Abstract
Vascular pythiosis is a rare, neglected, life-threatening disease with mortality of 100% in patients with incomplete surgical resection or patients with persistently elevated serum β-d-glucan (BDG). The study was conducted to understand the clinical outcomes of new treatment protocols and potential use of erythrocyte sedimentation rate (ESR) and c-reactive protein (CRP) as alternative monitoring tools, given recent favorable minimum inhibitory concentrations (MICs) of antibacterial agents and prohibitive cost of serum BDG in Thailand. A prospective cohort study of patients with vascular pythiosis was conducted between February 2019 and August 2020. After diagnosis, patients were followed at 0.5, 1, 1.5, 3, and 6 months. Descriptive statistics, Spearman’s correlation coefficient, and general linear model for longitudinal data were used. Amongst the cohort of ten vascular pythiosis patients, four had residual disease after surgery. Among four with residual disease, one developed disseminated disease and died, one developed relapse disease requiring surgery, and two were successfully managed with antimicrobial agents. The spearman’s correlation coefficients between BDG and ESR, and between BDG and CRP in patients without relapse or disseminated disease were 0.65 and 0.60, respectively. Tetracyclines and macrolides had most favorable minimum inhibitory concentrations and synergistic effects were observed in combinations of these two antibiotic classes. Adjunctive use of azithromycin and doxycycline preliminarily improved survival in vascular pythiosis patients with residual disease. Further studies are needed to understand the trends of ESR and CRP in this population.
Keywords: human pythiosis, P. insidiosum, vascular pythiosis, β-d-glucan, ESR, CRP
1. Introduction
Pythium insidiosum, an aquatic fungal-like pathogen, causes pythiosis in humans. Human pythiosis was first described in a Thai thalassemia patient in 1988 [1]. The clinical presentations are classified into four groups: vascular, ocular, subcutaneous/cutaneous, and disseminated infection [2]. Human pythiosis has also been reported from the United States of America, Canada, Brazil, Frances, Israel, India, China, and Australia [3,4,5,6,7,8,9]. In Thailand, vascular pythiosis is the most common form and it is associated with a mortality rate of 10–40%, depending on the rate of successful surgery [10,11,12,13]. Unfortunately, mortality was up to 100% in patients without complete surgical resection regardless of other adjunctive therapy [10,11,12,13]. Because of its low prevalence, a high index of suspicion is crucial in identifying disease, particularly in those with risk factors who present with arterial insufficiency or chronic lower extremity wounds [14]. Risk factors often include occupation in agriculture and/or underlying hemoglobinopathy, including thalassemia, in patients presenting from an endemic region [2,10,13,14]. The proposed mechanisms of infection in patients with hemoglobinopathy are interference of phagocytic activities of macrophages and neutrophils from iron overload, lower interferon gamma (IFN-γ) production in Thalassemia patients, and intrinsic virulence of P. insidiosum by carrying a gene encoding ferrochelatase [15,16,17].
Treatment strategies remain challenging, and radical surgery is the mainstay. Itraconazole plus terbinafine were formerly recommended as the backbone antimicrobial therapy along with radical surgery. This regimen was largely based on in vitro susceptibility data from Brazilian P. insidiosum isolates [18,19,20]. However, Thai P. insidiosum isolates had unfavorable antifungal minimum inhibitory concentrations (MICs) and antifungal agents did not improve survival outcomes in patients infected with P. insidiosum [10,13,21]. Recent study from the King Chulalongkorn Memorial Hospital (KCMH) pythiosis research group showed that Thai P. insidiosum isolates were susceptible to azithromycin and doxycycline [22,23]. Hence, the antimicrobial regimens under the KCMH research protocols were transitioned to itraconazole plus azithromycin; subsequently, doxycycline was added in the treatment protocol due to evidence of a synergistic effect with azithromycin and doxycycline in combination against Thai P. insidiosum isolates [22,23].
Early disease recognition and definitive surgical resection are associated with reduced mortality [10,13]. However, patients can have residual infection despite negative surgical margins. These patients typically demonstrate persistent elevation of the serum beta-d-glucan (BDG) postoperatively [13]. Therefore, serum BDG has been integrated into the KCMH vascular pythiosis research protocols for monitoring disease. Widespread use of serum BDG monitoring is limited in Thailand outside research protocols because the cost is prohibitive and the assay availability is limited to KCMH. This study was conducted to describe clinical outcomes of new treatment protocols incorporating surrogate markers, erythrocyte sedimentation rate (ESR) and c-reactive protein (CRP), as potential inexpensive monitoring tools which are more easily and globally accessible.
2. Patients and Methods
2.1. Patients and Study Design
This is a multicenter, prospective cohort study of vascular pythiosis patients who were treated according to the KCMH research treatment protocols with a combination of radical surgery and antimicrobial agents from February 2019 to August 2020. Given evidence of antimicrobial synergy between tetracyclines and macrolides, doxycycline was added into the antimicrobial treatment regimen starting in April 2019. Patients were eligible for the study if at least two pythiosis diagnostic criteria were met: (1) clinical presentation consistent with vascular pythiosis; (2) successful isolation of P. insidiosum with zoospore induction [24]; (3) positive P. insidiosum-specific antibody (Pi-Ab) by enzyme-linked immunosorbent assay (ELISA) [25,26]; (4) positive tissue polymerase chain reaction (PCR) for P. insidiosum using internal transcribed spacer or cytochrome oxidase II (PCR-ITS/COX2) primers [27,28,29]; (5) presence of sparsely septate hyphae consistent with P. insidiosum in tissue pathology. During the study period, clinical data and blood specimens were obtained at diagnosis, 0.5, 1, 1.5, 3, and 6 months. Subsequent clinic visits after 6 months were per clinical discretion if there was concern for persistent disease or relapsed infection. The study was approved by the Institutional Review Board at all study sites and informed consent was obtained from all patients.
