Abstract
Few papers discuss how the economic burden of patients with stroke receiving rehabilitation courses is related to post-acute care (PAC) programs. This is the first study to explore the economic burden of stroke patients receiving PAC rehabilitation and to evaluate the impact of multidisciplinary PAC programs on cost and functional status simultaneously. A total of 910 patients with stroke between March 2014 and October 2018 were separated into a PAC group (at two medical centers) and a non-PAC group (at three regional hospitals and one district hospital) by using propensity score matching (1:1). A cost–illness approach was employed to identify the cost categories for analysis in this study according to various perspectives. Total direct medical cost in the per-diem-based PAC cohort was statistically lower than that in the fee-for-service-based non-PAC cohort (p < 0.001) and annual per-patient economic burden of stroke patients receiving PAC rehabilitation is approximately US $354.3 million (in 2019, NT $30.5 = US $1). Additionally, the PAC cohort had statistical improvement in functional status vis-à-vis the non-PAC cohort and total score of each functional status before rehabilitation and was also statistically significant with its total score after one-year rehabilitation training (p < 0.001). Early stroke rehabilitation is important for restoring health, confidence, and safe-care abilities in these patients. Compared to the current stroke rehabilitation system, PAC rehabilitation shortened the waiting time for transfer to the rehabilitation ward and it was indicated as an efficient policy for treatment of stroke in saving medical cost and improving functional status.
Keywords: stroke, post-acute care, cost, functional status
1. Introduction
According to the latest global data from the World Health Organization (WHO), stroke is now the second-leading cause of death [1]. In Taiwan, data collected by the National Health Interview Survey revealed that the age-standardized incidence of a first-ever stroke reached as high as 320 per 100,000 population in 2012 [2]. In most developed countries, post-acute care (PAC) and long-term care systems provide support or back-up support for patients with stroke [3]. In the United States of America, for example, up to 62.6–74.5% patients with stroke undergo PAC rehabilitation after hospital discharge [4,5]. In Taiwan, stroke is the disease most likely to require a prolonged hospital stay, and up to 10.4% of stroke patients require a prolonged hospital stay in an acute care ward [6]. Hospital stays for stroke are prolonged in Taiwan for two reasons: the low copayment for hospitalization and the lack of long-term care systems [6,7].
In Taiwan, a Post-Acute Care–Cerebrovascular Diseases (PAC-CVD) program was implemented in 2014 to improve resource allocation and patient outcomes by transferring patients in post-acute phase of stroke from medical centers to community hospitals, including regional and district hospitals [3]. However, few studies have discussed the roles of PAC programs in the economic burden of stroke rehabilitation and in functional recovery in patients with stroke [8,9,10]. This study is, to our knowledge, the first to apply propensity score matching (PSM) in a natural experimental design to examine the longitudinal impacts of stroke PAC programs on medical utilization and functional status.
Therefore, this cohort study prospectively purposed to explore the economic burden of patients with stroke receiving post-acute care (PAC) rehabilitation and to explore the impact of multidisciplinary PAC programs on medical cost and functional status among patients with stroke.
2. Materials and Methods
2.1. The PAC Program
Multidisciplinary PAC stroke teams in Taiwan include neurologists, physiatrists, physiotherapists, occupational therapists, speech therapists, and nurses. The PAC rehabilitation program, which is prescribed by the physiatrist, comprises a complex program of universal activities performed at least three times per day. Depending on the condition of the patient, the components of the rehabilitation program may include facilitation, passive range of motion exercise, therapeutic exercise, bed mobility training, balance training, functional electric stimulation, training under suspension, ambulation training, transfer training, activities of daily living training, functional training, coordination training, posture training, speech training, and swallowing training. Hospitals receive a packaged and function-related reimbursement per day of rehabilitation. The maximal packaged reimbursement is NT $117.6 per day for high-intensity rehabilitation and NT $79.0 per day for general-intensity rehabilitation. The reimbursement covers all medical expenses for stroke care, including management of associated comorbidities/complications and stroke rehabilitation.
The traditional rehabilitation program received by the non-PAC cohort was identical to that received by the PAC cohort; however, physical therapy, occupational therapy, and speech/swallowing therapy were limited to once per day in the non-PAC cohort. Notably, the fiscal incentive for a medical center to transfer a patient to a regional or a district hospital is mitigated by several factors, including the PAC-CVD transfer policy, the willingness of the stroke patient (or family) to accept responsibility for PAC care, and whether the physician agrees to the transfer. These mitigating factors should be considered by health providers when setting policies for hospital stays after stroke. Health providers should also be mindful that a short length of stay (LOS) after stroke does not necessarily indicate a good outcome [11]. Another difference between this PAC-CVD program and the traditional program is that the reimbursement for inpatient stroke rehabilitation in the PAC-CVD program was on a per-diem basis whereas reimbursement for the traditional stroke rehabilitation program was on a fee-for-service basis.
