ×400) D-E. Representative immunohistochemical analysis of corresponding CAIX protein expression in placental parenchyma, showing intense CAIX immunoreactivity, localized to villous trophoblast, stromal and endothelial cells, in the anemic placental share (right), consistent with relative tissue hypoxia. F-G. Representative immunohistochemical analysis of CAIX protein expression in extravillous trophoblast, showing intense membranous immunoreactivity in the anemic placental share (right). (D-G: DAB-peroxidase system with hematoxylin counterstain, original magnification ×400).
3.3. Analysis of autophagy in TAPS placentas
Autophagy in the TAPS placentas was studied by Western blot analysis of microtubule-associated protein IA/IB light chain 3 (LC3) protein expression in placental lysates using an antibody that recognizes both LC3-I (unconjugated cytosolic form) and LC3-II (autophagosomal membrane-incorporated phosphatidylethanolamine-conjugated form) [45]. Immunoblot analysis of TAPS placental lysates revealed significantly higher levels of LC3 protein in the anemic twin territories (Fig. 3). Furthermore, the anemic placental shares consistently demonstrated two immunoreactive bands, corresponding to LC3-I and LC3-II, whereas the polycythemic shares contained very low levels of LC3-I with no discernible LC3-II. As conversion of LC3-I to LC3-II is recognized as a hallmark of autophagosome formation [1,46,47], these findings are indicative of autophagosome accumulation in the anemic shares of TAPS placentas.
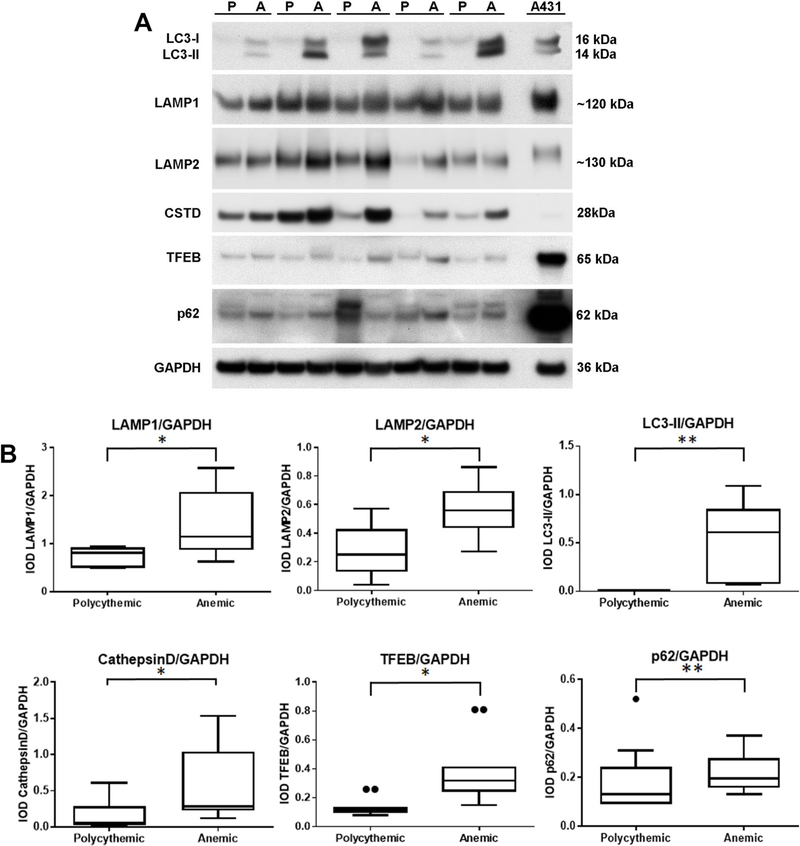
Fig. 3. Western blot analysis of autophagy-related protein expression in TAPS placentas.
A. Western blot analysis of lysosome- and autophagy-related protein expression in lysates of polycythemic (P) and corresponding anemic (A) territories of 5 representative TAPS placentas. GAPDH served as loading control. B. Densitometric analysis of Western blot. IOD: integrated optical density; *: P < 0.05; **: P < 0.01; ***: P < 0.001 (Wilcoxon matched-pairs signed rank test). LAMP: lysosome-associated membrane protein; LC3: microtubule-associated protein 1A/1B-light chain 3 (LC3-I: cytosolic form; LC3-II: phosphatidylethanolamine conjugate); CSTD: cathepsin D; TFEB: transcription factor EB; A431: human epidermoid carcinoma cell line (ATCC).
