Abstract
Simple Summary
Cysteine protease inhibitors (cystatins) are molecules that play key protective roles in protein degradation and are involved in the immunomodulation of host responses to parasites. Little is known about the cystatin gene repertoire, evolution, and lineage-specific adaptations of early-emerging metazoans. Using bioinformatics searches, we identified orthologues of cystatins in basal animal lineages including free-living and parasite taxa. We aimed to explore whether their cystatin gene repertoire and evolution follow similar patterns recognized for derived metazoans and whether the modifications are linked to the organism’s life history. We revealed that cysteine protease inhibitors from early-emerging animal groups are highly diverse, with modifications in gene organization and protein architecture. A new subtype of cystatins was discovered in the parasitic cnidarians, the Myxozoa, which has so far been only reported for a group of derived animals: trematode flukes. We set out hypotheses to describe the driving forces for the origins of this unique cystatin subtype and propose evolutionary scenarios elucidating the current existence of cystatins in the Metazoa, especially in their early-emerging lineages. Our research identified molecules for which future functional studies may help to identify their roles in host–parasite interactions and for the parasite itself.
Abstract
The evolutionary aspects of cystatins are greatly underexplored in early-emerging metazoans. Thus, we surveyed the gene organization, protein architecture, and phylogeny of cystatin homologues mined from 110 genomes and the transcriptomes of 58 basal metazoan species, encompassing free-living and parasite taxa of Porifera, Placozoa, Cnidaria (including Myxozoa), and Ctenophora. We found that the cystatin gene repertoire significantly differs among phyla, with stefins present in most of the investigated lineages but with type 2 cystatins missing in several basal metazoan groups. Similar to liver and intestinal flukes, myxozoan parasites possess atypical stefins with chimeric structure that combine motifs of classical stefins and type 2 cystatins. Other early metazoan taxa regardless of lifestyle have only the classical representation of cystatins and lack multi-domain ones. Our comprehensive phylogenetic analyses revealed that stefins and type 2 cystatins clustered into taxonomically defined clades with multiple independent paralogous groups, which probably arose due to gene duplications. The stefin clade split between the subclades of classical stefins and the atypical stefins of myxozoans and flukes. Atypical stefins represent key evolutionary innovations of the two parasite groups for which their origin might have been linked with ancestral gene chimerization, obligate parasitism, life cycle complexity, genome reduction, and host immunity.
Keywords: cysteine protease inhibitor, stefin, signal peptide, parasite, phylogenetic analysis, diversification, protein structure
1. Introduction
Members of the cystatin superfamily (I25), also known as cystatins, are major regulators of the activity of papain-like cysteine proteases and legumain-type proteases. These endogenous protease inhibitors are widely distributed in all domains of life. Based on sequence homology, the cystatin superfamily comprises three main sub-families: types 1 –3. Type 1 cystatins (stefins, I25A) are low-molecular-weight intracellular single-domain proteins generally lacking disulfide bonds and signal peptides. Within the stefins, an additional subtype, unusually featuring a signal peptide, is recognized exclusively in liver and intestinal flukes (Trematoda: Digenea) [1,2,3,4]. Type 2 cystatins (I25B) are mainly extracellular single-domain proteins containing two disulfide bonds and a signal peptide, while type 3 cystatins (kininogens, I25C) are large multi-domain proteins [5,6,7].
Cystatins are involved in the regulation of key physiological processes, such as the protection of cells from proteolysis [5,6,7]. In parasites, cystatins are involved in immunomodulation of host cells and represent important immunogenicity and pathogenicity factors involved in host–parasite interactions [8,9,10,11,12,13]. They facilitate parasite survival within the host [14,15] by inhibiting host proteases and by interfering with antigen processing and presentation [16,17,18,19]. The increased capacity of parasite cystatins to induce anti-inflammatory cytokine IL-10 [16,17,20] was suggested to be an adaptation to the parasitic lifestyle [21,22]. These molecules have been used as effective markers in diagnosis and vaccine development and as chemotherapeutic targets against various parasitic infections [23,24,25,26,27]. Recently, cystatins have also been suggested as candidates for immunotherapies for acute and chronic inflammatory diseases [28] and cancer [29].
The diversity and evolution of the cystatin superfamily is well-documented in prokaryotes and certain eukaryotic lineages [30,31,32,33]. Within the Metazoa, phylogenetic affinities and representation of cystatins have been investigated predominantly in the derived lineages (Bilateria) including both free-living [34] and parasite taxa, e.g., various helminth [2,10,35,36,37,38] and arthropod groups [39,40,41,42]. According to the current knowledge of cystatin evolution, the stefin and type 2 cystatin lineages were already present in the eukaryotic ancestor. Stefins have remained structurally similar to the ancestral form and are present across most metazoan groups. In contrast, type 2 cystatins greatly diversified, primarily in derived metazoans (Vertebrata), and have been lost entirely in some early (Placozoa) and derived (Acoela, Tardigrada, Hemichordata, etc.) metazoan groups. Multi-domain cystatins have been reported exclusively from several bilaterian groups [30]. Nevertheless, the picture of cystatin evolution in the Metazoa is incomplete, as early-emerging animal lineages (Porifera, Ctenophora, Placozoa, and Cnidaria) are greatly underexplored in this respect and completely underrepresented with regard to parasitic lineages. To date, only a few early-emerging animal taxa were included in the studies of cystatin evolution [30,34], from which none were parasites. Thus, further data are needed from these ancient animal lineages, including those with parasitic lifestyles, to improve our evolutionary understanding and to explore potential linkage to the organism’s life history.
Basal metazoans mostly comprise free-living species, but at least eight independent parasite lineages of early-emerging Metazoa are known, with all but one in Cnidaria [43]. Recently, next-generation sequencing (NGS) data have become available for three of these parasite lineages, i.e., two Edwardsiella spp. [44,45], Polypodium hydriforme [46,47], and several species of the large group Myxozoa [47,48,49,50,51,52,53,54]. While Edwardsiella and Polypodium parasitize their hosts exclusively during the larval stage of their development [45,55], the microscopic myxozoans are an entirely parasitic group with complex life cycles [56]. Members of the specious class Myxosporea are primarily known from fish (intermediate hosts) and annelids (definitive hosts). The more primitive Malacosporea use fish and freshwater bryozoans as hosts [57]. Myxozoans are an evolutionary old and fast-evolving group, evidenced by their highly derived morphology and seen in genome phylogenies [46,47,51,53,58]. Their genomes are the smallest amongst all animals due to evolutionary transition to parasitism and the associated extreme reduction of body complexity from free-living cnidarians [47].
To explore the gene repertoire, gene organization, and structural features of cystatins in phyla at the base of the metazoan tree, we mined 110 genomes and transcriptomes of 58 early-emerging animal species. We conducted phylogenetic analyses using newly obtained and published cystatin sequences of basal and derived metazoans to provide insights into the origin, evolution, and structural diversification of this group of inhibitors at the time when this superfamily emerged and expanded. We investigated the amino acid sequence structure and phylogenies of cystatins from free-living and parasite taxa to identify structural modifications, which potentially represent lineage-specific adaptations related to parasitic lifestyles. Our investigation thus aimed to reveal whether gene repertoire and evolution of cystatins in early-emerging animals follow similar patterns recognized for derived metazoans and whether the modifications are linked to the organism’s life history.
2. Materials and Methods
2.1. Data Mining
Representatives of basal metazoan lineages (Placozoa, Porifera, Ctenophora, and Cnidaria) were selected for mining of cystatin homologues from the NGS data available from GenBank, other public databases, or produced in our laboratories (110 datasets of 58 species; Table S1). The samples included free-living and parasite taxa, particularly Cnidaria, i.e., Polypodium hydriforme, Edwardsiella lineata, E. carnea, and all available myxozoans (13 species). We used published the cystatin homologues of metazoans from basal animal species [30,53] for the cystatin homology search. If no homologues were found in a species in the first blast, a repeated search was applied by utilizing the sequences of homologues found in related species in the first iteration as additional queries for the next search.
We used a combined search strategy using both motif- and sequence similarity-based methods to identify target molecules. The search was performed using the tBLASTn algorithm with the E-value cutoff set to 10−5. This relatively low threshold was selected based on low sequence similarity among our queries and subjects (approximately 30–40%). The mined sequences were examined for the presence of typical conserved motifs of the cystatin domain (G; Q-x-V-x-G; LP/PW; YF [30,59]), by searches against different databases (GenBank, MEROPS peptidase database [60]) including the reciprocal blast analysis, and by their positioning in our preliminary phylogenetic analyses. Detailed homology searches were performed using the hmmrscan algorithm of HmmerWeb version 2.32.0 software [61] against protein family databases: Pfam, CATH-Gene3D, PIRSF, Superfamily, TIGRFAM, and TreeFam. For parasites, the potential contaminating sequences of host or other origin were identified and filtered out by blast matches with reference NGS data and by phylogenetic clustering with contaminant species homologues.