2.2. Study Design and Sample
The study population included all patients who had undergone stroke (defined as ICD-9-CM-codes: 433.x, 434.x, and 436.x for ischemic stroke; 430 and 431 for hemorrhagic stroke) admission to a PAC ward at two medical centers and a non-PAC ward at three regional hospitals and one district hospital in south Taiwan between March 2014 and October 2018. The patients should meet the criteria including acute stroke, stroke onset day within 30 days, and Modified Rankin Scale (MRS) scores of 2 to 4, where MRS scores of 0, 1, 2, 3, 4, and 5 are interpreted as no symptoms, no significant disability, slight disability, moderate disability, moderately severe disability, and severe disability, respectively [12].
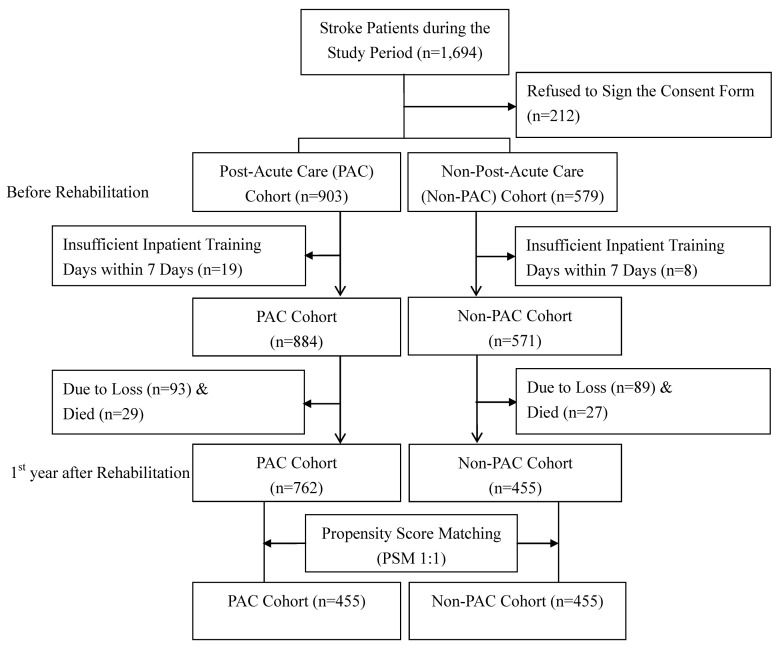
Potential selection bias was further minimized by using a PSM approach to select stroke patients in the PAC cohort used for comparisons. The present study used the caliper matching method (also known as the greedy algorithm) with 1 to 1 match between the PAC cohort (at two medical centers) and the non-PAC cohort (at three regional hospitals and one district hospital) based on the propensity score [13]. Thus, the PAC cohort of all participants with 455 patients yielded 455 patients in the non-PAC cohort for statistical analysis (Figure 1). These stroke patients completed the pre-rehabilitation and the first year after rehabilitation assessments in this study after providing written informed consent. The study protocol was approved by the local ethic committee of the Kaohsiung Medical University Hospital (KMUH-IRB-20140308). Participants gave written informed consent before data collection began.
Figure 1.
Flow chart of recruitment and study procedure.
2.3. Estimation of Cost
The cost analysis was performed from the hospital perspective, implying that direct medical costs were included in the cost analysis. Direct medical costs were costs of medical treatments for stroke patients in Taiwan. Cost items of the direct medical cost included diagnosis fee, ward fee, examination fee, medicine and pharmacy service fee, rehabilitation therapy fee, and others. In order to facilitate comparisons with other economic evaluations, all cost inputs were inflated to 2020 US dollars. All costs were discounted annually by 3%.
2.4. Instruments of Functional Status and Chart Review
The functional status measures included the Barthal Index (BI), Functional Oral Intake Scale (FOIS), Mini-Mental State Examination (MMSE), Instrumental Activities of Daily Living Scale (IADL), EuroQoL Quality of Life Scale (EQ5D), and Berg Balance Scale (BBS) and the Chinese version of these measures has been validated and extensively used in both clinical practice and health research [3,14].
The following patient data obtained by records review and questionnaire interview were tested as independent variables in this study: gender, age, Charlson Comorbidity Index (CCI), type of stroke, comorbid diseases (e.g., diabetes, hypertension, hyperlipidemia, and coronary artery disease), stroke history, tissue plasminogen activator (tPA), thrombectomy, medications (e.g., statins, antiplatelets, anticoagulants, and antihypertensives), acute care LOS, LOS during rehabilitation, and total score for each functional status measure before rehabilitation.
2.5. Statistical Analysis
The unit of analysis in this study was the individual stroke patient. Descriptive statistics were tabulated to depict the stroke patient demographics. Regarding total direct medical cost at hospitalization, the standard administrative claims data required by the Taiwan Bureau of National Health Insurance (BNHI) include fees for the following: physician, radiology, physical therapy, hospital room, pharmacy, laboratory, special materials, and others. Incidence-based approach was employed in estimating the per-patient economic burden of total direct medical cost in the PAC cohort and non-PAC cohort during year one after rehabilitation. To reflect changes in real dollar value, all dollar values were converted to their equivalent 2020 values; New Taiwan Dollar (NTD) values were then converted to USD values at the average exchange rate over the four-year period of 2015–2018 (in 2020, NT $30.5 = US $1).
