Abstract
Background
This paper aims to compare the efficacy and safety of recombinant human endostatin combined with chemotherapy in patients with squamous cell lung cancer (SqCLC).
Methods
We searched the Cochrane Library, PubMed, Embase, CNKI, Wanfang database, Metstr, VIP, and others and manually searched books and magazines until 2019 for articles about the efficacy and safety of recombinant human endostatin combined with chemotherapy in patients with SqCLC. A second search was conducted on the review literature. According to the criteria of the literature screen, the relevant randomized controlled trials (RCTs) and nonrandomized controlled trials (non-RCTs) of recombinant human endostatin combined with chemotherapy and chemotherapy alone in the treatment of SqCLC were included. After the data were extracted and analyzed, RevMan 5.3 software was used for meta-analysis for the outcome indicators. Then, heterogeneity tests and sensitivity analyses were carried out, and the publication bias of this study was tested in Stata 13.0 software. Six RCTs and eight non-RCTs were included. In total, 821 patients with SqCLC were included.
Results
The response rate (RR) was 2.12 (95% CI: 1.57–2.85, p < 0.00001). The disease control rate (DCR) was 2.38 (95% CI: 1.70–3.32, p < 0.00001). The difference between the two groups was statistically significant. Regarding safety, the incidence rates of the adverse reactions cardiotoxicity, leukopenia, thrombocytopenia, and gastrointestinal reactions were not significantly different between the two groups (OR = 1.70, 95% CI: 0.79–3.68; OR = 0.93, 95% CI: 0.61–1.42; OR = 1.08, 95% CI: 0.71–1.64; OR = 0.86, 95% CI: 0.56–1.30, respectively).
Conclusion
The combined treatment had a better therapeutic effect than chemotherapy alone. It did not increase the incidence of adverse reactions in the course of treatment.
Keywords: Recombinant human endostatin, Endostar, Endostar combined chemotherapy, Squamous cell lung cancer, Response rate, Disease control rate, Meta-analysis
Background
Lung cancer is one of the leading causes of cancer-associated deaths worldwide [1]. In 2018, there were an estimated 2.1 million new lung cancer cases and 1.8 million deaths worldwide, accounting for 11.6% and 18.4% of the cancer incidence and death, respectively. It is estimated that the number of patients diagnosed with lung cancer in the world within 5 years will be 2.13 million [2]. Non-small cell lung cancer (NSCLC) accounts for more than 80% of lung cancers. It can be divided into adenocarcinoma, SqCLC and large cell cancer, and SqCLC accounts for approximately 1/3 of NSCLC [3].
Surgery is still the main treatment for early SqCLC. Most patients have reached the advanced stage when they are diagnosed and so have missed the best time of surgery. At present, the preferred treatment of these cases is still based on chemotherapy and radiotherapy, but the overall prognosis is poor, so the treatment of SqCLC is urgent. Tyrosine kinase inhibitors (TKIs), such as gefitinib, erlotinib, and icotinib, have become the first-line treatment option for patients with driver gene-positive NSCLC. Compared with other types of lung cancer, SqCLC is more closely related to smoking. Due to the low gene mutation rate, the effect of molecular targeted drug therapy is not significant at present [4].
The growth, maintenance, and metastasis of solid tumors depend on the generation of new blood vessels. Angiogenesis in tumors is a multistep and complex process that is regulated by both angiogenesis-promoting factors and angiogenesis-inhibiting factors [5]. The angiogenesis-promoting and angiogenesis-inhibiting factors in solid tumors are in a dynamic balance when the tumor is in the dormant state. Professor Folkman of Harvard Medical School proposed the strategy of blocking tumor angiogenesis, then cutting off the tumor nutrition supply to kill it in the 1970s, which pioneered a therapeutic strategy to target tumor blood vessels [6]. The emergence and application of many anti-tumor angiogenesis drugs can break that dynamic balance and break the tumor out of its dormant state to inhibit its angiogenesis. Antiangiogenic therapy has gradually become an indispensable option in current cancer treatment. For example, bevacizumab and recombinant human endostatin combined with cytotoxic drugs are commonly administered in advanced NSCLC. Aflibercept has been approved for the second-line treatment of advanced colorectal cancer. Multitarget kinase inhibitors such as sorafenib, sunitinib, and pazopanib are commonly administered for advanced renal cell carcinoma. Because the first-line treatment for patients with advanced SqCLC is still based on platinum-based doublet chemotherapy, which is associated with poor survival, rare gene mutations, and poor effects when using TKIs, the proposal of anti-tumor angiogenesis theory provides a new research direction for patients with SqCLC.
Recombinant human endostatin (trade name: Endostar, code: YH-16) is endostatin with an additional nine-amino-acid sequence at its N terminus. It is more stable and more potent and appeared on the market in China in 2005 [7]. It is a vascular endothelial growth factor inhibitor that can specifically act on microvascular endothelial cells, thereby inhibiting tumor angiogenesis, cutting off the nutrient supply and the metastasis channels of tumors, and eventually inducing tumoral apoptosis. Several clinical trials have suggested that Endostar combined with chemotherapy for NSCLC, including SqCLC, has brought better results than therapy with the original endostatin. Since it came to market, it has been clinically applied for many solid tumors, such as lung cancer, malignant melanoma, osteosarcoma, and nasopharyngeal carcinoma. Bevacizumab combined with cytotoxic drugs is commonly used in the treatment of NSCLC and colorectal cancer. However, in a phase II clinical trial to evaluate the efficacy of bevacizumab for advanced NSCLC patients (Study AVF0757g) [8], 6 cases of pulmonary hemorrhage occurred, of which 5 cases were in the low-dose bevacizumab group and 4 cases were life-threatening. The study showed that pulmonary hemorrhage was related to the central location of the tumor, and the clinical manifestations of squamous cell lung cancer are often of the central type. Squamous cell lung cancer was classified as the main risk factor leading to hemorrhage, so patients with SqCLC were excluded from the clinical trials. As a result, the efficacy and safety of anti-tumor vascular-targeted drugs, including Endostar, in the treatment of patients with SqCLC is still under debate. This article aims to conduct a meta-analysis of the efficacy and safety of Endostar combined with chemotherapy for advanced SqCLC to provide a high-quality reference for future clinical work.
