Abstract
Background
X-linked hypophosphatemia (XLH) is a hereditary rare disease caused by loss-of-function mutations in PHEX gene leading tohypophosphatemia and high renal loss of phosphate. Rickets and growth retardation are the major manifestations of XLH in children, but there is a broad phenotypic variability. Few publications have reported large series of patients. Current data on the clinical spectrum of the disease, the correlation with the underlying gene mutations, and the long-term outcome of patients on conventional treatment are needed, particularly because of the recent availability of new specific medications to treat XLH.
Results
The RenalTube database was used to retrospectively analyze 48 Spanish patients (15 men) from 39 different families, ranging from 3 months to 8 years and 2 months of age at the time of diagnosis (median age of 2.0 years), and with XLH confirmed by genetic analysis. Bone deformities, radiological signs of active rickets and growth retardation were the most common findings at diagnosis. Mean (± SEM) height was − 1.89 ± 0.19 SDS and 55% (22/40) of patients had height SDS below—2. All cases had hypophosphatemia, serum phosphate being − 2.81 ± 0.11 SDS. Clinical manifestations and severity of the disease were similar in both genders. No genotype—phenotype correlation was found. Conventional treatment did not attenuate growth retardation after a median follow up of 7.42 years (IQR = 11.26; n = 26 patients) and failed to normalize serum concentrations of phosphate. Eleven patients had mild hyperparathyroidism and 8 patients nephrocalcinosis.
Conclusions
This study shows that growth retardation and rickets were the most prevalent clinical manifestations at diagnosis in a large series of Spanish pediatric patients with XLH confirmed by mutations in the PHEX gene. Traditional treatment with phosphate and vitamin D supplements did not improve height or corrected hypophosphatemia and was associated with a risk of hyperparathyroidism and nephrocalcinosis. The severity of the disease was similar in males and females.
Keywords: XLH, Inherited hypophosphatemia, Growth retardation, Bone deformities, Rickets
Background
X-linked hypophosphatemic rickets (XLH) (OMIM 307800) (ORPHA 89936) is the most common hereditary rickets [1–5] with an estimated prevalence of 1:20,000 [6, 7]. It follows an X-linked dominant transmission [8]. The disease is caused by a defective function of PHEX gene [1, 9–13], leading to elevated circulating concentrations of fibroblast growth factor 23 (FGF23) [14], relatively low levels of 1,25 dihydroxyvitamin d [1,25(OH)2D], hyperphosphaturia secondary to decreased proximal tubular reabsorption of phosphate and hypophosphatemia [8, 10, 15]. Classical, conventional treatment of XLH is based on the administration of phosphate supplements and 1-alpha hydroxylated derivates of vitamin D [16]. The wider availability of genetic studies and the recent development of an anti-FGF23 antibody, burosumab, as novel and promising therapy [10, 17] have resulted in a growing current interest for XLH.
We here report the clinical manifestations at diagnosis and follow-up of a large series of Spanish patients included in the online database RenalTube [18]. This study is justified at least by the following reasons: (1) XLH is a rare disease and few publications provide data on large series of patients; (2) XLH has a broad phenotypic variability and additional information is required to better characterize the clinical spectrum of the disease and to explain why the number of cases diagnosed usually does not correspond to the estimated prevalence of the disease; (3) It is important to share data of patients with genetically confirmed XLH in order to facilitate the finding of a potential phenotype—genotype correlation and to have current data that can be compared for the assessment of the new therapies.
Results
Forty-eight patients included in the RenalTube database with the diagnosis of XLH confirmed by defect-of-function variants found in the PHEX gene were analyzed. All variants had been identified as pathogenic. Sixteen patients (33%) had variants with strong evidence of pathogenicity (nonsense, frameshift, deletions) while the other 32 (67%) harbored variants with very strong evidence of pathogenicity (SNPs). Demographic and genetic data from patients are shown in Table 1. Patients were from 39 families and were being followed in pediatric nephrology units of 17 Spanish hospitals (Fig. 1). Fifteen patients were males and 33 females. Median age at diagnosis was 2.0 (IQR 2.6) years and the age ranged from 3 months to 8 years 2 months.
Table 1.
