Abstract
Objective
The objective of this study was to determine the proportion of extended spectrum β-lactamase producing gram-negative bacteria (ESBL-GNB) colonizing patients admitted at Mazimbu hospital and Morogoro Regional hospital, in Morogoro, Tanzania. Rectal colonization with ESBL-GNB increases the risks of developing bacterial infections by extra-intestinal pathogenic ESBL-GNB.
Results
Of the 285 patients investigated, 123 (43.2%) carried ESBL-GNB in their intestines. Five of the 123 ESBL positive patients were colonized with two different bacteria, making a total of 128 ESBL producing isolates. Escherichia coli (n = 95, 74.2%) formed the majority of ESBL isolates. The proportion of CTX-M-1 group genes among ESBL isolates tested was 94.9% (93/98). History of antibiotic use (OR: 1.83, 95% CI: 1.1–3.2, P = 0.03), being on antibiotic treatment (OR: 2.61, 95% CI: 1.5–4.53, P = 0.001), duration of hospital stay (OR: 1.2, 95% CI: 1.1–1.3, P < 0.001) and history of previous admission (OR: 2.24, 95% CI: 1.2–4.1, P = 0.009) independently predicted ESBL-GNB carriage.
Keywords: Antimicrobial stewardship, ESBL colonization, ESBL genes, Infection prevention and control
Introduction
Extended spectrum beta-lactamases (ESBLs) production, is the commonest mechanism of resistance to multiple broad-spectrum beta-lactams among gram-negative bacteria mainly members of the family Enterobacteriaceae [1, 2]. ESBL enzymes hydrolyze beta-lactam ring of the beta-lactams making these antibiotics ineffective against ESBL producing bacteria [3]. The blaCTX-M group out of other ESBL groups, is the commonest reported group of ESBL genes in different part of the World including in Tanzania [2, 4–7]. CTX-M enzymes effectively hydrolyzes third generation cephalosporins (3GCs) e.g., ceftriaxone and cefotaxime but not oxyimino-cephalosporins e.g., ceftazidime [8]. Although, some CTX-M members; CTX-M-15, -16 and -19 have been reported to hydrolyze ceftazidime activity [9–11].
Colonization with ESBL producing gram-negative bacteria (ESBL-GNB) increases the risk of developing multidrug resistant (MDR) bacterial infections e.g., bloodstream infection, urinary tract infection or wound infection [12]. Infections with MDR bacteria are associated with increased days of hospitalization, healthcare costs and mortalities from treatment failure and/or limited therapeutic options [13].
In Tanzania, previous studies from national and zonal referral hospitals have reported magnitudes of rectal/intestinal carriage of ESBL producing gram-negative bacteria (ESBL-GNB) ranging from 15% to 59.7% among hospitalized patients [14–17]. ESBL producing E. coli (ESBL-EC) and ESBL producing K. pneumoniae (ESBL-KP) are frequently reported with proportion ranging from 30% to 68.7% and 28.2% to 77.1%, respectively [14, 15, 17]. The magnitude of ESBL rectal colonization and associated factors among hospitalized patients in other tiers of the healthcare facilities like regional and district hospitals has not been well studied in developing countries including Tanzania. The objectives of this study was to determine the magnitude and factors associated with rectal colonization with ESBL producing gram-negative bacteria (ESBL-GNB) among hospitalized patients at Mazimbu hospital and Morogoro Regional hospital in Morogoro, Tanzania. Therefore, this study’s findings provide baseline information to improve measures of infections prevention and control (IPC).
Main text
Methods
Study design, population, duration and settings
This cross-sectional analytical study was conducted among patients admitted at Mazimbu hospital (~ 30 beds capacity) and Morogoro Regional hospital (~ 450 beds capacity) in Morogoro region, Tanzania between May and July 2017. A minimum sample size of 280 was obtained using Kish and Leslie formula (1965) and a prevalence of 24% [5]. Participants stayed ≥ 24 h in hospital wards were eligible to be enrolled in this study. A standardized data collection tool was used to collect socio-demographic and clinical associated data relevant to study’s objectives.