Methods
Search strategy
The current study was conducted on the basis of the PRISMA guidelines (the Preferred Reporting Item for Systematic Reviews and Meta-Analyses) and the PRISMA extension statement on meta-analyses to obtain accurate outcomes in clinical practice. The literature retrieval was carried out online from the Cochrane Library, PubMed, Embase, CNKI, Wanfang Chinese Database, Metstr, VIP, etc., and the retrieval of books and journals were completed manually. The comprehensive literature was searched twice. The search period was from the start of each database up to 2019. The search key words for Metstr were as follows: (“Recombinant human endostatin” OR “Endostar” OR “rh-endostatin” OR “ endostatin” OR “Endu”) AND (“squamous cell lung cancer” OR “SqCLC” OR “lung squamous cell carcinoma” OR “squamous cell lung carcinoma”) AND “chemotherapy.” The query for PubMed was (Recombinant human endostatin[MeSH Terms]) OR (Endostar[MeSH Terms]) OR (rh-endostatin[MeSH Terms]) OR (endostatin[MeSH Terms]) OR (Endu[MeSH Terms]) AND (squamous cell lung cancer) OR (SqCLC) OR (lung squamous cell carcinoma) OR (squamous cell lung carcinoma) OR (lung cancer) AND (chemotherapy) OR (Endostar combined chemotherapy) OR (chemotherapy group alone).
Inclusion and exclusion criteria
The following were the inclusion criteria: (a) patients: they were diagnosed with SqCLC or NSCLC (with specific SqCLC typing and efficacy analysis in the study) and TNM stage III or IV; (b) interventions: the patients in the control group were given chemotherapy, while the patients in the experimental group were treated with Endostar on the basis of the control treatment; and (c) outcome indicators: the total effective rate, disease control rate, and drug-related adverse reactions were extracted from the studies. Studies without a control group and studies in which patients had additional cancer, comorbidities, or organ dysfunction were excluded.
Data extraction
Two authors independently extracted the data. Disagreements were resolved by discussion until a consensus was reached or by consulting a third author.
The content extracted from each paper included the following: (1) study information: the authors and the year of publication; (2) the number of cases in the experimental group and control group; (3) the study indicators, including PFS; (4) the baseline characteristics, including sex, age, and chemotherapy.
Quality evaluation
The quality of the RCTs was evaluated on the modified Jadad scale [9]. This covers random sequence generation, allocation concealment, blinding, loss to follow-up, and withdrawal. The total score is seven, with four to seven indicating high-quality research and less than 4 being indicating low-quality research.
The quality scores of the non-RCTs were evaluated by the Newcastle-Ottawa Scale (NOS) [10].
Statistical analysis
RevMan 5.3 statistical software was used for the meta-analysis. The chi-square test was used to test the heterogeneity of the included studies. If p was > 0.10 and I2 was not more than 50%, there was no statistical heterogeneity among the results of the studies, and a fixed-effect model (Mantel-Haenszel method) was used for analysis; otherwise, a random-effect model was used. Dichotomous variables were analyzed by estimating odds ratios (ORs) with 95% confidence intervals (CIs) for interval estimation. p < 0.05 was statistically significant. The publication bias of the articles was analyzed using an inverted funnel plot [11]. Begg’s and Egger’s tests were performed using Stata 13.0 software to quantify the bias. When p > 0.05 for Begg’s test and p > 0.10 for Egger’s test, no significant publication bias was suggested.
Results
Study inclusion and characteristics of the literature
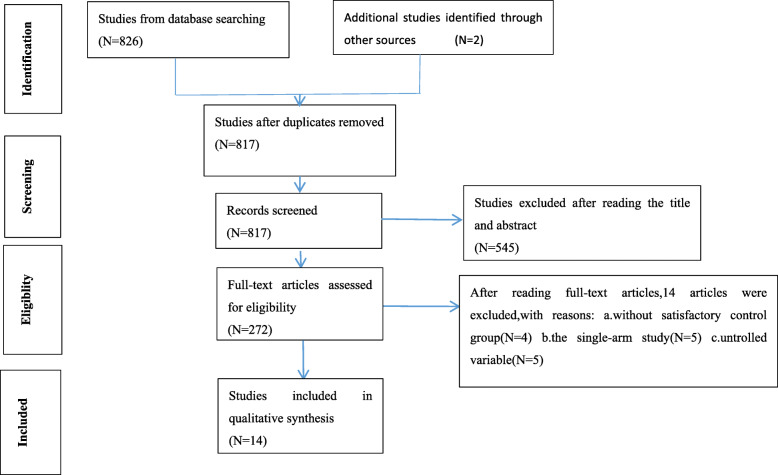
From the 828 articles originally returned by the keywords, 452 articles were rejected by browsing the titles. After reading the summary of each remaining article, 272 articles remained to be read in full. Fourteen articles were included in the study after reading the full text [12–25] (Fig. 1). The characteristics of the literature are given in Table 1.
Fig. 1.
The flow diagram of the meta-analysis
Table 1.