Effect size (ES) was calculated to directly compare the relative magnitude of change as measured by the two functional status measures. Thus, ES was calculated as the difference between the mean scores for two time intervals divided by the standard deviation of the score for the previous time interval [15]. Using this method of standardizing the extent of change measured by an instrument enabled comparisons between two instruments. An ES of 1.0 is equivalent to a change of one standard deviation in the sample. Effect sizes of 0.2, 0.5, and 0.8 are typically considered small, medium, and large changes, respectively. Differences in ES and associated 95% confidence intervals are also employed using bias-corrected and accelerated bootstrapping with 1000 replications [16]. Additionally, multiple linear regression analysis was carried out to determine the significant predictors of total direct medical costs and total score of each functional status measure of stroke patients after one-year rehabilitation. Log transformation was undertaken for total direct medical costs to reduce the skewness and number of outliers, and to improve normality, linearity, and homoscedasticity of residuals.
Standard regression diagnostics were used to check the assumptions for the regression analyses [17]. Statistical analyses were performed using Stata Statistical Package, version 13.0 (StataCorp LP, College Station, TX, USA). All tests were two-sided, and p values less than 0.05 were considered statistically significant.
3. Results
Table 1 compares the PAC cohort and the non-PAC cohort in terms of patient characteristics, and there were no statistically significant differences between these two cohorts after PSM. Most of the patients suffered from ischemic stroke, and among the common risk factors of stroke, over 50% of patients had a history of hypertension, hyperlipidemia, and diabetes mellitus. Furthermore, the annual total direct medical cost in the PAC cohort (per-diem-based LOS, mean US $5326.7, standard deviation (SD) US $1933.5) was significantly lower than that in the non-PAC cohort (fee-for-service-based LOS, mean US $10175.8, SD US $2377.9) (p < 0.001) (Table 2). The per-patient annual economic burden of total direct medical cost of stroke patients receiving PAC programs after rehabilitation is approximately to US $−354.6 million.
Table 1.
Stroke patient characteristics.
| Before Propensity Score Matching | After Propensity Score Matching | ||||||
|---|---|---|---|---|---|---|---|
| Variables | PAC Cohort (n = 903) |
Non-PAC Cohort (n = 579) | p Value | PAC Cohort (n = 455) |
Non-PAC Cohort (n = 455) | p Value | |
| Age, years | 61.9 ± 10.6 | 65.2 ± 11.9 | 0.152 | 65.0 ± 10.3 | 65.2 ± 10.7 | 0.934 | |
| Gender | Female | 269 (29.8%) | 261 (45.1%) | 0.146 | 192 (42.3%) | 190 (41.8%) | 0.684 |
| Male | 634 (70.2%) | 318 (54.9%) | 263 (57.7%) | 265 (58.2%) | |||
| Education, years | 8.7 ± 4.2 | 9.0 ± 4.8 | <0.001 | 8.8 ± 4.1 | 9.2 ± 4.3 | 0.888 | |
| BMI, kg/m2 | 24.1 ± 3.0 | 24.5 ± 3.8 | 0.968 | 24.2 ± 3.1 | 24.0 ± 3.4 | 0.949 | |
| Stroke type | Ischemic | 769 (85.2%) | 505 (87.2%) | 0.674 | 387 (85.1%) | 393 (86.3%) | 0.910 |
| Hemorrhagic | 134 (14.8%) | 74 (12.8%) | 68 (14.9%) | 62 (13.7%) | |||
| Hypertension | Yes | 634 (70.2%) | 420 (72.5%) | 0.487 | 328 (72.1%) | 329 (72.3%) | 0.991 |
| Hyperlipidemia | Yes | 480 (53.2%) | 340 (58.7%) | 0.311 | 236 (51.8%) | 240 (52.8%) | 0.807 |
| Diabetes mellitus | Yes | 461 (51.0%) | 306 (52.8%) | 0.570 | 236 (51.8%) | 239 (52.4%) | 0.990 |
| Atrial fibrillation | Yes | 75 (8.3%) | 48 (8.3%) | 0.989 | 38 (8.3%) | 37 (8.1%) | 0.934 |
| Previous stroke | Yes | 117 (13.0%) | 144 (24.9%) | <0.001 | 82 (18.0%) | 84 (18.4%) | 0.819 |
| Acute care LOS, days | 13.1 ± 27.3 | 24.5 ± 34.1 | <0.001 | 23.5 ± 11.4 | 24.0 ± 11.6 | 0.369 | |
| LOS during rehabilitation, days | 31.2 ± 17.5 | 37.0 ± 12.9 | <0.001 | 35.2 ± 12.1 | 36.3 ± 11.4 | 0.816 | |
| BI score before rehabilitation | 44.7 ± 23.5 | 46.0 ± 26.4 | 0.403 | 44.7 ± 20.2 | 46.0 ± 27.0 | 0.279 | |
| FOIS score before rehabilitation | 5.9 ± 1.8 | 6.1 ± 2.0 | 0.310 | 5.9 ± 2.0 | 6.1 ± 2.1 | 0.974 | |
