Abstract
Ageing is the greatest risk factor for most common chronic human diseases, and it therefore is a logical target for developing interventions to prevent, mitigate or reverse multiple age-related morbidities. Over the past two decades, genetic and pharmacologic interventions targeting conserved pathways of growth and metabolism have consistently led to substantial extension of the lifespan and healthspan in model organisms as diverse as nematodes, flies and mice. Recent genetic analysis of long-lived individuals is revealing common and rare variants enriched in these same conserved pathways that significantly correlate with longevity. In this Perspective, we summarize recent insights into the genetics of extreme human longevity and propose the use of this rare phenotype to identify genetic variants as molecular targets for gaining insight into the physiology of healthy ageing and the development of new therapies to extend the human healthspan.
The world’s population is ageing. Globally, the number of people 60 years or older reached 962 million in 2017, more than twice the number in 1980. In the future, this older segment of the world population is expected to double again by the year 2050, to approximately 2 billion. For the first time in human history, there will be more older people than adolescents and young adults combined1. In addition, people of highly advanced age compose an ever-growing fraction of the world’s population, and the number of people 80 years or older is projected to more than triple from 2017 to 2050, reaching 425 million1. This population ageing reflects progress in improving nutrition, living conditions, sanitation and health care2. Unfortunately, older people have a markedly greater risk of debilitating chronic diseases. More than 90% of individuals above 65 years of age have at least one chronic disease, such as cardiovascular disease, cancer, dementia, diabetes, osteoarthritis or osteoporosis, and >70% have at least two such conditions3,4. Thus, strategies to therapeutically target fundamental ageing mechanisms, as opposed to treating each age-associated disease separately, could have a tremendous effect on global health. Indeed, according to one estimate, a 2% delay in the progression of ageing processes would lead to an increase in 10 million healthy, as opposed to disabled, older people in the United States by 2050, thus resulting in healthcare cost savings of $7.1 trillion over 50 years (ref. 5). These numbers clearly demonstrate the socioeconomic effects of ageing and indicate the need for developing drugs and interventions to extend health into old age, that is, the healthspan.
Genetic approaches have been successfully used to extend the lifespan of model organisms, including yeast, worms, flies and rodents6. These studies have led to the identification of conserved genes and pathways controlling longevity and healthy ageing, which in turn have led to the identification of geroprotective drugs targeting these pathways. Recent human genetic studies suggest that the same conserved pathways may modulate lifespan and healthspan in humans. Most of these studies have used a candidate approach interrogating specific genes or pathways7,8. To advance this genetic discovery to identifying targets for slowing ageing in humans, a systematic approach is needed to discover the key genes and pathways that contribute to human longevity and healthy ageing. In this Perspective, we discuss how use of the extreme phenotype of long-lived individuals can enable the identification of genetic variants that may provide molecular targets for unravelling the physiology of healthy ageing and for developing therapies that prevent, ameliorate or attenuate multiple age-related illnesses in humans. After all, long-lived individuals, through their very existence, have established the physiological feasibility of living beyond the ninth decade in relatively good health and ending life without a period of protracted illness.
Healthy lifespan can be extended in model organisms
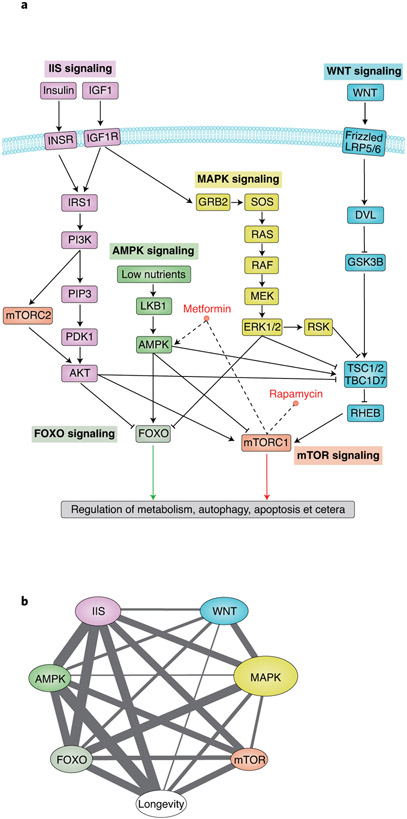
The lifespan of animal models of ageing, such as Caenorhabditis elegans, can be significantly extended by the mutation of single genes. For example, dampening insulin or insulin-like growth factor 1 (IGF-1) signalling (IIS) through weak mutations in daf-2, the worm ortholog of the human IGF-1 receptor (IGF-1R), nearly doubles the lifespan, possibly by increasing stress responses9. Modifying IIS also affects lifespan in other organisms, including yeast, flies and mice, probably because of the role of IIS in nutrient sensing6. Indeed, multiple similarities exist between the effects of IIS inhibition and those of dietary restriction, which is known to extend lifespan in multiple species10. Interventions in other pathways involved in growth, metabolism and nutrient sensing, such as dampening mechanistic target of rapamycin (mTOR) or activating AMP-activated protein kinase (AMPK), also extend lifespan11-13. These longevity pathways are intertwined, as evidenced by many connected effector proteins that interact either directly or through their network neighbors14 (Fig. 1 and Box 1). These signalling pathways are also connected to stress-response pathways, thus indicating the interplay between metabolism and molecular and cellular defences against damage.
Fig. 1 ∣. Examples of conserved pathways of ageing.
a, Interconnection among ageing pathways. Pathways are adapted from Kyoto Encyclopedia of Genes and Genomes (KEGG). Only key components of each pathway are shown. Arrows represent positive regulation, and bars represent negative regulation. b, Gene sharing among ageing pathways and longevity. Gene sets of these seven age-related pathways were collected from KEGG (as of 12 May, 2019) or MsigDB (v.6.2). Two pathways are connected if they share at least one gene. The size of the node and the width of the edge are proportional to the number of genes in the pathway and the number of shared genes between two pathways, respectively.
Box 1 ∣. Human ageing and metabolism.
Several pathways and regulators of metabolism have been identified that influence the lifespan and healthspan. These pathways and regulators perform nutrient sensing and highly conserved across species104. Examples of the major metabolic pathways implicated in regulating ageing are the IIS and AMPK-signalling pathways (Fig. 1a). Downstream of these signalling pathways are several key regulators of ageing-associated processes. These regulators include mTOR, forkhead box protein O (FOXO) and sirtuins, which control gene transcription and post-translational protein activity. Restriction of nutrients leads to lower secretion of insulin and IGF-1 and to the attenuation of IIS transmitted via the phosphatidylinositol 3-kinase and protein kinase B (PI3K–AKT) pathway105, thus resulting in downstream inhibition of mTOR106 and activation of FOXO107. Inhibition of mTOR complex 1 (mTORC1) enhances catabolic processes, such as autophagy108. FOXO proteins, a family of transcription factors, also regulate the transcription of autophagic genes109 as well as other genes that promote resistance to oxidative stress110. The AMPK pathway, in contrast, is activated under nutrient-restricted conditions, although with similar downstream effects. AMPK signalling inhibits mTOR and stimulates FOXO activity111. Furthermore, AMPK alters the metabolic environment of cells, thus increasing NAD+ levels112. The rise in NAD+ causes increased activity of SIRT1, a member of the sirtuin family of NAD-dependent deacytelases112. Activation of SIRT1 leads to deacetylation of FOXO, which in turn increases the DNA binding of FOXA and enhances transcriptional activation113. Decreased inflammation and enhanced cell survival are additional downstream effects of SIRT1 activation resulting from its inhibition of NF-κB, and p53 and BAX114. Thus, under a state of nutrient restriction, these highly interactive pathways and regulators together promote healthy ageing and longevity.
