Abstract
Given the drastic changes in our lifestyles and ecosystems worldwide, the potential health effects of natural environments have grown into a highly pervasive topic. Recent scientific findings suggest beneficial effects from nature exposure on human immune responses. This review aims at providing a comprehensive overview of literature published on immunomodulatory effects of nature exposure by inhalation of natural substances. A systematic database search was performed in SCOPUS and PubMed. The quality and potential bias of included studies (n = 33) were assessed by applying the EPHPP (Effective Public Health Practice Project) tool for human studies and the ARRIVE (Animal Research: Reporting of In Vivo Experiments) and SYRCLE (Systematic Review Centre for Laboratory Animal Experimentation) tools for animal studies. The synthesis of reviewed studies points to positive effects of nature exposure on immunological health parameters; such as anti-inflammatory, anti-allergic, anti-asthmatic effects or increased NK (natural killer) cell activity. Decreased expression of pro-inflammatory molecules, infiltration of leukocytes and release of cytotoxic mediators are outcomes that may serve as a baseline for further studies. However, partially weak study designs evoked uncertainties about outcome reproducibility and key questions remain open concerning effect sizes, duration of exposure and contributions of specific vegetation or ecosystem types.
Keywords: BVOCs, forest bathing, green-blue space, human health, immune system, inflammation, inhalation, natural environments, NK cells, terpenes
1. Introduction
During the last century, environmental degradation and urbanisation have caused drastic changes in our lifestyles and living environments [1,2]. Today, more than half of the world’s population live in urban areas [3], and advancements of the digital era have led to a substantial rise in screen time and time spent indoors along with a decline in outdoor activities, especially in the developed world [4]. This has caused a loss of interaction between humans and nature and a progressing feeling of disconnection from the natural world, which can be defined as everything that exists independently of human conduct [5].
The estrangement from nature and other modern lifestyle changes have considerable consequences for human health [6,7]. However, next to being a health resource, the natural environment today also poses substantial risks to human health, not least due to air pollution and contamination of land and water caused by human activity [8]. Toxic pollution ranges among the most prominent environmental health hazards and is responsible for one out of six deaths worldwide [9]; other environmental burdens of disease include exposure to extreme heat, noise, hazardous chemicals, electromagnetic fields and natural disasters [8]. In recent years, negative health effects related to climate change have also been observed [10], especially in urban areas that are particularly at risk of developing urban heat islands (UHI) due to the lack of natural environments. The impacts of the above-mentioned environmental stressors have led to a significant rise of preventable diseases, such as non-communicable diseases (NCDs), which are today the most frequent cause of death worldwide [11]. Globally, more than 20% of all mortalities could be avoided through healthier environments and almost two-thirds of these are related to NCDs [12]. Thus, the relationships between humans, the environment and health are complex and intertwined, and exposure to intact natural environments is connected to better human health on many levels.
A growing body of evidence suggests that various forms of being exposed to nature, such as living close to, frequenting or even looking at environments dominated by living material, are able to provide salutogenic effects on human health [4]. They range from beneficial psychological to physiological outcomes such as attention restoration, improved mood, lowered anxiety and decrease in depressive symptoms, improved cardiovascular, metabolic, oncogenic, respiratory and endocrine function as well as faster healing after surgery and longer life-expectancy [4,13,14,15,16,17,18,19,20]. Often, these benefits are attributed to indirect effects of nature exposure, such as increased physical activity, social interactions, positive mental effects and exposure to sunlight, but recent findings have also highlighted direct physiological mechanisms that are triggered by exposure to natural environments [13,16,17]. This review focuses on direct mechanisms by which nature can affect human health, more specifically on air-borne compounds emitted by natural environments that have the potential to modulate immunological responses when inhaled, such as biogenic volatile organic compounds (BVOCs), terpenes, essential oils, charged ions, pollen, fungi and bacteria.
1.1. Nature Exposure and Immune System Functioning
A limited set of studies have pointed to potential immunological benefits from exposure to natural environments [16,21,22]. By boosting immunological defence mechanisms, natural environments might be able to positively influence immunoregulatory pathways [16]. Immunological defence mechanisms are complex, highly specified and tightly regulated processes that fight foreign pathogens by inducing phagocytosis or apoptosis, producing cytokines or antibodies and releasing inflammatory or cytotoxic mediators [21,23]. During a lifespan, successful immune functioning is shaped by microorganisms we encounter in our environments, from other humans and animals, and is then continuously modified by our diets or medicinal use. By being exposed to a broad variety of organisms, the immune system learns to fine-tune the balance between attack and tolerance mechanisms, and is able to develop the regulatory pathways needed to avoid overshooting immune responses to self or harmless allergens [24,25].
1.2. Immunoregulation through Biodiversity
Natural environments are able to provide biologically and genetically diverse microbial inputs [26]. Enhanced hygiene, smaller family sizes, increased antibiotic use and lower exposure to food bacteria in today’s industrialised parts of the world increase the likelihood of acquiring an unfavourable microbiota prone to overreact to otherwise harmless organisms [24]. There is robust evidence that a limited gut microbial diversity leads to a higher prevalence of chronic inflammatory conditions such as inflammatory bowel diseases or obesity [24,25], and that reduced contact with “old friends” (bacteria and parasites common in the natural environment) increases the risk of developing asthma, allergies or other hypersensitivity diseases [24,27,28].
Advancing urbanisation and fragmentation of habitats along with the increase of immunological non-communicable diseases in developed countries led to the formulation of the biodiversity hypothesis [29]. It is based on the fact that nature is one of the richest sources of microbial input, and that reduced exposure to natural environments and biodiversity may adversely affect our microbiota and its immunomodulatory capacity [24]. The biodiversity encountered in natural environments not only comprises plant, animal, microbial and fungal varieties, but also the genetic variety of those species as well as the variety of ecosystems that serve as their habitats [26,27,29]. Healthy livelihoods depend on such bio-diverse, well-functioning environments being able to provide essential ecosystem services, regulate infectious disease reservoirs and transmission and serve as pool for potential medical treatments, amongst others [26]. Thus, biodiversity loss poses an acute threat to human health.
1.3. Immunoregulation through Inhalation of Air-Borne, Volatile Substances
Next to a diverse microbial input, natural environments are also a rich source of airborne substances such as BVOCs that are emitted by above- and below-ground vegetation, rivers and oceans, soils and other natural structures [30]. BVOCs are produced by terrestrial and marine vegetation and make up approximately two thirds of total volatile organic compounds (VOCs) currently emitted in the atmosphere, with forest ecosystems considered the largest emitters of BVOCs [31]. Since their emission is temperature- and light-sensitive, the amount and type of BVOC emitted varies strongly among species, diurnal and seasonal time points and geographic and climatic regions [30]. Next to methane and dimethyl sulphide (DMS) produced by oceanic plankton, the majority of emitted BVOCs belongs to the class of terpenoids [30].
The accredited anti-inflammatory effects of terpenes include both central and peripheral mechanisms. They encompass the reduction of pro-inflammatory cytokines, modulation of oxidative stress and inhibition of tissue infiltration by inflammatory cells, thereby being able to reduce both acute and chronic inflammatory responses in diverse pathological settings [32,33]. Moreover, terpenes can also exert immune-stimulatory effects such as increasing phagocytic activity, enhancing innate immune responses, repressing the expression of certain pro-inflammatory cytokines and increasing immunoglobulin levels [21]. The anti-tumour effects observed are mainly associated with inducing tumour cell apoptosis, inhibiting their proliferation and preventing metastasis [33]. Many of these effects are mediated by essential immunological cellular components, such as natural killer (NK) cells [33].
Besides terpenes, charged ions that occur in the air close to waterbodies might also have beneficial effects on immune functioning, especially in the respiratory tract [34,35]. Water spray is also a source of microbial input [24], and the inhalation of charged ions, airborne microbes and phytoncides emitted by trees is known to affect systemic immune responses in various ways [20].
By removing airborne pollutants, forest ecosystems are also responsible for health benefits resulting from improved air quality. Air pollution is estimated to cause 6.5 million annual premature deaths worldwide already today [9]. Dry deposition of particulate matter (PM) and absorption of gaseous pollutants by leaf stomata is able to remove up to 4 tons of airborne pollution per square mile and year [36]. This impacts acute and chronic immunological mechanisms by protecting against the development of respiratory diseases and significantly lowers mortality rates in the local population [37]. However, trees can also adversely affect air pollution. BVOCs are highly reactive molecules and can form secondary organic aerosols (SOA) with anthropogenic VOCs, thereby producing ozone [30]. SOAs directly affect the climate by scattering incoming solar radiation and acting as cloud condensation nuclei, thereby significantly changing the planet’s radiative balance and potentially leading to a net cooling effect by increasing cloud albedo [30,38]. This increased cloud cover may locally trap pollutants and lead to adverse health effects [15].
Thus, natural environments do not exclusively have beneficial effects on the immune system, but can sometimes even pose a threat to proper immune functioning. A wide range of microorganisms such as pollen grains, fungal spores, mycelium, algae and bacteria are produced by vegetation, especially grasses, and act as potential allergens and might therefore be harmful by causing or exacerbating allergic reactions [20,36]. A growing number of studies have tried to assess the effects of nature exposure on asthma and allergies; however, the overall outcome of these studies is inconsistent and ranges from positive and negative to no associations [13].
1.4. Health-Promoting Ecosystem Services and Their Effects on the Immune System
In order to understand the relationship between nature and immunological health in detail and to provide a thorough analysis of its long- and short-term co-benefits and potential adverse effects, it is important to consider the services that ecosystems provide either directly or indirectly for humans to sustain their lives and enhance their wellbeing. Many of these ecosystem services are health-supporting; they provide biodiversity and reduce harmful exposures, e.g. to extreme heat or air and water pollution [36]. The multitude and diversity of human health benefits observed from nature suggest a plurality of mechanisms that either stand side by side or interact in one broad pathway of action [16]. The immune system is a key player in maintaining physiological homeostasis and in sustaining health over disease in the human body. Current literature suggests that enhanced immune functioning can be the outcome of a vast majority of observed nature-related health effects [16]. It has therefore been postulated as a promising candidate that may incorporate many different health effects into one central pathway.
The aim of this review is to provide a comprehensive overview of literature published on the immunomodulatory effects on human health following exposure to natural environments. What distinguishes the paper at hand is its focus on inhalation as the only way of taking in the biogenic substances analysed. The goal was to define a baseline of reliable data that can be used as a starting point for future in-depth immunological research, to shed light on consistencies and potential discrepancies and to elucidate knowledge gaps in this field.
In order to establish a holistic perspective and stimulate a broad interdisciplinary research agenda on immunoregulation through nature exposure, different experimental setups were included in this review. Animal experiments represent an important data source that helps create both initial hypotheses as well as elucidate causal pathways through which nature unfolds its various health benefits. Therefore, both human as well as animal experimental studies were evaluated and rated for their scientific quality.
2. Methodology
The methodological approach for the present review followed the guidelines provided by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [39].
2.1. Search Strategy
A structured literature search was carried out in the databases Scopus and PubMed between February and March 2020 and included all articles published up to the search date. The search string was designed to combine different nature-based interventions with different immune-related physiological outcomes (using the Boolean operators AND and OR). A search for article titles including the following keywords was performed:
Nature OR “natural environment” OR forest* OR ecosystem* OR vegetation OR “green infrastructure” OR wood* OR greenness OR greenspace OR outdoor OR biodiversity OR shinrin yoku OR BVOC OR “biogenic volatile organic compound” OR “natural volatile organic compound” OR phytohormon* OR phytoncide* OR “plant gas” OR “essential oil” OR fragrance OR aromatherapy
AND
immun* OR inflamma* OR antiinflamma* OR interleukin* OR cytokin* OR allergen* OR asthma* OR physiologic* OR “NK cell” OR “natural killer“.
When possible, the search was limited to articles and conference papers, and excluded other document types.
The field of immunological health provisioning through nature exposure is multidisciplinary and entails studies with very different methodological approaches which yet have no common narrative, let alone keywords, methodological guidelines or shared objectives. This made it challenging to formulate a fitting keyword search that incorporated all possible wordings and headline formulations into one comprehensive search string and selectively targeted relevant studies in the wide-spanning field. Therefore, we included snowballing as additional search strategy by screening related reviews as well as references of the selected studies, which considerably expanded the results found (see limitations).
2.2. Study Selection
Articles retrieved from the database search were roughly screened according to title and abstract for meeting the eligibility criteria, which were defined as follows:
Analysed species were limited to mammals, and ranged from humans (no age or health status restrictions) to animal studies. In vitro studies on cell lines or primary cell material were excluded. A wide range of different nature exposures was considered in the inclusion criteria, such as all kinds of outdoor nature (urban nature, wilderness, green and blue spaces…), particles and gases released or produced by nature (BVOCs, pollen, fungi, moulds…) or man-made nature products (essential oils, fragrances, aromas, wood panels…). Excluded were foods, roots, traditional medicine, drugs and venoms. Only studies with no or light activities were included, since physical exercise is known to have immunological effects in itself [15,16]. Concerning the route of administration, only inhalation or olfactory stimulations were included. This likewise entailed being intentionally exposed to volatile substances in an experimental setting as well as normal breathing of ambient air while being exposed to natural environments, such as forest bathing or “Shinrin Yoku” (Japanese term for “taking in the forest atmosphere” [33,40]). Furthermore included were exposures to specific housing conditions or residential and recreational stays in nature. All other kinds of exposure (like simply viewing nature from inside) or administration routes (such as oral, topic or parenteral) were beyond the scope of this review and therefore not considered. Furthermore excluded were exposures specific to a certain situation or professional context, such as occupational wood dust exposure of forest workers or wood smoke from forest fires or stoves. Studies involving viral diseases and parasite infestations (e.g., Lyme disease, boreliose infections) were also not part of this review.
To be eligible, studies had to examine physiologic parameters attributable to immune system responses like cellular properties, cytokine and antibody levels, or disease-related outcomes serving as indicators for an immune response, e.g. respiratory symptoms, autoimmune conditions or allergic sensitisations. Due to limited resources, the language of included articles was limited to English and the study type to peer-reviewed intervention studies. None of the investigators were contacted, and no unpublished data was retrieved.
2.3. Quality Assessment
The included set of articles was divided into human and animal studies, and three distinct but specific quality assessment tools were applied. Each study was independently evaluated by two researchers. In case of disagreements, the individual assessments were discussed and a consensus was found between the researchers.
For human studies, the risk of bias was evaluated following the quality assessment tool developed by the Effective Public Health Practice Project (EPHPP) [41]. Based on six parameters (selection bias, study design, confounders, blinding, data collection methods and withdrawals/dropouts), the studies were rated and classified into overall strong (1), moderate (2) or weak (3). Scoring criteria followed the publicly available EPHPP dictionary. Two or more weak parameters classified a study as overall weakly designed. An overall moderate study may only be rated weak in one parameter, while an overall strong study required no weak parameters.
The quality assessment of animal studies was carried out according to the “Animals in Research: Reporting In Vivo Experiments” (ARRIVE) guidelines for good reporting practice in animal research [42]. These guidelines provide 20 questions concerning the quality of design and reporting in animal research and give a useful indication of the adoption of good scientific practise in animal studies. We rated each question with points from 1 (good/reported) to 3 (weak/not reported).
Additionally, we evaluated the risk of bias of each animal study based on the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) tool [43], which was adapted from the Cochrane Collaboration’s Risk of Bias (RoB) tool and developed specifically for the qualitative rating and systematic comparison of experimental animal studies. The SYRCLE tool addresses 10 different domains which are categorised into assessment of selection bias, performance bias, detection bias, attrition bias, reporting bias and other biases. By rating the potential biases of each individual study, we assessed the study to have a low, high or unclear risk of bias.