Sample collections and laboratory procedures
Sterile swabs (Mast Diagnostica GmbH, Germany) in Amies transport media were used to collect a single time rectal swab from participants. Then, transported to Microbiology laboratory at Morogoro Regional hospital within 4 h of collection for laboratory analysis. Screening of presumptive ESBL-GNB was done by direct inoculation of rectal swab samples on MacConkey agar (MCA; Oxoid, UK) plates supplemented with 2 µg/ml cefotaxime (MCA-C) incubated in ambient air at 37 °C for 24 h [18, 19]. CHROMagar ESBL plates (BD BBL™ CHROMagar™ ESBL, Germany) were used for primary identification while physiological and biochemical characteristics (lactose fermentation; production of CO2, H2S, indole, urease and oxidase; motility; and utilization of citrate) were used for secondary identification of isolates to species level as reported [20]. Discs combination method (ceftazidime 30 µg and cefotaxime 30 µg with and without clavulanic acid 10 µg) was used for phenotypic confirmation of ESBL production in E. coli, K. pneumoniae and K. oxytoca as recommended by Clinical and Laboratory Standards Institutes (CLSI) [21]. All isolates were archived in vials containing 20% glycerol in brain heart infusion (BHI; Oxoid, UK) broth and stored at − 40 °C untill molecular analysis.
Determination of minimum inhibitory concentration (MIC) of cefotaxime
The minimum inhibitory concentrations (MICs) of ESBL-GNB to cefotaxime were determined using agar incorporation method [22, 23] on Mueller Hinton agar (MHA; Oxoid, UK) plates supplemented with 4 µg/mL, 8 µg/mL and 16 µg/mL cefotaxime. Inoculated plates were incubated in ambient air at 37 °C for 18–24 h. The MICs were recorded as greater than the highest concentration tested or the lowest concentration when no growth occurs on any of the agar plates.
DNA extraction and molecular detection of blaCTX-M-1 group
Out of 128 isolates, 98 ESBL-GNB (73 E. coli, 18 K. pneumoniae and 7 K. oxytoca) were selected and successful recovered for molecular characterization of the blaCTX-M-1 group. Selection of isolates for molecular characterization was limited by the availability of PCR reagents. The isolates were sub-cultured on plain MCA (Oxoid, UK) plates followed by crude DNA extraction using boiling method as previously described [24]. Out of five major phylogenetic groups of CTX-M genes (CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, and CTX-M-25), we chose to test only for CTX-M-1 due to predominance of its members especially the blaCTX-M-15 and from insufficient resources. PCR amplifications of the blaCTX-M-1 group was carried out in thermal cycler machine (PCR Gene AmpR System) with primers CTX-M3G-F (5′-GTTACAATGTGTGAGAAGCAG) and CTX-M3G-R (5′-CCGTTTCCGCTATTACAAAC) and procedures reported previous [25]. PCR products were electrophoresed (at 110 V for 90 min) by using 2% agarose gel which was stained by SBR-Safe DNA gel stain (ThermoFisher Scientific, UK) and visualized under UV light.
Quality control
Known ESBL-GNB from [26] and E. coli ATCC 25922 were used as control organisms.
Data analysis
STATA software version 13.0 was used for data analysis as per objectives of this study.
Results
Socio-demographic and clinical characteristics of study participants
A total of 285 patients with median age (IQR: interquartile range) of 18 (3–34) years were enrolled with the majority (53%, n = 151) being males (Table 1).
Table 1.
