Abstract
Background
Between February and April 2016, a slight increase in mortality was observed in a colony consisting of 400 captive Seba’s short-tailed bats (Carollia perspicillata). These animals cohabited with other nocturnal animal species in a dome of a private zoo in Switzerland.
Results
Gross and histological analysis of two (14.3%) out of the 13 animals submitted for necropsy within this period revealed a necrosuppurative pneumonia, hepatitis, splenitis, enterocolitis, and endometritis, with abundant intralesional colonies of Gram-negative rods. Yersinia (Y.) pseudotuberculosis serotype O:1 and biotype 1 belonging to the sequence type ST90 was isolated from the affected organs in both animals. Following this diagnosis, ¼ of the colony (99 animals) was culled and submitted for gross and histopathological analysis, and a bacterial culture selective for Yersinia spp. of lung, liver, and spleen was performed. From these 99 animals, one gravid female was tested and found to be positive for Y. pseudotuberculosis in the absence of clinical symptoms and histopathological lesions. PCR analysis of altogether three bacterial isolates for virulence factors revealed the presence of the ail gene, and one isolate was also positive for the virF and yadA plasmid genes.
Conclusions
These findings suggest that Carollia perspicillata are susceptible to lethal yersiniosis but do not represent a regular reservoir for Y. pseudotuberculosis. Culling of ¼ of the population was sufficient to limit the spread of this infection among the colony. Moreover, no infections were detected in cohabitant nocturnal animals and caretakers, indicating that the zoonotic risk in this case was low.
Background
Yersinia (Y.) pseudotuberculosis is a zoonotic Gram-negative rod with a global distribution, which can cause disease in a broad range of mammalian and avian species [1]. This organism is transmitted faecal-orally, and ingestion of contaminated food or water is considered the major source of infection in humans and animals. Rodents and birds are considered to be reservoir hosts and display a milder or even asymptomatic course of infection [2]. Severe infection associated with high mortality has been described not only in hares during epizootics, but also in several zoo animals, including monkeys and larger rodents such as pacas and capybaras [2–4].
Bats act as important reservoirs for several bacterial, viral, protozoal and fungal pathogens, and their potential role in the transmission of zoonoses is receiving growing attention [5]. Following detection of Y. pseudotuberculosis in free-living bats (Myotis myotis) in Germany, a potential role of these animals as Yersinia spp. reservoirs is discussed [6, 7]. Moreover, two Y. pseudotuberculosis outbreaks in captive bat colonies of Egyptian rousette bats (Rousettus aegyptiacus) were associated with high morbidity and mortality rates [1, 8].
Carollia (C.) perspicillata is a common species naturally occurring in the Neotropics that belongs to the Phyllostomidae family. It is a medium sized bat, with a wingspan of about 30 cm for an adult, weight of approximately 20 g. The average lifespan in captivity is 12 years [9, 10]. Females reach sexual maturity at approximately 1 year of age and males become mature between 1 and 2 years [9]. They can have two litters per year in close synchrony with fruit availability [11].
The current report describes the detection of Y. pseudotuberculosis serotype O:1 infection in three out of 112 animals in an indoor-housed colony of captive C. perspicillata in a private zoo in Switzerland.
Results
Y. pseudotuberculosis infection is not associated with a statistically significant increase in mortality within C. perspicillata colony
A slight mortality increase among bats of this species was observed between February and April 2016 during a birth peak. Thirteen bats overall were submitted for necropsy. Some of these animals had been found dead and frozen shortly prior to February 2016. The majority of the necropsied bats were female (8.6%) and/or young animals (61.5%) (Table 1). Of these animals, one adult female was known to have aborted about a week prior to death (ID1). The other animal was an adult male (ID2) that displayed multifocal white foci in the liver measuring approximately 1–2 μm in diameter at necropsy, while the lungs were reddened and of increased consistency. Histopathological examination of both animals revealed a severe, multifocal to coalescing, acute, necrosuppurative hepatitis and pneumonia, with abundant intralesional bacterial rod colonies (Fig. 1a and b) that stained red in the Gram staining. Additional histopathological lesions included a severe necrosuppurative enterocolitis, lymphadenitis, splenitis, stomatitis and glossitis, as well as an endometritis in the female (Fig. 1c and d). In both animals (1,4; 3% of the examined animals), a high load of Y. pseudotuberculosis was cultivated from the liver, and a low bacterial load was isolated from the lungs and kidneys. In three of the remaining examined animals, a mild to moderate interstitial pneumonia (ID10, 12 and 13; 23% of the examined animals) was present. Three young bats were diagnosed with foetal atelectasis with intra-alveolar deposition of keratin scales (ID4, 6 and 7; 23% of the examined animals). One animal displayed multifocal acute haemorrhages in the musculature, representing trauma-associated lesions (ID6; 7.7% of the examined animals). The five remaining animals were inconspicuous both in the gross and histopathological analysis (ID 3, 5, 8, 9 and 11; 38.4% of the examined animals) (Table 1). Between January 2014 and April 2016, the average number of dead bats in this colony was 3.25 per month (min:0, max: 9), and the highest mortality rate registered was observed in July 2014 (n = 9, coefficient: 5.730, t-value = 3.627) (Table 2). The number of bats found dead at the time that Y. pseudotuberculosis was diagnosed within this colony did not differ significantly from the months measured before.
