Abstract
Over the past 20 years there has been a growing interest in the neural underpinnings of cost/benefit decision-making. Recent studies with animal models have made considerable advances in our understanding of how different prefrontal, striatal, limbic and monoaminergic circuits interact to promote efficient risk/reward decision-making, and how dysfunction in these circuits underlies aberrant decision-making observed in numerous psychiatric disorders. This review will highlight recent findings from studies exploring these questions using a variety of behavioural assays, as well as molecular, pharmacological, neurophysiological, and translational approaches. We begin with a discussion of how neural systems related to decision subcomponents may interact to generate more complex decisions involving risk and uncertainty. This is followed by an overview of interactions between prefrontal-amygdala-dopamine and habenular circuits in regulating choice between certain and uncertain rewards and how different modes of dopamine transmission may contribute to these processes. These data will be compared with results from other studies investigating the contribution of some of these systems to guiding decision-making related to rewards versus punishment. Lastly, we provide a brief summary of impairments in risk-related decision-making associated with psychiatric disorders, highlighting recent translational studies in laboratory animals.
Keywords: decision making, uncertainty, punishment, prefrontal cortex, nucleus accumbens, lateral habenula, dopamine, mania
Cost/benefit decision-making is a fundamental executive process that is common across species, ranging from worms, rodents, non-human primates and of course, humans. In particular, all organisms are faced on a daily basis with choices between options that differ in their expected reward and potentially negative consequences that may accompany those rewards. Thus, a system that integrates information related to risk and reward, as well as internal motivational drives and environmental factors, is crucial to be able to make adaptive decisions and guide subsequent behavior. In humans, most individuals are able to calculate the relative costs and benefits of options and make appropriate choices; however, maladaptive decision-making is a behavioral hallmark of several psychiatric conditions. For example, individuals diagnosed with substance use disorders display an increased propensity to engage in risky behavior, such as unprotected sex and intoxicated driving (Lejuez et al., 2005; Pulido et al., 2011). Other psychiatric conditions, such as anorexia and schizophrenia, are characterized by a pathological decrease in risk-taking behavior (Kaye et al., 2013; Reddy et al., 2014). Thus, a better understanding of the neurobiology underlying normal risky decision-making will provide insight into how these processes may go awry in pathological conditions.
Seminal work by Bechara and colleagues provided the first neurobiological clues as to how the brain mediates decision-making under risk. Using what has become a well-established behavioral assay of risky decision-making, the Iowa Gambling Task (IGT), Bechara et al. (1994, 1999) demonstrated that patients with prefrontal cortical damage were impaired in this task. In the IGT, subjects are asked to choose between different decks of cards, which differ in their long-term profitability. While healthy subjects choose cards that yield longer term payoffs, patients with prefrontal damage [encompassing the ventromedial and orbitofrontal (OFC) subregions] choose cards that yield a large immediate gain, but are accompanied by even larger losses in the long-term, indicating that damage to this brain region increases risky choice. Further insight into the role of the prefrontal cortex (PFC) in risky decision-making derives from more recent neuroimaging experiments in subjects diagnosed with psychiatric conditions characterized by pathological risk-taking behavior. Several studies have shown that substance abusers exhibit hypoactivation of various subregions of the PFC during decision-making (Fishbein et al., 2005; Crowley et al., 2010) and that this decreased functional activity is associated with preference for risky choices (Fishbein et al., 2005). Similarly, adults with attention deficit hyperactivity disorder (ADHD), a condition also associated with elevated risky decision-making (DeVito et al., 2008; Klein et al., 2012), have less extensive activation of the PFC relative to controls during risky decision-making (Ernst et al., 2003).
The PFC, as well as other brain regions implicated in risky decision-making, receives robust dopaminergic input from the ventral tegmental area (VTA). As such, alterations in dopamine (DA) signaling may have a deleterious impact on decision-making under risk. This is indirectly supported by studies that have assessed decision-making in individuals suffering from psychiatric conditions in which perturbations in the dopaminergic system are thought to be an underlying cause. For instance, stimulant abusers, schizophrenics, and patients with ADHD display maladaptive risky decision-making both in real world measures (Friedman, 1998; Lejuez et al., 2005; Klein et al., 2012; Ramos Olazagasti et al., 2013) and when assessed in laboratory gambling tasks (Rogers et al., 1999b; Bornovalova et al., 2005; Leland and Paulus, 2005; Schneider et al., 2012). As further evidence, when treated with DA agonists, patients with Parkinson’s disease and Restless Legs Syndrome can develop increased risk-taking (Dagher and Robbins, 2009), clearly indicating a role of DA in modulating risk-based decision-making. Finally, more recent studies have shown that poor performance in the IGT in pathological gamblers is associated with increased DA release in the ventral striatum (Linnet et al., 2011a, b). Altogether, these studies reveal the important contribution of the dopaminergic system to risky decision-making, and suggest that dysregulation in this system is a major cause of pathological risk-taking.
While informative, studies in humans are limited in their ability to determine how specific brain regions, and in particular, how neurochemical signaling within these regions, facilitate different component processes related to decision-making. Animal models have allowed researchers to address fundamental questions about the neural mechanisms supporting different forms of risky decision-making and how these systems may be impacted in pathological states. Critical to these questions, however, is understanding how different behavioral and cognitive elements of decision-making sculpt subsequent behavior. For example, making a decision between multiple options entails consideration of past outcomes, the relative value of the benefits associated with each option, and the valence and type of risk that may accompany those options, all of which must be integrated with other environmental and motivational factors in order to execute or inhibit behavior. The main endeavor of this review is to describe these processes and to highlight recent animal studies investigating the neural bases of different forms of risk-based decision-making. It begins with a discussion of the basic cognitive and neural systems underlying decision-making behaviors, followed by reviews of the neurobiology of two distinct animal models of decision-making involving different types of uncertainty or risk. Finally, the review extends this discussion to psychiatric conditions characterized by impaired risk-based decision-making, and highlights the necessity of assessing different components of decision-making so as to gain a more comprehensive understanding of these disorders.
Decision Making Is Supported by Multiple, Diverse Neural Systems
As alluded to previously, decision-making encompasses a complex conjunction of many behaviors. This is exemplified by the fact that there is an entire field related to sensorimotor decision-making, a close cousin of the type of motivational decision-making addressed here. The framework for sensorimotor decision-making is frequently rooted in neurophysiological studies, in which temporal components of behavior are well-controlled. These studies focus on the accumulation of information that ultimately allows a decision to be made and a response to be executed. For example, many studies have investigated decisions related to ambiguous visual stimuli, such as a field of dots in which some percentage (0% to 100%) is moving coherently in one direction while the others are moving randomly (Shadlen and Newsome, 1996). Subjects in this case must decide which direction the dots are moving by making an eye movement in that direction. Based on the percentage of motion coherence, the decision can be easier (100%) or harder (<25%). For many years, investigators have used neuronal recording, inactivation, and stimulation to interrogate the brain areas involved in this type of task, with a focus on determining at what point sensory information has accumulated such that the subject is able to make a decision. Work from many labs (Gold and Shadlen, 2007; Lee et al., 2007; Hayden and Pasternak, 2013; Romo, 2013; Shadlen and Kiani, 2013) has shown that neuronal activity, particularly in higher-level sensory and sensorimotor cortex, builds from cue onset and peaks at decision completion. This decision completion is demonstrated by the execution of a behavioral response, for example, a saccadic eye movement. Neural signals across multiple brain areas, including sensory, motor and prefrontal cortex (Uchida et al., 2006; Lee et al., 2007; Romo, 2013; Shadlen and Kiani, 2013), basal ganglia and midbrain (Felsen and Mainen, 2012; Ding and Gold, 2013), and neuromodulatory systems such as the locus coeruleus (Aston-Jones and Cohen, 2005) are involved in aspects of these relatively simple types of decision (e.g., deciding if the dots are moving left or right). Stimulation of these key regions can bias decisions depending on the area influenced (Salzman et al., 1990; Cohen and Newsome, 2004). Computational models (e.g.; drift diffusion model), have also been developed which accurately characterize this accumulation of neural information ultimately leading to a decision (Aston-Jones and Cohen, 2005; Shadlen and Kiani, 2013). Despite the relatively straightforward question (“is the cue going left or right?”), a comprehensive characterization of sensorimotor decision-making is still in development. However, the accumulated body of research has demonstrated that critical neural dynamics, such as escalation of neuronal activity with accumulating decision certainty (Gold and Shadlen, 2007), underlie many types of decisions and have allowed a close inspection of the neural components of the multifaceted aspects of decision-making (Hayden and Pasternak, 2013; Shadlen and Kiani, 2013).
One important implication from this growing body of work is that no one brain area independently regulates decision-making. Even the types of basic sensorimotor decisions described above require a wide range of neural systems, likely working in parallel. The complexity of neural signaling underlying simple perceptual decisions argues that cost/benefit decision-making, as discussed below, will involve equally, and likely more, diverse neural representations at the level of brain regions, neuromodulators, and neural circuits. In addition, complex decisions underlying risk and probability are all extensions of fundamental sensory, cognitive, emotional, and motor functions. These processes summate to allow risky decision-making, and some subset of these processes is disrupted in psychiatric and neurological diseases in which decision-making is impaired (Paulus, 2007). For example, when deciding between options associated with either high-risk or low-risk rewarded outcomes, a rat must have a neural representation of the location of each lever associated with these options. In addition, the rat must associate each potential response with a possible outcome, guided by memory, context and other cognitive factors (which lever is associated with which outcome and the history of outcomes after selecting that lever) as well as motivational and emotional states (hunger, fear of a negative outcome, etc.). A behavioral plan must then be developed and executed based on the accumulated information. At a relatively high level, this involves planning the execution of some behaviors while inhibiting others (e.g., directing behavior toward one option versus the other). After completion of the motor sequence, neural signals reflect acquisition and receipt of reward, a non-rewarded event, or some form of punishment, and there is an evaluatory component to behavior: did the expected outcome match the actual outcome? This information is factored into future planning by the animal to maximize rewards. If the probability of receiving a reward is low, the animal may switch strategies at the next opportunity and pursue higher probability rewards even if they may be smaller in magnitude (Cardinal, 2006; Floresco et al., 2008a). All of this information is integrated to bias action selection in what we consider cost/benefit decision-making.
When discussing disorders related to decision-making, it is equally important to consider the specific deficits exhibited by patients in order to precisely localize the source of the disease and address possible treatments. Thus, although patients suffering from psychiatric or neurological disease may present deficits in decision-making, these deficits could stem from a range of disordered subcomponents of decision-making, such as response planning or value representation. This conceptualization is very much in line with the National Institute of Mental Health’s recent emphasis on research domain criteria (RDoC), the goal of which is to emphasize neural systems related to specific behavioral and cognitive functions that cut across disorders, such as working memory, reward learning, or goal selection (http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml). In essence, this is an emphasis on behavioral (and cognitive) neuroscience as a way of furthering our understanding of mental disorders. The RDoC framework is also informative in the context of understanding the neural basis of complex behaviors such as decision-making. At the same time that we probe the neural systems specifically related to explicit decision-making tasks (see sections on uncertainty and punishment, below), investigations should dissect the fundamental behavioral and cognitive elements of decision-making and attempt to understand their relationships to neural function.
Thus, when attempting to characterize the neural correlates of decision-making, it is essential to consider the cognitive, emotional, motivational, and behavioral subprocesses that are combined to produce a decision. As noted above and elsewhere in this review, a diverse set of brain areas likely function together to guide risky decision-making. However, there is an additional critical issue to take into consideration: each of these neural systems does not perform a single function, but rather, may actually play multiple roles in tasks used to test decision-making. In both decision-related tasks and other types, these roles may even be opposite one another – signaling appetitive vs. aversive outcomes, for example. Hence, throughout this review, we will discuss, for example, what function the PFC, nucleus accumbens (NAc) or basolateral amygdala (BLA) plays in decision-making behavior. In reality, these areas and others can perform multiple functions that may be unrelated or even interfere with efficient decision-making.
One obvious example of a brain area involved in a wide array of behaviors (including risky decision-making), is the medial PFC (mPFC). The mPFC plays a major role in the regulation of decision-related behavior, as seen in the context of risky decision-making and related tasks in rodents (Floresco et al., 2008a; Jentsch et al., 2010; St Onge and Floresco, 2010a; Simon et al., 2011; St Onge et al., 2011; St Onge et al., 2012a; St Onge et al., 2012b), and more generally across a number of other tasks (Kesner and Churchwell, 2011; Euston et al., 2012; Cassaday et al., 2014). Broadly speaking, the mPFC in rodents is widely considered to subserve a variety of executive functions that are engaged during decision-making (Cardinal, 2006; St Onge and Floresco, 2010; Euston et al., 2012; Chudasama, 2011). These include working memory, attention, planning, impulse control, action/outcome monitoring and behavioral flexibility, etc. (De Bruin et al., 2000; Dalley et al., 2004; Kesner and Churchwell, 2011; Euston et al., 2012; Bissonette et al., 2013; Cassaday et al., 2014). In many ways, these cognitive phenomena are all root functions of decision-making. However, the mPFC has also been associated with fundamental behavioral processes such as the motivational states associated with rewards or punishments. In many cases, the functions ascribed to the mPFC seem at odds with one another. For example, some studies suggest that mPFC activity plays a role in regulating seeking of drug- or natural rewards (Tzschentke, 2000; Peters et al., 2009; Lasseter et al., 2010b; Ishikawa et al., 2008b, a) whereas other studies emphasize the mPFC in the representation of aversive stimuli and contexts as in fear conditioning (Morgan and LeDoux, 1995; Maren and Quirk, 2004; Peters et al., 2009; Sotres-Bayon and Quirk, 2010; Quirk and Mueller, 2008; Orsini et al., 2011). An important question, then, is what are the basic properties of the mPFC that allow such a diversity of behaviors and, consequently, play such an important role in decision-making?
Anatomical segregation of mPFC function - dichotomies and beyond
One possible explanation for these differential and even diverging properties of mPFC function lies in anatomical differences. The mPFC is made up of multiple subregions along the dorsal ventral axis (Heidbreder and Groenewegen, 2003; Vertes, 2004). In particular, the dorsal regions of the mPFC (typically the prelimbic cortex, PL, and sometimes including the anterior cingulate cortex, ACC) have been demonstrated to play a different, and sometimes even an opposite, role to the ventral mPFC (infralimbic cortex, IL) Killcross and Coutureau, 2003; Peters et al., 2009; Van den Oever et al., 2010; Euston et al., 2012; Gass and Chandler, 2013; Smith and Graybiel, 2013; Cassaday et al., 2014). In the context of fear conditioning, PL plays a more important role in encoding fear-related stimuli and associated behavioral responses (e.g., freezing). In contrast IL appears to play a more important role in inhibition of fear responses, particularly in the context of extinction learning (Morgan and LeDoux, 1995; Maren and Quirk, 2004; Peters et al., 2009; Sotres-Bayon and Quirk, 2010; Quirk and Mueller, 2008; Orsini et al., 2011). Similar dichotomies have been proposed in the realm of appetitive behaviors: PL appears to be more associated with driving responses to acquire reward whereas IL may be more associated with withholding responses (Ishikawa et al., 2008; Peters et al., 2009; Gass and Chandler, 2013). Consideration of this dichotomy is of particular interest in decision-making as virtually all decisions require a complex conjunction of response execution and response inhibition. In the context of the rodent studies described in this review, a risk-based decision ultimately comes about through execution of a response to the chosen option (e.g., a press on a “risky” lever) and inhibition of alternate responses (e.g., not pressing the “safe” lever). Although studies of risk-based decision-making incorporate additional parameters such as outcome prediction and evaluation, understanding how responses are executed and inhibited has the potential to provide fundamental insights into the nature of decision-making. A dichotomous model of mPFC function whereby dorsal and ventral mPFC compete for control over behavior would be an appealing way to explain the respective contributions of these areas to decision-making. Based on the results described above and elsewhere (Peters et al., 2009; Moorman et al., 2014) one possible model might be that PL drives active execution of behavior (reward-seeking or fear-related freezing) whereas IL supports inhibition of behavior (restraint from reward seeking or freezing during extinction).
