Abstract
Background
Coronavirus disease (COVID-19) is interfering heavily with the screening, diagnosis and treatment of cancer patients. Better knowledge of the seroprevalence and immune response after Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection in this population is important to manage them safely during the pandemic.
Methods
922 cancer patients, 100 non-cancer patients and 94 health care workers (HCW) attending the Multidisciplinary Oncology Unit of Antwerp University Hospital from 24th of March 2020 till 31st of May 2020, and the Oncology Unit of AZ Maria Middelares Hospital, Ghent, from 13th of April 2020 till 31st of May 2020 participated in the study. The Alinity® (A; Abbott) and Liaison® (D; DiaSorin) commercially available assays were used to measure SARS-CoV-2 IgG, while total SARS-CoV-2 Ig was measured by Elecsys® (R; Roche).
Results
In the overall study population IgG/total SARS-CoV-2 antibodies were found in respectively 32/998 (3.2%), 68/1020 (6.7%), 37/1010 (3.7%) and of individuals using the A, D or R test. Forty-six out of 618 (7.4%) persons had a positive SARS-CoV-2 polymerase chain reaction (RT-PCR) test. Seroprevalence in cancer patients (A:2.2%, D:6.2%, R:3.0%), did not significantly differ from that in non-cancer patients (A:1.1%, D:5.6%, R:0.0%), but was lower than the HCW (A:13%, D:12%, R:12%; respectively Fisher’s exact test p = 0.00001, p = 0.046, p = 0.0004). A positive SARS-CoV-2 RT-PCR was found in 6.8% of the cancer patients, 2.3% of the non-cancer patients and 28.1% of the HCW (Fisher’s exact test p = 0.0004). Correlation between absolute values of the different Ig tests was poor in the cancer population. Dichotomising a positive versus negative test result, the A and R test correlated well (kappa 0.82 p McNemar test = 0.344), while A and D and R and D did not (respectively kappa 0.49 and 0.57; result significantly different p McNemar test = <0.0001 for both). The rate of seroconversion (>75%) and median absolute antibody levels (A: 7.0 versus 4.7; D 74.0 versus 26.6, R: 16.34 versus 7.32; all >P Mann Whitney U test = 0.28) in cancer patients and HCW with a positive RT-PCR at least 7 days earlier did not show any differences. However, none (N = 0/4) of the patients with hematological tumours had seroconversion and absolute antibody levels remained much lower compared to patients with solid tumours (R: 0.1 versus 37.6, p 0.003; D 4.1 versus 158, p 0.008) or HCW (all p < 0.0001).
Conclusion
HCW were at high risk of being infected by SARS-CoV-2 during the first wave of the pandemic. Seroprevalence in cancer patients was low in the study period. Although Ig immune response in cancer patients with solid tumours does not differ from healthy volunteers, patients with hematological tumours have a very poor humoral immune response. This has to be taken into account in future vaccination programmes in this population. SARS-CoV-2 antibody tests have divergent results and seem to have little added value in the management of cancer patients.
Keywords: COVID-19, SARS-CoV-2, Cancer, Seroconversion, Immunoglobulin G, Health care workers
1. Introduction
COVID-19 is a disease caused by the SARS-CoV-2 Betacoronavirus. It was first reported after an outbreak of unusual pneumonia in the city of Wuhan, People’s Republic of China, on the 31st of December 2019 and shortly after declared to be a Public Health Emergency of International Concern [1]. Unfortunately, today, the virus is widely spread throughout the world and declared by the WHO as a pandemic [2]. There is a broad range of clinical presentations of SARS-CoV-2 viral infection varying from asymptomatic, sensation of a mild cold or flu to severe bilateral pneumonia and death [[1], [2], [3], [4]]. The evolution of COVID-19 depends not only on the pathogen but also on the genetic and epigenetic background of the host [5]. Apoptosis and/or dysfunctions of (cancer) immune cells and complex epigenetic reprogramming in immune and progenitor cells may contribute to the immunoparalysis and/or opportunistic co-infections in COVID-19 patients [5,6]. The mortality is the highest in the elderly and in people with a pre-existing condition such as cardiovascular disease, pulmonary disease, inflammatory disorders, hypertension, diabetes and particularly in cancer patients with active disease and (a history of) hematological tumours [6,7]. Ambiguity remains over differences in the expected acquired immunity and its duration in these different populations [6].
Understanding both the rate of asymptomatic infection and pre-existing immunity is key to assess the potential impact of the pandemic spread of this virus in a cancer patient community [8]. Important insights can be gained from serology studies to get data on the proportion of (asymptomatic) infected high-risk patients in a population [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33]]. In addition, patients and health care workers may be identified that are potentially protected from reinfection by neutralising antibodies, although currently, it is not certain if and how long immunity would be present [9]. On average IgM antibodies are detectable around 7 days after a SARS-CoV-2 infection. Immunoglobin G antibodies can be found in 75% of patients after 7 days and in about 90% after two weeks [8,14]. As IgA and IgM antibodies often lack specificity and have a shorter half-life, most serologic studies focus on IgG [8,9,[13], [14], [15], [16], [17], [18]]. The exact role of IgG antibodies in the immune response to SARS-CoV-2 is unclear, but these antibodies often have a virus neutralising capacity [9]. We present a prospective multicenter study evaluating three commercially available SARS-CoV-2 IgG/total Ig antibody tests on the prospectively collected serum of ambulatory cancer and non-cancer patients and HCW in two Belgian oncology units during the rise, nadir and decline of the first peak SARS-CoV-2 epidemic.
2. Materials and methods
2.1. Study objectives
The primary objective of the study was to document the prevalence of COVID-19 and seroprevalence of SARS-CoV-2 IgG/total Ig in ambulatory patients and a group of health care workers (HCW) in two oncology units during and after community spread of the virus. Three commonly used commercially available SARS-CoV-2 IgG/total IG tests for SARS-Cov-2 were compared in both populations in a real-life context to assess their reliability. Secondary additional translational research will be conducted on the samples, which is not part of this manuscript.
2.2. Sample collection
After written informed consent, an additional 10 ml lithium heparin plasma, a 10 ml EDTA-tube and a 4 ml serum sample were collected prospectively at every clinically indicated blood sampling in cancer and non-cancer patients attending the Multidisciplinary Oncology Unit of Antwerp University Hospital from 24th of March 2020 till 31st of May 2020, and the Oncology Unit of AZ Maria Middelares Hospital, Ghent from 13th of April 2020 till 31st of May 2020 (i.e. the early phase, peak period, and decline of the SARS-CoV-2 outbreak in the Flanders region). For participating paediatric cancer patients treated in the Antwerp University Hospital, the study blood sample volume (heparin plasma, EDTA and serum) was decided by the treating physician according to the body weight of the patient, ranging from 9.5 ml to 24 ml in total, and these patients were sampled at intervals of at least 30 days. In addition, HCW from both participating units were asked to voluntarily donate similar blood samples at time points 0, 1, 2 and 3 months after the start of the study. Samples from the Oncology Unit of AZ Maria Middelares Hospital, Ghent, were collected and labelled locally and transferred to the biobank of the Antwerp University Hospital. All samples were stored in the Biobank of Antwerp University Hospital at −20 °C. They received a unique code in order to allow linkage to ‘source data’.