Demographic and genetic data of 48 patients belonging to 39 families (Roman number indicates family)
| Patient | Relationship | Sex | cDNA mutation | Protein mutation | Variant type |
|---|---|---|---|---|---|
| I.1 | Index | F | c.758_759delTT | p.F26Cfs | P (PVS1) |
| I.2 | Sister | F | c.758_759delTT | p.F26Cfs | P (PVS1) |
| II.1 | Index | F | c.2223_2224delAC | p.A514Afs516X | P (PVS1) |
| III.1 | Index | F | c.1578_1579delAA | p.K299Nfs304X | P (PVS1) |
| IV.1 | Index | M | c.893A>T | p.N71I | P (PS1) |
| IV.2 | Brother | M | c.893A>T | p.N71I | P (PS1) |
| V.1 | Index | F | c.2633G>C | p.R651P | P (PS1) |
| VI.1 | Index | F | c.1885C>T | p.Q402X | P (PVS1) |
| VII.1 | Index | M | c.?-2664dup2949-? | Splice region variant | P (PVS1) |
| VII.2 | Brother | M | c.?-2664dup2949-? | Splice region variant | P (PVS1) |
| VII.3 | Daughter | F | c.?-2664dup2949-? | Splice region variant | P (PVS1) |
| VII.4 | Daughter | F | c.?-2664dup2949-? | Splice region variant | P (PVS1) |
| VIII.1 | Index | F | c.886insT | p.K69X | P (PVS1) |
| IX.1 | Index | F | c.2048G>A | p.W456X | P (PVS1) |
| X.1 | Index | F | g.22099152G>T | Splice region variant | P (PVS1) |
| XI.1 | Index | M | c.2648_?del | p.A656_?del | P (PVS1) |
| XII.1 | Index | F | c.2327_?del | p.R549_?del | P (PVS1) |
| XIII.1 | Index | F | g.22168393_delA | Splice region variant | P (PVS1) |
| XIV.1 | Index | F | c.1552C>T | p.R291X | P (PVS1) |
| XV.1 | Index | F | g.22190503G>A | Splice region variant | P (PVS1) |
| XVI.1 | Index | F | c.889_893delGTAAA | p.V70Sfs77X | P (PVS1) |
| XVII.1 | Index | F | c.2086_?del | p.A469_?del | P (PVS1) |
| XVIII.1 | Index | F | c.1180T>C | p.W167R | P (PS1) |
| XIX.1 | Index | F | c.2282C>T | p.P534L | P (PS1) |
| XX.1 | Index | F | c.2416G>A | p.G579R | P (PS1) |
| XXI.1 | Index | F | c.2920C>T | p.R747X | P (PVS1) |
| XXII.1 | Index | F | c.2920C>T | p.R747X | P (PVS1) |
| XXIII.1 | Index | M | c.1152delA | p.L157Lfs220X | P (PVS1) |
| XXIV.1 | Index | F | c.1572C>A,c.1580_1582delTGA | p.Y297X | P (PVS1) |
| XXV.1 | Index | F | g.22076478A>T | Splice region variant | P (PVS1) |
| XXVI.1 | Index | F | g.22190507G>A | Splice region variant | P (PVS1) |
| XXVII.1 | Index | F | c.889_893delGTAAA | p.V70Sfs77X | P (PVS1) |
| XXVII.2 | Father | M | c.889_893delGTAAA | p.V70Sfs77X | P (PVS1) |
| XXVIII.1 | Index | M | c.2879G>T | p.C733F | P (PS1) |
| XXVIII.2 | Sister | F | c.2879G>T | p.C733F | P (PS1) |
| XXIX.1 | Index | F | c.2642T>C | p.F654S | P (PS1) |
| XXX.1 | Index | M | c.2387T>G | p.L569R | P (PS1) |
| XXX.2 | Mother | F | c.2387T>G | p.L569R | P (PS1) |
| XXXI.1 | Index | F | c.2085G>C | p.K468N | P (PS1) |
| XXXII.1 | Index | M | c.2282C>T | p.P534L | P (PS1) |
| XXXIII.1 | Index | M | g.22033125T>G | Splice region variant | P (PVS1) |
| XXXIV.1 | Index | M | c.2380C>T | p.R567X | P (PVS1) |
| XXXV.1 | Index | M | c.2005G>T | p.V442P | P (PS1) |
| XXXV.2 | Mother | F | c.2005G>T | p.V442P | P (PS1) |
| XXXVI.1 | Index | F | c.2416G>A | G579R | P (PS1) |
| XXXVII.1 | Index | M | g.22099152G>A | Splice region variant | P (PVS1) |
| XXXVIII.1 | Index | F | c.2617delG | p.D646Ifs | P (PVS1) |
| XXXIX.1 | Index | M | c.1363_1364delTC | p.S228Pfs236X | P (PVS1) |
F: female, M: male
P: pathogenic
PVS1: very strong evidence of pathogenicity according to reference 19
PS1: strong evidence of pathogenicity according to reference 19
Fig. 1.
Geographical distribution of the Spanish hospitals participating in the study