Unfortunately, a clear dichotomy along these lines is complicated by diverging results. This is particularly noticeable in literature pertaining to appetitive motivation (Moorman et al., 2014). Thus, in some circumstances PL activation appears to promote the seeking of natural or drug rewards (Ishikawa et al., 2008b; McFarland et al., 2003; McLaughlin and See, 2003; McFarland et al., 2004; Hearing et al., 2008; Stefanik et al., 2013; Sparta et al., 2014), whereas in other contexts it plays an important role in inhibition of responses (Ishikawa et al., 2008b; Jonkman et al., 2009; Willcocks and McNally, 2013; Chen et al., 2013; Mihindou et al., 2013; Martin-Garcia et al., 2014, Broersen and Uylings, 1999; Risterucci et al., 2003; Narayanan et al., 2006; Narayanan and Laubach, 2006; Bari et al., 2011). Similarly, although IL has been characterized as suppressing behavior, particularly after extinction (Ishikawa et al., 2008b; Peters et al., 2008; Peters et al., 2009; LaLumiere et al., 2010; Rhodes and Killcross, 2007; Peters and De Vries, 2013; Van den Oever et al., 2013; Chudasama et al., 2003; Murphy et al., 2005; Murphy et al., 2012), the region is also important for driving the seeking of drugs or natural rewards (Bossert et al., 2011; Ishikawa et al., 2008b; Koya et al., 2009; Rogers et al., 2008; Sangha et al., 2014) in other contexts. In fact, neurons in both areas appear to be activated during both execution and inhibition of reward and drug-seeking (Burgos-Robles et al., 2013; Narayanan and Laubach, 2009; West et al., 2014). This lack of consistent association between region and function has profound relevance for the role of these areas in decision-making tasks, particularly those motivated by reward acquisition. In general, it underscores that there are additional levels of complexity that should be considered when ascribing functions to brain areas.
Lest we assume that this level of complexity is unique to the prefrontal cortex, it should be noted that there are striking degrees of functional heterogeneity in a wide range of brain areas frequently associated with decision-making behavior. For example, the BLA plays important roles in fear conditioning, appetitive motivation, salience encoding, memory, and a wide range of other cognitive and emotional functions (Janak and Tye, 2015). In some cases, these differences may be due to different functions being associated with different amygdala subregions. For example, whereas the rostral but not caudal BLA is important for responding to drugs of abuse but not food rewards (Kantak et al., 2002, McLaughlin and See, 2003), the caudal but not rostral BLA has been shown to be important for withholding responses to extinguished food-related cues (McLaughlin and Floresco, 2007). Even in the same region, neurons in the BLA sometimes the same neuron – fire in relation to both appetitive and aversive outcomes (Sangha et al., 2013) and during response execution and inhibition (Tye and Janak, 2007; Tye et al., 2010). In addition to the BLA, recent results have demonstrated that DA neurons in the VTA encode appetitive and aversive outcomes depending on anatomical connectivity (Brischoux et al., 2009; Matsumoto and Hikosaka, 2009b; Bromberg-Martin et al., 2010; Schultz, 2010; Lammel et al., 2014). Similarly, although it is well established that dopamine release in the NAc occurs during rewarding situations, aversive outcomes and their predictors can enhance ventral striatal DA transmission as well (Anstrom et al., 2009; Oleson et al., 2012; Wenzel et al., 2015).
These findings in mPFC and other brain areas underscore two points with respect to interpreting the function of brain regions in the context of decision-making and other complex behaviors. First, the behavioral context in which neural systems are tested makes an important contribution to interpretation. So, for example, it might be less useful to consider whether PL vs. IL encodes initiation vs. suppression of behaviors because these behavioral dimensions may be tangential to the main functions of these regions. Along these lines, there have been other frameworks proposed for mPFC subdivisions. A notable example that is supported by a considerable amount of data is the distinction between goal directed behavior (thought to be driven by dorsal mPFC) vs. habitual behavior (thought to be driven by ventral mPFC) (Balleine and Dickinson, 1998; Killcross and Coutureau, 2003; Balleine and O’Doherty, 2010; Smith et al., 2012). Of course we caution against shifting from one strict dichotomous assignment of function (Go vs. Stop) to another (Goal vs. Habit). However, it is certainly likely that one of the functions of PL is to drive goal directed behavior, etc., perhaps in conjunction with playing important roles in other behavioral parameters – fear expression, direction of attention, memory, and representation of context, for example. Rather than see these regional functions as absolute, it might be more valuable to consider each behavioral association as a member of a constellation of behaviors in which each area plays a role.
The second point is that within-area neural heterogeneity is likely an important contributor to the diversity of behaviors associated with these regions. One clear explanation for how brain regions as complex as the mPFC and amygdala can play a broad range of roles across behavior is through the presence of multiple, interdigitated networks within each region (Moorman et al., 2014; Riga et al., 2014; Janak and Tye, 2015). So, for example, PL neurons that project to the NAc may play an important role in appetitive motivated behaviors whereas PL neurons that project to the BLA may play a more important role in directing the expression of certain emotional responses. Of course, this characterization, while more sophisticated than asking what the PL “does” is still likely an oversimplification – there are also probably PL-BLA networks associated with appetitive motivation, for example. Conversely, afferent and neuromodulatory factors may play a role in defining functions. Prefrontal neurons with specific receptor subtypes or receiving afferent input from specific brain areas may play a vastly different role than members of other networks. Finally, it is important to remember that there are different phenotypes of neurons in almost all brain areas studied in the context of decision-making. In cortex, there are multiple subpopulations of both glutamatergic pyramidal and GABAergic neurons (Kvitsiani et al., 2013; Pi et al., 2013; van Aerde and Feldmeyer, 2013; Lee et al., 2014). In the NAc, there are subpopulations of medium spiny neurons (e.g., D1 vs. D2 receptor expressing) and multiple types of interneurons (Lenz and Lobo, 2013). In the VTA, there are complex conjunctions of dopamine, glutamate, and GABA, both across and even within neurons (Tritsch et al., 2012; Stamatakis et al., 2013; Morales and Root, 2014). In addition to network connectivity, it is crucial to incorporate diversity of neuronal phenotype into an overarching model of neural systems sculpting decision-making and related behaviors. By incorporating the confluence of network connectivity, neuronal phenotype, and possibly even other factors, we will begin to develop a more sophisticated understanding of the neural systems associated with specific behaviors, likely resolving many of the apparent contradictions described above.
It is essential to remind ourselves that decision-making is a complex behavior. This is particularly the case when we consider decisions made based on evaluation of risk, reward uncertainty and the likelihood of punishment, as discussed below. In considering how neural systems support decision-making, we must incorporate multiple levels of complexity, as discussed above. This complexity includes behavioral complexity – all of the cognitive and motivational components, each with neural systems supporting that specific function – as well as neural complexity. On the one hand, decision-making related behaviors are supported by interaction across a broad collection of brain areas, many of these are described below. On the other hand, each brain area performs multiple functions, including those unrelated to decision-making, likely supported by diverse ensembles of neurons within and across brain areas. Moving forward, merging a large-scale network view (the interaction of brain areas involved) with a more refined, neuronal network view (the interaction of specific populations of neurons within each region) will ultimately provide a crucial perspective on decision-making that either view alone will not permit. However, in order to know where to look for decision-related circuits, we must first have a well-characterized description of which brain regions and modulatory systems are even involved.
Neural circuitry mediating choice of uncertain rewards
A key component of more complex forms of reward-related decision-making entails monitoring the outcomes of one’s actions over time. Sometimes certain actions are rewarded, while other times they are not, and a decision-maker must keep track of how well his or her choices are paying off in order to maximize long-term gains. These decisions can be modulated further by information about the relative payoffs and risk associated with different actions. For example, imagine tracking a stock on a day its price increases after favorable news. With every uptick and downtick in price, you battle competing urges to either sell and put the proceeds into savings, or hold on and see if you can maximize your gains, and a variety of factors can influence your decision (historical performance of the stock, how much you stand to make, your individual tolerance for risk, etc). As another example, envision playing blackjack with a 7-deck shoe. The house odds on each hand are a little better than 50%, yet wins (or losses) often come in streaks. In lieu of counting cards, a typical player may keep rough estimates of how the last few hands have gone, factor that in with how much money has been won or lost during the session, and judge whether to continue gambling or if it is time to leave the table (hopefully with a profit, or at least minimizing the losses).
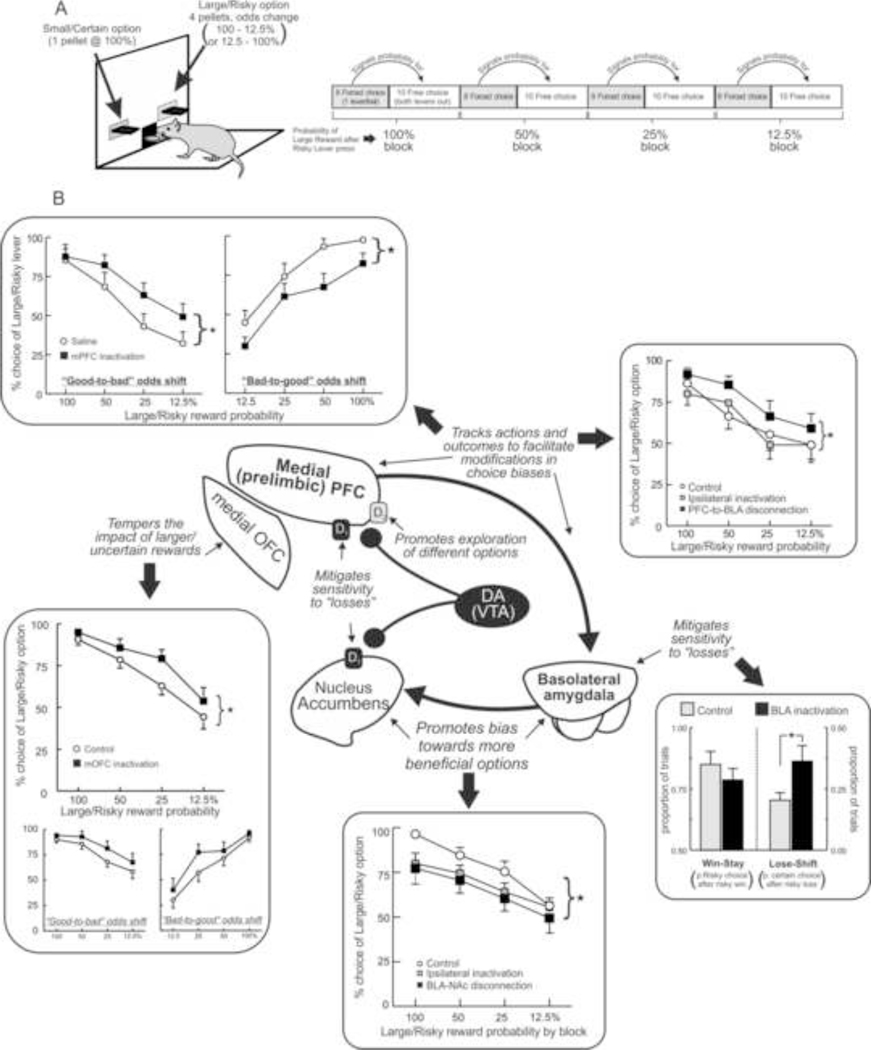
In laboratory animals, these types of processes can be modeled using tasks that require subjects to choose between options associated with different magnitudes of reward and varying probabilities of obtaining them. A series of studies by Floresco and colleagues have utilized a probabilistic discounting procedure wherein rats choose between a small/certain (1 reward pellet) and large/risky reward (4 pellets; Figure 1A). Over the course of a daily session, the probability of obtaining the larger reward changes in a systematic manner over blocks of free-choice trials, ranging from 100 to 12.5% either in a descending (“good-to-bad”) or ascending (“bad-to-good”) manner. Aside from blocks of forced-choice trials that precede free-choice periods, there are no explicit cues signaling changes in reward probabilities. Thus, over the course of a session, rats must keep track of their choices and their outcomes to ascertain when it may be more profitable to choose more risky or conservative. After approximately 20 days of training, rats display stable patterns of discounting, selecting the large reward more often when the probabilities are high (100–50%) and less so when the odds are lower. This extended training ensures that rats are familiar with both the basic reinforcement contingencies associated with the different options and with the changes in reward probabilities that occur during a session. Neural manipulations are then employed using a within-subjects design to probe how transient disruptions of different nodes within decision circuitry alter choice biases and ascertain how they may contribute to different processes related to risk/reward decision-making.
Figure 1:
A summary of some of the neural circuits involved in risk/reward decision-making involving choice between smaller certain and larger uncertain (or risky) rewards. (A) Depiction of the probabilistic discounting task used to asses risk/reward decision-making in rodents. (B) Summary of the dissociable functions of cortical, limbic and striatal nodes within DA circuitry in regulating probabilistic discounting, as inferred by inactivation and circuit disconnection studies. BLA inactivation reduces risky choice by increasing sensitivity to reward omissions (bottom right). Subcortical circuits incorporating the BLA and the NAc bias choices towards larger uncertain rewards, as disconnection between these regions reduces risky choice [bottom center: adapted from St Onge et al., (2012b)]. In contrast, the medial OFC tempers the impact that large, uncertain rewards exert over subsequent choices, as inactivation of this region increases risky choice [bottom left: adapted from Stopper et al., (2014a)]. Inactivation of the prelimbic region of the medial PFC impairs modifications in decision biases, affecting risky choice differentially depending on whether reward probabilities decrease or increase over time [top left: adapted from St Onge and Floresco, (2010)]. This function of the medial PFC is mediated via top-down interactions with the BLA; disconnection of descending PFC→BLA projections also impairs shifts in choice biases [top right: adapted from St Onge et al., (2012b)]. DA further refines risk/reward decision-making, with D1 receptors in the PFC and NAc reducing the impact of non-rewarded choices, and D2 receptors in the medial PFC facilitating adjustments in choice biases to promote exploration of different options (center). For all panels, stars denote significant differences between groups at p<0.05.
Amygdala-ventral striatal circuits bias choice towards larger, risky rewards
Studies using lesions or reversible inactivation of different brain nuclei have revealed a critical role for subcortical circuits linking the BLA and the NAc in biasing choice towards larger, more costly rewards. It has been shown repeatedly that disruption of BLA functioning shifts bias away from larger rewards when their delivery is i) delayed (Winstanley et al., 2004) ii) requires more effort to obtain them (Floresco and Ghods-Sharifi, 2007; Ghods-Sharifi et al., 2009; Ostrander et al., 2011) or iii) uncertain (Ghods-Sharifi et al., 2009). With respect to risk/reward decision-making, neural activity within the BLA appears to play a key role in mediating sensitivity to “losses”, as reductions in risky choice following inactivation of this nucleus are associated with an increased tendency to shift to smaller/certain rewards following a non-rewarded risky choice (Ghods-Sharifi et al., 2009; Figure 1B, bottom right). It is important to note, however, that the BLA does not appear to play a role in biasing choice towards larger, cost-free rewards vs smaller ones (Winstanley et al., 2004; Ghods-Sharifi et al., 2009; Ostrander et al., 2011), suggesting that it plays a more prominent role in guiding choice when a decision-maker must evaluate both the relative benefits and costs associated with different actions. This influence of the BLA appears to be mediated via interactions with the NAc, as lesions or inactivation of this nucleus also reduce preference for larger, more costly rewards (Cardinal et al., 2001; Ghods-Sharifi and Floresco, 2010; Stopper and Floresco, 2011). Interestingly, as opposed to decisions involving effort or delay-related costs, which recruit the lateral core region of the NAc, action selection involving reward uncertainty appears to be more dependent on activity within the medial shell region of this nucleus, although the core may also contribute to certain aspects of performance as well (Cardinal and Howes, 2005; Stopper and Floresco, 2011; Dalton et al., 2014). Moreover, unlike the BLA, which mediates sensitivity to reward omissions, inactivation of the NAc diminishes sensitivity to rewards, reducing the tendency to follow a rewarded risky choice with another risky choice (Stopper and Floresco, 2011). Thus, the BLA and NAc work in concert to promote biases towards larger uncertain rewards via somewhat different mechanisms, a notion supported by the findings that disrupting communication between these regions also reduces risky choice (Figure 1B, bottom) (St Onge et al., 2012b). As such, BLA-NAc circuity seems to help a decision-maker overcome uncertainty costs and provides a visceral, intuitive bias towards chasing larger payoffs.
Prefrontal regions mitigate and refine risk/reward decision-making
The drive provided by BLA-NAc circuitry towards potentially larger payoffs is in turn tempered by different regions of the OFC and mPFC, which may act as a brake on these impulses and help refine choice biases when reward probabilities change. Within the OFC, the medial portion plays a key role in mitigating the impact that larger, probabilistic rewards exert on subsequent choice behavior. Inactivation of this region leads to an increased tendency to select riskier options after these options have recently paid off, irrespective of whether the odds of obtaining the larger reward decreased or increased over a session (Figure 1B, bottom left; Stopper et al., 2014a). In contrast, disruption of lateral OFC function does not alter probabilistic discounting in well-trained animals (St Onge and Floresco, 2010). Instead the contribution of the lateral OFC to risk/reward decision-making may be more important during the initial learning of the relative magnitude and likelihood of rewards associated with different options (Mobini et al., 2002; Pais-Vieira et al., 2007; Zeeb and Winstanley, 2011).
