Abstract
Patient: Male, 19-year-old
Final Diagnosis: COVID-19
Symptoms: Cough • diarrhea • fever • shortness of breath
Medication: —
Clinical Procedure: —
Specialty: Immunology • Infectious Diseases • Pulmonology
Objective:
Rare co-existance of disease or pathology
Background:
Since the emergence of coronavirus disease 2019 (COVID-19), patients with the illness have presented with considerable variation in severity. Some infected individuals present mild or no symptoms, while others present severe illness with some fatal outcomes. Multiple lines of management have been suggested for critically ill patients, such as intravenous immunoglobulin (IVIG) and steroids. IVIG is the main treatment for patients with X-linked agammaglobulinemia. Multiple studies have reported that these patients have excellent outcomes when they contract COVID-19. This report describes the clinical course of COVID-19 pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a 19-year-old man on IVIG replacement therapy for X-linked agammaglobulinemia (XLA).
Case Report:
A patient with XLA receiving a monthly dose of IVIG and having bronchiectasis managed by prophylactic azithromycin presented with fever, shortness of breath, productive cough, and diarrhea. He was admitted to our hospital with SARS-CoV-2 infection. His treatment course for COVID-19 was uncomplicated and had excellent results. He completed a 10-day course of piperacillin/tazobactam and his symptoms resolved 3 days after admission, without complications, oxygen supplementation, or intensive care unit admission.
Conclusions:
Patients with XLA have weakened immunity and therefore may present with an infection as a first symptom. This report describes the mild course of COVID-19 pneumonia in an immunologically vulnerable patient with XLA who presented with SARS-CoV-2 infection while undergoing IVIG replacement therapy. Currently, IVIG is one of many supportive immune therapies undergoing clinical evaluation in patients with severe COVID-19.
Keywords: Agammaglobulinemia; COVID-19; Genetic Diseases, X-Linked; SARS Virus
Background
In December 2019, cases of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection first emerged in the city of Wuhan, China. Shortly afterward, the number of cases dramatically increased, and the disease rapidly spread worldwide [1]. The virus has a median incubation period of 5 days, ranging from 2 to 14 days [2]. Some infected individuals present mild or no symptoms, while others present severe illness with some fatal outcomes. The characteristic features in most patients include prodromal or flu-like symptoms, such as fever, cough, headache, fatigue, and breathlessness. In some patients, the disease can progress to more severe illness, including acute respiratory distress syndrome and multi-organ dysfunction [3].
The fatality of the disease is commonly related to the presence of comorbidities. Patients with chronic illnesses have a significantly higher fatality rate than do patients who are otherwise healthy [4]. Age also plays a crucial role in the severity of the disease, as older patients tend to have a higher risk of severe illness and intensive care unit admission [5].
It has been suggested that SARS-CoV-2 predominantly acts on lymphocytes, especially T cells, as demonstrated by the reduced lymphocyte values in most patients with COVID-19 [6]. Treatment with intravenous immunoglobulin (IVIG) and a short duration of steroids is recommended for severely ill patients with acute respiratory distress syndrome [3].
Therefore, this report describes the clinical course of COVID-19 pneumonia due to an infection with SARS-CoV-2 in a 19-year-old man on IVIG replacement therapy for X-linked agammaglobulinemia (XLA).
Case Report
We present a case of a 19-year-old man who is known to have XLA, having been diagnosed at the age of 4 years with XLA because of recurrent bacterial infections (Table 1 shows the diagnostic laboratory data), and is treated with monthly IVIG therapy, currently 70 g. He received his last dose 3 weeks before his presentation at our hospital. He also had asthma and bronchiectasis and has been treated with prophylactic azithromycin (500 mg every other day) since 2015.
Table 1.
Laboratory data concerning the diagnosis of X-linked agammaglobulinemia.