2.3. Inclusion criteria
All (cancer) patients having a routine blood sampling and HCW consenting to participate in the study in the Multidisciplinary Oncology unit of the Antwerp University Hospital, and the AZ Maria Middelares Hospital, Ghent. All paediatric cancer patients, having a routine blood sampling, whose parents or guardian consented to participate in the study in the Antwerp University Hospital at time points 0, 1, 2 and 3 months.
2.4. Exclusion criteria
Critically ill patients or volunteers in which the consenting procedure of taking additional blood samples is not ethically and/or clinically acceptable.
2.5. Treatment of the patients
As the SARS-CoV-2 antibody testing was performed as a batch in retrospect, the test results did not influence their clinical management. Several mitigating risk strategies were implemented in routine cancer care from 13th March onward. Non-urgent visits such as follow-ups were postponed or replaced by teleconsultation [10]. All oncologic systemic treatments were continued and administered on an outpatient basis when possible. A system for home monitoring for symptoms and treatment-related side effects (AMTRA, Remedus) and home blood sampling (BAPIC) was routinely offered to all patients [[10], [11], [12]]. Both BAPIC and AMTRA were used for systematic toxicity and SARS-CoV-2 symptom registration [11,12]. Visitation regulations were restrictive, and social distancing was practiced as much as possible [11]. From 31st March onward, mask wearing was obligatory for patients and health care providers. Additional throat washing for PCR testing against SARS-CoV-2 every fortnight was implemented from 14th April onward.
2.6. Accrual of clinical data
For the patients in the Antwerp University Hospital the information technology (IT) department used data mining of the electronic patient files of the hospital to register the following data for all cancer patients: COVID-19 PCR testing (positive/negative), type of cancer, stage of cancer (TNM), metastatic cancer (yes/no), relevant comorbidity disease (a. Diabetes, b. Hypertension, c. Inflammatory disorders, d. Other), type of oncology treatment (a. Chemotherapy, b. Endocrine therapy, c. Targeted therapy, d. Immunotherapy, e. Radiotherapy, f. Combination of above, g. No current treatment), World Health Organisation performance status, Body Mass Index, tumour marker (if available), CRP (as a marker of inflammation if available), white blood cell count (if available), lactate dehydrogenase (LDH), ferritin (as a marker of fibrosis and inflammation if available). The co-investigators in AZ Middelares accrued these data according to their local IT and confidentiality standards. Volunteering HCW were asked at the moment of the last blood sampling whether they had been absent during the last 3 months, whether they have attracted clinical COVID-19 and optional relevant concomitant disease.
2.7. Detection of SARS-CoV-2 RNA by RT-PCR
The Cobas SARs-CoV-2 test (Roche) was performed on an automated Cobas 6800 system. Classically taken nose/throat samples were used as the gold standard for an RT-PCR diagnosis of a SARS-CoV-2 infection. In 130 patients, additional mouthwashes were collected for RT-PCR.
2.8. SARS-CoV-2 immunoglobin G/total Ig antibody testing
The following tests were used: LIAISON® SARS-CoV-2 S1/S2 IgG test (DiaSorin, Saluggia, Italy) is a fully automated two-step chemiluminescent immunoassay to qualitatively detect IgG antibodies against the S1/S2 protein of SARS-Cov-2 [34]. Alinity SARS-CoV-2 IgG (Abbott, Chicago, IL, USA) is a high throughput immunoassay directed to IgG antibodies reactive against the nucleocapsid antigen of SARS-CoV-2, using a two-step sandwich immunoassay employing microparticle-bound antigen and acridinium-labelled human anti-IgG [35]. The total SARS-CoV-2 antibody test Elecsys® Anti-SARS-CoV-2 (Roche, Basel, Switzerland) is an immunoassay for the in vitro qualitative detection of antibodies (IgA, IgM and IgG) to SARS-CoV-2 in human serum and plasma on COBAS E immunoassay analyzers. It detects antibodies (isotype agnostic) reactive against SARS-CoV-2 nucleocapsid protein using a double-antigen sandwich immunoassay design employing ruthenium-labelled and biotin-labelled antigen [35]. Tests were validated internally before starting the study. As these were commercial tests, the threshold of the manufacturers was applied to consider a test positive (Abbott≥1.4, Roche≥1, DiaSorin >15 AU/ml). The collected blood samples were tested as a batch.
2.9. Data analysis
Data were stored in a main database and cleaned and validated manually. Descriptive statistics were reported per subgroup (pediatric patients, cancer, non-cancer patients and health care workers). Proportions of positive and negative tests were reported overall and per subgroup. As patients could have several tests at different dates, it was decided to declare a patient positive if at least one positive result was found on one of these dates. A patient was negative if test results on all dates were negative. Proportions were compared between subgroups using Fisher’s exact test. For the continuous test outcomes, a Kruskal–Wallis test (repeated measurements per subject were averaged) was performed. If there was an overall effect of group, two by two comparisons are made. For the analysis comparing antibody to PCR tests, we only considered the cancer patients and the volunteers as the other groups are too small. We defined day 0 as the day of the first positive PCR test, if available. In case of no positive PCR tests, day 0 is the day of the first PCR test. In the case of several positive PCR tests, day 0 is the day of the first PCR test. For the antibody tests only those that were taken at least 7 days after day 0 were considered. Tests were compared binary using a cross table, kappa and McNemar test, and continuously using a Spearman correlation. The analysis was done in R 3.5.2.
2.10. Ethical aspects
Informal authorisation to start the sample collecting after written informed consent was already given on 19th March 2020 by representatives of the Ethics Committee (CME) of Antwerp University Hospital, and the study was formally accepted by the CME of the Antwerp University Hospital on 30th March 2020 (EC number 20/13/156, internal EDGE 001070). The consent forms and results of the blood tests were handled under strict medical confidentiality according to the local regulations (‘Richtlijn tot bescherming van individuen betreffende het verwerken van persoonlijke gegevens’) and the European General Data Protection Regulation (EU2016/679) and its adaptation in the Belgian Law.
3. Results
3.1. Population characteristics
The dataset contained 3650 samples in 1115 different subjects: 13 children, 908 cancer and 100 non-cancer patients, and 94 HCW. Three hundred seventy-nine of the cancer patients (41.7%) had a hematological tumour, the others solid cancer (breast N = 163, lung N = 42, prostate cancer N = 29 being the most frequent tumour types). Six children had a malignant hematological cancer and 7 had a solid tumour. The HCW contained 91 non-oncological subjects, 2 oncological patients and 1 individual with a malignant hematological tumour. We considered the latter three in the cancer patient group. Details on the population characteristics are given in Table 1 . Cancer patients were older than the other groups (mean age 62.9 years versus non-cancer 54.4, children 9.6, and health care workers 40.1; Kruskal Wallis test: p < 0.0001). Four hundred and thirty cancer patients had advanced or metastatic disease. Cancer and non-cancer patients were more frequently diagnosed with hypertension, diabetes, other metabolic disease and cardiovascular disease compared to children and health care workers (Fisher’s exact test: p < 0.05). The cancer patients had recent (current or < 6 months) chemotherapy, endocrine treatment, radiotherapy, immunotherapy and targeted treatment in respectively 447 (49.2%), 59 (6.5%), 139 (15.3%), 144 (15.8%) and 348 (38.3%) of cases. Clinical COVID-19 severity according to World Organisation criteria was respectively mild in 6 (19.4%), moderate in 2 (6.5%), severe in 8 (25.8%) and critical in 15 (48.4%) of the cancer patients. One adult non-cancer patient had critical COVID-19, while 8 of the HCW (88.9%) had mild disease (11.1%) and 1 severe disease. In our population, 25 patients died, all cancer patients (2.8%) with advanced solid and/or hematological tumours.
