Abstract
Simple Summary
Chlamydia is a major pathogen of the Australian marsupial, the koala (Phascolarctos cinereus). One approach to improving this situation is to develop a vaccine. Human Chlamydia research suggests that an effective anti-chlamydial response will involve a balance between a cell-mediated Th1 response and a humoral Th2 responses, involving systemic IgG and mucosal IgA. Characterization of koalas with chlamydial disease suggests that increased expression for similar immunological pathways and monitoring of koalas’ post-vaccination can be successful and subsequently lead to improved vaccines. These findings offer optimism that a chlamydial vaccine for wider distribution to koalas is not far off.
Abstract
Chlamydia is a significant pathogen for many species, including the much-loved Australian marsupial, the koala (Phascolarctos cinereus). To combat this situation, focused research has gone into the development and refinement of a chlamydial vaccine for koalas. The foundation of this process has involved characterising the immune response of koalas to both natural chlamydial infection as well as vaccination. From parallels in human and mouse research, it is well-established that an effective anti-chlamydial response will involve a balance of cell-mediated Th1 responses involving interferon-gamma (IFN-γ), humoral Th2 responses involving systemic IgG and mucosal IgA, and inflammatory Th17 responses involving interleukin 17 (IL-17) and neutrophils. Characterisation of koalas with chlamydial disease has shown increased expression within all three of these major immunological pathways and monitoring of koalas’ post-vaccination has detected further enhancements to these key pathways. These findings offer optimism that a chlamydial vaccine for wider distribution to koalas is not far off. Recent advances in marsupial genetic knowledge and general nucleic acid assay technology have moved koala immunological research a step closer to other mammalian research systems. However, koala-specific reagents to directly assay cytokine levels and cell-surface markers are still needed to progress our understanding of koala immunology.
Keywords: koalas, vaccines, immunity, Chlamydia
1. Chlamydia and Koalas
Chlamydia are obligate intracellular bacteria recognised in a wide range of hosts. Traditionally identified and studied in birds, cattle, guinea pigs, sheep and humans [1], continued research has expanded the list of chlamydial hosts to include insects, amphibians, molluscs, arachnids, reptiles, fish, and amoeba, as well as mammals like pigs, goats, deer, cats, bats, possums, and koalas [2]. Chlamydial disease in the koala, Phascolarctos cinereus, has been particularly well studied, given the devastating toll it has taken on this iconic Australian marsupial (Figure 1) [3,4]. Chlamydial infection in koalas is dominated by the species Chlamydia pecorum. These infections lead to ocular and urogenital/reproductive diseases comparable to Chlamydia trachomatis infections in humans, which include keratoconjunctivitis and scarring in the eye leading to blindness and cystitis/nephritis and reproductive cysts in the urogenital and reproductive tracts, respectively, leading to severe pain and infertility [3,4]. In both humans and koalas, vaccination has been identified as the most promising avenue of control for this pathogen [5]. However, despite years of research, no commercial vaccine is available for either host. What research has achieved is a greater understanding of the immune response to chlamydial infection and vaccination, particularly in koalas, setting the stage for future success.
Figure 1.
Koala (Phascolarctos cinereus). Photo credit Bonnie Quigley.
Marsupials occupy a unique branch of the mammalian evolutionary tree, having diverged from their eutherian (placental) relatives ~148 million years ago [6]. Originally believed to have slower and less accentuated immune responses [7,8,9], it is now recognised that the immune system of marsupials is just as intricate and complex as that of their eutherian counterparts [10]. While study into the components and general development of the koala immune system has already spanned several decades of research (reviewed by [11]), the complete koala genome has only recently been sequenced [12]. This has meant that much of the foundational work carried out with koala immune genes and processes were based on concepts extrapolated from more characterised mammalian systems. Koala-specific reagents for experimentation have also been limited, given the non-model organism status of this marsupial. However, despite these limitations, chlamydial vaccine development for koalas has progressed over the last decade to generate research vaccine formulations with very promising efficacies [13,14,15]. Research has also highlighted many similarities in immune responses to Chlamydia infection between hosts, allowing for knowledge from one host to guide research in others. This has proven advantageous to the koala in the field of chlamydial immune responses and vaccine development.
2. Effective Chlamydial Immune Responses
Chlamydial infection, disease, and vaccine research, often from human or mouse studies, has established a solid framework for what immune responses are necessary to clear and prevent chlamydial infections. Additionally, chlamydial research in non-model systems, such as non-human primates and guinea pigs, deserves recognition for also advancing ocular chlamydial disease understanding and vaccine development [16]. Overall, it has become well-established that a combined cellular and humoral immune response is needed for complete protection from chlamydial infection and disease progression [17,18,19].