| Laboratory test | Result | Reference range |
|---|---|---|
| White blood count | 11.8 | 4.0–11.0×109/L |
| Hemoglobin | 13.7 | 11.5–16.5 g/dL |
| Platelet | 446 | 150–450×109/L |
| Neutrophils count | 5.30 | 2–7.5×109/L |
| Lymphocytes count | 4.11 | 1.5–4×109/L |
| CD3+ (T cells) | 98% | 67–76% |
| CD3+ CD4+ (T helpers) | 48% | 38–40% |
| CD3+ CD8+ (T suppressors) | 45% | 31–40% |
| CD19+ (B cells) | 0% | 11–16% |
| CD16+ CD56+ (natural killer cells) | 2% | 10–19% |
| CD3+ (T cells) | 4318.00 cells/mcL | 1100.00–1700.00 |
| CD3+ CD4+ (T helpers) | 2117.00 cells/mcL | 700.00–1100.00 cells/mcL |
| CD3+ CD8+ (T suppressors) | 2007.00 cells/mcL | 500.00–900.00 cells/mcL |
| CD19+ (B cells) | .0 cells/mcL | 200.0–400.0 cells/mcL |
| CD16+ CD56+ (natural killer cells) | 93.0 cells/mcL | 200.0–400.0 cells/mcL |
| Lymphocytes | 41.00% | 28.00–39.00% |
| CD4/CD8 ratio | 1.06 | 1.00–1.50 |
| Immunoglobulin G* | 7.44 g/L | 6.6–15.3 g/L |
| Immunoglobulin E | <25.0 IU/mL | 25–449.7 IU/mL |
| Immunoglobulin A | <0.05 g/L | 0.5–2.9 g/L |
| Immunoglobulin M | <0.05 g/L | 0.4–1.5 g/L |
CD – cluster of differentiation.
Patient is on regular intravenous immunoglobulin transfusion.
The patient presented with a fever which started 8 days before hospital presentation, which did not respond to antipyretics. It was accompanied by shortness of breath, a productive cough, and watery diarrhea 4 times a day. On physical examination, the patient was stable, with an oxygen saturation of 96% in ambient air. His breath sounds were decreased bilaterally in the lower lung field, with coarse crepitation, which was best heard in the left-lower zone. Initial laboratory blood test results revealed normal complete blood counts and renal and liver profiles. Other investigations showed a C-reactive protein level of 47.6 mg/L (range, 0–5 mg/L), D-dimer of 0.78 mg/L (range, 0–0.5 mg/L), and an erythrocyte sedimentation rate (ESR) of 43 mm/h (range, 0–20 mm/h). His ferritin, creatinine kinase, and procalcitonin levels were normal (Table 2). A chest X-ray showed bilateral bronchiectatic changes, with airspace opacity in the right-lower zone (Figure 1).
Table 2.
Patient’s initial blood work at the time of COVID-19 diagnosis.
| Laboratory test | Result | Reference range |
|---|---|---|
| White blood count | 9.9×109/L | 4.0–11.0×109/L |
| Hemoglobin | 14.5 g/dL | 11.5–16.5 g/dL |
| Platelet | 325×109/L | 150–450×109/L |
| Neutrophil count | 6.8×109/L | 2–7.5×109/L |
| Lymphocyte count | 2.15×109/L | 1.5–4×109/L |
| C-reactive protein | 47.6 mg/L | 0–5 mg/L |
| Erythrocyte sedimentation rate | 43 mm/h | 0–20 mm/h |
| Ferritin | 58 ug/L | 11–313 ug/L |
| Procalcitonin | 0.05 ug/L | ≥0.25 ug/L |
| Lactate dehydrogenase | 305 U/L | 100–217 U/L |
| D-dimer | 0.78 mg/L | 0–0.5 mg/L |
Figure 1.
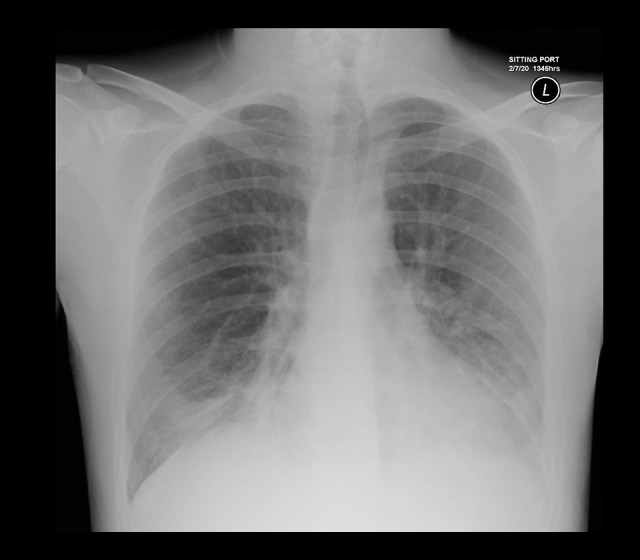
Patient’s initial chest X-ray showing airspace opacity in the right-lower zone.
