Abstract
Redox biology is a very quickly developing area of modern biological sciences, and roles of redox homeostasis in health and disease have recently received tremendous attention. There are a range of redox pairs in the cells/tissues responsible for redox homeostasis maintenance/regulation. In general, all redox elements are interconnected and regulated by various means, including antioxidant and vitagene networks. The redox status is responsible for maintenance of cell signaling and cell stress adaptation. Physiological roles of redox homeostasis maintenance in avian species, including poultry, have received limited attention and are poorly characterized. However, for the last 5 years, this topic attracted much attention, and a range of publications covered some related aspects. In fact, transcription factor Nrf2 was shown to be a master regulator of antioxidant defenses via activation of various vitagenes and other protective molecules to maintain redox homeostasis in cells/tissues. It was shown that Nrf2 is closely related to another transcription factor, namely, NF-κB, responsible for control of inflammation; however, its roles in poultry have not yet been characterized. Therefore, the aim of this review is to describe a current view on NF-κB functioning in poultry with a specific emphasis to its nutritional modulation under various stress conditions. In particular, on the one hand, it has been shown that, in many stress conditions in poultry, NF-κB activation can lead to increased synthesis of proinflammatory cytokines leading to systemic inflammation. On the other hand, there are a range of nutrients/supplements that can downregulate NF-κB and decrease the negative consequences of stress-related disturbances in redox homeostasis. In general, vitagene–NF-κB interactions in relation to redox balance homeostasis, immunity, and gut health in poultry production await further research.
Keywords: antioxidants, NF-κB, oxidative stress, poultry, redox balance
1. Introduction
Redox biology is a very quickly developing area of modern biological sciences, and roles of redox homeostasis in health and disease have recently received tremendous attention [1,2,3,4,5,6]. There are a range of redox pairs in cells/tissues responsible for redox homeostasis maintenance/regulation. They include, but are not limited to, NAD+/NADH, NADP+/NADPH, GSSH/GSH (glutathione system), Trxox/Trxred (thioredoxin system), protein thiolsox/protein thiolsred. It is believed that redox signaling is tightly integrated with various homeostatic mechanisms [7] and all redox elements are interconnected and regulated by various means, including antioxidant and vitagene networks [1]. The redox status is responsible for maintenance of cell signaling and cell stress adaptation. There are a range of redox sensors which determine redox imbalance and activate various pathways for its re-establishment. Among them are proteins Keap1, an inhibitor of Nrf2, and IκB, an inhibitor of NF-κB, which have received a lot of recent attention. Indeed, oxidation of SH groups in Cys of Keap1 or phosphorylation of IκB are important triggers for nuclear translocation and activation of Nrf2 and NF-κB—important players in the redox homeostasis regulation [6]. In particular, a recent model suggests regulation of all collaborating metabolic organs in the body through changes in circulating redox metabolites [5].
The physiological roles of redox homeostasis maintenance in avian species, including poultry, are poorly characterized. However, for the last 5 years, this topic attracted a lot of attention, and a range of publications covered some related aspects. Indeed, the redox system imbalance is shown to be associated with protein oxidation and impaired quality of poultry meat [8,9]. In broilers, subjected to dietary and heat stress, magnesium supplementation was indicated to improve redox status and meat quality [10]. The influence of selenium and selenoproteins in maintaining redox balance and immune responses of poultry and pigs was presented [11], and the effect of oxidative stress and redox disbalance on inflammation, including a detailed immune system investigation, was discussed [12,13]. Oxidative stress-related disturbances of the redox balance in the poultry gut have also been described [13,14,15,16]. The long-term effects of Ochratoxin A on the glutathione redox system in chickens have been investigated [17], and the protective effects of milk thistle on redox-homeostasis imbalance of duck liver imposed by mycotoxins [18] were shown. Furthermore, the detrimental effects of heavy metals (e.g., As) on redox imbalance in chickens have been reported [19]. Nutritional modulation of the antioxidant capacities and redox homeostasis in poultry by selenium [13,20], vitamin E [21], and carotenoids [22], including astaxanthin [23], has been described. Recently, the vitagene concept of stress adaptation was developed, and questions related to redox balance maintenance in poultry under various stress conditions were addressed [1]. In fact, the vitagene family includes superoxide dismutase (SOD), heat shock protein 70 (HSP70), heme oxygenase 1(HO-1), elements of thioredoxin and glutathione systems, and sirtuins [24,25,26]. Indeed, induction/activation of the aforementioned genes leading to synthesis/expression of protective molecules helps animals/poultry adapt to stress by using their internal resources to the maximum extent.
Furthermore, transcription factor Nrf2 was shown to be a master regulator of antioxidant defenses via activation of various vitagenes and other protective molecules to maintain redox homeostasis in cells/tissues [1,27]. It was shown that Nrf2 is closely related to another transcription factor, namely, NF-κB, responsible for control of inflammation; however, its roles in poultry are not yet characterized. The importance of understanding the molecular mechanisms of redox homeostasis maintenance and the regulatory roles of NF-κB in this process is related to several important issues in poultry production. Firstly, intensive poultry production is related to a variety of stresses which cannot be avoided because of price-sensitive production of meat and eggs [28,29,30]. Secondly, commercial poultry production is based mainly on large production units where several hundred birds are kept in a single room. In such conditions, immune protection against various microbial and viral diseases becomes an important issue, and several vaccinations during the production period take place [31]. Therefore, the important roles of NF-κB in maintaining the high immunocompetence of commercial birds deserve more attention. Because of growth-promoting antibiotic prohibition in poultry production in many countries with developed poultry production, poultry farmers face a lot of challenges associated with gut health problems and immunosuppression [32,33], where NF-κB is known to be involved. The global poultry industry faces challenges from Salmonella contamination of meat and eggs [34,35,36] and Campylobacter contamination of chicken meat [37], and understanding NF-κB functioning in poultry may help to develop effective protective measures against the aforementioned pathogens. Avian flu is also a great challenge for the global poultry industry [38], and understanding the involvement of NF-κB in antiviral immunity is on the agenda of many research centers worldwide. Furthermore, the avian immune system has a range of differences from the mammalian immune system (e.g., absence of lymph nodes, different antibody repertoire, absence of myeloperoxidase in macrophages, etc.) [39,40,41] and, therefore, understanding the regulatory functions of NF-κB would help to design various approaches for immunomodulation [42]. Lastly, there are a range of inflammation-associated conditions in poultry (see Section 8) where the crucial role of NF-κB is well known [43,44].
Therefore, the aim of this review is to provide and describe a current view on the NF-κB system functioning in poultry as an important part of redox balance maintenance mechanisms with a specific emphasis on the nutritional modulation of NF-κB under various stress conditions. For this purpose, a literature search using key words “NF-κB” and “poultry” or “chicken” was conducted using PubMed and Web of Science. Results of the analysis of relevant papers were included into the review. Since we were not able to find any reviews related to the roles of NF-κB in poultry, published in peer-reviewed journals, we also provide general information about the structure and functions of NF-κB on the basis of recent publications in this area.
2. Transcription Factor Nuclear Factor Kappa B
Nuclear factor kappa B (NF-κB) was discovered in the lab of the Nobel Prize winner, David Baltimore, as an inducible transcription factor in lymphocytes [45]. In general, NF-κB structure, functions, and regulation have been extensively characterized in recent comprehensive reviews [46,47,48]; however, in this review, the major emphasis is given to oxidative stress and the roles of NF-κB in the regulation of adaptive homeostasis in avian species.
The NF-κB family consists of several transcription factors characterized by Rel-homology domains (RHDs) that are able to bind to specific DNA sequences known as κB sites located in the promoter and enhancer regions of various genes [49]. It has been found that NF-κB is conserved across different phyla, and, in mammalian and avian species, it consists of a group of five related proteins (subunits) that are capable of binding to DNA: p50 (a 50 kDa protein also known as NF-κB1), p52 (known as NF-κB2), p65 (also known as RelA), c-Rel, and RelB. In fact, p65, RelB, and c-Rel are characterized by a C-terminal transcription activation domain (TAD) that participates in the positive regulation of gene expression [50]. Interestingly, the other NF-κB family members, NF-κB1 and NF-κB2, were shown to be synthetized as larger precursor proteins (p105 and p100) with subsequent proteolytic processing to p50 and p52 [47]. Transcription factor subunits of NF-κB, for example, p65, can combine to form hetero- and homodimers of different composition, defined as the NF-κB complex. As a result of binding to a variety of DNA sequences called κB sites, NF-κB can effectively regulate different gene targets [51].
By using two independent LC–MS/MS experiments, 365 NF-κB/RelA-associated proteins were identified [52]. The functional categories enriched in the newly identified NF-κB/RelA-associated proteins identified by the authors include DNA-binding factors and enzymes, RNA-binding factors and enzymes, nuclear matrix and cytoskeleton components, ribosome biogenesis, protein degradation, and mitochondrial proteins. For the maintenance of redox balance and development of adaptive homeostasis, the last category related to mitochondrial proteins, including antioxidant proteins, namely, peroxiredoxins 3 and 1 (PRDX3, and PRDX1), is of great importance. Functional analysis of the newly identified RelA-binding proteins conducted by the authors confirmed the complexity of the NF-κB actions and its involvement in the regulation of important biological functions including regulation of protein ubiquitination, chromatin organization, response to DNA damage stimulus, post-transcriptional regulation of gene expression, cell-cycle progress, and microtubule cytoskeleton translation [52]. Interestingly, an update on NF-κB and proteomics published a year earlier [53] indicated that stimulation of specific receptors by RNA, DNA, or lipopolysaccharide (LPS) is associated with the interactions of a number of unique proteins regulating NF-κB expression and activity, which activate a range of genes creating an adaptive response. Indeed, their roles in the maintenance of redox balance and the creation of adaptive homeostasis via modulating transcription factors and vitagenes deserve more attention.
Dimerization takes place at a region named the RHD, which is essential for DNA binding, dimerization, and interaction with the inhibitory κB (IκB) proteins [54,55]. The p65/50 dimer is considered to be the most important dimer activating transcription. Hence, RelA-deficient mice are shown to be embryonically lethal as a result of liver apoptosis [56].
As a result of action by various stimuli, IκB proteins are known to be rapidly phosphorylated by IκB kinase (IKK) on specific serine residues (e.g., Ser-32 and Ser-36 of IκBα; Ser-19 and Ser-23 of IκBβ), followed by ubiquitination (by E2- and E3-ligases), and degradation by the 26S proteasome [57]. To make signaling more effective, on the one hand, NF-κB is shown to encode gene effectors potentiating and amplifying its activation in a feedforward fashion. On the other hand, NF-κB activation is associated with programming default feedback mechanisms responsible for its automatic termination (post mission completion) by regulating the amount/activity/expression of negative effectors including microRNAs (miRNAs), decoy receptors, and anti-inflammatory cytokines, which can lead to the inhibition of the signaling pathways, inhibitory proteins IκBα and IκBε, etc. [58,59,60].
There are some similarities in the regulation of Nrf2 and NF-κB in biological systems. For example, in physiological resting conditions, NF-κB is known to be located in the cytoplasm of cells in an inactive state tightly bound to the inhibitory IκB proteins (e.g., IκBα, IκBβ, IκBγ, IκBδ, IκBε, etc.) preventing its binding to target sites. It seems likely that the IκB proteins are responsible for masking the DNA-binding domain of NF-κB/REL proteins, leading to their sequestering in the cytoplasm. Interestingly, IκB proteins are responsible for checking and controlling the pathway due to nuclear export signals and their ability to remove NF-κB proteins from the nucleus [59].
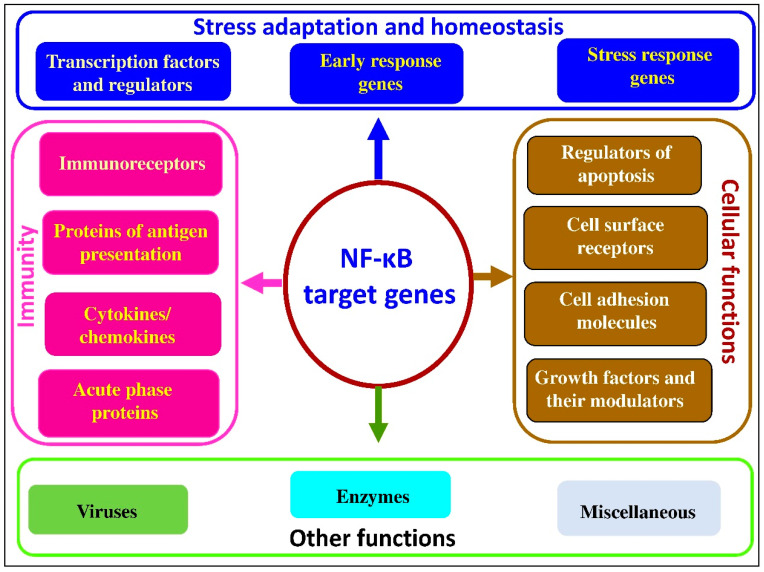
It seems likely that key steps in the NF-κB pathway include activation by the IκB kinase (IKK) complex, leading to the phosphorylation-induced proteasomal degradation of IκB proteins, dimer formation, and entering the nucleus, with subsequent binding to κB sites in promotor or enhancer regions of target genes [61]; along with other cofactors and histone acetyl transferases [62], it is also responsible for the transcription of more than 400 target genes regulating inflammation, immunity, apoptosis, stress adaptation, cell proliferation, and differentiation [63,64]. Major classes of target genes for NF-κB are shown in Figure 1.
Figure 1.
NF-κB target genes (adapted from [65]).
As can be seen from the data presented in Figure 1, NF-κB target genes can be divided into several groups. The main group includes genes directly related to immunity, including immunoreceptors, proteins involved in antigen presentation, cytokines/chemokines and their modulators, and acute phase proteins. The second group of genes regulated by NF-κB are associated with stress adaptation and homeostasis maintenance under various stress conditions, including transcription factors and regulators, stress response genes, and early response genes. The third group of NF-κB-regulated genes are responsible for the regulation of various cellular function, including regulators of apoptosis, cell-surface receptors, cell adhesion molecules, growth factors, and their modulators. There are also NF-κB-regulated genes related to viruses, enzymes, and some other important signaling molecules. Therefore, the great variety of NF-κB-regulated genes explains the pivotal roles of this transcription factor in major physiological and pathophysiological processes in mammalian and avian species.
Generally, the NF-κB system is tightly regulated and can be activated by more than 15 pathways, with the two most common pathways being canonical (classical) and noncanonical (alternative) pathways [57]. The canonical pathway (i.e., the classical pathway) is based primarily on usage of p50 (the product of p105) in conjunction with p65 (p65/p50 nuclear dimer), IKKβ, and NF-κB Essential Modulator (NEMO), and it can be activated by pro-inflammatory cytokine receptors (interleukin 1 receptor, IL-1R and tumor necrosis factor receptor, TNFR), by pattern recognition receptors including the Toll-like receptors (TLRs), and by various genotoxic agents. It seems likely that the NF-κB stimulation by various external and internal stressors takes place via the canonical pathway. This includes a response to genotoxic and oxidative stresses, caused by ultraviolet radiation, ionizing radiation, reactive oxygen species (ROS), hypoxia, and dysfunctional mitochondria or endoplasmic reticulum by activating NF-κB IKK-dependently, IKK-independently, or both [60,66,67]. In particular, NF-κB can be activated by DNA damage via the canonical pathway [68].
The noncanonical pathway (known as the alternative pathway) is associated with p52 (the product of p100), RelB, NF-κB-inducing kinase (NIK), and IKKα and it is known to be triggered by a range of stimuli, including lymphotoxin B receptor, B-cell activating factor receptor 3, cluster of differentiation 40 (CD40), and receptor activator of NF-κB ligand (RANKL). Therefore, ligand-induced activation of the aforementioned receptors leads to the activation of NF-κB-inducing kinase (NIK), which specifically activates IKK1, inducing the phosphorylation and proteolytic processing of p100 to p52, followed by heterodimer formation with RelB to regulate target gene expression [56]. It is established that the noncanonical NF-κB pathway is deeply involved in regulation of the immune system, including creation of the adaptive immune response [69]. This includes regulation of B-cell development and function, including differentiation into long-lived antibody-producing plasma cells and memory B cells (for a review, see [46,70]), both being an integral part of the humoral immune response. Since, in comparison to mammals, avian spices are characterized by a different set of immunoglobulin (Ig) classes (IgD and IgE molecules are absent in birds) and a different cytokine repertoire [41], understanding the molecular mechanisms underlying regulation of the noncanonical NF-κB pathway in birds is a priority for avian scientists. Furthermore, a range of vaccinations used in poultry production are based on humoral response activation and memory B-cell formation [31,71], and the noncanonical NF-κB pathway could be a target for improvement of vaccination efficacy. It should be mentioned that the noncanonical NF-κB pathway was also shown to be involved in T-cell development in the thymus, and in orchestrating the formation and maintenance of effector and memory T cells [65]. Indeed, the cell-mediated immunity based on T-cell activity is of paramount importance for poultry, including resistance to viral diseases and vaccination efficacy [72,73].
It is important to mention that canonical and noncanonical NF-κB pathways interact with each other. For example, in the classical NF-κB pathway, the first protein transcribed is IκBα. Therefore, it is believed that, in order to inhibit further transcription and restore the original latent state of NF-κB signaling, newly synthesized IκBα can enter the nucleus, remove NF-κB from DNA, and export the complex back to the cytoplasm [49]. Interestingly, the canonical NF-κB pathway is considered to be antiapoptotic, while the noncanonical pathway is proapoptotic [50].
It well known that NF-κB responds to a large variety of external and internal stress signals/stimuli including oxidative stress [62], playing essential roles in the development and maintenance of tissue homeostasis by regulating the transcription of an array of different genes, including proinflammatory cytokines, as well as adhesion molecules, antimicrobial peptides, and acute phase proteins [74,75,76]. In fact, NF-κB signaling can be considered as an emergency response system, since activation of NF-κB was shown to occur very quickly (within minutes) as a result of release from IκB or as a consequence of cleavage of the inhibitory ankyrin repeat domains of p100 and p105 [50]. Under physiological conditions, the majority of NF-κB-activated genes regulate biological processes associated with cell growth, protection, and repair. They are involved in T-cell maturation, DNA damage repair, tissue healing after injury, and orchestrating the fight against infections [60,67]. Indeed, it has been proven that activation of NF-κB is an evolutionarily conserved, effective mechanism of host defense against infection and stress [77]. However, excessive NF-κB activation in commercially relevant stress conditions in poultry and farm animal production systems can lead to detrimental consequences, including chronic inflammation, compromised health status, and decreased productive and reproductive performance. The repertoire of stimuli implicated in the NF-κB activation is very diverse and also includes inhibitory κB kinases, cell-surface receptors, and NF-κB-inducible inhibitor proteins (IB proteins), as well as factors regulating the post-translational modification of the Rel proteins, etc. [63,64,74,75,76]. Furthermore, p65 and p50 are the targets of many other post-translational modifications such as ubiquitination, acetylation, methylation, phosphorylation, oxidation/reduction, and prolyl-isomerization, leading to a change in NF-κB transcriptional activity due to affecting the interaction with DNA or as a result of changes in the protein–protein association of NF-κB [78].
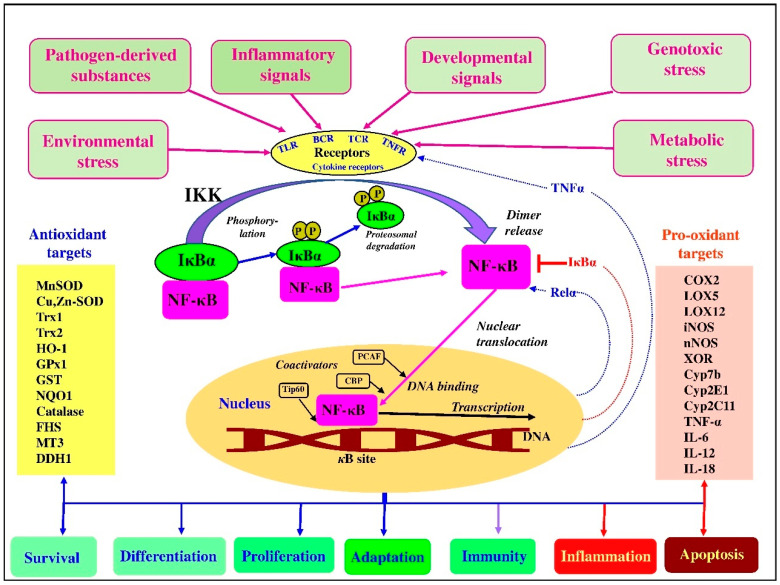
NF-κB signaling in numerous cell types is involved in the development of various metabolic disorders. In particular, it is thought that resident tissue cells activate NF-κB in response to stress associated with nutrient excesses [58]. Furthermore, oxidized lipids in the bloodstream can induce NF-κB in vascular endothelia, while, in adipocytes, hepatocytes, and neurons, NF-κB is induced by metabolic or oxidative stress in the ER due to overnutrition. Furthermore, an excess of free fatty acids could also activate NF-κB via TLR4 [58]. Some examples of activation of NF-κB associated with regulation of downstream transcriptional antioxidant and pro-oxidant targets in the canonical pathway are shown in Figure 2.
Figure 2.
Activation of NF-κB and regulation of downstream transcriptional antioxidant and pro-oxidant targets in the canonical pathway (adapted from [1,59,80,81,82,83,84]).
Depending on physiological context, the activation of NF-κB can have different consequences. Indeed, NF-κB does not function alone but is part of various networks, including crosstalk with other transcription factors (Nrf2; signal transducer and activator of transcription 3, STAT3; Forkhead box O3, FOXO3; etc.), upstream kinases, sirtuins, Wingless-related MMTV integration site 4 (Wnt4), ROS, p53, and miRNAS, which determine the pattern of its effects on the expression of a battery of various genes [48,60,67]. Furthermore, there are regulatory mechanisms coordinating NF-κB association with various important pathways [50]. It has been suggested to consider NF-κB as a stress response factor, since NF-κB signaling is condition-dependent, and NF-κB-dependent cell death or survival would depend on the stimulus and the cell type involved. It seems likely that this complexity is responsible for many apparent contradictions in the literature [79]. However, most research data indicate that NF-κB signaling pathway enables cells to maintain homeostasis and survive under various stress conditions, including genotoxic stress [60,67].
NF-κB is involved in the modulation of many different molecular events, including inflammation, immune function, cellular growth, and apoptosis [46,70]. There are a range of NF-κB activators, including pathogen-derived substances (LPS) and inflammatory signals (TNF-α, IL-1), as well as other signals recognized by various receptors, including TNFRs, TLRs, T-cell receptors (TCRs), B-cell receptors (BCRs), and cytokine receptors, which lead to an activation of IκB kinase (IKK) with subsequent phosphorylation of NF-κB inhibitor. This leads to proteasomal degradation of IκB. As a result, the released NF-κB migrates into the nucleus and binds with its corresponding DNA-responsive elements in the presence of coactivators. This results in the transcription of antioxidant (anti-inflammatory) or pro-oxidant (proinflammatory) mediators [46,85]. It is believed that p65 can induce the expression of both negative regulators (IκBα, IκBε, etc.) and positive regulators (Relα, TNFα, etc.) participating in tuning the NF-κB pathway [83]. It is important to mention that NF-κB can be directly activated or inhibited by ROS in a context-dependent manner, including levels of ROS, exposure, and cell type [59,86,87]. Indeed, ROS-mediated oxidation of redox-sensitive cysteine residues of NF-κB subunits was shown to have dual effects (inducing or inhibiting) on the NF-κB signaling depending on the level of ROS, the cell type, and the types of stimuli [81,88]. On the one hand, ROS can activate the NF-κB pathway by imposing disulfide bond formation between Cys54 and Cys347 in ΙΚΚγ [89]. On the other hand, ROS can have an opposite effect: inhibiting NF-κB activation as a result of restricting IκBα degradation, due to inactivation of the proteasome [90].
The NF-κB system integrates diverse upstream input signals (from various stresses to pathogen-related molecules) recognized by various receptors into varied downstream output responses. This function is mediated via promotion of the expression of a variety of genes responsible for the synthesis of antioxidant or prooxidant molecules, improving antioxidant defenses and redox homeostasis. Alternatively, NF-κB activation can also lead to synthesis of proinflammatory cytokines, imposing inflammation and causing detrimental health- and production-related consequences in poultry and farm animals. In particular, NF-κB and STAT3 regulate common processes and share regulatory binding sites of antiapoptotic, cell cycle and proliferation, tissue resistance, and repair genes. Furthermore, hypoxia-inducible factor (HIF) and NF-κB share common activating stimuli, regulators, and targets [61].
3. NF-κB and Oxidative Stress
Free-radical production is considered to be an important process in biological systems responsible for the antibacterial action of oxidative burst in phagocytes, cell signaling, and stress adaptation [7]. However, an excess of reactive oxygen and nitrogen species (RONS) due to high level of stress or a compromised antioxidant system leads to damages to major biological molecules (proteins, polyunsaturated fatty acids (PUFAs), DNA, etc.) associated with immunosuppression, gut health problems, and decreased productive and reproductive performance of poultry [30]. Therefore, a variety of protective mechanisms have been developed during evolution to deal with RONS excess, and many transcription factors are involved in this process via regulating vitagenes and a myriad of antioxidant enzymes in stress conditions [1].
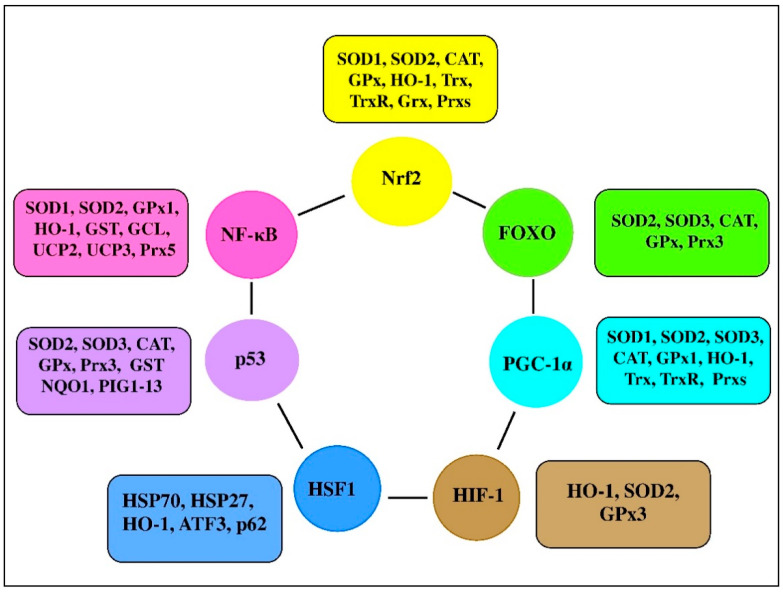
There are a range of transcription factors acting cooperatively with NF-κB. For-example, NF-κB and STAT3 are shown to regulate common pathways and share regulatory binding sites of various protective genes, while HIF and NF-κB are reported to share common activating stimuli, regulators, and targets [61]. Indeed, the redox balance is believed to be orchestrated by a range of transcription factors, including Nrf2, NF-κB, activator protein 1 (AP-1), FoxO, peroxisome proliferator-activated receptors (PPARs), peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α), p53, and mitogen-activated protein kinase (MAPK; Figure 3 [91,92]). It seems likely that transcription factors and vitagenes are involved in the regulation of redox status by effectively modulating the expression and activity of ROS-generating enzymes and antioxidant enzymes [93].
Figure 3.
Transcription factors and their clients involved in redox homeostasis regulation (adapted from [1,92,93,94]).
NF-κB has long been considered to be a prototypical proinflammatory signaling pathway stimulating the immune system in response to various stimuli, including physical, physiological, and/or oxidative stress. For example, NF-κB is a key target in receptor-independent hypothalamic microinflammation [95] associated with intracellular organelle stress, including RNA stress response [96], endoplasmic reticulum (ER) stress [97], and defective autophagy [98]. NF-κB is involved in the regulation of many important physiological processes; however, its overactivation has been shown to be associated with increased risk of disease, while NF-κB suppression is associated with risk reduction [63]. Taking the former into account, understanding the role of NF-κB signaling in stress adaptation awaits further investigation. For example, HO-1 can improve cell protection from apoptosis by stimulating free heme catabolism. Interestingly, the HO-1 promoter region contains an NF-κB responsive element and, therefore, HO-1 expression is regulated by NF-κB, as well as by other transcription factors [99]. A central role for NF-κB in regulating mitochondrial respiration has been suggested [100]. In fact, by controlling the balance between glycolysis and respiration for energy provision, NF-κB is involved in energy homeostasis and metabolic adaptation [101]. The authors suggested to consider NF-κB as an important checkpoint connecting cell activation and proliferation with energy sensing and metabolic homeostasis. Since mitochondria are the main ROS source in the cell, it could be that NF-κB signaling is involved in the regulation of ROS formation, detoxification, and the maintenance of redox homeostasis.
4. Nrf2 and NF-ĸB Interplay in Oxidative Stress
Proof of the interaction and cooperative action of Nrf2 and NF-κB was taken from experimental work with various model systems employing plant extracts, individual compounds in vitro and in vivo, pure chemicals, and some known toxicants [6]. In our recent review, a central role of Nrf2 in antioxidant defenses and vitagene regulation was described in detail [27], and it seems likely that, under oxidative stress, the transcription factors NF-κB and Nrf2 antagonize each other to coordinate a stress response [60,67,76]. For example, deletion of Nrf2 (Nrf2 knockout mice) enhanced inflammation, while Nrf2 upregulation was reported to decrease NF-ĸB-dependent proinflammatory and immune responses [62]. In fact, several known Nrf2 activators are able to inhibit the NF-κB pathway. There are many examples showing that activation and repression occur between members of the Nrf2 and NF-κB pathways through various mechanisms [102]. Some mechanisms of Nrf2–NF-κB interactions are summarized in Table 1.
Table 1.
