Abstract
Background
In view of the potential gravity of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection for patients with cancer, epidemiological data are vital to assess virus circulation among patients and staff of cancer centres. We performed a prospective study to investigate seroprevalence of SARS-CoV-2 antibodies among staff and patients with cancer at a large cancer centre, at the end of the period of first national lockdown in France and to determine factors associated with the risk of SARS-CoV-2 infection.
Methods
After the first lockdown, all medical and non-medical staff, as well as all patients attending the medical oncology department were invited to undergo serological testing for SARS-CoV-2 between 11 May and 30 June 2020. All participants were also invited to complete a questionnaire collecting data about their living and working conditions, and for patients, medical management during lockdown.
Findings
A total of 1,674 subjects (663 staff members, 1011 patients) were included. Seroprevalence was low in both staff (1.8%) and patients (1.7%), despite more features of high risk for severe forms among patients. None of the risk factors tested in our analysis (working or living conditions, comorbidities, management characteristics during lockdown) was found to be statistically associated with seroprevalence in either staff or patients. There was no significant difference in the proportion of symptomatic and asymptomatic subjects between staff and patients. Only fever, loss of smell, and loss of taste were significantly more frequent among seropositive patients, in both staff and patients.
Interpretation
We report very low seroprevalence of antibodies against SARS-CoV-2 in the staff (caregiving and non-caregiving) and patients of a large cancer care centre in which strict hygiene, personal protection, and social distancing measures were implemented.
Keywords: COVID-19, SARS-COV-2, Cancer center, Serology, Serodiagnosis, Antibody, Seroprevalence, Cancer patients, Healthcare workers, Staff
1. Introduction
In December 2019, China [1] and very soon, the rest of the world [2], were faced with an epidemic of coronavirus disease 2019 (COVID-19) caused by a new beta coronavirus, named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [3]. France was among the hardest hit countries in Europe, with more than 200,000 documented cases and more than 30,000 deaths at the end of the first period of national lockdown [4]. To limit the spread of the epidemic, the French government decided to implement a nationwide lockdown, which was in place from 17 March to 11 May 2020. During this period, and even now, French hospitals also took a number of measures to limit the spread of the virus within the hospital, to ensure continuity of care, but at the same time, to protect patients and staff as much as possible. Particular attention was paid from the outset to the most vulnerable patients and to subjects with comorbidities, including patients with cancer (whether on or off treatment). Indeed, these groups initially appeared to be at highest risk of developing the infection [5,6], particularly more severe, not to say fatal forms [[7], [8], [9], [10], [11], [12], [13]]. A retrospective series of 302 patients with cancer with suspected COVID-19 in France found that the death rate at 30 days after diagnosis was high in patients both with and without documented SARS-COV-2 on RT-PCR (Reverse Transcriptase-Polymerase Chain Reaction) [14]. Similarly, a recent prospective, observational study from a network of cancer centres in the United Kingdom reported that most patients with cancer had a mild disease course, but 28% died, suggesting high number of severe forms. The risk factors associated with mortality were age, male sex and comorbidities (hypertension, cardiovascular disease), whereas cytotoxic chemotherapy or other anticancer treatment was not associated with an increased risk of death [15]. Similar data have been published in French patients [16]. National and international recommendations have been issued regarding the optimal management of patients with cancer during the COVID-19 pandemic [[17], [18], [19], [20]]. Yet, while numerous publications have reported the prognosis of patients with cancer hospitalised for SARS-Cov-2 infection, few studies have investigated the incidence of infection in patients and staff of cancer care centres [21].
Moreover, these studies had potential for bias, notably due to the inclusion of a population of patients that is not representative of the wide diversity of cancer patients, or inclusion of only hospitalised patients, who potentially have more severe and advanced disease, and thus, a higher risk of death. Furthermore, in published studies to date, the diagnosis was made by RT-PCR, which is sensitive for the detection of the initial phase of infection, and also practical, notably in terms of conservation and transport of samples [9,22]. Conversely, it is quite possible that many asymptomatic or mildly symptomatic patients may have escaped diagnosis, contributing to the spread of the virus [[23], [24], [25]]. Compared with RT-PCR, serological tests in search of specific anti-SARS-CoV-2 antibodies (IgM and IgG) make it possible to study cumulative prevalence of infection, due to the persistence of circulating antibodies beyond the infectious period in patients who were infected, and even after recovery [23,26,27]. Evaluating the cumulative prevalence of SARS-CoV-2 infection is useful from an epidemiological point of view, to estimate the degree of dissemination of the virus, to highlight pathways of contamination and to study immunity in exposed populations [28,29].
In this cross-sectional study, performed shortly after the end of the first lockdown in France, we investigated seropositivity for SARS-CoV-2 in a large cancer care centre in one of the regions of France that was hardest hit by the epidemic (Burgundy-Franche-Comté, Eastern France) [4]. We aimed to assess seroprevalence in both staff (medical and non-medical) and patients (medical oncology patients) of a single, dedicated cancer centre, with the following objectives: (i) to describe the spread of infection among the different categories of staff and in a large population of patients being managed for cancer; (ii) using dedicated individual questionnaires, we sought to identify factors (professional, social, demographic, medical or related to living conditions during confinement) associated with SARS-CoV-2 infection in both groups; (iii) indirectly, we aimed to evaluate the efficacy of the preventive measures implemented during lockdown in the two groups who had different risks of SARS-CoV-2 infection and different risks of developing severe forms of the disease.
2. Methods
The location of the cancer care centre, epidemiology of SARS-CoV-2 in the region, and safety measures implemented at our institution are described in detail in the Supplemental Appendix.
2.1. Sampling strategy and sample size calculation
All employees, i.e. 860 staff members, were invited to participate in the study. Assuming at positivity rate of around 10% (according to the literature at the time of the study design), a non-response rate of 20%, a sample size of 860 would produce a 2-sided confidence interval (CI) with a precision of 2.3%.
