Abstract
Introduction: Recent studies suggest an overrepresentation of MGMT promoter methylated tumors in females with IDHwt glioblastoma (GBM) compared to males, with a subsequent better response to alkylating treatment. Methods: To reveal sex-bound associations that may have gone unnoticed in the original analysis, we re-analyzed two previously published clinical cohorts. One was the multicenter Nordic trial of elderly patients with GBM, randomizing patients into three different treatment arms, including 203 cases with known MGMT promoter methylation status. The other was a population-based study of 179 patients with IDHwt GBM, receiving concomittant radiotherapy and chemotherapy with temozolomide. Cohorts were stratified by sex to test the hypothesis that female sex in combination with MGMT promoter methylation constitutes a subgroup with more favorable outcome. Results: There was a significantly larger proportion of MGMT promoter methylation and better outcome for female patients with MGMT promoter methylated tumors. Results were confirmed in 257 TCGA-derived IDHwt GBM with known sex and MGMT status. Conclusions: These results confirm that patient sex in combination with MGMT promoter methylation is a key determinant in GBM to be considered prior to treatment decisions. Our study also illustrates the need for stratification to identify such sex-bound associations.
Keywords: glioblastoma, MGMT promoter methylation, survival, sex disparities
1. Introduction
Glioblastoma (GBM) is the most common malignant primary brain tumor in adults, occurring mostly in the fifth and sixth decade of life [1]. The incidence of GBM is around 3.2 per 100,000 inhabitants [2]. First line treatment consists of maximal safe surgery, followed by radiotherapy (RT) and chemotherapy [3]. In spite of multimodal strategies, the median survival of patients with IDHwt GBM is less than 18 months [2,4].
Similar to many other cancers, GBM occurs more frequently in the male population (male-to-female ratio 1.6:1) [5,6]. The potential influence of patient sex on the disease is intriguing and has received increased attention [7,8,9]. Of particular interest in this context are sex-bound disparities in the epigenetic regulation of glioma, and how they impact downstream gene expression. There is accumulating evidence that gliomas display sex-specific methylation patterns [10,11]. MGMT promoter methylation is more commonly found in females with GBM, with a subsequent better outcome after treatment with alkylating agent temozolomide (TMZ) [12,13,14,15]. Interestingly, this prognostic advantage for females is not present in gliomas of lower malignancy grade [16]. Since MGMT promoter methylation is the most powerful biomarker for response to alkylating agent treatment in GBM at present, these findings have important implications for tailored therapy and need confirmation by further studies [17].
To address this issue, we re-analyzed the datasets from two previously published GBM cohorts. Patient sex as a parameter was included in the original survival analyses, but no sub-analysis searching for specific interactions with regard to sex was made at that time. Based on the assumption that sex-bound associations may have gone unnoticed, we hypothesized that re-evaluation of data after stratification by sex may unravel differences in MGMT promoter methylation between female and male patients [18]. For confirmation of findings, a cohort of IDHwt GBM derived from The Cancer Genome Atlas (TCGA) was used.
2. Materials and Methods
2.1. Patient Cohorts
The first cohort was the NORDIC multicenter randomized study of elderly patients (60 years or older) with GBM diagnosis obtained by biopsy or tumor resection, and enrolled into a three-arm trial, comparing standard RT (60 Gy) with hypofractionated RT (34 Gy) or TMZ [19]. A total of 342 patients were included and randomized between three different treatment arms (temozolomide n = 119; hypofractionated RT n = 123, standard RT n = 100). MGMT methylation status, determined by methylation specific PCR, could be assessed for 203 patients, this being 59% of the total study cohort (Table 1). No follow-up was performed after publication of the study.
Table 1.