Characteristics of the included studies
| Studies | Number (C/E) | Age | Sex (F/M) | Regimens | PFS (C/E, months) |
|---|---|---|---|---|---|
| Wu F [12] | 25/25 | 37–74 | 15/35 | GNE vs GN | 5.72 ± 0.89/6.90 ± 1.15 |
| Song WC [13] | 30/30 | 33–74 | 26/34 | GPE vs GP | – |
| Wang ZF [14] | 30/34 | 35–70 | 3/61 | GPE vs GP | 7.35 ± 0.52/13.41 ± 1.23 |
| Chen Q [15] | 19/21 | 65–85 | – | GE vs G | 3.7/4 |
| Lv Y [16] | 10/10 | 60–83 | 4/16 | DPE vs DP | – |
| Yang L [17] | 37/35 | – | 13/59 | GPE vs GP | 5.5/7.0 |
| Zheng X [18] | 40/40 | 60–69 | 29/51 | GPE vs GP | 6.12 ± 1.78/9.51 ± 2.15 |
| Wang W [19] | 50/50 | 51–56 | 40/60 | PPE vs PP | 6.3/8.4 |
| Xun Yu [20] | 14/12 | 46.78–65.88 | – | GCE vs GC | 5.1 ± 0.6/8.2 ± 1.3 |
| Shi HL [21] | 6/3 | 37–74 | – | NPE vs NP | – |
| Yang L [22] | 10/24 | 33–78 | – | VPE vs VP | – |
| Wang QZ [23] | 29/29 | – | 12/46 | DPE vs DP | 4.97/7.17 |
| Zhang YY [24] | 26/27 | 53–54 | 6/53 | GPE vs GP | 6.5/8.3 |
| Hong S [25] | 52/98 | 32–80 | 14/136 | GPE vs GP | – |
Note: The dashes represent no data;
C the control group, E the experimental group, F female, M male; GNE gemcitabine + nedaplatin + Endostar, GN gemcitabine + nedaplatin, GPE gemcitabine + cisplatin + Endostar, GP gemcitabine + cisplatin, GE gemcitabine + Endostar, G gemcitabine, DPE docetaxel + cisplatin + Endostar, DP docetaxel + cisplatin, PPE paclitaxel + cisplatin + Endostar, PP paclitaxel + cisplatin, GCE gemcitabine + carboplatin + Endostar, GC gemcitabine + carboplatin, NPE norvincristine + cisplatin + Endostar, NP norvincristine + cisplatin, VPE vinorelbine + cisplatin + Endostar, VP vinorelbine + cisplatin
The quality assessment of studies
A total of 14 articles were identified in the study, including six RCTs [12–15, 23, 24] and eight non-RCTs [16–22, 25], which were scored using corresponding quality assessment criteria. Three of six RCTs had scores lower than four points and so were evaluated as low-quality research, and the others were high-quality research. The eight non-RCTs all had scores above 5, making them high-quality studies. The quality score sheets are shown in Tables 2 and 3. The risk assessment of the RCTs was performed using the Cochrane risk assessment tool, as illustrated in Fig. 2.
Table 2.
The quality assessment of the included RCTs
| Researcher | Year | Sequence generation | Allocation concealment | Blinding | Lost and withdrawal | Jadad |
|---|---|---|---|---|---|---|
| Wu F | 2018 | Sufficient (2) | Unclear (1) | Not mentioned (1) | No (0) | 4 |
| Song WC | 2018 | Insufficient (0) | Insufficient (0) | Not mentioned (1) | Yes (1) | 2 |
| Wang ZF | 2018 | Unclear (1) | Unclear (1) | Not mentioned (1) | No (0) | 3 |
| Chen Q | 2014 | Unclear (1) | Unclear (1) | Not mentioned (1) | Yes (1) | 4 |
| Wang QZ | 2019 | Unclear (1) | Unclear (1) | Not mentioned (1) | No (0) | 3 |
| Zhang YY | 2014 | Unclear (1) | Unclear (1) | Not mentioned (1) | Yes (1) | 4 |
Table 3.
The quality assessment of non-RCTs
| Researcher | Year | Population selection | Comparability between groups | Outcome data | Score |
|---|---|---|---|---|---|
| Lv Y | 2015 | 2 | 2 | 3 | 7 |
| Yang L | 2018 | 1 | 2 | 3 | 6 |
| Zheng X | 2018 | 2 | 2 | 3 | 7 |
| Wang W | 2017 | 2 | 2 | 3 | 7 |
| Xun Yu | 2018 | 1 | 2 | 3 | 6 |
| Shi HL | 2004 | 2 | 2 | 3 | 7 |
| Yang L | 2005 | 1 | 2 | 3 | 6 |
| Hong S | 2016 | 2 | 2 | 3 | 7 |
Fig. 2.
