Abstract
This report of the EFSA and the European Centre for Disease Prevention and Control presents the results of zoonoses monitoring activities carried out in 2019 in 36 European countries (28 Member States (MS) and eight non‐MS). The first and second most reported zoonoses in humans were campylobacteriosis and salmonellosis, respectively. The EU trend for confirmed human cases of these two diseases was stable (flat) during 2015–2019. The proportion of human salmonellosis cases due to Salmonella Enteritidis acquired in the EU was similar to that in 2017–2018. Of the 26 MS reporting on Salmonella control programmes in poultry, 18 met the reduction targets, whereas eight failed to meet at least one. The EU prevalence of Salmonella target serovar‐positive flocks has been stable since 2015 for breeding hens, laying hens, broilers and fattening turkeys, with fluctuations for breeding turkey flocks. Salmonella results from competent authorities for pig carcases and for poultry tested through national control programmes were more frequently positive than those from food business operators. Shiga toxin‐producing Escherichia coli (STEC) infection was the third most reported zoonosis in humans and increased from 2015 to 2019. Yersiniosis was the fourth most reported zoonosis in humans in 2019 with a stable trend in 2015–2019. The EU trend of confirmed listeriosis cases remained stable in 2015–2019 after a long period of increase. Listeria rarely exceeded the EU food safety limit tested in ready‐to‐eat food. In total, 5,175 food‐borne outbreaks were reported. Salmonella remained the most detected agent but the number of outbreaks due to S. Enteritidis decreased. Norovirus in fish and fishery products was the agent/food pair causing the highest number of strong‐evidence outbreaks. The report provides further updates on bovine tuberculosis, Brucella, Trichinella, Echinococcus, Toxoplasma, rabies, West Nile virus, Coxiella burnetii (Q fever) and tularaemia.
Keywords: Campylobacter, food‐borne outbreaks, Listeria, monitoring, parasites, Salmonella, zoonoses
Introduction
Legal basis of European Union‐coordinated zoonoses monitoring
The (European Union) EU system for monitoring and collection of information on zoonoses is based on the Zoonoses Directive 2003/99/EC1, which obliges EU Member States (MS) to collect relevant and, when applicable, comparable data on zoonoses, zoonotic agents, antimicrobial resistance and food‐borne outbreaks. In addition, MS shall assess trends and sources of these agents, as well as outbreaks in their territory, submitting an annual report each year by the end of May to the European Commission covering the data collected. The European Commission should subsequently forward these reports to the European Food Safety Authority (EFSA). EFSA is assigned the tasks of examining these data and publishing the EU Annual Summary Reports. In 2004, the European Commission entrusted EFSA with the task of setting up an electronic reporting system and database for monitoring zoonoses (EFSA Mandate No 2004‐0178).
Data collection on human diseases from MS is conducted in accordance with Decision 1082/2013/EU2 on serious cross‐border threats to health. This Decision replaced Decision 2119/98/EC on setting up a network for the epidemiological surveillance and control of communicable diseases in the EU in October 2013. The case definitions to be followed when reporting data on infectious diseases to the European Centre for Disease Prevention and Control (ECDC) are described in Decision 2018/945/EU3. ECDC has provided data on zoonotic infections in humans, as well as their analyses, for the EU Summary Reports since 2005. Since 2008, data on human cases have been received via The European Surveillance System (TESSy), maintained by ECDC.
Reporting requirements
According to List A of the Annex I of the Zoonoses Directive 2003/99/EC data on animals, food and feed must be reported on a mandatory basis for the following eight zoonotic agents: Salmonella, Campylobacter, Listeria monocytogenes, Shiga toxin‐producing Escherichia coli (STEC), Mycobacterium bovis, Brucella, Trichinella and Echinococcus. In addition and based on the epidemiological situations in the MS, data must be reported on the following agents and zoonoses (List B of the Annex I of the Zoonoses Directive): (i) viral zoonoses: calicivirus, hepatitis A virus, influenza virus, rabies, viruses transmitted by arthropods; (ii) bacterial zoonoses: borreliosis and agents thereof, botulism and agents thereof, leptospirosis and agents thereof, psittacosis and agents thereof, tuberculosis due to agents other than M. bovis, vibriosis and agents thereof, yersiniosis and agents thereof; (iii) parasitic zoonoses: anisakiasis and agents thereof, cryptosporidiosis and agents thereof, cysticercosis and agents thereof, toxoplasmosis and agents thereof; and (iv) other zoonoses and zoonotic agents such as Francisella, Cysticercus and Sarcocystis. Furthermore, MS provided data on certain other microbiological contaminants in foods: histamine, staphylococcal enterotoxins and Cronobacter sakazakii for which food safety criteria are set down in the EU legislation.
The general rules on monitoring of zoonoses and zoonotic agents in animals, food and feed are laid down in Article 4 of Chapter II ‘Monitoring of zoonoses and zoonotic agents’ of the Directive. Specific rules for coordinated monitoring programmes and for food business operators are, respectively, in Articles 5 and 6 of Chapter II. Specific rules for monitoring of antimicrobial resistance are in Article 7 of Chapter III ‘Antimicrobial resistance’, whereas rules for epidemiological investigation of food‐borne outbreaks are in Article 8 of Chapter IV ‘Food‐borne outbreaks’.
According to Article 9 of Chapter V ‘Exchange of information’ of the Directive, MS shall assess trends and sources of zoonoses, zoonotic agents and antimicrobial resistance in their territory and each MS shall send to the European Commission every year by the end of May a report on trends and sources of zoonoses, zoonotic agents and antimicrobial resistance, covering the data collected under Articles 4, 7 and 8 during the previous year. Reports, and any summaries of these, shall be made publicly available. The requirements for those MS‐specific reports are described in Parts A–D of Annex IV as regards the monitoring of zoonoses, zoonotic agents and antimicrobial resistance carried out in accordance with Article 4 or 7, and in Part E of Annex IV as regards the monitoring of food‐borne outbreaks carried out in accordance with Article 8.
Terms of Reference
In accordance with Article 9 of Directive 2003/99/EC, EFSA shall examine the submitted national reports and data of the EU MS 2019 zoonoses monitoring activities as described above and publish an EU Summary Report on the trends and sources of zoonoses, zoonotic agents and antimicrobial resistance in the EU.
The 2019 data on antimicrobial resistance in zoonotic agents submitted and validated by the MS are published in a separate EU Summary Report.
Data sources
Since 2019, the annual EU Summary Reports on zoonoses, zoonotic agents and food‐borne outbreaks have been renamed the ‘EU One Health Zoonoses summary report’ (EUOHZ), which is jointly drafted and co‐authored by EFSA and ECDC. The MS, other reporting countries, the European Commission, members of EFSA's Scientific Panels on Biological Hazards (BIOHAZ) and Animal Health and Welfare (AHAW) and the relevant European Union Reference Laboratories (EURLs) were consulted while preparing the present EU One Health Zoonoses 2019 report.
The efforts made by MS, the reporting non‐MS and the European Commission in the reporting of zoonoses data and in the preparation of this report are gratefully acknowledged.
The present EU One Health Zoonoses summary report focuses on the most relevant information on zoonoses and food‐borne outbreaks within the EU in 2019. If substantial changes compared with the previous year were observed, they have been reported. It is noteworthy that EFSA and ECDC were informed on the incompleteness of certain data provision by a few MS due to the COVID‐19 pandemic. The latter impacted on national resources allocated to zoonoses and food‐borne outbreaks data collection leading to a delay in reports from regional to national levels. Such incompleteness has been mentioned in a few chapters.
When the UK data were collected, the UK was an EU MS but as of 31 January 2020, it has become a third country.
Human 2019 data collection
The analyses of data from infections in humans in the EU Summary Report for 2019 were prepared by the Food‐ and Waterborne Diseases and Zoonoses (FWD) programme (brucellosis, campylobacteriosis, congenital toxoplasmosis, echinococcosis, listeriosis, salmonellosis, STEC infection, trichinellosis, yersiniosis), Emerging and Vector‐borne Diseases (EVD) programme (Q fever, rabies, tularaemia, West Nile virus (WNV) infection) and tuberculosis (TB) programme (TB due to Mycobacterium bovis and M. caprae) at the ECDC. Data were based on the data submitted via The European Surveillance System (TESSy), hosted at ECDC. Please note, as explained above, that the numbers presented in the report may differ from national reports due to differences in case definitions used at EU and national level or to different dates of data submission and extraction. The latter may also result in some divergence in case numbers presented in different ECDC reports.
TESSy is a software platform that has been operational since April 2008 and in which data on 56 diseases and special health issues are collected. Both aggregated and case‐based data were reported to TESSy. Although aggregated data did not include individual case‐based information, both reporting formats were included when possible to calculate number of cases and country‐specific notification rates. Human data used in the report were extracted from TESSy as of 7 September 2020 for FWD, as of 9 October 2020 for EVD (except for rabies as of 29 October) and as of 5 October 2020 for TB due to M. bovis and M. caprae. The denominators used for the calculation of the notification rates were the human population data from Eurostat 1 January 2020 update.
Data on human zoonoses cases were received from 28 MS and from two non‐MS (Iceland and Norway). Switzerland reported its data on human cases directly to EFSA. The human data for Switzerland include data from Liechtenstein.
Interpretation of the data should consider data quality issues and differences between MS surveillance systems, and therefore, comparisons between countries should be undertaken with caution.
Data collection on food, animals and feed and food‐borne outbreaks
For the year 2019, 28 MS submitted data and national zoonoses reports on monitoring results in food, animals, feed and food‐borne outbreaks. In addition, data and reports were submitted by four non‐MS and European Free Trade Association (EFTA) countries: Iceland, Norway, Switzerland and Liechtenstein.4 For some food, animal and feed matrices and food‐borne outbreaks, EFSA received data and reports from pre‐accession countries Albania, Bosnia and Herzegovina, the Republic of North Macedonia, Montenegro and Serbia. Data were submitted electronically to the EFSA zoonoses database, through EFSA's Data Collection Framework (DCF). MS could also update data from previous years, before 2019.
The deadline for data submission was 31 May 2020. Two data validation procedures were implemented, by 12 June 2020 and by 15 July 2020. Validated data on food, animals and feed used in the report were extracted from the EFSA zoonoses database on 27 July 2020.
The draft EU One Health Zoonoses Report was sent to MS for consultation on 7 December 2020 and comments were collected by 23 December 2020. The utmost effort was made to incorporate comments and data amendments within the available time frame. The report was finalised by 22 January 2021 and published online by EFSA and ECDC on 25 February 2021.
The detailed description of the terms used in the report is available in the EFSA's manuals for reporting on zoonoses (EFSA, 2020a,b).
The national zoonoses reports submitted in accordance with Directive 2003/99/EC are published on the EFSA website together with the EU One Health Zoonoses Report. They are available online at http://www.efsa.europa.eu/en/biological-hazards-data/reports.
Data analyses and presentation
Comparability and quality of the data
Humans
For data on human infections, please note that the numbers presented in this report may differ from national zoonoses reports due to differences in case definitions used at EU and national level or because of different dates of data submission and extraction. Results are generally not directly comparable between MS and sometimes not even between different years in one country.
Food–animals–feed and food‐borne outbreaks
For data on food, animals and feed please note that the numbers presented in this report may differ from national zoonoses reports due to different dates of data submission and extraction.
The data obtained in the EFSA DCF can vary according to the level of data quality and harmonisation. Therefore, the type of data analyses suggested by EFSA for each zoonosis and matrix (food, animals, feed or food‐borne outbreaks) sampling results strongly depended on this level of harmonisation and can either be a descriptive summary of submitted data, or the following up of trends (trend watching) or the (quantitative) analysis of trends. EFSA carried out data analyses according to Table 1 as adapted from Boelaert et al. (2016): food, animal, feed and food‐borne outbreaks data can be classified into three categories according to the zoonotic agent monitored and the design of the monitoring or surveillance carried out. It follows that these three distinct categories condition which type of data analyses can be implemented.
Table 1.
Categorisation of data used in EUOHZ 2019 (adapted from Boelaert et al., 2016)
| Category | Type of analyses | Type/comparability between MS | Examples |
|---|---|---|---|
| I |
Descriptive summaries at the national level and EU level EU trend watching (trend monitoring) Spatial and temporal trends analyses at the EU level |
Programmed harmonised monitoring or surveillance Comparable between MS; results at the EU level are interpretable |
Salmonella national control programmes in poultry; bovine tuberculosis; bovine and small ruminant brucellosis; Trichinella in pigs at slaughterhouse |
| II |
Descriptive summaries at national level and EU level EU trend watching (trend monitoring) No trend analysis at the EU level |
Not fully harmonised monitoring or surveillance Not fully comparable between MS; caution needed when interpreting results at the EU level |
Food‐borne outbreak data Monitoring of compliance with process hygiene and food safety criteria for Campylobacter, L. monocytogenes, Salmonella and E. coli in the context of Regulation (EC) No 2073/2005 Monitoring of rabies |
| III |
Descriptive summaries at national level and EU level No EU trend watching (trend monitoring) No trend analysis at the EU level |
Non‐harmonised monitoring or surveillance data with no (harmonised) reporting requirements Not comparable between MS; extreme caution needed when interpreting results at the EU level |
Campylobacter; Yersinia; Q fever; Francisella tularensis; West Nile virus; Taenia spp.; other zoonoses; Toxoplasma |
Rationale of the table of contents
Following the rationale of listing of zoonoses in Annex I of the Directive 2003/99/EC, of the mandatory reporting on food‐borne outbreaks and of the above‐mentioned categorisation of food, animal and feed data (Table 1), the following table of contents was implemented in this annual EUOHZ:
Zoonoses included in compulsory annual monitoring (Directive 2003/99 List A)
Campylobacter
Salmonella
Listeria
Shiga toxin‐producing Escherichia coli
Tuberculosis due to Mycobacterium bovis or Mycobacterium caprae
Brucella
Trichinella
Echinococcus
Food‐ and waterborne outbreaks (according to Directive 2003/99)
Zoonoses monitored according the epidemiological situation (Directive 2003/99 List B)
Yersinia
Toxoplasma gondii
Rabies
Q fever
West Nile virus
Tularaemia
Other zoonoses and zoonotic agents
Microbiological contaminants subject to food safety criteria (Regulation (EC) No 2073/2005)
A chapter on food‐borne outbreaks constitutes the second section of the EUOHZ. The data submitted to ECDC and to EFSA for List B zoonoses are rather unbalanced (varying numbers of reporting countries and varying data volumes across years) and are collected without harmonised sampling design. Therefore, these zoonoses only supported a simplified chapter structure underpinned by descriptive summarisation of submitted data. Moreover, links are provided to ECDC data published elsewhere in the Annual Epidemiological Reports.
Chapter sections
The EUOHZ 2019 presents a harmonised structure for each chapter, starting with the key facts. In addition, a section explains the monitoring and surveillance in the EU for the specific disease or for food‐borne outbreaks. A results section summarises the major findings of 2019 as regards trends and sources. A summary table displaying the data of the last 5 years (2015–2019) for human cases and for major animal and food matrices is presented. Each chapter also contains a discussion and ends with a list of related projects and links with useful information for the specific disease.
For each chapter, overview tables present reported data by any reporting country. However, for the tables summarising MS‐specific results and providing EU‐level results, unless stated otherwise, data from industry own check programmes and hazard analysis and critical control point (HACCP) sampling as well as data from suspect sampling, selective sampling and outbreak or clinical investigations are excluded. Moreover, regional data reported by countries without statistics at the national level were also excluded from these summary tables.
Data analyses
Statistical trend analyses in humans were carried out to evaluate the significance of temporal variations in the EU and the specifications of these analyses are explained in each separate chapter. The number of confirmed cases for the EU/EEA by month is presented as a trend figure. All countries that consistently reported cases – or reported zero cases over the whole reporting period – were included. The trend figure also shows a centred 12‐month moving average, illustrating the overall trend by smoothing seasonal and random variations. Also, in humans, the implemented general‐use statistical tests must be viewed as hypotheses generating, not as confirmatory, tests. Analyses other than trend analyses in humans are carried out for confirmed EU cases only (EEA cases were excluded).
Spatial trends in food and animals were visualised using R software (www.r-project.org); package ggplot2 as well as ArcGIS from the Economic and Social Research Institute (ESRI) were used to map the data. Choropleth maps with graduated colours over a continuous scale of values were used to map the proportion of positive sample units across the EU and other reporting countries. Statistical trend analysis of food‐borne outbreaks was performed to evaluate the significance of temporal variations at the single MS level over the period 2010–2019, as described in the food‐borne outbreaks chapter.
All summary tables and figures used to produce this report, and that are not displayed, are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at the general‐purpose open‐access repository zenodo at https://doi.org/10.5281/zenodo.4298993. All validated country‐specific data on food, animals, feed and food‐borne outbreaks are also available at the mentioned URL.
Summary human zoonoses data 2019
The numbers of confirmed human cases of 13 zoonoses presented in this report are summarised in Figure 1. In 2019, campylobacteriosis was the most commonly reported zoonosis, as it has been since 2005, representing 50% of all the reported cases. Campylobacteriosis was followed by other bacterial diseases; salmonellosis, STEC infections and yersiniosis in being the most frequently reported. Severity of the diseases was analysed based on hospitalisation and outcome of the reported cases (Table 2). Based on data on severity, listeriosis and West Nile virus infection were the two most severe diseases with the highest case fatality and the highest hospitalisation, respectively. Almost all confirmed cases with data available on hospitalisation for these two diseases were hospitalised. About one out of every fifth and one out of 10 confirmed listeriosis and WNV cases, respectively, with known data were fatal.
Figure 1.
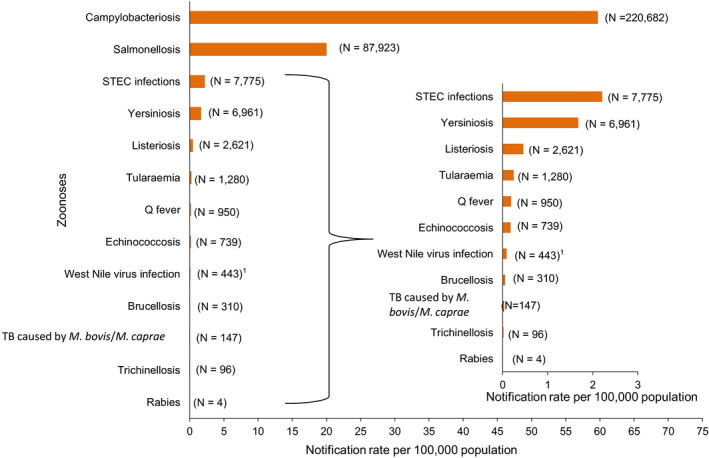
Reported numbers and notification rates of confirmed human zoonoses in the EU, 2019
-
Note: The total number of confirmed cases is indicated between parentheses at the end of each bar.1 Exception: West Nile virus infection for which the total number of cases was used.
Table 2.
Reported hospitalisations and case fatalities due to zoonoses in confirmed human cases in the EU, 2019
| Disease | Hospitalisation | Deaths | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of confirmed human cases | Status available (%) | Number of reporting MSb | Reported hospitalised cases | Proportion hospitalised (%) | Outcome available (%) | Number of reporting MSb | Reported deaths | Case fatality (%) | |
| Campylobacteriosis | 220,682 | 29.1 | 16 | 20,432 | 31.8 | 78.0 | 17 | 47 | 0.03 |
| Salmonellosis | 87,923 | 44.5 | 15 | 16,628 | 42.5 | 71.8 | 17 | 140 | 0.22 |
| STEC infections | 7,775 | 37.3 | 18 | 1,100 | 37.9 | 61.0 | 20 | 10 | 0.21 |
| Yersiniosis | 6,961 | 27.4 | 15 | 648 | 33.9 | 57.0 | 14 | 2 | 0.05 |
| Listeriosis | 2,621 | 51.1 | 19 | 1,234 | 92.1 | 65.1 | 20 | 300 | 17.6 |
| Tularaemia | 1,280 | 22.8 | 12 | 149 | 51.0 | 21.6 | 13 | 1 | 0.36 |
| Echinococcosis | 739 | 33.3 | 14 | 109 | 44.3 | 31.4 | 14 | 2 | 0.86 |
| Q fever | 950 | NAc | NA | NA | NA | 67.3 | 13 | 4 | 0.63 |
| West Nile virus infection a | 443 | 83.7 | 9 | 347 | 93.5 | 99.3 | 11 | 52 | 11.8 |
| Brucellosis | 310 | 44.5 | 11 | 98 | 71.0 | 36.8 | 12 | 2 | 1.75 |
| Trichinellosis | 96 | 16.7 | 5 | 6 | 37.5 | 25.0 | 7 | 1 | 4.20 |
| Rabies | 4 | NAc | NA | NA | NA | 75.0 | 3 | 3 | 100.0 |
MS: Member State.
Instead of confirmed human cases, the total number of human cases was included.
Not all countries observed cases for all diseases.
NA: Not applicable as the information is not collected for this disease.
Zoonoses included in compulsory annual monitoring (Directive 2003/99 List A)
1. Campylobacter
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
1.1. Key facts
Campylobacteriosis is the most commonly reported gastrointestinal infection in humans in the EU and has been so since 2005.
In 2019, the number of confirmed cases of human campylobacteriosis was 220,682 corresponding to an EU notification rate of 59.7 per 100,000 population, which is a decrease by 6.9% compared with the rate in 2018 (64.1 per 100,000 population).
The trend for campylobacteriosis in humans remained stable (flat) during 2015–2019.
Most cases (94.4%) with known origin of infection had acquired the infection in the EU.
In 2019, Campylobacter was the third most frequently reported causative agent of food‐borne outbreaks at EU level, by 18 MS, with 319 outbreaks reported to EFSA, involving 1,254 cases of illness, 125 hospitalisations and no deaths. Eighteen outbreaks were reported with strong‐evidence and 301 with weak evidence. The most common sources for the strong‐evidence campylobacteriosis food‐borne outbreaks were broiler meat and milk, as in previous years.
Seven MS reported monitoring results from official control samples collected in the context of the Campylobacter process hygiene criterion in force for food business operators. Of the 3,346 neck skin samples from chilled broiler carcases, 1,365 (41%) were Campylobacter‐positive and 506 (15%) exceeded the limit of 1,000 CFU/g. Seven MS reported such monitoring data based on sampling results collected from the food business operators. Of the 15,323 neck skin samples, 2,038 (13%) tested positive and 1,033 (7%) exceeded the limit of 1,000 CFU/g.
The proportion of Campylobacter‐positive samples within the categories ‘ready‐to-eat’ and ‘non ready‐to-eat’ food was 0.2% and 20.6% respectively. In 3,691 ‘ready‐to-eat’ food sampling units reported by eight MS, six Campylobacter‐positive units were detected; two from raw milk, two from ‘fruits, vegetables and juices’, one from salads and one from ‘other processed food products and prepared dishes’. From ‘non ready‐to-eat’ food, 16 MS reported data and ‘meat and meat products’ was the most contaminated food category followed by ‘milk and milk products’ and ‘fruits, vegetables and juices’, with 23.0%, 2.0% and 0.2% positive sampling units, respectively. Campylobacter was isolated from all fresh meat categories, with the highest percentage of Campylobacter‐positive sampling units being reported from fresh meat from turkeys and broilers; 33.0% and 29.6%, respectively.
Sixteen MS reported 2019 sampling results on Campylobacter in animals, mainly from broilers and bovine animals: the highest overall proportion of positives was observed in broilers (13%). Less samples were reported for pigs with a proportion of positives of 59%.
1.2. Surveillance and monitoring of Campylobacter in the EU
1.2.1. Humans
The notification of campylobacteriosis is mandatory in 21 EU MS, Iceland, Norway and Switzerland. In six MS, the notification is based on a voluntary system (Belgium, France, Greece, Italy, Luxembourg and the Netherlands) and in one country on another, unspecified system (the United Kingdom). Greece started to report campylobacteriosis data in 2018. The surveillance systems for campylobacteriosis cover the whole population in all MS except in four (France, Italy, the Netherlands and Spain). The estimated coverage of the surveillance system is 20% in France and 52% in the Netherlands. These estimated proportions of population coverage were used in the calculation of notification rates for these two MS. No estimates of population coverage in Italy and Spain were provided, so notification rates were not calculated for these two MS.
For 2019, Spain did not receive data from all regions due to COVID‐19, so the number of reported cases was lower than expected. The drop in cases in Luxembourg in 2019 is a surveillance artefact caused by a change to non‐culture methods in private laboratories, resulting in reduced numbers of isolates sent to the national reference laboratory. From March 2020, an electronic laboratory notification system has been in place in Luxembourg and the campylobacteriosis notifications are expected to increase as a result.
All countries reported case‐based data except Belgium, Bulgaria and Greece, which reported aggregated data. Both reporting formats were included to calculate annual numbers of cases and notification rates.
Diagnosis of human infection is generally based on culture from human stool samples and both culture and non‐culture methods (polymerase chain reaction (PCR)) are used for confirmation. Biochemical tests or molecular methods are used for species determination of isolates submitted to the National Public Health Reference Laboratories (NPHRL).
1.2.2. Food and animals
Monitoring of Campylobacter along the food chain is conducted during the primary production stage (farm animals), during harvest/slaughter and processing and at retail stages.
Campylobacter data in the context of Regulation (EC) No 2073/2005
A regulatory limit (microbiological process hygiene criterion (PHC)) for Campylobacter has been set for broiler carcases in Regulation (EC) No 2073/2005 (point 2.1.9 of Chapter 2 of Annex I). The Campylobacter PHC evaluates the counts above 1,000 CFU/g of Campylobacter on neck skins from broiler carcases after chilling, considering a set of 50 (pooled) samples derived from 10 consecutive sampling sessions. This criterion aims to stimulate action to lower the counts of Campylobacter on broiler carcases and to reduce the number of human campylobacteriosis cases due to the consumption or handling of chicken/broiler meat. This PHC has been in force since 1 January 2018. Food business operators (FBOp) shall use the criterion to validate and verify the correct functioning of their food safety management procedures based on HACCP principles and Good Manufacturing Practices (GMPs). FBOp must carry out corrective actions if the criterion target is exceeded. Official samples taken by the Competent Authorities (CA) serve the purpose of auditing the FBOp actions and ensure that the FBOp complies with regulatory requirements. Since 14 December 2019, the Commission Implementing Regulation (EU) 2019/6275 entered into force to harmonise the sampling within official control. Also, reporting of results became mandatory. According to this legislation, the CA has to verify whether the FBOp correctly implements and checks the PHC conducted on broiler carcases by choosing between two approaches: implementing ad hoc official samplings6 or collecting all information on the total number and the number of Campylobacter samples with more than 1,000 CFU/g taken by FBOp in accordance with Article 5 of Regulation (EC) No 2073/2005. These harmonised official control results, which became compulsory to report, will allow better trend watching and trend analyses than before (Table 1).
Official control results from tests for Campylobacter on chilled broiler carcases had the following specified options for the different data elements: sampler: ‘official sampling’ and/or ‘industry sampling’ and ‘HACCP and own check’ (self‐monitoring); sampling context: ‘surveillance, based on Regulation (EC) No 2073/2005’; sampling unit type: ‘single’; sampling strategy: ‘objective sampling’ and sampling stage: ‘slaughterhouse’.
Other monitoring data for food and animals
Campylobacter monitoring data at slaughter from poultry caeca as part of the annual antimicrobial resistance monitoring are collected in a more harmonised way.
Other monitoring data on Campylobacter from food and animals and submitted to EFSA according to Chapter II ‘Monitoring of zoonoses and zoonotic agents’ of the Zoonoses Directive 2003/99/EC are collected without harmonised design. These data have other specified options for the different data elements (including sampling context other than based on Regulation (EC) No 2073/2005) and allow for descriptive summaries at EU level to be made, but they do not support EU‐level trend analyses and trend watching (Table 1).
In 2019, general data on food and animals reported to EFSA by MS and non‐MS derived mainly from official sampling, industry sampling and from HACCP and own checks, in the context of national monitoring and surveillance and/or organised surveys. In addition, for animal data, other reported samples were from clinical investigations by private veterinarians and industry (artificial insemination centres).
The reported occurrence of Campylobacter in the most important food categories for the year 2019 and for the 4‐year period 2015–2018 was descriptively summarised, making a distinction between RTE and non‐RTE food. Data sets were extracted with ‘Objective sampling’ being specified as sampler strategy, which means that the reporting MS collected the samples according to a planned strategy based on the selection of a random sample, which is statistically representative of the population to be analysed.
Detection of Campylobacter in food and animals is generally based on culture and both biochemical and molecular methods (such as PCR) as well as matrix‐assisted laser desorption/ionisation, time‐of‐flight mass spectrometry (MALDI‐TOF MS) are used for confirmation and species identification.
1.2.3. Food‐borne outbreaks of campylobacteriosis
The reporting of food‐borne campylobacteriosis disease outbreaks in humans is mandatory according to the Zoonoses Directive 2003/99/EC.
1.3. Results
1.3.1. Overview of key statistics along the food chain, EU, 2015–2019
Table 3 summarises EU‐level statistics on human campylobacteriosis, and on the occurrence and prevalence of Campylobacter in food and animals, respectively, during 2015–2019. Food data of interest reported were classified into the major categories ‘meat and meat products’ and ‘milk and milk products’ and aggregated by year to obtain an annual overview of the volume of data submitted. The number of sampling units reported for 2019 for ‘meat and meat products’ increased substantially compared with 2018, which is likely due Commission Implementing Regulation (EU) 2019/627 prescribing compulsory reporting of PHC monitoring data (see above).
Table 3.
Summary of Campylobacter statistics related to humans and major food categories, EU, 2015–2019
| 2019 | 2018 | 2017 | 2016 | 2015 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 220,682 | 246,571 | 246,194 | 246,980 | 232,226 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 59.7 | 64.1 | 64.9 | 66.4 | 63.0 | ECDC |
| Number of reporting MS | 28 | 28 | 27 | 27 | 27 | ECDC |
| Infection acquired in the EU | 109,930 | 116,247 | 122,280 | 122,819 | 112,808 | ECDC |
| Infection acquired outside the EU | 6,513 | 7,685 | 6,583 | 5,966 | 6,444 | ECDC |
| Unknown travel status or unknown country of infection | 104,239 | 122,639 | 117,331 | 118,195 | 112,974 | ECDC |
| Number of food‐borne outbreak‐related cases | 1,254 | 2,365 | 3,608 | 4,645 | 1,483 | EFSA |
| Total number of food‐borne outbreaks | 319 | 537 | 395 | 474 | 397 | EFSA |
| Fooda | ||||||
| Meat and meat productsb | ||||||
| Number of sampling units | 58,050 | 26,514 | 21,521 | 18,253 | 16,752 | EFSA |
| Number of reporting MS | 24 | 26 | 22 | 21 | 21 | EFSA |
| Milk and milk productsc | ||||||
| Number of sampling units | 2,749 | 3,227 | 2,317 | 2,062 | 2,273 | EFSA |
| Number of reporting MS | 11 | 13 | 13 | 11 | 10 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member State.
The summary statistics, referring to MS, were obtained by summing all sampling units (single, batch, slaughter batch), sampling stage (farm, packing centre, automatic distribution system for raw milk, processing plant, cutting plant, slaughterhouse, catering, hospital or medical care facility, restaurant or cafe or pub or bar or hotel or catering service, retail, wholesale, unspecified), sampling strategies (census, convenience sampling, objective sampling and unspecified) and sampler (official sampling, official and industry sampling, private sampling, unspecified, not applicable).
Meat and meat products refer to carcases and fresh meat/ready‐to‐eat (RTE), cooked and fermented products.
Milk and milk products refer to raw and pasteurised milk and all dairy products including cheeses.
A more detailed description of the food‐borne outbreaks statistics is in the chapter on food‐borne outbreaks.
When the UK data were collected the UK was an EU MS but as of 31 January 2020 it has become a third country.
1.3.2. Human campylobacteriosis
For 2019, 220,682 confirmed cases of human campylobacteriosis were reported by 28 EU MS, corresponding to an EU notification rate of 59.7 cases per 100,000 population (Table 4). This is a decrease by 6.9% compared with 2018 (64.1 cases per 100,000 population).
Table 4.
Reported human cases of campylobacteriosis and notification rates per 100,000 population in the EU/EFTA, by country and year, 2015–2019
| Country | 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 6,573 | 6,573 | 74.2 | 7,999 | 90.7 | 7,204 | 82.1 | 7,083 | 81.4 | 6,258 | 72.9 |
| Belgium | Y | A | 7,337 | 7,337 | 64.0 | 8,086 | 70.9 | 8,649 | 76.2 | 10,055 | 88.9 | 9,066 | 80.7 |
| Bulgaria | Y | A | 231 | 229 | 3.3 | 191 | 2.7 | 195 | 2.7 | 202 | 2.8 | 227 | 3.2 |
| Croatia | Y | C | 1,732 | 1,722 | 42.2 | 1,965 | 47.9 | 1,686 | 40.6 | 1,524 | 36.4 | 1,393 | 33.0 |
| Cyprus | Y | C | 21 | 21 | 2.4 | 26 | 3.0 | 20 | 2.3 | 21 | 2.5 | 29 | 3.4 |
| Czechia | Y | C | 23,169 | 22,894 | 215.0 | 22,895 | 215.8 | 24,326 | 230.0 | 24,084 | 228.2 | 20,960 | 198.9 |
| Denmark | Y | C | 5,402 | 5,402 | 93.0 | 4,559 | 78.9 | 4,255 | 74.0 | 4,712 | 82.6 | 4,327 | 76.5 |
| Estonia | Y | C | 348 | 347 | 26.2 | 411 | 31.2 | 285 | 21.7 | 298 | 22.6 | 318 | 24.2 |
| Finland | Y | C | 4,382 | 4,382 | 79.4 | 5,099 | 92.5 | 4,289 | 77.9 | 4,637 | 84.5 | 4,588 | 83.8 |
| Franceb | N | C | 7,712 | 7,712 | 57.5 | 7,491 | 56.0 | 6,579 | 49.2 | 6,698 | 50.3 | 6,074 | 45.7 |
| Germany | Y | C | 61,526 | 61,254 | 73.8 | 67,585 | 81.6 | 69,251 | 83.9 | 73,736 | 89.7 | 69,921 | 86.1 |
| Greece | Y | A | 366 | 366 | 3.4 | 357 | 3.3 | . | . | . | . | . | . |
| Hungary | Y | C | 6,441 | 6,400 | 65.5 | 7,117 | 72.8 | 7,807 | 79.7 | 8,556 | 87.0 | 8,342 | 84.6 |
| Ireland | Y | C | 2,776 | 2,776 | 56.6 | 3,044 | 63.0 | 2,779 | 58.1 | 2,511 | 53.1 | 2,453 | 52.4 |
| Italyd | N | C | 1,633 | 1,633 | – | 1,356 | – | 1,060 | – | 1,057 | – | 1,014 | – |
| Latvia | Y | C | 133 | 133 | 6.9 | 87 | 4.5 | 59 | 3.0 | 90 | 4.6 | 74 | 3.7 |
| Lithuania | Y | C | 1,225 | 1,221 | 43.7 | 919 | 32.7 | 990 | 34.8 | 1,225 | 42.4 | 1,186 | 40.6 |
| Luxembourg | Y | C | 271 | 271 | 44.1 | 625 | 103.8 | 613 | 103.8 | 518 | 89.9 | 254 | 45.1 |
| Malta | Y | C | 298 | 278 | 56.3 | 333 | 70.0 | 231 | 50.2 | 212 | 47.1 | 248 | 56.4 |
| Netherlandsc | N | C | 3,415 | 3,415 | 34.1 | 3,091 | 34.6 | 2,890 | 32.5 | 3,383 | 38.3 | 3,778 | 43.0 |
| Poland | Y | C | 715 | 715 | 1.9 | 719 | 1.9 | 874 | 2.3 | 773 | 2.0 | 653 | 1.7 |
| Portugal | Y | C | 942 | 887 | 8.6 | 610 | 5.9 | 596 | 5.8 | 359 | 3.5 | 271 | 2.6 |
| Romania | Y | C | 805 | 805 | 4.1 | 573 | 2.9 | 467 | 2.4 | 517 | 2.6 | 311 | 1.6 |
| Slovakia | Y | C | 7,829 | 7,690 | 141.1 | 8,339 | 153.2 | 6,946 | 127.8 | 7,623 | 140.5 | 6,949 | 128.2 |
| Slovenia | Y | C | 1,085 | 1,085 | 52.1 | 1,305 | 63.1 | 1,408 | 68.2 | 1,642 | 79.5 | 1,328 | 64.4 |
| Spaind , f | N | C | 9,723 | 9,723 | – | 18,411 | – | 18,860 | – | 15,542 | – | 13,227 | – |
| Sweden | Y | C | 6,693 | 6,693 | 65.4 | 8,132 | 80.4 | 10,608 | 106.1 | 11,021 | 111.9 | 9,180 | 94.2 |
| United Kingdom | Y | C | 58,718 | 58,718 | 88.1 | 65,246 | 98.4 | 63,267 | 96.1 | 58,901 | 90.1 | 59,797 | 92.2 |
| EU Total | – | – | 221,501 | 220,682 | 59.7 | 246,571 | 64.1 | 246,194 | 64.9 | 246,980 | 66.4 | 232,226 | 63.0 |
| Iceland | Y | C | 136 | 136 | 38.1 | 145 | 41.6 | 119 | 35.2 | 128 | 38.5 | 119 | 36.2 |
| Norway | Y | C | 4,154 | 4,154 | 78.0 | 3,668 | 69.3 | 3,883 | 73.8 | 2,317 | 44.5 | 2,318 | 44.9 |
| Switzerlande | Y | C | 7,223 | 7,223 | 84.0 | 7,675 | 90.1 | 7,219 | 85.4 | 7,980 | 94.4 | 7,070 | 84.5 |
Y: yes; N: no; A: aggregated data; C: case‐based data.
Sentinel surveillance: notification rates calculated with estimated coverage of 20%.
Sentinel surveillance: notification rates calculated with estimated coverage 52%.
Sentinel surveillance; no information on estimated coverage. So, notification rate cannot be estimated.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
Data not complete in 2019, rate not calculated.
The highest country‐specific notification rates in 2019 were observed in Czechia (215.0 cases per 100,000), Slovakia (141.1), Denmark (93.0) and the United Kingdom (88.1). The lowest rates in 2019 were observed in Bulgaria, Cyprus, Greece, Latvia, Poland, Portugal and Romania (≤ 8.6 per 100,000).
Most (94.4%) of the campylobacteriosis cases reported with known origin were infected in the EU (Table 3). The highest proportions of domestic cases (> 97%) were reported in Czechia, Hungary, Latvia, Malta, Poland, Portugal, Romania and Slovakia. The highest proportions of travel‐associated cases were reported by the Nordic countries: Finland (77.8%), Denmark (44.1%), Sweden (56.3%), Iceland (57.0%) and Norway (54.8%). Among 14,501 travel‐associated cases with known country of infection in the MS, almost half of the cases (48.1%) were linked to travel within the EU, with most of the cases having acquired infections in Spain, Greece and Italy (13.9%, 4.1% and 3.6%, respectively). Turkey, Thailand and Morocco were the most often reported probable countries of infection outside the EU (8.2%, 7.8% and 4.9%, respectively).
Between 2015 and 2019, there was a clear seasonality in the number of confirmed campylobacteriosis cases reported in the EU/EEA, with peaks in the summer months. Annual winter peaks, albeit with lower numbers compared with summer, were also observed in January annually from 2012 to 2019. The EU/EEA trend was stable (flat) during 2015–2019 (Figure 2).
Figure 2.

Trend in reported confirmed human cases of campylobacteriosis in the EU/EEA, by month, 2015–2019
- Source(s): Austria, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Sweden and the United Kingdom. Belgium, Bulgaria, Croatia, Greece, Portugal and Spain did not report data to the level of detail required for the analysis.
Hungary was the only MS reporting decreasing (p < 0.01) trend, in the period 2015–2019. Four MS (Italy, Latvia, Portugal and Romania) reported increasing trends in the same time period.
Information on hospitalisation status was provided for 29.1% of all campylobacteriosis cases by 16 MS in 2019. Of cases with known hospitalisation status, 31.8% were hospitalised. The highest hospitalisation rates were reported in Cyprus, Latvia, Lithuania, Poland, Romania and the United Kingdom, where most reported cases were hospitalised.
The outcome was reported for 78.0% of all cases by 17 MS. Forty‐seven deaths due to campylobacteriosis were reported in 2019, resulting in an EU case fatality of 0.03%. This was similar to the average percentage of fatal outcome observed over the last 5 years.
Campylobacter species information was provided by 24 MS for 55.2% of confirmed cases reported in the EU, which was at the same level as in 2018. Of these, 83.1% were Campylobacter jejuni, 10.8% Campylobacter coli, 0.1% Campylobacter lari, 0.1% Campylobacter fetus and 0.1% Campylobacter upsaliensis. ‘Other’ Campylobacter species accounted for 5.8%, but the large majority of those cases were reported at the national level as ‘C. jejuni/C. coli/C. lari not differentiated’.
Human campylobacteriosis cases and cases associated with food‐borne outbreaks
Overall, for the year 2019, 94.5% of the number of reported human campylobacteriosis cases who acquired the infection in the EU (109,930; Table 3) were domestic (acquired within the home country) infections and 5.5% were acquired through travel in EU.
Campylobacter was the third most frequently reported causative agent for food‐borne outbreaks at the EU level, by 18 MS, with 319 outbreaks communicated to EFSA, 1,254 cases of illness, 125 hospitalisations and no deaths. Comparing the food‐borne outbreak cases (1,254), reported to EFSA, and cases of human campylobacteriosis acquired in the EU (109,930) considering also the proportion of unknown travel data (0.944 × 104,239) (Table 3), reported to ECDC, could suggest that overall in the EU, in 2019, only 0.6% of human campylobacteriosis cases would be reported through food‐borne outbreaks investigation. It is important to clarify that the case classification for reporting is different between these two databases. In TESSy, the cases reported are classified based on the EU case definition. All these cases visited a doctor and are either confirmed by a laboratory test (confirmed case) or not (probable case and classification is based on the clinical symptoms and epidemiological link). Cases that never visited a doctor are not reported to TESSy. Moreover, there may be missing probable cases in TESSy, as these data are not analysed or published and there is no incentive for reporting such cases. Information on which cases are linked to an outbreak and which not is also not systematically collected. In practice, the cases reported to TESSy are considered to be mostly sporadic cases. In food‐borne outbreaks, the human cases are the people involved in the outbreak as defined by the investigators (case definition), and cases must be linked, or probably linked, to the same food source (Directive 2003/99/EC). This can include both ill people (whether confirmed microbiologically or not) and people with confirmed asymptomatic infections (EFSA, 2014). Cases can be classified as confirmed or probable outbreak cases, but currently these specific classification data are not collected by EFSA.
C. jejuni and C. coli were identified in 72 and 7 outbreaks, respectively. However, most campylobacteriosis food‐borne outbreaks were reported without speciation information (240 outbreaks: 75.2%). Eighteen campylobacteriosis outbreaks were reported with strong‐evidence and 301 with weak evidence. Of the former outbreaks, eight were caused by broiler meat and three by milk. During 2010–2018, these were also the food vehicles causing most strong‐evidence campylobacteriosis food‐borne outbreaks. Further details and statistics on the campylobacteriosis food‐borne outbreaks for 2019 are in the food‐borne outbreaks chapter.
1.3.3. Campylobacter in food
Campylobacter data in the context of Regulation (EC) No 2073/2005
In total, seven MS (Bulgaria, Croatia, Cyprus, Estonia, Latvia, Romania and Spain) reported 2019 ad hoc official sampling results collected in the context of the Campylobacter PHC, which are quantitative data relating to neck skins from broiler carcases sampled at slaughterhouses. Of the 3,346 neck skin samples from chilled broiler carcases, 1,365 (41%) tested positive and 506 (15%) exceeded the limit of 1,000 CFU/g. However, the MS‐specific percentage of quantified results exceeding that limit varied widely and ranged from zero to 34%.
Seven MS (Denmark, Estonia, Germany, Ireland, Latvia, Romania and Sweden) reported 2019 Campylobacter PHC monitoring results collected from the FBOp. Of the 15,323 neck skin samples from chilled broiler carcases, 2,038 (13%) tested positive and 1,033 (7%) exceeded the limit of 1,000 CFU/g. The MS‐specific percentage of quantified results exceeding that limit varied from zero to 14%.
Other food monitoring data
Table 5 summarises the reported occurrence of Campylobacter in the most important food categories for the year 2019 and for the 4‐year period 2015–2018. Distinction is made between RTE, and non‐RTE food, and fresh meat.
Table 5.
Occurrence of Campylobacter in major food categories, EU
| Food | 2019 | 2015–2018 | ||||
|---|---|---|---|---|---|---|
| N reporting MS | N sampling units | Positive N (%) | N reporting MS | N sampling units | Positive N (%) | |
| RTE food | ||||||
| All | 8 | 3,691 | 6 (0.16) | 15 | 7,272 | 36 (0.50) |
| Meat and meat products | 6 | 328 | 0 | 9 | 1,040 | 27 (2.60) |
| Meat and meat products from broilers | 1 | 18 | 0 | 3 | 117 | 22 (18.80) |
| Milk and milk products | 6 | 821 | 2 (0.24) | 11 | 2,258 | 8 (0.35) |
| Milk | 5 | 204 | 2 (0.98) | 6 | 675 | 6 (0.89) |
| Raw milka | 4 | 185 | 2 (1.08) | 5 | 652 | 6 (0.92) |
| Cheese | 4 | 615 | 0 | 7 | 1,566 | 2 (0.13) |
| Dairy products excluding cheeses (butter, cream, ice cream, whey, yoghurt and fermented dairy products) | 2 | 3 | 0 | 4 | 71 | 0 |
| Fruits, vegetables and juices | 2 | 1,008 | 2 (0.20) | 4 | 1,119 | 1 (0.09) |
| Salads | 5 | 309 | 1 (0.32) | 2 | 30 | 0 |
| Other processed food products and prepared dishes | 4 | 1,002 | 1 (0.1) | 7 | 2,564 | 0 |
| Non‐RTE food | ||||||
| All | 16 | 26,687 | 5,504 (20.62) | 20 | 54,295 | 13,892 (25.59) |
| Meat and meat products | 15 | 23,837 | 5,475 (22.97) | 20 | 49,959 | 13,817 (27.66) |
| Fresh meat from broilers | 12 | 8,325 | 2,464 (29.60) | 19 | 31,665 | 12,210 (38.56) |
| Fresh meat from turkeys | 6 | 336 | 111 (33.04) | 8 | 3,384 | 824 (24.35) |
| Fresh meat from pigs | 3 | 135 | 6 (4.44) | 9 | 3,459 | 503 (14.54) |
| Fresh meat from bovine animals | 5 | 374 | 7 (1.87) | 9 | 3,959 | 468 (11.82) |
| Other fresh meat | 8 | 12,614 | 2,468 (19.57) | 12 | 4,130 | 668 (16.17) |
| Milk and milk products | 5 | 884 | 18 (2.04) | 9 | 1,552 | 39 (2.51) |
| Fruits, vegetables and juices | 5 | 512 | 1 (0.20) | 7 | 1,803 | 3 (0.17) |
| Other food | 6 | 1,454 | 10 (0.69) | 8 | 981 | 33 (3.36) |
The raw RTE milk sampling units are a subset of the RTE milk.
The proportion of Campylobacter‐positive samples within the RTE and non‐RTE categories was 0.2% and 20.6% respectively.
For 2019, most results from the 3,691 RTE food sampling units reported by eight MS originated from ‘fruits, vegetables and juices’ (27.3%), followed by ‘other processed food products and prepared dishes’ (27.1%), ‘milk and milk products’ (22.2%) and ‘meat and meat products’ (8.9%). In total, Campylobacter was detected in six RTE food samples: two from raw milk, two from ‘fruits, vegetables and juices’, one from salads and one from ‘other processed food products and prepared dishes’. During 2015–2018, in the RTE food category, 27 Campylobacter‐positive sampling units were reported from ‘meat and meat products’, in particular from broiler meat and broiler meat products, six from raw milk, two from cheeses and one from ‘fruits, vegetables and juices’.
Results reported by 16 MS for non‐RTE food show that ‘meat and meat products’ was the most contaminated food category as compared with ‘milk and milk products’ and ‘fruits, vegetables and juices’, in 2019. This was also the case for the years 2015–2018. Fifteen MS reported for 2019 results for fresh meat categories and all had some positive samples but the percentages of Campylobacter‐positive sampling units for fresh meat from broilers and turkeys were very high. This was also the case for the years 2015–2018.
1.3.4. Campylobacter in animals
In 2019, in total, 16 MS and four non‐MS reported monitoring data on Campylobacter in animals. Most samples originated from broilers and from bovine animals, and all proportions (%) of positive sampling units are displayed in Table 6.
Table 6.
Summary of Campylobacter statistics related to major animal species, reporting MS and non‐MS, EU, 2019
| N reporting MS/non‐MS | N tested unitsa, EU | Proportion (%) of positive sampling units, EU | |
|---|---|---|---|
| Animals | |||
| Broilers | 5/2 | 10,196 | 13.27 |
| Turkeys | 0/1 | – | – |
| Pigs | 7/1 | 1,125 | 58.58 |
| Bovine animalsb | 6/0 | 3,493 | 9.28 |
| Cats and dogs | 5/2 | 1,373 | 6.85 |
| Other animalsc | 7/3 | 3,024 | 12.63 |
MS: Member State.
The summary statistics were obtained summing all sampling units (single samples, batch samples, animals, slaughter animal batches and herds or flocks).
‘Artificial insemination stations’ in ‘sampling stage’ was not included in the count of the units tested.
Antelopes, badgers, birds, bison, budgerigars, canary, Cantabrian chamois, chinchillas, deer, dolphin, ferrets, foxes, geese, goats, guinea pigs, hamsters, hares, hedgehogs, lion, lynx, marten, minks, monkeys, night herons, oscine birds, other animals, parrots, peafowl, pheasants, pigeons, rabbits, raccoons, ratites (ostrich, emu, nandu), rats, reindeers, reptiles, rodents, sheep, snakes, domestic solipeds, Steinbock, turtles, water buffalos, wild boars, wild ducks, wolves and zoo animals.
1.4. Discussion
Campylobacteriosis has been the most commonly reported zoonosis in humans in the EU since 2005. Despite comprehensive surveillance and national coverage in most MS, reported cases represent only a small proportion of Campylobacter infections occurring in the EU (Teunis et al., 2013). There has been a significantly increasing trend in the number of cases at the EU level and at country level in half of the MS between 2009 and 2018. In the last 5 years from 2015 to 2019, the EU trend of confirmed cases has stabilised. In 2019, in two‐thirds of the MS, the number of confirmed campylobacteriosis cases decreased and the EU notification rate decreased by 6.9% compared with the rate in 2018. Despite this reduction, only one MS had a significant decreasing trend in the last 5 years. Four MS reported increasing trends, whereas most MS had stable, flat trends in 2015–2019. One MS notified that the reported number of campylobacteriosis cases is lower than expected as data were not received from all regions due to the COVID‐19 situation in 2020. It is not clear if, and to what extent, the pandemic situation had an effect on the decrease of notifications noted in several MS in 2019. In previous years, there has been a steady annual increase in reported cases in several countries. This may not only reflect changes in exposure but also improvements in surveillance systems, a better coverage of routine diagnostics across the country, requirement for medical laboratories to report positive test results and better knowledge and awareness among physicians. Almost half of the MS reported having the capacity to perform whole genome sequencing (WGS) on Campylobacter isolates (ECDC survey, 2020, data not published).
Campylobacter has a characteristic seasonality with a sharp increase of cases in the summer. Campylobacter tends to be more prevalent in humans during warmer seasons of the year; however, a smaller but distinct winter peak has become apparent in the past 8 years in the EU, including in 2019. Disease onsets of cases that were notified during winter peaks occurred predominantly in the three‐first calendar weeks of the year. This points towards exposures around Christmas and New Year. Winter peaks have been observed in Austria, Belgium, Finland, Germany, Luxembourg, the Netherlands, Switzerland and Sweden. Increased travel during the holiday season might be another explanation for the increase in many countries. In some countries with an observed winter peak, the consumption of meat fondue or table‐top grilling is popular during the festive season and could promote Campylobacter transmission (Bless et al., 2017).
In the EU, over 20,000 campylobacteriosis cases were hospitalised in 2019. This is the highest number of hospitalisations compared with all other food‐borne infections. The proportion of hospitalised campylobacteriosis cases was higher than expected in some MS, where all or most of the confirmed cases were hospitalised. These MS also reported the lowest notification rates, indicating that the surveillance is focusing mainly on hospitalised, i.e. severe cases. Hospitalisation status is ascertained and reported by hospitals, while for cases reported from other sources, e.g. laboratories, hospitalisation status is often missing. This can result in an overestimation of the proportion of hospitalised cases in some countries.
Broiler meat is considered the main source of human campylobacteriosis (EFSA BIOHAZ Panel, 2010). In 2011, EFSA published an opinion on ‘Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain’ (EFSA BIOHAZ Panel, 2011), which suggested the introduction of a microbiological criterion for Campylobacter on broiler carcases at the slaughterhouse. EFSA estimated that the public health risk from Campylobacter could be reduced by > 50% if no batches would exceed a critical limit of 1,000 CFU/g on neck and breast skin. This process hygiene criterion (PHC) has been in force for food business operators since 1 January 2018. Moreover, a 2012 EFSA opinion on the public health hazards to be covered by inspection of poultry meat identified the need to address Campylobacter as a high priority (EFSA BIOHAZ, CONTAM and AHAW Panels, 2012). In line with the high priority set by this EFSA opinion on poultry meat inspection, competent authorities ought to sample themselves for Campylobacter or carefully verify the implementation of the process hygiene criterion by the operator. Official samples taken by the competent authorities serve the purpose of auditing the food business operators’ actions and ensure that the food business operators comply with regulatory requirements. Since 14 December 2019, the Commission Implementing Regulation (EU) 2019/627 entered into force to harmonise the sampling within official control. Also, reporting of results became mandatory. Seven MS reported 2019 official control monitoring data from neck skin samples from chilled broiler carcases collected in the context of the Campylobacter PHC. Overall, one in six samples exceeded the limit of 1,000 CFU/g. Six MS reported such monitoring data based on sampling results collected from the food business operators and these data showed that one in 14 samples exceeded the limit of 1,000 CFU/g. Better populated EU summary tables with more complete data sets from all MS will in future allow better trend watching and trend analyses.
Other monitoring data on Campylobacter from food were submitted to EFSA according to Chapter II ‘Monitoring of zoonoses and zoonotic agents’ of the Zoonoses Directive 2003/99/EC. These data are collected without harmonised design between the MS. Eight MS reported monitoring data for RTE food and overall a few Campylobacter‐positive units were detected; in raw milk, ‘fruits, vegetables and juices’, salads and ‘other processed food products and prepared dishes’. Monitoring data considered were collected according to an ‘objective’ sampling strategy. Also considering the fact that for certain food categories, such as RTE milk, the overall sampling effort was small (five MS reporting 204 sample results) the finding of Campylobacter‐contaminated RTE food is of concern because it poses a direct risk to the consumer. No Campylobacter‐positive RTE meat and meat products were reported for 2019; however, the overall sampling effort was small (six MS, 328 sampling units). During 2015–2018, one in 40 RTE meat and meat products sampling units was reported positive, and for RTE meat and meat products from broilers, one in five was positive, albeit based on a small sample size (three MS, 117 samples). Quantitative data (counts) of Campylobacter are currently only collected in the context of the aforementioned PHC. Monitoring data for non‐RTE ‘meat and meat products’ showed that one in five samples were positive, for ‘milk and milk products’ one in 50 and for ‘fruits, vegetables and juices’ one in 500. Fifteen MS reported results for fresh meat categories and the overall percentage of Campylobacter‐positive sampling units for fresh meat from broilers and turkeys were very high, 32.10% and 33.04%, respectively.
In 2020, EFSA experts updated the 2011 scientific opinion (EFSA BIOHAZ Panel, 2011) using more recent scientific data and reviewed on‐farm control options for Campylobacter in broilers (EFSA BIOHAZ Panel, 2020a). The relative risk reduction in EU human campylobacteriosis attributable to broiler meat was estimated for on‐farm control options using population attributable fractions for interventions that reduce Campylobacter flock prevalence, updating the modelling approach for interventions that reduce caecal concentrations and reviewing scientific literature. The updated model resulted in lower estimates of impact of interventions (control options) than the model used in the 2011 opinion. A 3‐log10 reduction in broiler caecal concentrations was estimated to reduce the relative EU risk of human campylobacteriosis attributable to broiler meat by 58% compared with an estimate larger than 90% in the previous opinion.
1.5. Related projects and Internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Fact sheet on Campylobacter | https://www.cdc.gov/foodsafety/diseases/campylobacter/index.html |
| ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx | |
| EU case definition of campylobacteriosis | https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions | |
| Disease Programme on Emerging, Food‐ and Vector‐Borne Diseases | https://www.ecdc.europa.eu/en/about-us/who-we-are/units/disease-programmes-unit | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| World Health Organization – Campylobacter fact sheet | https://www.who.int/news-room/fact-sheets/detail/campylobacter | |
| Food and animals | European Union Reference Laboratory (EURL) for Campylobacter | http://www.sva.se/en/service-and-products/eurl-campylobacter |
| EFSA Scientific Opinion of the Panel on Biological Hazards (BIOHAZ) ‐ Quantification of the risk posed by broiler meat to human campylobacteriosis in the EU | http://www.efsa.europa.eu/en/efsajournal/pub/1437 | |
| EFSA Scientific Opinion of the Panel on Biological Hazards (BIOHAZ) ‐ Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain | https://www.efsa.europa.eu/en/efsajournal/pub/2105 | |
| EFSA Scientific Opinion of the Panel on Biological Hazards (BIOHAZ) ‐ Update and review of control options for Campylobacter in broilers at primary production | https://www.efsa.europa.eu/en/efsajournal/pub/6090 | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
2. Salmonella
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
2.1. Key facts
Salmonellosis is the second most commonly reported gastrointestinal infection in humans after campylobacteriosis, and an important cause of food‐borne outbreaks in the EU/EEA.
In 2019, 87,923 confirmed cases of salmonellosis in humans were reported with an EU notification rate of 20.0 cases per 100,000 population, which was at the same level as in 2018.
The trend for salmonellosis in humans has been stable (flat) over the last 5 years after a long period of a declining trend.
The trend of S. Enteritidis cases in humans acquired in the EU has stabilised in 2015–2019.
In total, 926 salmonellosis food‐borne outbreaks were reported by 23 EU MS in 2019, causing 9,169 illnesses, 1,915 hospitalisations (50.5% of all outbreak‐related hospitalisations) and seven deaths. Salmonella caused 17.9% of all food‐borne outbreaks during 2019. The vast majority (72.4%) of the salmonellosis food‐borne outbreaks were caused by S. Enteritidis. The four most implicated food vehicles in strong‐evidence salmonellosis food‐borne outbreaks were ‘eggs and egg products’, followed by ‘bakery products’, ‘pig meat and products thereof’ and ‘mixed food’, as in previous years.
Official control samples verifying compliance with food safety criteria according to Regulation (EC) No 2073/2005 found the highest percentages of Salmonella‐positive samples in poultry meat, including fresh meat (3.5%), minced meat and meat preparations intended to be eaten cooked (8.3%) and meat products intended to be eaten cooked (6.4%).
For 2019, 66,113 ‘ready‐to-eat’ and 191,181 and ‘non ready‐to-eat’ food sampling units were reported from 21 and 25 MS with 0.3% and 1.5% positive samples, respectively. Within the category of ‘ready‐to-eat’ food samples, positive samples were from divers food products; ‘meat and meat products’, ‘milk and milk products’, ‘fruits, vegetables and juices’, ‘fish and fishery products’, ‘spices and herbs’, ‘salads’, ‘other processed food products and prepared dishes’, ‘cereals and nuts’, ‘infant formulae and follow‐on formulae’, ‘other food’ and ‘cocoa and cocoa preparations, coffee and tea’. Within the category of ‘non ready‐to-eat’ food samples, positive samples originated also from divers food products and were mostly from ‘meat and meat products’, notably from fresh meat from broilers and from turkeys.
Significantly lower percentages of Salmonella‐positive pig carcases were reported, based on food business operators self‐monitoring data, compared with official control data from the competent authorities. The same observations were made for 2018 and 2017 data.
Eighteen of the 26 Member States reporting on Salmonella control programmes in poultry populations met all the reduction targets, compared to 14 in 2018. The number of MS that did not meet the Salmonella reduction targets was five in breeding flocks of Gallus gallus, four in laying hen flocks, one in broilers flocks, zero in flocks with breeding turkeys and one in fattening turkey flocks.
Among the target Salmonella serovars in the context of national control programmes in poultry, the reported flock prevalence was highest for S. Enteritidis in breeding flocks of Gallus gallus and laying hens. For broilers, the flock prevalence of S. Enteritidis and of S. Typhimurium were comparable, whereas for turkeys (both breeding and fattening flocks), the flock prevalence of S. Typhimurium was highest.
In the context of national control programmes in poultry, proportions of Salmonella target serovars‐positive broiler and fattening turkey flocks reported by food business operators was significantly lower than those reported by competent authorities.
A significant increase was noted in estimated Salmonella prevalence in breeding flocks of Gallus gallus, laying hens and breeding turkeys over the last 4–6 years. The trends in the prevalence of Salmonella target serovar‐positive flocks were, in contrast, quite stable (flat) since 2015 for all animal categories, with some fluctuations for breeding turkey flocks.
Of all serotyped Salmonella isolates reported by MS from food and animal sources, 70% originated from the broiler source, 12% from the pig source, while the laying hen and turkey sources accounted each for about 7% and isolates from the cattle source made up about 1%. The top five serovars responsible for human infections were distributed as follows among the serotyped isolates (17,176) from these food–animal sources: S. Infantis accounted for 29.7% of them, S. Enteritidis 6.9%, monophasic variant of S. Typhimurium 4.5%, S. Typhimurium 3.9% and S. Derby 3.7%.
Figure 3 summarises the main data reported in the Salmonella chapter and the major findings. It is a ‘graphical abstract’ presenting a global overview of the data mentioned in the Key facts section.
Figure 3.
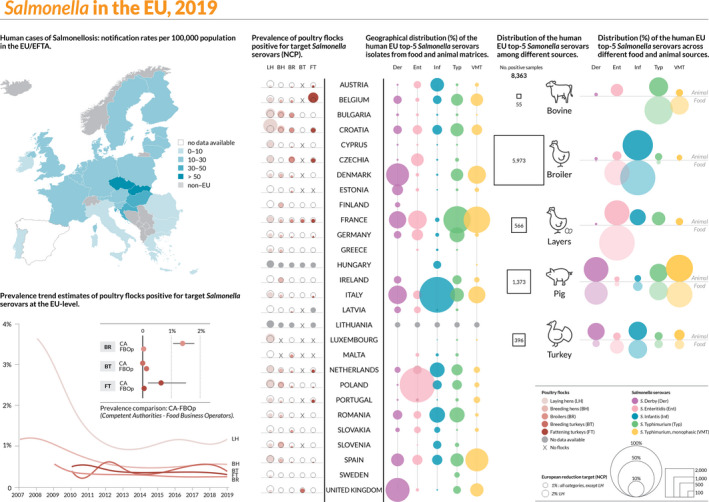
Salmonella summary infographic, EU/EFTA, 2019
- On the left side of the infographic are shown: (a) Map of the salmonellosis notification rates per 100,000 population in the EU/EFTA; (b) the single Member States’ prevalence in the context of national control programmes (NCP) in poultry compared with the European reduction target for laying hens (2%) and other poultry populations (1%); (c) the trends of the prevalence of poultry flocks positive for Salmonella target serovars in the context of NCP; (d) the comparisons between the results of the competent authorities (CA) and food business operators (FBOp) data in the context of the NCP; on the right side; (e) the distribution of the human top five Salmonella serovars coming from serotyped isolates from food and animal matrices reported by reporting MS, and (f) the distribution of human top five Salmonella serovars isolates according to different food and animal matrices.
2.2. Surveillance and monitoring of Salmonella in the EU
2.2.1. Humans
The notification of non‐typhoidal salmonellosis in humans is mandatory in 22 MS, Iceland, Norway and Switzerland, whereas in five MS reporting is based on a voluntary system (Belgium, France, Luxembourg and the Netherlands) or other systems (the United Kingdom). In the United Kingdom, although the reporting of food poisoning is mandatory, isolation and species identification of the causative organism is voluntary. The surveillance systems for salmonellosis cover the whole population in all MS except in France, the Netherlands and Spain. The estimated coverage of the surveillance system is 48% in France and 64% in the Netherlands. These proportions of populations were used in the calculation of country‐specific and EU‐level notification rates. No estimation for population coverage in Spain was provided, so the notification rate was not calculated. For 2019, Spain did not receive data from all regions that are normally reporting due to COVID‐19, and therefore, the case numbers are lower than expected. All countries reported case‐based data except Bulgaria, which reported aggregated data. Both reporting formats were included to calculate annual numbers of cases and notification rates.
Diagnosis of human Salmonella infections is generally carried out by culture from human stool samples. All countries, except Bulgaria perform serotyping of isolates.
2.2.2. Food, animals and feed
Monitoring of Salmonella along the food chain is conducted during preharvest (farm animals and their feed), processing (slaughterhouses and cutting plants) and post‐processing (wholesale, retail and catering) stages.
Salmonella monitoring data in the context of Regulation (EC) No 2073/2005
Regulatory limits (microbiological criteria) for Salmonella have been set for food specified in Regulation (EC) No 2073/2005 (Figure 4), which lays down Salmonella food safety criteria (FSC) and Salmonella PHC. Compliance with these criteria ought to be legally verified by the individual food business operator in the context of their own HACCP programmes, through self‐monitoring when implementing the general and specific hygiene measures of Regulation (EC) No 852/2002. Respect of the criteria should be guaranteed by the FBOp by preventive approaches (e.g. implementing good hygiene practices, GMPs and the application of risk management procedures based on HACCP). The collection of these data is not fully harmonised across MS, because the sampling objectives, the place of sampling and the applied sampling frequency vary or are interpreted differently between MS.
Figure 4.

The surveillance and monitoring of Salmonella in food, food‐producing animals and feed according to the sampling stage, the sampler, the objective of the sampling, the quality of data and the degree of harmonisation
The competent authority (CA), through official sampling or oversight of data, ensures that the food business operator (FBOp) complies with the regulatory requirements.
The Salmonella FSC prescribe that Salmonella is not detected in 25 or 10 g of different products (from 5 to 30 sampling units for the specified food categories) when they are on the market, during their shelf‐life. Moreover, according to Regulation (EC) No 1086/2011, in fresh poultry meat, the FSC prescribes that target serovars for poultry populations (S. Enteritidis and S. Typhimurium including monophasic S. Typhimurium) are ‘not detected in 25 g’. Salmonella PHC are regulated for carcases of pigs, cattle, sheep, goats, horses, broilers and turkeys, and evaluate the presence of Salmonella on a specific area of a tested carcass, or on a pooled sample of neck skin from broilers and turkeys, considering a set of 50 samples derived from 10 consecutive sampling sessions. Salmonella isolates collected from broilers and turkeys must be serotyped for the identification of S. Enteritidis and S. Typhimurium. Since 14 December 2019, the Commission Implementing Regulation (EU) 2019/6275 entered into force to harmonise the sampling within official control. Also, reporting of results became mandatory. According to this legislation, the CA has to verify whether the FBOp correctly implements and checks the PHC conducted on carcases (points 2.1.3, 2.1.4 and 2.1.5 of Chapter 2 of Annex I of Regulation (EC) No 2073/2005) by choosing between different approaches: implementing ad hoc official samplings6 and/or collecting all information on Salmonella‐positive samples from own checks by the FBOp and/or collecting information on Salmonella‐positive samples as part of national control programmes in the MS with special guarantees (Regulation (EC) No 853/2004). These harmonised official control results, which became compulsory to report, will allow better trend watching and trend analyses than before (Table 1).
Official control results from Salmonella had the following specified options for the different data elements; sampler: ‘official sampling’, except for pig carcases for which the sampler has to be labelled as ‘official, based on Regulation No 854/2004’ and/or ‘industry sampling’ and ‘HACCP and own check’ (self‐monitoring), for the PHC; sampling context: ‘surveillance, based on Regulation (EC) No 2073/2005’; sampling unit type: ‘single’; sampling strategy: ‘objective sampling’; and sampling stage: sampling units collected at the processing phase (e.g. slaughterhouse and cutting plant), or at the retail stage, identified as ‘catering’, ‘hospital or medical care facility’, ‘restaurant or cafe or pub or bar or hotel or catering service’ and ‘wholesale’.
Monitoring data for compliance with the Salmonella national control programmes in poultry
According to EU Regulation (EC) No 2160/2003 and its following amendments, MS have to set up Salmonella national control programmes (NCP) aimed at reducing the prevalence of Salmonella serovars that are considered relevant for public health (from this point forward termed target serovars), in certain animal populations. An overview of NCP for the poultry populations, relative targets to reach and serovars to be targeted is shown in Table 7.
Table 7.
Salmonella national control programmes in place in the poultry populations, targets to reach and reference legislation, EU
| Population | Maximum annual percentage (%) of flocks remaining positive | Target serovars | Legislation | Trade restrictions |
|---|---|---|---|---|
| Adult breeding hens (Gallus gallus) | 1 |
S. Enteritidis S. Typhimurium (including monophasic variants) S. Infantis S. Virchow S. Hadar |
Regulation (EC) No 200/2010 | Destruction or safe disposal of (hatching) eggs and birds (Annex II C of Regulation (EC) No 2160/2003 |
| Adult laying hens (Gallus gallus) | 2 | S. Enteritidis, S. Typhimurium (including monophasic variant) | Regulation (EC) No 517/2011 | Destruction or safe disposal of hens, marketing of eggs as class B (only for heat treated egg products) (Annex II D of Regulation (EC) No 2160/2003) |
| Broilers (Gallus gallus) | 1 | Regulation (EC) No 200/2012 | Absence in 25 g of fresh meat (point 1.28 of Annex I to Regulation (EC) No 2073/2005 | |
| Adult breeding turkeys (Meleagris gallopavo) | 1 | Regulation (EC) No 1190/2012 | Destruction or safe disposal of (hatching) eggs and birds (Annex II C of Regulation (EC) No 2160/2003) | |
| Fattening turkeys (Meleagris gallopavo) | 1 | Regulation (EC) No 1190/2012 | Absence in 25 g of fresh meat (point 1.28 of annex I to Regulation (EC) No 2073/2005) |
It is compulsory for MS to annually report results for Salmonella NCP and, in addition for broiler flocks and breeding and fattening turkey flocks, it is compulsory to report separate results for samplings conducted by CA and by FBOp. These NCP data allow data analyses such as assessing spatial and temporal trends at the EU level. They also allow for descriptive summaries at the EU level to be made and allow EU trends to be monitored (Table 1).
Other monitoring data for foods, animals and feed
Food, animal and feed monitoring data other from those described above are not collected in a harmonised way, because there are no requirements for sampling strategies, sampling methods, analytical tests or reporting. Still, the CA needs to report those according to Directive 2003/99/EC on the monitoring of zoonoses at the most appropriate stage of the food chain. The rationale for surveillance and monitoring of Salmonella in food‐producing animals, feed and food at different stages along the food chain is reported in Figure 4. There are also no harmonised rules for reporting these data. These data are summarised only and do not serve the purpose of trend watching or trend analyses (Table 1).
The reported occurrence of Salmonella in the most important food categories for the year 2019 and for the 4‐year period 2015–2018 was descriptively summarised making a distinction between RTE and non‐RTE food. Data sets were extracted with ‘objective sampling’ being specified as sampler strategy, which means that the reporting MS collected the samples according a planned strategy based on the selection of a random sample, which is statistically representative of the population to be analysed.
Reported Salmonella serovar data are also viewed as part of this category. MS are obliged to report the target serovars as part of the NCP in poultry populations, whereas for the other animal populations, serotyping is not mandatory and if it is performed, reporting of the serovar data is not mandatory either. Also, for the food sector, the FSC are the absence of Salmonella, except for fresh poultry meat, for which the criterion is limited to the absence of the target serovars. Therefore, some MS may decide to not report the presence of non‐target serovars, which would lead to a possible reporting bias for target serovars in poultry populations and for fresh poultry meat. Hence, the compulsory reporting of target serovars in the context of NCP and in the context of the FSC for fresh poultry meat guarantees the consistency of such data over many years and among MS but could result in an overestimation of these target serovars compared with the other serovars. For the remaining matrices, the serovar data collected could be strongly biased by what each MS serotyped and reported. Also, in this context, detection of Salmonella serovars other than those covered by the reduction targets does not in any way equate with a ‘Salmonella free’ finding.
2.2.3. Food‐borne outbreaks of salmonellosis
The reporting of food‐borne salmonellosis disease outbreaks in humans is mandatory according Zoonoses Directive 2003/99/EC.
2.3. Data analyses
2.3.1. Comparison between Competent Authority and Food Business Operator sampling results
Comparison of Salmonella results from CA and FBOp in the context of NCP for those programmes requiring separate reporting (NCP for broilers, fattening turkeys and breeding turkeys) as well as Salmonella PHC monitoring data from carcases (pigs), was carried out. The significance of differences was verified by the one‐tailed Fisher's exact probability test, in cases in which the expected values in any of the cells of a contingency table were below 5; otherwise the z‐statistic one‐tailed test was calculated. A p‐value < 0.107 was considered significant to consider every possible evidence of differences between FBOp and CA. Differences in official control sampling results by CA and self‐monitoring results by FBOp were expressed by exact binomial confidence interval (95% level).
R software (www.r-project.org) was used to conduct the above‐mentioned analyses.
2.3.2. Statistical trend analyses (methods) of poultry monitoring data
Statistical trend analyses were carried out with the objectives of evaluating the significance of temporal variations in the EU‐level flock prevalence of Salmonella and target Salmonella serovars in poultry since the start of the implementation of the NCP.
The tested flocks were either positive or negative for target serovars and Salmonella, and so the status of the flocks is a dichotomous outcome variable. Therefore, the binomial probability distribution for the response variable was assumed and the logit link function was computed in the model for the trend analysis. The logit is defined as the logarithm of p/(1 – p), where p/(1 – p) is the odds of being positive for Salmonella.
According to the temporal flock prevalence trends in the MS, polynomial or B‐spline basic models (in case of a supposed high degree of polynomial trend) for the logit of the probability of flocks being positive were fitted for the different poultry categories over the entire period of NCP implementation. Moreover, attention has been paid to the period after the achievement of the minimum prevalence reported to date, to capture any evidence of a significant increase in Salmonella prevalence. Marginal and conditional generalised linear models for repeated measures were used to perform these trend analyses. Details about the estimated parameters of the models, odds ratios, prevalence and graphical analyses (conditional and marginal) are reported in the supporting information to this report.
To investigate the EU‐level prevalence considering the relevant heterogeneity among MS for flock prevalence of Salmonella and target serovars over time, the results obtained using the conditional generalised mixed model for longitudinal binary data were summarised and discussed in the report, for all poultry categories covered by the NCP. To take account of the different levels (baselines) of risk of MS having positive flocks, but similar patterns over time, a random MS‐specific intercept effect was included in the model. To consider the trend over time, the variable ‘time’ was included in the model as a fixed effect. The correlation among repeated observations in the same MS in subsequent years was considered using a first autoregressive or exchangeable structure of the correlation matrix for the residuals. To evaluate the significance of the overall effect of fixed factors specified in the model, Type III F‐tests were applied, whereas the receiver operating characteristic (ROC) curve was used to assess the goodness of fit of the model. A p‐value < 0.10 was considered to be significant for both random and fixed effects.
GLIMMIX and SGPLOT procedures in SAS 9.4 software were used to fit the models and to produce the graphical outputs, respectively.
2.3.3. Descriptive analyses of Salmonella serovars
With the aim of evaluating the distribution of Salmonella serovars along the food chain and identifying the potential sources for human infections, descriptive analyses were made from serovar data on food and food‐producing animals for the most commonly reported Salmonella serovars from human cases acquired within the EU (domestically or during travel within the EU). For animal categories covered by the NCP, only serovar data reported in the context of these programmes were presented. For cattle, meat‐producing animals were considered, whereas for pigs, data from fattening animals were used. To interpret serovar data, it must be kept in mind that for NCP, mandatory reporting is limited to target serovars only and this could lead to a possible bias towards the reporting of these regulated serovars to the detriment of non‐regulated ones. For all the other animal species–food matrices the reporting of serovar data is carried out on a voluntary basis by the MS. Apart from possible reporting bias as regards serovars, the reporting on animal or food categories could also be unbalanced and specific sources (e.g. cattle) may be underrepresented.
Sankey diagrams were provided to show the most reported Salmonella serovars from humans in relation to their likely food and animal sources and in relation to the MS reporting them (geographical provenance). Stacked bar plots for each of the serovars of interest were prepared to show for each source the frequency of reporting in animal and food sources. Both graphical representations were performed using R software (www.r-project.org). The infographic, showing the most relevant data about Salmonella, was produced using Adobe Illustrator and InDesign.
2.4. Results
2.4.1. Overview of key statistics along the food chain, EU, 2015–2019
Table 8 summarises EU‐level statistics on human salmonellosis and on Salmonella in food and animals, respectively, during 2015–2019. Food data of interest reported were classified into the major categories and aggregated by year to obtain an annual overview of the volume of data submitted.
Table 8.
Summary of Salmonella statistics related to humans, major food categories and major animal species, EU, 2015–2019
| 2019 | 2018 | 2017 | 2016 | 2015 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 87,923 | 91,858 | 91,587 | 94,425 | 94,477 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 20.0 | 20.1 | 19.7 | 20.5 | 21.0 | ECDC |
| Number of reporting MS | 28 | 28 | 28 | 28 | 28 | ECDC |
| Infection acquired in the EU | 58,271 | 59,763 | 59,642 | 52,852 | 51,898 | ECDC |
| Infection acquired outside the EU | 6,343 | 6,376 | 6,001 | 6,466 | 6,830 | ECDC |
| Unknown travel status or unknown country of infection | 23,309 | 25,719 | 25,944 | 35,107 | 35,749 | ECDC |
| Number of outbreak‐related cases | 9,169 | 11,631 | 9,607 | 11,428 | 8,531 | EFSA |
| Total number of outbreaks | 926 | 1,588 | 1,241 | 1,372 | 1,216 | EFSA |
| Food | ||||||
| Meat and meat products | ||||||
| Number of sampling units | 525,704 | 433,197 | 380,000 | 285,564 | 211,072 | EFSA |
| Number of reporting countries | 28 | 28 | 28 | 27 | 27 | EFSA |
| Milk and milk products | ||||||
| Number of sampling units | 46,797 | 44,078 | 30,796 | 24,337 | 29,034 | EFSA |
| Number of reporting countries | 25 | 24 | 24 | 24 | 22 | EFSA |
| Fish and fishery products | ||||||
| Number of sampling units | 14,010 | 17,123 | 13,507 | 12,287 | 11,373 | EFSA |
| Number of reporting countries | 24 | 22 | 22 | 21 | 22 | EFSA |
| Eggs and egg products | ||||||
| Number of sampling units | 12,093 | 10,611 | 15,435 | 10,933 | 9,650 | EFSA |
| Number of reporting countries | 21 | 21 | 23 | 20 | 19 | EFSA |
| Fruits and vegetables (and juices) | ||||||
| Number of sampling units | 17,068 | 10,888 | 7,579 | 7,515 | 6,797 | EFSA |
| Number of reporting countries | 22 | 22 | 25 | 20 | 22 | EFSA |
| Animals | ||||||
| Gallus gallus (chicken) | ||||||
| Number of sampling units | 755,937 | 720,717 | 736,534 | 699,116 | 531,533 | EFSA |
| Number of reporting countries | 27 | 27 | 28 | 27 | 28 | EFSA |
| Turkeys | ||||||
| Number of sampling units | 65,960 | 68,009 | 74,739 | 79,245 | 56,569 | EFSA |
| Number of reporting countries | 23 | 24 | 26 | 24 | 24 | EFSA |
| Ducks and geese | ||||||
| Number of sampling units | 8,700 | 9,846 | 5,743 | 2,640 | 4,518 | EFSA |
| Number of reporting countries | 9 | 6 | 8 | 11 | 8 | EFSA |
| Pigs | ||||||
| Number of sampling units | 18,619 | 17,868 | 19,239 | 24,653 | 59,399 | EFSA |
| Number of reporting countries | 14 | 14 | 17 | 17 | 16 | EFSA |
| Bovine animals | ||||||
| Number of sampling units | 86,871 | 30,302 | 654,593 | 53,198 | 119,466 | EFSA |
| Number of reporting countries | 14 | 14 | 15 | 16 | 16 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member State.
More detailed descriptions of these statistics are in the results section of this chapter and in the chapter on FBOs.
When the UK data were collected, the UK was an EU MS but as of 31 January 2020, it has become a third country.
Humans
In 2019, the number of reported human salmonellosis cases acquired in the EU (i.e. by domestic infection and through travel within the EU) was at the same level as in 2018. The number of outbreak‐related cases and the total number of food‐borne salmonellosis outbreaks was lower in 2019 compared with 2018 and at a lower level compared with 2017 and previous years.
Food categories
The number of sampling units reported in 2019 for the different food categories was higher compared with 2018 and, in general, a constant increase was seen over the years (2015–2019). The only exception was for ‘fish and fish products’, for which the number of sampling units reported in 2019 decreased, compared with 2018, although it was higher than in the previous years (2015–2017). The number of reporting MS has been fairly stable over the years.
Animal categories
The number of sampling units related to animal categories fluctuated over the years, except for ‘Gallus gallus (chicken)’ for which the reported number of sampling units increased over the period 2015–2019. This fluctuation was very important for the category ‘bovine’, for which the number of sample units reported in 2019 was higher than 2018, but lower than the number of sample units reported especially in 2017, but also in 2015. For ‘pigs’, in the last year, there was an increase in the number of reported sample units, but it was comparable with 2017 and lower than in the two previous years (2015–2016). For the category ‘ducks and geese’, the number of flocks with monitoring data submitted to EFSA decreased compared with 2018 (even though the number of reporting countries increased), but it remained higher than the number of flocks reported in the previous years (2015–2017). For the category ‘turkeys’, the number of reported sample units remained rather stable over the years, although with a decrease in 2019 compared with 2018 in terms of reported sample units and reporting countries.
2.4.2. Human salmonellosis
In total, 90,105 human salmonellosis cases were reported by 28 EU MS in 2019. Of these, 87,923 were confirmed cases resulting in an EU notification rate of 20.0 cases per 100,000 population (Table 9). This was at the same level as in 2018 (20.1 cases per 100,000 population). As in the previous year, the highest notification rates in 2019 were reported by Czechia (122.2 cases per 100,000 population) and Slovakia (91.6 cases per 100,000 population), while the lowest rates were reported by Cyprus, Greece, Ireland, Italy, Portugal and Romania (≤ 7.1 cases per 100,000 population).
Table 9.
Reported human cases of salmonellosis and notification rates per 100,000 population in the EU/EFTA, by country and year, 2015–2019
| Country | 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 1,868 | 1,866 | 21.1 | 1,538 | 17.4 | 1,667 | 19.0 | 1,415 | 16.3 | 1,544 | 18.0 |
| Belgium | Y | C | 2,527 | 2,527 | 22.1 | 2,958 | 26.0 | 2,298 | 20.2 | 2,699 | 23.9 | 3,050 | 27.1 |
| Bulgaria | Y | A | 596 | 594 | 8.5 | 586 | 8.3 | 796 | 11.2 | 718 | 10.0 | 1,076 | 14.9 |
| Croatia | Y | C | 1,318 | 1,308 | 32.1 | 1,323 | 32.2 | 1,242 | 29.9 | 1,240 | 29.6 | 1,593 | 37.7 |
| Cyprus | Y | C | 62 | 62 | 7.1 | 44 | 5.1 | 59 | 6.9 | 77 | 9.1 | 65 | 7.7 |
| Czechia | Y | C | 13,306 | 13,009 | 122.2 | 10,901 | 102.7 | 11,473 | 108.5 | 11,610 | 110.0 | 12,408 | 117.7 |
| Denmark | Y | C | 1,119 | 1,119 | 19.3 | 1,168 | 20.2 | 1,067 | 18.6 | 1,081 | 18.9 | 925 | 16.3 |
| Estonia | Y | C | 154 | 150 | 11.3 | 314 | 23.8 | 265 | 20.1 | 351 | 26.7 | 112 | 8.5 |
| Finland | Y | C | 1,175 | 1,175 | 21.3 | 1,431 | 26.0 | 1,535 | 27.9 | 1,512 | 27.6 | 1,650 | 30.2 |
| Franceb | N | C | 8,935 | 8,935 | 27.8 | 8,936 | 27.8 | 7,993 | 24.9 | 8,876 | 27.7 | 10,305 | 32.3 |
| Germany | Y | C | 13,692 | 13,495 | 16.3 | 13,293 | 16.1 | 14,051 | 17.0 | 12,858 | 15.6 | 13,667 | 16.8 |
| Greece | Y | C | 642 | 642 | 6.0 | 640 | 6.0 | 672 | 6.2 | 735 | 6.8 | 466 | 4.3 |
| Hungary | Y | C | 5,172 | 4,452 | 45.6 | 4,161 | 42.6 | 3,922 | 40.0 | 4,722 | 48.0 | 4,894 | 49.7 |
| Ireland | Y | C | 356 | 347 | 7.1 | 352 | 7.3 | 379 | 7.9 | 299 | 6.3 | 270 | 5.8 |
| Italy | Y | C | 3,268 | 3,256 | 5.4 | 3,635 | 6.0 | 3,347 | 5.5 | 4,134 | 6.8 | 3,825 | 6.3 |
| Latvia | Y | C | 472 | 438 | 22.8 | 409 | 21.1 | 225 | 11.5 | 454 | 23.1 | 380 | 19.1 |
| Lithuania | Y | C | 745 | 736 | 26.3 | 779 | 27.7 | 1,005 | 35.3 | 1,076 | 37.3 | 1,082 | 37.0 |
| Luxembourg | Y | C | 131 | 131 | 21.3 | 135 | 22.4 | 118 | 20.0 | 108 | 18.7 | 106 | 18.8 |
| Malta | Y | C | 131 | 131 | 26.5 | 116 | 24.4 | 107 | 23.2 | 162 | 36.4 | 126 | 29.3 |
| Netherlandsc | N | C | 1,197 | 1,197 | 10.8 | 1,061 | 9.6 | 954 | 8.7 | 1,150 | 10.6 | 974 | 9.0 |
| Poland | Y | C | 8,919 | 8,373 | 22.0 | 9,064 | 23.9 | 8,921 | 23.5 | 9,718 | 25.6 | 8,245 | 21.7 |
| Portugal | Y | C | 500 | 432 | 4.2 | 302 | 2.9 | 462 | 4.5 | 376 | 3.6 | 325 | 3.1 |
| Romania | Y | C | 1,413 | 1,383 | 7.1 | 1,410 | 7.2 | 1,154 | 5.9 | 1,479 | 7.5 | 1,330 | 6.7 |
| Slovakia | Y | C | 5,234 | 4,992 | 91.6 | 6,791 | 124.8 | 5,789 | 106.5 | 5,299 | 97.7 | 4,841 | 89.3 |
| Slovenia | Y | C | 362 | 362 | 17.4 | 274 | 13.3 | 275 | 13.3 | 311 | 15.1 | 401 | 19.4 |
| Spaind , f | N | C | 5,103 | 5,103 | – | 8,730 | – | 9,426 | – | 9,818 | – | 9,015 | – |
| Sweden | Y | C | 1,990 | 1,990 | 19.5 | 2,041 | 20.2 | 2,280 | 22.8 | 2,247 | 22.8 | 2,312 | 23.7 |
| United Kingdom | Y | C | 9,718 | 9,718 | 14.6 | 9,466 | 14.3 | 10,105 | 15.3 | 9,900 | 15.1 | 9,490 | 14.6 |
| EU Total | – | – | 90,105 | 87,923 | 20.0 | 91,858 | 20.1 | 91,587 | 19.7 | 94,425 | 20.5 | 94,477 | 21.0 |
| Iceland | Y | C | 50 | 50 | 14.0 | 63 | 18.1 | 64 | 18.9 | 39 | 11.7 | 44 | 13.4 |
| Norway | Y | C | 1,093 | 1,092 | 20.5 | 961 | 18.2 | 992 | 18.9 | 865 | 16.6 | 928 | 18.0 |
| Switzerlande | Y | C | 1,547 | 1,547 | 18.0 | 1,467 | 17.2 | 1,848 | 21.9 | 1,517 | 17.9 | 1,375 | 16.4 |
Y: yes; N: no; A: aggregated data; C: case‐based data.
Sentinel system; notification rates calculated with an estimated population coverage of 48%.
Sentinel system; notification rates calculated with an estimated population coverage of 64%.
Sentinel surveillance; no information on estimated coverage 2015–2018. So, notification rate cannot be estimated.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
Data not complete in 2019, rate not calculated.
The proportion of domestic vs. travel‐associated cases varied markedly between countries, but most of the confirmed salmonellosis cases were acquired in the EU (66.3%), whereas 7.2% reported travel outside EU and 26.5% of infections were of unknown origin (Table 8). Considering all cases, the highest proportions of domestic cases over 95% were reported by Czechia, Hungary, Latvia, Lithuania, Malta, Portugal, Poland, Slovakia and Spain. The highest proportions of travel‐related cases were reported by five Nordic countries: Finland (78.5%), Denmark (64.2%), Sweden (60.9%), Iceland (66.7%) and Norway (76.1%). Among 7,900 travel‐associated cases with known information on probable country of infection, 80.3% of the cases represented travel outside EU. Turkey, Egypt, Thailand and India were the most frequently reported travel destinations outside EU (15.3%, 10.5%, 10.4% and 6.0%, respectively). In the EU, Spain and Greece were the most common travel destinations.
A seasonal trend was observed for confirmed salmonellosis cases in the EU/EEA in 2010–2019, with more cases reported during summer months (Figure 5). The overall EU/EEA trend for salmonellosis was stable (flat) in 2015–2019.
Figure 5.
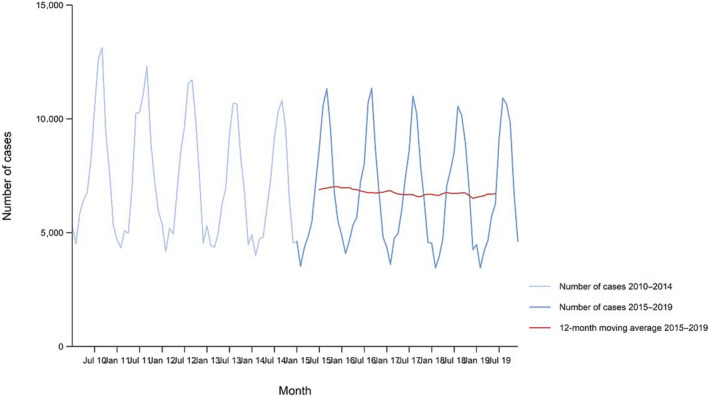
Trend in reported confirmed human cases of non‐typhoidal salmonellosis in the EU/EEA, by month, 2015–2019
- Source: Austria, Belgium, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Lithuania, Luxembourg, Latvia, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Sweden and the United Kingdom. Bulgaria, Croatia and Spain did not report data to the level of detail required for the analysis.
Finland was the only MS reporting a significantly decreasing trend (p < 0.01) in the last 5 years (2015–2019). An increasing trend was not observed in any MS in 2015–2019.
In total, 15 MS provided information on hospitalisation. The proportion of confirmed cases with known hospitalisation information was 44.5% at the EU level. Among these, the proportion of hospitalised cases was 42.5%, which was about at the same level as in 2018. The highest proportions of hospitalised cases were reported, as in previous years, in Cyprus, Greece, Lithuania, Poland and the United Kingdom, where most of the cases were hospitalised. The high proportion of hospitalised cases is probably due to surveillance focus on severe illnesses that require hospital care. Two of these countries also reported the lowest notification rates of salmonellosis, which indicates that the surveillance systems in these countries primarily capture the more severe cases.
Overall, 17 MS provided data on the outcome of salmonellosis and, among these, 11 MS reported 140 fatal cases resulting in an EU case fatality of 0.22%. Here, 46 fatal cases (32.9%) were reported by the United Kingdom.
Human serovar data are described in Section 2.4.6.
Human salmonellosis cases and cases associated with food‐borne outbreaks
In total, 87,923 confirmed human salmonellosis cases were reported to TESSy in 2019. Overall, 97.3% of the number of reported human salmonellosis cases who acquired the infection in the EU (58,271; Table 9) were domestic (acquired within the home country) infections and 2.7% were acquired through travel in EU.
Salmonella was identified overall by 23 MS in 926 FBOs that together affected 9,169 people in EU, with 1,915 hospitalised and seven deaths, as reported to EFSA. The vast majority (72.4%) of the salmonellosis FBOs were caused by S. Enteritidis. Comparing the FBOs outbreak cases (9,169) and confirmed cases human salmonellosis acquired in the EU (58,271) and also considering the estimated cases with unknown travel data (0.901 × 23,309) (Table 8) could suggest that overall in the EU in 2019 11.6% (9,169/79,292 × 100) of human salmonellosis cases would be reported through FBOs investigation. It is important to clarify that the case classification for reporting is different between these two databases. In TESSy, the cases reported are classified based on the EU case definition. All these cases visited a doctor and are either confirmed by a laboratory test (confirmed case) or not (probable case and classification is based on the clinical symptoms and epidemiological link). Cases that never visited a doctor are not reported to TESSy. Moreover, there may be missing probable cases in TESSy, as these data are not analysed or published and there is no incentive for reporting such cases. Information on which cases are linked to an outbreak and which not is also not systematically collected. In practice, the cases reported to TESSy are considered to be mostly sporadic cases. In food‐borne outbreaks, the human cases are the people involved in the outbreak as defined by the investigators (case definition), and cases must be linked, or probably linked, to the same food source (Directive 2003/99/EC). This can include both ill people (whether confirmed microbiologically or not) and people with confirmed asymptomatic infections (EFSA, 2014). Cases can be classified as confirmed or probable outbreak cases, but currently these specific classification data are not collected by EFSA.
For the 265 strong‐evidence outbreaks in EU in 2019 caused by Salmonella, 37.0% were caused by ‘eggs and egg products’, 11.7% by ‘bakery products’, 9.8% by ‘pig meat and products thereof’ and 8.7% by mixed food. Further details and statistics on the salmonellosis food‐borne outbreaks for 2019 are in the FBOs chapter.
2.4.3. Salmonella in food
Data collected in the context of Regulation (EC) No 2073/2005 on microbiological criteria
Food safety criteria
Considering data for samples collected at the retail stage, Salmonella‐positive samples from official controls were reported for ‘minced meat and meat preparations from poultry intended to be eaten cooked’ (8.3%, 60 out of 725), ‘meat products from poultry intended to be eaten cooked’ (6.4%, 14 out of 218), ‘fresh poultry meat’ (3.5%, 89 out of 2,533), ‘live bivalve molluscs and live echinoderms, tunicates and gastropods’ (2.3%, 4 out of 176), ‘RTE pre‐cut fruits and vegetables’ (2.2%, 10 out of 461), ‘ice cream’ (2.1%, 8 out 384), ‘dried infant formulae, dried diet foods for medical purpose, and dried follow‐on formulae’ (1.4%, 10 out of 718), ‘meat products intended to be eaten raw’ (1.3%, 6 out of 466), ‘minced meat and meat preparation from species other than poultry intended to be eaten cooked’ (1.0%, 29 out of 2,944), ‘RTE sprouted seeds’ (0.8%, 1 out of 133), ‘minced meat and meat preparation intended to be eaten raw’ (0.6%, 1 out of 158) and ‘cooked crustaceans and molluscan shellfish’ (0.3%, 1 out of 330). The percentage of positive samples for category ‘dried infant formulae, dried diet foods for medical purpose, and dried follow‐on formulae’ was strongly influenced by the subcategory ‘dried infant formulae’ with 10 out of 502 (1.99%) positive samples (all notified by Spain). Overviewing all poultry meat categories, there was an increase in the occurrence of Salmonella‐positive samples from 0.9% reported in 2018 to 8.3% in 2019 for ‘minced meat and meat preparations intended to be eaten cooked’, from 0% in 2018 to 6.4% in 2019 for ‘meat products from poultry intended to be eaten cooked’ and from 1.8% in 2018 to 3.5% in 2019 for ‘fresh poultry meat’. As defined by EU Regulation (EC) No 2073/2005, the microbiological criteria for fresh poultry meat targets positive samples for S. Enteritidis and S. Typhimurium and, according to these criteria, 29 out of the 1,345 samples (4.2%) were non‐compliant for the presence of these target serovars, according to data reported by four MS.
Considering data collected at production level (e.g. cutting and processing plants), ‘meat products from poultry meat intended to be eaten cooked’ category had a percentage of Salmonella‐positive samples of 27.8% (10 out of 36). Positive samples were also reported from ‘mechanically separated meat’ (9.2%, 6 out of 65), ‘fresh poultry meat’ (2.5%, 6 out of 292), ‘minced meat and meat preparations from poultry intended to be eaten cooked’ (2.1%, 16 out of 759), ‘cheese, butter and cream made from raw or low‐heat treated milk’ (0.7%, 8 out of 1,114), ‘meat products intended to be eaten raw’ (0.6%, 3 out of 482), ‘minced meat and meat preparations from species other than poultry intended to be eaten cooked’ (0.4%, 15 out of 3,399).
As also pointed out in previous years, data collected in the context of Regulation (EC) No 2073/2005 on microbiological criteria, which could serve the purpose of trend observation, were scarce and unrepresentative of the EU situation, because few data were reported for the specified food categories and regardless of the sampling stage.
Results are summarised in Figure 6.
Figure 6.
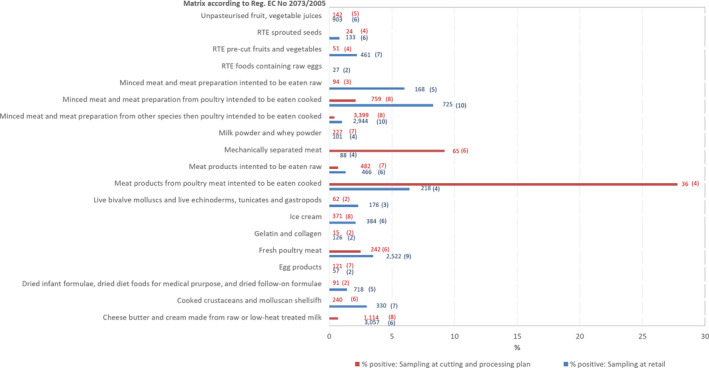
Summary of Salmonella monitoring results based on official control samples, by food category as defined by EU Regulation (EC) No 2073/2005 and by stage in the food chain, EU, 2019
- The number at the end of the bar indicates the number of tested samples and the number between brackets indicates the number of reporting MS for each food category and sampling stage.
Process hygiene criteria
As regards Salmonella PHC monitoring data from pig carcases collected at the slaughterhouse before chilling, 19 MS provided data. Four MS (Cyprus, Ireland, Slovakia and the United Kingdom) reported official control data only; seven MS (Austria, Denmark, Germany, France, Latvia, Portugal and Slovenia) self‐monitoring data only, from FBOp (Table 10) and eight MS (Belgium, Bulgaria, Estonia, Italy, Malta, the Netherlands, Poland and Spain) both samplers’ data. Considering pig carcass data sent by the latter eight MS, the percentage of Salmonella‐positive single samples from carcases was 3.88% (N = 15,745) for samples collected by CA and 1.11% (N = 35,765) for samples collected by FBOp. For Belgium, Estonia, Italy, the Netherlands, Poland and Spain, the percentage of positives based on official controls was significantly higher than that from self‐monitoring. Considering all Salmonella PHC monitoring data from pig carcases sent by the 19 MS, the percentage of Salmonella‐positive samples from carcases based on official controls was 3.15% (N = 22,271) and was significantly higher than that based on self‐monitoring (1.51%, N = 111,939). Comparing these data with the data collected in 2018, increase in prevalence was reported for samples collected by CA (2.69% in 2018) whereas it was similar for the situation reported by FBOp (1.57% in 2018). Spain reported an important increase in the percentage of positive official control samples in 2019 (17.57% of single samples from carcases were Salmonella‐positive) compared with 2018 (8.31%), whereas the opposite was reported for samples collected by FBOp (2.43% in 2019 and 5.06% in 2018).
Table 10.
Comparisons of proportions (%) of Salmonella‐positive single samples from pig carcases before chilling, by sampler, reporting MS, EU, 2019
| Country | Competent authorities (CA) | Food business operator (FBOp) | p‐value b | Interpretation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample weight | N samples Tested | N samples Positive | % samples positive | CI95 | Sample weight | N samples Tested | N samples Positive | % samples positive | CI95 | |||
| Austria | 400 cm2 | 5,633 | 5 | 0.09 | [0.03; 0.21] | |||||||
| Belgium | 600 cm2 | 1,049 | 65 | 6.20 | [4.81; 7.83] | 600 cm2 | 5,055 | 88 | 1.74 | [1.40; 2.14] | < 0.001 | CA > FBOp |
| Bulgaria | 400 cm2 | 2,094 | 0 | 0.00 | [0.00; 0.18]a | 400 cm2 | 337 | 0 | 0.00 | [0.00; 1.09]a | NS | |
| Cyprus | 400 cm2 | 6 | 0 | 0.00 | — | |||||||
| Denmark | 400 cm2 | 10,743 | 133 | 1.24 | [1.04; 1.46] | |||||||
| Estonia | 400 cm2 | 401 | 15 | 3.74 | [2.11; 6.09] | 400 cm2 | 1,666 | 2 | 0.12 | [0.01; 0.43] | < 0.001 | CA > FBOp |
| France | 400 cm2 | 14,409 | 651 | 4.52 | [4.18; 4.87] | |||||||
| Germany | 400 cm2 | 27,269 | 148 | 0.54 | [0.46; 6.37] | |||||||
| Ireland | 400 cm2 | 383 | 16 | 4.18 | [2.41; 6.70] | |||||||
| Italy | 400 cm2 | 6,186 | 235 | 3.80 | [3.34; 4.31] | 400 cm2 | 15,786 | 231 | 1.46 | [1.28; 1.66] | < 0.001 | CA > FBOp |
| Latvia | 400 cm2 | 606 | 0 | 0.00 | [0.00; 0.61]a | |||||||
| Malta | 400 cm2 | 60 | 5 | 8.33 | [2.76; 18.38] | 400 cm2 | 125 | 3 | 2.40 | [0.5; 6.85] | < 0.10 | CA > FBOp |
| Netherlands | 400 cm2 | 383 | 22 | 5.74 | [3.63; 8.57] | < 0.001 | CA > FBOp | |||||
| 100 cm2 | 9,613 | 272 | 2.83 | [2.51; 3.18] | ||||||||
| Poland | 400 cm2 | 4,189 | 26 | 0.62 | [0.41; 0.91] | 400 cm2 | 10,035 | 5 | 0.05 | [0.02; 0.12] | < 0.001 | CA > FBOp |
| Portugal | 400 cm2 | 6,806 | 76 | 1.12 | [0.88; 1.40] | |||||||
| Slovakia | 400 cm2 | 2,352 | 9 | 0.38 | [0.17; 0.72] | |||||||
| Slovenia | 400 cm2 | 1,095 | 11 | 1.00 | [0.50; 1.79] | |||||||
| Spain | 400 cm2 | 1,383 | 243 | 17.57 | [15.59; 19.68] | 400 cm2 | 2,761 | 67 | 2.43 | [1.88; 3.07] | < 0.001 | CA > FBOp |
| United Kingdom c | 400 cm2 | 3,785 | 65 | 1.72 | [1.33; 2.18] | |||||||
| Total | 22,271 | 701 | 3.15 | [2.92; 3.38] | 111,939 | 1,692 | 1.51 | [1.44; 1.58] | < 0.001 | CA > FBOp | ||
| Total d | 15,745 | 611 | 3.88 | [3.58; 4.19] | 35,765 | 396 | 1.11 | [1.00; 1.22] | < 0.001 | CA > FBOp | ||
MS: Member State.
One‐sided 97.5% confidence interval.
p‐value: NS, not significant.
The United Kingdom informed during the last phase of the production of this report of a reporting error and that samples had been taken by the food business operators.
Total number of samples considering only the MS that provided both CA and FBOp data (data in white rows).
Finland, Sweden and Norway, which are countries with special guarantees in relation to Salmonella on pig carcasses (according to Regulation (EU) No 853/2004), reported the following monitoring results: Finland five positive samples out of 6,507 tested (food business operator sampling), Sweden one positive out of 5,935 official control samples and Norway zero positive out of 3,314 official control samples tested.
As regards official control Salmonella PHC monitoring data from other animals than pigs, results from chilled carcases of broilers were reported by six MS (Bulgaria, Cyprus, Estonia, Latvia, Spain and Sweden) and only one single MS (Spain) provided data from chilled turkey carcases. The overall proportion of Salmonella‐positive broiler carcase samples was 9.8% (99 positive samples out of 1,012 tested carcases), whereas for turkey carcases it was 22% (11 positive samples out of 50 tested carcases). Additionally, Sweden, which has special guarantees in relation to Salmonella on broiler carcasses, reported zero positive out of 1,866 official control samples tested.
Finland, Sweden and Norway, which are countries with special guarantees in relation to Salmonella on pig carcasses (according to Regulation (EU) No 853/2004), reported the following monitoring results: Finland five positive samples out of 6,507 tested (food business operator sampling), Sweden one positive out of 5,935 official control samples and Norway zero positive out of 3,314 official control samples tested.
Occurrence in food
Monitoring data reported from food samples, which do not fit with the criteria described in the previous paragraphs, are described by merging investigations from all the monitoring and surveillance activities, from all the sampling stages (retail, slaughterhouse, processing, border inspection activities and unspecified) and from all the sampling units (single and batch).
Table 11 summarises the reported occurrence of Salmonella in the most important food categories for the year 2019 and for the four year–period 2015–2018. A distinction is made between RTE and non‐RTE food including fresh meat.
Table 11.
Occurrence of Salmonella in major food categories, EU
| 2019 | 2015–2018 | |||||
|---|---|---|---|---|---|---|
| Food | N reporting MS | N sampled units | Positive N (%) | N reporting MS | N sampled units | Positive N (%) |
| RTE food | ||||||
| All | 21 | 66,113 | 178 (0.27) | 24 | 198,922 | 542 (0.27) |
| Meat and meat products | 16 | 22,328 | 122 (0.55) | 21 | 46,115 | 200 (0.43) |
| Meat and meat products from broilers | 7 | 331 | 0 | 17 | 5,544 | 28 (0.51) |
| Meat and meat products from turkeys | 7 | 679 | 0 | 13 | 1,312 | 5 (0.38) |
| Meat and meat products from pigs | 14 | 7,307 | 24 (0.33) | 18 | 26,661 | 113 (0.42) |
| Meat and meat products from bovine animals | 10 | 1,154 | 1 (0.09) | 17 | 2,916 | 5 (0.17) |
| Mixed meat and meat products from bovine animals and pigs | 3 | 3,946 | 40 (1.01) | 4 | 272 | 8 (2.94) |
| Mixeda | 9 | 843 | 9 (1.07) | 13 | 2,808 | 7 (0.25) |
| Other meat and meat products | 11 | 8,068 | 48 (0.60) | 15 | 6,602 | 34 (0.52) |
| Milk and milk products | 18 | 19,929 | 24 (0.12) | 22 | 58,231 | 66 (0.11) |
| Milk | 8 | 616 | 1 (0.16) | 13 | 1,589 | 3 (0.19) |
| Raw milk | 3 | 258 | 0 | 5 | 864 | 0 |
| Cheese | 16 | 7,817 | 16 (0.21) | 22 | 26,612 | 42 (0.16) |
| Dairy products excluding cheeses (butter, cream, ice cream, whey, yoghurt and fermented dairy products) | 16 | 11,496 | 7 (0.06) | 20 | 30,030 | 21 (0.07) |
| Fruits, vegetables and juices | 11 | 2,052 | 1 (0.05) | 18 | 8,727 | 2 (0.02) |
| Fish and fishery products | 15 | 2,562 | 1 (0.04) | 21 | 11,604 | 12 (0.10) |
| Spices and herbs | 16 | 2,136 | 7 (0.33) | 18 | 4,399 | 50 (1.14) |
| Bakery products | 13 | 3,656 | 0 | 16 | 14,744 | 39 (0.27) |
| Salads | 10 | 3,695 | 2 (0.05) | 13 | 9,533 | 47 (0.49) |
| Other processed food products and prepared dishes | 14 | 7,197 | 11 (0.15) | 17 | 32,749 | 114 (0.35) |
| Eggs and egg products | 4 | 56 | 0 | 5 | 174 | 0 |
| Beverages, alcoholic | 1 | 5 | 0 | 2 | 14 | 0 |
| Cereals and nuts | 10 | 436 | 1 (0.23) | 11 | 1,322 | 1 (0.08) |
| Infant formulae and follow‐on formulae–RTE | 4 | 123 | 2 (1.63) | 8 | 576 | 0 |
| Other food | 7 | 84 | 1 (1.19) | 9 | 279 | 1 (0.36) |
| Cocoa and cocoa preparations, coffee and tea | 3 | 530 | 6 (1.13) | 6 | 919 | 0 |
| Non‐RTE food | ||||||
| All | 25 | 191,981 | 2,919 (1.52) | 26 | 569,789 | 11,448 (2.01) |
| Meat and meat products | 24 | 174,411 | 2,889 (1.66) | 25 | 499,648 | 11,118 (2.23) |
| Fresh meat from broilers | 15 | 23,580 | 1,805 (7.66) | 26 | 94,629 | 6,082 (6.43) |
| Fresh meat from turkeys | 12 | 4,417 | 160 (3.62) | 20 | 13,588 | 882 (6.49) |
| Fresh meat from pigs | 19 | 20,613 | 132 (0.64) | 25 | 111,106 | 1,372 (1.24) |
| Fresh meat from bovine animals | 13 | 18,377 | 36 (0.20) | 22 | 87,329 | 179 (0.21) |
| Other fresh meat | 15 | 42,998 | 687 (1.60) | 21 | 86,171 | 1,998 (2.32) |
| Milk and milk products | 8 | 1,390 | 0 | 15 | 3,324 | 11 (0.33) |
| Fruits, vegetables and juices | 16 | 4,955 | 4 (0.08) | 22 | 6,870 | 51 (0.74) |
| Fish and fishery products | 11 | 1,943 | 0 | 16 | 7,956 | 27 (0.34) |
| Eggs and egg products | 11 | 5,051 | 6 (0.12) | 20 | 26,392 | 113 (0.43) |
| Sprouts | 1 | 124 | 1 (0.84) | 11 | 1,505 | 3 (0.20) |
| Infant formulae | 9 | 562 | 10 (1.78) | 15 | 3,060 | 0 |
| Foodstuffs intended for special nutritional uses | 8 | 400 | 0 | 15 | 1,604 | 5 (0.31) |
| Cereals, dried seeds | 13 | 878 | 8 (0.91) | 16 | 3,149 | 79 (2.51) |
| Other processed food products and prepared dishes | 12 | 1,356 | 1 (0.07) | 19 | 12,989 | 16 (0.12) |
N: number
Meat consisting of ground meat other than beef and pork mixed together.
RTE food
For 2019, 66,113 RTE and 191,181 non‐RTE food sampling units were reported from 21 and 25 MS with 0.27% and 1.52% positive samples, respectively. Within the category of RTE food samples, positive samples were from divers food products; ‘meat and meat products’, ‘milk and milk products’, ‘fruits, vegetables and juices’, ‘fish and fishery products’, ‘spices and herbs’, ‘salads’, ‘other processed food products and prepared dishes’, ‘cereals and nuts’, ‘infant formulae and follow‐on formulae’, ‘other food’ and ‘cocoa and cocoa preparations, coffee and tea’, with the percentages of positive samples ranging from 0.04% in ‘fish and fishery products’ to 1.63% in ‘infant formulae and follow‐on formulae’.
Non‐RTE food
Within the category of non‐RTE food the highest percentage of positive samples was reported for ‘fresh meat from broilers’ (7.66%), ‘fresh meat from turkeys’ (3.62%), ‘infant formulae’ (1.78%) and ‘other fresh meat’ (1.60%). The number of sampling results for ‘meat and meat products’ was high both for RTE and no‐RTE food with 0.55% and 1.66% positive samples, respectively.
In the following descriptive analyses, food categories include RTE food and non‐RTE food.
Meat and meat products
A summary of results from the major meat and meat product categories and the sampling points is in Figure 7, considering all sampling units (single and batch). Considering the entire production chain for meat and meat products, the highest percentages of Salmonella‐positive samples were found for ‘Fresh broiler meat’ and ‘Fresh turkey meat’ (respectively, 7.66 and 5.38%). Salmonella‐positive samples of ‘Fresh broiler meat’ were collected mainly at the slaughterhouse, while for ‘Fresh turkey meat’, positive samples were both from slaughterhouses and cutting plants. For the other categories, 2.59% of the ‘Fresh poultry meat other than broiler and turkey’ samples were Salmonella‐positive, and these samples were reported mainly at the processing plants, as for ‘RTE minced meat, meat preparations and meat products from pig meat’.
Figure 7.