SIRT6 is another member of the sirtuin family with strong links to longevity. SIRT6 has been implicated in the control of glucose homeostasis, genome stability and silencing of repetitive elements115. Overexpression of SIRT6 leads to lifespan extension in mice116. Furthermore, a strong positive correlation has been identified between high SIRT6 enzymatic activity and maximum lifespan across mammalian species117. Although most support for the roles of these pathways and regulators in ageing has come from studies in model organisms, evidence of their effects on human ageing is rapidly accumulating. Decreased nutrient intake in humans through caloric restriction or condensed intake results in improved insulin signalling, decreased inflammation and stimulation of autophagy118. However, for many individuals, these lifestyle practices are challenging to durably sustain. Therefore, pharmacologic interventions that mimic the beneficial effects of these longevity-promoting pathways are needed, and some are already being tested in humans. A drug that decreases mTOR activity leads to enhanced immune response in older adults119, whereas supplementation with resveratrol, a sirtuin activator, improves metabolic measures in adults with diabetes120. Several activators of SIRT6 have recently been reported121. Despite these advances, progress has been slow. Genetic studies in people with extreme lifespans have the potential to accelerate the discovery of molecular targets with direct relevance to humans. Studies of long-lived individuals, including centenarians, have revealed that the genomes of these healthy agers are enriched in gene variants that attenuate IIS70,122-124 and contain polymorphisms in FOXO125. These findings substantiate the value of pursuing therapeutic strategies targeting these pathways discovered in model organisms, to achieve healthy ageing in humans.
Importantly, the genetic effects on healthspan and lifespan can be mimicked pharmacologically15. The most advanced example of this is the attenuation of TOR kinase activity by rapamycin, which significantly increases both lifespan and healthspan in mice16,17 and other model organisms18-20. To facilitate the identification of drugs that affect healthspan and lifespan, a decade ago, the US National Institute on Aging (NIA) established the Interventions Testing Program (ITP), a consortium of three centres that test drugs for their effects on the lifespan in mice21,22. To date, the ITP has shown that chronic treatment with rapamycin, 17α-oestradiol, nordihydroguaiaretic acid, acarbose, high-dose aspirin and low-dose metformin extend the lifespan, but often preferentially in male rather than female mice16,23. Among these compounds, metformin is an example of a drug that not only targets known ageing processes but also effectively protects humans against multiple age-related diseases24. The Targeting Aging with Metformin (TAME) clinical trial is poised to launch testing to determine whether metformin can delay the onset of age-related diseases in older people and may pave the way for the US Food and Drug Administration to consider ageing as a disease indication25.
Genetics of human ageing
Despite advances in identifying genes and pathways that can be targeted to increase the healthspan in model organisms, a key question remains as to whether these pathways are relevant in humans26,27. Even so, other pathways critical to ageing in our species are likely to exist. Indeed, humans are extremely long lived, and several major age-related diseases, such as Alzheimer’s disease and cardiovascular disease, are absent in most model organisms. Hence, to achieve the full beneficial potential of therapeutically targeting ageing, human genetics is required. In human studies, natural mutants, rather than targeted genetic engineering, must be used to identify genes and pathways regulating the lifespan. The clear place to start is studying long-lived individuals, particularly those extremely rare individuals who avoid disease and live to 100 years or older, that is, centenarians.
Lifespan refers to age at death, whereas longevity indicates survival into extreme old age. The natural lifespan in humans, even under optimal conditions in modern societies, varies considerably. Whereas environmental factors, including diet, physical activity, health habits, and psychosocial factors, are important, the human lifespan has a genetic component in cohorts of advanced age. This aspect was first demonstrated by a comparison of the survival of siblings of centenarians versus their siblings born at the same time as the centenarians but who died in their early seventies28. A heritable component of human longevity was later confirmed by studying sibships of long-lived people and comparing their survival to that in birth cohorts from the same geographical area29, and by comparing age at death between monozygotic and dizygotic twins30. Together, these studies have indicated that although as much as 25% of the variation in human lifespan may be due to genetic factors31, the genetic component of lifespan is particularly strong (35%) in the oldest old people28,32-36. Indeed, the offspring of centenarians have a lower prevalence of age-related diseases, as well as more beneficial or ‘youthful’ profiles for many metabolic and immune-related parameters than do age- and sex-matched controls37-40. A recent estimate of this prevalence, based on pedigree data from Ancestry. com public trees, is lower than 10%, possibly because of assortative mating around genetic and/or environmental lifespan-influencing factors41. Shared environment clearly plays a role in determining the average human lifespan42. However, the heritability of human longevity remains under study43.
Identification of the genetic factors that underlie extreme human lifespan should provide insights into the mechanisms of human longevity and disease resistance and may lead to the identification of novel targets for drugs and other treatment strategies to promote healthy ageing. As a complex trait, human longevity is likely to be influenced by different types of genetic variants and interactions among them, across the allele-frequency spectrum. Common variants associated with human longevity have been the focus of many recent genome-wide association studies (GWAS) using a variety of trait definitions and study designs (Supplementary Table 1) including: (1) exceptional longevity as an extreme binary phenotype in a case–control design; (2) parental lifespan as a continuous quantitative phenotype of individuals from a general population collected in large reference biobanks; (3) genome-wide scans informed by age-related diseases; and (4) an integrated approach combining several GWAS strategies. Genetic variants associated with age-related disease and traits are also likely to be associated with lifespan44 and the underlying ageing process45,46. These GWAS have identified more than 50 longevity-associated genetic loci of genome-wide significance (Supplementary Table 2), thus suggesting that human longevity is a polygenic trait influenced by many variants with small to modest effect sizes (Fig. 2). However, current GWAS based on widely used single-nucleotide-polymorphism arrays have three limitations. First, they cannot account for most of the genetic variance of complex traits, which consists of rare, not common, variants. This aspect is particularly important for rare phenotypes, such as extreme longevity. In general, human healthspan and lifespan are likely to be adversely affected by the germline burden of rare damaging variants47. Second, identifying the causal gene mutations from GWAS signals remains difficult48-50. Third, GWAS of human longevity have to date been predominantly based on populations of European ancestry. To fully understand the genetic architecture of human ageing, ancestrally diverse populations must be studied, while avoiding population stratification, which can confound associations between genotype and the trait of interest. This goal can be accomplished by cross-validating genetic variants between ancestrally different cohorts.