Regarding patients, we chose to include all patients attending our institution from 25 May to 30 June 2020, irrespective of cancer location, stage or treatment. We aimed to include about 1000 patients over the study period. Assuming a lower positivity rate of 5% (due to possibly lower exposure), and a non-response rate of 25%, this would produce a two-sided 95% CI with a precision of 1.6%.
2.2. Study questionnaires
The study was approved by the internal scientific committee of the Georges-Francois Leclerc Cancer Centre, and by its internal Ethics Committee, as well as by a national Ethics Committee (CPP Sud-Ouest et Outremer 1). The questionnaires destined for the staff and those for the patients were developed jointly by a group comprising oncologists, biologists, and epidemiologists from our centre, specifically for the purposes of this study.
For the staff, all employees of the centre received an email on their nominative work email address, providing information about the study and inviting them to participate. Blood tests for staff took place from 11 May 2020 (date of the end of national lockdown) to 25 May 2020.
For the patients, participation was proposed to all patients of the Medical Oncology department (patients seen in consultation, in the outpatient unit and in-patients) from 25 May to 30 June 2020. For all patients, data relating to their cancer and treatment were retrieved from the medical files.
2.3. Blood samples, serological tests and serum bank
All serum samples were analysed in the clinical biology unit of the Georges-Francois Leclerc cancer centre. We measured SARS-CoV-2 total antibodies on the fully-automated cobas e411 analyser (Roche Diagnostics) using the novel Elecsys® Anti-SARS-CoV-2 electrochemiluminescence immunoassay (Roche Diagnostics) for the qualitative detection of SARS-CoV-2 antibodies in human serum and plasma. The IVD CE-marked Elecsys® assay uses a modified double-antigen sandwich immunoassay using recombinant nucleocapsid protein (N), which is geared towards the detection of late, mature, high affinity antibodies independent of the subclass. It is a total SARS-CoV-2 antibody assay (IgA, IgM and IgG) detecting predominantly, but not exclusively, IgG. This test was validated (amongst others) by the French national reference centre on 21 May 2020 [30]. Measurement of anti–SARS-CoV-2 was performed following the manufacturer's instructions. Results are reported as numeric values in the form of a cutoff index (COI; signal sample/cutoff) and as a qualitative result, i.e. non-reactive (COI < 1.0; negative) or reactive (COI ≥ 1.0; positive).
2.4. Statistical analysis
Quantitative variables are described as mean ± standard deviation or median (range), and were dichotomised based on the median or a clinically relevant threshold. Qualitative variables are described as number (percentage). The number of missing data is indicated for each variable. The prevalence of seropositivity is expressed as a percentage with the associated 95% CI. Comparisons between groups were performed using the appropriate parametric or non-parametric tests, according to the number of groups compared and the normality (or not) of distributions in each group. Factors associated with seropositivity among staff members and among patients were investigated using univariate logistic regression. The small number of events precludes multivariate analysis. All analyses were performed by the Methodology & Biostatistics Unit of the Georges-Francois Leclerc cancer centre using SAS, version 9.4 (SAS Institute Inc., Cary, NC). A p-value <0.05 was considered statistically significant.
3. Results
The detailed characteristics and questionnaire results for the staff and patients are presented in Supplemental Appendix 1–3.
3.1. Characteristics of the study population: staff
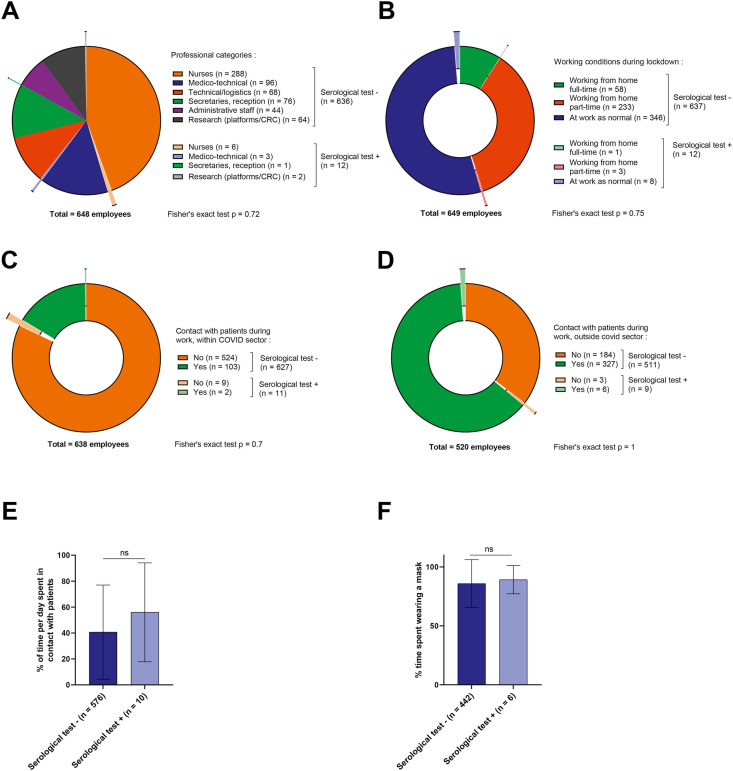
A total of 663 staff members (80.3% of all staff) completed the study questionnaire and had a blood sample for serological testing. The main characteristics of the participating staff members and their serological results are shown in Table 1 and Supplemental Appendix 1. Briefly, there were 522 (78.7%) women and 141 (21.3%) men. Average age was 38.5 (±11.6) years. The professional categories of employees are described in Fig. 1 A and Supplemental Appendix 1.
Table 1.