| EQ5D score before rehabilitation | 9.6 ± 1.9 | 8.9 ±1.9 | 0.593 | 9.6 ± 1.9 | 8.8 ± 2.0 | 0.712 | |
| IADL score before rehabilitation | 2.0 ± 1.3 | 2.3 ± 1.4 | 0.328 | 2.0 ± 1.1 | 3.1 ± 1.3 | 0.147 | |
| BBS score before rehabilitation | 20.6 ± 16.3 | 18.4 ± 15.2 | 0.486 | 20.6 ± 14.8 | 18.4 ± 14.0 | 0.392 | |
| MMSE score before rehabilitation | 20.8 ± 7.5 | 22.3 ± 6.9 | 0.213 | 21.8 ± 11.0 | 23.3 ± 9.2 | 0.259 | |
BMI, body mass index; LOS, length of stay; PAC, post-acute care; BI, Barthel Index; FOIS, Functional Oral Intake Scale; EQ5D, EuroQoL Quality of Life Scale; IADL, Instrumental Activities of Daily Living Scale; BBS, Berg Balance Scale; MMSE, Mini-Mental State Examination. Values are expressed as mean ± standard deviation or n (%).
Table 2.
Annual economic burdens of total direct medical cost per patient in PAC and non-PAC cohorts before and after one-year rehabilitation.
| Cost Components | PAC Cohort (n = 455) # | Non-PAC Cohort (n = 455) | Differences (PAC–non-PAC) |
Economic Burden * |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Diagnosis fee | 4139.5 ± 1798.1 | 1089.0 ± 157.3 | ||
| Ward fee | 2882.2 ± 560.9 | |||
| Examination fee | 1619.0 ± 373.9 | |||
| Medicine and pharmacy service fee | 450.1 ± 25.1 | |||
| Rehabilitation therapy fee | 1103.5 ± 273.6 | |||
| Other fees | 1785.9 ± 430.9 | |||
| Total direct medical cost during rehabilitation | 4139.5 ± 1798.1 | 8929.8 ± 1827.1 | −4790.3 ± 1805.7 | |
| Total direct medical cost after discharge | 1187.2 ± 1148.6 | 1246.0 ± 1203.6 | −58.8 ± 35.6 | |
| Total direct medical cost | 5326.7 ± 1933.5 | 10,175.8 ± 2377.9 | −4849.1 ± 2685.7 | −354,886,232.6 |
PAC, post-acute care; SD, standard deviation; ICER, incremental cost-effectiveness ratio. # Mean direct medical cost for the PAC cohort, hospitals will receive a packaged and function-related reimbursement by day, that is, a maximal packaged imbursement of US $117.6 per day for high-intensity rehabilitation or US $79.0 per day for general-intensity rehabilitation covering whole medical expenses for stroke care, managing associated comorbidities and complications, and rehabilitation. * Economic burden during 1 year after rehabilitation is US $4849.1 per patient × 318.2 patients per 100,000 person-year (age-standardized incidence of first-ever stroke) × 23,000,000 persons (Taiwan nationwide population). Therefore, annual per-patient economic burden of total direct medical cost approximately equals to US $354.6 million.
The effect size (ES) for total score of each functional status measure is shown as between 0.85 and −0.43 in PAC cohort and 0.04 to 0.71 in non-PAC cohort (Table 3). During the study period, both in the PAC cohort and non-PAC cohort, the IADL showed the largest improvement, but the EQ5D had least improvement. Furthermore, in comparison with the non-PAC cohort, the PAC cohort showed significant improvement among all functional status measures (p < 0.05).
Table 3.
Effect size (ES) and mean difference for total score of each functional status measure: comparison of post-acute care (PAC) and non-PAC cohorts.
| PAC Cohort (n = 455) | Non-PAC Cohort (n = 455) | PAC–non-PAC | |||||
|---|---|---|---|---|---|---|---|
| Measures | Before Rehabilitation | After 1-year Rehabilitation | ES of after Rehabilitation vs. before Rehabilitation | Before Rehabilitation | After 1-year Rehabilitation |
ES of after Rehabilitation vs. before Rehabilitation | Mean Difference (Estimate [95% C.I.]) * |
| BI | 44.7 | 64.0 | 0.82 | 46.0 | 63.3 | 0.65 | 0.17 (0.07, 0.27) |
| FOIS | 5.9 | 6.2 | 0.17 | 6.1 | 6.1 | 0.05 | 0.12 (0.09, 0.15) |
| EQ5D | 9.6 | 8.8 | −0.43 | 8.8 | 8.8 | 0.04 | −0.39 (−0.43, −0.34) |
| IADL | 2.0 | 3.1 | 0.85 | 3.1 | 3.3 | 0.71 | 0.14 (0.11, 0.16) |
| BBS | 20.6 | 29.9 | 0.57 | 18.4 | 24.9 | 0.42 | 0.13 (0.07, 0.19) |
| MMSE | 21.8 | 24.0 | 0.29 | 23.3 | 24.3 | 0.15 | 0.14 (0.11, 0.17) |
BI, Barthel Index; FOIS, Functional Oral Intake Scale; EQ5D, EuroQoL Quality of Life Scale; IADL, Instrumental Activities of Daily Living; BBS, Berg Balance Scale; MMSE, Mini-Mental State Examination. * Mean difference is presented as effect size (95% confidence intervals obtained by bootstrapping).