Table 1.
Characterization of C. perspicillata submitted to pathological and bacteriological examination between February and April 2016
| ID | Sex | Age group | Gross and histological findings | Bacteriological analysis |
|---|---|---|---|---|
| 1 | f | adult | Necrosuppurative metritis, pneumonia, hepatitis and splenitis |
Y. pseudotuberculosis positive (liver +++, lung +, kidney +) |
| 2 | m | adult | Necrosupppurative pneumonia, hepatitis, enterocolitis, splenitis, lymphadenitis, glossitis and stomatitis |
Y. pseudotuberculosis positive (liver +++, lung +, kidney +) |
| 3 | f | adult | Unremarkable | negative |
| 4 | f | young |
Fetal atelectasis with intraalveolar keratin scales |
negative |
| 5 | unknown | newborn | Unremarkable | negative |
| 6 | f | young |
1) Multifocal acute hemorrhages in the musculature 2) Fetal atelectasis with intraalveolar keratin scales |
negative |
| 7 | f | young |
Fetal atelectasis with intraalveolar keratin scales |
negative |
| 8 | f | young | Unremarkable | negative |
| 9 | f | young | Unremarkable | negative |
| 10 | f | young | Mild interstitial pneumonia | negative |
| 11 | f | young | Unremarkable so far as assessable due to severe autolysis | negative |
| 12 | f | young | Moderate interstitial pneumonia | negative |
| 13 | f | unknown | Moderate interstitial pneumonia | negative |
Fig. 1.
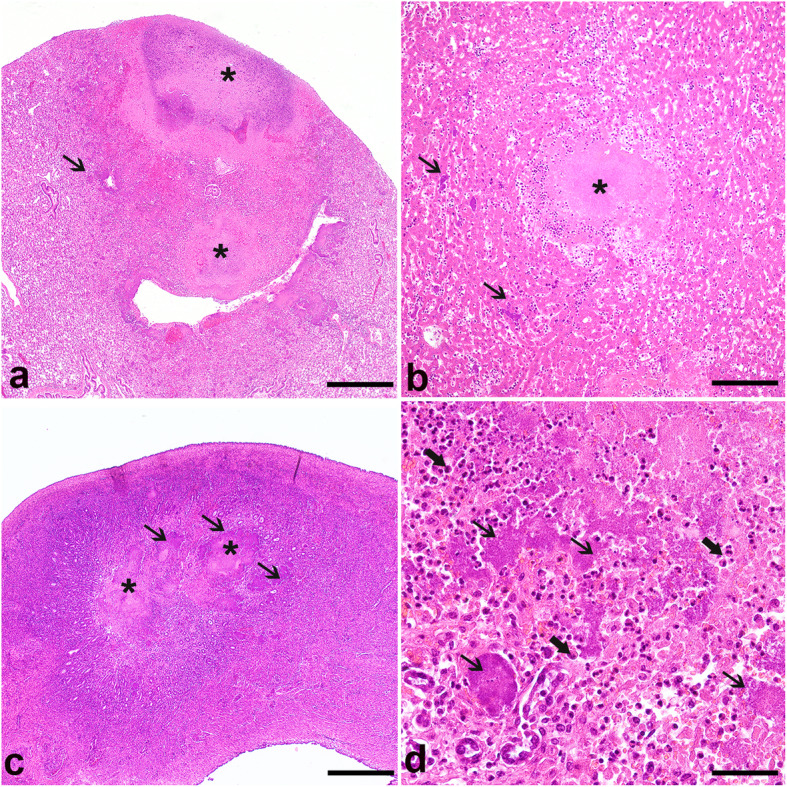
Necrosuppurative lesions identified histologically in the lung (a), liver (b), and uterus (c and d) from ID1. These were characterized by central necrotic areas (asterisks) surrounded by abundant intralesional colonies of bacterial rods (thin arrows) and variable numbers of degenerated neutrophils (thick arrows). HE, Bar 500 μm (a and c), 200 μm (b) and 50 μm (d), respectively
Table 2.