Clinical parameters of patients included in the three different cohorts.
| Total | Male | Female | p-Value | |
|---|---|---|---|---|
| 1st cohort (NORDIC trial) | 342 | 203 | 139 | |
| Known MGMT status | 203 | 117 | 86 | |
| Methylated MGMT promoter | 91 | 40 | 51 | 0.0004 |
| Unmethylated MGMT promoter | 112 | 77 | 35 | |
| 2nd cohort (population-based) | 179 | 112 | 67 | |
| Methylated MGMT promoter | 69 | 37 | 32 | 0.05 |
| Unmethylated MGMT promoter | 110 | 75 | 35 | |
| 3rd cohort (TCGA-derived) | 257 | 151 | 106 | |
| Patients treated with alkylating agent | 189 | 116 | 73 | |
| Methylated MGMT promoter | 87 | 43 | 44 | 0.001 |
| Unmethylated MGMT promoter | 102 | 73 | 29 |
The second cohort was a population-based cohort of 179 patients from South-East Sweden with IDHwt GBM and known MGMT methylation status, analyzed by pyrosequencing (Table 1). Patients were recruited from 2004 and onwards and followed until death or until last follow-up (1 December 2020) [20]. Histological diagnosis of IDHwt GBM was obtained by biopsy or tumor resection, and all patients received postoperative RT concomitant with TMZ.
As a third, confirmatory cohort, we searched TCGA for IDHwt GBM with known sex and MGMT methylation status, determined by bead-based microarray technology. We identified 257 patients (106 females, 151 males) of whom 189 received alkylating agent therapy, either with TMZ or a nitrosourea compound (Table 1).
2.2. Statistical Analyses
To test for sex-bound differences in the proportion of tumors with methylated MGMT promoter (mMGMT) versus (vs.) unmethylated MGMT promoter (uMGMT), the Pearson’s Chi2 test was used. Type I error was set at 5% and all tests were two-tailed. Overall survival was estimated by Kaplan–Meier method with a two-sided Log-rank test. For comparison of hazard ratios (HR) for relative risk of death, multivariate Cox regression was used.
3. Results
Table 1 shows the number of patients in the three cohorts and the parameters used for the present study.
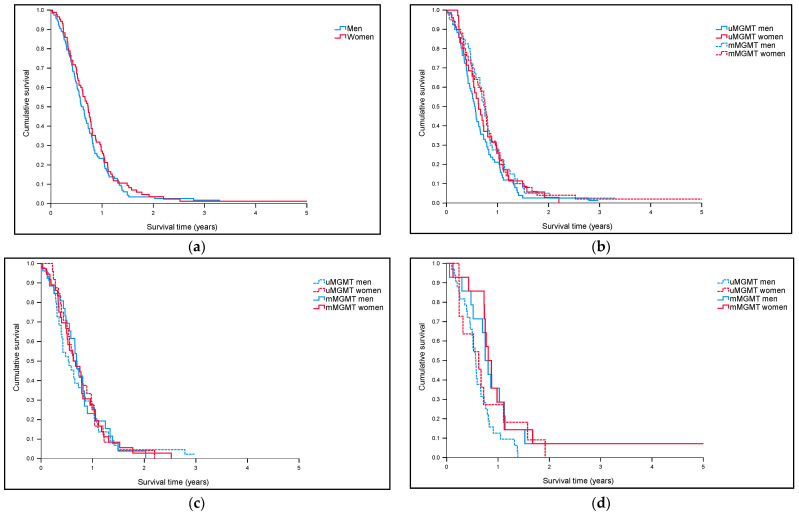
First Cohort (NORDIC trial): As shown in Table 1, the proportion of tumors with mMGMT was higher in females than in males (59% vs. 34%, p = 0.0004, Chi2 test). Median overall survival (MOS) for men was 7.0 months (CI 6.1–7.9) vs. 7.5 months (CI 6.4–8.6) for women in the whole cohort. In the subgroup of 203 patients with known MGMT status, MOS for men was 7.6 months (CI 6.5–8.6) vs. 8.7 months (CI 7.2–10.2) for women. There were no statistically significant differences in survival between the sexes in the whole cohort (Figure 1a) or according to MGMT status (Figure 1b).
Figure 1.
Overall survival in the NORDIC-trial (a) for men (n = 203) vs. women (n = 139) in the whole cohort (p = 0.37). (b) For men (n = 117) vs. women (n = 86) according to MGMT status (p = 0.40). (c) For men (n = 70) vs. women (n = 61) in the RT-arms (p = 1.0). (d) For men (n = 47) vs. women (n = 25) in the TMZ-arm of the trial (p = 0.056).