Possible mechanisms of Nrf2–NF-κB interactions.
| Mechanisms of Nrf2–NF-κB Interactions | References |
|---|---|
| Inhibiting effects of Nrf2 on NF-κB | |
| Decreasing the intracellular ROS levels. This inhibits oxidative stress-mediated NF-κB activation | [103] |
| Preventing the IκB-proteasomal degradation and inhibiting nuclear translocation of NF-κB. In Nrf2-deficient cells an inhibitor of NF-kB activity (IκB) is over-phosphorylated with rapid proteasomal degradation and increased NF-κB activity. Upregulation of Nrf2 induces increase heme oxygenase-1 (HO-1) levels and induce phase II enzymes expression blocking the degradation of IκB | [104,105,106,107] |
| Reducing p50 and p65 DNA binding. Nrf2 silencing enhanced p50 and p65 DNA binding and tumour necrosis factor (TNF)-α-induced proinflammatory gene expression | [108] |
| Preventing the recruitment of RNA polymerase II to start transcription of NF-κB-regulated genes. Nrf2 binds to regulatory regions of proinflammatory genes in an antioxidant-response element (ARE)-independent manner and prevents the recruitment of RNA polymerase II to start transcription of NF-κB-regulated genes | [109] |
| Competition between Nrf2 and p65 for binding to the transcriptional co-activator CBP-p300 complex. Overexpression of p65 limits the availability of CBR for Nrf2 interaction. Knockdown of p65 promotes Nrf2 complex formation with CBR | [110,111] |
| Degrading IKKβ through ubiquitination by Keap1 | [112] |
| Inhibiting effects of NF-κB on Nrf2 | |
| Inactivating Nrf2 by inducing cyclooxygenase 2 | [113,114] |
| Recruiting MAF BZIP Transcription Factor K (MafK)-associated histone deacetylase 3 (HDAC3) activity to the HO-1 enhancer and deacetylating CBP leading to a suppression of its co-activator activity | [115,116] |
| Interacting with CREB-binding protein, the competent Nrf2 coactivator, and inhibiting the transcription of genes regulated by Nrf2 | [111,117,118] |
| Decreasing free CBP, a transcriptional co-activator of Nrf2, and promoting phosphorylation of p65. Overexpression of p65 limits the availability of CBR for Nrf2 interaction. Knockdown of p65 promotes Nrf2 complex formation with CBR |
[111] |
| κB sites in proximal promoter of Nrf2 are believed to be subject to binding and transcription initiation by p65 | [119] |
There is accumulating evidence indicating that various nutrients with antioxidant (AO) activities could differently affect transcription factors: increasing expression of Nrf2 and simultaneously decreasing NF-κB expression and activity. This was proven in various model systems employing plant extracts and individual polyphenolics. Firstly, in in vitro systems, these include sinomenine [120], aloin [121], cannabisin F [122], urolithin B [123], 4-ethylguaiacol [124], gambogic acid [125], and the combination of ascorbic acid and rutin [126]. There are also a range of in vivo studies confirming that various plant-derived compounds, mainly polyphenols, decrease NF-κB and increase Nrf2 activity/expression in various model systems. These include peiminine [127], hesperetin [128], oxyresveratrol, resveratrol and mulberroside [129], salvianolic acid A [130], naringenin [131], δ-amyrone [132], chrysin, luteolin, apigenin, hesperetin and 3′, 4′-dimethoxy hesperetin [133], luteoloside [134], alpinetin [135], amygdalin [136], rosmarinic acid [137], chiisanoside [138], arbutin [139], and chicoric acid [140]. Moreover, the different direction of activation of Nrf2 and NF-κB was also shown to be a result of exposure to various toxic compounds. However, other agents and stimuli, including, but not limited to, ROS, LPS, flow shear stress, oxidized low-density lipoprotein, and cigarette smoke, were reported to activate both Nrf2 and NF-κB pathways [101,102].
The redox outcome of the NF-κB–Nrf2 interaction would depend on the activation/inhibition of various antioxidant and prooxidant enzymes. It is known that some antioxidant enzymes are dependent on both Nrf2 and NF-κB. For example, expression of HO-1 is shown to be regulated by Nrf2, NF-κB, and HIF-1α signaling [60,67]. On the one hand, HO-1 was shown to possess a functional ARE that is activated by Nrf2 [141]. On the other hand, HO-1 was shown to have a functional NF-κB site [142]. HO-1 is known to be the stress-inducible enzyme providing AO protection in vertebrate systems, participating in the maintenance of redox balance and being responsible for adaptation to oxidative, inflammatory, and cytotoxic stress [25]. Similarly, a key catalytic subunit of glutamate-cysteine ligase, the key enzyme of the cellular GSH biosynthetic pathway, also has an ARE and can be activated by Nrf2 [143], whereas it also possesses a κB site and can be induced by NF-κB [144]. Since GSH is a key physiological buffer responsible for the redox homeostasis [145], regulation of its synthesis via Nrf2 and NF-κB pathways is of great importance for redox homeostasis maintenance related to high immunocompetence. Furthermore, MnSOD is also a target for both NF-κB [146] and Nrf2 [27]. It is well established that MnSOD, a key enzyme of the first line of the AO network, is located in mitochondria and deals with major biological ROS, namely, superoxide radicals, and it is considered to be a major player in the establishment and maintenance of redox homeostasis [30]. Furthermore, glutathione peroxidase 1 (GPx1) and glutathione S-transferase (GST) expression and activities are also under strict control by NF-κB [81] and Nrf2 [13]. The important roles of these AO enzymes in AO defense and redox homeostasis have been previously discussed [13]. It seems likely that another redox balance regulator, namely, thioredoxin, is also regulated by NF-κB [146,147] and Nrf2 [25]. It is interesting that HO-1, SOD, and thioredoxins belong to the vitagene family responsible for stress adaptation and redox homeostasis [24].
It should also be mentioned that the NF-κB pathway can induce free-radical production via activating ROS-producing enzymes, including NADPH oxidase [148], cyclooxygenase-2 (COX-2) [102], cytochrome p450 enzymes, inducible nitric oxide synthase (iNOS), neuronal NOS (nNOS), and xanthine oxidase/dehydrogenase [81]. The impact of such ROS production on redox balance and adaptation to stress is still not well established; however, this complicates the interpretation of results related to NF-κB–Nrf2 interactions in biological systems under various stress conditions.
In many cases, activation of various transcription factors, including Nrf2, NF-κB, AP-1, HIF-1α, p53, PPAR-γ, and β-catenin/Wnt, was associated with the oxidative stress [149]. Therefore, a complex crosstalk between Nrf2 and NF-κB pathways under various stress conditions [62] further complicates interpretation of results related to the relative impact of each pathway on the regulation of stress adaptation. Indeed, as mentioned above, Nrf2 and NF-κB affect each other’s expression and activity to coordinate antioxidative and inflammatory responses; however, molecular mechanisms of this interconnection are not yet known [62].
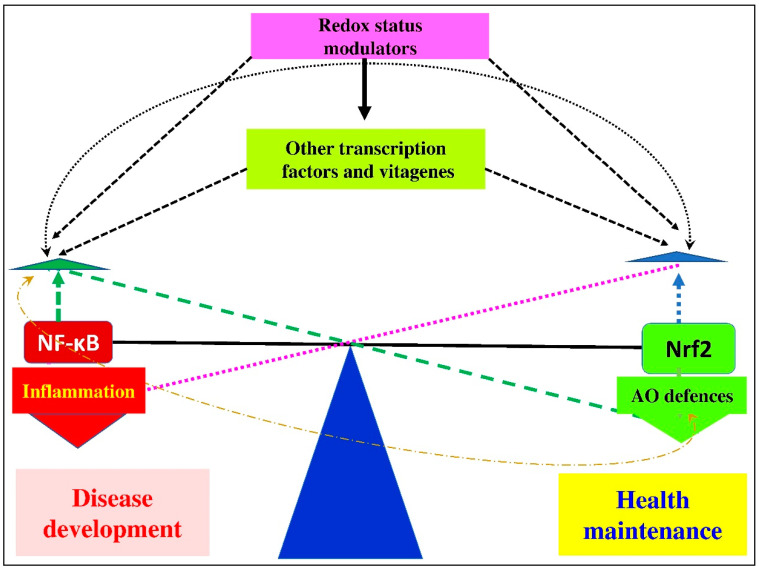
It is believed that condition-dependent, stress-associated changes in redox balance and in expressions/activities of transcription factors (e.g., Nrf2/Keap1 and NF-κB/IκB/IKK) are responsible for providing adaptive cell responses to a variety of stress stimuli through orchestrating the optimal expression of protective target genes [150]. A hypothetical scheme of the Nrf2–NF-κB crosstalk is shown in Figure 4.
Figure 4.
In physiological conditions, a delicate balance between Nrf2 and NF-κB expression in various tissues is well coordinated and maintained. It seems likely that increased NF-κB expression as a result of low/moderate stresses can lead to a simultaneous increase in the expression of Nrf2, leading to improved antioxidant defenses. At the same time, decreased NF-κB expression can be observed as a feedback mechanism. This balance is also regulated by other transcription factors and vitagenes. In the case of high oxidative stress, when the ability of the AO defense network to deal with RONS production is overwhelmed, the Nrf2/NF-κB balance would be broken. In such conditions, redox status would be compromised with detrimental consequences to animal health. Furthermore, the productive and reproductive performance of poultry and farm animals would be decreased.
5. NF-κB in Poultry Production
The regulatory roles of NF-κB in poultry are still poorly understood, but accumulating information clearly indicates that, similar to mammals, NF-κB is a main regulator of many important processes, including inflammation in avian species. In 1993, complementary DNA (cDNA) clones encoding the chicken NF-κB p65 subunit were isolated, and, according to the information provided by the authors, chicken NF-κB can be briefly characterized as follows [151]:
Chicken p65 was shown to be approximately 55% identical to the mouse and human p65 proteins. Similar to its mammalian counterpart, chicken p65 contains the Rel homology domain (RHD) in its N-terminal consisting of 286 amino acids and the putative transactivation domain in its C-terminal region;
It was proven that the RHD was highly conserved between the chicken and mammalian p65 proteins;
The highest expression of a 2.6 kb transcript of p65 was detected in the spleen. It was also detected in other organs;
A fusion protein containing the RHD of chicken p65 was reported to bind to a consensus kappa B-site;
p65 was shown to form one or more complexes with various cellular proteins, including p50, p105, and c-Rel in chicken spleen cells [151].
Furthermore, the cDNA clones encoding chicken p50B/p97 were isolated [152]. The amino-acid sequence of the precursor protein p97 was found to be characterized by a conserved structure. In particular, it was shown to have 86% identity in the RHD and lower (56%) identity in the ankyrin repeat domain (ARD) to human p50B/p97. Similar to previous findings, expression of this gene was also found to be highest in the chicken spleen [152]. In 1995 from a chicken genomic library, a clone containing the avian I kappa B-alpha gene was isolated [153]. Main characteristics of I kappa B-alpha can be summarized as follows: recognizable promoter elements (i.e., TATA and CAAT boxes) were not found in avian I kappa B-α. There were seven putative Rel/NF-kappa B binding sites in avian I kappa B-α. When transfected into cells which produce I kappa B-α, a CAT reporter construct containing the 5′ upstream region of I kappa B-α was expressed. The regulatory elements promoting I kappa B-α expression were identified within 1000 nt of the transcription start site. I kappa B-alpha was shown to be found as a single-copy gene per haploid genome. This gene was expressed in avian hematopoietic tissues and in lymphoid cells transformed by avian reticuloendotheliosis virus [153]. It was suggested that, similar to mammals, in chicken, p65 and c-Rel comprise components of the protein complexes that are able to bind to the kappa B-like sequence. This binding could lead to the progressively activated expression of the chicken lysozyme gene observed during the terminal differentiation of macrophages [154].
In 2001, Piffat et al. constructed and characterized a composite cDNA encoding most of the chicken RelB transcription factors [155], and their results can be summarized as follows: within the RH domain, chicken RelB (cRelB) protein was characterized by a high degree of sequence similarity to other vertebrate RelB proteins. However, outside this domain, cRelB was substantially less conserved. cRelB was found to be more widely expressed than mammalian RelB, and it was identified to have functional properties similar to other vertebrate RelB proteins. cRelB was reported to be unable to bind DNA in a homodimer form; however, it could form DNA-binding heterodimers with NF-kappaB p50 or p52. Overexpressed cRelB was shown to be present in the nucleus in chicken embryo fibroblasts. The nonconserved C-terminal sequences of cRelB contained a transactivation domain found in chicken and mouse fibroblasts [155]. A new isoform of chicken myeloid differentiation factor 88 (MyD88-2) expression was detected in a range of tissues tested and its overexpression was found to significantly induce the activation of NF-κB in vitro [156]. Recently the duck IKKα (duIKKα) gene was cloned and characterized. In fact, DuIKKα was reported to encode a protein containing 757 amino acids and having high sequence identities with the goose IKKα. Duck liver and heart were characterized by a high expression of duIKKα messenger RNA (mRNA), while its expression was reported in all tested tissues, including muscular stomach, spleen, heart, liver, lung, kidney, cerebellum, cerebrum, windpipe, muscle, glandular stomach, thymus, duodenum, cecum, pancreas, and bursa of Fabricius [157]. An important role of du IKKα in NF-κB regulation has been demonstrated by increasing or inhibiting expression of duIKKα. On the one hand, overexpression of duIKKα was shown to substantially increase NF-κB activity with subsequent induction of cytokines interferon beta (IFN-β), IL-1β, IL-6, and IL-8 in duck embryo fibroblasts. On the other hand, knockdown of duIKKα was found to significantly decrease LPS-, poly(I:C)-, poly(dA:dT)-, duck enteritis virus (DEV)-, or duck Tembusu virus (DTMUV)-induced NF-κB activation [157]. It seems likely that IKKα is evolutionarily conserved. In fact, phosphorylation of Ser176 and Ser180 in the active center of IKKα is believed to be vital to IKKα activation, and those Ser residues were shown to be well conserved among mammals, birds, and fish [157].
It was shown that the NF-κB family of transcription factors contribute to activation-induced cytidine deaminase-mediated gene conversion in chickens [158]. Gallus heat-shock cognate protein 70 was shown to regulate RelA/p65 gene expression induced by Apoptin, a nonstructural protein of chicken anemia virus [159]. In chicken heterophils, bacterial TLR agonists were indicated to activate NF-κB-mediated leukotriene B4 and prostaglandin E2 production [160]. A switchlike response in NF-κB activity is based on the existence of a threshold in the NF-κB signaling module, and phosphorylation of the Ser-578 residue of the scaffolding protein caspase recruitment domain (CARD)-containing protein 1 (CARMA1) was shown to account for the feedback [161]. It is known that tumor necrosis factor receptor-associated factors (TRAFs) are responsible for activation of various signaling cascades, being key regulatory proteins in NF-κB signaling pathways [162]. It seems likely that avian TRAFs play important roles in defending against both RNA and DNA virus infection. In fact, chicken TRAF3 (chTRAF3) was shown to encode a protein of 567 amino acids with high identity to TRAF3 homologs from mammals being abundantly expressed in the spleen, thymus, lung, and small intestine [163]. Of note, the authors showed that Newcastle disease virus F48E9 challenge was responsible for TRAF3 suppression in chicken embryo fibroblast cells. Recently, the full-length duck TRAF6 (duTRAF6) cDNA from embryo fibroblasts was cloned, and it was shown that duTRAF6 was widely expressed in different tissues. Interestingly, overexpression of duTRAF6 was found to activate NF-κB and induce interferon-β expression [164]. It has been shown that goose TRAF6 shared similar features with the TRAF6 of other avian species, being an essential regulator for inducing the activity of NF-κB and playing important roles in innate immune response [165]. The amino-acid sequence of pigeon FRAF6 (piTRAF6) was shown to share a strong identity with that of other birds. Furthermore, piTRAF6 expression was shown in all examined tissues, including heart, lung, spleen, thigh muscle, large intestine, caecum, kidney small intestine, brain, bursa of Fabricius, rib, and muscular stomach [166]. The heart was characterized by the highest level of piTRAF6 transcript, and the muscular stomach had the lowest level of piTAF6 transcript. On the one hand, overexpression of piTRAF6 was shown to induce NF-κB in a dose-dependent manner with increased IFN-β expression. On the other hand, piTRAF6 knockdown was reported to suppress NF-κB activation in HEK293T cells [166]. Furthermore, the pigeon TRAF3 (PiTRAF3) gene was reported to be highly expressed in the spleen, lung, kidney, brain, thymus, and muscle, while a moderate expression was observed in the small and large intestines, with relatively weak expression in the heart and liver [167].
Among the five major families of pattern recognition receptors (PRRs), Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), in particular, NOD1, recently received major attention in relation to their roles in avian immunity via NF-κB regulation. Indeed, NF-κB is considered to be the major transcription factor involved downstream of the TLR signaling pathway [168]. Avian TLRs are shown to be different from their mammalian counterparts: absence of TLR8 and TLR9, along with presence of TLR1La, TLR1Lb, TLR15, and TLR21 [169]. Therefore, in chickens, 10 TLR receptor genes were identified: TLR1LA, TLR1LB, TLR2B, TLR2A, TLR3, TLR4, TLR5, TLR7, TLR15 [170], and TLR21 [171]. Among them, TLR1LA, TLR1LB, TLR2A, TLR2B, TLR4, TLR5, and TLR15 are responsible for bacterial component (lipoproteins, peptidoglycans, LPS, and flagellin) detection, while TLR3 and TLR7 detect viruses (double-stranded RNA (dsRNA), single-stranded RNA (ssRNA), imidazoquinoline compounds), and TRL21 detects CpG oligodeoxynucleotides in viruses and bacteria [171]. Initially, it was reported that chicken TLR2 and TLR4 can mediate LPS-stimulated oxidative burst, while CD14 and TLR2 are involved in the mediation of lipoteichoic acid-stimulated oxidative burst in heterophils [172]. The tissue-specific expression of chicken TLRs (TLR2A, TLR3, TLR4, TLR5, TLR7, TLR15, and TLR21) during embryonic development was evaluated and early (third embryonic day) expression of all the TLR mRNAs was reported [173]. Furthermore, TLR1 (type 1 and 2), TLR2 (type 1 and 2), and TLRs 3–5, 7, 15, and 21 were shown to be expressed in the chicken follicular theca. The connection of the TLRs to NF-κB was proven experimentally; the expression of IL-1β, IL-6, chemotactic and angiogenic factor (CXCLi2), and IFN-β in tissues incubated with LPS was downregulated by an inhibitor of NF-κB [168].
It seems likely that NF-κB is involved in the activation of avian antimicrobial peptides. For example, chicken intestine defensins (e.g., AvBD13) were suggested to be endogenous ligands for TLR4 able to enhance the proliferation of monocytes via the NF-κB pathway [174]. It should be mentioned that cathelicidins (CATHs), short cationic host defense peptides, also act in close concert with NF-κB. Indeed, in macrophages primed by LPS, pigeon CATH2 was shown to act through MAPK and NF-κB signaling pathways to enhance expression of the anti-inflammatory cytokine, while downregulating the expressions of inducible nitric oxide synthase and proinflammatory cytokines and inhibiting the TLR4 pathway [175]. Furthermore, NK-lysin/granulysin (NKL), an antimicrobial cationic peptide expressed in natural killer cells and cytotoxic T lymphocytes, was identified in different avian species, including chicken, turkey, zebra finch, and quail, and the 5′ flanking region of quail NKL was shown to contain two NF-κB-binding sites [176], suggesting participation of NF-κB in regulation of NKL activity.
In hen vaginal cells, NF-κB was shown to be the transcription factor responsible for the expression of various proinflammatory cytokines and chemokines. In fact, in response to the ligands of TLR3, 4, and 21, increased expression of IL1B, IL6, and CXCLi2 was observed, while IL1B expression was found in response to the ligands of TLR5 and 7 [177]. The authors suggested that NF-κB-dependent expression of cytokines might provide the important defense capability of vaginal tissue to bacterial and viral infections. Activation of TLR3 was shown to induce the expression of NF-κB and the production of type-I interferon [178]. IFN-κ (a type I IFN) in both chicken and duck was found to be constitutively expressed in a range of tissues, including spleen, skin, lung, and peripheral blood mononuclear cells (PBMCs), and it could be induced after treatment with virus in PBMCs [179]. The duck TLR4 (duTLR4) gene was shown to be strongly expressed in the liver, kidney, spleen, intestine, and brain [180].
Goose TLR3 was shown to be analogous to mammalian TLR3 and recognized double-stranded RNA with subsequent activation of NF-κB [178]. In fact, the goose TLR3 gene was shown to encode a protein containing 896 amino acids, sharing 46.7–84.4% homology with other species with highest expression in the pancreas and lowest in the skin. The authors showed that geese infected with H5N1 were characterized by significant upregulation of TLR3 in various tissues, including the lung and brain [178]. The goose TLR5 (gTLR5) gene was shown to be expressed in all studied tissues, including high expression in the liver, spleen, and brain, moderate expression in kidney, lung, heart, bone marrow, small intestine, large intestine, and PBMCs, and minimal expression in the cecum [181]. It was also shown that gTLR5 can detect flagellin from Salmonella Typhimurium with subsequent NF-κB activation in HEK293 cells. It seems likely that there is a tissue-specific regulation of TLR expression in the process of orchestrating the immune response against bacterial pathogens [181]. Goose TLR2-1 was also shown to play an important role in the recognition of Mycoplasma fermentans lipopeptide, Mycoplasma gallisepticum (MG) and Salmonella enteritidis (SE), and it induced the activation of NF-κB [182]. Furthermore, in HEK293T cells, flagellin was shown to induce pigeon NF-κB via TLR5 activation. This was associated with significant upregulation of IL-1β, IL-8, TNF-α, and IFN-γ. Importantly, the levels of TLR5, NF-κB, IL-6, IL-8, chemokine ligand 5 (CCL5), and IFN-γ mRNA were significantly upregulated as a result of flagellin stimulation of pigeon splenic lymphocytes. As could be expected, goose TLR5 knockdown was shown to be associated with the significantly downregulated expression of NF-κB and related cytokines/chemokines [183]. Interestingly, the antiviral activity of pigeon IFN-α is believed to depend on the expression of NF-κB [184]. It is known that single-stranded viral RNAs and antiviral imidazoquinoline compounds can be recognized by TLR7 with subsequent NF-κB activation. Recently, it was shown that, in pigeon, agonist R848 (imidazoquinoline) can activate NF-κB via TLR7 [185].
It seems likely that chicken NOD1 activation in response to pathogenic invasion is of great importance for immune defense. In partridge chicken, NOD1 was shown to be widely distributed in various tissues, with the highest expression found in testes. Of note, as a result of S. enterica serovar Enteritidis infection, induced expression of chNOD1, as well as the effector molecule NF-κB, was observed in the spleen tissue [186]. Duck NOD1 (duNOD1) was shown to be widely distributed in various organs, including heart, liver, spleen, lung, kidney, cerebrum, cerebellum, colon, glandular stomach, thymus, and bursa of Fabricius tissue with the highest expression found in the liver. Of note, duNOD1 overexpression induced NF-κB, TNF-α, and IL-6 activation in duck embryo fibroblasts (DEFs), while silencing duNOD1 was indicated to decrease the activity of NF-κB in stimulated DEFs [187].
Chicken IL-26 was shown to regulate immune responses through the NF-κB and the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) Janus kinase signaling pathways [188]. Similarly, chicken IL-11 was shown to bind to IL-11R and activated the NF-κB, JAK/STAT, and MAPK signaling pathways, leading to modulation of T helper 1 (Th1)/Th17 and Th2 cytokine production in chicken cell lines [189]. Chicken interleukin-17B was shown to induce the NF-κB signaling pathway, leading to increased expression of proinflammatory cytokines playing a critical role in host defense against the bacterial pathogens [190]. In eukaryotic and prokaryotic expression systems, recombinant chicken TNF-α was generated to demonstrate its biological activity. In particular, as a result of binding to TNF-α receptor 1, the cytokine was shown to induce a complex signaling cascade leading to induction of the classical NF-κB pathway [191].
In Gaoyou duck skeletal muscle (Anas platyrhynchos domesticus), NF-κB motifs (binding sites) were identified, which are believed to be responsible for transcriptional regulation of the slow skeletal muscle troponin I (TNNI1) gene [192]. It seems likely that chicken NF-κB plays a central role in antiviral defense. In fact, chicken tracheal epithelial cells were shown to initiate effective antiviral responses after stimulation with TLR ligands as a result of interferon regulatory factor 7 (IRF7) and NF-κB signaling pathways associated with activation of other cells, such as macrophages [193].
Receptor activator of NF-κB ligand (RANKL), a new member of the chicken TNF superfamily, was recently identified and characterized [170]. Therefore, chicken RANKL (chRANKL), sharing ~59–62% identity with mammalian RANKL, was shown to be ubiquitously expressed in chicken tissues. In nonlymphoid tissues, chRANKL mRNA expression levels were shown to be highest in muscle, while, in lymphoid tissues, the highest RANKL expression was found to be in the thymus, followed by the upper gut and the bone marrow [194]. Recently identified and functionally characterized chicken leukocyte immunoglobulin-like receptor A5 (LILRA5) was reported to activate/induce NF-κB, as well as other immunoregulatory pathways [195].
6. Effect of Various Stress Factors on NF-κB Expression and Activity in Poultry
6.1. Thermal Stress
Continuous exposure of farm animals to an acute or gradual rise in habitat temperature was shown to induce oxidative stress leading to reduced survivability and longevity [196], reduced growth, decreased productive and reproductive performance, and compromised health in poultry [197,198]. Intestinal damages due to thermal stress could lead to redox balance disturbances and inflammatory reactions regulated via NF-κB [12]. It seems likely that NF-κB expression in thermally stressed birds is condition-dependent, including temperature, exposure duration, and bird’s age. On the one hand, when quails at the age of 20 weeks were heat-stressed (34 °C for 4 h per day for 20 consecutive days), liver IL-1β and TLR4 mRNA levels were significantly increased, while NF-κB mRNA levels were significantly decreased in comparison to the control group birds kept in normal physiological conditions [199]. Contrary to the former, heat stress (32 ± 1 °C, 6 h/day for 9 weeks) in 25 week old Roman egg-laying hens was shown to be associated with increased serum inflammatory cytokine (IL-1β, IL-6, and TNF-α) response as compared to control nonstressed birds. Furthermore, heat stress was also responsible for significantly increased proliferating cell nuclear antigen (PCNA), TLR4, and NF-κB protein expression [200]. The authors showed the protective anti-inflammatory effects of curcumin (100 and 200 mg/kg) in the heat-stressed layers. Similarly, in black-boned chickens exposed to circular heat stress, dietary supplementation with resveratrol (400 mg per kg) was shown to improve intestinal integrity and ameliorate the mRNA overexpression of HSP70, HSP90, and NF-κB on the 6th, 10th, and 15th days of stress [201].
It seems likely that cold stress can also impose oxidative stress and enhance in vivo proinflammatory cytokine gene expression in chickens [202]. In fact, the expression of inflammatory factors (iNOS, COX-2, NF-κB, TNF-α, and prostaglandin E synthases (PTGEs) were shown to be increased in chicken heart due to cold stress [203]. Under cold stress in quail, the SOD activity decreased, reflecting an oxidative stress state, while the mRNA expression of NF-κB increased in the duodenum, jejunum, and ileum [204]. The inflammatory factors (COX-2, PTGEs, iNOS, NF-κB, and TNF-α) and Hsp70 mRNA levels were shown to be increased in quail spleen as a result of the acute and chronic cold stress (12 ± 1 °C) compared with birds in the control groups [205]. Increased malondialdehyde (MDA) content and upregulation in HSP27, HSP40, HSP70, NF-κB, COX-2, PTGEs, iNOS, TNF-α, and IL-4 mRNAs, as well as in protein levels of HSP40, NF-κB, and iNOS, were observed in heart due to acute cold stress (7 °C for 24 h) in broiler chickens [206]. Therefore, both heat and cold stress in poultry could be responsible for oxidative stress and inflammation, NF-κB proven to play crucial roles in the regulation of those processes.
6.2. Mycotoxins
Mycotoxins are considered as major nutritional stress factors in poultry production [1] imposing oxidative stress, immunosuppression [207], and low-grade inflammatory response in the chicken intestine [44] and compromising intestinal barrier functions [208]. Among feed-contaminating mycotoxins, AFB1 is considered to be the most toxic mycotoxin. A low level of AFB1 in broiler diet (74 μg/kg) was shown to increase the serum levels of MDA, TNF-α, and IFN-γ. These changes were inhibited by alpha-lipoic acid (α-LA) dietary supplementation (300 mg/kg). Interestingly, the activities of total SOD and GPx and the expression of NF-κB p65 and HO-1 were not affected by AFB1 [209]. In a similar experiment, an AFB1-contaminated diet (74 μg/kg) fed to chickens was associated with upregulation of the proinflammatory cytokine IL-6 and an increase in the protein expressions of both NF-κB p65 and i-NOS in the liver. Those negative effects of dietary AFB1 were shown to be inhibited by dietary alpha-lipoic acid (300 mg/kg [210]).
In an experiment with chicken feed contaminated with 1 mg/kg AFB1 fed from day 1 until day 28, broilers exposed to AFB1 were characterized by increased serum concentrations and mRNA expressions of TNF-α, IFN-γ, IL-1β, IL-10, and IL-6 as compared to the control group. In addition, AFB1 caused increased degradation of the IκBα protein and significantly elevated the phosphorylation of NF-κB (p65). Furthermore, AFB1 was responsible for a significant reduction in the mRNA level and protein expression of the Nrf2 gene. As a result, the mRNA expression and protein expression level of Nrf2-dependent antioxidant genes (HO-1, GPx1, NQO1, and GCLC) in the AFB1 group were shown to be significantly downregulated [211]. Interestingly, the authors demonstrated that most aforementioned changes in NF-κB and Nrf2-related parameters were partly alleviated by feeding grape seed proanthocyanidin extract (250 mg/kg) simultaneously with AFB1.