Employees characteristics (N = 663).
| Characteristics | Positive serology (N = 12) | Negative serology (N = 651) | P value | Test | N (%) (N = 663) |
|---|---|---|---|---|---|
| Sex | 1 | Fisher | |||
| Female | 10 (83.3%) | 512 (78.6%) | 522 (78.7%) | ||
| Male | 2 (16.7%) | 139 (21.4%) | 141 (21.3%) | ||
| Age (years) | 0.3182 | Wilcoxon | |||
| N | 12 | 649 | 661 | ||
| Mean (std) | 35.3 (12.3) | 38.6 (11.6) | 38.5 (11.6) | ||
| Median [min - max] | 33.5 [21.0–55.0] | 37.0 [3.0–81.0] | 37.0 [3.0–81.0] | ||
| Place of residence | 0.5436 | Chi-square | |||
| Dijon | 4 (33.3%) | 273 (42.1%) | 277 (41.9%) | ||
| Outside of Dijon | 8 (66.7%) | 376 (57.9%) | 384 (58.1%) | ||
| Missing values | 0 | 2 | 2 |
Fig. 1.
Working conditions among employees. (A–D) Pie chart for professional categories of employees (A), working conditions during lockdown (B) and contact with patients during work within (C) or outside of (D) the COVID-19 sector. Light (dark) colours represent employees with positive (negative) COVID-19 tests at the time of study. (E) Bar chart showing the proportion of time employees spent in contact with patients per day, according to whether they had a positive (light) or negative (dark) test. ns: Wilcoxon test was not significant. (F) Pie chart showing whether employees wore a mask when in contact with patients. Light (dark) colours represent employees with positive (negative) COVID-19 tests at the time of study. COVID-19, coronavirus disease 2019.
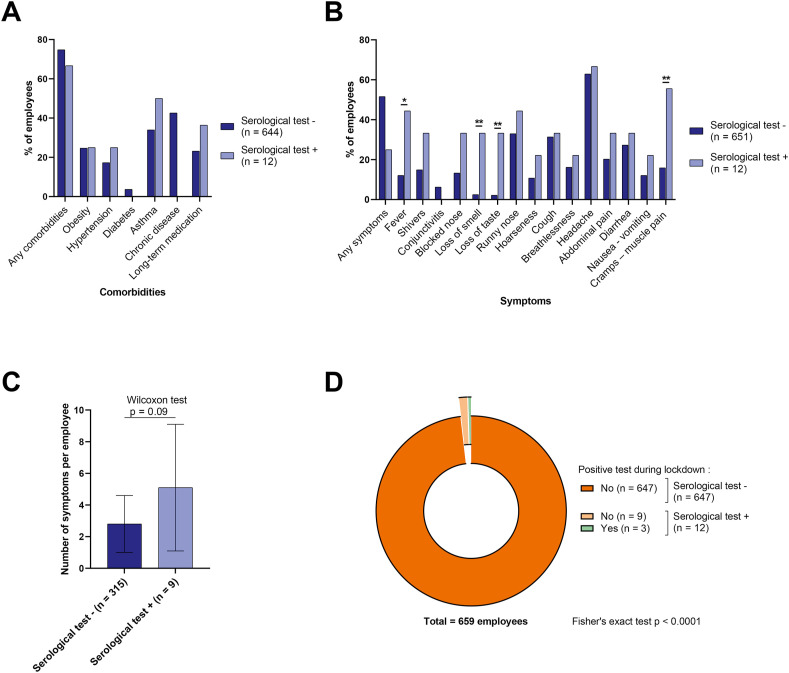
During lockdown, 9.1% of staff worked from home all the time, while 54.5% worked as usual at the cancer care centre and 36.4% worked partially from home (Fig. 1B). In total, 105 employees (15.8%) reported having worked in the dedicated COVID unit during lockdown (Fig. 1C). As regards exposure to risk of SARS-CoV-2 infection, 333 employees (50.2%) reported that they were in contact with patients during their routine work, outside the dedicated COVID unit (Fig. 1D). Among those who reported being in contact with patients during lockdown, 98% said that they wore a mask (on average, 86% of the time, Fig. 1F). Despite the comorbidities recorded among the staff members (Fig. 2 A and Supplemental Appendix 1), no employee had a severe form of COVID-19 infection during lockdown. A total of 324 employees (48.9%) reported at least one symptom (described in Fig. 2B and Supplemental Appendix 1).
Fig. 2.
Employees' living conditions and symptoms. (A–B) Bar chart showing comorbidities (A) and symptoms (B) among staff, according to whether they had positive (light) or negative (dark) tests. ∗Fisher's exact test p < 0.05, ∗∗Fisher's exact test p < 0.01. (C) Bar chart showing the number of symptoms per employee according to whether they had positive (light) or negative (dark) tests. (D) Pie chart for employees with positive tests during lockdown. Light (dark) colours represent employees with positive (negative) COVID-19 tests at the time of study. COVID-19, coronavirus disease 2019.
3.2. Seropositivity rates and factors associated with seropositivity among staff
In total, only 12 of the 663 employees were seropositive, yielding a seroprevalence of 1.8% (Supplemental Appendix 1). None of the seropositive employees had experienced severe COVID-19. Three of them (25%) had been tested positive during lockdown (by nasal swab and RT-PCR testing), and overall, they were the only 3 employees with a positive nasal swab test in the whole cohort of 663 participants (3/663: 0.45%) (Fig. 2D). Among the 12 seropositive employees, 6 were caregivers (3 physicians, 1 resident, 2 nurses), 3 were medico-technical staff (3 technicians from radiology/nuclear medicine), 1 secretary and 2 basic research staff members (Fig. 1A). A majority of them (66.7%) worked on-site as normal during lockdown (Fig. 1B). Only 2 of the seropositive employees had worked in the dedicated COVID unit in the centre (Fig. 1C). Of the 9 seropositive patients who worked in contact with patients during lockdown, all of them (100%) reported having worn a mask systematically when in contact with patients (Fig. 1F).