Summary of Salmonella monitoring results, by major meat and meat products categories and by sampling stage in the food chain, EU, 2019
Eggs and egg products
Austria, Bulgaria, Denmark, Germany, Italy, Poland, Portugal and Slovakia reported monitoring results for a total of 4,493 tested table egg sampling units and 6 (0.13%) were Salmonella‐positive: Austria, Germany and Italy found two positives each. As regards egg products, the same MS and Croatia, Cyprus, Lithuania and Spain reported data and overall two (0.16%) of the 1,246 sampling units collected were Salmonella‐positive and reported by Austria and Croatia.
Other foodstuffs
Results of 663 live bivalve molluscs sampling units were reported. Two (0.3%) were positive for Salmonella. Of the 7,462 units of fruit and vegetables tested, 0.10% were Salmonella‐positive.
2.4.4. Salmonella in animals
Poultry monitoring data according to the Salmonella national control programmes
Achievement of Salmonella reduction targets
Breeding flocks of Gallus gallus
In total, 24 MS and three non‐MS reported Salmonella NCP data from breeding flocks of Gallus gallus. Luxembourg and Malta do not have such flocks, whereas Hungary and Lithuania have flocks, but did not report any data. In the EU in 2019, Salmonella was found in 340 (2.34%) of the 14,513 flocks tested, compared with 2.03% in 2018 and 1.89% in 2017. The prevalence of flocks that were positive for any of the five target serovars (S. Enteritidis, S. Typhimurium including its monophasic variant, S. Virchow, S. Infantis and S. Hadar) was 0.62% in 2019 (or 90 flocks) compared with 0.54% in 2018. Therefore, 26.5% (90 of 340) of reported Salmonella‐positive breeding flocks were positive for target serovars. Eight MS and three non‐MS reported no flocks positive for target Salmonella serovars. All reporting countries except Bulgaria, Croatia, Ireland, Poland and Slovenia met the flock prevalence target of maximum 1% (Figure 8). It was the first time since 2017 that Bulgaria, Croatia, Ireland and Slovenia did not meet the target. The most frequently reported target serovar was S. Enteritidis (EU flock prevalence 0.36%), with 29 of the 53 positive flocks (54.7%) reported by Poland (Figure 9). The number of S. Enteritidis‐positive breeding flocks (53) increased compared with 2018 (36). Compared with the previous year, the number of S. Enteritidis‐positive flocks was similar for Poland (29 in 2019 and 26 in 2018), whereas the Netherlands reported nine positive flocks in 2019 and none in 2018. S. Typhimurium (including the monophasic variants) was the second most commonly reported target serovar (with 19 positive flocks, EU flock prevalence 0.13%) (Figure 10), followed by S. Infantis (EU flock prevalence 0.10%, 14 positive flocks) (Figure 11). Two flocks tested positive for S. Virchow (EU flock prevalence 0.01%) and were reported by Spain and additionally two other flocks tested positive for S. Hadar (EU flock prevalence 0.01%) and were reported by Denmark (1) and Poland (1).
Figure 8.
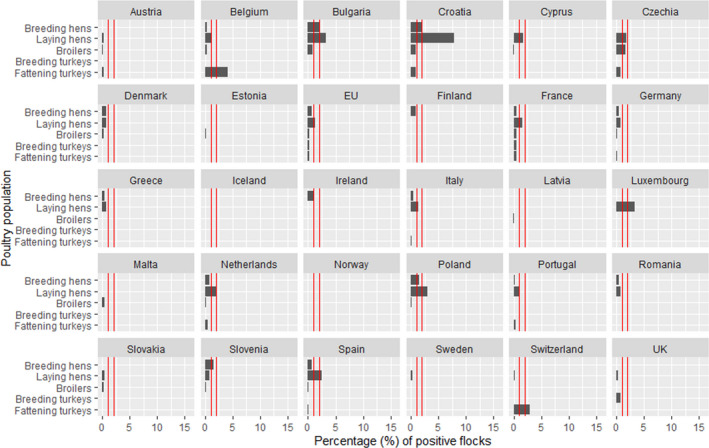
Prevalence of poultry flocks (breeding flocks of Gallus gallus, laying hens, broilers, breeding turkeys and fattening turkeys) positive for Salmonella target serovars, EU/EFTA, 2019
- Red vertical bars indicate the target to be reached, which was fixed at 1% for all poultry populations with the exception of laying hens for which it was 2% for all MS with the exception of Poland, for which it was 3.5%. Luxembourg met the target in laying hens (having less than 50 flocks with one positive for target serovars).
Figure 9.

Prevalence of S. Enteritidis‐positive breeding flocks of Gallus gallus during the production period, EU/EFTA, 2019
-
AL: Albania; BA: Bosnia and Herzegovina; ME: Montenegro; MK: Republic of North Macedonia; and SR: Serbia.Luxembourg and Malta do not have breeding flocks of Gallus gallus.
Figure 10.

Prevalence of S. Typhimurium‐positive (including monophasic variants) breeding flocks of Gallus gallus during the production period, EU/EFTA, 2019
-
AL: Albania; BA: Bosnia and Herzegovina; ME: Montenegro; MK: Republic of North Macedonia; and SR: Serbia.Luxembourg and Malta do not have breeding flocks of Gallus gallus.
Figure 11.
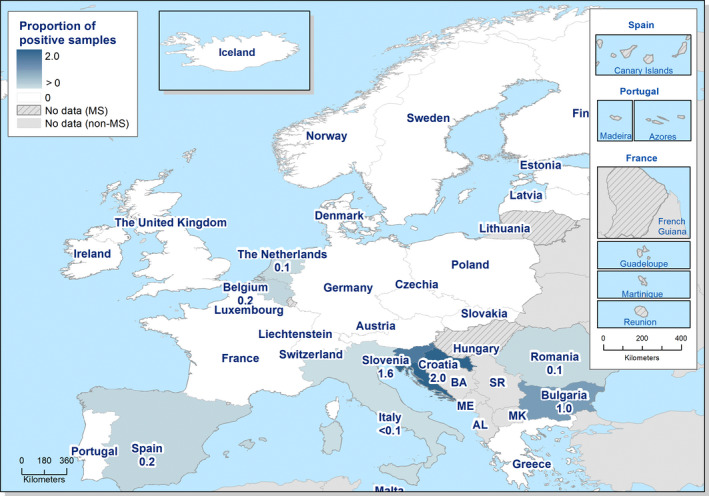
Prevalence of S. Infantis‐positive breeding flocks of Gallus gallus during the production period, EU/EFTA, 2019
- AL: Albania; BA: Bosnia and Herzegovina; ME: Montenegro; MK: Republic of North Macedonia; and SR: Serbia. Luxembourg and Malta do not have breeding flocks of Gallus gallus.
Flocks of laying hens
In total, 26 MS and three non‐MS reported Salmonella NCP data for laying hen flocks. No data were reported by Hungary and Lithuania. Salmonella was found in 1,529 or 3.9% of the flocks, compared with 4.04% in 2018. The EU prevalence of laying hen flocks that were positive for either of the two target serovars was 1.25% (N = 490), which was a slight increase compared with 2018, when 1.1% (N = 413) of the tested flocks were positive for target serovars. Therefore, 32% (490 of 1,529) of reported Salmonella‐positive laying hen flocks were positive for target serovars. Five MS and two non‐MS reported no Salmonella target serovar‐positive laying hen flocks. Four MS (Bulgaria, Croatia, Poland and Spain) did not meet their reduction target (Figure 8). Croatia and Poland also did not meet their reduction target in the previous years. The most frequently reported target serovar was S. Enteritidis (EU flock prevalence 0.95%) with 80.96% of the 373 S. Enteritidis‐positive flocks reported by six MS (France, Germany, Italy, the Netherlands, Poland and Spain) (Figure 12). For S. Typhimurium (including monophasic variants), 117 positive flocks were reported and 41.9% of them were reported by France (Figure 13).
Figure 12.
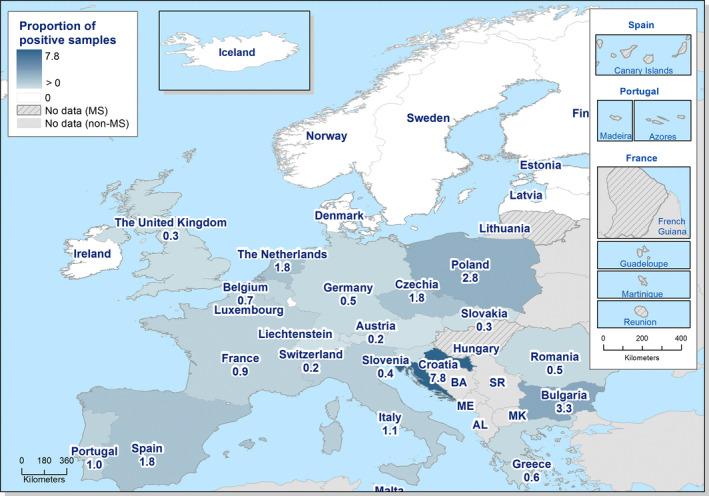
Prevalence of S. Enteritidis‐positive laying hen flocks of Gallus gallus during the production period, EU/EFTA, 2019
- AL: Albania; BA: Bosnia and Herzegovina; ME: Montenegro; MK: Republic of North Macedonia; and SR: Serbia.
Figure 13.
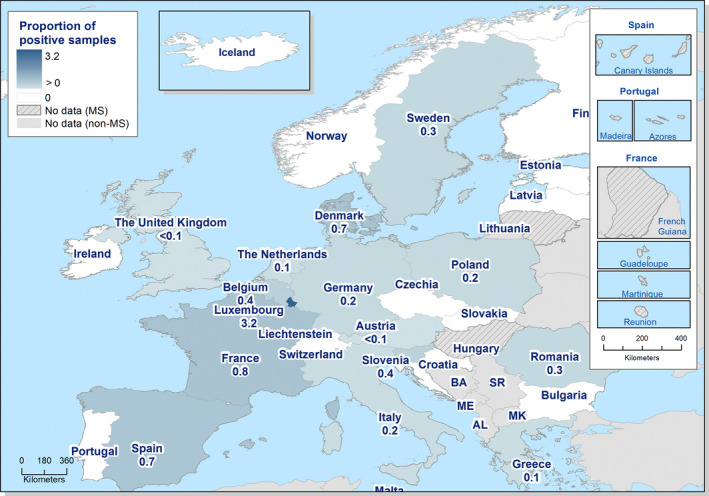
Prevalence of S. Typhimurium‐positive (including monophasic variants) laying hen flocks of Gallus gallus during the production period, EU/EFTA, 2019
- AL: Albania; BA: Bosnia and Herzegovina; ME: Montenegro; MK: Republic of North Macedonia; and SR: Serbia.
Broiler flocks
In total, 26 MS and three non‐MS reported Salmonella NCP data from broiler flocks. No data were reported by Hungary and Lithuania. Salmonella was found in 12,915 or 3.63% of the flocks compared with 3.49% in 2018. The EU prevalence of broiler flocks positive for either of the two target Salmonella serovars was 0.19% (corresponding to 698 flocks) like the previous 2 years (0.20% in 2018 and 0.19% in 2017). Therefore, 5.4% (698 of 12,915) of reported Salmonella‐positive broiler flocks were positive for target serovars. Five MS and three non‐MS reported no single Salmonella target serovar‐positive flock. All reporting MS met the target of 1% or less of broiler flocks positive for S. Enteritidis and/or S. Typhimurium, except Czechia (Figure 8), as in previous years. The EU flock prevalence was very similar for S. Typhimurium 0.099% (Figure 15) and S. Enteritidis 0.097% (Figure 14). Three MS (Czechia, France and Poland) accounted for 73.4% of the S. Enteritidis‐positive flocks and France accounted for 63.6% of the S. Typhimurium‐positive flocks.
Figure 15.
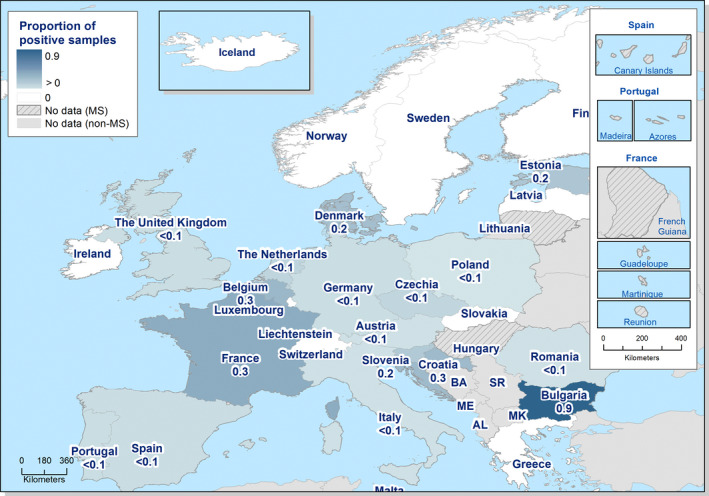
Prevalence of S. Typhimurium‐positive (including monophasic variants) broiler flocks of Gallus gallus before slaughter, EU/EFTA, 2019
- AL: Albania; BA: Bosnia and Herzegovina; ME: Montenegro; MK: Republic of North Macedonia; and SR: Serbia.
Figure 14.
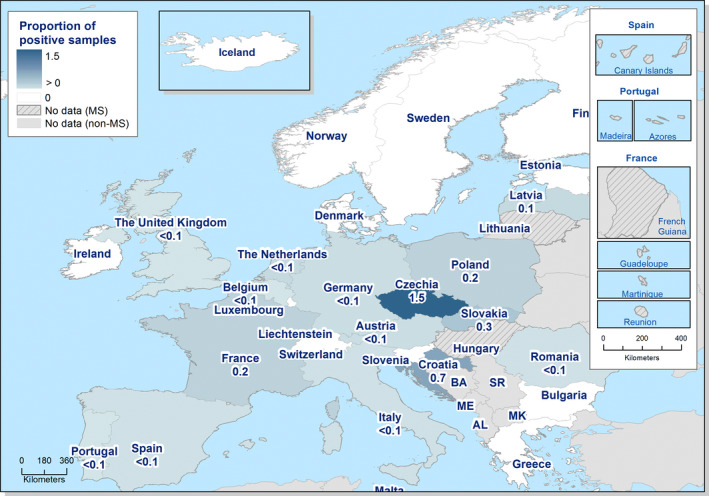
Prevalence of S. Enteritidis‐positive broiler flocks of Gallus gallus before slaughter, EU/EFTA, 2019
- AL: Albania; BA: Bosnia and Herzegovina; ME: Montenegro; MK: Republic of North Macedonia and SR: Serbia.
Reg (EC) No 200/2012 requires MS to report separately the results obtained by the FBOp and by the CA. Most MS (22) reported, additionally to the overall merged results, separate investigational results from the CA and the FBOp, from their broiler flocks. Four MS did not comply; France, Italy and the Netherlands only reported overall merged results and Croatia provided separate data for CA sampling only. Considering the data from the MS that reported separate results from both CA and FBOp, the prevalence of Salmonella target serovar‐positive flocks was, respectively, 1.60% (5,013 tested flocks by the CA) and 0.06% (241,344 tested flocks by FBOp). At the EU level, the prevalence of Salmonella target serovar‐positive broiler flocks obtained by the CA was significantly higher than that obtained from the FBOp’ self‐monitoring results. The same finding was also evident individually for Bulgaria, Czechia, Germany, Poland and Spain. For the remaining reporting MS, the differences between the results of both types of sampler were not significant or the sample sizes for one or both samplers were too low for analyses, or data were missing (Table 12).
Table 12.
Comparisons of prevalence of Salmonella target serovar‐positive broiler flocks, by sampler and by reporting MS, EU, 2019
| Country | Competent authority (CA) | Food business operator (FBOp) | p‐value a | Interpretation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N flocks Tested | N flocks positive to target serovars | % flocks positive to target serovars | CI95 | N flocks Tested | N flocks positive to target serovars | % flocks positive to target serovars | CI95 | |||
| Austria | 102 | 0 | 0.00 | [0.00; 3.55]a | 5,348 | 4 | 0.07 | [0.02; 0.19] | NS | |
| Belgium | 87 | 1 | 1.15 | [0.03; 6.24] | 9,016 | 24 | 0.27 | [0.17; 0.39] | NS | |
| Bulgaria | 230 | 2 | 0.87 | [0.11; 3.11] | 5,790 | 0 | 0.00 | [0.00; 0.06]a | < 0.01 | CA > FBOp |
| Croatia | 45 | 0 | 0.00 | [0.00; 7.87]a | ||||||
| Cyprus | 11 | 0 | 0.00 | – | 1,191 | 1 | 0.08 | [0.00; 0.47] | ||
| Czechia | 44 | 5 | 11.36 | [3.79; 24.56] | 4,739 | 70 | 1.48 | [1.15; 1.86] | < 0.001 | CA > FBOp |
| Denmark | 247 | 0 | 0.00 | [0.00; 1.48]a | 4,012 | 9 | 0.22 | [0.10; 0.43] | NS | |
| Estonia | 266 | 1 | 0.38 | [0.00; 2.08] | 477 | 0 | 0.00 | [0.00; 0.77]a | NS | |
| Finland | 513 | 0 | 0.00 | [0.00; 0.72]a | 3,443 | 0 | 0.00 | [0.00; 0.11]a | NS | |
| Germany | 301 | 8 | 2.66 | [1.15; 5.17] | 26,555 | 24 | 0.09 | [0.06; 0.13] | < 0.001 | CA > FBOp |
| Greece | 88 | 0 | 0.00 | [0.00; 4.10]a | 8,059 | 0 | 0.00 | [0.00; 0.05]a | NS | |
| Ireland | 111 | 0 | 0.00 | [0.00; 3.27]a | 4,122 | 0 | 0.00 | [0.00; 0.09]a | NS | |
| Latvia | 9 | 1 | 11.11 | – | 851 | 1 | 0.12 | [0.00; 0.65] | ||
| Luxembourg | 3 | 0 | 0.00 | – | 10 | 0 | 0.00 | – | ||
| Malta | 5 | 0 | 0.00 | – | 442 | 2 | 0.45 | [0.05; 1.62] | ||
| Poland | 1620 | 50 | 3.09 | [2.30; 4.05] | 43,894 | 30 | 0.07 | [0.05; 0.10] | < 0.001 | CA > FBOp |
| Portugal | 117 | 0 | 0.00 | [0.00; 3.10]a | 11,634 | 5 | 0.04 | [0.01; 0.10] | NS | |
| Romania | 354 | 0 | 0.00 | [0.00; 1.03]a | 12,442 | 4 | 0.03 | [0.00; 0.08] | NS | |
| Slovakia | 48 | 1 | 2.08 | [0.05; 11.06] | 2,803 | 8 | 0.29 | [0.12; 0.56] | NS | |
| Slovenia | 33 | 0 | 0.00 | [0.00; 10.58]a | 2,463 | 4 | 0.16 | [0.04; 0.42] | NS | |
| Spain | 517 | 10 | 1.93 | [0.93; 3.53] | 40,180 | 21 | 0.05 | [0.03; 0.08] | < 0.001 | CA > FBOp |
| Sweden | 129 | 0 | 0.00 | [0.00; 2.82]a | 4,373 | 0 | 0.00 | [0.00; 0.08]a | NS | |
| United Kingdom | 178 | 1 | 0.56 | [0.01; 3.09] | 54,239 | 16 | 0.03 | [0.02; 0.05] | < 0.1 | CA > FBOp |
| Total EU MS | 5,058 | 80 | 1.58 | [1.26; 1.96] | 246,083 | 223 | 0.09 | [0.08; 0.10] | < 0.001 | CA > FBOp |
| Total EU MS b | 5,013 | 80 | 1.60 | [1.27; 1.98] | 246,083 | 223 | 0.09 | [0.08; 0.10] | < 0.001 | CA > FBOp |
MS: Member State.
–, The confidence interval is not provided because of the small sample size.
One‐sided, 97.5% confidence interval; p‐value: NS: not significant.
Total number of flocks considering only the MS that provided both CA and FBOp data (data in white rows).
Breeding turkey flocks
For breeding turkeys, 13 MS and two non‐MS reported Salmonella NCP data. Although Hungary had breeding flocks of turkeys, they did not report such data. Salmonella was found in 85 (5.19%) of the 1,637 flocks tested, compared with 3.85% in 2018 and 2.63% in 2017. This increase is related to the marked increase of Salmonella‐positive breeding turkey flocks reported by Spain (30.43% of positive flocks in 2019 and 8.73% in 2018). In 2019, the prevalence of flocks positive for either of the two target Salmonella serovars was 0.30% (N = 5) compared with 0.47% and 0.50% in 2018 and 2017, respectively. The five target Salmonella serovar‐positive flocks were all positive for S. Typhimurium (Figure 16). Therefore, 5.9% (5 of 85) of reported Salmonella‐positive breeding turkey flocks were positive for S. Typhimurium. All reporting MS met the reduction target.
Figure 16.
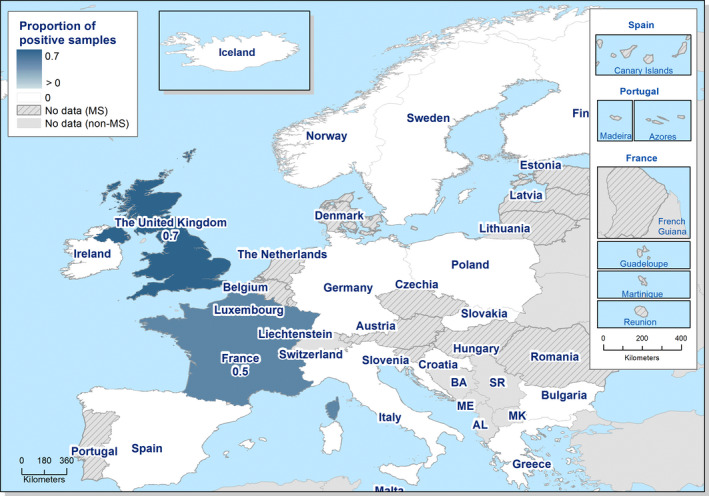
Prevalence of S. Typhimurium‐positive (including monophasic variants) turkey breeding flocks during the production period, EU/EFTA, 2019
-
AL: Albania; BA: Bosnia and Herzegovina; ME: Montenegro; MK: Republic of North Macedonia; and SR: Serbia.The following MS do not have turkey breeding flocks: Austria, Belgium, Cyprus, Czechia, Denmark, Estonia, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Portugal, Romania, Slovakia and Slovenia. Also the non‐MS Switzerland does not have such flocks.
Salmonella NCP monitoring data for turkey breeding flocks must be reported separately for investigations performed by CA and by FBOp, in addition to the overall merged results. Three MS (Croatia, France and Italy) did not comply with this reporting requirement, whereas 10 MS did (Table 13). The prevalence of Salmonella target serovar‐positive flocks based on official control samples and on self‐monitoring conducted by the FBOp were 0% (N = 544) and 0.28% (N = 721), respectively. All samples collected by CA and FBOp were negative, except for two isolates collected by FBOp and reported by the United Kingdom.
Table 13.
Comparisons of prevalence of Salmonella target serovar‐positive flocks of breeding turkeys, by sampler and by reporting MS, EU, 2019
| Country | Competent Authority (CA) | Food business operator (FBOp) | p‐value a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N flocks tested | N flocks positive to target serovars | % flocks positive to target serovars | CI95 | N flocks tested | N flocks positive to target serovars | % flocks positive to target serovars | CI95 | ||
| Bulgaria | 3 | 0 | 0.00 | – | 3 | 0 | 0.00 | – | |
| Finland | 7 | 0 | 0.00 | – | 7 | 0 | 0.00 | – | |
| Germany | 77 | 0 | 0.00 | [0.00; 4.68]a | 93 | 0 | 0.00 | [0.00; 3.89]a | NS |
| Greece | 2 | 0 | 0.00 | – | 8 | 0 | 0.00 | – | |
| Ireland | 6 | 0 | 0.00 | – | 6 | 0 | 0.00 | – | |
| Poland | 130 | 0 | 0.00 | [0.00; 2.80]a | 200 | 0 | 0.00 | [0.00; 1.83]a | NS |
| Slovakia | 37 | 0 | 0.00 | [0.00; 9.49]a | 37 | 0 | 0.00 | [0.00; 9.49]a | NS |
| Spain | 51 | 0 | 0.00 | [0.00; 6.98]a | 90 | 0 | 0.00 | [0.00; 4.02]a | NS |
| Sweden | 4 | 0 | 0.00 | – | 4 | 0 | 0.00 | – | |
| United Kingdom | 227 | 0 | 0.00 | [0.00; 1.61]a | 273 | 2 | 0.73 | [0.09; 2.62] | NS |
| Total EU MS | 544 | 0 | 0.00 | [0.00; 0.67] a | 721 | 2 | 0.28 | [0.03; 0.1] | NS |
MS: Member State.
–, The confidence interval is not provided because of the small sample size.
One‐sided, 97.5% confidence interval; p‐value: NS: not significant.
Fattening turkey flocks
For fattening turkey flocks, 22 MS and three non‐MS provided data. Hungary and Lithuania had flocks of fattening turkeys but did not report any data. In the EU in 2019, Salmonella was found in 2,241 or 5.84% of fattening turkey flocks compared with 6.32% in 2018. The EU prevalence of flocks positive for either of the two target Salmonella serovars was 0.24% (N = 93) (Figure 17), compared with 0.34% in 2018. Therefore, 4.1% (93 of 2,241) of reported Salmonella‐positive fattening turkey flocks were positive for either of the two target serovars. In total, 11 MS and two non‐MS reported no Salmonella target serovar‐positive flocks. Only Belgium did not meet the reduction target (Figure 8) of 1%. Belgium reported six S. Typhimurium‐positive flocks in 2019, similar to 2018. The EU flock prevalence was higher for S. Typhimurium (0.18%) than for S. Enteritidis (0.06%), with 56.99% of positive flocks for both serovars being reported by France, similar to the previous years.
Figure 17.
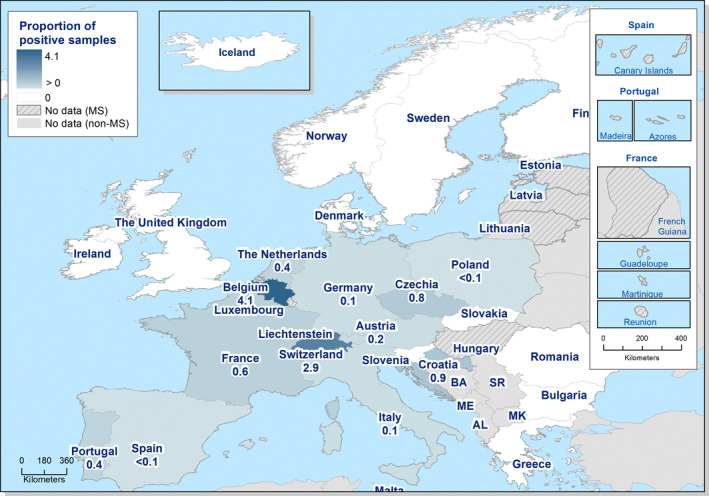
Prevalence of S. Enteritidis‐positive and/or S. Typhimurium‐positive (including monophasic variants) flocks of fattening turkeys before slaughter, EU/EFTA, 2019
-
AL: Albania; BA: Bosnia and Herzegovina; ME: Montenegro; MK: Republic of North Macedonia; and SR: Serbia.The following MS do not have turkey breeding flocks: Estonia, Latvia, Luxembourg and Malta.
Salmonella NCP monitoring data for turkey fattening flocks must be reported separately for investigations performed by CA and by FBOp, in addition to the overall merged results. Eighteen MS complied with the requirement, while four MS (Croatia, France, Italy and the Netherlands) did not send separate data from CA and FBOp. Considering all data sent, the percentages of target Salmonella‐positive flocks were, respectively, 0.64% (corresponding to 787 tested flocks) by the CA and 0.09% (corresponding to 22,299 tested flocks) by FBOp. The EU prevalence of Salmonella target serovar‐positive flocks based on official control samples (CA) was significantly higher than the FBOp’ self‐monitoring results. The same finding was also evident for data reported by Germany and Spain, like in 2018. In contrast, for the other MS that reported separate data from both CA and FBOp there were no significant differences between the two sampling categories (Table 14). Comparing data collected in 2019 with those reported in 2018, the prevalence of fattening turkey flocks positive for target serovars based on official control samples in 2019 (0.64%) was lower than the prevalence in 2018 for the same monitoring approach (2.07%).
Table 14.
Comparisons of prevalence of Salmonella target serovar‐positive flocks of fattening turkeys, by sampler and by reporting MS, EU, 2019
| Country | Competent authority (CA) | Food business operator (FBOp) | p‐value a | Interpretation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N flocks tested | N flocks positive to target serovars | % flocks positive to target serovars | CI95 | N flocks tested | N flocks positive to target serovars | % flocks positive to target serovars | CI95 | |||
| Austria | 20 | 0 | 0.00 | [0.00; 16.84]a | 431 | 1 | 0.23 | [0.00; 1.29] | NS | |
| Belgium | 4 | 0 | 0.00 | – | 147 | 6 | 4.08 | [1.51; 8.67] | ||
| Bulgaria | 2 | 0 | 0.00 | – | 4 | 0 | 0.00 | – | ||
| Cyprus | 4 | 0 | 0.00 | – | 4 | 0 | 0.00 | – | ||
| Czechia | 16 | 0 | 0.00 | [0.00; 20.50]a | 250 | 2 | 0.80 | [0.10; 2.86] | NS | |
| Denmark | 85 | 0 | 0.00 | [0.00; 4.25]a | 179 | 0 | 0.00 | [0.00; 2.04]a | NS | |
| Finland | 47 | 0 | 0.00 | [0.00; 7.55]a | 270 | 0 | 0.00 | [0.00; 1.36]a | NS | |
| Germany | 163 | 3 | 1.84 | [0.04; 5.28] | 4715 | 3 | 0.06 | [0.01; 0.19] | < 0.001 | CA > FBOp |
| Greece | 7 | 0 | 0.00 | – | 60 | 0 | 0.00 | [0.04; 5.96]a | ||
| Ireland | 59 | 0 | 0.00 | [0.00; 6.06]a | 566 | 0 | 0.00 | [0.06; 0.65]a | NS | |
| Poland | 149 | 0 | 0.00 | [0.00; 2.44]a | 6614 | 4 | 0.06 | [0.02; 0.15] | NS | |
| Portugal | 15 | 0 | 0.00 | [0.00; 21.80]a | 1159 | 5 | 0.43 | [0.14; 1.00]a | NS | |
| Romania | 33 | 0 | 0.00 | [0.00; 10.58]a | 977 | 0 | 0.00 | [0.00; 0.38]a | NS | |
| Slovakia | 6 | 0 | 0.00 | – | 57 | 0 | 0.00 | [0.00; 6.27]a | ||
| Slovenia | 7 | 0 | 0.00 | – | 109 | 0 | 0.00 | [0.00; 3.33]a | ||
| Spain | 92 | 2 | 2.17 | [0.26; 7.63] | 4259 | 0 | 0.00 | [0.00; 0.9]a | < 0.001 | CA > FBOp |
| Sweden | 30 | 0 | 0.00 | [0.00; 11.57]a | 121 | 0 | 0.00 | [0.00; 3.00]a | NS | |
| United Kingdom | 48 | 0 | 0.00 | [0.00; 7.40]a | 2377 | 0 | 0.00 | [0.00; 0.15]a | NS | |
| Total EU MS | 787 | 5 | 0.64 | [0.21; 1.48] | 22299 | 21 | 0.09 | [0.06; 0.14] | < 0.01 | CA > FBOp |
MS: Member State.
–, The confidence interval is not provided because of the small sample size.
One‐sided, 97.5% confidence interval; p‐value: NS: not significant.
Trends of Salmonella prevalence in poultry flocks
The trends in the EU flock prevalence of Salmonella target serovars in poultry flocks since the implementation of the EU‐wide NCP 2007–2019 are displayed in Figure 18.
Figure 18.
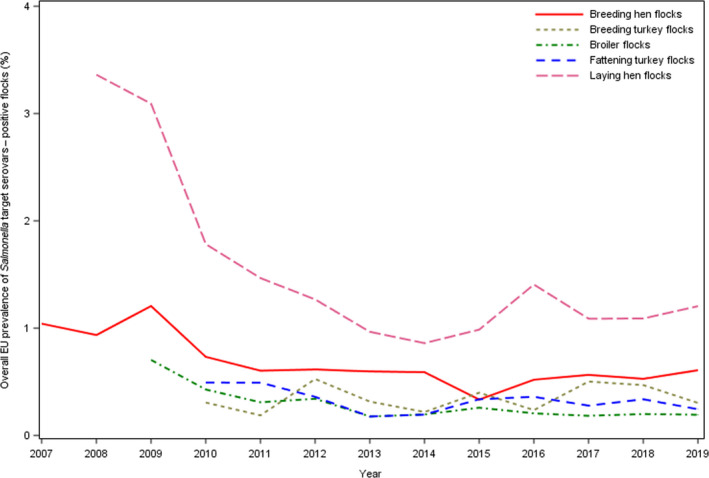
Overall reported percentage of poultry flocks positive for Salmonella target serovars relevant for public health in different poultry animal populations, reporting MS, EU, 2007–2019
In the supporting information to this report (‘Salmonella poultry outcome trends analyses’), the EU percentages of positive flocks for Salmonella, target and non‐target Salmonella serovars and S. Enteritidis over time are shown and compared for each poultry population covered by the NCP. Moreover, figures show the modelling of prevalence trends of Salmonella and target Salmonella serovars in poultry flocks. Detailed outputs of trend analyses (at subject level and at population level) are reported.
The apparent discrepancy between the percentage of positive flocks (both for target Salmonella serovars and for Salmonella, described above) and the estimated prevalence shown below is due to the fact that the first value is the ratio between all positive over all tested flocks, whereas the estimated prevalence is obtained by modelling the ratio between positive and tested flocks of each country, taking into account the variability among MS.
Breeding flocks of Gallus gallus
As observed during previous years, S. Enteritidis was by far the most common target serovar reported in 2019 in breeding flocks of Gallus gallus. Moreover, the temporal trend of S. Enteritidis in breeding Gallus gallus flocks was very similar to trends of the Salmonella target serovars, of Salmonella and of non‐target serovars.
The data used to model the trend in EU Salmonella flock prevalence for target serovars in breeding Gallus gallus for the period 2007–2019 were from 26 MS. Two MS (Estonia and Latvia) reported no single flock positive for target serovars during this entire period of implementation of NCP.
Since the beginning of the NCP, there has been an overall decreasing trend for the prevalence of breeding Gallus gallus flocks positive for target serovars (Figures 18 and 20); the prevalence estimated by modelling decreased from 1.10% CI95[0.62; 1.95] in 2007 to 0.38% CI95[0.28; 0.52] in 2015, the year in which the estimated prevalence achieved the minimum value. Over the next 4 years, the estimated prevalence slightly increased, reaching 0.46% CI95[0.35; 0.62] in 2019, but this increase was not statistically significant.
Figure 20.
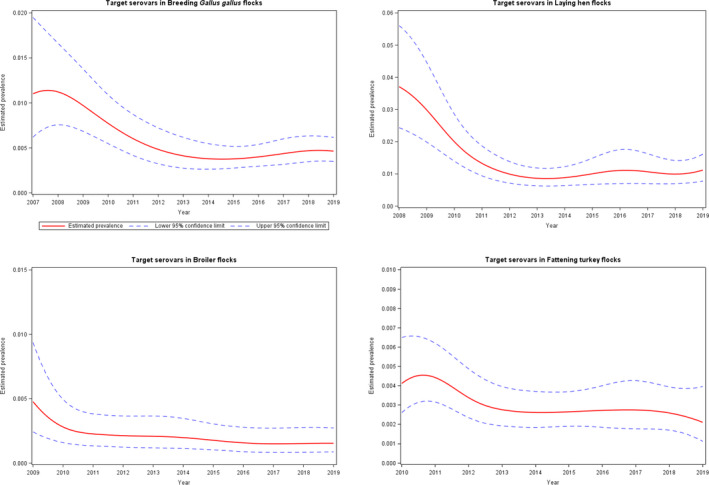
Estimates of the prevalence (represented as a probability taking any value between 0 and 1) of poultry flocks positive for Salmonella target serovars, at the EU level for different poultry populations, 2007–2019
After an initial fluctuation of the EU prevalence of Salmonella‐positive breeding flocks, the estimated prevalence reached the minimum value 1.2% CI95[0.76; 1.78] in 2015 and then it increased slightly to 1.76% CI95[1.23; 2.53] in 2019. This latter estimated prevalence was not significantly different from those of the previous 2 years, but it was significantly higher than the minimum prevalence estimated in 2015 (p‐value = 0.0701). Focusing the trend analysis modelling on the last 5 years confirmed that the estimated Salmonella flock prevalence in Gallus gallus breeding flocks has increased significantly and was significantly higher in 2019 compared with 2015 (p‐value = 0.042).
Flocks of laying hens
As observed during previous years in laying hen flocks, the temporal trends for S. Enteritidis, for target serovars, for non‐target serovars and for Salmonella were similar, because of its dominance, even though the prevalence differed.
Data used to model the trend in the EU Salmonella flock prevalence for target serovars in laying hen flocks over the period 2008–2019 were from all MS. No MS reported 0% prevalence for target serovars during this period. Since the beginning of the NCP, there has been a decreasing overall trend for the prevalence of flocks positive for target serovars (Figures 18 and 20); the prevalence estimated by modelling was 3.71% CI95[2.43; 5.61] in 2008 and decreased to reach the minimum value 0.86% CI95[0.63; 1.19] in 2014, with a steep downturn. From 2015 onwards, it increased slightly and stabilised to 1.1% CI95[0.77; 1.62] in 2019. This prevalence was not significantly different compared with the previous 2 years or compared with the minimum prevalence estimated in 2014.
The estimated EU Salmonella prevalence in laying hen flocks was 7.36% CI95[4.5; 11.83] in 2008 and decreased to 2.07% CI95[1.34; 3.19] in 2014, with a steep downturn. During the following years, it increased and reached 3.44% CI95[2.33; 5.06] in 2019. In 2019, the estimated Salmonella prevalence in laying hen flocks was not significantly different compared with the previous 2 years, but it was different compared with 2014, when the estimated prevalence reached the minimum value seen to date (p‐value = 0.0468). Focusing the trend analysis modelling on the last 6 years confirmed the prevalence of Salmonella in EU laying hen flocks has increased significantly, reaching a significantly higher prevalence in 2019 than in 2014 (p‐value = 0.075).
Figure 19 displays the EU S. Enteritidis flock prevalence in laying hens and the number of human cases due to S. Enteritidis infection acquired in the EU. The EU S. Enteritidis prevalence in laying hen flocks decreased from 2010 to 2014, after which it significantly increased during 2015 and 2016. It then decreased again and flattened out during 2017–2019 ranging from 0.86% to 0.95%. During 2010–2019, the number of human cases of S. Enteritidis infection acquired in the EU steadily increased and was highest during 2018 (32,727 cases) after a sharp decrease in human S. Enteritidis in 2013 (21,621 cases) compared with 2010.
Figure 19.
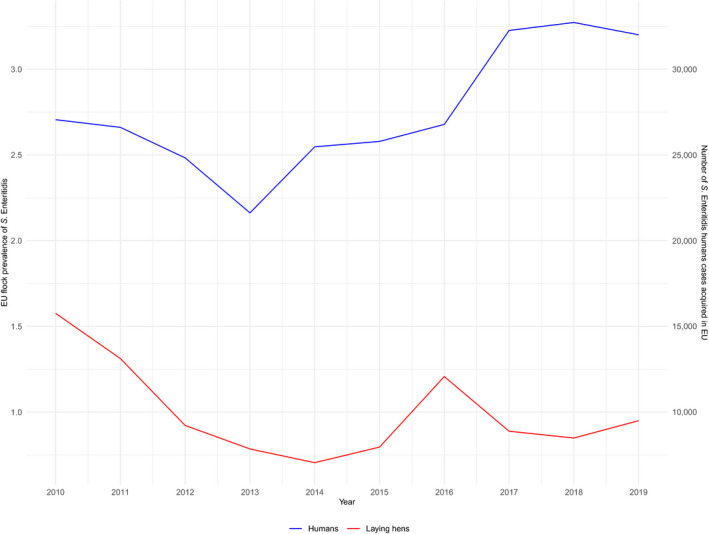
Percentage of laying hen flocks positive for S. Enteritidis and number of human salmonellosis cases due S. Enteritidis infection acquired in the EU, 2010–2019
Broiler flocks
As observed during previous years, in broiler flocks, the temporal trend of S. Enteritidis mimics that of the target serovars, because of its dominance. Moreover, the temporal trends of Salmonella and non‐target serovars are similar.
The data from 27 MS were used to model the trend in the EU Salmonella flock prevalence for target serovars in broilers flocks for the period 2009–2019. Finland reported no broiler flocks positive for Salmonella target serovars during this entire period, whereas Estonia notified its first positive flock for target serovars in 2019. From the beginning of the NCP, the flock prevalence for target serovars estimated by the model steeply decreased in the first time interval (until 2011) and then further decreased (Figures 18 and 20). The estimated prevalence was 0.48% CI95[0.24; 0.94] in 2009 and decreased to 0.15% CI95[0.09; 0.27] in 2019. This latter prevalence was not significantly different from that during the previous 2 years.
The EU prevalence of Salmonella‐positive broiler flocks estimated by modelling decreased from 2.9% CI95[1.44; 5.74] in 2009 to 1.3% CI95[0.73; 2.3] in 2015 and next increased again to 1.92% CI95[1.06; 3.47] in 2019. This increase was probably related to the increased reporting of non‐target serovars, in particular S. Infantis, the most frequently reported serovar from broiler flocks. Nevertheless, the estimated EU prevalence of Salmonella‐positive broiler flocks in 2019 was not significantly different to that of the previous 2 years or in 2015, when the estimated prevalence reached the minimum value. Focusing the trend analysis modelling on the last 5 years, the prevalence of Salmonella in broiler flocks was confirmed as increasing. However, the estimated Salmonella prevalence in EU broiler flocks in 2019 was not significantly higher than in 2015.
Breeding turkey flocks
In breeding turkey flocks, the temporal trends of S. Enteritidis and target serovars were similar, although with different prevalence, whereas the trends of Salmonella and non‐target serovars overlapped.
The data used to model the trend in EU Salmonella flock prevalence for target serovars in breeding turkey flocks for the period 2010–2019 were from 15 MS. Six MS reported no breeding turkey flocks positive for target Salmonella serovars over this entire period. The remaining MS had, from time to time, some positive flocks. The prevalence of Salmonella target serovar‐positive breeding turkey flocks fluctuated for the entire period around an estimated value of 0.35% CI95[0.28; 0.44].
After an initial fluctuation of the EU prevalence of Salmonella‐positive breeding turkey flocks from 7.7% CI95[3.36; 16.72] in 2010 to 1.33% CI95[0.69; 2.54] in 2016, when the estimate prevalence reached the lowest value seen in the entire study period, the estimated prevalence increased over time to 5.02% CI95[2.10; 11. 51] in 2019. This estimated prevalence in 2019 was not significantly different from the previous 2 years, but it was significantly higher than the estimated prevalence in 2016 (p‐value = 0.0468). Focusing the trend analysis modelling on the last 4 years results confirmed that the prevalence of Salmonella in breeding turkey flocks increased significantly, so the prevalence in 2019 was significantly higher than in 2016 (p‐value = 0.0249). This increase was probably related to the increased reporting of non‐target serovars.
Fattening turkey flocks
In fattening turkey flocks, the temporal trends of S. Enteritidis and the target serovars were different. Conversely, the temporal trends of Salmonella and non‐target serovars were very similar.
The data used to model the trend in the EU Salmonella flock prevalence for target serovars in fattening turkeys for the period 2010–2019 were from 25 MS. Two MS (Slovenia and Sweden) reported no single fattening turkey flock positive for target Salmonella serovars during this entire period. The Netherlands notified its first two positive flocks for target serovars in 2019. The estimated target serovar flock prevalence was 0.41% CI95[0.26; 0.64] in 2010, it decreased to 0.26% CI95[0.18; 0.37] in 2014 and decreased again to 0.21% CI95[0.11; 0.40] in 2019, after a slight increase from 2015 to 2017. Overall, the fattening turkey flock prevalence of target Salmonella serovars decreased slightly, but with small temporal fluctuations (Figures 18 and 20). Nevertheless, there were no significant differences in the estimated prevalence of the Salmonella target serovars in EU fattening turkey flocks in the last three years.
For this poultry category, after an initial fluctuation of the EU prevalence of Salmonella‐positive flocks from 5.9% CI95[1.44; 3.64] in 2010 to 2.1% CI95[1.06; 1.44] in 2015. In this last year, the estimate prevalence reached the lowest value and then it increased to 3.17% CI95[1.58; 6.23] in 2019. This increase was related to the increased reporting of non‐target serovars. Nevertheless, the prevalence in 2019 was not significantly different from those of the previous 2 years or from the minimum estimated prevalence in 2015. Focusing the trend analysis modelling on the last 5 years, a significant increasing trend of Salmonella prevalence in fattening turkey flocks was confirmed. However, the estimated prevalence of Salmonella in fattening turkey flocks in 2019 was not significantly higher than in 2015.
Salmonella monitoring data in other animals
Six MS (Estonia, Italy, Lithuania, Latvia, Poland and Sweden) and one non‐MS (Norway) reported monitoring data on Salmonella flock prevalence in ducks and geese for 2019. Of 8,343 flocks, 1.07% were positive for Salmonella, whereas 0.47% were positive for S. Enteritidis and/or S. Typhimurium.
In total, 15 MS and two non‐MS (Norway and Switzerland) reported data on Salmonella prevalence in pigs. Overall, 36.02% of the 66,624 reported sample units were positive for Salmonella. Among these, 72.3% (N = 48,184) were collected at the slaughterhouse and 49.07% were positive.
In cattle, based on data reported by 15 MS and four non‐MS at the EU level, the overall prevalence of Salmonella‐positive samples was 3.34% with 2,898 positive samples, whereas the prevalence of positive samples at the slaughterhouse was 7.76%.
2.4.5. Salmonella in feed
The overall prevalence of Salmonella‐positive units in ‘animal and vegetable derived feed’ supplies in 2019 in the EU was 2.46% of 29,111 reported units.
In compound feed (the finished feed for animals), the prevalence of Salmonella‐positive units in 2019 was 1.64% of 15,812 tested samples for poultry, 0.92% of 3,124 tested samples for cattle and 1.23% of 5,032 tested samples for pigs. As for feedingstuffs for animals other than pigs, cattle and poultry, the prevalence of Salmonella‐positive units in EU was 1.32% out of 9,686 tested samples. The prevalence of Salmonella‐positive sampling units for pet foods was 9.4% out of 3,448 tested samples.
2.4.6. Salmonella serovars in humans, food and animals
Humans
Serovars among all confirmed salmonellosis cases
For humans, information on Salmonella serovars was available for 90.2% of the total number of confirmed cases (79,300 cases out of 87,923) from 27 MS (Bulgaria did not report case‐based serovar data), Iceland and Norway. Data include all cases reported with serovar information regardless of the travel status. As in previous years, the three most commonly reported Salmonella serovars in 2019 were S. Enteritidis (50.3%), S. Typhimurium (11.9%) and monophasic S. Typhimurium (1,4,[5],12:i:‐) (8.2%), representing 70.3% of the 79,300 confirmed human cases with known serovar in 2019. The proportion of these three serovars was at the same level as in 2017 and 2018, as well as S. Infantis, which was the fourth most commonly reported serovar (Table 15). The fifth most common serovar S. Newport decreased by 20.0% compared with 2018. Serovar S. Mikawasima increased by 92.1% and 137.1% compared with 2018 and 2017, respectively. This serotype entered the top 20 list in 2019 and replaced serovar Brandenburg.
Table 15.
Distribution of reported confirmed cases of human salmonellosis in the EU/EEA, 2017–2019, by the 20 most frequent serovars in 2019
| Serovar | 2019 | 2018 | 2017 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | MSs | % | Cases | MSs | % | Cases | MSs | % | |
| Enteritidis* | 39,865 | 27 | 50.3 | 39,781 | 27 | 49.9 | 38,780 | 27 | 49.2 |
| Typhimurium* | 9,404 | 27 | 11.9 | 10,395 | 27 | 13.0 | 10,589 | 27 | 13.4 |
| Monophasic Typhimurium 1.4.[5].12:i:‐* | 6,491 | 18 | 8.2 | 6,427 | 17 | 8.1 | 6,322 | 16 | 8.0 |
| Infantis* | 1,924 | 26 | 2.4 | 1,859 | 26 | 2.3 | 1,803 | 26 | 2.3 |
| Newport | 870 | 24 | 1.1 | 1,086 | 21 | 1.4 | 920 | 24 | 1.2 |
| Derby | 721 | 23 | 0.9 | 710 | 23 | 0.9 | 612 | 23 | 0.8 |
| Stanley | 560 | 19 | 0.7 | 521 | 22 | 0.7 | 554 | 21 | 0.7 |
| Kentucky | 545 | 24 | 0.7 | 663 | 22 | 0.8 | 617 | 19 | 0.8 |
| Napoli | 508 | 18 | 0.6 | 457 | 15 | 0.6 | 406 | 17 | 0.5 |
| Agona | 503 | 20 | 0.6 | 602 | 18 | 0.8 | 645 | 20 | 0.8 |
| Virchow* | 477 | 21 | 0.6 | 541 | 24 | 0.7 | 510 | 21 | 0.6 |
| Coeln | 455 | 18 | 0.6 | 443 | 20 | 0.6 | 265 | 21 | 0.3 |
| Bovismorbificans | 454 | 19 | 0.6 | 465 | 18 | 0.6 | 344 | 20 | 0.4 |
| Java | 440 | 14 | 0.6 | 415 | 16 | 0.5 | 387 | 16 | 0.5 |
| Mikawasima | 415 | 15 | 0.5 | 216 | 13 | 0.3 | 175 | 13 | 0.2 |
| Chester | 350 | 17 | 0.4 | 369 | 19 | 0.5 | 329 | 18 | 0.4 |
| Bareilly | 321 | 17 | 0.4 | 299 | 16 | 0.4 | 427 | 18 | 0.5 |
| Saintpaul | 302 | 20 | 0.4 | 324 | 20 | 0.4 | 330 | 21 | 0.4 |
| Branderup | 300 | 18 | 0.4 | 259 | 17 | 0.3 | 260 | 18 | 0.3 |
| Hadar* | 298 | 17 | 0.4 | 312 | 20 | 0.4 | 334 | 19 | 0.4 |
| Other | 14,097 | – | 17.8 | 13,556 | – | 17.0 | 14,288 | – | 18.1 |
| Total | 79,300 | 27 | 100.0 | 79,700 | 27 | 100.0 | 78,897 | 27 | 100.0 |
MS: Member State.
Target Salmonella serovars in poultry populations. See Table 7 for details.
Source(s): 27 MS: Austria, Belgium, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, the United Kingdom; and two non‐MS: Iceland and Norway.
Serovars acquired in the EU
To estimate the impact of the Salmonella infections acquired at the EU level, serovar data were analysed for domestic and travel‐associated cases in which the probable country of infection was an EU MS. Information on Salmonella serovars with travel data was available from 24 MS, representing 74.8% of cases with known serovar data in 2019. Most cases (88.1%) with known data on serovar and travel were infected within the EU. Among the travel‐related cases, the most frequently reported travel destinations were Spain (28.9%), Greece (14.5%), Poland (9.9%), Italy (7.5%) and Croatia (6.9%), as in 2017–2018.
From reported cases of human salmonellosis acquired in the EU, S. Enteritidis dominated and almost two in three (61.6%) of the reported cases were infected by this serovar. Together with S. Typhimurium and monophasic S. Typhimurium 1,4,[5],12:i:‐, these three serovars represented 78.3% of the confirmed human cases acquired in the EU in 2019 (Table 16). S. Enteritidis cases were predominantly (93.1%) infected within EU. The proportion of S. Enteritidis was about at the same level as in 2017–2018. The proportion of S. Typhimurium and its monophasic variant strains 1,4,[5],12:i:‐ slightly decreased and S. Infantis and S. Derby remained at the same level as in 2018. Among the cases acquired in the EU, S. Newport has alternated between fifth and sixth places among the top six serovars.
Table 16.
Distribution of reported cases of human salmonellosis acquired in the EU, 2017–2019, by the six most frequently reported serovars in 2019
| Serovar | 2019 | 2018 | 2017 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | MSs | % | Cases | MSs | % | Cases | MSs | % | |
| Enteritidis | 32,010 | 24 | 61.6 | 32,727 | 24 | 60.9 | 32,262 | 25 | 61.2 |
| Typhimurium | 6,044 | 24 | 11.6 | 7,410 | 25 | 13.8 | 6,806 | 25 | 12.9 |
| Monophasic Typhimurium 1.4.[5].12:i:‐ | 2,688 | 17 | 5.2 | 2,553 | 17 | 4.7 | 2,096 | 16 | 4.0 |
| Infantis | 1,215 | 24 | 2.3 | 1,221 | 23 | 2.3 | 1,163 | 22 | 2.2 |
| Derby | 396 | 20 | 0.8 | 414 | 19 | 0.8 | 295 | 18 | 0.6 |
| Newport | 326 | 20 | 0.6 | 411 | 19 | 0.8 | 383 | 18 | 0.7 |
| Other | 9,322 | – | 17.9 | 9,047 | – | 16.8 | 9,723 | – | 18.4 |
| Total | 52,001 | 24 | 100.0 | 53,783 | 25 | 100.0 | 52,728 | 25 | 100.0 |
Source(s): 26 MS; Austria, Belgium, Croatia, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom.
A seasonal trend was observed for confirmed S. Enteritidis infections acquired in the EU in 2010–2019, with more cases reported during summer months. The trend from 2015 to 2019 was stable (flat) (Figure 21).
Figure 21.
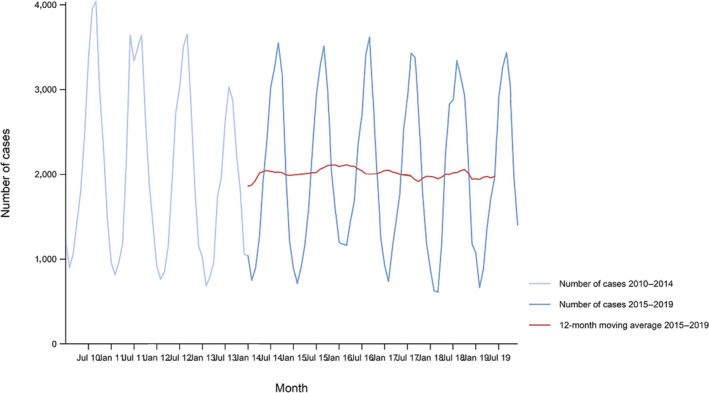
Trend in reported confirmed human cases of S. Enteritidis infections acquired in the EU, by month, 2015–2019
- Source(s): Austria, Czechia, Denmark, Estonia, Finland, Germany, Greece, Hungary, Ireland, Italy, Latvia, Malta, the Netherlands, Portugal, Slovakia, Spain, Sweden and the United Kingdom. Belgium, Bulgaria, Cyprus, Croatia, France, Lithuania, Luxembourg, Poland, Romania and Slovenia did not report data to the level of detail required for the analysis.
Malta was the only MS reporting a significantly decreasing (p < 0.01) trend of S. Enteritidis infections acquired within the EU over the last 5 years (2015–2019). A significant increasing trend was not observed in any MS for the last 5 years.
Food and animals
Descriptive analyses were made from food and animal data from 2019 for the five Salmonella serovars that were most frequently reported from cases of human salmonellosis acquired in the EU (Table 16). These top five serovars were S. Enteritidis, S. Typhimurium, monophasic S. Typhimurium, S. Infantis and S. Derby. Only isolates related to food‐producing animals and specific food matrices were aggregated into the following categories for further analyses: broiler flocks – broiler meat, laying hen flocks – eggs, fattening turkey flocks – turkey meat, pigs – pig meat and cattle – bovine meat. In total, 17,176 Salmonella serotyped isolates were reported that matched the mentioned inclusion criteria (Table 17).
Table 17.
Distribution of Salmonella isolates (number and percentage) with and without serotype identification among the different sources (food and animals), EU, 2019
| Broilers | Broiler meat | Bovine animals | Cattle meat | Pigs | Pig meat | Turkeys | Turkey meat | Laying hens of Gallus gallus | Eggs | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Salmonella units without serotyped isolate (N and %) | 2,308 | 500 | 72 | 39 | 23,455 | 1,732 | 1,213 | 144 | 272 | 2 | 29,737 |
| 7.76% | 1.68% | 0.24% | 0.13% | 78.87% | 5.82% | 4.08% | 0.48% | 0.91% | 0.01% | ||
| Salmonella units with serotyped isolate (N and %) | 10,632 | 1,820 | 196 | 22 | 465 | 1,588 | 1,041 | 144 | 1,258 | 10 | 17,176 |
| 61.90% | 10.60% | 1.14% | 0.13% | 2.71% | 9.25% | 6.06% | 0.84% | 7.32% | 0.06% |
Hence, more than 70% of these serotyped isolates were from broilers (both animals and food), pig sources accounted for about 12% of the serotyped isolates, laying hens and turkeys about 7% each (but for both species the vast majority of the isolates were from the animal sources), whereas serotyped isolates from cattle made up about 1% of the serotyped isolates.
The top‐five serovars responsible for human infections were distributed as follows among the serotyped isolates (17,176) from these food‐animal sources: S. Infantis accounted for 29.7% of them, S. Enteritidis 6.9%, monophasic variants of S. Typhimurium 4.5%, S. Typhimurium 3.9% and S. Derby 3.7%.
The Sankey diagram (Figure 22) illustrates how the EU top five Salmonella serovars in human salmonellosis cases acquired in the EU are associated with the most important animal species. S. Enteritidis was primarily associated with broiler sources (67.8% of the S. Enteritidis isolates were from broiler flocks and meat) and secondly with layers (26.7%). S. Typhimurium was mainly associated with pig, broiler and layer sources, respectively, 42%, 34.8% and 13.5%. Monophasic S. Typhimurium was associated mainly with pig (72.1%) and secondly with broiler (17.1%) sources. S. Infantis was mostly related to broiler sources (93.1%). S. Derby was primarily associated with pig (72%) and secondly with turkey (19.8%) sources. To interpret these data, it is important to be aware that the distribution of the serotyped isolates among the different sources is very heterogeneous in terms of number of isolates per species, as detailed above.
Figure 22.
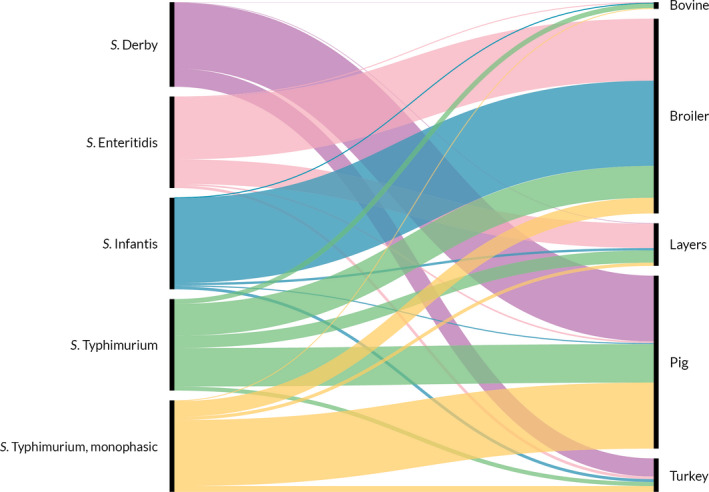
Sankey diagram of the distribution of the EU top five Salmonella serovars in human salmonellosis acquired in the EU, reported from specified food–animal categories, by food animal sources, EU, 2019
- The left side of the diagram shows the five most reported Salmonella serovars from human salmonellosis cases acquired in the EU: S. Enteritidis (pink), S. Typhimurium (green), monophasic S. Typhimurium (yellow), S. Infantis (blue) and S. Derby (violet). Animal and food data from the same source were merged: ‘broiler’ includes isolates from broiler flocks and broiler meat, ‘bovine’ includes isolates from bovines for meat production and bovine meat, ‘pig’ includes isolates from fattening pigs and pig meat, ‘turkey’ includes isolates from fattening turkey flocks and turkey meat and ‘layers’ includes isolates from laying hen flocks and eggs. The right side shows the five sources considered (broiler, bovine, pig, turkey and layers). The width of the coloured bands linking sources and serovars is proportional to the percentage of isolates of each serovar from each source.
The Sankey diagram in Figure 23 illustrates how the EU top five Salmonella serovars in human salmonellosis acquired in the EU were proportionally reported by the reporting MS from specified food–animal sources mentioned, in 2019. In this context too, the number of serotyped isolates reported by each MS is very heterogeneous which must be considered when interpreting the following data. Twenty‐seven MS reported the top‐five Salmonella serovars from the above sources. S. Enteritidis was widely reported by most MS, even though Poland accounted for the greatest percentage (49.6%) of the isolates, followed by France that reported 13.6% of the S. Enteritidis. Similarly, S. Typhimurium and monophasic S. Typhimurium isolates were reported by all MS, but the highest percentage of both serovars was reported by France, accounting for 29.4% and 27.8%, respectively. S. Infantis isolates were mostly reported by Italy (50.6%), whereas S. Derby was mostly reported, in decreasing order, by the United Kingdom (22.8%), Denmark (20.7%), Italy (13.5%) and France (11.8%).
Figure 23.
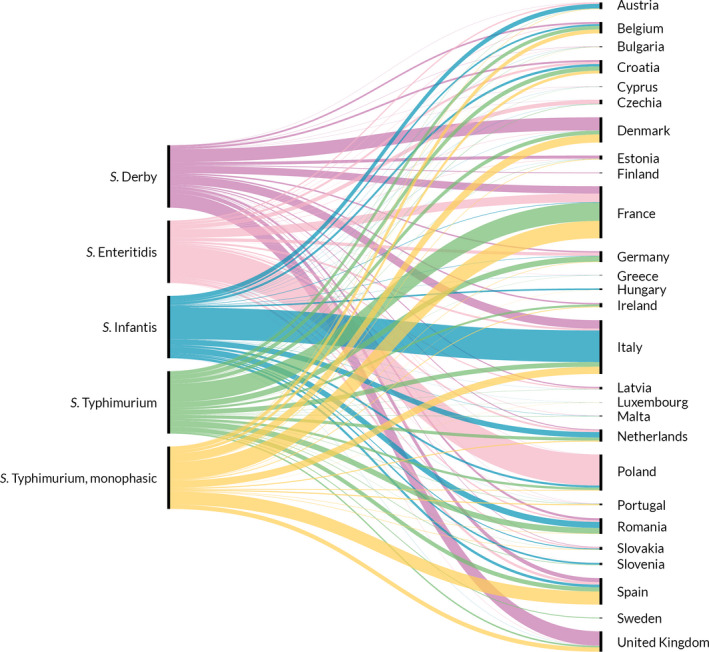
Sankey diagram of the distribution of the EU top five Salmonella serovars in human salmonellosis acquired in the EU and reported from specified food–animal categories, by reporting MS, EU, 2019
- The left side of the diagram shows the five most reported Salmonella serovars from human salmonellosis cases acquired in the EU: S. Enteritidis (pink), S. Typhimurium (green), monophasic S. Typhimurium (yellow), S. Infantis (blue) and S. Derby (violet). The right side shows the reporting MS. The width of the coloured bands linking MS and serovars is proportional to the percentage of isolates of each serovar reported by each MS.
EU top five Salmonella serovars: comparison of food and animal sources
Figure 24 shows the percentages of the EU top five Salmonella serovars in human salmonellosis acquired in the EU and reported from specified food and animal matrices, by food–animal category with isolates. Considering all poultry sources, S. Infantis was the most reported serovar, accounting for 5,043 of 14,905 (33.8%) serotyped isolates, followed by S. Enteritidis (1,156; 7.8%).
Figure 24.