6.3. Mineral Dietary Excess and Heavy-Metal Contamination
6.3.1. Mn, Cu, and NF-κB
Mn excess (600–1800 mg/kg feed) was shown to be associated with upregulated mRNA expression of TNF-α, COX-2, NF-κB, iNOS, and NO content in chicken testis on the 60th and 90th days [212]. The inflammatory response and the mitochondrial dynamics and apoptosis under Cu (300 mg/kg for 90 days) exposure in the heart of chickens were also investigated. It was shown that Cu exposure induced NF-κB-mediated pro-inflammatory cytokines, and the mitochondrial network was suggested to be considered as the cytosolic sensor responsible for the induction of NF-κB-mediated inflammatory responses under stress conditions [213].
In chickens, dietary Cu excess (220 and 330 mg of Cu/kg dry matter) was shown to increase the number and area of splenic corpuscles, as well as the ratio of cortex and medulla in the thymus and bursa of Fabricius. Furthermore, excessive Cu intake was associated with decreased AO defenses, indicated by the reduced activities of SOD, CAT, and GPx and increased content of MDA. There were also increased TNF-α, IL-1, and IL-1β concentrations, upregulated mRNA levels of TNF-α, IFN-γ, IL-1, IL-1β, IL-2, iNOS, COX-2, and NF-κB, and increased protein levels of TNF-α, IFN-γ, NF-κB, and p-NF-κB in immune organs due to Cu toxicity [214].
6.3.2. As and NF-κB
The proinflammatory activities of As were shown in different tissues of birds, including liver, heart, brain, muscles, and kidney. For example, in birds chronically treated with As2O3, the expression levels of NF-κB and IL-6, IL-8, and TNF-α (critical mediators in the inflammatory response) in the liver were shown to be increased [215]. Indeed, As2O3 exposure (7.5–30 mg/kg for 90 days) led to oxidative stress, inflammatory response, and histological and ultrastructural damage, as reflected by altered levels of cardiac enzymes in chicken heart tissues. In addition, the messenger RNA levels of NF-κB and inflammatory cytokines (TNF-α, COX-2, NOS, and PTGEs) significantly increased due to As2O3 intoxication [216]. Similarly, when As2O3 (1.25 mg/kg body weight (BW), corresponding to 15 mg/kg feed) was added to a basal diet and fed to male Hy-line chickens (1 day old) for 4, 8, and 12 weeks, the expression of TNF-α, NF-κB, and iNOS in chicken heart was shown to be increased compared with the corresponding control group [217].
Arsenic (7.5, 15, or 30 mg/kg feed) was shown to increase the expression of NF-κB and proinflammatory cytokine expression in Gallus gallus brain tissues including cerebrum, cerebellum, thalamus, brainstem, and myelencephalon [218]. The toxic effects of arsenic trioxide (As2O3, 7.5–30 mg/kg for 30–90 days) in the muscular tissues (wing, thigh, and pectoral) of chickens were also investigated. The results showed that As2O3 caused oxidative stress as indicated by decreased activities of AO enzymes (catalase (CAT) and GPx) and increased MDA content. There was a significant upregulation of the mRNA levels of NF-κB and inflammatory cytokines (TNF-α, COX-2, iNOS, and PTGEs) and heat-shock proteins (HSPs) in muscular tissue in the As2O3 exposure groups [219]. In Hy-line chickens, As2O3 exposure (7.5, 15, and 30 mg/kg diet) was shown to induce oxidative stress and inflammatory-mediated nephrotoxicity. In fact, elevated nuclear migration of NF-κB and inflammation-related phenotypes were observed. They led to marked renal injury and apoptosis through a mitochondrion-dependent pathway in chicken kidneys [220].
6.3.3. Cu, As, and NF-κB
Oxidative stress-induced skeletal muscle injury due to Cu2+ (300 mg/kg feed) and/or arsenite (2.5 mg/kg BW, corresponding 30 mg/kg feed) exposure in chickens was associated with inflammation in skeletal muscles induced via the NF-κB-mediated response pathway. Indeed, the increased protein and mRNA levels of NF-κB and TNF-α in skeletal muscles and the enhanced mRNA expressions of IL-1β, IL-6, and IL-12β were indicative of proinflammatory responses occurring due to Cu and/or As exposure [221]. Arsenic (30 mg/kg) and/or copper (300 mg/kg for 12 weeks) were shown to induce oxidative stress, inflammation, and autophagy in chicken brains. In fact, the mRNA levels and protein expressions of inflammation markers (NF-κB, TNF-α, COX-2, and PTGEs) were shown to be significantly increased due to As and Cu exposure [222]. Chicken exposure to As (30 mg/kg) and/or Cu (300 mg/kg for 4.8 and 12 weeks) was shown to lead to oxidative stress, inflammatory response (an increase in expression of NF-κB and its downstream inflammation-related genes), and liver damage through mitochondrial and death receptor-dependent pathways [223]. Arsenic trioxide (30 mg/kg) and/or copper sulfate (300 mg/kg) were added to the chicken basal diet for 12 weeks. Significantly reduced thymus weight and thymus index, hyperemia visible to the unaided eye, and inflammatory cell infiltration were observed. Concurrent administration of arsenic and copper significantly enhanced inflammation as indicated by increased levels of NF-κB, COX-2, iNOS, PTGEs, and proinflammatory cytokines in chicken thymus. Additionally, oxidative stress imposed by As and Cu was associated with elevation of the heat-shock protein levels [224].
Increased NF-κB expression and inflammation induction in chicken gizzard were also shown to be a result of As2O3 and/or CuSO4 dietary exposure [225]. Similarly, As and/or Cu exposure in the same doses was shown to induce immunotoxicity through triggering oxidative stress, inflammation (upregulation of NF-κB, inflammatory mediators, and proinflammation cytokines, accompanied by depletion of anti-inflammatory cytokines), and immune imbalance (decreased ratio of IFN-γ/IL-4 and increased level of IL-17) in the bursa of Fabricius of chicken [226]. In the chronic poisoning of Cu and/or As, inflammation occurs in the chicken thalamus as indicated by increased NF-κB expression, causing oxidative stress (MDA accumulation) and mitochondrial damage, leading to apoptosis [227]. Excessive intake of As (1.25 mg/kg BW) and/or Cu (CuSO4, 300 mg/kg feed) for 12 weeks was shown to lead to a significant reduction in the total antioxidant capacity (T-AOC), catalase level, and hydroxyl radical formation in chicken brain. In addition, an increase in the expression of HSPs and NF-κB, as well as NF-κB pathway-related proinflammatory mediators (COX-2, TNF-α, and iNOS), due to As/Cu intoxication was observed [228]. Therefore, the proinflammatory activities of Cu and As combinations were confirmed in the chicken liver, thymus, bursa of Fabricius, gizzard, thalamus, and brain.
6.3.4. Pb and NF-κB
Pb poisoning in chickens was shown to increase the mRNA expression of inflammation factors (NF-κB) and HSPs in chicken livers simultaneously with the induction of NO content and iNOS activity [229]. It was shown that Pb exposure was associated with increased Pb content in chicken serum, induced the NF-κB pathway, and increased the expression of selenoproteins in chicken neutrophils [230].
More data on Pb-associated modulation of the expression of NF-κB and related cytokines is subsequently discussed in the Se section.
6.3.5. Cd and NF-κB
It was shown that Cd significantly induced the expression of NF-κB, leading to activation of its downstream cytokines, IL-1β, TNF-α, and IL-6, in chicken peripheral blood lymphocytes [231]. As a result of CdCl2 (10 mg/kg feed) administration to chickens for 90 days, levels of NF-κB and phosphorylated c-Jun N-terminal kinase (p-JNK)/JNK in the spleen increased significantly, while those of mechanistic target of rapamycin (mTOR) and HSP70 decreased [232]. Exposure of 120 day old layers to Cd (150 mg/kg for 120 days) was associated with oxidative stress, increased NO production, iNOS activity, and increased expression of inflammatory factors (iNOS, NF-κB, TNF-α, and PTGE) and heat-shock proteins (HSPs 27, 40, 60, 70, and 90) in the liver tissues of birds [233]. In livers of duck exposed to a combination of molybdenum and cadmium, mRNA expression of Hsp60, Hsp70, Hsp90, TNF-α, NF-κB, and cyclooxygenase-2 (COX-2) was significantly upregulated [234]. Nickel chloride (NiCl2) was shown to cause inflammatory responses, indicated by activation of the NF-κB pathway and a reduction in the expression of anti-inflammatory mediators in broiler chicken kidney [235].
Cd exposure was associated with oxidative stress as indicated by increased MDA and reduced SOD and GPx in chicken peripheral blood lymphocytes. Interestingly, Astragalus polysaccharide was shown to inhibit Cd-induced cytotoxicity through regulation of NF-κB signaling [231]. It was shown that Agaricus blazei Murill polysaccharide (ABP) significantly reduced the accumulation of Cd in the chicken spleens and reduced the expression of NF-κB and its downstream inflammatory cytokines (IL-1β, IL-6, TNF-α, and IFN-β). Interestingly, ABP ameliorated the Cd-induced increase in protein levels of HSPs (HSP60, HSP70, and HSP90) in spleens. Furthermore, the activities of main antioxidant enzymes (SOD and GPx) significantly increased, while lipid peroxidation (MDA) decreased in the ABP + Cd group [236].
Therefore, as indicated by the above-presented data, the toxic effects of heavy metals (As, Pb, and Cd) and Cu excess in poultry have been associated with oxidative stress and increased expression and activity of NF-κB in various tissues, leading to inflammation. It seems likely that usage of various protective nutrients can prevent oxidative stress and control/decrease NF-κB expression. This can be demonstrated with plant polysaccharide or selenium (see Section 7.1) dietary supplementation.
6.4. Other Toxic Stress Factors
Hydrogen peroxide (H2O2) was shown to cause oxidative stress and impair redox status in farm animals [237] and poultry [238]; therefore, intraperitoneal injection of H2O2 can be used as an important model of oxidative stress in poultry.
Air quality, especially increased ammonia (NH3) and hydrogen sulfide (H2S) concentrations, is an important factor influencing poultry health and bird performance, including feed efficiency, growth rate, carcass quality, and susceptibility to diseases. Indeed, harmful concentrations of NH3 and H2S can suppress/dampen adaptive immune responses [239].
6.4.1. H2O2
Oxidative stress in chickens induced by H2O2 injection was shown to suppress NF-κB signal activation and initiate autophagy in breast muscles [240]. In an experiment, Arbor Acres chickens were grown for 42 days, and, on days 16 and 37 of growth, control chickens were injected with saline, while experimental chickens received an intraperitoneal injection of H2O2 with 0.74, 1.48, and 2.96 mM/kg BW.
It was shown that the two highest doses of H2O2 imposed oxidative stress (decreased SOD and GPx activity), disturbed the redox balance, and significantly decreased the expression of NF-κB and its subunits (p50 and p65) in the chicken liver on day 42, triggering apoptosis and autophagy [241]. Indeed, H2O2 is considered to be a central redox signaling molecule in physiological conditions, while increased concentrations of H2O2 (>100 nM) can cause oxidative stress [242].
6.4.2. NH3
Ammonia was shown to increase NF-κB expression in chicken trachea, associated with activation of downstream inflammation genes including iNOS and COX-2, reflecting a respiratory inflammation response [243]. The NH3-induced immunotoxic effects and inflammatory damage of broiler spleens were associated with the Th1/Th2 imbalance, NF-κB pathway, and compensatory response of HSPs. In particular, NH3 exposure led to inflammatory damage, indicated by decreased inflammation-related miRNAs (miR-133a and miR-6615), cytokines secreted by Th1 cells, and HO-1. Furthermore, the increased expression of two target genes of the two miRNAs, three cytokines secreted by Th2 cells, seven inflammation-related factors, and five heat-shock proteins was observed in broiler spleens due to NH3 exposure [244]. In a broiler model of ammonia exposure, it was shown that NH3 excess was associated with reduced breast weight and thigh weight, histopathological changes in kidney tissues, and increased iNOS activity and NO content. Furthermore, the mRNA and protein expression of inflammatory factors, including NF-κB, COX-2, prostaglandin E synthases, and iNOS, increased. At the same time, T helper 1 and regulatory T cytokines were shown to be downregulated with simultaneous upregulation of Th2 and Th17 cytokines [245]. A study was conducted to investigate NH3-induced inflammation in chicken bursa of Fabricius and thymus. Experimental chickens were divided into three groups: low (5.0 mg/m3), middle (10.0–15.0 mg/m3), and high (20.0–45.0 mg/m3) NH3-treated chickens. In comparison to the low NH3-treated group, high NH3 exposure was shown to induce inflammation associated with increased nuclear debris and vacuoles in the cortex and medulla of thymus and bursal follicles. Furthermore, reduced bursa of Fabricius and thymus index and increased NO content and iNOS activity due to high NH3 exposure for 14, 21, or 42 days were observed. Lastly, the inflammatory cytokine contents and mRNA levels of NF-κB, COX2, TNF-α, IL-6, IL-10, IL-1β, IL-18, TLR-2A, and iNOS were also increased in conditions of high NH3 exposure [246].
The effect of ammonia (1 mmol/L and 5 mmol/L) on chicken splenic lymphocyte apoptosis was studied. The results showed that NH3 exposure imposed oxidative stress, indicated by the increased release of calcium (Ca2+) and ROS from mitochondria. Furthermore, an increase in the mRNA levels of GPx, inflammation-related genes (NF-κB, COX-2, iNOS, TNF-α, and transforming growth factor-β (TGF-β)), and apoptosis-related genes (B-cell lymphoma 2, BCL-2; Bcl-2-associated X protein, BAX; cytochrome C, Caspase-9, and Caspase-3), and an increase in protein levels of NF-κB, iNOS, BAX, cytochrome C, Caspase-9, and Caspase-3 were also observed due to ammonia exposure. This was also associated with a decreased expression of GST and HO-1 in splenic lymphocytes exposed to ammonia [247]. In chickens, the spleen tissues were seriously injured due to high ammonia concentration (45 ppm from day 22 for 3 weeks) exposure. In the same group of birds, there was increased expression of IL-4, IL-6, and IFN-γ and decreased expression of IL-2 in the spleen, showing an imbalance in the Th1/Th2 response. Furthermore, the proinflammatory factors, including NF-κB, COX-2, iNOS, and prostaglandin E (PGE), were also upregulated in the high ammonia-exposed chickens [248].
6.4.3. H2S
It is known that the decomposition of sulfur-containing organics in poultry houses is responsible for the production of a large amount of H2S, a highly toxic air pollutant, having detrimental effects on poultry health and leading to extensive damage to the body. In poultry, H2S exposure is thought to damage the respiratory system and cause an inflammatory reaction. In particular, it was shown that H2S exposure can inhibit the anti-inflammatory and antioxidant effects of PPAR-γ/HO-1 and activate proinflammatory NF-κB pathway-related genes and downstream genes, leading to aggravation of pneumonia induced by LPS. In particular, the expression of IL-4, IL-6, TNF-α, and IL-1β was increased and that of IFN-γ decreased, and the level of PPAR-γ/HO-1 was significantly suppressed by H2S exposure. Furthermore, the increased expression of I-κB-β and NF-κB genes confirmed that the NF-κB pathway was activated, with subsequent activation of COX-2, PGE, and iNOS [249].
Fourteen day old chickens were exposed to 30 ppm H2S for 14 days, and inflammation and oxidative stress indices were determined in the lymphocytes from peripheral blood samples. An increase in the inflammatory response associated with upregulation of the heat-shock proteins, NF-κB, COX-2, and iNOS was detected in the H2S group in comparison to the control untreated chickens [250]. Furthermore, H2S exposure (0–3 weeks: 4 ppm, 4–6 weeks: 20 ppm of H2S gas) was shown to induce oxidative stress and energy metabolism dysfunction. It also led to necroptosis, activated the MAPK pathway, and triggered the NF-κB pathway associated with a promotion of inflammatory response in chicken spleens [251]. To study the immunotoxicity of H2S, 1 day old broiler chicks were exposed to atmospheric H2S for 42 days. As a result, H2S was shown to activate the TLR-7/MyD88/NF-κB pathway and the NOD-like receptor protein 3 (NLRP3) inflammasome to promote an inflammatory response, leading to tissue damage in broiler thymus and a Th1/Th2 imbalance. In fact, H2S was indicated to significantly induce IL-1β, IL-4, and IL-10 levels, and it downregulated IL-12 and IFN-γ. In addition, mitochondria were shown to be swollen, the chromatin was condensed, and nuclear structures were destroyed due to H2S exposure [252].
6.5. LPS-Induced Stress
The stimulating effect of LPS on NF-κB expression was shown in vitro in model systems and in vivo with poultry. For example, chicken thrombocytes responded to LPS through TLR4, MAP kinase, and NF-κB pathways associated with increased expression of IL-6 and cyclooxygenase-2 and enhanced production of prostaglandin E2 [253]. Similarly, in chicken thrombocytes, LPS-induced IL-6 production was shown to be mediated via activation of NF-κB, extracellular-signal-regulated kinase (ERK) ½, and MAPK [254]. Furthermore, LPS was shown to upregulate IL-6 and CXCLi2 gene expression in chicken heterophils via ERK1/2-dependent activation of AP-1 and NF-κB signaling pathways [255]. In laying hens, NF-κB was shown to participate in the induction of mucin expression by LPS in the vaginal mucosa, improving barrier function against infections [256]. The LPS challenge led to an increased mRNA abundance of TLR4, NF-κB, IL-1β, and IL-6 jejunal mucosa of broilers. However, these effects of the LPS administration were ameliorated by dietary Astragalus polysaccharide [257]. Salmonella LPS injection was found to induce liver damage as indicated by increased necrotic symptoms, severe fatty degeneration, increased alanine aminotransferase (ALT) activity, ballooning degeneration, congestion, and increased inflammatory cell infiltration in liver sinusoids. Significant upregulation in TLR4 expression and its downstream molecules (e.g., NF-κB, MyD88, TNF-α, IL-1β, and TGF-β), increased apoptosis, and decreased proliferation were also observed [258]. Acute spleen injury induced by LPS in young chicks was shown to be associated with significant upregulation of TLR4 at 36 h post LPS stimulation and a slight increase in the expression of NF-κB at 12 h post LPS treatment. The NF-κB-regulated cytokines (TNF-α and IL-6) were shown also to exhibit significant upregulation at 12 h following LPS stimulation [259]. The aforementioned data clearly indicate that LPS can activate NF-κB expression in vitro and in vivo.
An LPS-induced ileum injury model in chickens was established, and histological examination showed a fragmented structure of blood vessels in the ileum and presence of necrotic tissue in the lumen in the LPS-treated chickens. In the LPS group, the structure of the villi was chaotic with rough and uneven surface [238]. Moreover, in comparison to the control group, LPS (60 mg/kg) induced an increase in TLR4 protein expression levels and p65/p65 ratio, increased the mRNA expression of IL-6, IL-8, and TNF-α, and decreased the mRNA expression of IL-10 [260]. Dihydromyricetin (DHM), a natural flavonoid compound with anti-inflammatory activity (0.05% and 0.1%), was shown to have protective effects against LPS-induced inflammatory responses, including regulation of NF-κB expression [260]. Supplementation with leonurine hydrochloride (LH), an alkaloid isolated from Herba leonuri, attenuated LPS-induced intestinal inflammation and barrier dysfunction by significantly downregulating the mRNA expression of NF-κB, COX-2, and proinflammatory cytokines (TNF-α, IL-1β, and IL-6) in the jejunal mucosa. Furthermore, LH administration attenuated LPS-induced IκBα phosphorylation and nuclear translocation of NF-κB (p65) in the jejunal mucosa [261].
6.6. Diseases
Modern breeds/strains of commercially grown meat-type broiler chickens are characterized by increased body weight, improved meat yield, including the Pectoralis major (breast) muscle, improved feed conversion, and decreased time to processing. However, myopathies affecting meat quality, especially in the Pectoralis major muscle, are considered as a major challenge for modern broiler production. It seems likely that broiler breast muscle myopathies are associated with inflammation [262]. The NF-κB signaling pathway was found to be induced, the mRNA expression levels of downstream inflammatory mediators were increased, and TLR levels were upregulated in Pectoralis major of Wooden breast myopathy-affected broiler chickens [263]. The authors also showed that, in the serum of broilers with breast myopathies, contents of IL-1β, IL-8, and TNF-α were increased. At the same time, in breast muscle, the mRNA expression of inflammatory cytokines was dysregulated, showing association of this myopathy with an immune disorder and systemic inflammation response.
The regulatory roles of NF-κB in the development and pathogenesis of various bacterial and virus diseases were recently studied. In particular, the roles of NF-κB and inflammation in the pathogenesis of pathogenic Escherichia coli, various Salmonella species, and Mycoplasma gallisepticum, Eimeria tenella and Clostridium perfringens have received the most attention among bacterial diseases. Infectious bursal disease and Newcastle disease were on the frontline for understanding roles of inflammation and NF-κB in their pathology.
6.6.1. Escherichia coli
Escherichia coli is known to be a Gram-negative, facultative anaerobe bacterium belonging to the Enterobacteriaceae family [264]. Certain E. coli strains, known as “avian pathogenic E. coli” (APEC) are responsible for colibacillosis, one of the most important causes of chicken mortality in the poultry industry worldwide [265]. To explore the host–pathogen interaction, a response in global gene expression profiling of chicken type II pneumocytes (CP II cells), responsible for secreting surfactants and modulating lung immunity, to avian pathogenic Escherichia coli (APEC-O78) infection was determined. In fact, CP II cells were shown to respond to APEC infection with marked changes in the expression of 1390 genes (from 18,996 genes identified) with 803 downregulated mRNAs and 587 upregulated mRNAs [266]. The major enriched pathways were identified to be related to the NF-κB signaling pathway, apoptosis pathway, tight junction, and cytokine–cytokine receptor interaction. Furthermore, the top 15 upregulated biological process terms were found to include regulation of the Toll signaling pathway, apoptotic process, and intracellular signal transduction [244]. The expression of phosphorylated NF-κB p65 and phosphorylated IκB was significantly upregulated in the APEC-infected chicken type II pneumocytes compared with the control group. However, baicalin, a medicinal ingredient isolated from dry roots of Scutellaria baicalensis Georgi, was shown to significantly inhibit the expression of phosphorylated NF-κB p65 and phosphorylated IκB induced by APEC-O78 [267].
Furthermore, the protective effects of baicalin on avian pathogenic APEC-induced acute lung injury associated with NF-κB activation and inflammation in chicken were shown [268]. Artemisinin, a drug derived from the Asian plant Artemisia annua, was shown to alleviate Eimeria tenella infection in chickens as a result of facilitating the apoptosis of host cells and suppressing the inflammatory response by suppressing the increased mRNA expressions of NF-κB and interleukin-17A in ceca during infection [269]. Schizandrin, a group of bioactive chemical compounds found in Schisandra chinensis, was shown to attenuate inflammation induced by APEC-O78 in chicken type II pneumocytes by decreasing the levels of IL-1β, IL-8, IL-6, and TNF-α via its inhibitory effect on NF-κB and MAPK activation [270]. Dietary treatment with both live yeast and mannan oligosaccharide was shown to alleviate E. coli-induced increases in ileal Toll-like receptor 4, NF-κB, and IL-1 β expression in broilers [271].
6.6.2. Salmonella
Salmonella, a Gram-negative bacterium belonging to the Enterobacteriaceae family, is commonly found in the digestive tract of infected chickens. Furthermore, it is an important cause of foodborne human illnesses worldwide, and poultry meat is reported to be responsible for up to 25% of outbreaks caused by foodborne pathogens [272]. Infection of chicken TLR5 transfected cells with Salmonella enterica serovar Enteritidis was shown to activate NF-κB in a dose- and flagellin-dependent fashion [273]. In order to study the role of NF-κB in the signal transduction pathway of the Salmonella enteritidis-challenged cells, chicken macrophage HD11 cell line and small interfering RNAs (siRNA), specifically inhibiting NF-κB1 expression, were used. In particular, it was found that a 36% inhibition of NF-κB1 expression was associated with increased gene expression of both TLR4 and IL-6 at both 1 h and 4 h following Salmonella challenge [274]. TLR4 was shown to activate NF-κB signaling during cerebral ischemia–reperfusion, leading to increased secretion of inflammatory cytokines and damage of brain tissue [275]. Nucleotide-binding oligomerization domain-containing protein-1 (NOD1), known as a cytoplasmic pattern recognition receptor (PRR), is considered to be a key member of the NOD-like receptor (NLR) family. As a result of recognition of various pathogens by NLRs, NF-κB signaling is modulated, leading to induction of the host innate immune response. In fact, following S. enterica serovar Enteritidis infection, induced expression of chicken NOD1 and NF-κB was demonstrated [186]. In carrier chickens challenged with Salmonella enterica serovar Pullorum, upregulation of NF-κB and NRLC5 signaling pathways at different persistence periods was observed [276].
The Salmonella secreted factor L, a deubiquitinase that contributes to the virulence of Salmonella Typhimurium, was shown to suppress the intracellular NF-κB pathway associated with enhancement of the virulence of Salmonella Pullorum in a chicken model [277]. In chickens, Salmonella Typhimurium was shown to significantly reduce chicken performance, including the feed intake and body weight gain, detrimentally affecting the feed conversion ratio. At the same time, Salmonella infection induced the inflammatory expressions of NF-κB and MyD88 genes and decreased the expressions of claudin-1, occludin, and mucin-2 tight junction genes in the intestines. Furthermore, S. Typhimurium was reported to significantly decrease ileal bacterial diversity indices [278,279]. The invasion plasmid antigen J (IpaJ) from Salmonella Pullorum was reported to suppress NF-κB activation by inhibiting IκBα ubiquitination and modulating the subsequent inflammatory response [280].
6.6.3. Mycoplasma gallisepticum
Mycoplasma gallisepticum (MG), an avian pathogen, belonging to the class of Mollicutes, is known as the primary etiological agent of chicken chronic respiratory disease, causing inflammatory damage of the host respiratory system [281]. Initially, when live MG bacteria were incubated with primary chicken tracheal epithelial cells, inflammatory NF-κB-dependent genes were upregulated, while an NF-κB inhibitor abrogated the inflammatory response [282]. Furthermore, TLR2-2 and TLR6 were reported to be upregulated upon MG infection, followed by induction of the NF-κB-mediated inflammatory responses [283]. At the next stage of the research related to the relationship between MG infection and NF-κB expression, microRNas were employed. Indeed, noncoding RNAs, including microRNAs (miRNAs), are known to be involved in the regulation of various cellular processes including gene expression at the post-transcriptional level [284]. Among them, MiR-21 is an evolutionarily conserved miRNA found in a wide range of vertebrate species, including mammals and birds [285].
Recently, it was shown that, in order to provide an effective defense against MG infection, gga-miR-21 is involved in the activation of MAPKs and NF-κB signaling pathways, leading to increased production of inflammatory cytokines and suppressing cell apoptosis [286]. Similarly, upon MG infection, gga-miR-146c upregulation was shown to repress MMP16 expression and activate the TLR6/MyD88/NF-κB pathway. This was associated with inhibiting cell apoptosis and promoting cell proliferation, important events to defend against host MG infection [287]. Furthermore, upregulating gga-miR-16-5p was reported to decrease multiplication and cycle progression and increase apoptosis of MG-infected DF-1 cells, by inhibiting the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/NF-κB pathway to exert an anti-inflammatory effect [288]. In a model system with DF-1 cells in chicken embryo fibroblasts, gga-miR-146c was shown to activate the TLR6/MyD88/NF-κB pathway through targeting matrix metalloproteinase-16 (MMP16) to prevent MG infection in chickens [287]. Upon MG infection, upregulation of miR-130b-3p was shown to activate the PI3K/Akt/NF-κB pathway and induce cell proliferation as a result of downregulating phosphatase and tensin homolog (PTEN). Importantly, inhibition of miR-130b-3p led to the opposite results [289]. In DF-1 cells exposed to Mycoplasma gallisepticum, lipid-associated membrane proteins were reported to induce IL-1β production through the NF-κB pathway [290]. Indeed, MG infection was shown to trigger an inflammatory response through the TLR-2/MyD88/NF-κB signaling pathway, leading to tissue damage in chicken thymus [43,291].
Polydatin (PD), a resveratrol glycoside isolated from Polygonum cuspidatum, with prominent anti-inflammatory activity, was used as a therapeutic means against MG-induced inflammation in chickens. First, histopathological studies clearly showed that PD treatment (15, 30, and 45 mg/kg) was able to alleviate MG-induced pathological changes in the chicken embryonic lung. Second, PD treatment (15, 30, and 60 μg/mL) was shown to significantly suppress the expression of IL-6, IL-1β, and TNF-α induced by MG in chicken embryo fibroblast (DF-1) cells. Furthermore, the MG-induced levels of TLR6, MyD88, and NF-κB were also significantly decreased by PD treatment, which restrained the MG-induced NF-κB-p65 nuclear translocation [292].
As mentioned above, MG can target host cells and cause chronic respiratory disease in chicken. In fact, in chicken spleen and DF-1 cells, MD infection was shown to impose oxidative stress and inflammation. However, baicalin was reported to suppress TLR2–NF-κB signaling pathway by inhibiting the phosphorylation of p65 and IκB [293]. Interestingly, baicalin was reported to restore the mRNA expression of mitochondrial dynamics-related genes and maintain the balance between mitochondrial inner and outer membranes, as well as upregulate the Nrf2/HO-1 pathway and suppress the NF-κB pathway in the spleen of MG-infected chicken [294]. Similar protective effects of baicalin [295] and polydatin, a resveratrol glycoside isolated from Polygonum cuspidatum [292], against MG-induced inflammation injury in chicken embryonic lung were associated with their inhibition of the TLR6/MyD88/NF-κB pathway.