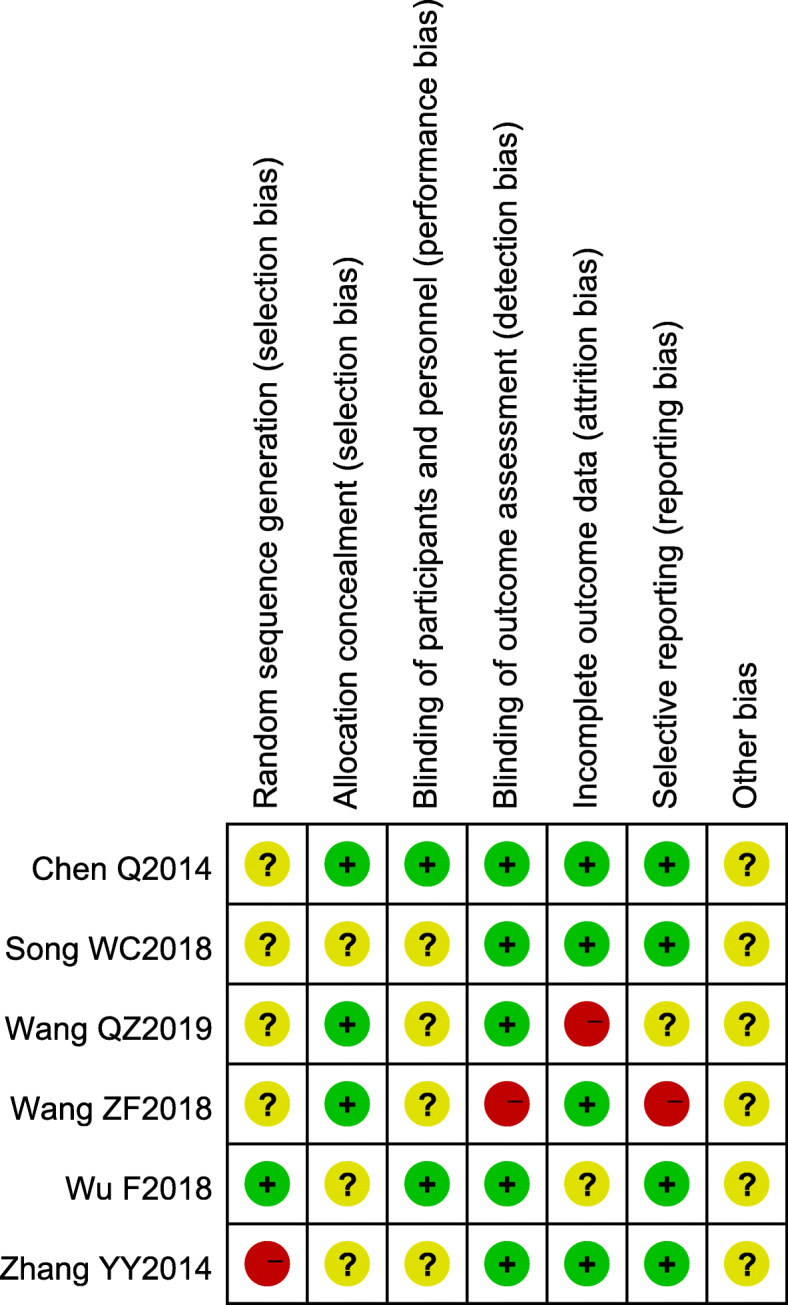
Individual risk of bias of the included RCT studies
The clinical efficacy and safety
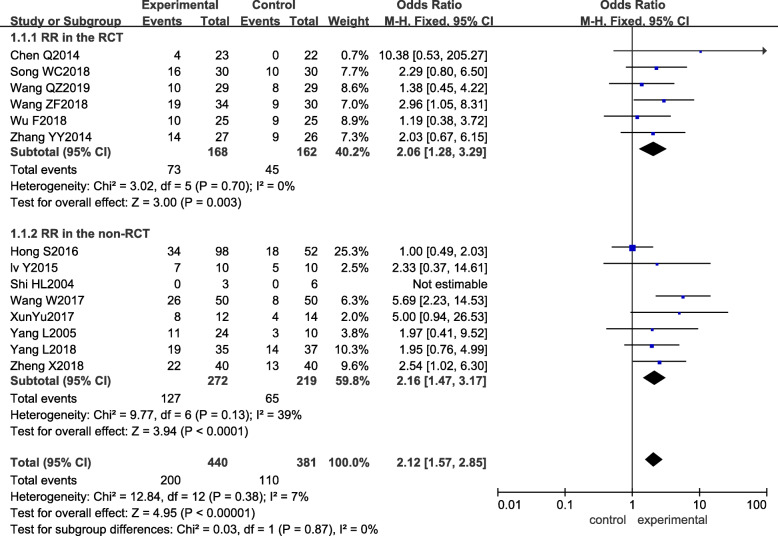
All studies reported the response rate (RR). There was no significant heterogeneity between studies, so a fixed-effect model was used for meta-analysis (p = 0.38, I2 = 7%). The difference in RR between the experimental group and the control group was statistically significant (ORmixed = 2.12, 95% CI: 1.57–2.85, and p < 0.00001), the RR of the experimental group being higher than that of the control group. The results of the subgroup analysis were the same as those obtained in the RCTs and non-RCTs, as shown in Fig. 3.
Fig. 3.
Forest plot of the experimental group vs the control group for response rate
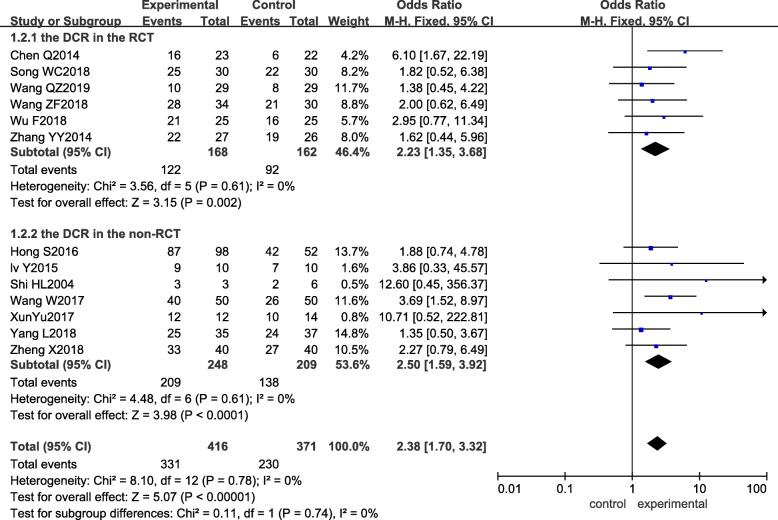
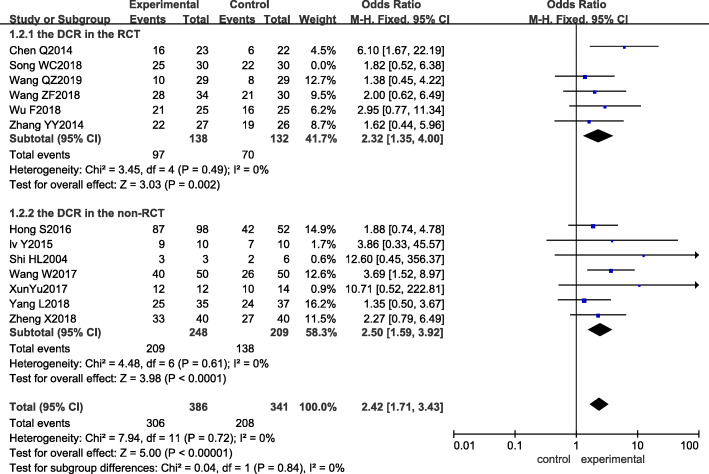
Among the 14 studies included, 13 studies [12–21, 23–25] reported the disease control rate (DCR) as an outcome indicator. The result of the heterogeneity test showed that I2 was 0%, and P was 0.78, which indicated that there was no significant heterogeneity among the studies. Meta-analysis with the fixed-effect model revealed that ORmixed = 2.38, 95% CI: 1.70–3.32, and p < 0.00001, suggesting that there was a significant difference between the two groups. The experimental group showed a better DCR than the control group. Subgroup analysis showed that both the RCT and non-RCT subgroups had higher DCRs, as shown in Fig. 4.