In a next step, we performed separate analyses for patients included in the RT arms (standard RT or hypofractionated RT) (n = 131) or the TMZ-arm (n = 72) of the trial. As expected, no sex-bound differences according to MGMT status were seen for patients included in the RT-arms (uMGMT men = 44 vs. uMGMT women = 24 vs. mMGMT men = 26 vs. mMGMT women = 37) (p = 1.0, Log-rank) (Figure 1c). Figure 1d, on the other hand, shows differences in survival between men and women included in the TMZ-arm (uMGMT men = 33 vs. uMGMT women = 11 vs. mMGMT men = 14 vs. mMGMT women = 14). As illustrated, MOS was longest for women (9.7 months, CI 7.5–11.9) and men (9.0 months, CI 7.4–10.6) with mMGMT, compared to women (7.5 months, CI 2.9–12.0) and men (6.8 months (CI 5.8–7.9) with uMGMT, although numbers were small and differences did not reach statistical significance (p = 0.056, Log-rank) (Figure 1d).
We used sex-specific multivariate Cox regression in the TMZ-arm (70 males, 47 with known MGMT status; 49 females, 25 with known MGMT status), to test the impact of surgery (biopsy vs. resection), performance status (PS) (WHO 0–1 vs. WHO 2–3), MGMT status (uMGMT vs. mMGMT) and dichotomized age on survival in males respectively females. Table S1 shows the results. In females, tumor resection was associated with longer survival (p = 0.017). In males, mMGMT was associated with longer survival (p = 0.013), together with PS WHO 0–1 (<0.0001).
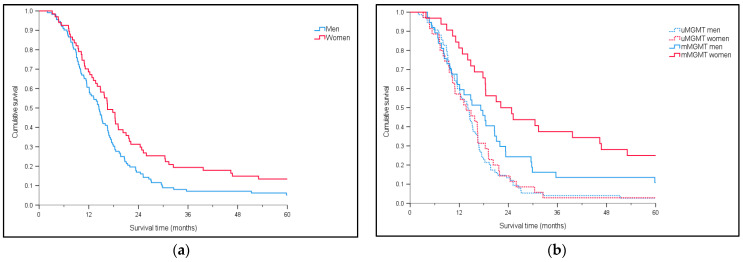
Second (population-based) cohort: As shown in Table 1, the proportion of mMGMT tumors was significantly higher in females compared to males (48% vs. 33%, p = 0.05, Chi-2 test) in the population-based cohort. The MOS in this cohort was 15.1 months (CI 13.5–16.8). Figure 2a shows a significantly shorter MOS for men (n = 112) than for women (n = 67) (p = 0.035, Log-rank). Figure 2b shows survival according to MGMT status (p = 0.0002, Log-rank), with longest survival for females with mMGMT (MOS females with mMGMT 22.1 months, CI 12.7–31.5; MOS males with mMGMT 17.3 months, CI 11.1–23.4). As expected, patients with uMGMT had poorest outcome (females with uMGMT 13.7 months, CI 8.1–19.2; males with uMGMT 14.1 months, CI 11.9–16.3).
Figure 2.
Overall survival in the population-based cohort (a) for men (n = 112) vs. women (n = 67) (p = 0.035). (b) For men (uMGMT = 75; mMGMT = 37) vs. women (uMGMT = 35; mMGMT = 32) according to MGMT status (p = 0.0002).
Table S2 shows the results of sex-specific multivariate survival analysis in cohort 2, with the variables surgery (biopsy vs. partial resection vs. radical resection), PS (WHO 0–1 vs. WHO 2–3), MGMT status (uMGMT vs. mMGMT) and dichotomized age entered in the Cox regression model. In females, mMGMT was associated with longer survival (p = 0.0014), together with radical resection. In males, mMGMT was associated with longer survival (p = 0.024).