Presenting manifestations are shown in Table 2 for each patient. Bone deformities and radiological signs of active rickets were the most frequent findings leading to diagnosis. Ten patients were diagnosed because of family screening. Age at diagnosis of these patients was no different from that of the rest of the series as it ranged from 0.5 to 8 years with a median age of 1.04 years.
Table 2.
Clinical manifestations at diagnosis
| Patient | Age at diagnosis | Bone deformities | Active rickets | Longitudinal growth retardation (≤ 2 SDS) | Dental problems |
|---|---|---|---|---|---|
| I.1 | 3 m | No | – | No | – |
| I.2 | 1 y | Yes | – | Yes | – |
| II.1 | 1 y 5 m | Yes | Yes | Yes | No |
| III.1 | 1 y 6 m | – | Yes | Yes | Yes |
| IV.1 | 1 y 4 m | No | No | Yes | Yes |
| IV.2 | 4 y | No | No | No | No |
| V.1 | 5 y | Yes | Yes | Yes | No |
| VI.1 | 2 y | Yes | No | No | No |
| VII.1 | 8 y | – | – | – | – |
| VII.2 | 2 y | – | Yes | – | No |
| VII.3 | 11 m | – | Yes | Yes | No |
| VII.4 | 1 y | – | – | Yes | – |
| VIII.1 | 4 y | Yes | Yes | No | No |
| IX.1 | 5 y | Yes | Yes | No | No |
| X.1 | 5 y | Yes | Yes | Yes | No |
| XI.1 | 2 y 3 m | Yes | Yes | Yes | No |
| XII.1 | 2 y | Yes | Yes | – | – |
| XIII.1 | 5 y | – | Yes | – | – |
| XIV.1 | 9 m | Yes | Yes | No | No |
| XV.1 | 1 y 1 m | Yes | Yes | Yes | – |
| XVI.1 | 4 y | Yes | – | Yes | No |
| XVII.1 | 5 y | – | – | Yes | – |
| XVIII.1 | 2 y | Yes | Yes | Yes | No |
| XIX.1 | 7 m | Yes | Yes | – | No |
| XX.1 | 2 y 1 m | Yes | – | Yes | – |
| XXI.1 | 4 y 5 m | Yes | Yes | No | No |
| XXII.1 | 4 y | Yes | Yes | Yes | No |
| XIII.1 | 2 y | Yes | Yes | No | No |
| XXIV.1 | 3 y | Yes | – | No | – |
| XXV.1 | 1 y 6 | Yes | Yes | No | No |
| XXVI.1 | 4 y | Yes | Yes | Yes | No |
| XXVII.1 | 6 m | Yes | Yes | No | No |
| XXVII.2 | 8 y | – | Yes | – | No |
| XXVIII.1 | 1 y 6 m | Yes | – | No | – |
| XXVIII.2 | 6 m | Yes | Yes | No | No |
| XXIX.1 | 1 y 9 m | Yes | Yes | Yes | No |
| XXX.1 | 1 y 1 m | Yes | Yes | Yes | – |
| XXX.2 | 1 y 6 m | – | Yes | – | No |
| XXXI.1 | 8 y2 m | Yes | Yes | Yes | No |
| XXXII.1 | 1 y 6 m | Yes | Yes | No | No |
| XXXIII.1 | 5 y | – | – | Yes | – |
| XXXIV.1 | 2 y 2 m | Yes | Yes | No | No |
| XXXV.1 | 7 m | Yes | Yes | No | – |
| XXXV.2 | 2 y 6 m | Yes | Yes | Yes | – |
| XXXVI.1 | 6 m | Yes | Yes | No | No |
| XXXVII.1 | 1 y 9 m | Yes | Yes | Yes | Yes |
| XXXVIII.1 | 1 y 6 m | Yes | Yes | No | No |
| XXXIX.1 | 2 y 2 m | Yes | Yes | – | No |
| Percentage of patients | P/A/U | P/A/U | P/A/U | P/A/U | |
| 73/6/21 | 73/6/21 | 46/38/17 | 6/63/31 | ||
m: month, y: year
SDS: standard deviation score. Dash: information in this field was missing from the database
P/A/U: present/absent/unreported
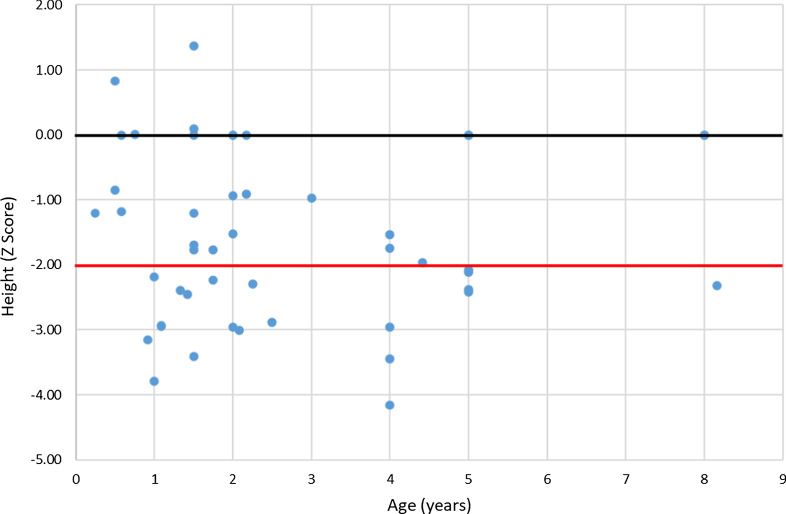
Twenty-two out of40 patients (55%) in whom the height was registered presented growth retardation (height ≤ 2 SDS). Patients’ height (X ± SEM) was − 1.89 ± 0.19 SDS (n = 40) (Fig. 2). In 87% (35/40) the height was below the 50th percentile. Weight was − 0.88 ± 0.14 SDS (n = 41) and body mass index 0.2 ± 0.15 SDS (n = 40).
Fig. 2.
Height at diagnosis (n = 40). Black line: 0 SD; red line: − 2 SD
Biochemical findings at diagnosis are shown in Table 3. Mean values (± SEM) of available data were serum phosphate 2.7 ± 0.1 mg/dl; − 2.81 ± 0.11 SDS, (n = 41), alkaline phosphatase (892 ± 84 mU/ml) (n = 39), 1,25(OH)2D62 ± 7 pg/ml (n = 34), parathyroid hormone (PTH)70 ± 7 pg/ml (n = 33), and tubular phosphate reabsorption (TPR) 69 ± 4% (n = 26).
Table 3.