Percentages of the EU top‐five Salmonella serovars in human salmonellosis acquired in the EU and reported from specified food–animal categories, by food–animal category with isolates, EU, 2019
- The percentages were calculated based on the total number of isolates serotyped for each of the five animal/food categories (bovine, broiler, layers, pig and turkey). The values at the top of each box are the numbers of Salmonella serovar isolates and the numbers in parentheses are the number of reporting MS, for animal matrices (grey) and food matrices (black). Each plot shows the percentage of isolates belonging to the reported serovar out of the total number of serotyped isolates.
S. Infantis was massively reported for broiler matrices, both from animals (36.3% of all serotyped isolates) and from food matrices (49.1%). It was also present, but to a lesser extent, in turkey flocks (13.3% of all serotyped isolates), turkey meat (13.9%) and in layer flocks (10.2%) (Figure 22). More than 50% of the S. Infantis isolated in 2019 from broilers was reported by Italy. Looking in detail at the serovar data from broiler flocks and focusing on the four MS that reported more than 75% of all serotyped isolates from this source (Italy 34.78%, France 19.4%, the United Kingdom 14.14% and the Netherlands 9.46%), the situation, in terms of reporting of S. Infantis, was very heterogeneous. Italy and the Netherlands reported 64.9% and 42.9%, respectively, of their serotyped isolates as S. Infantis. In contrast, the United Kingdom and France reported, respectively, none and less than 1% of the isolates belonging to this serovar from broiler flocks, whereas these two countries frequently reported other serovars in broiler flocks (e.g. S. Livingstone, S. Montevideo, S. Mbandaka, S. Kedougou). Irrespective of the situation in broilers, for most of the reporting MS, S. Infantis was the most common serovar reported from broiler meat (about one in two isolates were from this source).
S. Enteritidis accounted for 50% of all Salmonella isolates serotyped from eggs and 24.8% of the serotyped isolates from layer flocks. It also accounted for 25.2% of serotyped isolates from broiler meat. More than 50% of S. Enteritidis isolated in 2019 from these sources was reported by Poland. For the other sources, a very small number of S. Enteritidis isolates was reported.
For S. Typhimurium and its monophasic variants, they showed similar patterns, with S. Typhimurium accounting for 12.7% and 14% of the serotyped isolates from pig herds and pig meat and its monophasic variants accounting for 28.8% and 26.6% of serotyped isolates from these matrices, respectively. For bovine meat, 31.8% and 13.6% of serotyped isolates were S. Typhimurium and its monophasic variants, respectively.
Finally, S. Derby accounted for 24.1% of all the serotyped isolates from pigs and 21.3% of all serotyped pig meat isolates, while the percentages from turkey matrices were considerably lower (11.6% and 2.1% of all serotyped isolates from turkeys and turkey meat, respectively). Among the remaining animal/food categories, this serotype was rarely reported.
2.5. Discussion
Salmonellosis remains the second most common zoonosis in humans in the EU after campylobacteriosis. The previous decreasing trend of confirmed cases has stabilised since 2014 and, in 2019, the number of reported confirmed human cases and the EU notification rate were at the same level as in 2018. In 2019, only one MS (Finland) reported a decreasing trend in the last 5 years, whereas all other MS reported stable, flat trends during 2015–2019.
S. Enteritidis infections that were acquired within the EU also stabilised in 2015–2019, after several years of an increasing trend. S. Enteritidis infection is predominantly acquired in the EU, more frequently than other serovars. A large European multi‐country outbreak of S. Enteritidis associated with contaminated eggs from Poland was confirmed in 14 EU/EEA countries in 2016. Poland implemented control measures and the cases declined in 2017 but started to increase again at the end of the same year. It is likely that this multi‐country outbreak had already existed since 2012 and was still ongoing during 2019. Since 2016, the number of confirmed S. Enteritidis human cases has steadily increased and cases have been confirmed in 18 EU/EEA countries, with the most recent epidemiological update reported in February 2020 (ECDC, 2018; EFSA and ECDC, 2017a,b,c, 2018a,b, 2020a,b). In each year from 2016 to 2018, outbreak cases peaked in September, with large waves of cases reported between late spring and early autumn. Such a large seasonal increase was no longer observed in 2019. In this context, it is noteworthy that 54.7% of the S. Enteritidis‐positive breeding flocks of Gallus gallus were reported by Poland. All MS except Bulgaria, Croatia, Ireland, Poland and Slovenia met the flock prevalence target of maximum 1%. In laying hens, 80.9% of S. Enteritidis‐positive flocks were reported by six MS (France, Germany, Italy, the Netherlands, Poland and Spain) and Bulgaria, Croatia, Poland and Spain did not meet their reduction target, which was 2% flocks remaining positive for all MS except for Poland for which it was 3.5%.
The three most commonly reported serovars S. Enteritidis and S. Typhimurium (including monophasic variants) accounted for over 70% of human cases acquired in the EU. S. Infantis has been consistently the fourth most frequently reported serovar in the domestically acquired and travel‐associated human infections. As in previous year, serovars S. Derby and S. Newport were reported in almost equal numbers, being the fifth and sixth most frequently reported serovars in 2019. The EU trends for these six serovars have been stable in the last 5 years between 2015 and 2019.
Notification rates for salmonellosis in humans vary between MS, reflecting variations in, for example, quality, coverage and disease‐severity focus of the surveillance systems, practices in sampling and testing, disease prevalence in the food‐producing animal population, food and animal trade between MS and the proportion of travel‐associated cases. The hospitalisation rate varied from 23.5% to 96%. Countries reporting the lowest notification rates for salmonellosis had the highest proportions of hospitalisation, suggesting that the surveillance systems in these countries are focused on the most severe cases and underlining the variation in national surveillance systems.
Monitoring results for Salmonella contamination in food is in large part based on data collected in the context of Regulation (EC) No 2073/2005, which guarantees a certain level of harmonisation in terms of food categories considered, analytical methods used and sampling points. In this specific context, poultry meats (including fresh meat, minced meat, meat preparations and meat products) have been identified as the food categories for which Salmonella was most frequently reported, even though Salmonella national control programmes in poultry at the primary production level have been specifically implemented for several years (Antunes et al., 2016). Moreover, looking at FBOs, as in the previous years, egg and eggs products ranked first of food vehicles causing strong‐evidence salmonellosis FBOs. This matrix was implicated in 37% of such outbreaks.
Monitoring results for Salmonella contamination in RTE and non‐RTE food were also described for samples collected according to an ‘objective’ sampling strategy. The overall percentages of Salmonella‐positive samples for RTE and non‐RTE food were 0.27% and 1.52%, with ‘meat and meat products’ reported to have 0.55% and 1.66% positive samples, for the two categories, respectively. The findings of Salmonella‐contaminated RTE food is of concern because it poses a direct risk to the consumer. Another food category reported both within RTE and non‐RTE food was ‘infant formulae’ with 1.63% and 1.78% positive samples for RTE (N = 123) and non‐RTE products (N = 562). These findings merit attention because this product is intended for young, susceptible children. Outbreaks due to contaminated infant formula are reported and during 2019 a multi‐country outbreak associated with infant formula contaminated by Salmonella Poona involved three MS (France, Belgium and Luxembourg) affecting 32 infants and young children (EFSA and ECDC, 2019a). Analytical evidence linked that outbreak to another S. Poona outbreak relating to the same facility in 2010–2011, indicating a persistent source of contamination (Jones et al., 2019).
Control programmes in poultry at primary production level focus on serovars of particular relevance for public health (i.e. S. Enteritidis and S. Typhimurium), whereas data collected from poultry food categories refer to the genus Salmonella, regardless of serovar (with the only exception being fresh poultry meat). Trends for the target Salmonella serovar‐positive flocks have been quite constant (flat) over recent years for almost all poultry categories. The number of MS that did not meet the annual targets for the different poultry categories decreased in 2019 compared with 2018. Combining all these data, it seems that efforts aimed at control of S. Enteritidis and S. Typhimurium in poultry flocks have been partially effective. However, if we look at trends of Salmonella flock prevalence in poultry populations over the last 4–6 years, a significant increase was noted in breeding Gallus gallus, laying hens and breeding turkeys. These increasing trends for Salmonella can be partly explained by the emerging spread of certain clones in the different animal populations e.g. S. Infantis (EFSA BIOHAZ Panel, 2019).
S. Infantis is overall by far the most frequently reported serovar in broilers and their derived carcases. When considering the four countries reporting more than 75% of all reported serotyped isolates from the broiler source (in decreasing order: Italy, France, UK and the Netherlands), their reports on S. Infantis are very diverse. For Italy and the Netherlands, most of reported serovars from broiler flocks were S. Infantis, (64.9% and 42.9%, respectively), while UK and France reported almost no S. Infantis, but reported mainly isolates of S. Montevideo and S. Livingstone (France) and S. Kedougou and S. Mbandaka (UK). Other countries reporting a proportion of S. Infantis higher than 50% of the serotyped isolates, from broiler flocks, were Austria (75.2%), Slovakia (62.5%), Spain (60%) Croatia (54.1%) and Romania (53.6%). Caution is warranted when interpreting these data because the reporting of this serovar, as the other non‐target serovars, is not mandatory for broilers (reporting bias). Still, irrespective of MS‐specific reports for broiler flocks, S. Infantis was the most common serovar reported from broiler meat (about one in two isolates from this source), for most reporting MS. The recent epidemiological success of this serovar can be associated with its ability to enter and persist along the poultry food chain and this represents a growing risk for public health (Nagy et al., 2020). Moreover, the worldwide emergence of S. Infantis clones with enhanced epidemiological fitness has been attributed to the acquisition of a conjugative megaplasmid providing the bacteria with new resistance features, virulence‐associated properties, high tolerance to disinfectants and resistance to heavy metals (García‐Soto et al., 2020). S. Kentucky is another serovar that has undergone emergent spread both in humans and in the food chain, especially some clones (e.g. ST 198) characterised by resistance to multiple antimicrobials including some critically important ones (e.g. fluoroquinolones) (EFSA and ECDC, 2020b). This scenario has led France to include S. Kentucky among the regulated serovars in poultry, at national level.
It has been hypothesised that the recent spread of some serovars could be partly associated with to the regulatory policy addressing a limited selection of target serovars in the different poultry populations and that this surveillance approach could have allowed the expansion of other serovars that have found new niches in the poultry industry (EFSA BIOHAZ Panel, 2019).
As recently proposed by EFSA (EFSA BIOHAZ Panel, 2019), an alternative approach based on an ‘all serovars’ target for breeding flocks could be more effective. Moreover, this extended approach could be valuable in limiting the spread of emerging or re‐emerging serovars showing epidemic potential. Eventually this extended approach could have a direct effect in reducing the Salmonella prevalence in foodstuffs. However, this new extended target could be rather challenging for many MS and a good compromise could be a dual prevalence target for ‘all serovars’ and for ‘the selected/high priority serovars’ with different control measures and containment methods based on the identified serovar. Anyway, in 2019, S. Enteritidis remained the most common serovar in humans causing most FBOs. The flock prevalence of breeding Gallus gallus and laying hens was highest for S. Enteritidis, whereas for broilers the prevalence was at the same level as S. Typhimurium. These data indicate that it is important to prioritise attention on this serovar to avoid underestimating the risk posed by S. Enteritidis, especially in laying hens, where its true prevalence is likely to be substantially underestimated (EFSA BIOHAZ Panel, 2019), as this would have a direct effect on the control of most Salmonella cases in humans (De Cesare, 2018).
Salmonella was found in 2.46% tested units of ‘animal and vegetable derived feed’ supplies and 1.64% of the compound feed for poultry. These data demonstrated that feed remains a putative source of infections for poultry populations and finally for humans, although target serovars are not common in feed, but unfortunately, as for many other categories, prevalence data from feed are not representative of the EU situation since the number of serotyped isolates are very limited and are reported from few countries that vary over the years.
According to the legislation, the surveillance of Salmonella along the food chain is based on controls implemented by FBOp and CA. When there were data available to compare the Salmonella prevalence identified by the two systems, the percentage of Salmonella‐positive units reported by official controls was generally higher than that reported in the context of own check controls by FBOp. These differences can be related to the fact that the CA generally focuses their samplings on the most problematic herds/slaughterhouses (risk‐based approach). Anyway, this situation deserves attention as in the EU Salmonella surveillance at all levels of the food chain is primarily based on the controls conducted by FBOp. They are the cornerstone of the strategy and their control systems must be as effective as possible to guarantee proper surveillance of the pathogen. In light of this, comparative data collection (official controls vs. own checks) on sample sensitivity could also be considered for breeding and laying hen flocks.
Integrated surveillance based on the ‘One health’ approach combined with effective containment measures along the entire food chain (based on the application of biosecurity measures, effective surveillance and vaccination at the farm level, good manufacturing and hygienic practices during slaughtering, food processing, at retail and in the consumer phase) within integrated systems implemented by FBOp under the control of CAs are essential to control the spread of Salmonella, especially the most important current and emergent epidemic clones (Antunes et al., 2016; Campos et al., 2019).
2.6. Related projects and Internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definition of salmonellosis | https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions | |
| Disease Programme on Emerging, Food‐ and Vector‐Borne Diseases | https://www.ecdc.europa.eu/en/about-us/who-we-are/units/disease-programmes-unit | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| World Health Organization – Salmonella (non‐typhoidal) fact sheet | http://www.who.int/mediacentre/factsheets/fs139/en/ | |
| Food | European Union Reference Laboratory (EURL) for Salmonella | www.eurlsalmonella.eu |
| Microbiological criteria | https://ec.europa.eu/food/safety/biosafety/food_hygiene/microbiological_criteria_en | |
| Scientific Opinion on Public health risks of table eggs due to deterioration and development of pathogens | https://www.efsa.europa.eu/en/efsajournal/pub/3782 | |
| Scientific Opinion on the link between Salmonella criteria at different stages of the poultry production chain | https://www.efsa.europa.eu/en/efsajournal/pub/1545 | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports | |
| Animals | Control of Salmonella in animals | https://ec.europa.eu/food/safety/biosafety/food_borne_diseases/salmonella_en |
| General information on National Veterinary Programmes, in EU | https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en | |
| Scientific Opinion on Salmonella control in poultry flocks and its public health impact | https://www.efsa.europa.eu/en/efsajournal/pub/5596 | |
| Scientific Opinion on a quantitative estimation of the public health impact of setting a new target for the reduction of Salmonella in laying hens | http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2010.1546/abstract | |
| Scientific Opinion on public health impact of new target for the reduction of Salmonella in turkey flocks | https://www.efsa.europa.eu/en/efsajournal/pub/2616 | |
| Scientific Opinion on public health impact new target for the reduction of Salmonella in broiler flocks | https://www.efsa.europa.eu/en/efsajournal/pub/2106 | |
| Scientific Opinion on Salmonella in slaughter and breeder pigs | https://www.efsa.europa.eu/en/efsajournal/pub/1547 |
3. Listeria
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
3.1. Key facts
In 2019, 28 MS reported 2,621 confirmed invasive human cases of listeriosis with an EU notification rate of 0.46 cases per 100,000 population, which was at the same level as in 2018.
The EU trend of confirmed listeriosis cases remained stable (flat) in 2015–2019 after a long period of an increasing trend.
Listeria infections were most commonly reported in the age group over 64 years and particularly in the age group over 84 years.
The overall EU case fatality was high (17.6%) and increased compared with 2018 and 2017 (13.6% and 15.6%, respectively). This makes listeriosis one of the most serious food‐borne diseases under EU surveillance.
In 2019, the number of outbreaks caused by L. monocytogenes (n = 21) was 50% higher compared with 2018 (n = 14) and the related illnesses jumped from a total number of 748 cases reported at the EU level between 2010 and 2018 (83.4 annual cases on average) to 349 cases. This increase was mainly due to outbreaks in Spain, which reported 3 outbreaks, 225 cases, 131 hospitalisations and 3 deaths, compared with zero reported in 2018.
The occurrence of L. monocytogenes varied according to the RTE food category and the sampling stage. In all food categories covered by the Regulation (EC) No 2073/2005, the level of non‐satisfactory results remained low at retail (0.0% for hard cheeses to 2.1% for products of meat origin, fermented sausages). At processing, this level is systematically higher for all categories. The highest level was found, as previous year, for fish, with 5.8% unsatisfactory single units.
3.2. Surveillance and monitoring of Listeria monocytogenes in the EU
3.2.1. Humans
Surveillance of listeriosis in humans in the EU is based on invasive forms of L. monocytogenes infection, mostly manifested as septicaemia, meningitis or spontaneous abortion. Diagnosis of Listeria infections in humans is generally carried out by culture from blood, cerebrospinal fluid and vaginal swabs.
Notification of listeriosis in humans is mandatory in most EU MS, Iceland, Norway and Switzerland, except for three MS, where notification is based on a voluntary system (Luxembourg and the United Kingdom) and another, non‐specified system (Belgium). The surveillance systems for listeriosis cover the whole population in all MS, except in Belgium and Spain. Since 2015, the coverage of the surveillance system is estimated to be 80% in Belgium and this proportion of populations was used in the calculation of notification rates. No estimate for the population coverage was provided for Spain, so the notification rate was not calculated. For 2019, Spain did not receive data from all regions due to COVID‐19 so the case numbers might therefore not be complete. All countries reported case‐based data except Bulgaria, which reported aggregated data. Both reporting formats were included to calculate numbers of cases and notification rates.
3.2.2. Food, animals and feed
Monitoring of L. monocytogenes is conducted along the food chain during preharvest (e.g. animals at the farm and their feed), processing (e.g. cutting plant, slaughterhouses) and post‐processing (e.g. retail and catering). The public health risk of L. monocytogenes posed by RTE food also depends on the effectiveness of its control, which includes the implementation of Good Agricultural Practices (GAPs) at the farm level, the Good Manufacturing Practices (GMP) and HACCP programme during processing and retail in food business operators (FBOp). Regulation (EC) No 2073/20058 on microbiological criteria lays down the microbiological criteria and the implementing rules to be complied with by the FBOp when implementing the general and specific hygiene measures of Regulation (EC) No 852/2002. In this Regulation, RTE food is defined, as ‘Food intended by the producer or the manufacturer for direct human consumption without the need for cooking or other processing effective to cut out or reduce to acceptable level microorganisms of concern’. The National CAs conduct investigations (official sampling) to verify whether the FBOp implement correctly the legal framework of own check programmes (compliance with FSC, including for L. monocytogenes) as well as the analyses as part of HACCP (industry monitoring) according to the General Food Law principles.
The rationale for surveillance and monitoring of L. monocytogenes in animals, feed and food at the different stages along the food chain and the number of samples provided to EFSA for 2019 is shown in Figure 25. In 2019, 25 MS reported 218,439 samples tested for L. monocytogenes on different RTE food categories at retail or processing stages and 13 MS reported 22,135 samples tested at primary production level.
Figure 25.
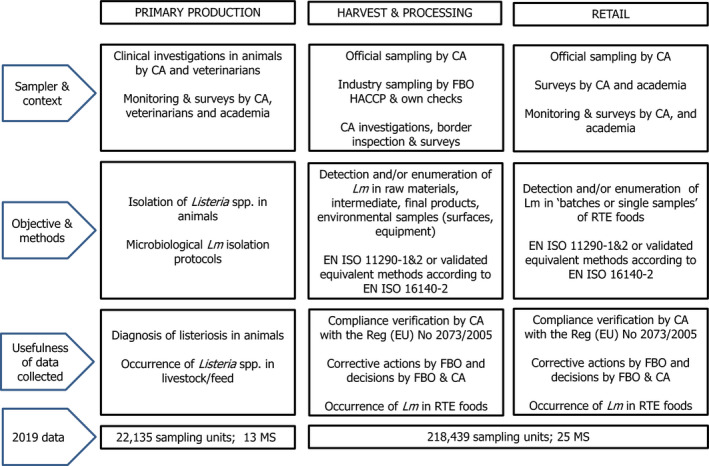
Overview of L. monocytogenes testing along the food chain according to the sampling stage, the sampler and the objective of the sampling
- CA: Competent Authority; FBOp: Food business operator; Lm: Listeria monocytogenes; MS: Member State; RTE: ready‐to‐eat.
Most of the monitoring data on L. monocytogenes in animals and feed provided are generated by non‐harmonised monitoring schemes across MS and for which mandatory reporting requirements do not exist. Among several transmission routes, listeriosis in animals can be acquired via the consumption of contaminated feed such as poor‐quality silage. Data on L. monocytogenes occurrence in feed are only collected as part of clinical investigations in farm animals. Hence, monitoring data on L. monocytogenes in animal feed are rarely available.
Reported data on L. monocytogenes in RTE food are, in the most part, food chain control data (official monitoring) and are collected by the CA conducting investigations to verify whether FBOp implement correctly the above‐mentioned FSC, which have been in force since January 2006. Data provided to EFSA within that context only allow a descriptive summary at the EU level and are not harmonised.
3.2.3. Food‐borne outbreaks of listeriosis
The reporting of food‐borne outbreaks is mandatory according to Zoonoses Directive 2003/99/EC and the reported data represent the most comprehensive set of data available at the EU level for assessing their burden – including those caused by L. monocytogenes. More details can be found in the chapter on food‐borne outbreaks.
3.3. Data analyses
The following two data streams were distinguished for summarising the information on L. monocytogenes in RTE foods.
3.3.1. Data of RTE food in the context of Regulation (EC) No 2073/2005 on microbiological criteria
The first stream of data is the official food chain control data; these data comprise samples collected by the CA as part of verification of the compliance of L. monocytogenes FSC listed in Regulation (EC) No 2073/2005 to verify whether FBOp implement correctly the legal framework of own check programmes as well as the analyses as part of HACCP according to the General Food Law principles. These data were filtered from the database using the criteria ‘official sampling’ for the sampler, ‘single units’ for the sampling unit and ‘objective sampling’ for the sampling strategy.
L. monocytogenes FSC of the Regulation (EC) No 2073/2005, which are to be complied with by FBOp and which are batch based, are specified by RTE food category, by sampling stage and are underpinned by the results of either the detection (ISO, 2017a) or enumeration (ISO, 2017b) analytical methods (Table 18).
Table 18.
L. monocytogenes FSC as described in Regulation (EC) No 2073/2005 for the different RTE categories across the food chain
| Sampling stage | RTE foods intended for infants and RTE foods for special medical purposes | Other RTE foods | |
|---|---|---|---|
| Able to support the growth of Lm | Unable to support the growth of Lm | ||
| Processinga | NA | Based on detection method: Lm not detected in 25 g of sample (n = 5, c = 0)c | NA |
| Retailb | Based on detection method: Lm not detected in 25 g of sample (n = 10, c = 0) | Based on enumeration method: limit of 100 CFU/g (n = 5, c = 0)d | Based on enumeration method: limit of 100 CFU/g (n = 5, c = 0) |
Lm: Listeria monocytogenes; NA: not applicable; RTE: ready‐to‐eat.
Before the food has left the immediate control of the food business operator who has produced it.
Products placed on the market during their shelf life.
n = number of units comprising the sample (number of sample units per food batch that are required for testing); c = the cmaximum allowable number of sample units yielding unsatisfactory test results. In a two‐class attributes sampling plan defined by n = 10, c = 0 and a microbiological limit of ‘not detected in 25 g’, in order for the food batch to be considered acceptable, L. monocytogenes must not be detected in qualitative (detection) analyses of 25‐g food test portions obtained from each one of 10 sample units taken from the batch. If even one of the sample units from the batch is found to contain L. monocytogenes (detected in 25 g), then the entire batch is deemed unacceptable. This criterion applies to products before they have left the immediate control of the producing food business operator, when he is not able to demonstrate, to the satisfaction of the competent authority, that the product will not exceed the limit of 100 CFU/g throughout the shelf‐life.
This criterion applies if the manufacturer is able to demonstrate, to the satisfaction of the competent authority, that the product will not exceed the limit 100 CFU/g throughout the shelf‐life. The operator may fix intermediate limits during the process that should be low enough to guarantee that the limit of 100 CFU/g is not exceeded at the end of the shelf‐life.
Data reported by MS were separated into the different categories of RTE food/sampling stages based on the assumptions described in the EU summary zoonoses and food‐borne outbreaks report of 2016.9 Briefly these assumptions are: all sampling units that were collected from ‘cutting plants’ and ‘processing plants’ were considered as units collected at the processing stage, while sampling units that were obtained from ‘catering’, ‘hospital or medical care facility’, ‘retail’, ‘wholesale’, ‘restaurant or cafe or pub or bar or hotel or catering service’, ‘border inspection activities’, ‘packing centre’ and ‘automatic distribution system for raw milk’ were considered as units collected at retail. When stage was ‘not available’, ‘unspecified’, data have also been considered as part of the retail stage. As no data on physicochemical parameters of the sampled foods such as pH, water activity (aw), levels and types of preservatives are provided to EFSA, it was considered that all RTE foods are able to support the growth of L. monocytogenes. So, the criterion applied for samples collected at the processing stage within the context of Regulation (EC) No 2073/2005 was ‘not detected in 25 g’. Two exceptions were applied for the ‘hard cheeses’ and ‘fermented sausages’, for which the criterion of ‘≤ 100 CFU/g’ was applied. EFSA assumes that ‘hard cheeses’ and ‘fermented sausages’ belong to the category of foods that are unable to support the growth of L. monocytogenes, because foods classified under these two categories of RTE products undergo ripening/fermentation and are expected to have low pH and moderate aw values. More information on the impact of RTE food processing, like fermentation and drying on pathogen loads in the RTE food can be found elsewhere (EFSA BIOHAZ Panel, 2018a). The RTE foods that are considered able to support the growth of L. monocytogenes are expected to have near‐neutral or moderately low pH and relatively high aw values or can be very heterogeneous in terms of their manufacturing technology and physicochemical characteristics. In assessing RTE food category ‘other dairy products’, EFSA is presenting the results in a conservative way by considering all ‘other dairy products’ as capable of supporting the growth of L. monocytogenes.
3.3.2. Other monitoring data for Listeria monocytogenes in RTE food
The second subset of data includes all monitoring and surveillance activities results reported by MS and non‐MS to assess the occurrence of L. monocytogenes in different RTE food categories. In this case, only the data retrieved using detection methods were used, as these have a higher sensitivity compared with the quantitative investigations (using L. monocytogenes enumeration methods). All levels of sampling unit (single and batches), sampling stage (processing and retail) and sampling context (surveillance, monitoring and surveillance – based on Regulation (EC) No 2073/2005) were considered. Data obtained from the sampling strategies ‘census sampling’, ‘convenient sampling’ and ‘objective sampling’ were used, excluding data reported from ‘suspect sampling’, ‘selective sampling’ and ‘other’ contexts. When the sampling strategy was not spelled out (either ‘not reported’, ‘not available’, not specified or ‘import sampling’), the data were included assuming that these would not fall into the category of suspect or selective sampling. All samplers’ data were included.
Specific graphs were prepared to illustrate the occurrence in different RTE food categories during the 2016–2019 period. Each point of these graphs represents the overall observed occurrence and the 2.5th and 97.5th percentiles of the uncertainty distributions of these occurrences. Data used for calculating uncertainty levels were the total number of samples (n) and the number of positive samples (s) observed. The uncertainty distributions were calculated with beta distribution beta (s + 1, n – s + 1) (Vose, 1998).
3.3.3. Monitoring data for Listeria monocytogenes in animals and feed
To describe the occurrence of L. monocytogenes in animals and feed, all the sampling strategies were included even data reported for ‘suspect sampling’ and ‘selective sampling’.
3.4. Results
3.4.1. Overview of key statistics along the food chain, EU, 2015–2019
Table 19 summarises EU‐level statistics on human listeriosis and on samples from RTE food tested for L. monocytogenes during 2015–2019. Food data of interest reported were classified into the major categories and aggregated by year to obtain an annual overview of the volume of data submitted. The sampling effort of the MS in 2019 for L. monocytogenes in some major RTE food categories can be found in Appendix A (Table A.1).
Table 19.
Summary statistics on human invasive L. monocytogenes infections and on sampled major RTE food categories in the EU, 2015–2019
| 2019 | 2018 | 2017 | 2016 | 2015 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 2,621 | 2,545 | 2,475 | 2,500 | 2,183 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.46 | 0.47 | 0.48 | 0.47 | 0.43 | ECDC |
| Number of reporting MS | 28 | 28 | 28 | 28 | 28 | ECDC |
| Infection acquired in the EU | 1,817 | 1,640 | 1,639 | 1,539 | 1,450 | ECDC |
| Infection acquired outside the EU | 12 | 8 | 4 | 6 | 7 | ECDC |
| Unknown travel status or unknown country of infection | 792 | 897 | 832 | 955 | 726 | ECDC |
| Number of outbreak‐related cases | 349 | 159 | 39 | 27 | 233 | ECDC |
| Total number of outbreaks | 21 | 14 | 10 | 6 | 15 | EFSA |
| RTE food categories a | ||||||
| RTE milk and milk products | N = 62,019; 23 MS | N = 59,313; 23 MS | N = 56,428; 25 MS | N = 34,850; 26 MS | N = 45,996; 24 MS | EFSA |
| RTE meat and meat products | N = 64,666; 22 MS | N = 57,861; 22 MS | N = 45,219; 24 MS | N = 25,195; 21 MS | N = 25,396; 22 MS | EFSA |
| RTE fish and fishery products | N = 13,376; 22 MS | N = 14,081; 22 MS | N = 12,604; 24 MS | N = 6,601; 23 MS | N = 7,986; 25 MS | EFSA |
| Other RTE food products | N = 76,657; 24 MS | N = 25,179; 22 MS | N = 23,915; 23 MS | N = 21,085; 22 MS | N = 25,544; 23 MS | EFSA |
| RTE foods intended for infants and for special medical purposes | N = 1,721; 18 MS | N = 1,663; 18 MS | N = 1,462; 20 MS | N = 1,274; 16 MS | N = 1,754; 12 MS | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States; RTE: ready‐to‐eat.
In 2019, as in previous years, the most sampled RTE food categories for L. monocytogenes detection and/or enumeration were ‘RTE meat and meat products’ (29.6% from total RTE food samples) and ‘RTE milk and milk products’ (28.4%). ‘RTE fish and fishery products’ samples represent 6.1% of the total reported by MS. The total number of sample units tested by MS increased by 38% in 2019 compared with 2018. This result is explained by an increase of 12% of the sampling units tested for ‘RTE meat and meat products’ and of 204% for ‘other RTE food products’. More specifically, a higher number of samples were tested for ‘bakery products’ (+75%), ‘broiler meat and meat products thereof’ (+304%) and fruit and vegetables (+79%). Romania contributed particularly to the increase for ‘other RTE food products’ (with 51,192 sampling units tested in this category in 2019).
Table A.1 in Appendix A contains the samples taken by country at processing and retail levels. 80% of ‘RTE milk and milk products’ data were provided in decreasing order by Italy, Poland, Bulgaria, Romania, Germany and the Netherlands. Similarly, 80% of ‘RTE meat and meat products’ were provided by Poland, Romania, Germany, Bulgaria and Belgium; 80% of ‘fish and fishery products’ were provided by Poland, Germany, Romania, France, Bulgaria, the Netherlands, Belgium and Italy. ‘Other RTE products’ were mainly reported by Romania (67% of the total reported in this category), Germany, Ireland and Spain. As previous years relatively few samples (0.8%) were reported for ‘RTE foods intended for infants and for medical purposes’; samples were mainly provided by Slovakia, Belgium, Ireland, Germany, the Netherlands and Italy.
When the UK data were collected, the UK was an EU MS but as of 31 January 2020, it has become a third country.
3.4.2. Human listeriosis
In 2019, 28 MS reported 2,621 confirmed cases of invasive listeriosis in humans (Table 20). The EU notification rate was 0.46 cases per 100,000 population, which was at the same level as in 2018 (0.47 cases per 100,000 population). The highest notification rates were observed for Estonia, Sweden, Denmark and Malta with 1.59, 1.10, 1.05 and 1.01 cases per 100,000 population, respectively. The lowest notification rates were reported by Bulgaria, Croatia, Cyprus and Romania (≤ 0.19 per 100,000).
Table 20.
Reported cases of human invasive listeriosis and notification rates per 100,000 population in the EU/EFTA, by country and year, 2015–2019
| Country | 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 38 | 38 | 0.43 | 27 | 0.31 | 32 | 0.36 | 46 | 0.53 | 38 | 0.44 |
| Belgiumb | Y | C | 66 | 66 | 0.72 | 74 | 0.81 | 73 | 0.80 | 103 | 1.14 | 83 | 0.74 |
| Bulgaria | Y | A | 14 | 13 | 0.19 | 9 | 0.13 | 13 | 0.18 | 5 | 0.07 | 5 | 0.07 |
| Croatia | Y | C | 7 | 6 | 0.15 | 4 | 0.10 | 8 | 0.19 | 4 | 0.10 | 2 | 0.05 |
| Cyprus | Y | C | 1 | 1 | 0.11 | 1 | 0.12 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czechia | Y | C | 29 | 27 | 0.25 | 31 | 0.29 | 30 | 0.28 | 47 | 0.45 | 36 | 0.34 |
| Denmark | Y | C | 61 | 61 | 1.05 | 49 | 0.85 | 58 | 1.01 | 40 | 0.70 | 44 | 0.78 |
| Estonia | Y | C | 21 | 21 | 1.59 | 27 | 2.05 | 4 | 0.30 | 9 | 0.68 | 11 | 0.84 |
| Finland | Y | C | 50 | 50 | 0.91 | 80 | 1.45 | 89 | 1.62 | 67 | 1.22 | 46 | 0.84 |
| France | Y | C | 373 | 373 | 0.56 | 338 | 0.51 | 370 | 0.55 | 375 | 0.56 | 412 | 0.62 |
| Germany | Y | C | 572 | 570 | 0.69 | 679 | 0.82 | 721 | 0.87 | 662 | 0.81 | 557 | 0.69 |
| Greece | Y | C | 10 | 10 | 0.09 | 19 | 0.18 | 20 | 0.19 | 20 | 0.19 | 31 | 0.29 |
| Hungary | Y | C | 39 | 39 | 0.40 | 24 | 0.25 | 36 | 0.37 | 25 | 0.25 | 37 | 0.38 |
| Ireland | Y | C | 17 | 17 | 0.35 | 21 | 0.43 | 14 | 0.29 | 13 | 0.28 | 19 | 0.41 |
| Italy | Y | C | 202 | 202 | 0.33 | 178 | 0.29 | 164 | 0.27 | 179 | 0.30 | 153 | 0.25 |
| Latvia | Y | C | 7 | 6 | 0.31 | 15 | 0.78 | 3 | 0.15 | 6 | 0.30 | 8 | 0.40 |
| Lithuania | Y | C | 6 | 6 | 0.21 | 20 | 0.71 | 9 | 0.32 | 10 | 0.35 | 5 | 0.17 |
| Luxembourg | Y | C | 3 | 3 | 0.49 | 5 | 0.83 | 5 | 0.85 | 2 | 0.35 | 0 | 0.00 |
| Malta | Y | C | 5 | 5 | 1.01 | 1 | 0.21 | 0 | 0.00 | 1 | 0.22 | 4 | 0.93 |
| Netherlands | Y | C | 103 | 103 | 0.60 | 69 | 0.40 | 108 | 0.63 | 89 | 0.52 | 71 | 0.42 |
| Poland | Y | C | 121 | 121 | 0.32 | 128 | 0.34 | 116 | 0.31 | 101 | 0.27 | 70 | 0.18 |
| Portugal | Y | C | 56 | 56 | 0.54 | 64 | 0.62 | 42 | 0.41 | 31 | 0.30 | 28 | 0.27 |
| Romania | Y | C | 18 | 17 | 0.09 | 28 | 0.14 | 10 | 0.05 | 9 | 0.05 | 12 | 0.06 |
| Slovakia | Y | C | 18 | 18 | 0.33 | 17 | 0.31 | 12 | 0.22 | 10 | 0.18 | 18 | 0.33 |
| Slovenia | Y | C | 20 | 20 | 0.96 | 10 | 0.48 | 13 | 0.63 | 15 | 0.73 | 13 | 0.63 |
| Spainc, e | N | C | 548 | 505 | – | 370 | – | 284 | – | 362 | – | 206 | – |
| Sweden | Y | C | 113 | 113 | 1.10 | 89 | 0.88 | 81 | 0.81 | 68 | 0.69 | 88 | 0.90 |
| United Kingdom | Y | C | 156 | 154 | 0.23 | 168 | 0.25 | 160 | 0.24 | 201 | 0.31 | 186 | 0.29 |
| EU Total | 2,674 | 2,621 | 0.46 | 2,545 | 0.47 | 2,475 | 0.48 | 2,500 | 0.47 | 2,183 | 0.43 | ||
| Iceland | Y | C | 4 | 4 | 1.12 | 2 | 0.57 | 6 | 1.77 | 0 | 0.00 | 0 | 0.00 |
| Norway | Y | C | 27 | 27 | 0.51 | 24 | 0.45 | 16 | 0.30 | 19 | 0.37 | 18 | 0.35 |
| Switzerlandd | Y | C | – | 36 | 0.42 | 52 | 0.61 | 45 | 0.53 | 50 | 0.59 | 54 | 0.65 |
–: Data not reported.
Y: yes; N: no; A: aggregated data; C: case‐based data.
Sentinel system; notification rates calculated with estimated population coverage of 80%.
Sentinel surveillance; no information on estimated coverage. So, the notification rate cannot be estimated.
Switzerland provided data directly to EFSA. The human data for Switzerland includes data from Liechtenstein.
Data were not complete in 2019, rate not calculated.
The majority (99.3%) of listeriosis cases with known origin of infection was reported to be acquired in the EU in 2019 (Table 19). Ten MS reported 28 travel‐associated listeriosis cases with known travel destination, 14 cases were travelled outside the EU and 14 cases within EU. The proportion of reported listeriosis cases without data on travel status or with unknown country of infection was 30.2% of all confirmed cases in 2019 (Table 19).
In the period 2010–2019, a seasonal pattern was observed in the listeriosis cases reported in the EU/EEA, with high summer peaks followed by smaller winter peaks. Over the 5‐year period during 2015–2019, the trend of confirmed listeriosis cases was stable (flat) (Figure 26).
Figure 26.
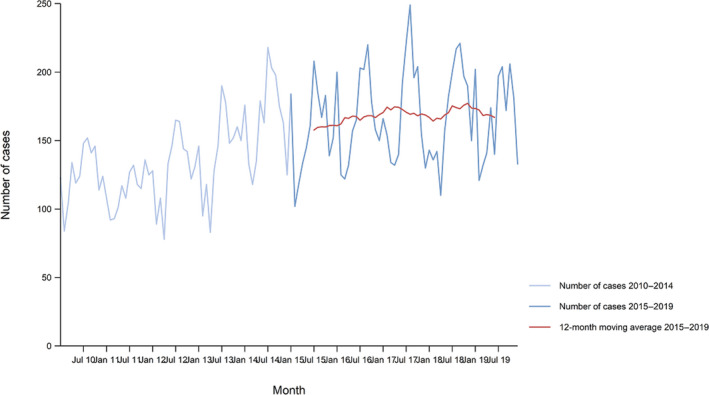
Trend in reported confirmed human cases of listeriosis in the EU/EEA, by month, 2015–2019
- Source: Austria, Belgium, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Malta, the Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Sweden and the United Kingdom. Bulgaria, Croatia, Luxembourg, Portugal and Spain did not report data to the level of detail required for the analysis.
Three MS (Estonia, Poland and Portugal) demonstrated a significantly increasing (p < 0.01) trend between 2015 and 2019. Greece was the only MS reporting a decreasing trend in the same time period.
Information on hospitalisation was provided by 19 MS for 51.1% of all confirmed cases in 2019. Among the cases with information on hospitalisation status, 92.1% were hospitalised. Listeriosis had the highest proportion of hospitalised cases of all zoonoses under EU surveillance.
The outcome was reported for 1,707 confirmed cases (65.1%). Twenty‐one MS reported 300 deaths with listeriosis in 2019. This represented a 31.0% increase compared with 2018 (229 deaths). There was a steady increase in the annual number of deaths between 2010 and 2019 (annual average: 217). The overall EU case fatality among cases with known outcome was 17.6% and increased from 13.6% and 15.6% in 2017 and 2018, respectively. France reported the highest number of fatal cases (56) followed by Spain (55) and Poland (54).
Listeria infections were most commonly reported in the age group over 64 years. At the EU level, the proportion of listeriosis cases in this age group has steadily increased from 56.1% in 2008 to 64.5% in 2019 and especially in the age group over 84 years, with an increase from 7.3% to 14.3% in the same time period. The case fatality was 19.5% and 23.0% in the age group 64–84 years and over 84 years, respectively, in 2019.
Human listeriosis cases and cases associated with food‐borne outbreaks
In total, 2,621 confirmed human listeriosis cases were reported to TESSy in 2019. Overall, there were 1,803 domestic (acquired within the home country) confirmed listeriosis cases reported to the TESSy, which was 99.3% of the number of reported human listeriosis cases infected in the EU (domestically or through travel within EU) during 2019 (Table 19).
Listeria monocytogenes was identified overall by 10 MS in nine strong‐evidence and 12 weak‐evidence food‐borne outbreaks that together affected 349 people in the EU (of which 207 in Spain), with 236 hospitalised and 31 deaths, as reported to EFSA. For nine strong‐evidence food‐borne outbreaks in the EU in 2019 caused by L. monocytogenes, three were caused by ‘meat and meat products’ (one reported with additional information ‘cold cuts’), two by ‘broiler meat and products thereof’ (with additional information ‘RTE meat products’ and ‘chicken mayo sandwich’) and one by each of the categories ‘bovine meat and products thereof’ (‘potted beef’), ‘pig meat and products thereof’ (no additional information), ‘mixed food’ (‘hummus and salads prepared in a small establishment’) and ‘vegetables and juices and other products thereof’ (‘black olives and other delicatessen products’). Previously, during 2010–2018, ‘mixed food’, ‘fish and fish products’ and ‘vegetables and juices and products thereof’ were the most frequently reported food matrices causing strong‐evidence listeriosis food‐borne outbreaks. Further details and statistics on the listeriosis food‐borne outbreaks for 2019 are in the food‐borne outbreaks chapter.
Comparing the food‐borne outbreak cases (349) and confirmed cases of human invasive listeriosis acquired in the EU (1,817) and considering also the estimated cases with unknown travel data (0.993 × 792) (Table 19) could suggest that overall in the EU in 2019 13.4% (349/2,604 × 100) of human listeriosis cases would be reported through food‐borne outbreak investigation. It is important to clarify that the case classification for reporting is different between these two databases. In TESSy, the cases reported are classified based on the EU case definition. All these cases visited a doctor and are either confirmed by a laboratory test (confirmed case) or not (probable case and classification is based on the clinical symptoms and epidemiological link). Also, surveillance of listeriosis in humans in the EU is based on invasive forms of L. monocytogenes infection, mostly manifested as septicaemia, meningitis or spontaneous abortion. Cases that never visited a doctor are not reported to TESSy. Moreover, there may be missing probable cases in TESSy, as these data are not analysed or published and there is no incentive for reporting such cases. Information on which cases are linked to an outbreak and which not is also not systematically collected. In practice, the cases reported to TESSy are considered to be mostly sporadic cases. In food‐borne outbreaks, the human cases are the people involved in the outbreak as defined by the investigators (case definition), and cases must be linked, or probably linked, to the same food source (Directive 2003/99/EC). This can include both ill people (whether confirmed microbiologically or not) and people with confirmed asymptomatic infections (EFSA, 2014). Cases can be classified as confirmed or probable outbreak cases, but currently these specific classification data are not collected by EFSA.
3.4.3. Listeria monocytogenes in food
Data on L . monocytogenes on RTE foods in the context of the Food Safety Criteria laid down in Regulation (EC) No 2073/2005
In total, 14 MS (BE, BG, CY, DK, EE, ES, GR, HR, LU, LV, RO, SI, SK) reported data according to the specifications mentioned above (Section 3.3.1) for 11 RTE food categories (Table 21).
Table 21.
Proportions (%) positive single samples from official sampling by Competent Authorities in the context of verification of the implementation by food business operators of the L. monocytogenes Food Safety Criteria according to Regulation (EC) No 2073/2005, EU, 2019
| RTE food categorya | Processing stageb | Retailc | ||
|---|---|---|---|---|
| Analytical methodd | ||||
| Detection | Enumeration | Detection | Enumeration | |
| Foods intended for infants and for medical purposes e : data reported from BE, CY, EE, ES, RO, SK and SI | 0.00 (N = 716; 7 MS)f | |||
| Fish g data reported from BE, BG, CY, DK, EE, ES, LV and SI | 5.8 (N = 469; 5 MS) | 1.9 (N = 571; 8 MS) | ||
| Fishery products h : data reported from AT, BE, BG, CY, DK, EE, ES, HR, LV, RO, SK and SI | 2.5 (N = 325; 9 MS) | 1.5 (N = 651; 11 MS) | ||
| Cheeses, soft and semi‐soft i : data reported from AT, BE, BG, CY, DK, EE, ES, HR, LU, RO and SK | 0.70 (N = 2,005; 9 MS) | 0.06 (N = 1,551; 9 MS) | ||
| Cheeses, hard j : data reported from AT, BG, CY, DK, EE, ES, HR, RO and SK | 8.9 (N = 79; 6 MS) | 0.00 (N = 90; 7 MS) | ||
| Cheeses, unspecified k : data reported from AT, BE, EE, ES, HR, GR and SI | 1.2 (N = 84; 5 MS) | 0.40q (N = 250; 3 MS) | ||
| Other dairy products (excluding cheeses) – entire category l : data reported from AT, BE, BG, CY, DK, HR, EE, ES, GR, RO, SK, SI | 0.30 (N = 671; 9 MS) | 0.00q (N = 829; 9 MS) | ||
| Milk m : data reported from AT, BG, EE, ES, HR, RO and SK | 1.2 (N = 84; 6 MS) | 0.00q (N = 31; 5 MS) | ||
| Products of meat origin, fermented sausages n : data reported from BE, BG, DK, EE, ES, HR and SK | 2.9q (N = 240; 6 MS) | 2.1q (N = 242; 6 MS) | ||
| Products of meat origin, other than fermented sausages o : Data reported from AT, BE, BG, CY, DK, EE, ES, HR, LU, LV, RO, SK and SI | 2.5 (N = 4,886; 10 MS) | 0.65q (N = 2,295; 12 MS) | ||
| Other products p : data reported from BE, BG, CY, DK, EE, ES, LV, RO, SK and SI | 0.20 (N = 2,036; 7 MS) | 0.23 (N = 5,585; 10 MS) | ||
MS: Member State; N: number of single samples tested.
Grey boxes are not applicable in relation to the analytical method for the specific food category and sampling stage in the context of Regulation (EC) No 2073/2005.
In the absence of relevant physicochemical data (pH, aw), EFSA assumes that foods listed under ‘fish and fishery products’, ‘soft and semi‐soft cheeses’, ‘unspecified cheeses’, ‘milk’, ‘products of meat origin other than fermented sausages’, ‘other dairy products’ and ‘other products’ belong to the category of foods that are able to support the growth of L. monocytogenes. EFSA assumes that ‘fermented sausages’ and ‘hard cheeses’ belong to the category of foods that are unable to support the growth of L. monocytogenes.
Includes sampling units that were collected from ‘cutting plants’ and ‘processing plants’.
Includes sampling units that were obtained from ‘catering’, ‘hospital or medical care facility’, ‘retail’, ‘wholesale’, ‘not available’, ‘unspecified’, ‘restaurant or cafe or pub or bar or hotel or catering service’, ‘automatic distribution system for raw milk’, ‘border inspection’ and ‘packing centre’.
The results from qualitative examinations using a detection method were used to assess the criterion of ‘not detected in 25 g’ and the results from quantitative analyses using an enumeration method were used to assess the criterion of ‘≤ 100 CFU/g’.
Includes ‘infant formula – dried’, ‘infant formula – RTE’, ‘infant formula – liquid’, ‘foodstuffs intended for special nutritional uses – dietary foods for special medical purposes’, ‘foodstuffs intended for special nutritional uses – RTE meal for infants and young children’ and ‘foodstuffs intended for special nutritional uses – processed cereal‐based food for infants and young children’.
Each cell contains the percentage (%) of non‐satisfactory samples (the detection of L. monocytogenes in 25‐g of sample for qualitative analyses or number of L. monocytogenes > 100 CFU/g for enumeration analyses) and in parenthesis the number of tested samples (single samples or batches) and the number of reporting MS.
Includes RTE fish that is ‘cooked’, ‘gravad/slightly salted’, ‘marinated’ or ‘smoked’.
Includes crustaceans, molluscan shellfish, fishery products unspecified, surimi, fishery products from fish species associated with a high amount of histidine and fish canned.
Includes ‘curd’, ‘fresh’ and ‘soft or semi‐soft’, cheeses made with milk from different species (‘cows’, ‘goats’, ‘sheep’, ‘mixed’ or ‘unspecified or other animal’).
Includes ‘hard’ cheeses made with milk from different species (‘cows’, ‘goats’, ‘sheep’, ‘mixed’, ‘unspecified’ or from other animals’).
Includes ‘unspecified’ cheeses made with milk from different species (‘cows’, ‘goats’, ‘sheep’, ‘mixed’, ‘unspecified’ or from other animals’).
Includes ‘butter’, ‘buttermilk’, ‘cheese analogue’, ‘cream’, ‘dairy desserts’, ‘dairy products, not specified’, ‘fermented dairy products’, ‘ice cream’, ‘milk‐based drinks’, ‘milk powder and whey powder’, ‘sour milk’ and ‘yoghurt’.
Includes milk (‘pasteurised’, ‘UHT’, or ‘raw, intended for direct human consumption’) from ‘cows’ or ‘sheep’. Raw milk and raw milk for the manufacture of raw and low heat‐treated products are not included.
Includes fermented sausages made from meat of different animal species (‘bovine animals’, ‘pig’, ‘mixed’, or ‘other animal species or unspecified’).
Includes ‘meat products’ (‘cooked ham’, ‘cooked, RTE’, ‘heat treated, RTE’, ‘raw and intended to be eaten raw’, ‘pâté’, ‘unspecified, RTE’ or ‘unspecified’) and meat preparations (‘intended to be eaten raw’) from different animal species (‘bovine animals’, ‘pigs’, poultry (‘broilers’, ‘duck’, ‘turkeys’, ‘unspecified’), ‘mixed’, ‘farmed game‐land mammals’, or ‘other animal species or not specified’).
Includes bakery products (‘cakes’, ‘desserts’, ‘pastry’), beverages, non‐alcoholic (‘soft drinks’), fruits (‘pre‐cut’, ‘products’), fruits and vegetables (‘pre‐cut’), juice (‘fruit juice’, ‘mixed juice’, ‘vegetable juice’), RTE salads (also those ‘containing mayonnaise’), seeds, sprouted (‘RTE’), soups (‘RTE’), spices and herbs (‘dried’), vegetables (‘pre‐cut’, ‘products’) and other processed food products and prepared dishes (‘unspecified’, ‘sandwiches’, ‘sushi’).
Includes data from Croatia that has only been reported as ≤ 100 CFU/g (and has not been reported as > 100 CFU/g although all negative).
At retail, depending on the RTE food category, 0.0–2.1% of single samples from official sampling were positive for L. monocytogenes, whereas at processing results ranged from 0.0% to 5.8%.
A lower overall proportion of positives was reported at retail level compared with processing stage for all RTE food categories.
Monitoring data for Listeria monocytogenes in RTE food
Details on the occurrence of L. monocytogenes in the main RTE food matrices in 2019 together with 2017 and 2018 results can be found in Appendix B (Table 69B). Below text summarises the results for the major food categories for the 2016–2019 period, considering all levels of sampling unit, sampling stage and sampling context.
Fish and fishery products, RTE
Over the 2016–2019 period, 24 MS and four non‐MS reported data on RTE fish and fishery products. A summary of the occurrence of L. monocytogenes‐positive units in RTE fish and fishery products in the EU over the period 2016–2019 is presented in Figure 27. For 2019, the overall occurrence of L. monocytogenes in RTE fish was 4.3% with Bulgaria, Germany, the Netherlands and Poland reporting more than 80% of the positive samples as in 2018. The overall occurrence of L. monocytogenes in RTE fishery products was 4.2% with Germany, Italy, Poland and Romania reporting more than 80% of positive samples. The occurrence by merging RTE fish and RTE fishery products was 4.3%, 2.7%, 5.3% and 4.7% for the period 2019–2016.
Figure 27.
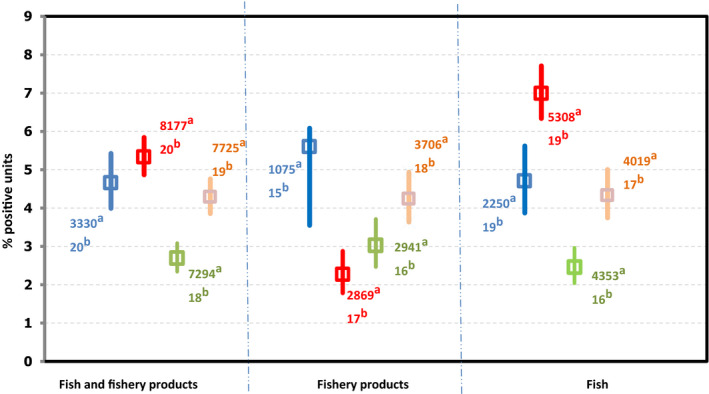
Proportion of L. monocytogenes‐positive sampling units (all sampling stages) in RTE fish and fishery products, EU, in 2016 (blue), 2017 (red) and 2018 (green) and 2019 (orange)
-
(a): Number of sampling units tested by the MS for the corresponding category and year.(b): Number of MS which have reported tested sampling units for the corresponding category and year.‘Fish, RTE’ includes data on ‘fish’ of the following types: ‘chilled’, ‘cooked‐chilled’, ‘gravad/slightly salted’, ‘marinated’ and ‘smoked – cold‐smoked’, ‘smoked – hot‐smoked’, ‘smoked’.‘Fishery products, RTE’ includes the following types: ‘crustaceans – prawns – cooked’, ‘crustaceans – lobsters – cooked’, ‘crustaceans – unspecified – cooked’, ‘crustaceans – shrimps – shelled, shucked and cooked’, ‘crustaceans – unspecified – shelled, shucked and cooked’, ‘crustaceans – shrimps – cooked’, ‘fish – fishery products from fish species associated with a high amount of histidine – not enzyme maturated’, ‘fish – fishery products from fish species associated with a high amount of histidine – which have undergone enzyme maturation treatment in brine’, ‘fishery products, unspecified – cooked’, ‘fishery products, unspecified – RTE – chilled’, ‘fishery products, unspecified – smoked’, ‘fishery products, unspecified – RTE’, ‘molluscan shellfish – shelled, shucked and cooked’, ‘molluscan shellfish – cooked’, ‘molluscan shellfish – cooked – frozen’, ‘Surimi – frozen’, ‘surimi – chilled’, ‘surimi’.
Meat and meat products, RTE
Over the 2016–2019 period, 26 MS and three non‐MS reported data from RTE meat products. Samples from pig meat were by far the main matrix tested in the EU. In 2019, 51.4% out of 56,070 samples were from pig meat. RTE meat from bovine, broilers and turkeys represented 3.6%, 8.7% and 0.2% of all tested samples, respectively. The remaining 36.0% of tested samples were from other animal species or unspecified, or mixed meat (20,195 samples). Combining all RTE meat product categories, the overall occurrence of L. monocytogenes in RTE meat products was 2.9% (1,634 positives out of 56,070). A summary of the proportion of L. monocytogenes‐positive units in RTE meat and meat products according to the main animal origin is presented in Figure 28.
Figure 28.
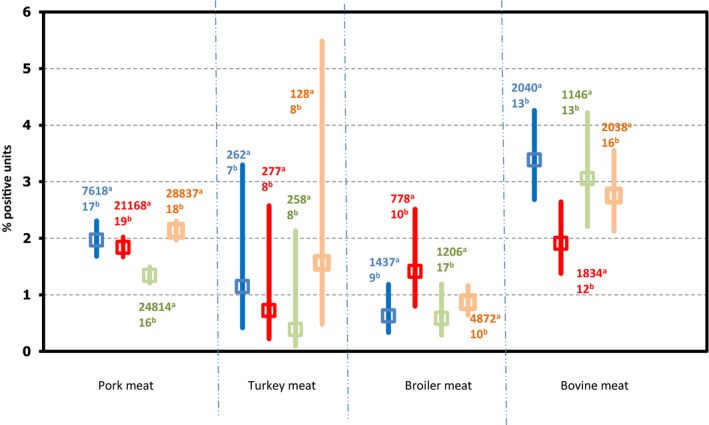
Proportion of L. monocytogenes‐positive sampling units (all sampling stages) in RTE meat and meat products (pork, turkey, broiler and beef), EU, in 2016 (blue), 2017 (red) and 2018 (green) and 2019 (orange)
-
(a): Number of samples tested by the MS for the corresponding category and year.(b): Number of MS which have reported tested samples for the corresponding category and year.Since data were mostly reported by a limited number of MS and are of a heterogeneous nature as these include various diverse subcategories, the findings presented in this figure may not be representative of the EU level or directly comparable across years. RTE pig meat products includes ‘meat from pig, meat products’ of the following types: ‘cooked ham’, ‘cooked, RTE’, ‘fermented sausages’, ‘pâté’, ‘raw and intended to be eaten raw’, ‘raw ham’, ‘unspecified, ready‐to‐eat’ and ‘ready‐to‐eat’ and ‘meat from pig – meat preparation’ of the following type ‘intended to be eaten raw’. ‘RTE turkey meat’ includes turkey ‘meat products’ of the following types: ‘cooked, RTE’, ‘ready‐to‐eat’ and ‘raw and intended to be eaten raw’. ‘RTE broiler meat’ includes broiler ‘meat products’ of the following types: ‘cooked, RTE’. ‘RTE bovine meat’ includes ‘meat from bovine animals, meat products’ of the following types: ‘cooked, RTE’, ‘fermented sausages’, ‘raw and intended to be eaten raw’, ‘pâté’; ‘ready‐to‐eat’; and ‘unspecified, RTE’; ‘meat from bovine animals, meat preparation’ of the following types: ‘intended to be eaten raw’ and ‘meat from bovine animals, minced meat’ of the following types: ‘intended to be eaten raw’.
Pig meat products, RTE. Sixteen MS (AT, BE, BG, CY, CZ, DE, DK, EE, ES, GR, HR, IT, LU, PL, PT, RO, SK) and one non‐MS (ME) reported 2019 data on RTE pig meat products and, overall, in the EU L. monocytogenes was detected in 2.1% of the 28,837 units tested. Poland and Romania provided data on 88.5% of tested samples in RTE pig meat.
Poultry meat products (broilers and turkeys). Nine MS (AT, BG, CY, DE, EE, ES, PL, RO, SI and SK) reported 2019 data on RTE broiler and turkey meat products. Overall, L. monocytogenes was detected in 0.9% of the 5,000 tested units in the EU. The detail of occurrence according to broiler or turkey is given in Figure 28.
Bovine meat products, RTE. Sixteen MS (AT, BE, BG, CY, CZ, DE, DK, ES, LU, NL, PT, RO, SK and SI) reported in 2019 data on RTE bovine meat products. Overall, L. monocytogenes was detected in 2.8% of the 2,038 units tested in the EU.
Milk and milk products, RTE
Over the 2016–2019 period, 22 MS and two non‐MS reported data from RTE milk and milk products.
Milk. Twelve MS (AT, BG, CY, CZ, DE, ES, HR, IT, NL, PL, RO and SK) reported 2019 data on RTE milk (‘pasteurised’, ‘UHT’ and ‘raw milk intended for direct human consumption’). Overall, L. monocytogenes was detected in 0.1% of the 2,292 units tested. Only two MS (NL and ES) out of the 10 reporting MS found positive samples.
Cheeses. Sixteen MS (AT, BE, BG, CY, DK, EE, DE, HR, IT, NL, PL, PT, RO, SK, ES and UK) and two non‐MS (ME and MK) reported 2019 data from L. monocytogenes detection in cheeses. Bulgaria, Germany, Italy, the Netherlands, Poland, Romania and Slovakia were the major contributor for all cheese samples tested (81.4%). Cheeses made from pasteurised cows’ milk represent more than 41.2% of samples collected and reported. Overall, considering all milk origin (species) and all types of cheeses L. monocytogenes was detected in 0.7% of the 9,660 cheese samples tested. A summary of the proportion of L. monocytogenes‐positive units for the various types of cheeses is presented in Figure 29.
Figure 29.
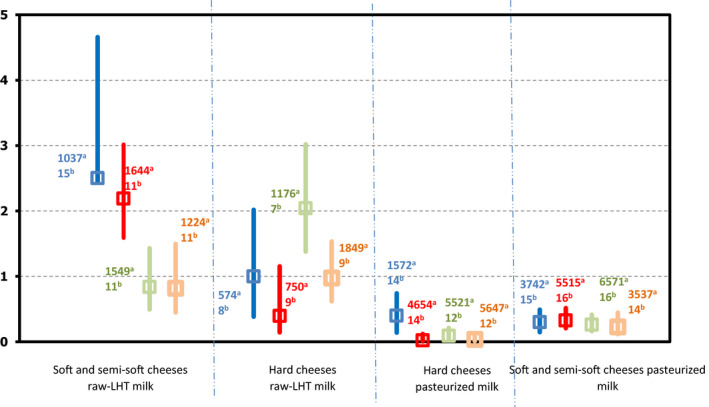
Proportion of L. monocytogenes‐positive sampling units (all sampling stages) in cheeses, EU, in 2016 (blue), 2017 (red) and 2018 (green) and 2019 (orange)
-
(a): Number of samples tested by the MS for the corresponding category and year.(b): Number of MS which have reported tested samples for the corresponding category and year.LHT: low heat treated. ‘Overall’ and the number of MS correspond to data across all major sampling stages (‘retail’ + ‘processing’ + ‘farm’ + ‘border inspection activities’ + ‘unspecified’). ‘Retail’ corresponds to data obtained from catering, hospital or medical care facilities, retail, wholesale and restaurants or cafes or pubs or bars or hotels or catering services. For each sampling stage (‘overall’, ‘retail’ and ‘processing’), data are pooled across both types of sampling units (‘single’ and ‘batch’). ‘Processing’ corresponds to data obtained from packing centres, cutting plants and processing plants. Since data were mostly reported by a limited number of MS, the findings presented in this figure may not be presentative of the EU level.‘Hard cheeses pasteurised milk’ and ‘hard cheeses from raw or low heat‐treated milk’ includes cheeses made from cows’ milk, sheep's milk, goats’ milk, mixed milk from cows, sheep and/or goats and unspecified milk or other animal milk.‘Soft and semi‐soft cheeses’ includes both soft and semi‐soft and fresh cheese made from cows’ milk, sheep's milk, goats’ milk, mixed milk from cows, sheep and/or goats and unspecified milk or other animal milk.
The 2019 prevalence of soft and semi‐soft cheeses (SSC) and hard cheeses (HC) made from raw‐low heat treated (LHT) milk were comparable and ranged between 0.9 and 1.0%. The 2019 prevalence of SSC and HC made from pasteurised milk were 0.3% and 0.04%, respectively. In general, considering the 2016–2019 time period, a higher prevalence in raw‐LHT cheeses (1.0% mean prevalence for HC and SSC) than in pasteurised cheeses (0.1% mean prevalence for HC and SSC) is observed.
Other RTE food products
In 2019, results from other RTE food product categories, such as ‘bakery products’, ‘fruit and vegetables’, ‘RTE salads’, ‘spices and herbs’, ‘sauces and dressings’ and ‘other processed food products and prepared dishes’ were reported.
For ‘bakery products’, samples testing using a detection method were reported by 11 MS. Overall, out of the 6,653 units of bakery products tested, 0.2% were found to be positive for L. monocytogenes, similar to 2018 results. Germany and Romania contributed to 80% of the samples taken in 2019.
In 2019, 17 MS provided data from investigations of L. monocytogenes on 2,357 units of ‘RTE fruit and vegetables’ tested using a detection method. The overall occurrence was of 1.7% (compared with 1.8% in 1,257 units tested in 2018). Bulgaria, Germany, Italy, Romania, Spain and the UK mainly contributed to the sampling effort with nearly 85% of the samples in 2019. The ‘RTE fruit and vegetables’ prevalence over the 2016–2019 period is presented in Figure 30.
Figure 30.
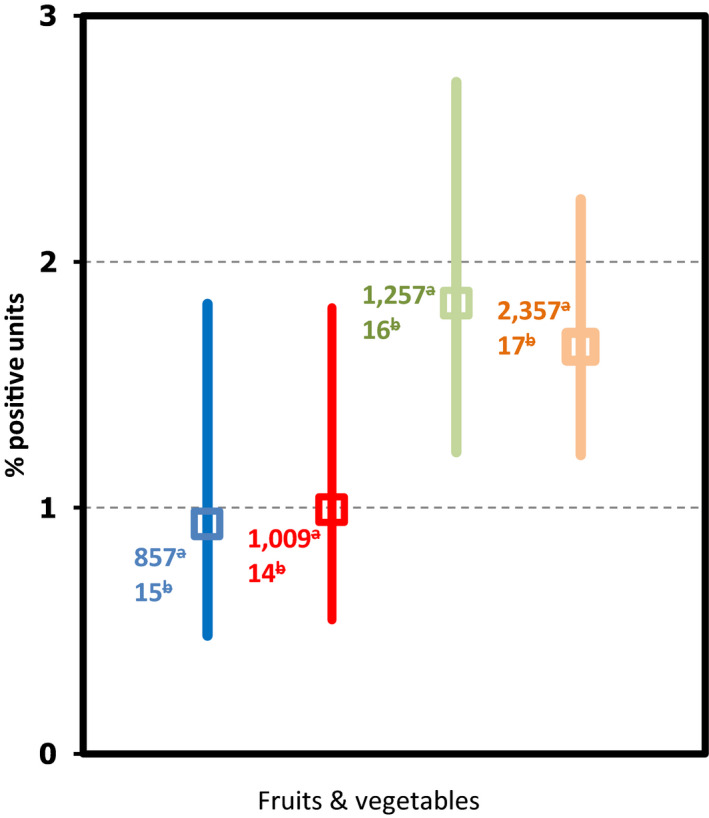
Proportion of L. monocytogenes‐positive sampling units (all sampling stages) in fruit and vegetables, EU, in 2016 (blue), 2017 (red) and 2018 (green) and 2019 (orange)
-
(a): Number of samples tested by the MS for the corresponding category and year.(b): Number of MS which have reported tested samples for the corresponding category and year.The fruit and vegetables group data provided included fruit juice, mixed juice, pre‐cut fruit and/or vegetables, fruit or vegetable products and the edible part of fruit.
For ‘RTE salads’, 3,138 samples were analysed and 109 samples (3.5%) were found to be positive by a detection method, while for ‘spices and herbs’, 291 samples were analysed and two samples (0.7%) were found positive. For ‘sauces and dressings’, 369 samples were analysed and one sample (0.3%) tested positive.
For ‘egg products’ and ‘confectionery and pastes’, respectively, 26 and 54 samples were analysed, and none was found positive by a detection method.
In ‘other processed food products and prepared dishes’ (unspecified, sushi or ices and similar frozen desserts), 14 MS submitted data. Overall, L. monocytogenes was detected in 0.3% of the 42,925 units tested with Romania reporting more than 90% of the samples.
3.4.4. Listeria spp. in animals
In 2019, 12 MS and two non‐MS reported data on several animal categories (food‐producing, wild‐, zoo‐ and pet animals, including birds) from different species. Reported data were mainly from animals (99%) compared with other sampling unit levels (‘herd/flock’ and ‘holding’). In the EU, the major animal data for Listeria testing concerned cattle (82%), sheep (11%) and pigs (3%). The sample size, as well as the sampling strategy and the proportion of positive samples, varied considerably among the reporting countries and animal species. Most EU data at the animal level were reported by two MS, the Netherlands (51%) and Ireland (38%).
In total, considering the three sampling units (animal, herd/flock and holding) together, MS reported 17,516 tested units for Listeria spp. and 246 (1.4%) were found to be positive. Among the positive units, 67 (27.2%) were reported as being positive for L. monocytogenes and only limited positive findings were reported as Listeria innocua (four units, 1.6%) and Listeria ivanovii (two units, 0.8%). As previous years, major positive findings (173 units, 70.3%) were reported as ‘other’ or ‘unspecified species’ for Listeria.
3.4.5. Listeria monocytogenes in feed
In 2019, only one MS (HR) reported a negative sample in soya‐derived feed material.
3.5. Discussion
EU surveillance of human listeriosis focuses on the severe, invasive form of the disease, which affects the following risk groups: elderly, immunocompromised people as well as pregnant women and infants. While still relatively rare with 2,621 confirmed cases in the EU (notification rate of 0.46 cases per 100,000 population) in 2019, it is one of the most serious food‐borne diseases under EU surveillance causing hospitalisation, high morbidity and mortality, particularly among the elderly. Confirmed human cases of invasive listeriosis have shown a significant increasing trend since EU surveillance was initiated in 2008. This trend stabilised in the EU as a whole over the last 5‐year period during 2015–2019 and in most MS, while three MS reported a significantly increasing trend. Most listeriosis cases — when this information was known — have been domestically acquired and few cases have been linked to travel, within or outside the EU. The number of cases acquired within the EU increased slowly in the last 5 years, as a smaller proportion of cases were reported with unknown information on travel status and country of infection in 2019.
Since the beginning of EU‐level surveillance, most listeriosis cases have been reported in people over 64 years of age. The number and proportion of cases reported for this age group have increased steadily from 2008 until 2017. Human cases almost doubled in the age group over 84 years in the same time period. The proportion of cases, however, slightly decreased in the age group over 64 years during the last 2 years in 2018–2019. This is particularly visible in the age group over 84 years. As in previous years, almost all reported listeriosis cases −with information on hospitalisation status− were hospitalised. In 2019, the overall EU case fatality among cases with known outcome was 17.6% and the number of deaths increased by 31% compared with 2018. Listeriosis continues to cause the highest number of fatal cases among food‐borne infections in the EU. The highest mortality was in age group over 84 years. The high incidence of Listeria infections in elderly may be partially explained by the ageing population in the EU and parallel increases in susceptibility due to underlying chronic diseases (EFSA BIOHAZ Panel, 2020b). As ageing of the populations will continue in most MS (EUROSTAT, 2020) in the coming years, it is important to raise awareness of listeriosis and the risk, especially to older people, associated with certain consumption habits and types of food (e.g. RTE fish products and frozen vegetables) (EFSA and ECDC, 2018a, 2019b; EFSA BIOHAZ Panel, 2018a, 2020b).
In 2019, the number of human cases reported as food‐borne outbreak cases (349) was 13.4% of the estimated number of the acquired cases of invasive human listeriosis in the EU (2,604 cases). Overall, L. monocytogenes was identified by 10 MS in nine strong‐evidence and 12 weak‐evidence food‐borne outbreaks that together affected 349 people in the EU, with 236 hospitalised and 31 deaths, as reported to EFSA. Outbreaks of listeriosis continue to occur – for strong‐evidence outbreaks – associated with several food vehicles including ‘meat and meat products’ (three strong‐evidence food‐borne outbreaks), ‘broiler meat and products thereof’ (two strong‐evidence food‐borne outbreaks) and ‘bovine meat and products thereof’, ‘pig meat and products thereof’, ‘mixed food’ and ‘vegetables and juices and other products thereof’ (each one strong‐evidence food‐borne outbreak). In six of these nine outbreaks, the food was RTE whereas for the remaining three no additional food vehicle information was provided.
Since 2016, MS continue to increase their sampling for most of the RTE food categories. The number of food samples tested was 38% higher in 2019 compared with 2018. This result is explained by an increase of 12% of the sampling units tested for ‘RTE meat and meat products’ and of 204% for ‘other RTE food products’. More specifically, a higher number of samples were tested for ‘bakery products’ (+75%), ‘broiler meat and meat products thereof’ (+304%) and fruit and vegetables (+79%). Most food samples collected at processing and retail were from RTE products of animal origin. The number of samples tested for fruits and vegetables has increased since 2016 (+189% between 2017 and 2019). This could be a result of the awareness of the multi‐country outbreak of L. monocytogenes ST6 over the period 2015–2018 caused by frozen vegetables. However, in 2019, this category still represents less than 2% of all food samples tested. EFSA published an opinion this year concluding that L. monocytogenes is the most relevant pathogen associated with blanched frozen vegetables. When these vegetables are consumed uncooked, the probability of illness per serving for the elderly (65–74 years old) population, is up to 3,600 times greater compared with those cooked, but still very likely lower than any of the evaluated RTE food categories. Routine monitoring programmes for L. monocytogenes should be designed following a risk‐based approach and regularly revised based on trend analysis, being food processing environment monitoring a key activity in the frozen vegetable industry (EFSA BIOHAZ Panel, 2020b).
The low number of data reported by MS in primary production (< 10% of the total reported data) reflects the absence of harmonised EU regulation in this sector. As previous years, in animals, an important proportion of isolates (70.3%) is reported as ‘unspecified Listeria spp.’ or ‘Listeria spp.’ and were not identified at the species level. Listeriosis in animals is, however, known to be almost exclusively caused by L. monocytogenes and L. ivanovii (ANSES, 2011).
In 2019, the occurrence of L. monocytogenes varied according to the RTE food category and ranged from 0.04% for ‘hard cheeses made from pasteurised milk’ up to 4.3% for ‘RTE fish’. Interpretation of trends for occurrence must be used with caution, since each year reporting data can vary according to the number of reporting MS, the food categories included in different contexts of the surveillance, the sampling efforts (sample size) and reporting attitude. Official sampling carried out by the CAs in the context of surveillance of the application of the FSC laid down in Regulation (EC) No 2073/2005 showed that the level of non‐satisfactory results remains low at retail (from 0.0% to 2.1%). For previous years, this level was however systematically higher at the processing stage compared with the retail stage.
New tools based on genotyping are now available to characterise isolates of L. monocytogenes. With these new developments in diagnostics and changes in the epidemiology of listeriosis outbreaks, the FAO/WHO JEMRA has launched in 2020 new work on L. monocytogenes in RTE foods. EFSA/ECDC surveillance data provide opportunities to validate the current risk assessment models for L. monocytogenes, assess their application to other food commodities and develop new management approaches to control L. monocytogenes. Combining such human, animal and food epidemiological data with molecular and genotyping data represents indeed an efficient tool to better understand the ecology of this pathogen among the different stages of the food chain and would improve the investigation of listeriosis outbreaks affecting one or several MS.
3.6. Related projects and Internet sources
| Subject | For more information see | |
|---|---|---|
| Human | ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definition of listeriosis | https://www.ecdc.europa.eu/en/surveillance‐and‐disease‐data/eu‐case‐definitions | |
| Disease Programme on Emerging, Food‐ and Vector‐Borne Diseases | https://www.ecdc.europa.eu/en/about-us/who-we-are/units/disease-programmes-unit | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| World Health Organisation ‐ listeriosis fact sheet | https://www.who.int/news-room/fact-sheets/detail/listeriosis | |
| Humans and food | Commission Regulation (EC) No 2073/2005 – Food Safety Criteria for L. monocytogenes in the EU | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073–20170101&rid=1 |
| EU Baseline Survey 2010–2011– part A; L. monocytogenes prevalence estimates (scientific report of EFSA) | https://www.efsa.europa.eu/en/efsajournal/pub/3241 | |
| EU Baseline Survey 2010–2011 – part B; analysis of factors related to prevalence and exploring compliance (scientific report of EFSA) | https://www.efsa.europa.eu/en/efsajournal/pub/3810 | |
| L. monocytogenes contamination of RTE foods and the risk for human health in the EU (Scientific Opinion) |
https://www.efsa.europa.eu/en/efsajournal/pub/5134 https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2018.5134 |
|
| The public health risk posed by L. monocytogenes in frozen fruit and vegetables including herbs, blanched during processing (Scientific Opinion) | https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2020.6092 | |
| Whole genome sequencing and metagenomics for outbreak investigation, source attribution and risk assessment of food‐borne microorganisms (Scientific Opinion) | https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2019.5898 | |
| Urgent scientific and technical assistance to provide recommendations for sampling and testing in the processing plants of frozen vegetables aiming at detecting L. monocytogenes (technical report) |
https://www.efsa.europa.eu/en/supporting/pub/en-1445 https://efsa.onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2018.EN-1445 |
|
| Closing gaps for performing a risk assessment on L. monocytogenes in RTE foods: activity 1, an extensive literature search and study selection with data extraction on L. monocytogenes in a wide range of RTE food (external scientific report) | https://www.efsa.europa.eu/en/supporting/pub/1141e | |
| Closing gaps for performing a risk assessment on L. monocytogenes in RTE foods: activity 2, a quantitative risk characterisation on L. monocytogenes in RTE foods; starting from the retail stage (external scientific report) | https://www.efsa.europa.eu/en/supporting/pub/1252e | |
| Closing gaps for performing a risk assessment on L. monocytogenes in RTE foods: activity 3, the comparison of isolates from different compartments along the food chain and from humans using whole genome sequencing (WGS) analysis (external scientific report) | https://www.efsa.europa.eu/en/supporting/pub/1151e | |
| Evaluation of listeriosis risk related with the consumption of non pre‐packaged RTE cooked meat products handled at retail stores in Greece (external scientific report) |
https://www.efsa.europa.eu/en/supporting/pub/en-1677 https://efsa.onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1677 |
|
| Quantitative assessment of relative risk to public health from food‐borne L. monocytogenes among selected categories of RTE foods | https://www.fda.gov/downloads/Food/FoodScienceResearch/UCM197330.pdf | |
| Risk assessment of L. monocytogenes in RTE foods: Technical report | http://www.fao.org/3/a-y5394e.pdf | |
| Risk assessment of L. monocytogenes in RTE foods – Interpretive summary | http://www.fao.org/fileadmin/templates/agns/pdf/jemra/mra4_en.pdf | |
| FSIS comparative risk assessment for L. monocytogenes in RTE meat and poultry deli meats | https://www.fsis.usda.gov/shared/PDF/ | |
| Interagency risk assessment: L. monocytogenes in retail delicatessens technical report | https://www.fsis.usda.gov/shared/PDF/Comparative_RA_Lm_Report_May2010.pdf | |
| Joint FAO/WHO Expert meeting on Microbiological Risk Assessment of L. monocytogenes in Ready‐to‐Eat (RTE) Food: Attribution, Characterisation and Monitoring | https://www.who.int/news-room/events/detail/2020/10/20/default-calendar/joint-fao-who-expert-meeting-on-microbiological-risk-assessment-of-listeria-monocytogenes-in-ready-to-eat-(rte) | |
| Guidance document on L. monocytogenes shelf‐life studies for RTE foods, under Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs | https://ec.europa.eu/food/sites/food/files/safety/docs/biosafety_fh_mc_guidance_document_lysteria.pdf | |
| EU Reference Laboratory activities and documents on L. monocytogenes for member laboratories | https://eurl-listeria.anses.fr/ | |
| Technical guidance document for conducting shelf‐life studies on L. monocytogenes in RTE foods (challenge testing and durability testing) | https://eurl-listeria.anses.fr/en/minisite/listeria/eurl-lm-technical-guidance-document-conducting-shelf-life-studies-listeria | |
| Guidelines on the application of general principles of food hygiene to the control of L. monocytogenes in foods | http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCAC%2BGL%2B61–2007%252FCXG_061e.pdf | |
| A public database of genome sequences, including L. monocytogenes sequences – GenomeTrakr | https://www.fda.gov/food/foodscienceresearch/wholegenomesequencingprogramwgs/ucm363134.htm | |
| The ECDC‐EFSA molecular typing database for European Union public health protection | https://euroreference.anses.fr/sites/default/files/17%2003%20ED%20ER%2002%201_RIZZI.pdf | |
| Comparison of the ISO method and three modifications of it for the enumeration of low concentrations of L. monocytogenes in naturally contaminated foods | https://euroreference.anses.fr/sites/default/files/3-Comparison.pdf | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports | |
| Animals | General overview of listeriosis in animals | http://www.merckvetmanual.com/generalised-conditions/listeriosis/overview-of-listeriosis |
| Overview and diagnosis of listeriosis in animals | http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.09.06_LISTERIA_MONO.pdf |
4. Shiga toxin‐producing Escherichia coli
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
4.1. Key facts
In 2019, 7,775 confirmed cases of Shiga toxin‐producing E. coli (STEC) infections in humans were reported at the EU level by 27 EU countries.
The EU notification rate was 2.2 cases per 100,000 population, which was similar to 2018.
The highest notification rates were reported in Ireland, Malta, Denmark and Sweden.
The EU/EEA trend has been increasing from 2015 to 2019.
STEC was the third most frequent bacterial agent detected in food‐borne outbreaks in the EU, with 42 outbreaks, 273 cases, 50 hospitalisations and 1 death reported in 2019.
The sources in the four strong‐evidence STEC food‐borne outbreaks during 2019 were ‘bovine meat and products thereof’ (two outbreaks), ‘milk’ and ‘tap water, including well water’ (one outbreak each). During 2010–2018, strong‐evidence STEC outbreaks were mostly caused by ‘bovine meat and products thereof’ (18), ‘tap water, including well water’ (16), ‘vegetables and juices and other products thereof’ (10) and milk (8) and cheese (8).
In 2019, 21 MS reported the presence of STEC in 2.8% of 20,395 food samples, compared with 2.4% in 2018.
Sprouted seeds were tested by six MS with no positive STEC results from 331 official samples. An EU regulation with a microbiological criterion for the presence of STEC in this food commodity has been in force since 2013.
Overall, STEC was most commonly found in meat of different types derived from different animal species (4.1% STEC‐positive), followed by ‘milk and dairy products’ (2.1%) while ‘fruits and vegetables’ was the least contaminated category (0.1%).
Sixteen MS tested 6,297 ?ready‐to-eat’ food samples for STEC of which 37 (0.6%) were found to be STEC‐positive, including 17 meat and meat product samples, 16 milk and milk product samples with 10 from cheese, two samples from spices and herbs, and one STEC‐positive sample from salads and ‘fruits, vegetables and juices’ each.
Of the isolates from food with the reported information on the serogroup 21.6% belonged to the ‘top‐five’ serogroups (O157, O26, O103, O111 and O145) in 2019 and more than half of all the remaining STEC belonged to the top‐20 STEC serogroups reported in human infections to ECDC in 2015–2018.
Most of the virulotypes of STEC isolates from food and animal were also identified in severe STEC infections in humans. This identification, however, was only carried out on 52.9% of the food isolates for the stx gene typing (stx1 and stx2) and stx gene subtyping was only done for 6.1% of the food isolates, and even less for animal isolates.
Testing of animal samples was still not widely carried out in the EU with 2,588 animal samples tested for STEC by nine MS in 2019.
4.2. Surveillance and monitoring of Shiga toxin‐producing Escherichia coli in the EU
4.2.1. Humans
The notification of STEC10 infections is mandatory in most EU MS, Iceland, Norway and Switzerland, except for four MS, where notification is based on a voluntary system (France, Luxembourg) or another system (Italy and the United Kingdom). In the United Kingdom, although the reporting of food poisoning is mandatory, isolation and specification of the organism is voluntary. The surveillance systems for STEC infections cover the whole population in all EU MS except for three MS (France, Italy and Spain). The notification rates were not calculated in these three countries for the following reasons: (a) in France, the STEC surveillance in humans is based on paediatric haemolytic uraemic syndrome (HUS) cases; (b) in Italy, STEC surveillance is sentinel and primarily based on the HUS cases reported through the national registry of HUS; (c) no estimation for population coverage of STEC cases was provided by Spain (until 2018). In Belgium, full national coverage was set up in 2015 and rates before then are not displayed. For 2019, Croatia did not report data, and in Spain, not all regions reported data for 2019 due to COVID‐19. Case numbers might therefore be lower than what could be expected. All countries report case‐based data except Bulgaria, which reported aggregated data. Both reporting formats were included to calculate numbers of cases and notification rates.
Diagnosis of human STEC infections is generally carried out by culture from stool samples and indirect diagnosis by the detection of antibodies against the O‐lipopolysaccharides of E. coli in serum from HUS cases. In addition, diagnosis by direct detection of free faecal Shiga toxin/verocytotoxin or the identification of the presence of stx1/vtx1 or stx2/vtx2 genes in stools by PCR without strain isolation is increasing.
4.2.2. Food and animals
STEC data in the context of Regulation (EC) No 2073/2005, STEC food safety criterion for sprouts at the retail level
Regulation (EC) No 2073/2005 sets a microbiological criterion for sprouted seeds (sprouts). According to this food safety criterion, the analytical results for sprouts placed on the market during their shelf‐life, based on the reference method ISO TS 13136:2012, shall be compliant with STEC O157, O26, O111, O103, O145 and O104:H4 ‘not detected in 25 g’.
Although the testing is intended to be mandatory, the sampling objectives and the sampling frequency applied varied or were interpreted differently between MS, making the data not fully harmonised. Data are also generated by the National CAs conducting inspections to verify whether the food business operators implement correctly the legal requirements (official monitoring data). The latter data are compliance checks and are not suitable for trend analyses, because a reference study population is mostly absent and because the sampling is risk based and so non‐representative (Boelaert et al., 2016).
Other STEC monitoring data from foods and animals
All the food and animal testing data, apart from those on sprouts testing produced in the context of the Regulation (EC) No 2073/2005, originate from the reporting obligations of MS under Directive 2003/99/EC (the zoonosis directive). Due to the absence in this Regulation of explicitly indicated sampling strategies, the data generated by MS are based on investigations with non‐harmonised sampling. Moreover, mainly for animal samples, they are obtained with different analytical methods. Therefore, STEC monitoring data according to Directive 2003/99/EC are not comparable between MS and preclude subsequent data analysis such as assessing temporal and spatial trends at the EU level.
In certain food categories, different sampling design and inaccuracies due to limited numbers of samples examined also preclude accurate prevalence estimation. Moreover, the use by MS of laboratory analytical methods that test only for E. coli O157 leads to inaccurate STEC prevalence estimations or STEC serogroup frequency distributions. While this problem affected less than 5% of food samples in 2019, these methods have been used to test more than 30% of the animal samples. Nevertheless, descriptive summaries of sample statistics at the EU level may be made and used to indicate the circulation of certain STEC types in food and animals, provided the mentioned relevant limitations of the data set are kept into consideration.
To improve the quality of the EU data on STEC monitoring of food and animals, EFSA issued technical specifications for harmonised monitoring and reporting of STEC in animals and foodstuffs in 2009 (EFSA, 2009a). With an additional Scientific Opinion, EFSA encouraged MS to extend the monitoring and report data on STEC serogroups (EFSA BIOHAZ Panel, 2013a). More recently, it has been recommended that the presence of the main virulence genes be reported, considering the most recent development in STEC testing and risk assessment (EFSA BIOHAZ Panel, 2020c; JEMRA FAO/WHO and NACMCF reports, see Section 4.6 for online reference of the last two reports). Finally, the latest published EFSA Scientific Opinion on the pathogenicity assessment of STEC presents important considerations related to the virulence of the different STEC types and underlines the importance of determining the virulence genes combinations (virulotypes) of the isolated STEC strains, with an emphasis on stx gene subtyping, which would facilitate a more precise assessment of the risk connected with different STEC isolates (EFSA BIOHAZ Panel, 2020c).
4.2.3. Food‐borne outbreaks of STEC infections
The reporting of food‐borne disease outbreaks of human STEC infections is mandatory according to Zoonoses Directive 2003/99/EC.
4.3. Data validation and analyses of monitoring data from food and animals
4.3.1. Data validation
The STEC monitoring data from food and animals reported for the year 2019 to EFSA were verified as regards their plausibility in line with the current knowledge.
The following plausibility criteria focused on the level of completion and coherence of the information and on the consistency of the laboratory results with the analytical method reported:
Plausibility of reported occurrence values with respect to the STEC epidemiology based on the updated scientific literature.
Consistency of the reported laboratory results with the purposes of the STEC monitoring data collection. An example of data not consistent with the objective of the collection and for this reason excluded from the analysis, is the reporting of E. coli indicators or pathogenic E. coli other than STEC.
Consistency of the reported laboratory results with the analytical method used for the analysis. An example may be the reporting of STEC O26 or other non‐O157 STEC serogroups for samples tested with the standard ISO 16654:2001 (ISO, 2001) or equivalent methods, which can only detect serogroup O157.
4.3.2. Data analysis
Occurrence in food and animals
The monitoring data on sprouts as part of Regulation (EC) No 2073/2005 were aggregated and summarised for trend watching according the following specified data elements (‘filters’); Sampling context: ‘surveillance, based on Regulation No 2073/2005’; Sampling unit type: ‘single’; Sampling stage: as appropriate; Sampling strategy: ‘objective sampling’, and Sampler: ‘official sampling’.
For the description of the occurrence of STEC‐positive samples in the different food categories, a subset of all validated monitoring data was used (N = 20,395). Data sets were extracted with ‘objective sampling’ being specified as sampler strategy, which means that the reporting MS collected the samples according a planned strategy based on the selection of random samples, which are statistically representative of the population to be analysed. Additionally, the data reported with a sampler ‘HACCP and own checks’ were excluded. For animal data (N = 1,802), the same filters applied.
Serogroups and virulence features in foods and animals
The full data set (N = 27,826) including also regionally only reported data (about 200 samples) was used instead for any other descriptive analysis of STEC findings in food and animals, primarily those on the serogroups and virulence genes’ frequency distribution, with the aim to describe the full range of STEC isolated from food and animals.
To descriptively analyse the reported STEC serogroups and virulence genes, the data were grouped according used test methods (Table 22):
-
a)
Methods aiming at detecting any STEC. This category includes the method ISO TS 13136:2012 (ISO, 2012) and other stx genes PCR‐based methods.
-
b)
Methods designed to detect only E. coli O157, such as method ISO 16654:2001 (ISO, 2001) and the equivalent methods NMKL 164:2005 (NMKL, 2005) and DIN 1067:2004–03 (DIN, 2004). One MS reported having used the AFNOR BIO 12/25 05/09 test method, which targets only E. coli O157. These results have been grouped together with the results based on the other E. coli O157‐specific methods.
Table 22.
Analytical methods from EFSA Catalogue browser (EFSA BIOHAZ Panel, 2019a) and the aggregation used to summarise the STEC monitoring results for food and animals, EU, 2015–2019
| Analytical methods for STEC in the catalogue | Method recoded for the analysis | |
|---|---|---|
| Food | Microbiological test ‐ ISO/PRF TS 13136 E.coli | ISO TS 13136:2012 |
| Real Time PCR (BAX): Detection of STEC and identifcation of serogroups O26, O111, O121, O145, O103 and O145 | ||
| ISO 16654:2001 or NMKL 164:2005 or DIN 10167 | ISO 16654:2001 | |
| BIO 12/25‐05/09, ELFA method for E. coli O157 | ||
| BAX‐based PCR and confirmation following AFNOR serological method. AFNOR validation certificate: QUA 18/04‐03/08 | ||
| Animals | In‐house real time PCR methods based on ISO/TS 13136:2012 | ISO TS 13136:2012 |
| Other methods based on PCR detection opf vtx genes | ||
| OIE mothod for E. coli O157 in animal faecal samples | ISO 16654:2001 |
EFSA (European Food Safety Authority), Ioannidou S, 2019. EFSA Catalogue Browser User Guide. EFSA supporting publication 2019:EN‐1726, 39 pp. https://doi.org/10.2903/sp.efsa.2019.EN1726
This disentanglement was necessary to minimise the impact of results based on E. coli O157‐specific methods, which do not allow identifying other STEC possibly present in the samples, on the analyses of the distribution of serogroups.
4.4. Results
4.4.1. Overview of key statistics along the food chain, EU, 2015–2019
Table 23 summarises EU‐level statistics on human STEC infections and on samples from food and animals tested for STEC, during 2015–2019. Food and animal data were classified into the major categories and aggregated by year to obtain an annual overview of the volume of data submitted, considering the information reported for laboratory analytical methods (Section 4.3.2) and not considering the information reported on the sampling strategies/contexts.
Table 23.
Summary of STEC statistics related to humans and to major food categories and major animal species, EU, 2015–2019
| 2019 | 2018 | 2017 | 2016 | 2015 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 7,775 | 8,161 | 5,958 | 6,474 | 5,929 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 2.21 | 2.28 | 1.67 | 1.79 | 1.65 | ECDC |
| Number of reporting MS | 27 | 28 | 28 | 28 | 28 | ECDC |
| Infection acquired in the EU | 4,835 | 5,783 | 4,747 | 4,037 | 3,991 | ECDC |
| Infection acquired outside the EU | 750 | 693 | 525 | 339 | 532 | ECDC |
| Unknown travel status or unknown country of infection | 2,190 | 1,685 | 686 | 2,098 | 1,406 | ECDC |
| Number of food‐borne outbreak‐related cases | 273 | 390 | 260 | 737 | 676 | EFSA |
| Total number of food‐borne outbreaks | 42 | 50 | 48 | 43 | 70 | EFSA |
| Food | ||||||
| All | ||||||
| Number of sampling units | 25,030 | 20,498 | 19,351 | 17,977 | 13,777 | EFSA |
| Number of reporting MS | 22 | 20 | 22 | 17 | 17 | EFSA |
| Meat and meat products | ||||||
| Number of sampling units | 14,110 | 9,250 | 10,706 | 8,771 | 7,865 | EFSA |
| Number of reporting MS | 20 | 17 | 18 | 17 | 15 | EFSA |
| Milk and milk products | ||||||
| Number of sampling units | 5,479 | 5,339 | 3,485 | 3,773 | 3,005 | EFSA |
| Number of reporting MS | 13 | 14 | 10 | 11 | 8 | EFSA |
| Fruits and vegetables (and juices) | ||||||
| Number of sampling units | 2,658 | 3,339 | 2,295 | 1,475 | 1,384 | EFSA |
| Number of reporting MS | 13 | 13 | 15 | 11 | 10 | EFSA |
| Animals | ||||||
| All | ||||||
| Number of sampling units | 2,588 | 1,631 | 2,217 | 1,892 | 884 | EFSA |
| Number of reporting MS | 9 | 5 | 7 | 6 | 4 | EFSA |
| Bovine animals | ||||||
| Number of sampling units | 1,615 | 1,112 | 1,681 | 1,230 | 266 | EFSA |
| Number of reporting MS | 7 | 5 | 6 | 5 | 3 | EFSA |
| Other ruminants a | ||||||
| Number of sampling units | 268 | 178 | 204 | 138 | 212 | EFSA |
| Number of reporting MS | 4 | 2 | 1 | 2 | 3 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member State; NA: Not available/not reported; STEC: Shiga toxin‐producing Escherichia coli.
sheep and goats, deer.
Humans
The proportion of human STEC cases infected domestically and through travel within the EU decreased since 2015 and increased slightly among the cases infected through travel outside the EU.
Food categories
For the year 2019, 22 MS provided results from analyses of 25,030 food units (batches or single samples).
The most recent source attribution analysis available for STEC underlined that ‘bovine meat and products thereof’, ‘milk and dairy products’ and ‘vegetables, fruit and products thereof’ were the vehicles most frequently implicated in STEC infections in the EU in the period 2012–2017 (inclusive) (EFSA BIOHAZ Panel, 2020c), confirming the results of previous JEMRA FAO/WHO and NACMCF reports (see Section 4.6 for online reference of these two reports). These categories were those most commonly tested in 2019 in the EU and represented the 89% of the total food sample units tested by 21 MS.
Animal categories
For the year 2019, 2,588 sampling units (single heads or herds or flocks) from animals were reported by nine MS. This number increased compared with the number of animals tested in 2018 (1,631). The proportion of animal samples tested for STEC and reported by EU MS in 2019 by the different analytical methods can be found in the supporting information to this report.
When the UK data were collected, the UK was an EU MS but as of 31 January 2020, it has become a third country.
4.4.2. STEC infections in humans
In 2019, 7,894 cases of STEC infections, including 7,775 confirmed cases, were reported in the EU (Table 24). Twenty‐four MS reported at least one confirmed STEC case and three MS reported zero cases. The EU notification rate was 2.2 cases per 100,000 population, which was similar to the level in 2018 (2.3 cases per 100,000 population). The highest country‐specific notification rates were observed in Ireland, Malta, Denmark and Sweden (16.3, 10.7, 10.7 and 7.4 cases per 100,000 population, respectively). Seven countries (Bulgaria, Cyprus, Greece, Lithuania, Poland, Portugal and Slovakia) reported ≤ 0.1 cases per 100,000 population.
Table 24.
Reported human cases of STEC infections and notification rates per 100,000 population in the EU/EFTA, by country and year, 2015–2019
| Country | 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 286 | 284 | 3.21 | 305 | 3.46 | 250 | 2.85 | 177 | 2.03 | 107 | 1.25 |
| Belgium | Y | C | 131 | 131 | 1.14 | 112 | 1.00 | 123 | 0.08 | 119 | 1.05 | 100 | 0.89 |
| Bulgaria | Y | A | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Croatia | Y | C | – | – | – | 10 | 0.24 | 7 | 0.17 | 9 | 0.21 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czechia | Y | C | 34 | 34 | 0.32 | 26 | 0.25 | 37 | 0.35 | 28 | 0.27 | 26 | 0.25 |
| Denmark | Y | C | 621 | 621 | 10.70 | 493 | 8.41 | 263 | 4.57 | 210 | 3.68 | 201 | 3.55 |
| Estonia | Y | C | 6 | 6 | 0.45 | 7 | 0.53 | 3 | 0.23 | 5 | 0.38 | 8 | 0.61 |
| Finland | Y | C | 311 | 311 | 5.64 | 210 | 3.81 | 123 | 2.24 | 139 | 2.53 | 74 | 1.35 |
| Franceb | N | C | 376 | 335 | – | 259 | – | 260 | – | 302 | – | 262 | – |
| Germany | Y | C | 1,932 | 1,907 | 2.30 | 2,226 | 2.69 | 2,065 | 2.50 | 1,843 | 2.24 | 1,616 | 1.99 |
| Greece | Y | C | 5 | 5 | 0.05 | 1 | 0.01 | 3 | 0.03 | 2 | 0.02 | 1 | 0.01 |
| Hungary | Y | C | 24 | 23 | 0.24 | 14 | 0.14 | 12 | 0.12 | 12 | 0.12 | 15 | 0.15 |
| Ireland | Y | C | 816 | 798 | 16.27 | 966 | 20.00 | 795 | 16.62 | 737 | 15.59 | 598 | 12.92 |
| Italyb | N | C | 87 | 59 | – | 73 | – | 92 | – | 78 | – | 59 | – |
| Latvia | Y | C | 48 | 48 | 2.50 | 3 | 0.16 | 1 | 0.05 | 1 | 0.05 | 4 | 0.20 |
| Lithuania | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 4 | 0.14 | 3 | 0.10 |
| Luxembourg | Y | C | 4 | 4 | 0.65 | 3 | 0.50 | 1 | 0.17 | 4 | 0.69 | 4 | 0.71 |
| Malta | Y | C | 53 | 53 | 10.74 | 41 | 8.62 | 9 | 1.96 | 4 | 0.89 | 4 | 0.93 |
| Netherlands | Y | C | 459 | 459 | 2.66 | 488 | 2.84 | 392 | 2.29 | 665 | 3.92 | 858 | 5.08 |
| Poland | Y | C | 17 | 14 | 0.04 | 6 | 0.01 | 4 | 0.01 | 4 | 0.01 | 0 | 0.00 |
| Portugal | Y | C | 1 | 1 | 0.01 | 2 | 0.02 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 |
| Romania | Y | C | 36 | 36 | 0.19 | 20 | 0.10 | 11 | 0.06 | 29 | 0.15 | 0 | 0.00 |
| Slovakia | Y | C | 3 | 3 | 0.06 | 12 | 0.22 | 3 | 0.06 | 2 | 0.04 | 1 | 0.02 |
| Slovenia | Y | C | 31 | 31 | 1.49 | 32 | 1.55 | 33 | 1.60 | 26 | 1.26 | 23 | 1.11 |
| Spainc , e | N | C | 270 | 269 | – | 126 | 0.28 | 86 | – | 69 | – | 86 | – |
| Sweden | Y | C | 756 | 756 | 7.39 | 892 | 8.81 | 504 | 5.04 | 638 | 6.48 | 551 | 5.65 |
| United Kingdom | Y | C | 1587 | 1587 | 2.38 | 1,840 | 2.78 | 993 | 1.51 | 1,367 | 2.09 | 1,328 | 2.05 |
| EU Total | 7,894 | 7,775 | 2.21 | 8,167 | 2.28 | 6,071 | 1.67 | 6,474 | 1.79 | 5,929 | 1.65 | ||
| Iceland | Y | C | 27 | 27 | 7.56 | 3 | 0.86 | 3 | 0.89 | 3 | 0.90 | 1 | 0.30 |
| Norway | Y | C | 511 | 511 | 9.59 | 494 | 9.33 | 381 | 7.25 | 239 | 4.59 | 221 | 4.28 |
| Switzerlandd | Y | C | 993 | – | 11.50 | 822 | 9.65 | 696 | 8.23 | 463 | 5.47 | 315 | 3.77 |
–: Data not reported.
Y: yes; N: no; A: aggregated data; C: case‐based data.
Sentinel surveillance; mainly cases with HUS are notified.
Sentinel surveillance; no information on estimated coverage. So, notification rate cannot be estimated.
Switzerland provided the data directly to EFSA. The human data for Switzerland includes data from Liechtenstein.
Data not complete in 2019, rate not calculated.
Most STEC cases reported were infected in the EU (62.2% domestic cases or travel in the EU, 9.7% travel outside EU and 28.2% of unknown travel history or unknown country of infection) (Table 23). Three Nordic countries – Finland, Sweden and Norway reported the highest proportion of travel‐associated cases (52.1, 44.4 and 37.9%, respectively). Among 1,034 travel‐associated cases with known probable country of infection, 72.5% of the cases involved travel outside the EU and 27.5% travel within the EU. Egypt was most frequently reported as the probable country of infection, followed by Turkey, Spain, Morocco, Italy and Thailand (14.7%, 13.4%, 5.0%, 3.3%, 3.1% and 3.1%, respectively).
There was a clear seasonal trend in confirmed STEC cases in the EU/EEA between 2010 and 2019, with more cases reported during the summer months (Figure 31). There was a significantly increasing trend (p < 0.01) for STEC in the EU/EEA in 2015–2019. Five MS (Austria, Denmark, Finland, Malta and Poland) reported significantly increasing trends (p < 0.01). One MS (the Netherlands) had a significantly decreasing (p < 0.01) trend over the same time period. This was due to a change in notification criteria in the Netherlands since 2016, where only acute infections with at least diarrhoea, vomiting and/or blood in stool have to be reported.
Figure 31.