The patient was admitted as a case of pneumonia, with suspicion of COVID-19. A nasal swab sample for COVID-19 testing was obtained, and he was started on piperacillin/tazobactam, alongside supportive measures. The next day, SARS-CoV-2 infection was confirmed using an approved diagnostic reverse transcription-polymerase chain reaction (RT-PCR) test, the RealStar® SARS-CoV-2 RT-PCR kit 1.0 (Altona Diagnostics, Hamburg, Germany). The patient’s condition improved 3 days after admission. After receiving his regular IVIG dose, he was transferred to an isolation facility designated for stable COVID-19 patients. The patient completed a 10-day course of piperacillin/tazobactam without complications, oxygen supplementation, or intensive care unit involvement. During hospitalization, the patient’s follow-up inflammatory markers had normalized, except for the ESR, which was 34 mm/h.
Discussion
Herein, we are discussing an unsophisticated course of COVID-19 pneumonia in a patient with XLA and its interlaced lines and supportive management. This case suggests that IVIG could be used for treating critically ill patients with COVID-19, particularly at early administration [7].
Bruton tyrosine kinase (BTK) is the main gene affected in XLA and is located on the X-chromosome [8]. BTK pathogenic mutations have a major effect in B-cell maturation, leading to a universal B-cell deficiency. The deficiency in antibody production is explained by the failure of B lymphocytes to mature and differentiate into plasma cells and the resulting abated immunoglobulin production [9]. Due to their weakened immunity, the first presentation of most patients with XLA is usually related to an infection, and the diagnosis is usually based on clinical manifestation, serum immunoglobulins, flow cytometry, and molecular studies [10,11]. Our patient was diagnosed based on recurrent bacterial infections, significantly low levels of immunoglobulin (Ig) G, IgA, and IgM, in addition to the absence of mature B lymphocytes in the peripheral circulation [12].
The replacement therapy for hypo-gammaglobulinemia in XLA is vital and life-saving [13]. However, in our patient, the COVID-19 symptoms were relatively mild, and minimal therapy was required. This suggests that a deficiency in B-cell immunity does not alter the response to COVID-19; instead, the host defense against SARS-CoV-2 may rely on T-cell immunity, which tends to decrease with aging, resulting in worse outcomes in elderly patients with COVID-19 [14]. Patients experiencing mild COVID-19 symptoms have markedly low T-cell counts and cluster of differentiation (CD) 8 levels, suggesting the ability of SARS-CoV-2 to directly infect lymphocytes [6]. In healthy people, the levels of cytotoxic T-cell activation markers, such as human leukocyte antigen-DR isotype (HLA-DR) and CD38, increase after SARS-CoV-2 infection [6].
The ability of patients with XLA to recover from COVID-19 suggests that B-cell immunity may have a role in protecting against SARS-CoV-2 infection; therefore, the outcomes of such patients could improve with antibody transfusion through convalescent plasma therapy [15,16]. Nevertheless, patients with XLA could have the advantage of avoiding the hyperinflammatory phase of COVID-19 that often results in death. However, not all patients progress to hyperinflammation, owing to the BTK deficiency in myeloid cells. BTK is expressed in myeloid cells and functions as a toll-like receptor aiding in the production of interleukin 6 and tumor necrosis factor alpha, 2 proinflammatory cytokines that are generated in enormous amounts in patients with COVID-19 [17]. A previous study reported 94 patients with primary immunodeficiency who were infected with SARS-CoV-2. Of the 94 patients, 6 had XLA, and all 6 of these patients had good outcomes [18]. Another study reported 7 patients with primary immunodeficiency, and 1 the patient who had XLA recovered after treatment without requiring admission to the intensive care unit [19].
Although the immune response to SARS-CoV-2 is not fully understood, early observations suggest the synthesis of antigen-specific cytotoxic T cells and neutralizing antibodies against the virus [6]. The clinical improvement in our patient, as in 2 other cases reported by Soresina et al, suggests that the human body can confront the virus in different ways, which require further observation and analysis.
Convalescent plasma has been administered to patients with COVID-19 as a treatment in early trials [16,20]. Immunoglobulin replacement therapy must be continued in patients with XLA, without altering the dose or timing of administration, unless otherwise specified, as it could lead to clinical improvement in these patients when they are infected with SARS-CoV-2 [21]. In a similar case published by Mira et al, a patient with XLA experienced a prompt improvement after the administration of convalescent plasma. However, as this finding does not confirm a cause-effect relationship, further investigations of hyperimmune plasma in such patients are needed [22].