Table 1.
Population characteristics: descriptive statistics per type of subjects.
| Cancer patients |
Non-cancer patients |
Children |
Health care workers |
|||||
|---|---|---|---|---|---|---|---|---|
| Total | n (%) or mean (SD) | Total | n (%) or mean (SD) | Total | n (%) or mean (SD) | Total | n (%) or mean (SD) | |
| Male gender | 908 | 507 (55.8) | 100 | 50 (50) | 13 | 2 (15.4) | 94 | 82 (87.2) |
| Age mean (SD) | 908 | 62.9 (13.1) | 100 | 54.4 (17.6) | 13 | 9.6 (6) | 94 | 40.1 (11.1) |
| Age median (IQR) | 908 | 64 (16) | 100 | 56 (28) | 13 | 9 (10) | 94 | 40 (18) |
| BCRP mean (SD)∗ | 906 | 18.8 (49.2) | 94 | 9.9 (24.3) | 13 | 17.5 (22.6) | 66 | 6 (5.7) |
| BCRP median (IQR) | 906 | 3.9 (5) | 94 | 3.9 (0.4) | 13 | 5.4 (21.7) | 66 | 3.9 (3.2) |
| CHOL mean (SD) | 467 | 180.9 (52.6) | 40 | 176.3 (44.9) | 2 | 105 (12.7) | 40 | 183.5 (35.8) |
| CHOL median (IQR) | 467 | 179 (68.5) | 40 | 167.5 (51) | 2 | 105 (9) | 40 | 176 (45.2) |
| BMI mean (SD) | 842 | 26 (5) | 72 | 27.6 (6.1) | 13 | 17.4 (2.4) | 28 | 23.8 (3.9) |
| BMI median (IQR) | 842 | 25.5 (6.3) | 72 | 26.2 (6) | 13 | 17.6 (3) | 28 | 22.9 (5.2) |
| Blood group | 570 | A 253 (44.4%) | 47 | A 16 (34.0%) | 11 | A 4 (36.3%) | 10 | A 8 (80%) |
| AB 13 (2.3%) | AB 0 (0%) | AB 0 (0%) | AB 0 (0%) | |||||
| B 47 (8.2%) | B 4 (8.5%) | B 0 (0%) | B 0 (0%) | |||||
| O 257 (45.1%) | O 27 (57.4%) | O 7 (63.6%) | O 2 (20%) | |||||
| WHO = 0 | 352 | 145 (41.2) | 8 | 5 (62.5) | NA | 2 | 2 (100) | |
| WHO = 1 | 352 | 180 (51.1) | 8 | 3 (37.5) | NA | 2 | 0 (0) | |
| WHO = 2 | 352 | 21 (6) | 8 | 0 (0) | NA | 2 | 0 (0) | |
| WHO = 3 | 352 | 6 (1.7) | 8 | 0 (0) | NA | 2 | 0 (0) | |
| Sleep apnea | 908 | 38 (4.2) | 100 | 8 (8) | 13 | 0 (0) | 94 | 1 (1.1) |
| Cardiovascular disease | 908 | 226 (24.9) | 100 | 27 (27) | 13 | 0 (0) | 94 | 4 (4.3) |
| Thromboembolic disease | 908 | 64 (7) | 100 | 5 (5) | 13 | 0 (0) | 94 | 1 (1.1) |
| Renal disease | 908 | 56 (6.2) | 100 | 8 (8) | 13 | 0 (0) | 94 | 1 (1.1) |
| Pulmonary disease | 908 | 128 (14.1) | 100 | 12 (12) | 13 | 2 (15.4) | 94 | 7 (7.4) |
| Diabetes | 908 | 82 (9) | 100 | 10 (10) | 13 | 0 (0) | 94 | 0 (0) |
| Metabolic disease | 908 | 163 (18) | 100 | 17 (17) | 13 | 0 (0) | 94 | 7 (7.4) |
| Hypertension | 908 | 208 (22.9) | 100 | 17 (17) | 13 | 0 (0) | 94 | 3 (3.2) |
| Infection | 908 | 155 (17.1) | 100 | 17 (17) | 13 | 4 (30.8) | 94 | 6 (6.4) |
| Allergic constitution | 908 | 44 (4.8) | 100 | 12 (12) | 13 | 0 (0) | 94 | 4 (4.3) |
| Hematologic disease | 908 | 378 (41.6) | 100 | 38 (38) | 13 | 6 (46.2) | 94 | 0 (0) |
| Gastrointestinal disease | 908 | 137 (15.1) | 100 | 19 (19) | 13 | 0 (0) | 94 | 7 (7.4) |
| Autoimmune disorder | 908 | 63 (6.9) | 100 | 16 (16) | 13 | 0 (0) | 94 | 4 (4.3) |
| Recent chemotherapy | 908 | 447 (49.2) | 100 | 5 (5) | 13 | 8 (61.5) | 94 | 0 (0) |
| Recent targeted therapy | 908 | 348 (38.3) | 100 | 10 (10) | 13 | 0 (0) | 94 | 0 (0) |
| Antihormonal treatment | 908 | 59 (6.5) | 100 | 0 (0) | 13 | 0 (0) | 94 | 0 (0) |
| Hereditary cancer | 908 | 4 (0.4) | 100 | 1 (1) | 13 | 0 (0) | 94 | 0 (0) |
| Transplant | 908 | 80 (8.8) | 100 | 3 (3) | 13 | 0 (0) | 94 | 0 (0) |
| Recent vaccination | 908 | 12 (1.3) | 100 | 1 (1) | 13 | 0 (0) | 94 | 1 (1.1) |
| Radiotherapy | 908 | 139 (15.3) | 100 | 0 (0) | 13 | 2 (15.4) | 94 | 0 (0) |
| Epileptic and neurologic disorder | 908 | 27 (3) | 100 | 7 (7) | 13 | 1 (7.7) | 94 | 1 (1.1) |
| Bone | 908 | 19 (2.1) | 100 | 3 (3) | 13 | 0 (0) | 94 | 0 (0) |
| Death during first wave COVID19 | 908 | 25 (2.8) | 100 | 0 (0) | 13 | 0 (0) | 94 | 0 (0) |
∗Cancer patients: BCRP<2.9 for 2 subjects, <4.0 for 590 subjects.
∗Non-cancer patients: BCRP<4.0 for 69 subjects.
∗Children: BCRP<4.0 for 5 subjects.
∗Health care workers: BCRP<2.9 for 21 subjects and <4.0 for 22 subjects.
Remark: For baseline C-reactive protein (BCRP), there were categories <2.9 and < 4.0 and they were recoded as 2.8 and 3.9 to make a mean. CHOL: cholesterol.