Trend in reported confirmed cases of human STEC infection in the EU/EEA, by month, 2015–2019
- Source: Austria, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Sweden and the United Kingdom. Belgium, Bulgaria, Czechia, Croatia, Portugal and Spain did not report data to the level of detail required for the analysis.
Eighteen MS provided information on hospitalisation for 37.3% of all confirmed STEC cases in the EU in 2019. Out of the 2,903 cases with known hospitalisation status, 37.9% were hospitalised. The highest proportions of hospitalised cases (80.0–100%) were reported in Estonia, Greece, Italy, Poland and Slovakia. The number of HUS cases (394) was about the same level as in 2018. HUS cases were reported in all age groups with the highest proportion of patients in the youngest age groups from 0 to 4 years (272 cases; 69.4%) to 5–14 years (75 cases; 19.1%). The most common serogroups among HUS cases were O26 (38.7%), O157 (23.0%), O80 (9.0%) and O145 (8.0%); while 4.7% were untypable.
In 2019, 10 deaths due to STEC infection were reported in the EU compared with 11 deaths in 2018. Six MS reported one to three fatal cases each and 14 MS reported no fatal cases. This resulted in an EU case fatality of 0.21% among the 4,739 confirmed cases with known outcome (61.0% of all reported confirmed cases). Deaths were reported in the age group 0–4 years (40%) and in all age groups over 25 years (60%). Half the deaths were associated with HUS. The serogroups and the stx gene subtypes associated with fatal cases were O157 (Stx2a), O145 (Stx1a, Stx2a) and O8 (Stx2d). For seven fatal cases, the serogroup was not specified.
Cases of STEC infections in humans associated with food‐borne outbreaks
Overall, for the year 2019, 94.1% of the 4,835 reported STEC infections in humans who acquired the infection in the EU (Table 23) were domestic (acquired within the home country) infections and 5.9% were acquired through travel in EU.
STEC was identified by 11 MS in 42 food‐borne outbreaks that together affected 273 people in EU, with 50 hospitalised and one death, as reported to EFSA. Comparing the food‐borne outbreaks cases (273), reported to EFSA, and cases of STEC infections in humans acquired in the EU (4,835) considering also the proportion of unknown travel data (0.865 × 2,190) (Table 23), reported to ECDC, could suggest that overall, in the EU in 2019 4.1% of human STEC cases could be reported through FBO investigations. It is important to clarify that the case classification for reporting is different between these two databases. In TESSy, the cases reported are classified based on the EU case definition. All these cases visited a doctor and are either confirmed by a laboratory test (confirmed case) or not (probable case and classification is based on the clinical symptoms and epidemiological link). Cases that never visited a doctor are not reported to TESSy. Moreover, there may be missing probable cases in TESSy, as these data are not analysed or published and there is no incentive for reporting such cases. Information on which cases are linked to an outbreak and which not is also not systematically collected. In practice, the cases reported to TESSy are considered to be mostly sporadic cases. In food‐borne outbreaks, the human cases are the people involved in the outbreak as defined by the investigators (case definition), and cases must be linked, or probably linked, to the same food source (Directive 2003/99/EC). This can include both ill people (whether confirmed microbiologically or not) and people with confirmed asymptomatic infections (EFSA, 2014). Cases can be classified as confirmed or probable outbreak cases, but currently these specific classification data are not collected by EFSA.
The sources in the four strong‐evidence STEC food‐borne outbreaks during 2019 were ‘bovine meat and products thereof’ (two outbreaks) and ‘milk’ and ‘tap water, including well water’ (one outbreak each). During 2010–2018, strong‐evidence STEC outbreaks were mostly caused by ‘bovine meat and products thereof’ (18), ‘tap water, including well water’ (16), ‘vegetables and juices and other products thereof’ (10) and milk (8) and cheese (8). Further details and statistics on the STEC food‐borne outbreaks for 2019 are in the FBO chapter.
Human serogroup and virulotype data are described in Section 4.4.5.
4.4.3. Occurrence of STEC in food
STEC data in the context of Regulation (EC) No 2073/2005 , STEC food safety criterion for sprouts at the retail level
As regards 2019 data for STEC on sprouted seeds in the context of Regulation No 2073/2005, 78 single samples taken at processing plant and 253 units sampled at retail by the CAs (official sampling) of six MS have been reported with no positive results. Out of the total 331 samples tested, approximately 70% were reported by two MS only (Belgium and Romania). In general, as noted in previous years, testing sprouted seeds is not widely applied at the EU level, although a microbiological criterion for this food commodity is laid down in EU regulation No 2073/2005.
Other STEC monitoring data from food
Overall, 564 (2.8%) out of 20,395 food sampling units reported by 21 MS and collected using an objective sampling strategy, were positive for STEC. For the years 2018, 2017, 2016 and 2015, the figures for STEC‐positive food samples were, respectively, 2.8%, 1.5%, 2.0% and 1.7%. In Table 25, those monitoring results are summarised and a distinction is made between RTE and non‐RTE food including fresh meat.
Table 25.
Occurrence of STEC in major food categories, EU
| 2019 | 2015–2018 | |||||
|---|---|---|---|---|---|---|
| Food | N reporting MS | N sampling units | Positive N (%) | N reporting MS | N sampling units | Positive N (%) |
| RTE food | ||||||
| All | 16 | 6,297 | 37 (0.59) | 19 | 16,727 | 145 (0.87) |
| Meat and meat products | 8 | 1,418 | 17 (1.20) | 10 | 3,848 | 51 (1.33) |
| Meat and meat products from bovine animals | 7 | 746 | 11 (1.48) | 9 | 2,224 | 33 (1.49) |
| Meat and meat products from pigs | 5 | 133 | 0 | 6 | 364 | 4 (1.10) |
| Other meat and meat products | 3 | 271 | 4 (1.48) | 4 | 744 | 6 (0.81) |
| Milk and milk products | 9 | 2,025 | 16 (0.79) | 12 | 5,717 | 78 (1.36) |
| Milk | 4 | 146 | 4 (2.74) | 5 | 682 | 21 (3.08) |
| Raw milk a | 3 | 139 | 4 (2.88) | 3 | 431 | 21 (4.87) |
| Cheese | 9 | 1,770 | 10 (0.57) | 12 | 4,654 | 55 (1.18) |
| Dairy products excluding cheeses (butter, cream, ice cream, whey, yoghurt and fermented dairy products) | 6 | 135 | 2 (1.48) | 6 | 438 | 2 (0.46) |
| Fruits, vegetables and juices | 9 | 1,290 | 1 (0.08) | 8 | 3,172 | 4 (0.13) |
| Spices and herbs | 5 | 685 | 2 (0.29) | 5 | 2,008 | 11 (0.55) |
| Salads | 2 | 285 | 1 (0.35) | 3 | 40 | 0 |
| Seeds, sprouted | 8 | 457 | 0 | 12 | 974 | 0 |
| Non‐RTE food | ||||||
| All | 20 | 13,997 | 527 (3.77) | 19 | 35,058 | 910 (2.60) |
| Meat and meat products | 18 | 11,350 | 479 (4.22) | 18 | 26,554 | 823 (3.10) |
| Fresh meat from bovine animals | 14 | 5,794 | 183 (3.16) | 15 | 9,394 | 175 (1.86) |
| Fresh meat from pigs | 4 | 119 | 8 (6.72) | 8 | 905 | 31 (3.43) |
| Fresh meat from goats | 2 | 18 | 3 (16.67) | 4 | 45 | 5 (11.11) |
| Fresh meat from sheep | 6 | 816 | 95 (11.64) | 7 | 2,036 | 213 (10.46) |
| Other fresh meat | 5 | 202 | 14 (6.93) | 5 | 2,083 | 91 (4.37) |
| Milk and milk products | 7 | 1,217 | 47 (3.86) | 8 | 2,311 | 52 (2.25) |
| Fruits, vegetables and juices | 8 | 926 | 1 (0.11) | 11 | 3,081 | 4 (0.13) |
The raw RTE milk sampling units are a subset of the RTE milk.
RTE food
As regards RTE food, most of the results of the 6,297 RTE food sampling units reported by 16 MS originated from ‘milk and milk products’ notably cheeses (32.2%), followed by ‘meat and meat products’ (22.5%), ‘fruits, vegetables and juices’ (20.5%) and ‘spices and herbs’ (10.9%). In total, 37 RTE food samples were found to be positive for STEC: 17 in ‘meat and meat products’ (notably of bovine origin), 16 in ‘milk and milk products’ particularly in cheeses, two in ‘spices and herbs’ and one in each of the categories ‘salads and fruits’ and ‘vegetables and juices’.
The analysis of RTE bovine meat products and meat preparations reported resulted in 1.48% positive samples out of 746 units tested by seven MS, while no positive samples were reported out of 113 units of RTE minced meat, meat preparations and meat products from pig meat, tested by five MS. Finally, 2.74% of the 146 RTE milk samples tested were STEC‐positive.
For the descriptive analysis of serogroups and virulence genes 6,757 sample units tested for STEC were available with 60 (0.9%) positive samples reported. The food categories concerned in this analysis included cheeses, sprouted seeds, spices and herbs, fruits and vegetables, meat products, fish and fishery products, raw milk and ‘others’. Overall, the 0.7% of the samples proved positive with the most contaminated commodities being meat products (1.2% of the samples in this commodity) and cheeses (0.9% of the samples in this category, see above).
Of all the STEC isolated from RTE food samples, only 16 were submitted with information on the serogroup. These included 11 different serogroups, with three STEC O157 isolated by one MS that used the ISO 16654:2001 method and three STEC O26. The characterisation of the virulence genes was carried out on 29 isolates for the stx genes (11 isolates with stx1, 12 with stx2 and six with stx1 and stx2) and on 18 for the presence of eae, while 16 isolates were provided with information on the type of stx gene and on the presence of the eae gene (four strains eae+; stx1+, two eae+; stx2+, three eae‐; stx1+, four eae‐; stx2+ and three eae‐; stx1+ and stx2+).
RTE and non‐RTE food
In the following descriptive analyses, food categories include RTE food and non‐RTE food.
Meat and meat products
Overall STEC contamination was detected in 494 (4.1%) out of 12,120 samples of meat and meat products reported by 16 MS.
Bovine meat. In 2019, 5,794 units of fresh bovine meat were tested for STEC by 14 MS with 3.2% of these being positive (Table 25). Most of the units were sampled at the slaughterhouse (63.4%), followed by retail sampling stage (28.2%). The samples taken at the retail outlet were the most contaminated with 4.0% of the samples being found positive for STEC, whereas at the slaughterhouse level, there were 2.5% positive tests out of 3,682 samples.
For the descriptive analysis of serogroups and virulence genes 198 isolates were available from 6,146 samples of bovine meat (fresh and other) tested by 15 MS. Information on the serogroup was reported by eight MS for 115 isolates (58.1%), which belonged to 39 different serogroups, among which the most frequently identified in 2019 were O13 (10 isolates) followed by O55 (eight isolates), O91 (seven isolates), O26, O174 and O113 (six isolates each) and others (Table 31). All the six most represented STEC serogroups identified in fresh bovine meat samples except the O13 serogroup were among the 20 most frequent serogroups reported in STEC from human disease in the EU in 2018 (EFSA and ECDC, 2019c). The analysis of the virulence genes of the isolated STEC included data reported by 12 MS and showed that 75.7% and 39.4% of the isolates were provided with information on the genes encoding the Shiga toxins (stx) and the intimin (eae), respectively, while 67 isolates were typed for both the genes. The latter isolates included 47 that were negative for the presence of the eae gene (12 with stx1, 19 with stx2 and 16 with both the stx1 and stx2) and 20 positives for eae (13 with stx1, four with stx2 and three with both stx1 and stx2).
Table 31.
Frequency distribution of non‐O157 STEC serogroups in food categories in reporting MS, 2019
| Food categorya | STEC isolates with serogroup reported | STEC serogroups | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of total STEC isolates with serogroup reported in the specific food category | ||||||||||||||||
| N | O26 | O103 | O145 | O111 | O146 | O91 | O13 | O113 | O55 | O174 | O8 | O116 | O6 | Other serogroups (list) | ||
| Bovine meat | 130 | 5.4 | 3.1 | 3.1 | 0.8 | 1.5 | 6.2 | 7.7 | 9.2 | 6.9 | 5.4 | 2.3 | 3.8 | 1.5 | 43.1 | (O100, O105, O109, O11, O117, O121, O127, O130, O136, O148, O149, O15, O150, O153, O155, O160, O168, O171, O177, O178, O179, O183, O185, O2, O22, O38, O43, O50, O7, O84, O88) |
| Ovine and goat meat | 44 | 6.8 | 4.5 | 0.0 | 0.0 | 22.7 | 2.3 | 0.0 | 9.1 | 2.3 | 0.0 | 9.1 | 0.0 | 4.5 | 38.6 | (O104, O108, O109, O128, O15, O176, O2, O32, O38, O78, O9, O98) |
| Other ruminants meatb | 6 | 0.0 | 0.0 | 0.0 | 0.0 | 16.7 | 16.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 66.7 | (O187, O21, O27, O43) |
| Pig meat | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Other meatc | 4 | 0.0 | 25.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 25.0 | 0.0 | 0.0 | 0.0 | 50.0 | (O102, O84) |
| Mixed meat | 2 | 0.0 | 50.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 50.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Milk and dairy productsd | 4 | 75.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 25.0 | (O181) |
| Raw milke | 1 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Fruit and vegetable | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | (O88) |
| Seedsf | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Other food | 2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | (O11, O88) |
| Total | 194 | 7.2 | 4.1 | 2.1 | 0.5 | 6.7 | 5.2 | 5.2 | 8.8 | 5.2 | 4.1 | 3.6 | 2.6 | 2.1 | 42.8 | (O100, O102, O104, O105, O108, O109, O11, O117, O121, O127, O128, O130, O136, O148, O149, O15, O150, O153, O155, O160, O168, O171, O176, O177, O178, O179, O181, O183, O185, O187, O2, O21, O22, O27, O32, O38, O43, O50, O7, O78, O84, O88, O9, O98) |
MS: Member State; N: number of samples; STEC: Shiga toxin‐producing Escherichia coli.
Non‐O157 STEC serogroups are listed according to their public health relevance as a cause of human infections in the EU (EFSA, 2009a).
Note: Only results from samples tested by the ISO TS 13136:2012 method were included.
The different meat categories presented in this table include all types of meat (not only fresh).
Includes meat from deer.
Includes meat from animals other than ruminants and pigs.
Includes any type of dairy product, cheese and milk other than raw milk.
Includes raw milk from different species, but most of tested samples and all the positive samples were from cows.
Includes sprouted seeds and dried seeds.
Ovine and goat meat. This food category is not widely tested at the EU level, reflecting the different eating habits of the different MS, particularly regarding goat meat. Conversely, small ruminants are important reservoir of STEC as reported in the literature (Persad and LeJeune, 2014). In 2019, six MS reported the results of investigation of 816 sample units of fresh ovine meat with 11.6% of these being STEC‐positive, whereas two MS reported on fresh goat meat with three STEC‐positive sampling units out of the 18 tested (Table 25).
For the descriptive analysis of serogroups and virulence genes 93 isolates were available from ovine and goat meat (fresh and other). Information on the serogroup was available for 42 strains and the most represented was O146 (10 isolates), followed by O26, O157 and O15 (three strains each) among the STEC O‐groups also represented amongst human isolates. The other isolates belonged to 17 other serogroups including some matching those isolated from human disease such as O113 and O91 (two and one isolate, respectively) (EFSA and ECDC, 2019c) (Table 32). Seventy‐five of the 93 STEC isolated from this food category in 2019 were provided with information on the presence of the Stx‐coding genes. Thirty‐five strains were stx1+, 18 and 22 isolates were stx2+ and stx1+ stx2+, respectively. In addition, 29 of these 75 isolates were provided with the information on the presence of the eae gene, which was present with stx1 in five isolates and with stx2 in four isolates.
Table 32.
Frequency distribution of non‐O157 STEC serogroups in animals in reporting MS, 2019
| Animal category | STEC isolates with serogroup reported | STEC serogroups | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of total STEC isolates with serogroup reported in the specific animal category | ||||||||||||||||
| N | O26 | O103 | O145 | O111 | O146 | O9 | O100 | O113 | O1 | O174 | O8 | O116 | O2 | Other serogroups (list) | ||
| Cattle | 6 | 16.7 | 0.0 | 16.7 | 16.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 50.0 | (O150, O168) |
| Goat and sheep | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | (O121) |
| Other ruminantsa | 10 | 0.0 | 10.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 60.0 | 0.0 | 0.0 | 0.0 | 30.0 | 0.0 | |
| Pigs | 47 | 0.0 | 0.0 | 0.0 | 0.0 | 4.3 | 8.5 | 27.7 | 4.3 | 2.1 | 2.1 | 29.8 | 0.0 | 4.3 | 17.0 | (O104, O115, O123, O159, O32, O45, O84) |
| Other animalsb | 3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 66.7 | 33.3 | (O24) |
| Total | 67 | 1.5 | 1.5 | 1.5 | 1.5 | 3.0 | 6.0 | 19.4 | 3.0 | 10.4 | 1.5 | 20.9 | 0.0 | 10.4 | 19.4 | (O104, O115, O121, O123, O150, O159, O168, O24, O32, O45, O84) |
MS: Member State; N: number of samples; STEC: Shiga toxin‐producing Escherichia coli.
Note: only results from samples tested by the ISO TS 13136:2012 method were included.
Includes deer and Cantabrian chamois.
Includes turtles, horses, donkeys, rats, lamas, ducks, dogs, cats, alpacas, wild boars, water buffalo, Steinbock, pigeons, hedgehogs, guinea pigs, fowl, foxes, ferrets, chinchillas, bison.
Meat from other ruminants. Only three MS provided information on the presence of STEC in fresh meat samples from deer. In total, 62 samples were taken and eight were found to be contaminated with STEC (12.9%). From monitoring results from fresh and other meat samples, six isolates were reported each belonging to a different serogroup, which included O91 and O146, both identified in STEC isolated from human disease (EFSA and ECDC, 2019c). Seven strains were reported with the information on the presence of the Stx‐coding genes and were all positive for stx2 but one strain which possessed stx1 and stx2. The same set of isolates was also provided with the information on the presence of the eae gene and all were negative.
Meat from other animal species. Four MS tested fresh pig meat in 2019 and reported data on 119 samples with eight of these being positive for the presence of STEC (6.7%) (Table 25). From monitoring results from fresh and other meat samples five of the positive samples contained STEC O157, all isolated from one MS that used the ISO 16654:2001 method. The remaining three isolates were reported as STEC of unspecified serogroup.
Five MS reported on the analyses carried out on 202 sample units of fresh meat from animal species other than bovine, ovine, goat, pigs and deer. These included samples taken from horses, rabbit, wild boars, poultry, wild and farmed game and unspecified meat. Fourteen samples were reported as STEC‐positive (6.93%). One of the isolates was an STEC O103 and another was an STEC O157. The remaining isolates were not provided with serogroup information. STEC O157 was isolated by one MS, which reported testing six samples of poultry meat at retail, all were tested using the ISO 16654:2001 method.
In 2019, one MS (Spain) presented data on the presence of STEC in fresh meat from broilers and turkeys. Thirty‐seven samples from broilers and 14 from turkey meat were tested, all using the ISO 16654:2001 method, with four E. coli O157 reported in fresh meat from broilers.
For the descriptive analysis of serogroups and virulence genes 2,560 sample results were available with 90 of them being positive for STEC. Information on the serogroup of the isolated STEC was provided for 17 isolates. Notably, 13 isolates were of the O157 serogroup, but all of these were from samples tested using the ISO 16654:2001 or equivalent methods. The remaining four belonged to different serogroups, which included STEC O103 and O174. Nine STEC isolates were reported with their stx genes profiles, four were stx2+, four were stx1+ and three stx1+; stx2+. The four isolates with the stx1‐coding genes were also positive for the presence of the eae gene.
Meat products and meat preparations. Meat products and meat preparations other than fresh were sampled in 2019 by 14 MS: 5,396 units were tested and 203 STEC strains isolated. In total, 691 samples were tested by three MS with the ISO 16654:2001 method, targeting E. coli O157, with seven positives. The remaining 4,705 units were all tested using the ISO TS 13136:2012 method or equivalent, which has a wider scope including all STEC, with 4.2% positives.
Eight MS reported, in 2019, the results from testing of 606 samples of meat preparations and meat products from mixed sources. In total, 15 non‐O157 STEC were isolated from the units tested representing 2.5% positivity. One MS reported 100 samples tested with the ISO 16654:2001 method with no positive results.
For the descriptive analysis of serogroups and virulence genes, 221 STEC isolates were available from 6,653 sample units (3.3%) of any meat products and meat preparations including those involving minced and mixed meats. The information on the serogroup was provided for 44 STEC strains, including 18 E. coli O157, seven of which had been detected using the ISO 16654:2001 method.
The analysis of the presence of the stx and eae genes could be carried out on 78 and 52 isolates with this information reported, respectively (35.3% and 23,1%). Out of the 37 eae‐positive isolates, six possessed the stx1 and four the stx2 genes, while, for the remaining 27, this information was not provided. Fourteen isolates were reported as being eae‐negative and fell into three groups based on the stx genes profiles: stx1+ (two isolates), stx2+; (nine isolates) and stx1+; stx2+ (three isolates).
Milk and milk products
Overall STEC was found in 61 (2.1%) out of 2,981 samples of milk and milk products reported by nine MS.
In 2019, eight MS reported on the testing of 1,216 sample units of raw cows’ milk with 48 positive units (3.9%). Information on the serogroup was provided for two isolates only (one STEC O26 and one O157).
Three MS reported monitoring results of 27 sample units of raw goats’ milk, while two MS reported only four samples of raw sheep milk. None of the samples tested was positive for STEC.
One MS reported monitoring results of STEC in 102 samples of raw milk from other or unspecified animal species. Four positive samples were reported as STEC of unspecified serogroup.
The presence of STEC in RTE dairy products other than milk and cheeses was reported by four MS, which tested 148 sample units of butter, cream, ice cream, whey, yoghurt and fermented dairy products. Overall found five isolates were found, one belonging to the O26 serogroup and the other four of a non‐specified serogroup.
For dairy products, in 2019, 2,696 cheese samples were tested for the presence of STEC, with 25 (0.9%) positive units.
For the descriptive analysis of serogroups and virulence genes, 5,479 sample results were available, of which cheese accounted for 56.9%, with 91 positives (1.7%). Only six STEC were typed for the serogroup and four were STEC O26, one was O157 and the remainder belonged to the O181 serogroup. Characterisation of the stx and eae gene profiles also involved a small number of isolates with 22 and 12 strains reported with this information in the data set, respectively. Nine isolates were provided with the data on both the presence of stx and eae genes and included one stx1 and eae positive, and two eae and stx2 positive isolates. Six eae negative strains included two stx1, two stx2 and two stx1 and stx2 isolates.
Vegetables and fruits
Overall STEC was found in two (0.1%) out of 2,171 samples of vegetables and fruits reported by nine MS. The positive records included two units of vegetables sampled at retail (leafy vegetables and pre‐cut vegetable products), reported by two MS and both were contaminated with STEC of non‐O157 serogroups.
Other foodstuffs
This category contains miscellaneous food commodities not included in the previously mentioned categories, which included cereals and meals, bakery products, non‐alcoholic beverages, cereals and meals, juices, live bivalve molluscs, fish and fishery products, sauces and dressing, dried seeds and fresh and dried spices and herbs, infant formula, coconuts products, shrimps, water, honey and others. For the whole category, 1,704 samples were analysed by 10 MS with three (0.18%) positive samples reported from salads (one unit) and spice and herbs (two units) (see above RTE food). The STEC strains identified in spices and herbs included one STEC O88:H25 stx1+ stx2+ and one other STEC strain for which no further information on the serogroup and virulence genes was reported, whereas one STEC O11:H5 possessing the stx2 gene was reported in salads.
4.4.4. Occurrence of STEC in animals
Animals are tested much less than food in the EU. In 2019, 2,588 sample units from animals (animals or herds or flocks) were tested for STEC by nine MS. Overall, the presence of STEC was reported in 14.1% of them, considering the full data set.
In total, 68.4% of the animal samples were tested using the ISO TS 13136:2012 method, while all the remaining samples were tested using methods targeting E. coli O157 only.
As observed in previous years, the highest proportion of animal sampling units tested in 2019 was related to cattle, with 1,493 tested (62.4% of animal samples) with 17.1% positives. As for the other categories, 53.8% of the 104 sampling units from pigs proved positive for STEC, followed by the small ruminants with 61 sheep and goat sample units (14.8% positives) and the 270 deer samples with 11 positive units.
The most relevant data reported on the animal categories are detailed below.
Cattle
Four MS reported the presence of STEC in 254 isolates (17%) out of 1,493 cattle sampling units. In total, 231 positive samples were detected out of 816 tested using the ISO TS 13136:2012 method or equivalent by three MS. Twenty‐three positive samples out of 677 samples were obtained using the OIE method for E. coli O157 by two MS.
The full data set (see Section 4.3.2, Data Analysis) included 276 STEC‐positive sample results out of 1,615 samples tested from cattle. These included 13 additional STEC O157, nine of which were detected using the OIE method for the E. coli O157, one STEC O26, one isolate of serogroup O111, one STEC O145 and others. The remaining isolates were reported without information on the serogroup. Only about 12% of the cattle isolates were provided with information on the virulence genes. The STEC isolates with a more complete set of features determined included one O26 stx2+ eae+, one O145 stx2+ eae+, one O111 stx1+ eae+, four O157 stx2+ eae+, 24 O157 stx1+ stx2+ eae+ and one O168 stx1+; stx2+; eae‐.
Sheep and goats
Two MS reported the analysis of 15 samples taken at a goat farm, with six positive results (40%).
By analysing the full data set, 61 samples from sheep and goats were reported from six MS. Nine positive samples yielded two STEC O157 and one STEC of O121 serogroup. The latter was also reported as possessing the stx2 and eae genes.
Pigs and other animal species
Pigs were tested by two MS, which tested six single animals and 85 herds and reported for the latter 50 positive herds (58.8%). The full data set contained six additional isolates out of 104 units tested. These were one STEC O1, one STEC O2, one STEC O45 and three STEC of non‐specified serogroup. The information on the stx genes was provided for 50 out of 56 strains and included 49 isolates positive for stx2 and one for stx1. The eae gene was not investigated in any of the pig isolates.
In 2019, one MS (IT) reported the presence of STEC in 317 sample units of Cantabrian chamois, deer, Steinbock and wild boar with 17 (5.4%) positives. One MS (NL) reported on the testing of 377 broilers with one positive. Analysis of the STEC serogroups, conducted using the full data set, revealed 25 STEC isolates. For 20 of these, information on the serogroup was provided. In detail, five STEC were of seven were STEC O157, six belonged to O1, five to O2, one O103, one O24, the latter with the virulence genes stx2+; eae+. The remaining isolates did not have any virulence genes characterisation data.
4.4.5. Serogroups and virulotypes of STEC in humans, food and animals
Humans
Data on STEC serogroups (based on the O antigen) were reported in 2019 by 24 MS. Serogroup data were available for 57.9% of the human confirmed cases, which was a slight decrease compared with in 2018 (61.6%). As in previous years, the most commonly reported serogroup was O157, accounting for 26.6% of the cases in humans with a known serogroup. Its proportion has been decreasing to less than half from 54.9% in 2012 to 26.6% in 2019. The proportion of the second most common serogroup O26 slightly decreased compared with 2018 but has steadily increased from 11.6% in 2012 to 16.0% in 2019. These two serogroups represented less than half (42.6%) of the total number of confirmed human cases with known serogroups in 2019 (Table 26). Serogroups O157 and O26 were followed by serogroups O146, O103, O91, O145 and O128 (the latter including variant O128ab). Three new serogroups (O27, O78 and O182) were added to and three serogroups (O5, O55 and O174) were dropped from the top 20 list in 2019. The proportion of serogroups other than O157 increased by 9.2% compared with 2018. The proportion of non‐typable STEC isolates increased by 15.0% (75 cases) representing 12.7% of the reported cases with known serogroup in 2019.
Table 26.
Distribution of the 20 most frequent serogroups reported in confirmed cases of human STEC infections in EU/EEA, 2017–2019
| Serogroup | 2019 | 2018 | 2017 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | MS | % | Cases | MS | % | Cases | MS | % | |
| O157 | 1,195 | 22 | 26.6 | 1,735 | 21 | 34.5 | 1,299 | 22 | 31.9 |
| O26 | 722 | 16 | 16.0 | 833 | 18 | 16.6 | 577 | 17 | 14.2 |
| NT1 | 572 | 11 | 12.7 | 497 | 9 | 9.9 | 493 | 10 | 12.1 |
| O146 | 220 | 11 | 4.9 | 179 | 9 | 3.6 | 139 | 8 | 3.4 |
| O103 | 213 | 13 | 4.7 | 233 | 14 | 4.6 | 245 | 13 | 6.0 |
| O91 | 181 | 12 | 4.0 | 192 | 10 | 3.8 | 178 | 12 | 4.4 |
| O145 | 162 | 11 | 3.6 | 158 | 12 | 3.1 | 147 | 12 | 3.6 |
| O1282 | 113 | 12 | 2.5 | 107 | 10 | 2.1 | 79 | 11 | 1.9 |
| O80 | 80 | 9 | 1.8 | 64 | 8 | 1.3 | 42 | 7 | 1.0 |
| O111 | 63 | 12 | 1.4 | 79 | 15 | 1.6 | 92 | 17 | 2.3 |
| O63 | 62 | 8 | 1.4 | 24 | 6 | 0.5 | 30 | 6 | 0.7 |
| O113 | 60 | 10 | 1.3 | 63 | 7 | 1.3 | 56 | 7 | 1.4 |
| O117 | 52 | 6 | 1.2 | 52 | 7 | 1.0 | 29 | 3 | 0.7 |
| O76 | 48 | 9 | 1.1 | 52 | 9 | 1.0 | 31 | 6 | 0.8 |
| O27 | 44 | 6 | 1.0 | 23 | 5 | 0.5 | 15 | 5 | 0.4 |
| O55 | 36 | 10 | 0.8 | 35 | 9 | 0.7 | 30 | 8 | 0.7 |
| O8 | 36 | 7 | 0.8 | 48 | 8 | 1.0 | 28 | 6 | 0.7 |
| O78 | 30 | 8 | 0.7 | 21 | 7 | 0.4 | 23 | 5 | 0.6 |
| O121 | 29 | 8 | 0.6 | 45 | 6 | 0.9 | 30 | 6 | 0.7 |
| O182 | 28 | 7 | 0.6 | 20 | 6 | 0.4 | 16 | 4 | 0.4 |
| Other | 554 | – | 12.3 | 573 | – | 11.4 | 488 | – | 12.0 |
| Total | 4,500 | 22 | 100.0 | 5,033 | 23 | 100.0 | 4,067 | 23 | 100.0 |
Non‐typable STEC include those strains in which the laboratory tried but was not able to define the O‐serogroup. This depends on how many sera/molecular tools are included in the typing panel.
Including O128ab.
Source: 24 MS and two non‐MS: Austria, Belgium, Croatia, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Luxembourg, Malta, the Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, the United Kingdom and Iceland and Norway.
Data on virulotypes (based on Shiga toxin genes stx1, stx2 and the intimin‐coding gene eae) were reported for 49.7% of confirmed STEC infections (7,775) in 2019 by 20 MS. This was a decrease compared with 2018 (62.3%). Virulence genes were reported for 51.4% of 1,853 severe STEC cases (hospitalised, bloody diarrhoea and/or HUS cases). Most isolates (91.2%) were subjected to subtyping of stx genes and 78.5% also had the information on the presence of the eae gene. The most commonly reported virulence gene combination was stx1‐/stx2+/eae+, accounting for 42.1% (399/948) of the severe human cases with known virulotypes (Table 28). The proportion of the second most common virulotype stx1+/stx2+/eae+ accounted for 30.1% (285/948) of the cases. Together these two virulotypes represented 72.2% of the total number of severe human cases with known virulotypes in 2019. The most common stx gene subtypes were stx1a (261/865; 30.2%), stx2a (222/865; 25.7%), stx2c (182/865; 21.0%) and stx2a;stx2c (100/865; 11.6%), representing 88.5% of the total number of subtypes in severe human cases (Table 29).
Table 28.
Virulotypes of the food, animal and human isolates causing severe infection (HUS, hospitalisation and bloody diarrhoea) in 2019 and comparison with those associated with severe disease in humans in 2012–2017, in EU. The stx genes are characterised at the type level (stx1 and stx2)
| Virulence genes profile | No of animal isolates in 2019^ | No of food isolates in 2019^ | No of human isolates in 2019 (%) | Relative frequency of the virulotype in* | ||
|---|---|---|---|---|---|---|
| HUS | Hospitalisation | Bloody diarrhoea | ||||
| stx2; eae+ | 8 | 13 | 399 (42.1) | 17.7 | 42.0 | 40.2 |
| stx2; stx1; eae+ | 26 | 3 | 285 (30.1) | 5.9 | 35.7 | 64.8 |
| stx2; eae‐ | ND | 42 | 90 (9.5) | 2.7 | 24.3 | 14.8 |
| stx1; eae+ | 1 | 25 | 88 (9.3) | 1.2 | 27.4 | 27.3 |
| stx1; eae‐ | ND | 25 | 44 (4.6) | 0.3 | 20.3 | 14.1 |
| stx2; stx1; eae‐ | 1 | 30 | 42 (4.4) | 1.4 | 15.3 | 19.4 |
| Total | 36 | 138 | 948 | |||
ND: Not detected.
Relative frequencies (%) of the different combinations of stx gene subtypes with or without the eae gene in STEC isolated from severe disease (TESSy data, 2012–2017). Data from: EFSA Journal 2020;18(1):5967.
Due to the low number of isolates virulotyped for food and animals only the number of isolates is displayed.
Table 29.
Stx‐coding genes profiles of the food and human isolates causing severe infection (HUS, hospitalisation and bloody diarrhoea) in 2019 and comparison with those associated with severe disease in humans in the 2012–2017, in EU. The stx genes are characterised at the subtype level
| Stx genes subtypes combinations | No of food isolates in 2019^ | No of human isolates in 2019 (%) | Relative frequency of the stx genes subtypes combinations in* | |||||
|---|---|---|---|---|---|---|---|---|
| HUS | Hospitalization | Bloody Diarrhea | ||||||
| eae+ | eae‐ | eae+ | eae‐ | eae+ | eae‐ | |||
| Stx1a | 10 | 261 (30.2) | 1.2 | 0.0 | 27.6 | 20.7 | 27.3 | 8.0 |
| Stx2a | 3 | 222 (25.7) | 27.4 | 10.4 | 56.4 | 32 | 58.4 | 26.3 |
| Stx2c | 2 | 182 (21.0) | 4.3 | 5.0 | 19.8 | NR | 23.9 | NR |
| Stx2c;Stx2a | 1 | 100 (11.6) | 29.0 | NR | 57.1 | NR | 65.5 | NR |
| Stx2d;Stx2a | 1 | ND | – | – | – | – | – | – |
| Stx2g;Stx2a | 2 | ND | – | – | – | – | – | – |
| Stx2b | 1 | 39 (4.5) | NR | 0.5 | NR | 21.3 | NR | 10.5 |
| stx1c | ND | 30 (3.5) | NR | 0.6 | NR | 18.9 | NR | 19.5 |
| Stx2d | 4 | 16 (1.8) | NR | 10.3 | NR | 33.3 | NR | 16.0 |
| Stx2f | ND | 8 (0.9) | 3.8 | NR | 21.0 | NR | 8.7 | NR |
| Stx1d | 1 | 3 (0.3) | – | – | – | – | – | – |
| Stx2c;Stx2a;Stx1a§ | 1 | ND | 20.8 | 4.5 | 59.3 | NR | 56.6 | NR |
| Stx2a;Stx1a | 8 | ND | 20.8 | 4.5 | 59.3 | NR | 56.6 | NR |
| Stx1a;Stx1c | ND | 1 (0.1) | – | – | – | – | – | – |
| Stx2e | ND | 1 (0.1) | NR | NR | NR | NR | 31.8 | |
| Stx2a;Stx2e | ND | 1 (0.1) | – | – | – | – | – | – |
| Stx2c;Stx2d | ND | 1 (0.1) | – | – | – | – | – | – |
| Stx2d;Stx2b | 2 | ND | – | – | – | – | – | – |
| Stx2d;Stx1a | 1 | ND | – | – | – | – | – | – |
| Stx2d;Stx2a;Stx1a | 2 | ND | – | – | – | – | – | – |
| Total | 39 | 865 | ||||||
NR: data present in the TESSy data set used, with less than 20 isolates.
ND: Not detected.
– : not present in the TESSy database in the 2012–2017 period.
Relative frequencies (%) of the different combinations of stx gene subtypes in STEC isolated from severe disease (TESSy data, 2012–2017). Human data from: EFSA Journal 2020;18(1):5967.
Due to the low number of isolates virulotyped for food only the number of isolates is displayed. Only six animal isolates were provided with the information on the stx gene subtypes and have not been included in this table.
Food
This section includes the analysis of the data present in the full data set (Section 4.3.2, Data Analysis), which contained 25,238 sample units tested of which 2.5% (641) were STEC‐positives.
For analysis of the distribution of STEC serogroups 25 of these 641 isolates, reported by five MS from 1,284 samples, could however not be used because they were obtained using the analytical method ISO 16654:2001 or equivalent methods, which aimed at detecting the serogroup O157 only, so introducing a bias in the descriptive analysis. In total, 23,954 (94.9%) food sample units were reported with analytical method ISO TS 13136:2012 and equivalent methods, which aimed at detecting all STEC, and 616 (2.6%) were STEC‐positive (Table 30). Of these 616 isolates, 212 (34.4%) were provided with the information on the serogroup, which were the data used for the description of STEC serogroups in food. Of these 212 isolates 45 (21.2%) belonged to the top five serogroups (O157, O26, O103, O111 and O145) while the remaining 167 isolates (78.8%) belonged to 53 different O‐groups (Table 31). All the top 20 STEC serogroups isolated from human infections were also found in the STEC isolated from food in 2019 with the exception of serogroups O80, O5 and O76 only found in food (Tables 26 and 27). For 404 (65.6%), STEC isolates the only information reported was that the isolate did not belong to the O157 serogroup (88 isolates: 14.3%) or that the serogroup was unspecified.
Table 30.
Proportion of positive samples for any STEC and STEC belonging to the ‘top‐five’ serogroups in food categories, in reporting MS, 2019
| Food categorya | Samples positive for | |||||
|---|---|---|---|---|---|---|
| Any STEC | O157 | O26 | O145 | O103 | O111 | |
| N | N | N | N | N | N | |
| Bovine meat | 315 | 14 | 7 | 4 | 4 | 1 |
| Ovine and goat meat | 102 | 3 | 3 | 0 | 2 | 0 |
| Other ruminants meatb | 10 | 0 | 0 | 0 | 0 | 0 |
| Pig meat | 54 | 0 | 0 | 0 | 0 | 0 |
| Other meatc | 21 | 0 | 0 | 0 | 1 | 0 |
| Mixed meat | 16 | 0 | 0 | 0 | 1 | 0 |
| Milk and dairy productsd | 39 | 0 | 3 | 0 | 0 | 0 |
| Raw milke | 52 | 1 | 1 | 0 | 0 | 0 |
| Fruit and vegetable | 2 | 0 | 0 | 0 | 0 | 0 |
| seedsf | 0 | 0 | 0 | 0 | 0 | 0 |
| Other food | 5 | 0 | 0 | 0 | 0 | 0 |
| Total | 616 | 18 | 14 | 4 | 8 | 1 |
MS: Member State; N: number of samples; STEC: Shiga toxin‐producing Escherichia coli.
Note: Only results from samples tested by the ISO TS 13136:2012 method were included.
The different meat categories presented in this table include all types of meat (not only fresh).
Includes meat from deer.
Includes meat from other animals (other than ruminants).
Includes any type of dairy product, cheese and milk other than raw milk.
Includes raw milk from different species, but most tested and all the positive samples were from cows.
Includes only sprouted seeds.
Table 27.
Distribution of the 20 most frequent serogroups reported in confirmed cases of human STEC infections and of STEC in food and in animals in EU/EEA, 2019
| Serogroup | Human | Food | Animals | ||||||
|---|---|---|---|---|---|---|---|---|---|
| STEC cases | MS | % | STEC‐positive | MS | % | STEC‐positive | MS | % | |
| O157 | 1,195 | 22 | 26.6 | 43 | 7 | 6.7 | 45 | 5 | 12.3 |
| O26 | 722 | 16 | 16.0 | 14 | 6 | 2.2 | 1 | 1 | 0.3 |
| NT1 | 572 | 11 | 12.7 | 316 | 10 | 49.1 | 253 | 4 | 69.1 |
| O146 | 220 | 11 | 4.9 | 13 | 2 | 2.0 | 2 | 1 | 0.5 |
| O103 | 213 | 13 | 4.7 | 8 | 6 | 1.2 | 1 | 1 | 0.3 |
| O91 | 181 | 12 | 4.0 | 10 | 4 | 1.6 | ND | 0.0 | |
| O145 | 162 | 11 | 3.6 | 4 | 1 | 0.6 | 1 | 1 | 0.3 |
| O1282 | 113 | 12 | 2.5 | 2 | 1 | 0.3 | ND | 0.0 | |
| O80 | 80 | 9 | 1.8 | ND | 0.0 | ND | 0.0 | ||
| O111 | 63 | 12 | 1.4 | 1 | 1 | 0.2 | 1 | 1 | 0.3 |
| O63 | 62 | 8 | 1.4 | ND | 0.0 | ND | 0.0 | ||
| O113 | 60 | 10 | 1.3 | 17 | 5 | 2.7 | 2 | 1 | 0.5 |
| O117 | 52 | 6 | 1.2 | 2 | 1 | 0.3 | ND | 0.0 | |
| O76 | 48 | 9 | 1.1 | ND | 0.0 | ND | 0.0 | ||
| O27 | 44 | 6 | 1.0 | ND | 0.0 | ND | 0.0 | ||
| O55 | 36 | 10 | 0.8 | 10 | 1 | 1.6 | ND | 0.0 | |
| O8 | 36 | 7 | 0.8 | 7 | 2 | 1.1 | 14 | 1 | 3.8 |
| O78 | 30 | 8 | 0.7 | ND | 0.0 | ND | 0.0 | ||
| O121 | 29 | 8 | 0.6 | 1 | 1 | 0.2 | 1 | 1 | 0.3 |
| O174 | ND | 0.0 | 8 | 2 | 1.2 | 1 | 1 | 0.3 | |
| O182 | 28 | 7 | 0.6 | ND | ND | ND | ND | ND | ND |
| Other | 554 | – | 12.3 | 97 | 6 | 15.1 | 43 | 3 | 11.7 |
| Total | 4,500 | 22 | 100.0 | 641 | 20 | 100.0 | 366 | 8 | 100.0 |
ND: not detected.
Non‐typable STEC include those strains in which the laboratory tried but was not able to define the O‐serogroup. This depends on how many sera/molecular tools are included in the typing panel.
Including O128ab.
For the characterisation of the virulence genes of STEC strains from food, 641 isolates were available. These data reported from food were still fragmented, as observed in the previous year (EFSA and ECDC, 2019c). Information on stx1 and/or stx2 was provided for 339 (52.9%) STEC strains. Only 185 (28.9%) were reported to have been tested for the presence of the eae gene. The combination of the stx and eae genes was available for 138 isolates (21.5%) (Table 28). Thirty‐nine STEC isolates (6.1%) were subjected to stx gene subtyping (Table 29) and for 11 (1.7%), the information on the presence of the eae genes was reported. Tables 28, 29 and 30 show the combinations of the virulence genes determined in the food isolates and their match with those found in the STEC isolated from severe human disease in the EU in 2012–2017, analysed in the latest pathogenicity assessment of STEC (EFSA BIOHAZ Panel, 2020c). Given the low number of food and animal isolates with the virulence genes characterised in 2019, the figures are displayed in terms of number of isolates instead of the relative frequency for each virulotype.
Animals
This section includes the analysis of the data present in the full data set (Section 4.3.2, Data Analysis), which contained 2,588 animal sample units tested of which 14.1% (366) were STEC‐positive.
For the analysis of the distribution of STEC serogroups, 108 (29.5%) STEC isolates with information on the serogroups was available. However, 41 of these 108 isolates could not be used because they were obtained with the analytical method ISO 16654:2001 or equivalent methods, which aim at detecting the serogroup O157 only, so introducing a bias in the descriptive analysis. The remaining 67 STEC isolates (18.3%) were obtained by using the ISO TS 13136:2012 method or equivalent, targeting any STEC, which were the data for the description of STEC serogroups (Table 32). Of these, eight (11.9%) belonged to the top five serogroups while the remaining 59 isolates (88%) belonged to 19 non‐top five serogroups, including 10 of the top 20 serogroups isolated from human disease in 2018 (EFSA and ECDC, 2019c) (Table 32).
For characterisation of the virulence genes of STEC strains from animals, 366 isolates were available but the virulence genes stx and eae were identified and typed only in a small proportion of the reported animal isolates. Out of the 366 STEC isolates reported, 92 (25.1%) were provided with the information on stx1 and/or stx2, but only 36 of these were reported together with the detection of the eae gene (Table 28). One MS also carried out stx gene subtyping and reported six STEC strains, four O157, one O26 and one O121, possessing the stx2c; stx2a combination, determined in the STEC O157 isolates and the stx2a subtype alone, found in the other two serogroups. All these isolates were also eae positive (Table 28).
All data provided by the reporting countries were used to generate an atlas of the STEC serogroups identified in the different food and animal categories for the years 2014–2019 (Figure 80C) and for 2019 (Figure 81C), and is shown in Appendix C. It must be emphasised that the differences in the sampling strategies and, to a lesser extent the analytical methods, applied by reporting countries did not allow confirmation of the existence of specific trends in the geographical distribution of STEC serogroups.
Figure C.1.

Frequency distributions of reported STEC serogroups in food and animals, in reporting MS during 2014–2019
- Note: The presence and absence of STEC serogroups in foods (left) and animals (right). Red boxes > 1%, orange boxes > 0.1% and ≤ 1%, yellow boxes > 0.0001% and ≤ 0.1% of positive samples. White boxes indicate absence of the serogroup. An E. coli O104:H4 stx2+ eae‐ was isolated from sprouted seeds in 2015.
Figure C.2.
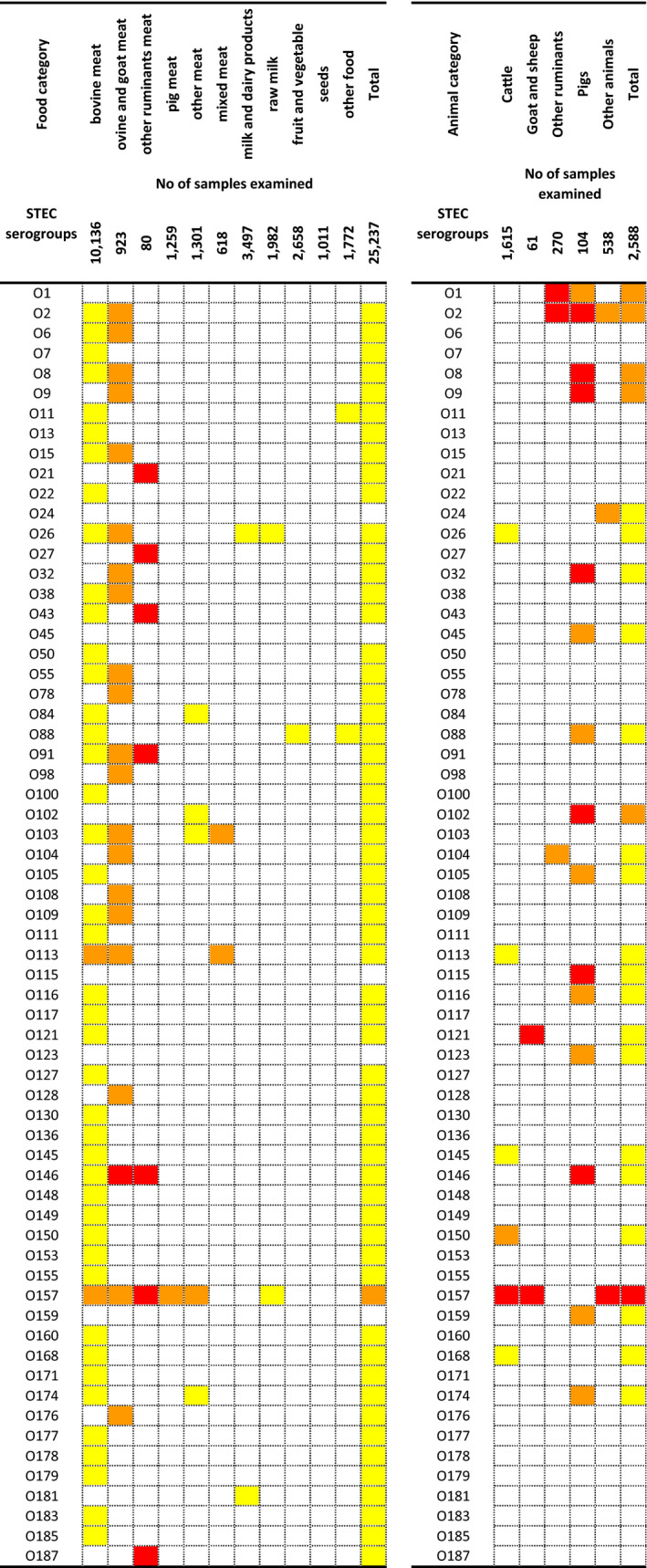
Relative presence of reported STEC serogroups in foods and animals, in reporting MS, 2019
-
Proportions of STEC serogroups: red boxes > 1%, orange boxes > 0.1% and ≤ 1%, yellow boxes > 0.0001% and ≤ 0.1% of positive samples. White boxes indicate absence of the serogroup.The food category ‘other ruminants’ meat’ includes meat from deer; ‘other meat’ includes meat from animals other than ruminants; ‘milk and dairy products’ include any type of dairy product, cheese and milk other than raw milk; ‘raw milk’ includes raw milk from different species, but most tested samples were from cows; ‘seeds’ includes mostly sprouted seeds, but dry seeds are also included.Source: Twenty‐two MS.The animal category ‘other ruminants’ includes deer; ‘other animals’ comprises pigeons, cats, chinchillas, dogs, ferrets, foxes, Gallus gallus, guinea pigs, hedgehogs, mice, rabbits, rats solipeds, water buffalos, weasels and wild boars.Source: nine MS.
4.5. Discussion
The number of cases and notification rate of human STEC infections increased notably in 2018, which made STEC the third most commonly reported zoonosis in EU. In 2019, the notification rate was at the same level as in 2018. The long‐term trend for human STEC infections showed an increase since 2010, which was mainly due to a large STEC outbreak in 2011. The notification rate stayed at a markedly higher level after the outbreak than before the outbreak. The overall trend of reported cases stabilised after the outbreak but has shown an increase again in the last 5 years during 2015–2019. Part of the observed increase may be explained by improved general awareness of STEC detection following the reporting of large STEC outbreaks. Other contributing factors could probably be the changes in laboratory techniques such as increased diagnostic use of multiplexed molecular assays (PCR) and direct DNA extraction from a specimen followed by isolation and further strain characterisation. More than half of MS national public health laboratories reported having the capacity to perform whole genome sequencing (WGS) for STEC isolates (EFSA BIOHAZ Panel, 2020c).
In 2019, 57.9% of the human confirmed cases have been reported with information on the serogroup. This was a slight decrease compared with 2018 when 61.6% of the human isolates had been serogrouped. As in previous years, the most commonly reported serogroup in human cases was O157, followed by O26. The proportion of serogroup O157, however, continued to decrease in 2019, whereas the proportion of non‐O157 STEC serogroups has increased over several years. Increasing numbers of laboratories were testing for serogroups other than O157 and there has been a shift in diagnostic methods, with PCR being more commonly used for detection of STEC cases in several MS. Serogroup O26 was the most commonly reported among HUS cases instead of serogroup O157, as it has been since 2016. Over half of the HUS cases caused by this serogroup were reported by two countries (France and Italy), where the surveillance of STEC infections is mainly based on detection of HUS cases.
The characterisation of the major virulence determinants such as the Shiga toxin‐coding genes (stx) and, to a lesser extent, the intimin‐coding eae gene has been indicated to have much more predictive power in terms of pathogenicity potential of STEC strains (EFSA BIOHAZ Panel, 2020c; and the JEMRA and NACMCF reports at Section 4.6 Internet sources) than the serogroups. In this respect, while the last pathogenicity assessment of STEC revolves around the statement that ‘all strains are pathogenic to humans, causing at least diarrhoea’, a deeper analysis of the virulence genes content, particularly the subtyping of the stx genes, allows identifying some virulence genes combinations (virulotypes), which have a higher frequency of association with severe disease in humans (EFSA BIOHAZ Panel, 2020c). About half of the STEC isolates from all human infections as well as severe human cases (hospitalised, bloody diarrhoea and HUS cases) were reported together with the information on the stx genes (stx1 or stx2) and for the presence of the intimin‐coding gene eae. Despite the decrease compared with 2018, when the highest number of virulence gene typing data was reported to TESSy, there has been a steady increase of reporting of stx and eae genes since 2012 (EFSA BIOHAZ Panel, 2020c). Most (> 90%) of the severe human cases were reported with information on stx gene subtypes and 78.5% with data on the presence of the eae gene in 2019. Based on the analysis of the stx subtypes reported to TESSy from 2012 to 2017, all STEC subtypes may be associated with severe illness, albeit at different frequencies. Although stx2a previously showed the highest rates of severe outcome, the stx1a was most frequently associated with severe illness outcomes in 2019 followed by stx2a. Of the STEC cases with known hospitalisation status, more than one‐third were hospitalised. Some countries reported very high proportions of hospitalised cases, but had notification rates that were among the lowest, indicating that the surveillance systems in these countries primarily captured the most severe cases. The age group most affected by STEC was infants and children up to 4 years of age, who accounted for two‐thirds of the cases of HUS. Most cases of deaths (60%) were, however, reported in age groups > 25 years. Half the deaths were reported to be associated with HUS.
In 2019, 22 EU MS reported monitoring results of STEC in 25,030 food samples. Not all reporting MS have tested all food categories equally. By aggregating the food samples into macro‐categories in 2019, the number of MS testing and reporting data on the presence of STEC in food ranged from 20 MS reporting the testing for STEC in meat samples to 16 MS and 13 MS testing vegetables (including seeds) and milk and dairy products, respectively. Sprouted seeds were tested by 15 MS, considering the full data set, a number slightly higher than that observed in 2018 (13 MS). Despite the existing microbiological criterion for the presence of STEC in seeds (EU Regulation No 209/2013), the sampling of this food category in the EU appears to be extremely low.
The analytical procedures for food testing in the EU are substantially harmonised. Twenty‐one out of the 22 MS reporting data have used the ISO TS 13136:2012 or equivalent methods. There was for 2019 still a residual amount of data being produced by some MS (five) for specific surveys using the ISO 16654:2001 or equivalent methods. These aimed at detecting the serogroup O157 only and do not give information on any other STEC serogroups possibly present in the sample. One MS reported food testing data only obtained with these methods. Additionally, the strategy that the methods for E. coli O157 are based on, revolves around the identification of the serogroup and does not include the determination of the stx gene or of the toxin produced. This laboratory analysis must be actively carried out by MS to confirm that isolated strains are actually STEC. This latter piece of information was missing in the 2019 data set for most O157 isolates.
The general extent of contamination of food with STEC observed, 2.8%, was in line with what has been determined in previous years. Monitoring results for STEC contamination in RTE food were described for samples collected according an ‘objective’ sampling strategy. STEC‐positive units were detected in the following RTE foods: in meat and meat products particularly in bovine meat, in milk and milk products notably cheeses, in spices and herbs, in salads and in fruits, vegetables and juices. Importantly, only one‐third of the MS or less reported data for certain food categories with a limited sampling effort for certain foods (e.g. two MS reporting 285 sample results for RTE salads). Nevertheless, the finding of STEC‐contaminated RTE food commodities is of concern as these are consumed without any treatment to reduce or eliminate the possible presence of the pathogen, posing a direct risk to the consumer.
As observed in previous years, different frequencies of contamination with STEC were found in the different major food categories, RTE and non‐RTE. The most contaminated food categories included commodities of animal origin, with fresh meat in particular. Small ruminants’ meat, including meat from sheep, goats and deer, was the food commodity presenting the highest values (11.6%, 16.7% and 12.9%, respectively). These frequencies, however, may reflect the effect of the few samples tested. As observed in 2018 data (EFSA and ECDC, 2019c), raw cows’ milk was the second food category with the highest STEC contamination frequency in 2019, with 3.9% STEC‐positive samples. Finally, the ‘vegetables and fruit’ food category was the less contaminated with 0.1% of positive samples.
The characterisation of the food STEC isolates is pivotal for the assessment of the risks for consumers posed by food. In this respect, determination of the serogroup is an important part of this process. Although the recent pathogenicity assessment of STEC (EFSA BIOHAZ Panel, 2020c) affirms that this feature is not an indication of pathogenicity, it still has some importance as an epidemiological marker, and it remains useful to observe the circulation of the different STEC types in food and human cases of disease. In 2019, 34.4% of the food isolates were provided with information on the serogroup, compared with 41.8% in 2018. Of these 21.6% belonged to the ‘top‐five’ serogroups (O157, O26, O103, O111 and O145) whereas more than half of all the remaining STEC belonged to the top 20 STEC serogroups reported in human infections to ECDC in 2015–2018 (EFSA and ECDC, 2019c; Table 27).
As for the animal monitoring results for 2019, overall, 14.1% of the samples were STEC‐positive, compared with 7.6% in 2018. However, the number of animal sampling units tested has been very low in the last years, biasing the estimates. In 2019, this high prevalence may be explained by a very high value of 58.8% STEC‐positive pig herds reported by one MS at the farm stage, but most of these are unlikely to involve zoonotic strains (Abubakar et al., 2017; Remfry et al., 2021). A large increase in the STEC‐positive samples, 17%, was also reported for cattle tested in 2019, compared with 3.1% in 2018, but only covered by data from four MS (three in 2018). The animal STEC strains were typed in a lower proportion than the food isolates, with 18.3% of the isolates obtained using the ISO TS 13136:2012 being serotyped.
The analysis of the presence and subtypes of virulence genes is important for pathogenicity assessment. Unfortunately, this level of characterisation is still far from being comprehensive for food and animal isolates and only 52.9% of the STEC isolated from food in 2019 have been reported together with the information on the stx gene types (stx1 or stx2) and only 28.9% have been tested for the presence of the intimin‐coding gene eae. These figures reduce dramatically to 6.1% and 1.7% when the information on the stx gene subtypes was considered, alone or together with the information on the presence of eae gene, respectively. As this typing and subtyping strategy represents the basis for molecular risk assessment of STEC circulating in the vehicles of infections, MS should be encouraged to expand the adoption of this approach.
The analysis of the STEC isolated from food in 2019 showed that many of the virulotypes identified matched those associated with the STEC strains isolated from severe disease (HUS, hospitalisation or bloody diarrhoea) in the EU in the period 2012–2017 (EFSA BIOHAZ Panel, 2020c).
Fewer animal STEC isolates were reported data on characterisation of the virulence genes as compared with food isolates. Only six animal isolates were subjected to stx gene subtyping by one MS. Nevertheless, also in this case, many of the virulotypes identified could find correspondence with the same feature of STEC isolated from human severe disease in the 2012–2017 time period (EFSA BIOHAZ Panel, 2020c).
The methodologies for typing and subtyping the virulence genes of STEC are available, including those based on WGS, and are supported by external quality assurance (EQA) at the EU National Reference Laboratories level by the EURL for E. coli through its annual inter‐laboratory studies scheme. For a greater adoption of the subtyping schemes for STEC to be achieved, it would be of fundamental importance that the cascade of methods distribution and EQA are disseminated down to the Official Laboratories level within the MS (EU Regulation 625/2017). This will provide a wider base of typing and subtyping data for food and animal STEC isolates enabling a deeper risk assessment of STEC in support of actions to be undertaken by the Competent Authorities to mitigate the impact of STEC on public health.
4.6. Related projects and Internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definition of STEC/VTEC infection | https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions | |
| Disease Programme on Emerging, Food‐ and Vector‐Borne Diseases | https://www.ecdc.europa.eu/en/about-us/who-we-are/units/disease-programmes-unit | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| World Health Organization – E. coli fact sheet | http://www.who.int/mediacentre/factsheets/fs125/en/ | |
| Food, animals | EFSA Scientific Opinion of the Panel on Biological Hazards (BIOHAZ) – Monitoring of verotoxigenic Escherichia coli (VTEC) and identification of human pathogenic VTEC types | http://www.efsa.europa.eu/en/efsajournal/pub/579 |
| Scientific Opinion of the Panel on Biological Hazards (BIOHAZ) – Monitoring of verotoxigenic Escherichia coli (VTEC) and identification of human pathogenic VTEC types | http://www.efsa.europa.eu/en/efsajournal/pub/579 | |
| VTEC‐seropathotype and scientific criteria for pathogenicity assessment | http://www.efsa.europa.eu/en/efsajournal/pub/3138 | |
| Pathogenicity assessment of Shiga toxin‐producing Escherichia coli (STEC) and the public health risk posed by contamination of food with STEC | https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2020.5967 | |
| JEMRA FAO/WHO report: Shiga toxin‐producing Escherichia coli (STEC) and food: attribution, characterisation and monitoring. Microbiological Risk Assessment Series. Rome | http://www.fao.org/documents/card/en/c/CA0032EN | |
| Public health advice on prevention of diarrhoeal illness with special focus on Shiga toxin‐producing Escherichia coli (STEC), also called verotoxin‐ producing E. coli (VTEC) or enterohaemorrhagic E. coli (EHEC) | http://www.efsa.europa.eu/en/press/news/110611 | |
| Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC | https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003L0099&from=EN | |
| Regulation (EC 209/2013) | http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32013R0209 | |
| EURL VTEC webpage: laboratory methods for STEC detection and typing | http://www.iss.it/vtec/index.php?lang=2&anno=2017&tipo=3 | |
| EURL VTEC webpage: Focus on‐STEC and other pathogenic E. coli | http://www.iss.it/vtec/index.php?lang=2&anno=2017&tipo=20# | |
| NACMCF report: Response to Questions Posed by the Food and Drug Administration Regarding Virulence Factors and Attributes that Define Food‐borne Shiga Toxin‐Producing Escherichia coli (STEC) as Severe Human Pathogens | https://www.fsis.usda.gov/wps/wcm/connect/981c8e0a-6a5b-45d1-a04d-1934463a666c/nacmcf-stec-2019.pdf?MOD=AJPERES | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
5. Tuberculosis due to Mycobacterium bovis or Mycobacterium caprae
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
5.1. Key facts
Tuberculosis due to Mycobacterium bovis or Mycobacterium caprae is a rare infection in humans in the EU, with 147 confirmed cases in humans reported in 2019.
The EU notification rate of M. bovis and M. caprae has ranged from 0.03 to 0.05 per 100,000 population between 2015 and 2019.
In 2019, the majority (69.4%) of M. bovis and M. caprae cases in humans was of EU origin (native cases and/or cases originating from other EU MS). Cases were more frequently reported by MS that were not officially bovine tuberculosis free (non‐OTF) compared with MS that were officially bovine tuberculosis free in cattle (OTF).
No food‐borne disease outbreak due to Mycobacterium spp. has ever been reported to EFSA since the start of the food‐borne outbreaks data collection in 2004 and this was also the case for 2019.
Fourteen MS reported to have detected bovine tuberculosis for the year 2019. As in previous years, it was heterogeneous and much spatially clustered with herd prevalence ranging from absence to 11.7% within England, in the United Kingdom.
Seventeen MS were officially bovine tuberculosis free in cattle (OTF) during 2019 and of the 11 non‐OTF MS four had OTF regions.
Overall, 143 (0.014%) bovine tuberculosis‐infected herds were reported in the OTF regions of 21 MS, which was a rare event, as in previous years.
In the non‐OTF regions of 11 MS, 16,277 (1.803%) cattle herds were reported positive for bovine tuberculosis for 2019. From 2010 to 2019, the overall annual number of positive cattle herds and the prevalence in these non‐OTF regions decreased, respectively, by 37.0% and 14.5%. Concomitantly, the total number of cattle herds in these regions reduced to about half. In recent years, the United Kingdom has reported in its non‐OTF regions an annual prevalence of bovine tuberculosis test‐positive herds of above 10% for Wales and for England, as well as for Northern Ireland; Greece, Ireland and Spain reported a low prevalence between 2 and 5%; whereas Italy and Portugal reported very low (< 1%) prevalence.
5.2. Surveillance and monitoring of tuberculosis due to M. bovis or M. caprae in the EU
5.2.1. Humans
The notification of tuberculosis in humans is mandatory in all EU MS, Iceland, Norway, Liechtenstein and Switzerland and covers the whole population. It has been possible to report M. caprae as a separate species since 2018. France did not report species‐specific data within the Mycobacterium tuberculosis complex for the human tuberculosis cases reported in 2019; therefore, no human M. bovis or M. caprae data are available for France. In addition, Latvia did not report any Mycobacterium tuberculosis complex data for 2018 or 2019. Countries may update their data retroactively, and therefore, reported numbers are subject to change in the future or may vary from numbers reported in previous reports.
The M. bovis and M. caprae notification rate was calculated using the combined population of the EU MS who reported data in 2019. The proportion of tuberculosis cases caused by M. bovis or M. caprae was calculated using the preliminary estimate of the total number of confirmed tuberculosis cases in 2019 among EU MS reporting species‐specific data.
As tuberculosis is a chronic disease with a long incubation period, it is not possible to assess travel‐associated cases in the same way as for diseases with acute onset. Instead, the distinction is made between individuals with the disease originating from an EU MS (cases of EU origin) and those originating from outside the EU (case originating outside of EU). In the analysis, origin is mainly based on the reported birthplace, except for cases from Austria, Belgium, Greece, Hungary and Poland, whose origin is based on their reported nationality.
The treatment outcome for tuberculosis due to M. bovis or M. caprae is assessed 1 year (12 months) after the case notification, as the shortest duration for treatment completion is 6 months according to the international treatment guidelines of tuberculosis.
5.2.2. Animals
Bovine tuberculosis monitoring data from bovine animals originating from the National Control and Eradication Programmes and/or Officially Free status
According to the Zoonoses Directive 2003/99/EC, MS must report bovine tuberculosis annual monitoring data. These data originate from national control and surveillance programmes implemented by the MS in accordance with EU legislation. The reports submitted by the MS are based on Council Directive 64/432/EEC and subsequent legislation and are essential for the assessment of the epidemiological situation in MS and MS regions, whether declared officially bovine tuberculosis free in cattle (OTF) or not yet declared OTF. Annual surveillance programmes are carried out in OTF regions to confirm freedom from bovine tuberculosis, whereas in all non‐OTF regions control and eradication programmes for bovine tuberculosis are in place. These data are comparable across MS because the monitoring schemes are harmonised, and the data collected and reported to EFSA originate from the census as the sampling frame. In addition to trend analysis both at the EU level and at MS level and to trend watching and descriptive summaries, these data may also be used to assess the impact of control and eradication programmes (Table 1).
EU MS also need to notify outbreaks of bovine tuberculosis in terrestrial animals from OTF regions to the EU Animal Disease Notification System (ADNS)11 and regular summaries are posted online.
For bovine tuberculosis cases, all tuberculosis cases irrespective of their causative agent (i.e. also including those caused by M. caprae) are included in the statistics provided by MS, as opposed to the procedure for the above‐mentioned statistics for humans, in which cases by M. bovis and M. caprae are separated. Based on the definition recommended by the bovine tuberculosis subgroup of the task force on monitoring animal disease eradication of the EU (SANCO/10200/2006), who made it explicit that all cases of tuberculosis in cattle due to a disease‐causing member of the M. tuberculosis complex are to be considered as a case of bovine tuberculosis, all available information on the specific bacterial species part of the M. tuberculosis complex recovered from cattle was taken into account to summarise the EU situation on bovine tuberculosis. A distinction is made descriptively, whenever possible, of reporting by MS on Mycobacterium tuberculosis complex, M. bovis and M. caprae.
Mycobacterium monitoring data from food and from animals other than bovine animals
Mycobacterium monitoring data from food and from animals other than bovine animals submitted to EFSA according the Zoonoses Directive 2003/99/EC and collected without harmonised design allow for descriptive summaries at the EU level to be made. They preclude trend analyses and trend watching at the EU level (Table 1).
5.2.3. Food‐borne outbreaks of tuberculosis due to M. bovis or M. caprae
The reporting of food‐borne outbreaks of tuberculosis due to M. bovis or M. caprae is mandatory according to the Zoonoses Directive 2003/99/EC.
5.3. Results
5.3.1. Overview of key statistics, EU, 2015–2019
Table 33 summarises EU‐level statistics on human tuberculosis due to M. bovis or M. caprae and on bovine tuberculosis, during 2015–2019. Further descriptions of findings can be found in the following sections.
Table 33.
Summary statistics on tuberculosis due to M. bovis or M. caprae related to humans and bovine animals, EU, 2015–2019
| 2019 | 2018 | 2017 | 2016 | 2015 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Number of confirmed M. bovis cases | 136 | 168 | 203 | 182 | 175 | ECDC |
| Number of confirmed M. caprae cases | 11 | 13 | 9 | 11 | 10 | ECDC |
| Total number of confirmed cases | 147 | 181 | 212 | 193 | 185 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.03 | 0.04 | 0.05 | 0.04 | 0.04 | ECDC |
| Number of EU MS that reported data on M. bovis or M. caprae cases | 26 | 26 | 27 | 27 | 27 | ECDC |
| M. bovis or M. caprae cases in individuals of EU origin | 102 | 105 | 141 | 109 | 118 | ECDC |
| M. bovis or M. caprae cases in individuals originating from outside EU | 39 | 67 | 62 | 72 | 57 | ECDC |
| M. bovis or M. caprae cases in individuals of unknown origin | 6 | 9 | 9 | 12 | 10 | ECDC |
| Total number of food‐borne outbreaks | 0 | 0 | 0 | 0 | 0 | EFSA |
| Number of outbreak‐related cases | 0 | 0 | 0 | 0 | 0 | EFSA |
| Bovine animals | ||||||
| Number of infected herds in OTF regions | 143 | 172 | 134 | 147 | 157 | EFSA |
| Number of reporting OTF MS | 17 | 17 | 18 | 18 | 18 | EFSA |
| Number of positive herds in non‐OTF regions | 16,277 | 18,801 | 18,857 | 17,421 | 17,477 | EFSA |
| Number of reporting non‐OTF MS | 11 | 11 | 10 | 10 | 10 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States; OTF: Officially bovine tuberculosis free (status on freedom from bovine tuberculosis, in cattle).
When the UK data were collected, the UK was an EU MS but as of 31 January 2020, it has become a third country.
5.3.2. Tuberculosis due to M. bovis and M. caprae in humans
In 2019, there were 147 confirmed human cases of tuberculosis due to M. bovis or M. caprae reported by 26 EU MS (Table 34). Of these cases, 136 were due to M. bovis and 11 were due to M. caprae. The 11 M. caprae cases were reported by Austria (n = 2), Germany (n = 3) and Spain (n = 6). Between 2015 and 2019, the number of M. caprae cases notified each year has ranged between nine (in 2017) and 13 (in 2018). Overall, M. bovis and M. caprae cases accounted for only 0.3% of total tuberculosis cases reported by the 26 EU MS with species‐specific data within the Mycobacterium tuberculosis complex in 2019.
Table 34.
Reported human cases of tuberculosis due to M. bovis or M. caprae and notification rates per 100,000 population in the EU/EFTA, by country and year, 2015–2019
| Country | 2019 | 2018 | 2017 | 2016 | 2015 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formatb | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria (OTF)c | Y | C | 3 | 0.03 | 2 | 0.02 | 2 | 0.02 | 3 | 0.03 | 6 | 0.07 |
| Belgium (OTF) | Y | C | 0 | 0.00 | 5 | 0.04 | 6 | 0.05 | 14 | 0.12 | 9 | 0.08 |
| Bulgaria | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 |
| Croatia | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czechia (OTF) | Y | C | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 1 | 0.01 | 1 | 0.01 |
| Denmark (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 1 | 0.02 | 2 | 0.04 | 0 | 0.00 |
| Estonia (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Finland (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Franced (OTF) | – | – | – | – | – | – | – | – | – | – | – | – |
| Germany (OTF) | Y | C | 48 | 0.06 | 64 | 0.08 | 47 | 0.06 | 60 | 0.07 | 53 | 0.07 |
| Greece | Y | C | 1 | 0.01 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 |
| Hungary (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Ireland | Y | C | 7 | 0.14 | 7 | 0.14 | 4 | 0.08 | 3 | 0.06 | 5 | 0.11 |
| Italye | Y | C | 11 | 0.02 | 17 | 0.03 | 21 | 0.03 | 13 | 0.02 | 17 | 0.03 |
| Latvia (OTF) | Y | C | – | – | – | – | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Lithuania (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Luxembourg (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Malta | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlands (OTF) | Y | C | 6 | 0.03 | 11 | 0.06 | 11 | 0.06 | 14 | 0.08 | 9 | 0.05 |
| Poland (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Portugalf | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Romania | Y | C | 1 | 0.01 | 0 | 0.00 | 2 | 0.01 | 2 | 0.01 | 0 | 0.00 |
| Slovakia (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Slovenia (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Spaing | Y | C | 32 | 0.07 | 46 | 0.10 | 73 | 0.16 | 39 | 0.08 | 41 | 0.09 |
| Sweden (OTF) | Y | C | 3 | 0.03 | 4 | 0.04 | 3 | 0.03 | 5 | 0.05 | 6 | 0.06 |
| United Kingdomh | Y | C | 35 | 0.05 | 24 | 0.04 | 41 | 0.06 | 37 | 0.06 | 37 | 0.06 |
| EU Total | 147 | 0.03 | 181 | 0.04 | 212 | 0.05 | 193 | 0.04 | 185 | 0.04 | ||
| Icelandi | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | – | – | 0 | 0.00 |
| Liechtenstein (OTF) | Y | C | – | – | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway (OTF) | Y | C | 1 | 0.02 | 0 | 0.00 | 3 | 0.06 | 5 | 0.10 | 1 | 0.02 |
| Switzerland (OTF)j | Y | C | – | – | 3 | 0.04 | 7 | 0.08 | 5 | 0.06 | 6 | 0.07 |
–: Data not reported.
Y: yes; N: no; –: no report.
A: aggregated data; C: case‐based data; –: no report.
OTF: Officially bovine tuberculosis free (status for freedom from bovine tuberculosis, in cattle) ‐ see Section 5.3.4.
Not reporting species of the M. tuberculosis complex.
In Italy, 8 regions and 14 provinces are OTF.
In Portugal, the whole of the Algarve is OTF.
In Spain, the province of Pontevedra and the Canary Islands are OTF.
In the United Kingdom, Scotland and the Isle of Man are OTF.
In Iceland, which has no special agreement on animal health status with the EU, the last outbreak of bovine tuberculosis was reported in 1959.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
Ten MS reported at least one confirmed case and 16 MS did not report any cases. The EU notification rate in 2019 was 0.03 cases per 100,000 population, which was slightly lower than the rate in the previous 4 years. The highest notification rate in 2019 was reported by Ireland (0.14 per 100,000), followed by Spain (0.07 per 100,000) (Table 34).
There were 17 EU MS that had OTF status (OTF, officially bovine tuberculosis free in cattle) in 2019, and, of these, 15 reported on species of the M. tuberculosis complex. The notification rate of human M. bovis and M. caprae cases among these 15 EU MS was 0.03 cases per 100,000 population. In the non‐OTF EU MS, the notification rate was 0.04 cases per 100,000 population.
Most cases, 69.4% (102/147), reported in 2019 were of EU origin (native cases and/or cases originating from other EU MS). The remaining cases originated from outside the EU (26.5%, n = 39), or had unknown origin (4.1%, n = 6) (Table 33). Cases were more likely to have originated from non‐OTF EU MS (66.7%, n = 68) than from OTF EU MS (33.3%, n = 34).
Treatment outcome after 12 months was reported for 90.1% (n = 164/181) of the human M. bovis and M. caprae cases reported in 2018. Among these cases, successful treatment was reported for 96 cases (58.5%), 22 cases (13.4%) died, five cases (3.0%) were still on treatment, one case (0.6%) was reported to have treatment failure and two cases (1.2%) were lost to follow‐up. The treatment outcome was not evaluated for 38 cases (23.2%).
Drug resistance to isoniazid and rifampicin among human M. bovis or M. caprae cases remained low in 2019; among 105 cases with test results reported for both isoniazid and rifampicin, only four were isoniazid‐resistant (3.8%). No multidrug‐resistant (resistance to rifampicin and isoniazid) cases were reported.
Figure 32 shows, for the year 2019, the number of confirmed tuberculosis cases due to M. bovis and to M. caprae in individuals of EU origin overlaid with the national aggregated herd prevalence of bovine tuberculosis.
Figure 32.
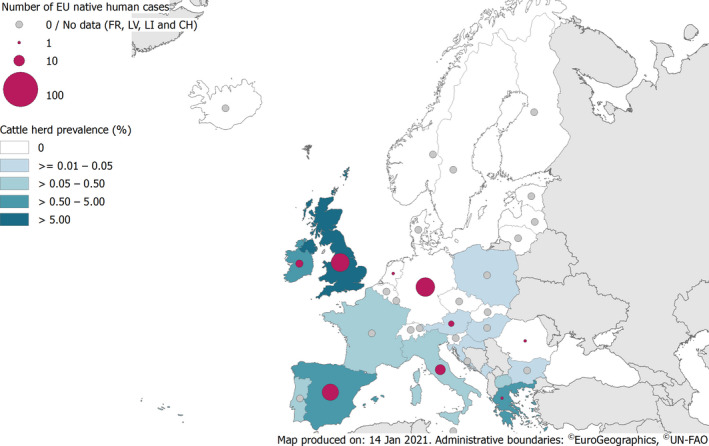
Number of confirmed tuberculosis cases due to M. bovis and to M. caprae in individuals of EU origin and national herd prevalence of bovine tuberculosis in cattle (ignoring OTF regions), EU/EFTA, 2019
- Data for EU/EEA human cases provided by ECDC.
Human tuberculosis cases associated with food‐borne outbreaks
No food‐borne disease outbreak due to Mycobacterium spp. was reported for 2019 in EU and no single such food‐borne outbreak has been reported to EFSA since the start of the food‐borne outbreaks reporting, in 2004.
5.3.3. Mycobacterium in food
No Mycobacterium monitoring data from food were submitted for the year 2019.
5.3.4. Bovine tuberculosis in animals
The country status on 31 December 2019 of freedom from bovine tuberculosis (OTF) is presented in Figure 33 and in Table 35. Seventeen MS were OTF during 2019. Of the 11 non‐OTF MS, four MS had OTF regions or provinces:
Italy: eight regions and 14 provinces;
Portugal: the Algarve region;
Spain: the province of Pontevedra and the Canary Islands;
the United Kingdom: Scotland and the Isle of Man.
Figure 33.
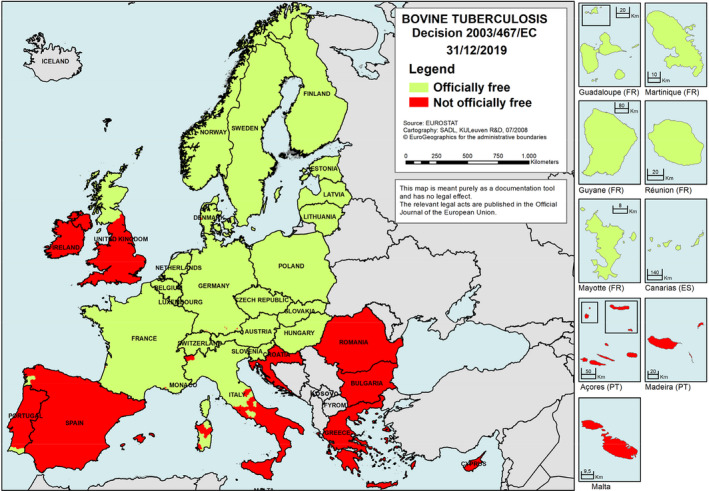
Status of countries on bovine tuberculosis, EU/EEA, 201912
Table 35.
Status of countries on bovine tuberculosis and related prevalence, EU, 2019
| Member state (MS) | OTF status | N (prevalence %) of infected herds in OTF regions | N (prevalence %) of positive herds in non‐OTF regions |
|---|---|---|---|
| Austria | OTF | 5 (0.009)a | –* |
| Belgium | OTF | 0 | – |
| Bulgaria | – | 7 (0.015) | |
| Croatia | – | 8 (0.038) | |
| Cyprus | – | 0 | |
| Czechia | OTF | 0 | – |
| Denmark | OTF | 0 | – |
| Estonia | OTF | 0 | – |
| Finland | OTF | 0 | – |
| France | OTF | 92 (0.055)b | – |
| Germany | OTF | 3 (0.002)c | – |
| Greece | – | 93 (0.517) | |
| Hungary | OTF | 4 (0.023)b | – |
| Ireland | – | 4,380 (3.946) | |
| Italy | 1 (0.002)b | 227 (0.455) | |
| Latvia | OTF | 0 | – |
| Lithuania | OTF | 0 | – |
| Luxembourg | OTF | 0 | – |
| Malta | – | 0 | |
| Netherlands | OTF | 0 | – |
| Poland | OTF | 26 (0.007)b | – |
| Portugal | 0 | 137 (0.383) | |
| Romania | – | 19 (0.004) | |
| Slovakia | OTF | 0 | – |
| Slovenia | OTF | 0 | – |
| Spain | 0 | 1,875 (1.712) | |
| Sweden | OTF | 0 | – |
| United Kingdom | 12 (0.094)b | 9,531 (11.287) | |
| EU Total | 143 (0.014) | 16,277 (1.803) |
Only M. caprae identified.
Only M. bovis identified.
One herd infected with M. bovis and two herds with M. caprae.
–: not applicable (no such regions).
OTF: Officially bovine tuberculosis free (status for freedom from bovine tuberculosis, in cattle).
 All regions of the MS are OTF.
All regions of the MS are OTF.
 Not all regions of the MS are OTF.
Not all regions of the MS are OTF.
 No region of the MS is OTF.
No region of the MS is OTF.
Seven non‐OTF MS had no OTF region: Bulgaria, Croatia, Cyprus, Greece, Ireland, Malta and Romania.
Norway and Switzerland were OTF, in accordance with EU legislation. Liechtenstein has the same status (OTF) as Switzerland. In Iceland, which has no special agreement on animal health status with the EU, the last outbreak of bovine tuberculosis was reported in 1959. Montenegro and the Republic of North Macedonia also reported data on bovine tuberculosis in their cattle.
During 2019, the overall EU proportion of cattle herds infected with, or positive for, bovine tuberculosis remained very low (0.8%, which was 16,420 out of 1,961,990 herds). Fourteen MS reported no case of bovine tuberculosis in cattle; Belgium, Cyprus, Czechia, Denmark, Estonia, Finland, Latvia, Lithuania, Luxembourg, the Netherlands, Malta, Slovakia, Slovenia and Sweden (Table 35). Bovine tuberculosis was reported by 14 MS and was heterogeneous and much spatially clustered with herd prevalence ranging from absence to 11.7% within the United Kingdom in England.
Officially bovine tuberculosis free (OTF) regions
In the OTF regions of the 21 MS with such regions, there were in total 1,059,412 cattle herds. Seven of these MS reported in total 143 (0.014% overall) bovine tuberculosis‐infected herds (Table 35), which is a rare event. Six MS reported infection with M. bovis (France, Germany, Hungary, Italy, Poland and UK). Austria reported herds infected with M. caprae. Additionally Austria and Germany reported herds infected with M. caprae. From 2010 to 2019, the overall annual number (prevalence) of cattle herds reported infected in the OTF regions decreased from 227 (0.016%) to 143 (0.013%), respectively (Figure 34). This was a proportional respective decrease by 37.0% and 14.5% in the annual number and prevalence of positive cattle herds, for that period 2010 to 2019. Concomitantly, the total number of cattle herds decreased by 26.4% from 1,439,899 in 2010 to 1,059,412 in 2019. When comparing 2019 with 2018 data, the annual number and prevalence of reported positive cattle herds proportionally decreased by 12.8% and 10.9%, respectively, whereas the total number of cattle herds decreased by 4.9%.
Figure 34.

Proportion of cattle herds infected with bovine tuberculosis in OTF regions, EU, 2010–2019
- OTF: Officially bovine tuberculosis free in cattle.
Non‐officially bovine tuberculosis free (non‐OTF) regions
During 2019, the 11 non‐OTF MS had in total 902,578 cattle herds in their non‐OTF regions. Nine of these MS reported in total 16,277 (1.803% overall) bovine tuberculosis‐positive herds (Table 35). Five of these non‐OTF MS (Ireland, Italy, Portugal, Spain and the United Kingdom) had their eradication programmes co‐financed by the EU. The number of positive herds out of all herds reported by these MS in non‐OTF regions was 4,380 (3.95%) in Ireland (5,573 in 2018), 227 (0.46%) in Italy (232 in 2018), 137 (0.38%) in Portugal (77 in 2018), 1,875 (1.71%) in Spain (2,384 in 2018) and 9,531 (11.29%) in the United Kingdom (10,359 in 2018). Reports concerned M. bovis except for Portugal and Spain reporting M. tuberculosis complex‐positive herds. Four of the six non‐co‐financed non‐OTF MS (Table 35) reported in total 127 bovine tuberculosis‐positive herds. Two of these MS reported infection with M. bovis (Bulgaria and Greece), whereas Romania reported herds infected with M. caprae and one herd infected with M. bovis. The fourth MS, Croatia, reported herds infected by M. tuberculosis complex and animals (slaughtered cattle) infected with M. bovis or M. caprae.
From 2010 to 2019, the overall annual number of reported positive cattle herds in the non‐OTF regions decreased from 17,814 to 16,277, respectively (Figure 35), whereas the prevalence increased from 1.0% to 1.8%. This was, respectively, a proportional decrease and increase by 8.6% and 72.1% of the annual number and prevalence of positive cattle herds, for that period 2010 to 2019. Concomitantly, the total number of cattle herds in those non‐OTF regions decreased by 44.9% from 1,638,694 in 2010 to 902,578 in 2019. When comparing 2019 with 2018 data, the annual number of positive cattle herds, the prevalence and the total number of cattle herds decreased by 14.2%, 11.8% and 4.4%, respectively.
Figure 35.

Proportion of cattle herds positive for bovine tuberculosis in non‐OTF regions, EU, 2010–2019
- OTF: Officially bovine tuberculosis free in cattle.
Figure 36 displays trends during 2004–2019 in the reported prevalence of bovine tuberculosis test‐positive cattle herds in non‐OTF regions of five non‐OTF co‐financed MS and of one non‐OTF not co‐financed MS, Greece. The United Kingdom has reported in recent years a decreasing annual prevalence of above 10% for Wales and for England, as well as for Northern Ireland. Greece, Ireland and Spain reported a low prevalence between 2 and 5%, during recent years. Italy and Portugal reported very low (< 1%) prevalence.
Figure 36.
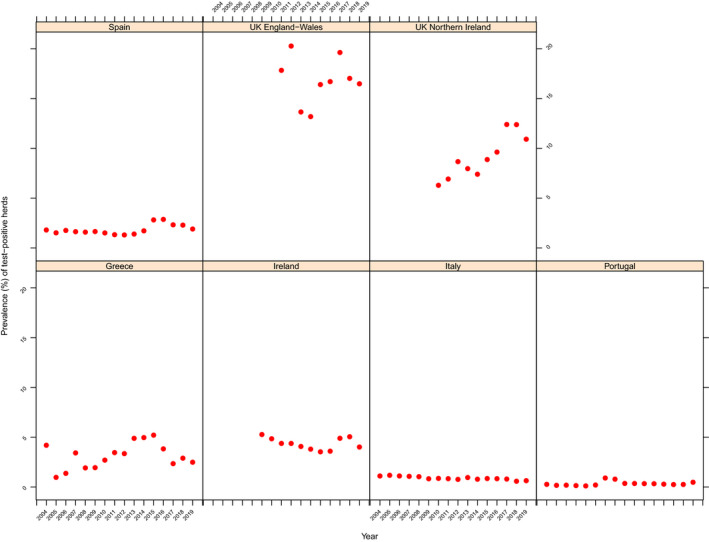
Prevalence of bovine tuberculosis test‐positive cattle herds in non‐OTF regions of five co‐financed non‐OTF MS and of one not co‐financed non‐OTF Member State Greece, 2004–2019
Non‐Member States and pre‐accession countries
Bovine tuberculosis was not detected in 2019 in the non‐MS Iceland, Norway, Switzerland and Liechtenstein. The Republic of North Macedonia and Montenegro, which are pre‐accession countries, submitted national monitoring data on bovine tuberculosis for the third and fourth consecutive year, respectively. The former reported 25 M. tuberculosis complex‐positive herds out of 17,201 (0.15%) compared with 58 (0.33%) in 2018, whereas Montenegro reported three M. bovis‐positive herds out of 22,983 (0.01%) compared with zero positives for the year 2018.
Complementary to 2019 reports from cattle, M. bovis was reported by countries in alpacas, badgers, cats, cattle, deer, dogs, foxes, goats, lamas, monkeys (a laboratory animal), pigs, sheep, wild boars and water buffalos. M. caprae was reported in cattle (reported by Croatia, slaughter animals) and farmed red deer.
5.4. Discussion
In the EU, tuberculosis due to M. bovis or M. caprae is rare in humans because of decades of disease control in cattle and routine pasteurisation of cow's milk. In 2019, human M. bovis and M. caprae cases represented only a small proportion (0.3%) of all notified human tuberculosis cases in the 26 EU MS that reported on the causative species. The notification rate of M. bovis and M. caprae in humans was slightly higher for the non‐OTF EU MS than in the OTF EU MS (0.04 vs. 0.03 per 100,000 population, respectively).
During 2019, the overall EU proportion of cattle herds infected with, or positive for, bovine tuberculosis was 0.8%. Bovine tuberculosis was reported by 14 MS and was heterogeneous and much spatially clustered with herd prevalence ranging from absence to 11.7% within the United Kingdom in England. This demonstrates that the situation in Europe on bovine tuberculosis infection, detection and control remained heterogeneous, as substantiated by EFSA (EFSA AHAW Panel, 2014).
Seventeen MS were OTF and in addition four non‐OTF MS had OTF regions. Twelve of these 21 MS reported no case of bovine tuberculosis in cattle. In these OTF regions, the detection during 2019 of bovine tuberculosis‐infected herds remained a rare event, as in the previous years. From 2010 to 2019, the overall annual number of infected cattle herds, the prevalence and the total number of cattle herds decreased.
All 11 non‐OTF MS except Cyprus and Malta detected bovine tuberculosis during 2019 in their non‐OTF regions and overall, about one in 50 herds were positive. When comparing 2019 with 2018 data, the overall annual number of positive cattle herds, the prevalence and the total number of cattle herds all decreased in these non‐OTF regions. When comparing 2010 to 2019 data, the overall annual number of reported positive cattle herds in these non‐OTF regions proportionally decreased by 8.6%, whereas the prevalence increased by 72.1%. Concomitantly, the total number of cattle herds in those non‐OTF regions was reduced to about half (decreased by 44.9%). This increase in prevalence can partly be explained by the increase in test‐positive cattle herds being detected in these regions along with an important decrease in the total number of cattle herds due to the gradual declaration of OTF status in regions within non‐OTF MS over this period. This overall increase can be further explained by specific trends in few non‐OTF MS during recent years. In the United Kingdom, M. bovis is widespread in England and Wales and in Northern Ireland and an epidemic in cattle has been ongoing for many years. A summary presentation on the situation can be found online.13 A major constraint to bovine tuberculosis eradication in cattle in those areas in which the infection is endemic in the Eurasian badger (Meles meles): this native wildlife species is a maintenance host of M. bovis. The challenge to successfully tackle bovine tuberculosis is also to address the reservoir of the disease in wildlife. Bovine tuberculosis remains one of the most serious and costly animal health problem for the UK cattle industry and taxpayer. Ireland also has for many years faced the challenge of containing the spread of bovine tuberculosis. It introduced a badger vaccination policy in 2018 and is also, among other control measures, reducing the badger population. A summary presentation on the situation in Ireland can be found online.14
Stagnating or increasing trends in the prevalence of bovine tuberculosis‐positive cattle herds demonstrate that control and eradication of this disease is a challenge, owing to the complex interactions between the pathogen, hosts and the local environments (EFSA AHAW Panel, 2014). MS‐specific evaluations of status, trends and of the relevance of bovine tuberculosis as a source of disease for humans can be found in the 2019 Annual National Zoonoses Country Reports referenced in Section 5.5.
In 2019, M. bovis was reported to be isolated – apart from bovine animals – from a wide range of animal species, both domestic and wild, reflecting that this causative agent of tuberculosis in cattle has a broad host range. M. caprae, recognised to cause bovine tuberculosis, was reported in cattle but also in farmed red deer.
5.5. Related projects and Internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definition of tuberculosis | https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions | |
| European Tuberculosis Surveillance Network | http://ecdc.europa.eu/en/healthtopics/Tuberculosis/european_tuberculosis_surveillance_network/Pages/index.aspx | |
| European Reference Laboratory Network for TB (ERLTB‐Net) | https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/erltb-net | |
| Food/Animals | European Union Reference Laboratory for Bovine Tuberculosis | https://www.visavet.es/bovinetuberculosis/ |
| Summary Presentations on the situation as regards bovine tuberculosis control and eradication programmes in MS; | https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en | |
| General information on EU Food Chain Funding | https://ec.europa.eu/food/funding_en | |
| General information on National Veterinary Programmes, in EU and Task Force on the eradication of animal diseases – bovine tuberculosis subgroup reports | https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en | |
| 2003/467/EC: Commission Decision of 23 June 2003 establishing the official tuberculosis, brucellosis and enzootic‐bovine‐leukosis‐free status of certain MS and regions of MS as regards bovine herds (text with EEA relevance) | https://eur-lex.europa.eu/eli/dec/2003/467/oj/eng | |
| Scientific Opinion of the EFSA Panel on Animal Health and Welfare (AHAW): Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): bovine tuberculosis | https://www.efsa.europa.eu/en/efsajournal/pub/4959 | |
| World Organisation for Animal Health, Summary of Information on Bovine Tuberculosis | http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/Disease_cards/BOVINE-TB-EN.pdf | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
6. Brucella
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
6.1. Key facts
In 2019, 310 confirmed brucellosis cases in humans were reported in the EU.
The EU notification rate was 0.06 cases per 100,000 population, which was the lowest notification rate reported since the beginning of the EU‐level surveillance.
There was a significantly declining EU/EEA trend in the number of confirmed brucellosis cases from 2015 to 2019.
Despite the declining trend, Greece reported the highest notification rate (0.61 cases per 100,000 population) of the domestic brucellosis cases in the EU followed by Portugal (0.32 cases per 100,000 population).
Most confirmed human cases (98 cases) were hospitalised and two deaths were reported in 2019.
One food‐borne brucellosis outbreak was reported for 2019 in EU, due to raw milk. During 2005–2018, there were 16 food‐borne outbreaks due to Brucella reported in EU, of which four were due to cheeses and 12 reported due to ‘unknown’ food.
Compared with 2018, the total number of Brucella‐positive or ‐infected cattle herds and sheep and goat flocks in the not officially free regions further decreased by 14% and by 27%, respectively.
In Croatia and Spain eradication of brucellosis in cattle, sheep and goats is within reach with almost no positive herds reported for these infections during recent years.
Brucellosis in cattle and in sheep and goats is still endemic in Italy, where the prevalence is highest in the southern region of Sicily, in Greece and in Portugal. In Italy and Portugal, the proportion of brucellosis‐positive cattle herds and sheep and goat flocks in not officially free regions decreased during recent years.
Greece reported the highest notification rate of confirmed cases in humans, 10 times higher than the EU average, while at the same time reporting an enzootic situation in animals: 2.8% infected cattle herds and 3.3% infected sheep and goats herds on the Greek islands whereas from Continental Greece data were lacking.
Brucellosis is still an animal health problem with public health relevance in southern Europe/in countries that are not officially free of brucellosis.
6.2. Surveillance and monitoring of Brucella in the EU
6.2.1. Humans
Notification of brucellosis in humans is mandatory in 26 MS, Iceland, Norway and Switzerland. In Denmark, no surveillance system is in place for brucellosis and the disease is not notifiable nor reported at the EU level. Belgium has another (not specified) system. The surveillance systems for brucellosis cover the whole population in all MS reporting cases. For 2019, Spain did not receive data from all regions and rates are therefore not displayed for this year. All countries reported case‐based data except Belgium and Bulgaria, which reported aggregated data. Both reporting formats were included to calculate numbers of cases, notification rates.
6.2.2. Food and animals
Brucella monitoring data from bovine animals and sheep and goats originating from the National Control and Eradication Programmes and/or Officially Free status
According to the Zoonoses Directive 2003/99/EC, MS must report bovine brucellosis and sheep and goat brucellosis annual monitoring data. These data originate from national control and surveillance programmes implemented by the MS in accordance with EU legislation. The reports submitted by the MS are based on Council Directive 64/432/EEC and subsequent legislation and are essential for the assessment of the epidemiological situation in MS and MS regions, whether declared officially brucellosis free in cattle (OBF) and/or officially B. melitensis free in sheep and goats (ObmF). Annual surveillance programmes are carried out in OBF regions to confirm freedom from bovine brucellosis and in ObmF regions freedom from B. melitensis in sheep and goats, whereas in all non‐OBF and non‐ObmF regions control and eradication programmes for brucellosis in cattle and in sheep and goats are in place. These data are comparable across MS because the monitoring schemes are harmonised, and the data collected and reported to EFSA originate from the census as sampling frame. In addition to trend analysis both at the EU level and at MS level and to trend watching and descriptive summaries, these data may also be used to assess the impact of control and eradication programmes (Table 1).
EU MS also need to notify outbreaks in terrestrial animals of bovine brucellosis and of caprine and ovine brucellosis (excluding Brucella ovis) in their OBF and/or ObmF regions to the EU ADNS12 and regular summaries are posted online.
Brucella monitoring data from food and animals other than bovine animals and sheep and goats
Brucella monitoring data from food and from animals other than bovine animals and sheep and goats, submitted to EFSA according to the Zoonoses Directive 2003/99/EC and collected without harmonised design allow for descriptive summaries at the EU level to be made. They preclude trend analyses and trend watching at the EU level (Table 1).
6.2.3. Food‐borne outbreaks of brucellosis
The reporting of food‐borne brucellosis outbreaks in humans is mandatory according to the Zoonoses Directive 2003/99/EC.
6.3. Results
6.3.1. Overview of key statistics, EU, 2015–2019
Table 36 summarises EU‐level statistics on human and animal brucellosis and on food investigated for Brucella, during 2015–2019. A more detailed description of these statistics is in the results section of this chapter and in the food‐borne outbreaks chapter.
Table 36.
Summary of Brucella statistics related to humans, major food categories and animal species, EU, 2015–2019
| 2019 | 2018 | 2017 | 2016 | 2015 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 310 | 358 | 378 | 530 | 437 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.06 | 0.08 | 0.09 | 0.11 | 0.09 | ECDC |
| Number of reporting MS | 27 | 26 | 26 | 27 | 27 | ECDC |
| Infection acquired in the EU | 126 | 133 | 148 | 180 | 176 | ECDC |
| Infection acquired outside the EU | 50 | 51 | 46 | 39 | 38 | ECDC |
| Unknown travel status or unknown country of infection | 134 | 174 | 184 | 311 | 223 | ECDC |
| Number of outbreak‐related cases | 2 | 0 | 2 | 0 | 2 | EFSA |
| Total number of outbreaks | 1 | 0 | 1 | 0 | 1 | EFSA |
| Food | ||||||
| Milk and milk products | ||||||
| Number of sampling units | 583 | 1,009 | 1,338 | 354 | 2,145 | EFSA |
| Number of reporting MS | 2 | 3 | 3 | 3 | 3 | EFSA |
| Animals | ||||||
| Bovine animals | ||||||
| Number of positive herds in OBF regions | 4 | 3 | 0 | 2 | 4 | EFSA |
| Number of reporting OBF MS | 20 | 20 | 20 | 19 | 19 | EFSA |
| Number of positive herds in non‐OBF regions | 485 | 563 | 648 | 808 | 938 | EFSA |
| Number of reporting non‐OBF MS | 8 | 8 | 8 | 9 | 9 | EFSA |
| Sheep and goats | ||||||
| Number of positive flocks in ObmF regions | 1 | 0 | 7 | 2 | 10 | EFSA |
| Number of reporting ObmF MS | 20 | 20 | 20 | 20 | 20 | EFSA |
| Number of positive flocks in non‐ObmF regions | 451 | 620 | 815 | 870 | 1,094 | EFSA |
| Number of reporting non‐ObmF MS | 8 | 8 | 8 | 8 | 8 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States; OBF/ObmF: Officially brucellosis free in cattle/Officially B. melitensis free in sheep and goats.
Reported food data of interest were categorised in the major category ‘milk and milk products’ and aggregated by year over the period 2015–2019 to obtain an overview, by year, of the amount of data sent. The numbers of sampling units reported, and the number of reporting MS are extremely low. The annual animal data statistics displayed in Table 36 include the numbers of OF MS and non‐OF MS and the number of flocks and herds remaining Brucella‐positive, during 2015–2019.
When the UK data were collected, the UK was an EU MS but as of 31 January 2020, it has become a third country.
6.3.2. Humans brucellosis
In 2019, 27 MS provided data and information on brucellosis in humans. In total, 319 cases were reported in the EU. These included 310 confirmed cases, which was a decrease by 13.4% compared with 2018. The notification rate was 0.06 cases per 100,000 population (Table 37), which represented 25% decrease compared with 2018. Nine MS (Bulgaria, Cyprus, Finland, Hungary, Ireland, Latvia, Lithuania, Luxembourg and Malta) and Iceland reported no human cases.
Table 37.
Reported human cases of brucellosis and notification rates per 100,000 population in the EU/EFTA, by country and year, 2015–2019
| Country | 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria (OBF/ObmF)b | Y | C | 6 | 6 | 0.07 | 7 | 0.08 | 6 | 0.07 | 4 | 0.05 | 1 | 0.01 |
| Belgium (OBF/ObmF) | Y | A | 3 | 3 | 0.03 | 9 | 0.08 | 8 | 0.07 | 4 | 0.04 | 9 | 0.08 |
| Bulgaria | Y | A | 0 | 0 | 0.00 | 1 | 0.01 | 2 | 0.03 | 0 | 0.00 | 36 | 0.50 |
| Croatia | Y | C | 3 | 3 | 0.07 | 3 | 0.07 | 1 | 0.00 | 2 | 0.05 | 0 | 0.00 |
| Cyprus (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.02 | 0 | 0.00 | 0 | 0.00 |
| Czechia (OBF/ObmF) | Y | C | 4 | 4 | 0.04 | 4 | 0.04 | 1 | 0.01 | 1 | 0.01 | 0 | 0.00 |
| Denmarkc (OBF/ObmF) | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia (OBF/ObmF) | Y | C | 1 | 1 | 0.08 | 1 | 0.08 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Finland (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 0.02 | 0 | 0.00 | 0 | 0.00 |
| Franced(OBF) | Y | C | 39 | 34 | 0.05 | 26 | 0.04 | 21 | 0.03 | 19 | 0.03 | 17 | 0.03 |
| Germany (OBF/ObmF) | Y | C | 37 | 37 | 0.04 | 37 | 0.04 | 41 | 0.05 | 36 | 0.04 | 44 | 0.05 |
| Greece | Y | C | 65 | 65 | 0.61 | 97 | 0.90 | 94 | 0.87 | 119 | 1.10 | 109 | 1.00 |
| Hungary (ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Ireland (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 2 | 0.04 | 2 | 0.04 | 0 | 0.00 |
| Italye | Y | C | 50 | 49 | 0.08 | 94 | 0.16 | 99 | 0.16 | 211 | 0.35 | 105 | 0.17 |
| Latvia (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Lithuania (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Luxembourg (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.17 | 0 | 0.00 |
| Malta (OBF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| The Netherlands (OBF/ObmF) | Y | C | 7 | 7 | 0.04 | 5 | 0.03 | 2 | 0.01 | 5 | 0.03 | 7 | 0.04 |
| Poland (OBF/ObmF) | Y | C | 2 | 2 | 0.01 | 0 | 0.00 | 2 | 0.01 | 3 | 0.01 | 4 | 0.01 |
| Portugalf | Y | C | 33 | 33 | 0.32 | 19 | 0.18 | 16 | 0.16 | 50 | 0.48 | 46 | 0.44 |
| Romania (OBF/ObmF) | Y | C | 1 | 1 | 0.01 | 1 | 0.01 | 3 | 0.02 | 1 | 0.01 | 0 | 0.00 |
| Slovakia (OBF/ObmF) | Y | C | 1 | 1 | 0.02 | 0 | 0.00 | 1 | 0.02 | 1 | 0.02 | 1 | 0.02 |
| Slovenia (OBF/ObmF) | Y | C | 6 | 6 | 0.29 | 3 | 0.15 | 1 | 0.05 | 1 | 0.05 | 0 | 0.00 |
| Spaing , k | Y | C | 23 | 20 | – | 40 | 0.09 | 63 | 0.14 | 37 | 0.08 | 33 | 0.07 |
| Sweden (OBF/ObmF) | Y | C | 14 | 14 | 0.14 | 11 | 0.11 | 14 | 0.14 | 19 | 0.19 | 13 | 0.13 |
| United Kingdomh (OBF/ObmF) | Y | C | 24 | 24 | 0.04 | – | – | – | – | 14 | 0.02 | 12 | 0.02 |
| EU Total | 319 | 310 | 0.06 | 358 | 0.08 | 378 | 0.09 | 530 | 0.11 | 437 | 0.09 | ||
| Icelandi | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway (OBF/ObmF) | Y | C | 4 | 4 | 0.08 | 3 | 0.06 | 3 | 0.01 | 4 | 0.08 | 2 | 0.04 |
| Switzerlandj (OBF/ObmF) | Y | C | – | 7 | 0.08 | 5 | 0.06 | 9 | 0.11 | 7 | 0.08 | 1 | 0.01 |
–: Data not reported.
Y: yes; N: no; A: aggregated data; C: case‐based data.
OBF/ObmF: Officially brucellosis free in cattle/Officially B. melitensis free in sheep and goats.
No surveillance system.
In France, all but one of the continental departments are ObmF.
In Italy, 11 regions and 9 provinces are OBF and 13 regions and 4 provinces are ObmF.
In Portugal, six islands of the Azores and the whole of the Algarve are OBF, whereas all nine Azores islands are ObmF.
In Spain, 15 autonomous communities and 4 provinces are OBF and 13 autonomous communities and 8 provinces are ObmF.
In the United Kingdom, England, Scotland, Wales, Northern Ireland and the Isle of Man are OBF.
In Iceland, which has no special agreement on animal health (status) with the EU, brucellosis (B. abortus, B. melitensis and B. suis) has never been reported.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
Data not complete in 2019, rate not calculated.
The highest notification rates of brucellosis were reported in two MS that were non‐OBF and/or non‐ObmF (Table 37): Greece and Portugal (0.61 and 0.32 cases per 100,000 population, respectively). The lowest notification rates were observed in OBF and ObmF MS where brucellosis cases were mainly travel‐associated. Slovenia and Sweden which have the OBF/ObmF status and had a relatively high notification rate (0.29 and 0.14 cases per 100,000 population, respectively) reported that the majority (≥ 75.0%) of confirmed brucellosis cases was travel associated. Most brucellosis cases (71.6%) with known data on travel were reported as being infected in the EU (Table 36). Among the 56 travel‐associated cases with known travel destination, 50 (89.3%) travelled outside EU. The most common travel destinations of the imported cases outside the EU were Iraq 12 cases (21.4%), Turkey 10 cases (17.9%), Bosnia and Herzegovina five cases (8.9%) and Egypt three cases (5.4%), respectively. In the EU, three cases reported travel to Spain and one case reported travel to Romania during the incubation period.
A clear seasonality was observed in the number of confirmed brucellosis cases in the EU/EEA with more cases reported from April to August. There was a significantly (p < 0.01) declining EU/EEA trend from 2015 to 2019 (Figure 37). Two MS (Greece and Italy) reported decreasing trend and two MS (Czechia and Slovenia) observed increasing trend (p < 0.01) from 2015 to 2019. A high increase in number of confirmed cases in 2016 at the EU level was mainly due to increase of reported cases in one MS (Italy).
Figure 37.
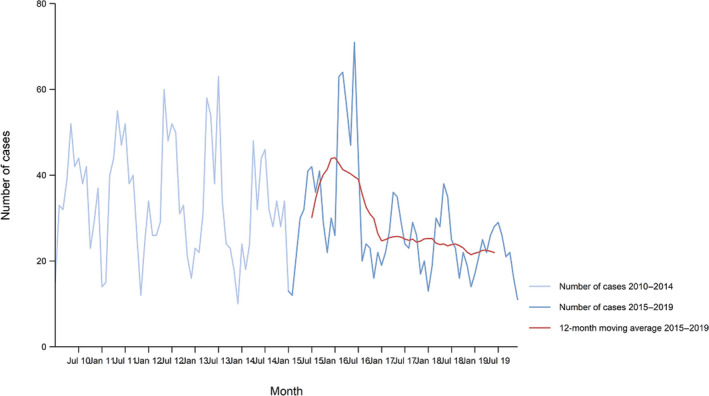
Trend in reported confirmed human cases of brucellosis in the EU/EEA, by month, 2015–2019
- Source: Austria, Cyprus, Czechia, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia and Sweden. Belgium, Bulgaria, Croatia, Luxembourg, Spain and the United Kingdom did not report data to the level of detail required for the analysis. Denmark does not have a surveillance system for this disease.
Eleven MS provided data on hospitalisation, accounting for 44.5% of confirmed cases in the EU. On average, 71.0% of the confirmed brucellosis cases with known status were hospitalised. In seven of the 11 countries reporting hospitalisation, the proportion of hospitalised cases ranged between 80% and 100%. Two deaths due to brucellosis were reported among 114 confirmed cases (36.8%) with outcome information by the 12 MS; one by the Netherlands and one by Spain in 2019.
Brucella species information was missing for 63.8% of the 310 confirmed cases reported in the EU. Of the 111 cases with known species, 105 (94.6%) were infected by B. melitensis, three (2.7%) by B. abortus and one (0.9%) by B. suis.
Figure 38 shows the number of domestically acquired (having not been outside the country of notification during the incubation period of the disease) confirmed brucellosis cases in humans overlaid with the national prevalence of Brucella‐positive cattle herds and sheep and goat flocks in EU/EFTA in 2019. The map shows that Greece, Portugal and Spain (human data not reported in all regions in 2019) have a higher number of domestically acquired confirmed brucellosis cases in humans and a higher prevalence of Brucella‐positive ruminant herds. Italy, which has reported a high number of human brucellosis cases over the years, did not report the origin of the infections in 2019.
Figure 38.

Number of domestically acquired confirmed brucellosis cases in humans and prevalence of Brucella test‐positive cattle herds and sheep and goat flocks, EU/EFTA, 2019
Human brucellosis cases associated with food‐borne outbreaks
Table 38 summarises reported brucellosis outbreaks data during 2005–2019, by MS and by incriminated food vehicle. Austria reported for the year 2019 one food‐borne outbreak due to Brucella melitensis in raw milk that was consumed in Turkey by the two affected persons.15
Table 38.
Distribution of food‐borne outbreaks caused by Brucella, by food vehicle, EU, 2005–2018
| Food vehicle | Year | Member State | Strength of evidence of outbreak * | N outbreaks | N human cases (illnesses) | N hospitalisations | N deaths |
|---|---|---|---|---|---|---|---|
| Cheese | 2008 | Greece (1), Spain (2) | Yes | 3 | 116 | 11 | 0 |
| Cheese | 2012 | France | Yes | 1 | 2 | 0 | 0 |
| Not available | 2012 | Greece | No | 4 | 14 | 11 | 0 |
| Not available | 2013 | Germany | No | 2 | 5 | 2 | 0 |
| Not available | 2013 | Greece | No | 2 | 5 | 5 | 0 |
| Unknown | 2014 | Germany | No | 2 | 7 | 5 | 1 |
| Unknown | 2015 | Germany | No | 1 | 2 | 1 | 0 |
| Unknown | 2017 | Germany | No | 1 | 2 | 1 | 0 |
| Raw milk | 2019 | Austria | Yes | 1 | 2 | 1 | 0 |
| Total | 17 | 155 | 37 | 1 |
‘Yes’ indicates reporting on a strong‐evidence food‐borne outbreak (= food‐borne outbreak when evidence implicating a particular food vehicle is strong); ‘No’ indicates reporting on a weak‐evidence food‐borne outbreak (= food‐borne outbreak when evidence implicating a particular food vehicle is weak or where no particular food vehicle was identified).
During 2005–2019, overall 17 brucellosis food‐borne outbreaks were reported, of which four with strong‐evidence were due to cheese, one with strong‐evidence due to raw milk and 12 with weak evidence due to ‘unknown’ food. Further details and statistics on the food‐borne outbreaks for 2019 are in the food‐borne outbreaks chapter.
6.3.3. Brucella in food
Very few 2019 Brucella monitoring data from food were submitted; in total from 586 milk and milk products sampling units, by Italy (78.8%, N = 462) and Portugal (21.2%, N = 124). In total, 15 Italian samples from a processing plant from ‘milk from other animal species or unspecified – pasteurised milk’ tested positive for Brucella spp. with reported species: B. abortus biovar 3, B. melitensis biovar 3 and Brucella unspecified spp.
6.3.4. Brucella in animals
Cattle
The country status on 31 December 2019 of freedom from bovine brucellosis (OBF) is presented in Figure 39 and in Table 39. Twenty MS were OBF in 2019. Of the eight non‐OBF MS, four had OBF regions:
in Italy: 11 regions and nine provinces;
in Portugal: the Algarve region and six of the nine islands of the Azores;
in Spain: 15 autonomous communities and four provinces
in the United Kingdom: England, Scotland, Wales, Northern Ireland and the Isle of Man (Channel Islands Jersey and Guernsey are non‐OBF).
Figure 39.
Status of countries on bovine brucellosis, EU/EEA, 201916
Table 39.
Status of countries on bovine brucellosis and related prevalence, EU, 2019
| Member State (MS) | Officially brucellosis free in cattle | N (prevalence %) of infected herds in OBF regions | N (prevalence %) of positive herds in non‐OBF regions |
|---|---|---|---|
| Austria | OBF | 1 (0.002)a | –* |
| Belgium | OBF | 0 | – |
| Bulgaria | – | 0 | |
| Croatia | – | 1 (0.005) | |
| Cyprus | OBF | 0 | – |
| Czechia | OBF | 0 | – |
| Denmark | OBF | 0 | – |
| Estonia | OBF | 0 | – |
| Finland | OBF | 0 | – |
| France | OBF | 0 | – |
| Germany | OBF | 0 | – |
| Greece | – | 85 (0.792) | |
| Hungary | – | 0 | |
| Ireland | OBF | 0 | – |
| Italy | 3 (0.005) | 361 (1.028) | |
| Latvia | OBF | 0 | – |
| Lithuania | OBF | 0 | – |
| Luxembourg | OBF | 0 | – |
| Malta | OBF | 0 | – |
| Netherlands | OBF | 0 | – |
| Poland | OBF | 0 | – |
| Portugal | 0 | 38 (0.113) | |
| Romania | OBF | 0 | – |
| Slovakia | OBF | 0 | – |
| Slovenia | OBF | 0 | – |
| Spain | 0 | 0 | |
| Sweden | OBF | 0 | – |
| United Kingdom b | 0 | 0 | |
| EU Total | 4 (< 0.001) | 485 (< 0.001) |
–: not applicable (no such regions).
B. melitensis
In the United Kingdom, England, Scotland, Wales, Northern Ireland and the Isle of Man are OBF.
OBF: Officially brucellosis free in cattle.
 All regions of the MS are OBF.
All regions of the MS are OBF.
 Not all regions of the MS are OBF.
Not all regions of the MS are OBF.
 No region of the MS is OBF.
No region of the MS is OBF.
Four non‐OBF MS had no OBF region: Bulgaria, Croatia, Greece and Hungary.
Norway, Switzerland and Liechtenstein were OBF in accordance with EU legislation. Iceland, which has no special agreement on animal health (status) with the EU, has never reported brucellosis due to B. abortus, B. melitensis or B. suis. Montenegro and the Republic of North Macedonia also reported data on brucellosis in their cattle.
During 2019, the overall EU proportion of cattle herds infected with, or positive for, bovine brucellosis remained a very rare event (0.025%, which was 489 out of 1,942,294 herds). Twenty‐three MS reported no case of brucellosis in cattle. Bovine brucellosis was reported by five MS: Austria, Croatia, Greece, Italy and Portugal (Table 39).
Regions officially brucellosis free in cattle (OBF)
In the OBF regions of the 24 MS with such regions, there were in total 1,650,343 cattle herds in 2019. Austria reported to have detected brucellosis due to B. melitensis in one cow in a herd in the context of a follow‐up investigation of an outbreak in 2018. Italy reported three positives herds. Bovine brucellosis was not detected in 2019 in the non‐MS: Iceland, Norway, Switzerland and Liechtenstein. During 2012–2019, there had been, respectively, nine, two, two, four, two, zero, three and four cattle herds reported infected in OBF regions in EU, meaning these were extremely rare events.
Regions non‐officially brucellosis free in cattle (non‐OBF)
During 2019, the eight non‐OBF MS had in total, 291,951 cattle herds in their non‐OBF regions and 485 (0.17%) were reported positive for brucellosis (Table 39). Three of these non‐OBF MS (Italy, Portugal and Spain) had their eradication programmes co‐financed by the EU. The number of positive herds out of all herds reported by these MS in non‐OBF regions was 361 in Italy (388 in 2018), 38 in Portugal (49 in 2018) and 0 in Spain (3 in 2018). Of the five non‐co‐financed non‐OBF MS, only Greece and Croatia reported positive herds, respectively, 85 (122 in 2018) and 1 (1 in 2018), respectively, whereas Bulgaria, Hungary and the United Kingdom did not report positive herds in 2019. No speciation of Brucella was reported.
In conclusion, in 2019, bovine brucellosis was mainly still present in a few MS, Greece, Italy and Portugal, in southern Europe. Sicily, in Italy, reported the highest regional prevalence in EU non‐OBF regions, with 2.3% positive herds.
From 2012 to 2019, the overall annual number of reported positive cattle herds in the non‐OBF regions decreased by 58.9% from 1,181 to 485, whereas the prevalence increased by 63.6% from 0.10% to 0.17% (Figure 40). The latter is due to the drastic decrease in the total number of cattle herds from 1,162,978 to 291,951 during the same period, i.e. a decrease of 74.9%.
Figure 40.
Proportion of Brucella‐positive cattle herds, in non‐OBF regions, EU, 2012–2019
- Non‐OBF: Non‐officially brucellosis free in cattle.
When comparing 2019 with 2018 data, the annual number of positive cattle herds, the prevalence and the total number of cattle herds decreased by 13.9%, 7.8% and 6.5%, respectively.
Figure 41 displays trends during 2004–2019 in the reported prevalence of brucellosis test‐positive cattle herds in non‐OBF regions of three non‐OBF co‐financed MS (Italy, Spain and Portugal) and of one non‐OBF not co‐financed MS, Greece. The prevalence in Greece showed a huge variation across the years from a minimum 2% in 2008 to a maximum 12% in 2012. The trend in prevalence in Italy is decreasing and was 1.3% for the year 2019. Portugal showed a prevalence consistently decreasing from about 2% to 0.14% for the year 2019. Spain reported for the last 4 years 2016, 2017, 2018 and 2019, respectively, 26, 21, 3 and zero positive herds, meaning that in the coming years, eradication of bovine brucellosis in Spain is within reach. This is also the case for Croatia that reported for the same years, respectively, 0, 0, 1 and 1 positive herds.
Figure 41.
Prevalence of Brucella test‐positive cattle herds, in non‐OBF regions of three co‐financed non‐OBF MS (Italy, Portugal and Spain) and of one not co‐financed non‐OBF MS Greece, 2004–2019
Non‐Member States and pre‐accession countries
Bovine brucellosis was not detected in 2019 in the non‐MS Iceland, Norway, Switzerland and Liechtenstein. The Republic of North Macedonia and Montenegro, which are pre‐accession countries, submitted national monitoring data on bovine brucellosis for the fourth consecutive year. The former reported 30 positives out of 17,201 herds (0.17%) compared with 55 (0.28%) in 2018, whereas Montenegro did not report any positive herd in the last 4 years, out of 22,983 cattle herds present in 2019 the country.
Sheep and goats
The country status on 31 December 2019 of freedom from ovine and caprine brucellosis by B. melitensis (ObmF) is presented in Figure 42 and in Table 40. Twenty MS were ObmF in 2019. Of the eight non‐OBF MS, four had ObmF regions:
in France: all but one of the continental departments in France (due to Rev.1 vaccination against Brucella ovis) are ObmF and no cases of brucellosis have been reported in small ruminants since 2003;
in Italy: 13 regions and four provinces;
in Portugal: the Azores region (all nine islands);
in Spain: 13 autonomous communities and eight provinces.
Figure 42.