The anemic territories of TAPS placentas also exhibited an expansion of the lysosome compartment, as demonstrated by significantly higher levels of the specific lysosome marker proteins, lysosome-associated membrane protein 1 (LAMP1) and LAMP2 [48,49] (Fig. 3). The accumulation of lysosomes and autophagosomes in anemic twin territories was associated with significantly higher levels of expression of other autophagy- and lysosome-related proteins, such as cathepsin D, a lysosomal aspartyl protease [50,51], and transcription factor EB (TFEB), a transcriptional regulator of lysosomal and autophagosome biogenesis and function [52–55] (Fig. 3).
Autophagosome accumulation, as observed in the anemic share of TAPS placentas, may result from enhanced autophagosome synthesis or reduced autophagosome turnover. To interpret the mechanism underlying the discordant LC3-II levels in TAPS placentas, we studied the protein expression of p62 (SQSTM1/sequestome 1). p62 acts as an adaptor molecule between poly-ubiquinated protein aggregates and LC3, thus targeting these aggregates for degradation at the autolysosome [56]. As p62 itself is degraded in the autolysosome, p62 accumulation is generally considered a reliable measure of defects in autophagy of ubiquinated aggregates [57]. The p62 protein levels were significantly higher in the anemic shares than in the polycythemic shares (P < 0.01; Wilcoxon Rank Sum Test) (Fig. 3), which, in association with elevated LC3-II levels, is suggestive of defects in autophagy in the anemic shares.
Immunohistochemical analysis of LAMP1, LAMP2 and LC3 expression confirmed the higher levels of these proteins in the anemic placental compartments (Fig. 4). LAMP1, LAMP2 and LC3 expression was cytoplasmic and localized to trophoblastic and mesenchymal stromal cells (Fig. 4). Immunostaining for p62, predominantly nuclear and localized to cytotrophoblastic and stromal cells, similarly was more intense in the anemic territory of the TAPS twin sets than in the polycythemic territories (Fig. 4).
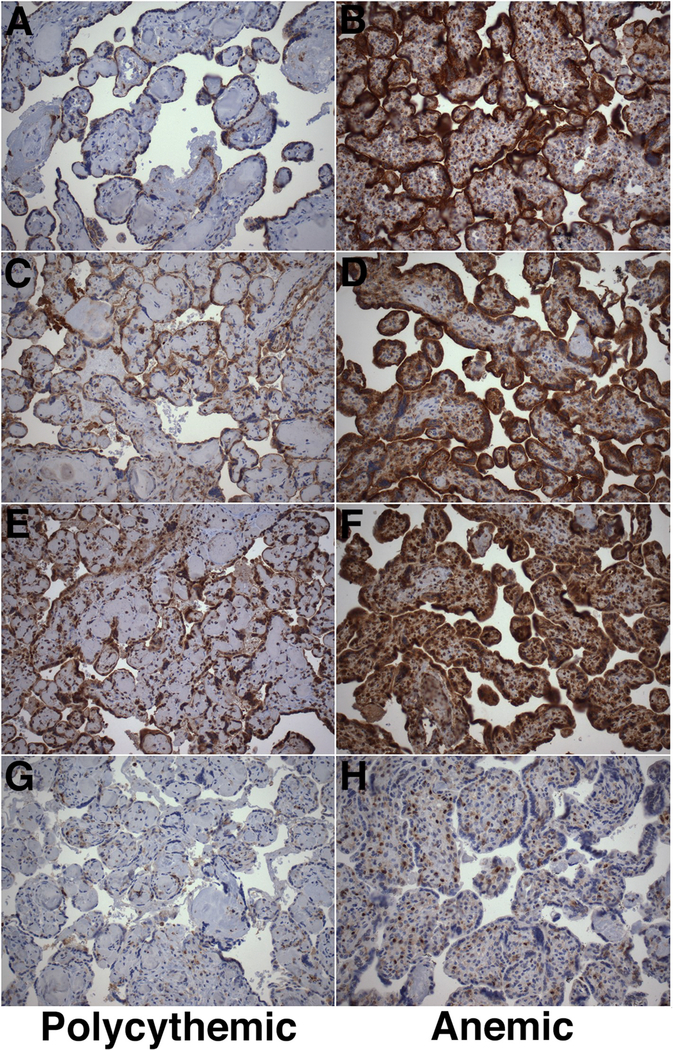
Fig. 4. Immunohistochemical analysis of autophagy-related protein expression in TAPS placentas.
