Abstract
Mycotoxins represent an assorted range of secondary fungal metabolites that extensively occur in numerous food and feed ingredients at any stage during pre- and post-harvest conditions. Zearalenone (ZEN), a mycotoxin categorized as a xenoestrogen poses structural similarity with natural estrogens that enables its binding to the estrogen receptors leading to hormonal misbalance and numerous reproductive diseases. ZEN is mainly found in crops belonging to temperate regions, primarily in maize and other cereal crops that form an important part of various food and feed. Because of the significant adverse effects of ZEN on both human and animal, there is an alarming need for effective detection, mitigation, and management strategies to assure food and feed safety and security. The present review tends to provide an updated overview of the different sources, occurrence and biosynthetic mechanisms of ZEN in various food and feed. It also provides insight to its harmful effects on human health and agriculture along with its effective detection, management, and control strategies.
Keywords: zearalenone, food and feed contamination, health issues, management strategies
1. Introduction
Extensive concerns have been raised over the years about the presence of fungal secondary metabolites in food and feed [1,2]. Amongst these secondary metabolites, mycotoxins are comprised of toxic metabolites of filamentous fungi that are chiefly produced by Aspergillus, Fusarium, and Penicillium species [3]. Mycotoxin contamination in food and feed cause acute and chronic mycotoxicosis, including teratogenic, carcinogenic, oestrogenic, neurotoxic, and immunosuppressive effects. Mycotoxins also serve as a crucial factor governing the safety for human consumption as they pose a serious threat to microbiological food safety and human health [3,4,5]. The incident of mycotoxin contamination may occur at any stages of culturing, harvesting and storage [6]. It is more protruding in areas with inefficient control over food quality, deprived production technologies, and poor storage surroundings that accelerate fungal growth and toxin production. Contamination of food and feed with mycotoxins has shown inevitable effects and raised widespread threats due to their less susceptible nature to any physical, chemical or thermal treatment [7].
Zearalenone (ZEN) is primarily produced by Fusarium graminearum and Fusarium culmorum and predominantly occurs in maize and other grain crops [7,8]. These species of Fusarium are generally found on plants primarily grown in temperate regions and contaminate foods of both plant and animal origin [1]. ZEN represents xenoestrogens having a chemical structure analogous to natural estrogens that permits its binding with estrogenic receptor sites leading to amplified estrogenicity. Exposure to this contaminant is accompanied by reduced levels of progesterone and serum testosterone in the bloodstream resulting in infertility and reduced incidences of pregnancy in animals like cows, pigs and rats [9,10]. It has also been shown to exert immunotoxic effects at low concentration levels. ZEN toxicity brings about numerous changes in the target cells by altering various metabolic events such as cell proliferation and apoptosis [11]. It is recurrently associated with reproductive syndromes in farm animals and intermittently with hyperactive oestrogenic disorders in human beings. There are numerous attestations of the fact that ZEN and its metabolites exert oestrogenic effects in pigs, sheep, and cattle amongst which pigs are the most susceptible to ZEN toxicity [12,13]. ZEN has been categorized as a Group 3 carcinogen by the International Agency for Research on Cancer (IARC) due to its unclassifiable carcinogenicity to humans with inadequate evidence [10]. However, owing to its continual incidence and extensive damage to both human and animal health, there is a need to adopt effective management strategies to control ZEN toxicity [14].
Insight of various immunotoxic and genotoxic effects of ZEN and its derivatives on human and animal health, there is an alarming attention towards the development of efficient and effective mitigation strategies against ZEN contamination [15]. As good storage and transportation facilities of agricultural commodities are not adequate to entirely hinder the occurrence of mycotoxin contamination in the food and feed chain, it is essential to embrace decent detection and decontamination strategies to mitigate the health risk and monetary losses [16,17,18]. This review tends to upgrade the information on various sources, chemistry, biosynthesis and occurrence of ZEN in food and feed. In addition to this, its impact on agriculture and human health along with probable detection and management strategies to safeguard the safety of food and feed will also be addressed in brief.
2. Major Source of Zearalenone
Fungal contamination and the subsequent production of mycotoxins are intrinsic to several food and feed across the globe [17,19]. Maize and other cereal crops such as barley, oats, rice, sorghum, rye and wheat, forming a comparatively major fragment of animal feed are primarily more susceptible to ZEN contamination [20]. Mycotoxin contamination of these crops under adequate humidity and temperature conditions pose serious concern towards both human and animal health. Also, foods prepared using the contaminated plant and animal products such as milk and meat products pose the utmost threat of mycotoxin contamination [21,22]. ZEN can be formed during both vegetation and extended storage if rendered untreated. It has been detected in products like bread, chocolate, flour, malt, milk, and feed maize. Given its hasty biotransformation and excretion by animals, the ingestion of this toxin together with meat is not very substantial [1]. Grains and vegetable protein in the animal feed serve as an essential source of nutrient for fungal growth rendering animal feed safety at risk [13]. The formation of mycotoxins in feed generally occurs during the pre-harvest stage and under inappropriate storage conditions [23]. Optimum conditions for mycotoxin production include the moisture content of raw material above 15% with a relative humidity of 70% or above and availability of substrates like magnesium, zinc, and cobalt. Other factors such as pH, optimum temperature (20–30 °C) and availability of oxygen also affect fungal growth [1].
3. Chemistry and Biosynthesis of Zearalenone
Zearalenone (earlier known as F-2 toxin) is a non-steroidal oestrogenic mycotoxin, chemically described as 6-[10-hydroxy-6-oxo-trans-1-undecenyl]-β-resorcyclic acid lactone. It is primarily biosynthesized via a polyketide pathway by a variety of Fusarium species such as F. graminearum, F. culmorum, and F. cerealis [12]. ZEN is formed as a result of successive reactions catalyzed by numerous multi-enzyme protein complexes that comprise polyketide synthases (PKSs). There are 15 PKSs that have been revealed in F. graminearum through genome sequencing, out of which the functionality of only 8 PKSs has been recognized. Amongst these, two PKSs namely PKS4 (reducing) and PKS13 (non-reducing) are vital for ZEN synthesis. These fungal PKS genes usually exist as a cluster that encrypts transcription factors, metabolic enzymes and transporters [24]. Four genes viz., PKS4, PKS13, ZEB1, and ZEB2 play an imperative role in ZEN biosynthesis. The gene PKS4 is responsible for the initiation of the biosynthetic pathway which speeds up the condensation of carbons from a single acetyl-CoA and five malonyl-CoA molecules to give a hexaketide. Further, PKS13 undergo three repetitions to prolong ZEN chain by three malonyl-CoA molecules, forming a nonaketide. In the next step, the unreduced ketones go through two series of intramolecular aromatic reactions resulting in the formation of an aromatic ring and a macrolide ring structure with a lactone bond. The final stage is catalyzed by ZEB1 to transform ZEL to ZEN [25].
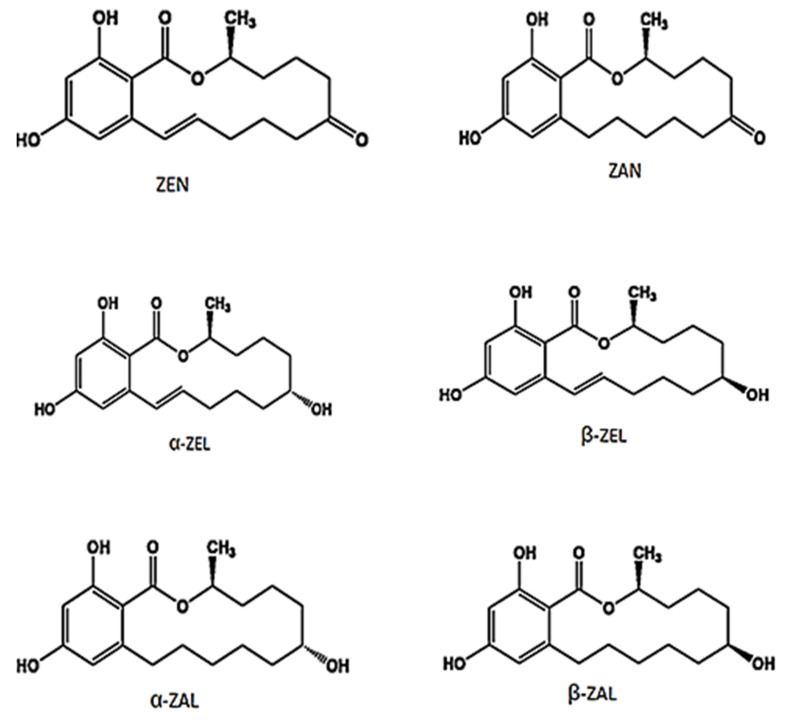
The metabolism of ZEN is poorly understood in humans [26], however, studies in animal model suggest that ZEN is metabolized primarily to α-zearalenol (α-ZEL) and β-zearalenol (β-ZEL) [27,28] and the ratio of their concentrations is dependent on the type of animal species. It is further reported that α-ZEL and β-ZEL may reduce to α-zearalanol (α-ZAL) and β-zearalanol (β-ZAL) [29,30]. α-ZAL is metabolized predominantly into β-ZAL and, to a lesser extent, into zearalanone (ZAN) [31]. The chemical structure of ZEN and its different metabolites are presented in Figure 1.
Figure 1.
Chemical structure of zearalenone (ZEN) and its different metabolites.
ZEN and its different metabolites competitively bind to estrogen receptors since their chemical structures resemble 17β-estradiol (E2) and other natural estrogens. Shier et al. [32] determined the relative estrogenicity of ZEN and its different metabolites compared to E2 (92% for α-ZEL, 18% for α-ZAL, 3.5% for β-ZAL, 1% for ZEN, and 0.44% for β-ZEL) based on a proliferation assay on MCF7 human breast cells. Further, α-ZEL compounds are 2–4 times as estrogenic as ZEN and β-ZEL [33,34]. Moreover, α-ZEL was reported even to be 17 times as strong as α-ethynyl estradiol based on estrogen receptor gene activation bioassays [35] and the relative binding affinities to estrogen receptors was in decreasing order: α-ZEL, ZEN, and β-ZEL [36,37,38].
4. Genes Responsible for Zearalenone Production
In fungal species, the genes responsible for the biosynthesis of secondary metabolites exist as clusters of one or more regulatory genes. The majority of clusters involved in polyketide biosynthesis comprises of a single PKS gene and numerous gene encrypting enzymes [39]. ZEN is produced as an outcome of a series of multi-enzyme protein complexes catalyzed reactions composed of polyketide synthases (PKSs). These fungal PKSs are large multi-domain enzymes (type-I PKSs) with a repetitious function. Four adjacent genes viz., PKS4, PKS13, ZEB1 and ZEB2 as stated earlier are essential for the biosynthesis of ZEN biosynthesis and form a gene cluster [25]. Among these, a non-reducing PKS13 is utilized during the biosynthesis due to the existence of ketone functional groups (as enol in resorcinol ring) in ZEN [40]. On the other hand, a reducing PKS4 gene of F. graminearum is crucial for the formation of ZEN [41] as it catalyzes a vital step in ZEN biosynthetic pathway and also regulates the expression of other genes indulged in the process [42]. ZEN is primarily a polyketide that is solely synthesized from acetate-malonate fragments. The inhibitory effect on ZEN production can be exerted by diminishing the mycelia biomass and through down-regulation of genes PKS4 and PKS13 that are responsible for ZEN synthesis [3].
5. Occurrence in Food and Feed
ZEN synthesized by various Fusarium species like F. cerealis, F. culmorum, F. crookwellense, F. equiseti, F. graminearum and F. semitectum are common contaminants of various food and feed worldwide [43]. ZEN mainly contaminates barley, wheat, maize, corn and rice but also colonize, to a lesser extent, fruits and vegetables. Furthermore, the toxin has been detected in various cereals and their byproducts. ZEN derivatives (α-ZEL, β-ZEL, α-ZAL and β-ZAL) can also be detected in various food and feed infected with Fusarium in the field [44]. The predominant feature of ZEN distribution in cereal grains and animal feed is its occurrence with other Fusarium toxins including trichothecenes and fumonisins [45]. The occurrence of ZEN in various food and feed around the world is presented in Table 1.
Table 1.
Occurrence of zearalenone in food and feed around the world.