Status of countries on ovine and caprine brucellosis, EU/EEA, 201917
Table 40.
Status of countries on ovine and caprine brucellosis and related prevalence, EU, 2019
| Member State (MS) | Officially brucellosis free in sheep and goats | N infected herds in ObmF regions | N (prevalence %) of positive herds in non‐ObmF regions |
|---|---|---|---|
| Austria | ObmF | 0 | –* |
| Belgium | ObmF | 0 | – |
| Bulgaria | – | 0 | |
| Croatia | – | 4 (0.018%) | |
| Cyprus | ObmF | 0 | – |
| Czechia | ObmF | 0 | – |
| Denmark | ObmF | 0 | – |
| Estonia | ObmF | 0 | – |
| Finland | ObmF | 0 | – |
| France | 0 | 0 | |
| Germany | ObmF | 0 | – |
| Greece | ‐ | 37 (0.167%) | |
| Hungary | ObmF | 0 | – |
| Ireland | ObmF | 0 | – |
| Italy | 1 (0.001%) | 206 (0.570%) | |
| Latvia | ObmF | 0 | – |
| Lithuania | ObmF | 0 | – |
| Luxembourg | ObmF | 0 | – |
| Malta | – | 0 | |
| Netherlands | ObmF | 0 | – |
| Poland | ObmF | 0 | – |
| Portugal | 0 | 203 (0.376%) | |
| Romania | ObmF | 0 | – |
| Slovakia | ObmF | 0 | – |
| Slovenia | ObmF | 0 | – |
| Spain | 0 | 1 (0.004%) | |
| Sweden | ObmF | 0 | – |
| United Kingdom | ObmF | 0 | – |
| EU Total | 1 (< 0.001) | 451 (0.210) |
–: Not applicable (no such regions).
>ObmF: Officially B. melitensis free in sheep and goats.
 All regions of the MS are OBF.
All regions of the MS are OBF.
 Not all regions of the MS are OBF.
Not all regions of the MS are OBF.
 No region of the MS is OBF.
No region of the MS is OBF.
Four non‐ObmF MS had no ObmF region: Bulgaria, Croatia, Greece and Malta.
Norway, Switzerland and Liechtenstein were ObmF in accordance with EU legislation. Iceland, which has no special agreement on animal health (status) with the EU, has never reported brucellosis due to B. abortus, B. melitensis or B. suis. Montenegro and the Republic of North Macedonia also reported data on brucellosis in their sheep and goat flocks.
During 2019, the overall EU proportion of sheep and goat flocks infected with or positive for B. melitensis remained a very rare event (0.04%, which was 452 out of 1,156,099 herds). Twenty‐three MS reported no case of B. melitensis brucellosis in sheep and goat flocks. B. melitensis cases in sheep and goats herds were reported by five MS: Croatia, Greece, Italy, Portugal and Spain (Table 40).
Regions officially B. melitensis free in sheep and goats (ObmF)
In the ObmF regions of the 24 MS with such regions, there were in total 941,317 sheep and goat flocks in 2019 and one case of brucellosis was reported in these herds during 2019, by Italy. B. melitensis was not reported in 2019 by the non‐MS: Iceland, Norway, Switzerland and Liechtenstein. During 2012–2019, there has been, respectively, 5, 4, 3, 10, 2, 7, 0 and 1 sheep and goat flocks reported infected in ObmF regions, meaning it was an extremely rare event.
Regions non‐officially B. melitensis free in sheep and goats (Non‐ObmF)
During 2019, the eight non‐ObmF MS had, in total, 214,782 sheep and goat flocks in their non‐ObmF regions and 451 (0.210%) were reported brucellosis‐positive (Table 40). Five of these non‐ObmF MS (Croatia, Greece, Italy, Portugal and Spain) had their eradication programmes co‐financed by the EU. The number of positive flocks/herds reported by these MS was: four in Croatia (nine in 2018), Greece 37 (36 in 2018), 206 in Italy (311 in 2018), 203 in Portugal (260 in 2018) and one in Spain (three in 2018). All three non‐co‐financed non‐ObmF MS (Bulgaria, France and Malta) reported zero positive cases in 2019.
In conclusion, in 2019, B. melitensis brucellosis in sheep and goat flocks was mainly still present in a few MS, Greece, Italy and Portugal, in southern Europe. Sicily, in Italy, reported the highest regional prevalence in EU non‐OBF regions, with 1.6% of positive herds.
From 2012 to 2019, the overall annual number of reported positive sheep and goat flocks in the non‐ObmF regions decreased by 73.4% from 1,693 to 451, whereas the prevalence decreased by 53.2% from 0.45% to 0.21% (Figure 43). The total number of sheep and goat flocks decreased by 43.1% from 377,690 to 214,782 during the same period.
Figure 43.
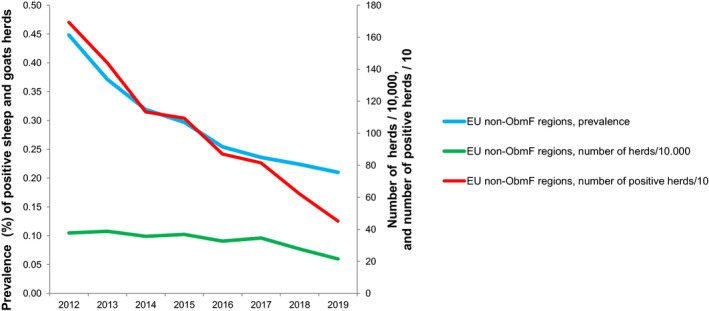
Proportion of brucellosis‐positive sheep flocks and goat flocks, in non‐ObmF regions, EU, 2012–2019
- Non‐ObmF: Non‐officially B. melitensis free in sheep and goats.
When comparing 2019 with 2018 data, the annual number of brucellosis‐positive sheep and goat flocks, the prevalence and the total number of herds, respectively, decreased by 27.3%, 6.3% and 22.4%.
Figure 44 displays trends during 2004–2019 in the reported prevalence of brucellosis test‐positive sheep and goat flocks in non‐ObmF regions of five non‐ObmF co‐financed MS. It is of note that, in 2016 and 2017 and 2019, only vaccination was co‐financed in Greece. Also, for Greece, the monitoring data reported on brucellosis in sheep and goats are exclusively from the eradication programme that runs in the Greek islands. The prevalence in Greece showed a huge variation across years from a minimum 0.4% in 2015 to a maximum of 8.6% in 2012.
Figure 44.

Prevalence of brucellosis test‐positive sheep and goat flocks, in non‐ObmF regions of five co‐financed non‐ObmF MS, 2004–2019
Italy and Portugal reported a low (> 1–10%) to very low (0.1–1%) prevalence during this period, decreasing for both MS. Croatia and Spain reported a very low prevalence (0.1–1%) to a rare detection (< 0.1%) and both decreasing. Croatia and Spain reported for the last 4 years 2016, 2017, 2018 and 2019, respectively, 8, 5, 9, 4 and 49, 18, 3, 1 B. melitensis‐positive herds, meaning that in the coming years eradication of sheep and goats brucellosis in Croatia and in Spain is within reach.
Non‐Member States ad pre‐accession countries
Brucellosis was not detected in sheep and goat flocks in 2019 in the non‐MS Iceland, Norway, Switzerland and Liechtenstein. The Republic of North Macedonia and Montenegro, which are pre‐accession countries, submitted national monitoring data on ovine and caprine brucellosis for the fourth consecutive year. The former reported 198 positives out of 6,696 herds (2.9%) compared with 112 (1.5%) in 2018, whereas Montenegro did not report any positive flock or herd in the last 3 years, out of 6,112 sheep flocks and goat flocks present in 2019 in the country.
Complementary to 2019 reports from cattle and from sheep and goats, Brucella species were reported from a wide range of animal species: Brucella unspecified species from ‘farmed animals’, dogs, pigs, rabbits and wild boars; B. suis from pigs and wild boars and notably biovar 2 from wild deer, wild hares, breeding pigs, pigs from mixed herds not raised under controlled housing conditions and wild boars; B. canis from dogs (pet) and B. pinnipedialis from wild seals.
6.4. Discussion
Brucellosis is a rare disease in the EU, although severe with most of the diagnosed human cases hospitalised. In 2019, the number of reported confirmed cases of brucellosis in humans and the EU notification rate was at the lowest level since the beginning of EU‐level surveillance in 2007. During 2019, the highest notification rates and most of the domestic brucellosis cases were reported from two MS, Greece and Portugal, that are not officially brucellosis free in cattle, sheep or goats. These two countries accounted for 32% of all confirmed brucellosis cases in the EU and consistently reported the highest notification rates within the EU despite the declining trends in Greece since 2014 and in Portugal since 2009. Greece continued reporting a notification rate over 10 times higher and Portugal over five times higher than the EU average. An outbreak of B. melitensis from home‐made fresh goat cheese, sold outside of the commercial circuit, was reported in the northern region of Portugal in 2018–2019 by Mendes et al. (2020). In Italy, a general decrease of cases has been notified in all regions in the last 20 years and its notification rate was for the first time in 2019 similar to the EU average. Brucellosis remains, however, an important health problem particularly in southern part of Italy, reporting 89% of the annual cases (Facciolà et al., 2018). Greece, Italy and Portugal were the southern European MS where bovine brucellosis and B. melitensis brucellosis in sheep and goat flocks were still present in 2019, with Sicily, in Italy, reporting the highest regional prevalence in bovine animals, and in sheep and goats. These findings underline that brucellosis is still an animal health problem with public health relevance in these southern European MS.
Bovine brucellosis and ovine and caprine brucellosis have been eradicated by most EU MS. In MS and regions officially free of brucellosis, no infected herds were reported for the year 2019, except for one B. melitensis–infected cattle herd in Austria and four positive herds in Italy (three in cattle and one in small ruminants). Reported food‐borne disease outbreaks due to Brucella have become rare in the EU. For the year 2019 one single food‐borne outbreak due to B. melitensis was reported by Austria, due to unpasteurised milk consumed in Turkey.16 As regards autochthonous human food‐borne illnesses in MS that are officially free of brucellosis, the question is raised as to the origin of these infections. Food‐borne exposure is normally limited to people consuming unpasteurised milk or dairy products from countries where brucellosis in animals is endemic. A recent study published by Jansen et al. (2019) based on samples from 2011 in Germany found Brucella‐positive raw milk cheeses were available at German retail level, so putting consumers at risk without travel history to endemic countries. The authors hypothesised that, in Germany, which is officially free of Brucella in cattle, sheep and goat populations, there are uncontrolled imports of cheese (from endemic regions) that do not comply with food safety standards. The above outbreak in northern Portugal was a further episode adding to the concern of illegal trade of raw milk cheese and challenging food safety standards in EU. As a result of the eradication of animal brucellosis in most EU MS, human brucellosis has become quite rare in northern and western Europe, where most cases are associated with travel outside EU. In some northern European countries (Germany, France, Sweden and Norway) an increased disease incidence may occur in recently arrived migrants (Garofolo et al., 2016; Mailles et al., 2016; Norman et al., 2016; Georgi et al., 2017; Johansen et al., 2018), a large part arriving from endemic countries (Africa, Middle East and Mediterranean countries). In France, a case report described the first case of brucellosis caused by an isolate whose genome is identical that of a frog isolate from Texas, demonstrating the zoonotic potential of amphibian‐type Brucella inopinata (Rouzic et al., 2020). This patient hospitalised with an altered general status, dyspnoea, night fever presented mediastinal lymphadenopathies, pulmonary condensations, emphysematous lesions and splenomegaly. Importantly, with such atypical Brucella, correct diagnosis cannot be performed using routine serological tests or identification methods.
Some MS were not officially free of bovine brucellosis and/or brucellosis in sheep and goats, and both infections were still mainly present in 2019 in Greece, Italy and Portugal. The highest regional prevalence for both infections was reported for Sicily, in southern Italy, representing an ongoing public health threat as evidenced by the fact that 89% of human cases in Italy are reported in Sicily (Facciolà et al., 2018). Greece and Portugal also reported the highest rates of confirmed human cases in 2019, respectively, 10 and 5 times higher than the EU average. At the same time, 2.8% cattle herds and 3.3% sheep and goat flocks were test‐positive on the Greek islands, being from mostly unvaccinated herds. From mainland Greece, where vaccination programmes are run against both brucellosis in cattle (in mountainous areas) and sheep and goats (on the mainland and some bigger islands), no animal test data were reported. Non‐food‐borne transmission of brucellosis to humans also occurs, through direct contact with infected animals. People working with farm animals, including farmers, livestock breeders, butchers, abattoir workers and veterinarians, are known to be at increased risk of brucellosis in the endemic countries. The largest proportion of the human cases in EU MS occurred in working‐age men, possibly indicating occupational exposure (ECDC, 2019). This finding is in agreement with a recent study in Greece by Fouskis et al. (2018), in which male patients were found to be related to high‐risk jobs and animal contact, while brucellosis in women was related to recent consumption of dairy products.
As compared with 2018, overall in the EU regions not officially free from bovine brucellosis the number of positive herds and the prevalence of bovine brucellosis decreased, respectively, by 14% and 8% in 2019, whereas in the regions not officially free from brucellosis in sheep and goats those proportions also decreased, respectively, by 27% and 6%. In Italy and Portugal, the prevalence of bovine, ovine and caprine brucellosis in not officially free regions has decreased in recent years. Croatia and Spain reported almost no positive herds during the last two years for these infections, meaning that in the coming years eradication of cattle and sheep and goat brucellosis is within reach. It is of note that compared with Spain, the situation is different for Croatia. In Croatia, cases in humans have been sporadic and low in prevalence and emerged only in animals and humans living close to the border of Bosnia and Herzegovina. In Bosnia and Herzegovina, the disease is enzootic.18 These findings support the assumption that the illegal import of animals is the main source of brucellosis in the country (Duvnjak et al., 2018). The most recent data on the incidence of brucellosis in humans in south‐east Europe (Balkan countries) proved the persistence of brucellosis in the area. Bulgaria reported re‐emergence of human brucellosis to the country, most probably related to import of infection from endemic areas in the near neighbouring countries, Greece and Macedonia (Karcheva et al., 2017).
In food, very few monitoring data were reported during these last years by the non‐OBF/ObmF MS Italy and Portugal. Italy reported, for 2019, positive findings in pasteurised milk ‘from other animal species or unspecified’ at processing plants. Greece did not submit food monitoring results for Brucella.
6.5. Related projects and Internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definition of brucellosis | https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions | |
| Disease Programme on Emerging, Food‐ and Vector‐Borne Diseases | https://www.ecdc.europa.eu/en/about-us/who-we-are/units/disease-programmes-unit | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| World Health Organization – brucellosis fact sheet | https://www.who.int/news-room/fact-sheets/detail/brucellosis | |
| Animals | EURL for Brucella | https://eurl-brucellosis.anses.fr/ |
| Summary Presentations on the situation as regards bovine brucellosis and brucellosis in sheep and goats’ control and eradication programmes in MS | https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en | |
| General information on EU Food Chain Funding | https://ec.europa.eu/food/funding_en | |
| 2003/467/EC: Commission Decision of 23 June 2003 establishing the official tuberculosis, brucellosis and enzootic‐bovine‐leukosis‐free status of certain MS and regions of MS as regards bovine herds | https://eur-lex.europa.eu/eli/dec/2003/467/oj/eng | |
| 93/52/EEC: Commission Decision of 21 December 1992 recording the compliance by certain MS or regions with the requirements for brucellosis (B. melitensis) and according them the status of a Member State or region officially free of the disease | https://eur-lex.europa.eu/eli/dec/1993/52/oj/eng | |
| General information on National Veterinary Programmes, in EU and Task Force on the eradication of animal diseases – Brucellosis subgroup reports | https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en | |
| EU approved and co‐financed veterinary programmes for bovine brucellosis and brucellosis in sheep and goats carried out by the MS | http://ec.europa.eu/dgs/health_food-safety/funding/cff/animal_health/vet_progs_en.htm | |
| World Organisation for Animal health, Summary of Information on Brucellosis | https://www.oie.int/en/animal-health-in-the-world/animal-diseases/Brucellosis/ | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
7. Trichinella
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
7.1. Key facts
In 2019, 96 confirmed cases of trichinellosis in humans were reported in the EU.
The EU notification rate increased to 0.02 cases per 100,000 population, compared with 2018 (0.01) but was generally low. Increase was mainly due to the increased number of confirmed cases in three MS (Bulgaria, Italy and Spain).
Bulgaria reported the highest EU notification rate (0.79 cases per 100,000 population).
Despite the increase in 2019, the trend in number of confirmed cases of trichinellosis in EU/EEA decreased significantly in the period 2015–2019.
The number of reported food‐borne trichinellosis outbreaks was 5, compared with 10 in 2018, with 44 illnesses, 12 hospitalised people and no deaths. Most outbreaks were caused by pig meat and products thereof, as during previous years.
Trichinella spiralis was the only species that was reported from confirmed human cases to TESSy. Species reported to EFSA from food were T. spiralis from pig meat and products thereof in one food‐borne outbreak in Croatia and one in Romania and T. britovi from other or mixed red meat and products thereof in one food‐borne outbreak in Italy.
In 2019, no infection with Trichinella was reported in tested fattening pigs (72.8 million) and breeding pigs (0.76 million) kept under controlled housing conditions, confirming that the farming conditions are the key factor to prevent infection with this zoonosis.
In pigs not kept under controlled housing conditions, 0.0016% (218 out of 139.6 million) fattening pigs and 0.00001% (1 out of 5.6 million) breeding pigs tested positive for Trichinella. Spain accounted for most of these positive pigs followed by Romania, Poland, Croatia, Bulgaria and France. As during 2014–2018, these Trichinella infections were from free‐range and backyard pigs reared in rural EU regions.
No Trichinella infection was observed in domestic solipeds in the EU in 2019, as during 2015–2018.
In total, 1,368 (0.08%) hunted wild boars tested positive. During 2015–2019, the reported EU prevalence of Trichinella‐positive wild boars fluctuated from one year to another, not exceeding 0.09%.
In 2019, the proportion of Trichinella‐positive red foxes (indicator animals) was 1.3%. During 2015–2019, the reported overall proportion fluctuated from one year to another, not exceeding 1.6%.
7.2. Surveillance and monitoring of Trichinella in the EU
7.2.1. Humans
The notification of Trichinella infections in humans is mandatory in all MS, Iceland, Norway and Switzerland, except in Belgium, France and the United Kingdom where surveillance systems are voluntary. No surveillance system for trichinellosis exists in Denmark. The surveillance systems for trichinellosis cover the whole population in all MS except in Belgium. All countries reported case‐based data except Belgium, Bulgaria and the Netherlands, which reported aggregated data. Both reporting formats were included to calculate numbers of cases and notification rates. For 2019, Belgium did not report data and Spain did not receive data from all regions due to COVID‐19. Rates are therefore not displayed for Spain for 2019.
In humans, diagnosis of Trichinella infections is primarily based on clinical signs and symptoms and serology (indirect enzyme‐linked immunosorbent assay (i‐ELISA) and western blot). Histopathology on muscle biopsies is very rarely performed.
7.2.2. Animals
Trichinella monitoring data from domestic pigs (both fattening and breeding animals), farmed wild boar and solipeds
According to the Commission Implementing Regulation (EU) 2015/137519, all Trichinella‐susceptible animals intended for human consumption in the EU, i.e. domestic pigs (both fattening and breeding animals), farmed wild boar and solipeds, should be tested for the presence of Trichinella larvae in the muscles unless carcases have undergone a freezing treatment (freezing inactivates the parasite). It follows that data on Trichinella infections in these animals are comparable across MS because the monitoring schemes are harmonised and the data collected and reported to EFSA originate from census sampling (Table 41). Domestic pigs, farmed and hunted wild boar and other wild animals (e.g. bears) that are not processed to be placed on the EU market (e.g. intended for own consumption) are exempted from the Commission Implementing Regulation (EU) 2015/1375 and their control falls under the national legislation. Commission Implementing Regulation (EU) 2015/1375 states that reporting of data for domestic pigs shall, at least, provide specific information related to the number of animals tested that were raised under controlled housing conditions as well as the number of breeding sows, boars and fattening pigs tested. Further, the regulation states that a negligible risk status for a country or region is no longer recognised.
Table 41.
Summary of Trichinella statistics related to humans and most important animal species, EU, 2015–2019
| 2019 | 2018 | 2017 | 2016 | 2015 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 96 | 66 | 168 | 101 | 155 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.02 | 0.01 | 0.03 | 0.02 | 0.03 | ECDC |
| Number of reporting MS | 26 | 27 | 27 | 27 | 27 | ECDC |
| Infections acquired in the EU | 26 | 18 | 81 | 53 | 126 | ECDC |
| Infections acquired outside the EU | 2 | 1 | 2 | 1 | 0 | ECDC |
| Unknown travel status or unknown country of infection | 68 | 47 | 85 | 47 | 29 | ECDC |
| Number of outbreak‐related cases | 44 | 114 | 199 | 27 | 123 | EFSA |
| Total number of outbreaks | 5 | 10 | 11 | 7 | 17 | EFSA |
| Animals | ||||||
| Domestic pigs RCHC a: | ||||||
| Number of unitsb tested | 73,633,900 | 77,794,786 | 72,227,074 | 62,594,969 | 55,329,437 | EFSA |
| % (N) positive units | 0 | 0 | 0 | < 0.0001 (31)c | 0 | EFSA |
| Number of reporting MS | 16 | 15 | 14 | 16 | 14 | EFSA |
| Domestic pigs NRCHC d: | ||||||
| Number of units tested | 145,176,068 | 152,922,322 | 124,689,434 | 124,496,074 | 53,136,580 | EFSA |
| % (N) positive units | 0.00015 (219) | 0.0003 (384) | 0.0002 (224) | 0.0002 (271) | 0.0003 (176) | EFSA |
| Number of reporting MS | 25 | 25 | 25 | 24 | 16 | EFSA |
| Farmed wild boar: | ||||||
| Number of units tested | 7,570 | 6,343 | 17,799 | 31,039 | 32,360 | EFSA |
| % (N) positive units | 0 | 0 | 0.7 (132) | 0.3 (90) | 0 | EFSA |
| Number of reporting MS | 7 | 7 | 8 | 8 | 9 | EFSA |
| Hunted wild boar: | ||||||
| Number of units tested | 1,757,383 | 1,465,788 | 1,389,905 | 1,400,393 | 875,539 | EFSA |
| % (N) positive units | 0.08 (1,368) | 0.09 (1,306) | 0.09 (1,228) | 0.05 (658) | 0.07 (600) | EFSA |
| Number of reporting MS | 23 | 23 | 22 | 20 | 20 | EFSA |
| Red foxes: | ||||||
| Number of animals tested | 6,696 | 6,612 | 6,486 | 7,785 | 7,902 | EFSA |
| % (N) positive units | 1.3 (89) | 1.6 (102) | 1.2 (79) | 0.9 (73) | 1.6 (130) | EFSA |
| Number of reporting MS | 10 | 10 | 11 | 12 | 11 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member State.
RCHC: raised under controlled housing conditions.
Units: animals and/or slaughter animal batches.
Romania reported 31 Trichinella‐positive fattening pigs from farms raised under controlled housing conditions, however these farms were not officially recognised in accordance with Article 8, Regulation (EU) 2015/1375, Annex IV, Chapter I.
NRCHC: not raised under controlled housing conditions.
Trichinella monitoring data from animals other than domestic pigs, farmed wild boar and solipeds
MS should monitor the circulation of these nematodes in the main natural reservoir hosts (carnivore and omnivore animals) to acquire information on the risk of transmission to domestic animals (and from these to humans) and on the introduction of new Trichinella species from non‐EU countries. However, monitoring data provided by the MS to EFSA are generated by non‐harmonised monitoring schemes across MS without mandatory reporting requirements. Wild animals are the main reservoir hosts of Trichinella, and their biology and ecology vary from one MS to another and from one region or habitat in the same MS to another due to the human and environmental impact on the ecosystems, resulting in different transmission patterns and prevalence of infection. Therefore, data from Trichinella in wild animals are not fully comparable between MS and the reported findings must be interpreted with caution. These data allow descriptive summaries at the EU level but preclude subsequent data analysis such as assessing temporal and spatial trends (Table 1).
7.2.3. Food‐borne outbreaks of trichinellosis
The reporting of food‐borne trichinellosis disease outbreaks in humans is mandatory according to Zoonoses Directive 2003/99/EC.
7.3. Results
7.3.1. Overview of key statistics along the food chain, EU, 2015–2019
Table 41 summarises EU‐level statistics on human trichinellosis and on Trichinella in animals, during 2015–2019. Animal data of interest reported were classified into categories and aggregated by year to obtain an annual overview of the volume of data submitted.
More detailed descriptions of these statistics are in the results section of this chapter and in the chapter on food‐borne outbreaks.
When the UK data were collected, the UK was an EU MS but as of 31 January 2020, it has become a third country.
7.3.2. Trichinellosis in humans
In 2019, 140 cases of trichinellosis, including 96 confirmed cases, were reported by 26 MS (Table 42). There was about 50% increase in case numbers and the EU notification rate doubled from 0.01 cases per 100,000 population in 2018 to 0.02 cases per 100,000 population in 2019. Despite the increased number of cases in 2019 compared with 2018, the number of cases was below the 5‐year average (117 cases). The increase was mainly due to the increased number of confirmed cases in three MS; Bulgaria (+10), Italy (+8) and Spain (+9). Together, these three countries accounted for 79.2% of all confirmed cases reported at the EU level in 2019. Bulgaria had the highest notification rate in the EU (0.79 cases per 100,000). Fourteen MS reported zero confirmed cases in 2019 including four MS (Cyprus, Finland, Luxembourg and Malta) that have never reported any trichinellosis cases.
Table 42.
Reported human cases of trichinellosis and notification rates per 100,000 population in the EU/EFTA, by country and year, 2015–2019
| Country | 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 1 | 1 | 0.01 | 2 | 0.02 | 3 | 0.03 | 2 | 0.0 | 0 | 0.00 |
| Belgiumb | Y | A | – | – | – | 0 | – | 0 | – | 0 | – | 0 | – |
| Bulgaria | Y | A | 55 | 55 | 0.79 | 45 | 0.64 | 55 | 0.77 | 35 | 0.49 | 22 | 0.31 |
| Croatia | Y | C | 3 | 3 | 0.07 | 0 | 0.00 | 21 | 0.51 | 5 | 0.12 | 3 | 0.07 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czechia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Denmarkc | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 2 | 0.15 |
| Finland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| France | Y | C | 3 | 2 | 0.00 | 0 | 0.00 | 8 | 0.01 | 3 | 0.00 | 3 | 0.00 |
| Germany | Y | C | 3 | 3 | 0.00 | 0 | 0.00 | 2 | 0.00 | 4 | 0.00 | 3 | 0.00 |
| Greece | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 |
| Hungary | Y | C | 0 | 0 | 0.00 | 2 | 0.02 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Ireland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Italy | Y | C | 10 | 10 | 0.02 | 2 | 0.00 | 4 | 0.01 | 5 | 0.01 | 36 | 0.06 |
| Latvia | Y | C | 1 | 1 | 0.05 | 1 | 0.05 | 1 | 0.05 | 1 | 0.05 | 4 | 0.20 |
| Lithuania | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 9 | 0.32 | 1 | 0.03 | 21 | 0.72 |
| Luxembourg | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Malta | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlands | Y | A | 1 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Poland | Y | C | 2 | 2 | 0.01 | 2 | 0.01 | 9 | 0.02 | 4 | 0.01 | 1 | 0.00 |
| Portugal | Y | C | 1 | 1 | 0.01 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 |
| Romania | Y | C | 21 | 6 | 0.03 | 10 | 0.05 | 48 | 0.24 | 26 | 0.13 | 55 | 0.28 |
| Slovakia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 0.02 | 1 | 0.02 | 1 | 0.02 |
| Slovenia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Spaine | Y | C | 39 | 11 | – | 2 | 0.00 | 5 | 0.01 | 12 | 0.03 | 3 | 0.01 |
| Sweden | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 2 | 0.02 | 1 | 0.01 |
| United Kingdom | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| EU Total | 140 | 96 | 0.02 | 66 | 0.01 | 168 | 0.03 | 101 | 0.02 | 155 | 0.03 | ||
| Iceland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0.0 | 0.00 |
| Norway | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0.0 | 0.00 |
| Switzerlandd | Y | C | – | 3 | 0.03 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 2 | 0.02 |
–: Data not reported.
Y: yes; N: no; A: aggregated data; C: case‐based data.
Sentinel surveillance, disease not under formal surveillance. Notification rate not calculated.
No surveillance system.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
Data not complete in 2019, rate not calculated.
In 2019, 26 cases (27.1%) of trichinellosis cases with known travel status and with known country of infection were reported to be acquired in the EU (Table 41). Four MS reported five travel‐associated trichinellosis cases of which two cases were infected outside the EU and one case infected within the EU. For 66 cases (68.7%), travel information was not reported.
The EU/EEA trend in confirmed cases of trichinellosis has substantially been influenced by a number of smaller and larger outbreaks, often with peaks in January–February (Figure 45). The EU/EEA trend was significantly declining in 2015–2019. Romania reported a decreasing trend and none of the MS observed significantly increasing trend during the same time period. Bulgaria, which reported most of the cases and highest notification rate in the EU in 2015–2019 was not included in the EU trend calculations since monthly data were not available.
Figure 45.
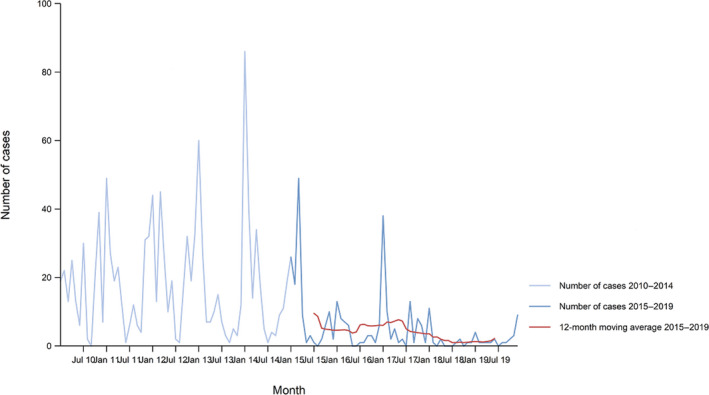
Trend in reported confirmed human cases of trichinellosis in the EU/EEA by month, 2015–2019
- Source: Austria, Cyprus, Czechia, Estonia, Finland, Germany, France, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Sweden and the United Kingdom. Belgium, Bulgaria, Croatia, Spain and Iceland did not report data to the level of detail required for the analysis. Denmark does not have any formal surveillance system for the disease.
Of the 12 MS reporting confirmed cases for 2019, five provided information on hospitalisation (16 cases, 16.7% of all confirmed cases reported in the EU). Among these, six cases (37.5%) were hospitalised, which was a decrease compared with 2018 (64.2%). Seven MS provided information on the outcome of their cases (24 cases, 25.0% of all confirmed cases). One death due to trichinellosis was reported in 2019 resulting in an EU case fatality of 4.2%.
Species information was available for 22 (22.9%) of the reported confirmed cases from six MS. The only species reported to TESSy from confirmed human cases was T. spiralis. Species reported to EFSA from food were T. spiralis from pig meat and products thereof in one food‐borne outbreak in Croatia and one in Romania and T. britovi from other or mixed red meat and products thereof in one food‐borne outbreak in Italy (see below).
Human trichinellosis cases associated with food‐borne outbreaks
Overall, for the year 2019, the number of reported human trichinellosis cases infected within the EU was 25, two cases contracted the infection outside EU and 68 cases were reported with unknown travel information (Table 41).
Overall Trichinella was identified by four MS in five outbreaks, which were all strong‐evidence outbreaks and which together affected 44 people in `EU, with 12 hospitalised and no deaths, as reported to EFSA. Comparing the number of food‐borne outbreak cases (44) reported to EFSA and the number of cases of human trichinellosis acquired in the EU (25) reported to ECDC, considering also the proportion of unknown travel data (0.926 × 68), reported to ECDC, could suggest that overall, in 2019, 50% of human trichinellosis cases in the EU would be reported through food‐borne outbreak investigation. In this context, it is important to clarify that the case classification for reporting is different between these two databases. In TESSy, the cases reported are classified based on the EU case definition. All these cases visited a doctor and are either confirmed by a laboratory test (confirmed case) or not (probable case and classification is based on the clinical symptoms and epidemiological link). Cases who never visited a doctor are not reported to TESSy. Moreover, probable cases may be missing in TESSy, as these data are not analysed or published and there is no incentive for reporting such cases. Information on which cases are linked to an outbreak and which not is also not systematically collected. In practice, the cases reported to TESSy are considered to be mostly sporadic cases. In food‐borne outbreaks, human cases are persons involved in the outbreak as defined by the investigators (case definition), and cases must be linked, or probably linked, to the same food source (Directive 2003/99/EC). This can include both ill people (whether confirmed microbiologically or not) and people with confirmed asymptomatic infections (EFSA, 2014). Cases can be classified as confirmed or probable outbreak cases, but currently these specific classification data are not collected by EFSA.
All five Trichinella food‐borne outbreaks (Tables 41 and 43) were reported as strong‐evidence outbreaks. They were reported by Bulgaria (two), Croatia (one), Italy (one) and Romania (one). Two food‐borne outbreaks reported by Bulgaria involved, in total, 27 people from which only one person needed hospitalisation and these food‐borne outbreaks were caused by unspecified Trichinella species. The two outbreaks reported by Croatia and Romania were caused by T. spiralis, involving three and five human cases, respectively, which needed hospitalisation. The food‐borne outbreak reported by Italy was caused by T. britovi and three out of nine people were hospitalised; the vehicle was wild boar meat products. Two food‐borne outbreaks reported by one non‐MS (Serbia) involved 27 people from which eight people were hospitalised and were caused by an unspecified Trichinella species. Trichinellosis food‐borne disease outbreaks were, during 2019, mostly caused by pig meat and products thereof (Figure 46 and Table 43), as during previous years (2010–2018). Further details and statistics on the trichinellosis food‐borne outbreaks for 2019 are in the food‐borne outbreaks chapter.
Table 43.
Distribution of strong‐evidence outbreaks caused by Trichinella, by food vehicle, by reporting MS, EU, 2010–2018 and 2019
| 2019 | 2010–2018 | |||||
|---|---|---|---|---|---|---|
| Food vehicle | Reporting Member State | N strong‐evidence food‐borne outbreaks | % of total | Reporting MS | N strong‐evidence food‐borne outbreaks | % of total |
| Pig meat and products thereof |
Bulgaria (2) Croatia (1) Romania (1) |
4 | 80.0 |
Romania (37) Lithuania (12) Croatia (5) Latvia (4) France (3) Belgium (1) Poland (1) Spain (1) |
73 | 73 |
| Other or mixed red meat and products thereof | Italy (1) | 1 | 20.0 |
Lithuania (6) Poland (6) Romania (3) Germany (1) Latvia (1) |
18 | 18 |
| Meat and meat products | – * | – | – |
Poland (5) Spain (2) Croatia (1) Germany (1) |
9 | 9 |
| Total | 5 | 100.0 | 100 | 100.0 | ||
No food‐borne outbreak during 2019 caused by Trichinella reported with this food vehicle incriminated.
Figure 46.
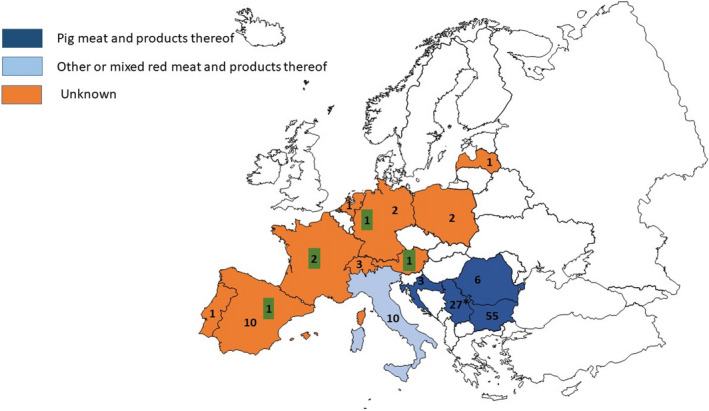
Total human cases in EU/EFTA and Serbia (ECDC data and EFSA food‐borne outbreaks data), 2019
- Countries that reported food‐borne human trichinellosis cases are coloured according the food vehicle causing the outbreaks (‘pig meat and products thereof’, ‘other or mixed red meat and products thereof’ or ‘unknown’ food vehicle) (data reported to EFSA). The numbers without green box indicate the number of domestic trichinellosis human cases and the numbers in a green box indicate the number of travel‐related trichinellosis human cases (data reported to ECDC except for Serbia (*) data reported to EFSA).
7.3.3. Trichinella infection in animals
Table 44 shows Trichinella summary monitoring results in domestic pigs and in farmed wild boar by housing conditions, for 2019. All pigs in mixed herds reported were not raised under controlled housing conditions.
Table 44.
Trichinella monitoring results in domestic pigs and in farmed wild boar in reporting MS and non‐MS, by housing conditions, EU, 2019
| No controlled housing conditions (NCHC) or not specified | Controlled housing conditions | |||||
|---|---|---|---|---|---|---|
| Country | Farmed wild boar | Fattening pigs | Breeding pigs | Pigs in mixed herds | Fattening pigs | Breeding pigs |
| Austria | 0/1,348 | 0/4,978,891 | 0/84,411 | – | – | – |
| Belgium | – | – | 0/3,294,615 | – | 0/4,117,021 | – |
| Bulgaria | – | 1/140a (0.71) | 0/86 | 0/582 | 0/82,702 | – |
| Croatia | – | 2/250,352 (< 0.01) | 1/4,404g (0.02) | – | 0/944,302 | 0/8,720h |
| Cyprus | – | 0/564,311 | 0/12,401 | – | – | – |
| Czechia | – | 0/2,340,037 | – | – | – | – |
| Denmark | 0/854 | 0/676,524 | 0/216,873 | – | 0/15,456,157 | 0/275,787h |
| Estonia | – | 0/391,602 | – | – | 0/48,759 | – |
| Finland | 0/263 | 0/1,787,431 | 0/33,346 | – | 0/444 | |
| France | 0/464 | 0/481,305 | 0/120,629 | 1/20,033 (< 0.01) | 0/22,997 | 0/187,407h |
| Germany | – | 0/53,561,424 | – | – | – | – |
| Greece | 0/2,036 | 0/1,051,473b | 0/21,892 | – | _ | |
| Hungary | – | 0/4,040,344 | 0/381,249 | – | – | – |
| Ireland | – | – | – | – | 0/3,354,931 | 0/91,401h |
| Italy | 0/1,884 | 0/259,351c | – | – | 0/9,780,920 | 0/129,528h |
| Latvia | – | 0/513,361 | _ | – | – | – |
| Lithuania | – | _ | – | – | 0/933,802 | |
| Luxembourg | – | 0/156,394 | – | – | – | – |
| Malta | – | 0/51,297 | 0/841 | – | – | – |
| Netherlands | – | – | – | – | 0/15,782,576 | – |
| Poland | – | – | – | 22/21,513,924 (< 0.01) | – | – |
| Portugal | – | 0/146,428 | 0/2,582 | – | 0/4,118,714 | 0/27,680h |
| Romania | – | 79/216,613 (0.036) | – | – | 0/4,231,267 | 0/11,235h |
| Slovakia | – | 0/694,619d | 0/13,282 | – | – | – |
| Slovenia | – | ‐ | – | 0/258,277 | – | – |
| Spain | – | 113/44,737,779e (< 0.01) | 0/910,339 | – | 0/6,163,002 | |
| Sweden | – | 0/470,951 | 0/32,416 | – | 0/1,459,867 | 0/23,197h |
| United Kingdom | 0/721 | 0/441,827 | 0/476,038 | – | 0/6,376,491 | 0/4,993 |
| EU Total | 0/7,570 | 195/117,812,454 (< 0.01) | 1/5,605,404 (< 0.01) | 23/21,792,816 (< 0.01) | 0/72,873,952 | 0/759,948 |
| Iceland | – | – | – | – | 0/78,625 | 0 |
| Norway | – | 0/1,622,000f | – | – | – | 0 |
| Switzerland | – | 0/2,285,231 | 0/30,099 | – | – | 0 |
| Total non‐MS | 0 | 0/3,907,231 | 0/30,099 | 0 | 0/78,625 | 0 |
| TOTAL EU + non‐EU EU MS | 0/7,570 | 195/121,686,061(< 0.01) | 1/5,634,521(< 0.01) | 23/21,792,816 (< 0.01) | 0/72,952,577 | 0/759,948 |
Including 1/98 (1.02%) pigs reported for own consumption.
Including 502 piglets and 2,269 pigs reported for own consumption.
Pigs reported for own consumption.
Including 820 pigs for own consumption.
Including 0/1,052,291 piglets, 1/21,232 (< 0.01%) pigs reported for own consumption, 112/62,215 (0.2%) wild pigs (free‐ranging pigs) and 48,118 slaughter animal batches.
Piglets.
Comprising 4,119 sows including one positive and 285 boars.
Including sows and boars.
In 2019, 31 countries (all 28 MS and 3 non‐MS) provided information on Trichinella in domestic animals (pigs and/or farmed wild boar). Six MS (Bulgaria, Croatia, France, Romania and Spain), as in 2018, reported positive findings in domestic pigs not raised under controlled housing conditions. No positive findings were found in farmed wild boars.
Sixteen MS (Belgium, Bulgaria, Croatia, Denmark, Estonia, Finland, France, Ireland, Italy, Latvia, the Netherlands, Portugal, Romania, Spain, Sweden and the United Kingdom) and one non‐MS (Iceland) reported data on breeding and fattening pigs raised under controlled housing conditions, no positive finding was reported.
In total, 72,873,952 fattening pigs and 759,948 breeding pigs from pigs kept under controlled housing conditions were tested for Trichinella spp. in 16 MS. None of these animals tested positive. Iceland tested 78,625 fattening pigs kept under controlled housing conditions and all were negative.
In 2019, 25 MS and two non‐MS reported data on breeding pigs, fattening pigs, pigs from mixed herds or on farmed wild boar that were not raised under controlled housing conditions and six MS reported positive findings among breeding pigs, fattening pigs and pigs from mixed herds. In total, one breeding pig (< 0.01%), 195 (< 0.01%) fattening pigs and 23 (< 0.01%) pigs from mixed herds were positive. Spain accounted for most positive pigs followed by Romania, Poland, Croatia, Bulgaria and France. As during 2014–2018, these Trichinella infections were from free‐range and backyard pigs reared in rural EU regions. All farmed wild boar (7,570) tested negative. Norway and Switzerland tested 3,907,231 fattening pigs not raised under controlled housing conditions and all tested negative. Two MS (Bulgaria and Croatia) reported data on food. Croatia reported eight positive units of meat from pig‐meat products out of 13 tested. Bulgaria reported one positive fresh raw sausage made with wild boar meat.
As shown in Figure 47 from 2012 to 2016 (5‐year period), Trichinella spp. were not reported in domestic pigs in 16 MS (Austria, Belgium, Cyprus, Czechia, Denmark, Estonia, Finland, Hungary, Ireland, Luxembourg, Malta, the Netherlands, Portugal, Slovenia, Sweden and the United Kingdom) while this was the case in the other 12 MS (Bulgaria, Croatia, France, Germany, Greece, Italy, Latvia, Lithuania, Poland, Romania, Slovakia and Spain). In 2017, 2018 and 2019, Trichinella spp. were only reported by six MS: Bulgaria, Croatia, France, Poland, Romania and Spain in 2017; Croatia, France, Italy, Poland, Romania and Spain in 2018; and Spain, Romania, Poland, Croatia, Bulgaria and France in 2019.
Figure 47.
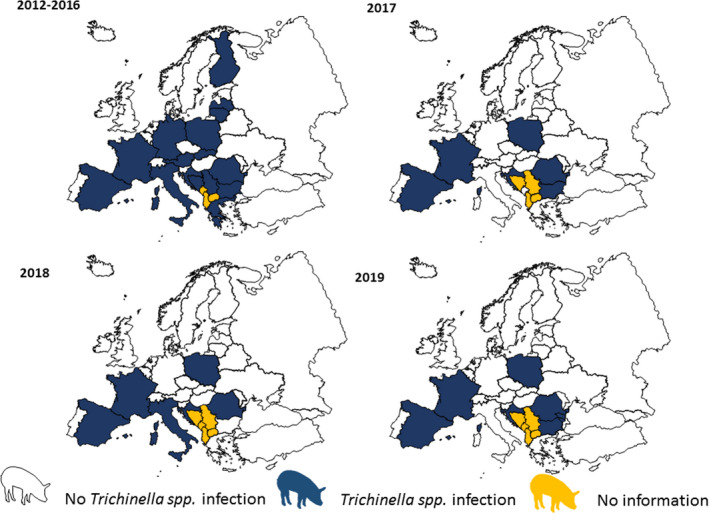
Trichinella spp. in domestic pigs and farmed wild boar, in EU/EFTA, 2012–2019
In 2019, as in the previous 4‐year period (2015–2018), no positive finding was reported in domestic solipeds (156,815 animals and 2,236 slaughter animal batches tested) and reported by 22 MS (Austria, Belgium, Bulgaria, Czechia, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Luxembourg, Malta, the Netherlands, Portugal, Romania, Slovenia, Spain, Sweden and the United Kingdom) and in two non‐MS (Iceland and Switzerland). Bulgaria reported two negative test results from fresh raw sausage made with horse meat.
Summary data for wild animals are given in Table 45. Seventeen MS (Austria, Bulgaria, Croatia, Czechia, Estonia, Finland, France, Germany, Hungary, Italy, Latvia, Poland, Romania, Slovakia, Slovenia, Spain and Sweden) and one non‐MS (Republic of North Macedonia) reported positive findings in hunted wild boar (1,378 positive findings out of 1,767,487 animals tested (< 0.007%). In total, 10 MS and one non‐MS reported data on Trichinella in red foxes (Vulpes vulpes) with, in total, 89 (1.3%) positive out of 6,697 tested animals. Eight MS reported data on Trichinella in brown bears (Ursus arctos) with 24 (3.08%) positive out of 779 tested in four MS. Six MS and one non‐MS reported data on Trichinella in other wild animals. Positive findings were detected in eight species (lynx, otter, wolverine, wolf, raccoon dog, eagle, polecat and jackal) from four MS and one non‐MS. The highest number of infected animals was observed in racoon dogs (41.2%) followed by wolverine (37.5%), lynxes (17.1%), wolves (14.6%), jackals (8.9%), polecats (11.1%), eagles (3.3%) and otter (1.6%).
Table 45.
Trichinella monitoring results in hunted wild boar or not specified wild boar, other wild animals and domestic solipeds, in reporting MS and non‐MS, EU, 2019
| Country | Positive/tested (% positive) | |||
|---|---|---|---|---|
| Hunted or not specified wild boar | Brown bears | Red foxes | Other wild animals and domestic solipeds | |
| Austria | 1/20,834 (< 0.01) | 0/2 (0) | 0/614 (0)a | |
| Belgium | 0/27,051 (0) | 0/27,669 (0)b | ||
| Bulgaria | 50/11,307 (0.44) | 0/21 (0)b | ||
| Croatia | 41/42,015 (0.1) | 3/63 (4.7) | – | 1/4 (25)c |
| Cyprus | – | – | 0/61 (0) | – |
| Czechia | 2/237,246 (< 0.01) | – | 2/2,848 (0.07) | 0/88 (0)b |
| Denmark | – | – | – | 0/1,321 (0)b |
| Estonia | 2/560 (0.36) | 12/45 (26.7) | – | 0/10 (0)b |
| Finland | 5/1,076 (0.5) | 6/279 (2.15) | 61/198 (30.8) | 194/563 (34.4)d |
| France | 2/44,950 (< 0.01) | – | – | 0/7,667 (0)b |
| Germany | 20/654,616 (< 0.01) | – | – | 0/5,120 (0)b |
| Greece | 0/4 (0) | – | – | |
| Hungary | 3/71,301 (< 0.01) | – | 15/820 (1.8) | 3/719 (0.4)e |
| Ireland | – | – | – | 0/5,499 (0)b |
| Italy | 5/172,847 (< 0.01) | 0/6 (0) | 5/2,260 (0.2) | 24/36,808 (0.06)f |
| Latvia | 26/4,133 (0.63) | – | – | 0/6 (0)b |
| Luxembourg | 0/5,501 (0) | – | 0/95 (0) | 0/14 (0)b |
| Malta | – | – | – | 0/1 (0)b |
| Netherlands | 0/5,012 (0) | – | – | 0/2,020 (0)b |
| Poland | 585/168,699 (0.34) | – | – | – |
| Portugal | 0/1,143 (0) | 0/890 (0)b | ||
| Romania | 196/17,550 (1.1) | 3/26 (11.5) | _ | 0/34,373 (0)b |
| Slovakia | 3/15,177 (0.02) | 0/4 (0) | 6/112 (5.3) | _ |
| Slovenia | 1/1,533 (0.6) | 0/124 (0) | – | 0/1,166 (0)b |
| Spain | 421/115,432 (0.36) | – | – | 0/25,305 (0)b |
| Sweden | 5/138,374 (< 0.01) | 0/232 (0) | 0/11 | 8/2,009 (0.4)g |
| United Kingdom | 0/1,022 (0) | 0/289 (0) | 0/21,852 (0)b | |
| EU Total | 1,368/1,757,383 (0.08) | 24/779 (3.08) | 89/6,696 (1.3) | 230/150,652 (0.15) |
|
Iceland Republic of North Macedonia |
–10/933 (1.1) | – | – | 0/8,202 (0)b |
| Switzerland | 0/9,171 (0) | – | 0/1 (0) | 3/1,560 (0.2)h |
| Total non‐EU | 10/10,104 (0.1) | – | 0/1 (0) | 3/9,762 (0.03) |
| Total EU and non‐EU | 1,378/1,767,487 (< 0.007) | 24/779 (3.08) | 89/6,697 (1.3) | 233/160,414 (0.14) |
Domestic horses.
Badgers.
0/9 beavers; 0/7 badgers; 1/30 (3.3%) eagles; 0/1 owls; 0/3 minks; 0/9 marten; 0/16 goshawk; 27/55 (49%) lynx; 1/50 (2%) otter; 1/1 polecats (100%); 133/323 (41.2%) raccoon dogs; 0/2 seals; 3/7 (42.8%) wolverine; 28/50 (56%) wolves; 0/1,096 domestic horses.
0/15 badgers; 3/30 (10%) jackals; 0/674 domestic horses.
0/484 badgers; 0/348 birds; 0/2 crows; 0/1 deer; 0/7 hedgehogs; 0/11 jackals; 0/1 lynx; 0/2 otter; 0/2 mouflon; 0/332 martens; 0/1 owls; 0/8 pool cats; 0/4 weasel; 0/5 wild cat; 24/304 (7.9%) wolves; 0/35,296 domestic horses.
0/8 badgers; 0/1 marten; 0/61 birds; 0/33 beavers; 6/130 lynx (4.6%); 0/11 seals; 0/1 raccoon dog; 2/14 (14.2%) wolves; 0/1 wolverine; 0/1,749 domestic horses.
0/2 badgers; 2/15 lynx (13%); 1/8 wolves (12.5%); 0/1,535 domestic horses.
0/45 badgers; 0/5 coypu; 0/564 domestic horses.
7.4. Discussion
Trichinellosis is a rare but serious human disease that is still present in low numbers in some of the EU MS. Half of the MS reported zero cases including four MS (Cyprus, Finland, Luxembourg and Malta) that have never reported any trichinellosis case since the beginning of the EU‐level surveillance in 2007.
The EU/EEA trend for trichinellosis has been greatly affected by the number and size of food‐borne outbreaks. The number of human cases and the EU notification rate have, however, been kept low in the last 5 years from 2015 to 2019 with the highest rate (0.03) reported in 2017 and 2015. In 2018, the lowest rate (0.01) was reported since the beginning of trichinellosis EU‐level surveillance in 2007. Despite the increase in cases and notification rate (0.02) in 2019 compared with 2018, the 5‐year trend from 2015 to 2019 was declining. The number of confirmed trichinellosis cases in 2019 was lower than the 5‐year average in the EU. The increase in 2019 was mainly due to the increase of domestic trichinellosis cases in three MS (Bulgaria, Italy and Spain). The main reason for this increase was the higher consumption of various home‐made pork products during winter as well as during the wild boar hunting season. Romania, which had experienced most Trichinella outbreaks in the previous years, reported fewer human cases in 2019 than in 2018. About one‐third of the confirmed cases were hospitalised, with one fatal outcome. This represents fewer hospitalisations compared with previous years.
In general, Trichinella infections in humans are linked to food‐borne outbreaks. In 2019, fewer human food‐borne cases (N = 44) were reported to EFSA (food‐borne outbreaks database) than confirmed sporadic cases (N = 96) reported to TESSy managed by ECDC. However, in 2017 and 2018, the former number was higher than the latter. Such discrepancies result from different case classification for reporting between the two databases. Spain was unable to report 2019 data to TESSy from all autonomous regions, due to COVID‐19. Given the pandemic emergency and related difficulties, exhaustive and accurate reporting – to ECDC and to EFSA – could have been challenging for other MS.
In 2019, five Trichinella outbreaks were reported by four MS (Bulgaria, Croatia, Italy and Romania, reporting rate < 0.01 outbreak per 100.000 population) and two outbreaks by one non‐MS (Serbia). In total, 44 patients were affected in the EU and 27 in the non‐EU MS of which almost half (20) were hospitalised. All outbreaks were reported with strong‐evidence and associated with ‘pig meat and products thereof’, except one which was associated with ‘other or mixed red meat and products thereof’. It is important to underline the reports of Trichinella‐positive domestic pigs by Bulgaria, Croatia and Romania that also reported food‐borne human cases (to EFSA food‐borne outbreaks database), and the reports by Poland and Spain that reported confirmed domestic human cases (to ECDC TESSy). By contrast, in other MS during the last years, there was an increasing number of pigs raised under controlled housing conditions and increased control at slaughtering of pigs that are not raised under controlled housing conditions. These measures, in combination with activities raising awareness about trichinellosis and farmers’ education, may have contributed to a reduction of the parasite biomass in the domestic habitat and the probability of acquiring an infection for humans (Figure 47).
In the EU, most pigs are subject to official meat inspection at slaughter in accordance with Regulation (EU) 2015/1375; only pigs slaughtered for own consumption are not covered by the Regulation. Around 218 million pigs were tested for Trichinella in MS and non‐MS in 2019, out of about 246 million reared pigs in the EU (Marquer et al., 2014), with only 219 positive animals, about 0.89 per million reared pigs. Only six out of 28 MS reported Trichinella in pigs in 2019, with an overall prevalence of 0.00001%. All positive findings were from pigs not raised under controlled housing conditions. In the EU, infected pigs are usually clustered in five MS (Bulgaria, Croatia, Poland, Romania and Spain) and sporadic infections are documented in other MS (Pozio, 2014). In 2019, Spain accounted for the highest number of positive domestic pigs (113) followed by Romania (79) Poland (22), Croatia (3), Bulgaria (1) and France (1). The reported number of Trichinella‐positive domestic pigs is likely to be an underestimation of the true number, as most pigs at risk for this infection are slaughtered at home without any veterinary control and recording. EFSA has identified that non‐controlled housing condition is a main risk factor for Trichinella infections in domestic pigs and the risk of Trichinella infection in pigs from well managed officially recognised controlled housing conditions is considered negligible (EFSA BIOHAZ, CONTAM and AHAW Panels, 2011; EFSA and ECDC, 2011).
In addition to domestic pigs, hunted wild boar are an important source of trichinellosis infections for humans. However, the prevalence of Trichinella spp. infections in this animal species has declined over the years due to the increased control for these pathogens. From 2012 to 2016, the prevalence of infection was reduced threefold (from 0.13% in 2012 to 0.05% in 2016) but increased up to 0.09% in 2018 in the hunted wild boar population. In 2019, a new decrease of the prevalence to 0.08% was recorded in this animal species. Trichinella spp. were not detected in farmed wild boar; however, the number of tested farmed wild boar decreased during the last years.
No positive finding was reported for solipeds in 2019. In the last 12 years, only four horses tested positive out of more than one million tested animals in 2008, 2010 and 2012 (EFSA and ECDC, 2009, 2010, 2011, 2012, 2013, 2014). This extremely low (< 0.001%) prevalence could be related to the effective control which, according to EFSA BIOHAZ Panel (2013b), should be maintained as long as there is no full and reliable traceability system in place.
Trichinella spp. circulate among wild animals in large parts of Europe. In 2019, seven MS and one non‐MS reported positive findings in wild animals (brown bears and wild animals different from foxes and wild boar). The reporting of negative findings in MS could be explained by insufficient number of surveys, inadequate sample size or, investigations in regions in which environmental conditions that do not favour the transmission of these zoonotic nematodes among wildlife.
Red foxes, having a large and widespread population, can be considered as the main natural reservoir of Trichinella in Europe. The prevalence decreased by twofold in the last 5 years (from 2% in 2013 to 1.1% in 2017) and then increased in 2018 (1.6%) and decreased again in 2019. In 2019, 10 MS and one non‐MS monitored Trichinella spp. infection in 6,697 red foxes and positive animals were detected in five MS. The proportion of positive samples from wildlife was higher in raccoon dogs, wolverine, lynxes, wolves and jackals, but their population size and distribution in Europe are generally limited to a few countries. Data from Trichinella in wild animals are not fully comparable between MS as neither harmonised monitoring schemes nor mandatory reporting requirements are in place and the reported findings must therefore be interpreted with caution. These data allow descriptive summaries at the EU level but preclude subsequent data analysis such as assessing temporal and spatial trends.
Identification of Trichinella larvae at the species level carried out in 2019 confirms that T. spiralis is more prevalent than T. britovi in pigs (Pozio et al., 2009). However, since T. spiralis is patchily distributed, T. britovi and Trichinella pseudospiralis were detected in pigs in some countries. Trichinella nativa has been documented in wild carnivores of Finland, Estonia and Sweden. T. pseudospiralis was documented in hunted wild boar, six lynxes and one eagle confirming its low prevalence in target animals (Pozio, 2016).
There is a relationship between unawareness and low‐income of consumers, living in rural areas, inadequacy of local veterinary meat inspection services and the occurrence of Trichinella in domestic animals in the EU and non‐EU countries (Pozio, 2014). The increasing number of wild boar and red foxes and the spread of the raccoon dog population from eastern to western Europe and that of the jackal from southern‐eastern to northern‐western Europe may increase the prevalence of Trichinella circulating among wild animals (Alban et al., 2011; Széll et al., 2013).
7.5. Related projects and Internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Fact sheet of trichinellosis | https://www.cdc.gov/parasites/trichinellosis/index.html |
| ECDC Surveillance Atlas of Infectious Diseases | http://ecdc.europa.eu/en/data‐tools/atlas/Pages/atlas.aspx | |
| EU case definition of trichinellosis | https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions | |
| Disease Programme on Emerging, Food‐ and Vector‐Borne Diseases | https://www.ecdc.europa.eu/en/about-us/who-we-are/units/disease-programmes-unit | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| FAO/WHO/OIE Guidelines for the surveillance, management, prevention and control of trichinellosis | http://www.trichinellosis.org/uploads/FAO-WHO-OIE_Guidelines.pdf | |
| International Commission on Trichinellosis | http://www.trichinellosis.org/ | |
| European Union Reference Laboratory for Parasites (humans and animals) | https://eurlp.iss.it | |
| Animals | World Organisation for Animal health, Summary of Information on Trichinellosis | http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/Disease_cards/TRICHI-EN.pdf |
| FAO/WHO/OIE Guidelines for the surveillance, management, prevention and control of trichinellosis | http://www.trichinellosis.org/uploads/FAO-WHO-OIE_Guidelines.pdf | |
| International Trichinella Reference Center | https://www.iss.it/site/Trichinella/ | |
| International Commission on Trichinellosis | http://www.trichinellosis.org/ | |
| Development of harmonised schemes for the monitoring and reporting of Trichinella in animals and foodstuffs in the European Union | http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/35e.pdf | |
| OIE Manual Chapter 2.1.16. Trichinellosis | https://web.oie.int/eng/normes/MMANUAL/2008/pdf/2.01.16_TRICHINELLOSIS.pdf | |
| Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015 laying down specific rules on official controls for Trichinella in meat | http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32015R1375 | |
| Pig farming in the European Union: considerable variations from one Member State to another | http://ec.europa.eu/eurostat/statistics-explained/index.php/Pig_farming_sector_-_statistical_portrait_2014 | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
8. Echinococcus
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
8.1. Key facts
In 2019, 751 confirmed human echinococcosis cases were reported in the EU.
The EU notification rate was 0.18 cases per 100,000 population, which was the lowest notification rate in the last 5 years.
Echinococcus granulosus accounted for 73.5% (408 cases) and Echinococcus multilocularis for 26.5% (147 cases).
The trends of human and animal infections caused by E. multilocularis or E. granulosus sensu lato (s.l.) did not show any significant increase or decrease in the EU/EEA in 2015–2019.
In total, 23 MS and two non‐MS provided 2019 monitoring data on Echinococcus in animals.
Thirteen MS and two non‐MS reported data on, respectively, 6,326 and 621 foxes that were examined for E. multilocularis. Seven MS and one non‐MS reported positive findings with an overall proportion of test‐positives of 12.9%.
Data for 2019 from Finland, Ireland, Malta, the United Kingdom and mainland Norway confirmed the free status of these countries for E. multilocularis in the context of Commission Delegated Regulation (EU) No 1152/2011.
For E. granulosus, 19 MS and two non‐MS reported data from around 113.76 million animals which were mainly domestic livestock (> 99%). The overall proportion of test‐positives was 0.15% and positives were reported by 11 MS. Positive samples were mainly from small ruminants (sheep and goats; 78.9%), whereas cattle constituted 9.8% of total positives and pigs 11.2% with most (85.4%) positive pigs reported by Poland.
8.2. Surveillance and monitoring of cystic and alveolar echinococcosis in humans and animals in the EU
8.2.1. Humans
Cases of both alveolar echinococcosis (AE) by E. multilocularis and cystic echinococcosis (CE) caused by E. granulosus sensu lato (s.l.) are listed with the common name ‘echinococcosis’ in the EU case definition, not distinguishing between these two diseases. AE and CE can be reported by species and since 2019 (2018 data) by clinical presentation of the disease into the ECDC TESSy database. The notification of echinococcosis in humans is mandatory in most MS, Iceland and Norway, except for Belgium, France, the Netherlands and the United Kingdom, where reporting is based on a voluntary surveillance system. Denmark and Italy have no surveillance system for echinococcosis. In Switzerland, echinococcosis in humans is not notifiable. The surveillance systems for echinococcosis cover the whole population in those MS where surveillance systems are in place. For 2019, Spain did not receive data from all regions due to COVID‐19 and the notification rate is therefore not displayed for this year. All countries reported case‐based data except Belgium, Bulgaria and the Netherlands, which reported aggregated data. Both reporting formats were included to calculate numbers of cases and notification rates.
An attempt to collect harmonised clinical data in the EU on a voluntary basis is currently undertaken by the European Register of Cystic Echinococcosis (ERCE) (Rossi et al., 2016, 2020; http://www.heracles-fp7.eu/erce.html) and in the past with the European (Alveolar) Echinococcosis Registry (EurEchinoReg) (Kern et al., 2003).
Estimates of the real burden of these diseases are extremely difficult to calculate because of the long incubation period (months or years) and the non‐specific symptoms. A recent cross‐sectional ultrasound‐based survey, conducted in Romania and Bulgaria, estimated around 45,000 human CE infections in rural areas of these two endemic European countries (Tamarozzi et al., 2018).
8.2.2. Animals
Echinococcus multilocularis in Europe is mainly transmitted to humans by a sylvatic cycle that is wildlife based (Casulli et al., 2019a). Intermediate hosts (IHs) for E. multilocularis are small rodents (microtine or arvicolid), while definitive hosts (DHs) are mainly red foxes and, to a lesser extent, other canids such as raccoon dogs, dogs, jackals and wolves. Echinococcus granulosus s.l. is a complex of species causing CE, in animals and humans. E. granulosus s.l. in Europe is mainly transmitted to humans by a pastoral cycle (Casulli et al., 2019b). IHs for E. granulosus s.l. are mainly livestock species (mainly sheep, secondarily pigs but also cattle and goats), while DHs are shepherd dogs (rarely wild canids). People become infected with AE and CE through the ingestion of eggs of the tapeworm prevalent in these DHs.
Surveillance for E. multilocularis in Europe is usually carried out on a voluntary basis, with the exception of the five reporting countries claiming to be free from this parasite according to the Commission Delegated Regulation (EU) 2018/772 supplementing Regulation (EU) No 576/201320. Surveillance is carried out in the main European DHs, the red fox (Vulpes vulpes). Four MS (Finland, Ireland, Malta and the United Kingdom) have demonstrated the absence of E. multilocularis through the implementation of an annual surveillance programme required in accordance with Regulation (EU) 2018/772. One EEA State, mainland Norway (Svalbard archipelago excluded), also implements a surveillance programme in line with Regulation (EU) 2018/772. In all other MS, data on E. multilocularis rely on whether findings are notifiable and if monitoring is in place or if studies on E. multilocularis are performed. As data on E. multilocularis in animals vary geographically (also within countries) and over time, reported cases of E. multilocularis are difficult to compare within and between countries. According to a recent meta‐analysis, based on studies published between 1900 and 2015, E. multilocularis has been documented in red foxes from 21 countries (Oksanen et al., 2016; Figure 48). Since 2015 and 2020, this parasite has been also found in foxes and golden jackals from Croatia and Hungary, respectively (Dušek et al., 2020; Balog et al., 2021).
Figure 48.
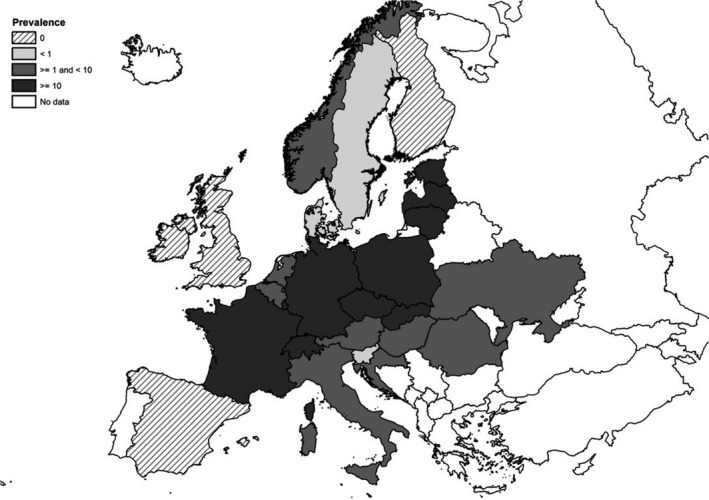
Pooled prevalence of Echinococcus multilocularis in red and Arctic foxes within the EU and adjacent countries at national level depicting current epidemiological situation in Europe (Oksanen et al., 2016)
Surveillance of E. granulosus s.l. is carried out in livestock IHs during slaughterhouse inspections. In particular, necropsy on sheep liver and lungs is used to detect the presence of parasitic cysts, while molecular PCR‐based methods are used to confirm and to identify genotype/species belonging to the Echinococcus genus (Siles‐Lucas et al., 2017). Although Regulation (EU) 2018/772 is in force for E. multilocularis, no specific EU Regulation is in place for detecting E. granulosus s.l. in animals or humans, therefore surveillance for the latter parasite depends on national regulations.
8.3. Results
8.3.1. Overview of key statistics, EU, 2015–2019
Table 46 summarises EU‐level statistics aggregated by year on cystic and AE in humans and on Echinococcus granulosus sensu lato and Echinococcus multilocularis in their most important definitive and intermediate animal hosts during 2015–2019.
Table 46.
Summary of echinococcosis in humans, of Echinococcus multilocularis and of Echinococcus granulosus sensu lato in most important definitive and intermediate animal hosts in the EU, 2015–2019
| 2019 | 2018 | 2017 | 2016 | 2015 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 751 | 810 | 850 | 844 | 887 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.18 | 0.21 | 0.19 | 0.22 | 0.20 | ECDC |
| Number of reporting MS | 26 | 25 | 26 | 25 | 26 | ECDC |
| Infection acquired in the EU | 173 | 149 | 169 | 122 | 172 | ECDC |
| Infection acquired outside the EU | 89 | 89 | 77 | 112 | 84 | ECDC |
| Unknown travel status or unknown country of infection | 489 | 572 | 604 | 610 | 631 | ECDC |
| Animals | ||||||
| Echinococcus multilocularis in red foxes | ||||||
| Number of animals tested | 6,326 | 6,566 | 7,148 | 4,561 | 5,371 | EFSA |
| % positive animals | 13.6 | 17.6 | 16.9 | 19.4 | 9.0 | EFSA |
| Number of reporting MS | 13 | 13 | 11 | 12 | 10 | EFSA |
| Echinococcus granulosus s.l. in dogs | ||||||
| Number of animals tested | 2,113 | 2,605 | 2,538 | 2,183 | 3,416 | EFSA |
| % positive animals | 0.2 | 0.1 | 0 | 0.4 | 0.2 | EFSA |
| Number of reporting MS | 6 | 6 | 7 | 5 | 7 | EFSA |
| Echinococcus granulosus s.l. in cattle | ||||||
| Number of animals tested | 10,956,692 | 9,920,338 | 9,834,374 | 7,746,553 | 6,539,857 | EFSA |
| % positive animals | 0.1 | 0.2 | 0.2 | 0.2 | 0.1 | EFSA |
| Number of reporting MS | 16 | 17 | 15 | 19 | 17 | EFSA |
| Echinococcus granulosus s.l. in sheep and goats | ||||||
| Number of animals tested | 36,891,061 | 38,870,644 | 38,278,897 | 12,159,745 | 7,067,952 | EFSA |
| % positive animals | 0.03 | 0.2 | 0.4 | 0.9 | 1.0 | EFSA |
| Number of reporting MS | 15 | 15 | 14 | 13 | 13 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
When the UK data were collected, the UK was an EU MS but as of 31 January 2020, it has become a third country.
8.3.2. Human echinococcosis
In 2019, 751 laboratory‐confirmed echinococcosis cases were reported in the EU by 26 MS (Table 47). Twenty‐three MS reported at least one confirmed case and three MS reported zero cases. The EU notification rate was 0.18 cases per 100,000 population, which was the lowest notification rate in the last five years. The highest notification rates were observed in Lithuania with 2.90 cases per 100,000 population, followed by Bulgaria with 2.76 and Austria and Latvia with 0.41 and 0.31 cases per 100,000 population, respectively.
Table 47.
Reported human cases of cystic and alveolar echinococcosis and notification rates per 100,000 population in the EU/EFTA, by country and year, 2015–2019
| Country | 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 36 | 36 | 0.41 | 46 | 0.52 | 50 | 0.57 | 26 | 0.30 | 8 | 0.09 |
| Belgium | Y | A | 20 | 20 | 0.17 | 14 | 0.12 | 12 | 0.11 | 17 | 0.15 | 9 | 0.08 |
| Bulgaria | Y | A | 193 | 193 | 2.76 | 206 | 2.92 | 218 | 3.07 | 269 | 3.76 | 313 | 4.35 |
| Croatia | Y | C | 4 | 3 | 0.07 | 4 | 0.10 | 15 | 0.36 | 9 | 0.21 | 7 | 0.17 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 2 | 0.24 |
| Czechia | Y | C | 1 | 1 | 0.01 | 4 | 0.04 | 1 | 0.01 | 4 | 0.04 | 3 | 0.03 |
| Denmarkb | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 2 | 2 | 0.15 | 0 | 0.00 | 1 | 0.08 | 0 | 0.00 | 0 | 0.00 |
| Finlandc | Y | C | 8 | 8 | 0.14 | 1 | 0.02 | 5 | 0.09 | 4 | 0.07 | 2 | 0.04 |
| France | Y | C | 45 | 45 | 0.07 | 62 | 0.09 | 53 | 0.08 | 38 | 0.06 | 48 | 0.07 |
| Germany | Y | C | 134 | 134 | 0.16 | 172 | 0.20 | 141 | 0.17 | 181 | 0.22 | 157 | 0.19 |
| Greece | Y | C | 7 | 7 | 0.07 | 11 | 0.10 | 15 | 0.14 | 18 | 0.17 | 13 | 0.12 |
| Hungary | Y | C | 10 | 10 | 0.10 | 9 | 0.09 | 14 | 0.14 | 5 | 0.05 | 2 | 0.02 |
| Irelandc | Y | C | 0 | 0 | 0.00 | 2 | 0.04 | 0 | 0.00 | 2 | 0.04 | 0 | 0.00 |
| Italyb | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Latvia | Y | C | 6 | 6 | 0.31 | 10 | 0.52 | 6 | 0.31 | 11 | 0.56 | 10 | 0.50 |
| Lithuania | Y | C | 81 | 81 | 2.90 | 50 | 1.78 | 53 | 1.86 | 26 | 0.90 | 33 | 1.13 |
| Luxembourg | Y | C | 1 | 1 | 0.16 | 0 | 0.00 | 2 | 0.34 | 0 | 0.00 | 0 | 0.00 |
| Maltac | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.22 | 0 | 0.00 |
| Netherlands | Y | A | 48 | 48 | 0.28 | 42 | 0.24 | 38 | 0.22 | 33 | 0.19 | 64 | 0.00 |
| Poland | Y | C | 70 | 70 | 0.18 | 51 | 0.13 | 75 | 0.20 | 64 | 0.17 | 47 | 0.12 |
| Portugal | Y | C | 5 | 5 | 0.05 | 9 | 0.09 | 2 | 0.02 | 2 | 0.02 | 4 | 0.04 |
| Romania | Y | C | 1 | 1 | 0.01 | 4 | 0.02 | 14 | 0.07 | 13 | 0.07 | 18 | 0.09 |
| Slovakia | Y | C | 11 | 11 | 0.20 | 10 | 0.18 | 7 | 0.13 | 4 | 0.07 | 5 | 0.09 |
| Slovenia | Y | C | 6 | 6 | 0.29 | 6 | 0.29 | 7 | 0.34 | 3 | 0.15 | 7 | 0.34 |
| Spaind | Y | C | 34 | 34 | – | 68 | 0.15 | 83 | 0.18 | 87 | 0.19 | 83 | 0.18 |
| Sweden | Y | C | 26 | 26 | 0.25 | 29 | 0.29 | 34 | 0.34 | 27 | 0.27 | 26 | 0.27 |
| United Kingdomc | Y | C | 3 | 3 | 0.00 | – | – | 4 | 0.01 | – | – | 26 | 0.04 |
| EU Total | 752 | 751 | 0.18 | 810 | 0.21 | 850 | 0.19 | 844 | 0.22 | 887 | 0.20 | ||
| Iceland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway | Y | C | 7 | 7 | 0.13 | 7 | 0.13 | 5 | 0.10 | 5 | 0.10 | 3 | 0.06 |
| Switzerland | – | – | – | – | – | – | – | – | – | – | – | – | – |
Y: yes; N: no; A: aggregated data; C: case‐based data.
No surveillance system.
Finland, Ireland, Malta, the United Kingdom and mainland Norway have been declared free of E. multilocularis.
Data not complete for 2019, rate not calculated.
–: Data no reported.
Most echinococcosis cases (65.1%) were reported without travel‐associated data, 23.0% were domestic or related to travel within the EU and 11.9% were associated travel outside the EU (Table 46). Seven MS (Czechia, Estonia, Hungary, Latvia, Lithuania, Romania and Slovakia) of the 15 MS reporting information on imported cases in 2019 notified all Echinococcus spp. infections as being domestically acquired. The highest proportion of travel‐related cases were reported by Finland (100%; eight cases), Luxembourg (100%; one case), Sweden (95.5%; 21 cases) and Norway (100%; seven cases). At a species level, E. multilocularis human infections were more often reported domestically acquired than E. granulosus s.l. human infections (85.1% vs. 34.9%). Among 112 travel‐associated cases of Echinococcus spp. with known origin of infection, majority (79.5%) were reported as originating from outside the EU. Syria, Iraq and Turkey were the most frequently reported probable country of infection, representing half (50.0%) of the imported cases in 2019. In EU, Bulgaria and Romania were reported as probable country of infection for 12 (10.5%) and six cases (5.3%), respectively.
In 2019, species information was provided for 555 confirmed echinococcosis cases (75.1%) by 16 MS. Human infections caused by E. multilocularis accounted for 147 cases (26.5%), which was at the same level as in 2018. The trend of human AE cases did not show any significant increase or decrease in 2015–2019 (Figure 49). For 10 MS with available data, Austria was the only country with an increasing trend from 2015 to 2019 and none of MS had a decreasing trend between 2015 and 2019.
Figure 49.
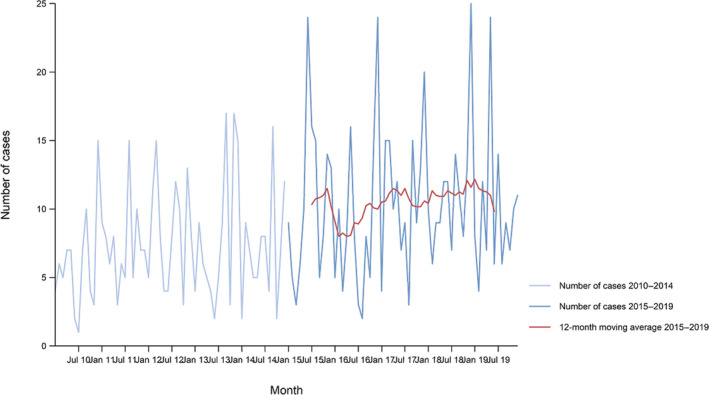
Trend in reported confirmed human cases of alveolar echinococcosis in the EU/EEA, by month, 2015–2019
- Source: Austria, France, Germany, Lithuania, Norway, Poland, Slovakia and Sweden. Belgium, Bulgaria, Croatia, Czechia, Cyprus, Denmark, Estonia, Finland, Greece, Hungary, Iceland, Italy, Ireland, Latvia, Luxembourg, Malta, the Netherlands, Portugal, Romania, Slovenia, Spain and the United Kingdom did not report data to the level of detail required for the analysis.
Human infections caused by E. granulosus s.l. accounted for 73.5% (408 cases) of those with species information available (555 confirmed cases). Almost half of the cases (47.3%; 193 cases) were from Bulgaria. The trend of cases of human CE did not show any significant increase or decrease in the EU/EEA in 2015–2019 (Figure 50).
Figure 50.

Trend in reported confirmed human cases of cystic echinococcosis in the EU/EEA, by month, 2015–2019
- Source: Austria, Czechia, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Latvia, Lithuania, Malta, Norway, Malta, Poland, Portugal, Romania, Slovakia, Slovenia, Spain and Sweden. Belgium, Bulgaria, Croatia, Cyprus, Denmark, Iceland, Italy Luxembourg, the Netherlands and the United Kingdom did not report data to the level of detail required for the analysis.
Lithuania and Finland reported an increasing trend and none of MS reported decreasing trend in 2015–2019. Bulgaria, which reported most of the human cases in the EU in 2010–2019 (all cases were caused by E. granulosus s.l.) was not included in the trend calculations as no monthly data were available. Cases from Bulgaria decreased by 33.7% from 291 cases to 193 cases in 2010–2019.
Fourteen MS provided information on hospitalisation, covering 32.8% of all confirmed cases of echinococcosis in the EU in 2019. The overall hospitalisation rate was 44.3%, which represents a continuous decrease during the last 10 years from 100% in 2008, when only hospitalised cases were reported. In 2019, the highest proportions of hospitalised cases (60–100%) were reported in Czechia, Greece, Poland, Portugal, Romania, Slovakia and Slovenia. More than half (56.4%) of human AE cases were hospitalised compared with about one‐third (36.4%) of human CE cases based on reporting by four and nine MS, respectively.
Information on the outcome of the cases was provided by 14 MS. One fatal case due to the infection by E. granulosus s.l. and one fatal case due to infection by E. multilocularis was reported in Portugal and Poland, respectively. This resulted in an EU case fatality of 0.86% among the 232 cases for which this information was reported (30.9% of all confirmed cases) in 2019.
8.3.3. Echinococcosis in animals
Table 48 summarises the most relevant DH and IH species tested for E. multilocularis, such as foxes, raccoon dogs, dogs, jackals, wolves, cats, beaver, voles, wild boar, coypu, squirrel, mice and pigs and results reported by MS and adjacent countries in 2019. In accordance with the Regulation (EU) 2018/772, surveillance of E. multilocularis is mainly focused on red foxes as DH.
Table 48.
Monitoring results of Echinococcus multilocularis in animals (wild and domestic) in EU/EFTA, 2019
| Country | Presence of Em/Egslb | Foxes | Racoon Dog | Wolves | Dogs | Cats | Jackals | Voles | Coypu | Beaver | Squirrel | Mice | Rodents | Pigs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Czechia | Em/Egsl | 596/2,848 (20.927%) | ||||||||||||
| Denmark | Em | 0/33 | 0/1 | 0/16,754,410 | ||||||||||
| Estonia | Em/Egsl | 0/558,717a | ||||||||||||
| Finlandd | Egsl | 0/198 | 0/325 | 0/1074 | ||||||||||
| France | Em/Egsl | 56/443 (12.641%) | 2/178 (1.124%) | 1/35 (2.857%) | 1/1 (100%) | 0/7 | 0/690 | |||||||
| Germany | Em | 67/441 (15.193%) | ||||||||||||
| Hungary | Em/Egsl | 38/795 (4.780%) | 2/22 (9.091%) | |||||||||||
| Irelandd | Egsl | 0/400 | ||||||||||||
| Italy | Em/Egsl | 0/17 | 0/17a | 0/10a | 0/1a | 143/5,124,363 (0.003%)a | ||||||||
| Luxembourg | Em | 18/94 (19.149%) | 0/125,996 | |||||||||||
| Poland | Em/Egsl | 76/240 (31.667%) | 15,959/21,513,924 (0.074%)a | |||||||||||
| Romania | Em/Egsl | 0/10a | 0/88a | |||||||||||
| Slovakia | Em/Egsl | 18/112 (16.071%) | 3/1,913 (0.157%) | 0/705a | 54/707,081 (0.008%)a | |||||||||
| Slovenia | Em/Egsl | 0/259,406a | ||||||||||||
| Sweden | Em/Egsl | 0/2 | 0/22a | 0/1a | 0/2,573,160a | |||||||||
| United Kingdomd | Egsl | 0/703 | ||||||||||||
| Total EU | 869/6,326 (13.737%) | 0/325 | 0/39 | 5/2,113 (0.237%) | 1/741 (0.135%) | 2/22 (9.091%) | 0/1,074 | 1/1 (100%) | 0/7 | 0/690 | 16,156/47,617,145 (0.034%) | |||
| Norwayc,d | Egsl | 0/543 | ||||||||||||
| Switzerland | Em | 31/78 (39.744%) | 2/4 (50%) | 13/40 (32.500%) | 0/1 | 2/2 (100%) | 0/1 | 1/1 (100%) | 7/7 (100%) | |||||
| Total EFTA | 31/621 (4.992%) | 2/4 (50%) | 13/40 (32.500%) | 0/1 | 2/2 (100%) | 0/1 | 1/1 (100%) | 7/7 (100%) | ||||||
| Total EU + EFTA | 900/6,947 (12.955%) | 0/325 | 2/43 (4.652%) | 18/2,153 (0.836%) | 1/742 (0.135%) | 2/22 (9.091%) | 0/1,074 | 1/1 (100%) | 2/9 (22.222%) | 0/1 | 1/1 (100%) | 0/690 | 16,163/47,617,152 (0.034%) |
Positive samples from dogs, cats, wolves and pigs without Echinococcus species information reported, were mentioned in the table only for countries with known circulation of both E. multilocularis and E. granulosus sensu lato.
Presence in the country of Echinococcus multilocularis (Em) and/or Echinococcus granulosus sensu lato (Egsl).
Mainland Norway (Svalbard archipelago excluded where E. multilocularis was documented).
Member States listed in the Annex to Commission Implementing Regulation (EU) 2018/878 concerning the application of preventive health measures for the control of Echinococcus multilocularis infection in dogs.
In total, 13 MS and two non‐MS (Norway and Switzerland) reported 2019 monitoring data on 6,326 and 621 foxes examined for E. multilocularis, respectively. Seven MS and one non‐MS (Switzerland) reported a total of 12.9% positive samples: Czechia (21%), France (12.6%), Germany (15.2%), Hungary (4.8%), Luxembourg (19.1%), Poland (31.7%), Slovakia (16.1%) and Switzerland (39.7%). Czechia (N = 596) reported most infected foxes in Europe accounting for 68.6% of the positive findings.
In addition to foxes, E. multilocularis has been reported in 18 dogs (two from France, three from Slovakia and 13 from Switzerland), two wolves from Switzerland, one cat from France, two jackals from Hungary, one coypu from France and two beavers and one mouse from Switzerland.
In total, 19 MS and two non‐MS reported data from 113,761,312 domestic and wild animals tested for E. granulosus s.l. of which > 99% were domestic animals (sheep, cattle, goats, pigs, horses, water buffalos, dogs and cats) (Table 49). A large proportion of these data were obtained from domestic livestock during meat inspection at the slaughterhouse. Wild animals tested included deer, moose, mouflons, wild boar, other wild ruminants and wolves. Eleven MS reported in total 167,003 (0.15%) positive samples mainly from domestic animals. These positive samples reported by Bulgaria, Greece, Italy, Poland, Slovakia, Spain and the United Kingdom were mainly from small ruminants (sheep and goats; N = 131,850; 78.9%) ranging from 0.02% to 4.8% positives. There were 16,298 positive cattle (9.8% of animals positive for E. granulosus s.l.) reported by Bulgaria, Greece, Hungary, Italy, Romania, Slovakia, Spain and UK and 18,696 positive pigs (11.2% of animals positive for E. granulosus s.l.), of which 85.4% were reported by Poland.
Table 49.
Monitoring results of Echinococcus granulosus sensu lato in animals (domestic and wild), EU/EFTA, 2019
| Country | Presence of Em/Egslb | Sheep | Sheep and goats | Goats | Water buffalos | Cattle (bovine animals) | Pigs | Wolves | Cats | Dogs | Wild boars | Other wild ruminants | Deer | Reindeer | Domestic solipeds | Moose | Mouflons | Alpaca |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgiumc | Em | 0/840,654 | ||||||||||||||||
| Bulgaria | Egsl | 1,833/235,286 (0.779%) | 947/29,274 (3.235%) | 337/1196,086 (0.028%) | ||||||||||||||
| Cyprus | Egsl | 0/11 | ||||||||||||||||
| Denmarkc | Em | 0/464,000 | ||||||||||||||||
| Estonia | Em/Egsl | 0/9,329 | 0/231 | 0/34,215 | 0/558,717a | 0/10 | ||||||||||||
| Finland | Egsl | 0/63,684 | 0/844 | 0/267,408 | 0/1,821,782 | 9/38 (23.684%) | 0/239 | 0/1,884 | 6/73,714 (0.008%) | 0/1,096 | 1/274 | |||||||
| Greece | Egsl | 8,261/541,514 (1.526%) | 2,078/135,957 (1.528%) | 280/30,330 (0.923%) | 2/173,652 (0.001%) | 0/111 | ||||||||||||
| Hungary | Em/Egsl | 0/1 | 1/3 (3.333%) | 35/84 (41.677%) | ||||||||||||||
| Italy | Em/Egsl | 55,177/1,138,798 (4.845%) | 872/55,529 (1.570%) | 30/35,906 (0.084%) | 2973/2,355,527 (0.126%) | 143/5,124,363 (0.003%)a | 0/17a | 0/1a | 0/10a | 8/40,654 (0.020%) | 0/758 | 1/7,100 (0.014%) | ||||||
| Latvia | Em/Egsl | 0/31,362 | 0/323 | 0/75,078 | 1/513,361 (0.0002%) | 0/60 | ||||||||||||
| Luxembourgc | Em | 0/26,818 | ||||||||||||||||
| Malta | Egsl | 0/6,434 | ||||||||||||||||
| Poland | Em/Egsl | 692/70,281 (0.985%) | 15,959/21,513,924 (0.074%)a | |||||||||||||||
| Romania | Em/Egsl | 0/29 | 0/3 | 18/24 (75.000%) | 0/88a | 0/10a | ||||||||||||
| Slovakia | Em/Egsl | 17/76,422 (0.022%) | 0/42 | 1/38,360 (0.003%) | 54/707,081 (0.008%)a | 0/705a | ||||||||||||
| Slovenia | Em/Egsl | 0/12,530 | 0/1,369 | 0/116,495 | 0/259,406a | 0/1,172 | ||||||||||||
| Spain | Egsl | 33,173/4,707,070 (0.705%) | 8,590/895,128 (0.960%) | 11,003/1,751,928 (0.628%) | 2,165/32,600,761 (0.007%) | 68/76,791 (0.088%) | 0/3,799 | 45/206,058 (0.022%) | 0/1,438 | 0/563 | ||||||||
| Sweden | Em/Egsl | 0/251,950 | 0/1,388 | 0/432,770 | 0/2,573,160a | 0/22a | 0/1a | 0/19,136 | 0/6,863 | 0/47,557 | 0/1,840 | |||||||
| United Kingdom | Egsl | 21,134/25,146,178 (0.084%) | 23/4,534 (0.507%) | 1,102/4,076,597 (0.027%) | ||||||||||||||
| Total EU | 118,454/32,055,582 (0.370%) | 1,833/235,286 (0.779%) | 11,563/1,095,348 (1.056%) | 30/35,906 (0.084%) | 16,325/9,698,827 (0.168%) | 18,696/67,042,465 (0.028%) | 9/77 (11.688%) | 0/706 | 0/21 | 76/117,795 (0.065%) | 0/3,799 | 45/215,563 (0.021%) | 6/121,271 (0.005%) | 1/5,716 (0.017%) | 1/274 (0.365%) | 0/574 | ||
| Norway | Egsl | 0/1,178,000 | 0/27,700 | 0/304,400 | 0/1,622,000 | 0/18 | ||||||||||||
| Switzerlandc | Em | 0/1 | 0/1 | 0/4 | 0/1 | |||||||||||||
| Total EFTA | 0/1,178,000 | 0/1 | 0/304,400 | 0/1,622,000 | 0/18 | 0/1 | 0/4 | 0/1 | ||||||||||
| Total EU + EFTA | 118,454/33,233,582 (0.356%) | 1,833/235,286 (0.779%) | 11,563/1,123,050 (1.030%) | 30/35,906 (0.084%) | 16,325/10,003,227 (0.163%) | 18,696/68,664,465 (0.027%) | 9/96 (9.375%) | 0/707 | 0/21 | 76/117,799 (0.065%) | 0/3,799 | 45/215,563 (0.021%) | 6/121,271 (0.005%) | 1/5,716 (0.017%) | 1/274 (0.365%) | 0/574 | 0/1 |
Positive samples from dogs, cats, wolves and pigs without Echinococcus species information reported, were mentioned in the table only for MS with known circulation of both Echinococcus multilocularis and Echinococcus granulosus sensu lato.
Presence in the country of Echinococcus multilocularis (Em) and/or Echinococcus granulosus sensu lato (Egsl).
Reporting countries with known circulation of Echinococcus multilocularis only and that tested suitable hosts for Echinococcus granulosus sensu lato.
Belgium, Cyprus, Denmark, Estonia, Ireland, Malta, Slovenia and Sweden did not report any positive finding of E. multilocularis or E. granulosus s.l. Austria, Croatia, Lithuania, Netherlands and Portugal did not report any animal monitoring data for E. multilocularis or E. granulosus s.l.
It should be emphasised that positive samples from dogs, cats, wolves and pigs without species specification were only mentioned in Table 48 and/or Table 49 for countries with known circulation of both E. granulosus s.l. and E. multilocularis. In fact, countries that are endemic for AE (i.e. Italy, Poland, Slovakia and Switzerland) reported 16,163 Echinococcus spp. positive pigs but the species identification was only reported by Switzerland, identifying E. multilocularis in seven pigs. The three mentioned MS endemic for AE (northern Italy, Poland and Slovakia) are also co‐endemic for CE. Pigs are good hosts for E. granulosus s.l., while E. multilocularis metacestodes in pigs are abortive and their presence is often used as sentinel for the presence of this parasite as demonstrated in Switzerland (Meyer et al., 2020). Hungary and Latvia reported 35 and 1 positive pigs, respectively, identifying the E. granulosus s.l. species.
Figures 51 and 52 show for the period between 2015 and 2019, respectively, the cumulative proportion of positive samples from different IHs of E. granulosus s.l. and its geographical distribution in the EU. Sheep contributed 64.5% (2015–2019) of all positive samples and these were reported from a few countries with large animal populations (Bulgaria, Greece, Italy, Poland, Spain and the UK). Positive cattle (8.6%; 2015–2019) were mainly reported by Bulgaria, Italy, Greece, Romania, Spain and UK. Positive pigs (16.9%; 2015–2019) were mainly reported by Bulgaria, Italy, Poland and Spain.
Figure 51.