Puerarin (PUE), an isoflavone found in a number of plants and herbs, was shown to inhibit MG-induced inflammation and apoptosis via suppressing the TLR6/MyD88/NF-κB signal pathway in chickens. In fact, compared to the MG-infected group, PUE was found to effectively inhibit the expression of MG-induced inflammatory genes, including TNF-α, IL-1β, IL-6, TLR6, MyD88, and NF-κB. In particular, PUE was reported to dose-dependently inhibit MG-induced NF-κB p65 translocation to the cell nucleus [296].
6.6.4. Eimeria tenella
Chicken coccidiosis is an enteric disease caused by Eimeria infection, leading to severe economic losses associated with immunosuppression and a high level of mortality in the poultry industry worldwide [297]. In fact, Eimeria tenella infection was shown to significantly increase the expression of NF-κB mRNA in chicken cecal tissue in vivo [269], and a similar increase in the expression level of NF-κB was observed in chicken intestinal epithelial cells in vitro after infection with E. tenella sporozoites [298].
There is a need for more research related to the regulatory roles of NF-κB pathway in the development of a chicken coccidiosis prevention strategy.
6.6.5. Clostridium perfringens
Clostridium perfringens-induced necrotic enteritis in chickens has become an economically significant problem for the broiler industry [299,300], especially at farms that have stopped the use of antibiotic growth promoters [301]. It is known that the Clostridium perfringens main cell-wall component, peptidoglycan, can be recognized by TLR2 with subsequent activation of the NF-κB signaling pathway to induce cytokine and chemokine production, leading to inflammation [302]. The authors conducted an in vitro study with primary intestinal epithelial cells to assess the chicken intestinal inflammatory responses to C. perfringens and showed increased cytokine expression related to NF-κB activation [302]. Furthermore, pathways affected by the infusion of C. perfringens culture supernatant in the duodenum of broilers included NF-κB signaling, death receptor signaling, and an inflammatory response [303].
Importantly, two Lactobacillus species were shown to reduce the growth of Clostridium perfringens and inhibit the upregulation of NF-κB p65 in C. perfringens-challenged chicken intestinal epithelial cells [304]. Indeed, inclusion of L. acidophilus into the chicken diet was shown to improve gut health and reduce the mortality of Clostridium-challenged broiler chicks suffering from necrotic enteritis [305].
6.6.6. Chlamydia psittaci
Chlamydia psittaci, a pathogen in poultry and pet birds, is known to have some protective mechanisms to cope with proinflammatory mediators during the early host response, leading to effective evasion and causing psittacosis/ornithosis [306]. The polymorphic membrane protein D (PmpD) is known as a highly conserved outer-membrane protein helping the pathogen to decrease immune system protection during Chlamydia psittaci infection. Therefore, the ability of the N-terminus of PmpD (PmpD-N) to regulate the functions of chicken macrophages was studied. In particular, it was shown that stimulation of HD11 macrophages with PmpD-N was associated with an increased secretion of the Th2 cytokines, IL-6, and IL-10 and upregulated expression of TLR2, TLR4, MyD88, and NF-κB. In great contrast, inhibition of TLR2, MyD88, and NF-κB in HD11 cells was reported to significantly decrease IL-6 and IL-10 cytokine levels associated with significantly enhanced NO production and phagocytosis [307]. The plasmid-encoded protein CPSIT_P7 of Chlamydia psittaci was shown to induce the TLR4/Mal/MyD88/NF-κB signaling axis and orchestrate the inflammatory cytokine response [308].
It is important to mention that, during host cell infection, NF-κB is activated by various pathogens, leading to the creation of a hostile environment for invading infectious agents; however, the pathogen can diminish the protective inflammatory response by blocking NF-κB translocation to the nucleus [309].
6.6.7. Infectious Bursal Disease
As mentioned above, NF-κB is involved in the pathogenesis of various virus-induced diseases. In fact, infectious bursal disease virus (IBDV) is known as the etiological agent of a highly transmissive and immunosuppressive disease detrimentally affecting domestic chickens (Gallus gallus) in commercial poultry production. Indeed, IBD (Gumboro disease) can cause high morbidity and mortality of infected birds, leading to major economic losses in the poultry industry worldwide. The danger of IBD is associated with its immunosuppressive action associated with a loss of IgM-bearing B lymphocytes and the destruction of the bursa of Fabricius [72]. There is some evidence indicating that IBDV infection can cause oxidative stress in chickens [310], but regulatory roles of the antioxidant defense network in IBD need further elucidation.
IBDV infection was found to induce spleen macrophage activation via p38 MAPK and NF-κB pathways [311]. However, the molecular mechanisms of IBDV development and pathogenicity are still poorly understood; nevertheless, poorly regulated cytokine production and B-cell depletion due to apoptosis are believed to be important contributing factors to the disease pathology and severity. In IBDV-infected chicken embryonic fibroblasts, a great number of target genes and inducers of NF-κB were reported to be upregulated, in comparison to noninfected cells. It could well be that IBDV may support its replication and facilitate viral spread by affecting host-cell survival and apoptosis through NF-κB activation [312]. Interestingly, exacerbated apoptosis of cells infected with IBD virus upon exposure to IFN-α was shown to be associated with double-stranded RNA-dependent protein kinase (PKR), TNF-α, and NF-κB expression. Indeed, their downregulation is reported to drastically reduce the extent of apoptosis [313]. Protocatechuic acid (PCA), a type of widely distributed naturally occurring phenolic acid, was found to activate NF-κB signal pathways in the early stage of IBDV infection, leading to apoptosis promotion [314].
6.6.8. Newcastle Disease
Newcastle disease (ND) is regarded as one of the most important avian diseases significantly affecting poultry production all over the world, being a great threat to the poultry industry [315]. It is well known that ND epidemics can cause high chicken mortality with great economic losses [315]. There is no effective treatment for the disease, and poultry producers rely on vaccination and strict biosecurity as vital measures for controlling the spread of the disease [316]. It is known that NDV can cause oxidative stress in poultry [310]; however, the roles of redox homeostasis in ND development are poorly understood. In fact, intense inflammatory responses leading to excessive cellular apoptosis and tissue damage were shown to be a result of Newcastle disease virus (NDV) infection in poultry. However, the molecular mechanisms of such actions have not been fully elucidated [317]. In NDV-infected chickens, glucocorticoid dexamethasone was shown to modulate NF-κB-dependent gene expression by upregulating FK506-binding protein 51 expression [318]. Furthermore, Newcastle disease virus-like particles were shown to induce dendritic cell maturation with synthesis of proinflammatory cytokines through the TLR4/NF-κB pathway [319]. Recently, it was shown that, during NFV infection, high-mobility group box 1 (HMGB1), a key member of the damage-associated molecular patterns (DAMPs), was responsible for NF-κB induction and a drastic increase in proinflammatory cytokine production in DF-1 and A549 cells [317]. It seems likely that activated NF-κB signaling can suppress NDV replication. This was shown experimentally with DF-1 cells (e.g., a chicken embryo fibroblast cell line) by using gga-miR-19b-3p, which enhanced NF-κB activity and led to increased inflammatory cytokine production and inhibition of NDV replication [320].
Expression of IFIT5 (interferon-induced protein with tetratricopeptide repeats 5) possessing antiviral activity and enhancing innate immunity was studied in chickens. The relative mRNA expression level of chicken IFIT5 (chIFIT5) was shown to be the highest in spleen, and the expression level of chIFIT5 was found to be significantly upregulated following NDV infection. In particular, it was shown that overexpression of chIFIT5 could promote IRF7- and NF-κB-mediated gene expression following NDV infection [321]. The DNA-sensing pathway is known to induce innate immune responses against DNA virus infection, with NF-κB signaling being critical for the establishment of innate immunity.
6.6.9. Other Viral Diseases
It seems likely that Marek disease virus (MDV) and reovirus infections also affect NF-κB signaling. In fact, Marek’s disease (MD) is a neoplastic virus disease infecting chickens and frequently causing cancers in animals [322]. It was shown that NF-κB is involved in MDV-induced neoplastic transformation of CD30-expressing chicken lymphocytes in vivo [323]. Furthermore, chicken MD virus RLORF4 (a MDV-specific gene) was shown to inhibit type I interferon production by antagonizing NF-κB activation. In fact, RLORF4 binds to the Rel homology domains of the NF-κB subunits p65 and p50, interrupting their translocation to the nuclei and, thus, inhibiting IFN-β production [324].
Avian reoviruses are important pathogens causing infectious arthritis/tenosynovitis, stunting syndrome, respiratory disease and enteric disease, immunosuppression, and malabsorption syndrome in poultry [325,326]. Thus, avian reovirus can cause oxidative stress and disturb redox homeostasis in poultry [310]. Avian reovirus S1133 in cell cultures, in the early stages of infection, was shown to induce Akt/NF-κB and STAT3 signaling, leading to an inflammatory response and delayed apoptosis [327]. Furthermore, in avian reovirus-infected chickens, the expression peak for NF-κB in peripheral blood lymphocytes was shown to occur at 3 days post infection (dpi). Similarly, IFN-α, IFN-β, and IL-12 expression levels also peaked at 3 dpi, while IFN-γ, IL-6, IL-17, and IL-18 expression reached a maximum level earlier (at 1 dpi), whereas IL-8 (5 dpi) and IL-1β and TNF-α (7 dpi) peaked later [328]. Recently, the phosphoproteomic responses of duck to reovirus infections in the spleen tissue were studied, and 16 proteins involved the intracellular signaling pathways of PRRs were shown to be phosphorylated proteins. In particular, changes in the phosphorylation levels of NF-κB, as well as MyD88, receptor interacting protein 1 (RIP1), MDA5, and IRF7, indicated an important role of protein phosphorylation in duck immune responses to viral antigens [329]. Pattern recognition receptor signaling during innate responses to influenza A viruses in the mallard duck was recently reviewed [330], and the fundamental roles of NF-κB in innate immune responses to duck Tembusu virus infection were discussed in detail [331].
As can be seen from the above-presented data, NF-κB plays a pivotal role in poultry protection against major microbial and viral diseases, by regulating immunity and inflammation; however, the molecular mechanisms of its regulation in avian species await further investigation.
7. Nutritional Modulation of NF-κB in Poultry
7.1. Selenium
There is a great body of evidence indicating that the micronutrient selenium (Se) and selenoproteins are involved in the regulation of inflammatory signaling pathways, including NF-κB signaling, implicated in the pathogenesis of various diseases [13,332]. As a part of 25 selenoproteins, Se is involved in antioxidant defenses and the maintenance of redox balance [13].
The literature data related to the effect of Se on NF-κB and inflammation can be divided into three groups. Firstly, the detrimental effects of Se deficiency or excess on NF-κB signaling were shown. Secondly, the protective effects of Se in Pb and Cd toxicity were described. Thirdly, in LPS-induced models of oxidative stress and inflammation, Se was shown to be protective.
7.1.1. Se Deficiency
Se deficiency in chickens was shown to lead to activation of the NF-κB pathway, with a change in selenoprotein gene expression resulting in kidney dysfunction [333]. Furthermore, Se deficiency was reported to attenuate chicken duodenal mucosal immunity via activation of the NF-κB signaling pathway. In particular, Se deficiency enhanced the phosphorylation of IκB-α and phosphorylation of kappa-B kinase subunit alpha (IKKα), as well as increased p50 and p65 DNA-binding activities. Furthermore, in Se deficiency, IKKα was elevated, but IκB-α was decreased [334]. The increasing levels of ROS in chicken duodenal mucosa due to Se deficiency could trigger NF-κB signal transduction [335]. In a recent experiment, the control group was fed a complete formula feed (0.2 mg Se/kg), while the experimental group of chickens was fed a self-made low-Se diet (0.004 mg/kg) for 15, 25, 35, 45, and 55 days. In chicken spleen at 15–45 days, the relative expression of TLR4 mRNA was shown to be increased due to Se deficiency. The relative expression of NF-κB mRNA in the experimental group was also increased in comparison to that in the control group at 15–45 days. The relative expression of IL-6 mRNA and the protein expression level of TLR4 in the experimental group were increased due to Se deficiency at 15–45 days of age [336]. The authors concluded that Se deficiency is associated with inflammatory injury as a result of the TLR4/TIR-domain-containing adapter-inducing interferon-β (TRIF)/NF-κB signaling pathway activation in chicken spleen.
Interestingly, the adverse effects of Se excess/toxicity (15 mg/kg Se for 45 days) on inflammatory and immune responses in chicken spleens were also associated with enhancement of the expression of NF-κB, iNOS, COX-2, PTGE, IL-6, TNF-α, and IL-4, but a depression of FOXP3 and IFN-γ [337]. However, Se dietary supplementation at 2 mg/kg did not affect the mRNA levels of NF-κB, COX-2, PTGEs, and TNF-α in chicken kidneys.
7.1.2. Se and Pb Toxicity
Dietary Se has been proven to alleviate the Pb-induced increase in NF-κB and HSP expression in chicken livers [229]. Importantly, Se supplementation (1 mg/kg diet) was shown to reduce Pb concentration in serum, partly mitigated the effect on the activation of the NF-κB pathway, and further enhanced selenoprotein expression induced by Pb exposure [230]. One week old male chickens were treated via drinking water with Pb (350 mg/L) and provided with dietary Se (1 mg/kg) or both Pb and Se. On the 4th, 8th, and 12th weeks, kidneys were used to assess oxidative stress indicators, relative expressions of cytokines, and other inflammatory factors. The results showed that Pb consumption imposed renal injuries associated with increased lipid peroxidation (MDA), as well as the content and expression of IL-1β, IL-6, IL-17, NLRP3, caspase-1, NF-κB, COX-2, TNF-α, and PTGEs, and with reduced GSH content, as well as GPx and SOD activities, in the chicken kidneys. Se administration was shown to alleviate the aforementioned changes [338].
Pb treatment (50 mg/kg for 90 days) was shown to compromise AO defenses by inhibiting the activities of SOD, GPx, and CAT, causing the accumulation of NO and MDA, leading to oxidative stress, which promoted the expression of MAPK/NF-κB pathway genes (ERK, JNK, P38, NF-κB, and TNF-α) and activated HSPs (HSP27, HSP40, HSP60, HSP70, and HSP90) in chicken spleen. However, Pb-caused necroptosis was inhibited by Se (2 mg/kg) co-treatment [339]. Selenium (sodium selenite, 1 μM) was shown to prevent lead (30 μM)-induced necroptosis by restoring antioxidant functions (SOD, GPx, CAT) and blocking the MAPK/NF-κB pathway (decreasing the expression of NF-κB and TNF-α) in chicken lymphocytes [340].
7.1.3. Se and Cd Toxicity
Dietary Cd (150 mg/kg for 90 days) was shown to increase the mRNA levels of NF-κB, COX-2, PTGEs, and TNF-α in chicken kidney. Interestingly, Se partly ameliorated the proinflammatory effects of Cd dietary supplementation [341]. Similarly, treatment with Se (2 mg/kg for 90 days) significantly alleviated Cd-induced hepatic toxicity (150 mg/kg) in laying hens, as evidenced by a reduction in Hsp60, Hsp70, Hsp90, NF-κB, COX-2, PTGEs, TNF-α, and IL-1β expression [342].
Chicken Cd exposure (150 mg/kg) was shown to activate inflammation-related genes including TNF-α, NF-κB, iNOS, COX-2, and prostaglandin E synthase (PTGEs) in chicken breast muscles [323,343]. Interestingly, Se (2 mg/kg as sodium selenite for 90 days) was reported to alleviate Cd-induced inflammation and meat quality deterioration via antioxidant and anti-inflammation action [343]. Supplementation with Se-yeast (0.5 mg/kg) was shown to have an antagonistic effect on Cd-induced inflammatory injury in chicken livers [233].
7.1.4. Se and LPS
By inhibiting the phosphorylation of NF-κB, Se was shown to reduce breast tissue inflammatory injury induced by LPS [344]. In laying hens, LPS stimulation (injected LPS into the abdominal cavity at the age of 8 months) imposed oxidative stress, indicated by decreased activity of SOD, GPx, and CAT, decreased GSH content, and increased H2O2 and MDA content in the chicken myocardium. LPS also increased the expression of p38 MAPK and NF-κB, as well as TNF-α, IL-1, PTGE, COX-2, and iNOS. Interestingly, the addition of dietary SeMet (0.5 mg/kg for 4 months) was found to alleviate the changes in the above inflammation indicators [345]. Similarly, SeMet (0.5 mg/kg) was shown to inhibit the LPS-induced inflammation of liver tissue via suppressing the TLR4–NF-κB–NLRP3 signaling pathway in chickens [346].
In a recent experiment with 46 week old ISA laying hens, birds were injected with LPS (200 mg/kg) intraperitoneally, and, after 5 h, the tracheal tissue was collected for various assays. In the LPS-treated group, the epithelial cells were shown to be degenerated with necrotic changes accompanied by inflammation. The expression of the NF-κB pathway and related inflammatory factors, including TNF-α, iNOS, NF-κB, COX-2, and PTGEs, was significantly increased in the trachea tissue due to LPS treatment. In such conditions, increased (from 0.2 up to 0.5 mg/kg) SeMet supplementation for 90 days showed anti-inflammatory effects [347].
7.2. Amino Acids
Dietary l-arginine supplementation (1.05–1.9%) was shown to attenuate the LPS-induced inflammatory response in broiler chickens, as evidenced by the decreased expression of IL-1β, TLR4, and PPAR-γ mRNA in the spleen, and IL-1β, IL-10, TLR4, and NF-κB mRNA in the cecal tonsils [348]. There were no significant interactions between immune stress caused by bovine serum albumin (BSA) and supplementation of threonine (0.49–0.76% for 21 days) for NF-κB gene expression in the jejunum or ileum of Pekin ducks [349].
Interestingly, NF-κB expression in the jejunum was twofold higher than that in the ileum. Leucine was reported to alleviate LPS-induced inflammatory responses as a result of downregulating the NF-κB signaling pathway. In particular, a model system employing the intestinal tissue from specific pathogen-free chick embryos cultured in the presence of LPS for 2 h was used. LPS was shown to increase the phosphorylation of NF-κB while decreasing the phosphorylation level of mTOR. In this system, leucine supplementation at 40 mM was reported to suppress the phosphorylation levels of NF-κB, while restoring the phosphorylation level of mTOR [350].
7.3. Phytogenic Supplements
Recently, various phytogenic supplements received tremendous attention in poultry and animal nutrition, and the molecular mechanisms of their protective actions in many cases were related to their antioxidant properties. However, our analysis of the current data in this area showed that polyphenolic compounds are poorly absorbed and their concentrations in target tissues are several orders lower than those used in in vitro studies [351]. Furthermore, their antioxidant properties are condition-dependent, and, in many cases, polyphenols could show pro-oxidant activities. Therefore, it was suggested that the polyphenolic effects on NF-κB and Nrf2 expression could be a major molecular mechanism of their protective action in various model systems and in poultry nutrition in general [351,352]. Data presented in Section 4 showing the activation effects of polyphenol compounds on Nrf2 expression and activity with simultaneous suppression of the NF-κB pathway confirmed that idea. There are also a range of publications showing protective effects of phytogenic supplements in poultry nutrition under various stress conditions.
In chickens receiving conventional vaccinations, the NF-κB gene mRNA relative expression in hepatocytes linearly decreased as a result of increasing resveratrol, a plant-derived polyphenolic compound, with a dietary concentration from 200 to 800 mg/kg of diet [353]. Similarly, dietary resveratrol (200–600 mg/kg) was shown to reduce the protein expression of NF-κB, HSP70, and HSP90 in the jejunal chicken villi after 15 days of heat stress [201]. Dietary resveratrol (400 mg/kg) was also shown to protect quail hepatocytes against heat stress by decreasing the expression of NF-κB, Hsp70, and Hsp90, and increasing the hepatic and SOD, CAT, and GSH-Px activities [354]. Daidzein (DA), a soy isoflavone, included into the breeder diet at 20 mg/kg was shown to activate the NF-κB, MAPK, and Toll-like receptor signaling pathways of the offspring broilers. Furthermore, DA promoted lymphocyte development and differentiation and downregulated the expression of genes regulating lymphocyte apoptosis. It also increased the proportion of B cells, leading to promotion of Ig secretion with increased serum IgA and IgG levels and serum ND virus antibody titers [355]. In healthy Arbor Acre broilers, quercetin supplementation (0.04% and 0.06% for 6 weeks) was shown to significantly increase the expression of TNF-α, TNF receptor associated factor-2 (TRAF-2), TNF receptor superfamily member 1B (TNFRSF1B), nuclear factor kappa-B p65 subunit (NF-κBp65), and interferon-γ (IFN-γ) mRNA, while expression of NF-κB inhibitor-alpha (IκB-α) mRNA was significantly decreased [356]. Ginsenosides, the major constituents of ginseng with unique biological activities, were shown to promote proliferation of chicken primordial germ cells through protein kinase C (PKC)-involved activation of NF-κB [357].
Tanshinone IIA (TIIA), a major lipophilic component extracted from the root of Salvia miltiorrhiza Bunge used in Chinese medicine, was shown to have a protective effect against pulmonary arterial hypertension-related inflammatory responses [358]. Treatment with an extract of Hypericum perforatum L., also known as Saint John’s wort, at doses of 480–120 mg/kg for 5 days was shown to reduce infectious bronchitis virus (IBV)-induced injury and reduced the mRNA expression level of IBV in the chicken trachea in vivo. In particular, the expression of IL-6, TNF-α, and NF-κB was shown to be significantly decreased, but mitochondrial antiviral signaling gene, IFN-α, and IFN-β mRNA levels were shown to be significantly induced in vitro and in vivo [359].
7.4. Other Nutrients and Probiotics
Retinoic acid, an active vitamin A metabolite, was indicated to activate the PI3K/Akt and NF-κB signaling pathways, leading to proliferation of the cultured chicken primordial germ cells [360]. In LPS-challenged chickens, increased vitamin E supplementation (50 mg/kg vs. 10 mg/kg) was shown to decrease the expression of nuclear NF-κB p65 and increase the levels of IκBα in the liver [361]. It seems likely that the inhibitory effects of vitamin E on NF-κB expression could also be seen in physiological conditions, without any stress challenges. For example, in broilers fed increased vitamin E levels (14.11–14.91 mg/kg vs. 4.38–4.63 mg/kg) for 21 or 42 days, liver NF-κB p65 levels were significantly decreased, whereas liver IκB-α levels were significantly increased [362]. The NF-κB DNA-binding activity in the high-density housing group was shown to increase significantly compared with the free-range and low-density housing birds. Dietary taurine (0.1%) was shown to significantly alleviate NF-κB DNA-binding activity in chicken liver [363] and layer oviduct tissue [364], which was initially increased due to high-density housing systems. Interestingly, in the chicken renal tissue, the same dose of dietary taurine was able to decrease the NF-κB DNA-binding activity only in the low-density housing environment [365].
Necrotic enteritis (NE) infection was shown to significantly upregulate the mRNA levels of the immune-related molecules TLR-2, IL-1β, IL-4, IL-10, IFN-γ, and iNOS and the growth factors TGF-β3 and insulin-like growth factor 2 (IGF2) in the jejunum of broiler chickens. However, NF-κB expression was not affected. Interestingly, compared with nonsupplemented groups, probiotic Enterococcus faecium NCIMB 11,181 was shown to ameliorate necrotic enteritis-induced intestinal barrier injury in broiler chickens and increased gene expression levels of TLR2, MyD88, NF-κB, IL-4, iNOS, TGF-β3, PI3K, IGF-2, glucagon-like peptide-2 (GLP-2), and epidermal growth factor receptor (EGFR). There was also a significant interactive effect of NE infection and E. faecium treatment on the mRNA expression of various immune-inflammation factors, including NF-κB [366]. A dendritic cell (DC)-targeted mucosal vaccine using Lactobacillus plantarum as an antigen delivery system against G57 virus infection was developed. The vaccine was shown to confer efficient protection against G57 H9N2 infection, due to activation of DCs by the TLR-induced NF-κB pathway. This was associated with improved DC migration and improving the presentation of immunogen to T and B lymphocytes, causing changes in T-cell polarization toward Th1, Th2, and regulatory T cells (Treg cells) and inducing more efficient mucosal and adaptive immunity responses [367]. Chickens infected with Salmonella enteritidis (0.5 mL of S. enteritidis bacterial suspension, 109 CFU/mL) through the oral cavity at 9 days of age) were administered with a probiotic Pediococcus pentosaceus microcapsule (1 g/kg). It was shown that the probiotic effects on proinflammatory indices were time-dependent; the probiotic significantly decreased the NF-κB expression in spleen and cecum samples at 1 dpi, whereas the difference disappeared at 3 dpi, and NF-κB expression was significantly increased in samples from chickens fed the probiotic at 7 dpi before disappearing again 14 dpi [368].
8. NF-κB and Inflammation in Poultry Production
Inflammation is known to be a highly orchestrated and tightly controlled protective mechanism dealing with infection and coordinating the repair and regeneration process. However, in the case of misregulation of inflammation or its chronic character, it can be responsible for severe tissue damage [369]. Therefore, elucidation of the molecular mechanisms underlying the regulation/control and resolution of inflammation is among the major topics of modern medical and veterinary sciences. Indeed, the relationship among inflammation, NF-κB signaling, and chronic diseases, including cancer [370], liver diseases [371], multiple sclerosis [372], immune-related disorders [69,373], and others [374], has received tremendous attention in recent years. Uncontrolled inflammation is shown to be associated with many widely occurring human diseases, including cardiovascular disease, neurodegenerative diseases, diabetes, obesity, asthma, arthritis, and periodontal diseases [375]. Therefore, modulating inflammation through the negative regulation of NF-κB signaling is an important approach in medical sciences and clinical practices [376].
In fact, the efficacy, duration, and outcomes of an inflammatory response in poultry were shown to be condition-dependent and determined by the triggering signal recognized by the innate immune receptors [377]. It seems likely that poultry metabolic diseases related to cardiovascular ailments, responsible for a major portion of the flock mortality, as well as musculoskeletal disorders responsible for slowing down chicken growth and causing lameness [378] and leg/bone disorders [379], are associated with increased and misregulated inflammatory responses. Intestinal health is known to depend on a range of host-related factors (immunity, mucosal barrier), as well as nutritional, microbial, and environmental challenges. Thus, intestinal dysbiosis, compromised mucosal barrier, and gut inflammation are among major issues in commercial poultry production systems after the ban on growth-promoting antimicrobials in animal feed [380]. Furthermore, non-nutritive dietary ingredients, biosecurity, immunology, vaccine technology, and genetics are suggested to play an important role in antibiotic-free poultry production [40].
It was hypothesized that subclinical gut disorders leading to a chronic low-level inflammatory response in the gut associated with oxidative stress and redox disbalance could result in the disruption of digestive function and poor immune competence [44]. Furthermore, the authors also suggested that chronic intestinal inflammation is responsible for the decreased performance and increased incidence of intestinal problems observed in poultry production. In fact, there are a range nutritional stress factors, including feed with a high content of nonstarch polysaccharides, crude protein excess, ingredient rancidity, or mycotoxin- and/or heavy-metal-contaminated feed, which could trigger gut inflammation in poultry species [30,44].
Inflammation and the susceptibility to inflammatory disorders are known to be regulated by nutrition, the gut microbiome, and genetics. In fact, altered nutrient status is shown to reprogram host inflammation via the gut microbiota [381]. Furthermore, the crosstalk between gut bacteria and host immunity is considered to be of great importance in intestinal inflammation [382], and the intestinal microbiota has emerged as a key player in metabolic inflammation and dysfunction [383]. The modulation of inflammatory responses associated with NF-κB regulation has been described for many nutrients, including selenium, taurine, carnitine, silymarin, and other nutrients [30]. Furthermore, commercially used feed additives, including probiotics, prebiotics, phytobiotics, organic acids, short- and medium-chain fatty acids, essential oils, and enzymes, can help maintain optimal host–microbiome interactions to support gut integrity and efficient growth and health [384,385].
NF-κB is known as a master regulator of the inflammatory response in the complex inflammatory network, playing a critical role in the host defense against pathogens, and it protects cells from apoptosis, as a result of triggering the upregulation of proinflammatory genes [386]. Furthermore, in acute inflammation, NF-κB, as a redox-sensitive transcription factor, was shown to participate in condition-dependent selective regulation of the expression of many target genes. They include proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-12), various antioxidants (e.g., glutamate cysteine ligase, SOD1, SOD2, and catalase) [386], proinflammatory enzymes (secretory phospholipase 2, sPLA2; COX-2, and iNOS), chemokines (macrophage inflammatory protein 1α (IP-1α), monocyte chemoattractant protein-1 (MCP1), growth factors, cell-cycle regulatory molecules, anti-inflammatory molecules, and adhesion molecules [387]. Under stress conditions, the NF-κB network is responsible for balancing the effect of various intracellular stress signals to maintain homeostasis and cell/tissue integrity [30]. Therefore, a better understanding of the condition-dependent molecular events/mechanisms determining the point at which NF-κB responses switch from being protective to become damaging is of great importance [372,388] and awaits further investigation.
9. Conclusions
Today, NF-κB is known as a redox-sensitive, inducible nuclear transcription factor that regulates the expression of a number of genes associated with important biological processes, including innate and adaptive immune responses, cell growth, maturation, survival, and adaptive homeostasis establishment via interactions with other transcription factors and vitagenes [1]. Indeed, NF-κB signaling is an important element in cell adaptation to a diverse array of environmental stimuli, including oxidative stress. These stimuli can be recognized by various receptors, and the subsequent response involves specific adapter proteins. Under normal physiological conditions, the antioxidant defense network and the optimized redox balance in the cell/tissue are responsible for orchestrating important biological processes associated with cell protection, repair, and growth. This includes T-cell maturation, fighting against infections, DNA damage repair, and tissue healing and integrity restoration after injury. However, under various stress conditions, associated with redox disbalance, aberrant NF-κB activation can lead to detrimental changes, including the development of many age-related diseases. In fact, it was reported that activation of NF-κB is an important mechanism of host defense against infection and stress [77]. Indeed, the results from gene knockout experiments clearly showed that mice deficient in NF-κB-mediated transcriptional regulation were susceptible to a variety of infections and were characterized by compromised immunity: specifically depressed immunoglobulin expression, defective humoral immune responses, and decreased responses to LPS [389]. In fact, it was recently proposed that NF-κB is a vital regulator of inflammation, indicating that the dynamical attributes and the composition of the nuclear NF-κB complexes cumulatively instruct context-specific inflammatory gene patterns [48]. A variety of endogenous and exogenous stimuli in poultry have been characterized, including danger, damage, and survival signals from viral and bacterial components, proinflammatory cytokines, DNA damage, growth factors, and other stressors. As mentioned above, the stimuli can be recognized by a range of various receptors with subsequent activation of NF-κB, along with involvement of an array of specific adapter proteins [60,67].