Representative immunohistochemical analysis of expression of LAMP1 (A–B), LAMP2 (C–D), LC3B (E–F) and p62 (G–H) in polycythemic (left) and corresponding anemic (right) territories of TAPS placentas. (DAB-peroxidase system with hematoxylin counterstain, original magnification ×200).
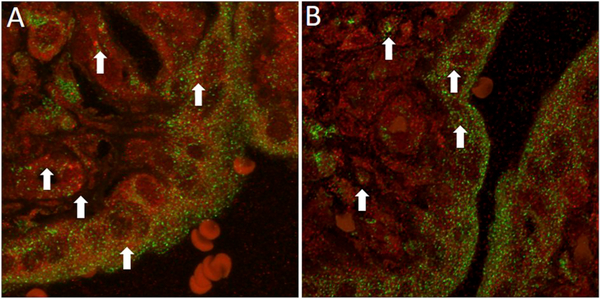
The occurrence of autophagosome/lysosome fusion was studied by combined LC3B/LAMP1 immunofluorescence studies. Scattered foci of apparent colocalization (yellow fluorescence) of LC3 (green fluorescence) and LAMP1 (red fluorescence), suggestive of autolysosome formation, were observed in both anemic and polycythemic twin territories (Fig. 5). Regions suggestive of colocalization of LAMP-associated and LC3-associated fluorochromes, based on the presence of yellow fluorescence, uniformly yielded a Pearson’s correlation coefficient r2 > 0.5, consistent with complete pixel-based colocalization and, therefore, consistent with fusion of autophagosomes and lysosomes. Although no formal quantitative analyses could be performed, the number of colocalization events appeared qualitatively similar in anemic and polycythemic placental shares.
Fig. 5. Combined immunofluorescence analysis of colocalization of LC3B and LAMP1 in TAPS placentas.
Confocal fluorescence microscopy of polycythemic (A) and anemic (B) territories of TAPS placenta subjected to combined anti-LC3B (red) and anti-LAMP1 (green) immunofluorescence, captured at the same settings. Arrows point to representative regions of colocalization of LC3B and LAMP1, suggestive of fusion of autophagosomes and lysosomes. In selected fields, colocalization was confirmed quantitatively using Pearson’s correlation coefficient analysis whereby a cutoff of r2 > 0.5 was used to indicate positive colocalization.
4. Discussion
In this study, we determined that the anemic and polycythemic placental territories from monochorionic pregnancies complicated by twin anemia-polycythemia sequence (TAPS) exhibit discordant expression of traditional autophagy- and lysosome-associated protein markers. Levels of microtubule-associated protein IA/IB light chain 3 (LC3) proteins, including the specific autophagosomal membrane-associated form, LC3-II [46,58–61], were significantly higher in the anemic twin shares than in the corresponding polycythemic shares, consistent with accumulation of autophagosomes. The anemic twin territories further displayed significantly higher levels of lysosome-associated membrane protein 1 (LAMP1) and LAMP2 [48,49], indicative of higher lysosome content, as well as higher levels of cathepsin D [50,51] and transcription factor EB (TFEB) [52–55], markers of lysosome and/or autophagosome function.
The expansion of the autophagosome compartment in the anemic shares of TAPS placentas, as demonstrated by increased LC3-II levels, might be interpreted as evidence of enhanced autophagic activity. However, LC3-II levels represent static autophagosomal biomarkers that fail to distinguish between increased autophagosome synthesis (increased on-rate) and reduced autophagosome turnover (decreased off-rate) [62–65]. When studying autophagy in vitro, these conditions can be distinguished by assays of autophagic flux, for instance, by comparing the amount of LC3-II generated in the presence or absence of lysosomal inhibitors [46,66]. We took the following alternative approaches to estimate autophagic activity in archival formalin-fixed, paraffin-embedded TAPS placentas. First, we studied the occurrence of autophagosome-lysosome fusion in TAPS placentas based on the colocalization of LC3 and LAMP1 proteins. While confocal microscopy allowed visualization of autophagosome-lysosome fusion in both anemic and polycythemic twin shares, no formal quantitation of LC3B/LAMP colocalization events could be performed.