2.1. Immunogenetics
For any adaptive immune response to be generated, an early key step is the presentation of chlamydial antigens to T cells via the major histocompatibility complex (MHC) or human leukocyte antigen (HLA) system. MHC molecules present antigens from either intracellular threats via class I molecules or externally phagocytosed antigens via class II molecules to T lymphocytes to initiate an adaptive immune response [20]. As an intracellular bacterium, Chlamydia has the potential to interact with both MHC classes. In humans, many studies have looked for associations between specific HLA alleles and susceptibility to chlamydial infection or complications [21]. Immunogenetic studies have found links between chlamydial infections/complications and HLA alleles from both classes, with examples including the presence of alleles from HLA class I A and C loci having significantly higher risk of C. trachomatis pelvic inflammatory disease [22] and the HLA class II HLA-DQB1*06 allele emerging as a significant risk marker for chlamydia reinfection in African American women [23]. These genetic links to infection outcome highlight that individuals within a population will mount slightly different responses to the same chlamydial infection, an important consideration during vaccine design.
2.2. Cell Mediated (Th1) Responses
Traditionally, the desired immune response to chlamydial infections has been identified as a T helper cell type 1 (Th1) cell-mediated response, with interferon gamma (IFN-γ) being the critical cytokine involved in chlamydial clearance [24]. IFN-γ directly affects the survival of Chlamydia through several mechanisms, including enhancing the engulfment and elimination of Chlamydia by macrophages [25], activating nitric oxide synthase (iNOS) to produce nitric oxide (NO) to inhibit chlamydial replication [26], and limiting both iron and tryptophan availability for Chlamydia growth by downregulating the transferrin receptor [27] and inducing indoleamine-2,3-dioxygenase (IDO) to degrade tryptophan [28], respectively. IFN-γ also affects the survival of Chlamydia by inducing T cells to differentiate into Th1 cells and inhibiting proliferation of the T helper cell type 2 (Th2) antibody response [29]. Along with a Th1 adaptive immune response involving CD4/CD8 T cells, an effective cellular response against Chlamydia also requires the recruitment of innate cells including macrophages, dendritic cells, and natural killer cells to the mucosal site of infection [17,24].
2.3. Antibody (Th2) Responses
While it is generally agreed that a Th1 cell-mediated response is necessary for Chlamydia control and protection, the distinct Th1/Th2 paradigm of host defence has encountered major challenges due to the reality that most antigens or vaccines (including chlamydial vaccines) induce mixed immune responses comprising of both humoral and cell- mediated effectors [30]. It has been shown that a robust and protective T-cell memory response against Chlamydia requires an effective primary antibody response characterised by specific antibody isotypes whose role is to modulate Th1 activation via Fc receptors that facilitate the rapid uptake, processing, and presentation of pathogen-derived antigens for an enhanced T-cell response [30]. Antibody mediated immunity is increasingly being recognised as necessary, with studies showing the appearance of serum antibodies strongly correlating with chlamydial clearance [31]. Specific examples include the presence of IgA within vaginal secretions correlating with chlamydial clearance [32] and the induction of anti-chlamydial IgG2a and IgA post-vaccination leading to a strong IgG2a recall response post-challenge [33]. Additionally, an important role for antibodies is emerging in the secondary memory response [34], with antibody-mediated neutralization and opsonization [35] and antibody-dependent cellular cytotoxicity (ADCC) [36] identified as important chlamydia control mechanisms.
2.4. Inflammatory/Neutrophil (Th17) Responses
The lineage of interleukin 17 (IL-17)-producing CD4 T helper (Th17) cells are also emerging as an important component of the anti-chlamydial response. Th17 cells produce pro-inflammatory cytokines, such as IL-17 and tumour necrosis factor alpha (TNF-α), to act on fibroblasts, macrophages, and endothelial and epithelial cells to recruit granulocytes (especially neutrophils) to the site of infection [29]. Once present, neutrophils in particular have been found to play a critical role in the control of Chlamydia in the early stages of infection [37].
2.5. Coordinated Responses
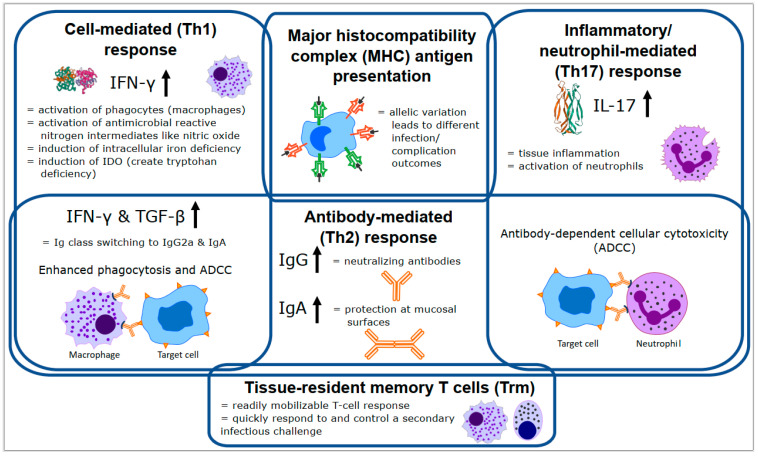
Finally, although traditional immunology divides immune responses into discreet Th1, Th2, and Th17 categories, several important anti-chlamydial mechanisms require coordinated action from multiple categories. Examples of these interactions can be seen in the critical antibody class switching to IgG2a and IgA induced by the Th1 cytokines, IFN-γ and transforming growth factor beta (TGF-β) [38] and role of anti-chlamydial antibodies in ADCC by both macrophages and neutrophils for chlamydial clearance [36,39]. In addition, the important role played by tissue-specific memory T cells (Trm), which are not easily categorised within the Th1/Th2/Th17 paradigms, is being increasingly recognised [40,41]. Clearly, many diverse and complex processes are needed to control chlamydial infection in the mammalian host (Figure 2).