Socio-demographic and clinical characteristics of study participants
| Variables | Frequency (n)/median (IQR) | Percentage (%) | |
|---|---|---|---|
| Median age (IQR) in years | 18 (3–34) | – | |
| Median days (IQR) of hospital stay | 1 (1–3) | – | |
| Median days (IQR) of antibiotic exposure | 2 (1–3) | – | |
| Gender | |||
| Males | 151 | 53 | |
| Females | 134 | 47 | |
| Hospital of admission | |||
| MH | 29 | 10.2 | |
| MRH | 256 | 89.8 | |
| Admitted ward during enrollment | |||
| Medical | 205 | 71.9 | |
| Surgical | 80 | 28.1 | |
| Antibiotics use past three months | |||
| No | 148 | 51.9 | |
| Yes | 137 | 48.1 | |
| Antibiotics use at enrollment | |||
| No | 107 | 37.5 | |
| Yes | 178 | 62.5 | |
| On β-lactams during sampling | |||
| No | 12 | 6.7 | |
| Yes | 166 | 93.3 | |
| Type of antibiotics used during sampling | |||
| Ciprofloxacin/gentamicin | 12 | 6.7 | |
| Penicillins | 95 | 53.4 | |
| Cephalosporins | 71 | 39.9 | |
| History of admission | |||
| No | 203 | 71.2 | |
| Yes | 82 | 28.2 | |
| Livestock keeping | |||
| No | 253 | 88.8 | |
| Yes | 32 | 11.2 | |
| HIV status | |||
| Negative | 283 | 99.3 | |
| Positive | 2 | 0.7 | |
MH Mazimbu Hospital, MRH Morogoro Regional Hospital
Carriage of ESBL-GNB, MICs, and harboring of blaCTX-M-1 group in ESBL-GNB
Of the 285 patients investigated, 123 (43.2%) were colonized by ESBL-GNB whereby five patients had two ESBL-GNB isolated from single rectal swab making a total of 128 isolates (Fig. 1). Of 128 ESBL confirmed isolates, 123 (96.1%) had a MIC of ≥ 16 µg/mL while the remaining 5 isolates had a MIC of ≥ 4 µg/mL. Ninety-nine ESBL-GNB tested, 93 (94.9%) carried blaCTX-M-1 group genes while the remaining five (all were Escherichia coli) had no blaCTX-M-1 group genes.
Fig. 1.
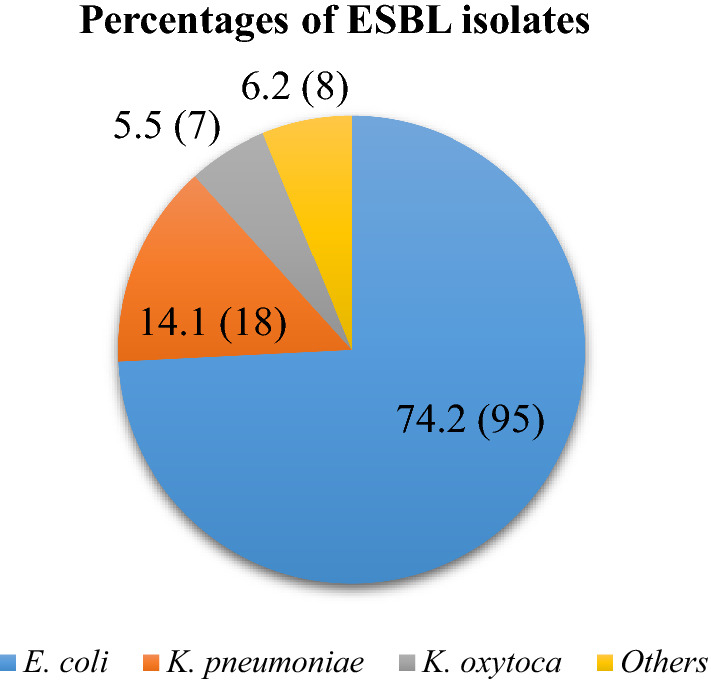
Genus and species of ESBL-GNB colonizing patients admitted at Mazimbu hospital and Morogoro regional hospital (Other isolates: C. freundii (n = 2), S. marcescens (n = 2), Shigella spp (n = 2), Providencia spp (n = 2) and Acinetobacter spp (n = 2))
Factors associated with rectal colonization by ESBL-GNB
On multivariable logistic regression analysis controlled by age and sex: history of antibiotic use (OR: 1.83, 95% CI: 1.1–3.2, P = 0.03), being on antibiotic treatment (OR: 2.61, 95% CI: 1.5–4.53, P = 0.001), duration of hospital stay (OR: 1.2, 95% CI: 1.1–1.3, P < 0.001) and history of previous hospital admission (OR: 2.24, 95% CI: 1.2–4.1, P = 0.009) were independently found to predict ESBL-PE GNB carriage (Table 2).
Table 2.
