Abstract
The brain needs more energy than other organs in the body. Mitochondria are the generator of vital power in the living organism. Not only do mitochondria sense signals from the outside of a cell, but they also orchestrate the cascade of subcellular events by supplying adenosine-5′-triphosphate (ATP), the biochemical energy. It is known that impaired mitochondrial function and oxidative stress contribute or lead to neuronal damage and degeneration of the brain. This mini-review focuses on addressing how mitochondrial dysfunction and oxidative stress are associated with the pathogenesis of neurodegenerative disorders including Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease, and Parkinson’s disease. In addition, we discuss state-of-the-art computational models of mitochondrial functions in relation to oxidative stress and neurodegeneration. Together, a better understanding of brain disease-specific mitochondrial dysfunction and oxidative stress can pave the way to developing antioxidant therapeutic strategies to ameliorate neuronal activity and prevent neurodegeneration.
Keywords: mitochondria, oxidative stress, antioxidants, Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease, Parkinson’s disease, computational modeling
1. Introduction
The brain uses around 20% of energy in the whole adult human body [1,2,3]. Because brain cells consume high levels of energy, they require a powerful generator of energy. The mitochondrion is a subcellular organelle and a power engine that produces adenosine-5′-triphosphate (ATP), the biochemical “energy currency” in cells. Glucose yields pyruvate (pyruvic acid) through the process of glycolysis. Pyruvate is transported to the mitochondria and generates hydrogen ions via a pathway of oxidative phosphorylation. Pyruvate dehydrogenase oxidizes pyruvate to form acetyl coenzyme A that is oxidized in the tricarboxylic acid cycle. The coenzymes released in these mentioned pathways, the reduced form of nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) are oxidized in the respiratory chain, resulting in the generation of a proton gradient between the inner and outer membrane of the mitochondria. An increase in electrochemical potential gradient by protons drives a membrane potential (negative inside) and a pH gradient (basic inside) that synthesizes ATP through F0F1-ATP synthase.
Mitochondria produce 80% or more of the reactive oxygen species (ROS) in brain cells. In general, oxidative stress is an indispensable process and an essential cellular event that occurs naturally [4]. Herein, an outstanding question arises: how does it become detrimental and toxic so as to damage macromolecules and many different types of cells in the brain [5,6]? A plausible answer may be that oxidative stress becomes toxic when it continuously accelerates and reaches beyond a threshold level. Produced by a leakage of electrons at the level of four complexes (I–IV), ROS, such as superoxide anion radical (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH) results in DNA oxidation, lipid peroxidation, and protein oxidation.
Superoxide dismutase 1 (SOD1) catalyzes the partitioning of two superoxide anion (2O2•−) and converts it into hydrogen peroxide and oxygen. Then, hydrogen peroxide is processed by catalase to produce two water molecules and oxygen. Aging and environmental stressors attenuate brain cell function to neutralize ROS and in consequence, oxidative stress disrupts cellular homeostasis and triggers the progression of neurodegeneration. The vicious cycle of oxidative stress is known to be the most common pathogenesis in neurodegenerative disorders including Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), Parkinson’s disease (PD), and others [3,7,8,9]. In this review, we discuss which molecules and stressors affect mitochondrial function and how oxidative stress is linked to the pathophysiology and neurodegeneration of AD, ALS, HD, and PD, respectively [3,7,8,9,10]. Furthermore, we present computational modeling studies on mitochondria-driven metabolic processes and oxidative stress.
2. Brain Disorder and Mitochondrial Dysfunction
2.1. Alzheimer’s Disease (AD)
Alzheimer’s disease (AD) is a common neurodegenerative disease characterized by impaired cognition and neuronal loss in aged people. Principal agents of AD pathology are attributed to the intracellular aggregation of hyperphosphorylated tau and the abnormal deposition of amyloid beta (Aβ), which may be oligomeric (oAβ) or aggregated into senile plaques. Recently, significant evidence of mitochondria as the direct targets for Aβ mediated neuronal-toxicity has been produced. Marked by a decrease in ATP production and an increase in oxidative stress elements such as ROS, mitochondrial dysfunction contributes to AD pathogenesis [10]. One area of interest is transcription factor p53, a vital component of aerobic respiration. Under normal circumstances, p53 trans-activates cytochrome oxidase 2 (SCOS), a subunit of cytochrome c oxidase (COX) or complex IV from the electron transport chain (ETC). However, it is well known that under pathogenic conditions, oAβ upregulates p53, leads to increased expression of SCOS, and increases ROS production [11]. Although additional p53 is recruited from the nucleus to the mitochondria to ensure cell survival, p53 dependent anti-oxidative capacities are greatly suppressed in AD, in which p53 will begin to exhibit prooxidative activities [12,13].
In AD mitochondria, antioxidant checkpoint mechanisms are significantly reduced. When returning to mitochondrial homeostasis, NAD-dependent deacetylase sirtuin-3 (SIRT3), a class III histone deacetylase localized in the inner mitochondrial membrane and matrix, decreases abundant p-53. Furthermore, when SIRT3 protein levels are reduced, Lee et al. discovered increased mito-p53 occupancy [14]. Recent RNA expression chip datasets have confirmed downregulated SIRT3 mRNA levels compared to controls in post-mortem AD brains and in 3xTG AD mice expressing microtubule associated protein tau (MAPT), amyloid-beta precursor protein (APP), and presenilin-1(PSEN1) transgenes [15]. Notably, AD patients exhibited decreased expression of mitochondrial DNA (mtDNA) encoded subunits of complex I (ND2 and ND4), nuclear DNA encoded subunits of complex I (NDUFA2), and nuclear DNA encoded subunits of complex IV (NDUFA4) [14,16,17]. Given that the mitochondrial respiratory complexes remain critical for normal ATP production, any subunit functional defects can reduce respiratory activity and increase ROS production.
Concurrently, mitochondria-specific Aβ and p53 accumulation revealed mitochondrial oxidative events as an element not only sufficient to induce pathological conditions but also result in neuronal death [12,18,19,20]. For instance, under oxidative stress, ROS damages nuclear DNA, triggering assembly of the DNA damage response network [20]. Especially when DNA double-stranded breaks (DBS) occur, p53 is phosphorylated by ATM serine/threonine kinase (ATM) [21]. However, depending on the extent of DNA damage, p53 will either drive gene expression towards the activation of cell cycle checkpoints or cell death checkpoints. It has been reported that p53 transactivates critical determinants of mitochondrial apoptosis such as Bax, Bak, Nova, and Puma while repressing those that are antiapoptotic such as Bcl-2, Bcl-X, and Mcl-1 [22]. p53 may also directly activate Bax and inhibit Bcl-2/-X in the mitochondria, driving the assembly of Bax/Bak lipid pores, which alters mitochondrial membrane potential (ΔΨ) and leads to mitochondrial apoptosis [23].
In addition, p53 also engages with the mitochondrial permeability transition pore via direct interaction with cyclophilin D (CypD) [24]. CypD regulates the opening of mitochondrial permeability transition pore by phosphorylating serine residue S191 [25]. Indeed, SIRT3 which deacetylates CypD is downregulated in AD [26]. Furthermore, oAβ-CypD complexes were found in AD brains and transgenic AD mice [27,28]. Mitochondrial permeability transition pore activation results in decreased ΔΨ. An influx of cytosolic molecules, induces mitochondrial swelling and eventual rupture of the outer membrane, ending in mitochondrial necrosis [29].
Overall, a loss of ΔΨ often activates both apoptotic/necrotic pathways as well as mitophagy in the same cell. Damaged mitochondria are selectively degraded by autophagy in an effort to prevent cell death. However, under pathogenic conditions, mitophagy or protective mechanisms are significantly compromised [30,31]. Simultaneously, impairment of mitochondrial trafficking has been reported in AD hippocampal neurons [32,33]. Mitochondria deficiency at the synapse, decreases available synaptic ATP essential for neurotransmission [18,34]. In combination, ranging from apoptosis/necrosis to mitophagy, mitochondrial dynamics remain as a complex interdependent system that is significantly disturbed, leading to neuronal death in the AD brain (Figure 1).
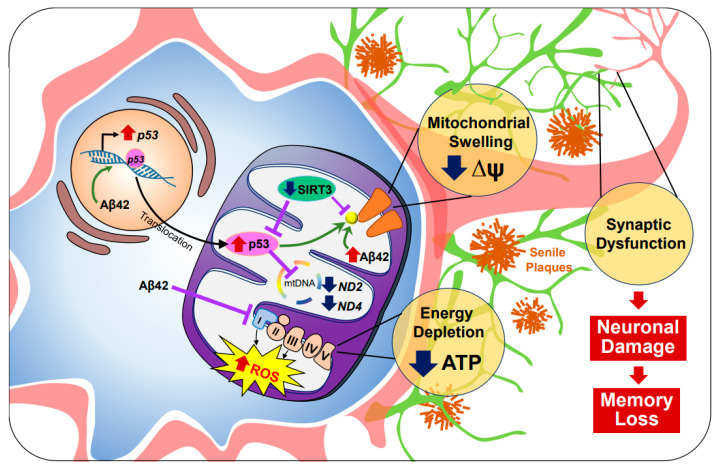
Figure 1.
Mitochondrial dysfunction in Alzheimer’s disease. Oligomeric amyloid beta (oAβ) accumulates intracellularly and triggers mitochondrial dysfunction in Alzheimer’s disease (AD). In the nucleus, oAβ upregulates the expression of p53. While stress signals are present, p53 is translocated to the mitochondria. Sirtuin-3 (SIRT3), which inhibits p53 as well as cyclophilin D (CypD) activity (yellow) functions as a reactive oxygen species (ROS) modulator, but is downregulated, tipping the balance of respiration in favor of ROS production. While oAβ inhibits electron transport chain activity, p53 also represses the production of ND2 and ND4, subunits of complex I, contributing towards overall energy depletion. Subsequently, low ATP levels disturb downstream processes, such as axonal transportation, resulting in synaptic dysfunction. Ultimately, over accumulated p53 and oAβ activates CypD, which prolongs mitochondrial permeability transition pore permeability, triggering cell death.
There still lacks a clear mechanism of the various elements that influence changes from normal conditions to abnormal conditions in AD mitochondria. Higher oAβ concentrations were correlated with lower SIRT3 levels in a dose-dependent manner, as shown on neuronal cells [35]. oAβ can also bind directly to and suppress complex I, accelerating Aβ toxicity [36,37,38]. This supports the notion that mitochondria are a site of a deleterious positive feed-back loop of toxic oAβ, exacerbating AD pathogenesis [39]. Moreover, studies have shown that Aβ jeopardizes neuronal viability via mitochondria in a non-cell autonomous manner. In microglia, the transcriptional upregulation of nucleotide-binding oligomerization domain (NOD)-like receptor protein (NLRP3) inflammasome components combined with the generation of Aβ induced ROS, triggers the assembly of the NLRP3 inflammasome. NLRP3 inflammasome activation is then amplified by cross seeding Aβ, which ultimately results in pyroptotic cell death (pyroptosis). Microglia releases free functional Aβ, consequently injuring neighboring neurons. In total, oAβ directly interacts with multiple downstream levels of mitochondria dysfunction in a cell specific manner. Thus, ameliorating suppressed anti-oxidative capacities in relation to Aβ toxicity in AD may be a potential therapeutic target warranting further investigation.