Poultry production was shown to be associated with a range of stresses, which can be divided into four major categories, namely, environmental, technological, nutritional, and internal/biological stresses [28,29]. It was proven that RONS excess and a compromised antioxidant defense network are responsible for compromised health, as well as the decreased productive and reproductive performance of growing broilers, laying hens and breeder birds [30,390]. Therefore, the antioxidant defense network in poultry is of great importance, and understanding the molecular mechanisms underlying its regulation to optimize redox balance in various tissues and in the body in general under commercially relevant stress conditions is an important topic of current research in avian biology and poultry health [26,30].
Our analysis of the recent literature related to the regulatory roles of NF-κB in poultry can be summarized as follows:
Similar to mammalian species, in poultry, NF-κB plays a central role in the regulation of many physiological and pathological processes.
In thermally stressed birds, NF-κB expression is condition-dependent, including temperature, exposure duration, and bird’s age.
The effects of dietary AFB1 on NF-κB expression in chicken liver are also condition-dependent. In general, AFB1 was shown to compromise AO defenses and increase proinflammatory cytokine production via NF-κB induction.
Mn or Cu excess in the chicken diet was shown to increase the expression of NF-κB in testes, heart, and immune organs.
The proinflammatory effects of heavy metals (As, Cd and Pb) in chickens were shown to be mediated via NF-κB pathway activation in various tissues.
Increased concentrations of NH3 and H2S (main environmental stressors in poultry production) in air during chicken housing were shown to impose oxidative stress and inflammatory responses via NF-κB activation.
The stimulating effect of LPS on NF-κB expression was shown in vitro in model systems and in vivo with poultry.
In many bacterial and viral diseases, NF-κB is activated to increase proinflammatory cytokine production and impose an inflammatory response to create a hostile environment for pathogens.
The main bacterial pathogens causing various diseases in poultry production, including Escherichia coli, various Salmonella species, Mycoplasma gallisepticum, Eimeria tenella, Clostridium perfringens, and Chlamydia psittaci, were shown to induce proinflammatory responses in birds associated with increased NF-κB expression and activity.
In model systems based on an investigation of gene expression changes due to various infections, it was proven that the development of viral diseases, including infectious bursal disease, Newcastle disease, Marek’s disease, and reovirus challenges, was associated with the induction of NF-κB and inflammatory responses.
Nutritional modulation of NF-κB expression and activity was shown to be achieved by using various antioxidants, including selenium, various polyphenols, taurine, retinoic acid, vitamin E, and some probiotics. In fact, under various stress conditions, these nutrients can ameliorate (partly or completely) the increased NF-κB expression and activity imposed by stressors.
An opportunity to use nutritional/pharmacological means to modulate NF-κB expression and activity should be exploited further to deal with inflammation-associated poultry disorders/diseases, including gut health problems associated with antibiotic-free poultry production. In fact, various nutrients, including taurine [391], carnitine [392,393,394], silymarin [352], vitamin E [21], selenium [13,20], carotenoids [22], and others [26], have been shown to affect antioxidant defenses and vitagenes, helping maintain the redox balance in various chicken tissues. There is a need for further research related to interactions of the antioxidant defense network with vitagenes and transcription factors, including NF-κB and Nrf2, which are responsible for the maintenance of redox homeostasis and stress adaptation under the commercial conditions of poultry production.
Acknowledgments
The authors acknowledge financial support in the form of a grant from the Government of the Russian Federation (Contract No. 14.W03.31.0013) to P.F.S. and I.I.K.
Abbreviations
| AFB1 | aflatoxin B1 |
| AKT | protein kinase B |
| ALT | alanine aminotransferase |
| AO | antioxidant |
| AP1 | transcription factor |
| APEC | avian pathogenic Escherichia coli |
| ARD | ankyrin repeat domain |
| ARE | antioxidant response element |
| ATF3 | activating transcription factor 3 |
| CAT | catalase |
| COX2 | cyclooxygenase-2 |
| CXCLi2 | recombinant chicken chemotactic and angiogenic factor 2 |
| Cyt C | cytochrome C |
| DC | dendritic cells |
| dpi | days post infection |
| HDAC3 | histone deacetylase3 |
| IBD | infectious bursal disease |
| IBDV | infectious bursal disease virus |
| IFIT5 | Interferon-induced protein with tetratricopeptide repeats 5 |
| iNOS | inducible nitric oxide synthases |
| IRF7 | interferon regulatory factor 7 |
| LOX5 | lipoxygenase 5 |
| NDV | Newcastle disease virus |
| PGC-1α | peroxisome proliferator-activated receptor coactivator |
| p53 | tumor protein p53 |
| PPAR | peroxisome proliferator-activated receptor |
| PTEN | phosphatase and tensin homolog |
| PUFAs | polyunsaturated fatty acids |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| FHS | follicle-stimulating hormone |
| FOXO3 | transcription factors |
| FOXP3 | forkhead box protein P3 |
| GCL | glutamyl-cysteine ligase |
| GCLC | catalytic subunit of GCL |
| GR | glutathione reductase |
| Grx | glutaredoxin |
| GSH | reduced glutathione |
| GPx | glutathione peroxidase |
| GST | glutathione S-transferase |
| HAT | histone acetyl transferase |
| HIF | hypoxia-inducible factor |
| HO-1 | heme oxygenase 1 |
| HS | heat stress |
| HSP | heat-shock protein |
| IκB | inhibitor of κB |
| IKK | IκB kinase |
| IL-1R | IL1 receptor |
| IFN-γ | interferon gamma |
| Keap1 | Kelch-like erythroid cell-derived protein with CNC homology (ECH)-associated protein 1 |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MDA | malondialdehyde |
| MIP-1α | macrophage inflammatory protein |
| MG | Mycoplasma gallisepticum |
| MMP16 | matrix metalloproteinase 16 |
| Msr | methionine sulfoxide reductase |
| MT3 | metallothionein 3 |
| NEMO | the regulatory subunit IKKγ |
| NF-κB | nuclear factor-κB |
| NLR | NOD-like receptor |
| NRLC5 | NOD-like receptor family CARD domain containing 5 |
| NQO1 | NAD(P)H:quinone dehydrogenase 1 |
| Nrf2 | nuclear factor-erythroid-2 (NF-E2) and related factor 2 |
| PCNA | proliferating cell nuclear antigen |
| PGC-1α | peroxisome proliferator-activated receptor-gamma coactivator 1α |
| PPARs | peroxisome proliferator-activated receptors |
| Prx | peroxiredoxin |
| PTGEs | prostaglandin E synthase |
| PGE | prostaglandin E |
| PRR | pattern recognition receptor |
| RANKL | receptor activator of NF-κB ligand |
| RHD | Rel homology domain |
| RONS | reactive oxygen and nitrogen species |
| ROS | reactive oxygen species |
| SeMet | selenomethionine |
| SOD | superoxide dismutase |
| sPLA2 | secretory phospholipase 2 |
| STAT3 | signal transducer and activator of transcription 3 |
| TGF-β | transforming growth factor beta |
| Th1 | T helper cell 1 |
| TLR | Toll-like receptor |
| TNF-α | tumor necrosis factor |
| TNFR | TNF receptor |
| TRAF6 | tumor necrosis factor receptor-associated factor 6 |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| Trx | thioredoxin |
| TrxR | thioredoxin reductase |
| UCP2 | uncoupling protein 2 |
| XOR | xanthine oxidoreductase |
| Wnt4 | signaling molecule (Wingless-related MMTV integration site 4) |
Author Contributions
P.F.S. wrote the manuscript; I.I.K. was involved in data analysis; M.T.K. was involved in data analysis and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
P.F.S. and I.I.K. were supported by a grant from the Government of the Russian Federation (Contract No. 14.W03.31.0013).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Surai P.F. Vitagenes in Avian Biology and Poultry Health. Wageningen Academic Publishers; Wageningen, The Netherlands: 2020. [Google Scholar]
- 2.Musaogullari A., Chai Y.C. Redox Regulation by Protein S-Glutathionylation: From Molecular Mechanisms to Implications in Health and Disease. Int. J. Mol. Sci. 2020;21:8113. doi: 10.3390/ijms21218113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun L., Wang X., Saredy J., Yuan Z., Yang X., Wang H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox. Biol. 2020;37:101759. doi: 10.1016/j.redox.2020.101759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francioso A., Baseggio Conrado A., Mosca L., Fontana M. Chemistry and Biochemistry of Sulfur Natural Compounds: Key Intermediates of Metabolism and Redox Biology. Oxid. Med. Cell Longev. 2020;2020:8294158. doi: 10.1155/2020/8294158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corkey B.E., Deeney J.T. The Redox Communication Network as a Regulator of Metabolism. Front. Physiol. 2020;11:567796. doi: 10.3389/fphys.2020.567796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saha S., Buttari B., Panieri E., Profumo E., Saso L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules. 2020;25:5474. doi: 10.3390/molecules25225474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell. Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho R.H., Ida E.I., Madruga M.S., Martínez S.L., Shimokomaki M., Estévez M. Underlying connections between the redox system imbalance, protein oxidation and impaired quality traits in pale, soft and exudative (PSE) poultry meat. Food Chem. 2017;215:129–137. doi: 10.1016/j.foodchem.2016.07.182. [DOI] [PubMed] [Google Scholar]
- 9.Pan X., Zhang L., Xing T., Li J., Gao F. The impaired redox status and activated Nrf2/ARE pathway in wooden breast myopathy in broiler chickens. Asian-Australas. J. Anim. Sci. 2020 doi: 10.5713/ajas.19.0953. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estevez M., Petracci M. Benefits of Magnesium Supplementation to Broiler Subjected to Dietary and Heat Stress: Improved Redox Status, Breast Quality and Decreased Myopathy Incidence. Antioxidants. 2019;8:456. doi: 10.3390/antiox8100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalgaard T.S., Briens M., Engberg R.M., Lauridsen C. The influence of selenium and selenoproteins on immune responses of poultry and pigs. Anim. Feed Sci. Technol. 2018;238:73–83. doi: 10.1016/j.anifeedsci.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauridsen C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019;98:4240–4246. doi: 10.3382/ps/pey407. [DOI] [PubMed] [Google Scholar]
- 13.Surai P.F. Selenium in Poultry Nutrition and Health. Wageningen Academic Publishers; Wageningen, The Netherlands: 2018. [Google Scholar]
- 14.Surai P.F., Fisinin V.I. Antioxidant-Prooxidant Balance in the Intestine: Applications in Chick Placement and Pig Weaning. J. Vet. Sci. Med. 2015;3:16. [Google Scholar]
- 15.Mishra B., Jha R. Oxidative Stress in the Poultry Gut: Potential Challenges and Interventions. Front. Vet. Sci. 2019;6:60. doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong Y., Lei J., Zhang B. Effects of dietary quercetin on the antioxidative status and cecal microbiota in broiler chickens fed with oxidized oil. Poult. Sci. 2020;99:4892–4903. doi: 10.1016/j.psj.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kövesi B., Cserháti M., Erdélyi M., Zándoki E., Mézes M., Balogh K. Long-Term Effects of Ochratoxin A on the Glutathione Redox System and Its Regulation in Chicken. Antioxidants. 2019;8:178. doi: 10.3390/antiox8060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egresi A., Süle K., Szentmihályi K., Blázovics A., Fehér E., Hagymási K., Fébel H. Impact of milk thistle (Silybum marianum) on the mycotoxin caused redox-homeostasis imbalance of ducks liver. Toxicon. 2020;187:181–187. doi: 10.1016/j.toxicon.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Zhao H., Wang Y., Guo M., Mu M., Xing M. Arsenic (III) and/or copper (II) induces oxidative stress in chicken brain and subsequent effects on mitochondrial homeostasis and autophagy. J. Inorg. Biochem. 2020;211:111201. doi: 10.1016/j.jinorgbio.2020.111201. [DOI] [PubMed] [Google Scholar]
- 20.Surai P.F., Kochish I.I. Nutritional modulation of the antioxidant capacities in poultry: The case of selenium. Poult. Sci. 2019;98:4231–4239. doi: 10.3382/ps/pey406. [DOI] [PubMed] [Google Scholar]
- 21.Surai P.F., Kochish I.I., Romanov M.N., Griffin D.K. Nutritional modulation of the antioxidant capacities in poultry: The case of vitamin E. Poult. Sci. 2019;98:4030–4041. doi: 10.3382/ps/pez072. [DOI] [PubMed] [Google Scholar]
- 22.Surai P.F., Kochish I.I. Pigments from Microalgae Handbook. Springer Nature Switzerland; Cham, Switzerland: 2020. Carotenoids in Aviculture; pp. 515–540. [Google Scholar]
- 23.Tolba S.A., Magnuson A.D., Sun T., Lei X.G. Dietary supplemental microalgal astaxanthin modulates molecular profiles of stress, inflammation, and lipid metabolism in broiler chickens and laying hens under high ambient temperatures. Poult. Sci. 2020;99:4853–4860. doi: 10.1016/j.psj.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surai P.F., Fisinin V.I. Vitagenes in poultry production. Part 3. Vitagene concept development. Worlds Poult. Sci. J. 2016;72:793–804. doi: 10.1017/S0043933916000751. [DOI] [Google Scholar]
- 25.Surai P.F., Fisinin V.I. Antioxidant system regulation: From vitamins to vitagenes. In: Watson R.R., de Meester F., editors. Handbook of Cholesterol. Wageningen Academic Publishers; Wageningen, The Netherlands: 2016. pp. 451–481. [Google Scholar]
- 26.Surai P.F., Kochish I.I., Fisinin V.I. Antioxidant systems in poultry biology: Nutritional modulation of vitagenes. Eur. J. Poult. Sci. 2017;81:1612–9199. [Google Scholar]
- 27.Surai P.F., Kochish I.I., Fisinin V.I., Kidd M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants. 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surai P.F., Fisinin V.I. Vitagenes in poultry production. Part 1. Technological and environmental stresses. Worlds Poult. Sci. J. 2016;72:721–733. doi: 10.1017/S0043933916000714. [DOI] [Google Scholar]
- 29.Surai P.F., Fisinin V.I. Vitagenes in poultry production. Part 2. Nutritional and internal stresses. Worlds Poult. Sci. J. 2016;72:761–772. doi: 10.1017/S0043933916000726. [DOI] [Google Scholar]
- 30.Surai P.F., Kochish I.I., Fisinin V.I., Grozina A.A., Shatskikh E.V. Molecular Mechanisms of Chicken Gut Health Maintenance: Role of Microbiota. Agricultural Technologies; Moscow, Russia: 2018. [Google Scholar]
- 31.Schijns V.E., van de Zande S., Lupiani B., Reddy S.M. Avian immunology. Academic Press; Cambridge, MA, USA: 2014. Practical aspects of poultry vaccination; pp. 345–362. [Google Scholar]
- 32.Cervantes H.M. Antibiotic-free poultry production: Is it sustainable? J. Appl. Poult. Res. 2015;24:91–97. doi: 10.3382/japr/pfv006. [DOI] [Google Scholar]
- 33.Kim W.H., Lillehoj H.S. Immunity, immunomodulation, and antibiotic alternatives to maximize the genetic potential of poultry for growth and disease response. Anim. Feed Sci. Technol. 2019;250:41–50. doi: 10.1016/j.anifeedsci.2018.09.016. [DOI] [Google Scholar]
- 34.Desin T.S., Köster W., Potter A.A. Salmonella vaccines in poultry: Past, present and future. Expert Rev. Vaccines. 2013;12:87–96. doi: 10.1586/erv.12.138. [DOI] [PubMed] [Google Scholar]
- 35.Guillén S., Marcén M., Álvarez I., Mañas P., Cebrián G. Stress resistance of emerging poultry-associated Salmonella serovars. Int. J. Food Microb. 2020;335:108884. doi: 10.1016/j.ijfoodmicro.2020.108884. [DOI] [PubMed] [Google Scholar]
- 36.Gast R.K., Porter Jr R.E. Salmonella infections. In: David E.S., Boulianne M., Logue C.M., McDougald L.R., Nair V., Suarez D.L., de Wit S., Grimes T., Johnson D., Kromm M., et al., editors. Diseases of Poultry. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2020. pp. 717–753. [Google Scholar]
- 37.Iannetti L., Neri D., Santarelli G.A., Cotturone G., Vulpiani M.P., Salini R., Antoci S., Di Serafino G., Di Giannatale E., Pomilio F., et al. Animal welfare and microbiological safety of poultry meat: Impact of different at-farm animal welfare levels on at-slaughterhouse Campylobacter and Salmonella contamination. Food Control. 2020;109:106921. doi: 10.1016/j.foodcont.2019.106921. [DOI] [Google Scholar]
- 38.Everest H., Hill S.C., Daines R., Sealy J.E., James J., Hansen R., Iqbal M. The evolution, spread and global threat of H6Nx avian influenza viruses. Viruses. 2020;12:673. doi: 10.3390/v12060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wigley P. Immunity to bacterial infection in the chicken. Dev. Comp. Immunol. 2013;41:413–417. doi: 10.1016/j.dci.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Korver D.R. Implications of changing immune function through nutrition in poultry. Anim. Feed Sci. Technol. 2012;173:54–64. doi: 10.1016/j.anifeedsci.2011.12.019. [DOI] [Google Scholar]
- 41.Kaspers B., Göbel T.W. The avian immune system. In: Ratcliffe M.J.H., editor. Encyclopaedia of Immunobiology. Volume 1. Elsevier; Amsterdam, The Netherlands: 2016. pp. 498–503. [Google Scholar]
- 42.Swaggerty C.L., Callaway T.R., Kogut M.H., Piva A., Grilli E. Modulation of the immune response to improve health and reduce foodborne pathogens in poultry. Microorganisms. 2019;7:65. doi: 10.3390/microorganisms7030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C., Li J., Zhang W., Shah S.W.A., Ishfaq M. Mycoplasma gallisepticum triggers immune damage in the chicken thymus by activating the TLR-2/MyD88/NF-κB signaling pathway and NLRP3 inflammasome. Vet. Res. 2020;51:1–13. doi: 10.1186/s13567-020-00777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cardoso Dal Pont G., Farnell M., Farnell Y., Kogut M.H. Dietary Factors as Triggers of Low-Grade Chronic Intestinal Inflammation in Poultry. Microorganisms. 2020;8:139. doi: 10.3390/microorganisms8010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 46.Yu H., Lin L., Zhang Z., Zhang H., Hu H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target Ther. 2020;5:209. doi: 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lambrou G.I., Hatziagapiou K., Vlahopoulos S. Inflammation and tissue homeostasis: The NF-κB system in physiology and malignant progression. Mol. Biol. Rep. 2020;47:4047–4063. doi: 10.1007/s11033-020-05410-w. [DOI] [PubMed] [Google Scholar]
- 48.Chawla M., Roy P., Basak S. Role of the NF-κB system in context-specific tuning of the inflammatory gene response. Cur. Opin. Immunol. 2020;68:21–27. doi: 10.1016/j.coi.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Kopitar-Jerala N. Innate Immune Response in Brain, NF-Kappa B Signaling and Cystatins. Front. Mol. Neurosci. 2015;8:73. doi: 10.3389/fnmol.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sehnert B., Burkhardt H., Dübel S., Voll R.E. Cell-Type Targeted NF-kappaB Inhibition for the Treatment of Inflammatory Diseases. Cells. 2020;9:1627. doi: 10.3390/cells9071627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grilli M., Memo M. Transcriptional pharmacology of neurodegenerative disorders: Novel venue towards neuroprotection against excitotoxicity? Mol. Psychiatry. 1997;2:192–194. doi: 10.1038/sj.mp.4000252. [DOI] [PubMed] [Google Scholar]
- 52.Li X., Zhao Y., Tian B., Jamaluddin M., Mitra A., Yang J., Rowicka M., Brasier A.R., Kudlicki A. Modulation of gene expression regulated by the transcription factor NF-κB/RelA. J. Biol. Chem. 2014;289:11927–11944. doi: 10.1074/jbc.M113.539965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niederberger E., Geisslinger G. Proteomics and NF-κB: An update. Expert Rev. Proteom. 2013;10:189–204. doi: 10.1586/epr.13.5. [DOI] [PubMed] [Google Scholar]
- 54.Wu J., Ding J., Yang J., Guo X., Zheng Y. MicroRNA Roles in the Nuclear Factor Kappa B Signaling Pathway in Cancer. Front. Immunol. 2018;9:546. doi: 10.3389/fimmu.2018.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thoma A., Lightfoot A.P. NF-kB and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. Adv. Exp. Med. Biol. 2018;1088:267–279. doi: 10.1007/978-981-13-1435-3_12. [DOI] [PubMed] [Google Scholar]
- 56.McGuire C., Prinz M., Beyaert R., van Loo G. Nuclear factor kappa B (NF-κB) in multiple sclerosis pathology. Trends Mol. Med. 2013;19:604–613. doi: 10.1016/j.molmed.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Awasthee N., Rai V., Chava S., Nallasamy P., Kunnumakkara A.B., Bishayee A., Chauhan S.C., Challagundla K.B., Gupta S.C. Targeting IκappaB kinases for cancer therapy. Semin. Cancer Biol. 2019;56:12–24. doi: 10.1016/j.semcancer.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker R.G., Hayden M.S., Ghosh S. NF-κB, inflammation, and metabolic disease. Cell. Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lingappan K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018;7:81–86. doi: 10.1016/j.cotox.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L., Yousefzadeh M.J., Suh Y., Niedernhofer L.J., Robbins P.D. Signal Transduction, Ageing and Disease. Subcell. Biochem. 2019;91:227–247. doi: 10.1007/978-981-13-3681-2_9. [DOI] [PubMed] [Google Scholar]
- 61.Patel M., Horgan P.G., McMillan D.C., Edwards J. NF-κB pathways in the development and progression of colorectal cancer. Transl. Res. 2018;197:43–56. doi: 10.1016/j.trsl.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Sivandzade F., Prasad S., Bhalerao A., Cucullo L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox. Biol. 2019;21:101059. doi: 10.1016/j.redox.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones S.V., Kounatidis I. Nuclear Factor-Kappa B and Alzheimer Disease, Unifying Genetic and Environmental Risk Factors from Cell to Humans. Front. Immunol. 2017;8:1805. doi: 10.3389/fimmu.2017.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayden M.S., Ghosh S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014;26:253–266. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.NF-kB Target Genes. [(accessed on 1 December 2020)]; Available online: www/bu.edu/nf-kb/gene-resources/target-genes/
- 66.Scott O., Roifman C.M. NF-κB pathway and the Goldilocks principle: Lessons from human disorders of immunity and inflammation. J. Allergy Clin. Immunol. 2019;143:1688–1701. doi: 10.1016/j.jaci.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 67.Zhang L., Xiao X., Arnold P.R., Li X.C. Transcriptional and epigenetic regulation of immune tolerance: Roles of the NF-κB family members. Cell Mol. Immunol. 2019;16:315–323. doi: 10.1038/s41423-019-0202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park Y.H. The nuclear factor-kappa B pathway and response to treatment in breast cancer. Pharmacogenomics. 2017;18:1697–1709. doi: 10.2217/pgs-2017-0044. [DOI] [PubMed] [Google Scholar]
- 69.Sun S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017;17:545–558. doi: 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giridharan S., Srinivasan M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018;11:407–419. doi: 10.2147/JIR.S140188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rabie N.S., Amin Girh Z. Bacterial vaccines in poultry. Bull. Natl. Res. Centre. 2020;44:15. doi: 10.1186/s42269-019-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gimeno I.M., Schat K.A. Virus-Induced Immunosuppression in Chickens. Avian Dis. 2018;62:272–285. doi: 10.1637/11841-041318-Review.1. [DOI] [PubMed] [Google Scholar]
- 73.Kim W.H., Chaudhari A.A., Lillehoj H.S. Involvement of T Cell Immunity in Avian Coccidiosis. Front. Immunol. 2019;10:2732. doi: 10.3389/fimmu.2019.02732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buelna-Chontal M., Zazueta C. Redox activation of Nrf2 & NF-κB: A double end sword? Cell Signal. 2013;25:2548–2557. doi: 10.1016/j.cellsig.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 75.Pedruzzi L.M., Stockler-Pinto M.B., Leite M., Jr., Mafra D. Nrf2-keap1 system versus NF-κB: The good and the evil in chronic kidney disease? Biochimie. 2012;94:2461–2466. doi: 10.1016/j.biochi.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 76.Tkach K.E., Oyler J.E., Altan-Bonnet G. Cracking the NF-κB code. Sci. Signal. 2014;7:pe5. doi: 10.1126/scisignal.2005108. [DOI] [PubMed] [Google Scholar]
- 77.Pal S., Bhattacharjee A., Ali A., Mandal N.C., Mandal S.C. Chronic inflammation and cancer: Potential chemoprevention through nuclear factor kappa B and p53 mutual antagonism. J. Inflamm. 2014;11:23. doi: 10.1186/1476-9255-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salles A., Romano A., Freudenthal R. Synaptic NF-kappa B pathway in neuronal plasticity and memory. J. Physiol. Paris. 2014;108:256–262. doi: 10.1016/j.jphysparis.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 79.Tilborghs S., Corthouts J., Verhoeven Y., Arias D., Rolfo C., Trinh X.B., van Dam P.A. The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit. Rev. Oncol. Hematol. 2017;120:141–150. doi: 10.1016/j.critrevonc.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 80.Hoffmann A., Baltimore D. Circuitry of nuclear factor κB signaling. Immunol. Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 81.Morgan M.J., Liu Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Jesús T.J., Ramakrishnan P. NF-κB c-Rel Dictates the Inflammatory Threshold by Acting as a Transcriptional Repressor. iScience. 2020;23:100876. doi: 10.1016/j.isci.2020.100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nelson R.H., Nelson D.E. Signal Distortion: How Intracellular Pathogens Alter Host Cell Fate by Modulating NF-κB Dynamics. Front. Immunol. 2018;9:2962. doi: 10.3389/fimmu.2018.02962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fusella F., Seclì L., Cannata C., Brancaccio M. The one thousand and one chaperones of the NF-κB pathway. Cell. Mol. Life Sci. 2020;77:2275–2288. doi: 10.1007/s00018-019-03402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Q., Lenardo M.J., Baltimore D. 30 years of NF-κB: A blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lepetsos P., Papavassiliou K.A., Papavassiliou A.G. Redox and NF-κB signaling in osteoarthritis. Free Rad. Biol. Med. 2019;132:90–100. doi: 10.1016/j.freeradbiomed.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 87.Gloire G., Legrand-Poels S., Piette J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 88.Gloire G., Piette J. Redox regulation of nuclear post-translational modifications during NF-kappaB activation. Antioxid. Redox Signal. 2009;11:2209–2222. doi: 10.1089/ars.2009.2463. [DOI] [PubMed] [Google Scholar]
- 89.Herscovitch M., Comb W., Ennis T., Coleman K., Yong S., Armstead B., Kalaitzidis D., Chandani S., Gilmore T.D. Intermolecular disulfide bond formation in the NEMO dimer requires Cys54 and Cys347. Biochem. Biophys. Res. Commun. 2008;367:103–108. doi: 10.1016/j.bbrc.2007.12.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu M., Bian Q., Liu Y., Fernandes A.F., Taylor A., Pereira P., Shang F. Sustained oxidative stress inhibits NF-κB activation partially via inactivating the proteasome. Free Rad. Biol. Med. 2009;46:62–69. doi: 10.1016/j.freeradbiomed.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lushchak V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011;153:175–190. doi: 10.1016/j.cbpc.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 92.Wang X., Hai C. Novel insights into redox system and the mechanism of redox regulation. Mol. Biol. Rep. 2016;43:607–628. doi: 10.1007/s11033-016-4022-y. [DOI] [PubMed] [Google Scholar]