Biochemical manifestations at diagnosis
| Patient | Serum phosphate | Serum alkaline phosphatases (mU/ml) | Serum 1,25(OH)2D (pg/ml) | Serum intact PTH (pg/ml) | TPR (%) | |
|---|---|---|---|---|---|---|
| mg/dl | SDS | |||||
| I.1 | 3.4 | − 2.21 | 1230 | 87 | – | – |
| I.2 | 3.8 | − 1.05 | 1916 | 76 | – | – |
| II.1 | 2.6 | − 2.68 | 695 | 57 | 81 | 79 |
| III.1 | 2.1 | − 3.36 | 1646 | 16 | 68 | 32 |
| IV.1 | 2.8 | − 2.41 | 378 | 73 | 29 | 78 |
| IV.2 | 3.0 | − 2.44 | 232 | 40 | 43 | 82 |
| V.1 | 2.1 | − 3.99 | 1513 | 147 | 78 | 85 |
| VI.1 | 2.6 | − 2.68 | 525 | 33 | 64 | 78 |
| VII.1 | – | – | – | – | – | – |
| VII.2 | 2.4 | − 2.95 | 516 | – | – | – |
| VII.3 | – | – | 991 | 28 | 30 | – |
| VII.4 | – | – | – | – | – | – |
| VIII.1 | 3.1 | − 2.27 | 598 | 55 | 39 | 38 |
| IX.1 | 3.0 | − 2.44 | 1824 | 47 | 59 | 67 |
| X.1 | 2.9 | − 2.61 | 639 | 33 | 136 | 74 |
| XI.1 | 2.3 | − 3.09 | – | – | 58 | – |
| XII.1 | 2.7 | − 2.54 | – | – | 48 | – |
| XIII.1 | – | – | – | – | – | – |
| XIV.1 | 3.3 | − 2.34 | 892 | 64 | 65 | 75 |
| XV.1 | 2.0 | − 3.49 | 704 | 20 | – | 39 |
| XVI.1 | 2.4 | − 3.47 | 571 | 108 | 71 | 26 |
| XVII.1 | 2.5 | − 3.30 | – | 28 | 30 | – |
| XVIII.1 | 2.3 | − 3.09 | 1864 | 31 | 64 | 73 |
| XIX.1 | – | – | – | – | – | – |
| XX.1 | 2.9 | − 2.27 | 446 | 88 | 116 | 93 |
| XXI.1 | 3.1 | − 2.27 | 470 | 56 | 49 | 86 |
| XXII.1 | 2.8 | − 2.78 | 697 | 74 | 32 | 76 |
| XXIII.1 | 2.1 | − 3.36 | 733 | – | 68 | 82 |
| XXIV.1 | 2.2 | − 3.22 | 514 | 61 | 78 | 58 |
| XXV.1 | 2.9 | − 2.27 | 1940 | 40 | 57 | 58 |
| XXVI.1 | 2.3 | − 3.64 | 423 | – | – | 82 |
| XXVII.1 | 2.9 | − 2.86 | 432 | 31 | 23 | – |
| XXVII.2 | – | – | – | – | – | – |
| XXVIII.1 | 3.0 | − 2.14 | 236 | 78 | 54 | – |
| XXVIII.2 | 3.3 | − 2.34 | 426 | 171 | 57 | 90 |
| XXIX.1 | 1.8 | − 3.77 | 1555 | – | – | – |
| XXX.1 | 3.1 | − 2.00 | 829 | 183 | 101 | 88 |
| XXX.2 | 2.2 | − 3.22 | 158 | 25 | – | – |
| XXXI.1 | – | – | – | – | – | – |
| XXXII.1 | 2.8 | − 2.41 | 692 | 41 | 111 | – |
| XXXIII.1 | 2.1 | − 3.99 | – | – | 76 | 54 |
| XXXIV.1 | 2.3 | − 3.09 | 856 | 64 | 101 | 46 |
| XXXV.1 | 3.5 | − 2.08 | 916 | – | 227 | 82 |
| XXXV.2 | 2.2 | − 3.22 | 1620 | – | – | 70 |
| XXXVI.1 | 2.8 | − 2.41 | 1093 | 65 | 66 | – |
| XXXVII.1 | 2.4 | − 2.95 | 1527 | 46 | 94 | – |
| XXXVIII.1 | 2.4 | − 2.95 | 856 | 40 | 58 | – |
| XXXIX.1 | 3.0 | − 2.14 | 759 | 51 | 43 | – |
1,25(OH)2D: 1,25 dihydroxyvitamin D. PTH: Parathyroid hormone
TPR: tubular phosphate reabsorption. Dash: information in this field was missing from the database
No differences were found between males and females for clinical manifestations, growth impairment or biochemical data at diagnosis. Likewise, no genotype–phenotype correlation was found. Actually, even patients within the same family presented different severity of clinical and biochemical manifestations.
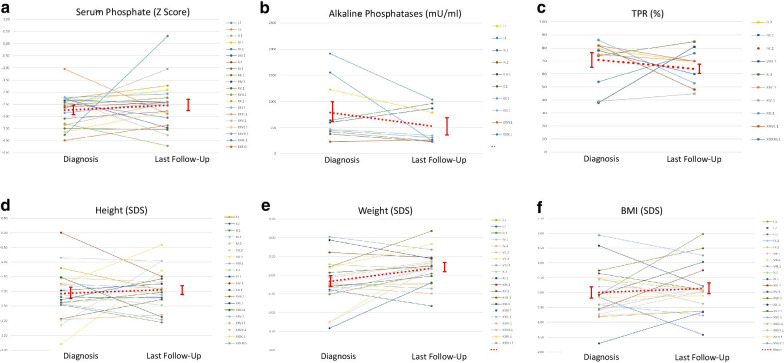
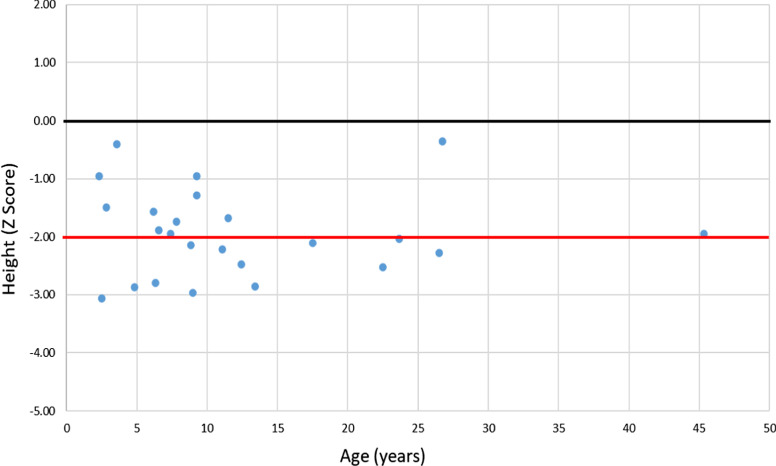
Growth and biochemical variables of 26 patients after a median follow uptime of 7.42 years (IQR = 11.26) are shown in Table 4, Figs. 3 and 4. Anthropometric data were − 1.94 ± 0.16 SDS for height (n = 24), − 0.82 ± 0.10 SDS for weight (n = 22) and 0.14 ± 0.19 SDS for BMI (n = 22). Comparison of data from patients with information both at diagnosis and last follow-up showed mean variations of 0.13 ± 0.23 SDS for height (p > 0.05) (n = 20), 0.35 ± 0.14 SDS for weight (p = 0.02) (n = 20) and 0.13 ± 0.20 SDS for BMI (p > 0.05) (n = 20).