2.2. Beta-d-Glucan, Erythrocyte Sedimentation Rate, and C-Reactive Protein
Serum BDG was evaluated using the Fungitell assay (Associates of Cape Cod, Inc., Falmouth, MA, USA) according to the manufacturer’s instruction. Samples with BDG levels < 31 pg/mL and >500 pg/mL although outside the Fungitell assay range (31–500 pg/mL) were statistically analyzed as 31 pg/mL and 500 pg/mL, respectively [30,31]. Serum ESR was tested by an automated analyzer (JOKOH Monitor-20, Tokyo, Japan) using an infrared barrier method. Normal range is 0–15 mm/h for males and 0–28 mm/h for females. Serum CRP was measured via immunoturbidimetric assay on a clinical chemistry analyzer (Abbott Alinity ci-series, Chicago, IL, USA, normal range 0–5 mg/L).
2.3. In Vitro Susceptibility
P. insidiosum was successfully isolated from eight patients. Broth microdilution was performed according to Clinical and Laboratory Standards Institute (CLSI) M38, third edition, Guidelines for Filamentous Fungi [32]. In vitro susceptibility was subsequently performed according to published methods and all assays were performed in triplicate [22,33,34,35]. We induced zoospores and diluted the inoculum (2 × 103 to 3 × 103 zoospores/mL) with Roswell Park Memorial Institute (RPMI) 1640 broth, pH 7.0 (with glucose and L-glutamine). The MICs were determined by direct visual observation of 100% inhibition of mycelium growth after incubation for 48 h. Eight P. insidiosum isolates were tested against eight antibacterial and four antifungal agents. The in vitro synergy of the two antimicrobial classes was determined according to the checkerboard technique [22,23]. The results were interpreted as synergism when the fractional inhibitory concentration index (FICI) ≤ 0.5; indifference when 0.5 < FICI < 4.0; antagonism when FICI > 4.0.
2.4. Statistical Analyses
The statistical analyses were conducted by SAS University edition (SAS Institute, Cary, NC, USA). Descriptive statistics were used to describe serum BDG, ESR, and CRP at each time point. General linear model for longitudinal data were used for the single group repeated measure design to understand the effect of time on the mean response of serum BDG, ESR, and CRP. Spearman’s correlation coefficient was used to evaluate correlation among serum BDG, ESR, and CRP. The general linear model for longitudinal data and Spearman’s correlation coefficient were only performed in patients without relapse or disseminated disease in this study given differences in the nature of the diseases.
3. Results
3.1. Patient Characteristics and Treatment Modalities
Ten patients met the diagnostic inclusion criteria and were enrolled into the study. Baseline patient characteristics, clinical presentation, and treatment modalities are summarized in Table 1. All patients had underlying thalassemia. Duration from the first medical encounter to radical surgery ranged from 1 to 60 days. Radical surgery was performed on all patients; however, four patients had residual disease after the first surgery (three without negative surgical margins and one with relapsed disease). Among three patients without negative surgical margins (cases 1, 2, and 9), one (case 9) was diagnosed with disseminated disease at the time of diagnosis and died 5 months after diagnosis. Case 1 and 2 were successfully managed with antimicrobial agents and did not require additional surgery. Case 8, with negative surgical margins, required a second surgery given clinical concern for relapsed disease and remained well through the end of the study period. All patients tolerated antimicrobial agents well without side effects during the study period.
Table 1.
Patient characteristics and treatment modalities.
| Case | Age (Years) /Sex |
Occupation | Clinical Presentations |
Arterial Involvement |
Surgery | Duration from First Medical Visit to Surgery (Days) | Free Surgical Margins Achievement |
Antimicrobial Agents | Follow-Up Duration (Months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 53/M | Farmer | Left leg pain 2 weeks | Left popliteal artery | Left AKA | 5 | No | Itraconazole Azithromycin |
6 |
| 2 | 52/M | Driver | Right leg pain, groin mass, cold skin, numbness 3 days | Right common iliac artery | Right AKA | 5 | No | Itraconazole Azithromycin Doxycycline |
6 |
| 3 | 69/M | Farmer | Left leg pain 2 weeks | Left tibial artery and left popliteal artery | Left AKA | 5 | Yes | Itraconazole Azithromycin |
6 |
| 4 | 34/M | Merchant | Right ankle swelling and tenderness 2 days | Right popliteal artery | Right BKA | 25 | Yes | Itraconazole Azithromycin |
8 |
| 5 | 50/F | Farmer | Right leg chronic wound 2 months | Right popliteal artery | Right BKA | 21 | Yes | Itraconazole Azithromycin |
8 |
| 6 | 53/M | Farmer | Left leg pain 1 day | Left femoral artery | Left AKA | 1 | Yes | Itraconazole Azithromycin Doxycycline |
6 |
| 7 | 49/M | Security guard | Left leg chronic wound 3 years | Cutaneous vessels | Left wide excision | 11 | Yes | Itraconazole Azithromycin Doxycycline |
6 |
| 8 | 45/M | Merchant | Right leg pain and chronic wound 2 months | Right femoral artery | Ligation of right external iliac artery | 6 | Yes | Itraconazole Azithromycin Doxycycline |
6 |
| 9 | 51/M | Potter | Right leg pain 2 months | Right femoral artery | Right hip disarticulation | 60 | No | Itraconazole Azithromycin Doxycycline |
5 |
| 10 | 53/M | Farmer | Left leg pain 2 months | Left anterior tibial artery | Left AKA | 7 | Yes | Itraconazole Azithromycin Doxycycline |
6 |
AKA: above knee amputation; BKA: below knee amputation; F: female; M: male.