- 93.Dayalan Naidu S., Kostov R.V., Dinkova-Kostova A.T. Transcription factors Hsf1 and Nrf2 engage in crosstalk for cytoprotection. Trends Pharmacol. Sci. 2015;36:6–14. doi: 10.1016/j.tips.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 94.Dengler V.L., Galbraith M., Espinosa J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014;49:1–15. doi: 10.3109/10409238.2013.838205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cai D., Khor S. “Hypothalamic Microinflammation” Paradigm in Aging and Metabolic Diseases. Cell Metab. 2019;30:19–35. doi: 10.1016/j.cmet.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 96.Yan J., Zhang H., Yin Y., Li J., Tang Y., Purkayastha S., Li L., Cai D. Obesity- and aging-induced excess of central transforming growth factor-beta potentiates diabetic development via an RNA stress response. Nat. Med. 2014;20:1001–1008. doi: 10.1038/nm.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meng Q., Cai D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF-kappaB pathway. J. Biol. Chem. 2011;286:32324–32332. doi: 10.1074/jbc.M111.254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kurata S., Matsumoto M., Tsuji Y., Nakajima H. Lipopolysaccharide activates transcription of the heme oxygenase gene in mouse M1 cells through oxidative activation of nuclear factor kappa B. Eur. J. Biochem. 1996;239:566–571. doi: 10.1111/j.1432-1033.1996.0566u.x. [DOI] [PubMed] [Google Scholar]
- 100.Tornatore L., Thotakura A.K., Bennett J., Moretti M., Franzoso G. The nuclear factor kappa B signaling pathway: Integrating metabolism with inflammation. Trends Cell. Biol. 2012;22:557–566. doi: 10.1016/j.tcb.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 101.Mauro C., Leow S.C., Anso E., Rocha S., Thotakura A.K., Tornatore L., Moretti M., De Smaele E., Beg A.A., Tergaonkar V., et al. NF-κB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat. Cell Biol. 2011;13:1272–1279. doi: 10.1038/ncb2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wakabayashi N., Slocum S.L., Skoko J.J., Shin S., Kensler T.W. When NRF2 talks, who’s listening? Antioxid. Redox Signal. 2020;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Soares M.P., Seldon M.P., Gregoire I.P., Vassilevskaia T., Berberat P.O., Yu J., Tsui T.Y., Bach F.H. Hemeoxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 104.Yerra V.G., Negi G., Sharma S.S., Kumar A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013;1:394–397. doi: 10.1016/j.redox.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thimmulappa R.K., Lee H., Rangasamy T., Reddy S.P., Yamamoto M., Kensler T.W., Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Investig. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen L.G., Zhang Y.Q., Wu Z.Z., Hsieh C.W., Chu C.S., Wung B.S. Peanut arachidin-1 enhances Nrf2-mediated protective mechanisms against TNF-α-induced ICAM-1 expression and NF-_B activation in endothelial cells. Int. J. Mol. Med. 2018;41:541–547. doi: 10.3892/ijmm.2017.3238. [DOI] [PubMed] [Google Scholar]
- 107.Bellezza I., Tucci A., Galli F., Grottelli S., Mierla A.L., Pilolli F., Minelli A. Inhibition of NF-κB nuclear translocation via HO-1 activation underlies α-tocopheryl succinate toxicity. J. Nutr. Biochem. 2012;23:1583–1591. doi: 10.1016/j.jnutbio.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 108.Rushworth S.A., Shah S., MacEwan D.J. TNF mediates the sustained activation of Nrf2 in human monocytes. J. Immunol. 2011;187:702–707. doi: 10.4049/jimmunol.1004117. [DOI] [PubMed] [Google Scholar]
- 109.Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., Tanaka N., Moriguchi T., Motohashi H., Nakayama K., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim S.W., Lee H.K., Shin J.H., Lee J.K. Up-down regulation of HO-1 and iNOS gene expressions by ethyl pyruvate via recruiting p300 to Nrf2 and depriving It from p65. Free Radic Biol Med. 2013;65:468–476. doi: 10.1016/j.freeradbiomed.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 111.Liu G.H., Qu J., Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 112.Lee D.F., Kuo H.P., Liu M., Chou C.K., Xia W., Du Y., Shen J., Chen C.T., Huo L., Hsu M.C., et al. KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol. Cell. 2009;36:131–140. doi: 10.1016/j.molcel.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Banning A., Brigelius-Flohé R. NF-kappaB, Nrf2, and HO-1 interplay in redox-regulated VCAM-1 expression. Antioxid. Redox Signal. 2005;7:889–899. doi: 10.1089/ars.2005.7.889. [DOI] [PubMed] [Google Scholar]
- 114.Brigelius-Flohé R., Flohé L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chuang H.C., Chang C.W., Chang G.D., Yao T.P., Chen H. Histone deacetylase 3 binds to and regulates the GCMa transcription factor. Nucl. Acids Res. 2006;34:1459. doi: 10.1093/nar/gkl048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hung H.L., Kim A.Y., Hong W., Rakowski C., Blobel G.A. Stimulation of NF-E2 DNA binding by CREB-binding protein (CBP)-mediated acetylation. J. Biol. Chem. 2001;276:10715. doi: 10.1074/jbc.M007846200. [DOI] [PubMed] [Google Scholar]
- 117.Ahmed S.M.U., Luo L., Namani A., Wang X.J., Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 118.Yang H., Magilnick N., Ou X., Lu S.C. Tumour necrosis factor α induces co-ordinated activation of rat GSH synthetic enzymes via nuclear factor κB and activator protein-1. Biochem. J. 2005;391:399–408. doi: 10.1042/BJ20050795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rushworth S.A., Zaitseva L., Murray M.Y., Shah N.M., Bowles K.M., MacEwan D.J. The high Nrf2 expression in human acute myeloid leukaemia is driven by NF-kappaB and underlies its chemo-resistance. Blood. 2012;120:5188–5198. doi: 10.1182/blood-2012-04-422121. [DOI] [PubMed] [Google Scholar]
- 120.Wu Y., Lin Z., Yan Z., Wang Z., Fu X., Yu K. Sinomenine contributes to the inhibition of the inflammatory response and the improvement of osteoarthritis in mouse-cartilage cells by acting on the Nrf2/HO-1 and NF-κB signaling pathways. Int. Immunopharmacol. 2019;75:105715. doi: 10.1016/j.intimp.2019.105715. [DOI] [PubMed] [Google Scholar]
- 121.Lee W., Yang S., Lee C., Park E.K., Kim K.M., Ku S.K., Bae J.S. Aloin reduces inflammatory gene iNOS via inhibition activity and p-STAT-1 and NF-κB. Food Chem. Toxicol. 2019;126:67–71. doi: 10.1016/j.fct.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 122.Wang J., Chen G., Shi T., Wang Y., Guan C. Possible treatment for cutaneous lichen planus: An in vitro anti-inflammatory role of Angelica polysaccharide in human keratinocytes HaCaT. Int. J. Immunopathol. Pharmacol. 2019;33:2058738418821837. doi: 10.1177/2058738418821837. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Lee G., Park J.S., Lee E.J., Ahn J.H., Kim H.S. Anti-inflammatory and antioxidant mechanisms of urolithin B in activated microglia. Phytomedicine. 2019;55:50–57. doi: 10.1016/j.phymed.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 124.Zhao D.R., Jiang Y.S., Sun J.Y., Li H.H., Luo X.L., Zhao M.M. Anti-inflammatory Mechanism Involved in 4-Ethylguaiacol-Mediated Inhibition of LPS-Induced Inflammation in THP-1 Cells. J Agric. Food Chem. 2019;67:1230–1243. doi: 10.1021/acs.jafc.8b06263. [DOI] [PubMed] [Google Scholar]
- 125.Ren J., Li L., Wang Y., Zhai J., Chen G., Hu K. Gambogic acid induces heme oxygenase-1 through Nrf2 signaling pathway and inhibits NF-κB and MAPK activation to reduce inflammation in LPS-activated RAW264.7 cells. Biomed. Pharmacother. 2019;109:555–562. doi: 10.1016/j.biopha.2018.10.112. [DOI] [PubMed] [Google Scholar]
- 126.Gęgotek A., Ambrożewicz E., Jastrząb A., Jarocka-Karpowicz I., Skrzydlewska E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch. Dermatol. Res. 2019;311:203–219. doi: 10.1007/s00403-019-01898-w. [DOI] [PubMed] [Google Scholar]
- 127.Luo Z., Zheng B., Jiang B., Xue X., Xue E., Zhou Y. Peiminine inhibits the IL-1β induced inflammatory response in mouse articular chondrocytes and ameliorates murine osteoarthritis. Food Funct. 2019;10:2198–2208. doi: 10.1039/C9FO00307J. [DOI] [PubMed] [Google Scholar]
- 128.Muhammad T., Ikram M., Ullah R., Rehman S.U., Kim M.O. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-κB Signaling. Nutrients. 2019;11:648. doi: 10.3390/nu11030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jia Y.N., Peng Y.L., Zhao Y.P., Cheng X.F., Zhou Y., Chai C.L., Zeng L.S., Pan M.H., Xu L. Comparison of the Hepatoprotective Effects of the Three Main Stilbenes from Mulberry Twigs. J Agric. Food Chem. 2019;67:5521–5529. doi: 10.1021/acs.jafc.8b07245. [DOI] [PubMed] [Google Scholar]
- 130.Zhang H.F., Wang J.H., Wang Y.L., Gao C., Gu Y.T., Huang J., Wang J.H., Zhang Z. Salvianolic Acid A Protects the Kidney against Oxidative Stress by Activating the Akt/GSK-3β/Nrf2 Signaling Pathway and Inhibiting the NF-κB Signaling Pathway in 5/6 Nephrectomized Rats. Oxid. Med. Cell Longev. 2019;2019:2853534. doi: 10.1155/2019/2853534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abdel-Magied N., Shedid S.M. The effect of naringenin on the role of nuclear factor (erythroid-derived 2)-like2 (Nrf2) and haem oxygenase 1 (HO-1) in reducing the risk of oxidative stress-related radiotoxicity in the spleen of rats. Environ. Toxicol. 2019;34:788–795. doi: 10.1002/tox.22745. [DOI] [PubMed] [Google Scholar]
- 132.Wang G.W., Zhang X.L., Wu Q.H., Jin Y.B., Ning C.T., Wang R., Mao J.X., Chen M. The hepatoprotective effects of Sedum sarmentosum extract and its isolated major constituent through Nrf2 activation and NF-κB inhibition. Phytomedicine. 2019;53:263–273. doi: 10.1016/j.phymed.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 133.He Y., Xia Z., Yu D., Wang J., Jin L., Huang D., Ye X., Li X., Zhang B. Hepatoprotective effects and structure-activity relationship of five flavonoids against lipopolysaccharide/d-galactosamine induced acute liver failure in mice. Int. Immunopharmacol. 2019;68:171–178. doi: 10.1016/j.intimp.2018.12.059. [DOI] [PubMed] [Google Scholar]
- 134.Li Z., Feng H., Wang Y., Shen B., Tian Y., Wu L., Zhang Q., Jin M., Liu G. Rosmarinic acid protects mice from lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting MAPKs/NF-κB and activating Nrf2/HO-1 signaling pathways. Int. Immunopharmacol. 2019;67:465–472. doi: 10.1016/j.intimp.2018.12.052. [DOI] [PubMed] [Google Scholar]
- 135.Liu T.G., Sha K.H., Zhang L.G., Liu X.X., Yang F., Cheng J.Y. Protective effects of alpinetin on lipopolysaccharide/d-Galactosamine-induced liver injury through inhibiting inflammatory and oxidative responses. Microb. Pathog. 2019;126:239–244. doi: 10.1016/j.micpath.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 136.Tang F., Fan K., Wang K., Bian C. Amygdalin attenuates acute liver injury induced by D-galactosamine and lipopolysaccharide by regulating the NLRP3, NF-κB and Nrf2/NQO1 signalling pathways. Biomed. Pharmacother. 2019;111:527–536. doi: 10.1016/j.biopha.2018.12.096. [DOI] [PubMed] [Google Scholar]
- 137.Li Q., Tian Z., Wang M., Kou J., Wang C., Rong X., Li J., Xie X., Pang X. Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARγ/Nrf2/NF-κB signaling pathway. Int. Immunopharmacol. 2019;66:309–316. doi: 10.1016/j.intimp.2018.11.044. [DOI] [PubMed] [Google Scholar]
- 138.Bian X., Liu X., Liu J., Zhao Y., Li H., Zhang L., Li P., Gao Y. Hepatoprotective effect of chiisanoside from Acanthopanax sessiliflorus against LPS/D-GalN-induced acute liver injury by inhibiting NF-κB and activating Nrf2/HO-1 signaling pathways. J. Sci. Food Agric. 2019;99:3283–3290. doi: 10.1002/jsfa.9541. [DOI] [PubMed] [Google Scholar]
- 139.Ye J., Guan M., Lu Y., Zhang D., Li C., Zhou C. Arbutin attenuates LPS-induced lung injury via Sirt1/ Nrf2/ NF-κBp65 pathway. Pulm. Pharmacol. Ther. 2019;54:53–59. doi: 10.1016/j.pupt.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 140.Ding H., Ci X., Cheng H., Yu Q., Li D. Chicoric acid alleviates lipopolysaccharide-induced acute lung injury in mice through anti-inflammatory and anti-oxidant activities. Int. Immunopharmacol. 2019;66:169–176. doi: 10.1016/j.intimp.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 141.Alam J., Stewart D., Touchard C., Boinapally S., Choi A.M., Cook J.L. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 142.Lavrovsky Y., Schwartzman M.L., Levere R.D., Kappas A., Abraham N.G. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc. Natl. Acad. Sci. USA. 1994;91:5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mulcahy R.T., Wartman M.A., Bailey H.H., Gipp J.J. Constitutive and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element = TRE sequence. J. Biol. Chem. 1997;272:7445–7454. doi: 10.1074/jbc.272.11.7445. [DOI] [PubMed] [Google Scholar]
- 144.Kimura T., Kawasaki Y., Okumura F., Sone T., Natsuki R., Isobe M. Ethanol-induced expression of glutamate-cysteine ligase catalytic subunit gene is mediated by NF-kappaB. Toxicol. Lett. 2009;185:110–115. doi: 10.1016/j.toxlet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 145.Muri J., Kopf M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-00478-8. in press. [DOI] [PubMed] [Google Scholar]
- 146.Kairisalo M., Korhonen L., Blomgren K., Lindholm D. X-linked inhibitor of apoptosis protein increases mitochondrial antioxidants through NF-kappaB activation. Biochem. Biophys. Res. Commun. 2007;364:138–144. doi: 10.1016/j.bbrc.2007.09.115. [DOI] [PubMed] [Google Scholar]
- 147.Djavaheri-Mergny M., Javelaud D., Wietzerbin J., Besançon F. NF-kappaB activation prevents apoptotic oxidative stress via an increase of both thioredoxin and MnSOD levels in TNFalpha-treated Ewing sarcoma cells. FEBS Lett. 2004;578:111–115. doi: 10.1016/j.febslet.2004.10.082. [DOI] [PubMed] [Google Scholar]
- 148.Anrather J., Racchumi G., Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J. Biol. Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- 149.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moldogazieva N.T., Mokhosoev I.M., Feldman N.B., Lutsenko S.V. ROS and RNS signalling: Adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic. Res. 2018;52:507–543. doi: 10.1080/10715762.2018.1457217. [DOI] [PubMed] [Google Scholar]
- 151.Ikeda T., Honjo K., Hirota Y., Onodera T. Isolation of the chicken NF-kappa B p65 subunit-encoding cDNA and characterization of its products. Gene. 1993;133:237–242. doi: 10.1016/0378-1119(93)90645-j. [DOI] [PubMed] [Google Scholar]
- 152.Ikeda T., Hirota Y., Onodera T. Isolation of a cDNA encoding the chicken p50B/p97 (Lyt-10) transcription factor. Gene. 1994;138:193–196. doi: 10.1016/0378-1119(94)90806-0. [DOI] [PubMed] [Google Scholar]
- 153.Krishnan V.A., Schatzle J.D., Hinojos C.M., Bose H.R., Jr. Structure and regulation of the gene encoding avian inhibitor of nuclear factor kappa B-alpha. Gene. 1995;166:261–266. doi: 10.1016/0378-1119(95)00547-1. [DOI] [PubMed] [Google Scholar]
- 154.Van Phi L. Transcriptional activation of the chicken lysozyme gene by NF-kappa Bp65 (RelA) and c-Rel, but not by NF-kappa Bp50. Biochem. J. 1996;313:39–44. doi: 10.1042/bj3130039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Piffat K.A., Hrdlicková R., Nehyba J., Ikeda T., Liss A., Huang S., Sif S., Gilmore T.D., Bose H.R., Jr. The chicken RelB transcription factor has transactivation sequences and a tissue-specific expression pattern that are distinct from mammalian RelB. Mol. Cell. Biol. Res. Commun. 2001;4:266–275. doi: 10.1006/mcbr.2001.0290. [DOI] [PubMed] [Google Scholar]
- 156.Qiu Y., Shen Y., Li X., Ding C., Ma Z. Molecular cloning and functional characterization of a novel isoform of chicken myeloid differentiation factor 88 (MyD88) Dev. Comp. Immunol. 2008;32:1522–1530. doi: 10.1016/j.dci.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 157.Zhou P., Zeng Y., Rao Z., Li Y., Zheng H., Luo R. Molecular characterization and functional analysis of duck IKKα. Dev. Comp. Immunol. 2021;115:103880. doi: 10.1016/j.dci.2020.103880. [DOI] [PubMed] [Google Scholar]
- 158.Kim Y., Tian M. NF-kappaB family of transcription factor facilitates gene conversion in chicken B cells. Mol. Immunol. 2009;46:3283–3291. doi: 10.1016/j.molimm.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 159.Chen K., Luo Z., Zheng S.J. Gallus Heat shock cognate protein 70, a novel binding partner of Apoptin. Virol. J. 2011;8:324. doi: 10.1186/1743-422X-8-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kogut M.H., He H., Genovese K.J. Bacterial toll-like receptor agonists induce sequential NF-κB-mediated leukotriene B4 and prostaglandin E2 production in chicken heterophils. Vet. Immunol. Immunopathol. 2012;145:159–170. doi: 10.1016/j.vetimm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 161.Shinohara H., Behar M., Inoue K., Hiroshima M., Yasuda T., Nagashima T., Kimura S., Sanjo H., Maeda S., Yumoto N., et al. Positive feedback within a kinase signaling complex functions as a switch mechanism for NF-κB activation. Science. 2014;344:760–764. doi: 10.1126/science.1250020. [DOI] [PubMed] [Google Scholar]
- 162.Tang X., Zhang L., Wei W. Roles of TRAFs in NF-κB signaling pathways mediated by BAFF. Immunol. Lett. 2018;196:113–118. doi: 10.1016/j.imlet.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 163.Yang H.L., Feng Z.Q., Zeng S.Q., Li S.M., Zhu Q., Liu Y.P. Molecular cloning and expression analysis of TRAF3 in chicken. Genet. Mol. Res. 2015;14:4408–4419. doi: 10.4238/2015.April.30.14. [DOI] [PubMed] [Google Scholar]
- 164.Zhai Y., Luo F., Chen Y., Zhou S., Li Z., Liu M., Bi D., Jin H. Molecular characterization and functional analysis of duck TRAF6. Dev. Comp. Immunol. 2015;49:1–6. doi: 10.1016/j.dci.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 165.Guo Y., Xu Y., Kang X., Meng C., Gu D., Zhou Y., Xiong D., Geng S., Jiao X., Pan Z. Molecular cloning and functional analysis of TRAF6 from Yangzhou great white goose Anser anser. Dev. Comp. Immunol. 2019;101:103435. doi: 10.1016/j.dci.2019.103435. [DOI] [PubMed] [Google Scholar]
- 166.Guo Y., Xu Y., Xiong D., Zhou Y., Kang X., Meng C., Gu D., Jiao X., Pan Z. Molecular characterisation, expression and functional feature of TRAF6 in the King pigeon (Columba livia) Innate Immun. 2020;26:490–504. doi: 10.1177/1753425920920930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou Y., Kang X., Xiong D., Zhu S., Zheng H., Xu Y., Guo Y., Pan Z., Jiao X. Molecular and functional characterization of pigeon (Columba livia) tumor necrosis factor receptor-associated factor 3. Dev. Comp. Immunol. 2017;69:51–59. doi: 10.1016/j.dci.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 168.Kang Y., Nii T., Isobe N., Yoshimura Y. Effects of TLR Ligands on the Expression of Cytokines and Possible Role of NFκB in its Process in the Theca of Chicken Follicles. J. Poult. Sci. 2018;55:288–300. doi: 10.2141/jpsa.0170217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen S., Cheng A., Wang M. Innate sensing of viruses by pattern recognition receptors in birds. Vet. Res. 2013;44:82. doi: 10.1186/1297-9716-44-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Downing T., Lloyd A.T., O’Farrelly C., Bradley D.G. The differential evolutionary dynamics of avian cytokine and TLR gene classes. J. Immunol. 2010;184:6993–7000. doi: 10.4049/jimmunol.0903092. [DOI] [PubMed] [Google Scholar]
- 171.Gillespie M., Shamovsky V., D’Eustachio P. Human and chicken TLR pathways: Manual curation and computer-based orthology analysis. Mamm. Genome. 2011;22:130–138. doi: 10.1007/s00335-010-9296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Farnell M.B., Crippen T.L., He H., Swaggerty C.L., Kogut M.H. Oxidative burst mediated by toll like receptors (TLR) and CD14 on avian heterophils stimulated with bacterial toll agonists. Dev. Comp. Immunol. 2003;27:423–429. doi: 10.1016/S0145-305X(02)00115-5. [DOI] [PubMed] [Google Scholar]
- 173.Kannaki T.R., Reddy M.R., Verma P.C., Shanmugam M. Differential Toll-like receptor (TLR) mRNA expression patterns during chicken embryological development. Anim. Biotechnol. 2015;26:130–135. doi: 10.1080/10495398.2014.939658. [DOI] [PubMed] [Google Scholar]
- 174.Yang Y., Jiang Y., Yin Q., Liang H., She R. Chicken intestine defensins activated murine peripheral blood mononuclear cells through the TLR4-NF-kappaB pathway. Vet. Immunol. Immunopathol. 2010;133:59–65. doi: 10.1016/j.vetimm.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 175.Yu H., Lu Y., Qiao X., Wei L., Fu T., Cai S., Wang C., Liu X., Zhong S., Wang Y. Novel Cathelicidins from Pigeon Highlights Evolutionary Convergence in Avain Cathelicidins and Functions in Modulation of Innate Immunity. Sci. Rep. 2015;5:11082. doi: 10.1038/srep11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ishige T., Hara H., Hirano T., Kono T., Hanzawa K. Basic characterization of avian NK-lysin (NKL) from the Japanese quail, Coturnix japonica. Anim. Sci. J. 2014;85:90–95. doi: 10.1111/asj.12138. [DOI] [PubMed] [Google Scholar]
- 177.Kamimura T., Isobe N., Yoshimura Y. Effects of inhibitors of transcription factors, nuclear factor-κB and activator protein 1, on the expression of proinflammatory cytokines and chemokines induced by stimulation with Toll-like receptor ligands in hen vaginal cells. Poult. Sci. 2017;96:723–730. doi: 10.3382/ps/pew366. [DOI] [PubMed] [Google Scholar]
- 178.Yong Y.H., Liu S.F., Hua G.H., Jia R.M., Gooneratne R., Zhao Y.T., Liao M., Ju X.H. Goose toll-like receptor 3 (TLR3) mediated IFN-γ and IL-6 in anti-H5N1 avian influenza virus response. Vet. Immunol. Immunopathol. 2018;197:31–38. doi: 10.1016/j.vetimm.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 179.Gao M., Guo Y., Du J., Song Z., Luo X., Wang J., Han W. Evolutional conservation of molecular structure and antiviral function of a type I interferon, IFN-kappa, in poultry. Dev. Comp. Immunol. 2018;89:44–53. doi: 10.1016/j.dci.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 180.Jia H., Li G., Li J., Tian Y., Wang D., Shen J., Tao Z., Xu J., Lu L. Cloning, expression and bioinformatics analysis of the duck TLR 4 gene. Br. Poult. Sci. 2012;53:190–197. doi: 10.1080/00071668.2012.674208. [DOI] [PubMed] [Google Scholar]
- 181.Fang Q., Pan Z., Geng S., Kang X., Huang J., Sun X., Li Q., Cai Y., Jiao X. Molecular cloning, characterization and expression of goose Toll-like receptor 5. Mol. Immunol. 2012;52:117–124. doi: 10.1016/j.molimm.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 182.Yong Y., Liu S., Hua G., Jia R., Zhao Y., Sun X., Liao M., Ju X. Identification and functional characterization of Toll-like receptor 2-1 in geese. BMC Vet. Res. 2015;11:108. doi: 10.1186/s12917-015-0420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xiong D., Song L., Pan Z., Jiao X. Molecular cloning, characterization, and functional analysis of pigeon (Columba livia) Toll-like receptor 5. Poult. Sci. 2018;97:4031–4039. doi: 10.3382/ps/pey244. [DOI] [PubMed] [Google Scholar]
- 184.Li S., Wang Y., Zhao H., Shao Y., Liu J., Xing M. Characterization, functional and signaling elucidation of pigeon (Columba livia) interferon-α: Knockdown p53 negatively modulates antiviral response. Dev. Comp. Immunol. 2019;90:29–40. doi: 10.1016/j.dci.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 185.Xiong D., Song L., Pan Z., Chen X., Geng S., Jiao X. Identification and immune functional characterization of pigeon TLR7. Int. J. Mol. Sci. 2015;16:8364–8381. doi: 10.3390/ijms16048364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tao Z.Y., Zhu C.H., Shi Z.H., Song C., Xu W.J., Song W.T., Zou J.M., Qin A.J. Molecular characterization, expression, and functional analysis of NOD1 in Qingyuan partridge chicken. Genet. Mol. Res. 2015;14:2691–2701. doi: 10.4238/2015.March.30.29. [DOI] [PubMed] [Google Scholar]
- 187.Li H., Jin H., Li Y., Liu D., Foda M.F., Jiang Y., Luo R. Molecular cloning and functional characterization of duck nucleotide-binding oligomerization domain 1 (NOD1) Dev. Comp. Immunol. 2017;74:82–89. doi: 10.1016/j.dci.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 188.Truong A.D., Hong Y., Hoang C.T., Lee J., Hong Y.H. Chicken IL-26 regulates immune responses through the JAK/STAT and NF-κB signaling pathways. Dev. Comp. Immunol. 2017;73:10–20. doi: 10.1016/j.dci.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 189.Truong A.D., Hong Y., Rengaraj D., Lee J., Lee K., Hong Y.H. Identification and functional characterization, including cytokine production modulation, of the novel chicken Interleukin-11. Dev. Comp. Immunol. 2018;87:51–63. doi: 10.1016/j.dci.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 190.Hoang C.T., Hong Y., Truong A.D., Lee J., Lee K., Hong Y.H. Molecular cloning of chicken interleukin-17B, which induces proinflammatory cytokines through activation of the NF-κB signaling pathway. Dev. Comp. Immunol. 2017;74:40–48. doi: 10.1016/j.dci.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 191.Rohde F., Schusser B., Hron T., Farkašová H., Plachý J., Härtle S., Hejnar J., Elleder D., Kaspers B. Characterization of Chicken Tumor Necrosis Factor-α, a Long Missed Cytokine in Birds. Front. Immunol. 2018;9:605. doi: 10.3389/fimmu.2018.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ji G.G., Shu J.T., Zhang M., Ju X.J., Shan Y.J., Liu Y.F., Tu Y.J. Transcriptional regulatory region and DNA methylation analysis of TNNI1 gene promoters in Gaoyou duck skeletal muscle (Anas platyrhynchos domestica) Br. Poult. Sci. 2019;60:202–208. doi: 10.1080/00071668.2019.1602250. [DOI] [PubMed] [Google Scholar]
- 193.Barjesteh N., Taha-Abdelaziz K., Kulkarni R.R., Sharif S. Innate antiviral responses are induced by TLR3 and TLR4 ligands in chicken tracheal epithelial cells: Communication between epithelial cells and macrophages. Virology. 2019;534:132–142. doi: 10.1016/j.virol.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 194.Sutton K.M., Hu T., Wu Z., Siklodi B., Vervelde L., Kaiser P. The functions of the avian receptor activator of NF-κB ligand (RANKL) and its receptors, RANK and osteoprotegerin, are evolutionarily conserved. Dev. Comp. Immunol. 2015;51:170–184. doi: 10.1016/j.dci.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 195.Truong A.D., Hong Y., Nguyen H.T., Nguyen C.T., Chu N.T., Tran H.T.T., Dang H.V., Lillehoj H.S., Hong Y.H. Molecular identification and characterisation of a novel chicken leukocyte immunoglobulin-like receptor A5. Br. Poult. Sci. 2020 doi: 10.1080/00071668.2020.1812524. in press. [DOI] [PubMed] [Google Scholar]
- 196.Paital B., Panda S.K., Hati A.K., Mohanty B., Mohapatra M.K., Kanungo S., Chainy G.B. Longevity of animals under reactive oxygen species stress and disease susceptibility due to global warming. World J. Biol. Chem. 2016;7:110–127. doi: 10.4331/wjbc.v7.i1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vandana G.D., Sejian V., Lees A.M., Pragna P., Silpa M.V., Maloney S.K. Heat stress and poultry production: Impact and amelioration. Int. J. Biometeorol. 2020 doi: 10.1007/s00484-020-02023-7. in press. [DOI] [PubMed] [Google Scholar]