There was no significant difference between seropositive and seronegative staff members in terms of staff category, working conditions (working from home or not), contact with patients, working in the dedicated COVID-19 unit, proportion of time in contact with patients during an average working day, or the proportion of time spent wearing a mask on an average working day (Fig. 1A–F and Supplemental Appendix 1). There was also no significant difference between seropositive and seronegative staff members in terms of sex, age, comorbidities, smoking status or chronic medication (Fig. 2A and Supplemental Appendix 1).
Regarding symptoms, there was a numerically higher rate of symptoms among seropositive employees (75% vs 48.4%; p = 0.06), but 25% of seropositive staff declared that they had experienced no symptoms. Among the symptoms recorded, only fever (p = 0.019), loss of smell (p = 0.0022), loss of taste (p = 0.0016) and muscle cramps or pains (p = 0.0087) were significantly more frequently present in seropositive staff. The total number of symptoms was also higher in seropositive employees (Fig. 2B and C).
By univariate analysis, none of the variables tested (namely sex, staff category, working in contact with patients, working in COVID unit, working from home, contact with known COVID-positive person) was statistically associated with positive SARS-CoV-2 serology (Supplemental Appendix 2), but given the lower than expected number of events, and the resulting lack of statistical power, no robust conclusions can be drawn from this analysis.
3.3. Characteristics of the study population: patients
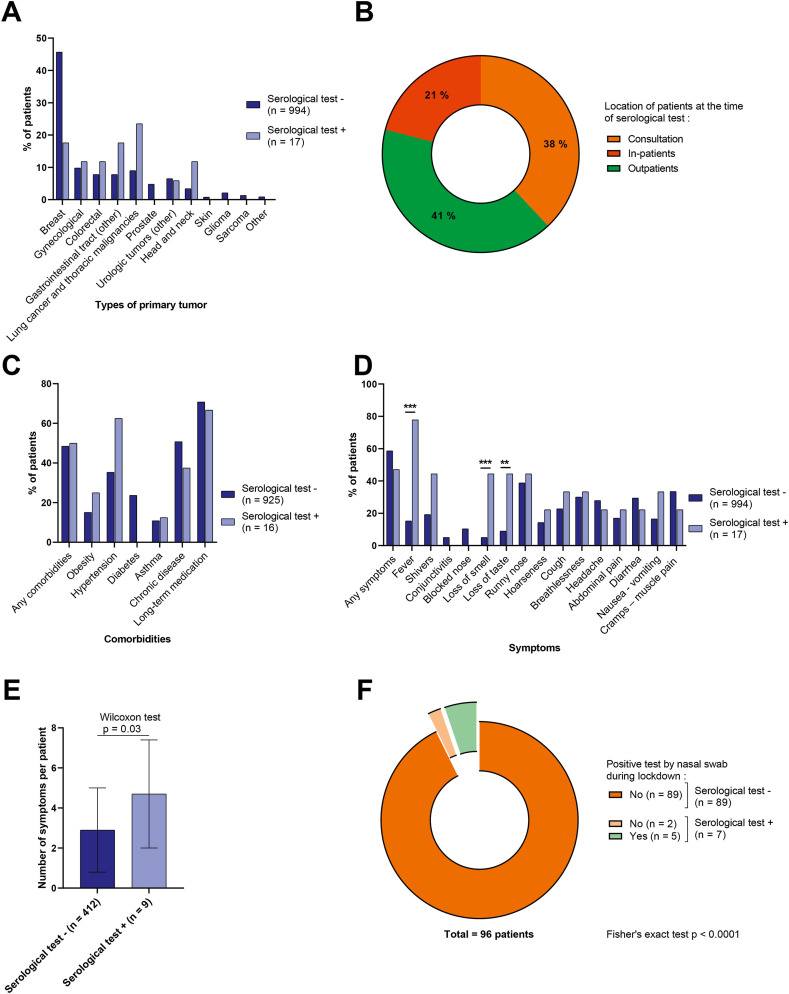
In total, 1011 patients from the Medical Oncology department were included, completed the study questionnaires, and had a blood sample taken for SARS-CoV-2 serological testing. The main characteristics of the patients included in the study are shown in Table 2 (and Supplemental Appendix 3). Regarding the type of cancer, breast cancer was the most frequent (45.2%), followed by digestive, urological and gynaecological tumours (Fig. 3 A). At the time of the blood test, 38% of patients were attending a consultation, 41% were attending the outpatient unit, and 21% were in-patients (Fig. 3B). Most were receiving chemotherapy (alone or in association with targeted therapy, immunotherapy or radiotherapy) (Table 2).
Table 2.
Patients characteristics (N = 1011).
| Characteristic | Positive serology (N = 17) | Negative serology (N = 994) | P value | Test | N (%) (N = 1011) |
|---|---|---|---|---|---|
| Sex | 0.3234 | Chi-square | |||
| Female | 10 (58.8%) | 695 (69.9%) | 705 (69.7%) | ||
| Male | 7 (41.2%) | 299 (30.1%) | 306 (30.3%) | ||
| Age | 0.4192 | Wilcoxon | |||
| N | 17 | 994 | 1011 | ||
| Mean (std) | 65.2 (12.3) | 63.1 (13.1) | 63.1 (13.0) | ||
| Median [min - max] | 68.0 [36.0–81.0] | 65.0 [24.0–95.0] | 65.0 [24.0–95.0] | ||
| Place of residence | 0.095 | Fisher | |||
| Dijon | 4 (25.0%) | 107 (11.0%) | 111 (11.2%) | ||
| Outside of Dijon | 12 (75.0%) | 864 (89.0%) | 876 (88.8%) | ||
| Missing values | 1 | 23 | 24 | ||
| Active anticancer treatment | 1 | Fisher | |||
| No | 2 (11.8%) | 132 (13.3%) | 134 (13.3%) | ||
| Yes | 15 (88.2%) | 860 (86.7%) | 875 (86.7%) | ||
| Missing values | 0 | 2 | 2 | ||
| Metastatic cancer | 0.7413 | Chi-square | |||
| No | 6 (35.3%) | 390 (39.2%) | 396 (39.2%) | ||
| Yes | 11 (64.7%) | 604 (60.8%) | 615 (60.8%) | ||
| Ongoing treatment | 0.4273 | Fisher | |||
| No systemic treatment | 2 (11.8%) | 132 (13.3%) | 134 (13.3%) | ||
| Chemotherapy | 7 (41.2%) | 357 (35.9%) | 364 (36%) | ||
| Targeted therapy | 1 (6%) | 162 (16.3%) | 163 (16%) | ||
| Immunotherapy | 3 (17.5%) | 88 (8.9%) | 91 (9%) | ||
| Endocrine therapy | 0 (0%) | 108 (10.9%) | 108 (10.7%) | ||
| Chemotherapy and targeted therapy | 3 (17.5%) | 97 (9.7%) | 100 (9.9%) | ||
| Chemotherapy and Immunotherapy | 1 (6%) | 31 (3.1%) | 32 (3.2%) | ||
| Radiotherapy | 0 (0%) | 12 (1.2%) | 12 (1.2%) | ||
| Other | 0 (0%) | 7 (0.7%) | 7 (0.7%) |
Fig. 3.