3.2. SARS-CoV-2 IgG/total antibody and PCR test results in the various populations
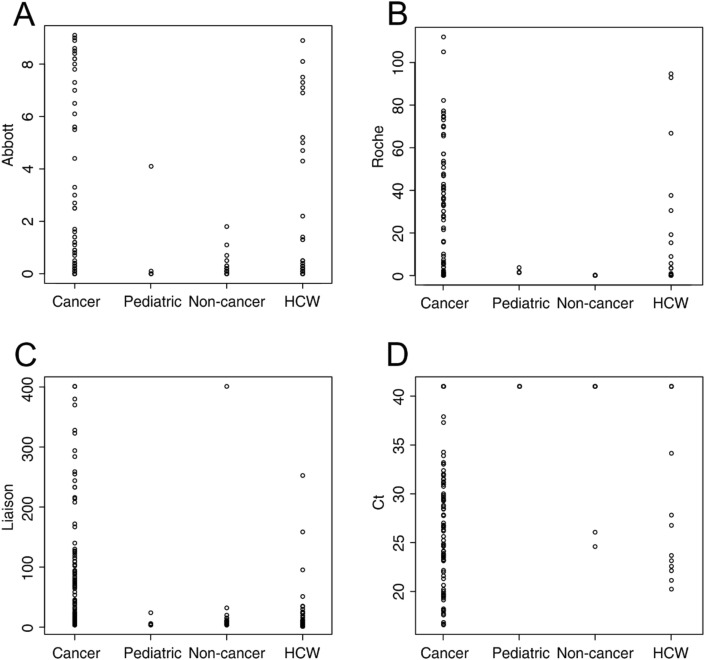
Test results for the entire population and different subgroups are given in Table 2 and in Fig. 1 . Comparing the cancer patients to the HCW (Fisher’s exact test) there was a significant difference for A (p < 0.0001), R (p = 0.007), D (p < 0.0001) SARS-CoV-2 antibody tests and RT-PCR (p < 0.0001). Non-cancer patients had lower rates of seroconversion compared to cancer patients and HCW for A and R (p < 0.043), a lower frequency of confirmatory positive RT-PCR (p < 0.0001).
Table 2.
IgG/total SARS-CoV-2 antibody and RT-PCR test results for the cancer, non-cancer, pediatric oncology patients and health care workers.
| Abbott | Roche | DiaSorin | PCRb | ||
|---|---|---|---|---|---|
| Overall | Total | 998 | 1010 | 1020 | 618 |
| Negative | 966 (96.8%) | 973 (96.3%) | 942 (92.4%) | 572 (92.6%) | |
| Positive | 32 (3.2%) | 37 (3.7%) | 68 (6.7%) | 46 (7.4%) | |
| Equivocala | 10 (1.0%) | ||||
| Adult cancer patients (Cancer) | Total | 805 | 820 | 827 | 530 |
| Negative | 787 (97.8%) | 795 (97.0%) | 768 (92.9%) | 494 (93.2%) | |
| Positive | 18 (2.2%) | 25 (3.0%) | 51 (6.2%) | 36 (6.8%) | |
| Equivocala | 8 (1.0%) | ||||
| Median value | 0 | 0.071 | 4.3 | 41 | |
| IQR | [0,0] | [0.070,0.073] | [3.7,5.9] | [40] | |
| Pediatric oncology patients | Total | 11 | 11 | 11 | 13 |
| Negative | 10 (90.9%) | 10 (90.9%) | 10 (90.9%) | 13 (100%) | |
| Positive | 1 (9.1%) | 1 (9.1%) | 1 (9.1%) | 0 (0%) | |
| Equivocal | 0 | ||||
| Adult non-cancer patients (Non-Cancer) | Total | 90 | 87 | 90 | 43 |
| Negative | 89 (98.9%) | 87 (100%) | 85 (94.4%) | 42 (97.7%) | |
| Positive | 1 (1.1%) | 0 (0%) | 5 (5.6%) | 1 (2.3%) | |
| Equivocala | 0 | ||||
| Median value | 0 | 0.072 | 4.8 | 41 | |
| IQR | [0,0] | [0.070, 0.074] | [3.7,6.3] | [41, 41] | |
| Health care workers (HCW) | Total | 92 | 92 | 92 | 32 |
| Negative | 80 (87.0%) | 81 (88.0%) | 79 (85.9%) | 23 (71.9%) | |
| Positive | 12 (13.0%) | 11 (12.0%) | 11 (12.0%) | 9 (28.1%) | |
| Equivocala | 2 (2.2%) | ||||
| Median value | 0 | 0.072 | 5.2 | 41 | |
| IQR | [0,0] | [0.070,0.077] | [4.3,7.4] | [32.6, 41] | |
| Statistics | |||||
| Overall p-value∗∗ | <0.0001 | 0.007 | <0.0001 | <0.0001 | |
| Cancer versus Non-Cancer | 0.038 | 0.035 | |||
| Cancer vs HCW | 0.0001 | 0.007 | <0.0001 | <0.0001 | |
| Non-Cancer versus HCW | 0.017 | 0.043 | <0.0001 | ||
IQR: interquartile range.
∗∗: Fisher’s exact test.
For the Liaison test a value of 12–15 AU/ml was considered equivocal.
Antibody testing and PCR testing were not done consistently simultaneously as, during the first wave, test strategies changed several times, driven by the availability of test-kits. Therefore PCR results should not be compared with IgG test results.
Fig. 1.
Dotplots of IgG/total Ig SARS-CoV-2 antibody levels (A–C) and polymerase chain reaction Ct levels (D) in cancer, non-cancer and pediatric patients and voluntary health care workers.
3.3. Correlations between the different SARS-CoV-2 IgG/total antibody tests
Correlation between the absolute values of the different SARS-CoV-2 IgG/total antibody tests is given in Table 3 . In HCW, the three tests correlated moderately well (kappa 0.39–0.53), while for the pediatric patients, only a correlation could be found between A and R (kappa 0.68). In cancer and non-cancer patients, a correlation between the three serological tests was absent or poor (kappa −0.18 to 17.0). Dichotomising results in positive versus negative, the A and R test correlated well (kappa 0.82, difference not significant p McNemar test = 0.344), while it remained poor comparing A and D, and R and D (respectively kappa 0.49 and 0.57; result significantly different p McNemar test = <0.0001 for both) (Supplementary Table S1 and Fig. 2).
Table 3.
Correlations between the absolute values of the different SARS-CoV-2 IgG/total antibody tests.
| Group | Tests | Correlation | n |
|---|---|---|---|
| Overall | A versus R | 0.21 | 991 |
| A versus D | 0.2 | 996 | |
| R versus D | 0.16 | 1007 | |
| Cancer patients | A versus R | 0.17 | 801 |
| A versus D | 0.13 | 804 | |
| R versus D | 0.15 | 818 | |
| Pediatric cancer patients | A versus R | 0.68 | 11 |
| A versus D | 0.39 | 11 | |
| R versus D | 0.22 | 11 | |
| Non-cancer patients | A versus R | 0.01 | 87 |
| A versus D | 0.16 | 89 | |
| R versus D | −0.08 | 86 | |
| Health care workers | A versus R | 0.48 | 92 |
| A versus D | 0.53 | 92 | |
| R versus D | 0.39 | 92 |
The Alinity® (A; Abbott) and Liaison® (D; DiaSorin) commercially available assays were used to measure SARS-CoV-2 IgG, while total SARS-CoV-2 Ig was measured by Elecsys® (R; Roche).