Mortality rate between January 2014 and April 2016 in C. perspicillata colony
| Month | Newborn | Young | Adult | Unknown | Total | Coefficient | t-value |
|---|---|---|---|---|---|---|---|
| Jan 14 | 2 | 1 | 3 | −0.192 | − 0.122 | ||
| Feb 14 | 1 | 1 | 2 | − 1.446 | − 0.916 | ||
| Mar 14 | 4 | 4 | 8 | 4.907 | 3.106 | ||
| Apr 14 | 1 | 1 | −2.358 | −1.493 | |||
| May 14 | 1 | 1 | −2.137 | −1.353 | |||
| Jun 14 | 1 | 1 | 1 | 3 | −0.402 | −0.255 | |
| Jul 14 | 2 | 2 | 2 | 3 | 9 | 5.73 | 3.627 |
| Aug 14 | 2 | 2 | 4 | 0.465 | 0.295 | ||
| Sep 14 | 4 | 4 | 0.642 | 0.406 | |||
| Oct 14 | 1 | 1 | 2 | 1 | 5 | 1.73 | 1.095 |
| Nov 14 | 1 | 2 | 3 | −0.314 | −0.199 | ||
| Dec 14 | 1 | 2 | 3 | −0.137 | −0.087 | ||
| Jan 15 | 1 | 1 | −2.137 | −1.353 | |||
| Feb 15 | 1 | 1 | −2.182 | −1.381 | |||
| Mar 15 | 2 | 2 | 4 | 0.907 | 0.574 | ||
| Apr 15 | 1 | 1 | 2 | −1.137 | −0.72 | ||
| May 15 | 0 | −3.182 | −2.014 | ||||
| Jun 15 | 1 | 2 | 3 | −0.049 | − 0.031 | ||
| Jul 15 | 2 | 2 | −1.137 | −0.72 | |||
| Aug 15 | 1 | 1 | −2.137 | −1.353 | |||
| Sep 15 | 1 | 1 | 2 | −1.137 | −0.72 | ||
| Oct 15 | 3 | 3 | −0.358 | −0.227 | |||
| Nov 15 | 2 | 1 | 4 | 7 | 3.818 | 2.417 | |
| Dec 15 | 2 | 2 | −1.491 | −0.943 | |||
| Jan 16 | 1 | 3 | 1 | 5 | 1.686 | 1.067 | |
| Feb 16 | 1 | 5 | 6 | 2.642 | 1.672 | ||
| Mar 16 | 3 | 1 | 4 | 0.686 | 0.434 | ||
| Apr 16 | 1 | 1 | 2 | −1.281 | −0.81 |
The coefficients and t-values of the outliers’ detection analysis was performed only on the total number of dead bats. The critical value was set at 3.5 and significant t-value (July 2014) is in bold. This finding was attributed to an ongoing birth peak in combination with increased stress due to an unusually high number of visitors
Low prevalence of Y. pseudotuberculosis infection in the C. perspicillata colony
Following Y. pseudotuberculosis detection within the colony, approximately ¼ of the C. perspicillata population was culled in June 2016. Out of the 99 clinically healthy animals that were captured and culled, Y. pseudotuberculosis was detected in a pooled organ sample comprising liver, lung and spleen from one clinically healthy female gravid bat in the absence of gross and histopathological lesions. Also, no signs of inflammation were detected macroscopically and histologically in any of the remaining 98 bats.
Y. pseudotuberculosis isolates from C. perspicillata are characterized as serotype O:1 and biotype 1
All three Y. pseudotuberculosis obtained isolates (16–1261/1, 16–1261/2 and 16–457/29) were characterized as serotype O:1 and biotype 1 following slide agglutination. They all belonged to the sequence type ST90 and harboured the chromosomal ail gene. In addition, the isolate 16–457/29 from the clinically healthy gravid female culled during depopulation was positive for the plasmid genes virF and yadA (Table 3).