Table 4.
Biochemical manifestations at last follow up
| Patient | Follow-up Time | Serum phosphate | Serum alkaline phosphatases (mU/ml) | Serum 1,25(OH)2D (pg/ml) |
Serum intact PTH (pg/ml) | TPR (%) | |
|---|---|---|---|---|---|---|---|
| mg/dl | SD (Z score) | ||||||
| I.1 | 7 y 2 m | 2.9 | − 2.07 | 787 | – | 30 | 72 |
| I.2 | 10 y 1 m | 2.7 | − 2.90 | 1035 | – | 35 | 50 |
| II.1 | 1 y 1 m | 3.8 | − 1.05 | – | – | 34 | 70 |
| III.1 | – | – | – | – | – | – | – |
| IV.1 | 7 y 6 m | 3.0 | − 1.91 | 231 | 78 | 32 | 60 |
| IV.2 | 7 y 6 m | 2.6 | − 3.06 | 266 | 73 | 44 | 48 |
| V.1 | – | – | – | – | – | – | – |
| VI.1 | – | – | – | – | – | – | – |
| VII.1 | – | – | – | – | – | – | – |
| VII.2 | – | – | – | – | – | – | – |
| VII.3 | 8 y 4 m | 1.9 | − 3.69 | – | – | 77 | 42 |
| VII.4 | 8 y 3 m | 1.8 | − 3.85 | 665 | – | 108 | 73 |
| VIII.1 | 2 y 7 m | 2.4 | − 3.47 | 868 | 15 | 84 | 81 |
| IX.1 | – | – | – | – | – | – | – |
| X.1 | 7 y 5 m | 3.0 | − 2.41 | 962 | 50 | 93 | 85 |
| XI.1 | 5 y 11 m | 2.8 | − 2.78 | 632 | 45 | 49 | 42 |
| XII.1 | 11 y 5 m | 2.8 | − 2.23 | 636 | – | 112 | 86 |
| XIII.1 | 17 y 6 m | 1.7 | − 4.22 | – | 29 | 86 | 64 |
| XIV.1 | 2 y 1 m | 2.8 | − 2.41 | – | 96 | 20 | 70 |
| XV.1 | 25 y 5 m | 2.0 | − 3.55 | – | 20 | 75 | 45 |
| XVI.1 | – | – | – | – | – | – | – |
| XVII.1 | 18 y 8 m | 1.7 | − 4.22 | 120 | 32 | 99 | 42 |
| XVIII.1 | – | – | – | – | – | – | – |
| XIX.1 | 26 y 2 m | 2.4 | − 2.66 | – | – | 200 | – |
| XX.1 | 1 y 9 m | 3.3 | − 1.73 | 312 | 72 | 53 | 45 |
| XXI.1 | 3 y 5 m | 2.6 | − 2.56 | 348 | 43 | 26 | 53 |
| XXII.1 | – | – | – | – | – | – | – |
| XXIII.1 | 2 y 10 m | 3.0 | − 2.44 | – | 29 | 28 | – |
| XXIV.1 | – | – | – | – | – | – | – |
| XXV.1 | 16 y | 1.9 | − 3.77 | – | – | 37 | – |
| XXVI.1 | 2 y 4 m | 2.8 | − 2.78 | 240 | 86 | 25 | 70 |
| XXVII.1 | 1 y 10 m | 2.8 | − 2.41 | – | 58 | 49 | 76 |
| XXVII.2 | 37 y 4 m | 2.3 | − 2.88 | – | – | 117 | 80 |
| XXVIII.1 | – | – | – | – | – | – | – |
| XXVIII.2 | – | – | – | – | – | – | – |
| XXIX.1 | 1 y 10 m | 4.8 | 0.31 | 244 | 68 | 73 | 89 |
| XXX.1 | – | – | – | – | – | – | – |
| XXX.2 | – | – | – | – | – | – | – |
| XXXI.1 | – | – | – | – | – | – | – |
| XXXII.1 | – | – | – | – | – | – | – |
| XXXIII.1 | 4 y | 2.1 | − 3.36 | – | – | 114 | 76 |
| XXXIV.1 | – | – | – | – | – | – | – |
| XXXV.1 | – | – | – | – | – | – | – |
| XXXV.2 | – | – | – | – | – | – | – |
| XXXVI.1 | – | – | – | – | – | – | – |
| XXXVII.1 | – | – | – | – | – | – | – |
| XXXVIII.1 | – | – | – | – | – | – | – |
| XXXIX.1 | – | – | – | – | – | – | – |
1,25(OH)2D: 1,25 dihydroxyvitamin D
m: month, y: year
PTH: parathyroid hormone
TPR: tubular phosphate reabsorption. Dash: information in this field was missing from the database
Fig. 3.