Fig. 2.
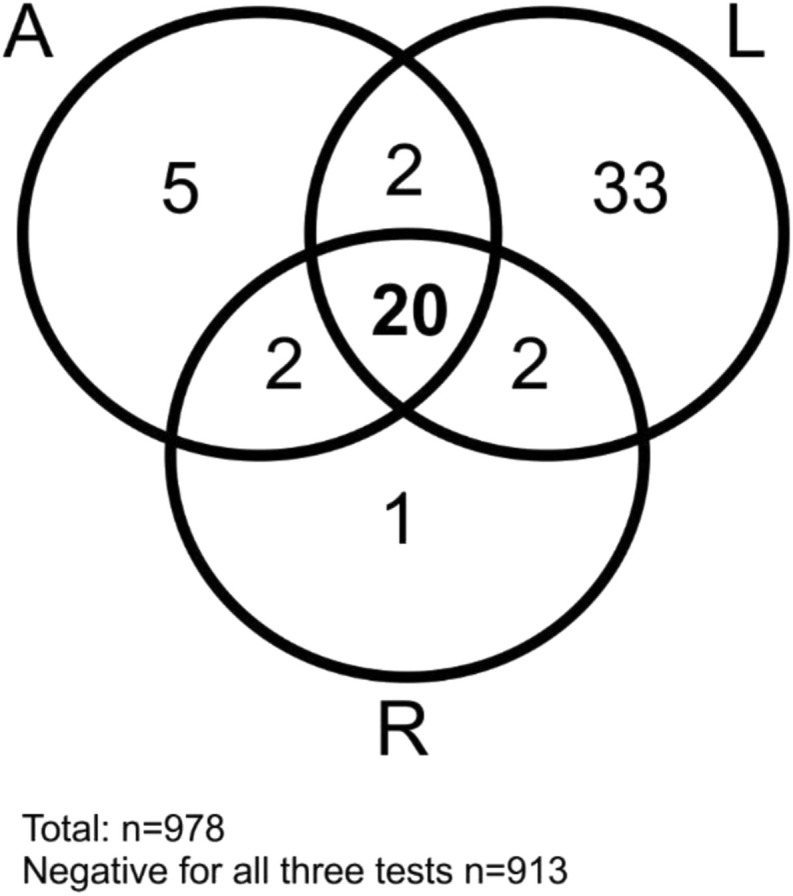
Summary of the concordance between the positive IgG/total Ig SARS-CoV-2 antibody test results in the entire population according to the Abbott, DiaSorin and Roche tests.
3.4. Detection of IgG IgG/total SARS-CoV-2 antibodies in subjects with positive RT-PCR test
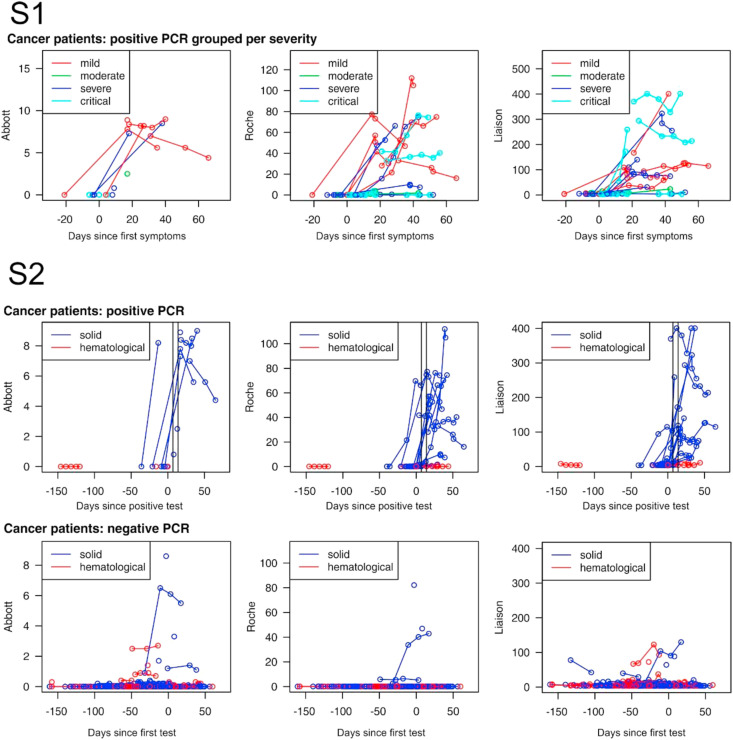
For the analysis, we only considered the cancer patients and the HCW as the other groups are too small. There is a significant correlation between the absolute antibody levels amongst the various serological tests (p-value <0.0001) for each of the comparisons (Fisher’s exact test). Seroconversion according to the A, R and D SARS-CoV-2 IgG/total antibody test at least 7 days after positive RT-PCR for SARS-CoV-2 was seen in respectively 7/9 (77%), 7/8 (87%), 6/8 (75%) of the HCWs, and 7/8 (87%), 17/22 (77%) and 7/8 (87%) of the cancer patients (all differences not significant). These were all detected in patients with solid tumours as no (0/4) seroconversions were seen in patients with hematological tumours Fig. 3 . The evolution of the repeated measurements split up for positive and negative PCR and severity of COVID-19 symptoms are, respectively, depicted in Fig. 3. Antibody levels according to the first date of COVID19 symptoms are given in Supplementary Fig. 1. Comparing the absolute antibody levels of the different Ig tests for the entire group of cancer patients or the cancer patients with solid tumours with HCWs at least 7 days after PCR positivity did not show any differences (Table 4 ). However, antibody levels were significantly lower in patients with hematological tumours compared to solid tumours (Mann–Whitney U test R: p = 0.003, L: p = 0.008) or HCW (Mann–Whitney U test all p < 0.0001) (Supplementary Fig. 2).
Fig. 3.
Evolution of the repeated SARS-CoV-2 IgG and total Ig antibody measurements split up for positive and negative RT-PCR at least 7 days earlier, and severity of COVID symptoms (S1) and hematological versus solid tumours (S2).
Table 4.
Absolute SARS-CoV-2 IgG/total antibody levels in the cancer patients, patients with hematological cancer, patients with solid cancer and voluntary health care workers.
| Abbott | Roche | DiaSorin | ||
|---|---|---|---|---|
| Cancer patients | Median | 7 | 16.64 | 74.02 |
| IQR | 3.6 | 51.9 | 126.3 | |
| n | 8 | 22 | 24 | |
| Solid cancer | Median | 7 | 37.6 | 89.4 |
| IQR | 3.6 | 56.2 | 158.5 | |
| n | 8 | 18 | 20 | |
| Hematological cancer (Hema) | Median | 0.1 | 5.2 | |
| IQR | 0.3 | 4.1 | ||
| n | 0 | 4 | 4 | |
| Health care workers (HCW) | Median | 4.7 | 7.3 | 26.6 |
| IQR | 4.9 | 36.1 | 27.0 | |
| n | 9 | 8 | 8 | |
| Statistics | ||||
| p-valuea Solid vs Hema | 0.003 | 0.008 | ||
| p-valuea Cancer versus VHW | 0.287 | 0.758 | 0.424 | |
Mann Whitney U test, n = number, IQR: interquartile range.