3.2. Serum Beta-d-Glucan, Erythrocyte Sedimentation Rate and C-Reactive Protein
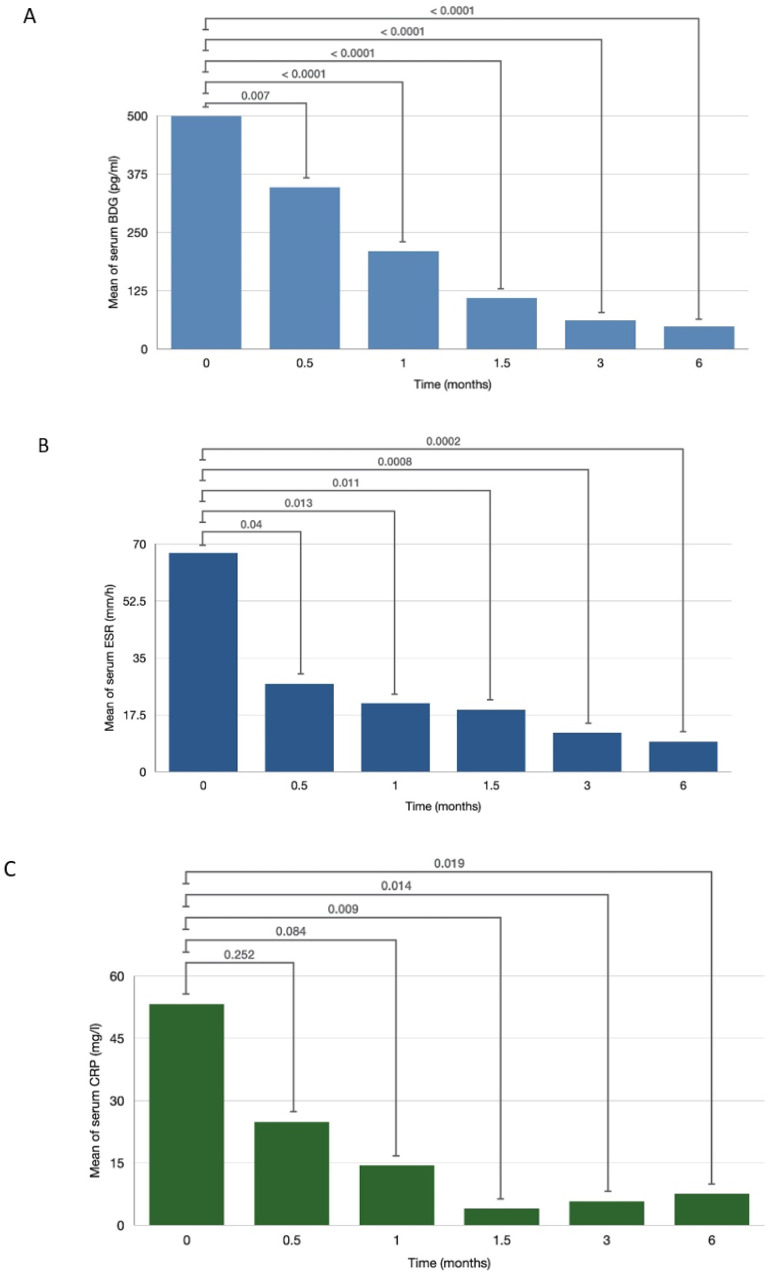
At the time of diagnosis, all patients had highly elevated serum BDG (>500 pg/mL), ESR (>60 mm/h) and CRP (>50 mg/L). In patients without relapsed or disseminated disease, the mean of serum BDG significantly decreased at 0.5 months after treatment initiation (347 vs. 500 pg/mL, p = 0.007). Mean serum BDG continued to decrease over time and was lower than the positive cut-off value (<80 pg/mL) by 3 months (Figure 1A). Similarly, among patients without relapsed or disseminated disease, the mean serum ESR significantly decreased at 0.5 months after treatment initiation (27.13 vs. 67.29 mm/h, p = 0.04). Mean serum ESR decreased linearly over time and had also normalized by 3 months (Figure 1B). The mean serum CRP also declined following treatment initiation, demonstrating a statistically significant decrease at 1.5 months (3.99 vs. 53.28 mg/L, p = 0.009) (Figure 1C). Spearman’s correlation coefficients between BDG and ESR, and between BDG and CRP were 0.65 and 0.60, respectively.
Figure 1.
Means of serum β-d-glucan (BDG) (A), serum erythrocyte sedimentation rate (ESR) (B), and serum C-reactive protein (CRP) (C) across different time points.