Third (TCGA-derived) cohort: Finally, we studied the correlation between MGMT status and sex in IDHwt GBM generated by TCGA Research Network: https://www.cancer.gov/tcga. A total of 151 males (55 mMGMT, 96 uMGMT) and 106 females (60 mMGMT, 46 uMGMT) were identified (Table 1). Consistent with reported findings, the proportion of mMGMT was significantly higher for women than for men (57% vs. 36%) (p = 0.001, Chi2 test). The MOS in this cohort was 12.9 months. Females with mMGMT had significantly longer MOS (17.2 months, CI 13.3–21.0) than females with uMGMT (11.8 months, CI 7.5–16.1), men with mMGMT (12.7 months, CI 8.6–16.8), and men with uMGMT (12.6 months, CI 11.1–14.1) (p = 0.01, Log-rank test; Figure S1a). Of the group of patients with mMGMT and treated with alkylating chemotherapy, either alone or in combination with RT, females had a statistically significant survival advantage (females 20.7 months, CI 19.3–30.0; males 15.8 months, CI 12.4–18.6) (p = 0.004, Log-rank) (Figure S1b).
4. Discussion
Sex-bound differences in susceptibility and survival of different cancer types are among the most consistent findings in cancer epidemiology that can be pivotal for developing a tailored therapy to cancer [7]. We tested the hypothesis that sex-associated disparities in MGMT promoter methylation may have gone unnoticed in the original analysis of two previously published clinical cohorts. For this purpose, we re-analyzed the datasets from the NORDIC trial of elderly GBM patients, and a population-based cohort of IDHwt GBM in our region receiving RT and chemotherapy. We found that approximately half of all women in both cohorts harbored mMGMT, while for men this proportion was around one third. These findings were robust and further confirmed by data from TCGA.
On top of sex-associated disparities in the proportion of mMGMT, there was a survival advantage for females in the population-based cohort, with longest survival in the subgroup of females with mMGMT. The results from TCGA database confirmed these data and showed a statically significant longer survival for females with mMGMT after alkylating agent treatment. For the NORDIC trial, no such survival differences were noted between men and women in the arm receiving TMZ treatment. This discrepancy was probably due to the low numbers (only 25 females with known MGMT status), reducing statistical power. Otherwise, sex-specific multivariate analysis confirmed mMGMT as a favorable prognostic factor in males (47 with known MGMT status) included in the TMZ-arm, and in males and females in the population-based cohort. Of the other established prognostic factors for GBM, tumor resection was associated with significantly longer survival for females in both cohorts. These findings give support for a favorable role of tumor resection on outcome also in the elderly population of GBM [21], and suggest that this benefit may not be similar for male and female patients. However, it should be noted that this is a preliminary observation that needs confirmation by larger studies. Also, different arrays to determine the MGMT promoter methylation status were used in the three cohorts, which may have affected the results.
Insight in the differences in epigenetic profiles between male and female patients will be vital for understanding the sex-bound discrepancies in gliomagenesis and prognosis, and may lead to improved treatments for both sexes. The study by Johansen and co-workers, exploring sex-specific gene methylation patterns in 587 glioma samples derived from TCGA, reported that the genes associated with hypermethylation in males with IDHwt GBM were enriched for cell cycle phase transition genes. In females, on the other hand, an enrichment of transcriptional regulators was seen, in agreement with the overrepresentation of mMGMT in females with GBM. Interestingly, methylation of the MGMT promoter does not seem to occur uniformly in a sex-bound fashion in all cancer types. A meta-analysis of the role of MGMT promoter methylation in small cell lung cancer showed a correlation with the clinical stage of this cancer type, but not with factors like sex, age, and smoking [22]. This suggests that the higher proportion of mMGMT and the better response to alkylating treatment seen in females with GBM is a tumor-specific phenomenon.
Our study confirms previous findings and exemplifies the need for stratification of the cohort by sex to unravel sex-bound differences that may otherwise go unnoticed. As pointed out by Dorak and Karpuzoglu [23], the concern over losing statistical power after stratification is not justified. On the contrary, by not splitting the sample into males and females there is a risk for not picking up gender-specific associations.