Figure 2.
Overview of the major immune responses and components important for controlling chlamydial infection (based on the mouse immune response). Details of each process are described in the text. Th = T helper cell, MHC = major histocompatibility complex, IFN-γ = interferon gamma, IL-17 = interleukin 17, TGF-β = transforming growth factor beta, ADCC = antibody-dependent cellular cytotoxicity, Ig = immunoglobulin, IDO = indoleamine-2,3-dioxygenase.
3. Chlamydial Infection, Disease, and Vaccine Responses in Koalas
It has been proposed that a successful Chlamydia vaccine for koalas will need to induce both cellular immune responses through up-regulation of IFN-γ and IL-17, as well as humoral immune responses that generate Chlamydia-specific plasma IgG and mucosal IgG and IgA responses with neutralizing capabilities [5]. As such, focused effort has gone into characterising these markers, among others, during both natural C. pecorum infection and post-C. pecorum vaccination in koalas.
3.1. Immunogenetics Related to Chlamydia in Koalas
The koala genome contains 23 MHC class I and 23 MHC class II genes and pseudogenes [12]. Examination of the class I genes determined that 11 genes are actively transcribed in the koala, with three genes ubiquitously expressed as classical class Ia genes (Phci-UA, UB and UC) and eight genes with tissue limited expressions as nonclassical class Ib genes (Phci-UD, UE, UF, UG, UH, UI, UJ and UK) [42]. Survey of the classical class I genes have thus far identified 21 UA, 5 UB, and 12 UC alleles for these genes in the koala population [43]. Even though the number of identified koala class I alleles is expected to increase as more koala populations are surveyed, these numbers are still quite small compared to the thousands of HLA class I alleles recognised in humans [44] (Table 1).
Table 1.
Major Histocompatibility Complex (MHC)/Human Leukocyte Antigen (HLA) alleles currently recognised in koalas and humans, respectively. Part A—MHC alleles in koalas, Part B—HLA alleles in humans (from [44]).
| A | Class | MHC Loci | Number of Alleles | B | Class | HLA Loci | Number of Alleles |
|---|---|---|---|---|---|---|---|
| Class I | UA | 21 | Class I | A | 2480 | ||
| UB | 5 | B | 3221 | ||||
| UC | 10 | C | 2196 | ||||
| Class II | DAα | 3 | E | 8 | |||
| DAβ | 44 | F | 4 | ||||
| DBα | 3 | G | 18 | ||||
| DBβ | 26 | Class II | DMα | 4 | |||
| DCα | No work yet done | DMβ | 7 | ||||
| DCβ | 3 | DOα | 3 | ||||
| DMα | No work yet done | DOβ | 5 | ||||
| DMβ | 4 | DPα1 | 22 | ||||
| DPβ1 | 591 | ||||||
| DQα1 | 34 | ||||||
| DQβ1 | 678 | ||||||
| DRα | 2 | ||||||
| DRβ1 | 1440 | ||||||
| DRβ3 | 106 | ||||||
| DRβ4 | 42 | ||||||
| DRβ5 | 39 |
Within the MHC class II gene family, four class II subfamilies are recognised, consisting of alpha and beta subunits of DA, DB, DC and DM [12,45,46]. Studies investigating the allelic diversity of class II subunits within DA and DB genes have found that the beta subfamilies (DAB and DBB) contain more allelic diversity than the alpha subfamilies (DAA and DAA), leading to more focus on the beta subfamilies in research studies [45]. As such, the current collection of sequenced koala MHC class II alleles stands at three DAA, 42 DAB, three DBA, 26 DBB, three DCB, and four DMB alleles, with DCA and DMA alleles yet to be characterised [43,47,48].
Several MHC allele associations have been made with koala chlamydial infections and disease progression. Within koalas from the mid-north coast of New South Wales, Australia, a higher proportion of Chlamydia-infected koalas were found to carry the DAB*10 allele relative to non-infected koalas [47]. In the same population, koalas possessing the DBB*04 allele had higher levels of Chlamydia heat shock protein 60 (c-hsp60) antibody levels than koalas without DBB*04 [47]. Moving further north to examine koalas already infected with Chlamydia in southeast Queensland, Australia, DAB*10 and DBB*04 alleles again emerged with significant associations, but to different circumstances [48]. In these infected koalas, both DAB*10 and UC*01:01 alleles were significantly more prevalent in infected koalas that did not progress to clinical disease, while DBB*04 and DCB*03 alleles were significantly more prevalent in infected koalas that did progress to clinical disease [48]. Finally, a modelling study that also looked at southeast Queensland koalas found that knowing the MHC class II DAB and DBB profiles of a koala improved the likelihood of predicting a koala’s chlamydial disease status and that koalas without DBB*03 were more likely to have clinical chlamydial disease [49]. Collectively, these studies suggest that MHC immunogenetics do play some role in the immune response to Chlamydia infection and progression to disease in koalas. However, as with most genetic associations, these links are complex and will require extensive study to be understood.