3. Results
3.1. Article Selection
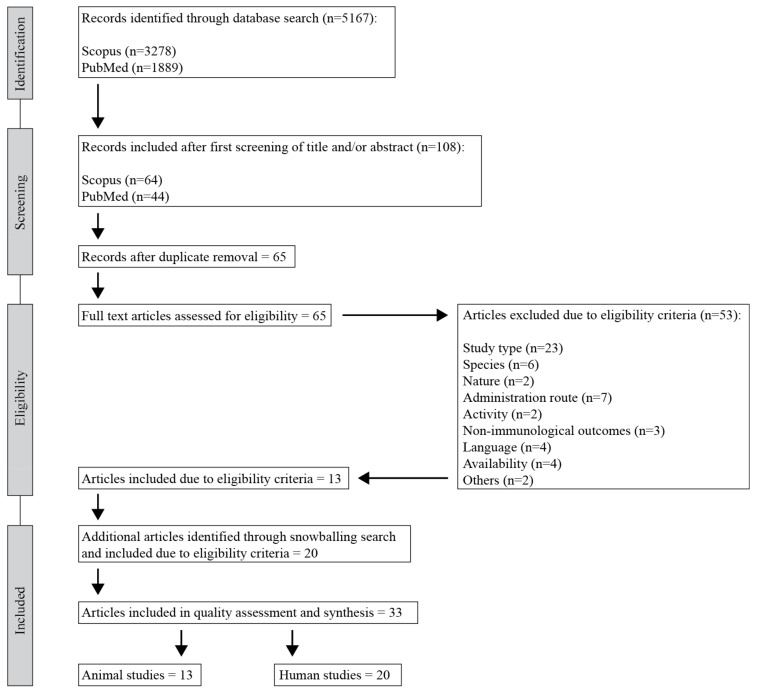
The initial database search returned 5167 records (3278 from Scopus and 1889 from PubMed). After a first screening of titles and abstracts and removal of duplicates, 65 studies remained for full-text analysis. Full-text reading resulted in a total of 13 studies that met all inclusion and exclusion criteria. Additional articles were identified through a snowballing search based on scanning references and reviews; 20 of those met the eligibility criteria and were therefore included in the final selection. Summing up, a total of 33 articles were included in this systematic review, comprising both human (n = 20) and animal (n = 13) intervention studies. Figure 1 illustrates the respective stages in the study selection process.
Figure 1.
Flowchart of study selection process (following PRISMA guidelines).
3.2. Characteristics of Included Studies
An overview of included human and animal studies is given in Table 1 and Table 2 and shows the year of publication, country of study origin, study design, sample size, sample characteristics, age and sex of sample, type of intervention and control and duration of intervention.
Table 1.
Characteristics of included human studies.
| Main Author | Year | Country | Study Design | Sample Size (Intervention/Control) | Sample Characteristics | Sample Age | Sample Sex | Intervention | Control | Duration |
|---|---|---|---|---|---|---|---|---|---|---|
| Forest bathing | ||||||||||
| Han et al. [45] | 2016 | South Korea | Pre-post (2 groups) CCT |
61 (33/28) | Adults with chronic pain | 25–49 | Mixed | Forest bathing (pine, oak maple forest) | Normal daily routine | 2 days |
| Im et al. [44] | 2016 | South Korea | Pre-post (2 groups crossover) RCT |
41 | Healthy students | 18–35 | Mixed | Forest environment (pine tree forest) |
Urban environment | 2 h |
| Jia et al. [46] | 2016 | China | Pre-post (2 groups) CCT |
18 (10/8) | COPD patients | 61–79 | Mixed | Forest bathing | Urban stay | 3 days |
| Kim et al. [52] | 2015 | South Korea | Pre-post (1 group) |
11 | Adults with breast cancer | 25–60 | Female | Forest therapy | / | 14 days |
| Li et al. [53] | 2007 | Japan | Pre-post (1 group) |
12 | Healthy adults (office workers) | 37–55 | Male | Forest bathing | / | 3 days (2–4 h/day) |
| Li et al. [55] | 2008a | Japan | Pre-post (1 group) |
13 | Healthy adults (nurses) | 25–43 | Female | Forest bathing | / | 3 days (2–4 h/day) |
| Li et al. [54] | 2008b | Japan | Pre-post (1 group crossover) |
12 | Healthy adults | 35–56 | Male | Forest bathing | Urban stay | 3 days (2–4 h/day) |
| Lyu et al. [47] | 2019 | China | Pre-post (2 groups) CCT |
60 (45/15) | Healthy adults | 19–24 | Male | Forest bathing (bamboo forest) | Urban stay | 3 days |
| Mao et al. [50] | 2012a | China | Pre-post (2 groups) CCT |
20 (10/10) | Healthy students | 20–21 | Male | Forest bathing (broad-leaved forest) | Urban stay | 2 days |
| Mao et al. [51] | 2012b | China | Pre-post (2 groups) CCT |
24 (12/12) | Elderly patients with hypertension | 60–75 | Mixed | Forest bathing (broad-leaved forest) | Urban stay | 7 days |
| Mao et al. [49] | 2017 | China | Pre-post (2 groups) CCT |
33 (23/10) | Elderly patients with chronic heart failure | 66–79 | Mixed | Forest bathing (broad-leaved forest) | Urban stay | 4 days |
| Mao et al. [48] | 2018 | China | Pre-post (2 groups) CCT |
20 (10/10) | Elderly patients with chronic heart failure | 66–79 | Mixed | Second forest bathing trip (broad-leaved forest) after 4 weeks break | Urban stay after previous forest bathing trip 4 weeks ago | 4 days |
| Seo et al. [56] | 2015 | South Korea | Pre-post (1 group) |
21 | Children with asthma | 7–12 | Mixed | Forest bathing (fir tree forest) |
/ | 4 days |
| Seo et al. [56] | 2015 | South Korea | Pre-post (1 group) |
27 | Children with atopic dermatitis | 7–12 | Mixed | Forest bathing (fir tree forest) |
/ | 4 days |
| Tsao et al. [57] | 2018 | Taiwan | Retrospective study (pre-post 2 groups) |
200 (90/110) | Healthy adults | 34–56 | Mixed | Forest workers | Urban residents | 1 year |
| Tsao et al. [57] | 2018 | Taiwan | Pre-post (1 group) |
11 | Healthy adults | / | / | Forest bathing (coniferous forest) | / | 5 days |
| BVOC inhalation | ||||||||||
| Li et al. [58] | 2009 | Japan | Pre-post (1 group) |
12 | Healthy adults | 37–60 | Male | Inhalation of phytoncides (vaporized hinoki cypress stem oil) in urban hotel room | / | 3 days |
| Fragrance inhalation | ||||||||||
| Kiecolt-Glaser et al. [59] | 2008 | Ohio, USA | Pre-post (1 group crossover) |
56 | Healthy adults | 18–43 | Mixed | Inhalation of fragrances (lavender, lemon) | Inhalation of water vapour | 1.25 h |
| Komori et al. [60] | 1995 | Japan | Pre-post (2 groups) |
20 (12/8) | Adults with depression | 26–53 | Male | Inhalation of citrus fragrance mix (limonene, citral, other EOs) | only positive control group | 4–11 weeks |
| Trellakis et al. [61] | 2012 | Germany | Pre-post (1 group crossover) |
32 | Healthy adults | 20–45 | Mixed | Inhalation of stimulant fragrances (grapefruit, fennel, pepper) | No fragrance exposure | 3 days (30 min/day) |
| Inhalation of relaxant fragrances (lavender, patchouli, rose) | ||||||||||
| Waterfall exposure | ||||||||||
| Gaisberger et al. [35] | 2012 | Austria | Pre-post (2 groups) CCT |
54 (27/27) | Children with allergic asthma | 8–15 | Mixed | Waterfall exposure (WF+) in national park | No waterfall exposure (WF-) in national park | 3 weeks (1 h/day) |
| Grafetstätter et al. [34] | 2017 | Austria | Pre-post (3 groups) CCT |
91 (33/32/26) | Adults with stress (Pre-treated with oral cholera vaccination) |
19–61 | Mixed | Hiking in national park with waterfall exposure (WF+) | Hiking in national park without waterfall exposure (WF-) | 1 week (1 h/day) |
Table 2.
Characteristics of included animal studies.
| Main Author | Year | Country | Study Design | Sample Size (Number per Group) | Sample Characteristics | Sample Age | Sample Sex | Intervention | Control | Duration |
|---|---|---|---|---|---|---|---|---|---|---|
| BVOC inhalation | ||||||||||
| Ahn et al. [62] | 2018a | South Korea |
Animal | 35 (7) | Mice Pre-treated with LPS |
7 weeks | Male | Housing with BVOC wood panels (C. obtusa, P. densiflora) LPS |
Housing without wood panels LPS |
4 weeks |
| Ahn et al. [63] | 2018b | South Korea |
Animal | 49 (7) | Mice Pre-treated with OVA |
5 weeks | / | Housing with BVOC wood panels (C. obtusa, P. densiflora, P. koraiensis, L.kaempferi) OVA |
Housing without wood panels OVA |
27 days |
| Yang et al. [64] | 2015 | South Korea |
Animal | / | Mice Dinitrochlorbenzene (DNCB)-induced atopic dermatitis (AD)-like disease model |
7 weeks | / | Exposure to BVOC (C. obtusa) | Exposure to vehicle | 8 weeks |
| Eucalyptol inhalation | ||||||||||
| Bastos et al. [74] | 2011 | Brazil | Animal | ca. 35 (7–10) |
Guineau pigs Pre-treated with OVA |
/ | Male | Eucalyptol (1,8-cineol) inhalation OVA |
Saline inhalation OVA |
15 min |
| Kennedy-Feitosa et al. [65] | 2019 | Brazil | Animal | 40 (10) | Mice Pre-exposed to cigarette smoke (CS) |
Male | Eucalyptol (1,8-cineol) inhalation CS |
Vehicle inhalation CS |
120 days (15 min/day) |
|
| Lee et al. [66] | 2016 | South Korea |
Animal | / | Mice Pre-sensitised to Der p (house dust mite allergen; HDM) |
6 weeks | Female | Eucalyptol (1,8-cineol) inhalation Der p |
Vehicle inhalation Der p |
/ |
| Limonene inhalation | ||||||||||
| Bibi et al. [67] | 2015 | Israel | Animal | 30 (10) | Mice Pre-treated with OVA |
8 weeks | Female | Housing with Limonene-treated wood bedding OVA |
Housing with untreated wood bedding OVA |
30 days |
| Hirota et al. [68] | 2012 | Japan | Animal | 30 (10) | Mice Pre-sensitised to Der f (house dust mite allergen; HDM) |
6 weeks | Male | Limonene inhalation Der f |
No inhalation Der f |
31 days |
| Keinan et al. [73] | 2005 | Israel | Animal | 40 (10) | Rats Pre-treated with OVA |
4 weeks | / | Limonene inhalation (ozone scavenger) Eucalyptol inhalation (inert to ozone) OVA |
No inhalation OVA |
1 week |
| Limonene/ozone inhalation | ||||||||||
| Hansen et al. [69] | 2013 | Denmark | Animal | ca. 40 (9–10) | Mice Pre-treated with OVA |
5-6 weeks | Female | Limonene inhalation Limonene + ozone inhalation OVA |
No inhalation Ozone inhalation OVA |
14 weeks |
| Hansen et al. [70] | 2016 | Denmark | Animal | 40 (10) | Mice Pre-treated with OVA |
6 weeks | Female | Limonene inhalation | Air inhalation | 3 days (60 min/day) |
| Linalool inhalation | ||||||||||
| Naka-mura et al. [72] | 2009 | Japan | Animal | 12 (4) | Rats Stressed by restraining in tube |
7–8 weeks | Male | Linalool inhalation Stress |
No inhalation Stress |
2 h |
| Fragrance inhalation | ||||||||||
| Fujiwara et al. [71] | 1998 | Japan | Animal | 84 (12) | Mice Stressed with high pressure |
8–10 weeks | Male | Fragrance exposure (lemon, oak moss, labdanum, tuberose) Stress |
No fragrance exposure Stress |
24 h |
3.2.1. Characteristics of Human Studies
Among the included human studies, only one study was designed as a randomised controlled trial (RCT) [44] and nine studies were controlled clinical trials (CCT) [34,35,45,46,47,48,49,50,51]. The rest were designed as one group pre-post intervention studies [52,53,54,55,56,57,58,59,60,61], three of which were carried out as one group crossover studies [55,59,61]. Sample sizes ranged between 11 and 200 subjects, most studies investigating 10–20 individuals per group. Participant characteristics ranged from healthy individuals to individuals suffering from diverse chronic conditions, and comprised children, adults and elderly people from both genders aged seven to 79 years. The types of interventions could roughly be divided into three experimental setups: forest bathing, experimental inhalation of BVOCs or fragrances and exposure to waterfalls. Forest bathing interventions and waterfall exposures were examined in 14 [44,45,46,47,48,49,50,52,53,54,55,56,57] and two [34,35] studies, respectively. Four studies analysed the effects of BVOC [58] or fragrance [59,60,61] inhalation on human subjects (Table 1).
3.2.2. Characteristics of Animal Studies
The majority of included animal studies were carried out in mice [62,63,64,65,66,67,68,69,70,71] (10 out of 13), while two studies used rats [72,73] and one study worked with guinea pigs [74]. All studies used a pre-treatment such as lipopolysaccharide (LPS), ovalbumin (OVA), Der p (Dermatophagoides pteronyssinus), Der f (Dermatophagoides farina) or other to experimentally induce an immune reaction which served as control condition for the actual intervention. Sample sizes ranged between 12 and 84 animals; most studies analysed 10 animals per group. Animals were between four and 10 weeks of age and comprised both genders. The types of interventions could be divided into the following experimental setups: inhalation of BVOCs [62,63,64], eucalyptol [65,66,74], limonene [67,68,73], mix of limonene/ozone [69,70], linalool [72] and other fragrances (lemon, oak moss, labdanum and tuberose) [71]. Three studies evaluated the effects of different housing conditions in laboratory animal cages equipped with different wood beddings [62,63,67] (Table 2).
3.3. Quality Assessment
3.3.1. Quality Assessment of Human Studies
For methodological quality assessment, we subjected all included human studies to a risk of bias assessment following the EPHPP quality assessment tool [41].
According to the EPHPP tool, one study was rated overall strong [61], four studies as overall moderate [48,49,52,56] and 15 studies got a weak overall score [34,35,44,45,46,47,50,51,52,53,54,55,57,58,59,60] (Table 3). The weak ratings were mainly due to lack of information on recruitment procedures, the use of self-referred or non-representative samples (selection bias) and missing information on blinding. Blinding was neither described for study assessors nor for participating subjects, the latter being hardly applicable in multi-day forest bathing studies where the exposure to environmental surroundings is obvious. In order to more representatively evaluate the methodological quality of included studies, we therefore chose to also provide an alternative overall score excluding the “blinding” parameter. This resulted in five studies being rated as overall strong, nine as moderate and six as weak (Table 3, last column). More information on scoring criteria is given in the Supplementary Material.
Table 3.