Proportion (%) Echinococcus granulosus s.l. test‐positive animals, by intermediate host species, EU, 2015–2019
- Total number of animals reported positive for Echinococcus granulosus s.l. was 977,697: number of positive sheep (N = 630,915), goats (N = 86,948), pigs (N = 165,572), cattle (N = 84,003), sheep and goats (N = 9,260), wild boars (N = 428), water buffalos (N = 406), domestic solipeds (N = 33), deer (N = 98), reindeer (N = 25), moose (N = 7) and mouflons (N = 2). Positive pigs could be overestimated in co‐endemic countries with Echinococcus multilocularis.
Figure 52.
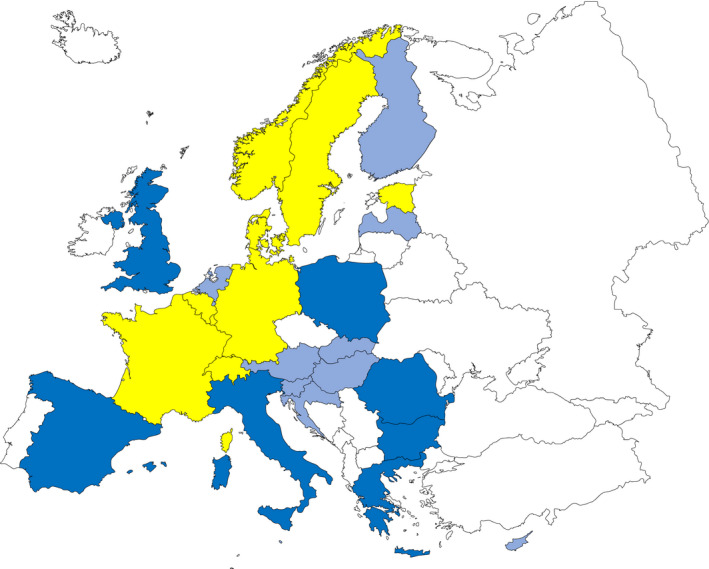
Proportion (%) Echinococcus granulosus s.l. test‐positive animals, by intermediate host species, EU, 2015–2019
-
Intermediate hosts included in map are cattle, deer, goats, horses, moose, mouflons, pigs, reindeer, sheep, water buffalos and wild boars.Legend: dark blue ≥ 500 positive cases; light blue < 500 cases; yellow = 0 cases reported; white = data not reported. Because of the co‐endemicity with Echinococcus multilocularis, pigs were excluded from Latvia, Hungary, Poland, Germany, Slovakia and Switzerland when Echinococcus species information was not reported.
As shown in Figure 52, Bulgaria, Greece, Italy, Poland, Romania, Spain and UK were the most endemic countries for Echinococcus granulosus s.l. in Europe during the period 2015–2019.
8.4. Discussion
The two parasitic diseases in humans, CE and AE, caused by E. granulosus s.l. and E. multilocularis, respectively, can be reported separately to ECDC TESSy database even though the EU case definition ‘echinococcosis’ does not differentiate between these two diseases. Most MS reported species information from 2008 to 2019. In addition, in 2018 and 2019, a few countries reported clinical presentation, which differentiates the two forms of the disease. Since the beginning of the surveillance of human echinococcosis in the EU in 2007, CE has been more frequently reported than AE, as expected by data reported in the scientific literature for Europe. The EU notification rate of confirmed human echinococcosis cases was stable and the trends for infections caused by E. granulosus s.l. and E. multilocularis did not show any significant increase or decrease in the last five years since 2015. In a few countries, the increase in the number of cases in the last few years could be explained by intensified surveillance and improved notification system for echinococcosis. The raised awareness of the disease among clinicians and immigration (people from endemic countries) may also have influenced the number of diagnosed cases in some countries (Richter et al., 2019). Distinction between infection with E. granulosus s.l. and E. multilocularis is needed because the two diseases require different clinical management and strategies for control. It should be also emphasised that the true prevalence of these diseases is extremely difficult to estimate due to the long incubation period (AE and CE), the high proportion of asymptomatic or paucisymptomatic carriers who never seek medical attention (CE) and the underreporting/misdiagnosed cases (AE and CE), factors, which contribute to their neglected status (Casulli, 2020). For these reasons, the patchy data reported by MS on the number of people with echinococcosis, currently represent the ‘tip of the iceberg’ of infections. The invisible portion includes asymptomatic carriers of CE and misdiagnosed cases of AE (Kern et al., 2017).
In animals, in 2019, 19 MS reported monitoring data on E. granulosus s.l., aetiological agent of CE and E. multilocularis, aetiological agent of AE. The highest number of animals infected with E. granulosus s.l. was reported in Bulgaria, Spain, Greece, Poland, Italy and UK and mainly observed in sheep. The highest number of animals (mainly foxes) infected with E. multilocularis was reported in Czechia, France, Germany, Hungary, Luxembourg, Poland, Slovakia and Switzerland. The surveillance of E. multilocularis in foxes is important to assess the prevalence in Europe, as the geographical distribution of E. multilocularis seems to have widened in the last decades. Whether the increased geographical distribution of E. multilocularis is due to an increased fox population in Europe (Oksanen et al., 2016), or to the expansion of their habitat to urban areas (Deplazes et al., 2004) or whether it reflects an increased surveillance effort, is difficult to disentangle, as there is a general lack of baseline data and standardised detection methods. Also, in animals, notification is a requirement for reliable data and information on parasite speciation is very important for risk management efforts as E. granulosus s.l. and E. multilocularis have a different epidemiology and pose different health risks for humans (Casulli, 2020). For E. granulosus s.l., a notification requirement would ensure that comparable data between MS are obtained from meat inspection of food‐producing animals. For E. multilocularis, a general notification requirement for all MS can be questioned, but it is required in countries free from this parasite, according to EU Regulation (EU) 2018/772.
In general, animal and human findings from 2019 seem similar to those of recent years. It should be emphasised that findings from most endemic countries fluctuated between years, but they reported positive findings in animals and humans in most years. Fluctuations in reported numbers of infected animals are probably associated with investigational efforts performed in a particular year, rather than reflecting a change in true prevalence. Moreover, it is unclear how the COVID‐19 pandemic is impacting on the diagnosis and notification of these chronic parasitic diseases at European level.
8.5. Related projects and Internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Fact sheet on echinococcosis | https://www.cdc.gov/parasites/echinococcosis/index.html |
| ECDC Surveillance Atlas of Infectious Diseases | http://ecdc.europa.eu/en/data-tools/atlas/Pages/atlas.aspx | |
| EU case definition of echinococcosis | https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions | |
| Disease Programme on Emerging, Food‐ and Vector‐Borne Diseases | https://www.ecdc.europa.eu/en/about-us/who-we-are/units/disease-programmes-unit | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) | https://doi.org/10.2903/j.efsa.2018.5495 | |
| World Health Organisation – Echinococcosis fact sheet | http://www.who.int/echinococcosis/en/ | |
| New approach needed to tackle parasitic liver disease in Europe and Turkey | http://www.who.int/neglected_diseases/news/new-approach-needeed-to-tackle-echinococcosis-europe/en/ | |
| Prevalence of abdominal cystic echinococcosis in rural Bulgaria, Romania and Turkey: a cross‐sectional, ultrasound‐based, population study | https://www.sciencedirect.com/science/article/pii/S1473309918302214?via%3Dihub | |
| Human cystic Echinococcosis ReseArch in CentraL and Eastern Societies (HERACLES project) | http://www.heracles-fp7.eu/index.html | |
| European Register of Cystic Echinococcosis (ERCE) | http://www.heracles-fp7.eu/erce.html | |
| Humans and animals | WHO/OIE Manual on Echinococcosis in Humans and Animals: a Public Health Problem of Global Concern | http://apps.who.int/iris/bitstream/10665/42427/1/929044522X.pdf |
| OIE Manual, Chapter 3.1.6. Echinococcosis (infection with Echinococcus granulosus and with E. multilocularis) | https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.06_ECHINOCOCCOSIS.pdf | |
| COMMISSION DELEGATED REGULATION (EU) No 1152/2011 (preventive health measures for the control of Echinococcus multilocularis infection in dogs) | http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1152 | |
| European Union Reference Laboratory for Parasites (humans and animals) | http://www.iss.it/crlp/ | |
| Animals | EFSA Scientific Opinion: Echinococcus multilocularis infection in animals (Panel on Animal Health and Welfare) | http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2015.4373/pdf |
| EFSA External Scientific Report: Echinococcus multilocularis infection in animals GP/EFSA/AHAW/2012/01 | http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2015.EN-882/pdf | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports | |
| MEME: Multi‐centre study on Echinococcus multilocularis and Echinococcus granulosus s.l. in Europe: development and harmonisation of diagnostic methods in the food chain (One Health EJP) | https://onehealthejp.eu/jrp-meme/ |
Food‐borne outbreaks (according to Directive 2003/99/EC)
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
1. Key facts
During 2019, 27 Member States reported 5,175 food‐borne outbreaks involving 49,463 cases of illness, 3,859 hospitalisations and 60 deaths. In addition, 117 outbreaks, 3,760 cases of illness and 158 hospitalisations were communicated by six non‐MS.
The health impact of food‐borne outbreaks in the EU was important in 2019 since 60 outbreak‐related deaths were reported; 20 more fatal cases than in 2018 (+50%).
A high number of deaths (N = 10) were registered in community settings such as ‘residential institution (nursing home or prison or boarding school)’. In addition, nearly 19% of cases involved in strong‐evidence outbreaks (2,407 cases) were exposed to contaminated foods in schools or kindergartens. These findings highlighted the need for attention to the high risk of vulnerable populations to food‐borne hazards.
The health burden of outbreaks caused by Listeria monocytogenes in the EU was remarkable since this agent was responsible for 349 cases of illness and more than 50% of total outbreak associated deaths (31 deaths; 10 deaths more than in 2018; 29 more than in 2017). Most of the deaths were due to the consumption of meat and meat products. The number of outbreaks, cases and hospitalisations associated with L. monocytogenes infection in the EU has continuously increased over the last four years.
Salmonella was the agent most identified in food‐borne outbreaks (N = 926), accounting for 17.9% of total outbreaks. Salmonella also caused the highest number of hospitalisations (n = 1,915; 49.6% of all outbreak‐associated hospitalisations). The number of notified outbreaks caused by S. Enteritidis (N = 439) reduced importantly in 2019 and was less than half of the number reported in 2018 (596 outbreaks less; 57.6% decrease). A reduction in the number of reported cases and hospitalisations associated with S. Enteritidis outbreaks was also observed in 2019 compared with 2018 (22.4% cases and 21.5% hospitalisations less than in 2018). Missing outbreak data from one MS, Slovakia, may have contributed to this drop.
At the EU level, the consumption of food of animal origin (‘fish and fishery products’, ‘eggs and egg products’, ‘meat and meat products’, ‘milk and milk products’) was associated with most of the food‐borne strong‐evidence outbreaks.
Outbreaks associated with the consumption of ‘crustaceans, shellfish, molluscs and products thereof’ increased markedly in the EU (by 80 outbreaks; 101.3% more than in 2018) even if this rise was entirely attributable to France which reported 129 outbreaks (81.1% of total outbreaks in the EU). Norovirus in ‘fish and fishery products’ was the agent/food pair causing the highest number of strong‐evidence outbreaks in the EU.
Salmonella in ‘mixed food’, norovirus in ‘fish and fishery products’ and Salmonella in ‘eggs and egg products’ were the agent/food pairs that caused the highest number of cases. Pairs with Salmonella in different types of food (‘eggs and egg products’, ‘mixed foods’, ‘meat and meat products’, ‘bakery products’, ‘buffet meals’) caused the highest numbers of hospitalisations.
Mixed foods (i.e. food resulting from mixing together multiple ingredients in the same preparation) were the foodstuff most frequently consumed by outbreaks cases. These mixed foods were associated with a wide range of causative agents including bacteria, viruses, bacterial toxins and histamine.
The number of outbreaks implicating food of non‐animal origin (FNAO) reported in 2019 was similar to those reported in recent years. Outbreaks by FNAO (mainly vegetables) were larger, on average, compared with outbreaks caused by food of animal origin and were associated with the widest variety of causative agents, mainly norovirus, Salmonella, Bacillus cereus and Cryptosporidium.
2. Surveillance and monitoring of food‐borne outbreaks in the EU
According to Directive 2003/99/EC, reporting information on food‐borne and waterborne outbreaks (FBOs) is mandatory for EU Member States (MS). EFSA is assigned the tasks of collecting, analysing and describing the data. The aim is to support characterising the epidemiology and the health impact of FBOs in the EU in the current year and the relative time trends. The main focus of the analysis is to provide a thorough description of the causative agents and the foodstuffs implicated in the FBOs, as well as to document the circumstances, the events and the potential risk factors that underlie the contamination of foodstuffs and the occurrence of the outbreaks. These data are collected annually by MS and reported to EFSA according to the standard defined by the EFSA Network for Zoonoses Monitoring Data and described in an updated technical document issued each year by EFSA (EFSA, 2020a,b). The current system is known as European Union Food‐borne Reporting System (EU‐FORS) and has been implemented since 2010.
The data collection includes any outbreaks deemed to implicate the consumption of food (including water) contaminated by either bacteria, viruses, parasites, algae, fungi and their products, such as toxins and biological amines (e.g. histamine). The reporting is not limited to the causative agents whose transmission to humans occurs primarily through food (e.g. Salmonella, L. monocytogenes), but also includes agents for which the food‐borne transmission is possible but usually accidental. Outbreaks caused by ingestion of drinking water are also deemed food‐borne as drinking water is defined as a food in Regulation 178/2002/EC.
Outbreaks are categorised as having ‘strong‐evidence’ or ‘weak evidence’ based on the strength of evidence implicating a suspected food vehicle as the cause of the outbreak (EFSA, 2014). The strength of evidence is a qualitative measure of the level of uncertainty which affects the likelihood that a food item is the vehicle of the outbreak. For strong‐evidence outbreaks, MS shall report a detailed data set describing the implicated food vehicle, contributory factors and source, whereas for weak‐evidence outbreaks this reporting is not compulsory. The evaluation of the strength of evidence implicating a suspected food vehicle in FBOs is based on the assessment of all available types of evidence related to illness and exposure information (i.e. microbiological, epidemiological, descriptive, environmental, based on traceability (tracing back/forward) of the investigated foodstuffs) and according to the EU‐FORS guidance and the last published manual for reporting on food‐borne and waterborne outbreaks (EFSA, 2014, 2020a,b).
Although the data reporting rules follow the same EFSA standard specifications as described above in all MS, the surveillance activities of FBOs are not fully harmonised. Differences in sensitivity and type of outbreaks under surveillance exist. For this reason, the difference in the numbers and types of reported FBOs, as well as in the causative agents and the type of outbreaks may not necessarily reflect the level of food safety in the MS. A description of the system in place for outbreak surveillance and reporting in the reporting countries is in the national zoonoses reports submitted in accordance with Directive 2003/99/EC, which are published on the EFSA website together with the EU One Health Zoonoses Report and are available online at http://www.efsa.europa.eu/en/biological-hazards-data/reports.
3. Data analyses
Key summary statistics for all reported FBOs are summarised in figures and tables. The impact of FBOs on public health is described in terms of total number of outbreaks and reporting rate (per 100,000 population), number of cases (of illnesses), number of hospitalisations (% of hospitalisation), number of deaths (% deaths), mean outbreak size (cases per outbreak) and range of cases per outbreak (minimum and maximum).
To limit the level of uncertainty, the description of food vehicles implicated in FBOs, the settings (places of exposure to contaminated food) and the risk factors refers to strong‐evidence food‐borne outbreaks only. However, the pattern of suspected food vehicles and settings is also summarised separately for weak‐evidence FBOs, based on the detailed data set that MS can report also for this type of FBO.
Causative agents, food vehicles and outbreak settings are summarised using multi‐level hierarchical categorisation to optimise the description of the findings. A priority is given to the description of FBOs caused by agents included in Annex IA of the Dir. 99/2003/CE (Brucella, Salmonella, Campylobacter, Listeria monocytogenes, Shiga toxin‐producing E. coli and Trichinella), as they are considered top‐priority pathogens at the EU level. Causative agents listed under Annex IB of the same Directive, with major epidemiological relevance (Calicivirus, hepatitis A virus, botulism and agents thereof, Yersinia, Cryptosporidium) were also described distinctly. The other causative agents are described either separately, where possible, or in homogeneous groups by type of agent. In this latter case, the agents included in each group are listed in tables and figures footnotes. Unknown agents are described separately.
Causative agents implicated in FBOs are grouped according either to taxonomy or the pathogenic mechanisms triggering illness in humans. In some circumstances (i.e. missing information) or to adjust the agents categorisation, further criteria have been applied as follows: any E. coli other than ‘Shiga toxin‐producing E. coli (STEC)’, have been categorised into a single ‘E. coli other than STEC’ group; ‘Bacillus cereus enterotoxins’ and ‘B. cereus’ were grouped into ‘B. cereus’ group; ‘Staphylococcus aureus’’, ‘Staphylococcus unspecified’ and ‘Staphylococcal enterotoxins’ have been grouped into the ‘S. aureus’ group together; ‘Clostridium unspecified’ and ‘C. perfringens’ were grouped into the ‘C. perfringens’ group; histamine and scombrotoxin have been grouped; ‘Calicivirus, unspecified’, ‘norovirus’ and ‘sapovirus’ have been grouped into the ‘norovirus and other Caliciviruses’ group; ‘hepatitis, unspecified’ and ‘hepatitis A’ have been grouped into ‘hepatitis A and other hepatitis virus, unspecified’ group.
Food vehicles have been grouped according to the general criteria adopted by EFSA for presenting data in this report. Places of exposures have been grouped according to the general characteristics and level of risk connected to the setting and the process behind food preparation.
In tables and figures, sums and proportions (%) are the basic statistics used to describe the reported counts (numbers) of outbreaks. The mean annual rate of reported outbreaks per 100,000 population (‘outbreak reporting rate’) is calculated to compare MS independently on demographic size and its variations over time. Data on resident population from Eurostat were used for this purpose (updated on 1 January 2020). Populations of MS not providing data on FBOs were excluded from this calculation.
Variations over time are described by comparison with different time frames. Data on food‐borne and waterborne outbreaks for 2018 differ from those published in the European Union One Health 2018 Zoonoses Report, due to a delay in reporting from one MS (the Netherlands). Short‐term variations are shown as absolute and relative (%) 2019/2018 difference. Long‐term variations are also described using years 2010–2019 as the comparative period. Frequency distributions and trends are visualised at the EU level. However, trend analysis is only performed at the single MS level, according to the rationale described in Boelaert et al. (2016) for data quality. Time trends have been tested for statistical significance over the period 2010–2019 using the Cox‐Stuart sign test, a non‐parametric test appropriate for limited numbers of observations (10 years at the maximum). P value < 0.05 was considered to identify a statistically significant trend, beyond chance. However, the detection of significant trends at the country level should be interpreted with caution since changes in the reporting specifications for FBOs were introduced in 2014 (EFSA, 2014). Sankey diagrams, which are available as supporting documents from the EFSA knowledge junction at zenodo (see link in the beginning of this chapter), were produced using the free software R version 3.5.3 (GNU project r‐project.org).
When the UK data were collected, the UK was an EU MS but as of 31 January 2020, it has become a third country.
4. Results and discussion
4.1. Overview of countries reporting food‐borne outbreak data, 2019
During 2019, 27 MS reported 5,175 FBOs, 49,463 cases of illness, 3,859 hospitalisations and 60 deaths. In addition, 117 FBOs, 3,760 cases of illness and 158 hospitalisations were communicated by six non‐MS (Iceland, Montenegro, Norway, Republic of North Macedonia, Serbia, Switzerland). Slovakia did not report data on FBOs.
The total number of outbreaks reported by each MS in 2019 varied importantly, with a small number of MS reporting most of the outbreaks. Altogether, FBOs reported by five countries (Belgium, France, the Netherlands, Poland and Spain) accounted for more than three‐quarters of total outbreaks (4,042 outbreaks; 78.1% of all outbreaks) and more than two‐thirds of total cases observed in the EU in 2019 (32,883 cases; 66.5% of all cases). The breakdown of FBOs by countries and by strength of evidence is reported in Table 50. In this table, the ‘outbreak reporting rate’ (per 100,000 population) describes how frequent was the reporting of FBOs in 2019, in EU/EFTA countries, regardless of the differently sized populations. The range of this value was huge, from 0.04 (Romania) to 9.12 (Malta) outbreaks (per 100,000 population) corresponding to a 253‐fold difference. The ‘mean outbreak size’ (i.e. the mean number of cases per outbreak) and the range of cases per outbreak is shown to characterise the pattern of FBOs reported to EFSA by MS and non‐MS. Altogether, these indicators provide evidence of the large variability among MS in the sensitivity of surveillance and the type of FBOs being monitored in each MS. As an example, household outbreaks (i.e. outbreaks in which all the human cases live in one single household) are usually small‐sized outbreaks. As not all MS report household outbreaks to EFSA, this may influence the mean outbreak size as well as the number of outbreaks. Details on the type of FBOs reported to EFSA, by country, is visualised in Figure 53.
Table 50.
Number of food‐borne outbreaks, human cases, hospitalisations and deaths, in reporting EU MS and non‐MS, 2019
| Country | Strong‐evidence outbreaks | Weak‐evidence outbreaks | Total outbreaks | Total cases | Mean outbreak size (cases/outbreak) and range (min–max) | Outbreak reporting rate (mean) per 100,000 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Cases | Hospitalised | Deaths | N | Cases | Hospitalised | Deaths | N | % of total | N | % of total | 2019 | 2010–2018 | ||
| Austria | 4 | 327 | 121 | 0 | 44 | 466 | 38 | 1 | 48 | 0.9 | 793 | 1.6 | 16.5 (2–350) | 0.54 | 1.38 |
| Belgium | 2 | 206 | 3 | 0 | 569 | 2,251 | 25 | 0 | 571 | 11.0 | 2,457 | 5.0 | 4.3 (2–203) | 4.98 | 2.80 |
| Bulgaria | 4 | 50 | 21 | 0 | 12 | 100 | 29 | 0 | 16 | 0.3 | 150 | 0.3 | 9.4 (4–19) | 0.23 | 0.18 |
| Croatia | 6 | 40 | 9 | 0 | 40 | 446 | 35 | 0 | 46 | 0.9 | 486 | 1.0 | 10.6 (2–96) | 1.13 | 1.15 |
| Cyprus | 0 | 0 | 0 | 0 | 2 | 24 | 5 | 0 | 2 | 0.0 | 24 | 0.0 | 12.0 (4–20) | 0.23 | 0.35 |
| Czechia | 6 | 416 | 56 | 0 | 18 | 475 | 20 | 0 | 24 | 0.5 | 891 | 1.8 | 37.1 (9–245) | 0.23 | 0.23 |
| Denmark | 16 | 707 | 1 | 0 | 35 | 1,230 | 1 | 0 | 51 | 1.0 | 1,937 | 3.9 | 38.0 (3–268) | 0.88 | 1.11 |
| Estonia | 2 | 12 | 9 | 0 | 11 | 39 | 17 | 0 | 13 | 0.3 | 51 | 0.1 | 3.9 (2–8) | 0.98 | 0.97 |
| Finland | 18 | 325 | 13 | 0 | 36 | 631 | 25 | 3 | 54 | 1.0 | 956 | 1.9 | 17.7 (3–94) | 0.98 | 0.90 |
| France | 232 | 2,796 | 106 | 12 | 1,553 | 12,881 | 495 | 3 | 1,785 | 34.4 | 15,677 | 31.7 | 8.8 (2–593) | 2.66 | 2.01 |
| Germany | 33 | 684 | 137 | 0 | 369 | 1,286 | 248 | 5 | 402 | 7.8 | 1,970 | 4.0 | 4.9 (2–75) | 0.48 | 0.50 |
| Greece | 2 | 696 | 33 | 0 | 4 | 111 | 6 | 0 | 6 | 0.1 | 807 | 1.6 | 134.5 (11–638) | 0.06 | 0.10 |
| Hungary | 16 | 1,135 | 150 | 0 | 19 | 869 | 17 | 0 | 35 | 0.7 | 2,004 | 4.1 | 57.3 (2–575) | 0.36 | 1.07 |
| Ireland | 0 | 0 | 0 | 0 | 25 | 193 | 6 | 0 | 25 | 0.5 | 193 | 0.4 | 7.7 (2–55) | 0.51 | 0.51 |
| Italy | 45 | 512 | 124 | 3 | 90 | 960 | 149 | 0 | 135 | 2.6 | 1,472 | 3.0 | 10.9 (2–199) | 0.22 | 0.32 |
| Latvia | 10 | 241 | 15 | 0 | 23 | 322 | 41 | 0 | 33 | 0.6 | 563 | 1.1 | 17.1 (2–51) | 1.72 | 12.7 |
| Lithuania | 1 | 23 | 1 | 0 | 54 | 312 | 195 | 0 | 55 | 1.1 | 335 | 0.7 | 6.1 (2–39) | 1.97 | 3.41 |
| Luxembourg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | 0 | 0.44 |
| Malta | 0 | 0 | 0 | 0 | 45 | 167 | 11 | 0 | 45 | 0.9 | 167 | 0.3 | 3.7 (2–18) | 9.12 | 8.50 |
| Netherlands | 8 | 232 | 36 | 6 | 727 | 2,826 | 6 | 0 | 735 | 14.2 | 3,058 | 6.2 | 4.2 (2–55) | 4.25 | 2.40 |
| Poland | 102 | 1,593 | 259 | 2 | 343 | 4,006 | 697 | 1 | 445 | 8.6 | 5,599 | 11.3 | 12.6 (2–237) | 1.17 | 1.22 |
| Portugal | 1 | 60 | 0 | 0 | 12 | 364 | 23 | 0 | 13 | 0.3 | 424 | 0.9 | 32.6 (2–138) | 0.13 | 0.14 |
| Romania | 5 | 218 | 85 | 0 | 2 | 29 | 17 | 0 | 7 | 0.1 | 247 | 0.5 | 35.3 (3–160) | 0.04 | 0.10 |
| Slovenia | 1 | 94 | 48 | 0 | 0 | 0 | 0 | 0 | 1 | < 0.1 | 94 | 0.2 | 94.0 (94–94) | 0.05 | 0.30 |
| Spain | 153 | 2,002 | 293 | 4 | 353 | 4,090 | 141 | 5 | 506 | 9.9 | 6,092 | 12.3 | 12.0 (2–207) | 1.08 | 1.10 |
| Sweden | 29 | 844 | 6 | 0 | 36 | 732 | 2 | 0 | 65 | 1.3 | 1,576 | 3.2 | 24.2 (2–150) | 0.64 | 2.84 |
| United Kingdom | 20 | 473 | 41 | 11 | 37 | 967 | 43 | 4 | 57 | 1.1 | 1,440 | 2.9 | 25.3 (2–152) | 0.09 | 0.10 |
| EU Total | 716 | 13,686 | 1,567 | 38 | 4,459 | 35,777 | 2,292 | 22 | 5,175 | 100 | 49,463 | 100 | 9.6 (2–638) | 1.02 | 1.08 |
| Iceland | 2 | 39 | 6 | 0 | 1 | 9 | 2 | 0 | 3 | – | 48 | – | 16.0 (9–24) | 0.84 | 1.56 |
| Montenegro | 1 | 14 | 0 | 0 | 2 | 83 | 0 | 0 | 3 | – | 97 | – | 32.3 (14–53) | 0.48 | 1.55 |
| Norway | 17 | 2,368 | 1 | 0 | 29 | 330 | 13 | 0 | 46 | – | 2,698 | – | 58.7 (2–2000) | 0.86 | 1.00 |
| Rep. of North Macedonia | 4 | 59 | 23 | 0 | 1 | 16 | 4 | 0 | 5 | – | 75 | – | 15.0 (3–29) | 0.24 | 1.40 |
| Serbia | 28 | 479 | 93 | 0 | 9 | 32 | 10 | 0 | 37 | – | 511 | – | 13.8 (2–91) | 0.53 | 0.77 |
| Switzerland | 5 | 94 | 1 | 0 | 18 | 237 | 5 | 0 | 23 | – | 331 | – | 14.4 (2–90) | 0.27 | 0.12 |
Figure 53.

Distribution of food‐borne outbreaks, by type of outbreak and country, in reporting EU MS and non‐MS, 2019
- Note: both strong‐evidence outbreaks and weak‐evidence outbreaks are considered in the figure.
The overall distribution of FBOs and outbreak cases reported by MS during 2010–2019 are plotted in Figures 54 and 55, respectively. For 2018, the numbers included in Figure 54 differs from those published in the European Union One Health 2018 Zoonoses Report, due to a delay in FBOs data reporting from one MS (the Netherlands). In 2019, the number of outbreaks reported in the EU was lower than in 2018 (727 outbreaks less; 12.3% less than in 2018). Cases of illness and hospitalisations also dropped, even if with different proportions. Cases decreased by 3.3% (1,708 cases less than in 2018) and hospitalisations by 20.0% (962 hospitalisations less than in 2018). The lack of 2019 FBOs data reporting from Slovakia may have substantially contributed to the reduction since this country had reported 522 outbreaks, 2,454 cases and 531 hospitalisations per year, on average in the five preceding years.
Figure 54.

Number of food‐borne outbreaks, by strength of evidence, in reporting EU MS, 2010–2019
-
Note: the number of MS reporting outbreaks is indicated in the bottom (N).Data on food‐borne outbreaks for 2018 differ from those published in the European Union One Health 2018 Zoonoses Report, due to a delay in reporting from one MS (the Netherlands).
Figure 55.
Number of illness cases in food‐borne outbreaks in reporting EU MS, 2010–2019
-
Note: the number of MS reporting outbreaks is indicated in the bottom (N).Cases involved in both strong‐evidence outbreaks and weak‐evidence outbreaks are counted in the figure.Data on food‐borne outbreaks for 2018 differ from those published in the European Union One Health 2018 Zoonoses Report, due to a delay in reporting from one MS (the Netherlands).
The health impact of FBOs in 2019 was remarkable since 60 outbreak‐related deaths were reported, 20 more fatal cases than in 2018 (50% more than in 2018). France and the United Kingdom reported each 15 deaths among outbreak cases which represents an important increase compared with the previous five years (3.8 and 4.2 mean deaths per year, in France and the United Kingdom, respectively). In France, 10 deaths were reported in outbreaks that occurred in a ‘residential institution (nursing home or prison or boarding school)’. These data call for attention to the increased risk of vulnerable populations to food‐borne hazards. In the United Kingdom, deaths were less clearly linked to specific settings. Most deaths were single cases involved in general dispersed outbreaks. However, seven deaths were reported from a single outbreak in hospital setting, which raises again the issue of the increased susceptibility to food‐borne hazards of vulnerable patients. Spain also reported a high number of deaths (N = 9) among outbreaks cases. Three of them were linked to large nation‐wide outbreaks by L. monocytogenes.
In 2019, strong‐evidence outbreaks (N = 716) were reported by 23 MS (all MS reporting data on FBO except Cyprus, Ireland and Malta) and accounted altogether for 13.8% of all outbreaks, which represents the highest proportion since 2010 (Table 50). At country level this proportion varied widely. For eight MS (Finland, Greece, Hungary, Italy, Romania, Slovenia, Sweden, the United Kingdom) strong‐evidence outbreaks accounted for more than a third of total reported FBOs. Interestingly, these MS also reported the smallest proportion of household outbreaks (Figure 53). In six MS (Austria, Belgium, Germany, Lithuania, the Netherlands, Portugal), strong‐evidence outbreaks did not exceed 10% of total FBOs. In addition, 57 strong‐evidence outbreaks were reported by the non‐MS countries which communicated to EFSA data on FBOs for 2019.
The annual variations (%) in the outbreak reporting rate at the EU and MS level are plotted in Figure 56. As the % variation is a relative measure of the increase or decrease in the frequency of FBOs reporting in 2019 compared with 2018, the figure allows a direct comparison between MS, regardless of the characteristics of FBOs surveillance.
Figure 56.
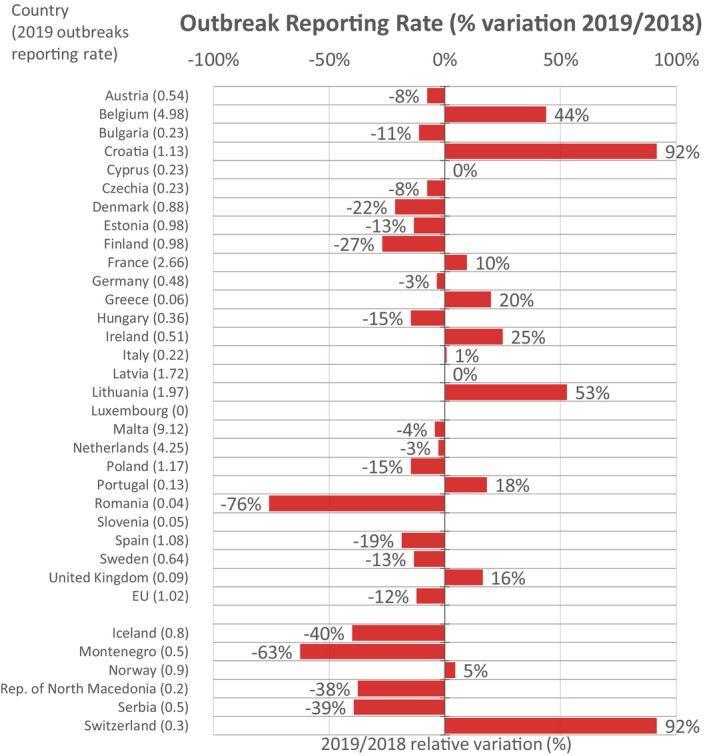
Yearly relative variation (%) of food‐borne outbreaks reporting rate (per 100,000 population) in 2019 compared with 2018, by country, in reporting EU MS and non‐MS
- For Slovenia the % variation cannot be calculated due to missing data reporting for 2018. Slovakia did not report data on outbreaks in 2019.
Seventeen MS (Austria, Bulgaria, Cyprus, Czechia, Estonia, France, Germany, Hungary, Italy, Latvia, Malta, the Netherlands, Poland, Portugal, Spain, Sweden, the United Kingdom) reported small variations with the outbreak reporting rate remaining relatively stable (i.e. below 20% increase). Eight MS reported large variations (≥ 20%) either increasing (Belgium, Croatia, Greece, Ireland, Lithuania) or decreasing (Denmark, Finland, Romania). Information provided by the reporting MS in their national zoonoses report21 may help understand whether recent changes in the FBOs surveillance might have contributed to these variations. In some MS, improvements of procedures for outbreak detection and investigation and/or increased awareness (sensitivity) of consumers may be the likely reasons for such rise.
Over the longer period (2010–2019), eight MS (Austria, Denmark, France, Hungary, Latvia, Lithuania, the Netherlands, the United Kingdom) and one non‐MS (Norway) reported statistically significant variations in the rate of outbreak reporting (Figure 57). Although these trends should be interpreted with caution for the reasons explained above in Section 3, it is important to disclose the country‐specific pattern of causative agents being monitored in outbreaks (Section 4.2) and their relative dynamics over time (Section 4.66), to unravel the components underlying these trends In Austria, Hungary and Lithuania outbreak trends are mainly influenced by specific agents’ variations over time, in particular Salmonella (section 4.66). For France and the United Kingdom, this is less evident. Trends observed for the Netherlands and to a lesser extent for Latvia seem to be driven by an increased reporting of small outbreaks of unknown aetiology.
Figure 57.
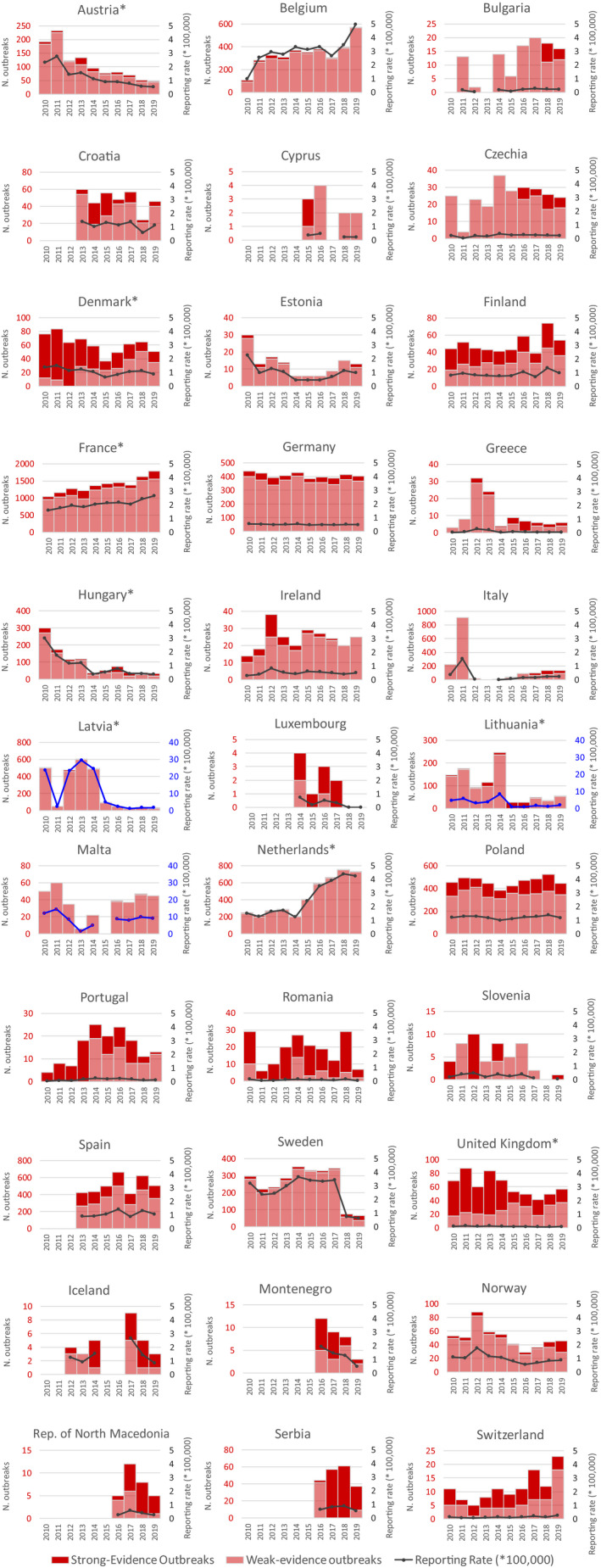
Trends of number of strong‐evidence and weak‐evidence outbreaks (left axis) and outbreak reporting rate (per 100,000) (right axis) in reporting EU MS and non‐MS, 2010–2019
-
Note: * indicates countries with a statistical significant trend (P < 0.05) over years.Blue colour for both the trend line and the secondary Y‐axis representing the FBO reporting rate was adopted for Latvia, Lithuania and Malta to highlight that the scale was different from the other countries. Slovakia did not report data on outbreaks in 2019.
The trends in the number of outbreaks reported by MS were mostly consistent with trends in the number of cases reported during 2010–2019 (data not shown), except for Austria and the United Kingdom. In Austria, 571 more cases than in 2018 were counted, corresponding to a 2.6‐fold increase. This rise was mainly due to two large general outbreaks caused by S. Enteritidis and norovirus in ‘eggs and egg products’, that each included more than 300 cases. In the United Kingdom, cases decreased over the years in parallel with the number of outbreaks, even if less markedly and with large yearly fluctuations.
4.2. Overview of causative agents in food‐borne outbreaks, 2019
In 2019, a causative agent was identified in 3,101 FBOs (59.9% of total outbreaks) causing 35,969 cases (72.7% of total cases), 3,290 hospitalisations (85.3% of total hospitalisations) and 54 deaths (90.0% of total deaths). Figure 58 shows the agents most frequently implicated in FBOs in the EU. For a high proportion of outbreaks (40.1%), the causative agent was ‘unknown’ or ‘unspecified’. The Netherlands (693 outbreaks), Belgium (554 outbreaks), France (288 outbreaks) and Spain (229 outbreaks) contributed most to this reporting (1,764 outbreaks altogether; 85.1% of outbreaks with ‘unknown’ or ‘unspecified’ causative agent). Bacteria were reported to have caused most outbreaks (N = 1,364; 26.4%) followed by bacterial toxins (N = 997; 19.3%), viruses (N = 554; 10.7%), other causative agents (N = 155; 3.0%) and parasites (N = 31; 0.6%).
Figure 58.
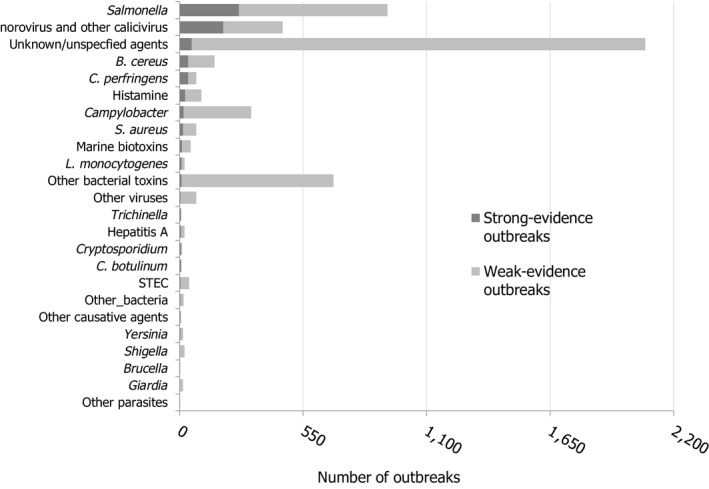
Distribution of strong‐ and weak‐evidence food‐borne outbreaks, per causative agent, in reporting EU MS, 2019
-
Note: Only FBOs reported by EU Member States are visualised in the figure. FBOs are sorted by number of strong‐evidence outbreaks.‘Hepatitis A’ includes also FBOs with causative agent encoded as ‘hepatitis virus, unspecified’. ‘Bacillus cereus’ includes FBOs with causative agent encoded as B. cereus enterotoxins. ‘Clostridium perfringens’ includes FBOs with causative agent encoded as Clostridium unspecified. ‘Staphylococcus aureus’ includes FBOs with causative agent encoded as Staphylococcus, unspecified’ or Staphylococcal enterotoxins. ‘Other bacteria’ includes Arcobacter butzleri, enteropathogenic Escherichia coli (EPEC), Enterotoxigenic Escherichia coli (ETEC), Escherichia coli, unspecified, Vibrio parahaemolyticus and other unspecified bacteria. ‘Other bacterial toxins’ includes FBOs by unspecified toxin‐producing bacteria.‘Other viruses’ includes adenovirus, flavivirus, hepatitis E virus, rotavirus and other viruses, unspecified. ‘Other causative agents’ includes atropine, mushroom toxins/mycotoxins and unspecified toxins.
Table 51 provides a detailed overview of the causative agents involved in FBOs and their overall impact on health in the EU in 2019. For each pathogens group and single causative agent, the proportion of hospitalisations and deaths among cases and the mean outbreak size facilitate description of the general characteristics of the FBOs and their impact on health. The highest proportion of hospitalisations and deaths were observed for outbreaks caused by bacteria. Salmonella was responsible for the highest number of hospitalisations (N = 1,915) and L. monocytogenes, alone, caused more than half of the fatal illnesses (N = 31). The number of deaths due to FBOs caused by L. monocytogenes doubled, compared with 2018 (10 deaths more than in 2018; 47.6% increase). Fatal cases also increased among outbreak cases caused by B. cereus (N = 7; 6 cases more than in 2018) mainly due to a single outbreak in France, with five fatal events reported among 17 cases. The breakdown of causative agents by countries is in Figure 59. Sankey diagrams by type of agent are included in the supplementary information.
Table 51.
Number of food‐borne outbreaks, human cases, hospitalisations and deaths, by causative agent, in reporting EU MS, 2019
| Type of agent | Outbreaks | Cases of illness | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strong‐evidence outbreaks | Weak‐evidence outbreaks | Total outbreaks | % of total | Reporting rate per 100,000 | Human cases | Mean outbreak size (cases/outbreaks) and range (min‐max) | Hospitalized | Deaths | ||||
| N | N | N | N | N | % of cases | N | % of cases | |||||
| Bacteria | Arcobacter | 0 | 1 | 1 | 0.0 | < 0.01 | 40 | 40.0 (2–40) | 0 | 0.0 | 0 | 0 |
| Brucella | 1 | 0 | 1 | 0.0 | < 0.01 | 2 | 2.0 (2–2) | 1 | 50.0 | 0 | 0 | |
| Campylobacter | 18 | 301 | 319 | 6.2 | 0.06 | 1,254 | 3.9 (2–91) | 125 | 10.0 | 0 | 0 | |
| Escherichia coli other than STEC | 3 | 7 | 10 | 0.2 | < 0.01 | 277 | 27.7 (4–130) | 9 | 3.2 | 0 | 0 | |
| Listeria monocytogenes | 9 | 12 | 21 | 0.4 | < 0.01 | 349 | 16.6 (2–207) | 236 | 67.6 | 31 | 8.9 | |
| Salmonella | 265 | 661 | 926 | 17.9 | 0.18 | 9,169 | 9.9 (2–575) | 1,915 | 20.9 | 7 | 0.1 | |
| Shigella | 2 | 20 | 22 | 0.4 | < 0.01 | 106 | 4.8 (2–20) | 19 | 17.9 | 0 | 0 | |
| STEC | 4 | 38 | 42 | 0.8 | 0.01 | 273 | 6.5 (2–29) | 50 | 18.3 | 1 | 0.4 | |
| Vibrio | 1 | 3 | 4 | 0.1 | < 0.01 | 15 | 3.8 (2–7) | 6 | 40.0 | 0 | 0 | |
| Yersinia | 3 | 12 | 15 | 0.3 | < 0.01 | 149 | 9.9 (2–37) | 14 | 9.4 | 0 | 0 | |
| Other bacteria, unspecified | 0 | 3 | 3 | 0.1 | < 0.01 | 33 | 11.0 (2–27) | 0 | 0 | 0 | 0 | |
| Subtotal | 306 | 1,058 | 1,364 | 26.4 | 0.27 | 11,667 | 8.6 (2–575) | 2,375 | 20.4 | 39 | 0.3 | |
| Bacterial toxins | B. cereus | 38 | 117 | 155 | 3.0 | 0.03 | 1,636 | 10.6 (2–141) | 44 | 2.7 | 7 | 0.4 |
| C. botulinum | 5 | 2 | 7 | 0.1 | < 0.01 | 17 | 2.4 (2–4) | 15 | 88.2 | 1 | 5.9 | |
| C. perfringens | 37 | 38 | 75 | 1.4 | 0.01 | 2,426 | 32.3 (3–268) | 27 | 1.1 | 3 | 0.1 | |
| S. aureus | 16 | 58 | 74 | 1.4 | 0.01 | 1,400 | 18.9 (2–380) | 141 | 10.1 | 0 | 0 | |
| Bacterial toxins, unspecified | 8 | 678 | 686 | 13.3 | 0.14 | 5,076 | 7.4 (2–264) | 134 | 2.6 | 3 | 0.1 | |
| Subtotal | 104 | 893 | 997 | 19.3 | 0.20 | 10,555 | 10.6 (2–380) | 361 | 3.4 | 14 | 0.1 | |
| Viruses | Adenovirus | 0 | 1 | 1 | 0.0 | < 0.01 | 8 | 8.0 (8–8) | 0 | 0 | 0 | 0 |
| Flavivirus including Tick–Borne Encephalitis virus | 2 | 1 | 3 | 0.1 | < 0.01 | 15 | 5.0 (2–8) | 12 | 80.0 | 0 | 0 | |
| Hepatitis A and other Hepatitis virus unspecified | 5 | 17 | 22 | 0.4 | < 0.01 | 135 | 6.1 (2–35) | 99 | 73.3 | 0 | 0 | |
| Hepatitis E | 0 | 3 | 3 | 0.1 | < 0.01 | 6 | 2.0 (2–2) | 1 | 16.7 | 0 | 0 | |
| Norovirus | 193 | 264 | 457 | 8.8 | 0.09 | 11,125 | 24.3 (2–638) | 279 | 2.5 | 0 | 0 | |
| Rotavirus | 0 | 8 | 8 | 0.2 | < 0.01 | 85 | 10.6 (7–20) | 51 | 60.0 | 0 | 0 | |
| Sapovirus | 0 | 1 | 1 | 0.0 | < 0.01 | 89 | 89.0 (89–89) | 0 | 0 | 0 | 0 | |
| Other viruses, unspecified | 1 | 58 | 59 | 1.1 | 0.01 | 764 | 12.9 (2–201) | 14 | 1.8 | 0 | 0 | |
| Subtotal | 201 | 353 | 554 | 10.7 | 0.11 | 12,227 | 22.1 (2–638) | 456 | 3.7 | 0 | 0 | |
| Parasites | Cryptosporidium | 5 | 6 | 11 | 0.2 | < 0.01 | 468 | 42.5 (2–122) | 4 | 0.9 | 0 | 0 |
| Giardia | 0 | 14 | 14 | 0.3 | < 0.01 | 233 | 16.6 (2–199) | 2 | 0.9 | 0 | 0 | |
| Trichinella | 5 | 0 | 5 | 0.1 | < 0.01 | 44 | 8.8 (3–14) | 12 | 27.3 | 0 | 0 | |
| Other parasites, unspecified | 0 | 1 | 1 | 0.0 | < 0.01 | 2 | 2.0 (2–2) | 0 | 0 | 0 | 0 | |
| Subtotal | 10 | 21 | 31 | 0.6 | 0.01 | 747 | 24.1 (2–199) | 18 | 2.4 | 0 | 0 | |
| Other causative agents | Histamine / Scombrotoxin | 25 | 71 | 96 | 1.9 | 0.02 | 428 | 4.5 (2–50) | 52 | 12.1 | 0 | 0 |
| Marine biotoxins | 10 | 38 | 48 | 0.9 | 0.01 | 214 | 4.5 (2–38) | 14 | 6.5 | 0 | 0 | |
| Mushroom toxins | 3 | 2 | 5 | 0.1 | < 0.01 | 43 | 8.6 (2–30) | 11 | 25.6 | 1 | 2.3 | |
| Other causative agent/Unspecified | 4 | 2 | 6 | 0.1 | < 0.01 | 88 | 14.7 (4–64) | 3 | 3.4 | 0 | 0 | |
| Subtotal | 42 | 113 | 155 | 3.0 | 0.03 | 773 | 5.0 (2–50) | 80 | 10.3 | 1 | 0.1 | |
| Unknown | Unknown / Unspecified | 53 | 2,021 | 2,074 | 40.1 | 0.41 | 13,494 | 6.5 (2–179) | 569 | 4.2 | 6 | < 0.1 |
| Total (EU) | 716 | 4,459 | 5,175 | 100.0 | 1.02 | 49,463 | 9.8 (2–638) | 3,859 | 7.8 | 60 | 0.1 | |
Note ‘Escherichia coli other than STEC’ includes Escherichia coli (unspecified), enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC).
‘Bacillus cereus’ also includes FBOs whose causative agent was encoded as B. cereus enterotoxins.
‘Clostridium perfringens’ also includes FBOs whose causative agent was encoded Clostridium unspecified.
‘Staphylococcus aureus’ also includes FBOs whose causative agent was encoded as either Staphylococcus, unspecified or Staphylococcal enterotoxins.
‘norovirus’ also includes FBOs whose causative agent was encoded as Calicivirus, unspecified.
‘Other causative agents’ includes atropine and unspecified toxins.
Figure 59.
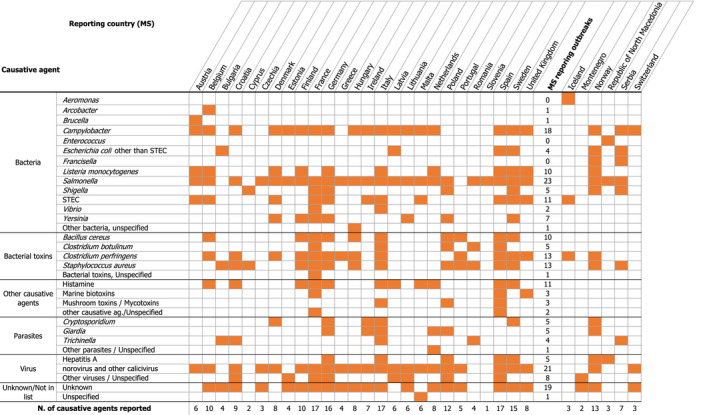
Overview of countries reporting data on food‐borne outbreaks, reporting EU MS and non‐MS, 2019
-
Note: the table may be read by column (country) or by row (causative agent). The number at each row end is the number of countries that reported for 2019 a given causative agent in outbreaks while the number at each column end are the numbers of causative agents identified in outbreaks by a given country in 2019. Luxembourg is not shown because no outbreaks were detected in 2019. Slovakia did not report data on outbreaks in 2019.‘Hepatitis A’ includes also FBOs with causative agent encoded as ‘hepatitis virus, unspecified’.‘B. cereus’ includes FBOs with causative agent encoded as B. cereus enterotoxins. ‘C. perfringens’ includes FBOs with causative agent encoded as Clostridium unspecified. ‘S. aureus’ includes FBOs with causative agent encoded as Staphylococcus, unspecified’ or Staphylococcal enterotoxins. ‘Other bacteria’ includes Arcobacter butzleri, enteropathogenic Escherichia coli (EPEC), enterotoxigenic Escherichia coli (ETEC), Escherichia coli, unspecified, Vibrio parahaemolyticus and other unspecified bacteria. ‘Other bacterial toxins’ includes FBOs by unspecified toxin‐producing bacteria.‘Other viruses’ includes adenovirus, flavivirus, hepatitis E virus, rotavirus and other viruses, unspecified. ‘Other causative agents’ includes atropine, mushroom toxins/mycotoxins and unspecified toxins.
As the monitoring and the reporting of food‐borne outbreaks among MS is poorly harmonised, the interpretation of pooled data at the EU level requires caution, as the situation at single MS level may differ importantly. The frequency distribution of the causative agents implicated in FBOs by MS is shown in Figure 60. The size and colour of each sector are proportional to the number of outbreaks and cases associated with each causative agent. The graphic aims to emphasise the major differences between MS in the causative agents being reported in FBOs rather than providing details. A graphical visualisation of the contribution (weight) of each MS to the number of FBOs reported at the EU level, by type of agent, is provided as supporting documents from the EFSA knowledge junction at zenodo (see link in the beginning of this chapter).
Figure 60.
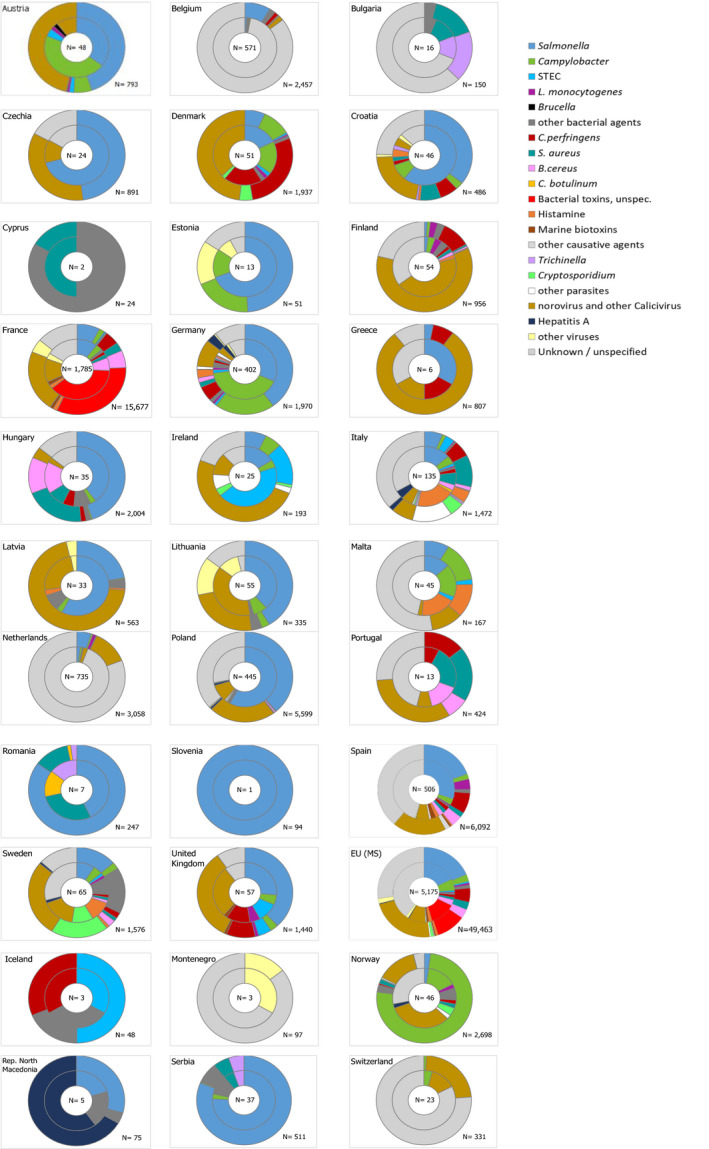
Frequency distribution of food‐borne outbreaks (internal circle) and human cases involved in outbreaks (external circle), by reporting EU MS and non‐MS (bottom figure), by causative agent, 2019
-
Causative agents are differently coloured. The size of each sector is proportional to the number of outbreaks (internal circle) and human cases (external circle) involved in outbreaks.‘Hepatitis A’ also includes FBOs with causative agent encoded as ‘hepatitis virus, unspecified’.‘B. cereus’ includes FBOs with causative agent encoded as B. cereus enterotoxins. ‘C. perfringens’ includes FBOs with causative agent encoded as Clostridium unspecified. ‘S. aureus’ includes FBOs with causative agent encoded as Staphylococcus, unspecified’ or Staphylococcal enterotoxins. ‘Other bacteria’ includes Arcobacter butzleri, Enteropathogenic Escherichia coli (EPEC), Enterotoxigenic Escherichia coli (ETEC), Escherichia coli, unspecified, Vibrio parahaemolyticus and other unspecified bacteria. ‘Other bacterial toxins’ includes FBOs by unspecified toxin‐producing bacteria.‘Other viruses’ includes adenovirus, flavivirus, Hepatitis E virus, rotavirus and other viruses, unspecified. ‘Other causative agents’ includes atropine, mushroom toxins/mycotoxins and unspecified toxins.
Information on the distribution of food vehicle implicated in the FBO by causative agent is presented in Section 4.3. Moreover, for the main causative agents, the ranking of food vehicles implicated in strong‐evidence outbreaks is described in tables in the supplementary information.
4.2.1. Bacteria
Salmonella
Salmonella was the agent most commonly identified in FBOs in the EU in 2019, accounting for 17.9% of total FBOs. Salmonella was also responsible for the highest number of hospitalisations (n = 1,915; 49.6% of all outbreak‐related hospitalisations). Outbreaks by Salmonella were reported by the greatest number of countries (23 MS and 7 non‐MS) (Figure 59). This agent was the most important cause of food‐borne outbreaks in 10 MS (Croatia, Czechia, Estonia, Greece, Hungary, Latvia, Lithuania, Poland, Romania, Slovenia) and one non‐MS (Iceland). Among the Salmonella outbreaks with available information on the serovar (N = 606), S. Enteritidis was the predominant serovar (n = 439; 72.4%) followed by S. Typhimurium (n = 85; 14.0%), monophasic S. Typhimurium (n = 12; 2%) and S. Infantis (n = 10; 1.7%).
At the EU level the number of S. Enteritidis outbreaks fell importantly in 2019 with less than half of outbreaks reported than in 2018 (596 outbreaks less: 57.6% reduction). Eleven MS (Austria, Bulgaria, Denmark, Estonia, France, Germany, Italy, Latvia, Poland, Spain, Sweden) communicated fewer outbreaks by S. Enteritidis than in 2018. Apart from this, the reduction in the number of S. Enteritidis outbreaks in 2019 is highly likely caused by the non‐data reporting of FBO data by Slovakia, which reported 505 FBOs caused by S. Enteritidis in 2018. A drop in the total number of cases (1,199 cases less; 18.3% reduction) and hospitalisations (265 hospitalisations less; 17.7% reduction) in S. Enteritidis outbreaks at the EU level was also observed, although this was less than the decrease in the number of FBOs. Outbreaks caused by S. Enteritidis were mainly small‐sized events involving less than 10 cases (N = 364; 83.0%). Only 10 large general outbreaks (> 100 cases, each) were reported in 2019 (2.3% of S. Enteritidis outbreaks) by five MS (Austria, Belgium, Hungary, Poland and Spain). In 2019, fewer outbreaks and related cases caused by S. Typhimurium and monophasic S. Typhimurium were reported (32 outbreaks fewer; 24.8% decrease compared with 2018 and 1,024 cases less, 56.5% less than in 2018). The drop was observed in almost all countries (Figure 61). The reporting of S. Infantis outbreaks in 2019 was similar to 2018, with 185 cases in 10 outbreaks from six countries (Croatia, France, Germany, Hungary, Italy, Latvia).
Figure 61.

Food‐borne outbreaks reported in 2019, by country and by causative agent and % of difference compared with 2018, reporting EU MS and non‐MS
-
Note: Both strong‐evidence outbreaks and weak‐evidence outbreaks are considered in the figure. Outbreaks caused by parasites are not shown due to paucity of data. Luxembourg is not shown since no outbreaks were detected in 2019. Slovakia did not report data on outbreaks in 2019.* % of variation could not be calculated as no outbreaks were reported in 2018. For Lithuania, the % of difference of FBOs by ‘norovirus and other calicivirus’ is + 2000%.‘Hepatitis A’ includes also FBOs with causative agent encoded as ‘hepatitis virus, unspecified’. ‘B. cereus’ includes FBOs with causative agent encoded as ‘B. cereus enterotoxins’. ‘C. perfringens’ includes FBOs with causative agent encoded as ‘Clostridium unspecified’. ‘S. aureus’ includes FBOs with causative agent encoded as ‘Staphylococcus, unspecified’ or ‘Staphylococcal enterotoxins’.
Altogether, the other 23 Salmonella serovars accounted for 36 outbreaks (5.9% of total Salmonella outbreaks with known serovar). The serovars of S. Coeln, S. Mikawasima, S. Agona, S. Muenchen, S. Poona, S. Newport were reported each in more than one outbreak. Other serovars (S. Bardo, S. Brandenburg, S. Bredeney, S. Chester, S. Derby, S. Duesseldorf, S. Indiana, S. Kentucky, S. London, S. Napoli, S. Paratyphi B, S. Saintpaul, S. Stanleyville, S. Strathcona, S. Virginia, S. Virchow, S. Javiana, Salmonella enterica 4,5,12:a:‐) were responsible for a single outbreak. S. Mikawasima caused a large multi‐country outbreak involving 152 cases in the United Kingdom, three in Denmark and 36 in Sweden. Overall, for 308 Salmonella outbreaks (33.3.% of all Salmonella outbreaks; 1 in 3), the serovar was not reported. The absence of this information introduces uncertainty in the identification of the most important sources of Salmonella at primary production level, given that the food vehicles implicated in Salmonella outbreaks differ importantly by serovar (Section 4.3). In addition, group B and group D Salmonella (Grimont and Weill, 2007), without full serotyping, were responsible for five and seven outbreaks, respectively.
Campylobacter
In 2019, Campylobacter was the fourth most reported causative agent for FBOs at the EU level, with 319 outbreaks communicated to EFSA (mostly weak‐evidence outbreaks), 1,254 cases of illness and 125 hospitalisations. Campylobacter was the leading causative agent in FBOs in Austria (22 outbreaks) and Germany (166 outbreaks). Campylobacter jejuni and C. coli were identified in 72 and 7 outbreaks, respectively. However, most Campylobacter outbreaks were reported without speciation information (240 outbreaks: 75.2%). Three MS (Germany, France and Austria) accounted for most of Campylobacter FBO reporting (N = 250; 78.4% of all Campylobacter outbreaks) in the EU. Outbreaks were predominantly small‐sized events of less than 10 cases (N = 298; 93.4%). However, single larger general outbreaks including up to 91 cases were reported by Denmark, France, Germany, Spain, Sweden and the United Kingdom. None of these were associated with C. coli.
Listeria monocytogenes
Outbreaks caused by Listeria monocytogenes in 2019 merit attention as they caused the highest burden in terms of deaths (N = 31; 51.7% of all outbreak associated deaths). In 2019, the number of outbreaks caused by L. monocytogenes (n = 21) was 50% higher compared with 2018 (n = 14) and the related illnesses jumped from a total number of 748 cases reported at the EU level between 2010 and 2018 (83.4 annual cases on average) to 349 cases. This increase was mainly due to outbreaks in Spain, which reported 3 outbreaks, 225 cases, 131 hospitalisations and 3 deaths, compared with zero reported in 2018. Most of the cases reported by Spain were associated with a community‐wide outbreak that was considered one of the largest L. monocytogenes outbreaks has ever occurred in the EU and which was linked to the consumption of contaminated meat and meat products (see dedicated text box).
The death toll linked to L. monocytogenes outbreaks was particularly high in the United Kingdom with 12 deaths among 17 outbreak related illnesses (seven deaths were reported from a single outbreak in hospital setting). Overall, in the EU the case fatality rate in L. monocytogenes outbreaks reported in 2019, 8.9%, was the highest among all causative agents implicated in FBOs.
Outbreak of Listeria monocytogenes infection associated with chilled roasted pork meat, Andalusia (Spain) 2019
In the summer of 2019, a large community‐wide outbreak caused by Listeria monocytogenes infection was detected in Andalusia (Spain). It started at the end of July 2019 with small household outbreaks in Andalusia and progressed up to involve 207 reported cases (189 confirmed cases with the human and food strains sharing the same sequences and 18 confirmed without human sequences available). Moreover, few cases were detected in other Spanish regions. Patients became infected through the consumption of chilled roasted pork meat contaminated with L. monocytogenes. Patients involved in the outbreak developed different clinical conditions, depending on the age, the health and pregnancy status and the presence of underlying conditions including involvement of the central nervous system, sepsis, stillbirth, abortion and preterm birth. Confirmed cases with a predominance of gastrointestinal symptoms presented an incubation period of 3 or less days, while in cases without gastroenteritis, the period of incubation was longer than 7 days. Cases needing hospitalisation were 131. Three deaths were reported among outbreak cases.
Based on the information available in the alert published by the Spanish Ministry of Health, the epidemiological investigation, the food analysis and the food business operator inspections made it possible to trace back the origin of the contamination to a single manufacturer located in Andalusia (Spain). Joint comparative analysis of the sequences obtained from the clinical isolates of L. monocytogenes and from the strains isolated from the food products and from the environment (contact surface) at the manufacturing plant revealed a close genetic similarity, so confirming the evidence obtained from the tracing‐back. The implicated products were withdrawn, and the manufacturing activity of the plant was suspended on August 14 and finally the products were recalled from the market on 16 August.
Consumers were warned about the risk posed by the consumption of chilled roasted pork products and products of the implicated brand through a public communication campaign. The EU Commission and the other EU MS were also alerted through the Rapid Alert System for Food and Feed (RASFF). Other information systems at the EU and international level were used by the Spanish Competent Authority to deliver information about the ongoing outbreak including the Early Warning and Response System (EWRS) managed by the EU Commission, the Epidemic Intelligence Information System (EPIS) managed by ECDC and the INFOSAN managed jointly by FAO and WHO. Two outbreak cases were communicated from abroad. Both cases were both linked to the consumption of meat products purchased in Andalusia. The distribution of the contaminated products, even if on a large scale, was limited to Spain and this explains the national dimension of the outbreak. Nonetheless, the outbreak had a remarkable impact on media and public opinion also outside Spain as it was considered one of the largest outbreaks of listeriosis that ever occurred in this country and in the EU.
The importance of multisectoral collaboration and prompt sharing of information and the need for strengthening the control of L. monocytogenes at all stages in the food manufacturing and distribution are key points of the lessons learned from this outbreak.
For more information on this outbreak:
World Health Organisation (WHO)
https://www.who.int/csr/don/16-September-2019-listeriosis-spain/en/
Spanish Ministry of Health
https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/listeriosis/home.htm
Shiga toxin‐producing E. coli (STEC)
Next to Salmonella and Campylobacter, Shiga toxin‐producing E. coli (STEC) were the third most frequent bacterial agents detected in FBOs in the EU, with 42 outbreaks, 273 cases, 50 and 1 death reported in 2019. Only four of these were classified as strong‐evidence outbreaks and for 17 outbreaks (40.5%) only, information on STEC serogroup was available. Although the STEC serogroup is no longer considered a valid predictor of the virulence, it plays an important role as a broad epidemiological marker. STEC O157, O26 and O145 were identified in nine, seven and one outbreaks in the EU, respectively. A single strong‐evidence outbreak caused by STEC O26 was also reported by Iceland. Like in recent years, in 2019 STEC have been the leading agents of food‐borne outbreaks in Ireland.
Shigella
Shigella was detected in 22 outbreaks, involving 106 cases and in 19 hospitalisations reported by MS (see Table 51 for details for EU reports), mostly small‐sized events. In addition, two non‐MS (Norway, Serbia) reported three outbreaks with 38 cases and four hospitalisations. Shigella sonnei was detected in four outbreaks (two of them were strong‐evidence outbreaks) reported by three MS (France, Poland, Sweden) and Norway. Shigella flexneri was detected in three outbreaks reported by Sweden and Serbia (all strong‐evidence outbreaks), respectively. Shigella flexneri serotype 3a was detected in a single medium‐size general outbreak in Sweden with 12 cases and four hospitalisations.
Yersinia
In 2019, outbreaks and illnesses by Yersinia (15 and 149, respectively) were reported by seven MS (Denmark, Finland, France, Germany, Lithuania, Poland, Sweden) in numbers close to recent years. Hospitalisations were reported for 14 cases. Yersinia enterocolitica was identified as the causative agent in all these outbreaks but one. Interestingly, two strong‐evidence outbreaks caused by Y. enterocolitica biotype 4 were part of the same single multi‐country outbreak linked to the consumption of food imported to both the Swedish and Danish markets.
Other bacterial agents
Among bacterial pathogens less frequently reported in food‐borne outbreaks, Arcobacter butzleri, previously named Campylobacter butzleri, was detected in a single weak‐evidence outbreak in Belgium involving 40 cases (no hospitalisations). Latvia, Spain and Sweden reported four outbreaks caused by enterotoxigenic E. coli (ETEC) which involved 199 cases and 7 hospitalisations. The largest event occurred in Sweden led to 130 notified cases. Another strong‐evidence general outbreak by enteropathogenic E. coli (EPEC) with 38 cases was also reported by Sweden. Latvia and Norway reported one single outbreak caused by EPEC, each. Four outbreaks caused by E. coli ‘unspecified’ (including two strong‐evidence outbreaks) were notified by Bulgaria, Spain and Serbia. Although the number of outbreaks caused by ETEC and EPEC is too small to draw conclusions on their trend over years, it is noteworthy that only four and three outbreaks caused by ETEC and EPEC, respectively, had been reported to EFSA by MS between 2010 and 2018. It is possible that the rise observed in 2019 may be linked to an improved capability to detect E. coli in food and to characterise E. coli pathogroups, even though no official methods exist for the detection of ETEC and EPEC in foodstuffs. Vibrio was identified in four small food‐borne outbreaks reported by France and Italy. The agent was identified as V. parahaemolyticus in all French outbreaks, while no information was available for the others. Francisella tularensis, the causative agent of human tularaemia, a severe condition characterised by multiple clinical symptoms, was reported in two strong‐evidence outbreaks in Norway and Serbia, causing 24 illnesses and 6 hospitalisations.
4.2.2. Bacterial toxins
Outbreaks caused by bacterial toxins represented an important proportion of all FBOs reported in the EU in 2019 (n = 997; 19.3% of all outbreaks) and were mostly classified as weak‐evidence outbreaks. These outbreaks caused a total of 10,555 cases, 361 hospitalisations and 14 deaths (Table 51). Outbreaks caused by bacterial toxins were mostly reported by France that communicated 876 outbreaks (87.9% of all outbreaks caused by bacterial toxins). In France, bacterial toxins were the leading cause of food‐borne outbreaks.
Bacillus cereus, Clostridium botulinum, Clostridium perfringens, Staphylococcus aureus
Toxins produced by B. cereus (155 outbreaks, 1,636 cases, 44 hospitalisations) were the agents most frequently reported at the EU level with a number of FBOs twice as much as the FBO numbers due to toxins produced by C. perfringens (75 outbreaks, 2,426 cases, 27 hospitalisations) or S. aureus (74 outbreaks, 1,400 cases, 141 hospitalisations).
Fourteen deaths were reported among food‐borne illnesses due to poisoning caused by bacterial toxins which correspond to a high proportion of all fatal cases reported in 2019 in FBOs (23.3%). Bacillus cereus was responsible for seven deaths. Five of them were associated with a single outbreak that occurred in a residential institution (nursing home or prison or boarding school) leading to 26 cases and 17 hospitalisations. Six deaths were caused by both C. perfringens and other undefined bacterial toxins. Clostridium botulinum (7 outbreaks, 17 cases and 15 hospitalisations in 2019) was responsible for one death.
In 2019, 16 strong‐evidence outbreaks, and 58 weak‐evidence outbreaks caused by S. aureus enterotoxins poisoning were reported by 13 MS (Bulgaria, Croatia, Cyprus, Finland, France, Germany, Hungary, Italy, Poland, Portugal, Romania, Spain, Sweden). Serbia and Norway also reported two strong‐ and one weak‐evidence outbreaks, respectively. Among MS, most of these outbreaks were reported as general outbreaks (N = 54) and involved overall 1,324 cases (94,6% of all outbreak cases caused by S. aureus). Two large outbreaks were reported, causing 380 illnesses in Hungary and causing 300 cases of illnesses including one hospitalisation in France. The most severe outbreak was described in Italy where 44 out of 70 cases (62%) needed hospitalisations. No deaths due to S. aureus poisoning was reported. The number of outbreaks caused by S. aureus poisoning showed a 35.7% drop in 2019 compared with 2018, mainly due to fewer outbreaks in France and Spain (17 and 18 outbreaks less).
Unspecified bacterial toxins
In many food poisonings attributed to the intake of bacterial toxins, the implicated agent was not identified but generically classified as ‘bacterial toxins, unspecified’ (n = 686; 68.9%). These events caused 5,076 illnesses and 134 hospitalisations. Such reporting was adopted by France only for the suspect cases identified on the basis of clinical signs, the median incubation time and types of consumed foods, when the pathogens and/or toxins were neither detected in human samples and/or food leftovers nor in food handling environment.
4.2.3. Viruses
A wide range of viruses were reported in FBOs in 2019, including adenovirus, flavivirus and Tick‐borne encephalitis virus, hepatitis A virus, hepatitis E virus, norovirus, sapovirus, rotavirus. Overall, 554 outbreaks caused by food‐borne viruses led to many illnesses (12,227 cases; 24.7% of all outbreaks cases). Nevertheless, no deaths were reported in FBOs caused by viruses and the number of hospitalisations (456 hospitalisations; 12% of the cases) was smaller, compared with FBOs caused by bacteria and other causative agents.
Norovirus
In 2019, norovirus (and other Calicivirus) was the second most frequently reported causative agent in FBOs in the EU and was reported by 21 MS (Figure 58). In four of these (Denmark, Finland, Lithuania, the United Kingdom) and one non‐MS (Norway) this agent was the leading cause of FBOs. Norovirus was associated with 457 outbreaks and, most importantly, with 11,125 related illnesses (22.5% of total cases) meaning one in five of all outbreak related illnesses in the EU. Norovirus was associated with large outbreaks (24.3 cases on average). In 2019, the number of outbreaks of medium size (involving between 10 and 100 cases) and large size (> 100 cases) were 204 and 14, respectively. Two very large outbreaks, reported by Greece and France, each involved more than 500 illnesses. Most norovirus outbreaks (N = 264; 57.8%) were general outbreaks; a proportion much higher than for other causative agents. In 2019, outbreaks caused by norovirus increased by 13.1% (53 outbreaks more than in 2018), with five countries contributing most to this rise, France (224 outbreaks more than in 2018), Lithuania (21 outbreaks more than in 2018), the Netherlands (17 outbreaks more than in 2018) and the United Kingdom (16 outbreaks more than in 2018).
Hepatitis A (including other hepatitis virus, unspecified) and flavivirus
In total, 22 hepatitis A (including other Hepatitis virus, unspecified) outbreaks involving 135 cases were reported in 2019 by five MS (Germany, Italy, Poland, Spain, Sweden). In addition, the Republic of North Macedonia and Norway also reported three and one outbreaks, respectively. Compared with 2018, the number of notified hepatitis A (including other hepatitis virus, unspecified) outbreaks decreased in the EU (36 outbreaks less; 62.1% decrease), mainly due to reduced reporting by Poland. hepatitis A outbreaks were characterised by a high percentage of cases needing hospitalisation (99 cases, 73.3% of cases).
Flaviviruses, including tick‐borne encephalitis virus was associated with an even higher proportion of hospitalisations (80% of cases) and detected in three outbreaks and 15 cases.
4.2.4. Parasites
The number of FBOs caused by parasites reported in 2019 was limited compared with the other agents (31 outbreaks in MS and five outbreaks in non‐MS) and fewer than in 2018.
Trichinella
Among Trichinella outbreaks (N = 5) in the EU, which was half of the number compared with 2018, T. spiralis accounted for two events (six outbreaks less than in 2018) and T. britovi was identified in a single outbreak reported by Italy. No information was available for the remaining Trichinella outbreaks reported by one MS (N = 2) and one non‐MS (N = 2).
Giardia
In 2019, Giardia caused most outbreaks (N = 14), involving parasites. Although there were five fewer outbreaks reported in 2019 compared with 2018, the total number of illnesses in 2019 increased fourfold, mainly due to a single large weak‐evidence outbreak caused by G. intestinalis (lamblia) reported by Italy, which resulted in 199 illnesses. G. intestinalis (lamblia) was identified in four outbreaks while no details on the species was provided for the remaining outbreaks.
Cryptosporidium
Cryptosporidium (11 outbreaks and 468 cases in 2019) was the only agent among parasites that caused more outbreaks (2 outbreaks more) and cases (425 cases more; 988.4% increase) than in 2018. Seven outbreaks with in total 304 notified cases were reported by Sweden after no reported outbreaks of cryptosporidiosis during the two former years. Overall, C. parvum was implicated in eight outbreaks while no information on the species was available for the other outbreaks.
4.2.5. Other causative agents
This group of outbreaks includes mainly events caused by ‘histamine’, ‘marine biotoxins’ and a few other chemical agents of biological origin that accidentally may contaminate food or its ingredients. The reporting of outbreaks caused by other causative agents is the least harmonised among MS. These agents are not regularly covered by the national outbreak surveillance programmes that in many MS only target infectious agents. Consequently, outbreaks communicated to EFSA are sparse and the importance of this type of food poisoning is highly likely underestimated at the EU level.
In 2019, 96 outbreaks caused by histamine were reported by 11 MS while only three MS reported 48 outbreaks caused by marine biotoxins (Table 51 and Figure 59). Histamine poisoning is usually associated with consumption of poor‐quality raw materials preserved in inadequate conditions during storage and preparation. France and Spain are the MS which contribute more regularly to the reporting of outbreaks involving marine biotoxins. These biotoxins are mainly produced by algae or phytoplankton and accumulate in fish and filter‐feeding molluscan shellfish. The toxins include also ciguatoxin, saxitoxin and its muscle‐paralyzing toxin, okadaic acid. Ciguatoxin, the causative agent of Ciguatera fish poisoning is characterised by gastrointestinal, neurological and/or generalised disturbances and occurs most commonly in fish from Pacific, Caribbean and Indian Ocean regions. In 2019, France reported 19 outbreaks caused by Ciguatoxin. In Spain, the number of outbreaks caused by marine biotoxins (N = 13) was higher than in 2018 (8 outbreaks more; 160% increase). The United Kingdom reported in 2019 a single outbreak with 13 illnesses involving okadaic acid, a heat stable toxin that can be found in various species of shellfish. Only two outbreaks caused by okadaic acid had been previously reported to EFSA in 2012, although the contamination of various type of shellfish by okadaic acid has not rarely been signalled through the Rapid Alert System for Food and Feed (RASFF) system.
4.2.6. Outbreaks caused by unknown/unspecified agents
Several reasons may explain the reporting of unknown/unspecified agents, including late reporting of illness, failure to detect causative agents in patients or in the food, unavailability of clinical or food samples (e.g. leftovers), delay in sample collection etc. For the same reasons, few outbreaks of unknown aetiology were classified as strong‐evidence outbreaks. In 2019, 2,074 food‐borne outbreaks of unknown aetiology accounted for 40.1% of total outbreaks and 27.3% of illnesses in the EU. At the country level, these proportions varied hugely. Outbreaks with unknown aetiology were mainly reported by Belgium and the Netherlands and these FBOs accounted for 1,274 outbreaks (60.1% of all outbreaks caused by unknown agents notified in the EU). They were mainly weak‐evidence, small‐sized (< 10 cases) events that included each, less than four cases, on average. In Belgium and in the Netherlands, this type of outbreak accounted for the majority the FBOs (Figure 60). These findings suggest that outbreaks caused by unknown agents occurred in confined contexts such as domestic settings or small groups, for which the identification of the link among cases was probably relatively easy. Conversely, 250 outbreaks with unknown aetiology involving each more than 10 cases (medium and large size outbreak) were reported by 15 MS. Not all MS, however, reported outbreaks of unknown aetiology to EFSA in 2019.
The short‐term relative variation (%) of the annual number of strong‐evidence and weak‐evidence outbreaks for specific causative agents and by MS are plotted in Figure 61.
4.3. Overview of food vehicles implicated in food‐borne outbreaks
This section aims to describe the characteristics of food vehicles that in 2019 were implicated in outbreaks in the European countries. The description of the implicated food vehicles relies on strong‐evidence outbreaks, because only for these events, the link between the consumption of foods and the illnesses was proved with minimal uncertainty. Strong‐evidence outbreaks represent a minority of all FBOs reported in 2019 (716 outbreaks, 13.8%).
4.3.1. Food vehicle in strong‐evidence outbreaks
The overview of the food vehicles implicated in strong‐evidence outbreaks and illnesses in the EU in 2019 is described in Table 52. For a correct interpretation of the data, it is worth remembering that the pattern of food vehicles implicated in outbreaks at the EU level, is highly influenced by those countries which contributed the most to strong‐evidence outbreaks data collection (Table 50). In 2019, these were France, Spain, Poland and Italy. Altogether these four MS provided information on almost three quarters of the total number of strong‐evidence outbreaks (532 outbreaks, 74.3% of strong‐evidence outbreaks), while the remaining outbreaks (184 outbreaks) were contributed by 19 MS altogether.
Table 52.
Frequency distribution of strong‐evidence food‐borne outbreaks, by food vehicle, in reporting EU MS, 2019
| Type of vehicle | Strong‐evidence outbreaks | Outbreak reporting rate per 100,000 | Rank | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N of outbreaks | % of total outbreaks | N of cases | % of total cases | 2019 | 2010–2018 (mean) | 2019 | 2010–2018 | ||
| Fish and fishery products | |||||||||
| Crustaceans, shellfish, molluscs and products thereof | 159 | 22.2 | 1,250 | 9.1 | 0.031 | 0.009 | 1 | 5 | |
| Fish and fish products | 34 | 4.7 | 360 | 2.6 | 0.007 | 0.011 | 9 | 3 | |
| Subtotal | 193 | 27.0 | 1,610 | 11.8 | 0.038 | 0.020 | – | – | |
| Meat and meat products | |||||||||
| Meat and meat products, unspecified | 41 | 5.7 | 770 | 5.6 | 0.008 | 0.003 | 5 | 13 | |
| Pig meat | 42 | 5.9 | 575 | 4.2 | 0.008 | 0.008 | 4 | 7 | |
| Poultry meat | 38 | 5.3 | 870 | 6.4 | 0.007 | 0.010 | 6 | 4 | |
| Bovine meat | 14 | 2.0 | 319 | 2.3 | 0.003 | 0.004 | 12 | 12 | |
| Sheep meat | 2 | 0.3 | 89 | 0.7 | < 0.001 | 0.000 | 22 | 22 | |
| Other or mixed red meat and products thereof | 14 | 2.0 | 112 | 0.8 | 0.003 | 0.003 | 13 | 14 | |
| Subtotal | 151 | 21.1 | 2,735 | 20.0 | 0.030 | 0.028 | – | – | |
| Eggs and egg products | 108 | 15.1 | 1,277 | 9.3 | 0.021 | 0.023 | 2 | 1 | |
| Mixed food | 81 | 11.3 | 3,079 | 22.5 | 0.016 | 0.018 | 3 | 2 | |
| Food of non‐animal origin | |||||||||
| Vegetables and juices and other products thereof | 30 | 4.2 | 836 | 6.1 | 0.006 | 0.007 | 10 | 9 | |
| Fruit, berries and juices and other products thereof | 8 | 1.1 | 96 | 0.7 | 0.002 | 0.002 | 17 | 18 | |
| Sweets and chocolate | 7 | 1.0 | 86 | 0.6 | 0.001 | 0.002 | 18 | 17 | |
| Cereal products including rice and seeds/pulses (nuts, almonds) | 5 | 0.7 | 53 | 0.4 | 0.001 | 0.002 | 19 | 19 | |
| Herbs and spices | 1 | 0.1 | 13 | 0.1 | < 0.001 | < 0.001 | 23 | 23 | |
| Subtotal | 51 | 7.1 | 1,084 | 7.9 | 0.010 | 0.011 | – | – | |
| Bakery products | 38 | 5.3 | 512 | 3.7 | 0.007 | 0.007 | 7 | 8 | |
| Milk and milk products | |||||||||
| Milk | 9 | 1.3 | 62 | 0.5 | 0.002 | 0.003 | 16 | 15 | |
| Cheese | 4 | 0.6 | 15 | 0.1 | 0.001 | 0.004 | 20 | 11 | |
| Dairy products (other than cheeses) | 4 | 0.6 | 10 | 0.1 | 0.001 | 0.001 | 21 | 20 | |
| Subtotal | 17 | 2.4 | 87 | 0.6 | 0.003 | 0.008 | – | – | |
| Other foods | |||||||||
| Other foods, unspecified | 15 | 2.1 | 338 | 2.5 | 0.003 | 0.009 | 11 | 6 | |
| Canned food products | 1 | 0.1 | 2 | < 0.1 | < 0.001 | < 0.001 | 24 | 24 | |
| Subtotal | 16 | 2.2 | 340 | 2.5 | 0.003 | 0.011 | – | – | |
| Buffet meals | 13 | 1.8 | 476 | 3.5 | 0.003 | 0.004 | 14 | 10 | |
| Water | Tap water, including well water | 11 | 1.5 | 1,170 | 8.5 | 0.002 | 0.003 | 15 | 16 |
| Unknown | 37 | 5.2 | 1,316 | 9.6 | 0.007 | 0.001 | 8 | 21 | |
| Total (EU) | 716 | 100 | 13,686 | 100.0 | 0.141 | 0.133 | – | – | |
Note: Single food items are consolidated into major groups according to their origin. The columns ‘outbreak reporting rate’ include the mean outbreak reporting rate per 100,000 for 2019 and for the previous years (2010–2018) for trend watching. The rank position of each food item provides a visual demonstration of the relative importance of the item, among all food vehicles implicated in food‐borne outbreaks, for the same year and period. ‘Pig meat’ includes pig meat and products thereof. ‘Poultry meat’ includes broiler meat (Gallus gallus) and products thereof, turkey meat and products thereof and other, mixed or unspecified poultry meat and products thereof. ‘Bovine meat’ includes bovine meat and products thereof. ‘Sheep meat’ includes sheep meat and products thereof.
Foods of animal origin
The consumption of food of animal origin (‘fish and fishery products’, ‘eggs and egg products’, ‘meat and meat products’, ‘milk and milk products’) was associated with most of the strong‐evidence FBOs (469 outbreaks; 65.5%) and illnesses (5,709 cases; 41.7%) reported in 2019. Food of animal origin was mainly implicated in outbreaks caused by Salmonella (182 outbreaks; 38.8% of all FBOs by food of animal origin), norovirus and other Calicivirus (148 outbreaks; 31.6%), histamine (21 outbreaks; 4.5%), C. perfringens (20 outbreaks; 4.3%) and Campylobacter (14 outbreaks; 3.0%) (Figure 62).
Figure 62.
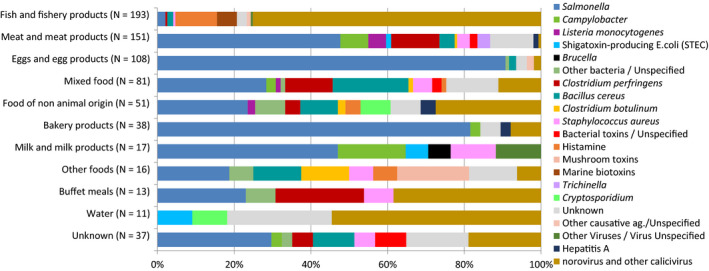
Frequency distribution of causative agents associated with strong‐evidence food‐borne outbreaks, by food vehicle, in reporting EU MS, 2019
-
Note: N = number of strong‐evidence outbreaks by food type.‘Hepatitis A’ includes also FBOs with causative agent encoded as ‘hepatitis virus, unspecified’. ‘B. cereus’ includes FBOs with causative agent encoded as ‘B. cereus enterotoxins’. ‘C. perfringens’ includes FBOs with causative agent encoded as ‘Clostridium unspecified’. ‘S. aureus’ includes FBOs with causative agent encoded as ‘Staphylococcus, unspecified’ or ‘Staphylococcal enterotoxins’. ‘Other bacteria’ includes enteropathogenic Escherichia coli (EPEC), enterotoxigenic Escherichia coli (ETEC), Escherichia coli, unspecified, Shigella, Vibrio parahaemolyticus, Yersinia and other unspecified bacteria. ‘Other bacterial toxins’ includes FBOs caused by unspecified toxin‐producing bacteria.‘Other viruses’ includes flavivirus and other unspecified viruses. ‘Other causative agents’ includes atropine, mushroom toxins/mycotoxins and unspecified toxins. ‘Fish and fishery products’ include ‘crustaceans, shellfish, molluscs and products thereof’, as well as ‘fish and fish products’. ‘Meat and meat products’ include bovine meat and products thereof, broiler meat (Gallus gallus) and products thereof, other or mixed red meat and products thereof, other, mixed or unspecified poultry meat and products thereof, pig meat and products thereof, sheep meat and products thereof, turkey meat and products thereof.‘Food of non‐animal origin’ includes cereal products including rice and seeds/pulses (nuts, almonds), fruit, berries and juices and other products thereof, herbs and spices, sweets and chocolate, vegetables and juices and other products thereof.’ ‘Milk and milk products’ include cheese, dairy products (other than cheeses), milk. ‘Other foods’ includes canned food products and other foods, unspecified.‘Water’ includes Tap water, including well water.
The importance of ‘crustaceans, shellfish, molluscs and products thereof’ (159 strong‐evidence outbreaks) increased substantially in the EU in 2019 (80 outbreaks more; 101.3% more than in 2018) and in particular in France which reported 129 outbreaks (81.1% of total outbreaks in the EU) compared with 32 in 2018 (287.5% increase). Almost all outbreaks caused by ‘crustaceans, shellfish, molluscs and products thereof’ reported by France involved norovirus (124 outbreaks, 756 cases). No increase was observed in the other MS (Croatia, Denmark, Finland, the Netherlands, Spain, Sweden or the United Kingdom) which reported similar outbreaks. In Sweden, many cases (N = 208) became infected following consumption of ‘crustaceans, shellfish, molluscs and products thereof’, oysters, contaminated with norovirus GI and GII. Two more outbreaks involving norovirus in oysters causing 126 cases of illness were reported by Norway. The increase observed in France in 2019 was the only driver of the overall rise of outbreaks by ‘fish and fishery products’ group, which was the food most frequently implicated in strong‐evidence outbreaks in the EU.
‘Eggs and egg products’, the next most frequently reported foodstuff, were implicated in 108 strong‐evidence outbreaks reported by 11 M (Austria, Croatia, France, Germany, Hungary, Italy, Latvia, the Netherlands, Poland, Spain and the United Kingdom). At the EU level, outbreaks linked to contaminated ‘eggs and egg products’ reduced by 24.5% in 2019 (35 outbreaks less than in 2018). The lack of data reporting by Slovakia may have contributed to this decrease. However, a significant drop was also observed for Italy, Poland and Spain. Germany and the United Kingdom were the only MS where outbreaks by ‘eggs and egg products’ increased. Consumption of contaminated ‘eggs and egg products’ has been often associated with very large EU‐wide outbreaks, such as the extensive outbreaks that in 2017, 2018 and 2019 involved many EU countries. In 2019, 20 medium‐sized outbreaks (including from 10 to 100 cases) and one single large outbreak (> 100 cases) linked to this food type caused 592 and 321 illnesses, respectively. At least six of these events were outbreaks dispersed in the EU, with cases scattered over large geographic areas including cross border zone. In these outbreaks, tracing of patients and the trace‐back of batches of ‘eggs and egg products’ delivered to the marketplaces as well as typing of human and food isolates by WGS have been successfully applied.
Outbreaks caused by ‘meat and meat products’ (151 outbreaks, Table 52) accounted for an important proportion of strong‐evidence outbreaks in the EU. In this group, outbreaks by ‘meat and meat products, unspecified’ (41 outbreaks), the item most frequently reported, had a twofold increase compared with 2018. This surge was mainly driven by Spain that reported 19 outbreaks in 2019 linked to this foodstuff (16 more than in 2018); bacterial toxins (3), Salmonella (3), L. monocytogenes (1) and ‘Unknown’ causative agent (12).
The number of outbreaks caused by ‘pig meat’ was stable in all the MS except France, which reported 19 outbreaks compared with five outbreaks in 2018. Sixteen of these were caused by Salmonella, including S. Typhimurium (12 outbreaks) and S. Infantis (two outbreaks). Outbreaks by ‘other or mixed red meat and products thereof’ also increased in France in 2019 (11 outbreaks, six more than in 2018). The implicated agent was mainly S. Typhimurium and its monophasic variants (eight outbreaks in total).
The consumption of ‘poultry meat’ was associated with many illnesses (N = 870) in strong‐evidence outbreaks in 2019. This foodstuff was mostly identified in Salmonella outbreaks (19 outbreaks), Campylobacter (eight outbreaks) and bacterial toxins other than C. botulinum (9 outbreaks). Overall, the reported number of outbreaks caused by ‘poultry meat’ (38 outbreaks) was rather stable at the EU level, even though seven MS (Denmark, Hungary, Finland, Latvia, the Netherlands, Spain and the United Kingdom) reported mild or moderate increases. Poland, in contrast, reported a reduction in outbreaks caused by ‘poultry meat’ (five outbreaks in 2019; eight less than in 2018) but this did not correspond to a parallel decrease in the number of illnesses. Denmark reported three outbreaks caused by C. jejuni in ‘poultry meat’ (115 cases involved) after 2 years with no reported outbreaks caused by ‘poultry meat’. The contamination was traced back to a slaughterhouse. The same agent/food pair was also implicated in the only outbreak caused by ‘poultry meat’ reported by Finland.
The number of strong‐evidence outbreaks associated with the consumption of ‘cheese’ decreased markedly, at the EU‐level and in all MS. There were 20 outbreaks in 2018 and four in 2019, which is the lowest number ever reported since the beginning of the FBOs data collection in the EU. Outbreaks caused by ‘milk’ were also less frequently reported in 2019 (nine outbreaks) mainly due to a remarkable decrease in milk‐borne outbreaks of Campylobacter in Germany (three outbreaks in 2019; 19 outbreaks in 2018; 10 outbreaks in 2017). The number of outbreaks implicating other dairy products (four outbreaks) did not substantially change in 2019 compared with previous years in the MS. Iceland reported a general outbreak connected with dairy product (ice cream) contaminated with STEC O26.
Foods of non‐animal origin
In 2019, 10 MS (Denmark, Finland, France, Germany, Italy, Latvia, the Netherlands, Poland, Spain, Sweden) reported 51 outbreaks associated with the consumption of FNAO. FNAO were mainly implicated in outbreaks caused by norovirus (14 outbreaks), Salmonella, (12 outbreaks), B. cereus (five outbreaks) and Cryptosporidium (four outbreaks). ‘Vegetables (and juice)’ (30 outbreaks) were the most frequently reported food vehicle of this group. Interestingly, the mean size of outbreaks associated with this food (21.2 cases/outbreak) was approximately twofold larger than outbreaks linked to consumption of food of animal origin (12.2 cases/outbreak). Various types of leafy‐green vegetables, olives, tomatoes, cucumbers and radish sprouts were the items described in this group. Vegetable‐associated outbreaks increased markedly in Sweden and less importantly in Italy and Latvia. Sweden was the MS reporting the highest number of the outbreaks caused by ‘vegetables (and juice)’ (seven outbreaks). Four of these were associated with the consumption of kale or vegetable juice, contaminated by Cryptosporidium parvum, with 223 cases notified. Another 132 cases of illness were caused by two Salmonella outbreaks associated with the consumption of various types of tomatoes. In Italy and Latvia outbreaks by vegetables were less clearly associated with a specific causative agent even if norovirus, as in many other countries, was mostly identified. In Denmark and Sweden, a single outbreak caused by Y. enterocolitica biotype 4 associated with the consumption of imported fresh green spinach contaminated at the primary production level led to 20 and 37 cases, respectively. In Spain, 50 cases were involved in an outbreak caused by vegetables (not specified) contaminated with Enterotoxigenic E. coli. ‘Fruits and juice’, in particular ‘frozen and fresh berries’, ‘pre‐cut melon’ and ‘dates’, were implicated in outbreaks caused by norovirus (four outbreaks), hepatitis A (one outbreak), Salmonella (two outbreaks) and B. cereus toxins (one outbreak). ‘Sweets and chocolate’ were mainly identified in Salmonella outbreaks (five outbreaks) and ‘cereal products including rice and seeds/pulses (nuts, almonds)’ in outbreaks caused by bacterial toxins (three outbreaks). The only outbreak associated with ‘herbs and spicy’ was reported by Italy and was caused by norovirus.
Mixed foods, bakery products, other foods, buffet meals
These foodstuffs include composite food resulting from the assembly of multiple ingredients or highly processed or manipulated foods. Interestingly, outbreaks associated with these foodstuffs were larger on average (29.8 cases/outbreak), than outbreaks associated with either food of animal origin (12.2 cases/outbreak) or FNAO (21.3 cases/outbreak). In 2019, the consumption of ‘mixed foods’ caused the highest number of cases of illness (N = 3,079, Table 52) in strong‐evidence outbreaks. This foodstuff was associated with a wide range of causative agents including bacteria (Salmonella, Campylobacter, L. monocytogenes, Shigella), norovirus, bacterial toxins (B. cereus, C. botulinum, C. perfringens, S. aureus) and histamine. Outbreaks caused by ‘mixed food’ were mainly general outbreaks and were reported by 14 MS. In Hungary, the consumption of various types of ‘mixed food’ was associated with five outbreaks that altogether involved 946 illnesses. The largest event (575 cases) was associated with various types of contaminated mixed food, also involving cross‐contamination, by S. Enteritidis. This was the largest outbreak by mixed food ever registered since the beginning of the surveillance of FBOs in the EU. Other seven large outbreaks (> 100 cases) linked to mixed food were reported by Belgium, Denmark, Hungary, Poland, Romania.
Outbreaks caused by ‘bakery products’ (38 outbreaks) were mostly associated with Salmonella (31 outbreaks) and were frequently linked to domestic settings (19 outbreaks). This finding suggests that improper food handling and poor storage habits in households may contribute to this type of outbreaks. Outbreaks caused by ‘other foods’ (16 outbreaks) halved in 2019 compared with 2018, mainly due to decreased reporting from France (zero reporting). Few details on the type of food were provided by the seven MS (Germany, Hungary, Italy, Poland, Romania, Spain, Sweden) that reported this food vehicle. In Poland, ‘other food, unspecified’ was associated with two outbreaks in ‘school or kindergarten’. In Germany, frozen Wakame algae was responsible for a community‐wide dispersed outbreak, with 53 cases of illness. In Sweden, salad dressing basil oil contaminated with EPEC caused 38 cases of illness.
‘Buffet meals’ related outbreaks decreased importantly in 2019 among strong‐evidence outbreaks and the reporting of this category (13 outbreaks) was quite sparse among MS.
The causative agents associated with the consumption of different type of food implicated in strong‐evidence FBOs are shown in the stacked bar chart in Figure 62. Sankey diagrams by food groups are included in the supplemental information.
4.3.2. Top‐10 agent/food pairs in strong‐evidence outbreaks associated with the highest impact on health in the EU, 2019
Tables 53–56 show the top 10 pairs of causative agents and food vehicles among outbreaks having the highest health impact in 2019 in the EU in terms of total outbreaks, cases, hospitalisations and deaths, respectively. The number of MS that reported outbreaks implicating each food/agent pair is also included in the tables, to indicate how common these types of outbreaks were in the EU MS. Indeed, MS that contribute the most to the data collection may influence the rank position of the pairs. The same information for the 2010–2018 period is also shown in parallel, for trend watching purposes.
Table 53.
Top‐10 pathogen/food vehicle pairs causing the highest number of strong‐evidence outbreaks in reporting EU MS, 2019
| 2019 | 2018–2010 | Evaluation (2019 vs. 2010–2018)b | ||||||
|---|---|---|---|---|---|---|---|---|
| Ranka | Causative agent | Food vehicle | Outbreaks (N) | Reporting MS (N outbreaks) | Ranka | Outbreaks (mean N/year) | Reporting MS (mean N/year) | |
| 1 | Norovirus and other Calicivirus | Fish and fishery products | 145 | France (124), Spain (7), Sweden (6), Finland (3), Denmark (2), United Kingdom (1), Croatia (1), Netherlands (1) | 4 | 25.9 | 6.3 | ↑↑ |
| 2 | Salmonella spp. | Eggs and egg products | 98 | Spain (44), Poland (26), France (9), Germany (6), United Kingdom (4), Netherlands (2), Hungary (2), Italy (2), Austria (1), Croatia (1), Latvia (1) | 1 | 103.0 | 9.8 | stable |
| 3 | Salmonella spp. | Meat and meat products | 72 | France (24), Poland (12), Spain (10), Hungary (7), Germany (5), United Kingdom (3), Denmark (2), Croatia (2), Latvia (2), Sweden (1), Netherlands (1), Czechia (1), Italy (1), Estonia (1) | 2 | 55.0 | 11.8 | ↑ |
| 4 | Salmonella spp. | Bakery products | 31 | Poland (26), Spain (3), Czechia (2) | 5 | 25.0 | 4.6 | stable |
| 5 | Salmonella spp. | Mixed food | 23 | Poland (12), Hungary (2), Spain (2), France (2), Germany (2), Belgium (1), Czechia (1), Romania (1) | 6 | 23.9 | 8.4 | stable |
| 6 | Histamine/Scombrotoxin | Fish and fishery products | 21 | Spain (6), Italy (4), France (3), Germany (3), Sweden (2), Finland (1), Netherlands (1), Latvia (1) | 3 | 32.9 | 6.9 | ↓ |
| 7 | Clostridium perfringens | Meat and meat products | 19 | France (5), Spain (4), Denmark (3), Italy (2), United Kingdom (2), Germany (1), Hungary (1), Greece (1) | 7 | 18.4 | 5.3 | stable |
| 8 | Bacillus cereus | Mixed food | 16 | Spain (5), France (4), Italy (2), Germany (2), Hungary (2), Sweden (1) | 17 | 10.3 | 4.6 | ↑↑ |
| 9 | Norovirus and other Calicivirus | Food of non‐ animal origin | 14 | Latvia (3), Poland (3), Italy (3), Germany (2), Finland (2), Netherlands (1) | 11 | 14.1 | 5.9 | stable |
| 10 | Salmonella spp. | Food of non‐animal origin | 12 | Poland (6), Latvia (3), Sweden (2), Finland (1) | 9 | 16.4 | 5.7 | ↓ |
Rank of the food vehicle based on the number of strong‐evidence FBOs in which the causative agent/food vehicle pair was implicated (rank 1 is the highest rank meaning the most commonly implicated). Strong‐evidence outbreaks with unknown causative agents are not included.
Single arrow indicates variations between 25% and 50%; double arrows indicate variations > 50%; ‘stable’ value indicates variations between −25% and +25%. ‘B. cereus’ includes FBOs with causative agent encoded as ‘B. cereus enterotoxins’. ‘C. perfringens’ includes FBOs with causative agent encoded as ‘Clostridium unspecified’. ‘Fish and fishery products’ include ‘crustaceans, shellfish, molluscs and products thereof’, as well as fish and fish products. ‘Food of non‐animal origin’ includes cereal products including rice and seeds/pulses (nuts, almonds), fruit, berries and juices and other products thereof, herbs and spices, sweets and chocolate, vegetables and juices and other products thereof.‘Meat and meat products’ include bovine meat and products thereof, broiler meat (Gallus gallus) and products thereof, other or mixed red meat and products thereof, other, mixed or unspecified poultry meat and products thereof, pig meat and products thereof, sheep meat and products thereof, turkey meat and products thereof.
Table 56.
Top nine pathogen/food vehicle pairs causing the highest number of deaths in strong‐evidence outbreaks, in reporting EU MS
| 2019 | 2010–2018 | Evaluation (2019 vs. 2010–2018)b | ||||||
|---|---|---|---|---|---|---|---|---|
| Ranka | Causative agent | Food vehicle | Deaths (N) | Reporting MS (no. of deaths) | Rank | Deaths (mean N/year) | Reporting MS (mean N/year) | |
| 1 | Listeria monocytogenes | Meat and meat products | 20 | United Kingdom (9), Netherlands (6), Spain (3), Italy (2) | 2 | 1.8 | 0.8 | ↑↑ |
| 2 | Clostridium perfringens | Food of non‐animal origin | 2 | France (2) | – | – | – | – |
| 3 | Bacterial toxins, unspecified | Mixed food | 1 | France (1) | – | – | – | – |
| 3 | Salmonella spp. | Eggs and egg products | 1 | United Kingdom (1) | 2 | 1.8 | 1.2 | ↓ |
| 3 | Bacillus cereus | Mixed food | 1 | Spain (1) | – | – | – | – |
| 3 | Shiga toxin‐producing E. coli (STEC) | Milk and milk products | 1 | United Kingdom (1) | 18 | 0.2 | 0.2 | ↑↑ |
| 3 | Clostridium botulinum | Other foods | 1 | Poland (1) | 10 | 0.4 | 0.3 | ↑↑ |
| 3 | Clostridium perfringens | Meat and meat products | 1 | Italy (1) | 6 | 0.9 | 0.7 | stable |
| 3 | Salmonella spp. | Meat and meat products | 1 | Poland (1) | 2 | 1.8 | 0.9 | ↓ |
Rank of the food vehicle based on the number of deaths in strong‐evidence FBOs in which the causative agent/food vehicle pair was implicated (rank 1 is the highest rank meaning the most commonly implicated). Strong‐evidence outbreaks with unknown causative agents are not included.
Single arrow indicates variations between 25% and 50%; double arrows indicate variations > 50%; ‘stable’ value indicates variations between −25% and +25%. ‘B. cereus’ includes FBOs with causative agent encoded as ‘B. cereus enterotoxins’. ‘C. perfringens’ includes FBOs with causative agent encoded as ‘Clostridium unspecified’. ‘Food of non‐animal origin’ includes cereal products including rice and seeds/pulses (nuts, almonds), fruit, berries and juices and other products thereof, herbs and spices, sweets and chocolate, vegetables and juices and other products thereof. ‘Meat and meat products’ include bovine meat and products thereof, broiler meat (Gallus gallus) and products thereof, other or mixed red meat and products thereof, other, mixed or unspecified poultry meat and products thereof, pig meat and products thereof, sheep meat and products thereof, turkey meat and products thereof. ‘Milk and milk products include cheese, dairy products (other than cheeses), milk.‘Other foods’ includes canned food products and other foods, unspecified.
Table 54.
Top‐10 pathogen/food vehicle pairs causing the highest number of cases in strong‐evidence outbreaks in reporting EU MS, 2019
| 2019 | 2018–2010 | Evaluation (2019 vs. 2010–2018)b | ||||||
|---|---|---|---|---|---|---|---|---|
| Ranka | Causative agent | Food vehicle | Cases (N) | Reporting MS (N cases) | Ranka | Cases (mean N/year) | Reporting MS (mean N/year) | |
| 1 | Salmonella spp. | Mixed food | 1,549 | Hungary (711), Poland (430), Belgium (203), Romania (160), Czechia (20), Spain (10), Germany (8), France (7) | 10 | 510.4 | 8.4 | ↑↑ |
| 2 | Norovirus and other Calicivirus | Fish and fishery products | 1,178 | France (756), Sweden (175), Spain (64), Denmark (59), United Kingdom (58), Finland (51), Croatia (8), Netherlands (7) | 14 | 359.3 | 6.3 | ↑↑ |
| 3 | Salmonella spp. | Eggs and egg products | 1,172 | Spain (359), Austria (321), Germany (151), Netherlands (104), United Kingdom (88), Poland (67), France (31), Italy (19), Hungary (13), Latvia (13), Croatia (6) | 3 | 1,160.4 | 9.7 | stable |
| 4 | Norovirus and other Calicivirus | Water | 984 | Greece (638), Czechia (268), Spain (60), Finland (18) | 6 | 832.6 | 3 | stable |
| 5 | Salmonella spp. | Meat and meat products | 950 | France (178), Hungary (152), Poland (113), United Kingdom (112), Germany (103), Spain (93), Czechia (74), Denmark (68), Latvia (21), Croatia (18), Netherlands (6), Sweden (5), Estonia (4), Italy (3) | 4 | 1,060.6 | 11.8 | stable |
| 6 | Clostridium perfringens | Meat and meat products | 589 | France (159), Spain (154), Denmark (74), Greece (58), United Kingdom (56), Italy (55), Hungary (21), Germany (12) | 7 | 679.3 | 5.2 | stable |
| 7 | Clostridium perfringens | Mixed food | 507 | Denmark (268), France (115), Portugal (60), Sweden (34), United Kingdom (30) | 12 | 392.9 | 4.3 | ↑ |
| 8 | Bacillus cereus | Mixed food | 431 | Spain (170), Hungary (155), Sweden (39), Germany (29), France (26), Italy (12) | 18 | 242.4 | 4.3 | ↑↑ |
| 9 | Salmonella spp. | Bakery products | 368 | Poland (300), Czechia (54), Spain (14) | 16 | 310.6 | 4.6 | stable |
| 10 | Norovirus and other Calicivirus | Food of non‐ animal origin | 337 | Latvia (144), Poland (73), Finland (39), Germany (36), Netherlands (23), Italy (22) | 2 | 1,737.8 | 5.9 | ↓↓ |
Rank of the food vehicle based on the number of cases of illness in strong‐evidence FBOs in which the causative agent/food vehicle pair was implicated (rank 1 is the highest rank meaning the most commonly implicated). Strong‐evidence outbreaks with unknown causative agents are not included.
Single arrow indicates variations between 25% and 50%; double arrows indicate variations > 50%; ‘stable’ value indicates variations between −25% and +25%. ‘B. cereus’ includes FBOs with causative agent encoded as ‘B. cereus enterotoxins’. ‘C. perfringens’ includes FBOs with causative agent encoded as ‘Clostridium unspecified’. ‘Fish and fishery products’ include ‘crustaceans, shellfish, molluscs and products thereof’, as well as ?fish and fish products’. ‘Food of non‐animal origin’ include cereal products including rice and seeds/pulses (nuts, almonds), fruit, berries and juices and other products thereof, herbs and spices, sweets and chocolate, vegetables and juices and other products thereof.‘Meat and meat products’ include bovine meat and products thereof, broiler meat (Gallus gallus) and products thereof, other or mixed red meat and products thereof, other, mixed or unspecified poultry meat and products thereof, pig meat and products thereof, sheep meat and products thereof, turkey meat and products thereof. ‘Water’ includes Tap water, including well water.
Table 55.
Top‐10 pathogen/food vehicle pairs causing the highest number of hospitalisations, in reporting EU MS, 2019
| 2019 | 2018–2010 | Evaluation (2019 vs 2010–2018)b | ||||||
|---|---|---|---|---|---|---|---|---|
| Ranka | Causative agent | Food vehicle | Hospitalisations (N) | Reporting MS (N hospitalisations) | Ranka | Hospitalisations (mean N/year) | Reporting MS (mean N/year) | |
| 1 | Salmonella spp. | Eggs and egg products | 351 | Austria (119), Spain (88), Germany (61), Poland (47), United Kingdom (13), France (12), Hungary (6), Italy (3), Latvia (2) | 2 | 275.2 | 9 | ↑ |
| 2 | Salmonella spp. | Mixed food | 194 | Hungary (109), Poland (48), Romania (27), Spain (4), Germany (4), Czechia (2) | 4 | 96.2 | 7.2 | ↑↑ |
| 3 | Listeria monocytogenes | Meat and meat products | 190 | Spain (131), Netherlands (34), United Kingdom (12), Finland (7), Italy (6) | 23 | 10.2 | 0.8 | ↑↑ |
| 4 | Salmonella spp. | Meat and meat products | 178 | Czechia (40), Poland (35), Hungary (33), Germany (26), France (19), Spain (15), Italy (3), Croatia (3), Estonia (2), Latvia (2) | 3 | 220.7 | 10 | stable |
| 5 | Salmonella spp. | Bakery products | 77 | Poland (63), Czechia (11), Spain (3) | 5 | 81.3 | 4.3 | stable |
| 6 | Salmonella spp. | Buffet meals | 65 | Spain (40), Romania (24), Lithuania (1) | 7 | 73.3 | 2.1 | stable |
| 7 | S. aureus | Mixed food | 53 | Italy (44), Germany (9) | 11 | 31.2 | 3.1 | ↑↑ |
| 8 | Salmonella spp. | Other foods | 33 | Romania (26), Poland (6), Spain (1) | 9 | 47.4 | 4 | ↓ |
| 9 | norovirus and other Calicivirus | Water | 26 | Greece (23), Czechia (3) | 56 | 2.7 | 0.8 | ↑↑ |
| 9 | Hepatitis A virus | Bakery products | 26 | Germany (26) | 29 | 6.8 | 0.2 | ↑↑ |
Rank of the food vehicle based on the number of hospitalisations in strong‐evidence FBOs in which the causative agent/food vehicle pair was implicated (rank 1 is the highest rank meaning the most commonly implicated). Strong‐evidence outbreaks with unknown causative agents are not included.
Single arrow indicates variations between 25% and 50%; double arrows indicate variations > 50%; ‘stable’ value indicates variations between −25% and +25%. ‘Hepatitis A’ includes also FBOs with causative agent encoded as ‘hepatitis virus, unspecified’. ‘S. aureus’ includes FBOs with causative agent encoded as ‘Staphylococcus, unspecified’ or ‘Staphylococcal enterotoxins’. ‘Meat and meat products’ include bovine meat and products thereof, broiler meat (Gallus gallus) and products thereof, other or mixed red meat and products thereof, other, mixed or unspecified poultry meat and products thereof, pig meat and products thereof, sheep meat and products thereof, turkey meat and products thereof.‘Other foods’ include canned food products and other foods, unspecified.‘Water’ includes Tap water, including well water.
4.3.3. Distribution of food vehicles implicated in strong‐evidence and weak‐evidence outbreaks caused by different causative agents
The description of foodstuffs most frequently implicated in food‐borne outbreaks provides useful indications about which sources at the primary production level or in the various sectors of food preparation should be targeted by control policies to reduce the public health impact of food‐borne pathogens in humans. For each causative agent, the food vehicles implicated in outbreaks in 2019 are described in Figure 63. In these figures, foodstuffs implicated in strong‐evidence FBOs (dark coloured bars on left) are matched in parallel with suspect foods implicated in weak‐evidence outbreaks (light coloured bars on the right). This visualisation allows presentation of the whole bulk of information provided by MS on food, but on the same time representing the different level of uncertainty affecting the findings. Data on foods implicated in weak‐evidence FBOs must be interpreted with caution, given the high level of uncertainty affecting evidence from weak‐evidence FBO.
Figure 63.