The PL region of the mPFC (anatomically homologous to Area 32 of the anterior cingulate cortex in primates) plays a more evaluative role in refining risk/reward decision biases. Inactivation of this region increased risky choice when reward shifted from good-to-bad (100→12.5%), but had the opposite effect when reward probabilities increased over a session (Figure 1B, top left; St Onge and Floresco, 2010). However, this region of the PFC does not appear to influence the direction of risky choice when reward probabilities remain static within a session. This combination of findings led to the conclusion that the PL region of the PFC guides decisions by monitoring actions and outcomes to track variations in reward probability and modify decision biases to promote optimal patterns of choice. These functions are in turn mediated via top-down control of the BLA. Disconnection of descending PFC→BLA pathway (but not ascending BLA→PFC projections) disrupted decision-making in a manner similar to bilateral inactivation of the mPFC (Figure 1B, top right; St Onge et al., 2012b). Under these conditions, animals were less sensitive to reward omissions, showing a decrease in lose-shift behavior. This collection of findings suggests that the PL region of the PFC appears to play a supervisory role during risk/reward decision-making, monitoring rewarded and non-rewarded actions over time. As the profitability of riskier options varies, the PFC aids in learning about changes in reward contingencies, which may facilitate shifts in decision biases. Alterations in decision biases are executed via descending pathways from PFC to BLA, which temper the influence that BLA-NAc circuitry exerts over the direction of choice. Thus, within these networks, subcortical circuits linking the BLA and NAc provide a visceral, intuitive bias towards options that may yield larger rewards (“go big”), whereas the frontal lobes aid in refining the choice biases to promote more optimal patterns of choice.
Dopamine
The influence that these limbic-cortico-striatal networks exert over action selection during risk/reward decision-making is further refined by DA transmission within forebrain terminal regions. Psychopharmacological studies have revealed key roles for mPFC and NAc D1 receptors in mitigating sensitivity to non-rewarded actions. Blockade of these receptors in either region reduces risky choice during probabilistic discounting, with these effects driven by increased lose-shift behavior (St Onge et al., 2011; Stopper et al., 2013). Thus, activity at these receptor sites promotes exploitation of more favorable situations by facilitating biases towards potentially more profitable options despite their uncertainty. In essence, D1 receptors aid in overcoming uncertainty costs and help a decision-maker keep an “eye on the prize”, maintaining choice biases even when risky choices do not always yield rewards. These functions may be mediated in part through modulation of BLA inputs to these regions, given that the BLA also mediates negative feedback sensitivity (Figure 1B) and D1 receptor activity can potentiate firing of NAc neurons driven by BLA inputs (Floresco et al., 2001; Floresco 2007).
In comparison to D1 receptor activity, D2 receptors in the PFC appear to facilitate modifications in decision biases and promote exploration of different options in response to changes in reward probabilities. Antagonism of these receptors retards shifts in choice biases and increases risky choice during probabilistic discounting when the odds vary from “good-to-bad”, similar to the effects of PFC inactivation (St Onge et al., 2011). The seemingly opposite effects of PFC D1 and D2 receptors may be mediated via actions of DA on separate populations of PFC pyramidal neurons that express one of these receptors exclusively (Gee et al., 2012; Seong and Carter, 2012), or their differential effects on the network activity of PFC neuronal populations (Durstewitz et al., 2000; Seamans and Yang, 2004). On the other hand, manipulation of NAc D2 receptors does not alter risky choice in this assay (Stopper et al., 2013). This is somewhat surprising, given that systemic D2 antagonists reduce preference for larger, uncertain rewards (St Onge and Floresco, 2009). In this regard, it is notable that separate populations of striatal neurons expressing D1 or D2 receptors have been proposed to regulate different patterns of behavior. D1-containing cells may be more important for promoting approach behaviors, whereas selective activation of neurons expressing D2 receptors can be aversive, suggesting that these cells may regulate avoidance behaviors (Lobo et al., 2010; Kravitz et al., 2012). Thus, the apparent lack of involvement of NAc D2 receptors in mediating probabilistic discounting may be related to the fact that in this assay, risky choices are not associated with any explicit punishments per se, but only the possibility of receiving a reward or not. As will be discussed in subsequent sections, striatal D2 receptors may play a more prominent role in regulating risky decision-making when certain actions may yield more preferred rewards but also deliver aversive consequences as well.
Tonic and phasic dopamine signaling and decision-making
Subsequent microdialysis studies provided additional insight into how fluctuations in PFC and NAc DA release relate to modifications in decision biases in well-trained rats performing the same probabilistic discounting task (St Onge et al., 2012a). Within the PFC, DA levels corresponded to changes in large/risky reward probabilities irrespective of whether the odds of obtaining the larger reward decreased or increased over a session. Thus, when the odds were initially 100% and then decreased across blocks, there was a robust initial increase in PFC DA efflux (~80–90% above baseline) that steadily declined over the session, and the opposite profile was observed when the odds shifted from “bad-to-good” over a session. This experiment included a key, yoked-reward control group consisting of rats that were not required to press any levers or make any decisions, but instead were trained to receive food delivered passively on a schedule matched to rats performing the decision-making task. Yoked rats displayed a near-identical profile of PFC DA efflux to that observed during decision-making, confirming that the fluctuations in PFC DA transmission during either condition corresponded primarily to changes in the relative rate of reward received. These findings suggest that DA input to the frontal lobes conveys information about changes in the relative amount of reward availability, and that dynamic fluctuations in mesocortical DA efflux may serve as a reward “running-rate meter,” informing the PFC about changes in reward rates that can aid in adjusting choice accordingly.
Within the NAc, fluctuations in tonic DA levels were also related to changes in reward availability over time. However, DA levels were higher in choice situations during periods when delivery of the larger reward was uncertain, compared to yoked animals that received the same amount of food delivered passively. Thus, rats that had to choose during periods when their actions might not yield a reward displayed higher DA levels compared to those that received the same amount of food but did not have to make any actions to receive it. In addition, changes in NAc DA corresponded very closely to changes in choice behavior, and DA levels were higher during free choice versus forced choice periods. Thus, variation in NAc DA signaling during risk/reward decision-making appears to integrate multiple types of information, including reward uncertainty, choice behavior, and changes in reward availability over time.
Drugs like amphetamine cause large increases in tonic DA efflux within the PFC and NAc. With respect to risk/reward decision-making, these treatments would be expected to occlude changes in these signals associated with variations in reward availability, leading to a more static tonic DA signal. In this regard, amphetamine markedly impairs shifts in risk/reward decision biases during probabilistic discounting, “locking in” choice preferences apparent during the start of a session, in a manner similar to PFC inactivation (St Onge and Floresco, 2009; St Onge et al., 2010). This combination of findings suggests that modifications in choice biases when reward probabilities are volatile may not be regulated as much by the absolute levels of tonic DA within forebrain regions, but rather may be driven primarily by fluctuations of these signals that occur over time.
DA signaling in the NAc is segregated into different compartments regulated by distinct modes of transmission (Grace, 1991; Floresco, 2007; Grace et al., 2007). Microdialysis techniques measure slower changing levels of extrasynaptic or “tonic” DA. In contrast, “phasic” signaling comprises a more spatially and temporally restricted signal (<1 s) mediated by burst firing of DA neurons. Recent in vivo voltammetry studies have revealed that different aspects of cost/benefit decision-making are associated with changes in phasic DA signaling within the NAc. Phasic DA signals track decision outcomes during risk/reward decision-making, wherein receipt of larger versus smaller rewards triggers proportional increases in DA, and reward omissions cause phasic suppressions or “dips” in DA levels (Sugam et al., 2012), in keeping with the idea that these signals encode reward prediction errors (Schultz, 2013). In addition, phasic DA signals prior to action selection appear to encode the expected availability of larger/more-preferred rewards (Day et al., 2010; Sugam et al., 2012).
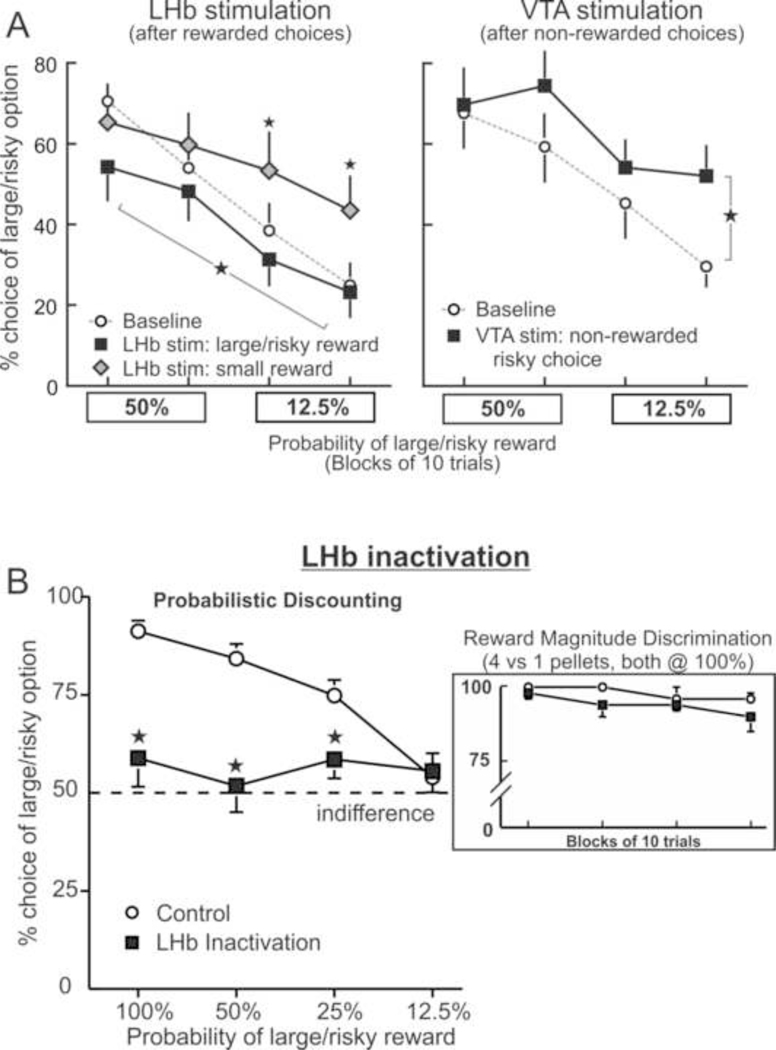
Pharmacological manipulations disrupt both tonic and phasic DA signaling, making it difficult to disentangle the specific contribution of pre-choice and outcome-related phasic DA signals to action selection during risk/reward decision-making, relative to slower-changing tonic DA levels. Circumvention of this issue may be achieved through the use of more temporally-precise manipulations to override natural phasic signals associated with different phases of decision-making. A recent study used this approach with rats well-trained on a modified version of probabilistic discounting task described previously (Stopper et al., 2014b). Brief trains of electrical stimulation were delivered to either the DA cell body region within the ventral tegmental area, or to the lateral habenula (LHb), a nucleus that exerts powerful inhibitory control over the firing of midbrain DA neurons. LHb stimulation induces robust suppression of DA neural firing that resembles phasic dips associated with reward omissions (Christoph et al., 1986; Ji and Shepard, 2007). This is mediated via disynaptic circuits linking excitatory LHb projections to the rostromedial tegmental nucleus (RMTg), which in turn sends inhibitory GABAergic projections onto DA neurons (Jhou et al., 2009; Lammel et al., 2012).
Stimulation of the LHb (or RMTg) coinciding with rewarded choices markedly affected subsequent action selection. When animals played risky and were rewarded, stimulation during reward delivery (i.e. periods associated with phasic increases in DA; Sugam et al., 2012) shifted bias towards the smaller/certain option (Figure 2A, left). Likewise, stimulation contingent with delivery of the smaller/certain reward caused the opposite effect, shifting biases towards the large/risky option. Notably, these manipulations did not affect preference for larger, certain rewards versus smaller ones, in keeping with the idea that DA does not play a role in mediating decisions based primarily on differences in reward magnitude. Conversely, when the VTA was stimulated following a non-rewarded risky choice (i.e. overriding DA phasic dips associated with these events), rats showed reduced sensitivity to reward omissions and an increase in risky choice, behaving as if the risky option was providing more reward than it actually was (Figure 2A, right). The findings that overriding phasic bursts and dips in DA activity associated with decision outcomes can redirect action selection provide compelling evidence that these signals convey short-term feedback information about recent action outcomes that can increase or decrease the likelihood that those actions are selected again (Schultz, 2013).
Figure 2.
Phasic DA signals and risk/reward decision-making (A) Suppression of outcome-related phasic DA signals via LHb stimulation altered choice biases. LHb during receipt of larger or smaller rewards (left) shifted bias towards the alternative option. Conversely, inducing phasic DA burst in DA activity non-rewarded risky choices (via VTA stimulation, right) increased risky choice. Adapted from Stopper et al. (2014b). (B) Inactivation of the LHb induced a massive disruption in decision-making, causing rats to be indifferent to either option irrespective of their relative value. However, these manipulations did not affect choice during simpler decisions when choosing between smaller vs. larger certain rewards of equal costs (inset). Adapted from Stopper and Floresco (2014). For all figures stars denote significant differences at p < 0.05.
In another experiment, LHb stimulation was given prior to rats making a choice, to ascertain whether these signals can influence action selection. This manipulation markedly increased choice latencies, suggestive of reduced incentive salience attributed to the reward-associated levers (Flagel et al., 2011; Danna et al., 2013). Suppression of pre-choice DA signals also altered choice patterns, in that it reduced selection of the more preferred of the two options, with this effect being most pronounced when rats normally preferred the option associated with the larger reward. Thus, phasic increases in DA occurring prior to action selection appear to aid in directing behavior towards more preferable rewards (Morris et al., 2006).
This collection of findings suggests that DA phasic bursts and dips, in conjunction with fluctuations in tonic DA signaling, play separate yet complementary roles that may form a system of reward checks and balances. Outcome-related phasic DA signals provide rapid feedback on whether or not recent actions were rewarded to quickly update a decision-maker’s framework for subsequent action selection. As these signals are integrated by other nodes of DA decision circuitry to establish and modify choice biases, phasic increases in DA prior to a choice promote expression of preferences for more desirable options. In comparison, slower fluctuations in tonic DA may provide a longer-term accounting of reward histories and average expected utility so that individual outcomes are not overemphasized, ensuring that ongoing decision-making proceeds in an efficient, adaptive and more rational manner.
The lateral habenula and subjective decision biases.
Stimulation of the LHb can be a useful tool to manipulate phasic bursts in DA neuron activity. With respect to the normal function of this nucleus, neurophysiological studies have revealed that LHb neurons encode negative reward prediction errors opposite to that displayed by DA neurons, exhibiting increased phasic firing in expectation of, or after, aversive events and reduced firing after positive outcomes (Matsumoto and Hikosaka, 2007, 2009a; Bromberg-Martin and Hikosaka, 2011). Importantly, glutamatergic neurons of the LHb display relatively high rates of spontaneous activity [>30 Hz; (Matsumoto and Hikosaka, 2007; Hong et al., 2011)]. As such, reductions in firing associated with rewarded outcomes would be expected to convey as much information to downstream structures as phasic increases in activity associated with reward omissions or other unpleasant events. Nevertheless, an impression that has emerged is that the LHb may serve as an “anti-reward” center that may underlie aversion or disappointment, a supposition supported by the findings that LHb stimulation promotes conditioned avoidance and reduces reward-related responding (Lammel et al., 2012; Stamatakis and Stuber, 2012). However, a limitation of these approaches is that they cannot readily identify how normal functioning of this nucleus contributes to overt behavior. Although prolonged stimulation of the LHb can induce aversive states, these studies are looking at only one side of the coin, as they cannot mimic the dynamic increases and decreases in phasic firing normally associated with aversive/rewarding events.
To explore how LHb signals may contribute to risk/reward decision-making, Stopper and Floresco (2014) investigated the effects of inactivation of this nucleus on probabilistic discounting. Given the general impression that the LHb conveys some form of “disappointment” signal that encodes reward omissions, combined with the observations that LHb inactivation increases mesolimbic DA release (Lecourtier et al., 2008), a parsimonious expectation would be that these manipulations might increase preference for the larger/uncertain reward. However, these manipulations induced a much more profound effect, in that they completely abolished any bias subjects had for either option (Figure 2B). Disruption of LHb signal outflow rendered animals absolutely indifferent to which option may be more preferable, inducing unbiased and random patterns of responding so that choice of either option did not differ from chance. Similar effects of LHb inactivation were observed in rats performing a delay discounting task in which they chose between either a small, immediate reward or larger, delayed reward (both delivered with 100% certainty). However, LHb inactivation did not affect preference for larger, cost free rewards vs smaller ones (Figure 2B, inset).