Quality assessment of human intervention studies following the EPHPP tool.
| Overall Score | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection Bias | Study Design | Con-founders | Blinding | Data Collection | Dropouts | Without Blinding * | |||
| Gaisberger et al. | 2012 | 2 | 1 | 1 | 3 | 1 | 3 | 3 | 2 |
| Grafetstätter et al. | 2017 | 3 | 1 | 2 | 3 | 1 | 3 | 3 | 3 |
| Han et al. | 2016 | 2 | 1 | 1 | 3 | 1 | 3 | 3 | 2 |
| Im et al. | 2016 | 2 | 1 | 1 | 3 | 1 | 3 | 3 | 2 |
| Jia et al. | 2016 | 3 | 1 | 1 | 3 | 1 | 3 | 3 | 3 |
| Kiecolt-Glaser et al. | 2008 | 2 | 2 | 3 | 1 | 3 | 1 | 3 | 3 |
| Kim et al. | 2015 | 2 | 2 | NA | 3 | 1 | 1 | 2 | 1 |
| Komori et al. | 1995 | 3 | 2 | 2 | 3 | 1 | 3 | 3 | 3 |
| Li et al. | 2007 | 3 | 2 | NA | 3 | 1 | 2 | 3 | 2 |
| Li et al. | 2008a | 3 | 2 | NA | 3 | 1 | 3 | 3 | 3 |
| Li et al. | 2008b | 3 | 2 | NA | 3 | 1 | 2 | 3 | 2 |
| Li et al. | 2009 | 3 | 2 | NA | 3 | 1 | 2 | 3 | 2 |
| Lyu et al. | 2019 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 2 |
| Mao et al. | 2012a | 3 | 1 | 1 | 3 | 1 | 2 | 3 | 2 |
| Mao et al. | 2012b | 3 | 1 | 1 | 3 | 1 | 2 | 3 | 2 |
| Mao et al. | 2017 | 2 | 1 | 1 | 3 | 1 | 1 | 2 | 1 |
| Mao et al. | 2018 | 2 | 1 | 1 | 3 | 1 | 1 | 2 | 1 |
| Seo et al. | 2015 | 2 | 2 | NA | 3 | 1 | 2 | 2 | 1 |
| Trellakis et al. | 2012 | 2 | 2 | NA | 2 | 1 | 1 | 1 | 1 |
| Tsao et al. | 2018 | 3 | 2 | 1 | 3 | 1 | 3 | 3 | 3 |
1 = strong (green), 2 = moderate (yellow), 3 = weak (red), NA = not applicable (one-group studies). An alternative overall score excludes the “blinding” category (*).
3.3.2. Quality Assessment of Animal Studies
All animal studies were evaluated following two standardised quality assessment guidelines: ARRIVE assessment tool [42] and SYRCLE’s risk of bias tool for animal studies [43]. The results from the ARRIVE assessment, entailing 20 categories, are summarised in Table 4. Specific information on assessment criteria for individual ratings are provided in the Supplementary Material.
Table 4.
Quality assessment of animal intervention studies following the ARRIVE guidelines.
| (a) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Title | Abstract | Introduction | Methods | ||||||||
| Background | Objectives and Hypotheses | Ethical Statement | Study Design (Number of Experimental Groups, Blinding, Experimental Unit) | Experimental Procedure | Animal Details (Species, Sex, Age, Source, Weight) | Housing and Husbandry Conditions | Sample size Calculation (Number per Group, Number of Independent Replicates) | ||||
| Ahn et al. | 2018a | 2 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 2 |
| Ahn et al. | 2018b | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 1 | 2 |
| Bastos et al. | 2011 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 3 | 3 |
| Bibi et al. | 2015 | 1 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 1 | 2 |
| Fujiwara et al. | 1998 | 1 | 1 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 1 |
| Hansen et al. | 2013 | 2 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 2 |
| Hansen et al. | 2016 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| Hirota et al. | 2012 | 1 | 2 | 2 | 3 | 2 | 1 | 1 | 1 | 1 | 2 |
| Keinan et al. | 2005 | 1 | 1 | 1 | 1 | 2 | 3 | 1 | 3 | 3 | 3 |
| Kennedy-Feitosa et al. | 2019 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 2 |
| Lee et al. | 2016 | 2 | 1 | 1 | 1 | 2 | 3 | 1 | 1 | 3 | 3 |
| Nakamura et al. | 2009 | 2 | 2 | 2 | 3 | 3 | 2 | 1 | 2 | 1 | 2 |
| Yang et al. | 2015 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 2 | 1 | 3 |
| (b) | |||||||||||
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | ||
| Methods | Results | Discussion | |||||||||
| Allocation to Experimen-tal Groups (Randomi-sation, Matching) | Experi-mental Outcomes | Statistical Analysis | Baseline Data Monitoring | Number of Animals Analysed | Outcome Report and Estimation (Individual Values, Standard Deviation) | Adverse Events | Interpreta-tion and Limitations | Generalis-ability and Translational Relevance | Funding | ||
| Ahn et al. | 2018a | 3 | 1 | 1 | 3 | 3 | 2 | 3 | 2 | 2 | 1 |
| Ahn et al. | 2018b | 3 | 1 | 1 | 3 | 3 | 2 | 3 | 2 | 2 | 3 |
| Bastos et al. | 2011 | 3 | 1 | 1 | 3 | 3 | 2 | 3 | 2 | 2 | 2 |
| Bibi et al. | 2015 | 3 | 1 | 1 | 3 | 1 | 2 | 3 | 2 | 1 | 1 |
| Fujiwara et al. | 1998 | 3 | 3 | 2 | 3 | 3 | 2 | 3 | 2 | 2 | 3 |
| Hansen et al. | 2013 | 3 | 1 | 1 | 1 | 2 | 1 | 3 | 1 | 1 | 1 |
| Hansen et al. | 2016 | 2 | 1 | 1 | 1 | 1 | 2 | 3 | 1 | 1 | 1 |
| Hirota et al. | 2012 | 2 | 1 | 1 | 3 | 1 | 2 | 3 | 2 | 2 | 2 |
| Keinan et al. | 2005 | 3 | 2 | 2 | 3 | 3 | 2 | 3 | 3 | 2 | 3 |
| Kennedy-Feitosa et al. | 2019 | 3 | 1 | 1 | 3 | 1 | 2 | 3 | 1 | 1 | 1 |
| Lee et al. | 2016 | 3 | 1 | 1 | 3 | 3 | 2 | 3 | 2 | 2 | 1 |
| Nakamura et al. | 2009 | 3 | 1 | 1 | 3 | 1 | 2 | 3 | 2 | 2 | 3 |
| Yang et al. | 2015 | 3 | 1 | 1 | 3 | 3 | 2 | 3 | 2 | 2 | 1 |
1 = reported/strong (green), 2 = moderate (yellow), 3 = not reported/weak (red).
Most studies provided a good to moderate title, abstract and introduction section. In the methods section, most studies met the criteria concerning the description of experimental procedures, animal details and housing and husbandry conditions (categories 7–9), while no study appropriately provided a calculation of sample sizes (category 10) or described the method of allocation to experimental groups (category 11). Almost all studies provided sufficient information regarding the experimental outcomes (category 12) and included a description of the statistical analysis of the results (category 13).
In the results section, we detected more reporting shortcomings than in the other categories. A failure in reporting group-specific baseline data of experimental animal characteristics (category 14) as well as reporting adverse events (category 17) was asserted in almost all studies. The majority of studies failed to monitor and report specific baseline characteristics such as body weight. Moreover, many studies did not report any information on the numbers of animals included in the final analysis (category 15), nor refer to drop out rates or reasons for exclusions. All studies included a measure of precision (e.g. bars representing standard deviations) in their outcome report (category 16), but only one study [69] also provided data on the number of individual data points within one group, which should be considered the gold standard of outcome reporting.
Regarding the discussion section, most studies interpreted the implications of their findings within the current scientific literature; however many failed to elaborate on study limitations (category 18) and generalisability of study outcomes (category 19), i.e. translation of outcomes into the human system.
A risk of bias assessment following the SYRCLE’s risk of bias (RoB) tool for animal studies [43] was conducted to complement the ARRIVE quality assessment. According to the SYRCLE RoB tool, many studies did not adequately report measures taken to reduce potential risk of biases in several categories. Randomisation and blinding of the experimental setup were rarely reported, and no study had performed a preceding calculation of sample sizes. All results of the SYRCLE risk of bias assessment are provided in the Supplementary Material (Table S1).
3.4. Outcomes and Synthesis
3.4.1. Outcomes of Human Studies
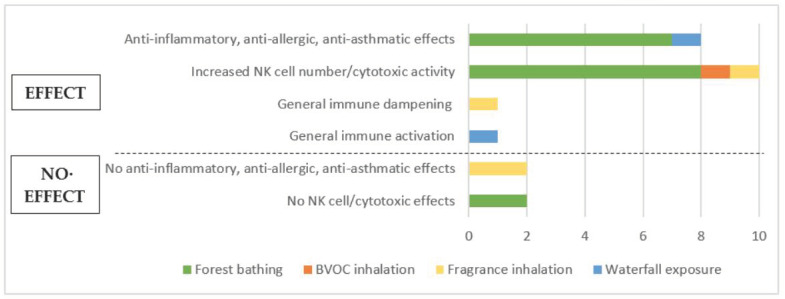
The majority of studies measured either anti-inflammatory or cytotoxic effects following nature exposure. Experimental parameters analysed were mainly expression of pro- or anti-inflammatory cytokines (mostly IL-6 and TNFα) in serum, numbers and percentages of immune cell subsets (mostly NK cells and T cells) and expression of cytotoxic mediators (perforin, granzyme A/B and granulysin) as well as cytotoxic NK cell activity. Some studies examined several outcomes. Overall, a positive effect was observed on most immunological parameters measured; four studies showed no significant changes. An overview of outcomes measured in studies with human subjects is presented in Figure 2.
Figure 2.
Overview of outcomes from human studies.
Most studies with human subjects examined the effects of forest bathing trips of varying lengths on different immunological parameters. Seven (out of 14) forest bathing studies analysed inflammatory cytokine expression and unanimously reported either a decrease in pro-inflammatory and/or an increase in anti-inflammatory cytokine levels [44,46,48,49,50,51,56], implicating anti-allergic or anti-asthmatic outcomes from being exposed to forest environments. One of the studies also evaluated changes in clinical scores of patients with asthma and atopic dermatitis after a forest trip and concluded a relief of clinical symptoms and beneficial effects of forest bathing on spirometric outcomes [56].
Ten (out of 14) forest bathing studies elucidated the effects on the distribution of immune cell subsets as well as on their distinct effector activities, with a special focus on NK cells. The majority of these studies (8 out of 10) reported an increase either in NK cell number or in NK cell activity [45,47,52,53,54,55,57], which was measured either directly by flow cytometry or indirectly by assessing the level of cytotoxic mediators circulating in the blood. Two forest bathing studies did not observe any significant changes in NK cell outcomes [46,50]. One additional study reported an increase in NK cell number and activity after the isolated inhalation of phytoncides under laboratory conditions [58], concluding that volatile substances released by plants are responsible for the effects observed in natural environments.
Three studies examined the outcomes of inhaling different fragrances (citrus mix, lavender, lemon, grapefruit, fennel, pepper, patchouli and rose) on immunological parameters such as cytokine and chemokine levels, cell ratios and strength of immune response to infection. Two of these studies reported no significant effect of fragrance exposure on levels of circulating cytokines and chemokines [59,61], while one of them observed a lower hypersensitivity to candida infection compared to control [59]. Another study reported beneficial effects on immune cell ratios after fragrance exposure [60].
Two studies looked at potential health effects of charged ions in ambient air in the vicinity of waterfalls. One of them detected significantly decreased pro-inflammatory cytokine levels combined with enhanced lung function and reduced clinical symptoms in children with allergic asthma, which was suggested to be due to an induction of circulating regulatory T cells [35]. The other study reported that exposure to waterfalls led to an activated immune system and also improved lung function [34].
Table 5 provides a comprehensive summary of all outcomes from human studies.
Table 5.
Outcomes of human studies. Significances are given with p < 0.05, p < 0.01 and p < 0.001; NS = not significant, NA = not applicable. A non-significant trend is described as “Decrease/Increase, NS”. If significances are not given, it is described as “Decrease/Increase, NA”.