Patients' living conditions and symptoms. (A) Bar chart showing the percentage of patients with positive (light) or negative (dark) tests according to the type of primary tumor. (B) Pie chart for patients' location at the time of blood test. (C–D) Bar chart showing the percentage of comorbidities (C) and symptoms (D) among patients according to whether they had positive (light) or negative (dark) tests. ∗∗Fisher's exact test p < 0.01, ∗∗∗Fisher's exact test p < 0.001. (E) Bar chart showing the number of symptoms per patient. (F) Pie chart for patients with positive tests during confinement. Light (dark) colours represent patients with positive (negative) COVID-19 tests at the time of study. COVID-19, coronavirus disease 2019.
Among the 1,011 patients included, more than half (51.4%) had one or more comorbidities (Fig. 3C and Supplemental Appendix 3). Overall, 421 patients (41.6%) reported having experienced one or more symptoms of SARS-CoV-2 (Fig. 3D and Supplemental Appendix 3). During lockdown, 28 patients (2.8%) reported that they had been in contact with a person known to have tested positive for SARS-CoV-2 by RT-PCR. Overall, 113 patients (11.4%) had RT-PCR testing of a nasal swab during lockdown, among these, 96 reported the results, and only 5 of these were positive (Fig. 3F).
3.4. Seropositivity rates and factors associated with seropositivity among patients
Among the 1,011 patients included, 17 (1.7%) were seropositive at the end of lockdown. Ten patients were women; 7 patients were men, and mean age was 65.2 years. None of these seropositive patients had a severe form of COVID-19, and only 3 of them required hospitalisation.
As for the employees, there was also no significant difference in terms of age, sex, comorbidities, smoking status or chronic (non-cancer) medication (Fig. 3C and Supplemental Appendix 3). Attendance at the cancer care centre during lockdown did not differ between seropositive and seronegative patients (Fig. 4 A), and there was no difference in terms of hospital admissions, consultations, radiotherapy, surgery or number of chemotherapy sessions performed (Supplemental Appendix 3). There was also no difference in the rate of cancellation or postponement of treatments or consultations during lockdown (Fig. 4B and C).
Fig. 4.
Follow-up of patients during lockdown. (A–C) Pie chart for patient visits to the center during lockdown based on whether they had positive (light) or negative (dark) tests (A), cancellation and/or postponement of treatment (B) or of consultations (C) during containment. Light (dark) colours represent patients with positive (negative) COVID-19 tests at the time of study. COVID-19, coronavirus disease 2019.
Regarding symptoms, seropositive patients did not have significantly more symptoms than seronegative patients (p = 0.34), and 47% of seropositive patients said they experienced no symptoms, whereas 41.4% of seronegative patients reported having had symptoms during lockdown (Fig. 3D). Among the symptoms reported, as for employees, fever (p < 0.0001), loss of smell (p = 0.001) and loss of taste (p = 0.0068) were significantly more frequent among seropositive patients, as was the overall number of symptoms reported (p = 0.03) (Fig. 3D and E).
Among the 7 seropositive patients who had undergone RT-PCR testing from a nasal swab during lockdown, 2 of them had received a negative result. Among seronegative patients who had had RT-PCR testing of a nasal swab during lockdown, none had a positive RT-PCR result (Fig. 3F).
By univariate analysis, none of the variables tested (i.e. sex, ongoing anticancer treatment, comorbidity, attendance at the cancer care centre during lockdown, cancellation or postponement of treatment or consultation) was significantly associated with seropositivity (Supplemental Appendix 4). Here again, given the lower than expected number of events, and the resulting lack of statistical power, no robust conclusion can be drawn from this analysis.
3.5. Comparison of characteristics between patients and staff
We compared the main characteristics associated with the risk of SARS-CoV-2 infection, as well as the symptoms presented, between staff and patients. The rate of seropositivity was the same in both groups, even though the patients were significantly older (p < 0.0001), with more men (p < 0.0001) and more frequent comorbidities (p < 0.0001), in particular more frequent hypertension, diabetes or chronic disease (other than cancer) (Supplemental Appendix 5). Patients more often lived alone during lockdown (p < 0.0001) and reported fewer contacts with known SARS-CoV-2–positive individuals (p < 0.0001) compared with staff members. Regarding symptoms, patients reported significantly fewer symptoms, but significantly more loss of taste, breathlessness and muscle pain. Conversely, the employees more frequently reported cough and headache.
The COI for seropositivity did not differ significantly between patients and staff, regardless of whether they had symptoms or not (Supplemental Fig. 1).
4. Discussion
To the best of our knowledge, this is the first prospective study of SARS-CoV-2 seroprevalence including all the employees, as well as medical oncology patients from a large cancer centre at the end of a period of national lockdown. Given the high risk of severe disease among patients with cancer, epidemiological data among patients with cancer and among those who care for them are of utmost importance.