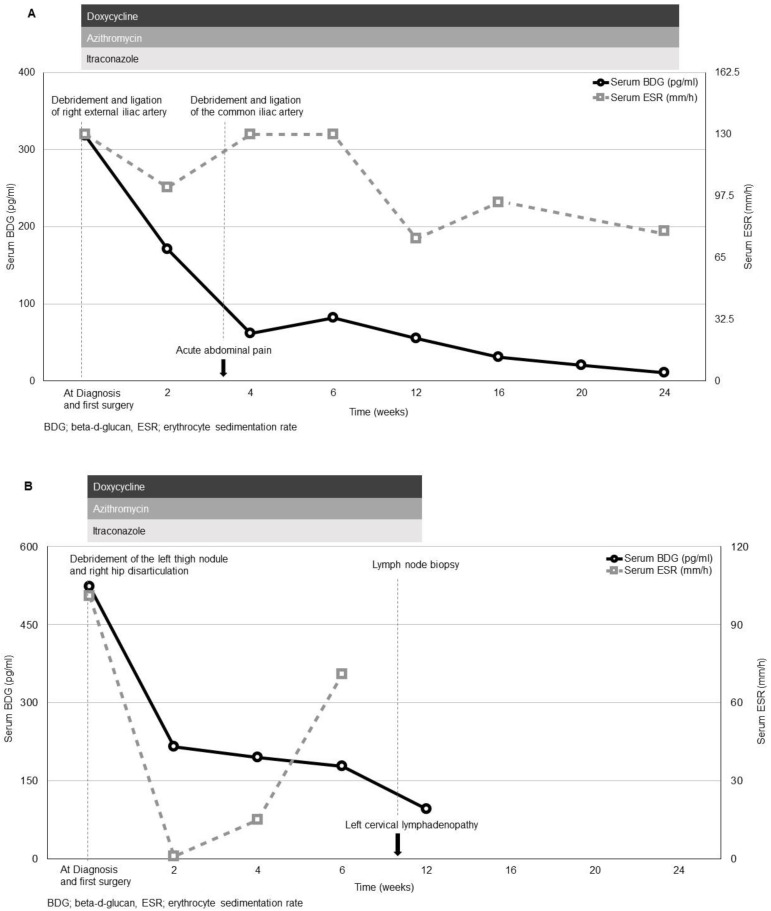
Case 8 with relapsed disease and case 9 with disseminated disease were separately described given their distinct features. In case 8, serum BDG, ESR, and CRP initially declined after the first surgery. However, the patient developed acute abdominal pain and a computed tomography angiography showed thickening wall of the right common iliac artery before the scheduled visit at 1 month. The patient underwent emergent debridement and ligation of the common iliac artery. The pathology did not reveal evidence of fungal disease. The patient’s ESR and CRP were checked at 1 month per study protocol and they were elevated after the surgery. After the second surgery, the patient did well clinically and patient’s serum BDG and CRP normalized at 6 months after diagnosis. However, the patient’s ESR remained elevated throughout the study period (Figure 2A).
Figure 2.
Clinical course and treatment modalities of relapsed disease (A) and disseminated disease (B).
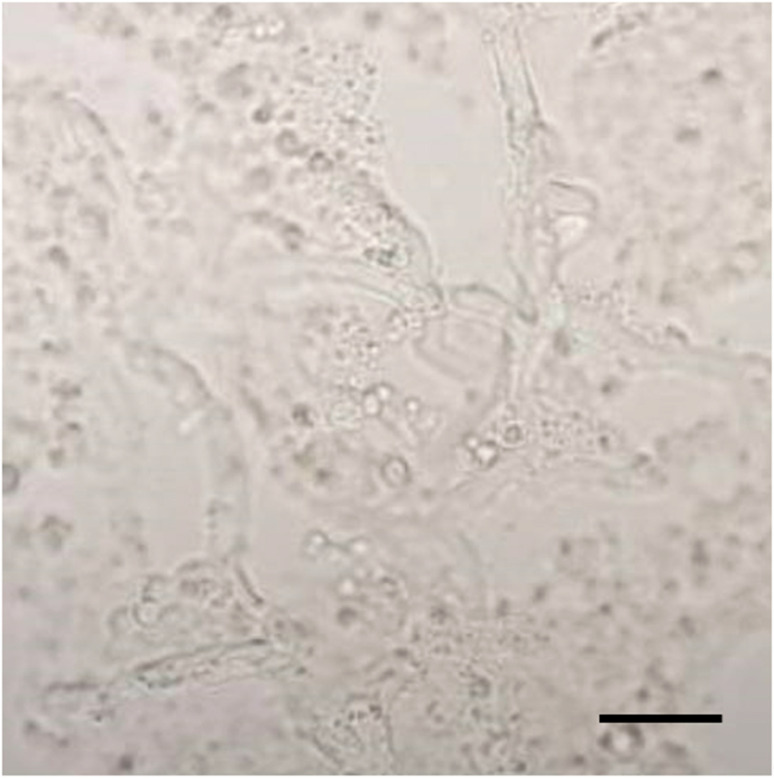
In case 9, serum BDG, ESR, and CRP initially declined after initial treatment (right hip disarticulation, wide excision of the subcutaneous nodule at the left thigh and antimicrobial therapy with itraconazole, azithromycin and doxycycline). The patient was diagnosed with disseminated pythiosis given presence of fungal elements from the right lower extremity vessels and from the skin lesion on the left thigh. Microscopic examination of the vascular tissue by using 10% potassium hydroxide preparation showed scarcely septate hyphae consistent with P. insidiosum (Figure 3). Computed tomography angiography at the time of diagnosis did not reveal disease involvement of the left lower extremity vessels. Prognosis was thoroughly discussed, and the patient elected to continue only medical therapy. Subsequent serum BDG, ESR, and CRP remained significantly elevated. Unfortunately, the patient developed left neck swelling and the lymph node biopsy showed fungal element consistent with P. insidiosum. Case 9 died at 5 months after initial diagnosis (Figure 2B).
Figure 3.
Direct examination of the vascular tissue by using potassium hydroxide preparation showed sparsely septate hyphae (×400; the bar scale is 20 µm).