2.2. Verification of Selected Cystatin Sequences
Full gene sequences of the cystatin superfamily homologues mined from unpublished genomes and transcriptomes (Table S1) were verified by PCR and by sequencing from DNA and/or cDNA templates using species-specific primers designed in this study, if possible, in the 5’and 3’UTR regions (details in Methods S1 and Table S2). PCR was used to obtain the stefin gene sequence of Buddenbrockia plumatellae (Methods S1 and Table S2) as mining of the published Buddenbrockia EST database (Table S1) did not return any cystatin hits. PCR-verified sequences were deposited in GenBank under the accession Nos. MT127416–MT127426 and MW498387–MW498390 (Table S2). The positions of the introns were assessed by comparison of the sequences of DNA vs. cDNA origin and, if possible, by comparing sequences mined from the genomic vs. transcriptomic data.
2.3. Phylogenetic Analyses
We compiled and analyzed a sequence dataset (128 taxa) of both newly identified and published cystatins. The dataset comprised Choanoflagellates (11 taxa), early-emerging animal lineages (58 taxa), more derived metazoans (58 taxa), and Giardia cystatin (outgroup) resembling the most ancestral eukaryotic cystatin [30] (Table S3). Ingroup taxa were represented by free-living species (72 taxa) and parasite species from basal metazoans (Polypodium hydriforme, Edwardsiella spp., and myxozoans; 16 taxa) and bilaterians (trematodes, cestodes, nematodes, monogeneans, ticks, and mites; 39 taxa) (Table S3). Multi-domain cystatins were omitted from the phylogenetic analyses due to difficulties with their alignment.
We aligned 329 amino acid cystatin sequences in MAFFT v7.017 implemented in Geneious v8.1.3 [62] using the iterative refinement by the G-INS-i strategy that resulted in an alignment of stefins and type 2 cystatins according to topological equivalence in tertiary structure (see Dataset S1 of [59]). Nonhomologous regions at the beginning (e.g., signal peptides) and end of the alignment were trimmed so the final alignment comprised 213 positions, principally corresponding to the conserved cystatin domain (Pfam: PF00031) (Dataset S2). The phylogenetic trees were reconstructed using the maximum likelihood (ML) and Bayesian inference (BI) methods. ML analysis was performed in RAxML v7.0.3 [63], using the WAG + G model selected by ModelFinder implemented in IQ-TREE webserver [64]. Indels were treated as unknown characters. Bootstraps were based on 1000 replicates. BI analysis was performed in MrBayes v3.2.7av1 [65] using the WAG model of evolution, with 24 million generations sampled at intervals of 1000 trees until the standard deviation from split frequencies was below 0.05. The burn-in period represented the default initial 25% of all generations. The trees were visualized in Geneious v8.1.3 and graphically modified in Adobe Illustrator CS5.
2.4. Comparison of the Protein Architecture
Cystatin diversity on the amino acid sequence level was analyzed in terms of critical motifs, amino acid conservation, presence/absence of a signal peptide and disulfide bridges, and modifications that might potentially affect the cystatin inhibitory activity. Disulfide bonds were predicted using the DISULFIND webserver [66]. Signal peptides were predicted using the SignalP v5.0 webserver [67]. Animal group-/cystatin type-specific sequence motifs were identified by comparison of primary structures of cystatin amino acid sequences in the WebLogo v3 software [68] using extractions of the alignment used for the phylogenetic analyses.
3. Results
In this study, we retrieved 184 homologues of cystatins (104 stefins and 80 type 2 cystatins) from the NGS data of early-emerging metazoans (Table S1). Of these, we verified with PCR the identity of 15 homologues mined from unpublished datasets (Table S2).
3.1. Cystatin Gene Repertoire and Diversity in Early-Emerging Metazoans
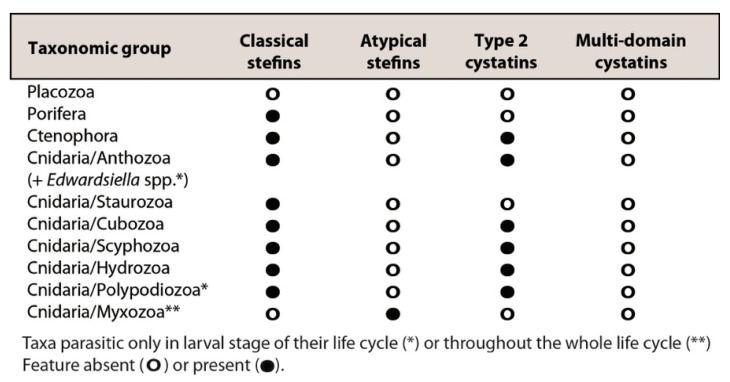
We observed a large variation in the representation of cystatin subclasses in the investigated animal groups (Figure 1). Type 2 cystatins were missing in several groups (Placozoa, Porifera, Cnidaria: Staurozoa, and Myxozoa). Stefins were present in all but one (Placozoa) basal metazoan lineage. We found a separate group of stefins in myxozoan parasites, designated here as “atypical stefins”, which differed in sequence structure to classical metazoan stefins (see Section 3.3 and Figure 2). No multi-domain cystatins could be identified in basal metazoans (Figure 1).
Figure 1.
Repertoire of cystatin superfamily genes in early-emerging metazoan lineages.
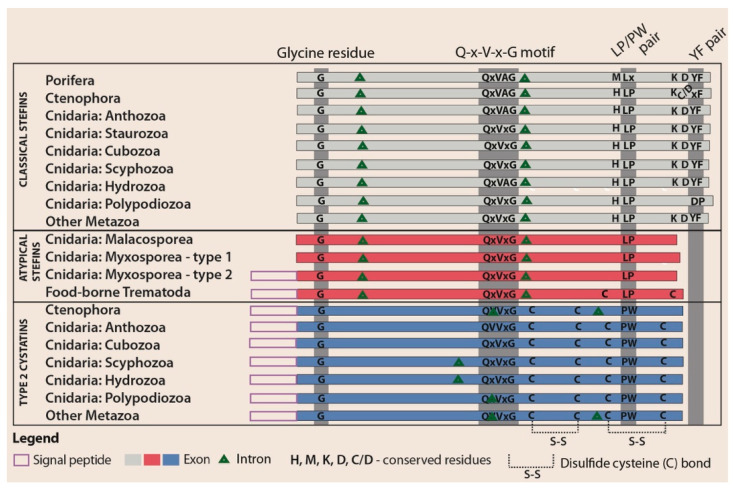
Figure 2.
Schematic comparison of the gene architecture and amino acid structure of metazoan cystatins: for myxozoans, information is shown separately for Malacosporea and Myxosporea.
We found that basal metazoans had a diverse cystatin gene repertoire: most investigated species had multiple cystatin homologues with significant sequence differences, even on the intraspecies level (i.e., several divergent sequences of type 2 cystatin and/or stefin homologues in the genome of a single species; Table S1). Exceptionally, certain species (e.g., cnidarians Ceratonova shasta and Physalia physalis) only possessed single type 2 cystatin and/or stefin homologue.
All mined cystatin homologues are listed either as new GenBank entries (for PCR-verified sequences mined from data unpublished at the time of the homology searches) or as Genbank contig/scaffold identifiers for sequences mined from published data (Table S2 and Dataset S1).