In Table 4, after adjusting for effective predictors, total direct medical cost in the PAC cohort was significantly lower than that in the non-PAC cohort (p < 0.001) and it is also shown that the PAC cohort had statistical improvement in functional status vis-à-vis the non-PAC cohort, and total score of each functional status before rehabilitation was also statistically significant with its total score after rehabilitation training (p < 0.001).
Table 4.
The coefficients of significant variables of multiple linear regression model of log of total direct medical cost and total score of each functional status measure after adjusting all effective predictors (n = 910).
| Variables | Cost | BI | FOIS | EQ5D | IADL | BBS | MMSE |
|---|---|---|---|---|---|---|---|
| Coefficient | Coefficient | Coefficient | Coefficient | Coefficient | Coefficient | Coefficient | |
| Study cohort (PAC vs. non-PAC) | −0.41 *** | 10.34 *** | 1.24 *** | −1.44 *** | 1.47 ** | 12.89 *** | 7.17 ** |
| BI score before rehabilitation | - | 0.74 *** | - | - | - | - | - |
| FOIS score before rehabilitation | - | - | 0.57 *** | - | - | - | - |
| EQ5D score before rehabilitation | - | - | - | 0.40 *** | - | - | - |
| IADL score before rehabilitation | - | - | - | - | 0.79 *** | - | - |
| BBS score before rehabilitation | - | - | - | - | - | 0.71 *** | - |
| MMSE score before rehabilitation | - | - | - | - | - | - | 0.74 *** |
# Adjusted factors included gender, age, Charlson Comorbidity Index (CCI), type of stroke, comorbid diseases (e.g., diabetes, hypertension, hyperlipidemia, and coronary artery disease), stroke history, tissue plasminogen activator (tPA), thrombectomy, medications (e.g., statins, antiplatelets, anticoagulants, and antihypertensives), acute care lengths of stay (LOS), and LOS during rehabilitation. * p < 0.05, ** p < 0.01, *** p < 0.001. PAC, post-acute care; BI, Barthel Index; FOIS, Functional Oral Intake Scale; EQ5D, EuroQoL Quality of Life Scale; IADL, Instrumental Activities of Daily Living; BBS, Berg Balance Scale; MMSE, Mini-Mental State Examination.
4. Discussion
Costs for stroke are related to the risk factors (such as hypertension, hyperlipidemia, diabetes mellitus, atrial fibrillation, and previous stroke history) and the social-economic status [9,10]. Without insurance coverage, managing stroke constitutes a huge direct-cost burden generally unaffordable by a stroke sufferer. Fattore et al. classified the cost into three categories as direct healthcare, direct non-healthcare (informal care costs and paid care costs), and productivity losses [18]. The major three cost components were informal care cost, initial hospitalization cost, and rehabilitation cost ranked in order. Moreover, to investigate the economic burden of stroke patients in the PAC rehabilitation program, this study utilized the 2018 data from BNHI to calculate the incidence of stroke. Additionally, health insurance claims data were used to estimate total direct medical cost. Taiwan has a single, national health insurance system; therefore, the national incidence of stroke could be estimated. This method has the added advantage of maintaining internal consistency. Although we used national health insurance claims data, the incidence rates reported here were similar to data from stroke registries.
A more intensive PAC program was incorporated into the Japanese medical insurance system after 2000. In Miyai’s study [19], the dose-dependent effect of hours of therapy on rehabilitation outcome after stroke was obvious. These patients received higher discharge motor and function levels with a shorter LOS. Under the current National Health Insurance system in Taiwan, all patients are limited to receive physical therapy, occupational therapy, and speech therapy once per day. In our study, patients received more rehabilitation programs to their level of tolerance. Patients could regain various functions earlier and complications were prevented or minimized. The per-diem reimbursement avoided the disadvantage of financial burden in fee-for-service reimbursement. Thus, the training program was efficient and economical.