Our study found a low rate of seropositivity for SARS-CoV-2 among both staff (medical and non-medical) and patients in our centre, and we failed to find any factor that was significantly associated with the risk of seropositivity, most likely due to a lack of power, given the lower than expected number of positive cases. Interestingly, this low seropositivity rate (<2% in all those tested) was found in both groups, even though the patients, most of whom were receiving ongoing cancer treatment, were generally older, more often male, with more comorbidities. The low seropositivity rate observed in our patients was probably not biased by uncounted patients who died of COVID-19. Indeed, as described in the Supplemental Appendix, our COVID-19 dedicated hospitalisation sector received all the patients from our cancer centre who were suspected of being infected, and only housed a total of 11 patients (all PCR-positive) during the period from 17 March to 11 May 2020. Six of these patients died of respiratory complications. The low seropositivity observed here is in line with, not to say lower than the projected rates for the general population in France at that time [31], even though serological testing generally reveals more cases than CT (Computed Tomography) scan or PCR testing.
The majority of papers published to date investigating SARS-CoV-2 infection rates in patients with cancer reported overall incidence, the clinical presentation of symptomatic forms, the incidence of severe forms (and risk factors thereof), as well as prognosis in this particularly vulnerable population. Because the clinical signs of mild or asymptomatic forms of COVID-19 are not specific, and similar to those of several other viruses responsible for seasonal respiratory infections, serological testing can help to diagnose subjects who remain asymptomatic at the acute phase, in addition to symptomatic individuals [32]. Mathematical models performed during the first wave estimated that only 2.9% of infected individuals in France were hospitalised [31]. Based on this estimation and on the reported cumulative cases of hospitalised patients with COVID-19 as of June 30th, 2020, we estimate that approximately 6% of the total population of our region (Burgundy-Franche-Comté) would have been infected when we finished our study. These estimates support the posit that seroprevalence was lower than expected in our 2 study populations.
A study performed in Wuhan, China between March and April 2020 reported that by oropharyngeal swab testing for SARS-CoV-2 nucleic acid by RT-PCR and/or serum specific antibody testing (IgM and IgG), the rate of asymptomatic infection was 2.9% in patients with cancer, and 2.1% among their caregivers [33], which is very close to the rates observed in our study. Another seroprevalence study in Wuhan found a seropositivity rate between 3.2 and 3.8%, with higher rates observed in those who attended the hospital more frequently [26].
Regarding the staff, published reports of SARS-CoV-2 seroprevalence mostly stemmed from caregivers directly involved in patient care, and also generally mixed groups of health professionals working in departments with very different clinical activity. Accordingly, published series from Europe from the first wave of the pandemic reported higher seroprevalence rates (at around 5–10%) than observed in our study [[34], [35], [36], [37], [38]], but with high proportions of asymptomatic subjects, as also observed in our cohort. The main factor associated with infection was generally the fact of working in contact with SARS-CoV-2–infected patients in dedicated COVID units [[35], [36], [37],39,40]. The fact that our institution implemented a protective ‘COVID-free’ policy, whereby we did not receive patients with suspected SARS-CoV-2 infection for first-line treatment, likely explains the low spread of infection among the caregiving and non-caregiving staff.
In our study, we did not identify any work-related or out-of-hospital factor that was statistically associated with positivity serology, which could also reflect the wide diversity of ways in which people may be contaminated (be it patients or employees).
We observed a low seropositivity rate in both employees and oncology patients, underlining that when there is a lockdown, and when strict hygiene measures are implemented in the hospital context, the spread of the virus is negligible among patients and among staff working in the hospital environment, even those who are in contact with patients. This suggests that with appropriate protective measures adequately implemented within hospitals welcoming patients with cancer, and by the general population, the delivery of care to patients with cancer may continue as normal, without any apparent excess risk of SARS-CoV-2 infection. Recently, a study of systematic screening for SARS-CoV-2 RNA by nasal swab and RT-PCR testing in patients with cancer at a large tertiary care hospital in Austria that implemented strict measures for hygiene, personal protection and social distancing, reported a very low rate (0.4%) of infection in patients [41]. Interestingly, the same team also reported low seropositivity in a small cohort of patients and health professionals in oncology (3.6% and 3.2%, respectively) [42]. The protective measures put in place were very similar to those implemented in our centre and demonstrate that it is possible to protect patients being treated for (or who were previously treated for) cancer, but also caregiving and non-caregiving staff at the height of the epidemic by means of simple measures, thus making it possible to continue delivering care to patients in need, in acceptable conditions of safety.
Regarding the utility of serological testing, beyond the epidemiological descriptive value, the persistence of antibodies in the long-term is still the subject of some debate, and prospective long-term follow-up of seropositive subjects is warranted to ascertain whether humoural immunity to SARS-CoV-2 remains present. Recent data seem to show lower seroconversion in cancer patients compared to healthcare workers [43], but the data published so far are discordant [44]. In our study, the COI for seropositivity (which does not, however, constitute an exact quantitative determination of antibodies) did not differ significantly between patients and staff, regardless of whether they had symptoms or not, which pleads in favour of a similar humoural response between these 2 populations. Furthermore, the level of neutralising antibody activity required to confer lasting immunity is also unknown. In this context, it is thus impossible to say with certainty whether the presence of antibodies against SARS-CoV-2 (especially at low levels) will protect against re-infection with the virus. Future studies are urgently needed to determine the threshold of antibodies, and the level of neutralising activity that are required to achieve protective immunity.
We confirm in this study that the symptoms experienced in SARS-CoV-2 are not specific, with a high proportion of seropositive subjects having experienced no symptoms, and conversely, numerous seronegative subjects who reported symptoms. This is was more predominant among the patients, in whom cancer may lead to the same respiratory or general signs as SARS-CoV-2 infection. Among the symptoms reported, only loss of taste and smell seem to be specifically associated with seropositivity, both among patients and employees, as has been widely reported among all the populations studied to date [45], including healthcare workers [[36], [37], [38]]. Apart from these two specific signs, our results highlight the difficulty of relying on the presence or absence of various symptoms as a means of identifying subjects who are infected, in a cancer context.