3.3. In Vitro Susceptibility
The in vitro susceptibility results are summarized in Table 2. Among eight antibacterial classes, tetracyclines (doxycycline, minocycline, and tigecycline) and macrolides (azithromycin and clarithromycin) had favorable MICs, compared to other classes. Synergistic effects between tetracyclines and macrolides were observed in all P. insidiosum isolates. Among seven antifungal agents, itraconazole showed favorable MICs, compared to other antifungal agents. No synergistic effects were observed with combinations of voriconazole/terbinafine and itraconazole/terbinafine. The P. insidiosum isolate from case 9 with disseminated disease had higher MIC values (doxycycline MIC 4, azithromycin MIC 2, itraconazole MIC 4, and voriconazole MIC 8).
Table 2.
In vitro susceptibility results of Pythium insidiosum isolates.
| Minimum Inhibitory Concentrations (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | Case 5 | Case 6 | Case 8 | Case 9 | Case 10 | ||
| Tetracyclines | Doxycycline | 4 | 1 | 4 | 2 | 2 | 4 | 4 | 2 |
| Minocycline | 1 | 1 | 0.5 | 1 | 0.25 | 1 | 1 | 2 | |
| Tigecycline | 1 | 1 | 0.5 | 1 | 1 | 1 | 2 | 1 | |
| Macrolides | Azithromycin | 4 | 2 | 2 | 2 | 2 | 2 | 2 | 4 |
| Clarithromycin | 2 | 1 | 1 | 1 | 0.125 | 1 | 2 | 1 | |
| Beta-lactams | Cefazolin | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 |
| ceftriaxone | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | |
| Ceftazidime | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | |
| Meropenem | 32 | 32 | >32 | 32 | 32 | >32 | >32 | >32 | |
| Oxazolidinone | Linezolid | 4 | 8 | 4 | 8 | 4 | 4 | 8 | 4 |
| Glycopeptide | Vancomycin | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 |
| Aminoglycosides | Amikacin | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 |
| Gentamicin | 32 | >32 | 16 | 32 | >32 | >32 | >32 | >32 | |
| Neomycin | 32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | |
| Streptomycin | 32 | >32 | >32 | 32 | 32 | 16 | >32 | 32 | |
| Tobramycin | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | |
| Quinolones | Ciprofloxacin | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 |
| Levofloxacin | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | |
| Moxifloxacin | 32 | 16 | >32 | 16 | 32 | 32 | >32 | 16 | |
| Polymyxins | Colistin (Polymyxin E) | 8 | 8 | 4 | 8 | >32 | 16 | 16 | 16 |
| Polymyxin B | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | |
| Combination | Azithromycin/Minocycline | S | S | S | S | S | S | S | S |
| Azithromycin/Tigecycline | S | S | S | S | S | S | S | S | |
| Clarithromycin/Minocycline | S | S | S | S | S | S | S | S | |
| Clarithromycin/Tigecycline | S | S | S | S | S | S | S | S | |
| Minocycline/Tigecycline | S | S | S | S | S | S | S | S | |
| Doxycycline/Azithromycin | S | S | S | S | S | S | S | S | |
| Doxycycline/Clarithromycin | S | S | S | S | S | S | S | S | |
| Doxycycline/Tigecycline | S | S | S | S | S | S | S | S | |
| Antifungal agents | Amphotericin B | 4 | 8 | 8 | 8 | 8 | 8 | 4 | 4 |
| Voriconazole | 2 | 4 | 2 | 2 | 4 | 4 | 8 | 4 | |
| Itraconazole | 2 | 2 | 1 | 1 | 2 | 2 | 4 | 4 | |
| Fluconazole | 2 | 4 | 2 | 2 | 4 | 2 | 4 | 8 | |
| Anidulafungin | 4 | 2 | 4 | 8 | 4 | 2 | 8 | 8 | |
| Caspofungin | 4 | 2 | 4 | 4 | 4 | 2 | 4 | 8 | |
| Terbinafine | 2 | 4 | 2 | 2 | 4 | 2 | 4 | 4 | |
| Voriconazole/Terbinafine | I | I | I | I | I | I | I | I | |
| Itraconazole/Terbinafine | I | I | I | I | I | I | I | I | |
The minimum inhibitory concentrations (MICs) of each agent were determined by 100% inhibition of mycelium by visual observation, comparted to the inhibition in the control wells (without antibiotics). S: synergism; I: indifference.
4. Discussion
We describe the first multicenter, prospective study to preliminarily report the potential clinical benefit of antibacterial agents and the use of ESR and CRP as monitoring tools in vascular pythiosis. All ten patients underwent radical surgery. Although, negative surgical margins were successfully obtained in seven patients, one death occurred in a patient with disseminated pythiosis. Despite lack of a control group and small sample size, the findings are encouraging. Adjunctive itraconazole, azithromycin, and doxycycline along with close monitoring of serum BDG for treatment response and early relapse detection likely provides survival benefit, particularly in those with residual disease. A multicenter, open-label, single arm clinical trial is being conducted in Thailand to fully understand the efficacy of the new treatment protocol and the study will be completed in 2023 (Thai Clinical Trial Registry number: TCTR20191217006).