Next, we estimated the extent of autophagosome degradation based on expression of p62/SQSTM1 (sequestome 1), an adaptor molecule implicated in the targeting of cargo for autophagosomes [56]. p62 itself is degraded by autophagy and its expression thus serves a selective marker of autophagosome degradation [56,66]: successful completion of the autophagy pathway is associated with decreased p62 levels, whereas p62 accumulation indicates reduced autophagosome turnover by blockage of the autophagy machinery [46]. In the present study, the autophagosome and lysosome accumulation in anemic twin shares was associated with significantly increased levels of p62, suggestive of inhibition of autophagic activity.
The exact mechanisms underlying the accumulation of autophagosomes and lysosomes in the anemic shares of TAPS placentas, at least partly attributable to reduced autophagosome turnover, remain undetermined. While autophagy may be influenced by many stressors, including nutritional deprivation, endoplasmic reticulum and redox stress, immune and infectious signals, and mitochondrial damage [67], hypoxia represents a likely candidate stimulus underlying autophagy discordance in TAPS placentas. In this study, we demonstrated that the anemic shares of TAPS placentas exhibit significant tissue hypoxia compared with their polycythemic counterparts, as supported by significantly higher placental levels of the endogenous hypoxia marker, carbonic anhydrase IX [39,42], and in concordance with the lower hemoglobin levels and increased numbers of circulating erythroid cells in these placental shares.
Attempts to reconcile our observations in TAPS placentas with in vitro studies describing the effects of hypoxia on LC3-II expression and/or autophagic activity in primary trophoblastic cells or trophoblast cell lines are complicated by the variable, often seemingly conflicting, results. Oh et al. [10] reported no significant change in LC3-II protein levels in the human choriocarcinoma JEG-3 cell line following exposure to hypoxia (O2 < 1%). Hung et al. [19] found a significant increase in LC3-II protein levels in primary human cytotrophoblastic cells following exposure to hypoxia (O2 2%). In the latter study, the hypoxia-induced LC3-II upregulation was further enhanced in the presence of the lysosomal inhibitor, bafilomycin A1, suggesting the basal rate of autophagy is increased by hypoxia in these cells [19]. Similarly, hypoxia exposure (6% O2) was associated with an increase in autophagic vacuoles in the BeWo choriocarcinoma cell line [20]. In a study by Chen et al. [23], in contrast, LC3-II protein levels in primary human trophoblastic cells were significantly decreased, rather than increased, following exposure to hypoxia (O2 < 1%). This decrease in LC3-II levels under hypoxic conditions was attributed to increased autophagic flux from increased degradation of LC3-II in the autophagosome, as LC3-II levels in the presence of bafilomycin were significantly higher in hypoxic conditions than in standard conditions [23]. As in vitro studies thus far have not provided a consensus on the effects of hypoxia on LC3-II expression (autophagosome content) and/or autophagic activity, studies as the current one, based on actual specimens under clinically relevant hypoxic conditions, may have a role in evaluating the in vivo validity of in vitro or in silico observations and speculations.
The exact clinical implications of the observed inhibition of autophagosome degradation and accompanying accumulation of autophagosomes in the anemic shares of TAPS placentas remain to be determined. In the present study, the birth weight of the anemic TAPS twins was significantly lower than that of the corresponding polycythemic twins, as is characteristic of TAPS pregnancies in general [32, 33]. It is tempting to speculate that the observed inhibition of placental autophagy may be implicated, at least in part, in the growth restriction of the anemic TAPS twins. By analogy, inhibition of placental autophagy was associated with lower fetal body weights in rodent models, both in uncomplicated pregnancies [68] and under preeclampsia-like stress conditions [69], suggesting autophagy may have a protective role for normal fetal growth [reviewed in Ref. [4]]. Similarly, inhibition of placental or trophoblast autophagy is described in pregnancies complicated by fetal growth restriction [64] and in pregnancies typically associated with fetal growth restriction, such as implantation disorders [15,70] and preeclampsia [14]. It must be emphasized, however, that the putative protective role of placental autophagy with respect to fetal growth and overall pregnancy outcome is certainly not universally accepted. In fact, some reports describe activation, rather than inhibition, of placental autophagy in association with fetal growth restriction and preeclampsia [reviewed in Ref. [4]].