Biochemical and growth data at diagnosis and last follow-up. TPR: tubular phosphate reabsorption. BMI: Body Mass Index. Mean values are connected by red dots line. Vertical bars represent ± SEM
Fig. 4.
Height at last follow-up (n = 24). Black line: 0 SD; red line: − 2 SD
Mean SDS for serum phosphate was − 2.72 ± 0.20 (n = 25). Alkaline phosphatases, 1,25 (OH)2D and PTH levels were 525 ± 82 mU/ml (n = 14), 53 ± 7 pg/ml (n = 15) and 68 ± 8 pg/ml (n = 26) respectively. Mean tubular phosphate reabsorption was 65 ± 3% (n = 22). Comparison between diagnosis and last follow-up data revealed a variation of 0.20 ± 0.28 SDS for serum phosphate (p > 0.05) (n = 20), 5 ± 7 pg/ml for 1,25 (OH)2D (p > 0.05) (n = 10), 0 ± 11 pg/ml for PTH (p > 0.05) (n = 16) and − 7 ± 8 for tubular phosphate reabsorption (p > 0.05) (n = 11).
Eight out of 24 patients with renal ultrasounds at last follow-up presented nephrocalcinosis (Table 5).
Table 5.
Treatment and clinical data at last follow-up
| Patient | Phosphorus element dose (mg/kg/day) | Vitamin D dose (µg/day) | Nephrocalcinosis |
|---|---|---|---|
| I.1 | 38a | 0.50c | No |
| I.2 | 40a | 0.50c | No |
| II.1 | 86a | 1.20d | Yes |
| III.1 | – | – | – |
| IV.1 | 83a | 0.60d | No |
| IV.2 | 62a | 1.00d | No |
| V.1 | – | – | – |
| VI.1 | – | – | – |
| VII.1 | – | – | No |
| VII.2 | – | – | – |
| VII.3 | 55b | 0.50c | Yes |
| VII.4 | 52b | 0.50c | Yes |
| VIII.1 | 90a | 1.50d | Yes |
| IX.1 | – | – | – |
| X.1 | 40a | 0.25d | No |
| XI.1 | 83a | 0.50c | No |
| XII.1 | 44b | 0.29d | No |
| XIII.1 | 41b | 0.25c | Yes |
| XIV.1 | 52a | 1.20d | – |
| XV.1 | 41b | 1.00c | No |
| XVI.1 | – | – | – |
| XVII.1 | 45b | 0.25c | Yes |
| XVIII.1 | – | – | – |
| XIX.1 | 27b | 0.50c | Yes |
| XX.1 | 32a | 0.50c | No |
| XXI.1 | 63a | 1.50d | No |
| XXII.1 | – | – | – |
| XXIII.1 | 65a | 1.10d | No |
| XXIV.1 | – | – | – |
| XXV.1 | 38a | 1.40c | – |
| XXVI.1 | 58b | 0.25c | No |
| XXVII.1 | 48b | 0.60d | No |
| XXVII.2 | – | – | – |
| XXVIII.1 | – | – | – |
| XXVIII.2 | – | – | – |
| XXIX.1 | – | 0.10d | No |
| XXX.1 | – | – | – |
| XXX.2 | – | – | Yes |
| XXXI.1 | – | – | – |
| XXXII.1 | – | – | – |
| XXXIII.1 | 49b | 0.75c | No |
| XXXIV.1 | – | – | – |
| XXXV.1 | – | – | – |
| XXXV.2 | – | – | – |
| XXXVI.1 | – | – | – |
| XXXVII.1 | – | – | – |
| XXXVIII.1 | – | – | – |
| XXXIX.1 | – | – | – |
aPhosphate was administered as a solution
bPhosphate was administered as tablets
cCorresponds to 1,25 dihydroxy vitamin D
dCorresponds to 1 hydroxy vitamin D
Discussion
This study provides a current description of the phenotypic characteristics of a large cohort of Caucasian pediatric patients with XLH genetically confirmed. The sample is a broad representation of the Spanish children with XLH, coming from several hospitals scattered through the country and provides data at diagnosis and after a median follow-up of 7.42 years. The study confirms that growth retardation, bone deformities and active lesions of rickets are the main presenting manifestations of the disease, within a wide spectrum of symptoms. No significant differences were found between males and females as for the severity of the disease. It is of interest that a broad spectrum of PHEX gene variants, all of them already described as pathogenic, was found and no mutation was specifically prevalent in Spanish population. All variants were classified as pathogenic according to the American College of Medical Genetics and Genomics consensus [19]. There was a high phenotypical variability even among family members harboring the same mutations, suggesting that other genes and environmental factors may affect the severity of XLH, as reported by other authors [20, 21].
In addition, the study shows that conventional treatment with phosphate supplements and vitamin D metabolites does not lead to persistent correction of hypophosphatemia or reduction of renal wasting of phosphate and does not modify the circulating levels of calcitriol. Unfortunately, this study does not provide information on circulating FGF23 levels, given its retrospective design. At the last follow-up visit, 11 out of 25 patients had serum PTH values mildly elevated. In XLH, development of hyperparathyroidism is thought to be related with the pharmacological administration of phosphate [22]. Eight patients developed nephrocalcinosis during the follow-up period, a finding linked to the administration of phosphate and vitamin D that usually does not result in subsequent clinical complications [16].