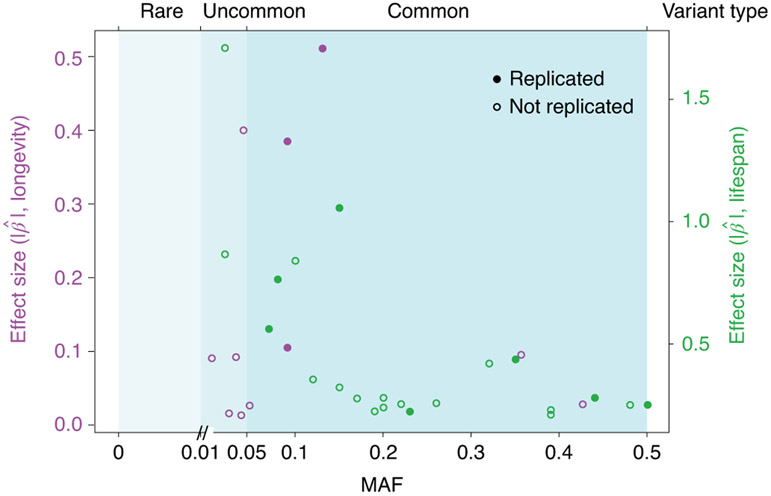
Fig. 2 ∣. Genetic architecture of human ageing.
The genetic architecture of human age-related phenotypes, because these are complex traits, is likely to include genetic variants across the allele frequency spectrum with different effect sizes. Common variants associated with human survival have been extensively searched for in many recent GWAS. Using the GWAS Catalog, we compiled minor-allele frequencies (MAFs) and effect sizes (estimated β values) of independent variants with genome-wide statistical significance (P < 5 10−8) from various studies of longevity and lifespan (Supplementary Table 2). Purple and green dots, with separate correspondingly coloured y axes, represent such longevity-associated and lifespan-associated variants, respectively. For lifespan, only variants with effect sizes available are included. On the basis of their MAFs, variants are separated into three types—rare (MAF < 1%), uncommon (1% ≤ MAF ≤ 5%) and common (MAF > 5%)—according to widely used, albeit arbitrary, criteria. Very few rare variants show significant association, because either they were not genotyped by single-nucleotide-polymorphism arrays used by current GWAS or the studies did not have sufficient power to detect genetic signals. As expected, there is a clear inverse relationship between the effect size and the allele frequency of complex-trait-associated variants.
Going to extremes: exceptional longevity for discovery of antiageing drug targets
Extremely long-lived individuals, such as centenarians, compose only a tiny proportion (~0.01–0.02%) of the United States population51, but their genes contain a biological blueprint for healthy ageing and longevity. Although advanced age is the major risk factor for most diseases affecting older adults, including cardiovascular disease, cancer, type 2 diabetes mellitus and Alzheimer’s disease, centenarians avoid the onset of these conditions by 20–30 years, and many remain disease free for the duration of their lifespan52-54. Importantly, the cost of end-of-life healthcare for centenarians is also substantially less than that for non-centenarians55, thus illustrating the lower burden of disease and less hospitalization. This extreme and extremely rare phenotype is ideal for the study of genetic variants that regulate healthspan and lifespan.
Genetic discovery using human populations typically involves thousands or even tens of thousands of individuals, whereas the size of a centenarian cohort is generally in the hundreds. In complex phenotypes, however, many different rare variants are likely to have large effects, whereas common variants have relatively minor effects. Thus, one strategy to overcome this power deficit is to sequence individuals at the extreme end of a phenotype distribution to search for rare variants with large effects. Identifying genes or gene products with large effects also makes sense in terms of identifying molecular targets for drug development. A successful example using this strategy has been the identification of proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9), an enzyme involved in cholesterol metabolism by regulating low-density lipoprotein (LDL)-receptor degradation, as a drug target to decrease plasma LDL cholesterol and the risk of coronary heart disease. PCSK9 was first connected to cholesterol metabolism through the study of a single family with autosomal-dominant hypercholesterolaemia caused by mutations in PCSK9 (ref. 56): one gene had a large effect. Subsequently, several healthy individuals with no circulating PCSK9, as a result of compound heterozygous loss-of-function mutations, were identified57,58. The finding that the few participants with loss-of-function mutations in PCSK9 had no adverse effects from being extremely hypocholesterolaemic throughout life and had a significantly lower risk of cardiovascular disease provided confidence to pursue the development of inhibitors of PCSK9. After successful clinical trials59,60, antibodies to PCSK9 have been approved, and an RNA-interference drug is under regulatory review, thus representing excellent examples of successful genomic medicine. Similarly to PGSCK9 mutations, rare mutations in the gene encoding cholesteryl ester transfer protein (CETP) have been linked to accelerated atherosclerosis, thus leading to the development of CETP inhibitors. However, these CETP inhibitors have not yet shown significant therapeutic effects towards atherosclerosis in clinical settings61. Clearly, the study of smaller cohorts of individuals with extreme phenotypes such as ultra-low plasma LDL cholesterol can be tremendously powerful and effective in drug development.
Because of its strong genetic component, extreme longevity lends itself to a similar approach to that in the PCSK9 example. Identifying genetic variants enriched in centenarians and possibly related to their extreme longevity, although more complicated than the case of PCSK9, may be a key strategy to develop the drug targets desperately needed for combating age-related multimorbidity. As a consequence of the enormous progress in DNA sequencing62, this search can now be accomplished in a straightforward manner by sequencing whole exomes and/or whole genomes of centenarian and control cohorts. In general, trait-associated rare variants are exceedingly difficult to identify, because of insufficient statistical power63, thus posing a major challenge in association studies64,65. To address this statistical issue, in addition to using exceptional longevity as an extreme phenotype, multiple steps must be taken to decrease false discovery and increase power, including (1) statistical analysis that integrates complementary genomic data (Box 2 and Fig. 3); (2) confirmation of a rare variant in a second cohort of long-lived individuals; (3) examination of the absence or depletion of the variant in a large control population; and (4) demonstration of functional effects of the identified longevity-associated genes and variants on various parameters relevant to health and longevity, including cellular and organismal resistance to stress and improved fitness. This combination of steps in the genetic analysis of long-lived individuals should enable the necessary discoveries of longevity-associated rare variants in genes that may potentially be targeted for drug development66,67.
Box 2 ∣. Genetic studies of complex human traits.