Our study has several strengths compared to other available data in the literature. First, we included more than 80% of all staff in our cancer care centre, including both caregivers and non-caregiving staff, employees who were in contact with patients as well as those who were not, and different levels of presence on site in the centre during national lockdown in France. Secondly, the diagnostic test used in our study is automated, reproducible, sensitive and specific (CV of 3.8% for positive internal control; sensitivity of 100% 14 days post-PCR confirmation; specificity greater than 99.8% with no cross-reaction with Coronavirus HKU1, NL63, 229E or OC43). These performances have been confirmed in numerous studies, including one head-to-head comparison of this test with other available immunoassays [46]. Moreover, the same assay was used for all the subjects included, both among the employees and the patients, which is not generally the case in studies using RT-PCR (because of changes in available tests during the epidemic) and/or CT scan (different machines and interpretation by different radiologists). Furthermore, our study was conducted prospectively in a large cohort of subjects with specific questionnaires developed ad hoc to obtain the same information for all participants. The study procedures (questionnaires and blood tests) were implemented among staff and patients over a short time period immediately after the end of the first lockdown, thus providing an accurate snapshot of the spread of the virus at that particular epidemiological timepoint. Our results thus stem from a more homogeneous patient population than included in previous reports in the literature, and a population that is representative of real-life management in oncology, rather than being limited to in-patients (who generally have more severe disease).
Conversely, our study also has some limitations. First, there was a low number seropositive participants, resulting in low power for the statistical analyses of factors associated with SARS-CoV-2 infection. This underlines the importance of large-scale pooling of serology data on SARS-CoV-2 to broaden our understanding of the epidemiology of this virus in hospital staff and among vulnerable individuals such as those with cancer. Second, the data recorded were self-reported and there may thus be potential for declaration bias. However, many of the variables reported here are not recorded in the patients’ medical files and therefore, a self-report questionnaire was the only way to access the information.
In conclusion, this prospective study shows that despite being geographically located in one of the regions hardest hit by the epidemic in France, the seropositivity rate for SARS-CoV-2 infection at the end of lockdown was very low in our cancer care centre, among both staff and medical oncology patients. The epidemiological data recorded in this study suggest that lockdown and strict application of hygiene measures, personal protection and social distancing were effective in our hospital, which was not a priority destination for patients infected with or suspected of COVID-19. These measures appear to have been effective during the first epidemic wave, and could guide recommendations in case of persistence of the epidemic, to enable for cancer centres to continue delivering care, while protecting patients and employees as much as possible.
Authors’ contributions
Sylvain Ladoire, Vincent Goussot, Emilie Redersdorff, Aurélie Bertaut, François Ghiringhelli performed literature search, study design, data collection, data analysis, data interpretation, and writing the paper
Sylvain Ladoire, Adele Cueff, Elise Ballot, Caroline Truntzer, made the figures
Siavoshe Ayati , Leila Bengrine-Lefevre, Nathalie Bremaud, Bruno Coudert, Isabelle Desmoulins, Laure Favier, Cléa Fraisse, Jean-David Fumet, Roxana Hanu, Audrey Hennequin, Alice Hervieu, Silvia Ilie, Courèche Kaderbhai, Aurélie Lagrange, Nils Martin, Irina Mazilu, Didier Mayeur, Rémi Palmier, Anne-Laure Simonet-Lamm, Julie Vincent, Sylvie Zanetta, performed data collection
Emilie Redersdorff, Charles Coutant, Laurent Arnould, organised the research work and the logistics
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Acknowledgements
Anti-SARS-CoV-2 immunoassays were funded by Roche Diagnostics France, but the company had no role in the study design and writing of the paper.
The authors thank Fiona Ecarnot, PhD (EA3920, University of Franche-Comté, Besançon, France) for translation and editorial assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2021.02.027.
Appendix A. Supplementary data
The following is/are the Supplementary data to this article:
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. Lond. Engl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skums P., Kirpich A., Baykal P.I., Zelikovsky A., Chowell G. Global transmission network of SARS-CoV-2: from outbreak to pandemic. medRxiv. 2020 doi: 10.1101/2020.03.22.20041145. 2020.03.22.20041145. [DOI] [Google Scholar]
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. Lond. Engl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.info coronavirus covid 19 - carte et donnees covid 19 en france. Gouvernement.fr https://www.gouvernement.fr/info-coronavirus/carte-et-donnees.