Distribution of food vehicles implicated in strong‐ and weak‐evidence food‐borne, by causative agents, in reporting EU MS, 2019
-
‘Hepatitis A’ includes also FBOs with causative agent encoded as ‘hepatitis virus, unspecified’.‘B. cereus’ includes FBOs with causative agent encoded as B. cereus enterotoxins. ‘C. perfringens’ includes FBOs with causative agent encoded as Clostridium unspecified. ‘S. aureus’ includes FBOs with causative agent encoded as Staphylococcus, unspecified’ or Staphylococcal enterotoxins.
In 2019, 21 MS reported information to EFSA on the suspected food vehicle in 1,960 weak‐evidence outbreaks (37.9% of all outbreaks). The ranking of the importance of food very consistent with the grading based on strong‐evidence outbreaks, for all the causative agents, with few exceptions.
In outbreaks caused by S. Typhimurium and monophasic S. Typhimurium, ‘eggs and egg products’ was the foodstuff most frequently reported, followed by ‘pig meat’. However, the link between the consumption of ‘eggs and egg products’ and the outbreaks was only supported by weak evidence (14 weak‐evidence outbreaks; 22% of weak‐evidence FBOs caused by S. Typhimurium and monophasic S. Typhimurium). This ranking was similar to that observed at the EU level for strong‐evidence outbreaks between 2010 and 2018. Discrepancies are also present in the ranking of items associated with outbreaks of STEC infection. Although ‘meat and meat products’ ranked first among strong‐evidence outbreaks (two strong‐evidence outbreak, four weak‐evidence outbreaks), ‘water’ was the source most frequently suspected (one strong‐evidence outbreak, 10 weak‐evidence outbreaks). This finding deserves attention because waterborne outbreaks caused by STEC, even severe and large events, have been reported in the literature due to contamination of either public or private drinking water, recreational water, lake, rivers, wells (Vanden Esschert et al., 2020). Interestingly, among all items described in STEC outbreaks in the last 10 years, water was the most frequently reported item (79 outbreaks between 2010 and 2018). Only a minority of these outbreaks (20 outbreaks; 25.3%) however were classified as strong‐evidence outbreaks. The lack of standard methods for the detection of STEC in water and the analytical difficulties connected with this matrix could be a reason to explain the low proportion of STEC waterborne outbreaks supported by strong‐evidence.
4.4. Overview of the places of exposure
The description of the settings of the outbreaks (places of exposure) characterises the stages of the food preparation chain where incidents leading to food contamination may have occurred and provides indications of where to plan risk mitigation strategies and control measures to prevent food‐borne illnesses. Figure 64 describes strong‐evidence FBOs’ characteristics by place of exposure. The analysis of the settings implicated in FBOs in 2019 has been limited to strong‐evidence outbreaks to avoid introducing the high‐level of uncertainty that affected weak‐evidence outbreaks reported by MS. This is evidenced by Figures 64 and 65 which show the ranking of the places of exposure implicated in strong‐ and weak‐evidence outbreaks, based on the number of outbreaks and cases of illness, respectively.
Figure 64.

Distribution of the number of strong‐ and weak‐evidence food‐borne outbreaks, by place of exposure (setting), in reporting EU MS, 2019
-
Note: Data on other settings (287) include: Camp or picnic (28), Farm (7), Others (243), Temporary mass catering (fairs or festivals) (9).N = number of outbreaks.
Figure 65.
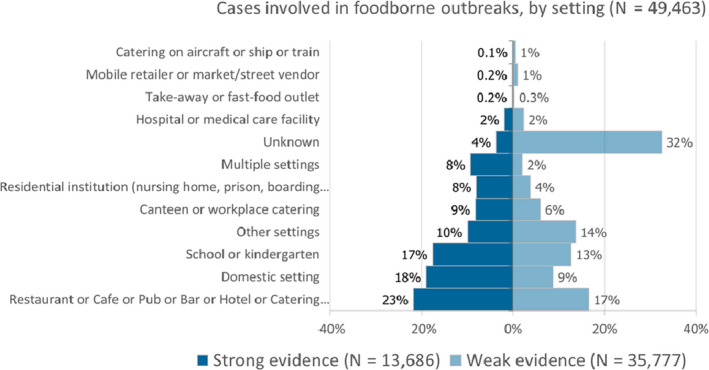
Distribution of the number of cases involved in strong‐ and weak‐evidence food‐borne outbreaks, by place of exposure (setting), in reporting EU MS, 2019
-
Note: Data on cases who became infected in other settings (6,252 cases) include: camp or picnic (776), farm (118), others (4,565), temporary mass catering (fairs or festivals) (793).N = number of cases.
In 2019, most of the strong‐evidence FBOs were from ‘domestic setting’ (N = 296), similarly to previous years. This number is probably underestimated given that only 10 MS among those reporting strong‐evidence outbreaks in 2019 (N = 23) communicated data on household outbreaks. Not surprisingly, most of the outbreaks in domestic settings are classified as ‘household outbreak’, meaning that all the human cases live in one single household (259 outbreaks; 87.5% of total outbreaks in domestic setting).
Table 57.
Frequency distribution of strong‐evidence food‐borne outbreaks by place of exposure (setting), in reporting EU, MS, 2019
| Type of setting | Strong‐evidence outbreaks | Reporting Rate per 100,000 | |||||
|---|---|---|---|---|---|---|---|
| Number of outbreaks | % of total | Number of human cases | % of total | 2019 | 2010–2018 (mean) | ||
| Domestic setting | 296 | 41.3 | 2,605 | 19.0 | 0.058 | 0.048 | |
| Canteen or catering at workplace, school, hospital, etc. | |||||||
| School or kindergarten | 32 | 4.5 | 2,407 | 17.6 | 0.006 | 0.009 | |
| Residential institution (nursing home or prison or boarding school) | 32 | 4.5 | 1,096 | 8.0 | 0.006 | 0.004 | |
| Canteen or workplace catering | 18 | 2.5 | 1,128 | 8.2 | 0.004 | 0.005 | |
| Hospital or medical care facility | 10 | 1.4 | 260 | 1.9 | 0.002 | 0.002 | |
| Catering on aircraft or ship or train | 1 | 0.1 | 10 | 0.1 | < 0.001 | 0.001 | |
| Subtotal | 93 | 13.0 | 4,901 | 35.8 | 0.018 | 0.021 | |
| Restaurant, pub, street vendors, take‐away, etc. | |||||||
| Restaurant or café or pub or bar or hotel or catering service | 195 | 27.2 | 2,978 | 21.8 | 0.038 | 0.032 | |
| Mobile retailer or market/street vendor | 7 | 1.0 | 26 | 0.2 | 0.001 | 0.001 | |
| Take‐away or fast‐food outlet | 3 | 0.4 | 31 | 0.2 | 0.001 | 0.001 | |
| Subtotal | 205 | 28.6 | 3,035 | 22.2 | 0.040 | 0.034 | |
| Other settings | |||||||
| Others | 48 | 6.7 | 873 | 6.4 | 0.009 | 0.008 | |
| Multiple places of exposure in one country | 32 | 4.5 | 1,214 | 8.9 | 0.006 | 0.001 | |
| Camp or picnic | 14 | 2.0 | 359 | 2.6 | 0.003 | 0.002 | |
| Farm | 5 | 0.7 | 103 | 0.8 | 0.001 | 0.001 | |
| Multiple places of exposure in more than one country | 3 | 0.4 | 62 | 0.5 | 0.001 | < 0.001 | |
| Temporary mass catering (fairs or festivals) | 2 | 0.3 | 25 | 0.2 | < 0.001 | 0.002 | |
| Subtotal | 104 | 14.5 | 2,636 | 19.3 | 0.020 | 0.016 | |
| Unknown | 18 | 2.5 | 509 | 3.7 | 0.004 | 0.014 | |
| Total (EU) | 716 | 100 | 13,686 | 100 | 0.141 | 0.133 | |
In ‘general outbreaks’ (i.e. outbreaks involving cases of more than one household), (431 outbreaks; 60.2% of strong‐evidence outbreaks), ‘restaurant, pub, street vendors, take‐away, etc.’ were the settings most frequently described (202 outbreaks; 46.9% of strong‐evidence general outbreaks), while ‘canteen or catering to workspace, school, hospital, etc.’ were the places where most cases became exposed to contaminated foods (4,899 cases; 39.3% of strong‐evidence general outbreaks). Outbreaks linked to ‘canteen or catering at workplace, school, hospital, etc’ were on average much larger (mean cases: 52.7) than those in the ‘restaurant, pub, street vendors, take‐away, etc’ (mean cases: 14.8 cases). In 2019, 12 large outbreaks connected to ‘canteen or catering to workspace, school, hospital, etc’ category were responsible altogether for 2,734 cases (20.0% of all cases involved in strong‐evidence outbreaks). Eight of these large outbreaks occurred in ‘school/kindergarten’ and were mainly associated with S. Enteritidis in mixed foods (5 outbreaks), including one outbreak reported by Hungary that involved 575 cases and 80 hospitalisations. The three other large outbreaks were caused by B. cereus toxins, due to inadequate heat treatment, by norovirus due to food contamination by food handlers and by an unknown agent.
Causative agents identified in strong‐evidence outbreaks in the different settings are described in Figure 66. The bar chart makes it possible to visualise the importance of causative agents in each group of settings. The findings refer to strong‐evidence outbreaks only, to reduce the degree of uncertainty characterising weak‐evidence outbreaks.
Figure 66.
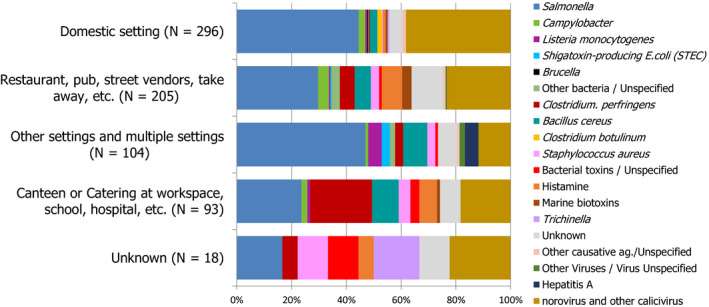
Distribution of strong‐evidence food‐borne outbreaks, by place of exposure (setting) and by causative agent, in reporting EU MS, 2019
-
Note: ‘Hepatitis A’ includes also FBOs with causative agent encoded as ‘hepatitis virus, unspecified’. ‘B. cereus’ includes FBOs with causative agent encoded as B. cereus enterotoxins. ‘C. perfringens’ includes FBOs with causative agent encoded as Clostridium unspecified. ‘S. aureus’ includes FBOs with causative agent encoded as Staphylococcus, unspecified’ or Staphylococcal enterotoxins. Other bacterial agents include enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC), Vibrio parahaemolyticus, Shigella and Yersinia.Other viruses include flavivirus (tick‐borne Encephalitis virus) and other unspecified viruses.Other causative agents include atropine, mushrooms toxins and other toxins, unspecified.‘Restaurant, pub, street vendors, take‐away, etc.’ includes: restaurant or café or pub or bar or hotel or catering service, mobile retailer or market/street vendor, take‐away or fast‐food outlet.‘Canteen or catering at workplace, school, hospital, etc.’ includes: school or kindergarten, residential institution (nursing home or prison or boarding school), canteen or workplace catering, hospital or medical care facility, catering on aircraft or ship or train.‘Other settings’ includes: camp or picnic, farm, multiple places of exposure in one country, multiple places of exposure in more than one country, other settings unspecified, temporary mass catering (fairs or festivals).
4.5. Contributing factors in strong‐evidence food‐borne outbreaks
Information on factors contributing to food contamination and outbreaks was available for a minority of food‐borne outbreaks (Figure 67). In household outbreaks the use of unprocessed contaminated ingredients was frequently reported (19 of 29 outbreaks with this information available). In general outbreaks, risk factors were documented in 167 strong‐evidence outbreaks (38.7% of strong‐evidence general outbreaks). Contamination by ‘food handlers’ was reported in 35 outbreaks in various settings and was mainly associated with norovirus (14 outbreaks; 16.9% of total strong‐evidence outbreaks caused by norovirus) and Salmonella (9 outbreaks; 6.3% of total strong‐evidence general outbreaks caused by this agent). ‘Cross‐contamination’ was identified in 39 outbreaks, mainly caused by Salmonella (15 outbreaks; 10.6% of total strong‐evidence general outbreaks caused by this agent) as well as in six and four outbreaks caused by Campylobacter and L. monocytogenes, respectively (40% and 44% of total strong‐evidence general outbreaks caused by these agents, each). ‘Inadequate heat treatment’ was identified in 45 outbreaks, mainly caused by C. perfringens toxins (14 strong‐evidence outbreaks; 37.8% of total strong‐evidence general outbreaks caused by this agent) and Salmonella (14 outbreaks; 9.9% of total strong‐evidence general outbreaks caused by this agent). In 30 outbreaks, mainly associated with either C. perfringens toxins (12 outbreaks; 33.3% of total strong‐evidence general outbreaks caused by this agent) or B. cereus, S. aureus and histamine, ‘time/temperature storage abuse’ was identified. ‘Inadequate chilling’ contributed to 24 outbreaks.
Figure 67.
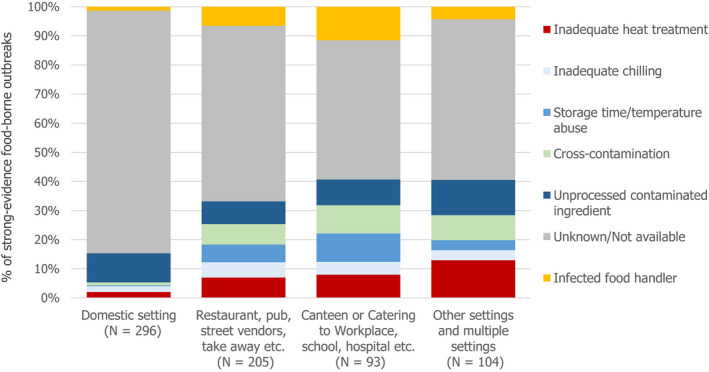
Frequency distribution of contributing factors in strong‐evidence food‐borne outbreaks, by place of exposure (setting), in reporting EU MS, 2019
-
Note: ‘Restaurant, pub, street vendors, take‐away, etc.’ includes; restaurant or café or pub or bar or hotel or catering service, mobile retailer or market/street vendor, take‐away or fast‐food outlet.‘Canteen or catering at workplace, school, hospital, etc.’ includes; school or kindergarten, residential institution (nursing home or prison or boarding school), canteen or workplace catering, hospital or medical care facility, catering on aircraft or ship or train.‘Other settings’ includes; camp or picnic, farm, multiple places of exposure in one country, multiple places of exposure in more than one country, other settings unspecified, temporary mass catering (fairs or festivals).
4.6. Temporal trends by causative agents 2010–2019
4.6.1. Temporal trend at the EU level
Figure 68 shows the number of FBOs reported by MS during 2010–2019, by causative agent, including strong‐evidence and weak‐evidence FBOs. The two graphs allow demonstration of the importance of the causative agents at the EU level, in terms of absolute number of FBOs and visualising the major differences among them. It is important to remember that the variations over years in the frequency distribution of causative agents may not reflect the true epidemiological pattern at the EU level as the collection of outbreak data is not fully harmonised among MS.
Figure 68.
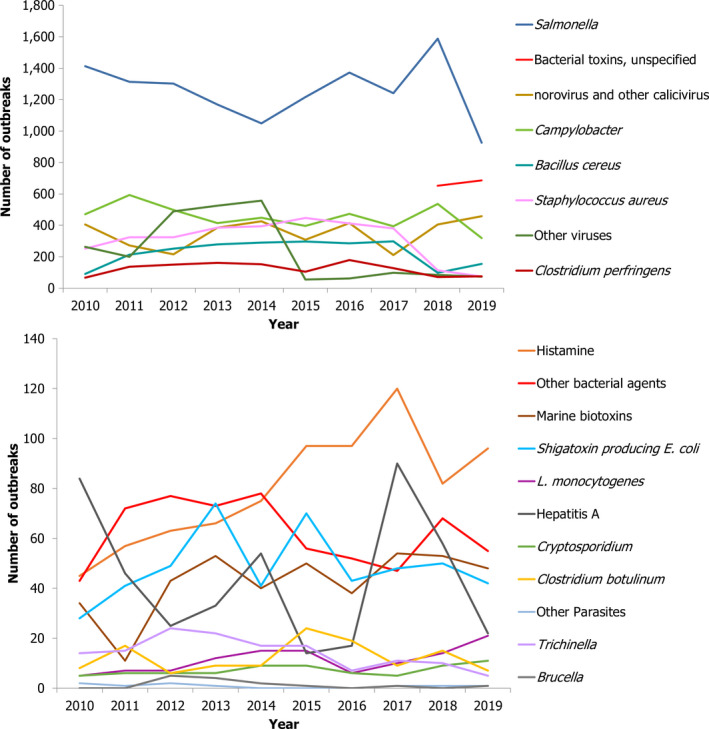
Number of food‐borne outbreaks, by causative agent, in reporting EU MS, 2010–2019
- Note: other bacterial agents include Aeromonas, Arcobacter, enteroaggregative E. coli (EAEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), E. coli unspecified, Francisella tularensis, Leptospira spp., Shigella spp., Streptococcus spp., Vibrio parahaemolyticus, Yersinia enterocolitica and other unspecified bacteria. ‘Hepatitis A’ includes also FBOs with causative agent encoded as ‘hepatitis virus, unspecified’. Other parasites include Anisakis, Cysticercus, Giardia and other unspecified parasites. Other causative agents include atropine, lectin, wax ester and other unspecified toxins.
4.6.2. Temporal country‐specific trends
Figure 69 shows the distribution of Salmonella outbreaks, including strong‐evidence and weak‐evidence ones, and the outbreak reporting rate (per 100,000) in MS and non‐MS during 2010–2019. The trend analysis showed a statistically significant decrease in the number of Salmonella outbreaks for three MS (Austria, Germany, Lithuania). The trend was primarily driven by S. Enteritidis outbreaks whose progressive decrease over the time period in question was also statistically significant in all the three MS, plus Hungary (Figure 70). Austria and Germany also reported significant decreasing trends for outbreaks caused by S. Typhimurium and monophasic S. Typhimurium. For Austria and Germany, the negative trend in Salmonella outbreaks matches with the corresponding significant negative time trend for the Salmonella outbreak cases (data not shown). In Lithuania outbreak illnesses also decreased, but by a lower proportion. For the other MS and non‐MS, no significant trends were observed for outbreaks caused by Salmonella spp. (all serovars), S. Enteritidis or S. Typhimurium, including its monophasic variants.
Figure 69.

Trends of number of Salmonella outbreaks (left axis) and Salmonella outbreak reporting rate (per 100,000 population) (right axis), reporting EU MS and non‐MS, 2010–2019
-
Note: The orange line (right axis) in the graphs represents the Salmonella outbreak reporting rate and was measured on the same scale for all MS, to allow a direct comparability among countries. The blue bars present the trend over years in terms of absolute numbers of Salmonella outbreaks, using for each country the most appropriate scale (left axis).* indicates countries with a statistically significant trend (p < 0.05) over several years.
Figure 70.
Trends in number of outbreaks (left axis) and outbreak reporting rate (per 100,000 population) (right axis) by causative agent, in reporting EU MS, 2010–2019. Only MS and causative agents with a statistically significant temporal trend are shown
- Note: only causative agents and countries with statistically significant trends and more than five outbreaks reported per year, on average, are visualised. ‘B. cereus’ includes FBOs with causative agent encoded as B. cereus enterotoxins. ‘Hepatitis A’ includes also FBOs with causative agent encoded as ‘hepatitis virus, unspecified’.
Other statistically significant trends in occurrence of FBOs by causative agents and MS are shown in Figure 70. Given the lack of specific control programmes it is difficult to unravel the reasons underlying these trends. Campylobacter outbreaks in Austria dropped significantly in recent years. However, no information on implicated food vehicles was available for most of these outbreaks (444 of the 499 outbreaks reported between 2010 and 2019). Similarly, reasons underlying the trends for outbreaks caused by bacterial toxins and histamine could not be readily elucidated, mainly because the circumstances leading to intake of toxins or histamine through food vary importantly and are highly dependent on the conditions and practices of food preparation and preservation in the close proximity of consumers. The increasing trend for Hepatitis A in Germany refers to a small number of outbreaks and does not match with a parallel increase in the number of Hepatitis A cases. Reasons underlying increasing or decreasing trends of outbreaks caused by unknown agents might reflect progressive changes in the sensitivity of outbreak surveillance due to variations in the criteria for outbreak definition or improved citizens’ engagement with FBOs surveillance.
4.6.3. Temporal trends by implicated food vehicles
Figure 71 displays country‐specific significant trends in the number of strong‐evidence outbreaks for specific food vehicles, during 2010–2019. Decreasing trends were noted for ‘eggs and egg products’ in France and Poland, ‘fish and fishery products’ in the United Kingdom, ‘meat and meat products’ in the United Kingdom and ‘mixed foods’ in Belgium, Germany and Denmark. The decreasing trend for outbreaks by ‘eggs and egg products’ was mainly driven by S. Enteritidis in Poland and by S. Enteritidis and other serovars in France. In both countries, the number of Salmonella outbreaks decreased progressively, although with large yearly fluctuations, especially in recent years, suggesting that the trend is not stable. This is a reason of concern also considering that eggs from Poland in recent years have been repeatedly implicated in large prolonged multi‐county outbreaks responsible for hundreds of cases reported in 18 MS. During 2010–2019, in Germany the reporting of outbreaks by ‘milk and milk products’ increased, even though in the most recent years a reverse trend was observed. This pattern was mainly guided by progressive variations in the number of Campylobacter outbreaks. Reasons explaining the trends for outbreaks for the other types of food are less evident.
Figure 71.
Trends for number of strong‐evidence outbreaks (left axis) and outbreak reporting rate (per 100,000 population) (right axis), by food vehicle, in reporting EU MS, 2010–2019
-
Note: only food vehicles and countries with statistically significant trends and more than five outbreaks reported per year, on average, are shown.‘Fish and fishery products’ include ‘crustaceans, shellfish, molluscs and products thereof’, as well as ‘fish and fish products’. ‘Meat and meat products’ include bovine meat and products thereof, broiler meat (Gallus gallus) and products thereof, other or mixed red meat and products thereof, other, mixed or unspecified poultry meat and products thereof, pig meat and products thereof, sheep meat and products thereof, turkey meat and products thereof. ‘Milk and milk products include cheese, dairy products (other than cheeses), milk. ‘Other foods’ includes canned food products and other foods, unspecified.
4.7. Waterborne outbreaks
Forty‐three waterborne outbreaks, meaning outbreaks associated with the consumption of ‘tap water, including well water’, were reported in 2019 by eight MS (Croatia, Czechia, Finland, France, Greece, Ireland, Italy, Spain). In addition, seven waterborne outbreaks were reported by four non‐MS (Norway, Republic of North Macedonia, Serbia, Switzerland). Overall, 11 waterborne outbreaks were reported as strong‐evidence outbreaks by five MS (Czechia, Finland, Greece, Italy, Spain) and involved 1,170 cases and 31 hospitalisations. Other seven strong‐evidence waterborne outbreaks, involving 2,219 cases and 21 hospitalisations were reported by four non‐MS (Norway, Rep. of North Macedonia, Serbia, Switzerland). Agents detected in strong‐evidence outbreaks in MS were ‘norovirus and other Calicivirus’ (six outbreaks), Cryptosporidium parvum (one outbreak) and STEC (one outbreak). The agent was unknown in three outbreaks. In non‐MS, the causative agents included norovirus (one outbreaks), hepatitis A virus (two outbreaks), F. tularensis (two outbreaks), Cryptosporidium (one outbreak) and C. jejuni (one outbreak). Waterborne outbreaks are often large or very large. In 2019, the mean number of cases in strong‐evidence waterborne outbreaks was 106 in MS and 304 in non‐MS. In Norway, C. jejuni was responsible for more than 2,000 cases in a single outbreak. Six outbreaks caused by ‘norovirus and other Calicivirus’ in MS resulted in 984 cases.
Most of the weak‐evidence waterborne outbreaks reported by eight MS were caused by STEC (10 outbreaks), norovirus and other Calicivirus (six outbreaks), Giardia (three outbreaks), Cryptosporidium (one outbreak), ‘bacterial toxins, unspecified’ (one outbreak), ‘virus, unspecified’ (one outbreak). For 10 weak‐evidence waterborne outbreaks the agent remained unknown. The mean outbreak size of waterborne outbreaks was 24.5.
4.8. Multi‐country food‐borne outbreaks
In 2019, ECDC and EFSA produced Joint Rapid Outbreak Assessments (ROA). These assessments concerned outbreaks caused by L. monocytogenes, Salmonella Poona and Salmonella Enteritidis. In all these outbreaks, clinical isolates were analysed using whole genome sequencing (WGS) allowing tracing of patients linked to the outbreak (and including them retrospectively) and assessing the extension of the outbreaks.
The first outbreak was caused by L. monocytogenes sequence type (ST) 1247, clonal complex (CC) 8, and included 22 notified cases in five EU countries (Denmark, Estonia, Finland, France and Sweden). Cases had occurred between July 2014 and February 2019. Evidence from epidemiological, microbiological, environmental and trace‐back investigations identified cold‐smoked fished products (cold‐smoked salmon and cold‐smoked trout), manufactured by an Estonian processing company, as the suspected source of the outbreak. Control measures were taken in the Estonian processing company and only batches that complied the food safety criterion (absence of L. monocytogenes in 25 g in cold‐smoked and salted product) were released onto the market (EFSA and ECDC, 2019b).
A second prolonged outbreak caused by L. monocytogenes IVb sequence type ST 6 was responsible for 21 cases in two MS (the Netherlands and Belgium). Cases were identified between October 2017 and August 2019. The close genetic similarity of the strains and the temporal distribution suggested that the cases were part of a common‐source food‐borne outbreak. Following the national investigation, various RTE meat products, all manufactured by a Dutch company, were found to be contaminated with L. monocytogenes showing high similarity with the outbreak strain. The company suspended the activities and a withdrawal and recall of all the RTE meat‐based products was implemented as control measure (EFSA and ECDC, 2019d).
Salmonella Poona was the causative agent of a multi‐country outbreak with 32 cases (infants and young children) reported by three MS (France, Belgium and Luxembourg) between August 2018 and February 2019. The link between the cases was established by a core genome Multilocus Sequence Typing (cgMLST) cluster analysis. Epidemiological evidence obtained from the children's parents of 30 out of 32 confirmed cases identified various infant formula products based on rice proteins as the potential vehicles of infection. All were manufactured by the same Spanish company and marketed by a French company. Environmental investigation in the manufacturing plant and food testing were carried out without findings of the bacterium. Nevertheless, a precautionary recall and withdrawal of the infant formula products of the same brand implicated in this outbreak was implemented (EFSA and ECDC, 2019a).
A multi‐country outbreak of S. Enteritidis was responsible for 1,041 confirmed cases and 615 probable cases from 2012 to 2019. The last ROA update, published in February 2020, provided information on cases communicated since November 2018, totalling 248 new cases. Of these, 166 were confirmed cases (including 42 historical‐confirmed cases) belonging to four distinct clusters identified by WGS and 82 (including 46 historical probable cases) were categorised as probable cases, belonging to six distinct MLVA profiles. This outbreak peaked between 2016 and 2018. Epidemiological, microbiological and food tracing investigations identified eggs from laying hen farms of a Polish consortium as the potential source of the outbreak. Control measures implemented at the farm and at the distribution level, including depopulation, cleaning and disinfection of the contaminated henhouses, however failed to limit the spread of the infection. The outbreak strain was persistently detected on the farms of the Polish consortium were positive during 2018–2019 for the outbreak strains, suggesting persistent contamination. To identify possible contamination at a higher level in the food chain, the feed supply chain as well as the origin of the animals were investigated up to parent stocks, but no significant information was obtained (EFSA and ECDC, 2020a).
5. Conclusions
5.1. Health impact, causative agents and trends
In 2019, the reporting of FBOs in the EU did not substantially change from previous years in terms of total outbreaks and illnesses. At country level, a large amount of variability was observed in the epidemiological indicators adopted to describe FBOs such as the reporting rate, the mean outbreak size, the type of outbreaks and their severity. This reflects both epidemiological differences and divergences in the approach and sensitivity of the surveillance of FBOs at the single country level.
Overall, in 2019 fatal illnesses (N = 60) due to FBOs increased by 50% compared with 2018. Most deaths were reported in settings such as ‘residential institution (nursing home or prison or boarding school) and hospital’. This finding calls for attention to the increased risk of vulnerable populations, including elderly and chronically ill patients to food‐borne hazards.
Another critical aspect emerging from the data analysis is the occurrence of FBOs outbreaks in schools and kindergartens. In 2019, almost 20% of cases involved in strong‐evidence general outbreaks (2,407 cases; 1 in 5) became exposed to contaminated foods in a school or kindergarten. In Hungary, a single outbreak of S. Enteritidis involved 575 individuals who had consumed different types of foodstuffs in these settings. Food‐borne outbreaks in schools/kindergartens are frequently reported in the literature and may be large or very large. In 2012, 11,000 people in Germany fell ill with gastroenteritis caused by norovirus, predominantly in schools and childcare facilities (Bernard et al. 2014). In 2019, school/kindergarten outbreaks were reported by 11 MS and involved wide range of causative agents. This suggests the need for strengthening the standard of hygiene and the procedures for food manufacturing and preparation, as well as the HACCP plans for such establishments.
In 2019, although Salmonella was confirmed as the most identified agent in FBOs in the EU/EFTA and it was responsible for the highest number of hospitalisations, L. monocytogenes caused more than 50% of total outbreak associated deaths (31 deaths; 10 deaths more than in 2018; 29 more than in 2017). This is a critical finding as outbreak‐associated cases and hospitalisations caused by L. monocytogenes have continuously increased over the last four years in the EU. A better tracing of patients, especially those affected by severe conditions and invasive listeriosis, resulting from the prompt application of WGS for the characterisation of L. monocytogenes clinical isolates may have contributed to improve the increase. Concern is also represented by the high epidemic potential of L. monocytogenes. In 2019, just after the end of the prolonged multi‐country outbreak by frozen vegetables (EFSA and ECDC, 2018a), L. monocytogenes was identified as the causative agent of multiple prolonged multi‐country outbreaks and was responsible in Spain of one of the largest outbreak ever recorded in the EU with 207 cases involved, 131 hospitalisations and 3 deaths. These findings need to be carefully considered, with particular attention to the large variety of food that may support the growth of L. monocytogenes and that have been implicated in community outbreaks in recent years (i.e. meat and meat products, cold meat, fish, cheese, vegetables).
5.2. Sources and places of exposures
As in recent years, most of the outbreaks in 2019 took place in a ‘domestic settings’ (N = 296; 41.3%). This proportion is probably underestimated since this setting is usually associated with ‘household’ outbreak but not all MS report ‘household’ outbreaks to EFSA. Such a finding reinforces the importance to continuing deliver recommendations to consumers on the correct mode of food preparation, storage and consumption. Among public settings, ‘restaurant, café, pub, bar, hotel catering service’ and ‘school or kindergarten’ are the places associated with the highest number of FBOs and cases, respectively.
The range of foodstuffs that have been identified or suspected in food‐borne outbreaks reported in 2019 closely reflects the known epidemiology of the implicated causative agents. The consumption of foods of animal origin was associated with most of the reported outbreaks, especially those caused by Salmonella and Campylobacter. Salmonella outbreaks caused by the consumption of eggs, meat or meat products accounted for more than twice as many of the outbreaks as those associated with all other items altogether, suggesting the need for continuing implementation of control actions at the primary production level. Notwithstanding, most of the cases involved in Salmonella outbreaks became infected after the ingestion of mixed foods, as well as of other highly manipulated foods such as bakery products, sweets and chocolate, revealing that errors during the preparation and/or the preservation of foods occur frequently.
Outbreaks linked to ‘crustaceans, shellfish, molluscs and products thereof’ increased markedly in 2019 due to outbreaks caused by ‘norovirus and other Calicivirus’ mainly reported from France. Highly manipulated foodstuffs such as mixed foods and buffet meals were also frequently implicated in norovirus outbreaks, in which the contamination of foodstuff by food handlers is very likely.
‘Mixed food’ is a miscellaneous group of foodstuffs which includes a large variety of multi‐element and multi‐origin ingredients. This heterogeneity makes it difficult to identify the primary source of contamination and the mechanisms leading to the contamination and/or cross‐contamination of the final preparation. In 2019, mixed foods were responsible for the highest number of illnesses in strong‐evidence outbreaks in the EU. Incidents leading to contamination of mixed foods (e.g. unhygienic manipulation of the ingredients by food handler, cross‐contamination, temperature abuse) frequently originate during the final preparation of the dishes close to the consumer in either restaurants, public settings or in the home. Preventive strategies in domestic settings require the engagement of citizens and media to deliver recommendations and promote education campaigns.
In 2019, vegetables were associated with the widest range of causative agents of FBOs including bacteria (Escherichia coli, L. monocytogenes, Salmonella, Yersinia), bacterial toxins (B. cereus, C. botulinum), histamine, parasite (Cryptosporidium), virus (hepatitis A, norovirus and other Calicivirus), in spite of the relatively small number of outbreaks reported. Salmonella outbreaks linked to vegetables and fruits should not be overlooked since these events were frequently responsible for a large number of illnesses. In this type of outbreak, mechanisms leading to food contamination are complex and may originate at various levels of the food chain from the growers in the pre‐harvest level up to the processing and retail chain. Water, especially irrigation water at the pre‐harvest level, or wash‐water, as well as wildlife, may play a critical role as a potential source of contamination by food‐borne pathogenic agents especially bacteria and viruses.
Others food/agent pairs that may merit special attention include enterotoxigenic E. coli (ETEC) and enteropathogenic E. coli (EPEC) in vegetables and ‘other foods’, respectively. Although the number of outbreaks caused by ETEC and EPEC reported in 2019 was limited, it is worth noting that the number of outbreaks implicating these agents was the highest since 2010.
In conclusion, to correctly understand the pattern of food vehicles and sources implicated in FBOs, it is important to appreciate that annual variations in the incidence of food‐borne illness depend not only on changes in the prevalence of foods contamination at the consumer level but also on the variations in the type of food being consumed and the consumption habits. Some food preparations, or novel foods or modes of consumption (e.g. delivered‐food, take‐away) may become progressively popular over the years leading to important changes in the pattern of exposure of consumers to food‐borne hazards. Demographic changes and increased susceptibility of vulnerable populations (e.g. the elderly, patients with chronic or immunosuppressive conditions, long‐term proton pump inhibitor users) should also be considered. Climate change may also play a part in increased exposure of foods to contamination, eating habits and multiplication of some bacterial pathogens in foodstuffs.
6. Related projects and internet sources
Zoonoses monitored according the epidemiological situation (Directive 2003/99 List B)
1. Yersinia
This chapter has a simplified structure underpinned by descriptive summarisation of submitted data (see rationale p. 16 of Introduction).
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. The human epidemiological yersiniosis data for 2019 are available at https://www.ecdc.europa.eu/en/publications-data/yersiniosis-annual-epidemiological-report-2019. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
1.1. Key facts
Yersiniosis was the fourth most commonly reported zoonosis in humans in 2019 with 6,961 confirmed cases reported in the EU.
The trend of human yersiniosis cases was stable (flat) in 2015–2019.
Overall, seven MS reported 149 food‐borne cases of yersiniosis and 15 outbreaks. These numbers were similar to those in recent years. In total three were reported with strong‐evidence, by Denmark and Sweden, caused by ‘vegetables and juices and other products thereof and by Finland, caused by ‘buffet meals’. During 2010–2019, the two food categories most reported to cause strong‐evidence food‐borne outbreaks of yersiniosis were ‘pig meat and products thereof’ (3) and ‘vegetables and juices and other products thereof’ (3).
Overall, 907 ‘ready‐to-eat’ food sampling units results were reported by four MS and 76 (8.4%) Yersinia enterocolitica‐positive units were detected: 75 from ‘meat and meat products’ (8.3% positives) and one from ‘other processed food products and prepared dishes’ (one positive sample out of two tested). The positive meat and meat product samples were almost all (71) from mixed meat from bovine animals and pigs and a few (4) were from mixed meat of other animals. For ‘non‐ready‐to-eat’ food 5 MS reported 1,191 sampling unit results and ‘meat and meat products’ and ‘milk and milk products’ were the contaminated food categories, for 2019. Four MS reported on results of fresh meat categories and most positive samples reported were from pig meat (3.3% positives).
1.2. Surveillance and monitoring of Yersinia in the EU
1.2.1. Humans
An overview of the national surveillance systems for human yersiniosis in 2019 is available at https://www.ecdc.europa.eu/en/publications-data/yersiniosis-annual-epidemiological-report-2019
1.2.2. Food and animals
Although the reporting of Yersinia occurrence or prevalence in food and animals is not mandatory, MS can report monitoring data on Yersinia to the European Commission in accordance with the Zoonoses Directive 2003/99/EC. The Directive specifies that, in addition to the number of zoonoses and zoonotic agents, for which monitoring is mandatory, zoonoses such as yersiniosis and agents thereof shall also be monitored when the epidemiological situation so warrants. At present, there is no harmonised surveillance of Yersinia in the EU for food or animals and Yersinia food and animal monitoring data submitted by the MS to EFSA are collected without harmonised design. These data allow for descriptive summaries at the EU level to be made but they preclude trend analyses and trend watching at the EU level (Table 1). A scientific report of EFSA suggested technical specifications for the harmonised monitoring and reporting of Y. enterocolitica in slaughter pigs in the EU (EFSA, 2009b).
The reported occurrence of Yersinia in major food categories for the year 2019 and for the four‐year period 2015–2018 was descriptively summarised making a distinction between RTE and non‐RTE food. Data sets were extracted with ‘objective sampling’ being specified as sampler strategy, which means that the reporting MS collected the samples according a planned strategy based on the selection of a random sample, which is statistically representative of the population to be analysed.
Biotype and serotype of Y. enterocolitica were rarely reported in 2019. Due to the relevance of certain pathoserotypes in the epidemiology of Y. enterocolitica, the access of typing information would be extremely important for a correct assessment of the public health significance and pathogenicity of Y. enterocolitica for humans.
1.2.3. Food‐borne outbreaks of yersiniosis
The reporting of food‐borne yersiniosis disease outbreaks in humans is mandatory according to the Zoonoses Directive 2003/99/EC.
When the UK data were collected the UK was an EU MS but as of 31 January 2020 it has become a third country.
1.3. Summary of the submitted data
1.3.1. Humans
The human data are available at: https://www.ecdc.europa.eu/en/publications-data/yersiniosis-annual-epidemiological-report-2019.
1.3.2. Human yersiniosis cases associated with food‐borne outbreaks
Seven MS reported 15 yersiniosis food‐borne outbreaks for the year 2019 (Denmark (1), Finland (2), France (3), Germany (4), Lithuania (1), Poland (2) and Sweden (2)), causing 149 illnesses, 14 hospitalisations and no deaths. These numbers were similar to recent years. Y. enterocolitica was identified as the causative agent in all these outbreaks but one. Three of these outbreaks were reported with strong‐evidence, by Denmark and Sweden, caused by ‘vegetables and juices and other products thereof’ and by Finland, caused by ‘buffet meals’ (Table 58). Interestingly, the two former strong‐evidence outbreaks, caused by Y. enterocolitica biotype 4, were part of the same single multi‐country outbreak linked to the consumption of food imported in both the Swedish and Danish markets.
Table 58.
Distribution of strong‐evidence yersiniosis food‐borne outbreaks, by food vehicle, EU, 2010–2019
| Food vehicle | Year | Member State | N outbreaks | N illnesses | N hospitalisations | N deaths |
|---|---|---|---|---|---|---|
| Pig meat and products thereof | 2011 | Denmark | 1 | 7 | 0 | 0 |
| Meat and meat products | 2013 | Austria | 1 | 2 | 0 | 0 |
| Milk | 2014 | Finland | 1 | 55 | 4 | 0 |
| Pig meat and products thereof | 2015 | Lithuania | 1 | 2 | 0 | 0 |
| Vegetables and juices and products thereof | 2016 | Finland | 1 | 20 | 2 | 0 |
| Mixed food | 2017 | Denmark (1), Poland (1) | 2 | Denmark (80), Poland (13) | Denmark (6), Poland (2) | 0 |
| Pig meat and products thereof | 2018 | Sweden | 1 | 6 | 0 | 0 |
| Buffet meals | 2019 | Finland | 1 | 3 | 0 | 0 |
| Vegetables and juices and other products thereof | Denmark, Sweden | 2 | Denmark (20), Sweden (37) | 0 | 0 | |
| Total | 11 | 245 | 14 | 0 |
The food categories most reported to cause strong‐evidence yersiniosis food‐borne outbreaks during 2010–2019 were ‘pig meat and products thereof’ and ‘vegetables and juices and other products thereof’ (three each). Further details and statistics on the yersiniosis food‐borne outbreaks for 2019 are in the food‐borne outbreaks chapter.
1.3.3. Yersinia enterocolitica in food and in animals
Table 59 summarises the reported occurrence of Yersinia enterocolitica in the most important food categories for the year 2019 and for the 4‐year period 2015–2018. Distinction is made between RTE and non‐RTE food including fresh meat.
Table 59.
Occurrence of Yersinia enterocolitica in major food categories, EU
| 2019 | 2015–2018 | |||||
|---|---|---|---|---|---|---|
| Food | N reporting MS | N sampling units | Positive N (%) | N reporting MS | N sampling units | Positive N (%) |
| RTE food | ||||||
| All | 4 | 907 | 76 (8.38) | 5 | 124 | 6 (4.84) |
| Meat and meat products | 3 | 901 | 75 (8.32) | 4 | 94 | 5 (5.32) |
| Meat and meat products from pigs | 2 | 17 | 0 | 2 | 32 | 0 |
| Mixed meat and meat products from bovine animals and pigs | 2 | 874 | 71 (8.12) | 0 | – | – |
| Mixed | 1 | 10 | 4 (40.00) | 1 | 50 | 5 (10.00) |
| Milk and milk products | 0 | – | – | 3 | 18 | 0 |
| Salads | 0 | – | – | 1 | 1 | 1 (100.00) |
| Other processed food products and prepared dishes | 1 | 2 | 1 (50.00) | 1 | 2 | 0 |
| Non‐RTE food | ||||||
| All | 5 | 1,191 | 105 (8.82) | 8 | 4,614 | 416 (9.02) |
| Meat and meat products | 4 | 1,066 | 85 (7.97) | 7 | 4,059 | 411 (10.13) |
| Fresh meat from pigs | 3 | 704 | 23 (3.27) | 7 | 1,364 | 171 (12.54) |
| Fresh meat from bovine animals | 1 | 10 | 1 (10.00) | 3 | 16 | 0 |
| Other fresh meat | 3 | 73 | 22 (30.14) | 3 | 144 | 8 (5.56) |
| Milk and milk products | 2 | 90 | 20 (22.22) | 2 | 36 | 4 (11.11) |
| Other food | 1 | 35 | 0 | 4 | 519 | 1 (0.19) |
N: number
For 2019, most results from the 907 RTE food sampling units reported by four MS originated from ‘meat and meat products’ (99.3%), whereas during 2015–2018, 14.5% of the sampling units were from ‘milk and milk products’ and 75.8% from ‘meat and meat products’.
In total 76 RTE food samples were found to be positive for Yersinia enterocolitica in 2019: 75 from ‘meat and meat products’ and one from ‘other processed food products and prepared dishes’. The positive meat and meat product samples were almost all (71) from mixed meat from bovine animals and pigs and a few (4) were from mixed meat of other animals. During 2015–2018, six Yersinia enterocolitica‐positive sampling units were reported for RTE food from ‘meat and meat products’ (five) and from salads (one). All five positive meat samples were from mixed meat of other animals. Monitoring data considered were collected according an ‘objective’ sampling strategy. Also considering that only few MS reported sampling results and that only a few results were reported for food categories other than meat and meat products, the finding of Yersinia enterocolitica‐contaminated RTE food is of concern because it poses a direct risk to the consumer.
Results reported by five MS for non‐RTE food show that ‘meat and Meat products’ and ‘milk and milk products’ were the contaminated food categories, for 2019 and during 2015–2018, during which also a contaminated ‘other processed food products and prepared dishes’ sample was reported. Four MS reported on results of fresh meat categories and overall, most positive samples reported were from pig meat, for 2019 and during 2015–2018.
Table 60 summarises the reported occurrence of Yersinia enterocolitica in animals for the year 2019. In 2019, five MS and two non‐MS reported animal monitoring data.
Table 60.
Summary of Yersinia enterocolitica statistics related to animal species, reporting MS and non‐MS, EU, 2019
| N reporting MS/non‐MS) | N tested unitsa, EU | N and Proportion (%) Yersinia enterocolitica‐positive units, EU | |
|---|---|---|---|
| Animals | |||
| Pigs | 5/1 | 2,561 | 3 (0.1) |
| Domestic livestock other than pigsb | 5/1 | 18,061 | 145 (0.8) |
| Other animal speciesc | 5/1 | 2,533 | 76 (3.0) |
MS: Member State.
The summary statistics were obtained summing all sampling units (single and batch samples).
Alpacas, cattle (bovine animals), Gallus gallus (fowl), goats, reindeers, sheep, domestic solipeds.
Badgers – wild, birds – wild, bison ‐ zoo animals, camels ‐ zoo animals, Cantabrian chamois – wild, cats, cats ‐ pet animals, chinchillas ‐ pet animal, deer, deer – wild, deer ‐ wild ‐ fallow deer, deer ‐ wild ‐ roe deer, dogs ‐ pet animals, ferrets – wild, foxes – wild, guinea pigs ‐ pet animals, hares, hares – wild, hedgehogs – wild, marten – wild, matrix, monkeys ‐ zoo animal, mouflons – wild, other animals ‐ exotic pet animals, otter – wild, parrots ‐ pet animals, pigeons, rabbits ‐ pet animals, raccoons, rats – wild, rodents – wild, squirrels, squirrels – wild, starlings, Steinbock – wild, turtles – wild, water buffalos, wild boars – wild, wolves – wild, zoo animals.
1.4. Related projects and Internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Fact sheet yersiniosis (Yersinia enterocolitica) | https://www.cdc.gov/yersinia/faq.html |
| ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx | |
| EU case definition of yersiniosis | https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions | |
| Disease Programme on Emerging, Food‐ and Vector‐Borne Diseases | https://www.ecdc.europa.eu/en/about-us/who-we-are/units/disease-programmes-unit | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| Food‐animals | Monitoring and identification of human enteropathogenic Yersinia spp. – Scientific Opinion of the Panel on Biological Hazards | https://www.efsa.europa.eu/en/efsajournal/pub/595 |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
2. Toxoplasma gondii
This chapter has a simplified structure underpinned by descriptive summarisation of submitted data (see rationale p. 16 of Introduction).
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
2.1. Key facts
Toxoplasma gondii is widely prevalent in humans and animals world‐wide. Virtually all warm‐blooded animals can act as IHs, but the life cycle is only completed in the DHs: cats and other felines, including lynx which is present in Europe.
Only congenital toxoplasmosis is reported to ECDC. There is two‐year delay in data reporting and the most recent epidemiological data, which pertain to the year 2018, are available at https://www.ecdc.europa.eu/en/publications-data/congenital-toxoplasmosis-annual-epidemiological-report-2018
In 2018, 208 confirmed cases of congenital toxoplasmosis were reported in the EU/EEA, with France accounting for 72.6% of all confirmed cases due to the active screening of pregnant women.
No food‐borne toxoplasmosis outbreak was reported in 2019 in EU and no such single food‐borne outbreak has ever been reported to EFSA since the start of its food‐borne outbreaks data collection in 2004.
In total, 13 MS and two non‐MS reported 2019 monitoring data on Toxoplasma infections in animals. Most animals tested were sheep and goats that also showed the highest overall prevalence of Toxoplasma infections in animals (13.5%) as reported by 12 MS. Most samples were obtained from clinical investigations. It is not possible to make a good estimate of the prevalence of Toxoplasma infections in animals due to the use of different diagnostic methods (indirect methods detecting antibodies vs. direct methods), the different sampling schemes in the MS and the lack of information on the animals’ age and rearing conditions.
2.2. Surveillance and monitoring of Toxoplasma in the EU
2.2.1. Humans
An overview of the national surveillance systems for human congenital toxoplasmosis is available at https://www.ecdc.europa.eu/en/publications-data/congenital-toxoplasmosis-annual-epidemiological-report-2018
2.2.2. Animals
No EU Regulation exists with relation to the surveillance and monitoring of Toxoplasma gondii in animals. Therefore, the available and reported information is strictly determined by national legislation and whether the countries have a mandatory reporting system after the detection of Toxoplasma gondii. The main animal species tested are small ruminants (goats and sheep), cattle, pigs and pet animals (cats and dogs) using samples from aborted animals (ruminants) or clinically suspected animals. Mainly blood samples but also samples from tissue and organs are analysed with either indirect methods to detect antibodies (ELISA, LAT, complement fixation test (CFT) and immunofluorescence assay (IFA)) or direct methods (PCR and immunohistochemistry (IHC)). As the surveillance of Toxoplasma in animals is not harmonised, data on Toxoplasma only allow descriptive summaries to be made at the EU level (Table 1). This is because the results submitted by different countries and from different regions within a country are mostly not directly comparable due to differences in sampling strategy, testing methods, as well as different sampling schemes. Both age of animals and production systems at farm level may influence the occurrence of Toxoplasma.
2.2.3. Food‐borne outbreaks of toxoplasmosis
The reporting of food‐borne toxoplasmosis disease outbreaks in humans is mandatory according the Zoonoses Directive 2003/99/EC.
When the UK data were collected the UK was an EU MS but as of 31 January 2020 it has become a third country.
2.3. Summary of submitted data
2.3.1. Humans
The human data are available at https://www.ecdc.europa.eu/en/publications-data/congenital-toxoplasmosis-annual-epidemiological-report-2018.
2.3.2. Human toxoplasmosis cases associated with food‐borne outbreaks
No food‐borne disease outbreak due to Toxoplasma was reported for 2019 in the EU and no single such food‐borne outbreak has been reported to EFSA since the start of the food‐borne outbreaks reporting, in 2004.
Available information discussed in the EFSA Scientific Opinion of food‐borne parasites (EFSA BIOHAZ Panel, 2018b) suggests that food‐borne transmission accounts for 40–60% of the T. gondii infections.
Food‐borne transmission of Toxoplasma gondii is possible via a range of routes, including consumption of undercooked meat or, to a lesser extent, unpasteurised milk, from an infected animal or via contamination with feline faeces. Although meat is considered to be the more usual source of food‐borne infection in Europe, based on risk factor studies, the exact contribution of different food‐borne routes is still a major research question.
2.3.3. Toxoplasma in food
One MS, Italy, submitted monitoring results for Toxoplasma gondii in food, like the previous two years. In total, 386 samples were reported from non‐RTE fish, meat products from pig, raw molluscan shellfish and from (RTE) honey and potable water.22 Thirty‐nine samples were positive (10.1%) and were from fish (nine), meat products from pig (25) and raw molluscan shellfish (five).
2.3.4. Toxoplasma in animals
Table 61 summarises statistics on Toxoplasma spp. occurrence in major animal species during 2015–2019. Animal data of interest reported were classified into the major categories and aggregated by year to obtain an annual overview of the volume of data submitted.
Table 61.
Summary of Toxoplasma spp. detected in major animal speciesa, EU, 2015–2019
| 2019 | 2018 | 2017 | 2016 | 2015 | |
|---|---|---|---|---|---|
| Small ruminants (animal level) | |||||
| Number of sampling units | 12,120 | 6,756 | 5,421 | 5,561 | 3,139 |
| Proportion of positive units (%) | 13.5 | 18.3 | 13.1 | 18.7 | 38.8 |
| Number of reporting MS | 12 | 12 | 12 | 12 | 11 |
| Cattle (animal level) | |||||
| Number of sampling units | 664 | 158 | 2,163 | 451 | 1,177 |
| Proportion of positive units (%) | 9.2 | 27.8 | 10.5 | 3.3 | 4.2 |
| Number of reporting MS | 6 | 6 | 7 | 8 | 7 |
For the summary statistics indirect and direct diagnostic methods were taken together to calculate proportion of positive units.
Thirteen MS (Austria, Finland, Germany, Greece, Hungary, Ireland, Italy, Latvia, the Netherlands, Romania, Slovakia, Spain and the United Kingdom) and two non‐MS (Norway and Switzerland) provided monitoring data on Toxoplasma in livestock (small ruminants, cattle, solipeds and pigs).
In small ruminants (sheep and goats), 12 MS (Austria, Finland, Germany, Greece, Hungary, Ireland, Italy, Latvia, the Netherlands, Slovakia, Spain and the United Kingdom) and two non‐MS (Norway, Switzerland) reported data. In total, 12,167 animals were tested and 1,648 were found to be positive (13.5%). In cattle, six MS (Austria, Germany, Ireland, Italy, Latvia and the United Kingdom) reported data on Toxoplasma‐specific antibodies. At animal level, about 9.2% tested seropositive. From pigs, four MS (Austria, Germany, Italy and Slovakia) reported monitoring data: in total 1,108 animals were tested and 130 (11.7%) were detected as positive. In pet animals (cats and dogs), nine MS (Austria, Finland, Germany, Hungary, Italy, Latvia and the United Kingdom) and one non‐MS (Switzerland) tested in total 3,169 animals (1,798 cats and 1,371 dogs) of which 323 were positive (10.2%) and obtained mainly from clinical investigations. Five MS (Austria, Finland, Germany, Italy and Slovakia) and one non‐MS (Switzerland) reported on testing for Toxoplasma in wildlife. In total, 833 animals (mainly from Italy) were tested and 164 were positive (19.7%).
The 2019 monitoring data reported by MS from animals show that Toxoplasma is present in most livestock species across the EU. The limitations of these surveillance data preclude any trend watching or trend analysis of prevalence in animals.
The current surveillance system of Toxoplasma in animals of EU is strongly affected by several important limitations: (i) small amount of tested animals; (ii) the use of different indirect and direct detection methods, which, in most cases have been not validated by an independent body; (iii) unknown age of tested animals; and (iv) no information on type of breeding. Furthermore, there is no relationship between the presence of anti‐Toxoplasma antibodies and infecting parasites in cattle and horses (Aroussi et al., 2015; Opsteegh et al., 2016). For pigs, poultry and small ruminants, serological methods could be useful for the detection of high‐risk animals/herds but not as an indicator of infection in individual animals, as the concordance between direct and indirect methods was estimated as low to moderate. All these limitations result in the lack of scientific value of data provided by MS and consequently of their use by the European Commission, MS and stakeholders. The data are mostly not directly comparable across MS.
2.4. Related projects and Internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Fact sheet toxoplasmosis | https://www.cdc.gov/parasites/toxoplasmosis/index.html |
| ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx | |
| EU case definition of congenital toxoplasmosis | https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions | |
| Disease Programme on Emerging, Food‐ and Vector‐Borne Diseases | https://www.ecdc.europa.eu/en/about-us/who-we-are/units/disease-programmes-unit | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| European Union Reference Laboratory for Parasites | http://www.iss.it/crlp/ | |
| Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV‐Exposed and HIV‐Infected Children | https://clinicalinfo.hiv.gov/en/guidelines/pediatric-opportunistic-infection/toxoplasmosis | |
| Animals | European Union Reference Laboratory for Parasites | http://www.iss.it/crlp/ |
| EFSA Scientific Opinion: Public health risks associated with food‐borne parasites | https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2018.5495 | |
| EFSA Scientific Opinion: Surveillance and monitoring of Toxoplasma in humans, food and animals | http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2007.583/epdf | |
| EFSA External Scientific Report: Relationship between seroprevalence in the main livestock species and presence of Toxoplasma gondii in meat (GP/EFSA/BIOHAZ/2013/01) An extensive literature review | http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2016.EN-996/pdf | |
| EFSA Supporting Publication: Experimental studies on Toxoplasma gondii in the main livestock species (GP/EFSA/BIOHAZ/2013/01) Final report. M. Opsteegh, G. Schares, R. Blaga and J. van der Giessen | https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/sp.efsa.2016.EN-995 | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports | |
| OIE Manual Chapter 2.9.9 Toxoplasmosis | http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.09.09_TOXO.pdf |
3. Rabies
This chapter has a simplified structure underpinned by descriptive summarisation of submitted data (see rationale p. 16 of Introduction).
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. The human epidemiological data for rabies for 2019 are available at https://www.ecdc.europa.eu/en/publications-data/rabies-annual-epidemiological-report-2019. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
3.1. Key facts
For 2019, EU MS reported four human Lyssavirus infections. Three human cases of travel‐related rabies were reported by Italy, Latvia and Spain with exposure in Tanzania, India and Morocco, respectively. One locally‐acquired case of European bat lyssavirus 1 (EBLV‐1) infection was reported by France. One travel‐related rabies case was reported by Norway with exposure in the Philippines.
In non‐flying terrestrial animals, five cases of rabies involving three foxes, one domestic and one wild animal were reported by two MS: Poland (two foxes) and Romania (one fox, one cow and a wild boar). The total number of reported rabies cases in foxes in the EU remains very low (N = 3) as in 2018 (N = 7) and 2017 (N = 2).
In 2019, six out the 18 reporting EU MS reported positive lyssavirus findings in bats, mainly of the European bat lyssavirus EBLV‐1 and EBLV‐2 species. In total, 39 cases were reported in bats.
3.2. Surveillance and monitoring of rabies in the EU
3.2.1. Humans
An overview of the national surveillance systems for human rabies in 2019 is available at: https://www.ecdc.europa.eu/en/publications-data/rabies-annual-epidemiological-report-2019
3.2.2. Animals
The aim of wildlife rabies surveillance is to demonstrate the absence of disease, or to identify its presence or distribution, to allow timely dissemination of information for integrated action among different sectors such as public health and veterinary sectors.
According to Regulation (EU) No 652/201423, multiannual programmes for eradication of rabies may be co‐financed by the EU. In 2019, 12 MS (Bulgaria, Croatia, Estonia, Finland, Greece, Hungary, Latvia, Lithuania, Poland, Romania, Slovakia and Slovenia) had approved eradication, control and surveillance programmes for rabies. A wildlife oral rabies vaccination campaign (ORV) is currently ongoing in these MS, as well as in some of the EU‐bordering countries. The surveillance of rabies is carried out by sampling and testing ‘indicator animals’; these are animals that are found dead in their natural habitat and/or suspected animals from wildlife and domestic species (foxes, badgers, raccoon dogs, dogs, cattle, cats, sheep, equines, goats, rabbits, etc.), i.e. animals showing neurological clinical signs or abnormal behaviour compatible with rabies.
The collection of healthy animals of the species targeted by oral vaccination (foxes, raccoon dogs and also golden jackals) is also valuable for monitoring the efficacy of the ORV campaign by determining the immunity and the oral vaccine bait uptake of animals.
Imported or travel‐related companion animals (mainly dogs and cats) from territories and non‐EU countries not included in Annex II of Regulation (EC) No 577/201324 are currently tested for rabies‐specific antibodies.
EU MS also need to notify outbreaks of infection with rabies virus in non‐flying terrestrial animals to the EU ADNS.12
3.3. Data analyses
In this report, the results of the surveillance activities for rabies are summarised for the indicator wild species such as foxes, raccoon dogs, raccoons (Procyon lotor) and other wild species (badgers, deer, marten, rodents, jackals, lynx, bears, hares, hedgehogs, minks, wolverine, wild boar, squirrels, ferrets, otter, polecat, etc.).
Separate tables for rabies surveillance in domestic carnivores (dogs and cats) and farmed animals (cattle, small ruminants, solipeds, pigs, rabbits, ferrets) were also produced to summarise the surveillance activities in the different MS. These summary tables are in the supporting information to this report.
All data were summarised (aggregated) at MS level; if MS reported data only at regional level or only for some regions, the total number of tested animals were not integrated in the summary tables or maps as it was not clear whether all regions in the MS were tested or not.
When the UK data were collected the UK was an EU MS but as of 31 January 2020 it has become a third country.
3.4. Summary of submitted data
3.4.1. Overview of statistics, EU, 2015–2019
Table 62 below summarises rabies EU‐level statistics in humans and in wild and domestic animals. For animals, the total number of samples taken from foxes, raccoon dogs, raccoon, dogs and bats, as well as the number of MS from which these samples originated, are shown. A significant reduction has been observed in the number of reported samples from foxes, the main reservoir, over the last 5 years at EU level. In 2019, the numbers of reported sampled foxes was halved compared with 2015.
Table 62.
Summary of rabies Lyssavirus statistics related to humans and main animal species, EU, 2015–2019
| 2019 | 2018 | 2017 | 2016 | 2015 | Data Source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 4 | 1 | 1 | 1 | 0 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ECDC |
| Number of reporting countries | 28 | 28 | 28 | 27 | 28 | ECDC |
| Infection acquired in the EU | 1 | 0 | 0 | 0 | – | ECDC |
| Infection acquired outside the EU | 3 | 1 | 1 | 1 | – | ECDC |
| Unknown travel status or unknown country of infection | 0 | 0 | 0 | 0 | – | ECDC |
| Animals | ||||||
| Foxes | ||||||
| Number of tested animalsa | 23,141 | 21,570 | 30,485 | 35,232 | 46,588 | EFSA |
| Number of reporting MS | 19 | 19 | 20 | 20 | 21 | EFSA |
| Raccoon dogs and raccoons | ||||||
| Number of tested raccoon dogs (raccoons) | 1,542 (6) | 1,358 (6) | 992 (12) | 1,169 (3) | 626 (11) | EFSA |
| Number of reporting MS | 9 | 9 | 9 | 7 | 7 | EFSA |
| Dogs | ||||||
| Number of tested animals | 1,901 | 2,097 | 2,334 | 2,469 | 2,784 | EFSA |
| Number of reporting MS | 22 | 23 | 22 | 24 | 22 | EFSA |
| Bats | ||||||
| Number of tested animals | 2,069 | 2,278 | 2,079 | 1,405 | 1,391 | EFSA |
| Number of reporting MS | 18 | 17 | 19 | 19 | 17 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States; NA: Not applicable.
The number of tested animals includes national statistics submitted by MS and not regional data that were submitted without a national summary.
3.4.2. Humans
The human data are available at: https://www.ecdc.europa.eu/sites/default/files/documents/rabies-annual-epidemiological-report-2019.pdf
3.4.3. Rabies in animals
Wildlife rabies
In 2019, in total, 23,141 foxes (Vulpes vulpes) were investigated by 19 MS. More than half of the tested samples (67.3%) were taken by three MS: Romania, Poland and Czechia. In total, three cases of rabies in foxes were detected in the EU: one case in Poland and two in Romania. The geographical distribution and number of cases in foxes, as well as a choropleth map of the total number of foxes sampled per MS are shown in Figure 72. Four non‐EU countries (Norway, Republic of North Macedonia, Serbia, Switzerland) reported 1,274 tested foxes. None of these countries reported positive cases for rabies.
Figure 72.
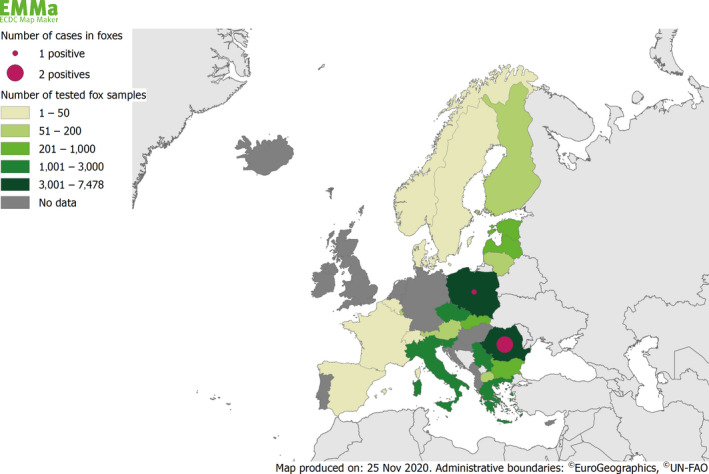
Choropleth map of the number of tested foxes and number and geographical distribution of the reported rabies cases in foxes, by reporting country, EU/EFTA, 2019
In 2019, 1,542 raccoon dogs and six raccoons were reported and tested for rabies by nine MS (Austria, Czechia, Estonia, Finland, Latvia, Lithuania, Poland, Slovakia and Spain). Most of these samples originated from raccoon dogs from three MS (Estonia, Finland and Latvia). All the samples tested were negative for rabies.
Fifteen MS reported results for 2,393 terrestrial wild animals other than foxes, raccoon dogs and raccoons. Almost half of these samples (45.2%) were reported by Bulgaria, with 1,077 of these originating from jackals. Other most tested species were badgers (452), martens (390), wolves (98) and roe deer (81). Other species tested included bears, deer, red deer, ferrets, hares, hedgehogs, lynx, mice, minks, moles, moose, otters, polecats, rats, rodents, squirrels, wild boars and wolverine. In 2019, one rabies positive result was reported in Romania in a wild boar.
In 2019, 18 MS and two non‐MS reported surveillance data on bats. In total, 2,069 bats were investigated in EU (Figure 73). Out of these, 39 samples tested positive in six MS: France (nine EBLV‐1), Germany (eight unspecified virus species), the Netherlands (five EBLV‐1), Poland (10 EBLV‐1), Spain (three EBLV‐1) and the United Kingdom (three EBLV‐1 and one EBLV‐2). Two non‐MS, Norway and Switzerland, tested five and 18 bats, respectively, with all samples being negative.
Figure 73.
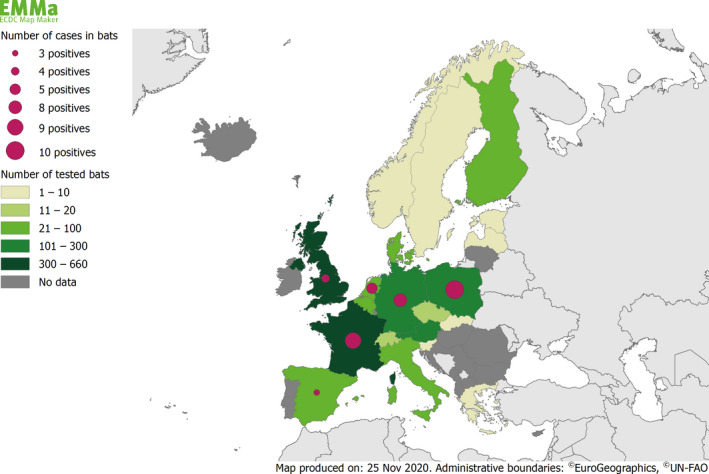
Choropleth map of the number of tested bats and number and geographical distribution of the reported rabies cases in bats (unspecified, EBLV‐1 and EBLV‐2), by reporting country, EU/EFTA, 2019
In conclusion, the results on bat rabies presented here (N = 39 positive cases) are in line with the previous years’ findings and confirm bats to be a reservoir for rabies, reaffirming in this way the public recommendation to handle bats with utmost caution, if at all. The public health hazard of bat rabies in Europe ought to not be underestimated.
Rabies cases in domestic animals
Romania reported one case of rabies (wild strain) in a cow in 2019 and was the only MS reporting a case in a domestic animal, like in 2018. In total, 404 samples from farmed animals were tested by 17 MS (reports included mainly cattle, small ruminants and domestic solipeds). The number of samples taken from domestic farmed animals in 2019 was lower than the number taken in the last four years.
No case of rabies was reported in 2019 in dogs and cats. Twenty‐two MS reported in total more than 4,000 tested samples for dogs (1,901) and cats (2,389). The numbers of samples reported for both species slightly decreased compared with 2018.
These results indicate that, as in the previous years, rabies still occurs in domestic animals in Eastern Europe, indicating the persistence of an active wildlife reservoir there as evidenced by the above‐mentioned results on cases of rabies in foxes (Poland and Romania) and wild boar (Romania).
Overall, the results from the rabies surveillance carried out by MS in 2019 highlight once more the very low number of positives rabies cases detected in non‐flying terrestrial animals in Europe (N = 5). Nonetheless, and as described in the report of the first meeting25 of the Standing Group of Experts on Rabies in Europe, in 2019, under the umbrella of The Global Framework for the Progressive Control of Transboundary Animal Diseases (GF‐TADs) cases can still appear in areas not far from the EU borders. Those experts also raised a concern in terms of rabies surveillance for in certain areas and strongly recommended an improvement of the surveillance in those areas. Appropriate surveillance is of paramount importance, particularly for MS countries close to rabies elimination (Cliquet et al., 2010; EFSA AHAW Panel, 2015). Although a reduction in the number of samples taken from foxes was observed (0), caution must be taken when interpreting this decrease in the sample size. As those reported numbers include monitoring and surveillance strategies and are aggregated at a country level, the decrease in sample size could be the result of a smaller number of suspect cases throughout Europe due to a decrease in prevalence. Nonetheless, MS, especially those with a recent history of rabies, should ensure that a robust surveillance programme is in place capable of the early detection of any potential cases of rabies in their territories.
3.5. Related projects and Internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Global Alliance for Rabies Control | https://rabiesalliance.org/world-rabies-day |
| Rabies surveillance blueprint | http://rabiessurveillanceblueprint.org/?lang=en | |
| EU case definitions of rabies | https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions | |
|
ECDC Surveillance Atlas of Infectious Diseases Disease Programme on Emerging, Food‐ and Vector‐Borne Diseases |
http://atlas.ecdc.europa.eu/public/index.aspx https://www.ecdc.europa.eu/en/about-us/who-we-are/units/disease-programmes-unit |
|
| Emerging Viral Diseases‐Expert Laboratory Network (EVD‐LabNet) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/evd-labnet | |
| World Health Organisation – Rabies fact sheet | http://www.who.int/mediacentre/factsheets/fs099/en/ | |
| Animals | EURL Rabies | https://eurl-rabies.anses.fr/ |
| Summary Presentations on the situation as regards Rabies veterinary programmes in MS | https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en | |
| General information on EU Food Chain Funding | https://ec.europa.eu/food/funding_en | |
| EU approved and co‐financed veterinary programmes for Rabies carried out by the MS | http://ec.europa.eu/dgs/health_food-safety/funding/cff/animal_health/vet_progs_en.htm | |
| World Health Organisation Rabies Bulletin Europe | http://www.who-rabies-bulletin.org/ | |
| EFSA Scientific Opinion on a request from the Commission regarding an assessment of the risk of rabies introduction into the UK, Ireland, Sweden, Malta, as a consequence of abandoning the serological test measuring protective antibodies to rabies | https://www.efsa.europa.eu/en/efsajournal/pub/436 | |
| EFSA Scientific Opinion ‘Update on oral vaccination of foxes and raccoon dogs against rabies’ | https://www.efsa.europa.eu/fr/efsajournal/pub/4164 | |
| World Organisation for Animal health, Questions and Answers on Rabies | http://www.oie.int/fileadmin/Home/fr/Animal_Health_in_the_World/docs/pdf/Portail_Rage/QA_Rage_EN.pdf | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
4. Q fever
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
4.1. Key facts
For 2019, 950 confirmed human cases of Q fever were reported in the EU. Spain reported the most cases (N = 332, more than one‐third of all confirmed cases) for 2019, followed by France and Germany (155 and 148 cases, respectively).
The EU notification rate in humans was 0.19 per 100,000 population, which is slightly higher than in 2018 (0.16 per 100,000 population), but comparable with the rates from 2015 to 2017 (0.18–0.19 per 100,000 population).
There was no statistically significant increase or decrease over the last 5 years (2015–2019) in confirmed Q fever cases in humans in the EU/EEA.
In animals, cattle and small ruminants are mostly sampled due to clinical investigations of animals suspected to be infected by C. burnetii. Because there is no compulsory harmonised monitoring or surveillance in animals in the EU, data reported to EFSA do not make it possible to follow or analyse trends for Q fever at the EU level or to compare national differences in proportions of test‐positive animals.
In total, 18 MS and four non‐MS reported 2019 data for C. burnetii from cattle, sheep and goats and several other domestic and wild animal species. The overall proportion of test‐positive animals in EU was 8.9% in sheep and goat (10.8% based on 2018 data), 5.3% in cattle (6.9% based on 2018 data) and 1% in other domestic and wild animals (2.7% based on 2018 data).
4.2. Surveillance and monitoring of Coxiella burnetii in the EU/EFTA
4.2.1. Humans
Q fever in humans is a mandatory notifiable disease at the EU level and cases are reported through TESSy. For 2019, 27 EU MS, Iceland, Norway and Switzerland provided information on Q fever in humans. Twenty‐three EU countries used the EU case definition, whereas Denmark, France, Germany and Italy used another case definition.
Reporting is mandatory in 25 EU countries and voluntary in France and the UK. Disease surveillance is comprehensive26 and mostly passive except in Czechia, Portugal and Slovakia. Data reporting is case based except from Belgium and Bulgaria.
4.2.2. Animals
At the EU level, there is no harmonised surveillance in place for Q fever in animals. The main animal species tested are cattle, goats and sheep. Samples are mostly blood samples, samples from foetus and stillborn animals and from organs or tissues of animals suspected of being infected by C. burnetii. In addition, other domestic and wild animal species were tested (samples taken from farms, zoos or natural habitat). Reporting on Q fever in animals is in most MS based on clinical investigation and monitoring. Few MS (Bulgaria, Belgium, Denmark, Romania and United Kingdom) and Norway implemented a planned surveillance in cattle and small ruminants by regularly sampling and analysing the presence of C. burnetii‐specific antibodies in blood and milk samples. Italy performed a systematic survey to estimate the national seroprevalence or to confirm the presence of C. burnetii in blood or organ/tissue samples from domestic and wild animals analysed mainly via ELISA.
Because Q fever monitoring data reported by MS to EFSA are generated by non‐harmonised monitoring schemes across MS with no mandatory reporting requirements, these data can only be used for descriptive summaries. Indeed, the results submitted by MS are mostly not directly comparable due to differences in sampling strategy, testing (laboratory analytical) methods, coverage of the monitoring and sensitivity of the surveillance for C. burnetii. They preclude additional data analyses such as following or assessing EU‐level temporal and spatial trends.
4.3. Results
4.3.1. Overview of key statistics, EU, 2015–2019
Table 63 summarises EU‐level statistics on Q fever in humans and in major animal species, respectively, during 2015–2019. Animal data of interest were classified into the major categories and aggregated by year to obtain an annual overview of the volume of data submitted.
Table 63.
Summary of Coxiella burnetii statistics related to humans and major animal speciesa, EU, 2015–2019
| 2019 | 2018 | 2017 | 2016 | 2015 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 950 | 789 | 882 | 975 | 822 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.19 | 0.16 | 0.18 | 0.19 | 0.18 | ECDC |
| Number of reporting EU MS | 27 | 27 | 27 | 27 | 26 | ECDC |
| Infection acquired in the EU | 809 | 628 | 718 | 713 | 550 | ECDC |
| Infection acquired outside the EU | 14 | 12 | 9 | 21 | 8 | ECDC |
| Unknown travel status or unknown country of infection | 127 | 149 | 155 | 241 | 264 | ECDC |
| Animals | ||||||
| Sheep and goats (animal level) | ||||||
| Number of sampling units | 4,828 | 6,386 | 4,245 | 8,323 | 10,054 | EFSA |
| % positive animals | 11.2 | 11.0 | 9.2 | 11.6 | 10.1 | EFSA |
| Number of reporting MS | 13 | 13 | 9 | 14 | 14 | EFSA |
| Cattle (animal level) | ||||||
| Number of sampling units | 13,809 | 23,461 | 16,272 | 18,496 | 44,235 | EFSA |
| % positive animals | 7.0 | 7.6 | 8.6 | 6.0 | 11.0 | EFSA |
| Number of reporting MS | 14 | 13 | 13 | 14 | 15 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
For the summary statistics indirect and direct diagnostic methods were taken together to calculate proportion of positive units.
When the UK data were collected the UK was an EU MS, but as of 31 January 2020, it has become a third country.
Humans
In 2019, the number of Q fever cases in humans who acquired the infection in the EU increased compared with 2018 and is the highest in the past five years. This might partly be due to the decreasing proportion of cases with unknown travel status or unknown country of infection.
Animal categories
In 2019, compared with the year 2018, the number of samples from animals submitted by EU MS from sheep and goats and from cattle decreased by 24.4% and by 41.1%, respectively. Since 2015, the number of submitted samples from animals has been decreasing, except for the year 2018 when samples collected increased. The overall proportions of positive samples ranged from 9.2% to 11.6% for sheep and goats and from 6.0% to 11.0% in cattle, during 2015–2019.
4.3.2. Coxiella burnetii in humans
Overall, 950 confirmed cases of Q fever were reported by 22 EU MS, eight cases were reported by Norway and 103 cases were reported by Switzerland (Table 64). For 2019, Spain was the country that reported most confirmed cases (N = 332), followed by France and Germany (155 and 148 cases, respectively).
Table 64.
Reported human cases of Q fever and notification rates per 100,000 population in the EU/EFTA, by country and year, 2015–2019
| Country | 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | Confirmed cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austriab | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Belgium | Y | A | 22 | 10 | 0.09 | 6 | 0.05 | 7 | 0.06 | 16 | 0.14 | 8 | 0.07 |
| Bulgaria | Y | A | 44 | 36 | 0.51 | 45 | 0.64 | 28 | 0.39 | 17 | 0.24 | 15 | 0.21 |
| Croatia | Y | C | 9 | 8 | 0.20 | 11 | 0.27 | 23 | 0.55 | 8 | 0.19 | 14 | 0.33 |
| Cyprus | Y | C | 1 | 1 | 0.11 | 0 | 0.00 | 3 | 0.35 | 2 | 0.24 | 4 | 0.47 |
| Czechia | Y | C | 1 | 1 | 0.01 | 1 | 0.01 | 0 | 0.00 | 2 | 0.02 | 1 | 0.01 |
| Denmark | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Estonia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Finland | Y | C | 2 | 2 | 0.04 | 2 | 0.04 | 4 | 0.07 | 2 | 0.04 | 3 | 0.05 |
| France | Y | C | 155 | 155 | 0.23 | 172 | 0.26 | 194 | 0.29 | 251 | 0.38 | 250 | 0.38 |
| Germany | Y | C | 150 | 148 | 0.18 | 90 | 0.11 | 107 | 0.13 | 270 | 0.33 | 310 | 0.38 |
| Greece | Y | C | 14 | 14 | 0.13 | 13 | 0.12 | 4 | 0.04 | 9 | 0.08 | 10 | 0.09 |
| Hungary | Y | C | 48 | 47 | 0.48 | 28 | 0.29 | 29 | 0.30 | 39 | 0.40 | 35 | 0.36 |
| Ireland | Y | C | 2 | 2 | 0.04 | 0 | 0.00 | 2 | 0.04 | 6 | 0.13 | 4 | 0.09 |
| Italy | Y | C | 6 | 6 | 0.01 | 1 | 0.00 | 7 | 0.01 | 3 | 0.00 | . | . |
| Latvia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.05 |
| Lithuania | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Luxembourg | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.18 |
| Malta | Y | C | 1 | 1 | 0.20 | 2 | 0.42 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlands | Y | C | 16 | 16 | 0.09 | 18 | 0.10 | 22 | 0.13 | 14 | 0.08 | 20 | 0.12 |
| Poland | Y | C | 4 | 4 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Portugal | Y | C | 32 | 32 | 0.31 | 36 | 0.35 | 48 | 0.47 | 17 | 0.16 | 20 | 0.19 |
| Romania | Y | C | 112 | 109 | 0.56 | 22 | 0.11 | 46 | 0.23 | 32 | 0.16 | 3 | 0.02 |
| Slovakia | Y | C | 1 | 1 | 0.02 | 2 | 0.04 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Slovenia | Y | C | 6 | 6 | 0.29 | 1 | 0.05 | 3 | 0.15 | 1 | 0.05 | 1 | 0.05 |
| Spain | Y | C | 415 | 332 | 0.71 | 313 | 0.67 | 333 | 0.72 | 249 | 0.54 | 97 | – |
| Sweden | Y | C | 11 | 10 | 0.10 | 7 | 0.07 | 1 | 0.01 | 3 | 0.03 | 4 | 0.04 |
| United Kingdom | Y | C | 9 | 9 | 0.01 | 19 | 0.03 | 21 | 0.03 | 34 | 0.05 | 21 | 0.03 |
| EU Total | 1061 | 950 | 0.19 | 789 | 0.16 | 882 | 0.18 | 975 | 0.19 | 822 | 0.18 | ||
| Iceland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway | Y | C | 8 | 8 | 0.15 | 5 | 0.09 | 4 | 0.08 | 2 | 0.04 | 1 | 0.02 |
| Switzerlandc | Y | C | – | 103 | 1.20 | 52 | 0.61 | 42 | 0.50 | 47 | 0.56 | 40 | 0.48 |
–: Data not reported.
Y: yes; N: no; A: aggregated data; C: case‐based data.
Not notifiable, no surveillance system exists.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
The number of confirmed Q fever cases in 2019 was higher than in 2018. The EU notification rate was 0.19 per 100,000 population, which is higher than in 2018 but comparable with the notification rates from 2015 to 2017. For 2019, the highest notification rate (0.71 cases per 100,000 population) was observed in Spain, followed by Romania (0.56), Bulgaria (0.51) and Hungary (0.48).
Six countries (Denmark, Estonia, Iceland, Latvia, Lithuania and Luxembourg) reported no human cases. A large majority (85.2%) of the Q fever cases were acquired in the EU (Table 64). In total, 14 travel‐associated cases were reported in people who had travelled to Bosnia and Herzegovina, Brazil, Egypt, Guinea‐Bissau, Kenya, Mauritius, Morocco, the Philippines, Senegal, Sri Lanka, Switzerland and Turkey.
Between 2007 and 2010, the Netherlands experienced a large outbreak with more than 4,000 human cases (Schneeberger et al., 2014). The number of cases in the Netherlands returned to the pre‐outbreak level in 2013 and has remained low since then.
Four deaths due to Q fever were reported for 2019 in the EU, all by Spain, resulting in an EU case fatality of 0.63% among the 639 confirmed cases with reported outcome.
In 2019, cases occurred during the whole year but with a seasonal increase between April and September when more than 60% of the cases were reported.
There was no statistically significant (p < 0.01) increase or decrease over the last 5 years (2015–2019) in confirmed Q fever cases in the EU/EEA (Figure 74). At the country level, Poland and Romania reported a significantly (p < 0.01) increasing trend and Germany and France a significantly decreasing trend in the past five years (2015–2019).
Figure 74.