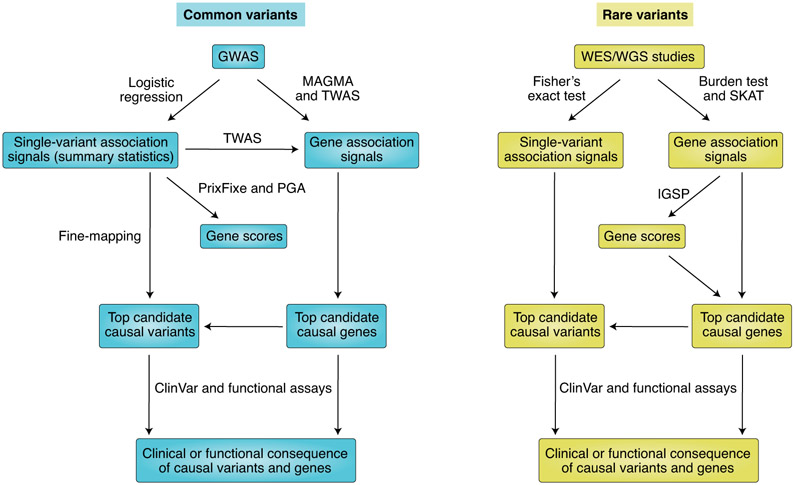
Different approaches are used to analyse common and rare variants in case–control association studies (Fig. 3). Common variants usually refer to variants with MAF >5% (or >1%) in the studied cohort or population. Direct trait association of individual common variants is usually examined by logistic regression with population-structure adjustment, if necessary. Different methods for post-GWAS analyses have been developed to uncover candidate causal variants and/or genes underlying trait-association signals from common variants. Fine-mapping analyses (for example, PAINTOR126 and eCAVIAR127) can prioritize causal variants in implicated risk loci and can be used to infer corresponding causal genes if methods integrate expression quantitative trait loci. Aggregation-based methods can have greater power to identify risk genes (for example, TWAS128), risk pathways (for example, ALIGATOR129) or risk gene modules (for example, dmGWAS130) without pinpointing casual variants. Many methods simply require summary statistics, but some approaches require genotype GWAS data (for example, MAGMA131). Other methods include network-based approaches, which can predict underlying causal genes given trait-associated variants (for example, PrixFixe132 and PGA133,134). Rare variants are variants with MAF <1% in the cohort or population. Trait association of individual rare variants can be examined by Fisher’s exact test. The burden test and SKAT are commonly used instead to test the collective association of a group of rare variants at the gene or gene-set level. The statistical power of such association tests may depend on the categories of tested variants. Selecting rare variants for testing can be based on their effects on coding sequences (for example, nonsense and frameshift variants are more likely to cause loss-of-function effects but are limited in number) or their functional scores from in silico prediction tools (for example, CADD135 and PrimateAI136). Another way to potentially improve statistical power is to weight rare variants in the burden test and SKAT with scores that are likely to reflect their functional effects. Common weighting schemes use allele frequency and/or scores from variant-scoring tools. A recently described related method integrates the burden test or SKAT results with gene network and phenotype data to predict causal genes137. All the aforementioned procedures can generate a list of causal-variant or causal-gene candidates. Human variant catalogues with clinical information (for example, ClinVar138) provide a convenient way to validate findings but have limitations due to their low coverage. Experimental validation of potential causal variants or genes with genome-editing tools (for example, CRISPR–Cas9) and functional assays are needed to demonstrate cause and effect, similarly to validating a disease-causing mutation.
Fig. 3 ∣. Discovery of causal variants and genes in genetic studies of complex human traits.
TWAS, transcriptome-wide association study; PGA, post-GWAS analysis; SKAT, sequence kernel association test; IGSP, integrated gene signal processing.
This approach essentially reflects an experiment of nature and uncovers specific targets ‘perturbed’ by ‘longevity’ alleles in rare individuals with long lifespans. Genetic variation acts as a natural version of a randomized control trial: alleles that perturb a particular target and are associated with a particular disease provide strong support for the therapeutic validity of the target and are a strong predictor of the success of clinical trials testing drugs able to perturb the target similarly68. Using drugs mimicking the effects of such alleles would be expected to establish causal relationships between targets and outcomes, thus eventually leading to pharmacological interventions in ageing. This novel pharmacological armamentarium may yield a true longevity dividend, as already demonstrated genetically. Some evidence has already indicated that this approach works. Through a resequencing approach of genes in the IIS pathway, the first conserved ageing pathway to be discovered, rare protective alleles have been found to be enriched in centenarians69,70. These longevity-associated rare alleles cause decreased IIS in cell models, thus demonstrating the mechanistic conservation of the pathway between invertebrate models and humans, and demonstrating the functional relevance of the positive associations between the variants and longevity. Approaches to decrease IIS therapeutically, particularly later in life, should have positive effects on human health. A recent preclinical study has shown that late-life targeting of IGF-1R by monoclonal antibodies significantly improves healthspan and lifespan in female mice, thereby underscoring how human genetic discoveries can be translated into immediate drug treatment71.
From genes to drugs: the road to healthy human ageing
With the identification of rare variants in long-lived individuals, there is now a need to functionally validate the variants (Fig. 4). Interpretation of variants on the basis of sequence information alone is limited, because classification of rare coding variants as causative mutations or neutral polymorphisms is challenging. One of the most successful approaches has been to assign the roles of variants in the context of protein–protein interactome networks72. The rationale of this approach is based on the finding that most proteins perform their functions through interacting with other proteins72,73. Many proteins are pleiotropic and perform diverse functions through interacting with multiple proteins74. Mutations in the same gene affecting different protein interactions can often lead to clinically distinct outcomes, whereas mutations affecting, the same interaction, for example, the binding interface of a protein, often lead to the same disorders75. Therefore, the protein interactome networks yield insights into the molecular mechanisms of disease-causing mutations75 and aid in identifying novel candidate genes and mutations72,73. On average, a protein interacts with more than five other protein partners in the human interactome network. The current version of the Human Gene Mutation Database76, the most comprehensive high-quality database for disease-associated genes and mutations, lists 3,667 disease-associated genes, 1,811 of which (49.4%) cause two or more clinically distinct disorders through different mutations on the same gene. Therefore, determining interaction-specific disruptions caused by rare missense variants is highly important.
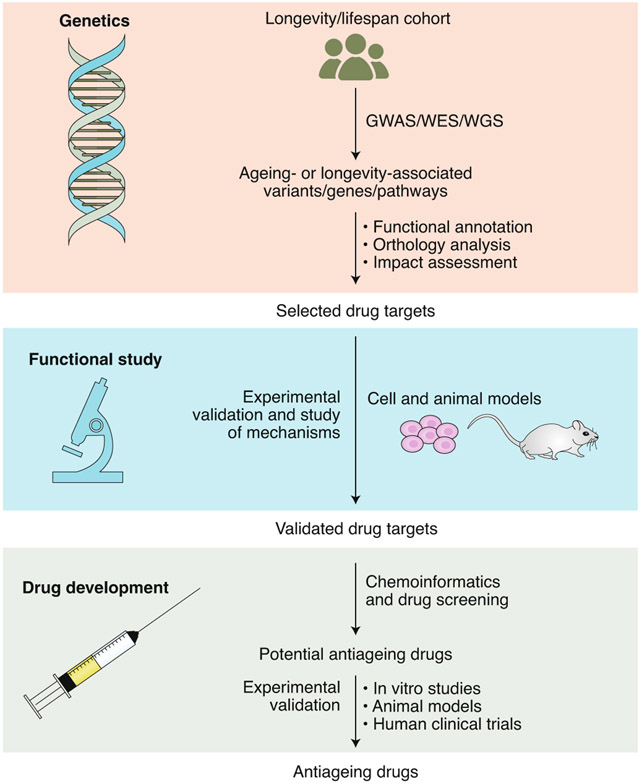
Fig. 4 ∣. An integrated approach to drug discovery for healthy human ageing, on the basis of the genomic analysis of extreme longevity.