Fig. 4.
Forest plot of the experimental group vs the control group for disease control rate
Adverse reactions (gastrointestinal reactions, cardiotoxicity, liver and kidney function damage, fatigue, leukopenia, thrombocytopenia, etc.) were also analyzed by meta-analysis. The fixed-effect model was used for the analysis of overall adverse event rates. The p values were greater than 0.05, as shown in Table 4. The incidence of adverse reactions in the two groups was almost the same, which was basically consistent with the findings of domestic and foreign studies [26, 27]. The incidence of grade III and IV adverse reactions was mentioned in four studies [12, 13, 23, 25], and the difference between the experimental group and the control group was not statistically significant (p > 0.05), further suggesting that Endostar is safe.
Table 4.
The meta-analysis of adverse reactions
| Adverse reaction | Number of included studies | Heterogeneity | Outcome model | OR | 95% CI | p | |
|---|---|---|---|---|---|---|---|
| P | I2 | ||||||
| Gastrointestinal reactions | 8 [12–14, 16–19, 24] | 0.83 | 0% | Fixed-effect model | 0.86 | (0.56,1.30) | 0.46 |
| Cardiotoxicity | 5 [12–14, 17, 24] | 0.68 | 0% | Fixed-effect model | 1.70 | (0.79,3.68) | 0.18 |
| Liver and kidney function damage | 4 [12, 17, 18, 24] | 0.75 | 0% | Fixed-effect model | 0.77 | (0.40,1.50) | 0.45 |
| Fatigue | 5 [13, 14, 17, 18, 24] | 0.59 | 0% | Fixed-effect model | 1.06 | (0.60,1.87) | 0.85 |
| Leukopenia | 6 [13, 14, 17–19, 24] | 0.99 | 0% | Fixed-effect model | 0.93 | (0.61,1.42) | 0.74 |
| Thrombocytopenia | 7 [12–14, 17–19, 24] | 0.99 | 0% | Fixed-effect model | 1.08 | (0.71,1.64) | 0.72 |
Sensitivity analysis
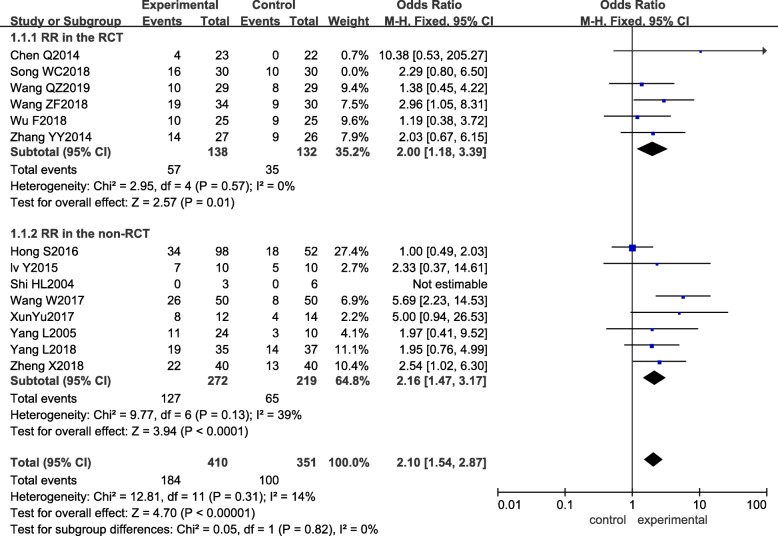
Sensitivity analysis was performed next. By removing each included study one by one and carrying out a pooled analysis, the obtained pooled effect OR and 95% CI still suggested that the experimental group had better RR and DCR, and the heterogeneity after individual study removal was similar to before, as seen in Tables 5 and 6 in detail. According to the results of the quality evaluation of the included studies, the Q value and I2 of the response rate and disease control rate after removal of Song WC [13], which had lower scores, were p = 0.31, I2 = 14%, and p = 0.72, I2=0%, respectively, showing no significant change in heterogeneity, meaning the results of this study were robust, as shown in Figs. 5 and 6.
Table 5.