4. Discussion
It remains an unanswered question how accurate serologic tests for SARS-CoV-2 are in different populations and how they can be used in clinical practice to benefit the patients [9,[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33]]. The majority of commercially available tests against SARS-CoV-2 IgG are directed to recombinant antigens covering the viral nucleocapsid protein (Abbott, Roche) or the S1/S2 domain of the spike protein (DiaSorin) [9]. They have been developed rapidly over the last half-year and were often introduced in clinical settings before being validated with samples in larger cohorts [9,[13], [14], [15], [16], [17], [18],[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]]. In a major effort, the National SARS-CoV-2 Serology Assay Evaluation Group performed a head-to-head assessment of SARS-CoV-2 IgG assay (Abbott, Chicago, IL, USA), LIAISON SARS-CoV-2 S1/S2 IgG assay (DiaSorin, Saluggia, Italy), Elecsys Anti-SARS-CoV-2 assay (Roche, Basel, Switzerland), SARS-CoV-2 Total assay (Siemens, Munich, Germany), and a novel 384-well ELISA (the Oxford immunoassay) [22]. They derived sensitivity and specificity from 976 pre-pandemic blood samples and 536 blood samples from patients with laboratory-confirmed SARS-CoV-2 infection, collected at least 20 days post symptom onset (collected between 1st Feb 2020, and May 31st, 2020). All assays achieved a sensitivity of at least 98% with thresholds optimised to achieve a specificity of at least 98% on samples taken 30 days or more post symptom onset. In a large study by Perkman et al. comparing the Abbott, Roche and DiaSorin tests, similar specificities were found, but in contrast to the manufacturer’s specifications, sensitivities only ranged from 83.1 to 89.2% [36]. It was shown in this study that at low seroprevalences, the minor differences in specificity resulted in profound discrepancies of positive predictive values: eg. at 1% seroprevalence: 52.3% (36.2–67.9), 77.6% (52.8–91.5), and 32.6% (23.6–43.1) for Abbott, Roche and DiaSorin, respectively. As in our study, a good level of agreement was found between the Roche and Abbott tests, but significant differences were noted with the DiaSorin test. These discrepancies may be partly explained by individual differences in the immune response against SARS-CoV-2 depending on the antigenic target: the S1/S2 antigen for DiaSorin versus the N antigen for the Roche and Abbott tests [34,35]. This may affect both the time frame and the extent of the antibody response. The Roche and Abbott tests probably have a high rate of similar epitope recognition. It has been hypothesised that the majority of produced antibodies are against the most abundant protein of a virus, which for SARS-CoV-2 is the N protein [9]. Boukli et al. reported a high incidence (10%) of false-positive results with the DiaSorin test, particularly in patients suffering from acute infectious conditions, such as Epstein–Barr virus (EBV) or hepatitis B virus (HBV) infections [37]. It is suggested that they result from nonspecific immune activation rather than cross-reactivity between non-SARS-CoV-2 antibodies and the SARS-CoV-2 proteins used in the assay. Strikingly a moderate correlation was found between the three serologic tests in the HCW, while it was very poor or absent in the other populations in our study. This deserves further attention as it remains unclear whether this is a true phenomenon induced by a certain disease or treatment-related factors, or just a statistical coincidence.
Estimates about seroprevalence of SARS-CoV-2 in cancer populations strongly depend on the type of serologic test used, the population tested (ambulatory versus hospitalised) and timing of testing, particularly related to the extent of the COVID-19 pandemic in the local general population [6]. A summary of studies performed in cancer populations is given in Table 5 . Immunoglobulin G/total antibodies directed to SARS-CoV-2 during the first wave of the SARS-CoV-2 pandemic were low in our cancer population and not above levels noted in the Belgian population in the same time interval (3.1–6.9%) [38]. A survey in our units showed that cancer patients protected themselves very well against SARS-CoV-2 by social distancing, isolation, wearing mouth masks and hand hygiene (unpublished data). This adds to the measures that were taken in our hospitals to provide cancer care as safe as possible [6,[10], [11], [12]]. The prevalence of SARS-CoV-2 antibodies among HCWs, which are presumed at higher risk for infection, has been increasingly investigated [39]. In a meta-analysis of 49 studies, including 127,480 health care workers overall seroprevalence of SARS-CoV-2 antibodies was 8.7% (95% CI: 6.7–10.9%) [40]. Major factors driving seropositivity are the type of hospital setting assessed, the job duties and patient contacts of the HCW, and the intensity of the COVID-19 pandemic in the area [41]. In a nationwide Scottish linkage cohorts study comprising 158,445 health care workers and 229,905 household members it was shown that health care workers facing patients and their household members respectively have a threefold and twofold increased risk of admission with serious COVID-19 [28]. Small studies in oncology units showed seroconversion rates of 5–7.5% amongst staff members [26,27]. Health care professionals had a significantly higher prevalence of SARS-CoV-2 antibodies, and RT-PCR confirmed SARS-CoV-2 infection compared to patients in our population. This can be explained by the lack of availability and suboptimal use of protective material until end-March 2020. We had an outbreak of COVID-19 in the oncology unit of the Antwerp University Hospital, affecting some patients, medical and nursing staff before masks and protective clothing became generally available and obligatory to be used. This was managed successfully by closing the unit for a week, testing all patients and health care professionals for SARS-CoV-2 by PCR, sending all asymptomatic staff on leave for a week and SARS-CoV-2 confirmed positive individuals for 2 weeks, isolating the involved patients and segregating pathways [10,12]. In fact, we did not have any problems afterwards; however, this highlights the importance of protective measures in this population.
Table 5.
Main characteristics of studies assessing SARS-CoV-2 IgG seropositivity in cancer patients and health care workers (HCW) in oncological units.
| First author | Region | Period | N | Antibody test | Seropositivity |
|
|---|---|---|---|---|---|---|
| Patients | HCW | |||||
| Cabezon-Gutierrez et al. | Torejon (Spain) | 1/6–18/6/2020 | 229/0 | Testsealabs® IgG/IgM | 29.0% | NA |
| Fuederer et al. | Vienna (Austria) | 21/3–4/6/2020 | 84/64 | Roche Elecsys®total Ig | 3.2% | 3.6% |
| Mara et al. | Italy | 30/3–11/5/2020 | 61/105 | Testsealabs® IgG/IgM | 87.9%a | 80.5%a |
| Van Dam et al. | Belgium | 24/3–31/5/2020 | 908/100 | Abbot | 2.2% | 13.0% |
| DiaSorin | 6.2% | 12.0% | ||||
| Roche | 3.0% | 12.0% | ||||
NA:not available.
After confirmed PCR.