- 198.Wasti S., Sah N., Mishra B. Impact of Heat Stress on Poultry Health and Performances, and Potential Mitigation Strategies. Animals. 2020;10:1266. doi: 10.3390/ani10081266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pu S., Usuda K., Nagaoka K., Gore A., Crews D., Watanabe G. The relation between liver damage and reproduction in female Japanese quail (Coturnix japonica) exposed to high ambient temperature. Poult. Sci. 2020;99:4586–4597. doi: 10.1016/j.psj.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nawab A., Li G., An L., Wu J., Chao L., Xiao M., Zhao Y., Birmani M.W., Ghani M.W. Effect of curcumin supplementation on TLR4 mediated non-specific immune responses in liver of laying hens under high-temperature conditions. J. Therm. Biol. 2019;84:384–397. doi: 10.1016/j.jtherbio.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 201.Liu L., Fu C., Yan M., Xie H., Li S., Yu Q., He S., He J. Resveratrol modulates intestinal morphology and HSP70/90, NF-κB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016;7:1329–1338. doi: 10.1039/C5FO01338K. [DOI] [PubMed] [Google Scholar]
- 202.Hangalapura B.N., Kaiser M.G., Poel J.J., Parmentier H.K., Lamont S.J. Cold stress equally enhances in vivo pro-inflammatory cytokine gene expression in chicken lines divergently selected for antibody responses. Dev. Comp. Immunol. 2006;30:503–511. doi: 10.1016/j.dci.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 203.Zhao F.Q., Zhang Z.W., Wang C., Zhang B., Yao H.D., Li S., Xu S.W. The role of heat shock proteins in inflammatory injury induced by cold stress in chicken hearts. Cell Stress Chaperones. 2013;18:773–783. doi: 10.1007/s12192-013-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fu J., Liu C.P., Zhang Z.W., Xing M.W., Xu S.W. Influence of inflammatory pathway markers on oxidative stress induced by cold stress in intestine of quails. Res. Vet. Sci. 2013;95:495–501. doi: 10.1016/j.rvsc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 205.Ren J., Liu C., Zhao D., Fu J. The role of heat shock protein 70 in oxidant stress and inflammatory injury in quail spleen induced by cold stress. Environ. Sci. Pollut. Res. Int. 2018;25:21011–21023. doi: 10.1007/s11356-018-2142-8. [DOI] [PubMed] [Google Scholar]
- 206.Wei H., Zhang R., Su Y., Bi Y., Li X., Zhang X., Li J., Bao J. Effects of Acute Cold Stress After Long-Term Cold Stimulation on Antioxidant Status, Heat Shock Proteins, Inflammation and Immune Cytokines in Broiler Heart. Front. Physiol. 2018;9:1589. doi: 10.3389/fphys.2018.01589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Surai P.F., Dvorska J.E. Effects of Mycotoxins on Antioxidant Status and Immunity. In: Diaz D.E., editor. The Mycotoxin Blue Book. Nottingham University Press; Nottingham, UK: 2005. pp. 93–137. [Google Scholar]
- 208.Gao Y., Meng L., Liu H., Wang J., Zheng N. The Compromised Intestinal Barrier Induced by Mycotoxins. Toxins. 2020;12:619. doi: 10.3390/toxins12100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Li Y., Ma Q.G., Zhao L.H., Wei H., Duan G.X., Zhang J.Y., Ji C. Effects of lipoic acid on immune function, the antioxidant defense system, and inflammation-related genes expression of broiler chickens fed aflatoxin contaminated diets. Int. J. Mol. Sci. 2014;15:5649–5662. doi: 10.3390/ijms15045649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ma Q., Li Y., Fan Y., Zhao L., Wei H., Ji C., Zhang J. Molecular Mechanisms of Lipoic Acid Protection against Aflatoxin B₁-Induced Liver Oxidative Damage and Inflammatory Responses in Broilers. Toxins. 2015;7:5435–5447. doi: 10.3390/toxins7124879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rajput S.A., Sun L., Zhang N.Y., Khalil M.M., Ling Z., Chong L., Wang S., Rajput I.R., Bloch D.M., Khan F.A., et al. Grape Seed Proanthocyanidin Extract Alleviates AflatoxinB₁-Induced Immunotoxicity and Oxidative Stress via Modulation of NF-κB and Nrf2 Signaling Pathways in Broilers. Toxins. 2019;11:23. doi: 10.3390/toxins11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Du Y., Zhu Y., Teng X., Zhang K., Teng X., Li S. Toxicological Effect of Manganese on NF-κB/iNOS-COX-2 Signaling Pathway in Chicken Testes. Biol. Trace Elem. Res. 2015;168:227–234. doi: 10.1007/s12011-015-0340-5. [DOI] [PubMed] [Google Scholar]
- 213.Li S., Zhao H., Wang Y., Shao Y., Li J., Liu J., Xing M. The inflammatory responses in Cu-mediated elemental imbalance is associated with mitochondrial fission and intrinsic apoptosis in Gallus gallus heart. Chemosphere. 2017;189:489–497. doi: 10.1016/j.chemosphere.2017.09.099. [DOI] [PubMed] [Google Scholar]
- 214.Yang F., Liao J., Yu W., Pei R., Qiao N., Han Q., Hu L., Li Y., Guo J., Pan J., et al. Copper induces oxidative stress with triggered NF-κB pathway leading to inflammatory responses in immune organs of chicken. Ecotoxicol. Environ. Saf. 2020;200:110715. doi: 10.1016/j.ecoenv.2020.110715. [DOI] [PubMed] [Google Scholar]
- 215.Zhang K., Zhao P., Guo G., Guo Y., Tian L., Sun X., Li S., He Y., Sun Y., Chai H., et al. Arsenic Trioxide Attenuates NF-κB and Cytokine mRNA Levels in the Livers of Cocks. Biol. Trace Elem. Res. 2016;170:432–437. doi: 10.1007/s12011-015-0455-8. [DOI] [PubMed] [Google Scholar]
- 216.Li S.W., Guo Y., He Y., Sun X., Zhao H.J., Wang Y., Wang Y.J., Xing M.W. Assessment of arsenic trioxide toxicity on cock muscular tissue: Alterations of oxidative damage parameters, inflammatory cytokines and heat shock proteins. Ecotoxicology. 2017;26:1078–1088. doi: 10.1007/s10646-017-1835-y. [DOI] [PubMed] [Google Scholar]
- 217.Li S., Wang Y., Zhao H., He Y., Li J., Jiang G., Xing M. NF-κB-mediated inflammation correlates with calcium overload under arsenic trioxide-induced myocardial damage in Gallus gallus. Chemosphere. 2017;185:618–627. doi: 10.1016/j.chemosphere.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 218.Sun X., He Y., Guo Y., Li S., Zhao H., Wang Y., Zhang J., Xing M. Arsenic affects inflammatory cytokine expression in Gallus gallus brain tissues. BMC Vet. Res. 2017;13:157. doi: 10.1186/s12917-017-1066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li S.W., Sun X., He Y., Guo Y., Zhao H.J., Hou Z.J., Xing M.W. Assessment of arsenic trioxide in the heart of Gallus gallus: Alterations of oxidative damage parameters, inflammatory cytokines, and cardiac enzymes. Environ. Sci. Pollut. Res. Int. 2017;24:5781–5790. doi: 10.1007/s11356-016-8223-7. [DOI] [PubMed] [Google Scholar]
- 220.Wang Y., Zhao H., Guo M., Shao Y., Liu J., Jiang G., Xing M. Arsenite renal apoptotic effects in chickens co-aggravated by oxidative stress and inflammatory response. Metallomics. 2018;10:1805–1813. doi: 10.1039/C8MT00234G. [DOI] [PubMed] [Google Scholar]
- 221.Zhao H., Wang Y., Shao Y., Liu J., Wang S., Xing M. Oxidative stress-induced skeletal muscle injury involves in NF-κB/p53-activated immunosuppression and apoptosis response in copper (II) or/and arsenite-exposed chicken. Chemosphere. 2018;210:76–84. doi: 10.1016/j.chemosphere.2018.06.165. [DOI] [PubMed] [Google Scholar]
- 222.Sun X., Li J., Zhao H., Wang Y., Liu J., Shao Y., Xue Y., Xing M. Synergistic effect of copper and arsenic upon oxidative stress, inflammation and autophagy alterations in brain tissues of Gallus gallus. J. Inorg. Biochem. 2018;178:54–62. doi: 10.1016/j.jinorgbio.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 223.Liu J., Zhao H., Wang Y., Shao Y., Zhang L., Xing M. Impacts of simultaneous exposure to arsenic (III) and copper (II) on inflammatory response, immune homeostasis, and heat shock response in chicken thymus. Int. Immunopharmacol. 2018;64:60–68. doi: 10.1016/j.intimp.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 224.Liu J., Zhao H., Wang Y., Shao Y., Li J., Xing M. Alterations of antioxidant indexes and inflammatory cytokine expression aggravated hepatocellular apoptosis through mitochondrial and death receptor-dependent pathways in Gallus gallus exposed to arsenic and copper. Environ. Sci. Pollut. Res. Int. 2018;25:15462–15473. doi: 10.1007/s11356-018-1757-0. [DOI] [PubMed] [Google Scholar]
- 225.Guo M., Zhao H., Wang Y., Liu J., Fei D., Yang X., Mu M., Xing M. Elemental imbalance elicited by arsenic and copper exposures leads to oxidative stress and immunotoxicity in chicken gizzard, activating the protective effects of heat shock proteins. Environ. Sci. Pollut. Res. Int. 2019;26:36343–36353. doi: 10.1007/s11356-019-06702-w. [DOI] [PubMed] [Google Scholar]
- 226.Liu J., Wang Y., Zhao H., Mu M., Guo M., Nie X., Sun Y., Xing M. Arsenic (III) or/and copper (II) exposure induce immunotoxicity through trigger oxidative stress, inflammation and immune imbalance in the bursa of chicken. Ecotoxicol. Environ. Saf. 2020;190:110127. doi: 10.1016/j.ecoenv.2019.110127. [DOI] [PubMed] [Google Scholar]
- 227.Wang D., Zong C., Cheng K. Chicken thalamic injury induced by copper (II) or / and arsenite exposure involves oxidative stress and inflammation-induced apoptosis. Ecotoxicol. Environ. Saf. 2020;197:110554. doi: 10.1016/j.ecoenv.2020.110554. [DOI] [PubMed] [Google Scholar]
- 228.Nie X., Wang Y., Zhao H., Guo M., Liu Y., Xing M. As3+ or/and Cu2+ exposure triggers oxidative stress imbalance, induces inflammatory response and apoptosis in chicken brain. Ecotoxicol. Environ. Saf. 2020;203:110993. doi: 10.1016/j.ecoenv.2020.110993. [DOI] [PubMed] [Google Scholar]
- 229.Wang H., Li S., Teng X. The antagonistic effect of selenium on lead-induced inflammatory factors and heat shock proteins mRNA expression in chicken livers. Biol. Trace Elem. Res. 2016;171:437–444. doi: 10.1007/s12011-015-0532-z. [DOI] [PubMed] [Google Scholar]
- 230.Li X., Xing M., Chen M., Zhao J., Fan R., Zhao X., Cao C., Yang J., Zhang Z., Xu S. Effects of selenium-lead interaction on the gene expression of inflammatory factors and selenoproteins in chicken neutrophils. Ecotoxicol. Environ. Saf. 2017;139:447–453. doi: 10.1016/j.ecoenv.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 231.Xie W., Ge M., Li G., Zhang L., Tang Z., Li R., Zhang R. Astragalus Polysaccharide Protect against Cadmium-Induced Cytotoxicity through the MDA5/NF-κB Pathway in Chicken Peripheral Blood Lymphocytes. Molecules. 2017;22:1610. doi: 10.3390/molecules22101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chen M., Li X., Fan R., Yang J., Jin X., Hamid S., Xu S. Cadmium induces BNIP3-dependent autophagy in chicken spleen by modulating miR-33-AMPK axis. Chemosphere. 2018;194:396–402. doi: 10.1016/j.chemosphere.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 233.Wang Y., Liu J., Chen R., Qi M., Tao D., Xu S. The Antagonistic Effects of Selenium Yeast (SeY) on Cadmium-Induced Inflammatory Factors and the Heat Shock Protein Expression Levels in Chicken Livers. Biol. Trace Elem. Res. 2020;198:260–268. doi: 10.1007/s12011-020-02039-5. [DOI] [PubMed] [Google Scholar]
- 234.Cao H., Gao F., Xia B., Zhang M., Liao Y., Yang Z., Hu G., Zhang C. Alterations in trace element levels and mRNA expression of Hsps and inflammatory cytokines in livers of duck exposed to molybdenum or/and cadmium. Ecotoxicol. Environ. Saf. 2016;125:93–101. doi: 10.1016/j.ecoenv.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 235.Guo H., Deng H., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Wu B., Chen K. Nickel chloride (NiCl2)-caused inflammatory responses via activation of NF-κB pathway and reduction of anti-inflammatory mediator expression in the kidney. Oncotarget. 2015;6:28607–28620. doi: 10.18632/oncotarget.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Xie W., Lv A., Li R., Tang Z., Ma D., Huang X., Zhang R., Ge M. Agaricus blazei Murill Polysaccharides Protect Against Cadmium-Induced Oxidative Stress and Inflammatory Damage in Chicken Spleens. Biol. Trace Elem. Res. 2018;184:247–258. doi: 10.1007/s12011-017-1189-6. [DOI] [PubMed] [Google Scholar]
- 237.Yin J., Duan J., Cui Z., Ren W., Li T., Yin Y. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Advances. 2015;5:15479–15486. doi: 10.1039/C4RA13557A. [DOI] [Google Scholar]
- 238.Chen Z., Xing T., Li J., Zhang L., Jiang Y., Gao F. Hydrogen peroxide-induced oxidative stress impairs redox status and damages aerobic metabolism of breast muscle in broilers. Poult. Sci. 2020 doi: 10.1016/j.psj.2020.11.029. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hofmann T., Schmucker S.S., Bessei W., Grashorn M., Stefanski V. Impact of Housing Environment on the Immune System in Chickens: A Review. Animals. 2020;10:1138. doi: 10.3390/ani10071138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen X., Zhang L., Li J., Gao F., Zhou G. Hydrogen Peroxide-Induced Change in Meat Quality of the Breast Muscle of Broilers Is Mediated by ROS Generation, Apoptosis, and Autophagy in the NF-κB Signal Pathway. J. Agric. Food Chem. 2017;65:3986–3994. doi: 10.1021/acs.jafc.7b01267. [DOI] [PubMed] [Google Scholar]
- 241.Chen X., Gu R., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Induction of nuclear factor-κB signal-mediated apoptosis and autophagy by reactive oxygen species is associated with hydrogen peroxide-impaired growth performance of broilers. Animal. 2018;12:2561–2570. doi: 10.1017/S1751731118000903. [DOI] [PubMed] [Google Scholar]
- 242.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Shi Q., Wang W., Chen M., Zhang H., Xu S. Ammonia induces Treg/Th1 imbalance with triggered NF-κB pathway leading to chicken respiratory inflammation response. Sci. Total Environ. 2019;659:354–362. doi: 10.1016/j.scitotenv.2018.12.375. [DOI] [PubMed] [Google Scholar]
- 244.An Y., Xing H., Zhang Y., Jia P., Gu X., Teng X. The evaluation of potential immunotoxicity induced by environmental pollutant ammonia in broilers. Poult. Sci. 2019;98:3165–3175. doi: 10.3382/ps/pez135. [DOI] [PubMed] [Google Scholar]
- 245.Han Q., Tong J., Sun Q., Teng X., Zhang H., Teng X. The involvement of miR-6615-5p/Smad7 axis and immune imbalance in ammonia-caused inflammatory injury via NF-κB pathway in broiler kidneys. Poult. Sci. 2020;99:5378–5388. doi: 10.1016/j.psj.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shah S., Ishfaq M., Nasrullah M., Qayum A., Akhtar M.U., Jo H., Hussain M., Teng X. Ammonia inhalation-induced inflammation and structural impairment in the bursa of fabricius and thymus of broilers through NF-κB signaling pathway. Environ. Sci. Pollut. Res. Int. 2020;27:11596–11607. doi: 10.1007/s11356-020-07743-2. [DOI] [PubMed] [Google Scholar]
- 247.Chen D., Ning F., Zhang J., Tang Y., Teng X. NF-κB pathway took part in the development of apoptosis mediated by miR-15a and oxidative stress via mitochondrial pathway in ammonia-treated chicken splenic lymphocytes. Sci. Total Environ. 2020;729:139017. doi: 10.1016/j.scitotenv.2020.139017. [DOI] [PubMed] [Google Scholar]
- 248.Zhao F., Qu J., Wang W., Li S., Xu S. The imbalance of Th1/Th2 triggers an inflammatory response in chicken spleens after ammonia exposure. Poult. Sci. 2020;99:3817–3822. doi: 10.1016/j.psj.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wang W., Chen M., Jin X., Li X., Yang Z., Lin H., Xu S. H(2)S induces Th1/Th2 imbalance with triggered NF-κB pathway to exacerbate LPS-induce chicken pneumonia response. Chemosphere. 2018;208:241–246. doi: 10.1016/j.chemosphere.2018.05.152. [DOI] [PubMed] [Google Scholar]
- 250.Chi Q., Chi X., Hu X., Wang S., Zhang H., Li S. The effects of atmospheric hydrogen sulfide on peripheral blood lymphocytes of chickens: Perspectives on inflammation, oxidative stress and energy metabolism. Environ. Res. 2018;167:1–6. doi: 10.1016/j.envres.2018.06.051. [DOI] [PubMed] [Google Scholar]
- 251.Chi Q., Wang D., Hu X., Li S., Li S. Hydrogen Sulfide Gas Exposure Induces Necroptosis and Promotes Inflammation through the MAPK/NF-κB Pathway in Broiler Spleen. Oxid. Med. Cell Longev. 2019;2019:8061823. doi: 10.1155/2019/8061823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hu X., Chi Q., Liu Q., Wang D., Zhang Y., Li S. Atmospheric H2S triggers immune damage by activating the TLR-7/MyD88/NF-κB pathway and NLRP3 inflammasome in broiler thymus. Chemosphere. 2019;237:124427. doi: 10.1016/j.chemosphere.2019.124427. [DOI] [PubMed] [Google Scholar]
- 253.Scott T., Owens M.D. Thrombocytes respond to lipopolysaccharide through Toll-like receptor-4, and MAP kinase and NF-kappaB pathways leading to expression of interleukin-6 and cyclooxygenase-2 with production of prostaglandin E2. Mol. Immunol. 2008;45:1001–1008. doi: 10.1016/j.molimm.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 254.Winkler C., Ferdous F., Dimmick M., Scott T. Lipopolysaccharide induced Interleukin-6 production is mediated through activation of ERK 1/2, p38 MAPK, MEK, and NFκB in chicken thrombocytes. Dev. Comp. Immunol. 2017;73:124–130. doi: 10.1016/j.dci.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 255.Kogut M.H., Genovese K.J., He H., Kaiser P. Flagellin and lipopolysaccharide up-regulation of IL-6 and CXCLi2 gene expression in chicken heterophils is mediated by ERK1/2-dependent activation of AP-1 and NF-kappaB signaling pathways. Innate Immun. 2008;14:213–222. doi: 10.1177/1753425908094416. [DOI] [PubMed] [Google Scholar]
- 256.Ariyadi B., Isobe N., Yoshimura Y. Toll-like receptor signaling for the induction of mucin expression by lipopolysaccharide in the hen vagina. Poult. Sci. 2014;93:673–679. doi: 10.3382/ps.2013-03667. [DOI] [PubMed] [Google Scholar]
- 257.Liu L., Shen J., Zhao C., Wang X., Yao J., Gong Y., Yang X. Dietary Astragalus polysaccharide alleviated immunological stress in broilers exposed to lipopolysaccharide. Int. J. Biol. Macromol. 2015;72:624–632. doi: 10.1016/j.ijbiomac.2014.08.057. [DOI] [PubMed] [Google Scholar]
- 258.Huang X.Y., Ansari A.R., Huang H.B., Zhao X., Li N.Y., Sun Z.J., Peng K.M., Zhong J., Liu H.Z. Lipopolysaccharide mediates immuno-pathological alterations in young chicken liver through TLR4 signaling. BMC Immunol. 2017;18:12. doi: 10.1186/s12865-017-0199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Li N., Ansari A.R., Sun Z., Huang H., Cui L., Hu Y., Zhao X., Zhong J., Abdel-Kafy E.M., Liu H. Toll like receptor 4 signaling pathway participated in Salmonella lipopolysaccharide-induced spleen injury in young chicks. Microb. Pathog. 2017;112:288–294. doi: 10.1016/j.micpath.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 260.Chang Y., Yuan L., Liu J., Muhammad I., Cao C., Shi C., Zhang Y., Li R., Li C., Liu F. Dihydromyricetin attenuates Escherichia coli lipopolysaccharide-induced ileum injury in chickens by inhibiting NLRP3 inflammasome and TLR4/NF-κB signalling pathway. Vet. Res. 2020;51:72. doi: 10.1186/s13567-020-00796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yang L., Liu G., Lian K., Qiao Y., Zhang B., Zhu X., Luo Y., Shang Y., Gu X.L. Dietary leonurine hydrochloride supplementation attenuates lipopolysaccharide challenge-induced intestinal inflammation and barrier dysfunction by inhibiting the NF-κB/MAPK signaling pathway in broilers. J. Anim. Sci. 2019;97:1679–1692. doi: 10.1093/jas/skz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Velleman S.G. Pectoralis Major (Breast) Muscle Extracellular Matrix Fibrillar Collagen Modifications Associated With the Wooden Breast Fibrotic Myopathy in Broilers. Front. Physiol. 2020;11:461. doi: 10.3389/fphys.2020.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Xing T., Luo D., Zhao X., Xu X., Li J., Zhang L., Gao F. Enhanced cytokine expression and upregulation of inflammatory signaling pathways in broiler chickens affected by wooden breast myopathy. J. Sci. Food Agric. 2020 doi: 10.1002/jsfa.10641. in press. [DOI] [PubMed] [Google Scholar]
- 264.Fancher C.A., Zhang L., Kiess A.S., Adhikari P.A., Dinh T., Sukumaran A.T. Avian Pathogenic Escherichia coli and Clostridium perfringens: Challenges in No Antibiotics Ever Broiler Production and Potential Solutions. Microorganisms. 2020;8:1533. doi: 10.3390/microorganisms8101533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Nhung N.T., Chansiripornchai N., Carrique-Mas J.J. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front. Vet. Sci. 2017;4:126. doi: 10.3389/fvets.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Peng L.Y., Cui Z.Q., Wu Z.M., Fu B.D., Yi P.F., Shen H.Q. RNA-seq profiles of chicken type II pneumocyte in response to Escherichia coli infection. PLoS ONE. 2019;14:e0217438. doi: 10.1371/journal.pone.0217438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Peng L.Y., Yuan M., Wu Z.M., Song K., Zhang C.L., An Q., Xia F., Yu J.L., Yi P.F., Fu B.D., et al. Anti-bacterial activity of baicalin against APEC through inhibition of quorum sensing and inflammatory responses. Sci. Rep. 2019;9:4063. doi: 10.1038/s41598-019-40684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Peng L.Y., Yuan M., Song K., Yu J.L., Li J.H., Huang J.N., Yi P.F., Fu B.D., Shen H.Q. Baicalin alleviated APEC-induced acute lung injury in chicken by inhibiting NF-κB pathway activation. Int. Immunopharmacol. 2019;72:467–472. doi: 10.1016/j.intimp.2019.04.046. [DOI] [PubMed] [Google Scholar]
- 269.Jiao J., Yang Y., Liu M., Li J., Cui Y., Yin S., Tao J. Artemisinin and Artemisia annua leaves alleviate Eimeria tenella infection by facilitating apoptosis of host cells and suppressing inflammatory response. Vet. Parasitol. 2018;254:172–177. doi: 10.1016/j.vetpar.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 270.Yuan M., Peng L.Y., Wu S.C., Li J.H., Song K., Chen S., Huang J.N., Yu J.L., An Q., Yi P.F., et al. Schizandrin attenuates inflammation induced by avian pathogenic Escherichia coli in chicken type II pneumocytes. Int. Immunopharmacol. 2020;81:106313. doi: 10.1016/j.intimp.2020.106313. [DOI] [PubMed] [Google Scholar]
- 271.Wang W., Li Z., Han Q., Guo Y., Zhang B., D’inca R. Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Br. J. Nutr. 2016;116:1878–1888. doi: 10.1017/S0007114516004116. [DOI] [PubMed] [Google Scholar]
- 272.Thames H.T., Sukumaran A.T. A Review of Salmonella and Campylobacter in Broiler Meat: Emerging Challenges and Food Safety Measures. Foods. 2020;9:776. doi: 10.3390/foods9060776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Keestra A.M., de Zoete M.R., van Aubel R.A., van Putten J.P. Functional characterization of chicken TLR5 reveals species-specific recognition of flagellin. Mol. Immunol. 2008;45:1298–1307. doi: 10.1016/j.molimm.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 274.Chiang H.I., Berghman L.R., Zhou H. Inhibition of NF-kB 1 (NF-kBp50) by RNA interference in chicken macrophage HD11 cell line challenged with Salmonellaenteritidis. Genet. Mol. Biol. 2009;32:507–515. doi: 10.1590/S1415-47572009000300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zhao H., Chen Z., Xie L.J., Liu G.F. Suppression of TLR4/NFκB signaling pathway improves cerebral ischemia-reperfusion injury in rats. Mol. Neurobiol. 2018;55:4311–4319. doi: 10.1007/s12035-017-0552-0. [DOI] [PubMed] [Google Scholar]
- 276.Liu X., Ma T., Wang H., Sheng Z., Dou X., Wang K., Li Z., Pan Z., Chang G., Chen G. Up-regulation of NLRC5 and NF-κB signaling pathway in carrier chickens challenged with Salmonella enterica Serovar Pullorum at different persistence periods. Indian J. Biochem. Biophys. 2015;52:132–139. [PubMed] [Google Scholar]
- 277.Geng S., Wang Y., Xue Y., Wang H., Cai Y., Zhang J., Barrow P., Pan Z., Jiao X. The SseL protein inhibits the intracellular NF-κB pathway to enhance the virulence of Salmonella Pullorum in a chicken model. Microb. Pathog. 2019;129:1–6. doi: 10.1016/j.micpath.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 278.He Y., Yang Y., Dong Y., Yan C., Zhang B. The Effects of Flavomycin and Colistin Sulfate Pre-Treatment on Ileal Bacterial Community Composition, the Response to Salmonella typhimurium and Host Gene Expression in Broiler Chickens. Microorganisms. 2019;7:574. doi: 10.3390/microorganisms7110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.He Y., Yang Y., Dong Y., Ito K., Zhang B. Highly nutritious diet resists Salmonella Typhimurium infections by improving intestinal microbiota and morphology in broiler chickens. Poult. Sci. 2020;99:7055–7065. doi: 10.1016/j.psj.2020.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Li Q., Xu L., Yin C., Liu Z., Li Y., Yuan Y., Hu Y., Jiao X. The Invasion Plasmid Antigen J (IpaJ) from Salmonella Inhibits NF-κB Activation by Suppressing IκBα Ubiquitination. Infect. Immun. 2020;88:e00875-19. doi: 10.1128/IAI.00875-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ishfaq M., Hu W., Khan M.Z., Ahmad I., Guo W., Li J. Current status of vaccine research, development, and challenges of vaccines for Mycoplasma gallisepticum. Poult. Sci. 2020;99:4195–4202. doi: 10.1016/j.psj.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Majumder S., Zappulla F., Silbart L.K. Mycoplasma gallisepticum lipid associated membrane proteins up-regulate inflammatory genes in chicken tracheal epithelial cells via TLR-2 ligation through an NF-κB dependent pathway. PLoS ONE. 2014;9:e112796. doi: 10.1371/journal.pone.0112796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Tian W., Zhao C., Hu Q., Sun J., Peng X. Roles of Toll-like receptors 2 and 6 in the inflammatory response to Mycoplasma gallisepticum infection in DF-1 cells and in chicken embryos. Dev. Comp. Immunol. 2016;59:39–47. doi: 10.1016/j.dci.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 284.Sheedy F.J. Turning 21: Induction of miR-21 as a Key Switch in the Inflammatory Response. Front. Immunol. 2015;6:19. doi: 10.3389/fimmu.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Krichevsky A.M., Gabriely G. miR-21: A small multi-faceted RNA. J. Cell. Mol. Med. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zhao Y., Zou M., Sun Y., Zhang K., Peng X. gga-miR-21 modulates Mycoplasma gallisepticum (HS strain)-Induced inflammation via targeting MAP3K1 and activating MAPKs and NF-κB pathways. Vet. Microbiol. 2019;237:108407. doi: 10.1016/j.vetmic.2019.108407. [DOI] [PubMed] [Google Scholar]
- 287.Zhang K., Han Y., Wang Z., Zhao Y., Fu Y., Peng X. gga-miR-146c Activates TLR6/MyD88/NF-κB Pathway through Targeting MMP16 to Prevent Mycoplasma Gallisepticum (HS Strain) Infection in Chickens. Cells. 2019;8:501. doi: 10.3390/cells8050501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhang K., Han Y., Zhao Y., Sun Y., Zou M., Fu Y., Peng X. Upregulated gga-miR-16-5p Inhibits the Proliferation Cycle and Promotes the Apoptosis of MG-Infected DF-1 Cells by Repressing PIK3R1-Mediated the PI3K/Akt/NF-κB Pathway to Exert Anti-Inflammatory Effect. Int. J. Mol. Sci. 2019;20:1036. doi: 10.3390/ijms20051036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Yuan B., Zou M., Zhao Y., Zhang K., Sun Y., Peng X. Up-Regulation of miR-130b-3p Activates the PTEN/PI3K/AKT/NF-κB Pathway to Defense against Mycoplasma gallisepticum (HS Strain) Infection of Chicken. Int. J. Mol. Sci. 2018;19:2172. doi: 10.3390/ijms19082172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Yu Y., Chen Y., Wang Y., Li Y., Zhang L., Xin J. TLR2/MyD88/NF-κB signaling pathway regulates IL-1β production in DF-1cells exposed to Mycoplasma gallisepticum LAMPs. Microb. Pathog. 2018;117:225–231. doi: 10.1016/j.micpath.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 291.Beaudet J., Tulman E.R., Pflaum K., Liao X., Kutish G.F., Szczepanek S.M., Silbart L.K., Geary S.J. Transcriptional Profiling of the Chicken Tracheal Response to Virulent Mycoplasma gallisepticum Strain Rlow. Infect. Immun. 2017;85:e00343-17. doi: 10.1128/IAI.00343-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zou M., Yang W., Niu L., Sun Y., Luo R., Wang Y., Peng X. Polydatin attenuates Mycoplasma gallisepticum (HS strain)-induced inflammation injury via inhibiting the TLR6/ MyD88/NF-κB pathway. Microb. Pathog. 2020;149:104552. doi: 10.1016/j.micpath.2020.104552. [DOI] [PubMed] [Google Scholar]