The massive disruption in decision biases induced by suppression of the LHb suggests that the differential signals it encodes about the expectation or occurrence of negative/positive events play a critical role in helping an organism make up its mind when faced with ambiguous decisions regarding the costs and benefits of different actions. Activity within this nucleus aids in biasing behavior from a point of indifference toward committing to choices that may yield outcomes perceived as more beneficial. Given the powerful influence this nucleus exerts over DA neural activity, expression of these subjective preferences is likely achieved through subsequent integration of these dynamic signals by regions downstream that include midbrain DA neurons. Thus, integration of differential LHb reward/aversion signals may provide a tone that is crucial for expression of preferences for one course of action over another. In turn, suppression of these signals would be expected to leave phasic (and tonic) DA signaling in disarray, rendering a decision-maker incapable of determining which option may be “better”, indicating the importance of these signals in guiding ongoing reward seeking.
The findings reviewed above have broadened our understanding of the relative contribution of different limbic, striatal, cortical and dopaminergic circuits to decision-making about uncertain rewards. However, it is important to emphasize that many real-life decisions that we face not only involve choices to maximize our payoffs, but often require us to avoid unpleasant consequences as well. In this regard, recent studies have shown that although there exists considerable overlap between the circuits that guide choice towards better rewards and those that move us away from aversive outcomes, there are considerable dissociations within the neural machinery that regulate these different types of decisions.
Neural circuitry mediating punishment-related decision-making
As described in the preceding section, many real-life decisions involve choices in which there is a risk that a desired outcome will be delivered (i.e., be omitted). In other situations, however, the “risk” does not solely involve reward omission, but may also involve the possibility of a distinct, adverse outcome (a punishment), which can offset the subjective value of the desired reward. For example, a compulsive gambler’s loss in a high-stakes game of blackjack represents not only a failure to win (reward omission), but also a set of punishing consequences resulting from the lost money (e.g., gambling debts, family discord, etc.). Similarly, the decision to purchase and consume cocaine will likely result in the desired outcome (a cocaine high), but may be accompanied by risks of medical or legal consequences. The presence of these potential punishments in the latter example will likely alter a cocaine user’s decision calculus, and may decrease the likelihood of future cocaine use, depending on the probability, magnitude, and timing of the punishment. Unlike reward omission, however, for which the value can be calculated as a function of reward probability, it is less clear how punishment value is calculated (as it involves a stimulus modality different from the reward), and how such punishment value is integrated with reward value to ultimately arrive at a decision. To begin to address these issues, Setlow and colleagues have employed a behavioral task in rats that involves choices between a small, “safe” food reward and a large, “risky” food reward accompanied by risk of punishment. This task is described below, along with data indicating important differences in the neural mechanisms underlying the effects of punishment vs. reward omission on choice behavior.
A rat model of decision-making under risk of punishment.
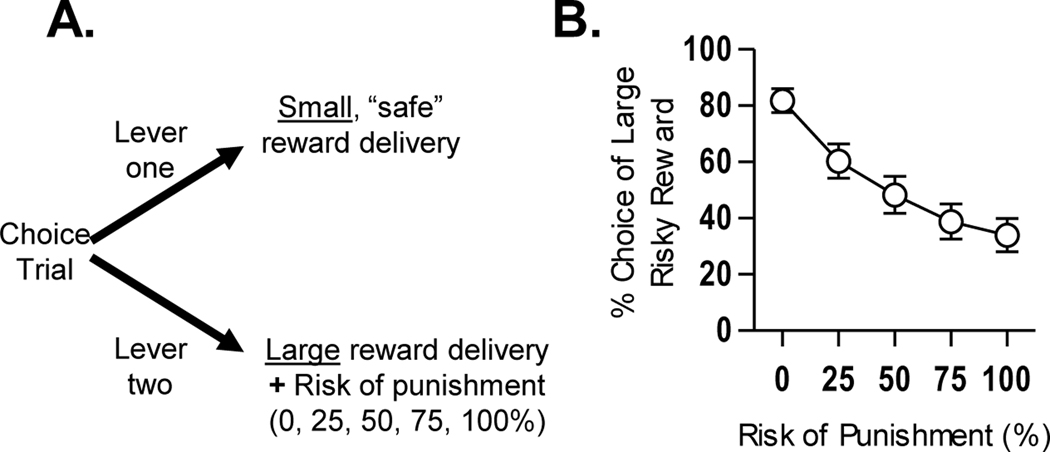
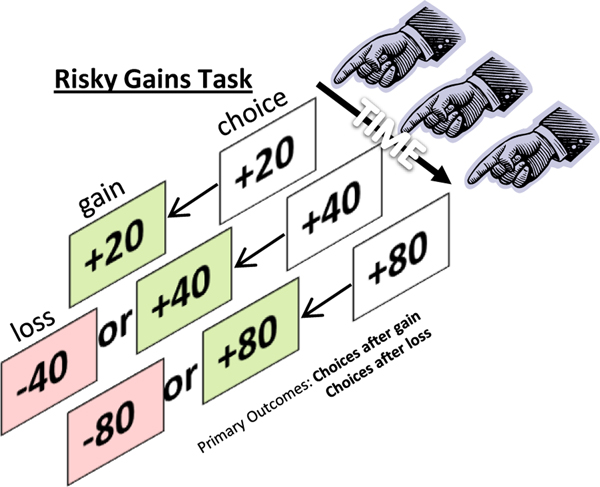
A number of behavioral tasks have been developed in rodents (largely in rats) to assess how factors such as effort, reward delay, and reward omission influence choice behavior (e.g. Evenden and Ryan, 1996; Cardinal and Howes, 2005; Floresco et al., 2008b; see preceding sections). These tasks employ the same basic design, in which rats make discrete-trial choices between two response options, one which yields a small reward and the other which yields a large reward accompanied by a variable response “cost” (effort, delay to reward delivery, probability of reward omission, etc.). The “Risky Decision-making Task” (RDT) uses this same design, but incorporates a variable probability of footshock punishment that accompanies delivery of the large reward (see Figure 3A). As shown in Figure 3B, rats tested in this task prefer the large reward when the probability of punishment is low, but shift their preference away from the large reward (and toward the small, safe reward) as the probability of punishment increases. The degree of preference for the large, “risky” reward is strongly dependent on shock intensity, with higher intensities resulting in decreased choice of the large reward (Simon et al., 2009; Cooper et al., 2014; Shimp et al., 2015). Somewhat surprisingly, the magnitude of the large reward appears to play less of a role in directing choice behavior in this task. In well-trained rats, varying the large reward magnitude from 2 to 5 food pellets does not shift preference for this reward (although decreasing its magnitude to 1 food pellet does sharply reduce preference for this reward, indicating maintained attention to reward magnitude; (Shimp et al., 2015; Orsini et al., 2015). These data are supported by the finding that reducing food motivation via pre-test satiation does not alter choice preference, although it does increase the number of omitted trials (Simon et al., 2009). In combination, these data suggest that, at least under these conditions, punishment plays a more significant role than reward motivation in guiding reward-related decision-making under risk of punishment.
Figure 3.
(A) Schematic of the risky decision-making task. Food-restricted rats are trained in operant chambers to make discrete-trial choices between two response levers. A press on one of the levers (“Lever one”) delivers a small, “safe” food reward (a single food pellet) and a press on the other (“Lever two”) delivers a large food reward (several food pellets) that is accompanied by a variable probability of footshock. Test sessions are organized into five blocks of trials, in which the probabilities of shock delivery are 0, 25, 50, 75, and 100% (usually in ascending order). Each block consists of 8–10 free-choice trials, which are preceded by 8 forced-choice trials used to familiarize rats with the probability of shock delivery for that block. The large reward is delivered after each choice of the large reward lever, irrespective of shock delivery [see Simon and Setlow (2012) for further details of task design]. (B) Mean (+/− SEM) performance of 42 male Long-Evans rats tested in the risky decision-making task. After 20–25 training sessions, rats on average prefer the large reward when the risk of accompanying shock is low, but shift their choices to the small, safe reward as the risk of shock increases.
Dopamine signaling and decision-making under risk of punishment.
As described throughout this review, DA signaling plays a central role in modulating decision-making. This role is usually framed in terms of reward [for example, by providing phasic signals that convey information regarding reward probability, timing, or magnitude (Schultz, 2013)]. However, there is considerable evidence that DA signaling is involved in processing aversive events as well (Brischoux et al., 2009; Bromberg-Martin et al., 2010; Badrinarayan et al., 2012; McCutcheon et al., 2012). In the RDT, acute systemic administration of amphetamine causes a dose-dependent decrease in rats’ choice of the large, risky reward (i.e., increased risk aversion) (Simon et al., 2009; Mitchell et al., 2011). This effect appears to be mediated by D2-like DA receptors, as it is blocked by co-administration of a D2-like (eticlopride), but not D1-like (SCH23390) antagonist, and mimicked by systemic administration of a D2-like (bromocriptine), but not D1-like (SKF81297) agonist (Simon et al., 2011). Further support for a role of D2 receptor signaling in risk aversion is provided by data showing that expression of D2 receptor messenger RNA (mRNA) in the striatum as assessed with in situ hybridization (which likely reflects post-synaptic receptors) is inversely related to risk-taking, such that greater preference for the large, risky reward is associated with lower D2 receptor mRNA expression (Simon et al., 2011; Mitchell et al., 2014). These mRNA data are consistent with the systemic behavioral pharmacological data (D2-like receptor activation reducing choice of the large reward associated with a potential footshock), and their functional significance is supported by the findings that direct administration of a D2-like agonist (quinpirole) into NAc reduces choice of the large, risky reward (Mitchell et al., 2014). Considered together, these data suggest that activation of (ventral) striatal D2 receptors biases choice behavior away from options associated with potential punishment.
Findings that D2 receptor activation decreases preference for rewards associated with potential punishment are consistent with a large literature implicating these receptors in performance of active avoidance tasks. In such tasks, rodents learn to make a response (a lever press or movement from one chamber to another) upon delivery of an auditory or visual signal in order to avoid a footshock punishment. D2 receptor antagonists reduce the number of successful avoidance responses, suggesting that activation of these receptors is necessary to motivate punishment avoidance (Salamone, 1994; Wadenberg and Hicks, 1999). This idea may appear to contradict recent theoretical proposals that reductions in D2 receptor activity (caused by phasic decreases in DA tone induced by aversive events) are critical for avoidance (Frank and Surmeier, 2009; Bromberg-Martin et al., 2010). A recent paper by Oleson et al. (2012), however, provides a potential resolution. These authors showed that during performance of a signaled avoidance task, anticipation or delivery of a footshock causes a transient decrease in DA release in ventral striatum measured with in vivo voltammetry, but anticipation of the shock causes an increase in DA release when that anticipation is followed by a successful avoidance response. In combination with data from the RDT and active avoidance tasks described above, these findings suggest that a transient increase in DA release (and consequent D2 receptor activation) in anticipation of an aversive outcome may promote avoidance of that outcome. In contrast, transient decreases in DA release may be more important for learning about aversive events than for actively addressing them. It will be of considerable interest in future work to determine how transient DA signals are affected in choice contexts that involve concurrent presentation of rewards and punishments (as in the RDT).
Aside from potentially distinct roles in learning versus performance of avoidance behavior, the role of D2 receptors in risky decision-making may depend on the nature of the risky outcome involved. In the probabilistic discounting task described above, systemic administration of a D2-like agonist increases choices of large, risky rewards, whereas the same drug decreases choice of large, risky rewards in the RDT (Simon et al., 2009; St Onge and Floresco, 2009). The difference in the direction of these effects may be due to the relative salience of the rewarding and aversive elements of each task. Killcross et al. (1997) showed that amphetamine potentiates the degree to which both appetitive and aversive cues control choice behavior, suggesting that the drug enhances the salience of these stimuli irrespective of their valence (positive or negative). As described above, choice preference in the RDT is largely directed by the magnitude and probability of the footshock punishment (Shimp et al., 2015), whereas in the probabilistic discounting task (which is very similar in structure and task demands) it seems likely that the reward plays a more salient role in choice behavior. Hence, the apparently opposite effects of D2-like receptor stimulation in these two otherwise similar risk-taking tasks may be due to the valence of the most salient feature of the tasks. In the RDT, D2 receptor activation most strongly potentiates the impact of the punishment (leading to greater risk aversion), whereas in the probabilistic discounting task, D2 receptor activation most strongly potentiates the impact of the large reward (leading to increased risky choice). A potential neural substrate for these effects lies in the recent discovery of a population of midbrain dopamine neurons that increases phasic activity in response to both appetitive and aversive stimuli (Matsumoto and Hikosaka, 2009b). Such neurons are proposed to encode the “motivational salience” of significant events, irrespective of their affective valence (Bromberg-Martin et al., 2010), and DA signals produced by these neurons could account for the effects of D2 receptor activation in both appetitive and aversive contexts. It should be noted, however, that the type of aversive outcome (reward omission vs. punishment) is likely not the sole determinant of the effects of dopaminergic manipulations on risky decision-making. For example, whereas D2-like agonist administration into NAc decreases choice of large, risky rewards in the RDT (Mitchell et al., 2014), this same manipulation has no effect in the probabilistic discounting task (Stopper et al., 2013). Moreover, research from several laboratories employing rodent versions of the Iowa Gambling Task, in which the risky, aversive outcome is a timeout period during which rewards cannot be earned, shows that systemic amphetamine administration reduces preference for risky in favor of safer options, which is similar to its effects in the RDT (Zeeb et al., 2009; van Enkhuizen et al., 2013a). Clearly, much remains to be understood regarding how different types of aversive outcomes are encoded in the context of risk taking tasks, and how such encoding is reflected at the neurobiological level.
Neural circuitry of decision-making under risk of punishment
Beyond dopamine signaling in the striatum, the neural mechanisms of decision-making under risk of punishment are only beginning to be investigated. However, recent data indicate roles for several ventral striatal afferent regions. The BLA sends a strong, direct projection to the NAc, and the two structures have been implicated as a functional circuit involved in several aspects of behavior (Everitt et al., 1991; Setlow et al., 2000; Floresco et al., 2001; Setlow et al., 2002; Stuber et al., 2011). The BLA plays a critical role in decision-making under risk of punishment as well, as lesions of this structure in well-trained rats increase choice of the large, risky reward in the RDT (Orsini et al., 2015). A series of control experiments show that this lesion effect is not due to altered reward sensitivity or impaired sensitivity to the footshock punishment, suggesting that the critical role for the BLA is in integrating the risk of punishment with reward magnitude information to guide adaptive choice behavior. In contrast to the BLA, lesions of the OFC in well-trained rats cause a decrease in choice of the large, risky reward in the RDT (Orsini et al. 2015). This effect does not appear to be due to increased anxiety or impaired sensitivity to the difference between the large and small reward [although see Kheramin et al. (2002)], but instead may result from an impaired ability to accurately calculate punishment probabilities, leading to a default strategy of punishment avoidance. Interestingly, both of these lesion effects are opposite of those observed in the probabilistic discounting task, in which BLA inactivation decreases and (medial) OFC increases choices of large rewards associated with risk of omission (Ghods-Sharifi et al., 2009; Stopper et al., 2014a). This pattern of differential results in the two tasks is consistent with the differential effects of dopaminergic manipulations described above, and further indicates that the neural systems supporting risky decision-making are engaged differently depending on the nature of the risk involved. The involvement of neural systems beyond BLA and OFC are only beginning to be evaluated. In situ hybridization data suggest that D2 receptor signaling in OFC and mPFC may be involved in the ability to flexibly adapt choice strategies in response to changing risks of punishment (Durstewitz and Seamans, 2008; Simon et al., 2011; see preceding section); however this hypothesis has yet to be tested experimentally in the RDT.
Future considerations for research on decision-making under risk of punishment
The findings described above suggest that decision-making under risk of punishment has unique features that render it at least partially distinct from decision-making involving other types of risks (particularly reward omission). These distinctions are of considerable interest from a basic science perspective; however, they represent a challenge for applying knowledge gained in these laboratory models to clinical settings. For example, if DA receptor activation can either increase or decrease preference for large, risky rewards depending on the type of risk involved (Simon et al., 2009; St Onge and Floresco, 2009), what is the predicted outcome of stimulant medications on real-world risky decision-making in patient populations (e.g., does it depend on the decision context and/or individual sensitivity to rewards vs. punishments?). One study indicates that methylphenidate administration in children with ADHD reduces risk-taking in the Cambridge Gambling Task, in which the “risk” is possible loss of points earned during the task (DeVito et al., 2008); however, to our knowledge, this issue has not been evaluated systematically.