| Food/Feed Matrix | Country | Range (μg/kg) | Detection Technique | Reference |
|---|---|---|---|---|
| Food | ||||
| Wheat | China | 10.1–3049 | LC–MS/MS | [56] |
| Malt | Botswana | 102–2213 | TLC and HPLC | [57] |
| Amaranthus | Argentina | 420–1980 | TLC | [58] |
| Cheese snacks | Iran | 1471 | HPLC-FD | [59] |
| Wheat | China | 5–1400 | HPLC | [60] |
| Corn byproducts | Germany | 369–1362 | GC-MS | [61] |
| Wheat | Romania | 327–1135 | LC-MS/MS | [62] |
| Corn | Thailand | 923 | HPLC-GC-MS | [63] |
| Corn flour | Iran | 889 | HPLC-FD | [59] |
| Corn | Germany | 48–860 | GC-MS | [61] |
| Maize | Tanzania | 729 | UHPLC/TOFMS | [64] |
| Corn | Brazil | 36.8–719 | LC-MS/MS | [65] |
| Corn | Brazil | 46.7–719 | HPLC | [66] |
| Barley | Brazil | 300–630 | LC-MS/MS | [67] |
| Durum wheat | Tunisia | 3–560 | HPLC | [68] |
| Corn | Philippines | 59–505 | HPLC-GC-MS | [63] |
| Maize | Italy | 453 | HPLC | [69] |
| Sorghum | USA | 443 | GC-MS | [70] |
| Wort | Botswana | 26–285 μg/L | TLC and HPLC | [57] |
| Wheat | Finland | 1.9–234 | LC-MS/MS | [71] |
| Wheat | Italy | 7–231 | HPLC-MS/MS | [72] |
| Maize | Iran | 100–212 | TLC | [73] |
| Soya meal | Germany | 51–211 | GC-MS | [61] |
| Beer | Botswana | 20–201 μg/L | TLC and HPLC | [57] |
| Barley foods | South Korea | 3.4–120 | ELISA and LC | [74] |
| Wheat | Germany | 17–104 | LC-MS | [75] |
| Wheat | Hungary | 50–98 | ELISA | [76] |
| Wheat | Kenya | 1–96 | ELISA | [77] |
| Corn foods | South Korea | 3.6–84 | ELISA and LC | [74] |
| Maize | Argentina | 0–83 | TLC | [78] |
| Oat | Finland | 76.9 | LC-MS/MS | [71] |
| Wheat bran | Germany | 3–67 | HPLC-FD | [79] |
| Barley | Czech Republic | 59.4 | HPLC-MS | [80] |
| Corn | Brazil | 55 | TLC | [81] |
| Maize | Italy | 53 | HPLC | [82] |
| Rice | South Korea | 21.7–47 | RP-HPLC-FLD | [83] |
| Paddy rice | Turkey | 42.9 | HPLC-PDA/HPLC-FLD | [20] |
| Barley malt | German federal states | 1.41–42.4 | LC-MS/MS | [84] |
| Barley | Lithuania | 10–41.4 | LC-MS/MS | [85] |
| Corn flour | UK | 6.5–40.8 | HPLC-FD | [86] |
| Maize | Botswana | 40 | HPLC | [87] |
| Wheat | Brazil | 40 | TLC | [81] |
| Corn flour | Germany | 2–40 | HPLC-FD | [79] |
| Maize | Poland | 18–39 | HPLC | [88] |
| Wheat | Syria | 4–34 | HPLC-MS/MS | [72] |
| Barley | Croatia | 32 | ELISA | [89] |
| Sorghum | Ethiopia | 19–32 | HPLC | [90] |
| Maize | Turkey | 28 | HPLC-PDA/HPLC-FLD | [20] |
| Breakfast cereal | Spain | 25 | HPLC | [91] |
| Oat | Germany | 21 | GC-MS | [61] |
| Wheat, corn, oat bran, oat products | Germany | 2–18 | HPLC-FD | [79] |
| Banana | India | 17 | HPLC | [92] |
| Red pepper | Germany | 2–17 | HPLC-FD | [79] |
| Maize | Morocco | 13.5–16.5 | HPLC | [93] |
| Chilli oil/chilli powder/chilli sauce | UK | 5–4/4.5–15.4/7.1 | HPLC-FD | [86] |
| Wheat | Germany | 15 | GC-MS | [61] |
| Gluten-free food | Germany | 2–14 | HPLC-FD | [79] |
| Barley | Finland | 13.7 | LC-MS/MS | [71] |
| Corn | Indonesia | 11–12 | HPLC-GC-MS | [94] |
| Milk | Egypt | 1.0–11.9 | HPLC–FLD | [21] |
| Curry powder | UK | 1.2–10.8 | HPLC-FD | [86] |
| Semolina | Germany | 2–9 | HPLC-FD | [79] |
| Garlic pickle | UK | 3–8 | HPLC-FD | [86] |
| Fennel | UK | 7 | HPLC-FD | [86] |
| Bean | Germany | 7 | HPLC-FD | [79] |
| Corn | Argentina | 3–7 | HPLC | [95] |
| Corn flakes | Qatar | 3.8–6.81 | HPLC | [96] |
| Coriander | UK | 3.6–6.7 | HPLC-FD | [86] |
| Canned foods | UK | 6.1 | HPLC-FD | [86] |
| Hazelnut | Germany | 6 | HPLC-FD | [79] |
| Corn | South Korea | 3.4–5.8 | ELISA and LC | [74] |
| Wheat | Sweden | 5 | HPLC/ESI-MS/MS | [97] |
| Pumpkin kernel | Germany | 4 | HPLC-FD | [79] |
| Sunflower seed | Germany | 2–4 | HPLC-FD | [79] |
| Wheat germ/corn flakes | Germany | 3/2–3 | HPLC-FD | [79] |
| Wheat flour | Turkey | 2.66 | HPLC-PDA/HPLC-FLD | [20] |
| Wheat | Qatar | 0.21–2.1 | HPLC | [96] |
| Potato products | Germany | 2 | HPLC-FD | [79] |
| Chicken meat | Pakistan | 0.85–1.83 | HPLC | [22] |
| Rice | Qatar | 0.18–1.4 | HPLC | [96] |
| Wheat | Turkey | 1.34 | HPLC-PDA/HPLC-FLD | [20] |
| Feed | ||||
| Complete feed | China | 10–3261.2 | HPLC-FD | [98] |
| Cow feeding stuffs | Argentina | 1200–3060 | HPLC | [99] |
| Pig complete feed (powder) | China | 10–835.4 | HPLC | [100] |
| Corn and poultry feed | Indonesia | 5.5–526 | ELISA and HPLC | [101] |
| Duck complete feed | China | 10–357.9 | HPLC | [100] |
| Cattle, poultry and swine feed | Poland | 0.07–349 | HPLC-MS/MS | [102] |
| Pig complete feed (pellets) | China | 10–329 | HPLC | [100] |
| Broiler feeds | Thailand | 2.22–263.51 | HPLC | [103] |
| Complete feed for pigs | Norway | 1.5–217.2 | HPLC-FLD | [104] |
| Swine feed | Hungary | 18–192 | ELISA | [105] |
| Sheep compound feed | Spain | 50–104.40 | UPLC–MS/MS and UPLC–QTOF–MS | [106] |
| Cattle compound feed | Spain | 88.2 | LC-MS | [107] |
| Poultry feed mixture | Slovakia | 3–86 | RP-HPLC-FLD | [108] |
| Fish feed | Europe | 67.9 | HPLC | [109] |
| Chicken feed | China | 61.59 | UPLC-MS/MS | [110] |
| Swine compound feed | Spain | 50 | UPLC–MS/MS and UPLC–QTOF–MS | [106] |
| Chicken feed | Botswana | 40 | HPLC | [87] |
| Pig feed | South Korea | 31.70 | HPLC | [13] |
| Poultry feed | South Korea | 0.24–26.80 | HPLC | [13] |
| Pig feed/cattle feed/rabbit feed | China | 18.78/14.43/10.46 | UPLC-MS/MS | [110] |
| Starter feed | India | 5.13–6.73 | HPLC and TLC | [111] |
| Compound feeds | South Africa | 0.56–1.85 | ELISA | [112] |
6. Effects on Agricultural Food and Feed
ZEN is found to cause contamination at different stages of food chain leading to adverse health effects in both human and animal [46,47]. ZEN is commonly found in animal feeds and grains stored improperly [48] which makes them undesirable for consumption [49]. The European Commission has limited the ZEN content in feed materials to 2 mg·kg−1, except that in maize, the proposed maximum limit is 3 mg·kg−1 [50]. A study by Jia et al. [51] observed the effect of two Fusarium toxins, ZEN and deoxynivalenol (DON), by feeding piglets with contaminated feed. The results showed intestinal inflammation, change in the population of gut microbiota and reduced expression of protein namely claudin-4, even when fed with a low dose of ZEN and DON. The deleterious effect of ZEN on pig’s health such as inflammation and changes in the morphology of intestine even with low doses has also been confirmed by other several studies [52,53,54]. Different ZEN derivatives such as α-ZEL and β-ZEL are found in crops like rice, soybean and maize besides their contamination in processed food products like flour, beer etc. [12,33]. Moreover, as processing methods are unable to completely degrade the toxin, therefore, a tolerable daily intake for human adults of 0.25 µg/kg by weight has been recommended by the European Food Safety Authority for ZEN. Further to minimize the health risk, the European Union has specified limits for ZEN in food products. For instance, the maximum permissible limit of ZEN in unprocessed cereals is 100–200 µg/kg while for processed cereals, the limit has been reduced to 75 µg/kg [55].
7. Effects of Environmental Factors on Zearalenone Production
The environmental conditions that affect the growth of Fusarium and thus the development of ZEN are precipitation, humidity, pH, temperature, composition of atmospheric gas and water activity (aw) [113]. aw is the ratio of water vapour pressure in food to pure water vapour, at constant temperature and pressure [114]. It is a key factor in determining the microbial load in food. Lowering aw in foods reduces microbial growth and chemical reactions, thereby, enhancing the shelf-life of food products [115]. Hence, aw is an indicator of water in food and its correlation with food quality, safety and stability [115].
Fusarium growth is promoted by relative humidity (90%), extended moisture period, moderate temperature (20–30 °C), degree of rainfall during pre-, post- and harvesting conditions as well as air currents [116]. The optimum temperature for Fusarium growth range between 25–30 °C and the optimum aw between 0.980–0.995 [117]. Piacentini et al. [67] suggested weather to be an important factor since warm and humid conditions favor the Fusarium growth and ZEN production during the flowering period. Pleadin et al. [118] showed that the mold growth and ZEN production was further enhanced by the change in humidity from high to extreme during the growth and harvesting period. The chances of crop contamination with ZEN increase under a prolonged period of cool and wet weather conditions in temperate regions [68]. Habschied et al. [119] studied the effect of incubation time, temperature and aw on ZEN concentration in crops. They found that at 20 °C, ZEN concentration in germ and bran increased with increase in incubation time whereas its concentration in flours increased with an increase in aw. Also, pH was observed to influence the ZEN production where alkaline pH favored the accumulation of the toxin at a lower incubation temperature (15 °C) [120]. The maximum production of ZEN was observed at pH 7 [121]. aw is another factor affecting ZEN accumulation where the production of ZEN was observed to be higher at 0.995 aw than at 0.950 aw, independent of temperature [122]. Further, Martins and Martins [123] observed that ZEN production was highest at 28 °C for 16 days followed by incubation at 12 °C (36.7 mg/kg) at the 35th day. Besides this, carbon dioxide level influenced the ZEN production by F. graminearum where maximum production occurred at 30 °C, 0.98 aw and 400 ppm of CO2 [124].
8. Mechanism of Toxicity and Health Effects of Zearalenone
The contamination of crops with primarily Fusarium species leads to the production of ZEN that contaminates various food and feed [125]. Exposure to ZEN can display both acute and chronic effects. The chronic ingestion includes low-dose intake for a long time resulting in decreased productivity and resistance to pathogens and serves as a major concern relating to human and animal health [126,127]. The toxicokinetic study of ZEN deals with the rate at which it enters, gets absorbed and metabolized in the body and excreted out [10]. Various studies performed on animals have indicated rapid and wide absorption of ZEN (e.g., 80–85% in pigs) by the gastrointestinal tract (GIT) [128]. The GIT serves as a key site for primary interaction with the mycotoxin and is frequently exposed to the toxic agent [129]. In view of this, further extensive studies have been conducted to study the role of GIT in primary immune defense during the last decades [130]. On oral administration by human or animal, ZEN is promptly absorbed by the intestine and breaks down into α- and β-ZEL by α- and β-hydroxysteroid dehydrogenase, respectively. Thus, intestinal epithelial cells are exposed to toxic substances that bring out structural changes in intestinal villi and augment lipid oxidation process resulting in oxidative stress in the intestine [126,131,132,133]. Apart from this, ZEN has shown a significant genotoxic potential [134] and can persuade oxidative DNA injury [135]. ZEN can also lead to DNA fragmentation, cell cycle arrest [136], micronuclei formation and chromosomal aberrations [137]. Further, it exerts nephrotoxic and hepatotoxic effects [138] and encourages the development of hepatocarcinoma [139,140].
The most common toxic effect of ZEN is related to reproductive disorders with pigs being the most affected [141]. Pigs are mostly used as the model for research since they possess similar digestive and immune system as humans [142]. ZEN is similar to that of β-estradiol, therefore, it activates estrogen receptors [130]. Further, ZEN binds with estrogen receptors resulting in hormonal imbalance and can lead to reproductive diseases [143,144]. Studies have shown that ZEN can induce apoptosis of ovarian granulosa cells which are crucial for the follicular development and ovulation [145,146]. ZEN is involved in human hyperestrogenic syndromes besides causing reproductive disorders in farm animals [140]. Further, histological alterations in reproductive organ with decreased intracellular connections in testes was observed in the ZEN-treated mice [9]. In addition, exposure to ZEN during pregnancy and lactation can exert either reversible or irreversible effects on the offspring [130]. Because of potent estrogenic activity, ZEN has been convicted to chiefly affect reproductive functions in females [147]. Studies have indicated that exposure to estrogenic mycotoxin results in advanced puberty time [148]. It also interrupts with the estrous cycle and results in reproductive complications during fertilization, implantation and embryo development [149,150]. Moreover, a study by Massart et al. [151] indicated the contamination of breast milk with ZEN after consumption of contaminated food by the women. Studies conducted so far have revealed that the concentration of 1.0 ppm ZEN in the diet may exert hyper-estrogenic effects in pigs and a further increase in concentration may cause complications with conception leading to miscarriage and other numerous diseases [152]. Further, Mauro et al. [153] measured the serum ZEN metabolites in 48 overweight or obese women to study the association with the food intake. The results indicated that the level of ZEN was found in nearly all the surveyed woman, but its concentration varied with meat intake and the body mass index. Natural incidence of ZEN contaminated food has been identified as the cause of alterations in female reproductive organs and associated health problems [1,154].
Estrogens perform diverse biological functions such as female sexual differentiation and development, bone density maintenance and neuroprotective effects. These effects are the result of interaction between estrogen and estrogen receptor (ER), which triggers the target gene expression that encodes a protein of important biological function. They are generally converted to estrogenically inactive metabolites and eliminated from the body through urine and/or faeces [155]. Certain undesirable metabolites are produced as a result of hydroxylation and amination reactions during the biotransformation process. The enzyme CYP P450 is believed to be responsible for this [156,157,158]. This enzyme is most active in liver and intestine and is also responsible for the synthesis of fatty acids and steroids. The first stage in the metabolism of estrogens is the hydroxylation catalyzed by cytochrome P450 (CYP) enzyme. A chief metabolite of estradiol, 2-hydroxyestradiol, is primarily catalyzed by CYP1A2 and CYP3A4 in the liver, and by CYP1A1 in extrahepatic tissues. Though CYP1B1 mainly targets tissues of mammary, ovary and uterus, it explicitly catalyzes the 4-hydroxylation of estradiol. The 4-hydroxyestradiol further creates free radicals due to reductive-oxidative cycling with the corresponding semiquinone and quinone forms ultimately leading to cell damage. Modification in the expression level of estrogen metabolizing CYP isoforms not merely modifies the intensity of the estrogen action but may also modify the profile of its physiological effect in the liver and target tissues. In general, some CYP isoforms are produced by the substrates themselves resulting in improved and enhanced elimination from the body [155]. Further, the human CYP1B1 is regulated through estrogen receptor by estradiol and hence, suggest that the CYP enzymes involved in estrogen metabolism by estrogen itself would be responsible for the homeostasis of estrogens at local organs [155].
The study on interaction of ZEN with cellular component plays an imperative role in understanding the toxicokinetics of the toxin. Albumin is abundantly present in the plasma protein of the blood. Human serum albumin (HSA) sustains the oncotic pressure and the pH in the human circulation. It also plays an important function in the formation of various complex endogenous and exogenous compounds [125]. Thus, HSA shows a critical role in the pharmacokinetics and toxicokinetics of toxins, contaminants and drugs. In view of the important role of albumin in toxicokinetics of different toxins, interaction of ZEN with albumin has high biological importance. Various methods such as spectroscopy, ultrafiltration and molecular modelling have been employed to study the interaction. Fluorescence spectroscopy reveals that HSA forms a complex with ZEN indicating interaction with a binding constant of logK = 5.1 [125]. The strong interaction of ZEN-HSA complex suggests the potential biological importance and promotes precise understanding of toxicokinetic behavior of ZEN.
The functioning of ZEN as an immunomodulator and immunotoxic compound has also been explored. The immune response due to inflammation utilizes cytokines as a modulator. Cytokines play a key role in inflammatory responses and modulate humoral and cellular immune responses. Any imbalance in the cytokine production could intensify inflammatory reactions and cause pathological problems. Numerous studies have revealed a negative impact of ZEN on cells like white blood cells, B-cells, immunoglobins, T-cells and cytokines that are produced as a response to infection and perform immune functions [159,160]. Salah-Abbès et al. [159] reported that Balb/c mice treated with ZEN (40 mg/kg) for two weeks showed a significant decrease of total white blood cells, immunoglobulin levels (IgG and IgM), B-cells, T-cell subtypes (CD3þ, CD4þ, CD8þ), NK cells as well as pro-inflammatory cytokines. Another study by Islam et al. [140] evaluated how the immune system of mice gets affected when exposed to ZEN for two weeks. The results broadly showed the impact on the mice’s immune system with a decrease in CDC cells (CD4+, CD8+ and CD11c+) of the spleen and mesenteric lymph nodes. Antibodies’ levels were also examined in the serum to determine the humoral response and an increase in IgE while a decrease in IgM was observed. These observations indicate the immunotoxic nature of ZEN, however, more research is required to understand the underlying molecular mechanism of how ZEN differentially regulates immune cells and influences inflammatory responses against pathogens [140]. Further, mechanisms underlying many of these effects remain unrevealed to date.