It remains controversial whether (some) cancer patients have a clinically important degree of immunosuppression related to their disease or treatment and have an adequate (IgG) immune response after a SARS-CoV-2 infection [6]. This is crucial for vaccine development and timing of vaccination in relation to oncologic treatment. Several small series have suggested that cancer patients had lower rates of seroconversion after clinical COVID-19 compared to non-cancer patients [[29], [30], [31], [32]], but this could not be confirmed by others [33]. In the study of Mara et al., specifically focusing on SARS-CoV-2 seroconversion in cancer patients and oncology health care workers, no difference in SARS-CoV-2 IgG positivity was observed between both groups using 2019-nCoV IgG/IgM Rapid Test Cassette: respectively 25/29 (87.9%) versus 25/33 (80.5%; p = 0.39) [25]. In addition, no differences in the time from SARS-CoV-2 diagnosis to IgG detection were seen comparing cancer patients with health care workers (23.0 versus 28.0 days, p = 0.21). Recent evidence has raised some new insights on the variable immune response of cancer patients. In a detailed translational study on 41 cancer (23 solid, 18 hematological) patients, Abdul Jawad et al. showed that SARS-CoV-2 exposed cancer patients with solid tumours develop immune response signatures similar to those of non-cancer patients [42]. However, this is not the case for patients with hematological malignancies that display heterogeneous humoral responses, an exhausted T-cell phenotype and a high prevalence of prolonged viral shedding. COVID-19 positive hematological cancer patients showed three phenotypes: (1) patients failing to mount an antibody response with prolonged viral shedding (2) patients mounting an antibody response but failing to clear the virus, and (3) those able to mount an antibody response and successfully clear virus. Although numbers are low, these observations were confirmed in our study. Patients with solid tumours had an antibody response that was comparable to that of healthy volunteers, while patients with hematological malignancies did not mount an antibody response in our population (Fig. 3). These patients were B-cell depleted or had a history of an autologous bone marrow transplant. In fact, at least 2 of these patients did not manage to clear the SARS-CoV-2 and had several episodes of hospital admission because of disease exacerbation. Hematological cancer patients often suffer from complex immunological dysregulation that can persist life-long. This probably explains their long-term increased risk of being hospitalised and death due to COVID-19 [7]. The effects of oncological treatment on antibody response and effectivity of vaccination against SARS-CoV-2 needs urgent prospective studies, particularly in B-Cell depleted patients on rituximab, autologous transplant patients and patients with extensive metastatic end-stage disease. Patients with solid and hematological malignancies should be regarded as different entities in future studies.
In our opinion, SARS-CoV-2 antibody testing has no added value in the management of cancer patients during the COVID-19 pandemic. Correlation between different commercial antibody tests is low, and the presence or absence of SARS-CoV-2 antibodies gives little information on the protection of patients against COVID-19. Our study highlights the importance of protecting the nursing and medical staff against a SARS-CoV-2 infection. The study has several limitations. It is a cross-sectional study, and blood samples were not taken at fixed time points. Clinical data were gathered retrospectively. Although it is a big study, luckily for the patients, the rate of seroconversion was low limiting the number of events. On the other hand, it is the first large study comparing three commercial tests for SARS-CoV-2 IgG/total Ig in a real-life ambulatory cancer population. More important, samples were not historic left-over samples but were collected prospectively and handled and stored with care, assuring optimal quality of the laboratory analysis. Additional studies looking at the immune response in a more detailed way on this material are planned in the future.
Authors’ contributions
MH and PVD conceptualised the paper. MH, ER and PVD analyzed the data. Drafting and revision of the manuscript was performed by all authors.
Funding
This was work supported by a ‘Kom Op Tegen Kanker’ grant (ref 000100470), a Bijzonder OnderzoeksFonds COVID19-Project grant (project ID: 42839) and a UZA Foundation grant (2020).
Ethical approval
Was formally accepted by the CME of the Antwerp University Hospital on 30th March 2020 (EC number 20/13/156, internal EDGE 001070).
Conflict of interest statement
MP is an advisor of Remedus; none of the other authors has a conflict of interest related to this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2021.02.024.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Fig. 1. Evolution of the repeated SARS-CoV-2 IgG and total Ig antibody measurements according to first date of symptoms befor. Supplementary Fig. 2. Absolute SARS-CoV-2 IgG/total antibody levels in the cancer patients, patients with hematological cancer, patients with solid cancer and voluntary health care workers. ∗Only measurable results were obtained for solid tumors in the Abbott test.
References
- 1.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robilotti E.V., Babady N.E., Mead P.A., Rolling T., Perez-Johnston R., Bernardes M., et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020 doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dam P., Huizing M., Mestach G., Dierckxsens S., Tjalma W., Trinh X.B., et al. SARS-CoV-2 and cancer: are they really partners in crime? Canc Treat Rev. 2020;89:102068. doi: 10.1016/j.ctrv.2020.102068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson E., Walker A.J., Bhaskaran K.J., Bacon S., Bates C., Morton C.E., et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million NHS patients. 2020. Online ahead of print. [DOI]
- 8.Theel E.S., Slev P., Wheeler S., Couturier M.R., Wong S.J., Kadkhoda K. The role of antibody testing for SARS-CoV-2: is there one ? J Clin Microbiol. 2020 Jul 23;58(8) doi: 10.1128/JCM.00797-20. Print 2020 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stromer A., Grobe O., Rose R., Fickenscher H., Lorentz T., Krumbholz A. Diagnostic accuracy of six commercial SAES-Cov-2 IgG/total antibody assays and identification of SARS-Cov-2 neutralizing antibodies in convalescent sera. MedRxiv. 2020 doi: 10.1101/2020.06.15.20131672. [DOI] [Google Scholar]
- 10.Peeters M., van Dam P., Rasschaert M.A., Vulsteke C., De Keersmaecker S., Croes L., et al. Prescreening for COVID-19 in patients receiving cancer treatment using a patient-reported outcome platform. ESMO Open. 2020;5(3) doi: 10.1136/esmoopen-2020-000817. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasschaert M., Vanclooster P.J., Mertens T., Roelant E., Lesage K., Prenen H., et al. The tele-transition of toxicity management in routine oncology care during the SARS-CoV-2 pandemic. Br J Canc. 2021 doi: 10.1038/s41416-020-01235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasschaert M., Vulsteke C., De Keersmaeker S., et al. AMTRA: a multicentered experience of a web-based monitoring and tailored toxicity management system for cancer patients. Support Care Canc. 2020 doi: 10.1007/s00520-020-05550-6. [DOI] [PubMed] [Google Scholar]
- 13.Cabezon-Gutierres L., Custodio-Cabello S., Palka-Kotolowska M., Oliveros-Acebes E., Garcia-Navarro M.J., Kosravi-Shahi P. Seroprevalence of SARs-CoV-2-specific antibodies in cancer outpatients in Madid (Spain): a single center, prospective cohort study and review of available data. Canc Treat Rev. 2020;90:102102. doi: 10.1016/j.ctrv.2020.102102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kontou P.I., Braliou G.G., Dimou N.O.L., Nikopulos G., Bagos P.G. Antibody tests in detecting SARS-CoV-2 infection: a metaanalysis. Diagnostics. 2020;10:319. doi: 10.3390/diagnostics10050319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsueh P.R., Huang L.M., Chen P.J., Kao C.L., Yang P.C. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect. 2004;10(12):1062–1066. doi: 10.1111/j.1469-0691.2004.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang F., Quan Y., Xin Z.T., Wrammert J., Ma M.J., Lv H., et al. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186:7264–7268. doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