5. Conclusions
Taken together, we provide further evidence for sex-bound disparities in the epigenetic regulation of IDHwt GBM, exemplified by the MGMT status of the tumor, which could contribute to a survival advantage for female patients. Our data illustrate the need for stratification by sex in clinical cohorts of GBM, where an unbalanced incidence of the disease between males and females may disguise gender-specific associations with survival.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/10/4/556/s1, Table S1: Multivariate Cox-regression in females and males in the TMZ-arm of cohort 1. Table S2: Multivariate Cox-regression in females and males in cohort 2. Figure S1a: Survival for men (n = 151) and women (n = 106) in 257 TCGA-derived IDHwt GBM with known MGMT status and sex (p = 0.01, Log-rank). Figure S1b: Survival for men (n = 116) and women (n = 73) in 189 TCGA-derived IDHwt GBM with known MGMT status and sex, treated with alkylating therapy (p = 0.004, Log-rank).
Author Contributions
Conceptualization, A.S., A.M. (Andreas Magnusson), and A.M. (Annika Malmström); methodology, A.S., M.L., A.M. (Andreas Magnusson), J.R., and A.M. (Annika Malmström); software, J.R.; validation, A.S., M.L., J.R., and A.M. (Annika Malmström); formal analysis, M.L. and J.R.; investigation, A.S.; resources, P.S. and A.M. (Annika Malmström); data curation, J.R.; writing—original draft preparation, A.S., M.L., A.M. (Andreas Magnusson), and A.M. (Annika Malmström); writing—review and editing, A.S., M.L., A.M. (Andreas Magnusson), P.S., and A.M. (Annika Malmström); visualization, M.L. and J.R.; supervision, A.S. and P.S.; project administration, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG 717021, ALFGBG 932533) and the Lions Cancer Foundation at Uppsala University Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Linköping University Hospital (EPN 99086; 2011/32-32; M167-07).
Informed Consent Statement
Consent according to ethical approval was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Thakkar J.P., Dolecek T.A., Horbinski C., Ostrom Q.T., Lightner D.D., Barnholtz-Sloan J.S., Villano J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014;23:1985–1996. doi: 10.1158/1055-9965.EPI-14-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R., Mason W.P., Van Den Bent M.J., Weller M., Fisher B., Taphoorn M.J.B., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Weller M., Le Rhun E., Preusser M., Tonn J.C., Roth P. How we treat glioblastoma. ESMO Open. 2019;4:e000520. doi: 10.1136/esmoopen-2019-000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrom Q.T., Gittleman H., Truitt G., Boscia A., Kruchko C., Barnholtz-Sloan J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro-Oncol. 2018;20:1–86. doi: 10.1093/neuonc/noy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrom Q.T., Rubin J.B., Lathia J.D., Berens M.E., Barnholtz-Sloan J.S. Females have the survival advantage in glioblastoma. Neuro-Oncol. 2018;20:576–577. doi: 10.1093/neuonc/noy002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matteoni S., Abbruzzese C., Villani V., Malorni W., Pace A., Matarrese P., Paggi M.G. The influence of patient sex on clinical approaches to malignant glioma. Cancer Lett. 2020;468:41–47. doi: 10.1016/j.canlet.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Ostrom Q.T., Coleman W., Huang W., Rubin J.B., Lathia J.D., Berens M.E., Speyer G., Liao P., Wrensch M.R., Eckel-Passow J.E., et al. Sex-specific gene and pathway modeling of inherited glioma risk. Neuro-Oncol. 2019;21:71–82. doi: 10.1093/neuonc/noy135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang W., Warrington N.M., Taylor S.J., Whitmire P., Carrasco E., Singleton K.W., Wu N., Lathia J.D., Berens M.E., Kim A.H., et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci. Transl. Med. 2019;11:eaao5253. doi: 10.1126/scitranslmed.aao5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen M.L., Stetson L.C., Vadmal V., Waite K., Berens M.E., Connor J.R., Lathia J., Rubin J.B., Barnholtz-Sloan J.S. Gliomas display distinct sex-based differential methylation patterns based on molecular subtype. Neuro-Oncol. Adv. 2020;2:vdaa002. doi: 10.1093/noajnl/vdaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majchrzak-Celińska A., Dybska E., Barciszewska A.M. DNA methylation analysis with methylation-sensitive high-resolution melting (MS-HRM) reveals gene panel for glioma characteristics. CNS Neurosci. Ther. 2020;26:1303–1314. doi: 10.1111/cns.