Limitations of this study remain to be acknowledged. First, analysis of placental autophagy in TAPS pregnancies was based on archival formalin-fixed and chemically preserved specimens, precluding formal analysis of the autophagic flux (defined as the rate of degradation of autophagosomes and their content within lysosomes with or without lysosome inhibition). Second, study of tissue oxygenation (hypoxia) was limited to Western blot and immunohistochemical analysis of carbonic anhydrase IX (CAIX) protein expression. While the increased CAIX expression in the anemic placental territories is in line with the markedly lower hemoglobin content and higher numbers of placental nucleated erythroid cells of the anemic twins, the exact performance of CAIX as marker of tissue hypoxia remains to be determined by formal systematic studies. Finally, this study focused on differential tissue oxygenation as dominant factor underlying the discordant autophagy in TAPS placental shares. Based on placental characteristics, such as more frequent peripheral cord insertion, it is possible that TAPS placental shares differ not only in oxygen content, but also in nutritional content. Differential nutritional supply may have contributed to the observed differential placental autophagy in TAPS pregnancies, in concordance with the in vitro effects of nutritional deprivation on autophagy markers in BeWo cells and human primary trophoblastic cells [20,25].
In summary, our findings demonstrate that TAPS placentas display intertwin autophagy discordance, suggestive of inhibition of autophagic activity in the territory of the anemic twin with associated accumulation of lysosomes and autophagosomes. In view of the known effects of hypoxia on autophagy in trophoblastic cells in vitro, and our observation of decreased tissue oxygenation in anemic twin territory, we speculate that the autophagy discordance of TAPS placentas may be attributable, at least in part, to differential tissue oxygenation. TAPS twin placentas thus provide an excellent model for study of the role and regulation of placental autophagy as adaptive stress response to intrauterine hypoxia and ultimately may contribute to a better understanding of the role of autophagy and its modulation in reproductive health.
Footnotes
Declaration of competing interest
We have no conflict of interest.
References
- [1].Mizushima N, Levine B, Autophagy in mammalian development and differentiation, Nat. Cell Biol. 12 (2010) 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Klionsky DJ, Emr SD, Autophagy as a regulated pathway of cellular degradation, Science 290 (2000) 1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jin M, Liu X, Klionsky DJ, SnapShot: selective autophagy, Cell 152 (2013), 368–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nakashima A, Aoki A, Kusabiraki T, Cheng SB, Sharma S, Saito S, Autophagy regulation in preeclampsia: pros and cons, J. Reprod. Immunol. 123 (2017) 17–23. [DOI] [PubMed] [Google Scholar]
- [5].Choi AM, Ryter SW, Levine B, Autophagy in human health and disease, N. Engl. J. Med. 368 (2013) 1845–1846. [DOI] [PubMed] [Google Scholar]
- [6].Mizumura K, Choi AM, Ryter SW, Emerging role of selective autophagy in human diseases, Front. Pharmacol. 5 (2014) 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mizushima N, Levine B, Cuervo AM, Klionsky DJ, Autophagy fights disease through cellular self-digestion, Nature 451 (2008) 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schneider JL, Cuervo AM, Autophagy and human disease: emerging themes, Curr. Opin. Genet. Dev. 26 (2014) 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Oh SY, Roh CR, Autophagy in the placenta, Obstetrics & gynecology science 60 (2017) 241–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Oh SY, Choi SJ, Kim KH, Cho EY, Kim JH, Roh CR, Autophagy-related proteins, LC3 and Beclin-1, in placentas from pregnancies complicated by preeclampsia, Reprod. Sci. 15 (2008) 912–920. [DOI] [PubMed] [Google Scholar]