3.2. Phylogeny of Metazoan Cystatins did not Mirror Animal Phylogeny and Was Highly Diverse within Individual Groups
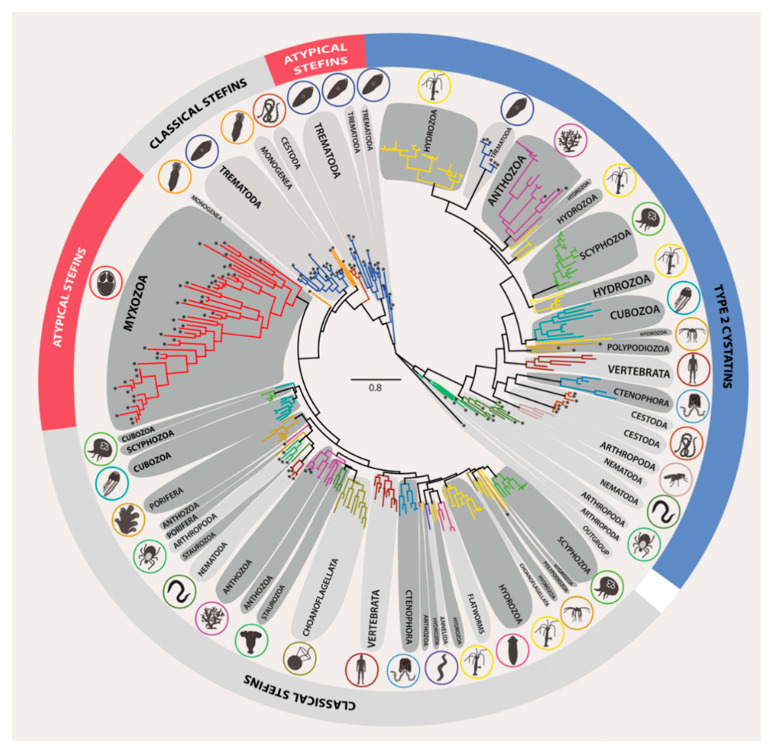
The clustering of cystatins in both ML and BI analyses did not follow the generally accepted trends of organismal phylogeny, as early-emerging animal lineages did not occupy basal positions in our trees (Figure 3 and Figure S1) as commonly known from other phylogenetically informative genes., e.g., 18S rDNA. Based on differences in the primary amino acid sequence structure, cystatins clustered into a single weakly supported stefin clade (ML/BI = 48/0.75) while the remainder of sequences clustered within the type 2 cystatin clade (ML/BI = 59/0.92). The stefin clade included a mixture of classical and atypical stefin subclades with weakly supported interrelationships. Classical stefin subclades comprised all Metazoa except the myxozoans, while atypical stefin subclades were formed exclusively of certain early-emerging (myxozoans) and more derived (trematodes) parasite animal lineages. Unlike other metazoan stefins, those of myxozoans were extremely divergent in their sequences, thus creating long branches (Figure 3 and Figure S1).
Figure 3.
Maximum likelihood phylogenetic tree of the cystatin superfamily represented in the Metazoa and Choanoflagellata. Giardia cystatin was used as the outgroup. The basal metazoan groups are depicted in dark grey shaded bubbles, while other groups are shaded light grey. Cystatin homologues from parasite taxa are labelled by asterisks at the tip of the nodes. The detailed ML and BI phylogenetic trees featuring taxa names and all nodal supports are provided in Figure S1.
Both stefins and type 2 cystatins clustered into subclades that paralleled classic taxonomy. However, interclade relationships of these taxonomy-defined subclades were weakly supported/not resolved due to polytomies (Figure 3 and Figure S1) and did not always reflect the organismal phylogeny. Indeed, some representatives formed monophyletic taxonomic groups, e.g., Ctenophora, Porifera, Cubozoa, and Myxozoa, while some cnidarian groups (Polypodiozoa, Staurozoa, Scyphozoa, Anthozoa, and Hydrozoa) split into multiple subclades not related by classic taxonomy. We attributed this splitting to (i) sequence differences among species of the same taxonomic group (e.g., hydrozoans Hydra, Podocoryna, Physalia, Velella, and Porpita spp. clustering into independent type 2 cystatin clades) or (ii) the occurrence of paralogues (e.g., stefin out-paralogues of the scyphozoan Aurelia aurita present in two distinct groups). Some of the out-paralogous clades additionally contained multiple sequences from single species (e.g., stefin in-paralogues of A. aurita ranging up to 37.7% amino acid sequence divergence) (Figure 3 and Figure S1).
3.3. Structural Modifications of Cystatin Homologues in Basal Metazoans
The results of structural analyses aimed at identifying intron number and positions, conserved regions, and the presence/absence of signal peptides and cysteine bonds were combined to create the comparative scheme of cystatin structural motifs (Figure 2).
We identified that introns in cystatin nucleotide sequences of early-emerging animal lineages varied in number (0–2) and location in type 2 cystatins while stefins contained two introns with conserved positions (Figure 2). All introns were delimited by typical GT/AG sequence boundaries at the exon–intron junction.
In amino acid sequence data from our alignment of basal animals (Dataset S2), we observed four conserved regions important for interaction with proteases, as found previously with derived metazoans [30,59]: (i) a glycine residue within the amino-terminal “trunk”, (ii) a Q-x-V-x-G motif within the first hairpin loop, (iii) the PW (type 2 cystatins) or LP (stefins) motifs within the second hairpin loop, and (iv) a tyrosine residue located in the relatively conserved D-x-L-x-Y-F carboxy terminus of stefins. We observed two novel conserved residues in stefins of both early-emerging and derived metazoans: (i) a histidine residue located seven amino acids before the LP pair towards the N-terminus and (ii) a lysine residue situated eight amino acids before the C-terminal conserved tyrosine residue (Figure 2 and Dataset S2).
While the amino acid structure of the first two key activity regions (G, Q-x-V-x-G) was conserved among all sequences, several modifications were seen in other regions. All sponge (Porifera) stefins showed a characteristic amino acid replacement of the LP pair to LD (or LS in one case). Substitution of the conserved C-terminal tyrosine residue by non-aromatic valine was observed in two homologues. The conserved histidine residue located near the LP pair was commonly replaced by methionine or, exceptionally, by histidine or tryptophan (both observed once) (Dataset S2).
Stefins and type 2 cystatins of comb jellies (Ctenophora) had the typical sequence motifs defined for these molecules in other metazoans. In a few taxa, we observed substitutions of the conserved cystatin PW pair by AW and stefin C-terminal tyrosine residue by proline or histidine (Dataset S2).
Cnidarian cysteine protease inhibitors showed a wide variety of modifications (Figure 2 and Dataset S2). Cubozoans were the only group for which conserved stefin regions had the classical organization, and their type 2 cystatins differed in a few cases by replacement of the PW motif with SW or KF. Stefins of hydrozoans and scyphozoans showed single substitutions of LP to FA or LK and single alterations of C-terminal tyrosine to aromatic phenylalanine, and many of the type 2 cystatins had substitutions of PW with AW, RW, or SW and exceptionally with PF or PL. Staurozoan stefins had C-terminus modifications, where the tyrosine residue was substituted with histidine or was missing. In anthozoans, type 2 cystatins had alterations of PW to SW or GW, while a few stefins had substitutions of LP with FD or FS and replacement of the C-terminal tyrosine residue to proline or histidine.
The most notable modification we found was in two parasitic cnidarian groups Polypodiozoa and Myxozoa. While the majority of type 2 cystatins of P. hydriforme had the typical conserved motifs, its stefin was highly modified, with the LP pair substituted with LS and the C-terminal conserved D-x-L-x-Y-F motif replaced with a completely different pattern lacking a tyrosine residue. While this stefin retained the histidine found near the LP pair, the conserved lysine located near the C-terminal was replaced by leucine. Myxozoans, as representatives of early-emerging metazoans, had atypical stefins, which completely lacked the D-x-L-x-Y-F region at the C-terminus and had the normally conserved histidine and lysine residues replaced by other residues. Stefins of an evolutionary older myxozoan subgroup, the Malacosporea, retained the original LP pair, but some stefins of the specious and more derived myxozoan group, the Myxosporea, had replaced this motif with LS, LY, LR, FK, or SA (Figure 2). We could not find classical stefins or type 2 cystatins in any myxozoan (Figure 1).
In general, signal peptides in cystatins of basal metazoan lineages followed established patterns: they were present in type 2 cystatins and absent in classical stefins. The only exceptions were some myxosporean stefins that carried a signal peptide (Figure 2). Interestingly, the pattern of signal peptide absence (in type 1 atypical stefins) or presence (in type 2 atypical stefins) was observed even at the intraspecies level. For example, some myxosporeans coded exclusively for stefins without a signal peptide (i.e., C. shasta and Sphaeromyxa zaharoni) while others presented multiple stefin genes that all carried the signal peptide (i.e., Myxobolus pendula and M. cerebralis) or had genes that represented both variations (i.e., Enteromyxum leei, E. scophthalmi, Kudoa iwatai, Thelohanellus kitauei, Myxidium lieberkuehni and Sphaerospora molnari) (Figure 2 and Dataset S1).
As typical for type 2 cystatins, two conserved disulfide bridges were identified in all basal metazoans with the exception of homologues found in ctenophorans, which were predicted to contain only one disulfide bridge. Classical stefins lacked disulfide bridges, as is typical for this cystatin subfamily type, and this pattern was also observed in atypical stefins (Figure 2).