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franceschi E., Tosoni A., Minichillo S., Depenni R., Paccapelo A., Bartolini S., Michiara M., Pavesi G., Urbini B., Crisi G., et al. The Prognostic Roles of Gender and O6-Methylguanine-DNA Methyltransferase Methylation Status in Glioblastoma Patients: The Female Power. World Neurosurg. 2018;112:e342–e347. doi: 10.1016/j.wneu.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 13.Schiffgens S., Wilkens L., Brandes A.A., Meier T., Franceschi E., Ermani M., Hartmann C., Sandalcioglu I.E., Dumitru C.A. Sex-specific clinicopathological significance of novel (Frizzled-7) and established (MGMT, IDH1) biomarkers in glioblastoma. Oncotarget. 2016;7:55169–55180. doi: 10.18632/oncotarget.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandith A.A., Qasim I., Zahoor W., Shah P., Bhat A.R., Sanadhya D., Shah Z.A., Naikoo N.A. Concordant association validates MGMT methylation and protein expression as favorable prognostic factors in glioma patients on alkylating chemotherapy (Temozolomide) Sci. Rep. 2018;8 doi: 10.1038/s41598-018-25169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell E.H., Zhang P., Fisher B.J., Macdonald D.R., McElroy J.P., Lesser G.J., Fleming J., Chakraborty A.R., Liu Z., Becker A.P., et al. Association of MGMT Promoter Methylation Status with Survival Outcomes in Patients with High-Risk Glioma Treated with Radiotherapy and Temozolomide: An Analysis from the NRG Oncology/RTOG 0424 Trial. JAMA Oncol. 2018;4:1405–1409. doi: 10.1001/jamaoncol.2018.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gittleman H., Ostrom Q.T., Stetson L.C., Waite K., Hodges T.R., Wright C.H., Wright J., Rubin J.B., Berens M.E., Lathia J., et al. Sex is an important prognostic factor for glioblastoma but not for nonglioblastoma. Neuro-Oncol. Pract. 2019;6:451–462. doi: 10.1093/nop/npz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gusyatiner O., Hegi M.E. Glioma epigenetics: From subclassification to novel treatment options. Semin. Cancer Biol. 2018;51:50–58. doi: 10.1016/j.semcancer.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Tevfik Dorak M., Karpuzoglu E. Gender differences in cancer susceptibility: An inadequately addressed issue. Front. Genet. 2012;3:1–11. doi: 10.3389/fgene.2012.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malmström A., Grønberg B.H., Marosi C., Stupp R., Frappaz D., Schultz H., Abacioglu U., Tavelin B., Lhermitte B., Hegi M.E., et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 20.Malmström A., Łysiak M., Åkesson L., Jakobsen I., Mudaisi M., Milos P., Hallbeck M., Fomichov V., Broholm H., Grunnet K., et al. ABCB1 single-nucleotide variants and survival in patients with glioblastoma treated with radiotherapy concomitant with temozolomide. Pharm. J. 2020;20:213–219. doi: 10.1038/s41397-019-0107-z. [DOI] [PubMed] [Google Scholar]
- 21.Conti Nibali M., Gay L.G., Sciortino T., Rossi M., Caroli M., Bello L., Riva M. Surgery for Glioblastoma in Elderly Patients. Neurosurg. Clin. N. Am. 2021;32:137–148. doi: 10.1016/j.nec.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Vaissière T., Hung R.J., Zaridze D., Moukeria A., Cuenin C., Fasolo V., Ferro G., Paliwal A., Hainaut P., Brennan P., et al. Quantitative analysis of DNA methylation profiles in lung cancer identifies aberrant DNA methylation of specific genes and its association with gender and cancer risk factors. Cancer Res. 2009;69:243–252. doi: 10.1158/0008-5472.CAN-08-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L., Wang Y., Liu F., Xu L., Peng F., Zhao N., Fu B., Zhu Z., Shi Y., Liu J., et al. A systematic review and meta-analysis: Association between MGMT hypermethylation and the clinicopathological characteristics of non-small-cell lung carcinoma. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-19949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.