Sensitivity analysis of response rate
| Removed study | p | I2 (%) | Odds ratio (95% CI) |
|---|---|---|---|
| – | 0.38 | 7 | 2.12 (1.57, 2.85) |
| Chen Q [15] | 0.39 | 6 | 2.06 (1.53, 2.78) |
| Song WC [13] | 0.31 | 14 | 2.10 (1.54, 2.87) |
| Wang ZF [14] | 0.34 | 11 | 2.06 (1.51, 2.80) |
| Wu F [12] | 0.38 | 7 | 2.21 (1.62, 3.01) |
| Wang QZ [23] | 0.34 | 10 | 2.19 (1.61, 2.98) |
| Zhang YY [24] | 0.30 | 14 | 2.13 (1.56, 2.89) |
| Lv Y [16] | 0.30 | 14 | 2.11 (1.56, 2.86) |
| Shi HL [21] | 0.38 | 7 | 2.12 (1.57, 2.85) |
| Wang W [19] | 0.72 | 0 | 1.88 (1.37, 2.58) |
| Xun Yu [20] | 0.38 | 6 | 2.06 (1.52, 2.79) |
| Yang L [22] | 0.30 | 14 | 2.12 (1.57, 2.88) |
| Yang L [17] | 0.31 | 14 | 2.14 (1.56, 2.92) |
| Zheng X [18] | 0.32 | 13 | 2.07 (1.51, 2.84) |
| Hong S [25] | 0.73 | 0 | 2.50 (1.80, 3.47) |
Table 6.
Sensitivity analysis of disease control rate
| Removed study | p | I2 (%) | Odds ratio (95% CI) |
|---|---|---|---|
| – | 0.78 | 0 | 2.38 (1.70, 3.32) |
| Chen Q [15] | 0.89 | 0 | 2.21 (1.56, 3.13) |
| Song WC [13] | 0.72 | 0 | 2.42 (1.71, 3.43) |
| Wang ZF [14] | 0.71 | 0 | 2.41 (1.70, 3.42) |
| Wu F [12] | 0.72 | 0 | 2.34 (1.66, 3.31) |
| Wang QZ [23] | 0.78 | 0 | 2.51 (1.76, 3.56) |
| Zhang YY [24] | 0.73 | 0 | 2.44 (1.73, 3.45) |
| Lv Y [16] | 0.72 | 0 | 2.35 (1.68, 3.30) |
| Shi HL [21] | 0.79 | 0 | 2.32 (1.66, 3.25) |
| Wang W [19] | 0.81 | 0 | 2.20 (1.53, 3.16) |
| Xun Yu [20] | 0.79 | 0 | 2.30 (1.64, 3.23) |
| Yang L [17] | 0.81 | 0 | 2.55 (1.79, 3.65) |
| Zheng X [18] | 0.70 | 0 | 2.83 (1.84, 4.35) |
| Hong S [25] | 0.72 | 0 | 2.39 (1.68, 3.40) |
Fig. 5.
Sensitivity of the experimental group vs the control group for response rate
Fig. 6.
Sensitivity of the experimental group vs the control group for disease control rate
Publication bias
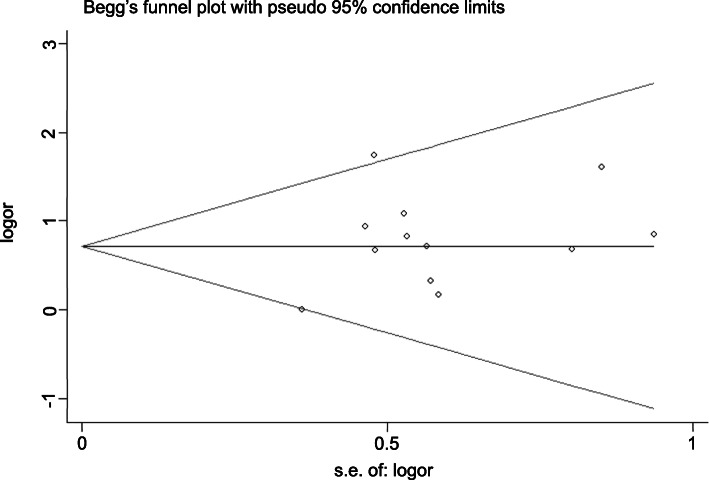
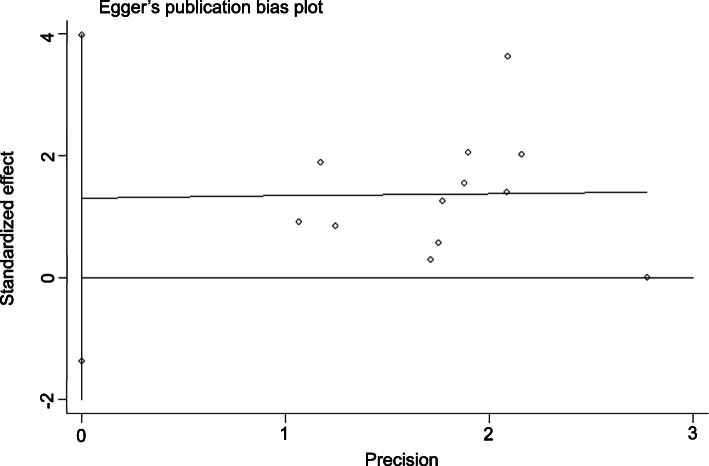
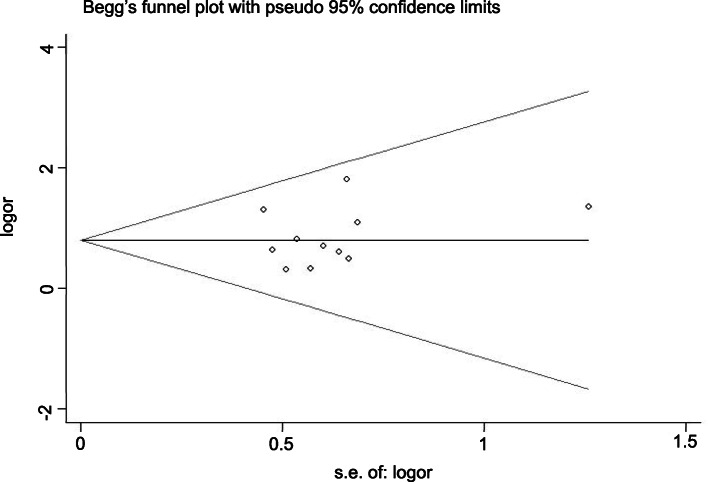
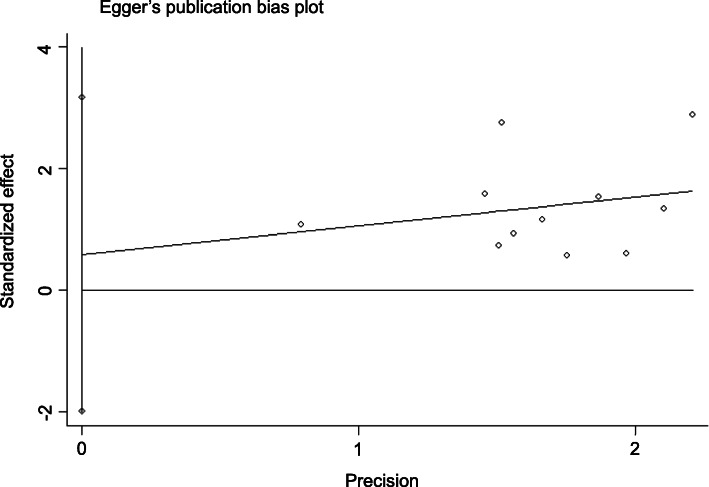
Publication bias analysis was performed using Stata 13.0 software. Begg’s test showed p = 0.945 > 0.05, and Egger’s test showed p = 0.302 > 0.10, suggesting that there was no significant publication bias in the analysis of RR. The DCR had p = 0.436 > 0.05 by Begg’s test and p = 0.615 > 0.10 by Egger’s test, suggesting that there was no significant bias in the analysis of DCR. We can conclude that the publication bias of this paper is small and that the included studies are comprehensive (Figs. 7, 8, 9, 10).
Fig. 7.
Begg’s funnel plot of the experimental group vs the control group for response rate
Fig. 8.
Egger’s publication bias plot of the experimental group vs the control group for response rate
Fig. 9.
Begg’s funnel plot of the experimental group vs the control group for disease control rate
Fig. 10.
Egger’s publication bias plot of the experimental group vs the control group for disease control rate
Discussion
Endostar is a new biological drug independently researched and developed in China and is the first anti-tumor vascular-targeted endothelial inhibitor in the world. It was approved by the State Food and Drug Administration (SFDA) for marketing in 2005 and approved for combination with vinorelbine and cisplatin for NSCLC in 2016. Studies have shown that Endostar can specifically inhibit the migration of endothelial cells and induce the apoptosis of endothelial cells, thereby targeting the inhibition of angiogenesis [7]. In China, many scholars have carried out meta-analyses on the efficacy of Endostar combined with chemotherapy for NSCLC. However, the biological behaviors of