3.2. Cell-Mediated Responses to Chlamydia in Koalas
Given the lack of koala-specific reagents to directly measure specific CD4 or CD8 T cell populations, the primary marker for evaluating Th1 responses in koalas has been to follow IFN-γ expression. In natural infection settings, peripheral blood mononuclear cells (PBMCs) from koalas with active chlamydial disease have been found to have higher expression of IFN-γ and TNF-α than koalas with asymptomatic chlamydial infection or no chlamydial infection/disease [50,51,52]. In large chlamydial vaccination studies of wild koalas, vaccination increases the level of IFN-γ detected [13,53,54]. This IFN-γ increase was seen in koalas regardless of whether the vaccine was formulated with C. pecorum major outer membrane protein (MOMP) [13,53] or polymorphic member protein (Pmp) [13] as the target chlamydial antigen. These studies indicate that, as in other hosts, IFN-γ production appears to be an important component of the koala anti-chlamydial and vaccine response.
Interestingly, despite the well-recognised importance of IFN-γ, there have also been smaller studies where positive vaccine outcomes were achieved and increases in IFN-γ levels could not be detected in koalas post-vaccination [14,15]. Although sample timing can always be a factor in detecting cytokine expression, the genetics of C. pecorum may also inform on the variable IFN-γ detection. As discussed above, a major mechanism of IFN-γ action against Chlamydia is the induced depletion of tryptophan [28]. For chlamydial species like C. trachomatis, which lack most of the tryptophan biosynthesis operon, growth is severely inhibited when IFN-γ is present [55]. However, C. pecorum possesses a nearly complete tryptophan biosynthesis operon [56,57] and is able to overcome IFN-γ mediated tryptophan depletion by utilising alternative precursors to sustain growth [55]. This suggests that IFN-γ mediated tryptophan depletion may not be as effective against C. pecorum as it is against other chlamydial species. It should still be acknowledged that IFN-γ affects Chlamydia through multiple mechanisms and remains an important cytokine for chlamydial clearance. However, C. pecorum control in koalas may require a coordinated response involving several anti-chlamydial mechanisms, with different studies detecting different mechanisms depending on the experimental design.
3.3. Antibody Responses to Chlamydia in Koalas
In a traditional Th2 response, IL-4, -6, -10, and -11 are the hallmark cytokines that initiate an antibody response and lead to the release of IL-4, -5, -9, -10, and -13, characteristic of the Th2 phenotype [29]. Within koalas with current chlamydial disease, significantly higher expression of IL-10 is detected in PBMCs compared to koalas with asymptomatic chlamydial infection and no chlamydial infection/disease [51]. This suggests that some level of Th2 response is generated during chlamydial disease in koalas.
In koala vaccination studies, antibody responses are typically measured as either total systemic anti-C. pecorum IgG in plasma or mucosal anti-C. pecorum IgG or IgA at the ocular or urogenital site. In every chlamydial vaccine trial in koalas where antibodies were measured, there has been a detectable increase in systemic IgG to C. pecorum post-vaccination, regardless of the vaccine formulation tested [5]. Characterisation of these systemic C. pecorum IgG antibodies has found that, (a) diverse MOMP genotypes, including genotypes not included as antigen in the vaccine, could be recognised [58], (b) vaccination induced a greater epitope recognition compared to natural infection (including to conserved regions of MOMP) [15,59,60] and that (c) vaccination increased the neutralisation effect of the antibodies generated [60]. Given the fact that C. pecorum is currently recognised to have 15 MOMP genotypes associated with koalas [4], achieving antibody responses to multiple MOMP genotype has offered promise that vaccination may induce heterologous protection. Focusing in on the mucosal epithelium, as the site of chlamydial infection, anti-C. pecorum mucosal IgG and IgA levels have also been found at higher levels post-vaccination [13,14,61]. This is an important parameter to consider in vaccine efficacy, as mucosal antibodies are part of the primary defence against infecting Chlamydia. Overall, chlamydial vaccination in koalas appears to generate a robust antibody response that can contribute to other aspects of the overall anti-Chlamydia immune response.
3.4. Inflammatory/Neutrophil Responses to Chlamydia in Koalas
The general state of tissue inflammation has come to be recognised as the result of Th17 cells producing IL-17, IL-6, IL-22, and TNF-α to activate several cell linages to recruit effector cells like neutrophils to the site of infection [29]. In koalas, when currently chlamydial diseased animals have been examined, significantly higher expression of IL-17A in PBMCs has been observed [52]. This has suggested that there is a role for the Th17 response in chlamydial disease progression and management in koalas.