Table 3.
Characterization of Y. pseudotuberculosis isolates obtained in the bacteriological analysis from the three infected C. perspicillata
| Isolate | 16–1261/1 | 16–1261/2 | 16–457/29 |
|---|---|---|---|
| (ID1) | (ID2) | ||
| Serotype | O:1 | O:1 | O:1 |
| Biotype | 1 | 1 | 1 |
| raffinose | – | – | – |
| melibiose | + | + | + |
| citrate | – | – | – |
| virF | – | – | + |
| yadA | – | – | + |
| ail | + | + | + |
| Sequence type | ST90 | ST90 | ST90 |
No indication of Y. pseudotuberculosis infection in cohabitant nocturnal animal species and caretakers
None of the nocturnal animals that inhabited the same dome as the C. perspicillata were sick, died or were euthanized between February and April 2016.
Moreover, Yersinia species were not diagnosed in any of the zoo animals submitted for necropsy and in the pooled faecal samples submitted for routine bacteriological analysis since April 2016 up to today. Also, no cases of Yersinia infection were detected among the animal caretakers.
Discussion
Close observation of an indoor-housed captive colony of C. perspicillata housed in a dome from a private zoo in Switzerland led to the detection of Y. pseudotuberculosis infection between February and April 2016. The infection occurred during a birth peak and was associated with a slight increase in the mortality rate with no significant statistical significance. The colony comprises higher percentages of immunocompromised animals during birth peaks, namely females in late pregnancy and juveniles. Moreover, higher animal densities lead to increased stress levels, which are known to increase the likelihood of intra- and interspecies transmission of viral infections [2]. It is likely that the same applies for bacterial infections. Overall, Y. pseudotuberculosis was isolated from three bats of the 112 examined animals (2.67%), two of which displayed severe histopathological lesions (ID1 and 2). Interestingly, ID1 was a female that had aborted approximately 1 week prior to death. This finding may indicate that Y. pseudotuberculosis may cause abortion in this bats species as it does in ewes and goats [12]. In addition, three of the examined bats (ID10, 12 and 13) displayed a mild to moderate interstitial pneumonia, but no Y. pseudotuberculosis, nor any other bacteria were isolated postmortem from these animals.
Ingestion of food contaminated with the feces of Yersinia reservoir animals such as wild rats and birds (pigeons, crows) represents a likely source of infection in zoological collections [13, 14]. However, similarly to other studies reporting outbreaks of yersiniosis in zoological collections, the source of Y. pseudotuberculosis infection in this case was not identified. Although this colony was kept in an indoor-housed dome, rodents and also birds, namely house sparrows (Passer domesticus), occasionally enter the facility. In addition, due to a rigorous health screening protocol and quarantine, it is unlikely that the cohabitant nocturnal species may have introduced the pathogens.
In the outbreaks previously described in captive colonies of R. aegyptiacus, 20–70% of the animals exhibited gross evidence of Y. pseudotuberculosis infection [1, 8]. In our case, the infection rate in C. perspicillata was rather low and there was no statistically significant increase in the mortality rate. This may suggest a lower disease susceptibility of C. perspicillata compared to R. aegyptiacus to Y. pseudotuberculosis infection. Alternatively, a variant outcome might be related to the expression of different virulence factors. One of the R. aegyptiacus outbreaks described in the literature was caused by an infection with Y. pseudotuberculosis serotype 4b expressing virF and inv genes, which are associated with increased virulence and invasiveness, respectively [8]. In addition, this strain harbored the superantigenic toxin YPMa, which is known to lead to acute systemic infection in humans [8]. The strain causing the other described outbreak in R. aegyptiacus was not further characterized [1]. In our case, we found differences between the isolates of the two sick animals (16–1261/1, 16–1261/2) and of the clinically healthy female gravid bat (16–457/29) concerning the virulence genes. In all three positive bats we isolated Y. pseudotuberculosis serotype O:1 and biotype 1 belonging to the sequence type ST90 and expressing the ail gene, but detection of the virF and YadA genes was restricted to isolate 16–457/29 from the clinically healthy bat. This finding indicates that subclinical Yersinia infections may occur in C. perspicillata comparably to what is described in rodents and birds [2].