Though conventional treatment has been described to heal active signs of rickets and may improve bone deformities [23], this study confirms that it does not lead to catch-up growth. Mean height Z score of the group of patients remained low, − 1.89 at diagnosis versus − 1.94 at the last visit, although Fig. 3 indicates that the individual patients’ response varied from marked improvement to worsening of growth impairment. Two patients, VII.3 and XV.1, transiently received growth hormone treatment and their heights improved + 2.19and + 0.66 SDS, respectively. It is of note that 4 out of 16 patients had BMI greater than + 1.00 SDS at the last follow-up visit. This percentage of 25% corresponds to the normal distribution of reference population and it indicates that tendency to overweight and obesity was not found in the group of XLH patients here reported, unlike other series that have recently drawn attention to these complications likely related with the sedentary life and restricted mobility of these patients [11]. In this regard, a slight but significant increase in weight was found during the follow-up period in our series.
Our study presents methodological limitations inherent to the retrospective analysis and to the fact that patients’ information was extracted from a database in which some data were missing and cannot be recovered. It is also of note the lack of information on the degree of adherence to medication of each patient as well as the different monitoring protocols among the participating centers. However, it is an observational clinical study describing a large cohort of Spanish pediatric patients with genetically confirmed XLH and it provides current and interesting information on the clinical and biochemical features of the disease, at diagnosis and follow-up after conventional treatment. Our findings could be used as reference for further studies using burosumab treatment.
Conclusions
This study confirms that growth retardation and rickets were the most prevalent clinical manifestations at diagnosis in a large series of Spanish pediatric patients with XLH confirmed by identification of pathogenic variants in the PHEX gene. Traditional treatment with phosphate supplements and calcitriol did not improve height or corrected hypophosphatemia and was associated with a risk of hyperparathyroidism and nephrocalcinosis. The severity of the disease was similar in males and females and no phenotype-genotype association was found.
Patients and methods
The RenalTube database including 48 patients, 15 males and 33 females, with the diagnosis of XLH confirmed by defect-of-function mutations found in the PHEX gene was retrospectively reviewed to obtain demographic information and clinical and biochemical manifestations at diagnosis and at the last annual follow-up. Genetic information was confirmed and formatted according to Genome Reference Consortium Human Build 38 patch release 13 (GRCh38.p13) [24]. Variants were analyzed in silico and classified according to recommendations from the consensus of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology [19] as pathogenic, likely pathogenic, benign or likely benign. Results for the age are presented as median and interquartile range (IQR). Other variables are presented for the group as mean (X) ± standard error of the mean (SEM). Z score (SDS) of anthropometric values was calculated using Spanish age and sex-matched reference values [25]. Patients with height ≤ 2 SDS were considered to have longitudinal growth retardation according to World Health Organization standards [26]. Reference values for biochemical parameters were obtained from the laboratory of the Hospital Universitario Central de Asturias (HUCA) [27].
All patients received treatment with phosphate supplementation (dose range of phosphorus element: 27–90 mg/kg/day at last follow-up) and vitamin D metabolites (dose range: 0.25–1.5 µg/day at last follow-up), according to the criteria and indications given by their physicians (Table 5). Two patients (VII.3 and XV.1) received growth hormone treatment. None of them received burosumab treatment.
Information in RenalTube database was downloaded and formatted to an Excel database. All fields but reasons for consultation and genetic information were multichoice or numeric format.
Chi squared test was used to analyze differences between sex for binary (Yes/No) fields (growth retardation, bone deformities). F-test was used to assess variance equality between sex for anthropometric and biochemical values. T-test was used to analyze differences between sex for anthropometric and biochemical values. Paired T-test for means was used to analyze differences between diagnosis and last follow-up for anthropometric and biochemical values. T-test for unequal variances was used to compare age at diagnosis for patients with and without family history of the disease.
Phenotype—genotype correlation was assessed by isolating the most severe phenotypes (lowest serum concentrations, most severe growth retardation, highest levels of alkaline phosphatases) and comparing genetic mutations in these patients looking for big deletions, SNPs with entirely different amino acids or nonsense mutations.
Acknowledgements
Not applicable.
Authors’ contributions
ER gathered, formattedand analyzed data from patients included in RenalTube database and was a major contributor in writing the manuscript. FS and HG contributed on analyzing data and were major contributors on writing the manuscript. Every other author introduced information of at least 1 patient from this study in RenalTube database. All authors read and approved the final manuscript.
Funding
This research has been partially funded by Kyowa Kirin Farmacéutica S.L.U., Fondo de Investigaciones Sanitarias (FIS) and Fundación Nutrición y Crecimiento (FUNDNYC).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Patients information has been processed via RenalTube database where patients signed their consent to participate in scientific studies.
Consent for publication
Patients or parents signed consent for publication of their data through RenalTube consent form.