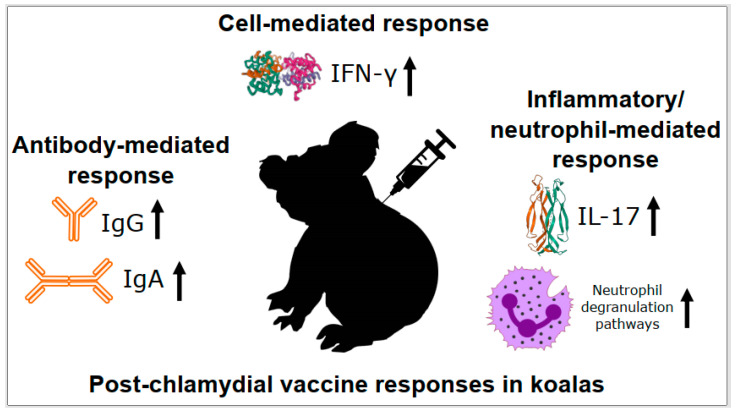
Examination of IL-17 expression during chlamydial vaccine trials in koalas has also supported a role for this immune response in C. pecorum disease and control. Levels of IL-17 expression have shown post-vaccination increases in wild koala chlamydial vaccine trials [13,53,54]. This has led to increased levels of IL-17 expression being strongly associated with decreases in urogenital chlamydial load and less chlamydial disease in these trials [54]. Looking globally, total transcriptome profiling of koala PBMCs one-month post-vaccination has further expanded links to inflammation-related pathways, with significant up-regulation of 26 genes involved in neutrophil degranulation detected [15]. Collectively, these results continue to support the multi-faceted anti-chlamydial immune response observed in other hosts, with aspects of the Th17 response having a place in vaccination responses in koalas (Figure 3).
Figure 3.
Post-chlamydial vaccine responses in koalas. Details of each process are described in the text. IFN-γ = interferon gamma, IL-17 = interleukin 17, Ig = immunoglobulin.
4. Future Directions
Our understanding of the koala immune system has advanced dramatically over the past decade. While the availability of the complete koala genome, as well as the genomes of closely related marsupials, has contributed greatly to this advancement, so has the extensive effort that has been expended characterising immune responses to chlamydial vaccine development in koalas. Studies continue to reveal similarities between koala immune responses and responses measured in other mammalian model systems. Methodologies utilizing nucleic acids (DNA and RNA) have flourished recently in koala immunology research, with the expanded genomic and transcriptomic data that has become available. The next big step forward will need to be the development of koala-specific reagents to investigate cytokines and specific cell-surface markers to the extents that are currently routine for human or mouse immunology research. The journey for an effective Chlamydia vaccine continues for both humans and koalas and progress is being made on both fronts. Expanding the repertoire of immunological assays available for koala vaccine research would allow discoveries in this marsupial to contribute back to overall understanding of anti-chlamydial immune responses, to the benefit of all species. That would be a successful outcome for everyone.
Acknowledgments
We thank the many groups that have supported our overall koala disease and vaccine research, including the Queensland Government (Department of Transport and Main Roads), the Moreton Bay Rail project team, the Queensland Department of Environment and Heritage Protection, the NSW Office of Planning, Industry and Environment, Moreton Bay Regional Council, Gold Coast City Council, Redland City Council, Gympie Regional Council, NSW Koala Strategy, Friends of the Koala—Lismore, Koala Action Inc., Royal Society of Queensland, Endeavour Veterinary Ecology, Australia Zoo Wildlife Hospital, Lone Pine Koala Sanctuary, Currumbin Wildlife Sanctuary, Wildlife HQ Zoo, and VIDO, Canada.