Finally, although progress has been made in elucidating the neurobehavioral mechanisms that guide decision-making under risk of punishment, there is much that remains unknown. In particular, it is as of yet largely unclear how risk of punishment is encoded at the circuit, neurochemical, and single-cell activity levels. Such information is important, as risky decision-making is dysregulated in a number of psychiatric disorders, including addiction, bipolar disorder, ADHD, and anorexia (see the following section for more on this topic). In this regard, studies using animal models will continue to provide insight into the pathophysiological mechanisms that underlies abnormal decision-making in these disorders. For example, a recent study showed that greater preference for large, risky rewards in the RDT during adolescence predicts greater cocaine self-administration during adulthood, and that cocaine self-administration itself can in turn cause further increases in preference for large, risky rewards (Mitchell et al., 2014). Such links between high levels of risk-taking behavior and drug use are evident in humans as well (Bechara et al., 2001; Bornovalova et al., 2005; Leland and Paulus, 2005; Gowin et al., 2013), and support the utility of modeling this type of decision-making in animals.
Insights into abnormal risky decision-making in humans from animal models
One of the key points in understanding the neural mechanisms underlying decision-making and risk-taking behavior in animals is how these mechanisms pertain to human performance in similar tasks. While there is an academic interest in understanding such mechanisms, there is also a very real-world need to understand them since they are deleteriously affected in numerous neuropsychiatric disorders (Paulus, 2007). The loss of optimal real-world decision-making can have dramatic effect on a sufferer’s everyday life, their ability to integrate into society, and chances for employment. The subsequent section will review some of the tasks used to measure decision-making in clinical populations, deleterious behaviors associated with these disorders, and links between these findings and those obtained from preclinical studies.
Assessing real-world decision making under reward and punishment using the Iowa Gambling Task
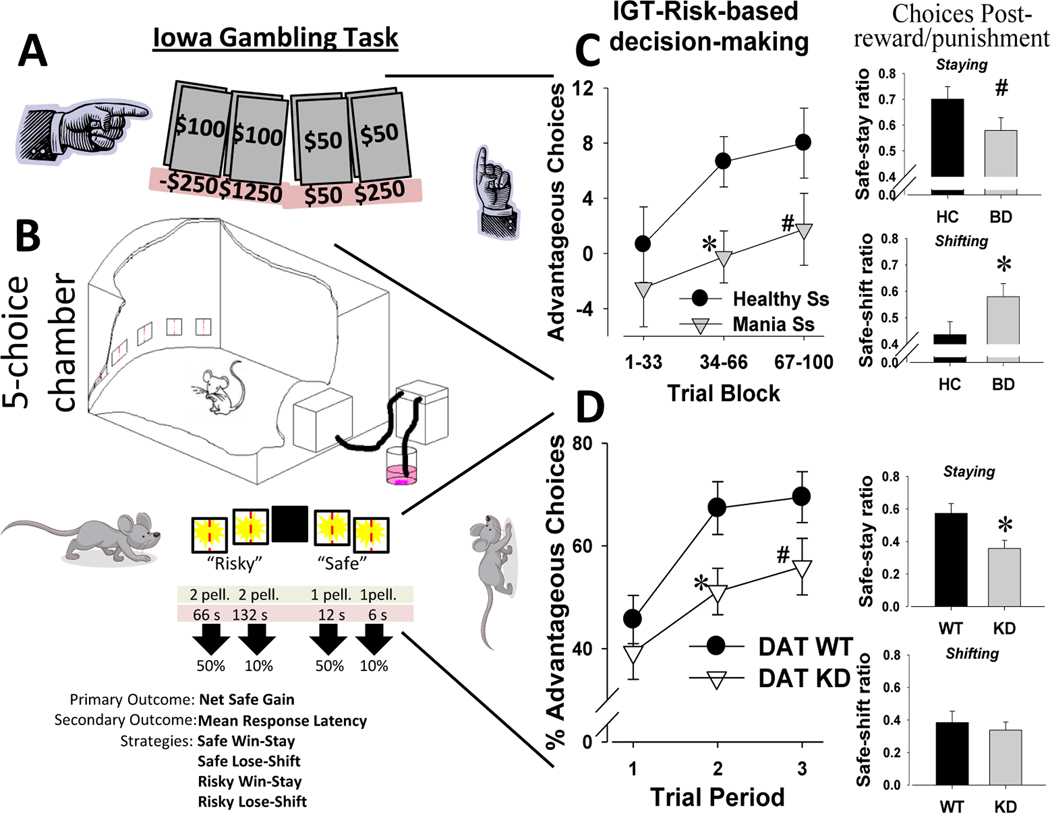
As described above, Bechara and colleagues provided the first neurobiological clues as to how the brain mediates decision-making under risk using the IGT (Bechara et al., 1994). The IGT assesses real-world decision-making by providing subjects with four card deck options mixed with varying reward (plus points), punishment (minus points), and ratio magnitudes (Bechara et al., 1994; Figure 4A). The IGT has become arguably the most popular paradigm to assess decision-making deficits in psychiatric patients (Toplak et al., 2010; Steingroever et al., 2013). The IGT decks are stacked so that selecting from the high reward but higher punishment (“risky”) sides result in overall loss over the 100 trials, whereas selecting from the low reward but lower punishment (“safe”) sides result in overall gains. While performing the IGT, subjects tend to initially select the risky decks but rapidly (often by trial 20) switch to selecting from the safe decks, although there is a great deal of variability between subjects (Steingroever et al., 2013; van Enkhuizen et al., 2014). The IGT can be extremely useful when assessing the ability of subjects to withhold responding directed towards highly rewarding stimuli in order to obtain even greater gains in the future. As such the IGT has been used to examine decision-making under risk in numerous clinical populations.
Figure 4.
Assessment of risky decision-making in the human and mouse Iowa Gambling Tasks (IGTs), recreating the reward-seeking behavior of bipolar mania. The Iowa Gambling Task (IGT) was originally designed by Bechara et al (1994) wherein 4 cards are presented (A), 2 with high-reward-high-punishment (loss) payouts (“risky” decks) and 2 with low-reward-low-punishment (loss) payouts (“safe” decks). The decks are stacked so that greater rewards are earned if the safe decks are chosen and healthy subjects rapidly start selecting from the safe decks after initial sampling. The rodent IGT can be assessed using 5-choice chambers (B) which have 5 holes recessed at the rear of the chamber with rewards delivered in the opposite side of the chamber. Four holes are illuminated from which the rodent can choose. Loss cannot be provided, but punishment comes in the form of lights in the holes being flashed for a certain time-period, during which no rewards can be gained. Primary outcomes for both tasks are net gain, represented by advantageous choices (C and D). Choice latency can also be measured. Important to understanding strategies underlying decision-making processes during the task, the likelihood of subjects repeating a safe choice after a reward on the safe side (safe win-stay), a risky choice after punishment following a safe-side choice (safe lose-shift), repeating a safe choice after rewarded choosing that side (risky win-stay), and a safe choice after punishment following a risky-side choice (risky lose-shift) can all be assessed. The similarity of task designs enables the assessment of mechanisms that might underlie abnormal decision-making in psychiatric patients. We have previously shown that patients with bipolar mania (BD) exhibit poor learning in the task compared with healthy subjects (HC; van Enkhuizen et al, 2014), consistent with other studies (C). Using these strategy assessment measures, it was clear that patients with BD preferred high reward sides after being given low rewards since they are less-likely to repeat preferences for low - but do so for high-rewards. This poor IGT learning performance was recreated in mice with reduced dopamine transporter (DAT) expression (knockdown; KD), compared to their wild type (WT) littermates (D). Importantly, the same pattern of reduced likelihood to stay at low rewards after receiving such a reward in BD subjects was also seen in DAT KD mice.
Initial studies utilizing the IGT demonstrated that people with lesions of the ventromedial PFC (vmPFC) and/or OFC exhibited impaired performance, responding to immediate rewards and punishments rather than gauging long-term benefits (Bechara et al., 1994; Bechara et al., 2000). In some instances, these types of patients exhibited poor learning for advantageous options in the IGT, even when repeatedly tested over 4 weeks (Bechara et al., 2000). A primary conclusion that arises from these studies is that this region of the frontal lobes plays a key role in guiding decision-making during probabilistic learning tests that include reward and punishments. This supposition is supported by preclinical investigations of OFC function in animals, revealing that OFC neural activity is sensitive to the properties of a reward such as size, probability, and delay (Roesch et al., 2006). It has thus been proposed that the OFC is a primary site for common currency computation (Pearson et al., 2014). The popularity of the IGT as an assay for decision-making has prompted a number of groups to develop similar task for use with rodents. When recreated for use in rodents using high and low reward and punishment probabilities across a single session (Rivalan et al., 2009; Figure 4B), more selective lesions of the OFC and prelimbic cortex in rats impaired choosing of advantageous options, primarily by causing inflexible behavior and a chance pattern of choice responding (Rivalan et al., 2011). Hence, the rat IGT exhibits some construct validity for the human IGT.
Impaired decision-making across neuropsychiatric populations
Impairments in IGT performance have been observed in numerous psychiatric disorders. For example, individuals diagnosed with pathological gambling (PG) disorder exhibited impaired IGT performance (Cavedini et al., 2002) as did cocaine abusers (Stout et al., 2004) and a variety of other neuropsychiatric groups (Steingroever et al., 2013). People with substance abuse disorders exhibited impaired IGT performance in the absence of impaired delay-dependent memory or executive functioning (Gonzalez et al., 2007; Quednow et al., 2007). Impaired decision-making on the IGT has also been observed in people with binge eating disorder and sufferers of alcoholism (Danner et al., 2012; Le Berre et al., 2014). Similarly, patients with schizophrenia also exhibit deficient IGT performance (Kim et al., 2009; Wasserman et al., 2012; Brambilla et al., 2013; Fond et al., 2013), although this finding has not been consistently observed (Turnbull et al., 2006). The IGT deficits of patients with schizophrenia appeared to be driven by altered learning of reward and punishment contingencies associated with different choices as they did show improved performance over the course of learning but at a slower rate (Kim et al., 2009; Brambilla et al., 2013; Fond et al., 2013). Learning on the IGT is largely driven by large punishments associated with the “bad” decks that bias choice toward advantageous options over time. In this regard, it has been well-established that schizophrenic patients show impairments in reward-associative learning (Waltz et al., 2007; Waltz and Gold, 2007; Gold et al., 2008). The fact that patients with schizophrenia do show some learning on the IGT suggests that impairments in risk-based decision making may be driven primarily by perturbed learning about rewards, whereas associative learning about punishments may be relatively spared.
People with bipolar disorder also exhibit IGT deficits during periods of mania (Clark et al., 2001; Adida et al., 2008; van Enkhuizen et al., 2014) and euthymia and depression (Adida et al., 2011). Although IGT deficits in bipolar patients with euthymia were not always replicated with smaller sample sizes (Edge et al., 2013), it is clear that during mania, patients exhibited poor IGT performance (van Enkhuizen et al., 2014). Importantly, when bipolar disorder patients are discharged but have a history of stimulant abuse, their IGT scores predicted future drug use within at least 4 weeks (Nejtek et al., 2013). Hence, the IGT predicts real world decision-making and can also be useful for gauging patient stability.
Investigating mechanisms underlying decision-making in response to gains and punishments
Data from the IGT can be scrutinized in greater detail than simply net gain over the session, thus providing greater insight into subjects’ decision-making processes. Post-hoc analyses of decision-making in other tasks have focused on subjects’ propensity to stick with the same choice after a reward (win-stay) or shift after a loss (lose-shift) (Paulus et al., 2003; Cassotti et al., 2011; St Onge et al., 2011; Stopper and Floresco, 2011; van den Bos et al., 2012). This form of analysis has also been more recently employed in human IGT studies (Worthy et al., 2013; van Enkhuizen et al., 2014). After receiving a punishment (loss) following a ‘safe’ choice, patients with bipolar mania were more likely to switch to the decks with higher rewards (Figure 4C). Likewise, mania patients were also less likely to select from the ‘safe’ side again after receiving a reward (win) from that side (van Enkhuizen et al., 2014). This post-hoc analysis of their decision-making in response to reward and punishment provided insight into why some patients exhibited poorer performance. Hence, mania patients appeared to prefer the high reward side as indicated by preferential switching to that side, likely finding the low-reward side not as appealing. Such deficits were also observed in euthymic bipolar disorder patients administered the DA D2-like agonist pramipexole (Burdick et al., 2014). Hence, consistent with descriptions above, DA receptor activity likely plays a role in modulating decision-making in the IGT. Interestingly however, when in a depressed state, bipolar patients exhibited poor IGT performance driven by increased sensitivity to punishment (Adida et al., 2011), although the mechanisms for this sensitivity remain unclear.
Insights into the potential neurochemical mechanisms that underlie altered decision-making in mania come from translational studies with rodents. Utilizing a similar single-session IGT paradigm in rodents, developed originally in rats (Rivalan et al., 2009), van Enkhuizen and colleagues demonstrated that either pharmacological (GBR 12909 treatment) or genetic (knockdown mice) reduction in dopamine transporter (DAT) function recreated the poor decision-making of patients with mania (van Enkhuizen et al., 2014). The impaired decision-making of both groups of mice was similarly driven by reduced probabilities of staying at the safe choices after a reward (Figure 4D). Further investigations are required into the mechanisms underlying choices made following reward or punishment presentation.
Other methods for assessing flexible decision-making across species
Other tasks exist which explicitly assess choices of subjects following reward or punishment presentation, wherein individual variation in the general population can be observed. For example, high thrill and attention seekers exhibited hypersensitivity to intense and rewarding stimuli but blunted sensitivity to punishing stimuli (Joseph et al., 2009). This self-report finding was supported with laboratory assessments using the Risky Gains Task [RGT; (Kruschwitz et al., 2012)]. In the RGT, subjects select rewards ranging from +20, +40, or +80 cents presented in sequence, but are warned that selecting +40 or +80 could result in a loss of 40 or 80 instead of a gain (Figure 5). The task is set up such that irrespective of their choice, the player receives the same level of reward so that the primary data of interest are choices that are made following rewarded or –punishment outcomes. High and low sensation seekers exhibited similar preferences for safe and risky options at baseline. After punishment however, low sensation seekers adopted a safer strategy while the behavior of high sensation seekers remained unchanged (Kruschwitz et al., 2012). Effectively, the high sensation seekers “chased the risk,” demonstrating the utility of decision-making assays such as the RGT to empirically characterize high vs. low sensation seekers. Given that sensation seeking and punishment have been directly linked to propensity for gambling (Navas et al., 2014), this approach may be useful for identifying people at high risk of developing a pathological gambling disorder. This differential behavior between high and low sensation seekers was linked to differences in neural activation in regions such as the NAc, implicated in risky decision-makers in rodent studies (see preceding sections). This finding complements those from rats in which expression of DA D2 receptor mRNA within this nucleus was inversely related to risky behavior in the RDT (Simon et al., 2011; Mitchell et al., 2014). Thus, these convergent data support the premise that variations in NAc functioning may underlie different patterns of risky versus safer decision-making and that pathophysiological alterations in this nucleus may contribute to abnormal decision-making observed in certain disorders.
Figure 5.
Assessment of risk decision-making in the human Risky Gains Task (RGT). The RGT has primarily been developed for using during functional magnetic resonance imaging studies. In this task, subjects are presented with a sequential series of options, +20, +40, and +80. If choosing +20, the subject receives 20 points 100% of the time. If the subject waits for +40 and selects this option, they will receive either +40 or −40 points. If the subject waits to +80, and chooses this option, they will receive +80 or −80 points. The computer ‘games the system’ so that the subjects will always receive the same total rewards. The primary outcome measures are strategy choices made at each level of gain or a loss.
Situations involving uncertainty typically require flexible decision-making in order to shift response biases upon changes in reinforcement contingencies. These functions can be assessed using probabilistic reversal learning tasks (Figure 6). In a typical variant of this procedure, “correct” choices are reinforced on 80% of trials and incorrect choices are reinforced on 20% of trials, with these contingencies reversing at some point during a session (Swainson et al., 2000). Poorer performance on this task predicted future losses accrued due to gambling in a student population (Navas et al., 2014). This finding suggested that impairments in behavioral flexibility may also contribute to pathological gambling disorders and that in combination with reduced sensitivity to punishment associated with thrill seeking, may synergistically increase risk of such disorders. Interestingly, chronic abusers of cocaine, but not amphetamine or opioids, exhibited normal initial learning but impaired reversal learning in this task as indicated by increased perseverative responses to previously rewarded stimuli. This deficit was unlikely due to poor learning from punishment or reward feedback but instead was more likely to reflect poor disengagement from previously rewarded stimuli contingencies (Ersche et al., 2008). Notably, probabilistic reversal learning tasks have also been developed for rodents (Bari et al., 2010; Amitai et al., 2013; Dalton et al., 2014) and primates (Rygulaet al., 2014) providing an opportunity for such behaviors to be examined across species.