3.4. Structural and Evolutionary Comparison of Cystatins of Parasitic vs. Free-Living Groups of Basal Metazoans: The Special Case of Myxozoa
The Myxozoa, the only obligate parasite group of early-emerging metazoans, possessed exclusively atypical stefins and lacked type 2 cystatins. Myxozoan stefins had characteristic differences in sequence to those of the other basal Metazoa (see Section 3.3) and clustered into a single clade with long branches (see Section 3.2). The two cnidarian lineages studied that are parasitic only in their larval stage (E. lineata, E. carnea, and P. hydriforme) possessed both classical stefins and type 2 cystatins, and no atypical stefins were found in these animals. Their stefins formed a distinct lineage that afterwards clustered with other cnidarians (P. hydriforme) or grouped with their respective anthozoan members (E. lineata and E. carnea). Similarly, clustering of “partially parasitic” cnidarians into distinct monotypic lineages (P. hydriforme) or within anthozoan lineages (E. lineata and E. carnea) was observed for type 2 cystatins (Figure 3 and Figure S1). No structurally unique molecules were identified in the entirely free-living basal metazoans.
4. Discussion
4.1. A Diverse Repertoire of Cystatins in Early-Emerging Metazoans
In this study, we identified the cystatin gene repertoire of early-emerging animals by mining homologues from 110 genomes and transcriptomes of basal Metazoa. We demonstrate that cystatins are widely distributed and highly diversified in current representatives of these ancient lineages and that the repertoire of cystatin superfamily genes differs widely among phyla. Each taxonomic group evolved multiple stefin and/or type 2 cystatin homologues, forming individual taxonomy-defined clades in the phylogeny. These paralogues probably arose by gene duplication [30] rather than by whole genome multiplication, as the only basal metazoans for which genome duplication has been suggested are members of the cnidarian genus Acropora [69]. Multiple out-paralogues found in cnidarians likely originated before cnidarian radiation while, in-paralogues, probably emerged after the speciation event. Bursts of sequence diversification likely gave rise to gene orthologues within each taxonomy-defined clade. These evolutionary processes resulted in the origin of diverse molecules with varying inhibitory and immunomodulatory functions in metazoans.
Some taxonomic groups had a wide diversity of cystatins, while others lacked some or all cystatin subfamilies. For example, genes coding for cystatins were not identified in the placozoan genomes: two different strains of Trichoplax sp. [70] and Hoilungia hongkongensis [71], which was in concordance with findings of Kordiš and Turk [30] from the related species Trichoplax adhaerens [70,72]. Evidently, cystatins are not essential for the survival of these simple organisms despite the presence of cysteine proteases in them [71,72]. We suggest that, in placozoans, the functions of cystatins have been replaced by other types of inhibitors, such as structurally unrelated equistatin or serpins [70,71,72]. These proteins have been recognized in other organisms as potent inhibitors of cysteine proteases [73] or cross-class inhibitors of them [74].
4.2. Atypical Stefins: A Unique Type of Cystatins in Myxozoans and Some Trematodes
Interestingly, we found a wide diversity of both classical and atypical stefins in basal metazoans and we suspect that these highly divergent homologues may have different or novel functions, as suggested for the highly divergent myxozoans serpins (serine protease inhibitors [75]). Stefins structurally similar to those of myxozoans have been identified only from more derived bilaterians: intestinal and liver flukes (Trematoda: Digenea) [1,2,3,4]. While myxozoans possess only atypical stefins, these trematodes have cystatins from several superfamily subclasses (classical and atypical stefins, multi-domain cystatins [1,2,3,4,22,76]). The atypical stefins presumably arose independently in the two groups, possibly as a result of pressures from a parasitic life history; however, other parasites do not possess these molecules. For example, parasitic metazoans (monogeneans, nematodes, cestodes, ticks, and mites) [9,36,37,39,42] including blood trematodes [35] have only classical stefins and type 2 cystatins. Similarly, cnidarians Polypodium and Edwardsiella spp. with parasitic larval and free-living adult stages also have only classical stefins and type 2 cystatins. Thus, atypical stefins represent key evolutionary innovations of myxozoans and intestinal and liver trematodes. The likely function of atypical stefins in myxozoans and trematodes is to control unwanted proteolysis by cysteine proteases expressed by different parasite life stages and by the host [1].
Structurally, atypical stefins contain the characteristic stefin LP pair, which is modified in certain myxozoan stefins. Importantly, all atypical stefins lack the conserved D-x-L-x-Y-F carboxy-terminal part that is generally found in classical stefins but is absent from type 2 cystatins (Figure 2). As the tyrosine residue of the D-x-L-x-Y-F region is important for stabilization of the stefin-protease complex [59,77], the absence of this motif along with the modifications in the conserved LP pair may have functional implications in atypical stefins, e.g., their capability to inhibit a broader range of proteases, including those of host origin. The function of this C-terminal modification warrants future experimental studies as demonstrated previously for stefin mutants [77,78].
Additionally, we identified that the atypical stefins of some myxozoans and all trematodes have a signal peptide typical for type 2 cystatins (Figure 2). In some taxa, this feature correlates with extracorporeal protein secretion [3,79,80,81], but this is not consistent across the Metazoa, as not all proteins that have a signal peptide are exported out of the organism [1] and a large proportion of excreted/secreted products do not have a signal peptide [1,4,37,79,81,82,83]. Secreted proteins, including cystatins, are crucial for parasite protection from degradation by host cysteine proteases and for modulation of host immunity [9,10,11,16,79]. We postulate that some myxozoan stefins are secretory proteins with immunomodulatory roles, as has been suggested for the atypical stefin of the myxosporean Thelohanellus kitauei [53,84]. These molecules are likely responsible for the increased expression of the anti-inflammatory IL-10 gene in fish hosts infected with different myxosporean species [85,86,87,88,89,90] as an increased production of IL-10 in hosts infected by other parasites is elicited by cystatin-induced immunomodulation [16,20]. Similar to other parasites [23,24,25,26,27], we suggest that these proteins should be further explored in myxozoans as vaccine targets due to the lack of efficient strategies against these parasites in fish destined for human consumption [91,92].
We propose two mechanisms for the origin of atypical stefins: (i) structural modification of ancestral classical stefins by the acquisition of a signal peptide in some homologues and loss of the C-terminal and its tyrosine residue, or (ii) combination of the features of classical stefins (LP pair) and type 2 cystatins (signal peptides, missing C-terminal part) in chimeric genes. Chimeric genes are important in the evolution of genetic novelty and can allow organisms to adapt to novel environments (or hosts) by acquisition of novel functions [93,94]. Both myxozoans and trematodes are obligate parasites with complex life cycles that involve vertebrate and invertebrate hosts [8,95]. The independent origins of their atypical stefins might have been associated with the transition of a presumably free-living ancestor to obligate parasitism and the associated challenges of surviving within one or more hosts. Extending a recent hypothesis that invertebrates were the initial hosts for myxozoans and fish were incorporated into life cycles later [96], we postulate that myxozoans reduced their type 2 cystatins in the invertebrate host in the course of genome reduction due to parasitism [47] since invertebrates have limited immune capacity [97]. Ancestral superfamily cystatin types were lost in myxozoans as their roles were replaced by atypical stefins, which in trematodes have been shown to inhibit both parasite and host proteases [2,3]. Then, under novel pressures in adopted fish hosts, myxozoans remodeled classical stefins into the atypical ones to interfere with specific immune responses of fish and to enable novel behaviors such as migration within host tissues.
4.3. Obstacles to Reconstruction of Cystatin Phylogenies
In our analyses, the reconstruction of evolutionary relationships in the cystatin superfamily had good support for the more recent clades but not for deeper evolutionary nodes, as reported previously for this group of genes [30,35]. Unstable topology, polytomies (in BI), and low nodal supports in our phylogeny were due to low informative content from short protein length and high sequence divergence in the chosen genes [30,35]. However, despite generally poor support, a clear separation of ingroup taxa into distinct lineages defined by taxonomy and protein architecture revealed remarkable trends in the evolution of cystatins in early-emerging metazoans, with structurally unique molecules of potentially novel functions. Different branches appear to have evolved at different rates [98], especially in fast-evolving myxozoans [46,47,51,53,58]. Fast rates of evolution of the myxozoan genes likely caused the extraordinary sequence divergence of myxozoan stefins, which may represent functional diversification of these molecules. Phylogenetic clustering of atypical stefins within the myxozoan clade seems to some extent to follow myxozoan speciation and differentiation into the well-known myxozoan lineages (oligochaete-infecting, polychaete-infecting, Sphaerospora sensu stricto, and malacosporeans [96]) Resolution of these established myxozoan clades is low due to the undersampling of taxa and is probably compounded by diversification including a wide functional range of atypical stefins, with or without signal peptides, and with each particular lineage displaying specific sequence motifs and potential host organ-specific protease expression.