Author Contributions
Conceptualization, B.L.Q. and P.T.; writing—original draft preparation, B.L.Q.; writing—review and editing, B.L.Q. and P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data referred to in the manuscript is already published.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shewen P.E. Chlamydial infection in animals: A review. Can. Vet. J. 1980;21:2–11. [PMC free article] [PubMed] [Google Scholar]
- 2.Collingro A., Köstlbacher S., Horn M. Chlamydiae in the Environment. Trends Microbiol. 2020;28:877–888. doi: 10.1016/j.tim.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Jelocnik M. Chlamydiae from down under: The curious cases of chlamydial infections in Australia. Microorganisms. 2019;7:602. doi: 10.3390/microorganisms7120602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quigley B.L., Timms P. Helping koalas battle disease—Recent advances in Chlamydia and koala retrovirus (KoRV) disease understanding and treatment in koalas. FEMS Microbiol. Rev. 2020;44:583–605. doi: 10.1093/femsre/fuaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips S., Quigley B.L., Timms P. Seventy years of Chlamydia vaccine research—Limitations of the past and directions for the future. Front. Microbiol. 2019;10:70. doi: 10.3389/fmicb.2019.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emonds O.R.B., Cardillo M., Jones K.E., MacPhee R.D., Beck R.M., Grenyer R., Price S.A., Vos R.A., Gittleman J.L., Purvis A. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. [DOI] [PubMed] [Google Scholar]
- 7.Jurd R.D. “Not proper mammals”: Immunity in monotremes and marsupials. Comp. Immunol. Microbiol. Infect. Dis. 1994;17:41–52. doi: 10.1016/0147-9571(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson R., Kotlarski I., Barton M. Koala lymphoid cells: Analysis of antigen-specific responses. Vet. Immunol. Immunopathol. 1992;33:237–247. doi: 10.1016/0165-2427(92)90184-R. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson R., Kotlarski I., Barton M. Further characterisation of the immune response of the koala. Vet. Immunol. Immunopathol. 1994;40:325–339. doi: 10.1016/0165-2427(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 10.Belov K., Miller R.D., Old J.M., Young L.J. Marsupial immunology bounding ahead. Aust. J. Zool. 2013;61:21–40. doi: 10.1071/ZO12111. [DOI] [Google Scholar]
- 11.Madden D., Whaite A., Jones E., Belov K., Timms P., Polkinghorne A. Koala immunology and infectious diseases: How much can the koala bear? Dev. Comp. Immunol. 2018;82:177–185. doi: 10.1016/j.dci.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Johnson R.N., O’Meally D., Chen Z., Etherington G.J., Ho S.Y.W., Nash W.J., Grueber C.E., Cheng Y., Whittington C.M., Dennison S., et al. Adaptation and conservation insights from the koala genome. Nat. Genet. 2018;50:1102–1111. doi: 10.1038/s41588-018-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desclozeaux M., Robbins A., Jelocnik M., Khan S.A., Hanger J., Gerdts V., Potter A., Polkinghorne A., Timms P. Immunization of a wild koala population with a recombinant Chlamydia pecorum Major Outer Membrane Protein (MOMP) or Polymorphic Membrane Protein (PMP) based vaccine: New insights into immune response, protection and clearance. PLoS ONE. 2017;12:e0178786. doi: 10.1371/journal.pone.0178786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyari S., Booth R., Quigley B.L., Waugh C.A., Timms P. Therapeutic effect of a Chlamydia pecorum recombinant major outer membrane protein vaccine on ocular disease in koalas (Phascolarctos cinereus) PLoS ONE. 2019;14:e0210245. doi: 10.1371/journal.pone.0210245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips S., Quigley B.L., Olagoke O., Booth R., Pyne M., Timms P. Vaccination of koalas during antibiotic treatment for Chlamydia-induced cystitis induces an improved antibody response to Chlamydia pecorum. Sci. Rep. 2020;10:10152. doi: 10.1038/s41598-020-67208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rank R.G., Whittum-Hudson J.A. Animal models for ocular infections. Methods Enzymol. 1994;235:69–83. doi: 10.1016/0076-6879(94)35132-5. [DOI] [PubMed] [Google Scholar]
- 17.Vasilevsky S., Greub G., Haefliger D.N., Baud D. Genital Chlamydia trachomatis: Understanding the roles of innate and adaptive immunity in vaccine research. Clin. Microbiol. Rev. 2014;27:346–370. doi: 10.1128/CMR.00105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hafner L., Beagley K., Timms P. Chlamydia trachomatis infection: Host immune responses and potential vaccines. Mucosal Immunol. 2008;1:116–130. doi: 10.1038/mi.2007.19. [DOI] [PubMed] [Google Scholar]
- 19.Redgrove K.A., McLaughlin E.A. The role of the immune response in Chlamydia trachomatis infection of the male genital tract: A double-edged sword. Front. Immunol. 2014;5:534. doi: 10.3389/fimmu.2014.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Punt J., Stranford S.A., Jones P.P., Owen J.A. Kuby Immunology. Mcmillian Education; New York, NY, USA: 2018. [Google Scholar]
- 21.Morré S.A., Karimi O., Ouburg S. Chlamydia trachomatis: Identification of susceptibility markers for ocular and sexually transmitted infection by immunogenetics. FEMS Immunol. Med. Microbiol. 2009;55:140–153. doi: 10.1111/j.1574-695X.2009.00536.x. [DOI] [PubMed] [Google Scholar]