Culling ¼ of the population seems to have been sufficient to control the spread of Y. pseudotuberculosis within this colony without subsequent culling or antibiotic treatment, possibly due to a reduction of stress levels. Rigorous rodent and bird control measures within the zoo area are the most appropriate prophylactic approach to prevent further cases of Yersinia infection. It is known that Y. pseudotuberculosis can survive and replicate in the soil and in aquatic environments outside of hosts for months to years [15], namely in association with entomopathogenic nematodes [16] or Acanthamoeba castellanii trophozoites [17]. However, it has been suggested that the reinfection risk is likely to strongly decrease following 9 months of persistence in the soil since Y. pseudotuberculosis loses its virulence genes following adaptation to a saprophytic lifestyle [18]. In fact, no further cases of Yersinia infection were identified in the C. perspicillata colony, in the cohabitant zoological species and in the animal caretakers since this event described here took place.
Conclusion
This report describes the occurrence of Y. pseudotuberculosis infections in an indoor-housed colony of C. perspicillata, which could be stopped by culling of ¼ of the colony. The above-described findings show that C. perspicillata are susceptible to infection with Y. pseudotuberculosis leading to lethal yersiniosis, but they don’t seem to represent a significant Y. pseudotuberculosis reservoir. Moreover, the infection risk for cohabitant nocturnal animals and the zoonotic potential seems to be low since no Yersinia infection has been diagnosed since April 2016 in this zoo.
Material and methods
This study did not require approval by an institutional and/or licensing committee since it was not an experimental study, but part of a clinical and pathological veterinary diagnostic case that occurred in a zoo setting. All described methods were carried out in accordance with relevant guidelines and regulations, including the ARRIVE guidelines for in vivo research [19] and the guidelines for the euthanasia of animals from the American Veterinary Medical Association [20].
Animals
At the time of the outbreak, the affected C. perspicillata colony comprised approximately 400 individuals of varying ages (Fig. 2). These animals were kept in a 40 m-diameter dome with an artificial cave for roosting and fed 3 times a day ad libitum. The bats also have access to the food plates of the cohabitant nocturnal animals. Food availability, temperature, and diurnal rhythm remained constant throughout the year. Due to the stable environmental conditions, birth events were spread over the entire year, with relative female reproductive synchronization. Inter-zoo exchanges and occasional population culling controlled population growth. Several other nocturnal animal species were present in the same dome, namely Brazilian porcupines (Coendou prehensilis), Lowland pacas (Cuniculus paca), night monkeys (Aotus lemurinus griseimembra), ocelots (Leopardus pardalis), and crab-eating raccoons (Procyon cancrivorus). The number of visitors walking through the cave varied significantly depending on seasonality and weather conditions. The health status of all animals present in the dome was continuously monitored, and all dead animals were routinely submitted for necropsy, histopathological and microbiological examination. All the bats that were culled for colony depopulation purposes following Y. pseudotuberculosis diagnosis within the colony were individually trapped, anesthetized with isoflurane (Nicholas Piramal I, Mumbai, India) using a rodent nosecone non-rebreathing system (Rothacher Medical, Heitenried, Switzerland), and humanly killed by cervical dislocation, in accordance with previously described protocols [21, 22].
Fig. 2.
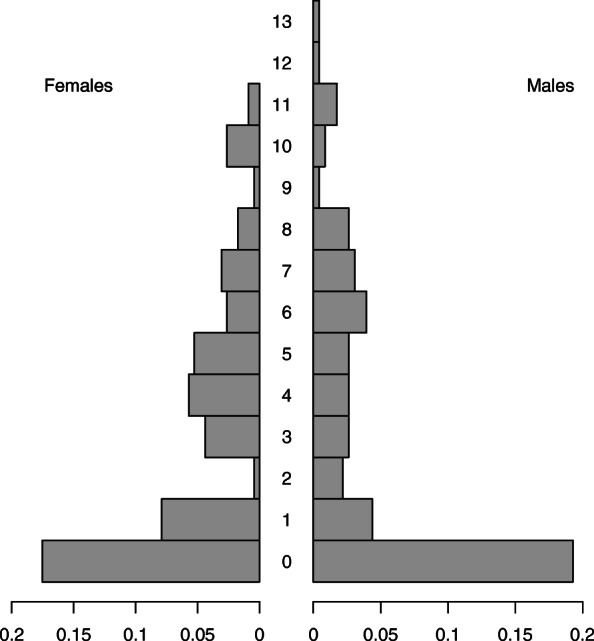
Age structure of the colony of captive C. perspicillata based on data from 2015. The age in years and the proportion of individuals within the population are represented on the y and x axes, respectively
Pathological examination
All necropsy and histopathological examinations carried out within the scope of this study were performed at the Institute for Animal Pathology, University of Bern, Switzerland. Each examined animal was weighed, and the reproductive status was recorded. A full necropsy was performed of each animal, and liver, lung, spleen and intestine were sampled for bacteriological analysis. Necropsy instruments were cleaned with 70% ethanol before and after each organ sampling to prevent cross contamination. All internal organs (liver, spleen pancreas, adrenals, reproductive tract, urinary bladder, trachea, lung, heart, thymus, salivary glands, lymph nodes, brain, skeletal muscle, skin, bone marrow, and gastro-intestinal tract) were sampled and immediately fixed in 4% neutral buffered formalin, embedded in paraffin, cut at 4 μm, and stained with haematoxylin and eosin (HE) according to routine laboratory procedures. Histopathological interpretation was performed by certified veterinary pathologists for all embedded organs.