| Outcome | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main Author |
Year | Title | Measure | Intervention (Compared to Baseline) | p-Value | Control (Compared to Baseline) | p-Value | Comparison (Intervention Compared to Control) | p-Value | Conclusion | Comment | |
| Forest bathing | ||||||||||||
| Han et al. [45] | 2016 | The effects of forest therapy on coping with chronic widespread pain | NK cell activity | Increase | p < 0.001 | NS | Increase | NA | NK cell activity increases after a forest bathing trip in adults with chronic pain. | Significant baseline differences between control and intervention group in NK cell activity. | ||
| Im et al. [44] | 2016 | Comparison of effect of two-hour exposure to forest and urban environments on cytokine, anti-oxidant and stress levels in young adults | IL-6 level in serum | NS | Pro-inflammatory cytokine level (IL-8 and TNFα, but not IL-6) is reduced in healthy students after a forest bathing trip. | No pre-intervention data shown. | ||||||
| IL-8 level in serum | Decrease | p < 0.001 | ||||||||||
| TNFα level in serum | Decrease | p < 0.001 | ||||||||||
| Glutathione peroxidase (GPx) level in serum | Increase | p < 0.05 | ||||||||||
| Jia et al. [46] |
2016 | Health effects of forest bathing trip on elderly patients with chronic obstructive pulmonary disease | CD8+ T cell number and % | NS | NS | NS | Forest bathing reduces pro-inflammatory cytokine levels, but not proportions of CD8+ T, NK or NKT cells or GrB expression in COPD patients. | Result on perforin expression is questionable, since there is also an effect in control group. | ||||
| NK cell number and % (CD3-CD56+) | NS | NS | NS | |||||||||
| NKT cell number and % (CD3+CD56+) | NS | NS | NS | |||||||||
| Perforin expression in CD8+ T cells (flow cytometry) |
Decrease | p < 0.001 | Decrease | NA | Decrease | p < 0.01 | ||||||
| Perforin expression in NK cells (flow cytometry) |
Decrease | p < 0.001 | Decrease | NA | NS | |||||||
| Perforin expression in NKT cells (flow cytometry) |
Decrease | p < 0.001 | Decrease | NA | Decrease | p < 0.05 | ||||||
| Granzyme B expression in CD8+ T cells (flow cytometry) |
NS | NS | NS | |||||||||
| Granzyme B expression in NK cells (flow cytometry) |
NS | NS | NS | |||||||||
| Granzyme B expression in NKT cells (flow cytometry) |
NS | NS | NS | |||||||||
| IL-6 level in serum | Decrease | p < 0.01 | NS | Decrease | p < 0.05 | |||||||
| IL-8 level in serum | Decrease | p < 0.05 | NS | Decrease | p < 0.01 | |||||||
| IFN-y level in serum | Decrease | p < 0.01 | NS | Decrease | p < 0.05 | |||||||
| IL-1b level in serum | NS | NS | Decrease | p < 0.05 | ||||||||
| CRP level in serum | NS | NS | Decrease | p < 0.05 | ||||||||
| TNFα level in serum | NS | NS | NS | |||||||||
| Kim et al. [52] | 2015 | Forest adjuvant anti-cancer therapy to enhance natural cytotoxicity in urban women with breast cancer: A preliminary prospective interventional study | NK cell number (CD3-CD56+) | Increase | p < 0.01 | Forest therapy enhances natural cytotoxicity in breast cancer patients by increasing NK cells and cytotoxic mediators. | ||||||
| Perforin level in serum (ELISA) | Increase | p < 0.02 | ||||||||||
| Granzyme B level in serum (ELISA) | Increase | p < 0.02 | ||||||||||
| Li et al. [53] | 2007 | Forest bathing enhances human natural killer activity and expression of anti-cancer proteins | NK cell number and % (CD16+) | Increase | p < 0.01 | Forest bathing enhances NK cell activity and numbers in healthy male adults. | ||||||
| Cytolytic NK cell activity (Cr-release assay) | Increase | p < 0.01 | ||||||||||
| % of T cells (CD3+) | Decrease | NA | ||||||||||
| % of perforin- expressing cells |
Increase | p < 0.01 | ||||||||||
| % of granzyme A/B- expressing cells |
Increase | p < 0.01 | ||||||||||
| % of granulysin-expressing cells | Increase | p < 0.01 | ||||||||||
| Li et al. [55] | 2008a | A forest bathing trip increases human natural killer activity and expression of anti-cancer proteins in female subjects | NK cell number and % (CD16+) | Increase | p < 0.01 | Forest bathing enhances NK cell activity and numbers in healthy female adults. | ||||||
| Cytolytic NK cell activity (Cr-release assay) | Increase | p < 0.01 | ||||||||||
| % of T cells (CD3+) | Decrease | p < 0.05 | ||||||||||
| % of perforin- expressing cells |
Increase | p < 0.01 | ||||||||||
| % of granzyme A/B- expressing cells |
Increase | p < 0.01 | ||||||||||
| % of granulysin- expressing cells |
Increase | p < 0.01 | ||||||||||
| Li et al. [54] | 2008b | Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins | NK cell number and % (CD16+) | Increase | p < 0.01 | NS | Increase | p < 0.05 | Forest bathing enhances NK cell activity and numbers in healthy adults. | |||
| Cytolytic NK cell activity (Cr-release assay) | Increase | p < 0.01 | NS | Increase | p < 0.05 | |||||||
| % of T cells (CD3+) | NS | NS | ||||||||||
| % of perforin- expressing cells |
Increase | p < 0.01 | NS | |||||||||
| % of granzyme A/B- expressing cells |
Increase | p < 0.01 | NS | |||||||||
| % of granulysin- expressing cells |
Increase | p < 0.01 | NS | |||||||||
| Lyu et al. [47] | 2019 | Benefits of a three-day bamboo forest therapy session on the psychophysiology and immune system responses of male college students | Cytolytic NK cell activity |
Increase | p < 0.05 | NS | NA | Forest bathing in a bamboo forest enhances NK cell activity and percentages in healthy adults. | ||||
| % of NK cells (CD16+CD56+) | Increase | p < 0.05 | NS | NA | ||||||||
| Perforin level (ELISA) | Increase | p < 0.05 | NS | NA | ||||||||
| Granulysin level (ELISA) | NS | NS | NA | |||||||||
| Granzyme A/B level (ELISA) | Increase | p < 0.05 | NS | NA | ||||||||
| Mao et al. [50] | 2012a | Effects of short-term forest bathing on human health in a broad-leaved evergreen forest in Zhejiang Province, China | IL-6 level in serum (radioimmunoassay) |
NS | Increase | NA | Decrease | p < 0.05 | Forest bathing decreases pro-inflammatory cytokine levels (IL-6 and TNFα) in healthy young adults but has no effect on immune cell distribution. | Questionable effect (increase) in control group compared to baseline for IL-6 and TNFα levels. | ||
| TNFα level in serum (radioimmunoassay) | Decrease | NA | Increase | NA | Decrease | p < 0.05 | ||||||
| HCRP level in serum | NS | |||||||||||
| % of B cells (CD5+CD19+) | Increase | p < 0.05 | No pre-intervention data shown for leukocyte distributions. | |||||||||
| % of T cells (CD3+) | NS | |||||||||||
| % of Th cells (CD3+CD4+) | NS | |||||||||||
| % of cytotoxic T cells (CD3+CD8+) | NS | |||||||||||
| % of NK cells (CD3-CD16+CD56+) |
NS | |||||||||||
| Mao et al. [51] | 2012b | Therapeutic effect of forest bathing on human hypertension in the elderly | IL-6 level in serum (radioimmunoassay) |
Decrease | p < 0.05 | NS | NS | Forest bathing decreases pro-inflammatory cytokine level (IL-6, but not TNFα) in elderly patients with hypertension. | ||||
| TNFα level in serum (radioimmunoassay) | NS | NS | NS | |||||||||
| Mao et al. [49] | 2017 | The salutary influence of forest bathing on elderly patients with chronic heart failure | IL-6 level in serum (ELISA) | NS | NS | Decrease | p < 0.05 | Forest bathing decreases pro-inflammatory cytokine level (IL-6, but not TNFα) in elderly patients with chronic heart failure. | No effect in intervention group compared to baseline. | |||
| TNFα level in serum (ELISA) | NS | NS | NS | |||||||||
| HCRP level in serum | NS | NS | NS | |||||||||
| Mao et al. [48] | 2018 | Additive benefits of twice forest bathing trips in elderly patients with chronic heart failure | IL-6 level in serum (ELISA) | NS | NS | NS | A second forest bathing trip further decreases pro-inflammatory cytokine level (TNFα, but not IL-6) in elderly patients with chronic heart failure. | |||||
| TNFα level in serum (ELISA) | Decrease | p < 0.05 | NS | Decrease | p < 0.05 | |||||||
| Seo et al. [56] | 2015 | Clinical and immunological effects of a forest trip in children with asthma and atopic dermatitis | Asthma: | Forest environment improves clinical symptoms in asthmatic children. | ||||||||
| Forced vital capacity (spirometry, FCV) |
Increase | p < 0.05 | ||||||||||
| Fractional exhaled nitric oxide (FeNO) | Decrease | NA | ||||||||||
| Seo et al. [56] | 2015 | Clinical and immunological effects of a forest trip in children with asthma and atopic dermatitis | Atopic dermatitis: | Forest environment improves clinical symptoms and has immunological effects in chronic allergic skin disease. | ||||||||
| Atopic dermatitis index (SCORAD) | Decrease | NA | ||||||||||
| Thymus and activation-regulated chemokine/CCL17 level | NS | |||||||||||
| Macrophage-derived chemokine/CCL22 level | Decrease | p < 0.01 | ||||||||||
| Tsao et al. (forest workers) [57] | 2018 | Health effects of a forest environment on natural killer cells in humans: an observational pilot study | % of NK cells in blood (CD3-CD56+) |
Increase | p < 0.05 | Living in a forest environment increases NK cell percentage, but not the amount of activated NK cells. | ||||||
| % of activated NK cells in blood (CD3-CD56+CD69+) | NS | |||||||||||
| Tsao et al. (forest bathing) [57] | 2018 | Health effects of a forest environment on natural killer cells in humans: an observational pilot study | % of NK cells in blood on d5 | NS | Short-term forest trip enhances fraction of activated NK cells in healthy adults, and effect lasts for at least 4 days. | |||||||
| % of activated NK cells in blood on d5 | Increase | p < 0.01 | ||||||||||
| % of NK cells in blood on d9 (4 days after intervention) | NS | |||||||||||
| % of activated NK cells in blood on d9 (4 days after intervention) | Increase | p < 0.01 | ||||||||||
| BVOC inhalation | ||||||||||||
| Li et al. [58] | 2009 | Effect of phytoncide from trees on human natural killer cell function | Cytolytic NK cell activity (Cr-release assay) | Increase | p < 0.05 | Phytoncide exposure enhances NK cell activity and % in healthy adults. | ||||||
| % of NK cells (CD16+) | Increase | p < 0.01 | ||||||||||
| % of T cells (CD3+) | Decrease | p < 0.01 | ||||||||||
| % of perforin- expressing cells |
Increase | p < 0.05 | ||||||||||
| % of granzyme A/B- expressing cells |
Increase |
p < 0.01 p < 0.05 |
||||||||||
| % of granulysin- expressing cells |
Increase | p < 0.05 | ||||||||||
| Fragrance inhalation | ||||||||||||
| Kiecolt-Glaser et al. [59] | 2008 | Olfactory influences on mood and autonomic, endocrine and immune function | Delayed hypersensitivity to candida (DTH) | Increase | NA | Increase | NA | Decrease | p = 0.02 (lavender) p = 0.06 (lemon) | Greater DTH response after water inhalation indicates better immune response than in fragrance groups, but no difference in cytokine levels detectable. | * Differing effects in blinded and informed groups for blastogenesis responses. | |
| PBL proliferation (blastogenesis) |
NA* | |||||||||||
| IL-6 level in PBLs (ELISA) | NS | |||||||||||
| IL-10 level in PBLs (ELISA) | NS | |||||||||||
| Komori et al. [60] | 1995 | Effects of citrus fragrance on immune function and depressive states | Deviation from normal CD4/CD8 ratio | Decrease | NA | Citrus fragrance has a beneficial effect on immune cell distribution in depressive patients and can reduce the dose antidepressants needed. | ||||||
| Deviation from normal NK cell activity (Cr-release assay) |
Decrease | NA | ||||||||||
| Trellakis et al. [61] | 2012 | Subconscious olfactory influences of stimulant and relaxant odors on immune function | IL-8 level in serum (ELISA) | NS | No significant effect of any stimulatory or relaxing fragrance exposure on immune parameters in healthy adults. | |||||||
| IL-6 level in serum (ELISA) | NS | |||||||||||
| TNFα level in serum (ELISA) | NS | |||||||||||
| CCL3 (MIP-1a) level in serum (ELISA) | NS | |||||||||||
| CCL4 (MIP-1b) level in serum (ELISA) | NS | |||||||||||
| CCL5 (RANTES) level in serum (ELISA) | NS | |||||||||||
| CXCL8 (IL-8) release by neutrophils | NS | |||||||||||
| Waterfall exposure | ||||||||||||
| Gaisberger et al. [35] | 2012 | Effects of ionized waterfall aerosol on pediatric allergic asthma | IL-5 level in serum (ELISpot) | Decrease | p < 0.05 | NS | Decrease | NS | Exposure to waterfalls reduces pro-inflammatory cytokines and allergic asthma symptoms, enhances lung function and induces Treg cells. | |||
| IL-10 level in serum (ELISpot) | Increase | p < 0.05 | NS | Increase | NS | |||||||
| IL-13 level in serum (ELISpot) | Decrease | p < 0.01 | Decrease | p < 0.01 | NS | |||||||
| IL-10 expression (PCR) | Increase | NA | Increase | NA | NS | |||||||
| IL-13 expression (PCR) | Decrease | NA | NS | NA | Decrease | p < 0.05 | ||||||
| IFNg expression (PCR) | Increase | NA | Increase | NA | NS | |||||||
| Treg cells (%) | Increase | p < 0.01 | Increase | p < 0.05 | NS | |||||||
| Eosinophilic cationic protein (ECP) levels in serum | Decrease | p < 0.05 | NS | NS | ||||||||
| Fractional exhaled nitric oxide (FeNO) at d20 | Decrease | p < 0.001 | Decrease | p < 0.001 | NA | |||||||
| Fractional exhaled nitric oxide (FeNO) at d80 | Decrease | p < 0.01 | NS | NA | ||||||||
| Peak expiratory flow rate (PEF) | Increase | p < 0.01 | Increase | p < 0.01 | NA | |||||||
| Other spirometric parameters (FEV, FEV%FVC, FEF25, FEF50, MMEF2575) | Increase |
p < 0.05 p < 0.01 |
NS | NA | ||||||||
| Grafetstätter et al. [34] | 2017 | Does waterfall aerosol influence mucosal immunity and chronic stress? A randomized controlled clinical trial | IgA level in saliva (d6) | Increase | NA | Increase | NA | Increase | p = 0.001 | Exposure to waterfalls activates the immune system and improves lung function. | ||
| IgA level in saliva (d66) | Increase | NA | NS | Increase | p < 0.05 | |||||||
| Peak expiratory flow rate (PEF) (d6) | Increase | p = 0.023 | Increase | NA | NS | |||||||
3.4.2. Synthesis of Human Studies
The synthesis of human studies analysing the immunological effects of forest bathing points to largely positive evidence of anti-inflammatory and anti-asthmatic effects along with a promising evidence of enhanced cytotoxicity stemming from increased NK cell levels or activities. However, the synthesis of anti-inflammatory effects was obscured by differing results derived from analysing cytokine levels, specifically IL-6 and TNFα. Some studies only observed an alteration in one of the cytokines and no change in the other, while other studies reported a change in the respective other cytokine value. Since the results reveal overall anti-inflammatory effects, the evidence base is still regarded as largely positive, but not entirely conclusive. Several forest bathing studies also measured immune cell subset distributions. While no significant changes in T cell numbers and/or percentages were observed, most studies showed an increase of NK cell levels along with elevated percentages of cells with cytotoxic content (perforin, granzyme A/B and granulysin).
Studies without control groups and/or without sufficient baseline values to adequately control for confounders were heavily represented among forest bathing studies. Almost half of the studies were carried out as one group pre-post intervention designs [52,53,54,55,56,57]; therefore, their findings have less strength but are backed up by similar results from the included CCT studies. Furthermore, a considerable number of forest bathing studies did not provide a thorough calculation of significances for certain outcome measures that were nevertheless interpreted by the study authors as showing an effect. Moreover, most studies comprised a low number of study participants (<30 subjects), which weakened the evidence base for the measured effects significantly. Only four forest bathing studies analysed larger group sizes [44,45,47,57]. One of them showed retrospective results from a big cohort of subjects living in a forest environment, but lacked baseline values of respective measures [57]. Three other studies with larger sample sizes either provided no pre-intervention data [44], no appropriate significance calculations [47] or ignored considerable baseline differences [45], which weakened their results. The findings on NK cell distributions were underlined by positive results from one laboratory experiment simulating BVOC exposure in natural environments [58]; however, this study provided no control group and must therefore be interpreted cautiously. Overall, the evidence base for the effects of forest bathing on immune functioning can be regarded as promising, despite substantial study design shortcomings.
The three studies analysing fragrance inhalation showed highly heterogeneous results that ranged from beneficial immune responses to no measurable effects at all. The different study designs and measured parameters resulted in differing outcomes, which barely suffice to formulate overall tendencies. It is noteworthy that the two studies describing certain positive effects were relatively old [59,60] and one of them used out-dated methods (for details, see Supplementary Material) [59]; accordingly, these results must be questioned in terms of reliability and validity.
Lastly, the two studies analysing the effects of waterfall exposure on immunological health provided good study designs with appropriate controls and group sizes as well as methodologically transparent approaches [34,35]. Thus, their results showing anti-inflammatory and anti-allergic outcomes as well as beneficial clinical outcomes provided a reliable evidence base which remains to be confirmed by a greater number of studies.
In conclusion, human studies provide a promising evidence base for immunomodulatory effects following exposure to natural environments, though general shortcomings in the study designs weaken their soundness.
3.5. Outcomes and Synthesis of Animal Studies
3.5.1. Outcomes of Animal Studies
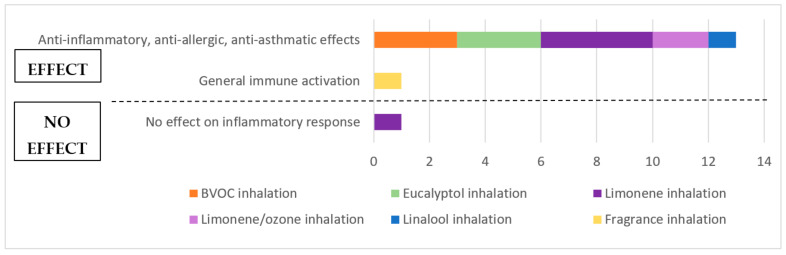
Most animal studies examined the effects of phytoncide or aroma inhalation on a pre-induced immune response. Experimental parameters analysed were levels of various cytokines and/or antibodies in sera and/or bronchoalveolar lavage fluids (BALF) of the respiratory tract, leukocyte numbers (mostly neutrophils, eosinophils, macrophages and lymphocytes) as well as histological or clinical symptoms such as cellular or structural changes, inflammatory cell infiltrations into lung tissue or respiratory markers. Some studies assessed several outcome measures. Overall, a positive effect was observed on most immunological parameters and only one study showed no significant changes. An overview of outcomes from animal studies is presented in Figure 3.