In our previous study, we have found that patients with persistently elevated serum BDG after surgery died within 3 months and this phenomenon occurred in patients with incomplete surgical resection or patients with relapsed disease regardless of antifungal therapy [13]. Interestingly, only the patient with disseminated pythiosis had persistently elevated serum BDG while the other three patients with residual disease (cases 1, 2, and 8) did well postoperatively. Based on our in vitro susceptibility results, tetracyclines and macrolides had 10–100 times lower MICs than antifungal agents in each P. insidiosum isolate and synergistic effects were observed in combinations of tetracyclines and macrolides in all P. insidiosum isolates. Similar to previous Thai P. insidiosum in vitro susceptibility results, no synergistic effects were observed with combinations of itraconazole/terbinafine or voriconazole/terbinafine [10,13,21]. We observed that the P. insidiosum isolate from the patient with disseminated disease had elevated MICs in all classes of antimicrobial agents which may explain why the patient did not respond to medical treatment. However, it is important to note that standardized MIC interpretations for P. insidiosum have not been established.
ESR and CRP are non-specific inflammatory markers used in monitoring various infections such as infective endocarditis and osteomyelitis [36,37,38,39,40]. ESR and CRP are inexpensive and accessible nationwide in Thailand. Given their established use in monitoring endovascular infections, we investigated their potential role in monitoring vascular pythiosis. Serum BDG demonstrated plausible correlation with ESR and CRP levels across time. We expect that the correlation coefficients would be higher in the larger study. Similar to our previous study of serial serum BDG monitoring [13], we believe serum ESR and CRP trends predict response to therapy.
Our main limitation is the small sample size in this rare disease. The correlation was only calculated from patients without relapsed or disseminated disease and no control arm was used. In clinical practice, ESR and CRP can be challenging to trend especially owing to inter-laboratory variation.
In summary, azithromycin and doxycycline should be considered in addition to surgery and antifungal agent(s) in vascular pythiosis treatment; however, antimicrobial regimens should be adjusted based on local susceptibility data. ESR and CRP demonstrate a preliminarily correlation with serum BDG in response to vascular pythiosis treatment in patients without relapse or disseminated disease. Further studies are needed to evaluate efficacy of treatment modalities and to understand patterns of ESR and CRP in pythiosis patients, particularly with relapse or disseminated disease.
Acknowledgments
The authors would like to thank the healthcare providers involved in patient care. The authors also would like to thank Santi Kulpatcharapong for graphic design in figures.
Author Contributions
Study design (P.T., N.C., R.P., N.W, A.C., N.P.); direct patient care and data collection (P.T., N.C., K.M., A.T., N.L., J.D., P.K., R.B., N.S., W.W.); data analysis (P.T., N.C., R.P., N.W., A.C., N.P.); manuscript writing (P.T., N.C., R.P., N.W., K.M., A.T., A.C., N.P.); critical review (P.T., N.C., R.P., N.W., K.M., A.T., N.L., J.D., P.K., R.B., N.S., W.W., A.C., N.P.) All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Health Systems Research Institute (grant number HSRI 63-003), Thailand. The funder had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Chulalongkorn University (673/61 on 12 February 2019 and 135/62 on 6 June 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study cannot be shared due to ethical, legal, and confidential issues in accordance with consent provided by participants.
Conflicts of Interest
N.W., R.P., and A.C. reported receiving grants from the Rachadapiseksompotch Fund, Chulalongkorn University. J.D. reports receiving a personal fee from Pfizer, MSD, and Janssen. N.P. reported receiving grants and salary support from the Health Systems Research Institute (Thailand), the Cystic Fibrosis Foundation, the National of Heart, Lung, and Blood Institute, and the Fisher Center Discovery Program, Johns Hopkins University. N.P. served on the Advisory Board for Shionogi Inc. No other conflict of interest to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sathapatayavongs B., Leelachaikul P., Prachaktam R., Atichartakarn V., Sriphojanart S., Trairatvorakul P., Jirasiritham S., Nontasut S., Eurvilaichit C., Flegel T. Human pythiosis associated with thalassemia hemoglobinopathy syndrome. J. Infect. Dis. 1989;159:274–280. doi: 10.1093/infdis/159.2.274. [DOI] [PubMed] [Google Scholar]
- 2.Krajaejun T., Sathapatayavongs B., Pracharktam R., Nitiyanant P., Leelachaikul P., Wanachiwanawin W., Chaiprasert A., Assanasen P., Saipetch M., Mootsikapun P., et al. Clinical and epidemiological analyses of human pythiosis in Thailand. Clin. Infect. Dis. 2006;43:569–576. doi: 10.1086/506353. [DOI] [PubMed] [Google Scholar]