Statistic evaluation of the mortality rate
An autoregressive model based on the monthly mortality recorded between January 2014 and April 2016 by the private zoo was used to analyse fluctuations in mortality rate. The “arima” function (order: 1,0,0) from R (version 3.2.3) was used to calculate the residuals and the function “locate.outliers” (package “tsouliers”). The critical value to consider t-values significant was set at 3.5 [23].
Microbiological examination
Bacterial cultures from the first 13 bats that died between February and April 2016 were performed immediately after necropsy at the Institute of Veterinary Bacteriology, University of Bern, Switzerland. A loopful of material for each organ was streaked on BD Trypticase Soy Agar with 5% Sheep Blood (TSA SB, Becton Dickinson and on Brolac Agar (Thermo Fisher Diagnostics AG, Pratteln Switzerland) or MacConkey agar (Thermo Fisher Diagnostics AG, Pratteln Switzerland) for the lungs. Plates were incubated aerobically at 37 °C up to 48 h. TSA SB plates ware incubated in a 5% CO2 atmosphere. Species identification was done by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI TOF MS, Bruker Daltonics GmbH, Bremen, Germany). Bacterial cultures from the 99 culled bats were performed immediately after necropsy at the Institute for Food Safety and Hygiene, Section of Veterinary Bacteriology, University of Zurich, Switzerland. The collected samples from liver, lung, and spleen were pooled and mashed via pestle homogenisation. A loopful of this mixture and the intestine were directly streaked onto a selective Yersinia agar (CIN agar; Thermo Fisher Diagnostics AG, Pratteln Switzerland) and incubated for approximately 48 h at 30 ± 1 °C. Additionally, a loopful of the mixture was inoculated in an enrichment-broth (EE-broth, Mossel; Thermo Fisher Diagnostics AG, Pratteln Switzerland), incubated overnight at 30 ± 1 °C, streaked onto CIN agar and again incubated overnight at 30 ± 1 °C. Suspicious colonies from the CIN agar were picked and tested for urease activity (Urea broth, Christensen and Maslen; Thermo Fisher Diagnostics AG, Pratteln Switzerland), which was incubated overnight at 30 ± 1 °C. If the urea broth was positive, subcultures were done onto Columbia blood agar containing sheep blood (Thermo Fisher Diagnostics AG, Pratteln Switzerland) at 30 ± 1 °C. Species identification was done by biochemical test using the automated VITEK® 2 Compact system (bioMérieux, Marcy l’Etoile, France).
In order to determine the serotype, biotype, sequence type and the expression of virulence genes virF, yadA and ail, the three isolates were further characterized at the Department of Food Hygiene and Environmental Health, Faculty of Veterinary Medicine, University of Helsinki, Finland. Serotyping of the isolates was performed by slide agglutination with commercial antisera O:1 - O:6 (Denka Seikan, Tokyo, Japan). The whole-genome sequencing was performed according to Joutsen et al. [24]. The sequence type was assigned using the multilocus sequence typing database described by Hall et al. [25]. The virF and yadA genes on the virulence plasmid and the chromosomal ail gene were detected by PCR according to Joutsen et al. [26].