WES, whole-exome sequencing; WGS, whole-genome sequencing.
For example, through experimentally and computationally integrated approaches, the functional effects of coding variants have been systematically investigated in the context of the human interactome networks through a series of agnostic functional assays in parallel75,77-80. This high-throughput pipeline, referred to as integrated protein interactome perturbation (InPOINT) screening (Fig. 5), has been used to identify causal coding variants (for example, missense mutations) that lead to changes in protein stability and protein interactome networks. Briefly, this InPOINT pipeline incorporates different high-throughput approaches: Clone-seq to generate specific mutant clones in a massively parallel manner79, a fluorescence-based assay to determine the variant’s effects on protein stability and protein interaction assays to examine a variant’s effect on specific protein–protein interactions81-83. Combining multiple assays ensures the quality of the prediction and practically eliminates false-positive results. More importantly, this strategy also provides several important insights into mechanisms, particularly that many coding mutations affect only a subset of specific protein–protein interactions, rather than all interactions, and that mutations in the same protein that disrupt different protein–protein interactions often lead to clinically distinct outcomes75,79,84,85.
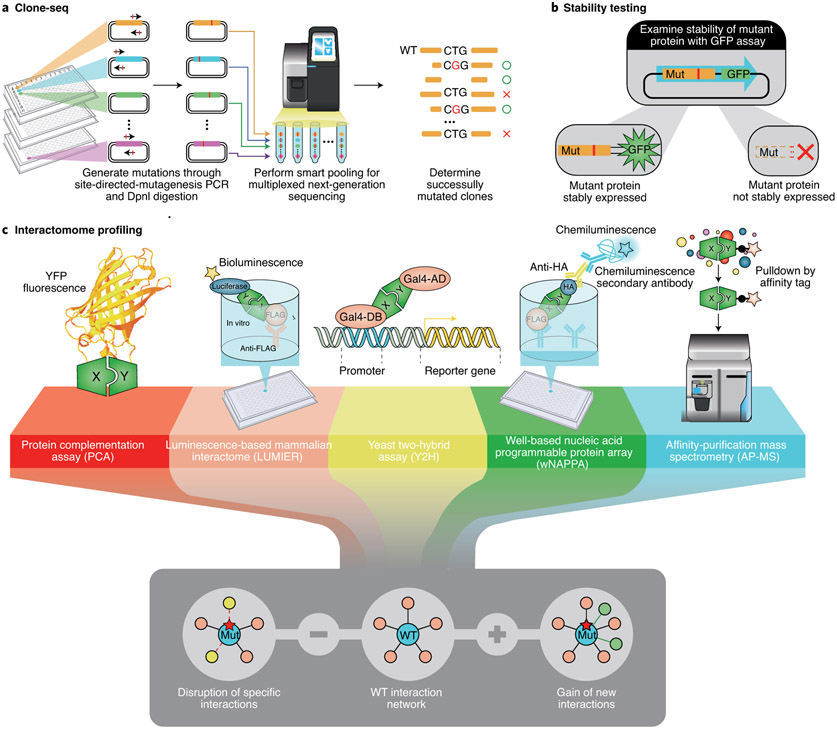
Fig. 5 ∣. Schematic illustration of the InPOINT pipeline.
a, Clone-seq. A massively parallel site-directed-mutagenesis assay to generate individual mutant clones of thousands of missense variants for downstream stability and interaction assays. b, Stability testing. A high-throughput YFP assay to detect mutant (Mut) stability relative to that of the wild-type (WT) protein. HA, haemagglutinin. c, Interactome profiling. A set of five complementary interaction assays to detect changes in specific interactions (loss of known interactions and gain of new interactions) for each mutant. Combining multiple assays ensures data quality and practically eliminates false-positive results.
Functional variants prioritized from the high-throughput molecular analysis can then be further tested for their effects on cellular outcomes, such as resistance to stress. Indeed, most longevity-associated genes identified in model organisms confer increased stress resistance, including the response to genotoxic or oxidative stress86-89. Such stress tests can be performed in a relatively high-throughput fashion in human cells with the variants introduced via gene-editing technology. The molecular effects of these variants can also be studied economically in human induced pluripotent stem cells (iPSCs) and their progeny to identify the functional outcomes of the sequence variants in a variety of cell types90. For example, iPSCs with a rare variant introduced via gene editing can be compared with the parental iPSCs for proliferation or differentiation capacity, thus creating embryonic fibroblasts, neurons, vascular smooth muscle cells and vascular endothelial cells. The iPSC-derived cell types can be treated with agents to induce a modest level of genotoxic or oxidative stress to measure the effects of the variants on markers of cell viability and resilience, including levels of reactive oxygen species, DNA-damage levels, mitochondrial function, senescence and cell signalling. This approach has been successfully used to document the role of the 9p21.3 cardiovascular disease locus on vascular smooth muscle cells derived from gene-edited iPSCs91.
Eventually, a candidate rare variant must be validated for its effects on healthspan and lifespan. This validation can be performed in rodents by introducing the rare coding variant into one allele of the gene. For example, mice and rats carrying an allele encoding the centenarian variant in the highly conserved Igf1r locus have been generated, and healthspan and lifespan studies are underway. However, this process is possible only if the centenarian rare variant is located in a conserved sequence, which is not always the case. If the rare variant is found in a domain not conserved between mice and humans, then the mouse model must be ‘humanized’ by replacing the mouse gene with the human gene, and the rare variant must be introduced into the humanized gene. Although time consuming, this approach documents the contribution of a specific centenarian variant to longevity. Often, preexisting data are available that predict successful outcomes. For example, genetic depletion of IGF-1, IGF-1R or growth hormones and growth-hormone receptors that function upstream of IGF-1 by using knockout alleles has been found to confer longevity in mice92.
If the variant is in a non-coding region of a gene, presumably in a promoter, enhancer or another regulatory region modulating expression with age or stress, then generating the appropriate mouse models to directly validate the effect of the variant on longevity is difficult. Instead, determining the effect of underexpression or overexpression of the gene by using mice heterozygous for the putative gene or carrying extra copies of the gene can be used to examine the roles of the gene in lifespan and healthspan. For example, because we had identified rare non-coding variants in several genes encoding components of the IκB kinase-NF-κB pathway (Y.S., unpublished data), we used mice heterozygous for the p65 (RelA) subunit of the transcription factor NF-κB to demonstrate that decreased NF-κB activity extends the healthspan93. Of note, this validation of rare variants in rodent models can be performed more rapidly if the analysis is performed in mouse models of accelerated ageing, such as the Ercc1−/Δ model of XFE progeria94 or mouse models of Hutchinson–Guilford progeria syndrome95.