Figure 3.
Overview of outcomes from animal studies.
By measuring a decrease in pro-inflammatory and/or an increase in anti-inflammatory (IL-10) cytokines as well as a reduced number of leukocytes, 13 animal studies reported anti-inflammatory, anti-allergic and/or anti-asthmatic effects following exposure to natural substances. All studies analysing BVOC [62,64,75] and eucalyptol [65,66,74] inhalation attested to protective and therapeutic anti-inflammatory effects of these substances in asthma- or allergy-challenged animals. The results for limonene were more heterogeneous, with four out of five studies observing potentially beneficial, anti-inflammatory effects in challenged animals [67,68,70,73], while one study showed no significant effects of limonene inhalation [69]. The mixture of limonene and ozone inhalation was able to protect from the adverse effects elicited by inhalation of only ozone in two studies [69,70]. Furthermore, inhalation of linalool was able to change the immune cell distribution of previously stressed rats, as one study reported [72]. Another study observed that exposure to certain fragrances was able to induce a general immune activation, which they diagnosed by measuring the number of plaque-forming cells in spleen and the thymic weight [71].
The findings of all animal studies are summarised in Table 6.
Table 6.
Outcomes of animal studies. Significances are given with p < 0.05, p < 0.01 and p < 0.001; NS = not significant, NA=not applicable. A non-significant trend is described as “Decrease/Increase, NS”. If significances are not given, it is described as “Decrease/Increase, NA”.
| Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Main Author | Year | Title | Measure | Pre-treatment | p-Value | Intervention (Compared to Pre-treatment) | p-Value | Conclusion | Comment |
| BVOC inhalation | |||||||||
| Ahn et al. [62] | 2018a | Alleviation effects of natural volatile organic compounds from Pinus densiflora and Chamaecyparis obtuda on systemic and pulmonary inflammation | IgE level in serum (ELISA) | Increase | p < 0.05 | Decrease | p < 0.05 | BVOCs (VOCCo, VOCPd) excert anti-inflammatory effects in mice. | |
| Prostaglandin E2 (PGE2) level in serum (ELISA) | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| COX-2 mRNA expression in PBMCs | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| TNFα mRNA expression in PBMCs | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| IL-1b mRNA expression in PBMCs | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| IL-13 mRNA expression in PBMCs | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| COX-2 mRNA expression in lung tissue | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| NF-kB mRNA expression in lung tissue | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| TNFα mRNA expression in lung tissue | Increase | p < 0.05 | NS | ||||||
| COX-2, NF-kB, IL-1b, TNFα, IL-13 mRNA in BALF cells | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| Ahn et al. [63] | 2018b | Anti-asthmatic effects of volatile organic compounds from Chamaecyparis obtusa, Pinus densiflora, Pinus koraiensis, and Larix kaempferi wood panels | Thickening of bronchiolar wall (hypertrophy) | Increase | NA | Decrease | NA | BVOCs (VOCCo, VOCPd, VOCPk, VOCLk) excert anti-allergic effects in asthmatic mice. | |
| IL-4 level in serum (ELISA) | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| TNFα level in serum (ELISA) | Increase | p < 0.05 | Decrease (C. obtusa) |
p < 0.05 | |||||
| IL-4 mRNA expression in bronchioles | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| IL-5 mRNA expression in bronchioles | Increase | p < 0.05 | NS | ||||||
| IL-9 mRNA expression in bronchioles | Increase | p < 0.05 | Decrease (C. obtusa) |
p < 0.05 | |||||
| IL-13 mRNA expression in bronchioles | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| Yang et al. [64] | 2015 | Estimation of the environmental effect of natural volatile organic compounds from Chamaecyparis obtusa and their effect on atopic dermatitis-like skin lesions in mice | IgE level in serum | Increase | p < 0.05 | Decrease | p < 0.05 | Exposure to BVOCs (C. obtusa) ameliorates inflammatory skin reactions in mice with atopic dermatitis. |
|
| Mast cell infiltration into skin lesions | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| IL-1b mRNA expression in skin lesions | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| IL-6 mRNA expression in skin lesions | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| Eucalyptol inhalation | |||||||||
| Bastos et al. [74] | 2011 | Inhaled 1,8-Cineole reduces inflammatory parameters in airways of ovalbumin-challenged guinea pigs | TNFα level in BALF (ELISA) | Increase | p < 0.05 | Decrease | NS | Eucalyptol (1,8-cineol) inhibits antigen-induced airway inflammation in guinea pigs. | |
| IL-1b level in BALF (ELISA) | Increase | p < 0.05 | Decrease | NS | |||||
| IL-10 level in BALF (ELISA) | Decrease | p < 0.05 | Increase | NS | |||||
| Leukocyte number in BALF (eosinophils and neutrophils) |
Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| MPO activity | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| Kennedy-Feitosa et al. [65] | 2019 | Eucalyptol promotes lung repair in mice following cigarette smoke-induced emphysema | TNFα level in lung tissue | Increase | p < 0.01 | Decrease | p < 0.01 | Eucalyptol reduces pro-inflammatory cytokines and neutrophil activation marker (MPO) after lung damage by cigarette smoke. | |
| IL-1b level in lung tissue | Increase | p < 0.01 | Decrease | p < 0.05 | |||||
| IL-6 level in lung tissue | Increase | p < 0.01 | Decrease | p < 0.01 | |||||
| TGFß-1 level in lung tissue | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| MPO activity in lung tissue | Increase | p < 0.01 | Decrease | p < 0.05 | |||||
| Lee et al. [66] | 2016 | Effect of 1,8-cineol in Dermatophagiodes pteronyssinus-stimulated bronchial epithelial cells and mouse model of asthma | IL-4 level in BALF (ELISA) | Increase | p < 0.01 | Decrease | p < 0.05 | Eucalyptol reduces pro-inflammatory cytokine expression (IL-4, IL-13, IL-17A) in house dust mite-allergic/asthmatic mice. | |
| IL-13 level in BALF (ELISA) | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| IL-17A level in BALF (ELISA) | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| Neutrophil number in BALF | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| Eosinophil number in BALF | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| Lymphocyte number in BALF | Increase | p < 0.05 | Decrease | p < 0.05 | |||||
| Der p-specific IgG1 in serum (ELISA) | Increase | p < 0.01 | Decrease | p < 0.05 | |||||
| Airway restriction (Penh) | Increase | p < 0.01 | Decrease | p < 0.05 | |||||
| Limonene inhalation | |||||||||
| Bibi et al. [67] | 2015 | Treatment of asthma by an ozone scavenger in a mouse model | Aldehyde (ozone oxydation product) levels in BALF | Increase | NA | Decrease | NA | Prophylactic limonene inhalation protects against allergic asthma in mice. | |
| Aldehyde (ozone oxydation product) levels in lung tissue | Increase | NA | Decrease | NA | |||||
| Aldehyde (ozone oxydation product) levels in spleen | Increase | NA | Decrease | NA | |||||
| Neutrophil number in BALF | Increase | NA | Decrease | p < 0.05 | |||||
| Eosinophil number in BALF | Increase | NA | NS | ||||||
| Infiltration of inflammatory cells into lung tissue | Increase | NA | Decrease | NA | |||||
| Hirota et al. [68] | 2012 | Limonene inhalation reduces allergic airway inflammation in Dermatophagoides farinae-treated mice | Der f-specific IgG in serum (ELISA) | Increase | p < 0.001 | Decrease | p < 0.01 | Limonene reduces pro-inflammatory cytokines and cell numbers in mice pre-sensitized to house dust mite allergen. | |
| Total IgE in serum (ELISA) | Increase | NS | Decrease | NS | |||||
| Eosinophil number in BALF | Increase | p < 0.001 | Decrease | p < 0.001 | |||||
| Lymphocyte number in BALF | Increase | p < 0.001 | Decrease | p < 0.05 | |||||
| Neutrophil number in BALF | Increase | p < 0.001 | Decrease | p < 0.05 | |||||
| Macrophage number in BALF | Increase | p < 0.001 | Decrease | p < 0.05 | |||||
| IL-5 level in BALF | Increase | p < 0.001 | Decrease | p < 0.001 | |||||
| IL-13 level in BALF | Increase | p < 0.001 | Decrease | p < 0.001 | |||||
| Eotaxin level in BALF | Increase | p < 0.001 | Decrease | p < 0.001 | |||||
| MCP-1 level in BALF | Increase | p < 0.001 | Decrease | p < 0.001 | |||||
| TGF-b level in BALF | Increase | p < 0.001 | Decrease | p < 0.05 | |||||
| IFNy level in BALF | Decrease | p < 0.01 | Increase | p < 0.05 | |||||
| Bronchorestriction with Ach | Increase | p < 0.01 | Decrease | p < 0.01 | |||||
| Keinan et al. [73] | 2005 | Natural ozone scavenger prevents asthma in sensitized rats |
Limonene
inhalation: |
Limonene reduces inflammatory cell infiltrates into lung tissue and improves airway restriction in lungs of rats with allergic asthma. | No significances provided in graphical outcome report | ||||
| Inflammatory cell infiltrates | Increase | NA | Decrease | NA | |||||
| Airway restriction (Penh) | Increase | NA | Decrease | NA | |||||
|
Eucalyptol
inhalation: |
Eucalyptol reduces inflammatory cell infiltrates into lung tissue, but to a lesser extent than limonene, but does not improve airway restriction. | ||||||||
| Inflammatory cell infiltrates | Increase | NA | Decrease | NA | |||||
| Airway restriction (Penh) | Increase | NA | NS | ||||||
| Limonene/ozone inhalation | |||||||||
| Hansen et al. [69] | 2013 | Adjuvant and inflammatory effects in mice after subchronic inhalation of allergen and ozone-initiated limonene reaction products |
Limonene
inhalation: |
Limonene inhalation has no significant effect on inflammatory response in pre-sensitized mice. | No naive (baseline before OVA-sensitisation) data shown. | ||||
| OVA-specific IgE in serum (ELISA) | Increase | NA | NS | ||||||
| Eosinophil number in BALF | NS | NS | |||||||
| Lymphocyte number in BALF | Increase | NA | NS | ||||||
| Neutrophil number in BALF | Increase | NA | NS | ||||||
| Macrophage number in BALF | Increase | NA | NS | ||||||
|
Ozone
inhalation: |
Limonene +
ozone inhalation: |
Limonene/ozone mixture reduces allergen-specific reactions in pre-sensitized mice. | No naive (baseline before OVA-sensitisation) data shown. | ||||||
| OVA-specific IgE in serum (ELISA) | Increase | NA | Increase | p < 0.05 | |||||
| Eosinophil number in BALF | Increase | NA | Decrease | p < 0.05 | |||||
| Lymphocyte number in BALF | Increase | NA | Decrease | NS | |||||
| Neutrophil number in BALF | Increase | NA | Decrease | p < 0.05 | |||||
| Macrophage number in BALF | Increase | NA | NS | ||||||
| Hansen et al. [70] | 2016 | Limonene and its ozone-initiated reaction products attenuate allergic lung inflammation in mice |
Air
inhalation: |
Limonene
inhalation: |
Limonene potentially reduces airway inflammation in allergic mice, however no significances given. |
Unclear graphical and verbal description of outcomes and signifi-cances.
No OVA-only control. No comparisons between relevant groups. |
|||
| OVA-specific IgE in serum (ELISA) | Increase | NA | Decrease | NA | |||||
| OVA-specific IgG1 in serum (ELISA) | Increase | NA | NS | ||||||
| Eosinophil number in BALF | Increase | NA | NS | ||||||
| Lymphocyte number in BALF | Increase | NA | Increase | NA | |||||
| Neutrophil number in BALF | Increase | NA | Decrease | NA | |||||
| Macrophage number in BALF | Increase | NA | Decrease | NA | |||||
| IL-5 expression in BALF | Increase | NA | Decrease | NA | |||||
|
Ozone
inhalation: |
Limonene +
ozone inhalation: |
Limonene/ozone mixture potentially attenuates allergic inflammation and ozone-induced pulmonary irritation in allergic mice. |
Unclear graphical and verbal description of outcomes and signifi-cances.
No OVA-only control. No comparisons between relevant groups. |
||||||
| OVA-specific IgE in serum (ELISA) | Increase | NA | Decrease | NA | |||||
| OVA-specific IgG1 in serum (ELISA) | Increase | NA | Increase | NA | |||||
| Eosinophil number in BALF | Increase | NA | Decrease | p < 0.05 | |||||
| Lymphocyte number in BALF | Increase | NA | Decrease | NA | |||||
| Neutrophil number in BALF | NS | NA | |||||||
| Macrophage number in BALF | Increase | NA | Decrease | NA | |||||
| IL-5 expression in BALF | Increase | NA | Decrease | NA | |||||
| Linalool inhalation | |||||||||
| Nakamura et al. [72] | 2009 | Stress repression in restrained rats by R-(-)-linalool inhalation and gene expression profiling of their whole blood cells | % of neutrophils | Increase | p < 0.05 | Decrease | NA | Linalool inhalation reverts stress-induced changes in neutrophil and lymphocyte fractions. | |
| % of lymphocytes | Decrease | p < 0.05 | Increase | NA | |||||
| Fragrance inhalation | |||||||||
| Fujiwara et al. [71] | 1998 | Effects of a long-term inhalation of fragrances on the stress-induced immunosuppression in mice |
Number of plaque forming cells (PFC)
in spleen: |
Exposure to natural fragrances reverses stress-induced thymic involution and activates the immune system. | |||||
| Lemon inhalation | Decrease | p < 0.05 | Increase | p < 0.05 | |||||
| Oakmoss inhalation | Decrease | p < 0.05 | Increase | p < 0.05 | |||||
| Labdanum inhalation | Decrease | p < 0.05 | Increase | p < 0.05 | |||||
| Tuberose inhalation | Decrease | p < 0.05 | Increase | p < 0.05 | |||||
| Thymic weight: | |||||||||
| Lemon inhalation | Decrease | p < 0.05 | Increase | p < 0.05 | |||||
| Oakmoss inhalation | Decrease | p < 0.05 | Increase | p < 0.05 | |||||
| Labdanum inhalation | Decrease | p < 0.05 | Increase | p < 0.05 | |||||
| Tuberose inhalation | Decrease | p < 0.05 | Increase | p < 0.05 | |||||
3.5.2. Synthesis of Animal Studies
Overall, animal studies point to largely coherent and unambiguous evidence for anti-inflammatory effects of phytoncide inhalation in immune-challenged animals. Most experimental setups could easily be compared to each other, since they were relatively homogeneous concerning their methodological approaches, sample sizes and outcome measures. Many studies examined a broad range of inflammatory cytokines and specific antibodies, some measuring both mRNA expression and protein levels. All studies reported widely concordant outcomes, as almost all interventions were able to reverse the inflammation induced by the distinct pre-treatments. Differences were found in the statistical analysis and evaluation of results, with some studies providing rigorous statistical significance calculations and others none. Especially the studies examining BVOC and eucalyptol inhalation comprised a good study design and statistical evaluation, rendering strong evidence for the beneficial effects of these treatments.
Out of the three studies examining limonene inhalation alone, two lacked significance calculations [67,73] while one provided good statistical evaluations [68]. This made it difficult to reliably conclude on the effects observed from limonene inhalation, but points to a positive immune response derived from these experiments.
In contrast, two studies analysing the combined effects of limonene and ozone inhalation in pre-sensitised animals failed to provide essential baseline values before sensitisation, did not show any sensitisation-only control values and lacked significances and statistical comparisons between relevant groups [69,70]. These extensive shortcomings made it impossible to draw any conclusions based on these experiments.
Lastly, single experiments measured the effects of linalool [72] and fragrance [71] inhalation, respectively. One of them used out-dated methods (see Supplementary Material for further details), which made the results less robust [71].