- 17.Kohmer N., Westhaus S., Rühl C., Ciesek S., Rabenau R.F. Clinical performance of different SARS-CoV-2 IgG antibody tests. J Med Virol. 2020 doi: 10.1002/jmv.26145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAndrews K.M., Adowlatshahi D.P., Dai J., Becker L.M., Hensel J., Snowdon L.M., et al. Heterogenous antibodies against SARS-CoV-2 spike receptor binding domain and nucleocapsid with implications for COV-19 immunity. JCI Insight. 2020;5(18) doi: 10.1172/jci.insight.14386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau C.S., Oh H.M., Hoo S.P., Liang Y.L., Phua S.K., Aw T.C. Performance of an automated chemiluminescence SARS-CoV-2 IgG assay. Clin Chim Acta. 2020;510:760–766. doi: 10.1016/j.cca.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan C.C., Parker K., Tesic V., Baldwin A., Tang N.Y., van Wijk X.M., et al. Analytical and clinical evaluation of the automated Elecsys anti-SARS-Cov-2 antibody assay on the Roche cobas e602 analyzer. Am J Clin Pathol. 2020:2020. doi: 10.1093/ajcp/aqaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau C.S., Hoo S.P., Yew S.F., Ong S.K., Lum L.T., Heng P.Y., et al. MedRxiv; 2020. Evaluation of the Roche Elecsys anti-SARS-CoV-2 assay. [Google Scholar]
- 22.The National SARS-CoV-2 Serology Assay Evaluation Group The performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet. 2020 doi: 10.1016/S1473-3099(20)30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan S.S., Saw S., Chew K.L., Wang C., Pajarillaga A., Khoo C., et al. Comparative clinical evaluation of the Roch Elecsys and Abbott SARS-CoV-2 serology assays for COVID-19. Arch Pathol Lab Med. 2020 doi: 10.5858/arpa.2020-0499-SA. [DOI] [PubMed] [Google Scholar]
- 24.Fuereder T., Berhoff A.S., Heller G., Haslacher H., Perkmann T., Strassl R., et al. Sars-Cov-2 seroprevalence in oncology health care professionals and patients with cancer at a tertiary care center during the COVID-19 pandemic. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marra A., Generali D., Zagami P., Cervoni V., Gandini S., Venturini S., et al. Seroconversion in patients with cancer and oncology health care workers infected by SARS-CoV-2. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.10.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sikora K., Barwick I., Hamilton C. Serological prevalence of antibodies to SARS-CoV-2 amongst cancer center staff. Medrxiv. 2020 doi: 10.1101/2020.05.16.2099408. [DOI] [Google Scholar]
- 27.Epstude J., Harsch I.A. Seroprevalence of COVID-19 antibodies in the cleaning and oncological staff of a municipal clinic. GMS Hyg Infect Control. 2020;15:Doc18. doi: 10.3205/dgkh000353. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah A.S., Wood R., Gribben C., Caldwell D., Bishop J., Weir A., et al. Risk of hospital admission with coronavirus disease 2019 in health care workers and their households: nationwide linkage cohort study. BMJ. 2020;371:m3582. doi: 10.1136/bmj.m3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solodky M.L., Galvez C., Russias B., Detourbet P., N'Guyen-Bonin V., Herr A.L., et al. Lower detection rates of SARS-COV2 antibodies in cancer patients vs healthcare workers after symptomatic COVID-19. Ann Oncol. 2020;S0923–7534(20) doi: 10.1016/j.annonc.2020.04.475. 39793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hempel L. Rare SARS-CoV-2 antibody development in cancer patients. Mdrix. 2020 doi: 10.21203/rs.3.rs-71560/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roeker L.E., Knorr D.A., Pessin M.S., Ramanathan L.V., Thompson M.C., Leslie L.A., et al. Anti-SARS-CoV-2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;234:3047–3049. doi: 10.1038/s41375-020-01030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T., Zeng G., Tao H., Shi H., COVID-19 in Cancer Patients Research Group. Wang T., et al. Low prevalence of IgG antibodies to SARS-CoV-2 in cancer patients with COVID-19. Int J Canc. 2020:33148. doi: 10.1002/ijc.33148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B., Van Oekelen O., Mouhiedidine T.H., De Valle D.M., Richter J., Cho H.J., et al. A tertiary center experience of multiple myeloma patients with COVD-19: lesson learned and the path forward. J Hematol Oncol. 2020;13:94. doi: 10.1186/s13045-020-00934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonelli F., Sarasini A., Zierold C., Calleri M., Bonetti A., Vismara C., et al. Clinical and analytical performance of an automated serological test that identifies S1/S2 neutralizing IgG in COVID-19 patients semiquantitatively. J Clin Microbiol. 2020 doi: 10.1128/JCM.01224-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harley K., Gunsolus I.L. Comparison of the clinical performances of the Abbott Alinity IgG, Abbott Architect IgM, and Roche Elecsys total SARS-CoV-2 antibody assays. J Clin Microbiol. 2021;59:e02104–e02120. doi: 10.1128/JCM.02104-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkmann T., Perkmann-Nagele N., Breyer M.K., Breyer-Kohansal R., Burghuber O.C., Hartl S., Aletaha D., Sieghart D., Quehenberger P., Marculescu R., Mucher P., Strass R., Wagner O.F., Binder C.J., Haslacher H. Side by side comparison of three fully automated SARS-CoV-2 antibody assays with a focus on specificity. medRxiv preprint. 2021 doi: 10.1101/2020.06.04.20117911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boukli N., Le Mene M., Schnuriger A., Cuervo N.S., Laroche C., Morand-Joubert L., et al. High incidence of false-positive results in patients with acute infections other than COVID-19 by the Liaison SARS-CoV-2 commercial chemiluminescent microparticle immunoassay for detection of IgG anti-SARS- CoV-2 antibodies. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.01352-20. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herzog S., De Bie J., Abrams S., Wouters I., Ekinci E., Patteet L., Coppens A., De Spiegeleer S., Beutels P., Van Damme P., Hens N., Theeten H. Seroprevalence of IgG antibodies against SARS coronavirus 2 in Belgium – a serial prospective cross-sectional nationwide study of residual samples. medRxiv preprint. 2020 doi: 10.1101/2020.06.08.20125179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brant-Zawadzki M., Fridman D., Robinson P.A., Zahn M., Chau C., German R., et al. SARS-CoV-2 antibody prevalence in health care workers: preliminary report of a single center study. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0240006. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galanis P., Vraka I., Fragkou D., Bilali A., Kaitelidpu D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in health care workers: a systematic review and metanalysis. J Hop Infect. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piccoli L., Ferrari P., Piumatti G., Jovic S., Fernadez Rodrigez B., Mele F., et al. Risk assessment and seroprevalence of SARS-CoV-2 infection in healthcare workers of COVID-19 and non-COVID-19 hospitals in southern Switzerland. Lancet Reg Health Eur. 2020 doi: 10.1016/j.lanepe.2020.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdul-Jawad S., Baù L., Alaguthurai T., Del Molino Del Barrio I., Laing A.G., Hayday T.S., et al. Acute immune signatures and their legacies in severe acute respiratory syndrome coronavirus-2 infected cancer patients. Canc Cell. 2020;S1535–6108(21):1–5. doi: 10.1016/j.ccell.2021.01.001. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Evolution of the repeated SARS-CoV-2 IgG and total Ig antibody measurements according to first date of symptoms befor. Supplementary Fig. 2. Absolute SARS-CoV-2 IgG/total antibody levels in the cancer patients, patients with hematological cancer, patients with solid cancer and voluntary health care workers. ∗Only measurable results were obtained for solid tumors in the Abbott test.