2.2. Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic lateral sclerosis (ALS) is an adult-onset fatal neurodegenerative disorder that affects the motor neurons located in the spinal cord, brain stem, and motor cortex. Progressive motor neuron loss leads to muscle weakness, then muscle atrophy, spasticity, eventual paralysis and finally death 3 to 5 years after diagnosis by denervation of the respiratory muscles [40]. ALS-associated mitochondrial dysfunction occurs at different levels, including ROS production, defective oxidative phosphorylation, oxidative stress, and defective mitochondrial dynamics [8]. Superoxide (O2−) and nitric oxide (NO), two key pathogenic components produced by the electron transport system (ETS), leads to oxidative damage such as DNA oxidation, protein oxidation, and lipid peroxidation [9,41]. High levels of O2− can interact with NO to form peroxynitrite (ONOO-) which may strengthen oxidative stress and trigger motor neuron damage [42,43,44]. Furthermore, our group has previously demonstrated that mitochondrial B-cell lymphoma-extra large (Bcl-xL) gene expression is increased in astrocytes in response to cellular stress. An increase in mitochondrial Bcl-xL prevents oxidative damage in astrocytes under ALS conditions in mutant (G93A SOD1) transgenic mice (Figure 2A) [45,46]. In the ALS model, excitatory amino acid transporter 2 (EAAT2) expression is reduced in reactive astrocytes, lowering glutamate uptake in the synaptic cleft. Subsequently, the high level of extracellular synaptic glutamate leads to excitotoxicity in the spinal cord of ALS patients [46,47].
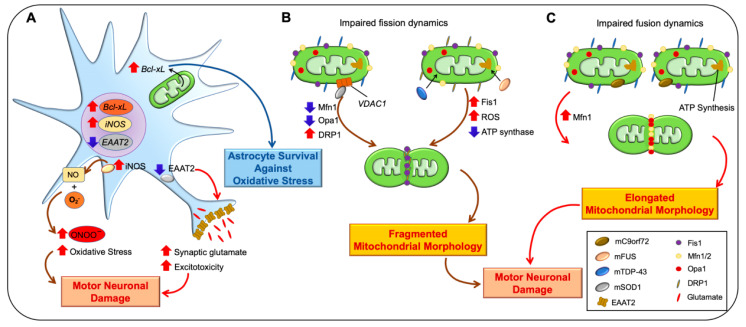
Figure 2.
Mitochondrial dysfunction in amyotrophic lateral sclerosis (ALS). (A) B-cell lymphoma-extra large (Bcl-xL) gene expression is increased in astrocytes in response to cellular stress. The increase of Bcl-xL prevents oxidative damage in astrocytes under ALS conditions. Furthermore, inducible nitric oxide synthase (iNOS) expression in reactive astrocytes facilitates an interaction of superoxide (O2−) with nitric oxide (NO) that forms peroxynitrite (ONOO-). Moreover, reduction of excitatory amino acid transporter 2 (EAAT2) levels inhibits glutamate uptake in the synaptic cleft, elevates the synaptic glutamate, and triggers motor neuronal damage and cell death. (B) Mutated FUS and mutated TAR DNA-binding protein 43 (mTDP-43) are localized to mitochondria, increase mitochondrial fission 1 protein (Fis1) and ROS levels, and subsequently decrease ATP synthesis activity. In addition, mutated superoxide dismutase 1 (SOD1), FUS, and TDP-43 impair mitochondrial fission dynamics and lead to mitochondrial fragmentation and apoptosis. (C) Mutated C9orf72 increase mitofusion-1 (Mfn1) and abnormal mitochondrial fusion.
Mitochondria are highly dynamic organelles whose structural alterations—mitochondrial fission and fusion—may contribute to the etiology of ALS (Figure 2B,C) [48]. Mitochondrial metabolites are shared and ΔΨ is dissipated during the fusion process. Motility acceleration and damaged part sequestration occurred during mitochondrial fission before mitochondrial disposal [49]. Mitochondrial fusion is governed by mitofusin1 (Mfn1), mitofusin2 (Mfn2), and optic atrophy protein1 (OPA1) [50]. Whereas dynamin-related protein1 (Drp1) and fission1 (Fis1) promote mitochondrial fission (Figure 2B,C) [51]. Disruption of mitochondrial cristae has been exhibited in C9orf72-related, mutated SOD1-, TARDBP-, and FUS-associated ALS cases [52,53,54,55]. Interestingly, either cellular stress or pathogenic-variant conditions facilitate trans-localization of FUS and TDP-43 to the mitochondria. They increase Fis1 levels that cause mitochondrial fragmentation, loss of membrane potential, and consequently an increase in ROS production and defective mitochondrial axonal transport (Figure 2B) [56]. Furthermore, FUS interacts with mitochondrial ATP synthase and reduces ATP production in mitochondria that cause a loss of mitochondrial cristae, and mitochondrial fragmentation [57]. Liu et al. demonstrated that the reduction of mitochondrial fusion proteins (Mfn1 and Opa1) and the steady-state activity of Drp1 and Fis1 are important for mitochondrial fission by altering the balance of mitochondrial morphology in ALS mutant (G93A SOD1) transgenic mice (Figure 2B) [58]. Notably, a dynamic change of fission and fusion is also shown in mC9orf72 fibroblasts with elevated MFN1 levels, resulting in an abnormal enlargement of mitochondrial morphology (Figure 2C) [53]. Together, given the contribution of mitochondrial dysfunction to the pathogenesis of ALS, identifying how mitochondrial dysfunctions are relevant to disease onset and progression will elucidate important targets for the development of neuroprotective therapies in ALS.
2.3. Huntington’s Disease (HD)
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disease caused by a polymorphic CAG repeat extension in exon 1 of the huntingtin gene, which encodes for mutant Huntingtin (mHTT) protein [59]. Although the exact function of mHTT is unknown, it interacts with other proteins involved in mitochondrial function, energy metabolism, calcium handling, and transcriptional regulation. Under HD conditions, mutant huntingtin (mHTT) protein becomes aggregated and generates nuclear inclusion bodies. They also make cytoplasmic aggregates that directly interact with mitochondrial elements [60]. It has previously been found that complex I activity was decreased in the platelets of HD patients [61,62]. In addition, a significant decrease in complexes III and IV activity was observed in the caudate and putamen of HD patients, which contributes to a reduction in ΔΨ [63,64,65]. Succinate dehydrogenase (SDH), a main component of Krebs cycle and complex II, was also markedly reduced in the caudate nucleus of HD brains [66]. SDH affects mitochondrial function, catalyzing the oxidation of succinate to fumarate in the Krebs cycle, reducing ubiquinone to ubiquinol. 3-nitropropionic acid (3-NP) and malonate act as an irreversible and reversible inhibitor of SDH, respectively [67,68,69]. In chemical models of HD, malonate and 3-NP decreases Krebs cycle, complex II, and mitochondrial respiratory chain complex activity. It is believed that malonate-induced energy deficits cause a partial membrane depolarization, resulting in excitatory toxicity by removing the voltage-dependent magnesium block of NMDA receptor-gated calcium channels [70,71,72].
In HD, mHTT inhibits protein importation in the mitochondria of isolated brains by overexpressing translocase from the inner membrane 23 (TIM23), leading to neuronal death in HD [73]. In addition, mHTT decreases ∆ψm stability, triggering impaired mitochondrial Ca2+ homeostasis, and abnormal mitochondrial trafficking [74]. It has been shown that mHTT enhances N-methyl-D-aspartate receptor (NMDAR), voltage-gated Ca2+ channel, and inositol trisphosphate receptor (InsP3R) activity in a HD transgenic (YAC128) mouse model [75,76,77,78]. Mitochondrial ATP production is significantly reduced in the muscles of both symptomatic HD patients and presymptomatic HD gene carriers [79]. Moreover, the generation of ROS highly impacts oxidative phosphorylation and lipid peroxidation in the mitochondria of HD patients. Lipid peroxidation and mitochondrial dysfunction are correlated in the striatum of HD patients and HD animal models [80,81]. Lipid peroxidation is a metabolic process in which ROS leads to the oxidative degradation of lipids. 4-hydroxynonenal (4-HNE), a lipid peroxidation product, is significantly elevated in the caudate and putamen of the human HD brain. Nordihydroguaiaretic acid (NDGA), a flavonoid and an antioxidant, decreased the levels of 4-HNE and mHTT and restored ΔΨ in R6/2 HD mice. Together, mHTT directly affects mitochondrial dysfunction and leads to neuronal death in HD (Figure 3). Therapeutic modulations of mitochondrial function and avoidance of oxidative stress are beneficial to HD [81]. In this regard, the development of antioxidant drugs for targeting mitochondria may expedite the treatment of HD.
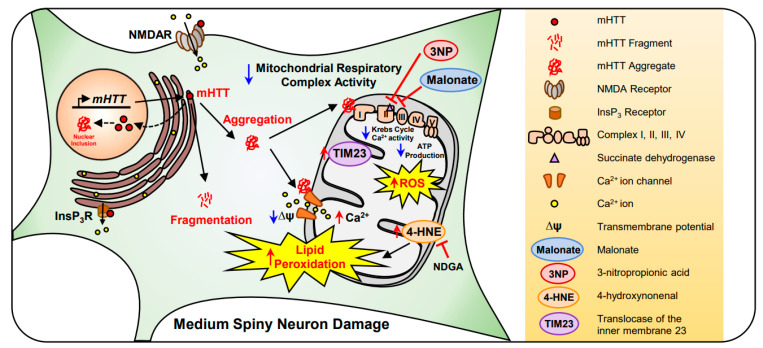
Figure 3.
Mitochondrial dysfunction and lipid peroxidation are found in the medium spiny neuron (MSN) of HD. mutant Huntingtin (mHTT) gene expresses mHTT protein in the MSN. mHTT inclusions are increased in the nucleus and cytosol. In the cytoplasm, both increased mHTT protein fragments and aggregates triggers neuronal damage. The aggregated form of mHTT decreases Krebs cycle and Ca2+ activity, resulting in a decrease in complex I–IV or mitochondrial respiratory complex activity. mHTT aggregation also decreases mitochondrial membrane potential (ΔΨ) and increases reactive oxygen species (ROS) production. As Ca2+ permeability increases, the mitochondrial membrane is hyperpolarized, triggering cell loss. In chemical models of Huntington’s disease (HD), malonate and 3-nitropropionic acid (3NP) inhibits succinate dehydrogenase (SDH) at complex II and, eventually, reduces ATP production via complex V. Lastly, the level of 4-hydroxynonenal (4-HNE), a neuronal lipid peroxidative damage marker, is elevated in HD and nordihydroguaiaretic acid (NDGA) reduces 4-HNE levels and prevents mitochondrial damage in the MSN.