After the functional effect of a sequence variant is confirmed through either transgenic or iPSC approaches, the next step is to identify novel compounds or existing drugs that mimic the effect of the variant on its cognate pathway or cell function. Phenotypic or fluorescence-based reporter assays in cells can be created to screen for compounds that mimic the effect of the genetic variant. For example, NF-κB reporters can be used to identify compounds that decrease NF-κB activation in response to stress. Indeed, compounds targeting the IκB kinase upstream of NF-κB extend healthspan in mice93. Another example is the IGF-1R coding variant found in centenarians, which acts in a dominant manner in activating some but not all downstream targets of the receptor. This selective activity of the IGF-1R variant can be screened with the appropriate combination of reporter constructs. Another example is the non-coding variants in FOXO3 associated with extreme longevity96,97. These variants appear to increase the level of FOXO3 promoter activity, at least under conditions of oxidative stress. The level of FOXO3 in the nucleus decreases with age in mice and worms. Thus, assays measuring changes in the level and subcellular localization of FOXO3 are needed. For example, knock-in of a fluorescent reporter into the FOXO3 locus to create a fusion protein expressed from the endogenous promoter has been used to screen for drugs that alter either the overall or nuclear level of FOXO3. Indeed, with FOXO3 fluorescence-based assays, a drug has been identified that increases nuclear localization of FOXO3 (ref. 98), and natural products such as astaxanthin, epigallocatechin gallate99, resveratrol and syringaresinol100 have been found to increase expression of FOXO3.
Summary and future prospects
In recent years, several breakthrough genetic discoveries in humans with extreme phenotypes have led to rapid and successful drug development57,101,102. In this Perspective, we propose the feasibility of applying this same concept to the development of novel gene-based therapeutics against ageing, that is, to increase human healthspan by interfering with pathways that collectively control ageing, the process that increases the risk of most chronic diseases to a greater extent than any other risk factor. Despite enormous improvements in human health over the past century, we remain far from a situation in which living to 100 years of age in fairly good health is the norm. To get closer to this state of good health, we propose that the genetics of extreme human longevity can be used as a blueprint, by using germline variants that have been found to be critical determinants of living a long and healthy life. Indeed, whereas the genetic component of human lifespan on average is not very strong, genetic variants, rather than merely being shared familial or environmental factors, are the critical determinants of extreme human longevity103. We argue that this aspect makes long-lived individuals exceptionally suitable as a source for discovery of genetic targets for new pharmaceutical approaches to modulate both conserved and non-conserved pathways of ageing in humans. Modulating such pathways has been conclusively shown to extend the lifespan and healthspan in model organisms, but other pathways, possibly specific to the human species, remain to be discovered. Given the increasing availability of whole-exome and whole-genome sequencing data from human populations, including centenarians49, rapid identification of rare coding variants that affect phenotypes of healthy ageing is now possible.
Supplementary Material
Acknowledgements
We thank M. Guo and R. Kohanski at NIA for their scientific input, which contributed to the concepts outlined in this Perspective. This work was supported by grant U19 AG056278 from the US National Institutes of Health.
Footnotes
Competing interests
J.V. is a founder of Singulomics Corp. P.R. and L.N. are co-founders of NRTK Biosciences. All other authors declare no competing interests.
Supplementary information is available for this paper at https://doi.org/10.1038/s42255-020-0247-0.
Peer review information Primary Handling Editor: Pooja Jha.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Population Ageing 2017 (United Nations, 2017). [Google Scholar]
- 2.Partridge L, Deelen J & Slagboom PE Facing up to the global challenges of ageing. Nature 561, 45–56 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Barnett K et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380, 37–43 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Marengoni A et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res. Rev 10, 430–439 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Goldman D The economic promise of delayed aging. Cold Spring Harb. Perspect. Med 6, a025072 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontana L, Partridge L & Longo VD Extending healthy life span: from yeast to humans. Science 328, 321–326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Spoel E et al. Association analysis of insulin-like growth factor-1 axis parameters with survival and functional status in nonagenarians of the Leiden Longevity Study. Aging (Albany N.Y.) 7, 956–963 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Passtoors WM et al. Gene expression analysis of mTOR pathway: association with human longevity. Aging Cell 12, 24–31 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Kenyon C, Chang J, Gensch E, Rudner A & Tabtiang R A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993). [DOI] [PubMed] [Google Scholar]
- 10.Fontana L & Partridge L Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vellai T et al. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426, 620 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Kapahi P et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol 14, 885–890 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson SC et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 342, 1524–1528 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q et al. Systems-level analysis of human aging genes shed new light on mechanisms of aging. Hum. Mol. Genet 25, 2934–2947 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson SC, Rabinovitch PS & Kaeberlein M mTOR is a key modulator of ageing and age-related disease. Nature 493, 338–345 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison DE et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson JE et al. Rapamycin slows aging in mice. Aging Cell 11, 675–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjedov I et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 11, 35–46 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powers RW III, Kaeberlein M, Caldwell SD, Kennedy BK & Fields S Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 20, 174–184 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robida-Stubbs S et al. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 15, 713–724 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller RA et al. An Aging Interventions Testing Program: study design and interim report. Aging Cell 6, 565–575 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Nadon NL et al. Design of aging intervention studies: the NIA interventions testing program. Age (Dordr.) 30, 187–199 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison DE et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 13, 273–282 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barzilai N, Crandall JP, Kritchevsky SB & Espeland MA Metformin as a tool to target aging. Cell Metab. 23, 1060–1065 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Justice JN et al. Development of clinical trials to extend healthy lifespan. Cardiovasc Endocrinol Metab 7, 80–83 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Magalhães JP Why genes extending lifespan in model organisms have not been consistently associated with human longevity and what it means to translation research. Cell Cycle 13, 2671–2673 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson SC, Dong X, Vijg J & Suh Y Genetic evidence for common pathways in human age-related diseases. Aging Cell 14, 809–817 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perls TT, Bubrick E, Wager CG, Vijg J & Kruglyak L Siblings of centenarians live longer. Lancet 351, 1560 (1998). [DOI] [PubMed] [Google Scholar]
- 29.Schoenmaker M et al. Evidence of genetic enrichment for exceptional survival using a family approach: the Leiden Longevity Study. Eur. J. Hum. Genet 14, 79–84 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Herskind AM et al. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870-1900. Hum. Genet 97, 319–323 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Christensen K, Johnson TE & Vaupel JW The quest for genetic determinants of human longevity: challenges and insights. Nat. Rev. Genet 7, 436–448 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murabito JM, Yuan R & Lunetta KL The search for longevity and healthy aging genes: insights from epidemiological studies and samples of long-lived individuals. J. Gerontol. A Biol. Sci. Med. Sci 67, 470–479 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perls TT et al. Life-long sustained mortality advantage of siblings of centenarians. Proc. Natl Acad. Sci. USA 99, 8442–8447 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robine JM & Allard M The oldest human. Science 279, 1834–1835 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Gögele M et al. Heritability analysis of life span in a semi-isolated population followed across four centuries reveals the presence of pleiotropy between life span and reproduction. J. Gerontol. A Biol. Sci. Med. Sci 66, 26–37 (2011). [DOI] [PubMed] [Google Scholar]