9. Effects of Processing on Zearalenone
The occurrence of ZEN in agricultural products is known well. Owing to its harmful impact on health, it is important to opt for measures that can mitigate the effect. Thermal processing methods including boiling (100–125 °C), baking, frying and extrusion cooking (150 °C or above) reduce the ZEN, however, the reduction depends on temperature, pH and duration of processing [161]. Pleadin et al. [161] analyzed these thermal processing methods where the extrusion cooking was found to be a superior method to reduce ZEN contamination (up to 75%) followed by roasting which decreased the level up to 40%. In addition, extrusion cooking reduced ZEN by 83% in corn-based products [12]. Besides these, food irradiation has been investigated in corn kernel and flour where degradation of ZEN increased with increased doses of irradiation [162,163]. Gamma radiation resulted in a significant reduction in ZEN and the degradation increased with an increase in the moisture content of the product [164]. However, irradiations produce harmful residues. Therefore, a new cost-effective and environment-friendly technique like cold atmospheric pressure plasma (CAP) generating reactive oxygen and nitrogen species (RONS) in short operating time with an ability to react with a large number of mycotoxin molecules leaving no residues, has been developed [165]. Thus, different processing methods can contribute to lower down the toxicity level in food products.
10. Detection Techniques
The traditional analytical methods of detecting ZEN include chromatographic methods like HPLC [166,167], LC-MS/MS [168,169], and GC-MS [170]. In addition, a new method for purifying ZEN from rice culture of Fusarium graminearum using macroporous resin column coupled with high-speed counter-current chromatography was developed by Wang et al. [171]. These analytical methods have high sensitivity and specificity; however, the major drawback underlies with tedious sample preparation, long analysis time, high cost as well as unsuitable for on-site rapid inspection. To overcome these limitations, immunoassay methods were developed for simple, rapid as well as in-field monitoring of large-scale sample screening and detection with low cost and high sensitivity [172,173,174].
The most widely used immunoassay methods include enzyme-linked immunosorbent assay (ELISA) [175], rapid immunochromatographic assays (ICA) [176], immunochip [177], immunosensor [178], fluorescence polarization immunoassays [179,180], lateral flow immunoassay (LFA) [181], multiplex dipstick immunoassay [182], and suspension array [183]. Though these methods have high sensitivity and specificity, they require skilled manpower to operate. Therefore, Kolosova et al. [181] developed user-friendly membrane-based LFA requiring less staff training and analysis time. Later, LFA based on highly sensitive anti-ZEN monoclonal antibody was developed for rapid detection of ZEN in food and feed samples [184]. Recently, Jin et al. [185] have developed a novel dual near-infrared fluorescence-based LFA to determine ZEN in maize.
In addition, Li et al. [186] developed a 3D printed smartphone-based detection device integrated with solid phase latex microsphere immunochromatography platform (SIAP) for detecting ZEN in cereals and feed. It is also coupled with a user-friendly Android App which is self-written for analyzing, reporting, and sharing the results. The cut-off values of SIAP for ZEN in cereals and feed were 2.5 and 3.0 μg/kg, respectively, while the detection limits of the SIAP detection system for ZEN in cereals and feed were 0.08 and 0.18 μg/kg, respectively [186]. Further, Ren et al. [187] developed an anti-idiotypic nanobody-phage display-mediated immuno-polymerase chain reaction (PD-IPCR) method for detecting ZEN in cereals. The primers for PCR amplification were designed using specific DNA sequences encoding anti-idiotypic nanobodies and the detection limit for total ZEN in a cereal sample was observed to be 0.09 ng/mL [187]. Furthermore, biosensors-based methods have immense potential for detecting ZEN in food and feed. Caglayan and Üstündağ [188] developed an aptamer assay using attenuated internal reflection ellipsometry (AIR-SE) for detecting ZEN in cereals. The AIR-SE linked with the signal amplification through surface plasmon resonance has been observed to be a highly sensitive analytical tool in bio-sensing for the selective detection of ZEN in cereal-based products. The method showed better performance with the limit of detection (LOD) of 0.08 ng/mL and detection range between 0.01 and 1000 ng/mL [188].
11. Masked Mycotoxins as a Major Concern in Detection
As per Rychlik et al. [189], the “matrix-associated” mycotoxins form covalent bonds, complexes and/or are dissolved or trapped in the matrix while the “modified mycotoxins” include both “biologically and chemically modified” mycotoxins. Further, the term “masked mycotoxins” is referred to as “biologically modified” mycotoxins conjugated by plants [189]. These “modified” forms remain undetected by the routine analysis techniques [190]. The most plentiful derivatives of ZEN include α-ZEL and β-ZEL. Three phases of chemical modifications of ZEN have been observed during the plant metabolism. Phase I involves reduction, oxidation, or acetylation of the parent mycotoxin into a derived molecule of a higher toxicity level (e.g., α-ZEL). Phase II consists of the enzymatic transformation of the reactive groups through conjugation such as glucosidation and sulfation to form more hydrophilic compounds which can facilitate the elimination of the masked mycotoxins [169,191,192]. Phase III consists of compartmentalization of mycotoxins into the vacuole of plant or binding to the cell wall [12,193,194,195].
ZEN is efficiently transformed to its glucose conjugate after its production by several Fusarium species during infection of cereals and maize [196]. ZEN is reduced to α-ZEL and β-ZEL and then produces glucose conjugates of the respective compounds, especially Z14G. Further, ZEN and its metabolites have been observed to be transformed into conjugated compounds like glucosides, malonylglucosides, dihexosides, and pentosylhexosides by Arabidopsis thaliana [197]. A study by Schneweis et al. [75] on ten wheat grain samples showed the relative proportion of Z14G to ZEN to be around 27%. Contrary to this, no traces of Z14G, α- or β-ZEL, α-zearalenol-14-β-d-glucopyranoside (α-ZELG) or β-zearalenol-14-β-dglucopyranoside (β-ZELG) were reported in 84 cereal-based products analysed by Vendl et al. [198]. However, zearalenone-14-sulfate (Z14S) was reported in different wheat-based products like flour, bread, biscuits, wheat flakes, bran flakes, muesli, crackers, and snack bars with the highest quantity being 6.1 µg/kg in bran flakes [198]. Huang et al. [199] developed a sensitive and rapid method of ultra-high performance liquid chromatography combined with electrospray ionization triple quadrupole tandem mass spectrometry (UHPLC–ESI–MS/MS) for the simultaneous determination of aflatoxin M1, ochratoxin A, ZEN and α-ZEL in milk. Similarly, Han et al. [200] and Belhassen et al. [201] developed a rapid and sensitive UHPLC–MS/MS method to simultaneously determine total ZEN (free + conjugated) and its five metabolites (α-ZEL, β-ZEL, α-ZAL, β-ZAL, and ZAN) content in traditional Chinese medicines and human urine samples, respectively.
Further, ZEN conjugates across different matrices are enlisted in Table 2. Mycotoxins, occurring in conjugated form, i.e., either in soluble or incorporated into/associated with/attached to macromolecules, can transform back into their parent forms during metabolization by living plants, fungi, and mammals or after food processing. Thereby, pose a serious concern for human and animal health [193,202,203,204,205,206,207]. Further, these transformations can be achieved by a hydrolytic process involving either alkaline, acidic or enzymatic methods [208,209]. Hence, these hydrolytic methods along with in vitro digestion followed by detection with chromatographic techniques like LC/MS/MS and confirmation by methods like ELISA can be applied for modified ZEN to ensure the safety of food and feed.
Table 2.
Zearalenone conjugates across different matrices.
| Origin | Conjugated Zearalenone | Matrix | Reference |
|---|---|---|---|
| Fungal conjugates | Zearalenone 4-sulfate | Fusarium graminearum, Rhizopus arrhizus | [191,210] |
| Zearalenone 4-glucoside | Rhizopus sp. | [211] | |
| Plant conjugates | α-/β-Zearalenol 4-glucoside | Maize cells | [212] |
| Zearalenone 4-glucoside | Maize cells, wheat | [75,196] | |
| Zearalenone dihexoside, α-Zearalenol dihexoside, β-Zearalenol dihexoside, Zearalenone malonylhexoside, α-Zearalenol malonylhexoside, β-Zearalenol malonylhexoside, Zearalenone pentosylhexoside, α-Zearalenol pentosylhexoside, β-Zearalenol pentosylhexoside |
Arabidopsis thaliana | [197] | |
| Palmitoyl zearalenone | Fusarium-infected banana | [92] | |
| Mammalian conjugates | Zearalenone 3-glucuronide | Urine | [213] |
| Zearalenone 4-sulfate | Urine | [214,215] |
12. Degradation Kinetics
The degradation methods have been categorized into physical, chemical, enzymatic and biological methods. Physical methods involve washing, sorting, grinding, hulling, adsorption, thermal treatment, and application of UV and gamma radiations [216]. The degradation of mycotoxin on increasing temperature and pH follows first-order reaction indicating greater destruction at high temperature and pH [217]. Further, adsorption methods adsorb mycotoxins directly and neutralize them, thus, making the mycotoxins ineffective [1]. Graphene oxide (GO) with amphiphilic didodecyl dimethyl ammonium bromide (DDAB) molecules has effectively adsorbed ZEN from maize oils [218]. Other adsorbents include organo-montmorillonites (OMts) [219], modified montmorillonites [220], montmorillonite clay with Cymbopogon citratus [221], organo-rectorites modified with different quaternary ammonium salts [222], organozeolites [223], talc and diatomaceous earth [224], activated carbon [225] and kaolin modified with octadecydimethilbenzyl ammonium [226].
Chemical methods are based on the application of chemicals such as ammonia, hydrogen peroxide, sodium hypochlorite and ozone [1]. Since ozone is safe, efficient and environmental friendly, it is widely used for detoxification of the toxin [227]. A study by Qi, et al. [228] indicated that ozone has the potential to significantly reduce ZEN in naturally contaminated corn without affecting its quality. Ozone reduces the toxicity of ZEN by forming degradation ozonolytic products which are less toxic than the original mycotoxin [229]. The reaction rate constant and degradation rate of ZEN increased with ozone concentration and treatment time [228] and this ZEN degradation by ozone followed first-order kinetic [230]. A study by Rogowska et al. [1] has suggested 83.9% ZEN degradation with the use of 10% hydrogen peroxide at 30 °C for 16 h. Su et al. [23] have also suggested the potential ability of vitamin C to reduce the toxicity of ZEN in animal models.
Both physical and chemical methods result in loss of nutrients and are regarded as expensive and ineffective, so biological strategies have been developed [216]. Biodegradation methods involve the use of microorganisms with high degradation efficiency, minimal environmental hazards with a lower reduction in nutritional and textural food quality [4]. The microorganisms degrade mycotoxin via two pathways: first through special structures in the cell wall which absorb the ZEN, reduce their exposure and thus achieve detoxification. Second through biotransformation of ZEN into less toxic compounds by metabolizing them [231]. Chlebicz and Śliżewska [232] have shown the detoxification properties of Lactobacillus and S. cerevisiae strains towards ZEN where the toxin decreases within 6 hours of incubation. S. cerevisiae degraded ZEN through intra- and extra-cellular production of enzyme which acted against oxidative stress and cell death and also assisted cell wall adsorption of toxin [216]. Further, Lactobacillus pentosus strains effectively reduced ZEN by binding 30–83% of ZEN and the binding capacity increased with increased ZEN concentration as determined by Freundlich isotherm [233]. In addition, Lactobacillus plantarum reduced ZEN by binding them into pellets [234]. The proteins in the extracellular extracts of Acinetobacter sp. SM04 and Bacillus natto CICC 24640 bring about the biodegradation of ZEN [235,236]. Streptomyces rimosus (K145, K189) reduces ZEN by producing toxin degrading enzymes [237]. Also, Bacillus strains RC1A, RC3A and RC6A degraded ZEN by extracellular metabolite, AHL enzyme [18]. Besides this, the combination of B. subtilis SP1, B. subtilis SP2, C. utilis and extracts of A. oryzae helps in biodegradation of ZEN [238]. Bacillus amyloliquefaciens ZDS-1, Bacillus subtilis, Bacillus velezensis Strain ANSB01E, Bacillus amyloliquefaciens H6, Aspergillus niger strain FS10, Lysinibacillus sp. ZJ-2016-1, Pseudomonas alcaliphila TH-C1, Pseudomonas plecoglossicida TH-L1, and Rhodococcus pyridinivorans K408 Strain also showed biodegradation of ZEN with a significant reduction in the mycotoxin level in food products either by adsorption of the toxin or through enzyme production which degraded the toxin [239,240,241,242,243,244]. Biodegradation using enzymes utilizes lactonohydrolase annotated as Zhd518 from E. coli and P. pastoris GSZ; lactonase from Gliocladium roseum, named ZENG; zearalenone lactonohydrolase (ZHD101) from Clonostachys rosea; laccase from Trametes versicolor; peroxidase (POD) from soya bean and rice bran; and a recombinant fusion enzyme (ZHDCP), a combination of hydrolase (ZHD) and carboxypeptidase (CP) having the potential to degrade the ZEN, hence, termed as ZEN-degrading enzymes [207,245,246,247,248].
13. Management and Control Strategies
Infection of crops by Fusarium growth and accumulation of ZEN has been a major concern for food and feed quality and safety, leading to economic losses. Hence, interventions to reduce contamination should be designed [249]. These interventions involve good agricultural practices (GAP’s) and good manufacturing practices (GMP’s) [250].
Pre-harvest strategies include weed eradication, soil analysis, application of herbicide, fungicide, and insecticide for pest and fungal eradication, seedbed treatment, decontamination of seeds, crop rotation, tillage and ploughing, fertilizers for nutrient enrichment, and genetically modified plants for mycotoxin suppression [50,251,252]. Tillage and ploughing in soil cultivation bring the contaminated part into the upper soil and can be removed so that the next crop is not infected [253]. Use of fertilizers especially nitrogen increases the growth of Fusarium along with the positive effect on plant growth and thus the contamination by mycotoxin increases [254]. Crop rotation helps in limiting the recontamination of crops in which Fusarium prone crops are cultivated with crops not prone to contamination [255]. Further, the strategies involving the cultivation of resistant varieties, excessive use of chemicals such as pesticides and insecticides, and extensive crop rotation programs have adverse effects on both human and environment. Therefore, novel strategies such as the use of biological control agents (BCA’s) which suppress the growth and colonization of harmful pathogens have been developed recently [256]. Abdallah et al. [257] have demonstrated that Epicoccum and Sordaria, endophytic fungi controlled the growth of Fusarium graminearum and hence, the production of ZEN in maize.
Post-harvest strategies include minimizing the time between harvesting and drying, efficient drying to a moisture content less than 14%, effective cleaning of maize before storage, hygiene and management of storage containers, clear specifications and traceability from field to store, absence of pests in storage areas, appropriate storage conditions in terms of temperature and moisture control and modified atmosphere storage using sulphur dioxide which is harmful to pathogenic microorganisms [258]. Other post-harvest strategies include physical, chemical, and biological decontamination of mycotoxin in agricultural products [250]. In the coming years, the foundation of such pre- and post-harvest strategies should be strengthened to prevent the mycotoxins from entering the human and animal food chain.
14. Conclusions
The pervasive contamination of various food and feed with mycotoxins poses serious threats to human and animal wellbeing as well as commercial trade across the globe. The instance of contamination can occur at any stage from pre- to post-harvest periods due to improper handling, storage, and distribution facilities. Insight of various deleterious effects of mycotoxin consumption such as teratogenic, carcinogenic, oestrogenic, and immunosuppressive effects, there is a need to develop efficient technologies for detection, decontamination, and management of these mycotoxins to prevent a threat to both human and animal with assured safety and security of food and feed. ZEN, an estrogenic mycotoxin, can contribute extensively to various hormone-dependent diseases. The development of effective neutralization and decontamination strategies for ZEN stands obligatory to mitigate its adverse effects as this toxin is highly resistant to various processing conditions. A comprehensive understanding of the biosynthetic mechanism stands essential for the development of effective control strategies. Various analytical methods such as HPLC, LC-MS/MS, and GC-MS have been developed for the detection of ZEN with different detection limits. Despite these, various other electrochemical, colorimetric, fluorometric, refractometric could be utilized for ZEN detection in food and feed. Furthermore, numerous physical, chemical and enzymatic methods could be used for decontamination of ZEN in food and feed for their safe consumption. Further advancement in these technologies and the adoption of novel detection and decontamination techniques is the need of the hour to embark the vision of attaining food and feed safety and security across the globe.