2.4. Parkinson’s Disease (PD)
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that is characterized by unusual motor, non-motor symptoms, and the loss of dopaminergic neurons [82]. The relation between mitochondrial damage and PD was first reported in the substantia nigra of PD patients in 1989 [83,84,85]. In the substantia nigra of the patient brain there is approximately a 35% deficiency in mitochondrial respiratory complex I [86]. This is supported by the administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a prodrug which inhibits complex I and causes oxidative stress damage in dopaminergic neurons [87].
In the dopaminergic neuron, gene mutations linked to mitochondrial dysfunction have been identified and their pathogenetic mechanisms have been studied in PD. For example, mutations of phosphatase and tensin homolog (PTEN)-induced kinase (PINK1), Parkin, and DJ-1 are closely associated with mitochondrial dysfunction and abnormal autophagy in PD patients [88,89]. Mutation of Omi/HtrA2 gene (G399S) results in a severe parkinsonian phenotype [90,91]. Moreover, glucocerebrosidase (GBA) L444P mutation causes a mitochondrial defect in primary neurons, affecting PD [92]. Together, it is apparent that gene mutations and oxidative stress are closely associated with dopaminergic neuronal damage and pathogenesis of PD.
Astrocytes provide structural and metabolic support to neurons, regulate blood flow, water transport, and synaptic transmission within the brain [93]. They also produce various neurotrophic molecules including glial-derived neurotrophic factor (GDNF) for the development and survival of dopaminergic neurons [94,95]. In contrast, reactive astrocytes trigger the disruption of the blood–brain barrier and affect the substantia nigra of PD patients [96]. Interestingly, our group recently found that reactive astrocytes elevate the activity of flavin-dependent monoamine oxidase B (MAO-B) and produce H2O2 in the substantia nigra of PD patients. This study shows a novel pathological mechanism of PD that reactive astrocytes release gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter, and the astrocytic tonic inhibition causes dopaminergic neuronal damage (Figure 4) [97]. This finding suggests that reactive astrocytes are involved in non-cell autonomous pathway of dopaminergic neuronal damage, and therapeutic regulation of this pathway may be applicable for improving neuropathological and behavioral abnormality in PD.
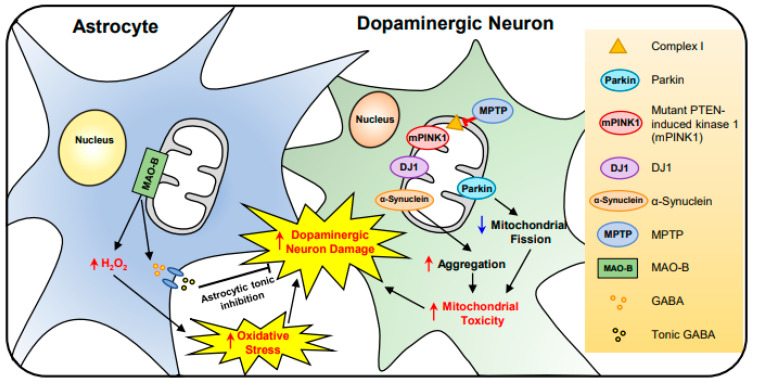
Figure 4.
The effect of mitochondrial dysfunction on astrocytes and neurons in Parkinson’s disease (PD). In the dopaminergic neuron, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) causes a parkinsonian syndrome characterized by a loss of dopamine-producing neurons. Autosomal recessive mutations in Parkin, Phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1), and DJ-1 increases mitochondrial toxicity. Thus, elevating α-Synuclein aggregation and mitochondrial degradation. On the other hand, in reactive astrocytes, monoamine oxidase B (MAO-B) produces GABA, which is released in a tonic manner, affecting neuronal degeneration. As a result, MAO-B activity also induces oxidative stress via H2O2 that ultimately triggers the damage of dopaminergic neurons.
3. Computational Modeling of Mitochondria in Brain Disorders
Computational modeling has been used to explore the role of mitochondrial dysfunction in neurodegenerative diseases. The nature of computational approaches allows zooming in the details of metabolic pathways within a mitochondrion or zooming out to describe the population dynamics of mitochondria. Computational models and simulations are especially useful for the understanding of the organelle’s function in a quantitative manner. Furthermore, they can provide information on the onset and progression of the diseases by bridging the gap between experimental studies.
3.1. Computational Modeling of Mitochondria
The main focus of the computational approaches at a single mitochondrion scale has been on the energy-producing machinery in a network of dynamic intracellular enzymatic reactions [98,99,100,101,102,103,104,105,106]. Traditionally, the computational modeling of this scale has been either thermodynamic, kinetic, or stoichiometric approaches [107]. Thermodynamic models, based on fundamental principles of thermodynamics, are simple approaches for the understanding of complex molecular pathways and for testing various hypotheses, but they lack mechanistic details [108,109,110,111,112]. To examine the biochemical details, one may use stoichiometric models [113,114,115], which, however, cannot offer information on transient behavior or regulatory interactions of metabolic pathways. Kinetic models can be used to describe transient behavior and regulatory interactions to unveil kinetics and mechanistic details of the pathways. However, when quantitative kinetic data from experiments is lacking, the model is only useful to understand the phenomenological behaviors of the pathways [103,116].
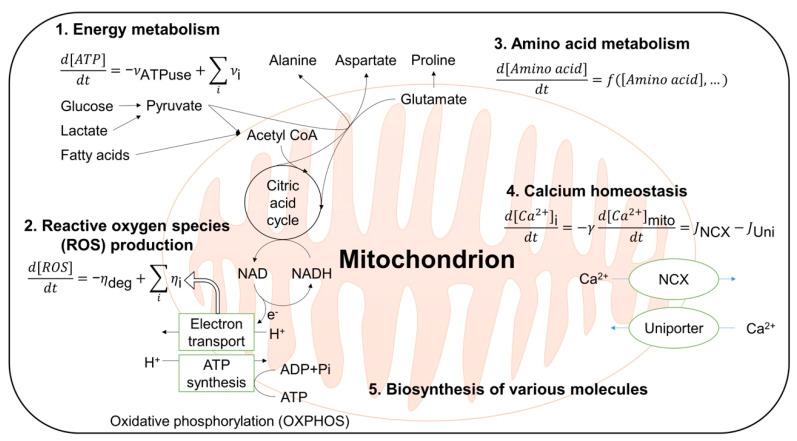
The modeling and simulations can examine not only the details of modular metabolic pathways of a mitochondrion, but can also be used to describe interactions of these pathways [117,118]. The major pathways (Figure 5) include energy metabolism [97,98,99,100,101,102,103,104,105], ROS production [117,118,119,120,121,122], amino acid metabolism [123], calcium homeostasis [124,125], and biosynthesis of various molecules [126] (Figure 5). Dysfunctions of these pathways are critical for the development of neurodegenerative diseases directly or indirectly via ROS-related pathways [19,127].
Figure 5.
Computational modeling in a single mitochondrion scale. The major roles of a mitochondrion are illustrated schematically and example equations are displayed. [X] represents the concentration of the molecule X. νATPuse is the rate of ATP usage, νi the ATP production rate of i-th pathway, ηdeg the rate of ROS degradation, ηi the ROS production rate of i-th pathway, γ the volume ratio of the mitochondrion to the cytoplasm, and J the flux of calcium ions through the channel in the subscript. f can be any function, usually polynomial function, of amino acid and other intermediates concentrations. NCX refers to the sodium–calcium exchanger.
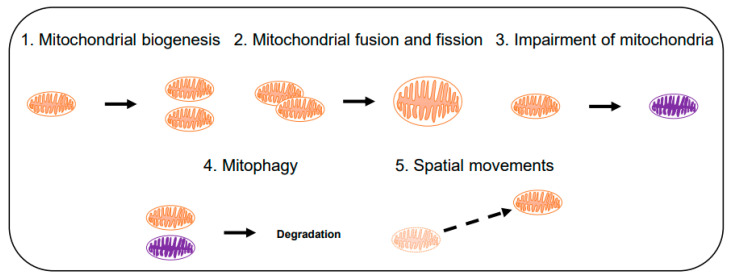
Figure 6 illustrates the representative computational modeling approaches for the population dynamics of mitochondria. Mitochondrial biogenesis (pathway 1 in Figure 6) and mitophagy (autophagic degradation of mitochondria; pathway 4) regulates the number of mitochondria in a cell. Mitophagy [128] occurs selectively to reduce the number of impaired mitochondria that are generated via pathway 3 [129]. Fusion (pathway 2, forward) helps mitochondria to survive against external stress and fission (pathway 2, backward) creates new mitochondria while keeping the quality of the population [130]. The spatial movement of mitochondria (pathway 5) helps mitochondria to enter other pathways like fusion and mitophagy. The mitochondrial population can also respond to stresses, such as hypoxia and calcium flux, by changing its spatial distribution [131]. Computational studies can explain how these pathways are related to the maintenance of mitochondrial functions against internal and external stressors [132,133,134,135,136,137,138,139,140].
Figure 6.
Computational modeling of mitochondria at a population scale. Major pathways for mitochondrial population dynamics are illustrated. Orange and purple colored mitochondria represent normal and impaired mitochondria, respectively.
The ATP synthase, also called the F1F0-ATPase, plays a critical role in mitochondrial function [141]. It is found deregulated in AD [142,143,144], which may lead to neuronal dysfunction [145,146], and it is linked to numerous other neurological diseases [147,148,149]. Modeling of the kinetics as well as the molecular dynamics may play an increasingly important role in understanding ATP synthase dysfunction in neurodegeneration.
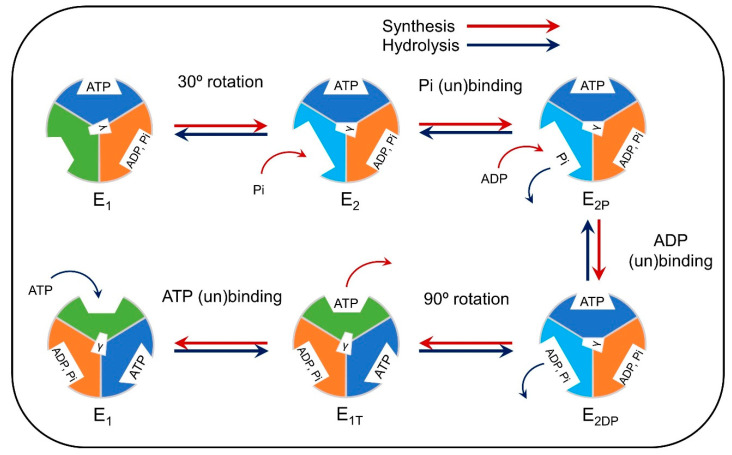
The ATP synthase uses the proton-motive force across the inner membrane to synthesize ATP from ADP and phosphate. It can also perform the opposite reaction, which results in ATP hydrolysis. Early kinetic models include Pietrobon and Caplan’s six-state model [150], which was incorporated in models of mitochondrial metabolism [102,103]. Gao et al. [151] incorporated structural details of the complex (Figure 7) [152,153,154,155]. Figure 7 shows a rotational cycle corresponding to the synthesis or hydrolysis of one ATP, and the three β subunits of the ATP synthase are drawn for each step of the cycle. The color and shape of the subunits correspond to their conformational states, which mediate substrate binding. The conformational changes of the β subunits are induced by the rotation of the γ subunit (whose orientation is depicted in the schematic), the rotation of which is driven by the proton-motive force [151].