- [11].Signorelli P, Avagliano L, Virgili E, Gagliostro V, Doi P, Braidotti P, Bulfamante GP, Ghidoni R, Marconi AM, Autophagy in term normal human placentas, Placenta 32 (2011) 482–485. [DOI] [PubMed] [Google Scholar]
- [12].Gong JS, Kim GJ, The role of autophagy in the placenta as a regulator of cell death, Clinical and experimental reproductive medicine 41 (2014) 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bildirici I, Longtine MS, Chen B, Nelson DM, Survival by self-destruction: a role for autophagy in the placenta? Placenta 33 (2012) 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nakashima A, Yamanaka-Tatematsu M, Fujita N, Koizumi K, Shima T, Yoshida T, Nikaido T, Okamoto A, Yoshimori T, Saito S, Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia, Autophagy 9 (2013) 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Saito S, Nakashima A, A review of the mechanism for poor placentation in early-onset preeclampsia: the role of autophagy in trophoblast invasion and vascular remodeling, J. Reprod. Immunol. 101–102 (2014) 80–88. [DOI] [PubMed] [Google Scholar]
- [16].Gao L, Qi HB, Kamana KC, Zhang XM, Zhang H, Baker PN, Excessive autophagy induces the failure of trophoblast invasion and vasculature: possible relevance to the pathogenesis of preeclampsia, J. Hypertens. 33 (2015) 106–117. [DOI] [PubMed] [Google Scholar]
- [17].Avagliano L, Terraneo L, Virgili E, Martinelli C, Doi P, Samaja M, Bulfamante GP, Marconi AM, Autophagy in normal and abnormal early human pregnancies, Reprod. Sci. 22 (2015) 838–844. [DOI] [PubMed] [Google Scholar]
- [18].Agrawal V, Jaiswal MK, Mallers T, Katara GK, Gilman-Sachs A, Beaman KD, Hirsch E, Altered autophagic flux enhances inflammatory responses during inflammation-induced preterm labor, Sci. Rep. 5 (2015) 9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hung TH, Chen SF, Lo LM, Li MJ, Yeh YL, Hsieh TT, Increased autophagy in placentas of intrauterine growth-restricted pregnancies, PLoS One 7 (2012), e40957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Curtis S, Jones CJ, Garrod A, Hulme CH, Heazell AE, Identification of autophagic vacuoles and regulators of autophagy in villous trophoblast from normal term pregnancies and in fetal growth restriction, J. Matern. Fetal Neonatal Med. 26 (2013) 339–346. [DOI] [PubMed] [Google Scholar]
- [21].Zhang QX, Na Q, Song W, Altered expression of mTOR and autophagy in term normal human placentas, Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie 58 (2017) 517–526. [PubMed] [Google Scholar]
- [22].Dimasuay KG, Gong L, Rosario F, McBryde E, Spelman T, Glazier J, Rogerson SJ, Beeson JG, Jansson T, Devenish RJ, Boeuf P, Impaired placental autophagy in placental malaria, PLoS One 12 (2017), e0187291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen B, Longtine MS, Nelson DM, Hypoxia induces autophagy in primary human trophoblasts, Endocrinology 153 (2012) 4946–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee Y, Jung J, Cho KJ, Lee SK, Park JW, Oh IH, Kim GJ, Increased SCF/c-kit by hypoxia promotes autophagy of human placental chorionic plate-derived mesenchymal stem cells via regulating the phosphorylation of mTOR, J. Cell. Biochem. 114 (2013) 79–88. [DOI] [PubMed] [Google Scholar]
- [25].Hung TH, Hsieh TT, Chen SF, Li MJ, Yeh YL, Autophagy in the human placenta throughout gestation, PLoS One 8 (2013), e83475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Avagliano L, Virgili E, Garo C, Quadrelli F, Doi P, Samaja M, Bulfamante GP, Marconi AM, Autophagy and human parturition: evaluation of LC3 expression in placenta from spontaneous or medically induced onset of labor, BioMed Res. Int. 2013 (2013) 689768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Doulaveris G, Orfanelli T, Benn K, Zervoudakis I, Skupski D, Witkin SS, A polymorphism in an autophagy-related gene, ATG16L1, influences time to delivery in women with an unfavorable cervix who require labor induction, J. Perinat. Med. 41 (2013) 411–414. [DOI] [PubMed] [Google Scholar]
- [28].Brickle A, Tran HT, Lim R, Liong S, Lappas M, Autophagy, which is decreased in labouring fetal membranes, regulates IL-1beta production via the inflammasome, Placenta 36 (2015) 1393–1404. [DOI] [PubMed] [Google Scholar]