Author Contributions
P.K. conceived and designed the manuscript; D.K.M., S.D., S.P., B.S., K.K.M., S.M., K.D., R.S. and M.K. wrote the manuscript; P.K. and D.K.M. helped in the editing of the manuscript; P.K. and A.K.M. critically reviewed the manuscript and did the required editing. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding received to support this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This review highlights the information on the occurrence of zearalenone across the world. In addition, it provides insight into the chemistry and biosynthesis, effects on human health, agricultural produce along with their detection and management strategies to ensure food and feed safety and security.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rogowska A., Pomastowski P., Sagandykova G., Buszewski B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon. 2019;162:46–56. doi: 10.1016/j.toxicon.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Kumar P., Mahato D.K., Kamle M., Mohanta T.K., Kang S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017;7:2170. doi: 10.3389/fmicb.2016.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sellamani M., Kalagatur N.K., Siddaiah C., Mudili V., Krishna K., Natarajan G., Rao Putcha V.L. Antifungal and zearalenone inhibitory activity of Pediococcus pentosaceus isolated from dairy products on Fusarium graminearum. Front. Microbiol. 2016;7:890. doi: 10.3389/fmicb.2016.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J., Xie Y. Review on microbial degradation of zearalenone and aflatoxins. Grain Oil Sci. Technol. 2020;3:117–125. doi: 10.1016/j.gaost.2020.05.002. [DOI] [Google Scholar]
- 5.Zhou Y., Zhang D., Sun D., Cui S. Zearalenone affects reproductive functions of male offspring via transgenerational cytotoxicity on spermatogonia in mouse. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020;234:108766. doi: 10.1016/j.cbpc.2020.108766. [DOI] [PubMed] [Google Scholar]
- 6.Marin S., Ramos A.J., Cano-Sancho G., Sanchis V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013;60:218–237. doi: 10.1016/j.fct.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 7.Al-Jaal B.A., Jaganjac M., Barcaru A., Horvatovich P., Latiff A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2018. Food Chem. Toxicol. 2019;129:211–228. doi: 10.1016/j.fct.2019.04.047. [DOI] [PubMed] [Google Scholar]
- 8.Caglayan M.O., Şahin S., Üstündağ Z. Detection strategies of Zearalenone for food safety: A review. Crit. Rev. Anal. Chem. 2020 doi: 10.1080/10408347.2020.1797468. [DOI] [PubMed] [Google Scholar]
- 9.Yang J.Y., Wang G.X., Liu J.L., Fan J.J., Cui S. Toxic effects of zearalenone and its derivatives α-zearalenol on male reproductive system in mice. Reprod. Toxicol. 2007;24:381–387. doi: 10.1016/j.reprotox.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Rai A., Das M., Tripathi A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2019;60:2710–2729. doi: 10.1080/10408398.2019.1655388. [DOI] [PubMed] [Google Scholar]
- 11.Taranu I., Braicu C., Marin D.E., Pistol G.C., Motiu M., Balacescu L., Neagoe I.B., Burlacu R. Exposure to zearalenone mycotoxin alters in vitro porcine intestinal epithelial cells by differential gene expression. Toxicol. Lett. 2015;232:310–325. doi: 10.1016/j.toxlet.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Zinedine A., Soriano J.M., Molto J.C., Manes J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007;45:1–18. doi: 10.1016/j.fct.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Chang H., Kim W., Park J.-H., Kim D., Kim C.-R., Chung S., Lee C. The occurrence of zearalenone in South Korean feedstuffs between 2009 and 2016. Toxins. 2017;9:223. doi: 10.3390/toxins9070223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fruhauf S., Novak B., Nagl V., Hackl M., Hartinger D., Rainer V., Labudová S., Adam G., Aleschko M., Moll W.-D. Biotransformation of the mycotoxin zearalenone to its metabolites hydrolyzed zearalenone (HZEN) and decarboxylated hydrolyzed zearalenone (DHZEN) diminishes its estrogenicity in vitro and in vivo. Toxins. 2019;11:481. doi: 10.3390/toxins11080481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayed Y., Ayed-Boussema I., Ouanes Z., Bacha H. In vitro and in vivo induction of chromosome aberrations by alpha-and beta-zearalenols: Comparison with zearalenone. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011;726:42–46. doi: 10.1016/j.mrgentox.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Afshar P., Shokrzadeh M., Raeisi S.N., Ghorbani-HasanSaraei A., Nasiraii L.R. Aflatoxins biodetoxification strategies based on probiotic bacteria. Toxicon. 2020;178:50–58. doi: 10.1016/j.toxicon.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Haque M.A., Wang Y., Shen Z., Li X., Saleemi M.K., He C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020;142:104095. doi: 10.1016/j.micpath.2020.104095. [DOI] [PubMed] [Google Scholar]
- 18.Pereyra M.L.G., Di Giacomo A.L., Lara A.L., Martínez M.P., Cavaglieri L. Aflatoxin-degrading Bacillus sp. strains degrade zearalenone and produce proteases, amylases and cellulases of agro-industrial interest. Toxicon. 2020;180:43–48. doi: 10.1016/j.toxicon.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Santos Pereira C., Cunha S.C., Fernandes J.O. Prevalent mycotoxins in animal feed: Occurrence and analytical methods. Toxins. 2019;11:290. doi: 10.3390/toxins11050290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golge O., Kabak B. Occurrence of deoxynivalenol and zearalenone in cereals and cereal products from Turkey. Food Control. 2020;110:106982. doi: 10.1016/j.foodcont.2019.106982. [DOI] [Google Scholar]
- 21.Abdallah M.F., Girgin G., Baydar T. Mycotoxin detection in maize, commercial feed, and raw dairy milk samples from Assiut City, Egypt. Vet. Sci. 2019;6:57. doi: 10.3390/vetsci6020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iqbal S.Z., Nisar S., Asi M.R., Jinap S. Natural incidence of aflatoxins, ochratoxin A and zearalenone in chicken meat and eggs. Food Control. 2014;43:98–103. doi: 10.1016/j.foodcont.2014.02.046. [DOI] [Google Scholar]
- 23.Su Y., Sun Y., Ju D., Chang S., Shi B., Shan A. The detoxification effect of vitamin C on zearalenone toxicity in piglets. Ecotoxicol. Environ. Saf. 2018;158:284–292. doi: 10.1016/j.ecoenv.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 24.Brown D.W., Butchko R.A.E., Baker S.E., Proctor R.H. Phylogenomic and functional domain analysis of polyketide synthases in Fusarium. Fungal Biol. 2012;116:318–331. doi: 10.1016/j.funbio.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Kim J.-E., Son H., Lee Y.-W. Biosynthetic mechanism and regulation of zearalenone in Fusarium graminearum. JSM Mycotoxins. 2018;68:1–6. doi: 10.2520/myco.68-1-2. [DOI] [Google Scholar]
- 26.Warth B., Sulyok M., Berthiller F., Schuhmacher R., Krska R. New insights into the human metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone. Toxicol. Lett. 2013;220:88–94. doi: 10.1016/j.toxlet.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Kuiper-Goodman T., Scott P.M., Watanabe H. Risk assessment of the mycotoxin zearalenone. Regul. Toxicol. Pharmacol. 1987;7:253–306. doi: 10.1016/0273-2300(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 28.Biehl M.L., Prelusky D.B., Koritz G.D., Hartin K.E., Buck W.B., Trenholm H.L. Biliary excretion and enterohepatic cycling of zearalenone in immature pigs. Toxicol. Appl. Pharmacol. 1993;121:152–159. doi: 10.1006/taap.1993.1140. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy D.G., Hewitt S.A., McEvoy J.D.G., Currie J.W., Cannavan A., Blanchflower W.J., Elliot C.T. Zeranol is formed from Fusarium spp. toxins in cattle in vivo. Food Addit. Contam. 1998;15:393–400. doi: 10.1080/02652039809374658. [DOI] [PubMed] [Google Scholar]
- 30.Miles C.O., Erasmuson A.F., Wilkins A.L., Towers N.R., Smith B.L., Garthwaite I., Scahill B.G., Hansen R.P. Ovine metabolism of zearalenone to α-zearalanol (zeranol) J. Agric. Food Chem. 1996;44:3244–3250. doi: 10.1021/jf9601325. [DOI] [Google Scholar]
- 31.Migdalof B.H., Dugger H.A., Heider J.G., Coombs R.A., Terry M.K. Biotransformation of zeranol: Disposition and metabolism in the female rat, rabbit, dog, monkey and man. Xenobiotica. 1983;13:209–221. doi: 10.3109/00498258309052257. [DOI] [PubMed] [Google Scholar]
- 32.Shier W.T., Shier A.C., Xie W., Mirocha C.J. Structure-activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon. 2001;39:1435–1438. doi: 10.1016/S0041-0101(00)00259-2. [DOI] [PubMed] [Google Scholar]
- 33.Richardson K.E., Hagler W.M., Mirocha C.J. Production of zearalenone. alpha.-and. beta.-zearalenol, and. alpha.-and. beta.-zearalanol by Fusarium spp. in rice culture. J. Agric. Food Chem. 1985;33:862–866. doi: 10.1021/jf00065a024. [DOI] [Google Scholar]
- 34.Celius T., Haugen T.B., Grotmol T., Walther B.T. A sensitive zonagenetic assay for rapid in vitro assessment of estrogenic potency of xenobiotics and mycotoxins. Environ. Health Perspect. 1999;107:63–68. doi: 10.1289/ehp.9910763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Guevel R., Pakdel F. Assessment of oestrogenic potency of chemicals used as growth promoter by in-vitro methods. Hum. Reprod. 2001;16:1030–1036. doi: 10.1093/humrep/16.5.1030. [DOI] [PubMed] [Google Scholar]
- 36.Eriksen G.S., Alexander J. Fusarium Toxins in Cereals: A Risk Assessment. Nordic Council of Ministers; Copenhagen, Denmark: 1998. pp. 7–58. [Google Scholar]
- 37.Miksicek R.J. Interaction of naturally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor. J. Steroid Biochem. Mol. Biol. 1994;49:153–160. doi: 10.1016/0960-0760(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 38.Fitzpatrick D.W., Picken C.A., Murphy L.C., Buhr M. Measurement of the relative binding affinity of zearalenone, alpha-zearalenol and beta-zearalenol for uterine and oviduct estrogen receptors in swine, rats and chickens: An indicator of estrogenic potencies. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 1989;94:691–694. doi: 10.1016/0742-8413(89)90133-3. [DOI] [PubMed] [Google Scholar]
- 39.Gaffoor I., Trail F. Characterization of two polyketide synthase genes involved in zearalenone biosynthesis in Gibberella zeae. Appl. Environ. Microbiol. 2006;72:1793–1799. doi: 10.1128/AEM.72.3.1793-1799.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huffman J., Gerber R., Du L. Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins. Biopolymers. 2010;93:764–776. doi: 10.1002/bip.21483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lysøe E., Klemsdal S.S., Bone K.R., Frandsen R.J.N., Johansen T., Thrane U., Giese H. The PKS4 gene of Fusarium graminearum is essential for zearalenone production. Appl. Environ. Microbiol. 2006;72:3924–3932. doi: 10.1128/AEM.00963-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng K., Wang Y., Yang P., Luo H., Bai Y., Shi P., Yuan T., Ma R., Yao B. Rapid detection and quantification of zearalenone-producing Fusarium species by targeting the zearalenone synthase gene PKS4. Food Control. 2010;21:207–211. doi: 10.1016/j.foodcont.2009.05.014. [DOI] [Google Scholar]
- 43.Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bottalico A., Visconti A., Logrieco A., Solfrizzo M., Mirocha C.J. Occurrence of zearalenols (diastereomeric mixture) in corn stalk rot and their production by associated Fusarium species. Appl. Environ. Microbiol. 1985;49:547–551. doi: 10.1128/AEM.49.3.547-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’mello J.P.F., Placinta C.M., Macdonald A.M.C. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999;80:183–205. doi: 10.1016/S0377-8401(99)00059-0. [DOI] [Google Scholar]
- 46.Romero A., Ares I., Ramos E., Castellano V., Martínez M., Martínez-Larrañaga M.-R., Anadón A., Martínez M.-A. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology. 2016;353:21–33. doi: 10.1016/j.tox.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Reddy K.E., Song J., Lee H.-J., Kim M., Kim D.-W., Jung H.J., Kim B., Lee Y., Yu D., Kim D.-W. Effects of high levels of deoxynivalenol and zearalenone on growth performance, and hematological and immunological parameters in pigs. Toxins. 2018;10:114. doi: 10.3390/toxins10030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li N., Liu X.-L., Zhang F.-L., Tian Y., Zhu M., Meng L.-Y., Dyce P.W., Shen W., Li L. Whole-transcriptome analysis of the toxic effects of zearalenone exposure on ceRNA networks in porcine granulosa cells. Environ. Pollut. 2020;261:114007. doi: 10.1016/j.envpol.2020.114007. [DOI] [PubMed] [Google Scholar]
- 49.Eskola M., Altieri A., Galobart J. Overview of the activities of the European Food Safety Authority on mycotoxins in food and feed. World Mycotoxin J. 2018;11:277–289. doi: 10.3920/WMJ2017.2270. [DOI] [Google Scholar]
- 50.European Commission Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (2006/576/EC) Off. J. Eur. Union. 2006;229:7–9. [Google Scholar]
- 51.Jia R., Liu W., Zhao L., Cao L., Shen Z. Low doses of individual and combined deoxynivalenol and zearalenone in naturally moldy diets impair intestinal functions via inducing inflammation and disrupting epithelial barrier in the intestine of piglets. Toxicol. Lett. 2020;333:159–169. doi: 10.1016/j.toxlet.2020.07.032. [DOI] [PubMed] [Google Scholar]
- 52.Lewczuk B., Przybylska-Gornowicz B., Gajęcka M., Targońska K., Ziółkowska N., Prusik M., Gajęcki M. Histological structure of duodenum in gilts receiving low doses of zearalenone and deoxynivalenol in feed. Exp. Toxicol. Pathol. 2016;68:157–166. doi: 10.1016/j.etp.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Pistol G.C., Gras M.A., Marin D.E., Israel-Roming F., Stancu M., Taranu I. Natural feed contaminant zearalenone decreases the expressions of important pro-and anti-inflammatory mediators and mitogen-activated protein kinase/NF-κB signalling molecules in pigs. Br. J. Nutr. 2014;111:452–464. doi: 10.1017/S0007114513002675. [DOI] [PubMed] [Google Scholar]