Factors associated with ESBL-GNB colonization
| Variable | All participants (N = 285) | ESBL-GNB positive colonization N = 123 (%) |
Univariable (P value) | Multivariable OR (95%CI) |
P value | |
|---|---|---|---|---|---|---|
| Median age (IQR) in years | 19 (IQR: 3–33) | 17 (IQR: 3–33) | 0.929 | 0.99 [0.99–1.01] | 1.000 | |
| Median (IQR) days in hospital | 1 (IQR: 1–2) | 2 (IQR: 1–4) | < 0.001 | 1.20 [1.08–1.33] | < 0.001 | |
| Antibiotic use past 3 months | ||||||
| No | 148 | 49 (33.1) | < 0.001 |
1 2.16 [1.22–3.84] |
0.009 | |
| Yes | 137 | 74 (54.0) | ||||
| Gender | ||||||
| Females | 134 | 51 (38.1) | 0.102 |
1 0.81 [0.47–1.39] |
0.443 | |
| Males | 151 | 72 (47.7) | ||||
| Type of ward of admission | ||||||
| Medical | 205 | 91 (44.4) | 0.502 |
1 1.24 [0.64–2.39] |
0.526 | |
| Surgical | 80 | 32 (40.0) | ||||
| On antibiotic use during sampling | ||||||
| No | 107 | 28 (26.2) | < 0.001 |
1 2.20 [1.23–3.95] |
0.008 | |
| Yes | 178 | 95 (53.4) | ||||
| Hospital admission past 3 months | ||||||
| No | 203 | 70 (34.5) | < 0.001 |
1 2.17 [1.17–4.01] |
0.013 | |
| Yes | 82 | 53 (64.6) | ||||
| Livestock keeping | ||||||
| No | 253 | 112 (44.2) | 0.287 |
1 0.51 [0.22–1.19] |
0.122 | |
| Yes | 32 | 11 (34.4) | ||||
Discussion
This study identified a high carriage of ESBL-GNB in Morogoro regional hospital and Mazimbu hospital. The overall prevalence (43.2%) observed in this study is comparable to a study in Gabon [27]. Although the carriage in our study is relatively lower compared to (50.4%) a study conducted at Tanzanian National Hospital, Muhimbili National hospital (MNH), in Dar es Salaam [28]. Being a national referral hospital, MNH receives patients with multiple antibiotics exposure from other healthcare facilities mainly regional and zonal referral hospitals, increasing the risk of carriage of ESBL-GNB.
E. coli followed by K. pneumoniae are predominant ESBL producers colonizing patients. Similar findings were reported previous in Ethiopia, Turkey and other regions of Tanzania [15, 16, 29, 30]. Pathogenic potential of E. coli (e.g., E. coli ST131) and K. pneumoniae (e.g., K. pneumoniae ST14), and frequent acquisition of conjugative plasmids encoding for antimicrobial resistance genes (ARGs i.e., ESBL genes) facilitates rapid exchange and dissemination of ARGs in E. coli and K. pneumoniae [31]. These isolates, ESBL-GNB, colonizing patients are potentially shaded of to contaminate patient’s immediate inanimate surroundings as previous reported [32, 33]. Thus increasing the risk of exogenous source of acquiring of healthcare associated infections (HCAIs) from ESBL-GNB among vulnerable patients (immunocompromised and critically ill) associated with increased mortality from treatment failures and limited antibiotic therapeutic options [34, 35]. Therefore, this study’s findings alert for the strengthening of infections prevention and control measures and AMR surveillance in line with the Tanzania National Action Plan in order to combat AMR in the country in all tiers of health facilities [36].
This study found high proportion (94.9%) of ESBL-GNB carrying CTX-M-1 group genes colonizing patients. The CTX-M-1 group genes particularly blaCTX-M-15 are predominantly reported in clinical, colonization and environment isolates in Tanzania and elsewhere [6–8, 15, 16, 37]. Horizontal gene transfer (HGT) of mobile genetic elements (MGEs) including plasmids, transposons, and intergrons facilitates rapid dissemination and spreading of CTX-M-1 group genes, mostly in E. coli and Klebsiella spp., [8, 38–40] in the hospital environment. These findings hint the possibility of the common genetic elements or resistant strains carrying CTX-M-1 genes in healthcare facilities in Tanzania, necessitating the strengthening of IPC and antimicrobial stewardship in Tanzania.