Competing interests
The study has been partially funded by Kyowa Kirin Farmacéutica S.L.U. This company produces the drug Crysvita® (burosumab). Nevertheless, no patients in this study had been or were being treated with burosumab at the time of data collection.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bastepe M, Jüppner H. Inherited hypophosphatemic disorders in children and the evolving mechanisms of phosphate regulation. Rev Endocr Metab Disord. 2008;9(2):171–180. doi: 10.1007/s11154-008-9075-3. [DOI] [PubMed] [Google Scholar]
- 2.Fuente R, Gil-Peña H, Claramunt-Taberner D, Hernández-Frías O, Fernández-Iglesias Á, Alonso-Durán L, et al. MAPK inhibition and growth hormone: a promising therapy in XLH. FASEB J. 2019;33(7):8349–8362. doi: 10.1096/fj.201802007R. [DOI] [PubMed] [Google Scholar]
- 3.Morey M, Castro-Feijóo L, Barreiro J, Cabanas P, Pombo M, Gil M, et al. Genetic diagnosis of X-linked dominant hypophosphatemic rickets in a cohort study: Tubular reabsorption of phosphate and 1,25(OH)2D serum levels are associated with PHEX mutation type. BMC Med Genet. 2011;12(1):116. doi: 10.1186/1471-2350-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C, Zhao Z, Sun Y, Xu L, JiaJue R, Cui L, et al. Clinical and genetic analysis in a large Chinese cohort of patients with X-linked hypophosphatemia. Bone. 2019;121:212–220. doi: 10.1016/j.bone.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Capelli S, Donghi V, Maruca K, Vezzoli G, Corbetta S, Brandi ML, et al. Clinical and molecular heterogeneity in a large series of patients with hypophosphatemic rickets. Bone. 2015;79:143–149. doi: 10.1016/j.bone.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 6.Francis F, Hennig S, Korn B, Reinhardt R, de Jong P, Poustka A, et al. A gene (PEX) with homologies to endopeptidases is mutated in patients with X–linked hypophosphatemic rickets. Nat Genet. 1995;11(2):130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 7.Fuente R, Gil-Peña H, Claramunt-Taberner D, Hernández O, Fernández-Iglesias A, Alonso-Durán L, et al. X-linked hypophosphatemia and growth. Rev Endocr Metab Disord. 2017 doi: 10.1007/s11154-017-9408-1. [DOI] [PubMed] [Google Scholar]
- 8.Gattineni J, Baum M. Genetic disorders of phosphate regulation. Pediatr Nephrol. 2012;27(9):1477–1487. doi: 10.1007/s00467-012-2103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bitzan M, Goodyer PR. Hypophosphatemic rickets. Pediatr Clin North Am. 2019;66(1):179–207. doi: 10.1016/j.pcl.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter TO, Whyte MP, Imel EA, Boot AM, Högler W, Linglart A, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378(21):1987–1998. doi: 10.1056/NEJMoa1714641. [DOI] [PubMed] [Google Scholar]
- 11.Zhukouskaya VV, Rothenbuhler A, Colao A, Di SC, Kamenický P, Trabado S, et al. Increased prevalence of overweight and obesity in children with x-linked hypophosphatemia. Endocr Connect. 2020;9(2):144–153. doi: 10.1530/EC-19-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emma F, Cappa M, Antoniazzi F, Bianchi ML, Chiodini I, Eller Vainicher C, et al. X-linked hypophosphatemic rickets: An Italian experts’ opinion survey. Ital J Pediatr. 2019;45(1):1–7. doi: 10.1186/s13052-019-0654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho HY, Lee BH, Kang JH, Ha IS, Cheong HI, Choi Y. A clinical and molecular genetic study of hypophosphatemic rickets in children. Pediatr Res. 2005;58(2):329–333. doi: 10.1203/01.PDR.0000169983.40758.7B. [DOI] [PubMed] [Google Scholar]
- 14.Endo I, Fukumoto S, Ozono K, Namba N, Inoue D, Okazaki R, et al. Nationwide survey of fibroblast growth factor 23 (FGF23)-related hypophosphatemic diseases in Japan: prevalence, biochemical data and treatment. Endocr J. 2015;62(9):811–816. doi: 10.1507/endocrj.EJ15-0275. [DOI] [PubMed] [Google Scholar]
- 15.Ariceta G, Langman CB. Growth in X-linked hypophosphatemic rickets. Eur J Pediatr. 2007;166(4):303–309. doi: 10.1007/s00431-006-0357-z. [DOI] [PubMed] [Google Scholar]
- 16.Haffner D, Emma F, Eastwood DM, Duplan MB, Bacchetta J, Schnabel D, et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol. 2019;15:435–455. doi: 10.1038/s41581-019-0152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Insogna KL, Briot K, Imel EA, Kamenický P, Ruppe MD, Portale AA, et al. A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res. 2018;33(8):1383–1393. doi: 10.1002/jbmr.3475. [DOI] [PubMed] [Google Scholar]
- 18.Renaltube. http://renaltube.com/en/
- 19.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding KR. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the american college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holm IA, Nelson AE, Robinson BG, Mason RS, Marsh DJ, Cowell CT, et al. Mutational analysis and genotype-phenotype correlation of the PHEX gene in X-linked hypophosphatemic rickets. J Clin Endocrinol Metab. 2001;86(8):3889–3899. doi: 10.1210/jcem.86.8.7761. [DOI] [PubMed] [Google Scholar]
- 21.Rafaelsen S, Johansson S, Ræder H, Bjerknes R. Hereditary hypophosphatemia in Norway: a retrospective population-based study of genotypes, phenotypes, and treatment complications. Eur J Endocrinol. 2016;174(2):125–136. doi: 10.1530/EJE-15-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothenbuhler A, Schnabel D, Högler W, Linglart A. Diagnosis, treatment-monitoring and follow-up of children and adolescents with X-linked hypophosphatemia (XLH) Metabolism. 2020;103:153892. doi: 10.1016/j.metabol.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter TO, Imel EA, Holm IA, et al. A clinician’s guide to X-linked hypophosphatemia. J Bone Min Res. 2011;26(7):1381–1388. doi: 10.1002/jbmr.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ensembl. https://www.ensembl.org/Homo_sapiens/Gene/Summary?db=core;g=ENSG00000102174;r=X:22032325-22251310
- 25.Endrocrinoped. http://www.webpediatrica.com/endocrinoped/antropometria.php
- 26.WHO. https://www.who.int/nutgrowthdb/about/introduction/en/index5.html
- 27.Servicio de Bioquímica Clínica (AGC-Laboratorio de Medicina). Hospital Universitario Central de Asturias. Valores bioquímica HUCA. Biblioteca De Pruebas Bioquimicas. 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.