- 5.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkrief A., Desilets A., Papneja N., Cvetkovic L., Groleau C., Lakehal Y.-A., et al. High mortality among hospital-acquired COVID-19 infection in patients with cancer: a multicentre observational cohort study. Eur J Cancer. 2020;139:181–187. doi: 10.1016/j.ejca.2020.08.017. Oxf. Engl. 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Joode K., Dumoulin D., Tol J., Westgeest H., Beerepoot L., van den Berkmortel F., et al. Dutch Oncology COVID-19 consortium: outcome of COVID-19 in patients with cancer in a nationwide cohort study. Eur J Cancer. 2020;141:171–184. doi: 10.1016/j.ejca.2020.09.027. Oxf. Engl. 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saini K.S., Tagliamento M., Lambertini M., McNally R., Romano M., Leone M., et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43–50. doi: 10.1016/j.ejca.2020.08.011. Oxf. Engl. 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assaad S., Avrillon V., Fournier M.-L., Mastroianni B., Russias B., Swalduz A., et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur J Cancer. 2020;135:251–259. doi: 10.1016/j.ejca.2020.05.028. Oxf. Engl. 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee L.Y., Cazier J.-B., Angelis V., Arnold R., Bisht V., Campton N.-A., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. Lond. Engl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lièvre A., Turpin A., Ray-Coquard I., Le Malicot K., Thariat J., Ahle G., et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19) Eur J Cancer. 2020;141:62–81. doi: 10.1016/j.ejca.2020.09.035. Oxf. Engl. 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Shamsi H.O., Alhazzani W, Alhuraiji A., Coomes E.-A, Chemaly R.-F., Almuhanna M, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncol. 2020;25:e936–e945. doi: 10.1634/theoncologist.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burki T.K. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. 2020;21:629–630. doi: 10.1016/S1470-2045(20)30217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueda M., Martins R., Hendrie P.-C., McDonnell T., Crews J.-R., Wrong T.-L., et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J Natl Compr Cancer Netw. 2020:1–4. doi: 10.6004/jnccn.2020.7560. [DOI] [PubMed] [Google Scholar]
- 20.Moujaess E., Kourie H.R., Ghosn M. Cancer patients and research during COVID-19 pandemic: a systematic review of current evidence. Crit Rev Oncol Hematol. 2020;150:102972. doi: 10.1016/j.critrevonc.2020.102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basse C., Diakite S., Servois V., Frelaut M., Noret A., Bellesoeur A., et al. Characteristics and outcome of SARS-CoV-2 infection in cancer patients. medRxiv. 2020 doi: 10.1101/2020.05.14.20101576. 2020.05.14.20101576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J., Liu X., Zhou D., Qiu G., Dai M., Yang Q., et al. Identification of RT-PCR-negative asymptomatic COVID-19 patients via serological testing. Front Public Health. 2020;8:267. doi: 10.3389/fpubh.2020.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection. Ann Intern Med. 2020 doi: 10.7326/M20-3012. [DOI] [PubMed] [Google Scholar]
- 25.Ooi E.E., Low J.G. Asymptomatic SARS-CoV-2 infection. Lancet Infect Dis. 2020;20:996–998. doi: 10.1016/S1473-3099(20)30460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X., Sun J., Nie S., Li H., Kong Y., Liang M., et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. 2020;26:1193–1195. doi: 10.1038/s41591-020-0949-6. [DOI] [PubMed] [Google Scholar]
- 27.Gudbjartsson D.F., Norddahl G., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020 doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koopmans M., Haagmans B. Assessing the extent of SARS-CoV-2 circulation through serological studies. Nat Med. 2020;26:1171–1172. doi: 10.1038/s41591-020-1018-x. [DOI] [PubMed] [Google Scholar]
- 29.Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 30.Plateforme COVID-19. https://covid-19.sante.gouv.fr/tests.
- 31.Salje H., Tran Kiem C., Lefrancq N., Courtejoie N., Bosetti P., Paireau J., et al. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369:208–211. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X., Fu B., Chen L., Feng Y. Serological tests facilitate identification of asymptomatic SARS-CoV-2 infection in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin P., Zeng R., Duan Y.-R., Zhang Y., Kuang X.-N., Zhang H.-F., et al. An analysis of cancer patients with asymptomatic infection of SARS-CoV-2 in a cancer center in Wuhan, China. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.07.008. Off. J. Eur. Soc. Med. Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Basteiro A.L., Moncunill G., Tortajada M., Vidal M., Guinovart C., Jiménez A., et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11:3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eyre D.W., Lumley S., O’Donnell D., Campbell M., Sims E., Lawson E., et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. eLife. 2020;9 doi: 10.7554/eLife.60675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iversen K., Bundgaard H., Hasselbalch R.-B., Kristensen J.-H., Nielsen P., Pries-Heje M., et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20:1401–1408. doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudberg A.-S., Havervall S., Manberg A., Jernbom Falk A., Aguilera K., Ng H., et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11:5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steensels D., Oris E., Coninx L., Nuyens D., Delforge M.-L., Vermeersch P., et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. J Am Med Assoc. 2020;324:195–197. doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korth J., Wilde B., Dolff S., Anastasiou O.-E., Krawczyk A., Jahn M., et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. doi: 10.1016/j.jcv.2020.104437. Off. Publ. Pan Am. Soc. Clin. Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stubblefield W.B., Talbot H.-K., Feldstein L., Tenforde M.-W., Rasheed M.-A.-U., Mills L., et al. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients - Nashville, Tennessee. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa936. Off. Publ. Infect. Dis. Soc. Am. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berghoff A.S., Gansterer M., Bathke A.-E., Trutschnig W., Hungerlander P., Berger J.M., et al. SARS-CoV-2 testing in patients with cancer treated at a tertiary care hospital during the COVID-19 pandemic. J. Clin. Oncol. 2020 doi: 10.1200/JCO.20.01442. Off. J. Am. Soc. Clin. Oncol. JCO2001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuereder T., Berghoff A.-S., Heller G., Haslacher H., Perkmann T., Strassl R., et al. SARS-CoV-2 seroprevalence in oncology healthcare professionals and patients with cancer at a tertiary care centre during the COVID-19 pandemic. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solodky M.L., Galvez C., Russias B., Detourbet P., N’Guyen-Bonin V., Herr A.-L., et al. Lower detection rates of SARS-COV2 antibodies in cancer patients versus health care workers after symptomatic COVID-19. Ann Oncol. 2020;31:1087–1088. doi: 10.1016/j.annonc.2020.04.475. Off. J. Eur. Soc. Med. Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marra A., Generali D., Zagami P., Cervoni V., Gandini S., Venturini S., et al. Seroconversion in patients with cancer and oncology health care workers infected by SARS-CoV-2. Ann Oncol. 2021;32:113–119. doi: 10.1016/j.annonc.2020.10.473. Off. J. Eur. Soc. Med. Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng X., Deng Y., Dai Z., Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol. 2020;41:102581. doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National SARS-CoV-2 Serology Assay Evaluation Group Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis. 2020;20:1390–1400. doi: 10.1016/S1473-3099(20)30634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.