4.4. Hypothetical Evolution of Metazoan Cystatins
From their comprehensive analysis of prokaryotic and eukaryotic cystatins, Kordiš and Turk [30] concluded that the ancestor of the cystatin superfamily was intracellular and lacked a signal peptide and disulfide bridges (similarly to extant stefins). Initial gene duplication produced two ancestral eukaryotic lineages, stefins and type 2 cystatins, with the latter evolving from the stefin-like ancestor by acquiring cysteine residues, disulfide bridges, and a signal peptide. Later gene and domain duplications combined with deletions and insertions of genetic material resulted in diverse single- and multi-domain proteins with or without disulfide bonds and glycosylations [30].
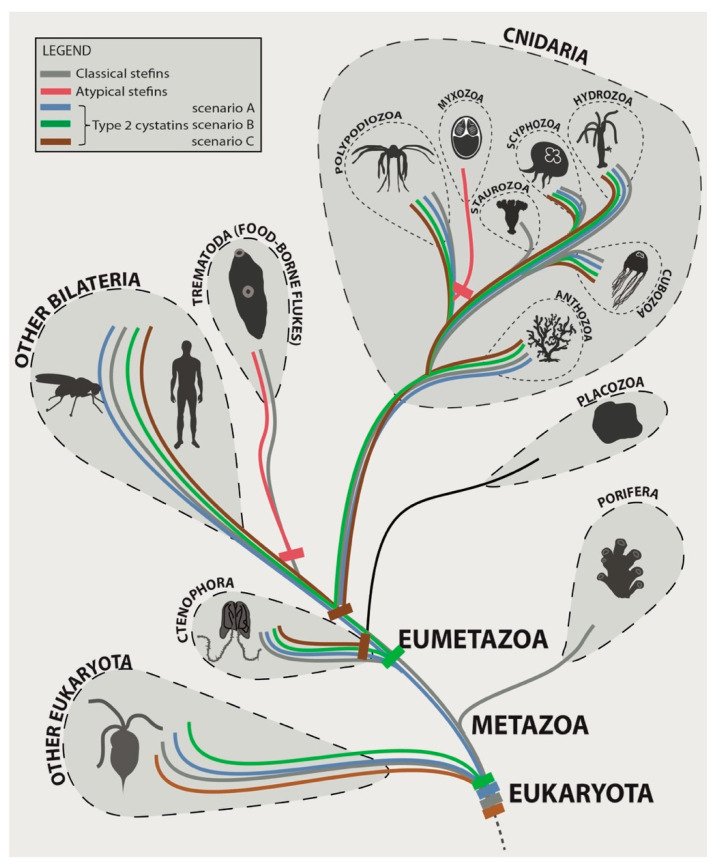
Based on our novel data and published records [30], we propose the following alternative scenarios for the evolution of single-domain cystatins in the Metazoa, with a focus on its basal lineages (Figure 4). After the split of the cystatin superfamily into the initial stefin and type 2 cystatin lineages in the ancestor of eukaryotes, stefins were retained in the common ancestor of the Metazoa and all its descendant lineages except Placozoa. Sequence diversification and lineage-specific adaptation of stefins occurred independently in parasites (Myxozoa and Trematoda), giving rise to atypical stefins. Type 2 cystatins were either (i) retained in the metazoan ancestor and its descendant lineages except for their independent loss in Porifera, Placozoa, some Cnidaria (Myxozoa, Staurozoa) and some bilaterians (liver and intestinal flukes) (scenario A in Figure 4); (ii) initially lost in the metazoan ancestor (or even before), reappeared in the ancestor of Eumetazoa, and were again lost in some descendant lineages (Placozoa, Myxozoa, Staurozoa, and some flukes) (scenario B in Figure 4); or (iii) initially lost in the metazoan ancestor (or even before) and reappeared independently in Ctenophora and in the ancestor of the lineage, leading to Cnidaria and Bilateria with some groups (Myxozoa, some flukes) having a secondary loss (scenario C in Figure 4).
Figure 4.
Hypothetical evolutionary scenarios of origins of stefins and type 2 cystatins in the Metazoa with a focus on early-emerging animal lineages: please note that the different colors for type 2 cystatins visualize the alternative scenarios of hypothetical evolution for the same cystatin subtype and not the sequence differences.
5. Conclusions
Stefins are considered relatively conserved genes with characteristics closer to the ancestral superfamily type, while type 2 cystatins are considered to have undergone a more complex and dynamic evolution through numerous gene and domain duplications [30]. In this study, we showed that early-emerging metazoan lineages were more prone to type 2 cystatin loss and that significant diversification of this type of molecules occurred only later in their evolution. Simultaneously, these ancient lineages retained stefin genes that were more dynamic with regard to structural modifications, with bursts of diversification creating atypical stefins in some obligate parasites with complex life cycles. Further research aimed at biochemical and functional characterization of the parasite cysteine protease inhibitors, especially of atypical stefins, is necessary to decipher the roles of these proteins in multi-host–parasite interactions. This knowledge will improve the understanding of the contributions of cystatins for parasite survival in the host causing disease and may reveal targets for therapeutant development against Myxozoa.
Acknowledgments
We thank to Baveesh Pudhuvai (BC CAS, Budweis, Czech Republic) for help in the PCR verification of Buddenbrockia stefin. We also thank Ivan Fiala (BC CAS, Budweis, Czech Republic) for providing suggestions to improve the manuscript and for sharing M. lieberkuehni and N. pickii transcriptomic data. We are grateful to Hanna Hartikainen (ETH Zurich, Switzerland) for sharing T. bryosalmonae genome data.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-7737/10/2/110/s1, Figure S1: Maximum likelihood and Bayesian trees with nodal supports; Table S1: Next-generation sequencing databases mined for cystatins; Table S2: List of myxozoan species with information on primers used for the PCR verification of stefin genes; Table S3: The list of groups/species used for phylogenetic analyses. Methods S1: Methodological details regarding the PCR verification of myxozoan stefins; Dataset S1: Fasta file of complete the amino acid sequences of cystatins present in the phylogenetic analyses; Dataset S2: Fasta-formatted alignment of the trimmed amino acid sequences of cystatins used for the phylogenetic analyses.