- 36.van den Berg N et al. Longevity defined as top 10% survivors and beyond is transmitted as a quantitative genetic trait. Nat. Commun 10, 35 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barzilai N, Gabriely I, Gabriely M, Iankowitz N & Sorkin JD Offspring of centenarians have a favorable lipid profile. J. Am. Geriatr. Soc 49, 76–79 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Newman AB et al. Health and function of participants in the Long Life Family Study: a comparison with other cohorts. Aging (Albany N.Y.) 3, 63–76 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deelen J et al. Employing biomarkers of healthy ageing for leveraging genetic studies into human longevity. Exp. Gerontol 82, 166–174 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Ash AS et al. Are members of long-lived families healthier than their equally long-lived peers? Evidence from the Long Life Family Study. J. Gerontol. A Biol. Sci. Med. Sci 70, 971–976 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruby JG et al. Estimates of the heritability of human longevity are substantially inflated due to assortative mating. Genetics 210, 1109–1124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarry V, Gagnon A & Bourbeau R Survival advantage of siblings and spouses of centenarians in 20th-century Quebec. Can. Stud. Popul 39, 67–78 (2012). [Google Scholar]
- 43.van den Berg N et al. Longevity Relatives Count score identifies heritable longevity carriers and suggests case improvement in genetic studies. Aging Cell 19, e13139 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDaid AF et al. Bayesian association scan reveals loci associated with human lifespan and linked biomarkers. Nat. Commun 8, 15842 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zenin A et al. Identification of 12 genetic loci associated with human healthspan. Commun. Biol 2, 41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandes M et al. Systematic analysis of the gerontome reveals links between aging and age-related diseases. Hum. Mol. Genet 25, 4804–4818 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shindyapina AV et al. Germline burden of rare damaging variants negatively affects human healthspan and lifespan. eLife 9, e53449 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cash TP et al. Exome sequencing of three cases of familial exceptional longevity. Aging Cell 13, 1087–1090 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nygaard HB et al. Whole exome sequencing of an exceptional longevity cohort. J. Gerontol. A Biol. Sci. Med. Sci 74, 1386–1390 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han J et al. Discovery of novel non-synonymous SNP variants in 988 candidate genes from 6 centenarians by target capture and next-generation sequencing. Mech. Ageing Dev 134, 478–485 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howden LM & Meyer JA Age and Sex Composition: 2010 (U.S. Census Bureau, 2011). [Google Scholar]
- 52.Andersen SL, Sebastiani P, Dworkis DA, Feldman L & Perls TT Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J. Gerontol. A Biol. Sci. Med. Sci 67, 395–405 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ismail K et al. Compression of morbidity is observed across cohorts with exceptional longevity. J. Am. Geriatr. Soc 64, 1583–1591 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sebastiani P et al. Families enriched for exceptional longevity also have increased health-span: findings from the Long Life Family Study. Front. Public Health 1, 38 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hazra NC, Rudisill C & Gulliford MC Determinants of health care costs in the senior elderly: age, comorbidity, impairment, or proximity to death? Eur. J. Health Econ 19, 831–842 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abifadel M et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet 34, 154–156 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Zhao Z et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet 79, 514–523 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hooper AJ, Marais AD, Tanyanyiwa DM & Burnett JR The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis 193, 445–448 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Fitzgerald K et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet 383, 60–68 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ridker PM et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N. Engl. J. Med 376, 1527–1539 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Tall AR & Rader DJ Trials and tribulations of CETP inhibitors. Circ. Res 122, 106–112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodwin S, McPherson JD & McCombie WR Coming of age: ten years of next-generation sequencing technologies. Nat. Rev. Genet 17, 333–351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gorlov IP, Gorlova OY, Sunyaev SR, Spitz MR & Amos CI Shifting paradigm of association studies: value of rare single-nucleotide polymorphisms. Am. J. Hum. Genet 82, 100–112 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kosmicki JA, Churchhouse CL, Rivas MA & Neale BM Discovery of rare variants for complex phenotypes. Hum. Genet 135, 625–634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicolae DL Association tests for rare variants. Annu. Rev. Genomics Hum. Genet 17, 117–130 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Finan C et al. The druggable genome and support for target identification and validation in drug development. Sci. Transl. Med 9, eaag1166 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oprea TI et al. Unexplored therapeutic opportunities in the human genome. Nat. Rev. Drug Discov 17, 317–332 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Plenge RM, Scolnick EM & Altshuler D Validating therapeutic targets through human genetics. Nat. Rev. Drug Discov 12, 581–594 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Tazearslan C, Huang J, Barzilai N & Suh Y Impaired IGF1R signaling in cells expressing longevity-associated human IGF1R alleles. Aging Cell 10, 551–554 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suh Y et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc. Natl Acad. Sci. USA 105, 3438–3442 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mao K et al. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat. Commun 9, 2394 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vidal M, Cusick ME & Barabasi AL Interactome networks and human disease. Cell 144, 986–998 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barabasi AL, Gulbahce N & Loscalzo J Network medicine: a network-based approach to human disease. Nat. Rev. Genet 12, 56–68 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pawson T & Nash P Protein-protein interactions define specificity in signal transduction. Genes Dev. 14, 1027–1047 (2000). [PubMed] [Google Scholar]
- 75.Wang X et al. Three-dimensional reconstruction of protein networks provides insight into human genetic disease. Nat. Biotechnol 30, 159–164 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stenson PD et al. Human Gene Mutation Database (HGMD): 2003 update. Hum. Mutat 21, 577–581 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Khurana E et al. Integrative annotation of variants from 1092 humans: application to cancer genomics. Science 342, 1235587 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guo Y et al. Dissecting disease inheritance modes in a three-dimensional protein network challenges the “guilt-by-association” principle. Am. J. Hum. Genet 93, 78–89 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei X et al. A massively parallel pipeline to clone DNA variants and examine molecular phenotypes of human disease mutations. PLoS Genet. 10, e1004819 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen S et al. An interactome perturbation framework prioritizes damaging missense mutations for developmental disorders. Nat. Genet 50, 1032–1040 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Braun P et al. An experimentally derived confidence score for binary protein-protein interactions. Nat. Meth 6, 91–97 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu H et al. High-quality binary protein interaction map of the yeast interactome network. Science 322, 104–110 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu H et al. Next-generation sequencing to generate interactome datasets. Nat. Methods 8, 478–480 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sahni N et al. Widespread macromolecular interaction perturbations in human genetic disorders. Cell 161, 647–660 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhong Q et al. Edgetic perturbation models of human inherited disorders. Mol. Syst. Biol 5, 321 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fabrizio P, Pozza E, Pletcher SD, Gendron CM & Longo VD Regulation of longevity and stress resistance by Sch9 in yeast. Science 292, 288–290 (2001). [DOI] [PubMed] [Google Scholar]