Figure 7.
Schematic of the reaction cycle of the F1 subcomplex for ATP synthesis and hydrolysis. The sequence of ATP synthesis is shown by red arrows, and ATP hydrolysis is indicated by blue arrows. After one cycle, the ATPase returns to an equivalent configuration but is rotated by 120°. The color and shape of each catalytic binding site indicate their structure with dark blue, orange, green, and light blue corresponding to structures βTP, βDP, βE, and βHC, respectively [14]. The sequence of ATP synthesis is shown by red arrows, and ATP hydrolysis is indicated by blue arrows. The orientation of the γ subunit is depicted at the center of the synthase.
Several partial high-resolution crystal structures of the mitochondrial F1F0-ATPase have been found for yeast and bovine heart [154,155,156,157,158], making possible molecular dynamics (MD) simulations that can help elucidate the workings of the ATPase in atomistic detail. MD simulations of the F1 subcomplex have provided insight into the transfer of mechanical energy by applying rotations on the γ subunit [159,160]. Simulations of the c10 ring of the F0 subcomplex provided insight into the proton binding mechanism [161,162]. Several simulations of the F0 motor investigated the ATP synthase’s relation to MPTP. An MD study of the c10 ring concluded that the lumen of the ring cannot be occupied by water and so it cannot be the MPTP [163]. However, a Ca2+ binding site on the β subunit of mitochondrial ATPase has been identified which could induce a conformational change transmitted to the lateral stalk and cause the permeability transition [164]. Recent advances in membrane molecular simulations using multi-scale methods have allowed simulations of a whole mitochondrial membrane [165], with promising future applications.
3.2. Application of Mitochondrial Modeling in Neurodegenerative Diseases
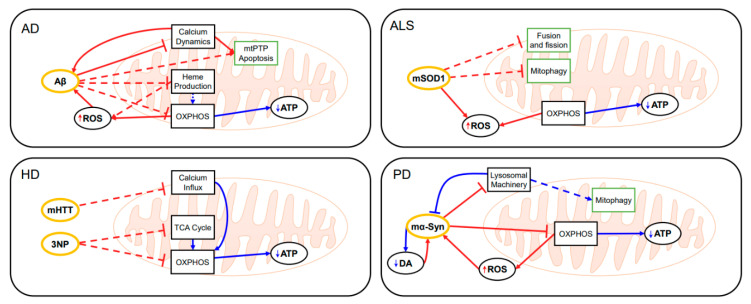
Computational modeling has been used to understand the roles of mitochondria in neurodegenerative diseases, including AD and PD (Figure 8) [49,166,167,168,169]. Among the computational studies of AD, the model of Hao et al. considered the effect of ROS, yet detailed mitochondrial ROS production mechanisms were omitted [170]. Their model of AD included neurons, astrocytes, microglia and peripheral macrophages, as well as amyloid beta aggregation and hyperphosphorylated tau proteins. The model was used to simulate the effect of drugs, which requires many resources to test without computational model, and suggested the efficient therapy for slowing the progression of AD. Ranjan et al. developed a computational model for the interaction among amyloid-beta, calcium signaling, and mitochondrial permeability transition pore (mtPTP) related cell apoptosis in AD [171]. The model was used to simulate calcium dynamics in the presence of amyloid beta and showed the increased concentration of intracellular calcium ions and dysregulation of Ca2+ channel receptors on the endoplasmic reticulum.
Figure 8.
Mitochondrial mechanisms of major neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS). Ovals and rectangles are molecules and pathways, respectively. Sharp/blunt arrows represent positive/negative interactions. Interactions that are enhanced in the disease condition are colored in blue, while the diminished are colored in red. Pathways in a single mitochondrion scale are colored in black and pathways in a population scale are colored in green. Interactions that have not been computationally modeled are represented in dashed lines. Aβ, amyloid-beta; ROS, reactive oxygen species; OXPHOS, oxidative phosphorylation; mtPTP, mitochondrial permeability transition pore; mα-Syn, mutant α-synuclein; DA, dopamine; TCA, tricarboxylic acid; mHTT, mutant huntingtin; 3NP, 3-nitropropionic acid; mSOD1, mutant superoxide dismutase 1.
Raichur et al. developed in 2006, one of the earliest computational models of PD focusing on the aggregation and clearance of mα-Syn as influenced by the ROS generation [166]. They showed that increased oxidative stress can generate a result similar to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which is known to induce PD. Cloutier et al. also developed the computational models of PD, of which the relation between mitochondrial ROS generation and α-synuclein aggregation was investigated [167,169]. They further extracted an equivalent two-state feedback motif from the model and proposed a principle for PD pathogenesis in the form of the two-state transition between “healthy” and “disease” states.
Accumulating evidence indicates that mitochondria may play an important role in HD and ALS [48,172] in addition to AD and PD as discussed above. Among them, autophagy is one of the most important and versatile mechanisms to understand the onset and progression of neurodegenerative diseases. Therefore, the computational modeling of autophagy [173,174,175,176,177] should be further developed to include detailed mitochondrial mechanisms. Further studies for computational modeling of the interaction between Aβ/mHTT/mSOD1/mα-Syn and mitochondria would offer a useful framework to understand the complex dynamics of the brain disease.
4. Conclusions
The energy transfer from non-living things to living organisms occurs through catabolism and anabolism of organic carbon molecules. Though the generation of ROS is an inevitable cellular event that happens during the catabolism in the mitochondria, apparently they become harmful factors to the brain, ultimately exacerbating neurodegeneration. By understanding how ROS-mediated oxidative stresses damages the brain, it will be possible to develop therapeutic strategies and antioxidant drugs to prevent neurodegeneration. To do so, future research will need to address several challenges as identified below.
First, it will be important to define the brain cell-type specific mechanisms of oxidative stress. Considering the fact that the brain consists of many different cell types such as neurons (excitatory neurons versus inhibitory neurons) and non-neuronal cells (astrocyte, microglia, oligodendrocyte, endothelial cells, pericytes, and ependymal cells), it is proposed that oxidative stress-responses are various and signal transduction pathways are differentially regulated in a brain cell type- and region-specific manner. In this paradigm, oxidative stress can be toxic or become pro-death signals to neurons while it can be pro-survival signals to non-neuronal cells. Indeed, gliogenesis, the generation of new astrocytes and microglia, is commonly found under oxidative stress-induced pathological conditions [178]. Accordingly, future study is required to determine what exact molecular pathways are triggered by oxidative stress and how these pathways influence the fate of neuron and glia selectively and respectively. It is also important to identify the threshold level of oxidative stress that ultimately causes autonomous cell death versus non-cell autonomous cell death in the brain. A growing body of evidence has proven that ROS from reactive astrocytes and microglia are detrimental to pyramidal neurons in the hippocampus, motor neurons in the spinal cords, medium spiny neurons in the striatum, and dopaminergic neurons in the substantia nigra [179].
Second, identification of bona fide early oxidative stress markers in the brain will expedite the diagnosis and treatment of neurodegenerative processes. Not only is the identification of specific molecules and signaling cascades crucial for understanding the pathogenesis of neurological disorders, but it is also pivotal in discovering treatments for oxidative damages in the brain. Recent findings show that mitochondria-dependent cellular events are emerging as potential therapeutic targets. The coping of acute oxidative stresses at the earlier time point may protect brain cells more effectively than that at a time point that is too late to balance the above threshold of oxidative damages, which could then become irreversible and degenerative. Perhaps constitutive generation of ROS can transform reactive glia to become foes of neurons and thereby propagate neuronal cytotoxicity throughout the brain [179]. In this paradigm, any therapeutic approaches to ameliorate oxidative stresses in neurodegenerative disorders should consider the time window and cell-type/brain-region specificity of drug effects.
Lastly, as we summarized in Section 3, useful computational models of mitochondria have been made over a wide range of scales. Some of these models have yet to be used to observe the mitochondria-dependent mechanisms of neurodegenerative diseases. The advance of computational modeling will expedite the understanding of the dynamic function of mitochondria and oxidative stress in neurodegenerative diseases. Together, we hope our review can accelerate modeling studies for the comprehensive understanding of neurodegenerative diseases, as well as contribute towards the development mitochondria-specific antioxidant therapeutics.
Acknowledgments
We thank Kowoon Choi and Jiwoo Choi for their assistance in preparing the manuscript.