- 22.Kimani J., Maclean I.W., Bwayo J.J., MacDonald K., Oyugi J., Maitha G.M., Peeling R.W., Cheang M., Nagelkerke N.J., Plummer F.A., et al. Risk factors for Chlamydia trachomatis pelvic inflammatory disease among sex workers in Nairobi, Kenya. J. Infect. Dis. 1996;173:1437–1444. doi: 10.1093/infdis/173.6.1437. [DOI] [PubMed] [Google Scholar]
- 23.Olson K.M., Tang J., Brown L., Press C.G., Geisler W.M. HLA-DQB1 * 06 is a risk marker for chlamydia reinfection in African American women. Genes Immun. 2019;20:69–73. doi: 10.1038/s41435-018-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunham R.C., Rey-Ladino J. Immunology of Chlamydia infection: Implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 25.Zhong G.M., de la Maza L.M. Activation of mouse peritoneal macrophages in vitro or in vivo by recombinant murine gamma interferon inhibits the growth of Chlamydia trachomatis serovar L1. Infect. Immun. 1988;56:3322–3325. doi: 10.1128/IAI.56.12.3322-3325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen B., Stout R., Campbell W.F. Nitric oxide production: A mechanism of Chlamydia trachomatis inhibition in interferon-gamma-treated RAW264.7 cells. FEMS Immunol. Med. Microbiol. 1996;14:109–120. doi: 10.1111/j.1574-695X.1996.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 27.Ryu S.Y., Jeong K.S., Kang B.N., Park S.J., Yoon W.K., Kim S.H., Kim T.H. Modulation of transferrin synthesis, transferrin receptor expression, iNOS expression and NO production in mouse macrophages by cytokines, either alone or in combination. Anticancer Res. 2000;20:3331–3338. [PubMed] [Google Scholar]
- 28.Akers J.C., Tan M. Molecular mechanism of tryptophan-dependent transcriptional regulation in Chlamydia trachomatis. J. Bacteriol. 2006;188:4236–4243. doi: 10.1128/JB.01660-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiko G.E., Horvat J.C., Beagley K.W., Hansbro P.M. Immunological decision-making: How does the immune system decide to mount a helper T-cell response? Immunology. 2008;123:326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Igietseme J.U., Eko F.O., He Q., Black C.M. Antibody regulation of T-cell immunity: Implications for vaccine strategies against intracellular pathogens. Expert Rev. Vaccines. 2004;3:23–34. doi: 10.1586/14760584.3.1.23. [DOI] [PubMed] [Google Scholar]
- 31.Casadevall A., Pirofski L.A. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv. Immunol. 2006;91:1–44. doi: 10.1016/S0065-2776(06)91001-3. [DOI] [PubMed] [Google Scholar]
- 32.Brunham R.C., Kuo C.C., Cles L., Holmes K.K. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect. Immun. 1983;39:1491–1494. doi: 10.1128/IAI.39.3.1491-1494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eko F.O., Ekong E., He Q., Black C.M., Igietseme J.U. Induction of immune memory by a multisubunit chlamydial vaccine. Vaccine. 2011;29:1472–1480. doi: 10.1016/j.vaccine.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison S.G., Morrison R.P. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J. Immunol. 2005;175:7536–7542. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartolini E., Ianni E., Frigimelica E., Petracca R., Galli G., Berlanda Scorza F., Norais N., Laera D., Giusti F., Pierleoni A., et al. Recombinant outer membrane vesicles carrying Chlamydia muridarum HtrA induce antibodies that neutralize chlamydial infection in vitro. J. Extracell. Vesicles. 2013;2:20181. doi: 10.3402/jev.v2i0.20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore T., Ananaba G.A., Bolier J., Bowers S., Belay T., Eko F.O., Igietseme J.U. Fc receptor regulation of protective immunity against Chlamydia trachomatis. Immunology. 2002;105:213–221. doi: 10.1046/j.0019-2805.2001.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barteneva N., Theodor I., Peterson E.M., de la Maza L.M. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect. Immun. 1996;64:4830–4833. doi: 10.1128/IAI.64.11.4830-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deenick E.K., Hasbold J., Hodgkin P.D. Decision criteria for resolving isotype switching conflicts by B cells. Eur. J. Immunol. 2005;35:2949–2955. doi: 10.1002/eji.200425719. [DOI] [PubMed] [Google Scholar]
- 39.Naglak E.K., Morrison S.G., Morrison R.P. Neutrophils are central to antibody-mediated protection against genital Chlamydia. Infect. Immun. 2017;85:e00409-17. doi: 10.1128/IAI.00409-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison S.G., Morrison R.P. In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infect. Immun. 2000;68:2870–2879. doi: 10.1128/IAI.68.5.2870-2879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson R.M., Brunham R.C. Tissue-Resident T cells as the central paradigm of Chlamydia immunity. Infect. Immun. 2016;84:868–873. doi: 10.1128/IAI.01378-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng Y., Polkinghorne A., Gillett A., Jones E.A., O’Meally D., Timms P., Belov K. Characterisation of MHC class I genes in the koala. Immunogenetics. 2018;70:125–133. doi: 10.1007/s00251-017-1018-2. [DOI] [PubMed] [Google Scholar]
- 43.Quigley B.L., Tzipori G., Nilsson K., Timms P. High throughput immunogenetic typing of koalas suggests possible link between MHC alleles and cancer. Immunogenetics. 2020;72:499–506. doi: 10.1007/s00251-020-01181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Punt J., Stranford S., Jones P., Owen J. Kuby Immunology. 8th ed. Volume 8. Macmillan Science and Education USA; New York, NY, USA: 2019. p. 944. [Google Scholar]