Author Contributions
Conceptualization, P.B.-S.; methodology, P.B.-S.; formal analysis: P.B.-S., J.K., O.P., M.N.F., and G.A.-B.; investigation: P.B.-S., S.D.A., O.P., A.P.-S., A.H., M.N.F., and G.A.-B.; resources: P.B.-S., A.S.H., O.P., J.W.H., and J.L.B.; data curation: P.B.-S. and O.P.; writing—original draft preparation: P.B.-S.; writing-review and editing: All authors; visualization: P.B.-S. and J.K.; supervision: P.B.-S.; funding acquisition: P.B.-S. and A.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Youth, and Sports of the Czech Republic, grant number LTAUSA17201; by the European Commission under the H2020 Programme—ParaFishControl, grant number 634429; by the Czech Science Foundation, grant number 19-28399X (to A. S. Holzer, G. Alama-Bermejo, and J. Kyslík) and 21-16565S and by the Czech Academy of Sciences and Hungarian Academy of Sciences, grant number MTA 19-07. This publication reflects the views of the authors only; the European Commission cannot be held responsible for any use which may be made of the information contained therein.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in Genbank (under the accession Nos. MT127416–MT127426 and MW498387–MW498390) and in the associated supplementary files.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cancela M., Corvo I., da Silva E., Teichmann A., Roche L., Diaz A., Tort J.F., Ferreira H.B., Zaha A. Functional characterization of single-domain cystatin-like cysteine proteinase inhibitors expressed by the trematode Fasciola hepatica. Parasitology. 2017;144:1695–1707. doi: 10.1017/S0031182017001093. [DOI] [PubMed] [Google Scholar]
- 2.Buša M., Matoušková Z., Řezáčová P., Bartošová-Sojková P., Štefanič S., Mareš M. Evolutionary upgrade of stefins for secretion in parasites; Proceedings of the 11th General Meeting of the International Proteolysis Society “Interfaces in Proteolysis”; Mariánské Lázně, Czech Republic. 29 September–4 October 2019. [Google Scholar]
- 3.Siricoon S., Grams S.V., Grams R. Efficient inhibition of cathepsin B by a secreted type 1 cystatin of Fasciola gigantica. Mol. Biochem. Parasit. 2012;186:126–133. doi: 10.1016/j.molbiopara.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Tarasuk M., Grams S.V., Viyanant V., Grams R. Type I cystatin (stefin) is a major component of Fasciola gigantica excretion/secretion product. Mol. Biochem. Parasit. 2009;167:60–71. doi: 10.1016/j.molbiopara.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Abrahamson M., Alvarez-Fernandez M., Nathanson C. Cystatins. Biochem. Soc. Symp. 2003;70:179–199. doi: 10.1042/bss0700179. [DOI] [PubMed] [Google Scholar]
- 6.Turk V., Stoka V., Turk D. Cystatins: Biochemical and structural properties, and medical relevance. Front. Biosci. 2008;13:5406–5420. doi: 10.2741/3089. [DOI] [PubMed] [Google Scholar]
- 7.Turk V., Turk D., Dolenc I., Stoka V. Characteristics, structure, and biological role of stefins (type-1 cystatins) of human, other mammals, and parasite origin. Acta Chim. Slov. 2019;66:5–17. doi: 10.17344/acsi.2018.4639. [DOI] [PubMed] [Google Scholar]
- 8.Ranasinghe S., McManus D. Protease inhibitors of parasitic flukes: Emerging roles in parasite survival and immune defence. Trends Parasitol. 2017;33:400–413. doi: 10.1016/j.pt.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Chmelař J., Kotál J., Langhansová H., Kotsyfakis M. Protease inhibitors in tick saliva: The role of serpins and cystatins in tick-host-pathogen interaction. Front. Cell. Infect. Microbiol. 2017;7:216. doi: 10.3389/fcimb.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory W.F., Maizels R.M. Cystatins from filarial parasites: Evolution, adaptation and function in the host-parasite relationship. Int. J. Biochem. Cell Biol. 2008;40:1389–1398. doi: 10.1016/j.biocel.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y.J., Wu L.Y., Liu X.C., Wang S., Ehsan M., Yan R.F., Song X.K., Xu L.X., Li X.R. Characterization of a secreted cystatin of the parasitic nematode Haemonchus contortus and its immune-modulatory effect on goat monocytes. Parasit. Vectors. 2017;10:425. doi: 10.1186/s13071-017-2368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotál J., Stergiou N., Buša M., Chlastáková A., Beránková Z., Řezáčová P., Langhansová H., Schwarz A., Calvo E., Kopecký J., et al. The structure and function of Iristatin, a novel immunosuppressive tick salivary cystatin. Cell Mol. Life Sci. 2019;76:2003–2013. doi: 10.1007/s00018-019-03034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz A., Valdés J.J., Kotsyfakis M. The role of cystatins in tick physiology and blood feeding. Ticks Tick Borne Dis. 2012;3:117–127. doi: 10.1016/j.ttbdis.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klotz C., Ziegler T., Figueiredo A.S., Rausch S., Hepworth M.R., Obsivac N., Sers C., Lang R., Hammerstein P., Lucius R., et al. A helminth immunomodulator exploits host signaling events to regulate cytokine production in macrophages. PLoS Pathog. 2011;7:e1001248. doi: 10.1371/journal.ppat.1001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maizels R.M., Gomez-Escobar N., Gregory W.F., Murray J., Zang X.X. Immune evasion genes from filarial nematodes. Int. J. Parasitol. 2001;31:889–898. doi: 10.1016/S0020-7519(01)00213-2. [DOI] [PubMed] [Google Scholar]
- 16.Klotz C., Ziegler T., Daniłowicz-Luebert E., Hartmann S. Cystatins of parasitic organisms. Adv. Exp. Med. Biol. 2011;712:208–221. doi: 10.1007/978-1-4419-8414-2_13. [DOI] [PubMed] [Google Scholar]
- 17.Khatri V., Chauhan N., Kalyanasundaram R. Parasite cystatin: Immunomodulatory molecule with therapeutic activity against immune mediated disorders. Pathogens. 2020;9:431. doi: 10.3390/pathogens9060431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopitar-Jerala N. The role of cystatins in cells of the immune system. FEBS Lett. 2006;580:6295–6301. doi: 10.1016/j.febslet.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 19.Salát J., Paesen G.C., Rezáčová P., Kotsyfakis M., Kovářová Z., Šanda M., Majtán J., Grunclová L., Horká H., Andersen J.F., et al. Crystal structure and functional characterization of an immunomodulatory salivary cystatin from the soft tick Ornithodoros moubata. Biochem. J. 2010;429:103–112. doi: 10.1042/BJ20100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartmann S., Lucius R. Modulation of host immune responses by nematode cystatins. Int. J. Parasitol. 2003;33:1291–1302. doi: 10.1016/S0020-7519(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 21.Schierack P., Lucius R., Sonnenburg B., Schilling K., Hartmann S. Parasite-specific immunomodulatory functions of filarial cystatin. Infect. Immun. 2003;71:2422–2429. doi: 10.1128/IAI.71.5.2422-2429.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaznadji E., Collins P., Dalton J.P., Bigot Y., Moire N. A new multi-domain member of the cystatin superfamily expressed by Fasciola hepatica. Int. J. Parasitol. 2005;35:1115–1125. doi: 10.1016/j.ijpara.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 23.McKerrow J., Engel J., Caffrey C. Cysteine protease inhibitors as chemotherapy for parasitic infections. Bioorg. Med. Chem. 1999;7:639–644. doi: 10.1016/S0968-0896(99)00008-5. [DOI] [PubMed] [Google Scholar]
- 24.Tang B., Liu M., Wang L., Yu S., Shi H., Boireau P., Cozma V., Wu X., Liu X. Characterisation of a high-frequency gene encoding a strongly antigenic cystatin-like protein from Trichinella spiralis at its early invasion stage. Parasit. Vectors. 2015;8:78. doi: 10.1186/s13071-015-0689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdulla M., Lim K., Sajid M., McKerrow J., Caffrey C. Schistosomiasis mansoni: Novel chemotherapy using a cysteine protease inhibitor. PLoS Med. 2007;4:130–138. doi: 10.1371/journal.pmed.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das P., Alam M., Paik D., Karmakar K., De T., Chakrahorti T. Protease inhibitors in potential drug development for leishmaniasis. Indian J. Biochem. Biol. 2013;50:363–376. [PubMed] [Google Scholar]
- 27.Duschak V. Targets and patented drugs for chemotherapy of Chagas disease in the last 15 years-period. Recent Pat. Antiinfect. Drug Discov. 2016;11:74–173. doi: 10.2174/1574891X11666161024165304. [DOI] [PubMed] [Google Scholar]
- 28.Smallwood T.B., Giacomin P.R., Loukas A., Mulvenna J.P., Clark R.J., Miles J.J. Helminth immunomodulation in autoimmune disease. Front. Immunol. 2017;8:453. doi: 10.3389/fimmu.2017.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breznik B., Mitrovic A., Lah T.T., Kos J. Cystatins in cancer progression: More than just cathepsin inhibitors. Biochimie. 2019;166:233–250. doi: 10.1016/j.biochi.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Kordiš D., Turk V. Phylogenomic analysis of the cystatin superfamily in eukaryotes and prokaryotes. BMC Evol. Biol. 2009;9:266. doi: 10.1186/1471-2148-9-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez M., Diaz I. The origin and evolution of plant cystatins and their target cysteine proteinases indicate a complex functional relationship. BMC Evol. Biol. 2008;8:198. doi: 10.1186/1471-2148-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown W.M., Dziegielewska K.M. Friends and relations of the cystatin superfamily—New members and their evolution. Protein Sci. 1997;6:5–12. doi: 10.1002/pro.5560060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawlings N.D., Barrett A.J. Evolution of proteins of the cystatin superfamily. J. Mol. Evol. 1990;30:60–71. doi: 10.1007/BF02102453. [DOI] [PubMed] [Google Scholar]