An organized, multidisciplinary team approach should be initiated early after the onset of acute stroke to minimize functional disability, prevent complications, and hence decrease prolonged hospital stays. According to the Get With The Guidelines–Stroke (GWTG-Stroke) program, among 616,982 adults with stroke diagnosis, almost 90% of them had documentation of an acute assessment for rehabilitation [20]. However, the inpatient stroke rehabilitation utilization in Taiwan was only 34.0% (33.0% for physical therapy, 19.6% for occupational therapy, and 5.3% for speech therapy), much lower than those observed in the United States, Canada, the United Kingdom, and Austria (59% to 75% for physical therapy, 16% to 39% for occupational therapy, and 10% to 23% for speech therapy) [7,21]. Furthermore, in some local hospitals, inpatient rehabilitation was carried out in the form of bedside programs, without rehabilitation facilities. It was noted that some stroke rehabilitation therapists in some local hospitals lacked experience; thus, it was necessary to qualify and classify the stroke rehabilitation provider by adjusting the payment system. Our study results revealed that early intensive stroke rehabilitation led to cost-effective and efficient outcomes. The multidisciplinary team approach was also important in achieving the outcomes.
A recently published study addressed the impact of female gender on stroke subtype, risk factors, severity, and outcome [22]. The authors concluded that Fragile X syndrome is a genetic condition known to increase the risk of cognitive impairment and socio-emotional challenges in affected males and females [23]. Another study by Arboix et al. reported that, compared to men with acute stroke, women with acute stroke have a significantly higher risk of death in the immediate post-stroke phase (13.5% vs. 10.8% in males; p = 0.006) and a significantly lower probability of early full neurological recovery (11.8% vs. 13.9% in males; p = 0.029) [22]. Theoretically, stroke should tend to occur at an older age in women compared to men. Since women are more likely to have cardioembolism-related risk factors, they are also more likely to have the cardioembolic stroke subtype, which may explain the poorer stroke outcomes reported in women compared to men [22,23]. Over the 24-year period of the current study, trends observed in women relative to men included older age, lower mortality, shorter hospitalization, and higher incidences of hypertension, atrial fibrillation, and cardioembolic infarction. In Kuptniratsaikul’s article review, the average LOS for inpatient rehabilitation after stroke was about a month, except for the USA (29.4 days in Thailand, 31.3 days in Ireland, 31.2 days in Switzerland, 37.1 days in Singapore, and 21.9 days in Texas) [24]. High economic burden from stroke was the most important reason for the shorter average LOS in the US. Few studies have discussed the inpatient rehabilitation after acute stroke [3,25]. The PAC programs shortened the LOS, especially shortening the days in waiting to transfer to the rehabilitation ward and reducing the readmission rates. Additionally, patients were transferred to PAC rehabilitation units as early as possible to maximize rehabilitation efficacy, to maximize the potential for functional restoration, and to minimize costs [3,25]. Early and intensive physical therapy, occupational therapy, and speech therapy are also important for successful rehabilitation of stroke patients. A collaborative effort by a multidisciplinary team was another important contributor to good outcomes. The Taiwan NHI system ensures that all hospitals that provide PAC receive the same reimbursement, regardless of care quality or hospital accreditation level.
In the study, this inpatient stroke rehabilitation program had four major differences from the current implementation policy. Firstly, the reimbursement was per diem but not fee-for-service. Secondly, patients could receive more intensive and more frequent rehabilitation programs. Thirdly, every patient should have his or her functional status re-evaluated every three weeks and these medical records should be sent to the NHI system. Fourthly, no matter the hospital accreditation level (medical center, regional hospital, or district hospital), the payment was all the same.
In Taiwan, stroke patients with prolonged hospital stay constitute only 10.4% of the total stroke patients but 38.9% of the total person–hospital bed days and 47.8% of the total in-hospital medical expenses [6]. Besides surgical intervention and mechanical ventilation use, rehabilitation need for physical/ADL dependency and speech/swallowing problems is a major cause to delay discharge from the hospital [26,27,28]. If stroke patients were referred successfully, the problems concerning prolonged hospital stay in medical centers would be resolved; however, a few stroke patients are transferred from medical centers to PAC program hospitals in the whole country. The major reasons why transfers from medical centers are limited include the following: stroke patients and their family have low confidence in local hospitals; some neurologists and neurosurgeons have limited knowledge of PAC programs; some local hospitals are not well prepared; and people treated for stroke in medical centers often insist on waiting for transfer to rehabilitation wards.
Although all research questions were adequately and satisfactorily addressed, three limitations are noted. Firstly, this study collected data from acute stroke patients from the stroke onset day within 30 days in two district hospitals, having one of the highest number of PAC-program stroke patients in Taiwan. Such a sample selection ensures that the limited experience of physicians and medical professionals does not significantly influence patient outcomes. Secondly, the etiology of ischemic stroke affects prognosis, outcome, and management. Trials of acute stroke therapies should investigate how responses are influenced by ischemic stroke subtype. The current study did not consider differences in ischemic stroke subtype, stroke risk, bleeding risk (i.e., CHA2DS2-VASc score and HAS-BLED score), or medications (i.e., statins, antiplatelets, anticoagulants, and antihypertensives). Additionally, we acknowledge that more data and longer survey time periods are needed to understand the complex patterns that emerged in the current analysis.