- 87.Wang HD, Kazemi-Esfarjani P & Benzer S Multiple-stress analysis for isolation of Drosophila longevity genes. Proc. Natl Acad. Sci. USA 101, 12610–12615 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muñoz MJ & Riddle DL Positive selection of Caenorhabditis elegans mutants with increased stress resistance and longevity. Genetics 163, 171–180 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Magalhães JP & Toussaint O GenAge: a genomic and proteomic network map of human ageing. FEBS Lett. 571, 243–247 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Soldner F & Jaenisch R Stem cells, genome editing, and the path to translational medicine. Cell 175, 615–632 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lo Sardo V et al. Unveiling the role of the most impactful cardiovascular risk locus through haplotype editing. Cell 175, 1796–1810.e1720 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aguiar-Oliveira MH & Bartke A Growth hormone deficiency: health and longevity. Endocr. Rev 40, 575–601 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tilstra JS et al. NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J. Clin. Invest 122, 2601–2612 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niedernhofer LJ et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444, 1038–1043 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Hambright WS, Niedernhofer LJ, Huard J & Robbins PD Murine models of accelerated aging and musculoskeletal disease. Bone 125, 122–127 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Willcox BJ et al. FOXO3A genotype is strongly associated with human longevity. Proc. Natl Acad. Sci. USA 105, 13987–13992 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morris BJ, Willcox DC, Donlon TA & Willcox BJ FOXO3: a major gene for human longevity: a mini-review. Gerontology 61, 515–525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cautain B et al. Discovery of a novel, isothiazolonaphthoquinone-based small molecule activator of FOXO nuclear-cytoplasmic shuttling. PLoS ONE 11, e0167491 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Belguise K, Guo S & Sonenshein GE Activation of FOXO3a by the green tea polyphenol epigallocatechin-3-gallate induces estrogen receptor alpha expression reversing invasive phenotype of breast cancer cells. Cancer Res. 67, 5763–5770 (2007). [DOI] [PubMed] [Google Scholar]
- 100.Cho S et al. Syringaresinol protects against hypoxia/reoxygenation-induced cardiomyocytes injury and death by destabilization of HIF-1α in a FOXO3-dependent mechanism. Oncotarget 6, 43–55 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bowman L et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N. Engl. J. Med 377, 1217–1227 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Hall SS Genetics: a gene of rare effect. Nature 496, 152–155 (2013). [DOI] [PubMed] [Google Scholar]
- 103.Rajpathak SN et al. Lifestyle factors of people with exceptional longevity. J. Am. Geriatr. Soc 59, 1509–1512 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bitto A, Wang AM, Bennett CF & Kaeberlein M Biochemical genetic pathways that modulate aging in multiple species. Cold Spring Harb. Perspect. Med 5, a025114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Taniguchi CM, Emanuelli B & Kahn CR Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol 7, 85–96 (2006). [DOI] [PubMed] [Google Scholar]
- 106.Kennedy BK & Lamming DW The mechanistic target of rapamycin: the grand conductor of metabolism and aging. Cell Metab. 23, 990–1003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kahn AJ FOXO3 and related transcription factors in development, aging, and exceptional longevity. J. Gerontol. A Biol. Sci. Med. Sci 70, 421–425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roczniak-Ferguson A et al. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal 5, ra42 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mammucari C et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 6, 458–471 (2007). [DOI] [PubMed] [Google Scholar]
- 110.Kops GJ et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419, 316–321 (2002). [DOI] [PubMed] [Google Scholar]
- 111.Greer EL et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J. Biol. Chem 282, 30107–30119 (2007). [DOI] [PubMed] [Google Scholar]
- 112.Cantó C et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brunet A et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 (2004). [DOI] [PubMed] [Google Scholar]
- 114.Yeung F et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tasselli L, Zheng W & Chua KF SIRT6: novel mechanisms and links to aging and disease. Trends Endocrinol. Metab 28, 168–185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roichman A et al. SIRT6 overexpression improves various aspects of mouse healthspan. J. Gerontol. A Biol. Sci. Med. Sci 72, 603–615 (2017). [DOI] [PubMed] [Google Scholar]
- 117.Tian X et al. SIRT6 is responsible for more efficient DNA double-strand break repair in long-lived species. Cell 177, 622–638.e622 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Di Francesco A, Di Germanio C, Bernier M & de Cabo R A time to fast. Science 362, 770–775 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mannick JB et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med 6, 268ra179 (2014). [DOI] [PubMed] [Google Scholar]
- 120.Timmers S et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 14, 612–622 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dai H, Sinclair DA, Ellis JL & Steegborn C Sirtuin activators and inhibitors: promises, achievements, and challenges. Pharmacol. Ther 188, 140–154 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Heemst D et al. Reduced insulin/IGF-1 signalling and human longevity. Aging Cell 4, 79–85 (2005). [DOI] [PubMed] [Google Scholar]
- 123.Milman S et al. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell 13, 769–771 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Deelen J et al. Gene set analysis of GWAS data for human longevity highlights the relevance of the insulin/IGF-1 signaling and telomere maintenance pathways. Age (Dordr.) 35, 235–249 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pawlikowska L et al. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell 8, 460–472 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kichaev G et al. Integrating functional data to prioritize causal variants in statistical fine-mapping studies. PLoS Genet. 10, e1004722 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hormozdiari F et al. Colocalization of GWAS and eQTL signals detects target genes. Am. J. Hum. Genet 99, 1245–1260 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gusev A et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat. Genet 48, 245–252 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Holmans P et al. Gene ontology analysis of GWA study data sets provides insights into the biology of bipolar disorder. Am. J. Hum. Genet 85, 13–24 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jia P, Zheng S, Long J, Zheng W & Zhao Z dmGWAS: dense module searching for genome-wide association studies in protein-protein interaction networks. Bioinformatics 27, 95–102 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Leeuw CA, Mooij JM, Heskes T & Posthuma D MAGMA: generalized gene-set analysis of GWAS data. PLOS Comput. Biol 11, e1004219 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Taşan M et al. Selecting causal genes from genome-wide association studies via functionally coherent subnetworks. Nat. Methods 12, 154–159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin JR et al. PGA: post-GWAS analysis for disease gene identification. Bioinformatics 34, 1786–1788 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin JR et al. Integrated Post-GWAS analysis sheds new light on the disease mechanisms of schizophrenia. Genetics 204, 1587–1600 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kircher M et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet 46, 310–315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sundaram L et al. Predicting the clinical impact of human mutation with deep neural networks. Nat. Genet 50, 1161–1170 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lin JR, Zhang Q, Cai Y, Morrow BE & Zhang ZD Integrated rare variant-based risk gene prioritization in disease case-control sequencing studies. PLoS Genet. 13, e1007142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Landrum MJ et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 42, D980–D985 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.