Hospital admission, previous and current antibiotic use, and longer hospital stay significantly predicted carriage of ESBL-GNB. These findings are in consistency with other studies [5, 16, 27]. Hospital admission and longer stays increases the odds of being exposed to antibiotics mostly beta-lactams i.e., ampicillin and ceftriaxone as they make first- and second-lines of therapy [41]. Therefore, increasing antimicrobial selection pressure favoring the proliferation of resistant bacterial strains colonizing patients’ gastro-intestinal tracts as observed in this study [42, 43].
Antibiotic exposure creates essential pressure which select the small fraction of resistant bacteria of the intestinal microbiota therefore giving rise to the emergency and establishment of an entirely resistant population of bacteria [42]. With poor IPC practices especially in low- and middle-income countries, these superbugs may be cross-transmitted between patients resulting to subsequent invasive infections such as BSIs, UTIs and SSTIs. Presence of these bacteria in the gut and environment may also result in exchange of resistance genes to the highly virulent bacteria making the infection difficult to treat hence high morbidity and mortality [42].
Limitations
From limited funds and resources: ESBL isolates were conventionally identified to possible genus and species; agar dilution method was used to determine MICs for cefotaxime only; and other ESBL alleles contributing about 5–10% of ESBL genes in our setting and genetic relatedness of ESBL isolates were not determined.
Acknowledgements
The authors acknowledge the assistance provided by the administration and health care workers of Morogoro regional hospital and Mazimbu District hospital.
Abbreviations
- ATCC
American Type Culture Collection
- BSIs
Bloodstream infections
- CI
Confidence interval
- DNA
Deoxyribose nucleic acid
- ESBL
Extended spectrum beta lactamase
- ESBL-EC
Extended spectrum beta lactamase producing Escherichia coli
- ESBL-GNB
Extended spectrum beta lactamase producing gram negative bacteria
- ESBL-KP
Extended spectrum beta lactamase producing Klebsiella pneumonia
- GNB
Gram negative bacteria
- HIV
Human immunodeficiency virus
- IPC
Infection prevention and control
- IQR
Interquartile range
- MCA
MacConkey agar
- MCA-C
MacConkey agar supplemented with cefotaxime 2 µg/mL
- MIC
Minimum inhibitory concentration
- MRRH
Morogoro Regional Referral Hospital
- OR
Odd ratio
- PCR
Polymerase chain reaction
- SSTIs
Skin and soft tissue infections
- UTI
Urinary tract infections
Authors’ contributions
NM, VS, MMM and SEM conceived and designed this study; EM, NM, and VS collected data and samples for this study; NM, VS, JS, EM, AC, MFM and LM performed laboratory procedures; NM, VS, and SEM analyzed and interpreted data; NM and VS wrote the first draft of the manuscript which was critically reviewed by JS and SEM. All authors read and approved the final manuscript.
Funding
The research was supported by grant from World Health Organization AGISAR Pilot Projects to SEM. Funder had no any role in conducting this study.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the department of Microbiology and Immunology repository of the Catholic University of Health and Allied Sciences-Bugando. The data can be obtained upon request to the Director of Research and Innovation of the Catholic University of Health and Allied Sciences.
Ethics approval and consent to participate
The protocols for the study were reviewed and approved by the Joint CUHAS/BMC ethics and scientific review committee (CREC/019/2014). Permissions were sought from administration of the Morogoro regional hospital and Mazimbu hospital. All participants aged above 18 years signed an informed written consent forms whereas for participants aged below 18 years their parents/caretakers consented on their behalf.
Consent for publication
Not applicable.