- 293.Wu Z., Chen C., Miao Y., Liu Y., Zhang Q., Li R., Ding L., Ishfaq M., Li J. Baicalin Attenuates Mycoplasma gallisepticum-Induced Inflammation via Inhibition of the TLR2-NF-κB Pathway in Chicken and DF-1 Cells. Infect. Drug Resist. 2019;12:3911–3923. doi: 10.2147/IDR.S231908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ishfaq M., Chen C., Bao J., Zhang W., Wu Z., Wang J., Liu Y., Tian E., Hamid S., Li R., et al. Baicalin ameliorates oxidative stress and apoptosis by restoring mitochondrial dynamics in the spleen of chickens via the opposite modulation of NF-κB and Nrf2/HO-1 signaling pathway during Mycoplasma gallisepticum infection. Poult. Sci. 2019;98:6296–6310. doi: 10.3382/ps/pez406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zou M., Yang L., Niu L., Zhao Y., Sun Y., Fu Y., Peng X. Baicalin ameliorates Mycoplasma gallisepticum-induced lung inflammation in chicken by inhibiting TLR6-mediated NF-κB signalling. Br. Poult. Sci. 2020 doi: 10.1080/00071668.2020.1847251. in press. [DOI] [PubMed] [Google Scholar]
- 296.Niu L., Luo R., Zou M., Sun Y., Fu Y., Wang Y., Peng X. Puerarin inhibits Mycoplasma gallisepticum (MG-HS)-induced inflammation and apoptosis via suppressing the TLR6/MyD88/NF-κB signal pathway in chicken. Int. Immunopharmacol. 2020;88:106993. doi: 10.1016/j.intimp.2020.106993. [DOI] [PubMed] [Google Scholar]
- 297.Fatoba A.J., Adeleke M.A. Diagnosis and control of chicken coccidiosis: A recent update. J. Parasit. Dis. 2018;42:483–493. doi: 10.1007/s12639-018-1048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Jin H., Haicheng Y., Caiyun Z., Yong Z., Jinrong W. The Expression of NF-kB Signaling Pathway Was Inhibited by Silencing TGF-b4 in Chicken IECs Infected with E. tenella. Braz. J. Poult. Sci. 2020 doi: 10.1590/1806-9061-2020-1338. in press. [DOI] [Google Scholar]
- 299.Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. (Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- 300.Mora Z.V., Macías-Rodríguez M.E., Arratia-Quijada J., Gonzalez-Torres Y.S., Nuño K., Villarruel-López A. Clostridium perfringens as Foodborne Pathogen in Broiler Production: Pathophysiology and Potential Strategies for Controlling Necrotic Enteritis. Animals. 2020;10:1718. doi: 10.3390/ani10091718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Van Immerseel F., De Buck J., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- 302.Guo S., Li C., Liu D., Guo Y. Inflammatory responses to a Clostridium perfringens type A strain and α-toxin in primary intestinal epithelial cells of chicken embryos. Avian Pathol. 2015;44:81–91. doi: 10.1080/03079457.2015.1005573. [DOI] [PubMed] [Google Scholar]
- 303.Athanasiadou S., Russell K.M., Kaiser P., Kanellos T., Burgess S.T., Mitchell M., Clutton E., Naylor S.W., Low C.J., Hutchings M.R., et al. Genome wide transcriptomic analysis identifies pathways affected by the infusion of Clostridium perfringens culture supernatant in the duodenum of broilers in situ. J. Anim. Sci. 2015;93:3152–3163. doi: 10.2527/jas.2014-8597. [DOI] [PubMed] [Google Scholar]
- 304.Guo S., Liu D., Zhang B., Li Z., Li Y., Ding B., Guo Y. Two Lactobacillus Species Inhibit the Growth and α-Toxin Production of Clostridium perfringens and Induced Proinflammatory Factors in Chicken Intestinal Epithelial Cells in Vitro. Front. Microbiol. 2017;8:2081. doi: 10.3389/fmicb.2017.02081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Li Z., Wang W., Liu D., Guo Y. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2018;9:25. doi: 10.1186/s40104-018-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Knittler M.R., Sachse K. Chlamydia psittaci: Update on an underestimated zoonotic agent. Pathog. Dis. 2015;73:1–15. doi: 10.1093/femspd/ftu007. [DOI] [PubMed] [Google Scholar]
- 307.Chu J., Li X., Qu G., Wang Y., Li Q., Guo Y., Hou L., Liu J., Eko F.O., He C. Chlamydia psittaci PmpD-N Exacerbated Chicken Macrophage Function by Triggering Th2 Polarization and the TLR2/MyD88/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2020;21:2003. doi: 10.3390/ijms21062003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Chen Q., Li Y., Yan X., Sun Z., Wang C., Liu S., Xiao J., Lu C., Wu Y. Chlamydia psittaci Plasmid-Encoded CPSIT_P7 Elicits Inflammatory Response in Human Monocytes via TLR4/Mal/MyD88/NF-κB Signaling Pathway. Front Microbiol. 2020;11:578009. doi: 10.3389/fmicb.2020.578009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.López-Osorio S., Chaparro-Gutiérrez J.J., Gómez-Osorio L.M. Overview of Poultry Eimeria Life Cycle and Host-Parasite Interactions. Front. Vet. Sci. 2020 doi: 10.3389/fvets.2020.00384. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Rehman Z.U., Meng C., Sun Y., Safdar A., Pasha R.H., Munir M., Ding C. Oxidative Stress in Poultry: Lessons from the Viral Infections. Oxid. Med. Cell Longev. 2018;2018:5123147. doi: 10.1155/2018/5123147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Khatri M., Sharma J.M. Infectious bursal disease virus infection induces macrophage activation via p38 MAPK and NF-kappaB pathways. Virus Res. 2006;118:70–77. doi: 10.1016/j.virusres.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 312.Wong R.T., Hon C.C., Zeng F., Leung F.C. Screening of differentially expressed transcripts in infectious bursal disease virus-induced apoptotic chicken embryonic fibroblasts by using cDNA microarrays. J. Gen. Virol. 2007;88:1785–1796. doi: 10.1099/vir.0.82619-0. [DOI] [PubMed] [Google Scholar]
- 313.Cubas-Gaona L.L., Diaz-Beneitez E., Ciscar M., Rodríguez J.F., Rodríguez D. Exacerbated Apoptosis of Cells Infected with Infectious Bursal Disease Virus upon Exposure to Interferon Alpha. J. Virol. 2018;92:e00364-18. doi: 10.1128/JVI.00364-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ou C., Wang Q., Wei X., Liu M., Liu X., He C. Pro-apoptosis effects of protocatechuic acid in the early stage of infectious bursal disease virus infection. Microb. Pathog. 2018;124:216–222. doi: 10.1016/j.micpath.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 315.Bello M.B., Yusoff K., Ideris A., Hair-Bejo M., Peeters B.P.H., Omar A.R. Diagnostic and Vaccination Approaches for Newcastle Disease Virus in Poultry: The Current and Emerging Perspectives. Biomed. Res. Int. 2018;2018:7278459. doi: 10.1155/2018/7278459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Mayers J., Mansfield K.L., Brown I.H. The role of vaccination in risk mitigation and control of Newcastle disease in poultry. Vaccine. 2017;35:5974–5980. doi: 10.1016/j.vaccine.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 317.Qu Y., Zhan Y., Yang S., Ren S., Qiu X., Rehamn Z.U., Tan L., Sun Y., Meng C., Song C., et al. Newcastle disease virus infection triggers HMGB1 release to promote the inflammatory response. Virology. 2018;525:19–31. doi: 10.1016/j.virol.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 318.Park J., Kim M., Na G., Jeon I., Kwon Y.K., Kim J.H., Youn H., Koo Y. Glucocorticoids modulate NF-kappaB-dependent gene expression by up-regulating FKBP51 expression in Newcastle disease virus-infected chickens. Mol. Cell. Endocrinol. 2007;278:7–17. doi: 10.1016/j.mce.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 319.Qian J., Xu X., Ding J., Yin R., Sun Y., Xue C., Wang J., Ding C., Yu S., Liu X., et al. Newcastle disease virus-like particles induce DC maturation through TLR4/NF-κB pathway and facilitate DC migration by CCR7-CCL19/CCL21 axis. Vet. Microbiol. 2017;203:158–166. doi: 10.1016/j.vetmic.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 320.Chen Y., Liu W., Xu H., Liu J., Deng Y., Cheng H., Zhan T., Lu X., Liao T., Guo L., et al. Gga-miR-19b-3p Inhibits Newcastle Disease Virus Replication by Suppressing Inflammatory Response via Targeting RNF11 and ZMYND11. Front Microbiol. 2019;10:2006. doi: 10.3389/fmicb.2019.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Li J.J., Yin Y., Yang H.L., Yang C.W., Yu C.L., Wang Y., Yin H.D., Lian T., Peng H., Zhu Q., et al. mRNA expression and functional analysis of chicken IFIT5 after infected with Newcastle disease virus. Infect. Genet. Evol. 2020;86:104585. doi: 10.1016/j.meegid.2020.104585. [DOI] [PubMed] [Google Scholar]
- 322.Bertzbach L.D., Conradie A.M., You Y., Kaufer B.B. Latest Insights into Marek’s Disease Virus Pathogenesis and Tumorigenesis. Cancers. 2020;12:647. doi: 10.3390/cancers12030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kumar S., Kunec D., Buza J.J., Chiang H.I., Zhou H., Subramaniam S., Pendarvis K., Cheng H.H., Burgess S.C. Nuclear Factor kappa B is central to Marek’s disease herpesvirus induced neoplastic transformation of CD30 expressing lymphocytes in-vivo. BMC Syst. Biol. 2012;6:123. doi: 10.1186/1752-0509-6-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Liu Y., Gao L., Xu Z., Luo D., Zhang Y., Gao Y., Liu C., Zhang Y., Qi X., Cui H., et al. Marek’s Disease Virus RLORF4 Inhibits Type I Interferon Production by Antagonizing NF-κB Activation. J. Virol. 2019;93:e01037-19. doi: 10.1128/JVI.01037-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Benavente J., Martínez-Costas J. Avian reovirus: Structure and biology. Virus Res. 2007;123:105–119. doi: 10.1016/j.virusres.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 326.De Carli S., Wolf J.M., Gräf T., Lehmann F.K., Fonseca A.S., Canal C.W., Lunge V.R., Ikuta N. Genotypic characterization and molecular evolution of avian reovirus in poultry flocks from Brazil. Avian Pathol. 2020;49:611–620. doi: 10.1080/03079457.2020.1804528. [DOI] [PubMed] [Google Scholar]
- 327.Lin P.Y., Liu H.J., Liao M.H., Chang C.D., Chang C.I., Cheng H.L., Lee J.W., Shih W.L. Activation of PI 3-kinase/Akt/NF-kappaB and Stat3 signaling by avian reovirus S1133 in the early stages of infection results in an inflammatory response and delayed apoptosis. Virology. 2010;400:104–114. doi: 10.1016/j.virol.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 328.Xie L., Xie Z., Wang S., Huang J., Deng X., Xie Z., Luo S., Zeng T., Zhang Y., Zhang M. Altered gene expression profiles of the MDA5 signaling pathway in peripheral blood lymphocytes of chickens infected with avian reovirus. Arch. Virol. 2019;164:2451–2458. doi: 10.1007/s00705-019-04340-8. [DOI] [PubMed] [Google Scholar]
- 329.Yun T., Hua J., Ye W., Ni Z., Chen L., Zhang C. The phosphoproteomic responses of duck (Cairna moschata) to classical/novel duck reovirus infections in the spleen tissue. Sci. Rep. 2020;10:15315. doi: 10.1038/s41598-020-72311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Campbell L.K., Magor K.E. Pattern Recognition Receptor Signaling and Innate Responses to Influenza A Viruses in the Mallard Duck, Compared to Humans and Chickens. Front. Cell. Infect. Microbiol. 2020;10:209. doi: 10.3389/fcimb.2020.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Li N., Zhao J., Yang Y., Zeng Y., Liu S. Innate immune responses to duck Tembusu virus infection. Vet. Res. 2020;51:87. doi: 10.1186/s13567-020-00814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Nettleford S.K., Prabhu K.S. Selenium and Selenoproteins in Gut Inflammation-A Review. Antioxidants. 2018;7:36. doi: 10.3390/antiox7030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zhang J.L., Xu B., Huang X.D., Gao Y.H., Chen Y., Shan A.S. Selenium Deficiency Affects the mRNA Expression of Inflammatory Factors and Selenoprotein Genes in the Kidneys of Broiler Chicks. Biol. Trace Elem. Res. 2016;171:201–207. doi: 10.1007/s12011-015-0512-3. [DOI] [PubMed] [Google Scholar]
- 334.Liu Z., Qu Y., Wang J., Wu R. Selenium Deficiency Attenuates Chicken Duodenal Mucosal Immunity via Activation of the NF-κb Signaling Pathway. Biol. Trace Elem. Res. 2016;172:465–473. doi: 10.1007/s12011-015-0589-8. [DOI] [PubMed] [Google Scholar]
- 335.Wang J., Liu Z., He X., Lian S., Liang J., Yu D., Sun D., Wu R. Selenium deficiency induces duodenal villi cell apoptosis via an oxidative stress-induced mitochondrial apoptosis pathway and an inflammatory signaling-induced death receptor pathway. Metallomics. 2018;10:1390–1400. doi: 10.1039/C8MT00142A. [DOI] [PubMed] [Google Scholar]
- 336.Zhang R., Guo R., Liu Q., Li G., Sun B., Huang X. Selenium Deficiency via the TLR4/TRIF/NF-κB Signaling Pathway Leading to Inflammatory Injury in Chicken Spleen. Biol. Trace Elem. Res. 2020 doi: 10.1007/s12011-020-02173-0. in press. [DOI] [PubMed] [Google Scholar]
- 337.Wang Y., Jiang L., He J., Hu M., Zeng F., Li Y., Tian H., Luo X. The Adverse Effects of Se Toxicity on Inflammatory and Immune Responses in Chicken Spleens. Biol. Trace Elem. Res. 2018;185:170–176. doi: 10.1007/s12011-017-1224-7. [DOI] [PubMed] [Google Scholar]
- 338.Huang H., Chen J., Sun Q., Liu Y., Tang Y., Teng X. NLRP3 inflammasome is involved in the mechanism of mitigative effect of selenium on lead-induced inflammatory damage in chicken kidneys. Environ. Sci. Pollut. Res. Int. 2020 doi: 10.1007/s11356-020-11322-w. in press. [DOI] [PubMed] [Google Scholar]
- 339.Jiayong Z., Shengchen W., Xiaofang H., Gang S., Shiwen X. The antagonistic effect of selenium on lead-induced necroptosis via MAPK/NF-κB pathway and HSPs activation in the chicken spleen. Ecotoxicol. Environ. Saf. 2020;204:111049. doi: 10.1016/j.ecoenv.2020.111049. [DOI] [PubMed] [Google Scholar]
- 340.Zhang J., Hao X., Xu S. Selenium Prevents Lead-Induced Necroptosis by Restoring Antioxidant Functions and Blocking MAPK/NF-κB Pathway in Chicken Lymphocytes. Biol. Trace Elem. Res. 2020;198:644–653. doi: 10.1007/s12011-020-02094-y. [DOI] [PubMed] [Google Scholar]
- 341.Wang X., Bao R., Fu J. The Antagonistic Effect of Selenium on Cadmium-Induced Damage and mRNA Levels of Selenoprotein Genes and Inflammatory Factors in Chicken Kidney Tissue. Biol. Trace Elem. Res. 2018;181:331–339. doi: 10.1007/s12011-017-1041-z. [DOI] [PubMed] [Google Scholar]
- 342.Zhang R., Liu Y., Xing L., Zhao N., Zheng Q., Li J., Bao J. The protective role of selenium against cadmium-induced hepatotoxicity in laying hens: Expression of Hsps and inflammation-related genes and modulation of elements homeostasis. Ecotoxicol. Environ. Saf. 2018;159:205–212. doi: 10.1016/j.ecoenv.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 343.Tang K.K., Li H.Q., Qu K.C., Fan R.F. Selenium alleviates cadmium-induced inflammation and meat quality degradation via antioxidant and anti-inflammation in chicken breast muscles. Environ. Sci. Pollut. Res. Int. 2019;26:23453–23459. doi: 10.1007/s11356-019-05675-0. [DOI] [PubMed] [Google Scholar]
- 344.Zhang W., Zhang R., Wang T., Jiang H., Guo M., Zhou E., Sun Y., Yang Z., Xu S., Cao Y., et al. Selenium inhibits LPS-induced pro-inflammatory gene expression by modulating MAPK and NF-κB signaling pathways in mouse mammary epithelial cells in primary culture. Inflammation. 2014;37:478–485. doi: 10.1007/s10753-013-9761-5. [DOI] [PubMed] [Google Scholar]
- 345.Liu J., Wang S., Zhang Q., Li X., Xu S. Selenomethionine alleviates LPS-induced chicken myocardial inflammation by regulating the miR-128-3p-p38 MAPK axis and oxidative stress. Metallomics. 2020;12:54–64. doi: 10.1039/C9MT00216B. [DOI] [PubMed] [Google Scholar]
- 346.Qu J., Wang W., Zhang Q., Li S. Inhibition of Lipopolysaccharide-Induced Inflammation of Chicken Liver Tissue by Selenomethionine via TLR4-NF-κB-NLRP3 Signaling Pathway. Biol. Trace Elem. Res. 2020;195:205–214. doi: 10.1007/s12011-019-01841-0. [DOI] [PubMed] [Google Scholar]
- 347.Shi X., Wang W., Zheng S., Zhang Q., Xu S. Selenomethionine relieves inflammation in the chicken trachea caused by LPS though inhibiting the NF-κB pathway. Biol. Trace Elem. Res. 2020;194:525–535. doi: 10.1007/s12011-019-01789-1. [DOI] [PubMed] [Google Scholar]
- 348.Tan J., Liu S., Guo Y., Applegate T.J., Eicher S.D. Dietary L-arginine supplementation attenuates lipopolysaccharide-induced inflammatory response in broiler chickens. Br. J. Nutr. 2014;111:1394–1404. doi: 10.1017/S0007114513003863. [DOI] [PubMed] [Google Scholar]
- 349.Bi Y., Nan X.M., Zheng S.S., Jiang L.S., Xiong B.H. Effects of dietary threonine and immune stress on growth performance, carcass trait, serum immune parameters, and intestinal muc2 and NF-κb gene expression in Pekin ducks from hatch to 21 days. Poult. Sci. 2018;97:177–187. doi: 10.3382/ps/pex283. [DOI] [PubMed] [Google Scholar]
- 350.Liu S.Q., Wang L.Y., Liu G.H., Tang D.Z., Fan X.X., Zhao J.P., Jiao H.C., Wang X.J., Sun S.H., Lin H. Leucine alters immunoglobulin a secretion and inflammatory cytokine expression induced by lipopolysaccharide via the nuclear factor-κB pathway in intestine of chicken embryos. Animal. 2018;12:1903–1911. doi: 10.1017/S1751731117003342. [DOI] [PubMed] [Google Scholar]
- 351.Surai P.F. Polyphenol compounds in the chicken/animal diet: From the past to the future. J. Anim. Physiol. Anim. Nutr. 2014;98:19–31. doi: 10.1111/jpn.12070. [DOI] [PubMed] [Google Scholar]
- 352.Surai P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants. 2015;4:204–247. doi: 10.3390/antiox4010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Zhang C., Tian Y., Yan F., Kang X., Han R., Sun G., Zhang H. Modulation of growth and immunity by dietary supplementation with resveratrol in young chickens receiving conventional vaccinations. Am. J. Vet. Res. 2014;75:752–759. doi: 10.2460/ajvr.75.8.752. [DOI] [PubMed] [Google Scholar]
- 354.Sahin K., Orhan C., Akdemir F.A., Tuzcu M., Iben C., Sahin N. Resveratrol protects quail hepatocytes against heat stress: Modulation of the Nrf2 transcription factor and heat shock proteins. J. Anim. Physiol. Anim. Nutr. 2012;96:66–74. doi: 10.1111/j.1439-0396.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 355.Fan H., Lv Z., Gan L., Guo Y. Transcriptomics-Related Mechanisms of Supplementing Laying Broiler Breeder Hens with Dietary Daidzein to Improve the Immune Function and Growth Performance of Offspring. J. Agric. Food Chem. 2018;66:2049–2060. doi: 10.1021/acs.jafc.7b06069. [DOI] [PubMed] [Google Scholar]
- 356.Yang J.X., Maria T.C., Zhou B., Xiao F.L., Wang M., Mao Y.J., Li Y. Quercetin improves immune function in Arbor Acre broilers through activation of NF-κB signaling pathway. Poult. Sci. 2020;99:906–913. doi: 10.1016/j.psj.2019.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Ge C., Zhang C., Ye J., Tang X., Wu Y. Ginbaltosides promote proliferation of chicken primordial germ cells via PKC-involved activation of NF-kappaB. Cell. Biol. Int. 2007;31:1251–1256. doi: 10.1016/j.cellbi.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 358.Lee M.T., Lin W.C., Wang S.Y., Lin L.J., Yu B., Lee T.T. Evaluation of potential antioxidant and anti-inflammatory effects of Antrodia cinnamomea powder and the underlying molecular mechanisms via Nrf2- and NF-κB-dominated pathways in broiler chickens. Poult. Sci. 2018;97:2419–2434. doi: 10.3382/ps/pey076. [DOI] [PubMed] [Google Scholar]
- 359.Chen H., Muhammad I., Zhang Y., Ren Y., Zhang R., Huang X., Diao L., Liu H., Li X., Sun X., et al. Antiviral Activity Against Infectious Bronchitis Virus and Bioactive Components of Hypericum perforatum L. Front. Pharmacol. 2019;10:1272. doi: 10.3389/fphar.2019.01272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Yu M., Ge C., Zeng W., Mi Y., Zhang C. Retinoic acid promotes proliferation of chicken primordial germ cells via activation of PI3K/Akt-mediated NF-κB signalling cascade. Cell Biol. Int. 2012;36:705–712. doi: 10.1042/CBI20110542. [DOI] [PubMed] [Google Scholar]
- 361.Zhang X., Zhong X., Zhou Y., Wang G., Du H., Wang T. Dietary RRR-α-tocopherol succinate attenuates lipopolysaccharide-induced inflammatory cytokines secretion in broiler chicks. Br. J. Nutr. 2010;104:1796–1805. doi: 10.1017/S0007114510002801. [DOI] [PubMed] [Google Scholar]
- 362.Zhan T., Han Y., Tang C., Zhao Q., Sun D., Li Y., Jia X., Zhou L., Zhang J. Metabolism and biological activity of α-tocopherol derived from vitamin E-enriched transgenic maize in broilers. J. Sci. Food. Agric. 2020;100:4319–4328. doi: 10.1002/jsfa.10480. [DOI] [PubMed] [Google Scholar]
- 363.Ma Z., Zhang J., Ma H., Dai B., Zheng L., Miao J., Zhang Y. The influence of dietary taurine and reduced housing density on hepatic functions in laying hens. Poult. Sci. 2014;93:1724–1736. doi: 10.3382/ps.2013-03654. [DOI] [PubMed] [Google Scholar]
- 364.Dai B., Zhang Y.S., Ma Z.L., Zheng L.H., Li S.J., Dou X.H., Gong J.S., Miao J.F. Influence of dietary taurine and housing density on oviduct function in laying hens. J. Zhejiang Univ.-SCIENCE B. 2015;16:456–464. doi: 10.1631/jzus.B1400256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Ma Z.L., Gao Y., Ma H.T., Zheng L.H., Dai B., Miao J.F., Zhang Y.S. Effects of taurine and housing density on renal function in laying hens. J. Zhejiang Univ.-SCIENCE B. 2016;17:952–964. doi: 10.1631/jzus.B1600014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Wu Y., Zhen W., Geng Y., Wang Z., Guo Y. Pretreatment with probiotic Enterococcus faecium NCIMB 11181 ameliorates necrotic enteritis-induced intestinal barrier injury in broiler chickens. Sci. Rep. 2019;9:10256. doi: 10.1038/s41598-019-46578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sun Y., Qian J., Xu X., Tang Y., Xu W., Yang W., Jiang Y., Yang G., Ding Z., Cong Y., et al. Dendritic cell-targeted recombinant Lactobacilli induce DC activation and elicit specific immune responses against G57 genotype of avian H9N2 influenza virus infection. Vet. Microbiol. 2018;223:9–20. doi: 10.1016/j.vetmic.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 368.Lan D., Xun X., Hu Y., Li N., Yang C., Jiang X., Liu Y. Research on the Effect of Pediococcus pentosaceus on Salmonella enteritidis-Infected Chicken. BioMed Res. Int. 2020;2020:6416451. doi: 10.1155/2020/6416451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Weavers H., Martin P. The cell biology of inflammation: From common traits to remarkable immunological adaptations. J. Cell Biol. 2020;219:e202004003. doi: 10.1083/jcb.202004003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Pflug K.M., Sitcheran R. Targeting NF-κB-Inducing Kinase (NIK) in Immunity, Inflammation, and Cancer. Int. J. Mol. Sci. 2020;21:8470. doi: 10.3390/ijms21228470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.de Gregorio E., Colell A., Morales A., Marí M. Relevance of SIRT1-NF-κB Axis as Therapeutic Target to Ameliorate Inflammation in Liver Disease. Int. J. Mol. Sci. 2020;21:3858. doi: 10.3390/ijms21113858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Zhou Y., Cui C., Ma X., Luo W., Zheng S.G., Qiu W. Nuclear Factor κB (NF-κB)-Mediated Inflammation in Multiple Sclerosis. Front. Immunol. 2020;11:391. doi: 10.3389/fimmu.2020.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Afonina I.S., Zhong Z., Karin M., Beyaert R. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat. Immunol. 2017;18:861–869. doi: 10.1038/ni.3772. [DOI] [PubMed] [Google Scholar]
- 374.Kunnumakkara A.B., Shabnam B., Girisa S., Harsha C., Banik K., Devi T.B., Choudhury R., Sahu H., Parama D., Sailo B.L., et al. Inflammation, NF-κB, and Chronic Diseases: How are They Linked? Crit. Rev. Immunol. 2020;40:1–39. doi: 10.1615/CritRevImmunol.2020033210. [DOI] [PubMed] [Google Scholar]
- 375.Serhan C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017;31:1273–1288. doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Rothschild D.E., McDaniel D.K., Ringel-Scaia V.M., Allen I.C. Modulating inflammation through the negative regulation of NF-κB signaling. J. Leukoc. Biol. 2018;103:1131–1150. doi: 10.1002/JLB.3MIR0817-346RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kogut M.H., Genovese K.J., Swaggerty C.L., He H., Broom L. Inflammatory phenotypes in the intestine of poultry: Not all inflammation is created equal. Poult. Sci. 2018;97:2339–2346. doi: 10.3382/ps/pey087. [DOI] [PubMed] [Google Scholar]
- 378.Julian R.J. Production and growth-related disorders and other metabolic diseases of poultry—A review. Vet. J. 2005;169:350–369. doi: 10.1016/j.tvjl.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 379.Kierończyk B., Rawski M., Józefiak D., Świątkiewicz S. Infectious and non-infectious factors associated with leg disorders in poultry—A review. Ann. Anim. Sci. 2017;17:645–669. doi: 10.1515/aoas-2016-0098. [DOI] [Google Scholar]
- 380.Ducatelle R., Goossens E., De Meyer F., Eeckhaut V., Antonissen G., Haesebrouck F., Van Immerseel F. Biomarkers for monitoring intestinal health in poultry: Present status and future perspectives. Vet. Res. 2018;49:43. doi: 10.1186/s13567-018-0538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Golonka R.M., Xiao X., Abokor A.A., Joe B., Vijay-Kumar M. Altered nutrient status reprograms host inflammation and metabolic health via gut microbiota. J. Nutr. Biochem. 2020 doi: 10.1016/j.jnutbio.2020.108360. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Yang Q., Wang Y., Jia A., Wang Y., Bi Y., Liu G. The crosstalk between gut bacteria and host immunity in intestinal inflammation. J. Cell. Physiol. 2020 doi: 10.1002/jcp.30024. in press. [DOI] [PubMed] [Google Scholar]
- 383.Tilg H., Zmora N., Adolph T.E., Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020;20:40–54. doi: 10.1038/s41577-019-0198-4. [DOI] [PubMed] [Google Scholar]
- 384.Broom L.J. Host–Microbe Interactions and Gut Health in Poultry—Focus on Innate Responses. Microorganisms. 2019;7:139. doi: 10.3390/microorganisms7050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Oviedo-Rondón E.O. Holistic view of intestinal health in poultry. Anim. Feed Sci. Technol. 2019;250:1–8. doi: 10.1016/j.anifeedsci.2019.01.009. [DOI] [Google Scholar]
- 386.Rius-Pérez S., Pérez S., Martí-Andrés P., Monsalve M., Sastre J. NF-κB Signaling complexes in acute inflammation. Antioxid. Redox Signal. 2019 doi: 10.1089/ars.2019.7975. in press. [DOI] [PubMed] [Google Scholar]
- 387.Farooqui A.A. Inflammation and Oxidative Stress in Neurological Disorders. Springer; Cham, Switzerland; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK: 2014. [Google Scholar]
- 388.Kircheis R., Haasbach E., Lueftenegger D., Heyken W.T., Ocker M., Planz O. NF-κB Pathway as a Potential Target for Treatment of Critical Stage COVID-19 Patients. Front. Immunol. 2020;11:598444. doi: 10.3389/fimmu.2020.598444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Sha W.C., Liou H.C., Tuomanen E.I., Baltimore D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 390.Surai P.F. Antioxidants in Poultry Nutrition and Reproduction: An Update. Antioxidants. 2020;9:105. doi: 10.3390/antiox9020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Surai P.F., Kochish I.I., Kidd M.T. Taurine in Poultry Nutrition. Anim. Feed Sci. Technol. 2020;260:114339. doi: 10.1016/j.anifeedsci.2019.114339. [DOI] [Google Scholar]
- 392.Surai P.F. Antioxidant action of carnitine: Molecular mechanisms and practical applications. EC Vet. Sci. 2015;2:66–84. [Google Scholar]
- 393.Surai P.F. Carnitine enigma: From antioxidant action to vitagene regulation. Part 1. Absorption, metabolism and antioxidant activities. J. Vet. Sci. Med. 2015;3:14. doi: 10.13188/2325-4645.1000017. [DOI] [Google Scholar]
- 394.Surai P.F. Carnitine enigma: From antioxidant action to vitagene regulation. Part 2. Transcription factors and practical applications. J. Vet. Sci. Med. 2015;3:17. doi: 10.13188/2325-4645.1000018. [DOI] [Google Scholar]