- [29].de Andrade Ramos BR, Witkin SS, The influence of oxidative stress and autophagy cross regulation on pregnancy outcome, Cell Stress & Chaperones 21 (2016) 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kanninen TT, Jayaram A, Jaffe Lifshitz S, Witkin SS, Altered autophagy induction by sera from pregnant women with pre-eclampsia: a case-control study, BJOG 121 (2014) 958–964. [DOI] [PubMed] [Google Scholar]
- [31].Akaishi R, Yamada T, Nakabayashi K, Nishihara H, Furuta I, Kojima T, Morikawa M, Fujita N, Minakami H, Autophagy in the placenta of women with hypertensive disorders in pregnancy, Placenta 35 (2014) 974–980. [DOI] [PubMed] [Google Scholar]
- [32].Robyr R, Lewi L, Salomon LJ, Yamamoto M, Bernard JP, Deprest J, Ville Y, Prevalence and management of late fetal complications following successful selective laser coagulation of chorionic plate anastomoses in twin-to-twin transfusion syndrome, Am. J. Obstet. Gynecol. 194 (2006) 796–803. [DOI] [PubMed] [Google Scholar]
- [33].Tollenaar LS, Slaghekke F, Middeldorp JM, Klumper FJ, Haak MC, Oepkes D, Lopriore E, Twin anemia polycythemia sequence: current views on pathogenesis, diagnostic criteria, perinatal management, and outcome, Twin Res. Hum. Genet.: the official journal of the International Society for Twin Studies 19 (2016) 222–233. [DOI] [PubMed] [Google Scholar]
- [34].Lopriore E, Slaghekke F, Oepkes D, Middeldorp JM, Vandenbussche FP, Walther FJ, Hematological characteristics in neonates with twin anemia-polycythemia sequence (TAPS), Prenat. Diagn. 30 (2010) 251–255. [DOI] [PubMed] [Google Scholar]
- [35].Couck I, Lewi L, The placenta in twin-to-twin transfusion syndrome and twin anemia polycythemia sequence, Twin Res. Hum. Genet. : the official journal of the International Society for Twin Studies 19 (2016) 184–190. [DOI] [PubMed] [Google Scholar]
- [36].De Paepe ME, Burke S, Luks FI, Pinar H, Singer DB, Demonstration of placental vascular anatomy in monochorionic twin gestations, Pediatr. Dev. Pathol. 5 (2002) 37–44. [DOI] [PubMed] [Google Scholar]
- [37].Paepe ME, Examination of the twin placenta, Semin. Perinatol. 39 (2015) 27–35. [DOI] [PubMed] [Google Scholar]
- [38].De Paepe ME, Gundogan F, Mao Q, Chu S, Shapiro S, Redness discordance in monochorionic twin placentas: correlation with clinical and placental findings, Placenta 60 (2017) 54–60. [DOI] [PubMed] [Google Scholar]
- [39].Ravishankar S, Bourjeily G, Lambert-Messerlian G, He M, De Paepe ME, Gundogan F, Evidence of placental hypoxia in maternal sleep disordered breathing, Pediatr. Dev. Pathol. 18 (2015) 380–386. [DOI] [PubMed] [Google Scholar]
- [40].Manders EMM, Verbeek FJ, Aten JA, Measurement of co-localization of objects in dual-colour confocal images, J. Microsc. 169 (1993) 375–382. [DOI] [PubMed] [Google Scholar]
- [41].Chu S, Mao Q, Shapiro S, De Paepe ME, Placental endoglin levels in diamniotic-monochorionic twin gestations: correlation with clinical and placental characteristics, Placenta 34 (2013) 261–268. [DOI] [PubMed] [Google Scholar]
- [42].Potter C, Harris AL, Hypoxia inducible carbonic anhydrase IX, marker of tumour hypoxia, survival pathway and therapy target, Cell Cycle 3 (2004) 164–167. [PubMed] [Google Scholar]
- [43].Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL, Hypoxia-inducible expression of tumor-associated carbonic anhydrases, Cancer Res. 60 (2000) 7075–7083. [PubMed] [Google Scholar]
- [44].Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL, New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation, Oncogene 29 (2010) 6509–6521. [DOI] [PubMed] [Google Scholar]
- [45].Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T, LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing, EMBO J. 19 (2000) 5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Klionsky DJ, et al. , Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition), Autophagy 12 (2016) 1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T, LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation, J. Cell Sci. 117 (2004) 2805–2812. [DOI] [PubMed] [Google Scholar]
