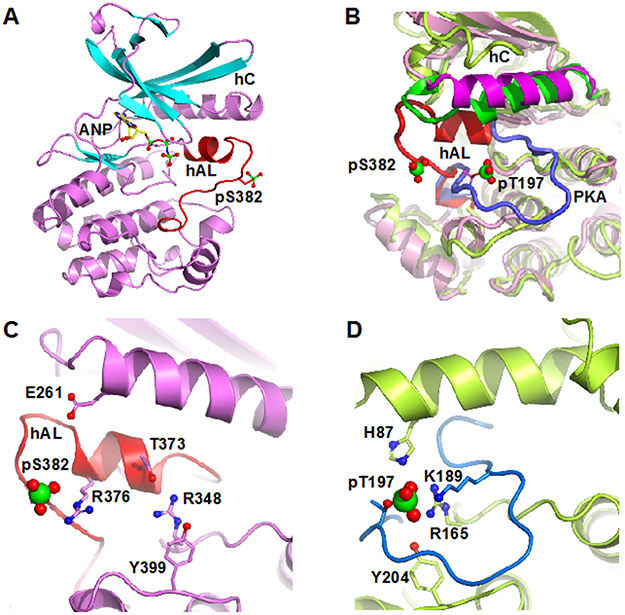
Figure 1.
Structure of pWNK1. (A) Overall structure of pWNK1. Helices are pink and β-strands are cyan, AMP-PNP (ANP) and the pS382 are shown in sticks. (B) Overlay of the structure of pWNK1 (PDB file 5W7T) with PKA (PDB file 1ATP). pWNK is magenta and its activation loop red; PKA is green, and it activation loop blue. (C) Unique conformation of the pWNK1 activation loop and unique interactions of pS382 with R376 in hAL, rather than R348 from the catalytic loop. (D) Interactions of the phosphorylation site in PKA from the same perspective as (C) showing canonical interactions with basic residues from the catalytic loop (R165), helix C (H87) and the activation loop (K189). Diagrams drawn in PyMOL 34