Acknowledgements
We would like to thank Manuela Bozzo, Bettina de Breuyn Dietler, and Erika Bürgi from the Institute of Animal Pathology, Vetsuisse Faculty, University of Bern, for their excellent technical assistance in histology; Irene Zühlke, Eve Tièche and Amandine Graber for their assistance in the capture and necropsy performance during the depopulation action during their student rotation at the Institute of Animal Pathology, Vetsuisse Faculty, University of Bern, and the team of the Papiliorama for the logistic support provided.
Abbreviations
- PCR
Polymerase chain reaction
- HE
Haematoxylin and eosin
Authors’ contributions
KH, IBV, MS, DW, CG, and NSR performed the necropsies and/or the histological analysis of the bats. MM and NJF performed the epidemiological analysis of the obtained data. SK, MS and SS executed the bacteriological analysis of the tissues obtained upon necropsy. PRvdB coordinated all operations performed within the private zoo. MFA performed the serotype and biotype determination, the sequence typing and the expression of virulence genes of the isolated Y. pseudotuberculosis strains. KH, IBV, MS, and NJF wrote the manuscript, which was reviewed and edited by all authors. All authors approved the final version of the manuscript.
Funding
The funding body had no role in any aspect of this study and no further external funding was used.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
This study did not require ethics approval by an institutional and/or licensing committee since it was not an experimental study, but part of a clinical and pathological veterinary diagnostic case that occurred in a zoo setting.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
K. Hahn and I. B. Veiga contributed equally to this work.
Contributor Information
K. Hahn, kershahn@googlemail.com
I. B. Veiga, Email: ines.veiga@vetsuisse.unibe.ch
M. Schediwy, Email: m.schediwy@kleintierpraxis-guemligen.ch
D. Wiederkehr, Email: danja.wiederkehr@bfh.ch
M. Meniri, Email: magali.meniri@yahoo.fr
M. Schneeberger, Email: marianne.schneeberger@vetbakt.uzh.ch
P. Rüegg-van den Broek, Email: peggy.vd.broek@papiliorama.ch.
C. Gurtner, Email: corinne.gurtner@vetsuisse.unibe.ch
N. J. Fasel, Email: fasel.nicolas@gmail.com
S. Kittl, Email: sonja.kittl@vetsuisse.unibe.ch
M. Fredriksson-Ahomaa, Email: maria.fredriksson-ahomaa@helsinki.fi
S. Schmitt, Email: sarah.schmitt@vetbakt.uzh.ch
N. Stokar-Regenscheit, Email: nadine.stokar@gmail.com
References
- 1.Childs-Sanford SE, Kollias GV, Abou-Madi N, McDonough PL, Garner MM, Mohammed HO. Yersinia pseudotuberculosis in a closed colony of Egyptian fruit bats (Rousettus aegyptiacus) J Zoo Wildl Med. 2009;40(1):8–14. doi: 10.1638/2007-0033.1. [DOI] [PubMed] [Google Scholar]
- 2.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006;19(3):531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bielli M, Lauzi S, Pratelli A, Martini M, Dall'Ara P, Bonizzi L. Pseudotuberculosis in marmosets, tamarins, and Goeldi's monkeys (Callithrichidae/Callimiconidae) housed at a European zoo. J Zoo Wildl Med. 1999;30(4):532–536. [PubMed] [Google Scholar]
- 4.Obwolo MJ. Yersiniosis in the Bristol zoo (1955-1974). Acta Zool Pathol Antverp. 1976;(64):81–90. [PubMed]
- 5.Luis AD, Hayman DT, O'Shea TJ, Cryan PM, Gilbert AT, Pulliam JR, et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc R Soc B Biol Sci. 2013;280(1756):20122753. doi: 10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muhldorfer K, Wibbelt G, Haensel J, Riehm J, Speck S. Yersinia species isolated from bats, Germany. Emerg Infect Dis. 2010;16(3):578–580. doi: 10.3201/eid1603.091035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muhldorfer K, Speck S, Kurth A, Lesnik R, Freuling C, Muller T, et al. Diseases and causes of death in European bats: dynamics in disease susceptibility and infection rates. PLoS One. 2011;6(12):e29773. [DOI] [PMC free article] [PubMed]