Figure 6:
Assessment of human and mouse probabilistic learning. During probabilistic learning, people are required to select an object based on its likelihood receiving reinforcement. One object is designated as a target while the other is designated as a non-target. Selection of the target is positively reinforced 80% of the time (“correct” for humans, a reward for animals), and not reinforced on the remaining 20% of trials (“incorrect” for humans, an omission of reward for animals), while for the non-target stimuli the contingencies were reversed. Once acquired, the contingencies are reversed. In rodents, this task can be recreated using two stimuli from 5-choice chambers. The primary outcome measures are trials to criterion. Stimuli designations can be reversed and thus, another outcome is the number of reversals achieved. Strategy assessment can also be conducted similarly to the IGT whereby the likelihood of subjects repeating a choice after a reward on the target side (target win-stay), shifting to the non-target after a loss on the target side (target lose-shift), repeating a non-target choice after being rewarded at that side (non-target win-stay), and shifting choice after punishment on the non-target side (non-target lose-shift) can be measured during the task.
Although the neural basis underlying reversal learning using deterministic outcomes has been studied in considerable detail in animals, there have been comparatively fewer preclinical studies investigating the mechanisms underlying probabilistic reversal learning. One important study revealed that alterations in 5-HT tone can have a pronounced impact on this form of flexibility. Acute citalopram increased lose-shift (punishment-sensitive) behavior, while chronic citalopram increased win-stay (reward-sensitive) behavior in both rats (Bari et al., 2010) and mice (Ineichen et al., 2012). These effects appear to be due to perturbations in 5-HT transmission within the OFC and BLA (Rygula et al., 2014). On the other hand, inactivation of the NAc shell impaired initial and reversal learning with probabilistic feedback, due to reduced win-stay behavior, while core inactivation produced only minimal effects on choice latencies (Dalton et al., 2014). Developmental manipulations can also affect performance in this task. Rearing rats in isolation impaired probabilistic learning between sessions, but not reversal learning within a session as is conducted in humans (Amitai et al., 2013). Hence, the probabilistic reversal learning task provides another opportunity to examine flexible decision-making across species, although the neural circuitry underlying this aspect of learning remains to be thoroughly elucidated.
Another methodology of assessing decision-making across species includes providing information on the relative probability (i.e. risk) and reward prior to making a choice (O’Neill and Schultz, 2010). The probability is altered such that the probability of obtaining a reward can be completely certain or very low. This pattern of presentation is similar in some respects to the probabilistic discounting task described previously, as varied probability of reward omission, rather than punishment, serves as the risk associated with the reward. In this assay, monkeys were provided information ahead of time as to the probability of reward. Certain OFC neurons encoded risk probabilities (O’Neill and Schultz, 2010). Similar types of encoding were observed in the rat OFC. When odor discriminanda are made difficult to distinguish, OFC neurons also identified risk (Kepecs et al., 2008), although it may also have encoded a combined reward/risk value (Roitman et al., 2010). In comparison, when a similar paradigm was used in humans wherein risk and reward values were explicitly signaled and varied from trial-to-trial, activation of the lateral OFC predicted risk but was not associated with reward (Tobler et al., 2007). Other areas that are important for encoding risk include the ventral striatum, mediodorsal thalamic nucleus, midbrain, and anterior insula (Preuschoff et al., 2006). Interestingly, one study reported that participants deemed inherent ‘risk-avoiders’ displayed increased lateral OFC activation when avoiding risk, but ‘risk-takers’ demonstrated mPFC activation when avoiding risks (Tobler et al., 2007). The given value of a reward can influence risk decisions, as described in Schultz et al, (2011). Note, however, that the computation of reward value is important given real-world scenarios of volatile probabilities of obtaining rewards or incurring losses, such as occurs in the IGT (Bechara et al., 1994; Must et al., 2013). Thus, one goal for future studies on abnormal decision-making is to identify whether impairments in decision-making associated with different psychiatric disorders are due to altered computation about the relative value of different rewards and/or the risks associated with obtaining them.
One paradigm utilized extensively in human testing is the Cambridge Gambling Task (CGT). This task requires subjects to choose between likely/low-reward-low-punishment vs. unlikely/high-reward-high-punishment options, with the probabilities of these outcomes explicitly presented to the subject prior to a choice. The primary outcomes are the speed of decision-making and the percent choice of low-reward outcomes across 5 reward-balanced conditions (Rogers et al., 1999a). Regional cerebral blood flow during choices increased in numerous prefrontal regions, including Brodmann areas 10, 11, and 47 (anterior of the middle frontal gyrus, medially in the orbital gyrus, and posteriorly in the anterior portion of the inferior frontal gyrus respectively). Interestingly, patients with dorsolateral and dorsomedial PFC lesions exhibited risk-preference choices in the IGT but not the CGT (Manes et al., 2002), suggesting task differentiation in assessing decision-making under risk. While there are some reports that children with ADHD exhibited reduced risk-taking in the CGT (Kroyzer et al., 2014), other findings demonstrated that children with ADHD exhibited increased risk-preference during CGT performance, which as ameliorated with methylphenidate treatment (DeVito et al., 2008). Hence, the CGT was sensitive to deficits in medication effects in children with ADHD. The CGT was also sensitive to impaired decision-making in sufferers of PG disorder (Kraplin et al., 2014), young (Ackerman et al., 2014) and old suicide attempters (Clark et al., 2011). To date, no rodent versions of the CGT exists but future studies could incorporate procedures that explicitly inform rodents about the likelihood of rewards or punishments, possibly by using operant chambers fitted with touchscreens that could display various stimuli associated with different outcome probabilities (Bussey et al., 2012).
Temporal discounting assesses another form of decision-making whereby reward valuation is modulated by temporal delay of reward arrival. These types of tasks have been used with both animals and humans and have proved useful in identifying differences in subjective reward valuations that may exist in certain patient populations. For example, individuals with PG exhibit a steeper temporal discounting profile whereby they select smaller immediate rewards over larger delayed rewards more frequently and faster than healthy subjects (Giorgetta et al., 2014). Note however, that differences in delay duration used in human (hypothetical seconds to months) vs. animal (seconds to minutes) delay discounting tasks may limit cross-species translational validity of such testing (McClure et al., 2007; Schultz, 2010). Nevertheless, another important aspect of such decision-making paradigms concerns temporal perception (Wittmann et al., 2007), in that the ability to perceive time is often altered in patients with schizophrenia, bipolar disorder, and other conditions (McDowell et al., 1996; Dale et al., 2010; Papageorgiou et al., 2013) and can be affected by treatments such as amphetamine (Fowler et al., 2009). Such changes in temporal perception in patients should be taken into account in future studies investigating mechanistic changes underlying decision-making deficits.
Identifying mechanisms to improve decision-making under risk will also be vital toward helping sufferers of poor decision-making in patient populations, either with cognitive behavioral and/or pharmacotheraputic treatments. Cognitive-behavioral interventions can improve symptom and consequence rating scores in sufferers of gambling disorders (Larimer et al., 2012), while modafinil can improve poor risk learning in individuals with PG (Zack and Poulos, 2009). At the preclinical level amphetamine improved conservative response strategies of mice and rats in the IGT (Zeeb et al., 2009; van Enkhuizen et al., 2013b) and it made rats less sensitive to changes in reward probabilities during a discounting task (St Onge and Floresco, 2009; St. Onge et al., 2010b). Confirming such effects in humans will be vital in future treatment development research.
Conclusions and future directions
As the field progresses, future translational studies evaluating risk-based decision-making will benefit from taking into account the risks associated with each choice. For example, a stock trader may avoid a stock because it could result in a large loss despite a potentially large gain, but go for a slightly smaller gain that is accompanied by very little risk of loss. Incorporation of conflict between rewards paired with the risk of punishment is common in many laboratory tests of risk-taking used in humans, such as the RGT, CGT, and the IGT (although some tasks use reward omission (see Table 1)), and findings highlighted in the sections above demonstrate that different neural mechanisms may underlie decision-making that involves risk of reward omission vs. risk of punishment (St. Onge & Floresco 2009; Simon et al. 2009; van Enkhuizen et al., 2013b; Orsini et al., 2015). Although the critical variables that define “punishment” vs. “omission” in these tasks are as of yet unclear, the results highlight the need for careful consideration when attempting to translate findings from any one task across species.
Table 1:
Comparison between some available decision-making tasks including the rodent decision-making task (RDT), risky gambling task (RGT), and Iowa Gambling Task (IGT)
| Paradigm | Species tested | Reward provided | Punishment utilized | Examples of varied findings in response to the same treatments across paradigms |
|---|---|---|---|---|
| Probabilistic Discounting | Rats | 1 vs. 4 pellets | No reward | Amphetamine increases preference for large uncertain rewards D2 antagonist reduce preference for large uncertain rewards (St. Onge et al, 2009) Basolateral amygdala inactivaiton reduces risk preference (Ghods-Sharifi et al., 2009) |
| RDT | Rat | 1 vs. 3 pellets | Electric shock | Amphetamine reduces preference for large, risky reward, blocked by D2 but not D1 antagonists (Simon et al., 2011) Basolateral amygdala lesions increase risk preference (Orsini et al., 2015) |
| RGT | Human | Gain of points | Loss of points | None |
| IGT | Human, Rat, Mouse | Low vs. high points, or 1 vs. 2 pellets | Low vs. high points loss, length (6–333s) of time outs | Amphetamine reduces preference for risky reward in rats and mice (Zeeb et al, 2010; van Enkhuizen et al, 2013a). |
| Probabilistic Learning | Human, Rat, Mouse | ‘correct’/ 1 pellet | No reward | Amphetamine enhances learning by increasing win-stay strategies |
The importance of consistency of testing across species in order to understand the neural mechanisms underlying a specific aspect of behavior is highlighted by the NIMH-led creation of the Research Domain Criteria (RDoC) strategy as a novel means to classify psychopathology based on dimensions of observable behavior and neurobiological measures (Cuthbert and Insel, 2010; Insel et al., 2010; Morris and Cuthbert, 2012). Using RDoCs, disorders will be classified by the behavior affected as opposed to criterion led by the Diagnostic and Statistical Manual of Mental Disorders (DSM), an approach that is understandable given the number of disorders that exhibit poor decision-making but for possibly different reasons (e.g., hypersensitivity to reward or punishment) that may be mediated by dissociable neural mechanisms. Two areas of particular interest are the Positive and Negative Valence domains. The Positive Valence domain includes the construct of approach motivation, specifically action selection/preference-based decision-making, as measured by the IGT. Hence, reward-driven decision-making (e.g., win-stay behavior) can be used as a method to investigate the neural mechanisms underlying one aspect of the Positive Valence domain. The Negative Valence domain includes the construct of loss, which includes bias toward negative information (sensitivity to punishment). Although in its infancy, the RDoC-style approach will likely greatly influence understanding of disordered decision-making in patient populations.
With respect to preclinical studies, another issue to consider when comparing effects in rats and mice is behavioral differences that exist across species. For example, good performance in mice correlates with minimizing punishment duration (van Enkhuizen et al., 2014) while rats tend to behave in a manner that maximizes reward (Rivalan et al., 2009). These differences likely result from their innate behaviors given that in the wild, mice are often preyed upon while rats are often predators (Young et al., 2013). Pearson et al (2014) highlight neuroethological contributions to decision-making as a vital component of understanding the neuroeconomics that contribute to such decision-making. Similarly, the importance of including emotion in understanding the decision-making process will be vital (Quartz, 2009), whereby choice will not always be driven by in the moment reactions, but also by historical information (e.g., a broker sticking with a stock that had been beneficial in the past, despite current fluctuations in price). The inclusion of emotional state, such as the effects of stress (Shafiei et al., 2012) into decision-making research will be critical to better understand psychiatric conditions, given that many psychiatric disorders are also associated with impairments in emotional regulation.
An ultimate goal of this review was to clarify how animal models can be useful for developing a more comprehensive understanding of the neural mechanisms underlying normal and abnormal decision-making. Future endeavors will require use of a battery of decision-making tasks that assess different subcomponents of decision making (Paulus, 2007). These components include varied reward and punishment value, probabilities of either reward or punishment, delay tolerance (temporal perception), and a method to examine reward and punishment sensitivities. The development of novel assays that more closely resemble the types of decisions faced by humans, in combination with pharmacological, neurophysiological and optogenetic approaches will undoubtedly yield powerful insight into how the brain solves these types of problems and the pathophysiological alterations that may underlie maladaptive decision making.
Highlights.