- 54.Przybylska-Gornowicz B., Tarasiuk M., Lewczuk B., Prusik M., Ziółkowska N., Zielonka Ł., Gajęcki M., Gajęcka M. The effects of low doses of two Fusarium toxins, zearalenone and deoxynivalenol, on the pig jejunum. A light and electron microscopic study. Toxins. 2015;7:4684–4705. doi: 10.3390/toxins7114684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011;9:2197. doi: 10.2903/j.efsa.2011.2197. [DOI] [Google Scholar]
- 56.Ji F., Xu J., Liu X., Yin X., Shi J. Natural occurrence of deoxynivalenol and zearalenone in wheat from Jiangsu province, China. Food Chem. 2014;157:393–397. doi: 10.1016/j.foodchem.2014.02.058. [DOI] [PubMed] [Google Scholar]
- 57.Nkwe D.O., Taylor J.E., Siame B.A. Fungi, aflatoxins, fumonisin B l and zearalenone contaminating sorghum-based traditional malt, wort and beer in Botswana. Mycopathologia. 2005;160:177–186. doi: 10.1007/s11046-005-6867-9. [DOI] [PubMed] [Google Scholar]
- 58.Bresler G., Vaamonde G., Brizzio S. Natural occurrence of zearalenone and toxicogenic fungi in amaranth grain. Int. J. Food Microbiol. 1991;13:75–80. doi: 10.1016/0168-1605(91)90139-G. [DOI] [PubMed] [Google Scholar]
- 59.Reza Oveisi M., Hajimahmoodi M., Memarian S., Sadeghi N., Shoeibi S. Determination of zearalenone in corn flour and a cheese snack product using high-performance liquid chromatography with fluorescence detection. Food Addit. Contam. 2005;22:443–448. doi: 10.1080/02652030500073709. [DOI] [PubMed] [Google Scholar]
- 60.Li F.-Q., Li Y.-W., Luo X.-Y., Yoshizawa T. Fusarium toxins in wheat from an area in Henan Province, PR China, with a previous human red mould intoxication episode. Food Addit. Contam. 2002;19:163–167. doi: 10.1080/02652030110070058. [DOI] [PubMed] [Google Scholar]
- 61.Schollenberger M., Müller H.-M., Rüfle M., Suchy S., Plank S., Drochner W. Natural occurrence of 16 Fusarium toxins in grains and feedstuffs of plant origin from Germany. Mycopathologia. 2006;161:43–52. doi: 10.1007/s11046-005-0199-7. [DOI] [PubMed] [Google Scholar]
- 62.Stanciu O., Juan C., Miere D., Loghin F., Mañes J. Occurrence and co-occurrence of Fusarium mycotoxins in wheat grains and wheat flour from Romania. Food Control. 2017;73:147–155. doi: 10.1016/j.foodcont.2016.07.042. [DOI] [Google Scholar]
- 63.Yamashita A., Yoshizawa T., Aiura Y., Sanchez P.C., Dizon E.I., Arim R.H., Sardjono. Fusarium mycotoxins (fumonisins, nivalenol, and zearalenone) and aflatoxins in corn from Southeast Asia. Biosci. Biotechnol. Biochem. 1995;59:1804–1807. doi: 10.1271/bbb.59.1804. [DOI] [PubMed] [Google Scholar]
- 64.Kamala A., Ortiz J., Kimanya M., Haesaert G., Donoso S., Tiisekwa B., De Meulenaer B. Multiple mycotoxin co-occurrence in maize grown in three agro-ecological zones of Tanzania. Food Control. 2015;54:208–215. doi: 10.1016/j.foodcont.2015.02.002. [DOI] [Google Scholar]
- 65.Vargas E.A., Preis R.A., Castro L., Silva C.M.G. Co-occurrence of aflatoxins B 1, B 2, G 1, G 2, zearalenone and fumonisin B 1 in Brazilian corn. Food Addit. Contam. 2001;18:981–986. doi: 10.1080/02652030110046190. [DOI] [PubMed] [Google Scholar]
- 66.Silva C.M.G., Vargas E.A. A survey of zearalenone in corn using Romer Mycosep™ 224 column and high performance liquid chromatography. Food Addit. Contam. 2001;18:39–45. doi: 10.1080/02652030010002649. [DOI] [PubMed] [Google Scholar]
- 67.Piacentini K.C., Rocha L., Savi G.D., Carnielli-Queiroz L., Almeida F., Minella E., Corrêa B. Occurrence of deoxynivalenol and zearalenone in brewing barley grains from Brazil. Mycotoxin Res. 2018;34:173–178. doi: 10.1007/s12550-018-0311-8. [DOI] [PubMed] [Google Scholar]
- 68.Zaied C., Zouaoui N., Bacha H., Abid S. Natural occurrence of zearalenone in Tunisian wheat grains. Food Control. 2012;25:773–777. doi: 10.1016/j.foodcont.2011.12.012. [DOI] [Google Scholar]
- 69.Pietri A., Bertuzzi T., Pallaroni L., Piva G. Occurrence of mycotoxins and ergosterol in maize harvested over 5 years in Northern Italy. Food Addit. Contam. 2004;21:479–487. doi: 10.1080/02652030410001662020. [DOI] [PubMed] [Google Scholar]
- 70.Hagler W.M., Jr., Bowman D.T., Babadoost M., Haney C.A., Swanson S.P. Aflatoxin, Zearalenone, and Deoxynivalenol in North Carolina Grain Sorghum 1. Crop Sci. 1987;27:1273–1278. doi: 10.2135/cropsci1987.0011183X002700060037x. [DOI] [Google Scholar]
- 71.Nathanail A.V., Syvähuoko J., Malachová A., Jestoi M., Varga E., Michlmayr H., Adam G., Sieviläinen E., Berthiller F., Peltonen K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015;407:4745–4755. doi: 10.1007/s00216-015-8676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alkadri D., Rubert J., Prodi A., Pisi A., Mañes J., Soler C. Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chem. 2014;157:111–118. doi: 10.1016/j.foodchem.2014.01.052. [DOI] [PubMed] [Google Scholar]
- 73.Hadiani M.R., Yazdanpanah H., Ghazi-Khansari M., Cheraghali A.M., Goodarzi M. Survey of the natural occurrence of zearalenone in maize from northern Iran by thin-layer chromatography densitometry. Food Addit. Contam. 2003;20:380–385. doi: 10.1080/0265203031000087968. [DOI] [PubMed] [Google Scholar]
- 74.Park J.W., Kim E.K., Shon D.H., Kim Y.B. Natural co-occurrence of aflatoxin B1, fumonisin B1 and ochratoxin A in barley and corn foods from Korea. Food Addit. Contam. 2002;19:1073–1080. doi: 10.1080/02652030210151840. [DOI] [PubMed] [Google Scholar]
- 75.Schneweis I., Meyer K., Engelhardt G., Bauer J. Occurrence of zearalenone-4-β-D-glucopyranoside in wheat. J. Agric. Food Chem. 2002;50:1736–1738. doi: 10.1021/jf010802t. [DOI] [PubMed] [Google Scholar]
- 76.Tima H., Brückner A., Mohácsi-Farkas C., Kiskó G. Fusarium mycotoxins in cereals harvested from Hungarian fields. Food Addit. Contam. Part B. 2016;9:127–131. doi: 10.1080/19393210.2016.1151948. [DOI] [PubMed] [Google Scholar]
- 77.Muthomi J.W., Ndung’u J.K., Gathumbi J.K., Mutitu E.W., Wagacha J.M. The occurrence of Fusarium species and mycotoxins in Kenyan wheat. Crop Prot. 2008;27:1215–1219. doi: 10.1016/j.cropro.2008.03.001. [DOI] [Google Scholar]
- 78.Garrido C., Pezzani C.H., Pacin A. Mycotoxins occurrence in Argentina’s maize (Zea mays L.), from 1999 to 2010. Food Control. 2012;25:660–665. doi: 10.1016/j.foodcont.2011.11.043. [DOI] [Google Scholar]
- 79.Schollenberger M., Müller H.-M., Rüfle M., Suchy S., Planck S., Drochner W. Survey of Fusarium toxins in foodstuffs of plant origin marketed in Germany. Int. J. Food Microbiol. 2005;97:317–326. doi: 10.1016/j.ijfoodmicro.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 80.Běláková S., Benešová K., Čáslavský J., Svoboda Z., Mikulíková R. The occurrence of the selected fusarium mycotoxins in czech malting barley. Food Control. 2014;37:93–98. doi: 10.1016/j.foodcont.2013.09.033. [DOI] [Google Scholar]
- 81.Almeida-Ferreira G.C., Barbosa-Tessmann I.P., Sega R., Machinski Junior M. Occurrence of zearalenone in wheat-and corn-based products commercialized in the State of Paraná, Brazil. Braz. J. Microbiol. 2013;44:371–375. doi: 10.1590/S1517-83822013005000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Camardo Leggieri M., Bertuzzi T., Pietri A., Battilani P. Mycotoxin occurrence in maize produced in Northern Italy over the years 2009–2011: Focus on the role of crop related factors. Phytopathol. Mediterr. 2015;54:212–221. doi: 10.14601/Phytopathol_Mediterr-14632. [DOI] [Google Scholar]
- 83.Park J.W., Choi S.-Y., Hwang H.-J., Kim Y.-B. Fungal mycoflora and mycotoxins in Korean polished rice destined for humans. Int. J. Food Microbiol. 2005;103:305–314. doi: 10.1016/j.ijfoodmicro.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 84.Habler K., Rychlik M. Multi-mycotoxin stable isotope dilution LC-MS/MS method for Fusarium toxins in cereals. Anal. Bioanal. Chem. 2016;408:307–317. doi: 10.1007/s00216-015-9110-7. [DOI] [PubMed] [Google Scholar]
- 85.Mankevičienė A., Butkutė B., Gaurilčikienė I., Dabkevičius Z., Supronienė S. Risk assessment of Fusarium mycotoxins in Lithuanian small cereal grains. Food Control. 2011;22:970–976. doi: 10.1016/j.foodcont.2010.12.004. [DOI] [Google Scholar]
- 86.Patel S., Hazel C.M., Winterton A.G.M., Mortby E. Survey of ethnic foods for mycotoxins. Food Addit. Contam. 1996;13:833–841. doi: 10.1080/02652039609374470. [DOI] [PubMed] [Google Scholar]
- 87.Siame B.A., Mpuchane S.F., Gashe B.A., Allotey J., Teffera G. Occurrence of aflatoxins, fumonisin B1, and zearalenone in foods and feeds in Botswana. J. Food Prot. 1998;61:1670–1673. doi: 10.4315/0362-028X-61.12.1670. [DOI] [PubMed] [Google Scholar]
- 88.Czembor E., Stępień Ł., Waśkiewicz A. Effect of environmental factors on Fusarium species and associated mycotoxins in maize grain grown in Poland. PLoS ONE. 2015;10:e0133644. doi: 10.1371/journal.pone.0133644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pleadin J., Vahčić N., Perši N., Ševelj D., Markov K., Frece J. Fusarium mycotoxins’ occurrence in cereals harvested from Croatian fields. Food Control. 2013;32:49–54. doi: 10.1016/j.foodcont.2012.12.002. [DOI] [Google Scholar]
- 90.Ayalew A., Fehrmann H., Lepschy J., Beck R., Abate D. Natural occurrence of mycotoxins in staple cereals from Ethiopia. Mycopathologia. 2006;162:57–63. doi: 10.1007/s11046-006-0027-8. [DOI] [PubMed] [Google Scholar]
- 91.Ibáñez-Vea M., Martínez R., González-Peñas E., Lizarraga E., de Cerain A.L. Co-occurrence of aflatoxins, ochratoxin A and zearalenone in breakfast cereals from Spanish market. Food Control. 2011;22:1949–1955. doi: 10.1016/j.foodcont.2011.05.008. [DOI] [Google Scholar]
- 92.Chakrabarti D.K., Ghosal S. Occurrence of free and conjugated 12, 13-epoxytrichothecenes and zearalenone in banana fruits infected with Fusarium moniliforme. Appl. Environ. Microbiol. 1986;51:217–219. doi: 10.1128/AEM.51.1.217-219.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zinedine A., Brera C., Elakhdari S., Catano C., Debegnach F., Angelini S., De Santis B., Faid M., Benlemlih M., Minardi V. Natural occurrence of mycotoxins in cereals and spices commercialized in Morocco. Food Control. 2006;17:868–874. doi: 10.1016/j.foodcont.2005.06.001. [DOI] [Google Scholar]
- 94.Ali N., Sardjono , Yamashita A., Yoshizawa T. Natural co-occurrence of aflatoxins and Fusarium mycotoxins (fumonisins, deoxynivalenol, nivalenol and zearalenone) in corn from Indonesia. Food Addit. Contam. 1998;15:377–384. doi: 10.1080/02652039809374655. [DOI] [PubMed] [Google Scholar]
- 95.Gonzalez H.H.L., Martinez E.J., Pacin A.M., Resnik S.L., Sydenham E.W. Natural co-occurrence of fumonisins, deoxynivalenol, zearalenone and aflatoxins in field trial corn in Argentina. Food Addit. Contam. 1999;16:565–569. doi: 10.1080/026520399283704. [DOI] [PubMed] [Google Scholar]
- 96.Abdulkadar A.H.W., Al-Ali A.A., Al-Kildi A.M., Al-Jedah J.H. Mycotoxins in food products available in Qatar. Food Control. 2004;15:543–548. doi: 10.1016/j.foodcont.2003.08.008. [DOI] [Google Scholar]
- 97.Lindblad M., Gidlund A., Sulyok M., Börjesson T., Krska R., Olsen M., Fredlund E. Deoxynivalenol and other selected Fusarium toxins in Swedish wheat—Occurrence and correlation to specific Fusarium species. Int. J. Food Microbiol. 2013;167:284–291. doi: 10.1016/j.ijfoodmicro.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 98.Liu J., Sun L., Zhang J., Guo J., Chen L., Qi D., Zhang N. Aflatoxin B1, zearalenone and deoxynivalenol in feed ingredients and complete feed from central China. Food Addit. Contam. Part B. 2016;9:91–97. doi: 10.1080/19393210.2016.1139003. [DOI] [PubMed] [Google Scholar]
- 99.Cavaglieri L.R., Gonzalez Pereyra L.M., Pereyra C.M., Magnoli C.E., Chulze S.N., Dalcero A.M. Proceedings of the International Conference. Fifth Framework Program. European Union Myco-Globe Project, Accra, Ghana, 13–16 September 2005. European Commission; Brussels, Belgium: 2005. Fungal and mycotoxin contamination of cow feeding stuffs in Argentina; pp. 13–16. [Google Scholar]
- 100.Wu L., Li J., Li Y., Li T., He Q., Tang Y., Liu H., Su Y., Yin Y., Liao P. Aflatoxin B 1, zearalenone and deoxynivalenol in feed ingredients and complete feed from different Province in China. J. Anim. Sci. Biotechnol. 2016;7:63. doi: 10.1186/s40104-016-0122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nuryono N., Noviandi C.T., Böhm J., Razzazi-Fazeli E. A limited survey of zearalenone in Indonesian maize-based food and feed by ELISA and high performance liquid chromatography. Food Control. 2005;16:65–71. doi: 10.1016/j.foodcont.2003.11.009. [DOI] [Google Scholar]
- 102.Kosicki R., Błajet-Kosicka A., Grajewski J., Twarużek M. Multiannual mycotoxin survey in feed materials and feedingstuffs. Anim. Feed Sci. Technol. 2016;215:165–180. doi: 10.1016/j.anifeedsci.2016.03.012. [DOI] [Google Scholar]
- 103.Kongkapan J., Poapolathep S., Isariyodom S., Kumagai S., Poapolathep A. Simultaneous detection of multiple mycotoxins in broiler feeds using a liquid chromatography tandem-mass spectrometry. J. Vet. Med. Sci. 2015;78:259–264. doi: 10.1292/jvms.15-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Janić Hajnal E., Kos J., Krulj J., Krstović S., Jajić I., Pezo L., Šarić B., Nedeljković N. Aflatoxins contamination of maize in Serbia: The impact of weather conditions in 2015. Food Addit. Contam. Part A. 2017;34:1999–2010. doi: 10.1080/19440049.2017.1331047. [DOI] [PubMed] [Google Scholar]
- 105.Tima H., Rácz A., Guld Z., Mohácsi-Farkas C., Kiskó G. Deoxynivalenol, zearalenone and T-2 in grain based swine feed in Hungary. Food Addit. Contam. Part B. 2016;9:275–280. doi: 10.1080/19393210.2016.1213318. [DOI] [PubMed] [Google Scholar]