- 8.Nakamura S, Settai S, Hayashidani H, Urabe T, Namai S, Une Y. Outbreak of yersiniosis in Egyptian rousette bats (Rousettus aegyptiacus) caused by Yersinia pseudotuberculosis serotype 4b. J Comp Pathol. 2013;148(4):410–413. doi: 10.1016/j.jcpa.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Cloutier D, Thomas DW. Carollia perspicillata. Mamm Species. 1992;417:1–9. [Google Scholar]
- 10.Jones KE, Bielby J, Cardillo M, Fritz SA, O'Dell J, Orme CDL, et al. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals: ecological archives E090-184. Ecology. 2009;90(9):2648. doi: 10.1890/08-1494.1. [DOI] [Google Scholar]
- 11.Fleming TH. The short-tailed fruit bat : a study in plant-animal interactions. Chicago II: University of Chicago Press; 1988. p. xvi, 365. [Google Scholar]
- 12.Giannitti F, Barr BC, Brito BP, Uzal FA, Villanueva M, Anderson M. Yersinia pseudotuberculosis infections in goats and other animals diagnosed at the California animal health and food safety laboratory system: 1990-2012. J Vet Diagn Invest. 2014;26(1):88–95. doi: 10.1177/1040638713516624. [DOI] [PubMed] [Google Scholar]
- 13.Baskin GB, Montali RJ, Bush M, Quan TJ, Smith E. Yersiniosis in captive exotic mammals. J Am Vet Med Assoc. 1977;171(9):908–912. [PubMed] [Google Scholar]
- 14.Otsuka Y, Okada Y, Makino S, Maruyama T. Isolation of Yersinia pseudotuberculosis from city-living crows captured in a zoo. J Vet Med Sci. 1994;56(4):785–786. doi: 10.1292/jvms.56.785. [DOI] [PubMed] [Google Scholar]
- 15.Gasper PW, Watson RP. Plague and yersiniosis. In: Williams ES, Barker IK, editors. Infectious diseases of wild mammals. 3. Ames: Iowa State University Press; 2001. pp. 313–329. [Google Scholar]
- 16.Gengler S, Laudisoit A, Batoko H, Wattiau P. Long-term persistence of Yersinia pseudotuberculosis in entomopathogenic nematodes. PLoS One. 2015;10(1):e0116818. doi: 10.1371/journal.pone.0116818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos-Montanez J, Benavides-Montano JA, Hinz AK, Vadyvaloo V. Yersinia pseudotuberculosis IP32953 survives and replicates in trophozoites and persists in cysts of Acanthamoeba castellanii. FEMS Microbiol Lett. 2015;362(13):fnv091. doi: 10.1093/femsle/fnv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buzoleva L, Somov GJ. Adaptation variability of Yersinia pseudotuberculosis during long-term persistence in soil. Bull Exp Biol Med. 2003;135(5):456–459. doi: 10.1023/A:1024915409187. [DOI] [PubMed] [Google Scholar]
- 19.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Bio. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Veterinary Medical Association (AVMA). AVMA guidelines for the euthanasia of animals: 2020 edition. Schaumburg: American Veterinary Medical Association. Available from https://www.avma.org/sites/default/files/2020-01/2020-Euthanasia-Final-1-17-20.pdf
- 21.Sikes RS. And the ACUCASM. 2016 guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal. 2016;97(3):663–688. doi: 10.1093/jmammal/gyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunz TH, Parsons S. Ecological and Behavioural methods for the study of bats. 2. Baltimore: John Hopkins University Press; 2009. [Google Scholar]
- 23.López-de-Lacalle J, López MJ. Package tsoutliers: detection of outliers in time series. R package version 0.6–8. 2019. [Google Scholar]
- 24.Joutsen S, Johansson P, Laukkanen-Ninios R, Björkroth J, Fredriksson-Ahomaa M. Two copies of the ail gene found in Yersinia enterocolitica and Yersinia kristensenii. Vet Microbiol. 2020;247:1–6. doi: 10.1016/j.vetmic.2020.108798. [DOI] [PubMed] [Google Scholar]
- 25.Hall M, Chattaway MA, Reuter S, Savin C, Strauch E, Carniel E, et al. Use of whole-genus genome sequence data to develop a multilocus sequence typing tool that accurately identifies Yersinia isolates to the species and subspecies levels. J Clin Microbiol. 2015;53(1):35–42. doi: 10.1128/JCM.02395-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joutsen S, Eklund KM, Laukkanen-Ninios R, Stephan R, Fredriksson-Ahomaa M. Sheep carrying pathogenic Yersinia enterocolitica bioserotypes 2/O:9 and 5/O:3 in the feces at slaughter. Vet Microbiol. 2016;197:78–82. doi: 10.1016/j.vetmic.2016.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