SqCLC and adenocarcinoma are completely different. There have been few studies on the meta-analysis of Endostar combined with chemotherapy in squamous cell lung cancer at home or abroad, so we carried out a meta-analysis to estimate its outcomes. In terms of efficacy, the difference between the experimental group and the control group was statistically significant (ORmixed=2.12, 95% CI: 1.57–2.85, and p < 0.00001), the RR of the experimental group being higher than that of the control group. With regard to DCR, the outcome was ORmixed = 2.38, 95% CI: 1.70–3.32, and p < 0.00001. The results were not significantly heterogeneous. According to sensitivity analysis, the results of this study are stable, and the publication bias is small. The included studies were comprehensive, so this meta-analysis provides a strong evidence-based foundation for the treatment of patients with advanced SqCLC in clinical practice. Common adverse reactions, consisting of myelosuppression, fatigue, cardiotoxicity, gastrointestinal reactions, and so forth, were mentioned, but there was no statistically significant difference between the experimental group and the control group. Five studies mentioned the condition of cardiotoxicity. A total of 302 patients were included in the meta-analysis of this condition under a fixed-effects model, and the OR was 1.70 (95% CI: 0.79–3.68, p = 0.18) (Table 4). Although the difference was not significant, it still suggested that patients with Endostar are more susceptible to cardiotoxicity. The clinical features showed increased heart rate, palpitations, changes in the T wave and ST-T segment of the electrocardiogram, etc. Therefore, more attention should be paid to the use of ECG monitoring and echocardiograms and closely monitoring patients’ heart function. Other, rare adverse reactions, such as peripheral neurotoxicity, hemorrhage, and diarrhea, are not mentioned in these studies but should not be neglected by clinicians.
Our research has some limitations. Randomized and nonrandomized controlled trials were included, and most RCT studies did not report how they created the random sequences and implemented blinding. Confusion bias inevitably arose. Endostar was administered intravenously in the two trials [15, 2 0], different from infusion pump administration in other trials. Therefore, the mode of administration, the sequence of administration, and the age and sex of patients could all have affected the result, but there was no corresponding data analysis. More factors affecting the efficacy also need to be studied. Miao M [28] suggested that the therapeutic effect, effective rate, and disease control rate after intravenous pump infusion of Endostar were all better than those after intravenous drip infusion (p < 0.05). Bone marrow suppression and cardiotoxicity in the pump infusion group were less than those in the intravenous drip group. In addition, the degree was alleviated. Therefore, intravenous pumping may be a better option for delivering Endostar clinically. Only two studies included in this paper were in English because Endostar has not come to any markets outside of China. This avoided the influence of gene polymorphism to some extent. Because of the limited number of studies included and the missing trials with negative results, a publication bias could be in play. Endostar, as a novel choice, still has many issues to be solved. More research with larger sample sizes and higher quality is needed to confirm that Endostar with chemotherapy can bring better efficacy and safety.