- [48].Kornfeld S, Mellman I, The biogenesis of lysosomes, Annu. Rev. Cell Biol. 5 (1989) 483–525. [DOI] [PubMed] [Google Scholar]
- [49].Eskelinen EL, Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy, Mol. Asp. Med. 27 (2006) 495–502. [DOI] [PubMed] [Google Scholar]
- [50].Varanou A, Withington SL, Lakasing L, Williamson C, Burton GJ, Hemberger M, The importance of cysteine cathepsin proteases for placental development, J. Mol. Med. (Berl.) 84 (2006) 305–317. [DOI] [PubMed] [Google Scholar]
- [51].Benes P, Vetvicka V, Fusek M, Cathepsin D–many functions of one aspartic protease, Crit. Rev. Oncol.-Hematol. 68 (2008) 12–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bajaj L, Lotfi P, Pal R, Ronza AD, Sharma J, Sardiello M, Lysosome biogenesis in health and disease, J. Neurochem. 148 (2019) 573–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A, A gene network regulating lysosomal biogenesis and function, Science 325 (2009) 473–477. [DOI] [PubMed] [Google Scholar]
- [54].Napolitano G, Ballabio A, TFEB at a glance, J. Cell Sci. 129 (2016) 2475–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A, TFEB links autophagy to lysosomal biogenesis, Science 332 (2011) 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T, p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy, J. Biol. Chem. 282 (2007) 24131–24145. [DOI] [PubMed] [Google Scholar]
- [57].Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K, Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice, Cell 131 (2007) 1149–1163. [DOI] [PubMed] [Google Scholar]
- [58].Xie Z, Klionsky DJ, Autophagosome formation: core machinery and adaptations, Nat. Cell Biol. 9 (2007) 1102–1109. [DOI] [PubMed] [Google Scholar]
- [59].Feng Y, He D, Yao Z, Klionsky DJ, The machinery of macroautophagy, Cell Res. 24 (2014) 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lee SJ, Ryter SW, Xu JF, Nakahira K, Kim HP, Choi AM, Kim YS, Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation, Am. J. Respir. Cell Mol. Biol. 45 (2011) 867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tanida I, Ueno T, Kominami E, LC3 conjugation system in mammalian autophagy, Int. J. Biochem. Cell Biol. 36 (2004) 2503–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Barth S, Glick D, Macleod KF, Autophagy: assays and artifacts, J. Pathol. 221 (2010) 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G, Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles, Nat. Rev. Drug Discov. 16 (2017) 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nakashima A, Aoki A, Kusabiraki T, Shima T, Yoshino O, Cheng SB, Sharma S, Saito S, Role of autophagy in oocytogenesis, embryogenesis, implantation, and pathophysiology of pre-eclampsia, J. Obstet. Gynaecol. Res. 43 (2017) 633–643. [DOI] [PubMed] [Google Scholar]
- [65].Mizushima N, Methods for monitoring autophagy, Int. J. Biochem. Cell Biol. 36 (2004) 2491–2502. [DOI] [PubMed] [Google Scholar]
- [66].Jiang P, Mizushima N, LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells, Methods 75 (2015) 13–18. [DOI] [PubMed] [Google Scholar]
- [67].Kroemer G, Marino G, Levine B, Autophagy and the integrated stress response, Mol. Cell 40 (2010) 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Muralimanoharan S, Gao X, Weintraub S, Myatt L, Maloyan A, Sexual dimorphism in activation of placental autophagy in obese women with evidence for fetal programming from a placenta-specific mouse model, Autophagy 12 (2016) 752–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kojima T, Yamada T, Akaishi R, Furuta I, Saitoh T, Nakabayashi K, Nakayama KI, Nakayama K, Akira S, Minakami H, Role of the Atg9a gene in intrauterine growth and survival of fetal mice, Reprod. Biol. 15 (2015) 131–138. [DOI] [PubMed] [Google Scholar]
- [70].Bainbridge SA, Roberts JM, von Versen-Hoynck F, Koch J, Edmunds L, Hubel CA, Uric acid attenuates trophoblast invasion and integration into endothelial cell monolayers, Am. J. Physiol. Cell Physiol. 297 (2009) C440–C450. [DOI] [PMC free article] [PubMed] [Google Scholar]