- 3.Triscott J.A., Weedon D., Cabana E. Human subcutaneous pythiosis. J. Cutan. Pathol. 1993;20:267–271. doi: 10.1111/j.1600-0560.1993.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 4.HHe H., Liu H., Chen X., Wu J., He M., Zhong X. Diagnosis and Treatment of Pythium Insidiosum Corneal Ulcer in a Chinese Child: A Case Report and Literature Review. Am. J. Case Rep. 2016;17:982–988. doi: 10.12659/AJCR.901158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilton R., Tepedino K., Glenn C.J., Merkel K.L. Swamp cancer: A case of human pythiosis and review of the literature. Br. J. Dermatol. 2016;175:394–397. doi: 10.1111/bjd.14520. [DOI] [PubMed] [Google Scholar]
- 6.Hung C., Leddin D. Keratitis caused by Pythium insidiosum in an immunosuppressed patient with Crohn’s disease. Clin. Gastroenterol. Hepatol. 2014;12:A21–A22. doi: 10.1016/j.cgh.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 7.FFranco D.M., Aronson J.F., Hawkins H.K., Gallagher J.J., Mendoza L., McGinnis M.R., Williams-Bouyer N. Systemic Pythium insidiosum in a pediatric burn patient. Burn J. Int. Soc. Burn Inj. 2010;36:e68–e71. doi: 10.1016/j.burns.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Bosco S., Bagagli E., Araújo J.P., Candeias J.M., Jr., de Franco M.F., Alencar Marques M.E., Mendoza L., de Camargo R.P., Alencar Marques S. Human pythiosis, Brazil. Emerg. Infect. Dis. 2005;11:715–718. doi: 10.3201/eid1105.040943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanhehco T.Y., Stacy R.C., Mendoza L., Durand M.L., Jakobiec F.A., Colby K.A. Pythium insidiosum keratitis in Israel. Eye Contact Lens Sci. Clin. Pract. 2011;37:96–98. doi: 10.1097/ICL.0b013e3182043114. [DOI] [PubMed] [Google Scholar]
- 10.Permpalung N., Worasilchai N., Plongla R., Upala S., Sanguankeo A., Paitoonpong L., Mendoza L., Chindamporn A. Treatment outcomes of surgery, antifungal therapy and immunotherapy in ocular and vascular human pythiosis: A retrospective study of 18 patients. J. Antimicrob. Chemother. 2015;70 doi: 10.1093/jac/dkv008. [DOI] [PubMed] [Google Scholar]
- 11.Reanpang T., Orrapin S., Orrapin S., Arworn S., Kattipatanapong T., Srisuwan T., Vanittanakom N., Lekawanvijit S.P., Rerkasem K. Vascular Pythiosis of the Lower Extremity in Northern Thailand: Ten Years’ Experience. Int. J. Low Extrem. Wounds. 2015;14:245–250. doi: 10.1177/1534734615599652. [DOI] [PubMed] [Google Scholar]
- 12.Sermsathanasawadi N., Praditsuktavorn B., Hongku K., Wongwanit C., Chinsakchai K., Ruangsetakit C., Hahtapornsawan S., Mutirangura P. Outcomes and factors influencing prognosis in patients with vascular pythiosis. J. Vasc. Surg. 2016;64:411–417. doi: 10.1016/j.jvs.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Worasilchai N., Permpalung N., Chongsathidkiet P., Leelahavanichkul A., Mendoza A.L., Palaga T., Reantragoon R., Finkelman M., Sutcharitchan P., Chindamporn A. Monitoring Anti-Pythium insidiosum IgG Antibodies and (1→3)-β-d-Glucan in Vascular Pythiosis. J. Clin. Microbiol. 2018;56 doi: 10.1128/JCM.00610-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Permpalung N., Worasilchai N., Chindamporn A. Human Pythiosis: Emergence of Fungal-Like Organism. Mycopathologia. 2020;185:801–812. doi: 10.1007/s11046-019-00412-0. [DOI] [PubMed] [Google Scholar]
- 15.de Sousa M. Immune cell functions in iron overload. Clin. Exp. Immunol. 1989;75:1–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Gordeuk V.R., Ballou S., Lozanski G., Brittenham G.M. Decreased concentrations of tumor necrosis factor-alpha in supernatants of monocytes from homozygotes for hereditary hemochromatosis. Blood. 1992;79:1855–1860. doi: 10.1182/blood.V79.7.1855.1855. [DOI] [PubMed] [Google Scholar]
- 17.Krajaejun T., Khositnithikul R., Lerksuthirat T., Lowhnoo T., Rujirawat T., Petchthong T., Yingyong W., Suriyaphol P., Smittipat N., Juthayothin T., et al. Expressed sequence tags reveal genetic diversity and putative virulence factors of the pathogenic oomycete Pythium insidiosum. Fungal Biol. 2011;115:683–696. doi: 10.1016/j.funbio.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Argenta J.S., Alves S.H., Silveira F., Maboni G., Zanette R.A., Cavalheiro A.S., Pereira P.L., Pereira D.I., Sallis E.S., Pötter L., et al. In vitro and in vivo susceptibility of two-drug and three-drug combinations of terbinafine, itraconazole, caspofungin, ibuprofen and fluvastatin against Pythium insidiosum. Vet. Microbiol. 2012;157:137–142. doi: 10.1016/j.vetmic.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Cavalheiro A.S., Maboni G., De Azevedo M.I., Argenta J.S., Pereira D.I.B., Spader T.B., Alves S.H., Santurio J.M. In Vitro activity of terbinafine combined with caspofungin and azoles against Pythium insidiosum. Antimicrob. Agents Chemother. 2009;53:2136–2138. doi: 10.1128/AAC.01506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shenep J.L., English B.K., Kaufman L., Pearson T.A., Thompson J.W., Kaufman R.A., Frisch G., Rinaldi M.G. Successful medical therapy for deeply invasive facial infection due to Pythium insidiosum in a child. Clin. Infect. Dis. 1998;27:1388–1393. doi: 10.1086/515042. [DOI] [PubMed] [Google Scholar]
- 21.Permpalung N., Worasilchai N., Manothummetha K., Torvorapanit P., Ratanawongphaibul K., Chuleerarux N., Plongla R., Chindamporn A. Clinical outcomes in ocular pythiosis patients treated with a combination therapy protocol in Thailand: A prospective study. Med. Mycol. 2019;57:923–928. doi: 10.1093/mmy/myz013. [DOI] [PubMed] [Google Scholar]