In addition, our patient was taking azithromycin as an antiinflammatory agent for bronchiectasis exacerbation. It has been speculated that azithromycin decreases virus entry into cells, which could explain why our patient did not have severe pneumonia [23,24]. Combining these interventions (azithromycin, convalescent plasma, and immunoglobulin therapy) could have contributed to this patient not requiring oxygen therapy or intensive care and to the lack of deterioration in his clinical status, as is the case in other patients with COVID-19.
One limitation of our study is that the outcome is multifactorial, and therefore no definite conclusion can be drawn regarding which treatments were beneficial or harmful. Moreover, it is a single case of a patient with XLA with COVID-19 who received IVIG and had no complications.
Conclusions
Our case suggests that high-risk patients with various infections can have an uncomplicated course of COVID-19, especially patients with XLA. IVIG could constitute a feasible approach for treating critical patients with SARS-CoV 2 infection. Additional randomized controlled trials must be conducted to confirm these findings, especially since patients with XLA have different genotypes and phenotypes than those of the healthy population. Currently, IVIG is one of many supportive immune therapies undergoing clinical evaluation in patients with severe COVID-19.
Footnotes
Conflict of Interest
None.
References:
- 1.Lu R, Zhao X, Li J, et al. Genomic characterization and epidemiology of 2019 novel coronavirus. Implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng ZJ, Shan J. 2019 novel coronavirus: Where we are and what we know. Infection. 2020;48(2):155–63. doi: 10.1007/s15010-020-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395(10223):507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention: [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China.] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–51. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Center for Disease Control Coronavirus Disease 2019 (COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html.
- 6.Vetrie D, Vorechovsky I, Sideras P. The gene involved in X-linked agammaglobulinemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361(6409):226–33. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 7.Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: A multicenter retrospective cohort study. Clin Transl Immunol. 2020;9(10):e1192. doi: 10.1002/cti2.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsukada S, Saffran DC, Rawlings DJ. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72(2):279–90. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 9.Lederman HM, Winkelstein JA. X-linked agammaglobulinemia: An analysis of 96 patients. Medicine. 1985;64(3):145–56. [PubMed] [Google Scholar]
- 10.Du RH, Liang LR, Yang CQ. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study [published correction appears in Eur Respir J, 2020;56(3):2050524] Eur Respir J. 2020;55(5):2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Sayed ZA, Abramova I, Aldave JC, et al. X-linked agammaglobulinemia (XLA):Phenotype, diagnosis, and therapeutic challenges around the world. World Allergy Organ J. 2019;12(3):100018. doi: 10.1016/j.waojou.2019.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lackey AE, Ahmad F. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. X-linked agammaglobulinemia. [PubMed] [Google Scholar]
- 13.Perez EE, Orange JS, et al. Update on the use of immunoglobulin in human disease: A review of evidence. J Allergy Clin Immunol. 2017;139(3Suppl.):S1–46. doi: 10.1016/j.jaci.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17(5):533–35. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490–96. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon SY, Kim EJ, Jung YS, et al. Post-donation COVID-19 identification in blood donors. Vox Sang. 2020;115(8):601–2. doi: 10.1111/vox.12925. [DOI] [PubMed] [Google Scholar]
- 17.Marron TU, Martinez-Gallo M, Yu JE, Cunningham-Rundles C. Toll-like receptor 4-, 7-, and 8-activated myeloid cells from patients with X-linked agammaglobulinemia produce enhanced inflammatory cytokines. J Allergy Clin Immunol. 2012;129:184–190. doi: 10.1016/j.jaci.2011.10.009. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyts I, Bucciol G, Quinti I, et al. Coronavirus Disease 2019 in patients with inborn errors of immunity: An international study. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.09.010. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinti I, Lougaris V, Milito C, et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146(1):211–13. doi: 10.1016/j.jaci.2020.04.013. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soresina A, Moratto D, Chiarini M, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol. 2020;31(5):565–69. doi: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130(6):2757–65. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mira E, Yarce OA, Ortega C, et al. Rapid recovery of a SARS-CoV-2-infected X-linked agammaglobulinemia patient after infusion of COVID-19 convalescent plasma. J Allergy Clin Immunol Pract. 2020;8(8):2793–95. doi: 10.1016/j.jaip.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71(15):732–39. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran DH, Sugamata R, Hirose T, et al. Azithromycin, a 15-membered macro-lide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process. J Antibiot (Tokyo) 2019;72(10):759–68. doi: 10.1038/s41429-019-0204-x. [DOI] [PubMed] [Google Scholar]