Author Contributions
Conceptualization, H.R.; writing—original draft preparation, J.W., H.C., Y.S., S.H.K., C.P., A.Y.-J., S.J.H., J.L., and H.R.; writing—review and editing, J.W., H.C., Y.S., J.L., and H.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Research Foundation (NRF) grant (NRF-2016M3C7A1904233, NRF-2018M3C7A1056894, and NRF-2020M3E5D9079742), the National Research Council of Science & Technology (NST) grant (No. CRC-15-04-KIST) from the Korea Ministry of Science, ICT and Future Planning (MSIP), and the grant (2E30954 and 2E30762) from Korea Institute of Science and Technology of South Korea. This study was also supported by NIH Grants (R01AG054156 to H.R. and R01NS109537 to J.L.).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kety S.S. Medicine. Springer; New York, NY, USA: 1957. The General Metabolism of the Brain In Vivo; pp. 211–237. [DOI] [Google Scholar]
- 2.Sokoloff L. The Metabolism of the Central Nervous System In Vivo. Volume 3. American Physiolgoical Society; Washington, DC, USA: 1960. pp. 1843–1864. [Google Scholar]
- 3.Camandola S., Mattson M.P. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 2017;36:1474–1492. doi: 10.15252/embj.201695810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman L.I., Schmidt T.R., Wildman D.E., Goodman M. Molecular Evolution of Aerobic Energy Metabolism in Primates. Mol. Phylogenet. Evol. 2001;18:26–36. doi: 10.1006/mpev.2000.0890. [DOI] [PubMed] [Google Scholar]
- 5.Harris J.J., Jolivet R., Attwell D. Synaptic Energy Use and Supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Hyder F., Rothman D.L., Bennett M.R. Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc. Natl. Acad. Sci. USA. 2013;110:3549–3554. doi: 10.1073/pnas.1214912110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferraiuolo L., Kirby J., Grierson A.J., Sendtner M., Shaw P.J. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011;7:616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- 8.Obrador E., Salvador R., López-Blanch R., Jihad-Jebbar A., Vallés S.L., Estrela J.M. Oxidative Stress, Neuroinflammation and Mitochondria in the Pathophysiology of Amyotrophic Lateral Sclerosis. Antioxidants. 2020;9:901. doi: 10.3390/antiox9090901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langley B., Ratan R.R. Oxidative stress-induced death in the nervous system: Cell cycle dependent or independent? J. Neurosci. Res. 2004;77:621–629. doi: 10.1002/jnr.20210. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Wang W., Li L., Perry G., Lee H.-G., Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2014;1842:1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohyagi Y., Asahara H., Chui D.-H., Tsuruta Y., Sakae N., Miyoshi K., Yamada T., Kikuchi H., Taniwaki T., Murai H., et al. Intracellular Aβ42 activates p53 promoter: A pathway to neurodegeneration in Alzheimer’s disease. FASEB J. 2004;19:255–257. doi: 10.1096/fj.04-2637fje. [DOI] [PubMed] [Google Scholar]
- 12.Dai C.-Q., Luo T.-T., Luo S.-C., Wang J.-Q., Wang S.-M., Bai Y.-H., Yang Y.-L., Wang Y.-Y. p53 and mitochondrial dysfunction: Novel insight of neurodegenerative diseases. J. Bioenerg. Biomembr. 2016;48:337–347. doi: 10.1007/s10863-016-9669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jembrek M.J., Slade N., Hof P.R., Šimić G. The interactions of p53 with tau and Aß as potential therapeutic targets for Alzheimer’s disease. Prog. Neurobiol. 2018;168:104–127. doi: 10.1016/j.pneurobio.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Lee J., Kim Y., Liu T., Hwang Y.J., Hyeon S.J., Im H., Lee K., Alvarez V.E., McKee A.C., Um S.-J., et al. SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer’s disease. Aging Cell. 2018;17:e12679. doi: 10.1111/acel.12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song S., Li B., Jia Z., Guo L. Sirtuin 3 mRNA Expression is Downregulated in the Brain Tissues of Alzheimer’s Disease Patients: A Bioinformatic and Data Mining Approach. Med. Sci. Monit. 2020;26:e923547. doi: 10.12659/MSM.923547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balsa E., Marco R., Perales-Clemente E., Szklarczyk R., Calvo E., Landázuri M.O., Enríquez J.A. NDUFA4 Is a Subunit of Complex IV of the Mammalian Electron Transport Chain. Cell Metab. 2012;16:378–386. doi: 10.1016/j.cmet.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Sze S.K., Park J.E., Sze S.K. Quantitative profiling brain proteomes revealed mitochondrial dysfunction in Alzheimer’s disease. Mol. Brain. 2019;12:8. doi: 10.1186/s13041-019-0430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J., Boo J.H., Ryu H. The failure of mitochondria leads to neurodegeneration: Do mitochondria need a jump start? Adv. Drug Deliv. Rev. 2009;61:1316–1323. doi: 10.1016/j.addr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cha M.-Y., Han S.-H., Son S.M., Hong H.-S., Choi Y.-J., Byun J., Mook-Jung I. Mitochondria-Specific Accumulation of Amyloid β Induces Mitochondrial Dysfunction Leading to Apoptotic Cell Death. PLoS ONE. 2012;7:e34929. doi: 10.1371/journal.pone.0034929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghavami S., Shojaei S., Yeganeh B., Ande S.R., Jangamreddy J.R., Mehrpour M., Christoffersson J., Chaabane W., Moghadam A.R., Kashani H.H., et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014;112:24–49. doi: 10.1016/j.pneurobio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Sharma V., Collins L.B., Chen T.H., Herr N., Takeda S., Sun W., Swenberg J.A., Nakamura J. Oxidative stress at low levels can induce clustered DNA lesions leading to NHEJ mediated mutations. Oncotarget. 2016;7:25377–25390. doi: 10.18632/oncotarget.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiloh Y., Ziv Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013;14:197–210. doi: 10.1038/nrm3546. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura Y., Shimohama S., Kamoshima W., Ota T., Matsuoka Y., Nomura Y., A Smith M., Perry G., Whitehouse P.J., Taniguchi T. Alteration of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer’s disease. Brain Res. 1998;780:260–269. doi: 10.1016/S0006-8993(97)01202-X. [DOI] [PubMed] [Google Scholar]
- 24.Vaseva A.V., Marchenko N.D., Ji K., Tsirka S.E., Holzmann S., Moll U.M. p53 Opens the Mitochondrial Permeability Transition Pore to Trigger Necrosis. Cell. 2012;149:1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurst S., Gonnot F., Dia M., Da Silva C.C., Gomez L., Sheu S.-S. Phosphorylation of cyclophilin D at serine 191 regulates mitochondrial permeability transition pore opening and cell death after ischemia-reperfusion. Cell Death Dis. 2020;11:66. doi: 10.1038/s41419-020-02864-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J., Xiang H., Liu J., Chen Y., He R.-R., Liu B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics. 2020;10:8315–8342. doi: 10.7150/thno.45922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du H., Guo L., Fang F., Chen D., A Sosunov A., McKhann G.M., Yan Y., Wang C., Zhang H., Molkentin J.D., et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du H., Guo L., Zhang W., Rydzewska M., Yan S.S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol. Aging. 2011;32:398–406. doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubli D.A., Gustafsson Å.B. Mitochondria and Mitophagy. Circ. Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X., Su B., Lee H.-G., Li X., Perry G., Smith M.A., Zhu X. Impaired Balance of Mitochondrial Fission and Fusion in Alzheimer’s Disease. J. Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakravorty A., Jetto C.T., Manjithaya R. Dysfunctional Mitochondria and Mitophagy as Drivers of Alzheimer’s Disease Pathogenesis. Front. Aging Neurosci. 2019;11:311. doi: 10.3389/fnagi.2019.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rui Y., Tiwari P., Xie Z.-P., Zheng J.Q. Acute Impairment of Mitochondrial Trafficking by beta-Amyloid Peptides in Hippocampal Neurons. J. Neurosci. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheng Z.-H., Cai Q. Mitochondrial transport in neurons: Impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 2012;13:77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalani K., Yan S.F., Yan S.S. Mitochondrial permeability transition pore: A potential drug target for neurodegeneration. Drug Discov. Today. 2018;23:1983–1989. doi: 10.1016/j.drudis.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin J., Han P., Song M., Nielsen M., Beach T.G., Serrano G.E., Liang W.S., Caselli R.J., Shi J. Amyloid-β Increases Tau by Mediating Sirtuin 3 in Alzheimer’s Disease. Mol. Neurobiol. 2018;55:8592–8601. doi: 10.1007/s12035-018-0977-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munguia M.E., Govezensky T., Martínez R., Manoutcharian K., Gevorkian G. Identification of amyloid-beta 1–42 binding protein fragments by screening of a human brain cDNA library. Neurosci. Lett. 2006;397:79–82. doi: 10.1016/j.neulet.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 37.Chen J.X., Du Yan S. Amyloid-β-Induced Mitochondrial Dysfunction. J. Alzheimer’s Dis. 2007;12:177–184. doi: 10.3233/JAD-2007-12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joh Y., Choi W.-S. Mitochondrial Complex I Inhibition Accelerates Amyloid Toxicity. Dev. Reprod. 2017;21:417–424. doi: 10.12717/DR.2017.21.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patten D.A., Germain M., Kelly M.A., Slack R.S. Reactive Oxygen Species: Stuck in the Middle of Neurodegeneration. J. Alzheimer’s Dis. 2010;20(Suppl. 2):S357–S367. doi: 10.3233/JAD-2010-100498. [DOI] [PubMed] [Google Scholar]