The review focuses on animal models of different aspects of risk-related decision making
PFC mechanisms of response selection are discussed
PFC-BLA-NAc-LHb and DA circuits interact to refine choice about uncertain rewards
Decisions about rewards vs punishment engage different mechanisms from those involving reward uncertainty
Preclinical research on this topic provides useful insight to abnormal decision making
Acknowledgements
Some of the work reviewed in this article was funded by a McKnight Brain Institute Fellowship to CAO, grants from the National Institutes of Health to DEM (R21-DA032005), BS (R01-DA024671), and JWY (R01-MH104344) and from the Canadian Institutes of Health Research to SBF (MOP 89861). The order of authorship was determined by academic rank.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman JP, McBee-Strayer SM, Mendoza K, Stevens J, Sheftall AH, Campo JV, Bridge JA (2014) Risk-Sensitive Decision-Making Deficit in Adolescent Suicide Attempters. Journal of child and adolescent psychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adida M, Clark L, Pomietto P, Kaladjian A, Besnier N, Azorin JM, Jeanningros R, Goodwin GM (2008) Lack of insight may predict impaired decision making in manic patients. Bipolar Disord 10:829–837. [DOI] [PubMed] [Google Scholar]
- Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, Mazzola-Pomietto P, Jeanningros R, Goodwin GM, Azorin JM, Courtet P (2011) Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry 70:357–365. [DOI] [PubMed] [Google Scholar]
- Amitai N, Young JW, Higa K, Sharp RF, Geyer MA, Powell SB (2013) Isolation rearing effects on probabilistic learning and cognitive flexibility in rats. Cogn Affect Behav Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstrom KK, Miczek KA, Budygin EA (2009) Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience 161:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual review of neuroscience 28:403–450. [DOI] [PubMed] [Google Scholar]
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ (2012) Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. The Journal of neuroscience : the official journal of the Society for Neuroscience 32:15779–15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A (1998) Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37:407–419. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP (2010) Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 35:48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW (2013) Inhibition and impulsivity: behavioral and neural basis of response control. Progress in neurobiology 108:44–79. [DOI] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW (2010) Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology 35:1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, Robbins TW (2011) Prefrontal and monoaminergic contributions to stop-signal task performance in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:9254–9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR (2000) Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 10:295–307. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW (1994) Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50:7–15. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP (1999) Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci 19:5473–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE (2001) Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 39:376–389. [DOI] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM, Roesch MR (2013) Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behavioural brain research 250:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez CW (2005) Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Experimental and clinical psychopharmacology 13:311–318. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y (2011) Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nature neuroscience 14:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P, Perlini C, Bellani M, Tomelleri L, Ferro A, Cerruti S, Marinelli V, Rambaldelli G, Christodoulou T, Jogia J, Dima D, Tansella M, Balestrieri M, Frangou S (2013) Increased salience of gains versus decreased associative learning differentiate bipolar disorder from schizophrenia during incentive decision making. Psychological medicine 43:571–580. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA (2009) Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proceedings of the National Academy of Sciences of the United States of America 106:4894–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broersen LM, Uylings HB (1999) Visual attention task performance in Wistar and Lister hooded rats: response inhibition deficits after medial prefrontal cortex lesions. Neuroscience 94:47–57. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O (2011) Lateral habenula neurons signal errors in the prediction of reward information. Nature neuroscience 14:1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O (2010) Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68:815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Braga RJ, Gopin CB, Malhotra AK (2014) Dopaminergic influences on emotional decision making in euthymic bipolar patients. Neuropsychopharmacology 39:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Bravo-Rivera H, Quirk GJ (2013) Prelimbic and infralimbic neurons signal distinct aspects of appetitive instrumental behavior. PloS one 8:e57575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM (2012) New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology 62:1191–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN (2006) Neural systems implicated in delayed and probabilistic reinforcement. Neural networks : the official journal of the International Neural Network Society 19:1277–1301. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Howes NJ (2005) Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC neuroscience 6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ (2001) Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292:2499–2501. [DOI] [PubMed] [Google Scholar]
- Cassaday HJ, Nelson AJ, Pezze MA (2014) From attention to memory along the dorsal-ventral axis of the medial prefrontal cortex: some methodological considerations. Frontiers in systems neuroscience 8:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassotti M, Houde O, Moutier S (2011) Developmental changes of win-stay and loss-shift strategies in decision making. Child Neuropsychol 17:400–411. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Riboldi G, Keller R, D’Annucci A, Bellodi L (2002) Frontal lobe dysfunction in pathological gambling patients. Biol Psychiatry 51:334–341. [DOI] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A (2013) Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496:359–362. [DOI] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS (1986) Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience 6:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y (2011) Animal models of prefrontal-executive function. Behavioral neuroscience 125:327–343. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW (2003) Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behavioural brain research 146:105–119. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM (2001) A neuropsychological investigation of prefrontal cortex involvement in acute mania. Am J Psychiatry 158:1605–1611. [DOI] [PubMed] [Google Scholar]
- Clark L, Dombrovski AY, Siegle GJ, Butters MA, Shollenberger CL, Sahakian BJ, Szanto K (2011) Impairment in risk-sensitive decision-making in older suicide attempters with depression. Psychology and aging 26:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Newsome WT (2004) What electrical microstimulation has revealed about the neural basis of cognition. Current opinion in neurobiology 14:169–177. [DOI] [PubMed] [Google Scholar]
- Cooper SE, Goings SP, Kim JY, Wood RI (2014) Testosterone enhances risk tolerance without altering motor impulsivity in male rats. Psychoneuroendocrinology 40:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, Du YP, Lejuez CW, Raymond KM, Banich MT (2010) Risky decisions and their consequences: neural processing by boys with Antisocial Substance Disorder. PloS one 5:e12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR (2010) Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull 36:1061–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A, Robbins TW (2009) Personality, addiction, dopamine: insights from Parkinson’s disease. Neuron 61:502–510. [DOI] [PubMed] [Google Scholar]
- Dale CL, Findlay AM, Adcock RA, Vertinski M, Fisher M, Genevsky A, Aldebot S, Subramaniam K, Luks TL, Simpson GV, Nagarajan SS, Vinogradov S (2010) Timing is everything: neural response dynamics during syllable processing and its relation to higher-order cognition in schizophrenia and healthy comparison subjects. Int J Psychophysiol 75:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience and biobehavioral reviews 28:771–784. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Phillips AG, Floresco SB (2014) Preferential involvement by nucleus accumbens shell in mediating probabilistic learning and reversal shifts. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:4618–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna CL, Shepard PD, Elmer GI (2013) The habenula governs the attribution of incentive salience to reward predictive cues. Frontiers in human neuroscience 7:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner UN, Ouwehand C, van Haastert NL, Hornsveld H, de Ridder DT (2012) Decision-making impairments in women with binge eating disorder in comparison with obese and normal weight women. Eur Eat Disord Rev 20:e56–62. [DOI] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, Carelli RM (2010) Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biological psychiatry 68:306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin JP, Feenstra MG, Broersen LM, Van Leeuwen M, Arens C, De Vries S, Joosten RN (2000) Role of the prefrontal cortex of the rat in learning and decision making: effects of transient inactivation. Progress in brain research 126:103–113. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Blackwell AD, Kent L, Ersche KD, Clark L, Salmond CH, Dezsery AM, Sahakian BJ (2008) The effects of methylphenidate on decision making in attention-deficit/hyperactivity disorder. Biol Psychiatry 64:636–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Gold JI (2013) The basal ganglia’s contributions to perceptual decision making. Neuron 79:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK (2008) The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatry 64:739–749. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ (2000) Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. Journal of neurophysiology 83:1733–1750. [DOI] [PubMed] [Google Scholar]
- Edge MD, Miller CJ, Muhtadie L, Johnson SL, Carver CS, Marquinez N, Gotlib IH (2013) People with bipolar I disorder report avoiding rewarding activities and dampening positive emotion. J Affect Disord 146:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, Contoreggi C, Leff M, Bolla K (2003) Neural substrates of decision making in adults with attention deficit hyperactivity disorder. The American journal of psychiatry 160:1061–1070. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ (2008) Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 197:421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL (2012) The role of medial prefrontal cortex in memory and decision making. Neuron 76:1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN (1996) The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology 128:161–170. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O’Brien A, Robbins TW (1991) The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience 42:1–18. [DOI] [PubMed] [Google Scholar]
- Felsen G, Mainen ZF (2012) Midbrain contributions to sensorimotor decision making. Journal of neurophysiology 108:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein DH, Eldreth DL, Hyde C, Matochik JA, London ED, Contoreggi C, Kurian V, Kimes AS, Breeden A, Grant S (2005) Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Brain research Cognitive brain research 23:119–136. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE (2011) A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience 196:80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB (2007) Dopaminergic regulation of limbic-striatal interplay. Journal of psychiatry & neuroscience : JPN 32:400–411. [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S (2007) Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cerebral cortex 17:251–260. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG (2001) Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 21:6370–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Onge JRS, Ghods-Sharifi S, Winstanley CA (2008a) Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cognitive Affective & Behavioral Neuroscience 8:375–389. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S (2008b) Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology 33:1966–1979. [DOI] [PubMed] [Google Scholar]
- Fond G, Bayard S, Capdevielle D, Del-Monte J, Mimoun N, Macgregor A, Boulenger JP, Gely-Nargeot MC, Raffard S (2013) A further evaluation of decision-making under risk and under ambiguity in schizophrenia. European archives of psychiatry and clinical neuroscience 263:249–257. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Pinkston J, Vorontsova E (2009) Timing and space usage are disrupted by amphetamine in rats maintained on DRL 24-s and DRL 72-s schedules of reinforcement. Psychopharmacology (Berl) 204:213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Surmeier DJ (2009) Do substantia nigra dopaminergic neurons differentiate between reward and punishment? Journal of molecular cell biology 1:15–16. [DOI] [PubMed] [Google Scholar]
- Friedman AS (1998) Substance use/abuse as a predictor to illegal and violent behavior: A review of the relevant literature. Aggress Violent Beh 3:339–355. [Google Scholar]
- Gass JT, Chandler LJ (2013) The Plasticity of Extinction: Contribution of the Prefrontal Cortex in Treating Addiction through Inhibitory Learning. Frontiers in psychiatry 4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee S, Ellwood I, Patel T, Luongo F, Deisseroth K, Sohal VS (2012) Synaptic activity unmasks dopamine D2 receptor modulation of a specific class of layer V pyramidal neurons in prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 32:4959–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Floresco SB (2010) Differential effects on effort discounting induced by inactivations of the nucleus accumbens core or shell. Behavioral neuroscience 124:179–191. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, St Onge JR, Floresco SB (2009) Fundamental contribution by the basolateral amygdala to different forms of decision making. The Journal of neuroscience : the official journal of the Society for Neuroscience 29:5251–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetta C, Grecucci A, Rattin A, Guerreschi C, Sanfey AG, Bonini N (2014) To play or not to play: a personal dilemma in pathological gambling. Psychiatry Res 219:562–569. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN (2007) The neural basis of decision making. Annual review of neuroscience 30:535–574. [DOI] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA (2008) Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull 34:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Bechara A, Martin EM (2007) Executive functions among individuals with methamphetamine or alcohol as drugs of choice: preliminary observations. J Clin Exp Neuropsychol 29:155–159. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Mackey S, Paulus MP (2013) Altered risk-related processing in substance users: Imbalance of pain and gain. Drug and alcohol dependence 132:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA (1991) Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 41:1–24. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ (2007) Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in neurosciences 30:220–227. [DOI] [PubMed] [Google Scholar]
- Hayden B, Pasternak T (2013) Linking neural activity to complex decisions. Visual neuroscience 30:331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Miller SW, See RE, McGinty JF (2008) Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology 198:77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ (2003) The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience & Biobehavioral Reviews 27:555–579. [DOI] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O (2011) Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:11457–11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ineichen C, Sigrist H, Spinelli S, Lesch KP, Sautter E, Seifritz E, Pryce CR (2012) Establishing a probabilistic reversal learning test in mice: evidence for the processes mediating reward-stay and punishment-shift behaviour and for their modulation by serotonin. Neuropharmacology 63:1012–1021. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P (2010) Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 167:748–751. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL (2008a) Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. The Journal of neuroscience : the official journal of the Society for Neuroscience 28:5088–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL (2008b) Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience 155:573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM (2015) From circuits to behaviour in the amygdala. Nature 517:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Woods JA, Groman SM, Seu E (2010) Behavioral characteristics and neural mechanisms mediating performance in a rodent version of the Balloon Analog Risk Task. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 35:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC (2009) The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61:786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD (2007) Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience 27:6923–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Mar AC, Dickinson A, Robbins TW, Everitt BJ (2009) The rat prelimbic cortex mediates inhibitory response control but not the consolidation of instrumental learning. Behavioral neuroscience 123:875–885. [DOI] [PubMed] [Google Scholar]
- Joseph JE, Liu X, Jiang Y, Lynam D, Kelly TH (2009) Neural correlates of emotional reactivity in sensation seeking. Psychol Sci 20:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB (2002) Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 22:1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A (2013) Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends in neurosciences 36:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Zariwala HA, Mainen ZF (2008) Neural correlates, computation and behavioural impact of decision confidence. Nature 455:227–231. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC (2011) An analysis of rat prefrontal cortex in mediating executive function. Neurobiology of learning and memory 96:417–431. [DOI] [PubMed] [Google Scholar]
- Kheramin D, Body S, Mobini S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM (2002) Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on inter-temporal choice: a quantitative analysis. Psychopharmacology (Berl) 165:9–17. [DOI] [PubMed] [Google Scholar]
- Killcross AS, Everitt BJ, Robbins TW (1997) Symmetrical effects of amphetamine and alpha-flupenthixol on conditioned punishment and conditioned reinforcement: Contrasts with midazolam. Psychopharmacology 129:141–152. [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E (2003) Coordination of actions and habits in the medial prefrontal cortex of rats. Cerebral cortex 13:400–408. [DOI] [PubMed] [Google Scholar]
- Kim YT, Lee KU, Lee SJ (2009) Deficit in decision-making in chronic, stable schizophrenia: from a reward and punishment perspective. Psychiatry investigation 6:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RG, Mannuzza S, Olazagasti MA, Roizen E, Hutchison JA, Lashua EC, Castellanos FX (2012) Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiatry 69:1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y (2009) Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology 56 Suppl 1:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraplin A, Dshemuchadse M, Behrendt S, Scherbaum S, Goschke T, Buhringer G (2014) Dysfunctional decision-making in pathological gambling: pattern specificity and the role of impulsivity. Psychiatry research 215:675–682. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC (2012) Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature neuroscience 15:816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroyzer N, Gross-Tsur V, Pollak Y (2014) Risk taking in adolescents with attention deficit hyperactivity disorder on a probabilistic choice task. The Journal of nervous and mental disease 202:247–252. [DOI] [PubMed] [Google Scholar]
- Kruschwitz JD, Simmons AN, Flagan T, Paulus MP (2012) Nothing to lose: processing blindness to potential losses drives thrill and adventure seekers. Neuroimage 59:2850–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A (2013) Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature 498:363–366.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW (2010) The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem 17:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC (2014) Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76 Pt B:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC (2012) Input-specific control of reward and aversion in the ventral tegmental area. Nature 491:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer ME, Neighbors C, Lostutter TW, Whiteside U, Cronce JM, Kaysen D, Walker DD (2012) Brief motivational feedback and cognitive behavioral interventions for prevention of disordered gambling: a randomized clinical trial. Addiction 107:1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA (2010) Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Current topics in behavioral neurosciences 3:101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Rauchs G, La Joie R, Mezenge F, Boudehent C, Vabret F, Segobin S, Viader F, Allain P, Eustache F, Pitel AL, Beaunieux H (2014) Impaired decision-making and brain shrinkage in alcoholism. Eur Psychiatry 29:125–133. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Defrancesco A, Moghaddam B (2008) Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. The European journal of neuroscience 27:1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Rushworth MF, Walton ME, Watanabe M, Sakagami M (2007) Functional specialization of the primate frontal cortex during decision making. The Journal of neuroscience : the official journal of the Society for Neuroscience 27:8170–8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AT, Vogt D, Rubenstein JL, Sohal VS (2014) A class of GABAergic neurons in the prefrontal cortex sends long-range projections to the nucleus accumbens and elicits acute avoidance behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:11519–11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Bornovalova MA, Daughters SB, Curtin JJ (2005) Differences in impulsivity and sexual risk behavior among inner-city crack/cocaine users and heroin users. Drug and alcohol dependence 77:169–175. [DOI] [PubMed] [Google Scholar]
- Leland DS, Paulus MP (2005) Increased risk-taking decision-making but not altered response to punishment in stimulant-using young adults. Drug and alcohol dependence 78:83–90. [DOI] [PubMed] [Google Scholar]
- Lenz JD, Lobo MK (2013) Optogenetic insights into striatal function and behavior. Behavioural brain research 255:44–54. [DOI] [PubMed] [Google Scholar]
- Linnet J, Moller A, Peterson E, Gjedde A, Doudet D (2011a) Dopamine release in ventral striatum during Iowa Gambling Task performance is associated with increased excitement levels in pathological gambling. Addiction 106:383–390. [DOI] [PubMed] [Google Scholar]
- Linnet J, Moller A, Peterson E, Gjedde A, Doudet D (2011b) Inverse association between dopaminergic neurotransmission and Iowa Gambling Task performance in pathological gamblers and healthy controls. Scandinavian journal of psychology 52:28–34. [DOI] [PubMed] [Google Scholar]
- Lobo DS, Souza RP, Tong RP, Casey DM, Hodgins DC, Smith GJ, Williams RJ, Schopflocher DP, Wood RT, el-Guebaly N, Kennedy JL (2010) Association of functional variants in the dopamine D2-like receptors with risk for gambling behaviour in healthy Caucasian subjects. Biological psychology 85:33–37. [DOI] [PubMed] [Google Scholar]
- Ma L, Hyman JM, Phillips AG, Seamans JK (2014) Tracking progress toward a goal in corticostriatal ensembles. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:2244–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T (2002) Decision-making processes following damage to the prefrontal cortex. Brain : a journal of neurology 125:624–639. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ (2004) Neuronal signalling of fear memory. Nature reviews Neuroscience 5:844–852. [DOI] [PubMed] [Google Scholar]