Conclusion
The results showed that Endostar combined with chemotherapy had better efficacy than chemotherapy alone. The adverse reactions were not significantly different between the two groups.
Acknowledgements
Not applicable.
Authors’ contributions
LF designed the study, and ZW drafted the manuscript. LJ and ZZ were responsible for the collection and analysis of the experimental data. SS, RD, YL, and QM revised the manuscript critically for important intellectual content. The authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University, China. Signed written informed consent was obtained from the patients and/or their guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li Feng and Zhicong Wang contributed equally to this work.
Contributor Information
Yibing Liu, Email: lyb.he@163.com.
Qingju Meng, Email: qingjumeng@163.com.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics, 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Ontario Health (Quality) Cell-free circulating tumour DNA blood testing to detect EGFR T790M mutation in people with advanced non–small cell lung cancer: a health technology assessment. Ont Health Technol Assess Ser. 2020;20:1–176. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang YC, Zhou Q, Wu YL. Emerging challenges of advanced squamous cell lung cancer. ESMO Open. 2016;1:e000129. doi: 10.1136/esmoopen-2016-000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/S0065-230X(08)60946-X. [DOI] [PubMed] [Google Scholar]
- 7.Jia H, Kling J. China offers alternative gateway for experimental drugs. Nat Biotechnol. 2006;24:117–8. [DOI] [PubMed]
- 8.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2542–2550. doi: 10.1200/jco.2004.22.90140.2542. [DOI] [PubMed] [Google Scholar]
- 9.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of non-randomized studies in meta-analysis. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 11.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu F, Wang XH, Chen L, et al. Clinical observation of continuous infusion of endostar combined with GP chemotherapy in the treatment of advanced squamous cell carcinoma of the lung. Contemp Med. 2018;24:34–36. [Google Scholar]
- 13.Song WC, Bao Y, Qian J. Study on clinical efficacy of endostar combined with Gemcitabine and Cisplatin in the treatment of advanced lung squamous cell carcinoma and effect on quality of life. Pract Cancer J. 2018;33:60–62. [Google Scholar]
- 14.Wang ZF. Effect of endostar combined first-line GP chemotherapy on survival time and quality of life in patients with advanced squamous cell lung carcinoma. Med Theory Pract. 2018;31:210–211. [Google Scholar]
- 15.Chen Q, Shi Q, Xie Q, et al. Comparison of gemcitabine alone and gemcitabine combined with Rh-endostatin as firstline treatment for elderly with advanced non-small cell lung cancer: a randomized controlled trial. Chin J Cancer Prev. 2014;6:265–270. [Google Scholar]
- 16.Lv Y, Jiang R, Ma CH, et al. Clinical observation of continuous intravenous infusion of recombinant human endostatin combined with window-stage arterial infusion chemotherapy for advanced squamous cell lung carcinoma. Zhongguo Fei Ai Za Zhi. 2015;18:500–504. doi: 10.3779/j.issn.1009-3419.2015.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Tang H, Wu YF, et al. Efficacy and safety of endostar combined with chemotherapy as the first-line treatment for advanced squamous cell lung cancer. Chin J Pract Med. 2018;45:4–7. [Google Scholar]
- 18.Zheng X, Jiang H, Yu X. The efficacy and impact on relevant indicators of recombinant human endostatin combined with chemotherapy on advanced lung squamous cell carcinoma. Med Clin Res. 2018;35:776–778. [Google Scholar]
- 19.Wang W, Ren BY. Therapeutic effect of recombinant human endostatin combined with TP in patients with advanced EGFR wild-type squamous cell lung cancer. Int J Oncol. 2017;44:745–748. [Google Scholar]
- 20.Yu X, Zhang L, Chen J. Effectiveness of treatment with endostatin in combination with gemcitabine, carboplatin, and gemcitabine in patients with advanced non-small cell lung cancer: a retrospective study. Open Med (Wars) 2018;13:142–147. doi: 10.1515/med-2018-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi HL, Xu LY, Liu Z. Phase II clinical trial of homemade human rh-endostatin in the treatment of patients with stage IIIB-IV non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2004;7:325–328. doi: 10.3779/j.issn.1009-3419.2004.04.13. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Wang JW, Cui CX, et al. Rh-endostatin (YH-16) in combination with vinorelbine and cisplatin for advanced non-small cell lung cancer: a multicenter phase II trial. Zhongguo Fei Ai Za Zhi. 2005;14:204–207. [Google Scholar]
- 23.Wang QZ, Cao J, Pu XX, et al. Study on the effectiveness and safety of continuous intravenous pump with endostar and the DP regimen in the treatment of advanced squamous non-small cell lung cancer. J Chengdu Med Coll. 2019;14:440–442. [Google Scholar]
- 24.Zhang YY. Efficacy and safety of combination of chemotherapy (gemcitabine and cisplatin) and recombinant human endostatin injection (endostar) in advanced primary squamous·cell lung cancer: Zhejiang University; 2014.
- 25.Shen H, Jing Z, Shan-Shan W, et al. Continuous administration of endostar plus GP chemotherapy in local advanced or metastatic lung squamous cell carcinoma. Acta Med Mediterr. 2016;32:57–62. [Google Scholar]
- 26.Dai WX, Wu ZY, Chen J, et al. Efficacy of recombinant human endostatin in the treatment of advanced non-small cell lung cancer. Lab Med Clin. 2015;12:1452–1453. [Google Scholar]
- 27.Kim JS, Kim ES, Liu D, et al. Prognostic implications of tumoral expression of insulin like growth factors 1 and 2 in patients with non-small-cell lung cancer. Clin Lung Cancer. 2014;15:213–221. doi: 10.1016/j.cllc.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miao M. Efficacy of two ways of infusion of Endostar combined with chemotherapy on advanced non small cell lung cancer. Clin Med. 2017;11:28–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.