- 34.Verdes A., Simpson D., Holford M. Are fireworms venomous? Evidence for the convergent evolution of toxin homologs in three species of fireworms (Annelida, Amphinomidae) Genome Biol. Evol. 2018;10:249–268. doi: 10.1093/gbe/evx279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuesta-Astroz Y., Scholte L.L.S., Pais F.S.M., Oliveira G., Nahum L.A. Evolutionary analysis of the cystatin family in three Schistosoma species. Front. Genet. 2014;5:206. doi: 10.3389/fgene.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo A.J. Comparative analysis of cystatin superfamily in Platyhelminths. PLoS ONE. 2015;10:e0124683. doi: 10.1371/journal.pone.0124683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ilgová J., Jedličková L., Dvořaková H., Benovics M., Mikeš L., Janda L., Vorel J., Roudnický P., Potěšil D., Zdráhal Z., et al. A novel type I cystatin of parasite origin with atypical legumain-binding domain. Sci. Rep. 2017;7:17526. doi: 10.1038/s41598-017-17598-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong X.W., Xu J., Song H.Y., Liu Y.C., Wu M.D., Zhang H.J., Jing B., Lai W.M., Gu X.B., Xie Y., et al. Molecular characterization of a Dirofilaria immitis cysteine protease inhibitor (cystatin) and its possible role in filarial immune evasion. Genes. 2019;10:300. doi: 10.3390/genes10040300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santamaria M.E., Hernandez-Crespo P., Ortego F., Grbic V., Grbic M., Diaz I., Martinez M. Cysteine peptidases and their inhibitors in Tetranychus urticae: A comparative genomic approach. BMC Genom. 2012;13:307. doi: 10.1186/1471-2164-13-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rangel C.K., Parizi L.F., Sabadin G.A., Costa E.P., Romeiro N.C., Isezaki M., Githaka N.W., Seixas A., Logullo C., Konnai S., et al. Molecular and structural characterization of novel cystatins from the taiga tick Ixodes persulcatus. Ticks Tick Borne Dis. 2017;8:432–441. doi: 10.1016/j.ttbdis.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Ibelli A.M.G., Hermance M.M., Kim T.K., Gonzalez C.L., Mulenga A. Bioinformatics and expression analyses of the Ixodes scapularis tick cystatin family. Exp. Appl. Acarol. 2013;60:41–53. doi: 10.1007/s10493-012-9613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alonso J., Martinez M. Insights into the molecular evolution of peptidase inhibitors in arthropods. PLoS ONE. 2017;12:e0187643. doi: 10.1371/journal.pone.0187643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinstein S.B., Kuris A.M. Independent origins of parasitism in Animalia. Biol. Lett. 2016;12:20160324. doi: 10.1098/rsbl.2016.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dnyansagar R., Zimmermann B., Moran Y., Praher D., Sundberg P., Moller L., Technau U. Dispersal and speciation: The cross Atlantic relationship of two parasitic cnidarians. Mol. Phylogenet. Evol. 2018;126:346–355. doi: 10.1016/j.ympev.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 45.Stefanik D.J., Lubinski T.J., Granger B.R., Byrd A.L., Reitzel A.M., DeFilippo L., Lorenc A., Finnerty J.R. Production of a reference transcriptome and transcriptomic database (EdwardsiellaBase) for the lined sea anemone, Edwardsiella lineata, a parasitic cnidarian. BMC Genom. 2014;15:71. doi: 10.1186/1471-2164-15-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shpirer E., Chang E.S., Diamant A., Rubinstein N., Cartwright P., Huchon D. Diversity and evolution of myxozoan minicollagens and nematogalectins. BMC Evol. Biol. 2014;14:205. doi: 10.1186/s12862-014-0205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang E.S., Neuhof M., Rubinstein N.D., Diamant A., Philippe H., Huchon D., Cartwright P. Genomic insights into the evolutionary origin of Myxozoa within Cnidaria. Proc. Natl. Acad. Sci. USA. 2015;112:14912–14917. doi: 10.1073/pnas.1511468112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar G., Abd-Elfattah A., El-Matbouli M. Identification of differentially expressed genes of brown trout (Salmo trutta) and rainbow trout (Oncorhynchus mykiss) in response to Tetracapsuloides bryosalmonae (Myxozoa) Parasitol. Res. 2015;114:929–939. doi: 10.1007/s00436-014-4258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiménez-Guri E., Philippe H., Okamura B., Holland P.W.H. Buddenbrockia is a cnidarian worm. Science. 2007;317:116–118. doi: 10.1126/science.1142024. [DOI] [PubMed] [Google Scholar]
- 50.Alama-Bermejo G., Meyer E., Atkinson S.D., Holzer A.S., Wiśniewska M.M., Kolísko M., Bartholomew J.L. Transcriptome-wide comparisons and virulence gene polymorphisms of host-associated genotypes of the cnidarian parasite Ceratonova shasta in salmonids. Genome Biol. Evol. 2020;12:1258–1276. doi: 10.1093/gbe/evaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nesnidal M., Helmkampf M., Bruchhaus I., El-Matbouli M., Hausdorf B. Agent of whirling disease meets orphan worm: Phylogenomic analyses firmly place Myxozoa in Cnidaria. PLoS ONE. 2013;8:e54576. doi: 10.1371/journal.pone.0054576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foox J., Ringuette M., Desser S.S., Siddall M.E. In silico hybridization enables transcriptomic illumination of the nature and evolution of Myxozoa. BMC Genom. 2015;16:840. doi: 10.1186/s12864-015-2039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y.L., Xiong J., Zhou Z.G., Huo F.M., Miao W., Ran C., Liu Y.C., Zhang J.Y., Feng J.M., Wang M., et al. The genome of the myxosporean Thelohanellus kitauei shows adaptations to nutrient acquisition within its fish host. Genome Biol. Evol. 2014;6:3182–3198. doi: 10.1093/gbe/evu247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartigan A., Kosakyan A., Pecková H., Eszterbauer E., Holzer A.S. Transcriptome of Sphaerospora molnari (Cnidaria, Myxosporea) blood stages provides proteolytic arsenal as potential therapeutic targets against sphaerosporosis in common carp. BMC Genom. 2020;21:404. doi: 10.1186/s12864-020-6705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evans N.M., Lindner A., Raikova E.V., Collins A.G., Cartwright P. Phylogenetic placement of the enigmatic parasite, Polypodium hydriforme, within the Phylum Cnidaria. BMC Evol. Biol. 2008;8:139. doi: 10.1186/1471-2148-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okamura B., Gruhl A. Myxozoan affinities and route to endoparasitism. In: Okamura B., Gruhl A., Bartholomew J.L., editors. Myxozoan Evolution, Ecology and Development. Springer; Cham, Switzerland: 2015. pp. 23–44. [Google Scholar]
- 57.Fiala I., Bartošová-Sojková P., Okamura B., Hartikainen H. Adaptive radiation and evolution within the Myxozoa. In: Okamura B., Gruhl A., Bartholomew J.L., editors. Myxozoan Evolution, Ecology and Development. Springer; Cham, Switzerland: 2015. pp. 69–84. [Google Scholar]
- 58.Feng J.M., Xiong J., Zhang J.Y., Yang Y.L., Yao B., Zhou Z.G., Miao W. New phylogenomic and comparative analyses provide corroborating evidence that Myxozoa is Cnidaria. Mol. Phylogenet. Evol. 2014;81:10–18. doi: 10.1016/j.ympev.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Stubbs M., Laber B., Bode W., Huber R., Jerala R., Lenarcic B., Turk V. The refined 2.4A X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: A novel type of proteinase inhibitor interaction. EMBO J. 1990;9:1939–1947. doi: 10.1002/j.1460-2075.1990.tb08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rawlings N.D., Alan J., Thomas P.D., Huang X.D., Bateman A., Finn R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018;46:D624–D632. doi: 10.1093/nar/gkx1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Potter S.C., Luciani A., Eddy S.R., Park Y., Lopez R., Finn R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018;46:W200–W204. doi: 10.1093/nar/gky448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trifinopoulos J., Nguyen L.T., von Haeseler A., Minh B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016;44:W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ceroni A., Passerini A., Vullo A., Frasconi P. DISULFIND: A disulfide bonding state and cysteine connectivity prediction server. Nucleic Acids Res. 2006;34:W177–W181. doi: 10.1093/nar/gkl266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Almagro Armenteros J.J., Tsirigos K.D., Sønderby C.K., Petersen T.N., Winther O., Brunak S., von Heijne G., Nielsen H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019;37:420–423. doi: 10.1038/s41587-019-0036-z. [DOI] [PubMed] [Google Scholar]