Competing interests
Authors declare no competing interests exist.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Teklu DS, Negeri AA, Legese MH, Bedada TL, Woldemariam HK, Tullu KD. Extended-spectrum beta-lactamase production and multi-drug resistance among Enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrob Resist Infect Contr. 2019;8(1):39. doi: 10.1186/s13756-019-0488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaikh S, Fatima J, Shakil S, Rizvi SMD, Kamal MA. Antibiotic resistance and extended spectrum beta-lactamases: types, epidemiology and treatment. Saudi J Biol Sci. 2015;22(1):90–101. doi: 10.1016/j.sjbs.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Bambeke F, Mingeot-Leclercq M-P, Glupczynski Y, Tulkens PM. Mechanisms of action. Infect Dis. 2017;2:1162–1180. doi: 10.1016/B978-0-7020-6285-8.00137-4. [DOI] [Google Scholar]
- 4.Moremi N, Claus H, Vogel U, Mshana SE. Faecal carriage of CTX-M extended-spectrum beta-lactamase-producing Enterobacteriaceae among street children dwelling in Mwanza city, Tanzania. PLoS ONE. 2017;12(9):e0184592. doi: 10.1371/journal.pone.0184592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moremi N, Claus H, Rutta L, Frosch M, Vogel U, Mshana S. High carriage rate of extended-spectrum beta-lactamase-producing Enterobacteriaceae among patients admitted for surgery in Tanzanian hospitals with a low rate of endogenous surgical site infections. J Hosp Infect. 2018;100(1):47–53. doi: 10.1016/j.jhin.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Moremi N, Manda EV, Falgenhauer L, Ghosh H, Imirzalioglu C, Matee M, Chakraborty T, Mshana SE. Predominance of CTX-M-15 among ESBL producers from environment and fish gut from the shores of Lake Victoria in Mwanza, Tanzania. Front Microbiol. 1862;2016:7. doi: 10.3389/fmicb.2016.01862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mshana SE, Falgenhauer L, Mirambo MM, Mushi MF, Moremi N, Julius R, Seni J, Imirzalioglu C, Matee M, Chakraborty T. Predictors of bla CTX-M-15 in varieties of Escherichia coli genotypes from humans in community settings in Mwanza, Tanzania. BMC Infect Dis. 2016;16(1):187. doi: 10.1186/s12879-016-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao W-H, Hu Z-Q. Epidemiology and genetics of CTX-M extended-spectrum β-lactamases in gram-negative bacteria. Crit Rev Microbiol. 2013;39(1):79–101. doi: 10.3109/1040841X.2012.691460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poirel L, Gniadkowski M, Nordmann P. Biochemical analysis of the ceftazidime-hydrolysing extended-spectrum β-lactamase CTX-M-15 and of its structurally related β-lactamase CTX-M-3. J Antimicrob Chemother. 2002;50(6):1031–1034. doi: 10.1093/jac/dkf240. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet R, Dutour C, Sampaio J, Chanal C, Sirot D, Labia R, De Champs C, Sirot J. Novel cefotaximase (CTX-M-16) with increased catalytic efficiency due to substitution Asp-240→ Gly. Antimicrob Agents Chemother. 2001;45(8):2269–2275. doi: 10.1128/AAC.45.8.2269-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poirel L, Naas T, Le Thomas I, Karim A, Bingen E, Nordmann P. CTX-M-type extended-spectrum β-lactamase that hydrolyzes ceftazidime through a single amino acid substitution in the omega loop. Antimicrob Agents Chemother. 2001;45(12):3355–3361. doi: 10.1128/AAC.45.12.3355-3361.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheikh A, Belefquih B, Chajai Y, Cheikhaoui Y, El Hassani A, Benouda A. Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs) colonization as a risk factor for developing ESBL infections in pediatric cardiac surgery patients: “retrospective cohort study”. BMC Infect Dis. 2017;17(1):1–6. doi: 10.1186/s12879-017-2346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters L, Olson L, Khu DT, Linnros S, Le NK, Hanberger H, Hoang NT, Tran DM, Larsson M. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: a cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. PLoS ONE. 2019;14(5):e0215666. doi: 10.1371/journal.pone.0215666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson E, Kayega J, Seni J, Mushi MF, Kidenya BR, Hokororo A, Zuechner A, Kihunrwa A, Mshana SE. Evaluation of existence and transmission of extended spectrum beta lactamase producing bacteria from post-delivery women to neonates at Bugando Medical Center, Mwanza-Tanzania. BMC Res Notes. 2014;7(1):279. doi: 10.1186/1756-0500-7-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marando R, Seni J, Mirambo MM, Falgenhauer L, Moremi N, Mushi MF, Kayange N, Manyama F, Imirzalioglu C, Chakraborty T. Predictors of the extended-spectrum-beta lactamases producing Enterobacteriaceae neonatal sepsis at a tertiary hospital, Tanzania. Int J Med Microbiol. 2018;308(7):803–811. doi: 10.1016/j.ijmm.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tellevik MG, Blomberg B, Kommedal Ø, Maselle SY, Langeland N, Moyo SJ. High prevalence of faecal carriage of ESBL-producing Enterobacteriaceae among children in Dar es Salaam, Tanzania. PLoS ONE. 2016;11(12):e0168064. doi: 10.1371/journal.pone.0168024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kibwana UO, Magijo M, Kamori D, Manyahi J. High fecal carriage of extended beta lactamase producing Enterobacteriaceae among adult patients admitted in Referral Hospitals in Dar es salaam, Tanzania. BMC Infect Dis. 2019;20:557. doi: 10.1186/s12879-020-05272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyambura Moremi HC, Vogel U, Mshana SE. Faecal carriage of CTX-M extended-spectrum beta-lactamase-producing Enterobacteriaceae among street children dwelling in Mwanza city, Tanzania. PLoS ONE. 2017;12(9):e0184592. doi: 10.1371/journal.pone.0184592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao SP, Rama PS, Gurushanthappa V, Manipura R, Srinivasan K. Extended-spectrum beta-lactamases producing Escherichia coli and Klebsiella pneumoniaei: a multi-centric study across Karnataka. J Lab Phys. 2014;6(01):007–013. doi: 10.4103/0974-2727.129083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koneman EW, Allen SD, Janda W, Schreckenberger P, Winn W. Diagnostic microbiology. The nonfermentative gram-negative bacilli. Philedelphia: Lippincott-Raven Publishers; 1997. pp. 253–320. [Google Scholar]
- 21.CLSI C. Performance standards for antimicrobial susceptibility testing. Clinical Lab Standards Institute 2016.
- 22.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(Suppl_1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 23.Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 24.Dashti AA, Jadaon MM, Abdulsamad AM, Dashti HM. Heat treatment of bacteria: a simple method of DNA extraction for molecular techniques. Kuwait Med J. 2009;41(2):117–122. [Google Scholar]
- 25.Pagani L, Dell'Amico E, Migliavacca R, D'Andrea MM, Giacobone E, Amicosante G, Romero E, Rossolini GM. Multiple CTX-M-type extended-spectrum β-lactamases in nosocomial isolates of Enterobacteriaceae from a hospital in northern Italy. J Clin Microbiol. 2003;41(9):4264–4269. doi: 10.1128/JCM.41.9.4264-4269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mshana SE, Falgenhauer L, Mirambo MM, Mushi MF, Moremi N, Julius R, Seni J, Imirzalioglu C, Matee M, Chakraborty T. Predictors of bl a CTX-M-15 in varieties of Escherichia coli genotypes from humans in community settings in Mwanza, Tanzania. BMC Infect Dis. 2016;16(1):187. doi: 10.1186/s12879-016-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaumburg F, Alabi A, Kokou C, Grobusch MP, Köck R, Kaba H, Becker K, Adegnika AA, Kremsner PG, Peters G. High burden of extended-spectrum β-lactamase-producing Enterobacteriaceae in Gabon. J Antimicrob Chemother. 2013;68(9):2140–2143. doi: 10.1093/jac/dkt164. [DOI] [PubMed] [Google Scholar]
- 28.Tellevik MG, Blomberg B, Kommedal Ø, Maselle SY, Langeland N, Moyo SJ. High prevalence of faecal carriage of ESBL-producing Enterobacteriaceae among children in Dar es Salaam, Tanzania. PLoS ONE. 2016;11(12):e0168024. doi: 10.1371/journal.pone.0168024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desta K, Woldeamanuel Y, Azazh A, Mohammod H, Desalegn D, Shimelis D, Gulilat D, Lamisso B, Makonnen E, Worku A: High gastrointestinal colonization rate with extended-Spectrum β-lactamase-producing Enterobacteriaceae in hospitalized patients: emergence of Carbapenemase-Producing K. pneumoniae in Ethiopia. PloS one 2016, 11(8). [DOI] [PMC free article] [PubMed]