- 40.Brown R.H., Al-Chalabi A. Amyotrophic Lateral Sclerosis. New Engl. J. Med. 2017;377:162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 41.Barber S.C., Shaw P.J. Oxidative stress in ALS: Key role in motor neuron injury and therapeutic target. Free. Radic. Biol. Med. 2010;48:629–641. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 42.Lee J., Ryu H., Ferrante R.J., Morris S.M., Jr., Ratan R.R. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc. Natl. Acad. Sci. USA. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J., Ryu H., Kowall N. Differential regulation of neuronal and inducible nitric oxide synthase (NOS) in the spinal cord of mutant SOD1 (G93A) ALS mice. Biochem. Biophys. Res. Commun. 2009;387:202–206. doi: 10.1016/j.bbrc.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almer G., Vukosavic S., Romero N., Przedborski S. Inducible Nitric Oxide Synthase Up-Regulation in a Transgenic Mouse Model of Familial Amyotrophic Lateral Sclerosis. J. Neurochem. 2002;72:2415–2425. doi: 10.1046/j.1471-4159.1999.0722415.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee J., Kannagi M., Ferrante R.J., Kowall N.W., Ryu H. Activation of Ets-2 by oxidative stress induces Bcl-xL expression and accounts for glial survival in amyotrophic lateral sclerosis. FASEB J. 2009;23:1739–1749. doi: 10.1096/fj.08-121046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J., Hyeon S.J., Im H., Ryu H., Kim Y., Ryu H. Astrocytes and Microglia as Non-cell Autonomous Players in the Pathogenesis of ALS. Exp. Neurobiol. 2016;25:233–240. doi: 10.5607/en.2016.25.5.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothstein J.D., Ba M.V.K., Levey A.I., Martin L.J., Kuncl R.W. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann. Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 48.Kodavati M., Wang H., Hegde M.L. Altered Mitochondrial Dynamics in Motor Neuron Disease: An Emerging Perspective. Cells. 2020;9:1065. doi: 10.3390/cells9041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith E.F., Shaw P.J., De Vos K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2019;710:132933. doi: 10.1016/j.neulet.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 50.Detmer S.A., Chan D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 51.Chan D.C. Fusion and Fission: Interlinked Processes Critical for Mitochondrial Health. Annu. Rev. Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 52.Deng J., Yang M., Chen Y., Chen X., Liu J., Sun S., Cheng H., Li Y., Bigio E.H., Mesulam M.M., et al. FUS Interacts with HSP60 to Promote Mitochondrial Damage. PLoS Genet. 2015;11:e1005357. doi: 10.1371/journal.pgen.1005357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Onesto E., Colombrita C., Gumina V., Borghi M.O., Dusi S., Doretti A., Fagiolari G., Invernizzi F., Moggio M., Tiranti V., et al. Gene-specific mitochondria dysfunctions in human TARDBP and C9ORF72 fibroblasts. Acta Neuropathol. Commun. 2016;4:47. doi: 10.1186/s40478-016-0316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dafinca R., Scaber J., Ababneh N., Lalic T., Weir G., Christian H., Vowles J., Douglas A.G.L., Fletcher-Jones A., Browne C., et al. C9orf72 Hexanucleotide Expansions Are Associated with Altered Endoplasmic Reticulum Calcium Homeostasis and Stress Granule Formation in Induced Pluripotent Stem Cell-Derived Neurons from Patients with Amyotrophic Lateral Sclerosis and Frontotemporal Demen. Stem Cells. 2016;34:2063–2078. doi: 10.1002/stem.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kirkinezos I.G., Bacman S.R., Hernandez D., Oca-Cossio J., Arias L.J., Perez-Pinzon M.A., Bradley W.G., Moraes C.T. Cytochrome c Association with the Inner Mitochondrial Membrane Is Impaired in the CNS of G93A-SOD1 Mice. J. Neurosci. 2005;25:164–172. doi: 10.1523/JNEUROSCI.3829-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Vos K.J., Mórotz G.M., Stoica R., Tudor E.L., Lau K.-F., Ackerley S., Warley A., Shaw C.E., Miller C.C.J. VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum. Mol. Genet. 2012;21:1299–1311. doi: 10.1093/hmg/ddr559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng J., Wang P., Chen X., Cheng H., Liu J., Fushimi K., Zhu L., Wu J.Y. FUS interacts with ATP synthase beta subunit and induces mitochondrial unfolded protein response in cellular and animal models. Proc. Natl. Acad. Sci. USA. 2018;115:E9678–E9686. doi: 10.1073/pnas.1806655115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu W., Yamashita T., Tian F., Morimoto N., Ikeda Y., Deguchi K., Abe K. Mitochondrial fusion and fission proteins expression dynamically change in a murine model of amyotrophic lateral sclerosis. Curr. Neurovascular Res. 2013;10:222–230. doi: 10.2174/15672026113109990060. [DOI] [PubMed] [Google Scholar]
- 59.Macdonald M.E., Ambrose C.M., Duyao M.P., Myers R.H., Lin C., Srinidhi L., Barnes G., Taylor S.A., James M., Groot N., et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- 60.DiFiglia M., Sapp E., Chase K.O., Davies S.W., Bates G.P., Vonsattel J.P., Aronin N. Aggregation of Huntingtin in Neuronal Intranuclear Inclusions and Dystrophic Neurites in Brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 61.Parker W.D., Boyson S.J., Luder A.S., Parks J.K. Evidence for a defect in NADH: Ubiquinone oxidoreductase (complex I) in Huntington’s disease. Neurology. 1990;40:1231. doi: 10.1212/WNL.40.8.1231. [DOI] [PubMed] [Google Scholar]
- 62.Arenas J., Campos Y., Ribacoba R., Martín M.A., Rubio J.C., Ablanedo P., Cabello A. Complex I Defect in muscle from patients with Huntington’s disease. Ann. Neurol. 1998;43:397–400. doi: 10.1002/ana.410430321. [DOI] [PubMed] [Google Scholar]
- 63.Browne S.E., Bowling A.C., MacGarvey U., Baik M.J., Berger S.C., Muquit M.M.K., Bird E.D., Beal M.F. Oxidative damage and metabolic dysfunction in Huntington’s disease: Selective vulnerability of the basal ganglia. Ann. Neurol. 1997;41:646–653. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- 64.Gu M., Gash M.T., Mann V.M., Javoy-Agid F., Cooper J.M., Schapira A.H.V. Mitochondrial defect in Huntington’s disease caudate nucleus. Ann. Neurol. 1996;39:385–389. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- 65.Mann V.M., Cooper J.M., Javoy-Agid F., Agid Y., Jenner P., Schapira A.H. Mitochondrial function and parental sex effect in Huntington’s disease. Lancet. 1990;336:749. doi: 10.1016/0140-6736(90)92242-A. [DOI] [PubMed] [Google Scholar]
- 66.Stahl W.L., Swanson P.D. Biochemical abnormalities in Huntington’s chorea brains. Neurology. 1974;24:813. doi: 10.1212/WNL.24.9.813. [DOI] [PubMed] [Google Scholar]
- 67.Kodsi M.H., Swerdlow N.R. Mitochondrial toxin 3-nitropropionic acid produces startle reflex abnormalities and striatal damage in rats that model some features of Huntington’s disease. Neurosci. Lett. 1997;231:103–107. doi: 10.1016/S0304-3940(97)00482-5. [DOI] [PubMed] [Google Scholar]
- 68.Beal M.F., Brouillet E., Jenkins B.G., Ferrante R.J., Kowall N.W., Miller J.M., Storey E., Srivastava R., Rosen B.R., Hyman B.T. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J. Neurosci. 1993;13:4181–4192. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brouillet E., Hantraye P., Ferrante R.J., Dolan R., Leroy-Willig A., Kowall N.W., Beal M.F. Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proc. Natl. Acad. Sci. USA. 1995;92:7105–7109. doi: 10.1073/pnas.92.15.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beal M.F. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann. Neurol. 1992;31:119–130. doi: 10.1002/ana.410310202. [DOI] [PubMed] [Google Scholar]
- 71.Beal M.F. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann. Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 72.Hansson O., Castilho R.F., Korhonen L., Lindholm D., Bates G.P., Brundin P. Partial resistance to malonate-induced striatal cell death in transgenic mouse models of Huntington’s disease is dependent on age and CAG repeat length. J. Neurochem. 2001;78:694–703. doi: 10.1046/j.1471-4159.2001.00482.x. [DOI] [PubMed] [Google Scholar]
- 73.Yano H., Baranov S.V., Baranova O.V., Kim J., Pan Y., Yablonska S., Carlisle D.L., Ferrante R.J., Kim A.H., Friedlander R.M. Inhibition of mitochondrial protein import by mutant huntingtin. Nat. Neurosci. 2014;17:822–831. doi: 10.1038/nn.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gellerich F.N., Gizatullina Z., Nguyen H.P., Trumbeckaite S., Vielhaber S., Seppet E., Zierz S., Landwehrmeyer B., Riess O., Von Hörsten S., et al. Impaired Regulation of Brain Mitochondria by Extramitochondrial Ca2+ in Transgenic Huntington Disease Rats. J. Biol. Chem. 2008;283:30715–30724. doi: 10.1074/jbc.M709555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fan M.M.Y., Fernandes H.B., Zhang L.Y.J., Hayden M.R., Raymond L.A. Altered NMDA Receptor Trafficking in a Yeast Artificial Chromosome Transgenic Mouse Model of Huntington’s Disease. J. Neurosci. 2007;27:3768–3779. doi: 10.1523/JNEUROSCI.4356-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shehadeh J., Fernandes H.B., Mullins M.M.Z., Graham R.K., Leavitt B.R., Hayden M.R., Raymond L.A. Striatal neuronal apoptosis is preferentially enhanced by NMDA receptor activation in YAC transgenic mouse model of Huntington disease. Neurobiol. Dis. 2006;21:392–403. doi: 10.1016/j.nbd.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 77.Fernandes H.B., Baimbridge K.G., Church J., Hayden M.R., Raymond L.A. Mitochondrial Sensitivity and Altered Calcium Handling Underlie Enhanced NMDA-Induced Apoptosis in YAC128 Model of Huntington’s Disease. J. Neurosci. 2007;27:13614–13623. doi: 10.1523/JNEUROSCI.3455-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang T.-S., Tu H., Chan E.Y., Maximov A., Wang Z., Wellington C.L., Hayden M.R., Bezprozvanny I. Huntingtin and Huntingtin-Associated Protein 1 Influence Neuronal Calcium Signaling Mediated by Inositol-(1,4,5) Triphosphate Receptor Type 1. Neuron. 2003;39:227–239. doi: 10.1016/S0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lodi R., Schapira A.H., Manners D., Styles P., Wood N.W., Taylor D.J., Warner T.T. Abnormal in vivo skeletal muscle energy metabolism in Huntington’s disease and dentatorubropallidoluysian atrophy. Ann. Neurol. 2000;48:72–76. doi: 10.1002/1531-8249(200007)48:1<72::AID-ANA11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 80.Mailloux R.J. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015;4:381–398. doi: 10.1016/j.redox.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee J., Kosaras B., Del Signore S.J., Cormier K., McKee A.C., Ratan R.R., Kowall N.W., Ryu H. Modulation of lipid peroxidation and mitochondrial function improves neuropathology in Huntington’s disease mice. Acta Neuropathol. 2010;121:487–498. doi: 10.1007/s00401-010-0788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kordower J.H., Olanow C.W., Dodiya H.B., Chu Y., Beach T.G., Adler C.H., Halliday G.M., Bartus R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jenner P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003;53(Suppl. 3):S26–S36; discussion S28–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 84.Schapira A.H.V., Cooper J.M., Dexter D., Jenner P., Clark J., Marsden C. MITOCHONDRIAL COMPLEX I DEFICIENCY IN PARKINSON’S DISEASE. Lancet. 1989;333:1269. doi: 10.1016/S0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 85.Gu M., Cooper J.M., Taanman J.-W., Schapira A.H.V. Mitochondrial DNA transmission of the mitochondrial defect in Parkinson’s disease. Ann. Neurol. 1998;44:177–186. doi: 10.1002/ana.410440207. [DOI] [PubMed] [Google Scholar]
- 86.Mann V.M., Cooper J.M., Daniel S.E., Srai K., Jenner P., Marsden C.D., Schapira A.H.V. Complex I, Iron, and ferritin in Parkinson’s disease substantia nigra. Ann. Neurol. 1994;36:876–881. doi: 10.1002/ana.410360612. [DOI] [PubMed] [Google Scholar]
- 87.Gerlach M., Riederer P., Przuntek H., Youdim M.B. MPTP mechanisms of neurotoxicity and their implications for Parkinson’s disease. Eur. J. Pharmacol. Mol. Pharmacol. 1991;208:273–286. doi: 10.1016/0922-4106(91)90073-Q. [DOI] [PubMed] [Google Scholar]
- 88.Jones N. PINK1 targets dysfunctional mitochondria for autophagy in Parkinson disease. Nat. Rev. Neurol. 2010;6:181. doi: 10.1038/nrneurol.2010.19. [DOI] [PubMed] [Google Scholar]