In conclusion, animal studies included in this review provide a solid evidence base for anti-inflammatory, anti-asthmatic and anti-allergic effects upon inhalation of nature-derived substances, especially BVOCs and eucalyptol.
4. Limitations of the Review
Limitations are found in the study selection process, which might be biased due to the interdisciplinarity of the topic and the difficulties generated in performing a fitting keyword search. Studies in the field of immunological health and nature have yet no common narrative, making it difficult to formulate a search string that selectively targeted studies from different disciplines. Also the variety of interventions and methodological approaches made it challenging to incorporate all possible wordings into one comprehensive search string. Especially within the field of animal studies, many studies focused on the analysis of a single substance and only included this specific term in their titles and keywords. This problem was handled by including snowballing as additional search strategy. Nevertheless, it cannot be ruled out that individual studies which would also meet this review’s inclusion criteria are missing from the present review. Furthermore, due to the large number of articles resulting from the initial keyword search, it was not possible to include more than two databases in the search. However, included databases gave entirely overlapping outputs, with Scopus being the database having most relevant hits.
Association studies were excluded from this review, since they require different quality assessment approaches and a separate synthesis, which was beyond the scope of the review. Nevertheless, the field provides a high amount of large and rigorously designed population-based association studies that render compelling insights into and provide supplementary evidence for nature’s effect on the immune system. It therefore remains an open task to systematically assess association studies published on this topic and evaluate their outcomes, which could subsequently be merged with the final synthesis of the review at hand.
5. Discussion
Until recently, scientific studies on nature and human health have largely been separated into traditional research fields such as environmental science, ecology, biology, geography, landscape architecture, medicine, psychology, epidemiology and public health. This has generated an impressive amount of data that is now starting to be fruitfully brought together in a holistic perspective to stimulate a broad, interdisciplinary research agenda on environmental health. A considerable number of reviews have emerged that illuminate connections between nature and many human health challenges [11,76,77,78]; however, studies focusing on immunological health benefits have so far been underrepresented. This review examined both human and animal studies and found a promising evidence base for immunomodulatory effects following exposure to natural volatile substances or environments, comprising anti-inflammatory, anti-asthmatic, anti-allergic and cytotoxic responses from inhalation of diverse nature-derived compounds.
Whether or not natural environments have the potential to alleviate or even prevent immunological health problems remains an open question that needs more investigation from a multi-dimensional perspective. Animal studies are a strong tool to formulate a robust initial starting point and can be used to back up findings from studies with human subjects. In this review they represent an important data source concerning changes in expression levels of immunological key molecules resulting from the inhalation of biogenic substances. They are used to dissect mRNA and protein expression of inflammatory molecules in various tissues (lung, bronchioles, skin) and not only in blood, as it is commonly done in human experiments. The advantage of animal experiments is that they can easily screen a set of potential hypotheses with relatively low effort and be carried out in standardised, controllable conditions. Unequivocally, translating results into the human setting is challenging, since the standardised experimental setups do not correspond to the multi-faceted aspects of human life. Further, the ethical aspects of using experimental animals need to be diligently balanced with the scientific gains and alternatives considered whenever possible. At present, laboratory experiments are fundamental in disentangling mechanistic pathways and establishing a data base that can eventually be tested in the human system. The positive evidence base derived from animal experiments included in the present review supports the notion that immune functioning might represent a direct, central pathway of how nature and health are connected.
5.1. A Baseline for Future Research
Next to providing a comprehensive literature overview, the goal of this review was to define a baseline of existing data for future research. However, since most included studies diverged in their methodological approaches and only performed a superficial screening of varying parameters, this baseline cannot be explicitly defined. Animal and human experiments analysed largely distinct parameters. While animal studies screened a wide range of different cytokines along with measuring detailed leukocyte subset distributions, most human studies did not provide such a thorough analysis of inflammatory parameters and focused more on specific cytokines and cytotoxic mediators. Thus, the human study outcomes are relatively fragmented and lack a comprehensive insight into the distribution of other immune cell types and properties. Therefore, data derived from included human and animal studies in this review can hardly be linked. Nevertheless, a clear anti-inflammatory and cytotoxic tendency can be observed in the majority of studies. Decreased expression levels of many pro-inflammatory molecules in various tissue and blood samples along with an infiltration of leukocyte subsets and an increase of NK cell activity and release of cytotoxic granules are results that may serve as a baseline for further studies.
5.2. Study Shortcomings and Recommendations for Future Study Designs
Overall, the synthesis of study findings carried out in this review presents some promising evidence for the positive influence of nature exposure on various aspects of immune functioning. However, considerable shortcomings in the design and conduct especially of included human studies weaken their solidity. Most studies examined only one independent replicate (trials were carried out once), leaving the study inherently prone to random and systematic errors that can only be ruled out by trial repetitions (at least three independent replicates). Moreover, the high number of human studies completely lacking controls gives rise to major concerns regarding the reproducibility and reliability of study outcomes. It has been shown that “within-subject” studies are susceptible to bias since an individual’s initial physiological outcome value can influence the extent and direction of post-intervention responses [79]. Furthermore, most studies neither monitored environmental conditions nor adjusted for potential changes in airborne parameters such as temperature, humidity or BVOC concentrations, which makes it hard to account for individual parameters that might influence study outcomes. The majority of animal studies did not show comparable shortcomings; however, selected studies lacked important baseline values as well as statistical comparisons between relevant groups. In general, included animal trials were also not repeated to generate three independent replicates, which again represents a main drawback concerning the solidity of outcomes.
Ideally, future studies should encompass relatable animal and human experiments including sufficient and adequate controls (placebo controls, controls of external conditions as well as positive and negative control groups). Moreover, they should calculate effect sizes and provide dose-response relationships. In order to guarantee outcome reproducibility, results should be consolidated by a minimum of three trial repetitions. Along with improving the rigor of study designs, the study field should be expanded to bigger group sizes and diverse environments and use more in-depth, state-of-the art analytical methods such as next generation sequencing and big data technologies.
5.3. What Is the Optimal Type, Length, Season and Dosage of Nature Exposure?
Studies included in this review originate from different research fields and approach the main question from distinct scientific angles. Although the included studies cover different target groups, exposure types and durations, some essential questions concerning the maximal efficacy of nature exposure still remain open. Which type of nature is best suited for governing positive immunoregulatory effects? How long should nature exposure last and which dosage do we need to ensure a prolonged, but safe, effect? When is the optimal timeframe for nature contact in order to gain the maximum effect?
A factor that might influence the efficacy and magnitude of immune response to natural environments is the type and variability of vegetation that humans are exposed to, also termed eco- or geodiversity [20]. This encompasses both diverse landscape types such as forests, meadows, mountains, coastal areas or oceans as well as the specific species that can be found in these habitats. Human studies in this review mainly analyse forest environments, but encompass a range of different forest types such as broad-leaved, coniferous or bamboo forests. Animal studies also highlight immunomodulatory properties of different BVOCs and natural fragrances, but fail to relate them to specific vegetation types in the natural world. Therefore, it cannot be concluded from the studies included here which type of nature is best at conferring immunological benefits. However, catalogues of different species’ BVOC emission rates from needles and leaves are available [80,81]. BVOC emission potentials depend on environmental factors such as temperature and light, and species-specific factors such as plant age, developmental stage, intercellular CO2 concentration, stomatal conductance, leaf structure and gas storage potential [31,82]. Seasonal and diurnal variability have also been observed, and summer seemed to be the best time for using forest environments for medical purposes due to highest temperatures and best light conditions [31,82]. Preliminary evidence points to a characteristic daily emission pattern which might at least apply on clear and calm days, while cloudy and windy conditions appear to be least beneficial for the uptake of BVOCs from forest environments [31,82], but further research is necessary to link these findings with policies that promote public health gains.
Concerning the duration of exposure, this review included studies with exposures ranging from a few hours to several days and weeks. Animal studies were especially useful to elucidate longer exposures of up to four months. However, no conclusion on minimal durations or dosage of nature exposure can be drawn due to the various experimental setups and substances tested. Along with the missing calculation of effect sizes, exposure characteristics need to be analysed in much more detail in order to correctly interpret the study results. Very limited research has so far been carried out to address the question of explicit exposure-response relationships between nature and human health, and no studies included in this review provided relevant answers on this topic. However, some large-scale, population-based research has tried to establish an association between the duration of nature exposure and observed health effects. One study showed that spending a minimum of two hours a week in nature improved overall health and wellbeing, with positive associations peaking at approximately four hours of exposure [83]. Another study examining the associations between frequency, duration and intensity of nature exposure observed a reduction in the prevalence of depression and high blood pressure following nature visits longer than 30 min a week [84]. However, these studies can only provide fractional answers and do not address immunological responses at all.
Another interesting question relates to the optimal exposure time point in life. The review at hand includes studies with adults, elderly and children, but none of the studies compared different exposure timeframes in different target groups; thus, this question cannot be answered here. However, association studies provide evidence for optimal time windows of nature exposure concerning immunological effects. Some studies show that very early exposures during pregnancy and childhood exhibit a greater immunomodulatory effect than exposures later in life [85,86]. Other studies report that exposures during late childhood up to the age of 10–15 years are associated with a lower risk of developing multiple sclerosis [87,88]. However, it seems that later exposures during adult life are also beneficial for certain immune parameters such as the immunoregulatory capacity of helminth infections [89]. To gain profound knowledge on the optimal exposure time point in life, longitudinal research monitoring immunological effects over longer timespans in different target groups is needed.
5.4. Understanding Biomedical Mechanisms
A wide range of studies exists that describes immunomodulatory effects of natural substances in vitro or in vivo by other administrative pathways than mere inhalation. Recently, the main molecular targets of terpenes in inflammatory diseases have been summarised and categorised into six groups [22]: inflammatory mediators (interleukins, TNFα, NO and COX2), transcription factors (NF-κB, Nrf2), signal transduction molecules (MAPK signalling molecules such as ERK, p38, JNK, but also STAT3, TRPVs, CB(2)R), oxidative stress (ROS, H2O2) and autophagy (by targeting apoptotic genes). Disentangling key molecular components and action pathways provides important information for understanding the biomedical mechanisms of how nature affects the immune system, which is essential for its effective and targeted application. However, most studies used parenteral, oral or topical administration, which possibly increases effect sizes compared to studies set in natural environments. Therefore, it remains an open task to verify this evidence in more natural interventions that mimic real life exposures. The review at hand focuses on studies that only use inhalation of volatile substances as administrative pathway. Advantages of this approach are a low implementation threshold and a relatively easy transferability into therapy and policy measures. However, smaller and less coherent outcomes stemming from experiments performed in natural environments should not be confused with evidence obtained from invasive administrations. The challenge of the former will be to define effect sizes and molecular action pathways as clearly and in as detailed a manner as in invasive drug studies.
5.5. Nature-Based Clinical Applications
Considering the wide range of potential health benefits that nature provides, possibilities of how to effectively harvest these natural goods for future therapeutic applications can be envisioned. Owing to the rapid rise of diseases related to misled immunoregulation and the frequent severe side effects of currently used immunomodulatory drugs [90,91], there is a growing need for discovering new therapeutic options that are better tolerated. Nature-based interventions might represent such an option; however, potential adverse effects need to be considered when using nature in therapeutic and preventive clinical applications or implementing it in policy recommendations. Many terpenes are not harmful themselves, but can easily oxidise upon air exposure and create allergenic or inflammatory secondary molecules. One study reported irritations of the respiratory tract in mice after exposure to oxidation products from α-pinene and d-limonene [92]. Another study analysing the association between indoor VOCs and lung function reported α-pinene as one of 10 substances negatively influencing human lung function [93]. Auto-oxidation of various terpenes such as α- and β-pinene, limonene, camphor and β-phellandrene has also been observed to have negative allergenic effects on atopic dermatitis [94,95,96]. Thus, the observed adverse effects are concentration- and exposure-dependent, and call for a detailed evaluation of the safe concentration range of terpenes. Furthermore, the use of terpenes through “milder” exposure routes such as forest bathing or nature trips may constitute a potentially safer therapeutic strategy than their direct intake or skin application. Considering the small amount of data that exists on the potential adverse effects of volatile biogenic substances, it is yet too early to draw any conclusion from these studies. Nevertheless, the synthesis of the studies included in this review supports the notion that breathing in nature-derived compounds is overall beneficial for reducing inflammation and promoting immune homeostasis.
6. Outlook
Besides the possible clinical use of nature, the findings of the studies in this review also point to potential benefits from promoting nature in official policy frameworks. Inhaling substances emitted from trees in close living proximity or being exposed to wood in daily housing environments display promising examples of co-benefits derived from sustainable developments tailored to support public health gains [13,36]. Possible policy measures could be to promote the conservation of natural environments, enforce the construction of houses from natural materials such as wood and enhance green infrastructure in urban regions. Green infrastructure could be a very effective and multifunctional measure to mitigate negative health impacts e.g., from urban heat islands in cities [36]. It may comprise vegetation planted in urban areas but also engineered structures that fulfil specific functions such as sustainable urban drainage systems (SUDS) [97], and may range from street trees, green roofs and walls, parks, allotment gardens to rain gardens and water basins. Pioneering policy recommendations have defined nature as a cost-effective planning tool for healthy cities [98]. Exposure to nature may therefore be an option to address a range of health challenges and be most effective if designed to harvest both direct as well as indirect benefits from nature (such as physical activity, social cohesion, improved mood, etc.).
Key environmental pressures, such as disruption of ecosystems, loss of biodiversity, habitat destruction, pollution and climate change are driven by human behaviour and threaten the resilience of natural systems [99]. Many studies have shown that these changes have a direct effect on living conditions on earth and significant implications for the public health agenda worldwide [2,11]. Human health is profoundly dependent on undamaged natural environments and functioning ecosystems that provide life supporting services and resources [1]. It is well known that climate change caused by human interventions in biogeochemical cycles negatively impacts ecological balances and concomitant ecosystem services [100]. The Lancet Countdown on Health and Climate Change has recently outlined pathways by which climate change will affect human health worldwide, spanning from exacerbation of existing health problems to introduction of new threats [10]. Anthropogenic climate change also has the potential to affect ecosystem services in terms of immunological health provisioning and regulation, especially by impacting the occurrence and spread of infectious diseases [10,101]. Examples are a change in the geographic range and resulting rise of vector-borne diseases [102], ancient viruses emerging from thawing permafrost [103,104] or the increase in allergenic pollen [105]. Mitigating climate change might therefore yield considerable co-benefits for human health, and joined-up policy making could be seen as a great opportunity for enforcing global health priorities in the 21st century. This study may contribute to a novel, uncommon argumentation in future nature protection and climate change mitigation debates and encourage green policy measures that benefit human and planetary health alike. At large, this review supports the notion that the conservation and creation of healthy human habitats along with the promotion of a nature-connected lifestyle create a new opportunity to support immunological health provisioning.
7. Conclusions
This systematic review gathers promising evidence that nature exposure influences measurable immunological parameters in healthy individuals as well as in people suffering from acute or chronic inflammatory conditions, and that inhaling certain volatile natural compounds can have a beneficial effect on the elicited immune response. According to the synthesis of the studies included in this review, nature exposure supports immunological homeostasis and might offer promising strategies for therapeutic and preventive clinical use. However, there is a lack of studies that rigorously address questions of selectivity, effectivity or adverse effects deriving from nature exposure, let alone providing mechanistic pathway analyses or a solid calculation of effect sizes. This is highly necessary to guarantee outcome reproducibility and safety of nature-based therapies for future broader applications. There is a need for expanding the study field to a larger scale and bigger study cohorts, including different study populations and environments, a standardised control for confounders and environmental conditions as well as the use of more in-depth, state-of-the-art analytical methods and tools. This is essential to draw adequate conclusions and envision a future potential for nature exposure in immunological disease prevention or treatment.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/18/4/1416/s1, Table S1: Risk of bias assessment of animal intervention studies following the SYRCLE guidelines.