- 30.ERDOĞAN DÇ, Cömert F, SEPETCİ EA, Köktürk F, Külah C: Fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp. in a Turkish community. Turkish journal of medical sciences 2017, 47(1):172–179. [DOI] [PubMed]
- 31.Isendahl J, Turlej-Rogacka A, Manjuba C, Rodrigues A, Giske CG, Naucler P. Fecal carriage of ESBL-producing E. coli and K. pneumoniae in children in Guinea-Bissau: a hospital-based cross-sectional study. PLoS ONE. 2012;7(12):e58981. doi: 10.1371/journal.pone.0051981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moremi N, Claus H, Silago V, Kabage P, Abednego R, Matee M, Vogel U, Mshana S. Hospital surface contamination with antimicrobial-resistant gram-negative organisms in Tanzanian regional and tertiary hospitals: the need to improve environmental cleaning. J Hosp Infect. 2019;102(1):98–100. doi: 10.1016/j.jhin.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Silago V, Kovacs D, Msanga DR, Seni J, Matthews L, Oravcová K, Zadoks RN, Lupindu AM, Hoza AS, Mshana SE. Bacteremia in critical care units at Bugando Medical Centre, Mwanza, Tanzania: the role of colonization and contaminated cots and mothers’ hands in cross-transmission of multidrug resistant gram-negative bacteria. Antimicrob Resist Infect Contr. 2020;9:1–14. doi: 10.1186/s13756-019-0662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Organization WH. Water, sanitation and hygiene in health care facilities: status in low and middle income countries and way forward. 2015.
- 35.Bouzid M, Cumming O, Hunter PR. What is the impact of water sanitation and hygiene in healthcare facilities on care seeking behaviour and patient satisfaction? A systematic review of the evidence from low-income and middle-income countries. BMJ Glob Health. 2018;3(3):e000648. doi: 10.1136/bmjgh-2017-000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.URT. The National Action Plan on Antimicrobial Resistance (2017–2022). In. The United Republic of Tanzania (URT): The Ministry of Health Community Development Gender Elderly and Children (MHCDGEC), Ministry of Agriculture, Livestock and Fisheries (MALF); 2017.
- 37.Mugnaioli C, Luzzaro F, De Luca F, Brigante G, Perilli M, Amicosante G, Stefani S, Toniolo A, Rossolini GM. CTX-M-type extended-spectrum β-lactamases in Italy: molecular epidemiology of an emerging countrywide problem. Antimicrob Agents Chemother. 2006;50(8):2700–2706. doi: 10.1128/AAC.00068-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peerayeh SN, Eslami M, Memariani M, Siadat SD. High prevalence of blaCTX-M-1 group extended-spectrum β-lactamase genes in Escherichia coli isolates from Tehran. Jundishapur J Microbiol 2013;6(7).
- 39.Rossolini G, Dandrea M, Mugnaioli C. The spread of CTX-M-type extended-spectrum β-lactamases. Clin Microbiol Infect. 2008;14:33–41. doi: 10.1111/j.1469-0691.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 40.Eckert C, Gautier V, Arlet G. DNA sequence analysis of the genetic environment of various bla CTX-M genes. J Antimicrob Chemother. 2006;57(1):14–23. doi: 10.1093/jac/dki398. [DOI] [PubMed] [Google Scholar]
- 41.Ministry of Health T. Standard Treatment Guidelines & National Essential Medicines List-Tanzania Mainland. 2017.
- 42.Karam G, Chastre J, Wilcox MH, Vincent J-L. Antibiotic strategies in the era of multidrug resistance. Crit Care. 2016;20(1):136. doi: 10.1186/s13054-016-1320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer E, Gastmeier P, Deja M, Schwab F. Antibiotic consumption and resistance: data from Europe and Germany. Int J Med Microbiol. 2013;303(6–7):388–395. doi: 10.1016/j.ijmm.2013.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the department of Microbiology and Immunology repository of the Catholic University of Health and Allied Sciences-Bugando. The data can be obtained upon request to the Director of Research and Innovation of the Catholic University of Health and Allied Sciences.