Trend in reported confirmed human cases of Q fever in the EU/EEA by month, 2015–2019
- Source: Cyprus, Czechia, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia and Sweden. Austria, Belgium, Bulgaria, Croatia, Denmark, Italy, Spain, Switzerland and the United Kingdom did not report data to the level of detail required for the analysis.
4.3.3. Coxiella burnetii in animals
Sixteen MS and three non‐MS provided data for sheep and goats, for 2019. In total, 4,384 holdings/flocks and 7,793 animals were tested of which, respectively, 6.6% and 8.8% tested positive for C. burnetii. Samples at animal level were mainly taken by Italy (n = 2,670), Norway (n = 2,282) and Netherlands (n = 1,150); Poland tested 79.3% of the holdings/flocks reported.
Seventeen MS and four non‐MS provided data for cattle for 2019. In total, 4,318 holdings/flocks and 19,035 animals were tested, of which, respectively, 10.2% and 5.3% tested positive. Belgium, Poland and Italy tested together 96.9% of the holdings/flocks; Italy, Czechia, Switzerland, Norway and Slovakia accounted for 82.2% of the tested animals.
Five MS and two non‐MS reported data on animals other than sheep, goats and cattle. In total, 302 animals and 37 holdings/flocks were tested from different domestic and wild animal species (Alpaca's, Alpine and Cantabrian chamois, antelopes, badgers, bears, bison, cats, deer, dogs, dolphin, dromedaries, foxes, hares, hedgehogs, horses, lamas, martens, mouflons, otter, parrots, pigeons, pigs, Steinbock, water buffalos, wild boars, wolves). Among all holding/flocks tested, three (with several animal species) out of 20 tested were reported positive by Cyprus (15%). Two dogs were reported test‐positive by Italy and one positive alpaca by Switzerland. Animal results were mainly submitted by Italy (n = 161; 27 different animal species), Slovakia (n = 60; hares and zoo animals) and Austria (n = 35; alpacas, Alpine chamois and pigs).
4.4. Discussion
Over the last five years (2015–2019), there was no statistically significant (p < 0.01) increase or decrease in confirmed Q fever cases in humans in the EU/EEA. While France and Germany reported most of the confirmed cases until 2016, Spain started to report the highest number of cases annually since 2017. The increase in the number of human cases reported by Spain is most likely explained by a change in their reporting system: from voluntary to mandatory. In 2019, Spain accounted for more than a third of the overall number of cases.
Case fatality increased between 2016 and 2018 from 0.39% to 1.92% but decreased to 0.63% in 2019.
The results obtained from animals — mainly from small ruminants and cattle — do not allow following or analysing trends for Q fever at the EU level, because the results submitted by MS are mostly not directly comparable due to differences in sampling strategy, testing methods, coverage of the monitoring and sensitivity of the surveillance for C. burnetii. The regional variability within Europe highlights the importance of understanding risk factors that may operate at a local scale and may be subtle (Georgiev et al., 2013).
4.5. Related projects and Internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | https://atlas.ecdc.europa.eu/public/index.aspx |
| EURL Q fever | https://www.anses.fr/fr/content/laboratoire‐de‐sophia‐antipolis | |
| EU case definition of Q‐fever | https://www.ecdc.europa.eu/en/surveillance‐and‐disease‐data/eu‐case‐definitions | |
| Disease Programme on Emerging, Food‐ and Vector‐Borne Diseases | https://www.ecdc.europa.eu/en/about-us/who-we-are/units/disease-programmes-unit | |
| Animals | World Organisation for Animal health, Summary of Information on Q Fever | https://www.oie.int/en/animal-health-in-the-world/animal-diseases/Q-Fever/ |
| EFSA: Scientific opinion on Q fever | http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2010.1595/full | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
5. West Nile virus
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
5.1. Key facts
For 2019, 443 WNV infections in humans were reported by 19 MS, of which 425 were locally acquired. Most locally acquired infections were reported by Greece, Romania and Italy, accounting, respectively, for 53%, 16% and 13% of the total number of reported infections in the EU. The EU notification rate per 100,000 population in 2019 was 0.09 compared with 0.32 in 2018.
There was no significant increase or decrease over the last 5 years (2015–2019) for WNV infections in humans in the EU/EEA.
For the year 2019, 16 MS submitted WNV monitoring and surveillance data from birds and equids to EFSA. Italy and Spain submitted, respectively, 69.4% and 14.7% of these data for birds, while for equids it was Spain and Greece that, respectively, submitted 30.4% and 23.1% of the data.
Eight MS reported 153 WNV outbreaks in birds (53) and equids (100) to ADNS. Germany and Greece reported, respectively, 52 and 1 outbreaks in birds. Germany and Greece reported the highest number of outbreaks among MS in equids, accounting, respectively, for 32% and 21% of the total number of outbreaks.
ADNS outbreaks data and surveillance data submitted to EFSA indicated WNV circulation during 2019 in countries in Central and Eastern Europe and in the Mediterranean basin. WNV infections of humans and equids now regularly occur in those countries.
5.2. Surveillance and monitoring of West Nile virus in the EU
West Nile fever, also known as ‘West Nile virus disease’, is an arboviral disease transmitted in natural conditions to humans and animals via infected mosquito bites (Diptera; Culicidae). The transmission period is typically between early or mid‐summer until the end of October when mosquitoes (predominantly Culex spp.) are most active and more abundant. The mosquitoes, in which the WNV replicates, acquire infection by feeding on viraemic birds. WNV is maintained in a bird–mosquito cycle, with birds acting as amplifying hosts. Apart from in humans, the virus can also emerge in equine species, which, as humans, are accidental hosts and which cannot in turn transmit the virus to the vectors. MS with areas that are typically prone to harbouring mosquitoes may be affected with both human cases and outbreaks in animals.
5.2.1. Humans
Human WNV infections data are collected through two complementary data collection processes. During the period of high mosquito abundance and activity (June–November), the MS report human infections timely to TESSy at ECDC (ECDC, 2020). Complementary to this real‐time data collection, an annual data collection is carried out. Countries who did not detect any infections during the year are asked to report ‘zero cases’; all other countries are encouraged to report complementary data on detected infections if considered relevant.
For 2019, 27 EU MS, Iceland and Norway reported information on WNV infections in humans to TESSy. The EU case definition was used by 26 countries. Germany did not specify which case definition was used and France and the United Kingdom used an alternative case definition. All reporting countries had a comprehensive surveillance system, except for Germany which did not specify the type of surveillance system. Reporting is compulsory in 26 EU/EEA countries, voluntary in France and the United Kingdom and not specified for Germany. Surveillance is passive, except in Czechia, Greece, Portugal, Slovakia and the United Kingdom. All countries have a national coverage of reporting and case‐based reporting.
5.2.2. Animals
According to Directive 2003/99/EC27, WNV infections in animals are not included in the zoonoses listed in Annex I, Part A of the Directive for which monitoring and surveillance activities as well as reporting are mandatory. Nevertheless, WNV is listed in Annex I, Part B (viruses transmitted by arthropods) to be monitored when to the epidemiological situation in a MS so warrants, in compliance with Article 4.1 of the same Directive. EFSA so is being provided with annual WNV monitoring data by MS that regularly or recently experienced WNV outbreaks (in animals or humans), or that are at high risk and having so put in place a surveillance system for early detection of the disease in animals. In addition to EU MS, Switzerland and Serbia submit reports on surveillance and monitoring activities in animals to EFSA. The heterogeneity in study designs and the variety of analytical methods used, make the reported WNV data from different countries not directly comparable. These data allow descriptive summaries at the EU level to be made (Tables 65 and 67). Proposals for harmonised schemes for monitoring and reporting of WNV in animals can be found in an External Scientific Report submitted to EFSA (Mannelli et al., 2012).
Table 65.
Summary of WNV infection statistics related to humans, birds and equids, in EU, 2015–2019
| 2019 | 2018 | 2017 | 2016 | 2015 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of cases | 443 | 1,615 | 208 | 240 | 128 | ECDC |
| Total number of cases/100,000 population (notification rates) | 0.09 | 0.32 | 0.05 | 0.06 | 0.03 | ECDC |
| Number of reporting MS | 27 | 26 | 26 | 26 | 26 | ECDC |
| Infection acquired in the EU | 435 | 1,573 | 205 | 227 | 122 | ECDC |
| Infection acquired outside the EU | 5 | 29 | 2 | 4 | 0 | ECDC |
| Unknown travel status or unknown country of infection | 3 | 13 | 1 | 9 | 6 | ECDC |
| Animals | ||||||
| Birds | ||||||
| Number of units tested | 14,922 | 14,216 | 11,525 | 8,258 | 8,594 | EFSA |
| Number of units positive for IgM by ELISA | 0 | 1 | 0 | 0 | 0 | EFSA |
| Number of units positive in PCR methods | 104 | 425 | 93 | 75 | 74 | EFSA |
| Number of units positive in seroneutralisation test | 3 | 0 | 56 | 70 | 9 | EFSA |
| Number of MS having reported surveillance/monitoring data to EFSA | 13 | 11 | 8 | 4 | 7 | EFSA |
| Number of outbreaks notified to the ADNS | 53 | 22 | 0 | 0 | 0 | ADNS |
| Number of MS having notified outbreaks to the ADNS | 2 | 6 | 0 | 0 | 0 | ADNS |
| Equids | ||||||
| Number of units tested | 5,563 | 13,785 | 11,670 | 9,949 | 12,961 | EFSA |
| Number of units positive for IgM by ELISA | 74 | 374 | 110 | 189 | 65 | EFSA |
| Number of units positive in PCR methods | 4 | 7 | 1 | 2 | 0 | EFSA |
| Number of units positive in seroneutralisation test | 22 | 9 | 25 | 52 | 5 | EFSA |
| Number of MS having reported surveillance/monitoring data to EFSA | 14 | 12 | 12 | 9 | 9 | EFSA |
| Number of outbreaks notified to the ADNS | 100 | 292 | 84 | 173 | 92 | ADNS |
| Number of MS having notified outbreaks to the ADNS | 8 | 10 | 7 | 5 | 6 | ADNS |
ADNS: Animal Disease Notification System; ECDC: European Centre for Disease Prevention and Control; ELISA: enzyme‐linked immunosorbent assay; EFSA: European Food Safety Authority; MS: Member States; PCR: polymerase chain reaction (for the identification of the virus genome).
Table 67.
Summary of the WNF surveillance/monitoring data submitted to the EFSA and the WNF outbreaks notified to the ADNS, by EU MS and non‐EU Countries in 2019. The percentages for each category are calculated only for EU MS out of the total numbers in EU level (EU Total) for each category the total number of units tested in EU, the total number of positive units in EU per analytical method and the total number of ADNS reported by EU MS
| Country (EU MS, non‐EU countries) | Birds | Equids | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Data on surveillance activities submitted to EFSA | N (%) outbreaks in ADNS | Data on surveillance activities submitted to EFSA | N (%) outbreaks in ADNS | |||||||
| N (%) units tested | N (%) units positive in ELISAa‐IgM | N (%) units positive in PCRb | N (%) units positive in seroneutralisation | N (%) units tested | N (%) units positive in ELISAa‐IgM | N (%) units positive in PCRb | N (%) units positive in seroneutralisation | |||
| EU MS | ||||||||||
| Austria | 20 (0.13) | – | 1 (0.96) | – | NR | 31 (0.56) | – | 4 (100) | – | 4 (4) |
| Bulgaria | 37 (0.25) | – | 0 | – | NR | 0 | – | – | – | NR |
| Cyprus | 382 (2.56) | 0 | – | – | NR | 111 (2) | 0 | – | – | NR |
| Czechia | 0 | – | – | – | NR | 782 (14.06) | – | – | 22(100) | NR |
| Denmark | 810 (5.43) | – | – | – | NR | 0 | – | – | – | NR |
| France | 38 (0.25) | – | 0 | – | NR | 81 (1.46) | 13 (17.57) | 0 | – | 13 (13) |
| Germany | NR | NR | NR | NR | 52 (98.11) | NR | NR | NR | NR | 32 (32) |
| Greece | 29 (0.19) | – | 1 (0.96) | – | 1 (1.89) | 1,285 (23.10) | 24 (32.43) | 0 | – | 21 (21) |
| Hungary | 27 (0.18) | – | 2 (1.92) | – | NR | 294 (5.28) | 20 (27.03) | 0 | – | 13 (13) |
| Italy | 10,362 (69.44) | – | 100 (96.15) | 3 (100) | NR | 1,012 (18.19) | 8 (10.81) | – | – | 8 (8) |
| Portugal | 0 | – | – | – | NR | 14 (0.25) | 4 (5.41) | 0 | 0 | 3 (3) |
| Romania | 217 (1.45) | – | – | – | NR | 156 (2.80) | 0 | – | – | NR |
| Slovakia | 0 | – | – | – | NR | 91 (1.64) | 0 | – | – | NR |
| Slovenia | 59 (0.40) | – | 0 | – | NR | 2 (0.04) | – | 0 | – | NR |
| Spain | 2,192 (14.69) | – | 0 | – | NR | 1,693 (30.43) | 5 (6.76) | – | – | 6 (6) |
| Sweden | 406 (2.72) | – | 0 | – | NR | 4 (0.07) | – | 0 | – | NR |
| United Kingdom | 343 (2.30) | – | 0 | – | NR | 7 (0.13) | 0 | – | – | NR |
| EU Total | 14,922 | 0 | 104 | 3 | 53 | 5,563 | 74 | 4 | 22 | 100 |
| Non‐EU Countries | ||||||||||
| Serbia | 585 | – | 15 | – | NR | 2,477 | 12 | 0 | – | NR |
| Switzerland | 5 | – | 0 | – | NR | 26 | 0 | 0 | – | NR |
NR: Not reported to EFSA or to ADNS. These countries have not submitted data for the WNF surveillance activities to EFSA or have not notified outbreaks in the ADNS.
– : Analytical method not used.
0 : Analytical method used with negative results.
ELISA: enzyme‐linked immunosorbent assay.
PCR: polymerase chain reaction (for identification of the virus genome).
Nonetheless, according to Council Directive 82/894/EEC28, it is mandatory for MS to notify outbreaks29 of WNF equine encephalomyelitis to the EU ADNS.12 Every week, each officially confirmed outbreak should be notified by the Veterinary Authority of the MS where it occurred, to all other countries that are connected to the ADNS application. Report summaries and annual reports on disease outbreaks are available online on the ADNS website.4 Moreover, animal WNF outbreak data reported to the World Organisation for Animal Health (OIE) are publicly available on the World Animal Health Information Database (WAHIS interface).
5.3. Results
5.3.1. Overview of key statistics, EU, 2015–2019
Table 65 summarises EU‐level WNV infection statistics on humans and on birds and equids, during 2015–2019. More detailed descriptions of these statistics are in the results section of this chapter.
When the UK data were collected, the UK was an EU MS, but as of 31 January 2020, it has become a third country.
5.3.2. West Nile virus infections in humans
WNV infections occur seasonally with most occurring in the summer and early autumn. In total, 443 infections were reported by 14 MS for 2019, of which 425 (96%) were locally acquired (acquired in the reporting country) as reported by 11 MS (Tables 65 and 66). Five infections were acquired outside the EU with information about exposure in Djibouti, Serbia, Tunisia, Turkey and the USA. Switzerland reported one infection that was acquired in Egypt.
Table 66.
Locally acquired human WNV infections and notification rates per 100,000 population in the EU/EFTA, by country and year, 2015–2019
| Country | 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Confirmed cases | Total cases and rates | Total cases and rates | Total cases and rates | Total cases and rates | Total cases and rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 4 | 4 | 0.0 | 21 | 0.2 | 6 | 0.1 | 5 | 0.1 | 6 | 0.1 |
| Belgium | Y | C | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Bulgaria | Y | C | 4 | 5 | 0.1 | 15 | 0.2 | 1 | 0.0 | 2 | 0.0 | 2 | 0.0 |
| Croatia | Y | – | 0 | 0 | 0.0 | 58 | 1.4 | 5 | 0.1 | 2 | 0.0 | 1 | 0.0 |
| Cyprus | Y | C | 18 | 23 | 2.6 | 1 | 0.1 | 0 | 0.0 | 1 | 0.1 | 0 | 0.0 |
| Czechia | Y | C | 1 | 1 | 0.0 | 5 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Denmarkb | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | – | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Finland | Y | – | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| France | Y | C | 1 | 2 | 0.0 | 27 | 0.0 | 2 | 0.0 | 0 | 0.0 | 1 | 0.0 |
| Germany | Y | C | 5 | 5 | 0.0 | 1 | 0.0 | – | – | – | – | – | – |
| Greece | Y | C | 89 | 227 | 2.1 | 315 | 2.9 | 48 | 0.4 | 0 | 0.0 | 0 | 0.0 |
| Hungary | Y | C | 34 | 36 | 0.4 | 215 | 2.2 | 20 | 0.2 | 44 | 0.4 | 18 | 0.2 |
| Ireland | Y | – | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Italy | Y | C | 54 | 54 | 0.1 | 610 | 1.0 | 53 | 0.1 | 76 | 0.1 | 61 | 0.1 |
| Latvia | Y | – | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Lithuania | Y | – | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Luxembourg | Y | – | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Malta | Y | – | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Netherlands | Y | – | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Poland | Y | – | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Portugal | Y | – | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.0 |
| Romania | Y | C | 60 | 67 | 0.3 | 277 | 1.4 | 66 | 0.3 | 93 | 0.5 | 32 | 0.2 |
| Slovakia | Y | C | 1 | 1 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Slovenia | Y | – | 0 | 0 | 0.0 | 4 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Spain | Y | – | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 0.0 | 0 | 0.0 |
| Sweden | Y | C | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| United Kingdom | Y | C | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| EU Total | 271 | 425 | 0.08 | 1549 | 0.31 | 201 | 0.05 | 226 | 0.1 | 122 | 0.03 | ||
| Iceland | Y | – | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | – | – | – | – |
| Norway | Y | – | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Switzerlandc | Y | C | 1 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
–: Data not reported.
Y: yes; N: no; A: aggregated data; C: case‐based data; –:no report.
Not notifiable, no surveillance system exists.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
For 2019, the 11 MS reporting locally acquired infections were Austria, Bulgaria, Cyprus, Czechia, France, Germany, Greece, Hungary, Italy, Romania and Slovakia. Slovakia reported locally acquired infections for the first time since 2015.
Most locally acquired infections were reported by Greece, Romania and Italy, accounting, respectively, for 53%, 16% and 13% of the total number of reported infections in the EU. The overall EU notification rate per 100,000 population in 2019 was 0.09 compared with 0.32 in 2018, which represents a 73% decrease of WNV infections compared with 2018.
There was no statistically significant (p < 0.01) increase or decrease over the last 5 years (2015–2019) for WNV infections in the EU/EEA (Figure 75). At the country level, Greece reported a significantly (p < 0.01) increasing trend in the past 5 years (2015–2019). In 2018, a large number of human WNV infections were reported in the EU/EEA, far exceeding the annual totals for the previous years. The notification rate for locally acquired WNV infections in the EU/EEA was almost eight times higher in 2018 compared with 2017. Almost all countries in 2018 reported their highest number of cases ever.
Figure 75.
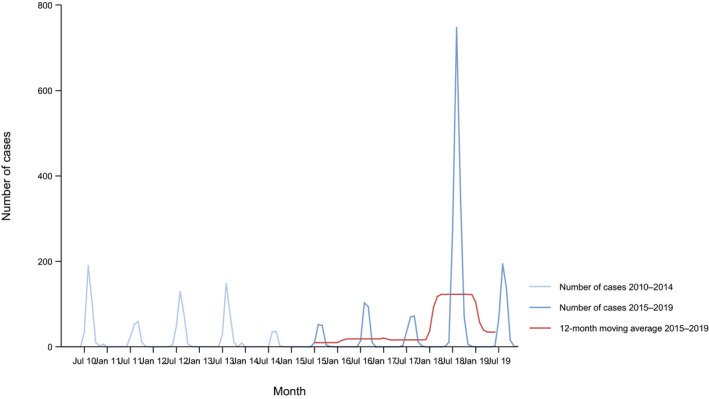
Trend in reported locally acquired human WNV infections in the EU/EEA, by month, 2015–2019
- Source: Austria, Belgium, Cyprus, Czechia, Estonia, Finland, France, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom. Bulgaria, Croatia, Denmark, Germany, Iceland and Portugal did not report data to the level of detail required for the analysis.
Nine EU MS reporting locally acquired infections provided data on the hospitalisation status of their cases. Among the cases with known hospitalisation status (86% of total infections) in 2019, 94% (N = 342) were hospitalised. Among the infections with known clinical manifestations (99.5% of total infections), 67% (N = 282) were neuroinvasive and 2% (N = 8) of infections were asymptomatic blood donors compared with 64% (N = 992) and 5% (N = 83) in 2018, respectively. The remaining 133 cases (31%) were cases with non‐neurological symptoms. Data on the outcome of infections were provided by 11 EU MS. For 2019, 52 deaths among cases with WNV infections were reported, compared with 166 in 2018. The case fatality in 2019 was 12% (11% in 2018) among all locally acquired WNV infections and 18% (16% in 2018) among locally acquired WNV infections with West Nile neuroinvasive disease (WNND).
During the WNV transmission season, weekly epidemiological WNV updates including the geographical distribution of human cases in the EU/EEA and EU neighbouring countries are published on the ECDC website (ECDC, 2020). These updates include a summary of the WNV transmission season, data from the ECDC Surveillance Atlas and three maps: (1) human WNV infections; (2) WNV outbreaks among equids and/or birds; and (3) combined distribution of WNV infections among humans and outbreaks among equids and/or birds. The latter map is in Figure 76.
Figure 76.

Distribution of West Nile virus infections among humans and outbreaks among equids and/or birds in the EU, transmission season 2019
- Source: TESSy and ADNS.
5.3.3. West Nile virus infections in animals
In relation to West Nile fever (WNF) in animals, there exist two sources of information mainly used for this report: the data of the annual surveillance and monitoring activities submitted to EFSA and the data of the outbreaks notified to the ADNS.30 Table 66 includes for each MS the jointly displayed data from both data sources. In some cases, their comparison may be subjected to some discrepancies and the following points should be taken under consideration for the interpretation: (i) the data on the surveillance and monitoring activities, submitted to the EFSA, include all the units that have been analysed with different types of methods; (ii) the data reported in ADNS include only the outbreaks for which the disease has been confirmed clinically and/or laboratory, either by the detection of IgM‐specific antibodies (indicator of recent infection with WNV) or by the detection of RNA particles via PCR‐based methods, as a result of the surveillance and monitoring activities and the investigation of suspected cases; (iii) an outbreak can refer to more than one affected animal if they constitute a unique epidemiological unit or/and are identified at the same location; (iv) the positive results of the surveillance data refer to the positive results of ELISA to detect IgM antibodies, to the seroneutralisation and the positive results of PCR methods to detect the virus genome; and (v) some countries have not submitted data either to the ADNS or to EFSA.
Annual results of the surveillance and monitoring activities
In 2019, according to the annual surveillance and monitoring data reported by 13 MS to EFSA, a total number of 14,922 samples from birds was tested for WNV, mostly wild birds but also fowl kept on farms (Tables 65 and 67). Two non‐EU Countries (Serbia and Switzerland) also reported to EFSA the results of 590 samples of birds tested for WNV (Table 67). The analytical methods used to underpin positive results in birds were mainly molecular methods based on PCR that detects the nucleic acid of WNV. In some cases, ELISA was the method used to detect immunoglobins IgG (Denmark and Romania) or IgM (Cyprus). Italy, in addition to PCR methods, reported positive results by seroneutralisation method. Bird species to be found positive were: doves, ducks, eagles, finches, flamingos, fowls (Gallus gallus), geese, gulls, hawks, herons, owls, pelicans, penguin, pheasants, pigeons, plovers, tits, birds of the family of Corvidae (e.g. crows, magpies, jays) and birds of the family of Psittacidae (e.g. parrots).
Furthermore, 14 MS reported to EFSA the results of 5,563 samples from equids, almost all from horses (Tables 65 and 67). Two non‐MS (Serbia and Switzerland) also reported to EFSA the results of 2,503 samples of equids tested for WNV. The analytical methods used to underpin positive results were mainly the IgM‐capture ELISA and the real‐time PCR. Czechia reported positivity to the seroneutralisation test. Positive animals to serological test were unvaccinated or had an unknown vaccination status.
During 2019, 153 WNF outbreaks in animals, both in equids (100) and birds (53) were notified to the ADNS by the Veterinary Authorities of eight MS (Table 67). The geographical distribution of these outbreaks is visualised in Figure 77.
Figure 77.
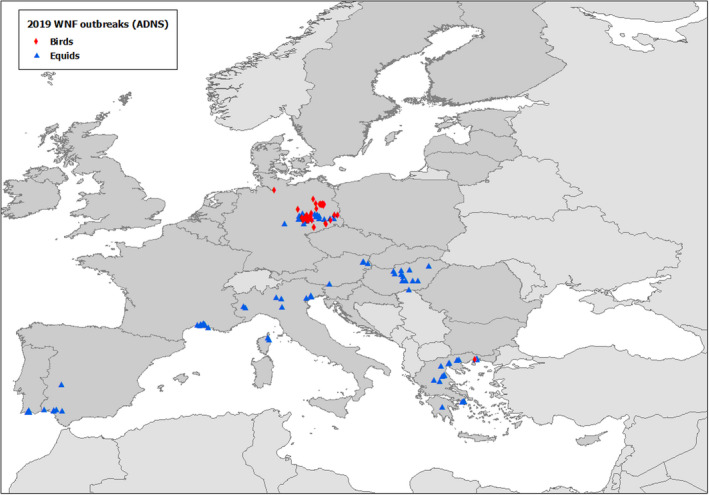
Geographical distribution of WNF outbreaks in equids (blue triangle) and birds (red rhombus) according to the notifications from the Veterinary Authorities, EU, 2019
- Source: ADNS, extracted on 1 September 2020.
Trends and seasonality of WNF in animals
During a seven‐year period 2013–2019, 927 WNF outbreaks have been notified by 12 EU MS, mainly in equids and birds and sporadically in other species.
Based on the date of confirmation notified in the ADNS, the number of WNF outbreaks (all species) per month, aggregated for all the EU MS, has been calculated for each year and presented in Figure 78.
Figure 78.

Monthly number of WNF outbreaks in all animal species based on notified date of confirmation, by month by year, 2013–2019, EU
- Source: ADNS, extracted on 1 September 2020.
The number of monthly WNF outbreaks, for each MS in all animal species for the seven years period 2013–2019 is presented in Figure 79.
Figure 79.
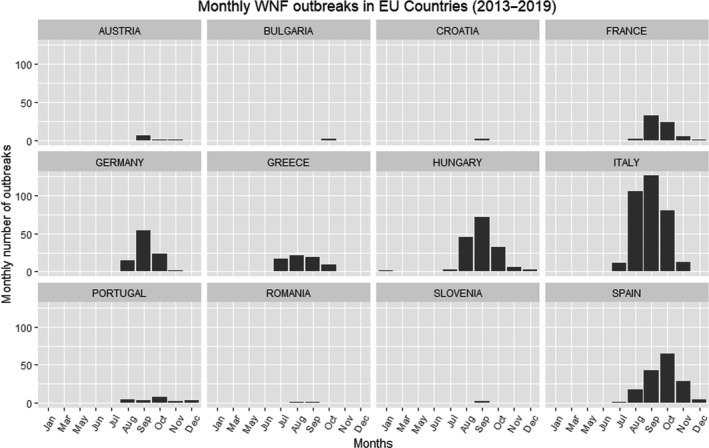
Monthly number of WNF outbreaks in all animal species based on notified date of confirmation, 2013–2109, EU
- Source: ADNS, extracted on 1 September 2020.
According the graphs in Figures 78 and 79, the occurrence of WNF in animals is seasonal with the outbreaks mainly confirmed during the summer and autumn (July–October), while some sporadic outbreaks are confirmed during winter months (November, December, January).
Out of the total number of the outbreaks in EU MS since 2013, 39% were confirmed in September, 23% in August, 26% in October, 6% in November and 4% in July. September looks like the month with the highest percentages of outbreaks for most of the MS: Germany (56.2%), France (50%), Hungary (43.97%), Italy (37.5%) and even in countries with very few outbreaks such as Austria (six out of eight), Croatia and Slovenia. In Spain and Portugal, respectively, 65% and 40% of the total amount of the outbreaks occurred in October. In Greece, it looks like most WNF outbreaks occurred earlier, with 25% of the outbreaks confirmed in July, 30.8% in August and 27.9% in September.
Evaluation of status on WNV and trends by the EU MS and non‐EU Countries
More information on the evaluation of the status as regards WNV and trends are in the national zoonoses reports submitted in accordance with Directive 2003/99/EC, which are published on the EFSA website (available online http://www.efsa.europa.eu/en/biological-hazards-data/reports) together with the EU One Health zoonoses report. Specific information on WNV in some countries was extracted by the above‐mentioned reports and are provided here below:
Czechia
… In total every year 783 horses are tested for antibodies against WNV by cELISA test with WNV antigen in the whole territory of Czechia. Virus neutralisation test is used to confirm the presence of antibodies against WNV. … In 2019, a total of 782 horses from entire Czechia were tested for the presence of antibodies against WNV. Samples that reacted positively in cELISA with WNV antigen were tested by virus neutralisation assay (VNT) for the presence of antibodies to WNV; 22 samples responded positively to VNT. …
France
…. After a first outbreak in the Camargue region (southern of France) in 1962, the virus remained undetected until an outbreak in the same region in 2000. Since then, outbreaks of various sizes and virus circulation have been detected in Camargue and other areas surrounding the Mediterranean Sea: 2003 (Var), 2004 (Camargue), 2006 (Pyrénées‐Orientales), 2009–2010 (serosurveys in birds, Camargue), 2015 (Camargue). In 2017, a human case in the department of Alpes‐Maritimes led to the detection of a subclinical infection in a horse in the same department. In total, 13 equines (Camargue and Corsica) and 27 human cases (mainly in the department of Alpes‐Maritimes and Corsica) were reported in 2019. …
Greece
… Since 2010, a surveillance programme for WNF is in place in Greece. West Nile Fever is a disease of mandatory declaration. …The West Nile Fever (WNF) surveillance programme of Greece consists of active surveillance in equine and wild avian populations and passive surveillance in wild and domestic birds, in Equidae and in other domestic animals sensitive to WNF. The purpose of this programme is to protect public and animal health by determining the origin and the possible reservoirs of the causative agent (WNV), which areas of Greece are endemic or of high risk for an outbreak of an epizootic/epidemic and the appropriate preventive measures for the spread of an outbreak of the disease. …
… The analytical methods used are: a. ID VET SCREEN COMPETITION ELISA and b. ID VET IgM CAPTURE ELISA for the differentiation of recent to past antibodies in all samples with a positive ELISA result. … Real time RT‐PCR tests were performed in samples from birds and in equidae samples with a positive IgM ELISA test. …
Italy
… In Italy since 2016 an integrated approach has been applied with the integration of veterinary and human surveillance activities in a unique national plan. Surveillance on animals and mosquitoes is focused on the early detection of the viral circulation. Since then WNV has been circulated every year in both the territories previously affected and in novel areas. To date, WNV circulation has been confirmed in 15 out of 20 Italian regions (Emilia Romagna, Veneto, Lombardy, Sardegna, Sicilia, Friuli Venezia Giulia, Piemonte, Molise, Toscana, Basilicata, Lazio, Puglia, Calabria, Marche and Liguria) in mosquitoes, birds and horses. …
WN viral genome has been detected by RT‐PCR in 21 collected wild birds during 2019. During the last epidemic season, infected birds were collected in Emilia Romagna, Sardegna, Veneto Piemonte and Lombardy regions. Genetic analyses of WNVs train confirmed the circulation of Lineage 2.
… WN viral genome has been detected by RT‐PCR in … 79 resident birds during 2019. During the last epidemic season infected birds among the resident species were collected in Emilia Romagna, Piemonte, Veneto, Sardegna and Lombardy regions. Genetic analyses of WNV strain confirmed the circulation of Lineage 2.
WNV infection has been confirmed in 30 horses in 2015, 51 in 2016, 93 in 2017, 235 during 2018. During the last epidemic season eight horses with neurological symptoms were identified in Piemonte, Emilia Romagna, Veneto and Lombardy regions. Genetic analyses of the WNV strain identified in a dead horse has been clustered in viral Lineage 2.
… In the last epidemic season, 51 positive mosquitoes pools were collected between July and September in Lombardy, Emilia Romagna, Veneto, Friuli Venezia Giulia and Piemonte regions. Genetic analyses of WNV strain confirmed the circulation of Lineage 2. …
Romania
… During 2019, active surveillance activities were foreseen in animals owned by humans confirmed with West Nile fever. In 14 counties, samples were taken in 29 backyards from birds (hens, geese and ducks) and Equidae (horses, donkeys). Two ELISA tests were used (IgG ELISA for birds and IgM ELISA for Equidae). Animals from 13 backyards were positive for West Nile virus antibodies. …
Slovakia
… West Nile Fever virus in horses was never isolated. Presence of virus was detected only serologically. In 2018 was one horse serologically positive for WNV and none in 2019.
According Plan of veterinary prevention and protection of state territory monitoring of the epidemiological situation is carried out through monitoring of West Nile virus fever antibodies in horses. Detection of post‐infection antibodies are performed within targeted intravital diagnostics in horses and the targeted intravital diagnosis of suspected CNS disease.
In horse holdings the breeding stallions prior to and after the completion of a mating season, mares prior to mating, sport and production horses used for the breeding and animals with suspicion of the disease of CNS are tested. Diagnostic/analytical methods used: ELISA IgM, ELISA IgG, Real‐time RT‐PCR. …
Switzerland
… In 2019, 26 horses were tested negative for WNV. In general horses should only be examined for WNV if they show neurological symptoms of unknown origin and if they were not vaccinated. In 2019 15 birds were tested for WNV using RT‐qPCR at the National Reference Center for Poultry and Rabbit Diseases, University of Zurich. 62 FTA‐cards which were placed in mosquito traps in the canton Ticino and in August and September 2019 were screened for Flavivirus and Alphavirus, all negative for WNV. The FTA‐cards contain a sugar solution. If consumed by the mosquitoes, the saliva of the mosquitoes, which might contain virus, gets into the FTA‐cards. The saliva contained virus is inactivated and fixed on the FTA‐card. Up to date there were no autochthonous cases of WNF reported. However, it cannot be excluded that WNV is circulating in Switzerland, especially in wild birds and mosquito populations. …
5.4. Discussion
A large number of human WNV infections had been reported in the EU/EEA for 2018 (n = 1,615), exceeding, by far, the total number from the previous 4 years. For 2019, reported human WNV infections decreased again in most countries (n = 443), although in Greece the number remained at a relatively high level (n = 227). For 2019, Cyprus reported 23 locally acquired human WNV infections, after previously having only reported one human WNV infection in 2016 and 2018, each. During 2019 Slovakia and Germany reported the first mosquito‐borne locally acquired human WNV infections. This was not unexpected as the presence of WNV among birds, equids and/or mosquitoes has been previously documented in those countries. All other human infections were reported in countries with known persistent transmission season in previous years. The case fatality among all locally acquired WNV infections, the case fatality among cases with WNND and the proportion of cases with WNND was slightly higher in 2019 compared with 2018.
In 2019, 16 MS have submitted to the EFSA data on surveillance activities on animals, while 8 MS notified outbreaks in animals to the ADNS. As during previous years, the 2019 data indicate WNV circulation in Central and Eastern Europe and in the Mediterranean basin: Austria, Czechia, France, Greece, Hungary, Italy, Portugal and Spain. Germany reported outbreaks of WNV in animals to the ADNS, as it did for the first time in 2018. During the previous years, it identified seropositivity during the surveillance activities. These reported observations are consistent with the OIE's conclusion that the occurrence of WNF in humans and animals along with bird and mosquito surveillance for WNV activity demonstrates that the virus range has dramatically expanded including North, Central and South America as well as Europe and countries facing the Mediterranean Basin (OIE, 2018).
The risk of WNV transmission is complex and multifactorial; it concerns the virus, the vectors, the animal reservoirs, the environmental conditions, the human behaviour and the density of human and animal populations. Preventing or reducing mosquito‐borne WNV transmission depends on successfully controlling the vector's abundance or interruption of human–vector contact. Human, animal and entomological WNF surveillance is crucial to allow the early detection of WNV infections in humans and take timely preventive measures. In horses, the development of WNV‐associated diseases is preventable with proper vaccination and protection against mosquito bites. It is important to take into consideration that the absence of cases and outbreaks does not imply the absence of the virus in the environment.
5.5. Related projects and Internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious Diseases | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definitions of West Nile virus infection | https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions | |
| Disease Programme on Emerging, Food‐ and Vector‐Borne Diseases | https://www.ecdc.europa.eu/en/about-us/who-we-are/units/disease-programmes-unit | |
| Emerging Viral Diseases‐Expert Laboratory Network (EVD‐LabNet) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/evd-labnet | |
| ECDC – Surveillance and disease data for West Nile virus infections | https://ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data | |
| World Health Organisation – West Nile virus fact sheet | http://www.who.int/mediacentre/factsheets/fs354/en/ | |
| ECDC – Fact sheet about West Nile virus infection | https://www.ecdc.europa.eu/en/west-nile-fever/facts/factsheet-about-west-nile-fever | |
| Animals | World Organisation for Animal Health (OIE), Summary of Information on West Nile fever | https://www.oie.int/animal-health-in-the-world/animal-diseases/west-nile-fever/ |
| OIE Reference Laboratory for West Nile Fever | http://www.izs.it/IZS/Centres_of_excellence/International_Centres/OIE_Reference_Laboratory_for_West_Nile_Fever | |
| EU Animal Disease Notification System (ADNS) | https://ec.europa.eu/food/animals/animal-diseases/not-system_en | |
| Vector‐borne diseases, Scientific Opinion of the Animal Health and Welfare Panel of EFSA, published 11 May 2017 | http://www.efsa.europa.eu/en/efsajournal/pub/4793 | |
| VectorNet, a joint initiative of the European Food Safety Authority (EFSA) and the European Centre for Disease Prevention and Control (ECDC), which started in May 2014. The project supports the collection of data on vectors and pathogens in vectors, related to both animal and human health | https://vectornet.ecdc.europa.eu/ | |
| An interactive presentation of WNF virus in Vector Born Diseases Story Maps application | https://efsa.maps.arcgis.com/apps/MapJournal/index.html?appid=512a03aa8df84d54a51bcb69d1b62735 | |
| Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law, Regulation (EU) No 2016/429): West Nile fever, Vector‐borne diseases, Scientific Opinion of the Animal Health and Welfare Panel of EFSA, published 8 August 2017 | http://www.efsa.europa.eu/en/efsajournal/pub/4955 | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports |
6. Tularaemia
This chapter has a simplified structure underpinned by descriptive summarisation of submitted data (see rationale p. 16 of Introduction).
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993. The human epidemiological data for tularaemia for 2019 are available at https://www.ecdc.europa.eu/en/publications-data/tularaemia-annual-epidemiological-report-2019. Summary statistics of human surveillance data with downloadable files are retrievable using ECDC's Surveillance Atlas of Infectious Diseases at http://atlas.ecdc.europa.eu/public/index.aspx
6.1. Key facts
For 2019, 1,649 human cases of tularaemia were reported in the EU, 1,280 (78%) of which were confirmed.
The EU notification rate for 2019 for human tularaemia cases was 0.25 cases per 100,000 population.
Two food‐borne disease outbreaks were reported for 2019 due to Francisella tularensis, both by non‐MS: one by Norway and one by Serbia. ‘Tap water, including well water’ was the incriminated food vehicle in both these strong‐evidence outbreaks, causing 36 illnesses from whom six were hospitalised, no deaths.
Tularaemia in animals is rarely reported in EU as submission of the data to EFSA is on voluntary basis. In 2019, two MS (Austria and Sweden) reported data on the occurrence of Francisella tularensis in hares. Sweden also reported cases in muskrats. One non‐MS (Switzerland) reported samples taken from wild species (hares, beavers, squirrels, hedgehogs, mice, deer, foxes and polecats) kept in zoos or from their natural habitat.
Two MS (Austria and Sweden) reported that 67 out of the 211 hares tested positive (31.7%) (17.9% in 2018). In Switzerland, the occurrence of Francisella tularensis in the tested hares was 87.1%.
6.2. Surveillance and monitoring of tularaemia in the EU
6.2.1. Humans
An overview of the national surveillance systems for tularaemia in humans in 2019 is available at https://www.ecdc.europa.eu/en/publications-data/tularaemia-annual-epidemiological-report-2019
6.2.2. Animals
Among EU MS, tularaemia in animals is not a reportable disease according to Council Directive 82/894/EEC on the notification of animal diseases within the EU amended and consolidated version 2013 01 01, but it is reportable to the OIE if a new disease event occurs in a country.
However, the notification is mandatory by national law in the Netherlands, Sweden, Iceland and Switzerland. The monitoring data from animals on Francisella tularensis are voluntarily submitted by MS and EFTA countries to EFSA. The data are collected without harmonised design at the EU level and only allow for descriptive summaries and not for trend analyses and trend watching (Table 1). Inference on the occurrence and prevalence of F. tularensis at animal level in the EU cannot be drawn from these monitoring data.
6.2.3. Food‐borne outbreaks of tularaemia
The reporting of food‐borne tularaemia disease outbreaks in humans is mandatory according the Zoonoses Directive 2003/99/EC.
When the UK data were collected the UK was an EU MS but as of 31 January 2020 it has become a third country.
6.3. Summary of submitted data
6.3.1. Humans
The human data are available at https://www.ecdc.europa.eu/en/publications-data/tularaemia-annual-epidemiological-report-2019.
6.3.2. Human cases associated with food‐borne outbreaks due to Francisella tularensis
Two food‐borne disease outbreaks were reported for 2019 due to Francisella tularensis, both by non‐MS: one by Norway and one by Serbia. ‘Tap water, including well water’ was the incriminated food vehicle in both these strong‐evidence outbreaks, causing 36 illnesses from whom six were hospitalised, no deaths. Previously Norway also reported one tularaemia waterborne outbreak in 2016 (6 illnesses from whom one was hospitalised, no deaths) and one tularaemia food‐borne outbreak in 2014 (due to unknown food, four illnesses, no hospitalisations and no deaths). In EU, during 2005–2017, there were three food‐borne outbreaks of tularaemia reported in EU, by Croatia (year 2015, five illnesses, three hospitalisations and no deaths), Germany (year 2016, six illnesses, two hospitalisations and no deaths) and France (year 2012, three illnesses, no hospitalisations and no deaths). France reported that outbreak with strong‐evidence as regards the incriminated food, which was ‘other, mixed or unspecified poultry meat and products thereof.
6.3.3. Tularaemia in animals
In 2019, two MS, Austria and Sweden, reported data on the occurrence of Francisella tularensis in hares (natural habitat) and overall, 67 out of the 211 were positive (31.7%). Sweden also reported data from 24 tested muskrats with eight positives.
The Swedish reports on hares were regarding 128 European brown hares and 48 mountain hares, of which 27 European brown hares and 31 mountain hares tested positive for F. tularensis subsp. holarctica.31 During the last two decades, in Sweden, the epidemiology of tularaemia has changed and the number of reported cases in animals, mainly European brown hares, infected south of the previous endemic region, has increased. In animals, outbreaks of tularaemia have in some countries been associated with rises in rodent and hare populations, but this has not been confirmed in Sweden.
The epidemiological role of the hare as a possible carrier of F. tularensis remains unclear. A recent study from Hestvik et al. (2019) found that all predator and scavenger species included in the study (brown bear (Ursus arctos), Eurasian lynx (Lynx lynx), raccoon dog (Nyctereutes procyonoides), red fox (Vulpes vulpes), wild boar (Sus scrofa), wolf (Canis lupus) and wolverine (Gulo gulo)) may serve as sentinels for tularaemia in Sweden as they found seropositive animals in all the species studied. At the same time, the role of these species as reservoir stays unclear.
Sweden has reported cases of tularaemia in humans and animals since 1931. Ever since the first Swedish tularaemia case was reported, endemic areas have been identified in northern and central Sweden.
Switzerland also reported samples taken from wild species (hares, beavers, squirrels, hedgehogs, mice, deer, foxes and polecats) kept in zoos or from their natural habitat. The occurrence of Francisella tularensis in the tested hares was 87.1%. One pet cat was also found positive. None of the other tested animal species (N = 10) in Switzerland tested positive.
Tularaemia has terrestrial and aquatic ecological cycles with an extensive host range among animals including vertebrates and invertebrates. Lagomorphs of the genus Lepus and small rodents are considered reservoirs, but antibodies against F. tularensis have been detected in other wild animals, such as red fox and wild boar and domestic animals such as cat and dog (Hestvik et al., 2015; Maurin and Gyuranecz, 2016). As for humans, the animal species susceptible to tularaemia may be infected either through the terrestrial or the aquatic cycle. A study performed in the Netherlands during an outbreak in hares in 2015 to assess potential reservoirs and transmission routes of F. tularensis showed the importance of the environmental surveillance of water and its valuable use to monitor this pathogen (Janse et al., 2015). Only Austria and Sweden reported data on hares obtained from passive surveillance. These data show that F. tularensis is still present in the wildlife and that hares (genus Lepus) are good indicator animals to monitor the occurrence. Wildlife may continue to play a role in the maintenance of F. tularensis in the ecological cycle and the occurrence of human cases. It is clear that Francisella spp. are widely present in the environment and a wide range of wild animals (such as hares), but also vectors (e.g. ticks as illustrated in the previous chapter) could be used to enforce passive surveillance in EU as they can be sources of infections in humans (WHO, 2007). Greater efforts are needed to assess the extent of the true animal reservoir population of F. tularensis and to assess the occurrence of this zoonotic pathogen in the EU animal reservoir populations including the environment.
6.4. Related projects and Internet sources
7. Other zoonoses and zoonotic agents
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993
In 2019, among others, data on Bacillus, Chlamydia, Clostridium, Cysticercus, Enterococcus, hepatitis A virus, Klebsiella, Leptospira, marine biotoxins, norovirus, Proteus, Sarcocystis, Shigella, coagulase‐positive Staphylococcus and tick‐borne encephalitis virus were reported to EFSA.
When the UK data were collected the UK was an EU MS but as of 31 January 2020 it has become a third country.
7.1. Bacillus in food and animals and B. cereus enterotoxins in foods
Slovenia submitted 2019 data on Bacillus cereus in food (N = 200) and Greece on Bacillus in animals (N = 8). Slovenia reported three positive samples (5.5%) out of 55 for ‘other processed food products and prepared dishes ‐ unspecified’ from Restaurant or Cafe or Pub or Bar or Hotel or Catering service. Greece reported a Bacillus‐positive goat from clinical investigation at farm. One non‐MS, the Republic of North Macedonia, also submitted food and animal testing results for Bacillus and all tested negative.
7.2. Chlamydia spp
Austria, Denmark and Greece reported in total 2,079 monitoring results for Chlamydia (Chlamydia/Chlamydophila psittaci) in animals. Overall 8.6% were positive and were from: birds, cattle, goats, Psittacidae, pigeons, pigs, sheep and wild ruminants.
7.3. Clostridium spp
Ireland and the non‐MS the Republic of North Macedonia submitted, in total, 260 sampling unit results for Clostridium with no positives found. Sampled foods were: bakery products, cereals and meals, cheeses made from cows’ milk, crustaceans, dairy products (excluding cheeses), fats and oils (excluding butter), fishery products, unspecified, fruits, honey, juice, meat and meat products, other processed food products and prepared dishes, RTE salads, sauce and dressings, soups, vegetables and water.
From animals Greece and the non‐MS the Republic of North Macedonia submitted overall 136 samples and both countries reported positive domestic livestock, in total 36.0%.
7.4. Enterococcus spp.
Bulgaria was the only MS that reported data on non‐pathogenic Enterococcus in 2019. None of the samples (potable water, N = 337 samples from own checks) taken at the processing level were positive.
7.5. Norovirus
Five MS (Croatia, France, Portugal, Romania and Slovenia) reported on the occurrence of norovirus in fruits and vegetables and other food of non‐animal origin (N = 1,097). France reported five norovirus‐positive samples from whole fruits, non‐pre‐cut fruits and vegetable leaves.
7.6. Proteus
Greece provided 2019 data from 136 animal samples (from cattle, goat and sheep) from clinical investigations tested for Proteus and 6.6% were positive.
7.7. Staphylococcus spp.
Bulgaria and Italy reported data on Staphylococcus spp. (S. aureus, S. intermedius and unspecified, excluding methicillin‐resistant S. aureus and staphylococcal enterotoxins) in various animal (N = 6,058) and food (N = 11,110) products sampling units. Overall, from animals 18.9% and from food 9.6% were reported positive. Positive tested foods were; cheeses, made from unspecified milk or other animal milk, ice‐cream, pre‐cut fruits and vegetables, meat products from broilers, meat preparation and meat products from other animal species or not specified, meat products from other animal species or not specified, other processed food products and prepared dishes (amongst other pasta), sauce and dressings, pastry, soft and semi‐soft cheeses made from cows’ milk, butter and pasteurised milk from other animal species or unspecified.
7.8. Tick‐borne encephalitis virus (TBE)
Slovenia reported test results for the presence of TBE of 20 batches of raw milk, from goats and from sheep and 30 batches of cheese from goat's milk and milk from sheep and none were positive.
7.9. Cysticercus, Sarcocystis and other parasites
Eight MS (Belgium, Bulgaria, Finland, Luxembourg, Malta, Slovenia, Spain and Sweden) submitted data (N = 52,167,264) on cysticerci (Taenia larvae) mainly based on reports from slaughterhouse surveillance, active monitoring or clinical investigations and overall 0.4% (210,455) were positive. Finland (N = 2,089,429 carcases from pigs, cattle and wild boar), Malta (N = 63,897 carcases from pigs, cattle, sheep and goats) and Sweden (3,005,930 carcases from pigs and cattle) reported no positive findings. Slovenia found eight (0.007% out of 116,495) positive cattle and no positive pigs. Bulgaria reported, respectively, < 0.001%, 0.47% and 0.06% positive pigs, sheep and goats and cattle out of 1,196,086, 235,286 and 29,274 examined. In Belgium, 1,075 out of the 840,654 cattle (0.13%) inspected at the slaughterhouse were positive. Luxembourg found 0.3% positive carcases from cattle out of 26,818 inspected. Spain provided data on cysticerci in various animal species: 74 (0.004%) out of 1,819,799 cattle, 0.004% out of 37,835,368 pigs, 5.2% out of 3,325,552 sheep and 2.9% out of 1,100,793 goats were positive for cysticerci. Finally, 38,917 wild boars and 127,264 deer were inspected at game handling establishment and one (0.003%) and one (0.001%) were positive for cysticerci, respectively. Examined carcases from 4,317 wild mouflons were all negative.
Estonia did not submit data for 2019 but informed that no cases of cysticerci of Taenia saginata and Taenia solium were detected during visual post‐mortem inspection at slaughterhouses of all slaughtered animals.
Belgium reported for 2019 840,654 bovine carcases from slaughterhouse inspection for the presence of Sarcocystis and 90 (0.01%) were positive.
7.10. Other
Of reported monitoring results for Leptospira, in total 6,746, Bulgaria found no positives out of 6,564 tested cattle and pigs, whereas Slovenia found 10 positive dogs in a total number of 182 samples from pets and domestic animals. For Shigella three food samples from Sweden were negative. For Vibrio, 326 food samples in total from Bulgaria, the Netherlands and Sweden, 32 were positive (9.8%). These positive results were from raw fish, from shrimps and from lobsters from third countries (border inspection activities). Out of the 535 samples tested (from fruits and vegetables) for hepatitis A virus (France, Romania and Sweden), no sample was positive. Out of a total of 136 cattle, sheep and goats tested for Klebsiella (Greece), one milk ewe was positive. Bulgaria reported monitoring results for marine biotoxins (N = 94) from raw molluscan shellfish with no positives.
Microbiological contaminants subject to food safety criteria (Regulation (EC) No 2073/2005)
Tables and figures that are not presented in this chapter are published as supporting information to this report and are available as downloadable files from the EFSA knowledge junction at zenodo https://doi.org/10.5281/zenodo.4298993
This chapter summarises the 2019 information provided by reporting countries on microbiological contaminants in foods: histamine, staphylococcal enterotoxins and Cronobacter sakazakii for which FSC are set down in the EU legislation (Regulation (EC) No 2073/2005).
As for food categories subject to FSC, EFSA used the following specific testing data in the context of Regulation (EC) No 2073/2005 for trend watching: Sampling context: surveillance, based on Regulation (EC) No 2073/2005; Sampling unit type: single; Sampling stage: catering, restaurant or cafe or pub or bar or hotel or catering service, wholesale, retail, hospital or medical care facility, conservation facilities; Sampling strategy: objective sampling; and Sampler: official sampling. Other data, having other specified options for the different data aspects (including sampling context other than based on Regulation (EC) No 2073/2005), are summarised only and do not serve the purpose of trend watching or trend analyses.
When the UK data were collected, the UK was an EU MS, but as of 31 January 2020, it has become a third country.
1. Histamine
Histamine is an endogenous compound of the human body that can also be introduced from external sources such as contaminated food. If histamine reaches a critical threshold, it can lead to symptoms such as skin flushing, rash, gastrointestinal complaints and throbbing headache. Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs defines FSC for histamine in food, at retail level, in two major food categories: ‘fishery products from fish species associated with a high amount of histidine’ (food category 1.25: n = 9; c = 2; m = 100 mg/kg; M = 200 mg/kg) and ‘Fishery products which have undergone enzyme maturation treatment in brine, manufactured from fish species associated with a high amount of histidine’ (food category 1.26: n = 9; c = 2; m = 200 mg/kg; M = 400 mg/kg).
For the year 2019, official control sample results (N = 1,020) for histamine in ‘fish, fishery products from fish species associated with a high amount of histidine’ were reported at retail by four MS (Romania, Slovakia, Slovenia and Spain) and overall two (0.2%) were reported with quantified results exceeding 200 mg/kg, whereas three samples (0.3%) were exceeding 100 mg/kg but did not exceed 200 mg/kg and 12 (1.2%) were below or equal to 100 mg/kg. Data (N = 38) for histamine in ‘Fishery products which have undergone enzyme maturation treatment in brine, manufactured from fish species associated with a high amount of histidine’ were reported at retail by two Romania and Spain and none were reported with quantified results exceeding 400 mg/kg, whereas one sample (2.6%) exceeded 200 mg/kg but did not exceed 400 mg/kg and the other samples were negative.
Czechia reported five official samples tested from ‘fish sauce produced by fermentation of fishery products’ and none was reported with a histamine quantified result above 400 mg/kg.
2. Staphylococcal enterotoxins
According the mentioned data elements, Romania and Spain reported in total 1,522 official control samples at the retail‐level, from cheeses, milk powder and whey powder. Three samples from Romania from hard cheeses made from cows’ milk were positive, sampled at ‘restaurant or cafe or pub or bar or hotel or catering service’.
3. Cronobacter sakazakii
Investigations according above data elements for Cronobacter in infant formula and dietary foods for special medical purposes were reported by four MS (Cyprus, Slovakia, Slovenia and Spain). In total, 198 single official control sample results were reported, and none was positive for Cronobacter spp.
Abbreviations
- ADNS
Animal Disease Notification System
- AE
alveolar echinococcosis
- AHAW
EFSA Panel on Animal Health and Welfare
- BIOHAZ
EFSA Panel on Biological Hazards
- CA
competent authority
- CE
cystic echinococcosis
- CFT
complement fixation test
- CFU
colony‐forming unit
- CONTAM
EFSA Panel on Contaminants in the Food Chain
- DCF
Data Collection Framework
- DH
definitive host
- EBLV
European bat lyssavirus
- ECDC
European Centre for Disease Prevention and Control
- EEA
European Economic Area
- EFTA
European Free Trade Association
- ELISA
Enzyme‐linked immunosorbent assay
- EPIS
Epidemic Intelligence Information System
- ESRI
Economic and Social Research Institute
- EU
European Union
- EURL
European Union Reference Laboratory
- EVD
Emerging and Vector‐borne Disease
- EWRS
Early Warning and Response System
- FAT
fluorescent antibody test
- FBO
food‐borne outbreak
- FBOp
food business operator
- FNAO
food of non‐animal origin
- FSC
food safety criteria
- g
gram
- GMP
Good Manufacturing Practices
- HACCP
hazard analysis and critical control point
- HC
hard cheeses
- HERACLES
Human cystic Echinococcosis ReseArch in CentraL and Eastern Societies
- HUS
haemolytic–uraemic syndrome
- i‐ELISA
indirect enzyme‐linked immunosorbent assay
- IFA
immunofluorescence assay
- IH
intermediate host
- IHC
immunohistochemistry
- ISO
International Organization for Standardization
- LHT
low heat‐treated
- MS
Member State
- N
number
- NCHC
no controlled housing conditions
- NMKL
Nordic Committee on Food Analysis
- NPHRL
National Public Health Reference Laboratory
- NT
not typable
- NCP
national control programmes
- OBF
official brucellosis‐free in cattle
- ObmF
official Brucella melitensis‐free in sheep and goats
- OIE
World Organisation for Animal Health
- ORV
oral rabies vaccination
- OTF
official tuberculosis‐free in cattle
- PCR
polymerase chain reaction
- PHC
process hygiene criteria
- RASFF
Rapid Alert System for Food and Feed
- ROA
Rapid Outbreak Assessments
- ROC
receiver operating characteristic
- RTE
ready‐to-eat
- RT‐PCR
reverse transcriptase‐polymerase chain reaction
- SSC
semi‐soft cheeses
- STEC
Shiga toxin‐producing Escherichia coli
- TBE
tick‐borne encephalitis
- TESSy
The European Surveillance System
- VTEC
verocytotoxigenic Escherichia coli
- WAHID
World Animal Health Information Database
- WGS
whole‐genome sequencing
- WHO
World Health Organisation
- WNF
West Nile fever
- WNV
West Nile virus
- WNND
West Nile neuroinvasive disease
Country codes
- Albania
AL
- Austria
AT
- Belgium
BE
- Bosnia and Herzegovina
BA
- Bulgaria
BG
- Croatia
HR
- Cyprus
CY
- Czechia
CZ
- Denmark
DK
- Estonia
EE
- Finland
FI
- Former
Yugoslav Republic of Macedonia, the MK
- France
FR
- Germany
DE
- Greece
GR
- Hungary
HU
- Iceland
IS
- Ireland
IE
- Italy
IT
- Latvia
LV
- Liechtenstein
LI
- Lithuania
LT
- Luxembourg
LU
- Montenegro
ME
- Malta
MT
- Netherlands
NL
- Norway
NO
- Poland
PL
- Portugal
PT
- Romania
RO
- Serbia
RS
- Slovakia
SK
- Slovenia
SI
- Spain
ES
- Sweden
SE
- Switzerland
CH
- United Kingdom
UK
Appendix A – Number of tested samples for the main ready‐to‐eat food categories, by reporting Member States and non‐Member States, EU, 2019
Table A.1.
Number of tested samples for the main ready‐to‐eat (RTE) food categories, by reporting MS and non‐MS, EU, 2019
| RTE milk and milk products | RTE fish and fishery products | RTE meat and meat products | Other RTE products | RTE food intended for infants and for medical purposes | |
|---|---|---|---|---|---|
| Austria | 1,005 | 200 | 735 | 1,214 | 76 |
| Belgium | 2,496 | 788 | 2,448 | 1,040 | 397 |
| Bulgaria | 8,042 | 1,097 | 2,521 | 1,414 | 14 |
| Croatia | 525 | 55 | 571 | 133 | 2 |
| Cyprus | 431 | 28 | 151 | 506 | 19 |
| Czechia | 159 | 35 | 107 | 703 | 17 |
| Denmark | 14 | 373 | 552 | 175 | – |
| Estonia | 100 | 126 | 150 | 114 | 2 |
| France | 1,914 | 1,321 | 2,124 | 2,312 | 36 |
| Germany | 6,621 | 1,924 | 5,082 | 6,398 | 164 |
| Greece | 156 | 22 | 61 | 81 | 5 |
| Ireland | 1,079 | 226 | 2,048 | 2,604 | 190 |
| Italy | 13,670 | 663 | 21 | 1,326 | 107 |
| Latvia | 50 | 150 | 85 | 30 | – |
| Lithuania | – | – | – | 15 | – |
| Luxembourg | – | – | 287 | – | – |
| Netherlands | 4,028 | 945 | 554 | 1,074 | 111 |
| Poland | 10,160 | 2,720 | 24,425 | 30 | – |
| Portugal | 591 | 146 | 257 | 1,331 | 47 |
| Romania | 7,254 | 1,550 | 19,123 | 51,192 | 10 |
| Slovakia | 2,024 | 435 | 1,889 | 1,694 | 472 |
| Slovenia | 90 | 33 | 65 | 235 | 10 |
| Spain | 987 | 508 | 1,410 | 2,533 | 42 |
| Sweden | 9 | 31 | – | 106 | – |
| United Kingdom | 614 | – | – | 397 | – |
| EU | 62,019 | 13,376 | 64,666 | 76,657 | 1,721 |
| Albania | 2 | 4 | 1 | – | – |
| Iceland | – | 5 | – | – | – |
| Montenegro | 1,596 | 26 | 285 | – | – |
| Republic of North Macedonia | 140 | – | 74 | – | – |
| Switzerland | 1,072 | – | – | – | – |
| Non‐EU | 2,810 | 35 | 360 | – | – |
| Total (EU and non‐EU) | 64,829 | 13,411 | 65,026 | 76,657 | 1,721 |
RTE: ready‐to‐eat; –: no data available.
For each food category, the number of samples reported in the table were obtained without exclusion criteria. Samples were tested by a detection method and/or an enumeration method.
Appendix B – ccurrence of L. monocytogenes at retail and processing stages combined in ready‐to‐eat food categories using a detection method, EU, 2017–2019
Table B.1.
Occurrence of L. monocytogenes at retail and processing stages combined in ready‐to‐eat (RTE) food categories using a detection method, EU, 2017–2019
| RTE food category | Food subcategories | Sampling unit | 2017 | 2018 | 2019 | |||
|---|---|---|---|---|---|---|---|---|
| N tested samples | Positive samples (%) | N tested samples | Positive samples (%) | N tested samples | Positive samples (%) | |||
| Fish and fishery products | Fish | Batch | 589 | 1.9 | 144 | 2.8 | 58 | 1.7 |
| Single | 4,719 | 7.6 | 4,209 | 2.5 | 3,961 | 4.4 | ||
| Fishery products | Batch | 519 | 0.8 | 420 | 0.2 | 25 | 0.0 | |
| Single | 2,350 | 2.6 | 2,521 | 3.5 | 3,681 | 4.3 | ||
| Milk | Pasteurised | Batch | 245 | 0 | 68 | 0.0 | 468 | 0.0 |
| Single | 1,924 | 2.9 | 1,879 | 0.1 | 1,500 | 0.1 | ||
| UHT | Batch | 8 | 0.0 | 7 | 0.0 | 0 | – | |
| Single | 10 | 0.0 | 29 | 0.0 | 115 | 0.0 | ||
| Raw, intended for direct human consumption | Batch | 69 | 0.0 | 55 | 1.8 | 144 | 0.7 | |
| Single | 148 | 2.7 | 281 | 6.1 | 60 | 0.0 | ||
| Hard cheeses from pasteurised milk | From cows’ milk | Batch | 3,166 | 0.0 | 2,431 | 0.2 | 1,932 | 0.1 |
| Single | 854 | 0.1 | 2,815 | 0.0 | 2,468 | 0.0 | ||
| From goats’ milk | Batch | 15 | 0.0 | 16 | 0.0 | 107 | 0.0 | |
| Single | 48 | 0.0 | 92 | 0.0 | 161 | 0.0 | ||
| From sheep milk | Batch | 47 | 0.0 | 9 | 0.0 | 4 | 0.0 | |
| Single | 12 | 0.0 | 118 | 0.0 | 110 | 0.0 | ||
| Hard cheeses from raw or low heat‐treated milk | From cows’ milk | Batch | 625 | 0.0 | 460 | 2.0 | 541 | 0.6 |
| Single | 90 | 2.2 | 485 | 2.1 | 988 | 0.8 | ||
| From goats’ milk | Batch | – | – | – | – | – | – | |
| Single | 5 | 0.0 | 22 | 0.0 | 29 | 3.5 | ||
| From sheep milk | Batch | 4 | 0.0 | – | – | – | – | |
| Single | 7 | 14.3 | 104 | 4.8 | 221 | 2.7 | ||
| Soft and semi‐soft cheeses from pasteurised milk (including fresh cheese) | From cows’ milk | Batch | 1,594 | 0.0 | 380 | 0.8 | 339 | 0.0 |
| Single | 2,487 | 0.7 | 4,935 | 0.3 | 3,304 | 0.4 | ||
| From goats’ milk | Batch | 240 | 0.0 | 25 | 0.0 | 30 | 0.0 | |
| Single | 410 | 0.0 | 341 | 0.0 | 53 | 0.0 | ||
| From sheep milk | Batch | 185 | 0.0 | 25 | 0.0 | – | – | |
| Single | 188 | 0.0 | 492 | 0.2 | 20 | 0.0 | ||
| Soft and semi‐soft cheeses from raw or low heat‐treated milk (including fresh cheese) | From cows’ milk | Batch | 150 | 0.7 | 148 | 0.7 | 130 | 0.0 |
| Single | 514 | 1.7 | 742 | 0.8 | 766 | 1.2 | ||
| From goats’ milk | Batch | 2 | 0.0 | – | – | – | – | |
| Single | 71 | 0.0 | 43 | 0.0 | 42 | 0.0 | ||
| From sheep milk | Batch | 7 | 0.0 | 60 | 0.0 | – | – | |
| Single | 843 | 3.1 | 452 | 0.2 | 461 | 1.5 | ||
| Meat products | From bovine animals | Batch | 285 | 2.8 | 7 | 0.0 | 3 | 0.0 |
| Single | 1,549 | 1.7 | 1,139 | 3.1 | 2,035 | 2.8 | ||
| From broilers | Batch | 347 | 0.0 | – | – | – | – | |
| Single | 431 | 2.6 | 1,206 | 0.6 | 4,872 | 0.9 | ||
| From turkeys | Batch | 27 | 0.0 | 142 | 0.0 | 3 | 0.0 | |
| Single | 250 | 0.8 | 116 | 0.9 | 125 | 1.6 | ||
| From pigs | Batch | 1,575 | 2.7 | 1,639 | 3.9 | 133 | 9.0 | |
| Single | 19,593 | 1.8 | 23,175 | 1.2 | 28,704 | 2.1 | ||
| Other RTE products | Saladsa | Batch | 349 | 0.0 | 79 | 2.5 | 47 | 0.0 |
| Single | 668 | 6.1 | 2,504 | 1.4 | 3,091 | 3.5 | ||
| Bakery productsb | Batch | 647 | 0.0 | 41 | 0.0 | 60 | 0.0 | |
| Single | 3,363 | 13.0 | 3,758 | 0.2 | 6,593 | 0.2 | ||
| Fruits and Vegetablesc | Batch | 258 | 0.8 | 41 | 0.0 | 66 | 0.0 | |
| Single | 751 | 1.1 | 1,216 | 1.9 | 2,291 | 1.7 | ||
| Sauces and dressingsd | Batch | 11 | 0.0 | 30 | 0.0 | – | – | |
| Single | 173 | 1.7 | 190 | 0.0 | 369 | 0.3 | ||
| Egg products | Batch | 3 | 0.0 | 3 | 0.0 | 3 | 0.0 | |
| Single | – | – | – | – | 23 | 0.0 | ||
| Confectionery products and pastese | Batch | 9 | 0.0 | – | – | 3 | 0.0 | |
| Single | 1 | 0.0 | 63 | 0.0 | 51 | 0.0 | ||
| Spices and herbsf | Batch | 4 | 0.0 | 13 | 0.0 | 2 | 0.0 | |
| Single | 44 | 0.0 | 108 | 0.0 | 289 | 0.7 | ||
| Other processed food products and prepared dishes | Batch | 276 | 0.0 | 31 | 0.0 | 154 | 2.0 | |
| Single | 2,456 | 1.0 | 2,077 | 0.8 | 42,771 | 0.3 | ||
UHT: ultrahigh temperature.
Includes RTE salads (containing mayonnaise).
Includes bread, cakes, desserts and pastry.
Includes fruits: edible part, pre‐cut, products, fruits and vegetables: pre‐cut, products, juice: fruit juice, mixed juice vegetable juice and vegetables: pre‐cut, products.
Includes sauces and dressings (containing mayonnaise).
Includes confectionery products and pastes such as chocolate‐based product and soft and hard candy.
Includes spices and herbs, either dried, fresh or frozen.
Includes for example ices and similar frozen desserts, pasta/rice salad, sandwiches, sushi.
Appendix C – Atlases of STEC serogroups: food and animals, EU, 2019
Suggested citation: EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) , 2021. The European Union One Health 2019 Zoonoses Report. EFSA Journal 2021;19(2):6406, 286 pp. 10.2903/j.efsa.2021.6406
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00001
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA and the ECDC wish to thank the members of the EFSA Scientific Network for Zoonoses Monitoring Data and of the ECDC Food and Waterborne Diseases and Zoonoses Network, the ECDC Emerging and Vector‐borne Diseases Network and the ECDC Tuberculosis Network, who provided the data and reviewed the report; the members of the Scientific Network for Zoonoses Monitoring Data for their endorsement of this scientific report; the EFSA staff members (Frank Boelaert, Anca Stoicescu, Giusi Amore, Winy Messens, Michaela Hempen, Valentina Rizzi, Sotiria‐Eleni Antoniou, Francesca Baldinelli, Elisabeth Dorbek‐Kolin and Yves Van der Stede), the ECDC staff members (Taina Niskanen, Joana Haussig, Marlena Kaczmarek and Joana Gomes Dias) and the EFSA contractors: the Istituto Zooprofilattico Sperimentale delle Venezie, Italy (and staff members: Lisa Barco, Marzia Mancin, Claudio Mantovani, Angelo Sardella, Pietro Antonelli, Marta Leati, Antonia Anna Lettini and Carmen Losasso), the Istituto Superiore di Sanita, Italy (and staff members: Stefano Morabito, Gaia Scavia, Arnold Knijn, Rosangela Tozzoli, Francesca Iacoponi, Ornella Moro, Michele Luca D'Errico, Antonietta Gattuso, Elisabetta Suffredini, Ilaria Di Bartolo, Elisabetta Delibato, Fabrizio Anniballi, Giovanni Ianiro and Ilaria Altieri), the European Union Reference Laboratory for Parasites (and staff members: Maria Angeles Gomez Morales, Adriano Casulli (who is also affiliated to the WHO Collaborating Centre for the Epidemiology, Detection and Control of Cystic and Alveolar Echinococcosis) and Simone Cacciò) and the European Union Reference Laboratory for Listeria monocytogenes (the French agency for food, environmental and occupational health safety (ANSES) and staff members Corinne Danan and Benjamin Felix), for the support provided to this scientific report.
Approved: 19 January 2021
Data: Tables and figures complementing the European Union One Health 2019 Zoonoses Report and cross‐referenced in the text are available on the Knowledge Junction community at: https://doi.org/10.5281/zenodo.4298993
This article was originally published on the EFSA website www.efsa.europa.eu on 25 February 2021 as part of EFSA’s urgent publication procedures.
Notes
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L 325, 12.12.2003, pp. 31–40.
Decision No 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross‐border threats to health and repealing Decision No 2119/98/EC. OJ L 293, 5.11.2013, pp. 1–15.
Commission Implementing Decision 2018/945/EU on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions. OJ L 170, 6.7.2018, pp. 1–74.
Based on the customs union treaty of the Principality of Liechtenstein with Switzerland, Liechtenstein is part of the Swiss customs territory. Due to the tight connection between the veterinary authorities of Liechtenstein and Switzerland as well as Liechtenstein's integration into the Swiss system in the veterinary field, in principal, all legislation, rules and data on contagious diseases are identical for both Switzerland and Liechtenstein. If not mentioned otherwise, the Swiss data include also the data from Liechtenstein.
COMMISSION IMPLEMENTING REGULATION (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as regards official controls.
Meaning official sampling using the same method and sampling area as food business operators. At least 49 random samples shall be taken in each slaughterhouse each year. This number of samples may be reduced in small slaughterhouses based on a risk evaluation.
Chapter 11.2 of Statistical Models in Epidemiology, Clayton D and Hills M (2013).
Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. OJ L 338, 22.12.2005, pp. 1–26 as amended by Commission Regulation (EC) No 2019/229 of 7 February 2019.
EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control), 2017. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2016. EFSA Journal 2017;15(12):5077, 228 pp. https://doi.org/10.2903/j.efsa.2017.5077
Also known as verotoxigenic, verocytotoxigenic, verotoxin‐producing, verocytotoxin‐producing E. coli (VTEC).
ADNS, the EU Animal Disease Notification System, see http://ec.europa.eu/food/animals/animal-diseases/not-system_en
Source: https://ec.europa.eu/food/sites/food/files/animals/docs/la_bovine_map_free-from_tuberc.pdf
United Kingdom: report on the implementation of the bovine tuberculosis eradication programme in 2018, SCoPAFF meeting, Brussels, 12–13 June 2019. Available Online (https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20190612_pres_bov-tub_gbr.pdf).
PAFF Committee meeting, Brussels, 24 September 2019. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20190924_bov-tub_erad_irl.pdf
Source: https://ec.europa.eu/food/sites/food/files/animals/docs/la_bovine_map_free-from_bov-bruc.pdf
Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015 laying down specific rules on official controls for Trichinella in meat (text with EEA relevance). OJ L 212, 11.8.2015, pp. 7–34.
Commission Delegated Regulation (EU) 2018/772 of 21 November 2017 supplementing Regulation (EU) No 576/2013 of the European Parliament and of the Council with regard to preventive health measures for the control of Echinococcus multilocularis infection in dogs, and repealing Delegated Regulation (EU) No 1152/2011 C/2017/7619 OJ L 130, 28.5.2018, p. 1–10.
Available online: http://www.efsa.europa.eu/en/biological-hazards-data/reports.
Additional information from Italian reference centre for Toxoplasma spp.: ‘Drinkable water samples were collected from municipal water supplies to assess the potential remaining contamination with oocysts shed by infected cats after the water undergoes treatment. The same applied to RTE honey samples tested to rule out any potential faecal contamination during extraction and processing.’
Regulation (EU) No 652/2014 of the European Parliament and of the Council of 15 May 2014 laying down provisions for the management of expenditure relating to the food chain, animal health and animal welfare, and relating to plant health and plant reproductive material, amending Council Directives 98/56/EC, 2000/29/EC and 2008/90/EC, Regulations (EC) No 178/2002, (EC) No 882/2004 and (EC) No 396/2005 of the European Parliament and of the Council, Directive 2009/128/EC of the European Parliament and of the Council and Regulation (EC) No 1107/2009 of the European Parliament and of the Council and repealing Council Decisions 66/399/EEC, 76/894/EEC and 2009/470/EC. OJ L 189, 27.6.2014, pp. 1–32.
Commission Implementing Regulation (EU) No 577/2013 of 28 June 2013 on the model identification documents for the non‐commercial movement of dogs, cats and ferrets, the establishment of lists of territories and third countries and the format, layout and language requirements of the declarations attesting compliance with certain conditions provided for in Regulation (EU) No. 576/2013 of the European Parliament and of the Council. OJ L 178, 28.6.2013, pp. 109–148.
1st meeting of the Standing Group of Experts on Rabies in Europe, Global framework for the progressive control of transboundary animal diseases (2019). Available online: https://rr-europe.oie.int/wp-content/uploads/2019/09/a_report_sge_rab1.pdf
(i) Comprehensive: All healthcare providers of at least one level of care in a defined geographical area, e.g. all general practitioners of the region, should report their cases. See: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.27.1900708
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. http://data.europa.eu/eli/dir/2003/99/oj
Council Directive 82/894/EEC of 21 December 1982 on the notification of animal diseases within the Community. http://data.europa.eu/eli/dir/1982/894/oj
Definitions of the terms outbreak and case (Article 4 Directive 82/894): ‘outbreak’ means the holding or place situated in the territory of the Community where animals are assembled and where one or more cases has or have been officially confirmed. While ‘case’ means the official confirmation of any of the diseases listed in Annex I of the Directive 82/894 in any animal or carcase.
The data extracted from the ADNS on September 1, 2020.
Swedish national zoonoses country report. Available online at http://www.efsa.europa.eu/en/biological-hazards-data/reports
References
- Abubakar RH, Madoroba E, Adenubi O, Morar‐Leather D and Fasina FO, 2017. Bacterial pathogens of pigs with particular reference to Escherichia coli: a systematic review and meta‐analysis. Journal of Veterinary Medicine and Animal Health, 9, 159–185. [Google Scholar]
- Alban L, Pozio E, Boes J, Boireau P, Boué F, Claes M, Cook AJC, Dorny P, Enemark HL, van der Giessen J, Hunt KR, Howell M, Kirjusina M, Nöckler K, Rossi P, Smith GC, Snow L, Taylor MA, Theodoropoulos G, Vallée I, Viera‐Pinto MM and Zimmer IA, 2011. Towards a standardised surveillance for Trichinella in the European Union. Preventive Veterinary Medicine, 99, 148–160. [DOI] [PubMed] [Google Scholar]
- ANSES , 2011. Data sheet on foodborne biological hazards – Listeria monocytogenes. Available online: https://www.anses.fr/en/system/files/MIC2011sa0171FiEN.pdf)
- Antunes P, Mourão J, Campos J and Peixe L, 2016. Salmonellosis: the role of poultry meat. Clinical Microbiology and Infection, 22, 110–121. 10.1016/j.cmi.2015.12.004 [DOI] [PubMed] [Google Scholar]
- Aroussi A, Vignoles P, Dalmay F, Wimel L, Darde M‐L, Mercier A and Ajzenberg D, 2015. Detection of Toxoplasma gondii DNA in horse meat from supermarkets in France and performance evaluation of two serological tests. Parasite, 22, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balog T, Nagy G, Halász T, Csányi E, Zomborszky Z and Csivincsik Á, 2021. The occurrence of Echinococcus spp. in golden jackal (Canis aureus) in southwestern Hungary: should we need to rethink its expansion? Parasitology International, 80, 102214. 10.1016/j.parint.2020.102214. Epub 2020 Oct 31. PMID: 33137507. [DOI] [PubMed] [Google Scholar]
- Bernard H, Faber M, Wilking H, Haller S, Höhle M, Schielke A, Ducomble T, Siffczyk C, Merbecks SS, Fricke G, Hamouda O, Stark K and Werber D; and the Outbreak Investigation Team , 2014. Large multistate outbreak of norovirus gastroenteritis associated with frozen strawberries, Germany, 2012. Eurosurveillance, 19, 20719. . PMID: 24602278 [DOI] [PubMed] [Google Scholar]
- Bless PJ, Schmutz C and Mäusezahl D, 2017. The recurrent campylobacteriosis epidemic over Christmas and New Year in European countries, 2006–2014. BMC Research Notes, 10, 266. 10.1186/s13104-0172587-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert F, Amore G, Van der Stede Y and Hugas M, 2016. EU‐wide monitoring of biological hazards along the food chain: achievements, challenges and EFSA vision for the future. Current Opinion in Food Science, 12, 56–72. [Google Scholar]
- Campos J, Mourão J, Peixe L and Antunes P, 2019. Non‐typhoidal Salmonella in the pig production chain: a comprehensive analysis of its impact on human health. Pathogens, 8, 19. 10.3390/pathogens8010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casulli A, 2020. Recognising the substantial burden of neglected pandemics cystic and alveolar echinococcosis. The Lancet. Global Health, 8, e470–e471. [DOI] [PubMed] [Google Scholar]
- Casulli A, Barth TFE and Tamarozzi F, 2019a. Echinococcus multilocularis . Trends in Parasitology, 35, 738–739. [DOI] [PubMed] [Google Scholar]
- Casulli A, Siles‐Lucas M and Tamarozzi F, 2019b. Echinococcus granulosus sensu lato . Trends in Parasitology, 35, 663–664. [DOI] [PubMed] [Google Scholar]
- Clayton C and Hills M, 1993. Statistical Models in Epidemiology. Oxford University Press, New York. 367 pp. [Google Scholar]
- Cliquet F, Freuling C, Smreczak M, Van der Poel WHM, Horton D, Fooks AR, Robardet E, Picard‐Meyer E and Müller T, 2010. Development of harmonised schemes for monitoring and reporting of rabies in animals in the European Union. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/sp.efsa.2010.EN-67
- De Cesare A, 2018. Salmonella in foods: a reemerging problem. Advances in Food and Nutrition Research, 86, 137–179. 10.1016/bs.afnr.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Deplazes P, Hegglin D, Gloor S and Romig T, 2004. Wilderness in the city: the urbanization of Echinococcus multilocularis . Trends in Parasitology, 20, 77–84. [DOI] [PubMed] [Google Scholar]
- DIN (Deutsches Institut für Normung e.V. German National Standard), 2004. DIN 10167:2004‐03. Nachweis von Escherichia coli O157 in Fleisch und Fleischerzeugnissen. [Detection of Escherichia coli O157 in meat and meat products.
- Dušek D, Vince A, Kurelac I, Papić N, Višković K, Deplazes P and Beck R, 2020. Human alveolar echinococcosis, Croatia. Emerging Infectious Diseases, 26, 364–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvnjak S, Racic I, Spicic S, Zdelar‐Tuk M, Reil I and Cvetnic Z, 2018. Molecular epidemiology of Brucella melitensis strains causing outbreaks in Croatia and Bosnia and Herzegovina. Acta Veterinaria Hungarica, 66, 177–188. 10.1556/004.2018.017 [DOI] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control), 2018. Multi‐country outbreak of Salmonella Enteritidis infections linked to Polish eggs – epidemiological update, 19 November, 2018. Stockholm: ECDC; 2018.
- ECDC (European Centre for Disease Prevention and Control), 2019. Brucellosis. In: ECDC. Annual epidemiological report for 2017. Stockholm: ECDC; 2019.
- ECDC (European Centre for Disease Prevention and Control), 2020. Weekly updates: 2020 West Nile virus transmission season. Available online: https://ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc
- EFSA (European Food Safety Authority), 2009a. Scientific Report of EFSA on technical specifications for the monitoring and reporting of verotoxigenic Escherichia coli (STEC) on animals and food (STEC surveys on animals and food). EFSA Journal 2009;7(11):1366, 43 pp. 10.2903/j.efsa.2009.1366 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009b. Technical specifications for harmonised national surveys of Yersinia enterocolitica in slaughter pigs on request of EFSA. EFSA Journal 2009; 7(11):1374, 23 pp. 10.2903/j.efsa.2009.1374. Available online: www.efsa.europa.eu [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Update of the technical specifications for harmonised reporting of food‐borne outbreaks through the European Union reporting system in accordance with Directive 2003/99/EC. EFSA Journal 2014;12(3):3598, 25 pp. 10.2903/j.efsa.2014.3598 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Beloeil P‐A, Bocca V, Boelaert F, Gibin D, Guerra B, Papanikolaou A and Stoicescu A‐V, 2020a. Zoonoses, antimicrobial resistance and foodborne outbreaks guidance for reporting 2019 data. EFSA supporting publication 2020;EN‐1792, 110 pp. 10.2903/sp.efsa.2020.EN-1792 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Boelaert F, Papanikolaou A, Rizzi V and Stoicescu A‐V, 2020b. Manual for reporting on zoonoses and zoonotic agents, within the framework of Directive 2003/99/EC, and on some other pathogenic microbiological agents for information derived from the year 2019. EFSA supporting publication 2020;EN‐1791, 63 pp. 10.2903/sp.efsa.2020.EN1791 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2014. Statement on a conceptual framework for bovine tuberculosis. EFSA Journal 2014;12(5):3711, 59 pp. 10.2903/j.efsa.2014.3711 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2015. Scientific Opinion – update on oral vaccination of foxes and raccoon dogs against rabies. EFSA Journal 2015;13(7):4164, 70 pp. 10.2903/j.efsa.2015.4164 [DOI] [Google Scholar]
- EFSA BIOHAZ, CONTAM and AHAW Panel (EFSA Panels on Biological Hazards, on Contaminants in the Food Chain, and on Animal Health and Welfare), 2011. Scientific Opinion on the public health hazards to be covered by inspection of meat (swine). EFSA Journal 2011;9(10):2351, 198 pp. 10.2903/j.efsa.2011.2351 [DOI] [Google Scholar]
- EFSA BIOHAZ, CONTAM and AHAW (Panels on Biological Hazards, on Contaminants in the Food Chain, and on Animal Health and Welfare), 2012. Scientific Opinion on the public health hazards to be covered by inspection of meat (poultry). EFSA Journal 2012;10(6):2741, 179 pp. 10.2903/j.efsa.2012.2741. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010. Scientific Opinion on quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA Journal 2010;8(1):1437, 89 pp. 10.2903/j.efsa.2010.1437. Available online: www.efsa.europa.eu [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011. Scientific Opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA Journal 2011;9(4):2105, 141 pp. 10.2903/j.efsa.2011.2105. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013a. Scientific Opinion on STEC‐seropathotype and scientific criteria regarding pathogenicity assessment. EFSA Journal 2013;11(4):3138, 106 pp. 10.2903/j.efsa.2013.3138. [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013b. Scientific Opinion on the public health hazards to be covered by inspection of meat (solipeds). EFSA Journal 2013;11(6):3263, 161 pp. 10.2903/j.efsa.2013.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2018a. Scientific Opinion on Listeria monocytogenes contamination of ready‐to‐eat (RTE) foods and the risk for human health in the EU. EFSA Journal 2018;16 (1):5134, 173 pp. 10.2903/j.efsa.2018.5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2018b. Scientific Opinion on the public health risks associated with food‐borne parasites. EFSA Journal 2018;16(12):5495, 113 pp. 10.2903/j.efsa. 2018.5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2020c. Pathogenicity assessment of Shiga toxin‐producing Escherichia coli (STEC) and the public health risk posed by contamination of food with STEC. EFSA Journal 2020;18(1):5967, 68 pp. 10.2903/j.efsa.2020.5967 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Allende A, Alvarez‐Ordonez A, Bolton D, Bover‐Cid S, Chemaly M, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Dewulf J, Hald T, Michel V, Niskanen T, Ricci A, Snary E, Boelaert F, Messens W and Davies R, 2019. Scientific Opinion on the Salmonella control in poultry flocks and its public health impact. EFSA Journal 2019;17(2):5596, 155 pp. 10.2903/j.efsa.2019.5596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Allende A, Alvarez‐Ordonez A, Bolton D, Bover‐Cid S, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Alter T, Crotta M, Ellis‐Iversen J, Hempen M, Messens W and Chemaly M, 2020a. Update and review of control options for Campylobacter in broilers at primary production. EFSA Journal 2020;18(4):6090, 89 pp. 10.2903/j.efsa.2020.6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Alvarez‐Ordóňez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Jordan K, Sampers I, Wagner M, Da Silva Felicio MT, Georgiadis M, Messens W, Mosbach‐Schulz O and Allende A, 2020b. Scientific Opinion on the public health risk posed by Listeria monocytogenes in frozen fruit and vegetables including herbs, blanched during processing. EFSA Journal 2020;18(4):6092, 102 pp. 10.2903/j.efsa.2020.6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2009. The Community summary report on trends and sources of zoonoses and zoonotic agents in the European Union in 2007. EFSA Journal 2009;7(1):223r, 350 pp. 10.2903/j.efsa.2009.223r [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2010. The Community summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in the European Union in 2008. EFSA Journal 2010;8(1):1496, 410 pp. 10.2903/j.efsa.2010.1496 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2011. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2009. EFSA Journal 2011;9:2090, 378 pp. 10.2903/j.efsa.2011.2090 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2012. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2010. EFSA Journal 2012;10(3):2597, 442 pp. 10.2903/j.efsa.2012.2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2013. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2011. EFSA Journal 2013;11(4):3129, 250 pp. 10.2903/j.efsa.2013.3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2014. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2012. EFSA Journal 2014;12(2):3547, 312 pp. 10.2903/j.efsa.2014.3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2015a. The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2013. EFSA Journal 2015;13(1):3991, 162 pp. 10.2903/j.efsa.2015.3991 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2015b. The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2014. EFSA Journal 2015;13(12):4329, 191 pp. 10.2903/j.efsa.2015.4329 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2016. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2015. EFSA Journal 2016;14(12):4634, 231 pp. 10.2903/j.efsa.2016.4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017a. The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2016. EFSA Journal 2017;15(12):5077, 228 pp. 10.2903/j.efsa.2017.5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017b. Multi‐country outbreak of Salmonella Enteritidis infections linked to Polish eggs. EFSA Supporting Publication 2017:EN‐1353, 21 pp. 10.2903/sp.efsa.2017.en-1353 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017c. Multi‐country outbreak of Salmonella Enteritidis phage type 8, MLVA profile 2–9‐7–3‐2 and 2–9‐6–3‐2 infections. EFSA supporting publication 2017;EN‐1188, 22 pp. 10.2903/sp.efsa.2017.en-1188 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018a. Multi‐country outbreak of Listeria monocytogenes serogroup IVb, multi‐locus sequence type 6, infections linked to frozen corn and possibly to other frozen vegetables – first update. EFSA supporting publication 2018;EN‐1448, 22 pp. 10.2903/sp.efsa.2018.en-1448 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018b. The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2017. EFSA Journal 2018;16(12):5500, 228 pp. 10.2903/j.efsa.2018.5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2019a. Multi‐country outbreak Salmonella Poona infections linked to consumption of infant formula. EFSA supporting publication 2019;EN‐1594, 18 pp. 10.2903/sp.efsa.2019.EN-1594 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2019b. Multi‐country outbreak of Listeria monocytogenes clonal complex 8 infections linked to consumption of cold‐smoked fish products. EFSA Journal 2019;EN‐1665, 22 pp. 10.2903/sp.efsa.112019.en-1665 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2019c. The European Union One Health 2018 Zoonoses Report. EFSA Journal 2019;17(12):5926, 276 pp. 10.2903/j.efsa.2019.5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2019d. Multi‐country outbreak of Listeria monocytogenes sequence type 6 infections linked to ready‐to‐eat meat products. Available online: 10.2903/sp.efsa.2019.en-1745 [DOI]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2020a.Multi‐country outbreak of Salmonella Enteritidis infections linked to eggs, third update – 6 February 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/multi-country-outbreak-salmonella-enteritidis-infections-linked-eggs
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2020b. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA Journal 2020;18(3):6007, 166 pp. 10.2903/j.efsa.2020.6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUROSTAT , 2020. Population structure and ageing. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Population_structure_and_ageing#The_share_of_elderly_people_continues_to_increase
- Facciolà A, Palamara MAR, D'Andrea G, Marano F, Magliarditi D, Puglisi G, Picerno I, Di Pietro A and Visalli G, 2018. Brucellosis is a public health problem in southern Italy: burden and epidemiological trend of human and animal disease. Journal of Infection and Public Health, 11, 861–866. 10.1016/j.jiph.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Fouskis I, Sandalakis V, Christidou A, Tsatsaris A, Tzanakis N, Tselentis Y and Psaroulaki A, 2018. The epidemiology of brucellosis in Greece, 2007–2012: a ‘One Health’ approach. Transactions of The Royal Society of Tropical Medicine and Hygiene, 112, 124–135. 10.1093/trstmh/try031 [DOI] [PubMed] [Google Scholar]
- García‐Soto S, Abdel‐Glil MY, Tomaso H, Linde J and Methner U, 2020. Emergence of multidrug‐resistant Salmonella enterica subspecies enterica Serovar Infantis of multilocus sequence type 2283 in German broiler farms. Frontiers in Microbiology, 11, 1741. 10.3389/fmicb.2020.01741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofolo G, Fasanella A, Di Giannatal E, Platone I, Sacchini L, Persiani T, Boskani T, Rizzardi K and Wahab T, 2016. Cases of human brucellosis in Sweden linked to Middle East and Africa. BMC Research Notes, 9, 277. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4869368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi E, Walter MC, Pfalzgraf M‐T, Northoff BH, Holdt LM, Scholz HC, et al., 2017. Whole genome sequencing of Brucella melitensis isolated from 57 patients in Germany reveals high diversity in strains from Middle East. PLoS ONE, 12, e0175425. 10.1371/journal.pone.0175425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev M, Afonso A, Neubauer H, Needham H, Thiery R, Rodolakis A, Roest H, Stark K, Stegeman J, Vellema P, van der Hoek W and More S, 2013. Q fever in humans and farm animals in four European countries, 1982 to 2010. Eurosurveillance, 18, pii: 20407. [PubMed] [Google Scholar]
- Grimont P and Weill F‐X, 2007. Antigenic formulae of the Salmonella serovars, 9th Edition. WHO Collaborating Centre for Reference and Research on Salmonella, Institute Pasteur, Paris, France. Available online: https://www.pasteur.fr/sites/default/files/veng_0.pdf [Google Scholar]
- Hestvik G, Warns‐Petit E, Smith LA, Fox NJ, Uhlhorn H, Artois M, Hannant D, Hutchings MR, Mattsson R, Yon L and Gavier‐Widen D, 2015. The status of tularemia in Europe in a one‐health context: a review. Epidemiology and Infection, 143, 2137–2160. 10.1017/s0950268814002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestvik G, Uhlhorn H, Koene M, Åkerström S, Malmsten A, Dahl F, Åhlén P‐A, Dalin A‐M and Gavier‐Widén D, 2019. Francisella tularensis in Swedish predators and scavengers. Epidemiology and Infection, 147(e293), 1–7. 10.1017/S0950268819001808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO (International Organization for Standardization), 2001. Microbiology of food and animal feeding stuffs – horizontal method for the detection of Escherichia coli O157. ISO, 16654, 2001 Available online: https://www.iso.org/standard/29821.html [DOI] [PubMed] [Google Scholar]
- ISO (International Organization for Standardization), 2012. Microbiology of food and animal feed. Real‐time polymerase chain reaction (PCR)‐based method for the detection of food‐borne pathogens. Horizontal method for the detection of shiga toxin‐producing Escherichia coli (STEC) and the determination of O157, O111, O26, O103 and O145 serogroups. ISO/TS 13136:2012. 22 pp. Available online: https://www.iso.org/standard/53328.html
- ISO (International Organization for Standardization), 2017a. ISO 11290‐1:2017. Microbiology of food and animal feeding stuffs. Horizontal method for the detection and enumeration of Listeria monocytogenes and Listeria spp. Part 1: Detection method. Available online: https://www.iso.org/standard/60313.html
- ISO (International Organization for Standardization), 2017b. ISO 11290‐2:2017. Microbiology of food and animal feeding stuffs. Horizontal method for the detection and enumeration of Listeria monocytogenes and Listeria spp. Part 2: Enumeration method. Available online: https://www.iso.org/standard/60314.html
- Janse I, Maas M, Rijks JM, Koene M, van der Plaats RQ, Engelsma M, van der Tas P, Braks M, Stroo A, Notermans DW, de Vries MC, Reubsaet F, Fanoy E, Swaan C, Kik MJ, IJzer J, Jaarsma RI, van Wieren S, de Roda‐Husman AM, van Passel M, Roest HJ and van der Giessen J, 2015. Environmental surveillance during an outbreak of tularaemia in hares, the Netherlands. Eurosurveillance Weekly, 22, pii: 30607. 10.2807/15607917.es.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen W, Linard C, Noll M, Nöckler K and Al Dahouk S, 2019. Brucella‐positive raw milk cheese sold on the inner European market: a public health threat due to illegal import? Food Control, 100, 130–137. [Google Scholar]
- Johansen TB, Scheffer L, Jensen VK, Bohlin J and Feruglio SL, 2018. Whole‐genome sequencing and antimicrobial resistance in Brucella melitensis from a Norwegian perspective. Science Reports, 8, 8538. 10.1038/s41598-018-26906-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, de la Gandara Pardos M, Herrera‐Leon L, Herrera‐Leon S, Varela Martinez C, Hureaux‐Roy R, Abdallah Y, Nisavanh A, Fabre L, Renaudat C, Mossong J, Mattheus W, Huard C, Le Borgne C, de Valk H, Weill F‐X and Jourdan‐Da Silva N, 2019. Outbreak of Salmonella enterica serotype Poona in infants linked to persistent Salmonella contamination in an infant formula manufacturing facility, France, August 2018 to February 2019. Eurosurveillance, 24, pii 1900161. 10.2807/1560-7917.es.2019.24.13.1900161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcheva MD, Birdanova VA and Alexandrova ML, 2017. Human brucellosis – new public health problem in Bulgaria. International Journal of Infectious Diseases and Therapy, 2, 66–71. 10.11648/j.ijidt. 20170204.11 [DOI] [Google Scholar]
- Kern P, Bardonnet K, Renner E, Auer H, Pawlowski Z, Ammann RW, Vuitton DA and Kern P, 2003. European echinococcosis registry: human alveolar echinococcosis, Europe, 1982–2000. Emerging Infectious Diseases, 9, 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P, Menezes da Silva A, Akhan O, Müllhaupt B, Vizcaychipi KA, Budke C and Vuitton DA, 2017. The echinococcoses: diagnosis, clinical management and burden of disease. Advances in Parasitology, 96, 259–369. 10.1016/bs.apar.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Mailles A, Garin‐Bastuji B, Lavigne JP, Jay M, Sotto A, Maurin M, Pelloux I, O'Callaghan D, Mick V, Vaillant V and De Valk H, 2016. Human brucellosis in France in the 21st century: results from national surveillance 20042013. Medecine et Maladies Infectieuses, 46, 411–418. 10.1016/j.medmal.2016.08.007 [DOI] [PubMed] [Google Scholar]
- Mannelli A, Martello E, Tomassone L, Calzolari M, Casalone C, De Meneghi D, Dottori M, Estrada‐Peña A, Fabbi M, Ferreri L, Ferroglio E, Luini M, Nicolau Solano S, Ortega C, Pautasso A, Prati P and Vesco U, 2012. Inventory of available data and data sources and proposal for data collection on vector‐borne zoonoses in animals. EFSA supporting publication 2012;EN‐234, 189 pp. Available online: http://www.efsa.europa.eu/en/supporting/doc/234e.pdf [Google Scholar]
- Marquer P, Rabade T and Forti R, 2014. Pig farming in the European Union: considerable variations from one Member State to another. Statistics in Focus 15/2014. ISSN: 2314‐9647.
- Maurin M and Gyuranecz M, 2016. Tularaemia: clinical aspects in Europe. Lancet Infectious Diseases, 16, 113–124. [DOI] [PubMed] [Google Scholar]
- Mendes A, Gomes B, Sousa L, Moreira H, Rosa I, Marques S, Machado E, Cruz Alves G and Neto M, 2020. Brucellosis: a rapid risk assessment by a regional outbreak team and its coordinated response with the Directorate‐General for Food and Veterinary, North region of Portugal, 2019. Zoonoses Public Health, 67, 587–590. 10.1111/zph.12692 [DOI] [PubMed] [Google Scholar]
- Meyer A, Olias P, Schüpbach G, Henzi M, Barmettler T, Hentrich B, Gottstein B and Frey CF, 2020. Combined cross‐sectional and case‐control study on Echinococcus multilocularis infection in pigs in Switzerland. Veterinary Parasitology: X, 4, 100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy T, Szmolka A, Wilk T, Kiss J, Szabó M, Pászti J, Nagy B and Olasz F, 2020. Comparative genome analysis of hungarian and global strains of Salmonella Infantis. Frontiers in Microbiology, 11, 539. 10.3389/fmicb.2020.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NMKL (Nordisk Metodikkomité for Næringsmidler – Nordic Committee on Food Analysis), 2005. NMKL No. 164, 2 Ed. 2005, Escherichia coli O157. Detection in food and feeding stuffs. Available online: http://www.nmkl.org/index.php?option=com_zoo&task=item&item_id=337&Itemid=319&lang=en [Google Scholar]
- Norman FF, Monge‐Maillo B, Chamorro‐Tojeiro S, Perez‐Molina J‐A and Lopez‐Velez R, 2016. Imported brucellosis: a case series and literature review. Travel Medicine and Infectious Disease, 14, 182–199. Available online: https://www.sciencedirect.com/science/article/pii/S1477893916300382?via%3Dihub [DOI] [PubMed] [Google Scholar]
- OIE (World Organisation for Animal Health), 2018. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Available online: https://www.oie.int/standard-setting/terrestrial-manual/ [Google Scholar]
- Oksanen A, Siles‐Lucas M, Karamon J, Possenti A, Conraths FJ, Romig T, Wysocki P, Mannocci A, Mipatrini D, La Torre G, Boufana B and Casulli A, 2016. The geographical distribution and prevalence of Echinococcus multilocularis in animals within the European Union and adjacent countries: a systematic review and meta‐analysis. Parasites & Vectors, 9, 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opsteegh M, Maas M, Schares G and van der Giessen J, 2016. Relationship between seroprevalence in the main livestock species and presence of Toxoplasma gondii in meat (GP/EFSA/BIOHAZ/2013/01). An extensive literature review. Final report. EFSA Supporting Publication 2016;EN‐996, 294 pp. Available online: http://online library.wiley.com/doi/10.2903/sp.efsa.2016.EN‐996/pdf [Google Scholar]
- Persad AK and LeJeune JT, 2014. Animal reservoirs of Shiga toxin‐producing Escherichia coli. Microbiology. Spectrum, 2, EHEC‐0027-2014. 10.1128/microbiolspec.EHEC-0027-2014 [DOI] [PubMed] [Google Scholar]
- Pozio E, 2014. Searching for Trichinella: not all pigs are created equal. Trends in Parasitology, 30, 4–11. [DOI] [PubMed] [Google Scholar]
- Pozio E, 2016. Trichinella pseudospiralis an elusive nematode. Veterinary Parasitology, 231, 97–101. [DOI] [PubMed] [Google Scholar]
- Pozio E, Rinaldi L, Marucci G, Musella V, Galati F, Cringoli G, Boireau P and La Rosa G, 2009. Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. International Journal for Parasitology, 39, 71–79. [DOI] [PubMed] [Google Scholar]
- Remfry SE, Amachawadi RG, Shi X, Bai J, Tokach MD, Dritz SS, Goodband RD, DeRouchey JM, Woodworth JC and Nagaraja TG, 2021. Shiga toxin‐producing Escherichia coli in Feces of Finisher Pigs: isolation, identification, and public health implications of major and minor serogroups. Journal of food protection, 84, 169–180. [DOI] [PubMed] [Google Scholar]
- Richter J, Esmann L, Lindner AK, Trebesch I, Equihua‐Martinez G, Niebank M, Georgi S, Schürmann D, Pfäfflin F, Pasić M and Gertler M, 2019. Cystic echinococcosis in unaccompanied minor refugees from Afghanistan and the Middle East to Germany, July 2016 through June 2017. European Journal of Epidemiology, 34, 611–612. [DOI] [PubMed] [Google Scholar]
- Rossi P, Tamarozzi F, Galati F, Pozio E, Akhan O, Cretu CM, Vutova K, Siles‐Lucas M, Brunetti E, Casulli A and HERACLES extended network, 2016. The first meeting of the European Register of Cystic Echinococcosis (ERCE). Parasite Vectors, 9, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P, Tamarozzi F, Galati F, Akhan O, Cretu CM, Vutova K, Siles‐Lucas M, Brunetti E, Casulli A and ERCE network, 2020. The European Register of Cystic Echinococcosis, ERCE: state‐of‐the‐art five years after its launch. Parasitology Vectors, 13, 236. 10.1186/s13071-020-04101-6. PMID: 32381109; PMCID: PMC7206799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzic N, Desmier L, Cariou M‐E, Gay E, Foster JT, Williamson CHD, Schmitt F, Le Henaff M, Le Coz A, Lorléac'h A, Lavigne J‐P, O'Callaghan D and Keriel A, 2020. First Case of Brucellosis Caused by an Amphibian‐type Brucella . Clinical Infectious Diseases, ciaa1082. 10.1093/cid/ciaa1082 [DOI] [PubMed] [Google Scholar]
- Schneeberger PM, Wintenberger C, Van der Hoek W and Stahl JP, 2014. Q fever in the Netherlands‐2007–2010: what we learned from the largest outbreak ever. Medecine et Maladies Infectieuses, 44, 339–353. [DOI] [PubMed] [Google Scholar]
- Siles‐Lucas M, Casulli A, Conraths FJ and Müller N, 2017. Laboratory diagnosis of Echinococcus spp. in human patients and infected animals. Advances in Parasitology, 96, 159–257. [DOI] [PubMed] [Google Scholar]
- Széll Z, Marucci G, Pozio E and Sréter T, 2013. Echinococcus multilocularis and Trichinella spiralis in golden jackals (Canis aureus) of Hungary. Veterinary Parasitology, 197, 393–396. [DOI] [PubMed] [Google Scholar]
- Tamarozzi F, Akhan O, Cretu CM, Vutova K, Akinci D, Chipeva R, Ciftci T, Constantin CM, Fabiani M, Golemanov B, Janta D, Mihailescu P, Muhtarov M, Orsten S, Petrutescu M, Pezzotti P, Popa AC, Popa LG, Popa MI, Velev V, Siles‐Lucas M, Brunetti E and Casulli A, 2018. Prevalence of abdominal cystic echinococcosis in rural Bulgaria, Romania, and Turkey: a cross‐sectional, ultrasound‐based, population study from the HERACLES project. Lancet Infectious Diseases, 18, 769–778. [DOI] [PubMed] [Google Scholar]
- Teunis PF, Falkenhorst G, Ang CW, Strid MA, De Valk H, Sadkowska‐Todys M, Zota L, Kuusi M, Rota MC, Simonsen JB, Mølbak K, Van Duynhoven YT and Van Pelt W, 2013. Campylobacter seroconversion rates in selected countries in the European Union. Epidemiology and Infection, 141, 2051–2057. 10.1017/S0950268812002774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Esschert KL, Mattioli MC, Hilborn ED, Roberts VA, Yu AT, Lamba K, Arzaga G, Zahn M, Marsh Z, Combes SM, Smith ES, Robinson TJ, Gretsch SR, Laco JP, Wikswo ME, Miller AD, Tack DM, Wade TJ and Hlavsa MC, 2020. Outbreaks associated with untreated recreational water – California, Maine, and Minnesota, 2018–2019. MMWR Morbidity and Mortalilty Weekly Report, 69, 781–783. 10.15585/mmwr.mm6925a3. PMID: 32584799; PMCID: PMC7316318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose DJ, 1998. The application of quantitative risk assessment to microbial food safety. Journal of Food Protection, 61, 640–648. 10.4315/0362-028X-61.5.640 [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2007. Epidemic and pandemic alert and response, 2007. Guidelines on tularaemia. Available online: https://www.cdc.gov/tularemia/resources/whotularemiamanual.pdf