Author Contributions
Each author made substantial contributions to the study. Conceptualisation: L.A., S.S.C. and U.K.S.; methodology: L.A. and S.S.C.; data retrieval and curation: L.A.; assessment: L.A. and S.S.C.; formal analysis: L.A. writing: L.A., S.S.C. and U.K.S.; project administration and funding acquisition: U.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the 15th of June Foundation, Denmark (grant number 2020-0374). The foundation had no involvement in the review.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
We have no archived datasets, since we did not produce any primary data in this review.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whitmee S., Haines A., Beyrer C., Boltz F., Capon A.G., De Souza Dias B.F.D.S., Ezeh A., Frumkin H., Gong P., Head P., et al. Safeguarding human health in the Anthropocene epoch: Report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet. 2015;386:1973–2028. doi: 10.1016/S0140-6736(15)60901-1. [DOI] [PubMed] [Google Scholar]
- 2.Myers S.S. Planetary health: Protecting human health on a rapidly changing planet. Lancet. 2017;390:2860–2868. doi: 10.1016/S0140-6736(17)32846-5. [DOI] [PubMed] [Google Scholar]
- 3.United Nations Departement of Economic and Social Affairs . Population Division World Urbanization Prospects: The 2018 Revision. UN DESA; New York, NY, USA: 2018. [Google Scholar]
- 4.Frumkin H., Bratman G.N., Breslow S.J., Cochran B., Jr., Khan P.H., Lawler J.J., Levin P.S., Tandon P.S., Varanasi U., Wolf K.L., et al. Nature Contact and Human Health: A Research Agenda. Environ. Health Perspect. 2017;125:075001. doi: 10.1289/EHP1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauesen L.M. Natural Environment BT. In: Idowu S.O., Capaldi N., Zu L., Das Gupta A., editors. Encyclopedia of Corporate Social Responsibility. Springer; Berlin/Heidelberg, Germany: 2013. pp. 1734–1742. [Google Scholar]
- 6.Mitten D., Brymer E. Encyclopedia of Teacher Education. Springer; Singapore: 2019. Outdoor and Environmental Education: Nature and Wellbeing; pp. 1–7. [DOI] [Google Scholar]
- 7.WHO . WHO Guidelines on Physical Activity and Sedentary Behaviour. WHO; Geneva, Switzerland: 2020. [Google Scholar]
- 8.European Environment Agency . Healthy Environment, Healthy Lives: How the Environment Influences Health and Well-being in Europe. EEA; Copenhagen, Denmark: 2020. [Google Scholar]
- 9.Landrigan P.J., Fuller R., Acosta N.J.R., Adeyi O., Arnold R., Basu N., Baldé A.B., Bertollini R., Bose-O’Reilly S., Boufford J.I., et al. The Lancet Commission on pollution and health. Lancet. 2018;391:462–512. doi: 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- 10.Watts N., Amann M., Ayeb-Karlsson S., Belesova K., Bouley T., Boykoff M., Byass P., Cai W., Campbell-Lendrum D., Chambers J., et al. The Lancet Countdown on health and climate change: From 25 years of inaction to a global transformation for public health. Lancet. 2018;391:581–630. doi: 10.1016/S0140-6736(17)32464-9. [DOI] [PubMed] [Google Scholar]
- 11.Frumkin H., Haines A. Global Environmental Change and Noncommunicable Disease Risks. Annu. Rev. Public Health. 2019;40:261–282. doi: 10.1146/annurev-publhealth-040218-043706. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization Preventing noncommunicable diseases (NCDs) by reducing environmental risk factors. 2017.
- 13.Fong K.C., Hart J.E., James P. A Review of Epidemiologic Studies on Greenness and Health: Updated Literature Through 2017. Curr. Environ. Health Rep. 2018;5:77–87. doi: 10.1007/s40572-018-0179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corazon S.S., Sidenius U., Poulsen D.V., Gramkow M.C., Stigsdotter U.K. Psycho-Physiological Stress Recovery in Outdoor Nature-Based Interventions: A Systematic Review of the Past Eight Years of Research. Int. J. Environ. Res. Public Health. 2019;16:1711. doi: 10.3390/ijerph16101711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartig T., Mitchell R., De Vries S., Frumkin H. Nature and Health. Annu. Rev. Public Health. 2014;35:207–228. doi: 10.1146/annurev-publhealth-032013-182443. [DOI] [PubMed] [Google Scholar]
- 16.Kuo M. How might contact with nature promote human health? Promising mechanisms and a possible central pathway. Front. Psychol. 2015;6:1093. doi: 10.3389/fpsyg.2015.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haluza D., Schönbauer R., Cervinka R. Green Perspectives for Public Health: A Narrative Review on the Physiological Effects of Experiencing Outdoor Nature. Int. J. Environ. Res. Public Health. 2014;11:5445–5461. doi: 10.3390/ijerph110505445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahan E.A., Estes D. The effect of contact with natural environments on positive and negative affect: A meta-analysis. J. Posit. Psychol. 2015;10:507–519. doi: 10.1080/17439760.2014.994224. [DOI] [Google Scholar]
- 19.Twohig-Bennett C., Jones A. The health benefits of the great outdoors: A systematic review and meta-analysis of greenspace exposure and health outcomes. Environ. Res. 2018;166:628–637. doi: 10.1016/j.envres.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig J., Logan A.C., Prescott S.L. Natural environments, nature relatedness and the ecological theater: Connecting satellites and sequencing to shinrin-yoku. J. Physiol. Anthr. 2016;35:1. doi: 10.1186/s40101-016-0083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim T., Song B., Cho K.S., Lee I. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020;21:2187. doi: 10.3390/ijms21062187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterfalvi A., Miko E., Nagy T., Reger B., Simon D., Miseta A., Czéh B., Szereday L. Much More Than a Pleasant Scent: A Review on Essential Oils Supporting the Immune System. Molecules. 2019;24:4530. doi: 10.3390/molecules24244530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janeway C.J., Travers P., Walport M. Immunobiology: The Immune System in Health and Disease. 5th ed. Garland Science; New York, NY, USA: 2001. [Google Scholar]
- 24.Rook G. Regulation of the immune system by biodiversity from the natural environment: An ecosystem service essential to health. Proc. Natl. Acad. Sci. USA. 2013;110:18360–18367. doi: 10.1073/pnas.1313731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aerts R., Honnay O., Van Nieuwenhuyse A. Biodiversity and human health: Mechanisms and evidence of the positive health effects of diversity in nature and green spaces. Br. Med Bull. 2018;127:5–22. doi: 10.1093/bmb/ldy021. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organisation . Connecting Global Priorities: Biodiversity and Human Health. A State of Knowledge Review. WHO; Geneva, Switzerland: 2015. [Google Scholar]
- 27.Hanski I., Von Hertzen L., Fyhrquist N., Koskinen K., Torppa K., Laatikainen T., Karisola P., Auvinen P., Paulin L., Mäkelä M.J., et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. USA. 2012;109:8334–8339. doi: 10.1073/pnas.1205624109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruokolainen L., Von Hertzen L.C., Fyhrquist N., Laatikainen T.J., Lehtomäki J., Auvinen P., Karvonen A.M., Hyvärinen A.M., Tillmann V., Niemelä O., et al. Green areas around homes reduce atopic sensitization in children. Allergy. 2015;70:195–202. doi: 10.1111/all.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Hertzen L., Hanski I., Haahtela T. Natural immunity. Biodiversity loss and inflammatory diseases are two global megatrends that might be related. EMBO Rep. 2011;12:1089–1093. doi: 10.1038/embor.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laothawornkitkul J., Taylor J.E., Paul N.D., Hewitt N. Biogenic volatile organic compounds in the Earth system: Tansley review. New Phytol. 2009;183:27–51. doi: 10.1111/j.1469-8137.2009.02859.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen J., Tang J., Yu X. Environmental and physiological controls on diurnal and seasonal patterns of biogenic volatile organic compound emissions from five dominant woody species under field conditions. Environ. Pollut. 2020;259:113955. doi: 10.1016/j.envpol.2020.113955. [DOI] [PubMed] [Google Scholar]
- 32.Quintans J.S., Shanmugan S., Heimfarth L., Araújo A.A.S., Almeida J.R.G.d.S., Picot L., Quintans-Júnior L.J. Monoterpenes modulating cytokines—A review. Food Chem. Toxicol. 2019;123:233–257. doi: 10.1016/j.fct.2018.10.058. [DOI] [PubMed] [Google Scholar]
- 33.Cho K.S., Lim Y.-R., Lee K., Lee J., Lee J.H., Lee I. Terpenes from Forests and Human Health. Toxicol. Res. 2017;33:97–106. doi: 10.5487/TR.2017.33.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grafetstätter C., Gaisberger M., Freidl J., Ritter M., Kolarž P., Pichler C., Thalhamer J., Hartl A. Does waterfall aerosol influence mucosal immunity and chronic stress? A randomized controlled clinical trial. J. Physiol. Anthr. 2017;36:10. doi: 10.1186/s40101-016-0117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaisberger M., Šanović R., Dobias H., Kolarž P., Moder A., Thalhamer J., Selimović A., Huttegger I., Ritter M., Hartl A. Effects of Ionized Waterfall Aerosol on Pediatric Allergic Asthma. J. Asthma. 2012;49:830–838. doi: 10.3109/02770903.2012.705408. [DOI] [PubMed] [Google Scholar]
- 36.Coutts C., Hahn M. Green Infrastructure, Ecosystem Services, and Human Health. Int. J. Environ. Res. Public Health. 2015;12:9768–9798. doi: 10.3390/ijerph120809768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organisation . Evolution of WHO Air Quality Guidelines: Past, Present and Future. WHO Regional Office for Europe; Copenhagen, Denmark: 2017. [Google Scholar]
- 38.Wu K., Yang X., Chen D., Gu S., Lu Y., Jiang Q., Wang K., Ou Y., Qian Y., Shao P., et al. Estimation of biogenic VOC emissions and their corresponding impact on ozone and secondary organic aerosol formation in China. Atm. Res. 2020;231:104656. doi: 10.1016/j.atmosres.2019.104656. [DOI] [Google Scholar]
- 39.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A., PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh B., Lee K.J., Zaslawski C., Yeung A., Rosenthal D., Larkey L., Back M. Health and well-being benefits of spending time in forests: Systematic review. Environ. Health Prev. Med. 2017;22:1–11. doi: 10.1186/s12199-017-0677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Effective Public Health Practice Project . Quality Assessment Tool for Quantitative Studies. Effective Public Health Practice Project; Hamilton, ON, USA: 1998. [Google Scholar]
- 42.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hooijmans C.R., Rovers M.M., De Vries R.B.M., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Im S.G., Choi H., Jeon Y.-H., Song M.-K., Kim W., Woo J.-M. Comparison of Effect of Two-Hour Exposure to Forest and Urban Environments on Cytokine, Anti-Oxidant, and Stress Levels in Young Adults. Int. J. Environ. Res. Public Health. 2016;13:625. doi: 10.3390/ijerph13070625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han J.-W., Choi H., Jeon Y.-H., Yoon C.-H., Woo J.-M., Kim W. The Effects of Forest Therapy on Coping with Chronic Widespread Pain: Physiological and Psychological Differences between Participants in a Forest Therapy Program and a Control Group. Int. J. Environ. Res. Public Health. 2016;13:255. doi: 10.3390/ijerph13030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia B.B., Yang Z.X., Mao G.X., Lyu Y.D., Wen X.L., Xu W.H., Lyu X.L., Cao Y.B., Wang G.F. Health Effect of Forest Bathing Trip on Elderly Patients with Chronic Obstructive Pulmonary Disease. Biomed. Environ. Sci. 2016;29:212–218. doi: 10.3967/bes2016.026. [DOI] [PubMed] [Google Scholar]
- 47.Lvu B., Zeng C., Xie S., Li D., Lin W., Li N., Jiang M., Liu S., Chen Q. Benefits of A Three-Day Bamboo Forest Therapy Session on the Psychophysiology and Immune System Responses of Male College Students. Int. J. Environ. Res. Public Health. 2019;16:4991. doi: 10.3390/ijerph16244991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mao G.X., Cao Y.B., Yang Y., Chen Z.M., Dong J.H., Chen S.S., Wu Q., Lyu X.L., Jia B.B., Yan J., et al. Additive Benefits of Twice Forest Bathing Trips in Elderly Patients with Chronic Heart Failure. Biomed. Environ. Sci. 2018;31:159–162. doi: 10.3967/bes2018.020. [DOI] [PubMed] [Google Scholar]
- 49.Mao G., Cao Y., Wang B., Wang S., Chen Z., Wang J., Xing W., Ren X., Lv X., Dong J., et al. The Salutary Influence of Forest Bathing on Elderly Patients with Chronic Heart Failure. Int. J. Environ. Res. Public Health. 2017;14:368. doi: 10.3390/ijerph14040368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao G.X., Lan X.G., Cao Y.B., Chen Z.M., He Z.H., Lv Y.D., Wang Y.Z., Hu X.L., Wang G.F., Yan J. Effects of short-term forest bathing on human health in a broad-leaved evergreen forest in Zhejiang Province, China. Biomed. Environ. Sci. 2012;25:317–324. doi: 10.3967/0895-3988.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Mao G.-X., Cao Y.-B., Lan X.-G., He Z.-H., Chen Z.-M., Wang Y.-Z., Hu X.-L., Lv Y.-D., Wang G.-F., Yan J. Therapeutic effect of forest bathing on human hypertension in the elderly. J. Cardiol. 2012;60:495–502. doi: 10.1016/j.jjcc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Kim B.J., Jeong H., Park S., Lee S. Forest adjuvant anti-cancer therapy to enhance natural cytotoxicity in urban women with breast cancer: A preliminary prospective interventional study. Eur. J. Integr. Med. 2015;7:474–478. doi: 10.1016/j.eujim.2015.06.004. [DOI] [Google Scholar]
- 53.Li Q., Morimoto K., Nakadai A., Inagaki H., Katsumata M., Shimizu T., Hirata Y., Hirata K., Suzuki H., Miyazaki Y., et al. Forest Bathing Enhances Human Natural Killer Activity and Expression of Anti-Cancer Proteins. Int. J. Immunopathol. Pharmacol. 2007;20:3–8. doi: 10.1177/03946320070200S202. [DOI] [PubMed] [Google Scholar]
- 54.Li Q., Morimoto K., Kobayashi M., Inagaki H., Katsumata M., Hirata Y., Hirata K., Suzuki H., Li Y., Wakayama Y., et al. Visiting a Forest, but Not a City, Increases Human Natural Killer Activity and Expression of Anti-Cancer Proteins. Int. J. Immunopathol. Pharmacol. 2008;21:117–127. doi: 10.1177/039463200802100113. [DOI] [PubMed] [Google Scholar]