- 68.Crooks G.E., Hon G., Chandonia J.M., Brenner S.E. WebLogo: A sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mao Y.F., Satoh N. A likely ancient genome duplication in the speciose reef-building coral genus, Acropora. Iscience. 2019;13:20–32. doi: 10.1016/j.isci.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamm K., Osigus H.J., Stadler P.F., DeSalle R., Schierwater B. Trichoplax genomes reveal profound admixture and suggest stable wild populations without bisexual reproduction. Sci. Rep. 2018;8:11168. doi: 10.1038/s41598-018-29400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eitel M., Francis W., Varoqueaux F., Daraspe J., Osigus H., Krebs S., Vargas S., Blum H., Williams G., Schierwater B., et al. Comparative genomics and the nature of placozoan species. PLoS Biol. 2018;16:e3000032. doi: 10.1371/journal.pbio.2005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Srivastava M., Begovic E., Chapman J., Putnam N., Hellsten U., Kawashima T., Kuo A., Mitros T., Salamov A., Carpenter M., et al. The Trichoplax genome and the nature of placozoans. Nature. 2008;454:955–960. doi: 10.1038/nature07191. [DOI] [PubMed] [Google Scholar]
- 73.Lenarcic B., Ritonja A., Strukelj B., Turk B., Turk V. Equistatin, a new inhibitor of cysteine proteinases from Actinia equina, is structurally related to thyroglobulin type-1 domain. J. Biol. Chem. 1997;272:13899–13903. doi: 10.1074/jbc.272.21.13899. [DOI] [PubMed] [Google Scholar]
- 74.Irving J.A., Pike R.N., Dai W.W., Bromme D., Worrall D.M., Silverman G.A., Coetzer T.H.T., Dennison C., Bottomley S.P., Whisstock J.C. Evidence that serpin architecture intrinsically supports papain-like cysteine protease inhibition: Engineering alpha(1)-antitrypsin to inhibit cathepsin proteases. Biochemistry. 2002;41:4998–5004. doi: 10.1021/bi0159985. [DOI] [PubMed] [Google Scholar]
- 75.Eszterbauer E., Sipos D., Kaján G.L., Szegö D., Fiala I., Holzer A.S., Bartošová-Sojková P. Genetic diversity of serine protease inhibitors in myxozoan (Cnidaria, Myxozoa) fish parasites. Microorganisms. 2020;8:1502. doi: 10.3390/microorganisms8101502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geadkaew A., Kosa N., Siricoon S., Grams S.V., Grams R. A 170 kDa multi-domain cystatin of Fasciola gigantica is active in the male reproductive system. Mol. Biochem. Parasit. 2014;196:100–107. doi: 10.1016/j.molbiopara.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Pol E., Bjork I. Importance of the second binding loop and the C-terminal end of cystatin B (stefin B) for inhibition of cysteine proteinases. Biochemistry. 1999;38:10519–10526. doi: 10.1021/bi990488k. [DOI] [PubMed] [Google Scholar]
- 78.Jerala R., Kroon-Zitko L., Kopitar M., Popovic T., Turk V. Deletion of the carboxy terminal part of stefin B does not have a major effect for binding to papain. Biomed. Biochim. Acta. 1991;50:627–629. [PubMed] [Google Scholar]
- 79.Hewitson J.P., Grainger J.R., Maizels R.M. Helminth immunoregulation: The role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasit. 2009;167:1–11. doi: 10.1016/j.molbiopara.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vendelova E., de Lima J.C., Lorenzatto K.R., Monteiro K.M., Mueller T., Veepaschit J., Grimm C., Brehm K., Hrčková G., Lutz M.B., et al. Proteomic analysis of excretory-secretory products of Mesocestoides corti metacestodes reveals potential suppressors of dendritic cell functions. PLoS Negl. Trop. Dis. 2016;10:e0005061. doi: 10.1371/journal.pntd.0005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Maggio L.S., Tirloni L., Pinto A.F.M., Diedrich J.K., Yates J.R., Benavides U., Carmona C., Vaz I.D., Berasain P. Across intra-mammalian stages of the liver fluke Fasciola hepatica: A proteomic study. Sci. Rep. 2016;6:32796. doi: 10.1038/srep32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang J.M., Lee K.H., Sohn W.M., Na B.K. Identification and functional characterization of CsStefin-1, a cysteine protease inhibitor of Clonorchis sinensis. Mol. Biochem. Parasit. 2011;177:126–134. doi: 10.1016/j.molbiopara.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 83.Cantacessi C., Mulvenna J., Young N.D., Kasny M., Horak P., Aziz A., Hofmann A., Loukas A., Gasser R.B. A deep exploration of the transcriptome and “excretory/secretory” proteome of adult Fascioloides magna. Mol. Cell. Proteomics. 2012;11:1340–1353. doi: 10.1074/mcp.M112.019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang F., Yang Y., Gao C., Yao Y., Xia R., Hu J., Ran C., Zhang Z., Zhou Z. Bioinformatics analysis and characterization of a secretory cystatin from Thelohanellus kitauei. AMB Express. 2020;10:116. doi: 10.1186/s13568-020-01052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Korytář T., Wiegertjes G.F., Zusková E., Tomanová A., Lisnerová M., Patra S., Sieranski V., Šíma R., Born-Torrijos A., Wentzel A.S., et al. The kinetics of cellular and humoral immune responses of common carp to presporogonic development of the myxozoan Sphaerospora molnari. Parasit. Vectors. 2019;12:208. doi: 10.1186/s13071-019-3462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bjork S.J., Zhang Y.A., Hurst C.N., Alonso-Naveiro M.E., Alexander J.D., Sunyer J.O., Bartholomew J.L. Defenses of susceptible and resistant Chinook salmon (Onchorhynchus tshawytscha) against the myxozoan parasite Ceratomyxa shasta. Fish Shellfish Immun. 2014;37:87–95. doi: 10.1016/j.fsi.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bailey C., Strepparava N., Wahli T., Segner H. Exploring the immune response, tolerance and resistance in proliferative kidney disease of salmonids. Dev. Comp. Immunol. 2019;90:165–175. doi: 10.1016/j.dci.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 88.Piazzon M.C., Estensoro I., Calduch-Giner J.A., del Pozo R., Picard-Sánchez A., Perez-Sánchez J., Sitjà-Bobadilla A. Hints on T cell responses in a fish-parasite model: Enteromyxum leei induces differential expression of T cell signature molecules depending on the organ and the infection status. Parasit. Vectors. 2018;11:443. doi: 10.1186/s13071-018-3007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pérez-Cordón G., Estensoro I., Benedito-Palos L., Calduch-Giner J.A., Sitjà-Bobadilla A., Pérez-Sanchez J. Interleukin gene expression is strongly modulated at the local level in a fish-parasite model. Fish Shellfish Immun. 2014;37:201–208. doi: 10.1016/j.fsi.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 90.Gorgoglione B., Wang T., Secombes C.J., Holland J.W. Immune gene expression profiling of proliferative kidney disease in rainbow trout Oncorhynchus mykiss reveals a dominance of anti-inflammatory, antibody and Th cell-like activities. Fish Shellfish Immun. 2013;34:1654. doi: 10.1016/j.fsi.2013.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sommerset I., Krossoy B., Biering E., Frost P. Vaccines for fish in aquaculture. Expert Rev. Vaccines. 2005;4:89–101. doi: 10.1586/14760584.4.1.89. [DOI] [PubMed] [Google Scholar]
- 92.Sitjà-Bobadilla A., Schmidt-Posthaus H., Wahli T., Holland J.W., Secombes C.J. Fish immune responses to Myxozoa. In: Okamura B., Gruhl A., Bartholomew J.L., editors. Myxozoan Evolution, Ecology and Development. Springer; Cham, Switzerland: 2015. pp. 253–280. [Google Scholar]
- 93.Rogers R.L., Hartl D.L. Chimeric genes as a source of rapid evolution in Drosophila melanogaster. Mol. Biol. Evol. 2012;29:517–529. doi: 10.1093/molbev/msr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rogers R.L., Bedford T., Hartl D.L. Formation and longevity of chimeric and duplicate genes in Drosophila melanogaster. Genetics. 2009;181:313–322. doi: 10.1534/genetics.108.091538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eszterbauer E., Atkinson S., Diamant A., Morris D.J., El-Matbouli M., Hartikainen H. Myxozoan life cycles: Practical approaches and insights. In: Okamura B., Gruhl A., Bartholomew J.L., editors. Myxozoan Evolution, Ecology and Development. Springer; Cham, Switzerland: 2015. pp. 175–198. [Google Scholar]
- 96.Holzer A.S., Bartošová-Sojková P., Born-Torrijos A., Lövy A., Hartigan A., Fiala I. The joint evolution of the Myxozoa and their alternate hosts: A cnidarian recipe for success and vast biodiversity. Mol. Ecol. 2018;27:1651–1666. doi: 10.1111/mec.14558. [DOI] [PubMed] [Google Scholar]
- 97.Melillo D., Marino R., Italiani P., Boraschi D. Innate immune memory in invertebrate metazoans: A critical appraisal. Front. Immunol. 2018;9:1915. doi: 10.3389/fimmu.2018.01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dickinson D.P. Salivary (SD-type) cystatins: Over one billion years in the making-but to what purpose? Crit. Rev. Oral Biol. Med. 2002;13:485–508. doi: 10.1177/154411130201300606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are openly available in Genbank (under the accession Nos. MT127416–MT127426 and MW498387–MW498390) and in the associated supplementary files.