In conclusion, improvements in mobility function were larger than improvements in emotional and mental function. Early PAC rehabilitation is important for restoring health, confidence, and safe-care abilities in these patients. Compared to the current stroke rehabilitation system, PAC rehabilitation achieved larger improvements in functional status while simultaneously reducing waiting time for transfer to rehabilitation ward, total LOS, and total direct medical costs. The PAC rehabilitation was effective training for rehabilitation of stroke patients. This study is the first to discuss the cost-effectiveness of intensive post-stroke rehabilitation under a per-diem payment system.
Author Contributions
Substantial contributions to conception and design by C.-C.C. and H.-Y.S.; acquisition of data: J.-J.W., C.-M.H., H.-F.L., H.-H.H., K.-W.H., H.-C.C. and S.-C.J.Y.; contributed to analysis and interpretation of data: C.-C.C. and H.-Y.S.; drafted the article: C.-C.C. and H.-Y.S. All authors revised the article critically for important intellectual content and final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by funding from the Chi-Mei Medical Center and the Kaohsiung Medical University Research Foundation (109 CM-KMU-08) and Ministry of Science and Technology (MOST 104-2410-H-037-006-SS2, MOST 106-2410-B-037-076, and MOST 108-2410-H-037-006-SS3) in Taiwan.
Institutional Review Board Statement
The study protocol was approved by the local ethic committee of the Kaohsiung Medical University Hospital (KMUH-IRB-20140308).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Werner R.M., Konetzka R.T. Trends in Post-Acute Care Use Among Medicare Beneficiaries: 2000 to 2015. JAMA. 2018;319:1616–1617. doi: 10.1001/jama.2018.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J., An Z., Li B., Yang L., Tu J., Gu H., Zhan C., Liu B., Su T.C., Ning X. Increasing stroke incidence and prevalence of risk factors in a low-income Chinese population. Neurology. 2015;84:374–381. doi: 10.1212/WNL.0000000000001175. [DOI] [PubMed] [Google Scholar]
- 3.Wang C.Y., Chen Y.R., Hong J.P., Chan C.C., Chang L.C., Shi H.Y. Rehabilitative post-acute care for stroke patients delivered by per-diem payment system in different hospitalization paths: A Taiwan pilot study. Int. J. Qual. Health Care. 2017;29:779–784. doi: 10.1093/intqhc/mzx102. [DOI] [PubMed] [Google Scholar]
- 4.McArthur K.S., Quinn T.J., Higgins P., Langhorne P. Post-acute care and secondary prevention after ischaemic stroke. BMJ. 2011;342:d2083. doi: 10.1136/bmj.d2083. [DOI] [PubMed] [Google Scholar]
- 5.Burke R.E., Juarez-Colunga E., Levy C., Prochazka A.V., Coleman E.A., Ginde A.A. Patient and Hospitalization Characteristics Associated with Increased Postacute Care Facility Discharges From US Hospitals. Med. Care. 2015;53:492–500. doi: 10.1097/MLR.0000000000000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H.C., Chang K.C., Lan C.F., Hong C.T., Huang Y.C., Chang M.L. Factors associated with prolonged hospital stay for acute stroke in Taiwan. Acta. Neurol. Taiwan. 2008;17:17–25. [PubMed] [Google Scholar]
- 7.Tseng M., Lin H. Readmission after hospitalization for stroke in Taiwan: Results from a national sample. J. Neurol. Sci. 2009;284:52–55. doi: 10.1016/j.jns.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Savic M., Cvjeticanin S., Lazovic M., Nikcevic L., Petronic I., Cirovic D., Nikolic D. Morphogenetic Variability as Potential Biomarker of Functional Outcome After Ischemic Stroke. Brain Sci. 2019;9:138. doi: 10.3390/brainsci9060138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajsic S., Gothe H., Borba H.H., Sroczynski G., Vujicic J., Toell T., Siebert U. Economic burden of stroke: A systematic review on post-stroke care. Eur. J. Health Econ. 2019;20:107–134. doi: 10.1007/s10198-018-0984-0. [DOI] [PubMed] [Google Scholar]
- 10.Smith S., Horgan F., Sexton E., Cowman. S., Hickey A., Kelly P., McGee H., Murphy S., O’Neill D., Royston M., et al. The cost of stroke and transient ischaemic attack in Ireland: A prevalence-based estimate. Age Ageing. 2012;41:332–338. doi: 10.1093/ageing/afr141. [DOI] [PubMed] [Google Scholar]
- 11.Lieber A.C., Hong E., Putrino D., Nistal D.A., Pan J.S., Kellner C.P. Nutrition, Energy Expenditure, Dysphagia, and Self-Efficacy in Stroke Rehabilitation: A Review of the Literature. Brain Sci. 2018;8:218. doi: 10.3390/brainsci8120218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fearon P., McArthur K.S., Garrity K., Graham L.J., McGroarty G., Vincent S., Quinn T.J. Prestroke modified rankin stroke scale has moderate interobserver reliability and validity in an acute stroke setting. Stroke. 2012;43:3184–3188. doi: 10.1161/STROKEAHA.112.670422. [DOI] [PubMed] [Google Scholar]