- 55.Li Q., Morimoto K., Kobayashi M., Inagaki H., Katsumata M., Hirata Y., Hirata K., Shimizu T., Li Y., Wakayama Y., et al. A forest bathing trip increases human natural killer activity and expression of anti-cancer proteins in female subjects. J. Biol. Regul. Homeost. Agents. 2008;22:45–55. [PubMed] [Google Scholar]
- 56.Seo S.C., Park S.J., Park C.-W., Yoon W.S., Choung J.T., Yoo Y. Clinical and immunological effects of a forest trip in children with asthma and atopic dermatitis. Iran. J. Allergy Asthma Immunol. 2015;14:28–36. [PubMed] [Google Scholar]
- 57.Tsao T.-M., Tsai M.-J., Hwang J.-S., Cheng W.-F., Wu C.-F., Chou C.-C., Su T.-C. Health effects of a forest environment on natural killer cells in humans: An observational pilot study. Oncotarget. 2018;9:16501–16511. doi: 10.18632/oncotarget.24741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Q., Kobayashi M., Wakayama Y., Inagaki H., Katsumata M., Hirata Y., Hirata K., Shimizu T., Kawada T., Park B., et al. Effect of Phytoncide from Trees on Human Natural Killer Cell Function. Int. J. Immunopathol. Pharmacol. 2009;22:951–959. doi: 10.1177/039463200902200410. [DOI] [PubMed] [Google Scholar]
- 59.Kiecolt-Glaser J.K., Graham J.E., Malarkey W.B., Porter K., Lemeshow S., Glaser R. Olfactory influences on mood and autonomic, endocrine, and immune function. Psychoneuroendocrinology. 2008;33:328–339. doi: 10.1016/j.psyneuen.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Komori T., Fujiwara R., Tanida M., Nomura J., Yokoyama M.M. Effects of Citrus Fragrance on Immune Function and Depressive States. Neuroimmunomodulation. 1995;2:174–180. doi: 10.1159/000096889. [DOI] [PubMed] [Google Scholar]
- 61.Trellakis S., Fischer C., Rydleuskaya A., Tagay S., Bruderek K., Greve J., Lang S., Brandau S. Subconscious olfactory influences of stimulant and relaxant odors on immune function. Eur. Arch. Oto-Rhino-Laryngol. 2011;269:1909–1916. doi: 10.1007/s00405-011-1876-4. [DOI] [PubMed] [Google Scholar]
- 62.Ahn C., Lee J.-H., Kim J.-W., Park M.-J., Lee S.-S., Jeung E.-B. Alleviation effects of natural volatile organic compounds from Pinus densiflora and Chamaecyparis obtusa on systemic and pulmonary inflammation. Biomed. Rep. 2018;9:405–414. doi: 10.3892/br.2018.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahn C., Jang Y.-J., Kim J.-W., Park M.-J., Yoo Y.-M., Jeung E.-B. Anti-asthmatic effects of volatile organic compounds from Chamaecyparis obtusa, Pinus densiflora, Pinus koraiensis, or Larix kaempferi wood panels. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2019;69:933–941. doi: 10.26402/jpp.2018.6.07. [DOI] [PubMed] [Google Scholar]
- 64.Yang H., Ahn C., Choi I.-G., Choi W.-S., Park M.-J., Lee S.-S., Choi D., Jeung E.-B. Estimation of the environmental effect of natural volatile organic compounds from Chamaecyparis obtusa and their effect on atopic dermatitis-like skin lesions in mice. Mol. Med. Rep. 2012;12:345–350. doi: 10.3892/mmr.2015.3431. [DOI] [PubMed] [Google Scholar]
- 65.Kennedy-Feitosa E., Cattani-Cavalieri I., Barroso M.V., Romana-Souza B., Brito-Gitirana L., Valença S.S. Eucalyptol promotes lung repair in mice following cigarette smoke-induced emphysema. Phytomedicine. 2019;55:70–79. doi: 10.1016/j.phymed.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Lee H.-S., Park D.-E., Song W.-J., Park H., Kang H., Cho S.-H., Sohn S.-W. Effect of 1.8-Cineole in Dermatophagoides pteronyssinus-Stimulated Bronchial Epithelial Cells and Mouse Model of Asthma. Biol. Pharm. Bull. 2016;39:946–952. doi: 10.1248/bpb.b15-00876. [DOI] [PubMed] [Google Scholar]
- 67.Bibi H., Reany O., Waisman D., Keinan E. Prophylactic treatment of asthma by an ozone scavenger in a mouse model. Bioorganic Med. Chem. Lett. 2015;25:342–346. doi: 10.1016/j.bmcl.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 68.Hirota R., Nakamura H., Bhatti S.A., Ngatu N.R., Muzembo B.A., Dumavibhat N., Eitoku M., Sawamura M., Suganuma N. Limonene inhalation reduces allergic airway inflammation inDermatophagoides farinae-treated mice. Inhal. Toxicol. 2012;24:373–381. doi: 10.3109/08958378.2012.675528. [DOI] [PubMed] [Google Scholar]
- 69.Hansen J.S., Nielsen G.D., Sørli J.B., Clausen P.A., Wolkoff P., Larsen S.T. Adjuvant and Inflammatory Effects in Mice After Subchronic Inhalation of Allergen and Ozone-Initiated Limonene Reaction Products. J. Toxicol. Environ. Health Part A. 2013;76:1085–1095. doi: 10.1080/15287394.2013.838915. [DOI] [PubMed] [Google Scholar]
- 70.Hansen J.S., Nørgaard A.W., Koponen I.K., Sørli J.B., Paidi M.D., Hansen S.W.K., Clausen P.A., Nielsen G.D., Wolkoff P., Larsen S.T. Limonene and its ozone-initiated reaction products attenuate allergic lung inflammation in mice. J. Immunotoxicol. 2016;13:793–803. doi: 10.1080/1547691X.2016.1195462. [DOI] [PubMed] [Google Scholar]
- 71.Fujiwara R., Komori T., Noda Y., Kuraoka T., Shibata H., Shizuya K., Miyahara S., Ohmori M., Nomura J., Yokoyama M.M. Effects of a long-term inhalation of fragrances on the stress-induced immunosuppression in mice. Neuroimmunomodulation. 1998;5:318–322. doi: 10.1159/000026351. [DOI] [PubMed] [Google Scholar]
- 72.Nakamura A., Fujiwara S., Matsumoto I., Abe K. Stress Repression in Restrained Rats by (R)-(−)-Linalool Inhalation and Gene Expression Profiling of Their Whole Blood Cells. J. Agric. Food Chem. 2009;57:5480–5485. doi: 10.1021/jf900420g. [DOI] [PubMed] [Google Scholar]
- 73.Keinan E., Alt A., Amir G., Bar-Yoseph R., Bibi H., Shoseyov D. Natural ozone scavenger prevents asthma in sensitized rats. Bioorg. Med. Chem. 2005;13:557–562. doi: 10.1016/j.bmc.2004.09.057. [DOI] [PubMed] [Google Scholar]
- 74.Bastos V.P., Gomes A.S., Lima F.J., Brito T.S., Soares P.M., Pinho J.P., Silva C.S., Santos A.A., Souza M.H., Magalhães P.J. Inhaled 1,8-Cineole Reduces Inflammatory Parameters in Airways of Ovalbumin-Challenged Guinea Pigs. Basic Clin. Pharmacol. Toxicol. 2010;108:34–39. doi: 10.1111/j.1742-7843.2010.00622.x. [DOI] [PubMed] [Google Scholar]
- 75.Lee J.-E., Lee E.-H., Park H.-J., Kim Y.-J., Jung H.-Y., Ahn D.-H., Cho Y.-J. Inhibition of inflammatory responses in lipopolysaccharide-induced RAW 264.7 cells byPinus densifloraroot extract. J. Appl. Biol. Chem. 2018;61:275–281. doi: 10.3839/jabc.2018.039. [DOI] [Google Scholar]
- 76.Wheeler B., Lovell R., Higgins S.L., White M.P., Alcock I., Osborne N.J., Husk K., Sabel C., Depledge M. Beyond greenspace: An ecological study of population general health and indicators of natural environment type and quality. Int. J. Health Geogr. 2015;14:1–17. doi: 10.1186/s12942-015-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.White M.P., Elliott L.R., Gascon M., Roberts B., Fleming L.E. Blue space, health and well-being: A narrative overview and synthesis of potential benefits. Environ. Res. 2020;191:110169. doi: 10.1016/j.envres.2020.110169. [DOI] [PubMed] [Google Scholar]
- 78.Gascon M., Zijlema W., Vert C., White M.P., Nieuwenhuijsen M.J. Outdoor blue spaces, human health and well-being: A systematic review of quantitative studies. Int. J. Hyg. Environ. Health. 2017;220:1207–1221. doi: 10.1016/j.ijheh.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 79.Song C., Ikei H., Miyazaki Y. Elucidation of a Physiological Adjustment Effect in a Forest Environment: A Pilot Study. Int. J. Environ. Res. Public Health. 2015;12:4247–4255. doi: 10.3390/ijerph120404247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Acosta Navarro J.C., Smolander S., Struthers H., Zorita E., Ekman A.M.L., Kaplan J.O., Guenther A., Arneth A., Riipinen I. Global emissions of terpenoid VOCs from terrestrial vegetation in the last millennium. J. Geophys. Res. Atmos. 2014;119:6867–6885. doi: 10.1002/2013JD021238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guenther A.B., Jiang X., Heald C.L., Sakulyanontvittaya T., Duhl T., Emmons L.K., Wang X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012;5:1471–1492. doi: 10.5194/gmd-5-1471-2012. [DOI] [Google Scholar]
- 82.Meneguzzo F., Albanese L., Bartolini G., Zabini F. Temporal and Spatial Variability of Volatile Organic Compounds in the Forest Atmosphere. Int. J. Environ. Res. Public Health. 2019;16:4915. doi: 10.3390/ijerph16244915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.White M.P., Alcock I., Grellier J., Wheeler B.W., Hartig T., Warber S.L., Bone A., Depledge M.H., Fleming L.E. Spending at least 120 minutes a week in nature is associated with good health and wellbeing. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-44097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shanahan D.F., Bush R., Gaston K.J., Lin B.B., Dean J., Barber E., Fuller R.A. Health Benefits from Nature Experiences Depend on Dose. Sci. Rep. 2016;6:28551. doi: 10.1038/srep28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Riedler J., Braun-Fahrländer C., Eder W., Schreuer M., Waser M., Maisch S., Carr D., Schierl R., Nowak D., Von Mutius E. Exposure to farming in early life and development of asthma and allergy: A cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 86.Ege M.J., Herzum I., Büchele G., Krauss-Etschmann S., Lauener R., Roponen M., Hyvärinen A., Vuitton D.A., Riedler J., Brunekreef B., et al. Prenatal exposure to a farm environment modifies atopic sensitization at birth. J. Allergy Clin. Immunol. 2008;122:407–412. doi: 10.1016/j.jaci.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 87.Gale C.R., Martyn C.N. Migrant studies in multiple sclerosis. Prog. Neurobiol. 1995;47:425–448. doi: 10.1016/0301-0082(95)80008-V. [DOI] [PubMed] [Google Scholar]
- 88.Ahlgren C., Odén A., Lycke J. A nationwide survey of the prevalence of multiple sclerosis in immigrant populations of Sweden. Mult. Scler. J. 2012;18:1099–1107. doi: 10.1177/1352458511433062. [DOI] [PubMed] [Google Scholar]
- 89.Correale J., Farez M.F. The impact of parasite infections on the course of multiple sclerosis. J. Neuroimmunol. 2011;233:6–11. doi: 10.1016/j.jneuroim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 90.Oray M., Abu Samra K., Ebrahimiadib N., Meese H., Foster C.S. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 2016;15:457–465. doi: 10.1517/14740338.2016.1140743. [DOI] [PubMed] [Google Scholar]
- 91.Michot J., Bigenwald C., Champiat S., Collins M., Carbonnel F., Postel-Vinay S., Berdelou A., Varga A., Bahleda R., Hollebecque A., et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 92.Rohr A.C., Wilkins C.K., Clausen P.A., Hammer M., Nielsen G.D., Wolkoff P., Spengler J.D. Upper airway and pulmonary effects of oxidation products of (+)- α -pinene, d -limonene and isoprene in balb/c mice. Inhal. Toxicol. 2002;14:663–684. doi: 10.1080/08958370290084575. [DOI] [PubMed] [Google Scholar]
- 93.Cakmak S., Dales R.E., Liu L., Kauri L.M., Lemieux C.L., Hebbern C., Zhu J. Residential exposure to volatile organic compounds and lung function: Results from a population-based cross-sectional survey. Environ. Pollut. 2014;194:145–151. doi: 10.1016/j.envpol.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 94.Wei Q., Wei C.-N., Harada K., Minamoto K., Okamoto Y., Otsuka M., Ueda A. Evaluation of Allergenicity of Constituents of Myoga Using the Murine Local Lymph Node Assay. Int. J. Immunopathol. Pharmacol. 2010;23:463–470. doi: 10.1177/039463201002300208. [DOI] [PubMed] [Google Scholar]
- 95.Pesonen M., Suomela S., Kuuliala O., Henriks-Eckerman M.-L., Aalto-Korte K. Occupational contact dermatitis caused byD-limonene. Contact Dermat. 2014;71:273–279. doi: 10.1111/cod.12287. [DOI] [PubMed] [Google Scholar]
- 96.Dharmagunawardena B., Takwale A., Sanders K.J., Cannan S., Rodger A., Ilchyshyn A. Gas chromatography: An investigative tool in multiple allergies to essential oils. Contact Dermat. 2002;47:288–292. doi: 10.1034/j.1600-0536.2002.470506.x. [DOI] [PubMed] [Google Scholar]
- 97.Kumar P., Druckman A., Gallagher J., Gatersleben B., Allison S., Eisenman T.S., Hoang U., Hama S., Tiwari A., Sharma A., et al. The nexus between air pollution, green infrastructure and human health. Environ. Int. 2019;133:105181. doi: 10.1016/j.envint.2019.105181. [DOI] [PubMed] [Google Scholar]
- 98.Shanahan D.F., Lin B.B., Bush R., Gaston K.J., Dean J.H., Barber E., Fuller R.A. Toward Improved Public Health Outcomes from Urban Nature. Am. J. Public Heal. 2015;105:470–477. doi: 10.2105/AJPH.2014.302324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steffen W., Broadgate W., Deutsch L., Gaffney O., Ludwig C. The trajectory of the Anthropocene: The Great Acceleration. Anthr. Rev. 2014;2:81–98. doi: 10.1177/2053019614564785. [DOI] [Google Scholar]
- 100.Maes J., Teller A., Nessi S., Bulgheroni C., Konti A., Sinkko T., Tonini D., Pant R. Mapping and Assessment of Ecosystems and Their Services: An EU Ecosystem Assessment. European Comission; Maastricht, The Netherlands: 2020. [Google Scholar]
- 101.Altizer S., Ostfeld R.S., Johnson P.T.J., Kutz S., Harvell C.D. Climate Change and Infectious Diseases: From Evidence to a Predictive Framework. Science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- 102.Rohr J.R., Dobson A.P., Johnson P.T., Kilpatrick A.M., Paull S.H., Raffel T.R., Ruiz-Moreno D., Thomas M.B. Frontiers in climate change–disease research. Trends Ecol. Evol. 2011;26:270–277. doi: 10.1016/j.tree.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Houwenhuyse S., Macke E., Reyserhove L., Bulteel L., Decaestecker E. Back to the future in a petri dish: Origin and impact of resurrected microbes in natural populations. Evol. Appl. 2018;11:29–41. doi: 10.1111/eva.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Legendre M., Bartoli J., Shmakova L., Jeudy S., Labadie K., Adrait A., Lescot M., Poirot O., Bertaux L., Bruley C., et al. Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a pandoravirus morphology. Proc. Natl. Acad. Sci. USA. 2014;111:4274–4279. doi: 10.1073/pnas.1320670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ziska L.H., Makra L., Harry S.K., Bruffaerts N., Hendrickx M., Coates F., Saarto A., Thibaudon M., Oliver G., Damialis A., et al. Temperature-related changes in airborne allergenic pollen abundance and seasonality across the northern hemisphere: A retrospective data analysis. Lancet Planet. Health. 2019;3:e124–e131. doi: 10.1016/S2542-5196(19)30015-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We have no archived datasets, since we did not produce any primary data in this review.