- 89.Moore D.J., Zhang L., Troncoso J., Lee M.K., Hattori N., Mizuno Y., Dawson T.M., Dawson V.L. Association of DJ-1 and parkin mediated by pathogenic DJ-1 mutations and oxidative stress. Hum. Mol. Genet. 2004;14:71–84. doi: 10.1093/hmg/ddi007. [DOI] [PubMed] [Google Scholar]
- 90.Strauss K.M., Martins L.M., Plun-Favreau H., Marx F.P., Kautzmann S., Berg D., Gasser T., Wszolek Z., Müller T., Bornemann A., et al. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum. Mol. Genet. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- 91.Martins L.M., Morrison A., Klupsch K., Fedele V., Moisoi N., Teismann P., Abuin A., Grau E., Geppert M., Livi G.P., et al. Neuroprotective Role of the Reaper-Related Serine Protease HtrA2/Omi Revealed by Targeted Deletion in Mice. Mol. Cell. Biol. 2004;24:9848–9862. doi: 10.1128/MCB.24.22.9848-9862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yun S.P., Kim D., Kim S., Kim S., Karuppagounder S.S., Kwon S.-H., Lee S., Kam T.-I., Lee S., Ham S., et al. α-Synuclein accumulation and GBA deficiency due to L444P GBA mutation contributes to MPTP-induced parkinsonism. Mol. Neurodegener. 2018;13:1–19. doi: 10.1186/s13024-017-0233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.SofroniewHarry M.V., Vinters H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2009;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin L.F., Doherty D.H., Lile J.D., Bektesh S., Collins F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 95.Scharr D.G., Sieber B.-A., Dreyfus C.F., Black I.B. Regional and Cell-Specific Expression of GDNF in Rat Brain. Exp. Neurol. 1993;124:368–371. doi: 10.1006/exnr.1993.1207. [DOI] [PubMed] [Google Scholar]
- 96.Gray M.T., Woulfe J.M. Striatal Blood–Brain Barrier Permeability in Parkinson’S Disease. Br. J. Pharmacol. 2015;35:747–750. doi: 10.1038/jcbfm.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heo J.Y., Nam M.-H., Yoon H.H., Kim J., Hwang Y.J., Won W., Woo D.H., Lee J.A., Park H.-J., Jo S., et al. Aberrant Tonic Inhibition of Dopaminergic Neuronal Activity Causes Motor Symptoms in Animal Models of Parkinson’s Disease. Curr. Biol. 2020;30:276–291.e9. doi: 10.1016/j.cub.2019.11.079. [DOI] [PubMed] [Google Scholar]
- 98.Chance E.M. A computer simulation of oxidative phosphorylation. Comput. Biomed. Res. 1967;1:251–264. doi: 10.1016/S0010-4809(67)80012-0. [DOI] [PubMed] [Google Scholar]
- 99.Bohnensack R. Control of energy transformation in mitochondria. Analysis by a quantitative model. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1981;634:203–218. doi: 10.1016/0005-2728(81)90139-0. [DOI] [PubMed] [Google Scholar]
- 100.Holzhütter H.-G., Henke W., Dubiel W., Gerber G. A mathematical model to study short-term regulation of mitochondrial energy transduction. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1985;810:252–268. doi: 10.1016/0005-2728(85)90140-9. [DOI] [PubMed] [Google Scholar]
- 101.Korzeniewski B., Froncisz W. An extended dynamic model of oxidative phosphorylation. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 1991;1060:210–223. doi: 10.1016/S0005-2728(09)91009-X. [DOI] [PubMed] [Google Scholar]
- 102.Magnus G., Keizer J. Minimal model of beta-cell mitochondrial Ca2+ handling. Am. J. Physiol. Physiol. 1997;273:C717–C733. doi: 10.1152/ajpcell.1997.273.2.C717. [DOI] [PubMed] [Google Scholar]
- 103.Cortassa S., Aon M.A., Marbán E., Winslow R.L., O’Rourke B. An Integrated Model of Cardiac Mitochondrial Energy Metabolism and Calcium Dynamics. Biophys. J. 2003;84:2734–2755. doi: 10.1016/S0006-3495(03)75079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beard D.A. A Biophysical Model of the Mitochondrial Respiratory System and Oxidative Phosphorylation. PLoS Comput. Biol. 2005;1:e36. doi: 10.1371/journal.pcbi.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu F., Yang F., Vinnakota K.C., Beard D.A. Computer Modeling of Mitochondrial Tricarboxylic Acid Cycle, Oxidative Phosphorylation, Metabolite Transport, and Electrophysiology. J. Biol. Chem. 2007;282:24525–24537. doi: 10.1074/jbc.M701024200. [DOI] [PubMed] [Google Scholar]
- 106.Bazil J.N., Buzzard G.T., Rundell A.E. Modeling Mitochondrial Bioenergetics with Integrated Volume Dynamics. PLoS Comput. Biol. 2010;6:e1000632. doi: 10.1371/journal.pcbi.1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cortassa S., Aon M.A. Computational Modeling of Mitochondrial Function. Toxic. Assess. 2011;810:311–326. doi: 10.1007/978-1-61779-382-0_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Westerhoff H.V. Thermodynamics and control of proton motive free-energy transduction. Biomed. Biochim. Acta. 1985;44:929–941. [PubMed] [Google Scholar]
- 109.Tager J., Wanders R., Groen A., Kunz W., Bohnensack R., Küster U., Letko G., Böhme G., Duszynski J., Wojtczak L. Control of mitochondrial respiration. FEBS Lett. 1983;151:1–9. doi: 10.1016/0014-5793(83)80330-5. [DOI] [PubMed] [Google Scholar]
- 110.Westerhoff H.V., Lolkema J.S., Otto R., Hellingwerf K.J. Thermodynamics of growth non-equilibrium thermodynamics of bacterial growth the phenomenological and the Mosaic approach. Biochim. Et Biophys. Acta (BBA) Rev. Bioenerg. 1982;683:181–220. doi: 10.1016/0304-4173(82)90001-5. [DOI] [PubMed] [Google Scholar]
- 111.Stucki J.W. The Optimal Efficiency and the Economic Degrees of Coupling of Oxidative Phosphorylation. JBIC J. Biol. Inorg. Chem. 1980;109:269–283. doi: 10.1111/j.1432-1033.1980.tb04792.x. [DOI] [PubMed] [Google Scholar]
- 112.Pietrobon D., Zoratti M., Azzone G.F., Caplan S.R. Intrinsic uncoupling of mitochondrial proton pumps. 2. Modeling studies. Biochemistry. 1986;25:767–775. doi: 10.1021/bi00352a005. [DOI] [PubMed] [Google Scholar]
- 113.Christensen B., Nielsen J. Metabolic network analysis. A powerful tool in metabolic engineering. Adv. Biochem. Eng. 2000;66:209–231. [PubMed] [Google Scholar]
- 114.Savinell J.M., Palsson B.O. Network analysis of intermediary metabolism using linear optimization. I. Development of mathematical formalism. J. Theor. Biol. 1992;154:421–454. doi: 10.1016/S0022-5193(05)80161-4. [DOI] [PubMed] [Google Scholar]
- 115.Cortassa S., Aon J.C., Aon M.A. Fluxes of carbon, phosphorylation, and redox intermediates during growth ofsaccharomyces cerevisiae on different carbon sources. Biotechnol. Bioeng. 1995;47:193–208. doi: 10.1002/bit.260470211. [DOI] [PubMed] [Google Scholar]
- 116.Mathematical models in molecular and cellular biology. Acta Appl. Math. 1985;4:267–268. doi: 10.1007/BF00052464. [DOI] [Google Scholar]
- 117.Cortassa S., Aon M.A., Winslow R.L., O’Rourke B. A Mitochondrial Oscillator Dependent on Reactive Oxygen Species. Biophys. J. 2004;87:2060–2073. doi: 10.1529/biophysj.104.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gauthier L.D., Greenstein J.L., O’Rourke B., Winslow R.L. An Integrated Mitochondrial ROS Production and Scavenging Model: Implications for Heart Failure. Biophys. J. 2013;105:2832–2842. doi: 10.1016/j.bpj.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Passos J.F., Nelson G., Wang C., Richter T., Simillion C., Proctor C.J., Miwa S., Olijslagers S., Hallinan J., Wipat A., et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kirkwood T.B.L., Kowald A. The free-radical theory of ageing–older, wiser and still alive: Modelling positional effects of the primary targets of ROS reveals new support. BioEssays. 2012;34:692–700. doi: 10.1002/bies.201200014. [DOI] [PubMed] [Google Scholar]
- 121.Pezze P.D., Nelson G., Otten E.G., Korolchuk V.I., Kirkwood T.B.L., Von Zglinicki T., Shanley D.P. Dynamic Modelling of Pathways to Cellular Senescence Reveals Strategies for Targeted Interventions. PLoS Comput. Biol. 2014;10:e1003728. doi: 10.1371/journal.pcbi.1003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Brito P.M., Antunes F. Estimation of kinetic parameters related to biochemical interactions between hydrogen peroxide and signal transduction proteins. Front. Chem. 2014;2:82. doi: 10.3389/fchem.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nilsson A., Haanstra J.R., Engqvist M.K., Gerding A., Bakker B.M., Klingmüller U., Teusink B., Nielsen J. Quantitative analysis of amino acid metabolism in liver cancer links glutamate excretion to nucleotide synthesis. Proc. Natl. Acad. Sci. USA. 2020;117:10294–10304. doi: 10.1073/pnas.1919250117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schuster S., Marhl M., Höfer T.T. Modelling of simple and complex calcium oscillations. From single-cell responses to intercellular signalling. JBIC J. Biol. Inorg. Chem. 2002;269:1333–1355. doi: 10.1046/j.0014-2956.2001.02720.x. [DOI] [PubMed] [Google Scholar]
- 125.Soman S., Keatinge M., Moein M., Da Costa M., Mortiboys H., Skupin A., Sugunan S., Bazala M., Kuznicki J., Bandmann O. Inhibition of the mitochondrial calcium uniporter rescues dopaminergic neurons inpink1−/−zebrafish. Eur. J. Neurosci. 2017;45:528–535. doi: 10.1111/ejn.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wofford J.D., Lindahl P.A. A mathematical model of iron import and trafficking in wild-type and Mrs3/4ΔΔ yeast cells. BMC Syst. Biol. 2019;13:23. doi: 10.1186/s12918-019-0702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Balaban R.S., Nemoto S., Finkel T. Mitochondria, Oxidants, and Aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 128.Melser S., Lavie J., Bénard G. Mitochondrial degradation and energy metabolism. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2015;1853:2812–2821. doi: 10.1016/j.bbamcr.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 129.Gibson G.E., Starkov A., Blass J.P., Ratan R.R., Beal M.F. Cause and consequence: Mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2010;1802:122–134. doi: 10.1016/j.bbadis.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Youle R.J., Van Der Bliek A.M. Mitochondrial Fission, Fusion, and Stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boland M.L., Chourasia A.H., MacLeod K.F. Mitochondrial Dysfunction in Cancer. Front. Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kowald A., Kirkwood T. Mitochondrial mutations, cellular instability and ageing: Modelling the population dynamics of mitochondria. Mutat. Res. 1993;295:93–103. doi: 10.1016/0921-8734(93)90011-Q. [DOI] [PubMed] [Google Scholar]
- 133.Kowald A., Kirkwood T. A network theory of ageing: The interactions of defective mitochondria, aberrant proteins, free radicals and scavengers in the ageing process. Mutat. Res. 1996;316:209–236. doi: 10.1016/S0921-8734(96)90005-3. [DOI] [PubMed] [Google Scholar]
- 134.Kowald A., Kirkwood T.B. Accumulation of Defective Mitochondria through Delayed Degradation of Damaged Organelles and Its Possible Role in the Ageing of Post-mitotic and Dividing Cells. J. Theor. Biol. 2000;202:145–160. doi: 10.1006/jtbi.1999.1046. [DOI] [PubMed] [Google Scholar]