- Martin-Garcia E, Courtin J, Renault P, Fiancette JF, Wurtz H, Simonnet A, Levet F, Herry C, Deroche-Gamonet V (2014) Frequency of cocaine self-administration influences drug seeking in the rat: optogenetic evidence for a role of the prelimbic cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 39:2317–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O (2007) Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447:1111–1115. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O (2009a) Representation of negative motivational value in the primate lateral habenula. Nature neuroscience 12:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O (2009b) Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459:837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD (2007) Time discounting for primary rewards. J Neurosci 27:5796–5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF (2012) Encoding of aversion by dopamine and the nucleus accumbens. Frontiers in neuroscience 6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA, Wixted JT (1996) Timing and amplitude of saccades during predictive saccadic tracking in schizophrenia. Psychophysiology 33:93–101. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 23:3531–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW (2004) Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 24:1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RJ, Floresco SB (2007) The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking behavior. Neuroscience 146:1484–1494. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE (2003) Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology 168:57–65. [DOI] [PubMed] [Google Scholar]
- Mihindou C, Guillem K, Navailles S, Vouillac C, Ahmed SH (2013) Discriminative inhibitory control of cocaine seeking involves the prelimbic prefrontal cortex. Biol Psychiatry 73:271–279. [DOI] [PubMed] [Google Scholar]
- Mitchell MR, Vokes CM, Blankenship AL, Simon NW, Setlow B (2011) Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology 218:703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Weiss VG, Beas BS, Morgan D, Bizon JL, Setlow B (2014) Adolescent risk taking, cocaine self-administration, and striatal dopamine signaling. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 39:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini D, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM (2002) Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 160:290–298. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G (2014) Orbitofrontal cortical neurons encode expectation-driven initiation of reward-seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:10234–10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, Aston-Jones G (2014) Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain research [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Root DH (2014) Glutamate neurons within the midbrain dopamine regions. Neuroscience 282C:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE (1995) Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral neuroscience 109:681–688. [DOI] [PubMed] [Google Scholar]
- Morris G, Nevet A, Arkadir D, Vaadia E, Bergman H (2006) Midbrain dopamine neurons encode decisions for future action. Nature neuroscience 9:1057–1063. [DOI] [PubMed] [Google Scholar]
- Morris SE, Cuthbert BN (2012) Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci 14:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ER, Dalley JW, Robbins TW (2005) Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology 179:99–107. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Fernando AB, Urcelay GP, Robinson ES, Mar AC, Theobald DE, Dalley JW, Robbins TW (2012) Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology 219:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must A, Horvath S, Nemeth VL, Janka Z (2013) The Iowa Gambling Task in depression - what have we learned about sub-optimal decision-making strategies? Front Psychol 4:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M (2006) Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron 52:921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M (2009) Delay activity in rodent frontal cortex during a simple reaction time task. Journal of neurophysiology 101:2859–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M (2006) Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience 139:865–876. [DOI] [PubMed] [Google Scholar]
- Navas JF, Torres A, Vilar R, Verdejo-Garcia A, Catena A, Perales JC (2014) Nonmonetary Decision-Making Indices Discriminate Between Different Behavioral Components of Gambling. J Gambl Stud. [DOI] [PubMed] [Google Scholar]
- Nejtek VA, Kaiser KA, Zhang B, Djokovic M (2013) Iowa Gambling Task scores predict future drug use in bipolar disorder outpatients with stimulant dependence. Psychiatry Res 210:871–879. [DOI] [PubMed] [Google Scholar]
- O’Neill M, Schultz W (2010) Coding of reward risk by orbitofrontal neurons is mostly distinct from coding of reward value. Neuron 68:789–800. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Gentry RN, Chioma VC, Cheer JF (2012) Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. The Journal of neuroscience : the official journal of the Society for Neuroscience 32:14804–14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Trotta RT, Bizon JL, Setlow B (2015) Dissociable roles for the basolateral amygdala and orbitofrontal cortex in decision-making under risk of punishment. The Journal of neuroscience: the official journal of the Society for Neuroscience 35:1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S (2011) Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:17269–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander S, Cazares VA, Kim C, Cheung S, Gonzalez I, Izquierdo A (2011) Orbitofrontal cortex and basolateral amygdala lesions result in suboptimal and dissociable reward choices on cue-guided effort in rats. Behavioral neuroscience 125:350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais-Vieira M, Lima D, Galhardo V (2007) Orbitofrontal cortex lesions disrupt risk assessment in a novel serial decision-making task for rats. Neuroscience 145:225–231. [DOI] [PubMed] [Google Scholar]
- Papageorgiou C, Karanasiou IS, Kapsali F, Stachtea X, Kyprianou M, Tsianaka EI, Karakatsanis NA, Rabavilas AD, Uzunoglu NK, Papadimitriou GN (2013) Temporal processing dysfunction in schizophrenia as measured by time interval discrimination and tempo reproduction tasks. Prog Neuropsychopharmacol Biol Psychiatry 40:173–179. [DOI] [PubMed] [Google Scholar]
- Paulus MP (2007) Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science 318:602–606. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB (2003) Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage 19:1439–1448. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Watson KK, Platt ML (2014) Decision making: the neuroethological turn. Neuron 82:950–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, De Vries TJ (2013) D-cycloserine administered directly to infralimbic medial prefrontal cortex enhances extinction memory in sucrose-seeking animals. Neuroscience 230:24–30. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 28:6046–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ (2009) Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A (2013) Cortical interneurons that specialize in disinhibitory control. Nature 503:521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, Bossaerts P, Quartz SR (2006) Neural differentiation of expected reward and risk in human subcortical structures. Neuron 51:381–390. [DOI] [PubMed] [Google Scholar]
- Pulido J, Barrio G, Lardelli P, Bravo MJ, Regidor E, de la Fuente L (2011) Association between cannabis and cocaine use, traffic injuries and use of protective devices. European Journal of Public Health 21:753–755. [DOI] [PubMed] [Google Scholar]
- Quartz SR (2009) Reason, emotion and decision-making: risk and reward computation with feeling. Trends Cogn Sci 13:209–215. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kuhn KU, Hoppe C, Westheide J, Maier W, Daum I, Wagner M (2007) Elevated impulsivity and impaired decision-making cognition in heavy users of MDMA (“Ecstasy”). Psychopharmacology (Berl) 189:517–530. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D (2008) Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 33:56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos Olazagasti MA, Klein RG, Mannuzza S, Belsky ER, Hutchison JA, Lashua-Shriftman EC, Castellanos FX (2013) Does childhood attention-deficit/hyperactivity disorder predict risk-taking and medical illnesses in adulthood? Journal of the American Academy of Child and Adolescent Psychiatry 52:153–162 e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LF, Lee J, Davis MC, Altshuler L, Glahn DC, Miklowitz DJ, Green MF (2014) Impulsivity and risk taking in bipolar disorder and schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 39:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SE, Killcross AS (2007) Lesions of rat infralimbic cortex enhance renewal of extinguished appetitive Pavlovian responding. The European journal of neuroscience 25:2498–2503. [DOI] [PubMed] [Google Scholar]
- Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van den Oever MC (2014) Optogenetic dissection of medial prefrontal cortex circuitry. Frontiers in systems neuroscience 8:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risterucci C, Terramorsi D, Nieoullon A, Amalric M (2003) Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. The European journal of neuroscience 17:1498–1508. [DOI] [PubMed] [Google Scholar]
- Rivalan M, Ahmed SH, Dellu-Hagedorn F (2009) Risk-prone individuals prefer the wrong options on a rat version of the Iowa Gambling Task. Biol Psychiatry 66:743–749. [DOI] [PubMed] [Google Scholar]
- Rivalan M, Coutureau E, Fitoussi A, Dellu-Hagedorn F (2011) Inter-individual decision-making differences in the effects of cingulate, orbitofrontal, and prelimbic cortex lesions in a rat gambling task. Front Behav Neurosci 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivalan M, Valton V, Series P, Marchand AR, Dellu-Hagedorn F (2013) Elucidating poor decision-making in a rat gambling task. PLoS One 8:e82052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Taylor AR, Schoenbaum G (2006) Encoding of time-discounted rewards in orbitofrontal cortex is independent of value representation. Neuron 51:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW (1999a) Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 19:9029–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW (1999b) Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 20:322–339. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Tiesinga PH, Roitman JD, Carelli RM (2010) Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning. Learn Mem 17:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R (2013) Conversion of sensory signals into perceptions, memories and decisions. Progress in neurobiology 103:1–2. [DOI] [PubMed] [Google Scholar]
- Rygula R, Clarke HF, Cardinal RN, Cockcroft GJ, Xia J, Dalley JW, Robbins TW, Roberts AC (2014) Role of central serotonin in anticipation of rewarding and punishing outcomes: effects of selective amygdala or orbitofrontal 5-HT depletion. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD (1994) The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behavioural brain research 61:117–133. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Britten KH, Newsome WT (1990) Cortical microstimulation influences perceptual judgements of motion direction. Nature 346:174–177. [DOI] [PubMed] [Google Scholar]
- Sangha S, Chadick JZ, Janak PH (2013) Safety encoding in the basal amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience 33:3744–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Robinson PD, Greba Q, Davies DA, Howland JG (2014) Alterations in reward, fear and safety cue discrimination after inactivation of the rat prelimbic and infralimbic cortices. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 39:2405–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S et al. (2012) Risk taking and the adolescent reward system: a potential common link to substance abuse. The American journal of psychiatry 169:39–46. [DOI] [PubMed] [Google Scholar]
- Schultz W (2010) Subjective neuronal coding of reward: temporal value discounting and risk. Eur J Neurosci 31:2124–2135. [DOI] [PubMed] [Google Scholar]
- Schultz W (2013) Updating dopamine reward signals. Current opinion in neurobiology 23:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, O’Neill M, Tobler PN, Kobayashi S (2011) Neuronal signals for reward risk in frontal cortex. Ann N Y Acad Sci 1239:109–117. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR (2004) The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Progress in neurobiology 74:1–58. [DOI] [PubMed] [Google Scholar]
- Seong HJ, Carter AG (2012) D1 receptor modulation of action potential firing in a subpopulation of layer 5 pyramidal neurons in the prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 32:10516–10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Roozendaal B, McGaugh JL (2000) Involvement of a basolateral amygdala complex-nucleus accumbens pathway in glucocorticoid-induced modulation of memory consolidation. The European journal of neuroscience 12:367–375. [DOI] [PubMed] [Google Scholar]
- Setlow B, Holland PC, Gallagher M (2002) Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive pavlovian second-order conditioned responses. Behavioral neuroscience 116:267–275. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT (1996) Motion perception: seeing and deciding. Proceedings of the National Academy of Sciences of the United States of America 93:628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Kiani R (2013) Decision making as a window on cognition. Neuron 80:791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiei N, Gray M, Viau V, Floresco SB (2012) Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology 37:2194–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp KG, Mitchell MR, Beas BS, Bizon JL, Setlow B (2015) Affective and cognitive mechanisms of risky decision making. Neurobiol Learn and Mem 117:60–70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Setlow B (2012) Modeling risky decision making in rodents. Methods Mol Biol 829:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B (2009) Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 34:2208–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, Banuelos C, Vokes CM, Taylor AB, Haberman RP, Bizon JL, Setlow B (2011) Dopaminergic modulation of risky decision-making. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:17460–17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Graybiel AM (2013) A Dual Operator View of Habitual Behavior Reflecting Cortical and Striatal Dynamics. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Virkud A, Deisseroth K, Graybiel AM (2012) Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America 109:18932–18937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ (2010) Prefrontal control of fear: more than just extinction. Current opinion in neurobiology 20:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Hovelso N, Mason AO, Kantak PA, Ung RL, Decot HK, Stuber GD (2014) Activation of prefrontal cortical parvalbumin interneurons facilitates extinction of reward-seeking behavior. The Journal of Neuroscience : the official journal of the Society for Neuroscience 34:3699–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB (2009) Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 34:681–697. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB (2010a) Prefrontal cortical contribution to risk-based decision making. Cerebral cortex 20:1816–1828. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Chiu YC, Floresco SB (2010b) Differential effects of dopaminergic manipulations on risky choice. Psychopharmacology 211:209–221. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Abhari H, Floresco SB (2011) Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. The Journal of Neuroscience : the official journal of the Society for Neuroscience 31:8625–8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Ahn S, Phillips AG, Floresco SB (2012a) Dynamic fluctuations in dopamine efflux in the prefrontal cortex and nucleus accumbens during risk-based decision making. The Journal of Neuroscience: the official journal of the Society for Neuroscience 32:16880–16891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Stopper CM, Zahm DS, Floresco SB (2012b) Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. The Journal of Neuroscience : the official journal of the Society for Neuroscience 32:2886–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, Stuber GD (2013) A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron 80:1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD (2012) Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nature neuroscience 15:1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, LaLumiere RT (2013) Optogenetic inhibition of cocaine seeking in rats. Addiction biology 18:50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingroever H, Wetzels R, Horstmann A, Neumann J, Wagenmakers EJ (2013) Performance of healthy participants on the Iowa Gambling Task. Psychological assessment 25:180–193. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB (2011) Contributions of the nucleus accumbens and its subregions to different aspects of risk-based decision making. Cognitive, affective & behavioral neuroscience 11:97–112. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Khayambashi S, Floresco SB (2013) Receptor-specific modulation of risk-based decision making by nucleus accumbens dopamine. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 38:715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Green EB, Floresco SB (2014a) Selective involvement by the medial orbitofrontal cortex in biasing risky, but not impulsive, choice. Cerebral cortex 24:154–162. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Tse MT, Montes DR, Wiedman CR, Floresco SB (2014b) Overriding phasic dopamine signals redirects action selection during risk/reward decision making. Neuron 84:177–189. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB (2014). What’s better for me? Fundamental role for lateral habenula in promoting subjective decision biases. Nat Neurosci 17:33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Busemeyer JR, Lin A, Grant SJ, Bonson KR (2004) Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychonomic bulletin & review 11:742–747. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A (2011) Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475:377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugam JA, Day JJ, Wightman RM, Carelli RM (2012) Phasic nucleus accumbens dopamine encodes risk-based decision-making behavior. Biological psychiatry 71:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW (2000) Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia 38:596–612. [DOI] [PubMed] [Google Scholar]
- Tobler PN, O’Doherty JP, Dolan RJ, Schultz W (2007) Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J Neurophysiol 97:1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak ME, Sorge GB, Benoit A, West RF, Stanovich KE (2010) Decision-making and cognitive abilities: A review of associations between Iowa Gambling Task performance, executive functions, and intelligence. Clin Psychol Rev 30:562–581. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL (2012) Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 490:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Janak PH (2007) Amygdala neurons differentially encode motivation and reinforcement. The Journal of neuroscience : the official journal of the Society for Neuroscience 27:3937–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Cone JJ, Schairer WW, Janak PH (2010) Amygdala neural encoding of the absence of reward during extinction. The Journal of neuroscience : the official journal of the Society for Neuroscience 30:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM (2000) The medial prefrontal cortex as a part of the brain reward system. Amino acids 19:211–219. [DOI] [PubMed] [Google Scholar]
- Turnbull OH, Evans CE, Kemish K, Park S, Bowman CH (2006) A novel set-shifting modification of the iowa gambling task: flexible emotion-based learning in schizophrenia. Neuropsychology 20:290–298. [DOI] [PubMed] [Google Scholar]
- Uchida N, Kepecs A, Mainen ZF (2006) Seeing at a glance, smelling in a whiff: rapid forms of perceptual decision making. Nature reviews Neuroscience 7:485–491. [DOI] [PubMed] [Google Scholar]
- van Aerde KI, Feldmeyer D (2013) Morphological and Physiological Characterization of Pyramidal Neuron Subtypes in Rat Medial Prefrontal Cortex. Cerebral cortex. [DOI] [PubMed] [Google Scholar]
- van den Bos R, Jolles J, van der Knaap L, Baars A, de Visser L (2012) Male and female Wistar rats differ in decision-making performance in a rodent version of the Iowa Gambling Task. Behav Brain Res 234:375–379. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ (2010) Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neuroscience and biobehavioral reviews 35:276–284. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Rotaru DC, Heinsbroek JA, Gouwenberg Y, Deisseroth K, Stuber GD, Mansvelder HD, Smit AB (2013) Ventromedial prefrontal cortex pyramidal cells have a temporal dynamic role in recall and extinction of cocaine-associated memory. The Journal of neuroscience : the official journal of the Society for Neuroscience 33:18225–18233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden CG, Buijs RM (2000) Functional neuroanatomy of the prefrontal cortex: autonomic interactions. Progress in brain research 126:49–62. [DOI] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Young JW (2013a) Differential effects of dopamine transporter inhibitors in the rodent Iowa gambling task: relevance to mania. Psychopharmacology 225:661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Young JW (2013b) Differential effects of dopamine transporter inhibitors in the rodent Iowa gambling task : Relevance to mania. Psychopharmacology (Berl) 225:661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Henry BL, Minassian A, Perry W, Milienne-Petiot M, Higa KK, Geyer MA, Young JW (2014) Reduced Dopamine Transporter Functioning Induces High-Reward Risk-Preference Consistent with Bipolar Disorder. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Hicks PB (1999) The conditioned avoidance response test re-evaluated: is it a sensitive test for the detection of potentially atypical antipsychotics? Neuroscience and biobehavioral reviews 23:851–862. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Gold JM (2007) Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res 93:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Robinson BM, Gold JM (2007) Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry 62:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman JI, Barry RJ, Bradford L, Delva NJ, Beninger RJ (2012) Probabilistic classification and gambling in patients with schizophrenia receiving medication: comparison of risperidone, olanzapine, clozapine and typical antipsychotics. Psychopharmacology 222:173–183. [DOI] [PubMed] [Google Scholar]
- Wenzel JM, Rauscher NA, Cheer JF, Oleson EB (2015) A Role for Phasic Dopamine Release within the Nucleus Accumbens in Encoding Aversion: A Review of the Neurochemical Literature. ACS chemical neuroscience 6:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, Saddoris MP, Kerfoot EC, Carelli RM (2014) Prelimbic and infralimbic cortical regions differentially encode cocaine-associated stimuli and cocaine-seeking before and following abstinence. The European journal of neuroscience 39:1891–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP (2013) The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. The European journal of neuroscience 37:259–268. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW (2004) Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. The Journal of neuroscience : the official journal of the Society for Neuroscience 24:4718–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Leland DS, Paulus MP (2007) Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res 179:643–653. [DOI] [PubMed] [Google Scholar]
- Worthy DA, Hawthorne MJ, Otto AR (2013) Heterogeneity of strategy use in the Iowa gambling task: a comparison of win-stay/lose-shift and reinforcement learning models. Psychon Bull Rev 20:364–371. [DOI] [PubMed] [Google Scholar]
- Young JW, Jentsch JD, Bussey TJ, Wallace TL, Hutcheson DM (2013) Consideration of species differences in developing novel molecules as cognition enhancers. Neurosci Biobehav Rev 37:2181–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack M, Poulos CX (2009) Effects of the atypical stimulant modafinil on a brief gambling episode in pathological gamblers with high vs. low impulsivity. J Psychopharmacol 23:660–671. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Winstanley CA (2011) Lesions of the basolateral amygdala and orbitofrontal cortex differentially affect acquisition and performance of a rodent gambling task. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:2197–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA (2009) Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 34:2329–2343. [DOI] [PubMed] [Google Scholar]