- 45.Lau Q., Jobbins S.E., Belov K., Higgins D.P. Characterisation of four major histocompatibility complex class II genes of the koala (Phascolarctos cinereus) Immunogenetics. 2013;65:37–46. doi: 10.1007/s00251-012-0658-5. [DOI] [PubMed] [Google Scholar]
- 46.Abts K.C., Ivy J.A., de Woody J.A. Demographic, environmental and genetic determinants of mating success in captive koalas (Phascolarctos cinereus) Zoo Biol. 2018;37:416–433. doi: 10.1002/zoo.21457. [DOI] [PubMed] [Google Scholar]
- 47.Lau Q., Griffith J.E., Higgins D.P. Identification of MHCII variants associated with chlamydial disease in the koala (Phascolarctos cinereus) PeerJ. 2014;2:e443. doi: 10.7717/peerj.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robbins A., Hanger J., Jelocnik M., Quigley B.L., Timms P. Koala immunogenetics and chlamydial strain type are more directly involved in chlamydial disease progression in koalas from two south east Queensland koala populations than koala retrovirus subtypes. Sci. Rep. 2020;10:15013. doi: 10.1038/s41598-020-72050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quigley B.L., Carver S., Hanger J., Vidgen M.E., Timms P. The relative contribution of causal factors in the transition from infection to clinical chlamydial disease. Sci. Rep. 2018;8:8893. doi: 10.1038/s41598-018-27253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathew M., Pavasovic A., Prentis P.J., Beagley K.W., Timms P., Polkinghorne A. Molecular characterisation and expression analysis of interferon gamma in response to natural Chlamydia infection in the koala, Phascolarctos cinereus. Gene. 2013;527:570–577. doi: 10.1016/j.gene.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 51.Mathew M., Beagley K.W., Timms P., Polkinghorne A. Preliminary characterisation of tumor necrosis factor alpha and interleukin-10 responses to Chlamydia pecorum infection in the koala (Phascolarctos cinereus) PLoS ONE. 2013;8:e59958. doi: 10.1371/journal.pone.0059958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathew M., Waugh C., Beagley K.W., Timms P., Polkinghorne A. Interleukin 17A is an immune marker for chlamydial disease severity and pathogenesis in the koala (Phascolarctos cinereus) Dev. Comp. Immunol. 2014;46:423–429. doi: 10.1016/j.dci.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 53.Waugh C., Khan S.A., Carver S., Hanger J., Loader J., Polkinghorne A., Beagley K., Timms P. A prototype recombinant-protein based Chlamydia pecorum vaccine results in reduced chlamydial burden and less clinical disease in free-ranging koalas (Phascolarctos cinereus) PLoS ONE. 2016;11:e0146934. doi: 10.1371/journal.pone.0146934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lizárraga D., Timms P., Quigley B.L., Hanger J., Carver S. Capturing complex vaccine-immune-disease relationships for free-ranging koalas: Higher chlamydial loads are associated with less IL17 expression and more chlamydial disease. Front. Vet. Sci. 2020;7:530686. doi: 10.3389/fvets.2020.530686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Islam M.M., Jelocnik M., Huston W.M., Timms P., Polkinghorne A. Characterization of the in vitro Chlamydia pecorum response to gamma interferon. Infect. Immun. 2018;86 doi: 10.1128/IAI.00714-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bachmann N.L., Fraser T.A., Bertelli C., Jelocnik M., Gillett A., Funnell O., Flanagan C., Myers G.S., Timms P., Polkinghorne A. Comparative genomics of koala, cattle and sheep strains of Chlamydia pecorum. BMC Genom. 2014;15:667. doi: 10.1186/1471-2164-15-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mojica S., Huot Creasy H., Daugherty S., Read T.D., Kim T., Kaltenboeck B., Bavoil P., Myers G. Gemone sequence of the obligate intracellular animal pathogen Chlamydia pecorum E58. J. Bacteriol. 2011;193:3690. doi: 10.1128/JB.00454-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kollipara A., Wan C., Rawlinson G., Brumm J., Nilsson K., Polkinghorne A., Beagley K., Timms P. Antigenic specificity of a monovalent versus polyvalent MOMP based Chlamydia pecorum vaccine in koalas (Phascolarctos cinereus) Vaccine. 2013;31:1217–1223. doi: 10.1016/j.vaccine.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 59.Kollipara A., Polkinghorne A., Beagley K.W., Timms P. Vaccination of koalas with a recombinant Chlamydia pecorum major outer membrane protein induces antibodies of different specificity compared to those following a natural live infection. PLoS ONE. 2013;8:e74808. doi: 10.1371/journal.pone.0074808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan S.A., Polkinghorne A., Waugh C., Hanger J., Loader J., Beagley K., Timms P. Humoral immune responses in koalas (Phascolarctos cinereus) either naturally infected with Chlamydia pecorum or following administration of a recombinant chlamydial major outer membrane protein vaccine. Vaccine. 2016;34:775–782. doi: 10.1016/j.vaccine.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 61.Nyari S., Khan S.A., Rawlinson G., Waugh C.A., Potter A., Gerdts V., Timms P. Vaccination of koalas (Phascolarctos cinereus) against Chlamydia pecorum using synthetic peptides derived from the major outer membrane protein. PLoS ONE. 2018;13:e0200112. doi: 10.1371/journal.pone.0200112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data referred to in the manuscript is already published.