- 135.Kowald A., Kirkwood T.B.L. Evolution of the mitochondrial fusion-fission cycle and its role in aging. Proc. Natl. Acad. Sci. USA. 2011;108:10237–10242. doi: 10.1073/pnas.1101604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mouli P.K., Twig G., Shirihai O.S. Frequency and Selectivity of Mitochondrial Fusion Are Key to Its Quality Maintenance Function. Biophys. J. 2009;96:3509–3518. doi: 10.1016/j.bpj.2008.12.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tam Z.Y., Gruber J., Halliwell B., Gunawan R. Mathematical Modeling of the Role of Mitochondrial Fusion and Fission in Mitochondrial DNA Maintenance. PLoS ONE. 2013;8:e76230. doi: 10.1371/journal.pone.0076230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Patel P.K., Shirihai O., Huang K.C. Optimal Dynamics for Quality Control in Spatially Distributed Mitochondrial Networks. PLoS Comput. Biol. 2013;9:e1003108. doi: 10.1371/journal.pcbi.1003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dalmasso G., Zapata P.A.M., Brady N.R., Hamacher-Brady A. Agent-Based Modeling of Mitochondria Links Sub-Cellular Dynamics to Cellular Homeostasis and Heterogeneity. PLoS ONE. 2017;12:e0168198. doi: 10.1371/journal.pone.0168198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kornick K., Bogner B., Sutter L., Das M. Population Dynamics of Mitochondria in Cells: A Minimal Mathematical Model. Front. Phys. 2019;7 doi: 10.3389/fphy.2019.00146. [DOI] [Google Scholar]
- 141.Boyer P.D. The Atp Synthase—A Splendid Molecular Machine. Annu. Rev. Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- 142.Liang W.S., Reiman E.M., Valla J., Dunckley T., Beach T.G., Grover A., Niedzielko T.L., Schneider L.E., Mastroeni D., Caselli R., et al. Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc. Natl. Acad. Sci. USA. 2008;105:4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Terni B., Boada J., Portero-Otin M., Pamplona R., Ferrer I. Mitochondrial ATP-Synthase in the Entorhinal Cortex Is a Target of Oxidative Stress at Stages I/II of Alzheimer’s Disease Pathology. Brain Pathol. 2010;20:222–233. doi: 10.1111/j.1750-3639.2009.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ding B., Xi Y., Gao M., Li Z., Xu C., Fan S., He W. Gene Expression Profiles of Entorhinal Cortex in Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dementiasr. 2014;29:526–532. doi: 10.1177/1533317514523487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Beck S.J., Guo L., Phensy A., Tian J., Wang L., Tandon N., Gauba E., Lu W., Pascual J.M., Kroener S., et al. Deregulation of mitochondrial F1FO-ATP synthase via OSCP in Alzheimer’s disease. Nat. Commun. 2016;7:11483. doi: 10.1038/ncomms11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen H., Tian J., Guo L., Du H. Caspase inhibition rescues F1Fo ATP synthase dysfunction-mediated dendritic spine elimination. Sci. Rep. 2020;10:17589. doi: 10.1038/s41598-020-74613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jonckheere A.I., Smeitink J.A.M., Rodenburg R.J.T. Mitochondrial ATP synthase: Architecture, function and pathology. J. Inherit. Metab. Dis. 2011;35:211–225. doi: 10.1007/s10545-011-9382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tebbenkamp A.T.N., Varela L., Choi J., Paredes M.I., Giani A.M., Song J.E., Sestan-Pesa M., Franjic D., Sousa A.M.M., Liu Z.-W., et al. The 7q11.23 Protein DNAJC30 Interacts with ATP Synthase and Links Mitochondria to Brain Development. Cell. 2018;175:1088–1104.e23. doi: 10.1016/j.cell.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sgarbi G., Baracca A., Lenaz G., Valentino M., Carelli V., Solaini G. Inefficient coupling between proton transport and ATP synthesis may be the pathogenic mechanism for NARP and Leigh syndrome resulting from the T8993G mutation in mtDNA. Biochem. J. 2006;395:493–500. doi: 10.1042/BJ20051748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pietrobon D., Caplan S.R. Flow-force relationships for a six-state proton pump model: Intrinsic uncoupling, kinetic equivalence of input and output forces, and domain of approximate linearity. Biochemistry. 1985;24:5764–5776. doi: 10.1021/bi00342a012. [DOI] [PubMed] [Google Scholar]
- 151.Gao Y.Q., Yang W., Karplus M. A Structure-Based Model for the Synthesis and Hydrolysis of ATP by F1-ATPase. Cell. 2005;123:195–205. doi: 10.1016/j.cell.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 152.Boyer P.D. The binding change mechanism for ATP synthase—Some probabilities and possibilities. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1993;1140:215–250. doi: 10.1016/0005-2728(93)90063-L. [DOI] [PubMed] [Google Scholar]
- 153.Noji H., Yasuda R., Yoshida M., Kinosita K., Jr. Direct observation of the rotation of F1-ATPase. Nat. Cell Biol. 1997;386:299–302. doi: 10.1038/386299a0. [DOI] [PubMed] [Google Scholar]
- 154.Abrahams J.P., Leslie A.G.W., Lutter R., Walker J.E. Structure at 2.8 Â resolution of F1-ATPase from bovine heart mitochondria. Nat. Cell Biol. 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 155.Menz R., Walker J.E., Leslie A.G. Structure of bovine mitochondrial F(1)-ATPase with nucleotide bound to all three catalytic sites: Implications for the mechanism of rotary catalysis. Cell. 2001;106:331–341. doi: 10.1016/S0092-8674(01)00452-4. [DOI] [PubMed] [Google Scholar]
- 156.Spikes T.E., Montgomery M.G., Walker J.E. Structure of the dimeric ATP synthase from bovine mitochondria. Proc. Natl. Acad. Sci. USA. 2020;117:23519–23526. doi: 10.1073/pnas.2013998117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Watt I.N., Montgomery M.G., Runswick M.J., Leslie A.G.W., Walker J.E. Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc. Natl. Acad. Sci. USA. 2010;107:16823–16827. doi: 10.1073/pnas.1011099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dautant A., Velours J., Giraud M.-F. Crystal Structure of the Mg·ADP-inhibited State of the Yeast F1c10-ATP Synthase. J. Biol. Chem. 2010;285:29502–29510. doi: 10.1074/jbc.M110.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ma J., Flynn T.C., Cui Q., Leslie A.G.W., E Walker J., Karplus M. A dynamic analysis of the rotation mechanism for conformational change in F(1)-ATPase. Structure. 2002;10:921–931. doi: 10.1016/S0969-2126(02)00789-X. [DOI] [PubMed] [Google Scholar]
- 160.Bockmann R.A., Grubmuller H. Nanoseconds molecular dynamics simulation of primary mechanical energy transfer steps in F1-ATP synthase. Nat. Genet. 2002;9:198–202. doi: 10.1038/nsb760. [DOI] [PubMed] [Google Scholar]
- 161.Symersky J., Pagadala V., Osowski D., Krah A., Meier T., Faraldo-Gómez J.D., Mueller D.M. Structure of the c10 ring of the yeast mitochondrial ATP synthase in the open conformation. Nat. Struct. Mol. Biol. 2012;19:485–491. doi: 10.1038/nsmb.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kubo S., Niina T., Takada S. Molecular dynamics simulation of proton-transfer coupled rotations in ATP synthase FO motor. Sci. Rep. 2020;10:8225. doi: 10.1038/s41598-020-65004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou W., Marinelli F., Nief C., Faraldo-Gómez J.D. Atomistic simulations indicate the c-subunit ring of the F1Fo ATP synthase is not the mitochondrial permeability transition pore. eLife. 2017;6:10580. doi: 10.7554/eLife.23781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Giorgio V., Burchell V., Schiavone M., Bassot C., Minervini G., Petronilli V., Argenton F., Forte M., E Tosatto S.C., Lippe G., et al. Ca2+ binding to F-ATP synthase β subunit triggers the mitochondrial permeability transition. EMBO Rep. 2017;18:1065–1076. doi: 10.15252/embr.201643354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pezeshkian W., König M., Wassenaar T.A., Marrink S.J. Backmapping triangulated surfaces to coarse-grained membrane models. Nat. Commun. 2020;11:2296. doi: 10.1038/s41467-020-16094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Raichur A., Vali S., Gorin F. Dynamic modeling of alpha-synuclein aggregation for the sporadic and genetic forms of Parkinson’s disease. Neuroscience. 2006;142:859–870. doi: 10.1016/j.neuroscience.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 167.Cloutier M., Wellstead P. Dynamic modelling of protein and oxidative metabolisms simulates the pathogenesis of Parkinson’s disease. IET Syst. Biol. 2012;6:65–72. doi: 10.1049/iet-syb.2011.0075. [DOI] [PubMed] [Google Scholar]
- 168.Cloutier M., Middleton R., Wellstead P. Feedback motif for the pathogenesis of Parkinson’s disease. IET Syst. Biol. 2012;6:86–93. doi: 10.1049/iet-syb.2011.0076. [DOI] [PubMed] [Google Scholar]
- 169.Bakshi S., Chelliah V., Chen C., Van Der Graaf P.H. Mathematical Biology Models of Parkinson’s Disease. CPT. 2019;8:77–86. doi: 10.1002/psp4.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hao W., Friedman A. Mathematical model on Alzheimer’s disease. BMC Syst. Biol. 2016;10:108. doi: 10.1186/s12918-016-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ranjan B., Chong K.H., Zheng J. Composite mathematical modeling of calcium signaling behind neuronal cell death in Alzheimer’s disease. BMC Syst. Biol. 2018;12:10. doi: 10.1186/s12918-018-0529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shi P., Gal J., Kwinter D.M., Liu X., Zhu H. Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2010;1802:45–51. doi: 10.1016/j.bbadis.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Han K., Kim J., Choi M.Y. Autophagy mediates phase transitions from cell death to life. Heliyon. 2015;1:e00027. doi: 10.1016/j.heliyon.2015.e00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Han K., Kim S.H., Choi M.Y. Computational modeling of the effects of autophagy on amyloid-β peptide levels. Theor. Biol. Med. Model. 2020;17:31. doi: 10.1186/s12976-020-00119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Han K., Kim J., Choi M.Y. Quantitative indices of autophagy activity from minimal models. Theor. Biol. Med. Model. 2014;11:31. doi: 10.1186/1742-4682-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Han K., Kim J., Choi M. Computer simulations unveil the dynamics of autophagy and its implications for the cellular qual-ity control. J. Biol. Syst. 2014;22:659–675. doi: 10.1142/S0218339014500260. [DOI] [Google Scholar]
- 177.Han K., Kwon H.W., Kang H., Kim J., Lee M.S., Choi M.Y. Dynamics of macroautophagy: Modeling and oscillatory be-havior. Phys. A. 2012 doi: 10.1016/j.physa.2011.08.046. [DOI] [Google Scholar]
- 178.Barbeito L., Pehar M., Cassina P., Vargas M.R., Peluffo H., Viera L., Estévez A.G., Beckman J.S. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res. Rev. 2004;47:263–274. doi: 10.1016/j.brainresrev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 179.Chun H., Im H., Kang Y.J., Kim Y., Shin J.H., Won W., Lim J., Ju Y., Park Y.M., Kim S., et al. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2− production. Nat. Neurosci. 2020;23:1555–1566. doi: 10.1038/s41593-020-00735-y. [DOI] [PubMed] [Google Scholar]