- 22.Worasilchai N., Chindamporn A., Plongla R., Torvorapanit P., Manothummetha K., Chuleerarux N., Permpalung N. In Vitro Susceptibility of Thai Pythium insidiosum Isolates to Antibacterial Agents. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.02099-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Susaengrat N., Torvorapanit P., Plongla R., Chuleerarux N., Manothummetha K., Tuangsirisup J., Worasilchai N., Chindamporn A., Permpalung N. Adjunctive antibacterial agents as a salvage therapy in relapsed vascular pythiosis patients. Int. J. Infect. Dis. 2019;88:27–30. doi: 10.1016/j.ijid.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 24.Vilela R., Mendoza L. Manual of Clinical Microbiology. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2015. Lacazia, Lagenidium, Pythium, and Rhinosporidium; pp. 2196–2208. [DOI] [Google Scholar]
- 25.Chindamporn A., Vilela R., Hoag K.A., Mendoza L. Antibodies in the sera of host species with pythiosis recognize a variety of unique immunogens in geographically divergent Pythium insidiosum strains. Clin. Vaccine Immunol. 2008;16:330–336. doi: 10.1128/CVI.00429-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krajaejun T., Kunakorn M., Niemhom S., Chongtrakool P., Pracharktam R. Development and evaluation of an in-house enzyme-linked immunosorbent assay for early diagnosis and monitoring of human pythiosis. Clin. Vaccine Immunol. 2002;9:378–382. doi: 10.1128/CDLI.9.2.378-382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worasilchai N., Chaumpluk P., Chakrabarti A., Chindamporn A. Differential diagnosis for pythiosis using thermophilic helicase DNA amplification and restriction fragment length polymorphism (tHDA-RFLP) Med. Mycol. 2017;56:216–224. doi: 10.1093/mmy/myx033. [DOI] [PubMed] [Google Scholar]
- 28.Worasilchai N., Permpalung N., Chindamporn A. High-resolution melting analysis: A novel approach for clade differentiation in Pythium insidiosum and pythiosis. Med. Mycol. 2017;56:868–876. doi: 10.1093/mmy/myx123. [DOI] [PubMed] [Google Scholar]
- 29.Kammarnjesadakul P., Palaga T., Sritunyalucksana K., Mendoza L., Krajaejun T., Vanittanakom N., Tongchusak S., Denduangboripant J., Chindamporn A. Phylogenetic analysis of Pythium insidiosum Thai strains using cytochrome oxidase II (COX II) DNA coding sequences and internal transcribed spacer regions (ITS) Med. Mycol. 2011;49:289–295. doi: 10.3109/13693786.2010.511282. [DOI] [PubMed] [Google Scholar]
- 30.Pickering J.W., Sant H.W., Bowles C.A.P., Roberts W.L., Woods G.L. Evaluation of a (1->3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. J. Clin. Microbiol. 2005;43:5957–5962. doi: 10.1128/JCM.43.12.5957-5962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran T., Beal S.G. Application of the 1,3-β-D-Glucan (Fungitell) Assay in the Diagnosis of Invasive Fungal Infections. Arch. Pathol. Lab. Med. 2016;140:181–185. doi: 10.5858/arpa.2014-0230-RS. [DOI] [PubMed] [Google Scholar]
- 32.Clinical and Laboratory Standards Institute . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, Approved Standard. 3rd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2017. Document M45. [Google Scholar]
- 33.Jesus F.P.K., Loreto E.S., Ferreiro L., Alves S.H., Driemeier D., Souza S.O., França R.T., Lopes S.T.A., Pilotto M.B., Ludwig A., et al. In Vitro and In Vivo Antimicrobial Activities of Minocycline in Combination with Azithromycin, Clarithromycin, or Tigecycline against Pythium insidiosum. Antimicrob. Agents Chemother. 2016;60:87–91. doi: 10.1128/AAC.01480-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loreto É.S., Mario D.A.N., Denardi L.B., Alves S.H., Santurio J.M. In vitro susceptibility of Pythium insidiosum to macrolides and tetracycline antibiotics. Antimicrob. Agents Chemother. 2011;55:3588–3590. doi: 10.1128/AAC.01586-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahl D.L., De Jesus F.P.K., Loreto É.S., Zanette R.A., Ferreiro L., Ben Pilotto M., Alves S.H., Santurio J.M. In vitro susceptibility of Pythium insidiosum isolates to aminoglycoside antibiotics and tigecycline. Antimicrob. Agents Chemother. 2012;56:4021–4023. doi: 10.1128/AAC.00073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen A.G., Espersen F., Skinhøj P., Frimodt-Møller N. Bacteremic Staphylococcus aureus spondylitis. Arch. Intern. Med. 1998;158:509–517. doi: 10.1001/archinte.158.5.509. [DOI] [PubMed] [Google Scholar]
- 37.Siemionow K., Steinmetz M., Bell G., Ilaslan H., McLain R.F. Identifying serious causes of back pain: Cancer, infection, fracture. Clevel. Clin. J. Med. 2008;75:557–566. doi: 10.3949/ccjm.75.8.557. [DOI] [PubMed] [Google Scholar]
- 38.Li J.S., Sexton D.J., Mick N., Nettles R., Fowler J.V.G., Ryan T., Bashore T., Corey G.R. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 39.Berbari E., Kanj S.S., Kowalski T.J., Darouiche R.O., Widmer A.F., Schmitt S.K., Hendershot E.F., Holtom P.D., Huddleston P.M., Petermann G.W., et al. Executive Summary: 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin. Infect. Dis. 2015;61:859–863. doi: 10.1093/cid/civ633. [DOI] [PubMed] [Google Scholar]
- 40.Habib G., Lancellotti P., Antunes M.J., Bongiorni M.G., Casalta J.-P., Del Zotti F., Dulgheru R., El Khoury G., Erba P.A., Iung B., et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur. Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study cannot be shared due to ethical, legal, and confidential issues in accordance with consent provided by participants.