- 106.Romera D., Mateo E.M., Mateo-Castro R., Gómez J.V., Gimeno-Adelantado J.V., Jiménez M. Determination of multiple mycotoxins in feedstuffs by combined use of UPLC–MS/MS and UPLC–QTOF–MS. Food Chem. 2018;267:140–148. doi: 10.1016/j.foodchem.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 107.Lee M.J., Kim H.J. Development of an immunoaffinity chromatography and LC-MS/MS method for the determination of 6 zearalenones in animal feed. PLoS ONE. 2018;13:e0193584. doi: 10.1371/journal.pone.0193584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Labuda R., Parich A., Berthiller F., Tančinová D. Incidence of trichothecenes and zearalenone in poultry feed mixtures from Slovakia. Int. J. Food Microbiol. 2005;105:19–25. doi: 10.1016/j.ijfoodmicro.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 109.Pietsch C., Kersten S., Burkhardt-Holm P., Valenta H., Dänicke S. Occurrence of deoxynivalenol and zearalenone in commercial fish feed: An initial study. Toxins. 2013;5:184–192. doi: 10.3390/toxins5010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu X., Hu R., Zhang Z., Li P., Zhang Q., Wang M. Development of a multiple immunoaffinity column for simultaneous determination of multiple mycotoxins in feeds using UPLC–MS/MS. Anal. Bioanal. Chem. 2016;408:6027–6036. doi: 10.1007/s00216-016-9626-5. [DOI] [PubMed] [Google Scholar]
- 111.Koteswara Rao V., Girisham S., Madhusudhan Reddy S. Prevalence of toxigenic Penicillium species associated with poultry house in Telangana, India. Arch. Environ. Occup. Health. 2016;71:353–361. doi: 10.1080/19338244.2016.1140627. [DOI] [PubMed] [Google Scholar]
- 112.Gizachew D., Szonyi B., Tegegne A., Hanson J., Grace D. Aflatoxin contamination of milk and dairy feeds in the Greater Addis Ababa milk shed, Ethiopia. Food Control. 2016;59:773–779. doi: 10.1016/j.foodcont.2015.06.060. [DOI] [Google Scholar]
- 113.Chilaka C.A., De Boevre M., Atanda O.O., De Saeger S. The status of Fusarium mycotoxins in sub-Saharan Africa: A review of emerging trends and post-harvest mitigation strategies towards food control. Toxins. 2017;9:19. doi: 10.3390/toxins9010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mathlouthi M. Water content, water activity, water structure and the stability of foodstuffs. Food Control. 2001;12:409–417. doi: 10.1016/S0956-7135(01)00032-9. [DOI] [Google Scholar]
- 115.Barbosa-Cánovas G.V., Fontana A.J., Jr., Schmidt S.J., Labuza T.P. Water Activity in Foods: Fundamentals and Applications. John Wiley & Sons; Hoboken, NJ, USA: 2020. [Google Scholar]
- 116.Stanciu O., Juan C., Berrada H., Miere D., Loghin F., Mañes J. Study on trichothecene and zearalenone presence in romanian wheat relative to weather conditions. Toxins. 2019;11:163. doi: 10.3390/toxins11030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mannaa M., Kim K.D. Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology. 2017;45:240–254. doi: 10.5941/MYCO.2017.45.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pleadin J., Frece J., Lešić T., Zadravec M., Vahčić N., Malenica Staver M., Markov K. Deoxynivalenol and zearalenone in unprocessed cereals and soybean from different cultivation regions in Croatia. Food Addit. Contam. Part B. 2017;10:268–274. doi: 10.1080/19393210.2017.1345991. [DOI] [PubMed] [Google Scholar]
- 119.Habschied K., Šarkanj B., Klapec T., Krstanović V. Distribution of zearalenone in malted barley fractions dependent on Fusarium graminearum growing conditions. Food Chem. 2011;129:329–332. doi: 10.1016/j.foodchem.2011.04.064. [DOI] [PubMed] [Google Scholar]
- 120.Wu L., Qiu L., Zhang H., Sun J., Hu X., Wang B. Optimization for the Production of Deoxynivalenoland Zearalenone by Fusarium graminearum Using Response Surface Methodology. Toxins. 2017;9:57. doi: 10.3390/toxins9020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hamed M.A., Abdel Ghany T.M., Elhussieny N.I., Nabih M.A. Exploration of fungal infection in agricultural grains, aflatoxin and zearalenone synthesis under pH stress. Int. J. Curr. Microbiol. App. Sci. 2016;5:1007–1017. doi: 10.20546/ijcmas.2016.504.115. [DOI] [Google Scholar]
- 122.Velluti A., Sanchis V., Ramos A.J., Turon C., Marin S. Impact of essential oils on growth rate, zearalenone and deoxynivalenol production by Fusarium graminearum under different temperature and water activity conditions in maize grain. J. Appl. Microbiol. 2004;96:716–724. doi: 10.1111/j.1365-2672.2004.02212.x. [DOI] [PubMed] [Google Scholar]
- 123.Martins M.L., Martins H.M. Influence of water activity, temperature and incubation time on the simultaneous production of deoxynivalenol and zearalenone in corn (Zea mays) by Fusarium graminearum. Food Chem. 2002;79:315–318. doi: 10.1016/S0308-8146(02)00147-4. [DOI] [Google Scholar]
- 124.Peter Mshelia L., Selamat J., Iskandar Putra Samsudin N., Rafii M.Y., Abdul Mutalib N.-A., Nordin N., Berthiller F. Effect of Temperature, Water Activity and Carbon Dioxide on Fungal Growth and Mycotoxin Production of Acclimatised Isolates of Fusarium verticillioides and F. graminearum. Toxins. 2020;12:478. doi: 10.3390/toxins12080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Poór M., Kunsági-Máté S., Bálint M., Hetényi C., Gerner Z., Lemli B. Interaction of mycotoxin zearalenone with human serum albumin. J. Photochem. Photobiol. B Biol. 2017;170:16–24. doi: 10.1016/j.jphotobiol.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 126.Grenier B., Applegate T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins. 2013;5:396–430. doi: 10.3390/toxins5020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheat S., Pinton P., Cossalter A.-M., Cognie J., Vilariño M., Callu P., Raymond-Letron I., Oswald I.P., Kolf-Clauw M. The mycotoxins deoxynivalenol and nivalenol show in vivo synergism on jejunum enterocytes apoptosis. Food Chem. Toxicol. 2016;87:45–54. doi: 10.1016/j.fct.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 128.Mally A., Solfrizzo M., Degen G.H. Biomonitoring of the mycotoxin Zearalenone: Current state-of-the art and application to human exposure assessment. Arch. Toxicol. 2016;90:1281–1292. doi: 10.1007/s00204-016-1704-0. [DOI] [PubMed] [Google Scholar]
- 129.Kollarczik B., Gareis M., Hanelt M. In vitro transformation of the Fusarium mycotoxins deoxynivalenol and zearalenone by the normal gut microflora of pigs. Nat. Toxins. 1994;2:105–110. doi: 10.1002/nt.2620020303. [DOI] [PubMed] [Google Scholar]
- 130.Braicu C., Selicean S., Cojocneanu-Petric R., Lajos R., Balacescu O., Taranu I., Marin D.E., Motiu M., Jurj A., Achimas-Cadariu P. Evaluation of cellular and molecular impact of zearalenone and Escherichia coli co-exposure on IPEC-1 cells using microarray technology. BMC Genom. 2016;17:576. doi: 10.1186/s12864-016-2830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Obremski K. Changes in Th1 and Th2 cytokine concentrations in ileal Peyer’s patches in gilts exposed to zearalenone. Pol. J. Vet. Sci. 2014;17:53–59. doi: 10.2478/pjvs-2014-0007. [DOI] [PubMed] [Google Scholar]
- 132.Cheng Q., Jiang S., Huang L., Ge J., Wang Y., Yang W. Zearalenone induced oxidative stress in the jejunum in postweaning gilts through modulation of the Keap1–Nrf2 signaling pathway and relevant genes. J. Anim. Sci. 2019;97:1722–1733. doi: 10.1093/jas/skz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gerez J.R., Pinton P., Callu P., Grosjean F., Oswald I.P., Bracarense A.P.F.L. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2015;67:89–98. doi: 10.1016/j.etp.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 134.Abbès S., Ouanes Z., Salah-Abbès J.B., Abdel-Wahhab M.A., Oueslati R., Bacha H. Preventive role of aluminosilicate clay against induction of micronuclei and chromosome aberrations in bone-marrow cells of Balb/c mice treated with Zearalenone. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis. 2007;631:85–92. doi: 10.1016/j.mrgentox.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 135.Fleck S.C., Hildebrand A.A., Müller E., Pfeiffer E., Metzler M. Genotoxicity and inactivation of catechol metabolites of the mycotoxin zearalenone. Mycotoxin Res. 2012;28:267–273. doi: 10.1007/s12550-012-0143-x. [DOI] [PubMed] [Google Scholar]
- 136.Abid-Essefi S., Baudrimont I., Hassen W., Ouanes Z., Mobio T.A., Anane R., Creppy E.E., Bacha H. DNA fragmentation, apoptosis and cell cycle arrest induced by zearalenone in cultured DOK, Vero and Caco-2 cells: Prevention by Vitamin E. Toxicology. 2003;192:237–248. doi: 10.1016/S0300-483X(03)00329-9. [DOI] [PubMed] [Google Scholar]
- 137.Ouanes Z., Ayed-Boussema I., Baati T., Creppy E.E., Bacha H. Zearalenone induces chromosome aberrations in mouse bone marrow: Preventive effect of 17β-estradiol, progesterone and Vitamin E. Mutat. Res. 2005;565:139–149. doi: 10.1016/j.mrgentox.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 138.Salah-Abbès J.B., Abbès S., Houas Z., Abdel-Wahhab M.A., Oueslati R. Zearalenone induces immunotoxicity in mice: Possible protective effects of radish extract (Raphanus sativus) J. Pharm. Pharmacol. 2008;60:761–770. doi: 10.1211/jpp.60.6.0012. [DOI] [PubMed] [Google Scholar]
- 139.Čonková E., Laciaková A., Pástorová B., Seidel H., Kováč G. The effect of zearalenone on some enzymatic parameters in rabbits. Toxicol. Lett. 2001;121:145–149. doi: 10.1016/S0378-4274(01)00312-5. [DOI] [PubMed] [Google Scholar]
- 140.Islam M.R., Kim J.W., Roh Y.-S., Kim J.-H., Han K.M., Kwon H.-J., Lim C.W., Kim B. Evaluation of immunomodulatory effects of zearalenone in mice. J. Immunotoxicol. 2017;14:125–136. doi: 10.1080/1547691X.2017.1340371. [DOI] [PubMed] [Google Scholar]
- 141.Zhou M., Yang L., Chen Y., Sun T., Wang N., Chen X., Yang Z., Ge J., Jiang S. Comparative study of stress response, growth and development of uteri in post-weaning gilts challenged with zearalenone and estradiol benzoate. J. Anim. Physiol. Anim. Nutr. 2019;103:1885–1894. doi: 10.1111/jpn.13195. [DOI] [PubMed] [Google Scholar]
- 142.Meurens F., Summerfield A., Nauwynck H., Saif L., Gerdts V. The pig: A model for human infectious diseases. Trends Microbiol. 2012;20:50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kowalska K., Habrowska-Górczyńska D.E., Piastowska-Ciesielska A.W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Pharmacol. 2016;48:141–149. doi: 10.1016/j.etap.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 144.Metzler M., Pfeiffer E., Hildebrand A. Zearalenone and its metabolites as endocrine disrupting chemicals. World Mycotoxin J. 2010;3:385–401. doi: 10.3920/WMJ2010.1244. [DOI] [Google Scholar]
- 145.Zhu L., Yuan H., Guo C., Lu Y., Deng S., Yang Y., Wei Q., Wen L., He Z. Zearalenone induces apoptosis and necrosis in porcine granulosa cells via a caspase-3-and caspase-9-dependent mitochondrial signaling pathway. J. Cell. Physiol. 2012;227:1814–1820. doi: 10.1002/jcp.22906. [DOI] [PubMed] [Google Scholar]
- 146.Zhang R.-Q., Sun X.-F., Wu R.-Y., Cheng S.-F., Zhang G.-L., Zhai Q.-Y., Liu X.-L., Zhao Y., Shen W., Li L. Zearalenone exposure elevated the expression of tumorigenesis genes in mouse ovarian granulosa cells. Toxicol. Appl. Pharmacol. 2018;356:191–203. doi: 10.1016/j.taap.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 147.Assumaidaee A.A.M., Ali N.M., Ahmed S.W. Zearalenone Mycotoxicosis: Pathophysiology and Immunotoxicity. Iraqi J. Vet. Med. 2020;44:29–38. doi: 10.30539/ijvm.v44i1.932. [DOI] [Google Scholar]
- 148.Massart F., Meucci V., Saggese G., Soldani G. High growth rate of girls with precocious puberty exposed to estrogenic mycotoxins. J. Pediatr. 2008;152:690–695. doi: 10.1016/j.jpeds.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 149.Yang Z., Xue K.S., Sun X., Williams P.L., Wang J.-S., Tang L. Toxicogenomic responses to zearalenone in Caenorhabditis elegans reveal possible molecular mechanisms of reproductive toxicity. Food Chem. Toxicol. 2018;122:49–58. doi: 10.1016/j.fct.2018.09.040. [DOI] [PubMed] [Google Scholar]
- 150.Yousef M.S., Takagi M., Talukder A.K., Marey M.A., Kowsar R., Abdel-Razek A.-R.K., Shimizu T., Fink-Gremmels J., Miyamoto A. Zearalenone (ZEN) disrupts the anti-inflammatory response of bovine oviductal epithelial cells to sperm in vitro. Reprod. Toxicol. 2017;74:158–163. doi: 10.1016/j.reprotox.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 151.Massart F., Micillo F., Rivezzi G., Perrone L., Baggiani A., Miccoli M., Meucci V. Zearalenone screening of human breast milk from the Naples area. Toxicol. Environ. Chem. 2016;98:128–136. doi: 10.1080/02772248.2015.1101112. [DOI] [Google Scholar]
- 152.Minervini F., Dell’Aquila M.E. Zearalenone and reproductive function in farm animals. Int. J. Mol. Sci. 2008;9:2570–2584. doi: 10.3390/ijms9122570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mauro T., Hao L., Pop L.C., Buckley B., Schneider S.H., Bandera E.V., Shapses S.A. Circulating zearalenone and its metabolites differ in women due to body mass index and food intake. Food Chem. Toxicol. 2018;116:227–232. doi: 10.1016/j.fct.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hueza I.M., Raspantini P.C.F., Raspantini L.E.R., Latorre A.O., Gornjak S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins. 2014;6:1080–1095. doi: 10.3390/toxins6031080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tsuchiya Y., Nakajima M., Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227:115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 156.Pfeiffer E., Hildebrand A., Damm G., Rapp A., Cramer B., Humpf H.U., Metzler M. Aromatic hydroxylation is a major metabolic pathway of the mycotoxin zearalenone in vitro. Mol. Nutr. Food Res. 2009;53:1123–1133. doi: 10.1002/mnfr.200800584. [DOI] [PubMed] [Google Scholar]
- 157.Kovacevic Z., Sahni S., Lok H., Davies M.J., Wink D.A., Richardson D.R. Regulation and control of nitric oxide (NO) in macrophages: Protecting the “professional killer cell” from its own cytotoxic arsenal via MRP1 and GSTP1. Biochim. Biophys. Acta. Gen. Subj. 2017;1861:995–999. doi: 10.1016/j.bbagen.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 158.Billat P.-A., Roger E., Faure S., Lagarce F. Models for drug absorption from the small intestine: Where are we and where are we going? Drug Discov. Today. 2017;22:761–775. doi: 10.1016/j.drudis.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 159.Salah-Abbès J.B., Abbès S., Ouanes Z., Houas Z., Abdel-Wahhab M.A., Bacha H., Oueslati R. Tunisian radish extract (Raphanus sativus) enhances the antioxidant status and protects against oxidative stress induced by zearalenone in Balb/c mice. J. Appl. Toxicol. 2008;28:6–14. doi: 10.1002/jat.1240. [DOI] [PubMed] [Google Scholar]