- 13.Mnatzaganian G., Davidson D.C., Hiller J.E., Ryan P. Propensity score matching and randomization. J. Clin. Epidemiol. 2015;68:760–768. doi: 10.1016/j.jclinepi.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Wang C.Y., Hsien H.H., Hung K.W., Lin H.F., Chiou H.Y., Yeh S.C., Yeh Y.J., Shi H.Y. Multidiscipline Stroke Post-Acute Care Transfer System: Propensity-Score-Based Comparison of Functional Status. J. Clin. Med. 2019;8:1233. doi: 10.3390/jcm8081233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazis L.E., Anderson J.J., Meenan R.F. Effect sizes for interpreting changes in health status. Med. Care. 1989;27:178–189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F., Wagner A.K., Soumerai S.B., Ross-Degnan D. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J. Clin. Epidemiol. 2009;62:143–148. doi: 10.1016/j.jclinepi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinbaum D.G., Kupper L.L., Muller K.E. Applied Regression Analysis and Other Multivariable Methods. PWS-KENT Publishing Co.; Boston, MA, USA: 1988. [Google Scholar]
- 18.Fattore G., Torbica A., Susi A., Giovanni A., Benelli G., Gozzo M., Toso V. The social and economic burden of stroke survivors in Italy: A prospective, incidence-based, multi-centre cost of illness study. BMC Neurol. 2012;12:137. doi: 10.1186/1471-2377-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyai I., Sonoda S., Nagai S., Takayama Y., Inoue Y., Kakehi A., Kurihara M., Ishikawa M. Results of new policies for inpatient rehabilitation coverage in Japan. Neurorehabil. Neural. Repair. 2011;25:540–547. doi: 10.1177/1545968311402696. [DOI] [PubMed] [Google Scholar]
- 20.Bettger J.A.P., Kaltenbach L., Reeves M.J., Smith E.E., Fonarow G.C., Schwamm L.H., Peterson E.D. Assessing stroke patients for rehabilitation during the acute hospitalization: Findings from the get with the guidelines-stroke program. Arch. Phys. Med. Rehabil. 2013;94:38–45. doi: 10.1016/j.apmr.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 21.McKevitt C., Coshall C., Tilling K., Wolfe. C. Are there inequalities in the provision of stroke care? Analysis of an inner-city stroke register. Stroke. 2005;36:315–320. doi: 10.1161/01.STR.0000152332.32267.19. [DOI] [PubMed] [Google Scholar]
- 22.Arboix A., Cartanyà A., Lowak M., García-Eroles L., Parra O., Oliveres M., Massons J. Gender differences and woman-specific trends in acute stroke: Results from a hospital-based registry (1986–2009) Clin. Neurol. Neurosurg. 2014;127:19–24. doi: 10.1016/j.clineuro.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Bartholomay K.L., Lee C.H., Bruno J.L., Lightbody A.A., Reiss A.L. Closing the Gender Gap in Fragile X Syndrome: Review on Females with FXS and Preliminary Research Findings. Brain Sci. 2019;9:11. doi: 10.3390/brainsci9010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuptniratsaikul V., Kovindha A., Massakulpan P., Permsirivanich W., Kuptniratsaikul P.S. Inpatient rehabilitation services for patients after stroke in Thailand: A multi-centre study. J. Rehabil. Med. 2009;41:684–686. doi: 10.2340/16501977-0399. [DOI] [PubMed] [Google Scholar]
- 25.Hammerbeck U., Gittins M., Vail A., Paley L., Tyson S.F., Bowen A. Spatial Neglect in Stroke: Identification, Disease Process and Association with Outcome During Inpatient Rehabilitation. Brain Sci. 2019;9:374. doi: 10.3390/brainsci9120374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jardim T.V., Mozaffarian D., Abrahams-Gessel S., Sy S., Lee Y., Liu J., Huang Y., Rehm C., Wilde P., Micha R., et al. Cardiometabolic disease costs associated with suboptimal diet in the United States: A cost analysis based on a microsimulation model. PLoS Med. 2019;16:e1002981. doi: 10.1371/journal.pmed.1002981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah S., Xian Y., Sheng S., Zachrison K.S., Saver J.L., Sheth K.N., Fonarow G.C., Schwamm L.H., Smith E.E. Use, Temporal Trends, and Outcomes of Endovascular Therapy After Interhospital Transfer in the United States. Circulation. 2019;139:1568–1577. doi: 10.1161/CIRCULATIONAHA.118.036509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bevilacqua R., Maranesi E., Riccardi G.R., Donna V.D., Pelliccioni P., Luzi R., Lattanzio F., Pelliccioni G. Non-Immersive Virtual Reality for Rehabilitation of the Older People: A Systematic Review into Efficacy and Effectiveness. J. Clin. Med. 2019;8:1882. doi: 10.3390/jcm8111882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.