- 160.Marin M.L., Murtha J., Dong W., Pestka J.J. Effects of mycotoxins on cytokine production and proliferation in EL-4 thymoma cells. J. Toxicol. Environ. Health. 1996;48:379–396. doi: 10.1080/009841096161267. [DOI] [PubMed] [Google Scholar]
- 161.Pleadin J., Babić J., Vulić A., Kudumija N., Aladić K., Kiš M., Jaki Tkalec V., Škrivanko M., Lolić M., Šubarić D. The effect of thermal processing on the reduction of deoxynivalenol and zearalenone cereal content. Croat. J. Food Sci. Technol. 2019;11:44–51. doi: 10.17508/CJFST.2019.11.1.06. [DOI] [Google Scholar]
- 162.Luo X., Qi L., Liu Y., Wang R., Yang D., Li K., Wang L., Li Y., Zhang Y., Chen Z. Effects of electron beam irradiation on zearalenone and ochratoxin a in naturally contaminated corn and corn quality parameters. Toxins. 2017;9:84. doi: 10.3390/toxins9030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Luo X., Zhai Y., Qi L., Pan L., Wang J., Xing J., Wang R., Wang L., Zhang Q., Yang K. Influences of Electron Beam Irradiation on the Physical and Chemical Properties of Zearalenone-and Ochratoxin A-Contaminated Corn and In Vivo Toxicity Assessment. Foods. 2020;9:376. doi: 10.3390/foods9030376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Calado T., Abrunhosa L., Cabo Verde S., Alté L., Venâncio A., Fernández-Cruz M.L. Effect of Gamma-Radiation on Zearalenone—Degradation, Cytotoxicity and Estrogenicity. Foods. 2020;9:1687. doi: 10.3390/foods9111687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hojnik N., Modic M., Tavčar-Kalcher G., Babič J., Walsh J.L., Cvelbar U. Mycotoxin Decontamination Efficacy of Atmospheric Pressure Air Plasma. Toxins. 2019;11:219. doi: 10.3390/toxins11040219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Drzymala S.S., Weiz S., Heinze J., Marten S., Prinz C., Zimathies A., Garbe L.-A., Koch M. Automated solid-phase extraction coupled online with HPLC-FLD for the quantification of zearalenone in edible oil. Anal. Bioanal. Chem. 2015;407:3489–3497. doi: 10.1007/s00216-015-8541-5. [DOI] [PubMed] [Google Scholar]
- 167.Ok H.E., Choi S.-W., Kim M., Chun H.S. HPLC and UPLC methods for the determination of zearalenone in noodles, cereal snacks and infant formula. Food Chem. 2014;163:252–257. doi: 10.1016/j.foodchem.2014.04.111. [DOI] [PubMed] [Google Scholar]
- 168.Senyuva H.Z., Gilbert J., Türköz G., Leeman D., Donnelly C. Analysis of Deoxynivalenol, Zearalenone, T-2, and HT-2 Toxins in Animal Feed by LC/MS/MS–A Critical Comparison of Immunoaffinity Column Cleanup with No Cleanup. J. AOAC Int. 2012;95:1701–1708. doi: 10.5740/jaoacint.11-523. [DOI] [PubMed] [Google Scholar]
- 169.Vendl O., Berthiller F., Crews C., Krska R. Simultaneous determination of deoxynivalenol, zearalenone, and their major masked metabolites in cereal-based food by LC–MS–MS. Anal. Bioanal. Chem. 2009;395:1347–1354. doi: 10.1007/s00216-009-2873-y. [DOI] [PubMed] [Google Scholar]
- 170.Rodríguez-Carrasco Y., Moltó J.C., Berrada H., Mañes J. A survey of trichothecenes, zearalenone and patulin in milled grain-based products using GC–MS/MS. Food Chem. 2014;146:212–219. doi: 10.1016/j.foodchem.2013.09.053. [DOI] [PubMed] [Google Scholar]
- 171.Wang G., Chen W., Hu J., Fan B., Shi J., Xu J. Preparative isolation and purification of zearalenone from rice culture by combined use of macroporous resin column and high-speed counter-current chromatography. J. Chromatogr. B. 2019;1110:43–50. doi: 10.1016/j.jchromb.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 172.Anfossi L., Di Nardo F., Cavalera S., Giovannoli C., Spano G., Speranskaya E.S., Goryacheva I.Y., Baggiani C. A lateral flow immunoassay for straightforward determination of fumonisin mycotoxins based on the quenching of the fluorescence of CdSe/ZnS quantum dots by gold and silver nanoparticles. Microchim. Acta. 2018;185:94. doi: 10.1007/s00604-017-2642-0. [DOI] [PubMed] [Google Scholar]
- 173.Yang Y., Li W., Shen P., Liu R., Li Y., Xu J., Zheng Q., Zhang Y., Li J., Zheng T. Aptamer fluorescence signal recovery screening for multiplex mycotoxins in cereal samples based on photonic crystal microsphere suspension array. Sens. Actuators B Chem. 2017;248:351–358. doi: 10.1016/j.snb.2017.04.004. [DOI] [Google Scholar]
- 174.Zhang X., Yu X., Wen K., Li C., Mujtaba Mari G., Jiang H., Shi W., Shen J., Wang Z. Multiplex lateral flow immunoassays based on amorphous carbon nanoparticles for detecting three Fusarium mycotoxins in maize. J. Agric. Food Chem. 2017;65:8063–8071. doi: 10.1021/acs.jafc.7b02827. [DOI] [PubMed] [Google Scholar]
- 175.Guan D., Li P., Zhang Q., Zhang W., Zhang D., Jiang J. An ultra-sensitive monoclonal antibody-based competitive enzyme immunoassay for aflatoxin M1 in milk and infant milk products. Food Chem. 2011;125:1359–1364. doi: 10.1016/j.foodchem.2010.10.006. [DOI] [Google Scholar]
- 176.Li X., Li P., Zhang Q., Li R., Zhang W., Zhang Z., Ding X., Tang X. Multi-component immunochromatographic assay for simultaneous detection of aflatoxin B1, ochratoxin A and zearalenone in agro-food. Biosens. Bioelectron. 2013;49:426–432. doi: 10.1016/j.bios.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 177.Wang Y., Liu N., Ning B., Liu M., Lv Z., Sun Z., Peng Y., Chen C., Li J., Gao Z. Simultaneous and rapid detection of six different mycotoxins using an immunochip. Biosens. Bioelectron. 2012;34:44–50. doi: 10.1016/j.bios.2011.12.057. [DOI] [PubMed] [Google Scholar]
- 178.Zhang J., Xia Y.-K., Chen M., Wu D.-Z., Cai S.-X., Liu M.-M., He W.-H., Chen J.-H. A fluorescent aptasensor based on DNA-scaffolded silver nanoclusters coupling with Zn (II)-ion signal-enhancement for simultaneous detection of OTA and AFB1. Sens. Actuators B Chem. 2016;235:79–85. doi: 10.1016/j.snb.2016.05.061. [DOI] [Google Scholar]
- 179.Li C., Mi T., Oliveri Conti G., Yu Q., Wen K., Shen J., Ferrante M., Wang Z. Development of a screening fluorescence polarization immunoassay for the simultaneous detection of fumonisins B1 and B2 in maize. J. Agric. Food Chem. 2015;63:4940–4946. doi: 10.1021/acs.jafc.5b01845. [DOI] [PubMed] [Google Scholar]
- 180.Li C., Wen K., Mi T., Zhang X., Zhang H., Zhang S., Shen J., Wang Z. A universal multi-wavelength fluorescence polarization immunoassay for multiplexed detection of mycotoxins in maize. Biosens. Bioelectron. 2016;79:258–265. doi: 10.1016/j.bios.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 181.Kolosova A.Y., De Saeger S., Sibanda L., Verheijen R., Van Peteghem C. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of zearalenone and deoxynivalenol. Anal. Bioanal. Chem. 2007;389:2103–2107. doi: 10.1007/s00216-007-1642-z. [DOI] [PubMed] [Google Scholar]
- 182.Lattanzio V.M., Nivarlet N. Oat. Humana Press; New York, NY, USA: 2017. Multiplex dipstick immunoassay for semiquantitative determination of fusarium mycotoxins in oat; pp. 137–142. [DOI] [PubMed] [Google Scholar]
- 183.Wang Y., Ning B., Peng Y., Bai J., Liu M., Fan X., Sun Z., Lv Z., Zhou C., Gao Z. Application of suspension array for simultaneous detection of four different mycotoxins in corn and peanut. Biosens. Bioelectron. 2013;41:391–396. doi: 10.1016/j.bios.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 184.Liu G., Han Z., Nie D., Yang J., Zhao Z., Zhang J., Li H., Liao Y., Song S., De Saeger S. Rapid and sensitive quantitation of zearalenone in food and feed by lateral flow immunoassay. Food Control. 2012;27:200–205. doi: 10.1016/j.foodcont.2012.03.023. [DOI] [Google Scholar]
- 185.Jin Y., Chen Q., Luo S., He L., Fan R., Zhang S., Yang C., Chen Y. Dual near-infrared fluorescence-based lateral flow immunosensor for the detection of zearalenone and deoxynivalenol in maize. Food Chem. 2020;336:127718. doi: 10.1016/j.foodchem.2020.127718. [DOI] [PubMed] [Google Scholar]
- 186.Li X., Wang J., Yi C., Jiang L., Wu J., Chen X., Shen X., Sun Y., Lei H. A smartphone-based quantitative detection device integrated with latex microsphere immunochromatography for on-site detection of zearalenone in cereals and feed. Sens. Actuators B Chem. 2019;290:170–179. doi: 10.1016/j.snb.2019.03.108. [DOI] [Google Scholar]
- 187.Ren X., Zhang Q., Wu W., Yan T., Tang X., Zhang W., Yu L., Li P. Anti-idiotypic nanobody-phage display-mediated real-time immuno-PCR for sensitive, simultaneous and quantitative detection of total aflatoxins and zearalenone in grains. Food Chem. 2019;297:124912. doi: 10.1016/j.foodchem.2019.05.186. [DOI] [PubMed] [Google Scholar]
- 188.Caglayan M.O., Üstündağ Z. Detection of zearalenone in an aptamer assay using attenuated internal reflection ellipsometry and it’s cereal sample applications. Food Chem. Toxicol. 2020;136:111081. doi: 10.1016/j.fct.2019.111081. [DOI] [PubMed] [Google Scholar]
- 189.Rychlik M., Humpf H.-U., Marko D., Dänicke S., Mally A., Berthiller F., Klaffke H., Lorenz N. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res. 2014;30:197–205. doi: 10.1007/s12550-014-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Berthiller F., Crews C., Dall’Asta C., Saeger S.D., Haesaert G., Karlovsky P., Oswald I.P., Seefelder W., Speijers G., Stroka J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013;57:165–186. doi: 10.1002/mnfr.201100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Plasencia J., Mirocha C.J. Isolation and characterization of zearalenone sulfate produced by Fusarium spp. Appl. Environ. Microbiol. 1991;57:146–150. doi: 10.1128/AEM.57.1.146-150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Poppenberger B., Berthiller F., Lucyshyn D., Sieberer T., Schuhmacher R., Krska R., Kuchler K., Glössl J., Luschnig C., Adam G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003;278:47905–47914. doi: 10.1074/jbc.M307552200. [DOI] [PubMed] [Google Scholar]
- 193.Berthiller F., Schuhmacher R., Adam G., Krska R. Formation, determination and significance of masked and other conjugated mycotoxins. Anal. Bioanal. Chem. 2009;395:1243–1252. doi: 10.1007/s00216-009-2874-x. [DOI] [PubMed] [Google Scholar]
- 194.Coleman J., Blake-Kalff M., Davies E. Detoxification of xenobiotics by plants: Chemical modification and vacuolar compartmentation. Trends Plant Sci. 1997;2:144–151. doi: 10.1016/S1360-1385(97)01019-4. [DOI] [Google Scholar]
- 195.He J., Zhou T., Young J.C., Boland G.J., Scott P.M. Chemical and biological transformations for detoxification of trichothecene mycotoxins in human and animal food chains: A review. Trends Food Sci. Technol. 2010;21:67–76. doi: 10.1016/j.tifs.2009.08.002. [DOI] [Google Scholar]
- 196.Engelhardt G., Zill G., Wohner B., Wallnöfer P.R. Transformation of the Fusarium mycotoxin zearalenone in maize cell suspension cultures. Naturwissenschaften. 1988;75:309–310. doi: 10.1007/BF00367324. [DOI] [PubMed] [Google Scholar]
- 197.Berthiller F., Werner U., Sulyok M., Krska R., Hauser M.-T., Schuhmacher R. Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) determination of phase II metabolites of the mycotoxin zearalenone in the model plant Arabidopsis thaliana. Food Addit. Contam. 2006;23:1194–1200. doi: 10.1080/02652030600778728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vendl O., Crews C., MacDonald S., Krska R., Berthiller F. Occurrence of free and conjugated Fusarium mycotoxins in cereal-based food. Food Addit. Contam. 2010;27:1148–1152. doi: 10.1080/19440041003801166. [DOI] [PubMed] [Google Scholar]
- 199.Huang L.C., Zheng N., Zheng B.Q., Wen F., Cheng J.B., Han R.W., Xu X.M., Li S.L., Wang J.Q. Simultaneous determination of aflatoxin M1, ochratoxin A, zearalenone and α-zearalenol in milk by UHPLC–MS/MS. Food Chem. 2014;146:242–249. doi: 10.1016/j.foodchem.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 200.Han Z., Ren Y., Zhou H., Luan L., Cai Z., Wu Y. A rapid method for simultaneous determination of zearalenone, α-zearalenol, β-zearalenol, zearalanone, α-zearalanol and β-zearalanol in traditional Chinese medicines by ultra-high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B. 2011;879:411–420. doi: 10.1016/j.jchromb.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 201.Belhassen H., Jiménez-Díaz I., Ghali R., Ghorbel H., Molina-Molina J.M., Olea N., Hedili A. Validation of a UHPLC–MS/MS method for quantification of zearalenone, α-zearalenol, β-zearalenol, α-zearalanol, β-zearalanol and zearalanone in human urine. J. Chromatogr. B. 2014;962:68–74. doi: 10.1016/j.jchromb.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 202.Grabley S., Gareis M., Böckers W., Thiem J. Glycosylation of mycotoxins. Synthesis. 1992;1992:1078–1080. doi: 10.1055/s-1992-26306. [DOI] [Google Scholar]
- 203.Berthiller F., Krska R., Domig K.J., Kneifel W., Juge N., Schuhmacher R., Adam G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011;206:264–267. doi: 10.1016/j.toxlet.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mahato D.K., Lee K.E., Kamle M., Devi S., Dewangan K., Kumar P., Kang S.G. Aflatoxins in food and feed: An overview on prevalence, detection and control strategies. Front. Microbiol. 2019;10:2266. doi: 10.3389/fmicb.2019.02266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kumar P., Mahato D.K., Sharma B., Borah R., Haque S., Mahmud M.M.C., Shah A.K., Rawal D., Bora H., Bui S. Ochratoxins in food and feed: Occurrence and its impact on human health and management strategies. Toxicon. 2020;187:151–162. doi: 10.1016/j.toxicon.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 206.Kamle M., Mahato D.K., Devi S., Lee K.E., Kang S.G., Kumar P. Fumonisins: Impact on agriculture, food, and human health and their management strategies. Toxins. 2019;11:328. doi: 10.3390/toxins11060328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang Z., Xu W., Wu H., Zhang W., Mu W. Identification of a Potent Enzyme for the Detoxification of Zearalenone. J. Agric. Food Chem. 2019;68:376–383. doi: 10.1021/acs.jafc.9b06223. [DOI] [PubMed] [Google Scholar]
- 208.Beloglazova N.V., De Boevre M., Goryacheva I.Y., Werbrouck S., Guo Y., De Saeger S. Immunochemical approach for zearalenone-4-glucoside determination. Talanta. 2013;106:422–430. doi: 10.1016/j.talanta.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 209.Vidal A., Marín S., Sanchis V., De Saeger S., De Boevre M. Hydrolysers of modified mycotoxins in maize: α-Amylase and cellulase induce an underestimation of the total aflatoxin content. Food Chem. 2018;248:86–92. doi: 10.1016/j.foodchem.2017.12.057. [DOI] [PubMed] [Google Scholar]
- 210.El-Sharkaway S.H., Selim M.I., Afifi M.S., Halaweish F.T. Microbial transformation of zearalenone to a zearalenone sulfate. Appl. Environ. Microbiol. 1991;57:549–552. doi: 10.1128/AEM.57.2.549-552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kamimura H. Conversion of zearalenone to zearalenone glycoside by Rhizopus sp. Appl. Environ. Microbiol. 1986;52:515–519. doi: 10.1128/AEM.52.3.515-519.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Boutigny A.-L., Richard-Forget F., Barreau C. Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. Eur. J. Plant Pathol. 2008;121:411–423. doi: 10.1007/s10658-007-9266-x. [DOI] [Google Scholar]
- 213.EFSA Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to zearalenone as undesirable substance in animal feed. EFSA J. 2004;89:1–35. doi: 10.2903/j.efsa.2004.89. [DOI] [Google Scholar]
- 214.Mirocha C.J., Pathre S.V., Robison T.S. Comparative metabolism of zearalenone and transmission into bovine milk. Food Cosmet. Toxicol. 1981;19:25–30. doi: 10.1016/0015-6264(81)90299-6. [DOI] [PubMed] [Google Scholar]
- 215.Olsen M., Mirocha C.J., Abbas H.K., Johansson B. Metabolism of high concentrations of dietary zearalenone by young male turkey poults. Poult. Sci. 1986;65:1905–1910. doi: 10.3382/ps.0651905. [DOI] [PubMed] [Google Scholar]
- 216.Zhang H., Dong M., Yang Q., Apaliya M.T., Li J., Zhang X. Biodegradation of zearalenone by Saccharomyces cerevisiae: Possible involvement of ZEN responsive proteins of the yeast. J. Proteom. 2016;143:416–423. doi: 10.1016/j.jprot.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 217.Numanoglu E., Yener S., Gökmen V., Uygun U., Koksel H. Modelling thermal degradation of zearalenone in maize bread during baking. Food Addit. Contam. Part A. 2013;30:528–533. doi: 10.1080/19440049.2012.751629. [DOI] [PubMed] [Google Scholar]
- 218.Bai X., Sun C., Xu J., Liu D., Han Y., Wu S., Luo X. Detoxification of zearalenone from corn oil by adsorption of functionalized GO systems. Appl. Surf. Sci. 2018;430:198–207. doi: 10.1016/j.apsusc.2017.06.055. [DOI] [Google Scholar]
- 219.Wang M., Yin L., Hu H., Selvaraj J.N., Zhou Y., Zhang G. Expression, functional analysis and mutation of a novel neutral zearalenone-degrading enzyme. Int. J. Biol. Macromol. 2018;118:1284–1292. doi: 10.1016/j.ijbiomac.2018.06.111. [DOI] [PubMed] [Google Scholar]
- 220.Wang G., Xi Y., Lian C., Sun Z., Zheng S. Simultaneous detoxification of polar aflatoxin B1 and weak polar zearalenone from simulated gastrointestinal tract by zwitterionic montmorillonites. J. Hazard. Mater. 2019;364:227–237. doi: 10.1016/j.jhazmat.2018.09.071. [DOI] [PubMed] [Google Scholar]
- 221.Olopade B.K., Oranusi S.U., Nwinyi O.C., Njobeh P.B., Lawal I.A. Modification of montmorillonite clay with Cymbopogon citratus for the decontamination of zearalenone in millet. AIMS Agric. Food. 2019;4:643–657. doi: 10.3934/agrfood.2019.3.643. [DOI] [Google Scholar]
- 222.Sun Z., Song A., Wang B., Wang G., Zheng S. Adsorption behaviors of aflatoxin B1 and zearalenone by organo-rectorite modified with quaternary ammonium salts. J. Mol. Liq. 2018;264:645–651. doi: 10.1016/j.molliq.2018.05.091. [DOI] [Google Scholar]
- 223.Marković M., Daković A., Rottinghaus G.E., Kragović M., Petković A., Krajišnik D., Milić J., Mercurio M., de Gennaro B. Adsorption of the mycotoxin zearalenone by clinoptilolite and phillipsite zeolites treated with cetylpyridinium surfactant. Colloids Surf. B Biointerfaces. 2017;151:324–332. doi: 10.1016/j.colsurfb.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 224.Sprynskyy M., Gadzała-Kopciuch R., Nowak K., Buszewski B. Removal of zearalenone toxin from synthetics gastric and body fluids using talc and diatomite: A batch kinetic study. Colloids Surf. B Biointerfaces. 2012;94:7–14. doi: 10.1016/j.colsurfb.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 225.Kalagatur N.K., Karthick K., Allen J.A., Nirmal Ghosh O.S., Chandranayaka S., Gupta V.K., Krishna K., Mudili V. Application of activated carbon derived from seed shells of Jatropha curcas for decontamination of zearalenone mycotoxin. Front. Pharmacol. 2017;8:760. doi: 10.3389/fphar.2017.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Spasojević M.P., Dakovic A., Rottinghaus G.E., Mihajlović A.S.R., Marković M.A., Krajišnik D.R. Zearalenone and ochratoxin A: Adsorption by kaolin modified with surfactant. Metall. Mater. Eng. 2019;25:39–45. doi: 10.30544/413. [DOI] [Google Scholar]
- 227.Yang K., Li K., Pan L., Luo X., Xing J., Wang J., Wang L., Wang R., Zhai Y., Chen Z. Effect of Ozone and Electron Beam Irradiation on Degradation of Zearalenone and Ochratoxin A. Toxins. 2020;12:138. doi: 10.3390/toxins12020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Qi L., Li Y., Luo X., Wang R., Zheng R., Wang L., Li Y., Yang D., Fang W., Chen Z. Detoxification of zearalenone and ochratoxin A by ozone and quality evaluation of ozonised corn. Food Addit. Contam. Part A. 2016;33:1700–1710. doi: 10.1080/19440049.2016.1232863. [DOI] [PubMed] [Google Scholar]
- 229.Xu Y., Wang Y., Ji J., Wu H., Pi F., Zhang Y., Sun X. Chemical and toxicological alterations of zearalenone under ozone treatment. Food Addit. Contam. Part A. 2019;36:163–174. doi: 10.1080/19440049.2018.1547425. [DOI] [PubMed] [Google Scholar]
- 230.Alexandre A.P.S., Castanha N., Costa N.S., Santos A.S., Badiale-Furlong E., Augusto P.E.D., Calori-Domingues M.A. Ozone technology to reduce zearalenone contamination in whole maize flour: Degradation kinetics and impact on quality. J. Sci. Food Agric. 2019;99:6814–6821. doi: 10.1002/jsfa.9966. [DOI] [PubMed] [Google Scholar]
- 231.Wang N., Wu W., Pan J., Long M. Detoxification strategies for zearalenone using microorganisms: A review. Microorganisms. 2019;7:208. doi: 10.3390/microorganisms7070208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chlebicz A., Śliżewska K. In vitro detoxification of aflatoxin B 1, deoxynivalenol, fumonisins, T-2 toxin and zearalenone by probiotic bacteria from genus Lactobacillus and Saccharomyces cerevisiae yeast. Probiotics Antimicrob. Proteins. 2020;12:289–301. doi: 10.1007/s12602-018-9512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sangsila A., Faucet-Marquis V., Pfohl-Leszkowicz A., Itsaranuwat P. Detoxification of zearalenone by Lactobacillus pentosus strains. Food Control. 2016;62:187–192. doi: 10.1016/j.foodcont.2015.10.031. [DOI] [Google Scholar]
- 234.Zhao L., Jin H., Lan J., Zhang R., Ren H., Zhang X., Yu G. Detoxification of zearalenone by three strains of Lactobacillus plantarum from fermented food in vitro. Food Control. 2015;54:158–164. doi: 10.1016/j.foodcont.2015.02.003. [DOI] [Google Scholar]
- 235.Yu Y., Qiu L., Wu H., Tang Y., Yu Y., Li X., Liu D. Degradation of zearalenone by the extracellular extracts of Acinetobacter sp. SM04 liquid cultures. Biodegradation. 2011;22:613–622. doi: 10.1007/s10532-010-9435-z. [DOI] [PubMed] [Google Scholar]
- 236.Tinyiro S.E., Wokadala C., Xu D., Yao W. Adsorption and degradation of zearalenone by Bacillus strains. Folia Microbiol. 2011;56:321. doi: 10.1007/s12223-011-0047-8. [DOI] [PubMed] [Google Scholar]
- 237.Harkai P., Szabó I., Cserháti M., Krifaton C., Risa A., Radó J., Balázs A., Berta K., Kriszt B. Biodegradation of aflatoxin-B1 and zearalenone by Streptomyces sp. collection. Int. Biodeterior. Biodegrad. 2016;108:48–56. doi: 10.1016/j.ibiod.2015.12.007. [DOI] [Google Scholar]
- 238.Liu C., Chang J., Wang P., Yin Q., Huang W., Dang X., Lu F., Gao T. Zearalenone biodegradation by the combination of probiotics with cell-free extracts of Aspergillus oryzae and its mycotoxin-alleviating effect on pig production performance. Toxins. 2019;11:552. doi: 10.3390/toxins11100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Xu J., Wang H., Zhu Z., Ji F., Yin X., Hong Q., Shi J. Isolation and characterization of Bacillus amyloliquefaciens ZDS-1: Exploring the degradation of Zearalenone by Bacillus spp. Food Control. 2016;68:244–250. doi: 10.1016/j.foodcont.2016.03.030. [DOI] [Google Scholar]
- 240.Cho K.J., Kang J.S., Cho W.T., Lee C.H., Ha J.K., Song K.B. In vitro degradation of zearalenone by Bacillus subtilis. Biotechnol. Lett. 2010;32:1921–1924. doi: 10.1007/s10529-010-0373-y. [DOI] [PubMed] [Google Scholar]
- 241.Guo Y., Zhou J., Tang Y., Ma Q., Zhang J., Ji C., Zhao L. Characterization and Genome Analysis of a Zearalenone-Degrading Bacillus velezensis Strain ANSB01E. Curr. Microbiol. 2020;77:273–278. doi: 10.1007/s00284-019-01811-8. [DOI] [PubMed] [Google Scholar]
- 242.Xu L., Sun X., Wan X., Li H., Yan F., Han R., Li H., Li Z., Tian Y., Liu X. Identification of a Bacillus amyloliquefaciens H6 Thioesterase Involved in Zearalenone Detoxification by Transcriptomic Analysis. J. Agric. Food Chem. 2020;68:10071–10080. doi: 10.1021/acs.jafc.0c03954. [DOI] [PubMed] [Google Scholar]
- 243.Sun X., He X., siyu Xue K., Li Y., Xu D., Qian H. Biological detoxification of zearalenone by Aspergillus niger strain FS10. Food Chem. Toxicol. 2014;72:76–82. doi: 10.1016/j.fct.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 244.Kriszt R., Krifaton C., Szoboszlay S., Cserháti M., Kriszt B., Kukolya J., Czéh Á., Fehér-Tóth S., Török L., Szőke Z. A new zearalenone biodegradation strategy using non-pathogenic Rhodococcus pyridinivorans K408 strain. PLoS ONE. 2012;7:e43608. doi: 10.1371/journal.pone.0043608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chang X., Liu H., Sun J., Wang J., Zhao C., Zhang W., Zhang J., Sun C. Zearalenone Removal from Corn Oil by an Enzymatic Strategy. Toxins. 2020;12:117. doi: 10.3390/toxins12020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kosawang C., Karlsson M., Vélëz H., Rasmussen P.H., Collinge D.B., Jensen B., Jensen D.F. Zearalenone detoxification by zearalenone hydrolase is important for the antagonistic ability of Clonostachys rosea against mycotoxigenic Fusarium graminearum. Fungal Biol. 2014;118:364–373. doi: 10.1016/j.funbio.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 247.Garcia S.O., Feltrin A.C.P., Garda-Buffon J. Zearalenone reduction by commercial peroxidase enzyme and peroxidases from soybean bran and rice bran. Food Addit. Contam. Part A. 2018;35:1819–1831. doi: 10.1080/19440049.2018.1486044. [DOI] [PubMed] [Google Scholar]
- 248.Azam M.S., Yu D., Liu N., Wu A. Degrading Ochratoxin A and Zearalenone Mycotoxins Using a Multifunctional Recombinant Enzyme. Toxins. 2019;11:301. doi: 10.3390/toxins11050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Janssen E.M., Mourits M.C.M., van der Fels-Klerx H.J., Lansink A.G.J.M.O. Factors underlying Dutch farmers’ intentions to adapt their agronomic management to reduce Fusarium species infection in wheat. PLoS ONE. 2020;15:e0237460. doi: 10.1371/journal.pone.0237460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Agriopoulou S., Stamatelopoulou E., Varzakas T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods. 2020;9:137. doi: 10.3390/foods9020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Alberts J.F., Lilly M., Rheeder J.P., Burger H.-M., Shephard G.S., Gelderblom W.C.A. Technological and community-based methods to reduce mycotoxin exposure. Food Control. 2017;73:101–109. doi: 10.1016/j.foodcont.2016.05.029. [DOI] [Google Scholar]
- 252.Adebiyi J.A., Kayitesi E., Adebo O.A., Changwa R., Njobeh P.B. Food fermentation and mycotoxin detoxification: An African perspective. Food Control. 2019;106:106731. doi: 10.1016/j.foodcont.2019.106731. [DOI] [Google Scholar]
- 253.Janssen E.M., Liu C., Van der Fels-Klerx12 H.J. Fusarium infection and trichothecenes in barley and its comparison with wheat. World Mycotoxin J. 2018;11:33–46. doi: 10.3920/WMJ2017.2255. [DOI] [Google Scholar]
- 254.Hofer K., Barmeier G., Schmidhalter U., Habler K., Rychlik M., Hückelhoven R., Hess M. Effect of nitrogen fertilization on Fusarium head blight in spring barley. Crop Prot. 2016;88:18–27. doi: 10.1016/j.cropro.2016.05.007. [DOI] [Google Scholar]
- 255.Schöneberg T., Martin C., Wettstein F.E., Bucheli T.D., Mascher F., Bertossa M., Musa T., Keller B., Vogelgsang S. Fusarium and mycotoxin spectra in Swiss barley are affected by various cropping techniques. Food Addit. Contam. Part A. 2016;33:1608–1619. doi: 10.1080/19440049.2016.1219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Abdallah M.F., Ameye M., De Saeger S., Audenaert K., Haesaert G. Mycotoxins-Impact and Management Strategies. IntechOpen; London, UK: 2018. Biological control of mycotoxigenic fungi and their toxins: An update for the pre-harvest approach. [Google Scholar]
- 257.Abdallah M.F., De Boevre M., Landschoot S., De Saeger S., Haesaert G., Audenaert K. Fungal endophytes control Fusarium graminearum and reduce trichothecenes and zearalenone in maize. Toxins. 2018;10:493. doi: 10.3390/toxins10120493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Magan N., Aldred D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007;119:131–139. doi: 10.1016/j.ijfoodmicro.2007.07.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.